Abstract
Background
Visceral crisis in metastatic breast cancer (MBC) is defined as severe organ dysfunction requiring rapidly efficacious therapy. Although weekly paclitaxel plus bevacizumab (wPTX + BV) achieves a high response rate in human epidermal growth factor receptor 2 (HER2)-negative MBC, the efficacy and safety of wPTX + BV for visceral crisis is unclear.
Methods
We retrospectively investigated patients with MBC with visceral crisis who received wPTX + BV. Visceral crisis was defined as follows: liver dysfunction (aspartate or alanine aminotransferase >200 U/L or total bilirubin >1.5 mg/dl), respiratory dysfunction (carcinomatous lymphangiomatosis, SpO2 <93% in ambient air or required thoracentesis), superior vena cava (SVC) syndrome, or bone marrow carcinomatosis. The primary outcome was the proportion of patients on-treatment with wPTX + BV after 12 weeks. We also investigated time to treatment failure (TTF), overall survival (OS), objective response rate (ORR), and adverse events.
Results
A total of 44 patients with respiratory dysfunction (n = 29), liver dysfunction (n = 10), bone marrow carcinomatosis (n = 7), and SVC syndrome (n = 2) were eligible for this investigation. The proportion of patients on-treatment with wPTX + BV after 12 weeks was 63% (30/44), and the other patients discontinued wPTX + BV because of adverse events (n = 5) and disease progression (n = 9). Median TTF and OS, and the ORR were 131 days and 323 days, and 41%, respectively. No treatment-related death occurred.
Conclusion: wPTX + BV achieved favorable efficacy and safety for treating patients with visceral crisis and may therefore be considered an option for the treatment of this acutely severe clinical condition.
Keywords: Breast cancer, Visceral crisis, Chemotherapy, Paclitaxel plus bevacizumab
Abbreviations: MBC, metastatic breast cancer; wPTX, weekly paclitaxel; BV, bevacizumab; HER2, human epidermal growth factor receptor 2; TTF, time to treatment failure; OS, overall survival; ORR, objective response rate; DCR, disease control rate; PS, performance status; AST, aspartate aminotransferase; ALT, alanine aminotransferase; LDH, lactate dehydrogenase; AE, adverse event; SVC, superior vena cava
Highlights
-
•
Visceral crisis is a severe organ dysfunction requiring rapidly efficacious therapy.
-
•
The efficacy of chemotherapy in visceral crisis is unclear.
-
•
Weekly paclitaxel plus bevacizumab (wPTX + BV) achieved favorable efficacy and safety.
-
•
wPTX + BV may be considered an option for breast cancer patients with visceral crisis.
1. Introduction
Breast cancer is the most prevalent malignancy among women worldwide, affecting 2,088,849 people in 2018, and has a consistently increasing prevalence [1]. Although breast cancer is curable when diagnosed during its early stages, metastatic or distantly recurrent breast cancer is incurable and accounts for 626,679 yearly deaths worldwide [1]. The characteristics of patients with metastatic or recurrent breast cancer are heterogeneous in that some patients remain in good condition for years with single endocrine therapy, while others require urgent chemotherapy because of impending organ failure caused by metastasis. The latter condition is often referred to as visceral crisis.
The 5th European School of Oncology (ESO)-European Society for Medical Oncology (ESMO) international consensus guidelines for advanced breast cancer (ABC 5) define visceral crisis as follows: “Visceral crisis is defined as severe organ dysfunction, as assessed by signs and symptoms, laboratory studies and rapid progression of disease. Visceral crisis is not the mere presence of visceral metastases but implies important organ compromise leading to a clinical indication for the most rapidly efficacious therapy.” [2]. This critical condition occurs in approximately 10%–15% of patients with advanced breast cancer who receive first-line systemic therapy [2]. Although clinical guidelines recommend considering chemotherapy for patients with visceral crisis regardless of hormone receptor status [2,3], chemotherapy regimens are not specified. A retrospective study found that the median survival of patients with visceral crisis was 4.7 weeks, and their response to chemotherapy was very poor [4]; therefore, the role of chemotherapy in this setting, per se, is not established.
