Abstract
Background
Heavy menstrual bleeding (HMB) is common in otherwise healthy women of reproductive age, and can affect physical health and quality of life. Surgery is usually a second‐line treatment of HMB. Endometrial resection/ablation (EA/ER) to remove or ablate the endometrium is less invasive than hysterectomy. Hysterectomy is the definitive treatment and can be via open (laparotomy) approach, or via minimally invasive approaches (vaginally or laparoscopically). Each approach has its own advantages and risk profile.
Objectives
To compare the effectiveness, acceptability and safety of endometrial resection or ablation versus different routes of hysterectomy (open, minimally invasive hysterectomy, or unspecified route) for the treatment of HMB.
Search methods
We searched the Cochrane Gynaecology and Fertility specialised register, CENTRAL, MEDLINE, Embase and PsycINFO (July 2020), and reference lists, grey literature and trial registers.
Selection criteria
Randomised controlled trials (RCTs) that compared techniques of endometrial resection/ablation with hysterectomy (by any technique) for the treatment of HMB in premenopausal women.
Data collection and analysis
We used standard methodological procedures expected by Cochrane.
Main results
We included 10 RCTs (1966 participants) comparing EA/ER to hysterectomy (open (abdominal), minimally invasive (laparoscopic or vaginal), or unspecified (or at surgeon's discretion) route of hysterectomy). The results were rated as moderate‐, low‐ and very low‐certainty evidence.
Endometrial resection/ablation versus open hysterectomy
We found two trials. Women having EA/ER are probably less likely to perceive an improvement in HMB compared to women having open hysterectomy (risk ratio (RR) 0.90, 95% confidence interval (CI) 0.84 to 0.95; 2 studies, 247 women; moderate‐certainty evidence) and probably have a 13% risk of requiring further surgery for treatment failure (compared to 0 on the open hysterectomy group; 2 studies, 247 women; moderate‐certainty evidence). Both treatments probably lead to similar quality of life at two years (mean difference (MD) –5.30, 95% CI –11.90 to 1.30; 1 study, 155 women; moderate‐certainty evidence) and satisfaction rate at one year (RR 0.91, 95% CI 0.82 to 1.00; 1 study, 194 women; moderate‐certainty evidence). There may be no difference in serious adverse events (RR 1.29, 95% CI 0.32 to 5.20; 2 studies, 247 women; low‐certainty evidence). EA/ER probably reduces time to return to normal activity compared to open hysterectomy (MD –21.00 days, 95% CI –24.78 to –17.22; 1 study, 197 women; moderate‐certainty evidence).
Endometrial resection/ablation versus minimally invasive hysterectomy
We found five trials. The proportion of women with perception of improvement in HMB at two years may be similar between groups (RR 0.97, 95% CI 0.90 to 1.04; 1 study, 79 women; low‐certainty evidence). Blood loss may be higher in the EA/ER group when assessed using the Pictorial Blood Assessment Chart (MD 44.00, 95% CI 36.09 to 51.91; 1 study, 68 women; low‐certainty evidence). Quality of life is probably lower in the EA/ER group compared to the minimally invasive hysterectomy group at two years according to the 36‐item Short Form (SF‐36) (MD –10.71, 95% CI –15.11 to –6.30; 2 studies, 145 women; moderate‐certainty evidence) and Menorrhagia Multi‐Attribute Scale (RR 0.82, 95% CI 0.70 to 0.95; 1 study, 616 women; moderate‐certainty evidence). EA/ER probably increases the risk of further surgery for HMB compared to minimally invasive hysterectomy (RR 7.70, 95% CI 2.54 to 23.32; 4 studies, 922 women; moderate‐certainty evidence) and treatments probably have similar rates of any serious adverse events (RR 0.75, 95% CI 0.35 to 1.59; 4 studies, 809 women; moderate‐certainty evidence). Women with EA/ER are probably less likely to be satisfied with treatment at one year (RR 0.90, 95% CI 0.85 to 0.94; 1 study, 558 women; moderate‐certainty evidence). We were unable to pool data for time to return to work or normal life because of extreme heterogeneity (99%); however, the three studies reporting this all had the same direction of effect favouring EA/ER.
Endometrial resection/ablation versus unspecified route of hysterectomy
We found three trials. EA/ER may lead to a lower perception of improvement in HMB compared to unspecified route of hysterectomy (RR 0.89, 95% CI 0.83 to 0.95; 2 studies, 403 women; low‐certainty evidence). Although EA/ER may lead to similar quality of life using the SF‐36 General Health Perception at two years' follow‐up (MD –1.90, 95% CI –8.67 to 4.87; 1 study, 209 women; low‐certainty evidence), the proportion of women with improvement in general health at one year may be lower (RR 0.85, 95% CI 0.77 to 0.95; 1 study, 185 women; low‐certainty evidence). EA/ER probably has a risk of 5.4% of requiring further surgery for treatment failure (compared to 0 with total hysterectomy; 2 studies, 374 women; moderate‐certainty evidence) and reduces the proportion of women with any serious adverse event (RR 0.21, 95% CI 0.06 to 0.80; 2 studies, 374 women; moderate‐certainty evidence). Both treatments probably lead to a similar satisfaction rate at one year' follow‐up (RR 0.96, 95% CI 0.88 to 1.04; 3 studies, 545 women; moderate‐certainty evidence). EA/ER may lead to shorter time to return to normal activity (MD –18.90 days, 95% CI –24.63 to –13.17; 1 study, 172 women; low‐certainty evidence).
Authors' conclusions
Endometrial resection/ablation (EA/ER) offers an alternative to hysterectomy as a surgical treatment for HMB.
Effectiveness varies with EA/ER compared to different hysterectomy approaches. The perception of improvement in HMB with EA/ER is probably lower compared to open and unspecified route of hysterectomy, but may be similar compared to minimally invasive. Quality of life with EA/ER is probably similar to open and unspecified route of hysterectomy, but lower compared to minimally invasive hysterectomy. Further surgery for treatment failure is probably more likely with EA/ER compared to all routes of hysterectomy.
Satisfaction rates also vary. EA/ER probably has a similar rate of satisfaction compared to open and unspecified route of hysterectomy, but a lower rate of satisfaction compared to minimally invasive hysterectomy. The proportion having any serious adverse event appears similar in all groups, but specific adverse events did reported difference between EA/ER and different routes. We were unable to draw conclusions about the time to return to normal activity, but the direction of effect suggests it is likely to be shorter with EA/ER.
Plain language summary
Is endometrial resection or ablation more effective, safer or more acceptable than different routes of hysterectomy?
Review question
This review compares the effectiveness, safety and acceptability of endometrial resection or ablation (EA/ER) compared to different routes of hysterectomy for the treatment of heavy menstrual bleeding (HMB).
Background
Surgical treatments for HMB include: endometrial resection or ablation (removal or destruction of the endometrium (inside lining) of the uterus (womb)) and hysterectomy (surgical removal of the uterus). Hysterectomy can be performed by different routes: by a surgical cut to the abdomen (open), by a minimally invasive procedure that can be by the vagina, or by laparoscopy (a 'keyhole' operation that involves very small surgical cuts to the abdomen). Hysterectomy is effective in permanently stopping HMB, but it halts fertility and is associated with the risks of major surgery, including infection and blood loss. Endometrial resection/ablation is performed by the vagina and cervix (entrance to the uterus).
Search date
In July 2020, we searched for studies that compared endometrial resection/ablation versus hysterectomy for the treatment of HMB. We included 10 studies involving 1966 women.
Study characteristics
We included only randomised controlled trials (RCTs; clinical studies where people are randomly put into one of two or more treatment groups) comparing endometrial ablation or resection and hysterectomy as treatment for HMB. The studies excluded women who had gone through the menopause or had cancer (or precancer) of the uterus.
Key results and conclusions
Women having EA/ER are probably less likely to perceive an improvement in HMB and more likely to require surgery for treatment failure compared to women having open hysterectomy. They probably have a similar quality of life and satisfaction rates, and may also have similar proportions of serious side effects. Some uncommon but important complications, such as infection and bleeding, are more common during open hysterectomy than with EA/ER.
Women having EA/ER may have a similar rate of perceiving HMB improvement, but may be less likely to have an objective decrease in blood loss, compared to women having minimally invasive hysterectomy. The EA/ER group probably have a lower quality of life and satisfaction rate. The rate of serious side effects is probably similar but women having EA/ER are probably at increased risk of surgery for treatment failure when compared to minimally invasive hysterectomy. The time taken for women to return to normal activity was shorter with EA/ER than with minimally invasive hysterectomy.
Women having EA/ER may be less likely to perceive an improvement in HMB and an improvement in general health compared to women having an unspecified route of hysterectomy (or at surgeon's discretion). EA/ER probably increases the chances of having surgery for treatment failure, but decreases the chances of any serious side effects and has shorter time to return to normal activity if compared to the unspecified route of hysterectomy.
Identifying harms
Both surgical treatments are generally safe, with low complication rates. There is no clear evidence of a difference on the total number of serious side effects between both surgical techniques. However, open hysterectomy and unspecified route of hysterectomy were both associated with a higher chance of individual complications such as infection and bleeding, whereas there was no difference in these outcomes when EA/ER was compared against minimally invasive hysterectomy.
Quality of the evidence
Evidence reported in this review ranged from very low to moderate quality, which suggests that further research may change the result. However, although most of the evidence is moderate, we do not think this will change with further studies. It is moderate because of high risk of differences between groups in the care provided, and in this case, blinding is not feasible due to the nature of the procedures.
Summary of findings
Background
Description of the condition
Heavy menstrual bleeding (HMB) is excessive blood loss that interferes with women's quality of life, either physical, psychological, material, or social (Munro 2011; NICE 2018). Many other terms are used as HMB synonyms, including menorrhagia, abnormal menstrual bleeding, abnormal uterine bleeding and disordered uterine bleeding. These terms have not always been universally defined, and there is considerable overlap and confusion between them in the existing literature (Woolcock 2008). In response to this confusion, the International Federation of Gynaecology and Obstetrics (FIGO) formally defined HMB as "the woman's perspective of increased menstrual volume, regardless of regularity, frequency, or duration" (Munro 2011).
HMB is a common presenting complaint for primary care services and a frequent reason for secondary referral, with 5% of women between 30 and 49 years of age seeking medical attention for the problem (Cooper 2011; Vessey 1992).
Personal perception is often what determines the need for treatment and assessment of outcomes afterwards.
Intervention for HMB should be aimed at correction of identified underlying causes, control of bleeding, amelioration of anaemia and improvement in quality‐of‐life measures, with the woman's contraceptive requirements or desire for future fertility considered, along with individual preferences for treatment.
Description of the intervention
Many well‐established treatment options for HMB are available, including hormonal and non‐hormonal medications, but, among women who do not desire future fertility or for whom medical treatment may be problematic, inconvenient or prolonged, surgical management may be preferred.
Until the mid‐1980s, hysterectomy was the only option for such women and 60% of women presenting with HMB during this time underwent hysterectomy as a first‐line treatment (NICE 2007). Hysterectomy can be performed via the open (laparotomy) approach, or via minimally invasive approaches such as vaginally or laparoscopically (including robotic surgery).
There are considerable differences between types of approach with regard to intraoperative and postoperative complications, duration of surgery, duration of hospital stay, duration of recovery and cost. In earlier versions of the review, we pooled the results for hysterectomy by all approaches, and, in the 2020 version of the review, we separated the comparisons into open hysterectomy versus endometrial resection/ablation and minimally invasive hysterectomy (vaginal and laparoscopic) versus endometrial resection/ablation.
Endometrial ablation or resection (EA/ER) are minimally invasive hysteroscopic surgical interventions for HMB involving the destruction (ablation) or removal (resection) of the endometrium. Since it was introduced in the 1990s, its popularity as an alternative to hysterectomy has grown (Fernandez 2011). An examination of the UK National Health Service (NHS) statistics in 2005 revealed that endometrial destruction was being performed more frequently than hysterectomy for benign HMB in the UK (Reid 2007). The trend seems to continue over time. A UK National HMB audit in 2019 reported that 40% of the women referred for HMB received surgery during the first year after their first outpatient clinic visit, with 57.8% receiving endometrial ablation and 37. 2% receiving hysterectomy (Geary 2019). In one long‐term study with up to 25 years' follow‐up in the UK, only 25% of the woman with an endometrial resection or ablation underwent a subsequent hysterectomy; and 75% of the surgeries were in the first five years of follow‐up (Kalampokas 2017), suggesting EA/ER may have a role in limiting the number of hysterectomies performed. However, this may also reflect progression through menopause for many of these women.
Endometrial resection/ablation techniques are classically divided, according to the requirement of hysteroscopy to perform the ablation, in first‐ and second‐generation techniques. First‐generation techniques require direct hysteroscopic vision throughout the procedure and include desiccation of the endometrium by way of rollerball endometrial ablation (REA) and transcervical resection of the endometrium (TCRE) with loop electrode. This can be useful to identify and remove other endometrial pathology such as polyps and fibroids. One potential complication of endometrial resection is systemic fluid overload due to absorption of hysteroscopic fluid; therefore, careful intraoperative input/output fluid balance requires attention and documentation. The efficacy and safety of first‐generation techniques are often operator dependent.
Newer technologies were subsequently introduced; second‐generation techniques are non‐hysteroscopic, considered easier to perform, equally effective and safe (Madhu 2009), reporting lower complication rates of around 1% for bipolar ablation (Athanatos 2015; Laberge 2015). All of these techniques, with the exception of hydrothermal ablation and endometrial laser intrauterine thermal therapy, involve performing surgery with a hysteroscope (i.e. without direct visualisation). The second‐generation techniques have allowed widespread use of EA, as they require less‐advanced hysteroscopic skill and do not necessitate intraoperative fluid balance. However, these procedures depend heavily on the surgical equipment itself for efficacy and safety. Newer techniques, sometimes called third generation, differ from the second because they have replaced latex with silicone in the balloon and have active fluid circulation, which enables the total endometrial surface to receive equal heat distribution (Kumar 2016).
There are some devices for EA that are currently out of the market for different reasons. Laser EA with the Neodymium was expensive and time consuming, so, although effective, have become obsolete (Munro 2018). The microwave endometrial ablation (MEA) and the Vesta balloon (radiofrequency ablation via electrodes on the surface of a balloon) were withdrawn from the market for commercial reasons (Arnold 2015). None of these devices were part of the comparisons included in this review.
How the intervention might work
Hysterectomy is regarded as 'definitive' treatment for HMB with guaranteed amenorrhoea and cessation of fertility and little need for future treatment. However, hysterectomy by any route is associated with all the complications of major surgery, including intraoperative bleeding, infection and venous thromboembolism. Other reported complications include urinary incontinence and dyspareunia. Urinary tract injuries are an unusual hysterectomy complication, having an overall incidence in laparoscopic hysterectomy of 0.73% (bladder 0.05% to 0.66%, ureter 0.02% to 0.4%) (Adelman 2014).
EA/ER are procedures that destroy (EA) or remove (ER) the endometrium, reducing menstrual bleeding. EA/ER is a less‐invasive surgical option for women with HMB, although a successful outcome after EA/ER is not guaranteed and further surgery is occasionally required (Bourdrez 2004; Nagele 1998). Endometrial resection/ablation is often chosen because of perceived shorter hospitalisation, quick return to normal functioning and avoidance of major surgery (Nagele 1998). Vaginal discharge and increased period pain are common complications following endometrial resection/ablation. Uncommon complications include intraoperative uterine perforation and associated pelvic organ injury from direct perforation or electrosurgical burns. Pregnancies occurring after EA/ER have been reported to be at higher risk of ectopic pregnancy, preterm birth and abnormality of placentation (Sharp 2012).
Why it is important to do this review
HMB is a common reason for women to seek treatment and many choose a surgical option as first‐ or second‐line management. Surgical methods for treatment of HMB show a trend in favour of endometrial resection/ablation and towards newer endometrial resection/ablation techniques. In the late 1980s and early 1990s, 60% of women with HMB who were referred to a gynaecologist in the UK were treated with hysterectomy (Coulter 1991), but an examination of NHS statistics in 2005 revealed that endometrial destruction was being performed more frequently than hysterectomy for benign HMB in the UK (Reid 2007).
Despite this trend, many women still express a preference for first‐line hysterectomy (Kennedy 2002), and rates of different methods of hysterectomy are evolving.
As surgical techniques for both hysterectomy and endometrial resection/ablation continue to evolve, corresponding changes in success rates, outcomes and complications will be noted, necessitating regular comparison and review.
Objectives
To compare the effectiveness, acceptability and safety of endometrial resection or ablation versus different routes of hysterectomy (open, minimally invasive hysterectomy, or unspecified route) for the treatment of HMB.
Methods
Criteria for considering studies for this review
Types of studies
Published and non‐published randomised controlled trials (RCTs) were eligible for inclusion. We excluded quasi‐randomised trials.
Types of participants
Source of recruitment
Primary care, family planning and specialist clinics.
Inclusion criterion
Women of reproductive years with HMB (including both heavy regular periods (menorrhagia) and heavy irregular periods (metrorrhagia)), measured objectively or subjectively.
Exclusion criteria
Postmenopausal bleeding (more than one year from the last period).
HMB caused by uterine malignancy or endometrial hyperplasia.
Iatrogenic causes of HMB (e.g. intrauterine coil devices).
Types of interventions
Endometrial resection/ablation (including first‐generation techniques, such as TCRE with loop electrode or rollerball; second‐generation techniques, such as EA by thermal balloon, microwave, thermal free‐fluid, radiofrequency and cryotherapy); and third‐generation EA techniques that are similar to the second‐generation techniques but have replaced latex with silicone in the balloon and have active fluid circulation.
Hysterectomy (by abdominal, vaginal and laparoscopic/laparoscopically assisted/robotic routes).
Types of outcome measures
Primary outcomes
Effectiveness (improvement in bleeding)
Woman's perception (proportion with improvement in bleeding symptoms).
Pictorial Blood Loss Assessment Chart (PBAC) score: a visual measure of amount of blood loss (clinically significant HMB correlates with a score greater than 100).*
Quality‐of‐life scores (continuous data).
Quality of life (proportion with improvement).
Requirement for further surgery for treatment failure.
*PBAC score: a score over 100 suggests significantly HMB. A reduction from over 100 to under 100 would be clinically significant at an individual level.
Acceptability
Proportion satisfied with treatment.
Safety (adverse outcomes)
Any serious adverse events (reported as any serious adverse event or any major complications).
Adverse events: short term (intraoperative and immediately postoperative).
Adverse events: long term (after hospital discharge).
Secondary outcomes
Duration of surgery.
Duration of hospital stay.
Time to return to normal activity.
Time to return to work.
Total health service cost per woman.
Total individual cost per woman.
Search methods for identification of studies
We searched all publications that would potentially describe RCTs comparing surgical techniques to resect or ablate the endometrium versus hysterectomy for the treatment of HMB. We consulted with the Cochrane Gynaecology and Fertility Group Information Specialist regarding our search strategies for identifying potential studies for inclusion.
Electronic searches
The original search was performed in 1999. Updated searches were performed in 2008, 2013, 2018 and 2020.
We searched the following databases:
the Gynaecology and Fertility Group's specialised register, ProCite platform (searched 29 July 2020; Appendix 1);
CENTRAL via the Cochrane Register of Studies Online (CRSO), Web platform (searched 29 July 2020; Appendix 2);
MEDLINE, Ovid platform (searched from 1946 to 29 July 2020; Appendix 3);
Embase, Ovid platform (searched from 1980 to 29 July 2020; Appendix 4);
PsycINFO, Ovid platform (searched from 1806 to 29 July 2020; Appendix 5).
Searching other resources
The principal review author searched the following trial registers for ongoing trials: ClinicalTrials.gov (a service of the US National Institutes of Health); and the World Health Organization International Trials Registry Platform search portal (www.who.int/trialsearch/Default.aspx) in July 2020 (Appendix 6).
We searched reference lists of all studies that appeared to meet the inclusion criteria of the review, other relevant studies and evidence‐based guidelines on the management of abnormal bleeding. We contacted experts in the field regarding additional studies.
Data collection and analysis
We performed statistical analysis in accordance with guidelines described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). When possible, we pooled outcomes statistically.
Selection of studies
Two review authors (AL and IC or AL and SS in 2008, RJF and AL in 2013, RJF and MBR in 2018 and 2020) independently selected studies. The final list of included studies reflected consensus between the two review authors. Details of the screening and selection process are shown in Figure 1. We collated multiple reports of the same study, so that each study rather than each report was the unit of interest in the review.
1.
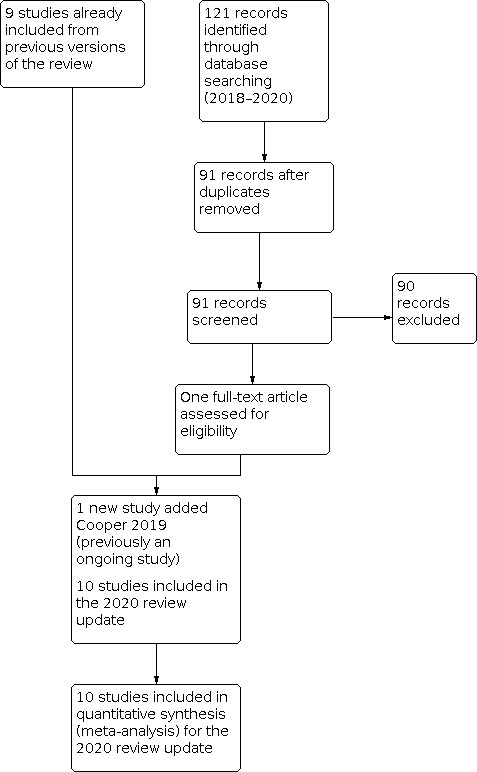
Study screening and selection process (2018 to 2020).
Data extraction and management
Two review authors (AL and IC in 2008, RJF and AL in 2013, MBR and RJF in 2019 and 2020) independently assessed the characteristics of the included studies using forms designed according to Cochrane guidelines and described in Higgins 2011.
When necessary, we sought additional information on trial methodology or original trial data from the principal or corresponding author of any trials that appeared to meet the eligibility criteria (see Acknowledgements section for details of the study authors who provided clarification of data beyond that reported in the publications).
We extracted quality criteria and methodological details from each study as follows.
Trial characteristics
Study design.
Numbers of women randomly assigned, excluded and lost to follow‐up.
Whether an intention‐to‐treat analysis was done.
Whether a power calculation was done.
Duration, timing and location of the study.
Number of centres.
Source of funding.
Characteristics of study participants
Age and any other recorded characteristics of participants in the study.
Inclusion criteria.
Exclusion criteria.
Type of surgery.
Source of participants.
Proportion participating of those eligible.
Interventions used
Type of endometrial destruction technique used and route of hysterectomy performed.
Outcomes
Methods used to evaluate menstrual symptoms (e.g. PBAC score, woman‐defined 'improvement in symptoms').
Methods used to measure requirement for further surgery for HMB.
Methods used to evaluate participant satisfaction and change in quality of life after surgery.
Methods used to record adverse surgical events.
Methods used to measure resource and individual costs.
Methods used to measure duration of surgery, hospital stay and recovery time.
Assessment of risk of bias in included studies
We assessed included trials for risk of bias using the 'Risk of bias tool' developed by Cochrane (Higgins 2011).
Sequence generation (whether the allocation sequence was adequately generated to produce comparable groups).
Allocation concealment (whether the allocation was adequately concealed).
Blinding of participants, personnel and outcome assessors (whether knowledge of the allocated intervention was adequately controlled during the study).
Incomplete outcome data (whether incomplete outcome data were adequately addressed).
Selective outcome reporting (whether reports of the study were free of the suggestion of selective outcome reporting).
Other sources of bias (whether the study was apparently free of other problems that could put it at high risk of bias, e.g. baseline imbalance, bias related to study design, early termination of the study).
Details of the risk of bias for each included study are displayed in the Characteristics of included studies table and are summarised in Figure 2 and Figure 3.
2.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
3.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Measures of treatment effect
For dichotomous data (e.g. number of adverse outcomes), we used the numbers of events in the control and intervention groups of each study to calculate risk ratios (RRs). For continuous data (e.g. PBAC score), if all studies reported the same outcomes, we calculated mean differences (MDs) between treatment groups. If studies reported similar outcomes on different scales, we calculated the standardised mean difference (SMD). We reversed the direction of effect of individual studies, if required, to ensure consistency across trials. We treated ordinal data (e.g. quality‐of‐life scores) as continuous data. We presented 95% confidence intervals (CIs) for all outcomes. When data needed to calculate RRs or MDs were not available, we utilised the most detailed numerical data available that facilitated similar analyses of included studies (e.g. test statistics, P values). We compared the magnitude and direction of effect reported by studies versus how they were presented in the review, while taking account of legitimate differences.
For dichotomous data (e.g. proportion of participants satisfied with their treatment), we expressed results of each study as an RR. For some dichotomous outcomes (e.g. proportion of participants requiring further surgery), a higher proportion represented a negative consequence of that treatment, and for other outcomes (e.g. proportion with improvement in menstrual blood loss), a higher proportion was considered a benefit of treatment. This approach to the categorising of outcomes should be noted when summary graphs for the meta‐analysis are viewed for assessment of benefits as opposed to harms of treatment. Thus, for some dichotomous outcomes, treatment benefit has been displayed as RRs and CIs to the left of the centre line, but for others, a treatment benefit has been shown to the right of the centre line. Each outcome was labelled for clarification.
