Abstract
Background
Changes to the method of payment for healthcare providers, including pay‐for‐performance schemes, are increasingly being used by governments, health insurers, and employers to help align financial incentives with health system goals. In this review we focused on changes to the method and level of payment for all types of healthcare providers in outpatient healthcare settings. Outpatient healthcare settings, broadly defined as 'out of hospital' care including primary care, are important for health systems in reducing the use of more expensive hospital services.
Objectives
To assess the impact of different payment methods for healthcare providers working in outpatient healthcare settings on the quantity and quality of health service provision, patient outcomes, healthcare provider outcomes, cost of service provision, and adverse effects.
Search methods
We searched CENTRAL, MEDLINE, Embase (searched 5 March 2019), and several other databases. In addition, we searched clinical trials platforms, grey literature, screened reference lists of included studies, did a cited reference search for included studies, and contacted study authors to identify additional studies. We screened records from an updated search in August 2020, with any potentially relevant studies categorised as awaiting classification.
Selection criteria
Randomised trials, non‐randomised trials, controlled before‐after studies, interrupted time series, and repeated measures studies that compared different payment methods for healthcare providers working in outpatient care settings.
Data collection and analysis
We used standard methodological procedures expected by Cochrane. We conducted a structured synthesis. We first categorised the payment methods comparisons and outcomes, and then described the effects of different types of payment methods on different outcome categories. Where feasible, we used meta‐analysis to synthesise the effects of payment interventions under the same category. Where it was not possible to perform meta‐analysis, we have reported means/medians and full ranges of the available point estimates. We have reported the risk ratio (RR) for dichotomous outcomes and the relative difference (as per cent change or mean difference (MD)) for continuous outcomes.
Main results
We included 27 studies in the review: 12 randomised trials, 13 controlled before‐and‐after studies, one interrupted time series, and one repeated measure study. Most healthcare providers were primary care physicians. Most of the payment methods were implemented by health insurance schemes in high‐income countries, with only one study from a low‐ or middle‐income country. The included studies were categorised into four groups based on comparisons of different payment methods.
(1) Pay for performance (P4P) plus existing payment methods comparedwith existing payment methods for healthcare providers working in outpatient healthcare settings
P4P incentives probably improve child immunisation status (RR 1.27, 95% confidence interval (CI) 1.19 to 1.36; 3760 patients; moderate‐certainty evidence) and may slightly increase the number of patients who are asked more detailed questions on their disease by their pharmacist (MD 1.24, 95% CI 0.93 to 1.54; 454 patients; low‐certainty evidence). P4P may slightly improve primary care physicians' prescribing of guideline‐recommended antihypertensive medicines compared with an existing payment method (RR 1.07, 95% CI 1.02 to 1.12; 362 patients; low‐certainty evidence). We are uncertain about the effects of extra P4P incentives on mean blood pressure reduction for patients and costs for providing services compared with an existing payment method (very low‐certainty evidence). Outcomes related to workload or other health professional outcomes were not reported in the included studies. One randomised trial found that compared to the control group, the performance of incentivised professionals was not sustained after the P4P intervention had ended.
(2) Fee for service (FFS) comparedwith existing payment methods for healthcare providers working in outpatient healthcare settings
We are uncertain about the effect of FFS on the quantity of health services delivered (outpatient visits and hospitalisations), patient health outcomes, and total drugs cost compared to an existing payment method due to very low‐certainty evidence. The quality of service provision and health professional outcomes were not reported in the included studies. One randomised trial reported that physicians paid via FFS may see more well patients than salaried physicians (low‐certainty evidence), possibly implying that more unnecessary services were delivered through FFS.
(3) FFS mixed with existing payment methods compared with existing payment methods for healthcare providers working in outpatient healthcare settings
FFS mixed payment method may increase the quantity of health services provided compared with an existing payment method (RR 1.37, 95% CI 1.07 to 1.76; low‐certainty evidence). We are uncertain about the effect of FFS mixed payment on quality of services provided, patient health outcomes, and health professional outcomes compared with an existing payment method due to very low‐certainty evidence. Cost outcomes and adverse effects were not reported in the included studies.
(4) Enhanced FFS compared with FFS for healthcare providers working in outpatient healthcare settings
Enhanced FFS (higher FFS payment) probably increases child immunisation rates (RR 1.25, 95% CI 1.06 to 1.48; moderate‐certainty evidence). We are uncertain whether higher FFS payment results in more primary care visits and about the effect of enhanced FFS on the net expenditure per year on covered children with regular FFS (very low‐certainty evidence). Quality of service provision, patient outcomes, health professional outcomes, and adverse effects were not reported in the included studies.
Authors' conclusions
For healthcare providers working in outpatient healthcare settings, P4P or an increase in FFS payment level probably increases the quantity of health service provision (moderate‐certainty evidence), and P4P may slightly improve the quality of service provision for targeted conditions (low‐certainty evidence). The effects of changes in payment methods on health outcomes is uncertain due to very low‐certainty evidence. Information to explore the influence of specific payment method design features, such as the size of incentives and type of performance measures, was insufficient. Furthermore, due to limited and very low‐certainty evidence, it is uncertain if changing payment models without including additional funding for professionals would have similar effects.
There is a need for further well‐conducted research on payment methods for healthcare providers working in outpatient healthcare settings in low‐ and middle‐income countries; more studies comparing the impacts of different designs of the same payment method; and studies that consider the unintended consequences of payment interventions.
Plain language summary
Payment methods for healthcare providers in outpatient healthcare settings
What is the aim of this review?
The aim of this Cochrane Review was to assess the effect of different payment methods for healthcare providers working in outpatient healthcare settings. The review authors collected and analysed all relevant studies to answer this question and found 27 studies.
Key messages
This review suggests that different payment methods can affect healthcare provider behaviour in both positive and negative ways. For instance, whilst healthcare providers may be encouraged to provide more of specific services, they may also be encouraged to provide unnecessary services. Considerable gaps remain in the understanding of how payment of healthcare providers affects healthcare services, healthcare providers’ work morale and workload, and patient health.
What was studied in the review?
Healthcare providers may be paid in different ways. Different payment methods can encourage healthcare providers to give patients the treatment they need in the best and most cost‐efficient way, but they can also encourage healthcare providers to offer poor‐quality, expensive, and unnecessary care, and to avoid certain treatments or certain types of patients. Different payment methods can also influence healthcare providers’ work morale and workload. And they can cost more or less for the healthcare system.
The review authors searched for studies on the effects of different payment methods for healthcare providers working in outpatient care. Outpatient care is where patients get health care from healthcare providers outside of hospitals and where there is no need for a bed. Healthcare centres, family planning centres, and dental clinics are all examples of outpatient facilities.
The payment methods the review authors were interested in were as follows.
‐ Pay‐for‐performance: healthcare providers are paid for carrying out certain tasks or reaching certain targets.
‐ Fee‐for‐service: healthcare providers are paid for each service they provide to the patient.
‐ Salary: healthcare providers are paid based on the time they spend at work.
‐ Capitation: healthcare providers are paid according to how many patients they have.
‐ A mix of these different approaches.
What are the main results of the review?
The review authors found 27 relevant studies. Most of the studies looked at primary healthcare doctors in high‐income countries.
When pay‐for‐performance plus other payment methods (including capitation, salary, and fee‐for‐service) is compared to other payment methods: healthcare providers probably provide more of certain services, including immunisations. They may also provide better‐quality care, including how some medicines are used, but these improvements may be reduced when the pay‐for‐performance payments end. Effects on patient health may be mixed. We are uncertain about the effect on healthcare providers’ work morale or workload, or on cost, because the evidence is missing or of very low certainty.
When fee‐for‐service methods are compared to other payment methods (such as capitation or salary): healthcare providers paid by fee‐for‐service may provide more unnecessary services than those paid by salary. We are uncertain about the effect on the quality or quantity of care, patient health, healthcare providers’ work morale or workload, or cost because the evidence is missing or of very low certainty.
When fee‐for‐service mixed with other payment methods (including fee‐for‐service plus capitation and fee‐for‐service plus salary) are compared to other payment methods: healthcare providers may provide more of specific services. We are uncertain about the effect on the quality of care, patient health, healthcare providers’ work morale or workload, cost, or unintended effects because the evidence is missing or of very low certainty.
When fee‐for‐service methods using a higher fee are compared to fee‐for‐service methods using a lower fee: healthcare providers probably provide more of certain services, including immunisations. We are uncertain if the higher fee has an impact on cost because the evidence is of very low certainty. We are uncertain about the effect on the quality of care, patient health, healthcare providers’ work morale or workload, or unintended effects because the evidence is missing.
How up‐to‐date is this review?
The review authors searched for studies that had been published up to 5 March 2019.
Summary of findings
Summary of findings 1. Pay for performance (P4P) plus existing payment methods compared with existing payment methods for outpatient healthcare providers.
| P4P plus existing payment methods compared with existing payment methods for outpatient healthcare providers | ||||||
|
Patient or population: healthcare providers working in outpatient healthcare settings Settings: Australia, Canada, India, Taiwan, the USA Intervention: P4P plus existing payment methods Comparison: existing payment methods (including capitation, FFS, and salary) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Results in words | No. of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with existing payment methods | Risk with P4P plus existing payment methods | |||||
| Quantity of service provision (dichotomous) ‐ child immunisation status up‐to‐date | 433 up‐to‐date per 1000 children | 550 up‐to‐date per 1000 children (515 to 588 children) | RR 1.27 (1.19 to 1.36) | P4P added to existing payment methods probably increases the number of children with up‐to‐date immunisation status, compared with an existing payment method. | 3760 children (2 RCTs) | ⊕⊕⊕⊝ MODERATE1 |
| Quantity of service provision (continuous) ‐ immunisation rates for patients aged 65 years or older | The mean immunisation rate with P4P plus existing payment methods was 0.34 per cent points higher (0.2 per cent points lower to 0.87 points higher) compared to existing payment methods. | MD 0.34 (−0.20 to 0.87) | We are uncertain of the effect of P4P added to existing payment methods on immunisation rates for the elderly. | 54 primary care practices (1 RCT) |
⊕⊝⊝⊝ VERY LOW2 | |
| Quantity of service provision (continuous) ‐ number of detailed disease‐related consultation services per 100 prescriptions | The mean number of consultation services with P4P plus existing payment methods was 1.24 per cent higher per 100 prescriptions (0.93 per cent points higher to 1.54 points higher) compared to existing payment methods. | MD 1.24 (0.93 to 1.54) | P4P added to existing payment methods may slightly increase the number of patients who are asked more detailed questions on their disease by their pharmacist, compared with an existing payment method. | 200 community pharmacies (1 RCT) | ⊕⊕⊝⊝ LOW3 | |
| Quality of service provision ‐ physician prescribing practices | Insufficient data to calculate | RR 1.07 (1.02 to 1.12) | P4P added to existing payment methods may slightly improve providers' prescribing of guideline‐recommended antihypertensive medicines compared with an existing payment method. | 362 people (1 RCT) |
⊕⊕⊝⊝ LOW4 | |
| Patient outcomes ‐ reduction in blood pressure | The mean blood pressure reduction with P4P plus existing payment methods was 0.07 mmHG greater (2.22 less to 2.37 greater) compared to existing payment methods. | MD 0.07 (ranged from −2.22 to 2.37) | We are uncertain of the effect of P4P added to existing payment methods on mean blood pressure reduction compared with an existing payment method. | 181 people (1 RCT) |
⊕⊝⊝⊝ VERY LOW5 |
|
| Quality of service provision and patient outcomes ‐ blood pressure management | Insufficient data to calculate | RR 1.13 (1.04 to 1.23) | P4P added to existing payment methods may improve blood pressure control or appropriate responses to patients with uncontrolled blood pressure, compared with an existing payment method. | 362 people (1 RCT) | ⊕⊕⊝⊝ LOW6 | |
| Healthcare provider outcomes (such as work morale or workload) | None of the included studies reported on healthcare provider outcomes. | ‐ | ‐ | |||
| Costs | We are uncertain of the costs of adding P4P to existing payment methods on expenditures for diabetes‐related services due to very low‐certainty evidence. | 1 CBA | ⊕⊝⊝⊝ VERY LOW7 |
|||
| Unintended or adverse effects | Insufficient data to calculate | RR 0.77 (0.71 to 0.82) | When the P4P intervention ended, there was an important reduction in performance (blood pressure control or appropriate responses to uncontrolled blood pressure) in the intervention group compared with the existing payment method. | 362 people (1 RCT) | ⊕⊕⊝⊝ LOW8 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CBA: controlled before‐after study; FFS: fee‐for‐service; MD: mean difference; P4P: pay for performance; RCT: randomised controlled trial; RR: risk ratio | ||||||
|
GRADE Working Group grades of evidence
High certainty: This research provides a very good indication of the likely effect. The likelihood that the effect will be substantially different** is low.
Moderate certainty: This research provides a good indication of the likely effect. The likelihood that the effect will be substantially different** is moderate.
Low certainty: This research provides some indication of the likely effect. However, the likelihood that it will be substantially different** is high.
Very low certainty: This research does not provide a reliable indication of the likely effect. The likelihood that the effect will be substantially different** is very high. ** Substantially different = a large enough difference that it might affect a decision | ||||||
1We rated two RCTs as unclear risk of bias (Fairbrother 1999; Fairbrother 2001), downgrading the certainty of the evidence one level because of limitation in study design.
2We rated one RCT as unclear risk of bias (Kouides 1998), downgrading the certainty of the evidence one level for limitation in study design, one level for indirectness (only one study targeting primary care physicians in the USA), and one level for imprecision (limited number of participants, and 95% CI overlaps no effect).
3We rated one RCT as unclear risk of bias (Christensen 2000), downgrading the certainty of the evidence one level for limitation in study design and one level for indirectness (only one study targeting community pharmacies in the USA).
4We rated one RCT as unclear risk of bias (Petersen 2013), downgrading the certainty of the evidence one level for limitation in study design and one level for indirectness (only one study targeting primary care physicians in the USA).
5We rated one RCT as unclear risk of bias (Houle 2016), downgrading the certainty of the evidence one level for limitation in study design, one level for indirectness (only one study targeting pharmacists in pharmacy practice in Canada), and one level for imprecision (study ended prior to enrolment of the full sample size of participants, and 95% CI overlaps no effect).
6We rated one RCT as unclear risk of bias (Petersen 2013), downgrading the certainty of the evidence one level for limitation in study design and one level for indirectness (only one study targeting primary care physicians in the USA).
7We rated one CBA as high risk of bias (Lee 2010). Initial rating of low certainty assigned due to non‐randomised study design and downgraded one level for further limitations in study design and one level for indirectness (only one study targeting pharmacists in community clinics physicians in Taiwan).
8We rated one RCT as unclear risk of bias (Petersen 2013), downgrading the certainty of the evidence one level for limitation in study design and one level for indirectness (only one study targeting primary care physicians in the USA).
Summary of findings 2. Fee‐for‐service (FFS) compared with existing payment methods for outpatient healthcare providers.
| FFS compared with existing payment methods for outpatient healthcare providers | ||||
|
Patient or population: healthcare providers working in outpatient healthcare settings Settings: the USA Intervention: FFS Comparison: existing payment method (input‐based payment ‐ capitation or salary) | ||||
| Outcomes | Impact | No. of participants (studies) | Certainty of the evidence (GRADE) | |
| Relative effect (range) | Results in words | |||
| Quantity of service provision ‐ number of outpatients visits, specialist visits, or hospitalisations | Median change = 10.44% (range: −460% to +175.65%) | We are uncertain of the effect of FFS payments on the number of patient visits to health facilities, compared with input‐based payment methods, due to very low‐certainty evidence. | 3 RCTs | ⊕⊝⊝⊝ VERY LOW1 |
| Quality of service provision | None of the included studies reported on the quality of services provided. | ‐ | ‐ | |
| Patient outcomes ‐ people with mental illness | Median change = −9.84% (range: −492% to +350%) | We are uncertain of the effect of FFS on patient outcomes for people with mental illness, compared with an input‐based payment method, due to very low‐certainty evidence. | 1 RCT | ⊕⊝⊝⊝ VERY LOW2 |
| Healthcare provider outcomes (such as work morale or workload) | None of the included studies reported on healthcare provider outcomes. | ‐ | ‐ | |
| Costs | We are uncertain of the effect of FFS on costs compared with an input‐based payment method (capitation) due to very low‐certainty evidence. | 2 CBAs | ⊕⊝⊝⊝ VERY LOW3 |
|
| Unintended or adverse effects | Physicians who receive FFS payments may see more well patients (potentially an indicator of unnecessary service provision) compared with physicians paid by salary. | 1 RCT | ⊕⊕⊝⊝ LOW4 | |
| CBA: controlled before‐after study; FFS: fee‐for‐service; RCT: randomised controlled trial | ||||
|
GRADE Working Group grades of evidence High certainty: This research provides a very good indication of the likely effect. The likelihood that the effect will be substantially different** is low. Moderate certainty: This research provides a good indication of the likely effect. The likelihood that the effect will be substantially different** is moderate. Low certainty: This research provides some indication of the likely effect. However, the likelihood that it will be substantially different** is high. Very low certainty: This research does not provide a reliable indication of the likely effect. The likelihood that the effect will be substantially different** is very high. ** Substantially different = a large enough difference that it might affect a decision | ||||
1We rated two RCTs as unclear risk of bias, Hickson 1987; Lurie 1992, and one RCT as high risk of bias (Davidson 1992), downgrading the certainty of the evidence two levels for limitation in study design and one level for imprecision.
2We rated one RCT as high risk of bias (Lurie 1992), downgrading the certainty of the evidence one level for limitation in study design, one level for indirectness (only one study targeting primary care physician for mental health care in the USA), and one level for imprecision.
3We rated two CBAs as unclear risk of bias (Yesalis 1980; Yesalis 1984. Initial rating of low certainty assigned due to non‐randomised study design and downgraded one level for further limitations in study design.
4We rated one RCT as unclear risk of bias (Hickson 1987), downgrading the certainty of the evidence one level for limitation in study design and one level for indirectness (only one study targeting paediatric residents in the USA).
Summary of findings 3. Fee‐for‐service (FFS) mixed with existing payment methods compared with existing payment method for outpatient healthcare providers.
| FFS mixed with existing payment methods compared with existing payment method for outpatient healthcare providers | ||||||
|
Patient or population: healthcare providers working in outpatient healthcare settings Settings: Australia, Canada, Denmark, Germany, the UK Intervention: FFS mixed with other payment methods Comparison: existing payment method (single payment method, including salary, capitation, and FFS) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Impact | No. of participants (studies) | Certainty of the evidence (GRADE) | ||
| Risk with existing payment | Risk with FFS mixed with other payment methods | Relative effect (95% CI) | Results in words | |||
| Quantity of service provision ‐ proportion of women/children receiving treatment | Insufficient data to calculate | RR 1.37 (1.07 to 1.76) | FFS mixed with other payment methods may increase the quantity of health services provided, compared with an existing payment method. | 2 RCTs | ⊕⊕⊝⊝ LOW1 | |
| Quality of service provision | We are uncertain of the effect of FFS mixed with other payment methods on the quality of service provided compared with an existing payment method due to very low‐certainty evidence. | 1 CBA | ⊕⊝⊝⊝ VERY LOW2 |
|||
| Patient outcomes ‐ satisfaction with care | We are uncertain of the effect of FFS mixed with other payment methods on patient outcomes compared with an existing payment method due to very low‐certainty evidence. | 1 RCT | ⊕⊝⊝⊝ VERY LOW3 |
|||
| Healthcare provider outcomes ‐ working hours and income | We are uncertain of the effect of FFS mixed with other payment methods on healthcare provider outcomes compared with an existing payment method due to very low‐certainty evidence. | 2 CBAs | ⊕⊝⊝⊝ VERY LOW4 |
|||
| Costs | None of the included studies reported on costs. | ‐ | ‐ | |||
| Unintended or adverse effects | None of the included studies reported on adverse effects. | ‐ | ‐ | |||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CBA: controlled before‐after study; FFS: fee‐for‐service; RCT: randomised controlled trial; RR: risk ratio | ||||||
|
GRADE Working Group grades of evidence High certainty: This research provides a very good indication of the likely effect. The likelihood that the effect will be substantially different** is low. Moderate certainty: This research provides a good indication of the likely effect. The likelihood that the effect will be substantially different** is moderate. Low certainty: This research provides some indication of the likely effect. However, the likelihood that it will be substantially different** is high. Very low certainty: This research does not provide a reliable indication of the likely effect. The likelihood that the effect will be substantially different** is very high. ** Substantially different = a large enough difference that it might affect a decision | ||||||
1We rated two RCTs as unclear risk of bias (Bilardi 2010; Clarkson 2008), downgrading the certainty of the evidence one level for limitations in study design and one level for imprecision due to very small numbers of participants in both studies.
2We rated one CBA as high risk of bias (Gosden 2003). Initial rating of low certainty assigned due to non‐randomised study design and downgraded one level for further limitations in study design and one level for indirectness (only one study targeting general practitioners in England).
3We rated one RCT as high risk of bias (Twardella 2007), downgrading the certainty of the evidence two levels for limitation in study design and one level for indirectness (only one study targeting general practitioners in Germany).
4We rated two CBAs as high risk of bias (Gosden 2003; Gray 2015. Initial rating of low certainty assigned due to non‐randomised study design and downgraded one level for further limitations in study design.
Summary of findings 4. Enhanced fee‐for‐service (FFS) compared with FFS for outpatient healthcare providers.
| Enhanced FFS compared with FFS for outpatient healthcare providers | ||||||
|
Patient or population: healthcare providers working in outpatient healthcare settings Settings: Germany, the USA Intervention: enhanced FFS with higher unit payment levels Comparison: FFS with normal unit payment levels | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Results in words | No. of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with FFS | Risk with Enhanced FFS | |||||
| Quantity of service provision ‐ child immunisation status up‐to‐date | 433 up‐to‐date per 1000 children | 541 up‐to‐date per 1000 children (459 to 640 children) | RR 1.25 (1.06 to 1.48) | Paying higher fees to healthcare providers for immunisations delivered probably increases the proportion of children aged 3 to 35 months whose immunisation is up‐to‐date, compared with the normal level of fees. | 2 RCTs | ⊕⊕⊕⊝ MODERATE1 |
| Quantity of service provision ‐ primary healthcare visits by children | We are uncertain of the effect of paying higher fees for service on the number of primary care visits by children compared with the normal level of fees due to very low‐certainty evidence. | 1 RCT | ⊕⊝⊝⊝ VERY LOW2 |
|||
| Quality of service provision | None of the included studies reported on quality of service provision. | ‐ | ‐ | |||
| Patient outcomes | None of the included studies reported on patient outcomes. | ‐ | ‐ | |||
| Healthcare provider outcomes (such as work morale or workload) | None of the included studies reported on healthcare provider outcomes. | ‐ | ‐ | |||
| Costs | We are uncertain if paying higher fees to healthcare providers for immunisations delivered influences the net expenditure per year on eligible children compared with the normal level of fees because the certainty of the evidence is very low. | 1 RCT | ⊕⊝⊝⊝ VERY LOW3 |
|||
| Unintended or adverse effects | None of the included studies reported on adverse effects. | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). FFS: fee‐for‐service; RCT: randomised controlled trial; RR: risk ratio | ||||||
|
GRADE Working Group grades of evidence High certainty: This research provides a very good indication of the likely effect. The likelihood that the effect will be substantially different** is low. Moderate certainty: This research provides a good indication of the likely effect. The likelihood that the effect will be substantially different** is moderate. Low certainty: This research provides some indication of the likely effect. However, the likelihood that it will be substantially different** is high. Very low certainty: This research does not provide a reliable indication of the likely effect. The likelihood that the effect will be substantially different** is very high. ** Substantially different = a large enough difference that it might affect a decision | ||||||
1We rated two RCTs as unclear risk of bias (Fairbrother 1999; Fairbrother 2001), downgrading the certainty of the evidence one level for limitation in study design.
2We rated one RCT as high risk of bias (Davidson 1992), downgrading the certainty of the evidence two levels for limitation in study design and one level for indirectness (only one study targeting primary care physicians in Suffolk County, New York).
3We rated one RCT as high risk of bias (Davidson 1992), downgrading the certainty of the evidence two levels for limitation in study design and one level for indirectness (only one study targeting primary care physicians in Suffolk County, New York).
Background
Description of the condition
Health care is a labour‐intensive service industry, so healthcare provision is directly influenced by healthcare providers' behaviours in delivering services. Examining optimal payment methods for healthcare providers is a key issue in ensuring care is delivered in a cost‐effective way to patients. Previous Cochrane Reviews have focused largely on physician payment methods (Flodgren 2011; Giuffrida 1999; Gosden 2000; Scott 2011). This review focused on payment methods for all healthcare providers in stand‐alone outpatient healthcare settings. Outpatient healthcare settings can vary considerably across countries, and are defined in this review as settings outside of inpatient hospitals for care where a bed is not required. They could include the private or public offices or clinics of primary care physicians and other specialists and healthcare providers. These may be stand‐alone buildings or could be adjacent to hospitals. Examples are care provided in stand‐alone community healthcare centres, clinics, urgent care centres, family planning centres, dental clinics, and allied health care (e.g. physiotherapy), but would exclude surgical day‐only procedures and emergency departments. The defining characteristics of outpatient care are the inclusion of services that involve a consultation with patients by healthcare providers, where they provide advice, prescriptions, immunisations, simple diagnostic tests, and some simple minor procedures and treatments that can be given in an office setting. Outpatient care may be the first point of contact for patients, or patients may be referred by other healthcare providers to outpatient care for more specialised care. Outpatient can play an important role in gatekeeping and rationing access to more expensive hospital care, and so can help reduce health expenditures.
Description of the intervention
Individual healthcare providers' behaviours directly influence the type, quantity, quality, and access to health services provided to patients. Several studies have summarised the determinants of healthcare providers' behaviours in health services delivery (Rowe 2005; WHO 2006). The method by which healthcare providers are remunerated (the payment method) has been frequently used by governments, health insurers, and employers to influence the behaviours of healthcare providers to meet specific objectives (Fairbrother 1999; Langenbrunner 2004). Payment methods refer to the way in which funds are received by a healthcare provider and used a personal income. The focus is on payment methods that influence health professional's personal income, rather than funding models aimed at healthcare organisations, although the two may interact. The most commonly used payment systems to remunerate healthcare providers are salary, capitation, fee‐for‐service, pay for performance, and mixed or blended systems of payment.
Salary: healthcare providers are paid based on the time spent at work. Salaried healthcare providers are usually employees of healthcare organisations, with these organisations paid using other methods such as global budget, FFS, and capitation.
Capitation: healthcare providers are paid a prospective fixed payment per period of time for each individual enrolled with the health professional.
Fee for service (FFS): healthcare providers are reimbursed based on each specific service, procedure, or visit provided, such as consultations, x‐ray tests, or surgical operations.
Pay for performance (P4P): the payment is directly linked to the achievement of specific behaviours defined in terms of the performance of healthcare providers. P4P can be used to pay individuals, groups of people, or organisations by government or insurers. Pay‐for‐performance schemes vary widely in terms of the types of performance that are targeted, how performance is measured, when payments for performance are paid, and the proportion of total reimbursements that is paid for performance (Witter 2012).
Blended or mixed payment: more than one of the above payment methods may be used at the same time, with a proportion of the health professional's income coming from each type of method.
How the intervention might work
The mechanism through which payment methods influence behaviour is through their effect on the income of healthcare providers. Earning income is a key driver of individual's consumption and leisure activities, and economic theory assumes that individuals are motivated only by monetary extrinsic rewards. Generally and in practice, extensions of this theory recognise that payment methods may have less of an impact if there are other sources of motivation from working, such as improving the health of patients. Payment to healthcare providers happens when funds are transferred from employers, patients, or insurers to individual healthcare providers in exchange for the provision of healthcare services. Each payment method may be part of a formal contract or agreement between the payor and payee, which may also specify working conditions, other in‐kind benefits, and detail about what is required in providing the services. Under each payment method, the expected total income must first be sufficient to encourage the health professional to work and enter into a contract. Payment methods in these contracts can be changed, or healthcare providers can move to a different job (contract) with a different payment method, raising the issue of selection bias when examining the effects of changes to payment methods.
Assuming income is sufficiently important to healthcare providers, the payment method will influence behaviour depending on the change to the unit of payment or a change to the level of payment for each unit. The unit of payment for those on salaries and who receive an hourly wage is hours worked, and income can be increased only by working more hours. The unit of payment in capitation payment is the number of enrolled patients, and so income can only be increased by increasing the number of enrolled patients. For those on FFS, income can rise only by increasing the number of services provided to existing or new patients. For those paid by P4P, income can only be increased by improvements in performance. In blended payment, a number of different units of payment are used, and so a range of behaviours can increase income.
Moving from one payment method to another, or changing the mix of payment methods in blended payment, will depend on the change to the unit of payment. With four different payment methods, moving from one to another defines many different combinations of comparators and interventions, each of which may have different effects on behaviour depending on the comparator and intervention units of payment. Under a new unit of payment, healthcare providers are assumed to adjust their work behaviours in ways that maximise their utility (including utility from the consumption of goods and services derived from their income, and utility from non‐work activities). A new payment method will provide the health professional with a different way to increase their income (and utility) depending on the unit of payment. healthcare providers may also reduce the costs of providing care to maximise their income, such as changes in skill mix, how they use their time (e.g. changes to consultation length), changes in effort, and changes to other practice costs (e.g. use of administrative staff). A change in payment methods could also lead healthcare providers to change jobs, which can influence patient access to care. The main hypotheses regarding the expected changes in behaviour from each payment method are outlined below, although the precise hypotheses will also depend on the existing (comparator) payment method, as well as the new payment method.
Salaried payment
Salaried payment focuses on the amount of time at work, usually in terms of hours. For a given number of hours worked, salaries are fixed in the short run, and do not provide any financial incentives for increased effort or increased quality of care or performance. In the longer run, financial incentives can encourage effort beyond some minimum to avoid being sacked and can also provide financial incentives to meet some level of performance to keep the job if on fixed‐term contract. In the longer run, salaried payment is also accompanied by different levels of salary (wages) for different levels of seniority (salary increments and promotion). Financial incentives for increased performance can be built into the salary scale (increments and promotion), driven by additional income from advancement up the salary scale. Progression up the salary scale may be automatic depending only on years of service, for example, or it may be based on a subjective measure of performance assessed in an annual performance review. This provides 'career' financial incentives to improve performance to obtain promotion and advancement.
Capitation
Payments are made per patient, and so capitation is normally accompanied by a system with patients registering/enrolling with a specific provider to receive care. Capitation is a prospective‐based payment system where healthcare providers can predict their income from each enrolled patient in advance. Capitation payment encourages healthcare providers to attract more patients, and so compete with other healthcare providers, such as by improving quality and access to care and those aspects of care that are valued by patients. With a fixed payment for each patient, the difference between the costs of treatment and the fixed payment defines the profit or income going to the health professional. More complex patients lead to higher costs and less profit. Capitation therefore includes an inbuilt incentive to minimise costs by selecting only the healthiest patients to enrol and treat, which can have adverse consequences for access to health care for those with more complex and costly conditions. Capitation may provide incentives to reduce costs by changing skill mix or providing short consultations that could be related to increased prescribing, fewer treatments provided, and more referrals compared to FFS and P4P. Though cost‐consciousness is important, it can also lead to lower quality and poor access if these are not separately monitored and rewarded (e.g. in a blended payment scheme that combines capitation and P4P). Capitation payments are usually 'risk‐adjusted', where higher payments are provided for patients with more complex/costly conditions. This reduces the incentives for patient selection (or 'cream skimming') and cutting costs that might reduce quality. It is also argued that capitation payment may provide incentives for healthcare providers to provide more preventive activities to patients so that they do not return in the future. This reduces future costs and increases provider income in the longer term, as patients make fewer visits.
Fee for service
This is likely to increase the number of services provided by healthcare providers compared to other payment methods, either through providing more services to each patient or by attracting more patients. FFS could lead to increased and less predictable health expenditures compared to other forms of payment depending on how fees are determined, as well as the overprovision of unnecessary services, including overdiagnosis. FFS also provides incentives for healthcare providers to provide treatment themselves rather than refer to others, especially compared with salaried or capitation payment.
Pay for performance
P4P payment is directly linked to performance targets that are often related to the type, mix, and quality of care delivered. P4P designs are complex and vary depending on how performance is measured, the level of the performance target, and many other components. Performance is usually defined in terms of the quality of care provided, which might include whether certain activities were performed or not (e.g. taking blood pressure) or the outcome of that activity (e.g. whether the measure of blood pressure is within accepted clinical guidelines). Payment may be made for each extra activity (e.g. each patient who immunised), or as a lump‐sum bonus for achieving a prespecified target. Financial penalties may also be used if targets are not met. The performance target for payment may be absolute (e.g. 80% of a target population being immunised) or relative to other healthcare providers (e.g. whether the provider is in the top quartile of immunisation rates across all providers), or based on the absolute (or relative) change in performance from one period to the next (Ogundeji 2016; Van Herck 2010). In practice, P4P rarely exists on its own, and is usually part of a blended payment method.
