Abstract
COVID-19 vaccination is recommended for multiple sclerosis patients. Disease-modifying therapies can influence the safety and efficacy of COVID-19 vaccines. RNA, DNA, protein, and inactivated vaccines are likely safe for multiple sclerosis patients. A few incidences of central demyelination were reported with viral vector vaccines, but their benefits likely outweigh their risks if alternatives are unavailable. Live-attenuated vaccines should be avoided whenever possible in treated patients. Interferon-beta, glatiramer acetate, teriflunomide, fumarates, and natalizumab are not expected to impact vaccine efficacy, while cell-depleting agents (ocrelizumab, rituximab, ofatumumab, alemtuzumab, and cladribine) and sphingosine-1-phosphate modulators will likely attenuate vaccine responses. Coordinating vaccine timing with dosing regimens for some therapies may optimize vaccine efficacy.
Graphical absract
1. Introduction
The coronavirus disease 2019 (COVID-19) pandemic, caused by the severe acute respiratory syndrome coronavirus type-2 (SARS-CoV-2), is a global public health emergency. Rapid delivery of safe and effective vaccines is therefore imperative. At the time of this writing, three vaccines to prevent COVID-19 have been approved by the U.S. Food and Drug Administration (FDA) (Different COVID-19 Vaccines | CDC [WWW Document], 2020), and several other vaccines have received approval in Europe, Asia, and the Middle East. The rapid development of COVID-19 vaccines is promising but raises questions about vaccine safety and efficacy in patients with immune-mediated conditions such as Multiple Sclerosis (MS) and the impact of immunosuppressive or immunomodulatory disease-modifying therapies (DMTs).
The National MS Society (NMSS) recently published guidance on COVID-19 mRNA vaccines that encouraged vaccination and emphasized safety in MS patients. Specific guidelines for vaccine timing in relation to dosing of individual DMTs were also included to maximize vaccine efficacy (MS Treatment Guidelines During the Coronavirus pandemic | National MS Society | National Multiple Sclerosis Society, 2020). However, the COVID-19 vaccine repertoire is becoming increasingly complex. Several vaccine subtypes with various mechanisms of action and diverse immunogenic properties are available worldwide, with many others in development. MS patients and clinicians around the world thus face complex questions regarding potential interactions between the various COVID-19 vaccines and different DMTs.
We previously evaluated the immune response against SARS-CoV-2 and its potential vaccines in relation to the mechanism of action of various DMTs (Zheng et al., 2020). In this review, we evaluate the safety and efficacy of current and emerging COVID-19 vaccines in MS patients as well as DMT-related implications. Not limited to FDA-approved vaccines, this review aims to offer guidance on vaccinating MS patients worldwide during the COVID-19 pandemic based on the mechanisms of action of each vaccine candidate and each available DMT.
2. mRNA VACCINES
2.1. mRNA-1273 (Moderna)
2.1.1. Vaccine profile and dosing
mRNA-1273 is a nucleoside-modified messenger RNA (mRNA) vaccine encoding the prefusion-stabilized spike glycoprotein of SARS-CoV-2 (Jackson et al., 2020). Two doses are administered intramuscularly 28 days apart (Baden et al., 2021).
2.1.2. Immunogenicity and efficacy
Phase-1 results demonstrated robust spike-specific binding and neutralizing antibodies after one dose, and two doses produced titers comparable to convalescent sera. This regimen generated strong CD4+ T-cell responses of a type-1 helper T (Th1) phenotype, with tumor necrosis factor-α (TNFα), interleukin-2 (IL-2), and interferon-γ (IFN-γ). There was minimal type-2 helper T-cell (Th2) cytokine expression (IL-4 and IL-13) observed. CD8+ T-cell responses were low (Anderson et al., 2020; Jackson et al., 2020). The Phase-3 trial found 94.1% efficacy for preventing symptomatic COVID-19 and 100% efficacy against severe illness (Baden et al., 2021).
2.2. BNT162b2 (Pfizer)
2.2.1. Vaccine profile and dosing
BNT162b2 is a nucleoside-modified mRNA vaccine encoding the SARS-CoV-2 spike glycoprotein and is administered intramuscularly as two doses given 21 days apart (Polack et al., 2020).
2.2.2. Immunogenicity and efficacy
The Phase-1 trial demonstrated antigen-binding neutralizing antibodies that increased with the second dose and exceeded those in convalescent sera (Walsh et al., 2020). Phase 1/2 trial pre-prints showed spike protein-specific CD4+ and early effector memory CD8+ T-cell responses. Expression of IFN-γ, IL-2, and low levels of IL-4 were detected, suggesting a Th1 phenotype (Pfizer and BioNTech Provide Data from German Phase 1/2 Study Further Characterizing Immune Response Following Immunization with Lead COVID-19 Vaccine Candidate BNT162b2 | Pfizer, 2020; Sahin et al., 2020). In the global Phase 2/3 study, BNT162b2 was 95% effective in preventing COVID-19 (Polack et al., 2020).
2.3. Expected safety of mRNA COVID-19 vaccines in MS patients
These mRNA vaccines do not contain live virus, do not integrate with the human genome, and cannot cause COVID-19 infection (Pardi et al., 2018). Side effects of mRNA-1273 (Baden et al., 2021) and BNT162b2 (Polack et al., 2020) were similar, including injection-site pain and short-lived febrile symptoms. Severe adverse events were rare and comparable in vaccine and placebo groups (Baden et al., 2021; Polack et al., 2020). Notably, Bell's palsy was found in three participants vaccinated with mRNA-1273 (Baden et al., 2021) and in four participants vaccinated with BNT162b2 (FDA, 2020).
One concern with mRNA vaccines (Edwards et al., 2017; Pepini et al., 2017) is the induction of type-I interferon responses, which are linked to inflammation and autoimmunity (Theofilopoulos et al., 2005). The use of modified nucleotide mRNA COVID-19 vaccines, however, may avoid this response (Jackson et al., 2020). The FDA also concluded that Bell's palsy cases likely represent chance occurrences, but a potential vaccine contribution cannot be eliminated (FDA, 2020; FDA, Cber, 2020). (10,16). In real-life clinical practice, no red flags have been observed in MS patients receiving mRNA vaccines to date. Nevertheless, because immunocompromised patients and those on immunomodulators were excluded from these trials, continued surveillance for immune-mediated adverse events is warranted until more safety data accumulate for mRNA vaccines in MS patients.
3. Viral vector vaccines
3.1. Ad26.COV2·S (Johnson & Johnson)
3.1.1. Vaccine profile and dosing
Ad26.COV2·S is a recombinant, replication-defective human adenovirus serotype-26 (Ad26) vector that encodes the stabilized SARS-CoV-2 spike protein (Sadoff et al., 2021). Ad26.COV2·S is administered as one intramuscular injection (Johnson and Johnson Announces Single-Shot Janssen COVID-19 Vaccine Candidate Met Primary Endpoints in Interim Analysis of its Phase 3 ENSEMBLE Trial | Johnson and Johnson [WWW Document], 2020).
3.1.2. Immunogenicity and efficacy
According to Phase 1-2a results, neutralizing and spike-binding antibodies were demonstrated in >90% of vaccinated participants. CD4+ T-cell responses with a Th1-skewed response were also found, and CD8+ T-cell responses were identified by IFN-γ or IL-2 expression (Sadoff et al., 2021). The Phase-3 trial showed 66% efficacy in preventing moderate to severe COVID-19 and 100% protection against hospitalizations and deaths (Johnson and Johnson Announces Single-Shot Janssen COVID-19 Vaccine Candidate Met Primary Endpoints in Interim Analysis of its Phase 3 ENSEMBLE Trial | Johnson and Johnson [WWW Document], 2020). Notably, participants with immune-mediated diseases were excluded.
3.2. ChAdOx1 nCoV-19 (AstraZeneca)
3.2.1. Vaccine profile and dosing
ChAdOx1 nCoV-19 is a replication-deficient chimpanzee adenovirus vector with the SARS-CoV-2 spike glycoprotein antigen gene (Folegatti et al., 2020). Two intramuscular injections are given 4–12 weeks apart in various ongoing trials (AZD1222 US Phase III trial met primary efficacy endpoint in preventing COVID-19 at interim analysis [WWW Document], 2020; Voysey et al., 2021).
3.2.2. Immunogenicity and efficacy
In the Phase 2/3 trial, ChAdOx1 nCoV-19 elicited spike-specific antibodies after the first dose that increased with the booster (Ramasamy et al., 2020). A single dose induced B-cell activation and spike-specific anti-IgA and IgG antibodies. T-cell responses were Th1-skewed, with IFN-γ, TNFα, and IL-2 secretion from CD4+ T cells observed. Robust cytotoxic CD8+ T cell responses were also found (Ewer et al., 2021). Interim results from four global trials showed an overall efficacy of 70%, with an efficacy of 62.1% in participants given two standard doses and 90% in those given a half dose followed by a standard dose. The cause of this discordance between dose and efficacy is unknown (Voysey et al., 2021). Phase-3 results from the U.S. showed a 76% efficacy against symptomatic COVID-19 and 100% efficacy against severe illness (AZD1222 US Phase III primary analysis confirms safety and efficacy [WWW Document], 2020).
3.3. Expected safety of viral vector COVID-19 vaccines in MS patients
These vaccines are non-replicating, non-integrating viral vectors that do not cause COVID-19 nor adenovirus infection (Understanding and Explaining Viral Vector COVID-19 Vaccines | CDC, 2020). Adverse events in Ad26.COV2·S (Sadoff et al., 2021) and ChAdOx1 (Ramasamy et al., 2020; Voysey et al., 2021) mainly involved injection-site pain and short-lived flu-like symptoms.
One case of a steroid-responsive spinal sensory MS relapse was reported five weeks after receiving Ad26.Cov2.S. Based on old demyelinating lesions detected on MRI, investigators determined this participant's MS was pre-existing and that the reaction was unrelated to vaccination (Sadoff et al., 2021).
Notably, three cases of transverse myelitis (TM) were identified in ChAdOx1 nCoV-19 trials. One case was observed 14 days after the second dose and was diagnosed as possibly vaccine-related short segment myelitis. Another case occurred 10 days after the first vaccination and was deemed unlikely to be related upon discovering pre-existing, undiagnosed MS. The third case was noted in a control participant. In addition, a small percentage of the vaccine (0.5%) and control (0.7%) groups reported neurological events, including gait disturbance, muscular weakness, peripheral neuropathy, sensory disturbance, and visual impairment. One case of celiac disease and one case of ankylosing spondylitis were also observed in the vaccine group. Three cases of facial paralysis and two cases of uveitis were reported in each group (Voysey et al., 2021).
The potentially vaccine-related case of TM and other cases of immune-mediated and neurological events in ChAdOx1 nCoV-19 recipients raise concern for MS patients. While there were no other similar events reported for Ad26.COV2·S, the MS relapse may favor a cautious approach toward this viral vector vaccine as well. Ad26.COV2·S may have a slightly safer profile over ChAdOx1 nCoV-19, however, and its single dose allows more flexibility with DMT regimens. The use of a simian adenovirus vector in ChAdOx1 nCoV-19 may explain the more frequent immune-mediated adverse events compared to Ad26.COV2·S.
While not specific to MS or the relationship to MS DMTs, it is important to recognize the potential rare risk of venous thrombosis with thrombocytopenia with both ChAdOx1 nCoV-19 and Ad26.COV2·S vaccination. A small number of ChAdOx1 nCoV-19 recipients in several European countries have experienced disseminated intravascular coagulation and cerebral venous sinus thrombosis with thrombocytopenia (COVID-19 Vaccine AstraZeneca: benefits still outweigh the risks despite possible link to rare blood clots with low blood platelets | European Medicines Agency [WWW Document], 2020). Moreover, a small number of female Ad26.COV2·S recipients in the U.S. have experienced cerebral venous sinus thrombosis and splanchnic vein thrombosis with reduction of platelets. An abnormal immune response to platelet factors may explain the pathophysiology behind these rare adverse events (COVID-19 Vaccine Janssen: EMA finds possible link to very rare cases of unusual blood clots with low blood platelets | European Medicines Agency [WWW Document], 2020). This adds to the spectrum of immune-mediated adverse events linked to COVID-19 viral vector vaccines. Moreover, the young age of those affected raises concern regarding the risk of immune-mediated reactions in the typical age group of MS patients. The stronger immune response in younger individuals is likely responsible for their susceptibility to vaccine reactions and increased risk of immune-mediated adverse events in general. Despite a possible connection between these viral vector COVID-19 vaccines and thromboembolic events, the European Medicines Agency and the U.S. FDA have emphasized that benefits of vaccination outweigh these risks (COVID-19 Vaccine Janssen: EMA finds possible link to very rare cases of unusual blood clots with low blood platelets | European Medicines Agency [WWW Document], 2020, FDA and CDC Lift Recommended Pause on Johnson and Johnson (Janssen) COVID-19 Vaccine Use Following Thorough Safety Review [WWW Document], 2020).
To date, there are no reports of viral vector vaccines in patients with immune-mediated diseases. However, further insight into their safety can be gleaned from studies on the approved Ebola vaccine (Pollard et al., 2020) and a human immunodeficiency virus (HIV) candidate vaccine (Baden et al., 2020) using the same Ad26 vector technology. One participant developed small fiber neuropathy two weeks after receiving the Ebola vaccine and one developed rheumatoid arthritis four months after receiving one of the HIV candidate vaccines. Both events were deemed possibly related to the respective vaccines (Baden et al., 2020; Pollard et al., 2020). Taken together, these adverse events along with the reported TM and MS events in the COVID-19 viral vector vaccine trials suggest a small potential for immune-mediated and neurological adverse events. Absence of prolonged follow-up data and exclusion of immunocompromised patients add uncertainty to their full safety profile in MS patients. Non-viral vector vaccines may be preferred for MS patients if available until additional safety data accumulate for viral vector vaccines. However, if no other COVID-19 vaccines are available, the benefits of viral vector vaccination likely outweigh the risk of acquiring COVID-19 infection in MS patients.
