Abstract
Background
In vitro fertilisation (IVF) or intracytoplasmic sperm injection (ICSI) treatments conventionally consist of a fresh embryo transfer, possibly followed by one or more cryopreserved embryo transfers in subsequent cycles. An alternative option is to freeze all suitable embryos and transfer cryopreserved embryos in subsequent cycles only, which is known as the 'freeze all' strategy. This is the first update of the Cochrane Review on this comparison.
Objectives
To evaluate the effectiveness and safety of the freeze all strategy compared to the conventional IVF/ICSI strategy in women undergoing assisted reproductive technology.
Search methods
We searched the Cochrane Gynaecology and Fertility Group Trials Register, CENTRAL, MEDLINE, Embase, PsycINFO, CINAHL, and two registers of ongoing trials from inception until 23 September 2020 for relevant studies, checked references of publications found, and contacted study authors to obtain additional data.
Selection criteria
Two review authors (TZ and MZ) independently selected studies for inclusion, assessed risk of bias, and extracted study data. We included randomised controlled trials comparing a 'freeze all' strategy with a conventional IVF/ICSI strategy including a fresh embryo transfer in women undergoing IVF or ICSI treatment.
Data collection and analysis
The primary outcomes were cumulative live birth rate and ovarian hyperstimulation syndrome (OHSS). Secondary outcomes included effectiveness outcomes (including ongoing pregnancy rate and clinical pregnancy rate), time to pregnancy and obstetric, perinatal and neonatal outcomes.
Main results
We included 15 studies in the systematic review and eight studies with a total of 4712 women in the meta‐analysis. The overall evidence was of moderate to low quality. We graded all the outcomes and downgraded due to serious risk of bias, serious imprecision and serious unexplained heterogeneity. Risk of bias was associated with unclear blinding of investigators for preliminary outcomes of the study during the interim analysis, unit of analysis error, and absence of adequate study termination rules. There was an absence of high‐quality evidence according to GRADE assessments for our primary outcomes, which is reflected in the cautious language below.
There is probably little or no difference in cumulative live birth rate between the 'freeze all' strategy and the conventional IVF/ICSI strategy (odds ratio (OR) 1.08, 95% CI 0.95 to 1.22; I2 = 0%; 8 RCTs, 4712 women; moderate‐quality evidence). This suggests that for a cumulative live birth rate of 58% following the conventional strategy, the cumulative live birth rate following the 'freeze all' strategy would be between 57% and 63%.
Women might develop less OHSS after the 'freeze all' strategy compared to the conventional IVF/ICSI strategy (OR 0.26, 95% CI 0.17 to 0.39; I2 = 0%; 6 RCTs, 4478 women; low‐quality evidence). These data suggest that for an OHSS rate of 3% following the conventional strategy, the rate following the 'freeze all' strategy would be 1%.
There is probably little or no difference between the two strategies in the cumulative ongoing pregnancy rate (OR 0.95, 95% CI 0.75 to 1.19; I2 = 31%; 4 RCTs, 1245 women; moderate‐quality evidence).
We could not analyse time to pregnancy; by design, time to pregnancy is shorter in the conventional strategy than in the 'freeze all' strategy when the cumulative live birth rate is comparable, as embryo transfer is delayed in a 'freeze all' strategy. We are uncertain whether the two strategies differ in cumulative miscarriage rate because the evidence is very low quality (Peto OR 1.06, 95% CI 0.72 to 1.55; I2 = 55%; 2 RCTs, 986 women; very low‐quality evidence) and cumulative multiple‐pregnancy rate (Peto OR 0.88, 95% CI 0.61 to 1.25; I2 = 63%; 2 RCTs, 986 women; very low‐quality evidence). The risk of hypertensive disorders of pregnancy (Peto OR 2.15, 95% CI 1.42 to 3.25; I2 = 29%; 3 RCTs, 3940 women; low‐quality evidence), having a large‐for‐gestational‐age baby (Peto OR 1.96, 95% CI 1.51 to 2.55; I2 = 0%; 3 RCTs, 3940 women; low‐quality evidence) and a higher birth weight of the children born (mean difference (MD) 127 g, 95% CI 77.1 to 177.8; I2 = 0%; 5 RCTs, 1607 singletons; moderate‐quality evidence) may be increased following the 'freeze all' strategy. We are uncertain whether the two strategies differ in the risk of having a small‐for‐gestational‐age baby because the evidence is low quality (Peto OR 0.82, 95% CI 0.65 to 1.05; I2 = 64%; 3 RCTs, 3940 women; low‐quality evidence).
Authors' conclusions
We found moderate‐quality evidence showing that one strategy is probably not superior to the other in terms of cumulative live birth rate and ongoing pregnancy rate. The risk of OHSS may be decreased in the 'freeze all' strategy. Based on the results of the included studies, we could not analyse time to pregnancy. It is likely to be shorter using a conventional IVF/ICSI strategy with fresh embryo transfer in the case of similar cumulative live birth rate, as embryo transfer is delayed in a 'freeze all' strategy. The risk of maternal hypertensive disorders of pregnancy, of having a large‐for‐gestational‐age baby and a higher birth weight of the children born may be increased following the 'freeze all' strategy. We are uncertain if 'freeze all' strategy reduces the risk of miscarriage, multiple pregnancy rate or having a small‐for‐gestational‐age baby compared to conventional IVF/ICSI.
Plain language summary
Fresh versus frozen embryo transfers for assisted reproduction
Review question
Is a freeze‐all strategy in IVF and ICSI treatments safe and effective in comparison to conventional IVF and ICSI treatment?
Background
Conventionally, in vitro fertilisation (IVF) or intracytoplasmic sperm injection (ICSI) treatments consist of a fresh embryo transfer directly after ovarian hyperstimulation, which is used in order to retrieve oocytes in the IVF/ICSI procedure. In the conventional IVF/ICSI treatment fresh embryo transfer is possibly followed by one or more frozen embryo transfers in subsequent cycles when enough embryos are available. Alternatively, one can opt to 'freeze all' suitable embryos, and transfer frozen embryos in subsequent cycles only, which is also known as the 'freeze all' strategy. In the "freeze all" strategy all embryos are frozen to be transferred at later time point when the ovaries are not stimulated. Therefore, this method could reduce the risk of ovarian hyperstimulation syndrome (OHSS, an overreaction to fertility drugs) as OHSS is more severe when pregnancy occurs. Furthermore, studies have suggested that a woman's hormonal response to fertility drugs could affect the lining of the womb making it difficult for an embryo to implant. Thus, it could be beneficial to freeze the embryos and transfer them later when the lining of the womb is not affected by fertility drugs.
In the past decade, an increasing number of clinics have applied the 'freeze all' strategy as a standard treatment strategy in their practice. In practice, the 'freeze all' strategy and the conventional strategy can vary technically.
We compared the effectiveness and safety of these treatment strategies in women undergoing assisted reproductive technology.
Study characteristics
We examined all research published in the scientific literature up to 23 September 2020.
We included 15 randomised controlled trials (experiments where each person has an equal chance of being chosen to receive the treatment or a comparator) in the review. We were able to combine and analyse the results of eight trials, with a total of 4712 women.
Key results
There is probably little or no difference in cumulative live birth rate and ongoing pregnancy rate between the 'freeze all' strategy and the conventional IVF/ICSI strategy. Our findings suggest that if the cumulative live birth rate is 58% following a conventional IVF/ICSI strategy, the rate following a 'freeze all' strategy would be between 57% and 63%. Not performing a fresh transfer, as is done in a freeze‐all strategy, might lower the OHSS risk for women at risk of OHSS. Our findings suggest that if the OHSS rate is 3% following a conventional IVF/ICSI strategy, the rate following a 'freeze all' strategy would be 1%. We are uncertain whether the 'freeze all' strategy has any effect on the risk of miscarriage, multiple pregnancy rate, and time to pregnancy compared to conventional IVF/ICSI.
We also evaluated differences in risks for mother and child. The 'freeze all' strategy may increase the risk of hypertensive disorders of pregnancy, the risk of having a large‐for‐gestational‐age baby, and may result in a higher birth weight of the children born. Caution is needed in drawing conclusions from this as the analysis is based on very low number of events.
Quality of the evidence
The evidence was of moderate quality for cumulative live birth rate and low quality for safety outcomes. The low quality was generally due to serious imprecision in view of the relatively few events, serious unexplained heterogeneity, meaning that the results across trials varied widely, and due to risk of bias within the included trials.
Summary of findings
Background
Description of the condition
Subfertility is defined as the failure to conceive after 12 months of regular unprotected intercourse (Van Voorhis 2007; Zegers‐Hochschild 2017). One in six couples experience subfertility at least once during their reproductive lifetime, and approximately 10% of couples worldwide are subfertile (CDC 2016; ESHRE 2018). Common causes of infertility include poor semen quality, obstruction of the fallopian tubes or absence of ovulation (Hull 1985). Poor semen quality can manifest itself as low sperm concentration, as low as no sperm (azoospermia), low motility, or low numbers of sperm with normal morphology. Fallopian tubes can be blocked or damaged by infection, for example, chlamydia, or there can be adhesions of the tubes or ovaries caused by surgery, infection or endometriosis. Couples who fail to conceive naturally are diagnosed as having unexplained infertility if no cause can be found after standard fertility tests. Numbers of couples with unexplained fertility have increased recently, which may be due to an increase in older women undergoing ART. Also genetic and environmental factors might play a role in unexplained infertility.
Description of the intervention
Assisted reproductive technology (ART) has rapidly evolved as an intervention to improve pregnancy rates. It is now estimated that more than 8 million babies have been born worldwide with assisted reproduction since the first ART baby was born in 1978 (ESHRE 2018). ART involves the handling of gametes and embryos outside the human body and consists of in vitro fertilisation (IVF) with or without intracytoplasmic sperm injection (ICSI). After fertilisation, fresh transfer of the morphologically best embryo(s) into the uterine cavity is performed. Embryos suitable for transfer, but not transferred fresh, are cryopreserved for future use. If a woman does not get pregnant after the fresh transfer or has a wish for a second child the frozen embryo(s) can be thawed and transferred.
In some cases, pregnancy after a fresh embryo transfer can lead to ovarian hyperstimulation syndrome (OHSS). OHSS is characterised by a fluid shift from blood vessels to the abdominal cavity, resulting in, for example, abdominal bloating, high risk of clots within the blood vessels (thrombosis) and decreased blood supply to important organs such as kidneys and liver. Severe OHSS is potentially life‐threatening and can lead to lethal complications. The development of OHSS is mainly an iatrogenic side effect of the high doses of gonadotropin used for ovarian stimulation, resulting in multi‐follicular growth. Multiple follicles will in their turn produce vascular endothelial growth factor, which induces hyperpermeability of ovarian blood vessels, leading to a fluid shift from the intravascular to the third space. The administration of human chorionic gonadotrophin (hCG) can trigger OHSS. Moreover, the extra hCG rise accompanying (multiple) pregnancy after a fresh embryo transfer can aggravate already existing OHSS or induce late‐onset OHSS (Mourad 2017; Youssef 2016).
In order to reduce OHSS by avoiding fresh embryo transfer, in 2011 Devroey and colleagues promoted the option to 'freeze all' suitable embryos after IVF/ICSI treatment, and transfer cryopreserved embryos in subsequent cycles only, which is also known as the 'freeze all' strategy (Devroey 2011).
Recent technical improvements in cryopreservation have led to increased chances of embryo survival after thawing and subsequently increased pregnancy rates per cryopreserved embryo transfer (CDC 2016; ESHRE 2018; Wong 2014). In fact, pregnancy rates after cryopreserved embryo transfer are now almost equal to pregnancy rates after fresh transfer when calculated per transfer. This has fuelled the idea that the 'freeze all' strategy might increase the cumulative live birth rate. Therefore, the 'freeze all' strategy has become a strategy that possibly increases effectiveness in IVF/ICSI treatment, with safety not its only objective (Devroey 2011; Griesinger 2011; Maheshwari 2013; Mastenbroek 2011; Roque 2019).
How the intervention might work
In contrast to the conventional strategy, in a 'freeze all' strategy there are no fresh embryo transfers in the cycle with ovarian stimulation with exogenous gonadotropins, but only cryopreserved embryo transfers in subsequent cycles without ovarian stimulation. This avoids possible adverse effects of ovarian stimulation on the endometrial environment.
During ovarian stimulation for IVF/ICSI, the development of multiple follicles leads to elevated oestradiol (Kosmas 2004), and progesterone levels (Venetis 2013; Venetis 2015). This endocrine milieu may reduce endometrial receptivity for the implanting embryo (Bourgain 2003; Kolibianakis 2002; Roque 2017; Fatemi 2015; Venetis 2013; Venetis 2016). Studies on the molecular level comparing stimulated with unstimulated endometrium samples have shown distinct gene‐expression profiles between the two conditions (Haouzi 2009; Van Vaerenbergh 2009; Fatemi 2015). Transfer of cryopreserved embryos only would thus circumvent a possible negative effect of gonadotropins on the endometrium in the cycle with ovarian stimulation, and consequently increase live birth rates, the main outcome of interest to subfertile couples.
Ovarian stimulation with exogenous gonadotropins in IVF increases the risk of OHSS when a pregnancy occurs right after the ovarian stimulation. Avoiding a pregnancy in the cycle with ovarian stimulation by only transferring cryopreserved embryos in subsequent unstimulated cycles would eliminate or significantly reduce the risks of OHSS.
In order to evaluate the efficacy of the 'freeze all' strategy, we have to compare the 'freeze all' cumulative live birth rate with the conventional IVF/ICSI strategy cumulative live birth rate. Currently, some studies primarily compare live birth rate after first transfer. This possibly shows differences in outcome for a stimulated versus unstimulated uterus, although this does not take the number of embryos that were thawed for transfer into account. For women, the live birth rate per first transfer is less relevant, since at the same time of first transfer in a 'freeze all' strategy, they would already have received the second transfer in a conventional IVF/ICSI strategy. Considering the important perspective of time, it would only be fair to compare cumulative live birth rate between groups instead of live birth rate after first transfer (Zaat 2019).
Why it is important to do this review
An increasing number of clinics apply the 'freeze all' strategy as a standard treatment strategy in their practice (Pereira 2016; Pereira 2019). Data from the Centers for Disease Control and Prevention (CDC) in the USA indicate that there has been a very steep rise of the 'freeze all' strategy from almost none of the cycles in 2007 to 25% of all IVF/ICSI cycles in 2016 (CDC 2016). Data from the European Society of Human Reproduction and Embryology (ESHRE) from the European IVF‐monitoring Consortium, presented provisionally for 2015 at last year's Annual Meeting, also revealed strong growth in the number of 'freeze all' cycles (up 7% on the previous year), and accounting for 15% of all IVF cycles in 2015 (Focus on Reproduction 2019). The Human Fertilisation & Embryology Authority (HFEA) of the UK states that 'freeze all' cycles have increased by 39% since 2014 (Focus on Reproduction 2019).
However, despite its increasing use, the relative effectiveness and safety of IVF treatment with the 'freeze all' strategy compared to the conventional IVF/ICSI strategy is unclear. It is important to do this review in order to evaluate the effectiveness and safety of the 'freeze all' strategy compared to the conventional IVF/ICSI strategy including fresh embryo transfer in women undergoing ART.
The previous version of this Cochrane review included data from 1892 women comparing a 'freeze all' strategy with a conventional IVF/ICSI strategy. Concerning effectiveness, moderate‐quality evidence showed that one strategy is not superior to the other in terms of cumulative live birth rate. With respect to safety, low‐quality evidence suggested that not performing a fresh transfer lowers the OHSS risk for women at risk of OHSS (Wong 2017).
Objectives
To evaluate the effectiveness and safety of the 'freeze all' strategy compared to the conventional IVF/ICSI strategy in women undergoing assisted reproductive technology.
Methods
Criteria for considering studies for this review
Types of studies
We included published RCTs comparing the 'freeze all' strategy with the conventional IVF/ICSI strategy with fresh embryo transfer regardless of the context of the evaluation (OHSS or susceptibility of the endometrium). We excluded quasi‐ and pseudo‐randomised controlled trials. We excluded trials published only as abstracts. We planned to include cross‐over trials for completeness, but would only pool the data from the first phase in the meta‐analysis (Vail 2003).
Types of participants
All women undergoing IVF or ICSI irrespectively of the reason for 'freeze all', the infertility factor, age, ethnicity, number of previous IVF cycles, type of stimulation protocol and type of embryo transfer protocol.
Types of interventions
Trials comparing the 'freeze all' strategy with transfer of cryopreserved embryos only versus the conventional IVF/ICSI strategy with transfer of fresh and subsequent cryopreserved embryos until a live birth occurred or until all embryos from the initial cycle were transferred.
Types of outcome measures
Primary outcomes
Effectiveness: cumulative live birth rate per randomised woman. That is, the rate of live birth following the transfer of all (fresh or cryopreserved) embryos within the time horizon of the follow‐up defined by the authors of the original study
Safety: OHSS per randomised woman
Secondary outcomes
Cumulative ongoing pregnancy rate, defined as the number of ongoing pregnancies per woman randomised (demonstrated by the presence of a gestational sac with fetal heartbeat on ultrasound at 10 to 12 weeks of gestation)
Cumulative clinical pregnancy rate, defined as the cumulative number of clinical pregnancies per woman randomised (demonstrated by a pregnancy confirmed by ultrasonographic visualisation of one or more gestational sacs)
Time to pregnancy, defined as the time between randomisation and ongoing pregnancy
-
Pregnancy outcomes and obstetric, perinatal and neonatal outcomes per woman.
Ectopic pregnancy, defined as a pregnancy in which implantation takes place outside the uterine cavity
Miscarriage rate, defined as the spontaneous demise of a pregnancy before the fetus reaches viability. The term therefore includes all pregnancy losses from the time of conception until 24 weeks of gestation
Multiple pregnancy rate, defined as presence of more than one sac at early pregnancy ultrasound six to eight weeks' gestation
Gestational diabetes mellitus
Hypertensive disorders of pregnancy, comprising pregnancy‐induced hypertension (PIH), pre‐eclampsia (PE) and haemolysis, elevated liver enzymes, and low platelets in the blood (HELLP syndrome)
Preterm delivery, defined as delivery more than 24 and less than 37 weeks of gestational age
Perinatal and neonatal death, defined as stillbirths and the death of a newborn within 28 days after delivery
Neonatal hospitalisation, defined as admission for longer than three days or admission to the neonatal intensive care unit (NICU)
Large for gestational age, defined as birth weight above 90th percentile
Small for gestational age, defined as birth weight below 10th percentile
Congenital abnormalities per live‐born children, defined as the number of congenital abnormalities at birth per live‐born children plus number of foetuses therapeutically terminated
Birth weight of babies born, per baby
We also provide multiple pregnancy rate and miscarriage rate per clinical pregnancy.
As an additional analysis we calculated the live birth rate per woman after first embryo transfer only.
Search methods for identification of studies
We searched for all published randomised controlled trials on the 'freeze all' strategy, without language or date restriction and in consultation with the Cochrane Gynaecology and Fertility (CGF) Information Specialist.
Electronic searches
We searched the following electronic databases, trials registers, and websites from their inception to 23 September 2020 in consultation with the CGF Information Specialist:
Cochrane Gynaecology and Fertility Group Specialised Register, Procite platform (searched 23 September 2020; Appendix 1);
CENTRAL via the Cochrane Central Register of Studies Online (CRSO), Web platform (searched 23 September 2020; Appendix 2);
MEDLINE, Ovid platform (searched 1946 to 23 September 2020; Appendix 3);
Embase, Ovid platform (searched 1980 to 23 September 2020; Appendix 4);
PsycINFO, Ovid platform (searched 1806 to 23 September 2020; Appendix 5);
CINAHL, Ebsco platform (searched 1961 to 23 September 2020; Appendix 6).
Other electronic sources of trials included:
-
trials registers for ongoing and registered trials:
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (clinicaltrials.gov/ct2/home; searched 23 September 2020; Appendix 7);
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (www.who.int/trialsearch/Default.aspx; searched 23 September 2020; Appendix 8);
DARE (Database of Abstracts of Reviews of Effects) in the Cochrane Library for reference lists from relevant non‐Cochrane reviews (onlinelibrary.wiley.com/o/cochrane/cochrane_cldare_articles_fs.html);
PubMed (www.ncbi.nlm.nih.gov/pubmed/).
Searching other resources
We examined the reference lists of eligible articles and contacted study authors where necessary to obtain additional relevant data and handsearched relevant journals and conference abstracts that were not covered in the CGF Register.
Data collection and analysis
Selection of studies
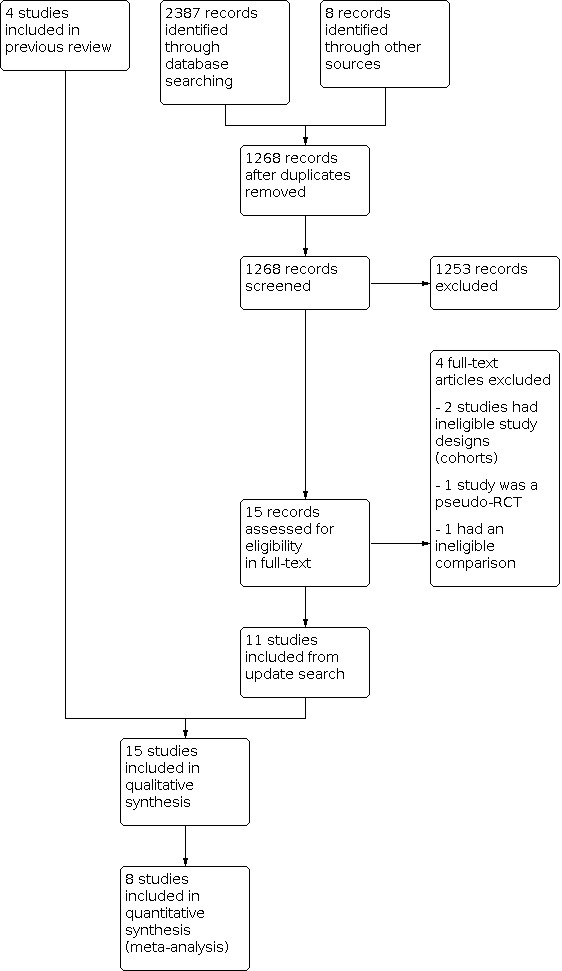
Two review authors (TZ and MZ) screened the titles and abstracts retrieved by the search and retrieved the full texts of all potentially eligible studies using Covidence. We independently examined these full‐text articles for compliance with the inclusion criteria and selected studies eligible for inclusion in the review. We corresponded with study investigators as required to clarify study eligibility. We resolved any disagreements about study eligibility by discussion or by consulting a third review author (SM). We documented the selection process with a PRISMA flow chart (Moher 2009; Figure 1).
1.
Data extraction and management
Two review authors (TZ and MZ) independently extracted data from the eligible studies using a data extraction form designed and pilot‐tested by the review authors. We resolved any discrepancies by discussion. The data extraction forms included methodological quality and allocation information. We included this information in the review and presented it in the Characteristics of included studies and Characteristics of excluded studies tables.
We corresponded with study investigators to request further data on methods or results, or both, as required. Whenever we did not receive a response within six weeks we sent a reminder email to the study authors.
Assessment of risk of bias in included studies
Two review authors (TZ and MZ) independently assessed the included studies for risk of bias using the Cochrane 'Risk of bias' assessment tool for the following domains (Higgins 2011).
Sequence generation
We allocated a low risk of bias if the investigators described a random component in the sequence generation process, such as:
using a computerised random number generator;
using a random numbers table.
Allocation concealment
We allocated a low risk of bias if the participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation:
central computer randomisation;
serially numbered, sealed, opaque envelopes.
Blinding
In this study design it was not possible to blind participants and clinicians and therefore the risk of performance bias will be low.
Completeness of outcome data
We allocated a low risk of bias if there were no missing data, which meant live birth rate and length of follow‐up were stated, loss to follow‐up was accounted for, and an intention‐to‐treat analysis had been carried out.
Selective outcome reporting
We allocated a low risk of bias if all of the study's primary, secondary, and additional outcomes that were of interest in the review had been reported in a prespecified way.
Other sources of bias
We allocated a low risk of bias if the study:
was free of commercial funding;
had no other source of bias identified (e.g. imbalance in prognostic factors at baseline).
Two review authors (TZ and MZ) assessed these domains and resolved any disagreements by consensus or by consulting a third review author (SM). We described the judgements and presented the conclusions in the 'Risk of bias' figures. We took into account all judgements in the interpretation of review findings.
Measures of treatment effect
For dichotomous data (e.g. live birth rates), we used the numbers of events in the 'freeze all' strategy and in the conventional IVF/ICSI strategy group of each study to calculate Mantel‐Haenszel odds ratios (ORs) with 95% confidence intervals (CI). We used Peto ORs where the event was very rare (less than 1%) or in the case of zero cell counts. For continuous data (e.g. birth weight), we calculated mean difference (MD) between treatment groups provided that the same measure was used. We reversed the direction of effect of individual studies if required to ensure consistency across studies. We treated ordinal data as continuous data. Where data to calculate ORs or MDs were not available, we utilised the most detailed numerical data available that would facilitate similar analyses of included studies (e.g. test statistics, P values). We compared the magnitude and direction of effect reported by studies with how they were presented in the review, taking into account legitimate differences.
We planned to analyse the outcome 'time to pregnancy' using hazard ratios (HRs). However, data were insufficient to conduct these analyses. Should more data become available in the future, we will conduct HR analyses of time to pregnancy in later updates of this review.
Unit of analysis issues
We performed the analyses with data per woman randomised, apart from birth weight, which we analysed per baby. If data of the primary analysis were reported per embryo, per oocyte, per cycle, or per transfer, we contacted the authors of the studies for per‐woman data for completeness.
We counted reported multiple live births as one live birth event.
We planned to include only first‐phase data from cross‐over trials.
We also performed secondary analyses for multiple pregnancy, miscarriage, pregnancy complications, and birth weight per pregnancy since these conditions only occur in pregnant women.
Dealing with missing data
We analysed the data on an intention‐to‐treat basis. In case of missing data we contacted authors of studies to request more data.
We assumed that live births had not occurred in women without a reported outcome. If studies reported sufficient detail to calculate MDs, but provided no information on associated standard deviations (SD), we assumed that the outcome had a SD equal to the highest SD from other studies within the same analysis.
Assessment of heterogeneity
We considered heterogeneity when the clinical and methodological characteristics of the included studies were sufficiently similar for a meta‐analysis to provide a clinically meaningful summary. We performed statistical analyses in accordance with the guidelines developed by Cochrane (Deeks 2020). We assessed heterogeneity between the results of different studies by the I2 statistic (Higgins 2003), considering an I2 value greater than 50% to indicate substantial heterogeneity (Deeks 2020).
Assessment of reporting biases
We aimed to minimise the potential impact of publication and reporting biases by performing a comprehensive search for eligible studies and looking for duplication of data. We planned to perform a funnel plot to investigate the possibility of small‐study effects if we included 10 or more studies in an analysis.
If included studies reported neither the primary outcome measure of live birth nor interim outcomes such as clinical pregnancy, we undertook informal assessment as to whether studies reporting the primary outcome measures reflected typical findings for the interim outcomes. We considered within‐study reporting bias by looking at the protocols.
We addressed the assessment of reporting biases in the Risk of bias in included studies section of the Results.
Data synthesis
We used Review Manager 5 software to perform the meta‐analyses with a fixed‐effect model to calculate pooled ORs and 95% CIs (Review Manager 2020).
