Abstract
Although opioids are potent analgesics, a consequence of chronic opioid use is hyperalgesia during withdrawal, which may contribute to opioid misuse. Dynorphin, the endogenous ligand of κ-opioid receptors (KORs), is upregulated in opioid-dependent rats and in animal models of chronic pain. However, the role of KORs in opioid withdrawal-induced hyperalgesia remains to be determined. We hypothesized that KOR antagonism would reverse opioid withdrawal-induced hyperalgesia in opioid-dependent rats. Male and female Wistar rats received daily injections of heroin (2–6 mg/kg, SC) and were tested for mechanical sensitivity in the electronic von Frey test 4–6 h into withdrawal. Female rats required significantly more heroin than male rats to reach comparable levels of both heroin-induced analgesia and hyperalgesia (6 mg/kg vs. 2 mg/kg). Once hyperalgesia was established, we tested the effects of the KOR antagonists nor-binaltorphimine (norBNI; 30 mg/kg, SC) and 5′-guanidinonaltrindole (5′GNTI; 30 mg/kg, SC). When the animals continued to receive their daily heroin treatment (or saline treatment in the repeated saline group) five times per week throughout the experiment, both KOR antagonists reversed heroin withdrawal-induced hyperalgesia. The anti-hyperalgesia effect of norBNI was more prolonged in males than in females (14 days vs. 7 days), whereas 5′GNTI had more prolonged effects in females than in males (14 days vs. 4 days). The behavioral effects of 5′GNTI coincided with higher 5′GNTI levels in the brain than in plasma when measured at 24 h, whereas 5′GNTI did not reverse hyperalgesia at 30 min posttreatment when 5′GNTI levels were higher in plasma than in the brain. Finally, we tested the effects of 5′GNTI on naloxone-induced and spontaneous signs of opioid withdrawal and found no effect in either male or female rats. These findings indicate a functional role for KORs in heroin withdrawal-induced hyperalgesia that is observed in rats of both sexes.
Keywords: Dynorphin, Negative affect, Opioid use disorder, Sex differences
1. Introduction
The United States is facing an opioid overdose epidemic. More than 70,000 people have died annually from drug overdose in the past 5 years, making it the leading injury-related cause of death. Two-thirds of drug overdose deaths involved an opioid, and the national prevalence is an estimated 14 opioid-related deaths per 100,000 people (Scholl et al., 2018; Wilson et al., 2020). There are still important gaps in our understanding of the contribution of biological sex to the epidemic (Becker and Mazure, 2019). Although women have historically used heroin at lower rates than men, the increase in heroin use has been faster in women than in men (Marsh et al., 2018). Men and women exhibit similar levels of opioid-induced analgesia (Pisanu et al., 2019), but the prevalence of opioid prescriptions is higher for women than for men (Frenk et al., 2019). Men report more positive subjective effects of acute opioid administration, whereas women report more negative subjective effects (Comer et al., 2009).
A consequence of chronic opioid use is withdrawal-induced hyperalgesia, reflected by a lowering of pain thresholds and a decrease in tolerance to pain. Opioid withdrawal-induced hyperalgesia is more prominent in patients with opioid use disorder than chronic pain (Higgins et al., 2019). Heroin users in acute withdrawal and ex-users in protracted abstinence also exhibit greater sensitivity to pain (Ren et al., 2009; Carcoba et al., 2011), which is hypothesized to contribute to drug seeking and taking and relapse. The influence of sex and gender on the prevalence of opioid withdrawal-induced hyperalgesia has not yet been investigated.
Studies in humans and laboratory animals have reported sex differences in opioid system function. A recent meta-analysis found little evidence of sex differences in opioid-induced analgesia (Pisanu et al., 2019). Some studies have reported greater analgesic effects that were produced by mixed μ-opioid receptor (MOR)/κ-opioid receptor (KOR) drugs in women (Gear et al., 1996, 1999; Mogil et al., 2003; Niesters et al., 2010). In rodents, males consistently exhibited greater MOR-mediated analgesia (for review, see Craft, 2003; Fillingin and Gear, 2004; Nasser and Afiy, 2019). Female mice have been shown to develop opioid withdrawal-induced hyperalgesia faster than males (Juni et al., 2008). Female rats exhibited hyperalgesia to subanalgesic doses of morphine, whereas males did not (Holtman and Wala, 2005). Evidence also indicates genomic-dependent (Taylor et al., 2020) and hormone-dependent KOR-mediated effects in female rats and mice (Rasakham and Liu-Chen, 2011; Abraham et al., 2018). Moreover, as recently highlighted by Becker and Chartoff (2019), we still have very limited knowledge of the role of sex, genetic, epigenetic, and pharmacological mechanisms that are associated with KOR function. These few and conflicting findings may suggest sex differences in the opioid system and nociception, which warrants further investigations of opioid withdrawal-induced hyperalgesia in both sexes.
Preclinical studies have shown that the dynorphin (endogenous KOR agonist)/KOR system is altered in both opioid dependence and pain. κ-Opioid receptors are Gi-coupled receptors that inhibit neurotransmitter release (Eguchi, 2004). The activation of KORs by dynorphin leads to a decrease in extracellular levels of dopamine (Shippenberg et al., 2007), γ-aminobutyric acid, and glutamate (Hjelmstad and Fields, 2003; Tejeda et al., 2017) in the nucleus accumbens and glutamate levels in the bed nucleus of the stria terminalis (Crowley et al., 2016). An increase in KOR binding was found in the central nucleus of the amygdala in chronic pain in male rats (Narita et al., 2006; Nation et al., 2018; Navratitlova et al., 2019) and mice (Narita et al., 2006). An increase in the expression of prodynorphin and G-protein activation by dynorphin was found in the nucleus accumbens in chronic pain in male mice (Liu et al., 2019; Meade et al., 2020). Also, allodynia was observed following the spinal administration of dynorphin in male and female rats (Ambriz-Tututi et al., 2011, Vanderah et al., 1996), mice (Laughlin et al., 1997), and male prodynorphin knockout mice did not exhibit hyperalgesia following nerve injury (Wang et al., 2001).
In addition, systemic (Liu et al., 2019), intra-amygdala (Nation et al., 2018), and intra-nucleus accumbens shell (Massaly et al., 2019) administration of KOR antagonists reversed negative emotional-like responses that were caused by chronic pain, such as anxiety-like and depression-like behaviors that were induced by peripheral nerve injury in male and female mice (Liu et al., 2019), reversed stress-induced loss of the diffuse noxious inhibitory control response (i.e., a pain-inhibiting response) in male morphine-primed rats (Nation et al., 2018), and reversed the motivation to earn a reward in male rodents (Massaly et al., 2019). κ-Opioid receptor antagonism reduced anhedonia-like responses that were associated with chemotherapy pain in male rats (Meade et al., 2020). Moreover, an increase in dynorphin expression was observed in the nucleus accumbens (Zhou et al., 2011) and spinal cord (Vanderah et al., 2000; Gardell et al., 2002, 2006) following sustained MOR activation by morphine, DAMGO, and oxymorphone in male rats.
In animal models associated with opioid addiction, prodynorphin immunoreactivity was reported to be elevated in the nucleus accumbens core in male opioid-dependent rats that were exposed to 12 h of intravenous heroin self-administration per day and euthanized 12 h into withdrawal and in nondependent male rats that were exposed to 1 h of intravenous heroin self-administration per day and euthanized 23 h into withdrawal (Schlosburg et al., 2013). An increase in prodynorphin immunoreactivity in the nucleus accumbens shell was observed only in male opioid-dependent rats (Schlosburg et al., 2013). Systemic or intra-nucleus accumbens shell norBNI administration reduced the development of addiction-like behavior and anxiety-like behavior during withdrawal in male rats (Schlosburg et al., 2013). These findings suggest that the dynorphin/KOR system plays a role in nociception and anxiety-, depression-, and addiction-like behavior. However, the role of the dynorphin/KOR system in opioid withdrawal-induced hyperalgesia remains to be explored.
In the present study, we hypothesized that male and female rats are differentially sensitive to opioid-induced analgesia, the development of opioid withdrawal-induced hyperalgesia, and the ability of KOR antagonists to reverse opioid withdrawal-induced hyperalgesia. To test this hypothesis, we evaluated the effects of the KOR antagonists norBNI and 5′-guanidinonaltrindole (5′GNTI) on opioid withdrawal-induced hyperalgesia and somatic signs of naloxone-precipitated and spontaneous heroin withdrawal to determine whether and the extent to which hyperalgesia and other signs of opioid withdrawal are mediated by KORs.
2. Results
2.1. Female rats exhibited less heroin-induced thermal antinociception than male rats
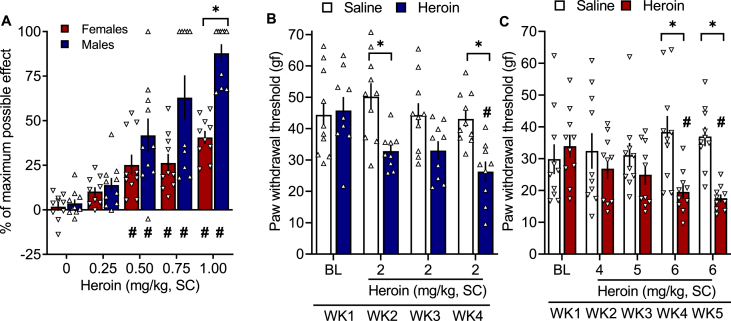
The two-way analysis of variance (ANOVA) revealed a significant sex × heroin dose interaction (F4,72 = 6.45, p = 0.0002). The Bonferroni post hoc test revealed significant antinociceptive effects of heroin at 0.5, 0.75, and 1 mg/kg in both males and females. Female rats exhibited significantly less thermal antinociception than male rats after treatment with heroin at 1 mg/kg (Fig. 1A).
Fig. 1.
