Abstract
Background
People with liver cirrhosis who have had one episode of variceal bleeding are at risk for repeated episodes of bleeding. Endoscopic intervention and portosystemic shunts are used to prevent further bleeding, but there is no consensus as to which approach is preferable.
Objectives
To compare the benefits and harms of shunts (surgical shunts (total shunt (TS), distal splenorenal shunt (DSRS), or transjugular intrahepatic portosystemic shunt (TIPS)) versus endoscopic intervention (endoscopic sclerotherapy or banding, or both) with or without medical treatment (non‐selective beta blockers or nitrates, or both) for prevention of variceal rebleeding in people with liver cirrhosis.
Search methods
We searched the CHBG Controlled Trials Register; CENTRAL, in the Cochrane Library; MEDLINE Ovid; Embase Ovid; LILACS (Bireme); Science Citation Index ‐ Expanded (Web of Science); and Conference Proceedings Citation Index ‐ Science (Web of Science); as well as conference proceedings and the references of trials identified until 22 June 2020. We contacted study investigators and industry researchers.
Selection criteria
Randomised clinical trials comparing shunts versus endoscopic interventions with or without medical treatment in people with cirrhosis who had recovered from a variceal haemorrhage.
Data collection and analysis
We used standard methodological procedures expected by Cochrane. When possible, we collected data to allow intention‐to‐treat analysis. For each outcome, we estimated a meta‐analysed estimate of treatment effect across trials (risk ratio for binary outcomes). We used random‐effects model meta‐analysis as our main analysis and as a means of presenting results. We reported differences in means for continuous outcomes without a meta‐analytic estimate due to high variability in their assessment among all trials. We assessed the certainty of evidence using GRADE.
Main results
We identified 27 randomised trials with 1828 participants. Three trials assessed TSs, five assessed DSRSs, and 19 trials assessed TIPSs. The endoscopic intervention was sclerotherapy in 16 trials, band ligation in eight trials, and a combination of band ligation and either sclerotherapy or glue injection in three trials. In eight trials, endoscopy was combined with beta blockers (in one trial plus isosorbide mononitrate). We judged all trials to be at high risk of bias. We assessed the certainty of evidence for all the outcome review results as very low (i.e. the true effects of the results are likely to be substantially different from the results of estimated effects). The very low evidence grading is due to the overall high risk of bias for all trials, and to imprecision and publication bias for some outcomes. Therefore, we are very uncertain whether portosystemic shunts versus endoscopy interventions with or without medical treatment have effects on all‐cause mortality (RR 0.99, 95% CI 0.86 to 1.13; 1828 participants; 27 trials), on rebleeding (RR 0.40, 95% CI 0.33 to 0.50; 1769 participants; 26 trials), on mortality due to rebleeding (RR 0.51, 95% CI 0.34 to 0.76; 1779 participants; 26 trials), and on occurrence of hepatic encephalopathy, both acute (RR 1.60, 95% CI 1.33 to 1.92; 1649 participants; 24 trials) and chronic (RR 2.51, 95% CI 1.38 to 4.55; 956 participants; 13 trials). No data were available regarding health‐related quality of life.
Analysing each modality of portosystemic shunts individually (i.e. TS, DSRS, and TIPS) versus endoscopic interventions with or without medical treatment, we are very uncertain if each type of shunt has effect on all‐cause mortality: TS, RR 0.46, 95% CI 0.19 to 1.13; 164 participants; 3 trials; DSRS, RR 0.93, 95% CI 0.65 to 1.33; 352 participants; 4 trials; and TIPS, RR 1.10, 95% CI 0.92 to 1.31; 1312 participants; 19 trial; on rebleeding: TS, RR 0.28, 95% CI 0.14 to 0.56; 127 participants; 2 trials; DSRS, RR 0.26, 95% CI 0.11 to 0.65; 330 participants; 5 trials; and TIPS, RR 0.44, 95% CI 0.36 to 0.55; 1312 participants; 19 trials; on mortality due to rebleeding: TS, RR 0.25, 95% CI 0.06 to 0.96; 164 participants; 3 trials; DSRS, RR 0.31, 95% CI 0.13 to 0.74; 352 participants; 5 trials; and TIPS, RR 0.65, 95% CI 0.40 to 1.04; 1263 participants; 18 trials; on acute hepatic encephalopathy: TS, RR 1.66, 95% CI 0.70 to 3.92; 115 participants; 2 trials; DSRS, RR 1.70, 95% CI 0.94 to 3.08; 287 participants; 4 trials, TIPS, RR 1.61, 95% CI 1.29 to 1.99; 1247 participants; 18 trials; and chronic hepatic encephalopathy: TS, Fisher's exact test P = 0.11; 69 participants; 1 trial; DSRS, RR 4.87, 95% CI 1.46 to 16.23; 170 participants; 2 trials; and TIPS, RR 1.88, 95% CI 0.93 to 3.80; 717 participants; 10 trials.
The proportion of participants with shunt occlusion or dysfunction was overall 37% (95% CI 33% to 40%). It was 3% (95% CI 0.8% to 10%) following TS, 7% (95% CI 3% to 13%) following DSRS, and 47.1% (95% CI 43% to 51%) following TIPS. Shunt dysfunction in trials utilising polytetrafluoroethylene‐covered stents was 17% (95% CI 11% to 24%).
Length of inpatient hospital stay and cost were not comparable across trials.
Funding was unclear in 16 trials; 11 trials were funded by government, local hospitals, or universities.
Authors' conclusions
Evidence on whether portosystemic shunts versus endoscopy interventions with or without medical treatment in people with cirrhosis and previous hypertensive portal bleeding have little or no effect on all‐cause mortality is very uncertain. Evidence on whether portosystemic shunts may reduce bleeding and mortality due to bleeding while increasing hepatic encephalopathy is also very uncertain. We need properly conducted trials to assess effects of these interventions not only on assessed outcomes, but also on quality of life, costs, and length of hospital stay.
Keywords: Humans; Bias; Cause of Death; Endoscopy; Endoscopy/methods; Esophageal and Gastric Varices; Esophageal and Gastric Varices/prevention & control; Esophageal and Gastric Varices/therapy; Gastrointestinal Hemorrhage; Gastrointestinal Hemorrhage/epidemiology; Gastrointestinal Hemorrhage/prevention & control; Gastrointestinal Hemorrhage/therapy; Hepatic Encephalopathy; Hepatic Encephalopathy/epidemiology; Hepatic Encephalopathy/etiology; Intention to Treat Analysis; Liver Cirrhosis; Liver Cirrhosis/complications; Portasystemic Shunt, Surgical; Portasystemic Shunt, Surgical/adverse effects; Portasystemic Shunt, Surgical/methods; Portasystemic Shunt, Transjugular Intrahepatic; Portasystemic Shunt, Transjugular Intrahepatic/adverse effects; Randomized Controlled Trials as Topic; Secondary Prevention; Splenorenal Shunt, Surgical; Splenorenal Shunt, Surgical/adverse effects
Plain language summary
Shunts compared with endoscopic intervention to prevent further episodes of variceal bleeding in people with liver cirrhosis
Background
People with scarring of the liver (cirrhosis) may develop high pressure in the portal vein (the vein that carries blood from the gut to the liver). This high pressure results in abnormally dilated veins (varices) in the gullet (oesophagus), in the stomach, or in the intestine, which may cause life‐threatening bleeding. People who have bled once are at high risk of bleeding in the future, so it is important to prevent further bleeding episodes in these people. Different treatment options are available to prevent further bleeding. One option is endoscopic treatment, which uses a flexible camera to examine the affected area and to seal varices with elastic bands, or to inject the varices with a substance to close the veins. A second option is 'shunting', which diverts blood flow away from the problematic vein, reducing pressure and thereby reducing the chance of bleeding. There are three main types of shunts: total shunt, distal splenorenal shunt, and transjugular intrahepatic portosystemic shunt. Total shunt and distal splenorenal shunt were more commonly used in the past and require invasive surgical procedures. TIPS are now much more commonly used, as they do not require invasive surgery.
Review question
The aim of this Cochrane Review was to compare shunts versus endoscopic treatments with or without further medications in people with liver cirrhosis who had previously bled from varices, by collecting and analysing all relevant studies in this topic area and by reviewing the evidence.
Study characteristics
In June 2020, we reviewed the evidence. We found 27 randomised clinical trials (trials where participants are allocated to groups at random) involving 1828 participants. Three trials investigated total shunt (164 participants); five trials investigated distal splenorenal shunt (352 participants); and 19 trials investigated transjugular intrahepatic portosystemic shunt (1312 participants). The source of funding was unclear in 16 trials. Eleven trials were funded by the government or received grants from local hospitals or universities.
Results
Evidence suggesting whether shunt treatments compared with endoscopic treatments with or without further medications alter the overall risk of death from any cause (all‐cause mortality), reduce the risk of bleeding from varices, or reduce the risk of dying from bleeding varices (death due to variceal bleeding) was very uncertain.
Evidence that people treated with shunts compared with endoscopic treatments with or without further medications are at increased risk of acute hepatic encephalopathy (brain dysfunction associated with liver disease) or chronic hepatic encephalopathy (brain dysfunction that occurs repeatedly or does not fully improve) was also very uncertain.
We could not conclude with certainty whether people treated with shunt stayed in hospital longer than people treated with endoscopy with or without further medications, or which treatment was more expensive, as we were not confident that combining the results from different studies would produce a meaningful result. No trials reported on the impact of treatments on patient quality of life.
Risk of bias
The results of our analyses must be interpreted with caution due to concerns about the quality of included trials. Weaknesses in the design of these studies could influence results, making them potentially misleading.
Conclusions
We cannot say for sure that portosystemic shunts when compared with endoscopic treatment associated sometimes with medical treatment modify the risk of overall death (all‐cause mortality), reduce the risk of repeated episodes of bleeding, or increase the risk of developing hepatic encephalopathy. We need properly conducted trials assessing important outcomes for people with cirrhosis and health providers.
Summary of findings
Summary of findings 1. Portosystemic shunts compared with endoscopic intervention with or without medical treatment for prevention of rebleeding in people with cirrhosis.
| Portosystemic shunts compared with endoscopic intervention with or without medical treatment for prevention of rebleeding in people with cirrhosis | ||||||
| Patient or population: participants with cirrhosis and with previous oesophagogastric variceal bleeding Setting: hospital; tertiary referral centres Intervention: shunt intervention (total shunt (TS), distal splenorenal shunt (DSRS), or transjugular intrahepatic portosystemic shunt (TIPS)) Comparison: endoscopic intervention with or without medical treatment | ||||||
| Outcomes | Illustrative comparative risks (95% CI) | Relative effect (95% CI) | № of participants (trials) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk with endoscopic intervention with or without medical treatment | Corresponding risk with shunts | |||||
|
All‐cause mortality Follow‐up: 32.9 months (range 11.7 to 98) |
Medium‐risk population | RR 0.99 (0.86 to 1.13) | 1828 (27 RCTs) |
⊕⊝⊝⊝ Very lowa |
||
| 288 per 1000 | 286 per 1000 (248 to 325) | |||||
|
Rebleeding Follow‐up: 33.8 months (range 13.5 to 98) |
Medium‐risk population | RR 0.40 (0.33 to 0.50) | 1769 (26 RCTs) | ⊕⊝⊝⊝ Very lowb | ||
| 432 per 1000 | 173 per 1000 (143 to 216) | |||||
| Health‐related quality of life | No data available | No data | ||||
|
Mortality due to rebleeding Follow‐up: 33.5 months (range 11.7 to 98) |
Medium‐risk population | RR 0.51 (0.34 to 0.76) | 1779 (26 RCTs) |
⊕⊝⊝⊝ Very lowc |
||
| 95 per 1000 | 48 per 1000 (32 to 72) | |||||
|
Acute hepatic encephalopathy Follow‐up: 33.8 months (range 13.5 to 98) |
Medium‐risk population | RR 1.60 (1.33 to 1.92) | 1649 (24 RCTs) | ⊕⊝⊝⊝ Very lowd | ||
| 185 per 1000 | 296 per 1000 (246 to 355) | |||||
|
Chronic hepatic encephalopathy Follow‐up: 28.5 months (range 13.5 to 98) |
Medium‐risk population | RR 2.51 (1.38 to 4.55) | 956 (13 RCTs) | ⊕⊝⊝⊝ Very lowe | ||
| 27 per 1000 | 68 per 1000 (37 to 123) | |||||
| The corresponding risk (risk of the intervention group) (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OIS: optimal information size; RCT: randomised clinical trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded three levels because of within‐study risk of bias: all trials were at overall high risk of bias (‐2 levels); publication bias (‐1 level). bDowngraded three levels because of within‐study risk of bias: all trials were at overall high risk of bias (‐2 levels); publication bias (‐1 level). cDowngraded four levels because of within‐study risk of bias: all trials were at overall high risk of bias (‐2 levels); imprecision: optimal information size (OIS) as calculated by GRADE was not met (‐1 level); publication bias (‐1 level). dDowngraded four levels because of within‐study risk of bias: all trials were at overall high risk of bias (‐2 levels); imprecision: OIS as calculated by GRADE was not met (‐1 level); publication bias (‐1 level). eDowngraded four levels because of within‐study risk of bias: all trials were at overall high risk of bias (‐2 levels); imprecision: OIS as calculated by GRADE was not met (‐1 level); publication bias (‐1 level).
Summary of findings 2. Total shunt compared with endoscopic intervention for prevention of rebleeding in people with cirrhosis.
| Total shunt compared with endoscopic intervention for prevention of rebleeding in people with cirrhosis | ||||||
| Patient or population: participants with cirrhosis and with previous oesophagogastric variceal bleeding Setting: hospital tertiary care centres Intervention: total shunt Comparison: endoscopic intervention | ||||||
| Outcomes | Illustrative comparative risks (95% CI) | Relative effect (95% CI) | № of participants (trials) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk with endoscopic intervention with or without medical treatment | Corresponding risk with total shunt | |||||
|
All‐cause mortality Follow‐up: 32.4 months (range 11.7 to 65.1) |
Medium‐risk population | RR 0.46 (0.19 to 1.13) | 164 (3 RCTs) | ⊕⊝⊝⊝ Very lowa | ||
| 198 per 1000 | 91 per 1000 (38 to 224) | |||||
|
Rebleeding Follow‐up: 42.8 months (range 20.4 to 65.1) |
Medium risk population | RR 0.28 (0.14 to 0.56) | 127 (2 RCTs) | ⊕⊝⊝⊝ Very lowb | ||
| 435 per 1000 | 122 per 1000 (61 to 244) | |||||
| Health‐related quality of life | No data available | No data | ||||
|
Mortality due to rebleeding Follow‐up: 32.4 months (range 11.7 to 65.1) |
Medium‐risk population | RR 0.25 (0.06 to 0.96) | 164 (3 RCTs) |
⊕⊝⊝⊝ Very lowc |
||
| 136 per 1000 | 34 per 1000 (8 to 131) | |||||
|
Acute hepatic encephalopathy Follow‐up: 42.8 months (range 20.4 to 65.1) |
Medium‐risk population | RR 1.66 (0.70 to 3.92) | 115 (2 RCTs) | ⊕⊝⊝⊝ Very lowd | ||
| 123 per 1000 | 204 per 1000 (86 to 482) | |||||
|
Chronic hepatic encephalopathy Follow‐up: 20.4 months |
69 (1 RCT) | There was a single trial with 3/34 events in total shunt group and 0/35 in endoscopy with or without drugs group | ||||
| The corresponding risk (risk of the intervention group) (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised clinical trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded three levels because of within‐study risk of bias: all trials were at overall high risk of bias (‐2 levels); imprecision: optimal information size (OIS) as calculated by GRADE was not met (‐1 level). bDowngraded three levels because of within‐study risk of bias: all trials were at overall high risk of bias (‐2 levels); imprecision: OIS as calculated by GRADE was not met (‐1 level). cDowngraded three levels because of within‐study risk of bias: all trials were at overall high risk of bias (‐2 levels); imprecision: OIS as calculated by GRADE was not met (‐1 level). dDowngraded four levels because of within‐study risk of bias: all trials were at overall high risk of bias (‐2 levels); imprecision: OIS as calculated by GRADE was not met; there were few events and the CI included appreciable benefit and harm (‐2 levels).
Summary of findings 3. Distal splenorenal shunt compared with endoscopic intervention for prevention of rebleeding in people with cirrhosis.
| Distal splenorenal shunt (DSRS) compared with endoscopic intervention for prevention of rebleeding in people with cirrhosis | ||||||
| Patient or population: participants with cirrhosis and with previous oesophagogastric variceal bleeding Setting: hospital tertiary care centres Intervention: DSRS Comparison: endoscopic intervention | ||||||
| Outcomes | Illustrative comparative risks (95% CI) | Relative effect (95% CI) | № of participants (trials) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk with endoscopic intervention | Corresponding risk with distal splenorenal shunt | |||||
|
All‐cause mortality Follow‐up: 68.6 months (range 27.1 to 98) |
Medium‐risk population | RR 0.93 (0.65 to 1.33) | 352 (5 RCTs) | ⊕⊝⊝⊝ Very lowa | ||
| 469 per 1000 | 436 per 1000 (305 to 624) | |||||
|
Rebleeding Follow‐up: 68.6 months (range 27.1 to 98) |
Medium‐risk population | RR 0.26 (0.11 to 0.65) | 330 (5 RCTs) | ⊕⊝⊝⊝ Very lowb | ||
| 458 per 1000 | 119 per 1000 (50 to 298) | |||||
| Health‐related quality of life | No data available | |||||
|
Mortality due to rebleeding Follow‐up: 68.6 months (range 27.1 to 98) |
Medium‐risk population | RR 0.31 (0.13 to 0.74) | 352 (5 RCTs) |
⊕⊝⊝⊝ Very lowc | ||
| 126 per 1000 | 39 per 1000 (16 to 93) | |||||
|
Acute hepatic encephalopathy Follow‐up: 68.6 months (range 27.1 to 98) |
Medium‐risk population | RR 1.70 (0.94 to 3.08) | 287 (4 RCTs) | ⊕⊝⊝⊝ Very lowd | ||
| 139 per 1000 | 236 per 1000 (131 to 428) | |||||
|
Chronic hepatic encephalopathy Follow‐up: 62.5 months (range 27.1 to 98) |
Medium‐risk population | RR 2.51 (1.38 to 4.55) | 170 (2 RCTs) | ⊕⊝⊝⊝ Very lowe | ||
| 27 per 1000 | 68 per 1000 (37 to 123) | |||||
| The corresponding risk (risk of the intervention group) (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised clinical trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded four levels because of within‐study risk of bias: all trials were at overall high risk of bias (‐2 levels); imprecision: optimal information size (OIS) as calculated by GRADE was not met; (‐1 levels); heterogeneity (‐1 level). bDowngraded four levels because of within‐study risk of bias: all trials were at overall high risk of bias (‐2 levels); imprecision: OIS as calculated by GRADE was not met (‐1 level); heterogeneity (‐1 level). cDowngraded three levels because of within‐study risk of bias: all trials were at overall high risk of bias (‐2 levels); imprecision: OIS as calculated by GRADE was not met (‐1 level). dDowngraded three levels because of within‐study risk of bias: all trials were at overall high risk of bias (‐2 levels); imprecision: OIS as calculated by GRADE was not met (‐1 level). eDowngraded three levels because of within‐study risk of bias: all trials were at overall high risk of bias (‐2 levels); imprecision: OIS as calculated by GRADE was not met (‐1 levels).
Summary of findings 4. Transjugular intrahepatic portosystemic shunt compared with endoscopic intervention with or without medical treatment for prevention of rebleeding in people with cirrhosis.
| Transjugular intrahepatic portosystemic shunt (TIPS) compared with endoscopic intervention with or without medical treatment for prevention of rebleeding in people with cirrhosis | ||||||
| Patient or population: participants with cirrhosis with previous oesophagogastric variceal bleeding Setting: tertiary care centres Intervention: TIPS Comparison: endoscopic intervention with or without medical treatment | ||||||
| Outcomes | Illustrative comparative risks (95% CI) | Relative effect (95% CI) | № of participants (trials) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk with endoscopic intervention with or without medical treatment | Corresponding risk with TIPS | |||||
|
All‐cause mortality Follow‐up: 24.5 months (range 13.5 to 46.2) |
Medium‐risk population | RR 1.10 (0.92 to 1.31) | 1312 (19 RCTs) |
⊕⊝⊝⊝ Very lowa | ||
| 252 per 1000 | 277 per 1000 (232 to 330) |
|||||
|
Rebleeding Follow‐up: 24.5 months (range 13.5 to 46.2) |
Medium‐risk population | RR 0.44 (0.36 to 0.55) | 1312 (19 RCTs) | ⊕⊝⊝⊝ Very lowb | ||
| 425 per 1000 | 187 per 1000 (153 to 234) | |||||
| Health‐related quality of life | No data available | |||||
|
Mortality due to rebleeding Follow‐up: 24.9 months (range 13.5 to 46.2) |
Medium‐risk population | RR 0.65 (0.40 to 1.04) | 1263 (18 RCTs) |
⊕⊝⊝⊝ Very lowc | ||
| 82 per 1000 | 53 per 1000 (33 to 85) |
|||||
|
Acute hepatic encephalopathy Follow‐up: 24.5 months (range 13.5 to 46.2) |
Medium‐risk population | RR 1.61 (1.29 to 1.99) | 1247 (18 RCTs) | ⊕⊝⊝⊝ Very lowd | ||
| 201 per 1000 | 324 per 1000 (259 to 400) | |||||
|
Chronic hepatic encephalopathy Follow‐up: 22.5 months (range 13.5 to 46.2) |
Medium‐risk population | RR 1.88 (0.93 to 3.80) | 717 (10 RCTs) | ⊕⊝⊝⊝ Very lowe | ||
| 28 per 1000 | 53 per 1000 (26 to 106) | |||||
| The corresponding risk (risk of the intervention group) (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RCT: randomised clinical trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty : We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded four levels because of within‐study risk of bias: all trials were at overall high risk of bias (‐2 levels); optimal information size (OIS) as calculated by GRADE was not met (‐1 level); publication bias (‐1 level). bDowngraded three levels because of within‐study risk of bias: all trials were at overall high risk of bias (‐2 levels); publication bias (‐1 level). cDowngraded four levels because of within‐study risk of bias: all trials were at overall high risk of bias (‐2 levels); OIS as calculated by GRADE was not met (‐1 level); publication bias (‐1 level). dDowngraded four levels because of within‐study risk of bias: all trials were at overall high risk of bias (‐2 levels); imprecision: OIS as calculated by GRADE was not met (‐1 level); publication bias (‐1 level). eDowngraded four levels because of within‐study risk of bias: all trials were at overall high risk of bias (‐2 levels); imprecision: OIS as calculated by GRADE was not met (‐1 level); publication bias (‐1 level).
Background
Description of the condition
Portal hypertension is a common complication of liver cirrhosis, usually defined as an increase in pressure within the portal venous system. Portal hypertension leads to development of portosystemic collateral vessels, and of these, gastro‐oesophageal varices are the most clinically relevant (Garcia‐Tsao 2007; Cordon 2012). At the time of diagnosis of cirrhosis, around 30% of people with cirrhosis have gastro‐oesophageal varices; 90% of people with cirrhosis will develop varices during their lifetime (D'Amico 2004; Cordon 2012). The presence and extent of varices are related to the severity of cirrhosis, and individuals with decompensated liver cirrhosis are at highest risk (Garcia‐Tsao 2007). Varices are at high risk of rupture, often resulting in catastrophic haemorrhage ― a major cause of death in people with cirrhotic liver disease. Other causes of bleeding related to portal hypertension in cirrhosis are hypertensive gastropathy and, less frequently, duodenopathy or colopathy. Improved treatment protocols have improved survival; however, mortality rates remain at around 15% to 20% for a first bleed (Cabonell 2004). Early and vigorous resuscitation and early endoscopy, preferably in specialist units, are essential for these individuals (Grace 1997; Herrera 2014). However, people who survive their first variceal bleed are at high risk of further episodes of bleeding ('rebleeding'). The risk of variceal rebleeding is up to 60% within one year, with mortality around 33% (Garcia‐Tsao 2007; Bari 2012). Risk factors specifically for rebleeding have not been well defined, and factors linked to the risk of initial bleeding include the size of varices, the appearance of varices (i.e. red wale marks), and variceal pressure (Zhao 2014).
Due to high risk of rebleeding, secondary prophylaxis is required for individuals with a history of variceal haemorrhage.
Many tools have been used to reduce the risk of rebleeding, such as surgical shunts to reduce portal hypertension, endoscopic obliteration of varices, and drugs like beta blockers (EASL 2018). Published guidelines recommend non‐selective beta‐blockers (NSBBs) and endoscopic band ligation (EBL) as preferable first‐line treatment for secondary prevention of variceal haemorrhage for cirrhotic portal hypertension (de Franchis 2015; Garcia‐Tsao 2017; EASL 2018) because combination treatment decreases the probability of rebleeding compared to monotherapy or either EBL or drug treatment. Recommendations are based on recent meta‐analyses showing that combining EBL with NSBBs reduces overall rebleeding, variceal rebleeding, and bleeding‐related mortality versus banding alone (Thiele 2012), and that adding NSBBs to EBL improves survival, whereas adding EBL to NSBBs has no effect on mortality (Puente 2014).
Description of the intervention
Portosystemic shunts represent an alternative approach for reducing portal hypertension and thereby the risk of rupture of varices. The role of portosystemic shunts, above all TIPSs in the last two decades, is usually limited to rescue treatment for acute persistent bleeding or rebleeding despite conventional treatment (i.e. endoscopic intervention and/or medical‐vasoactive drugs) and is limited for secondary prevention of bleeding. Current international guidelines suggest TIPSs for prevention of rebleeding in patients intolerant to beta blockers, or with contraindications to their use and/or concomitant refractory ascites (de Franchis 2015; EASL 2018), or in selected individuals due to patient choice (Tripathi 2015). However, shunting following the first episode of variceal bleeding could provide more effective treatment earlier in a person's disease pathway, potentially avoiding repeated admissions with variceal bleeding, and thereby possibly reducing mortality. In addition, portosystemic shunts confer the further advantage of being a 'once‐only treatment', potentially preventing repeated hospital visits.
Therapeutic portosystemic shunts are artificial conduits connecting the portal and systemic circulation; they may be inserted surgically or via interventional radiology. Shunts may be classified according to their haemodynamic consequences: the total surgical shunt (TS) has no prograde hepatopetal flow through the portal vein and, therefore, all portal blood is diverted into the systemic circulation (Collins 1995). In contrast, selective or partial shunts preserve pre‐existing hepatopetal portal vein flow (Collins 1995). The distal splenorenal shunt (DSRS) is a surgically placed selective shunt that has been associated with improved preservation of liver function and hence lower morbidity as compared to TS, although lower mortality has not been conclusively demonstrated (D'Amico 1995). The selectivity of DSRSs can be further improved if the venous collaterals between the splenic vein and the pancreas are disconnected ‐ an additional procedure that is particularly important for alcoholics (Warren 1986). Since the early 1990s, the radiologically placed transjugular intrahepatic portosystemic shunt (TIPS) has grown in popularity (LaBerge 1993). It is inserted radiologically by minimal access and can usually be placed more quickly than a surgical shunt (Brown 1997). In essence, it is a side‐to‐side portosystemic shunt. TIPSs can, however, lead to serious acute and chronic complications and small but significant mortality (Casado 1998). Stenosis and occlusion rates have been reported to exceed 75% at two years in randomised trials using TIPSs (Papatheodoridis 1999), although the use of polytetrafluoroethylene‐coated stents in modern practice greatly reduces rates of stent dysfunction (Bureau 2007).
Endoscopic treatments are well established, and various techniques may be practised. Among these interventions, sclerotherapy represents an approach by which varices are obliterated through injection of sclerosants (such as ethanolamine oleate, polidocanol, sodium morrhuate, or other agents) (Cordon 2012). A further alternative is endoscopic glue injection, whereby tissue adhesives (n‐butyl‐2‐cyanoacrylate (Histoacryl) or isobutyl‐2‐cyanoacrylate (Bucrylate)) are injected into varices (Cordon 2012) ‐ a technique that is of particular use in the management of gastric varices (Garcia‐Tsao 2007). An alternative is variceal band ligation ('variceal banding'): varices are obliterated by application of elastic bands via endoscopy (Cordon 2012; Tripathi 2015). It is important to note that effective endoscopic intervention generally requires multiple treatments; typically, varices are banded with an interval of one to four weeks until eradication of varices has been achieved (Garcia‐Tsao 2007; Tripathi 2015). Endoscopic intervention does carry risk of complications, such as provocation of further bleeding episodes, oesophageal ulcers, or bacteraemia (Terblanche 1983; McIntyre 1996; Cordon 2012). However, endoscopic techniques are well established, are usually well tolerated, and significantly reduce rates of rebleeding compared with controls (Graham 1981). Current consensus suggests that variceal banding is the endoscopic method of choice (Garcia‐Tsao 2007; de Franchis 2010; Tripathi 2015) because it is more effective and has lower complication rates than sclerotherapy (Laine 1995; Dai 2015).
Endoscopic treatments serve to locally obliterate varices. By obliterating varices, variceal rupture and haemorrhage may be prevented. However, endoscopic treatment alone does not combat portal hypertension; therefore, it does not target the underlying pathophysiology of varices formation (Villanueva 2008). As a result, endoscopic treatment is generally combined with pharmacological treatment, typically long‐term treatment with non‐cardioselective beta blockers (NSBBs), such as propranolol (Bernard 1997, Tripathi 2015), with or without nitrates. Beta blockade acts to reduce portal pressure by decreasing cardiac output (blockade of beta1 receptors), promoting splanchnic vasoconstriction (blockade of beta2 receptors), and reducing blood flow through collateral vessels (Garcia‐Tsao 2007; Villanueva 2008), hence reducing the risk of variceal bleeding. Combining pharmacological and endoscopic treatments has a synergistic effect by targeting both localised and decompressing varices (Bernard 1997;Villanueva 2008;Tripathi 2015).
How the intervention might work
Portosystemic shunts directly target portal hypertension. Portosystemic shunts act to divert blood from the portal circulation to the systemic circulation, decompressing varices and hence preventing rebleeding. By diverting blood flow, portosystemic shunts might provide a more effective treatment option than endoscopy.
Historically, surgical creation of a shunt (e.g. distal splenorenal shunt, portacaval shunt) was performed to control bleeding and prevent recurrent haemorrhage if other methods failed. However, placement of TIPSs has become the preferred intervention in this setting because covered stents have favourable long‐term patency and the risks and morbidity associated with major abdominal surgery are avoided.
Why it is important to do this review
This current review represents an update of a Cochrane Review first published in 2006 (Khan 2006), based on a protocol originally published in 1997 (Khan 1997).
As stated above, shunts provide a once‐only treatment modality for prevention of variceal rebleeding and could potentially be more effective than endoscopic treatment for preventing repeated episodes of variceal bleeding. However, portosystemic shunting does have potential drawbacks. Portosystemic shunting, particularly with surgical shunts, represents a more invasive option than endoscopic treatment. Also, by diverting blood flow away from the liver, portosystemic shunts carry increased risk of hepatic encephalopathy, and portosystemic shunts pose a risk for shunt failure or dysfunction (Luca 1999; Papatheodoridis 1999;Burroughs 2002; Garcia‐Tsao 2007). Therefore, it is important to ask whether a case can be made for more widespread use of shunting, and also to assess the relative safety and risk of complications of portosystemic shunting compared with endoscopic treatment.
In one meta‐analysis in Spina 1992a, DSRSs significantly reduced the risk of rebleeding compared to endoscopic sclerotherapy without increasing the risk of chronic hepatic encephalopathy. However, DSRSs did not significantly affect overall death risk. In D'Amico 1995, a comparison of TSs and DSRSs is reported without differences between the two treatments. Several published meta‐analyses have assessed TIPSs versus endoscopic intervention (Luca 1999; Papatheodoridis 1999;Burroughs 2002; Zheng 2008), all showing no differences in mortality, reduction in rebleeding, and increased incidence of hepatic encephalopathy. More recently, a multiple‐treatments meta‐analysis compared TIPSs, endoscopic treatment modalities, pharmacotherapies, and combination treatments (Shi 2013), showing that endoscopic band ligation combined with argon plasma coagulation resulted in the best profile of reduction in rebleeding rate and all‐cause mortality, and TIPSs had the greatest impact on reducing mortality rate due to rebleeding. Further, a 2019 meta‐analysis compared portosystemic shunts (including transjugular portosystemic shunt) to endoscopy, but in this review, authors also included trials utilising emergency shunts to treat active bleeding (Zhou 2019).
In the current work, we examined portosystemic shunts in the elective setting for patients with previous episodes of variceal bleeding. To comprehensively address the question, we have conducted a systematic review to compare shunts (TSs, DSRSs, and TIPSs) versus endoscopic interventions (sclerotherapy or banding, or both) with or without medical treatment for long‐term prophylaxis of rebleeding.
The current update, along with updated Cochrane standards, incorporates new trials (Lo 2007; Ferlitsch 2012; Luo 2015; Holster 2016; Lv 2018; Dunne 2020) and results of updated trials (Santambrogio 2006).
Objectives
To compare the benefits and harms of shunts (surgical shunts (total shunt (TSs), distal splenorenal shunt (DSRS), or transjugular intrahepatic portosystemic shunt (TIPS
)) versus endoscopic interventions (endoscopic sclerotherapy or banding, or both) with or without medical treatement (non‐selective beta blockers or nitrates, or both) for prevention of variceal rebleeding in people with liver cirrhosis.
Methods
Criteria for considering studies for this review
Types of studies
We endeavoured to identify all possible randomised clinical trials (published and unpublished) in which shunts were compared with endoscopic interventions with or without medical treatment.
Types of participants
People known to have cirrhosis who had bled from oesophagogastric varices but had subsequently stabilised (before randomisation), either spontaneously or via non‐surgical approaches, including vasoactive drugs or balloon tamponade, or endoscopic measures, or a combination of any two or three together.
Types of interventions
Surgical shunts (total shunt (TS), distal splenorenal shunt (DSRS), or transjugular intrahepatic portosystemic shunt (TIPS)) versus endoscopic interventions (endoscopic sclerotherapy or banding, or both) with or without concomitant long‐term medical treatment (e.g. non‐selective beta‐blockers, nitrates, both).
Types of outcome measures
All outcomes were evaluated at the maximum available follow‐up.
Primary outcomes
All‐cause mortality, defined as death due to any cause
Rebleeding, defined as a clinically significant episode of bleeding (i.e. requiring transfusion) from oesophagogastric varices or portal hypertensive gastropathy. The diagnosis should ideally have been confirmed by endoscopic examination to distinguish variceal bleeding or bleeding from portal hypertensive gastropathy from other causes of non‐portal hypertensive gastrointestinal haemorrhage
Health‐related quality of life, as measured by trial authors
Secondary outcomes
Mortality due to rebleeding, defined as death resulting from a further episode of bleeding from oesophagogastric varices or portal hypertensive gastropathy following the primary (index) bleeding
Acute hepatic encephalopathy, defined by classical signs detected on physical examination, signs unequivocally described by participants' relatives, psychometric testing, or electroencephalogram (EEG)
Chronic hepatic encephalopathy, defined by recurrent episodes of acute hepatic encephalopathy or inability of the individual to attain previous level of function because of post‐treatment hepatic encephalopathy
Complications, defined by untoward events reported by trial authors (aside from hepatic encephalopathy, which is reported separately)
Hospital stay, defined by total days spent in hospital when treatments are applied
Cost analysis, defined by actual financial costs of treatment complications of cirrhotic portal hypertension or of complications during the follow‐up period
Search methods for identification of studies
Electronic searches
We searched the Cochrane Hepato‐Biliary Group Controlled Trials Register (maintained and searched internally by the CHBG Information Specialist via the Cochrane Register of Studies Web; 22 June 2020); the Cochrane Central Register of Controlled Trials (CENTRAL; 2020, Issue 2), in the Cochrane Library (searched 22 June 2020); MEDLINE Ovid (1946 to 22 June 2020); Embase Ovid (1974 to 22 June 2020); Latin American Caribbean Health Sciences Literature (LILACS; Bireme; 1982 to 22 June 2020), and Science Citation Index ‐ Expanded (Web of Science; 1900 to 22 June 2020), as well as the Conference Proceedings Citation Index ‐ Science (Web of Science; 1990 to 25 February 2020) (Royle 2003). For the current update, we reviewed all records arising from Conference Proceedings Citation Index and LILACS, as these databases had not been included in the previous permutation of this review. CENTRAL, MEDLINE, Embase, and Science Citation Index ‐ Expanded had all been included in the previously published version of the review, and we reviewed all records from September 2006 (inclusive) onwards from these databases for the current update. We performed an all language search, evaluating only human studies. Appendix 1 presents the search strategies with time spans for the searches.
Searching other resources
We investigated the reference lists of identified trials for relevant trials. We searched conference proceedings/abstracts for European Association for the Study of the Liver (EASL), American Association for the Study of Liver Disease (AASLD), and British Society of Gastroenterology. When practicable, authors of studies identified to be pertinent were asked to review the list of identified trials and to add any unidentified trials. Manufacturers (TIPSs, pharmacological firms) were contacted. We also searched ClinicalTrials.gov to identify protocols and any ongoing trials.
Data collection and analysis
Selection of studies
Searches for the original updated review were conducted by at least two review authors (SK and CTS for the 1st version; and RGS, GP, and HR for the update), who independently applied the inclusion criteria to all identified studies. At least two review authors independently extracted data from publications of interest, and for the update, data were extracted in greater detail and were rechecked by RGS, GP, and HR. We selected studies for inclusion no matter whether they reported on outcomes of interest to our review. Unpublished data were sought by writing to study authors (see notes under Characteristics of included studies). Review authors collected data for intention‐to‐treat analysis. We resolved any discrepancies or differences among us by discussion.
Data extraction and management
We extracted data using standardised forms, which captured data related to participant characteristics. Three review authors (RGS, GP, and HR) independently extracted data. We resolved disagreements by discussion. Data extraction encompassed comparability between groups randomised to alternative treatments regarding baseline prognostic variables, including aetiology of cirrhosis; mean age; proportion of males/females; participants with Child‐Pugh stage A, B, or C (Pugh 1973); completeness and length of follow‐up of treatment groups and reasons for withdrawals; presence of, absence of, or unknown for‐profit support; and trial design, exclusions, losses to follow‐up, and cross‐over of patients. Review authors also extracted data of particular interest for shunt intervention, including whether assessments had been made to assess the suitability of shunt intervention, and whether splenopancreatic disconnection was undertaken in people undergoing DSRS. We also extracted data related to the timing and method of assessing shunt patency.
We collected data for all‐cause mortality, rebleeding, health‐related quality of life, death due to rebleeding, development of acute hepatic encephalopathy, development of chronic hepatic encephalopathy, complications, hospital stay, and financial cost. When a trial had more than two groups, we extracted data only from groups that corresponded to the treatments compared in this review.
When possible, we measured outcomes as 'time‐to‐event'. To prevent loss of data, we assessed outcomes as dichotomous variables, using raw incidence over the entire follow‐up period reported by study authors (or the longest time point reported whenever multiple time points were reported).
For the outcomes of health‐related quality of life, complications, in‐hospital stay, and cost, we extracted data directly 'as reported' by study authors.
Assessment of risk of bias in included studies
Two review authors (RS and GP) independently assessed the risk of bias of included studies. In light of changes to Cochrane methods, we updated the risk of bias assessment using an adapted Cochrane risk of bias tool (adapted from the Cochrane Handbook for Systematic Reviews of Interventions ‐ Higgins 2011; Higgins 2019 ‐ and the Cochrane Hepato-Biliary Group Module). Due to changes to the Cochrane risk of bias tool since conduct of the previous review, we reassessed the risk of bias in previously included trials, with any discrepancy resolved by discussion between review authors.
Allocation sequence generation
Low risk of bias: sequence generation was achieved using computer random number generation or a random numbers table. Drawing lots, tossing a coin, shuffling cards, and throwing dice were adequate if performed by an independent person not otherwise involved in the trial
Unclear risk of bias: the method of sequence generation was not specified
High risk of bias: the sequence generation method was not random
Allocation concealment
Low risk of bias: participant allocations could not have been foreseen in advance of, or during, enrolment. Allocation was controlled by a central and independent randomisation unit; or the allocation sequence was unknown to the investigators (e.g. the allocation sequence was hidden in sequentially numbered, opaque, and sealed envelopes)
Unclear risk of bias: the method used to conceal the allocation was not described, so that intervention allocations may have been foreseen in advance of, or during, enrolment
High risk of bias: the allocation sequence was likely to be known to investigators who assigned participants
Blinding of participants and treatment providers
Low risk of bias: any of the following ‐ no blinding or incomplete blinding, but review authors judge that the outcome is not influenced by lack of blinding; or blinding of participant and study personnel ensured, and it is unlikely that blinding could have been broken
Unclear risk of bias: insufficient information to permit judgement of 'low risk' or 'high risk'
High risk of bias: no blinding or incomplete blinding, and outcome likely to be influenced by lack of blinding; or, blinding of key study participants and personnel attempted, but likely that blinding could have been broken, and outcome is likely to be influenced by lack of blinding
Blinding of outcome assessment
Low risk of bias: any of the following: blinding of participants and key study personnel ensured, and unlikely that blinding could have been broken; or rarely no blinding or incomplete blinding, but review authors judged that the outcome was not likely to be influenced by lack of blinding
Unclear risk of bias: any of the following: insufficient information to permit judgement of 'low risk' or 'high risk'; or the trial did not address this outcome
High risk of bias: any of the following: no blinding of outcome assessment, and outcome measurement likely to be influenced by lack of blinding; or blinding of outcome assessment, but likely that blinding could have been broken, and the outcome measurement was likely to be influenced by lack of blinding
Incomplete outcome data
Low risk of bias: missing data were unlikely to make treatment effects depart from plausible values. The study used sufficient methods, such as multiple imputation, to handle missing data
Unclear risk of bias: information was insufficient to assess whether missing data in combination with the method used to handle missing data were likely to induce bias on the results
High risk of bias: results were likely to be biased due to missing data
Selective reporting
Low risk of bias: if the original trial protocol was available, outcomes should be those called for in that protocol. If the trial protocol was obtained from a trial registry, outcomes sought should have been those enumerated in the original protocol. If no protocol was available, the trial should have reported the following outcomes: all‐cause mortality, rebleeding, mortality due to rebleeding, complications, acute hepatic encephalopathy, and chronic hepatic encephalopathy
Unclear risk of bias: information insufficient to permit judgement of 'low risk' or 'high risk'
High risk: not all of the study's pre‐specified primary outcomes have been reported; one or more primary outcomes are reported via measurements, analyses, or subsets that were not pre‐specified; one or more primary outcomes were not pre‐specified; one or more outcomes of interest are reported incompletely; study fails to include results for a key outcome that would be expected to have been reported for such a study
Other bias
Low risk of bias: the trial appeared to be free of other factors that could put it at risk of bias
Unclear risk of bias: the trial may or may not have been free of other factors that could put it at risk of bias
High risk of bias: other factors in the trial could put it at risk of bias
Overall risk of bias
We assessed the overall risk of bias in a trial as:
low risk of bias: if all bias domains in a trial, as described in the above paragraphs, are judged at 'low risk of bias'; or
high risk of bias: if one or more of the bias domains in a trial, as described in the above paragraphs, are judged at 'unclear risk of bias' or 'high risk of bias'.
Measures of treatment effect
We assessed all outcomes through a combined analysis of all shunt types together ('shunt therapy pooled'; i.e. TS, DSRS, and TIPS combined) and repeated the analysis for each shunt type individually (TS, DSRS, TIPS). We calculated hazard ratios (HRs) for time‐to‐event outcomes and calculated risk ratios (RRs) for binary outcomes, and we planned to use mean differences (MDs) for continuous outcomes. For the outcomes all‐cause mortality, rebleeding, death due to rebleeding, development of acute hepatic encephalopathy, and development of chronic hepatic encephalopathy, when possible, we carried out analyses to allow reporting of time‐to‐event outcomes. Therefore, for each outcome in each comparison (shunts versus endoscopic intervention with or without medical treatment: TS versus endoscopic intervention with or without medical treatment; DSRS versus endoscopic intervention with or without medical treatment; TIPS versus endoscopic intervention with or without medical treatment), we planned to calculate a pooled estimate of treatment effect as an HR. If estimates of log HR and its variance were not quoted directly in trial reports, we used alternative aggregate data (e.g. log rank test P values) to estimate log(HR) and its variance utilising methods proposed by Parmar 1998 and Williamson 2002, and summarised by Tierney 2007. When possible, we planned to calculate variance and observed‐expected (O‐E) rank from reported HRs and confidence intervals (CIs) (Tierney 2007, Section 3 to 6). However, in most cases, we planned to estimate log(HR) and its variance and O‐E using the quoted P value of the log‐rank test (Tudur 2001, Section 2.3; Tierney 2007, Sections 7 to 9). If no P value was quoted for the log‐rank test, we planned to estimate log(HR) and its variance from Kaplan‐Meier survival curves (Tudur 2001, Sections 2.4 and 2.5; Tierney 2007, Section 10). Full details and discussion of the reliability of results are given in Tudur 2001.
We were not able to extract sufficient data to allow time‐to‐event analysis from all reports. Therefore, to prevent loss of data, we also reported the same outcomes (i.e. all‐cause mortality, rebleeding, death due to rebleeding, development of acute hepatic encephalopathy, and development of chronic hepatic encephalopathy) as dichotomous outcomes, using binary data to calculate the RR. We decided to report only the results of analysis of dichotomous data because they were available for all trials, and because we used dichotomous data to grade the evidence (see below) and to perform Trial Sequential Analysis, while avoiding redundant information. We planned to report results of time‐to‐event analysis if discrepancies with the main analysis were found.
For high variability on continuous outcomes (inpatient stay and costs), we did not meta‐analyse the results and reported mean differences for each trial when available.
To ensure consistency across studies identified in the initial review and in the current update, calculations from the initial review were reviewed and any discrepancies were resolved by discussion between review authors.
For this update, we decided to report 'financial cost' and 'length of hospital stay' as the raw data published by trialists in light of considerable inter‐trial variability in the definitions and methods used; therefore, overall, it was judged that reported data were not comparable across all trials.
Unit of analysis issues
Participants as randomised to an intervention group of a clinical trial are the unit of analysis. In trials of two–parallel group design, we compared the experimental intervention group versus the control. In trials with a parallel group design with more than two intervention groups of interest to our review, we planned to compare separately each of the experimental groups with each half of the control group.
To avoid repeated observations on trial participants, we used participant trial data at the longest follow‐up (Higgins 2011; Higgins 2019).
We identified no cluster‐randomised trials.
Dealing with missing data
Whenever possible, we performed all calculations according to intention‐to‐treat principles (i.e. with all randomised trial participants included in the analysis within the group into which they were randomised). In some trials, results were presented on a per‐protocol basis, or the given information was insufficient to assess whether data had truly been presented with use of the 'intention‐to‐treat' principle (GDEAIH 1995; García‐Villarreal 1999). When this was the case, we contacted study authors to retrieve pertinent data. As further information was not given, we used all data that were available to us.
Assessment of heterogeneity
We assessed heterogeneity by visual examination of forest plots and overlapping CIs, and through use of the I² statistic. The I² statistic was interpreted as follows: 0% to 40% heterogeneity may not be important; 30% to 60% moderate heterogeneity; 50% to 90% heterogeneity may be substantial; 75% to 100% heterogeneity may be considerable (Higgins 2011).
Assessment of reporting biases
Whenever we had 10 or more trials, we drew funnel plots to assess reporting biases from individual trials by plotting the risk ratio (RR) on a logarithmic scale against its standard error (Egger 1997; Higgins 2011; Higgins 2019). We examined the degree of asymmetry of the funnel plot.
Data synthesis
We performed meta‐analyses using the software package Review Manager 5.4 (Review Manager 2014). We used a random‐effects model meta‐analysis approach because we expected that the trials were heterogeneous. When data were available from only one trial, we used Fisher's exact test for dichotomous data (Fisher 1992). We planned to use Student's t‐test for continuous data such as 'health‐related quality of life' (Student 1908).
Subgroup analysis and investigation of heterogeneity
We performed all analyses with all shunt types but also with individual shunt types: TS, DSRS, and TIPS.
We planned subgroup analyses according to risk of bias, analysing separately randomised clinical trials at low risk of bias compared to trials at high risk of bias. We did this because trials at high risk of bias can overestimate the benefits and underestimate the harms.
We also analysed trials with for‐profit funding; without for‐profit support; and with unknown for‐profit support to evaluate whether for‐profit funding is associated with greater intervention benefit.
No other subgroup analyses were planned a priori, and none were undertaken due to the small sample size in each group.
Robustness of conclusions was assessed using sensitivity analyses (see below). When there was substantial heterogeneity, we considered and discussed the appropriateness of performing the meta‐analysis. We did not perform a meta‐analysis if viewed as inappropriate. We explored reasons for possible heterogeneity, while examining characteristics of trials.
Sensitivity analysis
We employed sensitivity analyses within each type of portosystemic shunt to test the robustness of our results.
Excluding trials examining participants with previous bleeding from gastric varices, as they were likely more difficult to treat with endoscopy.
Including only trials specifying use of polytetrafluorethylene (PTFE)‐covered TIPSs, as this could reduce the risk of occlusion of the stent.
Including only trials in which the PTFE‐covered TIPSs were not used, or in which the type of TIPS was not specified.
Including only trials combining endoscopic interventions with medical therapies, as these medical interventions could influence the effects of endoscopy.
Excluding trials combining endoscopic interventions with medical therapies to see if there are differences in the intervention effects.
Including only trials using endoscopic banding exclusively (i.e. excluding those using glue injection, sclerotherapy, or combination treatments) as use of the mentioned example interventions could modify effects of endoscopic intervention.
As further sensitivity analyses, we compared evaluation of imprecision with GRADE based on the GRADE Handbook, with GRADE based on our choice of plausible RRR and multiplicity correction, and according to our Trial Sequential Analysis (described below), with a similar choice of a plausible RRR and multiplicity correction, in addition to considering the choice of a meta‐analytic model and diversity (Jakobsen 2014; Castellini 2018; Gartlehner 2019).
Trial Sequential Analysis
Trial Sequential Analysis considers the choice of statistical model (fixed‐effect or random‐effects meta‐analysis) and diversity (Thorlund 2011; TSA 2011). We calculated the diversity‐adjusted required information size (DARIS, i.e. the number of participants needed in a meta‐analysis to detect or reject a certain intervention effect) (Brok 2008; Wetterslev 2008; Brok 2009; Wetterslev 2009; Thorlund 2010; Wetterslev 2017).
The underlying assumption of Trial Sequential Analysis is that testing for statistical significance may be performed each time a new trial is added to the meta‐analysis. We added trials according to the year of publication, and if more than one trial was published in a year, we added trials alphabetically according to the last name of the first author. On the basis of the DARIS, we constructed the trial sequential monitoring boundaries for benefit, harm, and futility (Wetterslev 2008; Wetterslev 2009; Thorlund 2011; Wetterslev 2017). These boundaries determine the statistical inference one may draw regarding the cumulative meta‐analysis that has not reached the DARIS; if the trial sequential monitoring boundary for benefit or harm is crossed before the DARIS is reached, firm evidence may be established and further trials may be superfluous. However, if the boundaries for benefit or harm are not crossed, it is most probably necessary to continue doing trials to detect or reject a certain intervention effect. However, if the cumulative Z‐curve crosses the trial sequential monitoring boundaries for futility, no additional trials may be needed.
In our Trial Sequential Analysis of the two primary dichotomous outcomes, we based the DARIS on event proportions in the control group assuming a plausible relative risk reduction (RRR) for all‐cause mortality of 10% and for rebleeding of 20%; risk of type I error of 2.5% due to the three primary outcomes (Jakobsen 2014); risk of type II error of 20%; and the diversity of trials included in the meta‐analysis. We repeated the analysis with a plausible RRR of 40% for rebleeding. For the continuous outcome, health‐related quality of life, we planned to estimate the DARIS using a minimal relevant difference of the standard deviation/2; type I error risk of 2.5% due to the three primary outcomes (Jakobsen 2014); risk of type II error of 20%; and diversity as estimated from trials in the meta‐analysis (Wetterslev 2009). We also calculated Trial Sequential Analysis–adjusted confidence intervals (CIs) (Thorlund 2011; Wetterslev 2017).
In our Trial Sequential Analysis of secondary outcomes, we based the DARIS for dichotomous outcomes on the event proportion in the control group; we made an assumption of an RRR of 20% for death due to rebleeding, development of acute hepatic encephalopathy, development of chronic hepatic encephalopathy and complications; type I error risk of 1.4% due to the six secondary outcomes (Jakobsen 2014); risk of type II error of 20%; and the diversity of trials included in the meta‐analysis. We repeated the analysis with a plausible RRR or increase of 40% for mortality due to rebleeding, development of acute hepatic encephalopathy, and development of chronic hepatic encephalopathy. For the continuous outcome, hospital stay and cost, we planned to estimate the DARIS using a minimal relevant difference of the standard deviation/2; type I error risk of 1.4% due to the six secondary outcomes (Jakobsen 2014); beta of 20%; and diversity as estimated from trials in the meta‐analysis (Wetterslev 2009).
We reported results of the comparison of GRADE and Trial Sequential Analysis. We downgraded imprecision in Trial Sequential Analysis by two levels if the accrued number of participants was below 50% of the diversity‐adjusted required information size (DARIS), and one level if between 50% and 100% of DARIS. We did not downgrade if the cumulative Z curve crossed the monitoring boundaries for benefit, harm, or futility, or if DARIS was reached.
A more detailed description of Trial Sequential Analysis and the software programme can be found at www.ctu.dk/tsa/ (Thorlund 2011).
'Summary of findings' tables and GRADE
We constructed 'Summary of findings' tables for the update of the review. We created 'Summary of findings' tables for the pooled analysis of all shunt interventions ('shunt therapy pooled') versus endoscopic intervention with or without medical treatment (Table 1), and we presented individual tables for TS (Table 2), DSRS (Table 3), and TIPS (Table 4) for the following outcomes: all‐cause mortality, rebleeding, health‐related quality of life, mortality due to rebleeding, acute hepatic encephalopathy, and chronic hepatic encephalopathy. We used dichotomous data to assess absolute effects.
We created 'Summary of findings' tables using GRADEpro software and GRADE Interactive software (www.gradepro.org; GRADEpro GDT), in accordance with Cochrane guidelines and the GRADE Handbook (Grade Handbook). GRADE appraises the certainty of evidence, assessing the degree to which we can be confident that the estimate of effect truly reflects the effect being assessed. The GRADE factors for assessing the evidence are trial risk of bias (methodological quality), indirectness of the evidence (population, intervention, control, outcomes), heterogeneity or inconsistency of results, imprecision of effect estimates (considering width of confidence intervals, optimal information size, and whether confidence intervals exclude important benefit or important harm), and possible publication bias (including use of funnel plots) (Grade Handbook). To calculate the optimal information size (OIS), we used the conservative estimates of a RRR of 25%, beta 20%, and alpha 0.05.
Overall, we graded the level of evidence as 'high', 'moderate', 'low', or 'very low' (Grade Handbook; GRADEpro GDT).
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Results
Description of studies
Results of the search
The updated search identified in total 210 records of possible interest for our review (Figure 1). Three additional records were identified through other sources (Figure 1).
1.
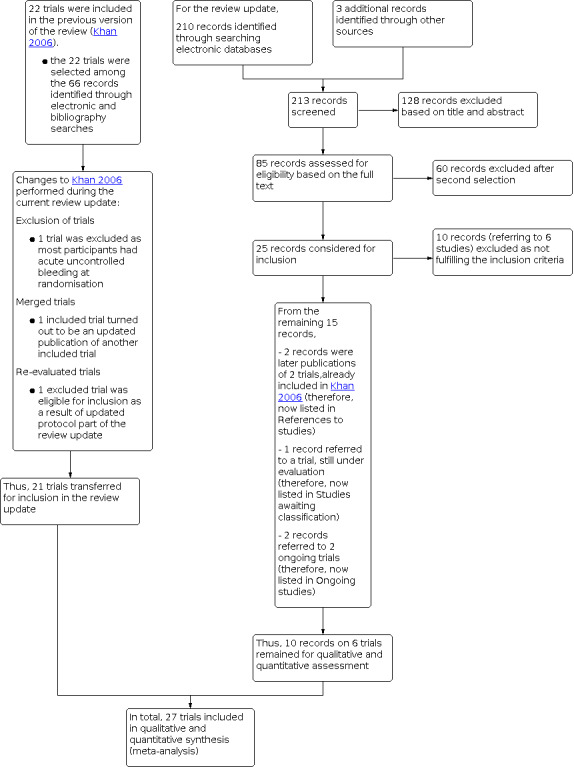
Study flow diagram. Searches performed up to June 2020
Of these 213 records, 25 were screened further for inclusion. We excluded ten records reporting six trials based on reading the full‐text publications, as they did not fulfil the inclusion criteria of our review (Orloff 2009; Garcia‐Pagan 2010; Li 2015; Sauerbruch 2015; Orloff 2015; Wang 2015). Two further records were later publications of two other trials already included in the previous review version (Merli 1998: a full report of a previous abstract; Santambrogio 2006: long‐term follow‐up of the Spina 1990 trial). We listed these two references within their trial identifiers in Characteristics of included studies. One record reported a randomised clinical trial (Lv 2019) which is under evaluation (Studies awaiting classification), because it is not clear if participants with active uncontrolled bleeding after randomisation were included. A letter was sent to the study authors to request more information. Two records described the protocols of two ongoing trials for which we did not find related publications at the time of our analysis (NCT02477384; NCT03094234) (Characteristics of ongoing studies). Ten records reported on six new trials, which we have included in the present review update (Lo 2007; Ferlitsch 2012; Luo 2015; Holster 2016; Lv 2018; Dunne 2020).
From the previous review version with 22 included trials, we had to reassess fulfilment of the inclusion criteria of two trial publications (Cello 1987; Sauer 1998). Most participants in the Cello 1987 trial had acute uncontrolled bleeding at randomisation; hence, we excluded the trial from the current review version. The trial publication Sauer 1998 and the trial publication Sauer 2002 seemed to be reports of the same trial, which was confirmed through personal communication with the trial authors (see the notes field of the Characteristics of included studies table). In addition, for the previous version of the review (Khan 2006), 14 trial publications have been excluded. Among these records, two publications (Rossi 1994 and Krieger 1997) were recognised as ancillary studies of trials that were already included, i.e.Sauer 1997 and Merli 1998. We listed these two references within their trial identifiers in Characteristics of included studies. One trial was reclassified and was added to the current version of the review (Urbistondo 1996).
For the current review version, we rechecked or updated extracted data with data found in the most recent multiple publications of five trials (Rikkers 1993; Merli 1998; Pomier‐Layrargues 2001; Sauer 2002; Santambrogio 2006). Furthermore, we found data on cost‐effectiveness presented in one conference abstract (see Holster 2016); and we extracted data on hepatic encephalopathy from Warren 1986 (see Henderson 1990), as the most recent publication ‐ Henderson 1990 ‐ did not report data on hepatic encephalopathy.
The Characteristics of excluded studies tables provides the reasons for exclusion of identified publications from the previous and current review versions (i.e. 18 trials with 24 references) (Cello 1982; Cello 1987; Escorsell 2002; Garcia‐Pagan 2010; Kitano 1992; Li 2015; Meddi 1999; Orloff 1994; Orloff 2009; Orloff 2015; Paquet 1990; Resnick 1974; Reynolds 1981; Sanyal 1994; Sauerbruch 2015; Terés 1987a; Tripathi 2001; Wang 2015).
As a result, 27 randomised clinical trials (three of which described results in abstract format (Korula 1987; GDEAIH 1995; Ferlitsch 2012) were included in this review update. The results of all trials were available in English.
Included studies
Trial design and setting
All of the 27 included trials were parallel‐group randomised clinical trials. These trials were carried out in 14 countries: the United States (n = 5) (Korula 1987; Henderson 1990; Rikkers 1993; Cello 1997; Sanyal 1997), Germany (n = 4) (Rossle 1997; Sauer 1997; Gülberg 2002; Sauer 2002), Spain (n = 4) (Terés 1987; Planas 1991; Cabrera 1996; García‐Villarreal 1999), Italy (n = 2) (Merli 1998; Santambrogio 2006), China (n = 2) (Luo 2015; Lv 2018), United Kingdom (n = 2) (Jalan 1997; Dunne 2020), France (n = 1) (GDEAIH 1995), Austria (n = 1) (Ferlitsch 2012), The Netherlands (n = 1) (Holster 2016), Sweden (n = 1) (Isaksson 1995), Canada (n = 1) (Pomier‐Layrargues 2001), Japan (n = 1) (Narahara 2001), Puerto Rico (n=1) (Urbistondo 1996), and Taiwan (n = 1) (Lo 2007).
Interventions
Three trials compared total shunt (TS) versus endoscopic intervention (164 participants) (Korula 1987; Planas 1991; Isaksson 1995), five trials compared distal splenorenal shunt (DSRS) versus endoscopic intervention (352 participants) (Terés 1987; Henderson 1990; Rikkers 1993; Santambrogio 2006; Urbistondo 1996). Ten trials compared transjugular intrahepatic portosystemic shunt (TIPS) versus endoscopic intervention (Cabrera 1996; Cello 1997; Jalan 1997; Sanyal 1997; Merli 1998; García‐Villarreal 1999; Narahara 2001; Pomier‐Layrargues 2001; Gülberg 2002; Lo 2007), and nine trials compared transjugular intrahepatic portosystemic shunt (TIPS) versus endoscopic intervention combined with beta blockers (GDEAIH 1995; Sauer 1997; Rossle 1997; Sauer 2002; Ferlitsch 2012; Luo 2015; Holster 2016; Lv 2018; Dunne 2020). The 19 TIPS trials included 1312 participants.
Comparisons
Eight trials employed banding in the endoscopic intervention group (Jalan 1997; Pomier‐Layrargues 2001; Gülberg 2002; Sauer 2002; Ferlitsch 2012; Luo 2015; Lv 2018; Dunne 2020). Sixteen trials employed endoscopic sclerotherapy (Korula 1987; Terés 1987; Henderson 1990; Planas 1991; Rikkers 1993; GDEAIH 1995; Isaksson 1995; Cabrera 1996; Urbistondo 1996; Cello 1997; Sanyal 1997; Sauer 1997; Merli 1998; García‐Villarreal 1999; Narahara 2001; Santambrogio 2006). Three trials used combinations of banding and either sclerotherapy or glue injection: one employed either sclerotherapy or band ligation (or combined sclerotherapy and banding) of oesophageal varices, with sclerotherapy of gastric varices (Rossle 1997); one treated oesophageal varices with banding and treated gastric varices with injection of cyanoacrylate/lipiodol (Holster 2016); one utilised glue injection of gastric varices, followed by banding when there were concomitant oesophageal varices (Lo 2007).
In summary, three trials compared TS versus sclerotherapy without drugs (Korula 1987; Planas 1991; Isaksson 1995), and five trials compared DSRSs with sclerotherapy without drugs (Terés 1987; Henderson 1990; Rikkers 1993; Urbistondo 1996; Santambrogio 2006). Among the 19 trials comparing TIPSs versus endoscopy, beta blockers were used in the endoscopic group in nine trials (two sclerotherapy (GDEAIH 1995; Sauer 1997), five band ligation (Sauer 2002; Ferlitsch 2012; Luo 2015; Lv 2018; Dunne 2020), and two band ligation and sclerotherapy (Rossle 1997; Holster 2016)). Ferlitsch 2012 specifically examined trial participants with rebleeding from oesophageal varices under sufficient pharmacological treatment (propranolol and isosorbide mononitrate) and continued medical treatment in the endoscopic banding group. In 10 trials, only endoscopic interventions were used in the control group (in three trials band ligation (Jalan 1997; Pomier‐Layrargues 2001; Gülberg 2002), in six trials sclerotherapy (Cabrera 1996; Cello 1997; Sanyal 1997; Merli 1998; García‐Villarreal 1999; Narahara 2001), and in one trial glue injection into gastric varices and band ligation of oesophageal varices were used (Lo 2007)).
Participants
Full details of the 27 included trials are given in the Characteristics of included studies tables. All trials included 1828 participants with cirrhosis, with a history of variceal bleeding. Twenty‐three publications reported on participant age: mean age in 22 of the trials was 53.5 years, and median age in one trial was 49 years for participants treated with TIPS and 46 years for those treated with endoscopic intervention. The same 23 publications reported on sex, with a predominance of men in all trials (68.9% of participants were male) (Korula 1987; Terés 1987; Planas 1991; Isaksson 1995; Cabrera 1996; Urbistondo 1996; Cello 1997; Jalan 1997; Rossle 1997; Sanyal 1997; Sauer 1997; Merli 1998; García‐Villarreal 1999; Narahara 2001; Pomier‐Layrargues 2001; Gülberg 2002; Sauer 2002; Santambrogio 2006; Lo 2007; Luo 2015; Holster 2016; Lv 2018; Dunne 2020).
Among trials assessing TSs, one trial included only participants with Child‐Pugh class A (Korula 1987), and two trials excluded participants in class C (Terés 1987; Planas 1991). Ferlitsch 2012 did not provide information on trial participants' Child‐Pugh class. Luo 2015 included participants in Child‐Pugh classes B and C.
All other trials included participants in all Child‐Pugh classes in different proportions. Merli 1998 excluded participants in Child‐Pugh class C with a score > 13. The Child‐Pugh score in the trials in which it was reported ranged from 6.6 in Rikkers 1993 to 9.8 in Pomier‐Layrargues 2001.
Urbistondo 1996 randomised participants in Child's classes A and B to DSRS, sclerotherapy, or propranolol, and participants in Child's C class to sclerotherapy or propranolol.
Eighteen trials specifically either included participants with oesophageal variceal bleeding only or excluded those with isolated gastric varices or gastric variceal bleeding (Henderson 1990; Rikkers 1993; GDEAIH 1995; Isaksson 1995; Cabrera 1996; Urbistondo 1996; Cello 1997; Jalan 1997; Sanyal 1997; Sauer 1997; Merli 1998; García‐Villarreal 1999; Gülberg 2002; Sauer 2002; Santambrogio 2006; Ferlitsch 2012; Luo 2015; Dunne 2020). One further trial excluded participants with 'large fundal varices' (Pomier‐Layrargues 2001). One trial was unique in focusing on gastric variceal bleeding (Lo 2007), excluding participants with acute oesophageal bleeds. One trial included participants with oesophageal or gastric variceal bleeding (or both) (Holster 2016). In four trial publications, the source of variceal bleeding was not specified (Korula 1987; Rossle 1997; Narahara 2001; Lv 2018), although Rossle 1997 discusses treatment of both oesophageal and gastric varices. Two trials included participants with both oesophageal and gastric variceal bleeding; however, participants with gastric varices were excluded from the endoscopic intervention group after randomisation (Terés 1987; Planas 1991).
Five trials did not provide information on the cause of cirrhosis (Korula 1987; Planas 1991; GDEAIH 1995; Isaksson 1995; Ferlitsch 2012). Of the remaining trials, alcohol was judged to be the specific cause of cirrhosis in 55.9% of trial participants (Terés 1987; Cabrera 1996; Urbistondo 1996; Cello 1997; Jalan 1997; Rossle 1997; Sanyal 1997; Sauer 1997; Merli 1998; García‐Villarreal 1999; Narahara 2001; Pomier‐Layrargues 2001; Gülberg 2002; Sauer 2002; Santambrogio 2006; Lo 2007; Luo 2015; Holster 2016; Lv 2018; Dunne 2020). The Merli 1998, Lo 2007, and Lv 2018 trials reported that alcohol contributed to liver cirrhosis in only 25.9%, 16.6%, and 2.0%, respectively, of trial participants. Viral aetiology was reported in 13 trials (Cello 1997; Jalan 1997; Rossle 1997; Sanyal 1997; Sauer 1997; Narahara 2001; Pomier‐Layrargues 2001; Gülberg 2002; Sauer 2002; Luo 2015; Holster 2016; Lv 2018; Dunne 2020), and this ranged from 3% in Dunne 2020 to 90% of participants in Lv 2018.
Portal vein thrombosis was an explicit exclusion criterion in at least 11 trials (Cello 1997; Jalan 1997; Sanyal 1997; Sauer 1997; García‐Villarreal 1999; Pomier‐Layrargues 2001; Gülberg 2002; Sauer 2002; Lo 2007; Holster 2016; Dunne 2020); three further trials excluded individuals with complete portal vein thrombosis or cavernous portal vein thrombosis (Rossle 1997; Merli 1998; Narahara 2001). In contrast, Luo 2015 and Lv 2018 specifically included only individuals with coexistent liver cirrhosis and portal vein thrombosis.
We provide full details of the included trials in the Characteristics of included studies tables.
For‐profit funding
Among 11 trials in which information on funding was reported, no trials were funded by industry or received any other type of for‐profit support. For the remaining 16 trials, no information on funding was available.
Total shunt (TS) versus endoscopic intervention
Isaksson 1995, which assessed TS versus endoscopic intervention, was funded by the National Research Council. For all other trials, no information on the source of funding was available (Korula 1987; Planas 1991).
Distal splenorenal shunt (DSRS) versus endoscopic intervention
Terés 1987,, Henderson 1990, and Rikkers 1993,,which assessed DSRS versus endoscopic intervention, were supported by grants provided by public research bodies. No information on the source of funding was available in Santambrogio 2006 nor Urbistondo 1996.
Transjugular intrahepatic portacaval shunt (TIPS) versus endoscopic intervention
Cabrera 1996 and Lo 2007, which assessed TIPS versus endoscopic intervention, were supported by local institutional grants: Pomier‐Layrargues 2001 and Holster 2016 were supported by national research organisations; and Cello 1997, Sanyal 1997, and Lv 2018 were supported by regional and institutional grants. The remaining 12 trials provided no information (GDEAIH 1995; Jalan 1997; Rossle 1997; Sauer 1997; Merli 1998; García‐Villarreal 1999; Narahara 2001; Gülberg 2002; Sauer 2002; Ferlitsch 2012; Luo 2015; Dunne 2020).
Excluded studies
Overall, we excluded 18 trials.
In brief, the reasons for exclusion of the six trials identified in the last search performed for the present review version were absence of randomised design (Li 2015; Wang 2015), comparisons not relevant to the review (Sauerbruch 2015), shunts and endoscopic interventions were performed as emergency treatment in an acute setting (Orloff 2009; Orloff 2015), and participants with uncontrolled bleeding were included (Garcia‐Pagan 2010).
We provide the reasons for exclusion in the Characteristics of excluded studies tables.
Risk of bias in included studies
Please see Figure 2 and Figure 3.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
Portosystemic shunts versus endoscopic intervention
As expected, due to the nature of the intervention, no trial blinded participants or personnel. We acknowledge that blinding could have been unrealistic in trials of surgical shunts versus endoscopic treatment. Perhaps evaluation of mortality was not affected, but assessment of the other outcomes could have been influenced. In addition, the rate of protocol deviations in each trial for non‐clinical reasons could have been influenced by knowledge of the assignment. We judged trials with a large number of protocol deviations to be at high risk of bias, as assignment of participants to treatments groups could have been distorted, whereas we judged trials with a small number of protocol deviations to be at unclear risk of bias.
For the domain 'other bias', we judged trials to be at high risk of bias if they reported a large number of participants being crossed‐over because of bleeding, and it is not known when the data were censored.
As we performed quantitative analysis according to shunt type (TS, DSRS, or TIPS), we also presented risk of bias according to shunt type to allow clearer conclusions when each shunt type was individually examined.
Total shunt (TS) versus endoscopic intervention
Random sequence generation and allocation concealment (selection bias)
All three trials had unclear generation of the randomisation sequence and unclear allocation concealment (Korula 1987; Planas 1991; Isaksson 1995) (Figure 2; Characteristics of included studies).
Blinding (performance bias and detection bias)
For blinding of participants and personnel (performance bias), two trials were at unclear risk of bias (Korula 1987; Isaksson 1995), whereas Planas 1991 was at high risk (Figure 2; Characteristics of included studies).
None of the trials reported blinding of outcome assessors (to minimise detection bias) (Korula 1987; Planas 1991; Isaksson 1995) (Figure 2; Characteristics of included studies).
Incomplete outcome data (attrition bias)
Two trials were at low risk of bias (Planas 1991; Korula 1987), and one trial was at high risk of bias (Isaksson 1995) (Figure 2; Characteristics of included studies).
Selective reporting (reporting bias)
Two trials were at low risk of bias (Isaksson 1995; Planas 1991), and one trial was at high risk risk of bias (Korula 1987) (Figure 2; Characteristics of included studies).
Other sources of bias
One trial was at low risk of bias (Isaksson 1995). One trial, published as a conference abstract, was at unclear risk of bias (Korula 1987). This is why we cannot exclude other potential sources of bias. One trial was at high risk of bias because of the large number of participants who were crossed‐over from one treatment group to another when a participant bled (Planas 1991). It is unknown whether they were censored at the time of cross‐over (Planas 1991) (Figure 2; Characteristics of included studies).
Distal splenorenal shunt (DSRS) versus endoscopic intervention
Random sequence generation and allocation concealment (selection bias)
For random sequence generation, four trials were at low risk of bias (Terés 1987; Rikkers 1993; Santambrogio 2006; Urbistondo 1996), and one trial was at unclear risk of bias (Henderson 1990) (Figure 2; Characteristics of included studies).
For allocation concealment, all five trials were at unclear risk of bias (Terés 1987; Henderson 1990; Rikkers 1993; Santambrogio 2006; Urbistondo 1996) (Figure 2; Characteristics of included studies).
Blinding (performance bias and detection bias)
For blinding of participants and personnel, four trials were at unclear risk of bias (Terés 1987; Henderson 1990; Rikkers 1993; Santambrogio 2006), whereas one trial was at high risk of bias (Urbistondo 1996) (Figure 2; Characteristics of included studies).
For blinding of outcome assessors, all five trials were at unclear risk of bias (Terés 1987; Henderson 1990; Rikkers 1993; Santambrogio 2006; Urbistondo 1996) (Figure 2; Characteristics of included studies).
Incomplete outcome data (attrition bias)
Three trials were at low risk of bias (Henderson 1990; Rikkers 1993; Santambrogio 2006), and two trials were at high risk of bias (Terés 1987; Urbistondo 1996) (Figure 2; Characteristics of included studies).
Selective reporting (reporting bias)
We judged all five trials to be at high risk of reporting bias (Terés 1987; Henderson 1990; Rikkers 1993; Santambrogio 2006; Urbistondo 1996) (Figure 2; Characteristics of included studies).
Other bias
One trial was at low risk (Santambrogio 2006), and four trials were at high risk of bias (Terés 1987; Henderson 1990; Rikkers 1993; Urbistondo 1996) (Figure 2; Characteristics of included studies).
Transjugular intrahepatic portacaval shunt (TIPS) versus endoscopic intervention
Random sequence generation and allocation concealment (selection bias)
For random sequence generation, 12 trials were at low risk of bias (Cabrera 1996; Rossle 1997; Sanyal 1997; Sauer 1997; García‐Villarreal 1999; Narahara 2001; Sauer 2002; Lo 2007; Luo 2015; Holster 2016; Lv 2018; Dunne 2020), and seven trials were at unclear risk of bias (GDEAIH 1995; Cello 1997; Jalan 1997; Merli 1998; Pomier‐Layrargues 2001; Gülberg 2002; Ferlitsch 2012) (Figure 2; Characteristics of included studies).
For allocation concealment, nine trials were at low risk of bias (Cello 1997; Sanyal 1997; Rossle 1997; Narahara 2001; Lo 2007; Luo 2015; Holster 2016; Lv 2018; Dunne 2020), and 10 trials were at unclear risk of bias (GDEAIH 1995; Cabrera 1996; Jalan 1997; Sauer 1997; Merli 1998; García‐Villarreal 1999; Pomier‐Layrargues 2001; Gülberg 2002; Sauer 2002; Ferlitsch 2012) (Figure 2; Characteristics of included studies).
Blinding (performance bias and detection bias)
For blinding of participants and personnel, all trials were at unclear risk of bias (GDEAIH 1995; Cabrera 1996; García‐Villarreal 1999; Cello 1997; Jalan 1997; Rossle 1997; Sanyal 1997; Sauer 1997; Merli 1998; Narahara 2001; Pomier‐Layrargues 2001; Gülberg 2002; Sauer 2002; Lo 2007; Ferlitsch 2012; Luo 2015; Holster 2016; Lv 2018; Dunne 2020) (Figure 2; Characteristics of included studies).
For blinding of outcome assessors, two trials were at low risk of bias (Lo 2007; Holster 2016). The other 17 trials were at unclear risk of bias (GDEAIH 1995; Cabrera 1996; García‐Villarreal 1999; Cello 1997; Jalan 1997; Rossle 1997; Sanyal 1997; Sauer 1997; Merli 1998; Narahara 2001; Pomier‐Layrargues 2001; Gülberg 2002; Sauer 2002; Ferlitsch 2012; Luo 2015; Lv 2018; Dunne 2020) (Figure 2; Characteristics of included studies).
Incomplete outcome data (attrition bias)
Fifteen trials were at low risk (Cabrera 1996; García‐Villarreal 1999; Jalan 1997; Rossle 1997; Sanyal 1997; Sauer 1997; Merli 1998; Narahara 2001; Gülberg 2002; Sauer 2002; Lo 2007; Luo 2015; Holster 2016; Lv 2018; Dunne 2020), and three trials were at unclear risk of bias (Cello 1997; Pomier‐Layrargues 2001; Ferlitsch 2012). One trial was at high risk of bias (GDEAIH 1995) (Figure 2; Characteristics of included studies).
Selective reporting (reporting bias)
Ten trials were at low risk of bias (Cabrera 1996; Jalan 1997; Sanyal 1997; Sauer 1997; Merli 1998; Rossle 1997; Narahara 2001; Lo 2007; Holster 2016; Lv 2018). Four trials were at unclear risk of bias (Gülberg 2002; Sauer 2002; Ferlitsch 2012; Dunne 2020). Five trials were at high risk of bias (GDEAIH 1995; Cello 1997; García‐Villarreal 1999; Pomier‐Layrargues 2001; Luo 2015) (Figure 2; Characteristics of included studies).
Other bias
Four trials were at low risk of bias (García‐Villarreal 1999; Narahara 2001; Lo 2007; Holster 2016), two trials were at unclear risk of bias (GDEAIH 1995; Sauer 2002), and 13 trials were at high risk of bias (Cabrera 1996; Cello 1997; Jalan 1997; Rossle 1997; Sanyal 1997; Sauer 1997; Merli 1998; Pomier‐Layrargues 2001; Gülberg 2002; Ferlitsch 2012; Luo 2015; Lv 2018; Dunne 2020) (Figure 2; Characteristics of included studies).
Overall judgement
We judged all trials to be at unclear or high risk of bias in at least one domain. As a result, all trials were at overall high risk of bias, which could result in systematic errors (Figure 2; Figure 3; Characteristics of included studies).
However, in Lo 2007 and Holster 2016, the judgement of high risk is based exclusively on lack of blinding of participants and personnel, which is not feasible for the kind of treatments, but did not exclude deviations of intended interventions (i.e. additional interventions given). In Narahara 2001, the judgement of high risk is based only on the lack of blinding of participants and personnel and of outcome assessors, which could influence all outcomes assessed.
We classified trials as having risk of 'other bias' when participants were crossed over to the alternative treatment after bleeding and it is not reported when they were censored. For this reason, rebleeding is not influenced by the cross‐over. On the contrary, mortality, mortality due to rebleeding, and hepatic encephalopathy could have been influenced by the cross‐over.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Results of the meta‐analysis of all shunt types (i.e. TS, DSRS, and TIPS) versus endoscopic intervention with or without medical treatment are summarised in Table 1.
Results of the meta‐analysis of total shunt (TS) versus endoscopic intervention with or without medical treatment are summarised in Table 2.
Results of the meta‐analysis of distal splenorenal shunt (DSRS) versus endoscopic intervention with or without medical treatment are summarised in Table 3.
Results of the meta‐analysis of transjugular intrahepatic portosystemic shunt (TIPS) versus endoscopic intervention with or without medical treatment are summarised in Table 4.
Time‐to‐event data for mortality could be obtained for only 23 trials, for rebleeding in only 16 trials, and for acute hepatic encephalopathy only in 12 trials. These results were similar to those obtained by the analysis of dichotomous data. No information on the other outcomes could be obtained. So, we decided to report only the dichotomous results. Morever, there was no pre‐defined time point for reporting time‐to‐event outcomes across all trials.
All shunt types (i.e. TS, DSRS, and TIPS) versus endoscopic treatment
All‐cause mortality
Twenty‐seven trials reported all‐cause mortality (3 trials using TS, 5 trials using DSRS, 19 trials using TIPS). Mortality data were available for time‐to‐event analysis in 23 trials (2 trials used TS, 4 trials used DSRS, and 17 trials used TIPS). When we meta‐analysed data for all shunt modalities, we found no evidence of a difference between shunts and endoscopic interventions with or without medical treatment in all‐cause mortality (risk ratio (RR) 0.99, 95% confidence interval (CI) 0.86 to 1.13; 1828 participants; 27 trials; I² = 3%; very low‐certainty evidence; Analysis 1.1). We downgraded the evidence by three levels because all trials were at overall high risk of bias (‐2 levels), and because of publication bias (‐1 level) (Table 1; Table 5; Figure 4).
1.1. Analysis.

