Abstract
Background
Belimumab, the first biologic approved for the treatment of systemic lupus erythematosus (SLE), has been shown to reduce autoantibody levels in people with SLE and help control disease activity.
Objectives
To assess the benefits and harms of belimumab (alone or in combination) in systematic lupus erythematosus.
Search methods
An Information Specialist carried out the searches of CENTRAL, MEDLINE, Embase, CINAHL, Web of Science, the World Health Organization (WHO) International Clinical Trials Registry Platform, and clinicaltrials.gov from inception to 25 September 2019. There were no language or date restrictions.
Selection criteria
We included randomized controlled trials (RCTs) or controlled clinical trials (CCTs) of belimumab (alone or in combination) compared to placebo/control treatment (immunosuppressive drugs, such as azathioprine, cyclosporine, mycophenolate mofetil or another biologic), in adults with SLE.
Data collection and analysis
We used standard methodologic procedures expected by Cochrane.
Main results
Six RCTs (2917 participants) qualified for quantitative analyses. All included studies were multicenter, international or US‐based. The age range of the included participants was 22 to 80 years; most were women; and study duration ranged from 84 days to 76 weeks. The risk of bias was generally low except for attrition bias, which was high in 67% of studies.
Compared to placebo, more participants on belimumab 10 mg/kg (Food and Drug Administration (FDA)‐approved dose) showed at least a 4‐point improvement (reduction) in Safety of Estrogen in Lupus National Assessment (SELENA) ‐ Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) score, a validated SLE disease activity index: (risk ratio (RR) 1.33, 95% confidence interval (CI) 1.22 to 1.45; 829/1589 in belimumab group and 424/1077 in placebo; I2= 0%; 4 RCTs; high‐certainty evidence).
Change in health‐related quality of life (HRQOL), assessed by Short Form‐36 Physical Component Summary score improvement (range 0 to 100), showed there was probably little or no difference between groups (mean difference 1.6 points, 95% CI 0.30 to 2.90; 401 in belimumab group and 400 in placebo; I2= 0%; 2 RCTs; moderate‐certainty evidence). The belimumab 10 mg/kg group showed greater improvement in glucocorticoid dose, with a higher proportion of participants reducing their dose by at least 50% compared to placebo (RR 1.59, 95% CI 1.17 to 2.15; 81/269 in belimumab group and 52/268 in placebo; I2= 0%; 2 RCTs; high‐certainty evidence).
The proportion of participants experiencing harm may not differ meaningfully between the belimumab 10 mg/kg and placebo groups: one or more serious adverse event (RR 0.87, 95% CI: 0.68 to 1.11; 238/1700 in belimumab group and 199/1190 in placebo; I2= 48%; 5 RCTs; low‐certainty evidence; ); one or more serious infection (RR 1.01, 95% CI: 0.66 to 1.54; 44/1230 in belimumab group and 40/955 in placebo; I2= 0%; 4 RCTs; moderate‐certainty evidence); and withdrawals due to adverse events (RR 0.82, 95% CI: 0.63 to 1.07; 113/1700 in belimumab group and 94/1190 in placebo; I2= 0%; 5 RCTs; moderate‐certainty evidence). Mortality was rare, and may not differ between belimumab 10 mg/kg and placebo (Peto odds ratio 1.15, 95% CI 0.41 to 3.25; 9/1714 in belimumab group and 6/1203 in placebo; I2= 4%; 6 RCTs; low‐certainty evidence).
Authors' conclusions
The six studies that provided evidence for benefits and harms of belimumab were well‐designed, high‐quality RCTs. At the FDA‐approved dose of 10 mg/kg, based on moderate to high‐certainty data, belimumab was probably associated with a clinically meaningful efficacy benefit compared to placebo in participants with SLE at 52 weeks. Evidence related to harms is inconclusive and mostly of moderate to low‐certainty evidence. More data are needed for the longer‐term efficacy of belimumab.
Keywords: Adult; Aged; Aged, 80 and over; Female; Humans; Male; Middle Aged; Young Adult; Antibodies, Monoclonal, Humanized; Antibodies, Monoclonal, Humanized/adverse effects; Antibodies, Monoclonal, Humanized/therapeutic use; Bias; Glucocorticoids; Glucocorticoids/therapeutic use; Immunosuppressive Agents; Immunosuppressive Agents/adverse effects; Immunosuppressive Agents/therapeutic use; Lupus Erythematosus, Systemic; Lupus Erythematosus, Systemic/drug therapy; Lupus Erythematosus, Systemic/mortality; Placebos; Placebos/therapeutic use; Quality of Life; Randomized Controlled Trials as Topic; Treatment Outcome
Plain language summary
What are the benefits and risks of belimumab for treating systemic lupus erythematosus (an autoimmune disease that affects the whole body)?
Why is this question important?
Systemic lupus erythematosus (SLE; or ‘lupus’) is a disease in which the body's immune (defense) system mistakenly attacks healthy tissue in many parts of the body. It is a long‐term disease (one that lasts longer than six weeks and is usually life‐long). Often, SLE causes pain in joints and muscles, and extreme tiredness. Symptoms can improve temporarily, or worsen suddenly (flares).
There is no cure for SLE. However, treatments can improve symptoms. Inflammation caused by lupus can affect the joints, skin, kidneys, blood cells, brain, heart, and lungs. Most people are treated with glucocorticoids (anti‐inflammation medicines; also called "steroids/prednisone/medrol/solumedrol") at some point in their disease course. These medicines reduce the swelling and pain associated with inflammation, but they can have serious adverse (unwanted) effects, such as kidney damage. This is why it is important to study other treatment options, such as belimumab.
Belimumab is a human protein (antibody) that is given as an injection to decrease inflammation. In people with SLE, belimumab prevents the development and survival of the cells that attack the body’s healthy tissues (B cells). The aim of belimumab is to reduce the number of B cells, so that people’s symptoms improve.
To find out about the benefits and risks of belimumab, we reviewed the evidence from research studies. In particular, we wanted to know how belimumab affects:
‐ disease intensity;
‐ health‐related quality of life;
‐ use of glucocorticoids; and
‐ adverse effects, including: serious effects (for example, diseases that affect kidneys or the nervous system), serious infections (since anti‐inflammation medicines reduce the body’s ability to fight infections), adverse effects that led people to withdraw from studies, and death.
How did we identify and evaluate the evidence?
First, we searched the medical literature for studies that compared belimumab against a placebo (dummy) treatment or another SLE treatment. We then compared the results and summarized the evidence from all the studies. Finally, we rated our confidence in the evidence, based on factors such as study methods and sizes, and the consistency of findings across studies.
What did we find?
We found six studies that involved a total of 2917 people (mostly women) aged between 22 and 80 years. People were followed for a minimum of 84 days and a maximum of 76 weeks. All studies compared belimumab against placebo.
Intensity of disease
Belimumab is better than placebo at reducing SLE intensity (4 studies, 2666 people). SLE became less intense in 52% of people treated with belimumab, compared to 39% of people treated with placebo.
Health‐related quality of life
The evidence suggests that there is probably little to no difference between belimumab and placebo for changes in health‐related quality of life (2 studies, 801 people). The quality of life scores reported by people treated with belimumab were only 1.6 points higher on average than those of people treated with placebo (scale: 0 to 100, higher scores = better quality of life).
Need for glucocorticoids (anti‐inflammation medicines)
Belimumab is more likely than placebo to reduce the amount of glucocorticoids required. Glucocorticoid doses were lowered by at least 50% in 30% of people treated with belimumab, compared to 19% of people treated with placebo (2 studies, 537 people).
Serious adverse events
The evidence suggests that there may be little to no difference in the numbers of serious adverse events attributable to belimumab or placebo (5 studies, 2890 people). We have little confidence in this finding, because it is based on relatively small numbers of adverse events and studies reported conflicting findings.
Serious infection
The evidence suggests that there is probably little to no difference in the numbers of serious infections attributable to belimumab or placebo (4 studies, 2185 people). We are only moderately confident about this finding, because it is based on small numbers of infections.
Adverse events causing people to withdraw from studies
The evidence suggests that there is probably little to no difference between belimumab and placebo in the numbers of adverse events that caused people to withdraw from studies (5 studies, 2890 people). We are only moderately confident about this finding, because it is based on relatively small numbers of adverse events.
Death
Few deaths (less than 1%) were reported in association with either belimumab or placebo. This suggests that there may be little to no difference in numbers of deaths between the two treatments, though, because death occurs rarely, it is difficult to be confident about this finding.
What does this mean?
When compared against placebo:
‐ belimumab is more likely to reduce SLE disease activity, and to reduce the amount of glucocorticoids needed;
‐ belimumab probably makes little to no difference to: health‐related quality of life improvement; numbers of serious adverse events; and deaths; and
‐ belimumab may make little to no difference to numbers of serious infections, and numbers of adverse events that cause people to withdraw from studies.
How‐up‐to date is this review?
The evidence in this Cochrane Review is current to September 2019.
