Abstract
Background
Dental professionals are well placed to help their patients stop using tobacco products. Large proportions of the population visit the dentist regularly. In addition, the adverse effects of tobacco use on oral health provide a context that dental professionals can use to motivate a quit attempt.
Objectives
To assess the effectiveness, adverse events and oral health effects of tobacco cessation interventions offered by dental professionals.
Search methods
We searched the Cochrane Tobacco Addiction Group's Specialised Register up to February 2020.
Selection criteria
We included randomised and quasi‐randomised clinical trials assessing tobacco cessation interventions conducted by dental professionals in the dental practice or community setting, with at least six months of follow‐up.
Data collection and analysis
Two review authors independently reviewed abstracts for potential inclusion and extracted data from included trials. We resolved disagreements by consensus. The primary outcome was abstinence from all tobacco use (e.g. cigarettes, smokeless tobacco) at the longest follow‐up, using the strictest definition of abstinence reported. Individual study effects and pooled effects were summarised as risk ratios (RR) and 95% confidence intervals (CI), using Mantel‐Haenszel random‐effects models to combine studies where appropriate. We assessed statistical heterogeneity with the I2 statistic. We summarised secondary outcomes narratively.
Main results
Twenty clinical trials involving 14,897 participants met the criteria for inclusion in this review. Sixteen studies assessed the effectiveness of interventions for tobacco‐use cessation in dental clinics and four assessed this in community (school or college) settings. Five studies included only smokeless tobacco users, and the remaining studies included either smoked tobacco users only, or a combination of both smoked and smokeless tobacco users. All studies employed behavioural interventions, with four offering nicotine treatment (nicotine replacement therapy (NRT) or e‐cigarettes) as part of the intervention. We judged three studies to be at low risk of bias, one to be at unclear risk of bias, and the remaining 16 studies to be at high risk of bias.
Compared with usual care, brief advice, very brief advice, or less active treatment, we found very low‐certainty evidence of benefit from behavioural support provided by dental professionals, comprising either one session (RR 1.86, 95% CI 1.01 to 3.41; I2 = 66%; four studies, n = 6328), or more than one session (RR 1.90, 95% CI 1.17 to 3.11; I2 = 61%; seven studies, n = 2639), on abstinence from tobacco use at least six months from baseline. We found moderate‐certainty evidence of benefit from behavioural interventions provided by dental professionals combined with the provision of NRT or e‐cigarettes, compared with no intervention, usual care, brief, or very brief advice only (RR 2.76, 95% CI 1.58 to 4.82; I2 = 0%; four studies, n = 1221). We did not detect a benefit from multiple‐session behavioural support provided by dental professionals delivered in a high school or college, instead of a dental setting (RR 1.51, 95% CI 0.86 to 2.65; I2 = 83%; three studies, n = 1020; very low‐certainty evidence). Only one study reported adverse events or oral health outcomes, making it difficult to draw any conclusions.
Authors' conclusions
There is very low‐certainty evidence that quit rates increase when dental professionals offer behavioural support to promote tobacco cessation. There is moderate‐certainty evidence that tobacco abstinence rates increase in cigarette smokers if dental professionals offer behavioural support combined with pharmacotherapy. Further evidence is required to be certain of the size of the benefit and whether adding pharmacological interventions is more effective than behavioural support alone. Future studies should use biochemical validation of abstinence so as to preclude the risk of detection bias. There is insufficient evidence on whether these interventions lead to adverse effects, but no reasons to suspect that these effects would be specific to interventions delivered by dental professionals. There was insufficient evidence that interventions affected oral health.
Keywords: Humans; Bias; Counseling; Dentists; Oral Health; Randomized Controlled Trials as Topic; Schools; Smoking Cessation; Smoking Cessation/methods; Smoking Cessation/psychology; Tobacco Use Cessation; Tobacco Use Cessation/methods; Tobacco Use Cessation/psychology; Tobacco, Smokeless; Tobacco, Smokeless/adverse effects; Universities
Plain language summary
Can dental professionals help people to stop smoking or using tobacco products?
Keeping your mouth healthy
Tobacco can be smoked, chewed or sniffed (as snuff). The best thing that people who use tobacco products can do for their health is to stop using them. This lowers the risk of lung cancer and other diseases, including mouth cancer and gum disease.
Many people visit a dental professional at least once a year; some may visit more often. Dental professionals could motivate people to stop using tobacco by telling them about the health risks of continuing and the health benefits of quitting. Dental professionals include:
· dentists;
· dental hygienists;
· dental therapists; and
· dental nurses (referred to as dental assistants in some countries).
Why we did this Cochrane Review
We wanted to find out if dental professionals could help people to stop using tobacco by offering them advice and support. We also wanted to know if support from dental professionals had any unwanted effects.
What did we do?
We searched for studies that tested whether advice and support from dental professionals helped people to stop smoking, chewing or sniffing tobacco.
We looked for randomised controlled studies, in which the people taking part were assigned to different treatment groups using chance to decide which people received support to stop using tobacco. This type of study usually gives reliable evidence about the effects of a treatment.
Search date: we included evidence published up to February 2020.
What we found
We found 20 studies in 14,897 people who used tobacco products (smoking, chewing or sniffing tobacco). The studies took place in the USA (13 studies), the UK (two studies), Sweden (two studies), Japan (one study), Malaysia (one study) and India (one study). Most studies (16) were in dental clinics and four were conducted in schools or colleges.
All studies used behavioural programmes to help people stop using tobacco; these programmes aimed to boost motivation and offer advice on stopping. Four studies also included offering people nicotine replacement therapy (NRT) or e‐cigarettes as well as a behavioural programme.
Nineteen studies were funded by government agencies or universities; one study reported that it received no funding.
For each type of behavioural programme tested, the studies measured how many people stopped smoking or using tobacco products for at least six months.
In all studies, the effect of receiving behavioural support from dental professionals was compared with:
· usual care (the studies did not state what this included);
· no support or advice;
· brief advice to stop smoking to improve health; or
· a less active form of behavioural support.
What are the main results of our review?
Behavioural programmes involving dental professionals and NRT or e‐cigarettes probably help more people to stop smoking. On average, 74 out of 1000 people stopped compared with 27 out of 1000 people who did not receive behavioural support (evidence from four studies in 1221 people).
Several sessions of behavioural programmes involving dental professionals may help people to stop using tobacco. On average,106 out of 1000 people stopped compared with 56 out of 1000 people who did not receive behavioural support (seven studies; 2639 people).
A single session of a behavioural programme may also help people to stop: on average, 45 out of 1000 people stopped compared with 24 out of 1000 who did not receive behavioural support (four studies; 6328 people).
We are uncertain about the effect of advice and support from dental professionals in settings other than a dental practice (such as in a school or college), because the studies that tested this were too small to show a reliable effect (three studies; 1020 people).
We are uncertain if behavioural programmes given by dental professionals had any unwanted effects, because only one study reported this information.
Our confidence in our results
We are moderately confident about the benefit of support from dental professionals plus NRT or e‐cigarettes. We are less confident about the benefits of one, or several, sessions of behavioural support from dental professionals.
We found weaknesses in the evidence. Some studies only asked people if they had stopped using tobacco, and did not use tests – such as testing their breath or saliva – to find out if they had stopped. Some studies did not describe clearly how they were conducted, or how they assigned people to the different groups. In some studies more than half of the people dropped out of the study before it ended.
Our results may change when more, high‐quality evidence becomes available.
Key messages
Advice and support from dental professionals that involves NRT or e‐cigarettes is more likely to help people to stop smoking.
Single or multiple sessions of advice and support may help people to stop smoking or using tobacco products.
Summary of findings
Summary of findings 1. Interventions delivered by dental professionals compared with control for tobacco cessation.
| Interventions delivered by dental professionals compared with no contact/intervention, usual care (non‐defined), very brief/brief advice or less treatment active controls for tobacco cessation | ||||||
| Patient or population: participants who used tobacco products Setting: dental clinic, community school, or college Intervention: tobacco cessation interventions delivered by dental professionals Comparison: no contact/intervention, usual care (non‐defined), very brief/brief advice or less treatment active controls | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no contact/intervention, usual care (non‐defined), very brief/brief advice or less treatment active controls | Risk with Interventions delivered by dental professionals | |||||
|
Multi‐session behavioural support versus usual care, brief advice, or very brief advice, or less active treatment Smoking cessation (≥ 6 months follow‐up) |
56 per 1000 | 106 per 1000 (65 to 173) | RR 1.90 (1.17 to 3.11) | 2639 (7 studies) | ⊕⊝⊝⊝ VERY LOW 1, 2, 3 | Intervention may help more people to quit |
|
Single session behavioural support versus usual care, brief advice, or very brief advice Smoking cessation (≥ 6 months follow‐up) |
24 per 1000 | 45 per 1000 (25 to 83) |
RR 1.86 (1.01 to 3.41) | 6328 (4 studies) | ⊕⊝⊝⊝ VERY LOW 2, 3, 4 |
Intervention may help more people to quit |
|
Behavioural intervention + NRT/e‐cigarette versus no intervention/usual care, brief advice, or very brief advice Smoking cessation (≥ 6 months follow‐up) |
27 per 1000 | 74 per 1000 (42 to 129) |
RR 2.76 (1.58 to 4.82) | 1221 (4 studies) | ⊕⊕⊕⊝ MODERATE 3, 5 |
Intervention probably helps more people to quit |
|
Behavioural support from dental professional at high school/college versus usual care/no intervention Smoking cessation (≥ 6 months follow‐up) |
260 per 1000 | 392 per 1000 (224 to 689) | RR 1.51 (0.86 to 2.65) | 1020 (3 RCTs) | ⊕⊝⊝⊝ VERY LOW 1, 2, 6 | Sensitivity analysis removing Gansky 2005 removes heterogeneity and demonstrates a benefit of intervention. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1Downgraded two levels because of very serious risk of bias: all studies were at high risk of bias.
2Downgraded one level because of inconsistency: substantial heterogeneity not accounted for by subgroup analysis
3Downgraded by one level because of imprecision: fewer than 300 events reported in analysis
4Downgraded one level because of risk of bias: only one study not at high risk of bias
5Not downgraded for risk of bias: two out of four studies at high risk of bias, but sensitivity analysis removing these studies did not substantially affect the result
6Downgraded two levels because of serious imprecision: fewer than 300 events reported in analysis and confidence intervals encompass both potential benefit and harm
Background
Description of the condition
Tobacco smoking is estimated to have been responsible for 100 million deaths worldwide in the 20th century, and it is predicted that this could reach one billion during the 21st century (WHO 2008). In addition to the well‐known harmful effects of smoking on respiratory and cardiovascular systems, tobacco use is a major risk factor for several oral diseases, including oral cancer and periodontitis (WHO 2017). The worldwide age‐standardised rate of oral cancer was 2.7 per 100,000 people in 2012 (Shield 2017) with the UK having 3700 cases in 2016 (Conway 2018). Smoking has been estimated to be responsible for up to 75% of these (Anantharaman 2011). Smoking cessation has positive effects on oral cancer risks, which reduce to the level of never‐smokers after 20 years (Marron 2010). Periodontitis (gum disease) is highly prevalent, with severe periodontitis being the sixth most prevalent health condition in the world, affecting approximately 11% of adults (Kassebaum 2014). Smoking is one of the biggest risk factors for disease development and progression, with smokers also having poorer responses to periodontal therapy (Chambrone 2013). Hence, smoking cessation has important roles in primary, secondary and tertiary prevention of oral diseases.
Description of the intervention
Dental professionals (including dentists, dental hygienists/therapists and dental nurses/assistants) can provide a range of tobacco use cessation interventions. Where appropriate training is available, interventions can focus on how to quit, advising that combining pharmacotherapy (including nicotine replacement therapy (NRT), varenicline and bupropion) and behavioural support is best. This approach has evidence of effectiveness in many settings (Stead 2016). Typically, behavioural support in the dental setting involves very brief advice interventions (e.g. the '3As' approach: Ask, Advise, Act); or brief advice interventions (e.g. the '5As' approach: Ask, Assess, Advise, Assist and Arrange); with several clinical guidelines recommending that dental professionals provide this support before referring people who smoke to another provider for further specialist support and/or pharmacotherapy (NICE 2018; PHE 2014; NCSCT 2012; ADA 2019).
How the intervention might work
Dental professionals are potentially in a unique position to support their tobacco‐using patients to quit. In many countries, large proportions of the population visit a dental professional on a regular basis throughout their life. With some dental diseases, such as periodontitis, regular supportive care can involve visits as frequently as every three months. There can be many 'teachable moments,' which can be powerful in initiating a quit attempt (e.g. tooth staining identification, periodontitis diagnosis or progression, tooth loss, oral cancer/pre‐cancer diagnosis). This regularity and relatively high frequency of interaction, as well as their credibility as a source of health advice, places dental professionals in a good position to deliver smoking cessation interventions.
Why it is important to do this review
Other Cochrane Tobacco Addiction reviews have evaluated behavioural and pharmacological interventions in a range of settings (Cahill 2014; Rigotti 2012; Stead 2013; Stead 2016). However, it is important to ascertain the effectiveness of these interventions in the dental setting or when delivered by dental professionals. The previous version of this review (Carr 2012) concluded that behavioural interventions delivered by dental professionals may increase quit rates in both cigarette and smokeless tobacco users. However, the review authors were unable to make conclusive recommendations about the type of interventions, due to limitations of the data.
Objectives
To assess the effectiveness, adverse events and oral health effects of tobacco cessation interventions offered by dental professionals.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs)
Cluster‐randomised controlled trials (cluster‐RCTs)
Quasi‐randomised controlled trials (quasi‐RCTs)
Types of participants
Tobacco users (smokers or smokeless tobacco users) of any age willing to enrol in a tobacco cessation trial. Users of electronic cigarettes (e‐cigarettes) were not considered tobacco users. We included studies that randomised dental professionals or practices, as well as those that randomised individual tobacco users, provided that the specific aim of the study was to examine the effect of the intervention on tobacco cessation. We did not include trials that randomised dental professionals or practices to receive an educational intervention. Health professional training interventions are reviewed separately (Carson 2012).
Types of interventions
We included any intervention to promote tobacco use cessation that involved a component delivered by a dentist, dental hygienist or therapist, dental nurse/assistant or dental practice office staff, delivered in either a dental or community setting. Interventions could include brief advice to quit, provision of self‐help materials, counselling, pharmacotherapy or any combination of these, or referral to other sources of support. Interventions directed at smokers, smokeless tobacco users, or both, were all eligible for inclusion.
Comparators
This review included trials that compared tobacco cessation interventions with any of the following comparators.
No intervention.
Wait‐list controls.
Usual care, including brief advice interventions.
Other active interventions (as defined above).
Types of outcome measures
Primary outcomes
Abstinence from tobacco (tobacco cigarettes, smokeless tobacco or all tobacco) at long‐term follow‐up (dichotomous)
To be eligible, studies had to report abstinence rates at least six months from baseline. We excluded trials that did not investigate tobacco‐use outcomes or did not have sufficiently long follow‐up. In trials with more than one measure of abstinence, we selected the measure with the longest follow‐up and the strictest definition, in line with the Russell Standard (West 2005). Therefore, we preferred biochemically validated over self‐reported abstinence, and prolonged or continuous abstinence over point prevalence abstinence. Abstinence rates were based on intention‐to‐treat analyses with drop‐outs and losses to follow‐up assumed to be continuing or relapsed tobacco users.
Secondary outcomes
Adverse events (including serious adverse events), as reported by the authors
Oral health outcome measures, as reported by the authors
Search methods for identification of studies
Electronic searches
To identify studies for this update, we searched the Cochrane Tobacco Addiction Group's Specialised Register on 21st February 2020. At the time of the updated search, the Register included the results of searches of the Cochrane Central Register of Controlled Trials (CENTRAL), Issue 1, 2020; MEDLINE (via OVID) to update 20200130; Embase (via OVID) to week 202005; PsycINFO (via OVID) to update 20200127; the US National Institutes of Health (NIH) trials registry at ClinicalTrials.gov; and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) to January 2020. See the Cochrane Tobacco Addiction Group website for full search strategies and a list of other resources searched to populate the Register.
See Appendix 1 for the search strategy used to search the Cochrane Tobacco Addiction Group's Specialised Register for this review.
Searching other resources
We contacted the authors of known unpublished trials. Searches of the clinical trials registers, ClinicalTrials.gov and the WHO's ICTRP, are carried out to populate the Tobacco Addiction Group's Specialised Register, and so were incorporated into our search.
Data collection and analysis
Selection of studies
Two review authors (RH and BH) independently checked the titles and abstracts of the studies generated by the search strategy for relevance. Any disagreements were resolved by discussion and inclusion of a third review author when required. We obtained full‐text versions of papers of the potentially relevant studies. Two review authors (RH and BH) then independently assessed the full‐text papers for inclusion in the review, with any disputes resolved by discussion with a third review author. We did not limit inclusion by language and planned to seek translations when necessary (this was not required).
Data extraction and management
For each included study, two review authors (RH and BH) independently extracted data, using a standardised electronic data collection form. Review authors then cross‐checked this information between themselves, and resolved disagreements through discussion. If review authors of this Cochrane Review were also authors of an included study, we ensured that the data extraction and risk of bias assessment were done by other review authors or other researchers (see Acknowledgements). We extracted the following information about each study, which is presented in the Characteristics of included studies tables.
Methods: study design; study location (i.e. country); study setting (e.g. dental practice); and study recruitment procedure.
Participants: number of participants (N); if this was a specialist population; if participants were selected based on motivation to quit; and participant characteristics (including gender, age, baseline average cigarettes/day, nicotine dependence, baseline motivation to quit and baseline self‐efficacy/confidence in quitting).
Interventions: comparator and intervention details including modality of support; details of provider training; overall contact time; number of sessions and use of pharmacotherapy.
Outcomes: definition of abstinence; longest follow‐up time; use of biochemical validation; oral health outcomes and adverse events.
Study funding sources and any reported author conflicts of interest.
Assessment of risk of bias in included studies
We assessed and reported the risk of bias of included studies, in accordance with the guidelines in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and specific 'Risk of bias' guidance developed by the Cochrane Tobacco Addiction Group. The latter states that performance bias (relating to the blinding of participants and providers) should not be assessed for behavioural interventions, as it is impossible to blind people to these types of interventions. Therefore, we reported on the following individual domains:
random sequence generation (selection bias);
allocation concealment (selection bias);
blinding of outcome assessment (detection bias);
incomplete outcome data (attrition bias); and
other sources of bias.
Two review authors (RH and BH) independently assessed risk of bias of included studies, with any disagreements resolved by discussion and inclusion of a third review author where required. A summary risk of bias judgement was derived for each study by applying an algorithm suggested in Section 8.7 (Table 8.7a) of the Cochrane Handbook (Higgins 2011). Specifically, if the judgement in at least one of these domains was 'high risk of bias', we determined the summary risk of bias to be high. If there were no judgements of 'high' risk, but the judgement in at least one domain was 'unclear risk of bias', then we determined the summary risk of bias to be unclear. We only judged the summary risk of bias to be 'low' if our judgements in all domains were 'low risk of bias'.
Measures of treatment effect
For tobacco use abstinence, we calculated a risk ratio (RR) and associated 95% confidence interval (CI) for the cessation outcome in each trial included in the meta‐analyses. We calculated RRs as follows: (number of participants abstinent from tobacco in the intervention group/number of participants in the intervention group)/(number of participants abstinent from tobacco in the control group/number of participants in the control group). The previous version of this review (Carr 2012) used odds ratios (OR), but in line with Cochrane policy, we used RR in this update. We used the same methods to calculate the RR and 95% CI for the numbers of participants experiencing adverse events for each study, distinguishing where possible between adverse events likely attributable to intervention or tobacco use cessation, and those likely attributable to the dental study context. For oral health outcomes, we calculated mean differences (MD) with 95% CI for individual studies, where the relevant data were presented.
Unit of analysis issues
The unit of analysis was the individual. Where we deemed it possible and appropriate to the structure of the analysis, we combined all relevant experimental intervention groups of a given multiple‐arm study into a single intervention group, and combined all relevant controls of that study into a single control group,
When extracting data from cluster‐RCTs, we considered whether study authors had made allowance for clustering in the data analysis reported, and were available, used data adjusted for clustering effects. Where studies reported analyses that accounted for the clustered study design, we estimated the effect on this basis. Where this was not possible and the information was not available from authors, we carried out an 'approximately correct' analysis, according to current guidelines (Higgins 2011). We imputed estimates of the intracluster correlation coefficient (ICC), either as reported in the study, using estimates derived from similar studies, or by using general recommendations from empirical research. If we had been unable to do this, we would have given the effect estimate as reported by the study but reported the unit of analysis error (this was not required in our review update).
Dealing with missing data
Where abstinence data were missing, we contacted the study authors for further information or clarifications. We calculated quit rates on an intention‐to‐treat basis, where participants lost to follow‐up were assumed to be smoking, excluding deaths from the denominator.
Assessment of heterogeneity
We assessed the characteristics of included studies to identify any clinical or methodological heterogeneity before pooling studies and conducting meta‐analyses. Where we deemed studies homogeneous enough to be meaningfully combined, we conducted a meta‐analysis, and we assessed statistical heterogeneity using the I2 statistic. We deemed an I2 of greater than 50% to indicate substantial heterogeneity.
Where there were enough data included in an analysis to draw meaningful conclusions, we conducted the subgroup and sensitivity analyses described below (Subgroup analysis and investigation of heterogeneity; Sensitivity analysis) to investigate any potential causes of observed heterogeneity.
Assessment of reporting biases
If we had meta‐analysed comparisons of abstinence rates in at least 10 studies, we planned to assess reporting bias, using funnel plots. Funnel plots illustrate the relationship between the effect estimates from individual studies against their size or precision. The greater the degree of asymmetry, the greater the risk of reporting bias.
Data synthesis
We conducted our analyses in RevMan 5 (Review Manager 2014). Where possible, we pooled studies for our tobacco cessation and adverse event outcomes, using Mantel‐Haenszel random‐effects models to generate pooled RRs with 95% CIs. Where the event was defined as tobacco cessation, an RR greater than one indicated that more people successfully quit in the treatment group than in the control group. Where the event was defined as the number of participants experiencing adverse events, an RR greater than one indicated that more people experienced adverse events in the treatment group than in the control group. In order to account for the clinical heterogeneity among studies, and to better understand the effect of intervention intensity and setting, we conducted separate analyses pooling studies testing:
multi‐session behavioural support;
single‐session behavioural support;
behavioural support plus pharmacotherapy; and
behavioural support provided by a dental professional outside of a dental context.
For oral health outcome measures, we planned to pool using an inverse variance random effects model.
Subgroup analysis and investigation of heterogeneity
We used subgroup analyses for the primary outcome (within each comparison) to explore the impact of:
type of comparison intervention (no intervention; usual care; less intense intervention);
type of tobacco use by study participants (smoked tobacco; smokeless tobacco; a combination);
different recruitment methods that may indicate different levels of motivation to quit among participants (not selected based on motivation; more likely motivated).
The method of recruitment is likely indicative of study participants' motivation to quit tobacco use, as people who smoke are unlikely to visit their dentist specifically for smoking cessation advice. Therefore, in studies where dental professionals recruited participants visiting their dental practice for a check‐up or oral health reasons, participants would not necessarily be motivated to quit smoking (and so motivation is likely to have varied across the sample). However, studies that recruited participants by advertising for people who used tobacco to join a trial of a tobacco‐use cessation intervention might be more likely to attract participants with a higher baseline motivation to quit.
Sensitivity analysis
We conducted the following sensitivity analyses:
removing studies deemed to be at high risk of bias;
removing Holliday 2019 from the analysis investigating behavioural support plus nicotine treatment, as Holliday 2019 provided e‐cigarettes, unlike the other studies that provided more traditional NRT;
removing Hanioka 2010 from the analysis investigating behavioural support plus nicotine treatment, as participants received multiple sessions with a dental professional, compared with the single session of support received in the other studies in this comparison; and
removing Gansky 2005 from the analysis investigating behavioural support delivered by dental professionals outside of a dental setting, as while the intervention comprised multiple sessions like the other studies in the comparison, only one was with a dental professional.
