Abstract
Despite aggressive multimodal treatment approaches, the prognosis for patients with diffuse gliomas remains disappointing. Glioma cells often extensively infiltrate in the surrounding brain parenchyma, a phenomenon that helps them to escape surgical removal, radiation exposure and chemotherapy. Moreover, conventional therapy is often associated with considerable local and systemic side effects. Therefore, the development of novel therapeutic approaches is essential to improve the outcome of these patients. Immunotherapy offers the opportunity to specifically target residual radio—and chemoresistant tumor cells without damaging healthy neighboring brain tissue. Significant progress has been made in recent years both in understanding the mechanisms of immune regulation in the central nervous system (CNS) as well as tumor‐induced and host‐mediated immunosuppression elicited by gliomas. In this review, after discussing the special requirements needed for the initiation and control of immune responses in the CNS, we focus on immunological phenomena observed in glioma patients, discuss different immunological approaches to attack glioma‐associated target structures and touch on further strategies to improve the efficacy of immunotherapy of gliomas.
Keywords: central nervous system, dendritic cell, glioma, immune escape, immunotherapy, vaccine
INTRODUCTION
Gliomas are the most common primary brain tumors in adults. They are categorized according to the World Health Organization (WHO) classification, most recently revised in 2007 (162). Most gliomas, the “diffuse gliomas,” are characterized by extensive infiltration of brain parenchyma. The diffuse gliomas can be subtyped as astrocytic, oligodendroglial and oligoastrocytic tumors, with a malignancy grade (low grade = WHO grade II; malignant = WHO grade III; highly malignant = WHO grade IV) assigned to each tumor (162). The most aggressive, that is WHO grade IV diffuse glioma, is glioblastoma (GBM). Although GBMs are generally considered purely astrocytic in nature, they in fact form a heterogeneous group of tumors, representing 15%–20% of all intracranial neoplasms and approximately 50% of the malignant gliomas. As most studies on the immunobiology and immunotherapy of gliomas were carried out on diffuse gliomas, especially GBMs, the present review focuses on these tumors as well. Currently the standard of care for patients with GBM after maximal tumor resection is concomitant chemoradiotherapy followed by adjuvant treatment with temozolomide (TMZ). The median overall survival for patients with GBM is 14.6 months and the overall survival rate is 27.2% at 2 years. After 3 years, nearly all patients have progressed, with mortality well over 90% after 5 years 249, 250. Factors associated with an increased risk of death are increased age, lower Karnofsky performance score (KPS), corticosteroid use, shorter time from original diagnosis to recurrence, tumor outside the frontal lobe, unmethylated O6‐methylguanine methyltransferase (MGMT)‐promotor status and the presence of tumor‐initiating glioma stem cells 40, 100, 196, 299. Overall, the prognosis for patients with GBM remains poor. It is thus essential to consider novel treatments in the hope of attacking residual radio‐ and chemoresistant tumor cells and to improve the survival of glioma patients. With increasing knowledge of central nervous system (CNS) immunity, immunotherapeutic approaches are promising options for the treatment of diffuse gliomas.
CENTRAL ROLE OF DENDRITIC CELLS (DCs) IN THE ACTIVATION OF THE IMMUNE SYSTEM
In general, the immune system is based on two distinct types of responses, the innate and the adaptive immune response. The innate immune system functions as a first line of defense against invading microorganisms. It discriminates between the different microbes by recognizing a set of conserved molecular structures, so called pathogen‐associated molecular patterns (PAMPs), shared by large groups of microorganisms. Cells that orchestrate the innate immune response are DCs, macrophages, monocytes, granulocytes and natural killer (NK) cells. The specific, adaptive immune system forms the second line of defense, which depends on the activity of effector T and B cells. It uniquely distinguishes distinct microorganisms and confers immunological memory. The specifity is mediated by membrane receptors bearing a great diversity of antigen binding sites.
DC subsets
DCs are the professional antigen‐presenting cells (APCs) of the immune system that instruct and control primary immune responses. Two major subtypes of DCs have been defined, myeloid and plasmacytoid DCs. Both DC subsets differ in morphology, tissue distribution, expression of markers and function (270). Plasmacytoid DCs are circulating cells with a plasmacytoid morphology that are capable of producing large amounts of type I interferons upon activation by microbial stimuli (242). In addition, they can differentiate into DCs that are capable of activating naive T cells against allo‐antigens (91) and exogenous antigens (22). Myeloid DCs can be further divided into migratory DCs, which serve as sentinels of the immune system in peripheral tissues and present foreign antigens to T cells after migration to the draining lymph nodes, and lymphoid‐tissue‐resident DCs that capture local (foreign and self‐) antigens and present them to local T cells (238).
Migratory DCs
At an immature stage, myeloid DCs take up tissue antigens, both soluble and particulate antigens, very efficiently. After challenge with microbial or inflammatory (“danger”‐associated) stimuli, DCs undergo a process of activation, also known as maturation. Several environmental stimuli, such as signals from pattern‐recognition receptors (PPR), inflammatory cytokines and members of the TNF superfamily (eg, CD40L, RANKL) have been shown to mediate DC activation (246). During DC maturation, captured antigens are processed and bound to both classical major histocompatibility complex (MHC) class I and class II molecules that are translocated to the cell surface, accompanied by an increased expression of costimulatory molecules and the secretion of cytokines that influence T cell proliferation and differentiation. In response to the appropriate stimuli, DCs also upregulate CC chemokine receptor‐7 (CCR7), a receptor that drives DC migration to the lymphoid vessels and T cell areas of secondary lymphoid organs (13). There, DCs are mature and are well‐equipped to attract and activate naive CD4+ and CD8+ T cells (3) In addition, DCs are also able to directly activate innate lymphocytes such as NK cells or NKT cells via non‐classical (CD1 family) MHC molecules presenting glycolipid structures 68, 72, thus providing a link between the adaptive and innate immune system (Figure 1).
Figure 1.
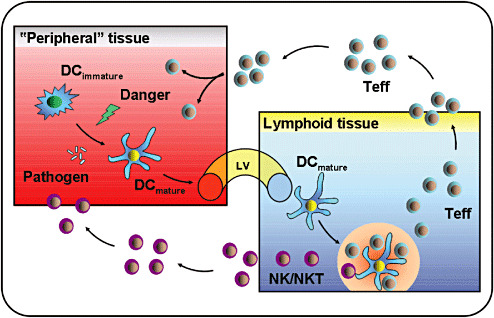
Central role of dendritic cells (DCs) in the initiation of an immune response. DCs are present in virtually all tissues and organs including the brain and continuously monitor their environment for the presence of danger, for example, invading microorganisms and tissue damage. Immature DCs are very efficient in antigen uptake, mediated by high endocytotic activity and expression of an array of cell surface receptors. Upon recognition of danger signals, DCs undergo maturation and migrate via lymph vessel (LV) to the draining lymphoid organs. Here, mature DCs interact with and activate naive lymphocytes specific for their cognate antigen and initiate a primary immune response. After expansion, activated T effector cells (Teffs) and natural killer/natural killer T cells (NK/NKT cells) exit the lymphoid tissue and home back via the blood stream to the site of antigen deposit to eliminate the threat in the endangered tissue (eg, brain). Under steady state conditions immature, non‐activated DCs contribute to immune homeostasis by deleting autoreactive T cells and inducing T‐cell tolerance, or leading to the generation of regulatory T cells (Tregs).
PPRs expressed by DCs
Four main PPRs expressed by DCs can be distinguished. These include Toll‐like receptors (TLRs), C‐type lectins, NOD‐like receptors and cytoplasmic RNA helicases 149, 268.
So far, the best‐characterized PPRs are the TLRs 128, 154. The TLR family in humans consists of 11 transmembrane members that are located on the cell surface (TLR1, 2, 4, 5, 6, 10 and 11) or within the endosomal compartments (TLR3, 7, 8 and 9) (Figure 2). Many specific ligands for TLRs have now been identified. TLR2 recognizes bacterial lipoproteins, peptidoglycan and lipoteichoic acid from Gram‐positive bacteria, and zymosam from fungi, and can associate with TLR1 and TLR6 for functional responses to mycobacterial lipopeptides. TLR3 recognizes viral double‐stranded (ds) RNA and synthetic dsRNAs, such as polyinosinic–polycytidylic acid (Poly I:C). TLR4 binds lipopolysaccharides (LPS) from Gram‐negative bacteria and viral envelope proteins, whereas TLR5 recognizes flagellin. TLR7 and TLR8 recognize viral single stranded RNA and synthetic molecules like imidazoquinoline or its derivates. TLR9 recognizes unmethylated CpG motifs within bacterial and viral DNA. The specific ligand of TLR10 is currently unknown. The recently discovered TLR11 recognizes uropathogenic bacteria 117, 254. TLR expression by DCs is dependent on the cellular subset and differentiation state. Myeloid DCs express virtually all TLRs with the exception of TLR9, which is selectively expressed by plasmacytoid DCs. In contrast to humans, TLR9 is expressed both by plasmacytoid and myeloid DCs in mice (212). Importantly, DC activation and cytokine production are strongly dependent on the type and combination of the TLRs that are triggered 121, 183. Consequently, the nature of specific T‐cell responses generated also depends on the different stimuli that a DC encounters locally (244).
Figure 2.
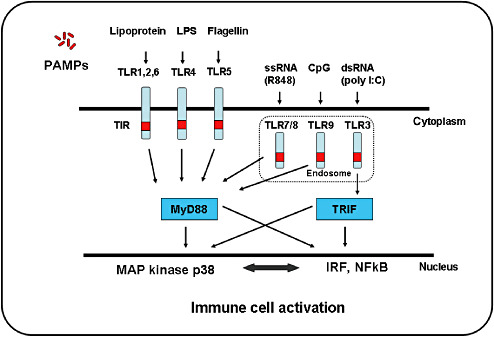
Toll‐like receptor (TLR)‐mediated recognition of pathogen‐associated molecular patterns (PAMPs) and signaling pathways. TLR1, 2, 4, 5 and 6 are expressed on the surface of dendritic cells, and are involved in detecting lipids, carbohydrates and protein ligands from extracellular pathogens. In contrast, TLR3, 7, 8 and 9 are mainly expressed in intracellular vesicles and function within the acidified endosomes to detect nucleic acids derived from viruses and bacteria. TLR2 recognizes bacterial lipoproteins, peptidoglycan and lipoteichoic acid from Gram‐positive bacteria, and zymosam from fungi, and can associate with TLR1 and TLR6 for functional responses to mycobacterial lipopeptides. TLR3 recognizes viral double‐stranded (ds) RNA and synthetic dsRNAs, such as polyinosinic‐polycytidylic acid (Poly I:C). TLR4 binds lipopolysaccharides (LPS) from Gram‐negative bacteria and viral envelope proteins, whereas TLR5 recognizes flagellin. TLR7 and TLR8 recognize viral single‐stranded (ss) RNA and synthetic molecules like imidazoquinoline or its derivates. TLR9 recognizes unmethylated CpG motifs within bacterial and viral DNA. TLR3 uses Toll/Interleukin‐1 receptor (TIR) domain‐containing adapter‐inducing interferon (TRIF), whereas all other TLRs use MyD88 as an adapter molecule. Both TRIF and MyD88‐dependent pathways lead to the activation of MAPK kinases such as p38 and the activation of nuclear factor‐κB (NF κB) and interferon (IFN)‐regulatory factors (IRFs) to induce type I IFN and inflammatory cytokines, respectively.
T‐cell polarization
Inside the lymph nodes, antigen‐loaded activated DCs interact with naive T cells and induce their differentiation into T effector cells. It is a unique feature of DCs to efficiently present captured antigens on MHC class‐II molecules to CD4+ T cells. Naive CD4+ T cells can be differentiated into either T‐helper type 1 (Th1), T‐helper type 2 (Th2) or TH17 cells. Th1 cells develop in the presence of interleukin 12 (IL‐12) and secrete IFN‐γ, whereas Th2 cells develop in the presence of IL‐4 and secrete IL‐4, IL‐5 and IL‐13 191, 256. For the development of TH17 cells from naive precursors a combination of IL‐6 and TGF‐β is required (23). Moreover, IL‐1 and IL‐21 seem to play important roles in promoting the induction of TH17 cells, whereas IL‐23 is vital for the expansion and survival of this population 24, 143, 148. In contrast to other APCs, DCs are also capable of presenting captured antigens on MHC class‐I molecules to CD8+ T cells, a process termed “cross‐presentation”5, 132. In vivo priming of CD8+ cytotoxic T lymphocytes (CTLs) generally requires the participation of CD4+ T‐helper cells, CD40‐CD40L interaction, innate lymphocytes or stimulation via certain TLRs 121, 160, 233. Besides effector T cells, DCs are also efficient at activating naturally occurring CD4+ regulatory T cells (Tregs) 14, 130, 295. In addition, DCs are able to induce several subsets of Tregs capable of controlling effector T cells, including induced Tregs, type 1 regulatory T cells (Tr1 cells) and Th3 cells. The regulatory T‐cell‐polarizing factors are IL‐10 and transforming growth factor‐β (TGF‐β) 44, 65, 273 (Figure 3).
Figure 3.
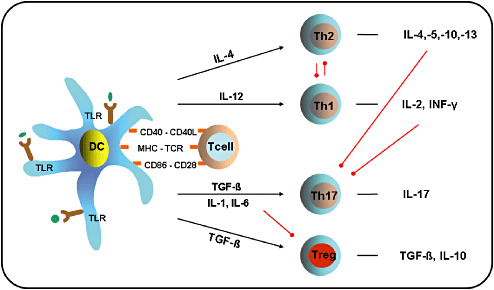
Dendritic cell (DC)‐mediated T‐cell polarization. After activation of Toll‐like receptors (TLRs) by pathogens (in green) DCs increase cell‐surface expression of MHC‐peptide complexes, upregulate costimulatory molecules (eg, CD86) and secrete immunomodulatory cytokines that direct T‐cell polarization. Ligation of distinct TLRs trigger differential cytokine production in DCs. Production of interleukin‐4 (IL‐4) promotes the development of T helper type 2 (Th2) cells, whereas the production of IL‐12 favors the development of Th1 cells. Th2 cells secrete IL‐4, IL‐5, IL‐10 and IL‐13, whereas Th1 cells secrete IL‐2 and IFN‐γ. IL‐4 and IL‐12 enhance the generation of their own Th‐subset and simultaneously inhibit the generation of the opposing subset. A combination of IL‐1 or IL‐6 and TGF‐s contributes to the development of Th17 cells from naive precursors, whereas TGF‐s alone promotes the development of regulatory T cells (Tregs). Both IL‐4 and IFN‐γ inhibit Th17 development. IL‐6 inhibits the development of Tregs. Black arrows indicate promoting activity, whereas red lines indicate inhibitory activity.
Naturally occurring CD4+ regulatory T cells
Naturally occurring CD4+ Tregs represent 5%–10% of the overall CD4+ T cell population and are generated in the thymus (223). They constitutively express the IL‐2 receptor α‐chain (CD25), cytotoxic lymphocyte‐associated antigen‐4 (CTLA‐4) and different members of the tumor necrosis factor (TNF) receptor superfamily such as the glucocorticoid‐induced TNF related protein (GITR) or Ox40 237, 265. Other candidate markers of Tregs have been described and currently include CD62L, CD103, CD122 and CD223 (178). So far the most specific marker for naturally occurring CD4+ Tregs is FoxP3, a member of the forkhead family of DNA‐binding transcription factors, which has been shown to be expressed specifically in mouse CD4+ Tregs and acts as a master switch in the regulation of their development and function 71, 105. Per se, Tregs are hyporesponsive to T‐cell receptor (TCR) stimulation in vitro, but high amounts of IL‐2 or stimuli bypassing the TCR can overcome their anergic state (224). After polyclonal or antigen‐specific TCR stimulation, Tregs potently suppress the proliferation and cytokine production of effector CD4+ and CD8+ T cells by inhibiting IL‐2 gene transcription. Once activated in an antigen‐specific manner, Tregs also suppress effector T cells, which are specific for other antigens. Furthermore, Tregs have been shown to inhibit the proliferation and antibody production of B cells and to profoundly suppress NK‐cell function 33, 77. Tregs show their suppressive effects by a complex and overlapping set of mechanisms (225) (Figure 4).
Figure 4.
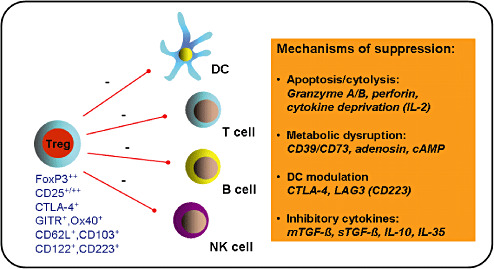
Regulatory T cells (Tregs) and immune cell suppression. Tregs are characterized by a set of markers (in blue), suppress different types of immune cells (red lines) and show their suppressive effects by a number of different mechanisms (orange box). Tregs have cytolytic activity and can kill target cells directly in a granzyme/perforin‐dependent manner or induce cytokine deprivation‐mediated apoptosis. Two ectoenzymes, CD39 and CD73, expressed on activated Tregs, induce the extracellular release of adenosine nucleosides that suppress effector T‐cell functions. Tregs also promote the transfer of the potent inhibitory second messenger cyclic adenosine monophosphate (cAMP) to effector T cells. In addition, Tregs can condition DCs, via a CTLA‐4:CD80/CD86‐dependent mechanism, to express indoleamine 2,3‐dioxygenase (IDO). Expression of the lymphocyte activation gene 3 (LAG3) on Tregs can further modulate the maturation and immunostimulatory capacity of DCs. Finally, potent inhibitory cytokines, produced by Tregs, such as membrane‐bound or soluble TGF‐s (mTGF‐s; sTGF‐s), IL‐10 or IL‐35, contribute to their suppressive function.