Weekly paclitaxel plus bevacizumab (paclitaxel days 1, 8, and 15 and bevacizumab days 1 and 15, q4 weeks) (wPTX + BV) achieved significantly longer progression-free survival (PFS) and a higher objective response rate (ORR) compared with wPTX alone in a phase III study (E2100) of patients with human epidermal growth factor receptor 2 (HER2)-negative metastatic or recurrent breast cancer (wPTX + BV versus [vs] wPTX, PFS 11.8 vs 5.8 months; ORR 36.9% vs 21.2%) [5]. In daily practice, wPTX + BV is often administered to patients with visceral crisis because it achieves a high response rate [5,6], and weekly administration appears suitable for patients with limited organ function. However, patients with visceral crisis are excluded from clinical trials, and our knowledge of the efficacy and safety of wPTX + BV in these patients is therefore limited.
To address this gap in our knowledge, here we retrospectively investigated the efficacy and safety of wPTX + BV administered to breast cancer patients with visceral crisis.
2. Patients and methods
2.1. Patients and treatment
We analyzed the records of 137 patients with metastatic or recurrent breast cancer who were treated with wPTX + BV from May 2013 to December 2019 at the National Cancer Center Hospital East, Japan. Patients’ data were collected from medical records, and patients selected for this study met the definition of visceral crisis as follows: severe liver dysfunction (aspartate aminotransferase [AST] >200 U/L, alanine aminotransferase [ALT] >200 U/L, or total bilirubin [T-bil] >1.5 mg/dl) caused by liver metastasis, respiratory dysfunction (carcinomatous lymphangiomatosis, SpO2 <93% in ambient air, or the requirement for thoracentesis before wPTX + BV), superior vena cava (SVC) syndrome, or bone marrow carcinomatosis. This definition was determined according to the consensus opinion of oncologists in our department who recommended that patients who met these criteria should be immediately administered the best efficacious chemotherapy. Patients’ other variables included serum lactate dehydrogenase (LDH), Eastern Cooperative Oncology Group (ECOG) performance status (PS), age, sex, histological type, histological grade, estrogen receptor (ER) status, progesterone receptor (PgR) status, metastatic site, and previous treatment for breast cancer. Patients with HER2-positive tumors were excluded.
The dosing schedule of wPTX + BV employed in our hospital was as follows: paclitaxel 80 mg/m2 or 90 mg/m2 infusion on days 1, 8, and 15 and bevacizumab 10 mg/kg infusion on days 1 and 15, every 4 weeks, unless specific reasons for modifying the dose/schedule exist. This treatment was repeated until disease progression, unacceptable toxicity, or the patient’s refusal to continue.
2.2. Data evaluation and statistical analysis
The primary outcome was the proportion of patients on-treatment with wPTX + BV after 12 weeks from the start of treatment. We further investigated time to treatment failure (TTF), overall survival (OS), the objective response rate (ORR), the disease control rate (DCR), and adverse events. For TTF, OS, and adverse event analyses, the data-cutoff date was July 1, 2020. TTF was defined as the interval between the start of wPTX + BV and the earliest date of treatment discontinuation, or death from any cause. OS was measured from the start of wPTX + BV therapy to the date of death from any cause. Tumor response was assessed for patients with measurable disease according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 [7]. We determined progressive disease (PD) by deterioration of clinical status definitively caused by disease progression, even when imaging studies were not performed. The DCR was defined as the proportion of patients who achieved a complete response, a partial response, or stable disease in response to wPTX + BV.
Patients’ survival and the proportion of those on-treatment with wPTX + BV after 12 weeks were estimated using Kaplan–Meier analysis [8]. A log-rank test was used to determine OS rates among subgroups. All statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan; http://www.jichi.ac.jp/saitama-sct/SaitamaHP.files/statmedEN.html) [9].
Adverse events were assessed using the Common Toxicity Criteria for Adverse Events, version 4.0.
2.3. Ethics
The Institutional Review Board (IRB) of the National Cancer Center approved this study (IRB number 2017-431), which was conducted in accordance with the principles stated in Japan’s Ethics Guidelines for Epidemiological Research. The IRB waived the requirement for obtaining written informed consent from the study’s subjects.
3. Results
3.1. Patients’ characteristics
Among 137 patients with metastatic or recurrent breast cancer treated with wPTX + BV from May 2013 to December 2019 at our hospital, 44 met the definition of visceral crisis, among which 35 (80%) were ER-positive, 9 (20%) were triple-negative, and 16 (36%) among them received perioperative taxane-based chemotherapy (Table 1). Visceral crisis was caused by respiratory dysfunction (n = 29, 66%), liver dysfunction (n = 10, 23%), bone marrow carcinomatosis (n = 7, 16%), and SVC syndrome (n = 2, 5%). Five patients met multiple criteria of visceral metastasis (Table 1). Thirty patients (68%) received wPTX + BV as first-line systemic therapy for metastatic or recurrent disease.
