Abstract
Background
Rehabilitation based upon research evidence gives stroke survivors the best chance of recovery. There is substantial research to guide practice in stroke rehabilitation, yet uptake of evidence by healthcare professionals is typically slow and patients often do not receive evidence‐based care. Implementation interventions are an important means to translate knowledge from research to practice and thus optimise the care and outcomes for stroke survivors. A synthesis of research evidence is required to guide the selection and use of implementation interventions in stroke rehabilitation.
Objectives
To assess the effects of implementation interventions to promote the uptake of evidence‐based practices (including clinical assessments and treatments recommended in evidence‐based guidelines) in stroke rehabilitation and to assess the effects of implementation interventions tailored to address identified barriers to change compared to non‐tailored interventions in stroke rehabilitation.
Search methods
We searched CENTRAL, MEDLINE, Embase, and eight other databases to 17 October 2019. We searched OpenGrey, performed citation tracking and reference checking for included studies and contacted authors of included studies to obtain further information and identify potentially relevant studies.
Selection criteria
We included individual and cluster randomised trials, non‐randomised trials, interrupted time series studies and controlled before‐after studies comparing an implementation intervention to no intervention or to another implementation approach in stroke rehabilitation. Participants were qualified healthcare professionals working in stroke rehabilitation and the patients they cared for. Studies were considered for inclusion regardless of date, language or publication status. Main outcomes were healthcare professional adherence to recommended treatment, patient adherence to recommended treatment, patient health status and well‐being, healthcare professional intention and satisfaction, resource use outcomes and adverse effects.
Data collection and analysis
Two review authors independently selected studies for inclusion, extracted data, and assessed risk of bias and certainty of evidence using GRADE. The primary comparison was any implementation intervention compared to no intervention.
Main results
Nine cluster randomised trials (12,428 patient participants) and three ongoing trials met our selection criteria. Five trials (8865 participants) compared an implementation intervention to no intervention, three trials (3150 participants) compared one implementation intervention to another implementation intervention, and one three‐arm trial (413 participants) compared two different implementation interventions to no intervention. Eight trials investigated multifaceted interventions; educational meetings and educational materials were the most common components. Six trials described tailoring the intervention content to identified barriers to change. Two trials focused on evidence‐based stroke rehabilitation in the acute setting, four focused on the subacute inpatient setting and three trials focused on stroke rehabilitation in the community setting.
We are uncertain if implementation interventions improve healthcare professional adherence to evidence‐based practice in stroke rehabilitation compared with no intervention as the certainty of the evidence was very low (risk ratio (RR) 1.19, 95% confidence interval (CI) 0.53 to 2.64; 2 trials, 39 clusters, 1455 patient participants; I2 = 0%). Low‐certainty evidence indicates implementation interventions in stroke rehabilitation may lead to little or no difference in patient adherence to recommended treatment (number of recommended performed outdoor journeys adjusted mean difference (MD) 0.5, 95% CI –1.8 to 2.8; 1 trial, 21 clusters, 100 participants) and patient psychological well‐being (standardised mean difference (SMD) –0.02, 95% CI –0.54 to 0.50; 2 trials, 65 clusters, 1273 participants; I2 = 0%) compared with no intervention. Moderate‐certainty evidence indicates implementation interventions in stroke rehabilitation probably lead to little or no difference in patient health‐related quality of life (MD 0.01, 95% CI –0.02 to 0.05; 2 trials, 65 clusters, 1242 participants; I2 = 0%) and activities of daily living (MD 0.29, 95% CI –0.16 to 0.73; 2 trials, 65 clusters, 1272 participants; I2 = 0%) compared with no intervention.
No studies reported the effects of implementation interventions in stroke rehabilitation on healthcare professional intention to change behaviour or satisfaction.
Five studies reported economic outcomes, with one study reporting cost‐effectiveness of the implementation intervention. However, this was assessed at high risk of bias. The other four studies did not demonstrate the cost‐effectiveness of interventions.
Tailoring interventions to identified barriers did not alter results.
We are uncertain of the effect of one implementation intervention versus another given the limited very low‐certainty evidence.
Authors' conclusions
We are uncertain if implementation interventions improve healthcare professional adherence to evidence‐based practice in stroke rehabilitation compared with no intervention as the certainty of the evidence is very low.
Keywords: Humans, Evidence-Based Medicine, Evidence-Based Medicine/education, Evidence-Based Medicine/methods, Evidence-Based Medicine/statistics & numerical data, Health Personnel, Health Personnel/education, Health Personnel/statistics & numerical data, Health Status, Patient Compliance, Patient Compliance/statistics & numerical data, Randomized Controlled Trials as Topic, Stroke Rehabilitation, Stroke Rehabilitation/methods, Stroke Rehabilitation/psychology
Plain language summary
Interventions to promote the use of evidence‐based practice in stroke rehabilitation
What is the aim of this review?
The aim of this Cochrane Review was to find out whether implementation strategies to encourage healthcare professionals to use evidence in stroke rehabilitation are effective. Examples of implementation strategies include education workshops, educational materials or providing feedback to healthcare professionals about their performance. The review authors collected and analysed all relevant studies to answer this question and found nine studies.
Key messages
We could not obtain a reliable estimate of the effect of implementation strategies in stroke rehabilitation on healthcare professional adherence to evidence‐based practice at 12 months because the evidence is of very low quality.
What was studied in the review?
Patients who have a stroke and participate in rehabilitation do not always receive treatments based on evidence. Considerable research has been conducted in stroke rehabilitation but this information does not easily translate to clinical practice or it takes a long time to be used by healthcare professionals. Strategies are needed to help healthcare professionals use best evidence when working with stroke survivors.
We included studies that compared a group of healthcare professionals receiving support to use evidence in stroke rehabilitation with another group who did not. We were interested to see whether healthcare professionals used more evidence in practice, whether patients adhered to evidence‐based recommendations from healthcare professionals, and whether patient health and well‐being improved.
What are the main results of the review?
We found nine studies from five countries; Australia, Canada, Malaysia, the UK and the US. Four studies reported on whether healthcare professionals increased their use of evidence in their work with stroke survivors. Studies compared healthcare professionals who received support to use evidence in stroke rehabilitation with healthcare professionals who did not receive support or received a different type of support.
We are uncertain if implementation strategies to support healthcare professionals to use evidence in stroke rehabilitation improve their practice compared to no support as the quality of the evidence is very low. The review found that strategies to encourage healthcare professionals to use evidence in stroke rehabilitation may make little or no difference to patient adherence to recommended treatment and patient psychological well‐being compared to no intervention (low‐quality evidence). Additionally, we found these strategies probably lead to little or no difference in patient health‐related quality of life and activities of daily living compared with no intervention (moderate certainty evidence).
We found no studies that reported healthcare professional intention to change their behaviour or satisfaction.
How up‐to‐date is this review?
The review authors searched for studies published up to 17 October 2019.
Summary of findings
Summary of findings 1. Implementation interventions compared with no intervention for promoting uptake of evidence‐based practices in stroke rehabilitation.
| Implementation interventions compared with no intervention for promoting uptake of evidence‐based practices in stroke rehabilitation | ||||||
|
Patient or population: healthcare professionals providing stroke rehabilitation Settings: inpatient, outpatient or community rehabilitation Intervention: any implementation intervention Comparison: control (no intervention) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies, clusters) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| No intervention | Implementation intervention | |||||
| Quality of care: healthcare professional adherence to EBP at 12 months | Study population stroke patients | RR 1.19 (0.53 to 2.64) | 1455 patient participants (2 trials, 39 clusters) | ⊕⊝⊝⊝ Very lowc,d | We are uncertain about the estimate of healthcare professional adherence to EBP at 12 months as the certainty of the evidence is very low. | |
| 5%a | 6% (2.7% to 13.2%) | |||||
| 33%b | 39.3% (17.5% to 87.1%) | |||||
| Patient adherence to recommended treatment: number of outdoor journeys per week at 6 months | 7.4 | 7.9 (5.6 to 10.2) | Adjusted MD 0.5 (–1.8 to 2.8) | 100 participants (1 trial, 21 clusters) | ⊕⊕⊝⊝ Lowe | Implementation interventions in stroke rehabilitation may lead to little or no difference in patient adherence to recommended treatment at 6 months, compared with no intervention. |
| Patient health status: HRQoL (EQ‐5D: –0.59 to 1, higher score better) at up to 6 months | 0.58f | 0.59 (0.56 to 0.63) | MD 0.01 (–0.02 to 0.05) | 1242 participants (2 trials, 65 clusters) | ⊕⊕⊕⊝ Moderateg | Implementation interventions in stroke rehabilitation probably lead to little or no difference in patient HRQoL at up to 6 months compared with no intervention. 4 trials assessed HRQoL using EQ‐5D but data from only 2 trials could be pooled. Findings across studies appeared consistent. |
| Patient health status: ADL (Barthel Index, 0–20, higher score = better) at up to 6 months | 15.8f | 16.09 (15.64 to 16.53) | MD 0.29 (–0.16 to 0.73) | 1272 participants (2 trials, 65 clusters) | ⊕⊕⊕⊝ Moderateg | Implementation interventions in stroke rehabilitation probably lead to little or no difference in patient function (ADL) at up to 6 months compared with no intervention. 4 trials assessed ADL using the Barthel Index but data from only 2 trials could be pooled. Findings across studies appeared consistent. |
| Patient health status: psychological well‐being (GHQ‐12, 0–36, higher score = worse) at up to 6 months | 14.9h | 14.69 (14.36 to 15.40) | SMD –0.02 (–0.54 to 0.50) | 1273 participants (2 trials, 65 clusters) | ⊕⊕⊝⊝ Lowi | Implementation interventions in stroke rehabilitation may lead to little or no difference in patient psychological well‐being at up to 6 months compared with no intervention. 3 trials reported psychological well‐being using different measures, data from 2 trials could be pooled. Findings across studies appeared consistent. |
| Health professional outcomes | No studies reported this outcome | |||||
| *The basis for the assumed risk (e.g. the mean control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ADL: activities of daily living; CI: confidence interval; EBP: evidence‐based practice; EQ‐5D: EuroQol 5‐dimension health state measure; GHQ‐12: General Health Questionnaire‐12; HRQoL: health‐related quality of life; MD: mean difference; SMD: standardised mean difference; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
a Adherence estimated from the control group value at 12 months in McCluskey 2016.
b Adherence estimated from the control group value at 12 months in Power 2014.
c Downgraded one level due to serious risk of bias; lack of blinding of personnel in both trials, outcome assessors not blinded and incomplete outcome data in one trial. d Downgraded two levels due to very serious imprecision; 95% confidence intervals wide. e Downgraded two levels due to very serious imprecision; suboptimal information size (one study with 100 participants) and 95% confidence intervals wide. f Estimated from the control group value at six months in Forster 2015. g Downgraded one level due to serious imprecision; suboptimal information size. h Estimated from the control group value on the GHQ‐12 at six months in Forster 2015. i Downgraded two levels due to serious indirectness; differences in outcome measures and uncertainty in whether the outcomes are assessing the same health issue.
Background
Description of the condition
Stroke is a leading cause of death and adult disability internationally (Feigin 2019). The Global Burden of Disease study reveals an increasing prevalence of stroke, caused by an epidemiological transition of increased risk factor prevalence and population ageing (Leyden 2013). There are 80.1 million prevalent cases of stroke globally (Johnson 2019); those experiencing resultant disability may have impairments in physical, sensory, cognitive or communication capacities. Poststroke disabilities negatively impact on quality of life and have major economic and societal costs (Cadilhac 2009).
Rehabilitation enables individuals with stroke to reach and maintain their optimal functional levels by providing skills and tools needed to attain independence and self‐determination (WHO 2015). The contemporary approach to stroke rehabilitation is being transformed by a greater understanding of the brain's ability to reorganise following injury (neuroplasticity), and previous timeframes for therapy and expected recovery are no longer restricted to immediately after stroke (Carey 2012; Korner‐Bitensky 2013). The setting for stroke rehabilitation can range from acute inpatient care to outpatient and community settings, and services are often provided to patients in the context of a multidisciplinary team, with goal‐setting a key feature (Langhorne 2011).
Though recent decades have seen important advances in the field of stroke with the emergence of strong evidence for stroke recovery (Langhorne 2011; Lindsay 2014), there have been significant delays in implementing evidence in clinical practice (Bayley 2012; Walker 2013), and stroke survivors often do not receive care based on the best available evidence (Hall 2013; Intercollegiate Stroke Working Party 2015; Stroke Foundation 2018).
Description of the intervention
Various implementation interventions can be used in stroke rehabilitation. Implementation interventions are strategies aimed at increasing the uptake of clinical research findings and other evidence‐based practices into routine healthcare practice. The Cochrane Effective Practice and Organisation of Care (EPOC) Group has categorised these interventions in a taxonomy of implementation strategies, financial arrangements, delivery arrangements and governance arrangements (EPOC 2015a). Examples of interventions relevant to this review include audit and feedback, educational meetings and local opinion leaders (implementation strategies), targeted financial incentives (financial arrangements) and care pathways for linking evidence to practice (delivery arrangements).
How the intervention might work
Implementation interventions aim to produce change in people's behaviour or the environments in which they operate, or both. Implementation interventions may target change at one or more levels (e.g. individual healthcare professionals, teams, organisations, systems) and may be tailored to overcome identified barriers to implementation (Baker 2015). For example, audit and feedback, which involves providing a summary of clinical performance to healthcare professionals over a specified period of time, is hypothesised to work by changing healthcare professionals' awareness and beliefs about their current practice and subsequent consequences, changing perceived subjective norms, self‐efficacy or by directing attention to a set of specific tasks (Ivers 2012). The use of opinion leaders is another implementation intervention, where an individual in a socially influential position within a system is able to promote and affect behavioural change through informal leadership. This implementation strategy is proposed to work via persuasive communication and interpersonal skills, where opinion leaders assist others to identify best‐practice evidence and then catalyse change (Flodgren 2019).
Why it is important to do this review
While evidence about the effects of various implementation interventions is growing (Arditi 2017; Baker 2015; Flodgren 2019; Forsetlund 2009; Giguère 2012; Ivers 2012; O'Brien 2007; Shojania 2009; Squires 2014), observed effects vary across different settings, healthcare professional groups and clinical areas making it difficult to determine which strategies are effective in stroke rehabilitation.
Only one systematic review of implementation interventions specific to stroke rehabilitation has been published to our knowledge (Bird 2019). However, this review has a number of methodological flaws that are likely to bias the results (e.g. synthesis based on vote counting of statistically significant studies, GRADE not appropriately applied to specific outcomes).
Synthesis of the available evidence conducted according to the guidelines recommended by Cochrane is warranted to produce reliable evidence of the effects of implementation interventions in stroke rehabilitation.
This review incorporates a previous Cochrane Review on in‐hospital care pathways for stroke (Kwan 2004), and complements another Cochrane Review on implementation interventions in acute stroke units (Luker 2017).
Objectives
To assess the effects of implementation interventions to promote the uptake of evidence‐based practices (including clinical assessments and treatments recommended in evidence‐based guidelines) in stroke rehabilitation and to assess the effects of implementation interventions tailored to address identified barriers to change compared to non‐tailored interventions in stroke rehabilitation.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised trials, including cluster and stepped wedge randomised trials; non‐randomised trials; interrupted time series studies and controlled before‐after studies. Randomised and non‐randomised trials were required to have at least two intervention and two control sites to be considered eligible for inclusion (EPOC 2016a). Interrupted time series studies were required to have a clearly defined intervention point, and at least three data points before and three after the intervention. Controlled before‐after studies were required to have contemporaneous data collection, and at least two intervention and two appropriate control sites. We decided to include non‐randomised study designs due to the acknowledged complexity of stroke rehabilitation interventions and the potential benefits of pragmatic controlled designs in implementation research (Glasgow 2013). Where available, we also included economic evaluations, such as cost‐effectiveness analyses, cost‐utility analyses and cost‐benefit analyses, conducted alongside the designs specified above.
We considered full‐text studies, conference abstracts and unpublished data, and reviewed studies irrespective of their publication status and language of publication.
Types of participants
Healthcare professionals
We included qualified healthcare professionals providing rehabilitation for stroke survivors and the patients they cared for. Examples of healthcare professionals involved in stroke rehabilitation include doctors, nurses, occupational therapists, physiotherapists, speech therapists, dieticians, social workers, psychologists and pharmacists; we considered studies with any healthcare professional working in the area of stroke for inclusion in the review. We excluded studies focused on entry‐level students (e.g. undergraduate students).
Defining therapy provided in stroke rehabilitation
We used the World Health Organization (WHO) definition of rehabilitation to guide inclusion, where rehabilitation is: "a process aimed at enabling (individuals) to reach and maintain their optimal physical, sensory, intellectual, psychological and social functional levels. Rehabilitation provides disabled people with the tools [strategies] they need to attain independence and self‐determination" (WHO 2015). It is recommended that stroke rehabilitation involves principles of individualised goal‐setting, the involvement of a multidisciplinary team, provision of education and encouragement of long‐term self‐management (Langhorne 2011). A range of therapies may be delivered in the context of stroke rehabilitation. Studies considered for inclusion involved provision of evidence‐based practices in stroke rehabilitation, as defined above, and were aimed at either the impairment level (e.g. muscle power and tone, cognitive processing, or speech and language deficits), the activity level (e.g. mobility, dressing or meal preparation) or the participation level (leisure activities or employment) (WHO 2011). Addressing psychosocial issues is also of importance in stroke rehabilitation (Dewey 2007), as such, we also considered for inclusion studies involving the provision of social or psychological‐based therapy (e.g. counselling).
Defining therapy settings in stroke rehabilitation
Stroke rehabilitation is care provided to a patient once they are medically stable; it may be commenced soon after stroke (24 to 48 hours) and occur in acute, subacute or community settings. We defined rehabilitation settings as the following (Turner‐Stokes 2015).
Inpatient settings: rehabilitation is provided in the context of 24‐hour care, in either a hospital ward or a specialist rehabilitation unit.
Outpatient or day treatment settings: rehabilitation is provided in a hospital context, a specialist subacute rehabilitation facility or a community venue.
Domiciliary or home‐based settings: rehabilitation is provided in a patient's home or local community.
We considered for inclusion qualified healthcare professionals delivering stroke rehabilitation and patients receiving stroke rehabilitation in any of the settings outlined above. Given the related Cochrane Review on implementation interventions in acute stroke units (Luker 2017), we excluded studies focused solely on acute stroke units from this review. Where studies reported on implementation interventions across settings (e.g. for healthcare professionals working in both acute stroke units and hospital rehabilitation wards), we extracted data from rehabilitation settings separate to acute stroke settings.
Types of interventions
We included interventions designed to increase the uptake of evidence‐based practices (including clinical assessments and treatments recommended in evidence‐based guidelines) in stroke rehabilitation, and to bring about changes in the behaviour of healthcare professionals or stroke services, or both. We used the EPOC taxonomy to categorise interventions (EPOC 2015a).
Examples include audit and feedback, educational materials, educational meetings, educational outreach visits, local opinion leaders and reminders.
We described interventions according to the Template for Intervention Description and Replication (TIDieR) (Hoffman 2014).
We compared implementation interventions to no intervention or to a different implementation intervention.
Types of outcome measures
Primary outcomes
-
Quality of care
Measures of healthcare professional adherence to evidence‐based practice, operationalised as the extent to which the healthcare professional gave recommended advice or delivered recommended interventions (e.g. as measured by a case note audit tool).
Secondary outcomes
-
Patient outcomes
Measures of patient health behaviour, that is, adherence to recommended treatment.
Measures of patient health status and well‐being, including physical health and treatment outcomes, psychological health and psychosocial outcomes (e.g. activity of daily living (ADL) measures, stroke impact scales, quality of life measures).
-
Healthcare professional outcomes
Measures of healthcare professional reported intention to change behaviour and other hypothesised mediators, including knowledge, attitudes, and beliefs and skills.
Measures of healthcare professional satisfaction.
-
Resource use outcomes
Economic outcomes, including, cost‐effectiveness, where effectiveness is measured according to the primary outcome (e.g. cost per quality‐adjusted life year (QALY) or disability‐adjusted life year (DALY)).
Resources needed to provide the intervention, for example, human resources, training, equipment and supplies.
Where available, we also evaluated economic measures related to the longer‐term consequences of successful or unsuccessful implementation.
-
Adverse effects/harms
We considered any undesirable effects reported in included studies.
We considered short‐term and long‐term outcomes, as available, to assess sustainability of any effects.
Search methods for identification of studies
Electronic searches
We developed a search strategy in consultation with the EPOC Information Specialist. We searched the following electronic databases on 17 October 2019:
the Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library (2019, Issue 10);
MEDLINE OvidSP (from 1946);
Embase OvidSP (from 1947);
PsycINFO OvidSP (from 1967);
CINAHL (Cumulative Index to Nursing and Allied Health Literature) EBSCOhost (from 1980);
PDQ‐Evidence (www.pdq-evidence.org).
The search strategies are shown in Appendix 1.
We used two methodological filters to limit results; the Cochrane Highly Sensitive Search Strategy (sensitivity‐ and precision‐maximising version) to identify randomised trials in MEDLINE (Higgins 2019), and an EPOC methodology filter to identify non‐randomised trial designs.
We searched the Cochrane Database of Systematic Reviews (CDSR) and the Database of Abstracts of Reviews of Effects (DARE) for related systematic reviews.
Searching other resources
Grey literature
We searched OpenGrey on 17 October 2019 to identify potentially relevant studies not indexed in the databases listed above (www.opengrey.eu/).
Trial registries
We searched the following registries for unpublished and in‐progress studies on 17 October 2019.
WHO International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/en/).
United States National Institutes of Health (NIH) Clinical Trials.gov (clinicaltrials.gov/).
Australian New Zealand Clinical Trials Registry (ANZCTR) (www.anzctr.org.au).
See Appendix 1 for terms used to search trial registries.
We also:
conducted citation tracking and reference checking on all included studies;
contacted study authors to obtain further information and identify unpublished data and studies;
reviewed all studies included in a previous review on in‐hospital care pathways for stroke (Kwan 2004), and conducted citation tracking for this review;
contacted authors known in the implementation science field in stroke rehabilitation regarding any other published or unpublished studies.
Data collection and analysis
Selection of studies
At least two of five review authors (LSC, MT, CN, EL, CM) independently screened all titles and abstracts identified from the search and coded them as potentially eligible or ineligible. At least two of three review authors (LSC, MT and CN) independently screened the full‐text versions of potentially eligible records and identified studies for inclusion and recorded reasons for exclusion of ineligible studies in the Characteristics of excluded studies table. We used Covidence for the screening of titles, abstracts and full‐text reports (Covidence 2018). We resolved any disagreements through discussion or consulting with a third review author (LMC, NAL or DO).
We recorded the process of study selection in a PRISMA flow diagram (Liberati 2009).
Data extraction and management
At least two of three review authors (LSC, CN, JH) independently extracted data from included studies using a modified version of the Cochrane EPOC data collection form (EPOC 2013). The form was piloted on a sample of included studies prior to use and changes were made to refine the questions used to guide data extraction. Categories of items extracted included: country, setting (including time poststroke), study design, characteristics of participants, characteristics of therapy, targeted behaviour change, implementation intervention/strategy (including rationale and theoretical underpinning, intervention components, mode/s of delivery, frequency, duration, provider characteristics and tailoring), the comparison intervention, outcomes, costs and adverse events. We prepared a TIDieR table for each included study. We resolved any disagreements in data extraction through discussion or by involving a third review author (LMC, NAL or DO).
Assessment of risk of bias in included studies
At least two of three review authors (LSC, CN, JH) independently assessed the risk of bias of each included study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019), and additional criteria specified by Cochrane EPOC (EPOC 2016b). We resolved any disagreements through discussion or by involving a third review author (DO or MT).
Randomised trials, non‐randomised trials and controlled before‐after studies
We assessed the risk of bias of randomised trials, non‐randomised trials and controlled before‐after studies according to the following domains.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective outcome reporting.
Other bias: recruitment bias, incorrect analysis.
We assessed four additional criteria specified by EPOC under 'other bias': similarity of baseline characteristics, similarity of baseline outcome measures, reliability of primary outcome measures and adequate protection against contamination (EPOC 2016b).
We judged each potential source of bias as high, low or unclear risk with justification for our judgement in the 'Risk of bias' table for each study. We represented the findings for each study and outcome in a 'Risk of bias' summary figure. When considering treatment effects, we considered the risk of bias for the studies that contributed to that outcome and incorporated this into our judgements about the certainty of the evidence.
Interrupted times series studies
We planned to assess the risk of bias of interrupted time series studies using the seven criteria specified by EPOC: intervention independent of other changes; shape of the intervention effect prespecified; intervention unlikely to affect data collection; knowledge of the allocated interventions adequately prevented during the study; incomplete outcome data adequately considered; selective outcome reporting; other bias (EPOC 2016b); however, we identified no eligible interrupted time series studies in the search. If the study ignored trend changes and conducted a simple t‐test of intervention periods without additional support for this decision, the study would not have been eligible for inclusion unless reanalysis was possible.
We assessed the methodological quality of economic evaluations using the 19‐item Consensus on Health Economic Criteria (CHEC) list (Evers 2005), and prepared a CHEC table for each included study.
Measures of treatment effect
Outcomes
We planned to report outcome data from different types of study designs separately; however, all eligible studies were cluster randomised trials. For each outcome category (e.g. healthcare professional adherence to evidence‐based practice, patient health behaviour, patient health status and well‐being, etc.), we included the outcome identified as the primary outcome by the study authors and checked this was consistent with trial protocols and trial registry entries. When the primary outcome was not specified, we used the outcome reported in the sample size calculation. When no sample size calculation was reported or multiple primary outcomes were specified, we ranked the effect estimates of the outcomes and selected the outcome with the median effect estimate. For dichotomous outcomes with the same comparison, we used the generic inverse variance method to compare transformed effect estimates on the logarithmic scale. For continuous outcomes, we used the mean difference (MD) (where the same tool was used to measure outcomes across studies) to compare effect sizes. We prepared a structured summary of results when meta‐analysis was not possible.
Measures of treatment effect for randomised trials, non‐randomised trials and controlled before‐after studies
For included outcomes, we prepared a structured summary of effects that included the intervention effect estimate, its 95% confidence interval (CI), P value and the method of statistical analysis used to calculate it. To make comparisons between studies, we planned to calculate risk ratios (RR) or adjusted risk differences with 95% CI for dichotomous outcomes and MDs (where the same tool was used to measure an outcome across studies) or standardised mean differences (SMD) with 95% CI for continuous outcomes. We used Cochrane's statistical software, Review Manager 5 to perform data analysis (Review Manager 2014).
Measures of treatment effect for interrupted time series studies
We planned to measure interrupted time series of trends before and after the intervention using regression analysis, with adjustment for autocorrelation. We planned to present results as changes along two dimensions: change in level and change in slope of the outcome, where change in level is the immediate effect of the intervention and change in slope is the change in trend from pre‐ to postintervention (EPOC 2015b). However, we identified no eligible interrupted time series studies.
Unit of analysis issues
We evaluated the analysis methods of clustered studies by determining the level of analysis (i.e. individual level or cluster level) and the use of statistical correction (i.e. generalised estimating equations, mixed models (random effects) and multilevel models). Where we identified unit‐of‐analysis problems, we conducted analyses adjusting for clustering. We sought estimates of intracluster correlation (ICC), an estimate of the similarity within and between clusters (Donner 1981), from study authors where these were not published. We planned to consider temporal trends in stepped wedge cluster randomised trials which may introduce the confounding effect of time (Hemming 2015), though we identified no eligible stepped wedge cluster randomised trials.
Dealing with missing data
We attempted to obtain additional necessary and unpublished information from the authors of included studies through personal communication. Communication with authors and access to additional outcome data is noted in the Characteristics of included studies table.
Assessment of heterogeneity
We assessed the clinical and methodological diversity of included studies in terms of participants, interventions, outcomes and study characteristics to determine whether a meta‐analysis was appropriate. Statistical heterogeneity was assessed by visual inspection of the forest plots and using the I2 test. We interpreted I2 values, consistent with Cochrane Handbook for Systematic Reviews of Interventions guidance (Higgins 2019), as follows: 0% to 40% might not be important; 30% to 60% may represent moderate heterogeneity; 50% to 90% may represent substantial heterogeneity; 75% to 100% represents considerable heterogeneity. We did not conduct a meta‐analysis when there was substantial or considerable heterogeneity (I2 > 50%).
Assessment of reporting biases
To assess outcome reporting bias, we checked trial protocols and online trial registries against published reports for discrepancies between planned and reported outcomes. We contacted authors for unpublished data, for example in the case of an included conference abstract without full published results.
If trial protocols were unavailable, we compared the outcomes reported in the methods and results sections of the trial reports. To assess small‐study effects, we planned to generate funnel plots for meta‐analyses including at least 10 trials of varying sizes. If there was asymmetry in the funnel plot, we planned to review the characteristics of the trials to assess whether the asymmetry was likely due to publication bias or other factors such as methodological or clinical diversity.
Data synthesis
For each comparison, we prepared a structured summary of effects, ordered by outcome. The tables reported summary data for intervention and control arms of included studies, the intervention effect estimates, 95% CIs, P values and statistical analyses used. Where possible, we pooled outcome data across studies. We used the generic inverse variance outcome type in Review Manager 5 to pool outcome data from studies reporting effect estimates and standard errors (SE) but not separate summary data for intervention and control arms (Review Manager 2014). We reported RRs with 95% CIs for dichotomous outcomes and MDs or SMDs with 95% CI for continuous outcomes. Expecting differences in effects across studies, we used random‐effects models. We presented a structured synthesis of effects where meta‐analysis was not possible.
'Summary of findings' table and GRADE
We created a 'Summary of findings' table for implementation interventions compared to no intervention using the following main outcomes:
healthcare professional adherence to evidence‐based practice (measure of quality of care);
patient adherence to recommended treatment (measure of patient health behaviour);
quality of life (measure of patient health status);
activities of daily living (ADL) (measure of patient health status);
psychological well‐being (measure of patient health status);
healthcare professional intention to change behaviour.
Two review authors (DO, LSC) independently assessed the certainty of the evidence for outcomes in the 'Summary of findings' table as high, moderate, low or very low certainty. We used the five GRADE considerations (risk of bias, consistency of effect, imprecision, indirectness and publication bias) and justified decisions to downgrade or upgrade the quality of studies in the footnotes of the table. We resolved any disagreements through discussion or consulting with a third review author (LMC). We developed the 'Summary of findings' table using GRADEpro software (GRADEpro 2015; Guyatt 2011).
Subgroup analysis and investigation of heterogeneity
We planned to conduct subgroup analyses to assess if there were differences in main outcomes for the primary comparison of implementation interventions versus no intervention according to:
study design (i.e. randomised trials, non‐randomised trials; interrupted time series studies, controlled before‐after studies);
intervention type according to EPOC taxonomy (EPOC 2015a);
population group (i.e. adults versus children; acute/subacute stroke survivors (sex months' poststroke or less) versus chronic stroke survivors (six months' poststroke or greater));
setting for stroke rehabilitation (i.e. acute setting, inpatient rehabilitation, outpatient or home‐based setting); and
tailored versus non‐tailored interventions.
Sensitivity analysis
We planned to conduct a sensitivity analysis to investigate the robustness of the main outcome effect estimates to potential risk of bias for the primary comparison of implementation interventions versus no intervention. We excluded studies judged at high or unclear risk of bias for allocation concealment (selection bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective outcome reporting (reporting bias) and unit of analysis issues from secondary analyses.
Results
Description of studies
Results of the search
The search identified 24,884 records. After removal of duplicates, we screened 17,869 records. We retrieved 169 studies for full‐text screening. We selected nine studies for inclusion (Abdul Aziz 2014; Forster 2013; Forster 2015; McCluskey 2016; Pennington 2005; Power 2014; Salbach 2017; Strasser 2008; Thomas 2015). We excluded 157 studies: 127 used ineligible study designs; nine did not evaluate implementation interventions; 15 were not concerned with stroke rehabilitation, two did not involve an evidence‐based practice and four were conducted in settings ineligible for this review (see a selection of excluded studies in the Characteristics of excluded studies table). A PRISMA flow diagram of the screening and selection process is presented in Figure 1.
1.
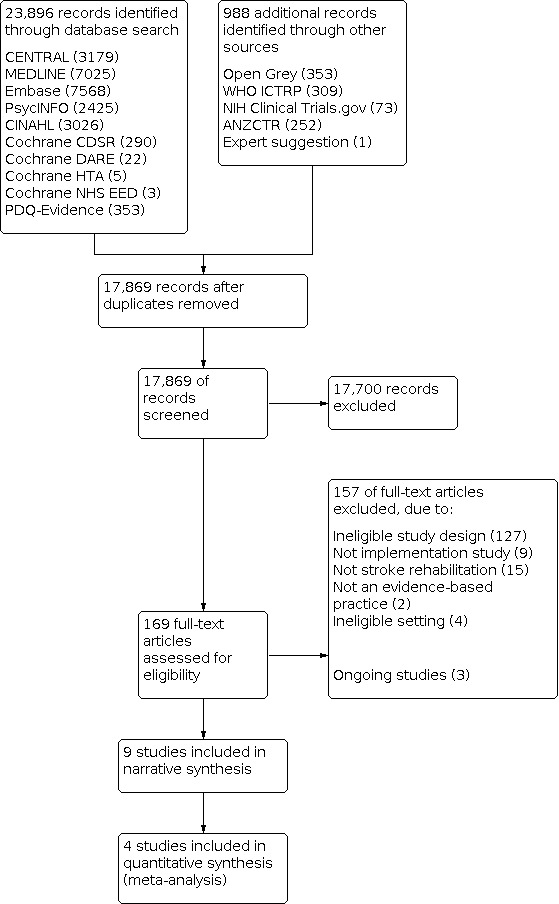
PRISMA flow chart.
Included studies
A full description of the nine included studies is provided in the Characteristics of included studies table.
Study design and setting
All nine studies were cluster randomised trials. Five studies were conducted in the UK (Forster 2013; Forster 2015; Pennington 2005; Power 2014; Thomas 2015), one in Australia (McCluskey 2016), one in Canada (Salbach 2017), one in Malaysia (Abdul Aziz 2014), and one in the US (Strasser 2008). Two studies focused on stroke rehabilitation in the acute setting (Pennington 2005; Power 2014), two studies provided rehabilitation in a subacute inpatient setting (Forster 2013; Salbach 2017), and three studies involved stroke rehabilitation in the community; either in an outpatient clinic or the patient's home (Abdul Aziz 2014; Forster 2015; McCluskey 2016). Two studies were set in both an acute and subacute inpatient rehabilitation setting (Strasser 2008; Thomas 2015). As per our protocol, studies occurring solely in acute stroke units were excluded given another Cochrane Review with this focus (Luker 2017). Results of seven studies were reported in peer‐reviewed publications (Forster 2013; Forster 2015; McCluskey 2016; Pennington 2005; Power 2014; Salbach 2017; Strasser 2008), one in a conference abstract (Abdul Aziz 2014), and one in a UK National Institute of Health Programme Grants report (Thomas 2015). All studies investigated the effects of implementation interventions. Two studies had a dual focus of investigating clinical effectiveness as well as the effect of an implementation intervention (Forster 2013; Forster 2015). The full results of two trials are not yet published (Abdul Aziz 2014; Salbach 2017); we obtained data from the authors (Abdul Aziz 2014), or an associated publication (Salbach 2017). All studies were published in English.
Ongoing studies
Three study protocols for ongoing trials are listed in Characteristics of ongoing studies table (Duncan 2017; McEwan 2015; NCT03807115).
Participants
Healthcare professionals
Healthcare professional participants included doctors, nurses, dieticians, occupational therapists, physiotherapists, speech pathologists, social workers and radiographers. Six studies involved a multidisciplinary team (Abdul Aziz 2014; Forster 2013; Power 2014; Salbach 2017; Strasser 2008; Thomas 2015), one involved two disciplines; occupational therapy and physiotherapy (McCluskey 2016), and two studies focused solely on speech pathologists (Pennington 2005) or stroke care co‐ordinators (Forster 2015). The characteristics of healthcare professionals participating in studies were not well described, with no study reporting sex or years of experience of participants. Only one study provided information on the number of healthcare professional participants (Strasser 2008; 464 healthcare professional participants).
Patients
Patient participants were people with stroke who ranged from being in the acute phase immediately poststroke (Power 2014) to a chronic phase where the median time poststroke was 2.25 years (Abdul Aziz 2014). Measures of central tendency for age were reported for all studies except two (Pennington 2005; Power 2014), and the mean or median age of stroke survivors was greater than 60 years in all studies reporting this information (range 60.2 to 72.5) . All studies reported the sex of patient participants except two (Abdul Aziz 2014; Pennington 2005). The numbers of male and female patient participants were generally balanced in the studies, except in one study where males comprised more than 95% of participants (Strasser 2008).
Targeted evidence‐based practices
The evidence‐based practices targeted by studies is detailed in Table 1 blow.
Evidence cited by authors for practices were stroke clinical practice guidelines (Forster 2015; McCluskey 2016; Pennington 2005; Salbach 2017), national stroke audits (Power 2014), Cochrane systematic reviews (Abdul Aziz 2014; Forster 2013; Thomas 2015), or a rehabilitation accreditation document. Strasser 2008 cited evidence for their targeted practice (effective multidisciplinary team functioning) from a rehabilitation standards manual, though this practice is also recommended in clinical practice guidelines.
Intervention
Comparison and control groups
Five studies compared an implementation intervention to no intervention (Abdul Aziz 2014; Power 2014; Forster 2013; Forster 2015; McCluskey 2016); three studies compared one implementation intervention to another (Pennington 2005; Salbach 2017; Strasser 2008); and one study used three trial arms, two arms involving different implementation interventions and one arm with no intervention (Thomas 2015) (Table 1 below).
Table 1 Interventions, comparisons and targeted evidence‐based practices in included studies
| Study ID | Implementation intervention | Control | Targeted evidence‐based practice |
| Abdul Aziz 2014 |
|
No intervention | For multidisciplinary teams to use a clinical care pathway in the community (iCaPPS: Integrated Care Pathway for managing poststroke patients) |
| Forster 2013 |
|
No intervention | For multidisciplinary teams to provide carer training to carers of people with recent stroke (LSCTC: the London Stroke Carers Training Course) |
| Forster 2015 |
|
No intervention | For stroke care co‐ordinators to use a new service model in the community (LoTS: the Longer‐term stroke care system) |
| McCluskey 2016 |
|
Copy of clinical practice guidelines (no intervention; guideline freely available) | For occupational therapists and physiotherapists to increase the number of community outings provided to stroke survivors during therapy |
| Pennington 2005 |
|
Education meeting (2.5‐day workshop, no theory) and tailoring | For speech and language therapists to adhere to clinical practice guidelines for poststroke dysphagia management (e.g. assessments including trial of food consistencies) |
| Power 2014 |
|
No intervention | For multidisciplinary teams to adhere to a 'bundle' of care involving 9 processes (e.g. timely assessment, goal setting) |
| Salbach 2017 |
|
Provision of clinical practice guideline (without treatment protocols) and educational materials | For multidisciplinary teams to provide 18 recommended treatments for physical rehabilitation (e.g. task‐specific training, functional electrical stimulation) |
| Strasser 2008 |
|
Audit and feedback | For multidisciplinary teams to function more effectively as a team |
| Thomas 2015 | 3‐armed trial Intervention A:
Intervention B:
|
No intervention | For multidisciplinary teams to provide a systematic voiding programme for poststroke incontinence |
Eight studies investigated implementation strategies (Forster 2013; Forster 2015; McCluskey 2016; Pennington 2005; Power 2014; Salbach 2017; Strasser 2008; Thomas 2015). The most commonly used implementation intervention was provision of education, either as single‐discipline educational meetings or interprofessional education meetings; all eight trials used this. Seven trials provided educational materials (Forster 2015; Forster 2013; McCluskey 2016; Pennington 2005; Salbach 2017; Strasser 2008; Thomas 2015), and three trials used audit and feedback (McCluskey 2016; Power 2014; Strasser 2008). Four trials used local opinion leaders (site leads, internal facilitators) (Forster 2013; Power 2014; Salbach 2017; Thomas 2015). Two trials used a form of community of practice, involving teleconferences or web‐based sharing sessions (Power 2014; Salbach 2017), though the authors did not use the term 'community of practice' in their description of this intervention. All studies used multifaceted interventions, except one (Abdul Aziz 2014). Two studies investigated the use of delivery arrangements (i.e. changes to how healthcare is organised and delivered (EPOC 2015a)) to promote uptake of evidence‐based practices (Abdul Aziz 2014; Thomas 2015). This included use of an integrated care pathway (Abdul Aziz 2014) and increasing healthcare staffing and availability of equipment (Thomas 2015). See additional Table 2 for TIDieR information available and Appendix 2 for TIDieR descriptions of interventions.
1. TIDieR information available across studies.
| Study | Information available | |||||||
|
Who provided |
How | Where |
When and how much |
Tailoring | Modification |
Strategies fidelity |
Extent fidelity |
|
| Abdul Aziz 2014 | √ | √ | √ | √ | X | X | X | X |
| Forster 2013 | √ | √ | X | √ | √ | X | √ | X |
| Forster 2015 | √ | √ | X | √ | X | X | X | X |
| McCluskey 2016 | √ | √ | √ | √ | √ | √ | X | X |
| Pennington 2005 | √ | √ | √ | √ | √ | X | X | X |
| Power 2014 | √ | X | X | √ | X | X | X | X |
| Salbach 2017 | X | √ | X | √ | √ | X | X | √ |
| Strasser 2008 | √ | √ | √ | √ | √ | X | √ | √ |
| Thomas 2015 | X | √ | X | X | √ | √ | √ | X |
√: yes; X: no; TIDieR: Template for Intervention Description and Replication.
Interventions tailored to identified barriers to change
Six studies used barrier identification and tailoring (Forster 2013; McCluskey 2016; Pennington 2005; Salbach 2017; Strasser 2008; Thomas 2015). Methods to identify barriers included interviews with participating healthcare professionals (McCluskey 2016; Thomas 2015), and focus groups with healthcare professionals and managers as part of a pilot study (Salbach 2017). Two studies used group discussion in training sessions to identify barriers (Forster 2013; Salbach 2017) while another asked team leaders to individually identify barriers and modify implementation action plans accordingly (Strasser 2008). One study used a combination of soft systems analysis, an evidence synthesis and interviews with healthcare professionals to identify barriers (Thomas 2015).
Four studies did not provide information on mapping barriers to change to implementation interventions (McCluskey 2016; Pennington 2005; Salbach 2017; Strasser 2008). Two studies used constructs in Normalisation Process Theory to explore barriers (Forster 2013; Thomas 2015).
Theoretical approaches
Five studies used theory or theoretical frameworks to inform design of implementation interventions: Normalisation Process Theory (Forster 2013; Thomas 2015); Roger's Diffusion of Innovation (Pennington 2005); the Knowledge to Action framework (Salbach 2017); and Lichstein's treatment implementation model (Strasser 2008). Three studies referred to a model or framework to guide the practice being implemented, for example the biopsychosocial model of health (Abdul Aziz 2014), the breakthrough series model (Power 2014), and the Medical Research Council framework (Forster 2015). One study did not report use of theory (McCluskey 2016).
Intervention fidelity
Four studies considered the fidelity of implementation interventions (Forster 2013; Thomas 2015; Salbach 2017; Strasser 2008). One study recorded programme deviations and difficulties with implementation (Thomas 2015). Salbach 2017 recorded the number of facilitators from sites attending training, while Forster 2013 recorded the number of cascade training sessions completed at sites. Strasser 2008 used a treatment implementation framework to promote fidelity and used records kept by research staff and questionnaires from healthcare professionals to monitor consistency of the implementation intervention across sites.
Forster 2013 reported a general measure of intervention fidelity and stated training was not cascaded among staff as widely as intended; 7/18 intervention clusters reported no cascade training occurred. Salbach 2017 reported 100% of facilitators from all sites attended the training workshop and Strasser 2008 reported 60% of sites documented implementing activities from training in their work environment.
No other study detailed whether the implementation intervention was delivered as planned.
Outcomes
Quality of care
Four studies reported quality of care measures, that is, healthcare professionals' adherence to recommended evidence‐based treatment (McCluskey 2016; Pennington 2005; Power 2014; Salbach 2017). McCluskey 2016 reported the proportion of patients receiving community outings during rehabilitation in accordance with clinical practice guidelines, measured through an audit of medical records. Pennington 2005 reported mean compliance with clinical practice guidelines for dysphagia management measured through an audit of case notes. Power 2014 reported compliance with acute and rehabilitation bundles of stroke care measured through an audit of patient registry information provided by sites. Salbach 2017 reported the number of times recommended treatments were implemented to address upper and lower extremity motor function, postural control and mobility, and used a self‐report checklist completed by healthcare professionals to measure this.
Patient outcomes
Measures of patient health behaviour
Only one study reported a measure of patient health behaviour, in the form of number of community outings taken per week by stroke survivors (McCluskey 2016). This outcome was measured through a patient self‐report diary.
Measures of patient health status and well‐being
Most studies reported one or more patient health status or well‐being measures. ADL was the most common, with five studies reporting this outcome using the Barthel Index (Abdul Aziz 2014; Forster 2013; Forster 2015; Thomas 2015), Nottingham Extended Activities of Daily Living (NEADL) scale (Forster 2013), Frenchay Activity Index (FAI) (Forster 2015), or the Functional Independence Measure (FIM) (Strasser 2008). Four studies measured health‐related quality of life using the EuroQol 5‐dimension health state measure (EQ‐5D) (Abdul Aziz 2014; Forster 2013; Forster 2015; Thomas 2015). Thomas 2015 investigated quality of life specific to continence status using the Incontinence Quality of Life (I‐QOL) measure; Thomas 2015 also measured continence using the International Consultation on Incontinence Questionnaire – Urinary Incontinence Short Form (ICIQ‐UI) and the Incontinence Severity Index (ISI). One study used the Stroke Impact Scale (SIS) (Forster 2013), and three studies measured psychological well‐being using the Patient Health Questionnaire‐9 (PHQ‐9), Two Questions With Help Questionnaire (TQWHQ) (Abdul Aziz 2014), General Health Questionnaire‐12 (GHQ‐12) (Forster 2015), and the Hospital Anxiety and Depression scale (HADS) (Forster 2013). One study measured patient cognition using the Modified Mini‐Mental Status Examination (M‐MMSE) and Elderly Cognitive Assessment Questionnaire (ECAQ) (Abdul Aziz 2014).
Two studies measured patient mobility using the Life‐Space Assessment (McCluskey 2016) and Six‐Minute Walk Test (6MWT) (Salbach 2017). One study measured upper limb function using the Box and Block Test (Salbach 2017).
Three studies reported death (Forster 2013; Forster 2015; Thomas 2015).
Healthcare professional outcomes
No studies reported healthcare professional intention to change behaviour or other hypothesised mediators of change, including knowledge, attitudes, beliefs or skills. No studies reported healthcare professional satisfaction.
Resource use outcomes
Five studies reported economic data (Abdul Aziz 2014; Forster 2013; Forster 2015; Pennington 2005; Thomas 2015). Economic analyses ranged from cost descriptions to cost‐effectiveness analyses. Four studies provided a comparison of costs per QALY (Abdul Aziz 2014; Forster 2013; Forster 2015; Thomas 2015). Three studies reported incremental cost‐effectiveness ratio (ICER) (Abdul Aziz 2014; Forster 2013; Thomas 2015). Four studies reported financial resources needed to provide the intervention, for example, for training (Forster 2013; Forster 2015; Pennington 2005; Thomas 2015).
Adverse effects
Three studies reported information on adverse effects (Forster 2013; Forster 2015; Thomas 2015).
Time points and follow‐up
Most studies used time points related to the implementation intervention; one study used time following a patient's stroke, for example, six, 12 and 52 weeks after stroke for assessment time points (Thomas 2015). Three studies reported outcomes at six months after delivery of the intervention (Abdul Aziz 2014; Forster 2015; Forster 2013), one at eight to 12 months (Pennington 2005), and five studies at 12 months (Forster 2013; Forster 2015; McCluskey 2016; Power 2014; Strasser 2008), though assessment periods (for example, a file audit) could have been over an extended timeframe. One study reported outcomes immediately after the intervention period, which lasted 16 months (Salbach 2017).
Excluded studies
The most common reason for excluding studies was ineligible study designs. A full description of notable excluded trials is provided in the Characteristics of excluded studies table.
Risk of bias in included studies
See 'Risk of bias' tables in Characteristics of included studies table, Figure 2 for a graph of risk of bias items presented as percentages across all included studies and Figure 3 for a summary of judgements about each risk of bias item.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Allocation was generally well described across studies. Random sequence generation was judged adequate (low risk) in all included studies, with methods such as simple coin toss, computer‐generated random numbers and statistical software such as Stata or R used. Five studies used stratification of clusters (e.g. by geographical region or quality of care) (Forster 2013; Forster 2015; Salbach 2017; Strasser 2008; Thomas 2015), and one study used a published peer‐reviewed algorithm with the aim of balanced cluster randomisation (Forster 2015). One study used minimisation, a form of covariate adaptive randomisation (McCluskey 2016).
Allocation concealment was of low risk in seven studies (Abdul Aziz 2014; Forster 2015; McCluskey 2016; Pennington 2005; Power 2014; Salbach 2017; Strasser 2008), with two studies having unclear risk (Forster 2013; Thomas 2015). Three studies described allocation as managed by an independent individual or randomisation service (Forster 2015; McCluskey 2016; Pennington 2005). Two studies used screening logs to monitor selection bias (Forster 2013; Forster 2015).
Blinding
Blinding of participants and personnel was at low risk of bias in two studies (Forster 2013; Forster 2015), high risk in five studies (McCluskey 2016; Pennington 2005; Power 2014; Salbach 2017; Thomas 2015), and unclear risk in two studies (Abdul Aziz 2014; Strasser 2008). The lack of clarity in blinding often resulted from the introduction of a new process of care or staff training; though authors stated participants were blinded, the change in procedures and practice reduced the likelihood of true blinding.
Blinding of outcome assessment was at low risk of bias in three studies (Forster 2013; Forster 2015; McCluskey 2016), high risk in five studies (Pennington 2005; Power 2014; Salbach 2017; Strasser 2008; Thomas 2015), and unclear risk in one study (Abdul Aziz 2014). Studies at high risk of bias used an unblinded primary assessor (e.g. due to funding restrictions, Pennington 2005). Studies at low risk used patient‐reported outcome measures, where the patients were blinded (Forster 2013; Forster 2015), or assessments were conducted by an individual blinded to cluster allocation (McCluskey 2016).
Incomplete outcome data
Seven studies were of low risk with either similar levels of missing data between intervention and control groups or proportions of missing data less than the effect size (Forster 2013, Forster 2015; McCluskey 2016; Pennington 2005; Salbach 2017; Strasser 2008; Thomas 2015). One study was of high risk of incomplete outcome data (Power 2014), with differences in missing data between intervention and control groups and incomplete site data not used in analysis. One study was of unclear risk with attrition not discussed or discernible (Abdul Aziz 2014).
Selective reporting
Three studies were of low risk with prespecified primary and secondary outcomes published in a protocol or documented on a trial registry before trial commencement (Forster 2015; McCluskey 2016; Thomas 2015). Four studies were at high risk of reporting bias with full results not published (Abdul Aziz 2014) or discrepancies between outcomes outlined in the study protocol and results paper (Forster 2013; Power 2014; Salbach 2017). The risk of selective reporting was unable to be determined in two studies (Pennington 2005; Strasser 2008).
Other potential sources of bias
Three studies were at low risk of other bias (Forster 2013; McCluskey 2016; Salbach 2017), three studies were at high risk (Abdul Aziz 2014; Pennington 2005; Thomas 2015), and three studies were of unclear risk. The following outlines components of bias contributing to overall judgement.
Recruitment bias
Eight studies were at low risk of recruitment bias (Forster 2013; Forster 2015; McCluskey 2016; Pennington 2005; Power 2014; Salbach 2017; Strasser 2008; Thomas 2015). One study was at high risk due to suggested differential recruitment between intervention and control groups (Abdul Aziz 2014).
Unit of analysis issues
Six studies used analysis methods that accounted for clustering with no suggested unit of analysis issues (Forster 2013; Forster 2015; McCluskey 2016; Power 2014; Salbach 2017; Thomas 2015). Five studies considered covariates such as patient characteristics and site locations in analyses (Forster 2013; Forster 2015; McCluskey 2016; Pennington 2005; Salbach 2017). Five studies reported ICCs (Forster 2013; Forster 2015; Power 2014; Salbach 2017; Thomas 2015). One study did not report on use of any statistical correction methods to account for clustering (Abdul Aziz 2014). In two studies, it was unclear whether analysis had accounted for clustering and unit of analysis issues could not be discounted (Pennington 2005; Strasser 2008). Unit of analysis issues were accounted for through sensitivity analysis.
Similar baseline characteristics
One study was of high risk of bias in this domain with differences in the characteristics of participants at baseline (Thomas 2015). For three studies, there was unclear risk of bias, either because baseline information was unavailable (Abdul Aziz 2014; Strasser 2008), or there were minor differences apparent (Pennington 2005). Five studies were of low risk in this domain as characteristics of participants at baseline were sufficiently similar (Forster 2013; Forster 2015; McCluskey 2016; Power 2014; Salbach 2017).
Similar baseline outcome measures
There were no studies of high risk in this domain. Four studies were of unclear risk, where information was not available on baseline outcome measures (Abdul Aziz 2014), or there were some differences between control and intervention groups but it was unclear if they were significant (Power 2014; Salbach 2017; Strasser 2008). The remainder of studies were of low risk, with similar baseline outcome measures or if imbalanced, an adjusted analysis was performed, such as an analysis of covariance (Forster 2013; Forster 2015; McCluskey 2016; Pennington 2005; Thomas 2015).
Reliable primary outcome measures
The majority of studies used validated primary outcomes and were rated low risk (Abdul Aziz 2014; Forster 2013; McCluskey 2016; Power 2014; Salbach 2017; Strasser 2008; Thomas 2015). Pennington 2005 was rated high risk due to using a tool developed by the authors that had low internal consistency based on published evaluation of reliability. One study was of unclear risk (Forster 2015).
Adequate protection against contamination
Most studies used allocation by cluster level, such as by hospital or public health centre, and were deemed at low risk of contamination (Abdul Aziz 2014; Forster 2013; Forster 2015; McCluskey 2016; Power 2014; Salbach 2017; Strasser 2008; Thomas 2015). There was only one study with unclear bias where the geographical location between clusters and possible communication between participants could not be established (Pennington 2005).
Effects of interventions
See: Table 1
Comparison 1: any implementation intervention versus no intervention
See Table 1 and Table 3 (Structured summary of effects; quality of care) and Table 4 (Structured summary of effects; patient outcomes).
2. Comparison 1: overview of results ordered by outcome, quality of care.
| Implementation intervention vs control | |||||||
| Quality of care | |||||||
| Outcome (scale details) | Intervention | Control | Time point | Effect estimate (metric) | 95% CI | Statistical test | P value |
| McCluskey 2016 | % (n) | % (n) | |||||
| Adherence to clinical practice guideline (patients receiving ≥ 4 escorted outdoor journeys) | 9 (164) | 5 (115) | 12 months | 4% (RD) | –9 to 17 | 0.54 | |
| Mean (n) | Mean (n) | Adjusted mean difference | |||||
| Adherence to clinical practice guideline (number of outdoor journeys patients received) | 1.1 (164) | 0.6 (115) | 12 months | 0.5 | –0.4 to 1.4 | 0.26 | |
| Power 2014 | % (n) | % (n) | |||||
| Adherence to bundle of care (patients receiving rehabilitation bundle e.g. MDT assessment, goal setting) | 46.2 (610) | 33.2 (566) | 12 months | 1.61 (OR) | 1.07 to 2.42 | ICC 0.197 | 0.023 |
CI: confidence interval; MDT: multidisciplinary team; n: number of participants; OR: odds ratio; RD: risk difference.
3. Comparison 1: overview of results ordered by outcome; patient outcomes.
| Implementation intervention vs control | |||||||||||||||||||||||
| Patient outcomes | |||||||||||||||||||||||
| Measures of patient health behaviour | |||||||||||||||||||||||
| McCluskey 2016 | Mean (SD), n | Mean (SD), n | Adjusted mean difference | ||||||||||||||||||||
| Number of outdoor journeys taken by patients outside of therapy per week | 9.0 (3.0), 55 | 7.4 (4.0), 60 | 6 months | 0.5 | –1.8 to 2.8 | 0.63 | |||||||||||||||||
| Measures of patient health status and well‐being | |||||||||||||||||||||||
| Quality of life | |||||||||||||||||||||||
| Study | Intervention | Control | Time point | Effect estimate (metric) | 95% CI | Statistical test | P value | ||||||||||||||||
| Abdul Aziz 2014 Change within groups | |||||||||||||||||||||||
| Median (IQR), n | Median (IQR), n | Mann Whitney | |||||||||||||||||||||
| EQ‐5D | 0.04 (0 to 0.13), 86 | 0.01 (0 to 0.05), 65 | 24 weeks | — | — | –0.386 | 0.699 | ||||||||||||||||
| Forster 2013 | Mean (SE), n | Mean (SE), n | Difference (SE) | ||||||||||||||||||||
| EQ‐5D | 0.44 (0.017), 319 | 0.44 (0.017), 334 | 6 months | –0.002 (0.0225) | –0.048 to 0.045 | ICC 0 | 0.946 | ||||||||||||||||
| Forster 2015 | Mean (SE), n | Mean (SE), n | |||||||||||||||||||||
| EQ‐5D | 0.55 (0.022), 301 | 0.58 (0.025), 288 | 6 months | 0.03 (0.025) | –0.02 to 0.08 | ICC 0.059 (I), 0.014 (C) | 0.252 | ||||||||||||||||
| Thomas 2015 | Intervention A | Control | Effect estimate (metric) | 95% CI | Statistical test | P value | |||||||||||||||||
| Median (IQR), n | Median (IQR), n | ||||||||||||||||||||||
| I‐QOL | 76.1 (42.5 to 94.3), 47 | 72.6 (58.3 to 83.0), 51 | 12 weeks | –5.5 (OR) | –24.1 to 13.1 | ICC 0.216 | — | ||||||||||||||||
| EQ‐5D | n | n | |||||||||||||||||||||
| EQ‐5D Mobility | 129 | 96 | 12 weeks | 0.92 (OR) | 0.52 to 1.62 | ICC 0 | — | ||||||||||||||||
| EQ‐5D Self‐care | 126 | 97 | 12 weeks | 0.45 (OR) | 0.26 to 0.79 | ICC 0 | — | ||||||||||||||||
| EQ‐5D Usual activity | 126 | 97 | 12 weeks | 0.49 (OR) | 0.27 to 0.90 | ICC 0 | — | ||||||||||||||||
| EQ‐5D Pain | 123 | 95 | 12 weeks | 0.73 (OR) | 0.43 to 1.23 | ICC 0 | — | ||||||||||||||||
| EQ‐5D Anxiety | 122 | 95 | 12 weeks | 0.67 (OR) | 0.39 to 1.13 | ICC 0 | — | ||||||||||||||||
| Thomas 2015 | Intervention B | Control | Effect estimate (metric) | 95% CI | Statistical test | P value | |||||||||||||||||
| Median (IQR), n | Median (IQR), n | ||||||||||||||||||||||
| I‐QOL | 67.1 (51.1–85.2), 35 | 72.6 (58.3–83.0), 51 | 12 weeks | –1.9 (OR) | –21.2 to 17.4 | ICC 0.216 | — | ||||||||||||||||
| EQ‐5D | n | n | |||||||||||||||||||||
| EQ‐5D Mobility | 92 | 96 | 12 weeks | 0.79 (OR) | 0.44 to 1.41 | ICC 0 | — | ||||||||||||||||
| EQ‐5D Self‐care | 92 | 97 | 12 weeks | 0.65 (OR) | 0.36 to 1.17 | ICC 0 | — | ||||||||||||||||
| EQ‐5D Usual activity | 91 | 97 | 12 weeks | 0.63 (OR) | 0.34 to 1.16 | ICC 0 | — | ||||||||||||||||
| EQ‐5D Pain | 93 | 95 | 12 weeks | 0.88 (OR) | 0.50 to 1.54 | ICC 0 | — | ||||||||||||||||
| EQ‐5D Anxiety | 92 | 95 | 12 weeks | 0.95 (OR) | 0.54 to 1.67 | ICC 0 | — | ||||||||||||||||
| Activities of daily living | |||||||||||||||||||||||
| Study | Intervention | Control | Time point | Effect estimate (metric) | 95% CI | Statistical test | P value | ||||||||||||||||
| Abdul Aziz 2014 Change within groups | |||||||||||||||||||||||
| Median (IQR), n | Median (IQR), n | Mann Whitney | |||||||||||||||||||||
| Barthel Index | 1.77 (0–5), 86 | 0.94 (0–5), 65 | 24 weeks | — | — | –1.197 | 0.231 | ||||||||||||||||
| Forster 2013 | Mean (SE), n | Mean (SE), n | Difference (SE) | ||||||||||||||||||||
| NEADL | 27.4 (1.00), 330 | 27.6 (0.99), 348 | 6 months | –0.2 (1.34) | –3.0 to 2.5 | ICC 0.027 | 0.866 | ||||||||||||||||
| Barthel Index | 14.2 (0.24), 323 | 14.1 (0.23), 346 | 6 months | 0.1 (0.31) | –0.6 to 0.7 | ICC 0 | 0.825 | ||||||||||||||||
| Forster 2015 | Mean (SE), n | Mean (SE), n | |||||||||||||||||||||
| Barthel Index | 15.3 (0.28), 307 | 15.8 (0.33), 296 | 6 months | 0.5 (0.33) | –0.2 to 1.1 | ICC 0 (I), 0.022 (C) | 0.133 | ||||||||||||||||
| Frenchay Activity Index | 18.0 (0.76), 304 | 19.0 (0.76), 293 | 6 months | 1.0 (0.80) | –0.6 to 2.5 | ICC 0.014 (I), 0 (C) | 0.229 | ||||||||||||||||
| Thomas 2015 | Intervention A | Control | |||||||||||||||||||||
| Median (IQR), n | Median (IQR), n | ||||||||||||||||||||||
| Barthel Index | 8 (4–13), 128 | 11 (4–16), 94 | 12 weeks | 0.71 (OR) | 0.46 to 1.11 | ICC 0 | — | ||||||||||||||||
| Intervention B | Control | ||||||||||||||||||||||
| Barthel Index | 11 (6–15), 95 | 11 (4–16), 94 | 12 weeks | 0.97 (OR) | 0.61 to 1.54 | ICC 0 | — | ||||||||||||||||
| Health status following stroke | |||||||||||||||||||||||
| Study | Intervention | Control | Time point | Effect estimate (metric) | 95% CI | Statistical test | P value | ||||||||||||||||
| Forster 2013 | Mean (SE), n | Mean (SE), n | Difference (SE) | ||||||||||||||||||||
| SIS | |||||||||||||||||||||||
| SIS Physical | 52.7 (1.10), 323 | 52.0 (1.08), 342 | 6 months | 0.7 (1.46) | –2.3 to 3.7 | ICC 0.001 | 0.641 | ||||||||||||||||
| SIS Memory | 70.1 (1.26), 317 | 70.4 (1.23), 343 | 6 months | –0.3 (1.66) | –3.7 to 3.1 | ICC 0 | 0.836 | ||||||||||||||||
| SIS Mood | 70.1 (0.99), 316 | 68.6 (0.96), 338 | 6 months | 1.5 (1.30) | –1.1 to 4.2 | ICC 0 | 0.244 | ||||||||||||||||
| SIS Communication | 80.1 (1.07), 321 | 80.9 (1.05), 340 | 6 months | –0.8 (1.41) | –3.6 to 2.1 | ICC 0 | 0.582 | ||||||||||||||||
| SIS Recover | 54.0 (1.72), 255 | 53.9 (1.67), 293 | 6 months | 0.1 (2.30) | –4.6 to 4.8 | ICC 0.038 | 0.974 | ||||||||||||||||
| SIS Social Participation | 49.5 (1.98), 307 | 50.6 (1.97), 329 | 6 months | –1.1 (2.67) | –6.6 to 4.4 | ICC 0.026 | 0.683 | ||||||||||||||||
| Cognition | |||||||||||||||||||||||
| Study | Intervention | Control | Time point | Effect estimate (metric) | 95% CI | Statistical test | P value | ||||||||||||||||
| Abdul Aziz 2014 Change within groups | |||||||||||||||||||||||
| Median (IQR), n | Median (IQR), n | Mann Whitney | |||||||||||||||||||||
| M‐MMSE | 0.3 (0 to 1.0), 86 | 1.34 (0 to 0.73), 65 | 24 weeks | — | — | –1.209 | 0.227 | ||||||||||||||||
| Elderly Cognitive Assessment Questionnaire | 0.6 (0 to 1.0), 86 | 0.33 (0 to 1.0), 65 | 24 weeks | — | — | –0.997 | 0.319 | ||||||||||||||||
| Mood/psychological well‐being | |||||||||||||||||||||||
| Study | Intervention | Control | Time point | Effect estimate (metric) | 95% CI | Statistical test |
P value | ||||||||||||||||
| Abdul Aziz 2014 Change within groups | |||||||||||||||||||||||
| Median (IQR), n | Median (IQR), n | Mann Whitney | |||||||||||||||||||||
| Mood measured by PHQ‐9 | 0 (–3.0 to 2.0), 86 | –0.92 (–3.0 to 1.0), 65 | 24 weeks | — | — | –0.190 | 0.848 | ||||||||||||||||
| % (n) | % (n) | ||||||||||||||||||||||
| Depression screen TQWHQ (% positive) | 18.6 (86) | 20 (65) | 24 weeks | — | — | Chi2 = 0.05, df = 1 | 0.829 | ||||||||||||||||
| Forster 2013 | Mean (SE), n | Mean (SE), n | Difference (SE) | ||||||||||||||||||||
| Anxiety measured by HADS | 6.7 (0.22), 323 | 6.6 (0.21), 340 | 6 months | 0.1 (0.29) | –0.5 to 0.7 | ICC 0 | 0.629 | ||||||||||||||||
| Depression measured by HADS | 7.3 (0.22), 323 | 7.2 (0.21), 341 | 6 months | 0.1 (0.29) | –0.5 to 0.7 | ICC 0 | 0.759 | ||||||||||||||||
| Forster 2015 | Mean (SE), n | Mean (SE), n | |||||||||||||||||||||
| Psychological well‐being measured by GHQ‐12 | 15.5 (0.60), 310 | 14.9 (0.60), 300 | 6 months | –0.6 (0.65) | –1.8 to 0.7 | ICC 0.025 (I), 0.013 (C) | 0.394 | ||||||||||||||||
| Mobility | |||||||||||||||||||||||
| Study | Intervention | Control | Time point | Effect estimate (metric) | 95% CI | Statistical test |
P value | ||||||||||||||||
| McCluskey 2016 | Mean (SD), n | Mean (SD), n | Adjusted mean difference | ||||||||||||||||||||
| Mobility measured by Life‐Space Assessment (0–120) | 61 (12), 55 | 51 (12), 60 | 6 months | 5 | –5 to 15 | 0.29 | |||||||||||||||||
| Continence | |||||||||||||||||||||||
| Study | Intervention | Control | Time point | Effect estimate (metric) | 95% CI | Statistical test |
P value | ||||||||||||||||
| Thomas 2015 | Intervention A | Control | |||||||||||||||||||||
| % (n) | % (n) | ||||||||||||||||||||||
| Incontinence (Y/N) measured by ICIQ‐UI Short Form | 59 (104) | 70 (80) | 12 weeks | 1.02 (OR) | 0.54 to 1.93 | ICC 0 | — | ||||||||||||||||
| Median (IQR), n | Median (IQR), n | ||||||||||||||||||||||
| ISI | 2.5 (0–8), 102 | 3 (0–6), 80 | 12 weeks | 0.86 (OR) | 0.50 to 1.50 | ICC 0 | — | ||||||||||||||||
| Thomas 2015 | Intervention B | Control | |||||||||||||||||||||
| % (n) | % (n) | ||||||||||||||||||||||
| ICIQ‐UI (Y/N) | 68 (86) | 70 (80) | 12 weeks | 1.06 (OR) | 0.54 to 2.09 | ICC 0 | — | ||||||||||||||||
| Median (IQR), n | Median (IQR), n | ||||||||||||||||||||||
| ISI | 4 (0–8), 86 | 3 (0–6), 80 | 12 weeks | 0.92 (OR) | 0.52 to 1.64 | ICC 0 | — | ||||||||||||||||
| Death | |||||||||||||||||||||||
| Study | Intervention | Control | Time point | Effect estimate (metric) | 95% CI | Statistical test |
P value | ||||||||||||||||
| Forster 2013 | % (n) | % (n) | |||||||||||||||||||||
| Death | 11.8 (450) | 11.5 (478) | 12 months | — | — | — | — | ||||||||||||||||
| Forster 2015 | % (n) | % (n) | |||||||||||||||||||||
| Death | 8.7 (401) | 8.0 (399) | 12 months | — | — | — | — | ||||||||||||||||
| Thomas 2015 | Intervention A | Control | |||||||||||||||||||||
| % (n) | % (n) | ||||||||||||||||||||||
| Death | 40 (152) | 29 (116) | 52 weeks | 1.20 (OR) | 0.72 to 2.00 | ICC 0 | — | ||||||||||||||||
| Thomas 2015 | Intervention B | Control | |||||||||||||||||||||
| % (n) | % (n) | ||||||||||||||||||||||
| Death | 29 (114) | 29 (116) | 52 weeks | 0.99 (OR) | 0.58 to 1.69 | ICC 0 | — | ||||||||||||||||
| Hospital readmission | |||||||||||||||||||||||
| Study | Intervention | Control | Time point | Effect estimate (metric) | 95% CI | Statistical test |
P value | ||||||||||||||||
| Forster 2015 | % (n) | % (n) | |||||||||||||||||||||
| Readmitted to hospital (% readmitted) | 24.2 (401) | 28.3 (399) | 6 months | — | — | — | — | ||||||||||||||||
C: control; CI: confidence interval; df: degrees of freedom; EQ‐5D: European Quality of Life‐5 Dimensions; GHQ‐12: General Health Questionnaire‐12; HADS: Hospital Anxiety and Depression Scale; I; intervention; I‐QOL: Incontinence Quality of Life; ICC: intraclass correlation coefficient; ICIQ‐UI: International Consultation on Incontinence Questionnaire – Urinary Incontinence; IQR: interquartile range; ISI: Incontinence Severity Index; M‐MMSE: Modified Mini‐Mental State Examination; n: number of participants; NEADL: Nottingham Extended Activities of Daily Living; OR: odds ratio; PHQ‐9: Patient Health Questionnaire‐9; SD: standard deviation; SE: standard error; SIS: Stroke Impact Scale; TQWHQ: Two Questions With Help Questionnaire; Y/N: yes/no.
Six trials compared any implementation intervention to no intervention (Abdul Aziz 2014; Forster 2013; Forster 2015; McCluskey 2016; Power 2014; Thomas 2015). See Description of studies for details of implementation interventions.
Quality of care: healthcare professional adherence to evidence‐based practice
Two trials reported measures of healthcare professional adherence to EBP at 12 months (McCluskey 2016; Power 2014). McCluskey 2016 reported the proportion of stroke survivors receiving rehabilitation for outdoor mobility in line with clinical practice guidelines. Power 2014 reported the proportion of patients receiving evidence‐based stroke care bundles. We pooled the outcome data from these trials using the generic inverse variance method.
We are uncertain about the estimate of healthcare professional adherence to EBP at 12 months and whether implementation interventions in stroke rehabilitation improve healthcare professional adherence to EBP compared with no intervention as the certainty of the evidence was very low (RR 1.19, 95% CI 0.53 to 2.64; 2 trials, 39 clusters, 1455 patient participants; I2 = 0%; Analysis 1.1; Figure 4). Healthcare professional adherence to EBP in the control group in McCluskey 2016 was 5%. The corresponding risk of adherence to EBP with intervention at 12 months was 6% (95% CI 2.7% to 13.2%). Healthcare professional adherence to EBP in the control group in Power 2014 was 33%. The corresponding risk of adherence to EBP with intervention at 12 months was 39.3% (95% CI 17.5% to 87.1%). We did not specify the smallest important difference for this outcome in our protocol. However, we assessed the importance of the effect and the precision of the estimate based on how likely it seemed to us that some people would make different decisions if the true effect was near one end or the other of the 95% CI. The certainty of the evidence was downgraded three levels due to serious risk of bias (lack of blinding of personnel in two trials; outcome assessors not blinded and incomplete outcome data in one trial) and very serious imprecision (95% CIs wide).
1.1. Analysis.

