Abstract
Background
Corticosteroids are routinely given to children undergoing cardiac surgery with cardiopulmonary bypass (CPB) in an attempt to ameliorate the inflammatory response. Their use is still controversial and the decision to administer the intervention can vary by centre and/or by individual doctors within that centre.
Objectives
This review is designed to assess the benefits and harms of prophylactic corticosteroids in children between birth and 18 years of age undergoing cardiac surgery with CPB.
Search methods
We searched CENTRAL, MEDLINE, Embase and Conference Proceedings Citation Index‐Science in June 2020. We also searched four clinical trials registers and conducted backward and forward citation searching of relevant articles.
Selection criteria
We included studies of prophylactic administration of corticosteroids, including single and multiple doses, and all types of corticosteroids administered via any route and at any time‐point in the perioperative period. We excluded studies if steroids were administered therapeutically. We included individually randomised controlled trials (RCTs), with two or more groups (e.g. multi‐drug or dose comparisons with a control group) but not ‘head‐to‐head' trials without a placebo or a group that did not receive corticosteroids. We included studies in children, from birth up to 18 years of age, including preterm infants, undergoing cardiac surgery with the use of CPB. We also excluded studies in patients undergoing heart or lung transplantation, or both; studies in patients already receiving corticosteroids; in patients with abnormalities of the hypothalamic‐pituitary‐adrenal axis; and in patients given steroids at the time of cardiac surgery for indications other than cardiac surgery.
Data collection and analysis
We used the Covidence systematic review manager to extract and manage data for the review. Two review authors independently assessed studies for inclusion, extracted data, and assessed risks of bias. We resolved disagreements by consensus or by consultation with a third review author. We assessed the certainty of evidence with GRADE.
Main results
We found 3748 studies, of which 888 were duplicate records. Two studies had the same clinical trial registration number, but reported different populations and interventions. We therefore included them as separate studies. We screened titles and abstracts of 2868 records and reviewed full text reports for 84 studies to determine eligibility. We extracted data for 13 studies. Pooled analyses are based on eight studies. We reported the remaining five studies narratively due to zero events for both intervention and placebo in the outcomes of interest. Therefore, the final meta‐analysis included eight studies with a combined population of 478 participants.
There was a low or unclear risk of bias across the domains. There was moderate certainty of evidence that corticosteroids do not change the risk of in‐hospital mortality (five RCTs; 313 participants; risk ratio (RR) 0.83, 95% confidence interval (CI) 0.33 to 2.07) for children undergoing cardiac surgery with CPB. There was high certainty of evidence that corticosteroids reduce the duration of mechanical ventilation (six RCTs; 421 participants; mean difference (MD) 11.37 hours lower, 95% CI ‐20.29 to ‐2.45) after the surgery. There was high‐certainty evidence that the intervention probably made little to no difference to the length of postoperative intensive care unit (ICU) stay (six RCTs; 421 participants; MD 0.28 days lower, 95% CI ‐0.79 to 0.24) and moderate‐certainty evidence that the intervention probably made little to no difference to the length of the postoperative hospital stay (one RCT; 176 participants; mean length of stay 22 days; MD ‐0.70 days, 95% CI ‐2.62 to 1.22). There was moderate certainty of evidence for no effect of the intervention on all‐cause mortality at the longest follow‐up (five RCTs; 313 participants; RR 0.83, 95% CI 0.33 to 2.07) or cardiovascular mortality at the longest follow‐up (three RCTs; 109 participants; RR 0.40, 95% CI 0.07 to 2.46). There was low certainty of evidence that corticosteroids probably make little to no difference to children separating from CPB (one RCT; 40 participants; RR 0.20, 95% CI 0.01 to 3.92). We were unable to report information regarding adverse events of the intervention due to the heterogeneity of reporting of outcomes.
We downgraded the certainty of evidence for several reasons, including imprecision due to small sample sizes, a single study providing data for an individual outcome, the inclusion of both appreciable benefit and harm in the confidence interval, and publication bias.
Authors' conclusions
Corticosteroids probably do not change the risk of mortality for children having heart surgery using CPB at any time point. They probably reduce the duration of postoperative ventilation in this context, but have little or no effect on the total length of postoperative ICU stay or total postoperative hospital stay. There was inconsistency in the adverse event outcomes reported which, consequently, could not be pooled. It is therefore impossible to provide any implications and policy‐makers will be unable to make any recommendations for practice without evidence about adverse effects. The review highlighted the need for well‐conducted RCTs powered for clinical outcomes to confirm or refute the effect of corticosteroids versus placebo in children having cardiac surgery with CPB. A core outcome set for adverse event reporting in the paediatric major surgery and intensive care setting is required.
Plain language summary
What are the benefits and risks of corticosteroids for preventing inflammation in children who undergo heart surgery involving a heart‐lung machine?
Why is this question important?
Children who are born with a heart defect, or who develop heart disease after birth, may need heart surgery. To operate, surgeons often need to stop the heart and lungs temporarily. To keep the child alive, they use a heart‐lung machine that takes over the work of the heart and lungs. The machine adds oxygen to the blood, removes carbon dioxide from it, and pumps the blood back into the child’s body.
We know that heart surgery involving a heart‐lung machine causes inflammation across the body. This can cause complications ranging from low blood pressure to major organ dysfunction. In some cases, patients may die.
Corticosteroids (a type of anti‐inflammation medicine) have been widely used to prevent inflammation in children who undergo heart surgery that requires a heart‐lung machine, but their benefits and risks are unclear. To find out whether they prevent inflammation, and whether they are associated with any unwanted effects (such as poor wound healing, increased risk of infection or increased risk of death), we reviewed the evidence from research studies.
How did we identify and evaluate the evidence?
First, we searched the medical literature for randomized controlled studies (studies in which people are randomly divided into different treatment groups), because these studies provide the most robust evidence about the effects of a treatment. We then compared the results and summarised the evidence from all the studies. Finally, we assessed how certain the evidence was. To do this, we considered factors such as the way studies were conducted, study sizes, and consistency of findings across studies. Based on our assessments, we categorised the evidence as being of very low, low, moderate or high certainty.
What did we find?
We found 13 studies that involved a total of 1087 children. The studies lasted for between 14 months and 30 months (duration was not reported for seven studies). Three corticosteroids were investigated: methylprednisolone (five studies), hydrocortisone (two studies) and dexamethasone (six studies). The studies compared these corticosteroids against a placebo (medicine that is exactly the same apart from it does not have the active medicine in it).
The evidence shows that:
‐ corticosteroids probably make little or no difference to the number of children who die in hospital after surgery (five studies, 313 children (participating in the studies), moderate‐certainty evidence);
‐ corticosteroids probably make little or no difference to the number of children who die from any cause (five studies, 313 children, moderate‐certainty evidence) or from heart and circulation problems specifically (three studies, 109 children, moderate‐certainty evidence) at the longest follow‐up time after surgery;
‐ corticosteroids may make little or no difference to whether children are taken off the heart‐lung machine after surgery (one study, 40 children, low‐certainty evidence).
‐ corticosteroids reduce the number of hours for which children need a breathing machine (six studies, 421 children, high‐certainty evidence);
‐ corticosteroids make little or no difference to the length of time children spend in the intensive care unit (six studies, 421 children, high‐certainty evidence);
‐ corticosteroids probably make little or no difference to the total length of time children spend in hospital after surgery (one study, 176 children, moderate‐certainty evidence).
It is unclear whether corticosteroids are associated with non‐fatal unwanted effects because the studies did not report on unwanted effects consistently.
What does this mean?
Giving corticosteroids to children who have heart surgery that requires a heart lung‐machine:
‐ probably makes little or no difference to the number who die after surgery at any point or from any cause;
‐ may make little to no difference to whether children are taken off the heart‐lung machine after surgery;
‐ probably reduces the length of time spent on the breathing machine after surgery, but this does not lead to a shorter stay in the intensive care unit or hospital.
Future studies need to collect information on non‐fatal unwanted effects in a standardised way, so that we can evaluate the risks of corticosteroids.
How‐up‐to date is this review?
The evidence in this Cochrane Review is current to June 2020.
Summary of findings
Summary of findings 1. Glucocorticoid compared to placebo for paediatric heart surgery with cardiopulmonary bypass.
| Glucocorticoid compared to Placebo for paediatric heart surgery with cardiopulmonary bypass | ||||||
| Patient or population: Children having heart surgery with cardiopulmonary bypass Setting: Hospitals Intervention: Glucocorticoid Comparison: Placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with glucocorticoid | |||||
| In‐hospital postoperative mortality | Study population | RR 0.83 (0.33 to 2.07) | 313 (5 RCTs) | ⊕⊕⊕⊝ MODERATE1 |
8 studies had zero events in both arms of the study. Note that these are the same studies as reported all‐cause mortality at longest follow‐up. | |
| 68 per 1,000 | 48 per 1,000 (20 to 116) | |||||
| Duration of postoperative mechanical ventilation | The mean duration of postoperative mechanical ventilation was 78.2 Hours | MD 11.37 Hours lower (20.29 Hours lower to 2.45 Hours lower) | ‐ | 421 (6 RCTs) | ⊕⊕⊕⊕ HIGH | |
| Length of postoperative ICU stay | The mean length of postoperative ICU stay was 7.67 Days | MD 0.28 Days lower (0.79 lower to 0.24 higher) | ‐ | 421 (6 RCTs) | ⊕⊕⊕⊕ HIGH | |
| Length of postoperative hospital stay | The mean length of postoperative hospital stay was 22 Days | MD 0.7 Days lower (2.62 lower to 1.22 higher) | ‐ | 176 (1 RCT) | ⊕⊕⊕⊝ MODERATE2 | |
| All‐cause mortality at longest follow‐up (longest follow‐up was "in‐hospital") | 68 per 1,000 | 48 per 1,000 (20 to 116) | RR 0.83 (0.33 to 2.07) | 313 (5 RCTs) | ⊕⊕⊕⊝ MODERATE1 |
8 studies had zero events in both arms of the study. Note that these are the same studies as reported in‐hospital postoperative mortality. |
| Cardiovascular mortality at longest follow‐up (longest follow‐up was "in‐hospital") | 93 per 1,000 | 37 per 1,000 (6 to 228) |
RR 0.40 (0.07 to 2.46) |
109 (3 RCTs) |
⊕⊕⊕⊝ MODERATE 3 | |
| Failure to separate from CPB | 100 per 1,000 | 20 per 1,000 (1 to 392) |
RR 0.20 (0.01 to 3.92) |
40 (1 RCT) |
⊕⊕⊝⊝ LOW 2, 4 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1Downgraded by one level for imprecision due to small sample sizes.
2Downgraded by one level for imprecision due to small sample size and only one study.
3Downgraded by one level for imprecision due to inclusion of both appreciable benefit and harm in the confidence interval.
4Downgraded by one level for publication bias where other studies excluded these patients from their analysis.
Background
Description of the condition
Paediatric heart surgery outcomes have improved markedly over time as a result of marginal gains in training, technology and safety systems (Brown 2015; Hoashi 2015; Jacobs 2016; NICOR 2016). This improvement has been particularly notable in the last 20 years: the publication of heart surgery outcomes after the Kennedy Inquiry in the UK has been associated with a large increase in survival for risk‐adjusted surgery (Kennedy 2001; Grant 2013). This has been mirrored worldwide (Brown 2015; Hoashi 2015; Jacobs 2016). One area where there is still controversy is corticosteroid use. Paediatric heart surgery with the use of cardiopulmonary bypass (CBP) results in a systemic inflammatory response. Corticosteroids have been widely used to mitigate the potentially deleterious effects of this response. The surgical intervention for which corticosteroids are used includes a variety of surgeries performed on the heart and great vessels. In most cases, this procedure aims to correct congenital heart diseases (i.e. heart malformations with which the child is born). In most cases (78% in the UK) (NICOR 2016), surgery will take place with the use of CBP, also known as the ‘heart‐lung machine.' Cannulae are placed in the child’s major blood vessels and blood is channeled out of the body, oxygen is added, carbon dioxide is removed and the blood is then pumped back to the child’s body. This allows the heart to be stopped and emptied of blood, which allows the surgeon to operate in a bloodless field on a non‐beating heart (Barry 2015). As a result, there is activation of white blood cells and platelets, as well as coagulation cascades (Tarnok 2001), with the end signalling due to cytokines. Endothelial permeability increases and parenchymal damage by free radicals occurs (Fudulu 2016; Pesonen 2016). Fluid leaks out of the circulation and into the tissues, blood vessels vasodilate, hypovolaemia occurs and thus blood pressure drops. Many of the complications of cardiac surgery, including multi‐organ failure and death, result from these mechanisms (Huffmyer 2015). Nevertheless, the impact of prophylactic corticosteroids on clinical outcomes following heart surgery on children remains unclear (Pasquali 2010; Keski‐Nisula 2013).
Description of the intervention
Corticosteroids are hormones produced by the adrenal glands of all mammals. In humans, the naturally‐occurring corticosteroid is called cortisol (hydrocortisone) (Gibbison 2013). Corticosteroids, at a molecular level, are composed of a steroid backbone plus various modifications to side‐chains which can change the activity of the molecule. These modified side‐chains are exploited by drug manufacturers to modify the different properties of corticosteroids. Corticosteroids are fat‐soluble and therefore can pass freely through cell walls to bind to their receptors, which are found inside the target cells. Once they bind to their receptor, they travel into the cell nucleus and act as a transcription factor, changing the expression of cellular proteins (Gibbison 2013). Synthetic and naturally‐occurring corticosteroids can be given either before, during or after cardiac surgery to elicit the beneficial effects described in the next section (Toledo‐Pereyra 1980; Pasquali 2010; Keski‐Nisula 2015). In this context, they are usually given as intravenous drugs and may be given as a bolus dose or by infusion. A variety of different steroid drugs are given. Frequently given drugs include dexamethasone (Lerzo 2011), methylprednisolone (Pasquali 2012), and hydrocortisone (Robert 2015). The dose given in this context is often equivalent to 10 to 20 times the total daily amount produced by adrenal glands in normal health.
How the intervention might work
Corticosteroids have several properties that make them attractive to give during the cardiac surgical perioperative period, their anti‐inflammatory potential being their most desired feature. Cardiac surgery, with or without the use of CPB, causes systemic inflammation by the earlier‐described mechanisms. This leads to poor perfusion which, coupled with the effects of inflammatory mediators that impact directly on the organs, can lead to organ dysfunction and potentially death (Medzhitov 2008). To the clinician, the most obvious organ dysfunction is altered haemodynamics, which is usually treated with inotropes and vasopressors postoperatively. The lungs are also frequently affected: the fluid that moves out of the vessels and into the lung tissue and alveoli (air spaces) can have a negative impact on ventilation and oxygenation, thus increasing the need for mechanical ventilatory support. Many studies have shown that corticosteroids reduce the concentrations and activity of inflammatory mediators after cardiac surgery and increase the concentrations of anti‐inflammatory mediators, both locally in the heart and systemically in the circulating plasma (Keski‐Nisula 2013; Graham 2014; Dreher 2015; Amanullah 2016). Inducing a shift of the inflammatory balance towards the anti‐inflammatory reaction is thought, by extrapolation, to reduce capillary leak, vasodilatation and organ dysfunction. Corticosteroids act directly to vasoconstrict arterioles, as well as increasing salt and water retention in the kidney. These properties can improve blood pressure and, potentially, organ perfusion in the short‐ and medium‐term. They also increase blood glucose levels by breaking down fats, proteins and carbohydrates into their constituent building blocks, which can be used for cellular energy.