Blended payments
Blended payments reduce the 'extremes' of single payment methods by providing a range of methods through which providers can increase their income, and can combine incentives to be cost‐conscious as well as maintain and improve quality (value‐based payment).
In addition, within each payment method the level of payment for each unit of payment (hours, patients, services, quality) may be decreased or increased. This may encourage more of the rewarded activity if the additional (marginal) income is greater than the additional (marginal) costs of increasing the activity, and also assuming that income is sufficiently important to the health professional compared to other sources of utility. The importance of income (monetary motivation) will likely vary across different types of healthcare providers leading to heterogenous effects of changes to payment methods.
Why it is important to do this review
Payment methods can change the behaviours of healthcare providers through financial incentives, and then influence number, mix, cost, and quality of care provided. The design of appropriate payment systems is a key issue for governments, health insurers, health organisation managers, and all relevant policymakers who expect the efficient use of limited funds in the health system.
This review focused on payment methods for individual healthcare providers. Other Cochrane Reviews examine payment methods for doctors across all types of setting (Flodgren 2011; Giuffrida 1999; Gosden 2000; Scott 2011), whilst this review includes all kinds of healthcare providers only in outpatient healthcare settings. Together with the Cochrane Reviews on payment methods for outpatient facilities (rather than individual healthcare providers) (Yuan 2017) and hospitals (Mathes 2014; Mathes 2019), this review contributes to evidence to encourage healthcare providers to provide high‐quality, efficient, and equitable health care to their patients.
Objectives
To assess the impact of different payment methods for healthcare providers working in outpatient healthcare settings on quantity and quality of health service provision, patient outcomes, healthcare provider outcomes, cost of service provision, and adverse effects.
Methods
Criteria for considering studies for this review
Types of studies
Randomised trials, including cluster‐randomised trials
Non‐randomised trials
-
Interrupted time series (ITS) and repeated measures studies with:
a clearly defined point in time when the intervention occurred;
at least three data points before and three data points after the intervention.
-
Controlled before‐after (CBA) studies with:
contemporaneous data collection;
a minimum of two intervention and two control sites.
Types of participants
Healthcare providers working in outpatient care facilities. Healthcare providers include, for example, primary care physicians and other non‐surgical specialists, dentists, midwives, nurses, or allied health (WHO 2006). Healthcare providers working in surgical day‐only procedures and emergency departments were excluded.
Types of interventions
The payment method is defined as the mechanism used to remunerate individual healthcare providers. Payment methods for healthcare providers include:
salary;
fee‐for‐service (FFS): healthcare providers are reimbursed based on specific items provided;
capitation: healthcare providers are paid a predetermined fixed rate in advance to provide a defined set of services for each enrolled individual for a fixed period;
pay for performance (P4P): the payment is directly linked to the performance of healthcare providers;
blended payments.
We included studies that evaluated changes from one type of payment method to another, changes to the design of a payment method, or changes to the level of payment. Any of these may change the level of provider income, or create different opportunities for the health professional to increase their income and change the care they provide.
We excluded studies of interventions that were primarily targeted at paying at practice or organisational level; in this review, we focused only on changes to payments made directly to healthcare providers. Another Cochrane Review has evaluated the payment at practice or organisational level (Yuan 2017). For example, the QOF (Quality and Outcomes Framework) applied in the UK is a pay‐for‐performance scheme delivering funding to general practices, not directly to general practitioners, and so studies evaluating the QOF were excluded from this review.
Types of outcome measures
Primary outcomes
The main behavior of healthcare providers is to provide health care to patients, so the primary outcomes included in this review are objective measures of health services provision and other measures which are closely related to the supply of health services in outpatient care facilities, including the following.
Service provision and process outcomes
-
Quantity of health services provided, including those:
measured as risk ratio (RR), e.g. for patients getting an aspirin prescription, for referring smokers to a quit line, for women having any prenatal care;
measured as mean difference (MD) or standardised mean difference (SMD), e.g. rate of screening service per 100 prescriptions, average length of service time.
-
Quality of health services provided, including those:
measured as RR, e.g. for physicians adhering to clinical guidelines;
measured as MD or SMD, e.g. the quality score for provision of certain health services.
-
-
Patient outcomes (the effect of changes in service provision or process outcomes on measures of length of life, quality of life, or clinical measures closely related to these outcomes)
-
Patients' intermediate and final health outcomes, including those:
measured as RR, e.g. if a smoker has sustained abstinence from smoking, if blood pressure has been controlled;
measured as MD or SMD, e.g. blood pressure level of patients with hypertension, health‐related quality of life, mortality.
-
-
Healthcare provider outcomes (the outcomes related to consequences on individual providers after delivering services), including those:
measured as RR, e.g. if individual professionals are satisfied with work or job turnover;
measured as MD or SMD, e.g. hours worked or the income level of healthcare providers.
-
Costs of delivering services, including those:
measured as MD or SMD, e.g. cost per service, administration costs, total cost for purchasers, changes in skill mix.
-
Unintended or adverse effects, including those:
measured as RR, e.g. patient selection and poorer access to care for disadvantaged populations;
measured as MD or SMD, e.g. the number of unnecessary services (overtreatment and overdiagnosis).
Secondary outcomes
Satisfaction of patients or other stakeholders; job mobility.
Search methods for identification of studies
Electronic searches
We searched the following databases.
Cochrane Database of Systematic Reviews, part of the Cochrane Library (www.cochranelibrary.com/) (searched 5 March 2019)
Database of Abstracts of Reviews of Effects, part of the Cochrane Library (www.cochranelibrary.com/) (searched 15 July 2017)
Cochrane Central Register of Controlled Trials (CENTRAL2019, Issue 3, part of the Cochrane Library (www.cochranelibrary.com/) (searched 5 March 2019)
MEDLINE and Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations and Daily 1946 to March 04, 2019, Ovid (searched 5 March 2019)
Embase 1974 to 2019 March 04, Ovid (searched 5 March 2019)
Web of Science, Conference Proceedings Citation Index‐Science, 1990 to present (ISI Web of Knowledge) (searched 5 March 2019)
PubMed, NLM (searched 10 December 2018)
Dissertations and Theses Database, 1861 to present, ProQuest (searched 10 December 2018)
EconLit, 1969 to present, ProQuest (searched 10 December 2018)
China National Knowledge Infrastructure (CHKD‐CNKI), 1915 to present (searched 10 December 2018)
Chinese Medicine Premier (Wanfang Data), 1988 to present (searched 10 December 2018)
IDEAS (Research Papers in Economics), 1927 to present (searched 30 December 2017)
POPLINE (Population Information Online), 1970 to present, K4Health (searched 30 December 2017)
The EPOC Information Specialist (TSC) helped develop some of the search strategies in consultation with the review authors.
Search strategies are comprised of keywords and controlled vocabulary terms. We applied no language limits. We searched all databases from database start date to date of search.
Searching other resources
Grey literature
We conducted a grey literature search to identify studies not indexed in the databases listed above.
OpenGrey (System for Information on Grey Literature in Europe) (www.opengrey.eu/) (searched 10 December 2018)
World Health Organization (WHO) (www.who.int/) (searched 17 November 2018)
World Bank (www.who.int/) /searched 17 November 2018)
Trial registries
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (clinicaltrials.gov/) (searched 27 June 2019)
World Health Organization International Clinical Trials Registry Platform (ICTRP) (www.who.int/clinical-trials-registry-platform) (searched 27 June 2019)
In addition, we:
searched reference lists of all relevant papers identified;
searched Web of Science Core collection; KCI‐Korean Journal Database; Russian Science Citation Index; SciELO Citation Index, Clarivate Analytics for papers that cited any of the included studies in this review (searched 8 February 2019);
searched PubMed for related citations to any studies to be included in the review;
contacted authors of relevant papers regarding any further published or unpublished work;
We re‐ran the search strategies in August 2020 and screened the identified records. Potentially relevant studies are awaiting classification and will be assessed at the next update.
All search strategies are provided in Appendix 1.
Data collection and analysis
Selection of studies
Two review authors scanned the titles and abstracts of all articles obtained from the search and retrieved the full text of articles deemed relevant. Two review authors independently assessed full texts of studies for inclusion. Any disagreements on inclusion were resolved by discussion with a third review author or EPOC editor.
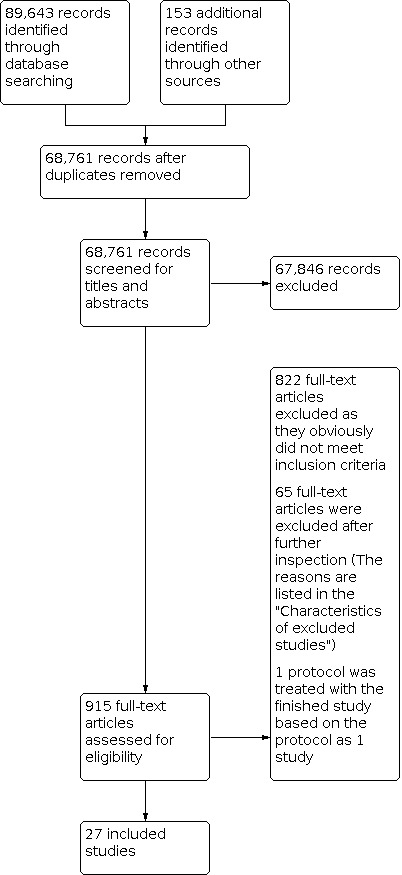
The screening process and results are reported in a PRISMA flow diagram (Figure 1). All included studies are described in the Characteristics of included studies table, even those for which useable results for reanalysis or synthesis were not reported. Studies that initially appeared to meet the inclusion criteria but that were excluded are described in the Characteristics of excluded studies table.
1.
Study flow diagram.
Data extraction and management
Two review authors independently carried out data extraction using a data extraction form adopted from the Cochrane good practice data collection form (EPOC 2013a). We extracted the following information.
General information, including title, reference details, author contact details, and publication type.
Participants and setting.
Study method.
Intervention groups, including payment method description, duration of intervention, if patients can choose providers, how purchasers monitored the implementation of payment.
Outcomes, including outcome measures, time points measured, unit of measurement, and person measuring outcomes.
Results, including result reported by authors, analysis method, unintended effects, if analysis required and possibility.
Any disagreements were resolved by discussion with a third review author or the EPOC contact editor. For ITS studies that reported time series data that were not appropriately analysed, we extracted and reanalysed the data as described in the EPOC resources for review authors (EPOC 2013b).
Assessment of risk of bias in included studies
We used the EPOC suggested 'Risk of bias' criteria to assess the risk of bias for each outcome in all included studies (EPOC 2013c). For each criterion, two review authors independently described what was reported in the study, commented on the description, and judged the risk of bias. Any unresolved disagreements were discussed with a third review author and, if consensus could not be reached, with the EPOC contact editor. We summarised the overall risk of bias across criteria for the outcomes of the included studies. For randomised trials, non‐randomised trials, and controlled before‐after studies, we primarily considered four criteria: baseline outcome measurements; baseline characteristics measurements; incomplete outcome data addressed; and protection against contamination. If these four criteria were all scored 'low risk of bias' for the outcome in a given study, the summary assessment was low risk of bias; if one or more key criteria were scored 'unclear', the summary assessment was unclear risk of bias; and if one or more key criteria were scored 'high risk of bias', the summary assessment was high risk of bias. For ITS studies, we primarily considered the following criteria when summarising the overall risk of bias: intervention independence, intervention affecting data collection, and incomplete outcome data addressed.
Measures of treatment effect
For randomised trials, non‐randomised trials, and controlled before‐after studies, we recorded or calculated risk ratio (RR) with 95% confidence intervals (CI) for dichotomous outcomes. If adjusted analysis was done, we reported the effect estimates provided by the study authors, also converting them into RR if possible. For continuous outcomes, we recorded or calculated mean difference (MD) with 95% CI if the studies to be synthesised had the same outcome measures. We calculated the standardised mean difference (SMD) with 95% CI if the studies to be synthesised assessed slightly different outcome measures within the same broad outcome category.
Data were insufficient data to permit statistical pooling for most outcomes. In order to facilitate comparison of the effect sizes of the included studies, we did the following: if the baseline levels were available, we reported the point estimates of absolute change adjusted for baseline differences or the relative change adjusted for baseline differences for all included outcome measures. If the baseline levels were not available, we reported the point estimates of absolute change and relative change for all included outcome measures.
The absolute change adjusted for baseline differences is defined as 'baseline‐post difference', and the formula is: (intervention group post level − intervention group baseline level) − (control group post level − control group baseline level). The relative change adjusted for baseline differences is calculated by the following formula: ((intervention group post level − intervention group baseline level) − (control group post level − control group baseline level))/(control group post level − control group baseline level).
For ITS and repeated measures studies, we reported the difference between the predicted value based on the pre‐intervention trend and the estimated value based on the change in level and post‐intervention trend at relevant time points (including immediately after the intervention (change in level), one year, two years, and three years).
Unit of analysis issues
This review analysed the impact on performance on the level of the individual, so the allocation and analysis unit should be aggregated to physicians. We planned to reanalyse studies that allocated clusters (e.g. clinics in one district) but that did not account for clustering if we were able to extract the intracluster coefficient. All included studies reported having accounted for and adjusting for clustering in their analysis.
Dealing with missing data
If any data needed for meta‐analysis or reanalysis (e.g. standard deviations, numbers of events, and subgroup analyses) were missing, we attempted to calculate them based on available data on the same outcome (e.g. calculation of standard deviation from CI). If these data were not available, we contacted the study authors to request the missing data. However, we did not receive responses from the relevant study authors and therefore reported the data that were available. For these studies, we reported the point estimate of the effect measures without CIs. Where data on subgroup analyses were missing, we were not able to include these subgroups in our analysis.
Assessment of heterogeneity
We conducted meta‐analysis to synthesise the effect measures of included studies if they met the following criteria.
Similar intervention payment method and comparison payment, based on the payment method categories described above.
Similar participants, e.g. the targets of the payment method were all primary care physicians.
The outcome measures fell into the same outcome category, e.g. the outcome measures were all health service provision process measures, or patient outcomes measures.
When the included studies were sufficiently similar based on the above criteria, we used the Chi2 test and I2 statistic to assess statistical heterogeneity. When the P value from a Chi2 test was smaller than 0.1, we interpreted this as an indication that the observed difference in results across studies was not compatible with chance alone. We used the I2 statistic to quantify the level of statistical heterogeneity.
As there is considerable heterogeneity in the design of payment methods, we attempted to explore this through the prespecified subgroup analyses (see Subgroup analysis and investigation of heterogeneity). We used caution in interpreting results from meta‐analyses with high levels of heterogeneity.
Assessment of reporting biases
We planned to use a funnel plot to examine asymmetry and assess the potential of any asymmetry being due to publication bias. However, due to the limited number of studies with the same outcomes in each comparison, we decided not to undertake funnel plots.
Data synthesis
We conducted a structured synthesis as described in the EPOC resources for review authors (EPOC 2013d). We first categorised the comparisons and outcomes, and then described the effects of different kinds of payment methods on different categories of outcomes. As mentioned above, there were two kinds of payment change with mechanisms for changing behaviour: additional incentives leading to an increase in total funding, or changes in the payment model without changes to the total funding received. These two mechanisms were assessed in different comparisons.
We synthesised the effects of studies that used the same type of study design. We conducted meta‐analysis for randomised trials, but not for controlled before‐after and interrupted time series studies due to insufficient data. For the meta‐analyses, we initially used a fixed‐effect model to pool data within a study if the study included more than one outcome indicator under the same category of outcome measures. We then used a random‐effects model for meta‐analysis across studies. This was because the payment method designs usually included several components; the payment methods conducted by different purchasers or in different areas were rarely exactly the same; and there were differences in outcome measures falling in the same outcome category. For randomised trials where the available data did not permit meta‐analysis, and for all controlled before‐after and interrupted time series studies, we reported the medians and full ranges of the available point estimates of effect sizes. We analysed these firstly within, and then secondly across, the studies for the same category of outcome measures. It was not possible to report an interquartile range due to the very limited number of data points.
Subgroup analysis and investigation of heterogeneity
In the protocol for this review, we hypothesised a series of factors that might affect the size of effects of payment methods and planned to perform subgroup analysis based on these factors, such as size of fee, duration of follow‐up, targeted population, if there were multiple providers, if there was monitoring of delivery of services, the frequency of monitoring, the frequency of payment, and baseline performance level. However, these subgroup analyses could not be conducted due to the limited number of included studies with similar comparisons and outcomes. Furthermore, the included studies reported limited detail on the characteristics of payment methods. 'Duration of follow‐up' and 'targeted population' were reported more often in the included studies, and these factors are presented in Table 5 and Table 6.
1. Outcome measures of included studies.
| Study | Outcomes | Length of observation |
| Quantity of health services provided | ||
| Bilardi 2010 |
|
12 months |
| Christensen 2000 |
|
20 months |
| Clarkson 2008 |
|
18 months |
| Davidson 1992 |
|
18 months |
| Fairbrother 1999 |
|
12 months |
| Fairbrother 2001 |
|
12 months |
| Flierman 1992 |
|
12 months |
| Gleeson 2017 |
|
12 months |
| Gosden 2003 |
|
18 months |
| Greene 2013 |
|
15 years |
| Hickson 1987 |
|
24 months |
| Kouides 1998 |
|
12 months |
| Krasnik 1990 |
|
9 months |
| Lee 2010 |
|
24 months |
| Li 2013 |
|
120 months |
| Lurie 1992 |
|
24 months |
| Twardella 2007 |
|
12 months |
| Yesalis 1980 |
|
36 months |
| Yesalis 1984 |
|
36 months |
| Young 2007 |
|
‐ |
| Quality of health services provided | ||
| Chung 2010 |
|
24 months |
| Gosden 2003 |
|
18 months |
| Petersen 2013 |
|
12 months |
| Young 2007 |
|
‐ |
| Young 2012 |
|
36 months |
| Patient outcomes | ||
| Gleeson 2017 |
|
‐ |
| Houle 2016 |
|
45 months |
| Jensen 2014 |
|
21 months |
| Lurie 1992 |
|
11 months |
| Petersen 2013 |
|
16 months |
| Singh 2015 |
|
12 months |
| Twardella 2007 |
|
12 months |
| Healthcare provider outcomes | ||
| Gosden 2003 |
|
18 months |
| Gray 2015 |
|
72 months |
| Costs | ||
| Davidson 1992 |
|
18 months |
| Lee 2010 |
|
24 months |
| Yesalis 1980 |
|
36 months |
| Yesalis 1984 |
|
36 months |
| Adverse effects | ||
| Hickson 1987 |
|
24 months |
| Petersen 2013 |
|
12 months |
LDL: low‐density lipoprotein UTD: up‐to‐date WHO: World Health Organization
2. Payment methods for the vulnerable populations.
| Target population | Payment methods | Outcomes |
| The elderly | Not reported in included studies | Not reported in included studies |
| The disabled | Not reported in included studies | Not reported in included studies |
| Minorities | Not reported in included studies | Not reported in included studies |
| People with low levels of education | Not reported in included studies | Not reported in included studies |
| Children | ||
| Clarkson 2008, cluster‐randomised trial | FFS remuneration | Children with 1 or more sealant per dentist |
| Davidson 1992, randomised trial | Capitation, FFS high rate compare with FFS (low rates) | Physician visits, hospitalisations |
| Jensen 2014, controlled before‐after study | Mixed system of capitation and FFS contracts | Birth weight, preterm birth, very preterm birth, fetal growth |
| Singh 2015, controlled before‐after study | Performance bonus | Weight, WHO malnourished status |
| Women | ||
| Bilardi 2010, cluster randomised trial | P4P (a small incentive payment per test) | Women being tested |
| People living in rural or remote areas | ||
| Yesalis 1980; Yesalis 1984, controlled before‐after study | Capitation compare with FFS | Rate of generic substitution per 100 prescriptions Percentage of Medicaid prescriptions classified as multi‐source drug products Numbers of prescriptions involving changes in labeller on refills (0 to 5 days) |
| Low‐income populations 2 | ||
| Christensen 2000, randomised trial, Medicaid recipients | Financial incentive (P4P) | Patients receiving cognitive services |
| Gleeson 2017, controlled before‐after study, Medicaid | P4P plus existing FFS compare with FFS | Adolescent well care, inactivated polio vaccine |
FFS: fee‐for‐service P4P: pay for performance WHO: World Health Organization
Sensitivity analysis
We planned to conduct a sensitivity analysis excluding studies with imputed data and studies with an overall high risk of bias. Due to the limited number of studies included for each comparison, and because there were no studies with an overall high risk of bias included in meta‐analysis, we did not conduct the sensitivity analysis as planned.
Summary of findings and assessment of the certainty of the evidence
We created 'Summary of findings' tables for the main intervention comparisons and most important outcomes: quantity and quality of health services provided, provider outcomes, costs, health outcomes, and adverse effects. For the comparisons and outcomes that included randomised trials, results were reported in the 'Summary of findings' table. For the comparisons and outcomes for which randomised trials were not found, we reported the results of controlled before‐after or interrupted time series studies where these were available. Two review authors independently assessed the certainty of the evidence as high, moderate, low, or very low using the five GRADE considerations (risk of bias, consistency of effect, imprecision, indirectness, publication bias) (Guyatt 2008). We used the methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of interventions,Higgins 2020, and the EPOC worksheets (EPOC 2013e), employing GRADEpro GDT software (GRADEpro GDT 2020). Any disagreements on certainty ratings were resolved by discussion. We provided justification for decisions to down‐ or upgrade the ratings using footnotes and made comments to aid readers' understanding of the review where necessary. We used plain language statements to report these findings in the review (EPOC 2018).
Results
Description of studies
See: Characteristics of included studies and Characteristics of excluded studies.
Results of the search
Search strategies yielded 89,643 references, which two review authors examined independently. We screened this large number of references because the searches were conducted without the study design filters in MEDLINE and Embase in order to also identify relevant studies for a larger scoping review on payment for health facilities or individual providers. We retrieved 915 full texts of articles regarded as potentially relevant, which two review authors read and evaluated independently. We initially assessed 92 full texts of studies as meeting the inclusion criteria for study designs and evaluating effectiveness of payment methods on healthcare providers working in outpatient health facilities. During data extraction we confirmed the inclusion of 27 studies which evaluated payment methods targeting individual healthcare providers in outpatient health facilities. See Figure 1.
We re‐ran the search strategies in August 2020. We screened the records identified by the search, and have listed two potentially relevant studies under Studies awaiting classification. We will assess these studies at the next update.
Included studies
The 27 included studies are described in detail in the Characteristics of included studies.
Study design
We included 27 studies (see Characteristics of included studies): 12 randomised trials (Bilardi 2010; Christensen 2000; Clarkson 2008; Davidson 1992; Fairbrother 1999; Fairbrother 2001; Hickson 1987; Houle 2016; Kouides 1998; Lurie 1992; Petersen 2013; Twardella 2007); 13 CBA studies (Chung 2010; Flierman 1992; Gleeson 2017; Gosden 2003; Gray 2015; Greene 2013; Jensen 2014; Krasnik 1990; Lee 2010; Li 2013; Singh 2015; Yesalis 1980; Yesalis 1984); and 2 ITS and repeated measure studies (Young 2012; Young 2007).
Participants and setting
The participants included healthcare providers working in outpatient care facilities. The majority of healthcare providers were primary care physicians (Bilardi 2010; Chung 2010; Davidson 1992; Fairbrother 1999; Fairbrother 2001; Flierman 1992; Gleeson 2017; Gosden 2003; Gray 2015; Greene 2013; Hickson 1987; Jensen 2014; Krasnik 1990; Lee 2010; Li 2013; Petersen 2013; Young 2007; Young 2012). Other participants included mental health physicians (Lurie 1992), pharmacists (Christensen 2000; Houle 2016; Yesalis 1980; Yesalis 1984), office staff (Kouides 1998), and health workers based in daycare centres for children (Singh 2015). Nine studies focused on vulnerable populations (Table 6), including children in four studies (Clarkson 2008; Davidson 1992; Jensen 2014; Singh 2015), women in one study (Bilardi 2010), people living in rural or remote areas in two studies (Yesalis 1980; Yesalis 1984), and low‐income populations in two studies (Christensen 2000; Gleeson 2017). Other vulnerable populations, such as people living with disabilities and minority groups, were not mentioned in the included studies.
Outpatient care centres were named differently in different health system settings, including private office‐based practices (Davidson 1992; Fairbrother 1999; Fairbrother 2001), medical practices (Twardella 2007), hospital outpatient clinics (Petersen 2013), community health centres (Christensen 2000; Houle 2016; Li 2013), and clinics (Bilardi 2010). Further information on these centres was not provided.
Most of the payment methods were implemented by health insurance schemes in high‐income countries or regions: three studies focused on P4P for general practitioners (GPs) in Australia, Bilardi 2010; Greene 2013, and Taiwan (Lee 2010); three studies evaluated P4P and capitation for family care physicians and GPs in Canada (Gray 2015; Houle 2016; Li 2013); one study evaluated P4P for GPs in Germany (Twardella 2007); three studies focused on changes of payment methods for GPs in Denmark (Flierman 1992; Jensen 2014; Krasnik 1990); and the remaining studies were conducted in the USA. Only one study focused on payment methods changed from fixed wages to performance pay for daycare centre health workers in India (Singh 2015).
Interventions and comparisons
We grouped the interventions and comparisons into four categories (see Table 7).
3. Interventions and comparisons in included studies.
| Comparison 1: P4P plus an existing payment method compared with an existing payment method | ||
| Study | Intervention | Comparison |
| Christensen 2000 | P4P + existing payment method | Capitation |
| Chung 2010 | P4P + existing payment method | Not described |
| Fairbrother 1999 | P4P + existing payment method | FFS |
| Fairbrother 2001 | P4P + existing payment method | FFS |
| Gleeson 2017 | P4P + existing payment method | FFS |
| Greene 2013 | P4P + existing payment method | FFS |
| Houle 2016 | P4P + existing payment method | FFS |
| Kouides 1998 | P4P + existing payment method | FFS |
| Lee 2010 | P4P + existing payment method | FFS |
| Li 2013 | P4P + existing payment method | FFS |
| Petersen 2013 | P4P + existing payment method | Not described |
| Singh 2015 | P4P + existing payment method | Salary |
| Young 2007 | P4P + existing payment method | Salary |
| Young 2012 | P4P + existing payment method | Salary |
| Comparison 2: FFS compared with existing payment methods | ||
| Study | Intervention | Comparison |
| Davidson 1992 | FFS | Capitation |
| Lurie 1992 | FFS | Capitation |
| Hickson 1987 | FFS | Salary |
| Yesalis 1984 | FFS | Capitation |
| Yesalis 1980 | FFS | Capitation |
| Comparison 3: FFS mixed with existing payment methods compared with existing payment methods | ||
| Study | Intervention | Comparison |
| Bilardi 2010 | FFS + existing payment method | Not explicitly described |
| Clarkson 2008 | FFS + capitation | Capitation |
| Twardella 2007 | FFS + existing payment method | Not explicitly described |
| Jensen 2014 | FFS + capitation | Capitation |
| Gosden 2003 | FFS + salary | Salary |
| Gray 2015 | FFS + capitation | FFS |
| Flierman 1992 | FFS + capitation | Capitation |
| Krasnik 1990 | FFS + capitation | Capitation |
| Comparison 4: Enhanced FFS compared with FFS | ||
| Study | Intervention | Comparison |
| Davidson 1992 | Increase in FFS per service payment rate | FFS |
| Fairbrother 1999 | Increase in FFS per service payment rate | FFS |
| Fairbrother 2001 | Increase in FFS per service payment rate | FFS |
FFS: fee‐for‐service P4P: pay for performance
1. P4P plus existing payment methods compared with existing payment methods
We included 14 studies that compared P4P added to an existing payment method versus an existing payment method (Christensen 2000; Chung 2010; Fairbrother 1999; Fairbrother 2001; Gleeson 2017; Greene 2013; Houle 2016; Kouides 1998; Lee 2010; Li 2013; Petersen 2013; Singh 2015; Young 2007; Young 2012). In this comparison, the purchasers of health services did not change the existing payment to providers, but used extra funds to pay health providers as incentives in order to motivate the provision of certain health services. The existing payment methods included capitation (Christensen 2000), FFS (Fairbrother 1999; Fairbrother 2001; Gleeson 2017; Greene 2013; Houle 2016; Kouides 1998; Lee 2010; Li 2013), and salary (Singh 2015; Young 2007; Young 2012). The authors of two studies did not explicitly describe the existing payment methods (Chung 2010; Petersen 2013).
2. FFS compared with existing payment methods
We included five studies that compared FFS to an existing payment method (Davidson 1992; Hickson 1987; Lurie 1992; Yesalis 1980; Yesalis 1984). The existing payments in this comparison included capitation, Davidson 1992; Lurie 1992; Yesalis 1980; Yesalis 1984, or salary (Hickson 1987), both of which are input‐based payment methods. In addition, and in contrast to comparison 1, the purchasers adjusted only the payment methods whilst maintaining the same level of total funding when moving to a FFS payment method from capitation or salary, or changing from capitation or salary to an FFS payment.
3. FFS mixed with existing payment methods compared with existing payment methods
We included eight studies that evaluated a mixed method of FFS compared with a single existing method (Bilardi 2010; Clarkson 2008; Flierman 1992; Gosden 2003; Gray 2015; Jensen 2014; Krasnik 1990; Twardella 2007)
The mixed methods included FFS mixed with capitation, Flierman 1992; Gray 2015; Jensen 2014; Krasnik 1990, and FFS mixed with salary (Gosden 2003). The single existing comparison payment methods included salary (Gosden 2003), capitation (Clarkson 2008; Flierman 1992; Jensen 2014; Krasnik 1990), and FFS (Gray 2015). In two studies (Bilardi 2010; Twardella 2007), the control payments were not explicitly described by the authors, but it is clear that the FFS payments received in the intervention group were added to an existing payment of some kind.
4. Enhanced FFS compared with FFS
We included three randomised trials that evaluated the effects of increasing the FFS fees paid per service provided (Davidson 1992; Fairbrother 1999; Fairbrother 2001).
Study outcomes
All outcome measures reported in the included studies are listed in Table 5.
Primary outcomes
Service provision process outcomes
Two categories of services provision process outcomes were included: quantity and quality of health services provided.
Quantity of health services provided
Twenty included studies evaluated the effects of payment on the quantity of health services provided, including medical admissions and physician visits (Davidson 1992; Hickson 1987; Lee 2010), testing services (Bilardi 2010; Gleeson 2017; Greene 2013), public health services such as immunisation (Fairbrother 1999; Fairbrother 2001; Gleeson 2017; Kouides 1998), and other preventive care (Li 2013), diabetes care (Lee 2010; Young 2007), cognitive services (Christensen 2000), dentist services (Clarkson 2008), diagnostic and curative care (Flierman 1992; Krasnik 1990), consultations (Gosden 2003; Krasnik 1990), referrals to specialists or hospital (Krasnik 1990; Lurie 1992), and drug prescriptions (Yesalis 1980; Yesalis 1984).
Quality of health services provided
Five included studies assessed the quality of health services (Chung 2010; Gosden 2003; Petersen 2013; Young 2007; Young 2012).
Patient outcomes
Patient outcome measures included behaviour change amongst patients and changes in their intermediate and final health status. Seven studies reported patient outcomes, including general health, physical function, psychiatric status (Lurie 1992), birth weight status (Jensen 2014; Singh 2015), self‐reported smoking cessation (Twardella 2007), psychiatric status (Gleeson 2017), community and role function (Lurie 1992), systolic blood pressure reductions (Houle 2016), and the proportion of people with blood pressure control (Petersen 2013).
Healthcare provider outcomes
Two studies assessed the workload or income of physicians following payment changes (Gosden 2003; Gray 2015).
Costs
Five included studies reported the costs of delivering health services. One randomised trial measured net expenditures per year of eligibility for the FFS group and the capitation group in the Medicaid programme (Davidson 1992). One CBA study compared the cost savings of generic substitution between the FFS year (year 1) and each of the capitation years (years 2 and 3) (Yesalis 1980; Yesalis 1984). Another CBA study measured the annual costs per person of diabetes‐related physician visits in the intervention group (Lee 2010).
Adverse effects
Purposefully selecting patients who will result in higher payments is a typical adverse effect of payment interventions, as this may result in unnecessary service provision and waste of resources. One randomised trial analysed changes in patient selection because of change of payments (Hickson 1987).
Another potential adverse effect is if the performance of healthcare providers who were motivated by an incentive intervention decreases rapidly after the incentive ends, compared to performance in the comparison group. One randomised trial evaluated whether changes in the performance of healthcare providers with regard to people with raised blood pressure could be sustained after the intervention ended (Petersen 2013).
Secondary outcomes
None of the included studies evaluated the effects of the interventions on the satisfaction of patients or other stakeholders or on job mobility.
Excluded studies
See: Characteristics of excluded studies.
We excluded 30 studies because they did not meet EPOC's recommended inclusion criteria for study designs; 26 studies because they focused on payments to healthcare facilities rather than targeting individual healthcare providers; and four studies because they did not focus on payment methods. One study was excluded because it did not measure any eligible outcomes.