4. Viral protein vaccines
4.1. NVX-CoV2373 (Novavax)
4.1.1. Vaccine profile and dosing
NVX-CoV2373 is a protein-based COVID-19 vaccine candidate with a modified prefusion SARS-CoV-2 spike protein created with recombinant nanoparticle technology and an adjuvant. In the Phase 1–2 trial, two doses were administered intramuscularly 21 days apart (Keech et al., 2020).
4.1.2. Immunogenicity and efficacy
Phase-1 results revealed that NVX-CoV2373 triggered robust anti-spike antibodies and neutralization responses that surpassed those of convalescent sera. It also elicited antigen-specific CD4+ T-cell responses shown by IFN-γ, IL-2, and TNF-α production. Th2 responses measured by IL-5 and IL-13 were minimal (Keech et al., 2020). Efficacies in the Phase-3 (Novavax Confirms High Levels of Efficacy Against Original and Variant COVID-19 Strains in United Kingdom and South Africa Trials, 2021) and 2b (Novavax COVID-19 Vaccine Demonstrates 89.3% Efficacy in UK Phase 3 Trial First to Demonstrate Clinical Efficacy Against COVID-19 and Both UK and South Africa Variants, 2021) trials were 96% and 60%, respectively, against COVID-19 infection, with 100% protection against severe disease. Phase 2b results are based on HIV-negative participants in South Africa, where the B.1.351 variant predominates (Novavax COVID-19 Vaccine Demonstrates 89.3% Efficacy in UK Phase 3 Trial First to Demonstrate Clinical Efficacy Against COVID-19 and Both UK and South Africa Variants, 2021).
4.2. Expected safety of viral protein vaccines in MS patients
Based on Phase-1 results, reactogenicity was absent or mild in participants vaccinated with NVX-CoV2373. A few participants had severe adverse events (headache, fatigue, fever, malaise, and/or liver enzyme elevation) (Keech et al., 2020). Phase-3 results suggest severe adverse events were rare and similar to the placebo group (Novavax COVID-19 Vaccine Demonstrates 89.3% Efficacy in UK Phase 3 Trial First to Demonstrate Clinical Efficacy Against COVID-19 and Both UK and South Africa Variants, 2021). There were no reports of serious immune-mediated or neurological events with NVX-CoV2373 (Keech et al., 2020). Several studies evaluating the efficacy of subunit vaccines in MS patients showed that patients on a variety of DMTs mount an immune response to these vaccines with no adverse events (Bar-Or et al., 2020; McCarthy et al., 2013; von Hehn et al., 2018). NVX-CoV2373 therefore may become a preferable vaccine for MS patients when available.
5. DNA vaccines
5.1. INO-4800 (Inovio)
5.1.1. Vaccine profile and dosing
INO-4800 contains a plasmid that expresses a synthetic, optimized DNA sequence of the SARS-CoV-2 spike glycoprotein. In a Phase-1 trial, participants received two intradermal doses four weeks apart (Tebas et al., 2021).
5.1.2. Immunogenicity and efficacy
Based on Phase-1 results, spike binding titers and/or neutralizing antibodies were found in 95% of participants. Cytokine production was robust from CD4+ and CD8+ T cells, including IFN-ɣ and TNF-α with or without IL-2. No increases in Th2 responses assessed by IL-4 production were found (Tebas et al., 2021). Notably, multi-cytokine production from CD8+ T cells reported in this study may surpass results from other vaccine studies, including those evaluating mRNA-1273 and ChAdOx1 nCoV-19 (Tebas et al., 2021). In other trials with similar DNA vaccines, immune responses lasting up to a year have been reported (Modjarrad et al., 2019; Tebas et al., 2019). No efficacy data is available at this time for NVX-CoV2373.
5.2. Expected safety of DNA-based COVID-19 vaccines in MS patients
According to Phase-1 results, all local and systemic events were mild, with no reported immune-mediated or neurological events with INO-4800. One mild nervous system disorder was reported and was not related to vaccination (Tebas et al., 2021).
The DNA plasmid does not interfere with or alter host cell DNA and does not contain a live virus (NEWS RELEASE INOVIO Doses First Subject in Phase 2 Segment of its INNOVATE Phase 2/3 Clinical Trial for INO-4800, its DNA Medicine to Prevent COVID-19, 2020). No DNA-based vaccines are currently approved for human use, making the safety profile for patients with immune-mediated diseases unclear. However, previous results of trials investigating DNA vaccines for Zika Virus (Tebas et al., 2017) and Middle East respiratory syndrome coronavirus (Modjarrad et al., 2019) reported no serious neurological or immunological adverse events. While Phase-1 results for INO-4800 were based on a small sample size and limited follow-up, the absence of severe neurological and immune-mediated adverse events is encouraging (Tebas et al., 2021). Larger, later-stage trials and long-term surveillance are still needed to assess safety in MS patients.
6. Inactivated virus vaccines
6.1. BBIBP-CorV (Sinopharm)
6.1.1. Vaccine profile and dosing
BBIBP-CorV is an inactivated virus vaccine formulated with an adjuvant and has 100% homology for the spike protein (Wang et al., 2020). Several countries are administering BBIBP-CorV in a two-dose regimen.
6.1.2. Immunogenicity and efficacy
Data regarding cellular immune responses in the Phase-1 trial are not available, but all participants developed neutralizing antibodies (Xia et al., 2021). Interim Phase-3 results from the United Arab Emirates demonstrated an 86% efficacy against COVID-19 infection (UAE Says Sinopharm Vaccine has 86% Efficacy Against COVID-19 | Reuters, 2020).
6.2. Expected safety of inactivated COVID-19 vaccines in MS patients
In Phase-1/2 trials for BBIBP-CorV, all adverse reactions were mild or moderate, and no reports of immune-mediated or neurological adverse events were published (Xia et al., 2021).
BBIBP-CorV is likely a suitable option for MS patients, given that several studies have demonstrated safety of various inactivated vaccines in MS patients on different DMTs (Bar-Or et al., 2013; Kaufman et al., 2014; Mehling et al., 2013; Metze et al., 2019; Olberg et al., 2018; Olberg et al., 2014; Schwid et al., 2005; Vågberg et al., 2012). Furthermore, the lack of serious neurological and immune-mediated adverse events from Phase 1/2 (Xia et al., 2021) supports the suspected safety of this vaccine candidate. Later stage trials are still needed to fully assess safety in MS patients.
7. Live-attenuated vaccines
7.1. MV-014-210 (Meissa)
7.1.1. Vaccine profile and dosing
MV-014-210 is a live-attenuated COVID-19 vaccine candidate. If shown to elicit a safe and efficacious immune response, MV-014-210 will offer the convenience of a single intranasal administration (Meissa Vaccines Provides a Pipeline Update on Vaccine Candidates for COVID-19 and RSV, 2020).
7.1.2. Immunogenicity and efficacy
No available data.
7.2. Expected safety of live-attenuated COVID-19 vaccines in MS patients
No safety data are currently available for MV-014-210. According to the American Academy of Neurology's (AAN) 2019 guidelines regarding vaccination and MS, live-attenuated vaccines should not generally be recommended to MS patients taking DMTs and those who recently used DMTs, given possible risks of infection associated with immunosuppressed patients (Farez et al., 2019). Indeed, package inserts of many DMTs advise against live vaccination during and shortly before treatment.
However, these guidelines also state that clinicians may recommend live-attenuated vaccines for MS patients taking DMTs if non-live vaccines are unavailable and the risk of infection is high (Farez et al., 2019). Given the number of non-live vaccines already approved and/or in late-stage trials, the use of live-attenuated COVID-19 vaccines in MS patients is not recommended, especially for those on cell-depleting DMTS.
8. Expected interaction of COVID-19 vaccines with MS DMTS
8.1. Interferon-Beta
Interferon-β (IFN-β) is an immunomodulator that increases expression of anti-inflammatory cells and decreases expression of pro-inflammatory cytokines (Kieseier, 2011). IFN-β agents are given intramuscularly or subcutaneously multiple times per week, weekly, or every two weeks.
Based on inactivated influenza vaccine studies, IFN-β does not seem to hamper the protective immune response to these inactivated vaccines in MS patients (Mehling et al., 2013; Metze et al., 2019; Olberg et al., 2018; Olberg et al., 2014; Schwid et al., 2005). Furthermore, in a study evaluating responses in patients taking IFN-β to the tetanus-diphtheria toxoid, pneumococcal polysaccharide, and meningococcal conjugate vaccines, 73% produced sufficient immune responses (von Hehn et al., 2018). Therefore, MS patients taking IFN-β will likely achieve adequate protection from inactivated and protein-based COVID-19 vaccines.
There are no studies evaluating mRNA, viral vector, and DNA vaccines in MS patients on DMTs, and thus the true efficacy of these COVID-19 vaccines in treated MS patients is unknown. Studies have shown, however, that IFN-β reduces levels of IFN-γ and TNFα (Salama et al., 2003), which help mediate Th1 responses to several COVID-19 vaccines. Nonetheless, because IFN-β does not deplete the lymphocytes that primarily mount immune responses to these vaccines, MS patients on IFN-β will likely achieve sufficient protection from the mentioned COVID-19 vaccines. In line with the 2019 AAN recommendations, live vaccines such as MV-014-210 should be avoided in treated MS patients unless absolutely necessary (Farez et al., 2019).
8.2. Glatiramer acetate
Glatiramer acetate (GA) is a mixture of synthetic polypeptides that was purported to compete with myelin antigens for interaction with antigen-presenting cells. Newer studies suggest the mechanism of action for GA includes immunomodulation of Th2, T-regulatory, antigen presenting, and B cells. GA-induced immunomodulation modifies the T-cell response against myelin and causes secretion of anti-inflammatory cytokines to reduce CNS inflammatory demyelination (Lalive et al., 2011). It is a subcutaneous injection given daily or three days a week.
One study found potentially attenuated immune responses to the influenza vaccine in MS patients taking GA (Pellegrino et al., 2014), but several other studies did not find a negative impact (Metze et al., 2019; Olberg et al., 2018). Overall, it seems likely that MS patients taking GA will mount an appropriate immune response to inactivated COVID-19 vaccines. As GA does not deplete lymphocytes, it is also unlikely to impact the protective immune response to mRNA, viral vector, DNA, and protein vaccines in MS patients. While there is no available data regarding MS patients taking GA and their responses to live-attenuated vaccines, live-attenuated COVID-19 vaccines are not preferred in line with the AAN recommendations (Farez et al., 2019).
8.3. Teriflunomide
Teriflunomide is a dihydroorotate dehydrogenase inhibitor that reduces replication of auto-reactive lymphocytes while preserving memory cells (Cross and Naismith, 2014). It is a daily oral medication.
Based on inactivated influenza and rabies vaccine studies, teriflunomide is unlikely to reduce the protective immune response against inactivated COVID-19 vaccines (Bar-Or et al., 2015; Bar-Or et al., 2013). In addition, successful development of anti-SARS-CoV-2 antibodies at levels comparable to patients not receiving immunotherapies was described in a patient taking teriflunomide after COVID-19 infection (Bollo et al., 2020). Teriflunomide reduces proliferation of auto-reactive lymphocytes and not lymphocytes that respond to foreign antigens such as the SARS-CoV-2 spike glycoprotein. Therefore, MS patients taking teriflunomide will likely mount an immune response to the mentioned mRNA, DNA, viral vector, and protein-based COVID-19 vaccines. Live-attenuated vaccines are not recommended during treatment and for 6 months after discontinuation (AUBAGIO® (teriflunomide) tablets, for oral use Prescribing Information, 2020), and therefore live-attenuated COVID-19 vaccines are not a preferable option for MS patients on teriflunomide.
8.4. Fumarates
Dimethyl fumarate (DMF) and diroximel fumarate share the active metabolite monomethyl fumarate (MMF). MMF has an unknown mechanism of action but is thought to cause immunomodulation by inhibiting the Nrf-2 protein, ultimately blocking inflammatory cascades (Cross and Naismith, 2014). One significant adverse effect is lymphopenia, which occurs in 37% of patients but is only severe in 8% of patients (Fox et al., 2016). Fumarates are taken orally twice a day.
One study assessing the immune response of MS patients on DMF to the tetanus-diphtheria toxoid, pneumococcal polysaccharide, and meningococcal conjugate vaccines found that DMF did not reduce T-cell dependent and humoral immune responses (von Hehn et al., 2018). It follows that that MS patients taking fumarates with normal lymphocyte counts will likely achieve sufficient protection from inactivated and protein-based COVID-19 vaccines. Furthermore, fumarates do not directly deplete lymphocytes and instead act mainly on downstream immune targets. Therefore, fumarates are unlikely to reduce the cellular immune response elicited by mRNA, DNA, and viral vector COVID-19 vaccines in non-lymphopenic patients. However, patients with moderate to severe fumarate-induced lymphopenia may not mount an adequate immune response to the mentioned COVID-19 vaccines. Accordingly, the absolute lymphocyte count should be checked before vaccination. Temporary interruption of fumarate treatment may be necessary in some patients to allow lymphocytic recovery prior to vaccination. While compatibility of fumarates with live-attenuated vaccines is unknown, they are not preferred based on AAN guidance (Farez et al., 2019).
8.5. Sphingosine-1-phosphate modulators
Sphingosine-1-phosphate (S1P) modulators prevent egress of T and B cells from lymph nodes. This change to lymphocyte trafficking reduces the number of lymphocytes available to the CNS and therefore decreases central inflammation. They do not directly deplete lymphocytes, however (Cross and Naismith, 2014). S1P modulators are taken orally once daily.