THE CNS—AN IMMUNOLOGICALLY SPECIALIZED SITE
In the past, the CNS has been described as an immunologically privileged site based on the presence of the blood–brain barrier (BBB), graft acceptance, lack of conventional lymphatics, low MHC expression and low T cell trafficking 41, 50. However, with recent advances in understanding CNS immunity it is more accurate to regard the CNS as an immunologically specialized site that can be divided into three different compartments: the brain parenchyma; the ventricles containing choroid plexus and cerebrospinal fluid; and the meninges. The immune reactivity of the ventricles and meninges is similar to that of the periphery, whereas immune responses are delayed or even abrogated within the brain parenchyma 17, 248, 280.
Initiation of an immune response in the CNS
Accumulating data suggest that DCs play a central role in the initiation of immune responses in the CNS 135, 198. DCs can be found in low numbers in the meninges, perivascular spaces, the choroid plexus and in the CSF where they reside in an immature state 169, 172, 197.
Under non‐inflammatory conditions, DCs present antigens derived from tissue debris and apoptotic cells in the absence of costimulatory molecules. Such antigen presentation by non‐activated DCs induces T‐cell anergy, T‐cell tolerance or the induction of Tregs 159, 236, 247. Moreover, it has been reported that non‐stimulated, brain‐derived DCs preferentially target B‐cell areas instead of T‐cell areas, again skewing the immune system towards a humoral response (96). Similarly, drainage of brain‐derived antigens was shown not to be sufficient to induce a Th1‐type and CTL response by itself, but leads to tolerance induction or skewing of the immune response towards a B cell and Th2‐type response 94, 95, 283. However, under a variety of inflammatory conditions, DCs are quickly recruited to the site of the brain lesion and appear in both the brain parenchyma and the cerebrospinal fluid in high numbers 69, 157, 235. Importantly, it was recently demonstrated that these DCs do not differentiate from CNS‐resident precursors like microglia cells, but are of peripheral origin (175). At the site of the lesion, inflammatory mediators and danger signals promote maturation and rerouting of DCs to the secondary lymphoid organs where they prime antigen‐specific immune responses.
Although conventional lymphatics are missing in the CNS, antigen‐loaded DCs have access to secondary lymphoid organs through several pathways. The most prominent one is the drainage of antigen‐loaded DCs along the subarachnoid space surrounding the olfactory bulbs through discrete channels in the cribriform plate into the lymphatics of the nasal submucosa and, subsequently, to the cervical lymph nodes. Regional lymph nodes that are associated with other cranial nerves might also be involved in the CNS drainage. With access to the cerebrospinal fluid and the perivascular spaces, antigen‐loaded DCs can also migrate out through the arachnoid villi into the venous blood 103, 208, 282.
These data further support recent analyses demonstrating that brain‐derived antigens can reach the cervical lymph nodes very quickly, but similar to the skin system, a second wave of incoming brain‐derived APCs, most likely DCs, is necessary for optimal T‐cell activation 80, 119, 135. After clonal expansion, activated antigen‐specific T cells preferentially home back to the site of antigen deposit in the brain 34, 35.
T cell trafficking into the CNS
In general, the traffic of leukocytes into the CNS is a highly regulated process. Resting T cells fail to enter the CNS, whereas activated T cells are able to penetrate the BBB under inflammatory conditions 279, 281. For CD4+ T cells, the activation‐stage rather than the antigen‐specificity determines BBB crossing (102). CNS recruitment of CD8+ T cells is regulated differently than CD4+ T cells. Recent data show that antigen specificity is a factor that governs CD8+ T cell infiltration into the brain and that this process is dependent on luminal expression of MHC class I by cerebral endothelium, but independent of antigen presentation by perivascular APCs (75).
The current paradigm of blood leukocyte transendothelial migration involves a sequential, multistep adhesion cascade between leukocyte and endothelial cell adhesion molecules 19, 63. T‐cell priming in CNS‐draining lymph nodes is usually associated with a rapid upregulation of α4 and β1 integrin (35). These α4β1 integrins have been shown to interact with VCAM‐1 on cerebral vascular endothelium and this interaction has been reported to facilitate CNS entry of encephalitogenic or virus‐specific T cells 39, 264. An important function for ALCAM in the recruitment of CD4+ T cells and monocytes across the BBB endothelium has recently been demonstrated (43). After CNS entry, antigen‐specific reactivation of effector T cells by perivascular APCs is needed for T‐cell persistence and amplification of T‐cell‐mediated immune responses in the CNS. In general, those T cells that have encountered their target antigen within the brain are retained for longer periods within the CNS (168).
After contact with perivascular APCs, effector T cells undergo rapid reactivation with renewed induction of activation markers such as CD25 and CD134 (OX‐40) 49, 90, 136. Moreover, CD8+ T cells further differentiate in the brain, showing enhanced IFN‐γ and granzyme B expression and induction of αEβ7 integrin, mediating increased adhesion to the brain parenchyma (168). Finally, effector T cells migrate to the site of the brain lesion towards a chemotactic gradient. Chemokines such as monocyte chemoattractant protein (MCP)‐1 also induce the expression of matrix metalloproteinases and other ectoenzymes that enable penetration of effector T cells into the brain parenchyma 6, 11.
Perivascular macrophages and parenchymal microglial cells
Next to the DCs, two other major populations of phagocytic cells of myeloid origin function as APCs in the CNS: perivascular macrophages and parenchymal microglia cells. Based on their morphology and immunophenotype, perivascular macrophages appear to be very similar to blood‐derived macrophages (228). Moreover, it has been shown that brain perivascular cells contain a population of replaceable, rather than resident phagocytic cells (18).
Microglial cells are the primary immunocompetent cells in the brain. In addition to their phagocytic function, they may also participate in the regulation of innate and adaptive immune responses. They show a very low turnover rate and remain in an undifferentiated state with little expression of MHC class II under steady‐state conditions. Upon activation, microglial cells can quickly upregulate their antigen presenting capabilities in response to inflammatory or microbial stimuli both in vitro and in vivo; they can also develop into more DC‐like cells 70, 195, 227. However, even upon full activation, the expression levels of MHC and costimulatory molecules on DC‐like microglial cells are much lower than generally found on DCs. Furthermore, CNS‐resident microglial cells purified from the inflamed CNS were found to be largely incapable of activating either naive or effector T cells (CD4+ T helper cells), in contrast to peripherally derived myeloid DCs 171, 175. In addition, there are currently no data available on the ability of microglial cells to migrate out of the CNS in significant numbers and present antigens in the draining lymph nodes. Instead of T‐cell priming, both cell types might play a direct role in reshaping the function CD8+ effector T cells or a more indirect role in the conditioning of the DC precursors to differentiate into DCs 136, 168.
Control of immune responses in the CNS
The time of onset, the intensity and duration of immune responses within the CNS have to be tightly controlled; otherwise permanent tissue damage can occur. Immunosuppressive Tregs have been shown to be essential for the control of immune pathology. It has been reported that, similar to effector T cells, Tregs traffic to the inflamed site within the CNS, become reactivated, and further expand in situ. Interestingly, it has been shown that Tregs are not functional at the disease peak of experimental autoimmune encephalomyelitis (EAE). However, in the recovery phase, Tregs regain suppressor activity and potently curb the autoimmune responses locally 144, 190. Moreover, it was recently demonstrated that CNS DCs show a dual role during the disease course of an EAE: these cells promote CNS inflammation at the onset of the disease, whereas by the peak of the disease, they lose their optimal T‐cell stimulatory capacity and support Treg‐mediated immunosuppression to limit the extent of CNS inflammation (58).
INTERACTION OF MALIGNANT GLIOMA AND THE IMMUNE SYSTEM
Immune cell infiltrates
The immune recognition of glial tumors arising within the brain is usually prevented at the earliest stages of neoplastic transformation where the tumor cells are totally contained within the normal brain parenchyma. As such, primary brain tumors present a unique challenge for CNS immunosurveillance. However, at later stages of tumor growth, when massive tissue damage with destruction of the BBB and tumor cell necrosis with antigen drainage to the periphery occurs, brain tumors become accessible to the peripheral immune system. In this case, albeit at varying degrees, immune cell infiltrates can regularly be found within malignant gliomas. The vast proportion of immune cells, mainly macrophages, microglial cells, DCs and T cells, is found at perivascular regions, but can also be observed throughout the tumor bed and within necrotic tissue regions 110, 199, 216, 272. Attraction of these immune cells is directed by a number of molecules, which are mainly produced by oxygen‐deprived tumor cells surrounding necrosis. Hypoxia inducible factor‐1α (HIF‐1α) has been identified as a key factor in gliomas promoting the release of chemoattractants, such as CXCL5, CXCL8/IL‐8 and CXCL12/SDF‐1 57, 253. Other chemoattractants, such as CCL2/MCP‐1, CCL3/MIP1‐α, CCL4/MIP1‐β, CCL22/MDC also contribute to the recruitment of immune cells to gliomas 56, 118, 252. However, early studies have already determined that the presence of immune cell infiltrates usually does not correlate with the clinical course of glioma patients or may even be a negative prognostic factor 217, 220, 221.
Immunosuppression elicited by malignant gliomas
Compelling evidence exists that immune activation within malignant gliomas is suppressed by the local tumor microenvironment 61, 274, 275 (Figure 5). Malignant gliomas are characterized by the presence of a large number of immunosuppressive soluble factors, such as vascular endothelial growth factor (VEGF), IL‐10, TGF‐β, whereas immunostimulatory molecules, such as IL‐12, IL‐18, INF‐γ, are lacking. In addition, immunosuppressive enzymes such as indoleamin 2,3‐dioxygenase (IDO), cyclooxygenase‐2 (COX‐2), arginase and nitric‐oxide synthase‐2 (NOS‐2) are overexpressed in glioma cells 28, 32, 42, 48, 83, 185. Moreover, many immunoinhibitory molecules are abundantly expressed on the surface of glioma cells. Non‐classical MHC ligands such as HLA‐G and HLA‐E 286, 290, co‐inhibitory molecules such as PD‐L1 (B7‐H1) 288, 289, minor brain gangliosides such as GM2 and GM3 (88), and galectin‐1 and galectin‐3, mammalian lectins with specificity to beta‐galactosides have been detected 74, 218. Furthermore, inhibitory cytokine signaling molecules such as signal transducers and activators of transcription 3 (Stat3) are known to be constitutively activated in several human glioma cell lines, promoting tumor cell growth and survival 2, 142, 207.
Figure 5.
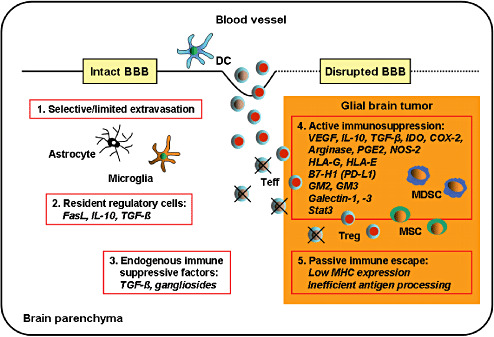
Immune escape mechanisms of diffuse gliomas. Once antigen‐specific effector T cells (Teffs) reach the central nervous system (CNS) parenchyma through the intact or disrupted blood–brain barrier (BBB) (1), they may face regulation by CNS‐residents cells such as astrocytes and microglial cells that are able to inhibit T‐cell proliferation and to induce T‐cell apoptosis by the secretion of immunosuppressive molecules and the expression of death receptors ligands such as FasL (2). Constitutive immunosuppressive factors in the brain parenchyma such as TGF‐s may also impede T‐cell differentiation or T‐cell effector functions (3). Within the tumor, Teffs are confronted with different subpopulations of immune suppressor cells that are attracted to the tumor site, such as regulatory T cells (Tregs), myeloid‐derived suppressor cells (MDSCs) and mesenchymal stem cells (MSCs). Moreover, tumor‐infiltrating Teffs are exposed to high concentrations of tumor‐derived soluble immunosuppressive factors as well as immunoinhibitory surface molecules expressed on the tumor cells (4). Finally, efficient immune recognition of glial brain tumor cells may be compromised by low MHC molecule expression and inefficient processing and presentation of tumor‐associated antigens (5). Adapted from Walker et al (2002) (274).
As a consequence, differentiation, maturation and function of tumor‐infiltrating DCs and other APCs, as well as the generation and activation of immune effector cells are all profoundly suppressed in the tumor microenvironment. In addition, the cytolytic activity of macrophages, NK cells and cytotoxic T cells is deeply disturbed 81, 111, 179, 239, 241, and other players in tumor‐induced immunosuppression are recruited to the tumor, preventing tumor‐specific immunity. Among those are myeloid‐derived suppressor cells, representing a phenotypically heterogeneous cell population that includes immature myelomonocytic cells, terminally differentiated monocytes and granulocytes 82, 203, 263, but also mesenchymal stem cells with immunosuppressive functions 26, 260. Recent studies clearly show that CD4+ Tregs infiltrate and fbaccumulate within gliomas 62, 125 and profoundly suppress antiglioma immune responses. Also, patients with malignant glioma show an elevated proportion of Tregs in peripheral blood in the setting of an overall diminished CD4+ T‐cell pool (66). This predominance of Tregs was shown to be responsible for the multiple immunological defects that have been previously described in glioma patients, including abnormal delayed hypersensitivity responses, depressed mitogen responsiveness of T and B cells, decreased antibody responses, and impaired T‐cell cytotoxicity (60).
GLIOMA‐ASSOCIATED TARGET STRUCTURES FOR IMMUNOTHERAPY
The ultimate goal of glioma immunotherapy is to induce prominent antitumor immune response leading to the complete eradication of malignant cells without eliciting serious negative side effects. An essential step in the development of immunotherapeutic strategies against malignant gliomas is the identification of suitable target structures. An ideal target for immunotherapy is an antigen that is specifically and stably expressed by the tumor, absent from normal tissues, and crucial for the survival of the cancer cell. Cancer/testis (CT) antigens have the potential to match such requirements. CT antigens are encoded by genes that are normally expressed only in the human germ line, but are aberrantly expressed in various tumor types. So far, more than 40 CT antigen family members have been identified, most of them in melanomas. Of note, these antigens are frequently found in only a relatively small proportion of tumor cells. Interestingly, cancer germline genes (CGGs) are frequently co‐expressed, and tumors that express them tend to express several CT antigens 93, 124.
CT antigens
The knowledge of tumor‐specific target structures for glioma immunotherapy is not far advanced. The fact that glial cells and melanocytes ontogenetically have a common neuroectodermal origin may explain the finding that melanoma‐associated CGGs such as the melanoma antigen‐encoding (MAGE) genes MAGE‐1 and MAGE‐3, MAGE‐E1 or GAGE‐1 151, 229, 230 can also be detected in malignant gliomas. Other CGGs, such as the expression of homo sapiens testis (HOM‐TES)‐14 [also known as stromal cell‐derived protein (SCP)‐1], synovial sarcoma X breakpoint (SSX)‐1, SSX‐2, SSX‐4, HOM‐TES‐85 and Sry‐related high‐mobility group (HMG) box‐containing gene (SOX)‐6 have also been described in malignant gliomas, although their antigenic determinants are not yet known 222, 261. Most studies have analyzed antigen expression at the mRNA level by reverse transcription polymerase chain reaction (RT–PCR). Protein expression or immunogenicity of CT antigens have only rarely been investigated in malignant gliomas (158). Efficient immune recognition of CT antigens on glioma cells may be compromised by low or absent expression of classical MHC molecules and inefficient processing and presentation of such antigens 64, 173.
Overexpressed self‐antigens
Further potential target structures with restricted protein expression include overexpressed self‐antigens, such as mutated versions of the epidermal growth factor receptor (EGFR), especially of variant III (EGFRvIII), the IL‐13 receptor α chain 2 (IL‐13Rα2) or the apoptosis inhibitor protein, survivin 109, 133, 287. Other tumor‐associated targets can be detected in malignant gliomas, such as the squamous cell carcinoma antigen recognized by T cells, SART1 and SART3 114, 180. Furthermore, transferrin receptors that are overexpressed on rapidly dividing cells, most notably on hematopoietic cells and various tumor cells, including GBM cells, are identified as target structures (210). Currently, much attention is drawn to viral antigens unique to human cytomegalovirus, which are expressed within the vast majority of high‐grade gliomas, but not within surrounding normal brain (176).
Differentiation antigens
Additional potential targets include differentiation antigens that are normally only seen at particular phases of cell differentiation, such as tyrosinase related protein (TRP)‐1 and TRP‐2 or gp100, but also antigen isolated from immunoselected melanoma (AIM)‐2, ADP ribosylation factor 4‐like protein ARF4L, and betaGlcNAc beta1, 3‐galactosyltransferase, polypeptide 3 (GALT3). However, as these antigens often demonstrate a broader tissue expression, their value as targets for antiglioma therapy might be limited 45, 158, 187, 230, 257.
Stromal antigens
Target structures within the tumor stroma have also been identified. One of the best examples is tenascin, a polymorphic glycoprotein of the extracellular matrix, which is expressed in about 80% of malignant gliomas, especially in GBM (20).
GLIOMA IMMUNOTHERAPY
For treatment strategies of gliomas, passive, adoptive and active immunotherapeutic approaches have been evaluated.
Passive immunotherapy
To target glioma‐specific structures, different monoclonal antibodies (mAbs) have been designed that are coupled to radionucleotides (radioimmunoconjugates) or exotoxins (immunotoxins) and are administrated locally. In some studies, a survival benefit in glioma patients has been documented. However, a disadvantage of this approach is that the penetration of these molecules into the tumor tissue is quite limited, because of high interstitial pressures in the tumor and surrounding tissue. High‐flow convention enhanced methods are needed to efficiently deliver these molecules to the more peripheral, diffusely infiltrative portions. Moreover, the effectiveness of the therapy seems to be restricted to those patients with a low tumor burden 29, 150, 277.