Summary of findings and assessment of the certainty of the evidence
Following standard Cochrane methodology (Schünemann 2017), we used the five GRADE considerations (risk of bias, inconsistency of effect, imprecision, indirectness and publication bias) to assess the certainty of the body of evidence for our primary outcome. Depending on our assessment of these considerations in each intervention comparison, we judged the certainty of evidence for this outcome to be 'high', 'moderate', 'low' or 'very low'. To present these judgements, we used GRADEpro GDT to create a GRADE 'Summary of findings' table with the following intervention comparisons: multi‐session behavioural support; single‐session behavioural support; behavioural support plus pharmacotherapy; and behavioural support provided by a dental professional outside of a dental context. We used these judgements to draw conclusions about the certainty of evidence within the text of the review.
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification; Characteristics of ongoing studies; Table 2; and Table 3, for additional details about studies.
1. Brief descriptions of cessation interventions.
| Study | Brief description of interventiona | Participant‐ or clinician‐centred intervention | Target of intervention‐ users? | Modality of intervention | Pharmacotherapy? |
| Andrews 1999 | Brief advice plus video‐based cessation program with phone follow‐up | Participant | Smokeless tobacco users | Face‐to‐face, telephone | No |
| Binnie 2007 | Counselling using the 5As plus nicotine replacement therapy | Participant | Cigarette users | Face‐to‐face | Yes, nicotine replacement therapy (patches or gum) |
| Cohen 1989 | Counselling with 4 steps and booklet provision plus | Participant | Cigarette users | Face‐to‐face | Yes, nicotine replacement therapy (gum) |
| Ebbert 2007 | Brief advice plus quit line referral | Participant | Cigarette users | Face‐to‐face, telephone | No |
| Gansky 2005 | School‐based intervention | Participant | Smokeless tobacco users | Face‐to‐face, internet | No |
| Gordon 2010a | 5As, discussion about pharmacotherapy and referral as needed | Participant | Intervention tailored to the type of tobacco use (cigarettes or smokeless) | Face‐to‐face | No |
| Gordon 2010b | 5As, nicotine replacement therapy and population‐specific printed material | Participant | Intervention tailored to the type of tobacco use (cigarettes or smokeless) | Face‐to‐face | Yes, nicotine replacement therapy (patches and lozenges) |
| Hanioka 2010 | Counselling at 6 visits and nicotine replacement therapy | Participant | Cigarette smokers | Face‐to‐face | Yes, nicotine replacement therapy (patches) |
| Holliday 2019 | 3As, offer of referral and e‐cigarette starter kit | Participant | Cigarette smokers | Face‐to‐face | Yes, e‐cigarette starter kit |
| Lando 2007 | Brief advice plus motivational interviewing | Participants | Cigarette smokers | Face‐to‐face, telephone | No |
| McClure 2018 | Oral health counselling integrated into quit line calls (4‐5 calls + 16 text messages) | Participants | Cigarette smokers | Telephone, internet, text message | Yes, nicotine replacement therapy starter kit |
| Nohlert 2013 | Counselling sessions (8 x 40 minutes) | Participants | Cigarette smokers | Face‐to‐face | No but pharmacological advice given |
| Selvamary 2016 | Health education including pamphlet and video plus cognitive behavioural therapy | Participants | Cigarette smokers | Face‐to‐face | No |
| Severson 1998 | Brief advice plus video‐based cessation program with phone follow‐up | Participants | Cigarette smokers | Face‐to‐face | No |
| Severson 2009 | Brief advice plus video‐based cessation program with phone follow‐up | Participants | Smokeless tobacco users | Telephone | No |
| Stevens 1995 | Brief advice plus video‐based cessation program with phone follow‐up | Participants | Smokeless tobacco users | Face‐to‐face, telephone | No |
| Virtanen 2015 | Brief advice (5As) plus quit line referral | Participants | Cigarette smokers | Face‐to‐face | No |
| Walsh 1999 | College‐based intervention | Participants | Smokeless tobacco users | Face‐to‐face, telephone | Yes, nicotine replacement therapy (gum) |
| Walsh 2003 | School‐based intervention | Participants | Smokeless tobacco users | Face‐to‐face, telephone | No |
| Yahya 2018 | Brief advice (5As) plus quit line referral | Participants | Cigarette smokers | Face‐to‐face | No |
aFor further details see Characteristics of included studies table.
2. Results data from included studies.
| Study ID | Intervention | Control | Abstinence definition | Notes |
| Andrews 1999 | 40/391 | 8/238 | 12 months, sustained, all tobacco | Cluster‐RCT, ICC 0.0009, 75 clusters (dental practices). Raw unadjusted data: Intervention 40/394, Control 8/239. Adjustments for cluster design:
|
| Binnie 2007 | 4/59 | 2/57 | 12 months, prolonged (repeated PP), all tobacco | Biochemically validated |
| Cohen 1989 | 7.7% | 3.1% | 12 months, PP, all tobacco | Not used in meta‐analysis as study arm denominator values not available |
| Ebbert 2007 | 15/61 | 6/22 | 6 months, PP, all tobacco | Cluster‐RCT, ICC 0.001, 8 clusters (dental practices). Raw unadjusted data: Intervention 15/60, Control 6/22. Adjustments for cluster design:
|
| Gansky 2005 | 84/233 | 106/288 | 12 months, 30 day PP, ST | Cluster‐RCT, ICC 0.0197. 52 colleges. Raw unadjusted data: Intervention 103/285, Control 130/352. Adjustments for cluster design:
|
| Gordon 2010a | 37/1175 | 6/402 | 12 months, sustained, all tobacco | Two intervention arms combined. Individual data: 5As (27/817) and 3As (24/793). Raw unadjusted data: Intervention 51/1610, Control 8/550. Cluster‐RCT, ICC 0.012. 68 clusters (dental practices) Adjustments for cluster design:
|
| Gordon 2010b | 28/530 | 8/439 | 7.5 months, prolonged, all tobacco | Cluster‐RCT, ICC 0.009, 14 clusters (dental clinics). Raw unadjusted data: Intervention 74/1394, Control 22/1155. Adjustments for cluster design:
|
| Hanioka 2010 | 12/33 | 3/23 | 12 months, continuous, all tobacco | |
| Holliday 2019 | 6/40 | 2/40 | 6 months, continuous, smoking | Biochemically validated |
| Lando 2007 | 4/61 | 7/63 | 12 months, PP (30 day), smoking | |
| McClure 2018 | 121/358 | 109/360 | 6 months, 7 day PP, smoking | |
| Nohlert 2013 | 18/150 | 6/150 | 12 months, sustained, smoking | |
| Selvamary 2016 | 14/100 | 1/100 | 6 months, continuous, all tobacco | Biochemically validated |
| Severson 1998 | 68/2624 | 31/1322 | 12 months, sustained, all tobacco | Two intervention arms combined. Individual data: enhanced intervention (35/1374), minimal intervention (34/1305). Cluster‐RCT, ICC 0.0004, 75 clusters (dental practices). Raw unadjusted data: Intervention 69/2679, Control 32/1350. Adjustments for cluster design:
|
| Severson 2009 | 53/392 | 22/393 | 6 months, prolonged, ST | |
| Stevens 1995 | 25/245 | 19/273 | ||
| Virtanen 2015 | 10/195 | 7/210 | 6 months, sustained for 3 months, all tobacco | Cluster‐RCT, ICC 0.01, 27 clusters (dental practices). Raw unadjusted data: Intervention 11/225, Control 8/242. Adjustments for cluster design:
|
| Walsh 1999 | 41/119 | 21/132 | 12 months, PP, ST | Cluster‐RCT, ICC 0.02, 16 clusters (colleges). Raw unadjusted data: Intervention 59/171, Control 30/189. Adjustments for cluster design:
|
| Walsh 2003 | 26/114 | 17/134 | 24 months, sustained, ST | Cluster‐RCT, ICC 0.04, 44 clusters (schools). Raw unadjusted data: Intervention 32/141, Control 21/166. Adjustments for cluster design:
|
| Yahya 2018 | 29/193 | 8/207 | 6 months, 30 day PP, smoking |
ICC: intracluster correlation coefficient; PP: point prevalence abstinence; RCT: randomised controlled trial; ST: sustained abstinence
Results of the search
The searches for this update of the review retrieved 216 unique records. After title and abstract screening, we classified 48 studies as potentially eligible for inclusion. After full‐text article screening, we identified five new studies that met inclusion criteria. We also found one new citation providing longer‐term follow‐up data for a previously included study (study ID changed from Nohlert 2009 to Nohlert 2013). Another study was excluded from the previous version of this review because of unavailable study arm denominator values (Cohen 1989). However, we chose to include Cohen 1989 in this update, though we excluded the study's data from meta‐analysis. In summary, we included 20 studies in this review: five new studies, 14 previously included studies (one of which was updated with new data from a more recent publication), and one study that was previously excluded but is now included. The flow of studies through the systematic review process for this update is shown in Figure 1.
1.
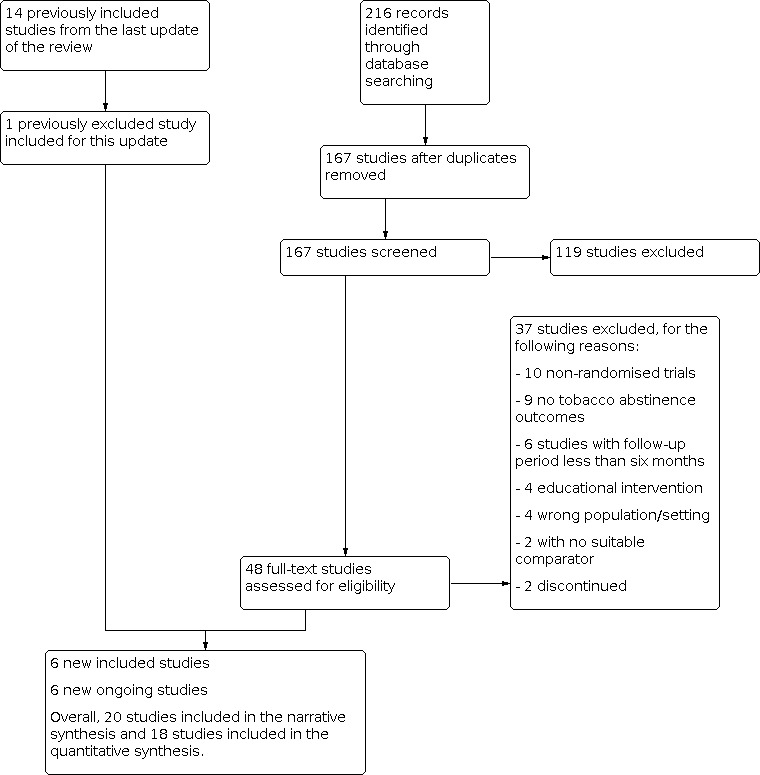
Study flow diagram for most recent update.
Included studies
This review includes 20 studies involving 14,897 participants. Details of included studies can be found in Characteristics of included studies. Although Andrews 1999 and Severson 1998 reported findings from the same trial, they are treated here as separate studies since Andrews 1999 focussed on outcomes in smokeless tobacco users and Severson 1998 focussed on outcomes in smokers. We did not include Cohen 1989 in any meta‐analysis because study arm denominator values were unavailable. We did not pool McClure 2018 in meta‐analysis because the study setting was too different from the other studies.
We classified 81 studies (from both the review's previous version and this update) as potentially relevant studies that did not meet all inclusion criteria. We list these studies in Characteristics of excluded studies, along with their reasons for exclusion.
Types of studies
We included ten RCTs in which the individual participant was the unit of randomisation (Binnie 2007; Hanioka 2010; Holliday 2019; Lando 2007; McClure 2018; Nohlert 2013; Selvamary 2016; Severson 2009; Stevens 1995; Yahya 2018). The remaining studies were cluster‐RCTs, using either the dental clinic (Andrews 1999; Cohen 1989; Ebbert 2007; Gordon 2010a; Gordon 2010b; Severson 1998; Virtanen 2015) or the school/college (Gansky 2005; Walsh 1999; Walsh 2003) as the unit of randomisation. All but one of the studies were funded by government or university agencies, with three of these being funded through a 'Tobacco Surtax Fund of the State of California' (Gansky 2005; Walsh 1999; Walsh 2003). One study reported receiving no funding (Selvamary 2016).
Types of participants and settings
Sixteen studies were conducted in a range of dental settings: twelve studies were conducted in dental clinic settings (Andrews 1999; Cohen 1989; Ebbert 2007; Gordon 2010a; Gordon 2010b; Hanioka 2010; Lando 2007Nohlert 2013; Severson 1998; Stevens 1995; Virtanen 2015: Yahya 2018); three were in hospital settings (Binnie 2007; Holliday 2019; Selvamary 2016); and one took place in military dental clinics (Severson 2009). Four studies were conducted in non‐dental settings. Three of these involved dental professionals providing interventions to athletes within high school or college settings (Gansky 2005; Walsh 1999; Walsh 2003) as a major part of the intervention. One study involved a 'quit line' (telephone help line) counsellor providing oral health promotion alongside quit line counselling (McClure 2018). Although this study did not involve dental professionals directly, we decided to include it, as dental professionals developed this intervention and trained the quit line counsellors to use it. However, we do report the results of McClure 2018 separately.
Nine studies targeted only cigarette smokers (Binnie 2007; Cohen 1989; Ebbert 2007; Hanioka 2010; Holliday 2019; Lando 2007; McClure 2018; Nohlert 2013; Yahya 2018). Five studies targeted smokeless tobacco users only (Gansky 2005; Severson 2009; Stevens 1995; Walsh 1999; Walsh 2003). Three studies included smokeless tobacco users as well as cigarette smokers and assessed abstinence from all tobacco (Gordon 2010a; Selvamary 2016; Virtanen 2015). One study targeted both cigarette smokers and smokeless tobacco users; the data for the two types of participant were reported separately and are treated in this review as two studies, with Severson 1998 covering smokers and Andrews 1999 covering smokeless tobacco users. Gordon 2010b included sole smokeless tobacco users and dual users of smoked and chewed tobacco, but only included sole smokers in the analysis because the proportion of sole smokeless tobacco users and dual users was low (2.4% and 1% respectively).
The majority of the studies did not select participants based on motivation to quit, except for three (Hanioka 2010 recruited participants willing to stop within one month; Selvamary 2016 recruited participants referred to a tobacco cessation programme; McClure 2018 recruited quit line callers).
Types of interventions
Table 2 provides a brief overview of the nature of cessation interventions used in the included studies. Interventions in the dental setting involved: brief advice plus quit line referral (Ebbert 2007; Virtanen 2015; Yahya 2018); brief advice plus motivational interviewing (Lando 2007); brief advice plus video‐based cessation programme with telephone follow‐up (Andrews 1999; Severson 1998; Severson 2009; Stevens 1995); health education, including pamphlet and video plus cognitive behavioural therapy (Selvamary 2016); counselling using the '5As' ('Ask, Advise, Assess, Assist, Arrange') plus NRT (Binnie 2007); 5As, NRT and population‐specific printed material (Gordon 2010b); 5As, discussion about pharmacotherapy and referral as needed (Gordon 2010a); counselling with four steps and booklet provision, plus nicotine replacement therapy (Cohen 1989); '3As' ('Ask, Advise, Act'), offer of referral and e‐cigarette starter kit (Holliday 2019); high‐intensity intervention (defined as five or more personal contacts) delivered with some form of NRT (Hanioka 2010); or without NRT, but with pharmacological advice (Nohlert 2013).
The three studies conducted in school/college settings involved a dental professional providing an examination and advice, and supplementing this with a range of components (e.g. follow‐up by the dental professional or athletic trainer, videos, newsletters and peer‐led support) (Gansky 2005; Walsh 1999; Walsh 2003). One study involved a quit line counsellor providing oral health promotion alongside quit‐line counselling (McClure 2018).
A range of comparator groups were used. Three studies had no contact or non‐intervention comparators (Gansky 2005; Hanioka 2010; Walsh 1999). Six studies had 'usual care' comparators but did not provide any further details about what this entailed (Andrews 1999; Gordon 2010a; Gordon 2010b; Severson 1998; Stevens 1995; Walsh 2003). Seven studies provided 'very brief advice' or 'brief advice' interventions as comparators (Binnie 2007; Ebbert 2007; Holliday 2019; Lando 2007; Severson 2009; Virtanen 2015; Yahya 2018). Three studies used less treatment active controls (McClure 2018; Nohlert 2013; Selvamary 2016). No comparator groups received pharmacotherapy.
Types of outcome measures
A wide range of abstinence definitions were used, either point prevalence (single or multiple) or continuous (sustained or prolonged abstinence). Biochemical validation of abstinence was used in six studies (Binnie 2007; Cohen 1989; Holliday 2019; Hanioka 2010; Selvamary 2016; Yahya 2018) with one study reportedly doing this on an 8% random sample (Walsh 2003). The strictest definition of abstinence was continuous smoking abstinence, with dropouts (or those with missing data) considered to have continued smoking or relapsed; this was used in three studies (Hanioka 2010; Holliday 2019; Selvamary 2016)
Two studies reported on oral health outcomes (Holliday 2019; McClure 2018). One of these studies reported whether or not participants received dental care in the last six months (McClure 2018), and the other study reported periodontal probing pocket depths, bleeding on probing, oral health‐related quality of life and a clinical oral dryness score (Holliday 2019). One study reported on adverse events (Holliday 2019).
In the majority of studies, participants were followed for a maximum of six months (Ebbert 2007; Holliday 2019; McClure 2018; Selvamary 2016; Severson 2009; Virtanen 2015; Yahya 2018) or 12 months (Andrews 1999; Binnie 2007; Cohen 1989; Gansky 2005; Gordon 2010a; Hanioka 2007; Lando 2007; Severson 1998; Stevens 1995; Walsh 1999). Other follow‐up periods included seven and one‐half months (Gordon 2010b), 24 months (Walsh 2003), and five to eight years (Nohlert 2013).
Excluded studies
We listed 81 studies in the Characteristics of excluded studies table with reasons provided. This combines 44 excluded studies from the previous version of this review (Carr 2012) with 37 excluded studies from this update. The most common reasons for exclusion were that the studies did not report appropriate smoking cessation outcomes, they followed participants for less than six months, or they were not RCTs.
Six ongoing studies are listed in the Ongoing studies table (the previous version of this review listed no ongoing studies).
Risk of bias in included studies
As demonstrated in the 'Risk of bias' summary figure (Figure 2), it was often difficult to assess bias using our criteria because there was insufficient information reported in the publications. For summary risk of bias judgements, as described in Assessment of risk of bias in included studies, we were able to judge that these conferred a low summary risk of bias for three studies (Holliday 2019; McClure 2018; Virtanen 2015). We assessed sixteen studies as being at high risk of bias (Andrews 1999; Binnie 2007; Cohen 1989; Ebbert 2007; Gansky 2005; Gordon 2010a; Gordon 2010b; Lando 2007; Nohlert 2013; Selvamary 2016; Severson 1998; Severson 2009; Stevens 1995; Walsh 1999; Walsh 2003; Yahya 2018), with the remaining study assessed to be at unclear risk of bias (Hanioka 2010).
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Rationale for 'Risk of bias' judgements for individual studies can be found in Characteristics of included studies.
Allocation
We assessed selection bias by investigating methods of random sequence generation and allocation concealment for each study. We rated nine studies at low risk of selection bias, with details being provided of an adequate sequence generation process and steps to ensure allocation concealment (Binnie 2007; Gansky 2005; Holliday 2019; McClure 2018; Nohlert 2013; Selvamary 2016; Severson 2009; Virtanen 2015; Walsh 1999). We rated two studies at high risk of selection bias (Lando 2007; Stevens 1995): one of these studies had both inadequate sequence generation processes and inadequate allocation concealment (Stevens 1995); the other provided details of adequate sequence generation but not allocation concealment (Lando 2007). The other studies had insufficient details to judge the risk of selection bias and were rated as being at unclear risk of bias (Andrews 1999; Cohen 1989; Ebbert 2007; Gordon 2010a; Gordon 2010b; Hanioka 2010; Severson 1998; Walsh 2003; Yahya 2018).
Incomplete outcome data
We assessed attrition bias by investigating the number of participants not followed up in each study according to the 'Risk of bias' guidance produced by the Cochrane Tobacco Addiction Group. We rated twelve studies at low risk of attrition bias (Andrews 1999; Gansky 2005; Gordon 2010a; Gordon 2010b; Hanioka 2010; Holliday 2019; McClure 2018; Nohlert 2013; Severson 2009; Virtanen 2015; Walsh 1999; Walsh 2003) meaning the overall number lost to follow‐up was clearly reported to be no more than 50%, and the difference in loss to follow‐up between groups was no greater than 20%. We rated five studies to be at high risk of bias (Binnie 2007; Cohen 1989; Selvamary 2016; Stevens 1995; Yahya 2018) because overall loss to follow‐up was more than 50%. Three studies were rated to be at unclear risk of attrition bias (Ebbert 2007; Lando 2007; Severson 1998) because the number lost to follow‐up in each group and/or sensitivity analyses were not reported.
Blinding of outcome assessment (detection bias)
We assessed detection bias by investigating blinding of the outcome measure, as recommended in the 'Risk of bias' guidance produced by the Cochrane Tobacco Addiction Group. Eight studies were rated to be at low risk of detection bias (Binnie 2007; Cohen 1989; Hanioka 2010; Holliday 2019; McClure 2018; Selvamary 2016; Virtanen 2015; Yahya 2018), because smoking status was measured objectively (i.e. biochemical validation) (Binnie 2007; Cohen 1989; Hanioka 2010; Holliday 2019; Selvamary 2016; Yahya 2018) or because although smoking status was not measured objectively, the study groups received similar amounts of face‐to‐face (or phone/text message) contact (McClure 2018; Virtanen 2015). We rated twelve studies to be at high risk of detection bias (Andrews 1999; Ebbert 2007; Gansky 2005; Gordon 2010a; Gordon 2010b; Lando 2007; Nohlert 2013; Severson 1998; Severson 2009; Stevens 1995; Walsh 1999; Walsh 2003) because smoking status was self‐reported and groups received different levels of contact.
Other potential sources of bias
We identified other potential sources of bias in Gansky 2005, where there was contamination bias from spillover of the cessation intervention to the control group (i.e. contamination of the control group with intervention information).
We planned to assess publication bias using a funnel plot. However, none of our analyses contained ten studies or more, the threshold set in our pre‐specified methods.
Effects of interventions
See: Table 1
Tobacco‐use cessation
Single session behavioural support
We pooled seven studies (n = 6328) testing behavioural interventions comprising a single session with a dental professional compared with usual care, brief advice, very brief advice, or less active treatment control. We found evidence of benefit from behavioural support (RR 1.86, 95% CI 1.01 to 3.34; Analysis 1.1), though there was substantial heterogeneity (I2 = 66%), which we could not explain through subgroup analysis. We conducted two subgroup analyses, one dividing studies by the intensity of control (usual care control, or brief or very brief advice control) for which we found no evidence of subgroup difference (P = 0.26; I2 = 21%), and one dividing studies by whether they included participants using smoked tobacco, smokeless tobacco, or both, for which we found no evidence of subgroup difference (P = 0.91; I2 = 0%; Analysis 5.1). We were unable to subgroup by level of motivation to quit, as no studies had recruitment methods that selected for motivation. We were unable to conduct our planned sensitivity analysis as all studies were at high risk of bias.