To aid interpretation, we translated findings for primary outcomes to absolute risks, expressed as percentages based on the 95% CIs. We combined results for continuous outcomes using MDs.
Prospectively, we planned to present the analyses as:
cumulative live birth rates for conventional IVF cycles and 'freeze all' cycles;
OHSS rate for conventional IVF cycles and 'freeze all' cycles;
cumulative rate for secondary outcomes for conventional IVF cycles and 'freeze all' cycles;
time to pregnancy.
We included an additional table (Table 3) with pregnancy and live birth rates for one IVF/ICSI cycle after the first cryopreserved embryo transfer in the 'freeze all' strategy versus one IVF/ICSI cycle after the first fresh embryo transfer in the conventional IVF/ICSI strategy. In the current literature it is usual to report on outcomes only after the first cryopreserved embryo transfer, but this comparison could easily result in the wrong conclusion (Zaat 2019).
1. Live birth rate after first transfer.
| Outcome | Number of studies | Number of participants | Analysis method | OR |
| Live birth ratea after first embryo transfer for all embryo stages of transfer | 13 | 7766 | Odds ratio (Mantel‐Haenszel, fixed‐effect, 95% confidence interval) | 1.17 (95% CI 1.06 to 1.28) |
aLive birth rate calculated after first transfer is added for illustrative purposes as this comparison is often reported in the literature. It possibly shows differences in outcome for a stimulated and an unstimulated uterus, although this does not take into account the number of embryos that were thawed for transfer. This outcome is less relevant for women undergoing treatment since at the same time of first transfer in a freeze‐all strategy, they would already have received the second transfer (as long as there was a sufficient number of embryos) in a conventional strategy that includes fresh transfer. Here, one could consider the result of the first embryo transfer in the frozen group against the combined outcomes of the fresh transfer and the first frozen‐thawed‐transfer in the fresh group. Therefore cumulative live birth rate is the relevant outcome for women.
For the calculated live birth rate after the first embryo transfer, we included the eight studies included for the primary outcome (Chen 2016; Ferraretti 1999; Santos‐Ribeiro 2020; Shapiro 2011a; Shapiro 2011b; Vuong 2018; Wei 2019; Wong 2021) and five additional RCTs: Aflatoonian 2018; Aghahosseini 2017; Coates 2017; Shi 2018; Stormlund 2020. We contacted the authors for additional information regarding cumulative data by email but we did not receive a response.
Aflatoonian 2018 compared live birth rate after the first embryo transfer between a freeze‐all strategy and a conventional strategy in high responders at risk for developing OHSS. Aghahosseini 2017 included infertile women who were candidates for an in vitro fertilisation (IVF) treatment with no further specification of age or type of responder. Coates 2017 excluded women with a suspected decreased ovarian reserve (based on serum follicle‐stimulating hormone and anti‐Müllerian hormone. Women in both groups underwent IVF with assisted hatching and pre‐implantation genetic screening. Shi 2018 compared live birth rate after the first embryo transfer between a freeze‐all strategy and a conventional strategy in young women with a regular menses and the reason for IVF procedure: tubal factor, male factor, or both. Stormlund 2020 compared live birth rate after the first embryo transfer between a freeze‐all strategy and a conventional strategy in young women with a regular menses and the reason for IVF procedure: male, tubal, uterine, or unexplained infertility.
Subgroup analysis and investigation of heterogeneity
We had planned to perform subanalyses on timing of cryopreservation (e.g. day of embryo development) and method of cryopreservation (e.g. slow freezing or vitrification). However, data were insufficient to conduct all planned subgroup analyses. Should more data become available in the future, we will conduct additional subgroup analyses in later updates of this review.
Sensitivity analysis
We conducted sensitivity analyses for the primary outcomes (cumulative live birth rate and OHSS). These analyses included consideration of whether the review conclusions would have differed if:
eligibility was restricted to studies without high risk of bias;
a random‐effects model had been adopted;
the summary effect measure was risk ratio rather than OR.
Summary of findings and assessment of the certainty of the evidence
We prepared a 'Summary of findings' table using GRADEpro GDT software and following Cochrane methods (Schünemann 2020). This table evaluates the overall quality of the body of evidence for the main review outcomes. Two review authors independently evaluated the overall quality of the evidence for the outcomes (live birth, OHSS, multiple pregnancy, miscarriage, pregnancy complications and time to pregnancy) using GRADE criteria (study limitations such as risk of bias, consistency of effect, imprecision, indirectness, and publication bias). We justified, documented, and took into account judgements about evidence quality (high, moderate, low, or very low) in the results for each outcome.
Results
Description of studies
Results of the search
Results of the previous search
Our searches on 14 November 2016 revealed 2401 reports, of which 785 were duplicates, leaving 1622 reports. After screening the title and abstract, we found 12 reports to be potentially eligible, and retrieved these reports in full text.
In the first version of our review (Wong 2017), we included four studies (Chen 2016; Ferraretti 1999; Shapiro 2011a; Shapiro 2011b). We excluded four studies: (Absalan 2013; Aflatoonian 2010; Boostanfar 2016; Yang 2015)). We classified one study as awaiting assessment because it did not clearly report the methods it used (Chandel 2016).
For the previous version of the review, we contacted the authors of three included studies reporting on the primary outcomes Ferraretti 1999; Shapiro 2011a; Shapiro 2011b and one excluded study, Absalan 2013, for missing data. We asked the study authors about these missing data and about bias (e.g. randomisation and blinding). One author did not reply to our request for information (Absalan 2013). The remaining authors very kindly responded to our request for additional information, and we were able to include these data in our analysis.
Results of the current search
Our searches on 23 September 2020 revealed 2395 reports, of which 1127 were duplicates, leaving 1268 reports. After screening the title and abstract, we found 13 reports to be potentially eligible, and retrieved these reports in full text.
We excluded three studies: one we considered not properly randomised (Magdi 2017); one randomised women to a different intervention that was not clear from the abstract (Simon 2020); and one was not the correct study design, which was not clear from the abstract screening (Beyer 2016).
Six studies were ongoing and awaiting data (ACTRN12612000422820; ACTRN12616000643471; ISRCTN61225414; NCT02133950; NCT02570386; NCT03349905).
We contacted the authors of five studies (Aflatoonian 2018; Aghahosseini 2017; Coates 2017; Shi 2018; Stormlund 2020), included in the additional analysis regarding live birth rate after first embryo transfer for cumulative live birth rate. None of the study authors responded to our request for this additional information.
We included 11 new randomised controlled trials (RCTs) in the update.
We reassessed Chandel 2016, which was awaiting assessment from the previous version of the review (Wong 2017). We have excluded this study because we did not consider it to be properly randomised.
The current review includes 15 studies in total: four studies from the first version of the review (Chen 2016; Ferraretti 1999; Shapiro 2011a; Shapiro 2011b), and 11 new studies (Aflatoonian 2018; Aghahosseini 2017; Coates 2017; Santos‐Ribeiro 2020; Shapiro 2016; Shi 2018; Stormlund 2020; Vuong 2018; Wei 2019; Wong 2021; Zhang 2018). We included eight studies in the meta‐analyses, which are the eight primary reports of the RCTs (Chen 2016; Ferraretti 1999; Santos‐Ribeiro 2020; Shapiro 2011a; Shapiro 2011b; Vuong 2018; Wei 2019; Wong 2021).
See the study flow diagram (Figure 1) and study tables (Characteristics of included studies; Characteristics of excluded studies; Characteristics of ongoing studies).
Included studies
Study design and setting
Of the 15 studies included in the systematic review, eight were the primary reports of the RCTs and had data on primary and secondary outcomes and therefore included in the meta‐analysis. Six of these eight were single‐centre studies, conducted in reproductive medical centres in Belgium, Italy, the Netherlands, USA and Vietnam (Ferraretti 1999; Santos‐Ribeiro 2020; Shapiro 2011a; Shapiro 2011b; Vuong 2018; Wong 2021), and two were multicentre studies conducted in reproductive medical centres throughout China (Chen 2016; Wei 2019).
We included two studies for secondary outcomes (Shapiro 2016; Zhang 2018). Both studies are follow‐up studies of included RCTs. Shapiro 2016 reports on the follow‐up data of Shapiro 2011a and Shapiro 2011b. Zhang 2018 reports on the follow‐up data of Chen 2016.
Thirteen studies supplied data for the additional analysis – live birth after a first embryo transplant (Aflatoonian 2018; Aghahosseini 2017; Chen 2016; Coates 2017; Ferraretti 1999; Santos‐Ribeiro 2020; Shapiro 2011a; Shapiro 2011b; Shi 2018; Stormlund 2020; Vuong 2018; Wei 2019; Wong 2021).
Participants
The eight studies reporting on the primary outcome enrolled a total of 4712 women, with 2342 women undergoing the 'freeze all' strategy and 2370 women undergoing the conventional IVF/ICSI strategy with fresh embryo transfer.
Ferraretti 1999 and Santos‐Ribeiro 2020 evaluated the 'freeze all' strategy in the context of prevention of OHSS. Shapiro 2011a; Shapiro 2011b; Vuong 2018; Wei 2019 and Wong 2021 evaluated the 'freeze all' strategy in the context of an offered approach to improve susceptibility of the endometrium. Chen 2016 evaluated the 'freeze all' strategy in the context of both an offered approach to improve susceptibility of the endometrium and prevention of OHSS.
The inclusion criteria of the two Shapiro studies were based on the number of antral follicles observed at baseline ultrasound examination: Shapiro 2011a included normal responders (8 to 15 antral follicles), and Shapiro 2011b included high responders (> 15 antral follicles). Ferraretti 1999 included women at risk of developing OHSS, based on level of estradiol (E2) and number of retrieved eggs (≥ 15 oocytes). Santos‐Ribeiro 2020 included women with an excessive response to ovarian stimulation (≥ 18 follicles measuring ≥ 11 mm on the day of the GnRH triggering). Chen 2016 included women with polycystic ovary syndrome. Vuong 2018 and Wei 2019 included women without polycystic ovary syndrome. Wong 2021 included women with any IVF indication, independent of the number of follicles or available embryos.
In all studies, the baseline characteristics were comparable between the two strategies.
The ages of the women included by Shapiro 2011a and Shapiro 2011b ranged from 18 to 41 years. The mean age for the women included in Ferraretti 1999 ranged from 31.4 to 31.6 years. Women in Chen 2016 were between the ages of 20 and 34 years. The mean age of women in Vuong 2018 was 32 years. In Wei 2019 the mean age of the participants was 28.8 years. The mean age for the women included in Santos‐Ribeiro 2020 ranged from 30.4 to 31.2 years. The mean age for the women included in Wong 2021 ranged from 35.1 to 35.2 years.
For details about the extra studies used for additional analysis (Aflatoonian 2018; Aghahosseini 2017; Coates 2017; Shi 2018; Stormlund 2020), see Characteristics of included studies.
Interventions
In Chen 2016, women received recombinant FSH at a daily dose of 112.5 IU for those weighing less than 60 kg and 150 IU for those weighing over 60 kg starting on day 2 or 3 of the menstrual cycle. This was adjusted following ovarian response. hCG could be added when considered appropriate. On the day of oocyte retrieval, women had to have more than 3 and fewer than 30 oocytes with a low risk of OHSS to be randomised. Intramuscular progesterone at a daily dose of 80 mg was administered for luteal‐phase support in the fresh‐transfer group. Embryos were cryopreserved at day 3 of development. Oral oestradiol valerate was used for endometrial preparation on day 2 or 3 of the second menstrual cycle after oocyte retrieval. Intramuscular progesterone (80 mg/day) was added when endometrial thickness reached 8 mm or more or at the physician’s discretion. On day 4 of progesterone administration, two day‐3 frozen embryos were thawed and transferred. Luteal‐phase support with oestradiol valerate and intramuscular progesterone for endometrium preparation continued until 10 weeks after conception.
Women in Ferraretti 1999 received a down‐regulation protocol with gonadotropin‐releasing hormone (GnRH) analogue (0.3 mg subcutaneous buserelin acetate (Suprefact) twice a day) and ovarian stimulation with urinary gonadotropin (4 ampoules of follicle‐stimulating hormone (FSH) on the first and second days of treatment, and 2 ampoules of FSH plus 2 ampoules of human menopausal gonadotropins on the third and fourth treatment days). This was followed by an adjusted dosage of gonadotropins according to the individual response measured by plasma concentration of E2 and follicular growth assessed by ultrasound (Ferraretti 1996). Human chorionic gonadotropin (hCG) was administered 34 to 36 hours before follicle aspiration followed by 20 g of intravenous albumin. Embryos were frozen at the pronuclear stage. All embryos were transferred at the early cleavage stage (day 3) in artificial cycles. The artificial cycle treatment included oral administration of oestradiol valerate, 2 mg daily for the first 5 days of the cycle; 4 mg/day from day 6 to day 10; 6 mg/day from day 11 to day 13; then 4 mg/day from day 14 onward. On day 15 of the cycle, 50 mg of progesterone in oil was administered daily, and on day 17 the dose was increased to 100 mg/day.
In women in Santos‐Ribeiro 2020, ovarian stimulation commenced after confirmation that the woman was not pregnant and that she had basal serum levels of estradiol (< 80 pg/mL) and progesterone (< 1.5 ng/mL). Treating physicians decided which exogenous gonadotropins should be used according to the woman’s profile and preference, including either recombinant FSH (Gonal‐FVR or Puregon) or highly purified urinary human menopausal gonadotropins (MenopurVR). All women included underwent exogenous ovarian stimulation using GnRH antagonist suppression from day 6 of stimulation onwards with daily injections of either ganirelix or cetrorelix. Final oocyte maturation was triggered with 0.2 mg triptorelin as soon as at least three follicles of larger than 17 mm were observed. A GnRH agonist was the preferred triggering agent for both groups in order to avoid the elevated risk of OHSS associated with hCG triggering in high responders. Oocyte retrieval was performed approximately 36 hours after the GnRH agonist administration. In the fresh transfer arm, following oocyte retrieval, intensified luteal phase support was provided with a single administration of 1500 IU of exogenous hCG approximately one hour after oocyte retrieval followed by 200 mg of vaginal micronized progesterone (UtrogestanVR) three times a day plus 2 mg of oral estradiol valerate (ProgynovaVR) twice daily. The embryo transfer was performed on day 3 or 5 of development with preference to the latter whenever at least four good‐quality embryos were available on day 3. The choice to transfer one or two embryos was decided by the clinician at consultation prior to commencing the ART treatment, mainly depending on the woman’s age and the number of embryos replaced in the previous treatment cycles, according to Belgian law. All remaining good‐quality embryos were vitrified. In the 'freeze all' arm, no fresh luteal phase support was provided following oocyte retrieval. Instead, all viable embryos were vitrified, preferably at blastocyst stage (day 5 or 6), according to the same, before‐mentioned threshold of good‐quality embryos available on day 3. Women started with exogenous hormone therapy for endometrial preparation in the next cycle. This therapy was initiated only after confirmation that the woman had basal serum levels of estradiol/progesterone and consisted of 2 mg of oral estradiol valerate (ProgynovaVR ) twice daily for seven days followed by three times a day for another six days. Endometrial development was assessed using ultrasound and if the endometrium was 7 mm or more, 200 mg of vaginal Utrogestan three times a day was added to the treatment scheme. The frozen embryo transfer was scheduled according to the developmental stage of the embryo.
In Shapiro 2011a and Shapiro 2011b, women received down‐regulation with a GnRH antagonist and a combination of recombinant FSH and highly purified urinary FSH. hCG was administered 34 to 36 hours prior to follicle aspiration. In those women with greater ovarian response, 4 mg leuprolide acetate was added concomitant to the hCG. Embryos were vitrified at the pronuclear stage. All embryos were transferred as blastocysts in artificial cycles. Women with fresh embryo transfers received 6.0 mg daily E2 and daily progesterone injections (100 mg), with progesterone supplementation beginning one to two days after follicle aspiration and E2 initiated as needed. Women with cryopreserved embryo transfers were down‐regulated with leuprolide acetate in a subsequent cycle and received oral 6.0 mg daily E2 and E2 patches as needed starting 10 to 14 days before thawing to achieve a target endometrial thickness of at least 8 mm. Daily progesterone injections (typically 100 mg) were started the day before thawing. In both groups, E2 and progesterone supplements were adjusted as needed to sustain serum levels of at least 200 pg/mL and 15 ng/mL, respectively, until increasing serum levels indicated placental production, at 9 to 10 weeks’ gestation.
Women in Vuong 2018 underwent ovarian hyperstimulation according to the protocol for the use of FSH and GnRH antagonists. The dose of recombinant FSH ranged from 150 to 300 IU per day, depending on the woman’s age, anti‐Müllerian hormone levels, and response to FSH in any prior IVF cycle. When the mean diameter of at least two leading follicles was 17 mm, 250 μg of recombinant hCG was administered, and oocyte retrieval was performed 36 hours later. Embryos were cryopreserved at day 3 of development. In the following cycle, the endometrium was prepared with the use of oral estradiol valerate at a dose of 8 mg per day, starting on the second or third day of the menstrual cycle. Endometrial thickness was monitored from day 6 onward, and vaginal progesterone at a dose of 800 mg per day was started when the endometrial thickness reached 8 mm or more. A maximum of two embryos of grade 1 or 2 were thawed on the day of embryo transfer, three days after the start of progesterone. Luteal‐phase support with oestradiol valerate and vaginal progesterone for endometrium preparation continued until seven weeks after conception. In Wei 2019 women were given GnRH antagonist (ganirelix) regimen for ovarian stimulation. Recombinant FSH (Puregon) was started on day 1 to 3 of the menstrual cycle. When at least two follicles were 18 mm or greater in mean diameter, hCG at a dose of 4000 IU to 10000 IU was administered to induce the final maturation of oocytes. Oocyte retrieval was done 34 hours to 36 hours after hCG injection. Luteal phase support was started from the day of oocyte retrieval with vaginal progesterone gel (Crinone) 90 mg per day and oral dydrogesterone (Duphaston) 10 mg twice daily. On day 3 of embryo culture, embryos were graded by morphological criteria. Women who had four or more high‐grade embryos were randomly assigned to the fresh or frozen blastocyst transfer group. In the 'freeze all' group, luteal phase support was stopped after randomisation. On day 3, embryos were removed from cleavage media and replaced in blastocyst media. All blastocysts were vitrified on day 5 or day 6 according to embryo development. At least 4 weeks later, the endometrium was prepared either with a natural cycle regimen or artificial cycle regimen, at the discretion of local investigators. For the natural ovulatory cycle regimen, ovulation was determined by ultrasound monitoring. Oral dydrogesterone (Duphaston) 10 mg three times daily was administered for luteal phase support after ovulation. A single cryopreserved blastocyst was transferred on the fifth day after ovulation. If pregnancy was achieved, luteal phase support was continued until 10 weeks’ gestation. For the artificial cycle regimen, oral oestradiol valerate (Progynova) at a dose of 4 mg to 8 mg daily was started on day 1 to 3 of the menstrual cycle. Vaginal progesterone gel (Crinone) 90 mg per day and oral dydrogesterone 10 mg twice daily were added when the endometrial thickness reached 7 mm or more. A single frozen‐thawed blastocyst was transferred on the fifth day after progesterone initiation. If pregnancy was achieved, oral oestradiol valerate was continued until eight weeks’ gestation, and vaginal progesterone gel and oral dydrogesterone were continued until 10 weeks’ gestation.
Women in Wong 2021 underwent pituitary downregulation with a long GnRH agonist protocol with or without oral contraceptive pill pre‐treatment. Ovarian stimulation was conducted with human menopausal gonadotrophin (Menopur) or recombinant FSH (Puregon or Gonal‐F) in women with polycystic ovary syndrome starting from the seventh day without oral contraceptive pill pre‐treatment. The starting dose depended on the antral follicle count. Ovarian stimulation was continued until three or more follicles with a diameter of 18 mm had developed. Ovulation was triggered with 5000 or 10,000 IU hCG (Pregnyl). A single embryo transfer was performed for women below 38 years of age and a double embryo transfer policy for women of 38 years of age and above, if two or more embryos were available. Embryos were cryopreserved on day 6 of culture. Women started with oral oestrogen supplementation of 6 mg daily on the first day of their first menstruation after the follicular aspiration. If the endometrium had reached 8 mm, women started vaginal progesterone of 600 mg daily and continued the oral oestrogen. At the seventh day of vaginal progesterone administration, the cryopreserved embryo transfer was performed. If pregnancy occurred, oestrogen and progesterone supplementation was continued until the eleventh week of gestation.
Outcomes
Data were extracted from study reports or provided by authors for the following outcomes.
Primary outcomes
Effectiveness: cumulative live birth rate per woman. Two studies did not report on live birth in their published article (Shapiro 2011a; Shapiro 2011b), but we were able to obtain these data by personal communication with the study authors. One study did not report on live birth rate after the first embryo transfer (Ferraretti 1999), but we were able to obtain these data by personal communication with the study authors. Chen 2016; Santos‐Ribeiro 2020; Vuong 2018; Wei 2019 and Wong 2021 reported these data. Five studies did not report on cumulative live birth rate but only on live birth rate after the first embryo transfer (Aflatoonian 2018; Aghahosseini 2017; Coates 2017; Shi 2018; Stormlund 2020). We contacted the authors for cumulative live birth rate but did not receive any response. We used these studies in the additional analysis on live birth rate per woman after the first embryo transfer.
Safety: OHSS. Two studies reported OHSS per woman if hospitalisation was required (Ferraretti 1999; Wong 2021). Two studies did not report on OHSS (Shapiro 2011a; Shapiro 2011b), but we were able to obtain these data by personal communication with the study authors. However, we did not include the data from these two studies in the analysis, as women with high risk of OHSS were excluded and received the 'freeze all' strategy as standard. Chen 2016; Santos‐Ribeiro 2020; Vuong 2018; Wei 2019 and Wong 2021 reported these data.
Secondary outcomes
Four studies reported ongoing pregnancy rate determined at 10 to 12 weeks of gestational age (Shapiro 2011a; Shapiro 2011b; Vuong 2018; Wong 2021).
Four studies reported cumulative clinical pregnancy rate (Ferraretti 1999; Santos‐Ribeiro 2020; Vuong 2018; Wong 2021).
Four of the studies reported time to pregnancy, each in a different way. Santos‐Ribeiro 2020 reported the mean time from randomisation to detection of clinical pregnancy after the first embryo transfer and the overall cumulative time to clinical pregnancy; Vuong 2018 reported on the median time to conception; Wei 2019 reported on the time to live birth; and Wong 2021 reported on the time to ongoing pregnancy.
-
The following studies reported obstetric, perinatal and neonatal complications per woman.
Two studies reported on cumulative ectopic pregnancy rate (Vuong 2018; Wong 2021). Five studies reported ectopic pregnancy after first embryo transfer (Chen 2016; Ferraretti 1999; Santos‐Ribeiro 2020; Vuong 2018; Wei 2019).
Two studies reported on cumulative miscarriage rate (Vuong 2018; Wong 2021). All eight studies reported the number of miscarriages after first embryo transfer (Chen 2016; Ferraretti 1999; Santos‐Ribeiro 2020; Shapiro 2011a; Shapiro 2011b; Vuong 2018; Wei 2019; Wong 2021).
Two studies reported on cumulative multiple‐pregnancy rate (Vuong 2018; Wong 2021). Five studies reported multiple‐pregnancy rate after first embryo transfer (Chen 2016; Shapiro 2011b; Vuong 2018; Wei 2019; Wong 2021).
One study reported on cumulative diabetes mellitus rate (Vuong 2018). Three studies reported on gestational diabetes mellitus after first embryo transfer (Vuong 2018; Wei 2019; Zhang 2018).
One study reported on cumulative rate of hypertensive disorders of pregnancy (Vuong 2018). Three studies reported on hypertensive disorders of pregnancy after first embryo transfer (Chen 2016; Vuong 2018; Wei 2019).
Two studies reported on cumulative rate of preterm delivery (Vuong 2018; Wong 2021). Three studies reported on preterm delivery after first embryo transfer (Chen 2016; Vuong 2018; Wei 2019).
Only one study reported on cumulative rate of perinatal and neonatal death (Vuong 2018). Two studies reported on perinatal and neonatal death after first embryo transfer (Chen 2016; Vuong 2018).
One study reported on cumulative rate of neonatal hospitalisation (Vuong 2018). Three studies reported on neonatal hospitalisation after first embryo transfer (Vuong 2018; Wei 2019; Zhang 2018).
One study reported on cumulative rate of large for gestational age (Vuong 2018). Three studies reported on the rate of babies who were large for gestational age after first embryo transfer (Vuong 2018; Wei 2019; Zhang 2018).
One study reported on cumulative rate of small for gestational age (Vuong 2018). Three studies reported on the rate of babies who were small for gestational age after first embryo transfer (Vuong 2018; Wei 2019; Zhang 2018).
None of the studies reported on the cumulative rate of babies born with congenital abnormalities. Three studies reported on the rate of babies born with congenital abnormalities after first embryo transfer (Chen 2016; Wei 2019; Wong 2021).
Five studies reported on the birth weight of babies born (Chen 2016; Shapiro 2016; Vuong 2018; Wei 2019; Wong 2021).
Excluded studies
We excluded eight potentially eligible studies from the review, for the following reasons.
Aflatoonian 2010: this study was retracted.
Absalan 2013: it was unclear whether this study was truly a RCT. This study compared the clinical and delivery rates between the 'freeze all' strategy and the conventional strategy in women at risk for OHSS. The abstract stated that women with OHSS were randomly divided into two groups, with fresh embryo transfer and with frozen transfer. However, the methods section does not mention the method of randomisation (sequence generation or allocation concealment) nor which method was used to divide women into the two groups. Nothing was reported on the occurrence of OHSS in these women. The study authors did not respond to our request for additional information.
Beyer 2016: this study was not the correct study design, which was not clear from the abstract screening.
Boostanfar 2016 randomised women to a different intervention that was not clear from the abstract.
Chandel 2016 randomised women but, based on results, switched women to the other randomisation group (pseudo‐RCT).
Magdi 2017 stated it was a prospective cohort study, however, randomly assigned women into two groups using a computer‐based Microsoft Excel spreadsheet. We did not consider the study to be a properly randomised RCT.
Simon 2020 randomised women to a different intervention that was not clear from the abstract.
Yang 2015: one‐third of all randomised women chose to be in group 3 (fresh transfer of a day‐3 embryo followed by cryopreserved embryos) after randomisation. We did not consider the study to be a properly randomised RCT.
Awaiting classification
Currently there are no studies awaiting classification.
Ongoing studies
We identified six ongoing studies from trials registers that may have results for inclusion in future versions of this review (ACTRN12612000422820; ACTRN12616000643471; ISRCTN61225414; NCT02133950; NCT02570386; NCT03349905). Note that studies that we did not include studies that were registered in the trials registers but that were not started or that were withdrawn or stopped.
Risk of bias in included studies
See the 'Risk of bias’ summary (Figure 2) and graph (Figure 3) for the included trials (eight RCTs for the primary outcomes, two follow‐up studies for the secondary outcomes and five extra for the additional analysis). See also Characteristics of included studies.
2.