Comparison 1: Portosystemic shunts versus endoscopic intervention with or without medical treatment, Outcome 1: All‐cause mortality
1. Comparison of imprecision by GRADE and Trial Sequential Analysis (TSA) in the comparison of portosystemic shunts versus endoscopic intervention with or without medical therapy for prevention of rebleeding in patients with cirrhosis.
| Comparison of imprecision by GRADE based on the GRADE Handbook, with GRADE based on our choice of plausible relative risk reduction (RRR) and multiplicity correction, and according to our Trial Sequential Analysis (TSA) based on our similar choice of plausible relative risk reduction and multiplicity correction while also considering choice of meta‐analytic model and diversity | |||||||
| Outcome | Proportion in control group | Relative risk reduction | Alpha | Beta | Diversity | Required information size | Downgrading of evidence for imprecision |
| All‐cause mortality ‐ GRADE Handbook | 28.8% | 25% | 5% | 20% | Not used | 1140 | Not downgraded |
| All‐cause mortality ‐ GRADE plausible RRR | 28.8% | 10% | 2.5% | 20% | Not used | 9108 | Downgraded 1 level |
| All‐cause mortality ‐ TSA | 28.8% | 10% | 2.5% | 20% | 0% | 9111 | Downgraded 2 levelsa |
| Rebleeding ‐ GRADE Handbook | 43.2% | 25% | 5% | 20% | Not used | 632 | Not downgraded |
| Rebleeding ‐ GRADE plausible RRR | 43.2% | 20% | 2.5% | 20% | Not used | 1208 | Not downgraded |
| Rebleeding ‐ GRADE plausible RRR | 43.2% | 40% | 2.5% | 20% | Not used | 286 | Not downgraded |
| Rebleeding ‐ TSA | 43.2% | 20% | 2.5% | 20% | 35% | 1854 | Not downgradedb |
| Rebleeding ‐ TSA | 43.2% | 40% | 2.5% | 20% | 35% | 441 | Not downgradedb |
| Health‐related quality of life ‐ GRADE Handbook | No data | ||||||
| Health‐related quality of life ‐ GRADE plausible RRR | No data | ||||||
| Health‐related quality of life ‐ TSA | No data | ||||||
| Mortality due to rebleeding ‐ GRADE Handbook | 9.5% | 25% | 5% | 20% | Not used | 4222 | Downgraded 1 level |
| Mortality due to rebleeding ‐ GRADE plausible RRR | 9.5% | 20% | 1.4% | 20% | Not used | 9426 | Downgraded 1 level |
| Mortality due to rebleeding ‐ GRADE plausible RRR | 9.5% | 40% | 1.4% | 20% | Not used | 2116 | Downgraded 1 level |
| Mortality due to rebleeding ‐ TSA | 9.5% | 20% | 1.4% | 20% | 0% | 9429 | Downgraded 2 levelsa |
| Mortality due to rebleeding ‐ TSA | 9.5% | 40% | 1.4% | 20% | 0% | 2117 | Not downgradedc |
| Acute hepatic encephalopathy ‐ GRADE Handbook | 18.5% | 25% | 5% | 20% | Not used | 1986 | Downgrade 1 level |
| Acute hepatic encephalopathy ‐ GRADE plausible RRR | 18.5% | 20% | 1.4% | 20% | Not used | 4410 | Downgrade 1 level |
| Acute hepatic encephalopathy ‐ GRADE plausible RRR | 18.5% | 40% | 1.4% | 20% | Not used | 972 | Not downgraded |
| Acute hepatic encephalopathy ‐ TSA | 18.5% | 20% | 1.4% | 20% | 14% | 5108 | Not downgradedd |
| Acute hepatic encephalopathy ‐ TSA | 18.5% | 40% | 1.4% | 20% | 14% | 1160 | Not downgradedd |
| Chronic hepatic encephalopathy ‐ GRADE Handbook | 2.7% | 25% | 5% | 20% | Not used | 15,644 | Downgraded 1 level |
| Chronic hepatic encephalopathy ‐ GRADE plausible RRR | 2.7% | 20% | 1.4% | 20% | Not used | 35,392 | Downgraded 1 level |
| Chronic hepatic encephalopathy ‐ GRADE plausible RRR | 2.7% | 40% | 1.4% | 20% | Not used | 7886 | Downgraded 1 level |
| Chronic hepatic encephalopathy ‐ TSA | 2.7% | 20% | 1.4% | 20% | 0% | 35,394 | Downgraded 2 levelse |
| Chronic hepatic encephalopathy ‐ TSA | 2.7% | 40% | 1.4% | 20% | 0% | 7888 | Downgraded 2 levelsa |
aThe Z‐curve did not reach 50% of the diversity‐adjusted required information size and did not cross any of the sequential boundaries for benefit, harm, or futility. bThe Z‐curve reached the monitoring boundary for benefit. cThe Z‐curve reached the monitoring boundary for futility. dThe Z‐ curve reached the monitoring boundary for harm. eThe TSA figure was not constructed due to too little information.
4.

Funnel plot of comparison: 1 Portosystemic shunt versus endoscopic intervention with or without medical treatment, outcome: 1.1 All‐cause mortality
Sensitivity analysis
Assessment of imprecision with Trial Sequential Analysis and comparison with GRADE
We compared the assessment of imprecision of intervention effects as assessed by GRADE and Trial Sequential Analysis by using the default relative risk reduction (RRR) of 25% for GRADE as suggested in the GRADE Handbook (Grade Handbook), along with the plausible RRR of 10% for GRADE and for Trial Sequential Analysis.
Trial Sequential Analysis of this comparison was constructed with 28.8% for all‐cause mortality in the endoscopic intervention with or without medical treatment group; an RRR of 10% with the portosystemic shunt; type I error of 2.5%; and type II error of 20% (80% power). There was no diversity (D² = 0%). The diversity‐adjusted required information size (DARIS) was 9111 participants. In Trial Sequential Analysis, the cumulative Z‐curve (blue line) did not approach the monitoring boundaries (red lines) for benefit, harm, or futility (figure not shown).
The GRADE optimal information size (OIS) was met by using the default RRR of 25% as suggested in the GRADE Handbook, but not when we used a more realistic RRR of 10% chosen by review authors for GRADE and Trial Sequential Analysis (Table 5).
Rebleeding
Across all shunt modalities, 26 trials reported on rebleeding (2 trials using TS, 5 trials using DSRS, 19 trials using TIPS). Data on rebleeding were available for time‐to‐event analysis in 16 trials (0 trials using TS, 1 trial using DSRS, 15 trials using TIPS). When all shunt modalities were meta‐analysed and compared with endoscopic interventions with or without medical treatment, shunt intervention reduced rebleeding (RR 0.40, 95% CI 0.33 to 0.50; 1769 participants; 26 trials; I² = 31%; very low‐certainty evidence; Analysis 1.2). We downgraded the evidence by three levels because all trials were at overall high risk of bias (‐2 levels), and because of publication bias (‐1 level) (Table 1; Table 5; Figure 5).
1.2. Analysis.

Comparison 1: Portosystemic shunts versus endoscopic intervention with or without medical treatment, Outcome 2: Rebleeding
5.

Funnel plot of comparison: 1 Portosystemic shunt versus endoscopic intervention with or without medical treatment, outcome: 1.2 Rebleeding
Sensitivity analysis
We compared the assessment of imprecision of intervention effects as assessed by GRADE and Trial Sequential Analysis by using the default RRR of 25% for GRADE as suggested in the GRADE Handbook, as well as a plausible RRR of 20% and 40% for GRADE and for Trial Sequential Analysis.
Trial Sequential Analysis of this comparison was constructed on a rebleeding risk of 43.2% in the endoscopic intervention with or without medical treatment group; type I error of 2.5%; and type II error of 20% (80% power). The cumulative Z‐curve crossed the monitoring boundary for benefit before reaching the required information size both when RRR with portosystemic shunt was set to 40% (D² = 35%, DARIS 441 participants), and when it was set to 20% (D² = 35%, DARIS 1854 participants) (figures not shown).
The OIS was met by using the default RRR of 25% as suggested in the GRADE Handbook, and when we used an RRR of 40% and 20% for GRADE and 40% for TSA, but not when the RRR was 20% for TSA (Table 5).
Health‐related quality of life
No trials examined health‐related quality of life.
Mortality due to rebleeding
We were able to extract data on death caused by rebleeding from 26 trials across all shunt modalities (3 trials using TS, 5 trials using DSRS, and 18 trials using TIPS). No data could be extracted to allow analysis of death due to rebleeding as a time‐to‐event outcome.
Portosystemic shunts reduced mortality due to rebleeding (RR 0.51, 95% CI 0.34 to 0.76; 1779 participants; 26 trials; I² = 0%; very low‐certainty evidence; Analysis 1.3). We downgraded the evidence by four levels because all trials were at overall high risk of bias (‐2 levels) due to imprecision, because OIS as calculated by GRADE was not met (‐1 level), and because of publication bias (‐1 level) (Table 1; Table 5; Figure 6).
1.3. Analysis.

Comparison 1: Portosystemic shunts versus endoscopic intervention with or without medical treatment, Outcome 3: Mortality due to rebleeding
6.

Funnel plot of comparison: 1 Portosystemic shunt versus endoscopic intervention with or without medical treatment, outcome: 1.3 Mortality due to rebleeding
Sensitivity analysis
Assessment of imprecision with Trial Sequential Analysis and comparison with GRADE
We compared the assessment of imprecision of intervention effects as assessed by GRADE and Trial Sequential Analysis by using the default RRR of 25% as suggested in the GRADE Handbook and the plausible RRR of 20% and 40% for GRADE and for Trial Sequential Analysis.
Trial Sequential Analysis of this comparison was constructed on mortality due to rebleeding risk of 9.5% in the endoscopic intervention with or without medical treatment group; type I error of 1.4%; and type II error of 20% (80% power). The cumulative Z‐curve reached the futility area before reaching the required information size when RRR with portosystemic shunt was set to 40% (D² = 0%, DARIS 2117 participants). When RRR was set to 20%, the cumulative Z‐curve did not approach the monitoring boundaries for benefit or harm or futility (D² = 0%; DARIS 9429 participants) (figures not shown).
The optimal information size was not reached by using the default RRR of 25% as suggested in the GRADE Handbook, by using an RRR of 40% and 20% for GRADE, or by using an RRR of 40% and 20% for Trial Sequential Analysis (Table 5).
Acute hepatic encephalopathy
Across all shunt modalities, 24 trials reported on development of acute hepatic encephalopathy as a dichotomous outcome (2 trials using TS, 4 trials using DSRS, 18 trials using TIPS). Portosystemic shunts increased acute hepatic encephalopathy (RR 1.60, 95% CI 1.33 to 1.92; 1649 participants; 24 trials; I² = 10%; Analysis 1.4; very low‐certainty evidence). We downgraded the evidence by four levels because all trials were at overall high risk of bias (‐2 levels), because of imprecision ‐ the OIS as calculated by GRADE was not met (‐1 level), and because of publication bias (Table 1; Table 5; Figure 7).
1.4. Analysis.

