Abstract
This study aimed to investigate the brain grey matter volume (GMV) related to problematic mobile phone use (PMPU), and whether these regions of GMV play a potential moderating role in the relationship between PMPU and depressive symptoms. We recruited 266 students who underwent magnetic resonance imaging (MRI) scanning. PMPU and depressive symptoms were assessed by a self-rating questionnaire for adolescent PMPU and patient health questionnaire-9, respectively. A multiple regression model was performed to detect GMV and white matter (WM) integrity associated with PMPU by voxel-based morphometry (VBM) and tract-based spatial statistics (TBSS) methods, and the moderating analysis was conducted by PROCESS using SPSS software. VBM analysis found an inverse correlation between the GMV of the anterior cingulate gyrus (ACC) and right fusiform gyrus (FFG) with PMPU (PFDR < 0.05), and TBSS analysis revealed that fractional anisotropy (FA) in the body of the corpus callosum was negatively correlated with PMPU. The correlation between PMPU and depressive symptoms was moderated by the GMV of the ACC. These results suggest that the GMV of the ACC and right FFG, as well as FA in the body of the corpus callosum, was related to PMPU, and we further found that increased GMV of the ACC could reduce the relationship between PMPU and depressive symptoms in college students.
Keywords: smartphone addiction, depression, moderating effect, adolescents, MRI
Introduction
With the rapid developments in electronic media experienced by younger generations, mobile phone use in children and adolescents has increased. Recent data from GlobalWebIndex (https://www.globalwebindex.com/) showed that the prevalence of mobile phone use is 90.8% globally, and there are 846.8 million mobile phone users—99.1% of the total population—in China (CNNIC, 2020). Despite many advantages of mobile phone use in daily life, there are potential negative consequences of mobile phone overuse. Problematic mobile phone use (PMPU) is a construct defined as excessive use, with features of craving, salience, tolerance, dependence and loss of control, leading to adverse health and functional consequences (Billieux et al., 2015; De-Sola Gutierrez et al., 2016; Lissak, 2018; Elhai et al., 2019). PMPU has also been called mobile phone dependence, mobile phone addiction and smartphone addiction (Derevensky et al., 2019).
Studies of the relationship between PMPU and mental health, especially those on depression, have received more attention in recent years (Demirci et al., 2015; Zou et al., 2019; Zhang et al., 2020). For example, a study by Ng et al. (2020) reported that PMPU was associated with depression. Similarly, studies of South Korean and American adolescents found that problematic use of smartphones was significantly associated with depression (Park et al., 2019; Grant et al., 2019). Moreover, a 3-year longitudinal study found that mobile phone dependence at year 1 significantly predicted depression at year 3 (Zhang et al., 2020). A systematic review of 10 studies by Elhai et al. (2017) revealed a significant association between problematic or general smartphone use and the severity of depression in different samples of adolescents and adults. However, little is known about the neurobiological mechanism underlying the relationship between PMPU and mental health symptoms.
Some studies have focused on the role of psychological factors, including life satisfaction, empathy, emotion and fear of missing out (Lachmann et al., 2018; Elhai et al., 2018, 2020). There is emerging evidence suggesting the involvement of brain structure and function in behavioural addictions. A recent meta-analytic study aimed to identify the functional and structural neural alterations in Internet gaming disorder (IGD), finding hyperactivation in the anterior cingulate gyrus (ACC) and posterior cingulate gyrus (PCC) caudate and posterior inferior frontal gyrus (IFG); hypoactivation in the anterior IFG and posterior insula; and reduced grey matter volume (GMV) in the ACC, orbitofrontal cortices, dorsolateral prefrontal cortices (PFCs) and premotor cortices in IGD compared to healthy controls (Yao et al., 2017). There have been a few studies of moderating effects, introducing brain structures to the relationship between PMPU and depressive symptoms. We hypothesized that GMV and white matter (WM) integrity were correlated with PMPU, which could moderate the association between PMPU and depressive symptoms.
The current study aimed to detect brain structural regions correlated with PMPU by voxel-based morphometry (VBM) and tract-based spatial statistics (TBSS) methods and to explore whether these regions moderate the association between PMPU and depressive symptom in college students.
Materials and methods
Participants
This study was performed between April 2019 and May 2019, and no examinations were conducted during this period. The study enrolled 268 college students from two schools and five different majors at Anhui Medical University. All of the participants provided written informed consent before the survey, and the study was approved by the Ethics Committee of Anhui Medical University. The survey collected basic information including age, gender, residential area (rural/urban), any siblings, perceived family income and academic performance.
Measures
Problematic mobile phone use.
