Abstract
Background
Primary febrile neutropenia (FN) prophylaxis with ciprofloxacin or granulocyte-colony stimulating factors (G-CSF) is recommended with docetaxel-cyclophosphamide (TC) chemotherapy for early-stage breast cancer (EBC). A pragmatic randomised trial compared the superiority of G-CSF to ciprofloxacin and a cost-utility analysis were conducted.
Methods
EBC patients receiving TC chemotherapy were randomised to ciprofloxacin or G-CSF. The primary outcome was a composite of FN and non-FN treatment-related hospitalisation. Secondary outcomes included; rates of FN, non-FN treatment-related hospitalisation, chemotherapy dose reductions/delays/discontinuations. Primary analysis was performed with the intention to treat population. Cost-utility analyses were conducted from the Canadian public payer perspective.
Results
458 eligible patients were randomised: 228 to ciprofloxacin and 230 to G-CSF. For the primary endpoint there was non-statistically significant difference (Risk difference = −6.7%, 95%CI = −13.5%–0.1%, p = 0.061) between ciprofloxacin patients (46,20.2%) and G-CSF (31,13.5%). Patients receiving ciprofloxacin were more likely to experience FN (36/228, 15.8% vs 13/230, 5.7%) than patients receiving G-CSF (p < 0.001). Non-FN treatment-related hospitalisation occurred in 40/228 (17.5%) of ciprofloxacin patients vs 28/230 (12.2%) of G-CSF patients (p = 0.12). There were no differences in other secondary outcomes. G-CSF was associated with an incremental cost-effectiveness ratio of C$1,760,796 per one quality-adjusted life year gained.
Conclusion
The primary endpoint of superiority of G-CSF over ciprofloxacin was not demonstrated. While there were reduced FN rates with G-CSF, there were no differences in chemotherapy dose delays/reductions or discontinuations. With the commonly used willingness to pay value of C$50,000/QALY, G-CSF use was not cost-effective compared to ciprofloxacin and deserves scrutiny from the payer perspective.
Keywords: Docetaxel-cyclophosphamide, Breast cancer, Febrile neutropenia, G-CSF, Ciprofloxacin
Highlights
-
•
Primary febrile neutropenia (FN) prophylaxis is indicated for docetaxel-cyclophosphamide (TC) chemotherapy.
-
•
In this multicentre trial 458 breast cancer patients receiving TC chemotherapy were randomised to ciprofloxacin or to G-CSF.
-
•
For the primary endpoint of FN and non-FN treatment-related hospitalizations, G-CSF was not superior over ciprofloxacin.
-
•
While there were reduced FN rates with G-CSF, the incremental cost-effectiveness ratio was C$1,760,796 per one QALYWALY gained.
1. Introduction
Docetaxel-cyclophosphamide (TC) chemotherapy is commonly used for early stage breast cancer (EBC) [1]. Due to the risk of neutropenia and associated consequences (including febrile neutropenia [FN], non-FN treatment-related hospitalisations, chemotherapy delays/dose reductions/discontinuation), evidence-based guidelines recommend that TC is co-administered with primary FN prophylaxis [[2], [3], [4], [5], [6]]. While ciprofloxacin was mainly used in the definitive trial [1], in clinical practice, granulocyte colony-stimulating factors (G-CSF) (e.g. filgrastim [Neupogen™, Grastofil™] [7] or peg-filgrastim [Neulasta™] are commonly used [1,8,9].
Despite differences in cost, route of administration and toxicity profiles between ciprofloxacin and G-CSF, the optimal choice of agent has never been definitively evaluated [[10], [11], [12]]. In the absence of prospective comparisons, the choice of agent should be based on a full discussion between the patient and health care provider. This discussion should reflect; drug side effects [13,14], subcutaneous administration of G-CSF, and expense (C$ 1920/cycle Neupogen, C$ 1440/cycle Grastofil and C$ 2380/cycle Neulasta™) [15,16]. In practice, however, the choice is usually based on physician preference [10].
We previously confirmed the feasibility of performing a randomised clinical trial using an oral consent methodology [17] for comparing ciprofloxacin with G-CSF in patients receiving TC [12]. This pilot study was then expanded to compare efficacy outcomes. We report on the expanded study. The primary outcome is a composite endpoint comprised of FN and non-FN treatment-related hospitalisations. This endpoint was chosen as a previous patient survey showed this was the outcome of greatest personal importance [10].
2. Methods
This open-label, superiority trial was performed at four Canadian cancer centres. Eligible patients had EBC, no prior history of chemotherapy, and were planned to receive 4 cycles of adjuvant TC chemotherapy. Exclusion criteria included contraindications to either G-CSF or ciprofloxacin. Patients were approached for study participation by their oncologist. The study was approved by the local Research Ethics Boards and registered on clinicaltrials.gov (NCT02816112) [18].
2.1. Randomisation
Consented patients were randomised 1:1 to ciprofloxacin (500 mg PO BID for 7–14 days starting 5 days after chemotherapy) or G-CSF (patients, with their physicians, could choose the type, dose, and duration of G-CSF use, including biosimilars) as primary FN prophylaxis for each chemotherapy cycle. Following confirmation of the feasibility of the oral consent methodology [12,19] (Version 28/Aug/2014, NCT02173262, n = 186), a protocol amendment (19/Oct/2016, NCT02816112) allowed study expansion to compare the efficacy of ciprofloxacin with G-CSF. Randomisation was performed using a permuted block design with blocks of variable sizes stratified by centre using a web-based application developed by The Ottawa Methods Centre.