Comparison 1: Portosystemic shunts versus endoscopic intervention with or without medical treatment, Outcome 4: Acute hepatic encephalopathy
7.

Funnel plot of comparison: 1 Portosystemic shunt versus endoscopic intervention with or without medical treatment, outcome: 1.4 Acute hepatic encephalopathy
Sensitivity analysis
Assessment of imprecision with Trial Sequential Analysis and comparison with GRADE
We compared the assessment of imprecision of intervention effects as assessed by GRADE and Trial Sequential Analysis by using the default RRR of 25% as suggested in the GRADE Handbook and the plausible RRR of 20% and 40% for GRADE and for Trial Sequential Analysis.
Trial Sequential Analysis of this comparison was constructed on an incidence of acute hepatic encephalopathy of 18.5% in the endoscopic intervention with or without medical treatment group; type I error of 1.4%; and type II error of 20% (80% power). The cumulative Z‐curve approached the monitoring boundary for harm before reaching the required information size when the RRR with portosystemic shunt was set to 40% (D² = 14%, DARIS 1160 participants), and when the RRR was set to 20% (D² = 14%, DARIS 5108 participants) (figures not shown).
The optimal information size was not reached by using the default RRR of 25% as suggested in the GRADE Handbook, by using an RRR of 20% for GRADE, or by using an RRR of 20% for Trial Sequential Analysis. It was reached with an RRR of 40% for GRADE and for Trial Sequential Analysis (Table 5).
Chronic hepatic encephalopathy
Across all shunt modalities, 13 trials reported on development of chronic hepatic encephalopathy (1 trial using TS, 2 trials using DSRS, 10 trials using TIPS). No time‐to‐event data could be extracted for chronic hepatic encephalopathy for any shunt type. Portosystemic shunts versus endoscopic intervention with or without medical treatment increased chronic hepatic encephalopathy (RR 2.51, 95% CI 1.38 to 4.55; 956 participants; 13 trials; I² = 0%; very low‐certainty evidence; Analysis 1.5). We downgraded the evidence by four levels because all trials were at overall high risk of bias (‐2 levels), because of imprecision ‐ the OIS as calculated by GRADE was not met (‐1 level), and because of publication bias (‐1 level) (Table 1; Table 5; Figure 8).
1.5. Analysis.

Comparison 1: Portosystemic shunts versus endoscopic intervention with or without medical treatment, Outcome 5: Chronic hepatic encephalopathy
8.

Funnel plot of comparison: 1 Portosystemic shunt versus endoscopic intervention with or without medical treatment, outcome: 1.5 Chronic hepatic encephalopathy
Sensitivity analysis
Assessment of imprecision with Trial Sequential Analysis and comparison with GRADE
We compared the assessment of imprecision of intervention effects as assessed by GRADE and Trial Sequential Analysis by using the default RRR of 25% as suggested in the GRADE Handbook and the plausible RRR of 20% and 40% for GRADE and for Trial Sequential Analysis.
Trial Sequential Analysis of this comparison was constructed on an incidence of chronic hepatic encephalopathy of 2.7% in the endoscopic intervention with or without medical treatment group; type I error of 1.4%; and type II error of 20% (80% power). The cumulative Z‐curve did not approach the monitoring boundaries for benefit, harm, or futility when RRR with portosystemic shunt was set to 40% (D² = 0%, DARIS 7888 participants). The Trial Sequential Analysis curve was not constructed due to little information (2.7%, DARIS) when RRR was set to 20% (D² = 0%, DARIS 35,394 participants) (figures not shown).
The required information size was not reached by using the default RRR of 25% as suggested in the GRADE Handbook, by using an RRR of 20% and 40% for GRADE, or by using an RRR of 20% and 40% for Trial Sequential Analysis (Table 5).
Complications
Full details of complications are summarised according to each individual shunt modality in Table 6, Table 7, and Table 8.
2. Total shunt versus endoscopic intervention: shunt surveillance and complications.
| Trial | Method of shunt surveillance | Incidence of shunt dysfunction | Other reported shunt complications or adverse eventsa | Other reported endoscopic or medical therapy complications or adverse eventsa |
| Isaksson 1995 | Angiography at 4 months, then ultrasound every 12 months | 1/24: acute shunt thrombosis (percentage and 95% CI 4.2%, 0.7% to 20%) | Oesophagitis 8 patients | Oesophageal stenosis 2 patients Oesophagitis 7 patients |
| Korula 1987 | Insufficient information | Insufficient information | Insufficient information | Insufficient information |
| Planas 1991 | Ultrasound 3 to 10 months after surgery or at the time of variceal rebleeding | 1/41: shunt thrombosis (percentage and 95% CI 2.4%, 0.4% to 13%) | Bleeding from lesions other than oesophageal or gastric varices 3 patients Other complications 7 patients (wound abscess 2, sepsis 1, pneumonia 2, chylous pleural effusion 1, cholestasis 1) |
Bleeding from lesions other than oesophageal or gastric varices 4 patients Other complications 12 patients (deep oesophageal ulcers 3, stenosis 1, pneumonia 1, transient dysphagia 4, fever 2, pleural effusion 1) |
aAcute hepatic encephalopathy, chronic hepatic encephalopathy, mortality, and shunt dysfunction not included, as reported elsewhere in this review.
3. Distal splenorenal shunt versus endoscopic intervention: shunt surveillance and complications.
| Trial | Method of shunt surveillance | Incidence of shunt dysfunction | Other reported shunt complications or adverse eventsa | Other reported endoscopic or medical therapy complications or adverse eventsa |
| Henderson 1990 | Insufficient information | Insufficient information | Insufficient information | Bleeding from deep oesophageal ulcers 2 patients Insufficient information on other complications or adverse events |
| Rikkers 1993 | Angiography at 3 months, then at 1, 3, and 6 years | 3/30 (percentage and 95% CI 10%, 3.5% to 26%) |
Insufficient information | Oesophageal stenosis 3 patients |
| Santambrogio 2006 | Angiography on 10th day, then at 1, 3, and 6 months after discharge, then at least twice yearly | 0/40 (percentage and 95% CI 0%, 0% to 9%) |
Insufficient information on surgical complications Bleeding from duodenal ulcers 5 patients Ascites 11 patients |
Bleeding from oesophageal ulcers 5 patients Bleeding from unknown sources 2 patients Dyspagia due to oesophageal ulcers 8 patients Oesophageal stenosis 5 patients Pleural effusion 1 patient Ascites 19 patients Liver failure 1 patient |
| Terés 1987 | Angiography, ultrasound, and/or isotopic splenoportography 7 to 10 months after surgery and when variceal rebleeding occurred | 5/43 (percentage and 95% CI 12%, 5% to 24%) |
Insufficient information on surgical complications Bleeding from sources other than varices (peptic ulcers or gastritis) 5 patients |
Bleeding from sources other than varices (peptic ulcers or gastritis) 7 patients Oesophageal ulcers 2 patients Oesophageal stenosis 3 patients Transient dysphagia 15 patients |
| Urbistondo 1996 | Insufficient information | Insufficient information | Insufficient information | Insufficient information |
aAcute hepatic encephalopathy, chronic hepatic encephalopathy, mortality, and shunt dysfunction not included, as reported elsewhere in this review.
4. Transjugular intrahepatic portosystemic shunt versus endoscopic intervention with and without medical treatments: shunt surveillance and complications.
| Trial | Stent type | Method of shunt surveillance | Incidence of shunt dysfunction | Other reported shunt complications or adverse eventsa | Other reported endoscopic or medical therapy complications or adverse eventsa |
| Cabrera 1996 | 10‐mm Wallstent endoprosthesis 15 patients Strecker stent 15 patients |
Angiography 6‐monthly and after any episode of ascites or variceal bleeding | 15/27 (percentage and 95% CI 55%, 37% to 72%) | Complications 7 patients (portal vein thrombosis 2, spontaneous bacterial peritonitis 2, haemobilia 1, sepsis 1, pneumonia 2, congestive heart failure 2) | Complications 11 patients (bleeding oesophageal ulcers 5, oesophageal stenosis 4, pneumonia 2, sepsis 1, spontaneous bacterial peritonitis 2) |
| Cello 1997 | Wallstents | Doppler ultrasound | 4/22 (percentage and 95% CI 18%, 7% to 38%) | Insufficient information | Insufficient information |
| Dunne 2020 | e‐PTFE‐covered stent (Viatorr TIPSS endoprosthesis, W.L. Gore & Associates, Inc., Newark, NJ, USA) | Doppler ultrasonography or TIPSS venography at 6 months and at 1 year | Insufficient information | No technical failures or major complications of the TIPSS procedure Causes of non‐variceal gastrointestinal rebleedingb: Banding ulcer 3 Mallory Weiss 2 Portal hypertensive gastropathy 1 Seizure 2, fractured bone 3, cellulitis 1, arrhythmia 1, urinary tract infection 1, alcohol hepatitis 1, left ventricular thrombus 1, pneumonia 2, spontaneous bacterial peritonitis 1, spontaneous retroperitoneal bleeding 1, hyponatraemia 1 |
No information on procedure‐related complications Causes of non‐variceal gastrointestinal rebleedingb: Banding ulcer 2 Mallory Weiss 1 Fractured bone 2, arrhythmia 1, abscess 1, compartment syndrome 1, urinary tract infection 1, alcohol hepatitis 2, spontaneous bacterial peritonitis 2 |
| Ferlitsch 2012 | PTFE‐coated TIPS (not specified) |
Insufficient information | Stent thrombosis 3 patients/21 (14%, 95% CI 5% to 35%) |
Insufficient Information | Insufficient Information |
| García‐Villarreal 1999 | Insufficient information | Angiography at 1 month and every 6 months thereafter, and if variceal bleeding or ascites occurred | 14/18 at 6 months (percentage and 95% CI 78%, 55% to 91%) 10/13 at 12 months (percentage and 95% CI 77%, 50% to 92%) |
Insufficient information | Chest discomfort almost all patients Oesophageal ulcer 5 patients Oesophageal stenosis 1 patient |
| GDEAIH 1995 | Insufficient information | Insufficient information | Insufficient information | Insufficient information | Insufficient information |
| Gülberg 2002 | Insufficient information | Doppler sonography at 1 month, at 3 months, and at 3‐monthly intervals thereafter | Insufficient information | Perforation of liver capsule with peritoneal haemorrhage (death) 1 patient | Perforation of oesophagus 1 patient |
| Holster 2016 | PTFE‐covered stent (Viatorr) |
Clinical evaluation every 6 weeks to 3 months. Duplex ultrasound undertaken when dysfunction suspected (new‐onset or progressive ascites, or variceal rebleeding) | 2/37: partial/complete occlusion (percentage and 95% CI 5%, 1.5% to 17.7%) | Severe adverse events 24 Number of complications or adverse eventsc: Bleeding from banding ulcer 1 Bleeding from other upper gastrointestinal sources 1 Ascites 5 Spontaneous bacterial peritonitis 2 Hepatocellular carcinoma or cholangiocarcinoma 2 Acute‐on‐chronic liver failure 4 Cholangitis 6 Alcoholic hepatitis 1 Sepsis 4 Cardiac event 1 Neurological disorder 1 Other complications 3 Severe itching 1 Gynaecomastia 1 |
Severe adverse events 24 patients Number of complications or adverse eventsc: Bleeding from banding ulcer 2 Bleeding from other upper gastrointestinal sources 1 Intra‐abdominal bleeding from collaterals 1 Laceration of hepatic artery (during TIPS placement) 1 Ascites 13 Spontaneous bacterial peritonitis 3 Hepatorenal syndrome 1 Hepatocellular carcinoma or cholangiocarcinoma 4 Acute‐on‐chronic liver failure 3 Cholangitis 2 Alcoholic hepatitis 2 Sepsis 1 Cardiac event 3 Neurological disorder 3 Other complications 3 Severe itching 1 |
| Jalan 1997 | 12‐mm expandable metal stent (Wallstent) | Duplex ultrasound at 1 week Angiography at 1 month, then at 6‐monthly intervals |
9/28: shunt thrombosis 6 patients (variceal rebleeding in 2 patients), pseudointimal hyperplasia 3 patients (percentage and 95% CI 32%, 18% to 51%) | Sepsis 3 patients (aspiration pneumonia 1 patient, staphylococcal septicaemia 2 patients) Perforation of liver capsule 1 patient Respiratory depression caused by sedation 1 patient |
Oesophageal ulcer 12 patients Bleeding from oesophageal ulcer 1 patient Aspiration pneumonia 2 patients Sepsis 4 patients (aspiration pneumonia 2 patients, spontaneous bacterial peritonitis 1 patient, central line‐related sepsis 1 patient) |
| Lo 2007 | Metallic endoprosthesis (Wallstent) | Doppler ultrasound at discharge and every 3 months, or when clinically indicated | 8/35 (percentage and 95% CI 23%, 12% to 39%) | Bacteremia 5 patients Hepatic failure 2 patients Bleeding from peptic ulcer 5 patients Bleeding from undetermined source 2 patients |
Refractory ulcer on gastric varices 3 patients Bacteraemia 10 patients Spontaneous bacterial peritonitis 1 patient Bleeding from peptic ulcer 1 patient Bleeding from undetermined source 1 patient |
| Luo 2015 | 10‐mm expanded PTFE‐covered stent (Fluency) |
Unclear whether routine surveillance was carried out | 11/37: stent occlusion 7 patients and stent stenosis 4 patients (percentage and 95% CI 30%, 17% to 46%) | No 'major' complications | Transient retrosternal pain 11 patients Post‐ligation bleeding ulcer 2 patients Bradycardia propranolol‐related 1 patient |
| Lv 2018 | 8‐mm PTFE‐covered stent (Fluency) | Doppler ultrasound and CT at 1, 3, and 6 months, then every 6 months or whenever clinical recurrence of portal hypertension | 1‐year and 2‐year shunt dysfunction 15% and 20%, respectively 4/24 shunt dysfunctiond (percentage and 95% CI 17%, 7% to 36%) |
Number of patients with complications or adverse events is not obtainablee Ascites 1 Hepatocellular carcinoma 2 Fatigue 2 Pneumonia 1 Intraperitoneal bleeding 1 Pulmonary embolism 1 Acute‐on‐chronic liver failure 1 Fever 2 Dizziness 1 Vomiting 1 Peripheral oedema 2 Mispuncture of bile duct 1 |
Number of patients with complications or adverse events is not obtainablee Ascites 3 Hepatic hydrothorax 1 Spontaneous bacterial peritonitis 1 Hepatorenal syndrome 1 Hepatocellular carcinoma 1 Bleeding from banding ulcer 1 Dysphagia 3 Deep venous thrombosis 1 Oesophageal stenosis 1 Haematuria 1 Chest pain after ligation 2 Fever 1 Fatigue 1 Dizzines 2 Abdominal pain 1 Diarrhoea 1 Peripheral oedema 1 Rash 1 |
| Merli 1998 | 10 × 52‐mm Wallstent or 10 × 70/80‐mm Nitinol Strecker stent | Duplex ultrasound 6‐monthly and angiography 6‐monthly or whenever clinically indicated | 21/33 (percentage and 95% CI 64%, 47% to 78%) | Haemolysis 1 patient Intrahepatic haematoma 1 patient Cardiac arrest 1 patient Pulmonary embolism 1 patient |
Sclerotherapy‐induced ulcer 2 patients Oesophageal stenosis 2 patients Aspiration pneumonia 1 patient Embolic stroke 1 patient |
| Narahara 2001 | Gianturco‐Rösch biliary expandable Z‐stent (21 patients), Spiral‐Z stent (4 patients), Wallstent (13 patients) | Duplex ultrasound every 3 months | 27/38 (percentage and 95% CI 71%, 55% to 83%) | Procedure‐related complications: Haemobilia 2 patients Segmental hepatic infarction 1 patient |
Procedure‐related complications: Dysphagia 2 patients Transient pleural effusion 6 patients Bleeding ulcer secondary to sclerotherapy 3 patients Oesophageal stenosis 1 patient |
| Pomier‐Layrargues 2001 | Insufficient information | Doppler ultrasound at 24 hours and then 3‐monthly for 2 years | 24/41 (percentage and 95% CI 58%, 43% to 72%) | Bleeding from non‐variceal sources 6 patients Causes of non‐variceal gastrointestinal rebleedingb: Oesophageal ulcer due to ligation 3 Oesophagitis 3 Haemobilia 1 Gastropathy 1 Peptic ulcer 2 No information on other complications or adverse events |
Bleeding from non‐variceal sources 9 patients Causes of non‐variceal gastrointestinal rebleedingb: Oesophageal ulcer due to ligation 7 Mallory‐Weiss 2 Oesophagitis 2 No information on other complications or adverse events |
| Rossle 1997 | Palmaz stent (39 patients, 92 stents), Memotherm stent (16 patients, 19 stents), Wallstent (6 patients, 6 stents) | Duplex ultrasound at 1, 3, 6, 9, and 12 months, then 6‐monthly or when needed for clinical reason | 18/61 (percentage and 95% CI 29%, 19% to 42%) | Stent migration or dislocation 2 patients Haemobilia 3 patients Haemoperitoneum 2 patients Bleeding in the liver 1 patient Sepsis 1 patient Bradycardia 4 patients Fever 11 patients Transient abdominal pain 6 patients Bradyarrhytmia due to stent migration into the right ventricle requiring pacemaker 1 patient |
Ulcer 8 patients Dysphagia 5 patients Mediastinitis 1 patient Hypopyon with eye enucleation (likely caused by sclerotherapy‐bacteraemia) 1 patient |
| Sanyal 1997 | Wallstent | Doppler ultrasound at day 1, at week 1, at 1 and 3 months, then 3‐monthly | 20/34 at 6 months (percentage and 95%CI 59%, 42% to 74%) | Haemolysis TIPS‐associated 5 patients (severe in 1 patient) Sepsis 6 patients Renal failure 1 patient |
Chest discomfort Oesophageal ulcer 22 patients Dysphagia + Stenosis 3 patients Dysphagia 2 patients Sepsis 2 patients Ascites 5 patients Seizures 1 patient Renal failure 2 patients Bleeding 1 patient |
| Sauer 1997 | Palmaz stent | Duplex ultrasound every 3 months and after variceal bleeding or other complications Angiography every 3 months and if dysfunction suspected |
32/42 at 2 years (percentage and 95% CI 76%, 61% to 86%) | Study authors did not report number of patients with adverse events but did report numbers of events: Stent dislocation 4 Renal failure 6 Pneumonia 5 Septicaemia 3 Liver failure 2 |
Study authors did not report number of patients with adverse events but did report numbers of events: Oesophageal ulcer 19 Bleeding ulcer 3 Post‐therapeutic haemorrhage 5 Renal failure 3 Pneumonia 6 Septicaemia 2 Liver failure 2 Heart failure propranolol‐related 1 |
| Sauer 2002 | Palmaz stent or Wallstent | Duplex sonography and angiography every 3 months in the first year, every 6 months in the second year, and at yearly intervals thereafter; also if shunt dysfunction suspected | Cumulative dysfunction: 38/43 (89%) during follow‐up | Pneumonia 4 patients Haemobilia 2 patients Stent dislocation 1 patient Septicaemia 3 patients |
Dysphagia 3 patients Pneumonia 3 patients Septicaemia 2 patients Post‐therapeutic haemorrhage 2 patients Severe side effects (type non‐specified) propranolol‐related 2 patients |
aAcute hepatic encephalopathy, chronic hepatic encephalopathy, mortality, and shunt dysfunction not included, as reported elsewhere in this review. bData for other complications and adverse events were extracted as numbers of events across all time points (< 2 years and ≥ 2 years combined).cData for other complications and adverse events were extracted as numbers of adverse events across all time points (< 6 months and > 2 months until end of follow‐up combined). dData extracted from plot in Lv 2018. eNumber of episodes.
PTFE: polytetrafluorethylene.
It was not possible to perform a meta‐analysis of all complications because trialists often reported the number of events and not the number of participants who had events. As some complications are treatment‐specific (e.g. shunt occlusion or thrombosis in shunt intervention, oesophageal ulcers or thoracic pain in endoscopic intervention), they cannot be compared in the two treatment groups. Below we report the number of cases of shunt dysfunction or occlusion (thrombosis) in the shunt group. For the endoscopy group, see details in Table 6, Table 7, and Table 8.
Shunt occlusion or dysfunction
The overall incidence of shunt occlusion or dysfunction was 37% (95% CI 33 to 40).
Inpatient stay
For the update of this review, we decided that because of the variability of definitions and of time periods measured, data for length of stay could not be combined across trials; therefore, we presented this outcome in a tabular format. The impact of shunt intervention on duration of inpatient stay remains unclear, and only limited conclusions may be drawn, with results summarised in Table 9.
5. Duration of hospital stay.
| Trial | Time measured |
Shunt group Mean (SD) or Median (range) [days] |
Endoscopic therapy with or without drugs group Mean (SD) or Median (range) [days] |
Statistical significance P value |
| Total shunt (TS) | ||||
| Planas 1991 | Index hospitalisation for treatment Total hospitalisation due to treatment or complications during follow‐up |
34.9 (14.1) 41.2 (13.1) |
22.3 (7.1) 38.1 (12.2) |
0.0001 NS |
| Isaksson 1995 | Total hospital stay including operation and readmission ICU stay |
34.5 (9 to 122) 4 (0 to 41) |
33 (15 to 64) 3.3 (0 to 16) |
NS NS |
| Distal splenorenal shunt (DSRS) | ||||
| Terés 1987 | Index hospitalisation for treatment Hospital stay for treatment of rebleeding and encephalopathy (shunt group)/treatment of rebleeding and sclerosis (sclerotherapy group) during follow‐up Hospital stay for treatment or for complications during follow‐up |
39.3 (12.8) 7.13 (16.1) 47.7 (20.9) |
23.5 (11.9) 16.5 (30.5) 51.5 (28.8) |
0.0001 0.027 NS |
| Transjugular intrahepatic portosystemic shunt (TIPS) | ||||
| Cabrera 1996 | Index hospitalisation for treatment Hospitalisation for rebleeding Hospitalisation for causes other than bleeding |
20.1 (3.1) 2.9 (6) 6.96 (14.7) |
17.7 (6.2) 7.7 (9.8) 4.45 (10.2) |
NS < 0.05 NS |
| Cello 1997 | Hospitalisation for variceal rebleeding Hospitalisation for any rebleeding Hospitalisation for portosystemic encephalopathy |
15.54 (2.13) 20.8 (3.2) 3.7 (1.7) |
16.92 (3.54) 20.1 (4.6) 1.2 (0.7) |
NS NS NS |
| García‐Villarreal 1999 | Hospitalisation for any reason after initial discharge Hospitalisation for variceal rebleeding |
20.9 (20.2) 1.5 (4.0) |
14.3 (18.7) 4.6 (7.3) |
NS < 0.05 |
| Jalan 1997 | Hospitalisation during follow‐up ICU stay Stay in the high‐dependency unit |
23.2 (15) 0.2 (0.8) 0.7 (1.3) |
31.2 (19) 1.15 (2.3) 3.1 (3.5) |
< 0.05 0.03 < 0.001 |
| Pomier‐Layrargues 2001 | Index hospitalisation Hospitalisation during 2‐year follow‐up |
15.8 (1.8) 45 (7) |
13.4 (2.4) 40 (6) |
NS NS |
| Rossle 1997 | Index hospitalisation (after randomisation) | 27 (17) | 34 (28) | NS |
| Sauer 2002 | Hospital stays per patient per year | 34.1 (30.2) | 19.8 (21.9) | < 0.05 |
| Lo 2007 | Index hospitalisation | 7.2 (5.3) | 8.7 (6.5) | NS |
| Holster 2016 | Index hospitalisation | 12.4 (11.2) | 8.8 (5.4) | 0.095 |
Data for length of hospital stay have been presented in a narrative/tabular format due to variation in time points measured between studies and resultant substantial heterogeneity.
Results are given as mean and SE. ICU: intensive care unit. NS: not statistically significant.
Full details are summarised according to each individual shunt modality (please see relevant sections).
Cost analysis
There was variation in the methods and definitions used to estimate cost of treatment, with results summarised in Table 10. Full details are summarised according to each individual shunt modality.
6. Cost of treatment: shunt versus endoscopic intervention with or without medical treatment.
| Trial | Costs measured | Outcomes reported | Shunt | Endoscopic therapy |
| Total shunt (TS) | ||||
| Planas 1991 | "Healthcare costs" (not defined) | Mean ± SD per patient |
USD 9761 ± 750 | USD 9047 ± 704 |
| Isaksson 1995 | Hospital costs included laboratory and X‐ray examinations, transfusions, drugs, grafts, hotel service, endoscopy investigations and treatment, and costs of surgical procedure (calculated according to Alinder G, et al. World J Surg 1985;9:329‐34) | Mean (range) per patient |
USD 12,949 (7802 to 64,835) | USD 12,027 (2525 to 39,018) |
| Distal splenorenal shunt (DSRS) | ||||
| Rikkers 1993 | Both initial and total medical costs for each therapy were calculated and compared. Initial medical costs were defined as those incurred during the hospitalisation in which shunt surgery was performed or sclerotherapy initiated. Total costs included initial hospitalisation, all subsequent hospitalisations required for treatment of recurrent haemorrhage and complications of therapy or chronic liver disease, and outpatient endoscopic evaluation with or without variceal sclerosis. Analysis included only patients treated at University hospitals. Data from Rikkers LF, Burnett DA, Volentine GD, Buchi KN, Cormier RA. Shunt surgery vs endoscopic sclerotherapy for long‐term treatment of variceal bleeding. Early results of a randomized trial. Ann Surg 1987;206(3):261‐71. PMID: 3307653 |
Total medical costs during mean follow‐up interval of 20 ± 5 and 24 ± 6 months for shunt and sclerotherapy patients Mean ± SEM |
USD 34,474 ± 5499 | USD 37,648 ± 6392 |
| Transjugular intrahepatic portosystemic shunt (TIPS) | ||||
| Cello 1997 | Total cost of health care per patient calculated as the sum of all real costs for inpatient and outpatient hospital care, including hospital expenditures and costs for professional services, and all outpatient costs for endoscopic sclerotherapy, doppler ultrasonography, and stent revision from day of randomisation until death or latest follow‐up | Mean ± SE per patient |
USD 29,790 ± 3422 | USD 27,540 ± 5088 |
| Jalan 1997 | Estimated cost of procedure (not including personnel) and costs of inpatient treatment | Mean per patient |
Procedure: GBP 2749 Hospital treatment: GBP 4310 Total: GBP 7059 |
Procedure: GBP 1422 Hospital treatment: GBP 7010 Total: GBP 8432 |
| Holster 2016 | Costs for admissions, consultations, initial treatment, imaging, diagnostics, transfusions, medications in the first year Abstract only: Harki J, Holster IL, Polinder S, Moelker A, van Buuren HR, Kuipers EJ, et al. Cost effectiveness of covered transjugular intrahepatic portosystemic shunt vs endoscopic treatment for secondary prevention of gastro‐oesophageal variceal bleeding, Journal of Hepatoloy 2016;64(2 Suppl):S265‐S266. |
Mean per patient |
EUR 27,746 | EUR 16,816 |
SD: standard deviation. SE: standard error. SEM: standard error of the mean.
Total shunt (TS) versus endoscopic intervention
All‐cause mortality
All‐cause mortality was reduced with total shunt (RR 0.46, 95% CI 0.19 to 1.13; 164 participants; 3 trials; I² = 1%; very low‐certainty evidence; Analysis 1.1.1). We downgraded the evidence by three levels because all trials were at overall high risk of bias (‐2 levels) and because of imprecision ‐ the OIS as calculated by GRADE was not met (‐1 level) (Table 2; Table 11).
7. Comparison of imprecision by GRADE and Trial Sequential Analysis (TSA) in the comparison of total shunt (TS) versus endoscopic intervention for prevention of rebleeding in people with cirrhosis.
| Comparison of imprecision evaluation with GRADE based on the GRADE Handbook, with GRADE based on our choice of plausible relative risk reduction (RRR) and multiplicity correction, and according to our Trial Sequential Analysis (TSA) based on our similar choice of plausible relative risk reduction and multiplicity correction, while considering choice of meta‐analytic model and diversity | |||||||
| Outcome | Proportion in control group | Relative risk reduction | Alpha | Beta | Diversity | Required information size | Downgrading of evidence for imprecision |
| All‐cause mortality ‐ GRADE Handbook | 19.7% | 25% | 5% | 20% | Not used | 1842 | Downgraded 1 level |
| All‐cause mortality ‐ GRADE plausible RRR | 19.7% | 10% | 2.5% | 20% | Not used | 14,902 | Downgraded 1 level |
| All‐cause mortality ‐ TSA | 19.7% | 10% | 2.5% | 20% | 0% | 14,904 | Downgraded 2 levelsa |
| Rebleeding ‐ GRADE Handbook | 43.5% | 25% | 5% | 20% | Not used | 628 | Downgraded 1 level |
| Rebleeding ‐ GRADE plausible RRR | 43.5% | 20% | 2.5% | 20% | Not used | 1196 | Downgraded 1 level |
| Rebleeding ‐ GRADE plausible RRR | 43.5% | 40% | 2.5% | 20% | Not used | 284 | Downgraded 1 level |
| Rebleeding ‐ TSA | 43.5% | 20% | 2.5% | 20% | 0% | 1197 | Downgraded 2 levelsb |
| Rebleeding ‐ TSA | 43.5% | 40% | 2.5% | 20% | 0% | 285 | Not downgradedc |
| Health‐related quality of life ‐ GRADE Handbook | No data | ||||||
| Health‐related quality of life ‐ GRADE plausible RRR | No data | ||||||
| Health‐related quality of life ‐ TSA | No data | ||||||
| Mortality due to rebleeding ‐ GRADE Handbook | 13.6% | 25% | 5% | 20% | Not used | 2846 | Downgraded 1 level |
| Mortality due to rebleeding ‐ GRADE plausible RRR | 13.6% | 20% | 1.4% | 20% | Not used | 6318 | Downgraded 1 level |
| Mortality due to rebleeding ‐ GRADE plausible RRR | 13.6% | 40% | 1.4% | 20% | Not used | 1424 | Downgraded 1 level |
| Mortality due to rebleeding ‐ TSA | 13.6% | 20% | 1.4% | 20% | 0% | 6321 | Downgraded 2 levelsa |
| Mortality due to rebleeding ‐ TSA | 13.6% | 40% | 1.4% | 20% | 0% | 1427 | Downgraded 2 levelsb |
| Acute hepatic encephalopathy ‐ GRADE Handbook | 12.3% | 25% | 5% | 20% | Not used | 3176 | Downgraded 2 levels |
| Acute hepatic encephalopathy ‐ GRADE plausible RRR | 12.3% | 20% | 1.4% | 20% | Not used | 7080 | Downgraded 2 levels |
| Acute hepatic encephalopathy ‐ GRADE plausible RRR | 12.3% | 40% | 1.4% | 20% | Not used | 1594 | Downgraded 2 levels |
| Acute hepatic encephalopathy ‐ TSA | 12.3% | 20% | 1.4% | 20% | 0% | 7082 | Downgraded 2 levelsa |
| Acute hepatic encephalopathy ‐ TSA | 12.3% | 40% | 1.4% | 20% | 0% | 1596 | Downgraded 2 levelsb |
| Chronic hepatic encephalopathy ‐ GRADE Handbookd | |||||||
| Chronic hepatic encephalopathy ‐ GRADE plausible RRRd | |||||||
| Chronic hepatic encephalopathy ‐ GRADE plausible RRRd | |||||||
| Chronic hepatic encephalopathy ‐ TSAd | |||||||
| Chronic hepatic encephalopathy ‐ TSAd | |||||||
aThe TSA curve was not constructed due to too little information. bThe Z‐curve did not did not reach 50% of the diversity‐adjusted required information size and did not cross any of the sequential boundaries for benefit, harm, or futility. cThe Z‐curve reached the monitoring boundary for benefit. dThere was a single trial with 3/34 events in the TS group and 0/35 in the endoscopy with or without drugs group.
Sensitivity analysis
We did not perform sensitivity analyses according to site of variceal bleeding, modality of endoscopy, or association with beta blockers because no trials were restricted to prevention of rebleeding of gastric varices; sclerotherapy was the endoscopic intervention in all trials; and no trials combined endoscopic intervention with medical treatment.
Assessment of imprecision with Trial Sequential Analysis and comparison with GRADE
We compared the assessment of imprecision of intervention effects as assessed by GRADE and Trial Sequential Analysis, by using the default RRR of 25% as suggested in the GRADE Handbook and the plausible RRR of 10% for GRADE and for Trial Sequential Analysis.
Trial Sequential Analysis was constructed with risk of 19.7% for all‐cause mortality in the endoscopic intervention group; an RRR of 10% with the TS; type I error of 2.5%; and type II error of 20% (80% power). There was no diversity (D² = 0%). The DARIS was 14,904 participants. Due to the fact that only 164 participants were recruited (which is 1.1% of the DARIS of 14,904 participants), the Trial Sequential Analysis figure was not drawn by the programme because too little information was available (figure not shown).
The optimal information size was not reached by using the default RRR of 25% as suggested in the GRADE Handbook or when we used a more realistic RRR of 10% as chosen by review authors for GRADE and for Trial Sequential Analysis (Table 11).
Rebleeding
Risk of rebleeding was reduced by TS (RR 0.28, 95% CI 0.14 to 0.56; 127 participants; 2 trials; I² = 0%; very low‐certainty evidence; Analysis 1.2.1). We downgraded the evidence by three levels because all trials were at overall high risk of bias (‐2 levels) and because of imprecision ‐ the OIS as calculated by GRADE was not met (‐1 level) (Table 2; Table 11).
Sensitivity analysis
We did not perform sensitivity analyses according to site of variceal bleeding, modality of endoscopy, or association with beta blockers because no trials were restricted to prevention of rebleeding of gastric varices; sclerotherapy was the endoscopic intervention in all trials, and no trials combined endoscopic intervention with medical treatment.
Assessment of imprecision with Trial Sequential Analysis and comparison with GRADE
We compared the assessment of imprecision of intervention effects as assessed by GRADE and Trial Sequential Analysis by using the default RRR of 25% as suggested in the GRADE Handbook and the plausible RRR of 20% and 40% for GRADE and for Trial Sequential Analysis.
Trial Sequential Analysis of this comparison was constructed on risk of rebleeding, with 43.5% in the endoscopic intervention group; type I error of 2.5%; and type II error of 20% (80% power). The cumulative Z‐curve crossed the monitoring boundary for benefit before reaching the required information size when RRR with TS was set to 40% (D² = 0%, DARIS 285 participants) ‐ not when RRR was set to 20% (D² = 0%, DARIS 1197 participants) (figures not shown).
The optimal information size was not reached by using the default RRR of 25% as suggested in the GRADE Handbook and an RRR of 20% and 40% as chosen by review authors for GRADE and TSA (Table 11).
Mortality due to rebleeding
Mortality due to rebleeding was reduced with TS (RR 0.25, 95% CI 0.06 to 0.96; 164 participants; 3 trials; I² = 0%; very low‐certainty evidence; Analysis 1.3.1). We downgraded the evidence by three levels because all trials were at overall high risk of bias (‐2 levels) and because of imprecision ‐ the OIS as calculated by GRADE was not met (‐1 level) (Table 2; Table 11).
Sensitivity analysis
We did not perform sensitivity analyses according to site of variceal bleeding, modality of endoscopy, or association with beta blockers because no trials were restricted to prevention of gastric variceal rebleeding; sclerotherapy was the endoscopic intervention in all trials, and no trials combined endoscopic intervention with medical treatment.
Assessment of imprecision with Trial Sequential Analysis and comparison with GRADE
We compared the assessment of imprecision of intervention effects as assessed by GRADE and Trial Sequential Analysis by using the RRR of 25% as suggested in the GRADE Handbook and the plausible RRR of 20% and 40% for GRADE and for Trial Sequential Analysis.
Trial Sequential Analysis of this comparison was constructed on a risk of mortality due to rebleeding of 13.6% in the endoscopic intervention group; type I error of 1.4%; and type II error of 20% (80% power). The cumulative Z‐curve did not cross the sequential boundaries for benefit, harm, or futility before reaching the required information size when RRR with TS was set to 40% (D² = 0%, DARIS 1427 participants); the Trial Sequential Analysis figure was not obtained due to the fact that only 164 participants were recruited (2.59% of the DARIS) when the RRR was set to 20% (D² = 0%, DARIS 6321 participants) (figures not shown).
The optimal information size was not reached by using the default RRR of 25% as suggested in the GRADE Handbook, by using an RRR of 40% and 20% for GRADE, or by using an RRR of 40% and 20 % for Trial Sequential Analysis (Table 11).
Acute encephalopathy
There was no difference between TS and endoscopic intervention in the development of acute hepatic encephalopathy (RR 1.66, 95% CI 0.70 to 3.92; 115 participants; 2 trials; I² = 0%; very low‐certainty evidence; Analysis 1.4.1). We downgraded the evidence by four levels because all trials were at overall high risk of bias (‐2 levels), because of imprecision ‐ the OIS as calculated by GRADE was not met ‐ and because there were very few events and CI included appreciable benefit and harm (‐2 levels) (Table 2; Table 11).
Sensitivity analysis
We did not perform sensitivity analyses according to site of variceal bleeding, modality of endoscopy, or association with beta blockers because no trials were restricted to prevention of gastric variceal rebleeding; sclerotherapy was the endoscopic intervention in all trials, and no trials combined endoscopic intervention with medical treatment.
Assessment of imprecision with Trial Sequential Analysis and comparison with GRADE
We compared the assessment of imprecision of intervention effects as assessed by GRADE and Trial Sequential Analysis by using the default RRR of 25% as suggested in the GRADE Handbook and the plausible RRR of 20% and 40% for GRADE and for Trial Sequential Analysis. Trial Sequential Analysis of this comparison was constructed on an incidence of acute encephalopathy of 12.3% in the endoscopic intervention group; type I error of 1.4%; and type II error of 20% (80% power). The cumulative Z‐curve did not reach the monitoring boundaries for benefit, harm, and futility when RRR with TS was set to 40% (D² = 0%, DARIS 1596 participants). When the RRR was set to 20% (D² = 0%, DARIS 7082 participants), the figure could not be constructed because of the small number of participants (1.62% of the DARIS). The optimal information size was not reached by using the default RRR of 25% as suggested in the GRADE Handbook,and the RRR of 20% and 40% for GRADE and for Trial Sequential Analysis (Table 11).
Chronic hepatic encephalopathy
Only one trial reported on development of chronic hepatic encephalopathy, with 3 out of 34 participants in the TS group compared with none out 35 in the endoscopic intervention group (Fisher's test P = 0.11; 69 participants) (Planas 1991).
Sensitivity analysis
We did not perform sensitivity analyses according to site of variceal bleeding, modality of endoscopy, or association with beta blockers because no trials were restricted to prevention of gastric variceal rebleeding; sclerotherapy was the endoscopic intervention in all trials, and no trials combined endoscopic intervention with medical treatment.
Complications
Complications are summarised in Table 6.
Two of the trials reported shunt occlusion or dysfunction, which developed in 3% (95% CI 0.8% to 10%) of participants (Planas 1991; Isaksson 1995).
Inpatient stay
Only Planas 1991 reported mean (± standard deviation) inpatient stay (days) (MD between groups 3.1 days, 95% CI ‐2.3 to 8.5 days).
Isaksson 1995 reported the median inpatient stay in the TS group as 34.5 days (range 9 to 122 days) and in the endoscopic intervention group as 33 days (range 15 to 64 days).
Cost analysis
Details of cost analyses are provided in Table 10. Two trials reported cost analyses (Planas 1991; Isaksson 1995). Isaksson 1995 calculated hospital costs by including costs of laboratory, radiology, transfusions, drugs, grafts, hotel service, endoscopy, and surgical procedures. Planas 1991 reported data but did not provide details on how costs were calculated. Both trials reported comparable costs between patients treated with TS and patients treated with endoscopic intervention.
Distal splenorenal shunt (DSRS) versus endoscopic intervention
All‐cause mortality
No differences were found between the two groups for all‐cause mortality (RR 0.93, 95% CI 0.65 to 1.33; 352 participants; 5 trials; I² = 56%; very low‐certainty evidence; Analysis 1.1.2). There was heterogeneity because two trials reported an increase in mortality with DSRS versus endoscopic intervention (Henderson 1990; Urbistondo 1996), whereas the other three trials reported a reduction (Terés 1987; Rikkers 1993; Santambrogio 2006). The large number of participants randomised to sclerotherapy who were treated by DSRS when they bled (35% in Henderson 1990) prevent us from considering this result as due to DSRS alone. In Urbistondo 1996, there was an unbalanced selection of participants because DSRS was performed in Child's class A and B, whereas sclerotherapy was performed also in Child's class C. We downgraded the evidence by four levels because all trials were at overall high risk of bias (‐2 levels), because of imprecision ‐ the OIS as calculated by GRADE was nor met (‐1 level), and because of heterogeneity (‐1 level) (Table 3; Table 12).
8. Comparison of imprecision by GRADE and Trial Sequential Analysis in the comparison of DSRS versus endoscopic intervention for prevention of rebleeding in people with cirrhosis.
| Comparison of imprecision evaluation with GRADE based on the GRADE Handbook, with GRADE based on our choice of plausible relative risk reduction (RRR) and multiplicity correction, and according to our Trial Sequential Analysis (TSA) based on our similar choice of plausible relative risk reduction and multiplicity correction, while considering choice of meta‐analytic model and diversity | |||||||
| Outcome | Proportion in control group | Relative risk reduction | Alpha | Beta | Diversity | Required information size | Downgrading of evidence for imprecision |
| All‐cause mortality ‐ GRADE Handbook | 46.8% | 25% | 5% | 20% | Not used | 554 | Downgraded 1 level |
| All‐cause mortality ‐ GRADE plausible RRR | 46.8% | 10% | 2.5% | 20% | Not used | 4284 | Downgraded 1 level |
| All‐cause mortality ‐ TSA | 46.8% | 10% | 2.5% | 20% | 68% | 13,538 | Downgraded 2 levelsa |
| Rebleeding ‐ GRADE Handbook | 45.8% | 25% | 5% | 20% | Not used | 574 | Downgraded 1 level |
| Rebleeding ‐ GRADE plausible RRR | 45.8% | 20% | 2.5% | 20% | Not used | 1096 | Downgraded 1 level |
| Rebleeding ‐ GRADE plausible RRR | 45.8% | 40% | 2.5% | 20% | Not used | 262 | Not downgraded |
| Rebleeding ‐ TSA | 45.8% | 20% | 2.5% | 20% | 72% | 3878 | Downgraded 2 levelsb |
| Rebleeding ‐ TSA | 45.8% | 40% | 2.5% | 20% | 72% | 928 | Downgraded 2 levelsb |
| Health‐related quality of life ‐ GRADE Handbook | No data | ||||||
| Health‐related quality of life ‐ GRADE plausible RRR | No data | ||||||
| Health‐related quality of life ‐ TSA | No data | ||||||
| Mortality due to rebleeding ‐ GRADE Handbook | 12.6% | 25% | 5% | 20% | Not used | 3102 | Downgraded 1 level |
| Mortality due to rebleeding ‐ GRADE plausible RRR | 12.6% | 20% | 1.4% | 20% | Not used | 6890 | Downgraded 1 level |
| Mortality due to rebleeding ‐ GRADE plausible RRR | 12.6% | 40% | 1.4% | 20% | Not used | 1552 | Downgraded 1 level |
| Mortality due to rebleeding ‐ TSA | 12.6% | 20% | 1.4% | 20% | 0% | 6892 | Downgraded 2 levelsb |
| Mortality due to rebleeding ‐ TSA | 12.6% | 40% | 1.4% | 20% | 0% | 1554 | Downgraded 2 levelsb |
| Acute hepatic encephalopathy ‐ GRADE Handbook | 13.9% | 25% | 5% | 20% | Not used | 2768 | Downgraded 1 level |
| Acute hepatic encephalopathy ‐ GRADE plausible RRR | 13.9% | 20% | 1.4% | 20% | Not used | 6164 | Downgraded 1 level |
| Acute hepatic encephalopathy ‐ GRADE plausible RRR | 13.9% | 40% | 1.4% | 20% | Not used | 1390 | Downgraded 1 level |
| Acute hepatic encephalopathy ‐ TSA | 13.9% | 20% | 1.4% | 20% | 26% | 8382 | Downgraded 2 levelsa |
| Acute hepatic encephalopathy ‐ TSA | 13.9% | 40% | 1.4% | 20% | 26% | 1892 | Downgraded 2 levelsb |
| Chronic hepatic encephalopathy ‐ GRADE Handbook | 3.4% | 25% | 5% | 20% | Not used | 12,542 | Downgraded 2 levels |
| Chronic hepatic encephalopathy ‐ GRADE plausible RRR | 3.4% | 20% | 1.4% | 20% | Not used | 27,924 | Downgraded 2 levels |
| Chronic hepatic encephalopathy ‐ GRADE plausible RRR | 3.4% | 40% | 1.4% | 20% | Not used | 6226 | Downgraded 2 levels |
| Chronic hepatic encephalopathy ‐ TSA | 3.4% | 20% | 1.4% | 20% | 0% | 27,926 | Downgraded 2 levelsa |
| Chronic hepatic encephalopathy ‐ TSA | 3.4% | 40% | 1.4% | 20% | 0% | 6228 | Downgraded 2 levelsa |
aThe TSA curve was not constructed due to too little information. bThe Z‐curve did not did not reach 50% of the diversity‐adjusted required information size and did not cross any of the sequential boundaries for benefit, harm, or futility.
Sensitivity analysis
We did not perform sensitivity analyses according to site of variceal bleeding, modality of endoscopy, or association with beta blockers because no trials were restricted to prevention of rebleeding of gastric varices; sclerotherapy was the endoscopic intervention in all trials, and no trials combined endoscopy with medical treatment.
Assessment of imprecision with Trial Sequential Analysis and comparison with GRADE
We compared the assessment of imprecision of intervention effects as assessed by GRADE and Trial Sequential Analysis by using the default RRR of 25% as suggested in the GRADE Handbook and the plausible RRR of 10% for GRADE and for Trial Sequential Analysis.
Trial Sequential Analysis of this comparison was constructed on a risk of all‐cause mortality of 46.8% in the endoscopic intervention group; an RRR of 10% with DSRS; type I error of 2.5%; and type II error of 20% (80% power). There was diversity (D² = 78%). The DARIS was 13,538 participants. Due to the fact that only 324 participants were recruited (which is 2.45% of the DARIS of 13,538 participants), the Trial Sequential Analysis figure was not constructed by the programme because too little information was available.The optimal information size was not reached by using the default RRR of 25% as suggested in the GRADE Handbook, or when we used a more realistic RRR of 10% chosen by review authors for GRADE and for Trial Sequential Analysis (Table 12).
Rebleeding
Rebleeding was reduced by DSRS (RR 0.26, 95% CI 0.11 to 0.65; 330 participants; 5 trials; I² = 66%); very low‐certainty evidence; Analysis 1.2.2). We downgraded the evidence by four levels because all trials were at overall high risk of bias (‐2 levels), because of imprecision ‐ the OIS as calculated by GRADE was not met (‐1 level), and because of heterogeneity (‐1 level) (Table 3; Table 12).
Sensitivity analysis
We did not perform sensitivity analyses according to site of variceal bleeding, modality of endoscopy, or association with beta blockers because no trials were restricted to prevention of rebleeding of gastric varices; sclerotherapy was the endoscopic intervention in all trials, and no trials combined endoscopic intervention with medical treatment.
Assessment of imprecision with Trial Sequential Analysis and comparison with GRADE
We compared the assessment of imprecision of intervention effects as assessed by GRADE and Trial Sequential Analysis by using the default RRR of 25% as suggested in the GRADE Handbook and the plausible RRR of 20% and 40% for GRADE and for Trial Sequential Analysis.
Trial Sequential Analysis of this comparison was constructed on a rebleeding risk of 45.8% in the endoscopic intervention group; type I error of 2.5%; and type II error of 20% (80% power). The cumulative Z‐curve crossed the monitoring boundary for benefit when RRR with DSRS was set to 40% (D² = 72%, DARIS 928 participants). When RRR was set to 20% (D² = 72%, DARIS 3878 participants), the cumulative Z‐curve did not cross the boundaries for benefit, harm, or futility (figures not shown). Overall, as the observed RRR greatly exceeded 40% (observed RRR of 81%), Trial Sequential Analysis would tend to support conclusions that DSRS shunt reduce the rate of rebleeding compared with endoscopic treatment.
The optimal information size was not reached by using the default RRR of 25% as suggested in the GRADE Handbook nor when we used an RRR of 20% for GRADE and an RRR of 20% and 40% for Trial Sequential Analysis. It was reached when we used a plausible RRR of 40% chosen by the review authors for GRADE (Table 12).
Mortality due to rebleeding
Cause of death was reported in four trials (Terés 1987; Henderson 1990; Rikkers 1993; Santambrogio 2006). Mortality from rebleeding was reduced by DSRS (RR 0.31, 95% CI 0.13 to 0.74; 352 participants; 5 trials; I² = 0%; very low‐certainty evidence; Analysis 1.3.2). All four trials included death from gastrointestinal bleeding of all causes (Terés 1987; Henderson 1990; Rikkers 1993; Santambrogio 2006). We downgraded the evidence by three levels because all trials were at overall high risk of bias (‐2 levels) and because of imprecision ‐ the OIS as calculated by GRADE was not met (‐1 level) (Table 3; Table 12).
Sensitivity analysis
We did not perform sensitivity analyses according to site of variceal bleeding, modality of endoscopy, or association with beta blockers because no trials were restricted to prevention of gastric variceal rebleeding; sclerotherapy was the endoscopic intervention in all trials, and no trials combined endoscopic intervention with medical treatment.
Assessment of imprecision with Trial Sequential Analysis and comparison with GRADE
We compared the assessment of imprecision of intervention effects as assessed by GRADE and Trial Sequential Analysis by using the default RRR of 25% as suggested in the GRADE Handbook and the plausible RRR of 40% and 20% for modified GRADE and for Trial Sequential Analysis.
Trial Sequential Analysis of this comparison was constructed on a risk of mortality due to rebleeding of 12.6% in the endoscopic intervention group; type I error of 1.4%; and type II error of 20% (80% power). The cumulative Z‐curve did not cross the monitoring boundaries before reaching the required information size when RRR with DSRS was set to 40% (D² = 0%, DARIS 1554 participants) nor when RRR was set to 20% (D² = 0%, DARIS 6892 participants) (figures not shown).
The optimal information size was not reached by using the default RRR of 25% as suggested in the GRADE Handbook, by using an RRR of 40% and 20% for GRADE, or by using an RRR of 40% and 20% for Trial Sequential Analysis (Table 12).
Acute hepatic encephalopathy
Acute hepatic encephalopathy was increased by DSRS (RR 1.70, 95% CI 0.94 to 3.08; 287 participants; 4 trials; I² = 25%; very low‐certainty evidence; Analysis 1.4.2). Henderson 1990 did not report data on hepatic encephalopathy; however, development of acute hepatic encephalopathy was reported in a preliminary analysis (see the reference to Warren 1985 in Henderson 1990). We downgraded the evidence by three levels because all trials were at overall high risk of bias (‐2 levels) and because of imprecision ‐ the OIS as calculated by GRADE was not met (‐1 level) (Table 3; Table 12).
Sensitivity analysis
We did not perform sensitivity analyses according to site of variceal bleeding, modality of endoscopy, or association with beta blockers because no trials were restricted to prevention of gastric variceal rebleeding; sclerotherapy was the endoscopic intervention in all trials, and no trials combined endoscopic intervention with medical treatment.
Assessment of imprecision with Trial Sequential Analysis and comparison with GRADE
We compared the assessment of imprecision of intervention effects as assessed by GRADE and Trial Sequential Analysis by using the default RRR of 25% as suggested in the GRADE Handbook and the plausible RRR of 20% and 40% for GRADE and for Trial Sequential Analysis.
Trial Sequential Analysis of this comparison was constructed on an incidence of acute hepatic encephalopathy of 13.9% in the endoscopic intervention group; type I error of 1.4%; and type II error of 20% (80% power). The cumulative Z‐curve did not reach the monitoring boundaries for harm, benefit, and futility when RRR with DSRS was set to 40% (D² = 26%, DARIS 1892 participants). When the RRR was set to 20% (D² = 26%, DARIS 8382 participants), the figure could not be constructed because of the small number of participants (figures not shown).
The optimal information size was not reached by using the default RRR of 25% as suggested in the GRADE Handbook, by using an RRR of 20% and 40% for GRADE, or by using an RRR of 40% and 20% for Trial Sequential Analysis (Table 12).
Chronic hepatic encephalopathy
Chronic hepatic encephalopathy was increased by DSRS (RR 4.87, 95% CI 1.46 to 16.23; 170 participants; 2 trials; I² = 0%; very low‐certainty evidence; Analysis 1.5.2). We downgraded the evidence by four levels because all trials were at overall high risk of bias (‐2 levels) and because of imprecision ‐ there were few events and CI included appreciable benefit and harm (‐2 levels) (Table 3; Table 12).
Sensitivity analysis
We did not perform sensitivity analyses according to site of variceal bleeding, modality of endoscopy, or association with beta blockers because no trials were restricted to prevention of gastric variceal rebleeding; sclerotherapy was the endoscopic intervention in all trials, and no trials combined endoscopic intervention with medical treatment.
Assessment of imprecision with Trial Sequential Analysis and comparison with GRADE
We compared the assessment of imprecision of intervention effects as assessed by GRADE and Trial Sequential Analysis by using the default RRR of 25% as suggested in the GRADE Handbook and the plausible RRR of 20% and 40% for GRADE and for Trial Sequential Analysis.
Trial Sequential Analysis of this comparison was constructed on an incidence of chronic hepatic encephalopathy of 3.4% in the endoscopic intervention group; type I error of 1.4%; and type II error of 20% (80% power). The cumulative Z‐curve was not constructed because too little information was available when the RRR was set to 40% (D² = 0%, DARIS 6228 participants) and to 20% (D² = 0%, DARIS 27,926 participants) (figures not shown).
The required information size was not reached by using the default RRR of 25% as suggested in the GRADE Handbook nor when we used an RRR of 20% or 40% for GRADE and Trial Sequential Analysis (Table 12).
Complications
Complications are summarised in Table 7. It is apparent that reporting of complications may be incomplete in all trials.
Shunt occlusion or dysfunction was reported in three trials (Terés 1987; Rikkers 1993; Santambrogio 2006). Shunt occlusion or dysfunction occurred in 8 out of 113 (7%), 95% CI 3% to 13%) participants treated by shunts.
Inpatient stay
Terés 1987 reported the number of inpatient days following randomisation (weighted mean difference (WMD) between groups ‐3.4 days, 95% CI ‐13.7 to 6.9 days) and was the only trialist to do so. Whilst participants treated with DSRS stayed significantly longer in hospital for initial treatment, participants treated with endoscopic intervention stayed significantly longer in hospital undergoing treatment during follow‐up after initial treatment. Therefore, overall, there was no significant difference in the total number of days spent in hospital ) (Table 9; Terés 1987).
Cost analysis
One trial provided a cost analysis in the preliminary report, but it included only participants who were admitted to the University Hospital and it did not include the full cohort of participants (Rikkers 1993). Whilst medical costs for the index hospitalisation were higher for participants treated with DSRS (mean ± SD for DSRS: USD 22,473 ± USD 3521) versus endoscopic intervention (USD 10,410 ± USD 1893), costs were comparable over a longer follow‐up period: DSRS (mean ± SD for DSRS: USD 34,474 ± USD 5499) versus endoscopic intervention (USD 37,648 ± USD 6392) (Table 10).
Transjugular intrahepatic portacaval shunt (TIPS) versus endoscopic intervention
All‐cause mortality
We found no evidence of a difference between TIPS and endoscopic intervention with or without medical treatment for all‐cause mortality (RR 1.10, 95% CI 0.92 to 1.31; 1312 participants; 19 trials; I² = 0%; very low‐certainty evidence; Analysis 1.1.3). We downgraded the evidence by four levels because all trials were at overall high risk of bias (‐2 levels), because of imprecision ‐ the OIS as calculated by GRADE was not met (‐1 level), and because of publication bias (‐1 level) (Table 4; Table 13; Figure 4).
9. Comparison of imprecision by GRADE and Trial Sequential Analysis in the comparison of TIPS versus endoscopic intervention with or without medical treatment for prevention of rebleeding in people with cirrhosis.
| Comparison of imprecision evaluation with GRADE based on the GRADE Handbook, with GRADE based on our choice of plausible relative risk reduction (RRR) and multiplicity correction, and according to our Trial Sequential Analysis (TSA) based on our similar choice of plausible relative risk reduction and multiplicity correction, while considering choice of meta‐analytic model and diversity | |||||||
| Outcome | Proportion in control group | Relative risk reduction | Alpha | Beta | Diversity | Required information size | Downgrading of evidence for imprecision |
| All‐cause mortality ‐ GRADE Handbook | 25.2% | 25% | 5% | 20% | Not used | 1358 | Downgraded 1 level |
| All‐cause mortality ‐ GRADE plausible RRR | 25.2% | 10% | 2.5% | 20% | Not used | 10,900 | Downgraded 1 level |
| All‐cause mortality ‐ TSA | 25.2% | 10% | 2.5% | 20% | 0% | 10,902 | Downgraded 2 levelsa |
| Rebleeding ‐ GRADE Handbook | 42.5% | 25% | 5% | 20% | Not used | 648 | Not downgraded |
| Rebleeding ‐ GRADE plausible RRR | 42.5% | 20% | 2.5% | 20% | Not used | 1242 | Not downgraded |
| Rebleeding ‐ GRADE plausible RRR | 42.5% | 40% | 2.5% | 20% | Not used | 294 | Not downgraded |
| Rebleeding ‐ TSA | 42.5% | 20% | 2.5% | 20% | 16% | 1474 | Not downgradedb |
| Rebleeding ‐ TSA | 42.5% | 40% | 2.5% | 20% | 16% | 351 | Not downgradedb |
| Health‐related quality of life ‐ GRADE Handbook | No data | ||||||
| Health‐related quality of life ‐ GRADE plausible RRR | No data | ||||||
| Health‐related quality of life ‐ TSA | No data | ||||||
| Mortality due to rebleeding ‐ GRADE Handbook | 8.2% | 25% | 5% | 20% | Not used | 4974 | Downgraded 1 level |
| Mortality due to rebleeding ‐ GRADE plausible RRR | 8.2% | 20% | 1.4% | 20% | Not used | 11,062 | Downgraded 1 level |
| Mortality due to rebleeding ‐ GRADE plausible RRR | 8.2% | 40% | 1.4% | 20% | Not used | 2478 | Downgraded 1 level |
| Mortality due to rebleeding ‐ TSA | 8.2% | 20% | 1.4% | 20% | 0% | 11,063 | Downgraded 2 levelsa |
| Mortality due to rebleeding ‐ TSA | 8.2% | 40% | 1.4% | 20% | 0% | 2481 | Not downgradedc |
| Acute hepatic encephalopathy ‐ GRADE Handbook | 20.1% | 25% | 5% | 20% | Not used | 1798 | Downgraded 1 level |
| Acute hepatic encephalopathy ‐ GRADE plausible RRR | 20.1% | 20% | 1.4% | 20% | Not used | 3990 | Downgraded 1 level |
| Acute hepatic encephalopathy ‐ GRADE plausible RRR | 20.1% | 40% | 1.4% | 20% | Not used | 906 | Not downgraded |
| Acute hepatic encephalopathy ‐ TSA | 20.1% | 20% | 1.4% | 20% | 25% | 5282 | Downgraded 2 levelsa |
| Acute hepatic encephalopathy ‐ TSA | 20.1% | 40% | 1.4% | 20% | 25% | 1217 | Not downgradedd |
| Chronic hepatic encephalopathy ‐ GRADE Handbook | 2.8% | 25% | 5% | 20% | Not used | 15,312 | Downgraded 1 level |
| Chronic hepatic encephalopathy ‐ GRADE plausible RRR | 2.8% | 20% | 1.4% | 20% | Not used | 34,096 | Downgraded 1 level |
| Chronic hepatic encephalopathy ‐ GRADE plausible RRR | 2.8% | 40% | 1.4% | 20% | Not used | 7598 | Downgraded 1 level |
| Chronic hepatic encephalopathy ‐ TSA | 2.8% | 20% | 1.4% | 20% | 0% | 34,099 | Downgraded 2 levelse |
| Chronic hepatic encephalopathy ‐ TSA | 2.8% | 40% | 1.4% | 20% | 0% | 7600 | Downgraded 2 levelsa |
aThe Z‐curve did not did not reach 50% of the diversity‐adjusted required information size and did not cross any of the sequential boundaries for benefit, harm, or futility. bThe Z‐curve reached the monitoring boundary for benefit. cThe Z‐curve reached the monitoring boundary for futility. dThe Z‐curve reached the monitoring boundary for harm. eThe TSA curve was not constructed due to too little information.
Sensitivity analysis
The results did not change by excluding the trial assessing prevention of gastric variceal rebleeding (Lo 2007) (RR 1.08, 95% CI 0.90 to 1.29; 1240 participants; 18 trials; I² = 0%); by including trials that have used only PTFE‐covered stents (RR 1.02, 95% CI 0.70 to 1.49; 292 participants; 5 trials; I² = 6%) (Ferlitsch 2012; Luo 2015; Holster 2016; Lv 2018; Dunne 2020); or by including trials that did not use PTFE‐covered stents or did not declare whether PTFE stents were covered or not (RR 1.13, 95% CI 0.93 to 1.39; 1020 participants; 14 trials; I² = 0%) (GDEAIH 1995; Cabrera 1996; Cello 1997; Jalan 1997; Rossle 1997; Sanyal 1997; Sauer 1997; Merli 1998; García‐Villarreal 1999; Narahara 2001; Pomier‐Layrargues 2001; Gülberg 2002; Sauer 2002; Lo 2007).
We did not observe a difference in all‐cause mortality when we excluded trials employing pharmacotherapy in addition to endoscopic intervention (RR 1.17, 95% CI 0.91 to 1.50; 661 participants; 10 trials; I² = 0%) (Cabrera 1996; Cello 1997; García‐Villarreal 1999; Gülberg 2002; Jalan 1997; Lo 2007; Merli 1998; Narahara 2001; Pomier‐Layrargues 2001; Sanyal 1997), nor when we restricted the analysis to trials that used only endoscopic banding with or without medical treatment (RR 1.00, 95% CI 0.76 to 1.31; 497 participants; 8 trials; I² = 0%) (Jalan 1997; Pomier‐Layrargues 2001; Gülberg 2002; Sauer 2002; Ferlitsch 2012; Luo 2015; Lv 2018; Dunne 2020), as well as to trials in which band ligation and beta blockers were combined (RR 1.02, 95% CI 0.73 to 1.43; 377 participants; 6 trials; I² = 0%) (Sauer 2002; Ferlitsch 2012; Luo 2015; Holster 2016; Lv 2018; Dunne 2020).
Assessment of imprecision with Trial Sequential Analysis and comparison with GRADE
We compared the assessment of imprecision of intervention effects as assessed by GRADE and Trial Sequential Analysis using the default RRR of 25% as suggested in the GRADE Handbook and the plausible RRR of 10% for GRADE and for Trial Sequential Analysis.
Trial Sequential Analysis of this comparison was constructed on an all‐cause mortality of 25.2% in the endoscopic intervention with or without medical treatment group; an RRR of 10% with TIPS, type I error of 2.5%; and type II error of 20% (80% power). There was no diversity (D² = 0%). The DARIS was 10,902 participants. The Z‐curve did not cross the sequential boundaries for benefit, harm, or futility (figure not shown).
The optimal information size was not reached by using the default RRR of 25% as suggested in the GRADE Handbook, nor when we used a more realistic RRR of 10% chosen by review authors for GRADE and Trial Sequential Analysis (Table 13).
Rebleeding
Rebleeding was reduced by TIPS (RR 0.44, 95% CI 0.36 to 0.55; 1312 participants; 19 trials; I² = 18%; very low‐certainty evidence; Analysis 1.2.3). We downgraded the evidence by three levels because all trials were at overall high risk of bias (‐2 levels), and because of publication bias (‐1 level) (Table 4; Table 13; Figure 5).
Sensivity analysis
The results did not change by excluding the only trial assessing prevention of gastric variceal rebleeding (Lo 2007) (RR 0.44, 95% CI 0.35 to 0.56; 1240 participants; 18 trials; I² = 22%); by including trials that specified only use of PTFE‐covered stents (RR 0.38, 95% CI 0.24 to 0.59; 292 participants; 5 trials; I² = 0%) (Ferlitsch 2012; Luo 2015; Holster 2016; Lv 2018; Dunne 2020); by including trials that did not specify the use of PTFE‐covered stents (RR 0.35, 95% CI 0.19 to 0.64; 234 participants;14 trials; I² = 15%) (GDEAIH 1995; Cabrera 1996; Cello 1997; Jalan 1997; Rossle 1997; Sanyal 1997; Sauer 1997; Merli 1998; García‐Villarreal 1999; Narahara 2001; Pomier‐Layrargues 2001; Gülberg 2002; Sauer 2002; Lo 2007); by excluding trials employing pharmacotherapy for the endoscopic intervention group (RR 0.45, 95% CI 0.33 to 0.62; 661 participants; 10 trials; I² = 26%) (Cabrera 1996; Cello 1997; García‐Villarreal 1999; Gülberg 2002; Jalan 1997; Lo 2007; Merli 1998; Narahara 2001; Pomier‐Layrargues 2001; Sanyal 1997); or by including trials that utilised only endoscopic banding with or without medical treatment (RR 0.43, 95% CI 0.31 to 0.60; 497 participants; 8 trials; I² = 4%) (Jalan 1997; Pomier‐Layrargues 2001; Gülberg 2002; Sauer 2002; Ferlitsch 2012; Luo 2015; Lv 2018; Dunne 2020), as well as trials that used only band ligation and beta blockers (RR 0.43, 95% CI 0.29 to 0.64; 377 participants; 6 trials; I² = 0%) (Sauer 2002; Ferlitsch 2012; Luo 2015; Holster 2016; Lv 2018; Dunne 2020).
Assessment of imprecision with Trial Sequential Analysis and comparison with GRADE
We compared the assessment of imprecision of intervention effects as assessed by GRADE and Trial Sequential Analysis by using the default RRR of 25% as suggested in the GRADE Handbook and the plausible RRR of 20% and 40% for GRADE and for Trial Sequential Analysis.
Trial Sequential Analysis of this comparison was constructed on a rebleeding risk of 42.5% for the endoscopic intervention with or without medical treatment group; type I error of 2.5%; and type II error of 20% (80% power). The cumulative Z‐curve crossed the monitoring boundary for benefit when RRR with TIPS was set to 40% (D² = 16 %, DARIS 351 participants), and when RRR was set to 20% (D² = 16%, DARIS 1474 participants) (figures not shown).
The optimal information size was reached by using the default RRR of 25% as suggested in the GRADE Handbook, and when we used an RRR of 40% and 20% for GRADE and an RRR of 40% for Trial Sequential Analysis. It was not reached with an RRR of 20% for Trial Sequential Analysis (Table 13).
Mortality due to rebleeding
There was no evidence of a difference between TIPS and endoscopic treatment with or without medical treatment (RR 0.65, 95% CI 0.40 to 1.04; 1263 participants; 18 trials; I² = 0%; very low‐certainty evidence; Analysis 1.3.3). In four trials, it is unclear whether deaths were due to variceal rebleeding or to other causes of gastrointestinal haemorrhage (GDEAIH 1995; Merli 1998; Luo 2015; Lv 2018). However exclusion of these trials had no substantial effect on results (RR 0.38, 95% CI 0.18 to 0.78; 937 participants; 13 trials; I² = 0%). We downgraded the evidence by four levels because all trials were at overall high risk of bias (‐2 levels), because of imprecision ‐ the OIS as calculated by GRADE was not met (‐1 level), and because of publication bias (‐1 level) (Table 4; Table 13; Figure 6).
Sensitivity analysis
The result was confirmed when we excluded the trial assessing prevention of rebleeding from gastric varices (Lo 2007) (RR 0.66, 95% CI 0.40 to 1.08; 1191 participants; 17 trials; I² = 1%); when we restricted analysis to trials that did not specify use of PTFE‐covered stents (GDEAIH 1995; Cabrera 1996; Jalan 1997; Rossle 1997; Sanyal 1997; Sauer 1997; Merli 1998; García‐Villarreal 1999; Narahara 2001; Pomier‐Layrargues 2001; Gülberg 2002; Sauer 2002; Lo 2007) (RR 0.57, 95% CI 0.31 to 1.03; 971 participants; 13 trials; I² = 14%); and when we excluded trials employing pharmacotherapy in the endoscopic intervention group (RR 0.51, 95% CI 0.24 to 1.10; 612 participants; 9 trials; I² = 11%) (Cabrera 1996; García‐Villarreal 1999; Gülberg 2002; Jalan 1997; Lo 2007; Merli 1998; Narahara 2001; Pomier‐Layrargues 2001; Sanyal 1997).
Analysis of trials that utilised only endoscopic banding (not in combination with other endoscopic therapies) confirmed the reduction in mortality due to rebleeding with TIPS (RR 0.41, 95% CI 0.17 to 1.00; 497 participants; 8 trials; I² = 0%) (Jalan 1997; Pomier‐Layrargues 2001; Gülberg 2002; Sauer 2002; Ferlitsch 2012; Luo 2015; Lv 2018; Dunne 2020).
There were no differences in mortality due to rebleeding between groups when we restricted the analysis to trials that specified use of PTFE‐covered TIPS (RR 0.59, 95% CI 0.18 to 1.99; 292 participants; 5 trials; I² = 0%) (Ferlitsch 2012; Luo 2015; Holster 2016; Lv 2018; Dunne 2020), and when we restricted the analysis to trials in which band ligation associated with beta blockers was used (RR 0.57, 95% CI 0.19 to 1.67; 377 participants; 6 trials; I² = 0%) (Sauer 2002; Ferlitsch 2012; Luo 2015; Holster 2016; Lv 2018; Dunne 2020).
Assessment of imprecision with Trial Sequential Analysis and comparison with GRADE
We compared the assessment of imprecision of intervention effects as assessed by GRADE and Trial Sequential Analysis by using the default RRR of 25% as suggested in the GRADE Handbook and the plausible RRR of 20% and 40% for GRADE and for Trial Sequential Analysis.
Trial Sequential Analysis of this comparison was constructed on a mortality due to rebleeding of 8.2% in the endoscopic intervention with or without medical treatment group; type I error of 1.4%, and type II error of 20% (80% power). The cumulative Z‐curve reached the futility area before reaching the required information size when RRR with TIPS was set to 40% (D² = 0%, DARIS 2481 participants); the cumulative Z‐curve did not approach the monitoring boundaries for benefit or harm or futility when the RRR was set to 20% (D² = 0%, DARIS 11,073 participants) (figures not shown).
The optimal information size was not reached by using the default RRR of 25% as suggested in the GRADE Handbook nor when we used an RRR of 40% and 20% for GRADE and Trial Sequential Analysis (Table 13).
Acute hepatic encephalopathy
We found evidence of an increase in acute hepatic encephalopathy with TIPS (RR 1.61, 95% CI 1.29 to 1.99; 1247 participants; 18 trials; I² = 20%; very low‐certainty evidence; Analysis 1.4.3). We downgraded the evidence by four levels because all trials were at overall high risk of bias (‐2 levels), because of imprecision ‐ the OIS as calculated by GRADE was not met (‐1 level), and because of publication bias (Table 4; Table 13; Figure 7) .
Sensitivity analysis
The increase in acute hepatic encephalopathy was confirmed by excluding the trial assessing prevention of gastric variceal rebleeding (Lo 2007) (RR 1.55, 95% CI 1.27 to 1.90; 1175 participants; 17 trials; I² = 9%); by excluding trials employing medical treatment in the endoscopic intervention group (RR 1.61, 95% CI 1.13 to 2.29; 661 participants; 10 trials; I² = 33%) (Cabrera 1996; Cello 1997; García‐Villarreal 1999; Gülberg 2002; Jalan 1997; Lo 2007; Merli 1998; Narahara 2001; Pomier‐Layrargues 2001; Sanyal 1997); by restricting analysis to trials that utilised only endoscopic banding (RR 1.27, 95% CI 0.97 to 1.67; 497 participants; 8 trials; I² = 0%) (Jalan 1997; Pomier‐Layrargues 2001; Gülberg 2002; Sauer 2002; Ferlitsch 2012; Luo 2015; Lv 2018; Dunne 2020); and by restricting the analysis to trials in which band ligation and beta blockers were used (RR 1.45, 95% CI 1.08 to 1.95; 377 participants; 6 trials; I² = 0%) (Sauer 2002; Ferlitsch 2012; Luo 2015; Holster 2016; Lv 2018; Dunne 2020).
The differences between groups were reduced when we restricted analysis to trials that specified use of only PTFE‐covered stents (RR 1.39, 95% CI 0.98 to 1.98; 292 participants; 5 trials; I² = 9%) (Ferlitsch 2012; Luo 2015; Holster 2016; Lv 2018; Dunne 2020), and differences were increased when we restricted analysis to trials that did not specify use of PTFE‐covered stents (RR 1.71, 95% CI 1.30 to 2.25; 955 participants; 13 trials; I² = 24%) (Cabrera 1996; Cello 1997; Jalan 1997; Rossle 1997; Sanyal 1997; Sauer 1997; Merli 1998; García‐Villarreal 1999; Narahara 2001; Pomier‐Layrargues 2001; Gülberg 2002; Sauer 2002; Lo 2007).
Assessment of imprecision with Trial Sequential Analysis and comparison with GRADE
We compared the assessment of imprecision of intervention effects as assessed by GRADE and Trial Sequential Analysis, by using the default RRR of 25% as suggested in the GRADE Handbook and the plausible RRR of 20% and 40% for modified GRADE and for Trial Sequential Analysis.
Trial Sequential Analysis of this comparison was constructed on an incidence of acute hepatic encephalopathy of 20.1% in the endoscopic intervention with or without medical treatment group; type I error of 1.4%; and type II error of 20% (80% power). The cumulative Z‐curve crossed the monitoring boundary for harm when RRR with TIPS was set to 40% (D² = 25%, DARIS 1217 participants). When the RRR was set to 20% (D² = 25%, DARIS 5282 participants), the cumulative Z‐curve did not approach the boundaries for benefit, harm, or futility (figures not shown).
The optimal information size was not reached by using the default RRR of 25% as suggested in the GRADE Handbook,nor by using a more realistic RRR of 20% for GRADE and 20% for Trial Sequential Analysis. It was reached with an RRR of 40% for GRADE and TSA (Table 13).
Chronic hepatic encephalopathy
Chronic hepatic encephalopathy was increased by TIPS (RR 1.88, 95% CI 0.93 to 3.80; 717 participants; 10 trials; I² = 0%; very low‐certainty evidence; Analysis 1.5.3). We downgraded the evidence by four levels because all trials were at overall high risk of bias (‐2 levels); because of imprecision ‐ the OIS as calculated by GRADE was not met (‐1 level), and because of publication bias (‐1 level) (Table 4; Table 13; Figure 8).
Sensitivity analysis
The result was confirmed when we excluded trials in which pharmacotherapy was combined with endoscopic intervention (RR 2.30, 95% CI 0.75 to 7.02; 301 participants; 5 trials; I² = 0%) (Cabrera 1996; García‐Villarreal 1999; Gülberg 2002; Jalan 1997; Pomier‐Layrargues 2001). No clear increase in chronic hepatic encephalopathy was noted when we restricted analysis to trials that utilised only endoscopic banding (RR 1.52, 95% CI 0.63 to 3.66; 399 participants; 6 trials; I² = 0%) (Jalan 1997; Pomier‐Layrargues 2001; Gülberg 2002; Sauer 2002; Luo 2015; Lv 2018); when we restricted the analysis to trials in which band ligation and beta blockers were used (RR 1.54, 95% CI 0.51 to 4.65; 207 participants; 3 trials; I² = 0%) (Sauer 2002; Luo 2015; Lv 2018), or when we restricted the analysis only to trials in which PTFE‐covered TIPS were used (RR 1.25, 95% CI 0.35 to 4.46; 122 participants; 2 trials; I² = 0%) (Luo 2015; Lv 2018).
Assessment of imprecision with Trial Sequential Analysis and comparison with GRADE
We compared the assessment of imprecision of intervention effects as assessed by GRADE and Trial Sequential Analysis by using the default RRR of 25% as suggested in the GRADE Handbook and the plausible RRR of 20% and 40% for GRADE and for Trial Sequential Analysis.
Trial Sequential Analysis of this comparison was constructed on an incidence of chronic hepatic encephalopathy of 2.8% in the endoscopic intervention with or without medical treatment group; type I error of 1.4%; and type II error of 20% (80% power). The cumulative Z‐curve did not approach the boundaries for benefit, harm, or futility when RRR with TIPS was set to 40% (D² = 0%, DARIS 7600 participants). When RRR was set to 20% (D² = 0%, DARIS 34,099 participants), the cumulative Z‐curve was not constructed due because we had insufficient information (2.1% of the DARIS) (figures not shown).
The required information size was not reached by using the default RRR of 25% as suggested in the GRADE Handbook, nor when we used an RRR of 20% and 40% for GRADE and Trial Sequential Analysis (Table 13).
Complications
Complications are summarised in Table 8.
Shunt occlusion or dysfunction was the most common problem following TIPS implantation. Rates of shunt occlusion or dysfunction were recorded in 16 trials, with a reported incidence varying from 8% to 89% (Cabrera 1996; Cello 1997; Jalan 1997; Rossle 1997; Sanyal 1997; Sauer 1997; Merli 1998; García‐Villarreal 1999; Narahara 2001; Pomier‐Layrargues 2001; Sauer 2002; Lo 2007; Ferlitsch 2012; Luo 2015; Holster 2016; Lv 2018).
Overall, across all trials utilising TIPS, the incidence of shunt occlusion or dysfunction was 47.1% (95% CI 43% to 51%) (Cabrera 1996; Cello 1997; Jalan 1997; Rossle 1997; Sanyal 1997; Sauer 1997; Merli 1998; García‐Villarreal 1999; Narahara 2001; Pomier‐Layrargues 2001; Sauer 2002; Lo 2007; Ferlitsch 2012; Luo 2015; Holster 2016; Lv 2018). There was substantial variation in shunt surveillance methods and protocols.
Four trials specified that PTFE‐covered stents were used (Ferlitsch 2012; Luo 2015; Holster 2016; Lv 2018); of these, three trials reported the incidence of shunt dysfunction (Luo 2015; Holster 2016; Lv 2018), and one reported the incidence of thrombosis (Ferlitsch 2012). Luo 2015 and Holster 2016 reported incidences of shunt dysfunction of 30% and 5%, respectively, whilst Lv 2018 reported shunt patency rates of 80% at two‐year follow‐up. When we pooled results, the incidence of shunt dysfunction or thrombosis in trials utilising PTFE‐covered stents was 17% (95% CI 11.0 to 24%) (Luo 2015; Holster 2016; Lv 2018).
The remaining trials appear to have used bare‐metal stents. The incidence of shunt dysfunction varied from 18% to 89% across trials that did not specify use of PTFE‐covered stents (12 trials) (Table 8). When we pooled results from these 12 trials, 55% (95% CI 50 to 59) of trial participants developed shunt dysfunction (Cabrera 1996; Cello 1997; Jalan 1997; Rossle 1997; Sanyal 1997; Sauer 1997; Merli 1998; García‐Villarreal 1999; Narahara 2001; Pomier‐Layrargues 2001; Sauer 2002; Lo 2007).
For the shunt group, few complications are reported, suggesting potential under‐reporting of complications.
For endoscopy, the most common complications were oesophageal ulcers and stenosis with or without bleeding and infection (Table 6; Table 7; Table 8).
In‐hospital stay
Nine trials reported on length of hospital stay (Cabrera 1996; García‐Villarreal 1999; Cello 1997; Jalan 1997; Rossle 1997; Pomier‐Layrargues 2001; Sauer 2002; Lo 2007; Holster 2016). The data (when reported) were found to be skewed, with significant statistical heterogeneity and variation in the time scales measured. Results are summarised in Table 9.
Cost
Three trials have reported costs of TIPS versus endoscopic intervention (Cello 1997; Jalan 1997; Holster 2016;Table 10).
Cello 1997 reported total costs from the day of randomisation to death or last follow‐up and reported comparable costs in both groups.
Jalan 1997 reported that the mean cost for the TIPS procedure was GBP 5782 per person (excluding personnel costs), and the mean cost for endoscopic intervention was GBP 4020 per person; Jalan 1997 also reported that the mean cost of inpatient treatment was GBP 7059 for TIPS and GBP 8432 for endoscopic intervention.
Although Cello 1997 and Jalan 1997 found comparable costs in both groups, Holster 2016 reported significantly higher financial costs of TIPS, with a mean cost of EUR 27,746 for TIPS versus a mean cost of EUR 16,816 for endoscopy over one year of follow‐up (including admissions, consultations, procedures, investigations, transfusions, and medications). However, it must be noted that we extracted cost‐effectiveness data from a conference abstract and noted that these data were not based on the full cohort of participants (Holster 2016).
Rebleeding from all causes
We added an unplanned analysis of gastrointestinal rebleeding from all causes (portal hypertension‐related and non‐portal hypertension‐related).
The rate of rebleeding was lower in the portosystemic shunts group than in the endoscopic intervention with or without medical treatment group for all three modalities of treatment: shunt intervention meta‐analysed together (RR 0.42, 95% CI 0.34 to 0.52; 1769 participants; 26 trials; I² = 42%; Analysis 1.6); TS (RR 0.34, 95% CI 0.19 to 0.61; 127 participants; 2 trials; I² = 0%; Analysis 1.6.1); DSRS (RR 0.35, 95% CI 0.18 to 0.68; 330 participants; 5 trials; I² = 63%; Analysis 1.6.2); TIPS (RR 0.45, 95% CI 0.35 to 0.56; 1312 participants; 19 trials; I² = 41%; Analysis 1.6.3).
1.6. Analysis.