The Self-rating Questionnaire for Adolescent Problematic Mobile Phone Use (SQAPMPU) (Tao et al., 2013) consists of 13 items (e.g. ‘I do not have enough time for sleeping due to the time I spend on my mobile phone’, ‘I think I need to spend more time on my mobile phone to be satisfied’ and ‘I feel lost without my mobile phone’) rated using a 5-point Likert scale (Not true at all = 1, Slightly true = 2, Moderately true = 3, Strongly true = 4 and Extremely true = 5), used to assess PMPU in the participants. It covers three dimensions: withdrawal symptoms, craving, and physical and mental health status. The total score ranges from 13 to 65; the Cronbach’s alpha coefficient was 0.90.
Patient health questionnaire-9.
The patient health questionnaire (PHQ)-9 is a 9-item self-reported questionnaire that assesses depressive symptoms over the previous 14 days (January and Chimbari, 2018). Items include ‘Little interest or pleasure in doing things’, ‘Feeling down, depressed, or hopeless’, and ‘Trouble falling or staying asleep, or sleeping too much’. Each item on the measure contains four options to describe how often each feeling occurred in the previous 14 days, including 0 (not at all), 1 (several days), 2 (more than half the days) and 3 (nearly every day). The total score ranges from 0 to 27 by totalling the scores of nine items, with higher scores indicating more severe depressive symptoms. In the present study, the Cronbach’s alpha coefficient was 0.87.
Image acquisition.
All of the MRI examinations were performed with 3.0 T Philips Ingenia CX scanner (Philips, Best, Netherlands) in the Ping An Healthcare Diagnostics Center (Hefei, Anhui, China). Polyurethane foam pads were used to minimize head motion and reduce scanner noise. The 3D high-resolution T1-weighted structural images were acquired by fast field echo technique with echo time (TE) = 3.2 ms, repetition time (TR) = 7.1 ms, field of view (FOV) = 256 × 256 mm2, slice thickness = 1 mm, voxel size 1 × 1 × 1 mm3 and number of slices = 180. The acquisition time was 5 min and 5 s. DTI images were acquired by echo planar imaging sequence with TE = 84 ms, TR = 8400 ms, FOV = 256 × 256 mm2, slice thickness = 3 mm, slice gap = 0, b = 0.1000 s/mm2 and direction = 32.
VBM analysis
Image processing was performed using statistical parametric mapping (SPM12, http://www.fil.ion.ucl.ac.uk/spm/). First, the structural images were segmented for GM, WM and cerebrospinal fluid by a unified segmentation model (Harrison et al., 2007). Subsequently, we performed the diffeomorphic anatomical registration using exponentiated lie algebra (DARTEL) method for registration of the GM template (Ashburner, 2007). Then, the images were normalized to the standard anatomic space of the Montreal Neurological Institute (MNI). Finally, the resulting images were modulated by the Jacobian determinants derived from the spatial normalization procedure and then smoothed with an isotropic Gaussian kernel of 6 mm full width at half maximum.
Voxel-based multiple regression analyses (based on general linear model) were performed by SPM12, with the voxel-wise GMV value as the dependent variable and SQAPMPU scores of PMPU as a covariate of interest. In addition, age and gender were used as external regressions to control their effects on both brain structure and PMPU. Intracranial volume (ICV) was not added as a covariate because there was no correlation between ICV and PMPU in this study (r = −0.06, P = 0.324). We excluded two participants due to missing SQAPMPU and PHQ-9 scores during this process. We used a cluster-extent-based threshold which was suggested by Woo et al. (2014), applying a primary significant threshold of uncorrected value of P < 0.001. Based on this height threshold, SPM12 provided the critical cluster size for non-stationary cluster extent correction with a false discovery rate (FDR) maintained at P < 0.05. We also extracted GMV from these brain regions to perform further moderating analyses.
TBSS analysis
DTI images were processed by the FMRIB Software Library (FSL 5.0) software package (http://www.fmrib.ox.ac.uk/fsl). Data preprocessing was as follows: (i) the eddy current correction within FMRIB’s Diffusion Toolbox (FDT) corrected was used for eddy currents and head motion; (ii) a brain mask was created by the Brain Extraction Tool (Smith, 2002) and (iii) FDT was used to fit the tensor model and to calculate the fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD) and radial diffusivity (RD) values.
Voxel-wise analysis of whole-brain WM multiple diffusion metrics (FA, MD, AD and RD) was performed by TBSS (Lee et al., 2007). In brief, all individuals’ FA maps were nonlinearly registered to a standard space at resolution of 1-mm isotropic voxels. Then, a mean FA image was created by averaging the aligned FA maps and thinned to create a mean FA skeleton using the default FA threshold of 0.2 to exclude non-WM voxels. Finally, each subject’s aligned FA map was projected onto the skeleton for further analyses.