Patients received ciprofloxacin or G-CSF (Neupogen™, Neulasta™: Amgen, Thousand Oaks CA, USA, or Grastofil™, Apotex, Toronto, Canada). Study drugs were funded through either Cancer Care Ontario (patients over 65) or through third party insurance co-pay programs (Ontario and Alberta). Follow-up visits occurred as per usual care. Outcome data were collected from case report forms and electronic health records.
2.2. Study hypotheses
The proportion of patients with the composite endpoint of FN or non-FN treatment-related hospitalisations will be lower for patients treated with G-CSF.
2.3. Outcomes
The primary outcome was the rate of a composite measure of documented, laboratory confirmed FN [20] or non-FN treatment-related hospital admission. Secondary outcomes included; FN rates, non-FN treatment-related hospitalisation rates, proportion of patients who required chemotherapy dose delays/reductions/discontinuation. Rates of laboratory confirmed Clostridium difficile infections or other microbiologic infections were collected.
2.4. Statistical analysis
Patient characteristics and outcomes were summarised using descriptive statistics. All outcomes were assessed from the date of randomisation until 28 days (inclusive) after the last cycle of TC chemotherapy. Primary analysis was based on the intention-to-treat (ITT) principle, which included all patients in the treatment group to which they were allocated, even if they did not receive treatment or were non-compliant. A supportive analysis was performed on the per protocol (PP) population, which included those who received initial treatment as per their randomised allocation. The primary analysis was based on a two-sided, Fisher’s exact test with statistical significance defined at the p = 0.05 level. Absolute risk difference and 95% confidence intervals were calculated between both treatment groups using the Score (Wilson) method. Pre-planned subgroup analyses included: treatment centre (Ottawa vs. others) and age (<65 vs. 65+). Logistic regression analyses, adjusted for stratification factors, were also performed. All statistical analysis was carried out using SAS version-9 or higher for Windows (Cary, NC) or R version 3.2.2 (www.r-project.org). or higher. Of note, the preliminary efficacy data of the feasibility study [12]. was not assessed to ensure the type I error was not impacted.
2.5. Sample size
An informal survey of medical oncologists from participating centres in February 2016 asked what they felt would be the minimally important effect of G-CSF needed to be deemed superior to ciprofloxacin. The published rates of FN in patients receiving primary FN prophylaxis cited in the survey ranged from 5% to 8% [[10], [11], [12]], thus, if the primary outcome rate in patients administered ciprofloxacin was estimated to be 8%, survey respondents identified that a rate of 2% or less of patients receiving G-CSF would be required to change practice universally. Hence, assuming primary outcome rates of 8% versus 2% for patients administered ciprofloxacin and G-CSF, 80% power and a two-sided alpha of 5%, the required overall sample size was 412 people (206 per treatment group). To account for 5% non-compliance with the protocol, the target sample size was 456 patients (228 per arm).
2.6. Health economic evaluation
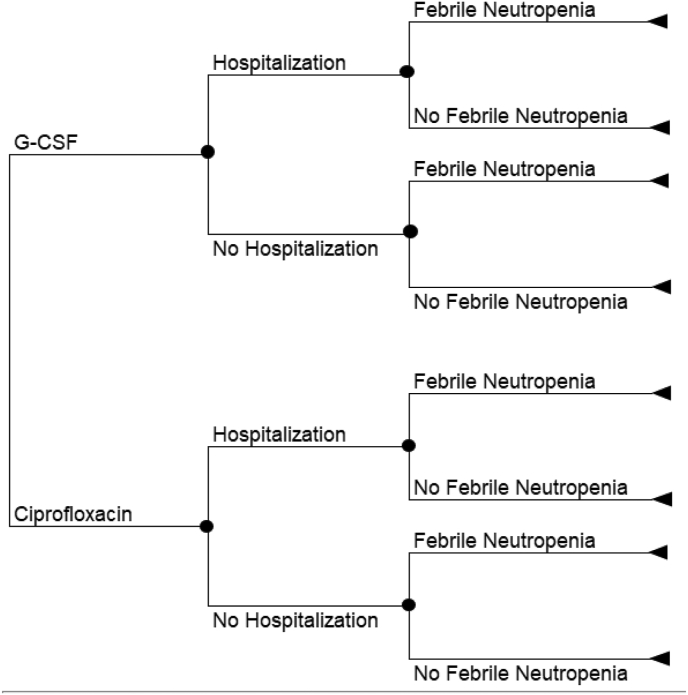
We conducted a cost-utility analysis utilising a decision tree and patient-level data from the trial (Fig. 1 and Supplemental Table 1). The time horizon of the model was three months to align with the trial follow-up, discounting was therefore not required. We did not use a lifetime horizon because G-CSF and ciprofloxacin are supportive care therapies in the breast cancer setting without survival benefits [21,22]. FN and non-FN treatment-related hospitalisations data were taken from the trial. Health outcome was shown as quality-adjusted life year (QALY), that was estimated based on the published health utility values and the area under the curve method. Costs were estimated from the Ontario Ministry of Health perspective and expressed in 2020 Canadian dollars (C$). Our model included the costs of G-CSF, ciprofloxacin, physicians, hospitalisation, inpatient and outpatient FN management. Incremental cost-effectiveness ratios (ICERs) were shown as the incremental cost per QALY gained. We performed a series of one-way and probabilistic sensitivity analyses (PSA) to assess the robustness of study results. A scenario analysis was carried out to assess the impact of the proportion of peg-filgrastim vs. filgrastim.