Comparison 1: Implementation intervention versus control, Outcome 1: Quality of care: healthcare professional adherence to evidence‐based practice (EBP) at 12 months
4.

Forest plot of comparison: 1 Implementation intervention versus control, outcome: 1.1 Quality of care: healthcare professional adherence to evidence‐based practice (EBP) at 12 months.
Subgroup analyses based on setting for stroke rehabilitation and tailoring did not substantially alter the results (Analysis 2.1; Analysis 3.1). Other planned subgroups based on study design, intervention category (according to EPOC taxonomy) and patient population (adults or children) could not be undertaken as both studies were cluster randomised trials of implementation interventions and one study did not report the age of patient participants (Power 2014). Sensitivity analysis based on excluding one trial (Power 2014) due to high risk of detection, attrition and reporting bias did not substantially alter the effect estimate (RR 1.05, 95% CI 0.21 to 5.24; 1 trial, 21 clusters, 279 patient participants; very low‐certainty evidence) (Analysis 5.1; Analysis 6.1; Analysis 7.1).
2.1. Analysis.

Comparison 2: Subgroup analysis: setting for stroke rehabilitation, Outcome 1: Quality of care: healthcare professional adherence to evidence‐based practice (EBP) at 12 months
3.1. Analysis.

Comparison 3: Subgroup analysis: tailored versus non‐tailored interventions, Outcome 1: Quality of care: healthcare professional adherence to evidence‐based practice (EBP) at 12 months
5.1. Analysis.

Comparison 5: Sensitivity analysis: low risk of detection bias, Outcome 1: Quality of care: healthcare professional adherence to evidence‐based practice (EBP) at 12 months
6.1. Analysis.

Comparison 6: Sensitivity analysis: low risk of attrition bias, Outcome 1: Quality of care: healthcare professional adherence to evidence‐based practice (EBP) at 12 months
7.1. Analysis.

Comparison 7: Sensitivity analysis: low risk of reporting bias, Outcome 1: Quality of care: healthcare professional adherence to evidence‐based practice (EBP) at 12 months
Patient health behaviour: adherence to recommended treatment
One trial reported patient adherence to recommended treatment at six months (McCluskey 2016). McCluskey 2016 reported the number of community outings undertaken by stroke survivors outside of therapy. Low‐certainty evidence indicated implementation interventions in stroke rehabilitation may lead to little or no difference in patient adherence to recommended treatment, as reflected by the number of outdoor journeys taken by patients at six months, compared with no intervention (adjusted MD 0.5, 95% CI –1.8 to 2.8; P = 0.63; 1 trial, 21 clusters, 100 participants). The mean number of outdoor journeys per week in the control group was 7.4. The mean number of outdoor journeys per week with intervention at six months was 7.9 (95% CI 5.6 to 10.2). The certainty of the evidence was downgraded two levels due to very serious imprecision (suboptimal information size arising from one study with 100 participants and wide 95% CI).
Patient health status and well‐being
Five trials reported at least one measure of patient health status or well‐being at up to six months (Abdul Aziz 2014; Forster 2013; Forster 2015; McCluskey 2016, Thomas 2015).
Health‐related quality of life
Four trials reported health‐related quality of life using the EQ‐5D (2292 participants) (Abdul Aziz 2014; Forster 2013; Forster 2015; Thomas 2015). One trial reported this outcome at three months (Thomas 2015), and three at six months (Abdul Aziz 2014; Forster 2013; Forster 2015). Additionally, Thomas 2015 used I‐QOL, a continence‐specific measure of quality of life, at three months.
We could only combine mean EQ‐5D data from two trials using the generic inverse variance method because the other trials reported median (Abdul Aziz 2014) or non‐aggregated (Thomas 2015) EQ‐5D scores. Table 4 provides a structured summary of effects from all four trials measuring health‐related quality of life.
Based on pooled data from Forster 2013 and Forster 2015, moderate‐certainty evidence indicates implementation interventions in stroke rehabilitation probably lead to little or no difference in patient health‐related quality of life as measured by the EQ‐5D at up to six months compared with no intervention (MD 0.01, 95% CI –0.02 to 0.05; 2 trials, 65 clusters, 1242 participants; I2 = 0%; Analysis 1.2). Mean EQ‐5D in the control group was 0.58 points and mean EQ‐5D with intervention at up to 6 months was 0.59 (95% CI 0.56 to 0.63). The certainty of the evidence was downgraded one level due to serious imprecision (suboptimal information size).
1.2. Analysis.

Comparison 1: Implementation intervention versus control, Outcome 2: Patient health status: quality of life at up to 6 months (EQ‐5D, summary index –0.59 to 1, higher score = better)
Subgroup analyses based on setting and tailoring did not substantially alter the results (Analysis 2.2; Analysis 3.2). Other planned subgroups based on study design, intervention category (according to EPOC taxonomy) and patient population (adults or children) could not be undertaken as both studies were cluster randomised trials of implementation interventions in adult stroke survivors. Sensitivity analysis based on excluding one trial due to unclear risk of selection bias did not substantially alter the effect estimate (MD 0.03, 95% CI –0.02 to 0.08; 1 trial, 29 clusters, 589 participants) (Forster 2013) (Analysis 4.2).
2.2. Analysis.

Comparison 2: Subgroup analysis: setting for stroke rehabilitation, Outcome 2: Patient health status: quality of life at up to 6 months (EQ‐5D, summary index –0.59 to 1, higher score = better)
3.2. Analysis.

Comparison 3: Subgroup analysis: tailored versus non‐tailored interventions, Outcome 2: Patient health status: quality of life at up to 6 months (EQ‐5D, summary index –0.59 to 1, higher score = better)
4.2. Analysis.

Comparison 4: Sensitivity analysis: low risk of selection bias, Outcome 2: Patient health status: quality of life at up to 6 months (EQ‐5D, summary index –0.59 to 1, higher score = better)
The findings from Abdul Aziz 2014 and Thomas 2015 appear to be consistent with Analysis 1.2. Abdul Aziz 2014 reported the median change in EQ‐5D scores at six months; 0.04 (IQR 0 to 0.13) in the implementation intervention group (86 participants) and 0.01 (IQR 0 to 0.05) in the control group (65 participants) (P = 0.699). Thomas 2015 reported effect estimates for five EQ‐5D subscales (mobility, self‐care, usual activity, pain, anxiety) but not overall EQ‐5D score at three months (Table 4). Thomas 2015 also reported the median scores for I‐QOL at three months; 76.1 (IQR 42.5 to 94.3) in the implementation intervention group (47 participants) and 72.6 (IQR 58.3 to 83.0) in the control group (51 participants) (odds ratio (OR) –5.5, 95% CI –24.1 to 13.1).
Activities of daily living
Four trials reported ADL status at up to six months (2292 participants) (Abdul Aziz 2014; Forster 2013; Forster 2015; Thomas 2015). All four trials used the Barthel Index, either at three months (Thomas 2015), or six months (Abdul Aziz 2014; Forster 2013; Forster 2015). Additionally, Forster 2013 used the NEADL scale and Forster 2015 used the FAI to measure ADL status, both at six months.
We could only pool mean Barthel Index data from two trials using the generic inverse variance method (Forster 2013; Forster 2015), as the other trials provided median scores (Thomas 2015) or median change scores between groups (Abdul Aziz 2014). Table 4 provides a structured summary of effects from all trials measuring ADL status.
Based on pooled data from Forster 2013 and Forster 2015, moderate‐certainty evidence indicates that implementation interventions in stroke rehabilitation probably leads to little or no difference in ADL status at up to six months compared with no intervention (MD 0.29, 95% CI –0.16 to 0.73; 2 trials, 65 clusters, 1272 participants; I2 = 17%; Analysis 1.3). Mean Barthel Index score in the control group was 15.8 points. Mean Barthel Index score with intervention at up to six months was 16.09 (95% CI 15.64 to 16.53). The certainty of the evidence was downgraded one level due to serious imprecision (suboptimal information size).
1.3. Analysis.