(A) Female rats exhibited lower heroin-induced thermal antinociception than male rats. Wistar rats received heroin doses of 0–1 mg/kg (1 ml/kg, SC), with one dose per day 15 min before the hot plate test (52.5 °C; 1 min cutoff time). All of the animals received each dose of heroin in a within-subjects Latin-square design. The percent maximal possible effect was based on the baseline measure collected prior to the heroin injection for each rat. The data are expressed as mean ± SEM and were analyzed using two-way repeated-measures ANOVA followed by the Bonferroni post hoc test. #p < 0.05, difference from respective 0 mg/kg; *p < 0.05, difference between males and females. n = 10/sex. (B) Male rats developed heroin withdrawal-induced hyperalgesia. Male Wistar rats received heroin (2 mg/kg, SC) or saline (1 ml/kg, SC) once per day, five times per week, and were tested once per week (WK) in the electronic von Frey test 4–6 h into withdrawal. The data are expressed as mean ± SEM and were analyzed using two-way repeated-measures ANOVA followed by Bonferroni post hoc test. *p < 0.05, difference between heroin and saline; #p < 0.05, difference from respective baseline (n = 9–10/group). gf, gram force. (C) Female rats required higher doses of heroin to develop heroin withdrawal-induced hyperalgesia. Female Wistar rats received heroin (4–6 mg/kg, SC) or saline (1 ml/kg, SC) once per day, five times per week, and were tested once per week in the electronic von Frey test 4–6 h into withdrawal. The data are expressed as mean ± SEM and were analyzed using two-way repeated-measures ANOVA followed by the Bonferroni post hoc test. *p < 0.05, difference between heroin and saline; #p < 0.05, difference from respective baseline (n = 9–10/group). gf, gram force.
2.2. Female rats require higher doses of heroin to develop withdrawal-induced hyperalgesia
Consistent with the lower sensitivity to heroin-induced thermal antinociception in female rats than in male rats, we found that females did not develop hyperalgesia at 2 mg/kg heroin (Fig. S1), a dose that produced hyperalgesia in males. Thus, we increased the dose from 2 mg/kg to 6 mg/kg, a dose that reliably produced hyperalgesia in females. Both male and female rats received their respective doses of heroin 5 days per week in the morning and were tested for hyperalgesia in the afternoon (4–6 h into withdrawal). The two-way repeated-measures ANOVA revealed a group × time interaction (F3,51 = 3.271, p = 0.03). The Bonferroni post hoc test revealed a significantly lower paw withdrawal threshold in male rats that were treated with heroin (Fig. 1B). In female rats, the two-way repeated-measures ANOVA revealed a significant group × dose interaction (F4,45 = 3.856, p = 0.009). The Bonferroni post hoc test showed a significant effect of repeated treatment with 6 mg/kg heroin compared with repeated saline and baseline (Fig. 1C).
2.2.1. A single treatment with norBNI reversed heroin withdrawal-induced hyperalgesia
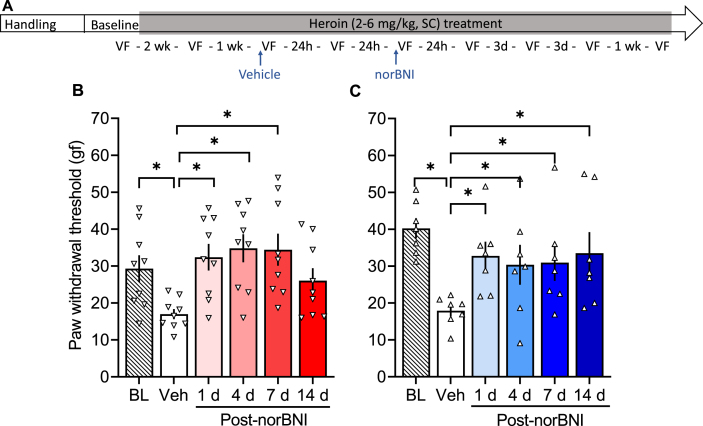
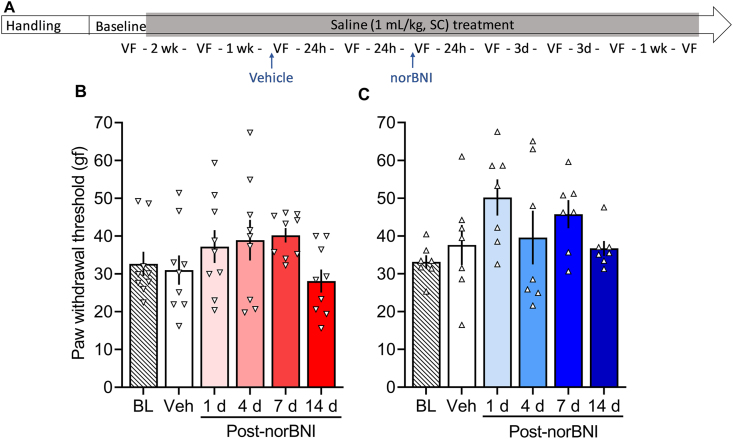
The KOR antagonist norBNI reversed opioid withdrawal-induced hyperalgesia in both females (F5,40 = 4.430, p = 0.003; Fig. 2B) and males (F5,30 = 5.167, p = 0.0015; Fig. 2C). In females, the measure taken 24 h after vehicle treatment was significantly lower than baseline (p < 0.05), indicating hyperalgesia. Measures that were taken 1, 4, and 7 days, but not 14 days, after norBNI treatment were significantly higher than the measure taken 24 h after vehicle treatment (p < 0.05). In males, the measure taken 24 h after vehicle treatment was significantly lower than baseline (p = 0.0001), and measures that were taken 1, 4, 7, and 14 days after norBNI treatment were significantly higher than vehicle (p < 0.05). Treatment with 30 mg/kg norBNI did not alter mechanical sensitivity in the saline-treated groups (males: F5,30 = 2.392, p = 0.06; females: F5,40 = 1.866, p = 0.12; Fig. 3B and C).
Fig. 2.
κ-Opioid receptor antagonist norBNI reversed heroin withdrawal-induced hyperalgesia. (A) Male and female Wistar rats were handled and habituated to subcutaneous (SC) saline injections for 1 week before the baseline (BL) paw withdrawal threshold measurement. All of the rats then received subcutaneous heroin injections once per day, five times per week. After 2 weeks of heroin treatment, the rats were re-tested in the electronic von Frey test. On the following week, all of the rats received vehicle and were tested 30 min (Table S1) and 24 h later (Veh). Next, all of the rats received a single injection of norBNI (30 mg/kg, SC) and were tested at 30 min (Table S1) and 1, 4, 7, and 14 days post-norBNI. Importantly, after their single norBNI injection, the rats continued to receive their daily heroin treatment five times per week throughout the experiment. We tested hyperalgesia 4–6 h after the heroin injection (i.e., acute withdrawal). (B) nor-Binaltorphimine reversed opioid withdrawal-induced hyperalgesia in females up to 7 days. (C) A single norBNI treatment reversed opioid withdrawal-induced hyperalgesia in males up to 14 days. The data are expressed as mean ± SEM and were analyzed using one-way repeated-measures ANOVA followed by the Bonferroni post hoc test. *p < 0.05, difference from Veh (n = 7–9/group). gf, gram force.
Fig. 3.
κ-Opioid receptor antagonist norBNI does not alter mechanical sensitivity in saline-treated rats. (A) Male and female Wistar rats were handled and habituated to subcutaneous (SC) saline injections for 1 week before the baseline (BL) paw withdrawal threshold measure. All of the rats then received subcutaneous saline injections once per day, five times per week. After 2 weeks of saline treatment, they were re-tested in the electronic von Frey test. On the following week, all of the rats received vehicle and were tested after 30 min (Table S1) and 24 h (Veh). Next, all of the rats received a single injection of norBNI (30 mg/kg, SC) and were tested at 30 min (Table S1) and 1, 4, 7, and 14 days post-norBNI. Importantly, after their single norBNI injection, the rats continued to receive their daily saline treatment five times per week throughout the experiment. We tested hyperalgesia 4–6 h after the saline injection. (B) Female rats received vehicle, which did not alter their paw withdrawal thresholds, followed by norBNI treatment. nor-Binaltorphimine did not alter mechanical sensitivity on any of the test days. (C) Male rats received vehicle, which did not alter their paw withdrawal thresholds, followed by a single norBNI treatment. nor-Binaltorphimine did not alter paw withdrawal thresholds in males. The data are expressed as mean ± SEM and were analyzed using one-way repeated-measures ANOVA. gf, gram force.
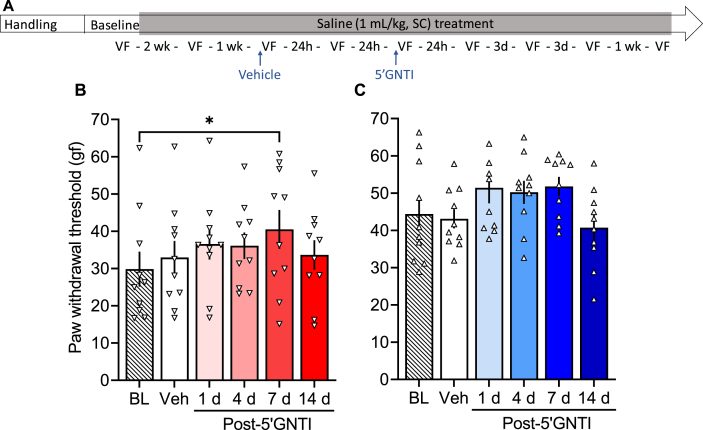
2.2.2. A single treatment with 5′GNTI reversed heroin withdrawal-induced hyperalgesia
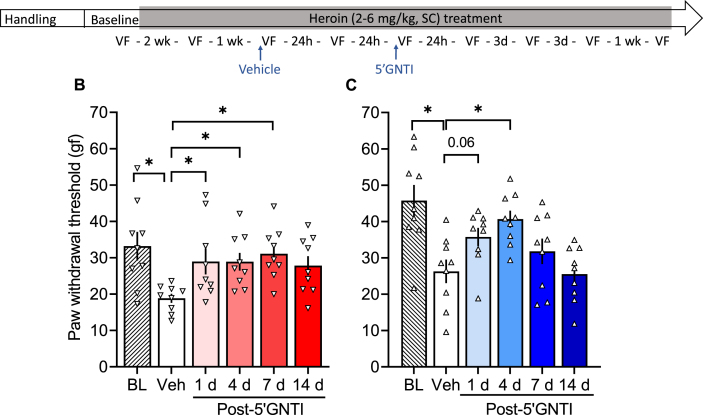
In a separate cohort of rats, we tested the effects of the selective KOR antagonist 5′GNTI on opioid withdrawal-induced hyperalgesia. 5′GNTI reversed heroin withdrawal-induced hyperalgesia in females (F5,40 = 4.141, p = 0.004; Fig. 4C) and males (F5,40 = 9.095, p < 0.0001; Fig. 4E). In females, the vehicle measure was significantly lower than baseline (p < 0.01) and measures that were taken 1, 4 and 7 days after 5′GNTI treatment were significantly higher than vehicle (p < 0.05). In males, the vehicle measure was significantly lower than baseline (p < 0.0001) and measures that were taken 4 days after 5′GNTI treatment were significantly higher than vehicle (p < 0.05). In females, a single treatment with 5′GNTI had an effect on the mechanical sensitivity (F5,45 = 2.447, p = 0.048; Fig. 5B), such that the baseline measure, but not the measure after vehicle, was significantly higher than the one taken 7 days post-5′GNTI. In males that were repeatedly treated with saline, there was no significant effect of 5′GNTI (F5,45 = 1.968, p = 0.10; Fig. 5C).