Fig. 1.
Decision tree structure.
3. Results
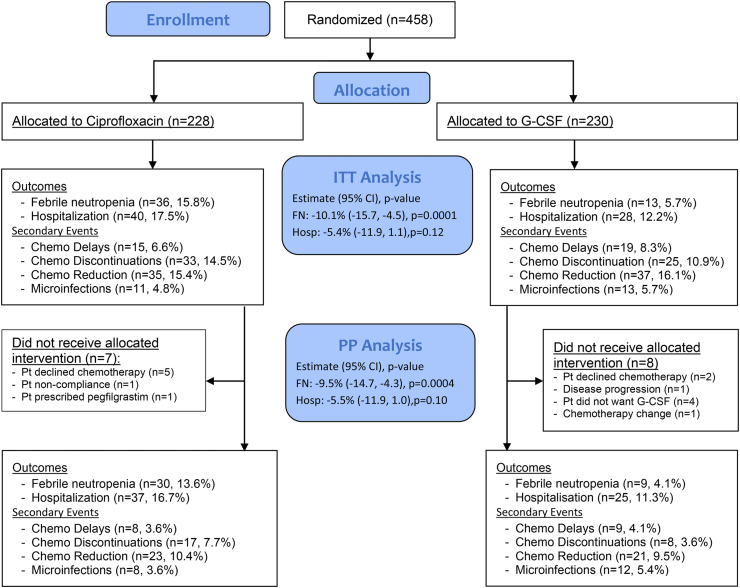
The trial enrolled from September 18, 2014 to November 19, 2019. Of 458 eligible patients, 228 were randomised to ciprofloxacin and 230 to G-CSF (CONSORT flow diagram, Fig. 2). Of 458 patients, 15 (7 ciprofloxacin; 8 G-CSF) withdrew prior to start of treatment (Fig. 2). In total, 443 (221 ciprofloxacin; 222 G-CSF) patients received treatment as part of this trial. Reasons for discontinuation of study treatments are shown in Fig. 2.
Fig. 2.
Study CONSORT diagram.
Baseline characteristics of the randomised patients are presented in Table 1. Median age was 58 years (range 30–84). The total number of chemotherapy cycles given were 819 in the ciprofloxacin arm and 849 in the G-CSF arm. Median (interquartile range) durations on study were 90 days (range 46–98) and 92 days (range 68–103) for ciprofloxacin and G-CSF patients, respectively.
Table 1.
Patient enrolment and baseline characteristics.
| Characteristic | Overall | Ciprofloxacin | G-CSF |
|---|---|---|---|
| N | 458 | 228 | 230 |
| Mean age (std dev) Median (range) |
57.7 (10.7) 58 (30, 84) |
59.0 (10.9) 60 (30, 84) |
56.4 (10.4) 57 (31, 81) |
| Type, dose and number of injections of G-CSF∗: n (%) | – | – | 147 |
| Pegfilgrastim (6 mg) Filgrastim (300mcg/5 d) Filgrastim (300mcg/7 d) Filgrastim (300mcg/10 d) Filgrastim (480mcg/5 d) Filgrastim (480mcg/7 d) Filgrastim (480mcg/10 d) Dose Unknown |
4 (2.7) 79 (53.7) 34 (23.1) 26 (17.7) 1 (0.7) 1 (0.7) 2 (1.4) 83 (36.1) |
||
|
Total Cycles of Treatment for all patients 0 1 2 3 4 |
15 (3.3) 26 (5.7) 12 (2.6) 18 (3.9) 387 (84.5) |
7 (3.1) 14 (6.1) 8 (3.5) 9 (4.0) 190 (83.3) |
8 (3.5) 12 (5.2) 4 (1.7) 9 (3.9) 197 (85.7) |
TC = docetaxel/cyclophosphamide.
∗ Data was available on the types of growth factors prescribed at one centre (Ottawa Hospital) only (n = 147). Biosimilar preparations of both filgrastim and pegfilgrastim were allowed.
3.1. Primary outcomes
FN occurred in 36 (15.8%) ciprofloxacin patients and 13 (5.7%) G-CSF patients (RD = −10.1%, 95%CI = −15.7% to −4.5%, p-value<0.001) (Table 2, Fig. 3). There were 40 (17.5%) ciprofloxacin patients who were hospitalised for any reason, compared with 28 (12.2%) G-CSF patients (RD = −5.4%, 95%CI = −11.9%–1.1%, p-value = 0.12). For the primary composite endpoint, a total of 46 (20.2%) ciprofloxacin patients experienced FN or were hospitalised, whereas 31 (13.5%) G-CSF patients experienced FN or were hospitalised. The risk difference was −6.7%, with a 95%CI of −13.5%–0.1%, p-value = 0.061 (Fig. 3, Table 2 and Supplemental Tables 2 and 3). Results were similar in the PP analysis (Supplemental Tables 2 and 3). After adjusting for age (>65 versus ≤ 65) and treatment centre, the odds ratio (95%CI) for developing FN or hospitalisation was 1.52 (95%CI 0.91 to 2.53; p = 0.11) for patients treated with ciprofloxacin. In other words, patients on the ciprofloxacin arm had increased odds of experiencing the primary outcome event, adjusted for stratification factors. Subgroup analyses are presented in Supplemental Tables 3 and 4
Table 2.