For other outcomes for which high values were considered a negative consequence of treatment (e.g. duration of surgery, length of hospital stay and time to return to work), evaluation of the summary graphs reveals that means and CIs to the left were considered a benefit of endometrial destruction.
With one exception, quality‐of‐life scores were entered as continuous data – mean plus standard deviation (SD) values after treatment. However, one study recorded the percentage mean change of EuroQol scores from baseline to four months after treatment. Some scales measured general quality‐of‐life summary score; others reported results separately in different categories, representing general quality of life but without a summary score; still others used more specific measurements of aspects of quality of life, such as anxiety, social adjustment and depression.
For some outcomes, women were assessed at different periods of follow‐up after surgery.
Menstrual symptoms were recorded at one, two, three and four years after surgery, PBAC scores at one and two years after surgery, and quality of life at four months and one, two and four years after treatment.
Adverse events were recorded before and after discharge from hospital.
Requirement for further surgery was measured separately within the first year and at two, three and four years after treatment.
Unit of analysis issues
The unit of analysis was the woman with HMB who was randomly assigned to endometrial resection/ablation or hysterectomy.
Dealing with missing data
We analysed data on an intention‐to‐treat basis as far as possible; otherwise, we only analysed available data. When we found that data were missing, we attempted to obtain this information from the original trial authors.
Assessment of heterogeneity
We examined heterogeneity (variations) between the results of different studies by visual inspection of scatter of data points on the graphs and overlap in their CIs. More formally, we examined the results of the I² statistic (a quantity that describes approximately the proportion of variation in point estimates that is due to heterogeneity rather than sampling error; Higgins 2008). When we found statistical heterogeneity to be very substantial (greater than 90%), we considered calculation of a summary effect measure to be inappropriate and we did not pool study data. Results of these studies can be viewed in forest plots that portray the range of values for comparison in each study.
Assessment of reporting biases
We aimed to avoid reporting bias by using a robust search strategy with no restrictions on language or publication forum. We identified multiple publications of the same study for several of the included studies and we referenced them as such. If we had found 10 or more studies to include in the analysis, we planned to use funnel plots to explore the possibility of small‐study effects.
Data synthesis
Combination of data was not always possible, as some outcomes were measured differently (e.g. some trials used PBAC score as a measure of improvement in bleeding, while others used proportion reporting improvement in bleeding symptoms, proportion with excessive bleeding, etc.). We displayed such data in the forest plots; however, often only one trial contributed data to each plot.
For continuous data (e.g. PBAC score), if all studies reported the same outcomes, we calculated MDs between treatment groups. We displayed continuous outcomes differently according to whether benefit or harm was measured. For most quality‐of‐life scores, a high score represented a benefit of treatment, but for the Hospital Anxiety and Depression Scale (HADS), a high score represented a greater degree of anxiety or depression. To present quality‐of‐life scales that differed in this way on the same graph, we displayed HADS scores as minus values, so that we could include all quality‐of‐life continuous outcomes in a single forest plot.
Subgroup analysis and investigation of heterogeneity
A priori, we planned to explore the possible contribution of differences in trial design to any heterogeneity identified in the manner described under Assessment of heterogeneity.
We considered whether the clinical and methodological characteristics of the included studies were sufficiently similar for meta‐analysis to provide a meaningful summary. Where meta‐analyses were able to be performed, we checked for heterogeneity by visually inspecting the forest plots for evidence of poor overlap of the 95% CIs. More formally, we used the Chi² test (with a P < 0.10 being evidence of significance) and the I² value. The Cochrane Handbook for Systematic Reviews of Interventions suggested an approximate guide for interpretation of I² values (Higgins 2011):
0% to 40% might not be important;
30% to 60% may represent moderate heterogeneity;
50% to 90% may represent substantial heterogeneity;
75% to 100% was considered substantial heterogeneity.
Sensitivity analysis
To assess the robustness of pooled estimates, we conducted the following sensitivity analyses for primary outcomes.
Estimates based on all relevant trials regardless of evidence of allocation concealment versus estimates based on trials that provided clear evidence that allocation was concealed.
Estimates based on all relevant trials regardless of missing data and loss to follow‐up versus estimates based on trials in which incomplete outcome assessment was not likely to cause bias.
Summary of findings and assessment of the certainty of the evidence
We generated 'Summary of findings' tables using GRADEpro software (GRADEpro GDT). These tables evaluate the overall certainty of the body of evidence for the main review outcomes: effectiveness (proportion with perception of improvement in bleeding symptoms, PBAC score, quality of life and requirement for further surgery for treatment failure), acceptability (proportion satisfied with treatment), safety (any serious adverse event) and time to return to normal activity using GRADE criteria. The time to return to normal activity was added on the 2020 update.
We justified, documented and incorporated judgements about evidence certainty (high, moderate, low or very low) into reporting of results for each of these outcomes.
Results
Description of studies
We included 10 RCTs comparing techniques of removal or ablation of endometrium versus hysterectomy by any route for the treatment of HMB. Four trials had multiple publications, each based on the same study population but providing assessment of different outcomes and different follow‐up times, as well as cost‐utility analyses of previously published data (Dickersin 2007; Dwyer 1993; Pinion 1994; Zupi 2003). We did not consider trials comparing different types of endometrial destruction in this review; these are assessed in another Cochrane Review (Bofill Rodriguez 2019).
Details of included studies can be found in the Characteristics of included studies tables.
Results of the search
The previous version of this review included nine studies. The 2020 search identified 93 records after removal of duplicates. We excluded 92 records after reading the titles or abstracts. We obtained the full text of one article for assessment (Cooper 2019). This article had been included as an ongoing study in the previous version of the review. We included this study resulting in 10 included trials within this review .
Included studies
Ten RCTs of endometrial resection/ablation versus hysterectomy with 1966 randomly assigned participants met the criteria for inclusion in the review, although not all participants contributed to the assessment of every outcome. We contacted six authors for further details: two replied (Crosignani 1997; Dickersin 2007); four did not (Dwyer 1993; Gannon 1991; Pinion 1994; Zupi 2003).
Study design
All included studies used a parallel‐group design. There were seven studies carried out at a single centre: three in Italy (Crosignani 1997; Sesti 2011; Zupi 2003); three in the UK (Dwyer 1993; Gannon 1991; Pinion 1994); and one in India (Jain 2016). There were two multicentre studies performed in the UK, one in nine centres (O'Connor 1997), and one in 31 centres (Cooper 2019). There was one study completed at 25 centres in the USA and Canada (Dickersin 2007).
Participants
All participants were premenopausal, had symptomatic HMB (regular or irregular prolonged or excessive bleeding) and were eligible for (i.e. had shown no response to medical treatment) or were awaiting hysterectomy. Participants in seven included studies had received a diagnosis of menorrhagia (heavy regular bleeding) (Crosignani 1997; Dwyer 1993; Gannon 1991; Jain 2016; O'Connor 1997; Sesti 2011; Zupi 2003), and participants in two studies had been diagnosed with dysfunctional uterine bleeding (defined as both regular and irregular ovulatory heavy bleeding and anovulatory abnormal bleeding not due to pathology) (Dickersin 2007; Pinion 1994).
Exclusion criteria included large fibroids (over 50% intramural extension (Crosignani 1997), or over 5 cm (Jain 2016)); large uterine size over 12 gestational weeks (Dwyer 1993; O'Connor 1997; Zupi 2003), or cavity over 11 cm (Cooper 2019); pelvic inflammatory disease (PID) (Gannon 1991); endometriosis (Gannon 1991); and abnormal pathology and contraindication for laparoscopy (Cooper 2019). Four studies excluded participants with submucosal fibroids (Crosignani 1997; Jain 2016; O'Connor 1997; Zupi 2003).
Interventions
Open hysterectomy
Two trials compared TCRE (ER) versus open hysterectomy (Dwyer 1993; Gannon 1991); Dwyer 1993 also included a preoperative procedure (medroxyprogesterone acetate injection four to six weeks before surgery) to reduce the thickness of the endometrium for participants undergoing endometrial resection.
Minimally invasive hysterectomy
One trial compared TCRE (ER) versus vaginal hysterectomy (Crosignani 1997)
One trial compared thermal balloon EA versus vaginal hysterectomy (Jain 2016).
Two studies compared second‐generation EA versus laparoscopic supracervical hysterectomy. One versus thermal balloon (Sesti 2011) and the second versus either thermal balloon or radiofrequency (Cooper 2019).
One study compared endometrial resection after preoperative gonadotrophin‐releasing hormone agonist (GnRHa) treatment versus laparoscopic supracervical hysterectomy (Zupi 2003).
Unspecified (or at surgeon's discretion) route of hysterectomy
One trial compared TCRE (ER) versus hysterectomy either abdominal (50%) or vaginal (50%) (O'Connor 1997).
One trial compared endometrial destruction after preoperative gonadotrophin‐releasing hormone (GnRHa) treatment (50% endometrial resection and 50% laser ablation) versus hysterectomy (88% abdominal or 12% vaginal) (Pinion 1994).
One trial compared endometrial resection or thermal balloon ablation (according to surgeon's choice) versus total hysterectomy (vaginal, laparoscopic or abdominal approach, according to surgeon's choice) (Dickersin 2007).
Outcomes
Follow‐up after surgery for all included studies ranged from four months to four years.
Seven studies assessed change in menstrual bleeding patterns (Crosignani 1997; Dickersin 2007; Dwyer 1993; Gannon 1991; Jain 2016; Pinion 1994; Sesti 2011); five of these assessed whether menorrhagia‐like symptoms had resolved (amount and frequency of bleeding), one study (in which participants had a complaint of dysfunctional bleeding) measured separate components of bleeding excess (excessive amount and duration) and another study used PBAC questionnaires.
Four studies assessed time to return to work in weeks (Crosignani 1997; Dwyer 1993; Gannon 1991; Pinion 1994), and one assessed it in days (Cooper 2019).
Two studies in which participants had a diagnosis of dysfunctional bleeding assessed whether an improvement in overall symptoms had occurred (recorded in one trial as 'problem solved') (Dickersin 2007; Pinion 1994).
Three studies measured costs of treatment (Dwyer 1993; Gannon 1991; Pinion 1994).
Five studies assessed satisfaction with surgery (Cooper 2019; Crosignani 1997; Dwyer 1993; O'Connor 1997; Pinion 1994) (although this was reported at different follow‐ups); six trials reported postoperative complications (Cooper 2019; Gannon 1991; Jain 2016; O'Connor 1997; Pinion 1994; Sesti 2011); and seven trials reported duration of surgery (Cooper 2019; Crosignani 1997; Dwyer 1993; Gannon 1991; Jain 2016; O'Connor 1997; Zupi 2003). Eight studies assessed hospital stay (Cooper 2019; Crosignani 1997; Dwyer 1993; Gannon 1991; Jain 2016; O'Connor 1997; Pinion 1994; Zupi 2003); and nine trials reported requirement for further surgery for treatment failure (Cooper 2019; Crosignani 1997; Dickersin 2007; Dwyer 1993; Gannon 1991; Jain 2016; O'Connor 1997; Pinion 1994; Zupi 2003).
Some outcomes measured time of surgery or recovery time and strictly speaking were time‐to‐event outcomes, such as duration of surgery, length of stay in hospital, and time to return to normal activities and work. However, these were analysed as continuous data, as all participants had initial and end values representing the time that had elapsed. Time‐to‐event analysis is mandatory when censoring is performed and only a subset of participants have an event, but the authors in this review considered comparison of means to be an acceptable analysis.
Eight studies assessed quality of life after surgery (Cooper 2019; Crosignani 1997; Dickersin 2007; Dwyer 1993; O'Connor 1997; Pinion 1994; Sesti 2011; Zupi 2003), but used several different scales. This review assessed quality of life (continuous data) as measured by the Golombok Rust Inventory of Marital State (one year after surgery), the 36‐item Short Form (SF‐36), the EuroQol Visual Analogue Scale, HADS, Sabbatsberg Sexual Rating (SSR) Scale (all one or two years after surgery) and the 12‐item Short Form (SF‐12) (in one trial that was reported on a suitable way). The SF‐36 is a generic measure of subjective health in the form of a profile with eight multi‐item dimensions (including physical and emotional role limitation, physical and social functioning, mental health, energy, pain and general health perception) developed in the USA and shown to be an acceptable tool when used by women with HMB (Coulter 1994; Ware 1993). The EuroQol health instrument is a generic single index measure of health‐related quality of life validated in several European countries, including the UK (Brazier 1993; EuroQol Group 1990). The Golombok Rust Inventory was modified by the investigators to obtain a brief measure of the overall quality of the marital relationship (Rust 1986), and the SSR Scale was designed to provide a self‐assessment of sexual functioning by women engaging in intercourse (Garrat 1995). HADS is a self‐assessment mood scale specifically designed to identify states of anxiety and depression and is regarded as a valid measure of the severity of these mood disorders (Zigmond 1983). Several other scales were used to evaluate aspects of quality of life in some of the trials, including the SF‐12, General Health Questionnaire, the Psychosocial Adjustment to Illness Scale, a psychiatric mood scale, a modified social adjustment scale and an unvalidated questionnaire, but we did not enter these outcomes into the review because the data were not obtained in a suitable form for inclusion in a meta‐analysis (quantitative data provided in graphical form indicated significant skew; data could not be obtained from study authors).
Three trials reported quality of life as dichotomous data (Cooper 2019; Dwyer 1993; Pinion 1994). The first study as proportion with improvement in pain at two years' follow‐up (Dwyer 1993), and the second study as proportion with improvement in general health at one and four years after surgery (Pinion 1994). The third study reported the proportion of women with a score of 100 in the Menorrhagia Multi‐Attribute Scale (MMAS) (the best score possible in MMAS) (Cooper 2019).
Five publications from three trials compared costs to the health service of the two techniques (Cameron 1996 and Aberdeen 1999 from the Pinion 1994 study; Gannon 1991; and Sculpher 1998 and Sculpher 1996 from the Dwyer 1993 study). Two of these trials had two publications reporting total health resource costs of the procedures (including the need for retreatment) at different follow‐up times (Cameron 1996 and Aberdeen 1999 (Pinion 1994); Sculpher 1993 and 1996 (Dwyer 1993)). One trial measured direct costs to the participant after one year (Cameron 1996 (Pinion 1994)).
Four publications from two trials calculated cost per participant based on resource use (Sculpher 1993 and Sculpher 1996 from Dwyer 1993, and Cameron 1996 and Aberdeen 1999 from Pinion 1994). The third trial calculated costs by summing the mean costs of variable resources and then adding a factor of 100% to allow for fixed costs (Gannon 1991).
Excluded studies
In the 2008 update of this review, two studies were retrieved for closer inspection and were excluded from the review (Lin 2006; Paddison 2003). One study had allocation according to date of admission and did not satisfy the criteria for true randomisation, and the other was a review of ablation versus hysterectomy.
There were no further studies retrieved for closer inspection but subsequently excluded in the 2013, 2019 and 2020 updates of the review.
Risk of bias in included studies
We assessed all included studies separately for risk of bias (Higgins 2011). We provide a summary of these assessments in Figure 2 and Figure 3.
Allocation
Seven included studies provided sufficient detail on the adequacy of the randomisation method and were at low risk of selection bias (Cooper 2019; Crosignani 1997; Dickersin 2007; Jain 2016; O'Connor 1997; Sesti 2011; Zupi 2003). The other three studies did not describe how randomisation was undertaken and were, therefore, at unclear risk of bias.
Seven studies provided sufficient details of allocation concealment and were at low risk of bias (Cooper 2019; Crosignani 1997; Dickersin 2007; Dwyer 1993; O'Connor 1997; Pinion 1994; Sesti 2011). Three studies provided insufficient details and were at unclear risk of bias (Gannon 1991; Jain 2016; Zupi 2003).
Blinding
Three of the more recent studies used single blinding for assessment of some outcomes (Dickersin 2007; Sesti 2011; Zupi 2003). The other seven studies did not appear to have any blinding of participants, investigators or assessors. All studies were, therefore, at high risk of performance and detection bias.
Incomplete outcome data
Two studies had withdrawals from the study or missing data were greater than 10% (or both), and there were no explanations enable judgement of whether this could have biased the results and were at unclear risk of attrition bias (Dickersin 2007; O'Connor 1997). One trial analysed primary outcomes by an intention‐to‐treat method (satisfaction rate, quality of life and bleeding outcomes), but analysed intraoperative and perioperative outcomes (adverse events, requirement for further surgery and hospital stay) according to surgery received (Dickersin 2007). This trial also reported outcomes at three and four years' follow‐up, but as women who were assigned later during the trial had shorter follow‐up, these assessments are likely to be underpowered. The other seven studies had withdrawals of less than 10% at the time of calculation of outcomes at the first time point and were at low risk of attrition bias. However, in studies with longer follow‐up, additional loss to follow‐up increased with the duration of the trial.
Selective reporting
Only one study provided sufficient information to indicate that it was free of selective outcome reporting was at low risk of reporting bias (Dickersin 2007). Before publication of the study results, an earlier publication provided details of the study protocol and changes made throughout the study (Dickersin 2007: 2003 paper on Dickersin 2007). Three studies reported all the outcomes that were previously specified and were at low risk of reporting bias (Cooper 2019; Jain 2016; Sesti 2011). The other studies provided no evidence of measures taken to prevent selective outcome reporting and were at unclear risk of bias.
Other potential sources of bias
In all the studies, groups were balanced at baseline. Six studies described the prior experience of the operating surgeon (Cooper 2019; Crosignani 1997; Gannon 1991; O'Connor 1997; Sesti 2011; Zupi 2003). However, the other studies did not report this and were considered at unclear risk of other potential sources of bias as a less‐experienced surgeon for either treatment group could affect results such as duration of surgery, adverse outcomes and, in the case of EA/ER, effectiveness.
Effects of interventions
See: Table 1; Table 2; Table 3
Summary of findings 1. Endometrial resection or ablation compared to open hysterectomy for heavy menstrual bleeding.
| Endometrial resection/ablation compared to open hysterectomy for HMB | ||||||
| Patient or population: HMB Setting: clinic Intervention: endometrial resection/ablation Comparison: open hysterectomy | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with open hysterectomy | Risk with endometrial resection/ablation | |||||
|
Effectiveness Woman's perception (proportion with improvement in bleeding symptoms) |
1000 per 1000 | 900 per 1000 (840 to 950) | RR 0.90 (0.84 to 0.95) | 247 (2 RCTs) | ⊕⊕⊕⊝ Moderatea | — |
| Quality of life SF‐36 at 2 years – general health perception | The mean quality‐of‐life scores (SF‐36, 2 years) general health perception was 74 | MD 5.3 lower (11.9 lower to 1.3 higher) | — | 155 (1 RCT) | ⊕⊕⊕⊝ Moderatea | Higher score reflects better quality of life. |
| Requirement for further surgery for treatment failure within 1 year after surgery | 0 per 1000 | Risk of having additional surgery in the EA group was 12.9% | — | 247 (2 RCTs) | ⊕⊕⊕⊝ Moderatea | The open hysterectomy (total) has no risk of further surgery for HMB (structural 0). |
|
Acceptability Proportion satisfied with treatment Follow‐up: 1 year |
937 per 1000 | 853 per 1000 (768 to 937) | RR 0.91 (0.82 to 1.00) | 194 (1 RCT) | ⊕⊕⊕⊝ Moderatea | — |
|
Safety Any serious adverse events |
24 per 1000 | 31 per 1000 (8 to 127) | RR 1.29 (0.32 to 5.20) | 247 (2 RCTs) | ⊕⊕⊝⊝ Lowa,b | — |
| Time to return to normal activity | The mean time to return to normal activity was 28 days | MD 21 days lower (24.78 lower to 17.22 lower) | — | 194 (1 RCT) | ⊕⊕⊕⊝ Moderatea | — |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; EA: endometrial ablation; HMB: heavy menstrual bleeding; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio; SF‐36: 36‐item Short Form. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level for risk of bias (performance). bDowngraded one level for inconsistency.
Summary of findings 2. Endometrial resection or ablation versus minimally invasive hysterectomy for heavy menstrual bleeding.
| Endometrial resection/ablation compared to minimally invasive hysterectomy for heavy menstrual bleeding | |||||||
| Patient or population: heavy menstrual bleeding Setting: clinic Intervention: endometrial resection/ablation Comparison: minimally invasive hysterectomy | |||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | ||
| Risk with minimally invasive hysterectomy | Risk with endometrial resection/ablation | ||||||
| Effectiveness |
Proportion of women with perception of improvement in bleeding symptoms Follow‐up: 2 years |
1000 per 1000 | 970 per 1000 (900 to 1000) | RR 0.97 (0.90 to 1.04) | 79 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | — |
|
PBAC score Follow‐up: 2 years |
The mean PBAC score (continuous data) ‐ At 2 years' follow‐up was 29 |
MD 44 higher (36.09 higher to 51.91 higher) | — | 68 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | — | |
| Quality of life |
SF‐36 general health perception Follow‐up: 2 years |
The mean quality‐of‐life scores (continuous data) – SF‐36 at 2 years – general health perception was 77.48 |
MD 10.71 lower (15.11 lower to 6.3 lower) | — | 145 (2 RCTs) | ⊕⊕⊕⊝ Moderateb | Higher score reflects better quality of life. |
|
Proportion with MMAS = 100 (best possible) 15 months after randomisations |
583 per 1000 | 478 per 1000 (408 to 553) | RR 0.82 (0.70 to 0.95) | 616 (1 RCT) | ⊕⊕⊕⊝ Moderateb | — | |
| Requirement for further surgery for treatment failure within 1 year after surgery | 4 per 1000 | 33 per 1000 (11 to 100) | RR 7.70 (2.54 to 23.32) | 922 (4 RCTs) | ⊕⊕⊕⊝ Moderateb | — | |
|
Acceptability Proportion satisfied with treatment Follow‐up: 1 year |
971 per 1000 | 874 per 1000 (826 to 913) | RR 0.90 (0.85 to 0.94) | 558 (1 RCT) | ⊕⊕⊕⊝ Moderateb | — | |
|
Safety Any serious adverse event |
37 per 1000 | 28 per 1000 (13 to 59) | RR 0.75 (0.35 to 1.59) | 809 (4 RCTs) | ⊕⊕⊕⊝ Moderateb | — | |
| Time to return to normal activity | We were unable to pool data because of extreme heterogeneity (99%). | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; MMAS: Menorrhagia Multi‐Attribute Scale; PBAC: Pictorial Blood Loss Assessment Chart; RCT: randomised controlled trial; RR: risk ratio; SF‐36: 36‐item Short Form. | |||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||||
aDowngraded one level for imprecision. One small trial. bDowngraded one level for risk of bias (performance).
Summary of findings 3. Endometrial resection/ablation versus unspecified (or at surgeon's discretion) route of hysterectomy for heavy menstrual bleeding.
| Endometrial resection/ablation compared to unspecified (or at surgeon's discretion) hysterectomy for heavy menstrual bleeding | ||||||
| Patient or population: heavy menstrual bleeding Setting: clinic Intervention: endometrial resection/ablation Comparison: surgeon's choice of route of hysterectomy | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with unspecified (or at surgeon's discretion) hysterectomy | Risk with endometrial resection/ablation | |||||
|
Effectiveness Proportion of women with perception of improvement of bleeding symptoms Follow‐up: 1 year |
965 per 1000 | 859 per 1000 (801 to 917) | RR 0.89 (0.83 to 0.95) | 403 (2 RCTs) | ⊕⊕⊝⊝ Lowa,b | — |
|
Quality of life SF‐36 general health perception Follow‐up: 2 years |
The mean quality‐of‐life scores (continuous data) – SF‐36 at 1 year – general health perception was 66.1 |
MD 1.9 lower (8.67 lower to 4.87 higher) | — | 209 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | Higher score reflects better quality of life. |
|
Proportion with improvement in general health Follow‐up: 1 year |
955 per 1000 | 812 per 1.000 (735 to 907) | RR 0.85 (0.77 to 0.95) | 185 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | — |
|
Requirement for further surgery for treatment failure Follow‐up: 1 year |
0 per 1000 | Risk of having additional surgery in the endometrial resection/ablation group was 5.4% | — | 374 (2 RCTs) | ⊕⊕⊕⊝ Moderatea | Total hysterectomy has no risk of further surgery for treatment failure (structural 0). |
|
Acceptability Proportion satisfied with treatment Follow‐up: 1 year |
773 per 1000 | 742 per 1000 (680 to 804) | RR 0.96 (0.88 to 1.04) | 545 (3 RCTs) | ⊕⊕⊕⊝ Moderatea | — |
|
Safety Any serious adverse event |
59 per 1000 | 12 per 1000 (4 to 47) | RR 0.21 (0.06 to 0.80) | 374 (2 RCTs) | ⊕⊕⊕⊝ Moderatea | — |
| Time to return to normal activity | The mean time to return to normal activity was 32 days | MD 18.9 days lower (24.63 lower to 13.17 lower) | — | 172 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | — |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio; SF‐36: 36‐item Short Form. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level for risk of bias (performance). bDowngraded one level for inconsistency, as it may present substantial heterogeneity.
We included 10 trials that allowed the following comparisons.
Comparison 1. Endometrial resection/ablation versus open hysterectomy.
Comparison 2. Endometrial resection/ablation versus minimally invasive hysterectomy.
Comparison 3. Endometrial resection/ablation versus unspecified (or at surgeon's discretion) route of hysterectomy.
1 Endometrial resection/ablation versus open hysterectomy
Two trials (247 women) compared TCRE (ER) versus abdominal hysterectomy (Table 1) (Dwyer 1993; Gannon 1991).
Primary outcomes
Effectiveness
1.1 Woman's perception (proportion with improvement in bleeding symptoms)
Both trials assessed whether bleeding symptoms were perceived as improved (Dwyer 1993; Gannon 1991). Women randomly assigned to EA/ER were less likely to show improvement in bleeding symptoms when compared with those randomly assigned to open hysterectomy at one year (RR 0.90, 95% CI 0.84 to 0.95; I² = 0%; 247 women; moderate‐certainty evidence) (Analysis 1.1).
1.1. Analysis.