Why it is important to do this review
Many corticosteroid studies are powered for and assess surrogate outcomes, such as inflammatory mediator levels, rather than objective clinical outcomes. Corticosteroids have several deleterious effects, which are traded off against the potentially beneficial effects outlined above. An increase in blood glucose, beneficial with respect to cellular energy, is associated with less favourable outcomes after cardiac surgery (Pasquali 2010). In the critically ill, corticosteroids impair wound healing and cause immunosuppression, which may allow secondary infections to develop (Pasquali 2012). Several studies also suggest that giving high‐dose corticosteroids to a child may impair long‐term cognitive development (Gibson 1993; Shinwell 2000; Yeh 2004). The neonatal population represents a group of particular interest, as the evidence for corticosteroid use has been inconclusive, and has possible life‐long harms. Despite the lack of certainty over its risks and benefits, its use is still common practice in many centres. There is no consensus about whether or not to give corticosteroids (Fudulu 2018), nor is there consensus about the type of corticosteroids, dose regimens or timing of when they may be beneficial, e.g. preoperatively versus intraoperatively versus postoperatively. There are no national or international guidelines pertaining to perioperative corticosteroid use in the paediatric cardiac surgery population. Practice varies both among and within institutions; patients in one hospital may or may not receive corticosteroids, depending on the treating clinician.
Objectives
To assess the benefits and harms of prophylactic corticosteroids in children between birth and 18 years of age undergoing cardiac surgery with CPB.
Methods
Criteria for considering studies for this review
Types of studies
We included individually randomised controlled trials (RCTs), including trials with more than two groups (e.g. multi‐drug or dose comparisons with a control group) but not ‘head‐to‐head' trials without a placebo or group not receiving corticosteroids. We specified in our review protocol that if we found any cross‐over randomised studies, we would include only the initial period in our analyses. This Cochrane Review examines the effect of prophylactic corticosteroids in the perioperative period. It would be extremely difficult or impossible to design a crossover study reporting clinical outcomes examining this intervention. As such, we did not expect to find these. We excluded cluster‐randomised controlled trials (cRCTs). They would be subject to significant bias in this context because the perioperative protocols and workloads would differ greatly among centres. In cRCTs, participants’ characteristics might appear to be well‐matched between groups but other factors, e.g. surgical techniques and postoperative care, would not. We included studies irrespective of their publication status. We considered including large, published registry studies as well as individually randomised RCTs, but there is likely to be a critical risk of confounding in these study groups. The factors that cause clinicians to prescribe steroids prophylactically are not captured due to the difficulty in defining and documenting those reasons. Also, publications arising from registries have reported the same outcomes as reported in RCTs (e.g. mortality and hospital length of stay). They would thus duplicate evidence for an outcome domain of interest, but the effect estimate would have lower certainty. There would be more justification in including such studies if they reported rare or long‐term outcomes such as cognitive function, which would complement outcomes reported in RCTs in an important way (Reeves 2013).
Types of participants
We included RCTs that recruited populations of children, from birth up to 18 years of age, including preterm infants undergoing cardiac surgery with the use of CPB. We excluded studies that included any participants with any of the following co‐morbidities or characteristics.
Undergoing heart or lung transplantation, or both
Already being treated with corticosteroids
With abnormalities of the hypothalamic‐pituitary‐adrenal (HPA) axis
Given steroids at the time of cardiac surgery for indications other than cardiac surgery (e.g. allergy, bronchoconstriction)
If findings were reported for an eligible subset of the trial population, we included the findings for the eligible subset. If any study included a subset of eligible participants but did not report findings for the eligible subset, we contacted the study authors to obtain patient‐level data or aggregated data for the eligible subset. If this was not possible, then we included a study if 80% or more of the participants satisfied our eligibility criteria. We set this threshold on the assumption that up to 20% of ineligible participants would not markedly bias the average estimate. We recognise that this rule represents an uncertain compromise. However, we believed at the outset that the number of potential studies (and therefore participants) was likely to be small. This approach trades off the risk of a slightly biased answer against an answer too imprecise to be useful.
Types of interventions
Corticosteroids had to be administered prophylactically, i.e. in anticipation of adverse effects of cardiac surgery, for an RCT to be eligible. The corticosteroids could have been administered at any point in the preoperative, intraoperative or postoperative period, but the time‐point and regimen must have been prespecified and given to all eligible participants randomised to the intervention group (apart from protocol deviations). We included studies that administered single or multiple doses and any type of corticosteroids administered by any route. Corticosteroid drugs considered were: hydrocortisone, dexamethasone, prednisolone, prednisone, methylprednisolone plus any other existing drugs or those in development. We excluded studies that evaluated the effectiveness of ‘rescue' corticosteroids (i.e. given in response to a clinical deterioration rather than prophylactically). However, we included studies that evaluated prophylactic corticosteroids and that allowed for ‘rescue' corticosteroids to be given to treat patients who deteriorated. We included trials that compared any corticosteroid with placebo or usual care without the use of corticosteroids. We also included multi‐group studies comparing multiple doses, drugs or regimen of corticosteroids against a placebo/no corticosteroids control.
Types of outcome measures
Reporting one or more of the outcomes of interest to the review was not an inclusion criterion for a trial to be included in the review. We decided study eligibility strictly according to the eligibility of the population studied and the intervention evaluated.
Primary outcomes
In‐hospital, postoperative mortality
Duration of postoperative mechanical ventilation (days)
An initial scoping search identified relatively few RCTs with relatively small numbers of participants. There was the possibility that there would not be sufficient power when the data were pooled to detect or exclude a clinically important difference in postoperative mortality, due to the low baseline mortality rate. Therefore, we included a second, continuous primary outcome which, if reported for a similar number of participants, would have greater power. Postoperative mechanical ventilation is an important outcome due to the risk of complications it confers, both directly attributable due to the intervention itself and indirectly, because a patient who is mechanically ventilated must be in treatment on an intensive care unit (a proxy marker for critical illness).
Secondary outcomes
Length of postoperative intensive care unit stay
Length of postoperative hospital stay
All‐cause mortality at longest follow‐up
Cardiovascular mortality at longest follow‐up
Duration of postoperative inotropes/vasopressors
Failure to separate from cardiopulmonary bypass
Adverse events
There is little consistency about the adverse events attributed to steroid use that clinicians regard as important in terms of both type of adverse event and the definitions and thresholds for reporting. Therefore, the adverse events that are reported by clinical trials are not consistent. They include outcomes such as infection, hyperglycaemia and poor wound healing. Such outcomes have never been universally defined. We anticipated that it might not be possible to synthesise adverse event data across the RCTs or even to tabulate event frequencies consistently across trials. We did, however, collect all available data and reviewed them. Where appropriate (similar definitions across studies) we attempted to pool this and report it in the meta‐analysis.
Search methods for identification of studies
Electronic searches
We identified potentially eligible trials by systematic searches of the following bibliographic databases on 11 June 2020:
Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library via CRS Web
Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, MEDLINE Daily and MEDLINE (Ovid, 1946 to 9 June 2020)
Embase (Ovid, 1974 to 10 June 2020)
CPCI‐S (Conference Proceedings Citation Index‐Science) on the Web of Science (Clarivate Analytics, 1990 to 11 June 2020)
We adapted the preliminary search strategy for MEDLINE (Ovid) (Appendix 1) for use in the other databases. We applied the Cochrane sensitivity‐maximising RCT filter to MEDLINE (Ovid) (Lefebvre 2011), and adaptations of it to the other databases, except CENTRAL. We also conducted a search in January 2020 of ClinicalTrials.gov (www.clinicaltrials.gov), the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) Search Portal (apps.who.int/trialsearch/), ISRCTN Registry (www.isrctn.com) and the European Union Clinical Trials Register (www.clinicaltrialsregister.eu) for ongoing or unpublished trials. We searched all databases from 2000 to the present without any restriction on language of publication or publication status. We excluded studies before 2000 because 30‐day mortality has declined remarkably in paediatric cardiac surgery (by around one‐quarter to one‐half in the UK, the USA and Japan) (Brown 2015; Hoashi 2015; Jacobs 2016; NICOR 2016). Studies before 2000 would be weighted disproportionately because of the higher death rates. Changes in clinical practice also make older studies less relevant to current practice. See also Sensitivity analysis. We did not perform a separate search for adverse effects of corticosteroid use in paediatric cardiac surgery. We expected the search strategy to capture studies reporting beneficial or detrimental outcomes with equal likelihood.
Searching other resources
We checked the reference lists of all included studies, and any relevant systematic reviews identified, for additional references to RCTs, and included them if eligible. We also examined any relevant retraction statements and errata that applied to included studies. We made every attempt to contact study authors for any missing data.
Data collection and analysis
Selection of studies
Two review authors (AWLS, KIAM) independently screened titles and abstracts retrieved by the literature searches to identify potentially eligible studies, and coded them as either ‘obtain full text' (eligible or potentially eligible/unclear) or ‘do not obtain full text.' If there were any disagreements, a third review author arbitrated (BCR). We retrieved the full‐text study reports and two review authors (BG, JCVS) screened full‐text articles to identify studies for inclusion. We listed all studies that were excluded after full‐text assessment with reasons for their exclusion. We resolved any disagreement through discussion. We identified duplicate reports of studies and collated multiple reports of the same study so that each study was the unit of interest in the review.
Data extraction and management
We used the Covidence systematic review manager for study screening and data extraction (Covidence 2020). Two review authors (DPF, JCVL) independently extracted study characteristics from included studies. Any differences in data extraction were resolved by a third review author (BG). The original extraction files were retained and a third consensus file produced with discrepancies resolved. We extracted the following data.
Methods: the total duration of the study, number of study centres and location, study setting, withdrawals and date of the study
Participants: the number randomised, the number lost to follow‐up/withdrawn, number analyzed, mean age, age range, sex, inclusion criteria and exclusion criteria. Where reported, we extracted the underlying cardiac pathology.
Interventions: intervention(s) and comparator
Outcomes: the primary and secondary outcomes of interest to the review, and time points at which they were reported
One review author (BG) transferred data into the Review Manager 5 (RevMan 5) file (RevMan 2014) and double‐checked that the data was entered correctly by comparing the data presented in the systematic review with the study reports.
Assessment of risk of bias in included studies
Two review authors (DPF, JCVL) independently assessed the risk of bias for each included study using the ‘Risk of bias' (RoB) tool Version 1.0 as described in Cochrane methods for individually RCTs (Higgins 2016; see Types of studies). We resolved any disagreements by discussion or by involving another review author (BG, BCR). We assessed the risk of bias according to the following domains.
Selection bias (random sequence allocation; allocation concealment)
Reporting bias (selective outcome reporting)
Performance bias (blinding of participants and personnel)
Detection bias (blinding in outcome assessment)
Attrition bias (incomplete outcome data)
Other sources of bias
For each outcome, the review authors judged the studies against the criteria in the tool (supported by quotes from the study where possible) and classified the risk of bias in each domain as high, low or unclear. We summarised the risk of bias judgements across different studies for each of the domains and overall. We entered review authors’ risk of bias judgements into Covidence, including free‐text explanations.
Assessment of bias in conducting the systematic review
We conducted the review according to the published protocol and report any deviations from it in the ‘Differences between protocol and review' section of the review.
Measures of treatment effect
We planned to analyze dichotomous data as odds ratios (OR) or risk ratios (RR) with 95% confidence intervals (CIs) and continuous data as mean difference (MD) or standardized mean difference (SMD) values with 95% CIs. During the review, we preferred RRs as we could calculate these from reported numerators and denominators. We used MD for all continuous outcomes. One study (Graham 2019) appropriately reported continuous outcomes as medians and interquartile ranges. We transformed these into means and standard deviations to allow assimilation of numbers, using the method of Hozo, Djulbegovic and Hozo (Hozo 2005). Had the numbers of studies doing this correctly been higher, we would have implemented a sensitivity analysis, excluding studies reporting these as a median. As this affected only one study, we did not conduct a sensitivity analysis.
Unit of analysis issues
If any multi‐arm studies met the inclusion criteria of this review, then we planned to merge studies where the participant had received the intervention of prophylactic corticosteroids (regardless of specific corticosteroid or dose). Data for the same outcome with similar follow‐up times were merged.
Dealing with missing data
We applied standard statistical formulae to calculate missing parameter estimates, wherever possible (e.g. using the RevMan 5 calculator to compute the standard deviation of an estimate from other report information such as the CI or exact P values). We attempted to contact investigators to verify key study characteristics and obtain missing numerical outcome data (e.g. when a study was identified only as an abstract).
Assessment of heterogeneity
We planned to use the I² statistic to describe heterogeneity among the treatment effects included in each analysis. We followed the guidance outlined in Section 9.5.2 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017)
0% to 40%: might not be important
30% to 60%: may represent moderate heterogeneity*
50% to 90%: may represent substantial heterogeneity*
75% to 100%: considerable heterogeneity
*The importance of the observed value of the I2 statistic depends on (i) magnitude and direction of effects and (ii) strength of evidence for heterogeneity. If our I2 statistic value indicated that heterogeneity were a possibility and either the Tau2 were greater than zero or the P value were low (less than 0.10), heterogeneity may be have been due to a factor other than chance. If observed, we intended to report substantial heterogeneity and explore possible causes by prespecified subgroup analyses (see Subgroup analysis and investigation of heterogeneity).
Assessment of reporting biases
For all analyses in which treatment effects from 10 or more RCTs were synthesised, we planned to create and examine a funnel plot to explore possible small study biases for the primary outcomes.
Data synthesis
We performed meta‐analyses only where this was meaningful i.e. if the treatments, participants and the underlying clinical question were similar enough for pooling to make sense.
We used random‐effects meta‐analytic models. We were evaluating a drug treatment, the effects of which should, in principle, be homogeneous; and thus we could have used a fixed‐effect model. However, given that we evaluated prophylactic corticosteroids, irrespective of specific drug, dose or timing, differences in treatment regimen could have plausibly introduced heterogeneity among reported treatment effects. A random‐effects model tends to make a pooled estimate more uncertain.
Subgroup analysis and investigation of heterogeneity
We planned to perform the following subgroup analysis where there was sufficient data:
Age: from birth to ≤ 30 days and from 30 days to 18 years
Route of administration (intravenous or oral)
Timing of administration (preoperative, intraoperative or postoperative).
Sensitivity analysis
We intended to carry out sensitivity analyses to assess the robustness of the results for the following categories:
Only including studies with a low risk of bias using the domains from the RoB 1.0 tool.
If we believed that there is large amount of missing data that would have lead to serious bias, then we planned to explore the impact of including such studies by a sensitivity analysis (Dealing with missing data).
As described above, the improvement in cardiac surgery mortality over the last two decades may have lead to a decrease in mortality for the control during the period specified by our search strategy (year 2000 to present, noting that surgery may have been done considerably earlier in a study published in 2000). If we observed this relationship, a further sensitivity analysis would have considered the impact of down‐weighting older studies (e.g. 2000 to 2005 versus 2006 to present) according to the mortality in the control group. To ensure that this sensitivity analysis had reasonable power, we would have needed to consider the distribution over time of participants in included RCTs as well as changes in mortality since 2000 to set a cut‐off for down‐weighting some studies. We considered that a demarcation between 2000 to 2005 and 2006 to present would be reasonable.
We did not carry out the planned sensitivity analyses because there was insufficient information to justify them. The further planned sensitivity analysis would have been based on down‐weighting older studies, which again we judged not justifiable given the small number of studies, and the fact that two‐thirds of studies (contributing over 80% of the weight in the primary analysis) were carried out in the last 10 years.
Reaching conclusions
We based our conclusions only on findings from the quantitative analyses of included studies and avoided making recommendations for practice. Similarly, implications for future research that we propose describe research questions based on the findings or absence of findings in relation to perceived clinical priorities. They also outline what the remaining uncertainties are in the area.
Summary of findings and assessment of the certainty of the evidence
We created a Summary of findings (SoF) table (‘Table 1') using the following outcomes.
In‐hospital, postoperative mortality
Duration of postoperative mechanical ventilation
Length of postoperative intensive care unit stay
Length of postoperative hospital stay
All‐cause mortality at longest follow‐up
Cardiovascular mortality at longest follow‐up
Duration of postoperative inotropes/vasopressors
Failure to separate from cardiopulmonary bypass
The above list does not include an explicit primary harm outcome for the reasons described above (see Types of outcome measures). Nevertheless, important harms might be expected to be reflected in the outcomes that steroids are hypothesised to benefit. We used the five GRADE considerations (study limitations, inconsistency of effect, imprecision, indirectness and publication bias) to assess the certainty of a body of evidence derived from studies contributing data to meta‐analyses for our prespecified outcomes. We used the methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017) using GRADEpro software (GRADEpro 2015). There was only one comparison (corticosteroids versus placebo) for which we generated an SoF table (‘Table 1'). We used footnotes to justify all decisions to downgrade the certainty of the evidence. We extracted study data, formatted our comparisons in data tables and prepared our SoF table (‘Table 1') before writing the results and conclusions of our review.