Fingolimod has been shown to dampen the cellular and humoral immune responses against vaccines, including in MS patients receiving the inactivated influenza (Kappos et al., 2015a; Metze et al., 2019; Olberg et al., 2018) and tetanus toxoid booster (Kappos et al., 2015b) vaccines. In addition, fingolimod has attenuated anti-SARS-CoV-2 antibodies in infected patients (Bollo et al., 2020). Furthermore, the AAN guidelines state that MS patients taking fingolimod have a decreased likelihood of achieving protection from the influenza vaccine (Farez et al., 2019).
Based on this evidence and the fact that S1P modulators reduce T and B cell infiltration into the CNS via lymphocyte trapping in secondary lymphoid tissues, it is likely that MS patients taking S1P modulators will produce an attenuated immune response to inactivated, mRNA, DNA, protein-based, and viral vector COVID-19 vaccines. For patients who receive the vaccine, it will be beneficial to check anti-spike protein neutralizing antibodies after vaccination to assess if an adequate immune response was mounted and to evaluate the need for a booster dose. With regards to live-attenuated vaccines in development, the prescribing information for fingolimod, siponimod, and ozanimod state that live-attenuated vaccines should be avoided during therapy and for 2 months, 4 weeks, or 3 months after discontinuation, respectively (Dosing | GILENYA® (fingolimod) | HCP, 2020; Relapsing MS Progression Treatment | MAYZENT® (siponimod) [WWW Document], 2020; ZEPOSIA, 2020). Unlike the fumarates, interruption of S1P modulator therapy to maximize benefit from the vaccine is not feasible because stopping this medication class may increase risk of severe MS rebound (Hatcher et al., 2016).
8.6. Natalizumab
Natalizumab is a monoclonal antibody (MAB) against alpha-4 integrin on the surface of leukocytes that blocks their interaction with vascular cell adhesion molecules and prevents leukocyte migration to the CNS. It is a non-depleting immunomodulator not associated with lymphopenia (Cross and Naismith, 2014). Its maintenance dose is administered as an intravenous infusion every 28 days.
MS patients on natalizumab receiving the inactivated influenza and tetanus toxoid vaccines mounted comparable immune responses to those not taking natalizumab (Kaufman et al., 2014; Vågberg et al., 2012). Other studies evaluating inactivated influenza vaccine responses suggested a reduced immune response in some MS patients treated with natalizumab (Metze et al., 2019; Olberg et al., 2018; Olberg et al., 2014). However, the AAN states there is insufficient evidence to determine if MS patients receiving natalizumab mount an appropriate versus attenuated immune response to these two vaccines (Farez et al., 2019).
Based on natalizumab's mechanism of action and data from vaccine studies, it seems likely that MS patients taking natalizumab will achieve protection—although perhaps slightly attenuated—from inactivated COVID-19 vaccines. Natalizumab is not a cell-depleting agent so it is also unlikely to impact mRNA, DNA, viral vector, or protein-based COVID-19 vaccines. The compatibility of natalizumab with live-attenuated vaccines is unknown, but given caution expressed by the AAN, live-attenuated COVID-19 vaccines are not preferred. It is unknown if the extended dosing interval adopted by some centers to reduce the risk of progressive multifocal leukoencephalopathy will affect vaccine efficacy. It is also unknown—but unlikely—that timing of vaccination relative to infusion timing impacts vaccine efficacy. Interruption of natalizumab treatment for vaccine administration is unlikely to influence vaccine efficacy and is not recommended since it may cause severe MS rebound (Rasenack and Derfuss, 2016).
8.7. Ocrelizumab
Ocrelizumab is a humanized anti-CD20 MAB that causes selective B-cell depletion (Cross and Naismith, 2014; Fox et al., 2019). Although it does not target plasma cells, prolonged depletion of memory B cells may cause hypogammaglobulinemia in some patients (Roberts et al., 2015). Its maintenance dose is administered as an intravenous infusion every six months.
By its effect on B cells, ocrelizumab can dampen the humoral response to vaccines. In addition, B cells act as antigen-presenting cells to T cells and are likely implicated in cell-mediated vaccine responses as well. Ocrelizumab has also been shown to cause near-complete depletion of CD20-expressing T cells within 2 weeks of administration in MS patients (Gingele et al., 2018). The duration of T cell versus B cell depletion is unclear, although one study found persistent and marked depletion of T cells in ocrelizumab-treated MS patients for at least 6 months (Capasso et al., 2021). Therefore, MS patients taking ocrelizumab may mount an attenuated humoral—and possibly cellular—immune response against COVID-19 vaccines. Moreover, a lack of anti-SARS-CoV-2 antibodies produced after COVID-19 infections has been reported in patients treated with ocrelizumab, supporting the notion that this DMT may dampen the protective immune response elicited by mRNA, DNA, viral vector, protein-based, and inactivated COVID-19 vaccines (Conte, 2020; Lucchini et al., 2020; Meca-Lallana et al., 2020; Thornton and Harel, 2020).
The VELOCE study investigated various vaccine responses in MS patients taking ocrelizumab 12 weeks after treatment. This study provided evidence that peripherally-depleted B cell patients mounted attenuated humoral responses to the inactivated influenza, tetanus toxoid, and pneumococcal polysaccharide vaccines. Markers of cellular immune response were not analyzed. The study concluded that despite the attenuated response, partial protection against vaccine-preventable infections is expected in patients taking ocrelizumab. The vaccine response in ocrelizumab patients with hypogammaglobulinemia was not reported (Bar-Or et al., 2020). Protein-based, inactivated, mRNA, DNA, and viral vector COVID-19 vaccines in patients taking ocrelizumab are therefore also likely to be partially efficacious in ocrelizumab-treated patients. Moreover, most viral vaccines, including COVID-19 vaccines, rely mainly on the cellular immune response (Clem, 2011), which is likely less affected by ocrelizumab compared to the humoral response.
Maximum attenuation of the immune response to COVID-19 vaccines is expected during maximum B cell depletion. Therefore, it is advisable to administer vaccines at least 12 weeks after ocrelizumab dosing and 4–6 weeks prior to the next infusion to optimize vaccine efficacy. This practice was adopted during the VELOCE study and is recommended by the NMSS (Bar-Or et al., 2020; MS Treatment Guidelines During the Coronavirus pandemic | National MS Society | National Multiple Sclerosis Society, 2020). It is important to note that B cell depletion may last longer than 12 weeks post-infusion, and memory B cell depletion may persist for after a year following treatment (Baker et al., 2020). Accordingly, if a patient on ocrelizumab decides to receive a COVID-19 vaccine, measuring levels of immunoglobulins and lymphocytes may help guide vaccination timing and modification of dosing intervals.
Depending on patient preferences and individual exposure risks, patients may choose to either modify vaccination timing based on their ocrelizumab dosing schedule or vice versa. For patients without flexibility in vaccine timing and those who choose not to delay vaccination, modification of ocrelizumab dosing should be considered to maximize vaccine efficacy. It is possible to postpone ocrelizumab dosing, especially in patients with prolonged B cell depletion, hypogammaglobulinemia, and less active disease (e.g. primary progressive MS). However, delaying the dose is not preferred in patients with significant B cell repletion toward the end of a treatment cycle and in those with highly active MS. All patients should be educated that non-live COVID-19 vaccines are expected to be safe and partially efficacious regardless of the timing of vaccine administration in relation to ocrelizumab dosing and that these recommendations are theoretical strategies to maximize benefit. If coordinating ideal timing is not possible, patients should proceed with vaccination at any time. Checking neutralizing antibodies post-vaccination should be considered to confirm vaccine efficacy and evaluate the need for a booster dose.
Finally, ocrelizumab is incompatible with live-attenuated vaccines. Patients who do choose a live-attenuated COVID-19 vaccine should receive the vaccine at least 4 weeks before starting ocrelizumab and avoid it after starting treatment (Genentech, Inc, 2020).
8.8. Rituximab
Rituximab is currently used off-label in some MS patients in the U.S. and is widely used among MS patients in other countries. Rituximab is a chimeric B-cell-depleting anti-CD20 MAB that exerts cell death through apoptosis, antibody-dependent cellular cytotoxicity and cell-mediated phagocytosis, and complement-dependent cytotoxicity. It is thought to elicit therapeutic benefits in MS via apoptosis of certain pro-inflammatory CD3+ T cells that express CD20 and via changes in B-cell-mediated cytokine and antibody production, antigen presentation, and T-cell activation. Hypogammaglobulinemia is commonly observed with chronic rituximab treatment, while late-onset neutropenia and agranulocytosis occur rarely. Although a rituximab dosing consensus for MS is lacking, some European countries are administering rituximab as an intravenous infusion every six months (Chisari et al., 2021).
While vaccine studies on rituximab-treated MS patients are unavailable, rituximab-mediated B and CD20-expressing T cell depletion is likely to dampen the protective immune response to vaccines. Of note, almost complete depletion of CD3+ CD20 T cells in rituximab-treated MS patients was reported to last for at least one year following rituximab treatment (Palanichamy et al., 2014). MS patients taking rituximab thus may mount an attenuated humoral and possibly cellular immune response against COVID-19 vaccines. A lack of anti-SARS-CoV-2 antibodies produced after COVID-19 infection in a lymphopenic rituximab-treated MS patient (Wurm et al., 2020) further suggests this DMT may hamper the protective immune response elicited by mRNA, DNA, viral vector, protein-based, and inactivated COVID-19 vaccines. Indeed, vaccine studies in patients treated with rituximab for cancer and rheumatological diseases suggest that an impaired immune response to various vaccines is likely. Among lymphopenic patients with lymphoma who received the influenza vaccine within 6 months of rituximab treatment, no protective immune response was detected (Yri et al., 2011). Another study assessing the efficacy of the pneumonia polysaccharide vaccine and the influenza conjugate vaccine in rituximab-treated patients with immune thrombocytopenia found reduced antibody responses for 6 months after treatment (Nazi et al., 2013).
Rituximab treatment typically results in almost-complete B cell depletion that begins two weeks after the infusion and lasts 6–12 months. Naive B-cell recovery typically occurs after 12 months, although the depletion of memory B cells may persist for several years. As with ocrelizumab, maximum attenuation of the immune response to vaccines is expected during maximum B cell depletion. Therefore, it is advisable to administer COVID-19 vaccines at least 3 to 6 months after rituximab dosing and at least 6 weeks prior to the next infusion to optimize vaccine efficacy. Live-attenuated vaccines are not recommended during rituximab treatment until complete B cell recovery (Chisari et al., 2021).
As with ocrelizumab, careful coordination of COVID-19 vaccination timing and rituximab dosing according to patient preferences, disease status, exposure risks, and vaccine access will help maximize vaccine efficacy. Moreover, measuring levels of immunoglobulins and lymphocytes may help guide vaccination timing and modification of dosing intervals. Checking neutralizing antibodies post-vaccination will also help assess vaccine efficacy and the need for a booster dose.
8.9. Ofatumumab
Ofatumumab is an anti-CD20 MAB administered as a monthly subcutaneous injection. B-cell depletion after ofatumumab injection is shorter than for ocrelizumab (Lulu and Waubant, 2013). It is expected to reduce the humoral, and to a lesser extent, the cellular vaccine response. Nevertheless, a case report has described an ofatumumab-treated MS patient with depleted B cells and normal immunoglobulin levels who developed anti-SARS-CoV-2 antibodies after asymptomatic COVID-19 infection (Flores-Gonzalez et al., 2021). To optimize COVID-19 vaccine response, patients may need to receive the vaccine at least one month after their latest ofatumumab dose and delay the subsequent ofatumumab dose one month after the second vaccine dose. This requires “skipping” two doses of ofatumumab, since most COVID-19 vaccines have a 3–4 week interval between doses. The single dose vaccine Ad26.COV2·S may be favorable as it requires only skipping one injection. The decision to delay ofatumumab dosing can be guided by B cell counts and the degree of MS activity. Delaying treatment is not preferred, however, in highly active MS patients and in those with early B cell repletion. In these cases, COVID-19 vaccines can be administered mid-cycle.
8.10. Alemtuzumab
Alemtuzumab is a humanized MAB that targets CD52 receptors on the surface of lymphocytes, causing generalized lymphopenia with a more pronounced effect on T cells (Cross and Naismith, 2014). Alemtuzumab also alters the innate immune response by acting on CD52-positive macrophages and dendritic cells (Gross et al., 2016). It is given intravenously in two courses one year apart. In some patients, alemtuzumab can reset the abnormal immune response after the second course, eliminating the need for lifelong treatment. Alemtuzumab-induced cytopenias affect T cells, B cells, and innate immune cells during the cell-depletion phase, which could reduce cellular and humoral immunity elicited by COVID-19 vaccination in the first 6 months during maximum lymphopenia (Gross et al., 2016). Due to early B-cell versus delayed T-cell recovery, vaccination six months after dosing may result in a relatively stronger humoral and weaker cellular immune response.
In a study testing vaccination responses in MS patients taking alemtuzumab, responses to tetanus-diphtheria toxoid, inactivated poliomyelitis, influenza, pneumococcal polysaccharide, and meningococcal conjugate vaccines were maintained yet somewhat reduced in those receiving vaccinations within 6 months of dosing (McCarthy et al., 2013). If vaccination can be delayed until at least 6 months after treatment, it is likely that MS patients taking alemtuzumab might generate an attenuated immune response to inactivated, protein, mRNA, DNA, and viral vector vaccines. Patients who have completed both courses of alemtuzumab with complete immune reconstitution are expected to mount full immune responses.
For vaccinated patients on alemtuzumab, it will be advisable to check neutralizing antibodies after vaccination and wait 4–6 weeks after vaccination before resuming therapy to ensure an adequate immune response was achieved. Alemtuzumab is incompatible with live vaccines 6 weeks before treatment, during treatment, and after recent treatment (LEMTRADA® (alemtuzumab) | Healthcare Professional Site, 2020).
