Abstract
AngioJet rheolytic thrombectomy, although a successful treatment modality for arterial thrombus removal and recanalization, has been shown to have increased rates of postoperative acute kidney injury (AKI) compared with other methods of treatment for acute limb ischemia. The postinterventional course of AKI can differ markedly from patient to patient, but typically resolves relatively quickly. Herein, we present a case of AKI secondary to AngioJet intervention that demonstrates an exceedingly prolonged but ultimately recoverable course with conservative management and without the need for renal replacement therapy.
Keywords: AngioJet, Acute kidney injury, AKI, Rheolytic, Thrombectomy
Acute kidney injury (AKI) in critically ill patients is often extrarenal in etiology and characterized by the rapid loss of kidney excretory function. Diagnosed by the accumulation of nitrogen metabolism end products, oliguria, or both, the pathogenesis of AKI secondary to extrarenal events is still debated.1 Clinically, it is defined as an increase in serum creatinine by 0.3 mg/dL or more within 48 hours, or an increase baseline creatinine by 1.5 times or greater.2
Acute limb ischemia requires prompt diagnosis and intervention for limb salvage owing to its associated mortality rate of 15% to 20% and risk of limb loss. The TOPAS and STILE trials have demonstrated lower rates of required amputations and open surgical revascularization using the current standard of treatment, catheter-directed thrombolysis (CDT).3, 4, 5 Percutaneous mechanical thrombectomy (PMT) for acute limb ischemia has been implicated as an extrarenal cause of AKI with intravascular hemolysis and subsequent oxidative damage to the kidney as the suggested mechanism of injury.5, 6, 7
Combining CDT and PMT, AngioJet (Boston Scientific, Marlborough, Mass) fragments and aspirates thrombus using high-pressure infusion of thrombolytic with rheolytic thrombectomy. This mechanism has been shown to remove up to 99% of thrombus and shorten hospital stays compared with CDT alone, but can lead to hemolysis with resultant postprocedural AKI.8 Although non-thrombectomy-related AKI has been well-classified as immediate, delayed, or nonrecoverable, few studies have detailed the recovery response in AKI from rheolytic thrombectomy.9, 10, 11, 12, 13 Herein, we detail a case of severe AKI with prolonged resolution after AngioJet rheolytic thrombectomy for acute-on-chronic lower extremity ischemia secondary to in-stent thrombosis.
Case report
A 54-year-old man with a past medical history significant for hypertension, coronary artery disease, prior stroke without residual deficit, deep venous thrombosis, peripheral arterial disease, and active smoking presented for evaluation of severe lifestyle-limiting and job-preclusive left leg claudication. He previously underwent bare metal stent placement to the left common iliac artery for thrombotic disease years prior at an outside institution. The patient was compliant with optimized medical therapy including aspirin 81 mg and apixaban 5 mg two times per day.
Clinical evaluation led to computed tomography angiography, which revealed a flush occlusion of the left common iliac artery likely secondary to in-stent restenosis with reconstitution of the distal external iliac artery and patent infrainguinal vessels. During this presentation, the lesion was treated with a 7 × 39 mm Viabahn VBX (W. L. Gore & Associates, Flagstaff, Ariz) covered stent postdilated with a 10-mm balloon in the left common iliac artery, with a kissing stent to the contralateral right common iliac artery. Further stenting to the left external iliac artery was performed with an 8 mm Innova stent (Boston Scientific) (Fig 1, A, B).
Fig 1.
A, Angiogram from initial presentation demonstrating flush occlusion of left common iliac artery inflow. B, Angiogram demonstrating return of inline flow after left common and external iliac stenting.
He was discharged on apixaban and aspirin with complete resolution of symptoms and reported smoking cessation and medication compliance in subsequent clinic appointments. Six months postoperatively, he re-presented with severe left lower extremity claudication that had progressed over 2 months. Thrombosis of aforementioned left iliac stents was suspected with an ankle-brachial index of 0.78 (right) and 0.37 (left); computed tomography angiography confirmed complete occlusion of the left iliac system with reconstitution at the iliofemoral junction and three-vessel runoff distally.
Via ipsilateral femoral access, the occlusion was treated with AngioJet Solent Proxi with tissue plasminogen activator (dose, 10 mg in 100 mL of 0.9% normal saline) in PowerPulse spray and aspiration modes. Angiography (using 110 mL of half-strength Optiray 320 contrast) demonstrated recanalization of the prior stent with a residual in-stent lesion in the distal bare metal stent that was not amenable to angioplasty or repeat AngioJet likely owing to chronic laminated thrombus. A self-expanding 8-mm Viabahn covered stent (W. L. Gore & Associates) excluded the aforementioned lesion (Fig 2, A, B).
Fig 2.