1.1. Analysis.

Comparison 1: Single‐session behavioural support (1 session) versus control: subgrouped by comparator, Outcome 1: Abstinence at 6+ months
5.1. Analysis.

Comparison 5: Single‐session behavioural support (1 session) versus control: subgrouped by tobacco‐use type, Outcome 1: Abstinence at 6+ months
Multi‐session behavioural support
We pooled four studies (n = 2639) testing behavioural interventions comprising more than one session with a dental professional compared with usual care, brief, or very brief advice. We found evidence of benefit from behavioural support (RR 1.90, 95% CI 1.17 to 3.11; Analysis 2.1), though there was substantial heterogeneity (I2 = 61%), which we could not explain through subgroup analysis. We conducted three subgroup analyses. First, we divided studies by the intensity of control (usual care control, or brief or very brief advice control) and found no evidence of subgroup difference (P = 0.87; I2 = 0%). Second, we divided studies by whether they included participants using smoked tobacco, smokeless tobacco, or both; we found no evidence of subgroup difference (P = 0.09; I2 = 57.6%; Analysis 6.1). Third, we divided studies by likely level of motivation to quit as indicated by the method of recruitment (not selected for motivation; more likely motivated); we found evidence of subgroup difference (P = 0.05; I2 = 74.6%; Analysis 7.1), but only one study fell into the 'more likely motivated' subgroup, and in both cases the subgroup effect estimates favoured the intervention. We were unable to conduct our planned sensitivity analysis as only one study was not at high risk of bias (Virtanen 2015).
2.1. Analysis.

Comparison 2: Multi‐session behavioural support (> 1 session) versus control: subgrouped by comparator, Outcome 1: Abstinence at 6+ months
6.1. Analysis.

Comparison 6: Multi‐session behavioural support (> 1 session) versus control: subgrouped by tobacco‐use type, Outcome 1: Abstinence at 6+ months
7.1. Analysis.

Comparison 7: Multi‐session behavioural support (> 1 session) versus control: subgrouped by motivation, Outcome 1: Abstinence at 6+ months
Behavioural support plus pharmacotherapy
We pooled four studies (n = 1221) testing behavioural interventions from a dental professional combined with the provision of NRT or e‐cigarettes, compared with no intervention, usual care, brief advice, or very brief advice, provided without NRT or e‐cigarettes. We found evidence of benefit from behavioural support and nicotine treatment (RR 2.76, 95% CI 1.58 to 4.82; Analysis 3.1), with no evidence of heterogeneity (I2 = 0%). As a result, our subgrouping by the intensity of the control group (usual care control, or brief or very brief advice control) found no evidence of a subgroup difference (P = 0.81; I2 = 0%), as did our subgrouping by likely level of motivation to quit (P = 0.98; I2 = 0%; Analysis 8.1). We were unable to subgroup by tobacco‐use type, as all studies were in smoked tobacco users. A sensitivity analysis removing two studies at high risk of bias still detected a benefit of behavioural support plus nicotine treatment (RR 2.86, 95% CI 1.14 to 7.18; I2 = 0%; two studies; n = 133). We also performed sensitivity analyses removing one study (Hanioka 2010) where participants received multiple sessions with a dental professional (RR 2.75, 95% CI 11.45 to 5.20; I2 = 0%; three studies; n = 1165), and removing the one study (Holliday 2019) that provided e‐cigarettes rather than NRT (RR 2.72, 95% CI 1.49 to 4.95; I2 = 0%; three studies; n = 1141). Both analyses still detected a similar benefit.
3.1. Analysis.

Comparison 3: Behavioural intervention + NRT/e‐cigarette versus control: subgrouped by comparator, Outcome 1: Abstinence at 6+ months
8.1. Analysis.

Comparison 8: Behavioural intervention + NRT/e‐cigarette versus control: subgrouped by motivation, Outcome 1: Abstinence at 6+ months
Behavioural support outside of a dental setting
We pooled three studies (n = 1020) testing the effect of multiple‐session behavioural interventions from dental professionals delivered in a high school or college instead of a dental setting. We did not find evidence of a benefit of the intervention (RR 1.51, 95% CI 0.86 to 2.65; Analysis 4.1). However, there was substantial heterogeneity (I2 = 83%). We were unable to subgroup by tobacco use type, as all studies were in smokeless tobacco users, and we were unable to subgroup by level of motivation to quit because no studies had recruitment methods that selected for motivation. We were unable to conduct our planned sensitivity analysis as all studies were at high risk of bias. We conducted a sensitivity analysis by removing Gansky 2005 because while the intervention comprised multiple sessions, only one was with a dental professional. Removing this study did change the result of the analysis, both detecting a benefit and removing the heterogeneity (RR 2.01, 95% CI 1.40 to 2.87; I2 = 0%; two studies; n = 499).
4.1. Analysis.

Comparison 4: Behavioural support from dental professional at high school/college versus usual care/no intervention, Outcome 1: Abstinence at 6+ months
We excluded McClure 2018 from this analysis because unlike the other non‐dental practice setting studies, this study was conducted over a smoking quit‐ line. Considered alone, this study did not detect a benefit of the intervention (RR 1.12, 95% CI 0.90 to 1.38; n = 718).
Adverse events
One study delivering an e‐cigarette intervention to smokers with periodontitis reported adverse events (Holliday 2019). Forty‐four percent of study participants reported an adverse event over the six months of the study, with 56 adverse events occurring in 35 participants. Most of the adverse events (toothache, dentine hypersensitivity, tooth loss and abscesses) were likely to be associated with the sequelae of severe periodontitis and were comparable across the study groups (RR 1.18, 95% CI 0.60 to 2.32; n = 80; Analysis 3.2.1). Five participants experienced adverse events less likely attributable to periodontitis (mouth ulceration or intra‐oral soft tissue soreness), with all of these occurring in the intervention group (RR 11.00, 95% CI 0.63 to 192.56; n = 80; Analysis 3.2.2). The authors discussed that these could have been associated with the e‐cigarette intervention (other forms of orally administered NRT have been associated with mouth soreness and ulceration; Hartmann‐Boyce 2018) or could be the result of the higher quit rate in the intervention group (mouth ulcers are a common result of stopping smoking, affecting two in five quitters; McRobbie 2004). The study reported no serious adverse events.
3.2. Analysis.

Comparison 3: Behavioural intervention + NRT/e‐cigarette versus control: subgrouped by comparator, Outcome 2: Adverse events
Oral health outcomes
It was not possible to perform a quantitative synthesis of the oral health outcomes since only one study reported them in detail (Holliday 2019), with one other study reporting whether participants had received dental care in the previous six months (McClure 2018). Holliday 2019 reported mean change from baseline to six months for mean probing pocket depths (MD ‐0.10, 95% CI ‐0.38 to 0.18; n = 58; Analysis 3.3.1), percentage of sites with pocket depths ≥ 5 mm (MD ‐2.20, 95% CI ‐9.07 to 4.67; n = 58; Analysis 3.3.2), percentage bleeding on probing (MD 4.10, 95% CI ‐2.87 to 11.07; n = 58; Analysis 3.3.3), oral dryness measured by the clinical oral dryness score (MD 0.40, 95% CI ‐0.35 to 1.15; n = 58; Analysis 3.3.4), and an oral health quality of life measure (OHQoL‐UK) (MD 1.40, 95% CI ‐5.90 to 8.70; n = 58; Analysis 3.4). In summary, participants showed improvements in all the oral health measures; improvements were similar in both the control and intervention groups. However, the study did not have sufficient power to detect differences between study arms.
3.3. Analysis.

Comparison 3: Behavioural intervention + NRT/e‐cigarette versus control: subgrouped by comparator, Outcome 3: Oral health outcomes
3.4. Analysis.

Comparison 3: Behavioural intervention + NRT/e‐cigarette versus control: subgrouped by comparator, Outcome 4: OHQoL‐UK
Discussion
Summary of main results
This systematic review provides evidence from 20 studies. Over 11,000 participants from 18 studies contributed to the meta‐analyses for the primary outcome of tobacco abstinence at six months or longer. We found evidence of very low to moderate certainty that dental professionals can successfully deliver tobacco cessation interventions to increase the chances of achieving long‐term tobacco‐use abstinence. These interventions included single and multi‐session behavioural support, and behavioural support with the addition of nicotine replacement therapy or e‐cigarettes. We did not find a benefit of multi‐session interventions delivered by dental professionals outside of a dental setting. However, this finding should be treated with caution, as the removal of one of the three studies, where only part of the intervention was delivered by a dental professional, resulted in a substantial change in the interpretation of results.
Where there were sufficient data available, we conducted subgroup analyses to attempt to explain heterogeneity in the results. We subgrouped by the comparator intervention received, whether studies included smoked or smokeless tobacco users, and the likely level of motivation to quit among participants, as indicated by the studies’ methods of participant recruitment. Only once did these analyses detect a subgroup difference; this should be treated with caution, as one of the subgroups only included one study.
Two studies reported on oral health outcomes, and only one of these reported findings in detail, meaning that we were not able to complete a quantitative synthesis of these data. The data from this single study showed that the control and intervention groups had similar improvements in oral health over the course of the study. Only one study reported on adverse events. Most of these adverse events appeared to be related to periodontitis or periodontal therapy. Some of the adverse events, such as mouth ulceration or soreness, could have been related to the nicotine in the intervention or as a common side‐effect of tobacco‐use cessation (McRobbie 2004).
Overall completeness and applicability of evidence
The majority of the studies (13 studies) were undertaken in the USA, with two undertaken in each of the UK and Sweden, and one in each of Japan, Malaysia and India. Given the significant differences in the healthcare systems and socioeconomic status in these different countries, this limits the generalisability of the results.
Although studies often described the practical details of how the interventions were delivered (e.g. telephone versus face‐to‐face, number of sessions, etc.) the details about specific behaviour change techniques used were often lacking. A recent systematic review and meta‐analysis of 142 smoking cessation trials found that reporting of the interventions was variable and incomplete (de Bruin 2020). Future trials should include comprehensive descriptions of the interventions delivered.
Quality of the evidence
We judged most studies (16 studies) to be at high risk of bias for a variety of reasons. The most common was a high risk of detection bias because tobacco‐use status was self‐reported and study groups received different levels of face‐to‐face contact. One study was at unclear risk of bias and three were at low risk of bias. The low risk of bias studies were all published in the last five years, potentially indicating an improvement in study conduct and reporting.
For the primary abstinence outcome, we assessed the certainty of the evidence for each of our analyses, using the GRADE system (Schünemann 2017). We judged the evidence for behavioural interventions comprising more than one session with a dental professional and behavioural interventions comprising a single session with a dental professional to be of very low certainty. In both cases, we downgraded the evidence due to risk of bias, inconsistency, and imprecision. We judged the evidence for behavioural interventions from a dental professional combined with the provision of NRT or e‐cigarettes to be of moderate certainty. We downgraded the evidence because of imprecision. We judged the evidence for multiple‐session behavioural interventions from dental professionals delivered in a high school or college instead of a dental setting to be of very low certainty. We downgraded the evidence because of risk of bias, inconsistency, and imprecision. Given the very low‐certainty evidence, future research is likely to substantially affect the conclusions of this review.
Subgroup analyses explored the impact of the varied comparator interventions on the pooled estimate for tobacco abstinence, but they did not explain the substantial heterogeneity we found in our analyses of single‐session and multi‐session behavioural interventions. However, it is worth noting that imprecise reporting in many studies concerning what treatment is involved in usual care may mean that some studies are misclassified in these subgroup analyses.
Potential biases in the review process
Cochrane methods are designed to minimise reviewer bias where possible. For example, at least two review authors independently conducted study selection, data extraction, and 'Risk of bias' assessments. A key possible limitation of the review is that we may have failed to identify all relevant research for inclusion in the review. However, given the nature of Cochrane methods, we are confident that any failures in identification of studies for inclusion will not be systematic, and therefore should not have a significant impact on the validity of our results.
Agreements and disagreements with other studies or reviews
We chose not to include interventions aimed at the training or provision of an educational intervention of or for dental health professionals. We felt that the inclusion of these studies would not address the objective of the review, which was to assess the effectiveness of the interventions and not the training of providers. The training of health professionals in smoking cessation is the topic of another Cochrane Review (Carson 2012). However, several studies are worthy of brief mention. Houston 2013 targeted dental providers through an internet‐based education system without finding a benefit for quit rates. Ray 2014 evaluated an electronic referral system for an online cessation resource, and while fewer referrals were made in the electronic referral group compared with those receiving paper‐based referrals, nearly four times as many people referred from the intervention group registered with the programme, and subsequent quit rates were four times higher. Ahmadian 2017 compared role play and problem‐based learning for teaching dental students tobacco cessation counselling skills and concluded that while both methods led to improved knowledge, attitude, and skills compared with before training, they could find no difference in quit rates between these methods. Albert 2004 evaluated the feasibility of face‐to‐face educational outreach visits to train dentists in tobacco cessation interventions; they noted significant barriers to implementing this. Walsh 2010 compared high‐ and low‐intensity training with or without reimbursement on dentist's attitudes and behaviours and found that while dentists in all intervention groups showed improvement in Assess, Assist and Arrange behaviours, participants whose dentists had received incentives were more likely to quit.
The findings of this review are in keeping with those of other related reviews. The effect sizes seen in our study are similar to those seen when medical physicians delivered smoking cessation advice (Stead 2013; RR 1.66, 95% CI 1.42 to 1.94) . When dental studies combined a method of behavioural support with nicotine treatment, we saw a larger treatment effect, which is in keeping with the evidence from other reviews (Cahill 2013; Hartmann‐Boyce 2019), although the numbers cannot be compared directly. Stead 2016 reviewed pharmacotherapy combined with behavioural treatment and found an RR of 1.83 (95% CI 1.68 to 1.98), which while being lower than the result seen in this review (RR 2.76, 95% CI 1.58 to 4.82), still agrees that there is a benefit of this kind of intervention.
Only one of the studies included in this review reported on the oral health response to smoking cessation (Holliday 2019). They did not detect a difference between control and intervention groups, but this was a small study and not powered to detect this outcome. Previous reviews on this topic have found that smoking cessation offered additional beneficial effects following non‐surgical periodontal therapy (Chambrone 2013).
Adverse events were reported by one study (Holliday 2019), with intra‐oral soreness and ulceration being associated with an e‐cigarette intervention group. Previous Cochrane Reviews on e‐cigarettes and NRT have reported similar localised irritation at the site of administration (Hartmann‐Boyce 2018; Hartmann‐Boyce 2020).
Authors' conclusions
Implications for practice.
There is very low‐certainty evidence that behavioural tobacco cessation interventions, delivered by dental professionals, can increase quit rates.
There is moderate‐certainty evidence that behavioural interventions combined with nicotine replacement, provided by dental professionals, may increase tobacco abstinence rates in cigarette smokers.
Implications for research.
Further well designed randomised controlled trials (RCTs) of smoking cessation interventions in dental settings are indicated. The evidence that pharmacological interventions delivered by dental professionals may be a particularly effective intervention suggests that future studies should explore this further. In particular, future studies should use biochemical validation of abstinence so as to preclude the risk of detection bias. Reporting more detail about specific behaviour change techniques used in study interventions would also help illuminate which components such interventions should contain. There has been very limited attention to cost‐effectiveness to date, and as the evidence regarding effectiveness grows, this will become more important to evaluate.
What's new
| Date | Event | Description |
|---|---|---|
| 1 February 2021 | New citation required but conclusions have not changed | Search updated February 2020, with new studies incorporated and methods and text updated to conform to new Cochrane standards |
| 21 February 2020 | New search has been performed | Title updated to reflect that not all studies are in dental settings. Search updated to February 2020. Five new studies included. Text and methodology updated to comply with the new editorial and methodological standards for Cochrane reviews ‐ see Differences between protocol and review for further details. Adverse events and oral health outcomes added and objectives restructured to account for this. |
History
Protocol first published: Issue 1, 2005 Review first published: Issue 1, 2006
| Date | Event | Description |
|---|---|---|
| 10 April 2012 | New citation required and conclusions have changed | Conclusions updated to include interventions among cigarette smokers as well as among smokeless tobacco users. New included studies increase strength of effect. |
| 10 April 2012 | New search has been performed | 8 new included studies added, evaluating interventions among cigarette smokers. |
| 22 February 2012 | New search has been performed | Updated search to November 2011 |
| 29 July 2008 | Amended | Converted to new review format. |
| 5 September 2006 | New search has been performed | Updated for issue 1 2007. No new studies identified. Two studies reviewed and added to excluded studies list. |
Acknowledgements
Due to competing interests for one included study (Holliday 2019), the data extraction and 'Risk of bias' assessment were completed by BH and Manás Dave, Academic Clinical Fellow in Oral Pathology, Manchester University, UK.
We would also like to acknowledge the support of the Cochrane Tobacco Addiction Group, specifically Nicola Lindson, who provided expert guidance during the review process. Our thanks to Dr Thomas James Lamont, University of Dundee, Scotland, and to another reviewer for conducting peer review, and to Sandra Wilcox for consumer review.
This project was supported by the National Institute for Health Research (NIHR), via a Clinical Lectureship (RH), Academic Clinical Fellowship (BH) and a Cochrane Infrastructure and Cochrane Programme grant funding to the Cochrane Tobacco Addiction Group. The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health and Social Care.
Appendices
Appendix 1. Search strategy: Cochrane Tobacco Addiction Group Specialised Register
1. dentist*:MH,EMT,KW,KY,XKY,TI,AB 2. dental*:MH,EMT,KW,KY,XKY,TI,AB 3. hygienist*:MH,EMT,KW,KY,XKY,TI,AB 4. oral health:MH,EMT,KW,KY,XKY,TI,AB 5. #1 OR #2 OR #3 OR #4 6. dentist*:MH,EMT,KW,KY,XKY,TI,AB 7. dental*:MH,EMT,KW,KY,XKY,TI,AB 8. hygienist*:MH,EMT,KW,KY,XKY,TI,AB 9. oral health:MH,EMT,KW,KY,XKY,TI,AB 10. #6 OR #7 OR #8 OR #9 11. #5 AND #10 AND INREGISTER
Data and analyses
Comparison 1. Single‐session behavioural support (1 session) versus control: subgrouped by comparator.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Abstinence at 6+ months | 4 | 6328 | Risk Ratio (M‐H, Random, 95% CI) | 1.86 [1.01, 3.41] |
| 1.1.1 Usual care control (not specified) | 2 | 5523 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [0.75, 2.49] |
| 1.1.2 Very brief or brief advice control | 2 | 805 | Risk Ratio (M‐H, Random, 95% CI) | 2.56 [1.03, 6.33] |
Comparison 2. Multi‐session behavioural support (> 1 session) versus control: subgrouped by comparator.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Abstinence at 6+ months | 7 | 2639 | Risk Ratio (M‐H, Random, 95% CI) | 1.90 [1.17, 3.11] |
| 2.1.1 Usual care control (not specified) | 2 | 1147 | Risk Ratio (M‐H, Random, 95% CI) | 2.03 [0.99, 4.17] |
| 2.1.2 Very brief or brief advice/less active treatment control | 5 | 1492 | Risk Ratio (M‐H, Random, 95% CI) | 1.86 [0.88, 3.93] |
Comparison 3. Behavioural intervention + NRT/e‐cigarette versus control: subgrouped by comparator.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Abstinence at 6+ months | 4 | 1221 | Risk Ratio (M‐H, Random, 95% CI) | 2.76 [1.58, 4.82] |
| 3.1.1 No intervention/usual care control | 2 | 1025 | Risk Ratio (M‐H, Random, 95% CI) | 2.86 [1.51, 5.44] |
| 3.1.2 Very brief or brief advice control | 2 | 196 | Risk Ratio (M‐H, Random, 95% CI) | 2.45 [0.79, 7.56] |
| 3.2 Adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.2.1 Likely sequelae of severe periodontitis | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.60, 2.32] |
| 3.2.2 Less likely attributable to periodontitis | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 11.00 [0.63, 192.56] |
| 3.3 Oral health outcomes | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.3.1 Mean probing pocket depths (mm) | 1 | 58 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.38, 0.18] |
| 3.3.2 Percentage of sites with pocket depths 5+ mm | 1 | 58 | Mean Difference (IV, Random, 95% CI) | ‐2.20 [‐9.07, 4.67] |
| 3.3.3 Percentage bleeding on probing | 1 | 58 | Mean Difference (IV, Random, 95% CI) | 4.10 [‐2.87, 11.07] |
| 3.3.4 Oral dryness | 1 | 58 | Mean Difference (IV, Random, 95% CI) | 0.40 [‐0.35, 1.15] |
| 3.4 OHQoL‐UK | 1 | 58 | Mean Difference (IV, Random, 95% CI) | 1.40 [‐5.90, 8.70] |
Comparison 4. Behavioural support from dental professional at high school/college versus usual care/no intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Abstinence at 6+ months | 3 | 1020 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [0.86, 2.65] |
Comparison 5. Single‐session behavioural support (1 session) versus control: subgrouped by tobacco‐use type.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Abstinence at 6+ months | 4 | 6328 | Risk Ratio (M‐H, Random, 95% CI) | 1.86 [1.01, 3.41] |
| 5.1.1 Smoked tobacco | 2 | 4346 | Risk Ratio (M‐H, Random, 95% CI) | 1.99 [0.58, 6.83] |
| 5.1.2 Smoked and smokeless tobacco | 2 | 1982 | Risk Ratio (M‐H, Random, 95% CI) | 1.83 [0.97, 3.45] |
Comparison 6. Multi‐session behavioural support (> 1 session) versus control: subgrouped by tobacco‐use type.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Abstinence at 6+ months | 7 | 2639 | Risk Ratio (M‐H, Random, 95% CI) | 1.90 [1.17, 3.11] |
| 6.1.1 Smoked tobacco | 3 | 507 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.47, 3.13] |
| 6.1.2 Smokeless tobacco | 3 | 1932 | Risk Ratio (M‐H, Random, 95% CI) | 2.15 [1.44, 3.20] |
| 6.1.3 Smoked and smokeless tobacco | 1 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 14.00 [1.88, 104.46] |
Comparison 7. Multi‐session behavioural support (> 1 session) versus control: subgrouped by motivation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Abstinence at 6+ months | 7 | 2639 | Risk Ratio (M‐H, Random, 95% CI) | 1.90 [1.17, 3.11] |
| 7.1.1 Not selected for motivation | 6 | 2439 | Risk Ratio (M‐H, Random, 95% CI) | 1.74 [1.11, 2.73] |
| 7.1.2 More likely motivated | 1 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 14.00 [1.88, 104.46] |
Comparison 8. Behavioural intervention + NRT/e‐cigarette versus control: subgrouped by motivation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Abstinence at 6+ months | 4 | 1221 | Risk Ratio (M‐H, Random, 95% CI) | 2.76 [1.58, 4.82] |
| 8.1.1 Not selected for motivation | 3 | 1165 | Risk Ratio (M‐H, Random, 95% CI) | 2.75 [1.45, 5.20] |
| 8.1.2 More likely motivated | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 2.79 [0.88, 8.78] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Andrews 1999.