Summary of findings
Summary of findings 1. Belimumab 10 mg/kg compared to placebo for systemic lupus erythematosus.
| Belimumab 10 mg/kg compared to placebo for systemic lupus erythematosus | ||||||
| Patient or population: people with active systemic lupus erythematosus (but not active nephritis or active central nervous system involvement) Setting: multicenter, international studies Intervention: belimumab 10 mg/kg (alone or in combination with other immunosuppressive drugs or another biologic) Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Belimumab 10 mg/kg | |||||
| Reduction of at least 4 points in SELENA‐SLEDAI at 52 weeks SELENA‐SLEDAI score, a validated marker of SLE disease activity (range 0 to 105; higher score = worse disease activity) Follow‐up: mean 52 weeks |
394 per 1000 | 524 per 1000 (480 to 571) | RR 1.33 (1.22 to 1.45) | 2666 (4 RCTs) | ⊕⊕⊕⊕ high | NNTB: 8 (95% CI 6 to 11) Absolute risk difference: 13% better (95% CI 8% to 17%) Relative percentage change: 33% better (95% CI 22% to 45%) |
| Change in HRQOL, on SF‐36 PCS score at 52 weeks Improvement in SF‐36 PCS score from baseline (range: 0 to 100); population mean 50; higher score = better HRQOL; MCID ~2.5 to 5 points) Follow‐up: mean 52 weeks |
The mean change in HRQOL on SF‐36 PCS score at 52 weeks in the control groups ranged from a 1.4 to 2.84 point improvement | The mean change in HRQOL on SF‐36 PCS score at 52 weeks in the intervention groups was 1.60 higher (0.30 lower to 2.90 higher) | MD 1.60 (0.30 to 2.90) | 801 (2 RCTs) | ⊕⊕⊕⊝ moderatea | Absolute risk difference: 1.6% higher (95% CI 0.3% to 2.9%) Relative percentage change: 3.2% higher (95% CI 0.6% to 5.8%) |
| Reduction of glucocorticoid dose by at least 50% Follow‐up: 52 weeks |
194 per 1000 | 309 per 1000 (227 to 417) |
RR 1.59 (1.17 to 2.15) | 537 (2 RCTs) | ⊕⊕⊕⊕ high | NNTB: 9 (95% CI 5 to 28) Absolute risk difference: 11% better (95% CI 4% to 18%) Relative percentage change: 59% better (95% CI 17% to 115%) |
| Participants with one or more serious adverse events Follow‐up: mean 52 to 76 weeks |
167 per 1000 | 145 per 1000 (114 to 185) | RR 0.87 (0.68 to 1.11) | 2890 (5 RCTs) | ⊕⊕⊝⊝ lowa,b | Absolute risk difference: 2% less (95% CI ‐6% to 2%) Relative percentage change: 13% more (95% CI ‐32% to 11%) |
| Participants with one or more serious infections Follow‐up: mean 52 to 76 weeks |
42 per 1000 | 42 per 1000 (28 to 65) | RR 1.01 (0.66 to 1.54) | 2185 (4 RCTs) | ⊕⊕⊕⊝ moderatea | Absolute risk difference: 0% (95% CI ‐1% to 1%) Relative percentage change: 1% fewer (95% CI ‐34% to 54%) |
| Withdrawals due to adverse events Follow‐up: mean 52 to 76 weeks | 79 per 1000 | 65 per 1000 (50 to 85) | RR 0.82 (0.63 to 1.07) | 2890 (5 studies) | ⊕⊕⊕⊝ moderatea | Absolute risk difference: 1% fewer (95% CI ‐3% to 1%) Relative percentage change: 18% fewer (95% CI ‐37% to 7%) |
| Number of participants who died during the study Follow‐up: mean 52 to 76 weeks |
5 per 1000 | 6 per 1000 (2 to 16) | Peto OR 1.15 (0.41 to 3.25) | 2917 (6 studies) | ⊕⊕⊝⊝ lowa,c | Absolute risk difference: 0% (95% CI ‐1% to 1%) Relative percentage change: 15% more (95% CI ‐59% to 221%) |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HRQOL: health‐related quality of life; MCID: minimal clinically important difference; MD: mean difference; NNTB: number needed to treat for an additional beneficial outcome; OR: odds ratio; PCS: Physical Component Score; RCT: randomized controlled trial; RR: risk ratio; SELENA‐SLEDAI: Safety of Estrogen in Lupus National Assessment (SELENA) ‐ Systemic Lupus Erythematosus Disease Activity Index (SLEDAI); SF‐36: 36‐item short‐form; | ||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded one level due to imprecision. The 95% confidence interval includes both no effect and appreciable benefit/harm exceeding a minimal clinically important difference. bDowngraded one level due to inconsistency, I2 = 48% cDowngraded one level due to imprecision. The total number of events was small (n = 15).
Background
Description of the condition
Lupus, also known as systemic lupus erythematosus (SLE), is a systemic autoimmune condition with multiple manifestations and a waxing and waning course. It is characterized by flares and periods of remission. In its severe form, it is a potentially life‐threatening condition that is difficult to treat. Many medications have been used for treatment in the past; however, few were approved for use by the US Food and Drug Administration (FDA). Several of the commonly used medications, including prednisone and cyclophosphamide, have significant adverse events. Recently belimumab, a biologic, was evaluated in people with SLE. Based on results of the randomized trials, it was approved by the FDA for the treatment of SLE. A Cochrane Review can summarize the benefits and harms data for people with SLE and providers.
Description of the intervention
Belimumab is a fully human monoclonal antibody that inhibits the B‐lymphocyte stimulator (BLyS). It is marketed by Glaxo SmithKline with the trade name Benlysta. BLyS, a cytokine member of the tumor necrosis factor (TNF) family, is a 285 amino‐acid cytokine and is also known as a B cell activation factor of the TNF family (BAFF) (Bossen 2006). Belimumab is indicated for use in people with active SLE. It is given as an intravenous infusion, usually over one hour, in a doctor's office. The dose given is 10 mg/kg at two‐week intervals for the first three doses and at four‐week intervals thereafter.
How the intervention might work
SLE, as a chronic inflammatory disorder, is thought to be driven by autoantibodies that target multiple organ systems including joints, skin, and kidneys. Although the disease is characterized by pathogenic autoantibodies that target specific tissues, many additional cell types (for example B cells, T cells) and cytokines (for example type I interferon (IFN‐I)‐a) are involved in the inflammatory response. B cells play a central role in the pathogenesis of SLE, mainly by producing autoantibodies but also by producing cytokines and by presenting antigens to T cells. SLE can result due to defective proteins that regulate T cells in the dysfunctional clearance of immune cells, suggesting that part of the pathology lies in loss of the immune tolerance and the persistence of attractive B‐ and T‐cell populations (Ravirajan 1996). On the other hand, dysregulation of the innate immune system also contributes to SLE. Immune complexes of autoantibodies with endogenous RNA and DNA can be taken up by plasmacytoid dendritic cells, which activate toll‐like receptor (TLR)7 and TLR9, respectively, and generate IFN‐I (Crow 2004). IFN‐I can then further activate the adaptive immunity by enhancing the antigen‐presenting function of monocytes and dendritic cells and activating B cells (Kim 2009). Thus, dysregulation of both adaptive and innate immunity plays a role in the pathogenesis of SLE.
BLyS is an important link between adaptive and innate immunity, thus playing a special role in SLE pathogenesis (Kim 2012). Many immune cells, such as monocytes and macrophages, dendritic cells, T cells, and activated neutrophils, have been implicated in the production of BLyS. BLyS plays a key role in B lymphocyte differentiation, survival, and activation (Crowley 2005). BLyS, along with a related cytokine (a proliferation‐inducing ligand (APRIL)) can bind to three membrane receptors on B cells: B cell maturation antigen (BCMA), transmembrane activator and calcium modulator and cyclophilin ligand interactor (TACI), and BAFF‐R (also known as BR3) (Litinskiy 2002). This interaction leads to activation of immature B lymphocytes into plasma cells. Belimumab is a fully human monoclonal antibody that inhibits the biological activity of BLyS, which is important for survival and development of B lymphocyte cells into antibody producing mature plasma B cells. SLE is associated with higher levels of BLyS, which contributes to the production of autoantibodies, a hallmark of the disease (Cheema 2001; Collins 2006; Petri 2008). Thus, the administration of belimumab can lead to decrease in the biologic/functional effects of BLyS.
Why it is important to do this review
Belimumab is the first biologic approved for use in SLE. A Cochrane systematic review will provide a comprehensive assessment of data from the randomized clinical trials of belimumab in SLE.
Objectives
To assess the benefits and harms of belimumab (alone or in combination) in systematic lupus erythematosus.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs) or controlled clinical trials (CCTs) were eligible for inclusion.
Types of participants
We included people with SLE who met the American College of Rheumatology classification criteria for SLE (Hochberg 1997).
Types of interventions
Belimumab, alone or in combination with other immunosuppressive drug (such as azathioprine, cyclosporine, mycophenolate mofetil) or another biologic, compared to placebo or other disease‐modifying anti‐rheumatic drugs (DMARDs), DMARD combinations, or biologics.
Types of outcome measures
Major outcomes
Outcomes as assessed by:
change in SLE scores on validated disease activity indices, the Systemic Lupus Erythematosus Disease Activity Index Safety of Estrogen in Lupus National Assessment (SELENA) Modification (SELENA‐SLEDAI) (Petri 2005), modified SELENA‐SLEDAI Flare Index (SFI) (Petri 1999; Petri 2005); British Isles Lupus Assessment Group index (BILAG) (Hay 1993; Isenberg 2000); or other similar validated indices;
quality of life, assessed by the Short‐Form 36 (SF‐36) Physical Component Score (PCS) or similar assessments;
reduction in glucocorticoid dose, defined as mean reduction of prednisone equivalent dose in mg/day, cumulative dose, percentage reduction or below a certain threshold (< 10 mg/day or < 7.5 mg/day), or percentage of participants off glucocorticoids
serious adverse events, number of serious adverse events (SAEs), or number of participants with one or more SAE;
serious infections;
withdrawals due to adverse events; and
death
Search methods for identification of studies
Electronic searches
The Information Specialist carried out searches of: Cochrane Central Register of Controlled Trials (CENTRAL; August 2019) in the Cochrane LibraryMEDLINE Ovid; Embase Classic + Embase; CINAHL (Cumulative Index to Nursing and Allied Health Literature), Web of Science, and the World Health Organization (WHO) International Clinical Trials Registry Platform. There were no language or date restrictions in the search for trials. The searches for all databases were from inception to 25 September 2019.