'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study
3.

'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies
Allocation
Sequence generation
Ferraretti 1999 did not describe the method of randomisation in the published article, but replied in a personal communication that the method of randomisation was performed with random sealed envelopes; we judged this study to be at unclear risk of this bias. Shapiro 2011a and Shapiro 2011b did not report on the method for random sequence generation; we judged these two studies at unclear risk of bias.
Sequence generation in Chen 2016 was well described; an online central randomisation system was used. We considered risk of selection bias related to sequence generation to be low. Vuong 2018 performed sequence generation randomly by an independent study co‐ordinator by means of block randomisation using a computer‐generated random list and therefore we considered risk of selection bias related to sequence generation to be low. Risk of selection bias related to sequence generation in Wei 2019 was also considered low based on the use of block randomisation using a computer‐generated random list. Santos‐Ribeiro 2020 randomised women by means of a computer‐generated randomisation list. Wong 2021 randomised women with an online randomisation program using block randomisation with a maximum block size of 6, stratified for age (18 years through 35 and 35 through 43 years) and study centre. Couples were allocated in a 1:1 ratio to the 'freeze all' strategy or the conventional strategy. We judged these two studies at low risk of bias.
Allocation concealment
Shapiro 2011a and Shapiro 2011b performed allocation concealment by drawing randomly among identical, opaque, unmarked, sealed envelopes, and we therefore judged both studies to be at low risk of selection bias related to allocation concealment. Chen 2016 used an online central randomisation system (www.medresman.org) to generate the assignment sequence automatically, which was unknown to the clinical investigators. Santos‐Ribeiro 2020 sealed each entry of the list in a sequentially numbered opaque envelope and allocated participants in that order. Participating physicians did not have access to the randomisation list. Wei 2019 used a sequence that was entered into their central online database, secured by username and password log‐in. Wong 2021 used a randomisation program to generate a unique study number with allocation code after entry of the participant’s date of birth and randomisation date. We also judged these four studies to be at low risk of bias.
The first author of the Ferraretti 1999 study provided additional information on allocation concealment. This study performed participant allocation by sealed envelopes, and we therefore judged it to be unclear risk of bias for this domain. Vuong 2018 randomised patients by means of block randomisation by an independent study co‐ordinator using a computer‐generated random list, there was no further explanation about allocation concealment. We also judged this study to be at unclear risk of bias.
Blinding
Performance bias
Blinding of doctors and participants was not possible due to the nature of the intervention. Therefore the risk of performance bias was low in all eight studies.
Detection bias
As described in the Methods section, blinding of the participant or the clinician is technically not possible due to the nature of the intervention in this study design. We felt that lack of blinding was not likely to influence findings for the primary outcomes live birth or OHSS. The risk of performance bias was low in all eight studies.
Incomplete outcome data
Three studies did not report intention‐to‐treat analysis in the methodological or analysis sections (Ferraretti 1999; Shapiro 2011a; Shapiro 2011b), while five studies did report intention‐to‐treat analysis (Chen 2016; Santos‐Ribeiro 2020; Vuong 2018; Wei 2019; Wong 2021).
We initially judged the studies by Shapiro 2011a and Shapiro 2011b to be at high risk of attrition bias. These studies did not take into account withdrawals or exclusions of randomised women in the reported analyses. Both studies also analysed the outcomes per embryo transferred instead of per woman. However, sufficient data were available for analysis per woman in meta‐analysis. We prespecified ongoing pregnancy as a viable pregnancy at 12 weeks' gestation. These two studies defined ongoing pregnancy at 10 weeks' gestation, which could slightly overestimate the results for this outcome. Taking these issues into account, we considered the risk of bias to be unclear in these two studies.
Ferraretti 1999; Santos‐Ribeiro 2020; Vuong 2018; Wei 2019 and Wong 2021 did analyse all randomised women. The risk of attrition bias was low.
Selective reporting
Four studies were registered in a prospective trials register under the following numbers: NCT01841528 (Chen 2016), NCT02471573 (Vuong 2018), ChiCTR‐IOR‐14005405 (Wei 2019) and NCT02148393 (Santos‐Ribeiro 2020), including an automatically indexed link on the published report on the study, and the study protocol was published beforehand (Chen 2016; Santos‐Ribeiro 2020; Vuong 2018; Wei 2019). Wong 2021 was registered in a prospective trials register with the trial number NTR3187. Prespecified outcomes were generally reported, although in Chen 2016 some prespecified outcomes (e.g. time to pregnancy) were missing from the report. Considering this, we judged Santos‐Ribeiro 2020; Vuong 2018; Wei 2019 and Wong 2021 to be at low risk of reporting bias and Chen 2016 to be at high risk of reporting bias. Two studies were registered in a prospective trials register with the respective trial numbers NCT00963625 and NCT00963079 (Shapiro 2011a; Shapiro 2011b). Data on the follow‐up of the studies were available in the trials register. The prespecified outcomes of interest were reported in the two studies, and we judged these studies to be at low risk of this bias. We could not assess reporting bias for Ferraretti 1999, as trials registers did not exist at that time, therefore the risk of reporting bias for this study was unclear.
Other potential sources of bias
We judged fourteen studies to be at unclear risk of other bias and Ferraretti 1999 to be at high risk of other bias. Three of the studies did not clearly report their prespecified criteria for early termination of their trial. Ferraretti 1999 did not prespecify rules as to when to terminate the study. In the two studies by Shapiro 2011a; Shapiro 2011b, an interim analysis was planned after 100 completed blastocyst transfers. While women were randomised, the interim analyses were based on completed blastocyst transfers (unit of analysis error). They did not report whether the interim analysis was performed by an independent committee that was blinded for the primary outcome. In addition, Shapiro 2011b terminated the study early after an interim analysis based on differences in embryo quality between the two strategies. This reason was not mentioned as one of the criteria to terminate the study. All three studies cryopreserved embryos at the two pro‐nucleate (2pn) stage with slow freezing, which is not currently a common freezing protocol in IVF centres.
After freezing and thawing, the eight studies transferred embryos at a different developmental stage: Chen 2016; Ferraretti 1999 and Vuong 2018 transferred cleavage embryos, and Santos‐Ribeiro 2020; Shapiro 2011a; Shapiro 2011b; Wei 2019 and Wong 2021 transferred blastocysts. Four studies reported a form of time to pregnancy: Vuong 2018 reported the median time to conception, Wei 2019 reported the mean time to live birth, Santos‐Ribeiro 2020 reported the mean time from randomisation to detection of clinical pregnancy after the first embryo transfer and Wong 2021 reported the time to ongoing pregnancy. None of the eight studies reported on blinding of doctors to interim analyses of outcomes of the study.
Effects of interventions
Summary of findings 1. Fresh compared to frozen embryo transfer (cumulatively) in assisted reproduction.
| Fresh compared to frozen embryo transfer (cumulatively) in assisted reproduction | ||||||
| Patient or population: women undergoing assisted reproduction Setting: assisted reproduction clinic Intervention: frozen embryo transfers only Comparison: fresh and frozen embryo transfers (conventional IVF) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with fresh and frozen embryo transfers | Risk with frozen embryo transfer only | |||||
| Live birth rate: cumulatively | 579 per 1000 | 589 per 1000 (567 to 627) | OR 1.08 (0.95 to 1.22) | 4712 (8 RCTs) | ⊕⊕⊕⊝ Moderatea | |
| OHSS: per cycle with ovarian hyperstimulation | 33 per 1000 | 9 per 1000 (6 to 13) | OR 0.26 (0.17 to 0.39) | 4478 (6 RCTs) | ⊕⊕⊝⊝ Lowa,b | |
| Ongoing pregnancy rate: cumulatively | 508 per 1000 | 495 per 1000 (436 to 551) | OR 0.95 (0.75 to 1.19) | 1245 (4 RCTs) | ⊕⊕⊕⊝ Moderatea | |
| Miscarriage rate: cumulatively | 118 per 1000 | 124 per 1000 (88 to 171) | OR 1.06 (0.72 to 1.55) | 986 (2 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c | |
| Multiple pregnancy rate: cumulatively | 156 per 1000 | 140 per 1000 (101 to 188) | OR 0.88 (0.61 to 1.25) | 986 (2 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c | |
| Time to pregnancy | Outcome could not be analysed. By design, time to pregnancy is shorter in the conventional strategy compared to the 'freeze all' strategy when the cumulative live birth rate is comparable, as embryo transfer is delayed in a 'freeze all' strategy. |
|||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; IVF: in vitro fertilisation; OHSS: ovarian hyperstimulation syndrome; OR: odds ratio; RCT: randomised controlled trial; RR: risk ratio | ||||||
|
GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded one level due to serious risk of bias associated with lack of power calculation (unclear what determined end of study) and/or use of interim analysis that was calculated per transfer (unit of analysis error) with absence of adequate stopping rules (possible overestimation of treatment effect). bDowngraded one level due to serious imprecision: event rate less than 300. cDowngraded one level due to serious unexplained heterogeneity.
Summary of findings 2. Fresh compared to frozen embryo transfers in assisted reproduction regarding pregnancy and neonatal outcomes.
| Fresh compared to frozen embryo transfers in assisted reproduction regarding pregnancy and neonatal outcomes (cumulatively and after first embryo transfer) | ||||||
| Patient or population: women undergoing assisted reproduction Setting: assisted reproduction clinic Intervention: frozen embryo transfers only Comparison: fresh and frozen embryo transfers (conventional IVF) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with fresh and frozen embryo transfers | Risk with frozen embryo transfer only | |||||
| Hypertensive disorders of pregnancy: cumulatively | 26 per 1000 | 18 per 1000 (7 to 46) | OR 0.70 (0.27 to 1.82) | 782 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | |
| Hypertensive disorders of pregnancy: after first ET | 15 per 1000 | 31 per 1000 (21 to 46) | OR 2.15 (1.42 to 3.25) | 3940 (3 RCTs) | ⊕⊕⊝⊝ Lowa,b | |
| Large for gestational age (birth weight above 90th percentile): cumulatively | 10 per 1000 | 20 per 1000 (6 to 60) | OR 1.97 (0.63 to 6.15) | 782 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | |
| Large for gestational age (birth weight above 90th percentile): after first ET | 42 per 1000 | 79 per 1000 (62 to 100) | OR 1.96 (1.51 to 2.55) | 3940 (3 RCTs) | ⊕⊕⊝⊝ Lowa,b,c | |
| Small for gestational age (birth weight below 10th percentile): cumulatively | 46 per 1000 | 17 per 1000 (8 to 37) | OR 0.36 (0.16 to 0.80) | 782 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | |
| Small for gestational age (birth weight below 10th percentile): after first ET | 82 per 1000 | 68 per 1000 (55 to 86) | OR 0.82 (0.65 to 1.05) | 3940 (3 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c | |
| Birth weight of babies born: singletons | MD 127.4 g higher (77.1 higher to 177.1 higher) | ‐ | 1607 (5 RCTs) | ⊕⊕⊕⊝ Moderatea | ||
| Birth weight of babies born: multiples | MD 49.5 g higher (21.1 lower to 120.1 higher) | ‐ | 804 (4 RCTs) | ⊕⊕⊝⊝ Lowa,c | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; IVF: in vitro fertilisation; MD: mean difference; OR: odds ratio; RCT: randomised controlled trial; RR: risk ratio | ||||||
|
GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded one level due to serious risk of bias associated with lack of power calculation (unclear what determined end of study) and/or use of interim analysis that was calculated per transfer (unit of analysis error) with absence of adequate stopping rules (possible overestimation of treatment effect). bDowngraded one level due to serious imprecision: event rate: less than 300. cDowngraded one level due to serious unexplained heterogeneity.
We included eight studies involving 4,712 women in this review. See Table 1.
1. Comparison of the 'freeze all' strategy versus the conventional IVF/ICSI strategy
1.1 Effectiveness: cumulative live birth rate per woman
All studies included in the meta‐analysis collected data on cumulative live birth rates (Chen 2016; Ferraretti 1999; Santos‐Ribeiro 2020; Shapiro 2011a; Shapiro 2011b; Vuong 2018; Wei 2019; Wong 2021).
There is probably little or no difference between the 'freeze all' strategy and the conventional IVF/ICSI strategy in cumulative live birth rate (OR 1.08, 95% CI 0.95 to 1.22; I2 = 0%; 8 RCTs, 4712 women; moderate‐quality evidence). The evidence suggests that for a cumulative live birth rate of 58% following the conventional strategy, the cumulative live birth rate following the 'freeze all' strategy would be between 57% and 63%.
As there was no indication of statistical heterogeneity an identical estimate for the OR was found when using the random‐effects model. The corresponding RR was 1.03 (95% CI 0.99 to 1.08; Table 4). A sensitivity analysis including only studies without risk of selection bias (Chen 2016; Santos‐Ribeiro 2020; Vuong 2018; Wei 2019; Wong 2021), found a comparable result (OR 1.09, 95% CI 0.95 to 1.23; I2 = 0%; 4 RCTs, 4328 women). A sensitivity analysis adopting a random‐effects model or using risk ratio did not lead to a change in result.
2. Sensitivity analysis for primary outcomes.
| Study (number of participants) | OR (95% CI) Fixed‐effect | OR (95% CI) Random‐effects | RR (95% CI) Fixed‐effect | RR (95% CI) Random‐effects |
|
Cumulative live birth rate Chen 2016 (1508) Ferraretti 1999 (125) Santos‐Ribeiro 2020 (184) Shapiro 2011a (103) Shapiro 2011b (122) Vuong 2018 (782) Wei 2019 (1650) Wong 2021 (204) |
1.08 (0.95 to 1.22) | 1.08 (0.95 to 1.22) | 1.03 (0.98 to 1.07) | 1.03 (0.99 to 1.08) |
|
OHSS Chen 2016 (1508) Ferraretti 1999 (125) Santos‐Ribeiro 2020 (184) Vuong 2018 (782) Wei 2019 (1650) Wong 2021 (204) |
0.26 (0.17 to 0.39) | 0.25 (0.13 to 0.46) | 0.22 (0.14 to 0.37) | 0.25 (0.14 to 0.44) |
| CI: confidence interval; OHSS: ovarian hyperstimulation syndrome; OR: odds ratio;RR: risk ratio | ||||
In the studies of Santos‐Ribeiro 2020; Shapiro 2011a there was lost to follow up for the primary outcome and therefore numbers of participants differ with the Characteristics tables.
There is also probably no difference between the two strategies in cumulative live birth rate when the studies are analysed per cleavage stage (OR 1.09, 95% CI 0.93 to 1.29; I2 = 0%; 3 RCTs, 2415 women; moderate‐quality evidence) or blastocyst transfer stage (OR 1.06, 95% CI 0.88 to 1.27; I2 =14%; 5 RCTs, 2297 women; moderate‐quality evidence; Analysis 1.1; Figure 4).
1.1. Analysis.

Comparison 1: Freeze‐all versus conventional IVF, outcomes per woman, Outcome 1: Cumulative live birth rate
4.

Forest plot of comparison 1. Freeze‐all versus conventional IVF, outcomes per woman, outcome 1.1 live birth rate
1.2 Safety: ovarian hyperstimulation syndrome (OHSS) per woman
Two studies reported on OHSS per woman if hospitalisation was required (Ferraretti 1999; Wong 2021). Two studies did not report on OHSS (Shapiro 2011a; Shapiro 2011b), but we were able to obtain these data by personal communication with the authors. However, we did not include the data from these two studies in the analysis, as women with high risk of OHSS were excluded and received the 'freeze all' strategy as standard. Chen 2016; Santos‐Ribeiro 2020; Vuong 2018 and Wei 2019 reported these data. The risk for developing OHSS may be lower after the 'freeze all' strategy compared to the conventional IVF/ICSI strategy (Peto OR 0.26, 95% CI 0.17 to 0.39; I2 = 0%; 6 RCTs, 4478 women; low‐quality evidence; Analysis 1.2; Figure 5). As there was no indication of statistical heterogeneity, we found an identical estimate for the OR when using the random‐effects model. The corresponding RR was 0.25 (95% CI 0.14 to 0.44; Table 4). A sensitivity analysis including only studies without risk of selection bias (Chen 2016; Santos‐Ribeiro 2020; Vuong 2018; Wei 2019; Wong 2021), found a comparable result (OR 0.27, 95% CI 0.18 to 0.40; I2 = 12%; 5 RCTs, 4354 women). A sensitivity analysis adopting a random‐effects model or using risk ratio did not lead to a change in result.
1.2. Analysis.

Comparison 1: Freeze‐all versus conventional IVF, outcomes per woman, Outcome 2: Ovarian hyperstimulation syndrome (OHSS)
5.

Forest plot of comparison 1. Freeze‐all versus conventional IVF, outcomes per woman, outcome 1.2 ovarian hyperstimulation syndrome (OHSS)
Secondary outcomes
1.3 Ongoing pregnancy rate per woman
Four studies reported on the cumulative ongoing pregnancy rates (Shapiro 2011a; Shapiro 2011b; Vuong 2018; Wong 2021). There was probably little or no difference between the two strategies in the cumulative ongoing pregnancy rate (OR 0.95, 95% CI 0.75 to 1.19; I2 = 31%; 4 RCTs, 1245 women; moderate‐quality evidence; Analysis 1.3).
1.3. Analysis.

Comparison 1: Freeze‐all versus conventional IVF, outcomes per woman, Outcome 3: Cumulative ongoing pregnancy rate
1.4 Clinical pregnancy rate per woman
Four studies reported on cumulative clinical pregnancy rate (Ferraretti 1999; Santos‐Ribeiro 2020; Vuong 2018; Wong 2021). There may be no difference between the two strategies in clinical pregnancy rate though the confidence interval of the estimate (OR 0.92, 95% CI 0.72 to 1.16; I2 = 35%; 4 RCTs; 1320 women; low‐quality evidence; Analysis 1.4).
1.4. Analysis.

Comparison 1: Freeze‐all versus conventional IVF, outcomes per woman, Outcome 4: Cumulative clinical pregnancy rate
Time to pregnancy
Four of the studies reported on time to pregnancy. Santos‐Ribeiro 2020 reported the mean time from randomisation to detection of clinical pregnancy after the first embryo transfer (average treatment effect 33.3 days, 95% CI 25.8 to 40.9; P < 0.01) and the overall cumulative time to clinical pregnancy (Cox regression hazard ratio 0.92, 95% CI 0.68 to 1.24; P = 0.59). Vuong 2018 reported median time to pregnancy of 3.6 months in the frozen embryo group and 2.2 months in the fresh embryo group (absolute difference 1.4 months, 95% CI 0.95 to 1.84; P < 0.001). Wei 2019 did not report time to live birth after randomisation but after embryo transfer and is therefore not a fair comparison. All three studies calculated time to pregnancy for the women who became pregnant and not for the entire study group. Therefore no valid analysis can be performed based the results of these three studies. It was not possible to calculate the median time to pregnancy for Wong 2021 due to the limited number of ongoing pregnancies.
Secondary outcomes per woman regarding obstetric, perinatal and neonatal complication
1.5 Ectopic pregnancy rate
Two studies reported on the cumulative ectopic pregnancy rate (Vuong 2018; Wong 2021). We are uncertain whether there is a difference between the two strategies in cumulative ectopic pregnancy rate (Peto OR 0.61, 95% CI 0.31 to 1.22; I2 = 0%; 2 RCTs. 986 women; low‐quality evidence; Analysis 1.5).
1.5. Analysis.