Clinical endpoints, Per Protocol Analysis.
| Outcome | Ciprofloxacin, N (%) |
G-CSF N (%) |
Risk Difference (95% CI) | p-value |
|---|---|---|---|---|
| N | 221 | 222 | ||
| Primary Endpoint | ||||
| FN or Treatment-related hospitalisation | 43 (19.5) | 29 (13.1) | −6.4% (−13.2, 0.5) | 0.073 |
| Secondary Endpoints | ||||
| Febrile Neutropenia | 36 (16.3) | 13 (5.9) | −10.4% (−16.2, −4.7) | <0.001 |
| Treatment-related Hospitalisation | 37 (16.7) | 25 (11.3) | −5.5% (−11.9, 1.0) | 0.10 |
| Chemotherapy dose reduction | 23 (10.4) | 21 (9.5) | −1.0% (−6.5, 4.6) | 0.75 |
| Chemotherapy discontinuation | 17 (7.7) | 8 (3.6) | −4.1% (−8.4, 0.2) | 0.067 |
| Chemotherapy dose delay | 8 (3.6) | 9 (4.1) | 0.4% (−3.1, 4.0) | 1.00 |
| Microinfection | 8 (3.6) | 12 (5.4) | 1.8% (−2.1, 5.7) | 0.49 |
| Any Event | 99 (44.8) | 76 (34.2) | −10.6% (−19.6, −1.5) | 0.026 |
FN=Febrile neutropenia.
Fig. 3.
Forest Plot of clinical endpoints, ITT Population.
Of the 36 patients in the ciprofloxacin group that experienced FN, 30 were hospitalised and 6 had outpatient management. Of 13 patients with FN while on G-CSF, 9 were hospitalised and 4 were managed as outpatients.
3.2. Cycle data
Events were most frequent during the first cycle of therapy (Table 3). Amongst ciprofloxacin patients, 10.4% and 11.3% respectively experienced FN or non-FN treatment-related hospitalisation during the first cycle, compared with 2.6% and 2.6% in the fourth cycle. Similarly, amongst G-CSF patients, 4.5% and 6.8% experienced FN or non-FN treatment-related hospitalisations during cycle 1, while 1.5% and 1.0% did so during cycle 4.
Table 3.
Clinical endpoints, by Cycle, ITT Analysis.
| Ciprofloxacin |
G-CSF |
|||||||
|---|---|---|---|---|---|---|---|---|
| Cycle | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 |
| n (%) | 221 | 203 | 199 | 196 | 222 | 214 | 211 | 202 |
| Febrile Neutropenia | 23 (10.4) | 17 (8.4) | 9 (4.5) | 5 (2.6) | 10 (4.5) | 8 (3.7) | 4 (1.9) | 3 (1.5) |
| Treatment-related Hospitalisation | 25 (11.3) | 10 (4.9) | 4 (2.0) | 5 (2.6) | 15 (6.8) | 8 (3.7) | 7 (3.3) | 2 (1.0) |
| Chemotherapy dose reduction | 0 | 19 (9.4) | 12 (6.0) | 6 (3.1) | 0 | 18 (8.4) | 12 (5.7) | 9 (4.5) |
| Chemotherapy discontinuation | 18 (8.1) | 4 (2.0) | 3 (1.5) | 8 (4.1) | 8 (3.6) | 3 (1.4) | 5 (2.4) | 9 (4.5) |
| Chemotherapy dose delay | 0 | 9 (4.4) | 5 (2.5) | 2 (1.0) | 0 | 5 (2.3) | 9 (4.3) | 6 (3.0) |
| Microinfection | 5 (2.3) | 5 (2.5) | 2 (1.0) | 1 (0.5) | 6 (2.7) | 6 (2.8) | 1 (0.5) | 1 (0.5) |
3.3. Secondary outcomes
Overall, 15.7% of patients experienced a dose reduction, 12.7% discontinued chemotherapy, and 7.4% experienced a dose delay. The most common reasons included non-neutropenic fever, peripheral neuropathy, and diarrhoea. No statistically significant difference was observed in the rates of chemotherapy dose reductions (p = 0.90), discontinuations (p = 0.26), dose delays (p = 0.59) or microinfections (p = 0.83) (Fig. 3 and Supplemental Table 3). Events were more common in earlier treatment cycles. In the ITT population, 24/458 (5.2%) patients experienced a laboratory confirmed microinfection. Three patients experienced C. difficile (2-ciprofloxacin; 1-G-CSF) and 1 G-CSF patient grew streptococci.
When combining all primary and secondary endpoints, 102/228 patients on ciprofloxacin (44.7%) had at least one primary or secondary endpoint event, compared with 78/230 (33.9%) of G-CSF patients (Fisher’s exact test, RD = −10.8%, 95%CI = −10.8% to −1.9%, p = 0.022) (Fig. 3).