A, Angiogram 6 months after the initial presentation demonstrating in-stent thrombosis of left common and external iliac arteries. B, Angiogram after AngioJet and tissue plasminogen activator spray with Viabahn stenting to left common iliac artery.
While concluding AngioJet therapy, the patient developed agitation, confusion, and progressively worsening bradycardia (heart rate of 30 bpm) with hypotension. Owing to transient asystole, cardiopulmonary resuscitation was instituted with a rapid return of spontaneous circulation after two chest compressions. The entirety of this episode lasted approximately 2 minutes, during which he was intubated for airway protection. A repeat angiogram showed no extravasation or other surgical explanation for this episode. This event led to early termination of the procedure (2.5-hour duration) and observation in the critical care unit. The patient was stabilized on norepinephrine (5 μg/min), and immediate postoperative laboratory tests revealed a serum creatinine of 1.5 mg/dL and AN estimated glomerular filtration rate OF 49 (baseline creatinine, 0.8 mg/dL [reference range, 0.5-1.4 mg/dL]; estimated glomerular filtration rate >60 mL/min/1.73 m2 [reference value, ≥60 mL/min/1.73 m2), initially thought to be due to transient hypotension and contrast-induced nephropathy. The patient was extubated on postoperative day 1 and weaned from all hemodynamic supportive infusions. His lower extremity symptoms resolved, although he disclosed he had not stopped smoking as previously reported.
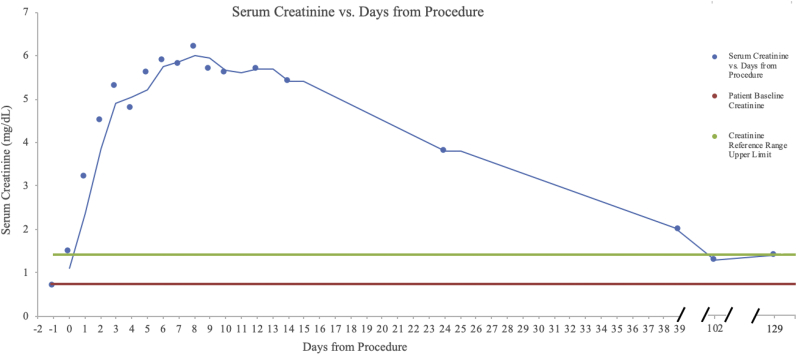
The patient maintained adequate daily urine output despite rising creatinine, which peaked at 6.2 mg/dL (glomerular filtration rate, 11 mL/min/1.73 m2; FeNa >1%) on postoperative day 8, with similarly normal electrolytes. The nephrology service was consulted for management of the nonoliguric AKI and he was maintained on intravenous fluids and oral sodium bicarbonate with daily laboratory monitoring. The creatinine eventually plateaued and on postoperative day 10 he was discharged home on therapeutic apixaban with outpatient follow-up and weekly laboratory monitoring. Because his urine output and serum electrolytes never became markedly abnormal, renal replacement therapy was never initiated. Approximately 3.5 months postoperatively, the patient's creatinine remained within normal limits (creatinine, 1.3 mg/dL; glomerular filtration rate, >60 mL/min/1.73 m2) with no prolonged symptoms of renal failure (Fig 3). He has now verifiably quit smoking and consented to this report of his clinical course.
Fig 3.
Patient creatinine after AngioJet intervention (day 0) with preoperative creatinine included (day –1).
Discussion
AngioJet for PMT has been associated with increased postoperative AKI when compared with CDT, with one study demonstrating a 2.46 times greater risk.14 Recovery from AKI after AngioJet has been previously reported as 5 days; however, the literature suggests a variable duration ranging 25 days to more than 5 months.9, 10, 11 In one reported case, no meaningful AKI recovery had been achieved within 5 months, necessitating continued hemodialysis.13 Our patient is one of few reported cases of prolonged AKI after AngioJet intervention, with a 3.5-month recovery of serum creatinine to within normal limits and has not returned to his baseline level more than 4 months post intervention.7,12,13 Our case was complicated by multiple factors that may have exacerbated the AKI; however, the clinical contribution is unknown given their brief duration.
Although acute tubular necrosis is a presumed mechanism of injury common to the aforementioned cases of AKI after AngioJet, patient presentation is variable. Patients displaying clinical signs of renal injury such as hematuria, hemoglobinuria, and oliguria have reportedly recovered renal function in less than one-half the time compared with our patient. Additionally, cases that reported prolonged AKI recovery after AngioJet have all cited the necessity of hemodialysis. Interestingly, our patient's creatinine peaked at 8.86 times greater than baseline with pertinent negatives including proteinuria, hematuria, and oliguria without requiring hemodialysis. To our knowledge, our patient displayed the longest known AKI recovery time after AngioJet thrombectomy that did not require renal replacement therapy or have disturbances in urine output/composition as reported in previous cases. This case supports the role of observation by a multispecialty team in cases of substantially prolonged recovery given continuously normal urine output and electrolyte levels.