Fig. 4.
κ-Opioid receptor antagonist 5′GNTI reversed heroin withdrawal-induced hyperalgesia. (A) Male and female Wistar rats were handled and habituated to subcutaneous (SC) saline injections for 1 week before the baseline (BL) paw withdrawal threshold measure. All of the rats then received a subcutaneous heroin injection once per day, five times per week. After 2 weeks of heroin treatment, they were re-tested in the electronic von Frey test. On the following week, all of the rats received vehicle and were tested after 30 min (data shown in Figs. 6A) and 24 h (Veh). Next, all of the rats received a single injection of 5′GNTI (30 mg/kg, SC) and were tested at 30 min (data shown in Fig. 6A) and 1, 4, 7, and 14 days post-5′GNTI. Importantly, after their single 5′GNTI injection, the rats continued to receive their daily heroin treatment five times per week throughout the experiment. We tested hyperalgesia 4–6 h after heroin administration (i.e., acute withdrawal). (B) Female rats received vehicle, which was significantly different from the baseline measure, followed by 5′GNTI treatment. 5′GNTI reversed opioid withdrawal-induced hyperalgesia in females up to 7 days. (C) Male rats received vehicle, which was significantly different from the baseline measure, followed by 5′GNTI treatment. 5′GNTI reversed opioid withdrawal-induced hyperalgesia in males up to 4 days. The data are expressed as mean ± SEM and were analyzed using one-way repeated-measures ANOVA followed by the Bonferroni post hoc test. *p < 0.05 different from Veh (n = 7–9/group). gf, gram force.
Fig. 5.
κ-Opioid receptor antagonist GNTI does not alter mechanical sensitivity in saline-treated rats. (A) Male and female Wistar rats were handled and habituated to subcutaneous (SC) saline injections for 1 week before the baseline (BL) paw withdrawal threshold measure. All of the rats then received subcutaneous saline injections once per day, five times per week. After 2 weeks of saline treatment, they were re-tested in the electronic von Frey test. On the following week, all of the rats received vehicle and were tested after 30 min (data shown in Fig. 6A) and 24 h (Veh). Next, all of the rats received a single injection of 5′GNTI (30 mg/kg, SC) and were tested at 30 min (data shown in Fig. 6A) and 1, 4, 7 and 14 days post-5′GNTI. Importantly, after their single 5′GNTI injection, the rats continued to receive their daily saline treatment five times per week throughout the experiment. We tested hyperalgesia 4–6 h after the saline injection. (B) Female rats received vehicle (Veh), which did not alter their paw withdrawal thresholds compared with baseline (BL), followed by 5′GNTI treatment. 5′GNTI increased mechanical sensitivity at day 7 compared with baseline in female rats. (C) Male rats received vehicle, which did not alter their paw withdrawal thresholds compared with BL, followed by 5′GNTI treatment, which did not alter their mechanical sensitivity. The data are expressed as mean ± SEM and were analyzed using one-way repeated-measures ANOVA followed by the Bonferroni post hoc test. *p < 0.05, different from baseline (n = 7–9/group). gf, gram force.
In a control experiment, we observed that a single treatment with vehicle (saline, 1 mL/kg, SC) did not change the maintenance of heroin withdrawal-induced mechanical hyperalgesia for 14 days (Fig. S2).
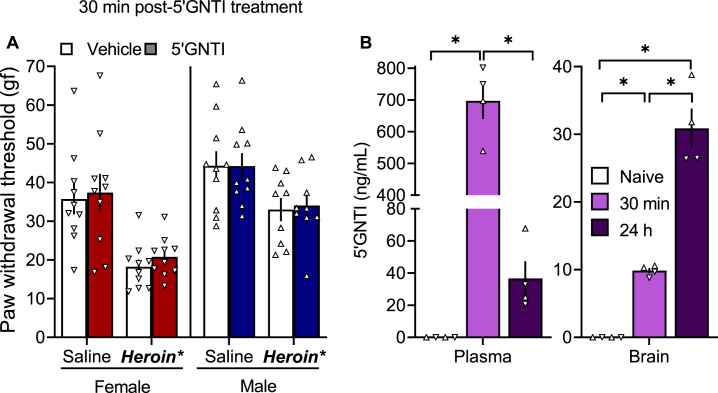
2.2.3. Blood and brain 5′GNTI levels after a single administration
5′GNTI did not alter paw withdrawal thresholds 30 min after treatment (Fig. 6A). We determined plasma and brain 5′GNTI levels 30 min or 24 h after treatment with 30 mg/kg 5′GNTI in male and female rats compared with naive controls (Fig. 6B). The ANOVA revealed a significant effect of time (F2,9 = 138.9, p < 0.0001) on plasma 5′GNTI levels. The Bonferroni post hoc test showed that plasma 5′GNTI levels at 30 min were significantly higher than the naive group (p < 0.0001) and compared with 24 h (p < 0.0001). The ANOVA also revealed a significant effect of time on brain 5′GNTI levels (F2,9 = 69.22, p < 0.0001). The Bonferroni post hoc test indicated that brain 5′GNTI levels at 24 h were significantly higher than at 30 min (p < 0.0001) and the naive group, and the levels at 30 min where significantly higher than in the naive group (p < 0.001).
Fig. 6.
(A) 5′GNTI had no effect on heroin withdrawal-induced hyperalgesia 30 min after treatment. Half of the rats received saline, and half received heroin (2–6 mg/kg, SC). Four hours later, all of the rats received vehicle and were tested after 30 min in the von Frey test. Two days later, the same rats received 5′GNTI (30 mg/kg, SC) and were tested 30 min later in the von Frey test. The data are expressed as mean ± SEM and were analyzed using a two-way ANOVA. *p < 0.05, main group effect. n = 9–10/group. gf, gram force. (B) Plasma and brain 5′GNTI levels. Wistar rats of both sexes received 5′GNTI (30 mg/kg, SC), and brain and plasma were collected 30 min or 24 h later. Samples were analyzed by mass spectrometry. The data are expressed as mean ± SEM and were analyzed using a one-way ANOVA followed by the Bonferroni post hoc test. ▲, males. ▼, females. *p < 0.05, difference between groups. n = 4/group.
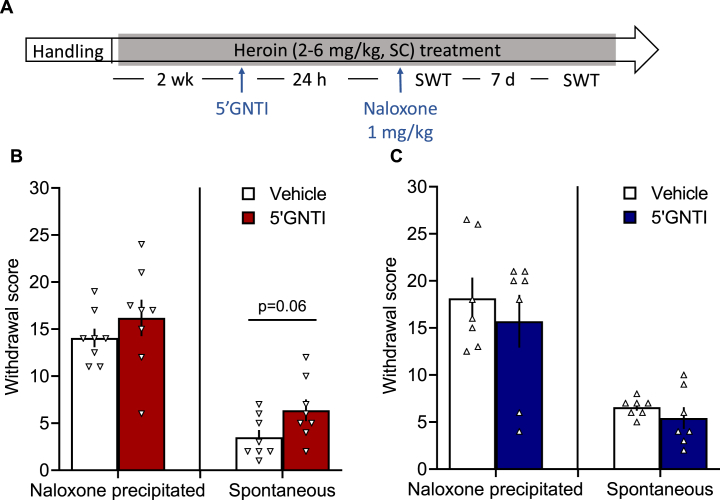
2.2.4. 5′GNTI did not block somatic signs of withdrawal
5′GNTI did not decrease somatic signs of naloxone-precipitated withdrawal (Fig. 7) in either male (T12 = 0.681; p > 0.05) or female rats (T14 = 0.978; p > 0.05). One week after 5′GNTI treatment, we also measured withdrawal scores during spontaneous withdrawal. No differences were found in either males (T12 = 0.945; p > 0.05) or females (T14 = 2.071, p = 0.06).
Fig. 7.
5′GNTI treatment did not affect somatic signs of naloxone-precipitated or spontaneous withdrawal. (A) All of the rats were handled and habituated to subcutaneous saline injections for 1 week. All of the rats then received heroin (2–6 mg/kg, SC) 5 days per week for 4 weeks. On the third week, half of the rats received vehicle, and half received 5′GNTI (30 mg/kg, SC). Twenty-four hours later, all of the rats received naloxone hydrochloride (1 mg/kg, SC) and were observed for signs of somatic withdrawal for 10 min. After 1 week, 4 h after the heroin injection, the rats were observed for signs of spontaneous withdrawal. Treatment with 5′GNTI in female (B) or male (C) rats did not change naloxone-precipitate or spontaneous signs of heroin withdrawal. The data are expressed as mean ± SEM and were analyzed using unpaired Student's t-test. n = 7–8/group. SWT, somatic withdrawal test.
3. Discussion
In the present study, we found that female rats were less sensitive to the analgesic effects of heroin, and higher doses of heroin were required to produce hyperalgesia in females compared with males. The antagonism of KORs with norBNI and 5′GNTI reversed opioid withdrawal-induced hyperalgesia in both heroin-dependent male and female rats in a sex-specific, time-dependent manner, without affecting somatic signs of withdrawal. We detected 5′GNTI in blood and brain following systemic administration, suggesting that it crosses the blood-brain barrier.