Table 1.
Patients’ characteristics.
| Age (years) | Median (range) | N (%) | |
|---|---|---|---|
| 60 (33–76) | |||
| ECOG-PS | 0 | 9 (20) | |
| 1 | 22 (50) | ||
| ≥2 | 13 (30) | ||
| Estrogen-receptor status | |||
| positive | 35 (80) | ||
| negative | 9 (20) | ||
| unknown | 0 | ||
| HER2 status | |||
| negative | 44 (100) | ||
| unknown | 0 | ||
| Triple negative | |||
| Yes | 9 (20) | ||
| No | 35 (80) | ||
| Previous (neo)adjuvant chemotherapy | |||
| None | 17 (39) | ||
| Anthracycline | 20 (45) | ||
| Taxane | 16 (36) | ||
| Site of disease | |||
| Liver | 22 (50) | ||
| Lung | 18 (41) | ||
| Bone | 30 (68) | ||
| Lymph node | 18 (41) | ||
| other | 2 (5) | ||
| Type of visceral crisis | |||
| Liver dysfunction | 10 (23) | ||
| Respiratory dysfunction | 29 (66) | ||
| SVC syndrome | 2 (5) | ||
| Bone marrow carcinomatosis | 7 (16) | ||
| Prior chemotherapy to advanced disease | |||
| none | 30 (68) | ||
| 1 line | 6 (14) | ||
| ≥2 lines | 8 (18) | ||
| Serum LDH | ≥300 | U/L | 24 (55) |
| <300 | U/L | 20 (45) | |
ECOG, European Clinical Oncology Group; PS, performance status.
The initial dose of PTX administered to 20 (45%) patients was reduced to <80 mg/m2 (Table 2). The reasons for dose reduction of PTX were liver dysfunction (n = 15), bone marrow carcinomatosis (n = 5), and/or poor performance status (n = 4). The dose of BV was not initially reduced for any patient.
Table 2.
Initial doses of wPTX + BV.
| Initial dose of PTX, BV | N (%) | |
|---|---|---|
| Paclitaxel | ||
| 20–25 | mg/m2 | 2 (5) |
| 40 | mg/m2 | 11 (25) |
| 60 | mg/m2 | 7 (15) |
| ≥80 | mg/m2 | 24 (55) |
| Bevacizumab | ||
| 10 | mg/kg | 44 (100) |
PTX, paclitaxel; BV, bevacizumab.
3.2. Efficacy of wPTX + BV for treating visceral crisis
With a median follow-up of 319 days (range, 260–442 days), 40 patients (91%) had terminated wPTX + BV by the data-cutoff date, among whom 27 (61%) and 13 (30%) discontinued wPTX + BV because of disease progression and adverse events, respectively; and 4 (9%) patients were on-treatment.
The proportion of patients on-treatment with wPTX + BV after 12 weeks was 68% (30/44). The reasons for early discontinuation of wPTX + BV within 12 weeks were adverse events (n = 5) and disease progression (n = 9). Adverse events causing early discontinuation of wPTX + BV included hypertension (n = 1), hypersensitivity reaction to PTX (n = 1), peripheral neuropathy (n = 1), pneumocystis pneumonia (n = 1), and pneumothorax (n = 1). The ORR of wPTX + BV was 41% and the DCR was 63%. Later lines of chemotherapy were administered to 27 of 44 (63%) patients; while 21 or 30 (70%) patients maintained on wPTX + BV for ≥12 weeks received subsequent chemotherapy, only 6 of 14 (42%) patients who discontinued wPTX + BV within 12 weeks did.
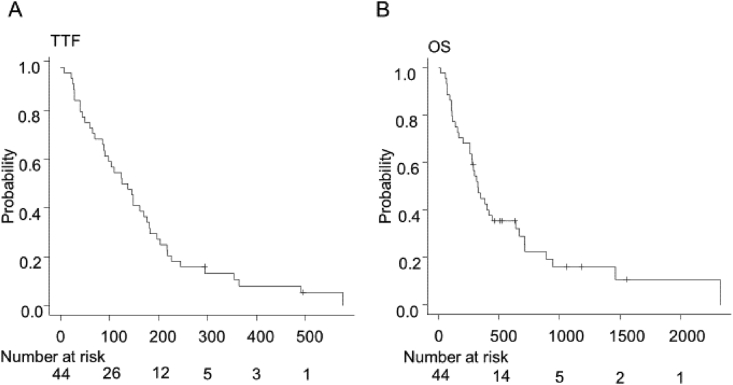
The median TTF and OS were 131 days (range 86–177 days) and 323 days (range 260–442 days), respectively (Fig. 1).
