Abstract
The effects of SARS-CoV-2 infection in the first trimester on the pregnant woman and the fetus remain unclear. We describe the complete follow-up of a pregnant woman with asymptomatic SARS-CoV-2 infection in the first trimester. The woman tested positive for SARS-CoV-2 viral RNA in nasopharyngeal swabs in her seventh week of gestation and was admitted to a local hospital for treatment. Although the woman had a BMI above 28 and a total gestational weight gain of 21 kg, no pregnancy complications or severe complications related to SARS-CoV-2 were reported. An ultrasound scan identified no fetal abnormalities at 22 weeks. The pregnancy ended at term (37 weeks), and the newborn's birth weight was 3100 g. Placental insufficiency was revealed by placental histology examination but this appeared not to be related to the SARS-CoV-2 infection. In-situ hybridisation and immunohistochemical tests for SARS-CoV-2 RNA, spike protein 1, and nucleocapsid proteins were negative. However, ACE-2 was positive in samples of the placenta, umbilical cord and fetal membrane. The baby was followed up through to 10 days after birth and grew normally. Our results suggest that asymptomatic SARS-CoV-2 infection in the first trimester of pregnancy might not have significant harmful effects on the mother and the developing fetus. This finding may be of interest to the general public, midwives and general practitioners. However, large population studies are needed to confirm our findings.
Keywords: SARS-CoV-2 infection, Pregnancy, First trimester, Placenta, Neonatal outcomes
Highlights
-
•
Effects of SARS-CoV-2 infection in first trimester on the pregnant woman and the foetus remain unclear.
-
•
We described a complete follow-up for a pregnant woman with asymptomatic SARS-CoV-2 infection in the first trimester.
-
•
Asymptomatic SARS-CoV-2 infection in early pregnancy might not have major harmful effects on the mother and the foetus
List of Abbreviations
| ACE2 | angiotensin-converting enzyme 2 protein |
| BMI | body mass index |
| GP | general practitioner |
| NP | nucleocapsid protein |
| SARS-CoV-2 | severe acute respiratory syndrome coronavirus 2 |
| S1 | spike protein 1 |
1. Introduction
Impacts of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection on pregnant women have been increasingly reported [1]. Although most cases are asymptomatic at admission, the psychological stress for pregnant women could be substantial [[2], [3], [4]]. Those infected in early pregnancy may choose to terminate the pregnancy because of the worry of detrimental effects on the fetus. However, this concern lacks a scientific basis. [5] To our knowledge, no complete follow-up data have been published regarding the direct effects of SARS-CoV-2 infection in the first trimester on maternal and neonatal outcomes (e.g. teratogenesis, in utero infection or placental dysfunction). In this report, we describe the complete clinical characteristics during pregnancy, at birth and ten days after delivery of asymptomatic SARS-CoV-2 infection in the first trimester. Our findings may be of interest to the general public, midwives and general practitioners (GPs).
2. Case Presentation
A pregnant woman tested positive for SARS-CoV-2 infection in her seventh week of gestation and was tracked throughout the pregnancy and through to six weeks after delivery at local hospitals in China. This primiparous pregnant woman had a pre-pregnancy body mass index (BMI) above 28 and no history of hypertension or diabetes. At 7+5 weeks of gestation, she was screened for SARS-CoV-2 infection because of close contact with a confirmed case. Her nasopharyngeal swabs tested positive in April 2020 (i.e. the early phase of the COVID-19 outbreak in Guangzhou, China). She was then admitted to a local hospital for treatment. Another nasal swab test for SARS-CoV-2 viral RNA confirmed she was infected, whereas the blood tests for IgM and IgG antibodies were negative (Table 1). At admission, the patient had decreased appetite but no fever, vomiting, myalgia, fatigue, diarrhoea, dyspnoea or dry cough; laboratory tests revealed increased levels of CRP (20.54 ml/l) and percentage of neutrophils (79.3%) and a decreased percentage of lymphocytes (16.6%). An ultrasound scan at 7+6 weeks of gestation did not reveal any pregnancy abnormalities. The patient was given oxygen therapy and Chinese traditional medicine. She did not have any severe complications related to SARS-CoV-2 during hospitalisation. Two nasal swabs tests at 8+1 weeks and 8+3 weeks were negative for SARS-CoV-2. The woman was discharged at 8+4 weeks and was transferred to a quarantine centre in the same hospital for two-week isolation. The SARS-CoV-2 IgM and IgG tested positive at 9+4 weeks and 10+2 weeks and turned negative at 14+3 weeks (Table 1).
Table 1.