Comparison 1: Freeze‐all versus conventional IVF, outcomes per woman, Outcome 5: Ectopic pregnancy
Five studies reported on ectopic pregnancy rate after the first embryo transfer (Chen 2016; Ferraretti 1999; Vuong 2018; Wei 2019; Santos‐Ribeiro 2020). We are uncertain whether there is a difference between the two strategies in ectopic rate after the first embryo transfer (Peto OR 0.64, 95% CI 0.39 to 1.06; I2 = 0%; 5 RCTs, 4274 women; low‐quality evidence; Analysis 1.5).
1.6 Miscarriage rate
Two studies reported on the cumulative miscarriage rate (Vuong 2018; Wong 2021). We are uncertain whether the two strategies differ in cumulative miscarriage rate (Peto OR 1.06, 95% CI 0.72 to 1.55; I2 = 55%; 2 RCTs, 986 women; very low‐quality evidence; Analysis 1.6).
1.6. Analysis.

Comparison 1: Freeze‐all versus conventional IVF, outcomes per woman, Outcome 6: Miscarriage
All eight studies reported on miscarriage rate after the first embryo transfer (Chen 2016; Ferraretti 1999; Santos‐Ribeiro 2020; Shapiro 2011a; Shapiro 2011b; Vuong 2018; Wei 2019; Wong 2021). We are uncertain about the existence of a difference between the two strategies in miscarriage rate after the first embryo transfer (Peto OR 0.90, 95% CI 0.76 to 1.07; I2 = 64%; 8 RCTs, 4569 women; very low‐quality evidence; Analysis 1.6).
1.7 Multiple pregnancy rate
Two studies reported on the cumulative multiple‐pregnancy rate (Vuong 2018; Wong 2021). We are uncertain whether the two strategies differ in cumulative multiple‐pregnancy rate (Peto OR 0.88, 95% CI 0.61 to 1.25; I2 = 63%; 2 RCTs. 986 women; very low‐quality evidence; Analysis 1.7).
1.7. Analysis.

Comparison 1: Freeze‐all versus conventional IVF, outcomes per woman, Outcome 7: Multiple pregnancy
Five studies reported on multiple‐pregnancy rate after the first embryo transfer (Chen 2016; Shapiro 2011b; Vuong 2018; Wei 2019; Wong 2021). We are uncertain whether the two strategies differ in multiple‐pregnancy rate after the first embryo transfer (Peto OR 1.18, 95% CI 0.96 to 1.45; I2 = 45%; 5 RCTs, 4266 women; very low‐quality evidence; Analysis 1.7).
1.8 Gestational diabetes
One study reported the cumulative rates of gestational diabetes (Vuong 2018), therefore pooling was not possible. We are uncertain whether the two strategies differ in cumulative rates of gestational diabetes (Peto OR 0.83, 95% CI 0.36 to 1.94; 1 RCT, 782 women; low‐quality evidence; Analysis 1.8).
1.8. Analysis.

Comparison 1: Freeze‐all versus conventional IVF, outcomes per woman, Outcome 8: Gestational diabetes mellitus
Three studies reported on the rate of gestational diabetes after the first embryo transfer (Vuong 2018; Wei 2019; Zhang 2018). We are uncertain whether the two strategies differ in gestational diabetes after the first embryo transfer (Peto OR 1.34, 95% CI 0.96 to 1.86; I2 = 20%; 3 RCTs, 3940 women; low‐quality evidence; Analysis 1.8).
1.9 Hypertensive disorders of pregnancy
One study reported the cumulative rates of hypertensive disorders of pregnancy (Vuong 2018), therefore pooling was not possible. We are uncertain whether the two strategies differ in cumulative rates of hypertensive disorders of pregnancy (Peto OR 0.70, 95% CI 0.27 to 1.82; 1 RCT, 782 women; low‐quality evidence; Analysis 1.9; Figure 6).
1.9. Analysis.

Comparison 1: Freeze‐all versus conventional IVF, outcomes per woman, Outcome 9: Hypertensive disorders of pregnancy
6.

Forest plot of comparison: 1 Freeze‐all versus conventional IVF, outcomes per woman, outcome 1.9 hypertensive disorders of pregnancy
Three studies reported on the rate of hypertensive disorders of pregnancy after the first embryo transfer (Chen 2016; Vuong 2018; Wei 2019). The risk of hypertensive disorders might be increased following the 'freeze all' strategy (Peto OR 2.15, 95% CI 1.42 to 3.25; I2 = 29%; 3 RCTs, 3940 women; low‐quality evidence; Analysis 1.9; Figure 6).
1.10 Preterm delivery (less than 37 weeks of gestational age)
Two studies reported the cumulative rates of preterm delivery (Vuong 2018; Wong 2021). We are uncertain whether the two strategies differ in cumulative rates of preterm delivery (Peto OR 0.62, 95% CI 0.39 to 0.99; I2 = 0%; 2 RCTs, 986 women; low‐quality evidence; Analysis 1.10).
1.10. Analysis.

Comparison 1: Freeze‐all versus conventional IVF, outcomes per woman, Outcome 10: Preterm delivery (< 37 weeks of gestational age)
Three studies reported on the rate of preterm delivery after the first embryo transfer (Chen 2016; Vuong 2018; Wei 2019). We are uncertain whether the two strategies differ in preterm delivery after the first embryo transfer (Peto OR 1.15, 95% CI 0.89 to 1.50; I2 = 0%; 3 RCTs, 3940 women; low‐quality evidence; Analysis 1.10).
1.11 Perinatal and neonatal death
One study reported the cumulative rates of perinatal and neonatal death (Vuong 2018), therefore pooling was not possible. Based on results from this solitary study (not meta‐analysis) we are uncertain whether the two strategies differ in cumulative rates of perinatal and neonatal death (Peto OR 0.13, 95% CI 0.01 to 1.30; 1 RCT, 782 women; very low‐quality evidence; Analysis 1.11).
1.11. Analysis.

Comparison 1: Freeze‐all versus conventional IVF, outcomes per woman, Outcome 11: Perinatal and neonatal death
Two studies reported on the rate of perinatal and neonatal death after the first embryo transfer (Chen 2016; Vuong 2018). We are uncertain whether the two strategies differ in perinatal and neonatal death after the first embryo transfer (Peto OR 2.27, 95% CI 0.65 to 7.84; I2 = 88%; 2 RCTs, 2290 women; very low‐quality evidence; Analysis 1.11).
1.12 Neonatal hospitalisation (for more than three days or NICU admission)
One study reported the cumulative rates of neonatal hospitalisation (Vuong 2018), therefore pooling was not possible. We are uncertain whether the two strategies differ in cumulative rates of neonatal hospitalisation (Peto OR 1.00, 95% CI 0.29 to 3.48; 1 RCT, 782 women; very low‐quality evidence; Analysis 1.12).
1.12. Analysis.

Comparison 1: Freeze‐all versus conventional IVF, outcomes per woman, Outcome 12: Neonatal hospitalisation (> 3 days or neonatal intensive care unit admission)
Three studies reported on the rate of neonatal hospitalisation after the first embryo transfer (Vuong 2018; Wei 2019; Zhang 2018). We are uncertain whether the two strategies differ in neonatal hospitalisation after the first embryo transfer (Peto OR 1.37, 95% CI 1.07 to 1.75; I2 = 0%; 3 RCTs, 3940 women; low‐quality evidence; Analysis 1.12).
1.13 Large for gestational age (birth weight above 90th percentile)
One study reported the cumulative rates of large‐for‐gestational‐age babies (Vuong 2018). Based on the results from this solitary study we are uncertain whether the two strategies differ in cumulative rates of having a large‐for‐gestational‐age baby (Peto OR 1.97, 95% CI 0.63 to 6.15; 1 RCT, 782 women; low‐quality evidence; Analysis 1.13; Figure 7).
1.13. Analysis.

Comparison 1: Freeze‐all versus conventional IVF, outcomes per woman, Outcome 13: Large for gestational age (birth weight > 90th percentile)
7.

Forest plot of comparison 1. Freeze‐all versus conventional IVF, outcomes per woman, outcome 1.13 large for gestational age (birth weight above 90th percentile)
Three studies reported on the rate of large for gestational age after the first embryo transfer (Vuong 2018; Wei 2019; Zhang 2018). Based on these studies the risk of having a large‐for‐gestational‐age baby might be increased following the 'freeze all' strategy (Peto OR 1.96, 95% CI 1.51 to 2.55; I2 = 0%; 3 RCTs, 3940 women; low‐quality evidence; Analysis 1.13; Figure 7).
1.14 Small for gestational age (birth weight below 10th percentile)
One study reported the cumulative rates of small for gestational age babies (Vuong 2018), therefore pooling was not possible. The results of this solitary study suggest that the cumulative risk of having a small‐for‐gestational‐age baby might be lower following the 'freeze all' strategy (Peto OR 0.36, 95% CI 0.16 to 0.80; 1 RCT, 782; low‐quality evidence; Analysis 1.14; Figure 8).
1.14. Analysis.

Comparison 1: Freeze‐all versus conventional IVF, outcomes per woman, Outcome 14: Small for gestational age (birth weight < 10th percentile)
8.

Forest plot of comparison 1. Freeze‐all versus conventional IVF, outcomes per woman, outcome 1.14 small for gestational age (birth weight below 10th percentile)
Three studies reported on the rate of small for gestational age after the first embryo transfer (Vuong 2018; Wei 2019; Zhang 2018). We are uncertain whether the two strategies differ in the risk of having a small‐for‐gestational‐age baby after the first embryo transfer (Peto OR 0.82, 95% CI 0.65 to 1.05; I2 = 64%; 3 RCTs, 3940 women; very low‐quality evidence; Analysis 1.14; Figure 8).
1.15 Congenital abnormalities
Three studies reported on congenital abnormalities (Chen 2016; Wei 2019; Wong 2021). We are uncertain whether the two strategies differ in rates of congenital abnormalities per live‐born children plus number of foetuses therapeutically terminated (OR 1.08, 95% CI 0.65 to 1.78; I2 = 0%; 3 RCTs, 1789 live‐born children plus number of foetuses therapeutically terminated; low‐quality evidence; Analysis 1.15).
1.15. Analysis.

Comparison 1: Freeze‐all versus conventional IVF, outcomes per woman, Outcome 15: Congenital abnormalities per live‐born children
1.16 Birth weight
Five studies reported on birth weight (Chen 2016; Shapiro 2016; Vuong 2018; Wei 2019; Wong 2021). The risk for having a higher birth weight of singleton babies born is probably increased following the 'freeze all' strategy (MD 127 g, 95% CI 77.1 to 177.8; I2 = 0%; 5 RCTs, 1607 singletons; moderate‐quality evidence).
We are uncertain whether birth weight of multiples is higher in the 'freeze all' strategy compared to the conventional IVF/ICSI strategy (MD 49.5, 95% CI −21.2 to 120.1; I2 = 17%; 4 RCTs, 804 children born from multiples; low‐quality evidence; Analysis 1.16; Figure 9).
1.16. Analysis.

Comparison 1: Freeze‐all versus conventional IVF, outcomes per woman, Outcome 16: Birth weight of babies born
9.

Forest plot of comparison 1. Freeze‐all versus conventional IVF, outcomes per woman, outcome 1.16 birth weight of babies born
Wong 2021 had three twin live‐born in the conventional IVF strategy and none in the 'freeze all' strategy and could therefore not be pooled in the analysis.
Other analyses
We also analysed the adverse events, multiple pregnancy and miscarriage per clinical pregnancy (Analysis 2.1; Analysis 2.2).
2.1. Analysis.

Comparison 2: Freeze‐all versus conventional IVF, adverse events per clinical pregnancy, Outcome 1: Multiple pregnancy: after first embryo transfer
2.2. Analysis.