Fig. 1.
Kaplan–Meier analysis of TTF (A) and OS (B). TTF, time to treatment failure; OS, overall survival.
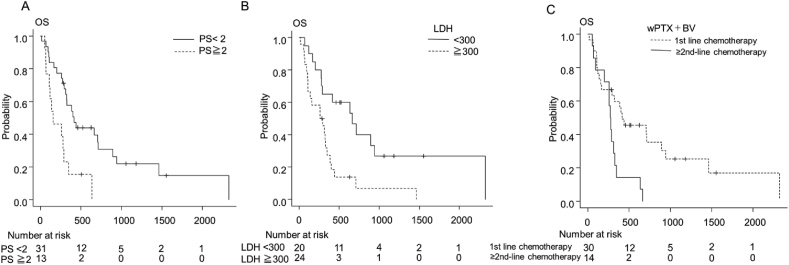
We performed univariate analysis to compare the OS and TTF rates of the subgroups as follows: PS, serum LDH, type of visceral crisis, previous taxane therapy, initial dose reduction of PTX, line of wPTX + BV, and breast cancer subtype (triple-negative breast cancer or not) (Table 3). Although OS was shorter in the PS ≥ 2, elevated serum LDH (≥300 U/L) and wPTX + BV as second- or later lines of chemotherapy subgroups (median OS, PS ≥ 2 vs PS 0–1, 155 days vs 416 days, p = 0.003) (median OS, ≥300 U/L vs < 300 U/L, 277 days vs 600 days, p = 0.003) (median OS, first-line vs second- or later lines, 416 days vs 277 days, p = 0.006) (Fig. 2), there were no significant differences between the other subgroups (Table 3). Furthermore, there was no significant difference in any subgroup analysis of TTF (Table 3). Multivariate analysis was not performed because of the small sample size, small number of events.
Table 3.
Univariate analyses of OS and TTF in subgroup subsets. OS, overall survival; PS, performance status; LDH, lactate dehydrogenase, SVC, superior vena cava; wPTX, weekly paclitaxel; BV, bevacizumab; CI, confidence interval.
| OS |
TTF |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Factor | number of patients | number of events | Median (days) | 95% CI | p | number of patients | number of events | Median (days) | 95% CI | p | |
| Performance status | 0–1 | 31 | 24 | 416 | 294–712 | 0.003 | 31 | 30 | 145 | 74–181 | 0.9 |
| >2 | 13 | 12 | 155 | 69–287 | 13 | 12 | 125 | 40–182 | |||
| Elevated LDH(>300U/L) | Yes | 24 | 22 | 278 | 107–348 | 0.003 | 24 | 23 | 125 | 40–181 | 0.83 |
| No | 20 | 14 | 666 | 275–943 | 20 | 19 | 149 | 65–197 | |||
| Liver dysfunction | Yes | 10 | 8 | 239 | 16–384 | 0.097 | 10 | 9 | 97 | 7–245 | 0.71 |
| No | 34 | 28 | 364 | 261–666 | 34 | 33 | 147 | 86–181 | |||
| Carcinomatous lymphangiomatosis or respiratory dysfunction | Yes | 29 | 24 | 328 | 260–666 | 0.45 | 29 | 29 | 137 | 70–170 | 0.14 |
| No | 15 | 12 | 323 | 93–712 | 15 | 13 | 125 | 25–245 | |||
| SVC syndrome | Yes | 2 | 1 | 279 | 279-∞ | 0.66 | 2 | 1 | 182 | 182-∞ | 0.11 |
| No | 42 | 35 | 323 | 260–442 | 42 | 40 | 125 | 86–162 | |||
| Bone marrow carcinomatosis | Yes | 7 | 7 | 139 | 16–712 | 0.29 | 7 | 7 | 90 | 7–226 | 0.81 |
| No | 37 | 29 | 348 | 275–637 | 37 | 35 | 137 | 89–177 | |||
| Triple-negative breast caner | Yes | 9 | 7 | 201 | 65-∞ | 0.23 | 9 | 8 | 125 | 28–203 | 0.95 |
| No | 35 | 29 | 384 | 275–666 | 35 | 34 | 137 | 86–177 | |||
| Dose reduction of wPTX | Yes | 20 | 18 | 287 | 106–384 | 0.05 | 20 | 19 | 124 | 74–217 | 0.83 |
| No | 24 | 18 | 481 | 260–891 | 24 | 23 | 141 | 60–181 | |||
| Previous taxane therapy | Yes | 23 | 19 | 384 | 261–666 | 0.8 | 23 | 22 | 137 | 90–177 | 0.75 |
| No | 21 | 17 | 287 | 107–891 | 21 | 20 | 125 | 60–197 | |||
| Line of wPTX + BV | 1st-line | 30 | 22 | 416 | 165–891 | 0.006 | 30 | 28 | 114 | 60–177 | 0.81 |
| 2nd- or later | 14 | 14 | 277 | 93–328 | 14 | 14 | 147 | 74–203 | |||
Fig. 2.