We used the MEDLINE search strategy (Appendix 1) and adapted it for other databases (CENTRAL, Appendix 2; Embase, Appendix 3; CINAHL, Appendix 4; Web of Science, Appendix 5; WHO International Clinical Trials Registry Platform, Appendix 6).
Searching other resources
We searched the reference lists of included studies to find additional articles of relevance.
Data collection and analysis
Selection of studies
Review authors independently reviewed titles and abstracts to find eligible studies. We discussed any disagreements and resolved them by consensus. We did not need to refer any to an adjudicator, since no disagreements remained after consensus.
Data extraction and management
Review authors independently extracted data from the included studies using standardized data extraction forms. The extracted data included study characteristics, study population characteristics, details about the interventions, funding sources, and outcomes of interest. When we needed more information, we contacted the authors of the studies. We extracted data from the published reports, including mean and standard deviation for continuous outcomes and the number of events and people at risk for dichotomous data. Whenever possible, we extracted the data based on an intention‐to‐treat analysis.
Assessment of risk of bias in included studies
Review authors independently assessed the risk of bias for each included trial using the recommendations in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2020). We assessed the risk of bias on each of the following criteria:
random sequence generation;
allocation concealment
blinding of participants and personnel;
blinding of outcome assessors;
incomplete outcome data;
selective outcome reporting; and
other bias
We assessed the risk of bias as: low risk, high risk, or unclear risk (either lack of information or uncertainty over the potential for bias). We also looked for other biases to see if the authors of the study had any vested interests. The review authors resolved any disagreements by discussion among themselves.
Measures of treatment effect
We analyzed dichotomous data as risk ratios (RR), or Peto odds ratios (OR) when the outcome was a rare event (approximately less than 10%), and used 95% confidence intervals (CIs). We analyzed continuous data as mean difference (MD) or standardized mean difference (SMD), and entered data presented as a scale with a consistent direction of effect across studies.
For dichotomous outcomes, we calculated the absolute risk difference using the risk difference statistic in RevMan 5, and expressed the result as a percentage. For continuous outcomes, we calculated the absolute risk difference as the improvement in the intervention group minus the improvement in the control group, in the original units, expressed as a percentage.
We calculated the relative percent change for dichotomous data as the risk ratio ‐ 1, and expressed it as a percentage. For continuous outcomes, we calculated the relative difference in the change from baseline as the absolute benefit divided by the baseline mean of the control group, expressed as a percentage.
Unit of analysis issues
We did not anticipate any unit of analysis issues. All studies used the participant as the unit of analysis. If a study had more than one treatment arm for belimumab, we used the treatment arm with the standard, approved dose of belimumab.
Dealing with missing data
In the case of significant missing data, we sent email queries to the authors requesting the missing data. When data for variability statistics (as standard deviations) were not available, we used the baseline standard deviation. We described when missingness was more than 20%. We did not plan to impute any missing data.
Assessment of heterogeneity
We assessed for clinical homogeneity by the following characteristics: age, gender, race, disease duration, number of immunosuppressives used or failed previously, and types of control interventions. To assess statistical heterogeneity quantitatively, we performed a Chi2 test and consider P < 0.10 as an indicator of potentially significant heterogeneity. To examine statistical heterogeneity qualitatively, we examined the I2 statistic. In interpreting the I2 statistic, we used the recommendation in the Cochrane Handbook for Systematic Reviews of Interventions, which indicates that 0% to 40% might not be important; 30% to 60% may represent moderate heterogeneity; 50% to 90% may represent substantial heterogeneity; and 75% to 100% considerable heterogeneity (Deeks 2011).
Assessment of reporting biases
We planned to make a funnel plot to assess reporting biases when performing an analysis on 10 or more studies. The funnel plot is a scatter plot with sample size on the y‐axis and the treatment effect on the x‐axis. An asymmetry in the funnel plot is indicative of reporting bias or other biases related to small study size. Since none of the comparisons had more than six studies, we could not construct a funnel plot for any comparisons.
Data synthesis
When feasible, based on the absence of substantial or considerable heterogeneity, we pooled data using meta‐analysis. We used the random‐effects model as our default model for pooling outcomes in the meta‐analysis, because this is a more conservative approach. We calculated the number needed to treat for an additional beneficial outcome (NNTB) or for an additional harmful outcome (NNTH) as the inverse of the absolute risk difference. We did this using the Visual Rx calculator for dichotomous outcomes (Cates 2004). We calculated NNTB/H for continuous measures with help from the Cochrane Musculoskeletal Group (CMSG) editorial office, using the Wells calculator (available at the CMSG Editorial office, musculoskeletal.cochrane.org/).
Subgroup analysis and investigation of heterogeneity
We planned the following subgroup analyses, pending data availability.
By disease severity: participants stratified by baseline SLE disease activity corresponding to mild versus moderate versus severe SLE (as per different cut‐offs on various scales). If data were scarce, we planned to collapse the moderate and severe categories.
By participant demographics: age (under 65 versus 65 years and above); race or ethnicity.
By concomitant prednisone use: dose of prednisone < 7.5 mg/day versus ≥ 7.5 to 10 mg/day equivalent.
Sensitivity analysis
We had planned to carry out sensitivity analyses to assess the impact of studies at low versus unclear risk of bias for allocation concealment and blinding. However, these were not feasible because the studies all had similar risks of bias for these domains. We also planned to undertake sensitivity analyses using fixed‐effect models; we have summarized these for each outcome.
Summary of findings and assessment of the certainty of the evidence
We constructed a 'Summary of findings' table (Table 1) in order to communicate the key outcomes of the review, as recommended by The Cochrane Handbook for Systematic Reviews of Interventions (Schunemann 2011a). We used GRADEpro GDT to provide an overall grading of the certainty of the evidence in the 'Summary of findings' table (Schunemann 2011b). Outcomes in the table included three beneficial outcomes (reduction in SELENA‐SLEDAI score, change in health‐related quality of life, and reduction of glucocorticoid dose) and four harmful outcomes (serious adverse events, serious infections, withdrawals due to adverse events, and deaths).
Results
Description of studies
Results of the search
Of the 1854 titles identified by searches, the review authors screened 1472 titles after de‐duplication and selected 31 articles for full‐text review (Figure 1). We contacted one author for missing information on random sequence generation (Wallace 2009). The author responded by email, indicating that computer‐randomized code was used for randomization. We excluded 25 studies due to various reasons, and included six in the quantitative analyses.
1.
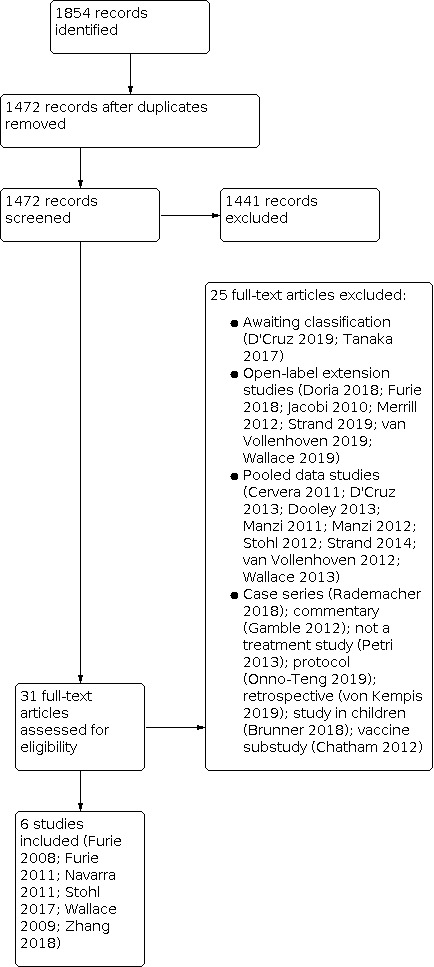
Study flow diagram.
Included studies
We included six original studies with 2917 participants in the analyses (Furie 2008; Furie 2011; Navarra 2011; Stohl 2017; Wallace 2009; Zhang 2018). A detailed description of the included studies is given in the 'Characteristics of included studies' table. All studies were RCTs and contributed to the meta‐analysis. These included BLISS‐52 (Navarra 2011) and BLISS‐76 (Furie 2011). One study was a phase‐I RCT (Furie 2008); the remaining studies were phase‐II and phase‐III RCTs.
All studies compared belimumab to placebo and some contained multiple dose comparisons (1, 4, 10 and 20 mg/kg in Furie 2008; 1, 4, and 10 mg/kg in Wallace 2009; 1 and 10 mg/kg in Furie 2011 and Navarra 2011). Zhang 2018 compared belimumab 10 mg/kg to placebo and Stohl 2017 looked at the comparable subcutaneous (SC) dose (200 mg). For the purpose of this review, we focused on that standard dose (10 mg/kg; 200 mg SC). Study duration was from 84 to 105 days for Furie 2008, 52 weeks for Navarra 2011, Stohl 2017, Wallace 2009, Zhang 2018, and 76 weeks for Furie 2011. The studies reported similar outcomes for benefits and harms except for Furie 2008, which reported primarily on safety and pharmacokinetics.
Excluded studies
Two studies were excluded from the current analysis and marked as awaiting classification (D'Cruz 2019; Tanaka 2017). An additional 23 studies were excluded for various reasons, as described in the 'Characteristics of excluded studies' table: open‐label extension studies (Doria 2018; Furie 2018; Jacobi 2010; Merrill 2012; Strand 2019; van Vollenhoven 2019; Wallace 2019); studies with pooled data (Cervera 2011; D'Cruz 2013; Dooley 2013; Manzi 2011; Stohl 2012; Strand 2014; van Vollenhoven 2012; Wallace 2013); case series (Rademacher 2018); commentary (Gamble 2012); not a belimumab treatment study (Petri 2013); protocol (Onno‐Teng 2019); retrospective analysis (von Kempis 2019); study in children (Brunner 2018) and vaccine substudy (Chatham 2012).