Our data provide evidence of the lower analgesic efficacy of heroin in female rats. The same doses of heroin in females produced 40% less thermal antinociception than in males in the hot plate test. Consistent with our results, Liu et al. (2018) reported that female mice exhibited less morphine-induced analgesia than male mice. These effects correlated with differential splicing of OPRM1, the gene that encodes the MOR, in several brain regions, particularly the thalamus. The lower effect of heroin in female rodents may have contributed to the previously reported increase in vaporized fentanyl self-administration in female mice compared with male mice (Moussawi et al., 2020) and increase in intravenous heroin self-administration in female mice compared with male mice (Towers et al., 2019) and rats (Cicero et al., 2003). Heroin is rapidly metabolized to 6-monoacetylmorphine (6-MAM) and morphine. 6-Monoacetylmorphine is the main active metabolite of heroin in the rat brain (Gottås et al., 2013; Schlosburg et al., 2013), and female mice have higher concentrations of 6-MAM in both brain and plasma following heroin administration (Hwang et al., 2019). The findings by Hwang et al. (2019) would be in contrast to our behavioral observations in rats (i.e., greater resistance to the effects of heroin in females). Additionally, opioid levels in the brain in female rats peaked earlier (15 min vs. 45 min) than in males following a heroin injection (Djurendic-Brenesel et al., 2010). Morphine is metabolized to morphine-6-glucuronide (M6G) and morphine-3-glucuronide (M3G; Gottås et al., 2013). Doyle and Murphy (2018) reported higher levels of M3G following morphine administration in female rats than in male rats. M3G exerts no analgesic effects per se, but it induces hyperalgesia in mice when administered alone (Juni et al., 2006; Roeckel et al., 2017; Blomqvist et al., 2020). Thus, higher levels of M3G in females may explain the lower analgesic potency of heroin in female rats but not their greater resistance to hyperalgesia. Note that saline injections had no effect on mechanical thresholds in females. Thus, the stress of injections appeared not to contribute to the resistance of females to develop heroin withdrawal-induced hyperalgesia. These findings suggest that sex differences in opioid-induced analgesia may be attributable to both pharmacokinetic and pharmacodynamic factors.
Opioid withdrawal-induced hyperalgesia has been shown to be MOR-dependent (Roeckel et al., 2017). The present study found that females required higher doses of heroin to exhibit hyperalgesia. Dynorphin has been shown to be upregulated in the spinal cord in opioid withdrawal-induced hyperalgesia and act through glutamate and N-methyl-D-aspartate (NMDA) receptors in the spinal cord to contribute to hyperalgesia (Ossipov et al., 1995, 2005; Celerier et al., 2001; Hemstapat et al., 2003). NMDA receptor-mediated opioid withdrawal-induced hyperalgesia was observed in ovariectomized mice but was absent in intact female mice, suggesting modulation by sexual hormones (Bryant et al., 2006; Arout et al., 2015). Other systems have been implicated in opioid withdrawal-induced hyperalgesia, including Toll-like receptor 4 (Hutchinson et al., 2010; Aguado et al., 2018) and corticotropin-releasing factor (Park et al., 2013), but the role of these systems in opioid withdrawal-induced hyperalgesia remained to be investigated in females.
The present study provided evidence that the dynorphin/KOR system is functionally involved in mechanical hyperalgesia that develops during withdrawal in heroin-dependent male and female rats. Both 5′GNTI and norBNI completely reversed opioid withdrawal-induced hyperalgesia in both sexes, whereas norBNI and 5′GNTI were mostly ineffective in altering pain mechanical sensitivity in male or female rats that were repeatedly treated with saline, indicating a specific effect of the dynorphin/KOR system on hyperalgesia in opioid dependent animals. This effect could be mediated spinally, given that dynorphin is upregulated in the spinal cord during opioid withdrawal-induced hyperalgesia (Rattan and Tejwani, 1997; Vanderah et al., 2000; Gardell et al., 2002, 2006). However, Obara et al. (2003) reported that intrathecal KOR antagonism enhanced rather than blocked allodynia after sciatic nerve injury and suggested that allodynia was mediated through a non-opioid effect of dynorphin (Obara et al., 2003).
nor-Binaltorphimine was developed by Portoghese et al. (1987). This KOR antagonist has transient MOR antagonist activity that fades after 4 h, whereas its KOR antagonist activity peaks at 24 h (Endoh et al., 1992), and some of its effects last for several weeks following a single treatment. Structure-activity relationship studies (Jones et al., 1998; Sharma et al., 2001) of norBNI led to the development of 5′GNTI, again by the Portoghese group. 5′GNTI is highly selectivite for KORs over other opioid receptors and four-times more potent than norBNI in vitro (Jones et al., 1998; Munro et al., 2013). Consistent with a delayed onset and long duration of action of norBNI and 5′GNTI, we found that 5′GNTI did not reverse opioid withdrawal-induced hyperalgesia when tested 30 min after treatment, whereas both norBNI and 5′GNTI reversed hyperalgesia 24 h after treatment and thereafter. The effects of norBNI and 5′GNTI in reversing hyperalgesia were long-lasting. In males, the anti-hyperalgesia effect of norBNI and 5′GNTI lasted for 14 days and 4 days, respectively. In females, the effect of norBNI and 5′GNTI lasted for 7 days. The long-lasting effect of norBNI in the present study is consistent with previous findings that a single administration of norBNI prevented the escalation and progression of dependence in male heroin- and methamphetamine-self-administering rats (Schlosburg et al., 2013; Whitfield et al., 2015). norBNI also has long-lasting (10–14 days) effects on anxiety-like (Knoll et al., 2007; Rogala et al., 2012) and anhedonia/depression-like (Todtenkopf et al., 2004; Chartoff et al., 2012; Mague et al., 2003; Laman-Marharg et al., 2018) behavior in male rodents. The effects of 5′GNTI have also been shown to be long-lasting (14 days) in male and female rhesus monkeys (Negus et al., 2002), blocking the KOR agonism-induced decrease in responding in a food-reinforcement assay. The long-lasting effects of norBNI and 5′GNTI have been hypothesized to involve various mechanisms (Munro et al., 2012), including long-lasting activation of the c-Jun N-terminal kinase 1 pathway (Bruchas et al., 2007; Melief et al., 2011). With regard to sex differences in the duration of the anti-hyperalgesia effect that was found in the present study, a possible explanation is that KOR antagonists may regulate the c-Jun N-terminal kinase 1 pathway differently in male and female mice as reported by Laman-Marharg et al. (2018). Remaining to be established is whether there are sex differences in the pharmacokinetics of norBNI and 5′GNTI, given that such studies have been performed in only male mice (Horan et al., 1992; Bruchas et al., 2007; Munro et al., 2012; Patkar et al., 2013).
norBNI has been shown to cross the blood-brain barrier (Munro et al., 2012; Patkar et al., 2013). Additionally, a single injection of norBNI at a dose of 4 μg/side in the shell of the nucleus accumbens blocked the escalation of heroin self-administration in male rats (Schlosburg et al., 2013), suggesting a central effect of KOR antagonism. However, Munro et al. (2012) did not detect 5′GNTI in the brain in male mice at doses of 10 mg/kg, suggesting that 5′GNTI did not cross the blood-brain barrier. However, we analyzed blood and brain samples from rats of both sexes 30 min and 24 h after treatment with 5′GNTI (30 mg/kg) and observed higher plasma 5′GNTI levels at 30 min than at 24 h and higher brain 5′GNTI levels at 24 h than at 30 min, suggesting that 5′GNTI also crosses the blood-brain barrier at this higher dose. The discrepancy between our findings and Munro et al. (2012) may be attributable to the higher sensitivity of our assay with a lower limit of detection (10 ng/ml vs. 120 ng/ml). Given that the Ki of 5′GNTI is 3 nM (Munro et al., 2013) and its molecular weight is 662.32 g/mol, a concentration of 2 ng/ml would be sufficient to block the receptor. Therefore, we suggest that the concentration of 5′GNTI that reached the brain (30 ng/ml) at 24 h is likely sufficient to elicit a pharmacological effect.
We found that 5′GNTI did not alter somatic signs of naloxone-precipitated or spontaneous withdrawal, suggesting a dissociation of the participation of KORs in opioid withdrawal-induced hyperalgesia and somatic signs of withdrawal. The data in the literature on the somatic signs of opioid withdrawal are conflicting. Some studies reported a decrease in opioid withdrawal signs following treatment with a KOR antagonist (Kelsey et al., 2015), whereas other studies reported an increase (Spanagel et al., 1994). The present results add to the literature that shows a lack of effect of KOR antagonism on somatic signs of opioid withdrawal in male and female rats.
κ-Opioid receptor antagonists have been proposed as a treatment for mood disorders (Carrol and Carlezon, 2013; Krystal et al., 2020), drug addiction (Chakvin and Koob, 2016; Helal et al., 2017), stress-related pain disorders, and neuropathic pain (Nation et al., 2018; Navratilova et al., 2019) by promoting resilience to stress. The reason for the failure of these compounds in clinical trials to date is unknown and may require testing hypotheses that involve specific behavioral symptoms rather than global disorder constructs (Kwako et al., 2017; Cuthbert, 2020). The present results suggest that KORs remain a viable target for medication development for elements of stress-related psychiatric disorders, such as depression, chronic pain, and addiction (Chavkin and Koob, 2016).
In summary, we found that female rats were more resistant to heroin-induced analgesia and hyperalgesia during withdrawal than male rats. κ-Opioid receptor antagonism reversed heroin withdrawal-induced hyperalgesia in both males and females, suggesting that the dynorphin/KOR system is functionally involved in this behavior. Thus, the dynorphin/KOR system is a promising target for improving our understanding of opioid withdrawal-induced hyperalgesia and its relationship with negative emotions, stress, and opioid addiction.
4. Methods
4.1. Animals
Eighty-four male and 72 female Wistar rats (175–225 g) were purchased from Charles River Laboratories (Kingston, NY, USA). They were acclimated to the animal facility for at least 2 weeks before the study. All of the rats were housed under a 12 h/12 h reverse light/dark cycle (lights on at 7:00 p.m. and off at 7:00 a.m.) with controlled temperature (22 °C ± 2 °C) and humidity (50–60%) and free access to water and food except during testing. All of the procedures were conducted according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the National Institute on Drug Abuse, Intramural Research Program, Animal Care and Use Committee.
4.2. Drugs
A 24 mg/ml stock solution of diamorphine hydrochloride (heroin; National Institute on Drug Abuse Drug Supply Program, National Institutes of Health, Baltimore, MD, USA) was diluted with sterile saline (Hospira, Lake Forest, IL, USA) at concentrations of 0.5–6 mg/ml norBinaltorphimine dihydrochloride-[4bS,8R,8aS,10aS,11R,14aS,19aR,20bR)-7,12-bis(cyclopropylmethyl)-5,6,7,8,9,10,11,12,13,14,19a,20b-dodecahydro-20H-4,8:11,15-dimethanobis (benzofuro)[2,3-a:3′,2′-i]dipyrido [4,3-b:3′,4′-h]carbazole-1,8a,10a,18-tetraol dihydrochloride] and 5′GNTI [1-((4bS,8R,8aS,14bR)-7-(cyclopropylmethyl)-1,8a-dihydroxy-5,6,7,8,8a,9,14,14b-octahydro-4,8-methanobenzofuro [2,3-a]pyrido [4,3-b]carbazol-11-yl)guanidine dihydrochloride] were synthetized at the National Institute on Drug Abuse Intramural Research Program (Rockville, MD, USA), and diluted with sterile saline.