Kaplan–Meier analysis of OS of subgroup subsets of PS (A), LDH (B), and lines of wPTX + BV (C). OS, overall survival; PS, performance status; LDH, lactate dehydrogenase; wPTX + BV, weekly paclitaxel plus bevacizumab.
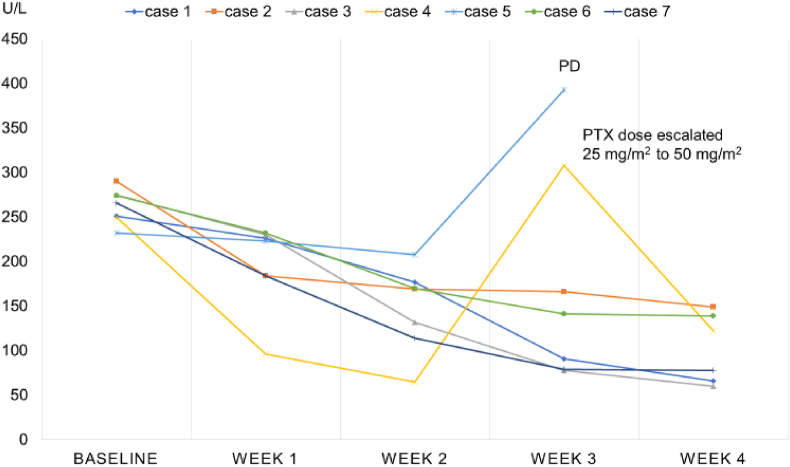
We estimated the time to response according to changes in serum AST or T-bil through initial doses of wPTX + BV in patients with elevated levels of these serum markers. In 7 patients with elevated AST, the reduction of AST was observed within the first 2 weeks after first induction with wPTX + BV (Fig. 3). AST was re-elevated after the third week in 2 patients; 1 was diagnosed with disease progression, and the other exhibited reduced AST levels again after dose escalation of PTX (Fig. 3). Similar changes were detected in 3 patients with elevated T-bil (data not shown).
Fig. 3.
AST values of patients with visceral crisis with extensive liver metastasis.
3.3. Safety
Adverse events are summarized in Table 4. Thirteen (30%) patients discontinued wPTX + BV because of adverse events. The adverse events that led to discontinuation of treatment were peripheral neuropathy (n = 6, 14%), hypertension (n = 1, 2%), hypersensitivity reaction to PTX (n = 1, 2%), gastric hemorrhage (n = 1, 2%), liver cirrhosis (n = 1, 2%), pneumocystis pneumonia (n = 1, 2%), pneumothorax (n = 1, 2%), and an unspecified AE (n = 1, 2%). The most common non-hematological adverse event was peripheral neuropathy (any grade [n = 28, 68%]). Grade 3 or higher non-hematological toxicities were hypertension (n = 5, 11%), peripheral neuropathy (n = 1, 2%), hypertension (n = 1, 2%), gastric hemorrhage (n = 1, 2%), pneumocystis pneumonia (n = 1, 2%), and pneumothorax (n = 1, 2%) (Table 4). Although grade 3 or higher hematological adverse events were experienced by 11 patients (25%) (Table 4), febrile neutropenia or treatment-related death did not occur.
Table 4.