To uncover whether WM integrity was correlated with PMPU, voxel-based multiple regression analyses (based on general linear model) were performed on the skeletonized DTI maps by permutation tests using threshold-free cluster enhancement (TFCE) option with the Randomise tool in FSL. The number of permutations was set to 5000. Correction for multiple comparisons used the family-wise error method. We also extracted the surviving results of DTI values and used them as a moderator.
Statistical analysis
The statistical analysis was conducted using SPSS software, version 23.0 (SPSS, Chicago, IL, USA). The descriptive statistics used were the mean (s.d.) and median for continuous variables and frequencies and percentages for categorical variables. A linear regression analysis was performed to explore the association between PMPU and depressive symptoms, including PHQ-9 scores as outcomes and SQAPMPU scores as predictors. A moderation analysis was performed with SQPMPU scores for PMPU as the independent variable, extracted GMV or microstructural integrity values that were strongly (PFDR < 0.05 for GMV and PFWE < 0.05 for microstructural integrity values) correlated with PMPU as moderators, and depressive symptoms as a dependent variable by the SPSS PROCESS macro (Preacher et al., 2007). Simple slopes showed the associations between PMPU and depressive symptoms at low (M − 1 s.d.) and high (M + 1 s.d.) levels of the moderator. The statistical significance was set at P < 0.05.
Results
Sample characteristics
The descriptive statistics of the sample characteristics are summarized in Table 1. We enrolled 268 participants at the beginning and excluded two students from the VBM and TBSS analyses. Finally, we included 266 students with a mean age (s.d.) of 19.02 (0.83) years old in this study. There were 60 men (22.6%), and 157 (59.0%) students came from rural areas. It was reported that 59 (22.2%) of the participants had siblings, and 196 (73.7%) students came from medium-income families. Moreover, 48 of them stated that they had poor academic performance in school. The SQAPMPU and PHQ-9 scores of the total participants were 24.63 ± 8.33 and 4.45 ± 4.08, respectively.
Table 1.
Demographic characteristics of participants
| n | Mean ± s.d./% | |
|---|---|---|
| Age | 266 | 19.02 ± 0.83 |
| Gender | ||
| Male | 60 | 22.6% |
| Female | 206 | 77.4% |
| Residential area | ||
| Rural | 157 | 59% |
| Urban | 109 | 41% |
| Any siblings | ||
| Yes | 59 | 22.2% |
| No | 207 | 77.8% |
| Perceived family income | ||
| Low | 55 | 20.6% |
| Medium | 196 | 73.7% |
| High | 15 | 5.7% |
| Academic performance | ||
| Poor | 48 | 18.0% |
| Medium | 167 | 62.8% |
| Good | 51 | 19.2% |
| Father’s educational level | ||
| Primary school or lower | 56 | 21.1% |
| Middle school | 182 | 68.4 |
| College or above | 28 | 10.5% |
| Mother’s educational level | ||
| Primary school or lower | 126 | 47.4% |
| Middle school | 127 | 47.7% |
| College or above | 13 | 4.9% |
| SQAPMPU scores | 266 | 24.63 ± 8.33 |
| PHQ-9 scores | 266 | 4.45 ± 4.08 |
VBM results
SQAPMPU score was negatively correlated with GMV of the ACC (coordinates: x = −8, y = 40, z = 10) and right fusiform gyrus (FFG) (coordinates: x = 24, y = 9, z = −45) (P < 0.05, FDR corrected, Figure 1, Table 2).
Fig. 1.

The blue-light colour indicates negative correlation of SQAPMPU score and GMV in the anterior cingulate gyrus (ACC) and right fusiform. The colour scale represents t values. The threshold for displaying was set to P < 0.05, FDR corrected. The scatterplots show the association between SQAPMPU score and GMV values that are extracted from significant clusters of each subject.
Table 2.
Brain regions in which GMV was significantly correlated with SQAPMPU scores
| Cluster | Region | Cluster size | Peak MNI(mm) | Peak t value | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| 1 | Anterior cingulate gyrus | 77 | −8 | 40 | 10 | 4.49 |
| 2 | Right fusiform gyrus | 25 | 24 | 9 | −45 | 3.81 |
Note: Height threshold P < 0.001, corrected for FDR, cluster size = 25.
TBSS results
TBSS analysis revealed that the FA in the body of the corpus callosum was negatively correlated with SQAPMPU score, and we did not find that MD, AD or RD was correlated with SQAPMPU score (Figure 2).
Fig. 2.