8.11. Cladribine
Cladribine is a purine nucleoside analog that inhibits DNA synthesis and repair in highly dividing cells, inducing B- and T-cell apoptosis with a more pronounced and longer effect on B cells. Lymphopenia occurs during the depletion phase followed by lymphocyte recovery over months. It has a limited effect on the innate immune system (Giovannoni, 2017). Cladribine is given as an oral treatment in two courses one year apart. Each course is composed of two 5-day cycles one month apart. Immune reconstitution occurs after the second course, and most patients will not require further treatment.
There are no relevant vaccine studies on patients taking cladribine to make robust efficacy claims. Case reports of antibody production after COVID-19 infection in MS patients taking cladribine are mixed. Some have described cladribine-treated MS patients who produced anti-SARS-CoV-2 antibodies with (De Angelis et al., 2020) and without (Celius, 2020) lymphopenia, and another described a cladribine-treated patient with no SARS-CoV-2 antibodies months after infection (Gelibter et al., 2021).
Nonetheless, cladribine-induced non-selective lymphopenia is likely to attenuate both the humoral and cellular immune responses to COVID-19 vaccines during the cell-depletion phase (Stuve et al., 2019). Vaccination may therefore be considered at least 6 months after the last treatment; however, vaccine efficacy may still be reduced and post-vaccination neutralizing antibodies should be checked. The cellular immune response is expected to be less affected in cladribine patients compared to alemtuzumab if vaccines are administered six months after dosing due to earlier T cell recovery. It may be reasonable for MS patients to delay their next course of cladribine 4–6 weeks after vaccination to achieve sufficient protection against COVID-19. Patients who have completed both courses of cladribine with complete immune reconstitution and no lymphopenia are expected to mount a full immune response to mRNA, DNA, viral vector, protein, and inactivated COVID-19 vaccines. Based on cladribine's prescribing information, patients should not receive live vaccines within 4–6 weeks before treatment or at any time during treatment (MAVENCLAD® (cladribine) Tablets, 2020).
9. Discussion
As the COVID-19 pandemic lingers and vaccines that prevent severe illness become increasingly available, choosing a safe and efficacious vaccine in the MS population is critical. Clinicians should devise the optimal strategy factoring a patient's preferences, MS status, medical history, and DMT class in the context of the COVID-19 vaccines available.
Despite concerns surrounding vaccines and immune-mediated diseases, the AAN's 2019 guidelines emphasize that there is no clear evidence that vaccination increases the risk of MS activity (Farez et al., 2019). Various vaccines have been implicated in rare monophasic demyelinating events, such as acute disseminated encephalomyelitis, optic neuritis, and transverse myelitis (Kumar et al., 2020). However, viral infections including COVID-19 are more strongly linked to immune-mediated neurological events (Guilmot et al., 2021), and therefore the benefits of COVID-19 vaccination outweigh the risks in MS patients.
While safety of COVID-19 inactivated and protein-based vaccines in MS patients can be inferred from studies of non-COVID-19 vaccines with similar properties, the novelty of mRNA, DNA, and viral vector COVID-19 vaccines makes it difficult to predict their safety in MS. Nevertheless, many MS patients have since received mRNA COVID-19 vaccines without red flags thus far. Based on safety data from clinical trials, no serious issues relevant to MS were reported with mRNA vaccines other than rare facial palsy cases. Likewise, the human viral vector vaccine Ad26.COV2·S showed a favorable safety profile with only a single report of MS relapse in an untreated patient. However, the simian viral vector vaccine ChAdOx1 nCoV-19 is linked to a probable vaccine-related transverse myelitis case and an MS relapse in an untreated patient. Both COVID-19 viral vector vaccines are also potentially linked to rare immune-mediated thromboembolic events in young vaccine recipients. Other viral vector vaccines have been associated with rare immune-mediated adverse events as well. Overall, if inactivated, protein-based, mRNA, or DNA vaccines are available, they may be anecdotally preferred over viral vector vaccines in MS patients for their lower immunogenicity and weaker association with immune-mediated adverse events. If only viral vector vaccines are available, benefits of vaccination still outweigh the risks of infection in MS patients. Ad26.COV2·S offers the benefit of a single dose, facilitating coordination with cell-depleting DMTs.
Because live-attenuated COVID-19 vaccines are contraindicated with certain DMTs and not preferred with most others, they should be avoided in treated MS patients if alternatives are available. In untreated MS patients, there is less concern of live vaccines causing symptomatic COVID-19 infection. However, there is concern for vaccine-induced relapse, as live vaccines usually produce stronger immune responses, especially in the absence of immunotherapy. Indeed, vaccine studies in neuromyelitis optica have shown increased risks of vaccine-related relapses in untreated patients while treated patients were protected (Mealy et al., 2018).
Studies demonstrating adequate immune responses elicited to inactivated and protein-based vaccines in MS patients on a variety of DMTs suggest that protein-based and inactivated COVID-19 vaccines are likely efficacious in treated MS patients. RNA, DNA, and viral vector vaccines are also expected to be effective in the MS population.
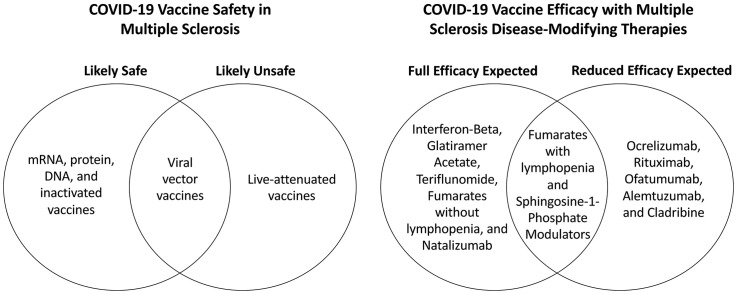
Many DMTs are not expected to significantly impact vaccine efficacy, including IFN-β, GA, teriflunomide, natalizumab, and the fumarates (without lymphopenia). The effect of fumarate-induced lymphopenia on vaccine efficacy is unknown, but attenuated vaccine responses in patients with moderate or severe lymphopenia is possible. Attenuated responses are also expected with S1P modulators due to reduction of circulating lymphocytes; however, treatment interruption is not recommended to avoid disease rebound.
MS patients taking cell-depleting agents (ocrelizumab, rituximab, ofatumumab, alemtuzumab, and cladribine) will likely have attenuated vaccine responses, especially if vaccinated during the maximum cell depletion period. Since these DMTs follow widely-spaced dosing regimens, coordinating vaccine timing to avoid the maximum cell depletion period may maximize vaccine efficacy. However, limited vaccine availability in various regions may pose difficulties for patients who wish to coordinate vaccination with treatment timing. It is also important to note that most of the vaccine studies mentioned in this review discuss humoral—rather than cellular—immune responses to vaccination in MS patients on a variety of DMTs. T-cell mediated responses are critical, however, for protection against viral infections such as COVID-19 and for mounting a protective immune response to the mentioned COVID-19 vaccines. Considering the implications of individual DMTs on the humoral versus cellular immune response to COVID-19 vaccines is thus relevant. In particular, ocrelizumab, rituximab, and ofatumumab are expected to more negatively impact the humoral response while alemtuzumab will likely more strongly weaken the cellular response. Cladribine is expected to elicit responses somewhere in the middle. MS patients on these DMTs should be advised that attenuated vaccine responses may still offer partial protection against COVID-19. Testing post-vaccination neutralizing antibodies will help assess immune responses, evaluate the need for boosters, and guide recommendations regarding continued masking and social distancing.
All patients should be educated that transient worsening of residual neurological symptoms after vaccination is possible but does not indicate a new relapse in most cases. They should also be assured that DMTs likely provide some protection against potential vaccine-related demyelinating or immune-mediated adverse events.
10. Conclusions
Non-live COVID-19 vaccines are likely safe and effective in MS patients on various DMTs. Demyelinating events are extremely rare with most COVID-19 vaccines and have mainly been reported with viral vector vaccines. Live-attenuated vaccines should be avoided as much as possible in treated MS patients. Attenuated but partially protective vaccine responses are expected in MS patients taking S1P modulators and cell-depleting DMTs. Other DMTs are not expected to significantly impact the efficacy of COVID-19 vaccines.
Table 1 summarizes the safety and efficacy of current and emerging COVID-19 vaccines in relation to various classes of DMTs for MS patients.
Table 1.
Current and emerging COVID-19 vaccines and their relevance to MS.
| COVID-19 vaccine | mRNA vaccines (Moderna, Pfizer) | Inactivated (Sinopharm), protein-based (Novavax), and DNA (Inovio) vaccines | Human viral vector vaccine (Johnson & Johnson) | Simian viral vector vaccine (AstraZeneca) | Live-attenuated vaccine (Meissa) |
|---|---|---|---|---|---|
| Number of doses | Two | Two | One | Two | One |
| Severe immune-mediated adverse events | Rare facial palsy | None reported | One report of MS relapse in an untreated patient | One report of vaccine-related TM and one report of MS relapse in an untreated patient | Unknown, but has a higher potential for immune-mediated adverse events and a higher risk of causing COVID-19 infection in immunocompromised patients |
| IFN-B, GA, teriflunomide, and natalizumab | No major safety or efficacy concerns | No major safety or efficacy concerns | No major safety or efficacy concerns | No major safety or efficacy concerns | Not preferred |
| Fumarates | Possible decreased vaccine efficacy in patients with severe lymphopenia Consider dose interruption in severely lymphopenic patients to allow for lymphocytic recovery prior to vaccination |
Possible decreased vaccine efficacy in patients with severe lymphopenia Consider dose interruption in severely lymphopenic patients to allow for lymphocytic recovery prior to vaccination |
Possible decreased vaccine efficacy in patients with severe lymphopenia Consider dose interruption in severely lymphopenic patients to allow for lymphocytic recovery prior to vaccination |
Possible decreased vaccine efficacy in patients with severe lymphopenia Consider dose interruption in severely lymphopenic patients to allow for lymphocytic recovery prior to vaccination |
Not preferred and may be especially dangerous in patients with severe lymphopenia |
| S1P modulators | Possible decreased cellular vaccine response Interruption of treatment not recommended to avoid MS rebound |
Possible decreased cellular vaccine response Interruption of treatment not recommended to avoid MS rebound |
Possible decreased cellular vaccine response Interruption of treatment not recommended to avoid MS rebound |
Possible decreased cellular vaccine response Interruption of treatment not recommended to avoid MS rebound |
Contraindicated |
| Ocrelizumab | Possible decreased humoral > cellular vaccine response Consider vaccination between month 3 and 5 of the treatment cycle to maximize vaccine efficacy Consider delaying infusion to one month after 2nd vaccine |
Possible decreased humoral > cellular vaccine response Consider vaccination between month 3 and 5 of the treatment cycle to maximize vaccine efficacy Consider delaying infusion to one month after 2nd vaccine |
Possible decreased humoral > cellular vaccine response Consider vaccination between month 3 and 5 of the treatment cycle to maximize vaccine efficacy Consider delaying infusion to one month after vaccination |
Possible decreased humoral > cellular vaccine response Consider vaccination between month 3 and 5 of the treatment cycle to maximize vaccine efficacy Consider delaying infusion to one month after 2nd vaccine |
Contraindicated |
| Ofatumumab | Possible decreased humoral > cellular vaccine response Consider vaccination one month after injection and delay next injection for one month after 2nd vaccine |
Possible decreased humoral > cellular vaccine response Consider vaccination one month after injection and delay next injection for one month after 2nd vaccine |
Possible decreased humoral > cellular vaccine response Consider vaccination one month after injection and delay next injection for one month after vaccination |
Possible decreased humoral > cellular vaccine response Consider vaccination one month after injection and delay next injection for one month after 2nd vaccine |
Contraindicated |
| Rituximab | Possible decreased humoral > cellular vaccine response Consider vaccination 3 to 6 months after injection and delay next injection for 4–6 weeks after 2nd vaccine |
Possible decreased humoral > cellular vaccine response Consider vaccination 3 to 6 months after injection and delay next injection for 4–6 weeks after 2nd vaccine |
Possible decreased humoral > cellular vaccine response Consider vaccination 3 to 6 months after injection and delay next injection for 4–6 weeks after vaccination |
Possible decreased humoral > cellular vaccine response Consider vaccination 3 to 6 months after injection and delay next injection for 4–6 weeks after 2nd vaccine |
Contraindicated |
| Alemtuzumab | Possible decreased cellular > humoral vaccine response Consider vaccination six months after completion of treatment course Consider delaying treatment course to one month after 2nd vaccine |
Possible decreased cellular > humoral vaccine response Consider vaccination six months after completion of treatment course Consider delaying treatment course to one month after 2nd vaccine |
Possible decreased cellular > humoral vaccine response Consider vaccination six months after completion of treatment course Consider delaying treatment course to one month after vaccination |
Possible decreased cellular > humoral vaccine response Consider vaccination six months after completion of treatment course Consider delaying treatment course to one month after 2nd vaccine |
Contraindicated |
| Cladribine | Possible decreased humoral > cellular vaccine response Consider vaccination six months after completion of the second treatment cycle Consider delaying treatment course to one month after 2nd vaccine |
Possible decreased humoral > cellular vaccine response Consider vaccination six months after completion of the second treatment cycle Consider delaying treatment course to one month after 2nd vaccine |
Possible decreased humoral > cellular vaccine response Consider vaccination six months after completion of the second treatment cycle Consider delaying treatment course to one month after vaccination |
Possible decreased humoral > cellular vaccine response Consider vaccination six months after completion of the second treatment cycle Consider delaying treatment course to one month after 2nd vaccine |
Contraindicated |
| Other considerations | Widely available vaccines and many MS patients received them without red flags | Inactivated and protein-based vaccines have a long track record of safety and compatibility with MS DMTs | Single dosing is convenient for patients on cell-depleting DMTs who wish to coordinate vaccine timing with DMT dosing to maximize vaccine efficacy | More reports of demyelinating side effects than other COVID-19 vaccines but they remain rare, isolated events | Lower risk for COVID-19 infection in untreated MS patients but possibly higher risk for inducing relapse |
Declaration of Competing Interest
Dr. Abboud is a consultant and speaker for Biogen, Genentech, Bristol Myer Squibb, Alexion, and Viela Bio. He receives research support to conduct clinical trials for Novartis, Genentech, Bristol Myer Squibb, and Sanofi Genzyme. Dr. Sokola has received consulting fees from Alexion. Hannah Kelly has no financial disclosures to report.