Adoptive immunotherapy
Alternatively, the adoptive transfer of ex vivo generated and activated effector cells into the resection cavity of patients with malignant gliomas has been extensively evaluated. Treatment approaches have differed in the types of cells administered, the route of administration, and the activation status of the cells. These approaches offer the advantage of circumventing deficits of antitumor effector cell populations in the host by providing optimal conditions for the culture and amplification of these cells in vitro, in the absence of tumor‐derived immunosuppressive factors. However, despite good tolerability, clinical efficacy has rarely been seen with the local administration of lymphokine‐activated killer cells (LAK‐cells) 27, 59, 126, mitogen‐activated killer cells (MAKs) 115, 129, NK cells, tumor‐infiltrating lymphocytes (TILs) and allogeneic or autologous, tumor‐specific cytototoxic T cells 139, 147, 200, 206, 291. Proinflammatory cytokines, such as IL‐2 have been coadministered locally to enhance antitumor responses, but has induced significant toxicity, particularly in patients with large tumor loads 16, 98.
Active immunotherapy
Active immunization of patients with malignant gliomas is regarded as a powerful strategy to induce potent antitumor response in vivo. As a vaccine, autologous inactivated tumor cells have been used, either gene‐modified or applied together with cytokine‐secreting fibroblasts 193, 245. Alternatively, tumor‐specific peptide vaccines targeting EGFRvIII have been administrated intradermally along with granulocyte macrophage colony‐stimulating factor (GM‐CSF) as an adjuvans (226). One of the most promising immunotherapeutic approaches to amplify tumor‐specific T‐cell responses is to use ex vivo generated, autologous tumor antigen‐loaded DCs as a vaccine (79). Several phase I/II studies have been carried out with this approach, and although these clinical trials differed in terms of DC generation, loading of tumor antigens and the route of application, robust intratumoral cytotoxic and memory T‐cell infiltration could be detected in patients who underwent resurgery after vaccination. DC vaccinations appeared to be mainly beneficial for patients with younger age, minimal residual tumor burden and expression of low levels of TGF‐β2 54, 156, 219, 292, 294, 296, 297. The principles and results of both peptide‐based vaccination and DC therapy against malignant gliomas are discussed in more detail in the companion articles of van Gool et al (267) and Choi et al (46).
ENHANCEMENT OF VACCINE IMMUNOGENICITY
Data from phase I/II studies support the view that strong glioma‐specific immune responses can be induced by a DC vaccine, but that therapy efficacy is still impeded by rapid tumor progression and a strong local immunosuppressive environment. To improve glioma immunotherapy, higher numbers and more potent glioma‐specific effector cells should be induced that home in to the CNS glioma site, including the peripheral, diffusely infiltrative areas (Figure 6).
Figure 6.
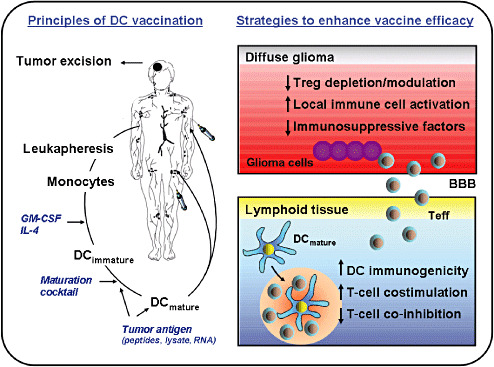
Principles of dendritic cell (DC) vaccination and further strategies to improve immunotherapy of diffuse gliomas. The most common method used to generate ex vivo DCs for vaccination is to culture autologous monocytes obtained from leukapheresis in the presence of granulocyte monocyte colony‐stimulating factor (GM‐CSF) and IL‐4. Following 5–7 days in culture, the monocytes differentiate into immature DCs. DC maturation is induced by culturing immature DCs for an additional 24–48 hours in the presence of a maturation cocktail, most commonly consisting of TNF‐α, IL‐1β, IL‐6 and PGE2, also known as monocyte‐conditioned medium. Tumor antigen loading with tumor lysate, tumor RNA or synthetic tumor‐specific peptides occurs at either the immature or mature DC stage. Finally, mature tumor antigen‐loaded DCs are injected back into patients. Critical issues to enhance the persistence of the vaccine‐induced immune responses for future immunotherapeutic approaches against diffuse gliomas are to improve the immunogenicity of a DC vaccine and to break the local immunosuppressive environment of diffuse gliomas. Toll‐like receptor (TLR) agonists, activation of costimulatory receptors, blockade of co‐inhibitory receptors and/or depletion or modulation of regulatory T cells (Treg) could all be used to intensify antiglioma immune responses induced by peripheral DC vaccinations. In addition, immunosuppressive molecules could be targeted directly within the tumor microenvironment to enhance treatment efficacy. Peri‐ or intratumoral administration of distinct TLR agonists might serve as an additional option to activate local immune cells and to potentiate antitumor immunity. Abbreviation: BBB = blood–brain barrier; Teff = T effector cells.
DC maturation
When using ex vivo generated DCs as a vaccine, the induction of effective immune responses is dependent upon proper DC maturation. The most widely used maturation cocktail in clinical DC trials consists of four reagents: TNF‐α; IL‐1β; IL‐6; and PGE2, also known as monocyte‐conditioned medium. A major disadvantage of this maturation cocktail is that the resulting DCs secrete little IL‐12, most likely as a result of the presence of PGE2 present during maturation 134, 213. Moreover, cytokine cocktail‐matured DCs were found to be more effective than immature DCs in expanding a population of immunosuppressive Tregs 55, 259.
An excellent method to enhance the immunogenicity of DCs is to include immune response modifiers, such as TLR agonists into the maturation cocktail for optimal DC activation. Several groups reported that DCs matured with more extensive maturation cocktails containing TLR‐agonists such as Poly I:C (TLR3) or R848 (TLR7/8) and cytokines such as TNF‐α, IL‐1β and INF‐γ show superior immunogenicity to standard cocktail matured DCs; this strategy enhanced T‐cell and NK‐cell responses, while decreasing the chance to induce Tregs. Addition of TLR‐agonists to the maturation cocktail also enabled the use of sufficiently low concentrations of PGE2 that the capacity of DCs to produce IL‐12 was not disturbed. Supplementation with PGE2 was essential to improve DC handling by reducing cell adherence and enhancing migratory capacity via upregulation of CCR7. Moreover, TLR triggered DCs retained a stable phenotype and IL‐12 producing capacity, even when exposed to TGF‐β, Th2 or Treg promoting environments 30, 73, 84, 166, 302. At present, a phase I/II‐study (NCT00766753) using TLR‐polarized DCs (type‐1 αDCs) loaded with glioma‐associated tumor antigens is being carried out in patients with recurrent malignant gliomas.
The importance of IL‐12 for an effective immunotherapy against gliomas has been demonstrated in several animal and human studies. Coadministration of IL‐12 with a DC vaccine was reported to enhance antitumor responses resulting in prolonged survival 116, 137, 293. In addition, IL‐12 was shown to directly inhibit Treg differentiation from naive T cells (278). Collectively, these data imply that mature DCs with retained capacity to produce IL‐12 are the most promising candidates for glioma immunotherapy and point towards an important role for TLR‐agonists in inducing potent anti‐glioma responses.
In addition, TLR‐agonists can be used as immune adjuvans. Pretreatment of the injection site with proinflammatory compounds or TLR‐agonists such as imiquimode (TLR7) have been shown to significantly enhance both the persistence and trafficking of DCs into the draining lymph nodes after vaccination, resulting in increased priming of tumor‐specific T cells 167, 181, 204.
Discovery of major glioma rejection antigens
To create a powerful DC vaccine, it is not only important to use proper maturation stimuli, but also to efficiently load DCs with relevant tumor antigens. A major challenge is to discover new, glioma‐specific tumor antigens that can be used for targeted immunotherapy without immunological side effects. Particular attention should be paid to detection of major glioma rejection antigens that are recognized by tumor‐infiltrating lymphocytes during endogenous antitumor responses, especially those expressed on tumor‐initiating glioma stem cells. Moreover, immunodominant CD4 and CD8 epitopes of these antigens should be defined to be able to generate tumor peptides that are presented both by MHC class I and class II molecules. Such information could be further used to create potent antitumor vaccines consisting of synthetic long peptides in combination with TLR‐agonists to elicit strong effector T‐cell responses 25, 243. In addition, large numbers of antigen‐specific effector T cells could be generated ex vivo that are adoptively transferred into lymphopenic patients followed by peptide‐pulsed DC vaccination. This approach has already resulted in effective antitumor responses against preestablished intracranial tumors in a preclinical model (205).
Irrespective of the detection of new glioma‐specific tumor antigens, future work should focus on the potential of tumor‐initiating glioma stem cells to provide tumor antigens for vaccination. The possibility of generating cancer stem cell lines from primary GBM patients has been demonstrated by several groups 21, 92, 155. These cancer stem cell lines could be used to specifically target glioma stem cells, for example, by replacing whole tumor lysate or total tumor RNA with lysate or RNA from glioma stem cells for loading DCs.
Further improvement could be obtained by immunizing not only against tumor cells, but also against the tumor stroma, which is important for tumor cell growth and survival. Despite the presence of tumor‐specific T cells, tumor cells can escape immune destruction as a result of inappropriate expression of MHC molecules or the presence of antigen‐loss variants. However, stromal cell subpopulations that can capture and present tumor‐derived antigens appear to show less immune escape mechanisms, and therefore might be a better target for immune effector cells (298).
Intensification of T‐cell costimulation
A powerful option to enhance the immunogenicity of a vaccine is to increase T‐cell costimulation. This can be achieved by coadministering agonistic antibodies specific for costimulatory molecules belonging to the TNF receptor superfamily, such as GITR, OX40 (CD134), 4‐1BB (CD137) and RANK 47, 89, 170, 237, 265. In murine glioma models some of these antibodies have exerted a promising effect 138, 140.
Alternatively, T‐cell costimulation can be increased by coadministering antagonistic antibodies specific for co‐inhibitory molecules expressed on T cells. For instance, many inhibitory pathways within the B7–CD28 family have been described that can attenuate T‐cell responses and promote T‐cell tolerance. CTLA‐4 and the programmed‐death (PD)‐1 receptor are two of the key negative regulators of T cell responses. Anti‐CTLA‐4 antibodies have been studied as potential therapeutics for glioma. We and others have shown that systemic CTLA‐4 blockade prolongs survival in treated mice, without eliciting signs of autoimmunity. Anti‐CTLA‐4 treatment reestablished normal CD4 counts and abrogated increased Treg fractions in the CD4 compartment observed in tumor‐bearing mice. Moreover, the CD4 T‐cell proliferative capacity could be restored and glioma‐specific antitumor immunity was enhanced 67, 85.
Recent data suggest that CTLA‐4 blockade can result in the effective treatment of tumors arising in the CNS. It was demonstrated that the systemic administration of a human mAb directed against CTLA‐4 (Ipilimumab, MDX‐010, Bristol‐Myers Squibb, New York, NY, USA and Medarex Inc. Princeton, NJ, USA) induced a significant clinical benefit in a female patient with metastatic melanoma to the CNS. Pathological examination of the brain metastasis following Ipilimumab treatment revealed abundant infiltrates of CD8+ lymphocytes and extensive tumor necrosis (104).
Counteracting Treg‐mediated immunosuppression
The immunogenicity of a vaccine can be further enhanced by removing suppression of Tregs. Especially in preclinical murine models, different strategies have been successfully used to eliminate Tregs such as CD25‐specific mAbs, immunotoxins or different chemotherapeutic agents.
We recently showed in a murine glioma model that depletion of Tregs by systemic administration of CD25‐specific mAbs can elicit an otherwise suppressed immune response against glioma‐specific tumor antigens, leading to the rejection of the tumors. Additional experiments revealed that combining Treg depletion with administration of CTLA‐4 mAbs further boosted glioma‐specific CD4 and CD8 effector T cells, as well as anti‐glioma IgG2a antibody titers in this model without any signs of autoimmunity (85). The potency of Tregs to suppress an effective anti‐glioma immune response was further demonstrated by the fact that mice were not protected from tumor outgrowth when vaccinated with tumor‐lysate pulsed DCs in a “simultaneous setting” where tumor cells and DCs are given at the same day. In contrast, depletion of Tregs before vaccination considerably increased the ability of vaccinated mice to survive the tumor challenge. Moreover, DC vaccination with anti‐CD25 treatment allowed the development of long‐lasting tumor protective immunity 87, 164. These results confirm that counteracting Treg‐mediated immunosuppression is an essential component in the success of anti‐glioma vaccines. However, a disadvantage of CD25 depletion is that treatment of mice with anti‐CD25 mAbs is only beneficial within a limited time window and its efficacy is dependent on tumor burden. Treg depletion after immunotherapy inhibited clonal expansion of tumor antigen specific T cells and reduced the efficacy of the vaccine (51).
In humans, several anti‐CD25 antibodies such as daclizumab have been developed and tested for their ability to deplete human Tregs. However, recent studies demonstrated that these antibodies failed to eliminate Tregs or did not affect their suppressive function 145, 271. Currently, two phase I/II randomized studies (NCT00626483, NCT00626015) are being carried out in patients with newly diagnosed GBM to determine if daclizumab inhibits the functional and numeric recovery of Tregs after therapeutic TMZ‐induced lymphopenia.
Besides CD25 mAb, a recombinant IL‐2 diphtheria toxin conjugate, DAB389IL‐2 (also known as denileukin diftitox or ONTAK) and a CD25‐specific immunotoxin (LMB‐2), which is formed of the single‐chain Fv fragment of the anti‐CD25 mAb with a recombinant form of the pseudomonas exotoxin, have been investigated for the elimination of Tregs. A few studies have shown a reduction in the number of circulating and tumor‐infiltrating Tregs after a single dose of ONTAK, with a more prominent effect after successive ONTAK treatments or administrations of LMB‐2. Moreover, ONTAK significantly improved the stimulation of tumor‐specific T cell responses after vaccination with tumor‐antigen loaded DCs 53, 165, 202. However, despite inducing a reduction in Treg cell numbers in vivo, ONTAK or LMB‐2 treatment have only resulted in limited objective clinical responses so far 146, 202.
Alternate chemotherapeutics are currently being investigated for their potency to selectively deplete Tregs 10, 76, 78. In a rat glioma model, oral administration of low‐dose metronomic TMZ has recently been shown to reduce circulating Tregs, associated with a suppression of their inhibitory functions on effector T cells in a rat glioma model. In contrast, high‐dose TMZ failed to significantly modulate Treg numbers or function (15). Similarly, a significant reduction of CD4+CD25+ T cells could be demonstrated with metronomic administration of TMZ in melanoma patients (251). Therefore, a low dose metronomic TMZ regime in association with a cancer vaccine could be an attractive strategy for the treatment of malignant glioma and might be superior in efficacy to conventional TMZ chemotherapy alone.
Another interesting approach to reduce the number of tumor‐infiltrating Tregs is to selectively block their trafficking to the tumor site. Treg subsets have been reported to differ in homing receptor expression and chemokine responsiveness 108, 189, 240. Recently, it was demonstrated that Tregs from GBM patients express significantly higher levels of CCR4 than those from healthy controls and they respond to both the recombinant human chemokines CCL2/MCP‐1 and CCL22/MDC in vitro. Moreover, chemotherapeutic agents such as TMZ and carmustine [3‐bis (2‐chloroethyl)‐1‐nitrosourea] were shown to reduce the production of CCL2 by glioma cells (131). Other inflammatory chemokine receptors involved in the attraction of Tregs are CCR2, CCR5 and CCR8 (113). Gliomas can synthesize and release CXCL12/SDF‐1 in a TGF‐β dependent manner (252). Interestingly, we observed CXCR4 expression on glioma‐infiltrating Tregs and its upregulation during tumor growth. Implementation of CXCR4 inhibitors may thus improve immunotherapeutic strategies against malignant gliomas (211).
BREAKING THE LOCAL IMMUNOSUPPRESSIVE ENVIRONMENT OF GLIOMAS
In order to enhance the persistence of the vaccine‐induced immune responses and to achieve optimal clinical benefit, apart from optimizing antiglioma vaccines, additional treatments need to be developed to break the local immunosuppressive environment of gliomas.
Targeting TGF‐β
A powerful approach to overcome glioma‐induced tolerance mechanisms involves targeting immunosuppressive molecules directly within the tumor microenvironment. In malignant gliomas, the three existing isoforms of TGF‐β in mammals, termed TGF‐β1, 2 and 3, are differentially expressed. High‐grade gliomas mostly overexpress the TGF‐β2 isoform, which is processed and secreted in large amounts in its active form (141). In recent years, different strategies have been developed for inhibiting TGF‐β. The most advanced in the clinical application is the use of a phosphorothioate‐modified antisense oligonucleotide (trabedersen, AP 12009, ANTISENSE Pharma GmbH, Regensburg, Germany), which is complementary to the mRNA encoding TGF‐β2 isoform 122, 123, 232. Several phase I/II‐studies have been carried out applying TGFβ2‐antisense oligonucleotides intratumorally through continuous high‐flow microperfusion [(convection‐enhanced delivery (CED)] in patients with high‐grade gliomas; these studies confirmed the safety of this approach, as well as long‐term clinical benefit in several individuals (97). A multinational phase III study (NCT00761280) comparing TGFβ2‐antisense‐oligonucleotides with standard chemotherapy (TMZ or BCNU) in adult patients with confirmed recurrent or refractory anaplastic astrocytoma (WHO grade III) is currently recruiting patients.
An alternative to the treatment with antisense oligonucleotides is to prevent downstream signaling by interfering with TGF‐β receptor kinase activities. Such kinase inhibitors have already been successfully tested in preclinical models, resulting in increased survival of treated animals, associated with pronounced tumor infiltration by NK‐cells, CD8+ T‐cells and macrophages 255, 262.
Strategies combining active immunotherapy with local TGF‐β blockade could thus further enhance therapy efficacy. In a preclinical study using a rat glioma model, the combination of intracranial TGF‐β2 antisense oligonucleotide administration and vaccination with irradiated glioma cells not only inhibited TGF‐β protein production in vivo, but also significantly prolonged survival times when compared with either vaccine alone or no therapy (161).
Targeting Stat3 and immunosuppressive enzymes
There is accumulating evidence from preclinical studies that targeting immunosuppressive molecules other than TGF‐β improves antitumor immunity against gliomas as well.