Risk of bias in included studies
The summary of risk of bias is shown in Figure 2 and Figure 3. Details are provided for each study as part of the 'Characteristics of included studies' table and briefly stated in the sections below.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
One study (Stohl 2017) was assessed as unclear for risk of selection bias due to allocation concealment. All other studies were determined to be low risk.
Blinding
All studies reported sufficient blinding and had low risk of performance and detection bias.
Incomplete outcome data
Two studies had low risk of attrition bias (Furie 2008; Wallace 2009). Four studies had high withdrawal rates (> 20%) or non‐balanced withdrawal rates, which could increase the risk of attrition bias (Furie 2011; Navarra 2011; Stohl 2017; Zhang 2018).
Selective reporting
All studies had low risk of reporting bias.
Other potential sources of bias
All studies had low risk of other potential sources of bias.
Effects of interventions
See: Table 1
Results described below and those in Table 1 looked at the standard dose of belimumab in SLE (10 mg/kg intravenous (IV); 200 mg subcutaneous (SC).
Belimumab 10 mg/kg vs placebo
Major outcomes
All studies provided data for this comparison. We summarize the main findings in the section below.
Reduction in SELENA‐SLEDAI score (at least a 4‐point reduction) at 52 weeks
Based on data from four studies (2666 participants) at 52 weeks, more participants in the belimumab group than in the placebo group showed improvement (at least a 4‐point reduction) in SELENA‐SLEDAI score (RR 1.33, 95% CI 1.22 to 1.45; I² = 0%). The absolute difference was 13% (95% CI 8% to 17%) better in the belimumab group with 829 of 1589 participants versus 424 of 1077 in the placebo group achieving this reduction. The NNTB was 8 (95% CI 6 to 11). The risk was the same in a fixed‐effect model (RR 1.33, 95% CI 1.22 to 1.46; I2=0%; Analysis 1.1).
1.1. Analysis.

Comparison 1: Belimumab 10 mg/kg vs placebo ‐ Major Outcomes, Outcome 1: Reduction of at least 4 points in SELENA‐SLEDAI at 52 weeks
Change in health‐related quality of life on SF‐36 PCS at 52 weeks
Based on data from two studies (801 participants), there was probably no difference in the health‐related quality of life as measured by change in SF‐36 PCS in participants in the belimumab group compared with the placebo group (MD 1.60, 95% CI 0.30 to 2.90; I2=0%; Analysis 1.2). The absolute risk difference was 1.60% (95% CI 0.30% to 2.0%) higher (better) in the belimumab group.
1.2. Analysis.

Comparison 1: Belimumab 10 mg/kg vs placebo ‐ Major Outcomes, Outcome 2: Change in health‐related quality of life, on Short‐Form 36 PCS score at 52 weeks
Reduction of glucocorticoid dose by 50% or more at 52 weeks
Based on data from two studies (537 participants), the belimumab group showed greater improvement in glucocorticoid dose, with a higher proportion of participants reducing their dose by at least 50%, compared to placebo (RR 1.59, 95% CI 1.17 to 2.15; I² = 0%; Analysis 1.3). The absolute risk difference was 11% (95% CI 4% to 18%) better in the belimumab group, with 81 of 269 participants in the belimumab group versus 52 of 268 in the placebo group having at least a 50% reduction in dose. The risk was similar in a fixed‐effect model (RR 1.58, 95% CI 1.17 to 2.14; I² = 0%).
1.3. Analysis.

Comparison 1: Belimumab 10 mg/kg vs placebo ‐ Major Outcomes, Outcome 3: Steriod sparing with prednisone dose reduced by 50% or more at week 52
Participants with one or more serious adverse events (SAEs)
Based on data from five studies (2890 participants), there may be little or no difference in the number of serious adverse events in the belimumab group compared with the placebo group (RR 0.87, 95% CI 0.68 to 1.11; I² = 48% (suggesting moderate inconsistency); Analysis 1.4). The absolute risk difference was 2% (95% CI ‐6% to 2%) less in the belimumab group with 238 of 1700 participants in the belimumab group versus 199 of 1190 in the placebo group experiencing at least one serious adverse event. The risk was the same in a fixed‐effect model (RR 0.87, 95% CI 0.74 to 1.04; I² = 48%).
1.4. Analysis.

Comparison 1: Belimumab 10 mg/kg vs placebo ‐ Major Outcomes, Outcome 4: Participants with at least one serious adverse event
Participants with one or more serious infections
Based on data from four studies (2185 participants), there was probably little or no difference in the number of serious infections in the belimumab group compared with the placebo group (RR 1.01, 95% CI 0.66 to 1.54; I² = 0%; Analysis 1.5). The absolute risk difference was 0% (95% CI ‐1% to 1%) with 44 of 1230 participants in the belimumab group versus 40 of 955 in the placebo group having at least one serious infection. The risk was the same in a fixed‐effect model.
1.5. Analysis.

Comparison 1: Belimumab 10 mg/kg vs placebo ‐ Major Outcomes, Outcome 5: Participants with at least one serious infection
Withdrawals due to adverse events (AEs)
Based on data from five studies (2890 participants), there was probably little or no difference in the proportion of participants who withdrew due to adverse events in the belimumab group compared with the placebo group (RR 0.82 (95% CI 0.63 to 1.07; I² = 0%; Analysis 1.6). The absolute risk difference was 1% (95% CI ‐3% to 1%) fewer in the belimumab group with 113 of 1700 participants in the belimumab group versus 94 of 1190 in the placebo group withdrawing due to adverse effects. The risk was similar in a fixed‐effect model (RR 0.83, 95% CI 0.64 to 1.08; I² = 0%).
1.6. Analysis.

Comparison 1: Belimumab 10 mg/kg vs placebo ‐ Major Outcomes, Outcome 6: Withdrawals due to adverse events
Deaths
Based on data from six studies (2917 participants), there may be little or no difference in the number of deaths in the belimumab group compared with the placebo group (Peto odds ratio (OR) 1.15, 95% CI 0.41 to 3.25; I² = 0%; Analysis 1.7). The absolute risk difference was 0% (95% CI ‐1% to 1%) with nine deaths out of 1714 participants in the belimumab group and six deaths out of 1203 participants in the placebo group.
1.7. Analysis.

Comparison 1: Belimumab 10 mg/kg vs placebo ‐ Major Outcomes, Outcome 7: Deaths
Subgroup and Sensitivity Analyses
We could not perform the planned subgroup analyses based on baseline SLE severity, age groups, and dose of prednisone, due to lack of available data. Neither could we undertake the planned sensitivity analyses about allocation concealment and blinding, as all studies contained similar low‐level risks in both domains. We did carry out the planned sensitivity analyses with fixed‐effect models, where appropriate, and summarized these within the main reporting of results for each outcome.
Discussion
Summary of main results
Major Outcomes
At the FDA‐approved dose of 10 mg/kg, belimumab was shown to improve some benefits compared to placebo in people with SLE, while evidence related to harms was inconclusive. Specifically, belimumab 10 mg/kg was associated with a higher likelihood of achieving at least a 4‐point improvement (reduction) in SELENA‐SLEDAI score, a validated SLE disease activity score (RR 1.33, 95% CI 1.22 to 1.45; I2 = 0%; high‐certainty evidence). More people taking belimumab than those taking placebo had an improvement (reduction) of glucocorticoid dose of at least 50% at 52 weeks (RR 1.59, 95% CI 1.17 to 2.15; I2 = 0%; high‐certainty evidence). Change in HRQOL, assessed by SF‐36 PCS score, showed an increased mean change in belimumab over placebo of 1.6 points (95% CI 0.30 to 2.90), which was probably not different and did not meet the clinically‐meaningful threshold of a 2.5 to 5‐point greater improvement (moderate‐certainty evidence; I2 = 0%). Moderate‐certainty evidence showed that there was probably little or no difference in the proportion of participants with one or more serious infections and in the proportion of participants who withdrew due to adverse events. There was low‐certainty evidence to suggest little or no difference in either the number of participants with one or more serious adverse events or in the number of deaths (which were rare).
Overall completeness and applicability of evidence
The evidence presented in this systematic review is current to 25 September 2019. The data presented here came from studies that were mostly of 52 weeks' duration, thus providing intermediate‐term outcomes for people with SLE. Our systematic review assessed the benefits and harms of the standard dose of belimumab (10 mg/kg) compared to placebo in people with SLE, who were recruited in multinational trials. Most trials were funded by Human Genome Sciences or GlaxoSmithKline, the developer of belimumab. They had similar designs, recruited similar participants, used similar interventions and reported similar outcome measures, thus allowing for meta‐analyses for several outcomes. Thus, the evidence here applies to a broad range of people with SLE seen in the clinical practice, as it relates to the short‐ to intermediate‐term benefits and harms of belimumab in the approved dose of 10 mg/kg, compared to placebo. However, these study findings do not apply to people with SLE who have active central nervous system disease or severe nephritis.
All trials compared belimumab to placebo, not an active comparator, so the efficacy of belimumab compared to other immunosuppressives or combinations of these drugs is unknown. In the absence of longer follow‐up studies, focusing on harms, the long‐term safety of belimumab is also unknown. Given the short‐term nature of these trials and the uncommon/rare occurrence of some harms, differences between belimumab and comparator are difficult to detect. Longer‐term follow‐up studies and registry data are needed to better understand uncommon/rare harms related to belimumab use. We planned several subgroup analyses according to baseline SLE severity, age groups and dose of prednisone. However, we could not perform any of these, since these data were not available.
We prespecified sensitivity analyses based on allocation concealment and blinding (low risk versus unclear risk) to try to more completely understand our results. Given that all studies were at low risk for blinding and only one study was at unclear risk for allocation concealment (Stohl 2017), we did not perform these sensitivity analyses.