Comparison 1: Endometrial resection/ablation versus open hysterectomy, Outcome 1: Woman's perception (proportion with improvement in bleeding symptoms)
1.2 Quality‐of‐life scores (continuous data)
One trial measured quality of life (Dwyer 1993). There appeared to be a benefit in pain at two years for the hysterectomy group (MD –9.90, 95% CI –17.63 to –2.17; 155 women; Analysis 1.2). For all other outcomes, there was no clear evidence of benefit or harm between TCRE and open hysterectomy at two years' follow‐up; perception of general health at two years (MD –5.30, 95% CI –11.90 to 1.30; 155 women; moderate‐certainty evidence); role limitation, physical at two years (MD 0.70, 95% CI –9.82 to 11.22); role limitation, emotional at two years (MD –6.20, 95% CI –15.87 to 3.47); social functioning at two years (MD –6.00, 95% CI –12.11 to 0.11); mental health at two years (MD –2.30, 95% CI –7.49 to 2.89); energy at two years (MD –1.50, 95% CI –8.18 to 5.18) and physical functioning at two years (MD –2.50, 95% CI –7.56 to 2.56).
1.2. Analysis.

Comparison 1: Endometrial resection/ablation versus open hysterectomy, Outcome 2: Quality‐of‐life scores (continuous data)
One trial assessed quality of life with the EuroQoL and reported no clear evidence of a difference at either one year after surgery (MD –7.00, 95% CI –17.29 to 3.29) or at two years after surgery (MD –1.50, 95% CI –6.29 to 3.29) (Dwyer 1993).
1.3 Quality of life (proportion with improvement)
According to one trial, there was no clear evidence of a difference in the proportion with improvement in quality of life in terms of pain at two years' follow‐up (RR (non‐event) 1.60, 95% CI 0.55 to 4.63; Analysis 1.3) (Dwyer 1993).
1.3. Analysis.

Comparison 1: Endometrial resection/ablation versus open hysterectomy, Outcome 3: Quality of life (proportion with improvement)
1.4 Requirement for further surgery for treatment failure
Risk of repeat surgery for treatment failure is zero when women have a total hysterectomy, this is a structural zero (will always be zero, and it is not random), as the bleeding was from the removed uterus, thus the calculation of RR may result in biased estimates (Sweeting 2004; Tang 2018). The risk of having a further surgery for treatment failure on the EA/ER group was 12.9% at one year (compared to 0 on the total hysterectomy group; I² = 0%; 2 studies, 247 women; moderate‐certainty evidence) (Dwyer 1993; Gannon 1991), and 33% at two years' follow‐up (1 trial, 196 women) (Dwyer 1993) (Analysis 1.4).
1.4. Analysis.

Comparison 1: Endometrial resection/ablation versus open hysterectomy, Outcome 4: Requirement for further surgery for treatment failure
Acceptability
1.5 Proportion satisfied with treatment
One trial (194 women) reported satisfaction (very or moderately satisfied) rates (Dwyer 1993). Compared to the open hysterectomy group, satisfaction rate was lower among women who had EA/ER at one year after surgery (RR 0.91, 95% CI 0.82 to 1.00) and two years after surgery (RR 0.82, 95% CI 0.73 to 0.93; moderate‐certainty evidence; Analysis 1.5).
1.5. Analysis.

Comparison 1: Endometrial resection/ablation versus open hysterectomy, Outcome 5: Proportion satisfied with treatment
Safety (adverse outcomes)
1.6 Any serious adverse events
Both studies reported adverse events (Dwyer 1993; Gannon 1991). There was no clear evidence of a difference for any serious adverse events (RR 1.29, 95% CI 0.32 to 5.20; I² = 59%; 2 studies, 247 women; low‐certainty evidence; Analysis 1.6).
1.6. Analysis.

Comparison 1: Endometrial resection/ablation versus open hysterectomy, Outcome 6: Any serious adverse event
1.7 Adverse events: short term (intraoperative and immediately postoperative)
There was clear evidence of a difference between procedures in terms of the following short‐term adverse effects favouring endometrial resection/ablation (Analysis 1.7):
1.7. Analysis.

Comparison 1: Endometrial resection/ablation versus open hysterectomy, Outcome 7: Adverse events – short term (intraoperative and immediate postoperative)
1.7.1 Sepsis (RR 0.07, 95% CI 0.02 to 0.25; I² = 0%; 2 studies; 247 women) (Dwyer 1993; Gannon 1991);
1.7.2 Pyrexia (RR 0.07, 95% CI 0.02 to 0.28; 1 study, 196 women) (Dwyer 1993);
1.7.3 Vault haematoma (RR 0.16, 95% CI 0.03 to 0.86; I² = 0%; 2 studies, 247 women) (Dwyer 1993; Gannon 1991);
There was no clear evidence of a difference in blood transfusion between groups in one trial.
1.7.4 Blood transfusion (RR 0.33, 95% CI 0.07 to 1.58; 196 women) (Dwyer 1993).
1.8 Adverse events: long term (after hospital discharge)
One trial reported long‐term adverse events, and there was no clear evidence of a difference between procedures (Analysis 1.8).
1.8. Analysis.

Comparison 1: Endometrial resection/ablation versus open hysterectomy, Outcome 8: Adverse events – long term (after hospital discharge)
1.8.1 Haematoma (RR 0.20, 95% CI 0.01 to 4.03; 196 women; Dwyer 1993);
1.8.2 Haemorrhage (RR 2.94, 95% CI 0.12 to 71.30; 196 women; Dwyer 1993).
Secondary outcomes
1.9 Duration of surgery
Both trials reported the duration of surgery (Dwyer 1993; Gannon 1991), and, even though there was very high heterogeneity (we could not pool the data), the direction of effect was the same and it was consistently longer on the open hysterectomy groups compared to the Endometrial resection/ablation group (Analysis 1.9). Reasons for heterogeneity may have included skills of the surgeons, type of anaesthesia, characteristics of the women and the method used to record duration of the surgery.
1.9. Analysis.

Comparison 1: Endometrial resection/ablation versus open hysterectomy, Outcome 9: Duration of surgery (minutes)
1.10 Duration of hospital stay
Both trials evaluated duration of hospital stay (Dwyer 1993; Gannon 1991). We noted a high heterogeneity in the results. Despite this, both studies reported shorter hospital stays for EA/ER compared to open hysterectomy (Analysis 1.10). Reasons for heterogeneity may have included hospital protocols for discharge, geographic conditions and time when the trial took place.
1.10. Analysis.

Comparison 1: Endometrial resection/ablation versus open hysterectomy, Outcome 10: Duration of hospital stay (days)
1.11 Time to return to normal activity
One trial reported time to return to normal activity (in days) (Dwyer 1993). There was clear evidence of a difference between groups favouring EA/ERor resection in time to return to normal activities compared to the open hysterectomy group (MD –21.00, 95% CI –24.78 to –17.22; Analysis 1.11).
1.11. Analysis.

Comparison 1: Endometrial resection/ablation versus open hysterectomy, Outcome 11: Time to return to normal activity (days)
1.12 Time to return to work
Both trials reported time to return work (Dwyer 1993; Gannon 1991). Time to return to work (in weeks) showed a high heterogeneity, but the direction of the effect presented a clear difference favouring the Endometrial resection/ablation compared to open hysterectomy (MD –7.72 days, 95% CI –8.05 to –7.38; I² = 81%; 245 women; Analysis 1.12). Reasons for heterogeneity may have included different protocols, types of job and geographical reasons to stay away from work.
1.12. Analysis.

Comparison 1: Endometrial resection/ablation versus open hysterectomy, Outcome 12: Time to return to work (weeks)
1.13 Total health service cost per woman
Both trials assessed the health service costs (Dwyer 1993; Gannon 1991). There was clear evidence of a difference between groups favouring Endometrial resection/ablation for the management of HMB at short‐term follow‐up and, in one trial (Dwyer 1993), the difference was still clear up to two years' follow‐up (Analysis 1.13).
1.13. Analysis.
Comparison 1: Endometrial resection/ablation versus open hysterectomy, Outcome 13: Total health service cost per woman
| Total health service cost per woman | |
| Study | Details |
| Dwyer 1993 |
Four months' follow‐up Mean resource cost per participant in 1991/1992 prices:
These costs were made up of preoperative, operative and postoperative costs; hotel costs; complications costs; retreatment and general practice costs. Mean difference in cost between groups: GBP –499.68 (95% confidence interval (CI) –567 to –432) Statistical test not specifically stated. Mean cost of resection at 4 months' follow‐up 53% of the cost of hysterectomy. 2.2 years' follow‐up Mean resource cost per participant in 1994 prices:
Costs made up of initial surgery, retreatment costs, other resource use after 4 months and hormone replacement therapy. Wilcoxon Rank Sum test used to test the difference between groups with a 5% significance level, P = 0.0001. Mean cost of resection at 2.2 years' follow‐up 71% of the cost of hysterectomy. |
| Gannon 1991 |
Initial costs (NHS) Mean cost per operation in 1991:
This cost was made up of: variable costs: mean cost of theatre consumables, staffing and maintenance in the operating theatre, marginal cost of a bed on the gynaecological ward; and fixed costs: capital depreciation, hospital staffing and energy. The difference between groups in resource cost was not assessed in a statistical test. |
2 Endometrial resection/ablation versus minimally invasive hysterectomy
Five trials (990 women) assessed endometrial resection/ablation versus minimally invasive hysterectomy (Table 2). Two compared second‐generation EA versus laparoscopic supracervical hysterectomy; one versus thermal balloon (Sesti 2011), and one versus either thermal balloon or radiofrequency (Cooper 2019). One compared TCRE (ER) versus vaginal hysterectomy (Crosignani 1997), one compared thermal balloon EA versus vaginal hysterectomy (Jain 2016) and one compared endometrial resection (after preoperative GnRHa) treatment versus laparoscopic supracervical hysterectomy (Zupi 2003).
Primary outcomes
Effectiveness
2.1 Woman's perception (proportion with improvement in bleeding symptoms)
One trial assessed the woman's perception of improvement in bleeding symptoms, reporting no clear evidence of a difference (RR 0.97, 95% CI 0.90 to 1.04; 79 women; low‐certainty evidence; Analysis 2.1) (Crosignani 1997).
2.1. Analysis.

Comparison 2: Endometrial resection/ablation versus minimally invasive hysterectomy, Outcome 1: Woman's perception (proportion with improvement in bleeding symptoms)
2.2 Pictorial Blood Loss Assessment Chart score
One trial assessed menstrual blood loss using the PBAC score (Sesti 2011). Compared with pretreatment scores, the PBAC score was clearly reduced in both groups at one and two years postoperatively; however, this finding overall favoured women randomly assigned to minimally invasive hysterectomy at one year (MD 24.40, 95% CI 16.01 to 32.79; 68 women; low‐certainty evidence), and even more so at two years (MD 44.00, 95% CI 36.09 to 51.91; 68 women) (Analysis 2.2).
2.2. Analysis.

Comparison 2: Endometrial resection/ablation versus minimally invasive hysterectomy, Outcome 2: PBAC score (continuous data)
2.3 Quality‐of‐life scores (continuous data)
Studies measured quality of life using several validated scales. It was not possible to combine scales, as the domains differed, measuring different aspects of quality of life.
There was a high heterogeneity with an I² statistic greater than 90%, which does not allow us to make any conclusion. There was no clear evidence of benefit or harm.
Some studies reported benefits for minimally invasive hysterectomy compared to EA/ER. One study (181 women) reported benefit favouring the minimally invasive group using the SF‐36 at one year' follow‐up for social functioning (MD –21.20, 95% CI –24.73 to –17.67), energy (MD –11.30, 95% CI –14.82 to –7.78), general health perception (MD –9.80, 95% CI –13.86 to –5.74) and physical functioning (MD –1.20, 95% CI –5.34 to 2.94) (Zupi 2003). Two studies (145 women) reported benefit favouring the minimally invasive surgery compared to EA/ER, using SF‐36, at two years for social functioning (MD –12.02, 95% CI –16.26 to –7.77; I² = 0%), general health perception (MD –10.71, 95% CI –15.11 to –6.30; I² = 0%), pain (MD –12.68, 95% CI –17.09 to –8.27; I² = 35%) and physical functioning (MD –15.59, 95% CI –20.47 to –10.72; I² = 89%) (Crosignani 1997; Sesti 2011).
In contrast, the pooled results of two studies (145 women) suggested benefits for EA/ER compared to minimally invasive hysterectomy, using the SF‐36, at two years on role limitation (emotional) (MD 15.41, 95% CI 9.97 to 20.85; I² = 89%). (Analysis 2.3) (Crosignani 1997; Sesti 2011).
2.3. Analysis.

Comparison 2: Endometrial resection/ablation versus minimally invasive hysterectomy, Outcome 3: Quality‐of‐life scores (continuous data)
There was no clear evidence of a difference, between EA/ER and minimally invasive hysterectomy, on the remaining parameters at one and two years.
The remaining parameters at one year using the SF‐36 were role limitation, physical (MD –0.80, 95% CI –4.99 to 3.39; 1 study, 181 women) (Zupi 2003), role limitation, emotional (MD –3.90, 95% CI –8.21 to 0.41; 1 study, 181 women) (Zupi 2003), mental health (MD –2.70, 95% CI –6.84 to 1.44; 1 study, 181 women) (Zupi 2003), pain (MD –1.50, 95% CI –6.05 to 3.05; 1 study, 181 women) (Zupi 2003); and using SF‐12 were physical score (MD –1.10, 95% CI –2.78 to 0.58; 1 study, 435 women) (Cooper 2019), and mental score (MD –1.90, 95% CI –4.00 to 0.20; 1 study, 435 women) (Cooper 2019).
The remaining parameters at two years' follow up were role limitation, physical (MD –4.12, 95% CI –9.58 to 1.35; I² = 0%; 2 studies, 145 women) (Crosignani 1997; Sesti 2011), SSR Scale (MD –3.70, 95% CI –11.17 to 3.77; 1 study, 77 women) (Crosignani 1997), total HADS scores (MD –1.50, 95% CI –4.32 to 1.32; 1 study, 77 women) (Crosignani 1997), anxiety HADS scores (MD –1.60, 95% CI –3.28 to 0.08; 1 study, 77 women) (Crosignani 1997), and depression HADS scores (MD –0.60, 95% CI –2.28 to 1.08; 1 study, 77 women) (Crosignani 1997).
Energy and mental health at two years follow up using the SF 36 were reported by two trials (Crosignani 1997; Sesti 2011), the results were inconclusive with heterogeneity over 90%. The mean difference between EA/ER and minimally invasive hysterectomy for energy was ‐11.30(95% CI ‐20.21 to 2.39) in Crosignani 1997 and 11.1 (95% CI 5.33 to 16.87 in Sesti 2011; and for mental health was ‐4.70(95% CI ‐13.33 to 3.93 in Crosignani 1997) and 13.50 (95% CI 8.35 to 18.65 in Sesti 2011.
2.4 Quality of life (proportion with improvement)
One trial reported quality of life measured using the MMAS (Cooper 2019), it was reported as the proportion of women with MMAS = 100 (the best score possible) at 15 months after randomisation (approximately 12 months after surgery). Women on the minimally invasive hysterectomy group were probably more likely to have a better quality of life measured with MMAS compared to the EA/ER group (RR 0.82, 95% CI 0.70 to 0.95; 616 women; moderate‐certainty evidence) (Analysis 2.4).
2.4. Analysis.