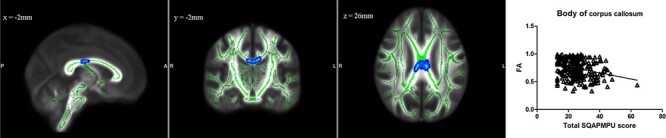
The blue-light blue colour indicates negative correlation between SQAPMPU score and FA in the body of corpus callosum (P < 0.05, TFCE corrected). Green represents mean FA skeleton of all participants. x, y, z is MNI coordinate. The scatterplots show the association between SQAPMPU score and FA values that are extracted from significant clusters of each subject.
Association of PMPU and depressive symptoms and the moderation analyses
Linear regression analyses showed significant relations between PMPU and depression [F(1264) = 137.31, P < 0.05, R2 = 0.34]. For moderation analysis, we found a significantly moderating effect of the GMV of the ACC on the relationship between PMPU and depressive symptoms (β = 0.274, ΔR2 = 0.018, P < 0.01) and did not find a moderate effect of any other PMPU-related GMVs or microstructural integrity values. Moreover, the ACC moderating effect was still significant after controlling for some sociodemographic variables (Table 3, Figures 3 and 4).
Table 3.
Results from the moderated regression analysis predicting depressive symptoms
| Predictors | Model 1 | Model 2 | |||||||||||
| β | SE | t | R 2 | ΔR2 | F | SE | t | R 2 | ΔR2 | F | |||
| 1 | PMPU | 0.274 | 0.026 | 10.561** | 0.363 | 0.018 | 7.444** | 0.279 | 0.027 | 10.381** | 0.370 | 0.016 | 6.462* |
| ACC | 0.929 | 1.078 | 0.862 | 1.028 | 1.119 | 0.919 | |||||||
| PMPU × ACC | −0.340 | 0.125 | −2.728** | −0.323 | 0.127 | −2.542* | |||||||
| 2 | PMPU | 0.273 | 0.026 | 10.519** | 0.351 | 0.009 | 3.437 | 0.278 | 0.027 | 10.338* | 0.359 | 0.007 | 2.725 |
| FFG_R | −0.311 | 1.834 | −0.170 | −0.347 | 1.973 | −0.176 | |||||||
| PMPU × FFG_R | −0.357 | 0.192 | −1.854 | −0.325 | 0.197 | −1.651 | |||||||
| 3 | PMPU | 0.295 | 0.026 | 11.570** | 0.346 | 0.001 | 0.345 | 0.299 | 0.026 | 11.373** | 0.355 | 0.001 | 0.340 |
| body of corpus callosum | 3.483 | 3.070 | 1.134 | 3.146 | 3.145 | 1.000 | |||||||
| PMPU × body of corpus callosum | 0.239 | 0.407 | 0.588 | 0.244 | 0.418 | 0.583 | |||||||
Note s: *P < 0.05, **P < 0.01, model 1 was not adjusted by any variables, model 2 was adjusted by age, gender, residential area, any siblings, perceived family income, academic performance and parent’s educational level. ACC, anterior cingulate gyrus; FFG, fusiform gyrus; PMPU, problematic mobile phone use; R, right.
Fig. 3.

Association between PMPU and depressive symptom was moderated by GMV of ACC. Path a illustrates the association between PMPU and GMV of ACC, Path b indicates the association between GMV of ACC and depressive symptom, Path c illustrates the association between PMPU and depressive symptom and Path c’ illustrates the association between PMPU and depressive symptom after being moderated by GMV of ACC.
Fig. 4.

GMV of ACC moderated the association between PMPU and depressive symptom. Simple slopes are plotted at low (M − 1 s.d.) and high (M + 1 s.d.) GMV of ACC.
Discussion
The present study sought to explore the moderate effects of brain structure on the relationship between PMPU and depressive symptoms in college students. In line with our hypothesis, PMPU was correlated with the GMV of the ACC, right FFG and FA in the body of the corpus callosum. Moreover, moderation analysis found that a greater GMV of the ACC could reduce the relationship between PMPU and depressive symptoms.
To detect PMPU-related brain structures, our VBM results were not consistent with three of the previous studies of PMPU, which found smaller GMVs in the right superior frontal gyrus, right IFG, bilateral thalamus, right lateral orbitofrontal cortex, left anterior insula, left inferior temporal cortex and left parahippocampal cortex of the PMPU group than healthy controls (Wang et al., 2016; Lee et al., 2019; Horvath et al., 2020). However, our findings of ACC-related PMPU were consistent with previous studies of IGD and Internet addiction (Zhou et al., 2011; Weinstein et al., 2017; Lee et al., 2018). Moreover, our results were also consistent with the findings of previous functional MRI study of IGD, which found alteration in brain activity of the ACC and right FFG (Ding et al., 2014; Jin et al., 2016; Qi et al., 2016).