Quality of the evidence
The certainty of the evidence for the three main beneficial outcomes was moderate (downgraded due to imprecision) to high. Certainty of evidence for the harmful outcomes was moderate (downgraded due to imprecision) to low (downgraded due to imprecision, inconsistency and small number of events). We noted imprecision of estimates for all four harmful outcomes and the HRQOL outcome.
Importantly, the risk of bias for the majority of the bias criteria was low for most included studies and unclear in only some instances. Three studies had high dropout rates (Furie 2011; Navarra 2011; Stohl 2017); however, the follow‐up in these studies was quite long at 52 to 76 weeks, so this dropout rate is somewhat explained by the length of RCT. With the exception of the study by Wallace 2009, all studies were funded by Human Genome Sciences/GlaxoSmithKline, the manufacturer of belimumab; most studies described their role.
Potential biases in the review process
We extracted the data for this systematic review and meta‐analysis using standard methodology. Two of the review authors (NS, JAS, AM, working in pairs) performed all data and 'risk of bias' assessments independently. All authors entered data into RevMan and cross‐checked the accuracy of each other's data entry.
Agreements and disagreements with other studies or reviews
There are few studies in the literature which have systematically assessed the effect of belimumab on people with SLE. Overall, the findings of our study compare favorably with other belimumab systematic reviews and meta‐analyses in the literature (Blair 2018; Boyce 2012; Burness 2011; Kandala 2013; Lee 2018). We found that major outcomes (reduction of at least four points in SELENA‐SLEDAI score and reduction of glucocorticoid dose by at least 50%) in our study showed improvements at week 52. Another review of belimumab use in SLE reported similar findings (Boyce 2012). Similar reviews (Burness 2011, Kandala 2013) have shown favorable results for all outcomes at week 52; however, the outcomes lost benefit at week 76.
Our analysis of harmful outcomes in belimumab was inconclusive. Efficacy and tolerability of belimumab were confirmed in a review by Burness 2011. The major belimumab trials were conducted in varied geographic regions and therefore were at risk of statistical heterogeneity. Our review only found moderate heterogeneity in one harmful outcome (participants with one or more serious adverse events). Kandala 2013 also reported negative test results for statistical heterogeneity. However, our review differs from Kandala 2013 in terms of quality evaluation of the included studies. The BLISS‐52 and BLISS‐76 had high withdrawal rates sufficient to have an effect on the effect measure. We therefore assessed them to have a high risk of attrition bias, whereas the previous meta‐analysis reported low risk for attrition bias.
Authors' conclusions
Implications for practice.
Belimumab 10 mg/kg probably shows benefit for reducing systemic lupus erythematosus (SLE) disease activity and glucocorticoid doses in SLE patients when compared to placebo. Evidence related to the increase in the risk of serious infections, serious adverse events, withdrawals due to adverse events or death was inconclusive. The certainty of evidence for all outcomes except serious adverse events and mortality was moderate to high. Longer‐term studies of belimumab are needed to confirm these results and better assess harms.
Implications for research.
The randomized controlled trials (RCTs) that provided evidence for benefits and harms of belimumab were well designed, of high quality and used validated outcomes. The trials' key primary outcomes were clinically relevant and they reported these in a transparent fashion. Harms assessment is always difficult in RCTs, unless they have a large sample size or longer follow‐up, and this limitation also applies to this systematic review. Open‐label follow‐up, registry and database studies, as well as RCTs with active comparators, can provide more knowledge regarding belimumab's benefits and harms. Longer‐term studies in subgroups of people categorized by SLE disease activity, prednisone dose, type of immunosuppressive and comparison to combination immunosuppressive regimens will further clarify the role that belimumab can play in the treatment of people with active SLE. Head‐to‐head active comparator trials should help in expanding this knowledge. Trials in people with active lupus nephritis, the most common visceral manifestation of SLE, may also help us to understand its role in the treatment of renal disease.
History
Protocol first published: Issue 7, 2013 Review first published: Issue 2, 2021
Acknowledgements
We thank Louise Falzon and Tamara Rader for developing the search strategy and performing the literature search. We thank Shahrzad Noobaloochi, Matthew Tucker and Margaret Tresler for review of the protocol and trial data abstraction. We thank Dr Winn Chatham for reviewing and providing critical comments.
Appendices
Appendix 1. Search strategy for MEDLINE
1. exp lupus erythematosus, cutaneous/ or exp lupus erythematosus, systemic/
2. lupus.tw.
3. sle.tw.
4.or/ 1‐3
5. Antibodies, Monoclonal/
6. Belimumab.tw.
7. Belimumabum.tw.
8. Benlysta.tw.
9. B‐lymphocyte stimulator$.tw.
10. BLyS.tw.
11. or/5‐10
12. 4 and 11
13. randomized controlled trial.pt.
14. controlled clinical trial.pt.
15. randomized.ab.
16. placebo.ab.
17. drug therapy.fs.
18. randomly.ab.
19. trial.ab.
20. groups.ab.
21. or/13‐20
22. (animals not (humans and animals)).sh.
23. 21 not 22
24. 12 and 23
Appendix 2. Search strategy for CENTRAL
#1 MeSH descriptor Lupus Erythematosus, Cutaneous explode all trees
#2 MeSH descriptor Lupus Erythematosus, Systemic explode all trees
#3 lupus:ti,ab
#4 sle:ti,ab
#5 (#1 OR #2 OR #3 OR #4)
#6 MeSH descriptor Antibodies, Monoclonal, this term only
#7 Belimumab:ti,ab
#8 Belimumabum:ti,ab
#9 Benlysta:ti,ab
#10 "B‐lymphocyte stimulator*":ti,ab
#11 BLyS:ti,ab
#12 (#6 OR #7 OR #8 OR #9 OR #10 OR #11)
#13 (#5 AND #11)
Appendix 3. Search strategy for EMBASE
1. exp lupus erythematosus/
2. lupus.tw.
3. sle.tw.
4. or/1‐3
5. belimumab/
6. belimumab.tw.
7. Belimumabum.tw.
8. Benlysta.tw.
9. B‐lymphocyte stimulator$.tw.
10. BLyS.tw.
11. or/5‐10
12. 4 and 11
13. (random$ or placebo$).ti,ab.
14. ((single$ or double$ or triple$ or treble$) and (blind$ or mask$)).ti,ab.
15. controlled clinical trial$.ti,ab.
16. RETRACTED ARTICLE/
17. or/13‐16
18. (animal$ not human$).sh,hw.
19. 17 not 18
20. 12 and 19
Appendix 4. Search strategy for CINAHL
S1 (MH "Lupus Erythematosus, Cutaneous") OR (MH "Lupus Erythematosus, Systemic+")
S2 TI lupus OR AB lupus
S3 TI sle OR AB sle
S4 (MH "Antibodies, Monoclonal")
S5 TI Belimumab OR AB Belimumab
S6 TI Belimumabum OR AB Belimumabum
S7 TI Benlysta OR AB Benlysta
S8 TI B‐lymphocyte stimulator* OR AB B‐lymphocyte stimulator*
S9 TI BLyS OR AB BLyS
S10 S1 or S2 or S3
S11 S4 or S5 or S6 or S7 or S8 or S9
Appendix 5. Search strategy for the Web of Science
Topic=(lupus OR sle)
AND
Topic=(Belimumab OR Belimumabum OR Benlysta OR B‐lymphocyte stimulator* OR BLyS)
Refined by document type (Meeting or Abstract)
Appendix 6. Search strategy for World Health Organization International Clinical Trials Registry Platform
Belimumab OR Belimumabum OR Benlysta OR B‐lymphocyte stimulator* OR BLyS in Intervention
Data and analyses
Comparison 1. Belimumab 10 mg/kg vs placebo ‐ Major Outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Reduction of at least 4 points in SELENA‐SLEDAI at 52 weeks | 4 | 2666 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [1.22, 1.45] |
| 1.2 Change in health‐related quality of life, on Short‐Form 36 PCS score at 52 weeks | 2 | 801 | Mean Difference (IV, Random, 95% CI) | 1.60 [0.30, 2.90] |
| 1.3 Steriod sparing with prednisone dose reduced by 50% or more at week 52 | 2 | 537 | Risk Ratio (M‐H, Random, 95% CI) | 1.59 [1.17, 2.15] |
| 1.4 Participants with at least one serious adverse event | 5 | 2890 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.68, 1.11] |
| 1.5 Participants with at least one serious infection | 4 | 2185 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.66, 1.54] |
| 1.6 Withdrawals due to adverse events | 5 | 2890 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.63, 1.07] |
| 1.7 Deaths | 6 | 2917 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.15 [0.41, 3.25] |
Characteristics of studies
Characteristics of included studies [author‐defined order]
Furie 2008.
| Study characteristics | ||
| Methods |
|
|
| Participants |
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Belimumab 1, 4, 10, or 20 mg/kg or placebo administered intravenously over at least 2 hours as a single infusion or 2 infusions 21 days apart | |
| Outcomes | 1. Adverse events 2. Serious Adverse events 3. SELENA‐SLEDAI score 4. Flare Index 5. Physician's Global Disease Assessment (PGA) 6. Short Form‐36 (SF‐36; version 2) Health Survey 7. Antibelimumab antibodies 8. CD20+ B cells and CD138+ plasmacytoid cells, anti‐dsDNA antibodies, ANAs, immunoglobulins (IgG, IgM, IgE, and IgA), and complement (C3 and C4). 9. Laboratory parameters (hematology, clinical chemistry, urinalysis) |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence generation based on correspondence with the lead author and his team. |
| Allocation concealment (selection bias) | Low risk | Central randomization based on correspondence with the lead author and his team. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants and personnel ensured based on correspondence with the lead author and his team. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment was ensured based on correspondence with the lead author and his team. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | Includes all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Furie 2011.