Comparison 2: Endometrial resection/ablation versus minimally invasive hysterectomy, Outcome 4: Proportion with MMAS = 100 (best possible) 15 months after randomisation
2.5 Quality of life at 14 years' follow‐up
One trial, using the SF‐12, reported physical and mental quality of life at 14 years' follow‐up (Zupi 2003), suggesting a higher quality of life in the minimally invasive hysterectomy group compared to the ablation group. There was clear evidence of a difference favouring minimally invasive surgery compared to EA/ER on the physical component score (ranged from 57.50 to 51.40 for minimally invasive hysterectomy and from 55.04 to 45.40 for ablation) and on the mental component (ranged from 56.80 to 50.40 for minimally invasive hysterectomy and from 51.90 to 36.10 for ablation). A higher score denotes a better quality of life. We could not enter these data in the meta‐analysis (Analysis 2.5).
2.5. Analysis.
Comparison 2: Endometrial resection/ablation versus minimally invasive hysterectomy, Outcome 5: Quality of life at 14 years' follow‐up
| Quality of life at 14 years' follow‐up | |||
| Study | Hysterectomy group | Endometrial ablation group | Heading 3 |
| Zupi 2003 | Physical score ranged from 57.5 to 51.4 Mental score ranged from 56.8 to 50.4 |
Physical score ranged from 55.04 to 45.4 Mental score ranged from 51.9 to 36.1 |
The difference was significant for both parameters physical and mental score. P < 0.0001 |
2.6 Requirement for further surgery for treatment failure
Studies reported risk of repeat surgery for failure of the initial surgical treatment at different follow‐up times. Four trials reported the requirement for further surgery at one year's follow‐up (Cooper 2019; Crosignani 1997; Jain 2016; Zupi 2003); three reported at two years' follow‐up (Crosignani 1997; Sesti 2011; Zupi 2003); and one reported at 14 years' follow‐up (Zupi 2003).
The risk of having a further surgery for treatment failure was more likely for EA/ER than for minimally invasive hysterectomy at all follow‐up periods (Analysis 2.6). We reported for this comparison the relative risk as women having minimally invasive hysterectomy may have a subtotal hysterectomy that could (exceptionally) lead to a treatment failure (this is not a structural zero as explained in 1.4 above).
2.6. Analysis.

Comparison 2: Endometrial resection/ablation versus minimally invasive hysterectomy, Outcome 6: Requirement for further surgery for treatment failure
2.6.1 At one year' follow‐up (RR 7.70, 95% CI 2.54 to 23.32; I² = 0%; 4 studies, 922 women) (Cooper 2019; Crosignani 1997; Jain 2016; Zupi 2003). There were only two surgeries in the hysterectomy group: one that was allocated to laparoscopic hysterectomy but received EA/ER and the second was a subtotal laparoscopic hysterectomy that required the cervix to be surgically removed for persistent bleeding.
2.6.2 At two years' follow‐up (RR 16.75, 95% CI 2.24 to 125.34; I² = 0%; 3 studies, 334 women) (Crosignani 1997; Sesti 2011; Zupi 2003).
2.6.3 At 14 years' follow‐up (RR 19.60, 95% CI 1.15 to 333.63; 1 study, 153 women) (Zupi 2003).
Acceptability
2.7 Proportion satisfied with treatment
One trial reported satisfaction rate (very or moderately satisfied) at one (Cooper 2019) and two years' follow‐up (Crosignani 1997). There was clear evidence of a difference in satisfaction rate favouring minimally invasive hysterectomy compared to EA/ER at one year (RR 0.90, 95% CI 0.85 to 0.94; 558 women) (Cooper 2019). At two years, there was no clear evidence of a difference between groups (RR 0.92, 95% CI 0.79 to 1.06; 77 women) (Crosignani 1997) (Analysis 2.7).
2.7. Analysis.

Comparison 2: Endometrial resection/ablation versus minimally invasive hysterectomy, Outcome 7: Proportion satisfied with treatment
Safety (adverse outcomes)
2.8 Any serious adverse events
All studies in this comparison reported adverse events. Four trials reported the number of women with any serious adverse events (Cooper 2019; Crosignani 1997; Jain 2016; Sesti 2011). One study reported only a list of adverse events, even though some serious adverse events were reported on the list, we did not include the data on the analysis due to the high risk of double counting (e.g. readmission, cardiorespiratory event and thromboembolic event, all in the same list, as it is unclear if that could be one or three women) (Zupi 2003) (Analysis 2.8).
2.8. Analysis.

Comparison 2: Endometrial resection/ablation versus minimally invasive hysterectomy, Outcome 8: Any serious adverse event
There was no clear evidence of a difference on the incidence of serious adverse events between groups (RR 0.75, 95% CI 0.35 to 1.59; I² = 0%; 4 studies, 809 women).
2.9 Adverse events: short term (intraoperative and immediately postoperative)
Three trials reported short‐term adverse effects (Cooper 2019; Jain 2016; Zupi 2003).
There was no clear evidence of a difference between EA/ER and minimally invasive hysterectomy in any of the adverse effects reported (Analysis 2.9).
2.9. Analysis.

Comparison 2: Endometrial resection/ablation versus minimally invasive hysterectomy, Outcome 9: Adverse events – short term (intraoperative and immediate postoperative)
2.9.1 Sepsis (RR 1.01, 95% CI 0.29 to 3.44; 1 study, 616 women) (Cooper 2019).
2.9.2 Blood transfusion (RR 0.68, 95% CI 0.11 to 4.08; I² = 30%; 3 studies, 837 women) (Cooper 2019; Jain 2016; Zupi 2003).
2.9.3 Pyrexia (RR 0.73, 95% CI 0.24 to 2.27; 2 studies, 797 women) (Cooper 2019; Zupi 2003).
2.9.4 Vault haematoma (RR 0.21, 95% CI 0.01 to 4.25; 1 study, 181 women) (Zupi 2003).
2.9.5 Fluid overload (RR 11.37, 95% CI 0.64 to 202.59; 1 study, 181 women) (Zupi 2003).
2.9.6 Haemorrhage (RR 0.86, 95% CI 0.27 to 2.74; 2 studies, 797 women) (Cooper 2019; Zupi 2003).
2.9.7 Perforation (RR 3.02, 95% CI 0.32 to 28.87; 1 study, 616 women) (Cooper 2019).
2.9.8 Laparotomy (RR 0.52, 95% CI 0.05 to 5.60; 1 study, 181 women) (Zupi 2003).
2.9.9 Cystotomy (bladder injury) (RR 0.34, 95% CI 0.01 to 8.20; 1 study, 616 women) (Cooper 2019).
2.9.10 Cervical laceration (RR 3.10, 95% CI 0.13 to 75.10; 1 study, 181 women) (Zupi 2003).
Adverse events: long term (after hospital discharge)
There were no reports about adverse events after hospital discharge for this comparison.
Secondary outcomes
2.10 Duration of surgery
All trials reported the duration of surgery. We were unable to pool the data due to a high heterogeneity (98%), but the direction of the effect was clear, and in general EA/ER reported shorter surgical times than minimally invasive hysterectomy, with an MD ranging from 30 minutes' to two hours' between trials. There was also a difference in operation time for each individual procedure, ranging the EA/ER from 13 to 44 minutes and the minimally invasive hysterectomy from 70 to 150 minutes. Reasons for heterogeneity may have included skills of the surgeons, type of anaesthesia, characteristics of the women and the method used to record duration of the surgery (Analysis 2.10).
2.10. Analysis.

Comparison 2: Endometrial resection/ablation versus minimally invasive hysterectomy, Outcome 10: Duration of surgery (minutes)
2.11 Duration of hospital stay
Three trials evaluated duration of hospital stay (Cooper 2019; Crosignani 1997; Zupi 2003). We did not pool data in this analysis as we noted a high degree of heterogeneity (100%) (Analysis 2.11). There was a wide range of differences in hospital stay for each individual procedure, ranging from 3 to 30 hours for EA/ER and from under 24 hours to five days for minimally invasive hysterectomy. Reasons for the heterogeneity may have included hospital protocols for discharge, geographic conditions and time when the trial took place (there was a clear difference on the time spent in hospital for minimally invasive hysterectomy in the older trial compared to more recent ones).
2.11. Analysis.

Comparison 2: Endometrial resection/ablation versus minimally invasive hysterectomy, Outcome 11: Duration of hospital stay (days)
Despite the high heterogeneity, all studies reported shorter hospital stays for EA/ER compared to hysterectomy.
2.12 Time to return to normal activity
Two trials reported time to return to normal activity (in days) (Crosignani 1997; Zupi 2003); one trial reported it as time to return to unpaid work (Cooper 2019) (Analysis 2.12). We do not include a pooled estimate in this analysis as we noted a high degree of heterogeneity (100%). The direction of the effect was consistent to having shorter periods of time to return to either normal life or to unpaid work with EA resection compared to minimally invasive hysterectomy. Causes of heterogeneity may have included hospital protocols, doctors' instructions and insurance allowance of time off work. The time to return to normal activities on the EA/ER group reported a range that was fairly similar between the trials (six days Cooper 2019; eight days Crosignani 1997; eight days Zupi 2003), but the results for the minimally invasive hysterectomy group reported a wide range (21 days Cooper 2019; 13 days Crosignani 1997; 10 days Zupi 2003).
2.12. Analysis.

Comparison 2: Endometrial resection/ablation versus minimally invasive hysterectomy, Outcome 12: Time to return to normal activity (days)
2.13 Time to return to work
Three trials reported time to return to work (in weeks) (Cooper 2019; Crosignani 1997; Zupi 2003) (Analysis 2.13). We were unable to pool the data due to a 100% heterogeneity. One trial reported that women returned to work at 3.5 days after either procedure showing no clear evidence of a difference between groups (Zupi 2003). This is markedly different to results in the other two studies reporting this outcome, reporting one (Cooper 2019) and two weeks (Crosignani 1997) for EA/ER; and four (Crosignani 1997) or five (Cooper 2019) weeks for minimally invasive hysterectomy.
2.13. Analysis.

Comparison 2: Endometrial resection/ablation versus minimally invasive hysterectomy, Outcome 13: Time to return to work (weeks)
Both data for time to return to unpaid work and time to return to work in Cooper 2019 were reported in median and interquartile range (IQR), we calculated the mean and SD according to Hozo 2005.
For both outcomes due to high heterogeneity, we tested the effect estimate by removing each study in turn; heterogeneity remained high: one was 83%, and the others were 98% and above.
2.14 Cost
None of the studies reported costs.
3 Endometrial resection/ablation versus unspecified (or at surgeon's discretion) route of hysterectomy
Three trials assessed EA/ER versus unspecified (or at surgeon's discretion) route of hysterectomy (Table 3): one compared TCRE versus hysterectomy (50% abdominal and 50% vaginal) (O'Connor 1997); one compared endometrial destruction after preoperative GnRH treatment (50% endometrial resection and 50% laser ablation) versus hysterectomy (88% abdominal and 12% vaginal) (Pinion 1994); and one compared endometrial resection or thermal balloon ablation (at surgeon's discretion) versus total hysterectomy (vaginal, laparoscopic or abdominal approach, at surgeon's discretion) (Dickersin 2007).
Primary outcomes
Effectiveness
3.1 Woman's perception (proportion with improvement in bleeding symptoms)
Two trials assessed whether bleeding symptoms were perceived as improved at one, two or four years' follow‐up (Dickersin 2007; Pinion 1994). Women on the unspecified (or at surgeon's discretion) route of hysterectomy were more likely to perceive an improvement in bleeding symptoms up to four years' follow‐up (Analysis 3.1).
3.1. Analysis.

Comparison 3: Endometrial resection/ablation versus unspecified (or at surgeon's discretion) route of hysterectomy, Outcome 1: Woman's perception (proportion with improvement in bleeding symptoms)
3.1.1 At one year' follow‐up (RR 0.89, 95% CI 0.83 to 0.95; I² = 72%; 2 studies, 403 women; low‐certainty evidence) (Dickersin 2007; Pinion 1994).
3.1.2 At two years' follow‐up (RR 0.90, 95% CI 0.82 to 0.99; 1 study, 213 women) (Dickersin 2007).
3.1.3 At four years' follow‐up (RR 0.93, 95% CI 0.88 to 0.99; I² = 79%; 2 studies, 237 women) (Dickersin 2007; Pinion 1994).
Pictorial Blood Loss Assessment Chart score
None of the trials reported of PBAC score.
3.2 Quality‐of‐life scores (continuous data)
Trials measured quality of life using several validated scales. It was not possible to combine scales, as the domains differed, measuring different aspects of quality of life (Analysis 3.2).
3.2. Analysis.

Comparison 3: Endometrial resection/ablation versus unspecified (or at surgeon's discretion) route of hysterectomy, Outcome 2: Quality‐of‐life scores (continuous data)
There was no evidence of clear difference between groups in all quality‐of‐life categories but one:
3.2.7 Energy at two years' follow‐up measured with SF‐36 (MD –15.60, 95% CI –22.47 to –8.73; 1 study, 213 women) (Dickersin 2007).
All the remaining parameters reported no clear evidence of a difference between the EA/ER group versus the unspecified (or at surgeon's discretion) route of hysterectomy.
3.2.1 Mental health at one year using the SF‐36 (MD 1.60, 95% CI –5.17 to 8.37; 1 study, 204 women) (Dickersin 2007).
3.2.2 Energy at one year using the SF‐36 (MD –2.30, 95% CI –20.90 to 16.30; 1 study, 204 women) (Dickersin 2007).
3.2.3 Pain at one year using the SF‐36 (MD –2.80, 95% CI –9.53 to 3.93; 1 study, 210 women) (Dickersin 2007).
3.2.4 General health perception at one year using the SF‐36 (MD –0.70, 95% CI –7.24 to 5.84; 1 study, 204 women) (Dickersin 2007).
3.2.5 Pain at two years using the SF‐36 (MD –2.40, 95% CI –8.86 to 4.06; 1 study, 213 women) (Dickersin 2007).
3.2.6 General health perception at two years using the SF‐36 (MD –1.90, 95% CI –8.67 to 4.87; 1 study, 209 women) (Dickersin 2007).
3.2.8 Golombok Rust Inventory of Marital State scores at one year after surgery (MD 0.00, 95% CI –1.75 to 1.75; 1 study, 182 women) (Pinion 1994).
3.2.9 EuroQol scores within one year after surgery (MD –2.00, 95% CI –7.90 to 3.90; 1 study, 210 women) (Dickersin 2007).
3.2.10 EuroQol scores at two years after surgery (MD –2.60, 95% CI –8.20 to 3.00; 1 study, 213 women) (Dickersin 2007).
3.2.11 Anxiety HADS scores at one year after surgery (MD –0.20, 95% CI –1.39 to 0.99; 1 study, 182 women) (Pinion 1994).
3.2.12 Depression HADS scores at one year after surgery (MD 0.00, 95% CI –0.09 to 0.09; 1 study, 182 women) (Pinion 1994).
3.3 Quality of life (proportion with improvement)
One trial reported the proportion of participants with improvement in general health at one and four years (Pinion 1994). A greater proportion of women who had undergone an unspecified (or at surgeon's discretion) route of hysterectomy reported an improvement in their general health one year after surgery when compared with women who received EA/ER (RR 0.85, 95% CI 0.77 to 0.95; 185 women); but at four years, this difference between groups had narrowed and there was no evidence of a clear difference (RR 0.89, 95% CI 0.80 to 1.00; 146 women) (Analysis 3.3).
3.3. Analysis.

Comparison 3: Endometrial resection/ablation versus unspecified (or at surgeon's discretion) route of hysterectomy, Outcome 3: Quality of life (proportion with improvement)
None of the studies reported quality of life using MMAS.
3.4 Requirement for further surgery for treatment failure
Studies reported risk of repeat surgery for failure of the initial surgical treatment at different follow‐up times. Two trials reported the requirement for further surgery at one year' follow‐up (O'Connor 1997; Pinion 1994); two reported at two years' follow‐up (Dickersin 2007; O'Connor 1997); one reported at three years' follow‐up (O'Connor 1997); and one at four years' follow‐up (Pinion 1994).
The risk of having a further surgery for treatment failure was more likely for EA/ER than for the unspecified (or at surgeon's discretion) route of hysterectomy at all follow‐up periods (Analysis 3.4).
3.4. Analysis.

Comparison 3: Endometrial resection/ablation versus unspecified (or at surgeon's discretion) route of hysterectomy, Outcome 4: Requirement for further surgery for treatment failure
3.4.1 Both trials reporting further surgery for treatment failure at one year included only total vaginal and abdominal hysterectomies. Risk of repeat surgery for treatment failure is zero when women have a total hysterectomy, this is a structural zero (will always be zero, and it is not random), as the bleeding was from the removed uterus, thus the calculation of RR may result in biased estimates (Sweeting 2004; Tang 2018). The risk of having a further surgery for treatment failure on the EA/ER group was 5.4% % at one year (I² = 0%; 2 studies, 374 women; moderate‐certainty evidence) (O'Connor 1997; Pinion 1994).
3.4.2 At two years' follow‐up (RR 35.70, 95% CI 5.30 to 240.55; I² = 0%; 2 studies, 400 women) (Dickersin 2007; O'Connor 1997).
3.4.3 At three years' follow‐up (RR 22.90, 95% CI 1.42 to 370.26; 1 study, 172 women) (O'Connor 1997).
3.4.4 At four years' follow‐up (RR 73.63, 95% CI 4.59 to 1181.42; 1 study, 197 women) (Pinion 1994).
One study reported three requirements for further surgery in the hysterectomy group (two oophorectomies and one cystoscopy for persistent urinary symptoms after the surgery), but no further surgery for HMB.
Acceptability
3.5 Proportion satisfied with treatment
Three trials reported satisfaction (very or moderately satisfied) rates (Dickersin 2007; O'Connor 1997; Pinion 1994). There was no clear evidence of a difference on the satisfaction rate between groups at any follow‐up time (Analysis 3.5).
3.5. Analysis.

Comparison 3: Endometrial resection/ablation versus unspecified (or at surgeon's discretion) route of hysterectomy, Outcome 5: Proportion satisfied with treatment
3.5.1 Within the first year (RR 0.96, 95% CI 0.88 to 1.04; I² = 0%; 3 studies, 545 women; moderate‐certainty evidence) (Dickersin 2007; O'Connor 1997; Pinion 1994).
3.5.2 At two years' follow‐up (RR 0.89, 95% CI 0.77 to 1.03; I² = 0%; 2 studies, 337 women) (Dickersin 2007; O'Connor 1997).
3.5.3 At four years' follow‐up (RR 0.89, 95% CI 0.77 to 1.03; I² = 0%; 2 studies, 246 women) (Dickersin 2007; Pinion 1994).
Safety (adverse events)
3.6 Any serious adverse events
All studies reported adverse events. Two reported the number of women with any serious adverse events (O'Connor 1997; Pinion 1994). One study reported only a list of adverse events, even though some serious adverse events were reported on the lists, we did not include the data on the analysis due to the high risk of double counting (e.g. readmission, cardiorespiratory event and thromboembolic event), as it is unclear if that could be one or three women) (Dickersin 2007).
There was clear evidence of a difference on serious adverse events favouring EA/ER compared with unspecified (or at surgeon's discretion) hysterectomy (RR 0.21, 95% CI 0.06 to 0.80; I² = 0%; 2 studies, 374 women; moderate‐certainty evidence; Analysis 3.6) (O'Connor 1997; Pinion 1994).
3.6. Analysis.

Comparison 3: Endometrial resection/ablation versus unspecified (or at surgeon's discretion) route of hysterectomy, Outcome 6: Any serious adverse event
3.7 Adverse events: short term (intraoperative and immediately postoperative)
Three trials reported short‐term adverse effects (Dickersin 2007; O'Connor 1997; Pinion 1994).
There was clear evidence of a difference between EA/ER and unspecified (or at surgeon's discretion) route of hysterectomy favouring EA/ER in the following adverse effects (Analysis 3.7).
3.7. Analysis.

Comparison 3: Endometrial resection/ablation versus unspecified (or at surgeon's discretion) route of hysterectomy, Outcome 7: Adverse events – short term (intraoperative and immediate postoperative)
3.7.1 Sepsis (RR 0.27, 95% CI 0.17 to 0.44; I² = 61%; 2 studies, 374 women) (O'Connor 1997; Pinion 1994).
3.7.2 Blood transfusion (RR 0.13, 95% CI 0.02 to 0.70; I² = 0%; 2 studies, 374 women) (O'Connor 1997; Pinion 1994).
3.7.3 Pyrexia (RR 0.21, 95% CI 0.08 to 0.61; 1 study, 228 women) (Dickersin 2007).
3.7.4 Vault haematoma (RR 0.08, 95% CI 0.02 to 0.42; I² = 0%; 2 studies, 430 women) (Dickersin 2007; Pinion 1994).
3.7.5 Wound haematoma (RR 0.03, 95% CI 0 to 0.53; 1 study, 202 women) (Pinion 1994).
However, there was clear evidence of a difference between EA/ER and unspecified (or at surgeon's discretion) route of hysterectomy favouring unspecified (or at surgeon's discretion) hysterectomy in the following adverse effects (Analysis 3.7).
3.7.6 Fluid overload (RR 8.59, 95% CI 1.59 to 46.36; I² = 0%; 2 studies, 430 women) (Dickersin 2007; Pinion 1994).
There was no clear evidence of a difference between groups for the following adverse effects (Analysis 3.7).
3.7.7 Haemorrhage (RR 0.61, 95% CI 0.24 to 1.57; I² = 68%; 2 studies, 374 women) (O'Connor 1997; Pinion 1994).
3.7.8 Anaesthetic complications (RR 0.18, 95% CI 0.01 to 3.80; 1 study, 202 women) (Pinion 1994).
3.7.9 Perforation (RR 5.05, 95% CI 0.61 to 42.16; I² = 0%; 2 studies, 430 women) (Dickersin 2007; Pinion 1994).
3.7.10 Gastrointestinal obstruction (RR 0.46, 95% CI 0.04 to 5.01; 1 study, 202 women) (Pinion 1994).
3.7.11 Laparotomy (RR 0.31, 95% CI 0.03 to 2.91; 1 study, 202 women) (Pinion 1994).
3.7.12 Cystotomy (bladder injury) (RR 0.21, 95% CI 0.01 to 4.42; 1 study, 228 women) (Dickersin 2007).
3.7.13 Cervical laceration (RR 3.22, 95% CI 0.13 to 78.13; 1 study, 228 women) (Dickersin 2007).
3.7.14 Cardiorespiratory event (RR 0.15, 95% CI 0.01 to 2.93; 1 study, 228 women) (Dickersin 2007).
3.7.15 Thromboembolic event (RR 0.21, 95% CI 0.01 to 4.42; 1 study, 228 women) (Dickersin 2007).
3.7.16 Readmission or return to surgery due to postoperative complications (RR 0.15, 95% CI 0.01 to 2.93; 1 study, 228 women) (Dickersin 2007).
3.8 Adverse events: long term (after hospital discharge)
One trial reported adverse events after hospital discharge (O'Connor 1997). There was clear evidence of a difference in sepsis rate between the two groups favouring EA/ER compared to unspecified (or at surgeon's discretion) route of hysterectomy for sepsis after discharge (RR 0.27, 95% CI 0.13 to 0.58; 172 women; Analysis 3.8) (O'Connor 1997).
3.8. Analysis.