Comparison 1: Implementation intervention versus control, Outcome 3: Patient health status: activities of daily living at up to 6 months (Barthel Index, 0–20, higher score = better)
Subgroup analyses based on setting and tailoring did not substantially alter the results (Analysis 2.3; Analysis 3.3). Other planned subgroup analyses based on study design, intervention category and patient population could not be undertaken as both studies were cluster randomised trials of implementation interventions in adult stroke survivors. Sensitivity analysis based on excluding Forster 2013 due to unclear risk of selection bias did not substantially alter the effect estimate (MD 0.50, 95% CI –0.15 to 1.15; 1 trial, 29 clusters, 603 participants) (Analysis 4.3).
2.3. Analysis.

Comparison 2: Subgroup analysis: setting for stroke rehabilitation, Outcome 3: Patient health status: activities of daily living at up to 6 months (Barthel Index, 0–20, higher score = better)
3.3. Analysis.

Comparison 3: Subgroup analysis: tailored versus non‐tailored interventions, Outcome 3: Patient health status: activities of daily living at up to 6 months (Barthel Index, 0–20, higher score = better)
4.3. Analysis.

Comparison 4: Sensitivity analysis: low risk of selection bias, Outcome 3: Patient health status: activities of daily living at up to 6 months (Barthel Index, 0–20, higher score = better)
The findings from Abdul Aziz 2014 and Thomas 2015 (both reported outcome data for Barthel Index) and Forster 2013 and Forster 2015 (additionally reported outcome data for NEADL and FAI scales respectively) appear to be consistent with the results of the meta‐analysis. Abdul Aziz 2014 reported median Barthel scores of 1.77 (IQR 0 to 5) in the implementation intervention group (86 participants) and 0.94 (IQR 0 to 5) with no intervention (65 participants) (P = 0.231) at six months. Thomas 2015 reported median Barthel scores of 11 (IQR 6 to 15) with a supported implementation group (95 participants) and 11 (IQR 4 to 16) with no intervention (94 participants) at three months (OR 0.97, 95% CI 0.61 to 1.54). Forster 2013 reported mean NEADL scores of 27.4 (SE 1.00) in the implementation intervention group (330 participants) and 27.6 (SE 0.99) with no intervention (348 participants) at six months (MD –0.2, 95% CI –3.0 to 2.5; P = 0.866). Forster 2015 reported mean FAI scores of 18.0 (SE 0.76) in the implementation intervention group (304 participants) and 19.0 (SE 0.76) with no intervention (293 participants) at six months (MD 1.0, 95% CI –0.6 to 2.5; P = 0.229).
Psychological well‐being
Three trials reported different measures of psychological well‐being at up to six months (1879 participants) (Abdul Aziz 2014; Forster 2013; Forster 2015). Abdul Aziz 2014 measured mood using the PHQ‐9 and depression using the TQWHQ; Forster 2013 measured anxiety and depression using the HADS scale and Forster 2015 measured psychological well‐being using the GHQ‐12.
We pooled data from two trials using the generic inverse variance method (Forster 2013; Forster 2015); the third trial was not included due to reporting only median change scores between groups (Abdul Aziz 2014). Forster 2013 reported HADS Anxiety and HADS Depression scores; we used HADS Depression scores in meta‐analysis as an outcome related to mood.
Based on pooled data from Forster 2013 and Forster 2015, low‐certainty evidence indicates implementation interventions in stroke rehabilitation may lead to little or no difference in psychological well‐being at up to six months compared with no intervention (SMD –0.02, 95% CI –0.54 to 0.50; 2 trials, 65 clusters, 1273 participants; I2 = 0%; Analysis 1.4). Mean psychological well‐being on the GHQ‐12 scale in the control group was 14.9 points (GHQ‐12 scores range from 12 to 48; higher scores indicate better psychological well‐being). The mean GHQ‐12 score with intervention at up to six months was 14.69 (95% CI 14.36 to 15.40) (SMD back transformed to GHQ‐12 scale). The certainty of the evidence was downgraded two levels due to indirectness (differences in outcome measures) and imprecision (suboptimal information size).
1.4. Analysis.

Comparison 1: Implementation intervention versus control, Outcome 4: Patient health status: psychological well‐being at 6 months
Subgroup analyses based on setting and tailoring did not substantially alter the results (Analysis 2.4; Analysis 3.4). Other planned subgroup analyses based on study design, intervention category and patient population could not be undertaken as both studies were cluster randomised trials of implementation interventions in adult stroke survivors. Sensitivity analysis based on excluding Forster 2013 due to unclear risk of selection bias did not substantially alter the results (SMD –0.60, 95% CI –1.87 to 0.67; 1 trial, 29 clusters, 610 participants) (Analysis 4.4).
2.4. Analysis.

Comparison 2: Subgroup analysis: setting for stroke rehabilitation, Outcome 4: Patient health status: psychological well‐being at 6 months
3.4. Analysis.

Comparison 3: Subgroup analysis: tailored versus non‐tailored interventions, Outcome 4: Patient health status: psychological well‐being at 6 months
4.4. Analysis.

Comparison 4: Sensitivity analysis: low risk of selection bias, Outcome 4: Patient health status: psychological well‐being at 6 months
The findings from Abdul Aziz 2014 and additional HADS Anxiety outcomes in Forster 2013 appear to be consistent with Analysis 1.4. Abdul Aziz 2014 reported a median PHQ‐9 change score of 0 (IQR –3.0 to 2.0) in the intervention group (86 participants) compared with –0.92 (IQR –3.0 to 1.0) in the control group (65 participants) (Mann Whitney –0.190; P = 0.848) and 18.6% of patients in the intervention group reported depressive symptoms on the TQWHQ compared with 20% in the control group at six months (Chi2 = 0.05, degrees of freedom (df) 1; P = 0.829). Forster 2013 reported mean HADS Anxiety scores of 6.7 (SE 0.22) in the intervention group (323 participants) and 6.6 (SE 0.21) in the control group (340 participants) at six months (MD 0.1, 95% CI –0.5 to 0.7; P = 0.629).
Table 4 provides a structured summary of effects from all trials measuring psychological well‐being.
Health status following stroke
One trial reported stroke impact using the SIS at six months (Forster 2013). Forster 2013 reported mean (and SE) scores for six SIS subscales (physical, memory, mood, communication, recover, social participation) (see Table 4 for effect estimates). Results from this trial indicate implementation interventions in stroke rehabilitation may result in little or no difference in stroke impact at six months compared with no intervention (1 trial, 18 clusters, 664 participants; low‐certainty evidence). The certainty of the evidence was downgraded two levels due to very serious imprecision (suboptimal information size arising from one study and wide CI).
Mobility status
One trial reported mobility status using the Life‐Space Assessment at six months (McCluskey 2016). Mean scores were 61 (standard deviation (SD) 12) in the intervention group (55 participants) and 51 (SD 12) in the control group (60 participants) (adjusted MD 5, 95% CI –5 to 15; P = 0.29). Based on data from this trial, implementation interventions in stroke rehabilitation may lead to little or no difference in patient mobility at six months compared with no intervention (1 trial, 21 clusters, 115 participants; low‐certainty evidence). The certainty of the evidence was downgraded two levels due to very serious imprecision (suboptimal information size arising from one study and wide CI).
Cognitive status
One trial reported median M‐MMSE scores of 0.3 (IQR 0 to 1.0) in the implementation intervention group (86 participants) and 1.34 (IQR 0 to 0.73) with no intervention (65 participants) at six months (P = 0.227), and median scores on the ECAQ of 0.6 (IQR 0 to 1.0) in the implementation intervention group and 0.33 (IQR 0 to 1.0) with no intervention (P = 0.319) at six months (Abdul Aziz 2014). Higher scores on the M‐MMSE and ECAQ indicate better cognitive status. Based on data from this trial, we are uncertain whether implementation interventions in stroke rehabilitation improve cognitive status at six months compared with no intervention as the certainty of the evidence was very low (1 trial, 10 clusters, 151 participants). The certainty of the evidence was downgraded three levels due to very serious risk of bias (high risk of selective outcome reporting, unclear blinding of personnel and outcome assessors and suspected unit of analysis issues) and very serious imprecision (suboptimal information size arising from one study with few participants and wide CI).
Continence status
One trial reported continence status at three months using the ICIQ‐UI and the ISI (Thomas 2015); 86 (68%) of patients in the implementation intervention group were incontinent at three months compared with 80 (70%) patients in the group with no intervention as measured on the ICIQ‐UI scale (OR 1.06, 95% CI 0.54 to 2.09). Median ISI scores were 4 (IQR 0 to 8) in the implementation intervention group and 3 (IQR 0 to 6) in the no treatment group (OR 0.92, 95% CI 0.52 to 1.64). Based on data from this trial, we are uncertain whether implementation interventions in stroke rehabilitation improve continence status at three months compared with no intervention as the certainty of the evidence was very low (1 trial, 12 clusters, 413 participants). The certainty of the evidence was downgraded three levels due to serious risk of bias (personnel and outcome assessors not blinded) and very serious imprecision (suboptimal information size arising from one study with few participants and wide CI).
Healthcare professional outcomes
No trials reported healthcare professional outcomes.
Resource use and costs
See Table 5 (Summary of included studies with economic data)
4. Economic evaluation: summary of included studies with economic data.
| Study | Type of economic evaluation | Key costs included | Health outcome | Resource use (intervention) | Resource use (control) | Time horizon | Main finding | Conclusion |
| Abdul Aziz 2014 | Cost‐effectiveness analysis | Intervention: Unspecified. Costs from 'provider and patient perspective' |
Patients: Quality of life (EQ‐5D) |
Total costs for 6 months of treatment with intervention = RM 893.75 (USD 271.12) Conversion using EPPI‐Centre Cost Converter (2019 – USD 676.48) |
Total costs of 6 months of conventional care = RM 408.47 (USD 123.91) Conversion using EPPI‐Centre Cost Converter (2019 – USD 309.17) |
6 months | Cost per QALY gained for intervention was RM 1625.00 (USD 492.95; converted 2019 USD 1229.96)), while conventional care was RM 1276.46 (USD 387.22; converted 2019 USD 966.15). The ICER was RM 2109.91 (USD 640.05; converted 2019 USD 1596.99). |
Cost‐effectivea |
| Forster 2013 | Cost‐effectiveness analysis | Intervention: Development and staff training costs Patient and carer resource use |
Patients: Self‐reported functional independence in extended activities of daily living Quality of life (EQ‐5D) Carers: Self‐reported carer burden |
Stroke admission and associated costs = GBP 13,127 Conversion using EPPI‐Centre Cost Converter (2019 – USD 20,794.60) Total health and social care costs for patients and societal costs = GBP 21,147 Conversion using EPPI‐Centre Cost Converter (2019 – USD 33,499.16) |
Stroke admission and associated costs = GBP 12,471 Conversion using EPPI‐Centre Cost Converter (2019 – USD 19,755.43) Total health and social care costs for patients and societal costs = GBP 21,147 Conversion using EPPI‐Centre Cost Converter (2019 – USD 33,499.16) |
6 months | No evidence of significant differences in QALYs between groups | Not cost‐effective |
| Forster 2015 | Cost‐effectiveness analysis | Intervention: Staff (stroke care co‐ordinator) inputs, total health and social care costs |
Patients: Psychological well‐being (GHQ‐12) Quality of life (EQ‐5D) |
Staff inputs = GBP 277 (mean, SD 207) Conversion using EPPI‐Centre Cost Converter (2019 – USD 429.55) Total health and societal care costs = GBP 3369 (mean, SD 4735) Conversion using EPPI‐Centre Cost Converter (2019 – USD 5224.33) |
Staff inputs = GBP 239 (mean, SD 146) Conversion using EPPI‐Centre Cost Converter (2019 – USD 370.62) Total health and societal care costs = GBP 3171 (mean, SD 5942) Conversion using EPPI‐Centre Cost Converter (2019 – USD 4917.29) |
6 months | Mean QALY gains similar between groups (0.27 intervention group vs 0.29 in control group; mean difference 0.004, 95% CI –0.02 to 0.01; P = 0.436) ICERs not calculated due to lack of statistical significance between groups |
Not cost‐effective |
| Pennington 2005 | Cost description | Intervention: Training costs |
No health/patient related outcomes reported Health professionals: adherence to clinical practice guidelines |
Total costs of training (Strategy A) = GBP 2001, EUR 2892, USD 3886 (SD GBP 502, EUR 726, USD 975) Conversion using EPPI‐Centre Cost Converter (2019 – USD 3743.40) |
Total costs of training (Strategy B) = GBP 3366, EUR 4866, USD 4119 (SD GBP 2121, EUR 3066, USD 4119) Conversion using EPPI‐Centre Cost Converter (2019 – USD 6297.00) |
Unclear | No relationship between costs and clinical outcome | N/A |
| Thomas 2015 | Cost‐utility analysis and cost‐effectiveness analysis | Intervention: In‐hospital costs Staff training, internal facilitators, staff time performing programme Posthospital costs Community health and social service input, admissions |
Patients: Continence status (ICIQ‐UI) Quality of life (I‐QOL and EQ‐5D) |
Intervention Mean cost per patient of staff training = GBP 13 (2019 conversion – USD 20.16) Mean total hospital and postdischarge costs = GBP 12423 (2019 conversion – USD 19264.43) Supported implementation Mean cost per patient of staff training = GBP 25 (2019 conversion – USD 38.77) Mean total hospital and postdischarge costs = GBP 10,913 (2019 conversion – USD 16922.87) |
Mean cost per patient of staff training = GBP 0 Mean total hospital and postdischarge costs = GBP 9563 (2019 conversion – USD 14,829.41) |
52 weeks | Mean QALY gains –0.33 usual care, –0.42 intervention group, –0.47 supported implementation group | Inconclusive due to data collection issues |
aAuthors did not complete statistical analysis on this comparison (they compared Intervention groups with control; three‐armed study).
CI: confidence interval; EQ‐5D: European Quality of Life‐5 Dimensions; GHQ‐12: General Health Questionnaire‐12; I‐QOL: Incontinence Quality of Life; ICER: incremental cost‐effectiveness ratio; ICIQ‐UI: International Consultation on Incontinence Questionnaire – Urinary Incontinence; QALY: quality‐adjusted life year; N/A: not applicable/available; SD: standard deviation.
Four studies reported resource use or costs (2292 participants) (Abdul Aziz 2014; Forster 2013; Forster 2015; Thomas 2015). Three studies conducted a cost‐effectiveness analysis comparing costs and QALYs between the implementation intervention group and control (Abdul Aziz 2014; Forster 2013; Forster 2015). Thomas 2015 conducted both cost‐utility analysis and cost‐effectiveness analyses.
We converted costs to a common currency (USD) and price year (2019) using an online cost converter to facilitate comparison of economic data across studies (EPPI‐Centre Cost Converter 2019).
Costs reported in studies included staff costs, such as training (Forster 2013; Forster 2015; Pennington 2005), and health and social care costs (Forster 2015; Thomas 2015). One study did not provide details of costs used in analysis (Abdul Aziz 2014).
Abdul Aziz 2014 reported the cost per QALY gained in the intervention group was USD 1229.96 and in the control group was USD 966.15. Forster 2015 found mean QALY gains of 0.27 in the intervention group and 0.29 in the control group (MD 0.004, 95% CI –0.02 to 0.01; P = 0.436). Thomas 2015 found mean QALY gains of –0.42 in the intervention group (intervention A), –0.47 in the supported intervention group (intervention B) and –0.33 in the control group.
Only one study reported cost‐effectiveness of an implementation intervention in stroke rehabilitation (a care pathway) (Abdul Aziz 2014). However, this study was assessed at high risk of reporting bias and had suspected unit of analysis issues. All other studies did not demonstrate cost‐effectiveness of the implementation intervention compared with control (Forster 2013; Forster 2015; Thomas 2015).
The quality of studies reporting economic data were assessed using the CHEC (Table 6; Table 7). Most studies reporting economic data provided well‐defined research questions, though the population was not clearly defined in two studies (Abdul Aziz 2014; Pennington 2005). Four studies used appropriate research designs to determine economic outcomes and the majority of studies reported outcomes appropriately in physical units (Abdul Aziz 2014; Forster 2013; Forster 2015; Thomas 2015). The study perspective (e.g. a societal perspective) was appropriate in three studies (Forster 2013; Forster 2015; Thomas 2015). As time horizons were less than 12 months in four studies, discounting was not performed or reported. None of the studies discussed ethical and distributional issues.
5. Overview of methodological quality (CHEC‐list) for included studies with economic data (Part 1).
| Checklist item | I. Is the study population clearly described? | 2. Are competing alternatives clearly described? | 3. Is a well‐defined research question posed in answerable form? | 4. Is the economic study design appropriate to the stated objective? | 5. Is the chosen time horizon appropriate to include relevant costs and consequences? | 6. Is the actual perspective chosen appropriate? | 7. Are all important and relevant costs for each alternative identified? | 8. Are all costs measured appropriately in physical units? | 9. Are costs valued appropriately? | 10. Are all important and relevant outcomes for each alternative identified? |
| Abdul Aziz 2014 | No | No | Yes | Yes | No | No | No | No | No | Yes |
| Forster 2013 | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Forster 2015 | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Pennington 2005 | No | Yes | Yes | No | No | No | No | No | No | No |
| Thomas 2015 | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
CHEC: Consensus on Health Economic Criteria.
6. Overview of methodological quality (CHEC‐list) for included studies with economic data (Part 2).
| Checklist item | 11. Are all outcomes measured appropriately? | 12. Are outcomes valued appropriately? | 13. Is an incremental analysis of costs and outcomes of alternatives performed? | 14. Are all future costs and outcomes discounted appropriately? | 15. Are all important variables, whose values are uncertain, appropriately subjected to sensitivity analysis? | 16. Do the conclusions follow from the data reported? | 17. Does the study discuss the generalisability of the results to other settings and patient/client groups? | 18. Does the article indicate that there is no potential conflict of interest of study researcher(s) and funder(s)? | 19. Are ethical and distributional issues discussed appropriately? |
| Abdul Aziz 2014 | Yes | No | Yes | Yes | No | No | No | No | No |
| Forster 2013 | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | No |
| Forster 2015 | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | No |
| Pennington 2005 | No | No | No | No | No | No | No | Yes | No |
| Thomas 2015 | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | No |
CHEC: Consensus on Health Economic Criteria.
See Appendix 3 for full details of CHEC assessment.
Adverse effects/harms
Three trials reported data on patient adverse effects/harms (Forster 2013, Forster 2015, Thomas 2015) .
Death
Three trials reported overall patient deaths at the conclusion of the trials (Forster 2013; Forster 2015; Thomas 2015). Forster 2013 reported 53 (11.8%) patients in the intervention group had died at 12 months compared to 55 (11.5%) patients with no intervention. Forster 2015 reported 35 (8.7%) patients in the intervention group had died at 12 months compared to 32 (8%) patients with no intervention. Thomas 2015 reported 28 (25%) patients in intervention group B had died at 52 weeks compared to 29 (25%) patients with no intervention (OR 0.99, 95% CI 0.58 to 1.69). We pooled the data from these trials as per guidance in Cochrane Handbook for Systematic Reviews of Interventions (Section 23.1.4.1; Higgins 2019). We applied a design effect to the number of participants and events in each trial to account for clustering.
Based on pooled data from these three trials, we are uncertain whether implementation interventions in stroke rehabilitation lead to fewer deaths at up to 12 months compared with no intervention as the certainty of the evidence was very low (RR 1.05, 95% CI 0.79 to 1.40; 3 trials, 77 clusters, 1958 participants; I2 =0%; Analysis 1.5). The certainty of the evidence was downgraded three levels due to very serious imprecision (wide 95% CI that included both an important reduction and increase in death) and serious risk of bias.
1.5. Analysis.