In addition, TBSS analysis found that FA of the body of the corpus callosum was negatively correlated with SQAPMPU score. Reduced WM integrity of the corpus callosum has been associated with many addictive behaviours (Pfefferbaum et al., 2010; Jeong et al., 2016). Our result was consistent with previous studies of Internet addiction, which found reduced FA in the corpus callosum in Internet-addicted adolescents compared to controls (Bi et al., 2015). Moreover, He et al. (2018) found the excessive social media use was correlated with abnormal WM integrity of the corpus callosum. Thus, it is possible that PMPU is related to inter-hemispheric communication deficits.
Nevertheless, there have been limited studies detecting the moderating effect of brain structures on the relationship between PMPU and depressive symptoms. Our results found that greater GMV of the ACC could reduce the relationship between PMPU and depressive symptoms in college students. In the context of the research, the current study expanded new evidence of brain structures playing a role in moderating the effect of the relationship between behaviour problems and emotional symptoms.
The ACC has been identified as a part of the reward system that encodes reward prediction error and plays a key role in attention and the motor control processes involved in behavioural adjustment (Hayden et al., 2011; Lee et al., 2018). Dong et al. (2011, 2012) revealed that IGD decreased activation in the ACC during a loss condition–based functional MRI study using the Probabilistic Guessing Task and STROOP Task, suggesting poor error monitoring and cognitive control. Therefore, altered GMV in the ACC, which contributes to rewards and cognitive control, could be associated not only with the pathophysiology of IGD, but also with PMPU.
There has been emerging evidence suggesting that rewards function is associated with affective disorders, such as depression, and is critical to the aetiology, development and pathophysiology of depression (Forbes, 2011; Forbes and Dahl, 2012). Previous studies identified decreased FA in the cingulum in adolescents with high familial risk for depression compared to adolescents with no family history of mood disorders (Huang et al., 2011; Keedwell et al., 2012). A recent meta-analysis found that ACC was consistently disrupted across a variety of task contexts for neural responses in adolescent depression (Miller et al., 2015). Moreover, Strikwerda-Brown et al. (2015) reported that weaker subgenual ACC functional connectivity with PCC, angular gyrus and dorsal prefrontal cortex at baseline, and weaker subgenual ACC functional connectivity with the dorsomedial prefrontal cortex at follow-up, were associated with worse depressive symptoms. In addition, ACC was regarded as a promising predictor for antidepression response from several studies (Dunlop et al., 2017; Godlewska et al., 2018; Wolke et al., 2019). Based on the above, it can be deduced that ACC might play a key role linking behavioural problems and mood disorders.
The current study had several limitations that should be acknowledged. First, there were some limitations in our measures of PMPU through well-validated self-reported questionnaires because there is no standardized measurement for the diagnosis of PMPU at present. Second, although this sample was relatively large, the cross-sectional design did not infer our findings’ stability, and we assume that the direction in behaviour to emotional symptoms supports the “displacement hypothesis” theory. (Boers et al., 2019). Longitudinal studies are needed in the future to detect the possible psychological and physiological mechanisms of the relationship between PMPU and depressive symptoms. Third, while only the brain structural results were reported in this study, we also got some brain functional results (not published) focusing on other aspects of PMPU. However, we believe that our findings could be helpful, at least in part from the neurobiological aspect, to have a moderating effect on the relationship between PMPU and depressive symptoms in college students.
This study demonstrated that PMPU was associated with depressive symptoms and this association was moderated by the GMV in the ACC, which was correlated with PMPU. In future, more longitudinal studies are needed to explore such moderating effects on the relationship between addictive behaviours and mental health.
Acknowledgements
We sincerely thank all the adolescents and their schools for kind participation, as well as staffs of the Ping An Healthcare Diagnostics Center.
Contributor Information
Liwei Zou, Department of Maternal, Child and Adolescent Health, School of Public Health, Anhui Medical University, Hefei, Anhui 230032, China; MOE Key Laboratory of Population Health Across Life Cycle, Hefei, Anhui 230032, China; Anhui Provincial Key Laboratory of Population Health and Aristogenics, Hefei, Anhui 230032, China.
Xiaoyan Wu, Department of Maternal, Child and Adolescent Health, School of Public Health, Anhui Medical University, Hefei, Anhui 230032, China; MOE Key Laboratory of Population Health Across Life Cycle, Hefei, Anhui 230032, China; Anhui Provincial Key Laboratory of Population Health and Aristogenics, Hefei, Anhui 230032, China; NHC Key Laboratory of Study on Abnormal Gametes and Reproductive Tract, Hefei, Anhui 230032, China.
Shuman Tao, Department of Nephrology, The Second Hospital of Anhui Medical University, Hefei, Anhui 230601, China.