References
- Anderson E.J., Rouphael N.G., Widge A.T., Jackson L.A., Roberts P.C., Makhene M., Chappell J.D., Denison M.R., Stevens L.J., Pruijssers A.J., McDermott A.B., Flach B., Lin B.C., Doria-Rose N.A., O’Dell S., Schmidt S.D., Corbett K.S., Swanson P.A., Padilla M., Neuzil K.M., Bennett H., Leav B., Makowski M., Albert J., Cross K., Edara V.V., Floyd K., Suthar M.S., Martinez D.R., Baric R., Buchanan W., Luke C.J., Phadke V.K., Rostad C.A., Ledgerwood J.E., Graham B.S., Beigel J.H. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N. Engl. J. Med. 2020;383:2427–2438. doi: 10.1056/NEJMoa2028436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AUBAGIO® (teriflunomide) tablets, for oral use Prescribing Information. 2020. [Google Scholar]
- AZD1222 US Phase III primary analysis confirms safety and efficacy [WWW Document] 2020. https://www.astrazeneca.com/media-centre/press-releases/2021/azd1222-us-phase-iii-primary-analysis-confirms-safety-and-efficacy.html URL. accessed 3.28.21.
- AZD1222 US Phase III trial met primary efficacy endpoint in preventing COVID-19 at interim analysis [WWW Document] 2020. https://www.astrazeneca.com/media-centre/press-releases/2021/astrazeneca-us-vaccine-trial-met-primary-endpoint.html URL. accessed 3.22.21.
- Baden L.R., Stieh D.J., Sarnecki M., Walsh S.R., Tomaras G.D., Kublin J.G., McElrath M.J., Alter G., Ferrari G., Montefiori D., Mann P., Nijs S., Callewaert K., Goepfert P., Edupuganti S., Karita E., Langedijk J.P., Wegmann F., Corey L., Pau M.G., Barouch D.H., Schuitemaker H., Tomaka F., Ake J.A., Buchbinder S., Buleza K., Cohen K.W., Crowell T.A., Euler Z., Frank I., Goedhart D., Keefer M., Kelly C., Mayer K., Nkolola J., Peter L., Robb M.L., Rouphael N., Scheppler L., Sobieszczyk M., Van Tieu H. Safety and immunogenicity of two heterologous HIV vaccine regimens in healthy, HIV-uninfected adults (TRAVERSE): a randomised, parallel-group, placebo-controlled, double-blind, phase 1/2a study. Lancet HIV. 2020;7 doi: 10.1016/S2352-3018(20)30229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., Diemert D., Spector S.A., Rouphael N., Creech C.B., McGettigan J., Khetan S., Segall N., Solis J., Brosz A., Fierro C., Schwartz H., Neuzil K., Corey L., Gilbert P., Janes H., Follmann D., Marovich M., Mascola J., Polakowski L., Ledgerwood J., Graham B.S., Bennett H., Pajon R., Knightly C., Leav B., Deng W., Zhou H., Han S., Ivarsson M., Miller J., Zaks T. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021;384 doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker D., Pryce G., James L.K., Marta M., Schmierer K. The ocrelizumab phase II extension trial suggests the potential to improve the risk: benefit balance in multiple sclerosis. Mult. Scler. Relat. Disord. 2020;44:102279. doi: 10.1016/j.msard.2020.102279. [DOI] [PubMed] [Google Scholar]
- Bar-Or A., Freedman M.S., Kremenchutzky M., Menguy-Vacheron F., Bauer D., Jodl S., Truffinet P., Benamor M., Chambers S., O’Connor P.W. Teriflunomide effect on immune response to influenza vaccine in patients with multiple sclerosis. Neurology. 2013;81:552–558. doi: 10.1212/WNL.0b013e31829e6fbf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Or A., Wiendl H., Miller B., Benamor M., Truffinet P., Church M., Menguy-Vacheron F. Randomized study of teriflunomide effects on immune responses to neoantigen and recall antigens. Neurol. - Neuroimmunol. Neuroinflamm. 2015:2. doi: 10.1212/NXI.0000000000000070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Or A., Calkwood J.C., Chognot C., Evershed J., Fox E.J., Herman A., Manfrini M., McNamara J., Robertson D.S., Stokmaier D., Wendt J.K., Winthrop K.L., Traboulsee A. Effect of ocrelizumab on vaccine responses in patients with multiple sclerosis. Neurology. 2020;95 doi: 10.1212/WNL.0000000000010380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollo L., Guerra T., Bavaro D.F., Monno L., Saracino A., Angarano G., Paolicelli D., Trojano M., Iaffaldano P. Seroconversion and indolent course of COVID-19 in patients with multiple sclerosis treated with fingolimod and teriflunomide. J. Neurol. Sci. 2020;416 doi: 10.1016/j.jns.2020.117011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capasso N., Nozzolillo A., Scalia G., Lanzillo R., Carotenuto A., De Angelis M., Petruzzo M., Saccà F., Russo C.V., Brescia Morra V., Moccia M. Ocrelizumab depletes T-lymphocytes more than rituximab in multiple sclerosis. Mult. Scler. Relat. Disord. 2021;49:102802. doi: 10.1016/j.msard.2021.102802. [DOI] [PubMed] [Google Scholar]
- Celius E.G. Normal antibody response after COVID-19 during treatment with cladribine. Mult. Scler. Relat. Disord. 2020;46 doi: 10.1016/j.msard.2020.102476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisari C.G., Sgarlata E., Arena S., Toscano S., Luca M., Patti F. Rituximab for the treatment of multiple sclerosis: a review. J. Neurol. 2021 doi: 10.1007/s00415-020-10362-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clem A. Fundamentals of vaccine immunology. J. Global Infect. Dis. 2011;3:73. doi: 10.4103/0974-777X.77299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conte W.L. Attenuation of antibody response to SARS-CoV-2 in a patient on ocrelizumab with hypogammaglobulinemia. Mult. Scler. Relat. Disord. 2020;44 doi: 10.1016/j.msard.2020.102315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COVID-19 Vaccine AstraZeneca: benefits still outweigh the risks despite possible link to rare blood clots with low blood platelets | European Medicines Agency [WWW Document] 2020. https://www.ema.europa.eu/en/news/covid-19-vaccine-astrazeneca-benefits-still-outweigh-risks-despite-possible-link-rare-blood-clots URL. accessed 3.22.21.
- COVID-19 Vaccine Janssen: EMA finds possible link to very rare cases of unusual blood clots with low blood platelets | European Medicines Agency [WWW Document] 2020. https://www.ema.europa.eu/en/news/covid-19-vaccine-janssen-ema-finds-possible-link-very-rare-cases-unusual-blood-clots-low-blood URL. accessed 4.21.21.
- Cross A.H., Naismith R.T. Established and novel disease-modifying treatments in multiple sclerosis. J. Intern. Med. 2014;275 doi: 10.1111/joim.12203. [DOI] [PubMed] [Google Scholar]
- De Angelis M., Petracca M., Lanzillo R., Brescia Morra V., Moccia M. Mild or no COVID-19 symptoms in cladribine-treated multiple sclerosis: two cases and implications for clinical practice. Mult. Scler. Relat. Disord. 2020;45 doi: 10.1016/j.msard.2020.102452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Different COVID-19 Vaccines | CDC [WWW Document] 2020. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines.html URL. accessed 2.28.21.
- Dosing | GILENYA® (fingolimod) | HCP, 2020.
- Edwards D.K., Jasny E., Yoon H., Horscroft N., Schanen B., Geter T., Fotin-Mleczek M., Petsch B., Wittman V. Adjuvant effects of a sequence-engineered mRNA vaccine: translational profiling demonstrates similar human and murine innate response. J. Transl. Med. 2017;15 doi: 10.1186/s12967-016-1111-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewer K.J., Barrett J.R., Belij-Rammerstorfer S., Sharpe H., Makinson R., Morter R., Flaxman A., Wright D., Bellamy D., Bittaye M., Dold C., Provine N.M., Aboagye J., Fowler J., Silk S.E., Alderson J., Aley P.K., Angus B., Berrie E., Bibi S., Cicconi P., Clutterbuck E.A., Chelysheva I., Folegatti P.M., Fuskova M., Green C.M., Jenkin D., Kerridge S., Lawrie A., Minassian A.M., Moore M., Mujadidi Y., Plested E., Poulton I., Ramasamy M.N., Robinson H., Song R., Snape M.D., Tarrant R., Voysey M., Watson M.E.E., Douglas A.D., Hill A.V.S., Gilbert S.C., Pollard A.J., Lambe T. T cell and antibody responses induced by a single dose of ChAdOx1 nCoV-19 (AZD1222) vaccine in a phase 1/2 clinical trial. Nat. Med. 2021;27:270–278. doi: 10.1038/s41591-020-01194-5. [DOI] [PubMed] [Google Scholar]
- Farez M.F., Correale J., Armstrong M.J., Rae-Grant A., Gloss D., Donley D., Holler-Managan Y., Kachuck N.J., Jeffery D., Beilman M., Gronseth G., Michelson D., Lee E., Cox J., Getchius T., Sejvar J., Narayanaswami P. Practice guideline update summary: vaccine-preventable infections and immunization in multiple sclerosis. Neurology. 2019;93 doi: 10.1212/WNL.0000000000008157. [DOI] [PubMed] [Google Scholar]
- FDA . 2020. Vaccines and Related Biological Products Advisory Committee December 10, 2020 Meeting Briefing Document- FDA. [Google Scholar]
- FDA and CDC Lift Recommended Pause on Johnson & Johnson (Janssen) COVID-19 Vaccine Use Following Thorough Safety Review [WWW Document] 2020. URL FDA NEWS RELEASE%0A%0AFDA and CDC Lift Recommended Pause on Johnson & Johnson (Janssen) COVID-19 Vaccine Use Following Thorough Safety Review. accessed 4.23.21. [Google Scholar]
- FDA, Cber . 2020. Vaccines and Related Biological Products Advisory Committee December 17, 2020 Meeting Briefing Document - FDA. [Google Scholar]
- Flores-Gonzalez R.E., Hernandez J., Tornes L., Rammohan K., Delgado S. Development of SARS-CoV-2 IgM and IgG antibodies in a relapsing multiple sclerosis patient on ofatumumab. Mult. Scler. Relat. Disord. 2021;49:102777. doi: 10.1016/j.msard.2021.102777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folegatti P.M., Ewer K.J., Aley P.K., Angus B., Becker S., Belij-Rammerstorfer S., Bellamy D., Bibi S., Bittaye M., Clutterbuck E.A., Dold C., Faust S.N., Finn A., Flaxman A.L., Hallis B., Heath P., Jenkin D., Lazarus R., Makinson R., Minassian A.M., Pollock K.M., Ramasamy M., Robinson H., Snape M., Tarrant R., Voysey M., Green C., Douglas A.D., Hill A.V.S., Lambe T., Gilbert S.C., Pollard A.J., Aboagye J., Adams K., Ali A., Allen E., Allison J.L., Anslow R., Arbe-Barnes E.H., Babbage G., Baillie K., Baker M., Baker N., Baker P., Baleanu I., Ballaminut J., Barnes E., Barrett J., Bates L., Batten A., Beadon K., Beckley R., Berrie E., Berry L., Beveridge A., Bewley K.R., Bijker E.M., Bingham T., Blackwell L., Blundell C.L., Bolam E., Boland E., Borthwick N., Bower T., Boyd A., Brenner T., Bright P.D., Brown-O’Sullivan C., Brunt E., Burbage J., Burge S., Buttigieg K.R., Byard N., Cabera Puig I., Calvert A., Camara S., Cao M., Cappuccini F., Carr M., Carroll M.W., Carter V., Cathie K., Challis R.J., Charlton S., Chelysheva I., Cho J.-S., Cicconi P., Cifuentes L., Clark H., Clark E., Cole T., Colin-Jones R., Conlon C.P., Cook A., Coombes N.S., Cooper R., Cosgrove C.A., Coy K., Crocker W.E.M., Cunningham C.J., Damratoski B.E., Dando L., Datoo M.S., Davies H., De Graaf H., Demissie T., Di Maso C., Dietrich I., Dong T., Donnellan F.R., Douglas N., Downing C., Drake J., Drake-Brockman R., Drury R.E., Dunachie S.J., Edwards N.J., Edwards F.D.L., Edwards C.J., Elias S.C., Elmore M.J., Emary K.R.W., English M.R., Fagerbrink S., Felle S., Feng S., Field S., Fixmer C., Fletcher C., Ford K.J., Fowler J., Fox P., Francis E., Frater J., Furze J., Fuskova M., Galiza E., Gbesemete D., Gilbride C., Godwin K., Gorini G., Goulston L., Grabau C., Gracie L., Gray Z., Guthrie L.B., Hackett M., Halwe S., Hamilton E., Hamlyn J., Hanumunthadu B., Harding I., Harris S.A., Harris A., Harrison D., Harrison C., Hart T.C., Haskell L., Hawkins S., Head I., Henry J.A., Hill J., Hodgson S.H.C., Hou M.M., Howe E., Howell N., Hutlin C., Ikram S., Isitt C., Iveson P., Jackson S., Jackson F., James S.W., Jenkins M., Jones E., Jones K., Jones C.E., Jones B., Kailath R., Karampatsas K., Keen J., Kelly S., Kelly D., Kerr D., Kerridge S., Khan L., Khan U., Killen A., Kinch J., King T.B., King L., King J., Kingham-Page L., Klenerman P., Knapper F., Knight J.C., Knott D., Koleva S., Kupke A., Larkworthy C.W., Larwood J.P.J., Laskey A., Lawrie A.M., Lee A., Ngan Lee K.Y., Lees E.A., Legge H., Lelliott A., Lemm N.