Comparison 1: Implementation intervention versus control, Outcome 5: Death
See also 'Overview of results ordered by outcome; patient outcomes' table (Table 4).
Hospitalisation
Two trials reported data on hospitalisation (1728 participants) (Forster 2013; Forster 2015).
One trial reported patient readmission to hospital at six months and found 97 (24.2%) patients in the intervention group had been readmitted for one night or more compared to 113 (28.3%) patients with no intervention (Forster 2015).
Forster 2013 did not report outcome data on readmission or institutionalisation rates but stated there were no statistically significant differences in hospital readmissions or institutionalisation rates between intervention and control groups. See 'Overview of results ordered by outcome; patient outcomes' table (Table 4).
We are uncertain whether implementation interventions in stroke rehabilitation reduce readmissions to hospital compared to no intervention as the certainty of the evidence was very low. The certainty of the evidence was downgraded three levels due to very serious imprecision (suboptimal information size and wide CI) and serious risk of bias (selective outcome reporting by one study (Forster 2013)).
Falls
Two trials reported data on falls (Forster 2013; Thomas 2015).
Forster 2013 documented the incidence of falls between groups at time of hospital discharge and reported 50 falls in intervention group (450 participants) compared with 42 falls in the control group (478 participants). In both groups, 35 patients fell more than once; the mean number of falls per patients who fell was 1.4 (SE 0.88) in the intervention group and 1.2 (SE 0.76) in the control group.
Thomas 2015 reported number of falls at 12‐month trial conclusion; there were 11 falls in intervention group A (164 participants), four falls in intervention group B (125 participants) and 16 falls in the control group (124 participants). These results include patients who had more than one fall.
We are uncertain whether implementation interventions in stroke rehabilitation reduce falls compared to no intervention as the certainty of the evidence was very low. The certainty of the evidence was downgraded three levels due to very serious imprecision (suboptimal information size and wide CI), serious risk of bias in one study and serious inconsistency (considerable inconsistency between studies, point estimates vary widely).
Other adverse effects
One trial reported other adverse effects; urinary tract infections and bladder catheterisation (Thomas 2015). The number of urinary tract infections in intervention group A (164 participants) was 18, intervention group B (125 participants) was 23 and in the control group (124 participants) was 13. These results included patients who had more than one urinary tract infection. Bladder catheterisation was required in four patients in intervention group A (164 participants), one in intervention group B (125 participants) and one patient in the control group (124 participants).
No trial reported adverse effects of interventions on healthcare professionals, for example, stress, burnout or sick leave.
We are uncertain whether implementation interventions in stroke rehabilitation reduce other adverse effects compared to no intervention as the certainty of the evidence was very low. The certainty of the evidence was downgraded three levels due to very serious imprecision (suboptimal information size) and serious risk of bias (lack of blinding of outcome assessment).
Tailored versus non‐tailored interventions
Subgroup analyses based on tailoring of implementation interventions to identified barriers to change did not alter the results for the main outcomes (Analysis 3.1; Analysis 3.2; Analysis 3.3; Analysis 3.4).
Comparison 2: one implementation intervention versus another implementation intervention
See 'Overview of results ordered by outcome; quality of care' (Table 8) and 'Overview of results ordered by outcome; patient outcomes' (Table 9).
7. Comparison 2: overview of results ordered by outcome; quality of care.
| One implementation intervention vs another implementation intervention | |||||||
| Quality of care | |||||||
| Outcome (scale details) | Intervention A | Intervention B | Time point | Effect estimate (metric) | 95% CI | Statistical test | P value |
| Pennington 2005 | Mean (SD), n | Mean (SD), n | |||||
| Adherence to clinical practice guidelines (composite score of compliance with actions, e.g. assessment, discussion of rehabilitation plan) | 71.5 (10.1) (342) | 72.7 (10.4) (375) | 8–12 months | — | — | — | |
| Salbach 2017 | Estimated % times implemented (n) | Estimated % times implemented (n) | Change in estimated % times implemented (unadjusted) (A) – (B) |
||||
| Adherence to recommended treatment (% of times treatment implemented) Sit‐to‐stand |
39.1 (276) | 33.6 (265) | 16 months | 21.4 | 10.4 to 32.4 | 0.028 (adjusted) | |
| LE ROM or stretching, or both | 10.5 (143) | 17.8 (135) | 16 months | –14.7 | –26.0 to 3.5 | ||
| Use of LE external support (i.e. brace) | 8.7 (276) | 17.4 (265) | 16 months | –0.9 | –8.7 to 6.9 | ||
| Task‐specific training (i.e. stairs) | 38.5 (143) | 37.8 (135) | 16 months | –4.8 | –20.6 to 11.0 | ||
| Training for sitting balance | 17.5 (143) | 25.2 (135) | 16 months | –14.6 | –28.2 to –1.0 | 0.037 (adjusted) | |
| Training for standing balance | 52.5 (143) | 60.0 (135) | 16 months | –22.8 | –39.4 to –6.2 | ||
| FES for LE | 0.7 (143) | 0.7 (135) | 16 months | –0.7 | –3.1 to 1.7 | ||
| Walking practice | 39.1 (276) | 32.8 (265) | 16 months | 22.0 | 11.2 to 32.8 | 0.043 (adjusted) | |
| Treadmill walking practice | 1.4 (143) | 5.2 (135) | 16 months | 0.3 | –6.4 to 7.1 | ||
| UE ROM or stretching, or both | 21.4 (276) | 25.3 (265) | 16 months | 5.2 | –4.4 to 14.7 | ||
| Interventions to prevent shoulder pain | 25.7 (276) | 21.1 (265) | 16 months | 4.9 | –5.1 to 14.9 | ||
| Task‐specific training | 40.9 (276) | 43.4 (265) | 16 months | 6.0 | –5.4 to 17.3 | ||
| Techniques to reduce hand oedema | 5.6 (143) | 8.9 (135) | 16 months | 0 | –9.6 to 8.8 | ||
| Ice/heat or soft tissue manage for shoulder | 2.8 (143) | 5.2 (135) | 16 months | 4.8 | –2.3 to 11.8 | ||
| FES for wrist/arm/shoulder | 1.4 (143) | 1.5 (135) | 16 months | 0.5 | –4.1 to 5.1 | ||
| Educate patient or carer on how to handle arm or shoulder | 9.4 (276) | 10.2 (265) | 16 months | 3.4 | –3.9 to 10.6 | ||
| UE constraint‐induced therapy | 0.7 (143) | 4.4 (135) | 16 months | 1.8 | –5.6 to 9.2 | ||
| Visual imagery to enhance arm recovery | 6.3 (143) | 5.2 (135) | 16 months | 3.5 | –3.7 to 10.8 | ||
CI: confidence interval; FES: functional electrical stimulation; LE: lower extremity; n: number of participants; ROM: range of movement; SD: standard deviation; UE: upper extremity.
8. Comparison 2: overview of results ordered by outcome; patient outcomes.
| One implementation intervention vs another implementation intervention | |||||||
| Quality of care | |||||||
| Patient outcomes | |||||||
| Measures of patient health behaviour | |||||||
| No studies reported this outcome | |||||||
| Measures of patient health status and well‐being | |||||||
| Quality of life | |||||||
|
Outcome (scale details) |
Intervention A | Intervention B | Time point | Effect estimate (metric) | 95% CI | Statistical test | P value |
| Thomas 2015 | n (%) | ||||||
| EQ‐5D Mobility No problems Some problems Confined to bed Self‐care No problems Some problems Unable to wash and dress Usual activities No problems Some problems Unable to perform Pain or discomfort None Moderate Extreme Anxiety or depression None Moderate Extreme |
n = 129 16 (12) 62 (48) 51 (40) n = 126 21 (17) 47 (37) 58 (46) n = 126 9 (7) 39 (31) 78 (62) n = 123 51 (41) 58 (47) 14 (11) n = 122 47 (39) 66 (54) 9 (7) |
n = 92 10 (11) 57 (62) 25 (27) n = 92 18 (20) 40 (43) 34 (37) n = 91 8 (9) 32 (35) 51 (56) n = 93 50 (54) 34 (37) 9 (10) n = 92 47 (51) 37 (40) 8 (9) |
12 weeks | ^ | ^ | ^ | ^ |
| Median (IQR), n | Median (IQR), n | ||||||
| Quality of life measured by I‐QOL |
67.1 (51.1–85.2), 35 | 76.1 (42.5–94.3), 47 | 12 weeks | ^ | ^ | ^ | ^ |
| ADL | |||||||
|
Outcome (scale details) |
Intervention A | Intervention B | Time point | Effect estimate (metric) | 95% CI | Statistical test | P value |
| Thomas 2015 | Median (IQR), n | Median (IQR), n | |||||
| ADL status measured by Barthel Index | 11 (6–15), 95 | 8 (4–13), 128 | 12 weeks | ^ | ^ | ^ | ^ |
| Strasser 2008 | % (n) | % (n) | |||||
| Functional improvement (Gain in FIM motor score > 23) | 47.2 (233) | 38.2 (346) | 12 months | Difference of differences 13.6 |
— | — | 0.032 |
| Mobility | |||||||
| Salbach 2017 | Intervention A mean (n) |
Intervention B mean (n) |
Time point | Effect estimate (metric) | 95% CI | ||
| Mobility measured by 6MWT | 250 m (410) | 190 m (367) | at discharge | 1.63 (OR) | 1.23 to 2.17 | ||
| Upper limb function | |||||||
| Salbach 2017 | Intervention A mean (n) |
Intervention B mean (n) |
|||||
| Upper limb function measured by Box and Block test | 27 blocks (410) | 29 blocks (367) | at discharge | 1.69 (OR) | 0.72 to 4.01 | ||
| Continence | |||||||
| Thomas 2015 | Intervention A | Intervention B | |||||
| Incontinence (Y/N) measured by ICIQ‐UI Short Form | 59 (104) | 68 (86) | 12 weeks | ^ | ^ | ^ | ^ |
| Incontinence Severity Index | 2.5 (0–8), 102 | 4 (0–8), 86 | 12 weeks | ^ | ^ | ^ | ^ |
| Return home | |||||||
| Strasser 2008 | % (n) | % (n) | Difference of differences | ||||
| % of patients returning home from hospital | 80.7 (233) | 72.8 (439) | 12 months | 5.5 | 0.257 | ||
| Length of hospital stay | |||||||
| Strasser 2008 | Mean (n) | Mean (n) | Difference of differences | ||||
| Length of hospital stay | 19.6 days (233) | 20.5 days (346) | 12 months | 3.0 days | 0.180 | ||
| Death | |||||||
| Thomas 2015 | Intervention A | Intervention B | |||||
| Death | % (n) | % (n) | |||||
| 40 (152) | 29 (114) | 52 weeks | ^ | ^ | ^ | ^ | |
^: authors did not complete statistical analysis on this comparison (they compared intervention groups with control; three‐armed study).
6MWT: Six‐Minute Walk Test; ADL: activities of daily living; CI: confidence interval; EQ‐5D: European Quality of Life‐5 Dimensions; FIM: Functional Independence Measure; I‐QOL: Incontinence Quality of Life; ICIQ‐UI: International Consultation on Incontinence Questionnaire – Urinary Incontinence; IQR: interquartile range; n: number of participants; OR: odds ratio; Y/N: yes/no.
Four trials compared one implementation intervention to another (see Description of studies and Table 2 (Pennington 2005; Salbach 2017; Strasser 2008; Thomas 2015). Three trials compared a tailored intervention to a non‐tailored intervention (Salbach 2017; Strasser 2008; Thomas 2015).
Quality of care: healthcare professional adherence to evidence‐based practice
Two trials reported measures of healthcare professional adherence to EBP at up to 16 months (Pennington 2005; Salbach 2017). Pennington 2005 compared a tailored education meeting (2.5‐day workshop; intervention A) with a tailored education meeting using Roger's Diffusions of Innovation theory (5‐day workshop; intervention B). Pennington 2005 reported mean adherence to clinical practice guidelines for dysphagia management. Salbach 2017 compared a facilitated implementation strategy involving education, tailoring, local opinion leaders and tailoring (intervention A) with a passive intervention providing clinical practice guidelines and educational materials (intervention B). Salbach 2017 reported change in percentage of patients receiving recommended physical rehabilitation treatments (e.g. lower extremity range of motion or stretching, or both). Intervention effect estimates were reported (see Table 8). Data could not be pooled due to the heterogeneity of outcomes measures and time points (six to eight months for Pennington 2005, 16 months for Salbach 2017).
Pennington 2005 reported mean adherence scores of 71.5 (SD 10.1) in intervention A versus 72.7 (SD 10.4) in intervention B. Salbach 2017 reported adherence rates for 18 rehabilitation treatments (see Table 8). The unadjusted compliance rates were reported for training sit‐to‐stand (39.1%, 95% CI 33.4 to 44.9 versus passive intervention group 33.6%, 95% CI 27.9 to 39.3) though the active implementation group had lower compliance for several rehabilitation treatments. Salbach 2017 only reported adjusted analyses, based on clustering at patient and provider levels and covariates, where there was a statistically significant improvement: sit to stand training and walking training in intervention A and standing balance training in intervention B.
Patient health behaviour: adherence to recommended treatment
No trials reported adherence to recommended treatment.
Patient health status and well‐being
Three trials reported at least one measure of patient health status and well‐being at up to 12 months (Salbach 2017; Strasser 2008; Thomas 2015).
Health‐related quality of life
One trial reported health‐related quality of life at three months using the I‐QOL to compare intervention A (47 participants) with intervention B (35 participants) (Thomas 2015). Intervention A had a median I‐QOL score of 76.1 (IQR 42.5 to 94.3) compared with a median of 67.1 (IQR 51.1 to 85.2) for intervention B.
Thomas 2015 also reported separate components of the EQ‐5D at 12 weeks to compare health‐related quality of life between intervention groups. No relative measure of treatment effect was reported for this comparison.
Table 9 provides a structured summary of quality of life outcomes for this comparison.
Activities of daily living
Two trials reported ADL status at up to 12 months (Strasser 2008; Thomas 2015). Thomas 2015 used the Barthel Index at three months to compare two different intervention groups; median Barthel Index scores reported were 8 (IQR 4 to 13) in intervention A (95 participants) and 11 (IQR 6 to 15) in intervention B (128 participants); no relative measure of treatment effect was reported for this comparison.
Strasser 2008 used motor items on the FIM at 12 months to compare two interventions designed to improve team functioning; scores in intervention A (233 participants) improved 47.2% compared with intervention group B (346 participants) at 38.2% (MD 13.6; P = 0.032); it should be noted there were suggested unit of analysis issues with this study.
Data could not be pooled due to the heterogeneity of interventions, outcomes measures and time points.
Mobility status
One trial reported mobility status using the 6MWT at 16 months (Salbach 2017). Mean score for intervention A (410 participants) was 250 m and intervention B (367 participants) was 190 m (OR 1.63, 95% CI 1.23 to 2.17).
Upper limb function
One trial reported upper limb function using the Box and Block Test at 16 months (Salbach 2017). Mean score for intervention A (410 participants) was 27 blocks and intervention B (367 participants) was 29 blocks (OR 1.69, 95% CI 0.72 to 4.01).
Continence status
One trial reported continence status at three months using the ICIQ‐UI (Thomas 2015). Intervention A (104 participants) was compared with Intervention B (86 participants). The percentage of stroke survivors with incontinence was 59% in intervention A and 68% in intervention B at three months; no relative measure of treatment effect was reported for this comparison.
Return home
One trial reported the percentage of patients returning home at 12 months (Strasser 2008).
Strasser 2008 found 80.7% of stroke patients in intervention group A (233 participants) returned home compared with 72.8% of stroke patients in another intervention group B (439 participants) (difference of differences 5.5; P = 0.257).
Healthcare professional outcomes
No trials reported healthcare professional outcomes.
Resource use and costs
One trial in this comparison reported healthcare resource use and costs (see Table 5 for economic data) (Thomas 2015).
Costs were converted to a common currency (USD) and price year (2019) using an online cost converter (EPPI‐Centre Cost Converter 2019).
Thomas 2015 conducted both cost‐utility analysis and cost‐effectiveness analyses; this trial found similar mean QALY gains for the implementation group (intervention A, mean –0.42) and the supported implementation group (intervention B, mean –0.47). Resource use costs were higher with intervention B (mean cost per patient of staff training USD 38.77) compared with intervention A (mean cost per patient of staff training USD 20.16).
The quality of this trial was assessed using the CHEC (Table 6; Table 7). A clear research question related to economic outcomes was not made in this trial though an appropriate research design was used to determine economic outcomes and these outcomes were reported appropriately in physical units. The study perspective was deemed appropriate. Ethical and distributional issues were not discussed.
Adverse effects/harms
Death
One trial reported deaths at 12‐month trial conclusion; 26% of patients had died in intervention group A (152 participants) compared with 25% of patients in intervention group B (114 participants) (Thomas 2015).
Falls
One trial reported number of falls; there were 11 falls in intervention group A (164 participants) compared with four falls in intervention group B (125 participants). These results include patients who had more than one fall.
Other adverse effects
One trial reported other adverse effects; specifically, urinary tract infections and bladder catheterisation (Thomas 2015). The number of urinary tract infections in intervention group A (164 participants) was 18 compared with 23 in intervention group B (125 participants). These results include patients who had more than one urinary tract infection. Bladder catheterisation was required in four patients in intervention group A (164 participants) and one in intervention group B (125 participants).
Discussion
Summary of main results
We found nine cluster‐randomised trials, including 12,428 participants, and three ongoing studies comparing an implementation intervention in stroke rehabilitation with no intervention or another implementation intervention. Most trials were susceptible to bias; in particular, performance (55%), detection (55%) and reporting (44%) biases.
We could not obtain a reliable estimate of the effect of implementation strategies in stroke rehabilitation on healthcare professional adherence to evidence‐based practice at 12 months because the evidence was of very low certainty. Low‐certainty evidence indicates implementation interventions in stroke rehabilitation may lead to little or no difference in patient adherence to recommended treatment and patient psychological well‐being. Moderate‐certainty evidence suggests implementation interventions probably lead to little or no difference in patient health‐related quality of life and ADL compared with no intervention. No studies reported the effects of implementation interventions in stroke rehabilitation on healthcare professional intention to change behaviour or satisfaction. Tailoring interventions to identified barriers did not alter results. Additionally, we are uncertain of the effect of one implementation intervention versus another given the limited evidence of very low certainty addressing this question.
In interpreting the results, it is important to be mindful of several factors. First, we did not prespecify a minimally importance difference in adherence to evidence‐based practice, which impacts interpretation of our primary outcome. Second, the number of studies included in this review was small and was likely to reflect the emerging state of implementation science in stroke rehabilitation. Only a small percentage of stroke rehabilitation research currently focuses on implementation (about 2.5%) (Lynch 2018). Future research attention and capital should be directed towards evaluating implementation interventions in high‐quality studies. Third, there was substantial variability in the implementation interventions and outcomes used across studies, with this heterogeneity especially evident in studies that compared two different implementation interventions.
The current review also incorporates a previous review on in‐hospital care pathways for stroke (Kwan 2004), as an integrated care pathway is a means of increasing uptake of evidence‐based practices in stroke rehabilitation. Many of the studies in the original Kwan 2004 review were ineligible for inclusion due to study design or setting. Our review found only one study involving a care pathway in stroke rehabilitation (Abdul Aziz 2014). This study was assessed as high risk of bias and did not measure our primary outcome of healthcare professional adherence to recommended treatment.
We found no serious adverse events in studies, though it should be noted six studies (66%) did not report these outcomes.
To complement our assessment of the effectiveness of implementation interventions to increase the uptake of evidence‐based practices in stroke rehabilitation, we sought to identify economic evaluations conducted alongside included studies. Our search identified five economic evaluations with one study reporting cost‐effectiveness of the implementation intervention (the care pathway). However, this study was at high risk of reporting bias and had suspected unit of analysis issues. All other studies did not demonstrate cost‐effectiveness of the interventions studied.
Overall completeness and applicability of evidence
We developed and conducted a comprehensive search of the literature in consultation with the Cochrane EPOC Information Specialist and screened 17,869 unique records for inclusion. We searched grey literature and included published and unpublished trials. Included studies were clinically heterogeneous and all but one assessed the effects of multifaceted implementation interventions. The studies targeted different evidence‐based practices; just under half of studies focused on treatments recommended in clinical practice guidelines and one third of studies focused on translating evidence from systematic reviews. Less than half of the included studies measured our primary outcome, healthcare professional adherence to recommended treatment. Most studies (77%) involved implementation interventions targeting two or more professional disciplines, likely reflecting the multidisciplinary nature of stroke rehabilitation. Characteristics of participating healthcare professionals were poorly reported; 88% did not report the number of healthcare professionals within clusters and no study reported demographic information (e.g. sex, years of experience). While 66% of studies reported using some form of barrier identification, the process of tailoring implementation interventions was not well described. Six studies compared tailored to non‐tailored interventions, 55% studies documented use of a theory for implementation, though the application of theory and description of how this practically guided the study was largely undiscussed.
Quality of the evidence
We are uncertain whether implementation interventions in stroke rehabilitation improve healthcare professional adherence to evidence‐based practice at 12 months compared to no intervention as the certainty of the evidence was very low. We downgraded the certainty of the evidence for our primary outcome three levels due to very serious imprecision and serious risk of bias. Very serious imprecision resulted from very wide 95% CIs and suboptimal information size and serious risk of bias resulted from lack of blinding of outcome assessors and incomplete data in one trial. This indicates future high‐quality research addressing this question is very likely to have an important impact on the effect estimate and our confidence in the findings.
We found low‐certainty evidence suggesting implementation interventions in stroke rehabilitation may lead to little or no difference in patient adherence to recommended treatment and patient psychological well‐being compared with no intervention. We downgraded the certainty of the evidence for patient adherence to recommended treatment by two levels due to very serious imprecision (suboptimal information size and wide 95% CIs) and two levels for patient psychological well‐being due to serious indirectness (differences in outcome measures). Moderate‐certainty evidence indicated implementation interventions in stroke rehabilitation probably lead to little or no difference in patient health‐related quality of life and ADL compared with no intervention. We downgraded the certainty of the evidence for these outcomes by one level due to serious imprecision (suboptimal information size).
Potential biases in the review process
We aimed to minimise bias at each stage of this review by conducting the review according to Cochrane Handbook for Systematic Reviews of Interventions guidance (Higgins 2019), and in accordance with our published protocol (Cahill 2017). To the best of our knowledge, we identified all relevant trials meeting the review's eligibility criteria through searching major electronic databases, trial registries and grey literature; reference checking; and contacting study authors and experts in the field. We located one unpublished study through a conference abstract, with further unpublished data provided by the authors and included in this review. The inclusion of unpublished data aimed to reduce the impact of publication bias. Two review authors independently screened, selected, extracted data, and judged risk of bias of studies and certainty of evidence. There were too few studies to formally assess the presence of publication bias. None of the review authors were involved in the conduct of the included studies.
Agreements and disagreements with other studies or reviews
To our knowledge, only one previous systematic review has investigated this research question (Bird 2019). Bird 2019 included single‐site randomised trials and used a broad definition of stroke rehabilitation encompassing acute stroke management and secondary stroke prevention by general practitioners. Five studies included in Bird 2019 are included in this review (McCluskey 2016; Pennington 2005; Power 2014; Salbach 2017; Strasser 2008). The remaining 11 studies in Bird 2019 were ineligible for inclusion in our review due to not meeting our definition of stroke rehabilitation or Cochrane EPOC recommended study designs (EPOC 2016a), which aim to minimise single‐site confounding.
Bird 2019 concluded that professional education alone is ineffective in changing healthcare professional adherence to evidence‐based practice but that multifaceted implementation interventions, specifically facilitation and tailoring, are effective when targeting fewer changes for healthcare professionals. Bird 2019 vote counted studies based on statistical significance and did not assess the certainty of the evidence using GRADE. Their approach is likely to lead to biased conclusions as underpowered studies are counted as not showing benefit and certainty of effect estimates is not assessed. In contrast, we could not obtain a reliable estimate of the effect of implementation strategies in stroke rehabilitation on healthcare professional adherence to evidence‐based practice because the evidence was of very low certainty. We did not identify any studies investigating the effect of education alone and found tailoring interventions to identified barriers to change did not alter the results. We pooled data in a meta‐analysis where possible and prepared structured summaries of effects for all included studies so that all relevant information could be considered when estimating treatment effects. We graded the certainty of the evidence for main outcomes for the primary comparison.
Authors' conclusions
Implications for practice.
Though knowledge about effective treatments for optimal stroke recovery is building rapidly, knowledge about how best to translate this evidence into routine stroke care is lacking. In this review, we could not obtain a reliable estimate of the effect of stroke rehabilitation implementation interventions on healthcare professional adherence to evidence‐based practice compared with no intervention as the certainty of the evidence is very low. Until this body of evidence matures, local implementation efforts should be informed by the broader body of evidence about the effects of implementation interventions (Baker 2015; Forsetlund 2009; Giguère 2012; Ivers 2012; O'Brien 2007), the likely mechanism of action of interventions and local factors influencing translation, including acceptability and feasibility of interventions.
Implications for research.
The full potential of stroke rehabilitation will only be realised through the sustained implementation of research knowledge into clinical practice. This review has compiled the evidence from studies of prespecified design that minimise the likelihood of bias; many studies investigating implementation in stroke rehabilitation were excluded from this review on the basis of study design. We believe the body of evidence for implementation interventions in stroke rehabilitation would be strengthened by the use of rigorous study designs that are adequately powered to detect clinically important differences in outcomes of interest. Future implementation studies should improve reporting of descriptions of interventions, including rationale for intervention design, methods used to identify barriers and tailor interventions to address barriers (where relevant), and extent of intervention fidelity. Reporting of methods and findings in studies should be consistent with the CONSORT Statement (Schulz 2010). Implementation studies should also measure the impact of implementation interventions on healthcare professional behaviour; only four studies included in this review reported this outcome. Measurement should also occur elsewhere along the pathway of behaviour change, where feasible, for example healthcare professional intention to change behaviour or other hypothesised mediators, patient behaviour, patient health outcomes and costs of delivering interventions.
Studies further evaluating implementation interventions are vital to ensure the benefits of research evidence are experienced by stroke survivors.
History
Protocol first published: Issue 3, 2017 Review first published: Issue 10, 2020
Acknowledgements
The authors would like to thank Emma Tavender and Clare Dooley from the Australasian Satellite of Cochrane EPOC for their advice in the early stages of the review and Paul Miller, EPOC Information Specialist, for his assistance with developing and running database searches. We acknowledge Luke Vale (EPOC economics editor) for advice regarding economic evaluation. We thank the following EPOC editors and peer reviewers for commenting on the review: Mary Ann O'Brien (EPOC Contact Editor), Simon Lewin (EPOC Co‐ordinating Editor), Ada Tang and Nancy Salbach. We thank Elizabeth Paulsen as our EPOC Managing Editor and Anne Lawson as copy‐editor.
National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Effective Practice and Organisation of Care (EPOC) Group. The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service (NHS) or the Department of Health.
The Australasian Satellite of the Cochrane EPOC Group receives in kind support from Cabrini Institute and Monash University.
Appendices
Appendix 1. Search strategies
The Cochrane Library (Wiley)
| No. | Search terms | Results |
| #1 | [mh stroke] or [mh "cerebrovascular disorders"] or [mh "brain ischemia"] or [mh "brain infarction"] or [mh "cerebral infarction"] or [mh "subarachnoid hemorrhage"] or [mh "intracranial hemorrhages"] | 13666 |
| #2 | (stroke or poststroke or (post NEXT stroke) or cerebrovasc* or brainvasc* or (cerebral NEXT vasc*) or cva* or apoplex* or sah):ti,ab | 53199 |
| #3 | ((brain* or cerebr* or cerebell* or intracran* or intracerebral) near/5 (isch?emi* or infarct* or thrombo* or emboli* or occlus*)):ti,ab | 8279 |
| #4 | ((brain* or cerebr* or cerebell*or intracerebral or intracranial or subarachnoid) near/5 (haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed*)):ti,ab | 6839 |
| #5 | (brain NEXT injur*):ti,ab | 5000 |
| #6 | {or #1‐#5} | 66348 |
| #7 | (post NEXT stroke or poststroke):ti,ab | 5032 |
| #8 | rehabilitat*:ti,ab | 30070 |
| #9 | [mh ^/RH] | 17290 |
| #10 | recover*:ti,ab | 54358 |
| #11 | [mh "recovery of function"] | 4829 |
| #12 | therap*:ti,ab | 358227 |
| #13 | {or #7‐#12} | 427940 |
| #14 | [mh "translational medical research"] | 119 |
| #15 | (knowledge near/2 (application or broke* or creation or diffus* or disseminat* or exchang* or implement* or management or mobili* or translat* or transfer* or uptak* or utili*)):ti,ab | 1040 |
| #16 | (evidence* near/2 (exchang* or translat* or transfer* or diffus* or disseminat* or exchang* or implement* or management or mobil* or uptak* or utili*)):ti,ab | 1800 |
| #17 | (kt near/2 (application or broke* or diffus* or disseminat* or decision* or exchang* or implement* or intervent* or mobili* or plan* or policy or policies or strateg* or translat* or transfer* or uptak* or utili*)):ti,ab | 198 |
| #18 | (research* near/2 (diffus* or disseminat* or exchang* or transfer* or translation* or application or implement* or mobil* or transfer* or uptak* or utili*)):ti,ab | 2120 |
| #19 | ((research or evidence) near/2 (action or practice)):ti,ab | 3114 |
| #20 | [mh "diffusion of innovation"] | 159 |
| #21 | ((evidence next base* or evidence next inform*) near/5 (decision* or plan* or policy or policies or practice or action*)):ti,ab | 1794 |
| #22 | implementation:ti,ab | 17927 |
| #23 | behavio?r next change:ti,ab | 4113 |
| #24 | [mh "organizational innovation"] | 111 |
| #25 | organi?ational next change?:ti,ab | 156 |
| #26 | complex next intervention?:ti,ab | 1028 |
| #27 | audit:ti,ab | 2933 |
| #28 | (barrier? and facilitator?):ti,ab | 1158 |
| #29 | (booklet* or brochure? or pamphlet? or (paper NEXT based) or printed material?):ti,ab | 6027 |
| #30 | decision next mak*:ti,ab or [mh "decision making"] | 11855 |
| #31 | ((change? or changing or improv* or effect* or influenc* or alter* or adapt* or amend* or modify* or adjust* or transform*) near/2 (policy or policies or process* or practic* or provider? or activit*)):ti,ab | 17160 |
| #32 | ((knowledge or evidence or quality or research or practice) near/2 gap?):ti,ab | 1222 |
| #33 | (education* near/3 (continuing or group? or outreach or plan* or practitioner? or program? or staff? or team?)):ti,ab | 13865 |
| #34 | ((evidence NEXT based) near/3 (algorithm? or evaluat* or guideline? or healthcare or implement* or improv* or intervention* or management or pathway? or plan? or practic* or program? or quality)):ti,ab | 5319 |
| #35 | (feedback or feed next back):ti,ab | 13986 |
| #36 | [mh "guideline adherence"] | 1006 |
| #37 | (guideline? near/3 (adher* or enforc* or influenc* or implement* or impact* or introduc* or uptake or follow)):ti,ab | 2484 |
| #38 | (incentiv* near/2 (economic or employee? or financ* or insurer? or insurance or market* or monetar* or pay* or plan? or practitioner? or program* or provider? or reimburs* or salary or salarie? or staff or team* or (value NEXT based))):ti,ab | 1426 |
| #39 | (collaborat* or (cross NEXT profession*) or intraprofession* or (intra NEXT profession*) or interprofession* or (inter NEXT profession*) or (skill near/2 mix*) or teambase? or (team NEXT based) or (inter NEXT disciplin*) or multidisciplin* or (multi NEXT disciplin*) or multiprofession*):ti,ab | 16949 |
| #40 | ((knowledge near/2 (transfer* or translation or shar* or exchan*)) or kt):ti,ab | 2085 |
| #41 | ((knowledge or evidence or practice) near/2 (gap? or barrier?)):ti,ab | 1154 |
| #42 | ((knowledge or evidence) near/2 synthesis):ti,ab | 265 |
| #43 | (opinion NEXT leader?):ti,ab | 207 |
| #44 | (outreach near/2 (communit* or plan? or program? or visit?)):ti,ab | 528 |
| #45 | ((policy or policies) near/2 (chang* or effect? or impact? or influenc*)):ti,ab | 425 |
| #46 | (quality near/2 (assurance or improvement? or initiativ* or plan* or program* or review or audit)):ti,ab | 6990 |
| #47 | (qi next (initiative? or intervention? or program* or plan* or audit)):ti,ab | 120 |
| #48 | ((change? or changing or improv* or effect* or influenc*) near/2 (policy or policies or practic* or provider?)):ti,ab | 5838 |
| #49 | ((journal next club?) or (clinical next librarian) or library or libraries or (answer next service?) or (information next science)):ti,ab | 6281 |
| #50 | (best NEXT practice):ti,ab | 1197 |
| #51 | (care NEXT pathway?):ti,ab | 576 |
| #52 | (project network next (technique? or diagram?)):ti,ab | 1 |
| #53 | {or #14‐#52} | 112302 |
| #54 | [mh "stroke rehabilitation"] | 2206 |
| #55 | #6 and #13 and #53 | 3403 |
| #56 | #53 and #54 | 508 |
| #57 | #55 or #56 | 3429 |
MEDLINE (Ovid) 1946 to 17 October 2019
| No. | Search terms | Results |
| 1 | exp stroke/ | 126055 |
| 2 | exp cerebrovascular disorders/ | 353346 |
| 3 | exp brain ischemia/ | 103799 |
| 4 | exp brain infarction/ | 35800 |
| 5 | exp cerebral infarction/ | 30699 |
| 6 | exp subarachnoid hemorrhage/ | 20550 |
| 7 | exp intracranial hemorrhages/ | 68810 |
| 8 | (stroke or poststroke or post‐stroke or cerebrovasc* or brainvasc* or cerebral vasc* or cva* or apoplex* or sah).ti,ab. | 284512 |
| 9 | ((brain* or cerebr* or cerebell* or intracran* or intracerebral) adj5 (isch?emi* or infarct* or thrombo* or emboli* or occlus*)).ti,ab. | 102898 |
| 10 | ((brain* or cerebr* or cerebell* or intracerebral or intracranial or subarachnoid) adj5 (haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed*)).ti,ab. | 63987 |
| 11 | (acquired brain injur* or brain injur*).ti,ab. | 61477 |
| 12 | or/1‐11 | 567494 |
| 13 | (post stroke or poststroke).ti,ab. | 13073 |
| 14 | rehabilitat*.ti,ab. | 156948 |
| 15 | rh.fs. | 192780 |
| 16 | recover*.ti,ab. | 630481 |
| 17 | exp recovery of function/ | 49075 |
| 18 | therap*.ti,ab. | 2630434 |
| 19 | or/13‐18 | 3426130 |
| 20 | exp translational medical research/ | 9903 |
| 21 | (knowledge adj2 (application or broke* or creation or diffus* or disseminat* or exchang* or implement* or management or mobili* or translat* or transfer* or uptak* or utili*)).ti,ab. | 14333 |
| 22 | (evidence* adj2 (exchang* or translat* or transfer* or diffus* or disseminat* or exchang* or implement* or management or mobil* or uptak* or utili*)).ti,ab. | 13774 |
| 23 | (kt adj2 (application or broke* or diffus* or disseminat* or decision* or exchang* or implement* or intervent* or mobili* or plan* or policy or policies or strateg* or translat* or transfer* or uptak* or utili*)).ti,ab. | 605 |
| 24 | (research* adj2 (diffus* or disseminat* or exchang* or transfer* or translation* or application or implement* or mobil* or transfer* or uptak* or utili*)).ti,ab. | 24574 |
| 25 | ((research or evidence) adj2 (action or practice)).ti,ab. | 39272 |
| 26 | exp "diffusion of innovation"/ | 19270 |
| 27 | ((evidence base* or evidence inform*) adj5 (decision* or plan* or policy or policies or practice or action*)).ti,ab. | 22349 |
| 28 | implementation.ti,ab. | 224288 |
| 29 | behavio?r change.ti,ab. | 12844 |
| 30 | exp organizational innovation/ | 26025 |
| 31 | organi?ational change?.ti,ab. | 2683 |
| 32 | complex intervention?.ti,ab. | 2324 |
| 33 | audit.ti,ab. | 32529 |
| 34 | (barrier? and facilitator?).ti,ab. | 9436 |
| 35 | (booklet* or brochure? or pamphlet? or paper‐based or printed material?).ti,ab. | 13956 |
| 36 | decision mak*.ti,ab. or exp decision making/ | 286431 |
| 37 | ((change? or changing or improv* or effect* or influenc* or alter* or adapt* or amend* or modify* or adjust* or transform*) adj2 (policy or policies or process* or practic* or provider? or activit*)).ti,ab. | 214129 |
| 38 | ((knowledge or evidence or quality or research or practice) adj2 gap?).ti,ab. | 21082 |
| 39 | (education* adj3 (continuing or group? or outreach or plan* or practitioner? or program? or staff? or team?)).ti,ab. | 75059 |
| 40 | (evidence‐based adj3 (algorithm? or evaluat* or guideline? or healthcare or implement* or improv* or intervention* or management or pathway? or plan? or practic* or program? or quality)).ti,ab. | 40746 |
| 41 | ((feedback or feed back) not feedback loop*).ti,ab. | 116871 |
| 42 | exp guideline adherence/ | 30650 |
| 43 | (guideline? adj3 (adher* or enforc* or influenc* or implement* or impact* or introduc* or uptake or follow)).ti,ab. | 20310 |
| 44 | (incentiv* adj2 (economic or employee? or financ* or insurer? or insurance or market* or monetar* or pay* or plan? or practitioner? or program* or provider? or reimburs* or salary or salarie? or staff or team* or value‐based)).ti,ab. | 9258 |
| 45 | (collaborat* or cross‐profession* or intraprofession* or intra‐profession* or interprofession* or inter‐profession* or (skill adj2 mix*) or teambase? or team‐based or inter disciplin* or multidisciplin* or multi disciplin* or multiprofession*).ti,ab. | 223634 |
| 46 | ((knowledge adj2 (transfer* or translation or shar* or exchan*)) or kt).ti,ab. | 18444 |
| 47 | ((knowledge or evidence or practice) adj2 (gap? or barrier?)).ti,ab. | 19033 |
| 48 | ((knowledge or evidence) adj2 synthesis).ti,ab. | 5175 |
| 49 | opinion leader?.ti,ab. | 1343 |
| 50 | (outreach adj2 (communit* or plan? or program? or visit?)).ti,ab. | 3414 |
| 51 | ((policy or policies) adj2 (chang* or effect? or impact? or influenc*)).ti,ab. | 11308 |
| 52 | (quality adj2 (assurance or improvement? or initiativ* or plan* or program* or review or audit)).ti,ab. | 74275 |
| 53 | (qi adj (initiative? or intervention? or program* or plan* or audit)).ti,ab. | 862 |
| 54 | ((change? or changing or improv* or effect* or influenc*) adj2 (policy or policies or practic* or provider?)).ti,ab. | 56365 |
| 55 | (journal club or clinical librarian or library or libraries or answer service* or information science).ti,ab. | 161048 |
| 56 | best practice.ti,ab. | 12416 |
| 57 | care pathway?.ti,ab. | 3897 |
| 58 | (project network adj (technique? or diagram?)).ti,ab. | 1 |
| 59 | or/20‐58 | 1482155 |
| 60 | 12 and 19 and 59 | 13009 |
| 61 | stroke rehabilitation/ | 12260 |
| 62 | stroke rehabilitation.ti,ab. | 3414 |
| 63 | or/61‐62 | 13607 |
| 64 | 63 and 59 | 2917 |
| 65 | 60 or 64 | 13329 |
| 66 | randomized controlled trial.pt. | 491454 |
| 67 | controlled clinical trial.pt. | 93316 |
| 68 | multicenter study.pt. | 258369 |
| 69 | pragmatic clinical trial.pt. | 1178 |
| 70 | (randomis* or randomiz* or randomly).ti,ab. | 853888 |
| 71 | groups.ab. | 1963833 |
| 72 | (trial or multicenter or multi center or multicentre or multi centre).ti. | 244485 |
| 73 | (intervention? or effect? or impact? or controlled or control group? or (before adj5 after) or (pre adj5 post) or ((pretest or pre test) and (posttest or post test)) or quasiexperiment* or quasi experiment* or pseudo experiment* or pseudoexperiment* or evaluat* or time series or time point? or repeated measur*).ti,ab. | 9203502 |
| 74 | non‐randomized controlled trials as topic/ | 553 |
| 75 | interrupted time series analysis/ | 677 |
| 76 | controlled before‐after studies/ | 434 |
| 77 | or/66‐76 | 1E+07 |
| 78 | exp animals/ | 2.3E+07 |
| 79 | humans/ | 1.8E+07 |
| 80 | 78 not (78 and 79) | 4628072 |
| 81 | review.pt. | 2565353 |
| 82 | meta analysis.pt. | 105857 |
| 83 | news.pt. | 197552 |
| 84 | comment.pt. | 808275 |
| 85 | editorial.pt. | 504990 |
| 86 | cochrane database of systematic reviews.jn. | 14547 |
| 87 | comment on.cm. | 808220 |
| 88 | (systematic review or literature review).ti. | 141692 |
| 89 | or/80‐88 | 8393027 |
| 90 | 77 not 89 | 7226905 |
| 91 | 65 and 90 | 6344 |
Embase (Ovid) 1946 to 17 October 2019
| No. | Search terms | Results |
| 1 | exp *cerebrovascular accident/ | 78276 |
| 2 | *cerebrovascular disease/ | 21975 |
| 3 | exp *brain ischemia/ | 89961 |
| 4 | exp *brain infarction/ | 27022 |
| 5 | *subarachnoid hemorrhage/ | 20187 |
| 6 | exp *brain haemorrhage/ | 51566 |
| 7 | (stroke or poststroke or post‐stroke or cerebrovasc* or brainvasc* or cerebral vasc* or cva* or apoplex* or sah).ti,ab. | 445890 |
| 8 | ((brain* or cerebr* or cerebell* or intracran* or intracerebral) adj5 (isch?emi* or infarct* or thrombo* or emboli* or occlus*)).ti,ab. | 145629 |
| 9 | ((brain* or cerebr* or cerebell* or intracerebral or intracranial or subarachnoid) adj5 (haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed*)).ti,ab. | 91813 |
| 10 | (acquired brain injur* or brain injur*).ti,ab. | 88324 |
| 11 | or/1‐10 | 674449 |
| 12 | (post stroke or poststroke).ti,ab. | 22249 |
| 13 | rehabilitat*.ti,ab. | 221273 |
| 14 | rh.fs. | 147772 |
| 15 | recover*.ti,ab. | 809132 |
| 16 | *convalescence/ | 6132 |
| 17 | therap*.ti,ab. | 3766222 |
| 18 | exp *rehabilitation/ | 107859 |
| 19 | or/12‐18 | 4719868 |
| 20 | *translational research/ | 6534 |
| 21 | (knowledge adj2 (application or broke* or creation or diffus* or disseminat* or exchang* or implement* or management or mobili* or translat* or transfer* or uptak* or utili*)).ti,ab. | 19250 |
| 22 | (evidence* adj2 (exchang* or translat* or transfer* or diffus* or disseminat* or exchang* or implement* or management or mobil* or uptak* or utili*)).ti,ab. | 18006 |
| 23 | (kt adj2 (application or broke* or diffus* or disseminat* or decision* or exchang* or implement* or intervent* or mobili* or plan* or policy or policies or strateg* or translat* or transfer* or uptak* or utili*)).ti,ab. | 797 |
| 24 | (research* adj2 (diffus* or disseminat* or exchang* or transfer* or translation* or application or implement* or mobil* or transfer* or uptak* or utili*)).ti,ab. | 32575 |
| 25 | ((research or evidence) adj2 (action or practice)).ti,ab. | 51416 |
| 26 | ((evidence base* or evidence inform*) adj5 (decision* or plan* or policy or policies or practice or action*)).ti,ab. | 28271 |
| 27 | implementation.ti,ab. | 300111 |
| 28 | behavio?r change.ti,ab. | 15755 |
| 29 | organi?ational change?.ti,ab. | 3265 |
| 30 | complex intervention?.ti,ab. | 3155 |
| 31 | audit.ti,ab. | 69577 |
| 32 | (barrier? and facilitator?).ti,ab. | 12154 |
| 33 | (booklet* or brochure? or pamphlet? or paper‐based or printed material?).ti,ab. | 19568 |
| 34 | decision mak*.ti,ab. or exp *decision making/ | 223833 |
| 35 | ((change? or changing or improv* or effect* or influenc* or alter* or adapt* or amend* or modify* or adjust* or transform*) adj2 (policy or policies or process* or practic* or provider? or activit*)).ti,ab. | 273287 |
| 36 | ((knowledge or evidence or quality or research or practice) adj2 gap?).ti,ab. | 26831 |
| 37 | (education* adj3 (continuing or group? or outreach or plan* or practitioner? or program? or staff? or team?)).ti,ab. | 99783 |
| 38 | (evidence‐based adj3 (algorithm? or evaluat* or guideline? or healthcare or implement* or improv* or intervention* or management or pathway? or plan? or practic* or program? or quality)).ti,ab. | 53858 |
| 39 | ((feedback or feed back) not feedback loop*).ti,ab. | 150981 |
| 40 | (guideline? adj3 (adher* or enforc* or influenc* or implement* or impact* or introduc* or uptake or follow)).ti,ab. | 34767 |
| 41 | (incentiv* adj2 (economic or employee? or financ* or insurer? or insurance or market* or monetar* or pay* or plan? or practitioner? or program* or provider? or reimburs* or salary or salarie? or staff or team* or value‐based)).ti,ab. | 11413 |
| 42 | (collaborat* or cross‐profession* or intraprofession* or intra‐profession* or interprofession* or inter‐profession* or (skill adj2 mix*) or teambase? or team‐based or inter disciplin* or multidisciplin* or multi disciplin* or multiprofession*).ti,ab. | 336277 |
| 43 | ((knowledge adj2 (transfer* or translation or shar* or exchan*)) or kt).ti,ab. | 25131 |
| 44 | ((knowledge or evidence or practice) adj2 (gap? or barrier?)).ti,ab. | 24616 |
| 45 | ((knowledge or evidence) adj2 synthesis).ti,ab. | 5860 |
| 46 | opinion leader?.ti,ab. | 1868 |
| 47 | (outreach adj2 (communit* or plan? or program? or visit?)).ti,ab. | 4721 |
| 48 | ((policy or policies) adj2 (chang* or effect? or impact? or influenc*)).ti,ab. | 13946 |
| 49 | (quality adj2 (assurance or improvement? or initiativ* or plan* or program* or review or audit)).ti,ab. | 113891 |
| 50 | (qi adj (initiative? or intervention? or program* or plan* or audit)).ti,ab. | 1630 |
| 51 | ((change? or changing or improv* or effect* or influenc*) adj2 (policy or policies or practic* or provider?)).ti,ab. | 75764 |
| 52 | (journal club or clinical librarian or library or libraries or answer service* or information science).ti,ab. | 188757 |
| 53 | best practice.ti,ab. | 20309 |
| 54 | care pathway?.ti,ab. | 7248 |
| 55 | (project network adj (technique? or diagram?)).ti,ab. | 1 |
| 56 | or/20‐55 | 1766033 |
| 57 | 11 and 19 and 56 | 21938 |
| 58 | randomized controlled trial/ | 576736 |
| 59 | controlled clinical trial/ | 465767 |
| 60 | quasi experimental study/ | 6077 |
| 61 | pretest posttest control group design/ | 422 |
| 62 | time series analysis/ | 24295 |
| 63 | experimental design/ | 17670 |
| 64 | multicenter study/ | 233309 |
| 65 | (randomis* or randomiz* or randomly).ti,ab. | 1205564 |
| 66 | groups.ab. | 2736983 |
| 67 | (trial or multicentre or multicenter or multi centre or multi center).ti. | 345636 |
| 68 | (intervention? or effect? or impact? or controlled or control group? or (before adj5 after) or (pre adj5 post) or ((pretest or pre test) and (posttest or post test)) or quasiexperiment* or quasi experiment* or pseudo experiment* or pseudoexperiment* or evaluat* or time series or time point? or repeated measur*).ti,ab. | 11800433 |
| 69 | or/58‐68 | 13164830 |
| 70 | (systematic review or literature review).ti. | 171764 |
| 71 | "cochrane database of systematic reviews".jn. | 13752 |
| 72 | exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ | 26617394 |
| 73 | human/ or normal human/ or human cell/ | 20344291 |
| 74 | 72 not (72 and 73) | 6333342 |
| 75 | 70 or 71 or 74 | 6517293 |
| 76 | 69 not 75 | 10134410 |
| 77 | 57 and 76 | 14539 |
| 78 | limit 77 to embase | 6044 |
CINAHL (EBSCOhost) 1980 to 17 October 2019
| No. | Search terms | Results |
| S1 | (MH "Stroke+") OR (MH "Cerebrovascular Disorders+") OR (MH "Cerebral Ischemia+") OR (MH "Intracranial Hemorrhage+") OR (MH "Subarachnoid Hemorrhage") | 57,317 |
| S2 | stroke or poststroke or post‐stroke or cerebrovasc* or brainvasc* or cerebral vasc* or cva* or apoplex* or sah | 67,524 |
| S3 | (brain* or cerebr* or cerebell* or intracran* or intracerebral) N5 (isch?emi* or infarct* or thrombo* or emboli* or occlus*) | 6,946 |
| S4 | (brain* or cerebr* or cerebell* or intracerebral or intracranial or subarachnoid) N5 (haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed*) | 9,543 |
| S5 | acquired brain injur* or brain injur* | 18,035 |
| S6 | S1 OR S2 OR S3 OR S4 OR S5 | 95,449 |
| S7 | post stroke or poststroke or rehabilitat* or recover* or therap* | 898,074 |
| S8 | (MH "Recovery") | 14,851 |
| S9 | (MH "Rehabilitation") | 11,428 |
| S10 | S7 OR S8 OR S9 | 898,074 |
| S11 | S6 AND S10 | 50,988 |
| S12 | knowledge N2 (application or broke* or creation or diffus* or disseminat* or exchang* or implement* or management or mobili* or translat* or transfer* or uptak* or utili*) | 5,964 |
| S13 | evidence* N2 (exchang* or translat* or transfer* or diffus* or disseminat* or exchang* or implement* or management or mobil* or uptak* or utili*) | 5,301 |
| S14 | kt N2 (application or broke* or diffus* or disseminat* or decision* or exchang* or implement* or intervent* or mobili* or plan* or policy or policies or strateg* or translat* or transfer* or uptak* or utili*) | 125 |
| S15 | research* N2 (diffus* or disseminat* or exchang* or transfer* or translation* or application or implement* or mobil* or transfer* or uptak* or utili*) | 8,482 |
| S16 | (research or evidence) N2 (action or practice) | 68,951 |
| S17 | (evidence base* or evidence inform*) N5 (decision* or plan* or policy or policies or practice or action*) | 48,951 |
| S18 | implementation or behavio?r change or organi?ational change? or complex intervention? or audit opinion leader? or best practice or care pathway? | 64,113 |
| S19 | (barrier? and facilitator?) | 2,309 |
| S20 | booklet* or brochure? or pamphlet? or paper‐based or printed material? | 4,398 |
| S21 | decision mak* | 78,556 |
| S22 | (MH "Decision Making+") | 63,660 |
| S23 | (change? or changing or improv* or effect* or influenc* or alter* or adapt* or amend* or modify* or adjust* or transform*) N2 (policy or policies or process* or practic* or provider? or activit*) | 41,385 |
| S24 | (knowledge or evidence or quality or research or practice) N2 gap? | 2,434 |
| S25 | education* N3 (continuing or group? or outreach or plan* or practitioner? or program? or staff? or team?) | 114,431 |
| S26 | evidence‐based N3 (algorithm? or evaluat* or guideline? or healthcare or implement* or improv* or intervention* or management or pathway? or plan? or practic* or program? or quality) | 50,721 |
| S27 | feedback or feed back | 16,619 |
| S28 | guideline? N3 (adher* or enforc* or influenc* or implement* or impact* or introduc* or uptake or follow) | 4,337 |
| S29 | incentiv* N2 (economic or employee? or financ* or insurer? or insurance or market* or monetar* or pay* or plan? or practitioner? or program* or provider? or reimburs* or salary or salarie? or staff or team* or value‐based) | 4,807 |
| S30 | collaborat* or cross‐profession* or intraprofession* or intra‐profession* or interprofession* or inter‐profession* or (skill N2 mix*) or teambase? or team‐based or inter disciplin* or multidisciplin* or multi disciplin* or multiprofession* | 107,663 |
| S31 | knowledge N2 (transfer* or translation or shar* or exchan*) or kt | 3,292 |
| S32 | (knowledge or evidence or practice) N2 (gap? or barrier?) | 3,171 |
| S33 | (knowledge or evidence) N2 synthesis | 903 |
| S34 | outreach N2 (communit* or plan? or program? or visit?) | 949 |
| S35 | (policy or policies) N2 (chang* or effect? or impact? or influenc*) | 4,199 |
| S36 | quality N2 (assurance or improvement? or initiativ* or plan* or program* or review or audit) | 20,543 |
| S37 | qi N (initiative? or intervention? or program* or plan* or audit) | 1 |
| S38 | (change? or changing or improv* or effect* or influenc*) N2 (policy or policies or practic* or provider?) | 20,915 |
| S39 | journal club or clinical librarian or library or libraries or answer service* or information science | 52,136 |
| S40 | project network N1 (technique? or diagram?) | 85 |
| S41 | S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 | 504,040 |
| S42 | S11 AND S41 | 8,592 |
| S43 | S42 Limiters ‐ Exclude MEDLINE records | 2,499 |
| S44 | PT randomized controlled trial | 30,875 |
| S45 | PT clinical trial | 52,908 |
| S46 | PT research | 996,041 |
| S47 | (MH "Randomized Controlled Trials") | 30,366 |
| S48 | (MH "Clinical Trials") | 87,660 |
| S49 | (MH "Intervention Trials") | 6,197 |
| S50 | (MH "Nonrandomized Trials") | 184 |
| S51 | (MH "Experimental Studies") | 15,269 |
| S52 | (MH "Pretest‐Posttest Design+") | 28,108 |
| S53 | (MH "Quasi‐Experimental Studies+") | 8,917 |
| S54 | (MH "Multicenter Studies") | 21,780 |
| S55 | (MH "Health Services Research") | 7,570 |
| S56 | TI ( randomis* or randomiz* or randomly) OR AB ( randomis* or randomiz* or randomly) | 120,589 |
| S57 | TI (trial or effect* or impact* or intervention* or before N5 after or pre N5 post or ((pretest or "pre test") and (posttest or "post test")) or quasiexperiment* or quasi W0 experiment* or pseudo experiment* or pseudoexperiment* or evaluat* or "time series" or time W0 point* or repeated W0 measur*) OR AB (trial or effect* or impact* or intervention* or before N5 after or pre N5 post or ((pretest or "pre test") and (posttest or "post test")) or quasiexperiment* or quasi W0 experiment* or pseudo experiment* or pseudoexperiment* or evaluat* or "time series" or time W0 point* or repeated W0 measur*) | 813,817 |
| S58 | S44 OR S45 OR S46 OR S47 OR S48 OR S49 OR S50 OR S51 OR S52 OR S53 OR S54 OR S55 OR S56 OR S57 | 1,350,912 |
| S59 | S43 AND S58 | 1,428 |
PsycINFO (Ovid) 1967 to 17 October 2019
| No. | Search terms | Results |
| 1 | exp cerebrovascular disorders/ | 23803 |
| 2 | exp cerebrovascular accidents/ | 17584 |
| 3 | exp cerebral ischemia/ | 4194 |
| 4 | exp cerebral hemorrhage/ | 1711 |
| 5 | exp subarachnoid hemorrhage/ | 635 |
| 6 | (stroke or poststroke or post‐stroke or cerebrovasc* or brainvasc* or cerebral vasc* or cva* or apoplex* or sah).ti,ab. | 31671 |
| 7 | ((brain* or cerebr* or cerebell* or intracran* or intracerebral) adj5 (isch?emi* or infarct* or thrombo* or emboli* or occlus*)).ti,ab. | 8369 |
| 8 | ((brain* or cerebr* or cerebell* or intracerebral or intracranial or subarachnoid) adj5 (haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed*)).ti,ab. | 3805 |
| 9 | (acquired brain injur* or brain injur*).ti,ab. | 21288 |
| 10 | or/1‐9 | 58113 |
| 11 | (post stroke or poststroke).ti,ab. | 3667 |
| 12 | (rehabilitat* or recover* or therap*).ti,ab. | 405369 |
| 13 | exp rehabilitation/ | 66470 |
| 14 | or/11‐13 | 434149 |
| 15 | 10 and 14 | 21965 |
| 16 | decision mak*.ti,ab. | 72792 |
| 17 | (knowledge adj2 (application or broke* or creation or diffus* or disseminat* or exchang* or implement* or management or mobili* or translat* or transfer* or uptak* or utili*)).ti,ab. | 10171 |
| 18 | (evidence* adj2 (exchang* or translat* or transfer* or diffus* or disseminat* or exchang* or implement* or management or mobil* or uptak* or utili*)).ti,ab. | 4196 |
| 19 | (kt adj2 (application or broke* or diffus* or disseminat* or decision* or exchang* or implement* or intervent* or mobili* or plan* or policy or policies or strateg* or translat* or transfer* or uptak* or utili*)).ti,ab. | 97 |
| 20 | (research* adj2 (diffus* or disseminat* or exchang* or transfer* or translation* or application or implement* or mobil* or transfer* or uptak* or utili*)).ti,ab. | 10797 |
| 21 | ((research or evidence) adj2 (action or practice)).ti,ab. | 37213 |
| 22 | ((evidence base* or evidence inform*) adj5 (decision* or plan* or policy or policies or practice or action*)).ti,ab. | 9230 |
| 23 | implementation.ti,ab. | 66790 |
| 24 | behavio?r change.ti,ab. | 8992 |
| 25 | organi?ational change?.ti,ab. | 5063 |
| 26 | complex intervention?.ti,ab. | 524 |
| 27 | audit.ti,ab. | 5239 |
| 28 | (barrier? and facilitator?).ti,ab. | 2818 |
| 29 | (booklet* or brochure? or pamphlet? or paper‐based or printed material?).ti,ab. | 4901 |
| 30 | ((change? or changing or improv* or effect* or influenc* or alter* or adapt* or amend* or modify* or adjust* or transform*) adj2 (policy or policies or process* or practic* or provider? or activit*)).ti,ab. | 71079 |
| 31 | ((knowledge or evidence or quality or research or practice) adj2 gap?).ti,ab. | 6221 |
| 32 | (education* adj3 (continuing or group? or outreach or plan* or practitioner? or program? or staff? or team?)).ti,ab. | 40719 |
| 33 | (evidence‐based adj3 (algorithm? or evaluat* or guideline? or healthcare or implement* or improv* or intervention* or management or pathway? or plan? or practic* or program? or quality)).ti,ab. | 16147 |
| 34 | ((feedback or feed back) not feedback loop*).ti,ab. | 52053 |
| 35 | (guideline? adj3 (adher* or enforc* or influenc* or implement* or impact* or introduc* or uptake or follow)).ti,ab. | 2846 |
| 36 | (incentiv* adj2 (economic or employee? or financ* or insurer? or insurance or market* or monetar* or pay* or plan? or practitioner? or program* or provider? or reimburs* or salary or salarie? or staff or team* or value‐based)).ti,ab. | 3543 |
| 37 | (collaborat* or cross‐profession* or intraprofession* or intra‐profession* or interprofession* or inter‐profession* or (skill adj2 mix*) or teambase? or team‐based or inter disciplin* or multidisciplin* or multi disciplin* or multiprofession*).ti,ab. | 83735 |
| 38 | ((knowledge adj2 (transfer* or translation or shar* or exchan*)) or kt).ti,ab. | 6971 |
| 39 | ((knowledge or evidence or practice) adj2 (gap? or barrier?)).ti,ab. | 4412 |
| 40 | ((knowledge or evidence) adj2 synthesis).ti,ab. | 658 |
| 41 | opinion leader?.ti,ab. | 514 |
| 42 | (outreach adj2 (communit* or plan? or program? or visit?)).ti,ab. | 1618 |
| 43 | ((policy or policies) adj2 (chang* or effect? or impact? or influenc*)).ti,ab. | 6624 |
| 44 | (quality adj2 (assurance or improvement? or initiativ* or plan* or program* or review or audit)).ti,ab. | 8746 |
| 45 | (qi adj (initiative? or intervention? or program* or plan* or audit)).ti,ab. | 100 |
| 46 | ((change? or changing or improv* or effect* or influenc*) adj2 (policy or policies or practic* or provider?)).ti,ab. | 26534 |
| 47 | (journal club or clinical librarian or library or libraries or answer service* or information science).ti,ab. | 8988 |
| 48 | best practice.ti,ab. | 4571 |
| 49 | care pathway?.ti,ab. | 558 |
| 50 | (project network adj (technique? or diagram?)).ti,ab. | 0 |
| 51 | exp decision making/ | 88097 |
| 52 | exp knowledge transfer/ | 2424 |
| 53 | evidence based practice/ | 14451 |
| 54 | or/16‐53 | 487429 |
| 55 | 15 and 54 | 2664 |
| 56 | (clinical trial or empirical study or experimental replication or followup study or longitudinal study or prospective study or quantitative study or treatment outcome).md. | 2077547 |
| 57 | experimental design/ | 10091 |
| 58 | between groups design/ | 107 |
| 59 | quantitative methods/ | 2732 |
| 60 | quasi experimental methods/ | 140 |
| 61 | (randomised or randomized or randomly or controlled or control group? or evaluat* or time series or time point or time points or quasi experiment* or quasiexperiment* or (before adj5 after) or (pre adj5 post) or ((pretest or pre test) and (posttest or post test)) or multicenter study or multicentre study or multi center study or multi centre study or repeated measur*).ti,ab. | 665794 |
| 62 | (trial or effect? or impact? or intervention?).ti. | 383025 |
| 63 | exp clinical trial/ | 10174 |
| 64 | ((clinical or control*) adj3 trial*).ti,ab. | 57155 |
| 65 | ((singl* or doubl* or trebl* or tripl*) adj5 (blind* or mask*)).ti,ab. | 22964 |
| 66 | (volunteer* or control group or controls).ti,ab. | 204890 |
| 67 | placebo/ or placebo*.ti,ab. | 35244 |
| 68 | pretesting/ | 228 |
| 69 | posttesting/ | 135 |
| 70 | repeated measures/ | 616 |
| 71 | time series/ | 1745 |
| 72 | or/56‐71 | 2410491 |
| 73 | 55 and 72 | 1943 |
ClinicalTrials.gov
| Search terms | RESULTS | |
| Condition / Disease: | stroke | |
| Intervention / Treatment: | implement OR implementation OR evidence OR knowledge OR complex | |
| Other Terms: | stroke rehabilitation | |
| Filter: | Interventional studies | |
| 40 |
WHO International Clinical Trials Registry Platform (ICTRP)
| No. | Search terms | RESULTS |
| stroke rehabilitation AND implement* | 116 | |
| stroke rehabilitation AND evidence | 88 | |
| stroke rehabilitation AND knowledge | 52 | |
| stroke rehabilitation AND complex | 42 |
The Australian New Zealand Clinical Trials Registry (ANZCTR)
| No. | Search term | RESULTS |
| stroke rehabilitation | 252 |
Appendix 2. Template for intervention description and replication (TIDieR) checklist for studies
Abdul Aziz 2014
| Author/year | Abdul Aziz 2014 |
| Brief name | iCaPPS |
| Recipient | Primary care team: Family Medical Specialists (FMSs), medical officers‐in‐charge, registered staff nurses or community nurses, physiotherapists and occupational therapists |
|
Why (rationale, theory or goal of elements essential to the intervention) |
The goal of the integrated care pathway for poststroke patients (iCaPPS) was to overcome fragmented care for a patient after discharge from hospital. By providing a set of steps related to 10 care issues, it was hoped multidisciplinary care would be co‐ordinated and provided more comprehensively. The authors noted integrated care pathways have been used widely in the management of acute stroke and based the need for the study on the disjointed and inconsistent care in the community. The pathway was developed by an expert panel of health professionals and was also aimed at increasing access to specialised stroke care services for patients. Evidence cited by authors for uptake: Cochrane Review; Kwan J. Care pathways for acute stroke care and rehabilitation: from theory to evidence. J Clin Neurosci. 2007;14(3):189–200. |