Results
Description of studies
We have presented the details of studies included in this review in the Characteristics of included studies, and reasons for exclusion in the Characteristics of excluded studies. We have detailed the status of ongoing trials in the Characteristics of ongoing studies.
Results of the search
We completed the search in June 2020. We retrieved 3748 records, of which 888 were reports of studies described in other publications (protocol papers, published conference abstracts or alternative analyses of the same trial data). Two studies that were included (Keski‐Nisula 2013 and Keski‐Nisula 2015) had the same clinical trial registration number, but different populations and interventions. We therefore treated them as separate studies. This led to 2868 records being screened, of which we excluded 2771 on the basis of their titles or abstracts. We reviewed the full texts of 83 studies (in 97 references) for eligibility and deemed 68 of these ineligible (76 references).
We took forward 13 studies (in 19 published references) for data extraction and RoB assessment. Five studies had zero events in both groups for all outcomes of interest and these were excluded from the quantitative analysis, leaving eight studies in the final quantitative meta‐analysis (See Characteristics of excluded studies).
Two studies (Bronicki 2000; Checchia 2003) 'overlapped' 11 participants (i.e. these participants were included in both studies). It was impossible to extract individual patient data from the studies. The corresponding author for both studies, although contacted, did not reply. Due to the small number of participants who were included in both studies and their likely small impact on the overall outcome, we treated each study as separate. We identified no studies that we could not classify as either included or excluded. The flowchart for the results of the search are presented in Figure 1.
1.
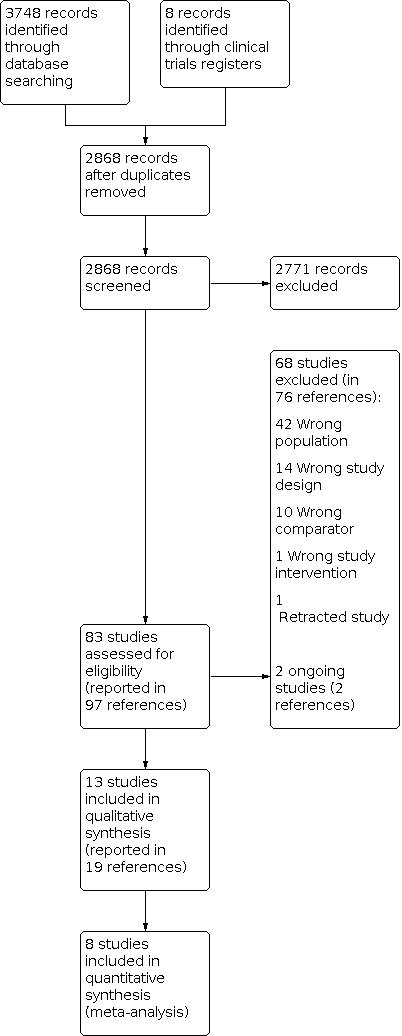
PRISMA diagram
Included studies
We extracted data for 13 studies (comprising 1087 participants), although data for five studies (609 participants) were not included in the final quantitative meta‐analysis due to zero event rates in both intervention and placebo groups for all the outcomes of interest. Therefore, the final meta‐analysis included eight studies and a combined population of 478 participants. They covered all age ranges and where reported (in 11 studies) the mean ages were less than five years old. Similarly the sex of the participants was variably reported, at a lower frequency than age. All studies reported including male and female participants. The studies were all in secondary care (either in the operating room and/or the intensive care unit) from all areas of the world and included a range of surgical procedures (see Included studies for more detail). Most studies had small sample sizes (the largest overall had 246 participants; the largest in the meta‐analysis had 190 participants; most other studies included fewer than 50 participants). Where reported, most studies were funded by non‐commercial organisations. All studies gave the drug intravenously. Five studies used methylprednisolone, two used hydrocortisone and six studies used dexamethasone; all compared the drug with a placebo which, where specifically stated, was normal saline. Of the studies in the meta‐analyses, four used methylprednisolone, two used hydrocortisone and two used dexamethasone. Studies administered the drug or placebo at various time‐points in the perioperative process.
Excluded studies
Of the ineligible studies, 42 had the wrong population (In 40 studies, an adult population),14 had the wrong study design,10 had the wrong comparator, one had the wrong intervention and one study had been retracted. See Characteristics of excluded studies.
We identified two ongoing studies. One is recruiting participants under the age of one year undergoing cardiac surgery with CPB and receiving prophylactic methylprednisolone or placebo (STRESS 2021); this study has a target sample size of 1200. It is not scheduled to complete recruiting until early‐ or mid‐2021.
Risk of bias in included studies
Figure 2 and Figure 3 summarise risk of bias in the included studies.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Four of the 13 studies had a low risk of bias for random sequence generation; all others had unclear risk of bias in this domain. Six studies had a low risk of bias for allocation concealment; two studies were at high risk of bias for this domain. All others had unclear risk of bias in this domain.
Blinding
Malagon 2005 and Amanullah 2016 had a high risk of bias for blinding of participants and personnel. Eight other studies had a low risk of bias and three studies had an unclear risk of bias for this domain. We assessed six studies to be at low risk of bias for blinding of outcome assessment. The other eight studies had unclear risk of bias in this domain.
Incomplete outcome data
Mott 2001 had a high risk of bias for incomplete outcome reporting, in that participants who died after randomization or did not separate from CPB respectively were excluded. Six studies had an unclear risk of bias and six had a low risk of bias for this domain.
Selective reporting
Most of the included studies (10/13) had a low risk of bias for this domain. All others were at unclear risk of bias for this domain.
Other potential sources of bias
Eleven studies were assessed as low risk for other potential sources of bias and two were at unclear risk of bias for this domain.
Effects of interventions
See: Table 1
See Table 1 for the main outcomes. The small number of studies did not support use of the I2 statistic (due to the statistic being biased under these conditions) and so this was not reported (von Hippel 2015). Although we pre‐specified subgroups, we did not conduct analyses, again due to the small number of studies in each group (Higgins 2017).
In‐hospital postoperative mortality
There was moderate certainty of evidence that perioperative corticosteroid changes the risk of in‐hospital mortality (RR 0.83, 95% CI 0.33 to 2.07; 313 participants; five studies; moderate certainty of evidence; Analysis 1.1). We observed no visual heterogeneity in the forest plots. All studies reported this outcome, but there were zero events in both groups for eight studies, comprising 733 participants (Amanullah 2016, Ando 2005, Dalili 2015, Heying 2012, Keski Nisula 2015, Lindberg 2003; Malagon 2005; Mott 2001). Therefore, we did not include them in the meta‐analysis. Five studies comprising 313 participants (Bronicki 2000, Checchia 2003, Graham 2019, Keski Nisula 2013 and Suominen 2017) were included for this outcome.
1.1. Analysis.

Comparison 1: Corticosteroid vs Placebo, Outcome 1: In‐hospital postoperative mortality
Duration of postoperative mechanical ventilation
Six studies (Ando 2005, Dalili 2015, Graham 2019, Keski Nisula 2013, Keski Nisula 2015 and Suominen 2017) were used for the meta‐analysis for this outcome. There was high‐certainty evidence that the intervention reduced the duration of mechanical ventilation (MD ‐11.37 hours, 95% CI ‐20.29 to ‐2.45; 421 participants; six studies; high certainty of evidence; Analysis 1.2). There was a small amount of observed heterogeneity in terms of the effect sizes for this outcome, but the direction of the effect was similar in all.
1.2. Analysis.

Comparison 1: Corticosteroid vs Placebo, Outcome 2: Duration of postoperative mechanical ventilation (hours)
Length of postoperative ICU stay
There was high‐certainty evidence that the intervention probably made little to no difference to the effect on the length of postoperative ICU stay (mean length of stay 7.67 days; MD ‐0.28 days; 95% CI ‐0.79 to 0.24; 421 participants; six studies; high certainty of evidence; Analysis 1.3). There was no visual heterogeneity observed on inspection of the forest plots. The studies included for this analysis were Ando 2005, Dalili 2015, Graham 2019, Keski Nisula 2013, Keski Nisula 2015 and Suominen 2017).
1.3. Analysis.

Comparison 1: Corticosteroid vs Placebo, Outcome 3: Length of postoperative ICU stay (days)
Length of postoperative hospital stay
Only one study (Graham 2019) examined the effect of the intervention on length of postoperative hospital stay. There was moderate certainty of evidence that the intervention had little to no effect on this outcome (mean length of stay 22 days; MD ‐0.7 days, 95%CI ‐2.62 to 1.22; 176 participants; one study; moderate certainty of evidence; Analysis 1.4).
1.4. Analysis.

Comparison 1: Corticosteroid vs Placebo, Outcome 4: Length of postoperative hospital stay
All‐cause mortality at longest follow‐up
In eight studies with 733 participants there were zero events for this outcome in both groups (Amanullah 2016, Ando 2005, Dalili 2015, Heying 2012, Keski Nisula 2015, Lindberg 2003; Malagon 2005; Mott 2001). Therefore, we did not include them in the meta‐analysis. Six studies comprising 353 participants (Bronicki 2000, Checchia 2003, Graham 2019, Keski Nisula 2013 and Suominen 2017) examined the effect of the intervention on all‐cause mortality at the longest follow‐up. There was moderate certainty of evidence that there was probably little to no difference in this outcome between intervention and the placebo (RR 0.83, 95% CI 0.33 to 2.07; 313 participants; five studies; moderate certainty of evidence; Analysis 1.5). There was no visual heterogeneity observed on inspection of the forest plots. The longest follow‐up for all included studies was "in‐hospital" and is therefore the same analysis as In‐hospital post‐operative mortality (Analysis 1.1
1.5. Analysis.

Comparison 1: Corticosteroid vs Placebo, Outcome 5: All‐cause mortality at longest follow‐up
Cardiovascular mortality at longest follow‐up
Three studies examined cardiovascular mortality (Bronicki 2000, Keski Nisula 2013 and Suominen 2017). There was moderate certainty of evidence that the intervention probably made little to no difference in the outcome between intervention and control groups (RR 0.40, 95% CI 0.07 to 2.46; 109 participants; three studies; moderate certainty of the evidence; Analysis 1.6). There was no visual heterogeneity observed on inspection of the forest plots. The longest follow‐up for this outcome was "in‐hospital".
1.6. Analysis.

Comparison 1: Corticosteroid vs Placebo, Outcome 6: Cardiovascular mortality at longest follow‐up
Duration of inotropes
We were unable to examine this outcome due to the variety of outcome measures that were used for reporting inotrope use.
Failure to separate from CPB
Only one study (Keski Nisula 2013) specifically reported failure to separate from CPB after randomization, with events. There was a low certainty of evidence that the intervention probably made little to no difference to the effect in this outcome (RR 0.20, 95% CI 0.01 to 3.92; 40 participants; one study; low certainty of evidence; Analysis 1.7).
1.7. Analysis.

Comparison 1: Corticosteroid vs Placebo, Outcome 7: Failure to separate from CPB
Discussion
Summary of main results
We found a moderate certainty of evidence that corticosteroids have any effect on the in‐hospital mortality in children having heart surgery with CPB. There was high‐certainty evidence that corticosteroids reduce the duration of postoperative ventilation (on average, by about 11 hours) but probably make little to no difference to the effect on the total length of postoperative ICU stay. There was also moderate‐certainty evidence that corticosteroids probably made little to no difference in the outcomes of total postoperative hospital stay, all‐cause mortality or cardiovascular mortality at longest follow‐up. There was low certainty of evidence that corticosteroids have any effect on failure to separate from CPB. See Table 1. We were unable to collect information regarding adverse events of the intervention due to the heterogeneity of outcomes reporting this outcome domain. The two potential short‐term adverse effects of corticosteroids in this setting are hyperglycaemia and infection. There was no standardized method of reporting these outcomes and thus they could not be combined. Methods of reporting inotrope and vasopressor use were also not standardized and for this reason, could not be pooled (or even reported in a useful way).
Overall completeness and applicability of evidence
The outcomes of the meta‐analysis are broadly applicable to all settings of paediatric cardiac surgery with cardiopulmonary bypass – with the caveats of reduced certainty of evidence where stated. The studies as a whole were designed using a method that was relevant to the primary outcomes of the review (i.e. mortality and duration of mechanical ventilation) and the majority of studies included in the synthesis were mainly from the USA and Northern Europe, although there were included studies from the Middle East and Asia (Dalili 2015 and Amanullah 2016). Studies investigated different drugs: methylprednisolone, hydrocortisone and dexamethasone. Many of the separate studies share authors (e.g. Bronicki 2000 / Checchia 2003 and Keski Nisula 2013 / Keski Nisula 2015 and Suominen 2017). The fact that these studies were most likely to be included in the quantitative review will tend to reduce the external validity of the results. The studies included in the meta‐analysis comprised a range of cardiac surgery procedures performed with CPB in all age ranges.
Quality of the evidence
We included 13 studies (1096 participants) in the synthesis, of which 8 studies (478 participants) contributed to the quantitative synthesis. Five studies (618 participants) were excluded from the quantitative synthesis because of zero event rates in both groups for dichotomous outcomes and did not report the continuous variable outcomes. This illustrates one of the issues with the studies contained within the review; most studies were small and even when pooled, the total number of participants may not be sufficient to demonstrate a difference in mortality due to the low event rate. This issue was predicted in our protocol and therefore we included a second primary outcome (postoperative mechanical ventilation) of a continuous nature that was more likely to give us the power to answer this question. We achieved this with high certainty.
Only one study (Graham 2019) was judged as at low risk of bias across all domains. The overall uncertain risk of bias in most studies, combined with the imprecision due to the small sample sizes of studies, resulted in our downgrading of evidence for most outcomes. The exceptions to this downgrading were duration of postoperative ventilation and length of postoperative ICU stay. Therefore, our overall judgement is that, apart from the latter two outcomes, the certainty of evidence is inadequate to draw firm conclusions. Furthermore, without pooled adverse event information, there is no information on which to make risk‐benefit judgments.
Potential biases in the review process
The potential sources of bias in the process of conducting the review were small. We may not have identified studies that were not in the English language but the number of such studies is likely to be very small. We searched all major databases but were not able to formally examine publication bias due to the small number of studies. Given the inconsistency of the results, it would subjectively appear that there is no important publication bias for this intervention. All relevant data were obtained, and it is unlikely that the methods used by the authors could have introduced bias.
We converted published medians and ranges within studies to means and SDs, using the method of Hozo and colleagues (Hozo 2005). We did this to allow their inclusion in the meta‐analysis, instead of being limited to a narrative summary. Only one study correctly used medians for length of stay and duration of mechanical ventilation. We combined data from this study with the others that did not use medians correctly. Whilst this is an approximation, we maintain that this remains relevant, since there was only one study for which this was done. Before doing so, we consulted the Cochrane Statistical Methods Group and received support for our approach.
Agreements and disagreements with other studies or reviews
One recent systematic review and meta‐analysis concerns the same intervention (Scrascia 2014). As with our review, Scrascia 2014 showed no statistical effect of corticosteroids on mortality. They also found no effect on postoperative mechanical ventilation or intensive care stay. The change in our meta‐analysis towards reduced postoperative mechanical ventilation is the result of including the study by Graham 2019. Without the inclusion of this study (as in Scrascia 2014) there would be no effect of corticosteroids on the outcome. This is due to the increased precision of a large study (i.e. reducing the confidence intervals), but the point estimates reported are consistent. Graham 2019 is the only study with a low risk of bias across all domains. The results of our meta‐analysis also concur with very large retrospective analyses of national databases, such as the one reported by Pasquali 2012 This analysis reported no effect of corticosteroids on mortality or length of stay outcomes.
Authors' conclusions
Implications for practice.