Comparison 1: Portosystemic shunts versus endoscopic intervention with or without medical treatment, Outcome 6: Rebleeding from all causes
Subgroup analyses
We were unable to perform subgroup analysis of trials according to risk of bias because all trials were at overall high risk of bias.
Subgroup analysis regarding funding
We found no evidence of differences between the subgroup of trials without for‐profit funding and the subgroup of trials in which the source of funding was unknown (no trials had definite for‐profit funding; see above for details) for all outcomes.
The test for subgroup differences showed no differences in:
all‐cause mortality (P = 0.25; I² = 24.8): trials without for‐profit funding (RR 1.12, 95% CI 0.85 to 1.49; 754 participants; 11 trials; I² = 39%; Analysis 2.1.1), trials with funding unknown (RR 0.93, 95% CI 0.78 to 1.10; 1074 participants; 16 trials; I² = 0%; Analysis 2.1.2);
rebleeding (P = 0.37; I² = 0%): trials without for‐profit funding (RR 0.36, 95% CI 0.25 to 0.51; 732 participants; 11 trials; I² = 37%; Analysis 2.2.1), trials with funding unknown (RR 0.44, 95% CI 0.33 to 0.58; 1037 participants; 15 trials; I² = 29%; Analysis 2.2.2);
mortality due to rebleeding (P = 0.61; I² = 0%): trials without for‐profit funding (RR 0.45, 95% CI 0.23 to 0.86; 705 participants; 10 trials; I² = 1%; Analysis 2.3.1), trials with funding unknown (RR 0.55, 95% CI 0.33 to 0.91; 1074 participants; 16 trials; I² = 0%; Analysis 2.3.2);
acute hepatic encephalopathy (P = 0.59%; I² = 0%): trials without for‐profit funding (RR 1.52, 95% CI 1.09 to 2.12; 705 participants; 11 trials; I² = 24%; Analysis 2.4.1), trials with funding unknown ( (RR 1.69, 95% CI 1.36 to 2.11; 944 participants; 13 trials; I² = 0%; Analysis 2.4.2); or
chronic hepatic encephalopathy (P = 0.61; I² = 0%): trials without for‐profit funding (RR 2.00, 95% CI 0.70 to 5.73; 282 participants; 4 trials; I² = 0%; Analysis 2.5.1), trials with funding unknown (RR 2.79, 95% CI 1.35 to 5.75; 674 participants; 9 trials; I² = 0%; Analysis 2.5.2).
2.1. Analysis.

Comparison 2: Portosystemic shunts versus endoscopic intervention with or without medical treatment regarding funding, Outcome 1: All‐cause mortality
2.2. Analysis.

Comparison 2: Portosystemic shunts versus endoscopic intervention with or without medical treatment regarding funding, Outcome 2: Rebleeding
2.3. Analysis.

Comparison 2: Portosystemic shunts versus endoscopic intervention with or without medical treatment regarding funding, Outcome 3: Mortality due to rebleeding
2.4. Analysis.

Comparison 2: Portosystemic shunts versus endoscopic intervention with or without medical treatment regarding funding, Outcome 4: Acute hepatic encephalopathy
2.5. Analysis.