| Study characteristics | ||
| Methods | Study Design: cluster‐randomised trial Location: USA Setting: dental practice Recruitment: dental offices in western Oregon, USA, from Medford to Portland, recruited by a letter, followed by a brief phone call. This procedure was supplemented by presentations at local dental and dental hygiene societies and study groups. |
|
| Participants | N = 633 (75 dental practices) Specialist population? no Participant characteristics: smokeless tobacco users ≥ 15 years of age Cigarettes/day: not reported Selected on motivation to quit? no |
|
| Interventions | Comparator: usual care. $2 bill (USD) as an incentive for returning forms and eligible for a USD 100 monthly lottery Modality of support: not specified Overall contact time: not specified Number of sessions: not specified Pharmacotherapy: not specified Intervention: direct advice to quit was given, relating this advice to oral health. A packet of written materials and a quit kit (e.g. sugarless candy and gum, flavoured toothpicks, rubber bands) was given to the participant. Asked the participant to set a quit date within 2 weeks of the visit. A motivational video was given to the participant. Participants were called within 2 weeks after the visit. Dentists were asked to briefly reinforce the advice given to the participant by the hygienist. Intervention provider: dental hygienist and dentist (training: 3 hour workshop) Modality of support: face‐to‐face Overall contact time: not reported Number of sessions: 1 Pharmacotherapy: not applicable |
|
| Outcomes | Definition of abstinence: 12 months 'sustained' abstinence from smokeless tobacco and all tobacco: subjects must have reported 7‐day point prevalence smokeless tobacco and all tobacco abstinence at both 3 months and 12 months. Longest follow‐up: 12 months Biochemical validation: none Oral health outcomes: not measured Adverse events: not reported |
|
| Funding source | QUOTE: "Supported in part by grant #1 R01 HL48768 from the National Heart, Lung and Blood Institute." | |
| Author conflicts of interest | No statement of declaration found | |
| Notes | • Data for smokers in the same trial reported in Severson 1998 • ICC calculated < 0.0009. • Cluster‐randomised controlled trial design accounted for in our analyses by 'approximately correct' analysis according to current guidelines (Higgins 2011) • Intervention group more likely to have previously been advised by a dental care provider to quit use of smokeless tobacco and were less likely to be unmarried • Study reports only those using smokeless tobacco |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | QUOTE: "Practices were blocked and randomised" (no further details given) |
| Allocation concealment (selection bias) | Unclear risk | QUOTE: "Practices were blocked and randomised" (no further details given) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Self‐reported abstinence rates reported and participants in the intervention arm are likely to have received longer personal contact time than the control arm. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 102/394 (26%) in intervention and 62/239 (26%) in the control group lost to follow‐up at 12 months |
Binnie 2007.
| Study characteristics | ||
| Methods | Study design: randomised controlled trial Location: UK Setting: hospital‐based periodontology clinic Recruitment: recruited new patients in a single hospital‐based periodontal clinic |
|
| Participants | N = 118 (59 randomised in the usual care arm but 1 died and 1 withdrew consent after completing baseline questionnaire but prior to attending any study visits) Specialised population? people with periodontal disease Participant characteristics: 82/116 (70.7%) female; average age 41.7 years; average cigarettes per day 20; nicotine dependence: Fagerstrom test for nicotine dependence 5.0; deprivation category 5‐7 (more deprived). 49% (57/116); motivation to quit: 'Stage of Change': pre‐contemplator 12.9% (15/116); contemplator 44.8% (52/116); preparation 42.2% (46/116). Selected on motivation to quit? no |
|
| Interventions | Common components of all trial arms: very brief advice to quit smoking by the examining consultant Comparator: as per the common component Comparator provider: participants in the comparator group received very brief advice to quit by a consultant (existing clinical care provider with no particular training in smoking cessation) Modality of support: face‐to‐face Overall contact time: very brief (time not specified) Number of sessions: 1 Pharmacotherapy: none Intervention: 5As intervention with free nicotine replacement therapy in the form of patches or gum. Particular emphasis on oral health aspects was developed for use by the dental hygienists Intervention provider: one of three dental hygienists with training (smoking cessation sessions (1 day equivalent, which covered epidemiology of tobacco use, nicotine dependence, basic smoking cessation skills, supporting the smoker), and training in nicotine replacement therapy (half day, which covered nicotine withdrawal, use of nicotine replacement therapy products, relevant clinical guidelines) and trial methodology (half day, which covered use of questionnaires in data collection, 5As methodology, salivary and carbon monoxide sampling) Modality of support: face‐to‐face Overall contact time: not reported Number of sessions: 1 Pharmacotherapy: free nicotine replacement therapy in the form of patches or gum |
|
| Outcomes | Definition of abstinence: point prevalence abstinence at 12 months validated by cotinine level (≤ 20 ng/ml) or carbon monoxide (≤ 8 PPM) Longest follow‐up: 12 months Biochemical validation: Yes, cotinine level (≤ 20 ng/ml) or carbon monoxide (≤ 8 PPM) Oral health outcomes: not reported Adverse events: not reported |
|
| Funding source | Nicotine replacement therapy was funded by the local NHS Smoking Cessation Services (Smoking Concerns, Glasgow, UK) but not reported whether any other bodies funded other aspects of the study. | |
| Author conflicts of interest | QUOTE: "VB has received funding from Pfizer for attending meetings, and has written articles on smoking cessation for GSK. None of the other authors have conflicting interests." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation process was set up by the project statistician and was implemented independently from the recruitment process. After recruitment, the patient's name was transcribed into a log book containing sequential patient log numbers and the allocated hygienist. |
| Allocation concealment (selection bias) | Low risk | After allocating the patient to a hygienist, the patient was allocated to either intervention or control group using the minimization method. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Abstinence data validated by cotinine or carbon monoxide level |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Overall attrition was 52.5% (if using 118 denominator). Differential rates between groups: Intervention had 44% attrition compared to 61% in control. |
Cohen 1989.
| Study characteristics | ||
| Methods | Study design: cluster‐randomised trial Location: USA Setting: private dental practice Recruitment: identified in private dental practices. When calling to confirm an appointment, the receptionist in each dental practice determined whether a patient was currently a cigarette smoker. |
|
| Participants | N = 374 (number in each arm unspecified) Specialised population? no Participant characteristics: 583/1,027 (56.8%) female; average age 37.1 (10.4) years; nicotine dependence: Fagerstrom test for nicotine dependence 5.5 (1.9); median amount of schooling completed: 1 year of college; average cigarettes per day: not reported; adults (18 to 64) smoking 1 ≤ cigarettes daily and had an "alveolar breath carbon monoxide determination" of > 8 PPM. Selected on motivation to quit? no |
|
| Interventions | Common components in all trial arms: All dentists either attended a 1 hour lecture or received personalised instructions on the medical consequences of smoking, the benefits of quitting and the evidence that physicians’ advice or nicotine‐containing chewing gum, or both can be effective in helping patients quit smoking. Dentists were suggested a four‐step protocol for counselling patients and gave them a booklet: 1. Ask patients about smoking; 2. Deliver a firm quit‐smoking message; 3. Mutually agree on a quit date; 4. Check on each patient’s progress at each regularly scheduled visit. Comparator: as per common components Comparator provider: dentist Modality of support: face‐to‐face Overall contact time: exact duration unspecified but likely to have been brief (a few minutes) Number of sessions: 1 Pharmacotherapy: none Intervention 1: dentists were told that their patients who had a fluorescent red sticker attached to their chart would be eligible for up to a 10 box supply of a nicotine chewing gum at no cost. Intervention provider: dentist Modality of support: face to face Overall contact time: exact duration unspecified but likely to have been brief (a few minutes) Number of sessions: 1 Pharmacotherapy: nicotine gum Intervention 2: two fluorescent stickers were placed on the charts to help dentists and their staff members to remember to follow the step‐care protocol: green sticker asked "did you talk to the patient today about smoking? yes or no"; orange sticker stated "the patient has agreed to the following quit date__". Intervention provider: dentist Modality of support: face‐to‐face Overall contact time: exact duration unspecified but likely to have been brief (a few minutes) Number of sessions: 1 Pharmacotherapy: none Intervention 3: dentists were told that their patients who had a fluorescent red sticker attached to their chart would be eligible for up to a 10 box supply of a nicotine chewing gum at no cost. Two fluorescent stickers were placed on the charts to help dentists and their staff members to remember to follow the step‐care protocol: green sticker asked "did you talk to the patient today about smoking? yes or no"; orange sticker stated "the patient has agreed to the following quit date__" Intervention provider: dentist Modality of support: face‐to‐face Overall contact time: exact duration unspecified but likely to have been brief (a few minutes) Number of sessions: 1 Pharmacotherapy: nicotine gum |
|
| Outcomes | Definition of abstinence: point prevalence abstinence at the follow‐up visit via carbon monoxide ≤ 8 PPM Longest follow‐up: 12 months Biochemical validation: carbon monoxide ≤ 8 PPM Oral health outcomes: not measured Adverse events: not reported |
|
| Funding source | QUOTE: "This research was supported by a grant from the National Cancer Institute, grant no. PHS 1 R01 CA38337." | |
| Author conflicts of interest | Lakeside Pharmaceuticals provided nicotine gum. None other stated. | |
| Notes | • New for 2020 update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | QUOTE: “Randomly assigned to one of four study groups" (no further details given) |
| Allocation concealment (selection bias) | Unclear risk | QUOTE: “Randomly assigned to one of four study groups" (no further details given) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation of abstinence |
| Incomplete outcome data (attrition bias) All outcomes | High risk | • Overall attrition was 58% • Dentists were the unit of randomization • N randomised was 50 dentists, 6 dropped out and analyses are based on 44 dentists. It is unspecified how many dentists dropped out from each arm • The total number of participants randomised is given as 1027 but unclear whether this was before dropout of 6 dentists. Also, the number of patients randomised or lost per group is not given |
Ebbert 2007.
| Study characteristics | ||
| Methods | Study design: cluster‐randomised controlled trial Location: USA Setting: dental practice Recruitment: recruited smokers attending general dental practices |
|
| Participants | N = 82 participants (8 practices) Specialised population? no Participant characteristics: 33/60 (55%) female; average age 43.8 years in 28/60 who completed questionnaire in the intervention group; nicotine dependence not reported; average cigarettes per day not reported. Selected on motivation to quit? no |
|
| Interventions | Common components in all trial arms: Brief (10 minutes) counselling from a dental hygienist and a dentist during the hygiene visit and National Cancer Institute brochure ("Clearing the air") provided. Comparator: brief counselling plus patient education brochure Comparator provider: dental hygienists (training provided by the research team but duration and modality unspecified) Modality of support: face‐to‐face, telephone Overall contact time: brief counselling 10 minutes Number of sessions: 1 Pharmacotherapy: none Intervention: after the dental hygiene visit, the dental office faxed the subject's contact information to the tobacco use quit line. A staff member from the quit line contacted the participant within 48 hours of receiving the fax and provided counselling (45 minutes) and set a quit date if the participant was willing. Further calls (20 minutes each) from a counsellor 1 and 2 weeks after the quit date. Additional calls made for up to 10 weeks after the dental visit if the participant requested them. Intervention provider: dental hygienists, dentists, counsellors (training provided by the research team but duration and modality unspecified). Modality of support: face‐to‐face, telephone Overall contact time: 75 minutes (brief counselling 10 minutes; initial session 45 minutes; subsequent session 20 minutes) Number of sessions: varied from 3 to 7 Pharmacotherapy: none |
|
| Outcomes | Definition of abstinence: self‐reported 7 day point prevalence abstinence Longest follow‐up: 6 months Biochemical validation: none Oral health outcomes: not measured Adverse events: not reported |
|
| Funding source | QUOTE: "This project was supported by the National Institute of Dental and Craniofacial Research grant DE16024‐02." | |
| Author conflicts of interest | No declaration of interests statement found in the paper | |
| Notes | • ICC 0.001 • Cluster‐randomised controlled trial design accounted for in our analyses by 'approximately correct' analysis according to current guidelines (Higgins 2011) • Quit line dose‐response trend noted |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random table used for random assignment of clinics to intervention or usual care (stated by the authors although information not found in the paper) |
| Allocation concealment (selection bias) | Unclear risk | Clinic allocation not concealed at time of participant recruitment, and lower participation rate amongst control clinic patients |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Smoking status was self‐reported and participants in the intervention arm received more personal contact than the usual care arm. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No attrition from 3 to 6 months in responders. Only 17 of 60 quit line subjects received any follow‐up counselling and 32 of the 60 in the quit line group received no quit line contact |
Gansky 2005.
| Study characteristics | ||
| Methods | Study design: cluster‐randomised controlled trial Location: USA Setting: colleges Recruitment: contacted athletic trainers at California colleges |
|
| Participants | N = 637 (52 colleges) Specialised population? baseball athletes Population characteristics: 0 (0%) female; age brackets provided: 17 to 18 years: 34% (534/1585); 19 to 20 years: 50% (777/1585); > 20 years: 16% (254/1585); nicotine dependence not reported; average cigarettes per day not reported; self‐efficacy ("how confident are you that you can quit for good in the next 2‐3 weeks?" with 4 response options): not at all/a little 28% (111/396); somewhat 26% (138/531); very 53% (280/528); male college baseball athletes who use smokeless tobacco; cigarettes/day: not reported. Selected on motivation to quit? no |
|
| Interventions | Comparator: no intervention Comparator provider: not applicable Modality of support: not applicable Overall contact time: not applicable Number of sessions: not applicable Pharmacotherapy: none Intervention: based upon the innovation theory and social learning theory. Video conference and follow‐up newsletter: three hours with athletic trainers/dentists/hygienists. Dental component: dentists/hygienists provided oral cancer screening, advised smokeless tobacco users to stop, identified oral lesions, provided self‐help guide, offered single 10 to 15 min individual counselling session focusing on smokeless tobacco addiction, set a quit date, developing a plan, training in action and thinking skills to get ready to quit and to prevent relapse. Athletic trainers follow‐up and referral: follow‐up by athletic trainers on quit date and three booster sessions one week apart. Peer‐led component: 50‐60 minute education meeting which included 3 components: two videos and slides of facial disfigurement. Intervention provider: ‘specially trained’ dentists or dental hygienists Modality of support: face‐to‐face, internet Overall contact time: 10 to 15 minutes Number of sessions: 1 Pharmacotherapy: none |
|
| Outcomes | Definition of abstinence: self‐reported 30‐day point prevalence smokeless tobacco abstinence Longest follow‐up: 12 months Biochemical validation: none Oral health outcomes: not measured Adverse events: not reported |
|
| Funding source | QUOTE: "This research was supported by the Tobacco Surtax Fund of the State of California (Grant 4RT‐0068) through the Tobacco‐Related Disease Research Program of the University of California." | |
| Author conflicts of interest | No declaration of interests statement found in the paper | |
| Notes | • Spillover of cessation intervention seen in control groups via athletic trainer activity. ICC: 0.0197 (the previous version of this review reported the ICC to be 0.0074 but this has been changed to 0.0197 in the updated review; the ICC of 0.0074 was reported for 'initiation' of smokeless tobacco use whereas the ICC of 0.0197 was reported for 'cessation' of smokeless tobacco). • Cluster‐randomised controlled trial design accounted for in our analyses by 'approximately correct' analysis according to current guidelines (Higgins 2011) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Cluster‐randomised: colleges stratified by tertiles of baseline smokeless tobacco use then within strata, colleges were randomised to intervention or control group. |
| Allocation concealment (selection bias) | Low risk | Intervention assignment determined by the allocation of the school (cluster) they attended; no differential recruitment suspected. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Self‐reported abstinence rate reported and it appears that only the intervention group received face‐to‐face intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 76% of smokeless tobacco users, 81% non‐users completed one year follow‐up [non‐significant after adjustment]; no differential drop‐out seen between groups. |
| Other bias | High risk | Spillover of cessation intervention seen in control group via athletic trainer activity. |
Gordon 2010a.
| Study characteristics | ||
| Methods | Study design: cluster‐randomised controlled trial Location: USA Setting: private dental clinic Recruitment: tobacco users in private practice clinics |
|
| Participants | N = 2160 (68 dental practices) Specialised population? no Population characteristics: 1296/2160 (60%) female; average age not reported; nicotine dependence not reported; average cigarettes per day not reported; readiness to quit assessed using contemplation ladder was on average 6/10; 1296/2160 (60%) had some college education; tobacco users attending 68 dental clinics in Mississippi. Selected on motivation to quit? no |
|
| Interventions | Comparator: practitioners provided usual tobacco‐use cessation services Comparator provider: “practitioners” with no specific training on smoking cessation Modality of support: face‐to‐face Overall contact time: not reported Number of sessions: not reported Pharmacotherapy: not reported Intervention 1: 3As intervention; ask, advise, arrange quit line referral using ‘fax‐to‐quit' referral form. Intervention provider: dentists and dental hygienists who had two hours of training workshop Modality of support: face‐to‐face Overall contact time: not reported Number of sessions: 1 Pharmacotherapy: none Intervention 2: 5As intervention; ask, advise, assess, assist, arrange counselling with quit line referral as an option at provider’s discretion Intervention provider: dentists and dental hygienists who have had three hours of training workshop Modality of support: face‐to‐face Overall contact time: not reported Number of sessions: 1 Pharmacotherapy: none |
|
| Outcomes | Definition of abstinence: self‐reported 12 months prolonged abstinence (9 months without tobacco use with 3 months grace period) Longest follow‐up: 12 months Biochemical validation: none Oral health outcomes: not measured Adverse events: not reported |
|
| Funding source | QUOTE: "This research was funded by grant R01 DA017972 from the National Institutes of Health, National Institute on Drug Abuse, to the Oregon Research Institute, Eugene." | |
| Author conflicts of interest | No declaration of interests statement found in the paper | |
| Notes | • The two behavioural interventions (3As and 5As) were combined to a single group for the meta‐analysis. • ICC 0.012 • Cluster‐randomised controlled trial design accounted for in our analyses by 'approximately correct' analysis according to current guidelines (Higgins 2011) • Significant impact on study by Hurricane Katrina. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | QUOTE: "Assigned each of them randomly" (no further details given) |
| Allocation concealment (selection bias) | Unclear risk | QUOTE: "Assigned each of them randomly" (no further details given) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Smoking status was self‐reported and participants in the intervention arms were likely to have received longer personal contact time than the usual care arm. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Overall attrition at 12 months was 28% and well balanced between arms (usual care 26.4%, 5 A’s 26.4%, 3 A’s 31.3%). |
Gordon 2010b.
| Study characteristics | ||
| Methods | Study design: cluster‐randomised controlled trial Location: USA Setting: community public health dental clinics Recruitment: smokers attending federally‐funded public health dental clinics in Mississippi, New York & Oregon |
|
| Participants | N = 2549 (14 dental clinics) Specialised population? no Population characteristics: 1508/2637 (57.2%) female; average age 40.5 (12.6); nicotine dependence not reported; average cigarettes per day 16.1 (10.4); 1906/2623 (72.3%) high school graduate or greater education level; readiness to quit scale (0 to 10) mean score 6.7 (2.7); adult smokers attending public dental clinics for non‐emergency visits. Selected on motivation to quit? no, but recruitment method is likely to have selected those more motivated. |
|
| Interventions | Comparator: tobacco cessation methods as standard practice Comparator provider: “practitioners” with no specific training on smoking cessation Modality of support: face‐to‐face Overall contact time: not reported Number of sessions: 1 Pharmacotherapy: none Intervention: brief ‘tailored’ tobacco advice following the 5As approach & nicotine replacement therapy. Self‐help material was tailored for type of tobacco used and race/ethnicity Intervention provider: dentists, dental hygienists, dental assistants with three hour training workshop Modality of support: face‐to‐face Overall contact time: not reported Number of sessions: 1 Pharmacotherapy: nicotine patches and lozenges |
|
| Outcomes | Definition of abstinence: prolonged defined as no tobacco use six months post‐enrolment plus a six‐week grace period Longest follow‐up: 7.5 months Biochemical validation: none Oral health outcomes: not measured Adverse events: not reported Prolonged abstinence at 7.5 months |
|
| Funding source | QUOTE: "This research was funded by grant from the National Institutes of Health, National Cancer Institute (grant R01‐CA107442)." | |
| Author conflicts of interest | No declaration of interests statement found in the paper | |
| Notes | • Did not use small participant group of smokeless tobacco only (2.4%) and smokeless tobacco/smoked tobacco (1%) users in analysis • ICC for prolonged abstinence at 7.5 months: 0.009 • Cluster‐randomised controlled trial design accounted for in our analyses by 'approximately correct' analysis according to current guidelines (Higgins 2011) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | QUOTE: "Stratified by size and patient ethnicity … and then randomised" (no further details given) |
| Allocation concealment (selection bias) | Unclear risk | QUOTE: "Stratified by size and patient ethnicity … and then randomised" (no further details given) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Smoking status was self‐reported and participants in the intervention arms are likely to have received longer personal contact time than the usual care arm. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Overall attrition 29%. Individual group attrition is reported (usual care = 26.1%; intervention = 30.7%) Women more likely to respond than men; respondents were older, smoked longer, and more educated; impact of responder profile for significant bias likely low. |
Hanioka 2010.
| Study characteristics | ||
| Methods | Study design: parallel randomised controlled trial Location: Japan Setting: dental clinic Recruitment: recruited adults willing to stop smoking within 1 month |
|
| Participants | N = 56 Specialised population? no Population characteristics: 16/56 (28.6%) female; average age 47.4; nicotine dependence: tobacco dependence screener: intervention 7.3; non‐intervention 6.0; motivation to quit: 100% willing to stop within 1 month; average cigarettes per day; intervention: 24.3 (95% CI 19.5 to 29.0); non‐intervention: 20.6 (95% CI 14.9 to 26.3). Selected on motivation to quit? yes |
|
| Interventions | Comparator: non‐intervention Comparator provider: dentists and dental hygienists with no specific training on smoking cessation Modality of support: not described Overall contact time: not reported Number of sessions: not reported Pharmacotherapy: not reported Intervention: behavioral and pharmacological (nicotine patch) relapse strategies; counselled at initial 2 visits and at weeks 2, 4, 8, 12 Intervention provider: dentists and dental hygienists with three‐hour training Modality of support: face‐to‐face Overall contact time: average duration of 116.2 minutes Number of sessions: average 4.4 sessions (week 2, 4, 8, 12 over 10 weeks) Pharmacotherapy: free nicotine patches and information about gum (which could be purchased in drug store) |
|
| Outcomes | Definition of abstinence: continuous abstinence validated by saliva cotinine level (< 20 ng/ml) at 12 months Longest follow‐up: 12 months Biochemical validation: validation by saliva cotinine < 20 ng/ml Oral health outcomes: not measured Adverse events: not reported |
|
| Funding source | QUOTE: "This study was supported by a Fukuoka Dental College Grant and by Grant‐in‐Aids for Cancer Research from the Japanese Ministry of Health, Labor and Welfare (13–3, 17–1)." | |
| Author conflicts of interest | No declaration of interests statement found in the paper | |
| Notes | Results for willing to quit cohort. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | QUOTE: "Modified random consent design… assigned to the intervention or non‐intervention groups according to an assignment card in an envelope provided a priori to clinics" (no further details provided) |
| Allocation concealment (selection bias) | Unclear risk | QUOTE: "Modified random consent design… assigned to the intervention or non‐intervention groups according to an assignment card in an envelope provided a priori to clinics" (no further details provided) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Abstinence rates were verified by salivary cotinine |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Overall attrition: 26.8%; intervention group: 24.2%; non‐intervention group: 30.4%. |
Holliday 2019.