It is impossible to provide any implications for practice without robust adverse event information to allow the risk trade‐off between benefits and harms. We are unable to do this for corticosteroids in the context of paediatric cardiac surgery with CPB.
Implications for research.
The evidence synthesised by this review for the most important outcome of mortality is uncertain due to imprecision. However, the low and declining mortality rate (Kennedy 2001; Grant 2013; Brown 2015; Hoashi 2015; Jacobs 2016) of paediatric cardiac surgery makes this outcome less useful than some composite measure of postoperative morbidity, both in the short term and long term. Our review has highlighted the need for adequately powered and well‐conducted RCTs to confirm or refute the effect of corticosteroids versus placebo in children having cardiac surgery with CPB. The studies should be large enough to separately analyze the outcomes of all children, as well as the neonatal and non‐neonatal populations. The studies should be powered for clinical outcomes rather than biochemical markers and would also need to collect robust adverse event information (including long‐term follow‐up of cognitive and educational outcomes), using a core outcome set, to allow robust decisions about their use to be made. We anticipate that the STRESS study (STRESS 2021) will have a major impact on the evidence base for this topic. This trial is due to finish recruiting in June 2021, and will include almost twice as many participants (1200) as are in our meta‐analysis.
History
Protocol first published: Issue 8, 2018 Review first published: Issue 10, 2020
Acknowledgements
We are grateful to Charlene Bridges (Information Specialist, Cochrane Heart Group) for performing an initial scoping search of manuscripts.
Paul Rival (Medical Student, University of Bristol) helped extract some information from the scoping search.
Vincent Cheng (University of Bristol) provided valuable skills moving data from Covidence to RevMan.
We are grateful to Eric M Graham, Matthew M Townsley, Kevin Hill, Eero Pesonen and Danial Sayyad whose peer review substantially improved the accuracy and clarity of this review.
Appendices
Appendix 1. Search strategies
CENTRAL
#1 MESH DESCRIPTOR Adrenal Cortex Hormones EXPLODE ALL AND CENTRAL:TARGET
#2 corticosteroid* AND CENTRAL:TARGET
#3 steroid* AND CENTRAL:TARGET
#4 corticoid* AND CENTRAL:TARGET
#5 MESH DESCRIPTOR Mineralocorticoids EXPLODE ALL AND CENTRAL:TARGET
#6 MESH DESCRIPTOR Glucocorticoids AND CENTRAL:TARGET
#7 glucocorticoid* AND CENTRAL:TARGET
#8 MESH DESCRIPTOR Hydrocortisone AND CENTRAL:TARGET
#9 hydrocortisone* AND CENTRAL:TARGET
#10 MESH DESCRIPTOR Dexamethasone AND CENTRAL:TARGET
#11 dexamethasone* AND CENTRAL:TARGET
#12 MESH DESCRIPTOR Methylprednisolone AND CENTRAL:TARGET
#13 methylprednisolone* AND CENTRAL:TARGET
#14 MESH DESCRIPTOR Prednisolone AND CENTRAL:TARGET
#15 prednisolone* AND CENTRAL:TARGET
#16 MESH DESCRIPTOR Prednisone AND CENTRAL:TARGET
#17 prednisone* AND CENTRAL:TARGET
#18 mineralocorticoid* AND CENTRAL:TARGET
#19 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18
#20 MESH DESCRIPTOR Thoracic Surgery AND CENTRAL:TARGET
#21 MESH DESCRIPTOR Cardiovascular Surgical Procedures EXPLODE ALL AND CENTRAL:TARGET
#22 MESH DESCRIPTOR Cardiac Surgical Procedures EXPLODE ALL AND CENTRAL:TARGET
#23 ((cardiac or cardiol* or heart) NEAR2 (surgery or surgeries or surgical or procedure* or operat*)) AND CENTRAL:TARGET
#24 MESH DESCRIPTOR Cardiopulmonary Bypass AND CENTRAL:TARGET
#25 cardiopulmonary bypass AND CENTRAL:TARGET
#26 cpb AND CENTRAL:TARGET
#27 heart NEAR3 bypass AND CENTRAL:TARGET
#28 cardiac NEAR3 bypass AND CENTRAL:TARGET
#29 MESH DESCRIPTOR Heart Defects, Congenital AND CENTRAL:TARGET
#30 #29 OR #28 OR #27 OR #26 OR #25 OR #23 OR #24 OR #22 OR #21 OR #20
#31 #30 AND #19
#32 >1999:YR AND CENTRAL:TARGET
#33 #31 AND #32
MEDLINE Ovid
1. exp Adrenal Cortex Hormones/
2. (corticosteroid* or steroid*).tw.
3. corticoid*.tw.
4. exp Mineralocorticoids/
5. Mineralocorticoid*.tw.
6. Glucocorticoids/
7. Glucocorticoid*.tw.
8. Hydrocortisone/
9. Hydrocortisone*.tw.
10. Dexamethasone/
11. Dexamethasone*.tw.
12. Methylprednisolone/
13. Methylprednisolone*.tw.
14. Prednisolone/
15. Prednisolone*.tw.
16. Prednisone/
17. Prednisone*.tw.
18. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17
19. Thoracic surgery/
20. exp Cardiovascular Surgical Procedures/
21. exp cardiac surgical procedures/
22. ((cardiac or cardiol* or heart) adj2 (surgery or surgeries or surgical or procedure* or operat*)).tw.
23. Cardiopulmonary Bypass/
24. cardiopulmonary bypass.tw.
25. cpb.tw.
26. (heart adj3 bypass).tw.
27. (cardiac adj3 bypass).tw.
28. Heart Defects, Congenital/
29. 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28
30. 18 and 29
31. randomized controlled trial.pt.
32. controlled clinical trial.pt.
33. randomized.ab.
34. placebo.ab.
35. drug therapy.fs.
36. randomly.ab.
37. trial.ab.
38. groups.ab.
39. 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38
40. exp animals/ not humans.sh.
41. 39 not 40
42. 30 and 41
43. limit 42 to yr="2000 ‐Current"
Embase Ovid
1. exp corticosteroid/
2. (corticosteroid* or steroid*).tw.
3. corticoid*.tw.
4. exp mineralocorticoid/
5. Mineralocorticoid*.tw.
6. glucocorticoid/
7. Glucocorticoid*.tw.
8. hydrocortisone/
9. Hydrocortisone*.tw.
10. dexamethasone/
11. Dexamethasone*.tw.
12. methylprednisolone/
13. Methylprednisolone*.tw.
14. prednisolone/
15. Prednisolone*.tw.
16. prednisone/
17. Prednisone*.tw.
18. or/1‐17
19. thorax surgery/
20. exp cardiovascular surgery/
21. ((cardiac or cardiol* or heart) adj2 (surgery or surgeries or surgical or procedure* or operat*)).tw.
22. cardiopulmonary bypass/
23. cardiopulmonary bypass.tw.
24. cpb.tw.
25. (heart adj3 bypass).tw.
26. (cardiac adj3 bypass).tw.
27. congenital heart malformation/
28. or/19‐27
29. 18 and 28
30. random$.tw.
31. factorial$.tw.
32. crossover$.tw.
33. cross over$.tw.
34. cross‐over$.tw.
35. placebo$.tw.
36. (doubl$ adj blind$).tw.
37. (singl$ adj blind$).tw.
38. assign$.tw.
39. allocat$.tw.
40. volunteer$.tw.
41. crossover procedure/
42. double blind procedure/
43. randomized controlled trial/
44. single blind procedure/
45. 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44
46. (animal/ or nonhuman/) not human/
47. 45 not 46
48. 29 and 47
49. limit 48 to yr="2000 ‐Current"
CPCI‐S
# 13 #12 AND #11 Indexes=CPCI‐S Timespan=2000‐2020
# 12 TS=(random* or blind* or allocat* or assign* or trial* or placebo* or crossover* or cross‐over*)
# 11 #10 AND #5
# 10 #9 OR #8 OR #7 OR #6
# 9 TS=(cardiac NEAR/3 bypass)
# 8 TS=(heart NEAR/3 bypass)
# 7 TS=("cardiopulmonary bypass" OR cpb)
# 6 TS=((cardiac or cardiol* or heart) NEAR/2 (surgery or surgeries or surgical or procedure* or operat*))
# 5 #4 OR #3 OR #2 OR #1
# 4 TS=(Mineralocorticoid* OR Glucocorticoid* OR Hydrocortisone* OR Dexamethasone* OR Methylprednisolone* OR Prednisolone* OR Prednisone*)
# 3 TS=corticoid*
# 2 TS=steroid*
# 1 TS=corticosteroid*
Trials Registers
(cardiac OR heart)
(steroid OR prednisolone)
(surgery OR surgical OR procedure)
Data and analyses
Comparison 1. Corticosteroid vs Placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 In‐hospital postoperative mortality | 5 | 313 | Risk Ratio (IV, Random, 95% CI) | 0.83 [0.33, 2.07] |
| 1.2 Duration of postoperative mechanical ventilation (hours) | 6 | 421 | Mean Difference (IV, Random, 95% CI) | ‐11.37 [‐20.29, ‐2.45] |
| 1.3 Length of postoperative ICU stay (days) | 6 | 421 | Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.79, 0.24] |
| 1.4 Length of postoperative hospital stay | 1 | 176 | Mean Difference (IV, Random, 95% CI) | ‐0.70 [‐2.62, 1.22] |
| 1.5 All‐cause mortality at longest follow‐up | 5 | 313 | Risk Ratio (IV, Random, 95% CI) | 0.83 [0.33, 2.07] |
| 1.6 Cardiovascular mortality at longest follow‐up | 3 | 109 | Risk Ratio (IV, Random, 95% CI) | 0.40 [0.07, 2.46] |
| 1.7 Failure to separate from CPB | 1 | 40 | Risk Ratio (IV, Random, 95% CI) | 0.20 [0.01, 3.92] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Amanullah 2016.
| Study characteristics | ||
| Methods |
Study design: RCT Study grouping: Parallel group Total Duration of Study: 2 years Number of study centres and location: 1 centre Study setting: PICU Withdrawals: None Study Date: April 2010 to April 2012 |
|
| Participants |
Baseline Characteristics Corticosteroid
Placebo
Overall
Included criteria: children between the ages of 1 month and 18 years undergoing their first elective cardiac surgery with cardiopulmonary bypass Exclusion criteria: PREOPERATIVELY Children with a history of premature birth (less than 28 weeks of gestation) Compromised immune system (known immunodeficiency or use of immunomodulatory therapy) PERIOPERATIVELY Those who perioperatively had two or more clinical or laboratory signs of active infection that were not attributable to any other cause:
INTRAOPERATIVELY Patients who required cardiopulmonary bypass for more than six hours or who required a second run of cardiopulmonary bypass during the same surgery. Patients who required medically appropriate steroid therapy during this time.Those who had to be taken back to the operating room for unforeseen complications.Those who expired before the completion of the 24 hour postoperative period. Pretreatment: nil |
|
| Interventions | Corticosteroid
Placebo
|
|
| Outcomes | Duration of postoperative mechanical ventilation
Length of postoperative intensive care unit stay
All‐cause mortality at longest follow‐up
Cardiovascular mortality at longest follow‐up
Duration of postoperative inotropes/vasopressors
Failure to separate from cardiopulmonary bypass
Adverse events
In‐hospital postoperative mortality
Length of postoperative hospital stay
|
|
| Identification |
Sponsorship source: University Research Council. Aga Khan University Country: Pakistan Setting: PICU Comments: NA Authors name: Muhammad M Amanullah Institution: The Aga Khan University Email: muneer.amanullah@aku.edu Address: Department of Surgery, The Aga Khan University Hospital, Stadium Road, PO Box 3500, Karachi 74800, Pakistan. Year: 2015 |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer generated randomization scheme |
| Allocation concealment (selection bias) | Low risk | "Randomisation was carried out by an independent statistician" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Anaesthesia resident prepared the syringes" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcomes reported |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No other bias |
Ando 2005.
| Study characteristics | ||
| Methods |
Study design: RCT Study grouping: Parallel group Duration of study: February 2002 to June 2004 Number of study centres: 1 Study setting: PICU ‐ Hospital Withdrawals: None reported Study date: 2005 |
|
| Participants |
Baseline characteristics Corticosteroid
Placebo
Overall
Included criteria: neonates within 28 days after birth undergoing complete biventricular repair Exclusion criteria: mechanical ventilation, evidence of infection, receiving more than renal dose dopamine (5mcg/kg/min), and genetic disorder or chromosomal abnormality. Pretreatment: no statistical differences |
|
| Interventions | Corticosteroid
Placebo
|
|
| Outcomes | All‐cause mortality at longest follow‐up
Length of postoperative hospital stay
Length of postoperative intensive care unit stay
Duration of postoperative inotropes/vasopressors
Duration of postoperative mechanical ventilation
Failure to separate from cardiopulmonary bypass
In‐hospital postoperative mortality
Cardiovascular mortality at longest follow‐up
Adverse events (Hypoglycaemia, Blood glucose level g/dL)
|
|
| Identification |
Sponsorship source: none reported Country: Japan Setting: PICU Comments: N/A Authors name: Makoto Ando Institution: Sakakibara Heart Institute, Email: maando@shi.heart.or.jp. Address: Department of Pediatric CardiacSurgery, Sakakibara Heart Institute, 3‐16‐1 Asahi‐cho, Fuchu‐si, Tokyo,183‐0003 Japan Year: 2005 |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Low risk | "Allocation was concealed to all clinical participants and data interpreter" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Allocation was concealed to all clinical participants and data interpreter ‐ clinical staff may have known" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcomes reported |
| Selective reporting (reporting bias) | Unclear risk | Not specified |
| Other bias | Low risk | No other bias |
Bronicki 2000.
| Study characteristics | ||
| Methods |
Study design: RCT Study grouping: Parallel group Duration of Study: not specified Number of study centres: 1 Study setting: PICU Withdrawals: unclear Study Date: 2000 |
|
| Participants |
Baseline characteristics Corticosteroid
Placebo
Overall
Included criteria: children undergoing open heart surgical procedures for congenital heart defects. Exclusion criteria: preoperative use of corticosteroids or nonsteroid anti‐inflammatory agents, isolated atrial septal defect, CPB time greater than 200 minutes, and aortic cross‐clamp time greater than 120 minutes. Pretreatment: similar at baseline |
|
| Interventions | Corticosteroid
Placebo
|
|
| Outcomes | Duration of postoperative mechanical ventilation
Length of postoperative intensive care unit stay
All‐cause mortality at longest follow‐up
Cardiovascular mortality at longest follow‐up
Duration of postoperative inotropes/vasopressors
Failure to separate from cardiopulmonary bypass
Adverse events (Wound healing dehiscence)
In‐hospital postoperative mortality
Length of postoperative hospital stay
|
|
| Identification |
Sponsorship source: not Specified Country: USA Setting: PICU Comments: N/A Authors name: Ronald A. Bronicki Institution: Northwestern University Medical School, Children’s Memorial Hospital Email: c‐backer@nwu.edu Address: Children’s Memorial Hospital, 2300 Children’s Plaza, m/c 22, Chicago, IL 60614 Year: 2000 |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not specified |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not specified |
| Selective reporting (reporting bias) | Unclear risk | Not specified |
| Other bias | Unclear risk | Unclear inclusion and exclusion criteria as well as how the participants were dealt with. |
Checchia 2003.
| Study characteristics | ||
| Methods |
Study design: RCT Study grouping: Parallel group Total duration of study: Not reported Number of study centres: 1 Study setting: Theatre/PICU Withdrawals: none reported Study Date: not reported |
|
| Participants |
Baseline characteristics Corticosteroid
Placebo
Overall
Included criteria: children undergoing open‐heart surgery for congenital heart disease. Exclusion criteria: patients undergoing repair of an isolated atrial septal defect, if there was a history of preoperative use of corticosteroid or nonsteroidal antiinflammatory agents, if they were febrile (38.5°C), or had an elevated white blood cell count (12,000 cells/mm3). Pretreatment: none |
|
| Interventions | Corticosteroid
Placebo
|
|
| Outcomes | All‐cause mortality at longest follow‐up
In‐hospital postoperative mortality
Length of postoperative intensive care unit stay
Duration of postoperative inotropes/vasopressors
Duration of postoperative mechanical ventilation
Length of postoperative hospital stay
Cardiovascular mortality at longest follow‐up
Failure to separate from cardiopulmonary bypass
Adverse effects
|
|
| Identification |
Sponsorship source: None reported Country: USA Setting: PICU Comments: NA Authors name: Paul A. Checchia Institution: Children’s Memorial Hospital, Chicago, USA Email: cbacker@childrensmemorial.org Address: Children’s Memorial Hospital, 2300 Children’sPlaza, m/c 22, Chicago, IL 60614 Year: 2003 |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were sequentially randomised" |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "all treating physicians were blinded" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not specified |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No other bias |
Dalili 2015.