Comparison 2: Portosystemic shunts versus endoscopic intervention with or without medical treatment regarding funding, Outcome 5: Chronic hepatic encephalopathy
Assessment for publication bias
The presence of publication bias was assessed by performing a dichotomous analysis. It was possible to assess it for all treatments together and for TIPS, given that there were at least 10 trials for TS and DSRS.
Asymmetry was present in the funnel plot, both for all portosystemic shunts analysed together, and for TIPS, which suggests that small trials favouring shunts are missing for analysis of all‐cause mortality (Figure 4), rebleeding (Figure 5), and mortality due to rebleeding (Figure 6) , and small trials favouring endoscopy are missing for analysis of development of acute hepatic encephalopathy (Figure 7) and for development of chronic hepatic encephalopathy (Figure 8).
Discussion
Summary of main results
In this systematic review we compared portosystemic shunting procedures (total shunt (TS), distal splenorenal shunt (DSRS), and transjugular intrahepatic portosystemic shunt (TIPS)) versus endoscopic interventions (endoscopic sclerotherapy or banding, or both) with or without medical treatment for prevention of rebleeding from oesophagogastric varices in people with cirrhosis. We included 27 trials with up to 1828 participants, which provided data for analyses.
Overall, we found very low‐certainty evidence suggesting that all modalities of shunt intervention compared with endoscopic intervention with or without medical treatment may make little or no difference in all‐cause mortality.
We found very low‐certainty evidence suggesting that shunts reduced the risk of rebleeding. Estimates for the risk of all‐cause mortality and rebleeding were precise, as optimal information sizes (OISs) were met and confidence intervals (CIs) were narrow. There was no heterogeneity. However, the overall high risk of bias of the included trials and the risk of publication bias are limitations that reduce the strength of the results of this review.
We found very low‐certainty evidence suggesting that shunts reduced mortality due to rebleeding, and that shunts increased the risk of acute and chronic hepatic encephalopathy. A summary of our results is reported in Table 1.
We found very low‐certainty evidence suggesting that DSRS and TIPS compared with endoscopic treatment with or without medical treatment may make little or no difference in all cause‐mortality, and that TS seemed to reduce mortality. We found very low‐certainty evidence suggesting that each shunt modality reduced rebleeding and mortality due to rebleeding. Although meta‐analysis did not demonstrate an impact of treatment with total shunt on the development of acute hepatic encephalopathy (very low‐certainty evidence), risk was increased with DSRS (very low‐certainty evidence) as with TIPS (very low‐certainty evidence). Chronic hepatic encephalopathy was not increased with TS but was increased with DSRS, and the tendency toward chronic hepatic encephalopathy was increased with TIPS (very low‐certainty evidence).
Differences between types of treatment in effects on all‐cause mortality and acute and chronic encephalopathy must be interpreted cautiously because of the low certainty of evidence, and because of the absence of statistical differences between them. When we analysed TS, DSRS, and TIPS as subgroups, the test for subgroup differences showed no differences between the three treatments on mortality (P = 0.14; I² = 48.8%; Analysis 1.1), acute encephalopathy (P = 0.98; I² = 0%; Analysis 1.4), or chronic encephalopathy (P = 0.31; I² = 13.5%; Analysis 1.5).
We found no substantial heterogeneity among the included trials. This was confirmed by the absence of differences in the main analyses. Sensitivity analysis performed in trials with TIPS, band ligation, medical treatment (beta blocker), and polytetrafluoroethylene (PFTE)‐covered stent did not show any changes in results.
Shunt dysfunction was a problem commonly encountered among individuals treated with TIPS. Summaries of our results comparing TS, DSRS, and TIPS versus endoscopic treatment with or without medical treatment are provided in Table 2, Table 3, and Table 4.
Overall completeness and applicability of evidence
Included trial participants had liver disease ranging from Child's class A to C, although the mean Child‐Pugh score, when reported, ranged between 6.6 and 9.8. This suggests that trials excluded participants with initial Child's class A and severe Class C. No analyses of the results according to liver function or grade of hypertension (based on variceal size or hepatic venous pressure gradient (HVPG)) are reported. Results show high variability in the number of previous bleedings and in time from the index bleeding; the start of prevention ranged from a few hours to several weeks. In addition, some of the included trials were conducted more than 30 years ago. Over the past decades, treatments such as orthotopic liver transplantation for advanced stages of cirrhosis and improved surgical, interventional, and endoscopic procedures have influenced the natural history of cirrhosis and have reduced the applicability of the results of old trials (usually assessing surgical procedures) to patients seen in current practice.
Quality of the evidence
Our current review has identified a number of methodological concerns. All trials were at overall high risk of bias. The a priori number of participants required for a meta‐analysis to be conclusive was reached for all‐cause mortality and rebleeding as shown by the meta‐analysis of all shunt treatments, and for re‐bleeding with TIPS as shown by the meta‐analysis of individual treatments, but not for the other outcomes nor for other individual treatments, meaning that risk of random error was high.
Our assessments of risk of bias reflect the lack of, or poor description of, trial design and performance, as well as incomplete reporting of results. We often failed in our attempts to obtain missing information from the authors of trial reports.
Regarding lack of blinding of participants and personnel, as we anticipated, blinding was impossible to perform in light of the nature of the interventions, but it could have influenced a shift to alternative treatments after randomisation, not due to medical or technical reasons.
Blinding of outcome assessors should have been achievable. Nonetheless, only two trials reported adequate blinding of outcome assessors (Holster 2016; Lo 2007), leaving the remaining trials at risk of detection and performance bias. A lack of blinding of outcome assessor (detection bias) may not affect the results of all‐cause mortality, but it could influence the results of other outcomes (e.g. hepatic encephalopathy, cause of mortality, etc), considering that outcomes can have a subjective component in the judgement (Savović 2018).
We found high risk of reporting bias. Most trials did not publish protocols, and not all of the outcomes of interest for the review were reported; in particular, the incidence of chronic hepatic encephalopathy was reported by only a relatively small number of trials.
Very few adverse events were reported in the shunt group in comparison with the endoscopic group, which could suggest selective under‐reporting of adverse events due to surgical treatment.
A large number of participants with treatment failure were crossed‐over to the other available treatment (mainly from endoscopic intervention to portosystemic shunts) after failure. In most cases, it is unclear if and when the data were censored. It is likely that this bias was relevant for all outcomes except rebleeding (this was the reason for the switch to alternative treatments). We classified this bias under 'other bias'.
The funnel plots in our review seem to suggest risk of publication bias. The visual inspection might give a misleading impression of the presence or absence of publication bias (Simmonds 2015) because there is concern that "visual interpretation of funnel plots is inherently subjective" (Higgins 2019). There is not an agreement on the use of statistical tools to interpret publication bias (Higgins 2019; Grade Handbook), and alternative hypotheses to explain the asymmetry in this review does not seem likely because there was no heterogeneity among the trials, asymmetry was present also for each type of treatment (TS, DSRS, TIPS), and if the number of trials for each type of shunt is low. There were no differences between the results with a random‐effects meta‐analysis and a fixed‐effect analysis (data not presented). We cannot exclude the role of chance completely, but we constructed funnel plots only for analyses with more than 10 trials. Publication bias seems to be the most likely explanation of the asymmetry also because a lot of the included trials are very old, being performed before the implementation of the trial registration initiative. Thus, a selective publication of studies was most likely to have taken place, also if it is not possible to exclude that the apparent asymmetry could be a result of factors other than publication bias, and that the direction of bias appears to vary.
All trials included in this review presented methodological weaknesses that increase bias risk and reduce the certainty of evidence. All of this has impacted the robustness of our conclusions.
Through our GRADE assessment, we considered the influence of the high risk of bias, imprecision, and publication bias in the trials on the trial outcome results. Whereas the precision of the results is high for rate of rebleeding (narrow CI and OIS were met), the limitations due to the overall low quality of trials and publication bias resulted in very low certainty of evidence rating for all outcomes and for each shunt modality.
No trial data on health‐related quality of life were reported; therefore, no evidence is available for this outcome. In addition, we were unable to draw clear conclusions with regards to cost and length of patient stay because data were variably reported, were highly heterogeneous, and were inconsistently collected.
It was not possible to perform meta‐analyses of complications because in most trials they were reported as single episodes, and the number of participants experiencing them was not reported.
Potential biases in the review process
We performed a comprehensive literature search for published and unpublished studies, and we combined electronic data searches with manual searches of the reference lists of identified trials as well as conference proceedings and abstract books from relevant national and international society meetings. We included trials regardless of their language of publication, and whether they reported data on the outcomes we needed. We contacted relevant study authors to request additional information.
Three review authors independently assessed study eligibility, extracted data, and assessed risk of bias in included trials, and we believe that this has reduced potential biases in the review process.
This systematic review represents an update of a previously published review, based on a protocol from 1997. Every effort has been made to update the review with up‐to‐date methods, whilst maintaining methods according to the published protocol when possible, hence avoid reporting bias in the current review. However, we acknowledge that changes in Cochrane guidance have resulted in minor deviations from the protocol.
Agreements and disagreements with other studies or reviews
The previous version of this review utilised a systematic approach to assess the effects of portosystemic shunts (TS, DSRS, and TIPS) versus endoscopic treatments (Khan 2006). Those review authors reported no differences in all‐cause mortality but a reduced incidence of rebleeding and an increased incidence of hepatic encephalopathy for all shunt modalities and with analysis of each modality individually for TIPS. Review results are consistent with our results.
The current version, updating data to 2020, has included more trials and, among these, trials that utilised PTFE‐covered stents and band ligation, which are judged better than uncovered stents and sclerotherapy, respectively. In a sensitivity analysis, the results did not change when trials using the modality of TIPS shunt and using endoscopic interventions were added. Second, more outcomes were assessed: among these, mortality due to rebleeding, which seems reduced by the portosystemic shunt in comparison with endoscopy with or without medical treatment. Whilst it is undoubtedly essential to examine the impact of treatments on health‐related quality of life, unfortunately our attempts to conduct this evaluation failed because none of the trials reported on it, suggesting that health‐related quality of life remains to be examined in future trials.
Moreover, although an assessment of risk of bias was reported in the previous version of this review, the methodological update of criteria according to Cochrane methods has been applied, and an assessment of the certainty of evidence via GRADE recommendations has been added.
Recently, another published meta‐analysis compared portosystemic shunts (including surgical portosystemic shunts and transjugular intrahepatic portosystemic shunt) versus endoscopic intervention (Zhou 2019). The authors of this meta‐analysis stated in the methods section that they assessed trials including participants with "at least one previous episode of gastroesophageal variceal bleeding that had subsequently stabilised, either spontaneously or by the use of non‐surgical therapies..."; however, it appears that they also included trials performed in emergency settings (Orloff 2009; Orloff 2015). In addition, they included trials comparing the surgical shunt to TIPS (Rosemurgy 2012). Therefore, our results represent a different clinical context. Our meta‐analysis is more homogeneous for settings of participants (elective prophylaxis of rebleeding) and comparisons (shunts versus endoscopy).
Two previous meta‐analyses compared uncovered TIPS versus endoscopic intervention (Luca 1999; Papatheodoridis 1999); these were updated by Burroughs 2002. All these meta‐analyses reported no differences in all‐cause mortality, a decrease in rebleeding risk, and an increase in hepatic encephalopathy. However, none of these meta‐analyses evaluated the certainty of evidence following stringent methods. In addition, these meta‐analyses included fewer trials, and given the years of publications, these meta‐analyses included fewer trials reporting on covered TIPS and/or band ligation with or without beta blockers.
In a multiple‐treatment meta‐analysis, Shi 2013 assessed several treatments to prevent rebleeding, including TIPS. Beta blockers combined with endoscopic injection sclerotherapy, and endoscopic banding ligation (EBL) combined with endoscopic injection sclerotherapy, were superior to beta blockers and endoscopic injection sclerotherapy in reducing rebleeding and mortality due to rebleeding; TIPS was more efficacious than beta blockers, endoscopic banding ligation, endoscopic injection sclerotherapy, beta blockers combined with isosorbide‐5‐mononitrate (5‐ISMN), and beta blockers combined with endoscopic injection sclerotherapy. TIPS had the greatest probability of reducing mortality due to rebleeding. Endoscopic banding ligation combined with endoscopic injection sclerotherapy was the best choice according to the cumulative probabilities of being among the three most efficacious interventions for the three outcomes examined in this review. In this meta‐analysis, trials in which drugs or treatments have been previously used were excluded. The list of included trials is not reported in Shi 2013, nor is it updated. Furthermore, the role of this method has yet to be validated.
Authors' conclusions
Implications for practice.
Overall, the very low certainty of evidence on the effects of shunts in comparison with endoscopic interventions with or without medical treatment prevents us from drawing definitive conclusions about the role of shunts in preventing rebleeding and mortality due to rebleeding. We are very uncertain whether shunts had an impact on mortality. Shunts are used at the cost of one having to undergo major surgery or a radiological interventional procedure (with its attendant risks) and the need for a specialised team, which could be different in different countries and between low‐ and high‐income countries. So, the decision on which treatment should be used to prevent further bleeding and the choice of the type of treatment to be used could be based on the physician's and the patient's values and preferences after they have understood the uncertainty on which these choices are based and the significant risks of publication bias behind these results.
Implications for research.
We propose adequately powered, adequately conducted, properly reported multi‐centre randomised clinical trials in this area. These trials should stratify patients at high risk of rebleeding and should consider an 'early' shunt procedure (in the early stage of the bleeding). The trials should consider to investigate hepatic venous pressure gradient (HVPG) as a prognostic variable at entry. The trials need to strive for outcome assessors blinded to intervention group. The issues of effects of interventions on quality of life and costs and their impact on length of hospital stay should also be adequately addressed. As patient recruitment will continue to be an impediment, the only way around this is the pooling of resources across different centres with similar interests. Furthermore, trial reporting should be done in such a way that it facilitates future meta‐analyses. These recommendations are not specific to the comparisons addressed here, but they have implications for randomised clinical trials examining the management of portal hypertension in general. Future trials should be designed according to the SPIRIT recommendations (www.spirit-statement.org), and trial details should be reported in keeping with the CONSORT recommendations (www.consort-statement.org).
What's new
| Date | Event | Description |
|---|---|---|
| 22 June 2020 | New search has been performed | This systematic review and meta‐analysis is an update of a previous version of the review, published in 2006 (Khan 2006). We used updated Cochrane methods during our work on the review. We added and analysed 2 additional outcomes: health‐related quality of life, and death due to rebleeding. We performed more sensitivity analyses, among these assessment of imprecision with Trial Sequential Analysis, and we compared the results with the assessment of imprecision with GRADE |
| 22 June 2020 | New citation required but conclusions have not changed | The conclusions in the current review and in the Khan 2006 review overlap regarding no differences between treatments in all‐cause mortality, reduction in rebleeding risk with portosystemic shunts, and increased acute and chronic hepatic encephalopathy with portosystemic shunts, but our assessment of the certainty of evidence is very low which means that the we are very uncertain in the results |
| 22 June 2020 | New search has been performed | We found 4 ongoing trials |
| 25 February 2019 | New search has been performed | Last search 22 June 2020. This updated review version includes 27 randomised clinical trials with 1828 participants compared to Khan 2006, which included 22 trials with 1409 participants. One trial included in the previous version was excluded from the current version because of the large number of participants with uncontrolled bleeding, and 2 trial references were confirmed to be reports of the same trial. One trial excluded in the previous version was included in the current version because it was judged eligible |
History
Protocol first published: Issue 4, 1997 Review first published: Issue 4, 2006
Notes
As new trials addressing the questions in the review are unlikely to be published within the next two years, if not significantly longer, we plan to update this review not before 2024. However, if contrary to our expectations, we identify trials that fulfil the inclusion criteria of this review before 2024, we will produce an updated version sooner.
Acknowledgements
We are deeply indebted to Christian Gluud, Dimitrinka Nikolova, and Sarah Louise Klingenberg of the Cochrane Hepato‐Biliary Group for their patience, help, and support.
We acknowledge Paula Williamson (PW) for developing and assessing the methods in version 1 of this review, reviewing the manuscript, and providing oversight for the meta‐analyses of version 1. We acknowledge Catrin Tudur Smith (CTS) for her assistance in developing methods and assessing the veracity of meta‐analyses and in reviewing the manuscript in version 1 of this review. We also acknowledge Stanford Wong (SW) and Sara Al‐Ansari (SA) for their work on an earlier permutation of this updated review. SA and CTS performed quality assessment, independently extracted data from eligible trials, provided statistical support, performed survival analyses, and contributed to writing of the review (version 1 only). PW supervised survival calculations and provided a methodological perspective (version 1 only).
Cochrane Review Group funding acknowledgement: the Danish State is the largest single funder of the Cochrane Hepato‐Biliary Group through its investment in the Copenhagen Trial Unit, Centre for Clinical Intervention Research, Rigshospitalet, Copenhagen University Hospital, Denmark.
Disclaimer: the views and opinions expressed in this review are those of the authors and do not necessarily reflect those of the Danish State or the Copenhagen Trial Unit.
Peer Reviewer: Chikwendu Jeffrey Ede, South Africa Contact Editor: Luit Penninga, Denmark; Stefano Trastulli, Italy Sign‐off Editor: Christian Gluud, Denmark Cochrane Abdomen and Endocrine Network Editor: Rachel Richardson, UK
Appendices
Appendix 1. Search strategies
| Database | Time span | Search strategy |
| The Cochrane Hepato‐Biliary Group Controlled Trials Register | June 2020 |
(40 records) (shunt* OR dsrs OR tips) AND (sclerotherap* OR band*) AND ('portal hypertension*' OR cirrho* OR varic*) AND prevent* AND rebleed* |
| Cochrane Central Register of Controlled Trials (CENTRAL), in the Cochrane Library | 2020, Issue 6 |
(46 records) #1 MeSH descriptor: [Portasystemic Shunt, Surgical] explode all trees #2 ((surgical or total or distal splenorenal or transjugular intrahepatic portosystemic) and shunt) or dsrs or tips #3 #1 or #2 #4 MeSH descriptor: [Sclerotherapy] explode all trees #5 endoscopic and (sclerotherap* or band*) #6 #4 or #5 #7 MeSH descriptor: [Hypertension, Portal] explode all trees #8 MeSH descriptor: [Liver Cirrhosis, Biliary] explode all trees #9 MeSH descriptor: [Esophageal and Gastric Varices] explode all trees #10 portal hypertension* or cirrho* or varic* #11 #7 or #8 or #9 or #10 #12 prevent* and rebleed* #13 #3 and #6 and #11 and #12 |
| MEDLINE Ovid | 1946 to June 2020 |
(47 records) 1. exp Portasystemic Shunt, Surgical/ 2. (((surgical or total or distal splenorenal or transjugular intrahepatic portosystemic) and shunt) or dsrs or tips).mp. [mp=title, abstract, original title, name of substance word, subject heading word, floating sub‐heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 3. 1 or 2 4. exp Sclerotherapy/ 5. (endoscopic and (sclerotherap* or band*)).mp. [mp=title, abstract, original title, name of substance word, subject heading word, floating sub‐heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 6. 4 or 5 7. exp Hypertension, Portal/ 8. exp Liver Cirrhosis, Biliary/ 9. exp "Esophageal and Gastric Varices"/ 10. (portal hypertension* or cirrho* or varic*).mp. [mp=title, abstract, original title, name of substance word, subject heading word, floating sub‐heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 11. 8 or 7 or 10 or 9 12. (prevent* and rebleed*).mp. [mp=title, abstract, original title, name of substance word, subject heading word, floating sub‐heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 13. 6 and 11 and 3 and 12 14. (randomized controlled trial or controlled clinical trial).pt. or clinical trials as topic.sh. or trial.ti. 15. (random* or blind* or placebo* or meta‐analys*).mp. [mp=title, abstract, original title, name of substance word, subject heading word, floating sub‐heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 16. 13 and (14 or 15) |
| Embase Ovid | 1974 to June 2020 |
(98 records) 1. exp Portosystemic Anastomosis/ 2. (((surgical or total or distal splenorenal or transjugular intrahepatic portosystemic) and shunt) or dsrs or tips).mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading word, candidate term word] 3. 1 or 2 4. exp SCLEROTHERAPY/ 5. (endoscopic and (sclerotherap* or band*)).mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading word, candidate term word] 6. 4 or 5 7. exp Portal Hypertension/ 8. exp Liver Cirrhosis/ 9. exp Esophagus Varices/ 10. (portal hypertension* or cirrho* or varic*).mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading word, candidate term word] 11. 8 or 7 or 10 or 9 12. (prevent* and rebleed*).mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading word, candidate term word] 13. 6 and 11 and 3 and 12 14. Randomized controlled trial/ or Controlled clinical study/ or trial.ti. 15. (random* or blind* or placebo* or meta‐analys*).mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading word, candidate term word] 16. 13 and (14 or 15) |
| LILACS (Bireme) | 1982 to June 2020 | (3 records) (((surgical or total or distal splenorenal or transjugular intrahepatic portosystemic) and shunt) or dsrs or tips) AND (endoscopic and (sclerotherap$ or band$)) [Words] and (portal hypertension$ or cirrho$ or varic$) [Words] and (prevent$ and rebleed$) [Words] |
| Science Citation Index ‐ EXPANDED (1900 to June 2020) and Conference Proceedings Citation Index – Science (1990 to June 2020) (Web of Science) | 1900 to June 2020 |
(108 records) #7 #6 AND #5 #6 TI=(random* or blind* or placebo* or meta‐analys* or trial*) OR TS=(random* or blind* or placebo* or meta‐analys*) #5 #4 AND #3 AND #2 AND #1 #4 TS=(prevent* and rebleed*) #3 TS=(portal hypertension* or cirrho* or varic*) #2 TS=(endoscopic and (sclerotherap* or band*)) #1 TS=(((surgical or total or distal splenorenal or transjugular intrahepatic portosystemic) and shunt) or dsrs or tips) |
Data and analyses
Comparison 1. Portosystemic shunts versus endoscopic intervention with or without medical treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 All‐cause mortality | 27 | 1828 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.86, 1.13] |
| 1.1.1 TS vs endoscopic intervention | 3 | 164 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.19, 1.13] |
| 1.1.2 DSRS vs endoscopic intervention | 5 | 352 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.65, 1.33] |
| 1.1.3 TIPS vs endoscopic intervention | 19 | 1312 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.92, 1.31] |
| 1.2 Rebleeding | 26 | 1769 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.33, 0.50] |
| 1.2.1 TS vs endoscopic intervention | 2 | 127 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.14, 0.56] |
| 1.2.2 DSRS vs endoscopic intervention | 5 | 330 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.11, 0.65] |
| 1.2.3 TIPS vs endoscopic intervention | 19 | 1312 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.36, 0.55] |
| 1.3 Mortality due to rebleeding | 26 | 1779 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.34, 0.76] |
| 1.3.1 TS vs endoscopic intervention | 3 | 164 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.06, 0.96] |
| 1.3.2 DSRS vs endoscopic intervention | 5 | 352 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.13, 0.74] |
| 1.3.3 TIPS vs endoscopic intervention | 18 | 1263 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.40, 1.04] |
| 1.4 Acute hepatic encephalopathy | 24 | 1649 | Risk Ratio (M‐H, Random, 95% CI) | 1.60 [1.33, 1.92] |
| 1.4.1 TS vs endoscopic intervention | 2 | 115 | Risk Ratio (M‐H, Random, 95% CI) | 1.66 [0.70, 3.92] |
| 1.4.2 DSRS vs endoscopic intervention | 4 | 287 | Risk Ratio (M‐H, Random, 95% CI) | 1.70 [0.94, 3.08] |
| 1.4.3 TIPS vs endoscopic intervention | 18 | 1247 | Risk Ratio (M‐H, Random, 95% CI) | 1.61 [1.29, 1.99] |
| 1.5 Chronic hepatic encephalopathy | 13 | 956 | Risk Ratio (M‐H, Random, 95% CI) | 2.51 [1.38, 4.55] |
| 1.5.1 TS vs endoscopic intervention | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 7.20 [0.39, 134.36] |
| 1.5.2 DSRS vs endoscopic intervention | 2 | 170 | Risk Ratio (M‐H, Random, 95% CI) | 4.87 [1.46, 16.23] |
| 1.5.3 TIPS vs endoscopic intervention | 10 | 717 | Risk Ratio (M‐H, Random, 95% CI) | 1.88 [0.93, 3.80] |
| 1.6 Rebleeding from all causes | 26 | 1769 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.34, 0.52] |
| 1.6.1 TS vs endoscopic intervention | 2 | 127 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.19, 0.61] |
| 1.6.2 DSRS vs endoscopic intervention | 5 | 330 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.18, 0.68] |
| 1.6.3 TIPS vs endoscopic intervention | 19 | 1312 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.35, 0.56] |
Comparison 2. Portosystemic shunts versus endoscopic intervention with or without medical treatment regarding funding.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 All‐cause mortality | 27 | 1828 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.86, 1.13] |
| 2.1.1 Trials without for‐profit funding | 11 | 754 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.85, 1.49] |
| 2.1.2 Trials with for‐profit funding or unknown | 16 | 1074 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.78, 1.10] |
| 2.2 Rebleeding | 26 | 1769 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.33, 0.50] |
| 2.2.1 Trials without for‐profit funding | 11 | 732 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.25, 0.51] |
| 2.2.2 Trials with for‐profit funding or unknown | 15 | 1037 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.33, 0.58] |
| 2.3 Mortality due to rebleeding | 26 | 1779 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.34, 0.76] |
| 2.3.1 Trials without for‐profit funding | 10 | 705 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.23, 0.86] |
| 2.3.2 Trials with for‐profit funding or unknown | 16 | 1074 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.33, 0.91] |
| 2.4 Acute hepatic encephalopathy | 24 | 1649 | Risk Ratio (M‐H, Random, 95% CI) | 1.60 [1.33, 1.92] |
| 2.4.1 Trials without for‐profit funding | 11 | 705 | Risk Ratio (M‐H, Random, 95% CI) | 1.52 [1.09, 2.12] |
| 2.4.2 Trials with for‐profit funding or unknown | 13 | 944 | Risk Ratio (M‐H, Random, 95% CI) | 1.69 [1.36, 2.11] |
| 2.5 Chronic hepatic encephalopathy | 13 | 956 | Risk Ratio (M‐H, Random, 95% CI) | 2.51 [1.38, 4.55] |
| 2.5.1 Trials without for‐profit funding | 4 | 282 | Risk Ratio (M‐H, Random, 95% CI) | 2.00 [0.70, 5.73] |
| 2.5.2 Trials with for‐profit funding or unknown | 9 | 674 | Risk Ratio (M‐H, Random, 95% CI) | 2.79 [1.35, 5.75] |
Characteristics of studies
Characteristics of included studies [author‐defined order]
Korula 1987.
| Study characteristics | ||
| Methods | Randomised controlled trial comparing surgical shunt with endoscopic sclerotherapy for prevention of variceal rebleeding in patients with Child's A cirrhosis Time from bleeding episode to randomisation: not mentioned Time from randomisation to treatment in days (mean): not mentioned Total number of participants evaluated: 55; found eligible: 37 Randomised to surgical shunt: 18; randomised to endoscopic sclerotherapy: 19 Adequate reasons provided for those not randomised: no information Follow‐up period months (mean, SD): surgical shunt group 13.1, 8.8; sclerotherapy group 10.5, 9.5 Deviation from intended interventions: 2 participants were not treated after randomisation (1 in shunt group and 1 in endoscopic group) Assessment of suitability for shunt carried out before randomisation: not mentioned Shunt patency assessed: no mention Method of Child's grading: Child‐Pugh Method of encephalopathy testing: not mentioned Rebleeding episodes endoscopically verified: no information |
|
| Participants | Inclusion criteria: all patients with cirrhotic portal hypertension (Child‐Pugh A class, Pugh modification < 8 points) with minimum of 2 variceal bleeding episodes who received less than 1 session of endoscopic sclerotherapy Exclusion criteria: not mentioned Aetiology: alcohol 16 patients in each group Baseline characteristic: similar in the 2 groups |
|
| Interventions | Shunt: TS (portacaval) (13 patients), DSRS (3 patients), mesocaval (1 patient) ET: sclerotherapy |
|
| Outcomes |
|
|
| Notes | Abstract only Funding: no information |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible. It is unclear if lack of blinding influenced the choice of treatments and the management of patients |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention of blinding. Judgement on all outcomes except mortality could be biased |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | High risk | Insufficient Information: information only on mortality and rebleeding. Not enough information on all outcomes |
| Other bias | Unclear risk | It is not possible to assess other bias because this was presented as an abstract |
Terés 1987.
| Study characteristics | ||
| Methods | Randomised controlled trial comparing distal splenorenal shunt with sclerotherapy for prevention of esophagogastric variceal rebleeding Intention‐to‐treat analysis Time from bleeding episode to randomisation: 10 to 15 days Time from randomisation to treatment in days: DSRS group 11 to 65 (mean 32.7, SD 17.2), sclerotherapy group 10 to 59 (mean 23.5, SD 15.1) Total number of patients evaluated: 189 Randomised to shunt surgery: 57; randomised to endoscopic sclerotherapy: 55 Deviation from intended interventions: 14 of the 57 patients assigned to the DSRS group (24.5%) and 4 of the 55 assigned to the endoscopic sclerotherapy group (7.3%) were excluded from the trial after randomisation before treatment for clinical reasons. Some reasons involved surgical considerations (i.e. splenic and vein thrombosis). Other reasons were participant related (4 refused surgery) Two patients in the endoscopic group were lost to follow‐up Mean follow‐up period in months (SD): shunt surgery 27.45 (15.6), endoscopic sclerotherapy 26.57 (16.9) Follow‐up range in months DSRS (1 to 58), endoscopic therapy (1 to 64) Assessment of suitability for shunt carried out before randomisation: no Method of Child's grading: Child‐Campbell Method of encephalopathy testing: clinical testing and history Rebleeding episodes endoscopically verified: yes Specified whether rebleeding episode clinically significant: not specified |
|
| Participants | Inclusion criteria: Child‐Campbell A and B cirrhotic patients with at least 1 episode of oesophageal or gastric variceal haemorrhage, documented by endoscopy, who were treated by conservative procedures (balloon tamponade or vasopressin) and achieved haemostasis Exclusion criteria: Child‐Campbell C patients, continual variceal bleeding despite medical treatment and balloon tamponade, early rebleeding between admission and randomisation Randomisation was done when the patient was stabilised ‐ between 10 and 15 days after cessation of the haemorrhage Child‐Cambell score: DSRS group 7.07 ± 1.17, sclerotherapy group 7.54 ± 1.38 Aetiology: alcohol 64% (DSRS group 51%, sclerotherapy group 78%) Child‐Campbell score greater and number of alcoholics greater in the endoscopic sclerotherapy group |
|
| Interventions | Shunt: DSRS (retroperitoneal approach) Endoscopic therapy: sclerotherapy, technique intravariceal, sclerosant 5% ethanolamine oleate (10 to 20 mL at each session), every week for an average of 4 weeks until varices disappeared or became of minimal size |
|
| Outcomes | Study authors reported planned outcomes:
Study authors reported complications |
|
| Notes | Funding: supported by Grants CAICYT (Comisión Asesora de Investigación Científica y Técnica) 1851/82 and 1853/82 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random table |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes. It is unknown if the envelopes were opaque and numbered |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible It is unclear if lack of blinding influenced the choice of treatments and the management of patients |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding not mentioned: assessment of outcomes, except for mortality, could be biased |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Study authors reported information on mortality for all randomised participants. They did not report information on the other outcomes of interest for excluded participants after randomisation |
| Selective reporting (reporting bias) | High risk | No pre‐published protocol is available Study authors report information on all key outcomes of interest in this review They reported complications in the endoscopic group but not in the shunt group |
| Other bias | High risk | In DSRS, 4 participants/6 who rebled were submitted to an alternative therapy: 1 to stapling transection; 3 to sclerotherapy In endoscopic group, 3 participants who rebled were treated: 1 by stapling transection, 2 by portacaval shunt Participants were not censored at the bleeding episode Cross‐over to alternative treatment could modify the effects of treatments |
Santambrogio 2006.
| Study characteristics | ||
| Methods | Randomised controlled trial comparing distal splenorenal shunt with endoscopic sclerotherapy for prevention of variceal rebleeding Time from bleeding episode to randomisation: when the patient achieved haemodynamic stability Time from randomisation to treatment: no longer than 24 hours Total number of patients evaluated: 282, of which 140 were eligible Adequate reasons provided for not randomising: yes Randomised to shunt surgery: 40; randomised to endoscopic sclerotherapy: 40 Long‐term follow‐up not complete in 2 DSRS and 3 endoscopic therapy patients Mean follow‐up period in months (mean, SD): shunt surgery group 109 ± 58, sclerotherapy group 87 ± 61 Protocol violation: DSRS group: 2 patients had TS for technical reasons; endoscopic therapy: 3 patients changed treatment (1 transplant, 2 portacaval shunt) Assessment of suitability for shunt carried out before randomisation: yes Method of Child's grading: Child‐Pugh Method of encephalopathy testing: mental status, asterixis, trail‐making tests, "cancelling A's" test, EEG Rebleeding episodes endoscopically verified: yes Specified whether rebleeding episode clinically significant: yes |
|
| Participants | Inclusion criteria: biopsy‐confirmed cirrhosis, endoscopically verified variceal bleed requiring at least 1 unit of blood transfusion, arrest of variceal haemorrhage spontaneously or by the use of drugs and/or tamponade and/or sclerotherapy, < 70 years old, good or moderate liver function as reflected by Child‐Pugh class A and B, patency of portal venous system and hepatopetal flow (according to Nordlinger's classification), eligible for shunt or sclerotherapy, absence of life‐threatening disease (e.g. tumour) and willingness to return for regular follow‐up Exclusion criteria: Budd‐Chiari syndrome, sclerosing cholangitis, > 70 years old, Child's class C, unsuitable splenic vein, gastric varices, chronic encephalopathy, severe ascites, associated disease, previous sclerotherapy, not willing Randomisation for assignment to groups was carried out when the patient was stabilized, which occurred no more than 24 hours before treatment Child‐Pugh class A/B (%): 37.5/62.5 (DSRS group 47.5/53.5, sclerotherapy group 27.5/72.5) Aetiology (%): alcohol 50, no alcohol 50 (DSRS group 35/65, sclerotherapy group 65/35) Patients in the endoscopic sclerotherapy group were slightly older and a larger number were alcoholics |
|
| Interventions | Shunt surgery: distal splenorenal shunt (Warren technique) with splenopancreatic disconnection in 18 patients; total shunt in 2 patients Endoscopic therapy: sclerotherapy, intravariceal and paravariceal technique, sclerosant 0.5% to 1% polidocanol and 0.5% methylene blue |
|
| Outcomes |
|
|
| Notes |
Santambrogio 2006 represents update of Spina 1990 Funding: no information Analysis of time to rebleeding appears to include 4 patients bleeding from duodenal ulcers in the shunt group and 1 patient bleeding from oesophageal ulcer and 2 from unknown causes in the endoscopic therapy group. Data for variceal bleeding and hypertensive gastropathy could be extracted only for assessment of raw numbers of patients experiencing rebleeding |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible. It is unclear if lack of blinding influenced the choice of treatments.and the management of patients |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding not mentioned. Judgement on all outcomes except mortality could be biased |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Mean follow‐up 109 months in DSRS group; 87 in endoscopic therapy group. Long‐term follow‐up complete in all patients, except 5. This was judged to be acceptable follow‐up by review authors in light of the extended follow‐up period |
| Selective reporting (reporting bias) | High risk | No pre‐published protocol available Study authors reported information on all key outcomes of interest in this review but provided incomplete reporting of surgical complications |
| Other bias | Low risk | Small number of cross‐over treatments: 2 participants in endoscopic group were treated during follow‐up with portacaval H‐graft shunt |
Henderson 1990.
| Study characteristics | ||
| Methods | Randomised controlled trial comparing distal splenorenal shunt with endoscopic variceal sclerosis to prevent variceal rebleeding in cirrhotic patients Time from bleeding episode to randomisation: not mentioned Time from randomisation to treatment: therapy was instituted within 48 hours of assignment for all patients except 2 in the shunt group Total number of patients evaluated: 420 Randomised to shunt: 35; randomised to endoscopic therapy: 37 Adequate reasons provided for those not randomised: yes One patient in the shunt group did not receive assigned treatment Surgical rescue was required in 35% of patients receiving sclerotherapy. Rescue surgery included distal splenorenal shunt in 8 patients, total portal systemic shunt in 2 patients, and splenectomy with devascularisation in 2 patients No losses to follow‐up Intention‐to‐treat analysis Median (range) follow‐up period in months: 61 (30 to 84) Assessment of suitability for shunt carried out before randomisation: no Method of Child's grading: single worst Child’s criterion Rebleeding episodes endoscopically verified: yes Specified whether rebleeding episode clinically significant: yes (Only 16 patients in the shunt group and 14 in the endoscopic therapy group were discharged alive after the initial hospitalisation) |
|
| Participants | Inclusion criteria: biopsy‐proven cirrhosis, endoscopic evidence of oesophageal variceal bleeding, suitability for DSRS established with angiography or sclerotherapy Exclusion criteria: living more than 200 miles from the base hospital, referred for specific therapy (surgery or sclerosis), previous long‐term sclerotherapy, emergent or urgent surgery, non‐cirrhotic variceal bleed Acute bleeding episode was managed as clinically indicated. After stabilisation of the patient's bleeding for 24 to 48 hours, the patient and his or her data were reviewed to assess eligibility Forty‐one participants (57%) were in Child's A and B classes (57% in each group); 31 participants (43%) were in class C (41% in each group) Aetiology: alcohol 57% in DSRS group and 62% in sclerotherapy group. No mention of HBV and HCV aetiology Patients comparable in terms of age, Child's class, and alcoholic status |
|
| Interventions | Surgical shunt: DSRS (Warren technique), with or without splenopancreatic disconnection Sclerotherapy: intravariceal and paravariceal injections of: 0.75 to 1.0% sodium tetradecyl sulphate or 1.5 to 2.0% sodium morrhuate, following a weekly, biweekly, then monthly schedule |
|
| Outcomes | Outcomes were pre‐defined at the beginning of the study:
Encephalopaty is reported in a previous report (Warren 1986) |
|
| Notes | Funding: supported by Public Health Service Research Grant AM 15736 and General Clinical Research Center Public Health Service Grant 5M01RR00039 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes. It is unknown if the envelopes were opaque and numbered |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible. It is unclear if lack of blinding influenced the choice of treatments.and the management of patients |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding was not mentioned. Judgement on all outcomes except mortality could be biased |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis. Median follow‐up 61 months (range 30 to 84 months). Telephone contact maintained: "in the latter years of the study, not every patient returned for follow‐up, but their status was confirmed by telephone interview" |
| Selective reporting (reporting bias) | High risk | No pre‐published protocol available "Endpoints were defined at the beginning of the study. Primary endpoints were death and failure of therapy"; "Secondary endpoints included rebleeding and a decline in either hepatic function or haemodynamics" Rates of encephalopathy were reported in preliminary analysis (Warren and colleagues) but not in final report. Not all key outcomes are reported |
| Other bias | High risk | Large number of cross‐over treatments. Twelve participants in endoscopic group received surgery for bleeding: 8 splenorenal shunt, 2 total portal shunt, 2 splenectomy with devascularisation |
Planas 1991.
| Study characteristics | ||
| Methods | Randomised controlled trial comparing portacaval shunt with endoscopic sclerotherapy for prevention of gastroesophageal variceal rebleeding Time from bleeding episode to randomisation: between 4 and 11 days in both groups Time from randomisation to treatment in days (mean, SD): shunt group 14.7, 6.3; endoscopic therapy group 7.2, 3.4 Total number of patients evaluated: 182 Randomised to shunt: 41; randomised to endoscopic sclerotherapy: 41 Adequate reasons provided for those not randomised: yes Seven patients in the shunt group and 6 in the endoscopic therapy group did not receive the allocated treatment One patient in each group was lost to follow‐up Intention‐to‐treat analysis Follow‐up period in months (mean, SD): shunt group 20.9, 13.9; endoscopic sclerotherapy group 20.8, 15 Assessment of suitability for shunt carried out before randomisation: no Method of Child's grading: Child‐Campbell Method of encephalopathy testing: clinical testing and history Rebleeding episodes endoscopically verified: yes Specified whether rebleeding episode clinically significant: not specified |
|
| Participants | Inclusion criteria: Child‐Campbell A and B cirrhotic patients following endoscopically proven gastroesophageal variceal haemorrhage Exclusion criteria: Child‐Campbell class C, uncontrollable haemorrhage or early rebleeding between admission and randomisation Randomisation was performed after the haemorrhage had stopped and the patient had been haemodynamically stable for 3 days Child‐Campbell score: shunt group 8.2 ± 1.3, sclerotherapy group 8.4 ± 1.5. Child‐Campbell class A/B (%): shunt group 39/61, sclerotherapy group 34/66 Aetiology (%): alcohol 61 in shunt group and 73 in sclerotherapy group. No mention of HCB and HBV aetiology Patient characteristics comparable, age slightly younger in the endoscopic sclerotherapy group |
|
| Interventions | Shunt: end‐to‐side portacaval shunt Sclerotherapy: intravariceal and paravariceal injections of 1% polidocanol (first 2 sessions performed weekly, and every 2 weeks afterwards, until obliteration) |
|
| Outcomes | Planned outcomes:
|
|
| Notes | Funding: no information | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible. It is unclear if lack of blinding influenced the choice of treatments and the management of patients Deviation from intended interventions: 4 participants in the shunt group refused surgery not for medical or technical reasons |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding not mentioned. Judgement on all outcomes except mortality could be biased |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 patient in each group lost to follow‐up, with censorship as appropriate (acceptable dropout rate < 5%). Results presented on both an intention‐to‐treat and a per‐protocol basis for all outcomes aside from chronic hepatic encephalopathy and length of hospitalisation |
| Selective reporting (reporting bias) | Low risk | No pre‐published protocol All outcomes of interest in this review are presented in the expected forms |
| Other bias | High risk | Large number of cross‐over treatments. In portacaval shunt group, 1 (2.4%) participant was crossed over to sclerotherapy and then to mesocaval shunt for repeated rebleeding. Five participants in endoscopic group (14%) were crossed over to portacaval shunt |
Rikkers 1993.
| Study characteristics | ||
| Methods | Randomised controlled trial comparing elective shunt surgery with endoscopic sclerotherapy for prevention of oesophageal variceal haemorrhage Time from bleeding episode to randomisation: not specified Time from randomisation to treatment: not specified Randomised to shunt surgery: 31; randomised to endoscopic sclerotherapy: 29. One patient switched to endoscopic sclerotherapy from shunt surgery after he withdrew consent; he was assessed as being randomised to endoscopic sclerotherapy Adequate reasons provided for those not randomised: not specified Deviation from intended interventions, small number: 1 patient who refused surgery was treated with endoscopic therapy and was included in the endoscopic therapy group for subsequent analysis (per‐protocol analysis) Two patients in each group lost to follow‐up Per‐protocol analysis, non‐intention‐to‐treat Follow‐up period in months (mean, SEM): shunt surgery group 85, 5; endoscopic sclerotherapy group 92, 7 Assessment of suitability for shunt carried out before randomisation: yes, with angiography Method of Child's grading: modified Child's with 4 parameters (serum albumin level, total bilirubin level, encephalopathy, ascites) Method of encephalopathy testing: clinical, EEG, psychometric (number connection test) Rebleeding episodes endoscopically verified: yes Specified whether rebleeding episode clinically significant: not specified |
|
| Participants | Inclusion criteria: portal hypertension secondary to cirrhosis, endoscopic documentation of acute or recent oesophageal variceal haemorrhage requiring a minimum transfusion of 3 U of blood, residence within 500 miles of Salt Lake City or Omaha, non‐operative control of acute variceal haemorrhage, patency of splenic and portal veins documented by selective angiography Exclusion criteria: not specified Child‐Pugh score (mean ± SEM): 6.6 ± 0.4 in each group. Child's class C (%): 33% in each group Aetiology (%): alcohol 83 in shunt group and 90 in sclerotherapy group. No mention of HBV and HCV aetiology Patients in the 2 groups were comparable at randomisation |
|
| Interventions | Shunt surgery: DSRS without splenopancreatic disconnection (n = 26), side‐to‐side portacaval shunt or Dacron graft interposition shunt (n = 3), end‐to‐side portacaval (n = 1) Endoscopic therapy: sclerotherapy, with intravariceal injections of 0.75% sodium tetradecyl sulphate and 50% dextrose or 5% sodium morrhuate, every 4 to 6 days until most varices were eradicated, then after 1 month, then as necessary at 6‐month intervals |
|
| Outcomes |
|
|