Comparison 2: Freeze‐all versus conventional IVF, adverse events per clinical pregnancy, Outcome 2: Miscarriage: after first embryo transfer
It is uncertain whether the two strategies differ in multiple pregnancy per clinical pregnancy after the first embryo transfer (OR 1.09, 95% CI 0.87 to 1.36; 5 RCTs, 2223 clinical pregnancies; I2 = 14%; Analysis 2.1). We are uncertain whether the two strategies differ in miscarriage rate per clinical pregnancy after the first embryo transfer (OR 0.82, 95% CI 0.66 to 1.02; I2 = 63%; 8 RCTs, 2451 clinical pregnancies; Analysis 2.2).
Additional analysis
We also calculated and presented the live birth rate after the first transfer (Table 3). Based on this calculation the live birth rate after the first transfer is increased following the 'freeze all' strategy (OR 1.17, 95% CI 1.06 to 1.28; 13 RCTs, 7766 women) for all stages of transfer.
Discussion
Summary of main results
Our findings suggest that the 'freeze all' strategy results in similar cumulative live birth rate but lower OHSS rate than the conventional IVF/ICSI strategy. We could not analyse time to pregnancy. We can assume that it is shorter with conventional IVF/ICSI strategy where cumulative live birth rate is similar, as embryo transfer is delayed in a 'freeze all' strategy (Zaat 2019). Low‐quality evidence suggests that the 'freeze all' strategy might be associated with increased risk of hypertensive disorders of pregnancy, increased risk of having a large‐for‐gestational‐age baby and a higher birth weight of singleton babies.
Overall completeness and applicability of evidence
All eight trials included in the meta‐analysis provided data on the primary outcome, live birth rate, but for OHSS we could use data from only five studies.
Four of these studies involved a small number of women. All studies in the meta‐analysis had specific and differing technical protocols, and studies had distinct inclusion criteria leading to the inclusion of select groups of women (women with a high risk of OHSS, good prognosis women, women with polycystic ovary syndrome, young women without polycystic ovary syndrome and in women with any IVF indication, including the women with possible low ovarian response). Four of the studies reported time to pregnancy, each in a different way and therefore we could not pool these outcomes. One of these three studies (Wei 2019), did not report time to live birth after randomisation but after embryo transfer and is therefore not a fair comparison. Time to a pregnancy leading to a live birth needs to be reported with the date of randomisation as a starting point.
Quality of the evidence
We rated the quality of evidence using GRADE methods and judged it to be moderate to low, due to serious risk of bias and (for some outcomes) serious imprecision. Risk of bias was associated with unclear blinding of investigators for preliminary outcomes of the study during the interim‐analysis, unit of analysis error, and absence of adequate study termination rules.
The eight included studies involved a total of 2342 women undergoing the 'freeze all' strategy and 2370 women undergoing the conventional IVF/ICSI strategy. Varying protocols between studies (some not common in routine practice), varying study population (select groups of women undergoing IVF), one study that did not report power calculation (Ferraretti 1999: unclear what determined the end of study), two studies that calculated interim analysis per transfer (Shapiro 2011a; Shapiro 2011b: unit of analysis error) with absence of adequate stopping rules (possible overestimation of treatment effect) and one study that reported cumulative live birth rate including a possible second ('freeze all' 5.9%, conventional IVF 6.4%) or third ('freeze all' 0.5%, conventional IVF 0.8%) retrieval cycle (Vuong 2018), resulted in an overall judgement of the evidence as moderate to low quality.
Our searches identified six ongoing studies. We anticipate that the evidence from these will provide a more definitive answer on the relative effectiveness and safety of a 'freeze all' strategy.
Potential biases in the review process
We tried to reduce potential bias in the review process to a minimum by identifying all eligible studies for inclusion in this meta‐analysis. We were able to retrieve additional information on three included trials where required, which helped us in providing accurate study outcomes. Unfortunately, we did not receive additional information regarding cumulative data for four other studies, therefore these studies could only be included in the additional analysis.
Agreements and disagreements with other studies or reviews
Cumulative live birth rate
Four out of eight studies included in the meta‐analysis in this review reported higher pregnancy or live birth rates in favour of the 'freeze all' strategy (Chen 2016; Shapiro 2011a; Shapiro 2011b; Wei 2019), while our review concluded that there was no difference in live birth rates between the strategies. This discrepancy in conclusion is attributed to the fact that these publications focused on outcomes that were reported after the first transfer, whereas in our review we focused on the cumulative live birth rate per woman randomised. In case cumulative live birth rates are comparable, as found in this review, then the difference between strategies could be time to pregnancy and possible differences in pregnancy and neonatal complications. For illustrative purposes we also calculated and presented the live birth rate after the first transfer (Table 3). Based on this calculation the live birth rate after the first transfer is increased following the 'freeze all' strategy (OR 1.17, 95% CI 1.06 to 1.28; 13 RCTs, 7766 women) for all stages of transfer. The live birth rate calculated after the first transfer possibly shows differences in outcome for a stimulated and an unstimulated uterus, although this does not take into account the number of embryos that were thawed for transfer.
For women, the live birth rate per first transfer is less relevant, since at the same time of first transfer in a 'freeze all' strategy, they would already have received the second transfer in a conventional IVF/ICSI strategy. Considering the important perspective of time, it would only be fair to compare cumulative live birth rate between groups instead of live birth rate after first transfer (Zaat 2019). In the additional calculation concerning live birth rate after the first transfer, we included a study in which women underwent preimplantation genetic testing for aneuploidies (PGT‐A) (Coates 2017). The use of PGT‐A could have an effect on the current comparison under evaluation, as PGT‐A affects the number of embryos available for transfer (Mastenbroek 2014). One out of eight included studies reported lower cumulative ongoing pregnancy rate or live birth rates for the 'freeze all' strategy than for conventional IVF/ICSI treatment including fresh transfer (Wong 2021). In this trial, an unselected cohort of couples undergoing IVF was selected, including women with poor prognosis. This could explain the difference from the other included trials, in which only women with a good prognosis (Santos‐Ribeiro 2020; Shapiro 2011a; Shapiro 2011b; Vuong 2018; Wei 2019), women with polycystic ovary syndrome (Chen 2016), and women with a high risk of OHSS were included (Ferraretti 1999). This should be taken into account when considering the 'freeze all' strategy, whether to use it in an unselected population or only in women with a good prognosis, with a minimum number of good‐quality embryos available. The current review does not provide an answer to this. Two recently published systematic reviews and meta‐analyses, Roque 2019 and Bosdou 2019 reported a higher live birth rate after the first transfer in the 'freeze all' strategy compared to conventional IVF/ICSI. No significant difference was found in cumulative live birth rate between both groups. In Roque 2019 this increased live birth rate was only found in hyper‐responders and in cycles with PGT‐A. However, no significant difference was found in cumulative live birth rate between both groups. Santos‐Ribeiro 2020; Wei 2019 and Wong 2021 had not been published when the review by Roque 2019 was written. In Bosdou 2019, high responders had a significantly higher probability of live birth in the 'freeze all' strategy based on the results after the first transfer. However, the probability of live birth was not significantly different between both groups in normal responders after the first embryo transfer. Bosdou and colleagues did include the Absalan 2013 study, which we excluded based on the unclear study design. No significant difference was found in cumulative live birth rate between both groups.
Ovarian hyperstimulation syndrome (OHSS)
The lower rate of OHSS found in our review is in agreement with previous systematic reviews and studies (Bosdou 2019; D'Angelo 2017; Evans 2014; Roque 2019; Takeshima 2016), and is to be expected. Avoiding a pregnancy in the initial cycle with ovarian stimulation would eliminate the residual risks of OHSS, and OHSS would therefore be self‐limiting. Mild OHSS symptoms can still occur as a result of a hCG trigger in the hyperstimulated cycle in the 'freeze all' strategy, but OHSS in its severe form should be rare and even close to zero when agonist trigger is used (D'Angelo 2017; Youssef 2016). All the included studies in our review used hCG trigger before oocyte aspiration. Although in agreement with previous studies and our expectations, the quality of evidence for the lower rate of OHSS in the 'freeze all' strategy was low. Definitions of OHSS vary widely in literature as in the studies included in this review. Therefore, this result should be interpreted with caution.
Time to pregnancy
Three of the studies reported on time to pregnancy, all in a different way. All three studies calculated time to pregnancy for the women who became pregnant and not for the entire study group. Therefore no valid analysis can be performed based on the results of these three studies. Reporting on time to pregnancy should be done based on the entire study group, including presenting Kaplan‐Meier curves, calculating hazard ratios, or calculating median survival times. By design, time to pregnancy is shorter in the conventional strategy than in the 'freeze all' strategy when the cumulative live birth rate is comparable, as embryo transfer is delayed in a 'freeze all' strategy (Zaat 2019).
Secondary outcomes concerning effectiveness
Although we reported pregnancy and live birth rates only cumulatively; for other outcomes, such as the number of multiples, the number of miscarriages and the obstetric, perinatal and neonatal outcomes, we reported the numbers cumulatively and also after the first embryo transfer. When the cumulative rates for these outcomes were reported in one or two of the included studies we also plotted the outcomes after first embryo transfer. Definitions used in the included studies for some of the secondary outcomes, especially miscarriages, differ between studies. The included data are insufficient to identify any differences in early or late miscarriage between both strategies.
Secondary outcomes concerning safety
In our review we found that women in the 'freeze all' strategy might have an increased risk of hypertensive disorders of pregnancy.
Several cohort studies (Ishihara 2014; Opdahl 2015; Sazonova 2012), and two recent meta‐analyses (Maheshwari 2018; Roque 2019), reported increased risk of hypertensive disorders of pregnancy after frozen embryo transfer compared to fresh embryo transfer. It is hypothesised that the risk of hypertensive disorders of pregnancy may relate to endometrial preparation with exogenous progesterone and prolonged estrogen use during artificial frozen embryo transfer cycles (Roque 2019). During the implantation period in early pregnancy, extravillous trophoblast cells are the key cells involved in uterine spiral arteriole remodelling during pregnancy, an event that is critical for a successful pregnancy outcome. Some studies suggest that aberrant progesterone levels in early pregnancy can lead to over‐invasion or an invasion defect of the extravillous trophoblast, which may possibly lead to serious complications such as hypertensive disorders of pregnancy (Esh‐Broder 2011; Schatz 2016).
Another hypothesis is that the increased risk of hypertensive disorders of pregnancy may be due to the missing circulating corpus luteum vasoactive products such as relaxin, vascular endothelial growth factor and angiogenic metabolites of estrogen. The absence of these vasoactive factors may lead to deficient circulatory adaptations during early gestation and therefore hypertensive disorders of pregnancy (Conrad 2011; Singh 2020; von Versen‐Höynck 2019a; von Versen‐Höynck 2019b). Recently, in line with this possible biological explanation, the results of two large cohort studies showed that the risk of hypertensive disorders of pregnancy is increased after cryopreserved embryo transfer in an artificial cycle compared to cryopreserved embryo transfer in a natural cycle (Ernstad 2019; Saito 2019).
In this review, seven of the included studies used only artificial cycles for frozen embryo transfer in the 'freeze all' strategy (Chen 2016; Ferraretti 1999; Santos‐Ribeiro 2020; Shapiro 2011a; Shapiro 2011b; Vuong 2018; Wong 2021), of which two studies (Chen 2016; Vuong 2018), reported on this outcome. Chen 2016 found a higher rate of pre‐eclampsia in the 'freeze all' strategy, Vuong 2018 found no difference between both groups. Frozen embryo transfers in the study by Wei 2019 were mainly performed in natural ovulatory cycles (63.7%) or in artificial cycles (36.4%); the type of treatment cycle was decided at the discretion of local physicians. Difference in hypertensive disorders of pregnancy between natural cycle frozen embryo transfer and artificial cycle embryo transfer was not reported. All seven studies used progesterone for luteal support in case of a pregnancy. Based on the results of the included studies in this review it is unclear whether the type of frozen embryo transfer and endometrial preparation is associated with increased risk of hypertensive disorders of pregnancy or whether there could be another explanation.
According to these studies, this over‐invasion or invasion defect of the extravillous trophoblast may also lead to abnormal placentation such as placenta accrete (Esh‐Broder 2011; Schatz 2016). In our current review we could not investigate whether the risk of abnormal placentation differs between the 'freeze all' strategy and the conventional IVF/ICSI strategy because none of the included RCTs reported on abnormal placentation. Regarding future research it would be of great interest to gain more knowledge about a possible difference between the strategies for this pregnancy complication.
The 'freeze all' strategy is associated with an increased risk of a higher birth weight in singleton babies. This finding is in agreement with a recently published meta‐analysis comparing frozen embryo transfer to fresh embryo transfer (Maheshwari 2012; Maheshwari 2018). The higher risk of large for gestational age after frozen embryo transfer is also applicable when compared to the general population (Luke 2017; Pinborg 2014; Spijkers 2017). It has been suggested that artificial endometrial preparation is associated with higher birth weights (Ernstad 2019; Roque 2019). In this review, seven of the included studies used only artificial cycles for frozen embryo transfer in the 'freeze all' strategy (Chen 2016; Ferraretti 1999; Santos‐Ribeiro 2020; Shapiro 2011a; Shapiro 2011b; Vuong 2018; Wong 2021), of which four studies (Chen 2016; Shapiro 2016; Wei 2019; Wong 2021), reported on this outcome. All of these five studies found a higher birth weight of babies born after the 'freeze all' strategy. Frozen embryo transfers in the study by Wei 2019 were mainly performed in natural ovulatory cycles (63.7%) or in artificial cycles (36.4%); the type of treatment cycle was decided at the discretion of local physicians. Difference in birth weight between natural cycle frozen embryo transfer and artificial cycle embryo transfer was not reported. All of the seven studies used progesterone for luteal support in case of a pregnancy. Based on the results of the included studies in this review it is unclear whether the type of endometrial preparation for frozen embryo transfer is associated with the increased risk of higher birth weight or whether there could be another explanation.
It has also been suggested that the freezing and thawing procedures or extended culture may play an independent role in the growth potential of the foetus due to epigenetic alterations at the early embryonic stages (Pinborg 2014).
The lower rate of small for gestational age in the 'freeze all' strategy in our review is in agreement with the findings of a recent meta‐analysis (Maheshwari 2018). It has been hypothesised that because of a state of hyperestrogenism ‐ due to hormonal stimulation of the ovaries ‐ at time of fresh embryo transfer abnormal endometrial angiogenesis occurs and may lead to reduced implantation as well as abnormal placentation. At the time of the frozen embryo transfer, the effect of the ovarian stimulation is worn off and therefore a frozen embryo transfer is performed in a more natural uterine environment compared to the fresh cycle (Healy 2010; Kansal Kalra 2011). However, this hypothesis would also account for findings such as more preterm deliveries and more low birth weight babies, which were not found in our meta‐analysis.
Authors' conclusions
Implications for practice.
We found moderate‐quality evidence showing that one strategy is probably not superior to the other in terms of cumulative live birth rate and ongoing pregnancy rate. The risk of ovarian hyperstimulation syndrome (OHSS) may be decreased in the 'freeze all' strategy. We could not pool data for time to pregnancy. We assume it is shorter using a conventional in vitro fertilisation (IVF) or intracytoplasmic sperm injection (ICSI) strategy in the case of similar cumulative live birth rate, as embryo transfer is delayed in a 'freeze all' strategy. The risk of maternal hypertensive disorders of pregnancy, of having a large‐for‐gestational‐age baby and a higher birth weight of the children born may be increased following the 'freeze all' strategy. We are uncertain if 'freeze all' strategy reduces the risk of miscarriage or multiple pregnancy rate compared to conventional IVF/ICSI with fresh embryo transfer.
Implications for research.
Based on moderate‐quality evidence we state that one strategy is probably not superior to the other in terms of cumulative live birth rate. In order to achieve high‐quality evidence, well designed randomised controlled trials (RCTs) reporting on cumulative live birth rate instead of live birth rate after the first transfer using adequate and universal reporting of outcomes should be performed. Time to a pregnancy leading to a live birth needs to be reported with the date of randomisation as the starting point, in a way that incorporates the follow‐up time of all randomised women. Time to pregnancy should be reported as Kaplan‐Meier curves, calculating hazard ratios, or calculating median survival times.
Regarding pregnancy outcomes and obstetric, perinatal and neonatal outcomes, future RCTs and large cohort studies should consider reporting neonatal outcomes not only per woman randomised but also per (live) birth per randomised arm. In this way, the crude rates become more comparable across studies, whereby better informed decisions can be made. To evaluate the effect of 'freeze all' on mothers and babies, an Individual Patient Data analysis including both RCTs and cohort studies may gain more insight into the differences in these outcomes we found in our review. Another possibility may be to use registries.
In future studies, participant characteristics (e.g. women with good prognosis versus poor prognosis), treatment characteristics (e.g. number of available embryos, number of embryos transferred, results for first and every subsequent transfer, time to pregnancy), and protocols used (e.g. timing/extended culture and method of cryopreservation, method of endometrial preparation for frozen embryo transfer) should be properly reported.
What's new
| Date | Event | Description |
|---|---|---|
| 23 September 2020 | New citation required but conclusions have not changed | The addition of the new studies has not led to change in conclusions |
| 23 September 2020 | New search has been performed | We updated the review. |
History
Protocol first published: Issue 7, 2014 Review first published: Issue 3, 2017
Acknowledgements
We would like to acknowledge the team at Cochrane Gynaecology and Fertility for their assistance, and especially Information Specialist Marian Showell for the literature search. We acknowledge the contribution of Kai Mee Wong to the previous version of this review. We would like to acknowledge the valuable feedback of our peer reviewers – Dr. Paraskevi Vogiatzi, Wellington Martins, Mamoona Javed and Jack Wilkinson. We would like to thank Kai Mee Wong and Sjoerd Repping for their contribution to previous versions of the review.
Appendices
Appendix 1. Cochrane Gynaecology and Fertility Group (CGFG) specialised register search strategy
Searched 23 September 2020
Procite platform
Keywords CONTAINS "cryopreservation" or "frozen embryo transfer" or "frozen embryos" or "frozen‐thawed cycle" or "frozen‐thawed embryo transfer" or "frozen‐thawed embryos" or "FET" or "cryopreserved embryos" or "cryopreserved‐thawed embryos" or "vitrified" or "vitrification" or "fresh v cryopreserved" or "freeze all" or "embryo vitrification" or "fresh versus frozen" or Title CONTAINS "cryopreservation" or "frozen embryo transfer" or "frozen embryos" or "frozen‐thawed cycle" or "frozen‐thawed embryo transfer" or "frozen‐thawed embryos" or "FET" or "cryopreserved embryos" or "cryopreserved‐thawed embryos" or "vitrified" or "vitrification" or "fresh v cryopreserved" or "freeze all" or "embryo vitrification" or "fresh versus frozen"
AND
Keywords CONTAINS "fresh" or "fresh blastocyst transfer" or "fresh cycle" or "fresh embryos" or "fresh v cryopreserved" or "fresh versus frozen" or Title CONTAINS "fresh" or "fresh blastocyst transfer" or "fresh cycle" or "fresh embryos" or "fresh v cryopreserved" or "fresh versus frozen"
117 records
Appendix 2. CENTRAL Register of Studies Online (CRSO) search strategy
Searched 23 September 2020
Web platform
#1 MESH DESCRIPTOR Embryo Transfer EXPLODE ALL TREES 1095 #2 MESH DESCRIPTOR Fertilization in Vitro EXPLODE ALL TREES 2060 #3 MESH DESCRIPTOR Sperm Injections, Intracytoplasmic EXPLODE ALL TREES 538 #4 embryo*: TI,AB,KY 7483 #5 (vitro fertili?ation):TI,AB,KY 3433 #6 ivf:TI,AB,KY 5639 #7 icsi:TI,AB,KY 2736 #8 (intracytoplasmic sperm injection*):TI,AB,KY 1925 #9 blastocyst*:TI,AB,KY 1284 #10 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 10823 #11 MESH DESCRIPTOR Cryopreservation EXPLODE ALL TREES 552 #12 MESH DESCRIPTOR Vitrification EXPLODE ALL TREES 42 #13 ((cryopreservat* or cryofixation or cryonic suspension)):TI,AB,KY 1011 #14 (freez* or frozen):TI,AB,KY 5794 #15 Vitrif*:TI,AB,KY 503 #16 Thaw*:TI,AB,KY 1117 #17 #11 OR #12 OR #13 OR #14 OR #15 OR #16 6782 #18 #10 AND #17 1692
Appendix 3. MEDLINE search strategy
Searched from 1946 until 23 September 2020
Ovid platform
1 exp Cryopreservation/ (37259) 2 exp Freezing/ (24066) 3 (cryopreservat$ or cryofixation or cryonic suspension).tw. (16154) 4 freez$.tw. (69378) 5 thaw$.tw. (26106) 6 exp Vitrification/ (1687) 7 Vitrif$.tw. (5311) 8 froze$.tw. (79302) 9 disengage$.tw. (5314) 10 or/1‐9 (178214) 11 exp embryo transfer/ or exp fertilization in vitro/ or exp sperm injections, intracytoplasmic/ or exp ovulation induction/ (49216) 12 embryo$.tw. (354686) 13 blastocyst$.tw. (22650) 14 vitro fertili?ation.tw. (23551) 15 ivf.tw. (24044) 16 icsi.tw. (8560) 17 intracytoplasmic sperm injection$.tw. (7331) 18 ovulation induc$.tw. (4181) 19 (ovar$ adj3 hyperstim$).tw. (5235) 20 (ovar$ adj3 stimulat$).tw. (8195) 21 exp Superovulation/ or Superovulat$.tw. (3960) 22 or/11‐21 (396906) 23 10 and 22 (14532) 24 randomized controlled trial.pt. (513703) 25 controlled clinical trial.pt. (93853) 26 randomized.ab. (493637) 27 placebo.tw. (217021) 28 clinical trials as topic.sh. (193027) 29 randomly.ab. (341750) 30 trial.ti. (225713) 31 (crossover or cross‐over or cross over).tw. (86227) 32 or/24‐31 (1346965) 33 exp animals/ not humans.sh. (4736958) 34 32 not 33 (1238835) 35 23 and 34 (793)
Appendix 4. Embase search strategy
Searched from 1980 until 23 September 2020
Ovid platform
1 exp Cryopreservation/ (41662) 2 exp Freezing/ (28402) 3 (cryopreservat$ or cryofixation or cryonic suspension).tw. (22526) 4 freez$.tw. (75296) 5 thaw$.tw. (33430) 6 exp Vitrification/ (6065) 7 Vitrif$.tw. (8400) 8 froze$.tw. (107125) 9 disengage$.tw. (6376) 10 or/1‐9 (216960) 11 exp embryo transfer/ or exp fertilization in vitro/ or exp intracytoplasmic sperm injection/ (70627) 12 in vitro fertili?ation.tw. (30829) 13 icsi.tw. (16299) 14 intracytoplasmic sperm injection$.tw. (9790) 15 (blastocyst adj2 transfer$).tw. (2451) 16 ivf.tw. (41016) 17 exp superovulation/ (2881) 18 superovulat$.tw. (3912) 19 exp ovulation induction/ (14554) 20 blastocyst$.tw. (30017) 21 embryo$.tw. (392171) 22 vitro fertili?ation.tw. (30870) 23 ovulation induc$.tw. (5650) 24 (ovar$ adj3 stimulat$).tw. (12319) 25 (ovar$ adj3 hyperstim$).tw. (7688) 26 or/11‐25 (449099) 27 10 and 26 (23805) 28 Clinical Trial/ (974756) 29 Randomized Controlled Trial/ (616533) 30 exp randomization/ (88043) 31 Single Blind Procedure/ (40177) 32 Double Blind Procedure/ (173052) 33 Crossover Procedure/ (64313) 34 Placebo/ (341414) 35 Randomi?ed controlled trial$.tw. (237064) 36 Rct.tw. (38374) 37 random allocation.tw. (2055) 38 randomly allocated.tw. (35997) 39 allocated randomly.tw. (2571) 40 (allocated adj2 random).tw. (826) 41 Single blind$.tw. (25257) 42 Double blind$.tw. (205370) 43 ((treble or triple) adj blind$).tw. (1191) 44 placebo$.tw. (307127) 45 prospective study/ (625354) 46 or/28‐45 (2232203) 47 case study/ (71852) 48 case report.tw. (411819) 49 abstract report/ or letter/ (1118194) 50 or/47‐49 (1590932) 51 46 not 50 (2177816) 52 27 and 51 (2370)
Appendix 5. PsycINFO search strategy
Searched from 1806 until 23 September 2020
Ovid platform
1 (cryopreservat$ or cryofixation or cryonic suspension).tw. (97) 2 freez$.tw. (4662) 3 thaw$.tw. (155) 4 Vitrif$.tw. (14) 5 froze$.tw. (1606) 6 disengage$.tw. (7954) 7 or/1‐6 (14202) 8 exp reproductive technology/ (1863) 9 icsi.tw. (74) 10 intracytoplasmic sperm injection$.tw. (57) 11 (blastocyst adj2 transfer$).tw. (4) 12 assisted reproduct$.tw. (998) 13 ovulation induc$.tw. (31) 14 (ovari$ adj2 stimulat$).tw. (58) 15 COH.tw. (132) 16 superovulat$.tw. (7) 17 infertil$.tw. (3600) 18 subfertil$.tw. (95) 19 (ovari$ adj2 induction).tw. (8) 20 ivf.tw. (576) 21 vitro fertili?ation.tw. (768) 22 (ovar$ adj3 hyperstimulat$).tw. (14) 23 or/8‐22 (5473) 24 7 and 23 (148) 25 random.tw. (59350) 26 control.tw. (450545) 27 double‐blind.tw. (23110) 28 clinical trials/ (11762) 29 placebo/ (5720) 30 exp Treatment/ (1056217) 31 or/25‐30 (1458404) 32 24 and 31 (66)
Appendix 6. CINAHL search strategy
Searched from 1961 to 23 September 2020
Ebsco platform
| # | Query | Results |
| S32 | S19 AND S31 | 310 |
| S31 | S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 | 1,355,174 |
| S30 | TX allocat* random* | 11,049 |
| S29 | (MH "Quantitative Studies") | 23,579 |
| S28 | (MH "Placebos") | 11,474 |
| S27 | TX placebo* | 59,611 |
| S26 | TX random* allocat* | 11,049 |
| S25 | (MH "Random Assignment") | 55,964 |
| S24 | TX randomi* control* trial* | 177,249 |
| S23 | TX ( (singl* n1 blind*) or (singl* n1 mask*) ) or TX ( (doubl* n1 blind*) or (doubl* n1 mask*) ) or TX ( (tripl* n1 blind*) or (tripl* n1 mask*) ) or TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) | 1,033,075 |
| S22 | TX clinic* n1 trial* | 252,733 |
| S21 | PT Clinical trial | 86,291 |
| S20 | (MH "Clinical Trials+") | 268,471 |
| S19 | S17 AND S18 | 1867 |
| S18 | S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 | 29,812 |
| S17 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 | 16,748 |
| S16 | TX ovulation induc* | 1732 |
| S15 | TX icsi | 1137 |
| S14 | TX ivf | 4531 |
| S13 | TX vitro fertili?ation | 6874 |
| S12 | TX blastocyst* | 2176 |
| S11 | TX embryo* | 22,594 |
| S10 | TX intracytoplasmic sperm injection* | 884 |
| S9 | (MM "Fertilization in Vitro") | 3392 |
| S8 | (MM "Embryo Transfer") | 1088 |
| S7 | TX disengage* | 2122 |
| S6 | TX frozen | 8196 |
| S5 | TX Vitrif* | 448 |
| S4 | TX thaw* | 1733 |
| S3 | TX freez* | 4646 |
| S2 | TX (cryopreservat* or cryofixation or cryonic suspension) | 2630 |
| S1 | (MH "Cryopreservation+") | 2443 |
Appendix 7. ClinicalTrials.gov search string
Web platform
Searched 23 September 2020
search terms
(IVF OR ICSI OR embryo transfer) AND (freeze‐all OR frozen thawed embryo transfer OR cryopreservation OR disengage)
Appendix 8. WHO ICTRP search string
Web platform
apps.who.int/trialsearch
Searched 23 September 2020
search terms
(IVF OR ICSI OR embryo transfer) AND (freeze‐all OR frozen thawed embryo transfer OR cryopreservation OR disengage)
Data and analyses
Comparison 1. Freeze‐all versus conventional IVF, outcomes per woman.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Cumulative live birth rate | 8 | 4712 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.95, 1.22] |
| 1.1.1 Live birth rate: cumulatively for cleavage stage transfer | 3 | 2415 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.93, 1.29] |
| 1.1.2 Live birth rate: cumulatively for blastocyst stage transfer | 5 | 2297 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.88, 1.27] |
| 1.2 Ovarian hyperstimulation syndrome (OHSS) | 6 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 1.2.1 Per cycle with ovarian hyperstimulation | 6 | 4478 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.26 [0.17, 0.39] |
| 1.3 Cumulative ongoing pregnancy rate | 4 | 1245 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.75, 1.19] |
| 1.3.1 Ongoing pregnancy rate: cumulatively for cleavage stage transfer | 1 | 782 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.78, 1.37] |
| 1.3.2 Ongoing pregnancy rate: cumulatively for blastocyst stage transfer | 3 | 463 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.54, 1.18] |
| 1.4 Cumulative clinical pregnancy rate | 4 | 1320 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.72, 1.16] |
| 1.4.1 Clinical pregnancy rate: cumulatively for cleavage stage transfer | 2 | 907 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.76, 1.32] |
| 1.4.2 Clinical pregnancy rate: cumulatively for blastocyst stage transfer | 2 | 413 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.44, 1.13] |
| 1.5 Ectopic pregnancy | 6 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 1.5.1 Ectopic pregnancy: cumulatively | 2 | 986 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.61 [0.31, 1.22] |
| 1.5.2 Ectopic pregnancy: after first embryo transfer | 5 | 4274 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.64 [0.39, 1.06] |
| 1.6 Miscarriage | 8 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 1.6.1 Miscarriage rate: cumulatively | 2 | 986 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.06 [0.72, 1.55] |
| 1.6.2 Miscarriage rate: after first embryo transfer | 8 | 4569 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.90 [0.76, 1.07] |
| 1.7 Multiple pregnancy | 5 | 5252 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.10 [0.92, 1.31] |
| 1.7.1 Multiple pregnancy rate: cumulatively | 2 | 986 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.88 [0.61, 1.25] |
| 1.7.2 Multiple pregnancy rate: after first embryo transfer | 5 | 4266 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.18 [0.96, 1.45] |
| 1.8 Gestational diabetes mellitus | 3 | 4722 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.26 [0.92, 1.71] |
| 1.8.1 Gestational diabetes mellitus: cumulatively | 1 | 782 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.83 [0.36, 1.94] |
| 1.8.2 Gestational diabetes mellitus: after first embryo transfer | 3 | 3940 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.34 [0.96, 1.86] |
| 1.9 Hypertensive disorders of pregnancy | 3 | 4722 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.80 [1.23, 2.64] |
| 1.9.1 Hypertensive disorders of pregnancy: cumulatively | 1 | 782 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.70 [0.27, 1.82] |
| 1.9.2 Hypertensive disorders of pregnancy: after first embryo transfer | 3 | 3940 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.15 [1.42, 3.25] |
| 1.10 Preterm delivery (< 37 weeks of gestational age) | 4 | 4926 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.00 [0.80, 1.25] |
| 1.10.1 Preterm delivery (< 37 weeks of gestational age): cumulatively | 2 | 986 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.62 [0.39, 0.99] |
| 1.10.2 Preterm delivery (< 37 weeks of gestational age): after first embryo transfer | 3 | 3940 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.15 [0.89, 1.50] |
| 1.11 Perinatal and neonatal death | 2 | 3072 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.18 [0.40, 3.51] |
| 1.11.1 Perinatal and neonatal death: cumulatively | 1 | 782 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.13 [0.01, 1.30] |
| 1.11.2 Perinatal and neonatal death: after first embryo transfer | 2 | 2290 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.27 [0.65, 7.84] |
| 1.12 Neonatal hospitalisation (> 3 days or neonatal intensive care unit admission) | 3 | 4722 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.35 [1.06, 1.72] |
| 1.12.1 Neonatal hospitalisation (> 3 days or neonatal intensive care unit admission): cumulatively | 1 | 782 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.00 [0.29, 3.48] |
| 1.12.2 Neonatal hospitalisation (> 3 days or neonatal intensive care unit admission): after first embryo transfer | 3 | 3940 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.37 [1.07, 1.75] |
| 1.13 Large for gestational age (birth weight > 90th percentile) | 3 | 4722 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.96 [1.52, 2.53] |
| 1.13.1 Large for gestational age (birth weight > 90th percentile): cumulatively | 1 | 782 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.97 [0.63, 6.15] |
| 1.13.2 Large for gestational age (birth weight > 90th percentile): after first embryo transfer | 3 | 3940 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.96 [1.51, 2.55] |
| 1.14 Small for gestational age (birth weight < 10th percentile) | 3 | 4722 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.77 [0.61, 0.97] |
| 1.14.1 Small for gestational age (birth weight < 10th percentile): cumulatively | 1 | 782 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.36 [0.16, 0.80] |
| 1.14.2 Small for gestational age (birth weight < 10th percentile): after first embryo transfer | 3 | 3940 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.82 [0.65, 1.05] |
| 1.15 Congenital abnormalities per live‐born children | 3 | 1789 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.08 [0.65, 1.78] |
| 1.16 Birth weight of babies born | 5 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.16.1 Birth weight of singletons | 5 | 1607 | Mean Difference (IV, Fixed, 95% CI) | 127.44 [77.08, 177.80] |
| 1.16.2 Birth weight of multiples | 4 | 804 | Mean Difference (IV, Fixed, 95% CI) | 49.46 [‐21.15, 120.08] |
Comparison 2. Freeze‐all versus conventional IVF, adverse events per clinical pregnancy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Multiple pregnancy: after first embryo transfer | 5 | 2223 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.87, 1.36] |
| 2.2 Miscarriage: after first embryo transfer | 8 | 2451 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.66, 1.02] |
Comparison 3. Additional analysis: freeze‐all versus conventional IVF, live birth rate after first transfer.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Additional analysis: live birth rate after first transfer per randomised woman | 13 | 7766 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.17 [1.06, 1.28] |
3.1. Analysis.