| Study characteristics | ||
| Methods |
Study design: RCT Study grouping: Parallel group Total duration of study: not reported Number of study centres: 1 Study setting: PICU Withdrawals: not reported |
|
| Participants |
Baseline characteristics Corticosteroid
Placebo
Overall
Included criteria: children aged 0 to 15 years who underwent total repair of Fallot's Tetralogy Exclusion criteria: not reported Pretreatment: no significantly differences between groups |
|
| Interventions | Corticosteroid
Placebo
|
|
| Outcomes | All‐cause mortality at longest follow‐up
Length of postoperative intensive care unit stay
Duration of postoperative inotropes/vasopressors
Duration of postoperative mechanical ventilation
Cardiovascular mortality at longest follow‐up
Adverse effects (Infection)
Failure to separate from cardiopulmonary bypass
In‐hospital postoperative mortality
Length of postoperative hospital stay
|
|
| Identification |
Sponsorship source: None reported Country: Iran Setting: PICU Comments: NA Authors name: Mohammad Dalili Institution: Rajale Cardiovascular Medical and Research Center, Iran University of Medical Sciences, Tehran, Iran. Email: drdalili@yahoo.com Address: Rajale Cardiovascular Medical and Research Center, Vali‐asr St., Niayesh Blvd, Tehran, IR Iran. Year: 2014 |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomised into 2 groups. Computer generated randomization specified |
| Allocation concealment (selection bias) | High risk | A placebo could not be prepared |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "all treating physicians were blinded" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Different staff |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not specified |
| Selective reporting (reporting bias) | Low risk | Specified outcomes reported |
| Other bias | Low risk | No other bias |
Graham 2019.
| Study characteristics | ||
| Methods |
Study design: RCT Study grouping: Parallel group Duration of study: 5 years Number of study centres: 2 Study date: June 2012 and ended in November 2017 Study setting: PICU Withdrawals: 14 |
|
| Participants |
Baseline Characteristics Corticosteroid
Placebo
Overall
Included criteria: infants ≤ 1 month (31 days) of age scheduled to undergo cardiac surgery with CPB. Exclusion criteria: prematurity (defined as < 37 weeks post gestational age) at the time of surgery, treatment with steroids in the 2 days prior to surgery, participation in research studies involving the evaluation of investigational drugs or vaccines within 30 days of randomization, suspected infection that would contraindicate steroid use (e.g. herpes), known hypersensitivity to methylprednisolone or other contraindication to steroid therapy (e.g. gastrointestinal bleeding), preoperative use of mechanical circulatory support or active resuscitation at the time of proposed randomization. Pretreatment: nil |
|
| Interventions | Corticosteroid
Placebo
|
|
| Outcomes | All‐cause mortality at longest follow‐up
Length of postoperative hospital stay
Length of postoperative intensive care unit stay
Duration of postoperative inotropes/vasopressors
Duration of postoperative mechanical ventilation
Failure to separate from cardiopulmonary bypass
In‐hospital postoperative mortality
Cardiovascular mortality at longest follow‐up
Adverse events
|
|
| Identification |
Sponsorship source: National Heart, Lung, and Blood Institute (NHLBI). Country: USA Setting: PICU Comments: none Authors name: Eric M. Graham Institution: Medical University of South Carolina Email: grahamem@musc.edu Address: Children’s Heart Center, 165 Ashley Ave; MSC 915, Charleston, SC 29425 Year: 2019 |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were assigned by permuted block randomization within strata according to the planned procedure being palliative or corrective and by the surgeon." |
| Allocation concealment (selection bias) | Low risk | "All patients, caregivers, health‐care providers, and investigation personnel were blinded to the treatment allocation until the close of the study." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Study drug was prepared and masked by the local investigational pharmacy and delivered to the operating room" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "All patients, caregivers, health‐care providers, and investigation personnel were blinded to the treatment allocation until the close of the study." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcomes reported |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No other bias |
Heying 2012.
| Study characteristics | ||
| Methods |
Study design: RCT Study grouping: Parallel group Duration of study: not stated Number of study centres: not reported Study date: not reported Study setting: PICU Withdrawals: none reported |
|
| Participants |
Baseline Characteristics Corticosteroid
Placebo
Overall
Included criteria: neonates (age, 8 to 21 days) diagnosed with TGA with or without ventricular septum defect scheduled for arterial switch operation Exclusion criteria: not reported Pretreatment: no differences |
|
| Interventions | Corticosteroid
Placebo
|
|
| Outcomes | All‐cause mortality at longest follow‐up
Length of postoperative hospital stay
Length of postoperative intensive care unit stay
Duration of postoperative inotropes/vasopressors
Duration of postoperative mechanical ventilation
Failure to separate from cardiopulmonary bypass
In‐hospital postoperative mortality
Cardiovascular mortality at longest follow‐up
Adverse events
|
|
| Identification |
Sponsorship source: Deutsche Forschungsgemeinschaft. Research Foundation Flanders (FWO, Klinische Doctoraatsbeurs), Belgium Country: Belgium and Germany Setting: PICU Comments: It is not clear if this is a single or multiple centre (Belgium and Germany) or single centre Authors name: Ruth Heying Institution: University Hospital Aachen, Aachen, Germany University Hospital Liège, Liège, Belgium German Heart Centre at the Technical University, Munich, Germany Email: ruth.heying@uzleuven.be Address: UZ Leuven,Herestraat 49, 3000 Leuven, Belgium Year: 2012 |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not specified |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "code was broken after data acquisition was completed." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcomes reported |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No other bias |
Keski Nisula 2013.
| Study characteristics | ||
| Methods |
Study design: RCT Study grouping: Parallel group Duration of study: not reported Number of study centres: not reported Study date: not reported Study setting: not reported Withdrawals: not reported |
|
| Participants |
Baseline Characteristics Corticosteroid
Placebo
Overall
Included criteria: neonates (age 28 days) undergoing open‐heart surgery Exclusion criteria: not reported Pretreatment: nil |
|
| Interventions | Corticosteroid
Placebo
|
|
| Outcomes |
All‐cause mortality at longest follow‐up
Length of postoperative hospital stay
Length of postoperative intensive care unit stay
Duration of postoperative inotropes/vasopressors
Duration of postoperative mechanical ventilation
Failure to separate from cardiopulmonary bypass
In‐hospital postoperative mortality
Cardiovascular mortality at longest follow‐up
Adverse events (hypoglycaemia, glucose (mmol/L)
|
|
| Identification |
Sponsorship source: The Foundation for Pediatric Research and Helsinki UniversityCentral Hospital Country: Finland Setting: PICU Comments: na Authors name: Juho Keski‐Nisula Institution: Children’s Hospital, Helsinki University Central Hospital. Email: juho.keski‐nisula@hus.fi Address: Department of Anesthesiaand Intensive Care, Children’s Hospital, Helsinki University CentralHospital, P.O B. 281, Stenbackinkatu 11, FI‐00029 HUS, Helsinki, Finland Year: 2013 |
|
| Notes | This study has the same ISCRTN number as Keski‐Nisula 2015 ‐ but a different population and intervention. Treated as separate studies. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "In this randomised (sealed envelope), double‐blind, placebo‐controlled investigation," |
| Allocation concealment (selection bias) | Low risk | "A pharmacist who was not involved in the care of the study patients prepared methylprednisolone and placebo solutions." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "All the syringes were covered with nontransparent paper foil" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcomes reported |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No other bias |
Keski Nisula 2015.
| Study characteristics | ||
| Methods |
Study design: RCT Study grouping: Parallel group Duration of study: not reported Number of study centres: 1 Study date: not reported Study setting: PICU Withdrawals: not reported |
|
| Participants |
Baseline Characteristics Corticosteroid
Placebo
Overall
Included criteria: children between 1 and 18 months of age undergoing VSD or AVSD repair Exclusion criteria:
Pretreatment: no significant difference |
|
| Interventions |
Intervention Characteristics Glucocorticoid
Placebo
|
|
| Outcomes | All‐cause mortality at longest follow‐up
Length of postoperative hospital stay
Length of postoperative intensive care unit stay
Duration of postoperative inotropes/vasopressors
Duration of postoperative mechanical ventilation
Failure to separate from cardiopulmonary bypass
In‐hospital postoperative mortality
Cardiovascular mortality at longest follow‐up
Adverse events (Infection)
|
|
| Identification |
Sponsorship source: Helsinki University Central Hospital Country: Finland Setting: PICU Comments: NA Authors name: Juho Keski‐Nisula Institution: Children’s Hospital, Helsinki University Central Hospital; Email: juho.keski‐nisula@hus.fi Address: Department of Anesthesiaand Intensive Care, Children’s Hospital, Helsinki University CentralHospital, PO Box 281, Stenbackinkatu 11, FI‐00029 HUS, Helsinki, Finland; Year: 2015 |
|
| Notes | This study has the same ISCRTN number as Keski‐Nisula 2013 ‐ but a different population and intervention. Treated as separate studies. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomised by sealed envelope equally into three study groups." |
| Allocation concealment (selection bias) | Low risk | "A pharmacist not involved in the care of the study patients prepared two syringes, which contained either MP or placebo (corresponding volume of saline solution);" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "these were subsequently covered by nontransparent paper. All the patients received two study drug syringes regardless of treatment." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "All study and clinical personnel were blinded to the treatment allocation until the study period ended" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not specified outcomes |
| Selective reporting (reporting bias) | Low risk | Appears to have reported all outcomes |
| Other bias | Low risk | No other bias |
Lindberg 2003.
| Study characteristics | ||
| Methods |
Study design: RCT Study grouping: Parallel group Duration of study: Not reported Number of study centres: 1 Study setting: PICU Withdrawals: 1 Study Date: Not clear |
|
| Participants |
Baseline Characteristics Corticosteroid
Placebo
Overall
Included criteria: children (>10 kg) about to have open heart surgery Exclusion criteria: not reported Pretreatment: no differences |
|
| Interventions | Corticosteroid
Placebo
|
|
| Outcomes | Duration of postoperative mechanical ventilation
Length of postoperative intensive care unit stay
All‐cause mortality at longest follow‐up
Cardiovascular mortality at longest follow‐up
Duration of postoperative inotropes/vasopressors
Failure to separate from cardiopulmonary bypass
Adverse events
In‐hospital postoperative mortality
Length of postoperative hospital stay
|
|
| Identification |
Sponsorship source: not reported Country: Sweden Setting: PICU Comments: none Authors name: L. Lindberg Institution: University Hospital, Lund, Sweden Email: larsolavlindberg@hotmail.com Address: Department of Pediatric Anesthesia and Intensive Care, University Hospital Lund,S‐221 85 Lund, Swede Year: 2003 |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The nature of the agent was masked." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Specified outcomes reported |
| Selective reporting (reporting bias) | Low risk | Specified outcomes reported |
| Other bias | Unclear risk | No other bias |
Malagon 2005.
| Study characteristics | ||
| Methods |
Study design: RCT Study grouping: Parallel group Duration of study: 14 months Number of study centres: 1 Study date: October 2003 and December 2004 Study setting: PICU Withdrawals: Not reported |
|
| Participants |
Baseline Characteristics Corticosteroid
Overall
Included criteria: pediatric patients with CPB for cardiac surgical procedures. Exclusion criteria: patients operated on without CPB. Pretreatment: nil |
|
| Interventions | Corticosteroid
|
|
| Outcomes | All‐cause mortality at longest follow‐up
Length of postoperative hospital stay
Length of postoperative intensive care unit stay
Duration of postoperative inotropes/vasopressors
Duration of postoperative mechanical ventilation
Failure to separate from cardiopulmonary bypass
In‐hospital postoperative mortality
Cardiovascular mortality at longest follow‐up
Adverse events
|
|
| Identification |
Sponsorship source: not reported Country: Netherlands Setting: PICU Comments: None Authors name: Ignacio Malagon Institution: Leiden University Medical Center Email: jmalagon@lumc.nl Address: Leiden University Medical Center,Albinusdreef 2, P.O. Box 9600,2300 RC Leiden, The Netherlands Year: 2005 |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomised using standard randomization tables" |
| Allocation concealment (selection bias) | High risk | "The use of placebo is not allowed by our hospital ethics committee." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "The use of placebo is not allowed by our hospital ethics committee." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "cTnT concentrations were measured by the hospital clinical chemistry laboratory, and the analysts were unaware of the conduct of this study." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not specified |
| Selective reporting (reporting bias) | Low risk | Appear to report all outcomes |
| Other bias | Low risk | No other bias |
Mott 2001.
| Study characteristics | ||
| Methods |
Study design: RCT Study grouping: Parallel group Duration of study: 30 months Number of study centres: 1 Study date: March 1996 to June 1998 Study setting: operating theatre and PICU Withdrawals: 20 (6 due to death, 14 due to protocol deviation) |
|
| Participants |
Baseline Characteristics Corticosteroid
Placebo
Overall
Included criteria: paediatric patients with CPB for cardiac surgical procedures. Exclusion criteria: Allergy to methylprednisolone, patients being treated with a steroid for chronic immune suppression, and patients with previously documented hematologic, hepatic or renal dysfunction. |
|
| Interventions | Corticosteroid
Placebo
|
|
| Outcomes | ||
| Identification |
Sponsorship source: not reported Country: USA Setting: operating theatre and PICU Comments: none Authors name: Antonio Mott Institution: Texas Childrens Hospital Email: AMott@bcm.tmc.edu Address: Section of Pediatric Cardiology, Texas Childrens Hospital, 6621 Fannin, MC#2‐2280, Houston. Texas 77030 Year: 2005 |
|
| Notes | No outcomes of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "each patient a study number from the randomisation table that was created by the study statistician." |
| Allocation concealment (selection bias) | Low risk | "Drugs delivered by pharmacist" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Non‐involved staff delivered drugs" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Patients who died were excluded after randomization |
| Selective reporting (reporting bias) | Low risk | Specified outcomes reported |
| Other bias | Low risk | No other bias |
Suominen 2017.