| Study characteristics | ||
| Methods |
|
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions | 1 mg/kg belimumab, 10 mg/kg belimumab, or placebo by intravenous (IV) infusion over 1 hour on days 0, 14, and 28 and every 28 days through week 72 | |
| Outcomes | Efficacy measures 1. SLE responder index (SRI) response rate 2. British Isles Lupus Assessment Group (BILAG) A organ domain score 3. Physician’s global assessment score 4. Physical component summary (PCS) score 5. Decrease in mean prednisone dose ≥ 25% from baseline 6. SLE flare index Biologic markers 1. Serum Ig, complement (C3 and C4), autoantibodies 2. Peripheral blood lymphocytes were collected at baseline and at weeks 8, 24, 52, and 76 to quantitate B and T cell subsets. Safety measures: 1. Adverse events (AEs) 2. Discontinuations due to AEs 3. Deaths 4. Malignant neoplasms 5. Infections 6. Infusion reactions 7. Laboratory abnormalities |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generation randomized using centralized interactive voice response system to 1 of 3 treatment groups in a 1:1:1 ratio. |
| Allocation concealment (selection bias) | Low risk | After screening, eligible participants were randomly assigned via a centralized interactive voice response system to 1 of 3 treatment groups in a 1:1:1 ratio. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | With the exception of unblinded site pharmacists or designees whose responsibilities were restricted solely to receiving, preparing, and dispensing the study agent, all study site personnel and participants, as well as sponsor and clinical research organization personnel, were blinded to trial agent assignments. Separate study monitors were responsible for the blinded (clinical) and unblinded (study agent preparation) components of the trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All study site personnel and participants, as well as sponsor and clinical research organization personnel, were blinded to trial agent assignments. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Proportion of missing outcomes high in both arms (20% in belimumab arm; 26% in placebo arm). |
| Selective reporting (reporting bias) | Low risk | Includes all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Navarra 2011.
| Study characteristics | ||
| Methods |
|
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions | 1 mg/kg belimumab, 10 mg/kg belimumab, or placebo by intravenous (IV) infusion over 1 hour on days 0, 14, and 28 and every 28 days through week 48 | |
| Outcomes | Efficacy measures 1. SLE responder index (SRI) response rate 2. British Isles Lupus Assessment Group (BILAG) A organ domain score 3. Physician’s global assessment score 4. Physical component summary (PCS) score 5. Decrease in mean prednisone dose ≥ 25% from baseline 6. SLE flare index Biologic markers 1. Serum Ig, complement (C3 and C4), autoantibodies Safety measures: 1. Adverse events (AEs) 2. Serious Adverse Events 3. Discontinuations due to AEs 4. Deaths 5. Malignant neoplasms 6. Infections 7. Infusion reactions 8. Laboratory abnormalities |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomized in a 1:1:1 ratio to placebo, or belimumab 1 mg/kg or 10 mg/kg and assigned to treatment by use of a central interactive voice response system. |
| Allocation concealment (selection bias) | Low risk | Participants who underwent all screening procedures and met the entry criteria were enrolled in the study and assigned to treatment by use of a central interactive voice response system, with the central randomization list provided by Human Genome Sciences. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants, investigators, study co‐ordinators, and sponsors were masked to treatment assignment during intravenous administration of the drug. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants, investigators, study co‐ordinators, and sponsors were masked to treatment assignment during assessment of the participants every 4 weeks during the 52‐week trial until the database was locked. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Proportion of missing outcomes high in both arms (17% in belimumab arm; 21% in placebo arm). |
| Selective reporting (reporting bias) | Low risk | Includes all expected outcomes, including those that were prespecified |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Stohl 2017.
| Study characteristics | ||
| Methods |
|
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions | Weekly doses of belimumab 200 mg or placebo administered SC with a prefilled syringe in addition to stable doses of standard SLE therapy. | |
| Outcomes | Efficacy measures 1. SLE responder index (SRI) response rate components including SELENA‐SLEDAI score 2. British Isles Lupus Assessment Group (BILAG) A organ domain score 3. Physician’s global assessment score 4. Decrease in mean prednisone dose ≥ 25% from baseline 5. SLE flare index Safety measures: 1. Adverse events (AEs) 2. Serious Adverse Events 3. Serious Infections 4. Discontinuations due to AEs 5. Deaths 6. AEs of special interest |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomized 2:1 to receive weekly doses of belimumab 200 mg or placebo administered SC with a prefilled syringe in addition to stable doses of standard SLE therapy. |
| Allocation concealment (selection bias) | Unclear risk | Does not state specifically that randomization was central. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind; dosing provided in pre‐filled syringe. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Study described as double‐blind. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Proportion of missing outcomes high in both arms (17% in belimumab arm; 24% in placebo arm). |
| Selective reporting (reporting bias) | Low risk | Includes all expected outcomes |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Wallace 2009.
| Study characteristics | ||
| Methods |
|
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions | 1, 4, or 10 mg/kg belimumab or placebo by intravenous infusion over 2 hours on days 0, 14, and 28, and then every 28 days for 52 weeks plus standard of care | |
| Outcomes | Efficacy measures 1. SLE responder index (SRI) response rate 2. British Isles Lupus Assessment Group (BILAG) A organ domain score 3. Physician’s global assessment score 4. Physical component summary (PCS) score 5. Decrease in prednisone dose 6. SLE flare index Biologic markers 1. Serum Ig, complement (C3 and C4), autoantibodies, serum BLyS levels, peripheral blood lymphocytes Safety measures: 1. Adverse events (AEs) 2. Serious Adverse Events 3. Discontinuations due to AEs 4. Deaths 5. Neoplams 6. Serious Infections 7. Infusion reactions 8. Laboratory abnormalities |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Lead author confirmed through email about random sequence generation. |
| Allocation concealment (selection bias) | Low risk | Lead author confirmed through email about central randomization. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants and personnel ensured as confirmed by lead author through email. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment was ensured as confirmed by the lead author through email. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data in both arms were < 20% and balanced across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | Includes all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Zhang 2018.
| Study characteristics | ||
| Methods |
|
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions | 10 mg/kg belimumab or placebo by intravenous (IV) infusion in addition to standard of care on days 0, 14, and 28 and then every 28 days through week 48 | |
| Outcomes | Efficacy measures 1. SLE responder index (SRI) response rate, including SELENA‐SLEDAI score 2. British Isles Lupus Assessment Group (BILAG) A organ domain score 3. Physician’s global assessment score 4. Physical component summary (PCS) score 5. SLE flare index 6. Decrease in prednisone dose Safety measures: 1. Adverse events (AEs) 2. Serious AEs 3. AEs of special interest 3. Discontinuations due to AEs 4. Deaths |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization schedules were created by study sponsor using validated software. |
| Allocation concealment (selection bias) | Low risk | Staff members preparing belimumab and placebo formulations, which were identical in appearance and labeled in a double‐blind manner, were not involved in other study activities. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study described as double‐blind. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Staff members preparing belimumab and placebo formulations, which were identical in appearance and labeled in a double‐blind manner, were not involved in other study activities. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Proportion of missing outcomes high in both arms (17% in belimumab arm; 24% in placebo arm). |
| Selective reporting (reporting bias) | Low risk | Includes all expected, prespecified outcomes. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
ACR: American College of Rheumatology AE: adverse event ANA: antinuclear antibodies anti‐dsDNA: anti‐double‐strandDNA BILAG: British Isles Lupus Assessment Group CNS: central nervous system Ig: immunoglobulin IV: intravenous IVIG: intravenous immunoglobulin M/F: male/female RCT: randomized controlled trial SC: subcutaneous SELENA‐SLEDAI: Safety of Estrogen in Lupus National Assessment (SELENA) ‐ Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) SLE: systemic lupus erythematosus TNF: tumor necrosis factor
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Brunner 2018 | Study in children |
| Cervera 2011 | Pooled data analysis |
| Chatham 2012 | Vaccine substudy |
| D'Cruz 2013 | Pooled data analysis |
| Dooley 2013 | Pooled data analysis |
| Doria 2018 | Open‐label extension study |
| Furie 2018 | Open‐label extension study |
| Gamble 2012 | Commentary |
| Jacobi 2010 | Open‐label extension study |
| Manzi 2011 | Pooled data analysis |
| Manzi 2012 | Pooled data analysis |
| Merrill 2012 | Open‐label extension study |
| Onno‐Teng 2019 | Protocol |
| Petri 2013 | Not a belimumab treatment study |
| Rademacher 2018 | Case series |
| Stohl 2012 | Pooled data analysis |
| Strand 2014 | Pooled data analysis |
| Strand 2019 | Open‐label extension study |
| van Vollenhoven 2012 | Pooled data analysis |
| van Vollenhoven 2019 | Open‐label extension study |
| von Kempis 2019 | Not a randomized controlled study, retrospective |
| Wallace 2013 | Pooled data analysis |
| Wallace 2019 | Open‐label extension study |
Characteristics of studies awaiting classification [ordered by study ID]
D'Cruz 2019.
| Methods | Multicenter, double‐blind, placebo‐controlled RCT, 52 weeks |
| Participants | People of self‐identified black race, age 18 years or over, with active SLE |
| Interventions | Monthly belimumab 10 mg/kg IV or placebo, plus standard of care |
| Outcomes | SLE Responder Index response rate with modified SLEDAI‐2K (S2K) scoring for proteinuria, 4‐point reduction in SELENA‐SLEDAI, time to first flare, reduction in prednisone dose by 25%, adverse events |
| Notes | N = 448 (97% female); study did not achieve primary endpoint but significant improvement in subgroups |
Tanaka 2017.
| Methods | Multicenter, placebo‐controlled RCT, 52 weeks |
| Participants | Age 18 or over with qualifying SELENA‐SLEDAI score |
| Interventions | IV belimumab 10 mg/kg or placebo every 28 days, plus standard SLE therapy |
| Outcomes | Reduction in steroid dose, adverse events |
| Notes | N = 677; belimumab effective in steroid reduction |
IV: intravenous RCT: randomized controlled trial SELENA‐SLEDAI: Safety of Estrogen in Lupus National Assessment (SELENA) ‐ Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) SLE: systemic lupus erythematosus SLEDAI‐2K: Safety of Estrogen in Lupus National Assessment (SELENA) ‐ Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) 2000
Differences between protocol and review
In order to harmonize outcomes with other reviews of interventions for SLE, an outcome focused on reduction of glucocorticoid dose by at least 50% was added to the 'Summary of Findings' table. This decision was approved by the Cochrane Musculoskeletal Group editors. The quality of life outcome reported was the Physical Component Score (PCS) of the SF‐36.