Comparison 3: Additional analysis: freeze‐all versus conventional IVF, live birth rate after first transfer, Outcome 1: Additional analysis: live birth rate after first transfer per randomised woman
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aflatoonian 2018.
| Study characteristics | ||
| Methods | Multicentre RCT Conducted: in 3 fertility clinics throughout Iran Enrolment: January 2014‐January 2017 Power calculation: stated Randomisation: computer‐generated random numbers in wrapped, unlabeled envelope each holding a unique number Timing randomisation: at day of oocyte retrieval Nature of intervention: day‐2 embryo cryopreservation by means of vitrification Follow‐up: LBR after the first ET |
|
| Participants | 240 women (121 freeze‐all, 119 control) Inclusion criteria:
Exclusion criteria were: women with < 14 and > 25 follicles ≥ 12 mm on the day of trigger, women with a previous history of OHSS development, endocrine disorders and > 40 years of age |
|
| Interventions | In the fresh transfer group, 2 embryos of good or excellent quality were transferred 48‐72 h after oocyte retrieval. In the fresh transfer group, 1500 IU hCG was administered on the day of ET. Moreover, progesterone suppositories 400 mg twice daily were administered vaginally, from the day of oocyte retrieval until the observation of fetal heart activity by ultrasound in the 8th week. In the freeze‐all group embryos were vitrified on day 2 after oocyte collection. The subsequent cycle was considered as a study cycle. The endometrium was artificially prepared prior to transfer in freeze‐all group. ET was performed 3 days after the beginning of progesterone administration. | |
| Outcomes | Primary outcome was clinical pregnancy, defined as observation of fetal heart activity by transvaginal ultrasonography 2‐3 weeks after positive β‐hCG. Secondary outcomes included chemical pregnancy, LBR, OHSS development and perinatal data. | |
| Notes | Funding: by Yazd Reproductive Sciences Institute. 2 of the authors reported conflicts of interest because they received unrestricted research grants from MSD, Merck and Ferring, as well as honoraria for lectures. We requested additional information regarding cumulative data from the study authors by email but we did not receive a response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | Wrapped, unlabeled envelope each holding a unique number |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of doctors and participants was not possible due to the nature of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessor blinding was not reported, however primary outcome is not likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were analysed for all randomised women. |
| Selective reporting (reporting bias) | Unclear risk | All registered outcomes were reported. Study was registered in a prospective trials register with the trial number: IRCT2016092224512N4. However, the study was registered while recruiting as it is stated in the trials register. |
| Other bias | Unclear risk | (Cumulative) data per subsequent menstrual or cryo‐transfer cycle not reported (relevant for time‐to‐pregnancy comparison and the related comparison of results after first transfer in frozen group vs results after first 2 transfers in fresh group). |
Aghahosseini 2017.
| Study characteristics | ||
| Methods | Multicentre RCT Conducted: in 2 fertility clinics in Teheran, Iran Enrolment: January 2016‐April 2016 Power calculation: stated Randomisation: computer‐generated random numbers in wrapped, unlabeled envelope each holding a unique number Timing randomisation: at day of oocyte retrieval, after retrieval Nature of intervention: blastocyst (day 5) cryopreservation by means of vitrification Follow‐up: LBR after the first ET |
|
| Participants | 72 women (36 freeze‐all, 36 control) Inclusion criteria:
Exclusion criteria were: uterine anomaly or previous uterine surgery, oocyte donation, azoospermia, severe endometriosis, previous chemotherapy or radiotherapy, conditions affecting the reproductive status. |
|
| Interventions | In the conventional IVF strategy group, a total of 1 or 2 blastocysts (grade A) were transferred at 5th day. Luteal phase support was carried out for all participants. In the freeze‐all strategy, all embryos were cryopreserved by vitrification and after 2 menstrual cycles, artificial endometrial preparation was performed. A total of 1 or 2 grade A thawed blastocysts were transferred. | |
| Outcomes | Primary outcome was clinical pregnancy, defined as a gestational sac with a live fetus on ultrasound 5 weeks after transfer. Secondary outcomes were not specified in the manuscript. | |
| Notes | Funding was not reported. We requested additional information regarding cumulative data from the authors by email but we did not receive a response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Authors state that women were randomly allocated using random allocation software, no further information provided. |
| Allocation concealment (selection bias) | Unclear risk | Authors state that women were randomly allocated using random allocation software, no further information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of doctors and participants was not possible due to the nature of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessor blinding was not reported, however primary outcome is not likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were analysed for all randomised women. |
| Selective reporting (reporting bias) | Unclear risk | Study was registered in a prospective trials register with the trial number: IRCT2016122131508N1. However, the study was registered while recruiting as it is stated in the trials register. |
| Other bias | Unclear risk | (Cumulative) data per subsequent menstrual or cryo‐transfer cycle not reported (relevant for time‐to‐pregnancy comparison and the related comparison of results after first transfer in frozen group vs results after first 2 transfers in fresh group). |
Chen 2016.
| Study characteristics | ||
| Methods | Multicentre RCT Conducted: in 14 reproductive medical centres throughout China Enrolment: June 2013‐May 2014 Power calculation: stated Randomisation: an online central randomisation system (www.medresman.org) was used Timing randomisation: at day of oocyte retrieval Nature of intervention: day‐3 embryo cryopreservation by means of vitrification. Local investigators had the option to transfer day‐2 embryos if there were < 3 embryos on day 2 Follow‐up: cumulative live birth (including all FETs performed within 12 months after the initial transfer) |
|
| Participants | 1508 women (746 freeze‐all, 762 control) Inclusion criteria:
Exclusion criteria: history of unilateral oophorectomy, recurrent spontaneous abortion (defined as ≥ 3 previous spontaneous pregnancy losses), congenital or acquired uterine malformations, abnormal results on parental karyotyping, or medical conditions that contraindicated ART or pregnancy |
|
| Interventions | For women who were assigned to the fresh embryo group, on day 3, 2 high‐quality embryos were picked out for fresh transfer and supernumerary embryos were transferred by means of vitrification. For women who were assigned to the FET group, there was no fresh transfer as all day‐3 embryos were cryopreserved for later transfer. Local investigators had the option to transfer day‐2 embryos if there were < 3 embryos on day 2. In cycles following the menstrual cycle with ovum pick‐up, after artificial endometrial preparation, on day 4 of the progesterone regimen, 2 day‐3 frozen embryos were thawed and transferred. |
|
| Outcomes | Primary outcome was a live birth, defined as delivery of any viable infant at ≥ 28 weeks of gestation during the first ET. Prespecified secondary outcomes included biochemical pregnancy, clinical pregnancy, ongoing pregnancy, singleton LBR, cLBR (including subsequent FET), pregnancy loss, moderate or severe OHSS, ectopic pregnancy, pregnancy and neonatal complications, and congenital anomalies. | |
| Notes | Funding: supported by a grant from the National Basic Research Program of China, by grants from the National Natural Science Foundation of China, and by grants from the Thousand Talents Program (to Drs. Legro and H. Zhang). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | An online central randomisation system (www.medresman.org) was used to automatically generate the assignment sequence |
| Allocation concealment (selection bias) | Low risk | Assignment sequence was unknown to the clinical investigators |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of doctors and participants was not possible due to the nature of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessor blinding was not reported, however primary outcome is not likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were analysed for all randomised women. |
| Selective reporting (reporting bias) | High risk | Some prespecified outcomes (e.g. time to pregnancy) were missing from the report |
| Other bias | Unclear risk | Not reported on blinding of doctors to interim analyses of outcomes of the study. Blinding of investigators was not reported (which is relevant for determining end of study). |
Coates 2017.
| Study characteristics | ||
| Methods | Single‐centre RCT Conducted: Oregon Reproductive Medicine Center Enrolment: December 2013‐August 2015 Power calculation: stated Randomisation: stratified block randomisation sequence was prepared by a professional third party (sealedenvelope.com). The allocation sequence was stratified for female age (< 35, 35–37, 38–40, and 41–42 years) and number of prior ART cycles (%2 or R3). Timing of randomisation: at time of hCG administration (trigger) Nature of intervention: day‐6 embryo cryopreservation by means of vitrification. Follow‐up: LBR after the first ET |
|
| Participants | 179 women (91 freeze‐all, 88 control) Inclusion criteria:
Exclusion criteria: the need to use surgically retrieved sperm (microsurgical epididymal sperm aspiration (MESA) or testicular sperm aspiration (TESA)), women using preimplantation genetic diagnosis for a single‐gene or chromosomal disorder, egg donor cycles, gender selection cycles, decreased ovarian reserve indicated by early follicular phase serum FSH level > 10 IU/L or random serum AMH level < 1 ng/mL, and any medical reasons occurring before recruitment that would not allow a participant to undergo a fresh ET such as the need for uterine surgery before transfer. |
|
| Interventions | Assisted hatching was performed on all embryos on day 3 after retrieval. The embryos were transferred back to culture media until day 5 or day 6 of development. Embryos were biopsied for PGS on day 5 or 6, based on the development of the hatching process. women had either 1 or 2 embryos transferred depending on availability of euploid embryos and woman's request. Women assigned to the conventional strategy received 1 or 2 fresh embryos on day 6, any remaining embryos were frozen. In the FET group, there was no fresh transfer and available embryos had been cryopreserved on day 6 by means vitrification. In menstrual cycles following the ovum pick‐up the endometrium was artificially prepared for transfer using estradiol and progesterone for transfer of a maximum of 2 embryos. |
|
| Outcomes | The primary outcomes were: implantation rates (number of gestational sacs divided by the number of embryos transferred per group), ongoing pregnancy rates (defined as a pregnancy beyond 8 weeks), and LBR after the first ET. No prespecified secondary outcomes were stated in the manuscript. In the trial registration the following secondary outcome was stated: determining retrospectively if mitochondrial DNA content is linked to implantation potential and if that is measurable by NGS. |
|
| Notes | Funding: supported by Life Technologies, Carlsbad, CA; Oregon Reproductive Medicine, Portland; and Reprogenetics, NJ We requested additional information regarding cumulative data from the authors by email but we did not receive a response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified block randomisation sequence was prepared by a professional third party (sealedenvelope.com). |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of doctors and participants was not possible due to the nature of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessor blinding was not reported, however primary outcome is not likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were analysed for all randomised women. |
| Selective reporting (reporting bias) | Unclear risk | Not all registered outcomes were reported (correlation of mitochondrial DNA and implantation) Not all reported outcomes were registered (ongoing pregnancy rates, and LBR after the first ET) Study was registered in a prospective trials register with the trial number: NCT02000349. |
| Other bias | Unclear risk | 3 of the authors disclosed to be co‐owner of Oregon Reproductive Medicine. 2 of the authors reported to be founding partner for Reprogenetics. (Cumulative) data per subsequent menstrual or cryo‐transfer cycle not reported (relevant for time‐to‐pregnancy comparison and the related comparison of results after first transfer in FET group vs results after first 2 transfers in fresh group). |
Ferraretti 1999.
| Study characteristics | ||
| Methods | Single‐centre RCT Conducted: in Italy Enrolment: January 1996‐July 1997 Power calculation: not reported Randomisation: allocation was performed with sealed envelopes Timing of randomisation: not reported Nature of intervention: slow freezing Follow‐up: until no cryopreserved embryos were left or delivery of child |
|
| Participants | 125 women (58 freeze‐all, 67 control) Inclusion criteria: all women with a high level of oestradiol the day of hCG administration (oestradiol ≥ 1500 pg/mL or ≥ 5.500 mmol/mL (conversion factor to SI unit 53.671)) and a high number of retrieved eggs (≥ 15 oocytes) |
|
| Interventions | Intervention: zygotes were cryopreserved, 3 or 4 zygotes were thawed and cultured for 36‐40 h before ET. If ≥ 2 zygotes did not cleave 24 h after being cultured, 1 or 2 additional zygotes were thawed. Control: zygotes were cultured for a subsequent 48 h, 3 or 4 fresh embryos were transferred, surplus embryos were cryopreserved |
|
| Outcomes | Clinical pregnancies: gestational sac and fetal heartbeat by ultrasound | |
| Notes | Funding was not reported. Additional information was obtained from the study authors by email. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information about method of randomisation available. Randomisation was used, but it is unclear how. |
| Allocation concealment (selection bias) | Unclear risk | No information about method of allocation concealment available. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of doctors and participants was not possible due to the nature of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessor blinding was not reported, however primary outcome is not likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were analysed for all randomised women. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. This trial was not registered because these registries did not exist at the time this study was performed. |
| Other bias | High risk | No power calculation reported. Unclear what determined the end of study. (Cumulative) data per subsequent menstrual or cryo‐transfer cycle not reported (relevant for time‐to‐pregnancy comparison and the related comparison of results after first transfer in FET group vs results after first 2 transfers in fresh group). |
Santos‐Ribeiro 2020.
| Study characteristics | ||
| Methods | Single‐centre RCT Conducted: in Belgium Enrolment: May 2014‐2017 Power calculation: stated Randomisation: performed using a computer‐generated randomisation list (SPSS Version 20VR, IBM Corporation, New York, USA). Each entry of the list was sealed in a sequentially numbered opaque envelope and allocated in that order to women. Participating physicians did not have access to the randomisation list. Timing of randomisation: prior to oocyte retrieval Nature of intervention: vitrification Follow‐up: 24 months after randomisation |
|
| Participants | 212 women (106 freeze‐all, 106 control) Inclusion criteria: women with an excessive response to ovarian stimulation (≥ 18 follicles measuring ≥ 11 mm on the day of the GnRH triggering), GnRH antagonist suppression, age between 18‐40 years, first/second ART cycle in the centre, planned placement of 1 or 2 blastocysts. |
|
| Interventions | Intervention: all viable embryos were vitrified, preferably at blastocyst stage (Day 5 or 6), according to the threshold of good‐quality embryos available on day 3. After thaw, 1 or 2 frozen embryo(s) were transferred, scheduled according to the developmental stage of the embryo. Control: fresh transfer of day 3 or 5 of development with preference to the latter whenever at least 4 good‐quality embryos were available on day 3. In women who were assigned to the fresh embryo group, luteal‐phase support was started immediately after oocyte retrieval and was continued until the day of serum hCG testing. On day 2 or 3 of the embryo culture, up to 2 embryos were selected and transferred. In women who were assigned to the FET group, all the embryos were vitrified. 2 good‐quality embryos were vitrified on day 2 or day 3, and the other embryos could be vitrified at the cleavage or blastocyst stage. At the second spontaneous menstrual cycle after oocyte retrieval, natural ovulation was monitored by means of ultrasonography. Luteal‐phase support was started from the day of ovulation. Up to two day 2 or day 3 frozen embryos were thawed and transferred 2 or 3 days, after ovulation. If the natural ovulation cycle was cancelled owing to anovulation or poor endometrial development, an artificial cycle was used for endometrial preparation in the next menstrual cycle. |
|
| Outcomes |
|
|
| Notes | Funding: this research received no specific grant from any funding agency in the public, commercial or not‐for‐profit sectors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | By means of a computer‐generated randomisation list (SPSS Version 20VR, IBM Corporation, New York, USA) |
| Allocation concealment (selection bias) | Low risk | Each entry of the list was sealed in a sequentially numbered opaque envelope and allocated in that order to women. Participating physicians did not have access to the randomisation list. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of doctors and participants was not possible due to the nature of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessor blinding was not reported, however primary outcome is not likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were analysed for all randomised women according to ITT. |
| Selective reporting (reporting bias) | Low risk | All registered outcomes reported. Study was registered in a prospective trials register with the trial number: NCT02148393. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Shapiro 2011a.
| Study characteristics | ||
| Methods | Single‐centre RCT Conducted: USA Enrolment: October 2007‐October 2010 Power calculation: stated. However, study was prematurely terminated after interim analysis Randomisation: performed by random drawing among identical, opaque, unmarked sealed envelopes Timing of randomisation: after oocyte retrieval Nature of intervention: slow freezing Follow‐up: clinical pregnancy after first ET |
|
| Participants | 137 women (70 freeze‐all, 67 fresh transfer) Inclusion criteria:
Exclusion criteria: genetic testing of embryos was excluded. |
|
| Interventions | Intervention: 2pn oocytes were frozen, and entire cohorts of frozen 2pn oocytes were thawed and subsequently cultured to the blastocyst stage. The morphologically best 1 or 2 blastocysts were transferred on the first day on which at least 1 good expanded blastocyst appeared. Supernumerary expanded blastocysts of high quality were cryopreserved. Control: fresh blastocysts transfer |
|
| Outcomes |
|
|
| Notes | Funding: research grant from the Investigator‐Initatiated trial research grant from Ferring Pharmaceuticals, Parsippany, NJ. Medications for this study were also provided by Ferring Pharmaceuticals. Time period was obtained from trials register Additional information was obtained from study authors by email. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not reported. |
| Allocation concealment (selection bias) | Low risk | Drawing randomly among identical, opaque, unmarked, sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of doctors and participants was not possible due to the nature of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessor blinding was not reported, however primary outcome is not likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Data were not reported for all women randomised, but per transfer. Dropouts and loss to follow‐up were not accounted for in the analysis. No ITT analysis was performed. Sufficient data available for analysis per woman in meta‐analysis. Ongoing pregnancy was determined at 10 weeks' gestation instead of 12 weeks' gestation. |
| Selective reporting (reporting bias) | Low risk | All registered outcomes reported Study was registered in a prospective trials register with the trial number: NCT00963625. |
| Other bias | Unclear risk | Study was pre‐terminated after interim analysis. Interim analysis was preplanned, but calculated per transfer (unit of analysis error) with a P value of 0.03, overestimating possible effects. (Cumulative) data per subsequent menstrual or cryo‐transfer cycle not reported (relevant for time‐to‐pregnancy comparison and the related comparison of results after first transfer in FET group vs results after first 2 transfers in fresh group). |
Shapiro 2011b.
| Study characteristics | ||
| Methods | Single‐centre RCT Conducted: USA Enrolment: July 2007‐July 2010 Power calculation: stated (referred to Shapiro 2011a). However, study was terminated because of differing embryo quality between the 2 groups. Randomisation: performed by random drawing among identical, opaque, unmarked, sealed envelopes Timing of randomisation: after oocyte retrieval Nature of intervention: slow freezing Follow‐up: clinical pregnancy after 1 ET |
|
| Participants | 122 women (60 freeze‐all, 62 control) Inclusion criteria:
Exclusion criteria: genetic testing of embryos was excluded. |
|
| Interventions | Intervention: 2pn oocytes were frozen, and entire cohorts of frozen 2pn oocytes were thawed and subsequently cultured to the blastocyst stage. The morphologically best 1 or 2 blastocysts were transferred on the first day on which at least 1 good expanded blastocyst appeared. Supernumerary expanded blastocysts of high quality were cryopreserved. Control: fresh blastocysts transfer |
|
| Outcomes |
|
|
| Notes | Funding: research grant from the Investigator‐Initatiated Studies Program of Merck Sharp & Dohme Time period was obtained from trial register. Additional information was obtained from study authors by email. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not reported. |
| Allocation concealment (selection bias) | Low risk | Drawing randomly among identical, opaque, unmarked, sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of doctors and participants was not possible due to the nature of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessor blinding was not reported, however primary outcome is not likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Data were not reported for all women randomised, but per transfer. Dropouts and loss to follow‐up were not accounted for in the analysis. No ITT analysis was performed. Sufficient data available for analysis per woman in meta‐analysis. Ongoing pregnancy was determined at 10 weeks' gestation instead of 12 weeks' gestation. |
| Selective reporting (reporting bias) | Low risk | All registered outcomes reported Study was registered in a prospective trials register with the trial number: NCT00963079. |
| Other bias | Unclear risk | Trial was pre‐terminated after interim analysis. Interim analysis was preplanned, but calculated per transfer (unit of analysis error) with a P value of 0.03, overestimating possible effects. Stopping rules for interim analysis (embryo quality) were unclear. (Cumulative) data per subsequent menstrual or cryo‐transfer cycle not reported (relevant for time‐to‐pregnancy comparison and the related comparison of results after first transfer in FET group vs results after first 2 transfers in fresh group). |
Shapiro 2016.
| Study characteristics | ||
| Methods | Post hoc analysis of the results of 2 single‐centre RCTs (Shapiro 2011a and Shapiro 2011b) Conducted: USA Enrolment: July 2007‐July 2010 Power calculation: stated (referred to Shapiro 2011a). However, study was terminated because of differing embryo quality between the 2 groups. Randomisation: performed by random drawing among identical, opaque, unmarked, sealed envelopes Timing of randomisation: after oocyte retrieval Nature of intervention: slow freezing Follow‐up: live birth, birth weight |
|
| Participants | The 2 combined RCTs included 259 women (130 freeze‐all, 129 control) Inclusion criteria:
Exclusion criteria: genetic testing of embryos was excluded. |
|
| Interventions | Intervention: 2pn oocytes were frozen, and entire cohorts of frozen 2pn oocytes were thawed and subsequently cultured to the blastocyst stage. The morphologically best 1 or 2 blastocysts were transferred on the first day on which at least 1 good expanded blastocyst appeared. Supernumerary expanded blastocysts of high quality were cryopreserved. Control: fresh blastocysts transfer |
|
| Outcomes |
|
|
| Notes | Funding was not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Drawing randomly among identical, opaque, unmarked, sealed envelopes. |
| Allocation concealment (selection bias) | Low risk | Drawing randomly among identical, opaque, unmarked, sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of doctors and participants was not possible due to the nature of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessor blinding was not reported, however primary outcome is not likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Data were not reported for all women randomised, but per transfer. Dropouts and loss to follow‐up were not accounted for in the analysis. No ITT analysis was performed. Sufficient data available for analysis per woman in meta‐analysis. Ongoing pregnancy was determined at 10 weeks' gestation instead of 12 weeks' gestation. |
| Selective reporting (reporting bias) | Low risk | All registered outcomes reported. Both studies used for this follow‐up study were registered in a prospective trials register with the trial numbers NCT00963625 and NCT00963079. |
| Other bias | Unclear risk | Trial was pre‐terminated after interim analysis. Interim analysis was preplanned, but calculated per transfer (unit of analysis error) with a P value of 0.03, overestimating possible effects. Stopping rules for interim analysis (embryo quality) were unclear. (Cumulative) data per subsequent menstrual or cryo‐transfer cycle not reported (relevant for time‐to‐pregnancy comparison and the related comparison of results after first transfer in FET group vs results after first 2 transfers in fresh group). |
Shi 2018.
| Study characteristics | ||
| Methods | Multicenter RCT Conducted: 20 reproductive medical centres throughout China Enrolment: March 2015‐March 2017 Power calculation: stated Randomisation: by means of an online central randomisation system (www.medresman.org) Timing of randomisation: on the day of oocyte retrieval Nature of intervention: day 2 or 3 embryo cryopreservation by means of vitrification Follow‐up: LBR after the first ET |
|
| Participants | 2157 women (1077 freeze‐all, 1080 control) Inclusion criteria:
Exclusion criteria: women with a history of unilateral oophorectomy, recurrent spontaneous abortion, diagnosis of the PCOS, or uterine abnormality (e.g. Müllerian duct anomaly, adenomyosis, submucous myoma, intra‐uterine adhesion, or scarred uterus) were excluded. Women were also excluded if they had a chronic medical condition that has been associated with adverse pregnancy outcomes, such as hypertension, symptomatic heart disease, diabetes mellitus, liver disease or dysfunction (according to the results of serum liver‐enzyme testing), renal disease or abnormal renal function, severe anaemia, history of deep venous thrombosis, pulmonary embolus, or cerebrovascular accident. All the couples were screened with the use of karyotyping, and those with an abnormal karyotype were excluded. |
|