-M., Lias A.M., Linder A., Lipworth S., Liu X., Liu S., Lopez Ramon R., Lwin M., Mabesa F., Madhavan M., Mallett G., Mansatta K., Marcal I., Marinou S., Marlow E., Marshall J.L., Martin J., McEwan J., McInroy L., Meddaugh G., Mentzer A.J., Mirtorabi N., Moore M., Moran E., Morey E., Morgan V., Morris S.J., Morrison H., Morshead G., Morter R., Mujadidi Y.F., Muller J., Munera-Huertas T., Munro C., Munro A., Murphy S., Munster V.J., Mweu P., Noé A., Nugent F.L., Nuthall E., O’Brien K., O’Connor D., Oguti B., Oliver J.L., Oliveira C., O’Reilly P.J., Osborn M., Osborne P., Owen C., Owens D., Owino N., Pacurar M., Parker K., Parracho H., Patrick-Smith M., Payne V., Pearce J., Peng Y., Peralta Alvarez M.P., Perring J., Pfafferott K., Pipini D., Plested E., Pluess-Hall H., Pollock K., Poulton I., Presland L., Provstgaard-Morys S., Pulido D., Radia K., Ramos Lopez F., Rand J., Ratcliffe H., Rawlinson T., Rhead S., Riddell A., Ritchie A.J., Roberts H., Robson J., Roche S., Rohde C., Rollier C.S., Romani R., Rudiansyah I., Saich S., Sajjad S., Salvador S., Sanchez Riera L., Sanders H., Sanders K., Sapaun S., Sayce C., Schofield E., Screaton G., Selby B., Semple C., Sharpe H.R., Shaik I., Shea A., Shelton H., Silk S., Silva-Reyes L., Skelly D.T., Smee H., Smith C.C., Smith D.J., Song R., Spencer A.J., Stafford E., Steele A., Stefanova E., Stockdale L., Szigeti A., Tahiri-Alaoui A., Tait M., Talbot H., Tanner R., Taylor I.J., Taylor V., Te Water Naude R., Thakur N., Themistocleous Y., Themistocleous A., Thomas M., Thomas T.M., Thompson A., Thomson-Hill S., Tomlins J., Tonks S., Towner J., Tran N., Tree J.A., Truby A., Turkentine K., Turner C., Turner N., Turner S., Tuthill T., Ulaszewska M., Varughese R., Van Doremalen N., Veighey K., Verheul M.K., Vichos I., Vitale E., Walker L., Watson M.E.E., Welham B., Wheat J., White C., White R., Worth A.T., Wright D., Wright S., Yao X.L., Yau Y. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396 doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox E.J., Buckle G.J., Singer B., Singh V., Boster A. Lymphopenia and DMTs for relapsing forms of MS. Neurol. Clin. Pract. 2019;9 doi: 10.1212/CPJ.0000000000000567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox R.J., Chan A., Gold R., Phillips J.T., Selmaj K., Chang I., Novas M., Rana J., Marantz J.L. Characterizing absolute lymphocyte count profiles in dimethyl fumarate–treated patients with MS. Neurol. Clin. Pract. 2016;6 doi: 10.1212/CPJ.0000000000000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelibter S., Orrico M., Filippi M., Moiola L. COVID-19 with no antibody response in a multiple sclerosis patient treated with cladribine: implication for vaccination program? Mult. Scler. Relat. Disord. 2021;49 doi: 10.1016/j.msard.2021.102775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genentech, Inc . 2020. Highlights of Prescribing Information. [Google Scholar]
- Gingele S., Jacobus T., Konen F., Hümmert M., Sühs K.-W., Schwenkenbecher P., Ahlbrecht J., Möhn N., Müschen L., Bönig L., Alvermann S., Schmidt R., Stangel M., Jacobs R., Skripuletz T. Ocrelizumab depletes CD20+ T cells in multiple sclerosis patients. Cells. 2018;8:12. doi: 10.3390/cells8010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannoni G. Cladribine to treat Relapsing forms of multiple sclerosis. Neurotherapeutics. 2017;14 doi: 10.1007/s13311-017-0573-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross C.C., Ahmetspahic D., Ruck T., Schulte-Mecklenbeck A., Schwarte K., Jörgens S., Scheu S., Windhagen S., Graefe B., Melzer N., Klotz L., Arolt V., Wiendl H., Meuth S.G., Alferink J. Alemtuzumab treatment alters circulating innate immune cells in multiple sclerosis. Neurol. - Neuroimmunol. Neuroinflamm. 2016;3 doi: 10.1212/NXI.0000000000000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilmot A., Maldonado Slootjes S., Sellimi A., Bronchain M., Hanseeuw B., Belkhir L., Yombi J.C., De Greef J., Pothen L., Yildiz H., Duprez T., Fillée C., Anantharajah A., Capes A., Hantson P., Jacquerye P., Raymackers J.-M., London F., El Sankari S., Ivanoiu A., Maggi P., van Pesch V. Immune-mediated neurological syndromes in SARS-CoV-2-infected patients. J. Neurol. 2021;268:751–757. doi: 10.1007/s00415-020-10108-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatcher S.E., Waubant E., Nourbakhsh B., Crabtree-Hartman E., Graves J.S. Rebound syndrome in patients with multiple sclerosis after cessation of Fingolimod treatment. JAMA Neurol. 2016;73:790. doi: 10.1001/jamaneurol.2016.0826. [DOI] [PubMed] [Google Scholar]
- Jackson L.A., Anderson E.J., Rouphael N.G., Roberts P.C., Makhene M., Coler R.N., McCullough M.P., Chappell J.D., Denison M.R., Stevens L.J., Pruijssers A.J., McDermott A., Flach B., Doria-Rose N.A., Corbett K.S., Morabito K.M., O’Dell S., Schmidt S.D., Swanson P.A., Padilla M., Mascola J.R., Neuzil K.M., Bennett H., Sun W., Peters E., Makowski M., Albert J., Cross K., Buchanan W., Pikaart-Tautges R., Ledgerwood J.E., Graham B.S., Beigel J.H. An mRNA vaccine against SARS-CoV-2 — preliminary report. N. Engl. J. Med. 2020;383 doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson & Johnson Announces Single-Shot Janssen COVID-19 Vaccine Candidate Met Primary Endpoints in Interim Analysis of its Phase 3 ENSEMBLE Trial | Johnson & Johnson [WWW Document] 2020. https://www.jnj.com/johnson-johnson-announces-single-shot-janssen-covid-19-vaccine-candidate-met-primary-endpoints-in-interim-analysis-of-its-phase-3-ensemble-trial URL. accessed 2.28.21.
- Kappos L., Mehling M., Arroyo R., Izquierdo G., Selmaj K., Curovic-Perisic V., Keil A., Bijarnia M., Singh A., von Rosenstiel P. Randomized trial of vaccination in fingolimod-treated patients with multiple sclerosis. Neurology. 2015:84. doi: 10.1212/WNL.0000000000001302. [DOI] [PubMed] [Google Scholar]
- Kappos L., Mehling M., Arroyo R., Izquierdo G., Selmaj K., Curovic-Perisic V., Keil A., Bijarnia M., Singh A., von Rosenstiel P. Randomized trial of vaccination in fingolimod-treated patients with multiple sclerosis. Neurology. 2015:84. doi: 10.1212/WNL.0000000000001302. [DOI] [PubMed] [Google Scholar]
- Kaufman M., Pardo G., Rossman H., Sweetser M.T., Forrestal F., Duda P. Natalizumab treatment shows no clinically meaningful effects on immunization responses in patients with relapsing-remitting multiple sclerosis. J. Neurol. Sci. 2014;341 doi: 10.1016/j.jns.2014.03.035. [DOI] [PubMed] [Google Scholar]
- Keech C., Albert G., Cho I., Robertson A., Reed P., Neal S., Plested J.S., Zhu M., Cloney-Clark S., Zhou H., Smith G., Patel N., Frieman M.B., Haupt R.E., Logue J., McGrath M., Weston S., Piedra P.A., Desai C., Callahan K., Lewis M., Price-Abbott P., Formica N., Shinde V., Fries L., Lickliter J.D., Griffin P., Wilkinson B., Glenn G.M. Phase 1–2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N. Engl. J. Med. 2020;383 doi: 10.1056/NEJMoa2026920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieseier B.C. The mechanism of action of interferon-β in Relapsing multiple sclerosis. CNS Drugs. 2011;25 doi: 10.2165/11591110-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Kumar N., Graven K., Joseph N.I., Johnson J., Fulton S., Hostoffer R., Abboud H. Case report: Postvaccination anti–myelin oligodendrocyte glycoprotein neuromyelitis optica spectrum disorder. Int. J. MS Care. 2020;22:85–90. doi: 10.7224/1537-2073.2018-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalive P.H., Neuhaus O., Benkhoucha M., Burger D., Hohlfeld R., Zamvil S.S., Weber M.S. Glatiramer acetate in the treatment of multiple sclerosis. CNS Drugs. 2011;25:401–414. doi: 10.2165/11588120-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEMTRADA® (alemtuzumab) | Healthcare Professional Site, 2020.
- Lucchini M., Bianco A., Del Giacomo P., De Fino C., Nociti V., Mirabella M. Is serological response to SARS-CoV-2 preserved in MS patients on ocrelizumab treatment? A case report. Mult. Scler. Relat. Disord. 2020;44 doi: 10.1016/j.msard.2020.102323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lulu S., Waubant E. Humoral-targeted immunotherapies in multiple sclerosis. Neurotherapeutics. 2013;10:34–43. doi: 10.1007/s13311-012-0164-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAVENCLAD® (cladribine) Tablets WWW Document. 2020. https://www.mavenclad.com/en?utm_source=google&utm_medium=cpc&utm_campaign=2020+Brand+Generic%3BS%3BPH%3BBR%3BNER%3BDTC%3BBR&utm_content=Generic_Prescribing_Exact&utm_term=cladribine+package+insert&gclid=CjwKCAiAm-2BBhANEiwAe7eyFDnjpv-2Djeqt3A_grHFCq83gou3rt_1cAGoqIi1o1Rpb7BxLv8U2xoCtXsQAvD_BwE&gclsrc=aw.ds URL. accessed 2.28.21.
- McCarthy C.L., Tuohy O., Compston D.A.S., Kumararatne D.S., Coles A.J., Jones J.L. Immune competence after alemtuzumab treatment of multiple sclerosis. Neurology. 2013;81 doi: 10.1212/WNL.0b013e3182a35215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mealy M.A., Cook L.J., Pache F., Velez D.L., Borisow N., Becker D., Arango J.A.J., Paul F., Levy M. Vaccines and the association with relapses in patients with neuromyelitis optica spectrum disorder. Mult. Scler. Relat. Disord. 2018;23:78–82. doi: 10.1016/j.msard.2018.05.003. [DOI] [PubMed] [Google Scholar]
- Meca-Lallana V., Aguirre C., Río B., Cardeñoso L., Alarcon T., Vivancos J. COVID-19 in 7 multiple sclerosis patients in treatment with ANTI-CD20 therapies. Mult. Scler. Relat. Disord. 2020;44 doi: 10.1016/j.msard.2020.102306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehling M., Fritz S., Hafner P., Eichin D., Yonekawa T., Klimkait T., Lindberg R.L.P., Kappos L., Hess C. Preserved antigen-specific immune response in patients with multiple sclerosis responding to IFNβ-Therapy. PLoS One. 2013:8. doi: 10.1371/journal.pone.0078532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissa Vaccines Provides a Pipeline Update on Vaccine Candidates for COVID-19 and RSV WWW Document. 2020. https://www.meissavaccines.com/post/meissa-vaccines-provides-a-pipeline-update-on-vaccine-candidates-for-covid-19-and-rsv URL. accessed 2.28.21.
- Metze C., Winkelmann A., Loebermann M., Hecker M., Schweiger B., Reisinger E.C., Zettl U.K. Immunogenicity and predictors of response to a single dose trivalent seasonal influenza vaccine in multiple sclerosis patients receiving disease-modifying therapies. CNS Neurosci. Ther. 2019;25 doi: 10.1111/cns.13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modjarrad K., Roberts C.C., Mills K.T., Castellano A.R., Paolino K., Muthumani K., Reuschel E.L., Robb M.L., Racine T., Oh M., Lamarre C., Zaidi F.I., Boyer J., Kudchodkar S.B., Jeong M., Darden J.M., Park Y.K., Scott P.T., Remigio C., Parikh A.P., Wise M.C., Patel A., Duperret E.K., Kim K.Y., Choi H., White S., Bagarazzi M., May J.M., Kane D., Lee H., Kobinger G., Michael N.L., Weiner D.B., Thomas S.J., Maslow J.N. Safety and immunogenicity of an anti-Middle East respiratory syndrome coronavirus DNA vaccine: a phase 1, open-label, single-arm, dose-escalation trial. Lancet Infect. Dis. 2019;19 doi: 10.1016/S1473-3099(19)30266-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MS Treatment Guidelines During the Coronavirus pandemic | National MS Society | National Multiple Sclerosis Society [WWW Document] 2020. https://www.nationalmssociety.org/coronavirus-covid-19-information/multiple-sclerosis-and-coronavirus/ms-treatment-guidelines-during-coronavirus URL. accessed 3.21.21.