Adverse events.
| N = 44 | All Grade | % | ≥Grade 3 | % | |
|---|---|---|---|---|---|
| Hematological AEs | Leukopenia | 7 | 16 | 2 | 5 |
| Neutropenia | 15 | 34 | 11 | 25 | |
| Anemia | 11 | 25 | 1 | 3 | |
| Thrombocytopenia | 3 | 6 | 1 | 3 | |
| Febrile neutropenia | 0 | 0 | 0 | 0 | |
| Non-hematological AEs | Peripheral neuropathy | 28 | 63 | 1 | 3 |
| Fatigue | 12 | 27 | 0 | 0 | |
| Arthralgia | 4 | 9 | 0 | 0 | |
| Myalgia | 3 | 7 | 0 | 0 | |
| Dysgeusia | 8 | 18 | 0 | 0 | |
| Hypertension | 15 | 34 | 5 | 11 | |
| Hypersensitivity reaction | 2 | 5 | 0 | 0 | |
| Nose bleeding | 6 | 13 | 0 | 0 | |
| Pneumonia | 1 | 2 | 1 | 2 | |
| Gastric hemorrhage | 1 | 2 | 1 | 2 | |
| Pneumothorax | 1 | 2 | 1 | 2 |
AEs, adverse events.
4. Discussion
Here we found that among 44 patients with metastatic or recurrent breast cancer patients with visceral crisis, 30 were maintained with wPTX + BV for at least 12 weeks, and 27 received later lines of chemotherapy. The median TTF and OS were 131 days and 323 days, respectively, and the ORR was 41%. Although 5 patients discontinued wPTX + BV because of adverse events within the initial 12 weeks, no treatment-related death occurred.
In the phase III E2100 study, the median OS and the ORR of the wPTX + BV arm were 26 months and 36.1%, respectively [5]. Compared with these findings, the ORR of our current cohort (41%) was similar, but the median OS was much shorter, 11.5 months [5]. These OS data, however, do not contradict the efficacy of wPTX + BV in the setting of visceral crisis because the prognosis of patients with visceral metastasis, particularly of those with visceral crisis, is inherently poor. For example, others found that the median OS of patients with visceral metastasis is 1.2–2.2 years [10,11]. In a retrospective study of 35 patients with visceral crisis, the median OS from diagnosis is 4.7 weeks [4]. All patients in the study, however, had PS = 2 (89%) or 3 (11%) [4], while only 13 of 44 (30%) had PS of ≥2 in our cohort. Nonetheless, because clinicians generally estimate the prognosis of patients such as those included in our study as ≤3 months and administering another treatment option at progression will likely not be possible, we believe it is encouraging that 68% of patients were maintained with wPTX + BV for ≥12 weeks and 63% subsequently were administered later-line therapy.
Visceral crisis is associated with impending organ failure caused by extensive metastasis. Therefore, an optimal chemotherapy regimen should include features as follows: 1) ability to induce a rapid tumor response, 2) manageable toxicity profile, and 3) adjustable dosing schedule. Here we show that the time to initial response of wPTX + BV, estimated according to the reduction of AST levels, was favorable, manifesting within 2 weeks of therapy (Fig. 3). The toxicity profile and incidence of patients with grade 3 or higher adverse events were comparable with those reported by other clinical trials of wPTX + BV administered to patients without visceral crisis [5,12,13]. Although we show here that 11% of patients discontinued wPTX + BV with adverse events within 12 weeks, treatment-related death did not occur. This favorable safety profile may be attributable to adjusting the dosing schedule of wPTX + BV. In fact, while a reduced initial dose of PTX was administered to 45% of patients (Table 2), the dose was adjusted according to weekly assessment of efficacy and drug-related adverse events (Fig. 3). Anthracyclines such as doxorubicin and epirubicin are options because of their promising antitumor activity, but they are most commonly administered every 3 weeks, which does not allow a clinician to adjust the dose each week. Weekly regimens of anthracyclines were previously tested and showed a good safety profile [[14], [15], [16]], indicating that they may serve as options in the setting of visceral crisis.
Our present data, taken together with those of others, support the conclusion that wPTX + BV serves as a beneficial treatment regimen for patients with visceral crisis. However, PS 2 or greater and elevated LDH were associated with poorer prognosis in the present study (Fig. 2). In the retrospective study of visceral crisis summarized above [4], all patients had PS 2 or 3, and 65% received chemotherapy, which did not confer a significant survival advantage compared with only supportive care (5.8 weeks vs 6.2 weeks). Therefore, careful consideration should be given to the indications for administering chemotherapy to patients with PS 2 or greater.