Comparison 3: Endometrial resection/ablation versus unspecified (or at surgeon's discretion) route of hysterectomy, Outcome 8: Adverse events – long term (after hospital discharge)
There was no clear evidence of a difference between groups after hospital discharge in terms of haematoma (RR 0.97, 95% CI 0.18 to 5.11; 172 women; Analysis 3.8) (O'Connor 1997).
There were no other adverse events reported.
Secondary outcomes
3.9 Duration of surgery
Two trials reported the duration of surgery (O'Connor 1997; Pinion 1994). There was clear evidence of a difference between groups, being longer for unspecified (or at surgeon's discretion) route of hysterectomy, ranging between an MD of 16 and 31 minutes in each trial. This outcome presented a high heterogeneity (88%). Reasons for heterogeneity may have included skills of the surgeons, type of anaesthesia, characteristics of the women and the method used to record duration of the surgery (Analysis 3.9).
3.9. Analysis.

Comparison 3: Endometrial resection/ablation versus unspecified (or at surgeon's discretion) route of hysterectomy, Outcome 9: Duration of surgery (minutes)
3.10 Duration of hospital stay
Three trials evaluated duration of hospital stay (Dickersin 2007; O'Connor 1997; Pinion 1994). We were unable to pool the data due to extreme heterogeneity (100%). Despite this, all studies reported shorter hospital stays for EA compared to unspecified (or at surgeon's discretion) route of hysterectomy. Reasons for the heterogeneity may have included hospital protocols for discharge, geographic conditions and time when the trial took place (there was a clear difference in the time spent in hospital for hysterectomy in older trials compared to more recent ones).
3.11 Time to return to normal activity
One trial reported time to return to normal activity (in days) (O'Connor 1997). There was clear evidence of a difference between groups favouring EA/ER compared to unspecified (or at surgeon's discretion) route of hysterectomy (MD –18.90 days, 95% CI –24.63 to –13.17; 172 women; low‐certainty evidence; Analysis 3.11) (O'Connor 1997).
3.11. Analysis.

Comparison 3: Endometrial resection/ablation versus unspecified (or at surgeon's discretion) route of hysterectomy, Outcome 11: Time to return to normal activity (days)
3.12 Time to return to work
One trial reported time to return to work (in weeks) (O'Connor 1997). There was clear evidence of a difference between groups favouring EA/ER compared to unspecified (or at surgeon's discretion) hysterectomy by four and a half weeks (MD –4.50 weeks, 95% CI –5.49 to –3.51; 172 women; Analysis 3.12) (O'Connor 1997).
3.12. Analysis.