Considering the suppressive role of signal transducers and activators of transcription 3 (Stat3) in antitumor immunity, selective inhibitors of Stat3 have been evaluated in murine glioma models and were shown to activate intratumoral macrophages and microglia, induce apoptosis in glioma cells, and inhibit tumor growth 112, 120, 300.
With regard to immunosuppressive enzymes, specific IDO inhibitors such as 1‐methyl‐l‐trypophan have been studied to break immune resistance of malignant gliomas through T‐cell inactivation caused by tryptophan depletion and metabolite accumulation 106, 177. Moreover, COX‐2 inhibitors have been shown to significantly reduce PGE2 levels in Cox2‐overexpressing gliomas, thereby abrogating the induction of IL‐10 and TGF‐β‐secreting Tr1 cells (4). Selective COX‐2 inhibitors also show antiproliferative and apoptosis‐inducing effects independent of their COX‐2 inhibitory activity, resulting in the reduction or inhibition of glioma growth in vivo 182, 234. Furthermore, antitumor responses could also be elicited by blocking arginase expression using COX‐2 inhibitors 214, 215. To restore T‐effector‐cell functions, selective iNOS inhibitors such as SD‐3651 might be attractive for the treatment of gliomas (186).
Local treatment of gliomas with TLR agonists
Local administration of immune response modifiers, such as TLR‐agonists are powerful tools to generate immune responses against antigens derived from the tumor in vivo. Pioneering experiments from Carpentier et al have shown that direct injections of synthetic phosphothioate‐stabilized CpG‐oligonucleotides (CpG‐ODNs) in neuroblastomas induced complete tumor rejection in the majority of mice and triggered long‐term immunity (37). Further studies have confirmed the antitumor effects of CpG‐ODN in different intracranial models of syngeneic glioma 38, 174. Recently, we could show in a murine glioma model that the therapeutic efficacy of CpG‐ODN is predominately mediated through a TLR9‐dependent immune activation leading to intratumoral accumulation of IFN‐γ producing CD4 and CD8 T cells, a marked increase in the ratio of CD4 effector T cells to Tregs, and long‐term protective immunity. Moreover, local administration of CpG‐ODNs (TLR9 agonist) was most efficient in inducing antitumor immunity as compared with other TLR‐agonists (86).
A phase I trial using convection‐enhanced intratumoral delivery of CPG‐ODN (CpG‐28) has already been completed in the setting of recurrent GBM and showed minor responses in two patients without showing serious adverse events (36). A randomized phase‐II trial with CpG‐ODN in GBM is still ongoing (NCT00190424). However, a major drawback of this approach is that TLR9 expression in humans is far more restricted and predominately confined to plasmacytoid DCs and B cells. Therefore, besides CpG‐ODN, it will also be interesting to explore local delivery of functionally similar agents, such as TLR7/8‐agonists to break local immunosuppression in glioma patients. Survival benefit was observed in therapeutic murine glioma models after intratumoral injection of synthetic TLR7/8‐agonists, such as R848 (resiquimod) or protamine‐protected mRNA 86, 231. Alternatively, recently identified phosphorothioate‐modified immunostimulatory RNA oligonucleotides might be attractive in this setting, as they proved to be potent inducers of INF‐α and TH1 cytokines via TLR 7/8 (secreted by DCs) and triggered an antigen‐specific cytotoxic T‐cell and IgG2a response (31).
In the future, it might be worthwhile to apply certain combinations of TLR agonists to further enhance the treatment efficacy. Synergy between different TLR agonists is now well documented 183, 276. Recent findings further imply that TLR agonists not only induce beneficial activating, but also potentially suppressive effector responses. Therefore, selective inhibition of immunosuppressive molecules induced during TLR immunotherapy might further enhance the efficacy of TLR‐agonists as tumor immunotherapeutics. Inhibition of IL‐10 with an anti‐IL‐10 receptor antibody combined with CpG‐ODN activation was shown to reverse the tolerogenic state of tumor‐infiltrating DCs and to augment their therapeutic efficacy in a mouse tumor model (269). Moreover, vaccinations with TLR‐matured DCs pulsed with tumor antigen combined with peri‐ or intratumoral injection of TLR‐agonists can potentiate antitumor immunity, as it was shown to eradicate large murine tumors resistant to chemotherapy (99).
It will be also interesting to evaluate stimulation of other innate immune receptors for glioma therapy. Recently, bifunctional RNA molecules that were designed to target key tumor survival factors and to activate cytosolic retinoic acid‐inducible gene‐I (RIG‐I)‐like helicases promoted strong antitumor effects in a mouse model of metastatic melanoma to the lung (201).
To move forward, however, additional studies are needed to define the protective mechanisms of action of distinct innate immune receptor agonists and their potential toxicity, because immune response modifiers that activate a wide range of innate and adaptive immune cells can have severe adverse effects, especially in the CNS.
Local glioma treatment with DCs
An alternative strategy to modify the tumor microenvironment is to inject DCs directly into the tumor bed. Intratumoral injections of DCs either transduced with INF‐α DNA or TLR‐polarized DC have been shown to enhance anti‐CNS immunity by promoting cross‐presentation of tumor‐associated antigens in draining lymph nodes and by enhancing CNS tumor homing of antigen‐specific type 1 CTLs through the induction of CXCL10 73, 258. Furthermore, intratumoral delivery of DCs enhances the antitumor efficacy of peripheral vaccines 73, 152, 194. Preliminary clinical results also confirm that intratumoral DC application in addition to intradermal DC injections are more beneficial in the treatment of glioma patients than intradermal DC injections alone (294).
COMBINATION OF IMMUNOTHERAPY AND RADIOCHEMOTHERAPY
Recent studies indicate that the clinical responsiveness of patients with malignant gliomas to chemotherapy is increased after DC vaccinations. Vaccinated patients receiving subsequent chemotherapy showed significantly longer times to tumor recurrence/progression and longer overall survival after chemotherapy than those who were treated only with chemotherapy. It has been postulated that therapeutic vaccinations increase the sensitivity to chemotherapy by eliminating chemoresistant tumor cells 284, 285.
There are a number of reasons to combine immunotherapeutic strategies with conventional therapeutic methods such as radiotherapy and chemotherapy 153, 266. Recent data show that tumor cell death triggered by chemotherapy or radiotherapy initiates an immunoadjuvant pathway that contributes to the success of cytotoxic treatments (301). Numerous endogenous danger signals transferred by dying tumor cells to innate immune effectors may account for the immunogenicity of tumor cell death (8). Both anthracyclines and alkylating chemotherapeutics such as TMZ have been shown to induce an immunogenic cell death by triggering innate receptors such as TLRs. As a consequence, the ability of DCs to present tumor antigens from dying tumor cells to T and B cells is enhanced 7, 52, 192. Moreover, activation of innate immune receptors leads to the upregulation of MHC molecules on tumor cells, thus increasing their sensitivity to T‐cell‐mediated killing 86, 184. In addition, radiotherapy and chemotherapy remove local suppressor cells, such as tumor‐specific Tregs or might affect their function, thus permitting a more effective T‐cell stimulation 188, 251. Furthermore, chemotherapy has been shown to induce lymphopenia, thereby allowing thymic‐independent antigen‐driven T‐cell regeneration within the context of T‐cell homeostasis 127, 163. The concept of tumor‐specific immunization at the time of immune reconstitution after chemotherapy has been successfully tested in different animal models and phase I/II clinical studies, demonstrating that the availability of tumor antigens during homeostatic T‐cell proliferation leads to effective antitumor immunity and enhanced memory T‐cell responses 9, 12, 107, 209.
Combination therapies integrating vaccination strategies into the standard chemoradiotherapy protocol are currently under evaluation. There is preliminary evidence of successful combination of immunotherapy and TMZ (101). However, administration of anti‐immunosuppressive therapy together with radiochemotherapy must be carefully timed so as to optimize the immune response. It is of critical importance to establish reliable, reproducible and quantitative assays to evaluate vaccine‐induced immune responses 1, 259. Ideally, pre‐ and postvaccination samples should be taken throughout the course of vaccination concomitantly with the evaluation of clinical parameters. Vaccine‐induced cellular and humoral immune responses should be monitored in different compartments, especially at the tumor site, in the circulation, and at the vaccination site [delayed‐type hypersensitivity (DTH) test biopsies]. Overall, the combination of radiotherapy, chemotherapy and immunotherapy has the potential for strong antitumoral activity against malignant glioma, when applied using a well‐designed strategy.
CONCLUSION
Although our understanding of glioma immunobiology is rapidly growing, the knowledge about the complex pathological interactions within the local tumor microenvironment is still limited. Further elucidation of the spatiotemporal organization of the different players in tumor‐induced immunosuppression is of utmost importance for improving intervention strategies that boost potent antitumor responses. Current vaccination protocols are still of limited efficacy for suppressing glioma growth. To achieve optimal clinical benefit, additional treatments need to be developed and combined with a vaccine to break the local immunosuppressive environment of gliomas without inducing autoimmunity. This will enhance the persistence of the vaccine‐induced immune responses and will increase the chance to eliminate diffusely infiltrating tumor cells embedded within the normal brain parenchyma. Moreover, understanding the details of immune evasion in individual patients is critical, because it will ultimately enable the development of tailored therapies that specifically overcome these mechanisms of resistance. Keeping in mind that many of these immune evasion strategies used by gliomas are also part of the regular defense mechanism of the brain to prevent autoimmunity, such therapies have to be monitored carefully to detect and limit immune responses that are harmful to normal brain tissue. With further unraveling of glioma immunobiology, immunotherapeutic strategies have the opportunity to become a standard component in the multimodal treatment of malignant gliomas.
ACKNOWLEDGMENTS
This work was supported by grants from the German Research Foundation and the Medical Faculty of the University of Regensburg (GR2089/1‐1 and ReForM program to O.M. Grauer), and by grants from the KWF/Dutch Cancer Society (KUN 2003‐2893 to G.J. Adema and KUN 2003‐2975 to P. Wesseling).
Disclosure of potential conflicts of interest: Authors have no relevant conflicts of interest to disclose.
REFERENCES
- 1. Aarntzen EH, Figdor CG, Adema GJ, Punt CJ, De Vries IJ (2008) Dendritic cell vaccination and immune monitoring. Cancer Immunol Immunother 57:1559–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Abou‐Ghazal M, Yang DS, Qiao W, Reina‐Ortiz C, Wei J, Kong LY et al (2008) The incidence, correlation with tumor‐infiltrating inflammation, and prognosis of phosphorylated STAT3 expression in human gliomas. Clin Cancer Res 14:8228–8235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Adema GJ, Hartgers F, Verstraten R, De Vries E, Marland G, Menon S et al (1997) A dendritic‐cell‐derived C‐C chemokine that preferentially attracts naive T cells. Nature 387:713–717. [DOI] [PubMed] [Google Scholar]
- 4. Akasaki Y, Liu G, Chung NH, Ehtesham M, Black KL, Yu JS (2004) Induction of a CD4+ T regulatory type 1 response by cyclooxygenase‐2‐overexpressing glioma. J Immunol 173:4352–4359. [DOI] [PubMed] [Google Scholar]
- 5. Albert ML, Sauter B, Bhardwaj N (1998) Dendritic cells acquire antigen from apoptotic cells and induce class I‐restricted CTLs. Nature 392:86–89. [DOI] [PubMed] [Google Scholar]
- 6. Anthony DC, Ferguson B, Matyzak MK, Miller KM, Esiri MM, Perry VH (1997) Differential matrix metalloproteinase expression in cases of multiple sclerosis and stroke. Neuropathol Appl Neurobiol 23:406–415. [PubMed] [Google Scholar]
- 7. Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A et al (2007) Toll‐like receptor 4‐dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med 13:1050–1059. [DOI] [PubMed] [Google Scholar]
- 8. Apetoh L, Tesniere A, Ghiringhelli F, Kroemer G, Zitvogel L (2008) Molecular interactions between dying tumor cells and the innate immune system determine the efficacy of conventional anticancer therapies. Cancer Res 68:4026–4030. [DOI] [PubMed] [Google Scholar]
- 9. Asavaroengchai W, Kotera Y, Mule JJ (2002) Tumor lysate‐pulsed dendritic cells can elicit an effective antitumor immune response during early lymphoid recovery. Proc Natl Acad Sci U S A 99:931–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Audia S, Nicolas A, Cathelin D, Larmonier N, Ferrand C, Foucher P et al (2007) Increase of CD4+ CD25+ regulatory T cells in the peripheral blood of patients with metastatic carcinoma: a Phase I clinical trial using cyclophosphamide and immunotherapy to eliminate CD4+ CD25+ T lymphocytes. Clin Exp Immunol 150:523–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Babcock AA, Kuziel WA, Rivest S, Owens T (2003) Chemokine expression by glial cells directs leukocytes to sites of axonal injury in the CNS. J Neurosci 23:7922–7930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Baccala R, Gonzalez‐Quintial R, Dummer W, Theofilopoulos AN (2005) Tumor immunity via homeostatic T cell proliferation: mechanistic aspects and clinical perspectives. Springer Semin Immunopathol 27:75–85. [DOI] [PubMed] [Google Scholar]
- 13. Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ et al (2000) Immunobiology of dendritic cells. Annu Rev Immunol 18:767–811. [DOI] [PubMed] [Google Scholar]
- 14. Banerjee DK, Dhodapkar MV, Matayeva E, Steinman RM, Dhodapkar KM (2006) Expansion of FOXP3high regulatory T cells by human dendritic cells (DCs) in vitro and after injection of cytokine‐matured DCs in myeloma patients. Blood 108:2655–2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Banissi C, Ghiringhelli F, Chen L, Carpentier AF (2009) Treg depletion with a low‐dose metronomic temozolomide regimen in a rat glioma model. Cancer Immunol Immunother Feb 17 [Epub ahead of print] DOI: 10.1007/s00262‐009‐0671‐1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Barba D, Saris SC, Holder C, Rosenberg SA, Oldfield EH (1989) Intratumoral LAK cell and interleukin‐2 therapy of human gliomas. J Neurosurg 70:175–182. [DOI] [PubMed] [Google Scholar]
- 17. Bechmann I (2005) Failed central nervous system regeneration: a downside of fimmune privilege? Neuromolecular Med 7:217–228. [DOI] [PubMed] [Google Scholar]
- 18. Bechmann I, Priller J, Kovac A, Bontert M, Wehner T, Klett FF et al (2001) Immune surveillance of mouse brain perivascular spaces by blood‐borne macrophages. Eur J Neurosci 14:1651–1658. [DOI] [PubMed] [Google Scholar]
- 19. Bechmann I, Galea I, Perry VH (2007) What is the blood‐brain barrier (not)? Trends Immunol 28:5–11. [DOI] [PubMed] [Google Scholar]
- 20. Behrem S, Zarkovic K, Eskinja N, Jonjic N (2005) Distribution pattern of tenascin‐C in glioblastoma: correlation with angiogenesis and tumor cell proliferation. Pathol Oncol Res 11:229–235. [DOI] [PubMed] [Google Scholar]
- 21. Beier D, Hau P, Proescholdt M, Lohmeier A, Wischhusen J, Oefner PJ et al (2007) CD133(+) and CD133(−) glioblastoma‐derived cancer stem cells show differential growth characteristics and molecular profiles. Cancer Res 67:4010–4015. [DOI] [PubMed] [Google Scholar]
- 22. Benitez‐Ribas D, Adema GJ, Winkels G, Klasen IS, Punt CJ, Figdor CG, De Vries IJ (2006) Plasmacytoid dendritic cells of melanoma patients present exogenous proteins to CD4+ T cells after Fc gamma RII‐mediated uptake. J Exp Med 203:1629–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M et al (2006) Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441:235–238. [DOI] [PubMed] [Google Scholar]