Risk of bias in included studies
The risk of bias for each of the included studies is shown in Characteristics of included studies.
Based on the protocol, we evaluated the included studies according to the following the criteria for randomised trials, non‐randomised trials, and controlled before‐after studies: baseline outcome measurements; baseline characteristic measurements; incomplete outcome data addressed; and protection against contamination. Of the 12 randomised trials, 10 trials were judged as at unclear risk of bias (Bilardi 2010; Christensen 2000; Clarkson 2008; Fairbrother 1999; Fairbrother 2001; Hickson 1987; Houle 2016; Kouides 1998; Lurie 1992; Petersen 2013), whilst the other two were assessed as at high risk of bias for all the primary outcomes (Davidson 1992; Twardella 2007). Of the 13 CBA studies, eight studies were judged as at unclear risk of bias (Chung 2010; Gleeson 2017; Greene 2013; Jensen 2014; Krasnik 1990; Singh 2015; Yesalis 1980; Yesalis 1984), and five studies were judged as at high risk of bias (Flierman 1992; Gosden 2003; Gray 2015; Lee 2010; Li 2013).
Based on the criteria for ITS studies (intervention independence, intervention affecting data collection, and incomplete outcome data addressed), we judged one ITS study, Young 2007, and one repeated measures study, Young 2012, as at unclear of risk of bias for the primary outcomes.
Allocation
Of the 12 randomised trials, we assessed four as at low risk of selection bias (Bilardi 2010; Clarkson 2008; Hickson 1987; Petersen 2013); one as at high risk of selection bias (Twardella 2007), and the others as having an unclear risk of selection bias (Christensen 2000; Davidson 1992; Fairbrother 1999; Fairbrother 2001; Houle 2016; Kouides 1998; Lurie 1992).
We judged all of the CBA studies, Chung 2010; Flierman 1992; Gleeson 2017; Gosden 2003; Gray 2015; Greene 2013; Jensen 2014; Krasnik 1990; Lee 2010; Li 2013; Singh 2015; Yesalis 1980; Yesalis 1984, and ITS studies, Young 2007; Young 2012, as having a high risk of selection bias, as participants were not allocated using randomised methods (see Table 8).
4. Distribution of risk of bias in included studies.
| Bias/study design | Randomised controlled trial | Controlled before‐after study | Interrupted time series |
| Allocation (selection bias) |
Unclear: Fairbrother 1999, Fairbrother 2001, Christensen 2000, Davidson 1992, Kouides 1998, Lurie 1992 High: Houle 2016, Twardella 2007 Low: Bilardi 2010, Hickson 1987, Petersen 2013, Clarkson 2008 |
High: Gosden 2003, Gray 2015, Chung 2010, Flierman 1992, Jensen 2014, Lee 2010, Li 2013, Singh 2015, Greene 2013, Yesalis 1980, Yesalis 1984, Krasnik 1990, Gleeson 2017 | High: Young 2012, Young 2007 |
| Blinding (performance bias and detection bias) |
Unclear: Fairbrother 1999, Fairbrother 2001, Hickson 1987, Kouides 1998 High: Lurie 1992 Low: Bilardi 2010, Christensen 2000, Clarkson 2008, Davidson 1992, Houle 2016, Petersen 2013, Twardella 2007 |
Unclear: Li 2013, Singh 2015 High: Gosden 2003 Low: Gleeson 2017, Gray 2015, Greene 2013, Chung 2010, Flierman 1992, Jensen 2014, Lee 2010, Yesalis 1980, Yesalis 1984, Krasnik 1990 |
Low: Young 2007, Young 2012 |
| Incomplete outcome data (attrition bias) |
Unclear: Bilardi 2010, Clarkson 2008 High: Twardella 2007 Low: Christensen 2000, Fairbrother 1999, Fairbrother 2001, Hickson 1987, Houle 2016, Kouides 1998, Lurie 1992, Petersen 2013, Davidson 1992 |
Unclear: Chung 2010, Gosden 2003, Lee 2010 Flierman 1992, Jensen 2014, Greene 2013, Yesalis 1980, Yesalis 1984, Gleeson 2017 High: Gray 2015 Low: Li 2013, Singh 2015, Krasnik 1990 |
Low: Young 2007, Young 2012 |
| Selective reporting (reporting bias) |
Unclear: Clarkson 2008 Low: Bilardi 2010, Christensen 2000, Davidson 1992, Fairbrother 1999, Fairbrother 2001, Hickson 1987, Houle 2016, Kouides 1998, Lurie 1992, Petersen 2013, Twardella 2007 |
Unclear: Chung 2010, Lee 2010, Greene 2013 Low: Gosden 2003, Gray 2015, Flierman 1992, Jensen 2014, Li 2013, Singh 2015, Yesalis 1980, Yesalis 1984, Krasnik 1990, Gleeson 2017 |
Low: Young 2007, Young 2012 |
Blinding
For performance bias and detection bias, we judged seven randomised trials as at low risk of bias (Bilardi 2010; Christensen 2000; Clarkson 2008; Davidson 1992; Houle 2016; Petersen 2013; Twardella 2007); one randomised trial as at high risk of bias (Lurie 1992); and the remaining randomised trials as having an unclear risk of bias. The majority of CBA studies were assessed as at low risk of bias (Chung 2010; Flierman 1992; Gleeson 2017; Gray 2015; Greene 2013; Jensen 2014; Krasnik 1990; Lee 2010; Yesalis 1980; Yesalis 1984); one was assessed as at high risk of bias (Gosden 2003); and two had an unclear risk of bias (Li 2013; Singh 2015). Both ITS studies were assessed as at low risk of bias (Young 2007; Young 2012). (See Table 8.)
Incomplete outcome data
For the randomised trials, we assessed only one study as at high risk of attrition bias (Twardella 2007); two at unclear risk of bias (Bilardi 2010; Clarkson 2008); and the remaining trials as at low risk of bias (Christensen 2000; Davidson 1992; Fairbrother 1999; Fairbrother 2001; Hickson 1987; Houle 2016; Kouides 1998; Lurie 1992; Petersen 2013). We assessed most of the CBA studies to be at unclear risk of attrition bias (Chung 2010; Flierman 1992; Gleeson 2017; Gosden 2003; Greene 2013; Jensen 2014; Lee 2010; Yesalis 1980; Yesalis 1984); one to be at high risk of bias (Gray 2015); and three as at low risk of bias (Krasnik 1990; Li 2013; Singh 2015). We assessed both ITS studies as at low risk of bias (Young 2007; Young 2012). (See Table 8.)
Selective reporting
We assessed most of the randomised trials as at low risk of reporting bias (Bilardi 2010; Christensen 2000; Davidson 1992; Fairbrother 1999; Fairbrother 2001; Hickson 1987; Houle 2016; Kouides 1998; Lurie 1992; Petersen 2013; Twardella 2007), except for one trial that was judged to be at unclear risk of bias (Clarkson 2008). We assessed most of the CBA studies as at low risk of reporting bias (Flierman 1992; Gleeson 2017; Gosden 2003; Gray 2015; Jensen 2014; Krasnik 1990; Lee 2010; Singh 2015; Yesalis 1980; Yesalis 1984), except for three studies judged as being at unclear risk of bias (Chung 2010; Greene 2013; Lee 2010). We assessed the two ITS studies as at low risk of reporting bias (Young 2007; Young 2012). (See Table 8.)
Other potential sources of bias
We did not identify any other potential sources of bias.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
We categorised the payment methods into four main comparisons. For the included randomised trials and CBA studies, the effect sizes of the payment methods were described by point estimates of absolute change and relative percentage changes (Table 9; Table 10). For the included ITS and repeated measures studies, the effects were reported by immediate change in level and change in trend (Table 11; Table 12).
5. Effect of all included randomised trials.
| Comparison 1: P4P plus existing payment methods compared with existing payment methods | ||||
| Study | Outcome measures | Control/baseline level | Absolute change | Relative change |
| Quantity of health services provided | ||||
| Fairbrother 1999 | UTD immunisation rate (adjusted by baseline) | 12.6% | 12.7% | +140.77% |
| Fairbrother 2001 | UTD immunisation rate (adjusted by baseline) | −2.5% | 8.4% | −336% |
| Kouides 1998 | Immunisation rate | 4.7 | 6.3% | +134.04% |
| Christensen 2000 | Documentation rate of CS per 100 Medicaid prescriptions | 0.67% | 0.92% | +137.31% |
| CS intervention time per 100 Medicaid prescriptions | 6.5% | 1.4% | +21.54% | |
| Synthesised effects inside the study (median) | ‐ | 1.16% | +79.43% | |
| Synthesised effect across the above 4 studies (median) | ‐ | ‐ | +134.04% | |
| Quality of health services provided | ||||
| Petersen 2013 | Use of guideline | 0.47% | 8.37% | +1780.85% |
| Patient outcomes | ||||
| Petersen 2013 | Blood pressure control or appropriate response | 4.35% | 4.72% | +108.51% |
| Houle 2016 | Systolic BP level reduction | 17.0 | 2.7 | +15.88% |
| Diastolic BP level reduction | 8.2 | −0.6 | −7.32% | |
| Synthesised effects inside the study (median) | ‐ | 1.05 | +4.28% | |
| Synthesised effect across the above 2 studies (median) | ‐ | ‐ | +15.88% | |
| Comparison 2: FFS compared with existing payment methods | ||||
| Study | Outcome measures | Control/baseline level | Absolute change | Relative change |
| Quantity of health services | ||||
| Davidson 1992 | Primary care physician visits | −0.33 | 0.36 | −61.02% |
| Non‐primary visit | −0.05 | 0.23 | −460% | |
| Clinic visits | −0.47 | −0.05 | +10.64% | |
| Hospitalisation | −0.042 | −0.0276 | +65.71% | |
| Synthesised effects inside the study (median) | ‐ | ‐ | −25.19% | |
| Lurie 1992 | Proportion of receiving outpatient | 61% | 10% | +16.39% |
| Over average inpatient admissions | 0.20 | 0.19 | +95% | |
| Proportion of outpatient chemical depending treatment | 4.5% | 5.8% | +128.89% | |
| Proportion of inpatient chemical depending treatment | 1.9% | 3.3% | +173.68% | |
| Length of stays | 1.56 | 2.74 | +175.64% | |
| Proportion of being refused | 17% | −5% | −29.41% | |
| Synthesised effects inside the study (median) | ‐ | ‐ | +111.95% | |
| Hickson 1987 | Average number of patient/physician | 43.4 | 11.7 | +26.96% |
| Average number of visits attended/physician | 111.6 | −6.8 | −6.09% | |
| % attended by primary physician | 86.6% | −8.3% | −9.58% | |
| Emergency visit | 0.12 | 0.1 | +83.33% | |
| Synthesised effects inside the study (median) | ‐ | ‐ | +10.44% | |
| Synthesised effect across the above 3 studies (median) | ‐ | ‐ | +10.44% | |
| Patient outcomes | ||||
| Lurie 1992 | Self‐rated health | 3.0% | −3.7% | +123.33% |
| General health status | 0.2 | −0.2 | +100.% | |
| Physical functioning index | 0 | 0 | 0 | |
| Social contact | −0.7 | 1.2 | −171.43% | |
| Mean Global Assessment Scale for psychiatric status | −1 | −0.3 | −30% | |
| Depression | −0.8 | 2.9 | −362.5% | |
| Anxiety | 0.2 | 0.7 | +350% | |
| Endogenous features | 0.7 | 0.5 | +71.43% | |
| Mania | 0.2 | −0.6 | −3% | |
| Delusions | 0.2 | 0 | 0 | |
| Miscellaneous | 0.1 | 0.4 | +80% | |
| Arrested | 0.3 | 1 | −333.33% | |
| Jailed | 0 | −1.1 | 0 | |
| Assaulted | 1.3 | −6.4 | −492.31% | |
| Suicide | −1.2 | 0.2 | −16.67% | |
| Living in sheltered setting | −1.9 | −3.1 | −147.62% | |
| With nights homeless during previous year | −0.3 | 1 | −333.33% | |
| Working in sheltered setting | −0.2 | 0.1 | −100% | |
| Synthesised effects inside the study (median) | ‐ | 0.05 | −9.84% | |
| Comparison 3: FFS mixed payment methods compared with single payment method | ||||
| Study | Outcome measures | Control/baseline level | Absolute change | Relative change |
| Quantity of health services provided | ||||
| Bilardi 2010 | Proportion of patients tested | 2.6% | −0.7% | −26.92% |
| Clarkson 2008 | Percentage of children with at least 1 sealant treatment per dentist | 26.3% | 6.6% | +25.1% |
| Synthesised effect across the above 2 studies (median) | ‐ | ‐ | −0.91% | |
| Patient outcomes | ||||
| Twardella 2007 | Self‐reported smoking abstinence | 0.12% | 0.03% | +25% |
| Comparison 4: Enhanced FFS compared with FFS | ||||
| Study | Outcome measures | Control/baseline level | Absolute change | Relative change |
| Quantity of health services | ||||
| Davidson 1992 | Primary care physician visits | −0.59 | 0.56 | −94.92% |
| Non‐primary visit | 0.19 | −0.37 | −194.74% | |
| Clinic visits | −0.34 | −0.18 | +52.94% | |
| Hospitalisation | −0.0312 | −0.0384 | +123.08% | |
| Synthesised effects inside the study (median) | ‐ | −0.1092 | −20.99% | |
| Fairbrother 1999 | UTD immunisation rate | 12.6% | −8.3 | −65.87% |
| Fairbrother 2001 | UTD immunisation rate | −2.5% | 9.9% | −396% |
| Synthesised effects across the above 3 studies (median) | ‐ | ‐ | −65.87% | |
BP: blood pressure CS: cognitive services FFS: fee‐for‐service P4P: pay for performance UTD: up‐to‐date
6. Effect measures of included CBA studies.
| Comparison 1: P4P plus existing payment methods compared with existing payment methods | ||||
| Study | Outcome measures | Control/baseline level | Absolute change | Relative change |
| Quantity of health services | ||||
| Li 2013 | Senior flu shots rate | 55.4% | 2.8% | +5.1% |
| Toddler immunisation rate | 54.3% | 1.1% | +2.0% | |
| Pap smears rate | 58.9% | 4.1% | +7.0% | |
| Mammogram rate | 64.6% | 1.8% | +2.8% | |
| Colorectal cancer screening rate | 15% | 8.5% | +57% | |
| Greene 2013 | The relationship between P4P and provision of diabetes test | ‐ | ‐ | ‐ |
| The relationship between P4P and cervical cancer screening | ‐ | ‐ | ‐ | |
| Lee 2010 | Number of essential exams/tests | 0.131 | 2.45 | +1870.23% |
| Number of diabetes‐related physician visits | 0.515 | 2.01 | +390.29% | |
| Number of diabetes‐related hospitalisations | 0.041 | −0.027 | −65.85% | |
| Gleeson 2017 | Incentivised measures (2 well care and 10 immunisation) | |||
| Proportion of adolescent well‐care visits | 0.6% | 1.7% | +283.33% | |
| Proportion of well‐child visits, 3 to 6 years | 1.1% | 0.7% | +63.64% | |
| Proportion of immunisations: adolescents | 12.9% | −2.1% | −16.28% | |
| Proportion of meningococcal immunisations | 12.1% | −1.3% | −10.74% | |
| Proportion of Td/Tdap immunisations | 15.4% | 0.1% | +0.65% | |
| Proportion of immunisations: children | 15.1% | 4.2% | +27.81% | |
| Proportion of DTP immunisations | 18.4% | 4.5% | +24.46% | |
| Proportion of hepatitis A immunisations | 10.6% | 0.2% | +1.89% | |
| Proportion of IPV immunisations | 23.1% | 5.5% | +23.81% | |
| Proportion of MMR immunisations | −0.1% | −0.5% | +500% | |
| Proportion of pneumococcal conjugate immunisations | 16.1% | 3.7% | +22.98% | |
| Proportion of varicella immunisations | 0.5% | −0.4% | +80% | |
| Unincentivised measures | ||||
| Proportion of ADHD maintenance | 4.4% | −0.6% | +13.84% | |
| Proportion of ADHD initiation | 4.8% | −2.5% | −52.08% | |
| Proportion of lead screening | 0.1% | 1.8% | +1800% | |
| Synthesised effect across the above 4 studies (median) | ‐ | ‐ | +22.98% | |
| Quality of health services provided | ||||
| Chung 2010 | Quality score for asthma controller prescribing | ‐ | ‐ | ‐ |
| Quality score for cervical cancer screening | ‐ | ‐ | ‐ | |
| Quality score for chlamydia screening | ‐ | ‐ | ‐ | |
| Cost | ||||
| Lee 2010 | Expenses for diabetes‐related physician visits | 1271 | 7191 | +565.77% |
| Expense for diabetes‐related inpatient services | 3627 | −3878 | −106.92% | |
| Expense for all diabetes‐related health services | 4898 | 3312 | +67.62% | |
| Synthesised effect inside the study (median) | ‐ | ‐ | +67.62% | |
| Patient outcomes | ||||
| Singh 2015 | Mean weight of the child | 0.261 | 0.003 | +1.1% |
| Mean grade (as measured in the Anganwadi) of the child | −0.047 | 0.018 | −38.3% | |
| Mean z‐score of the child | −0.057 | 0.002 | −3.5% | |
| Malnourished status of the child according to WHO | 0.019 | 0.013 | +68.42% | |
| Gleeson 2017 | Proportion of asthma, 12 to 18 years | −2.1% | 2.5% | −119.05% |
| Proportion of asthma, 5 to 11 years | −5.6% | 3.7% | −66.07% | |
| Proportion of pharyngitis | 0.3% | −2.1% | +700% | |
| Proportion of upper respiratory infection | 1.9% | −0.7% | −36.84% | |
| Proportion of influenza | 9.7% | 1.9% | +18.45% | |
| Proportion of rotavirus | 17.7% | 6.4% | +36.16% | |
| Synthesised effect across the above 2 studies (median) | ‐ | ‐ | −1.20% | |
| Comparison 2: FFS compared with existing payment methods | ||||
| Study | Outcome measures | Control/baseline level | Absolute change | Relative change |
| Quantity of health services | ||||
| Yesalis 1980 | Rate of generic substitution | 0.42% | 6.45% | +1535.71% |
| Percentage of Medicaid prescriptions classified as multi‐source drug products | −0.4% | −0.2% | +50% | |
| Numbers of prescriptions involving changes in labeller on refills (0 to 5 days) | 19 | −16 | −84.21% | |
| Yesalis 1984 | Mean rate of Medicaid generic substitution | 0.26% | 2.65% | +1019.23% |
| Mean rate of non‐Medicaid generic substitution | 0.28% | −0.08% | −28.57% | |
| Average days' therapy per recipient (institutional) | −1.2 | −6.21 | −517.5% | |
| Average days' therapy per recipient (non‐institutional) | 0.56 | −1.64 | −292.86% | |
| Synthesised effect across the above 2 studies (median) | ‐ | ‐ | −28.57% | |
| Comparison 3: FFS mixed payment methods compared with single payment method | ||||
| Study | Outcome measures | Control/baseline level | Absolute change | Relative change |
| Quantity of health services | ||||
| Krasnik 1990 | Number of face‐to‐face consultations per 1000 enlisted patients | 4.9 | −0.5 | −10.2% |
| Number of consultations by telephone per 1000 enlisted patients | 4 | 1.4 | +35% | |
| Number of renewal of prescriptions per 1000 enlisted patients | −7.4 | −27.4 | +370.27% | |
| Number of diagnostic services per 1000 enlisted patients | 7.6 | 51.9 | +682.89% | |
| Number of curative services per 1000 enlisted patients | 15 | 79.8 | +532% | |
| Number of referrals to specialist per 1000 enlisted patients | −1.2 | −21.1 | −1110.53% | |
| Number of referrals to hospital per 1000 enlisted patients | 2.1 | −33.7 | −1604.76% | |
| Gosden 2003 | Number of surgery consultations per GP per practice | −18.21 | 4.47 | −24.55% |
| Number of patients seen out‐of‐hours per GP per practice | −8.5 | 4.5 | −52.94% | |
| % consultations in which prescription given per GP per practice | 0.03% | −0.07 | −233.33% | |
| % consultations in which referral made per GP per practice | 0 | −0.01 | ‐ | |
| Total list size practice‐based | 305.2 | −213.4 | −69.92% | |
| List size per whole time practice‐based | −45.25 | 20.20 | −44.64% | |
| Cervical cytology (%) practice‐based | −1.8% | 0.38 | −21.11% | |
| Childhood immunisation (%) practice‐based | 0.35% | −1.08 | −240% | |
| Pre‐school booster (%) practice‐based | −1.69% | −3.08 | +133.33% | |
| Flierman 1992 | Blood sample | 0.22 | 0.04 | +18% |
| Cervical smear | −0.4 | −0.02 | +5% | |
| Pregnancy test | 0.09 | 0.01 | +11% | |
| Proctoscopy | 0.009 | 0.03 | +317% | |
| Electrocardiogram | 0.009 | 0.01 | +109% | |
| Haemoglobin measurement | 0.52 | 0.27 | +52% | |
| Blood glucose (photometer) | 0.02 | 0.12 | +585% | |
| Streptoculture or urine culture | 0.03 | 0.07 | +211% | |
| Inoculation for cultivation | 0.69 | 0.47 | +68% | |
| Urine test with sticks | 0.82 | 0.42 | +51% | |
| Urine microscopy | 0.89 | 0 | +53% | |
| Urine culture with sensitivity | 0.02 | 0.05 | +265% | |
| Removing warts | −0.11 | −0.12 | +114% | |
| Removing ear wax | 0.31 | 0.05 | +16% | |
| Removing corpora aliena from eye/ear/nose/throat | −0.024 | 0.01 | −41% | |
| Removing corpora aliena from skin/from under nail | 0.09 | 0.05 | +54% | |
| Incision or excision of abscess or tumour | 0.05 | 0.1 | +198% | |
| Treating a large wound | 0.06 | 0.04 | +66% | |
| Dressing an immobilising bandage | 0.05 | 0.08 | +173% | |
| Synthesised effect across the above 3 studies (median) | ‐ | ‐ | +51% | |
| Quality of health services provided | ||||
| Gosden 2003 | Access | −0.43 | −1.07 | +248.84% |
| Technical care | −0.74 | 1.28 | −172.97% | |
| Communication | 0.95 | −0.09 | −9.47% | |
| Interpersonal care | 0.69 | 0.18 | +26.09% | |
| Overall satisfaction | 2.41 | 2.36 | +97.93% | |
| Receptionists | −1.23 | 0.64 | −52.03% | |
| Continuity of care | −0.77 | 0.29 | −37.66% | |
| Trust in doctor | 0.51 | 0.57 | +111.76% | |
| Doctors' knowledge of patient | −0.08 | 1.18 | −14.75% | |
| Practice nursing | −0.61 | −0.23 | +37.7% | |
| Co‐ordination of care | 1.52 | 0.63 | +121.15% | |
| Appropriate referral | −0.58 | 0.91 | −156.9% | |
| Recommend to a friend | −0.61 | 1.71 | −280.33% | |
| Synthesised effect inside the study (median) | ‐ | ‐ | −9.47% | |
| Healthcare provider outcomes | ||||
| Gosden 2003 | Total daytime working times | −2.22 | 2.22 | −100% |
| Hours in surgery | −1.75 | 2.03 | −116% | |
| Consultation length (min) | 0.14 | −0.17 | −121.43% | |
| Practice administration (min) | −5.45 | −7.05 | +129.36% | |
| On‐call at weekend/night (h) | −4.13 | 5.42 | −131.23% | |
| Other activities (h) | −1.6 | 1.57 | −98.13% | |
| Gray 2015 | Annual incomes (FHN) | 1514 | 39962 | +2621.66% |
| Annual incomes (FHG) | 1514 | 13398 | +884.94% | |
| Synthesised effect across the above 2 studies (median) | ‐ | ‐ | −99.07% | |
| Patient outcomes | ||||
| Jensen 2014 | Average birth weight | 15 | 36 | +240% |
| Proportion of low birth weight | −0.3% | −0.4% | +133.33% | |
| Proportion of preterm birth: infants have a 1.9 percentage point (36.5% from the base of 5.2% births) higher probability of preterm birth | 0.9% | −1.7% | −188.89% | |
| Proportion of very preterm birth: no difference | 0.1% | −0.1% | −100% | |
| Mean rate of fetal growth | 0.32 | 0.92 | +287.5% | |
| Synthesised effect inside the study (median) | ‐ | ‐ | +133.33% | |
7. Effect measures of included interrupted time series and repeated measures studies (relative change).
| Study | Outcome measures | Control/baseline level | Absolute change | Relative change |
| Quantity of health services | ||||
| Young 2007 | Mean rate of glycated haemoglobin check | 56% | 7% | +12.5% |
| Mean rate of urinalysis check | 61% | 9% | +14.75% | |
| Mean rate of LDL check | 58% | 21% | +36.21% | |
| Mean rate of eye exams | 40% | 14% | +35% | |
| Quality of health services | ||||
| Young 2012 | Performance scores of eye examination | 0.1 | 0.11 | +110% |
| Performance scores of glycated haemoglobin test | 0.08 | 0.03 | +37.5% | |
| Performance scores of LDL screen | 0.14 | 0.04 | +28.57% | |
| Performance scores of nephropathy test | 0.09 | 0.03 | +33.33% | |
| Synthesised effect inside the study (median) | ‐ | ‐ | +34.17% | |
LDL: low‐density lipoprotein
8. Effect measures of included interrupted time series and repeated measures studies (change in level and trend).
| Comparison 1: P4P plus existing payment methods compared with existing payment methods | |||||
| Study | Immediate change in level | Change in trend |
Other effects results reported by authors |
||
| Estimate | Confidence interval | Estimate | Confidence interval | ||
| Young 2007, RM | |||||
| Adherence rate of glycated haemoglobin tests | 0.03 | 0.01 to 0.05 | 0.07 | 0.03 to 0.10 | ‐ |
| Adherence rate of urinalysis | 0.03 | 0.002 to 0.06 | 0.09 | 0.06 to 0.11 | ‐ |
| Adherence rate of lipoprotein density level screening | 0.05 | 0.02 to 0.08 | 0.2 | 0.18 to 0.24 | ‐ |
| Adherence rate of eye examination | 0.07 | 0.04 to 0.09 | 0.14 | 0.11 to 0.17 | ‐ |
| Young 2012, RM | |||||
| Performance scores of eye examination | 0.09 | 0.01 to 0.17 | 0.11 | 0.01 to 0.21 | ‐ |
| Performance scores of glycated haemoglobin tests | 0.04 | 0.01 to 0.07 | 0.03 | 0.02 to 0.04 | ‐ |
| Performance scores of lipoprotein density level screening | 0.03 | 0.02 to 0.04 | 0.04 | 0.01 to 0.07 | ‐ |
| Performance scores of nephropathy test | 0.02 | 0.01 to 0.03 | 0.03 | 0.01 to 0.05 | ‐ |
P4P: pay for performance RM: repeated measures study
COMPARISON 1: P4P plus existing payment methods compared with existing payment methods for healthcare providers working in outpatient healthcare settings
Fourteen studies compared P4P plus existing payment method with an existing payment method (Christensen 2000; Chung 2010; Fairbrother 1999; Fairbrother 2001; Gleeson 2017; Greene 2013; Houle 2016; Kouides 1998; Lee 2010; Li 2013; Petersen 2013; Singh 2015; Young 2007; Young 2012).
The outcomes measured in this comparison group included quantity of health service provision, quality of health service provision, patient outcomes, costs of service provision, and adverse effects. Healthcare provider outcomes and secondary outcomes were not reported.
See: Table 1; Figure 2; Figure 3.
2.

Forest plot of comparison 1: Comparison between P4P plus existing payment method and existing payment on the quantity of health services delivered by healthcare providers working in outpatient care facilities (immunisation coverage status for children). A risk ratio greater than 1 favours P4P plus existing payment method.
3.

Forest plot of comparison 1: Comparison between P4P plus existing payment methods and existing payment methods on the quantity of health services (services coverage rate). A standardised mean difference greater than 1 favours P4P plus existing payment method.
Effect on quantity of health service provision
Four randomised trials evaluated the effect of adding P4P on quantity of health services provided (Christensen 2000; Fairbrother 1999; Fairbrother 2001; Kouides 1998). Meta‐analysis of dichotomous data suggests that adding P4P probably increases the up‐to‐date immunisation status of children aged 3 to 35 months on Medicaid in the USA, compared with an existing payment method (risk ratio (RR) 1.27, 95% confidence interval (CI) 1.19 to 1.36; Analysis 1.1; moderate‐certainty evidence) (Fairbrother 1999; Fairbrother 2001). Analysis of continuous data suggests that an extra P4P intervention may slightly increase the number of patients who are asked more detailed questions by their pharmacist related to their disease (mean difference (MD) 1.24, 95% CI 0.93 to 1.54; Analysis 2.1; low‐certainty evidence) (Christensen 2000). We downgraded the certainty of evidence by one level for limitations in study design and one level for indirectness. We are uncertain if adding P4P improves immunisation rates amongst ambulatory Medicare patients aged 65 or older, compared with an existing payment method (MD 0.34, 95% CI −0.20 to 0.87; Analysis 2.1; very low‐certainty evidence) (Kouides 1998). The certainty of this evidence was further downgraded one level because of imprecision in addition to limitations in study design and indirectness.
1.1. Analysis.

Comparison 1: (Comparison 1) Effects of P4P plus existing payment methods compared to existing payment methods: dichotomous quantity of health services provided, Outcome 1: Quantity of health services provided (immunisation coverage status)
2.1. Analysis.

Comparison 2: (Comparison 1) Effects of P4P plus existing payment methods compared to existing payment methods: continuous quantity of health services provided, Outcome 1: Quantity of health services provided (services coverage rate)
Four CBA studies, Gleeson 2017; Greene 2013; Lee 2010; Li 2013, and one ITS study, Young 2007, also reported on quantity of health service provision. We are uncertain of the effect of P4P on quantity of health services reported in these studies due to very low‐certainty evidence. The results for the CBA and ITS studies are reported in Table 10 and Table 11.
Effect on quality of health service provision
One randomised trial found that P4P may slightly improve primary care physicians' prescribing practices for guideline‐recommended antihypertensive medicines for Medicare patients with hypertension, compared with an existing payment method (RR 1.07, 95% CI 1.02 to 1.12; Analysis 3.1; low‐certainty evidence) (Petersen 2013). We downgraded the certainty of evidence by one level for limitations in study design and one level for indirectness (only one study targeting primary care physicians in the USA).
3.1. Analysis.

Comparison 3: (Comparison 1) Effects of P4P plus existing payment methods compared to existing payment methods: dichotomous quality of health services provided, Outcome 1: Quality of health services provided of Petersen 2013 (use of guideline)
One CBA study, Chung 2010, and one ITS study, Young 2012, also reported on quality of health services provision. We are uncertain of the effect of P4P on the quality of screening services provided by primary health physicians (e.g. performance scores for eye examination, glycated haemoglobin tests, lipoprotein density level screening, and nephropathy test) due to very low‐certainty evidence. The results for the CBA and ITS studies are reported in Table 10 and Table 11.
Effect on patient outcomes
One randomised trial measured effect on health outcomes (Houle 2016). We are uncertain of the effect of P4P for pharmacists on mean blood pressure reduction of patients with above‐target blood pressure, compared with an existing payment method (MD 0.07 mmHG, 95% CI −2.22 to 2.37; Analysis 4.1; very low‐certainty evidence). The evidence is of very low certainty due to limitations in study design, indirectness, and imprecision.
4.1. Analysis.

Comparison 4: (Comparison 1) Effects of P4P plus existing payment methods compared to existing payment methods: continuous patient outcomes, Outcome 1: Health outcomes of Houle 2016 (blood pressure reduction (mmHG))
In addition, one randomised trial found that P4P may improve a mixed outcome measure of service provision and patient health outcomes (change in blood pressure control or appropriate response to patients with uncontrolled blood pressure), compared with an existing payment method (RR 1.13, 95% CI 1.04 to 1.23; Analysis 5.1; low‐certainty evidence) (Petersen 2013). We downgraded the certainty of evidence by one level for limitations in study design and one level for indirectness.
5.1. Analysis.

Comparison 5: (Comparison 1) Effects of P4P plus existing payment methods compared to existing payment methods: dichotomous mixed outcomes, Outcome 1: Mixed provision and health outcomes of Petersen 2013 (control of blood pressure or follow the guideline)
One CBA study reported health outcomes for children (Singh 2015). Because the certainty of the evidence is very low, we are uncertain of the effect of P4P on children's weight and grade of malnourishment (calculated according to the Indian Association of Paediatricians and the World Health Organization). The results are reported in Table 10.
Effect on cost
One CBA study with high risk of bias found that the net increase on expenditures on all diabetes‐related health services in the P4P programme was USD 104, a 67.62% increase compared with expenditures in the control group (P < 0.001) (Lee 2010). However, we are uncertain of the effect of P4P on expenditures for diabetes‐related services because the certainty of the evidence is very low due to study limitations and indirectness.