|
What (materials) (any physical or informational materials used in the intervention and where these can be accessed) |
iCaPPS itemised checklist (Example algorithm provided in Abdul Aziz 2017 Figure 4, pg. 8) |
|
What (procedures) (procedures, activities, processes, or a combination of these, used in the intervention, including enabling or support activities) |
The iCaPPS addresses care issues that may present once the patient is discharged from hospital and includes a detailed list of tasks for the primary care team to complete during each appointment, which included:
If the patient required speech and language therapy or occupational therapy or physiotherapy (or a combination) the iCaPPS‐Swallow and iCaPPS –Rehab algorithms were followed. Implementation of the care pathway not described. |
|
Who provided (expertise, background and any specific training given) |
The FMS was the main co‐ordinator to administer care as per the iCaPPS. Individuals involved in implementation of the care pathway not described. |
|
How (modes of delivery, f2f, internet etc. and whether provided individually or in a group) |
f2f contact between health professionals and individual stroke survivors. |
|
Where (type of location where intervention occurred, infrastructure or relevant features) |
Public community health centres |
|
When and how much (number of times the intervention was delivered, over what time period including number of sessions, their schedule, duration, intensity or dose) |
Individual appointments with patients up to 45 minutes, as example comprising a 30‐minute consultation with the FMS and 15‐minutes with registered nurse or community nurse. |
|
Tailoring (if intervention was planned to be personalised or adapted, then describe what, why, when and how) |
No tailoring of intervention (information from contact with trial author). |
|
Modification of intervention throughout trial (if intervention was modified during course of study, describe changes (what, why when and how) |
Not described. |
|
Strategies to improve or maintain intervention fidelity (how and by whom, and if any strategies were used to maintain or improve fidelity) |
No specific strategies described. |
|
Extent of intervention fidelity (If intervention adherence or fidelity assessed, describe extent to which intervention was delivered as planned) |
Not described. |
Forster 2013
| Author/year | Forster 2013 |
| Brief name | LSCTC |
| Recipient | Multidisciplinary team: physiotherapists, occupational therapists, speech and language therapists, nurses, dieticians, stroke co‐ordinators and stroke consultants |
|
Why (rationale, theory or goal of elements essential to the intervention) |
The goal of the LSCTC was to improve physical outcomes for stroke survivors and reduce carer burden through delivery of structured carer training programme by multidisciplinary staff. By training members of the multidisciplinary team it was hoped they would cascade the LSCTC training to other members of the stroke unit until it was embedded in routine care. The rationale for the study was based on a Cochrane Review of non‐pharmacological interventions for carers of stroke survivors which determined the LSCTC as the most promising intervention. This evidence was from a single randomised trial involving the LSCTC and the authors proposed a larger trial was required to investigate the benefits of the training when implemented as part of standard practice. Normalisation Process Theory was used to identify factors facilitating or presenting barriers to practice change. Core principles of the LSCTC also available (Forster 2015, Box 1) Evidence cited by authors for uptake: Single site randomised trial demonstrating effectiveness of the London Stroke Caregiver Training: Kalra L, Evans A, Perez I, et al. Training carers of stroke patients: randomised controlled trial. BMJ 2004; 328: 1099. Cochrane Review: Legg LA, Quinn TJ, Mahmood F, et al. Non‐pharmacological interventions for caregivers of stroke survivors. Cochrane Database Syst Rev 2011; 10: CD008179. |
|
What (materials) (any physical or informational materials used in the intervention and where these can be accessed) |
A copy of the LSCTC manual is available as a supplementary file in Forster 2015 and the training record is available in the appendix in the main study publication. |
|
What (procedures) (procedures, activities, processes, or a combination of these, used in the intervention, including enabling or support activities) |
Training session: 1‐day workshop attended by ≥ 2 staff members identified as key members from each participating. The first day focused on the role of the LSCTC champions and how the MDT teams could tailor core principles of the programme and tailor them to suit usual processes of care. Each of the 14 following training components were presented with discussion involving possible methods of delivery, how to assess competency and resources required to deliver.
Clinical champions: health professional who attended training and were tasked with cascading training were called 'Training Course Champions,' they were provided with information on how to asses competency and how to tailor core principles of the LSCTC to their sites. Ongoing support for champions following training not described. Cascade of training: staff attending the training days were tasked with cascading the LSCTS to other members of the multidisciplinary team by holding local training sessions. Resources for presentations (e.g. slides and recordings of talks) were provided. Follow‐up meeting: 1 month following training sessions, the champions and training team reconvened for a second training day to share experiences and discuss any difficulties. |
|
Who provided (expertise, background and any specific training given) |
Multidisciplinary research team developed and delivered training. Specific individuals delivering training not named. Training programme developed for multidisciplinary teams by Jayne Steadman, Anne Melbourn, Margreet Wittink and Anne Forster. |
|
How (modes of delivery, f2f, internet etc. and whether provided individually or in a group) |
Training sessions were presented f2f to a group of health professionals. A combination of presentations and group workshops were used. The training day was filmed and included on the training CD used to cascade training. |
|
Where (type of location where intervention occurred, infrastructure or relevant features) |
The same training sessions were repeated twice; once in Leeds (UK) and once in London (UK). |
|
When and how much (number of times the intervention was delivered, over what time period including number of sessions, their schedule, duration, intensity or dose) |
2 days of staff training, 1 month apart. |
|
Tailoring (if intervention was planned to be personalised or adapted, then describe what, why, when and how) |
At the training days, healthcare professionals discussed in working groups how the training could be provided in their own units, "highlighting potential barriers, challenges and approaches." |
|
Modification of intervention throughout trial (if intervention was modified during course of study, describe changes (what, why when and how) |
Not described. |
|
Strategies to improve or maintain intervention fidelity (how and by whom, and if any strategies were used to maintain or improve fidelity) |
Cascade training attendance, delivery and sessions at sites were recorded. Though compliance with the evidence‐based practice was evaluated, fidelity of the implementation intervention was not described. |
|
Extent of intervention fidelity (If intervention adherence or fidelity assessed, describe extent to which intervention was delivered as planned) |
Cascade training attendance, delivery and sessions at stroke units varied greatly with 7 units recording no total cascade training received and 1 unit up to 4060 minutes of cascade training received. |
Forster 2015
| Author/year | Forster 2015 |
| Brief name | System of Longer‐Term Stroke Care (LoTS) |
| Recipient | Stroke Care Coordinator Services (SCSs) |
|
Why (rationale, theory or goal of elements essential to the intervention) |
The aim was to introduce a new system of care to meet longer‐term problems experienced by stroke survivors and their carers. The rationale for the intervention was the gap in adequate services for longer‐term needs and the recommendations from stroke clinical guidelines to regard stroke as a long‐term condition, involving input from a Stroke Care Coordinator (SCC). The evidence‐based system of care (LoTS) was produced in line with the Medical Research Council Framework for the development and evaluation of complex interventions. By providing training in the new system of care to SCSs it was hoped the care plan containing a structured assessment with a goal and action planner would demonstrate effectiveness in clinical and cost effectiveness. Evidence cited by authors for uptake: Intercollegiate Stroke Working Party. National Clinical Guideline for Stroke. 4th ed. London: Royal College of Physicians; 2012. Authors stated: "National guidelines acknowledge that stroke should be regarded as a long‐term condition, and the role of a Stroke Care Coordinator (SCC) to facilitate inputs for community‐based patients with stroke and their families after initial (usually hospital‐based) treatment is a recommended policy." |
|
What (materials) (any physical or informational materials used in the intervention and where these can be accessed) |
|
|
What (procedures) (procedures, activities, processes, or a combination of these, used in the intervention, including enabling or support activities) |
Training Training in delivery of LoTS system of care was provided to SCSs through two centrally based Royal College of Nursing‐accredited training days. Training day 1: information covered:
Training day 2: covered:
The LoTS is a framework of 16 structured questions (16 assessment questions for patients and 13 assessment questions for carers) which link to evidence‐based treatment algorithms and reference guides. The system of care comprised the following components.
Assessment documentation is incorporated the patient details collected and a single care plan is created to replace currently used patient records. |
|
Who provided (expertise, background and any specific training given) |
Training provided to SCSs by Academic Unit of Elderly Care and Rehabilitation research team involved in the intervention's development, and clinicians involved in the pilot work. |
|
How (modes of delivery, f2f, internet etc. and whether provided individually or in a group) |
Training was presented f2f to a group of SCSs. |
|
Where (type of location where intervention occurred, infrastructure or relevant features) |
Location not specified. |
|
When and how much (number of times the intervention was delivered, over what time period including number of sessions, their schedule, duration, intensity or dose) |
2 × 1‐day workshops approximately 1 month apart. |
|
Tailoring (if intervention was planned to be personalised or adapted, then describe what, why, when and how) |
Not described. |
|
Modification of intervention throughout trial (if intervention was modified during course of study, describe changes (what, why when and how) |
Not described. |
|
Strategies to improve or maintain intervention fidelity (how and by whom, and if any strategies were used to maintain or improve fidelity) |
Though compliance with the evidence‐based practice was evaluated, fidelity of the implementation intervention was not described. |
|
Extent of intervention fidelity (If intervention adherence or fidelity assessed, describe extent to which intervention was delivered as planned) |
Not described. |
McCluskey 2016
| Author/year | McCluskey 2016 |
| Brief name | Out‐and‐About Program |
| Recipients | Occupational therapists and physiotherapists |
|
Why (rationale, theory or goal of elements essential to the intervention) |
The goal of the behaviour change programme was to increase the number of outings delivered to stroke survivors during outpatient stroke rehabilitation. The need for the study was based on stroke clinical practice guidelines recommending multiple escorted outdoor journeys for stroke survivors and research demonstrating stroke survivors did not receive this. By providing a behaviour change programme to staff it was hoped they would conduct more escorted therapy journeys with patients and these outings would increase the likelihood of patients taking more outdoor journeys in real life, ultimately increasing community participation. Evidence cited by authors for uptake: Logan PA, Gladman JRF, Avery A, Walker MF, Dyas J and Groom L. Randomised controlled trial of an occupational therapy intervention to increase outdoor mobility after stroke. Br Med J 2004; 329: 1372–1377. National Stroke Foundation. Clinical guidelines for stroke management 2010. Melbourne, Australia: National Stroke Foundation, 2010. Authors stated: "People faced with difficulties in community transport and mobility should undertake tailored strategies such as multiple escorted outdoor journeys (which may include practice crossing roads, visits to local shops, bus or train travel), help to resume driving, aids and equipment, and written information about local transport." |
|
What (materials) (any physical or informational materials used in the intervention and where these can be accessed) |
A copy of printed education materials can be accessed in the supplementary file in study publication. |
|
What (procedures) (procedures, activities, processes, or a combination of these, used in the intervention, including enabling or support activities) |
A copy of presentation slides, case studies and audit criteria can be accessed in the supplementary file in study publication. |
|
Who provided (expertise, background and any specific training given) |
Dr Annie McCluskey (first author) delivered all workshops. Annie McCluskey is an occupational therapist, health services researcher and educator with > 30 years' experience in stroke and brain injury rehabilitation. Printed educational materials were designed and prepared by Dr Annie McCluskey, Prof Louise Ada (physiotherapist) and Ms Aspasia Karageorge (psychology graduate). |
|
How (modes of delivery, f2f, internet etc. and whether provided individually or in a group) |
Workshops were presented f2f to a group of therapists and therapy assistants. |
|
Where (type of location where intervention occurred, infrastructure or relevant features) |
Workshops were held at each individual site. |
|
When and how much (number of times the intervention was delivered, over what time period including number of sessions, their schedule, duration, intensity or dose) |
Initial workshop (2 hours) and booster workshop (1 hour) 12 months later. |
|
Tailoring (if intervention was planned to be personalised or adapted, then describe what, why, when and how) |
20‐minute discussion with teams as part of workshops on barriers and enablers to change. No formal tailoring processes described. |
|
Modification of intervention throughout trial (if intervention was modified during course of study, describe changes (what, why when and how) |
High staff turnover (up to 50%) resulted in need for booster workshop at 12 months, this event was not planned in original protocol. |
|
Strategies to improve or maintain intervention fidelity (how and by whom, and if any strategies were used to maintain or improve fidelity) |
Not described. |
|
Extent of intervention fidelity (If intervention adherence or fidelity assessed, describe extent to which intervention was delivered as planned) |
Not described. |
Pennington 2005
| Author/year | Pennington 2005 |
| Brief name | Training strategies for speech and language therapists in dysphagia management. |
| Recipient | Speech and language therapists. |
|
Why (rationale, theory or goal of elements essential to the intervention) |
Focused on changing the practice of speech and language therapists in managing poststroke dysphagia by providing training workshops and management of change information, based on Roger's Diffusion of Innovation theory. The need for the study was based on a lack of research in the area of evidence‐based practice and behaviour change in allied health. The underlying assumption of the study is speech and language therapists were not using evidence in the form of stroke clinical practice guidelines in the area of dysphagia management. Evidence cited by authors for uptake: evidence not cited but training involved clinical practice guidelines: SIGN and RCP guidelines. |
|
What (materials) (any physical or informational materials used in the intervention and where these can be accessed) |
No physical materials described. Information from author, printed materials provided (no additional detail). |
|
What (procedures) (procedures, activities, processes, or a combination of these, used in the intervention, including enabling or support activities) |
Intervention A Workshop (2.5 days) involving:
Intervention B Workshop (5 days) involving:
2 speech and language therapists from each department received the training and were expected to cascade information across their department. |
|
Who provided (expertise, background and any specific training given) |
Training provided by researchers Lindsay Pennington and Hazel Roddam who are both speech and language therapists. |
|
How (modes of delivery, f2f, internet etc. and whether provided individually or in a group) |
f2f training workshops including short talks, group discussion, problem‐based learning and self‐directed study. |
|
Where (type of location where intervention occurred, infrastructure or relevant features) |
Training occurred at Manchester University. |
|
When and how much (number of times the intervention was delivered, over what time period including number of sessions, their schedule, duration, intensity or dose) |
Intervention A 2.5 days of training over 7 weeks Intervention B 5 days of training over 3 months |
|
Tailoring (if intervention was planned to be personalised or adapted, then describe what, why, when and how) |
Staff were asked during training session to choose a clinical guideline recommendation and draw up action plan for its implementation at their service. |
|
Modification of intervention throughout trial (if intervention was modified during course of study, describe changes (what, why when and how) |
Not described. |
|
Strategies to improve or maintain intervention fidelity (how and by whom, and if any strategies were used to maintain or improve fidelity) |
Not described. |
|
Extent of intervention fidelity (If intervention adherence or fidelity assessed, describe extent to which intervention was delivered as planned) |
Not described. |
Power 2014
| Author/year | Power 2014 |
| Brief name | Stroke 90:10 Quality Improvement Collaborative |
| Recipient | Multidisciplinary teams comprising radiographers, stroke co‐ordinators, specialist stroke nurses, occupational therapists, physiotherapists and health care assistants. |
|
Why (rationale, theory or goal of elements essential to the intervention) |
The goal was to reduce variation in compliance with recommended processes of care through a QIC focused on 9 indicators of quality care. The QIC was based on a Breakthrough series model and care processes were divided into 2 distinct care bundles, one for early hours care and the other rehabilitation following stroke. It was hoped that through implementation of the QIC with defined care processes the quality of stroke care provided would be improved. The underlying theory related to care bundles is that the groups of interrelated processes, when delivered as a whole, will achieve better results than the sum of parts. Evidence cited by authors for uptake: Authors stated evidence for QICs equivocal, citing: Schouten LM, Hulscher ME, van Everdingen JJ, Huijsman R, Grol RP: Evidence for the impact of quality improvement collaboratives: systematic review. BMJ 2008, 336(7659):1491–1494. |
|
What (materials) (any physical or informational materials used in the intervention and where these can be accessed) |
No physical or informational materials described. |
|
What (procedures) (procedures, activities, processes, or a combination of these, used in the intervention, including enabling or support activities) |
Hospital trusts received package of support from the programme office, which included:
Teams were asked to produce monthly reports to reflect their performance. Project director met with each hospital's chief executive and team to review progress twice. All participating hospitals committed to:
|
|
Who provided (expertise, background and any specific training given) |
First author (Maxine Power) met with hospital executives. Individuals who facilitated training sessions not named. |
|
How (modes of delivery, f2f, internet etc. and whether provided individually or in a group) |
Not described. Assume f2f in a group setting. |
|
Where (type of location where intervention occurred, infrastructure or relevant features) |
Not described. |
|
When and how much (number of times the intervention was delivered, over what time period including number of sessions, their schedule, duration, intensity or dose) |
Learning sessions 4 days in total. Weekly online sharing and learning sessions. |
|
Tailoring (if intervention was planned to be personalised or adapted, then describe what, why, when and how) |
Not described. |
|
Modification of intervention throughout trial (if intervention was modified during course of study, describe changes (what, why when and how) |
Not described. |
|
Strategies to improve or maintain intervention fidelity (how and by whom, and if any strategies were used to maintain or improve fidelity) |
Though compliance with the evidence‐based practice was evaluated, fidelity of the implementation intervention was not described. |
|
Extent of intervention fidelity (If intervention adherence or fidelity assessed, describe extent to which intervention was delivered as planned) |
Not described. |
Salbach 2017
| Author/year | Salbach 2017 |
| Brief name | SCORE‐IT |
| Recipient | Nurses, occupational therapists and physiotherapists |
|
Why (rationale, theory or goal of elements essential to the intervention) |
Focused on uptake of evidence‐based guidelines for the treatment for stroke patients in the areas of upper and lower extremity motor function, postural control and mobility. It was thought a facilitated, knowledge translation approach based on the Knowledge‐to‐Action framework would increase the adherence of staff to 18 recommended treatments based on clinical practice guidelines. Evidence cited by authors for uptake: Authors cited Canadian, Australian and US clinical practice guidelines: 1. Hebert D, Lindsay MP, McIntyre A, Kirton A, Rumney PG, Bagg S, et al. Canadian stroke best practice recommendations: stroke rehabilitation practice guidelines, update 2015. Int J Stroke. 2016;11:459–84. 2. National Stroke Foundation. Clinical guidelines for stroke management. 2010. informme.org.au/Guidelines/Clinical‐Guidelines‐for‐Stroke‐Management‐2010. 3. Winstein CJ, Stein J, Arena R, Bates B, Cherney LR, Cramer SC, et al. Guidelines for adult stroke rehabilitation and recovery: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2016;47(6):e98–e169. |
|
What (materials) (any physical or informational materials used in the intervention and where these can be accessed) |
|
|
What (procedures) (procedures, activities, processes, or a combination of these, used in the intervention, including enabling or support activities) |
2‐day workshop for facilitators, where facilitators received media releases for promoting guidelines to clinicians, slide presentation of the treatment protocols, and training in how to apply treatments and run small group education/training sessions. Local facilitators: 1 nurse and 1 therapist, funded for 4 hours per week of protected time to support guideline implementation over 16 months. Resources and support for facilitators; outline of strategies to foster guideline implementation, practice‐change tool kit, education in change management. Facilitators were then tasked with running local education sessions at their sites. Facilitators completed activities to compare current with recommended practice, identify barriers to practice change, and develop a plan that incorporated behavior change strategies to address local challenges to guideline implementation. Teleconferences and a web‐based platform were used for facilitators to communicate and share successful strategies. |
|
Who provided (expertise, background and any specific training given) |
Research team members (e.g. physicians, occupational therapists, physiotherapists) and healthcare professionals in clinical practice, with expertise with the recommended treatments and behaviour change strategies, delivered regional workshops (information from contact with trial author). |
|
How (modes of delivery, f2f, internet etc. and whether provided individually or in a group) |
f2f workshops, teleconferences and a web‐based platform were provided for facilitators to communicate (Information from contact with trial author). |
|
Where (type of location where intervention occurred, infrastructure or relevant features) |
Location for training not described. Internal facilitators worked with staff on‐site. |
|
When and how much (number of times the intervention was delivered, over what time period including number of sessions, their schedule, duration, intensity or dose) |
2‐day workshop. Each facilitator at each site (2 per site) was to spend 4 hours per week promoting guideline implementation. |
|
Tailoring (if intervention was planned to be personalised or adapted, then describe what, why, when and how) |
Intervention was tailored to address barriers and facilitators that arose from focus groups when the stroke rehabilitation guideline implementation was piloted at 5 inpatient rehabilitation hospitals in Canada. During workshop, training site facilitators completed an activity to compare current practice at their site with recommended practice, identify barriers to practice change and develop an implementation plan that incorporated behavior change strategies to address local challenges to implementation (information from contact with trial author). |
|
Modification of intervention throughout trial (if intervention was modified during course of study, describe changes (what, why when and how) |
Not described. |
|
Strategies to improve or maintain intervention fidelity (how and by whom, and if any strategies were used to maintain or improve fidelity) |
Not described. |
|
Extent of intervention fidelity (If intervention adherence or fidelity assessed, describe extent to which intervention was delivered as planned) |
Facilitators from all sites in the facilitated group attended the training workshop (Information from contact with trial author). |
Strasser 2008
| Author/year | Strasser 2008 |
| Brief name | Team training |
| Recipient | Multidisciplinary team: doctors, nurses, occupational therapists, speech‐language pathologists, physical therapists and social workers/case managers |
|
Why (rationale, theory or goal of elements essential to the intervention) |
Focused on effective functioning within a multidisciplinary team and the benefits of organised, co‐ordinated teams. By providing training to a team to increase skills in team effectiveness it was thought this would have a positive effect on the stroke survivors who were being treated by members of the team. The rationale for the intervention was the acceptance and endorsement of the role of the multidisciplinary rehabilitation and results from an observational study (conducted by the author) of characteristics of teams that predicted superior patient outcomes. Intervention was based on Lichstein's treatment implementation model. Evidence cited by authors for uptake: CARF – The Committee on Accreditation of Rehabilitation Facilities where team care regarded as an indicator of provider quality. |
|
What (materials) (any physical or informational materials used in the intervention and where these can be accessed) |
|
|
What (procedures) (procedures, activities, processes, or a combination of these, used in the intervention, including enabling or support activities) |
Team training intervention occurred in 3 phases.
Before the training, participants received workbooks containing an introductory letter, an overview of the workshop, published articles relevant to teamwork, patient vignettes and teamwork issues to be discussed in the workshop. Participants also engaged in formal social activities (a group dinner) during the training. A 'train the trainer' approach was used where 2 rehabilitation team leaders at each site received training to improve team functioning at their hospital. |
|
Who provided (expertise, background and any specific training given) |
Workshops conducted by research staff; an interdisciplinary team (a physiatrist, geriatric psychologist, rehabilitation psychologist, occupational therapist and research psychologist familiar with Veterans Affairs rehabilitation inpatient settings) led workshops. |
|
How (modes of delivery, f2f, internet etc. and whether provided individually or in a group) |
Off‐site, f2f workshops. Telephone and videoconference consultation. |
|
Where (type of location where intervention occurred, infrastructure or relevant features) |
Workshops held at Atlanta Veterans Affairs. |
|
When and how much (number of times the intervention was delivered, over what time period including number of sessions, their schedule, duration, intensity or dose) |
1 × 2.5‐day workshop (16 hours), written feedback 3–5 weeks after workshop, consultation 2–3 months after written feedback. |
|
Tailoring (if intervention was planned to be personalised or adapted, then describe what, why, when and how) |
Implementation action plans were modified according to perceived barriers by team leaders. |
|
Modification of intervention throughout trial (if intervention was modified during course of study, describe changes (what, why when and how) |
Not described. |
|
Strategies to improve or maintain intervention fidelity (how and by whom, and if any strategies were used to maintain or improve fidelity) |
A treatment implementation framework was used to measure and promote consistent and accurate presentation of the intervention. Feedback, individual consultations, detailed outlines of all intervention components and a timeline for delivery of interventions were used to increase consistency of workshop delivery. Research staff kept records of implementation activities, e.g. delivery of training materials, feedback documents to participants, participant attendance at workshops and consultation sessions with research staff. Questionnaires were used after workshops to determine participants receipt of information. A 15‐item questionnaire was sent to the primary contact at each site 2 months postintervention to report changes in team skills, new team behaviours and new programmes resulting from the training. |
|
Extent of intervention fidelity (If intervention adherence or fidelity assessed, describe extent to which intervention was delivered as planned) |
All sites received workshop materials before attending the workshop. 2 team leaders from 14/15 intervention sites attended the workshops. All sites received written documents relevant to the intervention. All sites received a minimum of 1 consultation from research staff, while most received 2–4 consultations. Questionnaires after workshops indicated participants strongly agreed (81%) or agreed (19%) that the workshops provided skills to enhance team functioning. Implementation of team activities were reported by 9/15 (60%) sites. These 9 sites reported implementing ≥ 1 changes in their work environment. |
Thomas 2015
| Author/year | Thomas 2015 |
| Brief name | ICONS |
| Recipient | Nurses |
|
Why (rationale, theory or goal of elements essential to the intervention) |
The goal of the intervention was to increase the delivery of a SVP by nursing staff for stroke survivors with urinary incontinence. The authors highlighted the evidence‐practice gap in the management of poststroke urinary incontinence (as demonstrated by a national audit) as rationale for the intervention and cited available clinical practice guidelines that are not incorporated into practice. Interventions compared were education regarding the SVP and education combined with facilitation. Normalisation process theory was the guiding theoretical approach and 16 normalisation process theory dimensions were considered to address barriers and enablers. Evidence cited by authors for uptake. Authors stated: the intervention in our programme will focus on conservative strategies shown to have some effect with participants in studies included in Cochrane systematic reviews but which have not had their effectiveness demonstrated with stroke patients. |
|
What (materials) (any physical or informational materials used in the intervention and where these can be accessed) |
Intervention A
Intervention B
|
|
What (procedures) (procedures, activities, processes, or a combination of these, used in the intervention, including enabling or support activities) |
Intervention A
Intervention B
|
|
Who provided (expertise, background and any specific training given) |
Not described |
|
How (modes of delivery, f2f, internet etc. and whether provided individually or in a group) |
Intervention A: training mainly web‐based, f2f sessions also offered to staff. Intervention B: training as per Intervention A. Educational outreach (external facilitators) provided support through a mixture of f2f meeting, teleconferences and e‐mail correspondence. |
|
Where (type of location where intervention occurred, infrastructure or relevant features) |
Not described. |
|
When and how much (number of times the intervention was delivered, over what time period including number of sessions, their schedule, duration, intensity or dose) |
Not described. |
|
Tailoring (if intervention was planned to be personalised or adapted, then describe what, why, when and how) |
Intervention was informed by the findings of an evidence synthesis on the barriers and enablers to successful implementation of conservative interventions for urinary incontinence completed during the programme's development phase. Semistructured interviews conducted with staff to identify barriers to successful implementation. Intervention B: ongoing facilitation efforts were to identify barriers and facilitators (as well as those identified during the case study) and address them. |
|
Modification of intervention throughout trial (if intervention was modified during course of study, describe changes (what, why when and how) |
Following findings of a case study the following changes were made to the implementation intervention:
|
|
Strategies to improve or maintain intervention fidelity (how and by whom, and if any strategies were used to maintain or improve fidelity) |
Trial manager's report from sites included a dimension on fidelity and asked for reports on deviations from programme and difficulties with implementation. |
|
Extent of intervention fidelity (If intervention adherence or fidelity assessed, describe extent to which intervention was delivered as planned) |
Not described. Though compliance with the evidence‐based practice was reported, fidelity of the implementation intervention was not described. |
| f2f: face‐to‐face; FMS: family medicine specialist; iCaPPS: Integrated Care Pathway for managing poststroke patients; LoTS: Longer‐Term Stroke Care; LSCTC: London Stroke Carers Training Course; n: number of participants; QIC: quality improvement collaborative; RCP: Royal College of Physicians; SCC: stroke care co‐ordinator; SIGN: Scottish Intercollegiate Guidelines Network; SVP: systematic voiding programme. | |
Appendix 3. Consensus on Health Economic Criteria (CHEC) list for studies reporting economic data
| Item | Yes/no | Comment |
| 1. Is the study population clearly described? | No | Population of stroke patients described in terms of inclusion and exclusion criteria though withdrawals/dropouts during follow‐up not stated. |
| 2. Are competing alternatives clearly described? | No | The control used was conventional care (usual care), authors stated there was no local guideline for care, conventional care usually follows the non‐communicable diseases clinic protocol – details of protocol not provided. |
| 3. Is a well‐defined research question posed in answerable form? | Yes | Research questions (posed in form of hypotheses) to:
|
| 4. Is the economic study design appropriate to the stated objective? | Yes | Cluster randomised trial design used, authors assessed cost per quality adjusted life year (QALY) gained and ICER. |
| 5. Is the chosen time horizon appropriate to include relevant costs and consequences? | No | Evaluated cost‐effectiveness at 6 months after introduction of pathway; this time horizon may not be sufficient to determine full benefit of iCaPPs (i.e. if most costs in introduction incurred in first few months and benefits to patients expected beyond 6‐month timeframe) |
| 6. Is the actual perspective chosen appropriate? | No | Authors stated costs were calculated from provider and patient perspective, rather than societal perspective. Details of costs related to iCaPPs implementation not provided. |
| 7. Are all important and relevant costs for each alternative identified? | No | Details of relevant costs for iCaPPs and conventional care not described. |
| 8. Are all costs measured appropriately in physical units? | No | Measurement of costs not described, authors only stated: "the step‐down and activity‐based‐costing (ABC) method" used. |
| 9. Are costs valued appropriately? | No | Information not provided, unable to determine whether costs match opportunity costs. |
| 10. Are all important and relevant outcomes for each alternative identified? | Yes | QoL identified as the primary outcome and Quality adjusted life years used for cost‐effectiveness analysis. |
| 11. Are all outcomes measured appropriately? | Yes | EQ‐5D (version not specified) used as primary outcome which is a tool validated for use with a stroke population. |
| 12. Are outcomes valued appropriately? | No | The method of outcome valuation was not clearly stated though EQ‐5D used so assume indirect utility assessment. |
| 13. Is an incremental analysis of costs and outcomes of alternatives performed? | Yes | ICER calculated and reported. |
| 14. Are all future costs and outcomes discounted appropriately? | Yes | Discounting only necessary if time‐horizon/follow‐up is longer than 12 months (in this study it was 6 months). |
| 15. Are all important variables, whose values are uncertain, appropriately subjected to sensitivity analysis? | No | Sensitivity analysis not reported. |
| 16. Do the conclusions follow from the data reported? | No | Authors concluded: "Managing post stroke patients using the iCaPPS protocol in the community cost 2.2 times more than conventional care although QALY scores improved." Authors stated iCaPPs is cost‐effective though detail other than total costs and QALYs gained not reported. Conclusions not drawn cautiously. |
| 17. Does the study discuss the generalisability of the results to other settings and patient/client groups? | No | No discussion of generalisability of results, conference abstract rather than full report. |
| 18. Does the article indicate that there is no potential conflict of interest of study researcher(s) and funder(s)? | No | Any potential conflict of interest not mentioned. |
| 19. Are ethical and distributional issues discussed appropriately? | No | Conference abstract, ethical and distributional issues not discussed. |
| Item | Yes/no | Comment |
| 1. Is the study population clearly described? | Yes | Clusters (stroke units) and patients described adequately, eligibility and dropout stated explicitly. |
| 2. Are competing alternatives clearly described? | No | Stated control group provided with 'usual care,' intensity, duration and frequency of intervention not described. |
| 3. Is a well‐defined research question posed in answerable form? | Yes | Clearly stated question in determining cost‐effectiveness of a training programme for carers of stroke patients. Identified alternatives being compared. |
| 4. Is the economic study design appropriate to the stated objective? | Yes | Cluster randomised trial using cost‐effectiveness analysis (primary economic evaluation) and cost‐utility analyses (secondary economic evaluation) to answer research question. |
| 5. Is the chosen time horizon appropriate to include relevant costs and consequences? | Yes | Evaluated cost‐effectiveness at 6 months primarily though also evaluated at 12‐month time point, which would have allowed additional adequate time for all relevant costs and benefits to become apparent. |
| 6. Is the actual perspective chosen appropriate? | Yes | 2 perspectives taken for patients and carers: health and social care cost perspective and a societal health perspective. |
| 7. Are all important and relevant costs for each alternative identified? | Yes | Health and social care costs included: nursing/residential care; hospital inpatient, outpatient, day hospital and accident and emergency services; primary care; community‐based health services; and social care services. Societal costs included all these categories plus informal care costs. |
| 8. Are all costs measured appropriately in physical units? | Yes | Resource use data collected using the Client Service Receipt Inventory. Intervention costs evaluated though project tasks (i.e. preparing and delivering 4 core training days). |
| 9. Are costs valued appropriately? | Yes | Sources of valuation described, an opportunity cost approach used involving valuing carer time according to opportunities they had forgone due to carer responsibilities. |
| 10. Are all important and relevant outcomes for each alternative identified? | Yes | All relevant outcomes (resource use, costs and QALYs) were reported in economic evaluation results. |
| 11. Are all outcomes measured appropriately? | Yes | Resource use and costs measured appropriately. NEADL used for cost‐effectiveness analysis for patient and CBS for carer and cost‐utility analysis based on QALYs. |
| 12. Are outcomes valued appropriately? | Yes | Indirect utility assessment using the EQ‐5D‐3L. Utility weights for the EQ‐5D‐3L were taken from a UK general population survey. |
| 13. Is an incremental analysis of costs and outcomes of alternatives performed? | Yes | Incremental analysis. ICER calculated. |
| 14. Are all future costs and outcomes discounted appropriately? | Yes | Stated 'discounting was not necessary' though further information not provided. Time horizon limited to 12 months, discounting only necessary if time horizon > 1 year. |
| 15. Are all important variables, whose values are uncertain, appropriately subjected to sensitivity analysis? | Yes | Sensitivity analysis conducted for:
|
| 16. Do the conclusions follow from the data reported? | Yes | Authors stated low probability of cost effectiveness based on QALYs. |
| 17. Does the study discuss the generalisability of the results to other settings and patient/client groups? | Yes | Generalisability discussed and authors stated results of TRACS should be generalisable to patients, carers and stroke rehabilitation units across the UK. |
| 18. Does the article indicate that there is no potential conflict of interest of study researcher(s) and funder(s)? |
Yes | Authors stated no conflicts of interest. |
| 19. Are ethical and distributional issues discussed appropriately? | No | Not discussed. |
| Item | Yes/no | Comment |
| I. Is the study population clearly described? | Yes | Clinical characteristics of participants and withdrawals stated and described explicitly. |
| 2. Are competing alternatives clearly described? | No | Usual care, authors state community‐based care determined by local policy and practices, no further information on timing, duration or frequency |
| 3. Is a well‐defined research question posed in answerable form? | Yes | Research question for economic evaluation stated: "Is SCC care under the new system of care cost‐effective compared with SCC care according to usual practice, from either a health and social care perspective or a societal perspective?" |
| 4. Is the economic study design appropriate to the stated objective? | Yes | Cluster randomised trial, using cost‐effectiveness analysis and cost‐utility analysis. |
| 5. Is the chosen time horizon appropriate to include relevant costs and consequences? | Yes | Evaluated cost‐effectiveness at 6 months (primary endpoint) though also explored outcomes at 12‐month time point, which would have allowed additional adequate time for all relevant costs and benefits to become apparent. |
| 6. Is the actual perspective chosen appropriate? | Yes | 2 perspectives taken for patients and carers: health and social care cost perspective and a societal perspective. |
| 7. Are all important and relevant costs for each alternative identified? | Yes | Unit costs of residential and nursing home stay, inpatient services, outpatient services, value of carer time, SCC time and stroke multidisciplinary meeting, total health and social care costs. |
| 8. Are all costs measured appropriately in physical units? | Yes | A Client Service Receipt Inventory specifically adapted for this study was used, based on versions used successfully in previous large stroke rehabilitation trials. |
| 9. Are costs valued appropriately? | Yes | Opportunity costs considered in unit costs in the form of wage cost and leisure time cost. |
| 10. Are all important and relevant outcomes for each alternative identified? | Yes | Economic evaluation was based on individual‐level data collected within the trial. It assessed cost‐effectiveness based on the GHQ‐12 and cost– utility based on quality‐adjusted life‐years (QALYs) derived from the EQ‐5D. |
| 11. Are all outcomes measured appropriately? | Yes | GHQ‐12 and EQ‐5D validated measures. |
| 12. Are outcomes valued appropriately? | Yes | Indirect utility assessment using the EQ‐5D, utility weights were taken from a UK population survey. |
| 13. Is an incremental analysis of costs and outcomes of alternatives performed? | No | No suggested between‐group differences for cost and outcomes, ICERs not calculated. |
| 14. Are all future costs and outcomes discounted appropriately? | Yes | Time horizon limited to 12 months, discounting only necessary if time horizon > 1 year. |
| 15. Are all important variables, whose values are uncertain, appropriately subjected to sensitivity analysis? | Yes | Sensitivity analysis conducted imputing missing health and social care costs and QALYs at 6 months. |
| 16. Do the conclusions follow from the data reported? | Yes | Authors reported no evidence of cost effectiveness in line with economic analysis results. |
| 17. Does the study discuss the generalisability of the results to other settings and patient/client groups? | Yes | Authors stated good generalisability: "The range of disparate geographical regions ensured a good representation of different healthcare settings optimizing generalizability." Authors also discussed keeping eligibility criteria to a minimum, to ensure the stroke patient population was representative of usual referrals to SCCs including patients with language and cognitive impairments. |
| 18. Does the article indicate that there is no potential conflict of interest of study researcher(s) and funder(s)? | Yes | Authors stated in 'Sources of Funding' that this is independent research. |
| 19. Are ethical and distributional issues discussed appropriately? | No | Socioeconomic status of patients/carers not discussed in detail, education level and main employment before stroke reported for stroke participants. |
| Item | Yes/no | Comment |
| 1. Is the study population clearly described? | No | Population was SLT departments. Information provided on: type of service (adult, or mixed adult and paediatric), number of therapists, number of units covered – though information missing on mean years of experience of therapists/age/sex. |
| 2. Are competing alternatives clearly described? | Yes | Study comparison between Strategy A and Strategy B in training speech and language therapists. Both strategies described. |
| 3. Is a well‐defined research question posed in answerable form? | Yes | Authors stated aim: "to evaluate the clinical and cost effectiveness of two training strategies to promote the use of research evidence in speech and language therapy (SLT) management post‐stroke." |
| 4. Is the economic study design appropriate to the stated objective? | No | A cost description – alongside a cluster randomised trial – is conducted which does not determine cost effectiveness of training strategies. |
| 5. Is the chosen time horizon appropriate to include relevant costs and consequences? | No | Time horizon unclear, not documented by authors. |
| 6. Is the actual perspective chosen appropriate? | No | Societal perspective not taken, training costs were sole costs considered. |
| 7. Are all important and relevant costs for each alternative identified? | No | 3 general categories of costs identified:
No details of costs within categories provided. |
| 8. Are all costs measured appropriately in physical units? | No | Training costs for attending training and rolling out the training were determined through semistructured interviews (no details of questions provided). |
| 9. Are costs valued appropriately? | No | Authors stated all items were valued at 2002 prices though details of valuation for each cost category not provided. |
| 10. Are all important and relevant outcomes for each alternative identified? | No | Main outcome was therapist adherence to clinical practice guidelines for poststroke dysphagia management; however, this outcome not directly linked to costs for analysis. |
| 11. Are all outcomes measured appropriately? | No | Given the research question aimed to determine cost‐effectiveness of training strategies, the cost description from semistructured interviews may not have been an adequate measurement approach. |
| 12. Are outcomes valued appropriately? | No | Method of outcome valuation not described. |
| 13. Is an incremental analysis of costs and outcomes of alternatives performed? | No | Incremental analysis not reported. |
| 14. Are all future costs and outcomes discounted appropriately? | No | Time horizon unclear and discounting not mentioned. |
| 15. Are all important variables, whose values are uncertain, appropriately subjected to sensitivity analysis? | No | Sensitivity analysis not reported. |
| 16. Do the conclusions follow from the data reported? | No | Authors did not make conclusions on cost‐effectiveness, only presented costs of training for Strategy A vs Strategy B. Authors stated "further analysis of individual departmental data showed no relationship between costs and clinical outcome" though details of analysis not provided. |
| 17. Does the study discuss the generalisability of the results to other settings and patient/client groups? | Yes | Authors state generalisability of study was enhanced by including departments in different geographical regions across 4 counties. |
| 18. Does the article indicate that there is no potential conflict of interest of study researcher(s) and funder(s)? |
Yes | It was stated that all authors were independent of the funding body for the study. |
| 19. Are ethical and distributional issues discussed appropriately? | No | Not discussed. |
| Item | Yes/no | Comment |
| 1. Is the study population clearly described? | Yes | Population were stroke services and stroke patients. Inclusion criteria described for both. Loss to follow‐up described at set time points. |
| 2. Are competing alternatives clearly described? | Yes | 3 arms of study (control, SVP and SVP + implementation programme) described in detail. |
| 3. Is a well‐defined research question posed in answerable form? | No | Authors stated 1 of the economic study aims as: to "describe the costs associated with the ICONS SVP and explore the data for evidence of potential cost‐effectiveness" – clear research question on economic outcomes not stated. |
| 4. Is the economic study design appropriate to the stated objective? | Yes | Cluster randomised trial using cost– utility analyses and cost‐effectiveness approaches |
| 5. Is the chosen time horizon appropriate to include relevant costs and consequences? | Yes | Authors stated "for the purpose of this exploratory trial the time horizon for the cost analysis will be from admission to the stroke unit to 52 weeks post stroke," this is a sufficient time for relevant costs to be evaluated. |
| 6. Is the actual perspective chosen appropriate? | Yes | Perspective of the UK NHS and Personal Social Services taken. |
| 7. Are all important and relevant costs for each alternative identified? | Yes | Costs considered for in‐hospital resources (staff training, internal facilitators, staff performing programme) posthospital resources (community health and social service input, admissions). |
| 8. Are all costs measured appropriately in physical units? | Yes | Data collection forms constructed to record data, e.g. amount of time spent in online training, facilitator number of site visits and travel costs. Also a postal questionnaire designed for self‐completion was sent to patients and carers. The postal questionnaire was used with patients to determine community health and social service input. |
| 9. Are costs valued appropriately? | Yes | Authors stated: "In order to value the cost of the time performing the programme we made estimates of the cost for a minute of staff time" |
| 10. Are all important and relevant outcomes for each alternative identified? | Yes | QoL and QALY calculation used via EQ‐5D and a continence‐specific QoL measure the I‐QOL. ISI and ICIQ‐SF also used to measure urinary frequency and symptom‐free days. |
| 11. Are all outcomes measured appropriately? | Yes | The EQ‐5D is a validated tool for use with stroke survivors to measure QoL. |
| 12. Are outcomes valued appropriately? | Yes | Indirect utility assessment using EQ‐5D. |
| 13. Is an incremental analysis of costs and outcomes of alternatives performed? | Yes | ICER calculated. |
| 14. Are all future costs and outcomes discounted appropriately? | Yes | Time horizon limited to 52 weeks, discounting only necessary if time horizon > 1 year. |
| 15. Are all important variables, whose values are uncertain, appropriately subjected to sensitivity analysis? | Yes | Sensitivity analysis performed, variables included cost per quality‐adjusted life‐year gained and cost per symptom‐free day . |
| 16. Do the conclusions follow from the data reported? | Yes | Due to study being exploratory, authors do not draw firm conclusions regarding cost‐effectiveness. |
| 17. Does the study discuss the generalisability of the results to other settings and patient/client groups? | No | Not discussed. |
| 18. Does the article indicate that there is no potential conflict of interest of study researcher(s) and funder(s)? |
Yes | Stated "Conflict of interest: none declared." |
| 19. Are ethical and distributional issues discussed appropriately? | No | Not discussed. |
| CBS: Carer Burden Scale; EQ‐5D: European Quality of Life‐5 Dimensions; EQ‐5D‐3L: 3‐level version of EQ‐5D; GHQ‐12: General Health Questionnaire‐12; I‐QOL; Incontinence Quality of Life; iCaPPS: integrated Care Pathway for Post Stroke; ICER: incremental cost‐effectiveness ratio; ICIQ‐SF: International Consultation on Incontinence Questionnaire Short Form; ISI: Incontinence Severity Index; NEADL: Nottingham Extended Activities of Daily Living; QALY: quality‐adjusted life year; QoL: quality of life; SCC: stroke care co‐ordinator; SLT: speech and language therapy; SVP: systematic voiding programme; TRACS: Training Caregivers After Stroke. | ||
Appendix 4. GRADE evidence profiles
| No of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other |
Certainty (overall score) |
| Outcome: quality of care: healthcare professional adherence to EBP | |||||||
| 2 | Cluster randomised trials | Serious Downgraded 1 level due to serious risk of bias; lack of blinding of personnel in both trials; outcome assessors not blinded and incomplete outcome data in 1 trial. |
None CIs overlap and I2 = 0 |
Not serious Adherence to EBP measured using file audit in both studies |
Serious Downgraded 1 level due to serious imprecision; 95% CIs wide. |
Not serious No serious concerns regarding publication bias |
⊕⊕⊝⊝ Low |
| Outcome: patient adherence to recommended treatment: number of outdoor journeys at 6 months | |||||||
| 1 | Cluster randomised trial | Not serious Low risk of bias |
None | None | Serious Downgraded 1 level due to suboptimal information size (1 study with 100 participants) and 95% CIs wide. |
Not serious No serious concerns regarding publication bias |
⊕⊕⊕⊝ Moderate |
| Outcome:measures of patient health status and well‐being: quality of Life (EQ‐5D) at up to 6 months | |||||||
| 2 | Cluster randomised trials | Not serious Low risk of bias |
Not serious | Not serious | None | Not serious No serious concerns regarding publication bias |
⊕⊕⊕⊕ High |
| Outcome:measures of patient health status and well‐being: patient psychological well‐being at up to 6 months | |||||||
| 2 | Cluster randomised trials | Not serious Low risk of bias |
Not serious | Serious Downgraded 1 level due to outcome dissimilarity. |
None | Not serious No serious concerns regarding publication bias |
⊕⊕⊕⊝ Moderate |
| CI: confidence interval; EBP: evidence‐based practice; EQ‐5D: European Quality of Life‐5 Dimensions. | |||||||
Data and analyses
Comparison 1. Implementation intervention versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Quality of care: healthcare professional adherence to evidence‐based practice (EBP) at 12 months | 2 | 1455 | Risk Ratio (IV, Random, 95% CI) | 1.19 [0.53, 2.64] |
| 1.2 Patient health status: quality of life at up to 6 months (EQ‐5D, summary index –0.59 to 1, higher score = better) | 2 | 1242 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.02, 0.05] |
| 1.3 Patient health status: activities of daily living at up to 6 months (Barthel Index, 0–20, higher score = better) | 2 | 1272 | Mean Difference (IV, Random, 95% CI) | 0.29 [‐0.16, 0.73] |
| 1.4 Patient health status: psychological well‐being at 6 months | 2 | 1274 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.54, 0.50] |
| 1.5 Death | 3 | 1311 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.79, 1.40] |
Comparison 2. Subgroup analysis: setting for stroke rehabilitation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Quality of care: healthcare professional adherence to evidence‐based practice (EBP) at 12 months | 2 | Risk Ratio (IV, Random, 95% CI) | 1.19 [0.53, 2.64] | |
| 2.2 Patient health status: quality of life at up to 6 months (EQ‐5D, summary index –0.59 to 1, higher score = better) | 2 | 1242 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.02, 0.05] |
| 2.2.1 Acute/subacute | 1 | 653 | Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐0.05, 0.04] |
| 2.2.2 Community | 1 | 589 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.02, 0.08] |
| 2.3 Patient health status: activities of daily living at up to 6 months (Barthel Index, 0–20, higher score = better) | 2 | 1272 | Mean Difference (IV, Random, 95% CI) | 0.29 [‐0.16, 0.73] |
| 2.3.1 Acute/subacute | 1 | 669 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.51, 0.71] |
| 2.3.2 Community | 1 | 603 | Mean Difference (IV, Random, 95% CI) | 0.50 [‐0.15, 1.15] |
| 2.4 Patient health status: psychological well‐being at 6 months | 2 | 1274 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.54, 0.50] |
| 2.4.1 Acute/subacute | 1 | 664 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.47, 0.67] |
| 2.4.2 Community | 1 | 610 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐1.87, 0.67] |
Comparison 3. Subgroup analysis: tailored versus non‐tailored interventions.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Quality of care: healthcare professional adherence to evidence‐based practice (EBP) at 12 months | 2 | Risk Ratio (IV, Random, 95% CI) | 1.19 [0.53, 2.64] | |
| 3.1.1 Tailored | 1 | Risk Ratio (IV, Random, 95% CI) | 1.05 [0.21, 5.24] | |
| 3.1.2 Non‐tailored | 1 | Risk Ratio (IV, Random, 95% CI) | 1.23 [0.49, 3.10] | |
| 3.2 Patient health status: quality of life at up to 6 months (EQ‐5D, summary index –0.59 to 1, higher score = better) | 2 | 1242 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.02, 0.05] |
| 3.2.1 Tailored | 1 | 653 | Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐0.05, 0.04] |
| 3.2.2 Non‐tailored | 1 | 589 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.02, 0.08] |
| 3.3 Patient health status: activities of daily living at up to 6 months (Barthel Index, 0–20, higher score = better) | 2 | 1272 | Mean Difference (IV, Random, 95% CI) | 0.29 [‐0.16, 0.73] |
| 3.3.1 Tailored | 1 | 669 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.51, 0.71] |
| 3.3.2 Non‐tailored | 1 | 603 | Mean Difference (IV, Random, 95% CI) | 0.50 [‐0.15, 1.15] |
| 3.4 Patient health status: psychological well‐being at 6 months | 2 | 1274 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.54, 0.50] |
| 3.4.1 Tailored | 1 | 664 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.47, 0.67] |
| 3.4.2 Non‐tailored | 1 | 610 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐1.87, 0.67] |
Comparison 4. Sensitivity analysis: low risk of selection bias.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Quality of care: healthcare professional adherence to evidence‐based practice (EBP) at 12 months | 2 | Risk Ratio (IV, Random, 95% CI) | 1.19 [0.53, 2.64] | |
| 4.1.1 Low risk | 2 | Risk Ratio (IV, Random, 95% CI) | 1.19 [0.53, 2.64] | |
| 4.1.2 Unclear risk | 0 | Risk Ratio (IV, Random, 95% CI) | Not estimable | |
| 4.1.3 High risk | 0 | Risk Ratio (IV, Random, 95% CI) | Not estimable | |
| 4.2 Patient health status: quality of life at up to 6 months (EQ‐5D, summary index –0.59 to 1, higher score = better) | 2 | 1242 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.02, 0.05] |
| 4.2.1 Low risk | 1 | 589 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.02, 0.08] |
| 4.2.2 Unclear risk | 1 | 653 | Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐0.05, 0.04] |
| 4.2.3 High risk | 0 | 0 | Mean Difference (IV, Random, 95% CI) | Not estimable |
| 4.3 Patient health status: activities of daily living at up to 6 months (Barthel Index, 0–20, higher score = better) | 2 | 1272 | Mean Difference (IV, Random, 95% CI) | 0.29 [‐0.16, 0.73] |
| 4.3.1 Low risk | 1 | 603 | Mean Difference (IV, Random, 95% CI) | 0.50 [‐0.15, 1.15] |
| 4.3.2 Unclear risk | 1 | 669 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.51, 0.71] |
| 4.3.3 High risk | 0 | 0 | Mean Difference (IV, Random, 95% CI) | Not estimable |
| 4.4 Patient health status: psychological well‐being at 6 months | 2 | 1274 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.54, 0.50] |
| 4.4.1 Low risk | 1 | 610 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐1.87, 0.67] |
| 4.4.2 Unclear risk | 1 | 664 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.47, 0.67] |
| 4.4.3 High risk | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | Not estimable |
4.1. Analysis.