There were some limitations to the present study. First, we conducted a retrospective study of patients treated at a single institution, and we did not include patients who underwent other treatments according to each clinician’s discretion or those with a similar or worse medical condition who did not receive chemotherapy. The selection of patients for treatment with wPTX + BV may have been biased, and our present cohort does not necessarily enhance the entire population of visceral crisis. In our cohort, 68% (30/44) of patients received wPTX + BV as first-line chemotherapy, because patents generally have better a chance of achieving a good response to chemotherapy compared with those pretreated with other chemotherapies. It is therefore impossible to conclude that wPTX + BV is the best regimen for visceral crisis, and other treatment options should be considered according to the clinical context of each patient. Moreover, although there is no single widely accepted definition of visceral crisis, the recently updated ESO-ESMO international consensus guidelines (ABC 5) provide specific examples of its clinical features as well as a descriptive definition (see Section 1) as follows: liver visceral crisis, rapidly increasing bilirubin >1.5 × the upper limit of normal in the absence of Gilbert’s syndrome or biliary tract obstruction and lung visceral crisis, rapidly increasing dyspnea at rest, not alleviated by drainage of pleural effusion [2]. Not all patients in our study exhibited these clinical features, although their conditions were consistent with what they describe as “impending visceral crisis”, where the criteria for visceral crisis are not yet met but, without rapidly efficacious measures, it is foreseen to happen [2], we recommend the administration of the most rapidly efficacious therapy which is available for treating patients with confirmed visceral crisis.
Second, the number of patients was relatively small, and heterogeneous types of visceral crisis were included, which restricted the power of univariate analyses and prevented the multivariate analyses. A larger sample size will make it possible to evaluate the efficacy of wPTX + BV for each breast cancer subtype. It is particularly important to test newer agents such as cyclin D kinase 4/6 inhibitors for the luminal type [[17], [18], [19]] and immune checkpoint inhibitors for triple-negative breast cancers [20] with visceral crisis. However, it will be challenging to verify the efficacy of wPTX + BV or any other treatment in patients with visceral crisis by randomized clinical trials because rapid introduction of treatment is required for highly frail and heterogenous patients. Therefore, we believe that our current study, to the best of our knowledge, represents the first thorough evaluation of the efficacy and safety of wPTX + BV applied to patients with visceral crisis and provides useful information for optimizing therapy.
5. Conclusions
wPTX + BV achieved favorable efficacy and safety for treating patients with visceral crisis as defined here. However, it should be noted that the majority (68%) of patients received wPTX + BV as first-line chemotherapy, and some patients remained in “impending visceral crisis” according to the definition by ABC 5 [2]. wPTX + BV should therefore be considered as an option for selected patients with an acutely severe clinical condition.
Compliance with ethical standards
Funding
This research did not receive a specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data statement: The data can be shared with the journal for review, if required.
Declaration of conflict of interest: Toru Mukohara received research funding from Daiichi-Sankyo, Sysmex, MSD, Pfizer, Sanofi, and Chugai Pharmaceuticals. The other authors declare that they have no conflicts of interest.
Ethics approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent: A waiver from the requirement to provide written informed consent was granted by the IRB of the National Cancer Center.
Acknowledgments
We thank Edanz Group (https://en-author-services.edanz.com/ac) for editing a draft of this manuscript.