Results of the patient's SARS-CoV-2 tests.
| Date | Sample type | Test | Results |
|---|---|---|---|
| 7+5 weeks of gestation | Nasal swab | Viral RNA | Positive |
| 7+6 weeks | Nasal swab | Viral RNA | Positive |
| 7+6 weeks | Blood | IgM and IgG | Both negative |
| 8+1 weeks | Nasal swab | Viral RNA | Negative |
| 8+3 weeks | Nasal swab | Viral RNA | Negative |
| 9+4 weeks | Blood | IgM and IgG | Both positive |
| 10+2 weeks | Blood | IgM and IgG | Both positive |
| 10+4 weeks | Faeces | Viral RNA | Negative |
| 10+5 weeks | Faeces | Viral RNA | Negative |
| 14+3 weeks | Blood | IgM and IgG | Both negative |
| 37+4 weeks (delivery admission) | Nasal swab | Viral RNA | Negative |
| Two days after delivery | Nasal swabs (mother and baby) | Viral RNA | Both negative |
After discharge from hospital, the pregnant woman attended regular antenatal visits (n = 10) at a local maternity hospital. Five ultrasound scans (from 22 weeks to 37 weeks) were performed to assess fetal growth, which was in the normal range (10th–90th percentile based on Intergrowth 21st standards). An ultrasound scan screening for fetal abnormalities was also carried out at 22+6 weeks and found null. The patient had a total gestational weight gain of 21 kg but no pregnancy complications (e.g. hypertensive disorders during pregnancy or gestational diabetes).
The woman was admitted to the maternity hospital for delivery with symptoms of premature rupture of membranes. She was given antibiotics. A baby girl was born via caesarean section. The gestational age at birth was 37+4 weeks, and the birth weight was 3100 g. Apgar scores were 9 and 10 at 1 and 5 min, respectively. No obvious abnormality was found visually.
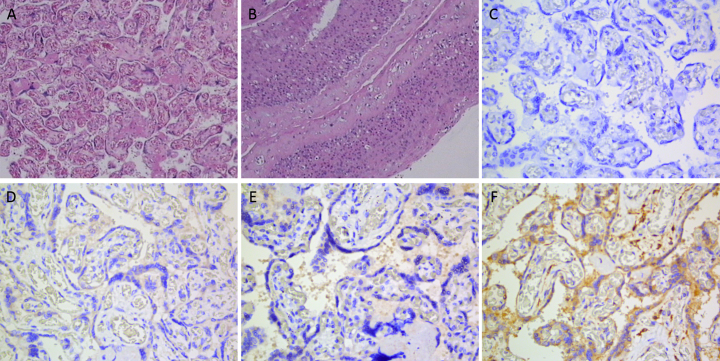
Placental histopathological examination revealed finer terminal villi, increased vascularisation and angiogenesis in majority villi (Figure 1A1), villous fibrosis (Figure 1A2) and an increased number of syncytium cell nodules (Figure 1A3). Endovasculitis of the stem villous vessels was also observed (Fig. 1B). These findings suggest placental insufficiency, which may affect blood flow perfusion and oxygen supply.
Fig. 1.
Placental examinations: Haematoxylin and eosin staining (panels A and B); SARS-CoV-2 RNA (panel C), S1 (panel D), NP (E) and ACE-2 proteins (F).
Tissues of the placenta, umbilical cord and fetal membrane were collected and tested for levels of SARS-CoV-2 RNA using in situ hybridisation, spike protein 1 (S1), nucleocapsid protein (NP) and angiotensin-converting enzyme 2 (ACE2) protein using immunohistochemical staining. Results showed SARS-CoV-2 RNA was negative (Fig. 1C), S1 and NP proteins were negative (Fig. 1D and E), whereas ACE-2 was positive (Fig. 1F).
The baby was seen by a GP 10 days after birth. She weighed 3.2 kg and was fed with breast milk and formula milk. Transcutaneous bilirubin measurement indicated jaundice (about 14 mg/dl). Other physical assessments did not reveal any abnormalities.
3. Discussion
We present the complete follow-up of a case of asymptomatic SARS-CoV-2 infection in the first trimester of pregnancy. Both pregnancy and obesity (BMI >28 kg/m2; based on Chinese standards [6]) are reportedly risk factors for severe complications [7]. The woman also had an excessive gestational weight gain, probably resulting from reduced physical activity (due to quarantine or lockdown). However, we did not observe significant adverse outcomes during pregnancy, at birth or in the neonatal period for this woman and her child. Changes in placental histology appeared not to be specific for SARS-CoV-2 infection and could be explained by maternal obesity [8,9].
The strength of this report is the complete follow-up of both mother and child from early pregnancy to the postnatal period. The main limitation is that we did not collect data on maternal and neonatal immune responses to SARS-CoV-2 (e.g. neutralising antibodies). Whether asymptomatic SARS-CoV-2 infection induces subtle immune alternation in the mother and the fetus warrants further investigation.