| Interventions | In women who were assigned to the fresh embryo group, luteal‐phase support with was started immediately after oocyte retrieval and was continued until the day of serum hCG testing. On day 2 or 3 of the embryo culture, up to 2 embryos were selected and transferred. In women who were assigned to the FET group, all the embryos were vitrified. 2 good‐quality embryos were vitrified on day 2 or day 3, and the other embryos could be vitrified at the cleavage or blastocyst stage. At the second spontaneous menstrual cycle after oocyte retrieval, natural ovulation was monitored by means of ultrasonography. Luteal‐phase support was started from the day of ovulation. Up to 2 day 2 or day 3 frozen embryos were thawed and transferred 2 or 3 days after ovulation. If the natural ovulation cycle was cancelled owing to anovulation or poor endometrial development, an artificial cycle was used for endometrial preparation in the next menstrual cycle. | |
| Outcomes | The primary outcome was: a live birth after the first ET. Prespecified secondary efficacy outcomes included biochemical pregnancy, implantation, clinical pregnancy, ongoing pregnancy, pregnancy loss, and birth weight. Safety outcomes included moderate or severe OHSS, ectopic pregnancy, congenital anomaly, and obstetric and perinatal complications (i.e. gestational diabetes, gestational hypertension, pre‐eclampsia, placenta praevia, placental abruption, preterm delivery, neonatal hospitalisation for > 3 days, and perinatal death). |
|
| Notes | Funded by the National Key Research and Development Program of China and the National Natural Science Foundation of China. We requested additional information regarding cumulative data from the authors by email but we did not receive a response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | By means of an online central randomisation system (www.medresman.org). |
| Allocation concealment (selection bias) | Low risk | By means of an online central randomisation system (www.medresman.org). The randomisation sequence was generated and kept by the data‐coordinating centre and was not accessible to the investigators who enrolled women. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of doctors and participants was not possible due to the nature of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessor blinding was not reported, however primary outcome is not likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were analysed for all randomised women. |
| Selective reporting (reporting bias) | Low risk | All registered outcomes were reported. Protocol publication. Study was registered in a prospective trials register with the trial number: ChiCTR‐IOR‐14005406. |
| Other bias | Unclear risk | (Cumulative) data per subsequent menstrual or cryo‐transfer cycle not reported (relevant for time‐to‐pregnancy comparison and the related comparison of results after first transfer in FET group vs results after first 2 transfers in fresh group). |
Stormlund 2020.
| Study characteristics | ||
| Methods | Multicenter RCT Conducted: 8 clinical sites in Denmark, Sweden and Spain Enrolment: May 2016‐September 2018 Power calculation: stated Randomisation: by means of a computerised randomisation program running a minimisation algorithm performed by a study nurse or non‐treating physician Timing of randomisation: on inclusion day (cycle day 2 or 3) before initiation of ovarian stimulation, and was blinded until the day of ovulation triggering so that ovarian stimulation was not influenced by the result Nature of intervention: day 5 or 6 embryo blastocyst vitrification (depending on embryo development) Follow‐up: LBR after the first ET, cumulative data will be accounted for in separate publications |
|
| Participants | 460 women (230 freeze‐all, 230 control) Inclusion criteria: were present during ovarian stimulation
Exclusion criteria: women with a diagnosis of endometriosis (stage III or IV), uterine abnormalities and submucosal fibroids, or dysregulated thyroid disease. Additionally, women with any severe comorbidity potentially associated with adverse pregnancy outcomes, such as insulin dependent or non‐insulin dependent diabetes mellitus, gastrointestinal, cardiovascular, pulmonary, liver, or kidney disease. Couples that required testicular sperm aspiration or oocyte donation were also excluded from participation. Women were only allowed to participate once. |
|
| Interventions | Final oocyte maturation was induced by administering 0.5 mg of a GnRH agonist (buserelin: Suprefact, Sanofi) in the FET group or 250 μg of hCG (choriogonadotropin alpha: Ovitrelle, Merck) in the fresh transfer group. Women randomised to the fresh transfer group received GnRH agonist triggering if > 18 follicles with a mean diameter > 11 mm were present on the day of ovulation triggering to prevent ovarian hyperstimulation syndrome as predefined in the protocol. Consequently, the first single blastocyst transfer was postponed to a subsequent modified natural FET cycle. All fertilised oocytes were cultured to the blastocyst stage and assessed according to the classification system by Gardner and Schoolcraft. Day‐5 blastocysts with a Gardner score of ≥ 3BB were considered to be good quality and suitable for transfer or vitrification. Additionally, day‐6 blastocysts with a Gardner score of ≥ 4BB were considered suitable for vitrification. If only suitable day‐6 blastocysts were present in the fresh transfer group, the first single blastocyst transfer was postponed until a subsequent modified natural FET cycle. In the fresh transfer group, the blastocyst with the highest ranking was transferred on day 5 of embryo culture. Luteal phase support was administered from day 2 after oocyte retrieval with vaginal progesterone and continued until a hCG test was performed 11 days after blastocyst transfer. In the FET group, blastocyst vitrification was done on day 5 or 6, depending on embryo development. The highest ranking blastocyst was graded and marked before vitrification using the same criteria as in the fresh transfer group. For FET, endometrial preparation was done in a modified natural cycle regimen, which meant that a single injection of 250 μg of hCG was administered as soon as the leading follicle was > 17 mm in the natural cycle. No luteal phase support was given. |
|
| Outcomes | The primary outcome was ongoing pregnancy rate per randomised participant, which also included natural conceptions (defined as a detectable fetal heart beat after 8 weeks of gestation). Ongoing pregnancy rate was recorded per randomised participant, per started stimulation, per oocyte retrieval, and per ET. Secondary outcomes were positive hCG rates (biochemical pregnancies), LBRs, pregnancy‐related complications, obstetric complications, and prevalence of OHSS, which included women who had ascites puncture and those admitted to hospital with the condition. For pregnancies that continued beyond 22 weeks, pregnancy‐related, obstetrical, and neonatal outcomes were recorded, including infants born small for gestational age or large for gestational age. Small for gestational age and large for gestational age were calculated from growth curves for Scandinavian children adjusted for sex and gestational age. Post hoc analysis was performed for selected obstetric outcomes (pregnancy‐induced hypertension, gestational diabetes, chorioamnionitis, postpartum haemorrhage, induction of birth, mode of birth (vaginal delivery or caesarean section), twin rates, and duration of hospital stay). |
|
| Notes | Funding: the study is part of the Reprounion collaborative study, co‐financed by the European Union, Interreg V ÖKS. We requested additional information regarding cumulative data from the authors, but we did not receive these data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Performed by a study nurse or non‐treating physician. Randomisation by using a computerised randomisation programme running a minimisation algorithm, initially seeded using a random block sequence for the first women. |
| Allocation concealment (selection bias) | Low risk | The random concealed allocation sequence was generated by statisticians from Statistika Konsultgruppen (Gothenburg, Sweden). Randomisation was done on inclusion day (cycle day 2 or 3) before initiation of ovarian stimulation, and was blinded until the day of ovulation triggering so that ovarian stimulation was not influenced by the result. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of doctors and participants was not possible due to the nature of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessor blinding was not reported, however primary outcome is not likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were analysed for all randomised women. |
| Selective reporting (reporting bias) | Low risk | All registered outcomes were reported. Protocol publication. Study was registered in a prospective trials register with the trial number: Clinicaltrials.gov NCT02746562. |
| Other bias | Unclear risk | (Cumulative) data per subsequent menstrual or cryo‐transfer cycle not reported (relevant for time‐to‐pregnancy comparison and the related comparison of results after first transfer in FET group vs results after first 2 transfers in fresh group). Cumulative LBRs including time to delivery in the cumulative cycles, detailed embryo data, and evaluation of cost effectiveness will be accounted for in separate publications. |
Vuong 2018.
| Study characteristics | ||
| Methods | Single‐centre RCT, My Duc Hospital, Ho Chi Minh City Conducted: Vietnam Enrolment: June 2015‐April 2016 Power calculation: stated Randomisation: performed by an independent study co‐ordinator by means of block randomisation using a computer‐generated random list Timing of randomisation: on day 3 after retrieval Nature of intervention: Cryotech vitrification method Follow‐up: cumulative ongoing pregnancy rate and LBR (including all FETs performed within the 12 months after the initial transfer) |
|
| Participants | 782 women (391 freeze‐all, 391 control)
Inclusion criteria:
Exlusion criteria: history of PCOS (based on the Rotterdam criteria), in vitro maturation with polycystic ovaries visible on the ultrasonography or cycle with oocyte donation. |
|
| Interventions | For women who were assigned to the fresh‐embryo group, a maximum of 2 grade 1 or 2 embryos were transferred on day 3. Any remaining grade 1 or 2 embryos, along with grade 3 embryos (if requested by the couple), were frozen and transferred in subsequent cycles if needed. In the FET group, there was no fresh transfer and all grade 1 and 2 embryos had been cryopreserved on day 3 by means of the Cryotech vitrification method. In menstrual cycles following the ovum pick‐up the endometrium was artificially prepared for transfer using estradiol and progesterone for transfer of a maximum of 2 embryos. |
|
| Outcomes | The primary outcome was ongoing pregnancy after the first ET. Prespecified secondary outcomes were the rates of implantation, clinical pregnancy, ectopic pregnancy, miscarriage, live birth, multiple pregnancy, vanishing‐twin pregnancy, and OHSS. Pregnancy complications for pregnancies that continued beyond 24 weeks, complications were recorded. Time to pregnancy was calculated in a post hoc analysis at 12 months after randomisation using the median to pregnancy. | |
| Notes | Funding: by My Duc Hospital | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Performed by an independent study co‐ordinator by means of block randomisation using a computer‐generated random list |
| Allocation concealment (selection bias) | Unclear risk | Randomised women by means of block randomisation by an independent study co‐ordinator using a computer‐generated random list, there was no further explanation about allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of doctors and participants was not possible due to the nature of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessor blinding was not reported, however primary outcome is not likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were analysed for all randomised women. |
| Selective reporting (reporting bias) | Low risk | All registered outcomes were reported. Protocol publication. Study was registered in a prospective trial register with the trial number: NCT02471573. |
| Other bias | Unclear risk | Not reported on blinding of doctors to interim analyses of outcomes of the study. Blinding of investigators was not reported (which is relevant for determining end of study). |
Wei 2019.
| Study characteristics | ||
| Methods | Multi‐centre RCT Conducted: in 21 academic fertility centres in China Enrolment: August 2016‐June 2017 Power calculation: stated Randomisation: performed by means of block randomisation using a computer‐generated random list Timing of randomisation: on day 3 after retrieval Nature of intervention: cryopreservation Follow‐up: cumulative ongoing pregnancy rate and LBR (including all FETs performed within the 12 months after the initial transfer) |
|
| Participants | 1650 women (825 freeze‐all, 825 control)
Inclusion criteria:
Exlusion criteria: women who were planning cycles of preimplantation genetic testing, women with diagnosis of a congenital or acquired uterine abnormality (such as a uterine malformation, adenomyosis, submucous myoma, or intrauterine adhesion), women also with medical conditions that are contraindications to IVF procedures or pregnancy (uncontrolled hypertension, known symptomatic heart disease, poorly controlled type 1 or type 2 diabetes, undiagnosed liver disease or dysfunction, renal disease, severe anaemia, history of deep venous thrombosis, history of pulmonary embolus, previous cerebrovascular accident, or history of cervical cancer, endometrial cancer, or breast cancer) |
|
| Interventions | For the fresh blastocyst transfer group, a single blastocyst was selected and transferred on day 5 of embryo culture (details of ET procedure are provided in the appendix). If ≥ 2 blastocysts were of equal grade, their early scores at cleavage stage were referred for the selection of the single blastocyst. Supernumerary embryos were frozen on day 5 or 6 according to embryo development. If pregnancy was achieved after fresh single blastocyst transfer, luteal phase support was continued until 10 weeks’ gestation. For women who were assigned to the fresh‐embryo group, a maximum of 2 grade 1 or 2 embryos were transferred on day 3. Any remaining grade 1 or 2 embryos, along with grade 3 embryos (if requested by the couple), were frozen and transferred in subsequent cycles if needed. For the FET group, all blastocysts were vitrified on day 5 or day 6 according to embryo development. Luteal phase support was stopped after randomisation. At least 4 weeks later, the endometrium was prepared either with a natural cycle regimen or a programmed cycle regimen, at the discretion of local investigators. For the natural ovulatory cycle regimen, ovulation was determined by ultrasound monitoring. |
|
| Outcomes | The primary outcome was singleton live birth rate. Prespecified secondary outcomes were rates of conception, clinical pregnancy, ongoing pregnancy, pregnancy loss, live birth, moderate and severe OHSS, ectopic pregnancy, pregnancy and perinatal complications, neonatal complication and other adverse events, and birth weight. A post‐hoc analysis was performed for the outcomes of small for gestational age (SGA), large for gestational age (LGA), the rate of cumulative live birth within 12 months after the first ET and the number of embryos remaining. The time to live birth was defined as after ET and not after randomisation which is not a fair comparison. |
|
| Notes | Funding: by The National Key Research and Development Program of China | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Perfomed by means of block randomisation using a computer‐generated random list. |
| Allocation concealment (selection bias) | Low risk | Used a sequence that was entered into the central online database, which was secured by the username and password log‐in. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of doctors and participants was not possible due to the nature of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessor blinding was not reported, however primary outcome is not likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were analysed for all randomised women. |
| Selective reporting (reporting bias) | Low risk | All registered outcomes were reported. Protocol publication. Study was registered in a prospective trial register with the trial number: ChiCTR‐IOR‐14005405. |
| Other bias | Unclear risk | Not reported on blinding of doctors to interim analyses of outcomes of the study. Blinding of investigators was not reported (which is relevant for determining end of study). |
Wong 2021.
| Study characteristics | ||
| Methods | Single‐centre RCT Conducted: in the Netherlands Enrolment: January 2013‐July 2015 Power calculation: stated Randomisation: performed by an independent study co‐ordinator by means of block randomisation using a computer‐generated random list Timing of randomisation: on day 3 after retrieval Nature of intervention: cryopreservation Follow‐up: cumulative ongoing pregnancy rate and LBR (including all FETs from 1 IVF cycle within 12 months after randomisation) |
|
| Participants | 204 (102 freeze‐all, 102 control)
Inclusion criteria:
Exlusion criteria: women undergoing a PGS cycle, women undergoing a modified natural cycle and couples with an HIV, HBV or HCV infection since all these cycles required modified IVF protocols. |
|
| Interventions | For both groups a single ET policy was adhered to for women < 38 years of age and a double ET policy for women of ≥ 38 years, if ≥ 2 embryos were available. For women who were assigned to the conventional (fresh) group, embryos at day 5 of culture were transferred. The morphologic best embryo(s) was transferred first. All surplus embryos were cryopreserved on day 6 of culture and were transferred in subsequent artificial cycles if needed. In the FET group, there was no fresh transfer and all embryos were cryopreserved on day 6 of culture. In menstrual cycles following the ovum pick‐up the endometrium was artificially prepared for transfer using estradiol and progesterone for transfer of a maximum of 2 embryos. |
|
| Outcomes | The primary outcome was cumulative ongoing pregnancy resulting from 1 IVF cycle within 12 months after randomisation. Prespecified secondary outcomes were time to pregnancy, defined as the time to ongoing pregnancy, from the date of randomisation to the date of ET that led to an ongoing pregnancy, live birth, defined as the delivery of a live fetus at ≥ 20 weeks of gestation, clinical pregnancy, defined as the presence of at least 1 intra‐uterine gestational sac at 7 weeks of gestation, biochemical pregnancy, defined as serum hCG > 2 IU/L. Safety outcomes were OHSS, multiple pregnancy, premature birth and congenital abnormalities. The study authors also reported on miscarriage rate, ectopic pregnancy rate and birth weight of the children born. | |
| Notes | Funding: by The Netherlands Organization for Health Research and Development | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation of women using an online randomisation program with block randomisation. |
| Allocation concealment (selection bias) | Low risk | Randomisation program generated a unique study number with allocation code after entry of the patient’s date of birth and randomisation date |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of doctors and participants was not possible due to the nature of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessor blinding was not reported, however primary outcome is not likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were analysed for all randomised women. |
| Selective reporting (reporting bias) | Low risk | All registered outcomes reported. Study was registered in a prospective trials register with the trial number: NTR3187 |
| Other bias | Unclear risk | Not reported on blinding of doctors to interim analyses of outcomes of the study. Blinding of investigators was not reported (which is relevant for determining end of study). |
Zhang 2018.
| Study characteristics | ||
| Methods | Secondary analysis of the results of a multicentre RCT (Chen 2016)
Conducted: in 14 reproductive medical centres throughout China
Enrolment: June 2013‐May 2014
Power calculation: stated (Chen 2016)
Randomisation: an online central randomisation system (www.medresman.org) was used. Timing of randomisation: after oocyte retrieval Nature of intervention: day‐3 embryo cryopreservation by means of vitrification. Local investigators had the option to transfer day‐2 embryos if there were < 3 embryos on day 2 Follow‐up: until delivery, cLBR, birth outcomes |
|
| Participants | 1508 women (746 freeze‐all, 762 control) Inclusion criteria:
Exclusion criteria: history of unilateral oophorectomy, recurrent spontaneous abortion (defined as ≥ 3 previous spontaneous pregnancy losses), congenital or acquired uterine malformations, abnormal results on parental karyotyping, or medical conditions that contraindicated assisted reproductive technology or pregnancy |
|
| Interventions | For women who were assigned to the fresh embryo group, on day 3, 2 high‐quality embryos were picked out for fresh transfer and supernumerary embryos were transferred by means of vitrification For women who were assigned to the FET group, there was no fresh transfer as all day‐3 embryos were cryopreserved for later transfer. Local investigators had the option to transfer day‐2 embryos if there were < 3 embryos on day 2. In cycles following the menstrual cycle with ovum pick‐up, on day 4 of the progesterone regimen, 2 day‐3 frozen embryos were thawed and transferred. | |
| Outcomes | Gestational diabetes mellitus, pre‐eclampsia, preterm birth, small for gestational age, and large for gestational age. All outcomes reported for first ET. | |
| Notes | Funding: by the National Basic Research Program of China; the State Key Program of National Natural Science Foundation of China; the National Natural Science Foundation of China; and the Thousand Talents Program | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | An online central randomisation system (www.medresman.org) was used to automatically generate the assignment sequence. |
| Allocation concealment (selection bias) | Low risk | Assignment sequence was unknown to the clinical investigators. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of doctors and participants was not possible due to the nature of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessor blinding was not reported, however primary outcome is not likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were analysed for all randomised women. |
| Selective reporting (reporting bias) | Low risk | All registered outcomes reported. The study used for this follow‐up study was registered in a prospective trials register with the trial number: NCT01841528. |
| Other bias | Unclear risk | (Cumulative) data regarding obstetric complications per subsequent menstrual or cryo‐transfer cycle not reported |
2pn: 2 pro‐nucleate; AMH: anti‐Müllerian hormone; ART: assisted reproductive technology; BMI: body mass index; cLBR: cumulative live birth rate; ET: embryo transfer; FET: frozen embryo transfer; FSH: follicle‐stimulating hormone; GnRH: gonadotropin‐releasing hormone; HBV: hepatitis B virus; hCG: human chorionic gonadotropin; HCV: hepatitis C virus; HSG: hysterosalpingogram; ICSI: intracytoplasmic sperm injection; ITT: intention‐to‐treat; IVF: in vitro fertilisation; LBR: live birth rate; NGS: next‐generation sequencing; OHSS: ovarian hyperstimulation syndrome; PCOS: polycystic ovary syndrome; PGS: pre‐implantation genetic screening; RCT: randomised controlled trial
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Absalan 2013 | Unclear from report whether study was a RCT, and the authors did not respond to our request for further information. |
| Aflatoonian 2010 | This article has been retracted from the literature at the request of the Editor and the ASRM Publications Committee. |
| Beyer 2016 | Wrong study design (retrospective cohort) |
| Boostanfar 2016 | Randomised a different intervention |
| Chandel 2016 | Wrong study design (pseudo‐RCT) |
| Magdi 2017 | Wrong study design (prospective cohort) |
| Simon 2020 | Wrong comparison |
| Yang 2015 | One‐third of participants chose to be in group 3 after randomisation. Not considered a properly randomised RCT. |
ASRM: American Society for Reproductive Medicine; RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
ACTRN12612000422820.
| Study name | A randomised study of IVF patients to assess whether freezing all of the embryos and transferring them in a later natural, unstimulated cycle results in a higher pregnancy rate than transferring an embryo 5 days after egg collection |
| Methods | RCT Target enrolment: 200 |
| Participants | Included:
Excluded:
|
| Interventions | Both study groups will undertake a stimulated IVF cycle. The first (intervention) group will have all embryos cryostored electively for transfer in a later natural menstrual cycle. The second group will have the best‐quality embryo transferred to the endometrial cavity fresh and all remaining embryos cryostored. The protocol for the second group is standard practice today. Both groups will undertake the same drug regimen, therefore there is no difference in drug intervention. |
| Outcomes |
|
| Starting date | 1 May 2012 |
| Contact information | Mark Livingstone: ecosse@ihug.com.au |
| Notes | www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=362361 |
ACTRN12616000643471.
| Study name | Comparison of the probability of live birth after elective freezing of all embryos versus standard fresh embryo transfer in patients undergoing in‐vitro fertilisation (IVF) |
| Methods | RCT Target enrolment: 400 |
| Participants | Women aged 18‐39 with indication for COS and IVF or ICSI with autologous gametes
Key inclusion criteria:
Key exclusion criteria:
|
| Interventions | Interventional freeze‐all group
Fresh transfer group
|
| Outcomes |
|
| Starting date | May 2016 |
| Contact information | Christos Venetis: c.venetis@unsw.edu.au |
| Notes | www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12616000643471 |
ISRCTN61225414.
| Study name | Freezing of embryos in assisted conception: a randomised controlled trial evaluating the clinical and cost‐effectiveness of a policy of freezing embryos followed by thawed frozen embryo transfer, compared with a policy of fresh embryo transfer in women undergoing in‐vitro fertilization |
| Methods | RCT Estimated enrolment: 1086 |
| Participants | Inclusion criteria
Exclusion criteria Couples in whom:
|
| Interventions | Intervention arm: all good‐quality embryos will be frozen and couples will undergo frozen‐thawed ET within 3 months of the egg collection process. Couples will attend a clinic visit and additional monitoring visits before FET is performed. Standard‐care arm: women will undergo fresh ET on day 3 or 5 (after egg collection). |
| Outcomes |
|
| Starting date | 1 March 2015 |
| Contact information | Christina Cole: christina.cole@npeu.oxa.c.uk |
| Notes | www.isrctn.com/ISRCTN61225414 |
NCT02133950.
| Study name | Efficacy study of segmentation of PGD treatment |
| Methods | RCT Estimated enrolment: 240 |
| Participants | Women aged 20‐40 Inclusion criteria:
Exclusion criteria:
|
| Interventions | Elective cryopreservation of available embryos after PGD PGD and elective fresh ET plus cryopreservation of supernumerary available embryos after PGD |
| Outcomes | cLBR of a single PGD treatment |
| Starting date | May 2014 |
| Contact information | Willem Verpoest, Centre for Reproductive Medicine UZ Brussel |
| Notes |
NCT02570386.
| Study name | Clinical effectiveness of frozen thawed embryo transfer compared to fresh embryo transfer |
| Methods | RCT Estimated enrolment: 800 |
| Participants | Women aged 18‐42 Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention: fresh ET will not be undertaken in this group. Embryos will be frozen by vitrification or slow freezing at cleavage or blastocyst stage according to standard agreed local protocols. Women will be contacted after 4 weeks and arrangements made for FET. Control: women allocated to the control arm will either undergo fresh ET at cleavage stage or extended culture and transfer at blastocyst stage according to local policy. A maximum of 2 embryos or blastocysts will be replaced according to the standard protocol under transabdominal ultrasound guidance. Luteal‐phase support is given according to local protocols. |
| Outcomes |
|
| Starting date | October 2015 |
| Contact information | Ernest HY Ng: nghye@hku.hk |
| Notes | clinicaltrials.gov/ct2/show/record/NCT02570386 |
NCT03349905.
| Study name | Deferred versus fresh embryo transfers (DEFETOSE) |
| Methods | RCT Estimated enrolment: 2294 |
| Participants | Women aged 18‐40 Inclusion criteria:
Exclusion criteria:
|
| Interventions | Fresh transfer
Deferred‐frozen ET
|
| Outcomes |
|
| Starting date | September 2018 |
| Contact information | Pietro SANTULLI, MD, PhD: pietro.santulli@aphp.fr Christelle AUGER: christelle.auger@aphp.fr |