Comparison 3: Endometrial resection/ablation versus unspecified (or at surgeon's discretion) route of hysterectomy, Outcome 12: Time to return to work (weeks)
3.13 Total health service cost per woman
One trial assessed the total health service cost per woman (Pinion 1994). There was clear evidence of a difference between groups favouring EA/ER for the management of HMB at short‐term follow‐up compared to unspecified (or at surgeon's discretion) route of hysterectomy (the costs of EA/ER were 76% to 80% of hysterectomy) (Pinion 1994). This difference continued over a prolonged follow‐up time, but the cost gap narrowed primarily because of the retreatment rate for women who underwent endometrial resection. By four years, ablation techniques cost between 5% and 11% less than a hysterectomy (Aberdeen 1999/Pinion 1994) (Analysis 3.13).
3.13. Analysis.
Comparison 3: Endometrial resection/ablation versus unspecified (or at surgeon's discretion) route of hysterectomy, Outcome 13: Total health service cost per woman
| Total health service cost per woman | |
| Study | Details |
| Pinion 1994 |
1 year' follow‐up Mean resource cost per participant in 1994:
No statistical test used to compare the difference between groups. Mean cost of endometrial resection at 1 year' follow‐up was 76% of the cost of hysterectomy. Mean cost of laser ablation at 1 year' follow‐up was 80% of the cost of hysterectomy. Costs based on preoperative costs, nights in hospital, theatre and ward costs, general practitioner and outpatient costs, retreatment costs and technical equipment costs. 4 years' follow‐up Mean resource cost per patient in 1994:
Included additional costs for retreatment or additional procedures arising between 1 and 4 years after surgery. Data reported as 1994 rates (discounted by 6%). Sensitivity analysis performed with variations in the discount rate. Cost of endometrial ablation techniques at 4 years reported as between 89% and 95% the costs of hysterectomy. |
3.14 Total individual cost per woman
One trial measured costs to the women (Cameron 1996/Pinion 1994). At one year' follow‐up, total personal costs, in terms of travel, loss of pay and child care, were higher for women who had a hysterectomy than for women who underwent EA/ER. However, women who had a hysterectomy estimated greater savings in the cost of sanitary protection when compared with those who underwent EA/ER(savings of GBP 85.10 per year with hysterectomy versus GBP 58.30 per year with EA/ER) (Analysis 3.14).
3.14. Analysis.
Comparison 3: Endometrial resection/ablation versus unspecified (or at surgeon's discretion) route of hysterectomy, Outcome 14: Total individual cost per woman
| Total individual cost per woman | |
| Study | Details |
| Pinion 1994 |
1 year' follow‐up Mean patient cost in in 1994:
Mean cost of hysteroscopic surgery was 29% the cost of hysterectomy. Costs estimated included loss of pay, child care and travel expenses. Student t test used to compare differences between surgery groups, P < 0.05. Mean annual savings from sanitary protection after treatment in 1994:
Student t test used to compare differences between surgery groups, P < 0.05. |
Funnel plots
We were unable to include enough studies in the review for funnel plots to have sufficient power to distinguish chance from true asymmetry.
Heterogeneity
We observed a high level of heterogeneity for some outcomes.
For many outcomes, only one trial contributed data, and analysis of heterogeneity was not applicable. However, several or all studies contributed data for four outcomes and we visually inspected the forest plots for these outcomes. The estimate for proportion requiring further surgery showed a low level of heterogeneity (all CIs overlapping and I² = 0% at one and two years' follow‐up). However, there was a very high degree of heterogeneity evident for outcomes such as duration of surgery (I² = 99%), time to return to work (I² = 100%) and time to return to normal activities (I² = 97%). As discussed in each comparison the reasons could be multiple.
All trials had similar risk profiles for most measures in the 'Risk of bias' table. Therefore, we assumed that there was no significant methodological heterogeneity present.
Sensitivity analysis
We performed an analysis to investigate the effect of clear evidence of allocation concealment on effect estimates. For six of the eight trials, there was clear evidence that allocation was concealed, but for two trials (Gannon 1991; Zupi 2003), this was not the case. When we included only trials with clear evidence of allocation concealment in the meta‐analysis, there was no significant change in the estimates compared with inclusion of all trials for primary outcomes.
We also performed an analysis to investigate the effect of missing data and loss to follow‐up on the effect estimates. Two of the included studies were at unclear risk of attrition bias (Dickersin 2007; O'Connor 1997). These trials contributed to most of the outcomes in Comparison 3 (EA/ER versus unspecified (or at surgeon's discretion) route of hysterectomy). When we included only trials that had a low risk of attrition bias and compared findings with the inclusion of all trials, we found no significant change in the estimates. For the outcomes, quality of life and satisfaction rates, there were only data from the trial(s) with unclear risk of attrition bias, so no comparison could be made.
Discussion
Summary of main results
This review assessed the benefits and harms of different surgical procedures for the treatment of HMB. In this 2020 update, we split the comparison of endometrial resection/ablation (EA/ER) versus hysterectomy into three separate comparisons; EA/ER versus open hysterectomy, EA/ER versus minimally invasive hysterectomy (laparoscopic and vaginal hysterectomy), and EA/ER versus unspecified (or at surgeon's discretion) route of hysterectomy. In the latter comparison, the included trials that did not specify the route of hysterectomy or the decision was left to the operator. This distinction was made as it is now clear that the safety profile, operating time and recovery times are individual to each surgical approach of hysterectomy and, therefore, the magnitude of the difference in these outcomes was likely to differ when each of these surgical approaches was compared against EA/ER (Aarts 2015; Garry 2005).
Table 1; Table 2; and Table 3 provide summaries of the results and the certainty of the evidence.
All surgical procedures are effective in treating HMB as all reduced bleeding symptoms. However open, minimally invasive and unspecified (or at surgeon's discretion) hysterectomy all compared more favourably in terms of effectiveness than EA/ER. This was reflected in the more favourable outcomes for hysterectomy such as woman's perception of symptoms, PBAC scores and proportion requiring further surgery in all three comparisons. The indication for further surgery in the hysterectomy groups was very exceptional (being in one case because the woman received a treatment different to the one she was allocated to (EA/ER instead of hysterectomy) and in the other because it was a subtotal hysterectomy with persistent bleeding (as the uterine cervix remained in place).
At most durations of follow‐up, regardless of how bleeding symptoms were measured, women in all the hysterectomy groups showed a greater reduction in symptoms than those in the EA/ER groups. For some outcomes, it was suggested that these differences were no longer experienced at longer durations of follow‐up; this might be due to several reasons including reduced numbers, retreatment in the EA/ER group and some women reaching menopause during the follow‐up period.
In terms of quality of life, although there appeared to be some differences for various parameters of quality of life in diverse scales, there was no definitive evidence of an established pattern emerging, making it difficult to reach conclusions. The general health perception assessed using the SF‐36 at two years is probably better with the minimally invasive hysterectomy compared to the EA/ER group, but it may be similar between groups when open hysterectomy or the unspecified (or at surgeon's discretion) route of hysterectomy are compared to EA/ER. Women with minimally invasive hysterectomy probably have better quality‐of‐life scores when measured using the MMAS.
The acceptability of the treatments, measured as the proportion satisfied with treatment at one year' follow‐up, is probably higher in the group having a minimally invasive hysterectomy compared to the EA/ER group, but it was probably similar when open hysterectomy or unspecified (or at surgeon's discretion) route of hysterectomy were compared to EA/ER.
All the surgical approaches to hysterectomy and all methods of EA/ER can be regarded as safe, with low numbers of serious adverse events for all procedures. When adverse events (any serious) were considered, there was evidence of a difference between EA/ER versus open hysterectomy, or EA/ER versus minimally invasive hysterectomy. This may be because serious adverse events are rare. When EA/ER was compared against unspecified (or at surgeon's discretion) approach to hysterectomy for this outcome, the comparison favoured EA/ER.
However, when considering individual adverse events, there were some clinically important differences. Open hysterectomy and unspecified (or at surgeon's discretion) hysterectomy were both associated with a higher chance of sepsis, pyrexia, wound infection, requirement for blood transfusion and vault haematoma, whereas there was no difference in these outcomes when comparing EA/ER against minimally invasive hysterectomy. This reflects findings from other publications that show a lower risk of infection and bleeding for minimally invasive hysterectomy than for open hysterectomy (Mahdi 2014).
Three trials reported that women in the EA/ER group were more likely to have fluid overload when compared against unspecified (or at surgeon's discretion) hysterectomy (Dickersin 2007; Pinion 1994; Zupi 2003). This is less likely to be a risk with more modern EA techniques that do not require the use of a hypotonic fluid medium for uterine distension, and it is a risk only associated with EA/ER and not with hysterectomy.
Although we were unable to pool the findings from the included studies due to high heterogeneity, almost all the individual trials consistently reported a shorter duration of surgery for EA/ER than hysterectomy in all three comparisons. Heterogeneity was highest in the minimally invasive hysterectomy versus EA/ER comparison, likely reflecting the longer learning curve required for laparoscopic hysterectomy, which was a novel technique at the time when some of these trials were undertaken.
Similarly, duration of hospital stay revealed high levels of heterogeneity when EA/ER was compared against all three surgical approaches to hysterectomy. Duration of hospital stay was likely to be affected by changes outside the control of investigators in all trials. Hospital policy for maximum stay can vary significantly between hospitals and could have accounted for the heterogeneity observed in the results for this outcome. Many hospitals worldwide are adopting a policy of 'enhanced recovery' following elective surgery, including shorter times to discharge for major operations such as hysterectomy (by all surgical approaches). This high level of heterogeneity was most pronounced in the EA/ER versus minimally invasive hysterectomy comparison, and may reflect that one trial (Crosignani 1997) was much older than the other two trials (Cooper 2019; Zupi 2003), both of which showed a much smaller magnitude of difference in duration of hospital stay when compared against EA/ER than when open hysterectomy was compared against EA/ER.
There were significant differences in time to return to normal activities and work in most of the trials, but the magnitude of these differences varied, depending on which mode of hysterectomy was being compared. The outcomes could not be pooled due to high heterogeneity. However, when compared against open hysterectomy or unspecified (or at surgeon's discretion) hysterectomy, the time to return to normal activity was clearly in favour of EA/ER, but when compared against minimally invasive hysterectomy, the difference was very small (1.5 days in Zupi 2003 and 5 days in Crosignani 1997). Similarly, the magnitude of difference in time to return to work was much smaller when comparing EA/ER versus minimally invasive hysterectomy than when comparing open or unspecified (or at surgeon's discretion) hysterectomy, and no different in one trial comparing EA/ER versus laparoscopic supracervical hysterectomy (Zupi 2003).
Evaluation of comparative costs between EA/ER and all surgical approaches to hysterectomy was affected by the increasing retreatment rate. Initially, treatment costs were much lower for women undergoing EA/ER than for those undergoing any surgical approach to hysterectomy, but the difference in costs between the groups narrowed over time because of the cost of retreatment. In one study with a minimum of four years' follow‐up, EA/ER was only 5% to 11% less costly than hysterectomy compared with 24% less costly at one year' follow‐up and 29% less costly at two years' follow‐up. Again 'enhanced recovery' policies may reflect a narrower difference in duration of hospital stay and, therefore, a corresponding narrower difference in patient costs.
As described previously, outcomes may be altered by the prior experience of the surgeon for all surgical procedures. A less‐experienced surgeon for any treatment group could alter results such as duration of surgery, adverse outcomes and, in the case of EA/ER, effectiveness.
Overall completeness and applicability of evidence
The included studies adequately addressed the review question: to assess the relative effectiveness, acceptability and safety of any technique of EA/ER and hysterectomy via any route for the treatment of HMB, as well as the secondary outcomes. The inclusion and exclusion criteria were met, and the interventions well described. Unfortunately, the outcomes (particularly measures of improvement in bleeding symptoms) were measured in several different ways, and it was not possible for us to pool all estimates, making meta‐analysis impossible for some outcomes.
Quality of the evidence
This review update included 10 studies with 1966 randomly assigned participants.
All studies were at high risk of performance/detection bias, as it is not feasible to blind women or surgeons to the type of operation that was performed. Several of the older studies were at high risk of selection bias.
As the studies used many different measurements for the outcomes (particularly for effectiveness of treatment), it was not possible to pool data for every outcome. However, the results were largely comparable; both treatments were considered effective and safe, with the same rate of serious adverse events, hysterectomy was generally superior in improving symptoms and in leading to less requirement for future surgery, and EA/ER was superior in terms of shorter operating times and faster return to activities/work.
For many outcomes, assessment of heterogeneity was not relevant, as only one trial contributed data. For outcomes that displayed a high level of clinical heterogeneity, this was explained in part by differences in outcomes for different modes of hysterectomy (further described under 'Sensitivity analysis').
Certainty of evidence was low to moderate, depending on the outcome. PBAC score, adverse events and proportion satisfied with treatment generated a moderate GRADE score, suggesting that further research may change the estimate. Outcomes that generated a low GRADE score included the PBAC score at one year' follow‐up and sepsis as an adverse event both for high risk of performance bias, and either imprecision or inconsistency. For these two outcomes, further evidence is likely to change the estimate.
Potential biases in the review process
Electronic searches in combination with handsearches performed by the review authors identified all relevant studies known to be available currently. Our inability to pool data for particular outcomes was likely to decrease the power of results; this may change if future studies are able to add data to the existing outcomes.
Agreements and disagreements with other studies or reviews
Bhattacharya 2011 performed a systematic review that compared clinical‐effectiveness and cost‐effectiveness analyses of hysterectomy, EA/ER and levonorgestrel‐releasing intrauterine device (LNG‐IUS) for HMB. These findings were in accordance with the findings of this review; satisfaction rates at 12 months were highest for hysterectomy, and rates of further surgery after EA/ER were comparable (8.5%). The authors also noted longer hospital stay and longer time to return to normal activities for hysterectomy.
Women who have undergone hysterectomy have experienced more postoperative complications than women who have received EA/ER, although for some types of complications, there were no differences. Because some complications are rare, these rates are best compared with those of large audits, such as the Mistletoe audit in the UK (Overton 1997). The low rate of complications for EA/ER in this review confirms the findings of the Mistletoe study.
A comparison of costs of EA/ER techniques versus those of hysterectomy cannot adequately provide information on the relative value in terms of money of these two surgical procedures. The availability of EA/ER as a treatment for HMB may result in an earlier recourse to surgery than a woman would have considered if the only surgery available was hysterectomy (Bridgman 1994; Coulter 1994), and this will have a significant impact on costs. Additional studies have provided assessments of cost‐effectiveness based on Dwyer 1993 through a cost‐utility analysis (Sculpher 1998); preference‐based treatment allocation (woman allocated to the treatment that she prefers) (Sculpher 1998); and four other cost‐utility analyses (Clegg 2007; Garside 2004; Roberts 2011; You 2006). Garside 2004 concluded that abdominal hysterectomy is likely to be more cost‐effective than endometrial resection if healthcare purchasers are willing to pay an additional cost of at least GBP 6500 per extra quality‐adjusted life‐year (QALY) generated by hysterectomy, although an area of uncertainty is attached to this conclusion in the form of variation in the parameters used in the analysis. Clegg 2007 found that a preference‐based treatment allocation was more cost‐effective than reliance on a single intervention for all women requiring surgery for HMB. Garside 2004 reported that hysterectomy was more expensive than EA but accrued more QALYs over 10 years. The incremental cost per QALY of hysterectomy compared with two second‐generation EA techniques was approximately GBP 2000. You 2006 also confirmed that hysterectomy was a more expensive option than EA (cost per woman: USD 6878 with hysterectomy versus USD 6185 with EA over five years) but was more effective (4.725 QALYs with hysterectomy versus 4.624 QALYs with EA). Roberts 2011 found that hysterectomy produced more QALYs relative to second‐generation EA, with an incremental cost‐effectiveness ratio of GBP 970 per additional QALY. Data from a cost‐utility analysis in Clegg 2007 contradicted this finding: the authors reported that second‐generation EA accrued marginally more QALYs than hysterectomy over five years (4.13 with EA versus 4.01 QALYs with hysterectomy). Evaluation of the comparative cost‐effectiveness of endometrial destruction techniques and hysterectomy is complex, and the simple conclusion that endometrial destruction is cheaper than hysterectomy, with the difference narrowing over time, may not represent an adequate economic assessment.
Overall, the review findings were consistent with currently accepted clinical advice and agree with current guidelines. National Institute for Health and Care Excellence (NICE) guidelines for menorrhagia suggest that hysterectomy should not be used as a first‐line treatment for benign HMB and should be considered only when other treatment options have failed, are contraindicated or are declined by the woman; when there is a wish for amenorrhoea; when the woman (who has been fully informed) requests it; when the woman no longer wishes to retain her uterus and fertility; or a combination of these (NICE 2007; NICE 2018). NICE 2007 and NICE 2018 guidelines are in agreement with the findings of this review with regard to potential complications associated with both modes of surgical treatment.
Authors' conclusions
Implications for practice.
Although hysterectomy is more effective at resolving bleeding problems and satisfaction rates are higher, endometrial destruction by ablation is an alternative to hysterectomy that could be offered to women with heavy menstrual bleeding.
Ablation techniques have high satisfaction rates, even though lower than hysterectomy at one and two years' follow‐up, with shorter operation time and hospital stay and earlier recovery. The shorter recovery varies according to the route of the hysterectomy. There was no difference in serious complications between procedures. Some complications were more frequent in the hysterectomy group such as sepsis, blood transfusion and haematoma (vault and wound). A preoperative discussion should focus on the possibility of further surgery being necessary for those women who choose endometrial destruction techniques for relief of their heavy menstrual bleeding.
The initial cost of endometrial destruction is clearly lower than that of hysterectomy, but because retreatment is often necessary, the cost difference narrows over time.
Minimally invasive hysterectomy (vaginal or laparoscopic) represents an improvement on several of the previously described disadvantages of hysterectomy, and some outcomes, such as duration of hospital stay, time to return to work and time to return to normal activities, have become more comparable with those of endometrial ablation. However, it should be noted that laparoscopic hysterectomy may be associated with longer operating time than other modes of hysterectomy and requires specific surgical expertise and equipment.
It is recommended that women be encouraged to play an active role in selecting the type of surgery, based on the personal value that they place on the advantages and disadvantages of each surgical approach.
Implications for research.
Additional trials are needed in the following areas.
Comparison of more recent types of hysterectomy (supracervical, vaginal and laparoscopically assisted vaginal) versus EA/ER techniques. One further trial in this category was added in the 2013 update (Sesti 2011); and another at the 2020 update (Cooper 2019); however, more data are needed to reduce heterogeneity for some outcomes. These trials did not reported data for cost comparisons, and this is likely to be important in making a treatment decision.
Comparison of second‐generation ablation methods versus hysterectomy. Evidence suggests that whereas first‐generation ablation methods did not have an impact on hysterectomy rates for heavy menstrual bleeding, second‐generation methods have overtaken first‐generation methods in the UK and are now the most common operation for heavy menstrual bleeding (Reid 2007). One further trial in this category was added in the 2013 update (Sesti 2011).
Trials with follow‐up of four years or longer to adequately assess the cost differential between the two types of surgery and the requirement for further surgical treatment in women randomly assigned to transcervical resection of the endometrium or to endometrial ablation, even though this may cause confusion with the proportion of women going through menopause.
Trials that incorporate 'enhanced recovery' programmes. In the future, findings of these trials may reflect shorter hospital stay for women undergoing hysterectomy, and therefore a smaller difference between the two procedures for this particular outcome.