3.4. Cost-utility results
G-CSF was associated with higher cost (C$4005 vs. C$2581) and improved QALYs (0.1783 vs. 0.1774) compared to ciprofloxacin, with an estimated ICER of C$1,760,796/QALY. The estimated ICERs increased with the larger proportions of peg-filgrastim (Table 4). The cost-effectiveness results were highly sensitive to the unit cost of G-CSF, the relative risk of FN, and the relative risk of treatment-related hospitalisation (Fig. 4). The higher cost of G-CSF, the less likely that G-CSF would be cost-effective. By contrast, the greater effectiveness of G-CSF in reducing FN or treatment-related hospitalisation, the more likely that G-CSF would be cost-effective. Results of the PSA show that G-CSF was associated with increased cost and improved QALYs in the majority of the 5000 simulations (Supplemental Fig. 1a). Regardless of the willingness-to-pay values, ciprofloxacin always had the greater probabilities of being a cost-effective option than G-CSF (Supplemental Fig. 1b).
Table 4.
Cost-utility analysis results.
| Analysis | Treatment | Average Cost (C$) |
Average QALYs | Incremental Cost (C$) |
Incremental QALYs | ICER, C$/QALY |
|---|---|---|---|---|---|---|
| Base case Pegfilgrastim 2.7% | G-CSF | 4005 | 0.1783 | 1424 | 0.0008 | 1,760,796 |
| Ciprofloxacin | 2581 | 0.1774 | ||||
| Scenario analysis Pegfilgrastim 0% |
G-CSF | 3881 | 0.1784 | 1298 | 0.0008 | 1,608,322 |
| Ciprofloxacin | 2583 | 0.1776 | ||||
| Scenario analysis Pegfilgrastim 25% |
G-CSF | 4355 | 0.1799 | 1779 | 0.0008 | 2,183,394 |
| Ciprofloxacin | 2576 | 0.1791 | ||||
| Scenario analysis Pegfilgrastim 50% |
G-CSF | 4800 | 0.1790 | 2232 | 0.0008 | 2,804,370 |
| Ciprofloxacin | 2568 | 0.1782 | ||||
| Scenario analysis Pegfilgrastim 75% |
G-CSF | 5221 | 0.1790 | 2636 | 0.0008 | 3,232,484 |
| Ciprofloxacin | 2585 | 0.1782 | ||||
| Scenario analysis Pegfilgrastim 100% |
G-CSF | 5611 | 0.1786 | 3036 | 0.0008 | 3,765,911 |
| Ciprofloxacin | 2576 | 0.1778 |
Fig. 4.
One-way sensitivity analysis results.
4. Discussion
TC chemotherapy is usually co-administered with primary FN prophylaxis. A systematic review showed FN rates were reduced from 27% to 5% by primary FN prophylaxis [11] but was limited by the retrospective nature of the trials involved. Hence the optimal form of prophylaxis is unknown [[10], [11], [12]]. Indeed, the original study leading to TC approval did not specify whether prophylaxis was used [1] and it was not until a subsequent publication when it became clear that most patients received prophylactic ciprofloxacin [23]. To our knowledge, this is the only randomised trial to prospectively address this important clinical question. In this study the primary outcome of a composite of FN and non-FN treatment-related hospitalisations was chosen because EBC patients have stated that both were the outcomes of greatest importance to them [10]. Our study did not demonstrate the superiority of G-CSF over ciprofloxacin. Our cost-utility analysis also showed that G-CSF was not cost-effective compared to ciprofloxacin at a commonly used willingness-to-pay threshold of C$50,000/QALY.
These finding are pertinent for several reasons. First, was a recent publication evaluating the omission of peg-filgrastim prophylaxis with the taxane component of dose-dense AC and paclitaxel [24]. In this single arm study omission of routine peg-filgrastim was safe and feasible and associated with a 95.7% reduction in the use of peg-filgrastim. Second, the report from the most recent ASCO Clinical Practice Guideline Update for the Use of WBC Growth Factors [25] states that, "Prophylactic use of CSFs to reduce the risk of FN is warranted when the risk of FN is approximately 20% or higher and no other equally effective and safe regimen that does not require CSFs is available”. Given that the rate of FN in the current study with ciprofloxacin use was 15%, this is below the 20% threshold stated in this guideline. Finally, with the commonly used willingness to pay value of C$50,000/QALY, the use of G-CSF was not cost-effective compared to ciprofloxacin and deserves great scrutiny from the payer perspective.
It is challenging to compare our findings with those in the literature, as past studies vary in the type of outcome they report (FN rates, neutropenia rates) and also in the type of analysis reported (by proportion of patients or per cycle of chemotherapy) [[2], [3], [4],9,23,[26], [27], [28], [29]]. As outcome events can be expressed by the rate in the study population overall, and by the total number of chemotherapy cycles received, we chose to present both formats [30]. With respect to study endpoints, TC is not an innocuous regimen even with G-CSF use, as 33.9% of G-CSF patients experienced at least one event related to the primary or secondary endpoint.