4.3. Experiment 1: heroin-induced analgesia dose-response curve
Heroin-induced analgesia was measured using a hot plate (Ugo Basile, Gemonio, Italy) at a fixed temperature of 52.5 °C. The rats were habituated to the apparatus at room temperature for 1 min before baseline measures were taken to minimize novelty-induced analgesia (Vendruscolo et al., 2004). For both baseline and antinociception testing, the rats were placed on the hot plate. The timer was triggered by a pedal. Once the rat exhibited nociceptive behavior, such as jumping, vocalization, tapping, or raising or licking the hind paw, the timer was stopped, and the rat was immediately removed from the hot plate. A 1-min cutoff time was used to avoid tissue damage in nonresponding animals.
We used a within-subjects Latin-square design to test heroin-induced analgesia at 0.0, 0.25, 0.50, 0.75, and 1.0 mg/kg (1 ml/kg, SC). All of the rats received every dose of heroin in a Latin-square design, 24 h apart, 15 min before the hot-plate test. Two rats of each sex started at one of the doses and progressed to the next higher dose on the next day until they were exposed to all of the doses (n = 10/sex). The data are expressed as a percentage of the maximum possible effect: %MPE = (latencyheroin – latencybaseline)/(cutoff – latencybaseline) × 100. The baseline latency was 7.8 ± 0.8 s for females and 8.0 ± 0.5 s for males.
4.4. Experiment 2: reversal of heroin withdrawal-induced hyperalgesia by systemic KOR antagonism
Mechanical hypersensitivity was evaluated using an electronic von Frey device (Ugo Basile, Gemonio, Italy). The rats were habituated to the testing room for at least 30 min and then acclimated to the apparatus for at least 15 min before testing. The apparatus consisted of an elevated platform (100 cm length × 50 cm width × 40 cm height) with a stainless-steel mesh floor with rectangular plastic compartments (27 cm × 16 cm × 13 cm) on top. To assess mechanical hypersensitivity, the mid-plantar area of each hind paw was stimulated twice with a rigid filament, with at least 30-s intervals between measurements. All of the measures were averaged and are expressed as grams force (gf). The animals were tested 4–6 h after the heroin or saline injection.
The rats were handled and habituated to the SC saline injections once per day for 1 week before the baseline measurement. Following the baseline measurement of mechanic sensitivity using the electronic von Frey device, the rats were split into two groups. One group received chronic saline injections (1 ml/kg, SC), and the other group received chronic heroin injections in the mornings, 5 days per week (Monday-Friday). The males received 2 mg/kg heroin (1 ml/kg, SC). The females started at 2 mg/kg heroin, and the dose was increased to 6 mg/kg at increments of 1 mg/kg per day and maintained at 6 mg/kg for the remainder of the study. All of the rats were tested once per week for mechanical sensitivity 4–6 h after heroin or saline administration (i.e., during acute heroin withdrawal). Once hyperalgesia was established in the chronic heroin group, we tested the effects of norBNI and 5′GNTI on hyperalgesia. Note that the rats continued to receive daily injections of heroin (or saline) during the post-norBNI or post-5′GNTI testing that occurred during heroin withdrawal (4–6 h after the heroin injection). We used a mixed between/within-subjects design, with group (chronic saline or chronic heroin) as the between-subjects factor and treatment (vehicle or KOR antagonist) as the within-subjects factor. All of the animals received a vehicle injection and were tested for hyperalgesia after 30 min and 24 h. The same rats received the KOR antagonist 48 h later and were tested in the von Frey test 30 min, 1 day, 4 days, 7 days, and 14 days after KOR antagonist treatment. Animals in an initial cohort received a single 30 mg/kg injection of norBNI (2 ml/kg, SC; females: n = 9/group; males: n = 7/group). Rats in a second cohort received a single 30 mg/kg injection of 5′GNTI (1 mg/ml, SC; repeated saline: n = 10/sex; repeated heroin: n = 9/sex). The rats were maintained on their ascribed injection regimen (i.e., chronic saline or chronic heroin) throughout this 14-day observation period. Body weights were recorded weekly. In a control experiment, the rats received a single injection of vehicle (sterile saline, Hospira, Lake Forest, IL, USA) instead of norBNI or 5′GNTI and were tested as above.
4.5. Experiment 3: measurement of blood and brain 5′GNTI levels
Serum and phosphate-buffered saline (PBS)-perfused brains samples were processed for the mass spectrometry identification of 5′GNTI. Brain tissue was homogenized in 2 ml of distilled water per gram of tissue. For extraction, 200 μl of brain homogenate or plasma was mixed with 600 μl of methanol. The samples were vortexed for 5 min and centrifuged at 15,600 rotations per minute at 4 °C for 15 min. The supernatant was transferred to a new microtube and evaporated at 50 °C under a nitrogen atmosphere. The samples were resuspended in 200 μl of 6% methanol in 0.1% formic acid for mass analysis. The samples were analyzed using a Dionex UltiMate 3000 high-performance liquid chromatography device coupled to an Orbitrap Velos with a heated electrospray ionization source (Thermo Scientific, San Jose, CA, USA). A Kinetex 2.6 μm C18 100 Å (100 × 2.1 mm) column (Phenomenex, Torrance, CA, USA) was used for liquid chromatography separation. The liquid chromatography conditions were the following: 30 °C column temperature, 0.1% formic acid mobile phase A (aqueous), methanol mobile phase B (organic), 0.3 ml/min flow rate, 6–100% gradient of mobile phase B in 8 min, and 5 μl sample injection volume. The electrospray ionization mass spectrometry parameters were the following: 4 kV source voltage (positive ion mode) and 60,000 m/Δm mass resolution. A standard curve was generated for 5′GNTI [M+2H]2+ from 5 to 1000 ng/ml. The limit of quantification was 10 ng/ml, and the limit of detection was 5 ng/ml.
The rats (n = 6 males, n = 6 females) received an SC injection of 30 mg/kg 5′GNTI. After 30 min (n = 4) or 24 h (n = 4), the rats were deeply anesthetized in an isoflurane-saturated chamber. The chest cavity was then opened, and blood was collected by cardiac puncture, followed by perfusion with PBS. The brains were collected and snap frozen in isopentane. A drug-naive group was included as a control (n = 4). Blood and brain samples were kept at −80 °C until the assay was performed.
4.6. Experiment 4: effect of KOR blockade on somatic signs of naloxone-precipitated withdrawal
Somatic withdrawal was scored as previously reported (Vendruscolo et al., 2018) by two experimenters who were blind to the rats’ group assignment. Two classes of somatic signs of opioid withdrawal were observed for 10 min. Graded signs included wet dog shakes (2 points for 1–2 occurrences and 4 points for ≥ 3 occurrences) and jump attempts (1 point for 2–4 occurrences, 2 points for 5–9 occurrences, and 3 points for ≥ 10 occurrences). Checked signs included abdominal spasms (2 points), genital area grooming (3 points), teeth chattering (2 points), vocalization upon touch (3 points), swallowing movements (2 points), ptosis (2 points), abnormal posture (3 points), defecation/diarrhea (2 points), and profuse salivation (7 points). Additionally, we recorded body weight loss 60 min after the naloxone injection (1 point per gram lost). The data are expressed as the total withdrawal score.
Male and female Wistar rats were treated 5 days per week (Monday-Friday) with heroin in the same manner as described in Experiment 2. After 3 weeks of repeated heroin injections, half of the rats were treated with vehicle, and the other half were treated with 5′GNTI (30 mg/kg, SC). Twenty hours after treatment, all of the rats received heroin followed 4 h later by naloxone (1 mg/kg, SC) and were observed for somatic signs of naloxone-precipitated withdrawal as described above. After one additional week of repeated heroin injections, the same rats were observed for somatic signs of spontaneous withdrawal (male: n = 7/group, female: n = 8/group).
4.7. Statistical analysis
The data are expressed as mean ± standard error of the mean (SEM). The data from Experiment 1 were analyzed using two-way repeated-measures ANOVA, with dose as the within-subjects factor and sex as the between-subjects factor, followed by the Bonferroni post hoc test when appropriate. In Experiment 2, the effect of treatment was analyzed separately by sex and group (repeated saline or repeated heroin) using a one-way repeated-measures ANOVA, with posttreatment time as the within-subjects factor. Bonferroni's post hoc test was used to compare baseline and different timepoints after KOR antagonist administration with vehicle. The data from Experiment 3 were analyzed using one-way ANOVA, with treatment as the between-subjects factor, followed by the Bonferroni post hoc test. The data from Experiment 4 were analyzed using unpaired t-tests separately by sex. Values of p < 0.05 were considered statistically significant.
CRediT authorship contribution statement
Renata C.N. Marchette: Formal analysis, Data curation, Methodology, performed the behavioral experiments, prepared the figures. Adriana Gregory-Flores: Formal analysis, Data curation, Writing – review & editing, performed the behavioral experiments. Brendan J. Tunstall: Writing – review & editing, performed the behavioral experiments. Erika R. Carlson: Writing – review & editing, performed the behavioral experiments, prepared the figures. Shelley N. Jackson: Formal analysis, Writing – review & editing, performed the mass spectrometry analysis. Agnieszka Sulima: Writing – review & editing, synthesized norBNI and 5′GNTI. Kenner C. Rice: Writing – review & editing. George F. Koob: Formal analysis, Data curation, Writing – review & editing, Methodology. Leandro F. Vendruscolo: Formal analysis, Data curation, Writing – original draft, designed the experiments. KCF, synthesized norBNI and 5′GNTI, RM, Writing – original draft.
Declaration of competing interest
The authors declare no conflicts of interest.
Acknowledgements
We thank Janaina Vendruscolo for technical assistance and Michael Arends for proofreading the manuscript. Heroin was provided by the National Institute on Drug Abuse Drug Supply Program and dispensed by the National Institute on Drug Abuse Intramural Research Program Pharmacy. This research was supported by the Intramural Research Programs of the National Institute on Drug Abuse and National Institute on Alcohol Abuse and Alcoholism. RCNM is a fellow of the Center for Compulsive Behaviors at the National Institutes of Health. BJT received funding from National Institutes of Health grant DA048530.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynstr.2021.100325.