| Study characteristics | ||
| Methods |
Study design: RCT Study grouping: Parallel group Duration of study: 30 Months Number of study centres: 1 Study date: April 2012 and October 2014 Study setting: PICU Withdrawals: 0 |
|
| Participants |
Baseline Characteristics Corticosteroid
Placebo
Overall
Included criteria: neonates (age 28 days) who were undergoing non emergency cardiac operations with cardiopulmonary bypass (CPB). Exclusion criteria: Symptoms related to prematurity or birth before 36 weeks of gestational age, chromosomal abnormalities, administration of corticosteroids before the operation, and the need of preoperative inotropic support other than milrinone Pretreatment: none |
|
| Interventions |
Intervention Characteristics Corticosteroid
Placebo
|
|
| Outcomes | All‐cause mortality at longest follow‐up
Length of postoperative hospital stay
Length of postoperative intensive care unit stay
Duration of postoperative inotropes/vasopressors
Duration of postoperative mechanical ventilation
Failure to separate from cardiopulmonary bypass
In‐hospital postoperative mortality
Cardiovascular mortality at longest follow‐up
Adverse events (insulin administration)
Adverse events (Wound infections)
Adverse events (Septic blood culture‐positive)
|
|
| Identification |
Sponsorship source: The Paulo Foundation (Tapani Tammiston rahasto 2012 to P.K.S) and the Foundation of Paediatric Research (130069 to P.K.S) Country: Finland Setting: PICU Comments: Nil Authors name: Pertti K. Suominen Institution: Children’s Hospital, Helsinki University Hospital Email: pertti.suominen@hus.fi Address: Department of Anesthesia and Intensive Care, Children’s Hospital, Helsinki University Hospital, University of Helsinki, Stenbackinkatu 11, FI‐00029 HUS Helsinki, Finland Year: 2017 |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomised by sealed envelope into two groups." |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "All study and clinical personnel were blinded to the treatment allocation until the study period ended" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "All study and clinical personnel were blinded to the treatment allocation until the study period ended." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not specified |
| Selective reporting (reporting bias) | Unclear risk | Not specified |
| Other bias | Low risk | No other bias |
Abbreviations: ASD: Atrial‐septal defect, VSD: Ventriculo‐septal defect, AVSD: Atrio‐ventriculo‐septal defect, CAVC: Complete atrio‐ventricular canal defect, TGA: Transposition of the great arteries, TAPVD: Total anomalous pulmonary venous drainage, PAPVD: Partial anomalous pulmonary venous drainage, MVR: Mitral valve repair/replacement, MVA: Mitral Valve Annuloplasty, TVR: Tricuspid valve replacement, TVA: Tricuspid valve annuloplasty, PS: Pulmonary stenosis, MAPCA: Major aorto‐pulmonary collateral arteries, CA: Coronary artery, TOF: Tetralogy of Fallot, RV: Right ventricle, PA: Pulmonary artery, HLHS: Hypoplastic left heart syndrome
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| AbbasiTashnizi 2013 | Wrong comparator |
| AbdEl Hakeem 2003 | Adult population |
| Alten 2011 | Wrong study design |
| Anic 2004 | Adult population |
| Brettner 2018 | Adult population |
| Bronicki 2012 | Wrong comparator |
| Bunge 2014 | Adult population |
| Carrel 2017 | Wrong study design |
| Celik 2004 | Adult population |
| Chaney 2001 | Adult population |
| Clarizia 2011 | Wrong study design |
| Corbi 2001 | Wrong study design |
| Crawford 2017 | Wrong study design |
| delaMotte 2014 | Adult population |
| Demir 2009 | Adult population |
| Dhar 2012 | Adult population |
| Dreher 2015 | Wrong study design |
| Ebrahimi 2010 | Adult population |
| Ebrahimi 2016 | Adult population |
| Fernandez 2011 | Wrong patient population |
| Fillinger 2002 | Adult population |
| Fontela 2011 | Wrong comparator |
| Garg 2014 | Wrong study design |
| Gessler 2005 | Wrong study design |
| Glumac 2017 | Adult population |
| Graham 2011 | Wrong comparator |
| Hauer 2012 | Adult population |
| Heckmann 2002 | Wrong study design |
| Juneja 2000 | Adult population |
| Keski Nisula 2016 | Wrong comparator |
| Keski‐Nisula 2020 | Wrong study design |
| Kilger 2003 | Adult population |
| Kilger 2011 | Wrong patient population |
| Liakopoulos 2007 | Adult population |
| Liu 2007 | Wrong comparator |
| Loef 2004 | Adult population |
| Maeda 2016 | Wrong study design |
| Malagon 2005a | Retracted Study |
| Mardani 2012 | Adult population |
| McClure 2017 | Adult population |
| Modan Moses 2010 | Wrong study design |
| Murphy 2011 | Adult population |
| Namdari 2011 | Adult population |
| NCT00293592 | Adult population |
| NCT00490828 | Adult population |
| NCT00879931 | Adult population |
| NCT01296074 | Adult population |
| NCT03002259 | Adult population |
| Oliver 2004 | Adult population |
| Ottens 2013 | Adult population |
| Paparella 2017 | Adult population |
| Poyrazoglu 2016 | Wrong study design |
| Rahman 2010 | Wrong comparator |
| Robert 2015 | Wrong study design |
| SanchezCanovas 2016 | Adult population |
| Santarpino 2009 | Adult population |
| Sauer 2013 | Adult population |
| Schelling 2004 | Adult population |
| Soltani 2013 | Wrong comparator |
| Suezawa 2013 | Adult population |
| Suominen 2005 | Wrong intervention |
| Varan 2002 | Wrong comparator |
| vonSpiegel 2002 | Adult population |
| Weis 2006 | Adult population |
| Whitlock 2006 | Adult population |
| Withington 2014 | Wrong comparator |
| Yared 2000 | Adult population |
| YasserMohamed 2009 | Adult population |
Characteristics of ongoing studies [ordered by study ID]
NCT02615262.
| Study name | Intraoperative Dexamethasone in Pediatric Cardiac Surgery |
| Methods | Parallel RCT |
| Participants | Children up to 12 months |
| Interventions | Dexamethasone 1 mg per 1 kg of body weight intravenously immediately after induction of anaesthesia |
| Outcomes | Major complications [ Time Frame: 30 days after surgery ] Composite of all‐cause death, myocardial infarction, need for extracorporeal membrane oxygenation implantation, cardiac arrest, acute renal failure (stage "injury" or higher according to pRIFLE scale), prolonged mechanical ventilation (> 24 hours), stroke, seizure, coma. |
| Starting date | December 2015 |
| Contact information | Vladimir Lomivorotov, PhD Novosibirsk Research Institute of Circulation Pathology |
| Notes |
STRESS 2021.
| Study name | STeroids to REduce Systemic Inflammation After Infant Heart Surgery (STRESS) |
| Methods | RCT |
| Participants | Age < 1 year at the time of surgery Undergoing heart surgery with CPB as part of standard clinical care |
| Interventions | Methylprednisolone IV preoperative and intraoperative |
| Outcomes | Primary Outcome Measures :
Secondary Outcome Measures :
|
| Starting date | October 2017 |
| Contact information | |
| Notes |
Differences between protocol and review
RoB 1 tool was used rather than RoB 2 as stated in the protocol due to RoB 2 not being implemented by the time it was required.
We did not search grey literature.
We used the Covidence systematic review manager. This changed the process required for many parts of the review. The system means that:
Data extraction and risk of bias assessment are done by the same people at the same time.
Only two people can do the processes of screening and data extraction at any one time
Only one person can resolve disputes
Therefore, only JCVL and BG undertook full text screening. We resolved disputes by consensus. JCVL and DPF extracted data and performed the assessment of risk of bias.
We transformed one study (Graham 2019) that had correctly used medians and range for length for continuous outcomes (e.g. length of stay). Other studies reported means and SD, even though they had non‐normal distributions.
We did not perform sensitivity analyses for the reasons outlined in the text.
We did not perform I2 statistical analysis due to the low number of studies and therefore the bias that this may cause under these conditions.
We did not perform subgroup analysis due to the low number of studies (< 10).
Contributions of authors
BG, JCVL, DPF, GPG, KIAM, BCR, AWLS and SLL wrote and edited the manuscript.
BG, JCVL, DPF, AWLS, and BCR assessed manuscripts for primary and secondary outcomes.
GPG, MAMA, SCS, GDA and SLL provided advice on outcomes.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research (NIHR), UK
This project was supported by the NIHR, via Cochrane Infrastructure funding to the Heart Group. The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, the NIHR, the NHS or the UK Department of Health
-
UK NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol, UK
This project is supported by the UK NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol. The views expressed in this publication are those of the review authors and not necessarily those of the NHS, the NIHR or the UK Department of Health
Declarations of interest
BG: Dr Gibbison's institution is in receipt of project grants from the UK National Institute of Health Research and the British Heart Foundation to carry out research surrounding the topics of cardiac surgery, perioperative care and perioperative hypothalamic‐pituitary‐adrenal function including corticosteroids.
JCVL: none known.
KIAM: none known.
DPF: none known.
MAMA: none known.
GPG: none known.
AWLS: none known.
SCS: none known.
SLL: none known.
GDA: none known.
BCR: Prof Barnaby Reeves is funded (both part salary and research consumables) in part by the Cardiovascular theme of the NIHR Bristol Biomedical Research Centre.
New
References
References to studies included in this review
Amanullah 2016 {published data only}
- Amanullah MM, Hamid M, Hanif HM, Muzaffar M, Siddiqui MT, Adhi F, et al. Effect of steroids on inflammatory markers and clinical parameters in congenital open heart surgery: a randomised controlled trial. Cardiology in the Young 2016;26(3):506-15. [DOI: ] [DOI] [PubMed] [Google Scholar]
Ando 2005 {published data only}
- Ando M, Park IS, Wada N, Takahashi Y. Steroid supplementation: a legitimate pharmacotherapy after neonatal open heart surgery. Annals of Thoracic Surgery 2005;80(5):1672-8; discussion 1678. [DOI] [PubMed] [Google Scholar]
Bronicki 2000 {published data only}
- Bronicki RA, Backer CL, Baden HP, Mavroudis C, Crawford SE, Green TP. Dexamethasone reduces the inflammatory response to cardiopulmonary bypass in children. Annals of Thoracic Surgery 2000;69(5):1490-5. [DOI] [PubMed] [Google Scholar]
Checchia 2003 {published data only}
- Checchia PA, Backer CL, Bronicki RA, Baden HP, Crawford SE, Green TP, et al. Dexamethasone reduces postoperative troponin levels in children undergoing cardiopulmonary bypass. Critical Care Medicine 2003;31(6):1742-5. [DOI] [PubMed] [Google Scholar]
Dalili 2015 {published data only}
- Dalili M, Vesal A, Tabib A, Khani-Tafti L, Hosseini S, Totonchi Z. Single dose corticosteroid therapy after surgical repair of Fallot's Tetralogy; A randomized controlled clinical trial. Research in Cardiovascular Medicine 2015;4(1):e25500. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Graham 2019 {published data only}
- Graham EM, Martin RH, Buckley JR, Zyblewski SC, Kavarana MN, Bradley SM, et al. Corticosteroid therapy in neonates undergoing cardiopulmonary bypass: randomized controlled trial. Journal of the American College of Cardiology 2019;74(5):659-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01579513. Corticosteroid therapy in neonates undergoing cardiopulmonary bypass. clinicaltrials.gov/ct2/show/NCT01579513 (first posted 18 April 2012).
Heying 2012 {published data only}
- Heying R, Wehage E, Schumacher K, Tassani P, Haas F, Lange R, et al. Dexamethasone pretreatment provides antiinflammatory and myocardial protection in neonatal arterial switch operation. Annals of Thoracic Surgery 2012;93(3):869-76. [DOI: ] [DOI] [PubMed] [Google Scholar]
- Heying R, Wehage E, Schumacher K, Tassani P, Haas F, Lange R, et al. Pre-treatment with dexamethasone provides antiinflammatory and myocardial protection in neonatal arterial switch operation: A prospective randomized double-blind controlled study. Acta Cardiologica 2012;67 (1):121-2. [DOI: ] [Google Scholar]
- Heying R, Wehage E, Tassani P, Haas F, Hess J, Seghaye MC. Effect of dexamethasone on the inflammatory response and myocardial protection during and after arterial switch operation in neonates: A double blind randomized controlled study. Cardiology in the Young 2010;2:S34-5. [DOI: ] [Google Scholar]
Keski Nisula 2013 {published data only}
- Keski-Nisula J, Pesonen E, Olkkola KT, Peltola K, Neuvonen PJ, Tuominen N, et al. Methylprednisolone in neonatal cardiac surgery: reduced inflammation without improved clinical outcome. Annals of Thoracic Surgery 2013;95(6):2126-32. [DOI: ] [DOI] [PubMed] [Google Scholar]
- Pesonen E, Keski-Nisula J, Andersson S, Palo R, Salminen J, Suominen PK. High-dose methylprednisolone and endothelial glycocalyx in paediatric heart surgery. Acta Anaesthesiologica Scandinavica 2016;60(10):1386-94. [PMID: ] [DOI] [PubMed] [Google Scholar]
Keski Nisula 2015 {published data only}
- Keski-Nisula J, Suominen PK, Olkkola KT, Peltola K, Neuvonen PJ, Tynkkynen P, et al. Effect of timing and route of methylprednisolone administration during pediatric cardiac surgical procedures. Annals of Thoracic Surgery 2015;99(1):180-5. [DOI: ] [DOI] [PubMed] [Google Scholar]
- Pesonen E, Keski-Nisula J, Andersson S, Palo R, Salminen J, Suominen PK. High-dose methylprednisolone and endothelial glycocalyx in paediatric heart surgery. Acta Anaesthesiologica Scandinavica 2016;60(10):1386-94. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lindberg 2003 {published data only}
- Lindberg L, Forsell C, Jogi P, Olsson AK. Effects of dexamethasone on clinical course, C-reactive protein, S100B protein and von Willebrand factor antigen after paediatric cardiac surgery. British Journal of Anaesthesia 2003;90(6):728-32. [DOI] [PubMed] [Google Scholar]
Malagon 2005 {published data only}
- Malagon I, Hogenbirk K, Pelt J, Hazekamp MG, Bovill JG. Effect of dexamethasone on postoperative cardiac troponin T production in pediatric cardiac surgery. Intensive Care Medicine 2005;31(10):1420-6. [DOI] [PubMed] [Google Scholar]
Mott 2001 {published data only}
- Mott AR, Fraser CD Jr Kusnoor AV, Giesecke NM, Reul GJ Jr Drescher KL, et al. The effect of short-term prophylactic methylprednisolone on the incidence and severity of postpericardiotomy syndrome in children undergoing cardiac surgery with cardiopulmonary bypass. Journal of the American College of Cardiology 2001;37(6):1700-6. [DOI] [PubMed] [Google Scholar]
Suominen 2017 {published data only}
- Jahnukainen T, Keski-Nisula J, Tainio J, Valkonen H, Patila T, Jalanko H, et al. Efficacy of corticosteroids in prevention of acute kidney injury in neonates undergoing cardiac surgery-A randomized controlled trial. Acta Anaesthesiologica Scandinavica 2018;17:17. [DOI: ] [DOI] [PubMed] [Google Scholar]
- Kaskinen A, Keski-Nisula J, Martelius L, Rautiainen P, Andersson S, Pitkanen OM. Systemic steroid therapy modifies postoperative lung injury after congenital cardiac surgery in neonates. Cardiology in the Young 2019;29(Supplement 1):S10-1. [Google Scholar]
- Suominen PK, Keski-Nisula J, Ojala T, Rautiainen P, Jahnukainen T, Hastbacka J, et al. Stress-dose corticosteroid versus placebo in neonatal cardiac operations: a randomized controlled trial. Annals of Thoracic Surgery 2017;104(4):1378-85. [DOI: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