Contributions of authors
JAS: duplicate abstract and title review; duplicate data abstraction; data entry into Revman; analyses and creation of GradePro table; wrote the first draft of the review
NS: duplicate abstract and title review; duplicate data abstraction; cross‐checking data entry into Revman; editing of the first draft of the review
AM: data entry into Revman, analyses and creation of GradePro table; cross‐checking data entry into Revman; revision and submission of the review
Sources of support
Internal sources
-
Birmingham VA Medical Center, USA
Dr Singh is supported by the resources and facilities of Birmingham Veterans Affairs Medical Center.
-
Division of Rheumatology, University of Alabama at Birmingham, Birmingham, AL, USA
Support for the Cochrane MSK Disease satellite center
External sources
No sources of support supplied
Declarations of interest
JAS: JAS has received consultant fees from Crealta/Horizon, Medisys, Fidia, UBM LLC, Trio Health, Medscape, WebMD, Adept Field Solutions, Clinical Care options, Clearview healthcare partners, Putnam associates, Focus forward, Navigant consulting, Spherix, Practice Point communications, the National Institutes of Health and the American College of Rheumatology. JAS owns stock options in TPT Global Tech, Vaxart pharmaceuticals and Charlotte’s Web Holdings, Inc. JAS previously owned stock options in Amarin, Viking and Moderna pharmaceuticals. JAS is on the speaker’s bureau of Simply Speaking. JAS is a member of the executive of Outcomes Measures in Rheumatology (OMERACT), an organization that develops outcome measures in rheumatology and receives arms‐length funding from 12 companies. JAS serves on the FDA Arthritis Advisory Committee. JAS is the chair of the Veterans Affairs Rheumatology Field Advisory Committee. JAS is the editor and the Director of the University of Alabama at Birmingham (UAB) Cochrane Musculoskeletal Group Satellite Center on Network Meta‐analysis. JAS previously served as a member of the following committees: member, the American College of Rheumatology's (ACR) Annual Meeting Planning Committee (AMPC) and Quality of Care Committees, the Chair of the ACR Meet‐the‐Professor, Workshop and Study Group Subcommittee and the co‐chair of the ACR Criteria and Response Criteria subcommittee.
NS: none
AM: none
New
References
References to studies included in this review
Furie 2008 {published data only}
- Furie R, Stohl W, Ginzler EM, Becker M, Mishra N, Chatham W, et al. Biologic activity and safety of belimumab, a neutralizing anti-B-lymphocyte stimulator (BLyS) monoclonal antibody: a phase I trial in patients with systemic lupus erythematosus. Arthritis Research and Therapy 2008;5:R109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Furie 2011 {published data only}
- Furie R, Petri M, Zamani O, Cervera R, Wallace DJ, Tegzova D, et al. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis and Rheumatism 2011;63(12):3918-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Navarra 2011 {published data only}
- Navarra SV, Guzman RM, Gallacher AE, Hall S, Levy RA, Jimenez RE, et al. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: A randomised, placebo-controlled, phase 3 trial. Lancet 2011;377(9767):721-31. [DOI] [PubMed] [Google Scholar]
Stohl 2017 {published data only}
- Stohl W, Schwarting A, Okada M, Scheinberg M, Doria A, Hammer A, et al. Efficacy and safety of subcutaneous belimumab in systemic lupus erythematosus. Arthritis and Rheumatism 2017;69(5):1016-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wallace 2009 {published data only}
- Wallace DJ, Stohl W, Furie RA, Lisse JR, McKay JD, Merrill JT, et al. A phase II, randomized, double-blind, placebo-controlled, dose-ranging study of belimumab in patients with active systemic lupus erythematosus. Arthritis and Rheumatism 2009;61(9):1168-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2018 {published data only}
- Zhang F, Bae S-C, Bass D, Chu M, Egginton S, Gordon D, et al. A pivotal phase III, randomised, placebo-controlled study of belimumab in patients with systemic lupus erythematosus located in China, Japan and South Korea. Annals of the Rheumatic Diseases 2018;77:355-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Brunner 2018 {published data only}
- Brunner HI, Abud-Mendoza C, Viola DO, Calvo Penades I, Levy D, Anton J, et al. Efficacy and safety of intravenous belimumab in children with systemic lupus erythematosus. Arthritis and Rheumatology 2018;70(Supplement 9):3225-6. [Google Scholar]
Cervera 2011 {published data only}
- Cervera R, Strand V, Levy RA, Petri M, Rudge H, Hough D, et al. Belimumab improved fatigue and SF-36 physical/mental component summary scores in SLE: BLISS-52/BLISS-76. Lupus 2011;20(4):425. [Google Scholar]
Chatham 2012 {published data only}
- Chatham WW, Wallace DJ, Stohl W, Latinis KM, Manzi S, McCune WJ, et al. Effect of belimumab on vaccine antigen antibodies to influenza, pneumococcal, and tetanus vaccines in patients with systemic lupus erythematosus in the BLISS-76 trial. Journal of Rheumatology 2012;39(8):1632-40. [DOI] [PubMed] [Google Scholar]
D'Cruz 2013 {published data only}
- D'Cruz D, Gladman D, Navarra SV, Sanchez-Guerrero J, Manzi S, Freimuth WW. Post Hoc British Isles Lupus Assessment Group Index musculoskeletal organ domain analysis of systemic lupus erythematosus patients in phase 3 belimumab trials. Lupus 2013;22(1):106. [Google Scholar]
Dooley 2013 {published data only}
- Dooley MA, Houssiau F, Aranow C, D'Cruz DP, Askanase A, Roth DA, et al. Effect of belimumab treatment on renal outcomes: results from the phase 3 belimumab clinical trials in patients with SLE. Lupus 2013;22(1):63-72. [DOI] [PubMed] [Google Scholar]
Doria 2018 {published data only}
- Doria A, Bass D, Schwarting A, Hammer A, Gordon D, Scheinberg M, et al. A 6-month open-label extension study of the safety and efficacy of subcutaneous belimumab in patients with systemic lupus erythematosus. Lupus 2018;27(9):1489-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Furie 2018 {published data only}
- Furie RA, Wallace DJ, Aranow C, Fettiplace J, Wilson B, Mistry P, et al. Long-term safety and efficacy of belimumab in patients with systemic lupus erythematosus: a continuation of a seventy-six-week phase III parent study in the United States. Arthritis and Rheumatology 2018;70(6):868-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gamble 2012 {published data only}
- Gamble RG, Dellavalle RP. A randomized controlled trial of belimumab for the treatment of active systemic lupus erythematosus. Archives of Dermatology 2012;148(3):376-8. [DOI] [PubMed] [Google Scholar]
Jacobi 2010 {published data only}
- Jacobi AM, Huang W, Wang T, Freimuth W, Sanz I, Furie R, et al. Effect of long-term belimumab treatment on B cells in systemic lupus erythematosus: extension of a phase II, double-blind, placebo-controlled, dose-ranging study. Arthritis and Rheumatism 2010;62(1):201-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Manzi 2011 {published data only}
- Manzi S, Gladman D, Navarra S, Sanchez-Guerrero J, D'Cruz D, Freimuth W, et al. Post Hoc British Isles Lupus Assessment Group Index mucocutaneous organ domain item analysis of systemic lupus erythematosus patients treated in phase 3 belimumab clinical trials.. Arthritis and Rheumatism 2011;63 (10 Suppl):S231. [Google Scholar]
Manzi 2012 {published data only}
- Manzi S, Sanchez-Guerrero J, Merrill JT, Furie R, Gladman D, Navarra SV, et al. Effects of belimumab, a B lymphocyte stimulator-specific inhibitor, on disease activity across multiple organ domains in patients with systemic lupus erythematosus: combined results from two phase III trials. Annals of the Rheumatic Diseases 2012;71(11):1833-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Merrill 2012 {published data only}
- Merrill JT, Ginzler EM, Wallace DJ, McKay JD, Lisse JR, Aranow C, et al. Long-term safety profile of belimumab plus standard therapy in patients with systemic lupus erythematosus. Arthritis and Rheumatism 2012;64(10):3364-73. [DOI] [PubMed] [Google Scholar]
Onno‐Teng 2019 {published data only}
- Teng YK, Bruce IN, Diamond B, Furie RA, Vollenhoven RF, Gordon D, et al. Phase III, multicentre, randomised, double-blind, placebo-controlled, 104-week study of subcutaneous belimumab administered in combination with rituximab in adults with systemic lupus erythematosus (SLE): BLISS-BELIEVE study protocol. BMJ Open 2019;9(3):e025687. [DOI] [PMC free article] [PubMed] [Google Scholar]
Petri 2013 {published data only}
- Petri MA, Van Vollenhoven RF, Buyon J, Levy RA, Navarra SV, Cervera R, et al. Baseline predictors of systemic lupus erythematosus flares: data from the combined placebo groups in the phase III belimumab trials. Arthritis and Rheumatism 2013;65(8):2143-53. [DOI] [PubMed] [Google Scholar]
Rademacher 2018 {published data only}
- Rademacher JG, Schrempf LE, Pluss M, Muller GA, Korsten P. Therapeutic efficacy of belimumab in addition to standard therapy for lupus nephritis and neuropsychiatric lupus-case series of routinely collected data at a single centre. Lupus Science and Medicine 2018;5(Supplement 1):A119-20. [Google Scholar]