Contributor Information
Renata C.N. Marchette, Email: renata.marchette@nih.gov.
Leandro F. Vendruscolo, Email: leandro.vendruscolo@nih.gov.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Abraham A.D., Schattauer S.S., Reichard K.L., Cohen J.H., Fontaine H.M., Song A.J., Johnson S.D., Land B.B., Chavkin C. Estrogen regulation of GRK2 inactivates kappa opioid receptor signaling mediating analgesia, but not aversion. J. Neurosci. 2018;38:8031–8043. doi: 10.1523/JNEUROSCI.0653-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguado D., Bustamante R., Gómez de Segura I.A. Toll-like receptor 4 deficient mice do not develop remifentanil-induced mechanical hyperalgesia: an experimental randomised animal study. Eur. J. Anaesthesiol. 2018;35:505–510. doi: 10.1097/EJA.0000000000000803. [DOI] [PubMed] [Google Scholar]
- Ambriz-Tututi M., Cruz S.L., Urquiza-Marín H., Granados-Soto V. Formalin-induced long-term secondary allodynia and hyperalgesia are maintained by descending facilitation. Pharmacol. Biochem. Behav. 2011;98:417–424. doi: 10.1016/j.pbb.2011.02.012. [DOI] [PubMed] [Google Scholar]
- Arout C.A., Caldwell M., Rossi G., Kest B. Spinal and supraspinal N-methyl-D-aspartate and melanocortin-1 receptors contribute to a qualitative sex difference in morphine-induced hyperalgesia. Physiol. Behav. 2015;147:364–372. doi: 10.1016/j.physbeh.2015.05.006. [DOI] [PubMed] [Google Scholar]
- Becker J.B., Chartoff E. Sex differences in neural mechanisms mediating reward and addiction. Neuropsychopharmacology. 2019;44(1):166–183. doi: 10.1038/s41386-018-0125-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomqvist K.J., Viisanen H., Ahlström F.H.G., Jokinen V., Sidorova Y.A., Suleymanova I., Rauhala P.V., Kalso E.A., Lilius T.O. Morphine-3-glucuronide causes antinociceptive cross-tolerance to morphine and increases spinal substance P expression. Eur. J. Pharmacol. 2020;875 doi: 10.1016/j.ejphar.2020.173021. 173021. [DOI] [PubMed] [Google Scholar]
- Becker J.B., Mazure C.M. The federal plan for health science and technology's response to the opioid crisis: understanding sex and gender differences as part of the solution is overlooked. Biol. Sex Differ. 2019;10(3) doi: 10.1186/s13293-018-0215-5. s13293-018-0215–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas M.R., Yang T., Schreiber S., DeFino M., Kwan S.C., Li S., Chavkin C. Long-Acting κ opioid antagonists disrupt receptor signaling and produce noncompetitive effects by activating C-jun N-terminal kinase. J. Biol. Chem. 2007;282:29803–29811. doi: 10.1074/jbc.M705540200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant C.D., Eitan S., Sinchak K., Fanselow M.S., Evans C.J. NMDA receptor antagonism disrupts the development of morphine analgesic tolerance in male, but not female C57BL/6J mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;291:R315–R326. doi: 10.1152/ajpregu.00831.2005. [DOI] [PubMed] [Google Scholar]
- Carcoba L.M., Contreras A.E., Cepeda-Benito A., Meagher M.W. Negative affect heightens opiate withdrawal-induced hyperalgesia in heroin dependent individuals. J. Addict. Dis. 2011;30:258–270. doi: 10.1080/10550887.2011.581985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll F.I., Carlezon W.A. Development of κ opioid receptor antagonists. J. Med. Chem. 2013;56:2178–2195. doi: 10.1021/jm301783x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Célèrier E., Laulin J.P., Corcuff J.B., Le Moal M., Simonnet G. Progressive enhancement of delayed hyperalgesia induced by repeated heroin administration: a sensitization process. J. Neurosci. 2001;21:4074–4080. doi: 10.1523/JNEUROSCI.21-11-04074.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartoff E., Sawyer A., Rachlin A., Potter D., Pliakas A., Carlezon W.A. Blockade of kappa opioid receptors attenuates the development of depressive-like behaviors induced by cocaine withdrawal in rats. Neuropharmacology. 2012;62:167–176. doi: 10.1016/j.neuropharm.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavkin C., Koob G.F. Dynorphin, dysphoria, and dependence: the stress of addiction. Neuropsychopharmacology. 2016;41:373–374. doi: 10.1038/npp.2015.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicero T.J., Aylward S.C., Meyer E.R. Gender differences in the intravenous self-administration of mu opiate agonists. Pharmacol. Biochem. Behav. 2003;74(3):541–549. doi: 10.1016/s0091-3057(02)01039-0. [DOI] [PubMed] [Google Scholar]
- Comer S.D., Cooper Z.D., Kowalczyk W.J., Sullivan M.A., Evans S.M., Bisaga A.M., Vosburg S.K. Evaluation of potential sex differences in the subjective and analgesic effects of morphine in normal, healthy volunteers. Psychopharmacology. 2009;208:45. doi: 10.1007/s00213-009-1703-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft R.M. Sex differences in opioid analgesia: “from mouse to man”. Clin. J. Pain. 2003;19:175–186. doi: 10.1097/00002508-200305000-00005. [DOI] [PubMed] [Google Scholar]
- Crowley N.A., Bloodgood D.W., Hardaway J.A., Kendra A.M., McCall J.G., Al-Hasani R., McCall N.M., Yu W., Schools Z.L., Krashes M.J., Lowell B.B., Whistler J.L., Bruchas M.R., Kash T.L. Dynorphin controls the gain of an amygdalar anxiety circuit. Cell Rep. 2016;14:2774–2783. doi: 10.1016/j.celrep.2016.02.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert B.N. The role of RDoC in future classification of mental disorders߭. Dialogues Clin. Neurosci. 2020;22(1):81–85. doi: 10.31887/DCNS.2020.22.1/bcuthbert. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djurendic-Brenesel M., Mimica-Dukic N., Pilija V., Tasic M. Gender-related differences in the pharmacokinetics of opiates. Forensic Sci. Int. 2010;194:28–33. doi: 10.1016/j.forsciint.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Doyle H.H., Murphy A.Z. Sex-dependent influences of morphine and its metabolites on pain sensitivity in the rat. Physiol. Behav. 2018;187:32–41. doi: 10.1016/j.physbeh.2017.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eguchi M. Recent advances in selective opioid receptor agonists and antagonists. Med. Res. Rev. 2004;24:182–212. doi: 10.1002/med.10059. [DOI] [PubMed] [Google Scholar]
- Endoh T., Matsuura H., Tanaka C., Nagase H. Nor-binaltorphimine: a potent and selective kappa-opioid receptor antagonist with long-lasting activity in vivo. Arch. Int. Pharmacodyn. Ther. 1992;316:30–42. [PubMed] [Google Scholar]
- Fillingim R.B., Gear R.W. Sex differences in opioid analgesia: clinical and experimental findings. Eur. J. Pain. 2004;8(5):413–425. doi: 10.1016/j.ejpain.2004.01.007. PMID: 15324773.Frenk, S.M., Gu, Q., Bohm, M.K., 2019. Prevalence of Prescription Opioid Analgesic Use Among Adults: United States, 2013–2016. [DOI] [PubMed] [Google Scholar]
- Frenk S.M., Gu Q., Bohm M.K. 2019. Prevalence of Prescription Opioid Analgesic Use Among Adults: United States, 2013–2016. [Google Scholar]
- Gardell L.R., Wang R., Burgess S.E., Ossipov M.H., Vanderah T.W., Malan T.P., Lai J., Porreca F. Sustained morphine exposure induces a spinal dynorphin-dependent enhancement of excitatory transmitter release from primary afferent fibers. J. Neurosci. 2002;22:6747–6755. doi: 10.1523/jneurosci.22-15-06747.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardell L.R., King T., Ossipov M.H., Rice K.C., Lai J., Vanderah T.W., Porreca F. Opioid receptor-mediated hyperalgesia and antinociceptive tolerance induced by sustained opiate delivery. Neurosci. Lett. 2006;396:44–49. doi: 10.1016/j.neulet.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Gear R.W., Gordon N.C., Heller P.H., Paul S., Miaskowski C., Levine J.D. Gender difference in analgesic response to the kappa-opioid pentazocine. Neurosci. Lett. 1996;205:207–209. doi: 10.1016/0304-3940(96)12402-2. [DOI] [PubMed] [Google Scholar]
- Gear R.W., Miaskowski C., Gordon N.C., Paul S.M., Heller P.H., Levine J.D. The kappa opioid nalbuphine produces gender- and dose-dependent analgesia and antianalgesia in patients with postoperative pain. Pain. 1999;83:339–345. doi: 10.1016/S0304-3959(99)00119-0. [DOI] [PubMed] [Google Scholar]
- Gottås A., Øiestad E.L., Boix F., Vindenes V., Ripel Å., Thaulow C.H., Mørland J. Levels of heroin and its metabolites in blood and brain extracellular fluid after i.v. heroin administration to freely moving rats. Br. J. Pharmacol. 2013;170:546–556. doi: 10.1111/bph.12305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helal M.A., Habib E.S., Chittiboyina A.G. Selective kappa opioid antagonists for treatment of addiction, are we there yet? Eur. J. Med. Chem. 2017;141:632–647. doi: 10.1016/j.ejmech.2017.10.012. [DOI] [PubMed] [Google Scholar]
- Hemstapat K., Monteith G.R., Smith D., Smith and M.T. Morphine-3-Glucuronide’s neuro-excitatory effects are mediated via indirect activation of N-Methyl-d-Aspartic acid receptors: mechanistic studies in embryonic cultured hippocampal neurones. Anesth. Analg. 2003;97:494–505. doi: 10.1213/01.ANE.0000059225.40049.99. [DOI] [PubMed] [Google Scholar]
- Higgins C., Smith B.H., Matthews K. Evidence of opioid-induced hyperalgesia in clinical populations after chronic opioid exposure: a systematic review and meta-analysis. Br. J. Anaesth. 2019;122:e114–e126. doi: 10.1016/j.bja.2018.09.019. [DOI] [PubMed] [Google Scholar]