What's new
| Date | Event | Description |
|---|---|---|
| 29 January 2021 | New citation required and conclusions have changed | The conclusions of this review have changed following the restructure of review comparisons and addition of 1 study (Cooper 2019). |
| 29 January 2021 | New search has been performed | The review was updated and restructured by splitting the comparison of endometrial resection and ablation (EA) vs hysterectomy into 3 comparisons: EA vs open hysterectomy, EA vs minimally invasive hysterectomy, and EA vs unspecified route of hysterectomy or at surgeon's discretion. |
History
Protocol first published: Issue 3, 1996 Review first published: Issue 2, 1999
| Date | Event | Description |
|---|---|---|
| 20 September 2019 | New citation required and conclusions have changed | The inclusion of data from 1 new study changed the conclusions of this review. |
| 20 September 2019 | New search has been performed | Updated; 1 new trial (Cooper 2019) added. |
| 1 November 2013 | New search has been performed | One new study added: Sesti 2011. |
| 1 November 2013 | New citation required but conclusions have not changed | One new study added: Sesti 2011. |
| 15 January 1999 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
The authors of the 2020 update acknowledge the contribution of Professor Cindy Farquhar and Dr Sasha Shepperd to previous versions of this review. They thank Gabriela Cooper, Andy Watson, Gaity Ahmad and Justin Clark for providing peer review comments. They also thank Marian Showell (Information Specialist) and Helen Nagels (Managing Editor) at the Cochrane Gynaecology and Fertility Group's editorial base for their time and support. Their thanks go also to Professor Cindy Farquhar and Dr Justin Clark for providing referee comment on the draft.
The authors of the 2013 update acknowledged the contribution of Dr Inez Cooke, who wrote the protocol for the 2008 review.
The review authors acknowledge the helpful comments of those who refereed previous versions of this review. We are especially grateful to Professor Pier Crosignani, Dr Kay Dickerson and Dr Sbracia, who answered queries and provided additional material for this review. Special thanks are also due to Helen Nagels, Managing Editor of the Menstrual Disorders and Subfertility Group, for her professionalism and help with the inevitable problems that arose; and to Marion Showell, Trials Search Co‐ordinator, for her assistance in identifying trials.
Appendices
Appendix 1. Cochrane Gynaecology and Fertility Group specialised register search strategy
ProCite platform
Searched 29 July 2020
Keywords CONTAINS "menorrhagia" or "heavy bleeding" or "heavy menstrual bleeding" or "heavy menstrual loss" or "dysfunctional uterine bleeding" or "dysfunctional bleeding" or Title CONTAINS "menorrhagia" or "heavy bleeding "or "heavy menstrual bleeding" or "heavy menstrual loss" or "dysfunctional uterine bleeding" or "dysfunctional bleeding"
AND
Keywords CONTAINS "Hysterectomy" or "Hysterectomy, abdominal" or "hysterectomy, laparoscopically assisted vaginal" or "Hysterectomy, subtotal" or "Hysterectomy, Vaginal" or "subtotal hysterectomy" or "abdominal hysterectomy" or "laparoscopic assisted vaginal hysterectomy" or "laparoscopic hysterectomy" or "vaginal hysterectomy" or "hysterectomy, laparoscopic" or Title CONTAINS "Hysterectomy" or "Hysterectomy, abdominal" or "hysterectomy, laparoscopically assisted vaginal" or "Hysterectomy, subtotal" or "Hysterectomy, Vaginal" or "subtotal hysterectomy" or "abdominal hysterectomy" or "laparoscopic assisted vaginal hysterectomy" or "laparoscopic hysterectomy" or "vaginal hysterectomy" or "hysterectomy, laparoscopic
(100 records)
Appendix 2. CENTRAL via the Cochrane Register of Studies Online (CRSO) search strategy
Web platform
Searched 29 July 2020
#1 MESH DESCRIPTOR menorrhagia EXPLODE ALL TREES 371
#2 menorrhag*:TI,AB,KY 946
#3 (menstrua* adj5 (bleed* or blood)):TI,AB,KY 1263
#4 (heavy adj5 menstrua*):TI,AB,KY 372
#5 (dysfunctional adj5 uter*):TI,AB,KY 184
#6 hypermenorrh*:TI,AB,KY 33
#7 #1 OR #2 OR #3 OR #4 OR #5 OR #6 1967
#8 MESH DESCRIPTOR Hysterectomy EXPLODE ALL TREES 1751
#9 hysterectom*:TI,AB,KY 6027
#10 (uter* adj5 excis*):TI,AB,KY 12
#11 #8 OR #9 OR #10 6036
#12 #7 AND #11 280
Appendix 3. MEDLINE search strategy
Ovid platform
Searched from 1946 to 29 July 2020
1 menorrhagia/ (4235) 2 menorrhag$.tw. (3321) 3 (menstrua$ adj5 (bleed$ or blood)).tw. (4864) 4 (heavy adj5 menstrua$).tw. (1118) 5 (dysfunctional adj5 uter$).tw. (1028) 6 hypermenorrh$.tw. (299) 7 or/1‐6 (10572) 8 exp hysterectomy/ or hysterectomy, vaginal/ (30707) 9 hysterectom$.tw. (36316) 10 (uter$ adj5 excis$).tw. (478) 11 or/8‐10 (48746) 12 7 and 11 (1783) 13 exp Electrocoagulation/ (11929) 14 exp Endometrium/su [Surgery] (1267) 15 Laser Coagulation/ (7558) 16 (endometri$ adj5 (ablat$ or excis$ or laser or electrocautery or destruct$ or radiofrequency)).tw. (2558) 17 (endometri$ adj5 resect$).tw. (1442) 18 (electrosurgery or thermal balloon or hypertherm$ or thermotherapy or photodynamic therapy or phototherapy or cryoablation or microwave ablation).tw. (70933) 19 (Thermachoice or rollerball).tw. (199) 20 or/13‐19 (93359) 21 12 and 20 (657) 22 randomized controlled trial.pt. (510180) 23 controlled clinical trial.pt. (93773) 24 randomized.ab. (487266) 25 placebo.tw. (215420) 26 clinical trials as topic.sh. (192241) 27 randomly.ab. (337802) 28 trial.ti. (222329) 29 (crossover or cross‐over or cross over).tw. (85521) 30 or/22‐29 (1334171) 31 exp animals/ not humans.sh. (4720834) 32 30 not 31 (1226757) 33 21 and 32 (100)
Appendix 4. Embase search strategy
Ovid platform
Searched from 1980 to 29 July 2020
1 menorrhagia/ (9678) 2 menorrhag$.tw. (5363) 3 (menstrua$ adj5 (bleed$ or blood)).tw. (6475) 4 (heavy adj5 menstrua$).tw. (1981) 5 (dysfunctional adj5 uter$).tw. (1239) 6 hypermenorrh$.tw. (416) 7 or/1‐6 (16136) 8 exp hysterectomy/ or hysterectomy, vaginal/ (69814) 9 hysterectom$.tw. (54719) 10 (uter$ adj5 excis$).tw. (692) 11 or/8‐10 (79142) 12 7 and 11 (3764) 13 electrosurgery/ or exp electrocoagulation/ or laser surgery/ (39881) 14 exp laser coagulation/ (20281) 15 endometrium ablation/ (2651) 16 (endometri$ adj5 (ablat$ or excis$ or laser or electrocautery or destruct$ or radiofrequency)).tw. (4551) 17 (endometri$ adj5 resect$).tw. (2470) 18 (electrosurgery or thermal balloon or hypertherm$ or thermotherapy or photodynamic therapy or phototherapy or cryoablation or microwave ablation).tw. (87557) 19 (Thermachoice or rollerball).tw. (325) 20 or/13‐19 (149764) 21 12 and 20 (1204) 22 Clinical Trial/ (969550) 23 Randomized Controlled Trial/ (609751) 24 exp randomization/ (87563) 25 Single Blind Procedure/ (39646) 26 Double Blind Procedure/ (171539) 27 Crossover Procedure/ (63757) 28 Placebo/ (339142) 29 Randomi?ed controlled trial$.tw. (233331) 30 Rct.tw. (37857) 31 random allocation.tw. (2029) 32 randomly allocated.tw. (35551) 33 allocated randomly.tw. (2558) 34 (allocated adj2 random).tw. (818) 35 Single blind$.tw. (24962) 36 Double blind$.tw. (203896) 37 ((treble or triple) adj blind$).tw. (1163) 38 placebo$.tw. (304749) 39 prospective study/ (615309) 40 or/22‐39 (2209791) 41 case study/ (70868) 42 case report.tw. (407312) 43 abstract report/ or letter/ (1108216) 44 or/41‐43 (1575613) 45 40 not 44 (2155908) 46 21 and 45 (294)
Appendix 5. PsycINFO search strategy
Ovid platform
Searched from 1806 to 29 July 2020
1 exp menstrual disorders/ (1265) 2 menorrhag$.tw. (87) 3 (menstrua$ adj5 (bleed$ or blood)).tw. (260) 4 (heavy adj5 menstrua$).tw. (33) 5 (dysfunctional adj5 uter$).tw. (26) 6 hypermenorrh$.tw. (2) 7 or/1‐6 (1561) 8 exp Hysterectomy/ (446) 9 hysterectom$.tw. (824) 10 (uter$ adj5 excis$).tw. (4) 11 or/8‐10 (850) 12 7 and 11 (35)
Appendix 6. Additional searches
Google, Google Scholar PubMed, and the trial registries, were searched using the key words 'endometrial ablation', 'endometrial resection', 'hysterectomy', 'heavy menstrual bleeding', 'menorrhagia', 'disordered uterine bleeding' and 'abnormal uterine bleeding'.
Data and analyses
Comparison 1. Endometrial resection/ablation versus open hysterectomy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Woman's perception (proportion with improvement in bleeding symptoms) | 2 | 247 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.84, 0.95] |
| 1.1.1 Within 1 year' follow‐up | 2 | 247 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.84, 0.95] |
| 1.2 Quality‐of‐life scores (continuous data) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.2.1 SF‐36 at 2 years – pain | 1 | 155 | Mean Difference (IV, Fixed, 95% CI) | ‐9.90 [‐17.63, ‐2.17] |
| 1.2.2 SF‐36 at 2 years – general health perception | 1 | 155 | Mean Difference (IV, Fixed, 95% CI) | ‐5.30 [‐11.90, 1.30] |
| 1.2.3 SF‐36 at 2 years – role limitation (physical) | 1 | 155 | Mean Difference (IV, Fixed, 95% CI) | 0.70 [‐9.82, 11.22] |
| 1.2.4 SF‐36 at 2 years – role limitation (emotional) | 1 | 155 | Mean Difference (IV, Fixed, 95% CI) | ‐6.20 [‐15.87, 3.47] |
| 1.2.5 SF‐36 at 2 years – social functioning | 1 | 155 | Mean Difference (IV, Fixed, 95% CI) | ‐6.00 [‐12.11, 0.11] |
| 1.2.6 SF‐36 at 2 years – mental health | 1 | 155 | Mean Difference (IV, Fixed, 95% CI) | ‐2.30 [‐7.49, 2.89] |
| 1.2.7 SF‐36 at 2 years – energy | 1 | 155 | Mean Difference (IV, Fixed, 95% CI) | ‐1.50 [‐8.18, 5.18] |
| 1.2.8 SF‐36 at 2 years – physical functioning | 1 | 155 | Mean Difference (IV, Fixed, 95% CI) | ‐2.50 [‐7.56, 2.56] |
| 1.2.9 EuroQol score within 1 year after surgery | 1 | 137 | Mean Difference (IV, Fixed, 95% CI) | ‐7.00 [‐17.29, 3.29] |
| 1.2.10 EuroQol scores at 2 years after surgery | 1 | 155 | Mean Difference (IV, Fixed, 95% CI) | ‐1.50 [‐6.29, 3.29] |
| 1.3 Quality of life (proportion with improvement) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.3.1 Proportion with improvement in pain 2 years after surgery | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.60 [0.55, 4.63] |
| 1.4 Requirement for further surgery for treatment failure | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.4.1 Within 1 year after surgery | 2 | 247 | Risk Ratio (M‐H, Fixed, 95% CI) | 17.03 [2.29, 126.72] |
| 1.4.2 At 2 years after surgery | 1 | 196 | Risk Ratio (M‐H, Fixed, 95% CI) | 63.70 [3.96, 1025.88] |
| 1.5 Proportion satisfied with treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.5.1 At 1 year' follow‐up | 1 | 194 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.82, 1.00] |
| 1.5.2 At 2 years' follow‐up | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.73, 0.93] |
| 1.6 Any serious adverse event | 2 | 247 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.32, 5.20] |
| 1.7 Adverse events – short term (intraoperative and immediate postoperative) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.7.1 Sepsis | 2 | 247 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.02, 0.25] |
| 1.7.2 Pyrexia | 1 | 196 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.02, 0.28] |
| 1.7.3 Vault haematoma | 2 | 247 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.03, 0.86] |
| 1.7.4 Blood transfusion | 1 | 196 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.07, 1.58] |
| 1.8 Adverse events – long term (after hospital discharge) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.8.1 Haematoma | 1 | 196 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.01, 4.03] |
| 1.8.2 Haemorrhage | 1 | 196 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.94 [0.12, 71.30] |
| 1.9 Duration of surgery (minutes) | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.10 Duration of hospital stay (days) | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.11 Time to return to normal activity (days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.12 Time to return to work (weeks) | 2 | 245 | Mean Difference (IV, Fixed, 95% CI) | ‐7.72 [‐8.05, ‐7.38] |
| 1.13 Total health service cost per woman | 2 | Other data | No numeric data |
Comparison 2. Endometrial resection/ablation versus minimally invasive hysterectomy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Woman's perception (proportion with improvement in bleeding symptoms) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1.1 At 2 years' follow‐up | 1 | 79 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.90, 1.04] |
| 2.2 PBAC score (continuous data) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.2.1 At 1 year' follow‐up | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | 24.40 [16.01, 32.79] |
| 2.2.2 At 2 years' follow‐up | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | 44.00 [36.09, 51.91] |
| 2.3 Quality‐of‐life scores (continuous data) | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.3.1 SF‐36 at 1 year – social functioning | 1 | 181 | Mean Difference (IV, Fixed, 95% CI) | ‐21.20 [‐24.73, ‐17.67] |
| 2.3.2 SF‐36 at 1 year – energy | 1 | 181 | Mean Difference (IV, Fixed, 95% CI) | ‐11.30 [‐14.82, ‐7.78] |
| 2.3.3 SF‐36 at 1 year – general health perception | 1 | 181 | Mean Difference (IV, Fixed, 95% CI) | ‐9.80 [‐13.86, ‐5.74] |
| 2.3.4 SF‐36 at 2 years – social functioning | 2 | 145 | Mean Difference (IV, Fixed, 95% CI) | ‐12.02 [‐16.26, ‐7.77] |
| 2.3.5 SF‐36 at 2 years – general health perception | 2 | 145 | Mean Difference (IV, Fixed, 95% CI) | ‐10.71 [‐15.11, ‐6.30] |
| 2.3.6 SF‐36 at 2 years – pain | 2 | 145 | Mean Difference (IV, Fixed, 95% CI) | ‐12.68 [‐17.09, ‐8.27] |
| 2.3.7 SF‐36 at 2 years – physical functioning | 2 | 145 | Mean Difference (IV, Fixed, 95% CI) | ‐15.59 [‐20.47, ‐10.72] |
| 2.3.8 SF‐36 at 2 years – role limitation (emotional) | 2 | 145 | Mean Difference (IV, Fixed, 95% CI) | 15.41 [9.97, 20.85] |
| 2.3.9 SF‐36 at 2 years – mental health | 2 | 145 | Mean Difference (IV, Fixed, 95% CI) | 8.72 [4.30, 13.14] |
| 2.3.10 SF‐36 at 1 year – role limitation (physical) | 1 | 181 | Mean Difference (IV, Fixed, 95% CI) | ‐0.80 [‐4.99, 3.39] |
| 2.3.11 SF‐36 at 1 year – role limitation (emotional) | 1 | 181 | Mean Difference (IV, Fixed, 95% CI) | ‐3.90 [‐8.21, 0.41] |
| 2.3.12 SF‐36 at 1 year – mental health | 1 | 181 | Mean Difference (IV, Fixed, 95% CI) | ‐2.70 [‐6.84, 1.44] |
| 2.3.13 SF‐36 at 1 year – pain | 1 | 181 | Mean Difference (IV, Fixed, 95% CI) | ‐1.50 [‐6.05, 3.05] |
| 2.3.14 SF‐36 at 1 year – physical functioning | 1 | 181 | Mean Difference (IV, Fixed, 95% CI) | ‐1.20 [‐5.34, 2.94] |
| 2.3.15 SF‐36 at 2 years – energy | 2 | 145 | Mean Difference (IV, Fixed, 95% CI) | 4.49 [‐0.36, 9.33] |
| 2.3.16 SF‐36 at 2 years – role limitation (physical) | 2 | 145 | Mean Difference (IV, Fixed, 95% CI) | ‐4.12 [‐9.58, 1.35] |
| 2.3.17 SSR score at 2 years after surgery | 1 | 77 | Mean Difference (IV, Fixed, 95% CI) | ‐3.70 [‐11.17, 3.77] |
| 2.3.18 Total HADS score at 2 years after surgery | 1 | 77 | Mean Difference (IV, Fixed, 95% CI) | ‐1.50 [‐4.32, 1.32] |
| 2.3.19 Anxiety HADS score at 2 years after surgery | 1 | 77 | Mean Difference (IV, Fixed, 95% CI) | ‐1.60 [‐3.28, 0.08] |
| 2.3.20 Depression HADS score at 2 years after surgery | 1 | 77 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐2.28, 1.08] |
| 2.3.21 SF‐12 physical score at 1 year' follow‐up | 1 | 435 | Mean Difference (IV, Fixed, 95% CI) | ‐1.10 [‐2.78, 0.58] |
| 2.3.22 SF‐12 mental score at 1 year' follow‐up | 1 | 435 | Mean Difference (IV, Fixed, 95% CI) | ‐1.90 [‐4.00, 0.20] |
| 2.4 Proportion with MMAS = 100 (best possible) 15 months after randomisation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.5 Quality of life at 14 years' follow‐up | 1 | Other data | No numeric data | |
| 2.6 Requirement for further surgery for treatment failure | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.6.1 Within 1 year after surgery | 4 | 922 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.70 [2.54, 23.32] |
| 2.6.2 At 2 years after surgery | 3 | 334 | Risk Ratio (M‐H, Fixed, 95% CI) | 16.75 [2.24, 125.34] |
| 2.6.3 At 14 years after surgery | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 19.60 [1.15, 333.63] |
| 2.7 Proportion satisfied with treatment | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.7.1 At 1 year' follow‐up | 1 | 558 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.85, 0.94] |
| 2.7.2 At 2 years' follow‐up | 1 | 77 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.79, 1.06] |
| 2.8 Any serious adverse event | 4 | 809 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.35, 1.59] |
| 2.9 Adverse events – short term (intraoperative and immediate postoperative) | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.9.1 Sepsis | 1 | 616 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.29, 3.44] |
| 2.9.2 Blood transfusion | 3 | 837 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.11, 4.08] |
| 2.9.3 Pyrexia | 2 | 797 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.24, 2.27] |
| 2.9.4 Vault haematoma | 1 | 181 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.01, 4.25] |
| 2.9.5 Fluid overload | 1 | 181 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.37 [0.64, 202.59] |
| 2.9.6 Haemorrhage | 2 | 797 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.27, 2.74] |
| 2.9.7 Perforation | 1 | 616 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.02 [0.32, 28.87] |
| 2.9.8 Laparotomy | 1 | 181 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.05, 5.60] |
| 2.9.9 Cystotomy | 1 | 616 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.20] |
| 2.9.10 Cervical laceration | 1 | 181 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.10 [0.13, 75.10] |
| 2.10 Duration of surgery (minutes) | 5 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.11 Duration of hospital stay (days) | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.12 Time to return to normal activity (days) | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.13 Time to return to work (weeks) | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only |
Comparison 3. Endometrial resection/ablation versus unspecified (or at surgeon's discretion) route of hysterectomy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Woman's perception (proportion with improvement in bleeding symptoms) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1.1 Within 1 year' follow‐up | 2 | 403 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.83, 0.95] |
| 3.1.2 At 2 years' follow‐up | 1 | 213 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.82, 0.99] |
| 3.1.3 At 4 years' follow‐up | 2 | 237 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.88, 0.99] |
| 3.2 Quality‐of‐life scores (continuous data) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.2.1 SF‐36 at 1 year – mental health | 1 | 204 | Mean Difference (IV, Fixed, 95% CI) | 1.60 [‐5.17, 8.37] |
| 3.2.2 SF‐36 at 1 year – energy | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐2.30 [‐20.90, 16.30] |
| 3.2.3 SF‐36 at 1 year – pain | 1 | 210 | Mean Difference (IV, Fixed, 95% CI) | ‐2.80 [‐9.53, 3.93] |
| 3.2.4 SF‐36 at 1 year – general health perception | 1 | 204 | Mean Difference (IV, Fixed, 95% CI) | ‐0.70 [‐7.24, 5.84] |
| 3.2.5 SF‐36 at 2 years – pain | 1 | 213 | Mean Difference (IV, Fixed, 95% CI) | ‐2.40 [‐8.86, 4.06] |
| 3.2.6 SF‐36 at 2 years – general health perception | 1 | 209 | Mean Difference (IV, Fixed, 95% CI) | ‐1.90 [‐8.67, 4.87] |
| 3.2.7 SF‐36 at 2 years – energy | 1 | 213 | Mean Difference (IV, Fixed, 95% CI) | ‐15.60 [‐22.47, ‐8.73] |
| 3.2.8 GR inventory scores at 1 year after surgery | 1 | 182 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐1.75, 1.75] |
| 3.2.9 EuroQol score within 1 year after surgery | 1 | 210 | Mean Difference (IV, Fixed, 95% CI) | ‐2.00 [‐7.90, 3.90] |
| 3.2.10 EuroQol scores at 2 years after surgery | 1 | 213 | Mean Difference (IV, Fixed, 95% CI) | ‐2.60 [‐8.20, 3.00] |
| 3.2.11 Anxiety HADS scores at 1 year after surgery | 1 | 182 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐1.39, 0.99] |
| 3.2.12 Depression HADS scores at 1 year after surgery | 1 | 182 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.09, 0.09] |
| 3.3 Quality of life (proportion with improvement) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.3.1 Proportion with improvement in general health 1 year after surgery | 1 | 185 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.77, 0.95] |
| 3.3.2 Proportion with improvement in general health 4 years after surgery | 1 | 146 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.80, 1.00] |
| 3.4 Requirement for further surgery for treatment failure | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.4.1 Within 1 year after surgery | 2 | 374 | Risk Ratio (M‐H, Fixed, 95% CI) | 28.19 [4.25, 187.22] |
| 3.4.2 At 2 years' follow‐up | 2 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 35.70 [5.30, 240.55] |
| 3.4.3 At 3 years' follow‐up | 1 | 172 | Risk Ratio (M‐H, Fixed, 95% CI) | 22.90 [1.42, 370.26] |
| 3.4.4 At 4 years' follow‐up | 1 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 73.63 [4.59, 1181.42] |
| 3.5 Proportion satisfied with treatment | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.5.1 At 1 year' follow‐up | 3 | 545 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.88, 1.04] |
| 3.5.2 At 2 years' follow‐up | 2 | 337 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.77, 1.03] |
| 3.5.3 At 4 years' follow‐up | 2 | 246 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.77, 1.03] |
| 3.6 Any serious adverse event | 2 | 374 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.06, 0.80] |
| 3.7 Adverse events – short term (intraoperative and immediate postoperative) | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.7.1 Sepsis | 2 | 374 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.17, 0.44] |
| 3.7.2 Blood transfusion | 2 | 374 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.02, 0.70] |
| 3.7.3 Pyrexia | 1 | 228 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.08, 0.61] |
| 3.7.4 Vault haematoma | 2 | 430 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.02, 0.42] |
| 3.7.5 Wound haematoma | 1 | 202 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.03 [0.00, 0.53] |
| 3.7.6 Fluid overload | 2 | 430 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.59 [1.59, 46.36] |
| 3.7.7 Haemorrhage | 2 | 374 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.24, 1.57] |
| 3.7.8 Anaesthetic | 1 | 202 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.01, 3.80] |
| 3.7.9 Perforation | 2 | 430 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.05 [0.61, 42.16] |
| 3.7.10 Gastrointestinal obstruction/ileus | 1 | 202 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.04, 5.01] |
| 3.7.11 Laparotomy | 1 | 202 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.03, 2.91] |
| 3.7.12 Cystotomy | 1 | 228 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.01, 4.42] |
| 3.7.13 Cervical laceration | 1 | 228 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.22 [0.13, 78.13] |
| 3.7.14 Cardiorespiratory event | 1 | 228 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.01, 2.93] |
| 3.7.15 Thromboembolic event | 1 | 228 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.01, 4.42] |
| 3.7.16 Readmission/return to surgery | 1 | 228 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.01, 2.93] |
| 3.8 Adverse events – long term (after hospital discharge) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.8.1 Sepsis | 1 | 172 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.13, 0.58] |
| 3.8.2 Haematoma | 1 | 172 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.18, 5.11] |
| 3.9 Duration of surgery (minutes) | 2 | 374 | Mean Difference (IV, Fixed, 95% CI) | ‐19.73 [‐24.35, ‐15.10] |
| 3.10 Duration of hospital stay (days) | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.11 Time to return to normal activity (days) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.12 Time to return to work (weeks) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.13 Total health service cost per woman | 1 | Other data | No numeric data | |
| 3.14 Total individual cost per woman | 1 | Other data | No numeric data |
3.10. Analysis.