There are limitations with the current trial. Due to the study’s pragmatic nature it did not employ a double-blind design. It might be viewed as both limitations and strengths that the duration of ciprofloxacin was between 7 and 14 days depending on local standards, while the type, dose and duration of G-CSF were left to patient and physician choice. This was done to reflect the realities of clinical practice in a real-world setting. Similarly, many physicians do not adjust the dose of G-CSF based on weight [[31], [32], [33]] or may use shorter durations than identified in prior studies [34]. Some studies cite patient age ≥65 as an important factor for increased risk of FN [3,[26], [27], [28]]. In our study the proportion of patients having FN was 35/339 (10.3%) vs. 14/119 (11.7%) for the <65 and ≥ 65 subgroups (p-value = 0.24), respectively. In the current study, 22.5% of patients were ≥65, compared with 16% in the definitive TC approval trial [1,23]. Our study also allowed patients to receive biosimilar formulations of filgrastim, again making the results more generalisable toward real-world practice.
Future studies are needed to evaluate primary FN prophylaxis from patient perspective, as there are considerable differences both in terms of toxicity and patient cost between these strategies. Growth factor support is expensive both in terms of direct (costs to the patient and/or the health care system) [6] and indirect (paying health care staff to administer and/or to teach patients to self-administer subcutaneous injections) costs. G-CSF use may also be associated with clinically important adverse effects including local and systemic pain syndrome. In addition, future studies could evaluate the effect of cost effectiveness of different G-CSF products. For example, differences in patients (dose and duration of G-CSF used), between agents (filgrastim vs pegfilgrastim), between biosimilars and non-biosimilars and between insurance companies and Provincial tender-based contracts could give interesting comparisons. Ciprofloxacin, while relatively inexpensive, is associated with its own adverse effects, such as C. difficile infection. With all of these factors considered, the ideal prophylaxis regimen needs to consider not only clinical efficacy but cost and patient comfort/acceptability [35].
5. Conclusion
We have demonstrated that while our primary outcome of FN and hospitalisations did not reach statistical significance, G-CSF was superior to ciprofloxacin for reducing FN rates. In addition, treatment related hospitalisations also favoured G-CSF, but was not statistically significant. Finally, the gains in clinical benefits of G-CSF did not justify its high cost.
Role of the funding source
This work was supported by the Ottawa Hospital Department of Medicine Patient Quality and Safety Committee PQ&I Project Grant with matched funding from the Ottawa Hospital Division of Medical Oncology. Additional funding was obtained from Cancer Care Ontario: Clinical Programs and Quality Initiatives 2017 and 2018 grants to support expansion of REaCT trials to other cancer centres in Ontario and from the Canadian Institute of Health Research: SPOR grant. The Rethinking Clinical Trials (REaCT) Program platform at the Ottawa Hospital is also supported by The Ottawa Hospital Foundation and its generous donors.
Trial registration
ClinicalTrials.gov: NCT02173262, NCT02816112;
Author contributions
MC, DF, AAJ, LV, BH and JH designed the study and prepared the protocol. MC, AAJ, JMJ, JPH, JM, XZ, TN, MKFI, MS, DS, BB, AA, LP, NN, JH collected the data. MC acted as principal investigator, MS and DS coordinated data entry and GP did the statistical analysis. KT performed a cost-utility analysis, MC, MS, KT, DS, LV and GP had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. MC, DF, MS, KT, DS, LV and GP wrote the manuscript. All authors were involved in the critical review of the manuscript and approved the final version.
Data sharing
The de-identified dataset is available upon request and approval from the REB Board of Record.
Ethics committee approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of each institutions Research Ethics Board and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all trial participants included in the study. All data has been anonymised to protect the identities of subjects involved in the research.
Declaration of competing interest
MC reports personal fees (honoraria) from Pfizer. AAJ reports personal fees (honoraria or travel funds) from Mylan, Teva, Purdue, BMS, BI, Genomic Health, PUMA, Pfizer, Roche, Astra Zeneca, Novartis and Eli Lilly, outside the submitted work. JPH reports institutional research funds from Roche and BMS, outside the submitted work. JM reports personal fees (honorarium) from Pfizer and share ownership in Pacylex Pharmaceutical Inc, SMHeart Card Inc, and illumiSonics, outside the submitted work. TN reports personal fees (honoraria) from ARIAD, Takeda and Boehringer-Ingelheim, outside the submitted work. AA reports personal fees (honorarium for advisory board participation) from Novartis, outside the submitted work. LP reports personal fees (honorarium from advisory board participation) from Serono Oncology, outside the submitted work. NN reports personal fees (honorarium) from Roche, and institutional research funds from Pfizer, outside the submitted work. BH reports consulting fees from Cornerstone Research, outside the submitted work. JFH reports consulting fees from BMS, Pfizer, Eli-Lilly, Novartis and Merck, outside the submitted work. All other authors declare no competing interests.