| Study characteristics | ||
| Methods | Study design: parallel randomised controlled trial Location: UK Setting: single centre (Newcastle Dental Hospital, UK) Recruitment: identified in clinics by a member of the existing clinical care team and recruited by a member of the research team at the Newcastle Dental Hospital or by general dental practitioners, therapists or hygienists in primary care. |
|
| Participants | N = 80 Specialised population? diagnosed with periodontitis, having interproximal probing pocket depths of ≥ 5 mm at ≥ 8 sites Population characteristics: 42/80 (52.5%) female; average age 44.3 (10.7); nicotine dependence: Fagerstrom test for nicotine dependence 5.0 (2.1); average cigarettes per day 17.4 (6.6); socioeconomic status: 22/80 (27%) working in a managerial or professional occupation, 20/80 (25%) working in a routine or manual occupation; 8/80 (10%) unemployed or not working for a year or more; adult smoker of burnt tobacco (≥ 10 factory‐made cigarettes/day or 7 g loose tobacco/day or 14 hand‐rolled cigarettes/day); not currently using an e‐cigarette or not having used one for more than two days in the last 30 days; a minimum of 16 natural teeth present, excluding third molars). Selected on motivation to quit? no |
|
| Interventions | Common components of all trial arms: Smoking cessation advice (ask, advise, act) delivered by a single treating dentist alongside their dental and periodontal treatment. Offer of referral to the ‘Newcastle Stop Smoking’ services. Standard non‐surgical periodontal therapy: oral hygiene instruction, full mouth debridement in line with local and international guidance on an individualised basis. Visit 2 was designated as the target quit date and was arranged after discussion with the participant with the recommendation that it was ideally within 4 weeks of Visit 1. Comparator: participants were asked to commit to not using an e‐cigarette for the duration of the study, especially during the first 4 weeks, and were invited to sign a commitment form agreeing to this Comparator provider: a single dentist member of the research team who completed National Centre for Smoking Cessation and Training electronic learning module for very brief advice on smoking cessation Modality of support: face‐to‐face Overall contact time: 3 minutes 12 seconds (mean) Number of sessions: 1 Pharmacotherapy: none Intervention: participants were offered e‐cigarette starter kit including a two‐week supply of e‐liquid (with a choice of flavour and nicotine strength), given information on where to buy more and were advised to use only the recommended brand of e‐liquid for the duration of the study Intervention provider: a single dentist member of the research team who completed National Centre for Smoking Cessation and Training electronic learning module for very brief advice on smoking cessation Modality of support: face‐to‐face Overall contact time: 9 minutes 29 seconds (mean) Number of sessions: 1 Pharmacotherapy: nicotine strength in e‐cigarette available in 0 mg/ml, 6 mg/ml, 12 mg/ml, 18 mg/ml. |
|
| Outcomes | Definition of abstinence: continuous six months' abstinence verified by expired air carbon monoxide < 10 PPM Longest follow‐up: 6 months Biochemical validation: expired air carbon monoxide < 10 PPM Oral health outcomes: measured probing pocket depths, bleeding on probing, oral health‐related quality of life, and clinical oral dryness score at baseline and at 6 months Adverse events: measured although not categorized into pre‐/post‐quit |
|
| Funding source | QUOTE: "Richard Holliday is funded by a National Institute for Health Research Doctoral Research Fellowship (DRF‐2015‐08‐077)." | |
| Author conflicts of interest | QUOTE: "The authors declare that they have no competing interests." | |
| Notes | New for 2020 update. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | QUOTE: "Randomised in a 1:1 ratio using random permuted blocks of variable size (2, 4, or 6)... randomization was performed using a secure password‐protected web‐based system" |
| Allocation concealment (selection bias) | Low risk | QUOTE: "Allocation schedule was generated by a statistician with no other involvement in the study" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Abstinence rates verified by expired air carbon monoxide levels |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 11 out of 40 participants (27.5%) lost to follow‐up in each arm |
Lando 2007.
| Study characteristics | ||
| Methods | Study design: parallel randomised controlled trial Location: USA Setting: dental clinics Recruitment: adolescents attending hygiene visits within multi‐clinic managed care organization |
|
| Participants | N = 124 Specialist population? no Participant characteristics: adolescents (14 to 17 years old) attending dental offices for hygiene care. Further demographic details not reported. Selected on motivation to quit? no |
|
| Interventions | Common components in all trial arms: provider advice Comparator: Usual care (provider advice) Comparator provider: dental hygienists and dentists Provider training: no Modality of support: face‐to‐face Overall contact time: up to 60 seconds Number of sessions: 1 Pharmacotherapy: none Intervention: provider advice plus motivational interviewing/follow‐up phone calls Intervention provider: dental hygienists and dentists Provider training: 20 hours Modality of support: face‐to‐face & telephone Overall contact time: 15 to 20 minutes face to face and 3 to 6 phone calls within 6 months Number of sessions: minimum 2, maximum 7 Pharmacotherapy: none |
|
| Outcomes | Definition of abstinence: self‐reported 30 day point prevalence Longest follow‐up: 12 months Biochemical validation: no Oral health outcomes: not reported Adverse events: not reported |
|
| Funding source | QUOTE: "This research was supported by a grant from the National Institute of Dental and Craniofacial Research (Grant R01 DE12677)." | |
| Author conflicts of interest | No declaration of interests statement found in the paper | |
| Notes | Significant process errors impacting recruitment and limiting amount of useful study data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | QUOTE: "Randomly assigned to either provider advice only or provider advice plus motivational interviewing. This was done using a table of random numbers in sequential order with odd numbers assigned to intervention and even numbers assigned to control". |
| Allocation concealment (selection bias) | High risk | Person responsible for assignment knew which group the participant would be assigned to. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Smoking status was self‐reported and participants in the intervention arm received more personal contact than the usual care arm. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Authors reported that administrative database problems had significant impact on study data. Overall attrition was 34.6% at 12 months. Attrition per arm was not reported. |
McClure 2018.
| Study characteristics | ||
| Methods | Study design: parallel randomised controlled trial Location: USA Setting: quit line Recruitment: invited callers to tobacco quit lines to be screened for eligibility. Adult (≥ 18 years) smokers who smoked ≥ 5 cigarettes per day and were ready to quit smoking; had no dental appointment in the previous or upcoming 6 months and had some natural teeth. |
|
| Participants | N = 718 Specialist population? no Participant characteristics: 443/718 (61.8%) female; average age: 44.3 (12.2); cigarettes per day: 19.1 (9.7); education: < high school 17.4% (123/718), general equivalency diploma or high school degree 37.6% (266/718), some college, technical or trade school 29.4% (201/718), ≥ college degree 15.1% (107/718); motivation to quit: 4.9 (0‐5 Likert scale); self‐efficacy: 4.5 (0 to 5 Likert scale). Selected on motivation to quit? yes, participants had already called quit line |
|
| Interventions | Common components in all trial arms: 4 to 5 planned calls (call 1: pre‐target quit date; call 2: quit date or 1 day post quit date; call 3: 1 to 2 weeks post quit date; call 4: 3 weeks after call 3; call 5: 3 weeks after call 4). Mailed smoking cessation guide, access to online cessation content. Encouraged callers to set a target quit date during their first contact, followed by subsequent calls to assist in quitting and remaining abstinent. 2‐ or 4‐week starter kit of nicotine replacement therapy. 16 attention‐matched text messages over 23 weeks ‐ with a focus on improving oral health ‐ text message 1: 1 day prior to quit date; text message 2 to 8: once a week; text message 9 to 16: every other week. Comparator: control intervention ‐ 16 attention‐matched text messages ‐ general health behaviour tips but not oral health messaging Comparator provider: quit line counsellor Provider training: none Modality of support: telephone, internet, text message Overall contact time: not reported Number of session: 4 to 5 calls, 16 text messages Pharmacotherapy: 2‐ or 4‐week starter kit of nicotine replacement therapy Intervention: 'Oral Health for Life' programme ‐ scripted oral health counselling integrated into each quit line call:
Intervention provider: quit line counsellor Provider training: two‐hour training on the intervention protocol and integration of the oral health counselling into standard cessation call Modality of support: telephone, internet, text message Overall contact time: not reported Number of session: 4 to 5 calls, 16 text messages Pharmacotherapy: 2‐ or 4‐week starter kit of nicotine replacement therapy |
|
| Outcomes | Definition of abstinence: self‐reported 7 day point prevalence of abstinence at 6 months Longest follow‐up: 6 months Biochemical validation: none Oral health outcomes: measured whether or not participants received dental care in the last 6 months Adverse events: not reported |
|
| Funding source | QUOTE: "This research was supported by the National Institute for Dental and Craniofacial Research (NIDCR; grant U01DE024462)." | |
| Author conflicts of interest | QUOTE: "The authors declare that they have no competing interests." | |
| Notes | New for 2020 update. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | QUOTE: “Randomised using an automated algorithm built into the quit line provider’s systems" |
| Allocation concealment (selection bias) | Low risk | Automated central system |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported abstinence outcomes, but there was no face‐to‐face contact and it is unlikely that the participants would have known about the intervention of the other group. Each counsellor provided either intervention or control |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 27.7% (99/358) in the intervention group and 27.5% (99/360) in the control group were lost to follow‐up |
Nohlert 2013.
| Study characteristics | ||
| Methods | Study design: parallel randomised controlled trial Location: Sweden Setting: mixed urban/rural general dental clinics Recruitment: smokers identified via dental and health care personnel screening over 18 months |
|
| Participants | N = 300 (12 died and 4 emigrated leaving 284 for follow‐up) Specialist population? no Participant characteristics: 226/284 (80%) female; average age: 48.6 (10.3) years; cigarettes per day: 106 (45); intention to quit: 4/278 (1%) not within six months, 139/278 (50%) within six months, 133/278 (48%) within one month, 2/278 (1%) trying just now. (Six did not complete the baseline questionnaire hence total of 278) Selected on motivation to quit? no |
|
| Interventions | Common components in all trial arms: smoking cessation date fixed for all participants in both groups in the first meeting. Comparator: low intensity intervention‐ 1 x 30 minutes counselling session explaining a self‐help program [eight week program]. Comparator provider: dental hygienists Provider training: yes Modality of support: face‐to‐face Overall contact time: 30 minutes Number of sessions: 1 Pharmacotherapy: none Intervention: high intensity intervention‐ 8 x 40 minute counselling sessions over 4 months; mixed behavioral, coaching, and pharmacological advice. Intervention provider: dental hygienists Provider training: yes Modality of support: face‐to‐face Overall contact time: 320 minutes Number of sessions: 8 Pharmacotherapy: none |
|
| Outcomes | Definition of abstinence: self‐reported sustained abstinence defined as quitting at the fixed smoking cessation date and not smoking at all since then Longest follow‐up: 5‐8 years Biochemical validation: no Oral health outcome: not reported Adverse events: not reported |
|
| Funding source | QUOTE: "This work was supported by grants from the Västmanland County Council, Sweden." | |
| Author conflicts of interest | QUOTE: "The authors declare that they have no competing interests." | |
| Notes | Did not exclude smokeless tobacco users from obtaining cessation support; smokers meeting inclusion criteria were randomised. New for 2020 update (replaced Nohlert 2009 used in previous version of this review). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed by an independent person using an envelope technique in blocks of four |
| Allocation concealment (selection bias) | Low risk | Assignment done through central location after consent/baseline questionnaire received |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Smoking status was self‐reported and participants in the intervention arms received more personal contact than the usual care arm. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 29% of high intensity treatment; 18% of low intensity treatment |
Selvamary 2016.
| Study characteristics | ||
| Methods | Study design: randomised controlled trial Location: India Setting: tobacco cessation centre of a dental hospital Recruitment: QUOTE: "those referred to the tobacco cessation centre" (no further details available) |
|
| Participants | N = 200 Specialist population? no Participant details: gender not reported; average age: 34.9 years in health education group and 35.6 in cognitive behavioural therapy group. Selected on motivation to quit? yes |
|
| Interventions | Common components in all trial arms: health education by eight custom‐made posters, a pamphlet and a video developed in local language and in English, explaining the epidemiology of tobacco‐related deaths, chemical contents, various forms of tobacco, legislation related to tobacco in India, addiction and benefits of quitting and instructions on how to quit. Comparator: health education‐ reinforcement of health education. Comparator provider: not reported Provider training: not reported Modality of support: face‐to‐face Overall contact time: not reported Number of sessions: 2 Pharmacotherapy: none Intervention: health education and cognitive behavioural therapy‐ cognitive behavioural therapy consisting of provision of cognition, counselling, motivational interviewing and relapse prevention strategies. Intervention provider: not reported Provider training: not reported Modality of support: face‐to‐face Overall contact time: not reported Number of sessions: 2 Pharmacotherapy: none |
|
| Outcomes | Definition of abstinence: self‐reported continuous abstinence verified by cotinine level Longest follow‐up: 6 months Biochemical validation: cotinine level (no cut‐off specified) Oral health outcomes: not reported Adverse events: not reported |
|
| Funding source | QUOTE: "Nil" | |
| Author conflicts of interest | QUOTE: "There are no conflicts of interest." | |
| Notes | New for the 2020 update. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | QUOTE: "Computer‐generated randomization" |
| Allocation concealment (selection bias) | Low risk | QUOTE: "Computer‐generated randomization" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Cotinine‐validated abstinence rate |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 79.0% (79/100) in the health education group and 57.0% (57/100) in the health education and cognitive behavioural therapy group were lost to follow‐up. The authors attributed the high rate of attrition to changes in contact details of the participants. No sensitivity analysis |
Severson 1998.
| Study characteristics | ||
| Methods | Study design: cluster‐randomised controlled trial Location: USA Setting: private dental practices Recruitment: hygiene patients in 75 private practices |
|
| Participants | N = 4029 (75 dental practices) Specialist population? no Population characteristics: cigarette smokers, ≥ 15 years of age. No further demographic details reported. Selected on motivation to quit? no |
|
| Interventions | Comparator: usual care Comparator provider: not reported Provider training: not reported Modality of support: not reported Overall contact time: not reported Number of sessions: not reported Pharmacotherapy: not reported Intervention 1: minimal intervention‐ determined tobacco use status from the patient's chart and health questionnaire; identified and recorded findings from the oral examination and related them to patient's tobacco use; gave advice to quit and relating advice to oral health; gave the patient a packet of materials that included pamphlets of health problems/ways to quit; a quit kit with sugarless candy and gum, flavoured toothpicks, and rubber bands. Intervention provider: primarily by dental hygienists but also dentists. Provider training: 3 hour training workshop Modality of support: face‐to‐face Overall contact time: not reported Number of sessions: single session Pharmacotherapy: nil Intervention 2: extended intervention‐ as per minimal intervention, plus asked the patient to set a quit date within 2 weeks of visit, gave the patient a motivational video, and called the patient within 2 weeks after the hygiene visit to ask if he/she read the materials, watched the video, and either quit or is now willing to set a quit date. Intervention provider: primarily by dental hygienists but also dentists Provider training: three hour training workshop Modality of support: face‐to‐face Overall contact time: not reported Number of sessions: single session Pharmacotherapy: none |
|
| Outcomes | Definition of abstinence: self‐reported 'sustained' abstinence from smokeless tobacco and all tobacco: subjects must have reported 7‐day point prevalence smokeless tobacco and all tobacco abstinence at both three months and 12 months Longest follow‐up: 12 months Biochemical validation: none Oral health outcomes: not reported Adverse events: not reported |
|
| Funding source | QUOTE: "This research was supported by grant 1 RO1 HL48768 from the National Heart, Lung, and Blood Institute." | |
| Author conflicts of interest | No declaration of interests statement found in the paper | |
| Notes | The two behavioural interventions (minimal and extended intervention) were combined to a single group for the meta‐analysis. Data for smokeless tobacco users reported in Andrews 1999. ICC for cigarette smoking was 0.00004. Cluster‐randomised controlled trial design accounted for in our analyses by 'approximately correct' analysis according to current guidelines (Higgins 2011) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | QUOTE: "Randomised the practices to one of three groups" (no further details given) |
| Allocation concealment (selection bias) | Unclear risk | QUOTE: "Randomised the practices to one of three groups" (no further details given) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Smoking status was self‐reported and participants in the intervention arms received more personal contact than the usual care arm. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 24.3% overall attrition. Attrition data by arm not reported. |
Severson 2009.
| Study characteristics | ||
| Methods | Study design: randomised controlled trial Location: USA Setting: military dental clinics Recruitment: active duty military smokeless tobacco users attending annual examination at military dental clinics, asked to participate irrespective of motivation to quit |
|
| Participants | N = 785 Specialist population? no Participant characteristics: 1/785 (0.1%) female, average age: 30.4 years; average days a smokeless tobacco tin lasts: 3.7. Selected on motivation to quit? no |
|
| Interventions | Comparator: usual care‐ recommendations to quit using smokeless tobacco and referral to extant local tobacco cessation programs Comparator provider: dental provider Provider training: none Modality of support: face‐to‐face Overall contact time: likely to be brief Number of sessions: single session Pharmacotherapy: none Intervention: minimal contact behavioral treatment consisting of smokeless tobacco cessation manual, videotape cessation guide tailored for military personnel, 3 x 15 minute telephone counselling. Sessions using motivational interviewing methods. Intervention provider: telephone counsellors Provider training: 8 hours training Modality of support: telephone Overall contact time: average duration 42.5 minutes Number of sessions: 3 Pharmacotherapy: none |
|
| Outcomes | Definition of abstinence: self‐reported prolonged abstinence of smokeless tobacco for six months Longest follow‐up: 6 months Biochemical validation: no Oral health outcomes: not reported Adverse events: not reported |
|
| Funding source | QUOTE: "Congressionally Directed Medical Research Program’s Peer Review Medical Research Program to HHS (DAMD17‐02‐2‐0)." | |
| Author conflicts of interest | No declaration of interests statement found in the paper | |
| Notes | Though minimal in face‐to‐face contact, which apparently occurred only at the annual evaluation session and then for recruitment, the intervention was not minimal in time expenditure. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | QUOTE: "Mailed completed enrolment material to Oregon Research Institute research project offices, whereupon participants were randomly assigned" (no further details provided) |
| Allocation concealment (selection bias) | Low risk | Allocation by a third party. QUOTE: "mailed completed enrolment material to Oregon Research Institute research project offices, whereupon participants were randomly assigned" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Status was self‐reported and participants in the intervention arm received more personal contact than the usual care arm. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Overall follow‐up: 72.7%. Incomplete data not different in terms of condition, race/ethnicity, rank, readiness to quit, age, first tobacco use, or time to first chew. Completed 6 months assessment ‐ intervention 69.9%, usual care 75.6% |
Stevens 1995.
| Study characteristics | ||
| Methods | Study design: quasi‐randomised controlled trial Location: USA Setting: dental clinics Recruitment: hygiene patients in prepaid group practice health maintenance organisation, smokeless tobacco users. |
|
| Participants | N = 518 Specialist population? no Participant characteristics: 0/518 (0%) female. No other demographic details reported. Selected on motivation to quit? no |
|
| Interventions | Comparator: usual care Comparator provider: dentists and dental hygienists Provider training: no Modality of support: not reported Overall contact time: not reported Number of sessions: not reported Pharmacotherapy: not reported Intervention: soft‐tissue exam, cleaning, patient education, feedback on oral health and advice on self‐care, report of keratotic lesions asking where tobacco was placed, hygienist‐directed advice to quit, dentists' strong advice to quit, nine‐minute video, setting a quit date, self‐help booklet, 24‐hour advice phone line, kit providing oral substitutes and tip sheets with advice on how to quit, one week follow‐up call by hygienist, plus monthly mailing of tip sheets and newsletter Intervention provider: dentists and dental hygienists. Provider training: two hour training then weekly visits by project staff member to provide ongoing training Modality of support: face‐to‐face, telephone Overall contact time: not reported Number of sessions: 2 Pharmacotherapy: none |
|
| Outcomes | Definition of abstinence: self‐reported sustained abstinence of all tobacco: subjects must have reported no tobacco use in the last 7 days at the 3 months and 12 months assessments (used in analyses) Longest follow‐up: 12 months Biochemical validation: no Oral health outcomes: not reported Adverse events: not reported |
|
| Funding source | QUOTE: "This work was supported by grant P01 CA 44648 from the National Cancer Institute." | |
| Author conflicts of interest | No declaration of interests statement found in the paper | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Pseudo‐randomised by clinic identification number QUOTE: "Those with an odd number were assigned to the control condition and those with an even number were assigned to the intervention condition." |
| Allocation concealment (selection bias) | High risk | Allocation not concealed at time of enrolment |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Status was self‐reported and participants in the intervention arm received more personal contact than the usual care arm. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High numbers lost to follow‐up: 51.9% (intervention) and 53.7% (control) |
Virtanen 2015.
| Study characteristics | ||
| Methods | Study design: cluster‐randomised controlled trial Location: Sweden Setting: dental clinics Recruitment: consecutive patients visiting dental clinic were asked to complete screening form |
|
| Participants | N = 467 (27 dental practices) Specialist population? no Participant characteristics: 171/467 (36.6%) female; average age 45.6 years; average cigarettes per day: 12.52; baseline intention to quit: within 6 months 18.9% (85/467), ≥ 6 months 18.7% (84/467), time undefined 62.4% (280/467); socioeconomic status by education: elementary 21.1% (90/467); secondary 61.3% (261/467); post‐secondary 17.6% (75/467); intention to quit: within 6 months 18.9% (85/467), ≥ 6 months 18.7% (84/467), time undefined 62.4% (280/467). Selected on motivation to quit? no |
|
| Interventions | Comparator: control ‐ usual practice (if any): QUOTE: "no advice was given to about 28% of the participants in the control condition. When given, it mainly consisted of information about consequence of tobacco use for oral health". Comparator provider: not recorded. QUOTE: "according to the usual praxis established at the clinic (if any)" Provider training: QUOTE: "half‐day workshop on the study protocol and data collection procedures, similar to staff in the intervention condition" Modality of support: face‐to‐face Overall contact time: if given, variable depending on the dentist's usual practice Number of sessions: 1 Pharmacotherapy: none Intervention: brief counselling‐ structured very brief advice based on the 5As, a leaflet about the quitting. Provider training: "one‐day workshop conducted by the two developers (of the intervention). Information was provided about tobacco harms, dependence and cessation. The counselling technique was demonstrated with the use of interactive teaching techniques such as role playing, films, simulations and group discussions. Study protocol and data collection procedures were illustrated at this stage". Intervention provider: dentist or dental hygienist Modality of support: face‐to‐face Overall contact time: 5 minutes Number of sessions: 1 Pharmacotherapy: none |
|
| Outcomes | Definition of abstinence: self‐reported sustained abstinence from all tobacco during the three months preceding the follow‐up survey Longest follow‐up: 6 months Biochemical validation: none Oral health outcomes: not reported Adverse events: not reported |
|
| Funding source | QUOTE: "The work of Maria R Galanti, Suvi Virtanen and Izla Rohyo was supported by grant awarded by the National Board of Health and Welfare. The work of Zangin Zeebariwas due as part of his employment at the Centre for Epidemiology and Community Medicine, Stockholm County Council. The funding agencies had no role in study design; collection, analysis, and interpretation of data; writing the report; and the decision to submit the report for publication." | |
| Author conflicts of interest | QUOTE: "Authors declared that they have no conflicts of interest. No financial disclosures were reported by the authors of this paper." | |
| Notes | New for 2020 update. ICC 0.01 Cluster‐randomised controlled trial design accounted for in our analyses by 'approximately correct' analysis according to current guidelines (Higgins 2011) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | QUOTE: "Computer‐generated random sequence" |
| Allocation concealment (selection bias) | Low risk | QUOTE: "The random allocation was performed after all clinics consented to enrolment; therefore it could not be foreseen at the time of acceptance" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported abstinence data but both groups had a similar amount of face‐to‐face contact. It is unlikely that the participants would have known about the intervention in the other group |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2.7% (6/225) in the intervention group and 3.7% (9/242) in the control group were lost to follow‐up. |
Walsh 1999.
| Study characteristics | ||
| Methods | Study design: cluster‐randomised controlled trial Location: USA Setting: college athletic facilities Recruitment: publicly‐supported colleges were contacted for permission to recruit athletes who were smokeless tobacco users |
|
| Participants | N = 360 (16 colleges) Specialist population? no Participant characteristics: QUOTE: "groups were similar with respect to demographic factors..." (data not shown) Selected on motivation to quit? no |
|
| Interventions | Comparator: control‐ no intervention Comparator provider: not applicable Provider training: not applicable Modality of support: not applicable Overall contact time: not applicable Number of sessions: not applicable Pharmacotherapy: not applicable Intervention: 3 to 5 minute dental exam, advice to quit, discussed smokeless tobacco‐related tissue changes, photographs of facial disfigurement due to oral cancer, self‐help guide, offered a 10 to 15 minute counselling session by the hygienist which included nicotine gum, review of addiction nature of smokeless tobacco and nicotine withdrawal, setting a quit date, developing a plan to quit, and identifying triggers for tobacco use. Phone calls were conducted by the hygienist on the quit date and 1 month later. Intervention provider: dentists and dental hygienists Provider training: one‐day workshop Modality of support: face‐to‐face, telephone Overall contact time: 28 to 45 minutes Number of sessions: 3 Pharmacotherapy: 2 mg nicotine gum |
|
| Outcomes | Definition of abstinence: self‐reported 30‐day point prevalence smokeless tobacco abstinence Longest follow‐up: 12 months Biochemical validation: none Oral health outcomes: not reported Adverse events: not reported |
|
| Funding source | QUOTE: "This research was supported by the Tobacco Surtax Fund of the State of California through the Tobacco‐Related Disease Research Program of the University of California." | |
| Author conflicts of interest | No declaration of interests statement found in the paper | |
| Notes | ICC: 0.02 Cluster‐randomised controlled trial design accounted for in our analyses by 'approximately correct' analysis according to current guidelines (Higgins 2011) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | QUOTE: "Pair‐matched randomization"; "Colleges were then pair matched by baseline prevalence of smokeless tobacco use, and 1 college from each pair was randomised to receive the intervention, while the other college in the pair received no intervention." (no further details given) |
| Allocation concealment (selection bias) | Low risk | Insufficient details provided to allow judgement. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Status was self‐reported and participants in the intervention arm received more personal contact than the usual care arm. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Overall attrition 7.2%; similar between groups: 10% (intervention) and 5% (control). |
Walsh 2003.