- Nazi I., Kelton J.G., Larché M., Snider D.P., Heddle N.M., Crowther M.A., Cook R.J., Tinmouth A.T., Mangel J., Arnold D.M. The effect of rituximab on vaccine responses in patients with immune thrombocytopenia. Blood. 2013;122:1946–1953. doi: 10.1182/blood-2013-04-494096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEWS RELEASE INOVIO Doses First Subject in Phase 2 Segment of its INNOVATE Phase 2/3 Clinical Trial for INO-4800, its DNA Medicine to Prevent COVID-19. 2020. [Google Scholar]
- Novavax Confirms High Levels of Efficacy Against Original and Variant COVID-19 Strains in United Kingdom and South Africa Trials. 2021. [Google Scholar]
- Novavax COVID-19 Vaccine Demonstrates 89.3% Efficacy in UK Phase 3 Trial First to Demonstrate Clinical Efficacy Against COVID-19 and Both UK and South Africa Variants. 2021. [Google Scholar]
- Olberg H.K., Cox R.J., Nostbakken J.K., Aarseth J.H., Vedeler C.A., Myhr K.-M. Immunotherapies influence the influenza vaccination response in multiple sclerosis patients: an explorative study. Mult. Scler. J. 2014;20 doi: 10.1177/1352458513513970. [DOI] [PubMed] [Google Scholar]
- Olberg H.K., Eide G.E., Cox R.J., Jul-Larsen Å., Lartey S.L., Vedeler C.A., Myhr K.-M. Antibody response to seasonal influenza vaccination in patients with multiple sclerosis receiving immunomodulatory therapy. Eur. J. Neurol. 2018;25 doi: 10.1111/ene.13537. [DOI] [PubMed] [Google Scholar]
- Palanichamy A., Jahn S., Nickles D., Derstine M., Abounasr A., Hauser S.L., Baranzini S.E., Leppert D., von Büdingen H.-C. Rituximab efficiently depletes increased CD20-expressing T cells in multiple sclerosis patients. J. Immunol. 2014;193:580–586. doi: 10.4049/jimmunol.1400118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardi N., Hogan M.J., Porter F.W., Weissman D. mRNA vaccines — a new era in vaccinology. Nat. Rev. Drug Discov. 2018;17 doi: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrino P., Carnovale C., Perrone V., Pozzi M., Antoniazzi S., Radice S., Clementi E. Efficacy of vaccination against influenza in patients with multiple sclerosis: the role of concomitant therapies. Vaccine. 2014;32 doi: 10.1016/j.vaccine.2014.06.068. [DOI] [PubMed] [Google Scholar]
- Pepini T., Pulichino A.-M., Carsillo T., Carlson A.L., Sari-Sarraf F., Ramsauer K., Debasitis J.C., Maruggi G., Otten G.R., Geall A.J., Yu D., Ulmer J.B., Iavarone C. Induction of an IFN-mediated antiviral response by a self-amplifying RNA vaccine: implications for vaccine design. J. Immunol. 2017;198 doi: 10.4049/jimmunol.1601877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfizer and BioNTech Provide Data from German Phase 1/2 Study Further Characterizing Immune Response Following Immunization with Lead COVID-19 Vaccine Candidate BNT162b2 | Pfizer [WWW Document] 2020. https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-provide-data-german-phase-12-study URL. accessed 2.28.21.
- Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Marc G.P., Moreira E.D., Zerbini C., Bailey R., Swanson K.A., Roychoudhury S., Koury K., Li P., Kalina W.V., Cooper D., Frenck R.W., Hammitt L.L., Türeci Ö., Nell H., Schaefer A., Ünal S., Tresnan D.B., Mather S., Dormitzer P.R., Şahin U., Jansen K.U., Gruber W.C. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020;383 doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard A.J., Launay O., Lelievre J.-D., Lacabaratz C., Grande S., Goldstein N., Robinson C., Gaddah A., Bockstal V., Wiedemann Aurelie, Leyssen M., Luhn K., Richert L., Bétard C., Gibani M.M., Clutterbuck E.A., Snape M.D., Levy Y., Douoguih M., Thiebaut R., McShane C., Callendret B., Dincq S., Ferrault C., Chai S.P., Gyselen M.P., van Looveren M., van Ballert S., de Cnodder T., Roza L., Forcheh C., Stevens K., Mastrandrea C., de Ridder S., Gundluru R., Swales N., Errijegers V., Willems W., Roorda V., Orzabal N., Assenberg M., Vialatte K., Remblier F., Porcar E., Ottavi A., Destandau E., Schwimmer C., Moinot L., Wallet C., Allais F., Savel H., Nedjaai N., Maugard A., Lenzi N., Loulergue P., Bahuaud M., Lainé F., Laviolle B., Boissel N., Thébault E., Vallée D., Nicolas J.-F., Gilbert S., Dahel K., Sagorny K., Lucht F., Paul S., Chanavat A.H., Charra F., Mutter C., Lambour M., Muller C., Hutt-Clauss A., Aranda O., Bernard L., Gissot V., Hallouin-Bernard M.-C., Goudeau A., Suzzoni S., Auostin E., Brick L., Lopez-Zaragoza J.-L., Melic G., Carvalho M., Chesnel C., Hocini H., Wiedemann Aurélie, Hanot L., Rieux V., Puri A., Adeloye T., Boyce M., Dennison J., Loewenstein I., Sahgal O., van den Berg F., Calvert W., Faldon M., McClain B., Newell M.-L., Molenberghs G. Safety and immunogenicity of a two-dose heterologous Ad26.ZEBOV and MVA-BN-Filo Ebola vaccine regimen in adults in Europe (EBOVAC2): a randomised, observer-blind, participant-blind, placebo-controlled, phase 2 trial. Lancet Infect. Dis. 2020 doi: 10.1016/S1473-3099(20)30476-X. [DOI] [PubMed] [Google Scholar]
- Ramasamy M.N., Minassian A.M., Ewer K.J., Flaxman A.L., Folegatti P.M., Owens D.R., Voysey M., Aley P.K., Angus B., Babbage G., Belij-Rammerstorfer S., Berry L., Bibi S., Bittaye M., Cathie K., Chappell H., Charlton S., Cicconi P., Clutterbuck E.A., Colin-Jones R., Dold C., Emary K.R.W., Fedosyuk S., Fuskova M., Gbesemete D., Green C., Hallis B., Hou M.M., Jenkin D., Joe C.C.D., Kelly E.J., Kerridge S., Lawrie A.M., Lelliott A., Lwin M.N., Makinson R., Marchevsky N.G., Mujadidi Y., Munro A.P.S., Pacurar M., Plested E., Rand J., Rawlinson T., Rhead S., Robinson H., Ritchie A.J., Ross-Russell A.L., Saich S., Singh N., Smith C.C., Snape M.D., Song R., Tarrant R., Themistocleous Y., Thomas K.M., Villafana T.L., Warren S.C., Watson M.E.E., Douglas A.D., Hill A.V.S., Lambe T., Gilbert S.C., Faust S.N., Pollard A.J., Aboagye J., Adams K., Ali A., Allen E.R., Allen L., Allison J.L., Andritsou F., Anslow R., Arbe-Barnes E.H., Baker M., Baker N., Baker P., Baleanu I., Barker D., Barnes E., Barrett J.R., Barrett K., Bates L., Batten A., Beadon K., Beckley R., Bellamy D., Berg A., Bermejo L., Berrie E., Beveridge A., Bewley K., Bijker E.M., Birch G., Blackwell L., Bletchly H., Blundell C.L., Blundell S.R., Bolam E., Boland E., Bormans D., Borthwick N., Boukas K., Bower T., Bowring F., Boyd A., Brenner T., Brown P., Brown-O’Sullivan C., Bruce S., Brunt E., Burbage J., Burgoyne J., Buttigieg K.R., Byard N., Cabera Puig I., Camara S., Cao M., Cappuccini F., Carr M., Carroll M.W., Cashen P., Cavey A., Chadwick J., Challis R., Chapman D., Charles D., Chelysheva I., Cho J.-S., Cifuentes L., Clark E., Collins S., Conlon C.P., Coombes N.S., Cooper R., Cooper C., Crocker W.E.M., Crosbie S., Cullen D., Cunningham C., Cuthbertson F., Datoo B.E., Dando L., Datoo M.S., Datta C., Davies H., Davies S., Davis E.J., Davis J., Dearlove D., Demissie T., Di Marco S., Di Maso C., DiTirro D., Docksey C., Dong T., Donnellan F.R., Douglas N., Downing C., Drake J., Drake-Brockman R., Drury R.E., Dunachie S.J., Edwards C.J., Edwards N.J., El Muhanna O., Elias S.C., Elliott R.S., Elmore M.J., English M.R., Felle S., Feng S., Ferreira Da Silva C., Field S., Fisher R., Fixmer C., Ford K.J., Fowler J., Francis E., Frater J., Furze J., Galian-Rubio P., Galloway C., Garlant H., Gavrila M., Gibbons F., Gibbons K., Gilbride C., Gill H., Godwin K., Gordon-Quayle K., Gorini G., Goulston L., Grabau C., Gracie L., Graham N., Greenwood N., Griffiths O., Gupta G., Hamilton E., Hanumunthadu B., Harris S.A., Harris T., Harrison D., Hart T.C., Hartnell B., Haskell L., Hawkins S., Henry J.A., Hermosin Herrera M., Hill D., Hill J., Hodges G., Hodgson S.H.C., Horton K.L., Howe E., Howell N., Howes J., Huang B., Humphreys J., Humphries H.E., Iveson P., Jackson F., Jackson S., Jauregui S., Jeffers H., Jones B., Jones C.E., Jones E., Jones K., Joshi A., Kailath R., Keen J., Kelly D.M., Kelly S., Kelly D., Kerr D., Khan L., Khozoee B., Killen A., Kinch J., King L.D.W., King T.B., Kingham L., Klenerman P., Knight J.C., Knott D., Koleva S., Lang G., Larkworthy C.W., Larwood J.P.J., Law R., Lee A., Lee K.Y.N., Lees E.A., Leung S., Li Y., Lias A.M., Linder A., Lipworth S., Liu S., Liu X., Lloyd S., Loew L., Lopez Ramon R., Madhavan M., Mainwaring D.O., Mallett G., Mansatta K., Marinou S., Marius P., Marlow E., Marriott P., Marshall J.L., Martin J., Masters S., McEwan J., McGlashan J.L., McInroy L., McRobert N., Megson C., Mentzer A.J., Mirtorabi N., Mitton C., Moore M., Moran M., Morey E., Morgans R., Morris S.J., Morrison H.M., Morshead G., Morter R., Moya N.A., Mukhopadhyay E., Muller J., Munro C., Murphy S., Mweu P., Noé A., Nugent F.L., O’Brien K., O’Connor D., Oguti B., Olchawski V., Oliveira C., O’Reilly P.J., Osborne P., Owen L., Owino N., Papageorgiou P., Parracho H., Parsons K., Patel B., Patrick-Smith M., Peng Y., Penn E.J., Peralta-Alvarez M.P., Perring J., Petropoulos C., Phillips D.J., Pipini D., Pollard S., Poulton I., Pratt D., Presland L., Proud P.C., Provstgaard-Morys S., Pueschel S., Pulido D., Rabara R., Radia K., Rajapaska D., Ramos Lopez F., Ratcliffe H., Rayhan S., Rees B., Reyes Pabon E., Roberts H., Robertson I., Roche S., Rollier C.S., Romani R., Rose Z., Rudiansyah I., Sabheha S., Salvador S., Sanders H., Sanders K., Satti I., Sayce C., Schmid A.B., Schofield E., Screaton G., Sedik C., Seddiqi S., Segireddy R.R., Selby B., Shaik I., Sharpe H.R., Shaw R., Shea A., Silk S., Silva-Reyes L., Skelly D.T., Smith D.J., Smith D.C., Smith N., Spencer A.J., Spoors L., Stafford E., Stamford I., Stockdale L., Stockley D., Stockwell L.V., Stokes M., Strickland L.H., Stuart A., Sulaiman S., Summerton E., Swash Z., Szigeti A., Tahiri-Alaoui A., Tanner R., Taylor I., Taylor K., Taylor U., te Water Naude R., Themistocleous A., Thomas M., Thomas T.M., Thompson A., Thompson K., Thornton-Jones V., Tinh L., Tomic A., Tonks S., Towner J., Tran N., Tree J.A., Truby A., Turner C., Turner R., Ulaszewska M., Varughese R., Verbart D., Verheul M.K., Vichos I., Walker L., Wand M.E., Watkins B., Welch J., West A.J., White C., White R., Williams P., Woodyer M., Worth A.T., Wright D., Wrin T., Yao X.L., Zbarcea D.-A., Zizi D. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2020;396 doi: 10.1016/S0140-6736(20)32466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasenack M., Derfuss T. Disease activity return after natalizumab cessation in multiple sclerosis. Expert. Rev. Neurother. 2016;16:587–594. doi: 10.1586/14737175.2016.1168295. [DOI] [PubMed] [Google Scholar]
- Relapsing MS Progression Treatment | MAYZENT® (siponimod) [WWW Document] 2020. https://www.mayzent.com/index.jsp?utm_source=google&utm_medium=paid&utm_campaign=Mayzent.com_Brand_Google_7.2020;S;PH;BR;NER;DTC;BR&utm_term=Generic_Phrase%7C siponimod&gclid=CjwKCAjwxuuCBhATEiwAIIIz0edX8Gu4Zlku2hKh5WpsVePM-AsSwBb08uJDgIMN_yTaUG3JKhlFxhoCcDwQAvD_BwE&gclsrc=aw.ds URL. accessed 3.24.21.