AbbasiTashnizi 2013 {published data only}
- Abbasi Tashnizi M, Soltani G, Moeinipour AA, Ayatollahi H, Tanha AS, Jarahi L, et al. Comparison between preoperative administration of methylprednisolone with its administration before and during congenital heart surgery on serum levels of IL-6 and IL-10. Iranian Red Crescent Medical Journal 2013;15(2):147-51. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
AbdEl Hakeem 2003 {published data only}
- Abd El-Hakeem EE, Zareh ZE. Effects of dexamethasone on the incidence of shivering and recovery in patients undergoing valve replacement surgery. Egyptian Journal of Anaesthesia 2003;19(4):361-70. [Google Scholar]
Alten 2011 {published data only}
- Alten JA, Robert S, Borasino S, Dabal RJ. Random cortisol poorly predicts low cardiac output syndrome (LCOS) severity and hemodynamic response to hydrocortisone (HC) after cardiopulmonary bypass (CPB). Pediatric Critical Care Medicine 2011;1:S98. [DOI: ] [Google Scholar]
Anic 2004 {published data only}
- Anic D, Gasparovic H, Ivancan V, Batinic D. Effects of corticosteroids on inflammatory response following cardiopulmonary bypass. Croatian Medical Journal 2004;45(2):158-61. [PubMed] [Google Scholar]
Brettner 2018 {published data only}
- Brettner F, Chappell D, Nebelsiek T, Hauer D, Schelling G, Becker BF, et al. Preinterventional hydrocortisone sustains the endothelial glycocalyx in cardiac surgery. Clinical hemorheology and microcirculation 2018;19:19. [DOI: ] [DOI] [PubMed] [Google Scholar]
Bronicki 2012 {published data only}
- Bronicki RA, Checchia PA, Stuart-Killion RB, Dixon DJ, Backer CL. The effects of multiple doses of glucocorticoids on the inflammatory response to cardiopulmonary bypass in children. World Journal for Pediatric & Congenital Heart Surgery 2012;3(4):439-45. [DOI: ] [DOI] [PubMed] [Google Scholar]
Bunge 2014 {published data only}
- Bunge JJ, Osch D, Dieleman JM, Jacob KA, Kluin J, van Dijk D, et al. Dexamethasone for the prevention of postpericardiotomy syndrome: A DExamethasone for Cardiac Surgery substudy. American Heart Journal 2014;168(1):126-31.e1. [DOI: ] [DOI] [PubMed] [Google Scholar]
Carrel 2017 {published data only}
- Carrel T, Englberger L. Steroids and cardiopulmonary bypass: a never-ending story. Seminars in Thoracic and Cardiovascular Surgery 2017;29(1):45-6. [DOI: ] [DOI] [PubMed] [Google Scholar]
Celik 2004 {published data only}
- Celik JB, Gormus N, Okesli S, Gormus ZI, Solak H. Methylprednisolone prevents inflammatory reaction occurring during cardiopulmonary bypass: effects on TNF-alpha, IL-6, IL-8, IL-10. Perfusion 2004;19(3):185-91. [DOI] [PubMed] [Google Scholar]
Chaney 2001 {published data only}
- Chaney MA, Durazo-Arvizu RA, Nikolov MP, Blakeman BP, Bakhos M. Methylprednisolone does not benefit patients undergoing coronary artery bypass grafting and early tracheal extubation. Journal of Thoracic and Cardiovascular Surgery 2001;121(3):561-9. [DOI] [PubMed] [Google Scholar]
Clarizia 2011 {published data only}
- Clarizia NA, Manlhiot C, Schwartz SM, Sivarajan VB, Maratta R, Holtby HM, Gruenwald CE, et al. Improved outcomes associated with intraoperative steroid use in high-risk pediatric cardiac surgery. Annals of Thoracic Surgery 2011;91(4):1222-7. [DOI: ] [DOI] [PubMed] [Google Scholar]
Corbi 2001 {published data only}
- Corbi PJ, Rahmati M, Lecron JC. Inflammatory response associated with cardiopulmonary bypass and effect of methylprednisolone. Journal of Thoracic and Cardiovascular Surgery 2001;122(5):1052-3. [DOI] [PubMed] [Google Scholar]
Crawford 2017 {published data only}
- Crawford JH, Hull MS, Borasino S, Steenwyk BL, Hock KM, Wall K, Alten JA. Adrenal insufficiency in neonates after cardiac surgery with cardiopulmonary bypass. Paediatric Anaesthesia 2017;27(1):77-84. [DOI: ] [DOI] [PubMed] [Google Scholar]
delaMotte 2014 {published data only}
- de la Motte L, Kehlet H, Vogt K, Nielsen CH, Groenvall JB, Nielsen HB, et al. Preoperative methylprednisolone enhances recovery after endovascular aortic repair: a randomized, double-blind, placebo-controlled clinical trial. Annals of Surgery 2014;260(3):540-8; discussion 548-9. [DOI: ] [DOI] [PubMed] [Google Scholar]
Demir 2009 {published data only}
- Demir T, Demir H, Tansel T, Kalko Y, Tireli E, Dayioglu E, et al. Influence of methylprednisolone on levels of neuron-specific enolase in cardiac surgery: a corticosteroid derivative to decrease possible neuronal damage. Journal of Cardiac Surgery 2009;24(4):397-403. [DOI: ] [DOI] [PubMed] [Google Scholar]
Dhar 2012 {published data only}
- Dhar S, Rahman Z, Hasan K, Hoque MF, Sultana A. Effect of pre-bypass methylprednisolone on post-operative renal function following correction of atrial septal defect under cardiopulmonary bypass. Mymensingh Medical Journal 2012;21(1):72-9. [PubMed] [Google Scholar]
Dreher 2015 {published data only}
- Dreher M, Glatz AC, Kennedy A, Rosenthal T, Gaynor JW. A single-center analysis of methylprednisolone use during pediatric cardiopulmonary bypass. Journal of Extra-corporeal Technology 2015;47(3):155-9. [PMC free article] [PubMed] [Google Scholar]
Ebrahimi 2010 {published data only}
- Ebrahimi L, Kheirandish M, Foroughi M, Aghaeii Pour M, Ahmadi Nejad M, Samarghandi N, et al. The effect of methylprednizolone on platelet aggregation during cardiopulmonary bypass surgery. Vox Sanguinis 2010;1:446. [DOI: ] [Google Scholar]
Ebrahimi 2016 {published data only}
- Ebrahimi L, Kheirandish M, Foroughi M. The effect of methylprednisolone treatment on fibrinolysis, the coagulation system, and blood loss in cardiac surgery. Turk 2016;46(6):1645-54. [DOI: ] [DOI] [PubMed] [Google Scholar]
Fernandez 2011 {published data only}
- Fernandez AB, Kilger E, Heyn J, Kur F, Vicol C, Reichart B, et al. Stress doses of hydrocortisone reduce severe systemic inflammatory response in patients undergoing cardiac surgery without cardiopulmonary bypass. Innovations: Technology and Techniques in Cardiothoracic and Vascular Surgery 2011;6(3):189. [DOI: ] [Google Scholar]
Fillinger 2002 {published data only}
- Fillinger MP, Rassias AJ, Guyre PM, Sanders JH, Beach M, Pahl J, et al. Glucocorticoid effects on the inflammatory and clinical responses to cardiac surgery. Journal of Cardiothoracic and Vascular Anesthesia 2002;16(2):163-9. [DOI] [PubMed] [Google Scholar]
Fontela 2011 {published data only}
- Fontela P, Harrington K, Lands L, Withington D. Does timing of steroid administration affect the inflammatory response to CPB in infants? A RCT. Paediatric Anaesthesia 2012;22(9):915. [DOI: ] [Google Scholar]
- Fontela P, Harrington K, Lands L, Withingto D. Does timing of steroid administration affect the inflammatory response to CPB? Pediatric Critical Care Medicine 2011;1:A12. [DOI: ] [Google Scholar]
Garg 2014 {published data only}
- Garg AX, Vincent J, Cuerden M, Parikh C, Devereaux PJ, Teoh K, et al. Steroids In caRdiac Surgery (SIRS) trial: acute kidney injury substudy protocol of an international randomised controlled trial. BMJ Open 2014;4(3):e004842. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gessler 2005 {published data only}
- Gessler P, Hohl V, Carrel T, Pfenninger J, Schmid ER, Baenziger O, et al. Administration of steroids in pediatric cardiac surgery: impact on clinical outcome and systemic inflammatory response. Pediatric Cardiology 2005;26(5):595-600. [DOI] [PubMed] [Google Scholar]
Glumac 2017 {published data only}
- Glumac S, Kardum G, Sodic L, Supe-Domic D, Karanovic N. Effects of dexamethasone on early cognitive decline after cardiac surgery: a randomised controlled trial. European Journal of Anaesthesiology 2017;34(11):776-84. [DOI: ] [DOI] [PubMed] [Google Scholar]
Graham 2011 {published data only}
- Graham EM, Atz AM, Butts RJ, Baker NL, Zyblewski SC, Deardorff RL, et al. Standardized preoperative corticosteroid treatment in neonates undergoing cardiac surgery - results from a randomized trial. Pediatric Critical Care Medicine 2011;1:S87. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham EM, Atz AM, Butts RJ, Baker NL, Zyblewski SC, Deardorff RL, et al. Standardized preoperative corticosteroid treatment in neonates undergoing cardiac surgery: results from a randomized trial. Journal of Thoracic and Cardiovascular Surgery 2011;142(6):1523-9. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham EM, Atz AM, McHugh KE, Butts RJ, Baker NL, Stroud RE, et al. Preoperative steroid treatment does not improve markers of inflammation after cardiac surgery in neonates: results from a randomized trial. Journal of Thoracic and Cardiovascular Surgery 2014;147(3):902-8. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hauer 2012 {published data only}
- Hauer D, Weis F, Campolongo P, Schopp M, Beiras-Fernandez A, Strewe C, et al. Glucocorticoid-endocannabinoid interaction in cardiac surgical patients: relationship to early cognitive dysfunction and late depression. Reviews in the Neurosciences 2012;23(5-6):681-90. [DOI] [PubMed] [Google Scholar]
Heckmann 2002 {published data only}
- Heckmann M, Pohlandt F. Hydrocortisone in preterm infants. Pediatrics 2002;109(6):1184-5; author reply 1184-5. [DOI] [PubMed] [Google Scholar]
Juneja 2000 {published data only}
- Juneja R, Dhar A, Raizada A, Mehta Y. Methylprednisolone-immunomodulation and outcome following CPB. British Journal of Anaesthesia 2000;84(Suppl 1):9. [Google Scholar]
Keski Nisula 2016 {published data only}
- Keski-Nisula J, Pesonen E, Olkkola KT, Ahlroth T, Puntila J, Andersson S, et al. High-dose methylprednisolone has no benefit over moderate dose for the correction of Tetralogy of Fallot. Annals of Thoracic Surgery 2016;102(3):870-6. [DOI: ] [DOI] [PubMed] [Google Scholar]
Keski‐Nisula 2020 {published data only}
- Keski-Nisula J, Arvola O, Jahnukainen T, Andersson S, Pesonen E. Reduction of inflammation by high-dose methylprednisolone does not attenuate oxidative stress in children undergoing bidirectional Glenn Procedure with or without aortic arch or pulmonary arterial repair. Journal of Cardiothoracic and Vascular Anesthesia 2020;34(6):1542-7. [DOI] [PubMed] [Google Scholar]
Kilger 2003 {published data only}
- Kilger E, Weis F, Briegel J, Frey L, Goetz AE, Reuter D, et al. Stress doses of hydrocortisone reduce severe systemic inflammatory response syndrome and improve early outcome in a risk group of patients after cardiac surgery. Critical Care Medicine 2003;31(4):1068-74. [DOI] [PubMed] [Google Scholar]
Kilger 2011 {published data only}
- Kilger E, Heyn J, Beiras-Fernandez A, Luchting B, Weis F. Stress doses of hydrocortisone reduce systemic inflammatory response in patients undergoing cardiac surgery without cardiopulmonary bypass. Minerva Anestesiologica 2011;77(3):268-74. [PubMed] [Google Scholar]
Liakopoulos 2007 {published data only}
- Liakopoulos OJ, Schmitto JD, Kazmaier S, Brauer A, Quintel M, Schoendube FA, Dorge H. Cardiopulmonary and systemic effects of methylprednisolone in patients undergoing cardiac surgery. Annals of Thoracic Surgery 2007;84(1):110-8; discussion 118-9. [DOI] [PubMed] [Google Scholar]
Liu 2007 {published data only}
- Liu J, Ji B, Long C, Li C, Feng Z. Comparative effectiveness of methylprednisolone and zero-balance ultrafiltration on inflammatory response after pediatric cardiopulmonary bypass. Artificial Organs 2007;31(7):571-5. [DOI] [PubMed] [Google Scholar]
Loef 2004 {published data only}
- Loef BG, Henning RH, Epema AH, Rietman GW, Oeveren W, Navis GJ, et al. Effect of dexamethasone on perioperative renal function impairment during cardiac surgery with cardiopulmonary bypass. British Journal of Anaesthesia 2004;93(6):793-8. [DOI] [PubMed] [Google Scholar]
Maeda 2016 {published data only}
- Maeda T, Takeuchi M, Tachibana K, Nishida T, Kagisaki K, Imanaka H. Steroids improve hemodynamics in infants with adrenal insufficiency after cardiac surgery. Journal of Cardiothoracic and Vascular Anesthesia 2016;30(4):936-41. [DOI: ] [DOI] [PubMed] [Google Scholar]
Malagon 2005a {published data only}
- Malagon I, Onkenhout W, Klok M, Linthorst L, van der Poel PF, Bovill JG, et al. Dexamethasone reduces gut permeability in pediatric cardiac surgery.[Retraction in J Thorac Cardiovasc Surg. 2007 Sep;134(3):771; PMID: 17726800]. Journal of Thoracic Cardiovascular Surgery 2005;130(2):265-71. [DOI] [PubMed] [Google Scholar]
Mardani 2012 {published data only}
- Mardani D, Bigdelian H. Prophylaxis of dexamethasone protects patients from further post-operative delirium after cardiac surgery: a randomized trial. Journal of Research in Medical Science 2013;18(2):137-43. [PMC free article] [PubMed] [Google Scholar]
- Mardani D, Bigdelian H. The effect of dexamethasone prophylaxis on postoperative delirium after cardiac surgery: a randomized trial. Journal of Research in Medical Sciences 2012;17(1 SPL.1):S113-9. [PMC free article] [PubMed] [Google Scholar]
McClure 2017 {published data only}
- McClure GR, Belley-Cote EP, Harlock J, Lamy A, Stacey M, Devereaux PJ, et al. Steroids in cardiac surgery (SIRS): infection substudy. European Heart Journal 2017;38(Suppl 1):1276. [DOI: ] [Google Scholar]
Modan Moses 2010 {published data only}
- Modan-Moses D, Kanety H, Dagan O, Ehrlich S, Lotan D, Pariente C, et al. Leptin and the post-operative inflammatory response. More insights into the correlation with the clinical course and glucocorticoid administration. Journal of Endocrinological Investigation 2010;33(10):701-6. [DOI: ] [DOI] [PubMed] [Google Scholar]
Murphy 2011 {published data only}
- Murphy G, Sherwani SS, Szokol J, Avram MJ. Small-dose dexamethasone improves patient-perceived quality of recovery after cardiac surgery. Anesthesia and Analgesia 2011;112(5 SUPPL. 1):S-41. [DOI: ] [Google Scholar]
- Murphy G, Sherwani SS, Szokol J, Avram MJ, Patel K. The effect of small-dose dexamethasone on clinical recovery after cardiac surgery. Anesthesia and Analgesia 2011;112(5 SUPPL. 1):S-40. [DOI: ] [Google Scholar]
- Murphy GS, Sherwani SS, Szokol JW, Avram MJ, Greenberg SB, Patel KM, et al. Small-dose dexamethasone improves quality of recovery scores after elective cardiac surgery: a randomized, double-blind, placebo-controlled study. Journal of Cardiothoracic and Vascular Anesthesia 2011;25(6):950-60. [DOI: ] [DOI] [PubMed] [Google Scholar]
Namdari 2011 {published data only}
- Namdari M, Ghafarzadeh M, Nikoo MA. Efficacy of intramuscular methyl prednisolone in preventing restenosis after coronary artery stenting with bare-metal stainless steel stent: a double-blind, randomised, controlled clinical trial. Cardiovascular Journal of Africa 2011;22(2):67-9. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT00293592 {published data only}
- NCT00293592. Dexamethasone for cardiac surgery trial. clinicaltrials.gov/ct2/show/NCT00293592 (first posted 17 February 2006).
NCT00490828 {published data only}
- Influence of hydrocortisone on immunologic markers and health care related quality of life in patients after cardiac surgery. clinicaltrials.gov/ct2/show/NCT00490828 (first posted 25 June 2007).
NCT00879931 {published data only}
- NCT00879931. Influence of corticoids on renal function in cardiac surgery. clinicaltrials.gov/show/nct00879931 (first posted 13 April 2009).
NCT01296074 {published data only}
- NCT01296074. Corticosteroid prophylaxis on the cardiopulmonary bypass-induced systemic inflammatory response. clinicaltrials.gov/show/nct01296074 (first posted 15 February 2011).
NCT03002259 {published data only}
- NCT03002259. Dexamethasone for cardiac surgery-II trial. clinicaltrials.gov/show/nct03002259 (first posted 23 December 2016).