Unintended or adverse effects
One randomised trial found that after the P4P intervention ends, and there are no extra financial rewards for performance, there may be an important reduction in patients with good blood pressure control as well as in whether primary care physicians respond appropriately to uncontrolled blood pressure in the intervention group, compared with the control group (low‐certainty evidence, downgraded one level for design limitations and one level for indirectness) (Petersen 2013).
COMPARISON 2: FFS compared with existing payment methods for healthcare providers working in outpatient healthcare settings
Five studies compared FFS to existing payment methods, including capitation, Davidson 1992; Lurie 1992; Yesalis 1980; Yesalis 1984, and salary, Hickson 1987.
The studies included in this comparison measured quantity of health service provision, patient outcomes, costs, and adverse effects. None of the studies reported quality of service provision, healthcare provider outcomes, and secondary outcomes.
See: Table 2.
Effect on quantity of health service provision
Three randomised trials measured outcomes related to the quantity of health service provision, including the number of outpatients visits, specialist visits, or hospitalisations (Davidson 1992; Hickson 1987; Lurie 1992). Although these outcomes all fall under the same outcome category for this review, the specific indicators and measurement methods used in the three studies differed. Due to heterogeneity and insufficient data for meta‐analysis, we used the median of relative changes to summarise the results. We are uncertain of the effect of FFS compared with existing payment methods on the number of patient visits to health facilities (median relative change: +10.44%; range: −460% to +175.64%; Table 9, 'Comparison 2 FFS compared with existing payment methods'; very low‐certainty evidence). We downgraded the certainty of evidence two levels for limitations in study design and one level for imprecision.
Two randomised trials found that office‐based physicians paid by capitation may have fewer numbers of outpatients visits relative to physicians paid by FFS (Davidson 1992; Lurie 1992). Lurie 1992 reported that fewer patients of physicians paid capitation may receive outpatient health care compared to patients in the FFS group (61% versus 71%). Davidson 1992 reported that compared to the reduction of non‐primary health visits in the capitation physician group, there may be an increase of non‐primary health visits in the FFS physician group (−0.05 versus 0.18). These results are consistent with the theory that the financial incentives in capitation will lead to cost containment through behaviours that reduce provision of outpatient and referral services.
Two CBA studies also reported on quantity of service provision, including the number of prescriptions of generically equivalent medicines with different prices (Yesalis 1980; Yesalis 1984). We are uncertain of the effect of FFS on the number of prescriptions dispensed by pharmacists, specifically the quantity of multi‐source products used (generic drug products with the comparable bioavailability from more than one labeller) due to very low‐certainty evidence. (See Table 10.)
Effect on patient outcomes
One randomised trial found inconsistent evidence on different health outcome indicators, including general self‐rated health status and some specific indicators of psychological health and behaviours, for Medicaid beneficiaries with chronic mental illness (Lurie 1992). Compared to patients in the FFS payment group, patients in a prepaid capitation plan had varied relative changes for these health outcome measures, ranging from −492% to +350%, and with a median relative change of −9.84%. However, we are uncertain of the effect of FFS on patient outcomes compared with a capitated payment method due to very low‐certainty evidence (downgraded one level for limitations in study design, one level for indirectness, and one level for imprecision).
Effect on cost
The two CBA studies reported effects on cost. We are uncertain of the effect of FFS on costs, compared with existing payment methods, due to very low‐certainty evidence (downgraded due to limitations in study design). Yesalis 1980 showed that there may be savings in the capitation group, compared to FFS, because of higher rates of prescribing low‐cost generic pharmacy substitutes by pharmacists (mean cost saving per generic substitution increasing from USD 0.24 to 2.73 in the Capitation group and decreasing from USD 1.04 to 0.88 in the FFS group). These differences were not found in Yesalis 1984, which was conducted in the expanded stage of the same capitation plan evaluated by Yesalis 1980.
Unintended or adverse effects
Hickson 1987 reported that physicians reimbursed by FFS may schedule more visits per patient and may see their patients more often than salaried physicians. Further analysis suggested that this may be due to FFS physicians seeing more "well patients" than salaried physicians, highlighting that more services that were unnecessary were delivered. The certainty of this evidence was low due to limitations in study design and indirectness.
COMPARISON 3: FFS mixed with existing payment methods compared with existing payment methods for healthcare providers working in outpatient healthcare settings
A total of three randomised trials, Bilardi 2010; Clarkson 2008; Twardella 2007, and five CBA studies, Flierman 1992; Gosden 2003; Gray 2015; Jensen 2014; Krasnik 1990, compared a mix of FFS and other payment methods with a single existing method. The mixed methods included FFS mixed with capitation, Flierman 1992; Gray 2015; Jensen 2014; Krasnik 1990, and FFS mixed with salary (Gosden 2003). The single existing payment methods included salary (Gosden 2003), capitation (Clarkson 2008; Flierman 1992; Jensen 2014; Krasnik 1990), and FFS (Gray 2015). In two studies (Bilardi 2010; Twardella 2007), the control payments were not explicitly described, but it was clear that the FFS payments in the intervention group were added to an existing payment. The FFS mixed payments intervention may be categorised into two types: in three studies (evaluated by three randomised trials; Bilardi 2010; Clarkson 2008; Twardella 2007), the payments constituted extra funding, in which insurers allocated additional funds to incentivise health providers (rewarding health providers for each instance of services they provided); and in five studies (evaluated by five CBA studies; Flierman 1992; Gosden 2003; Gray 2015; Jensen 2014; Krasnik 1990), the purchasers did not increase the total payment received by health providers, but just adjusted payment from a single type (capitation or salary or FFS) to the FFS mixed payment method.
The eight included studies for this comparison measured quantity of health service provision, quality of health service provision, patient outcomes, and healthcare provider outcomes. None of the included studies reported costs, adverse effects, and secondary outcomes.
4.

Forest plot of comparison 2: Comparison between FFS mixed payment and single payment on quantity of services delivered by healthcare providers working in outpatient care facilities (services coverage status). A risk ratio greater than 1 favours FFS mixed payment.
Effect on quantity of health service provision
Two randomised trials evaluated the quantity of health services provided (Bilardi 2010; Clarkson 2008), including the proportion of women tested for chlamydia and of children with at least one dental sealant treatment. Meta‐analysis of the data from these trials suggests that the extra funding paid by FFS may increase the quantity of health service provision compared with a single payment method (RR 1.37, 95% CI 1.07 to 1.76; Analysis 6.1; low‐certainty evidence). We assessed the evidence as of low certainty owing to unclear risk of bias, downgrading one level for limitations in study design and one level for imprecision (very small numbers of participants in both studies).
6.1. Analysis.

Comparison 6: (Comparison 3) Effects of FFS mixed with existing payment methods compared to single payment method: dichotomous quantity of health services provided, Outcome 1: Quantity of health services provided (services coverage status)
Two CBA studies also reported on quantity of health service provision (Flierman 1992; Krasnik 1990). We are uncertain of the effect of FFS mixed with other payments on the numbers of face‐to‐face consultations, consultations by telephone, diagnostic services, and curative services, compared with a single payment method due to very low‐certainty evidence. The effect measures for these studies are reported in Table 10.
Effect on quality of health service provision
In one CBA study (Gosden 2003), quality of services was measured through patients' satisfaction with different aspects of care, such as access, technical care, communication, interpersonal care, etc. Although satisfaction was rated higher for three out of 13 quality aspects in FFS mixed practices and 10 out of 13 quality aspects in salaried practices, we are uncertain of the effects of FFS mixed with other payments on the quality of health services provided compared with a single payment method due to very low‐certainty evidence.
Effect on patient outcomes
One randomised trial evaluated one patient behaviour change outcome: the prevalence of smoking abstinence at 12 months (Twardella 2007). We are uncertain of the effect of FFS mixed with other payments on patient behaviour change outcomes due to very low‐certainty evidence (RR 1.20, 95% CI 0.70 to 2.08; Analysis 7.1), downgraded for limitations in study design and indirectness.
7.1. Analysis.

Comparison 7: (Comparison 3) Effects of FFS mixed with existing payment methods compared to single payment method: dichotomous quality of health services provided, Outcome 1: Quality of health services provided (patients' behaviour change)
One CBA study also reported effect on patient outcomes (Jensen 2014). We are uncertain of the effect of FFS mixed with other payments on infant health outcomes such as birth weight, preterm birth, and rate of fetal growth due to very low‐certainty evidence. The effect measures for this study are reported in Table 10.
Effect on healthcare provider outcomes
Two CBA studies assessed the effects of FFS mixed with other payments on healthcare provider outcomes (Gosden 2003; Gray 2015).
Gray 2015 compared the annual income of family physicians in Ontario, Canada. Their findings suggest that adopting a mixed payment model may increase the incomes of family physicians compared with those paid by pure FFS (physicians who adopted the mixed‐payment approach of mainly FFS and a small portion of capitation gained between 28% and 33% in terms of their real salaries). It is not clear whether the working times of physicians who were paid by standard contract (FFS mixed with capitation) were longer than those paid by salary alone, due to very wide confidence intervals around the estimate (2.22 hours longer for the standard contract (95% CI −8.69 to 13.14 hours)) (Gosden 2003). These physicians were rewarded financially for increasing their patient list size through capitation payments. Overall, we are uncertain of the effect of FFS mixed with other payment methods on healthcare provider outcomes compared with an existing payment method due to very low‐certainty evidence (downgraded for limitations in study design).
COMPARISON 4: Enhanced FFS compared with FFS for healthcare providers working in outpatient healthcare settings
Three randomised trials evaluated the effects of increasing the FFS unit payment rate on quantity of health service provision and costs of service provision (Davidson 1992; Fairbrother 1999; Fairbrother 2001). None of the included studies reported quality of health service provision, patient health outcomes, healthcare provider outcomes, adverse effects, and secondary outcomes.
5.

Forest plot of comparison 4: Comparison between enhanced FFS and FFS on quantity of services delivered by healthcare providers working in outpatient care facilities (immunisation coverage status for children). A risk ratio greater than 1 favours enhanced FFS.
Effect on quantity of health service provision
Two randomised trials evaluated if enhanced FFS to physicians affects the immunisation rates of covered children (Fairbrother 1999; Fairbrother 2001). Fairbrother 2001 is a follow‐up study to Fairbrother 1999, but is treated here as a separate study because, after the first stage of the study (reported in 1999), the allocation of physicians to different payments was changed. Meta‐analysis of these two studies shows that enhanced FFS probably increases the proportion of children aged 3 to 35 months on Medicaid whose immunisation status is up‐to‐date, compared with FFS (RR 1.25, 95% CI 1.06 to 1.48; Analysis 8.1; moderate‐certainty evidence, downgraded one level for limitations in study design).
8.1. Analysis.

Comparison 8: (Comparison 4) Effects of enhanced FFS compared to FFS: dichotomous quantity of health services provided, Outcome 1: Quantity of health services provided (immunisation coverage status)
One randomised trial compared the effect of different FFS rates on utilisation of health services for children on Medicaid, but the data provided were insufficient to include in the meta‐analysis reported above (Davidson 1992). We are uncertain of the effect of paying higher fees for service on the number of primary care visits by children compared with the normal level of fees due to very low‐certainty evidence. The adjusted analysis reported by the study authors showed that children in the high‐rate FFS group may have 0.77 to 0.92 more primary care visits per year, compared to those in the low‐rate FFS group (evidence downgraded two levels due to high risk of bias and one level due to indirectness).
Effect on cost
It is uncertain if the enhanced FFS increased net expenditure per year on eligible children compared with regular FFS. Davidson 1992 showed that net expenditure per year of eligibility was estimated at USD 56.19 higher for children under the enhanced FFS programme than would have been the case under the regular FFS within the Medicaid programme; however, the certainty of the evidence was downgraded to very low due to limitations in study design and indirectness.
Discussion
Summary of main results
See Table 1; Table 2; Table 3; Table 4.
The included studies covered different kinds of payments to individual healthcare providers working in outpatient healthcare settings: salary, capitation systems, FFS systems, P4P, and mixed payment method system. We evaluated four major kinds of payment comparisons, which are described below.
The first comparison was P4P plus existing payment methods for healthcare providers working in outpatient healthcare settings compared with existing payment methods which included capitation, FFS, and salary. Extra funding paid by P4P probably increases up‐to‐date immunisation coverage for children between 3 and 35 months on Medicaid (RR 1.27, 95% CI 1.19 to 1.36; moderate‐certainty evidence). We are uncertain of the effects of adding P4P to existing payment methods on influenza vaccination rates amongst the outpatient elderly, compared with existing payment methods (MD 0.34, 95% CI −0.20 to 0.87; very low‐certainty evidence). Extra P4P incentives may result in a slight increase in pharmacists asking more detailed questions on patients' diseases (MD 1.24, 95% CI 0.93 to 1.54; low‐certainty evidence). It is uncertain if adding P4P incentives to existing payment methods could change blood pressure control (MD 0.07, 95% CI −2.22 to 2.37; very low‐certainty evidence).
The second comparison was FFS compared with existing payment methods which were capitation and salary. The measurements of quantity of services and patient health outcomes for the studies in this comparison were heterogeneous, and it is uncertain if FFS results in an increase of outpatients and inpatients provision (median of relative change being +10.44%, ranging from −460% to 175.65%; very low‐certainty evidence). We are also uncertain of the effect of FFS on patient health outcomes compared with capitation or salary (median of relative change being −9.84%, ranging from −492% to 350%; very low‐certainty evidence).
The third comparison was FFS mixed payment methods compared with a single payment method, with most of the mixed payment methods being FFS plus capitation. FFS mixed payment methods (i.e. portion of income paid by FFS) may increase the quantity of certain testing services for women and children (RR 1.37, 95% CI 1.07 to 1.76; low‐certainty evidence). Based on evidence from one randomised trial, we are uncertain of the effect of FFS mixed payment methods on patient outcomes, measured as smoking abstinence behaviour (RR 1.20, 95% CI 0.70 to 2.08; very low‐certainty evidence).
The fourth comparison was changes of the design of a payment method, that is an increase in FFS fees paid per service provided. Two randomised trials evaluated the effect of increasing FFS rate levels on the immunisation status of children, finding that compared with the low‐rate group, the high‐rate FFS group probably had higher immunisation rates (RR 1.25, 95% CI 1.06 to 1.48; moderate‐certainty evidence).
Overall completeness and applicability of evidence
The participants included in this review were different kinds of healthcare providers working in outpatient care facilities. They included primary care physicians or family physicians (19 studies), mental health physicians (1 study), pharmacists (4 studies), dentists (1 study), physician or office staff (1 study), and healthcare providers working in daycare centres (1 study). Outpatient care facilities included private office‐based practice; public clinics and health centres; hospital outpatient clinics; community health centres; general clinics; and primary care practices.
The studies included in this review involved the full range of payment methods for individual healthcare providers: salary, FFS, capitation, P4P, and blended payment. We furthermore included studies of changes in the design of one form of payment (FFS). Nevertheless, there are many possible comparisons across these payment methods as well as possible changes to these payment methods for which eligible studies were not identified. The comparisons we included and evaluated in this review do not therefore provide a complete evidence base for policymaking, especially considering the variations in existing payment methods for healthcare providers in outpatient care facilities across countries.
With regard to outcomes, most of the included studies evaluated the quantity of health services provision and patient health outcomes. Several included studies also assessed the effects of payments on the quality of health services. A limited number of studies evaluated the effects on the cost of service delivery, and only one included study examined physician satisfaction. Only two studies assessed adverse effects of payment interventions.
Most of included studies were implemented by health insurance schemes and purchasers in high‐income countries. More evidence from low‐ and middle‐income countries is therefore needed.
None of the outcomes in this review were assessed as providing high‐certainty evidence, and only limited evidence of moderate certainty was identified, which has some important implications for policymaking. Firstly, most of the robust evidence provided in this review was on the effects of payment interventions involving extra funding to provide incentives for healthcare providers. We found little evidence on the effects of changes in payment models within existing levels of funding. This will restrict the applicability of the review findings in settings with limited resources where additional funding may not be readily available. Secondly, even though we found evidence that financial incentives through P4P or enhanced FFS may provide some benefits, theoretically the size of effects is still dependent on many specific aspects of the design of these incentives programmes (Ogundeji 2016; Van Herck 2010). This includes, for example, the type of performance target, performance measurement methods, etc. However, details of this type were seldom reported in the included studies. In addition, the limited number of included studies in each comparison prevented us from conducting subgroup analyses regarding the design features of payment schemes.
Certainty of the evidence
The overall certainty of evidence on the effects of P4P plus existing payment methods compared with existing payment methods was moderate. A common problem of the 14 randomised trials included in this comparison was inadequate description of the random allocation method.
The overall certainty of evidence on the effects of FFS compared with the existing payment methods was very low. We were unable to pool data from the three randomised trials in this comparison due to heterogeneous outcome measures and insufficient data. The three trials also had design limitations including providing insufficient information on protection against contamination and on methods of random allocation.
Three randomised trials and five CBA studies compared the effects of FFS mixed with existing payment methods versus single payment methods such as salary, capitation, or FFS. The overall certainty of evidence from the randomised trials was low; common design limitations included insufficient information on protection against contamination and on methods of random allocation.
Three randomised trials evaluated changes in the design of FFS payments. The overall certainty of the evidence on effects of increasing FFS unit payment level was moderate, due to design limitations including providing insufficient information on the random allocation method and how the study was protected against contamination.
Potential biases in the review process
We carried out an extensive search of the literature to ensure that all relevant studies were identified, yet the possibility remains that some unpublished studies could have been missed. We contacted the study authors for missing data during the data analysis process, but at the time this review was completed had not received any responses.
Agreements and disagreements with other studies or reviews
Previous reviews have focused only on physician payment methods and conducted a narrative synthesis of results. In this review we focused on payment methods for all healthcare providers in outpatient healthcare settings, and on how all types of payment methods affect the behaviours of these healthcare providers. We categorised the outcome indicators as follows: quantity and quality of health services provision; patient health outcomes; costs of health care; healthcare provider outcomes; and patient satisfaction. These outcome groups reflect the different likely effects of provider payments. We have also conducted meta‐analysis where feasible and appropriate.
There are several Cochrane Reviews on payment methods, but these have different PICOs to this review (Table 13). Giuffrida 1999 focuses only on target payments; Gosden 2000 compared different payment methods which could affect the clinical behaviour of primary care physicians based on few included studies; Scott 2011 expanded the interventions to all types of financial incentives and narrowed the outcome of interest to quality of health care; Witter 2012 evaluated paying‐for‐performance schemes targeting different levels of providers in low‐ and middle‐income countries; Brocklehurst 2013 focused on clinical activity undertaken by primary care dentists; and Flodgren 2011 conducted an overview of reviews evaluating the effectiveness of financial incentives in changing healthcare provider behaviour and patient outcomes. In addition, there are several non‐Cochrane systematic reviews with a focus on payment methods (Chaix 2000; Mendelson 2017; Petersen 2006). As almost all of the reviews described above are very out‐of‐date, the current update which includes newly published primary studies was needed.
9. Published Cochrane Reviews on payment.
| Review | Focus | Key findings |
| Giuffrida 1999 | Focuses on “target payments” ‐ linking payment to a specific level of activity or quality | Linking payment to physicians' target behaviours was associated the increased immunisation rates. |
| Gosden 2000 | Focuses on the effects of different payment methods on the clinical behaviours of primary care physicians | Fee‐for‐service resulted in a higher number of service provision process outcomes compared with fixed payment. |
| Scott 2011 | Includes all kinds of payment changes, but focuses on outcomes related to quality of health care | Linking financial incentives directly to quality of primary care physicians had moderate positive effects. |
| Witter 2012 | Focuses on paying‐for‐performance schemes targeting different levels of healthcare providers in low‐ and middle‐income countries | There were mixed results on the effects of performance‐based funding on service provision and health outcome, and the effects of paying for performance depended on intervention design. |
| Brocklehurst 2013 | Focuses on participants and outcomes related to clinical activities undertaken by primary care dentists | Limited included studies provided low‐/very low‐evidence showing financial incentives within remuneration systems may produce changes to clinical activity undertaken by primary care dentists. |
| Flodgren 2011 | An overview of reviews evaluating the effectiveness of all kinds of payments at both the individual and organisation level on changing healthcare provider behaviour and patient outcomes | Financial incentives may be effective in changing healthcare provider practice. The evidence has serious methodological limitations and is also very limited in its completeness and generalisability. |
Authors' conclusions
Implications for practice.
Adding pay for performance (P4P) to existing payment methods and increasing fee‐for‐service (FFS) payment levels (enhanced FFS) for healthcare providers working in outpatient healthcare settings probably increases the quantity of targeted health services provision (with moderate‐certainty evidence), whilst adding P4P to existing payment methods may slightly improve the quality of targeted service provision (with low‐certainty evidence). However, we are uncertain of the effects of changes in payment methods on patient behaviour or their health outcomes, based on low‐ or very low‐certainty evidence. If policymakers intend to incentivise outpatient healthcare providers to supply particular essential health and public health services, they might consider implementing P4P or increasing the unit payment rate of FFS. There is also some evidence that P4P may be an option if policymakers are considering ways of improving the quality of health services delivered by outpatient healthcare providers. Where policymakers identify that services of low value are being overprovided, they may want consider switching away from FFS payment.
We also found potential adverse effects of financial incentives: the review suggests that when the financial rewards used to encourage the provision of certain services are stopped, the improved performance of incentivised physicians could not be sustained and may decrease more for this group than for physicians who were never incentivised (Petersen 2013). Considering that the sustainability of financial incentive programmes is difficult due to the need for additional funding, our finding on the adverse effects of stopping incentives suggests that payers may need to be both cautious in initiating these schemes and to consider if they can be sustained in the longer term. In addition, P4P schemes have been found by some qualitative studies to have negative impacts on medical professionalism and clinical autonomy, and to encourage healthcare providers to prioritise their own pay rather than patients' best interests (Gillam 2012; Hendrickson 2008; Lester 2013). Policymakers or health services purchasers should keep in mind that other non‐financial interventions, such as educational meetings, audit and feedback, and promoting interprofessional collaboration (Ivers 2012; O'Brien 2007; Reeves 2013), are alternatives for changing the behaviours of healthcare providers.
Implications for research.
Whilst we included a fairly large number of studies in this review, we have identified a number of important research areas for future studies, as described below.
Most of the studies included in this review were conducted in high‐income countries. Well‐conducted comparative evaluations of payment method options for outpatient care professionals in low‐ and middle‐income countries are needed.
Few studies included in this review analysed the cost‐effectiveness of the payment changes evaluated; this needs to be built into future studies.
Few studies targeted non‐physician healthcare providers in outpatient healthcare settings. Given the wide range of healthcare providers working in these settings, studies of other kinds of professional providers would be helpful.
Further well‐conducted studies to compare or evaluate the effects of different designs of the same payment method (e.g. different designs of P4P incentives programmes or FFS models) are needed.
There is a need for studies with theory‐informed qualitative components that explore why particular payment interventions work or not, and under what circumstances, as well as evaluating the mechanisms through which payment interventions impact on the behaviours of individual professionals.
As payment method interventions may have unintended consequences (e.g. P4P schemes leading to the neglect of non‐incentivised services), studies evaluating these adverse effects/unintended consequences are needed. Such evidence could support the better design of payment methods for healthcare providers.
Both researchers and policymakers would benefit from future studies that consistently reported information on health system contexts in the study settings and detailed descriptions of the payment interventions used.
History
Protocol first published: Issue 9, 2015 Review first published: Issue 1, 2021
Notes
Acknowledgements
This review was funded by the Alliance for Health Policy and Systems Research, World Health Organization, and Shandong Social Science Planning (18CZKJ22). Technical support was provided through the Norwegian Satellite of Cochrane Effective Practice and Organisation of Care (EPOC). Thanks to Marit Johansen for assisting in designing the search strategy and citation search. Thanks to Chris Rose for assisting in data analysis. Particular thanks to Elizabeth Paulsen and Simon Lewin for their considerable support at all stages of the review process.
The Norwegian Satellite of Cochrane Effective Practice and Organisation of Care (EPOC) receives funding from the Norwegian Agency for Development Cooperation (Norad), via the Norwegian Institute of Public Health, to support review authors in the production of their reviews.