- Roberts D.M., Jones R.B., Smith R.M., Alberici F., Kumaratne D.S., Burns S., Jayne D.R.W. Rituximab-associated hypogammaglobulinemia: incidence, predictors and outcomes in patients with multi-system autoimmune disease. J. Autoimmun. 2015;57 doi: 10.1016/j.jaut.2014.11.009. [DOI] [PubMed] [Google Scholar]
- Sadoff J., Le Gars M., Shukarev G., Heerwegh D., Truyers C., de Groot A.M., Stoop J., Tete S., Van Damme W., Leroux-Roels I., Berghmans P.-J., Kimmel M., Van Damme P., de Hoon J., Smith W., Stephenson K.E., De Rosa S.C., Cohen K.W., McElrath M.J., Cormier E., Scheper G., Barouch D.H., Hendriks J., Struyf F., Douoguih M., Van Hoof J., Schuitemaker H. Interim results of a phase 1–2a Trial of Ad26.COV2.S Covid-19 vaccine. N. Engl. J. Med. 2021 doi: 10.1056/NEJMoa2034201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin U., Muik A., Vogler I., Derhovanessian E., Kranz L.M., Vormehr M., Quandt J., Bidmon N., Ulges A., Baum A., Pascal K., Maurus D., Brachtendorf S., Lörks V., Sikorski J., Koch P., Hilker R., Becker D., Eller A.K., Grützner J., Tonigold M., Boesler C., Rosenbaum C., Heesen L., Kühnle M.C., Poran A., Dong J.Z., Luxemburger U., Kemmer-Brück A., Langer D., Bexon M., Bolte S., Palanche T., Schultz A., Baumann S., Mahiny A.J., Boros G., Reinholz J., Szabó G.T., Karikó K., Shi P.Y., Fontes-Garfias C., Perez J.L., Cutler M., Cooper D., Kyratsous C.A., Dormitzer P.R., Jansen K.U., Türeci Ö. BNT162b2 induces SARS-CoV-2-neutralising antibodies and T cells in humans. medRxiv. 2020 doi: 10.1101/2020.12.09.20245175. [DOI] [Google Scholar]
- Salama H.H., Kolar O.J., Zang Y.C., Zhang J. Effects of combination therapy of beta-interferon 1a and prednisone on serum immunologic markers in patients with multiple sclerosis. Mult. Scler. J. 2003;9 doi: 10.1191/1352458503ms865oa. [DOI] [PubMed] [Google Scholar]
- Schwid S.R., Decker M.D., Lopez-Bresnahan M. Immune response to influenza vaccine is maintained in patients with multiple sclerosis receiving interferon beta-1a. Neurology. 2005;65 doi: 10.1212/01.wnl.0000188901.12700.e0. [DOI] [PubMed] [Google Scholar]
- Stuve O., Soerensen P.S., Leist T., Giovannoni G., Hyvert Y., Damian D., Dangond F., Boschert U. Effects of cladribine tablets on lymphocyte subsets in patients with multiple sclerosis: an extended analysis of surface markers. Ther. Adv. Neurol. Disord. 2019;12 doi: 10.1177/1756286419854986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tebas P., Roberts C.C., Muthumani K., Reuschel E.L., Kudchodkar S.B., Zaidi F.I., White S., Khan A.S., Racine T., Choi H., Boyer J., Park Y.K., Trottier S., Remigio C., Krieger D., Spruill S.E., Bagarazzi M., Kobinger G.P., Weiner D.B., Maslow J.N. Safety and immunogenicity of an anti–Zika virus DNA vaccine — preliminary report. N. Engl. J. Med. 2017 doi: 10.1056/NEJMoa1708120. NEJMoa1708120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tebas P., Kraynyak K.A., Patel A., Maslow J.N., Morrow M.P., Sylvester A.J., Knoblock D., Gillespie E., Amante D., Racine T., McMullan T., Jeong M., Roberts C.C., Park Y.K., Boyer J., Broderick K.E., Kobinger G.P., Bagarazzi M., Weiner D.B., Sardesai N.Y., White S.M. Intradermal SynCon® Ebola GP DNA vaccine is temperature stable and safely demonstrates cellular and Humoral immunogenicity advantages in healthy volunteers. J. Infect. Dis. 2019;220 doi: 10.1093/infdis/jiz132. [DOI] [PubMed] [Google Scholar]
- Tebas P., Yang S., Boyer J.D., Reuschel E.L., Patel A., Christensen-Quick A., Andrade V.M., Morrow M.P., Kraynyak K., Agnes J., Purwar M., Sylvester A., Pawlicki J., Gillespie E., Maricic I., Zaidi F.I., Kim K.Y., Dia Y., Frase D., Pezzoli P., Schultheis K., Smith T.R.F., Ramos S.J., McMullan T., Buttigieg K., Carroll M.W., Ervin J., Diehl M.C., Blackwood E., Mammen M.P., Lee J., Dallas M.J., Brown A.S., Shea J.E., Kim J.J., Weiner D.B., Broderick K.E., Humeau L.M. Safety and immunogenicity of INO-4800 DNA vaccine against SARS-CoV-2: A preliminary report of an open-label, Phase 1 clinical trial. EClinicalMedicine. 2021;31 doi: 10.1016/j.eclinm.2020.100689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theofilopoulos A.N., Baccala R., Beutler B., Kono D.H. Type I INTERFERONS (α/β) in immunity and autoimmunity. Annu. Rev. Immunol. 2005;23 doi: 10.1146/annurev.immunol.23.021704.115843. [DOI] [PubMed] [Google Scholar]
- Thornton J.R., Harel A. Negative SARS-CoV-2 antibody testing following COVID-19 infection in two MS patients treated with ocrelizumab. Mult. Scler. Relat. Disord. 2020;44 doi: 10.1016/j.msard.2020.102341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UAE Says Sinopharm Vaccine has 86% Efficacy Against COVID-19 | Reuters, 2020.
- Understanding and Explaining Viral Vector COVID-19 Vaccines | CDC [WWW Document] 2020. https://www.cdc.gov/vaccines/covid-19/hcp/viral-vector-vaccine-basics.html URL. accessed 2.28.21.
- Vågberg M., Kumlin U., Svenningsson A. Humoral immune response to influenza vaccine in natalizumab-treated MS patients. Neurol. Res. 2012;34 doi: 10.1179/1743132812Y.0000000059. [DOI] [PubMed] [Google Scholar]
- von Hehn C., Howard J., Liu S., Meka V., Pultz J., Mehta D., Prada C., Ray S., Edwards M.R., Sheikh S.I. Immune response to vaccines is maintained in patients treated with dimethyl fumarate. Neurol. - Neuroimmunol. Neuroinflamm. 2018:5. doi: 10.1212/NXI.0000000000000409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., Angus B., Baillie V.L., Barnabas S.L., Bhorat Q.E., Bibi S., Briner C., Cicconi P., Collins A.M., Colin-Jones R., Cutland C.L., Darton T.C., Dheda K., Duncan C.J.A., Emary K.R.W., Ewer K.J., Fairlie L., Faust S.N., Feng S., Ferreira D.M., Finn A., Goodman A.L., Green C.M., Green C.A., Heath P.T., Hill C., Hill H., Hirsch I., Hodgson S.H.C., Izu A., Jackson S., Jenkin D., Joe C.C.D., Kerridge S., Koen A., Kwatra G., Lazarus R., Lawrie A.M., Lelliott A., Libri V., Lillie P.J., Mallory R., Mendes A.V.A., Milan E.P., Minassian A.M., McGregor A., Morrison H., Mujadidi Y.F., Nana A., O’Reilly P.J., Padayachee S.D., Pittella A., Plested E., Pollock K.M., Ramasamy M.N., Rhead S., Schwarzbold A.V., Singh N., Smith A., Song R., Snape M.D., Sprinz E., Sutherland R.K., Tarrant R., Thomson E.C., Török M.E., Toshner M., Turner D.P.J., Vekemans J., Villafana T.L., Watson M.E.E., Williams C.J., Douglas A.D., Hill A.V.S., Lambe T., Gilbert S.C., Pollard A.J., Aban M., Abayomi F., Abeyskera K., Aboagye J., Adam M., Adams K., Adamson J., Adelaja Y.A., Adewetan G., Adlou S., Ahmed K., Akhalwaya Y., Akhalwaya S., Alcock A., Ali A., Allen E.R., Allen L., Almeida T.C.D.S.C., Alves M.P.S., Amorim F., Andritsou F., Anslow R., Appleby M., Arbe-Barnes E.H., Ariaans M.P., Arns B., Arruda L., Azi P., Azi L., Babbage G., Bailey C., Baker K.F., Baker M., Baker N., Baker P., Baldwin L., Baleanu I., Bandeira D., Bara A., Barbosa M.A.S., Barker D., Barlow G.D., Barnes E., Barr A.S., Barrett J.R., Barrett J., Bates L., Batten A., Beadon K., Beales E., Beckley R., Belij-Rammerstorfer S., Bell J., Bellamy D., Bellei N., Belton S., Berg A., Bermejo L., Berrie E., Berry L., Berzenyi D., Beveridge A., Bewley K.R., Bexhell H., Bhikha S., Bhorat A.E., Bhorat Z.E., Bijker E., Birch G., Birch S., Bird A., Bird O., Bisnauthsing K., Bittaye M., Blackstone K., Blackwell L., Bletchly H., Blundell C.L., Blundell S.R., Bodalia P., Boettger B.C., Bolam E., Boland E., Bormans D., Borthwick N., Bowring F., Boyd A., Bradley P., Brenner T., Brown P., Brown C., Brown-O’Sullivan C., Bruce S., Brunt E., Buchan R., Budd W., Bulbulia Y.A., Bull M., Burbage J., Burhan H., Burn A., Buttigieg K.R., Byard N., Cabera Puig I., Calderon G., Calvert A., Camara S., Cao M., Cappuccini F., Cardoso J.R., Carr M., Carroll M.W., Carson-Stevens A., de Carvalho Y.M., Carvalho J.A.M., Casey H.R., Cashen P., Castro T., Castro L.C., Cathie K., Cavey A., Cerbino-Neto J., Chadwick J., Chapman D., Charlton S., Chelysheva I., Chester O., Chita S., Cho J.-S., Cifuentes L., Clark E., Clark M., Clarke A., Clutterbuck E.A., Collins S.L.K., Conlon C.P., Connarty S., Coombes N., Cooper C., Cooper R., Cornelissen L., Corrah T., Cosgrove C., Cox T., Crocker W.E.M., Crosbie S., Cullen L., Cullen D., Cunha D.R.M.F., Cunningham C., Cuthbertson F.C., Da Guarda S.N.F., da Silva L.P., Damratoski B.E., Danos Z., Dantas M.T.D.C., Darroch P., Datoo M.S., Datta C., Davids M., Davies S.L., Davies H., Davis E., Davis Judith, Davis John, De Nobrega M.M.D., De Oliveira Kalid L.M., Dearlove D., Demissie T., Desai A., Di Marco S., Di Maso C., Dinelli M.I.S., Dinesh T., Docksey C., Dold C., Dong T., Donnellan F.R., Dos Santos T., dos Santos T.G., Dos Santos E.P., Douglas N., Downing C., Drake J., Drake-Brockman R., Driver K., Drury R., Dunachie S.J., Durham B.S., Dutra L., Easom N.J.W., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397 doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh E.E., Frenck R.W., Falsey A.R., Kitchin N., Absalon J., Gurtman A., Lockhart S., Neuzil K., Mulligan M.J., Bailey R., Swanson K.A., Li P., Koury K., Kalina W., Cooper D., Fontes-Garfias C., Shi P.-Y., Türeci Ö., Tompkins K.R., Lyke K.E., Raabe V., Dormitzer P.R., Jansen K.U., Şahin U., Gruber W.C. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N. Engl. J. Med. 2020;383 doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Zhang Y., Huang B., Deng W., Quan Y., Wang W., Xu W., Zhao Y., Li N., Zhang J., Liang H., Bao L., Xu Y., Ding L., Zhou W., Gao H., Liu J., Niu P., Zhao L., Zhen W., Fu H., Yu S., Zhang Z., Xu G., Li C., Lou Z., Xu M., Qin C., Wu G., Gao G.F., Tan W., Yang X. Development of an inactivated vaccine candidate, BBIBP-CorV, with potent protection against SARS-CoV-2. Cell. 2020;182 doi: 10.1016/j.cell.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurm H., Attfield K., Iversen A.K., Gold R., Fugger L., Haghikia A. Recovery from COVID-19 in a B-cell-depleted multiple sclerosis patient. Mult. Scler. J. 2020;26:1261–1264. doi: 10.1177/1352458520943791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia S., Zhang Y., Wang Y., Wang Hui, Yang Yunkai, Gao G.F., Tan W., Wu G., Xu M., Lou Z., Huang W., Xu W., Huang B., Wang Huijuan, Wang Wei, Zhang W., Li N., Xie Z., Ding L., You W., Zhao Y., Yang Xuqin, Liu Y., Wang Q., Huang L., Yang Yongli, Xu G., Luo B., Wang Wenling, Liu P., Guo W., Yang Xiaoming. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect. Dis. 2021;21 doi: 10.1016/S1473-3099(20)30831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yri O.E., Torfoss D., Hungnes O., Tierens A., Waalen K., Nordøy T., Dudman S., Kilander A., Wader K.F., Østenstad B., Ekanger R., Meyer P., Kolstad A. Rituximab blocks protective serologic response to influenza A (H1N1) 2009 vaccination in lymphoma patients during or within 6 months after treatment. Blood. 2011;118:6769–6771. doi: 10.1182/blood-2011-08-372649. [DOI] [PubMed] [Google Scholar]
- ZEPOSIA (ozanimod) - An Oral Medication for Relapsing Forms of MS [WWW Document] 2020. https://www.zeposia.com/ URL. accessed 3.24.21.
- Zheng C., Kar I., Chen C.K., Sau C., Woodson S., Serra A., Abboud H. Multiple sclerosis disease-modifying therapy and the COVID-19 pandemic: implications on the risk of infection and future vaccination. CNS Drugs. 2020;34 doi: 10.1007/s40263-020-00756-y. [DOI] [PMC free article] [PubMed] [Google Scholar]