Stohl 2012 {published data only}
- Stohl W, Hiepe F, Latinis KM, Thomas M, Scheinberg MA, Clarke A, et al. Belimumab reduces autoantibodies, normalizes low complement levels, and reduces select B cell populations in patients with systemic lupus erythematosus. Arthritis and Rheumatism 2012;64(7):2328-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Strand 2014 {published data only}
- Strand V, Levy RA, Cervera R, Petri MA, Birch H, Freimuth WW, et al. Improvements in health-related quality of life with belimumab, a B-lymphocyte stimulator-specific inhibitor, in patients with autoantibody-positive systemic lupus erythematosus from the randomised controlled BLISS trials. Annals of the Rheumatic Diseases May 2014;73(5):838-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Strand 2019 {published data only}
- Strand V, Berry P, Lin X, Asukai Y, Punwaney R, Ramachandran S. Long-term impact of belimumab on health-related quality of life and fatigue in patients with systemic lupus erythematosus: six years of treatment. Arthritis Care and Research 2019;71(6):829-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Vollenhoven 2012 {published data only}
- Vollenhoven RF, Petri MA, Cervera R, Roth DA, Ji BN, Kleoudis CS, et al. Belimumab in the treatment of systemic lupus erythematosus: high disease activity predictors of response. Annals of the Rheumatic Diseases 2012;71(8):1343-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Vollenhoven 2019 {published data only}
- Vollenhoven RF, Navarra SV, Levy RA, Thomas M, Heath A, Lustine T, et al. Long-term safety and limited organ damage in patients with systemic lupus erythematosus treated with belimumab: a Phase III study extension. Rheumatology 2019;59(2):281-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
von Kempis 2019 {published data only}
- Kempis J, Duetsch S, Reuschling N, Villiger R, Villiger PM, Vallelian F, et al. Clinical outcomes in patients with systemic lupus erythematosus treated with belimumab in clinical practice settings: a retrospective analysis of results from the OBSErve study in Switzerland. Swiss Medical Weekly 2019;149:w20022. [DOI] [PubMed] [Google Scholar]
Wallace 2013 {published data only}
- Wallace DJ, Navarra S, Petri MA, Gallacher A, Thomas M, Furie R, et al. Safety profile of belimumab: pooled data from placebo-controlled phase 2 and 3 studies in patients with systemic lupus erythematosus. Lupus 2013;22(2):144-54. [DOI] [PubMed] [Google Scholar]
Wallace 2019 {published data only}
- Wallace DJ, Ginzler EM, Merrill JT, Furie RA, Stohl W, Chatham WW, et al. Safety and efficacy of belimumab plus standard therapy for up to thirteen years in patients with systemic lupus erythematosus. Arthritis and Rheumatology 2019;71(7):1125-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
D'Cruz 2019 {published data only}
- D'Cruz D, Maksimowicz-McKinnon K, Oates J, Santiago MB, Bass D, Burriss S, et al. Efficacy and safety of belimumab in patients of black race with systemic lupus erythematosus: Results from the embrace study. Lupus Science and Medicine 2019;6(Supplement 1):A149-50. [Google Scholar]
Tanaka 2017 {published data only}
- Tanaka Y, Bass D, Chu M, Egginton S, Ji B, Pobiner B, et al. Effects of belimumab on corticosteroid use in a pivotal phase III, randomised, placebo controlled study in subjects with systemic lupus erythematosus in North East Asia. Lupus Science and Medicine 2017;4(Supplement 1):A45-6. [Google Scholar]
Additional references
Blair 2018
- Blair HA, Duggan ST. Belimumab: a review in systemic lupus erythematosus. Drugs 2018;78(3):355-66. [DOI] [PubMed] [Google Scholar]
Bossen 2006
- Bossen C, Schneider P. BAFF, APRIL and their receptors: structure, function and signaling. Seminars in Immunology 2006;18(5):263-75. [DOI] [PubMed] [Google Scholar]
Boyce 2012
- Boyce EG, Fusco BE. Belimumab: review of use in systemic lupus erythematosus. Clinical Therapeutics 2012;34(5):1006-22. [DOI] [PubMed] [Google Scholar]
Burness 2011
- Burness CB, McCormack PL. Belimimab: in systemic lupus erythematosus. Drugs 2011;71(18):2435-44. [DOI] [PubMed] [Google Scholar]
Cates 2004
- Cates C. EBM Web Site. Visual Rx Version 3 (accessed October 2011) [Computer program]. Available from: www.nntonline.net/visualrx 2004.
Cheema 2001
- Cheema GS, Roschke V, Hilbert DM, Stohl W. Elevated serum B lymphocyte stimulator levels in patients with systemic immune-based rheumatic diseases. Arthritis and Rheumatism 2001;44:1313–9. [DOI] [PubMed] [Google Scholar]
Collins 2006
- Collins CE, Gavin AL, Migone T-S, Hilbert DM, Nemazee D, Stohl W. B lymphocyte stimulator (BLyS) isoforms in systemic lupus erythematosus: disease activity correlates better with blood leukocyte BLyS mRNA levels than with plasma BLyS protein levels. Arthritis Research and Therapy 2006;8:R6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Crow 2004
- Crow M, Kirou KA. Interferon-alpha in systemic lupus erythematosus. Current Opinion in Rheumatology 2004;16:541-7. [DOI] [PubMed] [Google Scholar]
Crowley 2005
- Crowley JE, Treml LS, Stadanlick JE, Carpenter E, Cancro MP. Homeostatic niche specification among naive and activated B cells: a growing role for the BLyS family of receptors and ligands. Seminars in Immunology 2005;17(3):193-9. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JP, Altman DG, editor(s). Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from: www.cochrane-handbook.org.
GRADEpro GDT [Computer program]
- GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), Accessed October 2019. Available at gradepro.org.
Hay 1993
- Hay EM, Bacon PA, Gordon C, Isenberg DA, Maddison P, Snaith ML, et al. The BILAG index: a reliable and valid instrument for measuring clinical disease activity in systemic lupus erythematosus. Quarterly Journal of Medicine 1993;86(7):447-58. [PubMed] [Google Scholar]
Higgins 2020
- Higgins JPT, Savović J, Page MJ, Elbers RG, Sterne JAC. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020.. Available from www.training.cochrane.org/handbook..
Hochberg 1997
Isenberg 2000
- Isenberg DA, Gordon C. From BILAG to BLIPS - disease activity assessment in lupus past, present and future. Lupus 2000;9(9):651-4. [DOI] [PubMed] [Google Scholar]
Kandala 2013
- Kandala NB, Connock M, Grove A, Sutcliffe P, Mohiuddin S, Hartley L, et al. Belimumab: a technological advance for systemic lupus erythematosus patients? Report of a systematic review and meta-analysis. BMJ Open 2013;3(7):1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2009
- Kim WU, Sreih A, Bucala R. Toll-like receptors in systemic lupus erythematosus prospects for therapeutic intervention. Autoimmunity Reviews 2009;8:204–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2012
- Kim SS, Kirou KA, Erkan D. Belimumab in systemic lupus erythematosus: an update for clinicians. Therapeutic Advances in Chronic Disease 2012;3(1):11-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2018
- Lee YH, Song GG. Comparative efficacy and safety of intravenous or subcutaneous belimumab in combination with standard therapy in patients with active systemic lupus erythematosus: a Bayesian network meta-analysis of randomized controlled trials. Lupus 2018;27(1):112-119. [DOI] [PubMed] [Google Scholar]
Litinskiy 2002
- Litinskiy MB, Nardelli B, Hilbert DM, He B, Schaffer A, Casali P, et al. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nature Immunology 2002;3:822–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Petri 1999
- Petri M, Buyon J, Kim M. Classification and definition of major flares in SLE clinical trials. Lupus 1999;8(8):685-91. [DOI] [PubMed] [Google Scholar]
Petri 2005
- Petri M, Kim MY, Kalunian KC, Grossman J, Hahn BH, Sammaritano LR, et al. Combined oral contraceptives in women with systemic lupus erythematosus. New England Journal of Medicine 2005;353(24):2550-8. [DOI] [PubMed] [Google Scholar]
Petri 2008
- Petri M, Stohl W, Chatham W, McCune WJ, Chevrier M, Ryel J, et al. Association of plasma B-lymphocyte stimulator (BLyS) levels and disease activity in systemic lupus erythematosus. Arthritis and Rheumatism 2008;58:2453–9. [DOI] [PubMed] [Google Scholar]
Ravirajan 1996
- Ravirajan CT, Sarraf CE, Anilkumar TV, Golding MC, Alison MR, Isenberg D. An analysis of apoptosis in lymphoid organs and lupus disease in murine systemic lupus erythematosus (SLE). Clinical and Experimental Immunology 1996;105:306–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schunemann 2011a
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and ‘Summary of findings' tables. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Schunemann 2011b
- Schünemann HJ, Oxman AD, Vist GE, Higgins JP, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
References to other published versions of this review
Singh 2013
- Singh JA, Noorbaloochi S, Tucker MD. Belimumab for systemic lupus erythematosus.. Cochrane Database of Systematic Reviews 2013, Issue 7. Art. No: CD010668. [DOI: 10.1002/14651858.CD010668] [DOI] [PMC free article] [PubMed] [Google Scholar]