| Notes | Portacaval shunts were performed on 3 patients with medically intractable ascites and on 1 with massive rebleeding; SPD was not used in any patients Rikkers 1987 reported early results of the same study Funding: supported by Public Health Service Grant #5 ROI DK35168 and General Clinical Research Center Public Health Service Grant #5MOIRR0004 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Efron's biased coin design based on 3 liver function strata and types of hospital |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible. It is unclear if lack of blinding influenced the choice of treatments and the management of patients |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information. Judgement on all outcomes except mortality could be biased |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Adequate follow‐up: survival status of all patients was known; late postoperative evaluations were not possible for 2 patients in each group (total of 60 patients randomised) Dropouts: 7% |
| Selective reporting (reporting bias) | High risk | No pre‐published protocol available. Study authors did not report all key outcomes: surgical complications were not reported |
| Other bias | High risk | Large number of cross‐over treatments. In shunt group, 2 participants (6.7%) underwent shunt reoperation for rebleeding (1 end‐to‐side portacaval shunt, 1 interposition meso‐renal shunt). In endoscopic group, 5 participants (16.6%) underwent emergency salvage operations (4 end‐to‐side portacaval shunts, 1 splenectomy + oesophagogastric devascularisation) |
GDEAIH 1995.
| Study characteristics | ||
| Methods | Randomised controlled trial comparing transjugular intrahepatic portosystemic shunt with sclerotherapy + propranolol for prevention of variceal rebleeding in patients with Child's C cirrhosis Randomised to TIPS: 32; endoscopic therapy + propranolol: 33 Time from bleeding episode to randomisation: not specified Time from randomisation to treatment: TIPS performed within 4 days after bleeding; time to treatment not specified for sclerotherapy In 2 patients; TIPS could not be performed (1 technical failure, 1 portal vein thrombosis). These 2 patients were treated with sclerotherapy + propranolol Adequate reasons provided for those not randomised: no information Follow‐up: 1 year or until death or liver transplantation 53 patients completed the study. All patients were included in analysis Mean follow‐up period in months (SE): not specified Assessment of suitability for shunt carried out before randomisation: unclear Method of Child's grading: unclear Method of encephalopathy testing: not applicable Rebleeding episodes endoscopically verified: not specified. Rebleeding defined as any digestive haemorrhage Specified whether rebleeding episode clinically significant: yes – necessitating transfusion of 2 or more units of blood |
|
| Participants | Inclusion criteria: Child's C cirrhotic patients presenting with oesophageal variceal bleeding Exclusion criteria: not specified Aetiology (%): alcohol 94 Patients were treated with emergency sclerotherapy before randomisation Patients were treated with sclerotherapy before randomisation |
|
| Interventions | TIPS: type not specified Endoscopic therapy: sclerotherapy + propranolol |
|
| Outcomes |
|
|
| Notes | Abstract only Funding: no information |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible. It is unclear if lack of blinding influenced the choice of treatments and the management of patients |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information. Judgement on all outcomes except mortality could be biased |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only preliminary results reported. All randomised patients were included in the analysis; however study authors stated that "so far 53 patients have completed the study". No information on remaining participants |
| Selective reporting (reporting bias) | High risk | No pre‐published protocol available Study authors reported on not all of the key outcomes of interest in the review |
| Other bias | Unclear risk | Abstract only In TIPS group, 2 participants were crossed over to endoscopy treatment for rebleeding |
García‐Villarreal 1999.
| Study characteristics | ||
| Methods | Randomised controlled trial comparing transjugular intrahepatic portosystemic shunt vs endoscopic sclerotherapy for prevention of variceal rebleeding after recent variceal haemorrhage
Time from bleeding episode to randomisation: after a period of 24 hours of stability Time from randomisation to assigned therapy: 3 days; in case of rebleeding after randomisation, assigned therapy was applied immediately Time from variceal bleeding to therapy in days, mean (SD): TIPS 5.4 (2.1), sclerotherapy 5.6 (2.2) 22 patients randomised in TIPS group, 24 patients in endoscopic therapy group Results presented according to intention‐to‐treat Follow‐up period in days (mean, SD): TIPS 760, 390; endoscopic therapy 503, 463 Assessment of suitability for shunt carried out before randomisation: not specified One participant in endoscopic group crossed to TIPS after 2 rebleeding episodes 1 patient lost to follow‐up, and 1 patient in each group left the study Shunt patency assessed with portography at 1 month and then every 6 months Method of Child's grading: Child‐Pugh Method of encephalopathy testing: Parson‐Smith criteria Rebleeding episodes endoscopically verified: yes Specified whether rebleeding episode clinically significant: not specified |
|
| Participants | Inclusion criteria: endoscopically proven oesophageal variceal bleeding; diagnosis of cirrhosis based on clinical history and laboratory, ultrasonography, and/or liver biopsy findings; age between 18 and 75 years; informed consent from patient or next of kin when encephalopathy was present Exclusion criteria: history of chronic encephalopathy, portal vein thrombosis, hepatocellular carcinoma, end‐stage liver disease defined by the presence of more than 1 of the following parameters: prothrombin index < 35%, bilirubin > 5 mg/dL, plasma creatinine > 3 mg/dL, and follow‐up not possible Child‐Pugh score (mean ± SD): TIPS group 8.6 ± 2.2, sclerotherapy group 8.8 ± 2.2. Child‐Pugh class A/B/C (%): TIPS group 23/45/32, sclerotherapy group 12/58/30 Aetiology (%): alcohol 68 in TIPS group, 75 in sclerotherapy group. No mention of HBV and HCV aetiology Both groups were comparable with respect to age, gender, aetiology. Endoscopic group had a significantly greater proportion of patients with pre‐existing encephalopathy who were comparable in terms of Child‐Pugh class and score All patients presenting with variceal haemorrhage were treated with endoscopic sclerotherapy (within 4 hours of admission) and vasoactive drugs and balloon tamponade when necessary |
|
| Interventions | TIPS: wall stent endoprosthesis Sclerotherapy: intravariceal injections of 0.5 to 1 mL of 5% ethanolamine oleate (for a total of 12 to 20 mL per session), repeated every 7 to 10 days until obliteration |
|
| Outcomes | Pre‐planned outcomes:
|
|
| Notes | Funding: no information | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible. It is unclear if lack of blinding influenced the choice of treatments.and the management of patients |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient Information. Judgement on all outcomes except mortality could be biased |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One patient lost to follow‐up, 1 patient in each group decided to leave the study Small number of dropouts (5%) |
| Selective reporting (reporting bias) | High risk | No pre‐published protocol available Planned outcomes: "primary endpoint was variceal rebleeding. Secondary outcomes were survival and hepatic encephalopathy. Other parameters that reflect the benefit of therapy such as rebleeding index, days spent in hospital and cause of death were studied". No report of complications for shunt group |
| Other bias | Low risk | In endoscopic group, only 1 participant who had treatment failure was switched to TIPS |
Cabrera 1996.
| Study characteristics | ||
| Methods | Randomised controlled trial comparing transjugular intrahepatic portosystemic shunt vs sclerotherapy for elective treatment of oesophageal variceal haemorrhage Time from bleeding episode to randomisation: 3 days after bleeding was controlled Time from randomisation to treatment in days (mean, SD): TIPS 8.4, 3.6; endoscopic therapy 2.7, 3.2 Total number of patients evaluated and found eligible: 63 (90 evaluated) Randomised to TIPS: 31 participants; randomised to endoscopic therapy: 32 participants 1 participant from each group died before treatment Adequate reasons provided for those not randomised: yes Nine patients in the endoscopic therapy group were crossed over to TIPS during follow‐up There were no losses to follow‐up Intention‐to‐treat analysis Follow‐up period, days (mean, SD): TIPS 452, 298 (range 30 to 1020); endoscopic therapy 455, 298 (range 70 to 951) Assessment of suitability for shunt carried out before randomisation: no Shunt patency assessed with angiography at 6 months or at the time of rebleeding Method of Child's grading: Child‐Pugh Method of encephalopathy testing: clinical Rebleeding episodes endoscopically verified: yes Specified whether rebleeding episode clinically significant: yes |
|
| Participants | Inclusion criteria: all cirrhotic patients admitted with an episode of acute oesophageal variceal bleeding Exclusion criteria: presence of gastric varices with active bleeding or with stigmata of recent haemorrhage at first emergency endoscopy, episodes of chronic hepatic encephalopathy, severe acute alcoholic hepatitis (prothrombin time < 30%), hepatic encephalopathy and/or functional renal failure in an alcoholic cirrhotic patient, end‐stage cirrhosis (≥ 2 of the following parameters: prothrombin time < 35%, plasma creatinine > 2 mg/dL, bilirubin > 5 mg/dL, age > 75 years), neoplastic disease, septicaemia, portal vein thrombosis Active bleeding episode was treated with intravenous somatostatin infusion (250 microg/h after initial bolus of 1 microg/kg body weight) for 48 hours. If bleeding was not controlled, a Sengstaken‐Blakemore tube was placed Child‐Pugh score (mean ± SD): TIPS group 7.1 ± 1.59, sclerotherapy group 7.22 ± 1.75. Child‐Pugh Class A/B/C (%): TIPS group 45/42/13, sclerotherapy group 44/50/6 Aetiology (%): alcohol 64.4 in TIPS group and 72 in sclerotherapy group. No mention of HBV and HCV aetiology The 2 groups were comparable in terms of age, sex, aetiology of cirrhosis, Child‐Pugh classification |
|
| Interventions | TIPS: Wallstent endoprosthesis (Schneider Europe, Bulach, Switzerland) or Strecker stent (Meditech, Watertown, MA, USA) Sclerotherapy: intravariceal and paravariceal injections of 1% polidocanol (2 to 4 mL/injection, with total injection of sclerosant between 10 and 30 mL) with the use of videoendoscopy, weekly for the first month, and between 1 and 3 months thereafter until obliteration |
|
| Outcomes |
|
|
| Notes | Long‐term follow‐up; published as abstract in Hepato‐Gastroenterology 1998, Third International Congress of Hepato‐Pancreato‐Biliary Association Funding: supported in part by a grant from Fundacíon Universitaria de Las Palmas |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible. It is unclear if lack of blinding influenced the choice of treatments and the management of patients |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient Information. Judgement on all outcomes except mortality could be biased |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts are reported |
| Selective reporting (reporting bias) | Low risk | No pre‐published protocol Study authors report all key outcomes of interest in the review |
| Other bias | High risk | Large number of cross‐overs. Nine participants randomised to endoscopic intervention were treated by TIPS after rebleeding |
Isaksson 1995.
| Study characteristics | ||
| Methods | Randomised controlled trial comparing mesocaval shunt with endoscopic sclerotherapy for prevention of oesophageal variceal rebleeding Time from bleeding episode to randomisation and treatment: not specified (interval between index sclerotherapy and subsequent elective therapy was 78 days, not different from interval from index sclerotherapy to shunt surgery) Total number of patients evaluated: 228 Randomised to shunt surgery: 24; randomised to endoscopic sclerotherapy: 21 Adequate reasons provided for those not randomised: yes No patient was crossed over No losses to follow‐up Intention‐to‐treat analysis Follow‐up period in months (mean): shunt group 69.5, endoscopic therapy group 60.2 Assessment of suitability for shunt carried out before randomisation: not specified Method of Child's grading: Child's (version not specified) Method of encephalopathy testing: clinical and psychometric testing Rebleeding episodes endoscopically verified: yes Specified whether rebleeding episode clinically significant: yes Isaksson and colleagues were unable to assess encephalopathy in 7/24 patients in the shunt group and in 5/21 patients in the endoscopic therapy group |
|
| Participants | Inclusion criteria: age between 20 and 75 years, endoscopically verified oesophageal varices as the source of bleeding, portal hypertension, biopsy‐confirmed cirrhosis Exclusion criteria: not specified All patients underwent emergency endoscopy to verify bleeding oesophageal varices. Thereafter, patients were stabilised by vasopressin treatment and usually initial sclerotherapy; In some cases, balloon tamponade was applied Child‐Pugh class A/B/C (%): 15/56/29 Aetiology (%): alcohol 81 in shunt group, 67 in sclerotherapy group Participants in the two arms were comparable in terms of age and Child's status, but slightly more participants with alcohol abuse were included in the endoscopic sclerotherapy group. |
|
| Interventions | Shunt surgery: interposition polytetrafluoroethylene (Gore‐Tex, W.L. Gore & Associates Inc., Medical Products, Falgstaff, AZ, USA) 14‐mm mesocaval shunt Sclerotherapy: submucosal and paravariceal injections of 1% ethoxy‐sclerol (hydroxy‐poly‐etoxi‐dodecan), twice the first week, then once a month until most varices were eradicated |
|
| Outcomes |
|
|
| Notes | Funding: supported by grant number 4X‐9489 from the Swedish Medical Research Council | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | Closed envelopes; it is unknown if the envelopes were opaque and numbered |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible. It is unclear if lack of blinding influenced the choice of treatments and the management of patients |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient Information. Judgement on all outcomes except mortality could be biased |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No dropouts, but not all patients were fully assessed for hepatic encephalopathy (no information on 27% of participants) |
| Selective reporting (reporting bias) | Low risk | No pre‐published protocol. All key outcomes are reported |
| Other bias | Low risk | No additional sources of bias identified |
Rossle 1997.
| Study characteristics | ||
| Methods | Randomised controlled trial comparing transjugular intrahepatic portosystemic shunt with endoscopy + propranolol for prevention of variceal rebleeding Time from bleeding episode to randomisation in hours (SD): TIPS group 6.3 (5.5), endoscopic therapy + propranolol group 4.4 (5.0) Time from randomisation to treatment: 48 hours Total number of patients evaluated and found eligible: 126 (assessed patients: 190) Randomised to TIPS: 61; randomised to endoscopic therapy + propranolol: 65 Adequate reasons provided for those not randomised: not individually specified One patient in TIPS group and 3 in endoscopic therapy group were lost to follow‐up. Nine patients were crossed over from endoscopic therapy to TIPS during follow‐up Intention‐to‐treat analysis Follow‐up period in months (median, interquartile range): TIPS group 14, 8 to 23; endoscopic therapy + propranolol group 13, 8 to 25 Assessment of suitability for shunt carried out before randomisation: not mentioned Shunt patency assessed with Duplex ultrasound at 1, 3, 6, 9, and 12 months, then every 6 months Method of Child's grading: Child‐Pugh Method of encephalopathy testing: clinical testing, trail‐making test, mental state examination Rebleeding episodes endoscopically verified: yes Specified whether rebleeding episode clinically significant: yes |
|
| Participants | Inclusion criteria: liver cirrhosis, variceal bleeding (confirmed endoscopically) within 2 weeks before randomisation, age over 18 years Exclusion criteria: hepatic encephalopathy grades 3 and 4, liver insufficiency (bilirubin > 5 mg/dL ‐ except patients with primary biliary cirrhosis), cavernomatous portal vein thrombosis, advanced malignancy, contraindications to propranolol (severe heart insufficiency, obstructive lung disease, severe hypotension), bleeding emergency Patients with acute bleeding at admission were treated by injection of polidocanol (59 patients), a mixture of n‐butyl‐2‐cyanoacrilate and lipiodol (5 patients) or fibrin glue (4 patients), or polidocanol + bucrylate (8 patients) to stop the bleeding. In 6 patients in whom endoscopic treatment failed, a Sengkstaken tube was put in place Treatment was assigned to patients within 24 hours; treatment was started within 28 hours after admission Child‐Pugh class A/B/C (%): TIPS group 28/54/18, endoscopic group 34/48/18 Aetiology (%): alcohol 69 in TIPS group and 65 in endoscopic group; viral 18 in TIPS group and 15 in endoscopic group; alcohol + viral 5 in TIPS group and 6 in endoscopic group; miscellaneous 8 in TIPS group and 15 in endoscopic group Patients in the 2 groups were comparable |
|
| Interventions | TIPS: Palmaz stent (n = 39), Memotherm stent (n = 16), Wallstent (n = 6). In patients with huge varices or in whom variceal perfusion persisted after creation of the shunt, embolisation was done Endoscopic therapy: sclerotherapy with injections of polidocanol (16 mL per session) or banding ligation (3.2 rubber bands) at intervals of 2 to 5 days until eradication of the varices was achieved or at least 6 treatment sessions were applied. Gastric varices were treated by intravariceal injection of bucrylate/lipiodol Thirty‐three patients were treated with sclerotherapy only, 31 had a combination of sclerotherapy and banding ligation, and 1 patient had banding ligation only. Propranolol was given at a dose of 63 (SD 33) mg/d to decrease heart rate by 25%; propanolol was taken by 44 of the 65 patients who had endoscopic treatment (17 patients were not compliant, and in 4 patients, medication was withdrawn because of severe side effects) |
|
| Outcomes | Planned primary outcome was rebleeding from varices Secondary endpoints were death, bleeding from non‐variceal sources, procedure‐related complications, and hepatic encephalopathy. Hospital stay was reported |
|
| Notes | Funding: no information | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | Study authors stated that "study groups read by person not involved in the clinical setting. Randomization sequence could not be previewed" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible. It is unclear if lack of blinding influenced the choice of treatments and the management of patients |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding not mentioned. Judgement on all outcomes except mortality could be biased |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1/65 patients in the shunt group and 3/62 in the endoscopic group were lost to follow‐up (< 5% in both groups) Intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | No pre‐published protocol available All key outcomes are reported as defined in the methods |
| Other bias | High risk | Large number of cross‐over treatments: 9 out of 61 participants (14.7%) in the endoscopic group were crossed over to TIPS as rescue treatment |
Sauer 1997.
| Study characteristics | ||
| Methods | Randomised controlled trial comparing transjugular intrahepatic portosystemic shunt vs sclerotherapy + propranolol for variceal rebleeding Time from bleeding episode to randomisation in days (mean, SD): TIPS group 1.1, 1.1; endoscopic therapy + propranolol group 1.2, 0.9 Time from randomisation to treatment in days (mean, SD): TIPS group 3.4, 2.8; endoscopic therapy + propranolol group 2.9, 3.8 Total number of patients evaluated and found eligible: 83 (98 assessed for eligibility). Randomised to TIPS: 42; randomised to endoscopic therapy + propranolol: 41 Adequate reasons provided for those not randomised: yes No losses to follow‐up Five patients in endoscopic therapy + propranolol group were crossed over to TIPS during follow‐up Intention‐to‐treat analysis Method of Child's grading: Child‐Pugh Method of encephalopathy testing: clinical and trail‐marking tests Rebleeding episodes endoscopically verified: yes Specified whether rebleeding episode was clinically significant: no Median observation time in years: TIPS group 1.6, endoscopic therapy + propranolol group 1.45 Assessment of suitability for shunt carried out before randomisation: not mentioned Shunt patency assessed with duplex ultrasound and angiography at 3‐monthly intervals |
|
| Participants | Inclusion criteria: cirrhosis and acute oesophageal haemorrhage Exclusion criteria (≥ 1 of the following): gastric varices, prior endoscopic or surgical treatment of varices, portal venous thrombosis, neoplastic disease and/or severe comorbid conditions that would limit patient life span to < 6 months, septicaemia, uncontrolled bleeding requiring emergency TIPS procedure, contraindications for beta blockers (cardiovascular failure, respiratory failure, insulin‐dependent diabetes mellitus) Active bleeding was primarily treated by injection sclerotherapy. Initial success was defined as cessation of bleeding longer than 24 hours together with stable vital signs. Patients received a Sengstaken‐Blakemore tube if active bleeding persisted despite endoscopic treatment, or if there was evidence of bleeding within 24 hours after a bleeding‐free interval. When balloon tamponade failed to control bleeding, patients additionally received intravenous octreotide Randomisation was performed within 1 to 3 days after variceal bleeding which was controlled within 24 hours after admission Child‐Pugh score (mean ± SD): TIPS group 7.76 ± 2.29, sclerotherapy group 8.26 ± 2.46. Child‐Pugh class A/B/C (%): TIPS group 36/43/21, sclerotherapy group 29/44/27 Aetiology (%): alcohol 60 in TIPS group and 63 in sclerotherapy group; viral 31 in TIPS group and 24 in sclerotherapy group; others 9 in TIPS group and 12 in sclerotherapy group Both groups were comparable regarding patient data at entry (slightly younger patients in the TIPS group) |
|
| Interventions | TIPS: Palmaz stents Sclerotherapy + beta blocker: intravariceal and paravariceal injections of 5% ethanolamine oleate (2 to 3 mL/injection, with a total injection of sclerosant between 10 and 30 mL), at weekly intervals for the first month and between 1 and 3 months thereafter until obliteration. Propranolol was given twice daily at oral doses that reduced resting heart rate by 25% |
|
| Outcomes |
|
|
| Notes | Funding: no information | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasibIle. It is unclear if lack of blinding influenced the choice of treatments and the management of patients |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding not mentioned. Judgement on all outcomes except mortality could be biased |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All outcomes reported as defined in methods |
| Other bias | High risk | Large number of cross‐over treatments: 5 out of 41 participants were crossed over from endoscopic therapy to TIPS during follow‐up |
Sanyal 1997.
| Study characteristics | ||
| Methods | Randomised controlled trial comparing transjugular intrahepatic portosystemic shunt with endoscopic sclerotherapy for prevention of variceal rebleeding Time from bleeding episode to randomisation: clinical stability for at least 72 hours following a variceal bleed Time from randomisation to treatment: within 72 hours Total number of patients evaluated and found eligible: 80 (132 patients evaluated) Randomised to TIPS: 41, randomised to endoscopic therapy: 39 Adequate reasons provided for those not randomised: yes Two patients in TIPS group and 1 in endoscopic therapy group lost to follow‐up Six patients in endoscopic therapy group were crossed over to TIPS during follow‐up Five patients in the TIPS group and 3 in the endoscopic therapy group underwent liver transplantation, censored at the time of transplantation Intention to treat analysis Follow‐up period in days (median): TIPS 956, endoscopic therapy 990 Assessment of suitability for shunt carried out before randomisation: yes Shunt patency assessed with duplex ultrasound at 1 week, at 1 and 3 months, then every 3 months; angiography carried out at 6‐monthly intervals Method of Child's grading: Child‐Pugh Method of encephalopathy testing: not mentioned Rebleeding episodes endoscopically verified: yes Specified whether rebleeding episode clinically significant: yes |
|
| Participants | Inclusion criteria: clinical stability in the absence of rebleeding 72 hours following an oesophageal variceal bleed Exclusion criteria: portal venous thrombosis, hepatoma, end‐stage cancer or systemic disease that would limit the patient life span to less than 1 year, failure to obtain informed consent, pregnancy, history of non‐compliance with treatment Child‐Pugh class A/B/C (%): TIPS group 17/32/51, sclerotherapy group 15/38/47 Aetiology (%): alcohol 39 in TIPS group and 44 in sclerotherapy group; HCV 37 in TIPS group and 41 in sclerotherapy group; HBV 7 in TIPS group and 5 in sclerotherapy group; other 17 in TIPS group and 10 in sclerotherapy group Groups were similar with respect to patient characteristics at study entry |
|
| Interventions | TIPS: Wallstents (Schneider, Inc., Plymouth, MN, USA) Sclerotherapy: intravariceal injections of 5% sodium morrhuate, 12 to 20 mL per session, then every 2 to 3 weeks until varices obliteration Patients on beta blocker before randomisation were asked to stop taking it before study entry |
|
| Outcomes |
Planned outcomes Primary outcomes: rebleeding and survival. Secondary outcomes: complications (including encephalopathy) and rate of re‐hospitalisation |
|
| Notes | Funding: grant support in part by an award from the National Institutes of Health to the Clinical Research Centre at the Medical College of Virginia (RR‐00065) and by an award from the American College of Gastroenterology | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation code |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible It is unclear if lack of blinding influenced the choice of treatments and the management of patients |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding not mentioned. Judgement on all outcomes except mortality could be biased |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Two patients in TIPS group and 1 in endoscopic therapy group lost to follow‐up. Dropout < 5% |
| Selective reporting (reporting bias) | Low risk | No pre‐published protocol available All outcomes reported as defined in methods |
| Other bias | High risk | Large number of cross‐over treatments: 6 out of 30 (15%) participants in endoscopic group were crossed over to TIPS during follow‐up |
Jalan 1997.
| Study characteristics | ||
| Methods | Randomised controlled trial comparing transjugular intrahepatic portosystemic shunt with variceal band ligation for prevention of rebleeding from oesophageal varices Time from bleeding episode to randomisation (mean, SD): TIPS group 1.2, 0.3 days; endoscopic group 1.5, 0.5 days Time from randomisation to treatment in days (mean, SD): TIPS 2.2, 0.2; variceal banding 2.4, 0.2 Total number of patients evaluated and found eligible: 61 (105 evaluated) Randomised to TIPS: 31, randomised to endoscopic therapy: 27 Adequate reasons provided for those not randomised: yes Three patients in the TIPS group did not receive the allocated treatment; 6 patients in the endoscopic therapy group were crossed over to TIPS during follow‐up No losses to follow‐up Intention‐to‐treat analysis Follow‐up period months (mean, SD): TIPS 15.7, 10.2; endoscopic therapy 16.8, 10.9 Assessment of suitability for shunt carried out before randomisation: yes Shunt patency assessed with duplex ultrasonography and portography at 1 week and at 1 month, then every 6 months Method of Child's grading: Child‐Pugh Method of encephalopathy testing: Parson‐Smith criteria Rebleeding episodes endoscopically verified: yes Specified whether rebleeding episode clinically significant: yes |
|
| Participants | Inclusion criteria: all cirrhotic patients between 18 and 75 years of age who presented with a first (index) episode of oesophageal variceal bleeding Exclusion criteria: age < 18 years, rebleeding from varices within 24 hours of initial endoscopy, bleeding from ectopic varices, previous endoscopic treatment for variceal bleeding, hepatorenal failure, hepatic or extrahepatic malignancy, portal vein thrombosis, failure to give informed consent Patients with active bleeding underwent an upper gastrointestinal endoscopy within 6 hours of admission. If patients were bleeding actively from varices, endoscopic variceal sclerotherapy was performed; otherwise, they underwent variceal band ligation Patients were randomised 24 hours after their first endoscopic treatment if no further haemorrhage occurred Aetiology (%): alcohol 84 in TIPS group and 78 in endoscopic group; HCV/HBV 6 in TIPS group and 4 in endoscopic group; PBC 6 in TIPS group and 11 in endoscopic group; cryptogenetic 3 in TIPS group and 4 in endoscopic group Child‐Pugh class A/B/C (%): TIPS group 6/45/49, endoscopic group 18/33/49 Patient characteristics similar in the 2 groups |
|
| Interventions | TIPS: Wallstent Endoscopic therapy: variceal banding ligation, single application, every week until variceal eradication, then at 3 and 6 months, and at 6‐monthly intervals thereafter |
|
| Outcomes |
|
|
| Notes | Funding: no information | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes in batches of 25. It is unknown if the envelopes were also opaque and numbered |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible. It is unclear if lack of blinding influenced the choice of treatments and the management of patients |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding not mentioned. Judgement on all outcomes except mortality could be biased |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts. Similar length of follow‐up in both groups |
| Selective reporting (reporting bias) | Low risk | No pre‐published protocol available. All key outcomes of interest in the review are reported as planned in the methods section |
| Other bias | High risk | Large number of cross‐over treatments: 8 out of 27 participants (30%) in endoscopic group were crossed over to TIPS group for failure of treatment during follow‐up (7 participants) or 8 days after randomisation (1 participant) |
Cello 1997.
| Study characteristics | ||
| Methods | Randomised controlled trial comparing transjugular intrahepatic portosystemic shunt with endoscopic sclerotherapy after initial sclerotherapy in patients with acute large oesophageal variceal haemorrhage Time from admission to randomisation (mean, SE): TIPS 37.4, 4.7 hours, endoscopic therapy 35.4, 5.6 hours Time from randomisation to treatment (mean, SE): TIPS 59.5, 6.7 hours Total number of patients evaluated: 299. Randomised to TIPS 24; randomised to endoscopic therapy 25 Adequate reasons provided for those not randomised. Reasons mentioned but numbers not provided One patient assigned to TIPS received sclerotherapy because stent placement was technically impossible Six patients treated by sclerotherapy were crossed over to TIPS because of recurrent variceal haemorrhage that did not respond to sclerotherapy (failure of treatment) during follow‐up Follow‐up period in days (mean, SE): TIPS 575, 109; endoscopic therapy 567, 104 Assessment of suitability for shunt carried out before randomisation: yes Shunt patency assessed with duplex ultrasound Method of Child's grading: Child‐Pugh; however, patients were not stratified according to the Child‐Pugh system Method of encephalopathy testing: clinical Rebleeding episodes endoscopically verified: yes Specified whether rebleeding episode clinically significant: yes |
|
| Participants | Inclusion criteria: all patients with massive or submassive acute gastrointestinal tract haemorrhage from large oesophageal varices were admitted Exclusion criteria (≥ 1 of the following): prisoners, < 18 or > 75 years of age, cerebrovascular accident 3 months before the onset of bleeding, refusal to accept blood products, gastric variceal haemorrhage, ECG changes compatible with myocardial infarction, PO2 < 70 mmHg or arterial pH ≤ 7.20 on room air at the time of evaluation for eligibility, serum creatinine ≥ micromol/L, serum bilirubin ≥ 7 mg/dL, prothrombin time at least 5 seconds longer than control, platelet count < 50 × 10⁹/L, grade IV encephalopathy, cancer other than skin cancer, AIDS or advanced AIDS complex, sepsis, pneumonia, peritonitis, alcoholic hepatitis (clinical evidence only), thrombosis of portal, hepatic, or inferior vena caval veins All patients received endoscopic sclerotherapy at the time of the initial endoscopic procedure that established the source of haemorrhage as oesophageal varices Active haemorrhage at randomisation (%): TIPS group 21, sclerotherapy group 28. Shock (systolic blood pressure ≤ 80 mmHg) (%): TIPS group 4, sclerotherapy group 20 Child‐Pugh score (mean ± SE): TIPS group 9.0 ± 0.4, sclerotherapy group 7.8 ± 0.5 Aetiology (%): alcoholism 67 in TIPS group and 68 in sclerotherapy group Patients across the 2 strata were comparable in terms of clinical and laboratory variables, except for Child‐Pugh score and blood transfusion requirements, which were higher in the TIPS group than in the sclerotherapy group |
|
| Interventions | TIPS: Wall stent (Schneider, Inc., Minneapolis, MN, USA) within 48 hours of randomisation Sclerotherapy: injection of 0.5 to 2.0 mL ethanolamine oleate solution per varix. Sclerotherapy was repeated every 2 to 7 days during initial hospitalisation and weekly after discharge |
|
| Outcomes | Study authors planned to analyse:
|
|
| Notes | Grant support: in part by National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases Liver Core Center grant P30 DK26743 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Low risk | Serially numbered, sealed, opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible. It is unclear if lack of blinding influenced the choice of treatments and the management of patients |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding not mentioned. Judgement on all outcomes except mortality could be biased |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Intention‐to‐treat. Similar length of follow‐up in both groups No information on dropouts |
| Selective reporting (reporting bias) | High risk | Not all key outcomes are reported (no chronic hepatic encephalopathy) |
| Other bias | High risk | Large number of cross‐over treatments: 6 out of 25 (24%) participants treated by sclerotherapy were crossed over to TIPS because of recurrent variceal haemorrhage that did not respond to sclerotherapy (failure of treatment) |
Merli 1998.
| Study characteristics | ||
| Methods | Randomised controlled trial comparing transjugular portosystemic shunt vs endoscopic sclerotherapy for prevention of recurrent variceal bleeding in cirrhotic patients
Time from bleeding episode to randomisation: patients were randomised to 3 separate strata according to the interval between bleeding and the time of randomisation: (I) 1 to 7 days, (II)1 to 6 weeks, (III) 7 weeks to 6 months
Time from randomisation to treatment: active bleeding had to have been controlled for a minimum of 24 hours Time between index bleeding and randomisation in days (mean, SE): stratum I: TIPS group 3.5, 0.5 vs sclerotherapy group 3.1, 0.5; stratum II: TIPS group 21, 3.4 vs sclerotherapy group 19, 2.8; stratum III: TIPS group 63, 16 vs sclerotherapy group 65, 8 Total number of patients evaluated and found eligible: 82 (120 evaluated) Randomised to TIPS: 39 patients; randomised to endoscopic therapy: 43 patients. One patient in the TIPS group was erroneously randomised (bleeding had not stopped before randomisation) Adequate reasons provided for those not randomised: yes Five patients in TIPS group and 4 patients in endoscopic therapy group did not receive allocated treatment: 1 participant in each group refused treatment, 1 in TIPS group and 2 in endoscopic group died before treatment was provided after randomisation; 3 in TIPS group and 1 in sclerotherapy group could not be treated for technical reasons One patient in each group lost to follow‐up In the TIPS group, 2 participants were crossed over to endoscopic therapy and 1 to portacaval shunt. During follow‐up for treatment failure In the endoscopic group, 6 participants were crossed over to TIPS and 1 to portacaval shunt during follow‐up for treatment failure Intention‐to‐treat analysis but 1 patient erroneously assigned to TIPS and excluded from analysis Follow‐up period in weeks (mean, SE): TIPS 73.9, 7.3; endoscopic therapy 77.7, 7.12 Assessment of suitability for shunt carried out before randomisation: yes Shunt patency assessed with duplex ultrasound at 6 months, or when shunt malfunction was suspected Method of Child's grading: Child‐Pugh Method of encephalopathy testing: Parsons‐Smith criteria Rebleeding episodes endoscopically verified: yes Specified whether rebleeding episode clinically significant: yes |
|
| Participants | Inclusion criteria: cirrhotic patients with active or recent (< 24 weeks) oesophageal variceal bleeding (proven or presumed) Exclusion criteria: complete portal vein thrombosis, previous episode(s) of chronic recurrent hepatic encephalopathy, advanced hepatocellular carcinoma, previous multiple sessions of sclerotherapy, ongoing pharmacological prophylaxis of rebleeding (1 emergency session during acute bleeding phase was permissible), severe cardiovascular contraindications, concomitant morbid condition(s) with life expectancy < 1 year Patients admitted for active bleeding were included only after bleeding had stopped for at least 24 hours, and when they were haemodynamically stable Aetiology (%): alcohol 16 in TIPS group and 35 in sclerotherapy group; alcohol + HCV 5 in TIPS group and 5 in sclerotherapy group; alcohol + HBV 3 in TIPS group and 0 in sclerotherapy group; viral (HBV or HCV or HBV + HCV) 71 in TIPS group and 44 in sclerotherapy group Child‐Pugh class A/B/C (%): TIPS group 34/53/13, sclerotherapy group 30/58/12 Patient characteristics comparable other than for alcoholics, which were more numerous in the endoscopic group |
|
| Interventions | TIPS: Wallstent (Schneider Europe AG, Zurich, Switzerland) or Nitinol Strecker stent (Ultraflex Biliry Stent System, Meditech, Boston Scientific Co., Natick, MA, USA) Endoscopic sclerotherapy: 1% to 2% polidocanol, every 7 to 10 days until eradication |
|
| Outcomes |
|
|
| Notes | Funding: no information | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes. It is unknown if the envelopes were also opaque and numbered |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible. It is unclear if lack of blinding influenced the choice of treatments and the management of patients |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding not mentioned. Judgement on all outcomes except mortality could be biased |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Small number of dropouts: 1 patient lost to follow‐up in each group |
| Selective reporting (reporting bias) | Low risk | No published protocol available All key outcomes of interest in the review were planned and reported |
| Other bias | High risk | Large number of cross‐over treatments during follow‐up for treatment failure. In the TIPS group, 3 out of 31 (9.6%) participants were crossed over to alternative treatment (2 to endoscopic therapy and 1 to portacaval shunt). In the endoscopic group, 7 out of 43 (16%) participants were crossed over (6 to TIPS and 1 to portacaval shunt) |
Narahara 2001.
| Study characteristics | ||
| Methods | Randomised controlled trial comparing transjugular intrahepatic portosystemic shunt with endoscopic sclerotherapy for long‐term management of patients with cirrhosis after recent variceal haemorrhage Time from bleeding episode to randomisation in days (mean, SEM): TIPS 19.1, 2; endoscopic therapy 17.9, 1.9 Time from randomisation to treatment (mean, SD): not clear Total number of patients evaluated: 101 Randomised to TIPS 38; randomised to endoscopic therapy 40 Adequate reasons provided for those not randomised: yes One patient in the TIPS group had to be treated by endoscopic sclerotherapy during follow‐up. Two patients in endoscopic therapy group were crossed over to TIPS during follow‐up Follow‐up period in days (mean, SEM): TIPS 1116, 92; endoscopic therapy 1047, 102 Assessment of suitability for shunt carried out before randomisation: no Shunt patency assessed with duplex ultrasonography every 3 months Method of Child's grading: Child‐Pugh; however, patients were not stratified according to the Child‐Pugh system Method of encephalopathy testing: Parson‐Smith criteria Rebleeding episodes endoscopically verified: yes Specified whether rebleeding episode clinically significant: not stated |
|
| Participants | Inclusion criteria: cirrhosis with recent variceal haemorrhage, clinical stability at randomisation (no hematemesis and melena, stable haemoglobin concentration, and no need for transfusion for at least 5 days), age between 20 and 69 years Exclusion criteria: hepatocellular carcinoma, episodes of chronic encephalopathy, complete portal vein thrombosis with cavernomatous transformation, Child‐Pugh ≥ 13, serum creatinine ≥ 2.5 mg/dL, serum bilirubin ≥ 5 mg/dL, active infection, severe cardiopulmonary disease Active bleeding was treated with intravenous vasopressin + nitroglycerin infusion and/or a single session of endoscopic sclerotherapy or endoscopic variceal ligation. Balloon tamponade with a Sengstaken‐Blackemore tube was used when necessary Aetiology (%): alcohol 24 in TIPS group and 42 in sclerotherapy group; HCV 53 in TIPS group and 40 in sclerotherapy group; HBV 10 in TIPS group and 10 in sclerotherapy group Child‐Pugh score (mean ± SEM): TIPS group 6.8 ± 0.3, sclerotherapy group 7.4 ± 0.3 |
|
| Interventions | TIPS: Gianturco‐Rösch biliary expandable Z‐stents (Cook) in 21 patients, Spiral‐Z stents (Cook) in 4 patients, Wallstents (Schneider, Inc., Plymouth, MN, USA) in 13 patients with final stent diameters of 8 and 10 mm Endoscopic therapy: sclerotherapy using 5% ethanolamine oleate, repeated weekly until varices eradication |
|
| Outcomes | Planned outcomes
|
|
| Notes | Funding: no information | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible. It is unclear if lack of blinding influenced the choice of treatments and the management of patients |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding not mentioned. Judgement on all outcomes except mortality could be biased |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | No published protocol was available. Study authors reported all planned key outcomes of interest for this review |
| Other bias | Low risk | Small number of participants (< 5%) were crossed over during follow‐up for treatment failure: 1 out of 38 participants in the TIPS group was treated by endoscopic sclerotherapy during follow‐up, 2 out of 40 participants in the endoscopic therapy group were crossed over to TIPS |
Pomier‐Layrargues 2001.
| Study characteristics | ||
| Methods | Randomised controlled trial comparing transjugular intrahepatic portosystemic shunt with endoscopic variceal ligation for prevention of variceal rebleeding in patients with cirrhosis Time from bleeding to randomisation (hours): TIPS: 44, endoscopic therapy: 42 Time from randomisation to TIPS procedure (mean, SD): 13, 11 hours 158 patients evaluated, reasons provided for those excluded: yes Patients randomised to TIPS 41; patients randomised to endoscopic therapy 39 Follow‐up period in days (mean): TIPS 678, endoscopic therapy 581 Assessment of suitability for shunt carried out before randomisation: yes Shunt patency assessed with duplex doppler ultrasonography at 24 hours, then 3‐monthly Two patients in the TIPS group and 4 in the endoscopic therapy group underwent liver transplantation. Eight patients were crossed over from endoscopic therapy to TIPS for uncontrolled rebleeding Intention‐to‐treat analysis Method of Child's grading: Child‐Pugh Method of encephalopathy testing: clinical Rebleeding episodes endoscopically verified: yes Specified whether clinically significant: yes |
|
| Participants | Inclusion criteria: cirrhosis (according to clinical findings, laboratory data, ultrasonography, and/or liver biopsy), Child‐Pugh score 7 to 12 (TIPS 9.6, endoscopy 9.8), age between 18 and 75 years, episode of variceal bleeding demonstrated by upper gastrointestinal endoscopy performed within 72 hours of the bleeding episode Exclusion criteria: portal vein thrombosis, previous endoscopic therapy within 3 months, previous shunt surgery, bleeding from large fundal varices, hepatocellular carcinoma, cardiac failure, renal failure, respiratory failure, non‐compliance, sepsis, uncontrolled bleeding, wait list for emergency liver transplantation, neurologic comorbidities, anticoagulation, extrahepatic biliary obstruction, refusal to participate Initial control of bleeding episode was obtained by 1 session of endoscopic ligation or sclerotherapy and/or balloon tamponade and/or octreotide infusion Randomisation was carried out only after the initial haemorrhagic episode had been controlled and haemodynamic status had been stable for at least 24 hours Severity of index bleed and clinical and biochemical parameters were non‐significantly different in the 2 groups with the exception of serum bilirubin level, which was significantly higher in the ligation group Aetiology (%): alcohol 61 in TIPS group and 61 in endoscopic group; HCV 2 in TIPS group and 8 in endoscopic group; HBV 7 in TIPS group and 5 in endoscopic group; PBC 2 in TIPS group and 8 in endoscopic group; sclerosing cholangitis 7 in TIPS group and 3 in endoscopic group; haemochromatosis 12 in TIPS group and 3 in endoscopic group; cryptogenetic 7 in TIPS group and 10 in endoscopic group |
|
| Interventions | TIPS: type not specified Endoscopic therapy: variceal band ligation on days 1 and 10, then every 3 to 4 weeks until obliteration |
|
| Outcomes |
|
|
| Notes | (P‐Layrargues 1997 represents duplicate publication of P‐Layragues 2001) Funding: supported in part by a grant from the Medical Research Council of Canada (UI 11508) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes. It is unknown if the envelopes were also opaque and numbered |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible. It is unclear if lack of blinding influenced the choice of treatments and the management of patients |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding not mentioned. Judgement on all outcomes except mortality could be biased |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information on dropouts |
| Selective reporting (reporting bias) | High risk | No pre‐published protocol. Not all key outcomes of interest for the review are reported |
| Other bias | High risk | Large number of cross‐over treatments: 8 out of 39 (20%) participants were crossed over from endoscopic therapy to TIPS for uncontrolled rebleeding |
Gülberg 2002.
| Study characteristics | ||
| Methods | Randomised controlled trial comparing transjugular intrahepatic portosystemic shunt with endoscopic variceal band ligation for prevention of variceal rebleeding in cirrhotic patients Time from bleeding episode to randomisation in days (mean, SE): TIPS group 13, 3; endoscopic therapy group 14, 3 Time from randomisation to treatment: unclear Total number of patients evaluated: 86 Randomised to TIPS: 28; randomised to endoscopic therapy: 26 Adequate reasons provided for those not randomised: yes Two patients in TIPS group did not receive the allocated treatment (TIPS not feasible); 1 of them was treated endoscopically; in the other, band ligation failed to prevent early rebleeding and the patient underwent shunt surgery. Four patients in endoscopic therapy group were crossed over to TIPS Follow‐up period in years (median): TIPS group 1.8; endoscopic therapy group 2.0 Assessment of suitability for shunt carried out before randomisation: yes Shunt patency assessed with duplex ultrasound Method of Child's grading: Child‐Pugh (patients were stratified according to the Child‐Pugh system) Method of encephalopathy testing: clinical Rebleeding episodes endoscopically verified: yes Specified whether rebleeding episode clinically significant: yes |
|
| Participants | Inclusion criteria: age > 18 years, endoscopic evidence of oesophageal variceal bleeding within 2 months before randomisation, stable haemodynamic condition, no transfusions during the preceding 24 hours Exclusion criteria: isolated gastric varices, index bleeding from gastric varices, large or diffuse liver tumours, hepatocellular carcinoma, liver transplantation intended in 6 months, hepatic encephalopathy ≥ grade 2 before the bleeding episode, severely compromised liver function (Child Pugh score ≥ 13 or bilirubin > 5 mg/dL with the exception of cholestatic liver disease, where a prothrombin index > 40% and serum albumin > 2.8 mg/dL were required for inclusion in the study), extrahepatic cholestasis, heart failure NYHA III or IV, sepsis, multi‐organ failure, anticipation of technical contraindications to one of the procedures before randomisation Child‐Pugh class A/B/C (%): TIPS group 39/55/7, endoscopic group 38/46/16 Aetiology (%): alcohol 76 in TIPS group and 89 in endoscopic group; viral 11 in TIPS group and 11 in endoscopic group; other 13 in TIPS group and 0 in endoscopic group |
|
| Interventions | TIPS: expandable 8 to 10 mm stents Endoscopic therapy: variceal band ligation, every week The 2 groups were comparable regarding baseline characteristics except for number of previous bleeding episodes, which was higher in the TIPS group |
|
| Outcomes | Preplanned outcomes
|
|
| Notes | Funding: no information | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes. It is unknown if the envelopes were also opaque and numbered |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible. It is unclear if lack of blinding influenced the choice of treatments and the management of patients |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding not mentioned. Judgement on all outcomes except mortality could be biased |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis. Reasons given for protocol violations. All patients included in analysis |
| Selective reporting (reporting bias) | Unclear risk | No pre‐published protocol. All planned primary and secondary outcomes plus procedure‐related complications are reported. Liver‐related complications are not reported |
| Other bias | High risk | Large number of cross‐over treatments: 4 out of 26 (15.3%) participants in endoscopic therapy group were crossed over to TIPS: 1 participant before the first ligation as an emergency procedure, 2 for recurrent bleeding, the fourth for perforation of the oesophagus |
Sauer 2002.
| Study characteristics | ||