Oliver 2004 {published data only}
- Oliver WC Jr Nuttall GA, Orszulak TA, Bamlet WR, Abel MD, Ereth MH, et al. Hemofiltration but not steroids results in earlier tracheal extubation following cardiopulmonary bypass: a prospective, randomized double-blind trial. Anesthesiology 2004;101(2):327-39. [DOI] [PubMed] [Google Scholar]
Ottens 2013 {published data only}
- Ottens TH, Dieleman JM, Van Dijk D, Nijsten MW, Van Der Maaten JM. The influence of dexamethasone on intraoperative and postoperative lactate levels and glycaemic control in cardiac surgery patients. Applied Cardiopulmonary Pathophysiology 2013;17(2):185-6. [Google Scholar]
Paparella 2017 {published data only}
- Paparella D, Parolari A, Rotunno C, Vincent J, Myasoedova V, Guida P, et al. The effects of steroids on coagulation dysfunction induced by cardiopulmonary bypass: a Steroids in Cardiac Surgery (SIRS) trial substudy. Seminars in Thoracic and Cardiovascular Surgery 2017;29(1):35-44. [DOI: ] [DOI] [PubMed] [Google Scholar]
Poyrazoglu 2016 {published data only}
- Poyrazoglu HH, Duman Z, Demir S, Avsar MK, Atalay A, Ciftci B, et al. Investigating the impacts of preoperative steroid treatment on tumor necrosis factor-alpha and duration of extubation time underwent ventricular septal defect surgery. Balkan Medical Journal 2016;33(2):158-63. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rahman 2010 {published data only}
- Rahman Z, Dhar S, Hasan K, Sultana A. Effect of pre-bypass methylprednisolone on post-operative renal function in cardiac ICU following correction of atrial septal deffect under cardiopulmonary bypass. Intensive Care Medicine 2010;2):S316. [DOI: ] [PubMed] [Google Scholar]
Robert 2015 {published data only}
- Robert SM, Borasino S, Dabal RJ, Cleveland DC, Hock KM, Alten JA. Effect of postoperative steroids on cardiovascular/respiratory function in neonates undergoing cardiopulmonary bypass. Paediatric Critical Care Medicine 2015;16(7):629-36. [NCT01595386] [DOI] [PubMed] [Google Scholar]
- Robert SM, Borasino S, Dabal RJ, Cleveland DC, Hock KM, Alten JA. Postoperative hydrocortisone infusion reduces the prevalence of low cardiac output syndrome after neonatal cardiopulmonary bypass. Pediatric Critical Care Medicine 2015;16(7):629-36. [DOI: ] [DOI] [PubMed] [Google Scholar]
SanchezCanovas 2016 {published data only}
- Sanchez Canovas S, Garcia Candel A. Methylprednisolone in patients undergoing cardiopulmonary bypass (SIRS): a randomised, double-blind, placebo-controlled trial. Cirugia Cardiovascular 2016;23(5):270-1. [DOI: ] [Google Scholar]
Santarpino 2009 {published data only}
- Santarpino G, Caroleo S, Onorati F, Rubino AS, Dardano A, Gulletta E, et al. Inflammatory response after cardiopulmonary bypass: a randomized comparison between conventional hemofiltration and steroids. Journal of Cardiovascular Surgery 2009;50(4):555-64. [PubMed] [Google Scholar]
Sauer 2013 {published data only}
- Sauer AC, Slooter AJ, Veldhuijzen DS, Van Eijk MM, Van Dijk D. Intraoperative dexamethasone and delirium after cardiac surgery: a randomized clinical trial. Critical Care 2013;2):S148. [DOI: ] [DOI] [PubMed] [Google Scholar]
Schelling 2004 {published data only}
- Schelling G, Kilger E, Roozendaal B, de Quervain DJ, Briegel J, Dagge A, et al. Stress doses of hydrocortisone, traumatic memories, and symptoms of posttraumatic stress disorder in patients after cardiac surgery: a randomized study. Bioligal Psychiatry 2004;55(6):627-33. [DOI] [PubMed] [Google Scholar]
Soltani 2013 {published data only}
- Soltani G, Abbasi Tashnizi M, Moeinipour AA, Ganjifard M, Esfahanizadeh J, Sepehri Shamloo A, et al. Comparing the effect of preoperative administration of methylprednisolone and its administration before and during surgery on the clinical outcome in pediatric open heart surgeries. Iranian Red Crecent Medical Journal 2013;15(6):483-7. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Suezawa 2013 {published data only}
- Suezawa T, Aoki A, Kotani M, Tago M, Kobayashi O, Hirasaki A, et al. Clinical benefits of methylprednisolone in off-pump coronary artery bypass surgery. General Thoracic and Cardiovascular Surgery 2013;61(8):455-9. [DOI: ] [DOI] [PubMed] [Google Scholar]
Suominen 2005 {published data only}
- Suominen PK, Dickerson HA, Moffett BS, Ranta SO, Mott AR, Price JF, et al. Hemodynamic effects of rescue protocol hydrocortisone in neonates with low cardiac output syndrome after cardiac surgery. Pediatric Critical Care Medicine 2005;6(6):655-9. [DOI] [PubMed] [Google Scholar]
Varan 2002 {published data only}
- Varan B, Tokel K, Mercan S, Donmez A, Aslamaci S. Systemic inflammatory response related to cardiopulmonary bypass and its modification by methyl prednisolone: high dose versus low dose. Pediatric Cardiology 2002;23(4):437-41. [DOI] [PubMed] [Google Scholar]
vonSpiegel 2002 {published data only}
- von Spiegel T, Giannaris S, Wietasch GJ, Schroeder S, Buhre W, Schorn B, et al. Effects of dexamethasone on intravascular and extravascular fluid balance in patients undergoing coronary bypass surgery with cardiopulmonary bypass. Anesthesiology 2002;96(4):827-34. [DOI] [PubMed] [Google Scholar]
Weis 2006 {published data only}
- Weis F, Kilger E, Roozendaal B, Quervain DJ, Lamm P, Schmidt M, et al. Stress doses of hydrocortisone reduce chronic stress symptoms and improve health-related quality of life in high-risk patients after cardiac surgery: a randomized study. Journal of Thoracic and Cardiovascular Surgery 2006;131(2):277-82. [DOI] [PubMed] [Google Scholar]
- Weis F, Beiras-Fernandez A, Schelling G, Briegel J, Lang P, Hauer D, et al. Stress doses of hydrocortisone in high-risk patients undergoing cardiac surgery: effects on interleukin-6 to interleukin-10 ratio and early outcome. Critical Care Medicine 2009;37(5):1685-90. [DOI: ] [DOI] [PubMed] [Google Scholar]
Whitlock 2006 {published data only}
- Whitlock RP, Young E, Noora J, Farrokhyar F, Blackall M, Teoh KH. Pulse low dose steroids attenuate post-cardiopulmonary bypass SIRS; SIRS I. Journal of Surgery Research 2006;132(2):188-94. [DOI] [PubMed] [Google Scholar]
Withington 2014 {published data only}
- Withington DE, Fontela PS, Harrington KP, Tchervenkov C, Lands LC. A comparison of three dose timings of methylprednisolone in infant cardiopulmonary bypass. Springerplus 2014;3:484. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yared 2000 {published data only}
- Yared JP, Starr NJ, Torres FK, Bashour CA, Bourdakos G, Piedmonte M, et al. Effects of single dose, postinduction dexamethasone on recovery after cardiac surgery. Annals of Thoracic Surgery 2000;69(5):1420-4. [DOI] [PubMed] [Google Scholar]
YasserMohamed 2009 {published data only}
- Yasser Mohamed A, Elmistekawy E, El-Serogy H. Effects of dexamethasone on pulmonary and renal functions in patients undergoing CABG with cardiopulmonary bypass. Seminars in Cardiothoracic and Vascular Anesthesia 2009;13(4):231-7. [DOI: ] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT02615262 {published data only}
- NCT02615262. Intraoperative dexamethasone in pediatric cardiac surgery. clinicaltrials.gov/ct2/show/NCT02615262 (first posted 26 November 2015).
STRESS 2021 {published and unpublished data}
- Hill KD, Baldwin HS, Bichel DP, Butts RJ, Chamberlain RC, Ellis AM, et al. Rationale and design of the steroids to reduce systemic inflammation after infant heart surgery (STRESS) trial. American Heart Journal 2020;220:192-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Barry 2015
- Barry AE, Chaney MA, London MJ. Anesthetic management during cardiopulmonary bypass: a systematic review. Anesthesia and Analgesia 2015;120(4):749-69. [PMID: ] [DOI] [PubMed] [Google Scholar]
Brown 2015
- Brown KL, Crowe S, Franklin R, McLean A, Cunningham D, Barron D, et al. Trends in 30-day mortality rate and case mix for paediatric cardiac surgery in the UK between 2000 and 2010. Open Heart 2015;2(1):e000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
Covidence 2020 [Computer program]
- Veritas Health Innovation. Avaialble at covidence.org Covidence. Version accessed prior to 16 September 2020. Melbourne, Australia: Veritas Health Innovation. Avaialble at covidence.org.
Fudulu 2016
- Fudulu D, Angelini G. Oxidative stress after surgery on the immature heart. Oxidative Medicine and Cellular Longevity 2016;2016:1971452. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fudulu 2018
- Fudulu D, Schadenberg A, Gibbison B, Jenkins I, Lightman SL, Angelini GD, et al. Corticosteroids and other anti-inflammatory strategies in pediatric heart surgery: a national survey of practice. World Journal for Pediatric and Congenital Heart Surgery 2018;9(3):289-93. [DOI] [PubMed] [Google Scholar]
Gibbison 2013
- Gibbison B, Angelini GD, Lightman SL. Dynamic output and control of the hypothalamic-pituitary-adrenal axis in critical illness and major surgery. British Journal of Anaesthesia 2013;111(3):347-60. [PMID: ] [DOI] [PubMed] [Google Scholar]
Gibson 1993
- Gibson AT, Pearse RG, Wales JK. Growth retardation after dexamethasone administration: assessment by knemometry. Archives of Disease in Childhood 1993;69(5):505-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro 2015 [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed 01 June 2018. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Graham 2014
- Graham EM, Atz AM, McHugh KE, Butts RJ, Baker NL, Stroud RE, et al. Preoperative steroid treatment does not improve markers of inflammation after cardiac surgery in neonates: results from a randomized trial. Journal of Thoracic and Cardiovascular Surgery 2014;147(3):902-8. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Grant 2013
- Grant SW, Hickey GL, Cosgriff R, Cooper G, Deanfield J, Roxburgh J, et al. Creating transparency in UK adult cardiac surgery data. Heart 2013;99(15):1067-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Higgins 2016
- Higgins JP, Sterne JAC, Savović J, Page MJ, Hróbjartsson A, Boutron I, et al. A revised tool for assessing risk of bias in randomized trials. Cochrane Database of Systematic Reviews 2016;10(Suppl 1):29-31. [DOI: 10.1002/14651858.CD201601] [DOI]
Higgins 2017
- Higgins JP, Thomas J, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). London: Cochrane, 2017. [Google Scholar]
Hoashi 2015
- Hoashi T, Miyata H, Murakami A, Hirata Y, Hirose K, Matsumura G, et al. The current trends of mortality following congenital heart surgery: the Japan Congenital Cardiovascular Surgery Database. Interactive Cardiovascular and Thoracic Surgery 2015;21(2):151-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Hozo 2005
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC medical research methodology 2005 April 30;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Huffmyer 2015
- Huffmyer JL, Groves DS. Pulmonary complications of cardiopulmonary bypass. Best Practice & Research. Clinical Anaesthesiology 2015;29(2):163-75. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jacobs 2016
- Jacobs JP, He X, Mayer JE Jr, Austin EH 3rd, Quintessenza JA, Karl TR, et al. Mortality trends in pediatric and congenital heart surgery: an analysis of The Society of Thoracic Surgeons congenital heart surgery database. Annals of Thoracic Surgery 2016;102(4):1345-52. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kennedy 2001
- Kennedy I. The report of the public inquiry into children’s heart surgery at the Bristol Royal Infirmary 1984-1995. UK National Archives 2001. webarchive.nationalarchives.gov.uk/20090811143822/http://www.bristol-inquiry.org.uk/final_report/the_report.pdf (accessed 01 May 2018).
Keski‐Nisula 2013
- Keski-Nisula J, Pesonen E, Olkkola KT, Peltola K, Neuvonen PJ, Tuominen N, et al. Methylprednisolone in neonatal cardiac surgery: reduced inflammation without improved clinical outcome. Annals of Thoracic Surgery 2013;95(6):2126-32. [PMID: ] [DOI] [PubMed] [Google Scholar]
Keski‐Nisula 2015
- Keski-Nisula J, Suominen PK, Olkkola KT, Peltola K, Neuvonen PJ, Tynkkynen P, et al. Effect of timing and route of methylprednisolone administration during pediatric cardiac surgical procedures. Annals of Thoracic Surgery 2015;99(1):180-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Lerzo 2011
- Lerzo F, Peri G, Doni A, Bocca P, Morandi F, Pistorio A, et al. Dexamethasone prophylaxis in pediatric open heart surgery is associated with increased blood long pentraxin PTX3: potential clinical implications. Clinical & Developmental Immunology 2011;2011:730828. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Medzhitov 2008
- Medzhitov R. Origin and physiological roles of inflammation. Nature 2008;454(7203):428-35. [PMID: ] [DOI] [PubMed] [Google Scholar]
NICOR 2016
- National Institute for Cardiovascular Outcomes Research (NICOR) Web Portal. Available at: nicor4.nicor.org.uk (accessed 10 July 2017).
Pasquali 2010
- Pasquali SK, Hall M, Li JS, Peterson ED, Jaggers J, Lodge AJ, et al. Corticosteroids and outcome in children undergoing congenital heart surgery: analysis of the Pediatric Health Information Systems database. Circulation 2010;122(21):2123-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pasquali 2012
- Pasquali SK, Li JS, He X, Jacobs ML, O'Brien SM, Hall M, et al. Perioperative methylprednisolone and outcome in neonates undergoing heart surgery. Pediatrics 2012;129(2):e385-91. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pesonen 2016
- Pesonen E, Keski-Nisula J, Andersson S, Palo R, Salminen J, Suominen PK. High-dose methylprednisolone and endothelial glycocalyx in paediatric heart surgery. Acta Anaesthesiologica Scandinavica 2016;60(10):1386-94. [PMID: ] [DOI] [PubMed] [Google Scholar]
Reeves 2013
- Reeves BC, Higgins JP, Ramsay C, Tugwell P, Wells G. An introduction to methodological issues when including non-randomized studies in systematic reviews on the effects of interventions. Research Synthesis Methods 2013;4:1-11. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Scrascia 2014
- Scrascia G, Rotunno C, Guida P, Amorese L, Polieri D, Codazzi D, et al. Perioperative steroids administration in pediatric cardiac surgery: a meta-analysis of randomized controlled trials. Pediatric Critical Care Medicine 2014;15(5):435-42. [DOI] [PubMed] [Google Scholar]
Shinwell 2000
- Shinwell ES, Karplus M, Reich D, Weintraub Z, Blazer S, Bader D, et al. Early postnatal dexamethasone treatment and increased incidence of cerebral palsy. Archives of Disease in Childhood. Fetal and Neonatal Edition 2000;83(3):F177-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tarnok 2001
- Tarnok A, Schneider P. Pediatric cardiac surgery with cardiopulmonary bypass: pathways contributing to transient systemic immune suppression. Shock 2001;16(Suppl 1):24-32. [PMID: ] [DOI] [PubMed] [Google Scholar]
Toledo‐Pereyra 1980
- Toledo-Pereyra LH, Lin CY, Kundler H, Replogle RL. Steroids in heart surgery: a clinical double-blind and randomized study. American Surgeon 1980;46(3):155-60. [PMID: ] [PubMed] [Google Scholar]
von Hippel 2015
- Hippel PT. The heterogeneity statistic I(2) can be biased in small meta-analyses. BMC medical research methodology 2015/04/14;15:35-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yeh 2004
- Yeh TF, Lin YJ, Lin HC, Huang CC, Hsieh WS, Lin CH, et al. Outcomes at school age after postnatal dexamethasone therapy for lung disease of prematurity. New England Journal of Medicine 2004;350(13):1304-13. [DOI] [PubMed] [Google Scholar]