| Study characteristics | ||
| Methods | Study design: cluster‐randomised controlled trial Location: USA Setting: high schools Recruitment: principals from randomly selected high schools were contacted. High school baseball team members who use smokeless tobacco were recruited. |
|
| Participants | N = 307 (44 schools) Specialist population? baseball team members Participant characteristics: 0/307 (0%) female; average age not reported; 77/166 in the control and 54/141 in the intervention groups used smokeless tobacco daily; self‐efficacy: not at all/a little: 105, somewhat confident: 57, very confident: 123. Selected on motivation to quit? no |
|
| Interventions | Comparator: control‐ usual care. Comparator provider: not applicable Provider training: not applicable Modality of support: not applicable Overall contact time: not applicable Number of sessions: not applicable Pharmacotherapy: not applicable Intervention: Peer‐led component: 50 to 60 minute educational meeting with videotape and discussion, slide presentation, small‐group discussion on tobacco industry advertising. Dental‐component: oral cancer screening in school environment by dental hygienist, advice to quit, identified oral findings related to tobacco use, self‐help guide for quitting, offered 15 minute counselling in groups, dental hygienists made 5 to 10 minute follow‐up call. Intervention provider: dental hygienist Provider training: one‐day training workshop Modality of support: face‐to‐face and telephone Overall contact time: 55 to 70 minutes Number of sessions: 2 Pharmacotherapy: none |
|
| Outcomes | Definition of abstinence: no current smokeless tobacco use self‐reported at both the 1 month and the 1 year outcome assessments Longest follow‐up: 24 months Biochemical validation: 8% random sample tested using salivary cotinine but 24 months results self‐reported Oral health outcomes: not reported Adverse events: not reported |
|
| Funding source | QUOTE: "This research was supported by the Tobacco Surtax Fund of the State of California (Grant No. 4RT‐0068) through the Tobacco‐Related Disease Research Program of the University California and by the National Cancer Institute (Grant No. CA 67654)." | |
| Author conflicts of interest | No declaration of interests statement found in the paper | |
| Notes |
Walsh 2003 was the main trial report but only reported 12 months findings. Abstinence at 24 months reported in Gansky 2002 was used in the meta‐analysis. ICC 0.04 Cluster‐randomised controlled trial design accounted for in our analyses by 'approximately correct' analysis according to current guidelines (Higgins 2011). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | QUOTE: "Selected randomly from a list of all public California high schools" (no further details given) |
| Allocation concealment (selection bias) | Unclear risk | QUOTE: "Selected randomly from a list of all public California high schools" (no further details given) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Status was self‐reported and participants in the intervention arm received more personal contact than the usual care arm. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reported < 10% loss to follow‐up |
Yahya 2018.
| Study characteristics | ||
| Methods | Study design: cluster‐randomised controlled trial Location: Malaysia Setting: dental clinics Recruitment: patients at dental clinics recruited by dental public health (DPH) specialists |
|
| Participants | N = 400 Specialist population? no Participant characteristics: 3/400 (0.75%) female; age: 5As group 26.07 ± 12.12 years, brief advice group 35.38 ± 10.24 y; average cigarettes per day: 5As group 9.94 ± 8.3, brief advice group 11.60 ± 7.86; Fagerstrom test for nicotine dependence: very low dependence 59% (236/400), low dependence 24% (96/400), moderate dependence 7.3% (29/400), high dependence 8% (32/400). Selected on motivation to quit: no |
|
| Interventions | Comparator: brief advice‐ a brief advice message to quit smoking (1 to 5 min) regardless of at which stage they are in the stage of change. Comparator provider: DPH specialists Provider training: QUOTE: "a smoking cessation training workshop was held to train and standardize the DPH specialists in each intervention group on two separate dates. After the training, all dental public health specialists involved were briefed on the clinical trial protocols. The specialists received kits containing tobacco use assessment sheets, pamphlets and carbon monoxide monitors after the training in order to conduct the intervention in their respective dental clinics" Modality of support: face‐to‐face Overall contact time: 1 to 5 minutes Number of sessions: 1 Pharmacotherapy: none Intervention: Provided the 5As intervention developed by Fiore et al. (2008). Patients who were at the pre‐contemplation and contemplation stages of change received the 5Rs strategies (Fiore et al. 2008). Patients in the preparation stage were advised on the behavioral strategies that would help them cope with withdrawal symptoms and prevent relapses. Self‐help pamphlets. Encouraged to set a quit date within two weeks of their first visit. Intervention provider: DPH specialists Provider training: QUOTE: "A smoking cessation training workshop was held to train and standardize the DPH specialists in each intervention group on two separate dates. After the training, all dental public health specialists involved were briefed on the clinical trial protocols. The specialists received kits containing tobacco use assessment sheets, pamphlets and carbon monoxide monitors after the training in order to conduct the intervention in their respective dental clinics" Modality of support: face to face Overall contact time: not reported Number of sessions: 1 Pharmacotherapy: none |
|
| Outcomes | Definition of abstinence: continuous abstinence of 30 days verified by carbon monoxide level ≤ 6 PPM Longest follow‐up: 6 months Biochemical validation: carbon monoxide 6 PPM Oral health outcomes: not reported Adverse events: not reported |
|
| Funding source | This research project was funded by the University of Malaya Postgraduate Research Fund (Reference Number: PG075‐2013A) | |
| Author conflicts of interest | No declaration of interests statement found in the paper | |
| Notes | New for 2020 update. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | QUOTE: "Randomised equally into two intervention groups by the drawing of lots" |
| Allocation concealment (selection bias) | Unclear risk | QUOTE: "Randomised equally into two intervention groups by the drawing of lots" (no further details given) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Abstinence rates validated by carbon monoxide level |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 66.8% (129/193) in the 5As group and 61.8% (128/207) in the brief advice group were lost to follow‐up. |
ICC: intracluster correlation coefficient; PPM: parts per million
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ahmadian 2017 | Educational intervention for dental students. |
| Albert 2004 | No tobacco use outcomes reported. Study assessed the effectiveness of academic detailing. |
| Amemori 2013 | No tobacco use outcomes reported. Intervention to increase delivery of counselling. |
| Barker 1995 | Not a randomised controlled trial. School‐wide tobacco cessation effort. |
| Barker 2001 | Not a randomised controlled trial. Survey of cessation practice behavior of hygienists and dentists. |
| Barnfather 2005 | Short follow‐up (8 weeks). Intervention included exam and counselling for both arms, with point‐of‐care test for salivary nicotine as the exposure variable. |
| Binnie 2003 | 3‐month outcomes only. Randomised controlled trial assessing the effectiveness of smoking cessation counselling and nicotine replacement delivered by dental hygienists. |
| Boundouki 2004 | Not a randomised controlled trial. Use of a patient‐information leaflet to improve knowledge of mouth cancer. |
| Campbell 1997 | No tobacco use outcomes reported. This report describes the recruitment strategy and response rate for a 3 year randomised controlled trial to test the effectiveness of a dissemination strategy aimed at improving the tobacco cessation services offered by rural dental practices. |
| Christen 1984 | 15‐week outcomes only. Assessed the efficacy of nicotine gum versus advice to quit and videotape. |
| Christen 1985 | Not a randomised controlled trial. Assessed nicotine effects on oral health. |
| Cooper 1989 | Not a randomised controlled trial. Hospital‐based smoking cessation program using behavioral modification and pharmacotherapy. |
| Ebn 2014 | Follow‐up less than 6 months following baseline. |
| Gelskey 2002 | Not a randomised controlled trial. No tobacco cessation outcomes. Study of tobacco use cessation counselling by oral health professionals. |
| Glasgow 1993 | Description of efforts to biochemically validate self‐reports of smoking cessation from participants in four large‐scale randomised trials, one of which was undertaken in dental settings (Stevens 1995). |
| Gonseth 2010 | No comparator group. |
| Gordon 2002 | Not a randomised controlled trial. Assessed the effectiveness of tobacco use counselling through public health dental clinics. |
| Gordon 2005a | Not a randomised controlled trial. Assessed the effectiveness of a behavioral intervention delivered through public health dental clinics. |
| Gordon 2005b | No tobacco use outcomes. Compared different methods of training on hygienist's tobacco use cessation activities. |
| Gordon 2007 | Follow‐up less than six months from baseline. |
| Gorin 2004 | Meta‐analysis included 5 dental intervention studies of 3 months' duration and Stevens 1995, which is included in the review. |
| Gould 1998 | Not a randomised controlled trial. Survey of participants in an NCI training program for delivering tobacco use interventions. |
| Greene 1994 | 3‐month outcomes only. Assessed the effectiveness of two interventions for smokeless tobacco cessation. |
| Gritz 1993 | Hospital‐based study assessing the impact of tobacco use counselling on head and neck cancer patients. Only 7/110 health care professionals were dental providers. |
| Guglielmetti 2014 | Not a randomised controlled trial. |
| Hanioka 2007 | The effectiveness of intervention was evaluated with respect to attempts to quit and progression through the stages of behavioral changes involved in quitting using the standardised questionnaire ‐ not abstinence. |
| Hedman 2010 | Not a randomised controlled trial. |
| Hedman 2015 | The vast majority of participants were non‐smokers. |
| Houston 2008 | No tobacco use outcomes reported. Assessed an internet‐delivered intervention to increase implementation of brief provider advice. |
| Houston 2013 | Educational intervention targeted at dental professionals. |
| Hovell 1995 | No tobacco use outcomes reported. Assessed the distribution of anti‐tobacco materials in orthodontic offices. |
| Hovell 2001 | Not a randomised controlled trial. Assessed the effectiveness of a behavioral intervention delivered by orthodontists in preventing pre‐teens from initiating tobacco use. |
| Hovell 2018 | No assessment of tobacco use outcomes. |
| ISRCTN19783832 | Study tested training dental professionals rather than a specific intervention |
| Jain 2015 | Follow‐up less than six months from baseline. |
| Johnston 1996 | Not a randomised controlled trial. The questionnaire was being developed as part of a two‐year randomised controlled trial of the effect of a multifaceted oral health education program on tobacco use among elementary school children in Ontario, Canada. This is a report of pretest evaluation for the questionnaire. |
| Jones 1993 | Not a randomised controlled trial. Baseline survey of tobacco use cessation activity and attitudes in community practices. |
| Kentala 1999 | Prevention study. Assessed the effectiveness of behavioral counselling on preventing or treating adolescent smoking. |
| Kirkwood 2001 | Four‐week outcomes only. Assessed the efficacy of a smoking deterrent mouthwash. No tobacco use outcomes reported. |
| Kirkwood 2002 | Four‐week outcomes only. Assessed the efficacy of a smoking deterrent breath spray. Outcome was smoking reduction not cessation. |
| Koerber 2003 | No tobacco use outcomes reported. Assessed the effects of teaching dental students brief motivational interviewing. |
| La Torre 2019 | Patient population was healthcare students. Intervention not delivered by a dental professional or in a dental setting. |
| Little 2009 | No tobacco use outcomes reported. Evaluated assisted referral. |
| Maassen 2008 | Not a randomised controlled trial. Study sought to determine guideline implementation parameters in a trial of 12 dental practices ‐ measured patient receptiveness to cessation advice. |
| Macgregor 1996 | Not a randomised controlled trial. Evaluated the effectiveness of dental health advice for a reduction in cigarette smoking. |
| Masouredis 1997 | 3‐month outcomes only. Assessed the effectiveness of a smokeless tobacco intervention in colleges. |
| Matias 2013 | Not a randomised controlled trial. |
| McRee 2012 | Study groups were 1. Tobacco & Alcohol; 2. Tobacco only; 3. Alcohol only; 4. Wait‐list control. Participants in groups 1 and 2 received the same tobacco intervention. |
| Montini 2013 | Not a randomised controlled trial. |
| Morgan 2000 | Not a randomised controlled trial. Recommendations for oral health professionals for addressing patient tobacco use. |
| Myers 2018 | Not a randomised controlled trial. |
| Nasry 2006 | Not a randomised controlled trial. Single cohort of smokers in a periodontal clinic provided counselling and pharmacotherapy as needed. |
| NCI 1994 | Collection of monographs addressing smoking cessation in medical and dental environments. Data from primary literature are covered elsewhere in this review. See Cohen 1989, Gritz 1993. |
| NCI 1995 | Intervention not confined to the dental setting. Community‐based interventions with communities as the unit of randomization. Tobacco control activities were promoted through medical and dental office settings. |
| NCT00907309 | Study discontinued. Author (retired) confirmed that this study could not continue due to funding issues. |
| NCT01275391 | Study discontinued. Author (retired) confirmed that this study could not continue due to funding issues. |
| NCT02188563 | Intervention not delivered by a dental professional or in a dental setting. Also, follow‐up less than six months. |
| NCT02570646 | Not a randomised controlled trial. |
| NCT02737176 | Non‐randomised design. |
| NCT03276819 | Cohort study investigating biomarkers predictive of malignant transformation or second primary tumour. |
| NCT03579355 | Quasi‐experimental controlled study conducted on non‐smokers (middle school students). |
| NCT03656874 | Smoking cessation was not an outcome measure. |
| O'Keefe 1995 | Not a randomised controlled trial. Study of dental practitioner compliance with tobacco use intervention training. |
| Olson 1985 | 15‐week outcomes only of salivary parameters among smokers using nicotine‐containing chewing gum. No tobacco cessation outcomes. |
| Ostroff 2014 | Author confirmed that they did not have resources to collect smoking cessation data. |
| Pai 2012 | Author confirmed that they do not have access to the raw data to make smoking abstinence data available. |
| Raja 2014 | Follow‐up less than six months from baseline. |
| Raja 2016 | Follow‐up less than six months from baseline. |
| Ray 2014 | Study testing modality of referral systems |
| Rindal 2013 | No tobacco use outcomes. Intervention to increase tobacco treatment delivery. |
| Rozi 2019 | No assessment of tobacco use outcomes. |
| Schoonheim‐Klein 2013 | Not a randomised controlled trial. |
| Secker‐Walker 1988 | Not a randomised controlled trial. Pilot study of smoking cessation advice among patients in a periodontal practice. |
| Shelley 2011 | Assessed the impact of an intervention on provider adherence to tobacco use treatment guidelines. |
| Shelley 2012 | Follow‐up less than six months from baseline. |
| Smith 1998 | Not a randomised controlled trial. Case series of smoking cessation programs conducted in dental practices in the UK. |
| Stoops 2009 | Follow‐up less than six months from baseline. |
| Walsh 2007 | Educational intervention for dental students. |
| Walsh 2010 | No tobacco use outcomes. |
| Williams 2002 | Abstract unavailable. No additional information supplied by author. |
| Wood 1997 | Not a randomised controlled trial. 3‐month data only. Office‐based training in tobacco cessation for dentists. |
Characteristics of ongoing studies [ordered by study ID]
CTRI/2018/02/011846.
| Study name | Tobacco Cessation Program Conducted At Government Dental College, Kottayam, Kerala |
| Methods | Study design: Randomised parallel group controlled trial Location: India Setting: Government Dental College |
| Participants | Recruitment: Attending outpatient department Goal N = 160 Specialised population? no Eligibility criteria: tobacco chewers and smokers attending the out patient departments of Government Dental College, give consent, have a Fagerstrom score of 6 or less, not attending other tobacco cessation programs, not with existence of psychiatric disorders or drug addiction. |
| Interventions | Comparator: brief anti‐tobacco advice group Intervention: motivational interviewing group |
| Outcomes | Definition of abstinence: unclear Longest follow‐up: six months |
| Starting date | March 2018 |
| Contact information | Dr Arun Rao |
| Notes |
CTRI/2018/03/012867.
| Study name | Effectiveness of Behavioral Counselling with Nicotine Gum versus Behavioral Counselling alone for quitting tobacco usage among patients at a Tobacco Cessation Clinic in Delhi. |
| Methods | Study design: Randomised parallel group controlled trial Location: India Setting: Tobacco Cessation Clinic |
| Participants | Recruitment: Unclear Goal N = 120 Specialised population? no Eligibility criteria: daily tobacco user, reporting to the tobacco cessation clinic, give informed consent, able to read and write the local language, have a medium nicotine dependence score as per Fagerstrom scale for smoking and Modified Fagertrom scale for smokeless tobacco. |
| Interventions | Comparator: behavioural counselling alone Intervention: behavioural counselling with nicotine gum |
| Outcomes | Definition of abstinence: not specified Longest follow‐up: six months |
| Starting date | July 2017 |
| Contact information | Dr Vikrant Mohanty |
| Notes |
La Torre 2013.
| Study name | Randomized controlled trial on the promotion of healthy lifestyles among adolescents in the orthodontic setting |
| Methods | Study design: randomised controlled trial Location: Italy Setting: complex unit of orthodontics |
| Participants | Recruitment: patients in the complex unit of orthodontics Goal N = not reported Specialised population? no Eligibility criteria: adolescents aged 10 to 14 |
| Interventions | Comparator: unclear Intervention: · Deterring adolescents from smoking · Discouraging the use and abuse of alcoholic beverages · Encouraging adherence to the Mediterranean style diet |
| Outcomes | Definition of abstinence: not reported Longest follow‐up: three years |
| Starting date | October 2019‐ contacted authors who advise the study has completed and is being prepared for publication. |
| Contact information | Dr La Torre |
| Notes |
NCT00591175.
| Study name | A Randomized Clinical Trial Assessing Smoking Cessation Interventions In Dental Clinic Smokers |
| Methods | Study design: randomised controlled trial Location: USA Setting: New York University College of Dentistry |
| Participants | Recruitment: recruited patients at the New York University College of Dentistry N = 1072 participants Specialised population? no Eligibility criteria: patient seeking routine dental care at New York University College of Dentistry and meets medical clearance for routine care; active smokers (active smoking as self‐reported regular use of at least 10 cigarettes per day); able to provide a telephone number or collateral contact information where they can be reached over the subsequent 12 months; fluent in English or Spanish |
| Interventions | Comparator: standard care by dentist Intervention 1: dental hygienist provided counselling and brief questionnaire Intervention 2: hygienist provided counselling with personalized risk communication and brief questionnaire |
| Outcomes | Definition of abstinence: abstinence validated by salivary cotinine at 12 months Longest follow‐up: 12 months |
| Starting date | December 2001 |
| Contact information | Jamie Ostroff, Memorial Sloan Kettering Cancer Center, New York, United States. |
| Notes | Estimated Study Completion Date: December 2020 |
NCT01846910.
| Study name | Prepare to Quit ‐ A Randomized Clinical Trial to Help People Quit Smoking in a Community Dental Clinic Setting |
| Methods | Study design: randomised controlled trial Location: Canada Setting: dental clinic |
| Participants | Recruitment: recruited patients in a community dental clinic N = 156 participants Specialised population? no Eligibility criteria: current smoker; 18 years or older; dental clinic patient; interested in quitting smoking |
| Interventions | Common components in all trial arms: Motivational Counseling Normally three counselling sessions. Session #1: Introductions; discuss habits, feelings, pros and cons of smoking; degree of addiction; previous quit attempts and relapse; reinforce patient confidence; open‐ended questions to elicit self‐efficacy; homework includes Pack‐Track and Why Test. Session #2: Discuss smoking patterns, triggers, and risk situations; discuss methods to resist triggers, need for medications; prepare environment and back‐up plan; discuss quit date; homework to review self‐help workbook. Session #3: Reinforce previous concepts; review resistance plan; discuss and arrange pharmaceutical cessation aids; set quit date; homework to finalize all self/home preparations; offer tooth polishing on quit date; follow‐up by telephone or appointment. Other Name: Motivational Interviewing Intervention 1: motivational counselling (as above) Intervention 2: motivational counselling and tooth whitening incentive 1. A dental check‐up will ensure the absence of contraindications to tooth whitening (cavities, soft tissue pathology, severe periodontitis); 2. Removal of calculus, plaque, or stains with a dental polishing; 3. Treatment time is approximately 1 hour total; 4. Lips are retracted, tongue protector is placed and protective eyewear used; 5. Saliva is suctioned and teeth are air dried; 6. A liquid rubber dam material is applied to protect gums and cured by visible light; 7. The bleaching material is placed on the enamel surface of the involved teeth and is replaced if drying occurs; 8. After 15 minutes, the bleach is suctioned, mouth rinsed and process repeated; 9. Three to four applications are typically used. |
| Outcomes | Definition of abstinence: abstinence from tobacco use biochemically verified by carbon monoxide breath test at 12 months Longest follow‐up: 12 months |
| Starting date | October 2013 |
| Contact information | Dr Douglas James Brothwell |
| Notes | Estimated Study Completion Date: August 2019 |
NCT02582008.
| Study name | Bupropion Hydrochloride or Patient's Choice for Smoking Cessation in Patients With Squamous Cell Head and Neck Cancer Undergoing Radiation Therapy With or Without Chemotherapy |
| Methods | Study design: randomised controlled trial Location: USA Setting: Wake Forest University Health Sciences |
| Participants | Recruitment: recruited patients with confirmed squamous cell carcinoma of the nasopharynx, oropharynx, larynx, hypopharynx, oral cavity Goal N = not reported but so far 9 participants recruited Specialised population? Yes, head and neck cancer patients scheduled to received radiotherapy or chemoradiotherapy Eligibility criteria: • Patients must have histologically or cytologically confirmed squamous cell carcinoma of the nasopharynx, oropharynx, larynx, hypopharynx, oral cavity • Patients must be scheduled to receive radiotherapy or combined chemotherapy and radiotherapy as definitive treatment or surgery with planned adjuvant radiotherapy or combined chemotherapy and radiotherapy treatment post‐surgery per surgeon or Head and Neck Cancer Tumor Board decision • Patients should receive their definitive treatment at Wake Forest University Cancer Center or at Medical University of South Carolina Cancer Center • Patients must be active smokers (defined as smoking any cigarette, cigar or pipe in the last 30 days) • Ability to understand and the willingness to sign an Institutional Review Board ‐approved informed consent document |
| Interventions | Intervention 1: Patients receive bupropion hydrochloride by mouth for three days and then twice daily for up to one year post radiotherapy/combined chemotherapy and radiotherapy Intervention 2: Patients receive smoking cessation treatment tailored to individual smokers based on preference, smoking history and contra‐indications. Patients are given the choice of one of the National Comprehensive Cancer Network‐recommended first‐line pharmacotherapy options for smoking cessation comprised of varenicline by mouth daily for 1 week and then twice daily for 12 weeks or combination of nicotine patch and acute nicotine replacement therapy for 12 weeks. Treatment continues in the absence of disease progression or unacceptable toxicity. Treatment with varenicline or nicotine replacement therapy can be extended up to 6 months to 1 year as needed. |
| Outcomes | Definition of abstinence: Not reported Longest follow‐up: 12 months |
| Starting date | January 2016 |
| Contact information | Mercedes Porosnicu, Wake Forest University Health Sciences |
| Notes | Estimated Study Completion Date: September 2020 |
Differences between protocol and review
Title updated to reflect that not all studies are in dental settings.
We chose not to include interventions aimed at the training/provision of an educational intervention of/for dental health professionals in the current version of the review, although we have mentioned them in the discussion. We felt these did not address the objective of the review which was to assess the effectiveness of the interventions and not the training of providers. The training of health professionals in smoking cessation has been the topic of a previous Cochrane Review (Carson 2012).