- 24. Bettelli E, Korn T, Kuchroo VK (2007) Th17: the third member of the effector T cell trilogy. Curr Opin Immunol 19:652–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bijker MS, Van Den Eeden SJ, Franken KL, Melief CJ, Van Der Burg SH, Offringa R (2008) Superior induction of anti‐tumor CTL immunity by extended peptide vaccines involves prolonged, DC‐focused antigen presentation. Eur J Immunol 38:1033–1042. [DOI] [PubMed] [Google Scholar]
- 26. Birnbaum T, Roider J, Schankin CJ, Padovan CS, Schichor C, Goldbrunner R, Straube A (2007) Malignant gliomas actively recruit bone marrow stromal cells by secreting angiogenic cytokines. J Neurooncol 83:241–247. [DOI] [PubMed] [Google Scholar]
- 27. Blancher A, Roubinet F, Grancher AS, Tremoulet M, Bonate A, Delisle MB et al (1993) Local immunotherapy of recurrent glioblastoma multiforme by intracerebral perfusion of interleukin‐2 and LAK cells. Eur Cytokine Netw 4:331–341. [PubMed] [Google Scholar]
- 28. Bodmer S, Strommer K, Frei K, Siepl C, De Tribolet N, Heid I, Fontana A (1989) Immunosuppression and transforming growth factor‐beta in glioblastoma. Preferential production of transforming growth factor‐beta 2. J Immunol 143:3222–3229. [PubMed] [Google Scholar]
- 29. Boskovitz A, Wikstrand CJ, Kuan CT, Zalutsky MR, Reardon DA, Bigner DD (2004) Monoclonal antibodies for brain tumour treatment. Expert Opin Biol Ther 4:1453–1471. [DOI] [PubMed] [Google Scholar]
- 30. Boullart AC, Aarntzen EH, Verdijk P, Jacobs JF, Schuurhuis DH, Benitez‐Ribas D et al (2008) Maturation of monocyte‐derived dendritic cells with Toll‐like receptor 3 and 7/8 ligands combined with prostaglandin E(2) results in high interleukin‐12 production and cell migration. Cancer Immunol Immunother 57:1589–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bourquin C, Schmidt L, Hornung V, Wurzenberger C, Anz D, Sandholzer N et al (2007) Immunostimulatory RNA oligonucleotides trigger an antigen‐specific cytotoxic T‐cell and IgG2a response. Blood 109:2953–2960. [DOI] [PubMed] [Google Scholar]
- 32. Buccoliero AM, Caldarella A, Gheri CF, Taddei A, Paglierani M, Pepi M et al (2006) Inducible cyclooxygenase (COX‐2) in glioblastoma–clinical and immunohistochemical (COX‐2‐VEGF) correlations. Clin Neuropathol 25:59–66. [PubMed] [Google Scholar]
- 33. Bystry RS, Aluvihare V, Welch KA, Kallikourdis M, Betz AG (2001) B cells and professional APCs recruit regulatory T cells via CCL4. Nat Immunol 2:1126–1132. [DOI] [PubMed] [Google Scholar]
- 34. Calzascia T, Di Berardino‐Besson W, Wilmotte R, Masson F, De Tribolet N, Dietrich PY, Walker PR (2003) Cutting edge: cross‐presentation as a mechanism for efficient recruitment of tumor‐specific CTL to the brain. J Immunol 171:2187–2191. [DOI] [PubMed] [Google Scholar]
- 35. Calzascia T, Masson F, Di Berardino‐Besson W, Contassot E, Wilmotte R, Aurrand‐Lions M et al (2005) Homing phenotypes of tumor‐specific CD8 T cells are predetermined at the tumor site by crosspresenting APCs. Immunity 22:175–184. [DOI] [PubMed] [Google Scholar]
- 36. Carpentier A, Laigle‐Donadey F, Zohar S, Capelle L, Behin A, Tibi A et al (2006) Phase 1 trial of a CpG oligodeoxynucleotide for patients with recurrent glioblastoma. Neuro-oncol 8:60–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Carpentier AF, Chen L, Maltonti F, Delattre JY (1999) Oligodeoxynucleotides containing CpG motifs can induce rejection of a neuroblastoma in mice. Cancer Res 59:5429–5432. [PubMed] [Google Scholar]
- 38. Carpentier AF, Xie J, Mokhtari K, Delattre JY (2000) Successful treatment of intracranial gliomas in rat by oligodeoxynucleotides containing CpG motifs. Clin Cancer Res 6:2469–2473. [PubMed] [Google Scholar]
- 39. Carrithers MD, Visintin I, Kang SJ, Janeway CA, Jr. (2000) Differential adhesion molecule requirements for immune surveillance and inflammatory recruitment. Brain 123:1092–1101. [DOI] [PubMed] [Google Scholar]
- 40. Carson KA, Grossman SA, Fisher JD, Shaw EG (2007) Prognostic factors for survival in adult patients with recurrent glioma enrolled onto the new approaches to brain tumor therapy CNS consortium phase I and II clinical trials. J Clin Oncol 25:2601–2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Carson MJ, Doose JM, Melchior B, Schmid CD, Ploix CC (2006) CNS immune privilege: hiding in plain sight. Immunol Rev 213:48–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Castelli MG, Chiabrando C, Fanelli R, Martelli L, Butti G, Gaetani P, Paoletti P (1989) Prostaglandin and thromboxane synthesis by human intracranial tumors. Cancer Res 49:1505–1508. [PubMed] [Google Scholar]
- 43. Cayrol R, Wosik K, Berard JL, Dodelet‐Devillers A, Ifergan I, Kebir H et al (2008) Activated leukocyte cell adhesion molecule promotes leukocyte trafficking into the central nervous system. Nat Immunol 9:137–145. [DOI] [PubMed] [Google Scholar]
- 44. Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N et al (2003) Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF‐beta induction of transcription factor Foxp3. J Exp Med 198:1875–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chi DD, Merchant RE, Rand R, Conrad AJ, Garrison D, Turner R et al (1997) Molecular detection of tumor‐associated antigens shared by human cutaneous melanomas and gliomas. AmJPathol 150:2143–2152. [PMC free article] [PubMed] [Google Scholar]
- 46. Choi BD, Archer GE, Mitchell DA, Heimberger AB, McLendon RE, Bigner DD, Sampson JH (2009) EGFRvIII‐targeted peptide vaccine therapy in patients with malignant glioma. Brain Pathol 19:713–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Choi BK, Bae JS, Choi EM, Kang WJ, Sakaguchi S, Vinay DS, Kwon BS (2004) 4‐1BB‐dependent inhibition of immunosuppression by activated CD4+CD25+ T cells. J Leukoc Biol 75:785–791. [DOI] [PubMed] [Google Scholar]
- 48. Cobbs CS, Brenman JE, Aldape KD, Bredt DS, Israel MA (1995) Expression of nitric oxide synthase in human central nervous system tumors. Cancer Res 55:727–730. [PubMed] [Google Scholar]
- 49. Cross AH, Dolich S, Raine CS (1990) Antigen processing of myelin basic protein is required prior to recognition by T cells inducing EAE. Cell Immunol 129:22–31. [DOI] [PubMed] [Google Scholar]
- 50. Cserr HF, DePasquale M, Harling‐Berg CJ, Park JT, Knopf PM (1992) Afferent and efferent arms of the humoral immune response to CSF‐administered albumins in a rat model with normal blood‐brain barrier permeability. J Neuroimmunol 41:195–202. [DOI] [PubMed] [Google Scholar]
- 51. Curtin JF, Candolfi M, Fakhouri TM, Liu C, Alden A, Edwards M et al (2008) Treg depletion inhibits efficacy of cancer immunotherapy: implications for clinical trials. PLoS ONE 3:e1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Curtin JF, Liu N, Candolfi M, Xiong W, Assi H, Yagiz K et al (2009) HMGB1 mediates endogenous TLR2 activation and brain tumor regression. PLoS Med 6:e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Dannull J, Su Z, Rizzieri D, Yang BK, Coleman D, Yancey D et al (2005) Enhancement of vaccine‐mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J Clin Invest 115:3623–3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. De Vleeschouwer S, Fieuws S, Rutkowski S, Van Calenbergh F, Van Loon J, Goffin J et al (2008) Postoperative adjuvant dendritic cell‐based immunotherapy in patients with relapsed glioblastoma multiforme. Clin Cancer Res 14:3098–3104. [DOI] [PubMed] [Google Scholar]
- 55. De Vries IJ, Lesterhuis WJ, Scharenborg NM, Engelen LP, Ruiter DJ, Gerritsen MJ et al (2003) Maturation of dendritic cells is a prerequisite for inducing immune responses in advanced melanoma patients. Clin Cancer Res 9:5091–5100. [PubMed] [Google Scholar]
- 56. Desbaillets I, Tada M, De Tribolet N, Diserens AC, Hamou MF, Van Meir EG (1994) Human astrocytomas and glioblastomas express monocyte chemoattractant protein‐1 (MCP‐1) in vivo and in vitro . Int J Cancer 58:240–247. [DOI] [PubMed] [Google Scholar]
- 57. Desbaillets I, Diserens AC, Tribolet N, Hamou MF, Van Meir EG (1997) Upregulation of interleukin 8 by oxygen‐deprived cells in glioblastoma suggests a role in leukocyte activation, chemotaxis, and angiogenesis. J Exp Med 186:1201–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Deshpande P, King IL, Segal BM (2007) Cutting edge: CNS CD11c+ cells from mice with encephalomyelitis polarize Th17 cells and support CD25+CD4+ T cell‐mediated immunosuppression, suggesting dual roles in the disease process. J Immunol 178:6695–6699. [DOI] [PubMed] [Google Scholar]
- 59. Dillman RO, Duma CM, Schiltz PM, DePriest C, Ellis RA, Okamoto K et al (2004) Intracavitary placement of autologous lymphokine‐activated killer (LAK) cells after resection of recurrent glioblastoma. J Immunother 27:398–404. [DOI] [PubMed] [Google Scholar]
- 60. Dix AR, Brooks WH, Roszman TL, Morford LA (1999) Immune defects observed in patients with primary malignant brain tumors. J Neuroimmunol 100:216–232. [DOI] [PubMed] [Google Scholar]
- 61. Dunn GP, Dunn IF, Curry WT (2007) Focus on TILs: prognostic significance of tumor infiltrating lymphocytes in human glioma. Cancer Immun 7:12. [PMC free article] [PubMed] [Google Scholar]
- 62. El Andaloussi A, Lesniak MS (2006) An increase in CD4+CD25+FOXP3+ regulatory T cells in tumor‐infiltrating lymphocytes of human glioblastoma multiforme. Neuro-oncol 8:234–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Engelhardt B, Wolburg H (2004) Mini‐review: transendothelial migration of leukocytes: through the front door or around the side of the house? Eur J Immunol 34:2955–2963. [DOI] [PubMed] [Google Scholar]
- 64. Facoetti A, Nano R, Zelini P, Morbini P, Benericetti E, Ceroni M et al (2005) Human leukocyte antigen and antigen processing machinery component defects in astrocytic tumors. Clin Cancer Res 11:8304–8311. [DOI] [PubMed] [Google Scholar]
- 65. Fantini MC, Becker C, Monteleone G, Pallone F, Galle PR, Neurath MF (2004) Cutting edge: TGF‐beta induces a regulatory phenotype in CD4+CD25− T cells through Foxp3 induction and down‐regulation of Smad7. J Immunol 172:5149–5153. [DOI] [PubMed] [Google Scholar]
- 66. Fecci PE, Mitchell DA, Whitesides JF, Xie W, Friedman AH, Archer GE et al (2006) Increased regulatory T‐cell fraction amidst a diminished CD4 compartment explains cellular immune defects in patients with malignant glioma. Cancer Res 66:3294–3302. [DOI] [PubMed] [Google Scholar]
- 67. Fecci PE, Ochiai H, Mitchell DA, Grossi PM, Sweeney AE, Archer GE et al (2007) Systemic CTLA‐4 blockade ameliorates glioma‐induced changes to the CD4+ T cell compartment without affecting regulatory T‐cell function. Clin Cancer Res 13:2158–2167. [DOI] [PubMed] [Google Scholar]
- 68. Fernandez NC, Lozier A, Flament C, Ricciardi‐Castagnoli P, Bellet D, Suter M et al (1999) Dendritic cells directly trigger NK cell functions: cross‐talk relevant in innate anti‐tumor immune responses in vivo . Nat Med 5:405–411. [DOI] [PubMed] [Google Scholar]
- 69. Fischer HG, Bonifas U, Reichmann G (2000) Phenotype and functions of brain dendritic cells emerging during chronic infection of mice with Toxoplasma gondii. J Immunol 164:4826–4834. [DOI] [PubMed] [Google Scholar]
- 70. Fischer HG, Reichmann G (2001) Brain dendritic cells and macrophages/microglia in central nervous system inflammation. J Immunol 166:2717–2726. [DOI] [PubMed] [Google Scholar]
- 71. Fontenot JD, Gavin MA, Rudensky AY (2003) Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol 4:330–336. [DOI] [PubMed] [Google Scholar]
- 72. Fujii S, Shimizu K, Smith C, Bonifaz L, Steinman RM (2003) Activation of natural killer T cells by alpha‐galactosylceramide rapidly induces the full maturation of dendritic cells in vivo and thereby acts as an adjuvant for combined CD4 and CD8 T cell immunity to a coadministered protein. J Exp Med 198:267–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Fujita M, Zhu X, Ueda R, Sasaki K, Kohanbash G, Kastenhuber ER et al (2009) Effective immunotherapy against murine gliomas using type 1 polarizing dendritic cells–significant roles of CXCL10. Cancer Res 69:1587–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Fukumori T, Takenaka Y, Yoshii T, Kim HR, Hogan V, Inohara H et al (2003) CD29 and CD7 mediate galectin‐3‐induced type II T‐cell apoptosis. Cancer Res 63:8302–8311. [PubMed] [Google Scholar]
- 75. Galea I, Bernardes‐Silva M, Forse PA, Van Rooijen N, Liblau RS, Perry VH (2007) An antigen‐specific pathway for CD8 T cells across the blood‐brain barrier. J Exp Med 204:2023–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ghiringhelli F, Larmonier N, Schmitt E, Parcellier A, Cathelin D, Garrido C et al (2004) CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur J Immunol 34:336–344. [DOI] [PubMed] [Google Scholar]
- 77. Ghiringhelli F, Menard C, Terme M, Flament C, Taieb J, Chaput N et al (2005) CD4+CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor‐beta‐dependent manner. J Exp Med 202:1075–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ghiringhelli F, Menard C, Puig PE, Ladoire S, Roux S, Martin F et al (2007) Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother 56:641–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Gilboa E (2007) DC‐based cancer vaccines. J Clin Invest 117:1195–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Gordon LB, Knopf PM, Cserr HF (1992) Ovalbumin is more immunogenic when introduced into brain or cerebrospinal fluid than into extracerebral sites. J Neuroimmunol 40:81–87. [DOI] [PubMed] [Google Scholar]
- 81. Gorelik L, Constant S, Flavell RA (2002) Mechanism of transforming growth factor beta‐induced inhibition of T helper type 1 differentiation. J Exp Med 195:1499–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Graf MR, Sauer JT, Merchant RE (2005) Tumor infiltration by myeloid suppressor cells in response to T cell activation in rat gliomas. J Neurooncol 73:29–36. [DOI] [PubMed] [Google Scholar]
- 83. Grant RS, Naif H, Espinosa M, Kapoor V (2000) IDO induction in IFN‐gamma activated astroglia: a role in improving cell viability during oxidative stress. Redox Rep 5:101–104. [DOI] [PubMed] [Google Scholar]
- 84. Grauer O, Poschl P, Lohmeier A, Adema GJ, Bogdahn U (2007) Toll‐like receptor triggered dendritic cell maturation and IL‐12 secretion are necessary to overcome T‐cell inhibition by glioma‐associated TGF‐beta2. J Neurooncol 82:151–161. [DOI] [PubMed] [Google Scholar]
- 85. Grauer OM, Nierkens S, Bennink E, Toonen LW, Boon L, Wesseling P et al (2007) CD4+FoxP3+ regulatory T cells gradually accumulate in gliomas during tumor growth and efficiently suppress antiglioma immune responses in vivo . Int J Cancer 121:95–105. [DOI] [PubMed] [Google Scholar]
- 86. Grauer OM, Molling JW, Bennink E, Toonen LW, Sutmuller RP, Nierkens S, Adema GJ (2008) TLR ligands in the local treatment of established intracerebral murine gliomas. J Immunol 181:6720–6729. [DOI] [PubMed] [Google Scholar]
- 87. Grauer OM, Sutmuller RP, Van Maren W, Jacobs JF, Bennink E, Toonen LW et al (2008) Elimination of regulatory T cells is essential for an effective vaccination with tumor lysate‐pulsed dendritic cells in a murine glioma model. Int J Cancer 122:1794–1802. [DOI] [PubMed] [Google Scholar]
- 88. Grayson G, Ladisch S (1992) Immunosuppression by human gangliosides. II. Carbohydrate structure and inhibition of human NK activity. Cell Immunol 139:18–29. [DOI] [PubMed] [Google Scholar]
- 89. Green EA, Choi Y, Flavell RA (2002) Pancreatic lymph node‐derived CD4(+)CD25(+) Treg cells: highly potent regulators of diabetes that require TRANCE‐RANK signals. Immunity 16:183–191. [DOI] [PubMed] [Google Scholar]
- 90. Greter M, Heppner FL, Lemos MP, Odermatt BM, Goebels N, Laufer T et al (2005) Dendritic cells permit immune invasion of the CNS in an animal model of multiple sclerosis. Nat Med 11:328–334. [DOI] [PubMed] [Google Scholar]
- 91. Grouard G, Rissoan MC, Filgueira L, Durand I, Banchereau J, Liu YJ (1997) The enigmatic plasmacytoid T cells develop into dendritic cells with interleukin (IL)‐3 and CD40‐ligand. J Exp Med 185:1101–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Gunther HS, Schmidt NO, Phillips HS, Kemming D, Kharbanda S, Soriano R et al (2008) Glioblastoma‐derived stem cell‐enriched cultures form distinct subgroups according to molecular and phenotypic criteria. Oncogene 27:2897–2909. [DOI] [PubMed] [Google Scholar]