- Hjelmstad G.O., Fields H.L. Kappa opioid receptor activation in the nucleus accumbens inhibits glutamate and GABA release through different mechanisms. J. Neurophysiol. 2003;89:2389–2395. doi: 10.1152/jn.01115.2002. [DOI] [PubMed] [Google Scholar]
- Holtman J., Wala E. Characterization of morphine-induced hyperalgesia in male and female rats. Pain. 2005;114:62–70. doi: 10.1016/j.pain.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Horan P., Taylor J., Yamamura H.I., Porreca F. Extremely long-lasting antagonistic actions of nor-binaltorphimine (nor-BNI) in the mouse tail-flick test. J. Pharmacol. Exp. Therapeut. 1992;260:1237–1243. [PubMed] [Google Scholar]
- Hutchinson M.R., Zhang Y., Shridhar M., Evans J.H., Buchanan M.M., Zhao T.X., Slivka P.F., Coats B.D., Rezvani N., Wieseler J., Hughes T.S., Landgraf K.E., Chan S., Fong S., Phipps S., Falke J.J., Leinwand L.A., Maier S.F., Yin H., Rice K.C., Watkins L.R. Evidence that opioids may have toll-like receptor 4 and MD-2 effects. Brain Behav. Immun. 2010;24:83–95. doi: 10.1016/j.bbi.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang C.S., Smith L.C., Wenthur C.J., Ellis B., Zhou B., Janda K.D. Heroin vaccine: using titer, affinity, and antinociception as metrics when examining sex and strain differences. Vaccine. 2019;37:4155–4163. doi: 10.1016/j.vaccine.2019.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R.M., Hjorth S.A., Schwartz T.W., Portoghese P.S. Mutational evidence for a common κ antagonist binding pocket in the wild-type κ and mutant μ[K303E] opioid receptors †. J. Med. Chem. 1998;41:4911–4914. doi: 10.1021/jm9805182. [DOI] [PubMed] [Google Scholar]
- Juni A., Klein G., Kest B. Morphine hyperalgesia in mice is unrelated to opioid activity, analgesia, or tolerance: evidence for multiple diverse hyperalgesic systems. Brain Res. 2006;1070:35–44. doi: 10.1016/j.brainres.2005.11.054. [DOI] [PubMed] [Google Scholar]
- Juni A., Klein G., Kowalczyk B., Ragnauth A., Kest B. Sex differences in hyperalgesia during morphine infusion: effect of gonadectomy and estrogen treatment. Neuropharmacology. 2008;54:1264–1270. doi: 10.1016/j.neuropharm.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Kelsey J.E., Verhaak A.M.S., Schierberl K.C. The kappa-opioid receptor antagonist, nor-binaltorphimine (nor-BNI), decreases morphine withdrawal and the consequent conditioned place aversion in rats. Behav. Brain Res. 2015;283:16–21. doi: 10.1016/j.bbr.2015.01.008. [DOI] [PubMed] [Google Scholar]
- Knoll A.T., Meloni E.G., Thomas J.B., Carroll F.I., Carlezon W.A. Anxiolytic-like effects of kappa-opioid receptor antagonists in models of unlearned and learned fear in rats. J. Pharmacol. Exp. Therapeut. 2007;323:838–845. doi: 10.1124/jpet.107.127415. [DOI] [PubMed] [Google Scholar]
- Krystal A.D., Pizzagalli D.A., Smoski M., Mathew S.J., Nurnberger J., Jr., Lisanby S.H., Iosifescu D., Murrough J.W., Yang H., Weiner R.D., Calabrese J.R., Sanacora G., Hermes G., Keefe R.S.E., Song A., Goodman W., Szabo S.T., Whitton A.E., Gao K., Potter W.Z. A randomized proof-of-mechanism trial applying the 'fast-fail' approach to evaluating κ-opioid antagonism as a treatment for anhedonia. Nat. Med. 2020;26(5):760–768. doi: 10.1038/s41591-020-0806-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwako L.E., Momenan R., Litten R.Z., Koob G.F., Goldman D. Reply to: neuroclinical assessment of addiction needs to incorporate decision-making measures and ecological validity. Biol. Psychiatr. 2017;81(7):e55. doi: 10.1016/j.biopsych.2016.08.029. Epub 2016 Aug 30. PMID: 27776735. [DOI] [PubMed] [Google Scholar]
- Laman-Maharg A., Williams A.V., Zufelt M.D., Minie V.A., Ramos-Maciel S., Hao R., Ordoñes Sanchez E., Copeland T., Silverman J.L., Leigh A., Snyder R., Carroll F.I., Fennell T.R., Trainor B.C. Sex differences in the effects of a kappa opioid receptor antagonist in the forced swim test. Front. Pharmacol. 2018;9:93. doi: 10.3389/fphar.2018.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughlin T.M., Vanderah T.W., Lashbrook J., Nichols M.L., Ossipov M., Porreca F., Wilcox G.L. Spinally administered dynorphin A produces long-lasting allodynia: involvement of NMDA but not opioid receptors. Pain. 1997;72:253–260. doi: 10.1016/S0304-3959(97)00046-8. [DOI] [PubMed] [Google Scholar]
- Liu A., Zhang H., Qin F., Wang Qisheng, Sun Q., Xie S., Wang Qian, Tang Z., Lu Z. Sex associated differential expressions of the alternatively spliced variants mRNA of OPRM1 in brain regions of C57bl/6 mouse. Chem. Pharm. Bull. 2018;50:1441–1459. doi: 10.1159/000494644. [DOI] [PubMed] [Google Scholar]
- Liu S. Steve, Pickens S., Burma N.E., Ibarra-Lecue I., Yang H., Xue L., Cook C., Hakimian J.K., Severino A.L., Lueptow L., Komarek K., Taylor A.M.W., Olmstead M.C., Carroll F.I., Bass C.E., Andrews A.M., Walwyn W., Trang T., Evans C.J., Leslie F.M., Cahill C.M. Kappa opioid receptors drive a tonic aversive component of chronic pain. J. Neurosci. 2019;39:4162–4178. doi: 10.1523/JNEUROSCI.0274-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mague S.D., Pliakas A.M., Todtenkopf M.S., Tomasiewicz H.C., Zhang Y., Stevens W.C., Jones R.M., Portoghese P.S., Carlezon W.A. Antidepressant-like effects of kappa-opioid receptor antagonists in the forced swim test in rats. J. Pharmacol. Exp. Therapeut. 2003;305:323–330. doi: 10.1124/jpet.102.046433. [DOI] [PubMed] [Google Scholar]
- Marsh J.C., Park K., Lin Y.-A., Bersamira C. Gender differences in trends for heroin use and nonmedical prescription opioid use, 2007-2014. J. Subst. Abuse Treat. 2018;87:79–85. doi: 10.1016/j.jsat.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massaly N., Copits B.A., Wilson-Poe A.R., Hipólito L., Markovic T., Yoon H.J., Liu S., Walicki M.C., Bhatti D.L., Sirohi S., Klaas A., Walker B.M., Neve R., Cahill C.M., Shoghi K.I., Gereau R.W., McCall J.G., Al-Hasani R., Bruchas M.R., Morón J.A. Pain-induced negative affect is mediated via recruitment of the nucleus accumbens kappa opioid system. Neuron. 2019;102:564–573. doi: 10.1016/j.neuron.2019.02.029. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meade J.A., Alkhlaif Y., Contreras K.M., Obeng S., Toma W., Sim-Selley L.J., Selley D.E., Damaj M.I. Kappa opioid receptors mediate an initial aversive component of paclitaxel-induced neuropathy. Psychopharmacology. 2020;237:2777–2793. doi: 10.1007/s00213-020-05572-2. [DOI] [PubMed] [Google Scholar]
- Melief E.J., Miyatake M., Carroll F.I., Béguin C., Carlezon W.A., Cohen B.M., Grimwood S., Mitch C.H., Rorick-Kehn L., Chavkin C. Duration of action of a broad range of selective κ-opioid receptor antagonists is positively correlated with c-Jun N-terminal kinase-1 activation. Mol. Pharmacol. 2011;80:920–929. doi: 10.1124/mol.111.074195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogil J.S., Wilson S.G., Chesler E.J., Rankin A.L., Nemmani K.V.S., Lariviere W.R., Groce M.K., Wallace M.R., Kaplan L., Staud R., Ness T.J., Glover T.L., Stankova M., Mayorov A., Hruby V.J., Grisel J.E., Fillingim R.B. The melanocortin-1 receptor gene mediates female-specific mechanisms of analgesia in mice and humans. Proc. Natl. Acad. Sci. Unit. States Am. 2003;100:4867–4872. doi: 10.1073/pnas.0730053100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussawi K., Ortiz M.M., Gantz S.C., Tunstall B.J., Marchette R.C.N., Bonci A., Koob G.F., Vendruscolo L.F. Fentanyl vapor self-administration model in mice to study opioid addiction. Sci Adv. 2020;6(32) doi: 10.1126/sciadv.abc0413. eabc0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro T.A., Berry L.M., Van’t Veer A., Béguin C., Carroll F.I., Zhao Z., Carlezon W.A., Cohen B.M. Long-acting κ opioid antagonists nor-BNI, GNTI and JDTic: pharmacokinetics in mice and lipophilicity. BMC Pharmacol. 2012;12:5. doi: 10.1186/1471-2210-12-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro T.A., Huang X.-P., Inglese C., Perrone M.G., Veer A.V., Carroll F.I., Béguin C., Jr W.A.C., Colabufo N.A., Cohen B.M., Roth B.L. Selective κ opioid antagonists nor-BNI, GNTI and JDTic have low affinities for non-opioid receptors and transporters. PloS One. 2013;8 doi: 10.1371/journal.pone.0070701. e70701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita M., Kaneko C., Miyoshi K., Nagumo Y., Kuzumaki N., Nakajima M., Nanjo K., Matsuzawa K., Yamazaki M., Suzuki T. Chronic pain induces anxiety with concomitant changes in opioidergic function in the amygdala. Neuropsychopharmacology. 2006;31:739–750. doi: 10.1038/sj.npp.1300858. [DOI] [PubMed] [Google Scholar]
- Nasser S.A., Afify E.A. Sex differences in pain and opioid mediated antinociception: modulatory role of gonadal hormones. Life Sci. 2019;237 doi: 10.1016/j.lfs.2019.116926. 116926. [DOI] [PubMed] [Google Scholar]