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Cardoso F., Paluch-Shimon S., Senkus E., Curigliano G., Aapro M.S., Andre F. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5) Ann Oncol. 2020;31:1623–1649. doi: 10.1016/j.annonc.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Partridge A.H., Rumble R.B., Carey L.A., Come S.E., Davidson N.E., Di Leo A. Chemotherapy and targeted therapy for women with human epidermal growth factor receptor 2-negative (or unknown) advanced breast cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2014;32:3307–3329. doi: 10.1200/JCO.2014.56.7479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sbitti Y., Slimani K., Debbagh A., Mokhlis A., Kadiri H., Laraqui A. Visceral crisis means short survival among patients with luminal A metastatic breast cancer: a retrospective cohort study. World J Oncol. 2017;8:105–109. doi: 10.14740/wjon1043w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller K., Wang M., Gralow J., Dickler M., Cobleigh M., Perez E.A. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 6.Aogi K., Masuda N., Ohno S., Oda T., Iwata H., Kashiwaba M. First-line bevacizumab in combination with weekly paclitaxel for metastatic breast cancer: efficacy and safety results from a large, open-label, single-arm Japanese study. Breast Canc Res Treat. 2011;129:829–838. doi: 10.1007/s10549-011-1685-x. [DOI] [PubMed] [Google Scholar]
- 7.Eisenhauer E.A., Therasse P., Bogaerts J., Schwartz L.H., Sargent D., Ford R. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Canc. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 8.Rich J.T., Neely J.G., Paniello R.C., Voelker C.C.J., Nussenbaum B., Wang E.W. A practical guide to understanding Kaplan-Meier curves. Otolaryngol Head Neck Surg. 2010;143:331–336. doi: 10.1016/j.otohns.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanda Y. Investigation of the freely available easy-to-use software ’EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kast K., Link T., Friedrich K., Petzold A., Niedostatek A., Schoffer O. Impact of breast cancer subtypes and patterns of metastasis on outcome. Breast Canc Res Treat. 2015;150:621–629. doi: 10.1007/s10549-015-3341-3. [DOI] [PubMed] [Google Scholar]
- 11.Zeichner S.B., Herna S., Mani A., Ambros T., Montero A.J., Mahtani R.L. Survival of patients with de-novo metastatic breast cancer: analysis of data from a large breast cancer-specific private practice, a university-based cancer center and review of the literature. Breast Canc Res Treat. 2015;153:617–624. doi: 10.1007/s10549-015-3564-3. [DOI] [PubMed] [Google Scholar]
- 12.Miles D., Cameron D., Bondarenko I., Manzyuk L., Alcedo J.C., Lopez R.I. Bevacizumab plus paclitaxel versus placebo plus paclitaxel as first-line therapy for HER2-negative metastatic breast cancer (MERiDiAN): a double-blind placebo-controlled randomised phase III trial with prospective biomarker evaluation. Eur J Canc. 2017;70:146–155. doi: 10.1016/j.ejca.2016.09.024. [DOI] [PubMed] [Google Scholar]
- 13.Masuda N., Takahashi M., Nakagami K., Okumura Y., Nakayama T., Sato N. First-line bevacizumab plus paclitaxel in Japanese patients with HER2-negative metastatic breast cancer: subgroup results from the randomized Phase III MERiDiAN trial. Jpn J Clin Oncol. 2017;47:385–392. doi: 10.1093/jjco/hyx001. [DOI] [PubMed] [Google Scholar]
- 14.Jones W.G. Effective palliation of advanced breast cancer with weekly low dose epirubicin. Eur J Cancer Clin Oncol. 1989;25:357–360. doi: 10.1016/0277-5379(89)90030-8. [DOI] [PubMed] [Google Scholar]
- 15.Sigurdsson H., Johansson-Terje I., Aspegren K., Landberg T., Andersson T., Borgstrom S. Weekly-dose doxorubicin (WDA) in advanced breast cancer. Radiother Oncol. 1986;7:133–139. doi: 10.1016/s0167-8140(86)80092-5. [DOI] [PubMed] [Google Scholar]
- 16.Masci G., Gandini C., Zuradelli M., Losurdo A., Torrisi R., Rota S. Weekly non-pegylated liposomal doxorubicin chemotherapy in heavily pre-treated patients with metastatic breast cancer. Anticancer Res. 2013;33:4603–4609. [PubMed] [Google Scholar]
- 17.Finn R.S., Martin M., Rugo H.S., Jones S., Im S.A., Gelmon K. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375:1925–1936. doi: 10.1056/NEJMoa1607303. [DOI] [PubMed] [Google Scholar]
- 18.Goetz M.P., Toi M., Campone M., Sohn J., Paluch-Shimon S., Huober J. Monarch 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol. 2017;35:3638–3646. doi: 10.1200/JCO.2017.75.6155. [DOI] [PubMed] [Google Scholar]
- 19.Hortobagyi G.N., Stemmer S.M., Burris H.A., Yap Y.S., Sonke G.S., Paluch-Shimon S. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med. 2016;375:1738–1748. doi: 10.1056/NEJMoa1609709. [DOI] [PubMed] [Google Scholar]
- 20.Schmid P., Adams S., Rugo H.S., Schneeweiss A., Barrios C.H., Iwata H. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379:2108–2121. doi: 10.1056/NEJMoa1809615. [DOI] [PubMed] [Google Scholar]