- 93. Gure AO, Chua R, Williamson B, Gonen M, Ferrera CA, Gnjatic S et al (2005) Cancer‐testis genes are coordinately expressed and are markers of poor outcome in non‐small cell lung cancer. Clin Cancer Res 11:8055–8062. [DOI] [PubMed] [Google Scholar]
- 94. Harling‐Berg CJ, Knopf PM, Cserr HF (1991) Myelin basic protein infused into cerebrospinal fluid suppresses experimental autoimmune encephalomyelitis. J Neuroimmunol 35:45–51. [DOI] [PubMed] [Google Scholar]
- 95. Harling‐Berg CJ, Park TJ, Knopf PM (1999) Role of the cervical lymphatics in the Th2‐type hierarchy of CNS immune regulation. J Neuroimmunol 101:111–127. [DOI] [PubMed] [Google Scholar]
- 96. Hatterer E, Davoust N, Didier‐Bazes M, Vuaillat C, Malcus C, Belin MF, Nataf S (2006) How to drain without lymphatics? Dendritic cells migrate from the cerebrospinal fluid to the B‐cell follicles of cervical lymph nodes. Blood 107:806–812. [DOI] [PubMed] [Google Scholar]
- 97. Hau P, Jachimczak P, Schlingensiepen R, Schulmeyer F, Jauch T, Steinbrecher A et al (2007) Inhibition of TGF‐beta2 with AP 12009 in recurrent malignant gliomas: from preclinical to phase I/II studies. Oligonucleotides 17:201–212. [DOI] [PubMed] [Google Scholar]
- 98. Hayes RL, Koslow M, Hiesiger EM, Hymes KB, Hochster HS, Moore EJ et al (1995) Improved long term survival after intracavitary interleukin‐2 and lymphokine‐activated killer cells for adults with recurrent malignant glioma. Cancer 76:840–852. [DOI] [PubMed] [Google Scholar]
- 99. Heckelsmiller K, Beck S, Rall K, Sipos B, Schlamp A, Tuma E et al (2002) Combined dendritic cell‐ and CpG oligonucleotide‐based immune therapy cures large murine tumors that resist chemotherapy. Eur J Immunol 32:3235–3245. [DOI] [PubMed] [Google Scholar]
- 100. Hegi ME, Diserens AC, Gorlia T, Hamou MF, De Tribolet N, Weller M et al (2005) MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 352:997–1003. [DOI] [PubMed] [Google Scholar]
- 101. Heimberger AB, Sun W, Hussain SF, Dey M, Crutcher L, Aldape K et al (2008) Immunological responses in a patient with glioblastoma multiforme treated with sequential courses of temozolomide and immunotherapy: case study. Neuro Oncol 10:98–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Hickey WF, Hsu BL, Kimura H (1991) T‐lymphocyte entry into the central nervous system. J Neurosci Res 28:254–260. [DOI] [PubMed] [Google Scholar]
- 103. Hochmeister S, Zeitelhofer M, Bauer J, Nicolussi EM, Fischer MT, Heinke B et al (2008) After injection into the striatum, in vitro‐differentiated microglia‐ and bone marrow‐derived dendritic cells can leave the central nervous system via the blood stream. Am J Pathol 173:1669–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Hodi FS, Oble DA, Drappatz J, Velazquez EF, Ramaiya N, Ramakrishna N et al (2008) CTLA‐4 blockade with ipilimumab induces significant clinical benefit in a female with melanoma metastases to the CNS. Nat Clin Pract Oncol 5:557–561. [DOI] [PubMed] [Google Scholar]
- 105. Hori S, Takahashi T, Sakaguchi S (2003) Control of autoimmunity by naturally arising regulatory CD4+ T cells. Adv Immunol 81:331–371. [DOI] [PubMed] [Google Scholar]
- 106. Hou DY, Muller AJ, Sharma MD, DuHadaway J, Banerjee T, Johnson M et al (2007) Inhibition of indoleamine 2,3‐dioxygenase in dendritic cells by stereoisomers of 1‐methyl‐tryptophan correlates with antitumor responses. Cancer Res 67:792–801. [DOI] [PubMed] [Google Scholar]
- 107. Hu HM, Poehlein CH, Urba WJ, Fox BA (2002) Development of antitumor immune responses in reconstituted lymphopenic hosts. Cancer Res 62:3914–3919. [PubMed] [Google Scholar]
- 108. Huehn J, Siegmund K, Hamann A (2005) Migration rules: functional properties of naive and effector/memory‐like regulatory T cell subsets. Curr Top Microbiol Immunol 293:89–114. [DOI] [PubMed] [Google Scholar]
- 109. Husain SR, Puri RK (2003) Interleukin‐13 receptor‐directed cytotoxin for malignant glioma therapy: from bench to bedside. J Neurooncol 65:37–48. [DOI] [PubMed] [Google Scholar]
- 110. Hussain SF, Yang D, Suki D, Aldape K, Grimm E, Heimberger AB (2006) The role of human glioma‐infiltrating microglia/macrophages in mediating antitumor immune responses. Neuro-oncol 8:261–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Hussain SF, Yang D, Suki D, Grimm E, Heimberger AB (2006) Innate immune functions of microglia isolated from human glioma patients. J Transl Med 4:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Hussain SF, Kong LY, Jordan J, Conrad C, Madden T, Fokt I et al (2007) A novel small molecule inhibitor of signal transducers and activators of transcription 3 reverses immune tolerance in malignant glioma patients. Cancer Res 67:9630–9636. [DOI] [PubMed] [Google Scholar]
- 113. Iellem A, Mariani M, Lang R, Recalde H, Panina‐Bordignon P, Sinigaglia F, D'Ambrosio D (2001) Unique chemotactic response profile and specific expression of chemokine receptors CCR4 and CCR8 by CD4(+)CD25(+) regulatory T cells. J Exp Med 194:847–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Imaizumi T, Kuramoto T, Matsunaga K, Shichijo S, Yutani S, Shigemori M et al (1999) Expression of the tumor‐rejection antigen SART1 in brain tumors. Int J Cancer 83:760–764. [DOI] [PubMed] [Google Scholar]
- 115. Ingram M, Buckwalter JG, Jacques DB, Freshwater DB, Abts RM, Techy GB et al (1990) Immunotherapy for recurrent malignant glioma: an interim report on survival. Neurol Res 12:265–273. [DOI] [PubMed] [Google Scholar]
- 116. Insug O, Ku G, Ertl HC, Blaszczyk‐Thurin M (2002) A dendritic cell vaccine induces protective immunity to intracranial growth of glioma. Anticancer Res 22(2A):613–621. [PubMed] [Google Scholar]
- 117. Ishii KJ, Coban C, Akira S (2005) Manifold mechanisms of toll‐like receptor‐ligand recognition. J Clin Immunol 25:511–521. [DOI] [PubMed] [Google Scholar]
- 118. Ishii N, Tada M, Sakuma S, Sawamura Y, Shinohe Y, Abe H (1998) Human astrocytoma cells are capable of producing macrophage inflammatory protein‐1beta. J Neurooncol 37:17–23. [DOI] [PubMed] [Google Scholar]
- 119. Itano AA, McSorley SJ, Reinhardt RL, Ehst BD, Ingulli E, Rudensky AY, Jenkins MK (2003) Distinct dendritic cell populations sequentially present antigen to CD4 T cells and stimulate different aspects of cell‐mediated immunity. Immunity 19:47–57. [DOI] [PubMed] [Google Scholar]
- 120. Iwamaru A, Szymanski S, Iwado E, Aoki H, Yokoyama T, Fokt I et al (2007) A novel inhibitor of the STAT3 pathway induces apoptosis in malignant glioma cells both in vitro and in vivo . Oncogene 26:2435–2444. [DOI] [PubMed] [Google Scholar]
- 121. Iwasaki A, Medzhitov R (2004) Toll‐like receptor control of the adaptive immune responses. Nat Immunol 5:987–995. [DOI] [PubMed] [Google Scholar]
- 122. Jachimczak P, Bogdahn U, Schneider J, Behl C, Meixensberger J, Apfel R et al (1993) The effect of transforming growth factor‐beta 2‐specific phosphorothioate‐anti‐sense oligodeoxynucleotides in reversing cellular immunosuppression in malignant glioma. J Neurosurg 78:944–951. [DOI] [PubMed] [Google Scholar]
- 123. Jachimczak P, Hessdorfer B, Fabel‐Schulte K, Wismeth C, Brysch W, Schlingensiepen KH et al (1996) Transforming growth factor‐beta‐mediated autocrine growth regulation of gliomas as detected with phosphorothioate antisense oligonucleotides. Int J Cancer 65:332–337. [DOI] [PubMed] [Google Scholar]
- 124. Jacobs JF, Grauer OM, Brasseur F, Hoogerbrugge PM, Wesseling P, Gidding CE et al (2008) Selective cancer‐germline gene expression in pediatric brain tumors. J Neurooncol 88:273–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Jacobs JF, Idema AJ, Bol K, Nierkens S, Grauer OM, Wesseling P et al (2008) Regulatory T cells and the PD‐L1/PD‐1 pathway mediate immune suppression in malignant human brain tumors. Neuro-Oncology Nov 21 [Epub ahead of print] DOI: 10.1215/15228517‐2008‐104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Jacobs SK, Wilson DJ, Kornblith PL, Grimm EA (1986) Interleukin‐2 or autologous lymphokine‐activated killer cell treatment of malignant glioma: phase I trial. Cancer Res 46:2101–2104. [PubMed] [Google Scholar]
- 127. Jameson SC (2002) Maintaining the norm: T‐cell homeostasis. Nat Rev Immunol 2:547–556. [DOI] [PubMed] [Google Scholar]
- 128. Janeway CA, Jr. , Medzhitov R (2002) Innate immune recognition. Annu Rev Immunol 20:197–216. [DOI] [PubMed] [Google Scholar]
- 129. Jeffes EW, 3rd , Beamer YB, Jacques S, Silberman RS, Vayuvegula B, Gupta S et al (1993) Therapy of recurrent high grade gliomas with surgery, and autologous mitogen activated IL‐2 stimulated killer (MAK) lymphocytes: I. Enhancement of MAK lytic activity and cytokine production by PHA and clinical use of PHA. J Neurooncol 15:141–155. [DOI] [PubMed] [Google Scholar]
- 130. Jonuleit H, Schmitt E, Schuler G, Knop J, Enk AH (2000) Induction of interleukin 10‐producing, nonproliferating CD4(+) T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J Exp Med 192:1213–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Jordan JT, Sun W, Hussain SF, Deangulo G, Prabhu SS, Heimberger AB (2008) Preferential migration of regulatory T cells mediated by glioma‐secreted chemokines can be blocked with chemotherapy. Cancer Immunol Immunother 57:123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Jung S, Unutmaz D, Wong P, Sano G, De los Santos K, Sparwasser T et al (2002) In vivo depletion of CD11c(+) dendritic cells abrogates priming of CD8(+) T cells by exogenous cell‐associated antigens. Immunity 17:211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Kajiwara Y, Yamasaki F, Hama S, Yahara K, Yoshioka H, Sugiyama K et al (2003) Expression of survivin in astrocytic tumors: correlation with malignant grade and prognosis. Cancer 97:1077–1083. [DOI] [PubMed] [Google Scholar]
- 134. Kalinski P, Schuitemaker JH, Hilkens CM, Wierenga EA, Kapsenberg ML (1999) Final maturation of dendritic cells is associated with impaired responsiveness to IFN‐gamma and to bacterial IL‐12 inducers: decreased ability of mature dendritic cells to produce IL‐12 during the interaction with Th cells. J Immunol 162:3231–3236. [PubMed] [Google Scholar]
- 135. Karman J, Ling C, Sandor M, Fabry Z (2004) Initiation of immune responses in brain is promoted by local dendritic cells. J Immunol 173:2353–2361. [DOI] [PubMed] [Google Scholar]
- 136. Karman J, Chu HH, Co DO, Seroogy CM, Sandor M, Fabry Z (2006) Dendritic cells amplify T cell‐mediated immune responses in the central nervous system. J Immunol 177:7750–7760. [DOI] [PubMed] [Google Scholar]
- 137. Kikuchi T, Akasaki Y, Abe T, Fukuda T, Saotome H, Ryan JL et al (2004) Vaccination of glioma patients with fusions of dendritic and glioma cells and recombinant human interleukin 12. J Immunother 27:452–459. [DOI] [PubMed] [Google Scholar]
- 138. Kim JA, Averbook BJ, Chambers K, Rothchild K, Kjaergaard J, Papay R, Shu S (2001) Divergent effects of 4‐1BB antibodies on antitumor immunity and on tumor‐reactive T‐cell generation. Cancer Res 61:2031–2037. [PubMed] [Google Scholar]
- 139. Kitahara T, Watanabe O, Yamaura A, Makino H, Watanabe T, Suzuki G, Okumura K (1987) Establishment of interleukin 2 dependent cytotoxic T lymphocyte cell line specific for autologous brain tumor and its intracranial administration for therapy of the tumor. J Neurooncol 4:329–336. [DOI] [PubMed] [Google Scholar]
- 140. Kjaergaard J, Wang LX, Kuriyama H, Shu S, Plautz GE (2005) Active immunotherapy for advanced intracranial murine tumors by using dendritic cell‐tumor cell fusion vaccines. J Neurosurg 103:156–164. [DOI] [PubMed] [Google Scholar]
- 141. Kjellman C, Olofsson SP, Hansson O, Von Schantz T, Lindvall M, Nilsson I et al (2000) Expression of TGF‐beta isoforms, TGF‐beta receptors, and SMAD molecules at different stages of human glioma. Int J Cancer 89:251–258. [DOI] [PubMed] [Google Scholar]
- 142. Konnikova L, Kotecki M, Kruger MM, Cochran BH (2003) Knockdown of STAT3 expression by RNAi induces apoptosis in astrocytoma cells. BMC Cancer 3:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB et al (2007) IL‐21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature 448:484–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Korn T, Reddy J, Gao W, Bettelli E, Awasthi A, Petersen TR et al (2007) Myelin‐specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat Med 13:423–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Kreijveld E, Koenen HJ, Klasen IS, Hilbrands LB, Joosten I (2007) Following anti‐CD25 treatment, a functional CD4+CD25+ regulatory T‐cell pool is present in renal transplant recipients. Am J Transplant 7:249–255. [DOI] [PubMed] [Google Scholar]
- 146. Kreitman RJ (2004) Recombinant immunotoxins for the treatment of haematological malignancies. Expert Opin Biol Ther 4:1115–1128. [DOI] [PubMed] [Google Scholar]
- 147. Kruse CA, Cepeda L, Owens B, Johnson SD, Stears J, Lillehei KO (1997) Treatment of recurrent glioma with intracavitary alloreactive cytotoxic T lymphocytes and interleukin‐2. Cancer Immunol Immunother 45:77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Kryczek I, Wei S, Vatan L, Escara‐Wilke J, Szeliga W, Keller ET, Zou W (2007) Cutting edge: opposite effects of IL‐1 and IL‐2 on the regulation of IL‐17+ T cell pool IL‐1 subverts IL‐2‐mediated suppression. J Immunol 179:1423–1426. [DOI] [PubMed] [Google Scholar]
- 149. Kumar H, Kawai T, Akira S (2009) Pathogen recognition in the innate immune response. Biochem J 420:1–16. [DOI] [PubMed] [Google Scholar]
- 150. Kunwar S (2003) Convection enhanced delivery of IL13‐PE38QQR for treatment of recurrent malignant glioma: presentation of interim findings from ongoing phase 1 studies. Acta Neurochir Suppl 88:105–111. [DOI] [PubMed] [Google Scholar]
- 151. Kuramoto T (1997) Detection of MAGE‐1 tumor antigen in brain tumor. Kurume Med J 44:43–51. [DOI] [PubMed] [Google Scholar]
- 152. Kuwashima N, Nishimura F, Eguchi J, Sato H, Hatano M, Tsugawa T et al (2005) Delivery of dendritic cells engineered to secrete IFN‐alpha into central nervous system tumors enhances the efficacy of peripheral tumor cell vaccines: dependence on apoptotic pathways. J Immunol 175:2730–2740. [DOI] [PubMed] [Google Scholar]
- 153. Lake RA, Robinson BW (2005) Immunotherapy and chemotherapy—a practical partnership. Nat Rev Cancer 5:397–405. [DOI] [PubMed] [Google Scholar]
- 154. Lee HK, Iwasaki A (2007) Innate control of adaptive immunity: dendritic cells and beyond. Semin Immunol 19:48–55. [DOI] [PubMed] [Google Scholar]
- 155. Lee J, Kotliarova S, Kotliarov Y, Li A, Su Q, Donin NM et al (2006) Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum‐cultured cell lines. Cancer Cell 9:391–403. [DOI] [PubMed] [Google Scholar]
- 156. Liau LM, Prins RM, Kiertscher SM, Odesa SK, Kremen TJ, Giovannone AJ et al (2005) Dendritic cell vaccination in glioblastoma patients induces systemic and intracranial T‐cell responses modulated by the local central nervous system tumor microenvironment. Clin Cancer Res 11:5515–5525. [DOI] [PubMed] [Google Scholar]
- 157. Ling C, Sandor M, Fabry Z (2003) In situ processing and distribution of intracerebrally injected OVA in the CNS. J Neuroimmunol 141:90–98. [DOI] [PubMed] [Google Scholar]
- 158. Liu G, Ying H, Zeng G, Wheeler CJ, Black KL, Yu JS (2004) HER‐2, gp100, and MAGE‐1 are expressed in human glioblastoma and recognized by cytotoxic T cells. Cancer Res 64:4980–4986. [DOI] [PubMed] [Google Scholar]
- 159. Liu K, Iyoda T, Saternus M, Kimura Y, Inaba K, Steinman RM (2002) Immune tolerance after delivery of dying cells to dendritic cells in situ . J Exp Med 196:1091–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Liu K, Idoyaga J, Charalambous A, Fujii S, Bonito A, Mordoh J et al (2005) Innate NKT lymphocytes confer superior adaptive immunity via tumor‐capturing dendritic cells. J Exp Med 202:1507–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Liu Y, Wang Q, Kleinschmidt‐DeMasters BK, Franzusoff A, Ng KY, Lillehei KO (2007) TGF‐beta2 inhibition augments the effect of tumor vaccine and improves the survival of animals with pre‐established brain tumors. J Neurooncol 81:149–162. [DOI] [PubMed] [Google Scholar]