We updated the 'Risk of bias' assessment in line with guidance from the Cochrane Tobacco Addiction Group and the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Adverse event and oral health data were added as primary outcomes, and the objectives restructured to account for this.
We restructured the abstinence analyses to explore the effect of differences in intervention intensity and comparator type.
We used RR rather than OR (used in the previous version of this review) in line with Cochrane policy.
Further information extracted from studies: author conflict of interests and sources of funding.
When studies reported on multiple groups, we chose to combine relevant groups into a single group. In the current review this applies to two studies: Gordon 2010a evaluated two behavioural interventions (3As and 5As) which we combined to a single group (the previous version of this review used the 3As group only); Severson 1998 evaluated a minimal and an enhanced intervention and again we combined these to a single group (the previous version of this review used the enhanced intervention group only). This is in line with the recommended method described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
The authors of this updated review extracted the data for all included studies, including those included in the previous version of the review (Carr 2012). Hence, there are minor differences in some data. For example, Gansky 2005 was previously reported to have an ICC of 0.0074 but this has been changed to 0.0197 in the updated review (the ICC of 0.0074 was reported for 'initiation' of smokeless tobacco use whereas the ICC of 0.0197 was reported for 'cessation' of smokeless tobacco).
'Summary of findings' table added with GRADE assessments.
Contributions of authors
Completed updated search: JLB
Selected which studies to include: RH, BH.
Extracted data into Review Manager: RH, BH.
Carried out the analysis: RH, JLB
Interpreted the analysis: all authors.
Drafted the final review: all authors.
RH is the guarantor of the review.
Sources of support
Internal sources
-
National Institute for Health Research, UK
RH is funded by the National Institute for Health Research (NIHR) as a Clinical Lecturer. BS is funded by the NIHR as an Academic Clinical Fellow. JLB is funded via Cochrane Infrastructure and Cochrane Programme grant funding to the Cochrane Tobacco Addiction Group. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
-
Newcastle University, UK
Computer use, database access, library services.
External sources
No sources of support supplied
Declarations of interest
RH, EM, PP are authors of one of the studies included in the review (Holliday 2019).
BH declares no competing interests.
JLB: none known
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Andrews 1999 {published data only}
- *.Andrews JA, Severson H, Lichtenstein E, Gordon JS, Barckley MF. Evaluation of a dental office tobacco cessation program: effects on smokeless tobacco use. Annals of Behavioral Medicine 1999;21:48-53. [DOI] [PubMed] [Google Scholar]
- Gordon JS, Severson HH. Tobacco cessation through dental office settings. Journal of Dental Education 2001;65:354-63. [PubMed] [Google Scholar]
- Severson HH, Andrews JA, Lichtenstein E, Gordon JS, Barckley MF. Using the hygiene visit to deliver a tobacco cessation program: results of a randomized clinical trial. Journal of the American Dental Association 1998;129:993-9. [DOI] [PubMed] [Google Scholar]
Binnie 2007 {published data only}
- Binnie VI, McHugh S, Jenkins W, Borland W, MacPherson LM. A randomised controlled trial of a smoking cessation intervention delivered by dental hygienists: A feasibility study. BMC Oral Health 2007;7:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cohen 1989 {published data only}
- Cohen SJ, Christen AG, Katz BP, Drook CA, Davis BJ, Smith DM, et al. Counseling medical and dental patients about cigarette smoking: the impact of nicotine gum and chart reminders. American Journal of Public Health 1987;77:313-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Cohen SJ, Stookey GK, Katz BP, Drook CA, Christen AG. Helping smokers quit: a randomized controlled trial with private practice dentists. Journal of the American Dental Association 1989;118:41-5. [DOI] [PubMed] [Google Scholar]
Ebbert 2007 {published data only}
- Ebbert JO, Carr AB, Patten CA, Morris RA, Schroeder DR. Tobacco use quit line enrollment through dental practices: a pilot study. Journal of the American Dental Association 2007;138:595-601. [DOI] [PubMed] [Google Scholar]
Gansky 2005 {published data only}
- Ellison J, Gansky SA, Kavanagh C, Rudy D, et al. An athletic trainer-mediated spit tobacco prevention and cessation program (IADR San Diego 2002 abstracts). Journal of Dental Research 2002;81(March, Special Issue A):A-494 (Abs 4047). [Google Scholar]
- *.Gansky SA, Ellison JA, Rudy D, Bergert N, Letendre MA, Nelson L, et al. Cluster randomized controlled trial of an athletic trainer-directed spit (smokeless) tobacco intervention for college baseball athletes: results after one year. Journal of Athletic Training 2005;40:76-87. [PMC free article] [PubMed] [Google Scholar]
Gordon 2010a {published data only}
- Gordon JS, Andrews JA, Crews KM, Payne TJ, Severson HH, Lichtenstein E. Do faxed quit line referrals add value to dental office-based tobacco-use cessation interventions? Journal of the American Dental Association 2010;141:1000-7. [CENTRAL: 814382] [30282] [6840] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gordon 2010b {published data only}
- Gordon JS, Albert DA, Andrews JA, Crews KM, Payne TJ, Severson HH. Tobacco cessation in public dental clinics: short-term outcomes (PA14-5). In: Society for Research on Nicotine and Tobacco 14th Annual Meeting February 26-March 1, Portland, Oregon. 2008.
- *.Gordon JS, Andrews JA, Albert DA, Crews KM, Payne TJ, Severson HH. Tobacco cessation via public dental clinics: results of a randomized trial. American Journal of Public Health 2010;100:1307-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hanioka 2010 {published data only}
- Hanioka T, Ojima M, Tanaka H, Naito M, Hamajima N, Matsuse R. Intensive smoking-cessation intervention in the dental setting. Journal of Dental Research 2010;89:66-70. [DOI] [PubMed] [Google Scholar]
Holliday 2019 {published data only}
- Holliday R, Preshaw PM, Ryan V, Sniehotta FF, McDonald S, Bauld L, et al. A feasibility study with embedded pilot randomised controlled trial and process evaluation of electronic cigarettes for smoking cessation in patients with periodontitis. Pilot and Feasibility Studies 2019;5:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lando 2007 {published data only}
- Lando HA, Hennrikus D, Boyle R, Lazovich D, Stafne E, Rindal B. Promoting tobacco abstinence among older adolescents in dental clinics. Journal of Smoking Cessation 2007;2:23-30. [Google Scholar]
McClure 2018 {published data only}
- McClure JB, Bush T, Anderson ML, Blasi P, Thompson E, Nelson J, et al. Oral health promotion and smoking cessation program delivered via tobacco quit lines: the oral health 4 life trial. American Journal of Public Health 2018;108:689-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nohlert 2013 {published data only}
- *.Nohlert E, Ohrvik J, Tegelberg A, Tillgren P, Helgason AR. Long-term follow-up of a high- and low-intensity smoking cessation intervention in a dental setting - a randomized trial. BMC Public Health 2013;13:592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nohlert E, Tegelberg A, Tillgren P, Johansson P, Rosenblad A, Helgason AR. Comparison of a high and a low intensity smoking cessation intervention in a dentistry setting in Sweden: a randomized trial. BMC Public Health 2009;9:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
Selvamary 2016 {published data only}
- Selvamary AL, Aswath Narayanan MB, Doss J, Ramesh Kumar SG. Effectiveness of cognitive behavior therapy in tobacco cessation at a dental setting: a hospital-based randomized controlled trial. Journal of Indian Association of Public Health 2016;14:370-6. [Google Scholar]
Severson 1998 {published data only}
- Gordon JS, Severson HH. Tobacco cessation through dental office settings. Journal of Dental Education 2001;65:354-63. [PubMed] [Google Scholar]
- Severson HH, Andrews JA, Lichtenstein E, Gordon J. A dental office intervention for smokeless tobacco cessation. In: 10th World Conference on Tobacco or Health 24-28 August Beijing Abstract book. 1997.
- *.Severson HH, Andrews JA, Lichtenstein E, Gordon JS, Barckley MF. Using the hygiene visit to deliver a tobacco cessation program: results of a randomized clinical trial. Journal of the American Dental Association 1998;129:993-9. [DOI] [PubMed] [Google Scholar]
- Severson HH, Andrews JA, Lichtenstein E, Gordon JS. Smokeless tobacco cessation through dental offices: An intervention that works (AADR Abstract No. 1206). Journal of Dental Research 1988;77(AADR Abstracts):256. [Google Scholar]
Severson 2009 {published data only}
- Severson HH, Gordon J, Andrews J, Peterson AL, Cigrang J. Evaluating motivational interview phone support for smokeless tobacco cessation with military personnel (PA10-6). In: Society for Research on Nicotine and Tobacco 12th Annual Meeting February 15-18, Orlando, Florida. 2006.
- *.Severson HH, Peterson AL, Andrews JA, Gordon JS, Cigrang JA, Danaher BG, et al. Smokeless tobacco cessation in military personnel: a randomized controlled trial. Nicotine & Tobacco Research 2009;11:730-38. [DOI] [PubMed] [Google Scholar]
Stevens 1995 {published data only}
- Gordon JS, Severson HH. Tobacco cessation through dental office settings. Journal of Dental Education 2001;65:354-63. [PubMed] [Google Scholar]
- Little SJ, Stevens VJ, Severson HH, Lichtenstein E. Effective smokeless tobacco intervention for dental hygiene patients. Journal of Dental Hygiene 1992;66(4):185-90. [PubMed] [Google Scholar]
- *.Stevens VJ, Severson H, Lichtenstein E, Little SJ, Leben J. Making the most of a teachable moment: a smokeless-tobacco cessation intervention in the dental office. American Journal of Public Health 1995;85:231-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Virtanen 2015 {published data only}
- Oncioiu SI, Franchetti-Pardo L, Virtanen SE, Faggiano F, Galanti MR. Beyond intention-to-treat: the effect of brief counseling for tobacco cessation in secondary analyses for a cluster randomized controlled trial in Swedish dental clinics. Contemporary Clinical Trials Communication 2017;5:92-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Virtanen SE, Zeebari Z, Rohyo I, Galanti MR. Evaluation of a brief counseling for tobacco cessation in dental clinics among Swedish smokers and snus users. A cluster randomized controlled trial (the FRITT study). Preventive Medicine 2015;70:26-32. [DOI] [PubMed] [Google Scholar]
Walsh 1999 {published data only}
- Walsh MM, Hilton JF, Masouredis C, Gee L, Chesney MA, Ernster VL. A randomized controlled intervention trial to promote spit tobacco cessation among college athletes. In: Journal of Dental Research. Vol. Special Issue - Abstracts of Papers (73rd General Session and Exhibition of the International Association for Dental Research June 28-July 1, 1995, Singapore). 1995:458 (Abs No 463).
- *.Walsh MM, Hilton JF, Masouredis CM, Gee L, Chesney MA, Ernster VL. Smokeless tobacco cessation intervention for college athletes: results after 1 year. American Journal of Public Health 1999;89:228-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Walsh 2003 {published data only}
- Gansky SA, Ellison JA, Kavanagh C, Hilton JF, Walsh MM. Oral screening and brief spit tobacco cessation counseling: a review and findings. Journal of Dental Education 2002;66:1088-98. [PubMed] [Google Scholar]
- Walsh MM, Ellison J, Hilton JF, Kavanagh C, Chesney M, Ernster V. Spit tobacco cessation: high school baseball athletes, 2 year findings. Journal of Dental Research 2001;80(January 2001 Special Issue AADR Abstracts):116 (Abstract 645). [Google Scholar]
- *.Walsh MM, Hilton JF, Ellison JA, Gee L, Chesney MA, Tomar SL, et al. Spit (smokeless) tobacco intervention for high school athletes: results after 1 year. Addictive Behaviors 2003;28:1095-113. [DOI] [PubMed] [Google Scholar]
Yahya 2018 {published data only}16325841
- Yahya NA, Saub R, Nor MM. A randomized control trial of smoking cessation interventions conducted by dentists. Sains Malaysiana 2018;47:131-140. [Google Scholar]
References to studies excluded from this review
Ahmadian 2017 {published data only}
- Ahmadian M, Khami MR, Ahamdi AE, Razeghi S, Yazdani R. Effectiveness of two interactive educational methods to teach tobacco cessation counseling for senior dental students. European Journal of Dentistry 2017;11:287-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Albert 2004 {published data only}
- Albert DA, Anluwalia KP, Ward A, Sadowsky D. The use of 'academic detailing' to promote tobacco-use cessation counseling in dental offices. Journal of the American Dental Association 2004;135:1700-6. [DOI] [PubMed] [Google Scholar]
Amemori 2013 {published data only}15427433
- Amemori M, Korhonen T, Kinnunen T, Michie S, Murtomaa H. Enhancing implementation of tobacco use prevention and cessation counselling guideline among dental providers: a cluster randomised controlled trial. Implementation Science 2011;6:13. [CENTRAL: 858582] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amemori M, Korhonen T, Michie S, Murtomaa H, Kinnunen TH. Implementation of tobacco use cessation counseling among oral health professionals in Finland. Journal of Public Health Dentistry 2013;73:230-6. [DOI] [PubMed] [Google Scholar]
- *.Amemori M, Virtanen J, Korhonen T, Kinnunen TH, Murtomaa H. Impact of educational intervention on implementation of tobacco counselling among oral health professionals: A cluster-randomized community trial. Community Dentistry and Oral Epidemiology 2013;41:120-9. [CENTRAL: 853503] [PMID: ] [DOI] [PubMed] [Google Scholar]
Barker 1995 {published data only}
- Barker GJ, Taylor TS, Barker BF. Implementation of a tobacco cessation program in the student clinics. Journal of Dental Education 1995;59:850-5. [PubMed] [Google Scholar]
Barker 2001 {published data only}
- Barker GJ, Williams KB, Taylor TS, Barker BF. Practice behaviors of alumni trained as students in tobacco use cessation interventions. Journal of Dental Hygiene 2001;75:165-9. [PubMed] [Google Scholar]
Barnfather 2005 {published data only}
- Barnfather KD, Cope GF, Chapple IL. Effect of incorporating a 10 minute point of care test for salivary nicotine metabolites into a general practice based smoking cessation programme: randomised controlled trial. BMJ 2005;331:979-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Binnie 2003 {published data only}
- Binnie V, McHugh S, MacPherson LM, Jenkins W, et al. Dental hygienists' delivery of smoking cessation: 3-month outcomes. Journal of Dental Research 2003;82(Special Issue B, June):B-373. [Google Scholar]
Boundouki 2004 {published data only}
- Boundouki G, Humphris G, Field A. Knowledge of oral cancer, distress and screening intentions: longer term effects of a patient information leaflet. Patient Education & Counseling 2004;53:71-7. [DOI] [PubMed] [Google Scholar]
Campbell 1997 {published data only}
- Campbell HS, Petty TL, Meadows LM, Simpson L, Jennett PA. Improving tobacco cessation services in rural dental offices - recruitment to a randomized controlled trial. Canadian Journal of Community Dentistry 1997;12:15-22. [Google Scholar]
Christen 1984 {published data only}
- Christen AG, McDonald JL Jr, Olson BL, Drook CA, Stookey GK. Efficacy of nicotine chewing gum in facilitating smoking cessation. Journal of the American Dental Association 1984;108:594-7. [DOI] [PubMed] [Google Scholar]
Christen 1985 {published data only}
- Christen AG, Beiswanger BB, Mallatt ME, Tomich CE, Drook CA, McDonald JL Jr, et al. Effects of nicotine-containing chewing gum on oral soft and hard tissues: A clinical study. Oral Surgery, Oral Medicine and Oral Pathology 1985;59:37-42. [DOI] [PubMed] [Google Scholar]
Cooper 1989 {published data only}
- Cooper TM, Clayton RR. Stop-smoking program using nicotine reduction therapy and behavior modification for heavy smokers. Journal of the American Dental Association 1989;118:47-51. [DOI] [PubMed] [Google Scholar]
Ebn 2014 {published data only}
- Ebn Ahmady A, Homayoun A, Lando HA, Haghpanah F, Khoshnevisan MH. Patients' attitudes towards the role of dentists in tobacco cessation counselling after a brief and simple intervention. Eastern Mediterranean Health Journal 2014;20:82-9. [PubMed] [Google Scholar]
Gelskey 2002 {published data only}
- Gelskey SC. Impact of a dental/dental hygiene tobacco-use cessation curriculum on practice. Journal of Dental Education 2002;66:1074-8. [PubMed] [Google Scholar]
Glasgow 1993 {published data only}
- Glasgow RE, Mullooly JP, Vogt TM, Stevens VJ, Lichtenstein E, Hollis JF, et al. Biochemical validation of smoking status: Pros, cons, and data from four low-intensity intervention trials. Addictive Behaviors 1993;18:511-27. [DOI] [PubMed] [Google Scholar]
Gonseth 2010 {published data only}
- Goneth S, Abarca M, Madrid C, Cornuz J. A pilot study combining individual-based smoking cessation counseling, pharmacotherapy, and dental hygiene intervention. BMC Public Health 2010;10:348. [DOI] [PMC free article] [PubMed]
Gordon 2002 {published data only}
- Gordon JS, Andrews JA, Severson HH, Stewart DCL. Tobacco cessation via public health dental clinics: preliminary results. In: Society for Research on Nicotine and Tobacco 8th Annual Meeting Rapid Communications Posters, Savannah, Georgia. 2002:RP-64.
Gordon 2005a {published data only}
- Gordon JS, Andrews JA, Lichtenstein E, Severson HH. The impact of a brief tobacco cessation intervention in public health dental clinics. Journal of the American Dental Association 2005;136:179-86. [DOI] [PubMed] [Google Scholar]
Gordon 2005b {published data only}
- Akers L, Gordon JS, Andrews JA, Barckley M, Lichtenstein E, Severson HH. Cost effectiveness of changing health professionals' behavior: Training dental hygienists in brief interventions for smokeless tobacco cessation. Preventive Medicine 2006;43:482-7. [DOI] [PubMed] [Google Scholar]
- *.Gordon JS, Andrews JA, Lichtenstein E, Severson HH, Akers L. Disseminating a smokeless tobacco cessation intervention model to dental hygienists: A randomized comparison of personalized instruction and self-study methods. Health Psychology 2005;24:447-55. [DOI] [PubMed] [Google Scholar]
Gordon 2007 {published data only}
- Gordon JS, Andrews JA, Crews KM, Payne TJ, Severson HH. The 5A's vs 3A's plus proactive quitline referral in private practice dental offices: preliminary results. Tobacco Control 2007;16:285-8. [DOI] [PMC free article] [PubMed]
Gorin 2004 {published data only}
- Gorin SS, Heck JE. Meta-analysis of the efficacy of tobacco counseling by health care providers. Cancer Epidemiology, Biomarkers & Prevention 2004;13:2012-22. [PubMed] [Google Scholar]
Gould 1998 {published data only}
- Gould KA, Eickhoff-Shemek JM, Stacy RD, Mecklenburg RE. The impact of National Cancer Institute training on clinical tobacco use cessation: services by oral health teams. Journal of the American Dental Association 1998;129:1442-9. [DOI] [PubMed] [Google Scholar]
Greene 1994 {published data only}
- Greene JC, Walsh MM, Masouredis C. A program to help major league baseball players quit using spit tobacco. Journal of the American Dental Association 1994;125:559-68. [DOI] [PubMed] [Google Scholar]
Gritz 1993 {published data only}
- *.Gritz ER, Carr CR, Rapkin D, Abemayor E, Chang LJ, Wong WK, et al. Predictors of long-term smoking cessation in head and neck cancer patients. Cancer Epidemiology, Biomarkers & Prevention 1993;2:261-70. [PubMed] [Google Scholar]
- Gritz ER, Carr CR, Rapkin DA, Chang C, Beumer J, Ward PH. A smoking cessation intervention for head and neck cancer patients: trial design, patient accrual, and characteristics. Cancer Epidemiology, Biomarkers & Prevention 1991;1:67-73. [PubMed] [Google Scholar]
Guglielmetti 2014 {published data only}
- Guglielmetti MR, Rosa EF, Lourencao DS, Inoue G, Gomes EF, De Micheli G, et al. Detection and quantification of periodontal pathogens in smokers and never-smokers with chronic periodontitis by real-time polymerase chain reaction. Journal of Periodontology 2014;85:1450-7. [DOI] [PubMed] [Google Scholar]
Hanioka 2007 {published data only}
- Hanioka T, Ojima M, Hamajima N, Naito M. Patient feedback as a motivating force to quit smoking. Community Dentistry & Oral Epidemiology 2007;35:310-7. [DOI] [PubMed] [Google Scholar]
Hedman 2010 {published data only}
- Hedman E, Riis U, Gabre P. The impact of behavioural interventions on young people's attitudes toward tobacco use. Oral Health & Preventive Dentistry 2010;8:23-32. [PubMed] [Google Scholar]
Hedman 2015 {published data only}
- Hedman E, Gabre P, Birkhed D. Dental hygienists working in schools - a two-year oral health intervention programme in Swedish secondary schools. Oral Health & Preventive Dentistry 2015;13:177-88. [DOI] [PubMed] [Google Scholar]
Houston 2008 {published data only}
- Houston TK, Richman JS, Coley HL, Ray MN, Allison JJ, Gilbert GH, et al. Does delayed measurement affect patient reports of provider performance? Implications for performance measurement of medical assistance with tobacco cessation: a Dental PBRN study. BMC Health Services Research 2008;8:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Houston TK, Richman JS, Ray MN, Allison JJ, Gilbert GH, Shewchuk RM, et al. Internet delivered support for tobacco control in dental practice: randomized controlled trial. Journal of Medical Internet Research 2008;10:e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Houston 2013 {published data only}
- Houston TK, Delaughter KL, Ray MN, Gilbert GH, Allison JJ, Kiefe CI, et al. Cluster-randomized trial of a web-assisted tobacco quality improvement intervention of subsequent patient tobacco product use: a National Dental PBRN study. BMC Oral Health 2013;13:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hovell 1995 {published data only}
- Hovell MF, Russos S, Beckhelm MK, Jones JA, Burkham-Kreitner SM, Slymen DJ, et al. Compliance with primary prevention in private practice: creating a tobacco-free environment. American Journal of Preventive Medicine 1995;11:288-93. [PubMed] [Google Scholar]
Hovell 2001 {published data only}
- Hovell MF, Jones JA, Adams MA. The feasibility and efficacy of tobacco use prevention in orthodontics. Journal of Dental Education 2001;65:348-53. [PubMed] [Google Scholar]
Hovell 2018 {published data only}
- Hovell MF, Schmitz KE, Liles S, Robusto K, Hofstetter CR, Nichols JF, Rock CL, Irvin V, Parker MS, Surillo SA, Noel D. A randomized controlled trial of orthodontist-based brief advice to prevent child obesity. Contemporary Clinical Trials 2018;70:53-61. [DOI] [PubMed] [Google Scholar]
ISRCTN19783832 {published data only}19783832
- ISRCTN19783832. Implementation of a smoking cessation protocol in dental practices. doi.org/10.1186/ISRCTN19783832 (first received April 2006). [ISRCTN19783832]
Jain 2015 {published data only}
- Jain R, Jhanjee S, Jain V, Gupta T, Mittal S, Chauhan P, et al. Biochemical validation of self-reported smokeless tobacco abstinence among smokeless tobacco users: results from a clinical trial of Varenicline in India. Journal of Psychoactive Drugs 2015;47:331-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Johnston 1996 {published data only}
- Johnston DW, O'Shea RM. Testing an evaluation instrument in a tobacco use intervention study. Journal of Dental Research 1996;75(5 (Divisional Abstracts)):1241 (Abstract 16). [Google Scholar]
Jones 1993 {published data only}
- Jones RB, Pomrehn PR, Mecklenburg RE, Lindsay EA, Manley M, Ockene JK. The COMMIT dental model: tobacco control practices and attitudes. Journal of the American Dental Association 1993;124:92-104; discussion 106-8. [DOI] [PubMed] [Google Scholar]
Kentala 1999 {published data only}
- *.Kentala J, Utriainen P, Pahkala K, Mattila K. Can brief intervention through community dental care have an effect on adolescent smoking? Preventive Medicine 1999;29:107-11. [DOI] [PubMed] [Google Scholar]
- Saari AJ, Kentala J, Mattila KJ. Long-term effectiveness of adolescent brief tobacco intervention: a follow-up study. BMC Research Notes 2012;5:101. [CENTRAL: 863865] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kirkwood 2001 {published data only}
- Kirkwood KL, Ciancio SG, Mather ML. Clinical evaluation of a smoking deterrent mouthwash. Journal of Dental Research 2001;80(January 2001 Special Issue AADR Abstracts):223 (Abstract 1504). [Google Scholar]
Kirkwood 2002 {published data only}
- Kirkwood KL, Ciancio SG, Mather ML. Clinical evaluation of a smoking deterrent breathspray compared to a placebo spray. Journal of Dental Research 2002;81(March 2002 Special Issue A):A-295 (Abstract 2328). [Google Scholar]
Koerber 2003 {published data only}
- Koerber A, Crawford J, O'Connell K. The effects of teaching dental students brief motivational interviewing for smoking-cessation counseling: a pilot study. Journal of Dental Education 2003;67:439-47. [PubMed] [Google Scholar]
La Torre 2019 {published data only}
- La Torre G, D'Egidio V, Patrissi R, Chiarini M, DE Vivo G, Mannocci A, et al. Effectiveness of a training course on smoking cessation knowledge and behaviour for health profession students: the SISMA project. Journal of Preventive Medicine and Hygiene 2019;60(2):E119-23. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Little 2009 {published data only}
- Fellows JL, Hollis JF, Fleming C, Little SJ, Snyder J. Randomized trial of a tobacco "Assisted Referral" programme in a large dental office (RPOS 3-69). In: Society for Research on Nicotine and Tobacco 12th Annual Meeting February 15-18, Orlando, Florida. 2006.