Conclusions
Our case contributes to the limited literature on the potential prolongation and recovery of AKI after AngioJet thrombectomy. It also suggests that clinical signs of renal injury such as hematuria, hemoglobinuria, and oliguria are not necessarily indicative of predicted length of recovery.
Footnotes
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Bellomo R., Kellum J.A., Ronco C. Acute kidney injury. Lancet. 2012;380:756–766. doi: 10.1016/S0140-6736(11)61454-2. [DOI] [PubMed] [Google Scholar]
- 2.KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:19. [Google Scholar]
- 3.Ouriel K., Veith F.J., Sasahara A.A. A comparison of recombinant urokinase with vascular surgery as initial treatment for acute arterial occlusion of the legs. Thrombolysis or Peripheral Arterial Surgery (TOPAS) Investigators. N Engl J Med. 1998;338:1105–1111. doi: 10.1056/NEJM199804163381603. [DOI] [PubMed] [Google Scholar]
- 4.Darwood R., Berridge D.C., Kessel D.O., Robertson I., Forster R. Surgery versus thrombolysis for initial management of acute limb ischaemia. Cochrane Database of Systematic Reviews. 2018;8:CD002784. doi: 10.1002/14651858.CD002784.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Güneş Y., Sincer I., Erdal E. Catheter-directed intra-arterial thrombolysis for lower extremity arterial occlusions. Alt ekstremite arteriyel tıkanıkları için kateter aracılı intra-arteryal tromboliz tedavisi. Anatol J Cardiol. 2019;22:54–59. doi: 10.14744/AnatolJCardiol.2019.63296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borgia F., Di Serafino L., Sannino A., Gargiulo G., Schiattarella G.G., De Laurentis M. AngioJet rheolytic thrombectomy for acute superficial femoral artery stent or femoropopliteal by-pass thrombosis. Monaldi Arch Chest Dis. 2010;74:76–81. doi: 10.4081/monaldi.2010.271. [DOI] [PubMed] [Google Scholar]
- 7.Esteras R., Cannata-Ortiz P., del Palacio-Tamarit M., Guerrero-Hue M., García-Caballero C., Egido J. Podocyte and tubular involvement in AngioJet-induced kidney injury. Clin Kidney J. 2019;14:424–428. doi: 10.1093/ckj/sfz104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muli Jogi R.K., Damodharan K., Leong H.L., Swee Tan A.C., Chandramohan S., Venkatanarasimha N.K.K. Catheter-directed thrombolysis versus percutaneous mechanical thrombectomy in the management of acute limb ischemia: a single center review. CVIR Endovasc. 2018;1:35. doi: 10.1186/s42155-018-0041-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morrow K.L., Kim A.H., Plato S.A., 2nd, Shevitz A.J., Goldstone J., Baele H. Increased risk of renal dysfunction with percutaneous mechanical thrombectomy compared with catheter-directed thrombolysis. J Vasc Surg. 2017;65:1460–1466. doi: 10.1016/j.jvs.2016.09.047. [DOI] [PubMed] [Google Scholar]
- 10.Dukkipati R., Yang E.H., Adler S., Vintch J. Acute kidney injury caused by intravascular hemolysis after mechanical thrombectomy. Nat Rev Nephrol. 2009;5:112–116. doi: 10.1038/ncpneph1019. [DOI] [PubMed] [Google Scholar]
- 11.Escobar G.A., Burks D., Abate M.R., Faramawi M.F., Ali A.T., Lyons L.C. Risk of acute kidney injury after percutaneous pharmacomechanical thrombectomy using AngioJet in venous and arterial thrombosis. Ann Vasc Surg. 2017;42:238–245. doi: 10.1016/j.avsg.2016.12.018. [DOI] [PubMed] [Google Scholar]
- 12.Sebastian H., Tam N., Karumathil M. AngioJetTM rheolytic thrombectomy induced intravascular haemolysis leading to acute kidney injury requiring dialysis. J Clin Nephrol. 2018;2:25–28. [Google Scholar]
- 13.Mathews J.C., Pillai U., Lacasse A. Prolonged renal failure post-percutaneous mechanical thrombectomy. NDT Plus. 2011;4:241–243. doi: 10.1093/ndtplus/sfr050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shen Y., Wang X., Jin S., Zhang R., Zhao W., Chen G. Increased risk of acute kidney injury with percutaneous mechanical thrombectomy using AngioJet compared with catheter-directed thrombolysis. J Vasc Surg Venous Lymphat Disord. 2019;7:29–37. doi: 10.1016/j.jvsv.2018.06.016. [DOI] [PubMed] [Google Scholar]