Yajuan Yang, School of Nursing, Anhui Medical University, Hefei, Anhui 230601, China.
Qingjun Zhang, Ping An Healthcare Diagnostics Center, Hefei, Anhui 230000, China.
Xuedong Hong, Ping An Healthcare Diagnostics Center, Hefei, Anhui 230000, China.
Yang Xie, Department of Maternal, Child and Adolescent Health, School of Public Health, Anhui Medical University, Hefei, Anhui 230032, China; MOE Key Laboratory of Population Health Across Life Cycle, Hefei, Anhui 230032, China; Anhui Provincial Key Laboratory of Population Health and Aristogenics, Hefei, Anhui 230032, China.
Tingting Li, Department of Maternal, Child and Adolescent Health, School of Public Health, Anhui Medical University, Hefei, Anhui 230032, China; MOE Key Laboratory of Population Health Across Life Cycle, Hefei, Anhui 230032, China; Anhui Provincial Key Laboratory of Population Health and Aristogenics, Hefei, Anhui 230032, China.
Suisheng Zheng, Ping An Healthcare Diagnostics Center, Hefei, Anhui 230000, China.
Fangbiao Tao, Department of Maternal, Child and Adolescent Health, School of Public Health, Anhui Medical University, Hefei, Anhui 230032, China; MOE Key Laboratory of Population Health Across Life Cycle, Hefei, Anhui 230032, China; Anhui Provincial Key Laboratory of Population Health and Aristogenics, Hefei, Anhui 230032, China; NHC Key Laboratory of Study on Abnormal Gametes and Reproductive Tract, Hefei, Anhui 230032, China.
Funding
This study was supported by the National Natural Science Foundation of China (81773455 and 81803257) and Scientific Research of BSKY from Anhui Medical University (XJ201824).
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Ashburner, J. (2007). A fast diffeomorphic image registration algorithm. NeuroImage, 38(1), 95–113. [DOI] [PubMed] [Google Scholar]
- Bi, Y., Yuan, K., Feng, D., et al. (2015). Disrupted inter-hemispheric functional and structural coupling in Internet addiction adolescents. Psychiatry Research, 234(2), 157–63. [DOI] [PubMed] [Google Scholar]
- Billieux, J., Philippot, P., Schmid, C., Maurage, P., De Mol, J., Van der Linden, M. (2015). Is dysfunctional use of the mobile phone a behavioural addiction? Confronting symptom-based versus process-based approaches. Clinical Psychology and Psychotherapy, 22(5), 460–8. [DOI] [PubMed] [Google Scholar]
- Boers, E., Afzali, M.H., Newton, N., Conrod, P. (2019). Association of screen time and depression in adolescence. JAMA Pediatrics, 173(9), 853–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CNNIC . (2020). The 45th China statistical report on Internet development. https://www.cnnic.com.cn/
- Demirci, K., Akgonul, M., Akpinar, A. (2015). Relationship of smartphone use severity with sleep quality, depression, and anxiety in university students. Journal of Behavioral Addictions, 4(2), 85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derevensky, J.L., Hayman, V., Lynette, G. (2019). Behavioral addictions: excessive gambling, gaming, Internet, and smartphone use among children and adolescents. Pediatric Clinics ofNorth America, 66(6), 1163–82. [DOI] [PubMed] [Google Scholar]
- De-Sola Gutierrez, J., Rodriguez de Fonseca, F., Rubio, G. (2016). Cell-phone addiction: a review. Frontiers inPsychiatry, 7, 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding, W.N., Sun, J.H., Sun, Y.W., et al. (2014). Trait impulsivity and impaired prefrontal impulse inhibition function in adolescents with Internet gaming addiction revealed by a Go/No-Go fMRI study. Behavioral andBrain Functions, 10, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, G., Huang, J., Du, X. (2011). Enhanced reward sensitivity and decreased loss sensitivity in Internet addicts: an fMRI study during a guessing task. Journal of Psychiatric Research, 45(11), 1525–9. [DOI] [PubMed] [Google Scholar]
- Dong, G., Devito, E.E., Du, X., Cui, Z. (2012). Impaired inhibitory control in ‘Internet addiction disorder’: a functional magnetic resonance imaging study. Psychiatry Research, 203(2–3), 153–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop, B.W., Rajendra, J.K., Craighead, W.E., et al. (2017). Functional connectivity of the subcallosal cingulate cortex and differential outcomes to treatment with cognitive-behavioral therapy or antidepressant medication for major depressive disorder. American Journal ofPsychiatry, 174(6), 533–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elhai, J.D., Dvorak, R.D., Levine, J.C., Hall, B.J. (2017). Problematic smartphone use: a conceptual overview and systematic review of relations with anxiety and depression psychopathology. Journal of Affective Disorders, 207, 251–9. [DOI] [PubMed] [Google Scholar]