- 162. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A et al (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol (Berl) 114:97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Mackall CL, Bare CV, Granger LA, Sharrow SO, Titus JA, Gress RE (1996) Thymic‐independent T cell regeneration occurs via antigen‐driven expansion of peripheral T cells resulting in a repertoire that is limited in diversity and prone to skewing. J Immunol 156:4609–4616. [PubMed] [Google Scholar]
- 164. Maes W, Rosas GG, Verbinnen B, Boon L, De Vleeschouwer S, Ceuppens JL, Van Gool SW (2009) DC vaccination with anti‐CD25 treatment leads to long‐term immunity against experimental glioma. Neuro Oncol March 31 [Epub ahead of print] DOI: 10.1215/15228517‐2009‐004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Mahnke K, Schonfeld K, Fondel S, Ring S, Karakhanova S, Wiedemeyer K et al (2007) Depletion of CD4+CD25+ human regulatory T cells in vivo: kinetics of Treg depletion and alterations in immune functions in vivo and in vitro . Int J Cancer 120:2723–2733. [DOI] [PubMed] [Google Scholar]
- 166. Mailliard RB, Wankowicz‐Kalinska A, Cai Q, Wesa A, Hilkens CM, Kapsenberg ML et al (2004) alpha‐type‐1 polarized dendritic cells: a novel immunization tool with optimized CTL‐inducing activity. Cancer Res 64:5934–5937. [DOI] [PubMed] [Google Scholar]
- 167. MartIn‐Fontecha A, Sebastiani S, Hopken UE, Uguccioni M, Lipp M, Lanzavecchia A, Sallusto F (2003) Regulation of dendritic cell migration to the draining lymph node: impact on T lymphocyte traffic and priming. J Exp Med 198:615–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Masson F, Calzascia T, Di Berardino‐Besson W, De Tribolet N, Dietrich PY, Walker PR (2007) Brain microenvironment promotes the final functional maturation of tumor‐specific effector CD8+ T cells. J Immunol 179:845–853. [DOI] [PubMed] [Google Scholar]
- 169. Matyszak MK, Perry VH (1996) The potential role of dendritic cells in immune‐mediated inflammatory diseases in the central nervous system. Neuroscience 74:599–608. [DOI] [PubMed] [Google Scholar]
- 170. McHugh RS, Whitters MJ, Piccirillo CA, Young DA, Shevach EM, Collins M, Byrne MC (2002) CD4(+)CD25(+) immunoregulatory T cells: gene expression analysis reveals a functional role for the glucocorticoid‐induced TNF receptor. Immunity 16:311–323. [DOI] [PubMed] [Google Scholar]
- 171. McMahon EJ, Bailey SL, Castenada CV, Waldner H, Miller SD (2005) Epitope spreading initiates in the CNS in two mouse models of multiple sclerosis. Nat Med 11:335–339. [DOI] [PubMed] [Google Scholar]
- 172. McMahon EJ, Bailey SL, Miller SD (2006) CNS dendritic cells: critical participants in CNS inflammation? Neurochem Int 49:195–203. [DOI] [PubMed] [Google Scholar]
- 173. Mehling M, Simon P, Mittelbronn M, Meyermann R, Ferrone S, Weller M, Wiendl H (2007) WHO grade associated downregulation of MHC class I antigen‐processing machinery components in human astrocytomas: does it reflect a potential immune escape mechanism? Acta Neuropathol 114:111–119. [DOI] [PubMed] [Google Scholar]
- 174. Meng Y, Carpentier AF, Chen L, Boisserie G, Simon JM, Mazeron JJ, Delattre JY (2005) Successful combination of local CpG‐ODN and radiotherapy in malignant glioma. Int J Cancer 116:992–997. [DOI] [PubMed] [Google Scholar]
- 175. Miller SD, McMahon EJ, Schreiner B, Bailey SL (2007) Antigen presentation in the CNS by myeloid dendritic cells drives progression of relapsing experimental autoimmune encephalomyelitis. Ann N Y Acad Sci 1103:179–191. [DOI] [PubMed] [Google Scholar]
- 176. Mitchell DA, Xie W, Schmittling R, Learn C, Friedman A, McLendon RE, Sampson JH (2008) Sensitive detection of human cytomegalovirus in tumors and peripheral blood of patients diagnosed with glioblastoma. Neuro Oncol 10:10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Miyazaki T, Moritake K, Yamada K, Hara N, Osago H, Shibata T et al (2009) Indoleamine 2,3‐dioxygenase as a new target for malignant glioma therapy. J Neurosurg Jan 23 [Epub ahead of print] DOI: 10.3171/2008.10.JNS081141. [DOI] [PubMed] [Google Scholar]
- 178. Mottet C, Golshayan D (2007) CD4+CD25+Foxp3+ regulatory T cells: from basic research to potential therapeutic use. Swiss Med Wkly 137:625–634. [DOI] [PubMed] [Google Scholar]
- 179. Munn DH, Sharma MD, Lee JR, Jhaver KG, Johnson TS, Keskin DB et al (2002) Potential regulatory function of human dendritic cells expressing indoleamine 2,3‐dioxygenase. Science 297:1867–1870. [DOI] [PubMed] [Google Scholar]
- 180. Murayama K, Kobayashi T, Imaizumi T, Matsunaga K, Kuramoto T, Shigemori M et al (2000) Expression of the SART3 tumor‐rejection antigen in brain tumors and induction of cytotoxic T lymphocytes by its peptides. J Immunother 23:511–518. [DOI] [PubMed] [Google Scholar]
- 181. Nair S, McLaughlin C, Weizer A, Su Z, Boczkowski D, Dannull J et al (2003) Injection of immature dendritic cells into adjuvant‐treated skin obviates the need for ex vivo maturation. J Immunol 171:6275–6282. [DOI] [PubMed] [Google Scholar]
- 182. Nam DH, Park K, Park C, Im YH, Kim MH, Lee S et al (2004) Intracranial inhibition of glioma cell growth by cyclooxygenase‐2 inhibitor celecoxib. Oncol Rep 11:263–268. [PubMed] [Google Scholar]
- 183. Napolitani G, Rinaldi A, Bertoni F, Sallusto F, Lanzavecchia A (2005) Selected Toll‐like receptor agonist combinations synergistically trigger a T helper type 1‐polarizing program in dendritic cells. Nat Immunol 6:769–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Newcomb EW, Demaria S, Lukyanov Y, Shao Y, Schnee T, Kawashima N et al (2006) The combination of ionizing radiation and peripheral vaccination produces long‐term survival of mice bearing established invasive GL261 gliomas. Clin Cancer Res 12:4730–4737. [DOI] [PubMed] [Google Scholar]
- 185. Nitta T, Hishii M, Sato K, Okumura K (1994) Selective expression of interleukin‐10 gene within glioblastoma multiforme. Brain Res 649:122–128. [DOI] [PubMed] [Google Scholar]
- 186. Njoku C, Self SE, Ruiz P, Hofbauer AF, Gilkeson GS, Oates JC (2008) Inducible nitric oxide synthase inhibitor SD‐3651 reduces proteinuria in mrl/lpr mice deficient in the nos2 gene. J Investig Med 56:911–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Nonaka Y, Tsuda N, Shichijo S, Ito M, Maeda Y, Harada M et al (2002) Recognition of ADP‐ribosylation factor 4‐like by HLA‐A2‐restricted and tumor‐reactive cytotoxic T lymphocytes from patients with brain tumors. Tissue Antigens 60:319–327. [DOI] [PubMed] [Google Scholar]
- 188. North RJ (1984) Gamma‐irradiation facilitates the expression of adoptive immunity against established tumors by eliminating suppressor T cells. Cancer Immunol Immunother 16:175–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Nummer D, Suri‐Payer E, Schmitz‐Winnenthal H, Bonertz A, Galindo L, Antolovich D et al (2007) Role of tumor endothelium in CD4+ CD25+ regulatory T cell infiltration of human pancreatic carcinoma. J Natl Cancer Inst 99:1188–1199. [DOI] [PubMed] [Google Scholar]
- 190. O'Connor RA, Malpass KH, Anderton SM (2007) The inflamed central nervous system drives the activation and rapid proliferation of Foxp3+ regulatory T cells. J Immunol 179:958–966. [DOI] [PubMed] [Google Scholar]
- 191. O'Garra A (1998) Cytokines induce the development of functionally heterogeneous T helper cell subsets. Immunity 8:275–283. [DOI] [PubMed] [Google Scholar]
- 192. Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL et al (2007) Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med 13:54–61. [DOI] [PubMed] [Google Scholar]
- 193. Okada H, Lieberman FS, Edington HD, Witham TF, Wargo MJ, Cai Q et al (2003) Autologous glioma cell vaccine admixed with interleukin‐4 gene transfected fibroblasts in the treatment of recurrent glioblastoma: preliminary observations in a patient with a favorable response to therapy. J Neurooncol 64:13–20. [DOI] [PubMed] [Google Scholar]
- 194. Okada H, Tsugawa T, Sato H, Kuwashima N, Gambotto A, Okada K et al (2004) Delivery of interferon‐alpha transfected dendritic cells into central nervous system tumors enhances the antitumor efficacy of peripheral peptide‐based vaccines. Cancer Res 64:5830–5838. [DOI] [PubMed] [Google Scholar]
- 195. Olson JK, Miller SD (2004) Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J Immunol 173:3916–3924. [DOI] [PubMed] [Google Scholar]
- 196. Pallini R, Ricci‐Vitiani L, Banna GL, Signore M, Lombardi D, Todaro M et al (2008) Cancer stem cell analysis and clinical outcome in patients with glioblastoma multiforme. Clin Cancer Res 14:8205–8212. [DOI] [PubMed] [Google Scholar]
- 197. Pashenkov M, Huang YM, Kostulas V, Haglund M, Soderstrom M, Link H (2001) Two subsets of dendritic cells are present in human cerebrospinal fluid. Brain 124:480–492. [DOI] [PubMed] [Google Scholar]
- 198. Pashenkov M, Teleshova N, Link H (2003) Inflammation in the central nervous system: the role for dendritic cells. Brain Pathol 13:23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Perrin G, Schnuriger V, Quiquerez AL, Saas P, Pannetier C, De Tribolet N et al (1999) Astrocytoma infiltrating lymphocytes include major T cell clonal expansions confined to the CD8 subset. Int Immunol 11:1337–1350. [DOI] [PubMed] [Google Scholar]
- 200. Plautz GE, Miller DW, Barnett GH, Stevens GH, Maffett S, Kim J et al (2000) T cell adoptive immunotherapy of newly diagnosed gliomas. Clin Cancer Res 6:2209–2218. [PubMed] [Google Scholar]
- 201. Poeck H, Besch R, Maihoefer C, Renn M, Tormo D, Morskaya SS et al (2008) 5′‐Triphosphate‐siRNA: turning gene silencing and Rig‐I activation against melanoma. Nat Med 14:1256–1263. [DOI] [PubMed] [Google Scholar]
- 202. Powell DJ, Jr. , Felipe‐Silva A, Merino MJ, Ahmadzadeh M, Allen T, Levy C et al (2007) Administration of a CD25‐directed immunotoxin, LMB‐2, to patients with metastatic melanoma induces a selective partial reduction in regulatory T cells in vivo . J Immunol 179:4919–4928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Prins RM, Scott GP, Merchant RE, Graf MR (2002) Irradiated tumor cell vaccine for treatment of an established glioma. II. Expansion of myeloid suppressor cells that promote tumor progression. Cancer Immunol Immunother 51:190–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Prins RM, Craft N, Bruhn KW, Khan‐Farooqi H, Koya RC, Stripecke R et al (2006) The TLR‐7 agonist, imiquimod, enhances dendritic cell survival and promotes tumor antigen‐specific T cell priming: relation to central nervous system antitumor immunity. J Immunol 176:157–164. [DOI] [PubMed] [Google Scholar]
- 205. Prins RM, Shu CJ, Radu CG, Vo DD, Khan‐Farooqi H, Soto H et al (2008) Anti‐tumor activity and trafficking of self, tumor‐specific T cells against tumors located in the brain. Cancer Immunol Immunother 57:1279–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Quattrocchi KB, Miller CH, Cush S, Bernard SA, Dull ST, Smith M et al (1999) Pilot study of local autologous tumor infiltrating lymphocytes for the treatment of recurrent malignant gliomas. J Neurooncol 45:141–157. [DOI] [PubMed] [Google Scholar]
- 207. Rahaman SO, Vogelbaum MA, Haque SJ (2005) Aberrant Stat3 signaling by interleukin‐4 in malignant glioma cells: involvement of IL‐13Ralpha2. Cancer Res 65:2956–2963. [DOI] [PubMed] [Google Scholar]
- 208. Ransohoff RM, Kivisakk P, Kidd G (2003) Three or more routes for leukocyte migration into the central nervous system. Nat Rev Immunol 3:569–581. [DOI] [PubMed] [Google Scholar]
- 209. Rapoport AP, Stadtmauer EA, Aqui N, Badros A, Cotte J, Chrisley L et al (2005) Restoration of immunity in lymphopenic individuals with cancer by vaccination and adoptive T‐cell transfer. Nat Med 11:1230–1237. [DOI] [PubMed] [Google Scholar]
- 210. Recht L, Torres CO, Smith TW, Raso V, Griffin TW (1990) Transferrin receptor in normal and neoplastic brain tissue: implications for brain‐tumor immunotherapy. J Neurosurg 72:941–945. [DOI] [PubMed] [Google Scholar]
- 211. Redjal N, Chan JA, Segal RA, Kung AL (2006) CXCR4 inhibition synergizes with cytotoxic chemotherapy in gliomas. Clin Cancer Res 12:6765–6771. [DOI] [PubMed] [Google Scholar]
- 212. Reis e Sousa C (2004) Toll‐like receptors and dendritic cells: for whom the bug tolls. Semin Immunol 16:27–34. [DOI] [PubMed] [Google Scholar]
- 213. Reis e Sousa C, Yap G, Schulz O, Rogers N, Schito M, Aliberti J et al (1999) Paralysis of dendritic cell IL‐12 production by microbial products prevents infection‐induced immunopathology. Immunity 11:637–647. [DOI] [PubMed] [Google Scholar]
- 214. Rodriguez PC, Quiceno DG, Zabaleta J, Ortiz B, Zea AH, Piazuelo MB et al (2004) Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T‐cell receptor expression and antigen‐specific T‐cell responses. Cancer Res 64:5839–5849. [DOI] [PubMed] [Google Scholar]
- 215. Rodriguez PC, Hernandez CP, Quiceno D, Dubinett SM, Zabaleta J, Ochoa JB et al (2005) Arginase I in myeloid suppressor cells is induced by COX‐2 in lung carcinoma. J Exp Med 202:931–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Roggendorf W, Strupp S, Paulus W (1996) Distribution and characterization of microglia/macrophages in human brain tumors. Acta Neuropathol 92:288–293. [DOI] [PubMed] [Google Scholar]
- 217. Rossi ML, Hughes JT, Esiri MM, Coakham HB, Brownell DB (1987) Immunohistological study of mononuclear cell infiltrate in malignant gliomas. Acta Neuropathol 74:269–277. [DOI] [PubMed] [Google Scholar]
- 218. Rubinstein N, Alvarez M, Zwirner NW, Toscano MA, Ilarregui JM, Bravo A et al (2004) Targeted inhibition of galectin‐1 gene expression in tumor cells results in heightened T cell‐mediated rejection; A potential mechanism of tumor‐immune privilege. Cancer Cell 5:241–251. [DOI] [PubMed] [Google Scholar]
- 219. Rutkowski S, De Vleeschouwer S, Kaempgen E, Wolff JE, Kuhl J, Demaerel P et al (2004) Surgery and adjuvant dendritic cell‐based tumour vaccination for patients with relapsed malignant glioma, a feasibility study. Br J Cancer 91:1656–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Safdari H, Hochberg FH, Richardson EP, Jr. (1979) Histological correlations with survival in malignant gliomas. Acta Neurochir Suppl (Wien) 28:485–488. [PubMed] [Google Scholar]
- 221. Safdari H, Hochberg FH, Richardson EP, Jr. (1985) Prognostic value of round cell (lymphocyte) infiltration in malignant gliomas. Surg Neurol 23:221–226. [DOI] [PubMed] [Google Scholar]
- 222. Sahin U, Koslowski M, Tureci O, Eberle T, Zwick C, Romeike B et al (2000) Expression of cancer testis genes in human brain tumors. ClinCancer Res 6:3916–3922. [PubMed] [Google Scholar]
- 223. Sakaguchi S (2004) Naturally arising CD4+ regulatory T cells for immunologic self‐tolerance and negative control of immune responses. Annu Rev Immunol 22:531–562. [DOI] [PubMed] [Google Scholar]
- 224. Sakaguchi S (2005) Naturally arising Foxp3‐expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non‐self. Nat Immunol 6:345–352. [DOI] [PubMed] [Google Scholar]
- 225. Sakaguchi S (2008) Regulatory T cells in the past and for the future. Eur J Immunol 38:901–937. [DOI] [PubMed] [Google Scholar]
- 226. Sampson JH, Archer GE, Mitchell DA, Heimberger AB, Bigner DD (2008) Tumor‐specific immunotherapy targeting the EGFRvIII mutation in patients with malignant glioma. Semin Immunol 20:267–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Santambrogio L, Belyanskaya SL, Fischer FR, Cipriani B, Brosnan CF, Ricciardi‐Castagnoli P et al (2001) Developmental plasticity of CNS microglia. Proc Natl Acad Sci U S A 98:6295–6300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Sasaki A, Nakazato Y, Ogawa A, Sugihara S (1996) The immunophenotype of perivascular cells in the human brain. Pathol Int 46:15–23. [DOI] [PubMed] [Google Scholar]
- 229. Sasaki M, Nakahira K, Kawano Y, Katakura H, Yoshimine T, Shimizu K et al (2001) MAGE‐E1, a new member of the melanoma‐associated antigen gene family and its expression in human glioma. Cancer Res 61:4809–4814. [PubMed] [Google Scholar]
- 230. Scarcella DL, Chow CW, Gonzales MF, Economou C, Brasseur F, Ashley DM (1999) Expression of MAGE and GAGE in high‐grade brain tumors: a potential target for specific immunotherapy and diagnostic markers. Clin Cancer Res 5:335–341. [PubMed] [Google Scholar]
- 231. Scheel B, Aulwurm S, Probst J, Stitz L, Hoerr I, Rammensee HG et al (2006) Therapeutic anti‐tumor immunity triggered by injections of immunostimulating single‐stranded RNA. Eur J Immunol 36:2807–2816. [DOI] [PubMed] [Google Scholar]