- Nation K.M., De Felice M., Hernandez P.I., Dodick D.W., Neugebauer V., Navratilova E., Porreca F. Lateralized kappa opioid receptor signaling from the amygdala central nucleus promotes stress-induced functional pain. Pain. 2018;159:919–928. doi: 10.1097/j.pain.0000000000001167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navratilova E., Ji G., Phelps C., Qu C., Hein M., Yakhnitsa V., Neugebauer V., Porreca F. Kappa opioid signaling in the central nucleus of the amygdala promotes disinhibition and aversiveness of chronic neuropathic pain. Pain. 2019;160:824–832. doi: 10.1097/j.pain.0000000000001458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus S.S., Mello N.K., Linsenmayer D.C., Jones R.M., Portoghese P.S. Kappa opioid antagonist effects of the novel kappa antagonist 5’-guanidinonaltrindole (GNTI) in an assay of schedule-controlled behavior in rhesus monkeys. Psychopharmacology (Berlin) 2002;163:412–419. doi: 10.1007/s00213-002-1038-x. [DOI] [PubMed] [Google Scholar]
- Niesters M., Dahan A., Kest B., Zacny J., Stijnen T., Aarts L., Sarton E. Do sex differences exist in opioid analgesia? A systematic review and meta-analysis of human experimental and clinical studies. Pain. 2010;151:61–68. doi: 10.1016/j.pain.2010.06.012. [DOI] [PubMed] [Google Scholar]
- Obara I., Mika J., Schäfer M.K.-H., Przewlocka B. Antagonists of the κ-opioid receptor enhance allodynia in rats and mice after sciatic nerve ligation. Br. J. Pharmacol. 2003;140:538–546. doi: 10.1038/sj.bjp.0705427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossipov M.H., Lopez Y., Nichols M.L., Bian D., Porreca F. The loss of antinociceptive efficacy of spinal morphine in rats with nerve ligation injury is prevented by reducing spinal afferent drive. Neurosci. Lett. 1995;199:87–90. doi: 10.1016/0304-3940(95)12022-v. [DOI] [PubMed] [Google Scholar]
- Ossipov M.H., Lai J., King T., Vanderah T.W., Porreca F. Underlying mechanisms of pronociceptive consequences of prolonged morphine exposure. Biopolymers. 2005;80:319–324. doi: 10.1002/bip.20254. [DOI] [PubMed] [Google Scholar]
- Park P.E., Schlosburg J.E., Vendruscolo L.F., Schulteis G., Edwards S., Koob G.F. Chronic CRF1 receptor blockade reduces heroin intake escalation and dependence-induced hyperalgesia. Addiction Biol. 2013;20:275–284. doi: 10.1111/adb.12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patkar K.A., Wu J., Ganno M.L., Singh H.D., Ross N.C., Rasakham K., Toll L., McLaughlin J.P. Physical presence of nor-binaltorphimine in mouse brain over 21 Days after a single administration corresponds to its long-lasting antagonistic effect on κ -opioid receptors. J. Pharmacol. Exp. Therapeut. 2013;346:545–554. doi: 10.1124/jpet.113.206086. [DOI] [PubMed] [Google Scholar]
- Pisanu C., Franconi F., Gessa G.L., Mameli S., Pisanu G.M., Campesi I., Leggio L., Agabio R. Sex differences in the response to opioids for pain relief: a systematic review and meta-analysis. Pharmacol. Res. 2019;148 doi: 10.1016/j.phrs.2019.104447. 104447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portoghese P.S., Lipkowski A.W., Takemori A.E. Binaltorphimine and nor-binaltorphimine, potent and selective k-opioid receptor antagonists. Life Sci. 1987;40:1287–1292. doi: 10.1016/0024-3205(87)90585-6. [DOI] [PubMed] [Google Scholar]
- Rasakham K., Liu-Chen L.-Y. Sex differences in kappa opioid pharmacology. Life Sci. 2011;88:2–16. doi: 10.1016/j.lfs.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattan A.K., Tejwani G.A. Effect of chronic treatment with morphine, midazolam and both together on dynorphin(1-13) levels in the rat. Brain Res. 1997;754:239–244. doi: 10.1016/s0006-8993(97)00084-x. [DOI] [PubMed] [Google Scholar]
- Ren Z.-Y., Shi J., Epstein D.H., Wang J., Lu L. Abnormal pain response in pain-sensitive opiate addicts after prolonged abstinence predicts increased drug craving. Psychopharmacology. 2009;204:423–429. doi: 10.1007/s00213-009-1472-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeckel L.-A., Utard V., Reiss D., Mouheiche J., Maurin H., Robé A., Audouard E., Wood J.N., Goumon Y., Simonin F., Gaveriaux-Ruff C. Morphine-induced hyperalgesia involves mu opioid receptors and the metabolite morphine-3-glucuronide. Sci. Rep. 2017;7:1–15. doi: 10.1038/s41598-017-11120-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogala B., Li Y., Li S., Chen X., Kirouac G.J. Effects of a post-shock injection of the kappa opioid receptor antagonist norbinaltorphimine (norBNI) on fear and anxiety in rats. PloS One. 2012;7 doi: 10.1371/journal.pone.0049669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosburg J.E., Whitfield T.W., Park P.E., Crawford E.F., George O., Vendruscolo L.F., Koob G.F. Long-Term antagonism of κ opioid receptors prevents escalation of and increased motivation for heroin intake. J. Neurosci. 2013;33:19384–19392. doi: 10.1523/JNEUROSCI.1979-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl L., Seth P., Kariisa M., Wilson N., Baldwin G. Drug and opioid-involved overdose deaths - United States, 2013-2017. MMWR Morb. Mortal. Wkly. Rep. 2018;67:1419–1427. doi: 10.15585/mmwr.mm675152e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S.K., Jones R.M., Metzger T.G., Ferguson D.M., Portoghese P.S. Transformation of a kappa-opioid receptor antagonist to a kappa-agonist by transfer of a guanidinium group from the 5’- to 6’-position of naltrindole. J. Med. Chem. 2001;44:2073–2079. doi: 10.1021/jm010095v. [DOI] [PubMed] [Google Scholar]
- Shippenberg T.S., Zapata A., Chefer V.I. Dynorphin and the pathophysiology of drug addiction. Pharmacol. Therapeut. 2007;116:306–321. doi: 10.1016/j.pharmthera.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R., Almeida O.F., Bartl C., Shippenberg T.S. Endogenous kappa-opioid systems in opiate withdrawal: role in aversion and accompanying changes in mesolimbic dopamine release. Psychopharmacology (Berlin) 1994;115:121–127. doi: 10.1007/BF02244761. [DOI] [PubMed] [Google Scholar]
- Taylor A.M.W., Chadwick C.I., Mehrabani S., Hrncir H., Arnold A.P., Evans C.J. Sex differences in kappa opioid receptor antinociception is influenced by the number of X chromosomes in mouse. J. Neurosci. Res. 2020 doi: 10.1002/jnr.24704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejeda H.A., Wu J., Kornspun A.R., Pignatelli M., Kashtelyan V., Krashes M.J., Lowell B.B., Carlezon W.A., Bonci A. Pathway- and cell-specific kappa-opioid receptor modulation of excitation-inhibition balance differentially gates D1 and D2 accumbens neuron activity. Neuron. 2017;93:147–163. doi: 10.1016/j.neuron.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todtenkopf M.S., Marcus J.F., Portoghese P.S., Carlezon W.A. Effects of kappa-opioid receptor ligands on intracranial self-stimulation in rats. Psychopharmacology (Berlin) 2004;172:463–470. doi: 10.1007/s00213-003-1680-y. [DOI] [PubMed] [Google Scholar]
- Towers E.B., Tunstall B.J., McCracken M.L., Vendruscolo L.F., Koob G.F. Male and female mice develop escalation of heroin intake and dependence following extended access. Neuropharmacology. 2019;151:189–194. doi: 10.1016/j.neuropharm.2019.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderah T.W., Laughlin T., Lashbrook J.M., Nichols M.L., Wilcox G.L., Ossipov M.H., Malan P.T., Porreca F. vol. 68. 1996. Single Intrathecal Injections of Dynorphin A or Des-Tyr-Dynorphins Produce Long-Lasting Allodynia in Rats: Blockade by MK-801 but Not Naloxone; pp. 275–281. Pain. [DOI] [PubMed] [Google Scholar]
- Vanderah T.W., Gardell L.R., Burgess S.E., Ibrahim M., Dogrul A., Zhong C.-M., Zhang E.-T., Malan T.P., Ossipov M.H., Lai J., Porreca F. Dynorphin promotes abnormal pain and spinal opioid antinociceptive tolerance. J. Neurosci. 2000;20:7074–7079. doi: 10.1523/JNEUROSCI.20-18-07074.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendruscolo J.C.M., Tunstall B.J., Carmack S.A., Schmeichel B.E., Lowery-Gionta E.G., Cole M., George O., Vandewater S.A., Taffe M.A., Koob G.F., Vendruscolo L.F. Compulsive-like sufentanil vapor self-administration in rats. Neuropsychopharmacology. 2018;43:801–809. doi: 10.1038/npp.2017.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendruscolo L.F., Pamplona F.A., Takahashi R.N. Strain and sex differences in the expression of nociceptive behavior and stress-induced analgesia in rats. Brain Res. 2004;1030:277–283. doi: 10.1016/j.brainres.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Wang Z., Gardell L.R., Ossipov M.H., Vanderah T.W., Brennan M.B., Hochgeschwender U., Hruby V.J., Malan T.P., Lai J., Porreca F. Pronociceptive actions of dynorphin maintain chronic neuropathic pain. J. Neurosci. 2001;21:1779–1786. doi: 10.1523/JNEUROSCI.21-05-01779.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield T.W., Schlosburg J.E., Wee S., Gould A., George O., Grant Y., Zamora-Martinez E.R., Edwards S., Crawford E., Vendruscolo L.F., Koob G.F. κ Opioid receptors in the nucleus accumbens shell mediate escalation of methamphetamine intake. J. Neurosci. 2015;35:4296–4305. doi: 10.1523/JNEUROSCI.1978-13.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson N. Drug and opioid-involved overdose deaths — United States, 2017–2018. MMWR Morb. Mortal. Wkly. Rep. 2020;69 doi: 10.15585/mmwr.mm6911a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q., Carlsson A., Hallberg M., Nyberg F. Substance P N-terminal fragment SP(1–7) attenuates chronic morphine tolerance and affects dynorphin B and nociceptin in rats. Peptides. 2011;32:1661–1665. doi: 10.1016/j.peptides.2011.06.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.