| Notes | https://clinicaltrials.gov/ct2/show/NCT03349905 |
AFC: antral follicle count; AMH: anti‐Müllerian hormone; ART: assisted reproductive technology; BMI: body mass index; COS: controlled ovarian stimulation; ET: embryo transfer; FET: frozen embryo transfer; FSH: follicle‐stimulating hormone; GnRH: gonadotropin‐releasing hormone; HBV: hepatitis B virus; hCG: human chorionic gonadotropin; HCV: hepatitis C virus; ICM: inner cell mass; ICSI: intracytoplasmic sperm injection; HSG: hysterosalpingogram; IVF: in vitro fertilisation; LH: luteinising hormone; OHSS: ovarian hyperstimulation syndrome; PCOS: polycystic ovary syndrome; PGD: pre‐implantation genetic diagnosis; PGS: pre‐implantation genetic screening; PRL: prolactin; r‐hCG: recombinant human chorionic gonadotropin; RCT: randomised controlled trial; SHBG: sex hormone‐binding globulin; TSH: thyroid‐stimulating hormone
Differences between protocol and review
For the primary version of the review we added a method of analysing time to pregnancy (by hazard ratios), as this was not reported in the protocol; in the event, no data were available for this outcome. For the primary version of the review we performed a subgroup analysis by timing of embryo transfer for the primary outcome of cumulative live birth. For the primary version of the review we changed the unit of analysis for birth weight (from per woman to per baby). For the primary version of the review we added some details to the section specifying our plans for the summary of findings table. For the primary version of the review congenital disorders, defined as the number of congenital abnormalities at birth, were reported per live‐born children plus number of foetuses therapeutically terminated in stead of per all clinical pregnancies.
For the 2021 update we added a method of analysing time to pregnancy, eventually this method was not possible given the difference in the studies included in this outcome.
For the 2021 update we extended the secondary outcomes regarding pregnancy outcomes and obstetric, perinatal and neonatal outcomes per woman.
For the 2021 update we added a second table with Summary of Findings concerning obstetric en neonatal safety outcomes.
All studies reported sufficient detail to calculate mean differences and standard deviations (SDs) and therefore we did not have to impute data on basis of the assumption that the outcome had a SD equal to the highest SD from other studies within the same analysis as mentioned in the methods section.
Contributions of authors
Tjitske Zaat, Miriam Zagers, Femke Mol, Madelon van Wely and Sebastiaan Mastenbroek updated the search and adjusted the review. Previously, Kai Mee Wong, Sjoerd Repping, and Sebastiaan Mastenbroek developed the concept of the review. Mariëtte Goddijn provided feedback on the review.
Sources of support
Internal sources
None, Other
External sources
None, Other
Declarations of interest
Tjitske Zaat: none known Miriam Zagers: none known Madelon van Wely: is author of the previous version of this review (Wong 2017), and is author of one of the included studies (Wong 2021). Femke Mol: is author of the previous version of this review (Wong 2017), and is author of one of the included studies (Wong 2021). Mariëtte Goddijn: none known Sebastiaan Mastenbroek is author of the previous version of this review (Wong 2017), and is author of one of the included studies (Wong 2021).
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Aflatoonian 2018 {published data only (unpublished sought but not used)}
- Aflatoonian A, Mansoori-Torshizi M, Mojtahedi MF, Aflatoonian B, Khalili MA, Amir-Arjmand MH, et al. Fresh versus frozen embryo transfer after gonadotropin releasing hormone agonist trigger in gonadotropin releasing hormone antagonist cycles among high responder women: a randomized, multi-center study. International Journal of Reproductive BioMedicine 2018;16(1):9-18. [PMC free article] [PubMed] [Google Scholar]
Aghahosseini 2017 {published data only (unpublished sought but not used)}
- Aghahosseini M, Aleyasin A, Sarfjoo FS, Mahdavi A, Yaraghi M, Saeedabadi H. In vitro fertilization outcome in frozen versus fresh embryo transfer in women with elevated progesterone level on the day of HCG injection: an RCT. International Journal of Reproductive BioMedicine 2017;15(12):757-62. [PMC free article] [PubMed] [Google Scholar]
Chen 2016 {published data only}
- Chen ZJ, Shi Y, Sun Y, Zhang B, Liang X, Cao Y, et al. Fresh versus frozen embryos for infertility in the polycystic ovary syndrome. New England Journal of Medicine 2016;375(6):523-33. [DOI: 10.1056/NEJMoa1513873] [DOI] [PubMed] [Google Scholar]
- Zhang B, Wei D, Legro RS, Shi Y, Li J, Zhang L, et al. Obstetric complications after frozen versus fresh embryo transfer in women with polycystic ovary syndrome: results from a randomized trial. Fertility and Sterility 2018;109:324–9. [DOI: ] [DOI] [PubMed] [Google Scholar]
Coates 2017 {published data only (unpublished sought but not used)}
- Coates A, Kung A, Mounts E, Hesla J, Bankowski B, Barbieri E, et al. Optimal euploid embryo transfer strategy, fresh versus frozen, after preimplantation genetic screening with next generation sequencing: a randomized controlled trial. Fertility and Sterility 2017;107(3):723-30. [DOI] [PubMed] [Google Scholar]
Ferraretti 1999 {published and unpublished data}
- Ferraretti AP, Gianaroli L, Magli C, Fortini D, Selman HA, Feliciani E. Elective cryopreservation of all pronucleate embryos in women at risk of ovarian hyperstimulation syndrome: efficiency and safety. Human Reproduction 1999;14:1457-60. [DOI: 10.1093/humrep/14.6.1457] [PMID: 10357958 ] [DOI] [PubMed] [Google Scholar]
Santos‐Ribeiro 2020 {published data only}
- Santos-Ribeiro S, Mackens S, Popovic-Todorovic B, Racca A, Polyzos NP, Van Landuyt L, et al. The freeze-all strategy versus agonist triggering with low-dose hCG for luteal phase support in IVF/ICSI for high responders: a randomized controlled trial. Human Reproduction 2020;35(12):2808-18. [DOI: 10.1093/humrep/deaa226] [DOI] [PubMed] [Google Scholar]
Shapiro 2011a {published and unpublished data}
- Shapiro BS, Daneshmand ST, Bedient CE, Garner FC. Comparison of birth weights in patients randomly assigned to fresh or frozen-thawed embryo transfer. Fertility and Sterility 2016;106:317–21. [DOI: ] [DOI] [PubMed] [Google Scholar]
- Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Hudson C, Thomas S. Evidence of impaired endometrial receptivity after ovarian stimulation for in vitro fertilization: a prospective randomized trial comparing fresh and frozen-thawed embryo transfer in normal responders. Fertility and Sterility 2011;96:344-8. [DOI: 10.1016/j.fertnstert.2011.05.050] [PMID: 21737072 ] [DOI] [PubMed] [Google Scholar]
Shapiro 2011b {published and unpublished data}
- Shapiro BS, Daneshmand ST, Bedient CE, Garner FC. Comparison of birth weights inpatients randomly assigned to fresh or frozen-thawed embryo transfer. Fertility and Sterility 2016;106:317–21. [DOI: ] [DOI] [PubMed] [Google Scholar]
- Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Hudson C, Thomas S. Evidence of impaired endometrial receptivity after ovarian stimulation for in vitro fertilization: a prospective randomized trial comparing fresh and frozen-thawed embryo transfers in high responders. Fertility and Sterility 2011;96:516-8. [DOI: 10.1016/j.fertnstert.2011.02.059] [PMID: 21737071 ] [DOI] [PubMed] [Google Scholar]
Shapiro 2016 {published data only}
- Shapiro BS, Daneshmand ST, Bedient CE, Garner FC. Comparison of birth weights inpatients randomly assigned to fresh or frozen-thawed embryo transfer. Fertility and Sterility 2016;106:317-21. [DOI] [PubMed] [Google Scholar]
Shi 2018 {published data only (unpublished sought but not used)}
- Shi Y, Sun Y, Hao C, Zhang H, Wei D, Zhang Y, et al. Transfer of fresh versus frozen embryos in ovulatory women. New England Journal of Medicine 2018;378:126-38. [DOI] [PubMed] [Google Scholar]
Stormlund 2020 {published data only}
- Stormlund S, Sopa N, Zedeler A, Bogstad J, Prætorius L, Svarre Nielsen H, et al. Freeze-all versus fresh blastocyst transfer strategy during in vitro fertilisation in women with regular menstrual cycles: multicentre randomised controlled trial. BMJ 2020;370:m2519. [DOI: 10.1136/bmj.m2519] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vuong 2018 {published data only}
- Vuong LN, Dang VQ, Ho TM, Huynh BG, Ha DT, Pham TD, et al. IVF transfer of fresh or frozen embryos in women without polycystic ovaries. New England Journal of Medicine 2018;378(2):137-47. [DOI: 10.1056/NEJMoa1703768] [DOI] [PubMed] [Google Scholar]
Wei 2019 {published data only}
- Wei D, Liu J-Y, Sun Y, Shi Y, Zhang B, Liu J-Q, et al. Frozen versus fresh single blastocyst transfer in ovulatory women: a multicentre, randomised controlled trial. Lancet 2019;393(10178):1310-18. [DOI] [PubMed] [Google Scholar]
Wong 2021 {unpublished data only}
- Wong KM, Van Wely M, Verhoeve HR, Kaaijk EM, Mol F, Van der Veen F, et al. Transfer of fresh or frozen embryos: a randomised controlled trial. Human Reproduction 2021:deaa305. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2018 {published data only}
- Zhang B, Wei D, Legro RS, Shi Y, Li J, Zhang L, et al. Obstetric complications after frozen versus fresh embryo transfer in women with polycystic ovary syndrome: results from a randomized trial. Fertility and Sterility 2018;109:324-9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Absalan 2013 {published and unpublished data}
- Absalan F, Ghannadi A, Kazerooni M. Reproductive outcome following thawed embryo transfer in management of ovarian hyperstimulation syndrome. Journal of Reproduction and Infertility 2013;14(3):133-7. [PMID: 24163797 ] [PMC free article] [PubMed] [Google Scholar]
Aflatoonian 2010 {published data only}
- Aflatoonian A, Oskouian H, Ahmadi S, Oskouian L. Can fresh embryo transfers be replaced by cryopreserved-thawed embryo transfers in assisted reproductive cycles? A randomized controlled trial. Journal of Assisted Reproduction and Genetics 2010;27:357-63. [DOI: 10.1007/s10815-010-9412-9] [PMID: 20373015 ] [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Editor and the ASRM Publications Committee. Retraction note to: Can fresh embryo transfers be replaced by cryopreserved-thawed embryo transfers in assisted reproductive cycles? A randomized controlled trial. Journal of Assisted Reproduction and Genetics 2013;30(9):1245. [DOI: ] [PMID: 23975193 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Beyer 2016 {published data only}
- Beyer DA, Griesinger G. Vitrified-warmed embryo transfer is associated with mean higher singleton birth weight compared to fresh embryo transfer. European Journal of Obstetrics & Gynecology and Reproductive Biology 2016;203:104–7. [DOI] [PubMed] [Google Scholar]
Boostanfar 2016 {published data only}
- Boostanfar R, Gates D, Guan Y, Gordon K, McCrary Sisk C, Stegmann B. Efficacy and safety of frozen-thawed embryo transfer in women aged 35 to 42 years from the PURSUE randomized clinical trial. Fertility and Sterility 2016;106(2):300-5. [DOI] [PubMed] [Google Scholar]
Chandel 2016 {published data only}
- Chandel NP, Bhat VV, Bhat BS, Chandel SS. Outcome analysis of day-3 frozen embryo transfer v/s fresh embryo transfer in infertility: a prospective therapeutic study in Indian scenario. Journal of Obstetrics and Gynecology of India 2016;66(5):345–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Magdi 2017 {published data only}
- Magdi Y, El-Damen A, Fathi AM, Abdelaziz AM, Youssef MA, Abd-Allah AA, et al. Revisiting the management of recurrent implantation failure through freeze-all policy. Fertility and Sterility 2017;108(1):72-7. [DOI] [PubMed] [Google Scholar]
Simon 2020 {published data only}
- Simón C, Gómez C, Cabanillas S, Vladimirov I, Castillón G, Giles J, et al, for the ERA-RCT Study Consortium Group. A 5-year multicentre randomized controlled trial comparing personalized, frozen and fresh blastocyst transfer in IVF. Reproductive BioMedicine Online 2020;41(3):402-15. [DOI: https://doi.org/10.1016/j.rbmo.2020.06.002 1472- 6483/] [DOI] [PubMed] [Google Scholar]
Yang 2015 {published data only}
- Yang S, Pang T, Li R, Yang R, Zhen X, Chen X, et al. The individualized choice of embryo transfer timing for patients with elevated serum progesterone level on the HCG day in IVF/ICSI cycles: a prospective randomized clinical study. Gynecological Endocrinology 2015;31(5):355-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
ACTRN12612000422820 {unpublished data only}
- ACTRN12612000422820. A randomised controlled trial to determine the effect of elective embryo cryopreservation and subsequent transfer in a natural menstrual cycle on clinical pregnancy rates in infertile females. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=362361 (first received 11 April 2012).
ACTRN12616000643471 {unpublished data only}
- ACTRN12616000643471. Fresh vs. elective frozen embryo transfer after IVF: a randomised controlled trial. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=370373 (first received 24 March 2016).
ISRCTN61225414 {unpublished data only}
- ISRCTN61225414. Freezing of embryos in assisted conception: a randomised controlled trial evaluating the clinical and cost-effectiveness of a policy of freezing embryos followed by thawed frozen embryo transfer, compared with a policy of fresh embryo transfer in women undergoing in-vitro fertilization. www.isrctn.com/ISRCTN61225414 (first received 24 December 2015).
NCT02133950 {unpublished data only}
- NCT02133950. Efficacy study of segmentation of PGD treatment. clinicaltrials.gov/ct2/show/NCT02133950 (first received 6 May 2014).
NCT02570386 {unpublished data only}
- NCT02570386. Clinical effectiveness of frozen thawed embryo transfer compared to fresh embryo transfer. clinicaltrials.gov/ct2/show/record/NCT02570386 (first received 29 September 2015).
NCT03349905 {published data only}
- NCT03349905. Deferred versus fresh embryo transfers (DEFETOSE). clinicaltrials.gov/ct2/show/NCT03349905 (first received September 2018).
Additional references
Bosdou 2019
- Bosdou JK, Venetis CA, Tarlatzis BC, Grimbizis GF, Kolibianakis EM. Higher probability of live-birth in high, but not normal, responders after first frozen-embryo transfer in a freeze-only cycle strategy compared to fresh-embryo transfer: a meta-analysis. Human Reproduction 2019;34:491-505. [DOI] [PubMed] [Google Scholar]
Bourgain 2003
- Bourgain C, Devroey P. The endometrium in stimulated cycles for IVF. Human Reproduction Update 2003;9:515-22. [DOI] [PubMed] [Google Scholar]
CDC 2016
- Centers for Disease Control and Prevention. Assisted Reproductive Technology Report; 2016. cdc.gov/nchs/fastats/infertility.htm (accessed 15 July 2016).
Conrad 2011
- Conrad KP. Emerging role of relaxin in the maternal adaptations to normal pregnancy: implications for preeclampsia. Seminars in Nephrology 2011;31:15-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Covidence [Computer program]
- Veritas Health Innovation Covidence. Version accessed after 23 September 2020. Melbourne, Australia: Veritas Health Innovation. Available at covidence.org.
D'Angelo 2017
- D'Angelo A, Amso NN, Hassan R. Coasting (withholding gonadotrophins) for preventing ovarian hyperstimulation syndrome. Cochrane Database of Systematic Reviews 2017, Issue 5. Art. No: CD002811. [DOI: 10.1002/14651858.CD002811.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2020
- Deeks JJ, Higgins JP, Altman DG (editors). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
Devroey 2011
- Devroey P, Polyzos NP, Blockeel C. An OHSS-free clinic by segmentation of IVF treatment. Human Reproduction 2011;26:2593-7. [DOI] [PubMed] [Google Scholar]
Ernstad 2019
- Ginström Ernstad E, Wennerholm U-B, Khatibi A, Petzold M, Christina Bergh C. Neonatal and maternal outcome after frozen embryo transfer: increased risks in programmed cycles. American Journal of Obstetrics & Gynecology 2019;221(2):126.e1-126.e18. [DOI] [PubMed] [Google Scholar]
Esh‐Broder 2011
- Esh-Broder E, Ariel I, Abas-Bashir N, Bdolah Y, Celnikier DH. Placenta accreta is associated with IVF pregnancies: a retrospective chart review. BJOG 2011;118:1084–9. [DOI] [PubMed] [Google Scholar]
ESHRE 2018
- European Society of Human Reproduction and Embryology (ESHRE). ART Fact Sheet; 2018. eshre.eu/~/media/sitecore-files/Guidelines/ART-fact-sheet_vFebr18_VG.pdf?la=en (accessed before 26 January 2021).
Evans 2014
- Evans J, Hannan NJ, Edgell TA, Vollenhoven BJ, Lutjen PJ, Osianlis T, et al. Fresh versus frozen embryo transfer: backing clinical decisions with scientific and clinical evidence. Human Reproduction Update 2014;20:808-21. [DOI] [PubMed] [Google Scholar]
Fatemi 2015
- Fatemi HM, Van Vaerenbergh I. Significance of premature progesterone rise in IVF. Current Opinion in Obstetrics & Gynecology 2015;3:242-8. [DOI] [PubMed] [Google Scholar]
Ferraretti 1996
- Ferraretti AP, Magli C, Feliciani E, Montanaro N, Gianaroli L. Relationship of timing of agonist administration in the cycle phase to the ovarian response to gonadotropins in the long down-regulation protocols for assisted reproductive technologies. Fertility and Sterility 1996;65(1):114-21. [DOI] [PubMed] [Google Scholar]
Focus on Reproduction 2019
- Focus on Reproduction, ESHRE. The number of freeze-all cycles in IVF continues to increase 'dramatically'. focusonreproduction.eu/article/ESHRE-News-Freeze-all-2 (accessed before 26 January 2021).
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed 18 november 2019. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Griesinger 2011
- Griesinger G, Schultz L, Bauer T, Broessner A, Frambach T, Kissler S. Ovarian hyperstimulation syndrome prevention by gonadotropin-releasing hormone agonist triggering of final oocyte maturation in a gonadotropin-releasing hormone antagonist protocol in combination with a "freeze-all" strategy: a prospective multicentric study. Fertility and Sterility 2011;6:2029-33, 2033 e1. [DOI] [PubMed] [Google Scholar]
Haouzi 2009
- Haouzi D, Assou S, Mahmoud K, Tondeur S, Reme T, Hedon B, et al. Gene expression profile of human endometrial receptivity: comparison between natural and stimulated cycles for the same patients. Human Reproduction 2009;24:1436-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Healy 2010
- Healy DL, Breheny S, Halliday J, Jaques A, Rushford D, Garrett C, et al. Prevalence and risk factors for obstetric haemorrhage in 6730 singleton births after assisted reproductive technology in Victoria Australia. Human Reproduction 2009;25:265-74. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Sterne JA (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Hull 1985
- Hull MG, Glazener CM, Kelly NJ, Conway DI, Foster PA, Hinton RA, et al. Population study of causes, treatment, and outcome of infertility. British Medical Journal (Clinical Research Ed.) 1985;291:1693-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ishihara 2014
- Ishihara O, Araki R, Kuwahara A, Itakura A, Saito H, Adamson GD. Impact of frozen-thawed single-blastocyst transfer on maternal and neonatal outcome: an analysis of 277,042 single-embryo transfer cycles from 2008 to 2010 in Japan. Fertility and Sterility 2013;101:128-33. [DOI] [PubMed] [Google Scholar]
Kansal Kalra 2011
- Kansal Kalra S, Ratcliffe SJ, Milman L, Gracia CR, Coutifaris C, Barnhart KT. Perinatal morbidity after in vitro fertilization is lower with frozen embryo transfer. Fertility and Sterility 2011;95:548–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kolibianakis 2002
- Kolibianakis E, Bourgain C, Albano C, Osmanagaoglu K, Smitz J, Van Steirteghem A, et al. Effect of ovarian stimulation with recombinant follicle-stimulating hormone, gonadotropin releasing hormone antagonists, and human chorionic gonadotropin on endometrial maturation on the day of oocyte pick-up. Fertility and Sterility 2002;78:1025-9. [DOI] [PubMed] [Google Scholar]
Kosmas 2004
- Kosmas IP, Kolibianakis EM, Devroey P. Association of estradiol levels on the day of hCG administration and pregnancy achievement in IVF: a systematic review. Human Reproduction 2004;19:2446-53. [DOI] [PubMed] [Google Scholar]
Luke 2017
- Luke B, Brown MB, Wantman E, Stern JE, Toner JP, Coddington CC 3rd. Increased risk of large-for-gestational age birthweight in singleton siblings conceived with in vitro fertilization in frozen versus fresh cycles. Journal of Assisted Reproduction Genetics 2016;34:191-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maheshwari 2012
- Maheshwari A, Pandey S, Shetty A, Hamilton M, Bhattacharya S. Obstetric and perinatal outcomes in singleton pregnancies resulting from the transfer of frozen thawed versus fresh embryos generated through in vitro fertilization treatment: a systematic review and meta-analysis. Fertility and Sterility 2012;98:368-77. [DOI] [PubMed] [Google Scholar]
Maheshwari 2013
- Maheshwari A, Bhattacharya S. Elective frozen replacement cycles for all: ready for prime time? Human Reproduction 2013;28:6-9. [DOI] [PubMed] [Google Scholar]
Maheshwari 2018
- Maheshwari A, Pandey S, Amalraj Raja E, Shetty A, Hamilton M, Bhattacharya S. Is frozen embryo transfer better for mothers and babies? Can cumulative meta-analysis provide a definitive answer? Human Reproduction Update 2017;24:35-58. [DOI] [PubMed] [Google Scholar]
Mastenbroek 2011
- Mastenbroek S, Van der Veen F, Aflatoonian A, Shapiro B, Bossuyt P, Repping S. Embryo selection in IVF. Human Reproduction 2011;26:964-6. [DOI] [PubMed] [Google Scholar]
Mastenbroek 2014
- Mastenbroek S, Repping S. Preimplantation genetic screening: back to the future. Human Reproduction 2014;29:1846-50. [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG. The PRISMA Group (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. PLoS Medicine 6;7:e1000097. [DOI: 10.1371/journal.pmed1000097] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mourad 2017
- Mourad S, Brown J, Farquhar C. Interventions for the prevention of OHSS in ART cycles: an overview of Cochrane reviews. Cochrane Database of Systematic Reviews 2017, Issue 1. Art. No: CD012103. [DOI: 10.1002/14651858.CD012103.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Opdahl 2015
- Opdahl S, Henningsen AA, Tiitinen A, Bergh C, Pinborg A, Romundstad P, et al. Risk of hypertensive disorders in pregnancies following assisted reproductive technology: a cohort study from the CoNARTaS group. Human Reproduction 2015;30:1724-31. [DOI] [PubMed] [Google Scholar]
Pereira 2016
- Pereira N, Rosenwaks Z. A fresh(er) perspective on frozen embryo transfers. Ferility and Sterility 2016;106:257-8. [DOI] [PubMed] [Google Scholar]
Pereira 2019
- Pereira N, Petrini AC, Hancock KL, Rosenwaks Z. Fresh or frozen embryo transfer in in vitro fertilization: an update. Clinical Obstetrics and Gynecology 2019;62:293-9. [DOI] [PubMed] [Google Scholar]
Pinborg 2014
- Pinborg A, Henningsen AA, Loft A, Malchau SS, Forman J, Andersen AN. Large baby syndrome in singletons born after frozen embryo transfer (FET): is it due to maternal factors or the cryotechnique? Human Reproduction 2014;29:618-27. [DOI] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: The Cochrane Collaboration, 2020.
RevMan Web 2019 [Computer program]
- The Cochrane Collaboration Review Manager Web (RevMan Web). The Cochrane Collaboration, 2019. Available at revman.cochrane.org.
Roque 2017
- Roque M, Valle M, Kostolias A, Sampaio M, Geber S. Freeze-all cycle in reproductive medicine: current perspectives. JBRA Assisted Reproduction 2017;21(1):49-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Roque 2019
- Roque M, Valle M, Sampaio M, Geber S. Obstetric outcomes after fresh versus frozen-thawed embryo transfers: a systematic review and meta-analysis. JBRA Assisted Reproduction 2018;22:253-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Saito 2019
- Saito K, Kuwahara A, Ishikawa T, Morisaki N, Miyado M, Miyado K, et al. Endometrial preparation methods for frozen-thawed embryo transfer are associated with altered risks of hypertensive disorders of pregnancy, placenta accreta, and gestational diabetes mellitus. Human Reproduction 2019;34(8):1567-75. [DOI] [PubMed] [Google Scholar]
Sazonova 2012
- Sazonova A, Kallen K, Thurin-Kjellberg A, Wennerholm UB, Bergh C. Obstetric outcome in singletons after in vitro fertilization with cryopreserved/thawed embryos. Human Reproduction 2012;27:1343-50. [DOI] [PubMed] [Google Scholar]
Schatz 2016
- Schatz F, Guzeloglu-Kayisli O, Arlier S, Kayisli UA, Lockwood CJ. The role of decidual cells in uterine hemostasis, menstruation, inflammation, adverse pregnancy outcomes and abnormal uterine bleeding. Human Reproduction Update 2016;4:497-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2020
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
Singh 2020
- Singh B, Reschke L, Segars J, Baker VL. Frozen-thawed embryo transfer: the potential importance of the corpus luteum in preventing obstetrical complications. Fertility and Sterility 2020;113:252-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Spijkers 2017
- Spijkers S, Lens JW, Schats R, Lambalk CB. Fresh and frozen-thawed embryo transfer compared to natural conception: differences in perinatal outcome. Gynecologic and Obstetric Investigation 2017;82:538-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Takeshima 2016
- Takeshima K, Jwa SC, Saito H, Nakaza A, Kuwahara A, Ishihara O, et al. Impact of single embryo transfer policy on perinatal outcomes in fresh and frozen cycles-analysis of the Japanese assisted reproduction technology registry between 2007 and 2012. Fertility and Sterility 2016;105:337-46. [DOI] [PubMed] [Google Scholar]
Vail 2003
- Vail A, Gardener E. Common statistical errors in the design and analysis of subfertility trials. Human Reproduction 2003;18:1000-4. [DOI] [PubMed] [Google Scholar]
Van Vaerenbergh 2009
- Van Vaerenbergh I, Van Lommel L, Ghislain V, In't Veld P, Schuit F, Fatemi H, et al. In GnRH antagonist/rec-FSH stimulated cycles, advanced endometrial maturation on the day of oocyte retrieval correlates with altered gene expression. Human Reproduction 2009;24:1085-91. [DOI] [PubMed] [Google Scholar]
Van Voorhis 2007
- Van Voorhis BJ. Clinical practice. In vitro fertilization. New England Journal of Medicine 2007;356:379-86. [DOI] [PubMed] [Google Scholar]
Venetis 2013
- Venetis CA, Kolibianakis EM, Bosdou JK, Tarlatzis BC. Progesterone elevation and probability of pregnancy after IVF: a systematic review and meta-analysis of over 60 000 cycles. Human Reproduction Update 2013;19:433-57. [DOI] [PubMed] [Google Scholar]
Venetis 2015
- Venetis CA, Kolibianakis EM, Bosdou JK, Lainas GT, Sfontouris IA, Tarlatzis BC, et al. Estimating the net effect of progesterone elevation on the day of hCG on live birth rates after IVF: a cohort analysis of 3296 IVF cycles. Human Reproduction 2015;30:684-91. [DOI] [PubMed] [Google Scholar]
Venetis 2016
- Venetis CA, Kolibianakis EM, Bosdou JK, Lainas GT, Sfontouris IA, Tarlatzis BC, et al. Basal serum progesterone and history of elevated progesterone on the day of hCG administration are significant predictors of late follicular progesterone elevation in GnRH antagonist IVF cycles. Human Reproduction 2016;31:1859-65. [DOI] [PubMed] [Google Scholar]
von Versen‐Höynck 2019a
- Versen-Höynck F, Schaub AM, Chi Y-Y, Chiu K-H, Liu J, Lingis M, et al. Increased preeclampsia risk and reduced aortic compliance with in vitro fertilization cycles in the absence of a corpus luteum. Hypertension 2019;73:640-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
von Versen‐Höynck 2019b
- Versen-Höynck F, Strauch NK, Liu J, Chi YY, Keller-Woods M, Conrad KP, et al. Effect of mode of conception on maternal serum relaxin, creatinine, and sodium concentrations in an infertile population. Reproductive Sciences (Thousand Oaks, Calif.) 2019;26:412-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wong 2014
- Wong KM, Mastenbroek S, Repping S. Cryopreservation of human embryos and its contribution to IVF success rates. Fertility and Sterility 2014;102(1):19-26. [DOI] [PubMed] [Google Scholar]
Youssef 2016
- Youssef MA, Mourad S. Volume expanders for the prevention of ovarian hyperstimulation syndrome. Cochrane Database of Systematic Reviews 2016, Issue 8. Art. No: CD001302. [DOI: 10.1002/14651858.CD001302.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zaat 2019
- Zaat T, Mol F, Van Wely M, Wilkinson J, Mastenbroek S. Fresh versus frozen blastocyst transfer. Lancet 2019;394(10204):1227. [DOI: ] [DOI] [PubMed] [Google Scholar]
Zegers‐Hochschild 2017
- Zegers-Hochschild F, Adamson GD, Dyer S, Racowsky C, Mouzon J, Sokol R, et al. The international glossary on infertility and fertility care, 2017. Human Reproduction 2017;32:1786-801. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Wong 2017
- Wong KM, Van Wely M, Mol F, Repping S, Mastenbroek S. Fresh versus frozen embryo transfers in assisted reproduction. Cochrane Database of Systematic Reviews 2017, Issue 3. Art. No: CD011184. [DOI: 10.1002/14651858.CD011184.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