- 232. Schlingensiepen KH, Schlingensiepen R, Steinbrecher A, Hau P, Bogdahn U, Fischer‐Blass B, Jachimczak P (2006) Targeted tumor therapy with the TGF‐beta2 antisense compound AP 12009. Cytokine Growth Factor Rev 17:129–139. [DOI] [PubMed] [Google Scholar]
- 233. Schoenberger SP, Toes RE, Van Der Voort EI, Offringa R, Melief CJ (1998) T‐cell help for cytotoxic T lymphocytes is mediated by CD40‐CD40L interactions. Nature 393:480–483. [DOI] [PubMed] [Google Scholar]
- 234. Schonthal AH (2006) Antitumor properties of dimethyl‐celecoxib, a derivative of celecoxib that does not inhibit cyclooxygenase‐2: implications for glioma therapy. Neurosurg Focus 20:E21. [DOI] [PubMed] [Google Scholar]
- 235. Serafini B, Columba‐Cabezas S, Di Rosa F, Aloisi F (2000) Intracerebral recruitment and maturation of dendritic cells in the onset and progression of experimental autoimmune encephalomyelitis. Am J Pathol 157:1991–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Serot JM, Bene MC, Foliguet B, Faure GC (2000) Monocyte‐derived IL‐10‐secreting dendritic cells in choroid plexus epithelium. J Neuroimmunol 105:115–119. [DOI] [PubMed] [Google Scholar]
- 237. Shimizu J, Yamazaki S, Takahashi T, Ishida Y, Sakaguchi S (2002) Stimulation of CD25(+)CD4(+) regulatory T cells through GITR breaks immunological self‐tolerance. Nat Immunol 3:135–142. [DOI] [PubMed] [Google Scholar]
- 238. Shortman K, Naik SH (2007) Steady‐state and inflammatory dendritic‐cell development. Nat Rev Immunol 7:19–30. [DOI] [PubMed] [Google Scholar]
- 239. Shurin GV, Shurin MR, Bykovskaia S, Shogan J, Lotze MT, Barksdale EM, Jr. (2001) Neuroblastoma‐derived gangliosides inhibit dendritic cell generation and function. Cancer Res 61:363–369. [PubMed] [Google Scholar]
- 240. Siegmund K, Feuerer M, Siewert C, Ghani S, Haubold U, Dankof A et al (2005) Migration matters: regulatory T‐cell compartmentalization determines suppressive activity in vivo . Blood 106:3097–3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Sombroek CC, Stam AG, Masterson AJ, Lougheed SM, Schakel MJ, Meijer CJ et al (2002) Prostanoids play a major role in the primary tumor‐induced inhibition of dendritic cell differentiation. J Immunol 168:4333–4343. [DOI] [PubMed] [Google Scholar]
- 242. Soumelis V, Liu YJ (2006) From plasmacytoid to dendritic cell: morphological and functional switches during plasmacytoid pre‐dendritic cell differentiation. Eur J Immunol 36:2286–2292. [DOI] [PubMed] [Google Scholar]
- 243. Speetjens FM, Kuppen PJ, Welters MJ, Essahsah F, Voet van den Brink AM, Lantrua MG et al (2009) Induction of p53‐specific immunity by a p53 synthetic long peptide vaccine in patients treated for metastatic colorectal cancer. Clin Cancer Res 15:1086–1095. [DOI] [PubMed] [Google Scholar]
- 244. Sporri R, Reis e Sousa C (2005) Inflammatory mediators are insufficient for full dendritic cell activation and promote expansion of CD4+ T cell populations lacking helper function. Nat Immunol 6:163–170. [DOI] [PubMed] [Google Scholar]
- 245. Steiner HH, Bonsanto MM, Beckhove P, Brysch M, Geletneky K, Ahmadi R et al (2004) Antitumor vaccination of patients with glioblastoma multiforme: a pilot study to assess feasibility, safety, and clinical benefit. J Clin Oncol 22:4272–4281. [DOI] [PubMed] [Google Scholar]
- 246. Steinman RM (2006) Linking innate to adaptive immunity through dendritic cells. Novartis Found Symp 279:101–109. discussion 9–13, 216–9. [PubMed] [Google Scholar]
- 247. Steinman RM, Hawiger D, Liu K, Bonifaz L, Bonnyay D, Mahnke K et al (2003) Dendritic cell function in vivo during the steady state: a role in peripheral tolerance. Ann N Y Acad Sci 987:15–25. [DOI] [PubMed] [Google Scholar]
- 248. Stevenson PG, Hawke S, Sloan DJ, Bangham CR (1997) The immunogenicity of intracerebral virus infection depends on anatomical site. J Virol 71:145–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Stupp R, Mason WP, Van Den Bent MJ, Weller M, Fisher B, Taphoorn MJ et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996. [DOI] [PubMed] [Google Scholar]
- 250. Stupp R, Hegi ME, Mason WP, Van Den Bent MJ, Taphoorn MJ, Janzer RC et al (2009) Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5‐year analysis of the EORTC‐NCIC trial. Lancet Oncol 10:459–466. [DOI] [PubMed] [Google Scholar]
- 251. Su YB, Sohn S, Krown SE, Livingston PO, Wolchok JD, Quinn C et al (2004) Selective CD4+ lymphopenia in melanoma patients treated with temozolomide: a toxicity with therapeutic implications. J Clin Oncol 22:610–616. [DOI] [PubMed] [Google Scholar]
- 252. Tabatabai G, Bahr O, Mohle R, Eyupoglu IY, Boehmler AM, Wischhusen J et al (2005) Lessons from the bone marrow: how malignant glioma cells attract adult haematopoietic progenitor cells. Brain 128:2200–2211. [DOI] [PubMed] [Google Scholar]
- 253. Tabatabai G, Frank B, Mohle R, Weller M, Wick W (2006) Irradiation and hypoxia promote homing of haematopoietic progenitor cells towards gliomas by TGF‐beta‐dependent HIF‐1alpha‐mediated induction of CXCL12. Brain 129:2426–2435. [DOI] [PubMed] [Google Scholar]
- 254. Takeda K, Kaisho T, Akira S (2003) Toll‐like receptors. Annu Rev Immunol 21:335–376. [DOI] [PubMed] [Google Scholar]
- 255. Tran TT, Uhl M, Ma JY, Janssen L, Sriram V, Aulwurm S et al (2007) Inhibiting TGF‐beta signaling restores immune surveillance in the SMA‐560 glioma model. Neuro Oncol 9:259–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Trinchieri G (1993) Interleukin‐12 and its role in the generation of TH1 cells. Immunol Today 14:335–338. [DOI] [PubMed] [Google Scholar]
- 257. Tsuda N, Nonaka Y, Shichijo S, Yamada A, Ito M, Maeda Y et al (2002) UDP‐Gal: betaGlcNAc beta1, 3‐galactosyltransferase, polypeptide 3 (GALT3) is a tumour antigen recognised by HLA‐A2‐restricted cytotoxic T lymphocytes from patients with brain tumour. Br J Cancer 87:1006–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Tsugawa T, Kuwashima N, Sato H, Fellows‐Mayle WK, Dusak JE, Okada K et al (2004) Sequential delivery of interferon‐alpha gene and DCs to intracranial gliomas promotes an effective antitumor response. Gene Ther 11:1551–1558. [DOI] [PubMed] [Google Scholar]
- 259. Tuyaerts S, Aerts JL, Corthals J, Neyns B, Heirman C, Breckpot K et al (2007) Current approaches in dendritic cell generation and future implications for cancer immunotherapy. Cancer Immunol Immunother 56:1513–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Uccelli A, Pistoia V, Moretta L (2007) Mesenchymal stem cells: a new strategy for immunosuppression? Trends Immunol 28:219–226. [DOI] [PubMed] [Google Scholar]
- 261. Ueda R, Iizuka Y, Yoshida K, Kawase T, Kawakami Y, Toda M (2004) Identification of a human glioma antigen, SOX6, recognized by patients' sera. Oncogene 23:1420–1427. [DOI] [PubMed] [Google Scholar]
- 262. Uhl M, Aulwurm S, Wischhusen J, Weiler M, Ma JY, Almirez R et al (2004) SD‐208, a novel transforming growth factor beta receptor I kinase inhibitor, inhibits growth and invasiveness and enhances immunogenicity of murine and human glioma cells in vitro and in vivo . Cancer Res 64:7954–7961. [DOI] [PubMed] [Google Scholar]
- 263. Umemura N, Saio M, Suwa T, Kitoh Y, Bai J, Nonaka K et al (2008) Tumor‐infiltrating myeloid‐derived suppressor cells are pleiotropic‐inflamed monocytes/macrophages that bear M1‐ and M2‐type characteristics. J Leukoc Biol 83:1136–1144. [DOI] [PubMed] [Google Scholar]
- 264. Vajkoczy P, Laschinger M, Engelhardt B (2001) Alpha4‐integrin‐VCAM‐1 binding mediates G protein‐independent capture of encephalitogenic T cell blasts to CNS white matter microvessels. J Clin Invest 108:557–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Valzasina B, Guiducci C, Dislich H, Killeen N, Weinberg AD, Colombo MP (2005) Triggering of OX40 (CD134) on CD4(+)CD25+ T cells blocks their inhibitory activity: a novel regulatory role for OX40 and its comparison with GITR. Blood 105:2845–2851. [DOI] [PubMed] [Google Scholar]
- 266. Van Der Most RG, Currie A, Robinson BW, Lake RA (2006) Cranking the immunologic engine with chemotherapy: using context to drive tumor antigen cross‐presentation towards useful antitumor immunity. Cancer Res 66:601–604. [DOI] [PubMed] [Google Scholar]
- 267. Van Gool SW, Maes M, Ardon H, Verschuere T, De Vleeschouwer S (2009) Dendritic cell therapy of gliomas. Brain Pathol 19:694–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Van Kooyk Y (2008) C‐type lectins on dendritic cells: key modulators for the induction of immune responses. Biochem Soc Trans 36:1478–1481. [DOI] [PubMed] [Google Scholar]
- 269. Vicari AP, Chiodoni C, Vaure C, Ait‐Yahia S, Dercamp C, Matsos F et al (2002) Reversal of tumor‐induced dendritic cell paralysis by CpG immunostimulatory oligonucleotide and anti‐interleukin 10 receptor antibody. J Exp Med 196:541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Villadangos JA, Schnorrer P (2007) Intrinsic and cooperative antigen‐presenting functions of dendritic‐cell subsets in vivo . Nat Rev Immunol 7:543–555. [DOI] [PubMed] [Google Scholar]
- 271. Vlad G, Ho EK, Vasilescu ER, Fan J, Liu Z, Cai JW et al (2007) Anti‐CD25 treatment and FOXP3‐positive regulatory T cells in heart transplantation. Transpl Immunol 18:13–21. [DOI] [PubMed] [Google Scholar]
- 272. Von Hanwehr RI, Hofman FM, Taylor CR, Apuzzo ML (1984) Mononuclear lymphoid populations infiltrating the microenvironment of primary CNS tumors. Characterization of cell subsets with monoclonal antibodies. J Neurosurg 60:1138–1147. [DOI] [PubMed] [Google Scholar]
- 273. Wakkach A, Fournier N, Brun V, Breittmayer JP, Cottrez F, Groux H (2003) Characterization of dendritic cells that induce tolerance and T regulatory 1 cell differentiation in vivo . Immunity 18:605–617. [DOI] [PubMed] [Google Scholar]
- 274. Walker PR, Calzascia T, Dietrich PY (2002) All in the head: obstacles for immune rejection of brain tumours. Immunology 107:28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Walker PR, Calzascia T, De Tribolet N, Dietrich PY (2003) T‐cell immune responses in the brain and their relevance for cerebral malignancies. Brain Res Brain Res Rev 42:97–122. [DOI] [PubMed] [Google Scholar]
- 276. Warger T, Osterloh P, Rechtsteiner G, Fassbender M, Heib V, Schmid B et al (2006) Synergistic activation of dendritic cells by combined Toll‐like receptor ligation induces superior CTL responses in vivo . Blood 108:544–550. [DOI] [PubMed] [Google Scholar]
- 277. Weaver M, Laske DW (2003) Transferrin receptor ligand‐targeted toxin conjugate (Tf‐CRM107) for therapy of malignant gliomas. J Neurooncol 65:3–13. [DOI] [PubMed] [Google Scholar]
- 278. Wei J, Duramad O, Perng OA, Reiner SL, Liu YJ, Qin FX (2007) Antagonistic nature of T helper 1/2 developmental programs in opposing peripheral induction of Foxp3+ regulatory T cells. Proc Natl Acad Sci U S A 104:18169–18174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Wekerle H (1993) T‐cell autoimmunity in the central nervous system. Intervirology 35:95–100. [DOI] [PubMed] [Google Scholar]
- 280. Wekerle H (2006) Breaking ignorance: the case of the brain. Curr Top Microbiol Immunol 305:25–50. [DOI] [PubMed] [Google Scholar]
- 281. Wekerle H, Lassmann H, Linington C, Meyermann R (1986) Cellular immune reactivity within the CNS. Trends Neurosci 9:271–277. [Google Scholar]
- 282. Weller RO, Djuanda E, Yow HY, Carare RO (2009) Lymphatic drainage of the brain and the pathophysiology of neurological disease. Acta Neuropathol 117:1–14. [DOI] [PubMed] [Google Scholar]
- 283. Wenkel H, Streilein JW, Young MJ (2000) Systemic immune deviation in the brain that does not depend on the integrity of the blood‐brain barrier. J Immunol 164:5125–5131. [DOI] [PubMed] [Google Scholar]
- 284. Wheeler CJ, Das A, Liu G, Yu JS, Black KL (2004) Clinical responsiveness of glioblastoma multiforme to chemotherapy after vaccination. Clin Cancer Res 10:5316–5326. [DOI] [PubMed] [Google Scholar]
- 285. Wheeler CJ, Black KL, Liu G, Mazer M, Zhang XX, Pepkowitz S et al (2008) Vaccination elicits correlated immune and clinical responses in glioblastoma multiforme patients. Cancer Res 68:5955–5964. [DOI] [PubMed] [Google Scholar]
- 286. Wiendl H, Mitsdoerffer M, Hofmeister V, Wischhusen J, Bornemann A, Meyermann R et al (2002) A functional role of HLA‐G expression in human gliomas: an alternative strategy of immune escape. J Immunol 168:4772–4780. [DOI] [PubMed] [Google Scholar]
- 287. Wikstrand CJ, Reist CJ, Archer GE, Zalutsky MR, Bigner DD (1998) The class III variant of the epidermal growth factor receptor (EGFRvIII): characterization and utilization as an immunotherapeutic target. J Neurovirol 4:148–158. [DOI] [PubMed] [Google Scholar]
- 288. Wilmotte R, Burkhardt K, Kindler V, Belkouch MC, Dussex G, Tribolet N et al (2005) B7‐homolog 1 expression by human glioma: a new mechanism of immune evasion. Neuroreport 16:1081–1085. [DOI] [PubMed] [Google Scholar]
- 289. Wintterle S, Schreiner B, Mitsdoerffer M, Schneider D, Chen L, Meyermann R et al (2003) Expression of the B7‐related molecule B7‐H1 by glioma cells: a potential mechanism of immune paralysis. Cancer Res 63:7462–7467. [PubMed] [Google Scholar]
- 290. Wischhusen J, Friese MA, Mittelbronn M, Meyermann R, Weller M (2005) HLA‐E protects glioma cells from NKG2D‐mediated immune responses in vitro: implications for immune escape in vivo . J Neuropathol Exp Neurol 64:523–528. [DOI] [PubMed] [Google Scholar]
- 291. Wood GW, Holladay FP, Turner T, Wang YY, Chiga M (2000) A pilot study of autologous cancer cell vaccination and cellular immunotherapy using anti‐CD3 stimulated lymphocytes in patients with recurrent grade III/IV astrocytoma. J Neurooncol 48:113–120. [DOI] [PubMed] [Google Scholar]
- 292. Yamanaka R, Abe T, Yajima N, Tsuchiya N, Homma J, Kobayashi T et al (2003) Vaccination of recurrent glioma patients with tumour lysate‐pulsed dendritic cells elicits immune responses: results of a clinical phase I/II trial. Br J Cancer 89:1172–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Yamanaka R, Tsuchiya N, Yajima N, Honma J, Hasegawa H, Tanaka R et al (2003) Induction of an antitumor immunological response by an intratumoral injection of dendritic cells pulsed with genetically engineered Semliki Forest virus to produce interleukin‐18 combined with the systemic administration of interleukin‐12. J Neurosurg 99:746–753. [DOI] [PubMed] [Google Scholar]
- 294. Yamanaka R, Homma J, Yajima N, Tsuchiya N, Sano M, Kobayashi T et al (2005) Clinical evaluation of dendritic cell vaccination for patients with recurrent glioma: results of a clinical phase I/II trial. Clin Cancer Res 11:4160–4167. [DOI] [PubMed] [Google Scholar]
- 295. Yamazaki S, Iyoda T, Tarbell K, Olson K, Velinzon K, Inaba K, Steinman RM (2003) Direct expansion of functional CD25+ CD4+ regulatory T cells by antigen‐processing dendritic cells. J Exp Med 198:235–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296. Yu JS, Wheeler CJ, Zeltzer PM, Ying H, Finger DN, Lee PK et al (2001) Vaccination of malignant glioma patients with peptide‐pulsed dendritic cells elicits systemic cytotoxicity and intracranial T‐cell infiltration. Cancer Res 61:842–847. [PubMed] [Google Scholar]
- 297. Yu JS, Liu G, Ying H, Yong WH, Black KL, Wheeler CJ (2004) Vaccination with tumor lysate‐pulsed dendritic cells elicits antigen‐specific, cytotoxic T‐cells in patients with malignant glioma. Cancer Res 64:4973–4979. [DOI] [PubMed] [Google Scholar]
- 298. Yu P, Rowley DA, Fu YX, Schreiber H (2006) The role of stroma in immune recognition and destruction of well‐established solid tumors. Curr Opin Immunol 18:226–231. [DOI] [PubMed] [Google Scholar]
- 299. Zeppernick F, Ahmadi R, Campos B, Dictus C, Helmke BM, Becker N et al (2008) Stem cell marker CD133 affects clinical outcome in glioma patients. Clin Cancer Res 14:123–129. [DOI] [PubMed] [Google Scholar]
- 300. Zhang L, Alizadeh D, Van Handel M, Kortylewski M, Yu H, Badie B (2009) Stat3 inhibition activates tumor macrophages and abrogates glioma growth in mice. Glia March 20 [Epub ahead of print] DOI: 10.1002/glia.20863. [DOI] [PubMed] [Google Scholar]
- 301. Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G (2008) Immunological aspects of cancer chemotherapy. Nat Rev Immunol 8:59–73. [DOI] [PubMed] [Google Scholar]
- 302. Zobywalski A, Javorovic M, Frankenberger B, Pohla H, Kremmer E, Bigalke I, Schendel DJ (2007) Generation of clinical grade dendritic cells with capacity to produce biologically active IL‐12p70. J Transl Med 5:18. [DOI] [PMC free article] [PubMed] [Google Scholar]