- *.Little SJ, Hollis JF, Fellows JL, Snyder JJ, Dickerson JF. Implementing a tobacco assisted referral program in dental practices. Journal of Public Health Dentistry 2009;69:149-55. [DOI] [PubMed] [Google Scholar]
Maassen 2008 {published data only}
- Maassen IT, Jacobs JE, Plasschaert AJ, Allard RH, Schattenberg G, Hilberink SR. [Stop smoking advice for patients who smoke: feasible in the dental practice?]. [Dutch]. Nederlands Tijdschrift Voor Tandheelkunde 2008;115(9):460-5. [PubMed] [Google Scholar]
Macgregor 1996 {published data only}
- Macgregor ID. Efficacy of dental health advice as an aid to reducing cigarette smoking. British Dental Journal 1996;180:292-6. [DOI] [PubMed] [Google Scholar]
Masouredis 1997 {published data only}
- Masouredis CM, Hilton JF, Grady D, Gee L, Chesney M, Hengl L, et al. A spit tobacco cessation intervention for college athletes: three-month results. Advances in Dental Research 1997;11(3):354-9. [DOI] [PubMed] [Google Scholar]
Matias 2013 {published data only}
- Matias MA, Steindl SR, Plonka KA, Pukkallus M, Palmer J, Holcombe T, et al. Do school based anti-smoking campaigns delivered by oral health therapists work? Australian Dental Journal 2013;58:301-5. [DOI] [PubMed] [Google Scholar]
McRee 2012 {published data only}
- McRee BG, Babor T, Del Boca F, Vendetti J, Oncken C, Bailit H, et al. Screening and brief intervention for patients with tobacco and at-risk alcohol use in a dental setting. Addiction Science and Clinical Practice 2012;72:5922. [Google Scholar]
Montini 2013 {published data only}
- Montini T, Schenkel AB, Shelley DR. Feasibility of a computerized clinical decision support system for treating tobacco use in dental clinics. Journal of Dental Education 2013;77:458-62. [PubMed] [Google Scholar]
Morgan 2000 {published data only}
- Morgan ML. Are you doing your part to help stamp out tobacco use? Journal of the Oklahoma Dental Association 2000;90:30-3. [PubMed] [Google Scholar]
Myers 2018 {published data only}
- Myers VS, Rotz ME, Boyd M, Lykon JL, Waldron EM, Theodorou J. Impact of a novel interprofessional dental and pharmacy student tobacco cessation education programme on dental patient outcomes. Journal of Interprofessional Care 2018;32:52-62. [DOI] [PubMed] [Google Scholar]
Nasry 2006 {published data only}
- Nasry HA, Preshaw PM, Stacey F, Heasman L, Swan M, Heasman PA. Smoking cessation advice for patients with chronic periodontitis. British Dental Journal 2006;200(5):272-5. [DOI] [PubMed] [Google Scholar]
NCI 1994 {published data only}
- NCI (National Cancer Institute). Tobacco and the Clinician: Interventions for Medical and Dental Practice. Smoking and Tobacco Control Monograph 5 1994.
NCI 1995 {published data only}
- NCI (National Cancer Institute). Smoking and Tobacco Control Monograph 6. Bethesda MD: National Cancer Institute, 1995. [Google Scholar]
NCT00907309 {published data only}
- NCT00907309. Dental and Medical Office iMET to Reduce Teen Tobacco, Alcohol, and Drug Use. clinicaltrials.gov/ct2/show/NCT00907309 (first received 22 May 2009). [NCT00907309]
NCT01275391 {published data only}
- NCT01275391. cSBIRT to reduce teen tobacco, alcohol and drug use. clinicaltrials.gov/ct2/show/NCT01275391 (first received 12 January 2011).
NCT02188563 {published data only}
- NCT02188563. A comprehensive smoking cessation intervention duration radiation for upper aerodigestive cancers. ClinicalTrials.gov/show/NCT02188563 (first received 11 July 2014). [NCT02188563]
NCT02570646 {published data only}
- NCT02570646. QuitAdvisorDDS: A point-of-care tobacco cessation tool for dental settings. clinicaltrials.gov/ct2/show/NCT02570646 (first received 7 October 2015). [NCT02570646]
NCT02737176 {published data only}
- NCT02737176. Tobacco cessation intervention study for oral diseases (TISOD). clinicaltrials.gov/ct2/show/NCT02737176 (first received 13 April 2016). [NCT02737176]
NCT03276819 {published data only}
- NCT03276819. A clinical and biological umbrella protocol for smoker or non-smoker patients with OPML or HNSCC. ClinicalTrials.gov/show/NCT03276819 (first received 8 September 2017). [NCT03276819]
NCT03579355 {published data only}
- NCT03579355. Effectiveness of a school-based tobacco prevention program for middle school students in Saudi Arabia. clinicaltrials.gov/ct2/show/NCT03579355 (first received 6 July 2018). [NCT03579355]
NCT03656874 {published data only}
- NCT03656874. Engaging patients in tobacco cessation resources in dental settings. clinicaltrials.gov/ct2/show/NCT03656874 (first received September 2018). [NCT03656874]
O'Keefe 1995 {published data only}
- O'Keefe J, Lessio A, Kassirer B. A pilot smoking cessation program involving dental offices in the borough of East York, Ontario: an initial evaluation. Journal of the Canadian Dental Association 1995;61:65-7. [PubMed] [Google Scholar]
Olson 1985 {published data only}
- Olson BL, McDonald JL, Gleason MJ, Stookey GK, Schemehorn BR, Drook CA, et al. Comparisons of various salivary parameters in smokers before and after the use of a nicotine-containing chewing gum. Journal of Dental Research 1985;64:826-30. [DOI] [PubMed] [Google Scholar]
Ostroff 2014 {published data only}
- Ostroff JS, Li Y, Shelley DR. Dentists United to Extinguish Tobacco (DUET): a study protocol for a cluster randomized, controlled trial for enhancing implementation of clinical practice guidelines for treating tobacco dependence in dental care settings. Implementation Science 2014;9:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelley DR, Kyriakos C, Campo A, Li Y, Khalife D, Ostroff J. An analysis of adaptations to multi-level intervention strategies to enhance implementation of clinical practice guidelines for treating tobacco use in dental care settings. Contemporary Clinical Trials Communication 2018;11:142-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pai 2012 {published data only}
- Pai A, Prasad S. Attempting tobacco cessation-an oral physician's perspective. Asian Pacific Journal of Cancer Prevention 2012;13:4973-7. [DOI] [PubMed] [Google Scholar]
Raja 2014 {published data only}
- Raja M, Saha S, Mohd S, Narang R, Reddy LV, Kumari M. Cognitive behavioural therapy versus basic health education for tobacco cessation among tobacco users: a randomized clinical trial. Journal of Clinical and Diagnostic Research 2014;8:ZC47-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Raja 2016 {published data only}
- Raja M, Saha S, Krishna-Reddy V, Mohd S, Narang R, Sood P. Effectiveness of oral health education versus nicotine replacement therapy for tobacco cessation- a parallel randomized clinical trial. Journal of Clinical and Experimental Dentistry 2016;8:e64-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ray 2014 {published data only}
- Ray MN, Funkhouser E, Williams JH, Sadasivam RS, Gilbert GH, Coley HL, et al. Smoking-cessation e-referrals: a national dental practice-based research network randomized controlled trial. American Journal of Preventive Medicine 2014;46:158-65. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rindal 2013 {published data only}
- Kottke TE, Brad Rindal D, Rush WA, Asche SE, Enstad CJ. Persistence of smoking-cessation decision support use in a dental practice. American Journal of Preventive Medicine 2015;48(6):722-8. [CENTRAL: 1077092] [EMBASE: 2015785386] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Rindal D, Rush WA, Schleyer T, Kirshner M, Boyle R, Thoele MJ, et al. Effectiveness of computer-assisted guidance for tobacco dependence in dental offices [abstract]. In: Proceedings of the 41st Annual Meeting & Exhibition of the American Association for Dental Research & the 36th Annual Meeting of the Canadian Association for Dental Research; 2012, Mar 21-24; Tampa, Florida, USA. 2012:Abstract no: 619. [CENTRAL: 921934]
- *.Rindal DB, Rush WA, Schleyer TK, Kirshner M, Boyle RG, Thoele MJ, et al. Computer-assisted guidance for dental office tobacco-cessation counseling: a randomized controlled trial. American Journal of Preventive Medicine 2013;44(3):260-4. [CENTRAL: 853436] [EMBASE: 2013118212] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush WA, Schleyer TK, Kirshner M, Boyle R, Thoele MJ, Lenton PA, et al. Integrating tobacco dependence counseling into electronic dental records: a multi-method approach. Journal of Dental Education 2014;78:31-9. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Rozi 2019 {published data only}
- Rozi S, Zahid N, Roome T, Lakhdir MPA, Sawani S, Razzak A, Butt ZA. Effectiveness of a school based smokeless tobacco intervention: a cluster randomized trial. Journal of Community Health 2019;44:1098-110. [DOI] [PubMed] [Google Scholar]
Schoonheim‐Klein 2013 {published data only}
- Schoonheim-Klein M, Gresnigt C, Velden U. Influence of dental education in motivational interviewing on the efficacy of interventions for smoking cessation. European Journal of Dental Education 2013;17:e28-33. [DOI] [PubMed] [Google Scholar]
Secker‐Walker 1988 {published data only}
- Secker-Walker RH, Solomon LJ, Haugh LD, Welsh D, Tatro M, Witham L, et al. Smoking cessation advice delivered by the dental hygienist. A pilot study. Dental Hygiene (Chicago) 1988;62:186-92. [PubMed] [Google Scholar]
Shelley 2011 {published data only}
- Shelley D, Anno J, Tseng TY, Calip G, Wedeles J, Lloyd M, et al. Implementing tobacco use treatment guidelines in public health dental clinics in New York City. Journal of Dental Education 2011;75:527-33. [PubMed] [Google Scholar]
Shelley 2012 {published data only}
- Shelley D, Zraik D, Tseng TY, Jannat-Khah DP, Pae-Cho C, Moon L. Effect of short acting vs long acting nicotine replacement therapy among a low SES population of light smokers (POS3-77). In: Society for Research on Nicotine and Tobacco 18th Annual Meeting March 13-16, 2012, Houston, Texas. 2012:113.
Smith 1998 {published data only}
- Smith SE, Warnakulasuriya KA, Johnson NW, Cooper DJ, Feyerabend C, Belcher M. A smoking cessation programme conducted through dental practices in the UK. British Dental Journal 1998;185:299-303. [DOI] [PubMed] [Google Scholar]
Stoops 2009 {published data only}
- Stoops WW, Dallery J, Fields NM, Nuzzo PA, Schoenberg NE, Martin CA, et al. An internet-based abstinence reinforcement smoking cessation intervention in rural smokers. Drug and Alcohol Dependence 2009;105:56-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Walsh 2007 {published data only}
- Walsh SE, Singleton JA, Worth CT, Krugler J, Moore R, Wesley GC, et al. Tobacco cessation counseling training with standardized patients. Journal of Dental Education 2007;71:1171-8. [PubMed] [Google Scholar]
Walsh 2010 {published data only}
- Walsh MM, Belek MG, Prakash P, Grimes B, Heckman B, Kaufman N, et al. Changing dentists' tobacco control attitudes and behaviors. (PA9-2). In: Society for Research on Nicotine and Tobacco 16th Annual Meeting February 24-27, Baltimore, MD. 2010.
Williams 2002 {published data only}
- Williams NJ. Comparison of effectiveness of a dental school based nicotine dependence program to dental hygiene student lead tobacco intervention counseling. In: Society for Research on Nicotine and Tobacco 8th Annual Meeting, Savannah, Georgia. 2002:Sym 6a.
Wood 1997 {published data only}
- Wood GJ, Cecchini JJ, Nathason N, Hiroshige K. Office-based training in tobacco cessation for dental professionals. Journal of the American Dental Association 1997;128:216-24. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
CTRI/2018/02/011846 {published data only}
- CTRI/2018/02/011846. Tobacco Cessation Program Conducted At Government Dental College, Kottayam, Kerala. http://www.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2018/02/011846 2018 (first received 12 February 2018). [CTRI/2018/02/011846]
CTRI/2018/03/012867 {published data only}
- CTRI/2018/03/012867. Effectiveness of Behavioral Counselling with Nicotine Gum versus Behavioral Counselling alone for quitting tobacco usage among patients at a Tobacco Cessation Clinic in Delhi. http://www.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2018/03/012867 2018 (first received 26 March 2018). [CTRI/2018/03/012867]
La Torre 2013 {published data only}
- La Torre G, Rossini G, Saulle R, Mannocci A, Di Thiene D, Mauro V, et al. Randomized controlled trial on the promotion of healthy lifestyles among adolescents in the orthodontic setting: Study protocol. [Italian] [Trial clinico randomizzato sulla promozione di corretti stili di vita tra gli adolescenti in ambito ortodontico: Protocollo dello studio]. Clinica Terapeutica 2013;164(Suppl 4):e301-4. [CENTRAL: 875688] [EMBASE: 2013659295] [DOI] [PubMed] [Google Scholar]
NCT00591175 {published data only}
- NCT00591175. A Randomized Clinical Trial Assessing Smoking Cessation Interventions In Dental Clinic Smokers. https://clinicaltrials.gov/ct2/show/NCT00591175 (first received 11 January 2008). [NCT00591175]
NCT01846910 {published data only}
- NCT01846910. Prepare to Quit - A Randomized Clinical Trial to Help People Quit Smoking in a Community Dental Clinic Setting. Http://clinicaltrials.gov/show/NCT01846910 2013.
NCT02582008 {published data only}
- NCT02582008. Bupropion Hydrochloride or Patient's Choice for Smoking Cessation in Patients With Squamous Cell Head and Neck Cancer Undergoing Radiation Therapy With or Without Chemotherapy. clinicaltrials.gov/ct2/show/NCT02582008 (first received 21 October 2015). [NCT02582008]
Additional references
ADA 2019
- American Dental Association. Tobacco use and cessation - cessation counseling. 2019. Available at www.ada.org/en/member-center/oral-health-topics/tobacco-use-and-cessation (accessed prior to 3 February 2021).
Anantharaman 2011
- Anantharaman D, Marron M, Lagiou P, Samoli E, Ahrens W, Pohlabeln H. Population attributable risk of tobacco and alcohol for upper aerodigestive tract cancer. International Journal of Epidemiology 2010;39:182-96. [DOI] [PubMed] [Google Scholar]
Cahill 2013
- Cahill K, Stevens S, Perera R, Lancaster T. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database of Systematic Reviews 2013, Issue 5. Art. No: CD009329. [DOI: 10.1002/14651858.CD009329.pub2] [CD009329] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cahill 2014
- Cahill K, Lancaster T. Workplace interventions for smoking cessation. Cochrane Database of Systematic Reviews 2014, Issue 2. Art. No: CD003440. [DOI: 10.1002/14651858.CD003440.pub4] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Carson 2012
- Carson KV, Verbiest MEA, Crone MR, Brinn MP, Esterman AJ, Assendelft WJJ, et al. Training health professionals in smoking cessation. Cochrane Database of Systematic Reviews 2012, Issue 5. Art. No: CD000214. [DOI: 10.1002/14651858.CD000214.pub2] [CD000214] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chambrone 2013
- Chambrone L, Preshaw PM, Rosa EF, Heasman PA, Romito GA, Pannuti CM, et al. Effects of smoking cessation on the outcomes of non-surgical periodontal therapy: a systematic review and individual patient data meta-analysis. Journal of Clinical Periodontology 2013;40(6):607-15. [PMID: ] [DOI] [PubMed] [Google Scholar]
Conway 2018
- Conway DI, Purkayastha M, Chestnutt IG. The changing epidemiology of oral cancer: definitions, trends and risk factors. British Dental Journal 10/11/2018;225(9):867-873. [DOI] [PubMed] [Google Scholar]
de Bruin 2020
- Bruin M, Black N, Javornik N, Viechtbauer W, Eisma M, Hartmann-Boyce J, et al. Underreporting of the active content of behavioural interventions: a systematic review and meta-analysis of randomised trials of smoking cessation interventions. Health Psychology Review 2020:1-19. [DOI: 10.1080/17437199.2019.1709098] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), accessed 12 December 2020. Available at gradepro.org.
Hartmann‐Boyce 2018
- Hartmann-Boyce J, Chepkin SC, Ye W, Bullen C, Lancaster T. Nicotine replacement therapy versus control for smoking cessation. Cochrane Database of Systematic Reviews 2018, Issue 5. Art. No: CD000146. [DOI: 10.1002/14651858.CD000146.pub5] [CD000146] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hartmann‐Boyce 2019
- Hartmann-Boyce J, Hong B, Livingstone-Banks J, Wheat H, Fanshawe TR. Additional behavioural support as an adjunct to pharmacotherapy for smoking cessation. Cochrane Database of Systematic Reviews 2019, Issue 6. Art. No: CD009670. [DOI: 10.1002/14651858.CD009670.pub4] [CD009670] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hartmann‐Boyce 2020
- Hartmann-Boyce J, McRobbie H, Lindson N, Bullen C, Begh R, Theodoulou A, et al. Electronic cigarettes for smoking cessation. Cochrane Database of Systematic Reviews 2020, Issue 10. Art. No: CD010216. [DOI: 10.1002/14651858.CD010216.pub4] [CD010216] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration (Available from www.cochrane-handbook.org), 2011. [Google Scholar]
Kassebaum 2014
- Kassebaum NJ, Bernabe E, Dahiya M, Bhandari B, Murray CJ, Marcenes W. Global burden of severe periodontitis in 1990-2010: a systematic review and meta-regression. Journal of Dental Research 2014;93(11):1045-53. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marron 2010
- Marron M, Boffetta P, Zhang Z-F, Zaridze D, Wunsch-Filho V, Winn DM, et al. Cessation of alcohol drinking, tobacco smoking and the reversal of head and neck cancer risk. International Journal of Epidemiology 2010;39:182-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
McRobbie 2004
- McRobbie H, Hajek P, Gillison, F. The relationship between smoking cessation and mouth ulcers. Nicotine & Tobacco Research 2004;6(4):655-9. [DOI] [PubMed] [Google Scholar]
NCSCT 2012
- National Centre for Smoking cessation and Training. Very brief advice on smoking for dental patients. 2012. Available at www.ncsct.co.uk/usr/pub/NCSCT%20dental%20VBA.pdf (accessed prior to 3 February 2021).
NICE 2018
- The National Institute for Health and Care Excellence. Stop smoking interventions and services (NG92). 2018. Available at www.nice.org.uk/guidance/ng92 (accessed prior to 3 February 2021).
Nohlert 2009
- Nohlert E, Tegelberg A, Tillgren P, Johansson P, Rosenblad A, Helgason AR. Comparison of a high and a low intensity smoking cessation intervention in a dentistry setting in Sweden - a randomized trial. BMC Public Health 2009;9:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
PHE 2014
- Public Health England. Delivering better oral health: an evidence-based toolkit for prevention. Third edition (2017). Available at assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/605266/Delivering_better_oral_health.pdf (accessed prior to 3 February 2021).
Review Manager 2014 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.4.1. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rigotti 2012
- Rigotti NA, Clair C, Munafo MR, Stead LF. Interventions for smoking cessation in hospitalised patients. Cochrane Database of Systematic Reviews 2012, Issue 5. Art. No: CD001837. [DOI: 10.1002/14651858.CD001837.pub3] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2017
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Akl E, et al on behalf of the Cochrane GRADEing Methods Group and the Cochrane Statistical Methods Group. Chapter 11: Completing ‘Summary of findings’ tables and grading the confidence in or quality of the evidence. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from www.training.cochrane.org/handbook.
Shield 2017
- Shield KD, Ferlay J, Jemal A, Sankaranarayanan R, Chaturvedi AK, Bray F, et al. The global incidence of lip, oral cavity, and pharyngeal cancers by subsite in 2012. CA: A Cancer Journal for Clinicians 2017;67(1):51-64. [DOI] [PubMed] [Google Scholar]
Stead 2013
- Stead LF, Buitrago D, Preciado N, Sanchez G, Hartmann-Boyce J, Lancaster T. Physician advice for smoking cessation. Cochrane Database of Systematic Reviews 2013, Issue 5. Art. No: CD000165. [DOI: 10.1002/14651858.CD000165.pub4] [CD000165] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stead 2016
- Stead LF, Koilpillai P, Fanshawe TR, Lancaster T. Combined pharmacotherapy and behavioural interventions for smoking cessation. Cochrane Database of Systematic Reviews 2016, Issue 3. Art. No: CD008286. [DOI: 10.1002/14651858.CD008286.pub3] [CD008286] [DOI] [PMC free article] [PubMed] [Google Scholar]
West 2005
- West R, Hajek P, Stead L, Stapleton J. Outcome criteria in smoking cessation trials: proposal for a common standard. Addiction 2005;100(3):299-303. [DOI] [PubMed] [Google Scholar]
WHO 2008
- World Health Organization. WHO report on the global tobacco epidemic, 2008: the MPOWER package. Geneva 2008;ISBN 978 92 3 159628 2.
WHO 2017
- World Health Organization. WHO monograph on tobacco cessation and oral health integration. 2017. Available at apps.who.int/iris/bitstream/handle/10665/255692/9789241512671-eng.pdf (accessed prior to 3 February 2021).
References to other published versions of this review
Carr 2006
- Carr A, Ebbert J. Interventions for tobacco cessation in the dental setting. Cochrane Database of Systematic Reviews 2006, Issue 1. Art. No: CD005084. [DOI: 10.1002/14651858.CD005084.pub2] [DOI] [PubMed] [Google Scholar]
Carr 2012
- Carr AB, Ebbert J. Interventions for tobacco cessation in the dental setting. Cochrane Database of Systematic Reviews 2012, Issue 6. Art. No: CD005084. [DOI: 10.1002/14651858.CD005084.pub3] [CD005084] [DOI] [PMC free article] [PubMed] [Google Scholar]