Appendices
Appendix 1. Search strategies
Cochrane Database of Systematic Reviews, part of the Cochrane Library (www.cochranelibrary.com/) (searched 5 March 2019) Database of Abstracts of Reviews of Effects, part of the Cochrane Library (www.cochranelibrary.com/) (searched 15 July 2017)
| ID | Search |
| #1 | MeSH descriptor: [Physician Incentive Plans] this term only |
| #2 | MeSH descriptor: [Group Practice, Prepaid] this term only |
| #3 | MeSH descriptor: [Reimbursement Mechanisms] this term only |
| #4 | MeSH descriptor: [Reimbursement, Incentive] this term only |
| #5 | MeSH descriptor: [Prospective Payment System] this term only |
| #6 | MeSH descriptor: [Single‐Payer System] this term only |
| #7 | MeSH descriptor: [Prepaid Health Plans] this term only |
| #8 | MeSH descriptor: [Capitation Fee] this term only |
| #9 | MeSH descriptor: [Fees and Charges] this term only |
| #10 | MeSH descriptor: [Fee‐for‐Service Plans] this term only |
| #11 | MeSH descriptor: [Salaries and Fringe Benefits] this term only |
| #12 | MeSH descriptor: [Remuneration] this term only |
| #13 | MeSH descriptor: [Value‐Based Purchasing] this term only |
| #14 | {or #1‐#13} |
| #15 | (reimbursement or economic next incentive* or financial next incentive* or monetary next incentive* or economic next reward* or financial next reward* or monetary next reward* or incentive next payment* or payment next incentive* or performance next bonus* or bonus next payment* or "bonus for practice" or target next payment* or conditional next payment* or performance payment* or "pay for procedure" or "paid for procedure" or "paying for procedure" or "pay for performance" or "paid for performance" or "paying for performance" or p4p or performance next based next payment* or "performance based subsidy" or "performance based subsidies" or "performance based financing" or result* next based next financing or result* next based next funding or result* next based next payment* or "input based financing" or "input based funding" or "output based financing" or "output based funding" or remunerate or remuneration or capitation or "capitated financing" or "fee for service" or "fee for services" or salary or salaries or salaried next contract* or prepaid next plan* or prepaid next health next plan* or prepaid next healthcare next plan* or prepaid next health next care next plan* or pre next paid next plan* or pre next paid next health next plan* or pre next paid next healthcare next plan* or pre next paid next health next care next plan* or prepaid next service* or prepaid next health next service* or prepaid next healthcare next service* or prepaid next health next care next service* or pre next paid next service* or pre next paid next health next service* or pre next paid next healthcare next service* or pre next paid next health next care next service* or "prepaid care" or "pre paid care" or "prepaid healthcare" or "pre paid healthcare" or "prepaid health care" or "pre paid health care" or prospective next payment* or retrospective next payment* or payment near/2 method* or payment near/2 mechanism* or payment near/2 system*):ti,ab |
| #16 | MeSH descriptor: [Physicians, Primary Care] this term only |
| #17 | MeSH descriptor: [Physicians, Family] this term only |
| #18 | MeSH descriptor: [General Practitioners] this term only |
| #19 | MeSH descriptor: [Family Nurse Practitioners] this term only |
| #20 | MeSH descriptor: [Nurses, Community Health] this term only |
| #21 | MeSH descriptor: [Primary Health Care] this term only |
| #22 | MeSH descriptor: [Ambulatory Care] this term only |
| #23 | MeSH descriptor: [Family Practice] this term only |
| #24 | MeSH descriptor: [Family Nursing] this term only |
| #25 | MeSH descriptor: [General Practice] this term only |
| #26 | MeSH descriptor: [General Practice, Dental] this term only |
| #27 | MeSH descriptor: [Private Practice] this term only |
| #28 | MeSH descriptor: [Group Practice] this term only |
| #29 | MeSH descriptor: [Group Practice, Dental] this term only |
| #30 | MeSH descriptor: [Office Visits] this term only |
| #31 | MeSH descriptor: [Ambulatory Care Facilities] this term only |
| #32 | MeSH descriptor: [Community Health Centers] this term only |
| #33 | MeSH descriptor: [Community Mental Health Centers] this term only |
| #34 | MeSH descriptor: [Outpatient Clinics, Hospital] this term only |
| #35 | MeSH descriptor: [Community Health Services] this term only |
| #36 | MeSH descriptor: [Community Health Nursing] this term only |
| #37 | MeSH descriptor: [Community Mental Health Services] this term only |
| #38 | MeSH descriptor: [Community Pharmacy Services] this term only |
| #39 | MeSH descriptor: [Dental Health Services] this term only |
| #40 | MeSH descriptor: [Quality of Health Care] this term only |
| #41 | MeSH descriptor: [Quality Improvement] this term only |
| #42 | MeSH descriptor: [Quality Assurance, Health Care] this term only |
| #43 | MeSH descriptor: [Quality Indicators, Health Care] this term only |
| #44 | MeSH descriptor: [Health Services] this term only |
| #45 | MeSH descriptor: [Health Services Administration] this term only |
| #46 | MeSH descriptor: [Practice Patterns, Physicians'] this term only |
| #47 | MeSH descriptor: [Practice Patterns, Nurses'] this term only |
| #48 | MeSH descriptor: [Practice Patterns, Dentists'] this term only |
| #49 | (family next physician* or family next practitioner* or family next clinician* or family next doctor* or general next practitioner* or community next physician* or community next pharmac* or dentist* or pharmacist* or family next practice* or general next practice* or private next practice* or group next practice* or "dental practice" or "primary care" or "primary healthcare" or "primary health care" or "ambulatory care" or ambulatory next patient* or outpatient* or community next service* or "community care" or "community healthcare" or "community health care" or "ambulatory facility" or "ambulatory care facility" or "ambulatory facilities" or "ambulatory care facilities" or ambulatory next clinic* or community next clinic* or community next health* next clinic* or community next health next care next clinic* or community next health* next center* or community next health next care next center* or community next health* next centre* or community next health next care next centre? or dental next clinic* or health* next service* or health next care next service* or dental next service* or pharmacy next service* or quality next improvement* or "quality of care" or "quality of health care" or "health care quality"):ti,ab |
| #50 | {or #16‐#49} |
| #51 | #15 and #50 |
| #52 | #14 or #51 |
Cochrane Central Register of Controlled Trials (CENTRAL) Issue 3 2019, Cochrane Library (searched 5 March 2019)
| ID | Search | Hits |
| #1 | MeSH descriptor: [Physician Incentive Plans] this term only | 14 |
| #2 | MeSH descriptor: [Group Practice, Prepaid] this term only | 9 |
| #3 | MeSH descriptor: [Reimbursement Mechanisms] this term only | 47 |
| #4 | MeSH descriptor: [Reimbursement, Incentive] this term only | 73 |
| #5 | MeSH descriptor: [Prospective Payment System] this term only | 2 |
| #6 | MeSH descriptor: [Single‐Payer System] this term only | 2 |
| #7 | MeSH descriptor: [Prepaid Health Plans] this term only | 6 |
| #8 | MeSH descriptor: [Capitation Fee] this term only | 32 |
| #9 | MeSH descriptor: [Fees and Charges] this term only | 55 |
| #10 | MeSH descriptor: [Fee‐for‐Service Plans] this term only | 36 |
| #11 | MeSH descriptor: [Salaries and Fringe Benefits] this term only | 45 |
| #12 | MeSH descriptor: [Remuneration] this term only | 10 |
| #13 | MeSH descriptor: [Value‐Based Purchasing] this term only | 1 |
| #14 | {or #1‐#13} | 285 |
| #15 | (reimbursement or economic next incentive* or financial next incentive* or monetary next incentive* or economic next reward* or financial next reward* or monetary next reward* or incentive next payment* or payment next incentive* or performance next bonus* or bonus next payment* or "bonus for practice" or target next payment* or conditional next payment* or performance payment* or "pay for procedure" or "paid for procedure" or "paying for procedure" or "pay for performance" or "paid for performance" or "paying for performance" or p4p or performance next based next payment* or "performance based subsidy" or "performance based subsidies" or "performance based financing" or result* next based next financing or result* next based next funding or result* next based next payment* or "input based financing" or "input based funding" or "output based financing" or "output based funding" or remunerate or remuneration or capitation or "capitated financing" or "fee for service" or "fee for services" or salary or salaries or salaried next contract* or prepaid next plan* or prepaid next health next plan* or prepaid next healthcare next plan* or prepaid next health next care next plan* or pre next paid next plan* or pre next paid next health next plan* or pre next paid next healthcare next plan* or pre next paid next health next care next plan* or prepaid next service* or prepaid next health next service* or prepaid next healthcare next service* or prepaid next health next care next service* or pre next paid next service* or pre next paid next health next service* or pre next paid next healthcare next service* or pre next paid next health next care next service* or "prepaid care" or "pre paid care" or "prepaid healthcare" or "pre paid healthcare" or "prepaid health care" or "pre paid health care" or prospective next payment* or retrospective next payment* or payment near/2 method* or payment near/2 mechanism* or payment near/2 system*):ti,ab | 2344 |
| #16 | MeSH descriptor: [Physicians, Primary Care] this term only | 140 |
| #17 | MeSH descriptor: [Physicians, Family] this term only | 443 |
| #18 | MeSH descriptor: [General Practitioners] this term only | 230 |
| #19 | MeSH descriptor: [Family Nurse Practitioners] this term only | 1 |
| #20 | MeSH descriptor: [Nurses, Community Health] this term only | 12 |
| #21 | MeSH descriptor: [Primary Health Care] this term only | 3719 |
| #22 | MeSH descriptor: [Ambulatory Care] this term only | 3079 |
| #23 | MeSH descriptor: [Family Practice] this term only | 1963 |
| #24 | MeSH descriptor: [Family Nursing] this term only | 35 |
| #25 | MeSH descriptor: [General Practice] this term only | 422 |
| #26 | MeSH descriptor: [General Practice, Dental] this term only | 52 |
| #27 | MeSH descriptor: [Private Practice] this term only | 70 |
| #28 | MeSH descriptor: [Group Practice] this term only | 35 |
| #29 | MeSH descriptor: [Group Practice, Dental] this term only | 0 |
| #30 | MeSH descriptor: [Office Visits] this term only | 430 |
| #31 | MeSH descriptor: [Ambulatory Care Facilities] this term only | 432 |
| #32 | MeSH descriptor: [Community Health Centers] this term only | 193 |
| #33 | MeSH descriptor: [Community Mental Health Centers] this term only | 109 |
| #34 | MeSH descriptor: [Outpatient Clinics, Hospital] this term only | 572 |
| #35 | MeSH descriptor: [Community Health Services] this term only | 934 |
| #36 | MeSH descriptor: [Community Health Nursing] this term only | 333 |
| #37 | MeSH descriptor: [Community Mental Health Services] this term only | 680 |
| #38 | MeSH descriptor: [Community Pharmacy Services] this term only | 236 |
| #39 | MeSH descriptor: [Dental Health Services] this term only | 24 |
| #40 | MeSH descriptor: [Quality of Health Care] this term only | 798 |
| #41 | MeSH descriptor: [Quality Improvement] this term only | 564 |
| #42 | MeSH descriptor: [Quality Assurance, Health Care] this term only | 612 |
| #43 | MeSH descriptor: [Quality Indicators, Health Care] this term only | 205 |
| #44 | MeSH descriptor: [Health Services] this term only | 427 |
| #45 | MeSH descriptor: [Health Services Administration] this term only | 6 |
| #46 | MeSH descriptor: [Practice Patterns, Physicians'] this term only | 1164 |
| #47 | MeSH descriptor: [Practice Patterns, Nurses'] this term only | 133 |
| #48 | MeSH descriptor: [Practice Patterns, Dentists'] this term only | 21 |
| #49 | (family next physician* or family next practitioner* or family next clinician* or family next doctor* or general next practitioner* or community next physician* or community next pharmac* or dentist* or pharmacist* or family next practice* or general next practice* or private next practice* or group next practice* or "dental practice" or "primary care" or "primary healthcare" or "primary health care" or "ambulatory care" or ambulatory next patient* or outpatient* or community next service* or "community care" or "community healthcare" or "community health care" or "ambulatory facility" or "ambulatory care facility" or "ambulatory facilities" or "ambulatory care facilities" or ambulatory next clinic* or community next clinic* or community next health* next clinic* or community next health next care next clinic* or community next health* next center* or community next health next care next center* or community next health* next centre* or community next health next care next centre? or dental next clinic* or health* next service* or health next care next service* or dental next service* or pharmacy next service* or quality next improvement* or "quality of care" or "quality of health care" or "health care quality"):ti,ab | 60135 |
| #50 | {or #16‐#49} | 64834 |
| #51 | #15 and #50 | 767 |
| #52 | #14 or #51 in Trials | 894 |
MEDLINE and Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations and Daily 1946 to March 04, 2019, Ovid (searched 5 March 2019)
| # | Searches | Results |
| 1 | Physician Incentive Plans/ec [Economics] | 590 |
| 2 | Group Practice, Prepaid/ | 551 |
| 3 | or/1‐2 [PAYMENT & PRACTITIONERS/PRACTICE mixed] | 1138 |
| 4 | Physician Incentive Plans/ or Reimbursement Mechanisms/ or Reimbursement Incentive/ or Prospective Payment System/ or Single‐Payer System/ or Prepaid Health Plans/ or Capitation Fee/ or "Fees and Charges"/ or Fee‐for‐Service Plans/ or "Salaries and Fringe Benefits"/ or Remuneration/ or Value‐Based Purchasing/ | 52017 |
| 5 | (reimbursement or economic incentive? or financial incentive? or monetary incentive? or economic reward? or financial reward? or monetary reward? or incentive payment? or payment incentive? or performance bonus* or bonus payment? or bonus for practice or target payment? or conditional payment? or performance payment? or pay for procedure or paid for procedure or paying for procedure or pay for performance or paid for performance or paying for performance or p4p or performance based payment? or performance based subsidy or performance based subsidies or performance based financing or result? based financing or result? based funding or result? based payment? or input based financing or input based funding or output based financing or output based funding or remunerate or remuneration or capitation or capitated financing or fee for service? or salary or salaries or salaried contract? or prepaid plan? or prepaid health plan? or prepaid healthcare plan? or prepaid health care plan? or pre paid plan? or pre paid health plan? or pre paid healthcare plan? or pre paid health care plan? or prepaid service? or prepaid health service? or prepaid healthcare service? or prepaid health care service? or pre paid service? or pre paid health service? or pre paid healthcare service? or pre paid health care service? or prepaid care or pre paid care or prepaid healthcare or pre paid healthcare or prepaid health care or pre paid health care or prospective payment? or retrospective payment? or (payment adj2 method?) or (payment adj2 mechanism?) or (payment adj2 system?)).ti,ab,kf. | 48165 |
| 6 | or/4‐5 [PAYMENT METHODS] | 85413 |
| 7 | Physicians, Primary Care/ | 2845 |
| 8 | Physicians, Family/ | 15945 |
| 9 | General Practitioners/ | 6711 |
| 10 | Family Nurse Practitioners/ | 37 |
| 11 | Nurses, Community Health/ | 715 |
| 12 | or/7‐11 [OUTPATIENT HEALTH PROFESSIONALS] | 26063 |
| 13 | Primary Health Care/ | 70902 |
| 14 | Ambulatory Care/ | 41074 |
| 15 | Family Practice/ | 64286 |
| 16 | Family Nursing/ | 1372 |
| 17 | General Practice/ | 12042 |
| 18 | General Practice, Dental/ | 4725 |
| 19 | Private Practice/ | 8072 |
| 20 | Group Practice/ | 7800 |
| 21 | Group Practice, Dental/ | 612 |
| 22 | Office Visits/ | 6560 |
| 23 | or/13‐22 [OUTPATIENT CARE / PRACTICE] | 200140 |
| 24 | Ambulatory Care Facilities/ | 17510 |
| 25 | Community Health Centers/ | 6788 |
| 26 | Community Mental Health Centers/ | 2871 |
| 27 | Outpatient Clinics, Hospital/ | 15358 |
| 28 | or/24‐27 [OUTPATIENT FACILITIES] | 41997 |
| 29 | Community Health Services/ | 30386 |
| 30 | Community Health Nursing/ | 19219 |
| 31 | Community Mental Health Services/ | 18014 |
| 32 | Community Pharmacy Services/ | 4126 |
| 33 | Dental Health Services/ | 3990 |
| 34 | or/29‐33 [OUTPATIENT SERVICES] | 73883 |
| 35 | Quality of Health Care/ | 68748 |
| 36 | Quality Improvement/ | 19322 |
| 37 | Quality Assurance, Health Care/ | 54666 |
| 38 | Quality Indicators, Health Care/ | 14391 |
| 39 | or/35‐38 [QUALITY] | 147946 |
| 40 | Health Services/ | 24233 |
| 41 | Health Services Administration/ | 4378 |
| 42 | or/40‐41 [HEALTH SERVISES] | 27801 |
| 43 | Practice Patterns, Physicians'/ | 54398 |
| 44 | Practice Patterns, Nurses'/ | 2305 |
| 45 | Practice Patterns, Dentists'/ | 2182 |
| 46 | or/43‐45 [PRACTICE PATTERNS] | 58613 |
| 47 | (family physician? or family practitioner? or family clinician? or family doctor? or general practitioner? or community physician? or family practice? or family nursing or community health nursing or general practice? or private practice? or group practice? or dental practice? or primary care or primary healthcare or primary health care or ambulatory care or ambulatory patient? or outpatient? or community service? or community care or community healthcare or community health care or ambulatory facility or ambulatory care facility or ambulatory facilities or ambulatory care facilities or ambulatory clinic? or community clinic? or community health* clinic? or community health care clinic? or community health* center? or community health care center? or community health* centre? or community health care centre? or dental clinic? or dental service? or pharmac* service? or community pharmac* or quality improvement? or quality of care or quality of health care or health care quality or health* service? or health care service?).ti,ab,kf. | 573686 |
| 48 | 12 or 23 or 28 or 34 or 39 or 42 or 46 or 47 [MeSH: OUTPATIENT PROFESSIONALS / CARE / PRACTICE / FACILITIES / SERVICES / QUALITY / HEALTH SERVICES / PRACTICE PATTERNS OR text words] | 874687 |
| 49 | 6 and 48 | 25684 |
| 50 | 3 or 49 | 26308 |
| 51 | randomized controlled trial.pt. | 476954 |
| 52 | controlled clinical trial.pt. | 92938 |
| 53 | multicenter study.pt. | 245956 |
| 54 | pragmatic clinical trial.pt. | 981 |
| 55 | non‐randomized controlled trials as topic/ | 457 |
| 56 | interrupted time series analysis/ | 539 |
| 57 | controlled before‐after studies/ | 374 |
| 58 | (randomis* or randomiz* or randomly).ti,ab. | 815615 |
| 59 | groups.ab. | 1886058 |
| 60 | (trial or multicenter or multi center or multicentre or multi centre).ti. | 230785 |
| 61 | (intervention? or effect? or impact? or controlled or control group? or (before adj5 after) or (pre adj5 post) or ((pretest or pre test) and (posttest or post test)) or quasiexperiment* or quasi experiment* or pseudo experiment* or pseudoexperiment* or evaluat* or time series or time point? or repeated measur*).ti,ab. | 8846181 |
| 62 | or/51‐61 | 9867943 |
| 63 | exp Animals/ | 22127115 |
| 64 | Humans/ | 17574668 |
| 65 | 63 not (63 and 64) | 4552447 |
| 66 | (review or meta analysis or news or comment or editorial).pt. or cochrane database of systematic reviews.jn. or comment on.cm. or systematic review.ti. or literature review.ti. | 3804659 |
| 67 | 62 not (65 or 66) | 6926753 |
| 68 | 50 and 67 | 9399 |
Embase 1974 to 2019 March 04, Ovid (searched 5 March 2019)
| # | Searches | Results |
| 1 | medical fee/ | 13185 |
| 2 | capitation fee/ | 3746 |
| 3 | prospective payment/ | 8259 |
| 4 | reimbursement/ | 52616 |
| 5 | fee for service/ | 5851 |
| 6 | (capitation or reimbursement or fee for service? or prospective payment? or retrospective payment? or target payment? or pay* for performance or p4p or performance payment? or performance based pay* or performance based financing or financial incentive? or economic incentive? or monetary incentive? or prepaid health* plan? or pre paid health* plan? or prepaied health care plan? or pre paied health care plan? or (payment? adj2 method?) or (payment? adj2 mechanism?) or (payment? adj2 system?)).ti,ab,od,ct,kw. | 86582 |
| 7 | or/1‐6 [PAYMENT METHIODS] | 97015 |
| 8 | ambulatory care/ | 33832 |
| 9 | primary health care/ | 60691 |
| 10 | primary medical care/ | 94258 |
| 11 | outpatient care/ | 32813 |
| 12 | outpatient department/ | 56842 |
| 13 | general practitioner/ | 89987 |
| 14 | general practice/ | 74378 |
| 15 | community care/ | 51541 |
| 16 | (family physician? or family practitioner? or family clinician? or family doctor? or general practitioner? or community physician? or family practice? or general practice? or primary care or primary healthcare or primary health care or ambulatory care or ambulatory patient? or outpatient? or (community adj3 service?) or (community adj3 care) or ambulatory facility or ambulatory care facility or ambulatory facilities or ambulatory care facilities or ambulatory clinic? or (community adj3 clinic?) or (community adj3 center?) or (community adj3 centre?) or dental clinic? or dental service? or pharmac* service? or community pharmac*).ti,ab,od,ct,kw. | 674465 |
| 17 | or/8‐16 [OUTPATIENT] | 753505 |
| 18 | 7 and 17 | 17829 |
| 19 | (performance based financing or (pay* and performance) or target payment? or financial incentive? or incentive payment? or capitation or capitated financing or capitated payment? or reimbursement or remuneration system? or fee for service? or (pay* and behaviour) or (pay* and behavior)).ti. [TERMS TAKEN FROM TITLES OF INCLUDED STUDIES] | 10822 |
| 20 | 18 or 19 | 26880 |
| 21 | Randomized Controlled Trial/ | 534657 |
| 22 | Quasi Experimental Study/ | 5333 |
| 23 | Pretest Posttest Control Group Design/ | 367 |
| 24 | Time Series Analysis/ | 22359 |
| 25 | (randomis* or randomiz* or randomly or controlled or control group? or (before adj5 after) or (pre adj5 post) or ((pretest or pre test) and (posttest or post test)) or quasiexperiment* or quasi experiment* or evaluat* or time series or time point? or repeated measur* or groups).ti,ab. | 7648001 |
| 26 | (trial or intervention? or effect? or impact?).ti. | 2454297 |
| 27 | or/21‐26 | 9085911 |
| 28 | exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ | 25442079 |
| 29 | human/ or normal human/ or human cell/ | 19376288 |
| 30 | 28 not (28 and 29) | 6119288 |
| 31 | (systematic review or literature review).ti. | 150895 |
| 32 | "cochrane database of systematic reviews".jn. | 13058 |
| 33 | 27 not (30 or 31 or 32) | 7133848 |
| 34 | 20 and 33 | 8335 |
| 35 | ("Health Policy, Economics and Management" or "Public Health, Social Medicine and Epidemiology").ec. | 2719999 |
| 36 | 34 and 35 | 4112 |
| 37 | limit 36 to embase | 4100 |
Web of Science, Conference Proceedings Citation Index‐Science, 1990 to present (ISI Web of Knowledge) (searched 5 March 2019)
#1 TS= (wage OR wages OR salary OR salaries OR salaried OR “fringe benefit” OR “fringe benefits” OR capitation OR “fee for service” OR “fee for services” OR fee‐for‐service OR “prospective payment system” OR “retrospective payment system” OR “single payer” OR “reimbursement mechanisms” OR “reimbursement mechanism” OR “incentive reimbursements” OR “incentive reimbursement” OR “pay for performance” OR p4p OR “physician incentive plans” OR “physician incentive plan” OR “employee incentive plans” OR “employee incentive plan” OR remuneration OR remunerations OR “mixed payment systems”)
#2 TS= (“outpatient clinics” OR “outpatient clinic” OR “urgent care centers” OR “urgent care center” OR “urgent care clinics” OR “urgent care clinic” OR “family planning centers” OR “family planning center” OR “ambulatory health centers” OR “ambulatory health center” OR “Abortion centers” OR “abortion center” OR “Abortion clinics” OR “abortion clinic” OR “Hospital outpatient clinics” OR “hospital outpatient clinic” OR “community health center” OR “community health centers” OR “dental clinic” OR “dental clinics” OR “substance abuse treatment centers” OR “Substance abuse treatment center” OR “community mental health centers” OR “community mental health center” OR “child guidance clinics” OR “child guidance clinic” OR “maternal‐child health centers” OR “maternal‐child health center” OR “pain clinics” OR “pain clinic” OR surgicenters OR surgicenter)
#3 TS= (“health professionals” OR “health professional” OR “health care providers” OR “health care provider” OR “health care providers” OR “health care provider” OR “paramedical personnel” OR “allied Health personnel” OR physicians OR “health workers” OR “health worker” OR “general practitioner” OR “general practitioners” OR nurse OR nurses OR “emergency medical technicians” OR “emergency medical technician” OR “operating room technicians” OR “operating room technician” OR Pharmacists aides OR “physical therapist assistants” OR “physical therapist assistant” OR “physical therapist assistants” OR “dental staff” OR dentist OR dentists OR pharmacists OR pharmacist OR “medical staff” OR “medical staffs” OR caregivers OR caregiver)
#4 #2 AND #3
#5 #1 AND #4
#6 (letter[PT] OR news item[PT] OR editorial material[PT] OR REVIEW[PT] OR MEETING ABSTRACT[PT] OR CORRECTION OR BOOK CHAPTER[PT])
#7 #5 NOT #6
International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/en) (searched 27 June 2019)
Advanced search:
4 individual strategies searched In Title OR Intervention, with Recruitment status: All
1. payment method OR payment methods OR reimbursement method OR reimbursement methods OR incentive plan OR incentive plans OR remuneration system OR remuneration systems
2. economic incentive OR economic incentives OR financial incentive OR financial incentives OR monetary incentive OR monetary incentives OR reimbursement incentive OR reimbursement incentives
3. pay for performance OR paying for performance OR p4p OR performance based payment OR performance based payments
4. capitation OR capitated financing OR fee for service OR fee for services OR prepaid health plan OR prepaid health plans OR pre‐paid health plan OR pre‐paid health plans
ClinicalTrials.gov (www.clinicaltrials.gov) (searched 27 June 2019)
Advanced Search – Other terms – Study type: Interventional studies
1. "payment method" OR "payment methods" OR "reimbursement method" OR "reimbursement methods" OR "incentive plan" OR "incentive plans" OR "remuneration system" OR "remuneration systems"
2. ("economic incentive" OR "economic incentives" OR "financial incentive" OR "financial incentives") AND ("primary care" OR "primary health care" OR "primary healthcare" OR ambulatory OR outpatient OR outpatients)
3. ("monetary incentive" OR "monetary incentives" OR "reimbursement incentive" OR "reimbursement incentives") AND ("primary care" OR "primary health care" OR "primary healthcare" OR ambulatory OR outpatient OR outpatients)
4. "pay for performance" OR "paying for performance" OR p4p OR "performance based payment" OR "performance based payments"
5. "capitation" OR "capitated financing" OR "fee for service" OR "fee for services" OR "prepaid health plan" OR "prepaid health plans" OR "pre‐paid health plan" OR "pre‐paid health plans"
Dissertations and Theses Database, 1861 to present, ProQuest (searched 10 December 2018)
#1 Search ti(wage OR wages OR salary OR salaries OR salaried OR "fringe benefit" OR "fringe benefits" OR capitation OR "fee for service" OR "fee for services" OR fee‐for‐service OR "prospective payment system" OR "retrospective payment system" OR "single payer" OR "reimbursement mechanisms" OR "reimbursement mechanism" OR "incentive reimbursements" OR "incentive reimbursement" OR "pay for performance" OR p4p OR "physician incentive plans" OR "physician incentive plan" OR "employee incentive plans" OR "employee incentive plan" OR remuneration OR remunerations OR " mixed payment systems") OR ab(wage OR wages OR salary OR salaries OR salaried OR "fringe benefit" OR "fringe benefits" OR capitation OR "fee for service" OR "fee for services" OR fee‐for‐service OR "prospective payment system" OR "retrospective payment system" OR "single payer" OR "reimbursement mechanisms" OR "reimbursement mechanism" OR "incentive reimbursements" OR "incentive reimbursement" OR "pay for performance" OR p4p OR "physician incentive plans" OR "physician incentive plan" OR "employee incentive plans" OR "employee incentive plan" OR remuneration OR remunerations OR " mixed payment systems")
#2 ti("outpatient clinics" OR "outpatient clinic" "urgent care centers" OR "urgent care center" OR "urgent care clinics" OR "urgent care clinic" OR "family planning centers" OR "family planning center" OR "ambulatory health centers" OR "ambulatory health center" OR "abortion centers" OR "abortion center" OR "abortion clinics" OR "abortion clinic" OR" hospital outpatient clinics" OR "hospital outpatient clinic" OR" community health center" OR "community health centers" OR "dental clinic" OR "dental clinics" OR "substance abuse treatment centers" OR "substance abuse treatment center" OR "community mental health centers" OR "community mental health center" OR "child guidance clinics" OR "child guidance clinic" OR "maternal‐child health centers" OR "maternal‐child health center" OR "pain clinics" OR "pain clinic" OR surgicenters OR surgicenter) OR ab("outpatient clinics" OR "outpatient clinic" "urgent care centers" OR "urgent care center" OR "urgent care clinics" OR "urgent care clinic" OR "family planning centers" OR "family planning center" OR "ambulatory health centers" OR "ambulatory health center" OR "abortion centers" OR "abortion center" OR "abortion clinics" OR "abortion clinic" OR" hospital outpatient clinics" OR "hospital outpatient clinic" OR" community health center" OR "community health centers" OR "dental clinic" OR "dental clinics" OR "substance abuse treatment centers" OR "substance abuse treatment center" OR "community mental health centers" OR "community mental health center" OR "child guidance clinics" OR "child guidance clinic" OR "maternal‐child health centers" OR "maternal‐child health center" OR "pain clinics" OR "pain clinic" OR surgicenters OR surgicenter)
#3 ti("health professionals" OR "health professional" OR" health care providers" OR "health care provider" OR "healthcare providers" OR "healthcare provider" OR" paramedical personnel" OR "allied health personnel" OR physicians OR "health workers" OR "health worker" OR "general practitioner" OR "general practitioners" OR nurse OR nurses OR "emergency medical technicians" OR "emergency medical technician" OR "operating room technicians" OR "operating room technician" OR "pharmacists aides" OR "physical therapist assistant" OR "physical therapist assistants" OR dentist OR dentists OR pharmacists OR pharmacist OR "medical staff" OR "medical staffs" OR caregivers OR caregiver) OR ab("health professionals" OR "health professional" OR" health care providers" OR "health care provider" OR "healthcare providers" OR "healthcare provider" OR" paramedical personnel" OR "allied health personnel" OR physicians OR "health workers" OR "health worker" OR "general practitioner" OR "general practitioners" OR nurse OR nurses OR "emergency medical technicians" OR "emergency medical technician" OR "operating room technicians" OR "operating room technician" OR "pharmacists aides" OR "physical therapist assistant" OR "physical therapist assistants" OR dentist OR dentists OR pharmacists OR pharmacist OR "medical staff" OR "medical staffs" OR caregivers OR caregiver)
#4 #2 AND #3
#5 #1 AND #4
EconLit, 1969 to present, ProQuest (searched 10 December 2018)
((ti(payment OR payout OR disbursement) AND ti(method* OR system* OR scheme*)) OR (AB(payment OR payout OR disbursement) NEAR/4 AB(method* OR system* OR scheme*)) OR (ti(pay*) AND ti(advance OR prospect* OR retrospect*)) OR (AB(pay*) NEAR/4 AB(advance OR prospect* OR retrospect*)) OR (ti(fixed) AND ti(pay* OR amount* OR fee OR fees OR fund*)) OR (AB(fixed) NEAR/4 AB(pay* OR amount* OR fee OR fees OR fund*)) OR (ti("global budget" OR "global budgets" OR "global payment" OR "global payments" OR "line‐item budget" OR "line‐item budgets" OR "budget payment" OR "budget payments") OR ab("global budget" OR "global budgets" OR "global payment" OR "global payments" OR "line‐item budget" OR "line‐item budgets" OR "budget payment" OR "budget payments")) OR (ti(capitation OR capitated) OR ab(capitation OR capitated)) OR (ti("fee for service" OR "fee for services" OR "pay for performance" OR p4p OR "target pay" OR "target payment" OR "target payments") OR ab("fee for service" OR "fee for services" OR "pay for performance" OR p4p OR "target pay" OR "target payment" OR "target payments")) OR (ti(result* OR performance OR output OR out‐put) AND ti(pay* OR financing)) OR (AB(result* OR performance OR output OR out‐put) NEAR/4 AB(pay* OR financing)) OR (ti(payment* OR monetary OR economic OR financial OR reimbursement) AND ti(incentive*)) OR (AB(payment* OR monetary OR economic OR financial OR reimbursement) NEAR/4 AB(incentive*)) OR (ti("single payer") AND ti(system* OR plan*)) OR (AB("single payer") NEAR/4 AB(system* OR plan*)) OR (ti(reimbursement OR "case based" OR case‐based) AND ti(mechanism* OR pay*)) OR (AB(reimbursement OR "case based" OR case‐based) NEAR/4 AB(mechanism* OR pay*)) OR (ti(remuneration OR remunerate OR remunerates OR remunerating OR prepay* OR "pre‐payment" OR prepaid) OR ab(remuneration OR remunerate OR remunerates OR remunerating OR prepay* OR "pre‐payment" OR prepaid)) OR (ti(combined OR mixed OR bundle*) AND ti(pay* OR funding*)) OR (AB(combined OR mixed OR bundle*) NEAR/4 AB(pay* OR funding*)) OR (ti(salary OR salaries OR salaried OR wage OR wages OR "fringe benefit" OR "fringe benefits") OR ab(salary OR salaries OR salaried OR wage OR wages OR "fringe benefit" OR "fringe benefits"))) AND (((ti("ambulatory care" OR "ambulatory health care" OR "ambulatory healthcare") OR ab("ambulatory care" OR "ambulatory health care" OR "ambulatory healthcare")) OR (ti("primary care" OR "primary health care" OR "primary healthcare") OR ab("primary care" OR "primary health care" OR "primary healthcare")) OR (ti("public health" OR "public healthcare") OR ab("public health" OR "public healthcare")) OR (ti("child health" OR "maternal health" OR "mental health" OR "family planning" OR abortion OR preventive OR dental OR "free standing") AND ti(service* OR facility OR facilities OR clinic* OR center* OR centre*)) OR (ab("child health" OR "maternal health" OR "mental health" OR "family planning" OR abortion OR preventive OR dental OR "free standing") NEAR ab(service* OR facility OR facilities OR clinic* OR center* OR centre*)) OR (ti(community) AND ti(health OR "health care" OR healthcare)) OR (ab(community) NEAR ab(health OR "health care" OR healthcare)) OR (ti(outpatient or outpatients) OR ab(outpatient or outpatients)) OR (ti("general practice" OR "general practices" OR "family practice" OR "family practices") OR ab("general practice" OR "general practices" OR "family practice" OR "family practices"))) AND ((ti("general practitioner" OR "general practitioners" OR "family practitioner" OR "family practitioners") OR ab("general practitioner" OR "general practitioners" OR "family practitioner" OR "family practitioners")) OR (ti("family planning" OR preventive OR dental) AND ti(service* OR facility OR facilities OR clinic* OR center* OR centre*)) OR (ab("family planning" OR preventive OR dental) NEAR ab(service* OR facility OR facilities OR clinic* OR center* OR centre*)) OR (ti("medical staff" OR physician OR physicians OR "family planning personnel" OR "family planning worker" OR "family planning workers" OR "doctor" OR "doctors" OR nurse OR nurses OR "nursing staff" OR "dental staff" OR dentist*) OR ab("medical staff" OR physician OR physicians OR "family planning personnel" OR "family planning worker" OR "family planning workers" OR "doctor" OR "doctors" OR nurse OR nurses OR "nursing staff" OR "dental staff" OR dentist*)) OR (ti(health OR healthcare OR "health care") AND ti(provider* OR worker* OR professional*)) OR (ab(health OR healthcare OR "health care") NEAR ab(provider* OR worker* OR professional*))))
Chinese Medicine Premier (Wanfang Data), 1988 to present (searched 10 December 2018)
(主题=支付方式+支付制度+支付体系+支付方法+预付+后付+总额预算+总额预付+条目预算+按人头+按项目支付+绩效支付+病种支付+病历支付+酬劳+补偿+绩效工资+工资制) AND (主题=门诊+公共卫生+妇幼+孕产妇+计划生育+口腔+牙科+精神卫生+基层+初级卫生保健+初级卫生服务+社区+全科+家庭医生+家庭医师+家庭医疗+医生+医师+护士+牙医+卫生工作者+医疗工作人员+医疗工作者)
China National Knowledge Infrastructure (CHKD‐CNKI), (1915 to present (searched 10 December 2018)
1: (主题=支付方式+支付制度+支付体系+支付方法+预付+后付+预算+按人头+按项目+绩效支付+病种支付+病历支付+酬劳+补偿+绩效工资+工资制) AND (主题=门诊+公共卫生+妇幼+儿童+孕妇+产妇+孕产妇+计划生育+口腔+牙科+精神卫生+基层+初级卫生保健+初级卫生服务+社区+全科+家庭医生+家庭医师+家庭医疗+预防+医生+医师+护士+牙医+卫生工作人员+卫生工作者+医疗工作人员+医疗工作者)AND (主题=评价+干预)
2: (主题=支付方式+支付制度+支付体系+支付方法+预付+后付+预算+按人头+按项目+绩效支付+病种支付+病历支付+酬劳+补偿+绩效工资+工资制) AND (主题=门诊+公共卫生+妇幼+儿童+孕妇+产妇+孕产妇+计划生育+口腔+牙科+精神卫生+基层+初级卫生保健+初级卫生服务+社区+全科+家庭医生+家庭医师+家庭医疗+预防+医生+医师+护士+牙医+卫生工作人员+卫生工作者+医疗工作人员+医疗工作者) AND (主题=试验+准试验)
3: (主题=支付方式+支付制度+支付体系+支付方法+预付+后付+预算+按人头+按项目+绩效支付+病种支付+病历支付+酬劳+补偿+绩效工资+工资制) AND (主题=门诊+公共卫生+妇幼+儿童+孕妇+产妇+孕产妇+计划生育+口腔+牙科+精神卫生+基层+初级卫生保健+初级卫生服务+社区+全科+家庭医生+家庭医师+家庭医疗+预防+医生+医师+护士+牙医+卫生工作人员+卫生工作者+医疗工作人员+医疗工作者) AND (主题=对照+时间序列)
4: 1 OR 2 OR 3
OpenGrey (www.opengrey.eu/) (searched 10 December 2018)
("payment method" OR "payment methods" OR"payment system" OR "payment mechanism" OR capitation OR "fee for service" OR "fee for services" OR "fee‐for‐service" OR "fee‐for‐services" OR "pay for performance" OR p4p OR "pay‐for‐performance" OR "performance‐related pay" OR "performance‐related payment" OR "payment for performance" OR "performance based payment" OR "performance‐based payment" OR salary) AND ((ambulatory OR primary OR public OR child OR maternal OR mental OR preventive OR "community health" OR dental OR clinics OR outpatient OR outpatients) AND (physician OR physicians OR doctor OR doctors OR nurse OR nurses OR dentists OR dentist))
WHO website (https://www.who.int/) (searched 17 November 2018)
wage OR wages OR salary OR salaries OR salaried OR "fringe benefit" OR "fringe benefits" OR "global budget" OR "global budgets" OR "line item budgets" OR "line‐item budgets" OR capitation OR "fee for service" OR "fee for services" OR fee‐for‐service OR "case‐based reimbursement" OR "prospective payment system" OR "retrospective payment system" OR "single payer" OR "reimbursement mechanisms" OR "reimbursement mechanism" OR "incentive reimbursements" OR "incentive reimbursement" OR "pay for performance" OR p4p OR "physician incentive plans" OR "physician incentive plan" OR "employee incentive plans" OR "employee incentive plan" OR remuneration OR remunerations OR "mixed payment systems"
World Bank website (www.worldbank.org/) (searched 17 November 2018)
wage OR wages OR salary OR salaries OR salaried OR "fringe benefit" OR "fringe benefits" OR "global budget" OR "global budgets" OR "line item budgets" OR "line‐item budgets" OR capitation OR "fee for service" OR "fee for services" OR fee‐for‐service OR "case‐based reimbursement" OR "prospective payment system" OR "retrospective payment system" OR "single payer" OR "reimbursement mechanisms" OR "reimbursement mechanism" OR "incentive reimbursements" OR "incentive reimbursement" OR "pay for performance" OR p4p OR "physician incentive plans" OR "physician incentive plan" OR "employee incentive plans" OR "employee incentive plan" OR remuneration OR remunerations OR "mixed payment systems"
IDEAS (Research Papers in Economics) 1927 to present (searched 30 December 2017)
("payment method" | "payment methods" |"payment system" | "payment mechanism" |"global budget" | "line‐item budget" | capitation | "fee for service" | "fee for services" | "fee‐for‐service" | "fee‐for‐services" | "pay for performance" | p4p | "pay‐for‐performance" | "performance‐related pay" | "performance‐related payment" | "payment for performance" | "performance based payment" | "performance‐based payment" | salary) + (ambulatory | primary | public | child | maternal | mental | preventive | "community health" | dental | clinics | outpatient | outpatients | physician | physicians | doctor | doctors | nurse | nurses | dentists | dentist)
POPLINE (Population Information Online) 1970 to present, K4Health (searched 30 December 2017
(wage OR wages OR salary OR salaries OR salaried OR "fringe benefit" OR "fringe benefits" OR "global budget" OR "global budgets" OR "line item budgets" OR "line‐item budgets" OR capitation OR "fee for service" OR "fee for services" OR fee‐for‐service OR "case‐based reimbursement" OR "prospective payment system" OR "retrospective payment system" OR "single payer" OR "reimbursement mechanisms" OR "reimbursement mechanism" OR "incentive reimbursements" OR "incentive reimbursement" OR "pay for performance" OR p4p OR "physician incentive plans" OR "physician incentive plan" OR "employee incentive plans" OR "employee incentive plan" OR remuneration OR remunerations OR " mixed payment systems") AND (("outpatient clinics" OR "outpatient clinic" "urgent care centers" OR "urgent care center" "urgent care clinics" OR "urgent care clinic" OR "family planning centers" OR "family planning center" OR "ambulatory health centers" OR "ambulatory health center" OR "abortion centers" OR "abortion center" OR "abortion clinics" OR "abortion clinic" OR" hospital outpatient clinics" OR "hospital outpatient clinic" OR" community health center" OR "community health centers" OR "dental clinic" OR "dental clinics" OR "substance abuse treatment centers" OR "substance abuse treatment center" OR "community mental health centers" OR "community mental health center" OR "child guidance clinics" OR "child guidance clinic" OR "maternal‐child health centers" OR "maternal‐child health center" OR "pain clinics" OR "pain clinic" OR surgicenters OR surgicenter)OR ("health professionals" OR "health professional" OR" health care providers" OR "health care provider" OR "healthcare providers" OR "healthcare provider" OR" paramedical personnel" OR "allied health personnel" OR physicians OR "health workers" OR "health worker" OR "general practitioner" OR "general practitioners" OR nurse OR nurses OR "emergency medical technicians" OR "emergency medical technician" OR "operating room technicians" OR "operating room technician" OR "pharmacists aides" OR "physical therapist assistant" OR "physical therapist assistants" OR dentist OR dentists OR pharmacists OR pharmacist OR "medical staff" OR "medical staffs" OR caregivers OR caregiver))
Data and analyses
Comparison 1. (Comparison 1) Effects of P4P plus existing payment methods compared to existing payment methods: dichotomous quantity of health services provided.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Quantity of health services provided (immunisation coverage status) | 2 | 3760 | Risk Ratio (IV, Random, 95% CI) | 1.27 [1.19, 1.36] |
Comparison 2. (Comparison 1) Effects of P4P plus existing payment methods compared to existing payment methods: continuous quantity of health services provided.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Quantity of health services provided (services coverage rate) | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 3. (Comparison 1) Effects of P4P plus existing payment methods compared to existing payment methods: dichotomous quality of health services provided.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Quality of health services provided of Petersen 2013 (use of guideline) | 1 | Risk Ratio (IV, Fixed, 95% CI) | 1.07 [1.02, 1.12] |
Comparison 4. (Comparison 1) Effects of P4P plus existing payment methods compared to existing payment methods: continuous patient outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Health outcomes of Houle 2016 (blood pressure reduction (mmHG)) | 1 | 362 | Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐2.22, 2.37] |
Comparison 5. (Comparison 1) Effects of P4P plus existing payment methods compared to existing payment methods: dichotomous mixed outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Mixed provision and health outcomes of Petersen 2013 (control of blood pressure or follow the guideline) | 1 | Risk Ratio (IV, Fixed, 95% CI) | 1.13 [1.04, 1.23] |
Comparison 6. (Comparison 3) Effects of FFS mixed with existing payment methods compared to single payment method: dichotomous quantity of health services provided.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Quantity of health services provided (services coverage status) | 2 | 192 | Risk Ratio (IV, Random, 95% CI) | 1.37 [1.07, 1.76] |
Comparison 7. (Comparison 3) Effects of FFS mixed with existing payment methods compared to single payment method: dichotomous quality of health services provided.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Quality of health services provided (patients' behaviour change) | 1 | Risk Ratio (IV, Fixed, 95% CI) | 1.20 [0.70, 2.08] |
Comparison 8. (Comparison 4) Effects of enhanced FFS compared to FFS: dichotomous quantity of health services provided.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Quantity of health services provided (immunisation coverage status) | 2 | 3160 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [1.06, 1.48] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bilardi 2010.