Comparison 4: Sensitivity analysis: low risk of selection bias, Outcome 1: Quality of care: healthcare professional adherence to evidence‐based practice (EBP) at 12 months
Comparison 5. Sensitivity analysis: low risk of detection bias.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Quality of care: healthcare professional adherence to evidence‐based practice (EBP) at 12 months | 2 | 1455 | Risk Ratio (IV, Random, 95% CI) | 1.19 [0.53, 2.64] |
| 5.1.1 Low risk | 1 | 279 | Risk Ratio (IV, Random, 95% CI) | 1.05 [0.21, 5.24] |
| 5.1.2 Unclear risk | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | Not estimable |
| 5.1.3 High risk | 1 | 1176 | Risk Ratio (IV, Random, 95% CI) | 1.23 [0.49, 3.10] |
| 5.2 Patient health status: quality of life at up to 6 months (EQ‐5D, summary index –0.59 to 1, higher score = better) | 2 | 1242 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.02, 0.05] |
| 5.2.1 Low risk | 2 | 1242 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.02, 0.05] |
| 5.2.2 Unclear risk | 0 | 0 | Mean Difference (IV, Random, 95% CI) | Not estimable |
| 5.2.3 High risk | 0 | 0 | Mean Difference (IV, Random, 95% CI) | Not estimable |
| 5.3 Patient health status: activities of daily living at up to 6 months (Barthel Index, 0–20, higher score = better) | 2 | 1272 | Mean Difference (IV, Random, 95% CI) | 0.29 [‐0.16, 0.73] |
| 5.3.1 Low risk | 2 | 1272 | Mean Difference (IV, Random, 95% CI) | 0.29 [‐0.16, 0.73] |
| 5.3.2 Unclear risk | 0 | 0 | Mean Difference (IV, Random, 95% CI) | Not estimable |
| 5.3.3 High risk | 0 | 0 | Mean Difference (IV, Random, 95% CI) | Not estimable |
| 5.4 Patient health status: psychological well‐being at 6 months | 2 | 1274 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.54, 0.50] |
| 5.4.1 Low risk | 2 | 1274 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.54, 0.50] |
| 5.4.2 Unclear risk | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | Not estimable |
| 5.4.3 High risk | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | Not estimable |
5.2. Analysis.