4. Conclusions
Our case report does not support the concern that asymptomatic SARS-CoV-2 infection in the first trimester of pregnancy has significant harmful effects on the mother and the developing fetus. However, large population studies are needed to confirm our findings.
Acknowledgments
Contributors
Jian-Rong He collected clinical data, cleaned and analysed the data, interpreted the results and wrote the original draft of the manuscript.
Yan-Hua Xiao performed RT-PCR, in situ hybridisation and immunohistochemical tests and interpreted the results.
Wen Ding collected clinical data and biospecimens.
Ya-Ling Shi performed RT-PCR, in situ hybridisation and immunohistochemical tests and interpreted the results.
Xi He performed RT-PCR, in situ hybridisation and immunohistochemical tests and interpreted the results.
Xiao-Dan Liu collected clinical data and biospecimens.
Guo-Zheng Zhang collected clinical data and biospecimens.
Sha-Sha Li performed RT-PCR, in situ hybridisation and immunohistochemical tests and interpreted the results.
Jin-Qing Su performed RT-PCR, in situ hybridisation and immunohistochemical tests and interpreted the results.
Li Liang collected clinical data and biospecimens.
Liang Zeng performed the placental histopathological examination and interpreted the results.
Fang Li conceptualised and designed the study, coordinated data and biospecimen collection and interpreted the results.
Xiu Qiu conceptualised and designed the study, coordinated data and biospecimen collection and interpreted the results.
All authors critically reviewed and revised the manuscript and approved the final version for submission.
Conflict of Interest
The authors declare that they have no conflict of interest regarding the publication of this case report.
Funding
This study was financially supported by the Department of Science and Technology of Guangdong Province (project numbers, 2019B030316014, 2019B020227001 and 2020B111108001). There was no role of the funding body in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Patient Consent
Obtained.
Provenance and Peer Review
This case report was peer reviewed.
Contributor Information
Fang Li, Email: gz8hlf@126.com.
Xiu Qiu, Email: xiu.qiu@bigcs.org.
References
- 1.Allotey J., Stallings E., Bonet M., Yap M., Chatterjee S., Kew T. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370:m3320. doi: 10.1136/bmj.m3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Overbeck G., Graungaard A.H., Rasmussen I.S., Andersen J.H., Ertmann R.K., Kragstrup J. Pregnant women’s concerns and antenatal care during COVID-19 lockdown of the Danish society. Dan Med J. 2020;67(12) [PubMed] [Google Scholar]
- 3.Medina-Jimenez V., Bermudez-Rojas M.L., Murillo-Bargas H., Rivera-Camarillo A.C., Munoz-Acosta J., Ramirez-Abarca T.G. The impact of the COVID-19 pandemic on depression and stress levels in pregnant women: a national survey during the COVID-19 pandemic in Mexico. J. Matern. Fetal Neonatal Med. 2020:1–3. doi: 10.1080/14767058.2020.1851675. [DOI] [PubMed] [Google Scholar]
- 4.Lebel C., MacKinnon A., Bagshawe M., Tomfohr-Madsen L., Giesbrecht G. Corrigendum to elevated depression and anxiety symptoms among pregnant individuals during the COVID-19 pandemic journal of affective disorders 277 (2020) 5-13. J. Affect. Disord. 2020;279:377–379. doi: 10.1016/j.jad.2020.07.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kotlyar A.M., Grechukhina O., Chen A., Popkhadze S., Grimshaw A., Tal O. Vertical transmission of coronavirus disease 2019: a systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2021;224(1):35–53. doi: 10.1016/j.ajog.2020.07.049. (e33) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen C., Lu F.C. The guidelines for prevention and control of overweight and obesity in Chinese adults. Biomed. Environ. Sci. 2004;17(Suppl):1–36. [PubMed] [Google Scholar]
- 7.Gao Y.D., Ding M., Dong X., Zhang J.J., Kursat Azkur A., Azkur D. Risk factors for severe and critically ill COVID-19 patients: a review. Allergy. 2021;76(2):428–455. doi: 10.1111/all.14657. [DOI] [PubMed] [Google Scholar]
- 8.Kelly A.C., Powell T.L., Jansson T. Placental function in maternal obesity. Clin Sci (Lond). 2020;134(8):961–984. doi: 10.1042/CS20190266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kretschmer T., Turnwald E.M., Janoschek R., Zentis P., Bae-Gartz I., van Beers T. Maternal high fat diet-induced obesity affects trophoblast differentiation and placental function in mice. Biol. Reprod. 2020;103(6):1260–1274. doi: 10.1093/biolre/ioaa166. [DOI] [PubMed] [Google Scholar]