| Study characteristics | ||
| Methods | Cluster‐randomised trial | |
| Participants | General practitioners | |
| Interventions | Victoria, Australia Intervention: pay for performance (a small incentive payment per test): receive a AUD 5 payment per chlamydia test for testing 16‐ to 24‐year‐old women for chlamydia Control: receive no payment for testing 16‐ to 24‐year‐old women for chlamydia Year 2008 to 2009, intervention happened in 2008 |
|
| Outcomes |
Quantity of health services provided Women tested |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Using a predetermined randomisation sequence prepared by the trial statistician |
| Allocation concealment (selection bias) | Low risk | The allocation was not revealed to staff at either of the paired practices until representatives from both had completed the pre‐trial requirements (questionnaire, audit, education session). General practitioners in the practice were then contacted by letter to inform them of the allocation relevant to their practice and reminded of testing payment or non‐payment. |
| Baseline outcome measurements similar | Unclear risk | No comparison on the baseline outcome measurements was reported. |
| Baseline characteristics similar | Low risk | An imbalanced distribution of variables was also evident at trial commencement, with GPs in the control group more likely to be younger and to have a postgraduate qualification. But the adjusted analysis controlling the characteristics variables was conducted. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information |
| Knowledge of the allocated interventions adequately prevented | Low risk | The allocation was not revealed to staff at either of the paired practices until representatives from both had completed the pre‐trial requirements (questionnaire, audit, education session). GPs in the practice were then contacted by letter to inform them of the allocation relevant to their practice and reminded of testing payment or non‐payment. |
| Study adequately protected against contamination | Low risk | Mid‐trial, GPs in the intervention group received a letter to remind them of the incentive offered for chlamydia testing. They were not provided with any information about the number of tests performed to date nor the amount of money they had accrued through testing. Payment was made to GPs at the end of the trial period. |
| Selective reporting (reporting bias) | Low risk | No missing outcome data |
| Other bias | Low risk | |
Christensen 2000.
| Study characteristics | ||
| Methods | Randomised trial | |
| Participants | Community pharmacies | |
| Interventions | State of Washington, US Intervention: financial incentive (Pay‐for‐performance). All pharmacies agreed to document cognitive services (CS) for Medicaid recipients in exchange for a modest monthly fee (USD 40). Additionally, study group pharmacies were eligible to be reimbursed for each CS documented and billed to Medicaid. They were compensated at the rate of USD 4.00 for interventions up to 6 minutes in duration, and USD 6.00 for interventions of 6 minutes or longer. 110 community pharmacies Control: pharmacies agreed to document CS for Medicaid recipients in exchange for a modest monthly fee (USD 40). 90 community pharmacies First study month to 20th study month, intervention happened in 1990 |
|
| Outcomes |
Quantity of health services provided Documentation rate Frequency of cognitive services (CS) Frequency of CS interventions Primary results of CS interventions CS intervention rates per 100 prescriptions Patients receiving CS, by problem type Pharmacists self‐reported time per CS |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomised cluster sampling methodology is described in an earlier article. |
| Allocation concealment (selection bias) | Unclear risk | No more information in the text |
| Baseline outcome measurements similar | Unclear risk | No comparison on the baseline outcome measurements was reported. |
| Baseline characteristics similar | Unclear risk | No comparison on the baseline characteristics was reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The missing data are lower than 0.1%. |
| Knowledge of the allocated interventions adequately prevented | Low risk | The outcomes are objective. |
| Study adequately protected against contamination | Low risk | Allocation was by county and country, and it is unlikely that the control group received the intervention. |
| Selective reporting (reporting bias) | Low risk | Assessment of the cost impact of these changes is reported separately. |
| Other bias | High risk | It is possible that pharmacists performed but did not document CS except in response to the financial incentive. And any event‐based reimbursement system should recognise the need to compensate pharmacists more for longer intervention times and for multiple interventions. |
Chung 2010.
| Study characteristics | ||
| Methods | Controlled before‐after study | |
| Participants | Primary care physicians | |
| Interventions | California, USA Intervention:physician‐specific P4P plus existing payment (salary based on relative value units of service). The bonus amount was based on individual physicians' performance on 15 ambulatory quality measures, with a composite score calculated using an algorithm developed by the incentive programme leadership. The physicians set targets for each measure. Physicians received varying points for achieving minimal, average, and stretch goals based on the percentage of their patients achieving the target. The bonus was based on the percentage of potentially achievable points actually earned. The maximum achievable bonus was USD 5000/year, or about 2% of the primary care physicians annual salary. Control: existing payment (salary based on relative value units of service). The other 2 physician groups continued using the previous P4P measures for group‐level or department‐level performance, but no group‐level or department‐level bonuses were distributed to individual physicians. Years 2005 to 2007, intervention happened in 2007 |
|
| Outcomes |
Quality of health services provided Quality score for asthma controller prescribing: no difference Quality score for cervical cancer screening: no difference Quality score for chlamydia screening: no difference |
|
| Notes | There are only figures for the quality score in the control group but no specific data. Controlled before‐after study with high risk of bias | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Control before‐after study rated as 'high risk' for this domain. |
| Allocation concealment (selection bias) | High risk | This is a controlled before‐after study (years 1998 to 2001, intervention happened in May 1999). |
| Baseline outcome measurements similar | Unclear risk | No comparison on the baseline outcome measurements was reported. |
| Baseline characteristics similar | Low risk | All 3 physician groups, located at clinics in adjacent counties, had a roughly similar mix of primary care and specialty physicians and served patients of similar demographic composition. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Amongst 179 physicians, 167 were included in the study, whilst 12 had insufficient qualifying patients for various reasons. Most physicians (152 in 2005 and 169 in 2006) also had data for the equivalent measures in the previous years; 148 had data for all 3 years |
| Knowledge of the allocated interventions adequately prevented | Low risk | The outcomes are objective. |
| Study adequately protected against contamination | Low risk | Allocation was by county, and it is unlikely that the control group received the intervention. |
| Selective reporting (reporting bias) | Unclear risk | Not specified in the paper |
| Other bias | High risk | Did not have a contemporaneous comparison group at the same study site receiving only performance reporting or group‐level incentives |
Clarkson 2008.
| Study characteristics | ||
| Methods | Cluster‐randomised trial | |
| Participants | Dentists | |
| Interventions | Scotland Intervention 1: fee‐for‐service remuneration: GBP 6.80 for each second permanent molar fissure sealed during a 6‐month period. The level of the fee was set so that it was consistent with the fee level payable through the normal National Health Service system for a restorative fissure sealant application and for preventive sealing of third permanent molars. Intervention 2: education regarding evidence‐based practice Intervention 3: both fee‐for‐service and education Control: no specific intervention Year September 2003 to February 2004, intervention happened in 2003 |
|
| Outcomes |
Quantity of health services provided Children with 1 or more sealant per dentist |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sampling, randomisation, and analysis were conducted at arm's length from the study base by the Health Services Research Unit, University of Aberdeen. |
| Allocation concealment (selection bias) | Low risk | Sampling, randomisation, and analysis were conducted at arm's length from the study base by the Health Services Research Unit, University of Aberdeen. |
| Baseline outcome measurements similar | Low risk | There was a lower baseline of sealant treatment of second permanent molars in all the intervention arms. No other significant baseline differences in practice or practitioner characteristics were found. |
| Baseline characteristics similar | Low risk | There was a lower baseline of sealant treatment of second permanent molars in all the intervention arms. No other significant baseline differences in practice or practitioner characteristics were found. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There does not appear to be an imbalance of missing data across the fee‐for‐service and control arms (e.g. 4 dentists in both the fee‐for‐service and control arms were lost to follow‐up), nor an imbalance in the reasons behind missing data in these arms (e.g. 7.1% of children were excluded from the fee‐for‐service arm and 10.4% of children were excluded in the control arm because they did not have erupted second permanent molars). However, the authors do not appear to have conducted statistical analyses to check for possible imbalances that may have occurred, particularly between the arm where dentists received fee‐for‐service remuneration and education (6 dentists were lost to follow‐up in this arm) and the education arm (2 dentists were lost to follow‐up in this arm). The data were analysed using the intention‐to‐treat principle. For example, dentists who did not attend the education intervention were mailed the course material and retained in the study on an intention‐to‐treat basis, thus reducing the use of incomplete outcome data. |
| Knowledge of the allocated interventions adequately prevented | Low risk | The outcomes are objective. |
| Study adequately protected against contamination | Low risk | The risk of contamination is not a concern as dentists (rather than patients) were randomised, and it is unlikely that communication between dentists in the different arms could have occurred as a maximum of 1 dentist per dental practice was selected. |
| Selective reporting (reporting bias) | Unclear risk | No protocol is cited, and it is not stated whether all the prespecified primary outcomes have been reported. |
Davidson 1992.
| Study characteristics | ||
| Methods | Randomised trial | |
| Participants | Primary care physicians (PCPs) | |
| Interventions | Suffolk County, New York, USA Intervention (1): capitation. Capitation PCPs were paid USD 6 for 13‐ to 18‐year‐olds; USD 8.50 for 6‐ to 12‐year‐olds; USD 13.50 for 3‐ to 5‐year‐olds; and USD 18.50 for children 2 years or younger. Each month USD 25 was set aside for each child. Intervention (2): Fee‐for‐Service (high rates). PCPs were paid a fee for comprehensive exams (including treatment), routine office visits, initial hospital visits, and follow‐up hospital visits. Control:Fee‐for‐service (low rates). PCPs were paid a fee for the same services as the high‐rate group, but the fee was approximately half the size. Years 1983 to 1985, intervention happened in 1983 |
|
| Outcomes |
Quantity of health services provided Utilisation Primary care physician visits Non‐primary care physician visits Hospitalisations Costs Expenditures |
|
| Notes | No details on the characteristics or number of PCPs were reported or compared across the 2 groups. The authors did not compare participating PCPs with those who did not participate. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No specific random component is described. |
| Allocation concealment (selection bias) | Low risk | The primary care physicians signed up for the demonstration without knowing whether they would be prepaid or paid fee‐for‐service at market‐level rates. |
| Baseline outcome measurements similar | High risk | Utilisation data for the 6 months prior to the programme showed baseline differences amongst groups. |
| Baseline characteristics similar | High risk | There is no report of characteristics of providers in the text or tables. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Knowledge of the allocated interventions adequately prevented | Low risk | The outcomes are objective. |
| Study adequately protected against contamination | Low risk | The comparison group consisted of a sample of children in the demonstration area who had not been asked to join the programme. |
| Selective reporting (reporting bias) | Low risk | The outcomes mentioned in the methods were reported. |
| Other bias | Unclear risk | No more information |
Fairbrother 1999.
| Study characteristics | ||
| Methods | Randomised trial | |
| Participants | Paediatricians and family practice physicians | |
| Interventions | New York City Intervention: new P4P. Bonus and feedback: physicians assigned to the bonus‐with‐feedback group received USD 1000 for a 20% improvement from baseline, USD 2500 for a 40% improvement from baseline, and USD 5000 for reaching 80% coverage irrespective of baseline performance level. Control 1: FFS. Enhanced fee‐for‐service with feedback (EFF): physicians assigned to the EFF group received USD 5 for each vaccine that they administered within 30 days of its coming due and USD 15 for each visit at which all due vaccines were administered. Control 2: feedback only. The control group received feedback on their performance with respect to lead and anaemia screenings. The feedback included overall up‐to‐date screening rates, rates by patient age groups, and comparisons with peer performance. Control 3: existing P4P Year 1995 to 1996, intervention happened in 1995 |
|
| Outcomes |
Quantity of health services provided Up‐to‐date immunisation coverage rate Missed opportunities to immunise Average number of immunisations per child given inside versus outside the practice |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No specific randomised method |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Baseline outcome measurements similar | Low risk | The 3 measures of immunisation performance used varied significantly at baseline. |
| Baseline characteristics similar | Low risk | The characteristics of the practices from which sample children were drawn did not vary significantly across study groups. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data are complete. |
| Knowledge of the allocated interventions adequately prevented | Unclear risk | No information |
| Study adequately protected against contamination | Unclear risk | No information |
| Selective reporting (reporting bias) | Low risk | All the outcome indicators were reported. |
| Other bias | Unclear risk | Not found |
Fairbrother 2001.
| Study characteristics | ||
| Methods | Randomised trial | |
| Participants | Private practice physicians | |
| Interventions | New York City Intervention: new P4P. Bonus and feedback: physicians assigned to the bonus‐with‐feedback group received at each data collection point USD 1000 and USD 2500 for 30% and 45% improvements during time 1, respectively; USD 5000 for reaching 80% up‐to‐date (UTD) coverage irrespective of time 1 performance level; and USD 7500 for reaching 90% UTD coverage. Control 1: FFS. Enhanced fee‐for‐service with feedback (EFF): physicians assigned to the EFF group received USD 5 for each vaccine that they administered within 30 days of its coming due and USD 15 for each visit at which all due vaccines were administered. Control 2: existing P4P. The control group received feedback on their performance with respect to lead and anaemia screenings. The feedback included overall UTD screening rates, rates by patient age groups, and comparisons with peer performance. Year 1997 to 1998, intervention happened in 1997 |
|
| Outcomes |
Quantity of health services provided UTD immunisation coverage rate Missed opportunities to immunise UTD levels |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No specific randomisation method |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Baseline outcome measurements similar | Low risk | Children in the 3 study groups did not differ at time 1 on any of the 3 outcome variables. |
| Baseline characteristics similar | Low risk | The characteristics of the practices from which sample children were drawn did not differ significantly amongst study groups. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data are complete. |
| Knowledge of the allocated interventions adequately prevented | Unclear risk | No information |
| Study adequately protected against contamination | Unclear risk | No information |
| Selective reporting (reporting bias) | Low risk | All the outcome indicators were reported. |
| Other bias | Unclear risk | Not found |
Flierman 1992.
| Study characteristics | ||
| Methods | Controlled before‐after study | |
| Participants | General practitioners | |
| Interventions | Copenhagen City and Copenhagen County, Denmark Intervention: GPs in Copenhagen changed from capitation to a mix of one‐half capitation and one‐half fee for service in October 1987. Control: GPs in Copenhagen County paid by capitation. Year March 1987 to November 1988, intervention happened in October 1987 |
|
| Outcomes |
Quantity of health care: changes in numbers of services per week per 1000 registered patients performed. Amongst these 19 services, 18 tend to increase in number more strongly in Copenhagen City than they do in Copenhagen County. For 8 amongst the 18, the difference is significant on at least a 5% level. The relative increases vary from 5% for taking a cervical smear to 585% for blood glucose measurement, and of the significant ones the lowest relative increase is 51% for urine tests with sticks. Physician services Diagnostic Blood sample: +18 P > 0.10 Cervical smear: +5 P > 0.10 Pregnancy test: +11 P > 0.10 Proctoscopy: +317 P > 0.10 Electrocardiogram: +109 P > 0.10 Haemoglobin measurement: +52 P < 0.10 Blood glucose (photometer): +585 P < 0.01 Streptoculture or urine culture: +211 P < 0.10 Inoculation for cultivation: +68 P < 0.10 Urine test with sticks: +51 P < 0.05 Urine microscopy: +53 P > 0.10 Urine culture with sensitivity: +265 P > 0.10 Curative Removing warts: +114 P < 0.10 Removing ear wax: +16 P > 0.10 Removing corpora aliena from eye/ear/nose/throat: −41 P > 0.10 Removing corpora aliena from skin/from under nail: +54 P > 0.10 Incision or excision of abscess or tumour: +198 P < 0.05 Treating a large wound: +66 P > 0.10 Dressing an immobilising bandage: +173 P < 0.10 |
|
| Notes | Proportional changes and relative change about the physician service | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | This study was designed as controlled before‐after. |
| Allocation concealment (selection bias) | High risk | This study was designed as controlled before‐after. |
| Baseline outcome measurements similar | High risk | The baseline outcome is different between the 2 groups. |
| Baseline characteristics similar | Unclear risk | No description |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No description |
| Knowledge of the allocated interventions adequately prevented | Low risk | The outcome indicators are objective. |
| Study adequately protected against contamination | Low risk | The allocation was by city and county, and it was unlikely that the control group received the intervention. |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes in the methods section are reported in the results section. |
| Other bias | Unclear risk | No description |
Gleeson 2017.
| Study characteristics | ||
| Methods | Controlled before‐after study | |
| Participants | Primary care physicians | |
| Interventions | Central and Southeast Ohio, USA Intervention: P4P plus existing FFS. Practices received USD 0.50 per member per quarter if they accepted at least 500 Medicaid members per physician averaged across the practice. They received an additional USD 0.50 per member per quarter if they completed a "Partners for Kids" program ‐approved Maintenance of Certification programme or were recognised by the National Committee for Quality Assurance as a patient‐centred medical home. Finally, the bulk of the incentive funds were dedicated to a select list of Healthcare Effectiveness Data Information Set measures. The quality payments (USD 40.18 in 2012 and USD 41.39 in 2013) were made per successful patient and were paid to the patient’s attributed physician group. Control: existing Fee‐for‐Service 1 January 2010 to 31 December 2013, intervention happened in 2012 to 2013 |
|
| Outcomes |
Quantity of health care Incentivised measures (2 well care, 2 asthma, and 10 immunisation): adolescent well care OR 1.05, 99.88% CI 1.02 to 1.08; inactivated polio vaccine OR 1.14, 99.88% CI 1.07 to 1.21 Un‐incentivised measures (2 acute illness, 2 attention‐deficit/hyperactivity disorder, 2 immunisation, and a screening test): hepatitis A vaccine OR 0.34, 99.88% CI 0.31 to 0.37 |
|
| Notes | Change OR to RR if necessary. Detailed quantitative results for each outcome require reading of figures. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | This is a controlled before‐after study. Physicians were not randomised into the incentivised, national children's hospital, and non‐member groups. |
| Allocation concealment (selection bias) | High risk | This is a controlled before‐after study. Physicians were not randomised into the incentivised, national children's hospital, and non‐member groups. |
| Baseline outcome measurements similar | Unclear risk | No description |
| Baseline characteristics similar | Low risk | The proportions of patient counts and the patient‐years over the study period were nearly identical, with a trend toward smaller patient volumes at the older age groups and all models adjusted for the patients’ ages. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There were incomplete outcome data in this study, but the author did not describe the effect and adequately addressed. |
| Knowledge of the allocated interventions adequately prevented | Low risk | The outcomes are objective. |
| Study adequately protected against contamination | Low risk | All physicians in a practice fell into the same incentive condition. |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes in the methods section are reported in the results section. |
| Other bias | Unclear risk | No description |
Gosden 2003.
| Study characteristics | ||
| Methods | Controlled before‐after study | |
| Participants | General practitioners | |
| Interventions | England Intervention:salary contract. GPs can be employed on a salaried basis to provide ‘personal medical services’ (PMS) alongside other services needed in the locality. These salaried schemes were intended to improve GP recruitment and retention and quality of care, particularly in underserved areas. Control: capitation and FFS. Standard contract GPs (GMS) are financially rewarded for increasing patient list size (through capitation payments) and for providing specific types of services (through target payments). Years 1998 to 2001, intervention happened in 1999 May |
|
| Outcomes |
Healthcare provider outcomes: Working times: control group longer 2.22 (−8.69, 13.14), and times on 5 specific activities were analysed Quantity of health care outcome: Number of surgery consultations per GP per practice: intervention group more 4.47 (−36.55, 45.5) Number of patients seen out‐of‐hours per GP per practice: intervention group more 4.5 (−1300.46, 1309.46) % consultations in which prescription given per GP per practice: intervention group less −0.07 (−0.20, 0.05) % consultations in which referral made per GP per practice: intervention group less −0.01 (−0.07, 0.05) Total list size practice‐based: intervention group less −213.4 (−942.11, 515.31) List size per whole time practice‐based: intervention group more increase 20.20 (−294.60, 334.99) Cervical cytology (%) practice‐based: intervention group higher 0.38 (−14.90, 15.67) Childhood immunisation (%) practice‐based: intervention group lower −1.08 (−17.95, 15.80) Pre‐school booster (%) practice‐based: intervention group lower −3.08 (−9.63, 3.47) Quality of health care outcome (patients' assessment of quality of care) Access: −1.07 (−4.84, 2.69) Technical care: 1.28 (−4.62, 7.18) Communication: −0.09 (−6.84, 6.66) Interpersonal care: 0.18 (−6.26, 6.62) Overall satisfaction: 2.36 (−6.12, 1.40) Receptionists: 0.64 (−4.81, 6.09) Continuity of care: 0.29 (−5.37, 5.95) Trust in doctor: 0.57 (−3.88, 5.03) Doctors’ knowledge of patient: 1.18 (−3.88, 6.23) Practice nursing: −0.23 (−4.3, 3.83) Co‐ordination of care: 0.63 (−13.43, 14.69) Appropriate referral: 0.91 (−5.31, 7.12) Recommend to a friend: 1.71 (−5.79, 9.21) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | This is a controlled before‐after study (years 1998 to 2001, intervention happened in 1999 May). |
| Allocation concealment (selection bias) | High risk | This is a controlled before‐after study (years 1998 to 2001, intervention happened in 1999 May). |
| Baseline outcome measurements similar | Unclear risk | No description |
| Baseline characteristics similar | High risk | Doctors in GMS and PMS practices differed in their age and gender distributions. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No description |
| Knowledge of the allocated interventions adequately prevented | High risk | Not all the outcome indicators are objective. |
| Study adequately protected against contamination | Low risk | The allocation was by practice, and it is unlikely that the control group received the intervention. |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes in the methods section are reported in the results section. |
| Other bias | Unclear risk | No description |
Gray 2015.
| Study characteristics | ||
| Methods | Controlled before‐after study | |
| Participants | Family physicians | |
| Interventions | Ontario, Canada Intervention 1: Family Health Group (FHG, a small capitation payment and mostly FFS) model Intervention 2: Family Health Network (FHN, mostly on capitation payments for rostered patients) model Control: fee‐for‐service Year 2000 to year 2006, intervention happened in year 2004 |
|
| Outcomes | Healthcare provider outcomes: annual incomes, compared with the control group of family physicians who practised within the FFS model throughout interval of observation. Those who adopted the FHN model gained between 28% and 33% in terms of their real salaries in 2004 compared with the control group. Those who adopted the FHG model gained between 12% and 18%. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | This is a controlled before‐after study. |
| Allocation concealment (selection bias) | Unclear risk | It is reported that the investigators were not aware of the physician’s selection of payment scheme. The physicians decided whether or not to join the new payment scheme. The study tried to compare the comparability of groups, but there may be some bias from unobserved differences amongst physicians choosing different payment schemes. |
| Baseline outcome measurements similar | Low risk | It is reported that the mean incomes of different groups were similar during the earlier stage before changing payment scheme. The data sources of all groups for the years 2000 to 2004 were from income data that were declared to the Canada Revenue Agency. |
| Baseline characteristics similar | Unclear risk | The pre‐intervention comparability analysis reported that the treatment groups resembled the control group fairly for the attributes of the patient population. However, because the selection of those participating in the new payment scheme was dependent on physicians, there may be some unobserved differences. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The overall response rate of survey for income and workload was 20.1%. Due to confidentiality concerns, the link between indicators of workload and/or patient composition to income data at the micro‐level could not be conducted. Futhermore, the data sources for incomes in different stages were different: reporting data in the pre‐intervention stage and survey data in the post‐intervention stage. |
| Knowledge of the allocated interventions adequately prevented | Low risk | The indicator is objective. |
| Study adequately protected against contamination | Low risk | Family physicians were allocated by their own payment scheme. |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes in the methods section are reported in the results section. |
| Other bias | High risk | Theres is serious bias in this study due to physicians' selection of payment scheme and the different data sources at different stages. |
Greene 2013.
| Study characteristics | ||
| Methods | Controlled before‐after study | |
| Participants | General practitioner practices | |
| Interventions | Australia Intervention: P4P plus existing FFS. The voluntary programme pays GPs AUD 40 and AUD 100 in addition to fee‐for‐service payment for providing patients recommended diabetes and asthma treatment over a year, and AUD 35 for screening women for cervical cancer who have not been screened in 4 years. Extra incentives for rural practice loading, 15% increase for practising in large rural centres, and 50% increase for practising in very remote areas. Control: existing FFS Years 1995 to 2010, intervention happened in 2001 |
|
| Outcomes |
Quantity of health services provided The relationship between P4P and provision of diabetes test: incidence RR is 1.0 (P > 0.05) for glycated haemoglobin and 1.07 (P > 0.05) for microalbumin tests. The relationship between P4P and cervical cancer screening: incidence RR is 1.01 (P > 0.05) for diagnostic screening and 1.07 (P > 0.05) for treatment screening. |
|
| Notes | No numeric details to permit analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before‐after study, no random allocation process |
| Allocation concealment (selection bias) | High risk | This is a controlled before‐after study. |
| Baseline outcome measurements similar | Unclear risk | No comparison of outcome measurements was reported. |
| Baseline characteristics similar | Unclear risk | The comparisons on baseline characteristics are not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All indicators are reported. |
| Knowledge of the allocated interventions adequately prevented | Low risk | Outcomes were objective. |
| Study adequately protected against contamination | Low risk | It is not likely that the control group received or was influenced by the payment intervention. |
| Selective reporting (reporting bias) | Unclear risk | Due to the large number of outcome measures, the possibility of selective reporting cannot be ruled out. |
| Other bias | Unclear risk | No more information |
Hickson 1987.
| Study characteristics | ||
| Methods | Randomised trial | |
| Participants | Primary care physicians (PCPs) | |
| Interventions | Continuity Clinic, USA Intervention payment:FFS group received USD 2 per visit Control payment: Salary group received USD 20 per month Year 1983 to 1984, intervention happened in 1983 |
|
| Outcomes |
Quantity of health services provided Patients enrolled per PCP Patient visits attended per PCP Percentage visits attended by patient’s primary physician (continuity) Emergency room visits Scheduled visits Completed visits Sick, primary visits Sick follow‐up visit Well‐child visits The number of recommended visits missed The number of visits in excess of the recommended Adverse effects The number of well patients |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Paediatric residents were initially matched for year of training and the day of the week their clinics were held. Each pair was randomised by the flip of a coin. |
| Allocation concealment (selection bias) | Low risk | Patients are recruited by residents from the health unit or assigned by the secretary. No attempts were made by the faculty or clinic staff to adjust the size of these groups. |
| Baseline outcome measurements similar | Unclear risk | No comparison of outcome measurements was reported. |
| Baseline characteristics similar | Low risk | No significant PCP differences in time since graduation, gender, interest in continuity. No significant patient differences in salary group, mother's age, number of children at home, number of fathers in home. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Knowledge of the allocated interventions adequately prevented | Low risk | Outcomes are objective. |
| Study adequately protected against contamination | Unclear risk | No more information |
| Selective reporting (reporting bias) | Low risk | No reporting bias |
| Other bias | Unclear risk | No more information |
Houle 2016.
| Study characteristics | ||
| Methods | Randomised trial | |
| Participants | Pharmacists | |
| Interventions | Alberta, Canada Intervention: P4P. Included FFS payments plus incentives of CAD 125 and CAD 250 for each patient who reached 50% and 100% of the blood pressure target, respectively. Control: FFS. Pharmacists received CAD 150 for the initial visit and CAD 75 for follow‐up visits. Years 2009 to 2013, intervention happened in 2013 |
|
| Outcomes |
Patient outcomes Systolic blood pressure Diastolic blood pressure The proportion of patients achieving target blood pressure |
|
| Notes | Both continuous and dichotomous data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No specific random component is described. |
| Allocation concealment (selection bias) | Unclear risk | Pharmacist participants in the study specified at the onset of the study whether payments should be provided in their name or to the employing business/organisation. |
| Baseline outcome measurements similar | Low risk | Outcomes were similar at baseline. |
| Baseline characteristics similar | Low risk | Baseline characteristics were similar, and the characteristics were controlled in the analysis. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcomes are mentioned. |
| Knowledge of the allocated interventions adequately prevented | Low risk | The outcomes are objective. |
| Study adequately protected against contamination | Unclear risk | The pharmacists included in this study came from a variety of practice settings, ranging from independently owned pharmacies to chain pharmacies, hospital practice, or family health team practice. Performance payments in the P4P arm may therefore not have always been directed to the pharmacist providing the care. This means that some pharmacists in the P4P arm may not have received the intervention. |
| Selective reporting (reporting bias) | Low risk | The outcomes mentioned in the methods were reported in the results. |
| Other bias | Low risk | No more information in text |
Jensen 2014.