Acknowledgements
We are grateful for patients and their families for their assistance with this study, as well as physicians for approaching patients. Highest accrual by physician was: Clemons (215), Hilton (54), Meza-Junco (29), Price-Hiller (26), Joy (24), Mackey (17), Ng (16), Tonkin (16), Zhu (15), Awan (7), Ibrahim (6) and Verma (5).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2021.03.012.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Jones S.E., Savin M.A., Holmes F.A. Phase III trial comparing doxorubicin plus cyclophosphamide with docetaxel plus cyclophosphamide as adjuvant therapy for operable breast cancer. J Clin Oncol. 2006;24(34):5381–5387. doi: 10.1200/JCO.2006.06.5391. [DOI] [PubMed] [Google Scholar]
- 2.Aapro M.S., Bohlius J., Cameron D.A. Update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Canc. 2010;47(1):8–32. doi: 10.1016/j.ejca.2010.10.013. 2011. [DOI] [PubMed] [Google Scholar]
- 3.Younis T., Rayson D., Thompson K. Primary G-CSF prophylaxis for adjuvant TC or FEC-D chemotherapy outside of clinical trial settings: a systematic review and meta-analysis. Support Care Canc. 2012;20(10):2523–2530. doi: 10.1007/s00520-011-1375-6. [DOI] [PubMed] [Google Scholar]
- 4.Smith T.J., Bohlke K., Lyman G.H. Recommendations for the use of WBC growth factors: American society of clinical Oncology clinical practice guideline update. J Clin Oncol. 2015;33(28):3199–3212. doi: 10.1200/JCO.2015.62.3488. [DOI] [PubMed] [Google Scholar]
- 5.National comprehensive cancer network clinical practice guidelines in Oncology (NCCN Guidelines)®: breast cancer version 2. 2017. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.CCO Drug Formulary. GCSF Working Group . 2016. Cancer care Ontario GCSF recommendations.https://www.cancercareontario.ca/en/guidelines-advice/types-of-cancer/38561 [Google Scholar]
- 7.Amgen Canada Inc. Product monograph (filgrastim) http://pi.amgen.com/∼/media/amgen/repositorysites/pi-amgen-com/neupogen/neupogen_pi_hcp_english.ashx
- 8.Amgen Canada Inc. Product monograph (pegfilgrastim) http://pi.amgen.com/∼/media/amgen/repositorysites/pi-amgen-com/neulasta/neulasta_pi_hcp_english.ashx
- 9.Altwairgi A.K., Hopman W.M., Mates M. Real-world impact of granulocyte-colony stimulating factor on febrile neutropenia. Curr Oncol. 2013;20(3):e171–e179. doi: 10.3747/co.20.1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hilton J., Vandermeer L., Sienkiewicz M. Filgrastim use in patients receiving chemotherapy for early-stage breast cancer-a survey of physicians and patients. Support Care Canc. 2018;26(7):2323–2331. doi: 10.1007/s00520-018-4074-8. [DOI] [PubMed] [Google Scholar]
- 11.Fernandes R., Mazzarello S., Stober C. Optimal primary febrile neutropenia prophylaxis for patients receiving docetaxel-cyclophosphamide chemotherapy for breast cancer: a systematic review. Breast Canc Res Treat. 2017;161(1):1–10. doi: 10.1007/s10549-016-4028-0. [DOI] [PubMed] [Google Scholar]
- 12.Clemons M., Mazzarello S., Hilton J. Feasibility of using a pragmatic trials model to compare two primary febrile neutropenia prophylaxis regimens (ciprofloxacin versus G-CSF) in patients receiving docetaxel-cyclophosphamide chemotherapy for breast cancer (REaCT-TC) Support Care Canc. 2019;27(4):1345–1354. doi: 10.1007/s00520-018-4408-6. [DOI] [PubMed] [Google Scholar]
- 13.Fernandes R., Mazzarello S., Joy A.A. Taxane acute pain syndrome (TAPS) in patients receiving chemotherapy for breast or prostate cancer: a prospective multi-center study. Support Care Canc. 2018;26(9):3073–3081. doi: 10.1007/s00520-018-4161-x. [DOI] [PubMed] [Google Scholar]
- 14.Fernandes R., Mazzarello S., Majeed H. Treatment of taxane acute pain syndrome (TAPS) in cancer patients receiving taxane-based chemotherapy-a systematic review. Support Care Canc. 2016;24(4):1583–1594. doi: 10.1007/s00520-015-2941-0. [DOI] [PubMed] [Google Scholar]
- 15.CADTH C.A.D.T.H. CADTH; 2016. Canadian drug expert committee final recommendation: filgrastim (Grastofil - Apotex Inc.) indications: preventions or treatment of neutropenia. [Google Scholar]
- 16.Neulasta - Board Staff Statement of Allegations (Severed). http://www.pmprb-cepmb.gc.ca/view.asp?ccid=838 (September 28, 2016; [date last accessed)].