- Elhai, J.D., Hall, B.J., Erwin, M.C. (2018). Emotion regulation’s relationships with depression, anxiety and stress due to imagined smartphone and social media loss. Psychiatry Research, 261, 28–34. [DOI] [PubMed] [Google Scholar]
- Elhai, J.D., Levine, J.C., Hall, B.J. (2019). The relationship between anxiety symptom severity and problematic smartphone use: a review of the literature and conceptual frameworks. Journal ofAnxiety Disorders, 62, 45–52. [DOI] [PubMed] [Google Scholar]
- Elhai, J.D., Yang, H., Fang, J., Bai, X., Hall, B.J. (2020). Depression and anxiety symptoms are related to problematic smartphone use severity in Chinese young adults: fear of missing out as a mediator. Addictive Behaviors, 101, 105962. [DOI] [PubMed] [Google Scholar]
- Forbes, E.E. (2011). fMRI studies of reward processing in adolescent depression. Neuropsychopharmacology, 36(1), 372–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes, E.E., Dahl, R.E. (2012). Research review: altered reward function in adolescent depression: what, when and how? Journal ofChild Psychology and Psychiatry, 53(1), 3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godlewska, B.R., Browning, M., Norbury, R., Igoumenou, A., Cowen, P.J., Harmer, C.J. (2018). Predicting treatment response in depression: the role of anterior cingulate cortex. International Journal of Neuropsychopharmacology, 21(11), 988–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant, J.E., Lust, K., Chamberlain, S.R. (2019). Problematic smartphone use associated with greater alcohol consumption, mental health issues, poorer academic performance, and impulsivity. Journal of Behavioral Addictions, 8(2), 335–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison, L.M., Penny, W., Ashburner, J., Trujillo-Barreto, N., Friston, K.J. (2007). Diffusion-based spatial priors for imaging. NeuroImage, 38(4), 677–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden, B.Y., Heilbronner, S.R., Pearson, J.M., Platt, M.L. (2011). Surprise signals in anterior cingulate cortex: neuronal encoding of unsigned reward prediction errors driving adjustment in behavior. Journal of Neuroscience, 31(11), 4178–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, Q., Turel, O., Bechara, A. (2018). Association of excessive social media use with abnormal white matter integrity of the corpus callosum. Psychiatry Research, 278, 42–7. [DOI] [PubMed] [Google Scholar]
- Horvath, J., Mundinger, C., Schmitgen, M.M., et al. (2020). Structural and functional correlates of smartphone addiction. Addictive Behaviors, 105, 106334. [DOI] [PubMed] [Google Scholar]
- Huang, H., Fan, X., Williamson, D.E., Rao, U. (2011). White matter changes in healthy adolescents at familial risk for unipolar depression: a diffusion tensor imaging study. Neuropsychopharmacology, 36(3), 684–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- January, J., Chimbari, M.J. (2018). Study protocol on criterion validation of Edinburgh Postnatal Depression Scale (EPDS), Patient Health Questionnaire (PHQ-9) and Centre for Epidemiological Studies-Depression (CES-D) screening tools among rural postnatal women; a cross-sectional study. BMJ Open, 8(4), e019085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong, B.S., Han, D.H., Kim, S.M., Lee, S.W., Renshaw, P.F. (2016). White matter connectivity and Internet gaming disorder. Addiction Biology, 21(3), 732–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin, C., Zhang, T., Cai, C., et al. (2016). Abnormal prefrontal cortex resting state functional connectivity and severity of Internet gaming disorder. Brain Imaging and Behavior, 10(3), 719–29. [DOI] [PubMed] [Google Scholar]
- Keedwell, P.A., Chapman, R., Christiansen, K., Richardson, H., Evans, J., Jones, D.K. (2012). Cingulum white matter in young women at risk of depression: the effect of family history and anhedonia. BiologicalPsychiatry, 72(4), 296–302. [DOI] [PubMed] [Google Scholar]
- Lachmann, B., Sindermann, C., Sariyska, R.Y., et al. (2018). The role of empathy and life satisfaction in Internet and smartphone use disorder. Frontiers in Psychology, 9, 398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, D., Park, J., Namkoong, K., Kim, I.Y., Jung, Y.C. (2018). Gray matter differences in the anterior cingulate and orbitofrontal cortex of young adults with Internet gaming disorder: surface-based morphometry. Journal of Behavioral Addictions, 7(1), 21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, D., Namkoong, K., Lee, J., Lee, B.O., Jung, Y.C. (2019). Lateral orbitofrontal gray matter abnormalities in subjects with problematic smartphone use. Journal of Behavioral Addictions, 8(3), 404–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, H., Kim, M.S., Son, H.K., Ahn, S., Kim, J.S., Kim, Y.H. (2007). Discriminating power of socio-demographic and psychological variables on addictive use of cellular phones among middle school students. Taehan Kanho Hakhoe Chi, 37(6), 957–65. [DOI] [PubMed] [Google Scholar]