| Study characteristics | ||
| Methods | Controlled before‐after study | |
| Participants | General practitioners | |
| Interventions | Denmark Intervention: mixed system of capitation and FFS contracts. GPs in Copenhagen moved from pure capitation to a mix of one‐third capitation and two‐thirds FFS contracts. Control: pure capitation contracts Years 1984 to 1988, intervention happened in 1987 |
|
| Outcomes |
Patient outcomes Birth weight: 1.0% lower under the capitation system, P > 0.05 Low birth weight: no difference Preterm birth: infants have a 1.9 percentage point (36.5% from the base of 5.2% births) Higher probability of preterm birth Very preterm birth: no difference Fetal growth: infants born under capitation contracts have 0.8 g per week |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | The allocation is not made by the investigator. |
| Allocation concealment (selection bias) | Unclear risk | The allocation is not made by the investigator. |
| Baseline outcome measurements similar | Unclear risk | No more information in text |
| Baseline characteristics similar | Low risk | The baseline characteristics are not similar, but the results changed only marginally when the author excluded these variables from the model. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No more information in text |
| Knowledge of the allocated interventions adequately prevented | Low risk | The outcomes are objective. |
| Study adequately protected against contamination | Low risk | Children are automatically assigned to their mother’s GP, and the possibility of reallocation within a municipality without moving occurs only annually. |
| Selective reporting (reporting bias) | Low risk | The outcomes mentioned in the methods were reported in the results. |
| Other bias | Low risk | No more information in text |
Kouides 1998.
| Study characteristics | ||
| Methods | Randomised trial | |
| Participants | Primary care physicians | |
| Interventions | Monroe County, NY (USA) Intervention payment: P4P + FFS. PCPs in the intervention group received a fee for each immunisation of USD 8 and an additional 10% (USD 0.8) or 20% (USD 1.6) reimbursement per shot according to whether they immunised 70% or 85% (respectively) of the eligible population. Control payment: FFS. PCPs in the control group received only the fee for each immunisation of USD 8. Year 1990 to 1991, intervention happened in 1990 |
|
| Outcomes |
Quantity of health services provided Mean influenza vaccination rate in the intervention period (1991) Change in influenza vaccination rate from baseline year (between 1991 and 1990) Overall influenza vaccination rate: sum of all immunisations given divided by the sum of eligible patients in the intervention period (1991) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, but no specific method mentioned |
| Allocation concealment (selection bias) | Low risk | The practising physicians who participated in the demonstration received free influenza vaccine and a USD 8 administration fee for influenza immunisation of Medicare patients. |
| Baseline outcome measurements similar | Low risk | The proportion of people immunised at baseline (1990) was calculated for each group, and there were no differences between the incentive and control groups. |
| Baseline characteristics similar | Unclear risk | A limited number of characteristics were listed and compared, including number of the elderly in practice, the setting of practice (private, clinics, or Health Management Organisation), and no differences were found. It is not clear whether there were other differences considering the limited number of practices (26) allocated in this study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Knowledge of the allocated interventions adequately prevented | Unclear risk | No information |
| Study adequately protected against contamination | Low risk | To prevent contamination, the unit of randomisation was the office practice so that all physicians in a given practice were in either the incentive or control group. |
| Selective reporting (reporting bias) | Low risk | No reporting bias |
| Other bias | Unclear risk | No information |
Krasnik 1990.
| Study characteristics | ||
| Methods | Controlled before‐after study | |
| Participants | General practitioners | |
| Interventions | Copenhagen City and Copenhagen County, Denmark Intervention: GPs in Copenhagen City changed from capitation to part fee‐per‐item basis and part capitation in October 1987. Control: GPs in Copenhagen County paid FFS and capitation. Year March 1987 to November 1988, intervention happened in October 1987 |
|
| Outcomes |
Quantity of health care outcome Number of contacts (consultations face‐to‐face and by telephone, renewals of prescriptions) Number of activities (diagnostic and curative services, and specialist and hospital referrals) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | This is a controlled before‐after study (Year March 1987 to November 1988, intervention happened in October 1987). |
| Allocation concealment (selection bias) | High risk | This is a controlled before‐after study (Year March 1987 to November 1988, intervention happened in October 1987). |
| Baseline outcome measurements similar | Unclear risk | No comparison of outcome measurements was reported. |
| Baseline characteristics similar | Low risk | No significant differences between groups of PCPs and patients |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The missing data were unlikely to bias the results. |
| Knowledge of the allocated interventions adequately prevented | Low risk | The outcome indicators are objective. |
| Study adequately protected against contamination | Low risk | The allocation was by city and county, and it was unlikely that the control group received the intervention. |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes in the methods section are reported in the results section. |
| Other bias | Unclear risk | No description |
Lee 2010.
| Study characteristics | ||
| Methods | Controlled before‐after study | |
| Participants | Physicians | |
| Interventions | Taiwan Intervention: P4P plus existing FFS. In addition to regular reimbursement for healthcare services such as physician visits, medications, physical exams, and laboratory tests, the P4P programme compensates participating clinicians additional “enlarged physician fees” and “case management fees". The case management fees cover the following 3 types of services: initial enrolment visit, comprehensive follow‐up visits, and an annual evaluation visit. Control: existing FFS Years 2004 to 2006, intervention happened in 2006 |
|
| Outcomes |
Quantity of health services provided Number of essential exams/test: 2.450 (0.019) P < 0.001 Number of diabetes‐related physician visits: 2.010 (0.069) P < 0.001 Number of diabetes‐related hospitalisations: −0.027 (0.009) P = 0.003 Cost Expenses for diabetes‐related physician visits: 7191 (208) P < 0.001 Expenses for diabetes‐related inpatient services: −3878 (716) P < 0.001 Expenses for all diabetes‐related health services: 3312 (764) P < 0.001 |
|
| Notes | High risk of bias | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before‐after study, no random allocation process |
| Allocation concealment (selection bias) | High risk | This is a controlled before‐after study (Years 2004 to 2006, intervention happened in 2006). |
| Baseline outcome measurements similar | Low risk | For essential exams/tests: before the P4P programme, the average number of essential exams/tests performed in a year was similar between groups. However, for other indicators there were no relevant comparisons. |
| Baseline characteristics similar | High risk | There was a greater percentage of women in the intervention group (53.6%) than in the comparison group (51.0%). Intervention group patients were younger than comparison group patients (mean age 61.5 years vs 63.4 years). Patients in the intervention group were more likely to have a Charlson Comorbidity Index score greater than 2 (54.0% vs 48.8%, P < 0.001), implying that the intervention group was not healthier than the comparison group. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Many indicators are reported; it is difficult to assess their completeness and how denominators have changed over the study period. |
| Knowledge of the allocated interventions adequately prevented | Low risk | Outcomes were objective. |
| Study adequately protected against contamination | Low risk | It is not likely that the control group received or was influence by the payment intervention. |
| Selective reporting (reporting bias) | Unclear risk | Due to the large number of outcome measures, the possibility of selective reporting cannot be ruled out. |
| Other bias | Unclear risk | No more information |
Li 2013.
| Study characteristics | ||
| Methods | Controlled before‐after study | |
| Participants | General practitioners | |
| Interventions | Ontario, Canada Incentive group: P4P plus existing payment Comparison group: existing payment (blend of FFS and capitation) Years 1998 to 2008, intervention happened in 2003 |
|
| Outcomes |
Quantity of health services provided (Difference‐in‐difference with physician‐specific fixed‐effect model) Senior flu shots rate: marginal effect 2.8 percentage point increase (P = 0.007, N = 19,866), 5.1% increase over the base compliance level Toddler immunisation rate: marginal effect 1.1 percentage point increase (P = 0.012, N = 16,826) Pap smears rate: marginal effect 4.1 percentage point increase (P = 0.005, N = 19,926), 7.0% increase over the base compliance level Mammograms rate: marginal effect 1.8 percentage point increase (P = 0.005, N = 19,888), 2.8% increase over the base compliance level Colorectal cancer screenings rate: marginal effect 8.5 percentage point increase (P = 0.007, N = 19,918), 57% increase over the base compliance level |
|
| Notes | High risk of bias | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | The allocation is not made by the investigator. |
| Allocation concealment (selection bias) | High risk | The allocation is not made by the investigator. |
| Baseline outcome measurements similar | Unclear risk | No comparison of outcome measurements was reported. |
| Baseline characteristics similar | High risk | At baseline, the GPs in the incentive group were younger than those in the comparison group, had fewer years of practice experience, were more likely to be female, worked more days per year, practised more intensively, had larger patient populations with slightly more females and infants, were more likely to be located in urban areas, and were more homogenous (as indicated by smaller standard deviations). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data |
| Knowledge of the allocated interventions adequately prevented | Low risk | The outcomes are objective. |
| Study adequately protected against contamination | Unclear risk | No more information in text |
| Selective reporting (reporting bias) | Low risk | The outcomes mentioned in the methods were reported in the results. |
| Other bias | Low risk | No more information in text |
Lurie 1992.
| Study characteristics | ||
| Methods | Randomised trial | |
| Participants | Mental health physician | |
| Interventions | Hennepin County, Minnesota (USA) Intervention: FFS Control: prepaid (capitation) Years 1987 to 1989, intervention happened in 1987 |
|
| Outcomes |
Quantity of service provision Referrals to specialists Patient outcomes General health Physical function Social health Psychiatric status Community and role function |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | It is reported that all eligible clients were identified and randomised to the experimental (prepaid) group, with an equal random sample of clients remaining in FFS Medicaid. However, no specific random method was described. |
| Allocation concealment (selection bias) | Low risk | The unit of allocation was groups. |
| Baseline outcome measurements similar | Unclear risk | No comparison of baseline outcome measurements was reported. |
| Baseline characteristics similar | Low risk | There were no significant differences in characteristics between the prepaid and FFS populations at baseline. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The incomplete outcome data did not affect the outcome. |
| Knowledge of the allocated interventions adequately prevented | High risk | Once randomised to prepaid care, clients were given a choice of 4 health plans. An independent broker educated clients about the plans and encouraged them to choose 1. |
| Study adequately protected against contamination | Unclear risk | No information in the text |
| Selective reporting (reporting bias) | Low risk | No important results were ignored. |
| Other bias | Unclear risk | No information in the text |
Petersen 2013.
| Study characteristics | ||
| Methods | Randomised trial | |
| Participants | Primary care physicians | |
| Interventions | USA, many states Intervention: pay for performance plus existing salary payment: participants earned incentives for achieving JNC 7 guideline. The reward was USD 9.10 for each successful measure. Control 1: education: participants attended webinars beginning in February 2008 that reviewed the guidelines from the 'Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7)' Control 2: audit and feedback: customised audit and feedback reports detailing performance for each period and the next period’s performance goals were posted to the study’s secure website Control 3: only salary payment to physician in practice Year 2007 to 2008, intervention happened in 2007 |
|
| Outcomes |
Quality of service provision Use of guideline‐recommended antihypertensive medicines Patient outcomes 24 months after interventions Number of patients achieving guideline‐recommended blood pressure thresholds or receiving an appropriate response to uncontrolled blood pressure Number of patients prescribed guideline‐recommended medications Number of patients who developed hypotension Unintended outcomes Adverse effects Reduction in blood pressure control and appropriate response to uncontrolled blood pressure after the intervention had ended |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A data analyst assigned a uniform random number to each of the possible allocations using SAS version 9.1.3 (SAS Institute) and selected the 1 with the highest random number. |
| Allocation concealment (selection bias) | Low risk | Cluster randomised by hospitals, and the sequence and allocation happened at the same time |
| Baseline outcome measurements similar | Unclear risk | No comparison analysis on baseline outcomes, and from descriptive data they seemed to be different. At the same time the sample of hospitals (12) for randomised allocation was limited. The results were based on analysis adjusted by baseline level. |
| Baseline characteristics similar | Unclear risk | There were no significant differences in the distributions of physician sex, race, years practising since completing residency, or patient characteristics. There were significant differences across groups in characteristics of the hospitals where the participants worked, including whether they were teaching hospitals (P < 0.001), whether they were an antihypertensive programme sites (P < 0.001), and whether they were in the southern or northern USA (P = 0.04). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The dropout rates of participants were not high (1/19, 4/24, 2/44, 1/20 excluded during the study process |
| Knowledge of the allocated interventions adequately prevented | Low risk | All outcomes were objective. |
| Study adequately protected against contamination | Low risk | This study was cluster randomised by hospital to avoid contamination of the intervention; all participants at a given hospital belonged to the same intervention group. |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in the methods section were reported in the results section. |
| Other bias | Low risk | |
Singh 2015.
| Study characteristics | ||
| Methods | Controlled before‐after study | |
| Participants | Health workers in daycare centres for children | |
| Interventions | India Intervention 1: incentive treatment. Fixed wages plus performance bonus (Rupees100*n, n = number of children with nutrition grade improved − number of children with grade declined) Intervention 2: recipe treatment. Provided mothers with information without incentivising the workers Intervention 3: combined treatment. Intervention 1 + intervention 2 Control: no bonus incentive (only wage) or information Years 2009 to 2011, intervention happened in 2010 |
|
| Outcomes |
Patient outcomes (DID analysis with control for factors of workers and household) Weight: 3 g increase (Standard Deviation 0.070, P > 0.1) Grade (as measured in the Anganwadi): increase by 0.018 (Standard Deviation 0.040, P > 0.1) z‐score: increase by 0.002 (Standard Deviation 0.034, P > 0.1) World Health Organisation malnourished status: increase by 1.3 percentage points (Standard Deviation 0.023, P > 0.1) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before‐after study |
| Allocation concealment (selection bias) | High risk | Controlled before‐after study |
| Baseline outcome measurements similar | Low risk | The baseline malnutrition rates of the different groups were similar. |
| Baseline characteristics similar | Low risk | The key characteristic variables were compared, and the normalised differences did not exceed a quarter in the key variables. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | It is evident from the table that attrition rates are actually lower in three intervention groups and control group. |
| Knowledge of the allocated interventions adequately prevented | Low risk | For the purpose of the experiment, change in weight‐for‐age grade was therefore used as the criterion for evaluating performance of an Anganwadi worker. |
| Study adequately protected against contamination | Unclear risk | No more information |
| Selective reporting (reporting bias) | Low risk | No missing outcome data |
| Other bias | Unclear risk | No more information |
Twardella 2007.
| Study characteristics | ||
| Methods | Randomised trial | |
| Participants | General practitioners | |
| Interventions |
Financial incentive characteristics GPs were assured a financial remuneration of Euro 130 after study completion for each study participant they recruited who was "smoke free" at 12 months follow‐up. GPs could offer to their patients cost‐free prescriptions (up to Euro 130) for drugs proved effective in supporting smoking cessation. Type of intervention The practices were randomised into 4 experimental groups:
Year 2002 to 2003, intervention happened in 2002 |
|
| Outcomes |
Patient outcome Self‐reported smoking abstinence obtained at 12 months follow‐up and validated by serum cotinine |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | The GPs participating in this trial were not a random selection but a selected group recruited from participants of a survey on promotion of smoking cessation. This group might be characterised by an increased commitment to the issue of smoking cessation and to improving their quality of cessation promotion. |
| Allocation concealment (selection bias) | High risk | Owing to the nature of the interventions, GPs and participants could not be blinded to the intervention. |
| Baseline outcome measurements similar | Unclear risk | The study has no baseline measure of outcome. |
| Baseline characteristics similar | Low risk | Participants did not differ substantially at baseline by intervention arm. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Missing outcome data likely to be related to true outcome. Reasons for the 13 withdrawals from the study were not given by the authors and were likely to be related to GP practices or patient characteristics. |
| Knowledge of the allocated interventions adequately prevented | Low risk | Blood samples collected in the follow‐up 12 months after recruitment were sent to a central laboratory, and cotinine levels in serum were determined in a blinded fashion by radio immunoassay, according to the manufacturer instructions (Immunodiagnostic, Bensheim, Germany). |
| Study adequately protected against contamination | Low risk | Allocation was by practice, and it is unlikely that the control group received the intervention. |
| Selective reporting (reporting bias) | Low risk | The outcomes mentioned in the methods section were reported in the results section. |
| Other bias | Low risk | No more information |
Yesalis 1980.
| Study characteristics | ||
| Methods | Controlled before‐after study | |
| Participants | Pharmacists | |
| Interventions | Rural counties in Iowa (USA) Intervention: capitation Control: fee for service Under the current fee‐for‐service system in the Medicaid programme, the pharmacist receives payment for the cost of ingredients as well as a professional fee for each prescription filled. Year 1 to year 3, intervention happened in year 2 |
|
| Outcomes |
Quantity of health services provided Rate of generic substitution per 100 prescriptions: change from 0.38 to 7.25 vs change from 0.12 to 0.54 Percentage of Medicaid prescriptions classified as multi‐source drug products: change from 47.6 to 47.0 vs change from 49.3 to 48.9 Numbers of prescriptions involving changes in labeller on refills (0 to 5 days): change from 6 to 9 vs change from 2 to 22 Cost Mean cost saving per generic substitution between control and experimental pharmacies: change from 0.24 to 2.73 vs change from 1.04 to 0.88 |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | This is a controlled before‐after study. |
| Allocation concealment (selection bias) | High risk | This is a controlled before‐after study. |
| Baseline outcome measurements similar | Low risk | There were no significant differences between experimental and control counties. |
| Baseline characteristics similar | Low risk | Demographics such as age, sex, number of pharmacists and number of physicians per capita are comparable amongst the experimental and control counties, Iowa, and the nation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No description |
| Knowledge of the allocated interventions adequately prevented | Low risk | The outcome indicators are objective. |
| Study adequately protected against contamination | Low risk | The allocation was by counties, and it is unlikely that the control group received the intervention. |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes in the methods section are reported in the results section. |
| Other bias | High risk | No chain pharmacies were present in either the experimental or control countries. |
Yesalis 1984.
| Study characteristics | ||
| Methods | Controlled before‐after study | |
| Participants | Pharmacists | |
| Interventions | Iowa (USA) Intervention: capitation. Pharmacists were paid 80% of projected drug expenditures in advance based on the types of Medicaid eligible who chose them as their providers. The remaining 20% was withheld in an escrow account to be used for supplemental, emergency, and bonus payments. Control: fee for service. The fee‐for‐service system under Medicaid usually covers ingredient costs plus a fixed professional dispensing fee. Under the current fee‐for‐service system in the Medicaid programme, the pharmacist receives payment for the cost of ingredients as well as a professional fee for each prescription filled. |
|
| Outcomes |
Quantity of health services provided Generic substitution: the dramatic increase in the use of generic products happened in pilot stage. Pharmacists in the expanded capitation programme showed no statistically significant increase in generic substitution with either the Medicaid or non‐Medicaid patient population. Quantities of drugs dispensed per prescription: no significant changes Changing the type of drugs dispensed within a therapeutic category: no significant changes Switching to non‐prescription (Over the Counter) drugs: no significant changes Average number of prescriptions per eligible: no significant changes Average days' therapy per recipient: fewer days in capitation group Costs Cost saving from generic substitution |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | This is a controlled before‐after study. |
| Allocation concealment (selection bias) | High risk | This is a controlled before‐after study. |
| Baseline outcome measurements similar | Low risk | Differences between experimental and control counties were not significant. |
| Baseline characteristics similar | Low risk | Patient demographic characteristics in the control and experimental counties and the state and the nation are reasonably similar. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No more information in text |
| Knowledge of the allocated interventions adequately prevented | Low risk | The outcome indicators are objective. |
| Study adequately protected against contamination | Low risk | The allocation was by counties, and it is unlikely that the control group received the intervention. |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes in the methods section are reported in the results section. |
| Other bias | Unclear risk | No more information in text |
Young 2007.
| Study characteristics | ||
| Methods | Interrupted time series study | |
| Participants | 334 primary health physicians members of the Rochester Individual Practice Association (RIPA) | |
| Interventions | Rochester, New York (USA) Interventions: pay for performance. In 2001, RIPA began the process of establishing a pay‐for‐performance programme as part of its contract with Excellus Health Plan to provide professional services for the approximately 450,000 individuals enrolled in Excellus’ Blue Choice Health Maintenance Organization (HMO) insurance product. Each RIPA primary care physician was eligible for reward payments of up to approximately USD 15,000 depending on his or her relative ranking on a composite performance measure for the diabetes‐related tests and screens. Control: no explicit information, but may be salary. RIPA served as an umbrella organisation for its member physicians with responsibilities for contracting with health plans on behalf of its member physicians and developing arrangements by which member physicians were reimbursed for their services. Years 1999 to 2004, intervention happed in 2002, 3 time points for pre‐/postintervention |
|
| Outcomes |
Quality of health care provision 4 diabetes performance measures: glycated haemoglobin check, urinalysis, lipoprotein density level check, eye exam. Glycated haemoglobin check: increase in level without significance, but post‐trend did not differ from the pre‐trend Urinalysis: increase in level without significance, but post‐trend did not differ from the pre‐trend LDL check: increase in level without significance, but post‐trend did not differ from the pre‐trend Eye exams: increase in levels (7 percentage point increase after 1 year of intervention, but the increase did not persist), post‐trend did not differ from the pre‐trend |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Intervention independent of other changes (ITS) | Low risk | The increase in the eye examination score of intervention programme (Rochester (New York) Individual Practice Association) was contrary to the trends observed in the eye examination scores in Health Employer Data Information System nationally and statewide during this period, which were largely flat from 2000 to 2002. The observed increase thus did not appear to be attributable to a secular trend. |
| Shape of the intervention effect pre‐specified (ITS) | Low risk | The point of analysis is clear, the intervention start time: 2002. |
| Intervention unlikely to affect data collection (ITS) | Unclear risk | The data used for analysis in this paper were from patient electronic records, which were not influenced by payment reform. However, it is unclear if GPs' recording was changed with the start of the new payment policy. |
| Knowledge of the allocated interventions adequately prevented (ITS) | Low risk | Outcomes were objective. |
| Incomplete outcome data adequately addressed (ITS) | Low risk | No missing outcome data |
| Study free from selective outcome reporting (ITS) | Low risk | All outcomes mentioned in the methods section were reported in the results section. |
| Other risk of bias (ITS) | Low risk | The study included physicians who were members of the health plan for all 6 years, and so does not account for the potential selection bias if poorly performing physicians chose not to participate or withdraw. |
Young 2012.
| Study characteristics | ||
| Methods | Repeated measures study | |
| Participants | General practitioner practices | |
| Interventions | Rochester, New York (USA) Interventions: pay for performance. In 2001, Rochester Individual Practice Association (RIPA) began the process of establishing a pay‐for‐performance programme as part of its contract with Excellus Health Plan to provide professional services for the approximately 450,000 individuals enrolled in Excellus’ Blue Choice Health Maintenance Organization (HMO) insurance product. Each RIPA primary care physician was eligible for reward payments of up to approximately USD 15,000 depending on his or her relative ranking on a composite performance measure for the diabetes‐related tests and screens. Control: no explicit information, but may be salary. RIPA served as an umbrella organisation for its member physicians with responsibilities for contracting with health plans on behalf of its member physicians and developing arrangements by which member physicians were reimbursed for their services. Years 1999 to 2004, intervention happened in 2002, 3 time points for pre‐/postintervention |
|
| Outcomes |
Quality of health care provision Diabetes‐related composite measure performance score (4 diabetes performance measures include glycated haemoglobin testing, lipoprotein density level screening, nephropathy, eye examination): Repeated Measure regression model controlling all factors showed that P4P was related to 0.100 (Standard Error 0.032, P < 0.01) higher score. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Intervention independent of other changes (ITS) | Low risk | As noted earlier, the study sample consisted of physicians who had received reports regarding their relative performance on the diabetes‐related measures both during the baseline period and after introduction of the incentive. As such, a strength of the study design is that the participants did not experience any changes in performance goals or performance‐related information during the study period. The key relevant change was the introduction of the financial incentive. |
| Shape of the intervention effect pre‐specified (ITS) | Low risk | The point of analysis is clear, the intervention start time: 2002. |
| Intervention unlikely to affect data collection (ITS) | Unclear risk | The data used for analysis in this paper were from patient electronic records, which were not influenced by payment reform. However, it is unclear if GPs' recording was changed with the start of the new payment policy. |
| Knowledge of the allocated interventions adequately prevented (ITS) | Low risk | Outcomes were objective. |
| Incomplete outcome data adequately addressed (ITS) | Low risk | No missing outcome data |
| Study free from selective outcome reporting (ITS) | Low risk | All outcomes mentioned in the methods section were reported in the results. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abelsen 2015 | This is a cross‐sectional study. |
| Agee 2014 | Just a comparative study |
| Allard 2014 | This study just used a model of physician treatment and referral decisions under endogenous payment form in the flavour of Allard and colleagues. |
| Allen 2016 | Not a Controlled before‐after study (CBA) study design: although it uses difference‐in‐differences model, study lacks a control group |
| Alshamsan 2012 | The provider is institution. |
| An 2008 | Study participants are clinics. |
| Arrowsmith 2014 | The provider is institution. |
| Baker 2005 | This study is not an Randomised Controlled Trial (RCT), CBA, or Interrupted Time Series (ITS). |
| Barnato 2017 | The target of the intervention in this study is the patient. |
| Basu 2016 | The outcome index concerns the impact on financial outcome. |
| Boyden 2000 | The provider is institution. |
| Broadway 2017 | This study is not an RCT, CBA, or ITS; it uses a structure, discrete choice model. |
| Carey 1990 | Not control study |
| Chen 2016 | Not a CBA study design; control group was created by matching methods to conduct difference‐in‐differences analysis |
| Clancy 1988 | Not control study |
| Coleman 2007 | Not a CBA study, only a before‐and‐after study |
| Davies 1986 | Provider is insurance plan. |
| Douven 2015 | Not an ITS study design because it does not have a clearly defined point in time; only time trends are described |
| Engineer 2016 | The participants of pay for performance in this study are primary care facilities. |
| Erickson 2016 | In this study the participants are physicians in haemodialysis facility and their patients, but only patients are described and there is no more information about the physicians. |
| Erickson 2017 | The intervention in this study is for inpatients. |
| Erus 2017 | Not designed as a CBA study, no control group |
| Feng 2015 | Not a CBA study design, but rather a before‐after comparative study without control group |
| Gallagher 2015 | The provider is institution. |
| Giguere 2015 | The intervention of this study is not related to payment methods. |
| Greene 2013 | The interventions are for both general practitioners and general practices. |
| Hamilton 2016 | Not a CBA study design, but rather a before‐after comparative study without control group |
| Han 2015 | This study does not have at least 3 time points before and 3 after the intervention. An interrupted time‐series analysis allows separate analysis of immediate effects and monthly trends following policy implementation, but it is shown through 3 periods. |
| Hickey 2015 | Not a CBA study design; only 1 site |
| Hysong 2017 | The participants of the study are not ambulatory care professionals. |
| Jones 2015 | Not a CBA study design; no control group |
| Kiran 2015 | Not a CBA study design. It does not have a clearly defined point in time, but just describes the change trend of different physicians payment methods. |
| Kliner 2015 | Not a CBA study design. The intervention was only performed in a regional hospital, which made it difficult to attribute any observed differences to the intervention rather than to other site‐specific variables. |
| Lagarde 2016 | The participants in this study are not in outpatient care facilities. |
| Lee 2010 | Participants of this study were patients. Although the physicians could enrol individual patients in the programme and receive regular reimbursement, there is no description of physicians, and not all the physicians come from outpatient facilities. |
| Lee 2015 | The participants of this study are patients. |
| Lezzi 2014 | Not an RCT, CBA, or ITS study design; a panel count data model is used |
| Lix 2016 | This study is not designed as a CBA, but rather a retrospective case‐control study. |
| Maini 2014 | The participants of this study are facilities, and the intervention objects are patients. |
| Merilind 2015 | This study is not designed as an ITS. It compared childhood immunisation coverage rates of all Estonian family physicians in 2 groups, joined and not joined to the quality system, during the observation period of 2006 to 2012. Also, the family physicians had joined the quality system and were not assigned by investigators in 2006. |
| Michel 2015 | Cross‐sectional study |
| Mullen 2009 | Provider is not clear. |
| Murray 1992 | Cross‐sectional study |
| Odesjo 2015 | The participants of this study are practices. |
| Olivier 2015 | Not a CBA study design; no control group |
| Petersen 2016 | The participants in this study are in‐hospital, and the incentives are for both physicians and facility. |
| Ritchie 1992 | Not an ITS study; it only measured 7 quarters after intervention |
| Robertson 2017 | This study used quality measure, plan‐do‐study‐act, root‐cause analyse, lean six sigma. |
| Roski 2003 | The participants of the study are clinics. |
| Rudasingwa 2017 | The participants of this study are healthcare facilities. |
| Shelley 2012 | Not about payment methods |
| Shen 2003 | The provider is not clear. |
| Shen 2017a | The participants of the study are health centres. |
| Shen 2017b | The participants of the study are not ambulatory care professionals. |
| Sicsic 2015 | Not a CBA study; only a before‐and‐after study |
| Simonsen 2017 | The intervention in this study is not related to payment methods. |
| Simpson 2011 | The participants of this study were patients. Physicians are only mentioned in the background (intervention can increase a doctor's income). |
| Stearns 1992 | Not a CBA study; only a before‐and‐after study |
| Sun 2016 | The participants of this study are healthcare facilities. |
| To 2015 | Cross‐sectional study |
| van Dijk 2014 | Not a CBA study design, just a simple before‐after comparative study |
| van Dijk 2015 | Not a CBA study; no control group |
| Vats 2014 | The participants of this study are primary care practices. |
| Wei 2015 | The intervention in this study was just an assumption. |
| White 2006 | The participant is not health care professionals in ambulatory care facilities. |
Characteristics of studies awaiting classification [ordered by study ID]
Lavergne 2018.
| Methods | Interrupted time series study |
| Participants | Primary care physicians |
| Interventions | British Columbia, Canada Intervention: Pay‐for‐performance plus existing payment. Incentive payments targeting chronic disease management, including CAD 75 for diabetes (later increased to CAD 125), CAD 50 for hypertension, and CAD 125 for Chronic Obstructive Pulmonary Disease per patient; and the payment was based on performance for which the physicians needed to submit the charts for patients. Tha charts need include documentation of relevant guideline indicated processes of care and flow sheets or care plan templates for each condition should be available as part of billing guides for performance check and payment purpose. Control: existing payment, the province‐wide fee‐for‐service system |
| Outcomes | Primary care visits: number of visits with any primary care physician Continuity of care: percent of primary care visits across the whole study population in each month that occurred with patients' usual providers of care assigned over the preceding year Testing and pharmaceutical dispensing: rates of anatomic therapeutic chemical testing and antihypertensive dispensing Hospitalization: all acute admissions, hospital admissions through the emergency department, and admissions for selected conditions, including acute myocardial infarction, stroke and heart failure among hypertension patients, and Chronic Obstructive Pulmonary Disease patients Health care spending: total constant dollar spending, and spending on primary care physicians, medical specialists, surgical specialists, laboratory services, imaging, pharmaceutical use, acute care, and day surgery |
| Notes |
Navathe 2019.
| Methods | Cluster randomised trial |
| Participants | Physicians |
| Interventions | Illinosisi, US Intervention 1: Enhanced Pay‐for‐performance. Providing maximum Pay‐for‐perfomrance bonuses larger than previous years by a mean of USD 3355 per physician, an approximately 32% increase in bonus size and an increase of USD 16 per patient. Intervention 2: Enhanced Pay‐for‐performance plus loss aversion. Prefunded incentives are put in a virtual health system bank account in the physician name. If at the end of intervention year physicians earned less pay‐for‐performance bonus than were placed in the virtual accounts, the physician were required to pay back funds. Intervention 3: Enhanced Pay‐for‐performance plus increased social pressure. Providing increased proportion of pay‐for‐performance bonus determined by group performance from 30% to 50%. |
| Outcomes | The proportion of 20 evidence‐based quality measures achieved at the patient level |
| Notes |
Characteristics of ongoing studies [ordered by study ID]
NCT02731716.
| Study name | Transforming primary care payment in Hawaii |
| Methods | Randomised trial |
| Participants | Primary care providers |
| Interventions |
Intervention 1:Pay‐for‐performance (P4P). A quality incentive payment based upon attainment of 16 quality metrics, and a possible bonus payment for savings in total cost of care at the provider organisation level. Intervention 2:P4P + social comparisons. Providers will also receive weekly emails that will show comparisons of their own performance against their peers within the same provider organisation on specific quality measures and total cost of care. Intervention 3:P4P + social comparisons + Hemoglobin A1c member/provider incentive. There is also a shared incentive between the member and the provider. The member incentive will be a payment made to diabetic patients with an A1C of greater than or equal to 9% who experience a reduction of at least 0.5%. Each participating member and primary care providers can receive up to USD 75 per quarter for A1C reduction. Control:FFS. |
| Outcomes |
Quantity of health care provided Improvement in Hemoglobin A1c among poorly controlled diabetics Quality of health care provided Improvement in provider performance Cost Primary care spending |
| Starting date | April 2016 |
| Contact information | ClinicalTrials.gov Identifier: NCT02731716 |
| Notes |
Differences between protocol and review
One new author was added, Lu Zhang, who assisted in applying the inclusion criteria and extracting the data for the review.
We changed the title of the review from 'Payment methods for ambulatory care health professionals' to 'Payment methods for healthcare providers working in outpatient healthcare settings' based on the comments of an expert group.
Contributions of authors
All review authors have contributed to the production of the review. Liying Jia, Beibei Yuan, and Qingyue Meng drafted and amended the protocol. Liying Jia, Beibei Yuan, Minxuan Xu, and Lu Zhang applied the inclusion criteria, assessed the risk of bias, and extracted data for the included studies. Liying Jia and Lu Zhang prepared the report, and the other review authors commented on it. Anthony Scott commented on the protocol and several drafts of the review.
Declarations of interest
Liying Jia: None known.
Qingyue Meng: None known.