| Methods | Randomised controlled trial comparing transjugular intrahepatic portosystemic shunt with endoscopic variceal ligation + propranolol for prevention of recurrent variceal bleeding Time from bleeding episode to randomisation in days, mean (SD): TIPS group 1.2 (1.3), endoscopic group + propranolol 1.3 (0.9) Time from randomisation to treatment in days, mean (SD): TIPS group 3.1 (2.1), endoscopic group + propranolol 2.4 (1.8) Total number of patients evaluated and found eligible: 85 (assessed for eligibility 112) Randomised to TIPS: 43 patients; randomised to endoscopic therapy + propranolol: 42 patients Adequate reasons provided for those not randomised: yes One patient in the shunt group and 2 in the endoscopic group were lost to follow‐up. Data were censored at time of last examination One patient in the TIPS group was crossed over to endoscopic therapy + propranolol. Three patients in the endoscopic therapy group were crossed over to TIPS during follow‐up Intention‐to‐treat analysis Assessment of suitability for shunt carried out before randomisation: no Shunt patency assessed with duplex ultrasound or angiography at 3‐monthly intervals Method of Child's grading: Child‐Pugh Method of encephalopathy testing: clinical Rebleeding episodes endoscopically verified: yes Specified whether rebleeding episode clinically significant: yes Mean (SD) observation time in years: TIPS 4.1 (0.26), endoscopic therapy + propranolol 3.6 (0.25) Assessment of suitability for shunt carried out before randomisation: not mentioned |
|
| Participants | Inclusion criteria: cirrhosis and acute first oesophageal haemorrhage controlled within 24 hours after admission Exclusion criteria: gastric varices, prior endoscopic or surgical treatment of varices, portal vein thrombosis, neoplastic disease and/or systemic disease that would limit the patient's life span to less than 6 months, hepatic encephalopathy grade 3 or 4, septicaemia, uncontrolled bleeding that required an emergency TIPS procedure, contraindications for propranolol (such as severe heart insufficiency, obstructive pulmonary disease, severe hypotension) Active bleeding was primarily treated by sclerotherapy. Control of bleeding was defined as cessation of bleeding for longer than 24 hours. If active bleeding persisted despite endoscopic treatment, patients received a Sengstaken‐Blakemore tube and/or intravenous octreotide The accepted interval after control of variceal bleeding was up to 3 days Both groups were comparable regarding clinical characteristics Child‐Pugh score (mean ± SD): TIPS group 7.9 ± 2.1, endoscopic group 8.2 ± 2.0. Child‐Pugh class A/B/C (%): TIPS group 35/37/28, endoscopic group 24/45/31 Aetiology (%): alcohol 67 in TIPS group and 57 in endoscopic group; viral 21 in TIPS group and 29 in endoscopic group; others 12 in TIPS group and 14 in endoscopic group |
|
| Interventions | TIPS: Palmaz stents (Johnson and Johnson Interventional Systems, Warren, NJ, USA) or Wallstents (Schneider, Minneapolis, MN, USA) Endoscopic therapy: variceal band ligation at intervals of 1 to 2 weeks until disappearance of varices + propranolol twice daily at oral doses that reduced the resting heart rate by approximately 25% |
|
| Outcomes | Pre‐planned outcomes:
|
|
| Notes | Personal communication with Peter Sauer via email 14/03/2016 Funding: no information |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible. It is unclear if lack of blinding influenced the choice of treatments and the management of patients |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding not mentioned. Judgement on all outcomes except mortality could be biased |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis. Mean follow‐up 4.1 years for TIPS and 3.64 for endoscopic band ligation. One patient in shunt group and 2 in endoscopic band ligation group lost to follow‐up, with censoring appropriate. Data were censored at the time of last examination |
| Selective reporting (reporting bias) | Unclear risk | All primary and secondary outcomes plus procedure‐related complications are reported. Liver‐related complications are not reported |
| Other bias | Unclear risk | One out of 43 (2.5%) participants in TIPS group were crossed over to endoscopic therapy + propranolol. Three out of 42 (7%) participants in endoscopic therapy group were crossed over to TIPS during follow‐up. It is not clear if the data were censored at the time of cross‐over. The total number of participants who switched was low, but in the single arm of endoscopy, the number was high |
Lo 2007.
| Study characteristics | ||
| Methods | Randomised controlled trial of transjugular intrahepatic portosystemic shunt vs cyanoacrylate injection for prevention of gastric variceal rebleeding Time from bleeding episode to randomisation in days: once bleeding had been controlled for 3 days Time from randomisation to treatment in days (SD): not mentioned Total number of patients evaluated and found eligible: 77 (460 assessed for eligibility), 3 patients eligible but not randomised (refusal to participate) Randomisation: 37 randomised to TIPS, 37 randomised to endoscopic therapy; 2 randomised to TIPS refused allocated intervention ‐ not included in evaluation Loss to follow‐up: 1 patient in TIPS group, 6 patients in endoscopic therapy group. Telephone contact maintained, included in analysis Assessment of suitability for shunt carried out before randomisation: not mentioned Shunt patency assessed using doppler ultrasound before discharge and every 3 months, or when clinically indicated Method of Child's grading: Child‐Pugh score Method of encephalopathy testing: clinical (altered consciousness and elevated arterial ammonia levels requiring treatment) Rebleeding episodes endoscopically verified: yes Specified whether rebleeding episode clinically significant: yes Median observation time in months: TIPS 33 (range 3 to 46), endoscopic therapy 32 (range 1 to 50) Note: time to rebleeding was presented for both gastric variceal rebleeding and upper gastrointestinal rebleeding. Gastric variceal rebleeding was used |
|
| Participants | Inclusion criteria: liver cirrhosis (diagnosis based on liver biopsy or clinical, laboratory, and imaging studies); acute gastric variceal bleeding with haematemesis or melaena (or both) and fall in haemoglobin level Exclusion criteria: age < 20; age > 75; acute bleeding from oesophageal varices; serum bilirubin > 10 mg/dL; hepatic encephalopathy; hepatocellular carcinoma, uraemia or other debilitating disease; previous specific treatment of gastric varices; uncontrolled acute gastric variceal bleeding; portal vein thrombosis; pregnancy; refusal to participate; death within 72 hours of admission Child‐Pugh score (mean ± SD): TIPS group 7.8 ± 1.8, endoscopic group 7.6 ± 1.7. Child‐Pugh class A/B/C (%): TIPS group 26/57/17, endoscopic group 32/51/17 Aetiology (%): alcohol 11 in TIPS group and 22 in endoscopic group; HBV 34 in TIPS group and 32 in endoscopic group; HCV 37 in TIPS group and 30 in endoscopic group; HBV + HCV 14 in TIPS group and 8 in endoscopic group; cryptogenetic 3 in TIPS group and 8 in endoscopic group Patients suspected of gastroesophageal variceal bleeding received somatostatin. Endoscopy was performed within 24 hours of admission, and cyanoacrylate glue injection was instituted when acute gastric variceal bleeding was noted. Randomisation was performed after acute gastric variceal bleeding had been controlled for 3 days |
|
| Interventions | TIPS: metallic endoprosthesis 10 × 68 to 91 mm (Wallstent, Boston Scientific, Galway, Ireland) Endoscopic therapy: n‐butyl‐2‐cyanoacrylate injection (Histoacril; B. Braun, Melsungen AG, Germany) mixed with lipiodol (Lipiodol Ultra‐Fluid, Guerbet, Aulnay sos Bois, France), followed by banding if concomitant prominent oesophageal varices. Endoscopic obturation was performed at intervals of 4 weeks until obliteration |
|
| Outcomes | Pre‐planned outcomes
|
|
| Notes | Funding: supported by internal hospital grant (VGHKS 90‐14) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of random numbers. A study nurse generated the allocation sequence |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible. It is unclear if lack of blinding influenced the choice of treatments and the management of patients |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The doctors who evaluated outcomes were blinded to group assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Six participants in the endoscopic group and 1 in the TIPS group were lost to‐follow‐up (included in analysis; telephone contact maintained). The 2 participants randomised to TIPS who refused the allocated intervention were not included in the evaluation |
| Selective reporting (reporting bias) | Low risk | No pre‐published protocol All key primary and secondary outcomes were reported |
| Other bias | Low risk | No additional sources of bias identified |
Ferlitsch 2012.
| Study characteristics | ||
| Methods | Randomised controlled trial comparing transjugular intrahepatic portosystemic shunt with endoscopic band ligation in cirrhotic patients with recurrent variceal bleeding not responding to pharmacological therapy Time from bleeding episode to randomisation: median to inclusion 20 days in clinical non‐responders, 87 days in HVPG non‐responders Time from randomisation to treatment: not reported Total number of patients evaluated: not reported Randomised to TIPS: 21; randomised to endoscopic + medical therapy: 19 For 1 participant, TIPS placement was impossible and endoscopic treatment was applied Two participants were crossed over to endoscopic + medical therapy group for rebleeding from stent thrombosis 3 months after TIPS implantation (1) and for encephalopathy that required TIPS occlusion during follow‐up. Three participants in endoscopic + medical therapy group were crossed over to TIPS during follow‐up (1 participant for rebleeding and 2 for ascites) Intention‐to‐treat analysis Rebleeding episodes endoscopically verified: unclear Specified whether rebleeding episode clinically significant: not mentioned Median follow‐up: 30 months (95% CI 20 to 40) Assessment of suitability for shunt carried out before randomisation: not mentioned Shunt patency assessment: not mentioned Method of Cirrhosis grading: Child‐Pugh Method of encephalopathy testing: not mentioned |
|
| Participants | Inclusion criteria: rebleeding from oesophageal varices under sufficient pharmacological therapy (clinical non‐responders); recurrent variceal bleeding as hepatic venous pressure gradient (HVPG) in non‐responders to minimum of 80 mg propranolol and 40 mg isosorbide mononitrate (ISMN) (HVPG non‐responders) In the pre‐published protocol: NCT00570973 Inclusion criteria: age ≥ 18 years, liver cirrhosis, bleeding from oesophageal varices within last 6 months, sufficient medical therapy (≥ 80 mg propranolol per day), signed written informed consent Exclusion criteria: bleeding of gastric varices, poral vein thrombosis, insufficient medical therapy (< 80 mg propranolol per day), exclusion criteria for TIPS/band ligation (anatomy; impaired coagulation parameters; severe encephalopathy; severe liver failure (bilirubin > 10 mg/dL); congestive heart failure; pulmonary hypertension; polycystic liver disease; presence or suspicion of active systemic, biliary, or ascitic fluid infection; known cavernous portal vein occlusion; Budd‐Chiari syndrome Child‐Pugh score and class not reported Aetiology not reported |
|
| Interventions | TIPS: PTFE‐coated stent Endoscopic band ligation repeated until eradication of varices + continuation of medical therapy |
|
| Outcomes |
Pre‐published protocol Primary outcome: variceal rebleeding (time frame: 2 years) Secondary outcome: survival (time frame: 2 years) Study authors report:
|
|
| Notes | Clinicaltrials.gov NCT 00570973 No study results posted on ClinicalTrials.gov Study start date: November 2004 Abstract only Funding: no information ‐ trials registry states sponsor is University of Vienna |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible. It is unclear if lack of blinding influenced the choice of treatments and the management of patients |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding not mentioned. Judgement on all outcomes except mortality could be biased |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information |
| Selective reporting (reporting bias) | Unclear risk | Pre‐published protocol. All planned outcomes reported. Complications of interest for the review not reported |
| Other bias | High risk | Abstract only Large number of participants were crossed over during follow‐up. Three out of 21 (14%) participants in TIPS group were crossed over to endoscopic + medical therapy group (reasons given). Three out of 19 (15.7%) participants in endoscopic + medical therapy group were crossed over to TIPS group (reasons given). It is not reported when the data were censored |
Luo 2015.
| Study characteristics | ||
| Methods | Randomised controlled trial comparing transjugular portosystemic shunt with or without variceal embolisation with endoscopic band ligation + propranolol for prevention of oesophageal variceal rebleeding in patients with advanced cirrhosis and portal vein thrombosis Time from bleeding episode to randomisation: unclear Time from randomisation to treatment: unclear Total number of patients evaluated and found eligible: 73 (114 assessed for eligibility) Randomised to TIPS: 37; randomised to endoscopic therapy + propranolol: 36 Nine participants in endoscopic therapy + propranolol group were crossed over to TIPS during follow‐up One participant in TIPS group and 2 in endoscopic therapy + propranolol group lost to follow‐up Intention‐to‐treat analysis Follow‐up period in months (mean ± SD): TIPS 22.8 ± 7.7, endoscopic therapy + propranolol 20.9 ± 8.9 Assessment of suitability for shunt carried out before randomisation: unclear Shunt patency assessment: angiography (direct portography) Unclear whether routine assessment of shunt patency was undertaken Method of Child's grading: Child‐Pugh Method of encephalopathy testing: not specified Rebleeding episodes endoscopically verified: ? yes ‐ variceal bleeding specified Specified whether rebleeding episode clinically significant: not specified |
|
| Participants | Inclusion criteria: advanced cirrhosis (Child‐Pugh score 7 to 13), portal vein thrombosis, age 18 to 70 years, previous episode of variceal bleeding Exclusion criteria: portal vein thrombosis ≤ 25% within vessel lumen, limited thrombosis within intrahepatic portal branch, portal cavernoma, gastric varices, hepatocellular carcinoma, previous endoscopic treatment of varices within 3 months, contraindications to TIPS, endoscopic band ligation or propranolol Child‐Pugh class B/C (%): TIPS group 68/32, endoscopic group 67/33. Child‐Pugh score (mean ± SD): TIPS group 8.76 ± 1.70, endoscopic group 8.89 ± 1.77. MELD score (mean ± SD): TIPS group 14.2 ± 6.5, endoscopic group 15.9 ± 5.7 Aetiology (%): HBV 73 in TIPS group and 67 in endoscopic group; HCV 8 in TIPS group and 6 in endoscopic group; alcohol 5 in TIPS group and 11 in endoscopic group; other 13 in TIPS group and 17 in endoscopic group |
|
| Interventions | TIPS: polytetrafluoroethylene‐covered stents (Fluency; C.R. Bard, Murray Hill, NJ, USA) with embolisation with coils of portosystemic collateral veins if observed at post‐TIPS portography. Warfarin was administered for 6 months after recanalisation of the portal venous system, with a target international normalised ratio of 2 to 3 Endoscopic therapy: band ligation every 4 to 6 weeks until varices were eradicated + propranolol (starting dose 20 mg/d, with increase by 20 to 40 mg/d every week, either until reduction of resting heart rate of 25% was achieved or up to the maximum dose was tolerated). Immediately after variceal eradication, warfarin was prescribed and was continued for an additional 6 months after recanalisation of the portal venous system, with a target international normalised ratio of 2 to 3 |
|
| Outcomes | Pre‐planned outcomes
|
|
| Notes | Randomised clinical trial registered in the Chinese Clinical Trial Registry: ChiCTR‐TRC‐11001577 Funding: no information |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Consecutive numbers generated by computer |
| Allocation concealment (selection bias) | Low risk | Allocated random digit numbers |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible. It is unclear if lack of blinding influenced the choice of treatments and the management of patients |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding not mentioned. Judgement on all outcomes except mortality could be biased |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 73 participants randomised; 3 lost to‐follow‐up after a mean of 7 months (1 in TIPS group, 2 in endoscopic band ligation group) |
| Selective reporting (reporting bias) | High risk | Trial registered (ChiCTR‐TRC‐11001577). All primary and secondary outcomes reported as pre‐specified Complications are not pre‐defined. Study authors reported only major complications for TIPS, while reporting minor complications for endoscopic treatment + propranolol |
| Other bias | High risk | The number of participants who were crossed over during follow‐up is high: 9 out of 37 (25%) participants in endoscopic therapy + propranolol group were crossed over to TIPS. It is not clear if the data are censored |
Holster 2016.
| Study characteristics | ||
| Methods | Randomised controlled trial comparing covered transjugular intrahepatic portosystemic shunt vs endoscopic therapy + beta blocker for prevention of variceal rebleeding Time from bleeding episode to randomisation: median 4 days (IQR 1 to 7) Time from randomisation to treatment: median time from bleeding to TIPS 6 days (IQR 3 to 9) Total number of patients evaluated and found eligible: 72 (174 patients were admitted for acute variceal bleeding and evaluated: 40 did not fulfil the inclusion criteria; 62 met 1 or more exclusion criteria; of these, 24 were unable/unwilling to give informed consent) Randomised to TIPS 37; randomised to endoscopic therapy + beta blocker 35 Four patients randomised to TIPS were treated by endoscopic therapy + beta blocker (advanced HCC diagnosed after randomisation; technical infeasibility due to Budd‐Chiari syndrome; peri‐procedural ventricular fibrillation). Two participants were crossed over to endoscopy because of closure of TIPS due to severe untreatable hepatic encephalopathy. Six patients randomised to endoscopic therapy + beta blocker were crossed over to TIPS (recurrent/uncontrollable variceal rebleeding; refractory ascites) Two patients in the TIPS group and 4 in the endoscopic therapy + beta blocker group were lost to follow‐up. Twelve patients were censored due to liver transplantation Note that original sample size was calculated at 124 patients; study authors stated that due to a greater than expected benefit of early TIPS in rebleeding, the sample size was reduced to 72 patients Intention‐to‐treat analysis and per‐protocol presented Follow‐up period in months (median): 23.4 months (IQR 6.9 to 38.5) Assessment of suitability for shunt carried out before randomisation: unclear Shunt patency assessment: TIPS function assessed by clinical evaluation every 6 weeks to 3 months. Duplex ultrasound undertaken when signs of possible dysfunction were present (new onset/progressive hepatic encephalopathy or ascites). Liver ultrasound every 6 months Method of Child's grading: Child‐Pugh Method of encephalopathy testing: not specified Rebleeding episodes endoscopically verified: not specified Specified whether rebleeding clinically significant: yes |
|
| Participants | Inclusion criteria: patients with cirrhosis, age 18 to 75 years, first or second episode of endoscopically documented oesophageal or gastric variceal bleeding Exclusion criteria: serious or refractory hepatic encephalopathy unrelated to gastrointestinal bleeding; history of significant heart failure (NYHA III and IV); portal hypertension from cause other than liver disease (e.g. portal or splenic vein thrombosis); previous TIPS; advanced HCC; Child‐Pugh score > 13; sepsis and/or multi‐organ failure; inability/unwillingness to give informed consent After stabilisation and successful endoscopic haemostasis, patients were randomly assigned to TIPS placement or long‐term endoscopic therapy (variceal ligation or injection therapy) + beta blocker, preferably within 1 to 2 days after admission. Initial stabilisation included broad‐spectrum antibiotics, vasoactive drugs (octreotide, terlipressin, or somatostatin), fluid and packed cell administration, and endoscopic treatment according to international consensus guidelines. Endoscopic treatment of oesophageal varices consisted of endoscopic variceal ligation; gastric varices were injected with cyanoacrylate glue with lipiodol Child‐Pugh score (mean ± SD): TIPS group 7.5 ± 2.0, endoscopic group 7.3 ± 1.9. Child‐Pugh class A/B/C (%): TIPS group 35/51/14, endoscopic group 37/51/11. MELD score (mean ± SD): TIPS group 13.5 ± 6.3, endoscopic group 12.7 ± 3.8 Aetiology (%): alcohol 35 in TIPS group and 51 in endoscopic group; HBV/HCV 19 in TIPS group and 3 in endoscopic group; alcohol + HBV/HCV 8 in TIPS group and 8 in endoscopic group; autoimmune liver/biliary disease 24 in TIPS group and 26 in endoscopic group; other 14 in TIPS group and 11 in endoscopic group |
|
| Interventions | TIPS: PTFE‐covered stents (Viatorr; W.L. Gore and Associates, Flagstaff, AZ, USA); embolisation of left gastric (coronary) vein or other collaterals was considered when there was evidence of active variceal bleeding and marked collateral filling on portography Endoscopic therapy: endoscopic band ligation of oesophageal varices; cyanolacrylate injection of gastric varices + non‐selective beta blocker (preferably slow‐release propranolol, titrated to maximum tolerated dose aiming to decrease the heart rate in rest by 25%, with a lower limit of 50 beats per minute) unless a contraindication was present |
|
| Outcomes | Protocol planned outcomes
|
|
| Notes | Dutch trial register: www.trialregister.nl; No.: NTR973 (https://www.trialregister.nl/trial/948) Study authors emailed 29/05/2016 and 26/06/2016. Reply received 05/07/2016 Funding: financial support provided by ZON‐MW, The Netherlands Organization for Health Research and Development (project no.: 80‐007029‐98‐07046). Study authors stated that "the funding source did not have influence on study design, data collection, analysis, and interpretation of the data, writing of the report, nor the decision to submit for publication" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated with the use of concealed block size of 4 stratified by Child‐Pugh class |
| Allocation concealment (selection bias) | Low risk | Randomisation assigned through a permanently available central telephone system to receive further therapy |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible. It is unclear if lack of blinding influenced the choice of treatments and the management of patients |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes scored centrally by 2 physicians blinded to allocated treatment, with a third consulted if no consensus was reached |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Two patients in the TIPS group and 4 in the endoscopic therapy + beta blocker group were lost to‐follow‐up (< 10%) Study authors performed intention‐to‐treat analysis and as‐treated analysis |
| Selective reporting (reporting bias) | Low risk | Study authors reported all pre‐planned outcomes. Cost‐effectiveness was reported in conference abstracts but not for full cohort in the final paper |
| Other bias | Low risk | Two out of 37 (5.4%) participants in TIPS group were crossed over to endoscopy + beta blocker because of closure of TIPS due to severe untreatable hepatic encephalopathy. Six out of 35 (17%) participants in the endoscopic group were crossed over to TIPS because of recurrent/uncontrollable variceal rebleeding or refractory ascites. These participants were censored at the moment they switched therapy |
Lv 2018.
| Study characteristics | ||
| Methods | Randomised controlled trial comparing transjugular intrahepatic portosystemic shunt vs endoscopic band ligation + propranolol for prevention of variceal rebleeding in patients with cirrhosis and portal vein thrombosis Inclusion of all randomised participants at evaluation: no (3 patients excluded early after randomisation due to non‐cirrhotic portal hypertension) Time from bleeding episode to randomisation: patients with endoscopy‐proven variceal bleeding in past 6 weeks included Time from randomisation to treatment: within 48 hours Total number of patients evaluated and found eligible: 52 (156 patients assessed for eligibility; 67 did not meet the inclusion criteria and 46 met the exclusion criteria) Randomised to TIPS: 25; randomised to endoscopic band ligation + drug: 27. One TIPS patient excluded after randomisation due to non‐cirrhotic hypertension; 2 endoscopic band ligation + drug patients excluded after randomisation due to non‐cirrhotic hypertension (excluded from intention‐to‐treat analysis) One participant randomised to endoscopy withdrew consent and was treated by TIPS One participant allocated to TIPS group was treated by endoscopic band ligation + drug due to technical failure of TIPS Four participants treated with endoscopy were crossed over to TIPS due to uncontrolled variceal bleeding or refractory ascites (included in intention‐to‐treat analysis) (extensive thrombosis) Rebleeding episodes endoscopically verified: no, according to Baveno V consensus Specified whether rebleeding episode clinically significant: not specified, but possible because study authors refer to Baveno V Assessment of suitability for shunt carried out before randomisation: at enrolment, doppler ultrasound, abdominal CT Shunt patency assessment: doppler ultrasound and CT evaluations Method of cirrhosis grading: Child‐Pugh Method of encephalopathy testing: clinical Follow‐up, months (median, IQR): TIPS group 30.9, 21.6 to 42.5; endoscopic band ligation + drug group 30.4, 24.6 to 39.0. No participants were lost to follow‐up |
|
| Participants | Inclusion criteria: liver cirrhosis (diagnosed by clinical finding, laboratory tests, imaging, or liver biopsy); portal vein thrombosis; > 50% portal vein trunk lumen; history of endoscopy‐proven variceal bleeding (gastric or oesophageal) in the past 6 weeks; age 18 to 75 years Exclusion criteria: uncontrolled active variceal bleeding; fibrotic cord; previous endoscopic bad ligation + non‐selective beta blockers, TIPS or shunt surgery; renal insufficiency (serum creatinine > 170 micromol/L); severe liver insufficiency; severe cardiopulmonary disease; uncontrolled systemic infection or sepsis; hepatocellular carcinoma or other extrahepatic malignancy; contraindications to propranolol, anticoagulation, or TIPS; HIV infection; pregnant or breastfeeding Patients presenting with acute bleeding were screened on day 6 after successful treatment of the index bleeding with vasoactive drugs (terlipressin or somatostatin), antibiotics, and endoscopic treatment for 5 days. Those who failed to achieve primary haemostasis during acute bleeding were excluded. Patients with a history of recent variceal bleeding were screened on day 1 after hospital admission, and those who previously had received more than 1 session of ligation/sclerotherapy and non‐selective beta blocker were excluded Baseline characteristics comparable between study groups Child‐Pugh score (median, IQR): TIPS group 7, 6 to 8; endoscopic group 7, 6 to 8. Child‐Pugh class A/B/C (%): TIPS group 38/55/8, endoscopic group 40/56/4 Aetiology (%): alcohol 4 in TIPS group and 0 in endoscopic group; HBV 83 in TIPS group and 88 in endoscopic group; HCV 4 in TIPS group and 0 in endoscopic group; autoimmune 4 in TIPS group and 4 in endoscopic group; HBV + autoimmune 0 in TIPS group and 4 in endoscopic group; cryptogenetic 4 in TIPS group and 4 in endoscopic group |
|
| Interventions | TIPS group: polytetrafluoroethylene‐covered stents (Fluency; Bard Peripheral Vascular, Tempe, AZ, USA). Local thrombolysis with bolus infusion of urokinase was performed for 3 days in patients with occlusive thrombus remaining in the superior mesenteric vein and/or the splenic vein after stent insertion. TIPS + anticoagulants (5 patients had local thrombolysis; 7 patients had collateral embolisation) Endoscopic band ligation + drug group: endoscopic band ligation scheduled every 1 to 2 weeks until variceal eradication + propranolol at initial dose of 20 mg twice daily, then with increasing doses until 55 beats per minute or a 25% decrease in heart rate was achieved In both groups, anticoagulant therapy was given TIPS group: intravenous heparin for 5 days, followed by warfarin for 6 months or until portal vein thrombosis complete recanalisation achieved EBL + drug group: intravenous heparin for 5 days followed by warfarin for 6 months or until portal vein thrombosis complete recanalisation. Warfarin was commenced when variceal eradication was achieved or "risk of variceal bleeding was thought to be low after careful evaluation by the investigators" |
|
| Outcomes | Planned outcomes in the published protocol
|
|
| Notes | Amendments to study protocol
Trial registered with ClinicalTrials.gov under NCT01326949 Funding: supported by grants from the Optimized Overall Project of Shaanxi Province (2013KTCL03‐05) and the Boost Program of Xijing Hospital (XJZT11Z07) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Eligible patients were randomised in a 1:1 ratio, stratified according to Child‐Pugh class (A or B/C) and degree of PVT (partial or complete obstruction) using a web‐based allocation system (http://openrct.fmmu.edu.cn) with Pocock and Simon’s minimisation method |
| Allocation concealment (selection bias) | Low risk | Randomisation performed within 24 hours after enrolment by a clinical research co‐ordinator who was not involved in the clinical setting nor in data analysis |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible. It is unclear if lack of blinding influenced the choice of treatments and the management of patients |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding not mentioned. Judgement on all outcomes except mortality could be biased |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participants lost at follow‐up |
| Selective reporting (reporting bias) | Low risk | All outcomes reported as specified in protocol and trial registry |
| Other bias | High risk | The number of participants who were crossed over is high: 4 out of 25 (16%) participants treated with endoscopy were crossed to TIPS due to uncontrolled variceal bleeding or refractory ascites. It is not clear if the data are censored |
Dunne 2020.
| Study characteristics | ||
| Methods | Randomised clinical trial evaluating early transjugular intrahepatic portosystemic shunt (TIPSS) vs standard of care in patients with cirrhosis and oesophageal variceal bleeding Time from bleeding episode to randomisation and time from randomisation to treatment: for those patients randomised to early TIPSS, the aim was to perform TIPSS within 72 hours after initial endoscopy. Mean time from endoscopy to TIPSS placement for all participants was 65 ± 37 hours. Ten participants received TIPSS placement outside the 72‐hour window due to a delay in randomisation, with mean time from endoscopy to randomisation of 37 ± 22 hours. In comparison, the remaining 13 participants who received TIPSS placement within the 72‐hour window had mean time from endoscopy to randomisation of 18 ± 12 hours. Of the 23 participants who received TIPSS placement, 22 received it within 72 hours of randomisation, rather than from endoscopy Total number of patients evaluated and found eligible: 59 (206 patients assessed for eligibility; 147 were excluded); 1 withdrew consent Randomised to TIPS: 29; randomised to endoscopic therapy + beta blocker. Of the 29 participants randomised to the early TIPSS group, 6 participants did not undergo TIPSS placement due to logistical and practical issues. Of the 29 participants randomised to the standard of care group, 1 underwent liver transplantation and 2 underwent rescue TIPSS because of rebleeding during follow‐up Intention‐to‐treat analysis and per‐protocol presented No participants were lost to follow‐up Assessment of suitability for shunt carried out before randomisation: not specified Shunt patency assessment: TIPSS patency was checked at 6 months and at 1 year using doppler ultrasonography or TIPSS venography. If TIPSS dysfunction was confirmed, balloon angioplasty was performed or a further e‐PTFE‐covered stent was placed Method of Child's grading: Child‐Pugh Method of encephalopathy testing: not reported Rebleeding episodes endoscopically verified: yes Specified whether rebleeding clinically significant: not specified |
|
| Participants | Inclusion criteria: patients with liver cirrhosis presenting with acute oesophageal variceal bleeding and subsequent haemostasis following treatment with vasoactive drugs and endoscopic band ligation Exclusion criteria: younger than 18 or older than 75 years of age, pregnancy, Child‐Pugh score < 8 and > 13, inability to control bleeding at index endoscopy, previous porto‐systemic shunt or TIPSS, previous pharmacotherapy and endoscopic band ligation, bleeding from isolated gastric or ectopic varices, known portal vein thrombosis, active cancer including hepatocellular carcinoma, recurrent hospital admissions with encephalopathy After endoscopic haemostasis was achieved, consenting participants were randomised 1:1 to early TIPSS or to standard of care (continued endoscopic band ligation sessions ± pharmacotherapy) Pre‐endoscopic management included use of antibiotics and vasoactive drugs (terlipressin 2 mg QDS) unless contraindicated. Policy was to perform endoscopy within 12 hours of presentation. During endoscopy, band ligation was performed to gain haemostasis for actively bleeding varices or to treat pre‐existing varices with high‐risk stigmata of recent bleeding such as red spots or fibrin plugs There were no differences in baseline characteristics between the 2 treatment groups at study entry Causes of cirrhosis (%): ALD 97 and 90, NAFLD 3 and 7, viral 0 and 3 Child‐Pugh score, mean (SD): 9.8 (1.2) and 9.8 (1.5). Child‐Pugh class B/C (%): 45/55 and 41/59. MELD score, mean (SD): 17 (3.4) and 17 (3.8) |
|
| Interventions | Early TIPSS group: TIPSS (e‐polytetrafluoroethylene (e‐PTFE)‐covered stents (Viatorr TIPSS endoprosthesis; W.L. Gore & Associates, Inc., Newark, NJ, USA)) within 72 hours after initial endoscopy. Terlipressin was continued until TIPSS was performed, and antibiotics were continued for 5 to 7 days. TIPSS were initially dilated to 8 or 9 mm. If the portal pressure gradient did not decrease to below 12 mmHg, the stent was dilated further to 9 or 10 mm Endoscopic intervention with medical therapy group: terlipressin continued for up to 5 days + antibiotics for 5 to 7 days + outpatient endoscopic variceal band ligation programme (endoscopy at 2‐ to 4‐weekly intervals until variceal eradication, then repeat endoscopy in 3‐, then 6‐monthly intervals). Carvedilol was commenced before discharge from hospital at a dose of 6.25 mg and was titrated thereafter, depending on participant tolerability |
|
| Outcomes | Primary outcome was 1‐year survival Secondary outcomes included survival at 6 weeks; rates of early rebleeding (within 6 weeks); late rebleeding (between 6 weeks and 1 year); development of hepatic encephalopathy Subsidiary outcomes: development of new ascites, number of days in the intensive care unit, hospital attendances (including to the endoscopy unit), use of alternative treatments including beta blockers, safety profile |
|
| Notes | ClinicalTrials.gov reference: NCT02377141 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A 24‐hour web‐based randomisation service [https://www. aleaclinical.eu (ALEA Clinical, Abcoude, The Netherlands)] was used |
| Allocation concealment (selection bias) | Low risk | A 24‐hour web‐based randomisation service [https://www. aleaclinical.eu (ALEA Clinical, Abcoude, The Netherlands)] was used |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible. It is unclear if lack of blinding influenced the choice of treatments and the management of patients |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding not mentioned. Judgement on all outcomes except mortality could be biased |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcome data are reported |
| Selective reporting (reporting bias) | Unclear risk | A pre‐published protocol was available. Protocol‐planned outcomes were reported. Not all key outcomes of interest in the review were reported |
| Other bias | High risk | In the TIPS groups, 6 participants did not undergo TIPSS placement due to logistical and practical issues. Reasons for this are not specified |
Urbistondo 1996.
| Study characteristics | ||
| Methods | Randomised controlled trial comparing distal splenorenal shunt (DSRS), propranolol (P), and endoscopic sclerotherapy (ES) for prevention of oesophageal variceal rebleeding in alcoholic cirrhosis Time from bleeding episode to randomisation: no later than 4 weeks after discharge from hospital for the bleeding episode Time from randomisation to treatment: no later than 2 weeks after randomisation Total number of patients evaluated and found eligible: 43 (58 patients evaluated for eligibility; 15 patients excluded: 10 refused participation and 5 did not return for further evaluation) Randomised to DSRS (only patients in Child's class A or B): 15; randomised to P: 15; randomised to ES: 13 Four participants randomised to DSRS group refused and opted for ES group Intention‐to‐treat analysis and per‐protocol presented Follow‐up period in months (mean): 23.2 (16.4). Lost to follow‐up: DSRS group 5, ES group 6, P group 8 Assessment of suitability for shunt carried out before randomisation: yes Shunt patency assessment: not specified Method of Child's grading: Child‐Pugh Method of encephalopathy testing: not reported Rebleeding episodes endoscopically verified: yes Specified whether rebleeding clinically significant: yes |
|
| Participants | Inclusion criteria: alcoholic cirrhosis, previous episode of endoscopically proven oesophageal variceal bleeding for which no treatment was applied Exclusion criteria: not specified Baseline characteristics: Child class A/B/C: DSRS group 11/4/0, ES group 6/6/1. |
|
| Interventions | DSRS group ‐ distal splenorenal shunt after splenoportogram documented a patent and adequate vascular system ES group ‐ endoscopic sclerotherapy using a solution of 1:1 3% sodium tetradecyl sulfate and 5% dextrose, intravariceal injection in distal 6 cm of the oesophagus using a 23 G needle, 1 to 2 mL of sclerosant per injection with a total of 12 to 16 mL per session. Sclerotherapy was performed twice the first week, then weekly until total obliteration of the varices was achieved. After total obliteration, endoscopy was repeated every 3 months for the first year, and every 6 months for the duration of the study. If non‐bleeding varices were found, sclerotherapy was performed again until re‐obliteration following the same weekly scheme P group ‐ patients underwent hepatic‐portal pressure gradient measurement. Propranolol was given orally at a starting dose of 60 mg/d (single dose), and was titrated to obtain a 25% reduction in heart rate from the baseline or less than 60 beats per minute. Hepatic‐portal pressure gradient was measured again at 90 to 120 days after start of therapy. Response to propranolol was defined as a 10% decrease in the gradient |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of random numbers |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible. It is unclear if lack of blinding influenced the choice of treatments and the management of patients. 4 (26%) participants randomised to surgery refused it and opted for sclerotherapy |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding not mentioned. Judgement on all outcomes except mortality could be biased |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 5/15 (33%) participants were randomised to surgery; 6/13 (46%) were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Not all outcomes of interest in the review are reported |
| Other bias | High risk | Unbalanced randomisation between the 2 groups: participants in Child's class A and B were randomised to surgery, sclerotherapy, or propranolol (third arm not of interest in this review). Participants in Child's class C were randomised only to sclerotherapy or propranolol. So there is bias in selection of participants |
ALD: alcoholic liver disease.
CT: computed tomography.
DSRS: distal splenorenal shunt.
ECG: electrocardiogram.
EEG: electroencephalogram.
HBV: hepatitis B virus.
HCC: hepatocellular carcinoma.
HCV: hepatitis C virus.
HVPG: hepatic venous pressure gradient.
IQR: interquartile ratio.
ISMN: isosorbide mononitrate.
MELD: Model for End‐Stage Liver Disease.
NAFLD: non‐alcoholic fatty liver disease.
NYHA: New York Heart Association.
OLT: ortotopic liver transplantation.
P: propranolol.
PBC: braided power shunt.
PO2: partial pressure of oxygen.
PTFE: polytetrafluoroethylene.
PVT: portal vein thrombosis.
SD: standard deviation.
SE: standard error.
SEM: standard error of the mean.
TIPS: transjugular intrahepatic portosystemic shunt.
TS: total shunt.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Cello 1982 | Thirteen Child's class C patients with variceal haemorrhage requiring 6 or more units of blood were randomly assigned to 2 groups: endoscopic sclerotherapy using 5% sodium morrhuate and oesophageal transection/re‐anastomosis employing the EEA Auto Suture stapling instrument. Results were compared retrospectively to those of a separate group of 20 patients who had received total shunts |
| Cello 1987 | Randomised controlled trial comparing portacaval shunt with endoscopic sclerotherapy for patients with severe cirrhosis and acute variceal haemorrhage. Variceal bleeding not controlled before randomisation |
| Escorsell 2002 | Ninety‐one Child‐Pugh class B/C cirrhotic patients surviving their first episode of variceal bleeding were randomised to receive TIPS or medical therapy (propranolol + isosorbide‐5‐mononitrate) to prevent variceal rebleeding. Endoscopic therapy (banding ligation preferably) was used only acutely to treat index bleeding |
| Garcia‐Pagan 2010 | Sixty‐three patients with cirrhosis and acute variceal bleeding who had been treated with vasoactive drugs + endoscopic therapy were randomly assigned within 24 hours after admission to treatment with a polytetrafluoroethylene‐covered stent within 72 hours after randomisation (early‐TIPS group, 32 patients) or continuation of vasoactive drug therapy, followed after 3 to 5 days by treatment with propranolol or nadolol and long‐term endoscopic band ligation, with insertion of a TIPS if needed as rescue therapy (pharmacotherapy–EBL group, 31 patients). Variceal bleeding not controlled before randomisation |
| Kitano 1992 | Ninety‐six patients with good liver function (Child class A or B) and oesophageal varices were randomly assigned to 1 of 3 groups given different treatments: endoscopic sclerotherapy (n = 32), oesophageal transection (n = 32), or distal splenorenal shunt (n = 32). Patients who had not previously bled from varices were included. Only 35/96 (36%) participants had had at least 1 endoscopically proven episode of variceal bleeding |
| Li 2015 | Non‐randomised prospective study enrolling 109 cirrhotic patients with oesophageal variceal bleeding non‐responders to pharmacological therapy based on HVPG measurement who were divided into 2 groups: 55 patients were treated with endoscopic variceal ligation and non‐selective beta blocker; 54 patients were treated with endoscopic variceal ligation and non‐selective beta blocker if HVPG ≤ 16 mmHg, with percutaneous transhepatic variceal embolisation if HVPG > 16 mmHg and ≤ 20 mmHg, or with transjugular intrahepatic portosystemic shunt if HVPG > 20 mmHg |
| Meddi 1999 | Cost analysis study comparing the cumulative cost of the first 18‐month period in a group of participants selected from a multi‐centre randomised controlled trial comparing transjugular intrahepatic portosystemic shunt vs endoscopic sclerotherapy to prevent variceal rebleeding. Possible overlap of previously published results |
| Orloff 1994 | Prospective randomised trial conducted in unselected, consecutive patients with bleeding oesophageal varices resulting from cirrhosis comparing emergency portacaval shunt performed within 8 hours of initial contact (21 patients) with emergency medical therapy (intravenous vasopressin and oesophageal balloon tamponade) followed in 9 to 30 days by elective portacaval shunt in survivors (22 patients). Variceal bleeding not controlled before randomisation. Endoscopic therapy not used in the medically treated group of patients |
| Orloff 2009 | Randomised trial that compared endoscopic sclerotherapy with emergency portacaval shunt in cirrhotic patients with acute variceal haemorrhage. Variceal bleeding not controlled before randomisation |
| Orloff 2015 | Randomised trials of endoscopic therapy and transjugular intrahepatic portosystemic shunt vs portacaval shunt for emergency and elective treatment of bleeding gastric varices in cirrhosis |
| Paquet 1990 | Non‐randomised study comparing endoscopic sclerotherapy and surgical shunt (narrow‐lumen mesocaval interposition shunt and distal splenorenal shunt) for treatment of acute or recurrent haemorrhage from oesophagogastric varices |
| Resnick 1974 | Prospective controlled trial comparing medical therapy, end‐side portacaval shunt, and side‐side portacaval shunt. Endoscopic therapy not employed in the medically treated group |
| Reynolds 1981 | Randomised controlled trial of medical therapy vs end‐to‐side portacaval shunt in 89 patients with alcoholic liver disease and at least 1 severe upper gastrointestinal haemorrhage thought to be from oesophageal varices. Endoscopic therapy not employed in the medically treated group |
| Sanyal 1994 | Non‐randomised study evaluating prospectively portosystemic encephalopathy in 30 patients undergoing transjugular intrahepatic portosystemic shunts, compared with 25 patients concurrently undergoing sclerotherapy. Study outcome measures are not a subject of this review |
| Sauerbruch 2015 | Multi‐centre, open‐label randomised clinical trial of patients with cirrhosis (92% Child's class A or B, 70% alcoholic) comparing more than 5 days after variceal haemorrhage the insertion of a small covered transjugular intrahepatic portosystemic stent shunt (TIPS) (8 mm; n = 90) with medical therapy (propranolol and isosorbide‐5‐mononitrate; n = 95). In the medical group, patients with an adequate reduction in HVPG (responders) remained on the drugs, whereas non‐responders underwent only variceal band ligation |
| Terés 1987a | Randomised controlled trial in 70 consecutive cirrhotic patients with persistent or recurrent variceal bleeding comparing portacaval shunt with stapler transection in patients with low surgical risk, and stapler transection with endoscopic sclerotherapy in patients with high surgical risk |
| Tripathi 2001 | Randomised controlled trial comparing transjugular intrahepatic portosystemic stent‐shunt vs transjugular intrahepatic portosystemic stent‐shunt and variceal band ligation for prevention of variceal rebleeding |
| Wang 2015 | Non‐randomised trial. Retrospective study comparing transjugular intrahepatic portosystemic shunt and endoscopic band ligation in patients with cirrhosis and portal vein thrombosis |
HVPG: hepatic venous pressure gradient. RCT: randomised clinical trial. TIPS: transjugular intrahepatic portosystemic shunt.
Characteristics of studies awaiting classification [ordered by study ID]
Lv 2019.
| Methods | Randomised controlled trial comparing early TIPS with covered stents vs standard treatment for acute variceal bleeding in patients with advanced cirrhosis Time from bleeding episode to randomisation (hours): TIPS group 24.3 (16.1), endoscopic intervention with NSBB 24.7 (19.5) Time from randomisation to treatment: early TIPS performed within 72 hours (preferably within the first 24 hours) after diagnostic endoscopy (45 patients within 24 hours, 28 in 24 to 48 hours, and 10 in 48 to 72 hours) Total number of patients evaluated and found eligible: 132 (317 assessed for eligibility: 101 did not meet inclusion criteria; 140 met exclusion criteria) Randomised to TIPS: 86; randomised to endoscopic therapy + beta blocker: 46. After randomisation, 3 patients were excluded due to non‐cirrhotic portal hypertension (1 in each group) and hepatocellular carcinoma (1 in the early TIPS group). Therefore, the intention‐to‐treat population consisted of 84 patients in the early TIPS group and 45 in the control group. One patient allocated to the early TIPS group died before TIPS placement, and 1 patient assigned to the control group withdrew consent before administration of propranolol; thus, 83 patients in the early TIPS group and 44 in the control group were included in the per‐protocol population Intention‐to‐treat analysis and per‐protocol analysis presented Follow‐up period in months (median): 24.0 (IQR 18.1 to 24.0) in the early TIPS group and 24.0 (9.0 to 24.0) in the control group No participants were lost to follow‐up Assessment of suitability for shunt carried out before randomisation: not specified Shunt patency assessment: TIPS revision with angioplasty or another stent placement was done when portal hypertensive complications re‐emerged or when doppler ultrasonography indicated shunt dysfunction (i.e. reduction in portal blood flow velocity > 50% or < 28 cm/s, or reversion of blood flow direction within the intrahepatic branches) Method of Child's grading: Child‐Pugh Method of encephalopathy testing: West‐Haven criteria |
| Participants | Inclusion criteria: liver cirrhosis (diagnosed based on clinical presentation, laboratory tests, images, or liver biopsies); age 18 to 75 years; endoscopy‐proven acute variceal bleeding according to Baveno 2 definitions; Child‐Pugh class B or C (< 14 points) Exclusion criteria: uncontrolled bleeding before randomisation; bleeding from isolated gastric or ectopic varices; severe cardiopulmonary disease; spontaneous recurrent hepatic encephalopathy; complete portal vein thrombosis or cavernoma; creatinine > 3 mg/dL; hepatocellular carcinoma or other extrahepatic malignancy; uncontrolled infection or sepsis; previous treatment with a surgical shunt, TIPS, or combined therapy with non‐selective beta blockers + endoscopic band ligation; contraindications to TIPS; pregnancy or breastfeeding; declining to participate or unable to give informed consent Baseline characteristics were comparable between study groups |
| Interventions | TIPS group: an 8‐mm covered stent (Fluency; Bard Peripheral Vascular, Tempe, AZ, USA) dilated to 8 mm Control group: vasoactive drugs for up to 5 days. At day 6, propranolol was started at an initial dose of 20 mg twice daily, then was titrated to reduce the resting heart rate by 25% but not below 55 beats per minute. An elective session of endoscopic band ligation was done within 7 to 14 days after initial endoscopic treatment, then every 14 days (plus or minus 3 days) thereafter until variceal eradication was achieved. Endoscopic band ligation was done with multi‐band devices (Wilson‐Cook Medical; Winston‐Salem, NC, USA). Once variceal eradication was achieved, monitoring endoscopy was done every 6 months. Additional sessions of ligation were done if varices reappeared In both groups, vasoactive drugs (octreotide, somatostatin, or terlipressin) or endoscopic band ligation (sclerotherapy if technically difficult or not feasible) within 12 hours of admission and prophylactic antibiotics were used to control the initial bleeding episode |
| Outcomes | Primary endpoint: transplantation‐free survival Secondary endpoints: failure to control bleeding or rebleeding defined as per recommendations of the Baveno V workshop; new or worsening ascites defined as an increase of ≥ 1 point in the ultrasound ascites score (0 = none, 1 = mild, 2 = moderate, 3 = massive) or sustained ascites up to a volume requiring paracentesis; overt hepatic encephalopathy, diagnosed and graded according to the West Haven criteria; other complications of portal hypertension and adverse events |