Comparison 5: Sensitivity analysis: low risk of detection bias, Outcome 2: Patient health status: quality of life at up to 6 months (EQ‐5D, summary index –0.59 to 1, higher score = better)
5.3. Analysis.

Comparison 5: Sensitivity analysis: low risk of detection bias, Outcome 3: Patient health status: activities of daily living at up to 6 months (Barthel Index, 0–20, higher score = better)
5.4. Analysis.

Comparison 5: Sensitivity analysis: low risk of detection bias, Outcome 4: Patient health status: psychological well‐being at 6 months
Comparison 6. Sensitivity analysis: low risk of attrition bias.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Quality of care: healthcare professional adherence to evidence‐based practice (EBP) at 12 months | 2 | Risk Ratio (IV, Random, 95% CI) | 1.19 [0.53, 2.64] | |
| 6.1.1 Low risk | 1 | Risk Ratio (IV, Random, 95% CI) | 1.05 [0.21, 5.24] | |
| 6.1.2 Unclear risk | 0 | Risk Ratio (IV, Random, 95% CI) | Not estimable | |
| 6.1.3 High risk | 1 | Risk Ratio (IV, Random, 95% CI) | 1.23 [0.49, 3.10] | |
| 6.2 Patient health status: quality of life at up to 6 months (EQ‐5D, summary index –0.59 to 1, higher score = better) | 2 | 1242 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.02, 0.05] |
| 6.2.1 Low risk | 2 | 1242 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.02, 0.05] |
| 6.2.2 Unclear risk | 0 | 0 | Mean Difference (IV, Random, 95% CI) | Not estimable |
| 6.2.3 High risk | 0 | 0 | Mean Difference (IV, Random, 95% CI) | Not estimable |
| 6.3 Patient health status: activities of daily living at up to 6 months (Barthel Index, 0–20, higher score = better) | 2 | 1272 | Mean Difference (IV, Random, 95% CI) | 0.29 [‐0.16, 0.73] |
| 6.3.1 Low risk | 2 | 1272 | Mean Difference (IV, Random, 95% CI) | 0.29 [‐0.16, 0.73] |
| 6.3.2 Unclear risk | 0 | 0 | Mean Difference (IV, Random, 95% CI) | Not estimable |
| 6.3.3 High risk | 0 | 0 | Mean Difference (IV, Random, 95% CI) | Not estimable |
| 6.4 Patient health status: psychological well‐being at 6 months | 2 | 1274 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.54, 0.50] |
| 6.4.1 Low risk | 2 | 1274 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.54, 0.50] |
| 6.4.2 Unclear risk | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | Not estimable |
| 6.4.3 High risk | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | Not estimable |
6.2. Analysis.

Comparison 6: Sensitivity analysis: low risk of attrition bias, Outcome 2: Patient health status: quality of life at up to 6 months (EQ‐5D, summary index –0.59 to 1, higher score = better)
6.3. Analysis.

Comparison 6: Sensitivity analysis: low risk of attrition bias, Outcome 3: Patient health status: activities of daily living at up to 6 months (Barthel Index, 0–20, higher score = better)
6.4. Analysis.

Comparison 6: Sensitivity analysis: low risk of attrition bias, Outcome 4: Patient health status: psychological well‐being at 6 months
Comparison 7. Sensitivity analysis: low risk of reporting bias.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Quality of care: healthcare professional adherence to evidence‐based practice (EBP) at 12 months | 2 | Risk Ratio (IV, Random, 95% CI) | 1.19 [0.53, 2.64] | |
| 7.1.1 Low risk | 1 | Risk Ratio (IV, Random, 95% CI) | 1.05 [0.21, 5.24] | |
| 7.1.2 Unclear risk | 0 | Risk Ratio (IV, Random, 95% CI) | Not estimable | |
| 7.1.3 High risk | 1 | Risk Ratio (IV, Random, 95% CI) | 1.23 [0.49, 3.10] | |
| 7.2 Patient health status: quality of life at up to 6 months (EQ‐5D, summary index –0.59 to 1, higher score = better) | 2 | 1242 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.02, 0.05] |
| 7.2.1 Low risk | 1 | 589 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.02, 0.08] |
| 7.2.2 Unclear risk | 0 | 0 | Mean Difference (IV, Random, 95% CI) | Not estimable |
| 7.2.3 High risk | 1 | 653 | Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐0.05, 0.04] |
| 7.3 Patient health status: activities of daily living at up to 6 months (Barthel Index, 0–20, higher score = better) | 2 | 1272 | Mean Difference (IV, Random, 95% CI) | 0.29 [‐0.16, 0.73] |
| 7.3.1 Low risk | 1 | 603 | Mean Difference (IV, Random, 95% CI) | 0.50 [‐0.15, 1.15] |
| 7.3.2 Unclear risk | 0 | 0 | Mean Difference (IV, Random, 95% CI) | Not estimable |
| 7.3.3 High risk | 1 | 669 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.51, 0.71] |
| 7.4 Patient health status: psychological well‐being at 6 months | 2 | 1274 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.54, 0.50] |
| 7.4.1 Low risk | 1 | 610 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐1.87, 0.67] |
| 7.4.2 Unclear risk | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | Not estimable |
| 7.4.3 High risk | 1 | 664 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.47, 0.67] |
7.2. Analysis.

Comparison 7: Sensitivity analysis: low risk of reporting bias, Outcome 2: Patient health status: quality of life at up to 6 months (EQ‐5D, summary index –0.59 to 1, higher score = better)
7.3. Analysis.

Comparison 7: Sensitivity analysis: low risk of reporting bias, Outcome 3: Patient health status: activities of daily living at up to 6 months (Barthel Index, 0–20, higher score = better)
7.4. Analysis.

Comparison 7: Sensitivity analysis: low risk of reporting bias, Outcome 4: Patient health status: psychological well‐being at 6 months
Comparison 8. Sensitivity analysis: low risk of unit of analysis issues.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Quality of care: healthcare professional adherence to evidence‐based practice (EBP) at 12 months | 2 | Risk Ratio (IV, Random, 95% CI) | 1.19 [0.53, 2.64] | |
| 8.1.1 Low risk | 2 | Risk Ratio (IV, Random, 95% CI) | 1.19 [0.53, 2.64] | |
| 8.1.2 Unclear risk | 0 | Risk Ratio (IV, Random, 95% CI) | Not estimable | |
| 8.1.3 High risk | 0 | Risk Ratio (IV, Random, 95% CI) | Not estimable | |
| 8.2 Patient health status: quality of life at up to 6 months (EQ‐5D, summary index –0.59 to 1, higher score = better) | 2 | 1242 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.02, 0.05] |
| 8.2.1 Low risk | 2 | 1242 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.02, 0.05] |
| 8.2.2 Unclear risk | 0 | 0 | Mean Difference (IV, Random, 95% CI) | Not estimable |
| 8.2.3 High risk | 0 | 0 | Mean Difference (IV, Random, 95% CI) | Not estimable |
| 8.3 Patient health status: activities of daily living at up to 6 months (Barthel Index, 0–20, higher score = better) | 2 | 1272 | Mean Difference (IV, Random, 95% CI) | 0.29 [‐0.16, 0.73] |
| 8.3.1 Low risk | 2 | 1272 | Mean Difference (IV, Random, 95% CI) | 0.29 [‐0.16, 0.73] |
| 8.3.2 Unclear risk | 0 | 0 | Mean Difference (IV, Random, 95% CI) | Not estimable |
| 8.3.3 High risk | 0 | 0 | Mean Difference (IV, Random, 95% CI) | Not estimable |
| 8.4 Patient health status: psychological well‐being at 6 months | 2 | 1274 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.54, 0.50] |
| 8.4.1 Low risk | 2 | 1274 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.54, 0.50] |
| 8.4.2 Unclear risk | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | Not estimable |
| 8.4.3 High risk | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | Not estimable |
8.1. Analysis.

Comparison 8: Sensitivity analysis: low risk of unit of analysis issues, Outcome 1: Quality of care: healthcare professional adherence to evidence‐based practice (EBP) at 12 months
8.2. Analysis.

Comparison 8: Sensitivity analysis: low risk of unit of analysis issues, Outcome 2: Patient health status: quality of life at up to 6 months (EQ‐5D, summary index –0.59 to 1, higher score = better)
8.3. Analysis.

Comparison 8: Sensitivity analysis: low risk of unit of analysis issues, Outcome 3: Patient health status: activities of daily living at up to 6 months (Barthel Index, 0–20, higher score = better)
8.4. Analysis.