- Lissak, G. (2018). Adverse physiological and psychological effects of screen time on children and adolescents: literature review and case study. Environmental Research, 164, 149–57. [DOI] [PubMed] [Google Scholar]
- Miller, C.H., Hamilton, J.P., Sacchet, M.D., Gotlib, I.H. (2015). Meta-analysis of functional neuroimaging of major depressive disorder in youth. JAMA Psychiatry, 72(10), 1045–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng, K.C., Wu, L.H., Lam, H.Y., et al. (2020). The relationships between mobile phone use and depressive symptoms, bodily pain, and daytime sleepiness in Hong Kong secondary school students. Addictive Behaviors, 101, 105975. [DOI] [PubMed] [Google Scholar]
- Park, S.Y., Yang, S., Shin, C.S., Jang, H., Park, S.Y. (2019). Long-term symptoms of mobile phone use on mobile phone addiction and depression among Korean adolescents. International Journal of Environmental Research andPublic Health, 16(19), 3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum, A., Rosenbloom, M.J., Fama, R., Sassoon, S.A., Sullivan, E.V. (2010). Transcallosal white matter degradation detected with quantitative fiber tracking in alcoholic men and women: selective relations to dissociable functions. Alcoholism, Clinical and Experimental Research, 34(7), 1201–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher, K.J., Rucker, D.D., Hayes, A.F. (2007). Addressing moderated mediation hypotheses: theory, methods, and prescriptions. Multivariate Behavioral Research, 42(1), 185–227. [DOI] [PubMed] [Google Scholar]
- Qi, X., Yang, Y., Dai, S., et al. (2016). Effects of outcome on the covariance between risk level and brain activity in adolescents with Internet gaming disorder. NeuroImage: Clinical, 12, 845–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, S.M. (2002). Fast robust automated brain extraction. HumanBrain Mapping, 17(3), 143–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strikwerda-Brown, C., Davey, C.G., Whittle, S., et al. (2015). Mapping the relationship between subgenual cingulate cortex functional connectivity and depressive symptoms across adolescence. Social Cognitive and Affective Neuroscience, 10(7), 961–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao, S., Fu, J., Wang, H., Hao, J., Tao, F. (2013). The development of self-rating questionnaire for adolescent problematic mobile phone use and the psychometric evaluation in undergraduates. Chinese Journal of School Health, 34(1), 26–9. [Google Scholar]
- Wang, Y., Zou, Z., Song, H., et al. (2016). Altered gray matter volume and white matter integrity in college students with mobile phone dependence. Frontiers in Psychology, 7, 597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein, A., Livny, A., Weizman, A. (2017). New developments in brain research of Internet and gaming disorder. Neuroscience and Biobehavioral Reviews, 75, 314–30. [DOI] [PubMed] [Google Scholar]
- Wolke, S.A., Mehta, M.A., O’Daly, O., et al. (2019). Modulation of anterior cingulate cortex reward and penalty signalling in medication-naive young-adult subjects with depressive symptoms following acute dose lurasidone. Psychological Medicine, 49(8), 1365–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo, C.W., Krishnan, A., Wager, T.D. (2014). Cluster-extent based thresholding in fMRI analyses: pitfalls and recommendations. NeuroImage, 91, 412–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao, Y.W., Liu, L., Ma, S.S., et al. (2017). Functional and structural neural alterations in Internet gaming disorder: a systematic review and meta-analysis. Neuroscience and Biobehavioral Reviews, 83, 313–24. [DOI] [PubMed] [Google Scholar]
- Zhang, G., Yang, X., Tu, X., Ding, N., Lau, J.T.F. (2020). Prospective relationships between mobile phone dependence and mental health status among Chinese undergraduate students with college adjustment as a mediator. Journal of Affective Disorders, 260, 498–505. [DOI] [PubMed] [Google Scholar]
- Zhou, Y., Lin, F.C., Du, Y.S., et al. (2011). Gray matter abnormalities in Internet addiction: a voxel-based morphometry study. European Journal of Radiology, 79(1), 92–5. [DOI] [PubMed] [Google Scholar]
- Zou, L., Wu, X., Tao, S., et al. (2019). Mediating effect of sleep quality on the relationship between problematic mobile phone use and depressive symptoms in college students. Frontiers inPsychiatry, 10, 822. [DOI] [PMC free article] [PubMed] [Google Scholar]