- 17.Basulaiman B., Awan A.A., Fergusson D. Creating a pragmatic trials program for breast cancer patients: rethinking Clinical Trials (REaCT) Breast Canc Res Treat. 2019 doi: 10.1007/s10549-019-05274-0. [DOI] [PubMed] [Google Scholar]
- 18.ClinicalTrials.gov. A Multi-Cantre Study to Compare Granulocyte-colony Stimulating Factors to Antibiotics for Primary Prophylaxis of Taxotere/Cyclophosphamide-Induced Febrile Neutropenia REaCT-TC2. https://register.clinicaltrials.gov/prs/app/action/SelectProtocol?sid=S0006DI3&selectaction=Edit&uid=U0001VRU&ts=50&cx=-ochws7. [DOI] [PMC free article] [PubMed]
- 19.Kim S.Y., Miller F.G. Informed consent for pragmatic trials--the integrated consent model. N Engl J Med. 2014;370(8):769–772. doi: 10.1056/NEJMhle1312508. [DOI] [PubMed] [Google Scholar]
- 20.de Naurois J., Novitzky-Basso I., Gill M.J. Management of febrile neutropenia: ESMO clinical practice guidelines. Ann Oncol. 2010;21(Suppl 5):v252–v256. doi: 10.1093/annonc/mdq196. [DOI] [PubMed] [Google Scholar]
- 21.Sung L., Nathan P.C., Alibhai S.M. Meta-analysis: effect of prophylactic hematopoietic colony-stimulating factors on mortality and outcomes of infection. Ann Intern Med. 2007;147(6):400–411. doi: 10.7326/0003-4819-147-6-200709180-00010. [DOI] [PubMed] [Google Scholar]
- 22.Lyman G., Yau L., Nakov R. Overall survival and risk of second malignancies with cancer chemotherapy and G-CSF support. Ann Oncol. 2018;29(9):1903–1910. doi: 10.1093/annonc/mdy311. [DOI] [PubMed] [Google Scholar]
- 23.Jones A., Barlow M., Barrett-Lee P. Management of cardiac health in trastuzumab-treated patients with breast cancer: updated United Kingdom National Cancer Research Institute recommendations for monitoring. Br J Canc. 2009;100(5):684. doi: 10.1038/sj.bjc.6604909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vaz-Luis I., Barroso-Sousa R., Di Meglio A. Avoiding peg-filgrastim prophylaxis during the paclitaxel portion of the dose-dense doxorubicin-cyclophosphamide and paclitaxel regimen: a prospective study. J Clin Oncol. 2020 doi: 10.1200/jco.19.02484:Jco1902484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith T.J., Bohlke K., Lyman G.H. Recommendations for the use of WBC growth factors: American society of clinical Oncology clinical practice guideline update. J Clin Oncol. 2015;33(28):3199–3212. doi: 10.1200/JCO.2015.62.3488. [DOI] [PubMed] [Google Scholar]
- 26.Madarnas Y., Dent S.F., Husain S.F. Real-world experience with adjuvant fec-d chemotherapy in four Ontario regional cancer centres. Curr Oncol. 2011;18(3):119–125. doi: 10.3747/co.v18i3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vandenberg T., Younus J., Al-Khayyat S. Febrile neutropenia rates with adjuvant docetaxel and cyclophosphamide chemotherapy in early breast cancer: discrepancy between published reports and community practice-a retrospective analysis. Curr Oncol. 2010;17(2):2–3. doi: 10.3747/co.v17i2.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soong D., Haj R., Leung M.G. High rate of febrile neutropenia in patients with operable breast cancer receiving docetaxel and cyclophosphamide. J Clin Oncol. 2009;27(26):e101–e102. doi: 10.1200/JCO.2009.23.0508. [DOI] [PubMed] [Google Scholar]
- 29.Weycker D., Barron R., Edelsberg J. Risk and consequences of chemotherapy-induced neutropenic complications in patients receiving daily filgrastim: the importance of duration of prophylaxis. BMC Health Serv Res. 2014;14:189. doi: 10.1186/1472-6963-14-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fernandes R., Mazzarello S., Stober C. Primary febrile neutropenia prophylaxis for patients who receive FEC-D chemotherapy for breast cancer: a systematic review. J Glob Oncol. 2018;(4):1–8. doi: 10.1200/jgo.2016.008540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen B., Nagai S., Armitage J.O. Regulatory and clinical experiences with biosimilar filgrastim in the US, the European Union, Japan, and Canada. Oncol. 2019;24(4):537. doi: 10.1634/theoncologist.2018-0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Collins J.M., Fleming G.F., Christ T.N. Comparison of the incidence of febrile neutropenia in obese and normal weight breast cancer patients receiving myelosuppressive chemotherapy and prophylactic pegfilgrastim. J Oncol Pharm Pract. 2019;25(5):1112–1118. doi: 10.1177/1078155218776471. [DOI] [PubMed] [Google Scholar]
- 33.Buchner A., Lammerich A., Abdolzade-Bavil A. Lipegfilgrastim: pharmacodynamics and pharmacokinetics for body-weight-adjusted and 6 mg fixed doses in two randomized studies in healthy volunteers. Curr Med Res Opin. 2014;30(12):2523–2533. doi: 10.1185/03007995.2014.962131. [DOI] [PubMed] [Google Scholar]
- 34.Clemons M., Fergusson D., Simos D. A multicentre, randomised trial comparing schedules of G-CSF (filgrastim) administration for primary prophylaxis of chemotherapy-induced febrile neutropenia in early stage breast cancer. Ann Oncol. 2020;31(7):951–957. doi: 10.1016/j.annonc.2020.04.005. [DOI] [PubMed] [Google Scholar]
- 35.Fraser J., Steele N., Al Zaman A. Are patients in clinical trials representative of the general population? Dose intensity and toxicities associated with FE100C-D chemotherapy in a non-trial population of node positive breast cancer patients compared with PACS-01 trial group. Eur J Canc. 2011;47(2):215–220. doi: 10.1016/j.ejca.2010.10.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.