Anthony Scott: None known.
Beibei Yuan: None known.
Lu Zhang: None known.
New
References
References to studies included in this review
Bilardi 2010 {published data only}
- Bilardi JE, Fairley CK, Temple-Smith MJ, Pirotta MV, McNamee KM, Bourke S, et al. Incentive payments to general practitioners aimed at increasing opportunistic testing of young women for chlamydia: a pilot cluster randomised controlled trial. BMC Public Health 2010;10(70):1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Christensen 2000 {published data only}
- Christensen DB, Neil N, Fassett WE, Smith DH, Holmes G, Stergachis A. Frequency and characteristics of cognitive services provided in response to a financial incentive. Journal of the American Pharmaceutical Association 2000;40:609-17. [DOI] [PubMed] [Google Scholar]
Chung 2010 {published data only}
- Chung S, Palaniappan L, Wong E, Rubin H, Luft H. Does the frequency of pay-for-performance payment matter? Experience from a randomized trial. Health Services Research 2010;45(2):553-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Clarkson 2008 {published data only}
- Clarkson JE, Turner S, Grimshaw JM, Ramsay CR, Johnston M, Scott A, et al. Changing clinicians’ behavior: a randomized controlled trial of fees and education. Journal of Dental Research 2008;87(7):640-4. [DOI] [PubMed] [Google Scholar]
Davidson 1992 {published data only}
- Davidson SM, Manheim LM, Werner SM, Hohlen MM, Yudkowsky BK, Fleming GV. Prepayment with office-based physicians in publicly funded programs: results from the Children’s Medicaid Program. Pediatrics 1992;89:761-7. [PubMed] [Google Scholar]
Fairbrother 1999 {published data only}
- Fairbrother G, Hanson KL, Friedman S, Butts GC. The impact of physician bonuses, enhanced fees, and feedback on childhood immunization coverage rates. American Journal of Public Health 1999;89(2):171-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fairbrother 2001 {published data only}
- Fairbrother G, Siegel MJ, Friedman S, Kory PD, Butts GC. Impact of financial incentives on documented immunization rates in the inner city: results of a randomized controlled trial. Ambulatory Pediatrics 2001;1(4):206-12. [DOI] [PubMed] [Google Scholar]
Flierman 1992 {published data only}
- Flierman H, Groenewegen P. Introducing fees for services with professional uncertainty. Health Care Financing Review 1992;14(1):107-15. [PMC free article] [PubMed] [Google Scholar]
Gleeson 2017 {published data only}
- Gleeson S, Kelleher K, Gardner W. Evaluating a pay-for-performance program for Medicaid children in an accountable care organization. JAMA Pediatrics 2017;170(3):259-66. [DOI] [PubMed] [Google Scholar]
Gosden 2003 {published data only}
- Gosden T, Sibbald B, Williams J, Petchey R, Leese B. Paying doctors by salary: a controlled study of general practitioner behaviour in England. Health Policy 2003;64(3):415-23. [DOI] [PubMed] [Google Scholar]
Gray 2015 {published data only}
- Gray D, Hogg W, Green M, Zhang Y. Did family physicians who opted into a new payment model receive an offer they should not refuse? Experimental evidence from Ontario. Canadian Public Policy-analyse De Politiques 2015;41(2):151-65. [Google Scholar]
Greene 2013 {published data only}
- Greene J. An examination of pay-for-performance in general practice in Australia. Health Services Research 2013;48(4):1415-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hickson 1987 {published data only}
- Hickson GB, Altemeier WA, Perrin JM. Physician reimbursement by salary or fee-for-service: effect on physician practice behavior in a randomized prospective study. Pediatrics 1987;80(3):344-50. [PubMed] [Google Scholar]
Houle 2016 {published data only}
- Houle SK, Charrois TL, McAlister FA, Kolber MR, Rosenthal MM, Lewanczuk R, et al. Pay-for-performance remuneration for pharmacist prescribers' management of hypertension: a substudy of the RxACTION trial. Canadian Pharmaceutical Journal 2016;149:345-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jensen 2014 {published data only}
- Jensen VM. Happy doctor makes happy baby? Incentivizing physicians improves quality of prenatal care. Review of Economics and Statistics 2014;96(5):838-48. [Google Scholar]
Kouides 1998 {published data only}
- Kouides R, Bennet N, Lewis B, Cappuccio J, Barker W, LaForce M. Performance based physician reimbursement and influenza immunisation rates in the elderly. American Journal of Preventive Medicine 1998;14:89-95. [DOI] [PubMed] [Google Scholar]
Krasnik 1990 {published data only}
- Krasnik A, Groenewegen PP, Pedersen PA, Scholten PV, Mooney G, Gottschau A, et al. Changing remuneration systems: effects on activity in general practice. BMJ 1990;300:1698-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2010 {published data only}
- Lee TT, Cheng SH, Chen CC, Lai MS. A pay-for-performance program for diabetes care in Taiwan: a preliminary assessment. American Journal of Managed Care 2010;16(1):65-9. [PubMed] [Google Scholar]
Li 2013 {published data only}
- Li J, Hurley J, DeCicca P, Buckley G. Physician response to pay-for-performance: evidence from a natural experiment. Health Economics 2014;23(8):962-78. [DOI] [PubMed] [Google Scholar]
Lurie 1992 {published data only}
- Lurie N, Moscovice IS, Finch M, Christianson JB, Popkin MK. Does capitation affect the health of the chronically mentally iII? Results from a randomized trial. JAMA 1992;267:3300-4. [PubMed] [Google Scholar]
Petersen 2013 {published data only}
- Petersen LA, Simpson K, Pietz K, Urech TH, Hysong SJ, Profit J, et al. Effects of individual physician-level and practice-level financial incentives. JAMA 2013;310(10):1042-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Singh 2015 {published data only}
- Singh P. Performance pay and information: reducing child under nutrition in India. Journal of Economic Behavior & Organization 2015;112:141-63. [Google Scholar]
Twardella 2007 {published data only}
- Twardella D, Brenner H. Effects of practitioner education, practitioner payment and reimbursement of patients' drug costs on smoking cessation in primary care: a cluster randomised trial. Tobacco Control 2007;16(1):15-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yesalis 1980 {published data only}
- Yesalis CE 3rd, Norwood GJ, Lipson DP, Helling DK, Fisher WP, Burmeister LF. Capitation payment for pharmacy services: impact on generic substitution. Medical Care 1980;18(8):816-28. [DOI] [PubMed] [Google Scholar]
Yesalis 1984 {published data only}
- Yesalis CE 3rd, Norwood GJ, Helling DK, Lipson DP, Mahrenholz RJ, Burmeister LF, et al. Capitation payment for pharmacy services. II. Impact on costs. Medical Care 1984;22(8):746-54. [DOI] [PubMed] [Google Scholar]
Young 2007 {published data only}
- Young GJ, Meterko M, Beckman H, Baker E, White B, Sautter KM, et al. Effects of paying physicians based on their relative performance for quality. Journal of General Internal Medicine 2007;22(6):872-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Young 2012 {published data only}
- Young GJ, Beckman H, Baker E. Financial incentives, professional values and performance: a study of pay-for-performance in a professional organization. Journal of Organizational Behavior 2012;33(7):964-83. [Google Scholar]
References to studies excluded from this review
Abelsen 2015 {published data only}
- Abelsen B, Olsen JA. Young doctors’ preferences for payment systems: the influence of gender and personality traits. Human Resources for Health 2015;13(69):1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Agee 2014 {published data only}
- Agee MD, Gates Z. The impact of an insurance administration–free primary care office on hospital admissions: a community level comparison with traditional fee-for-service family practice groups. Journal of Primary Care & Community Health 2014;5(3):202-7. [DOI] [PubMed] [Google Scholar]
Allard 2014 {published data only}
- Allard M, Jelovac I, Léger PT. Payment mechanism and GP self-selection: capitation versus fee for service. International Journal of Health Care Finance and Economics 2014;14:143-60. [DOI] [PubMed] [Google Scholar]
Allen 2016 {published data only}
- Allen T, Whittaker W, Sutton M. Does the proportion of pay linked to performance affect the job satisfaction of general practitioners? Social Science & Medicine 2017;173:9-17. [DOI] [PubMed] [Google Scholar]
Alshamsan 2012 {published data only}
- Alshamsan R, Lee JT, Majeed A, Netuveli G, Millett C. Effect of a UK pay-for-performance program on ethnic disparities in diabetes outcomes: interrupted time series analysis. Annals of Family Medicine 2012;10(3):228-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
An 2008 {published data only}
- An LC, Bluhm JH, Foldes SS, Alesci NL, Klatt CM, Center BA , et al. A randomised trial of a pay-for-performance program targeting clinician referral to a state tobacco quit line. Archives of Internal Medicine 2008;168(18):1993-9. [DOI] [PubMed] [Google Scholar]
Arrowsmith 2014 {published data only}
- Arrowsmith ME, Majeed A, Lee JT, Saxena S. Impact of pay for performance on prescribing of long-acting reversible contraception in primary care: an interrupted time series study. PLOS ONE 2014;9(4):1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Baker 2005 {published data only}
- Baker G, Carter B. Provider pay-for-performance incentive programs: 2004 national study results. San Francisco: Med-Vantage, Inc, 2005. [Google Scholar]
Barnato 2017 {published data only}
- Barnato AE, Moore R, Moore CG, Kohatsu ND, Sudore RL. Financial incentives to increase advance care planning among Medicaid beneficiaries: lessons learned from two pragmatic randomized trials. Journal of Pain and Symptom Management 2017;10.1016:1-11. [DOI] [PubMed] [Google Scholar]
Basu 2016 {published data only}
- Basu S, Phillips RS, Song Z, Landon BE, Bitton A. Effects of new funding models for patient-centered medical homes on primary care practice finances and services: results of a microsimulation model. Annals of Family Medicine 2016;14(5):404-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boyden 2000 {published data only}
- Boyden A, Carter R. The appropriate use of financial incentives to encourage preventative care in general practice. Centre for Health Program Evaluation 2000;18:1-25. [Google Scholar]
Broadway 2017 {published data only}
- Broadway B, Kalb G, Li J, Scott A. Do financial Incentives influence GPs' decisions to do after-hours work? A discrete choice labour supply model. Health Economics 2017;26(12):e52-66. [DOI] [PubMed] [Google Scholar]
Carey 1990 {published data only}
- Carey TS, Weis K. Diagnostic testing and return visits for acute problems in prepaid, case-managed Medicaid plans compared with fee-for-service. Archives of Internal Medicine 1990;150:2369-72. [PubMed] [Google Scholar]
Chen 2016 {published data only}
- Chen HJ, Huang N, Chen LS, Chou YJ, Li CP, Wu CY, et al. Does pay-for-performance program increase providers adherence to guidelines for managing hepatitis B and hepatitis C virus infection in Taiwan? PLOS ONE 2016;11(8):1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Clancy 1988 {published data only}
- Clancy CM, Hillner BE. Physicians as gatekeepers: the impact of financial incentives. Archives of Internal Medicine 1989;149:917-20. [DOI] [PubMed] [Google Scholar]
Coleman 2007 {published data only}
- Coleman T, Lewis S, Richard H, Christopher S. Impact of contractual financial incentives on the ascertainment and management of smoking in primary care. Addiction 2007;102:803-8. [DOI] [PubMed] [Google Scholar]
Davies 1986 {published data only}
- Davies AR, Ware JE Jr, Brook RH, Peterson JR, Newhouse JP. Consumer acceptance of prepaid and fee-for-service medical care: results from a randomized controlled trial. Health Services Research 1986;21(3):429-52. [PMC free article] [PubMed] [Google Scholar]
Douven 2015 {published data only}
- Douven R, Mocking R, Mosca I. The effect of physician remuneration on regional variation in hospital treatments. International Journal of Health Economics Management 2015;15:215-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Engineer 2016 {published data only}
- Engineer CY, Dale E, Agarwal A, Agarwal A, Alonge O, Edward A, et al. Effectiveness of a pay-for-performance intervention to improve maternal and child health services in Afghanistan: a cluster-randomized trial. International Journal of Epidemiology 2016;45(2):451-9. [DOI] [PubMed] [Google Scholar]
Erickson 2016 {published data only}
- Erickson KF, Winkelmayer WC, Chertow GM, Bhattacharya J. Effects of physician payment reform on provision of home dialysis. American Journal of Managed Care 2016;22(6):215-23. [PMC free article] [PubMed] [Google Scholar]
Erickson 2017 {published data only}
- Erickson KF, Winkelmayer WC, Chertow GM, Bhattacharya J. Hemodialysis hospitalizations and readmissions: the effects of payment reform. American Journal of Kidney Diseases 2017;69(2):237-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Erus 2017 {published data only}
- Erus B, Hatipoglu O. Physician payment schemes and physician productivity: analysis of Turkish healthcare reforms. Health Policy 2017;121:553-7. [DOI] [PubMed] [Google Scholar]
Feng 2015 {published data only}
- Feng Y, Mab A, Farrar S, Sutton M. The tougher the better: an economic analysis of increased payment thresholds on the performance of general practices. Health Economics 2015;24:353-71. [DOI] [PubMed] [Google Scholar]
Gallagher 2015 {published data only}
- Gallagher N, Cardwell C, Hughes C, O'Reilly D. Increase in the pharmacological management of Type 2 diabetes with pay-for-performance in primary care in the UK. Diabetic Medicine 2015;32:62-8. [DOI] [PubMed] [Google Scholar]
Giguere 2015 {published data only}
- Giguere AM, Labrecque M, Borduas F, Rouleau M. Effectiveness of monetary incentives to recruit family physicians as study subjects: a randomized controlled trial. BMC Research Notes 2015;8(15):1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Greene 2013 {published data only}
- Greene J. An examination of pay-for-performance in general practice in Australia. Health Services Research 2013;48(4):1415-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hamilton 2016 {published data only}
- Hamilton FL, Laverty AA, Huckvale K, Car J, Majeed A, Millett C. Financial incentives and inequalities in smoking cessation interventions in primary care: before-and-after study. Nicotine & Tobacco Research 2016;18(3):341-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Han 2015 {published data only}
- Han E, Chae SM, Kim NS, Park S. Effects of pharmaceutical cost containment policies on doctors' prescribing behavior: focus on antibiotics. Health Policy 2015;119:1245-54. [DOI] [PubMed] [Google Scholar]
Hickey 2015 {published data only}
- Hickey RG, Coe PF, Buchko BL, Woods AB. A comparison of salary-wage and hourly-wage acute care nursing units. JONA 2015;45(5):250-3. [DOI] [PubMed] [Google Scholar]
Hysong 2017 {published data only}
- Hysong SJ, SoRelle R, Broussard Smitham K, Petersen LA. Reports of unintended consequences of financial incentives to improve management of hypertension. PLOS ONE 2017;12(9):e0184856. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jones 2015 {published data only}
- Jones WS, Mi X, Qualls LG, Vemulapalli S, Peterson ED, Patel MR, et al. Trends in settings for peripheral vascular intervention and the effect of changes in the outpatient prospective payment system. Journal of the American College of Cardiology 2015;65(9):920-7. [DOI] [PubMed] [Google Scholar]
Kiran 2015 {published data only}
- Kiran T, Kopp A, Moineddin R, Glazier RH. Longitudinal evaluation of physician payment reform and team-based care for chronic disease management and prevention. CMAJ 2015;187(17):e494-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kliner 2015 {published data only}
- Kliner M, Canaan M, Ndwandwe SZ, Busulwa F, Welfare W, Richardson M, et al. Effects of financial incentives for treatment supporters on tuberculosis treatment outcomes in Swaziland: a pragmatic interventional study. Infectious Diseases of Poverty 2015;4(29):1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lagarde 2016 {published data only}
- Lagarde M, Blaauw D. Physicians' responses to financial and social incentives: a medically framed real effort experiment. Social Science & Medicine 2017;179:147-59. [DOI] [PubMed] [Google Scholar]
Lee 2010 {published data only}
- Lee TT, Cheng SH, Chen CC, Lai MS. A pay-for-performance program for diabetes care in Taiwan: a preliminary assessment. American Journal of Managed Care 2010;16(1):65-9. [PubMed] [Google Scholar]
Lee 2015 {published data only}
- Lee CY, Chi MJ, Yang SL, Lo HY, Cheng SH. Using financial incentives to improve the care of tuberculosis patients. American Journal of Managed Care 2015;21(1):35-42. [PubMed] [Google Scholar]
Lezzi 2014 {published data only}
- Lezzi E, Bruni ML, Ugolini C. The role of GP's compensation schemes in diabetes care: evidence from panel data. Journal of Health Economics 2014;34:104-20. [DOI] [PubMed] [Google Scholar]
Lix 2016 {published data only}
- Lix LM, Kuwornu JP, Kroeker K, Kephart G, Sikdar KC, Smith M, et al. Estimating the completeness of physician billing claims for diabetes case ascertainment using population-based prescription drug data. Research, Policy and Practice 2016;36(3):54-60. [PMC free article] [PubMed] [Google Scholar]
Maini 2014 {published data only}
- Maini R, Bergh RV, Griensven GV, Katie TS, Janet O, Daniel C, et al. Picking up the bill - improving health-care utilisation in the Democratic Republic of Congo through user fee subsidisation: a before and after study. BMC Health Services Research 2014;14(504):1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Merilind 2015 {published data only}
- Merilind E, Salupere R, Västra K, Kalda R. The influence of performance-based payment on childhood immunisation coverage. Health Policy 2015;119:770-7. [DOI] [PubMed] [Google Scholar]
Michel 2015 {published data only}
- Michel AL, Ventelou B. The true impact of the French pay-for-performance program on physicians’ benzodiazepines prescription behavior. European Journal of Health Economics 2016;17:723-32. [DOI] [PubMed] [Google Scholar]
Mullen 2009 {published data only}
- Mullen KJ, Frank RG, Rosenthal MB. Can you get what you pay for? Pay-for-performance and the quality of healthcare providers. National Bureau of Economic Research 2009;41(1):1-50. [DOI] [PubMed] [Google Scholar]
Murray 1992 {published data only}
- Murray JP, Greenfield S, Kaplan SH, Yano EM. Ambulatory testing for capitation and fee-for-service patients in the same practice setting: relationship to outcomes. Medical Care 1992;30(3):252-61. [DOI] [PubMed] [Google Scholar]
Odesjo 2015 {published data only}
- Ödesjö H, Anell A, Gudbjörnsdottir S, Thorn J, Björck S. Short-term effects of a pay-for-performance programme for diabetes in a primary care setting: an observational study. Scandinavian Journal of Primary Health Care 2015;33(4):291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Olivier 2015 {published data only}
- Olivier SL, Jonathan S. Impact of a pay for performance programme on French GPs' consultation length. Health Policy 2015;119:417-26. [DOI] [PubMed] [Google Scholar]
Petersen 2016 {published data only}
- Petersen LA, Ramos KS, Pietz K, Woodard LD. Impact of a pay-for-performance program on care for black patients with hypertension: important answers in the era of the Affordable Care Act. Health Services Research 52;3:1138-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ritchie 1992 {published data only}
- Ritchie L, Bisset A, Russell D, Leslie V, Thomson I. Primary and pre-school immunisation in Grampian: progress and the 1990 contract. BMJ 1992;304:816-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Robertson 2017 {published data only}
- Robertson-Cooper H, Neaderhiser B, Happe LE, Beveridge RA. Family physician readiness for value-based payments: does ownership status matter? Population Health Management 2017;20(5):357-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Roski 2003 {published data only}
- Roski J, Jeddeloh R, An L, Lando H, Hannan P, Hall C, et al. The impact of financial incentives and a patient registry on preventive care quality: increasing provider adherence to evidence-based smoking cessation practice guidelines. Preventative Medicine 2003;36(3):291-9. [DOI] [PubMed] [Google Scholar]
Rudasingwa 2017 {published data only}
- Rudasingwa M, Soeters R, Basenya O. The effect of performance-based financing on maternal healthcare use in Burundi: a two-wave pooled cross-sectional analysis. Global Health Action 2017;10:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shelley 2012 {published data only}
- Shelley D, Anno J, Tseng TY, Calip G, Wedeles J, Lloyd M, Wolff MS. Implementing Tobacco Use Treatment Guidelines in Dental Public Health Clinics. J Dent Educ 2011 Apr.;75(4):527-33. [PubMed] [Google Scholar]
Shen 2003 {published data only}
- Shen YJ. Selection incentives in a performance-based contracting system. Health Services Research 2003;38(2):535-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shen 2017a {published data only}
- Shen GC, Nguyen HT, Das A, Sachingongu N, Chansa C, Qamruddin J, et al. Incentives to change: effects of performance-based financing on health workers in Zambia. Human Resources for Health 2017;15(20):1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shen 2017b {published data only}
- Shen GC, Nguyen HT, Das A, Sachingongu N, Chansa C, Qamruddin J, et al. Incentives to change: effects of performance-based financing on health workers in Zambia. Human Resources for Health 2017;15(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sicsic 2015 {published data only}
- Sicsic J, Franc C. Impact assessment of a pay-for-performance program on breast cancer screening in France using micro data. European Journal of Health Economics 2017;18:609-21. [DOI] [PubMed] [Google Scholar]
Simonsen 2017 {published data only}
- Simonsen S, Heinskou T, Sørensen P, Folke S, Lau ME. Personality disorders: patient characteristics and level of outpatient treatment service. Nordic Journal of Psychiatry 2017;71(5):325-31. [DOI] [PubMed] [Google Scholar]
Simpson 2011 {published data only}
- Simpson CR, Hannaford PC, Ritchie LD, Sheikh A, Williams D. Impact of the pay-for-performance contract and the management of hypertension in Scottish primary care: a 6-year population-based repeated cross-sectional study. British Journal of General Practice 2011;61(588):443-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stearns 1992 {published data only}
- Stearns SC, Wolfe BL, Kindig DA. Physician responses to fee-for-service and capitation payment. Inquiry 1992;29(4):416-25. [PubMed] [Google Scholar]
Sun 2016 {published data only}
- Sun X, Liu X, Sun Q, Yip W, Wagstaff A, Meng Q. The impact of a pay-for-performance scheme on prescription quality in rural China. Health Economics 2016;25:706-22. [DOI] [PubMed] [Google Scholar]
To 2015 {published data only}
- To T, Guan J, Zhu J, Lougheed MD, Kaplan A, Tamari I, et al. Quality of asthma care under different primary care models in Canada: a population-based study. BMC Family Practice 2015;16(19):1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Dijk 2014 {published data only}
- Dijk CE, Verheij RA, te Brake H, Spreeuwenberg P, Groenewegen PP, Bakker DH. Changes in the remuneration system for general practitioners: effects on contact type and consultation length. European Journal of Health Economics 2014;15:83-91. [DOI] [PubMed] [Google Scholar]
van Dijk 2015 {published data only}
- Cheryl LD, Marc NE, Brett AE. Pay-for-performance schemes that use patient and provider categories would reduce payment disparities. Health Affairs 2015;34(1):134-42. [DOI] [PubMed] [Google Scholar]
Vats 2014 {published data only}
- Vats S. Role of information, cost-sharing, and payment reforms in healthcare markets. Boston University Theses & Dissertations, 2014. [Google Scholar]
Wei 2015 {published data only}
- Wei X, Li H, Yang N, Wong SY, Chong MC, Shi L, et al. Changes in the perceived quality of primary care in Shanghai and Shenzhen, China: a difference-in-difference analysis. Bulletin of the World Health Organization 2015;93:407-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
White 2006 {published data only}
- White E. Employers Increasingly Favor Bonuses to Raises. The Wall Street Journal 2006;Aug. 28:https://www.wsj.com/articles/SB115673064517347005. [Google Scholar]
References to studies awaiting assessment
Lavergne 2018 {published data only}
- Lavergne MR, Law MR, Peterson S, Garrison S, Hurley J, Cheng L, et al. Effect of incentive payments on chronic disease management and health services use in British Columbia, Canada: interrupted time series analysis. Health Policy 2018;122:157-64. [DOI] [PubMed] [Google Scholar]
Navathe 2019 {published data only}
- Navathe AS, Volpp KG, Caldarella KL, Bond A, Troxel AB, Zhu J, et al. Effect of financial bonus size, loss aversion, and increased social pressure on physician pay-for-performance: a randomized clinical trial and cohort study. JAMA Network Open 2019;2:e187950. [DOI: 10.1001/jamanetworkopen.2018.7950] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
NCT02731716 {published data only}
- NCT02731716. Transforming primary care payment in Hawaii. clinicaltrials.gov/show/NCT02731716 (first received 28 March 2016).
Additional references
Brocklehurst 2013
- Brocklehurst P, Price J, Glenny AM, Tickle M, Birch S, Mertz E, et al. The effect of different methods of remuneration on the behaviour of primary care dentists. Cochrane Database of Systematic Reviews 2013, Issue 11. Art. No: CD009853. [DOI: 10.1002/14651858.CD009853.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chaix 2000
- Chaix-Couturier C, Durand-Zaleski I, Jolly D, Durieux P. Effects of financial incentives on medical practice: results from a systematic review of the literature and methodological issues. International Journal for Quality in Health Care 2000;12:133-42. [DOI] [PubMed] [Google Scholar]
EPOC 2013a
- Cochrane Effective Practice and Organisation of Care (EPOC). Good practice data extraction form. EPOC resources for review authors, 2013. epoc.cochrane.org/epoc-resources-review-authors (accessed 27 April 2015).
EPOC 2013b
- Cochrane Effective Practice and Organisation of Care (EPOC). Interrupted time series (ITS) analyses. EPOC resources for review authors, 2013. epoc.cochrane.org/epoc-resources-review-authors (accessed 27 April 2015).
EPOC 2013c
- Cochrane Effective Practice and Organisation of Care (EPOC). Suggested risk of bias criteria for EPOC reviews. EPOC resources for review authors, 2013. epoc.cochrane.org/epoc-resources-review-authors (accessed 27 April 2015).
EPOC 2013d
- Cochrane Effective Practice and Organisation of Care (EPOC). Synthesising results when it does not make sense to do a meta-analysis. EPOC resources for review authors, 2013. epoc.cochrane.org/epoc-resources-review-authors (accessed 27 April 2015).
EPOC 2013e
- Cochrane Effective Practice and Organisation of Care (EPOC). Worksheets for preparing summary of findings tables using GRADE. EPOC resources for authors, 2013. epoc.cochrane.org/epoc-resources-review-authors (accessed 27 April 2015).
EPOC 2018
- Cochrane Effective Practice and Organisation of Care (EPOC). How to report the effects of an intervention. EPOC resources for authors, 2018. epoc.cochrane.org/epoc-resources-review-authors (accessed prior to 11 December 2020).
Flodgren 2011
- Flodgren G, Eccles MP, Shepperd S, Scott A, Parmelli E, Beyer FR. An overview of reviews evaluating the effectiveness of financial incentives in changing health care professional behaviours and patient outcomes. Cochrane Database of Systematic Reviews 2011, Issue 7. Art. No: CD009255. [DOI: 10.1002/14651858.CD009255] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gillam 2012
- Gillam SJ, Siriwardena AN, Steel N. Pay-for-performance in the United Kingdom: impact of the quality and outcomes framework: a systematic review. Annals of Family Medicine 2012;10(5):461-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Giuffrida 1999
- Giuffrida A, Gosden T, Forland F, Kristiansen IS, Sergison M, Leese B, et al. Target payments in primary care: effects on professional practice and health care outcomes. Cochrane Database of Systematic Reviews 2000, Issue 3. Art. No: CD000531. [DOI: 10.1002/14651858.CD000531] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gosden 2000
- Gosden T, Forland F, Kristiansen IS, Sutton M, Leese B, Giuffrida A, et al. Capitation, salary, fee-for-service and mixed systems of payment: effects on the behaviour of primary care physicians. Cochrane Database of Systematic Reviews 2000, Issue 3. Art. No: CD002215. [DOI: 10.1002/14651858.CD002215] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT 2020 [Computer program]
- GRADEpro GDT: GRADEpro Guideline Development Tool. McMaster University (developed by Evidence Prime, Inc.), 2020. Available from gradepro.org.
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck-Ytter Y, Schunemann HJ. What is ’quality of evidence’ and why is it important to clinicians? BMJ 2008;336:995-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hendrickson 2008
- Hendrickson MA. Pay for performance and medical professionalism. Quality Management in Health Care 2008;17(1):9-18. [DOI] [PubMed] [Google Scholar]
Higgins 2020
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available from training.cochrane.org/handbook.
Ivers 2012
- Ivers N, Jamtvedt G, Flottorp S, Young JM, Odgaard‐Jensen J, French SD, et al. Audit and feedback: effects on professional practice and healthcare outcomes. Cochrane Database of Systematic Reviews 2012, Issue 6. Art. No: CD000259. [DOI: 10.1002/14651858.CD000259.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Langenbrunner 2004
- Langenbrunner J, Liu X. How to pay? Understanding and using incentives. Health, Nutrition and Population (HNP) discussion paper. The World Bank 2004;https://openknowledge.worldbank.org/handle/10986/13674 License: CC BY 3.0 IGO.
Lester 2013
- Lester H, Matharu T, Mohammed MA, Lester D, Foskett-Tharby R. Implementation of pay for performance in primary care: a qualitative study 8 years after introduction. British Journal of General Practice 2013;63(611):e408-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mathes 2014
- Mathes T, Pieper D, Mosch CG, Jaschinski T, Eikermann M. Payment methods for hospitals. Cochrane Database of Systematic Reviews 2014, Issue 6. Art. No: CD011156. [DOI: 10.1002/14651858.CD011156] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mathes 2019
- Mathes T, Pieper D, Morche J, Polus S, Jaschinski T, Eikermann M. Pay for performance for hospitals. Cochrane Database of Systematic Reviews 2019, Issue Issue 7. Art. No: CD011156. [DOI: 10.1002/14651858.CD011156.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mendelson 2017
- Mendelson A, Kondo K, Damberg C, Low A, Motúapuaka M, Freeman M, et al. The effects of pay-for-performance programs on health, health care use, and processes of care: a systematic review. Annals of Internal Medicine 2017;166:341–53. [DOI] [PubMed] [Google Scholar]
O'Brien 2007
- O'Brien MA, Rogers S, Jamtvedt G, Oxman AD, Odgaard-Jensen J, Kristoffersen DT, et al. Educational outreach visits: effects on professional practice and health care outcomes. Cochrane Database of Systematic Reviews 2007, Issue 4. Art. No: CD000409. [DOI: 10.1002/14651858.CD000409.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ogundeji 2016
- Ogundeji YK, Bland JM, Sheldon TA. The effectiveness of payment for performance in health care: a meta-analysis and exploration of variation in outcomes. Health Policy 2016;120(10):1141-50. [DOI] [PubMed] [Google Scholar]
Petersen 2006
- Petersen LA, Woodard LD, Urech T, Daw C, Sookanan S. Does pay-for-performance improve the quality of health care? Annals of Internal Medicine 2006;145:265-72. [DOI] [PubMed] [Google Scholar]
Reeves 2013
- Reeves S, Perrier L, Goldman J, Freeth D, Zwarenstein M. Interprofessional education: effects on professional practice and healthcare outcomes. Cochrane Database of Systematic Reviews 2013, Issue 3. Art. No: CD002213. [DOI: 10.1002/14651858.CD002213.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rowe 2005
- Rowe AK, Savigny D, Lanata CF, Victora CG. How can we achieve and maintain high-quality performance of health workers in low-resource settings? Lancet 2005;366(9490):1026-35. [DOI] [PubMed] [Google Scholar]
Scott 2011
- Scott A, Sivey P, Ait Ouakrim D, Willenberg L, Naccarella L, Furler J, et al. The effect of financial incentives on the quality of health care provided by primary care physicians. Cochrane Database of Systematic Reviews 2011, Issue 9. Art. No: CD008451. [DOI: 10.1002/14651858.CD008451.pub2] [DOI] [PubMed] [Google Scholar]
Van Herck 2010
- Van Herck P, De Smedt D, Annemans L, Remmen R, Rosenthal MB, Sermeus W. Systematic review: effects, design choices, and context of pay-for-performance in health care. BMC Health Services Research 2010;10:247. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2006
- World Health Organization. The World Health Report 2006: working together for health. Geneva: World Health Organization, 2006. [DOI: 10.1080/13576280600937911] [DOI] [Google Scholar]
Witter 2012
- Witter S, Fretheim A, Kessy FL, Lindahl AK. Paying for performance to improve the delivery of health interventions in low- and middle-income countries. Cochrane Database of Systematic Reviews 2012, Issue 2. Art. No: CD007899. [DOI: 10.1002/14651858.CD007899.pub2] [DOI] [PubMed] [Google Scholar]
Yuan 2017
- Yuan B, He L, Meng Q, Jia L. Payment methods for outpatient care facilities. Cochrane Database of Systematic Reviews 2017, Issue 3. Art. No: CD011153. [DOI: 10.1002/14651858.CD011153.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Jia 2015
- Jia L, Yuan B, Meng Q, Scott A. Payment methods for ambulatory care health professionals. Cochrane Database of Systematic Reviews 2015, Issue 9. Art. No: CD011865. [DOI: 10.1002/14651858.CD011865] [DOI] [Google Scholar]