| Notes | This study is registered with ClinicalTrials.gov, number NCT01370161, and is completed. Funding: National Natural Science Foundation of China, National Key Technology R&D Program, Optimized Overall Project of Shaanxi Province, Boost Program of Xijing Hospital This study is under evaluation, because in the title it is reported that it is:"A randomised controlled trial comparing early‐TIPS with covered stents versus standard treatment for acute variceal bleeding in patients with advanced cirrhosis". But it is not clear if they treat only patients with a controlled bleeding or not |
IQR: interquartile ratio. NSBB: non‐selective beta blocker. TIPS: transjugular intrahepatic portosystemic shunt.
Characteristics of ongoing studies [ordered by study ID]
NCT02477384.
| Study name | A randomised, controlled trial comparing 8 mm transjugular intrahepatic portosystemic shunt vs endoscopic variceal ligation + propranolol for prevention of variceal rebleeding |
| Methods | Study type: interventional Study phase: not applicable Study design: parallel assignment, open label Estimated enrolment: 100 participants |
| Participants | Inclusion criteria: cirrhosis, patients who have bled from oesophageal varices (≥ 5 days and ≤ 28 days), Child‐Pugh B or Child‐Pugh C ≤ 13 Exclusion criteria: gastric varices, non‐cirrhotic portal hypertension, portal vein thrombosis, hepatic encephalopathy, total bilirubin ≥ 51.3 μmol/L, previous treatment of TIPS or surgery, malignancy including hepatocellular carcinoma, contraindications to TIPS, contraindications to EVL, contraindications to propranolol, renal replacement therapy, cardiorespiratory failure, pregnancy, patients not giving informed consent for endoscopic procedures |
| Interventions | Active comparator: 8 mm TIPS
Patients in this group would have undergone TIPS placement with 8‐mm‐diameter ePTFE‐covered stents
Interventions:
Procedure: 8 mm TIPS
Device: 8 mm ePTFE‐covered stent Active comparator: EVL + propranolol Patients in this group would have undergone sequential endoscopic variceal ligation and propranolol treatment Interventions: Procedure: endoscopic variceal ligation Drug: propranolol |
| Outcomes | Primary outcome: variceal rebleeding rate (time frame: 3 years) Secondary outcomes: hepatic encephalopathy rate (time frame: 3 years), number of participants with improving or worsening hepatic function (time frame: 3 years), TIPS dysfunction rate (time frame: 3 years), incidence of complications (time frame: 3 years), number of participants with improving or worsening quality of life (time frame: 3 years), mortality rate (time frame: 3 years) |
| Starting date | June 2015 |
| Contact information | Luo Xuefeng, MD West China Hospital |
| Notes | Estimated study completion date: June 2020 |
NCT03094234.
| Study name | Randomised, controlled trial comparing 8‐mm TIPS vs endoscopic variceal ligation + propranolol for prevention of variceal rebleeding in patients with Child's A cirrhosis |
| Methods | Study type: interventional Study phase: not applicable Study design: parallel assignment, single masking (care provider) Estimated enrolment: 72 participants |
| Participants | Inclusion criteria: cirrhosis patients who had bled from oesophageal varices (≥ 5 days and ≤ 28 days), Child‐Pugh A Exclusion criteria: gastric varices; non‐cirrhotic portal hypertension; portal vein thrombosis; hepatic encephalopathy; total bilirubin ≥ 51.3 micromol/L; previous TIPS or surgery; malignancy including hepatocellular carcinoma; contraindications to TIPS, EVL, or propranolol; renal replacement therapy; cardiorespiratory failure; pregnancy; patients not giving informed consent for endoscopic procedure |
| Interventions | Active comparator: 8‐mm TIPS
Patients in this group would have undergone TIPS placement with 8‐mm‐diameter ePTFE‐covered stents
Intervention: Device: 8‐mm TIPS Active comparator: EVL + propranolol Patients in this group would have undergone sequential endoscopic variceal ligation and propranolol treatment Interventions: Procedure: endoscopic variceal ligation (EVL) Drug: propranolol |
| Outcomes | Primary outcome: variceal rebleeding rate (time frame: 3 years) Secondary outcomes: hepatic encephalopathy rate (time frame: 3 years), TIPS dysfunction rate (time frame: 3 years), incidence of complications (time frame: 3 years), mortality rate (time frame: 3 years) |
| Starting date | 28 April 2017 |
| Contact information | Iuo Xuefeng, MD West China Hospital |
| Notes | Estimated study completion date: 28 March 2020 |
EVL: endoscopic variceal ligation.
PTFE: polytetrafluoroethylene.
TIPS: transjugular intrahepatic portosystemic shunt.
Differences between protocol and review
This systematic review represents an update of a previously published review, based on a protocol from 1997. Changes in Cochrane guidance have resulted in minor deviations from the protocol, which were necessary to bring the current review up to current standards. In addition, several analyses have been added in response to feedback received during the peer review process.
The protocol portion of the review was updated according to updated Cochrane and CHBG methods and requirements and GRADE methods.
We changed the title of the review from "Portosystemic shunts versus endoscopic therapy for variceal rebleeding in patients with cirrhosis" to "Portosystemic shunts versus endoscopic intervention with or without medical intervention for prevention of rebleeding in people with cirrhosis" to outline that endoscopic treatment was associated with medical treatment in some trials.
We added outcomes (health‐related quality of life, death due to rebleeding) following comments made by editors during the review process.
In response to comments by editors during the review process, we have reported all time‐to‐event outcomes as dichotomous outcomes (mortality was planned to be presented as a time‐to‐event outcome alone).
We promoted mortality to a primary outcome in accordance with Cochrane guidelines, and we renamed 'all‐cause mortality' to differentiate if from death due to rebleeding.
We used risk ratios (RRs) instead of odds ratios (ORs) because the RR is more appropriate when there are not few events, and because it is more easily understandable.
We updated risk of bias assessments in keeping with updated Cochrane guidelines.
We added risk of bias summary figures in keeping with updated Cochrane guidelines.
We added 'Summary of findings' tables in accordance with updated Cochrane guidelines, including calculation of optimal information size.
We added GRADE assessment in accordance with updated Cochrane guidelines, including calculation of optimal information size.
We added information about study participants (age, sex, alcohol status) according to updated Cochrane guidelines.
We added Trial Sequential Analysis in accordance with updated Cochrane Hepato‐Biliary Group guidelines, which we used to compare imprecision according to GRADE instructions.
We added a study flow diagram in line with updated Cochrane guidelines.
We searched LILACS and the Conference Proceedings Citation Index in line with updated Cochrane Hepato‐Biliary Group guidelines.
We performed additional sensitivity analyses of included trials,
We reported financial costs and length of hospital stay as raw outcomes, as reported by trialists, in light of considerable variability of methods and definitions used by trial authors.
We performed subgroup analysis according to for‐profit funding.
Contributions of authors
RGS updated the protocol according to recent Cochrane methods and instructions, selected trials for inclusion, rechecked data extracted and extracted further data, added and analysed two additional outcomes, modified and added information, performed meta‐analysis and Trial Sequential Analysis (TSA), updated references, and rewrote the review.
GP updated the protocol, selected trials for inclusion, rechecked data extracted and extracted further data, added and analysed the two additional outcomes, modified and added information, performed meta‐analysis and TSA analysis, updated references, and revised the review.
HLR performed the literature search (2018 and 2019), identified relevant studies, used the trial data extraction protocol, extracted data for individual trials, and performed and updated all meta‐analyses as well as time‐to‐event meta‐analyses according to previously published guidance (developed by the group who published the the last review in 2006). This manuscript was rewritten and updated.
NRB assisted in the literature search (2014), extracted data, identified developments since the last review, and assisted in updating the current manuscript.
MOW assisted in preparing the manuscript and critically reviewing the outcomes, meta‐analyses, and methods.
RS was responsible for placing the current version in its true context with regards to the most recently published literature (including observational and randomised or synthesised evidence). RS was instrumental in updating the whole text and determining implications for future research and applicability to practice sections.
SK conceived the current version (2014‐2019), developed and updated data extraction protocols for individual trials, extracted data for surgical shunt comparisons, provided liaison between study authors, acted as the final arbiter of interpretation and directed the team, and was responsible for overall organisation and oversight of the project.
Sources of support
Internal sources
RS is supported by a National Institute for Health Research Senior Investigator Award, UK
External sources
European Commission (BMH4‐CT96‐0373), Belgium
Declarations of interest
RGS: nothing to declare. GP: nothing to declare. HLR: nothing to declare. NRB: nothing to declare. MOW: nothing to declare. RS: nothing to declare. SK: nothing to declare.
Rosa Simonetti shares first authorship with Giovanni Perricone and Helen Robbins as all three have worked together on the review and contributed equally.
Giovanni Perricone shares first authorship with Helen Robbins and Rosa Simonetti as all three have worked together on the review and contributed equally.
Helen Robbins shares first authorship with Rosa Simonetti and Giovanni Perricone as all three have worked together on the review and contributed equally.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Cabrera 1996 {published data only}
- Cabrera J, Maynar M, Granados R, Gorriz E, Reyes R, Pulido-Duque JM, et al. Transjugular intrahepatic portosystemic shunt versus sclerotherapy in the elective treatment of variceal hemorrhage. Gastroenterology 1996;110(3):832-9. [DOI: 10.1053/gast.1996.v110.pm8608893] [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cello 1997 {published data only}
- Cello JP, Ring EJ, Olcott EW, Koch J, Gordon R, Sandhu J, et al. Endoscopic sclerotherapy compared with percutaneous transjugular Intrahepatic portosystemic shunt after initial sclerotherapy in patients with acute variceal haemorrhage. A randomised controlled trial. Annals of Internal Medicine 1997;126(11):858-65. [DOI: 10.7326/0003-4819-126-11-199706010-00002] [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dunne 2020 {published data only}
- Dunne PDJ, Sinha R, Stanley AJ, Lachlan N, Ireland H, Shams A, et al. Randomised clinical trial: standard of care versus early-transjugular intrahepatic porto-systemic shunt (TIPSS) in patients with cirrhosis and oesophageal variceal bleeding. Alimentary Pharmacology & Therapeutics 2020;52(1):98-106. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ferlitsch 2012 {published data only}
- Ferlitsch A, Ulbrich G, Maieron A, Salzl P, Reiberger T, Payer BA, et al. A randomized, controlled, multicentre trial comparing endoscopic band ligation versus tips in cirrhotic patients with recurrent variceal bleeding non-responding to pharmacological therapy. Journal of Hepatology 2012;56(Suppl 2):S555. [Google Scholar]
García‐Villarreal 1999 {published data only}
- García-Villarreal L, Martinez-Lagares F, Sierra A, Guevara C, Marrero JM, Jiménez E, et al. Transjugular Intrahepatic portosystemic shunt versus prevention of rebleeding after recent variceal hemorrhage. Hepatology (Baltimore, Md.) 1999;29(1):27-32. [DOI: 10.1002/hep.510290125] [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
GDEAIH 1995 {published data only}
- Groupe d'Etude de Anastomoses Intra-Hepatiques. TIPS versus sclerotherapy + propranolol in the prevention of variceal rebleeding: preliminary results of a multicenter randomised trial. Hepatology (Baltimore, Md.) 1995;22(S4):297A. [Google Scholar]
Gülberg 2002 {published data only}
- Gülberg N, Schepke G, Geigenberger G, Holl J, Brensing KA, Waggershauser T, et al. Transjugular intrahepatic portosystemic shunting is not superior to endoscopic variceal band ligation for prevention of variceal rebleeding in cirrhotic patients: a randomized, controlled trial. Scandinavian Journal of Gastroenterology 2002;37(3):338-43. [DOI: 10.1080/003655202317284255] [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Henderson 1990 {published data only}
- *.Henderson JM, Kutner MH, Millikan WJ, Galambos JT, Riepe SP, Brooks S, et al. Endoscopic variceal sclerosis compared with distal splenorenal shunt to prevent recurrent variceal bleeding in cirrhosis, a prospective randomized trial. Annals of Internal Medicine 1990;112(4):262-9. [DOI: 10.7326/0003-4819-112-4-262] [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Warren WD, Henderson JM, Millikan WJ, Galambos JT, Brooks WS, Riepe SP, et al. Distal splenorenal shunt versus endoscopic sclerotherapy for long-term management of variceal bleeding: preliminary report of a prospective, randomised trial. Annals of Surgery 1986;203(5):454-62. [DOI: 10.1097/00000658-198605000-00002] [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Holster 2016 {published data only}
- Harki J, Holster IL, Polinder S, Moelker A, Van Buuren HR, Kuipers EJ, et al. Cost effectiveness of covered transjugular intrahepatic portosystemic shunt (TIPS) versus endoscopic treatment for secondary prevention of gastro-oesophageal variceal rebleeding. Gastroenterology 2016;150(4 Suppl 1):S1160. [Google Scholar]
- Harki J, Holster LI, Polinder S, Moelker A, Van Buuren HR, Kuipers EJ, et al. Cost effectiveness of covered transjugular intrahepatic portosystemic shunt versus endoscopic treatment for secondary prevention of gastro-oesophageal variceal bleeding. Journal of Hepatology 2016;64(2 Suppl):S265-6. [DOI: ] [Google Scholar]
- Holster IL, Moelker A, Tjwa ET, Wils A, Kupers EJ, Pattynama P, et al. Early transjugular intrahepatic portosystemic shunt (TIPS) as compared to endoscopic treatment reduces rebleeding but not mortality in cirrhotic patients with a 1st or 2nd episode of variceal bleeding: a multicentre randomized controlled trial. United European Gastroenterology Journal 2013;1(S1):A84. [Google Scholar]
- *.Holster IL, Tjwa ETTL, Moelker A, Wils A, Hansen BE, Vermeijden JR, et al. Covered transjugular intrahepatic portosystemic shunt versus endoscopic therapy + ß-blocker for prevention of variceal rebleeding. Hepatology (Baltimore, Md.) 2016;63(2):581-9. [DOI: 10.1002/hep.28318] [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Isaksson 1995 {published data only}
- Isaksson B, Jeppsson B, Bengtsson F, Hannesson P, Herlin P, Bengmark S. Mesocaval shunt or repeated sclerotherapy: effects on rebleeding and encephalopathy - a randomized trial. Surgery 1995;117(5):498-504. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jalan 1997 {published data only}
- Jalan R, Forrest EH, Redhead DN, Dillon JF, Bzeizi KI, Finlayson NDC, et al. TIPSS vs variceal band ligation in the secondary prevention of variceal hemorrhage in cirrhosis: preliminary results of a randomised, controlled study. Hepatology (Baltimore, Md.) 1995;22(4):251A. [Google Scholar]
- *.Jalan R, Forrest EH, Stanley AJ, Redhead DN, Forbes J, Dillon JF, et al. A randomized trial comparing transjugular intrahepatic portosystemic stent-shunt with variceal band ligation in the prevention of rebleeding from esophageal varices. Hepatology (Baltimore, Md.) 1997;26(5):1115-22. [DOI: 10.1002/hep.510260505] [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Korula 1987 {published data only}
- Korula J, Yelin A, Yamada S, Weiner J, Cohen H, Reynolds TB. A prospective randomised controlled comparison of chronic endoscopic variceal sclerotherapy and portalsystemic shunt for variceal hemorrhage in Child's Class A cirrhotics: a preliminary report. Gastroenterology 1987;92:1745. [Google Scholar]
Lo 2007 {published data only}
- Lo GH, Liang HL, Chen WC, Chen MH, Lai KH, Hsu PI, et al. A prospective, randomized controlled trial of transjugular intrahepatic portosystemic shunt versus cyanoacrylate injection in the prevention of gastric variceal rebleeding. Endoscopy 2007;39(8):679-85. [DOI: 10.1055/s-2007-966591] [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Luo 2015 {published data only}
- Luo X, Wang Z, Tsauo J, Zhou B, Zhang H, Li Z. Advanced cirrhosis combined with portal vein thrombosis: a randomized trial of TIPS versus endoscopic band ligation plus propranolol for the prevention of recurrent esophageal variceal bleeding. Radiology 2015;276(1):286-93. [DOI: 10.1148/radiol.15141252] [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lv 2018 {published data only}
- *.Lv Y, Qi X, He C, Wang Z, Yin Z, Niu J, et al. Covered TIPS versus endoscopic band ligation plus propranolol for the prevention of variceal rebleeding in cirrhotic patients with portal vein thrombosis: a randomised controlled trial. Gut 2018;67(12):2156-68. [DOI: 10.1136/ gutjnl-2017-314634] [MEDLINE: ] [DOI] [PubMed]
- Qi XS, He CY, Yin ZX, Wang ZY, Zhang HB, Zia JL, et al. Transjugular intrahepatic portosystemic shunt for the prevention of variceal rebleeding in cirrhotic patients with portal vein thrombosis: study protocol for a randomised controlled trial. BMJ Open 2013;3(7):1162-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Merli 1998 {published data only}
- Merli M, Riggio O, Capoccaccia L, Ziparo V, Bolognese A, Rossi P, et al. Transjugular intrahepatic portosystemic shunt (TIPS) vs endoscopic sclerotherapy (ES) in preventing variceal rebleeding preliminary results of a randomized controlled trial. Hepatology (Baltimore, Md.) 1994;20(4):A107. [Google Scholar]
- *.Merli M, Salerno F, Riggio O, Franchis R, Fiaccadori F, Meddi P, et al. Transjugular intrahepatic portosystemic shunt versus endoscopic sclerotherapy for the prevention of variceal bleeding in cirrhosis: a randomized multicenter trial. Gruppo Italiano Studio TIPS (G.I.S.T.). Hepatology (Baltimore, Md.) 1998;27(1):48-53. [DOI: 10.1002/hep.510270109] [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Rossi P, Maccioni F, Salvatori FM, Bezzi M, Gandini R, Broglia L, et al. Transjugular intrahepatic portosystemic shunt (TIPS): indications and results after 22 months of experience [Derivazione porto-sistemica intraepatica transgiugulare (TIPS): indicazioni e risultati dopo 22 anni di esperienza]. Radiologica Medica 1994;87(5):585-96. [MEDLINE: ] [PubMed] [Google Scholar]
Narahara 2001 {published data only}
- Narahara Y, Kanazawa H, Kawamata H, Tada N, Saitoh H, Matsuzaka S, et al. A randomized clinical trial comparing transjugular intrahepatic portosystemic shunt with endoscopic sclerotherapy in the long-term management of patients with cirrhosis after recent variceal hemorrhage. Hepatology Research 2001;21(3):189-98. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Planas 1991 {published data only}
- Planas R, Boix J, Broggi M, Cabre E, Gomes-Vieira MC, Morillas R, et al. Portacaval shunt versus endoscopic sclerotherapy in the elective treatment of variceal hemorrhage. Gastroenterology 1991;100(4):1078-86. [DOI: 10.1016/0016-5085(91)90285-s] [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pomier‐Layrargues 2001 {published data only}
- Pomier-Layrargues G, Dufresne MP, Buli B, Lambert J, Fenyves D, Willems B, et al. TIPS versus endoscopic variceal ligation in the prevention of variceal rebleeding in cirrhotic patients: a comparative randomized clinical trial (interm analysis). Hepatology (Baltimore, Md.) 1997;26(4):137A. [Google Scholar]
- *.Pomier-Layrargues G, Villeneuve JP, Deschenes M, Bui B, Perreault P, Fenyves D, et al. Transjugular intrahepatic portosystemic shunt (TIPS) versus endoscopic variceal ligation in the prevention of variceal rebleeding in patients with cirrhosis: a randomised trial. Gut 2001;48(3):390-6. [DOI: 10.1136/gut.48.3.390] [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rikkers 1993 {published data only}
- Rikkers LF, Burnett DA, Volentine GD, Buchi KN, Cormier RA. Shunt surgery versus endoscopic sclerotherapy for long-term treatment of variceal bleeding. Early results of a randomized trial. Annals of Surgery 1987;206(3):261-71. [DOI: 10.1097/00000658-198709000-00004] [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Rikkers LF, Jin G, Burnett DA, Buchi KN, Cormier RA. Shunt surgery versus endoscopic sclerotherapy for variceal hemorrhage: late results of a randomized trial. American Journal of Surgery 1993;165(1):27-32. [DOI: 10.1016/s0002-9610(05)80400-3] [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rossle 1997 {published data only}
- Rossle M, Deibert P, Haag K, Ochs A, Olschewski M, Siegerstetter V, et al. Randomised trial of transjugular intrahepatic portosystemic shunt versus endoscopy plus propranolol for prevention of variceal rebleeding. Lancet 1997;349(9058):1043-9. [DOI: 10.1016/s0140-6736(96)08189-5] [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Santambrogio 2006 {published data only}
- *.Santambrogio R, Opocher E, Costa M, Bruno S, Caretti AP, Spina GP. Natural history of a randomized trial comparing distal spleno-renal shunt with endoscopic sclerotherapy in the prevention of variceal rebleeding: a lesson from the past. World Journal of Gastroenterology 2006;12(39):6331-8. [DOI: 10.3748/wjg.v12.i39.6331] [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spina GP, Santambrogrio R, Opocher E, Cosentino F, Zambelli A, Passoni GR, et al. Distal splenorenal shunt versus endoscopic sclerotherapy in the prevention of variceal rebleeding. First stage of a randomized, controlled trial. Annals of Surgery 1990;211(2):178-86. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sanyal 1997 {published data only}
- Sanyal AJ, Freedman AM, Luketic VA, Purdum PP 3rd, Shiffman ML, Cole PE, et al. Transjugular intrahepatic portosystemic shunts compared with endoscopic sclerotherapy for the prevention of recurrent variceal hemorrhage. A randomized, controlled trial. Annals of Internal Medicine 1997;126(11):849-57. [DOI: 10.7326/0003-4819-126-11-199706010-00001] [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sauer 1997 {published data only}
- Krieger S, Jauss M, Jansen O, Stiehl A, Sauer P, Geissler M, et al. MRI findings in chronic hepatic encephalopathy depend on portosystemic shunt: results of a controlled prospective clinical investigation. Journal of Hepatology 1997;27(1):121-6. [DOI: 10.1016/s0168-8278(97)80290-5] [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- *.Sauer P, Theilmann L, Stremmel W, Benz C, Richter GM, Stiehl A. Transjugular intrahepatic portosystemic stent shunt versus sclerotherapy plus propranolol for variceal rebleeding. Gastroenterology 1997;113(5):1623-31. [DOI: 10.1053/gast.1997.v113.pm9352865] [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sauer 2002 {published data only}
- Sauer P, Benz C, Theilmann G, Richter W, Stremmel W, Stiehl A. Transjugular intrahepatic portosystemic stent shunt (TIPS) vs. endoscopic banding in the prevention of variceal rebleeding: final results of a randomized study. Gastroenterology 1998;114(4):A1334. [Google Scholar]
- Sauer P, Hansmann J, Richter G, Stremmel W, Stiehl A. Transjugular intrahepatic portosystemic stent shunt (TIPS) vs. endoscopic banding in the prevention of variceal rebleeding: a long-term randomized trial. Gastroenterology 2000;118(4 Pt 2):A1485. [Google Scholar]
- *.Sauer P, Hansmann J, Richter GM, Stremmel W, Stiehl A. Endoscopic variceal ligation plus propranolol versus transjugular intrahepatic portosystemic stent shunt: a long-term randomized trial. Endoscopy 2002;34(9):690-7. [DOI: 10.1055/s-2002-33565] [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Sauer P, Theilmann L, Benz C, Richter G, Stremmel W, Stiehl A. Transjugular intrahepatic portosystemic stent shunt (TIPS) vs. endoscopic banding in the prevention of variceal rebleeding: interim analysis of a randomized study. Gastroenterology 1997;112(4):A1374. [DOI] [PubMed] [Google Scholar]
Terés 1987 {published data only}
- Terés J, Bordas JM, Bravo D, Visa J, Grande L, Garcia-Valdecasas JC, et al. Sclerotherapy vs. distal splenorenal shunt in the elective treatment of variceal hemorrhage: a randomized controlled trial. Hepatology (Baltimore, Md.) 1987;7(3):430-6. [DOI: DOI: 10.1002/hep.1840070303] [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Urbistondo 1996 {published data only}
- Urbistondo M, Torres EA, Castro F, Oharriz J, Medina R, Molina A, et al. Prevention of recurrent esophageal bleeding and survival in patients with alcoholic cirrhosis: a randomized study. Puerto Rico Health Sciences Journal 1996;15(3):195-9. [MEDLINE: ] [PubMed] [Google Scholar]
References to studies excluded from this review
Cello 1982 {published data only}
- Cello JP, Crass RC, Trunkey DD. Endoscopic sclerotherapy versus esophageal transection of Child's class C patients with variceal hemorrhage. Comparison with results of portacaval shunt: preliminary report. Surgery 1982;91(3):333-8. [MEDLINE: ] [PubMed] [Google Scholar]
Cello 1987 {published data only}
- Cello JP, Grendell JH, Crass RA, Trunkey DD, Cobb EE, Heibron DC. Endoscopic sclerothearpy versus portacaval shunt in patients with severe cirrhosis and variceal hemorrhage. New England Journal of Medicine 1984;311(25):1589-94. [DOI: 10.1056/NEJM198412203112501] [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- *.Cello J P, Grendell JH, Crass RA, Weber TE, Trunkey DD. Endoscopic sclerotherapy versus portacaval shunt in patients with severe cirrhosis and acute variceal hemorrhage. Long-term follow-up. New England Journal of Medicine 1987;316(1):11-5. [DOI: 10.1056/NEJM198701013160103] [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Escorsell 2002 {published data only}
- Escorsell A, Bañares R, García-Pagán JC, Gilabert R, Moitinho E, Piqueras B, et al. TIPS versus drug therapy in preventing variceal rebleeding in advanced cirrhosis: a randomized controlled trial. Hepatology (Baltimore, Md.) 2002;35(2):385-92. [DOI: 10.1053/jhep.2002.30418] [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Garcia‐Pagan 2010 {published data only}
- García-Pagán JC, Caca K, Bureau C , Laleman W, Appenrodt B, Luca A, et al. Early use of TIPS in patients with cirrhosis and variceal bleeding. New England Journal of Medicine 2010;362(25):2370-9. [DOI: 10.1056/NEJMoa0910102.] [MEDLINE: ] [ISRCTN58150114] [DOI] [PubMed] [Google Scholar]
Kitano 1992 {published data only}
- Kitano S, Iso Y, Hashizume M, Yamaga H, Koyanagi N, Wada H, et al. Sclerotherapy vs. esophageal transection vs. distal splenorenal shunt for the clinical management of esophageal varices in patients with child class A and B liver function: a prospective randomized trial. Hepatology (Baltimore, Md.) 1992;15(1):63-8. [DOI: 10.1002/hep.1840150113] [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Li 2015 {published data only}
- Li GQ, Yang B, Liu J, Wang GC, Yuan HP, Zhao JR, et al. Hepatic venous pressure gradient is a useful predictor in guiding treatment on prevention of variceal rebleeding in cirrhosis. International Journal of Clinical and Experimental Medicine 2015;8(10):19709-16. [MEDLINE: ] [PMC free article] [PubMed] [Google Scholar]
Meddi 1999 {published data only}
- Meddi P, Merli M, Lionetti R, De Santis A, Valeriano V, Masini A, et al. Cost analysis for the prevention of variceal rebleeding: a comparison between transjugular intrahepatic portosystemic shunt and endoscopic sclerotherapy in a selected group of Italian cirrhotic patients. Hepatology (Baltimore, Md.) 1999;29(4):1074-7. [DOI: 10.1002/hep.510290411] [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Orloff 1994 {published data only}
- Orloff MJ, Bell RH, Orloff MS, Hardison WGM, Greenburg AG. Prospective randomized trial of emergency portacaval shunt and emergency medical therapy in unselected cirrhotic patients with bleeding varices. Hepatology (Baltimore, Md.) 1994;20(4 Pt 1):863-72. [DOI: 10.1002/hep.1840200414] [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Orloff 2009 {published data only}
- Orloff M, Isenberg J, Wheeler H, Haynes K, Jinich-Brook H, Rapier R, et al. Portal-systemic encephalopathy in a randomized controlled trial of endoscopic sclerotherapy versus emergency portacaval shunt treatment of acutely bleeding esophageal varices in cirrhosis. Annals of Surgery 2009;250(4):598-610. [DOI: 10.1097/SLA.0b013e3181b73126] [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Orloff MJ, Isenberg JI, Wheeler HO, Haynes KS, Jinich-Brook H, Rapier R, et al. Direct costs of care in a randomized controlled trial of endoscopic sclerotherapy versus emergency portacaval shunt for bleeding esophageal varices in cirrhosis - Part 4. Journal of Gastrointestinal Surgery 2011;15(1):38-47. [DOI: 10.1007/s11605-010-1332-6] [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orloff MJ, Isenberg JI, Wheeler HO, Haynes KS, Jinich-Brook H, Rapier R, et al. Emergency portacaval shunt versus rescue portacaval shunt in a randomized controlled trial of emergency treatment of acutely bleeding esophageal varices in cirrhosis - Part 3. Journal of Gastrointestinal Surgery 2010;14(11):1782-95. [DOI: 10.1007/s11605-010-1279-7] [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orloff MJ. Fifty-three years' experience with randomized clinical trials of emergency portacaval shunt for bleeding esophageal varices in cirrhosis: 1958 - 2011. Journal of the American Medical Association Surgery 2014;149(2):155-69. [DOI: 10.1001/jamasurg.2013.4045] [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Orloff 2015 {published data only}
- Orloff MJ, Hye RJ, Wheeler HO, Isenberg JI, Haynes KS, Vaida F, et al. Randomized trials of endoscopic therapy and transjugular intrahepatic portosystemic shunt versus portacaval shunt for emergency and elective treatment of bleeding gastric varices in cirrhosis. Surgery 2015;157(6):1028-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orloff MJ, Isenberg JI, Wheeler HO, Haynes KS, Jinich-Brook H, Rapier R, et al. Randomized trial of emergency endoscopic sclerotherapy versus emergency portacaval shunt for acutely bleeding esophageal varices in cirrhosis. Journal of the American College of Surgeons 2009;209(1):25-40. [DOI: 10.1016/j.jamcollsurg.2009.02.059] [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Paquet 1990 {published data only}
- Paquet KJ, Lazar A, Gad HA. Surgical measures in recurrent hemorrhage from esophageal and gastric varices after sclerotherapy - a prospective study [Chirurgische Massnahmen bei Rezidivblutungen aus Osophagus- und Magenvarizen nach Sklerosierungstherapie - eine prospektive Studie]. Langenbecks Archiv für Chirurgie. Supplement II, Verhandlungen der Deutschen Gesellschaft für Chirurgie 1990;Suppl II:397-402. [MEDLINE: ] [PubMed] [Google Scholar]
Resnick 1974 {published data only}
- Resnick RH, Iber FL, Ishihara AM, Chalmers TC, Zimmerman H. A controlled study of the therapeutic portacaval shunt. Gastroenterology 1974;6(5):843-57. [MEDLINE: ] [PubMed] [Google Scholar]
Reynolds 1981 {published data only}
- Reynolds TB, Donovan AJ, Mikkelsen WP, Redeker AG, Turrill FL, Weiner JM. Results of a 12-year randomized trial of portacaval shunt in patients with alcoholic liver disease and bleeding varices. Gastroenterology 1981;80(5 Pt 1):1005-11. [MEDLINE: ] [PubMed] [Google Scholar]
Sanyal 1994 {published data only}
- *.Sanyal AJ, Freedman AM, Shiffman ML, Purdum PP 3rd, Luketic VA, Cheatham AK. Portosystemic encephalopathy after transjugular intrahepatic portosystemic shunt: results of a prospective controlled study. Hepatology (Baltimore, Md.) 1994;20(1 Pt 1):46-55. [DOI: 10.1016/0270-9139(94)90133-3] [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Sauer P, Theilmann L, Stremmel W, Stiehl A. Hepatic encephalopathy after transjugular intrahepatic portosystemic stent shunt (TIPS) [Hepatische Enzephalopathie nach transjugularem intrahepatischem porto-systemischem Stent-Shunt (TIPS)]. Zeitschrift für Gastroenterologie 1995;33(5):277-8. [MEDLINE: ] [PubMed] [Google Scholar]
Sauerbruch 2015 {published data only}16334693
- Sauerbruch T, Mengel M, Dollinger M, Zipprich A, Rossle M, Panther E, et al, German Study Group for Prophaxis of Variceal Rebleeding. Prevention of rebleeding from esophageal varices in patients with cirrhosis receiving small-diameter stents versus hemodynamically controlled medical therapy. Gastroenterology 2015;149(3):660-8. [MEDLINE: 10.1053/j.gastro.2015.05.011] [PMID: ] [DOI] [PubMed] [Google Scholar]
Terés 1987a {published data only}
- Terés J, Baroni R, Bordas JM, Visa J, Pera C, Rodes J. Randomized trial of portacaval shunt, stapling transection and endoscopic sclerotherapy in uncontrolled variceal bleeding. Journal of Hepatology 1987;4(2):159-67. [DOI: 10.1016/s0168-8278(87)80075-2] [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tripathi 2001 {published data only}
- Tripathi D, Helmy A, Lui HF, Stanley AJ, Forrest E, Redhead DN, et al. Randomized controlled trial of transjugular intra-hepatic portosystemic stent-shunt (TIPSS) versus TIPSS and variceal band ligation (VBL) in the prevention of variceal re-bleeding. Journal of Hepatology 2001;34(Suppl 1):69-70. [DOI: 10.1016/S0168-8278(01)81114-4] [DOI] [Google Scholar]
Wang 2015 {published data only}
- Wang Z, Zhao H, Wang X, Zhang H, Jiang M, Tsauo J, et al. Clinical outcome comparison between TIPS and EBL in patients with cirrhosis and portal vein thrombosis. Abdominal Imaging 2015;40(6):1813-20. [DOI: 10.1007/s00261-014-0320-9] [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Lv 2019 {published data only}
- Lv Y, Yang Z, Liu L, Li K, He C, Wang Z, et al. Early TIPS with covered stents versus standard treatment for acute variceal bleeding in patients with advanced cirrhosis: a randomised controlled trial. The Lancet Gastroenterology and Hepatology 2019;4(8):587-98. [DOI: 10.1016/S2468-1253(19)30090-1] [MEDLINE: ] [NCT01370161] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT02477384 {published data only}
- NCT02477384. 8mm-TIPS versus endoscopic variceal ligation (EVL) plus propranolol for prevention of variceal rebleeding [Randomised trial of 8mm transjugular intrahepatic portosystemic shunt versus endoscopic variceal ligation plus propranolol for prevention of variceal rebleeding]. clinicaltrials.gov/ct2/show/study/NCT02477384 (first received 15 June 2015).
NCT03094234 {published data only}
- NCT03094234. 8mm-TIPS versus endoscopic variceal ligation (EVL) plus propranolol for prevention of variceal rebleeding in patients with child A cirrhosis. clinicaltrials.gov/ct2/show/study/NCT03094234 (first received 23 March 2017).
Additional references
Bari 2012
- Bari K, Garcia-Tsao G. Treatment of portal hypertension. World Journal of Gastroenterology 2012;18(11):1166-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bernard 1997
- Bernard B, Lebrec D, Mathurin P, Opolon P, Poynard T. Propranolol and sclerotherapy in the prevention of gastrointestinal rebleeding in patients with cirrhosis: a meta-analysis. Journal of Hepatology 1997;26(2):312-24. [DOI: 10.1016/s0168-8278(97)80047-5] [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Brok 2008
- Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta-analyses. Journal of Clinical Epidemiology 2008;61(8):763-9. [DOI: 10.1016/j.jclinepi.2007.10.007] [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Brok 2009
- Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta-analyses may be inconclusive - trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta-analyses. International Journal of Epidemiology 2009;38(1):287-98. [DOI: 10.1093/ije/dyn188] [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Brown 1997
- Brown RS Jr, Lake JR. Transjugular intrahepatic portosystemic shunt as a form of treatment for portal hypertension: indications and contraindications. Advances in Internal Medicine 1997;42:485-504. [MEDLINE: ] [PubMed] [Google Scholar]
Bureau 2007
- Bureau C , Pagan JC, Layrargues GP, Metivier S, Bellot P, Perreault P, et al. Patency of stents covered with polytetrafluoroethylene in patients treated by transjugular intrahepatic portosystemic shunts: long-term results of a randomized multicentre study. Liver International 2007;27(6):742-7. [DOI: 10.1111/j.1478-3231.2007.01522.x] [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Burroughs 2002
- Burroughs AK, Vangeli M. Transjugular Intrahepatic portosystemic shunt versus endoscopic therapy: randomized trials for secondary prophylaxis of variceal bleeding: an updated meta-analysis. Scandinavian Journal of Gastroenterology 2002;37(3):249-52. [DOI: 10.1080/003655202317284138] [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cabonell 2004
- Carbonell N, Pauwels A, Serfaty L, Fourdan O, Levy VG, Poupon R. Improved survival after variceal bleeding in patients with cirrhosis over the past two decades. Hepatology (Baltimore, Md.) 2004;40(3):652-9. [DOI: 10.1002/hep.20339] [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Casado 1998
- Casado M, Bosch J, Garcia-Pagan JC, Bru C, Banares R, Bandi JC, et al. Clinical events after transjugular intrahepatic portosystemic shunt: correlation with hemodynamic findings. Gastroenterology 1998;114(6):1296-303. [MEDLINE: ] [10.1016/s0016-5085(98)70436-6] [DOI] [PubMed] [Google Scholar]
Castellini 2018
- Castellini G, Bruschettini M, Gianola S, Gluud C, Moja L. Assessing imprecision in Cochrane systematic reviews: a comparison of GRADE and Trial Sequential Analysis. Systematic Reviews 2018;7(1):110. [MEDLINE: ] [10.1186/s13643-018-0770-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Collins 1995
- Collins JC, Sarfeh J. Surgical management of portal hypertension. Western Journal of Medicine 1995;162(6):527-35. [MEDLINE: ] [PMC free article] [PubMed] [Google Scholar]
Cordon 2012
- Cordon JP, Torres CF, Garcia AB, Rodriguez FG, Suarez de Parga JM. Endoscopic management of esophageal varices. World Journal of Gastrointestinal Endoscopy 2012;4(7):312-22. [DOI: 10.4253/wjge.v4.i7.312] [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
D'Amico 1995
- D'Amico G, Pagliaro L, Bosch J. The treatment of portal hypertension: a meta-analytic review. Hepatology (Baltimore, Md.) 1995;22(1):332-54. [DOI: 10.1002/hep.1840220145] [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
D'Amico 2004
- D'Amico G. Esophageal varices: from appearance to rupture; natural history and prognostic indicators. In: Groszmann RJ, Bosch J (Editors). Portal Hypertension in the 21st Century. King's College London, UK: Springer, Kluwer Academic Publishers, 2004.
Dai 2015
- Dai C, Liu WX, Jiang M, Sun MJ. Endoscopic variceal ligation compared with endoscopic injection sclerotherapy for treatment of esophageal variceal hemorrhage: a meta-analysis. World Journal of Gastroenterology 2015;21(8):2534-41. [DOI: 10.3748/wjg.v21.i8.2534] [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
de Franchis 2010
- Franchis R, Braveno V Faculty. Revising consensus in portal hypertension: report of the Baverno V Consensus Workshop on Methodology of Diagnosis and Therapy in Portal Hypertension. Journal of Hepatology 2010;53(4):762-8. [DOI: 10.1016/j.jhep.2010.06.004] [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
de Franchis 2015
- Franchis R, Baveno VI Faculty. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. Journal of Hepatology 2015;63(3):743-52. [DOI: 10.1016/j.jhep.2015.05.022] [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
EASL 2018
- European Association for the Study of the Liver. EASL clinical practice guidelines for the management of patients with decompensated cirrhosis. Journal of Hepatology 2018;69(2):406-60. [DOI: 10.1016/j.jhep.2018.03.024] [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Phillips AN. Meta-analysis: principles and procedure. BMJ (Clinical Research Ed.) 1997;315(7121):1533-7. [DOI: 10.1136/bmj.315.7121.1533] [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Garcia‐Tsao 2007
- Garcia-Tsao G, Sanyal AJ, Grace ND, Carey WD, The Practice Guidelines Committee of the American Association of the Study of Liver Diseases, The Practice Parameters Committee of the American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology (Baltimore, Md.) 2007;46(3):922-38. [DOI: 10.1002/hep.21907] [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Garcia‐Tsao 2017
- Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the Study of Liver Diseases. Hepatology (Baltimore, Md.) 2017;65(1):310-35. [DOI: 10.1002/hep.28906] [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gartlehner 2019
- Gartlehner G, Nussbaumer-Streit B, Wagner G, Patel S, Swinson-Evans T, Dobrescu A, et al. Increased risks for random errors are common in outcomes graded as high certainty of evidence. Journal of Clinical Epidemiology 2019;106:50-9. [DOI: 10.1016/j.jclinepi.2018.10.009] [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Grace 1997
- Grace ND. Diagnosis and treatment of gastrointestinal bleeding secondary to portal hypertension. American Journal of Gastroenterology 1997;92(7):1081-91. [MEDLINE: ] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed 4 April 2020. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Graham 1981
- Graham DY, Smith JL. The course of patients after variceal haemorrhage. Gastroenterology 1981;80(4):800-9. [MEDLINE: ] [PubMed] [Google Scholar]
Herrera 2014
- Herrera JL. Management of acute variceal bleeding. Clinics in Liver Disease 2014;18(2):347-57. [DOI: 10.1016/j.cld.2014.01.001] [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2019
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated August 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Jakobsen 2014
- Jakobsen J, Wetterslev J, Winkel P, Lange T, Gluud C. Thresholds for statistical and clinical significance in systematic reviews with meta-analytic methods. BMC Medical Research Methodology 2014;14:120. [DOI: 10.1186/1471-2288-14-120] [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
LaBerge 1993
- LaBerge JM, Ring EJ, Gordon RL, Lake JR, Doherty MM, Somberg KA, et al. Creation of transjugular intrahepatic portosystemic shunts with the wall stent endoprosthesis: results in 100 patients. Radiology 1993;187(2):413-20. [DOI: 10.1148/radiology.187.2.8475283] [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Laine 1995
- Laine L, Cook D. Endoscopic ligation compared with sclerotherapy for treatment of esophageal variceal bleeding. A meta-analysis. Annals of Internal Medicine 1995;123(4):280-7. [DOI: 10.7326/0003-4819-123-4-199508150-00007] [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Luca 1999
- Luca A, D'Amica G, La Galla R, Midiri M, Morabito A, Pagliaro L. TIPS for prevention of recurrent bleeding in patients with cirrhosis: meta-analysis of randomized clinical trials. Radiology 1999;212(2):411-21. [DOI: 10.1148/radiology.212.2.r99au46411] [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
McIntyre 1996
- McIntyre N, Burroughs AK. Cirrhosis and portal hypertension. In: Weatherall DJ, Ledingham JGG, Warrell DA, editors(s). Oxford Textbook of Medicine. London, UK: Churchill Livingstone, 1996:2085-100. [Google Scholar]
Papatheodoridis 1999
- Papatheodoridis GV, Goulis J, Leandro G, Patch D, Burroughs AK. Transjugular intrahepatic portosystemic shunt compared with endoscopic treatment for prevention of variceal rebleeding: a meta-analysis. Hepatology (Baltimore, Md.) 1999;30(3):612-22. [DOI: 10.1002/hep.510300316] [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MKB, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815-34. [DOI: ] [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Puente 2014
- Puente A, Hernandez-Gea V, Graupera I, Roque M, Colomo A, Poca M, et al. Drugs plus ligation to prevent rebleeding in cirrhosis: an updated systematic review. Liver International 2014;34(6):823-33. [DOI: 10.1111/liv.12452] [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pugh 1973
- Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. British Journal of Surgery 1973;60(8):646-9. [MEDLINE: ] [10.1002/bjs.1800600817] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014. https://community.cochrane.org/help/tools-and-software/revman-5.
Rosemurgy 2012
- Rosemurgy AS, Frohman HA, Teta AF, Luberice K, Ross SB. Prosthetic H-graft portacaval shunts vs transjugular intrahepatic portosystemic stent shunts: 18-year follow-up of a randomized trial. Journal of the American College of Surgeons 2012;214(4):445-53. [DOI: 10.1016/j.jamcollsurg.2011.12.042] [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Royle 2003
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591-603. [DOI: 10.1017/s0266462303000552] [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sauer 1998
- Sauer P, Benz C, Theilmann G, Richter W, Stremmel W, Stiehl A. Tranjugular intrahepatic portosystemic stent shunt (TIPS) vs. endoscopic banding in the prevention of variceal rebleeding: final results of a randomized study. Gastroenterology 1998;114(1):A1334. [Google Scholar]
Savović 2018
- Savović J, Turner RM, Mawdsley D, Jones HE, Beynon R, Higgins JPT, et al. Association between risk-of-bias assessments and results of randomized trials in Cochrane Reviews: The ROBES Meta-Epidemiologic Study. American Journal of Epidemiology 2018;187(5):1113-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shi 2013
- Shi KQ, Liu WY, Pan ZZ, Lin ZF, Chen SL, Chen YP, et al. Secondary prophylaxis of variceal bleeding for cirrhotic patients: a multiple-treatments meta-analysis. European Journal of Clinical Investigation 2013;43(8):844-54. [DOI: 10.1111/eci.12115] [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Simmonds 2015
- Simmonds M. Quantifying the risk of error when interpreting funnel plots. Systematic Reviews 2015;4(24):1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Spina 1992a
- Spina GP, Henderson JM, Rikkers LF, Teres J, Burroughs AK, Conn HO, et al. Distal spleno-renal shunt versus endoscopic sclerotherapy in the prevention of variceal rebleeding. A meta-analysis of 4 randomized clinical trials. Journal of Hepatology 1992;16(3):338-45. [DOI: 10.1016/s0168-8278(05)80666-x] [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Terblanche 1983
- Terblanche J, Bornman PC, Kahn D, Jonker MA, Campbell JA, Wright J, et al. Failure of repeated injection sclerotherapy to improve long-term survival after oesophageal variceal bleeding. A five-year prospective controlled clinical trial. Lancet 1983;2(8363):1328-32. [DOI: 10.1016/s0140-6736(83)91090-5] [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Thiele 2012
- Thiele M, Krag A, Rohde U, Gluud LL. Meta-analysis: banding ligation and medical interventions for the prevention of rebleeding from oesophageal varices. Alimentary Pharmacology & Therapeutics 2012;35(10):1155–65. [DOI: 10.1111/j.1365-2036.2012.05074.x] [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Thorlund 2010
- Thorlund K, Anema A, Mills E. Interpreting meta-analysis according to the adequacy of sample size. An example using isoniazid chemoprophylaxis for tuberculosis in purified protein derivative negative HIV-infected individuals. Clinical Epidemiology 2010;2:57-66. [MEDLINE: ] [10.2147/clep.s9242] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thorlund 2011
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User manual for Trial Sequential Analysis (TSA). ctu.dk/tsa/files/tsa_manual.pdf 2011 (accessed June 2019).
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [DOI: 10.1186/1745-6215-8-16] [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tripathi 2015
- Tripathi D, Stanley AJ, Hayes PC, Patch D, Millson C, Mehrzad H, et al. UK Guidelines on the management of variceal haemorrhage in cirrhotic patients. Gut 2015;64(11):1680-704. [DOI: 10.1136/gutjnl-2015-309262] [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
TSA 2011 [Computer program]
- TSA - Trial Sequential Analysis. Version 0.9.5.10 Beta. Copenhagen: Copenhagen Trial Unit, 2011. www.ctu.dk/tsa/downloads.aspx.
Tudur 2001
- Tudur C, Williamson PR, Khan S, Best LY. The value of the aggregate data approach in meta-analysis with time-to-event outcomes. Journal of the Royal Statistical Society: Series A (Statistics in Society) 2001;2(164):357-70. [DOI: 10.1111/1467-985X.00207] [DOI] [Google Scholar]
Villanueva 2008
- Villanueva C, Balanzo J. Variceal bleeding: pharmacological treatment and prophylactic strategies. Drugs 2008;68(16):2303-24. [DOI: 10.2165/0003495-200868160-00004] [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Warren 1986
- Warren WD, Millikan WJ Jr, Henderson JM, Abu-Elmagd KM, Galloway JR, Shires GT 3rd, et al. Splenopancreatic disconnection. Improved selectivity of distal splenorenal shunt. Annals of Surgery 1986;204(4):346-55. [DOI: 10.1097/00000658-198610000-00002] [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wetterslev 2008
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta-analysis. Journal of Clinical Epidemiology 2008;61(1):64-75. [DOI: 10.1016/j.jclinepi.2007.03.013] [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wetterslev 2009
- Wetterslev J, Thorlund K, Brok J, Gluud C. Estimating required information size by quantifying diversity in a random-effects meta-analysis. BMC Medical Research Methodology 2009;9:86. [DOI: 10.1186/1471-2288-9-86] [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wetterslev 2017
- Wetterslev J, Jakobsen JC, Gluud C. Trial Sequential Analysis in systematic reviews with meta-analysis. BMC Medical Research Methodology 2017;17(1):39. [DOI: 10.1186/s12874-017-0315-7] [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Williamson 2002
- Williamson PR, Tudur Smith C, Hutton JL, Marson AG. Aggregate data meta-analysis with time-to-event outcomes. Statistics in Medicine 2002;21(22):3337-51. [DOI: 10.1002/sim.1303] [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Zhao 2014
- Zhao JR, Wang GC, Hu JH, Zhang CQ. Risk factors for early rebleeding and mortality in acute variceal hemorrhage. World Journal of Gastroenterology 2014;20(47):17941-8. [DOI: 10.3748/wjg.v20.i47.17941] [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zheng 2008
- Zheng M, Chen Y, Bai J, Zeng Q, You J, Jin R, et al. Transjugular intrahepatic portosystemic shunt versus endoscopic therapy in the secondary prophylaxis of variceal rebleeding in cirrhotic patients: meta-analysis update. Journal of Clinical Gastroenterology 2008;42(5):507-15. [DOI: 10.1097/MCG.0b013e31815576e6] [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Zhou 2019
- Zhou GP, Sun LY, Wei L, Qu W, Zeng ZG, Liu Y, et al. Comparision between portosystemic shunts and endoscopic therapy for prevention of variceal re-bleeding: a systematic review and meta-analysis. Chinese Medical Journal 2019;132(9):1087-99. [DOI: 10.1097/CM9.0000000000000212] [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Khan 1997
- Khan SA, Williamson P, Sutton R, Tudur C. Portosystemic shunts versus endoscopic therapy for variceal rebleeding in patients with cirrhosis. Cochrane Database of Systematic Reviews 1997, Issue 4. Art. No: CD000553. [DOI: 10.1002/14651858.CD000553] [DOI] [PMC free article] [PubMed] [Google Scholar]
Khan 2006
- Khan SA, Tudur Smith C, Williamson PR, Sutton R. Portosystemic shunts versus endoscopic therapy for variceal rebleeding in patients with cirrhosis. Cochrane Database of Systematic Reviews 2006, Issue 4. Art. No: CD000553. [DOI: 10.1002/14651858.CD000553.pub2] [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]