Comparison 3: Endometrial resection/ablation versus unspecified (or at surgeon's discretion) route of hysterectomy, Outcome 10: Duration of hospital stay (days)
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Cooper 2019.
| Study characteristics | ||
| Methods | Multicentre randomised controlled trial Setting: 31 hospitals in the UK May 2014 to September 2017 |
|
| Participants | Women aged < 50 years with no desire for more children referred to a gynaecologist for surgical treatment of HMB Inclusion criteria: eligibility for EA (fibroids < 3 cm, uterine cavity size < 11 cm, and absence of endometrial pathology on biopsy); normal cervical cytology Exclusion criteria: previous EA or laparoscopic surgery; unable to give informed consent or complete trial paperwork |
|
| Interventions | Treatment 1: laparoscopic supracervical hysterectomy; total randomised: 309 women Treatment 2: second‐generation EA (either thermal balloon or radiofrequency); total randomised: 307 women |
|
| Outcomes | Primary outcomes
Secondary outcomes Patient reported
Clinical
Economic
|
|
| Notes | Funding: UK National Institute for Health Research Health Technology Assessment Programme ISRCTN49013893; prospective registration 28 January 2014 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Telephone system or internet application with minimisation algorithm. |
| Allocation concealment (selection bias) | Low risk | Women were randomly assigned by either an Interactive Voice Response telephone system or an internet‐based application with a minimisation algorithm based on centre and age group. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Surgeons and participants could not be blinded to the allocated procedure because of the nature of the interventions. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropouts, similar in both groups. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported as per protocol. |
| Other bias | Low risk | No differences between groups at baseline. Funding: UK National Institute for Health Research Health Technology Assessment Programme. |
Crosignani 1997.
| Study characteristics | ||
| Methods | Randomisation by computer‐generated sequence using numbered opaque sealed envelopes Single‐centre, parallel‐group with no blinding Setting: outpatient clinic in Milan, Italy Number of women randomly assigned: 92 Number of withdrawals: 7 (4 in the resection group: 1 refused treatment, 1 underwent surgery in another hospital, 1 did not want further treatments and 1 was lost to follow‐up before surgery; 3 in the hysterectomy group: 1 refused treatment, 1 had surgery in another hospital and 1 decided on long‐term medical treatment) Power calculation for sample size performed and analysis by intention to treat |
|
| Participants | Women aged 42–49 years, with menorrhagia not responding to medical treatment and requiring hysterectomy Inclusion criteria: aged ≤ 50 years; mobile uterus with volume < 12 weeks in gestational size and < 380 mL on ultrasound; negative cervical smear; no evidence of atypical hyperplasia at endometrial biopsy; no adnexal tumours at clinical and ultrasound examination Exclusion criteria: known PID or endometriosis; urinary stress incontinence; moderate/severe genital prolapse; clotting disorders; use of IUD or drugs that may affect MBL; unstable general conditions; submucous myomas > 3 cm in diameter or > 50% intramural extension |
|
| Interventions | Treatment 1: hysteroscopic endometrial resection; total randomised: 41 women Treatment 2: vaginal hysterectomy total randomised: 44 women Duration: 2 years' follow‐up Prior experience of the surgeon not mentioned |
|
| Outcomes |
|
|
| Notes | Author contacted for additional information and reply received. Source of funding not reported No information stated regarding trial registration |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation sequence. |
| Allocation concealment (selection bias) | Low risk | Numbered opaque sealed envelopes. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not feasible for a comparison of surgical techniques. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Clear reasons given for withdrawal – appeared balanced in numbers between groups. |
| Selective reporting (reporting bias) | Unclear risk | Unclear – no protocol identified. Study did not measure adverse events. |
| Other bias | Low risk | Groups appeared balanced at baseline (although the table did not include dropouts), and no other biases identified |
Dickersin 2007.
| Study characteristics | ||
| Methods | Randomisation by computer‐generated permuted blocks of random size stratified by age and site Multicentre, parallel‐group, single‐blinded (only assessors) Setting: 25 clinical centres in the USA and Canada Number of women randomly assigned: 237 Number of withdrawals: at 1 year: 16 in EA group, 11 in hysterectomy group; at 2 years: 17 in EA group, 7 in hysterectomy group Power calculation for sample size (90% power based on quality‐of‐life measures) but recruitment did not quite achieve the required sample size Analysis by intention to treat for primary bleeding and satisfaction outcomes. Analysis not by intention to treat for assessment of intraoperative and perioperative events |
|
| Participants | Women with dysfunctional bleeding (not explained by pathology, drugs, etc.), most of whom aged < 45 years of age (85%) Inclusion criteria: aged ≥ 18 years; premenopausal; dysfunctional uterine bleeding for ≥ 6 months (defined as ≥ 1 of excess duration, amount or unpredictability); refractory to medical treatment for ≥ 3 months Exclusion criteria: postmenopausal; bilateral oophorectomy; pregnant; wishing to retain fertility; refusal to consider surgery |
|
| Interventions | Treatment 1: resectoscopic EA with electrodesiccation/coagulation or vaporisation or ablation with thermal balloon: 123 women Treatment 2: vaginal, laparoscopic, or abdominal hysterectomy under general or regional anaesthesia: 114 women In both groups, women > 45 years were allowed oophorectomy Duration of trial: enrolment was staggered, with some women having data for 5 years Prior experience of the surgeon not mentioned |
|
| Outcomes |
|
|
| Notes | Author contacted for clarification of some points and reply received. Source of funding: AHRQ and Brown Medical School Trial registration: retrospective registration 14 June 2005; NCT00114088 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation schedule (computer‐generated permuted blocks) developed by the Co‐ordinating Centre and stratified by participating centre. |
| Allocation concealment (selection bias) | Low risk | Central allocation. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Single blinding for interviewers requesting information on bleeding history, QoL, etc. But women providing this information knew of their assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No reasons given for dropouts. |
| Selective reporting (reporting bias) | Low risk | Protocol published with details on prespecified outcomes. |
| Other bias | Unclear risk | Groups appeared balanced at baseline. However, some outcomes (intraoperative and perioperative events, including adverse events) reported according to surgery received. Surgeons' previous experience was not reported. |
Dwyer 1993.
| Study characteristics | ||
| Methods | Randomisation by sealed numbered envelopes in variable blocks of 20, 30 and 50 Single‐centre, parallel‐group, no blinding Setting: outpatient gynaecology clinic at a teaching hospital in Bristol, UK Number of women randomly assigned: 200 Number of withdrawals: 4 (3 from hysterectomy group and 1 from resection group) Power calculation for sample size performed but intention‐to‐treat analysis not reported |
|
| Participants | Women with mean age of 40 years Inclusion criteria: < 52 years of age; complaint of menorrhagia that could not be controlled by conservative means; candidates for abdominal hysterectomy Exclusion criteria: uterine size ≤ 12 gestational weeks; additional symptoms or other pathology; making hysterectomy the preferred treatment |
|
| Interventions | Treatment 1: endometrial resection (TCRE) (women received medroxyprogesterone acetate injection four to six weeks before surgery): 99 women Treatment 2: abdominal hysterectomy: 97 women Duration: 4 months' follow‐up, 2.8 years' follow‐up Prior experience of the surgeon not mentioned |
|
| Outcomes |
|
|
| Notes | 3 publications used the same study population: Dwyer 1993; Sculpher 1993; Sculpher 1996. Dr Dwyer contacted for additional information but no reply received. Source of funding: South West Regional Health Authority No information stated regarding trial registration |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation sequence not described. |
| Allocation concealment (selection bias) | Low risk | Sealed numbered envelopes in variable blocks. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not feasible for a comparison of surgical techniques. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts from trial minimal and not likely to bias results. |
| Selective reporting (reporting bias) | Unclear risk | No prior protocol identified. |
| Other bias | Unclear risk | Groups appeared balanced at baseline. Surgeons' previous experience was not reported. |
Gannon 1991.
| Study characteristics | ||
| Methods | Randomisation by sealed envelope Single‐centre, parallel‐group, no blinding Setting: Royal Berkshire Hospital in Reading, UK Number of women randomly assigned: 54 Number of withdrawals: 3 (1 from the resection group who wanted hysterectomy, 1 from the hysterectomy group who wanted resection, and 1 from the hysterectomy group who postponed surgery and was treated elsewhere) Power calculation and intention‐to‐treat analysis not reported |
|
| Participants | Women with median age 40 years awaiting abdominal hysterectomy for menorrhagia Exclusion criteria: leiomyomata; endometrial or cervical neoplasia; concomitant ovarian pathology, PID or endometriosis |
|
| Interventions | Treatment 1: endometrial resection (TCRE): 25 women Treatment 2: abdominal hysterectomy: 26 women Duration: mean 12 months' follow‐up Procedures performed by 'experienced surgeons on routine operating lists' Endometrial resection group given an intramuscular injection of medroxyprogesterone acetate 150 mg 4–6 weeks before surgery to reduce endometrial thickness. |
|
| Outcomes |
|
|
| Notes | Author contacted but no reply received. Source of funding: not reported. No information stated regarding trial registration |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation sequence not described. |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelope but no other safeguards described. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not feasible for a comparison of surgical techniques. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts minimal and not likely to cause bias. |
| Selective reporting (reporting bias) | Unclear risk | No prior protocol identified. |
| Other bias | Low risk | Groups appeared balanced at baseline, although very few parameters examined. |
Jain 2016.
| Study characteristics | ||
| Methods | Randomisation by sealed envelope Single‐centre, parallel‐group, no blinding Setting: Department of Obstetrics and Gynecology, University College of Medical Sciences and Guru Teg Bahadur Hospital, Delhi, India Number of women randomly assigned: 40 Number of withdrawals: 0 1 November 2012 to 31 October 2014 |
|
| Participants | 40 women randomised Inclusion criteria: women aged > 40 years; no desire for future childbearing; HMB PBAC score ≥ 100; uterine size up to 14 weeks of pregnancy; leiomyomas ≤ 5 cm in diameter; uterocervical length ≤ 12 cm Exclusion criteria: acute PID or pelvic pathology (e.g. adenomyosis, gynaecologic cancers (including endometrial malignancy)); atypical endometrial hyperplasia; submucosal leiomyomas |
|
| Interventions | Treatment 1: thermal balloon ablation: 20 woman Treatment 2: vaginal hysterectomy: 20 woman |
|
| Outcomes |
|
|
| Notes | Source of funding: not reported Declared no conflicts of interest Clinical Trials Registry India: retrospective registration 26 July 2016; CTRI/2016/07/007119 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants randomly allocated into 2 groups (thermal balloon ablation and vaginal hysterectomy) in a 1:1 ratio using computer‐generated random number tables. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding: participants, investigators and data analysts were not blinded to group assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts. |
| Selective reporting (reporting bias) | Low risk | All the outcomes previously specified were reported. |
| Other bias | Unclear risk | Surgeons' previous experience was not reported. Source of funding: not reported. Declared no conflicts of interest. |
O'Connor 1997.
| Study characteristics | ||
| Methods | Randomisation at a ratio of 2:1 from a computer‐generated random number sequence, with the code kept at the Royal Free Hospital Multicentre, parallel‐group with no blinding Setting: centres in King's Lynn, Portsmouth, Plymouth, London, Bradford, Chelmsford, Northampton, Birmingham and Exeter, UK Number of women randomly assigned: 202 women Number of withdrawals: 30 (26 before surgery and 4 lost to follow‐up) Power calculation for sample size performed and analysis by intention to treat Source of funding: Medical Research Council |
|
| Participants | Women aged 30–50 years with symptomatic menorrhagia that required hysterectomy Inclusion criteria: aged 30–50 years; decision to have no more children; regular menstrual cycles 21–35 days; each period lasting < 50% of the cycle; documented evidence of normal endometrial histology within the previous 12 months and normal cervical smear within the previous 3 years Exclusion criteria: serious intercurrent illness; intermenstrual or postcoital bleeding; uterine size corresponding to pregnancy > 12 weeks' gestation; submucous fibroids > 5 cm in diameter, adnexal tenderness suggestive of PID or endometriosis; major uterovaginal prolapse or severe urinary symptoms; severe premenstrual syndrome or menopausal symptoms |
|
| Interventions | Treatment 1: transcervical endometrial resection (TCRE): 116 women Treatment 2: hysterectomy: 56 women (28 abdominal, 28 vaginal) Duration: median 2 years' follow‐up Surgeons performing TCRE required prior experience of ≥ 20 procedures. Surgeons performing hysterectomy were 'experienced' or were supervised by an experienced surgeon |
|
| Outcomes |
|
|
| Notes | No information stated regarding trial registration | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number sequence. |
| Allocation concealment (selection bias) | Low risk | Code to randomisation kept separate, with telephoning for next assignment. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not feasible for a comparison of surgical techniques. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropouts > 10% and reasons not given. |
| Selective reporting (reporting bias) | Unclear risk | No prior protocol identified. |
| Other bias | Low risk | Groups appeared balanced at baseline. No other potential bias identified. |
Pinion 1994.
| Study characteristics | ||
| Methods | Randomisation, ratio of 2:1:1 (hysterectomy:resection:ablation), by a series of numbered opaque envelopes in a random order Single‐centre, parallel‐group, no blinding Setting: general gynaecology clinic of the Aberdeen Royal Infirmary in Scotland, UK Number of participants randomly assigned: 204 Number of withdrawals: 6 (2 before surgery and 4 refused the allocated treatment) Power calculation for sample size performed and analysis by intention to treat |
|
| Participants | Women with mean age 40 years, eligible to undergo hysterectomy for menorrhagia Inclusion criteria: aged < 50 years; weight < 100 kg; clinical diagnosis of dysfunctional uterine bleeding; uterus < 10 weeks of gestational size; normal endometrial histology Exclusion criteria: none reported |
|
| Interventions | Treatment 1: laser ablation (1st generation) (53 women) or endometrial resection (TCRE). Women received gonadotrophin releasing hormone
agonist analogue (Goserelin) five weeks preoperatively (52 women) Treatment 2: hysterectomy (abdominal (87 women), vaginal (12 women) Duration: 4 years' follow‐up Prior experience of the surgeon not mentioned |
|
| Outcomes |
|
|
| Notes | Author contacted for additional data but no reply received. This trial has 4 publications using the same study population and assessing different outcomes. Data usually available only for the laser ablation and endometrial resection combined. Source of funding: Scottish Office Home and Health Dept and ICI (Zeneca) No information stated regarding trial registration |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation sequence not described. |
| Allocation concealment (selection bias) | Low risk | Series of numbered opaque envelopes. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not feasible for a comparison of surgical techniques. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts minimal and unlikely to cause bias. |
| Selective reporting (reporting bias) | Unclear risk | No prior protocol identified. |
| Other bias | Unclear risk | Groups appeared balanced at baseline. Surgeons' previous experience was not reported. |
Sesti 2011.
| Study characteristics | ||
| Methods | Randomisation by computer‐generated sequence of serially numbered opaque envelopes Single‐centre parallel‐group with blinding of assessors Setting: Italy Number of women randomly assigned: 68 Number of withdrawals: 0 Power calculation for sample size performed and analysis by intention to treat |
|
| Participants | Women aged 35–50 years with HMB, who had failed appropriate first‐line oral medical therapy and required surgical treatment Inclusion criteria: PBAC score ≥ 100 (mean of 2 consecutive cycles); completed family; normal smear; pelvic ultrasound scan and endometrial biopsy Exclusion criteria: previous endometrial resection/ablation; previous levonorgestrel IUD; any uterine pathology on pelvic ultrasound scan or hysteroscopy; any pathology where hysterectomy was indicated; uninvestigated abnormal bleeding or postmenopausal bleeding |
|
| Interventions | Treatment 1: EA via Thermachoice III thermal balloon: 34 women Treatment 2: laparoscopic subtotal hysterectomy: 34 women 24 months' follow‐up All surgery was performed by the same 2 surgeons; however, prior experience of the surgeon not mentioned |
|
| Outcomes | Primary outcome:
Secondary outcomes:
|
|
| Notes | Information included in the study was sufficient for meta‐analysis – not necessary to contact authors. Source of funding: Italian Ministry of University. No information stated regarding trial registration |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation sequence. |
| Allocation concealment (selection bias) | Low risk | Serially numbered, opaque sealed envelopes. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not feasible for a comparison of surgical techniques. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All women accounted for in groups to which they were randomly assigned. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported. |
| Other bias | Low risk | Groups balanced at baseline. No other potential bias identified. All procedures were performed by the same surgeons using an identical technique. |
Zupi 2003.
| Study characteristics | ||
| Methods | Computer‐generated randomisation number sequence Single‐centre, parallel‐group, single‐blind (assessors of SF‐36 results) Setting: Departmental O and G Clinic in the Tor Vergarta University of Rome, Italy Number of women randomly assigned: 203 Number of women analysed: 181 Power calculation for sample size (power of 0.8 for difference in satisfaction rate of 50% between groups) Analysis by intention to treat Recruited between March 1995 and February 1997 |
|
| Participants | Women with mean age of 43 years with menometrorrhagia unresponsive to medical treatment Inclusion criteria: aged < 50 years; weight < 100 kg; not seeking contraception; normal endometrial histology and Pap smear within previous 6 months; uterus < 12 weeks of pregnancy in size; no submucosal fibroids, adnexal masses, or endometriosis Exclusion criteria: none reported |
|
| Interventions | Treatment 1: pretreatment with GnRHa 1 month before surgery, then hysteroscopic endometrial resection (TCRE): 89 women Treatment 2: laparoscopic supracervical hysterectomy: 92 women Duration: 2 years (follow‐up at 3 months, 1 and 2 years) All surgeons were proficient in both endometrial resection and laparoscopic hysterectomy. |
|
| Outcomes |
|
|
| Notes | Source of funding: not reported. No information stated regarding trial registration |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number sequence. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not feasible for a comparison of surgical techniques. Single blinding of assessors who administered the SF‐36, but women who provided the answers knew of their assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts minimal and unlikely to cause bias. |
| Selective reporting (reporting bias) | Unclear risk | No prior protocol identified. |
| Other bias | Low risk | Groups appeared balanced at baseline and no other potential bias identified. |
EA: endometrial ablation; GnRHa: gonadotrophin‐releasing hormone agonists; HMB: heavy menstrual bleeding; IUD: intrauterine device; MBL: menstrual blood loss; MMAS: Menorrhagia Multi‐Attribute Scale; NRS: Numerical Rating Scale; PBAC: Pictorial Blood Assessment Chart; PID: pelvic inflammatory disease; QALY: quality‐adjusted life year; QoL: quality of life; SF‐12: 12‐item Short Form; SF‐36: 36‐item Short Form; TCRE: transcervical resection of the endometrium; UFS‐QoL: Uterine Fibroid Symptom and Health Related Quality of Life Questionnaire; VAS: visual analogue scale (pain).
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Lin 2006 | Not an RCT. |
| Paddison 2003 | Not an RCT. |
RCT: randomised controlled trial.
Differences between protocol and review
In the protocol and previous versions of the review the outcome 'Further gynaecological surgery' included any further gynaecological surgery, meaning if the woman had an oophorectomy or a cystoscopy this was valid as further surgery. In the 2019 update, the outcome was limited to further gynaecological surgery for heavy menstrual bleeding, and the data extraction was rechecked and updated.
In the 2020 update, we changed quality of life to be part of effectiveness (primary outcome) as currently the definition of heavy menstrual bleeding is based on quality of life. We added a new outcome measure for safety (adverse outcome): 'any serious adverse event' as the adverse events commonly related to both techniques are specific, thus difficult to compare one by one and thus clinically unhelpful. We added time to return to normal activity to the 'Summary of findings' tables. We also made changes to the comparisons compared to earlier versions of the review: we divided hysterectomy into three groups: open, minimally invasive and unspecified (or at surgeon's discretion) route of hysterectomy.
For clarification, we confirm that prespecified sensitivity analyses in the 2020 update were of primary analyses, as has been the case in previous versions of this review.
Contributions of authors
2020 version of the review
MBR: performed selection of trials, data extraction, data entry and prepared all versions of drafts and the final version of the review for comments from the other review authors.
AL: commented on the final version of the review.
RJF: performed selection of trials, data extraction, and commented on drafts and final version of the review.
Sources of support
Internal sources
The authors received no sources of support for this Cochrane Review, Other
External sources
The authors received no sources of support for this Cochrane Review, Other
Declarations of interest
MBR: none.
AL: none.
RJF: none.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Cooper 2019 {published data only}
- Cooper K, Breeman S, Scott N, Scotland G, Clark J, Hawe J, et al, on behalf of the HEALTH Study Group. Laparoscopic supracervical hysterectomy versus endometrial ablation for women with heavy menstrual bleeding (HEALTH): a parallel-group, open-label, randomised controlled trial. Lancet 2019;394(10207):1425-36. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper K, McCormack K, Breeman S, Wood J, Scott NW, Clark J, et al. HEALTH: laparoscopic supracervical hysterectomy versus second-generation endometrial ablation for the treatment of heavy menstrual bleeding: study protocol for a randomised controlled trial. Trials 2018;19(1):63. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Crosignani 1997 {published data only}
- Crosignani PG, Vercellini P, Apolone G, De Giorgi O, Cortesi I, Meschia M. Endometrial resection versus vaginal hysterectomy for menorrhagia: long-term clinical and quality-of-life outcomes. American Journal of Obstetrics and Gynecology 1997;177(1):95-101. [DOI] [PubMed] [Google Scholar]
Dickersin 2007 {published data only}
- Dickersin K, Munro M, Langenberg P, Scherer R, Frick KD, Weber AM, et al. Surgical treatments outcomes project for dysfunctional uterine bleeding (STOP-DUB): design and methods. Controlled Clinical Trials 2003;24(5):591-609. [DOI] [PubMed] [Google Scholar]
- Dickersin K, Munro MG, Clark M, Langenberg P, Scherer R, Frick K, et al. Hysterectomy compared with endometrial ablation for dysfunctional uterine bleeding. Obstetrics and Gynecology 2007;110(6):1279-89. [DOI] [PubMed] [Google Scholar]
- Munro M, Dickerson K, Clark M, Langenberg P, Scherer R, Frick K. The Surgical Treatments Outcomes Project for Dysfunctional Uterine Bleeding: summary of an Agency for Health Research and Quality-sponsored randomized trial of endometrial ablation versus hysterectomy for women with heavy menstrual bleeding. Menopause 2011;18(4):451-8. [DOI] [PubMed] [Google Scholar]
Dwyer 1993 {published data only}
- Dwyer N, Hutton J, Stirrat GM. Randomised controlled trial comparing endometrial resection with abdominal hysterectomy for the surgical treatment of menorrhagia. British Journal of Obstetrics and Gynaecology 1993;100(3):237-43. [DOI] [PubMed] [Google Scholar]
- Sculpher M. A cost-utility analysis of abdominal hysterectomy versus transcervical endometrial resection for the surgical treatment of menorrhagia. International Journal of Technology Assessment in Health Care 1998;14(2):302-19. [DOI] [PubMed] [Google Scholar]
- Sculpher MJ, Bryan S, Dwyer N, Hutton J, Stirrat GM. An economic evaluation of transcervical endometrial resection versus abdominal hysterectomy for the treatment of menorrhagia. British Journal of Obstetrics and Gynaecology 1993;100(3):244-52. [DOI] [PubMed] [Google Scholar]
- Sculpher MJ, Dwyer N, Byford S, Stirrat GM. Randomised trial comparing hysterectomy and transcervical endometrial resection: effect on health related quality of life and costs two years after surgery. British Journal of Obstetrics and Gynaecology 1996;103(2):142-9. [DOI] [PubMed] [Google Scholar]
Gannon 1991 {published data only}
- Gannon MJ, Holt EM, Fairbank J, Fitzgerald M, Milne MA, Crystal AM, et al. A randomised trial comparing endometrial resection and abdominal hysterectomy for the treatment of menorrhagia. BMJ (Clinical Research Ed.) 1991;303(6814):1362-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jain 2016 {published data only}
- Jain P, Rajaram S, Gupta B, Goel N, Srivastava H. Randomized controlled trial of thermal balloon ablation versus vaginal hysterectomy for leiomyoma-induced heavy menstrual bleeding. International Journal of Gynaecology and Obstetrics 2016;135(2):140-4. [doi.org/10.1016/j.ijgo.2016.04.020] [DOI] [PubMed] [Google Scholar]
O'Connor 1997 {published data only}
- O'Connor H, Broadbent JA, Magos AL, McPherson K. Medical Research Council randomised trial of endometrial resection versus hysterectomy in management of menorrhagia. Lancet 1997;349(9056):897-901. [DOI] [PubMed] [Google Scholar]
Pinion 1994 {published data only}
- Aberdeen Endometrial Ablation Trials Group. A randomised trial of endometrial ablation versus hysterectomy for the treatment of dysfunctional uterine bleeding: outcome at four years. British Journal of Obstetrics and Gynaecology 1999;106(4):360-6. [DOI] [PubMed] [Google Scholar]
- Alexander DA, Naji AA, Pinion SB, Mollison J, Kitchener HC, Parkin DE, et al. Randomised trial comparing hysterectomy with endometrial ablation for dysfunctional uterine bleeding: psychiatric and psychosocial aspects. BMJ (Clinical Research Ed.) 1996;312(7026):280-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron IM, Mollison J, Pinion SB, Atherton-Naji A, Buckingham K, Torgerson D. A cost comparison of hysterectomy and hysteroscopic surgery for the treatment of menorrhagia. European Journal of Obstetrics and Gynaecology 1996;70(1):87-92. [DOI] [PubMed] [Google Scholar]
- Pinion SB, Parkin DE, Abramovich DR, Naji A, Alexander DA, Russell IT, et al. Randomised trial of hysterectomy, endometrial laser ablation, and transcervical endometrial resection for dysfunctional uterine bleeding. BMJ (Clinical Research Ed.) 1994;309(6960):979-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sesti 2011 {published data only}
- Sesti F, Ruggeri V, Pietropolli A, Piancatelli R, Piccione E. Thermal balloon ablation versus laparoscopic supracervical hysterectomy for the surgical treatment of heavy menstrual bleeding: a randomized study. Journal of Obstetrics and Gynaecology Research 2011;37(11):1650-7. [DOI] [PubMed] [Google Scholar]
Zupi 2003 {published data only}
- Centini G, Lazzeri L, Finco A, Afors K, Zupi E. Quality of life and risk of reintervention comparing endometrial ablation and laparoscopic subtotal hysterectomy: a 15 years follow up study. Gynecological Surgery 2015;12(Suppl 1):S11. [Google Scholar]
- Zupi E, Centini G, Lazzeri L, Finco A, Exacoustos C. Hysteroscopic endometrial resection versus laparoscopic supracervical hysterectomy for abnormal uterine bleeding: long term follow-up of a prospective randomised trial. Journal of Minimally Invasive Gynecology 2015;22(5):841-5. [DOI: 10.1016/j.jmig.2015.08.108] [DOI] [PubMed] [Google Scholar]
- Zupi E, Zullo F, Marconi D, Sbracia M, Pellicano M, Solima E, et al. Hysteroscopic endometrial resection versus laparoscopic supracervical hysterectomy for menorrhagia: a prospective randomised trial. American Journal of Obstetrics and Gynecology 2003;188(1):7-12. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Lin 2006 {published data only}
- Lin H. Comparison between microwave endometrial ablation and total hysterectomy. Chinese Medical Journal 2006;119(14):1195-7. [PubMed] [Google Scholar]
Paddison 2003 {published data only}
- Paddison K. Menorrhagia: endometrial ablation or hysterectomy. Nursing Standard 2003;18(1):33-7. [DOI] [PubMed] [Google Scholar]
Additional references
Aarts 2015
- Aarts J, Nieboer T, Johnson N, Tavender E, Garry R, Mol B. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database of Systematic Reviews 2015, Issue 8. Art. No: CD003677. [DOI: 10.1002/14651858.CD003677.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Adelman 2014
- Adelman MR, Bardsley TR, Sharp HT. Urinary tract injuries in laparoscopic hysterectomy: a systematic review. Journal of Minimally Invasive Gynecology 2014;21(4):558-66. [DOI: 10.1016/j.jmig.2014.01.006] [DOI] [PubMed] [Google Scholar]
Arnold 2015
- Arnold A, Abbott J. Endometrial ablation. Obstetrics and Gynaecology Magazine 2015;17(4):-. [www.ogmagazine.org.au/17/4-17/endometrial-ablation/]
Athanatos 2015
- Athanatos D, Pados G, Venetis CA, Stamatopoulos P, Rousso D, Tsolakidis D. Novasure impedance control system versus microwave endometrial ablation for the treatment of dysfunctional uterine bleeding: a double-blind, randomized controlled trial. Clinical and Experimental Obstetrics & Gynecology 2015;42(3):347-51. [PMID: ] [PubMed] [Google Scholar]
Bhattacharya 2011
- Bhattacharya S, Middleton L, Tsourapas A, Champaneria R, Daniels J, Roberts T, et al. Hysterectomy, endometrial ablation and Mirena for heavy menstrual bleeding: a systematic review of clinical effectiveness and cost-effectiveness analysis. Health Technology Assessment 2011;19:1-252. [DOI] [PMC free article] [PubMed]
Bofill Rodriguez 2019
- Bofill Rodriguez M, Lethaby A, Grigore M, Brown J, Hickey M, Farquhar C. Endometrial resection and ablation techniques for heavy menstrual bleeding. Cochrane Database of Systematic Reviews 2019, Issue 1. Art. No: CD001501. [DOI: 10.1002/14651858.CD001501.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bourdrez 2004
- Bourdrez P, Bongers MY, Mol BW. Treatment of dysfunctional uterine bleeding: patient preferences for endometrial ablation, a levonorgestrel-releasing intrauterine device, or hysterectomy. Fertility and Sterility 2004;82(1):160-6. [DOI] [PubMed] [Google Scholar]
Brazier 1993
- Brazier JE, Jones NM, Kind P. Testing the validity of the EuroQol and comparing it with the SF-36 health survey questionnaire. Quality of Life Research 1993;2(3):169-80. [DOI] [PubMed] [Google Scholar]
Bridgman 1994
- Bridgman SA. Increasing operative rates for dysfunctional uterine bleeding after endometrial ablation (letter). Lancet 1994;344:893. [DOI] [PubMed] [Google Scholar]
Clegg 2007
- Clegg JP, Guest JF, Hurskainen R. Cost-utility of levonorgestrel intrauterine system compared with hysterectomy and second generation endometrial ablative techniques in managing patients with menorrhagia in the UK. Current Medical Research and Opinion 2007;23(7):1673-48. [DOI] [PubMed] [Google Scholar]
Cooper 2011
- Cooper K, Lee A, Chien P, Raja E, Timmaraju V, Bhattacharya S. Outcomes following hysterectomy or endometrial ablation for heavy menstrual bleeding: retrospective analysis of hospital episode statistics in Scotland. British Journal of Obstetrics and Gynaecology 2011;118(10):1171-9. [DOI] [PubMed] [Google Scholar]
Coulter 1991
- Coulter A, Bradlow J, Agass M, Martin-Bates C, Tullock A. Outcomes of referrals to gynaecology outpatient clinics for menstrual problems: an audit of general practice records. British Journal of Obstetrics and Gynaecology 1991;98(8):789-96. [DOI] [PubMed] [Google Scholar]
Coulter 1994
- Coulter A. Trends in gynaecological surgery (letter). Lancet 1994;344:1367. [DOI] [PubMed] [Google Scholar]
EuroQol Group 1990
- EuroQol Group. EuroQol – a new facility for the measurement of health-related quality of life. Health Policy (Amsterdam, Netherlands) 1990;16(3):199-208. [DOI] [PubMed] [Google Scholar]
Fernandez 2011
- Fernandez H. Update on the management of menometrorrhagia: new surgical approaches. Gynecological Endocrinology 2011;Suppl 1:1131-6. [DOI] [PubMed] [Google Scholar]
Garrat 1995
- Garrat AM, Torgerson DJ, Wyness J, Hall MH, Reid DM. Measuring sexual functioning in premenopausal women. British Journal of Obstetrics and Gynaecology 1995;102(4):311-6. [DOI] [PubMed] [Google Scholar]
Garry 2005
- Garry R, Fountain J, Brown J, Manca A, Mason S, Sculpher M, et al. EVALUATE hysterectomy trial: a multicentre randomised trial comparing abdominal, vaginal and laparoscopic methods of hysterectomy. Health Technology Assessment 2005;8(26):1-154. [DOI: ] [DOI] [PubMed] [Google Scholar]
Garside 2004
- Garside R, Stein K, Wyatt K, Round A, Pitt M. A cost-utility analysis of microwave and thermal balloon endometrial ablation techniques for the treatment of heavy menstrual bleeding. British Journal of Obstetrics and Gynaecology 2004;111(10):1103-14. [DOI] [PubMed] [Google Scholar]
Geary 2019
- Geary R, Gurol-Urganci I, Kiran A, Cromwell D Bansi-Matharu L, Shakespeare J et al. Factors associated with receiving surgical treatment for menorrhagia in England and Wales: findings from a cohort study of the National Heavy Menstrual Bleeding Audit. BMJ 2019;9(2):e024260. [DOI: 10.1136/bmjopen-2018-024260] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed prior to 17 July 2019. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Higgins 2008
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2008;327(7414):557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Hozo 2005
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Medical Research Methodology 2005;5:13. [DOI: 10.1186/1471-2288-5-13] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kalampokas 2017
- Kalampokas E, McRobbie S, Payne F, Parkin DE. Long-term incidence of hysterectomy following endometrial resection or endometrial ablation for heavy menstrual bleeding. International Journal of Gynaecology and Obstetrics 2017;139(1):61-4. [DOI: 10.1002/ijgo.12259] [DOI] [PubMed] [Google Scholar]
Kennedy 2002
- Kennedy AD, Sculpher MJ, Coulter A, Dwyer N, Rees M, Abrams KR, et al. Effects of decision aids for menorrhagia on treatment choices, health outcomes and costs. JAMA 2002;288(21):2701-8. [DOI] [PubMed] [Google Scholar]
Kumar 2016
- Kumar V, Chodankar R, Gupta JK. Endometrial ablation for heavy menstrual bleeding. Women's Health (London, England) 2016;12(1):45-52. [DOI: 10.2217/whe.15.86] [DOI] [PMC free article] [PubMed] [Google Scholar]
Laberge 2015
- Laberge P, Leyland N, Murji A, Fortin C, Martyn P, Vilos G, et al. Endometrial ablation in the management of abnormal uterine bleeding. Journal d'Obstetrique et Gynecologie du Canada : JOGC [Journal of Obstetrics and Gynaecology Canada : JOGC] 2015;37(4):362-79. [PMID: ] [DOI] [PubMed] [Google Scholar]
Madhu 2009
- Madhu CK, Nattey J, Naeem T. Second generation endometrial ablation techniques: an audit of clinical practice. Archives of Gynecology and Obstetrics 2009;280(4):599-602. [DOI: 10.1007/s00404-009-0982-7] [DOI] [PubMed] [Google Scholar]
Mahdi 2014
- Mahdi H, Goodrich S, Lockhart D, DeBernardo R, Moslemi-Kebria M. Predictors of surgical site infection in women undergoing hysterectomy for benign gynecologic disease: a multicenter analysis using the National Surgical Quality Improvement Program data. Journal of Minimally Invasive Gynaecology 2014;21(5):901-9. [DOI] [PubMed] [Google Scholar]
Munro 2011
- Munro MG, Critchley HO, Broder MS, Fraser IS. FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. International Journal of Gynaecology and Obstetrics 2011;113(1):3-13. [DOI] [PubMed] [Google Scholar]
Munro 2018
- Munro M. Endometrial ablation. Best Practice & Research Clinical Obstetrics & Gynaecology 2018;46:120-39. [DOI: 10.1016/j.bpobgyn.2017.10.003] [DOI] [PubMed] [Google Scholar]
Nagele 1998
- Nagele F, Rubinger T, Magos A. Why do women choose endometrial ablation rather than hysterectomy? Fertility and Sterility 1998;69(6):1063-6. [DOI] [PubMed] [Google Scholar]
NICE 2007
- National Collaborating Centre for Women's and Children's Health. Heavy Menstrual Bleeding. London: RCOG Press, 2007. [PubMed] [Google Scholar]
NICE 2018
- NICE guideline. Heavy menstrual bleeding: assessment and management. www.nice.org.uk/guidance/ng88 (accessed prior to 21 January 2020).
Overton 1997
- Overton C, Hargreaves J, Maresh M. A national survey of the complications of endometrial destruction for menstrual disorders: the MISTLETOE study. British Journal of Obstetrics and Gynaecology 1997;104(12):1351-9. [DOI] [PubMed] [Google Scholar]
Reid 2007
- Reid PC. Endometrial ablation in England – coming of age? An examination of hospital episode statistics 1989/1990 to 2004/2005. European Journal of Obstetrics and Gynecology 2007;135(2):191-4. [DOI] [PubMed] [Google Scholar]
Roberts 2011
- Roberts T, Tsourapas A, Champaneria R, Daniels J, Cooper K, Bhattacharya S, et al. Hysterectomy, endometrial ablation, and levonorgestrel releasing intrauterine system (Mirena) for treatment of heavy menstrual bleeding: cost effectiveness analysis. BMJ 2011;342:d2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rust 1986
- Rust J, Bennum I, Crowe M, Golombok S. The construction and validation of the Golombok Rust Inventory of Marital State. Sexual and Marital Therapy 1986;1:34-40. [Google Scholar]
Sculpher 1998
- Sculpher M. The cost-effectiveness of preference-based treatment allocation: the case of hysterectomy versus endometrial resection in the treatment of menorrhagia. Health Economics 1998;7(2):129-42. [DOI] [PubMed] [Google Scholar]
Sharp 2012
- Sharp HT. Endometrial ablation: postoperative complications. American Journal of Obstetrics and Gynecology 2012;207(4):242-7. [DOI] [PubMed] [Google Scholar]
Sweeting 2004
- Sweeting M, Sutton A, Lambert P. What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data. Statistics in Medicine 2004;23(9):1361-75. [DOI: 10.1002/sim.1761] [DOI] [PubMed] [Google Scholar]
Tang 2018
- Tang W, He H, Wang WJ, Chen DG. Untangle the structural and random zeros in statistical modelings. Journal of applied statistics 2018;45(9):1714-33. [DOI: 10.1080/02664763.2017.1391180] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vessey 1992
- Vessey M, Villard-Mackintosh L, McPherson K, Coulter A, Yeates D. The epidemiology of hysterectomy: findings in a large cohort study. British Journal of Obstetrics and Gynaecology 1992;99(5):402-7. [DOI] [PubMed] [Google Scholar]
Ware 1993
- Ware JE, Snow KK, Kosinski M, Gandek BG. SF-36 Health survey: manual and interpretation guide. In: The Health Institute. Boston (MA): New England Medical Centre, 1993. [Google Scholar]
Woolcock 2008
- Woolcock JG, Critchley HO, Munro MG, Broder MS, Fraser IS. Review of the confusion in current and historical terminology and definitions for disturbances in menstrual bleeding. Fertility and Sterility 2008;90(6):2269. [DOI] [PubMed] [Google Scholar]
You 2006
- You JH, Sahota DS, Yuen PM. A cost-utility analysis of hysterectomy, endometrial resection and ablation and medical therapy for menorrhagia. Human Reproduction (Oxford, England) 2006;21(7):1878-83. [DOI] [PubMed] [Google Scholar]
Zigmond 1983
- Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatrica Scandinavica 1983;67(6):361-70. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Cooke 1996
- Cooke I, Shepperd S. Comparison of the effectiveness of endometrial resection and ablation to reduce heavy menstrual bleeding versus hysterectomy. Cochrane Database of Systematic Reviews 1996, Issue 2. Art. No: CD000329. [DOI: 10.1002/14651858.CD000329] [DOI] [PubMed] [Google Scholar]
Fergusson 2013
- Fergusson RJ, Lethaby A, Shepperd S, Farquhar C. Endometrial resection and ablation versus hysterectomy for heavy menstrual bleeding. Cochrane Database of Systematic Reviews 2013, Issue 11. Art. No: CD000329. [DOI: 10.1002/14651858.CD000329.pub2] [DOI] [PubMed] [Google Scholar]
Fergusson 2019
- Fergusson RJ, Bofill Rodriguez M, Lethaby A, Farquhar C. Endometrial resection and ablation versus hysterectomy for heavy menstrual bleeding. Cochrane Database of Systematic Reviews 2019, Issue 8. Art. No: CD000329. [DOI: 10.1002/14651858.CD000329.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lethaby 1999
- Lethaby A, Shepperd S, Cooke I, Farquhar C. Endometrial resection and ablation versus hysterectomy for heavy menstrual bleeding. Cochrane Database of Systematic Reviews 1999, Issue 2. Art. No: CD000329. [DOI: 10.1002/14651858.CD000329] [DOI] [PubMed] [Google Scholar]
Lethaby 2009
- Lethaby A, Shepperd S, Farquhar C, Cooke I. Endometrial resection and ablation versus hysterectomy for heavy menstrual bleeding. Cochrane Database of Systematic Reviews 2009, Issue 3. Art. No: CD000329. [DOI: 10.1002/14651858.CD000329] [DOI] [PubMed] [Google Scholar]