Comparison 8: Sensitivity analysis: low risk of unit of analysis issues, Outcome 4: Patient health status: psychological well‐being at 6 months
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abdul Aziz 2014.
| Study characteristics | ||
| Methods |
Aim of study: to evaluate the cost‐effectiveness of an integrated care pathway (iCaPPS) in improving quality of life for stroke survivors Study design: cluster randomised trial Unit of randomisation: public health centres Mean cluster size: each cluster comprised 3 healthcare professionals (information from contact with trial author) Unit of analysis: patient Sample size calculation: study was designed with 80% power to detect a difference of 15% between mean primary outcomes (EQ‐5D) scores between intervention and control groups. Based on these calculations, a minimum sample of 65 per arm was required, with no allowance made for dropouts as ITT analysis was intended. |
|
| Participants |
Healthcare professionals: 10 public health centres each with primary care teams comprising doctors, nurses, physiotherapists and occupational therapists. Demographics of staff within clusters not provided Patients: stroke survivors (n = 151) Intervention group: n = 86 Control group: n = 65 Mean age of stroke survivors 60.2 (SD 9.5) years, median duration poststroke 2.25 (IQR 5.1) years Sex and ethnicity: not described Setting: community public health centres Country: Malaysia |
|
| Interventions |
Targeted behaviour change: for staff to deliver iCaPPS, a comprehensive poststroke care pathway for stroke survivors. Theory used: Biopsychosocial Model of Health used to guide long‐term stroke care, no implementation theory used (information from contact with trial author). Category of implementation intervention: delivery arrangements Multifaceted intervention: no Tailoring to barriers: no (information from contact with trial author) Description of intervention: integrated care pathway (see Appendix 2 for full details of intervention) Delivery: components of pathways delivered to patients face‐to‐face, no information about delivery with healthcare professionals Frequency: information not provided Duration of intervention: 6 months Control: no intervention |
|
| Outcomes |
Main outcome: Patient outcomes: quality of life measured with EQ‐5D‐3L Secondary outcomes: measurement of blood pressure, cholesterol, triglycerides, haemoglobin A1c, fasting blood sugar, TQWHQ, PHQ‐9, Barthel Index, ECAQ and M‐MMSE Follow‐up: 6 months after introduction of pathway Loss of clusters and individuals: no (information from contact with trial author) Adjusted for clustering for each outcome: no (information from contact with trial author) Method of cluster adjustment for each outcome: information not provided ICC reported for each outcome: information not provided |
|
| Notes |
Outcomes used in this review: quality of life measured with EQ‐5D, PHQ‐9, Barthel Index, M‐MMSE, TQWHQ, ECAQ Unit of analysis error: yes, no report of analysis accounting for clustering Ethical approval and informed consent obtained: yes. Ethics approval obtained and informed written consent was obtained from all participants. Funding source: National University of Malaysia Research University Grant Declarations of interest: none declared though only abstract publication. First author was involved in the development of the care pathway implemented (the iCaPPS) Contact with author? yes Additional outcome data provided from author? yes Trial registration: ACTRN12616001322426 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Simple randomisation of clusters using coin toss method. |
| Allocation concealment (selection bias) | Low risk | Central randomisation at health centre level using coin toss. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Patients receiving treatment were blinded. Authors stated staff administering the treatment were blinded; however, given a new procedure (pathway) was being used by staff, the means of blinding was unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Some outcomes used self‐report of blinded patients, other outcomes were those administered by staff; it was unclear whether staff completing these assessments were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Full results not published or provided, missing data could not be determined; loss of clusters not reported. |
| Selective reporting (reporting bias) | High risk | Retrospectively registered on ANZCTR.org.au, full research results not published or provided. |
| Other bias | High risk | Recruitment bias: high risk, each arm was originally planned to have ≥ 65 patients; the intervention arm ultimately had 86 patients. Some suggestion of potential recruitment bias at the cluster or patient level. Incorrect analysis: high risk, unclear whether clustering accounted for in analysis. Similar baseline characteristics: unclear risk, information not available. Similar baseline outcome measures: unclear risk, baseline assessment of primary outcome not available. Reliability of primary outcome measures: low risk, psychometric properties of primary outcome (EQ‐5D) has been demonstrated in stroke patients. Adequate protection against contamination: low risk, allocation by health centre. |
Forster 2013.
| Study characteristics | ||
| Methods |
Aim of study: an effectiveness‐implementation hybrid trial assessing clinical effectiveness and implementation. Authors wanted to determine the effectiveness of implementing a training programme for carers (the LSCTC) in improving physical and psychological outcomes for stroke patients and reducing carer burden. Study design: cluster randomised trial Unit of randomisation: stroke rehabilitation unit Mean cluster size: information not available Unit of analysis: patient Sample size calculation: study was designed with 90% power to detect a 6‐point difference on the primary outcome between experimental and control groups at a 5% significance level. It was calculated 36 units providing 25 patients would be required to achieve 450 patients per group. Sample size incorporated an inflation factor of 1.9 due to clustering (cluster size of 19 after loss to follow‐up; ICC ≤ 0.05) and 25% loss to follow‐up. To preserve the final power of 90%, the trial sample size was increased from 900 to 950 patient and carer dyads, allowing up to 35 dyads per stroke unit to compensate for lower recruitment at some centres. |
|
| Participants |
Healthcare professionals: 36 stroke rehabilitation units. Staff involved in study included physiotherapists, occupational therapists, speech and language therapists, nurses, dieticians, stroke co‐ordinators and stroke consultants. Number of staff involved in initial training was 56. Demographics of staff within clusters not provided. Patients: stroke survivors (n = 928) Experimental group: n = 450, 57% men, mean age 71.0 (SD 12.76) Control group: n = 478, 55% men, mean age 71.3 (SD 12.18) Ethnicity: Intervention group: white 95.3% Control group: white 92.9% Setting: inpatient stroke rehabilitation units Country: UK |
|
| Interventions |
Targeted behaviour change: for staff to provide carer training (the LSCTC) to the carers of people with recent stroke Theory used: Normalisation Process Theory Category of implementation intervention: implementation strategies Multifacetedintervention (yes/no): yes Tailoring to barriers: yes Staff were asked during training sessions to discuss in groups how the training could be provided in their own units, highlighting potential barriers, challenges and approaches. Description of intervention: interprofessional education, educational materials, local opinion leaders, cascade method of implementation (see Appendix 2 for full details of intervention) Delivery: face‐to‐face training in group setting Frequency: 2 days of training, 1 month apart Duration of intervention: 4–6 months after training Control: no intervention |
|
| Outcomes |
Main outcome: Patient outcomes: functional independence measured by the NEADL assessment Carer outcomes: CBS Secondary outcomes: Patient outcomes:
Carer outcomes:
Follow‐up: 6 and 12 months after recruitment Loss of clusters and individuals: no clusters lost to follow‐up Intervention group: 146 patients (32.4%) and 329 (34.4%) carers lost to follow‐up Control group: 145 patients (30.3%) and 164 (34.3%) carers lost to follow‐up Adjusted for clustering for each outcome: yes Method of cluster adjustment for each outcome: 2‐level hierarchical model used to compare intervention and control groups with patients nested within stroke units ICC reported for each outcome: yes, for primary and secondary outcomes |
|
| Notes |
Outcomes used in this review: functional independence measured by the NEADL assessment, EQ‐5D, HADS, SIS, death We pooled death rates from this trial as per guidance in the Cochrane Handbook for Systematic Reviews of Interventions Section 23.1.4.1 and applied a design effect to the number of participants and events to account for the clustering. Unit of analysis error: no Ethical approval and informed consent obtained: yes. Ethics approval through Leeds Research Ethics Committee (07/Q1205/12). All participants provided written informed consent. Funding source: MRC Declarations of interest: none declared or detected Contact with author? yes Additional outcome data provided from author? no Trial registration: ISRCTN 49208824 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stroke units were randomly assigned (1:1) to groups (process not described), stratified by geographical region and quality of care. These covariates were balanced by block randomisation (size of 2). |
| Allocation concealment (selection bias) | Unclear risk | Cluster randomisation was done centrally at a CTRU. Screening logs taken by research staff to monitor selection bias but review and discussion not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Stroke survivors and carers were blinded to stroke unit allocation. Staff were likely aware of their allocation as those working in intervention sites received training in delivery of the LSCTC and those in control sites were asked to continue care as recommended in national guidelines. However, the outcomes are unlikely to be influenced by incomplete blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were self‐report measures from patients and carers who were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data were balanced in numbers across groups and for similar reasons. No clusters lost to follow‐up. |
| Selective reporting (reporting bias) | High risk | Some secondary outcomes proposed in trial registry are incompletely reported (e.g. hospital readmission and institutionalisation). |
| Other bias | Low risk | Recruitment bias: low risk Incorrect analysis: low risk Similar baseline characteristics: low risk, the groups were sufficiently similar and balanced at baseline, with similar mean age, sex, functional independence before stroke and language impairment. Similar baseline outcome measures: low risk, similar baseline outcome measures between groups for patients and carers. Reliability of primary outcome measures: low risk, The NEADL has confirmed validity, reliability and demonstrated responsiveness to change. Adequate protection against contamination: low risk, allocation by stroke unit, stratification by geographical region in random. |
Forster 2015.
| Study characteristics | ||
| Methods |
Aim of study: an effectiveness‐implementation hybrid trial assessing clinical effectiveness and implementation. Authors wanted to determine the effectiveness of implementing a new postdischarge system of care (LoTS) targeting longer‐term problems experienced by stroke survivors and their carers. Study design: cluster randomised trial Unit of randomisation: stroke care co‐ordinator service Mean cluster size: intervention 26.7 patients (range 2–45), control 28.5 (range 15–46) Unit of analysis: patient Sample size calculation: study was designed with 90% power at 5% significance to detect a clinically relevant difference of 2.5 points in the primary outcome (GHQ‐12) based on a previous study. 40 clusters involving 800 patients overall (20 patients per cluster) was determined to be needed, accounting for an estimated 25% loss to follow‐up and clustering; an inflation factor of 1.95 was derived from the maximum cluster size of 20 and an ICC > 0.05. The study was able to identify 32 eligible clusters willing to participate, so planned for each cluster to recruit 25 patients providing 88% power, assuming equal cluster size and ≤ 25% loss to follow‐up. To minimise unequal recruitment clusters, maximum number of patients per service was capped at 45. |
|
| Participants |
Healthcare professionals: 29 clusters of stroke care co‐ordinators or stroke care co‐ordinator services. Number and demographics of staff within clusters not provided. 15 intervention clusters, 14 control clusters Patients: stroke survivors (n = 800) Experimental group: n = 401, 53.6% men, mean age 70.9 (SD 13.18) years Control group: n = 399, 54.6% men, mean age 72.5 (SD 12.84) years Ethnicity: Intervention group: white 96.8% Control group: white 97.5% Carers: n = 208 Experimental group: n = 108, 32.5% men, mean age 61.0 (SD 15.02) years Control group: n = 100, 32% men, mean age 61.4 (SD 14.07) years Setting: community‐based setting: outpatients and home‐based care Country: UK |
|
| Interventions |
Targeted behaviour change: for staff to use a new service model (the LoTS system) with stroke survivors living in the community Theory used: the MRC Framework was used for the evaluation of a complex intervention. No implementation theory mentioned. Category of implementation intervention: delivery arrangements and implementation strategies Multifacetedintervention: yes Tailoring to barriers: not described Description of intervention: co‐ordination of care and management of care processes, interprofessional education, educational materials (see Appendix 2 for full details of intervention) Delivery: centrally based, face‐to‐face training by experienced clinicians Frequency: 2 × 1‐day workshops approximately 1 month apart Duration of intervention: 4‐month period of 'hands‐on experience' following training (or 12 months LoTS care delivery to patients) Control: no intervention; care delivered as usual by stroke care co‐ordinators as determined by local policy and practices |
|
| Outcomes |
Main outcome: Patient outcomes: improved stroke survivor psychological well‐being measured by the GHQ‐12 Secondary outcome: Patient outcomes:
Carer outcomes:
Follow‐up: 6 and 12 months after recruitment Loss of clusters and individuals: no clusters lost to follow‐up once participation began. Patients lost to follow‐up at 6 and 12 months: Intervention group: 6 months: n = 91 (22.7%), 12 months: 120 patients (32.8%) Control group: 6 months: n = 99 (24.8%), 12 months: 131 patients (29.9%) Adjusted for clustering for each outcome: yes Method of cluster adjustment for each outcome: 2‐level linear model used for primary and secondary analysis, models were adjusted for patient‐level covariates and stroke unit‐level covariates. ICC reported for each outcome: yes, for primary and secondary outcomes |
|
| Notes |
Outcomes used in this review: EQ‐5D, GHQ‐12, Barthel Index, FAI, death, hospital readmission We pooled death rates from this trial as per guidance in the Cochrane Handbook for Systematic Reviews of Interventions Section 23.1.4.1 and applied a design effect to the number of participants and events to account for the clustering (Higgins 2019). Unit of analysis error: no Ethical approval and informed consent obtained: yes, ethics approval from Leeds West and Scotland Research Ethics Committees. Written informed consent obtained from patients and carers (if appropriate) Funding source: NIHR (Programme Grants for Applied Research Programmed; RP‐PG‐0606‐1128) and The Stroke Association (TSA 2006/15). Declarations of interest: none declared or detected. Authors stated in 'Sources of funding' this is independent research. Contact with author? yes Additional outcome data provided from author? no Trial registration: ISRCTN67932305 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stroke units were randomly assigned (1:1) to groups (process not described), stratified by quality of stroke unit, annual number of referrals, stroke care co‐ordinators working alone or within community‐based team. Clusters randomised in 2 phases due to delays in research approval. Method of obtaining balanced randomisation based on a peer‐reviewed published algorithm for cluster randomisation. |
| Allocation concealment (selection bias) | Low risk | Cluster randomisation was performed centrally by a CTRU. Recruitment of trial participants was by independent research staff who were blinded to which group they were recruiting to. Screening and recruitment data were collected and analysed. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | States stroke care co‐ordinators were unaware which of their patients had consented to participate. Stroke participants and their carers likely blinded though not specifically reported. Intervention (care plan) was designed so that it replaced previous patient documentation and became embedded in standard practice; participants were new stroke patients so likely not to notice change in standard practice. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were self‐report measures from patients and carers who were likely blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No clusters lost to follow‐up. Response rates for patient‐reported outcomes were comparable: 75.2% in control and 77.3% in intervention at 6 months and 67.2% in control and 70.1% in intervention at 12 months. |
| Selective reporting (reporting bias) | Low risk | Prospectively registered trial with published protocol, prespecified primary and secondary outcomes reported. |
| Other bias | Unclear risk | Recruitment bias: low risk Incorrect analysis: low risk Similar baseline characteristics: low risk, baseline characteristics of patients balanced across control and intervention groups. Differences between groups in language or cognitive impairment and length of inpatient stay, which authors noted. Language and cognitive impairment were accounted for in statistical modelling. Similar baseline outcome measures: low risk, some imbalance in baseline outcome measures between intervention and control groups; however, the authors used a multilevel statistical model to adjust for patient‐level covariates, e.g. baseline Barthel Index and GHQ‐12 score. Reliability of primary outcome measures: unclear risk, validity of GHQ‐12 version not fully established in a stroke population, authors only stated measure was short and easy to complete and was consistent with the high prevalence of psychological symptoms after stroke. Adequate protection against contamination: allocation by stroke care co‐ordinator service. Stratification addressed co‐ordinators working individually or within a team. |
McCluskey 2016.
| Study characteristics | ||
| Methods |
Aim of study: to determine the effectiveness of a behaviour change programme (Out‐and‐About) to increase community outings taken by stroke survivors Study design: cluster randomised trial Unit of randomisation: rehabilitation team Mean cluster size: 22 teams, median of 3 therapists per team (IQR 2–13) Unit of analysis: patient Sample size calculation: study was designed with 80% power to detect a 20% difference between experimental and control groups regarding percentage of patients receiving ≥ 4 outings during therapy. An ICC of 0.04 was used to adjust for the effects of clustering and ≥ 20 clusters involving 15 patients in each cluster were determined to be needed (2 sided, 5% significance level). |
|
| Participants |
Healthcare professionals: 22 community teams comprised of occupational therapists and physiotherapists. Number and demographics of therapists within clusters not provided. Patients: stroke survivors (n = 542) Preintervention Experimental group: n = 164, 55% men, mean age 67 (SD 16) years Control group: n = 115, 56% men, mean age 67 (SD 14) years Postintervention Experimental group: n = 164, 62% men, mean age 68 (SD 14) years Control group: n = 115, 59% men, mean age 67 (SD 15) years Ethnicity: not described Setting: community rehabilitation centres providing outpatient, day therapy or home‐based rehabilitation Country: Australia |
|
| Interventions |
Targeted behaviour change: for staff to increase the number of outings provided to stroke survivors during therapy Theory used: none Category of implementation intervention: implementation strategies Multifacetedintervention: yes Tailoring to barriers: yes Barriers at sites identified through 20‐minute discussion with therapists, strategies for overcoming barriers discussed at time of training sessions Description of intervention: interprofessional education, educational materials including clinical practice guidelines, audit and feedback (see Appendix 2 for full details of intervention) Delivery: on‐site, face‐to‐face training workshops conducted by the lead author Frequency: initial 2‐hour workshop, audit and feedback, printed materials and barrier identification, 12 months later booster session for 9/11 intervention teams Duration of intervention: 12 months Control: control group provided with a copy of Australian stroke clinical practice guidelines |
|
| Outcomes |
Main outcome: Quality of care: percentage of stroke survivors receiving ≥ 4 outings during therapy Secondary outcomes: Quality of care: number of outdoor‐related sessions delivered by occupational therapists and physiotherapists during therapy Patient outcomes: number of outings undertaken by stroke survivors outside of therapy, Life‐Space Assessment Follow‐up: stroke survivor files reaudited at 12 months, observed stroke survivor group completed self‐report diary at 6 months Loss of clusters and individuals: 1/22 cluster lost to follow‐up due to service cessation, 15/115 stroke survivors lost to follow‐up in observed sample Adjusted for clustering for each outcome: mean difference between experimental and control groups adjusted for cluster randomisation Method of cluster adjustment for each outcome: mixed models ICC reported for each outcome: no Outcomes used in this review: percentage of stroke survivors receiving ≥ 4 outings during therapy, number of outdoor‐related sessions delivered by occupational therapists and physiotherapists during therapy, Life‐Space Assessment |
|
| Notes |
Outcomes used in this review:
Unit of analysis error: no Ethical approval and informed consent obtained: ethics approval obtained from university and local ethics committees. Informed consent not specifically reported though inclusion criteria for stroke survivors was ability to give informed consent. Funding source: government source, NHMRC Declarations of interest: none declared or detected Contact with author? yes. Erratum to original data in Tables 3 and 4 in original publication provided by authors (McCluskey 2016) Additional outcome data provided from author? no Trial registration: ACTRN12611000554965 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | An 'independent randomisation service' was used to allocate clusters to experimental or control groups. Minimisation was used, a form of covariate adaptive randomisation to ensure a balance of variables; location (centre or home‐based teams), funding (public or private), volume of caseload and level of outings at baseline. |
| Allocation concealment (selection bias) | Low risk | Authors stated 'concealed allocation.' As minimisation was used to randomise teams the sites would have been recruited before the random sequence was generated, reducing selection bias. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Personnel delivering the intervention were not blinded. Blinding of therapist participants was attempted, but possible the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding (quote: "to optimize blinding of therapists, only team leaders were privy to study aims"). Unclear whether patient participants were blinded (they provided informed consent to complete self‐report outcome measures). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Patient files were audited by an individual blinded to team allocation. Therapists documenting in files may have been unblinded but this is unlikely to affect reporting of outcome (whether a community outing occurred or not). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up in outcome data of primary outcome due to method (audit of patient files). Loss to follow‐up of observed stroke survivors of 13% in both intervention and control groups, this population related to secondary outcomes only. Staff turnover high (quite "up to 50%") during study but did not impact data due to patient measures used as outcomes. 1 cluster lost to follow‐up due to service cessation. |
| Selective reporting (reporting bias) | Low risk | Reports primary and secondary outcome data as outlined in published protocol. |
| Other bias | Low risk | Recruitment bias: low risk Incorrect analysis: low risk Similar baseline characteristics: low risk, adequate similarity; stroke survivors similar in terms of age, sex and stroke severity between groups. Characteristics of teams similar at baseline in terms of funding, minor differences between groups in location, control group had more outpatient settings than intervention group. Similar baseline outcome measures: low risk, control group providing less outings at baseline but direction of potential bias unlikely to impact results. Reliability of primary outcome measures: low risk, audit involved a count of escorted journeys from review of medical files. Authors note they considered the impact of therapists not documenting patient journeys however they were confident these events were 'novel, time‐consuming' events reported in detail. Adequate protection against contamination: low risk, allocation was by community rehabilitation teams. Unlikely that the control group received the intervention. |
Pennington 2005.
| Study characteristics | ||
| Methods |
Aim of study: to determine the clinical and cost‐effectiveness to 2 training strategies to promote the use of research evidence by speech and language therapists to manage poststroke dysphagia Study design: cluster randomised trial Unit of randomisation: speech and language therapy departments Mean cluster size: strategy A 20.9 staff (SD 12.4), strategy B 22.2 staff (SD 9.6) Unit of analysis: patient Sample size calculation: calculations made on baseline adherence to clinical guidelines of 74% (SD 1.6%) and assumption of 24 departments participating; a mean of 50 patients records per department was required to detect a standardised difference of 0.24 based on type 1 error of 5% and type 2 errors of 20%. Authors estimated intradepartmental correlation as 0.1, resulting in an ability to detect a larger standardised difference between strategies of 0.55, or a change of nearly 5% in summary adherence score. |
|
| Participants |
Healthcare professionals: 17 speech and language therapy departments comprising speech and language therapists. Number and demographics of therapists within clusters not provided. Patients: stroke survivors (n = 1470) Intervention A: n = 708 Intervention B: n = 762 Patient demographic information (e.g. sex/age/ethnicity) not provided Setting: hospitals providing inpatient services to adults and children Country: England |
|
| Interventions |
Targeted behaviour change: for speech and language therapists to adhere to clinical practice guidelines for poststroke dysphagia management Theory used: Roger's Diffusion of Innovations Category of implementation intervention: implementation strategies Multifacetedintervention: yes Tailoring to barriers: not formally done though staff were asked during training session to choose a clinical guideline recommendation and draw up action plan for its implementation at their service. Description of intervention: interprofessional education (see Appendix 2 for full details of intervention) Description of implementation strategy/strategies: Intervention A Training workshops Covering topics of: clinical governance, evidence‐based healthcare, critical appraisal of studies, clinical guidelines for poststroke dysphagia management Mode of delivery: short talks, group discussion, problem‐based learning, self‐directed study, action plans by participants for guideline implementation Intervention B Training workshops The same as Intervention A with additional training on Roger's Diffusion of Innovation model. Mode of delivery: the same as Intervention A with additional components of considering the characteristics of the intended user of guidelines, identifying leaders for change and materials needed. Delivery: off‐site face‐to‐face training workshops conducted by authors Frequency: Intervention A 2.5 days of training over 7 weeks Intervention B 5 days of training over 3 months Duration of intervention: 8–12 months (this was time point of reassessment, intervention period unclear) Control: no control, intervention A compared with intervention B |
|
| Outcomes |
Main outcome: Patient outcomes: none reported Quality of care: adherence to clinical practice guidelines for dysphagia management Secondary outcome: incremental cost of increased adherence to clinical guidelines Follow‐up: 8–12 months after training Loss of clusters and individuals: 1 cluster withdrew from trial due to staff shortages Adjusted for clustering for each outcome: ? no Method of cluster adjustment for each outcome: not done ICC reported for each outcome: no |
|
| Notes |
Outcomes used in this review: adherence to clinical practice guidelines for dysphagia management Unit of analysis error:? yes Ethical approval and informed consent obtained: no. Authors stated the study was an audit of case management by a UK NHS ethics committee, which did not require ethical approval. Funding source: UK NHS Research and Development programme funding Declarations of interest: none declared or detected Contact with author? yes Additional outcome data provided from author? no Trial registration: N/A |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers were used to allocate clusters to the 2 different interventions. This was carried out by a health statistician who was independent of the trial and blind to its aims. |
| Allocation concealment (selection bias) | Low risk | Allocation was conducted by a health statistician who was independent of the trial and blind to its aims. The research team were notified via email of the allocation of each department. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Personnel delivering intervention not blinded. Unclear whether therapist participants were blinded to group allocation, and outcomes likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Primary assessor was not blinded due to funding restrictions. A second reviewer, who was blinded to allocation, independently audited 10% of cases from each department to check primary assessor coding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Adherence outcome data provided for each department. 1 department withdrew before the start of the intervention. No loss of clusters once intervention commenced, pre‐ and postdata collected for all departments. |
| Selective reporting (reporting bias) | Unclear risk | No published protocol and no trial registry to determine if outcomes reported were the intended measures. |
| Other bias | High risk | Recruitment bias: low risk Incorrect analysis: unclear whether clustering accounted for in analysis. Similar baseline characteristics: unclear risk, notable differences in the number of reported poststroke dysphagia referrals per month between intervention groups. Similar baseline outcome measures: low risk, similar baseline adherence scores between groups: mean 73.2 in intervention A group and mean 72.2 in intervention B group. Reliability of primary outcome measures: high risk. Audit tool developed by researchers and a consensus group. A published evaluation of the reliability of the tool found low internal consistency and advised caution with the use of a composite score of adherence. Adequate protection against contamination: unclear risk, efforts made to reduce risk of contamination, e.g. managers and participants asked not to discuss content of training outside of departments. Departments in the pilot study who were part of the same organisation were allocated as a single unit to reduce contamination. All clusters were from 1 geographical region in the UK (North‐West England). |
Power 2014.
| Study characteristics | ||
| Methods |
Aim of study: to determine whether a quality improvement collaborative (Stroke 90:10) involving 2 evidence‐based bundles of care could improve reliability of stroke care Study design: cluster randomised trial with interrupted time series design Unit of randomisation: hospitals Mean cluster size: information not available Unit of analysis: patient Sample size calculation: study was designed with 90% power at 0.05 significance with minimum detectable difference of 25% for bundle 1 (early hours) and 35% for bundle 2 (rehabilitation) in relative increased uptake between the control and intervention. Using data from a national stroke audit, the ICC was estimated at 0.149 for bundle 1 and 0.217 for bundle 2. It was estimated that for improvement in bundle 1 to reach significance, 24 hospitals (12 in either arm) would be required, and for improvement in bundle 2 to reach significance, 19 hospitals (10 in either arm) would be required. |
|
| Participants |
Healthcare professionals: 21 hospital trusts with multidisciplinary teams comprising radiographers, stroke co‐ordinators, specialist stroke nurses, occupational therapists, physiotherapists and healthcare assistants. Number, disciplines and demographics of therapists within clusters not provided. Patients: stroke survivors (n = 6592) Experimental group: n = 3533, 48.2% men, mean age not available Control group: n = 3059, 47.3% men, mean age not available Ethnicity: not described Setting: inpatient wards in hospitals Country: UK |
|
| Interventions |
Targeted behaviour change: for multidisciplinary teams to adhere to a 'bundle' of care involving 9 processes (e.g. timely assessment and goal setting) Theory used: quality improvement collaborative based on the Breakthrough Series (BTS) model. No implementation theory mentioned. Category of implementation intervention: implementation strategies Multifacetedintervention: yes Tailoring to barriers: not described Description of intervention: intervention involving interprofessional education, continuous quality improvement, audit and feedback (see Appendix 2 for full details of intervention) Delivery: assumed face‐to‐face training sessions (not specified) and face‐to‐face mentoring visits. Web‐based portal used for access to 90:10 project director and improvement advisor. Frequency: 1 × 2‐day and 2 × 1‐day learning sessions, 90 days apart. Weekly online sharing and learning sessions Duration of intervention: 12 months Control: no intervention. The control groups participated in the 90:10 quality improvement collaborative 1 year after the intervention group, with the intervention group helping the control group to learn. |
|
| Outcomes |
Main outcome: Patient outcomes: none reported Quality of care: compliance with care bundle 1 (early hours) and compliance with care bundle 2 (rehabilitation) Secondary outcomes:
Follow‐up: percentage compliance with bundle at baseline and 12 months Loss of clusters and individuals: 3 clusters lost following randomisation, 2 withdrawn by senior leaders after randomisation and 1 merged with another organisation. More patient records unavailable in control group (723 records) compared with intervention group (244 records). Adjusted for clustering for each outcome: yes Method of cluster adjustment for each outcome: logistic regression model was fitted with a random‐effects term to take account of clustering at hospital level. ICC reported for each outcome: yes |
|
| Notes |
Outcomes used in this review: compliance with care bundle 2 (rehabilitation) Unit of analysis error: no Ethical approval and informed consent obtained: ethics approval was obtained from Tameside & Glossop Local Research Ethics Committee (Ref: 08/H1013/55). Authors did not report on informed consent. Funding source: The Health Foundation (charity no. 286967). Declarations of interest: 2 authors worked for the Health Foundation and contributed to the design of the study but had no undue influence over the data or interpretation. Contact with author? no (contact attempted, no response) Additional outcome data provided from author? no This review used rehabilitation bundle results, acute bundle results are included in another review (Luker 2017). Trial registration: ISRCTN13893902 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Hospitals were stratified by performance and within each group, a computer‐generated list was used to randomly allocate hospitals to intervention or control group. |
| Allocation concealment (selection bias) | Low risk | Central service decided on allocation after hospitals were recruited. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Staff participants were unblinded. Unclear whether patient participants were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessment data collected by staff at participating sites who were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss of clusters: 6/24 (25%) clusters not included in final analysis due to withdrawal, cluster loss equal in both groups. Missing patient data in groups were different (11% for intervention group vs 23% for control group), many more patient medical records unavailable in control group (723) compared to intervention (244). Some sites with incomplete data were not included in analysis. |
| Selective reporting (reporting bias) | High risk | Trial registered retrospectively, some outcomes proposed in protocol (e.g. Modified Rankin Scale and length of stay) were not reported. |
| Other bias | Unclear risk | Recruitment bias: low risk Incorrect analysis: low risk Similar baseline characteristics: low risk, no significant baseline differences between groups, similar attributes across intervention and control groups for sex, comorbidities and risk factors. More patients in intervention group had comorbidity from myocardial infarction, unlikely to have significant impact on study. Similar baseline outcome measures: unclear risk, baseline outcome measures for some components appeared different between groups although not clear if significant. Possible cluster‐level differences in practice at baseline. Reliability of primary outcome measures: low risk, outcome measure was from medical record check regarding compliance with aspects of bundle, straightforward yes/no answer whether each process element was met. Method the same as process used in national audit. Adequate protection against contamination: low risk, allocation by hospital reduced likelihood of contamination. Though all sites were geographically in the Northwest of England, there is no indication of contact between clusters. |
Salbach 2017.
| Study characteristics | ||
| Methods |
Aim of study: to implement 2 KT interventions and evaluate whether a multimodal, facilitated KT approach to implementing a stroke rehabilitation guideline was more likely than passive strategies to improve patient function in the inpatient rehabilitation setting Study design: cluster randomised trial Unit of randomisation: stroke rehabilitation unit Mean cluster size: mean cluster sizes preintervention: Intervention group: 15 (SD 8) Control group: 10 (SD 7) Unit of analysis: healthcare professional Sample size calculation: No calculation for sample size except post hoc power calculations. Analysis of treatment implementation by nurses and therapists: given 1381 observations, mean patient‐level ICC 0.12 and mean cluster size of 8 observations per patient, with 375 observations per group (2‐sided alpha = 0.05) and a baseline implementation rate of 30%, there was 80% power to detect a between‐group difference of 10% in the rate of treatment implementation. Analysis of treatment implementation by therapists alone: given 547 observations, mean patient‐level ICC of 0.09 and mean cluster size of 4 observations per patient, with 431 independent observations (215 per group) (2‐sided alpha = 0.05) and a baseline implementation rate of 10%, there was 80% power to detect a between‐group difference of 10% in the rate of treatment implementation. |
|
| Participants |
Healthcare professionals: 20 rehabilitation units comprising nurses, occupational therapists and physiotherapists. Number and demographics of staff within clusters not provided. Patients: stroke survivors (n = 312) Intervention group: n = 169 Control group: n = 143 For 7 treatments implemented by nurses and therapists: Facilitated intervention group preintervention: median 62 (IQR 57–77) years, 69% men Facilitated intervention group postintervention: median 68 (IQR 60–78) years, 65% men Passive intervention group preintervention: median 71 (IQR 62–79) years, 52% men Passive intervention postintervention: median 72 (IQR 65–79) years, 57% men For 11 treatments implemented by therapists: Facilitated intervention group preintervention: median 64 (IQR 57–77) years, 68% men Facilitated intervention group postintervention: median 68 (IQR 60–78) years, 65% men Passive intervention preintervention: median 73 (IQR 62–79) years, 54% men Passive intervention postintervention: median 72 (IQR 64–79) years, 55% men Ethnicity: not described Setting: inpatient rehabilitation Country: Canada |
|
| Interventions |
Targeted behaviour change: for staff to increase uptake of best practice recommendations for physical rehabilitation and implement 18 recommended treatments Theory used: intervention development was guided by the KTA process Category of implementation intervention: implementation strategies Multifacetedintervention (yes/no): yes Tailoring to barriers: yes Intervention was tailored to address barriers and facilitators that arose from focus groups when implementation was piloted at 5 inpatient rehabilitation hospitals. During workshop training, site facilitators completed an activity to compare current practice at their site with recommended practice, identify barriers to practice change and develop an implementation plan that incorporated behaviour change strategies to address local challenges to implementation. Description of intervention: educational workshops, interprofessional education, educational materials, clinical practice guidelines with treatment protocols, local opinion leaders, communities of practice (see Appendix 2 for full details of intervention) Delivery: workshops were delivered face‐to‐face, teleconferences and a web‐based platform were provided for facilitators to communicate Frequency: 2‐day workshop. Each facilitator at each site (2 per site) was to spend 4 hours per week promoting guideline implementation Duration of intervention: 16 months Control: control group received a passive KT intervention; they were provided with a version of the SCORE (Systematic COronary Risk Evaluation) guideline without treatment protocols, and a handbook and educational DVD on the use of standardised assessment tools poststroke. |
|
| Outcomes |
Main outcome: Patient outcomes: 6MWT and Box and Block Test (outcomes from a conference abstract as full results are unpublished) Quality of care: change in the percentage of patients for which stroke teams implemented each recommended treatment pre‐ to postintervention Secondary outcome: not reported Follow‐up: preintervention and postintervention (measured after treatment sessions for 2 weeks at each time point, intervention lasted 16 months) Loss of clusters and individuals: 3 clusters excluded due to lack of data Facilitated intervention arm: 4 providers, 4 patients and 43 forms removed because of missing provider ID or patient FIM data Passive intervention arm: 3 providers, 1 patient and 6 forms removed because of missing provider ID or patient FIM data Adjusted for clustering for each outcome: yes Method of cluster adjustment for each outcome: a random‐effects logistic regression analysis was carried out in SAS v9.3 to account for clustering effects at hospital, provider and patient level and covariates ICC reported for each outcome: yes |
|
| Notes |
Outcomes used in this review: change in the percentage of patients for which stroke teams implemented each recommended treatment pre‐ to postintervention, 6MWT and Box and Block Test Unit of analysis error: no Ethical approval and informed consent obtained: yes. The ethics board at each site and affiliated university approved the study protocol. Informed consent was obtained from staff working on each stroke rehabilitation unit. Funding source: study was funded by a grant from the Canadian Stroke Network. Study analysis was funded by the Toronto Rehabilitation Institute University Health Network Declarations of interest: none declared or detected Contact with author? yes Additional outcome data provided from author? no Trial registration: NCT00359593 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Hospitals were stratified by language (English/French) by a biostatistician not involved in study recruitment or data collection. Statistical software (R) was used for stratification and to randomly assign clusters using 1:1 allocation. |
| Allocation concealment (selection bias) | Low risk | Allocation was done centrally, site staff informed of allocation after preintervention data were already collected. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | After preintervention data collection, researchers and study participants were aware of allocated groups. It is unclear whether patients were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessors were unblinded and used a self‐report checklist of their own performance. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss of clusters: 3 sites excluded (1 in facilitated and 2 in passive intervention) after allocation and preintervention due to technical issues and could not/did not have data to provide. No clusters lost after intervention. Only completed checklists were analysed. 43 forms removed in intervention group due to missing provider ID or patient FIM data. Checklist completion by providers between groups appeared similar postintervention. |
| Selective reporting (reporting bias) | High risk | Outcomes listed in trial registry are different to process measures reported as this study is not the main results paper (results paper in press). |
| Other bias | Low risk | Recruitment bias: low risk Incorrect analysis: low risk Similar baseline characteristics: low risk, site characteristics similar between the 2 intervention arms. Similar baseline outcome measures: preintervention percentages implemented (outcome measures) varied between groups but was not discussed. Unsure if significantly different. Reliability of primary outcome measures: low risk. Self‐report measures of practice may be vulnerable to over‐reporting, authors of the study noted this as a limitation though this would be expected to affect both groups similarly, as site staff were not aware of the study's hypothesis. Adequate protection against contamination: low risk, geographical separation between sites, the study was conducted in 20 sites in 19 cities across Canada (confirmed with trial authors). |
Strasser 2008.
| Study characteristics | ||
| Methods |
Aim of study: to determine the effectiveness of a team training intervention in improving patient outcomes Study design: cluster randomised trial Unit of randomisation: rehabilitation unit Mean cluster size: information not available Unit of analysis: patient Sample size calculation: study was designed with 80% power to detect a difference of 6 points in primary outcome measure (motor FIM) between intervention and control groups. ICC in motor FIM score gain was estimated to be 4.6%, (SD 12.8%) using data from 45 rehabilitation sites participating in a previous observational study by author. Assuming 20 patients per site, it was anticipated 28 sites would be required. |
|
| Participants |
Healthcare professionals: 31 rehabilitation teams, comprising 464 staff of 6 disciplines; medicine, nursing, occupational therapy, speech‐language pathology, physiotherapy and social work/case management. Demographics of staff within all clusters not provided. Patients: stroke survivors (n = 1368) < 90 days' poststroke Preintervention Experimental group: n = 350, 98.3% men, mean age 65.9 (SD 11.4) years Control group: n = 439, 96.8% men, mean age 66.6 (SD 12.0) years Postintervention Experimental group: n = 233, 96.6% men, mean age 67.6 (SD 11.1) years Control group: n = 346, 97.4% men, mean age 66.9 (SD 11.9) years Ethnicity: Preintervention Experimental group: white 57.7%, black 30.9%, Hispanic 8.9%, Asian 0.6%, Native American 0.6%, other/unknown 1.5% Control group: white 55.6%; black 24.6%; Hispanic 17.1%; Asian 1.1%; Native American 1.1%, other/unknown 0.4% Postintervention Experimental group: white 62.7%; black 22.7%; Hispanic 9.4%; Asian 0.9%; Native American 0.9%; other/unknown 3.4% Control group: white 53.8%; black 24.6%; Hispanic 19.9%; Asian 0.6%; Native American 0.3%; other/unknown 0.9% Setting: inpatient (acute or subacute) Country: USA |
|
| Interventions |
Targeted behaviour change: for multidisciplinary staff to function more effectively as a team Theory used: Lichstein's treatment implementation model Category of implementation intervention: delivery arrangement intervention/implementation strategies Multifacetedintervention: yes Tailoring to barriers: yes Implementation action plans were modified according to perceived barriers by team leaders Description of intervention: interprofessional education, local opinion leaders, audit and feedback (see Appendix 2 for full details of intervention) Delivery: off‐site, face‐to‐face workshops conducted by research staff Frequency: 1 × 2.5‐day workshop (16 hours), written feedback 3–5 weeks after workshop, consultation 2–3 months after written feedback Duration of intervention: 6 months Control: received summary of performance on process measures taken pre‐ and postintervention |
|
| Outcomes |
Main outcome: Patient outcomes: functional improvement measured by change in the motor items of Functional Independence Measure (FIM) Quality of care outcomes: not reported Secondary outcome: patient length of stay and percentage discharged home from inpatient rehabilitation Follow‐up: 12‐months after completion of the intervention Loss of clusters and individuals: 4/31 clusters lost due to incomplete ethics process (1) and unreported data (3) Adjusted for clustering for each outcome: yes Method of cluster adjustment for each outcome: cluster‐adjusted Chi2 t‐tests used in analysis ICC reported for each outcome: no |
|
| Notes |
Outcomes used in this review:
Unit of analysis error: no Ethical approval and informed consent obtained (yes/no): site ethics obtained from local Veterans Affairs research committees and institutional review boards. Informed consent not reported. Funding source: supported by Veterans Administration Rehabilitation Research and Development Service Declarations of interest: no conflict of interest reported. Some evidence cited to support implementation research was author's own work. Contact with author? yes Additional outcome data provided from author? no Trial registration: NCT00237757 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Computer' used to randomise sites to intervention or control groups (process not described) Clusters were stratified into 4 strata according to volume of patients and FIM scores. Each stratum 'force randomised' to have 4 sites in 1 arm. |
| Allocation concealment (selection bias) | Low risk | Concealment of allocation prior to assignment not described, computer used for randomisation. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Information on blinding not available. Staff likely unblinded due to participating or not participating in training. Patients likely blinded to group allocation as data taken from an outcomes database (Veterans Affairs Functional Status Outcomes Database) and was information collected in usual practice. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | FIM scores assessed by staff who were likely unblinded to group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 clusters lost overall following randomisation (3 in control, 1 in intervention). 2/16 control sites did not report postintervention data, only patients with complete data (FIM) were eligible for inclusion so no patient loss to follow‐up. The proportion of missing data was less than the effect size and therefore unlikely to overturn the study result. |
| Selective reporting (reporting bias) | Unclear risk | No published protocol, study registered retrospectively on ClinicalTrials.gov. Secondary outcomes not mentioned in registered project details. |
| Other bias | Unclear risk | Recruitment bias: low risk Incorrect analysis: unclear whether clustering accounted for in analysis. Similar baseline characteristics: unclear risk, comparable characteristics of sites from available information. Details of sites and composition of teams not provided. Similar baseline outcome measures: unclear risk, control sites had patients with a lower initial motor FIM score (mean: 43.5 control group vs 48.2 intervention group) at baseline. Reliability of primary outcome measures: low risk. FIM has demonstrated reliability and validity, though could be questioned as an adequate measure of team effectiveness. Adequate protection against contamination: low risk, allocation was by rehabilitation unit at separate hospitals. |
Thomas 2015.
| Study characteristics | ||
| Methods |
Aim of study: to assess the feasibility of a full‐scale cluster randomised trial and conduct a preliminary evaluation of supported implementation of a systematic voiding programme for incontinence compared to usual care Study design: cluster randomised trial Unit of randomisation: stroke service Mean cluster size: information not available. 4 clusters in each arm. Unit of analysis: patient Sample size calculation: sample size chosen pragmatically rather than based on formal sample size calculation. The aim was to balance practicalities and the need for 'reasonable precision' in the estimation of effects to inform the sample size calculation. Expected to recruit 780 patients across the 12 services. |
|
| Participants |
Healthcare professionals: 12 stroke services comprising nurses (ranging in grade from healthcare assistants to ward managers), occupational therapists and physiotherapists. Demographics of staff within clusters not provided. Patients: stroke survivors (n = 413) Intervention A: n = 164, 52% men, median age 77 (IQR 68–83) years Intervention B: n = 125, 42% men, median age 81 (IQR 74–85) years Control: n = 124, 41% men, median age 80 (IQR 72–86) years Ethnicity: Intervention A: white 96% Intervention B: white 95% Control: white 99% Setting: inpatient stroke units (acute and rehabilitation) Country: UK |
|
| Interventions |
Targeted behaviour change: for staff to deliver a systematic voiding programme with stroke survivors with incontinence Theory used: Normalisation Process Theory Category of implementation intervention: delivery arrangements and implementation strategies Multifacetedintervention: yes Tailoring to barriers: yes Semistructured interviews conducted with staff to identify barriers to successful implementation Description of intervention: Intervention A: interprofessional education, educational materials Intervention B: interprofessional education, educational materials, local opinion leaders, educational outreach visits (See Appendix 2 for full details of intervention) Delivery: Intervention A: training mainly web‐based, face‐to‐face sessions also offered to staff Intervention B: training as per Intervention A. Educational outreach (External facilitators) provided support through a mixture of face‐to‐face meeting, teleconferences and e‐mail correspondence Frequency: information not available Duration of intervention: unclear Control: no intervention |
|
| Outcomes |
Main outcome: Patient outcomes: presence or absence of urinary incontinence measured by ICIQ‐UI Short Form Secondary outcomes:
Follow‐up: 6, 12 and 52 weeks' poststroke Loss of clusters and individuals: no clusters lost to follow‐up. Adjusted for clustering for each outcome: authors stated little or no evidence of clustering effects, with ICC estimates mostly being very close to 0 Method of cluster adjustment for each outcome: mixed‐effects modelling for continuous, ordinal and dichotomous outcomes were used to compare the 2 groups on primary outcome data and account for clustering ICC reported for each outcome: yes |
|
| Notes |
Outcomes used in this review:
We pooled death rates from this trial as per guidance in the Cochrane Handbook for Systematic Reviews of Interventions Section 23.1.4.1 and applied a design effect to the number of participants and events to account for the clustering. Unit of analysis error: no Ethical approval and informed consent obtained (yes/no): yes. Local research ethical approval was granted by Bolton Research Ethics Committee (09/H1009/15). Approval was also obtained from the University of Central Lancashire Faculty of Health and Social Care Ethics Committee (FHEC) (CA 138). Informed consent obtained from all patients. Funding source: National Institute for Health Research Programme Grants for Applied Research programme. Declarations of interest: 1 author was a member of the National Institute for Health Research (NIHR) Programme Grants for Applied Research subpanel and a member of the NIHR Journals Editorial Board. Contact with author? yes Additional outcome data provided from author? no Trial registration: ISRCTN08609907 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Trial arms stratified into 4 strata, randomisation schedule was then generated using block randomisation to allocate 1 site to each arm within each stratum, using computer software package Stata. |
| Allocation concealment (selection bias) | Unclear risk | Allocation based on clusters, stroke services not informed of intervention allocation until all services recruited. Within each stratum, stroke services were not aware of their allocation until all within that stratum were recruited for trial. However, when 2 sites required substitution, the rest of the stratum were already aware of their allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | After recruitment, all patients, recruiting staff and stroke services were aware of allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Research nurses completed outcome measures, they were not blinded. They originally planned to collect data in sites other than their own to assure blinding; however, this was ultimately not possible due to geographical location. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No clusters lost. Attrition rates between groups were similar at 12 weeks (the primary point of analysis) (24.2% Intervention A, 24% Intervention B, 26.5% control). Overall response rate for questionnaires were similar (97% Intervention A, 92% Intervention B, 98% control). |
| Selective reporting (reporting bias) | Low risk | Published protocol, all proposed primary and secondary outcomes reported. |
| Other bias | High risk | Recruitment bias: low risk Incorrect analysis: low risk Similar baseline characteristics: high risk, some differences in baseline characteristics, e.g. more males in intervention group A (52%) compared with control group (41%) and intervention group B (42%). The proportion of participants with no symptoms on the modified Rankin Scale was slightly higher in the control group (42%) compared with intervention group A (33%) and intervention group B (27%). There were also fewer patients with the most severe stroke subtype (total anterior circulation syndrome) in the control group (29.8%) compared with intervention group A (48.8%) and intervention group B (54.4%). Authors discussed that there may have been some consent and recruitment bias by research nurses. Similar baseline outcome measures: low risk, similar continence status at baseline between groups (% incontinent at baseline: 85% intervention group A, 89% intervention group B, 90% control group). Reliability of primary outcome measures: low risk, ICIQ‐UI Short Form is considered to have acceptable reliability, validity and responsiveness. The authors conducted a preliminary validation of the tool with 6 stroke survivors and found it appropriate. Adequate protection against contamination: low risk, allocation by stroke service. |
CBS: Carer Burden Scale; CTRU: Clinical Trials Research Unit; ECAQ: Elderly Cognitive Assessment Questionnaire; EQ‐5D: European Quality of Life‐5 Dimensions; EQ‐5D‐3L: 3‐level version of EQ‐5D; FAI: Frenchay Activities Index; GHQ‐12: General Health Questionnaire‐12; HADS: Hospital Anxiety and Depression Scale; iCaPPES: integrated Care Pathway for Post Stroke; ICC: intraclass correlation coefficient; ICIQ‐UI: International Consultation on Incontinence Questionnaire – Urinary Incontinence; IQR: interquartile range; ISI: Incontinence Severity Index; ITT: intention to treat; KT: knowledge translation; KTA: Knowledge to Action; LoTS: Longer‐Term Stroke; LSCTC: London Stroke Carers Training Course; LUNS: Longer‐term Unmet Needs after Stroke; M‐MMSE: Modified Mini‐Mental State Examination; MRC: Medical Research Council; n: number of participants; NEADL: Nottingham Extended Activities of Daily Living; NHMRC: National Health and Medical Research Council; NHS: National Health Service; PHQ‐9: Patient Health Questionnaire‐9; SD: standard deviation; SIS: Stroke Impact Scale; TQWHQ: Two Questions With Help Questionnaire.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ab Malik 2017 | Not stroke rehabilitation. |
| Allen 2002 | Ineligible study design. Randomised trial at 1 site. |
| Allen 2009 | Ineligible study design. Randomised trial at 1 site. |
| Banta 2012 | Ineligible study design. Before‐after study at 1 site. |
| Barrett 2016 | Ineligible study design. Before‐after study at 1 site. |
| Bates 2000 | Ineligible study design. Before‐after study at 1 site. |
| Beckerman 2004 | Not an implementation study. |
| Bjartmarz 2017 | Ineligible study design. Uncontrolled before‐after study. |
| Bland 2013 | Ineligible study design. Retrospective cohort study. |
| Booth 2005 | Ineligible study design. Non‐randomised trial with 1 intervention and 1 control site. |
| Burton 2005 | Not an implementation study. |
| Cheung 2012 | Ineligible study design. Uncontrolled before‐after study. |
| Connell 2016 | Ineligible study design. Uncontrolled before‐after study. |
| Fedai 2006 | Study design. Uncontrolled before‐after study. |
| Fisher 2014 | Study design. Uncontrolled before‐after study. |
| Glegg 2017 | Does not meet protocol definition of stroke rehabilitation. |
| Horton 2016 | Ineligible study design. Non‐randomised trial with 1 intervention and 1 control site. |
| Jensen 2015 | Ineligible study design. Uncontrolled before‐after study. |
| Jolliffe 2019 | Ineligible study design. Cluster randomised trial at 3 sites. |
| Jones 1998 | Does not meet definition of evidence‐based practice. |
| Jones 2005 | Does not meet definition of evidence‐based practice. |
| Kristensen 2014 | Ineligible study design. Uncontrolled study at 2 sites. |
| Lakshminarayan 2009 | Did not meet protocol definition of stroke rehabilitation. |
| Levac 2016a | Ineligible study design. Uncontrolled before‐after study. |
| Levac 2016b | Ineligible study design. Uncontrolled before‐after study. |
| Lynch 2016 | Does not meet protocol definition of stroke rehabilitation. |
| Markle‐Reid 2011 | Not an implementation study. |
| McEwan 2019 | Ineligible study design. Uncontrolled before‐after study. |
| Middleton 2011 | Did not meet protocol definition of stroke rehabilitation. |
| O'Connor 2009 | Ineligible study design. Uncontrolled before‐after study. |
| Pandey 2006 | Did not meet protocol definition of stroke rehabilitation. |
| Panella 2012 | Did not meet protocol definition of stroke rehabilitation. |
| Perry 2000 | Ineligible study design. Uncontrolled before‐after study. |
| Petzold 2011 | Ineligible study design. Uncontrolled repeated measures study. |
| Ranta 2015 | Did not meet protocol definition of stroke rehabilitation. |
| Richardson 2011 | Ineligible study design. Uncontrolled before‐after study. |
| Sulch 2000 | Ineligible study design. Randomised trial involving only 1 site. |
| Van Peppen 2009 | Ineligible study design. |
| Vratsistas‐Curto 2017 | Ineligible study design. Uncontrolled before‐after study. |
| Wielaert 2018 | Ineligible study design. Uncontrolled before‐after study. |
| Wilks 2014 | Ineligible study design. Non‐randomised trial at 1 site. |
| Willems 2016 | Ineligible study design. Uncontrolled before‐after study. |
| Williams 2016 | Did not meet protocol definition of stroke rehabilitation. |
Characteristics of ongoing studies [ordered by study ID]
Duncan 2017.
| Study name | Early supported discharge for improving functional outcomes after stroke. The Comprehensive Post‐Acute Stroke Services (COMPASS) study |
| Methods | Cluster‐randomised pragmatic trial |
| Participants | English and Spanish speaking stroke patients aged > 18 years with diagnosis of ischaemic stroke, haemorrhagic stroke or transient ischaemic attack who are discharged home from participating hospitals |
| Interventions | COMPASS Intervention A postacute co‐ordinator will visit each patient prior to discharge from the hospital. Patient will receive a follow‐up telephone call 2 days after having been discharged. 7–14 days after discharge, the patient will attend postacute stroke clinic visit and receive an assessment from an advanced practice provider, a brief patient‐reported functional assessment to generate an individualised care plan, and referrals from an advanced practice provider. The patient's primary carer will be assessed to ensure availability and ability to support the patient and the carer's ability to cope with the new challenges of caring. Patient will receive a call at 30 and 60 days' postdischarge for follow‐up of functional status, recovery, risk factor management and their access or utilisation of recommended services. |
| Outcomes | Primary outcome: Stroke Impact Scale‐16 |
| Starting date | July 2016 |
| Contact information | Principal Investigator: Pamela Duncan, Wake Forest University Health Sciences |
| Notes | ClincalTrials.gov Identifier: NCT02588664 COMPASS Study website: www.nccompass-study.org/ Expected study completion: November 2020 |
McEwen 2015.
| Study name | A multifaceted knowledge translation approach to support persons with stroke and cognitive impairment: evaluation protocol |
| Methods | 3 inter‐related studies, 1 an interrupted time series with 28 time points prior to intervention and 15 time points after intervention |
| Participants | 5 inpatient stroke rehabilitation units; study will involve occupational therapists, physiotherapists and speech pathologists. Data will be collected from data from patients aged ≥ 18 years who have completed inpatient rehabilitation with a primary diagnosis of stroke. 34 stroke participants expected. |
| Interventions | The implementation intervention will involve educational meetings (on the COOP approach), implementation facilitators and a virtual community of practice. |
| Outcomes | Stroke unit level data
|
| Starting date | 1 January 2016 |
| Contact information | Lead author: sara.mcewen@utoronto.ca |
| Notes | ClinicalTrials.gov Identifier: NCT02597569 Study completion date listed as 31 August 2018 First publication from this study listed in excluded studies table (McEwan 2019). |
NCT03807115.
| Study name | Effects of an educational intervention on rehabilitation clinicians' practices for health‐related outcomes after stroke |
| Methods | Pilot stepped‐wedge cluster randomised trial |
| Participants | Occupational therapists and physiotherapists with a minimum of 1 year of clinical experience, working in an in‐patient stroke rehabilitation centre in Canada. Patients with a documented walking deficit (documented in patient's chart) and who are on the caseload of ≥ 1 participating clinician. |
| Interventions | Implementation of stroke mobility guidelines which will involve: delivery of weekly online educational capsules on 4 evidence‐based stroke recommendations (motor imagery/mental practice, rhythmic auditory stimulation gait therapy, task‐oriented training including fitness and mobility exercises, and aerobic training) plus feedback on participant's awareness, agreement, satisfaction with, and perceived value of the content, perceived implementation success and facilitators and barriers encountered. |
| Outcomes | Primary outcomes:
|
| Starting date | 9 September 2019 |
| Contact information | Principal investigator: Aliki Thomas, McGill University Project contact: Heather Owens, heather.owens@mcgill.ca |
| Notes | Expected study completion date: 31 March 2020 |
Differences between protocol and review
Randomised studies with only one intervention and control site were excluded. This is consistent with Cochrane EPOC guidance.
Patients were included as a participant group as their health outcomes are an important measure of uptake of evidence‐based practices by healthcare professionals.
Four review authors (CN, EL, CM JH) were added to the author group, their roles are reflected in the Contributions of authors section.
Contributions of authors
LSC: conceived the review.
LSC, LMC, NAL and DO: designed the review.
DO: conceived the protocol outline.
LSC: led the writing of the protocol.
LSC, MT, CN, EL and CM: screening of titles for inclusion.
LSC, CN, JH and MT: data extraction, 'risk of bias' assessment.
LSC and DO: grading of the evidence.
LSC and DO: led the writing of the review.
LMC, DO, NAL, MT, CN, CM, EL and JH: provided critical feedback on drafts of the protocol and review.
Sources of support
Internal sources
-
La Trobe University, Australia
LSC and MT were supported by Postgraduate Scholarships
LMC was supported by a Building Healthy Communities Grant (#1023514)
External sources
-
James S. McDonnell Foundation 21st Century Science Initiative in Cognitive Rehabilitation, USA
LMC was supported by a Collaborative Award (#220020413)
-
National Health and Medical Research Council, Australia
LMC was supported by a NHMRC Partnership Grant (#1134495); A network of sites and 'up‐skilled' therapists to deliver best practice stroke rehabilitation of the upper limb. LMC was also supported by a Project Grant (#1022694) and Career Development Award (#307905)
NAL was supported by a Translating Research into Practice (TRIP) Fellowship (#1112158)
DO was supported by a Translating Research into Practice Fellowship (APP1168749)
-
Australian Research Council, Australia
LMC was supported by a Future Fellowship (#FT0992299)
-
Victorian Government, Australia
LMC was supported by a Victorian Government Operational Infrastructure Support Program
Declarations of interest
LSC: supported by an Australian Government Research Training Program Scholarship and received a small grant from La Trobe University for travel costs to attend an interstate Cochrane Training Workshop.
LMC: none.
NAL: none.
MT: supported by an Australian Government Research Training Program Scholarship
CN: none.
EL: none.
CM: none.
JH: assistant managing editor with the Cochrane EPOC Group but not involved in editorial decisions regarding this review.
DO: editor with the Cochrane EPOC Group and director of the Australasian EPOC Satellite but not involved in editorial decisions regarding this review.
New
References
References to studies included in this review
Abdul Aziz 2014 {published and unpublished data}
- Abdul Aziz AF, Ali MF, Che' Man Z, Nordin NA. The screening for post stroke dementia among stroke survivors residing at home in the community: findings from the iCaPPS trial in peninsular Malaysia. Cerebrovascular Diseases 2016;42(Suppl 1):1-57. [Google Scholar]
- *.Abdul Aziz AF, Muhammad Nur A, Sulong S, Aljunid S. The integrated care pathway for managing post stroke (ICaPPS) patients in the community: a cost-effectiveness analysis. Value in Healthcare 2014;17:A719-A813. [DOI] [PubMed] [Google Scholar]
- Abdul Aziz AF, Nordin NA, Ali MF, Aziz NA, Sulong S, Aljunid SM. The integrated care pathway for post stroke patients (iCaPPS): a shared care approach between stakeholders in areas with limited access to specialist stroke care services. BMC Health Services Research 2017;17(1):1-11. [DOI: 10.1186/s12913-016-1963-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
Forster 2013 {published data only}49208824
- Clarke DJ, Godfrey M, Hawkins R, Sadler E, Harding G, Forster A, et al. Implementing a training intervention to support caregivers after stroke: a process evaluation examining the initiation and embedding of programme change. Implementation Science 2013;8(1):96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke DJ, Hawkins R, Sadler E, Harding G, McKevitt C, Godfrey M, et al. Introducing structured caregiver training in stroke care: findings from the TRACS process evaluation study. BMJ Open 2014;4(4):e004473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster A, Dickerson J, Melbourn A, Steadman J, Wittink M, Young J, et al. TRACS trial collaboration. The development and implementation of the structured training programme for caregivers of inpatients after stroke (TRACS) intervention: the London Stroke Carers Training Course. Clinical Rehabilitation 2015;29(3):211-20. [DOI] [PubMed] [Google Scholar]
- Forster A, Dickerson J, Young J, Patel A, Kalra L, Nixon J, et al. A cluster randomised controlled trial and economic evaluation of a structured training programme for caregivers of inpatients after stroke: the TRACS trial. Health Technology Assessment 2013;17(46):1-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Forster A, Dickerson J, Young J, Patel A, Kalra L, Nixon J, et al. A structured training programme for caregivers of inpatients after stroke (TRACS): a cluster randomised controlled trial and cost-effectiveness analysis. Lancet 2013;382(9910):2069-76. [DOI] [PubMed] [Google Scholar]
- Forster A, Young J, Nixon J, Kalra L, Smithard D, Patel A, et al. A cluster randomized controlled trial of a structured training programme for caregivers of inpatients after stroke (TRACS). International Journal of Stroke 2011;7(1):94-9. [DOI] [PubMed] [Google Scholar]
Forster 2015 {published data only}67932305
- Forster A, Mellish K, Farrin A, Bhakta B, House A, Hewison J, et al. Development and evaluation of tools and an intervention to improve patient- and carer-centred outcomes in Longer-Term Stroke care and exploration of adjustment post stroke: the LoTS care research programme. Programme Grants for Applied Research 2014;2(6):1-262. [PubMed] [Google Scholar]
- *.Forster A, Young J, Chapman K, Nixon J, Patel A, Holloway I, et al. Cluster randomized controlled trial clinical and cost-effectiveness of a system of longer-term stroke care. Stroke 2015;46:2212-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster A, Young J, Nixon J, Chapman K, Murray J, Patel A, et al. Protocol of a cluster randomized trial evaluation of a patient and carer-centered system of longer-term stroke care (LoTS care). International Journal of Stroke 2015;10(2):259-63. [DOI] [PubMed] [Google Scholar]
McCluskey 2016 {published data only}
- *.McCluskey A, Ada L, Kelly PJ, Middleton S, Goodall S, Grimshaw J, et al. A behavior change program to increase outings delivered during therapy to stroke survivors by community rehabilitation teams: the Out-and-About trial. International Journal of Stroke 2016;11(4):425-37. [DOI] [PubMed] [Google Scholar]
- McCluskey A, Ada L, Kelly PJ, Middleton S, Goodall S, Grimshaw J, et al. Corrigendum: a behavior change program to increase outings delivered during therapy to stroke survivors by community rehabilitation teams: the Out-and-About trial. International Journal of Stroke 2019. [DOI: 10.1177/1747493019888613] [DOI] [PubMed]
- McCluskey A, Ada L, Middleton S, Kelly PJ, Goodall S, Grimshaw JM, et al. Improving quality of life by increasing outings after stroke: study protocol for the Out-and-About trial. International Journal of Stroke 2013;8(1):54-8. [DOI] [PubMed] [Google Scholar]
Pennington 2005 {published data only}
- Burton C, Pennington L, Roddam, H, Russell I, Russell D, Krawczyk K, et. Assessing adherence to the evidence base in the management of poststroke dysphagia. Clinical Rehabilitation 2006;20:46-51. [DOI] [PubMed] [Google Scholar]
- *.Pennington L, Roddam H, Burton C, Russell I, Godfrey C, Russell D. Promoting research use in speech and language therapy: a cluster randomized controlled trial to compare the clinical effectiveness and costs of two training strategies. Clinical Rehabilitation 2005;19:387-97. [DOI] [PubMed] [Google Scholar]
Power 2014 {published data only}13893902
- Carter P, Ozieranski P, McNicol S, Power M, Dixon-Woods M. How collaborative are quality improvement collaboratives: a qualitative study in stroke care. Implementation Science 2014;9(32):1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Power M, Tyrrell PJ, Rudd AG, Tully MP, Dalton D, Marshall M, et al. Did a quality improvement collaborative make stroke care better? A cluster randomized trial. Implementation Science 2014;9(1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Salbach 2017 {published data only (unpublished sought but not used)}
- Bayley M, Mayo NE, Richards C, Eng J, Wood-Dauphinee S. Stroke Canada optimization of rehabilitation by evidence (score): detailed analysis of the effect on outcomes of practice by guidelines vs. practice by outcomes, a clustered randomized trial. Stroke 2012;43(11):E119-20. [Google Scholar]
- Munce SE, Graham ID, Salbach NM, Jaglal SB, Richards CL, Eng JJ, et al. Perspectives of health care professionals on the facilitators and barriers to the implementation of a stroke rehabilitation guidelines cluster randomized controlled trial. BMC Health Services Research 2017;17(1):440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Salbach NM, Wood-Dauphinee S, Desrosiers J, Eng JJ, Graham ID, Jaglal SB, et al. Facilitated interprofessional implementation of a physical rehabilitation guideline for stroke in inpatient settings: process evaluation of a cluster randomized trial. Implementation Science 2017;12(1):100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Strasser 2008 {published data only}
- Stevens AB, Strasser DC, Uomoto J, Bowen SE, Falconer JA. Utility of treatment implementation methods in clinical trial with rehabilitation teams. Journal of Rehabilitation Research and Development 2007;44(4):537. [DOI] [PubMed] [Google Scholar]
- Strasser DC, Burridge AB, Falconer JA, Herrin J, Uomoto J. Measuring team process for quality improvement. Topics in stroke rehabilitation 2010;17(4):282-93. [DOI] [PubMed] [Google Scholar]
- *.Strasser DC, Falconer JA, Stevens AB, Uomoto JM, Herrin J, Bowen SE, et al. Team training and stroke rehabilitation outcomes: a cluster randomized trial. Archives of Physical Medicine and Rehabilitation 2008;89(1):10-5. [DOI] [PubMed] [Google Scholar]
Thomas 2015 {published data only}ISRCTN08609907
- French B, Thomas LH, Harrison J, Burton CR, Forshaw D, Booth J, et al, ICONS project team and the ICONS Patient, Public and Carer Involvement Groups. Implementing a systematic voiding program for patients with urinary incontinence after stroke. Qualitative Health Research 2016;26(10):1393-408. [DOI] [PubMed] [Google Scholar]
- Thomas LH, French B, Burton CR, Sutton C, Forshaw D, Dickinson H, et al. Evaluating a systematic voiding programme for patients with urinary incontinence after stroke in secondary care using soft systems analysis and Normalisation Process Theory: findings from the ICONS case study phase. International Journal of Nursing Studies 2014;51(10):1308-20. [DOI] [PubMed] [Google Scholar]
- *.Thomas LH, French B, Sutton CJ, Forshaw D, Leathley MJ, Burton CR, et al. Identifying Continence OptioNs after Stroke (ICONS): an evidence synthesis, case study and exploratory cluster randomised controlled trial of the introduction of a systematic voiding programme for patients with urinary incontinence after stroke in secondary care. Programme Grants for Applied Research 2015;3:1. [PubMed]
- Thomas LH, Watkins CL, French B, Sutton C, Forshaw D, Cheater F, et al. Study protocol: ICONS: Identifying continence options after stroke: a randomised trial. Trials 2011;12(1):131. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Ab Malik 2017 {published data only}
- Ab Malik N, Mohamad Yatim S, Lam OL, Jin L, McGrath CP. Effectiveness of a web-based health education program to promote oral hygiene care among stroke survivors: randomized controlled trial. Journal of Medical Internet Research 2017;19(3):e87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Allen 2002 {published data only}
- Allen KR, Hazelett S, Jarjoura D, Wickstrom GC, Hua K, Weinhardt J, et al. Effectiveness of a postdischarge care management model for stroke and transient ischemic attack: a randomized trial. Journal of Stroke and Cerebrovascular Diseases 2002;11(2):88-98. [DOI] [PubMed] [Google Scholar]
Allen 2009 {published data only}
- Allen K, Hazelett S, Jarjoura D, Hua K, Wright K, Weinhardt J, et al. A randomized trial testing the superiority of a postdischarge care management model for stroke survivors. Journal of Stroke and Cerebrovascular Diseases 2009;18(6):443-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Banta 2012 {published data only}
- Banta M, Chan A, Devers A, Vaught J, Wilks M. Comparison of acute rehabilitation outcomes in stroke survivors before and after implementation of a clinical practice guideline for walking recovery. Brain Injury 2012;26(4-5):515. [Google Scholar]
Barrett 2016 {published data only}
- Barrett A, Chen P, Hreha K, Alban F, Gocon C, Santos C, et al. Knowledge translation in right brain rehabilitation: a feasibility study of prism treatment after acute stroke. Neurology 2016;86(16 Suppl 1):6. [Google Scholar]
Bates 2000 {published data only}
- Bates BE, Stineman MG. Outcome indicators for stroke: application of an algorithm treatment across the continuum of postacute rehabilitation services. Archives of Physical Medicine and Rehabilitation 2000;81(11):1468-78. [DOI] [PubMed] [Google Scholar]
Beckerman 2004 {published data only}
- Beckerman H, Roelofsen E, Knol D, Lankhorst G. The value of the Rehabilitation Activities Profile (RAP) as a quality sub-system in rehabilitation medicine. Disability and Rehabilitation 2004;26(7):387-400. [DOI] [PubMed] [Google Scholar]
Bjartmarz 2017 {published data only}
- Bjartmarz I, Jonsdottir H, Hafsteinsdottir TB. Implementation and feasibility of the stroke nursing guideline in the care of patients with stroke: a mixed methods study. BMC Nursing 2017;16(1):72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bland 2013 {published data only}
- Bland MD, Sturmoski A, Whitson M, Harris H, Connor LT, Fucetola R, et al. Clinician adherence to a standardized assessment battery across settings and disciplines in a poststroke rehabilitation population. Archives of Physical Medicine and Rehabilitation 2013;94(6):1048-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Booth 2005 {published data only}
- Booth J, Hillier VF, Waters KR, Davidson I. Effects of a stroke rehabilitation education programme for nurses. Journal of Advanced Nursing 2005;49(5):465-73. [DOI] [PubMed] [Google Scholar]
Burton 2005 {published data only}
- Burton C, Gibbon B. Expanding the role of the stroke nurse: a pragmatic clinical trial. Journal of Advanced Nursing 2005;52(6):640-50. [DOI] [PubMed] [Google Scholar]
Cheung 2012 {published data only}
- Cheung D, McKellar J, Parsons J, Lowe M, Willems J, Heus L, et al. Community re-engagement and interprofessional education: the impact on health care providers and persons living with stroke. Topics in Stroke Rehabilitation 2012;19(1):63-74. [DOI] [PubMed] [Google Scholar]
Connell 2016 {published data only}
- Connell LA, McMahon NE, Tyson SF, Watkins CL, Eng JJ. Case series of a knowledge translation intervention to increase upper limb exercise in stroke rehabilitation. Physical Therapy 2016;96(12):1930-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fedai 2006 {published data only}
- Fedai T, Ulas UH, Kavuncubasi S, Demir C, Teke A, Bakir B, et al. The use of clinical pathways in the management of acute stroke patients. Neurology Psychiatry and Brain Research 2006;13(3):115-20. [Google Scholar]
Fisher 2014 {published data only}
- Fisher AR. Development of clinical practice guidelines for urinary continence care of adult stroke survivors in acute and rehabilitation settings. Canadian Journal of Neuroscience Nursing 2014;36(3):16-31. [PubMed] [Google Scholar]
Glegg 2017 {published data only}
- Glegg SM, Holsti L, Stanton S, Hanna S, Velikonja D, Ansley B, et al. Evaluating change in virtual reality adoption for brain injury rehabilitation following knowledge translation. Disability & Rehabilitation: Assistive Technology 2017;12(3):217-26. [DOI] [PubMed] [Google Scholar]
Horton 2016 {published data only}
- Horton S, Clark A, Barton G, Lane K, Pomeroy VM. Methodological issues in the design and evaluation of supported communication for aphasia training: a cluster-controlled feasibility study. BMJ Open 2016;6(4):e011207. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jensen 2015 {published data only}
- Jensen LR, Løvholt AP, Sørensen IR, Blüdnikow AM, Iversen HK, Hougaard A, et al. Implementation of supported conversation for communication between nursing staff and in-hospital patients with aphasia. Aphasiology 2015;29(1):57-80. [Google Scholar]
Jolliffe 2019 {published data only}
- Jolliffe L, Hoffman T, Churilov L, Lannin L. Feasibility of an upper limb implementation package for neurological rehabilitation: a pilot clustered longitudinal cohort study. Australian Occupational Therapy Journal 2019;66(S1):1-149. [Google Scholar]
Jones 1998 {published data only}
- Jones A, Carr EK, Newham DJ, Wilson-Barnett J. Positioning of stroke patients: evaluation of a teaching intervention with nurses. Stroke 1998;29(8):1612-7. [DOI] [PubMed] [Google Scholar]
Jones 2005 {published data only}
- Jones A, Tilling K, Wilson-Barnett J, Newham DJ, Wolfe CD. Effect of recommended positioning on stroke outcome at six months: a randomized controlled trial. Clinical Rehabilitation 2005;19(2):138-45. [DOI] [PubMed] [Google Scholar]
Kristensen 2014 {published data only}
- Kristensen H, Hounsgaard L. Evaluating the impact of audits and feedback as methods for implementation of evidence in stroke rehabilitation. British Journal of Occupational Therapy 2014;77(5):251-9. [Google Scholar]
Lakshminarayan 2009 {published data only}
- Lakshminarayan K, Vazquez G, Borbas C, Luepker RV, Duval S, Anderson DC. A group-randomized trial to improve stroke care in the community. Stroke 2009;40(4):e126-e7. [Google Scholar]
Levac 2016a {published data only}
- Levac DE, Glegg SM, Sveistrup H, Colquhoun H, Miller P, Finestone H, et al. Promoting therapists' use of motor learning strategies within virtual reality-based stroke rehabilitation. PloS One 2016;11(12):e0168311. [DOI] [PMC free article] [PubMed] [Google Scholar]
Levac 2016b {published data only}
- Levac D, Glegg SM, Sveistrup H, Colquhoun H, Miller PA, Finestone H, et al. A knowledge translation intervention to enhance clinical application of a virtual reality system in stroke rehabilitation. BMC Health Services Research 2016;16(1):557. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lynch 2016 {published data only}
- Lynch EA, Cadilhac DA, Luker JA, Hillier SL. Education-only versus a multifaceted intervention for improving assessment of rehabilitation needs after stroke; a cluster randomised trial. Implementation Science 2016;11(1):120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Markle‐Reid 2011 {published data only}
- Markle-Reid M, Orridge C, Weir R, Browne G, Gafni A, Lewis M, et al. Interprofessional stroke rehabilitation for stroke survivors using home care. Canadian Journal of Neuroscience Nursing 2011;38(2):317-34. [DOI] [PubMed] [Google Scholar]
McEwan 2019 {published data only}
- McEwen SE, Donald M, Jutzi K, Allen KA, Avery L, Dawson DR, et al. Implementing a function-based cognitive strategy intervention within inter-professional stroke rehabilitation teams: changes in provider knowledge, self-efficacy and practice. PloS One 2019;14(3):e0212988. [DOI] [PMC free article] [PubMed] [Google Scholar]
Middleton 2011 {published data only}
- Middleton S, McElduff P, Ward J, Grimshaw JM, Dale S, D'Este C, et al. Implementation of evidence-based treatment protocols to manage fever, hyperglycaemia, and swallowing dysfunction in acute stroke (QASC): a cluster randomised controlled trial. Lancet 2011;378(9804):1699-706. [DOI] [PubMed] [Google Scholar]
O'Connor 2009 {published data only}
- O'Connor DA, Moss S. Quality indicators for stroke – the influence of stroke champions on improving outcomes in multi-disciplinary team (MDT) goal planning. International Journal of Stroke 2009;4:37. [Google Scholar]
Pandey 2006 {published data only}
- Pandey DK, Cursio JF, Investigators CS . Data feedback for quality improvement of stroke care: CAPTURE stroke experience. American Journal of Preventive Medicine 2006;31(6 Suppl 2):S224-9. [DOI] [PubMed] [Google Scholar]
Panella 2012 {published data only}
- Panella M, Marchisio S, Brambilla R, Vanhaecht K, Di Stanislao F. A cluster randomized trial to assess the effect of clinical pathways for patients with stroke: results of the clinical pathways for effective and appropriate care study. BMC Medicine 2012;10:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Perry 2000 {published data only}
- Perry L, McLaren SM. An evaluation of implementation of evidence-based guidelines for dysphagia screening and assessment following acute stroke: phase 2 of an evidence-based practice project. Journal of Clinical Excellence 2000;2(3):147-56. [Google Scholar]
Petzold 2011 {published data only}
- Petzold A, Korner-Bitensky N, Salbach N, Ahmed S, Menon A, Ogourtsova T. Increasing knowledge of best practices in occupational therapists treating post-stroke unilateral spatial neglect: a pilot study. Stroke 2011;42(11):e618. [DOI] [PubMed] [Google Scholar]
Ranta 2015 {published data only}
- Ranta A, Dovey S, Weatherall M, O'Dea D, Gommans J, Tilyard M. Cluster randomized controlled trial of TIA electronic decision support in primary care. Neurology 2015;84(15):1545-51. [DOI] [PubMed] [Google Scholar]
Richardson 2011 {published data only}
- Richardson J, Paul V, Wilkins S, Letts L, Bosch J, Wishart L. An evidence based intervention for stroke rehabilitation in the home environment: a knowledge translation study. Physiotherapy 2011;97:eS1046-eS7. [Google Scholar]
Sulch 2000 {published data only}
- Sulch D, Perez I, Melbourn A, Kalra L. Randomized controlled trial of integrated (managed) care pathway for stroke rehabilitation. Stroke 2000;31(8):1929-34. [DOI] [PubMed] [Google Scholar]
Van Peppen 2009 {published data only}
- Van Peppen RP, Schuurmans MJ, Stutterheim EC, Lindeman E, Van Meeteren NL. Promoting the use of outcome measures by an educational programme for physiotherapists in stroke rehabilitation: a pilot randomized controlled trial. Clinical Rehabilitation 2009;23(11):1005-17. [DOI] [PubMed] [Google Scholar]
Vratsistas‐Curto 2017 {published data only}
- Vratsistas-Curto A, McCluskey A, Schurr K. Use of audit, feedback and education increased guideline implementation in a multidisciplinary stroke unit. BMJ Open 2017;6(2):e000212. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wielaert 2018 {published data only}
- Wielaert S, de Sandt-Koenderman M, Dammers N, Sage K. ImPACT: a multifaceted implementation for conversation partner training in aphasia in Dutch rehabilitation settings. Disability and Rehabilitation 2018;40(1):76-89. [DOI] [PubMed] [Google Scholar]
Wilks 2014 {published data only}
- Wilks M, Devers A, Pidcoe P. Implementation of a clinical practice guideline for walking recovery: standardized users improve knowledge translation. Archives of Physical Medicine and Rehabilitation 2014;95(10):e24. [Google Scholar]
Willems 2016 {published data only}
- Willems M, Schroder C, Weijden T, Post MW, Visser-Meily AM. Encouraging post-stroke patients to be active seems possible: results of an intervention study with knowledge brokers. Disability and Rehabilitation 2016;38(17):1748-55. [DOI] [PubMed] [Google Scholar]
Williams 2016 {published data only}
- Williams L, Daggett V, Slaven JE, Yu Z, Sager D, Myers J, et al. A cluster-randomised quality improvement study to improve two inpatient stroke quality indicators. BMJ Quality & Safety 2016;25(4):257-64. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Duncan 2017 {published data only}
- Duncan PW, Bushnell CD, Rosamond WD, Jones Berkeley SB, Gesell SB, D'Agostino RB, et al. The Comprehensive Post-Acute Stroke Services (COMPASS) study: design and methods for a cluster-randomized pragmatic trial. BMC Neurology 2017;17(1):1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
McEwen 2015 {published data only}
- McEwen SE, Donald M, Dawson D, Egan MY, Hunt A, Quant S, et al. A multi-faceted knowledge translation approach to support persons with stroke and cognitive impairment: evaluation protocol. Implementation Science 2015;10(1):157. [DOI: 10.1186/s13012-015-0346-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT03807115 {published data only}
- NCT03807115. Effects of an educational intervention on rehabilitation clinicians' practices for health-related outcomes after stroke. clinicaltrials.gov/show/NCT3807115 (first received 16 January 2019).
Additional references
Arditi 2017
- Arditi C, Rège-Walther M, Durieux P, Burnand B. Computer-generated reminders delivered on paper to healthcare professionals: effects on professional practice and healthcare outcomes. Cochrane Database of Systematic Reviews 2017, Issue 7. Art. No: CD001175. [DOI: 10.1002/14651858.CD001175.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Baker 2015
- Baker R, Camosso-Stefinovic J, Gillies C, Shaw JE, Cheater F, Flottrop S, et al. Tailored interventions to address determinants of practice. Cochrane Database of Systematic Reviews 2015, Issue 4. Art. No: CD005470. [DOI: 10.1002/14651858.CD005470.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bayley 2012
- Bayley MT, Hurdowar A, Richards CL, Korner-Bitensky N, Wood-Dauphinee S, Eng JJ, et al. Barriers to implementation of stroke rehabilitation evidence: findings from a multi-site pilot project. Disability and Rehabilitation 2012;34(19):1633-8. [DOI] [PubMed] [Google Scholar]
Bird 2019
- Bird ML, Miller T, Connell LA, Eng JJ. Moving stroke rehabilitation evidence into practice: a systematic review of randomised controlled trials. Clinical Rehabilitation 2019;33(10):1586-95. [DOI: 10.1177/0269215519847253] [DOI] [PubMed] [Google Scholar]
Cadilhac 2009
- Cadilhac DA, Carter R, Thrift AG, Dewey HM. Estimating the long-term costs of ischemic and hemorrhagic stroke for Australia: new evidence derived from the North East Melbourne Stroke Incidence Study (NEMESIS). Stroke 2009;40(3):915-21. [DOI] [PubMed] [Google Scholar]
Carey 2012
- Carey LM. Stroke Rehabilitation: Insights from Neuroscience and Imaging. New York (NY): Oxford University Press, 2012. [Google Scholar]
Covidence 2018 [Computer program]
- Covidence. Melbourne, Australia: Veritas Health Innovation, 2018. Available at www.covidence.org.
Dewey 2007
- Dewey HH, Sherry LJ, Collier JM. Stroke rehabilitation 2007: what should it be? International Journal of Stroke 2007;2(3):191-200. [DOI] [PubMed] [Google Scholar]
Donner 1981
- Donner A, Birkett N, Buck C. Randomization by cluster sample size requirements and analysis. American Journal of Epidemiology 1981;114(6):906-14. [DOI] [PubMed] [Google Scholar]
EPOC 2013
- Effective Practice and Organisation of Care (EPOC). Data collection form. EPOC resources for review authors. Available from epoc.cochrane.org/epoc-specific-resources-review-authors 2013.
EPOC 2015a
- Effective Practice and Organisation of Care (EPOC). EPOC taxonomy. Available from epoc.cochrane.org/epoc-taxonomy 2015.
EPOC 2015b
- Effective Practice and Organisation of Care (EPOC). Interrupted time series (ITS) analyses. EPOC resources for review authors. Available from epoc.cochrane.org/epoc-specific-resources-review-authors 2015.
EPOC 2016a
- Effective Practice and Organisation of Care (EPOC). What study designs should be included in an EPOC review? EPOC resources for review authors. Available from epoc.cochrane.org/epoc-specific-resources-review-authors 2016.
EPOC 2016b
- Effective Practice and Organisation of Care (EPOC). Suggested risk of bias criteria for EPOC reviews. EPOC resources for review authors. Available from epoc.cochrane.org/epoc-specific-resources-review-authors 2016.
EPPI‐Centre Cost Converter 2019
- Shemilt I, James T, Morciano M. A web-based tool for adjusting costs to a specific target currency and price year. eppi.ioe.ac.uk/costconversion/default.aspx (accessed prior to 5 October 2020).
Evers 2005
- Evers S, Goossens M, De Vet H, Van Tulder M, Ament A. Criteria list for assessment of methodological quality of economic evaluations: consensus on health economic criteria. International Journal of Technology Assessment in Health Care 2005;1(21):240-5. [PubMed] [Google Scholar]
Feigin 2019
- Feigin VL. Anthology of stroke epidemiology in the 20th and 21st centuries: assessing the past, the present, and envisioning the future. International Journal of Stroke 2019;14(3):223-37. [DOI] [PubMed] [Google Scholar]
Flodgren 2019
- Flodgren G, O'Brien MA, Parmelli E, Grimshaw JM. Local opinion leaders: effects on professional practice and healthcare outcomes. Cochrane Database of Systematic Reviews 2019, Issue 6. Art. No: CD000125. [DOI: 10.1002/14651858.CD000125.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Forsetlund 2009
- Forsetlund L, Bjorndal A, Rashidian A, Jamtvedt G, O'Brien MA, Wolf FM, et al. Continuing education meetings and workshops: effects on professional practice and health care outcomes. Cochrane Database of Systematic Reviews 2009, Issue 2. Art. No: CD003030. [DOI: 10.1002/14651858.CD003030.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Giguère 2012
- Giguère A, Légaré F, Grimshaw J, Turcotte S, Fiander M, Grudniewicz A, et al. Printed educational materials: effects on professional practice and healthcare outcomes. Cochrane Database of Systematic Reviews 2012, Issue 10. Art. No: CD004398. [DOI: 10.1002/14651858.CD004398.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Glasgow 2013
- Glasgow RE. What does it mean to be pragmatic? Pragmatic methods, measures, and models to facilitate research translation. Health Education & Behavior 2013;40(3):257-65. [DOI] [PubMed] [Google Scholar]
GRADEpro 2015 [Computer program]
- GRADEpro GDT. Hamilton (ON): GRADE Working Group, McMaster University, 2015. Available at gradepro.org.
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction – GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64:383-94. [DOI] [PubMed] [Google Scholar]
Hall 2013
- Hall R, Khan F, O'Callaghan C, Kapral MK, Hodwitz K, Kapila S, et al. Ontario stroke evaluation report 2013 spotlight on secondary prevention and care: Toronto. www.ices.on.ca/~/media/Files/Atlases-Reports/2013/Stroke-evaluation-report/Full-report.ashx (accessed 1 September 2018).
Hemming 2015
- Hemming K, Haines TP, Chilton PJ, Girling AJ, Lilford RJ. The stepped wedge cluster randomised trial: rationale, design, analysis, and reporting. BMJ 2015;6(350):391. [DOI] [PubMed] [Google Scholar]
Higgins 2019
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane 2019. Available from www.training.cochrane.org/handbook. [DOI] [PMC free article] [PubMed]
Hoffman 2014
- Hoffman T, Glasziou P, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348:1687. [DOI] [PubMed] [Google Scholar]
Intercollegiate Stroke Working Party 2015
- Intercollegiate Stroke Working Party. Sentinel Stroke National Audit Programme (SSNAP) post-acute stroke service commissioning audit. www.strokeaudit.org/results/PostAcute.aspx (accessed 13 April 2016).
Ivers 2012
- Ivers N, Jamtvedt G, Flottorp S, Young JM, Odgaard-Jensen J, French SD, et al. Audit and feedback: effects on professional practice and healthcare outcomes. Cochrane Database of Systematic Reviews 2012, Issue 6. Art. No: CD000259. [DOI: 10.1002/14651858.CD000259.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Johnson 2019
- Johnson CO, Nguyen M, Roth GA, Nichols E, Alam T, Abate D and the GBD 2016 Stroke Collaborators. Global, regional, and national burden of stroke, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurology 2019;18(5):439-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Korner‐Bitensky 2013
- Korner-Bitensky N. When does stroke rehabilitation end? International Journal of Stroke 2013;8(1):8-10. [DOI] [PubMed] [Google Scholar]
Kwan 2004
- Kwan J, Sandercock PA. In-hospital care pathways for stroke. Cochrane Database of Systematic Reviews 2004, Issue 4. Art. No: CD002924. [DOI: 10.1002/14651858.CD002924.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Langhorne 2011
- Langhorne P, Bernhardt J, Kwakkel G. Stroke rehabilitation. Lancet 2011;377(9778):1693-702. [DOI] [PubMed] [Google Scholar]
Leyden 2013
- Leyden JM, Kleinig TJ, Newbury J, Castle S, Cranefield J, Anderson CS, et al. Adelaide stroke incidence study: declining stroke rates but many preventable cardioembolic strokes. Stroke 2013;44(5):1226-31. [DOI] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Medicine 2009;6(7):e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lindsay 2014
- Lindsay P, Furie KL, Davis SM, Donnan GA, Norrving B. World Stroke Organization global stroke services guidelines and action plan. International Journal of Stroke 2014;9(A100):4-13. [DOI] [PubMed] [Google Scholar]
Luker 2017
- Luker JA, Bernhardt J, Graham ID, Middleton S, Lynch E, Thayabaraanthan T, et al. Interventions for the uptake of evidence-based recommendations in acute stroke settings. Cochrane Database of Systematic Reviews 2017, Issue 1. Art. No: CD012520. [DOI: 10.1002/14651858.CD012520] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lynch 2018
- Lynch, EA, Chesworth, BM, Connell, LA. Implementation – the missing link in the research translation pipeline: is it any wonder no one ever implements evidence-based practice? Neurorehabilitation and Neural Repair 2018;32(9):751-61. [DOI: 10.1177/1545968318777844] [DOI] [PubMed] [Google Scholar]
McEwan 2015
- McEwen SE, Donald M, Dawson D, Egan MY, Hunt A, Quant S, et al. A multi-faceted knowledge translation approach to support persons with stroke and cognitive impairment: evaluation protocol. Implementation Science 2015;10(1):157. [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Brien 2007
- O'Brien MA, Rogers S, Jamtvedt G, Oxman AD, Odgaard‐Jensen J, Kristoffersen DT, et al. Educational outreach visits: effects on professional practice and health care outcomes. Cochrane Database of Systematic Reviews 2007, Issue 4. Art. No: CD000409. [DOI: 10.1002/14651858.CD000409.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schulz 2010
- Schulz KF, Altman DG, Moher D, Consort Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. Trials 2010;11(1):32. [PMC free article] [PubMed] [Google Scholar]
Shojania 2009
- Shojania KG, Jennings A, Mayhew A, Ramsay CR, Eccles MP, Grimshaw J. The effects of on-screen, point of care computer reminders on processes and outcomes of care. Cochrane Database of Systematic Reviews 2009, Issue 3. Art. No: CD001096. [DOI: 10.1002/14651858.CD001096.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Squires 2014
- Squires JE, Sullivan K, Eccles MP, Worswick J, Grimshaw JM. Are multifaceted interventions more effective than single-component interventions in changing health-care professionals' behaviours? An overview of systematic reviews. Implementation Science 2014;9(1):152. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stroke Foundation 2018
- Stroke Foundation. National stroke audit – rehabilitation services report. www.informme.org.au/stroke-data/Rehabilitation-audits (accessed 11 February 2020).
Turner‐Stokes 2015
- Turner-Stokes L, Pick A, Nair A, Disler PB, Wade DT. Multi-disciplinary rehabilitation for acquired brain injury in adults of working age. Cochrane Database of Systematic Reviews 2015, Issue 12. Art. No: CD004170. [DOI: 10.1002/14651858.CD004170.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Walker 2013
- Walker MF, Fisher RJ, Korner-Bitensky N, McCluskey A, Carey LM. From what we know to what we do: translating stroke rehabilitation research into practice. International Journal of Stroke 2013;8(1):11-7. [DOI] [PubMed] [Google Scholar]
WHO 2011
- World Health Organization. Global burden of stroke. www.who.int/cardiovascular_diseases/en/cvd_atlas_15_burden_stroke.pdf?ua=1 (accessed 31 July 2018).
WHO 2015
- World Health Organization. Health topics: rehabilitation. www.who.int/topics/rehabilitation/en/2015 (accessed 16 August 2018).
References to other published versions of this review
Cahill 2017
- Cahill LS, Carey LM, Lannin NA, Turville M, O'Connor D. Implementation interventions to promote the uptake of evidence-based practices in stroke rehabilitation. Cochrane Database of Systematic Reviews 2017, Issue 3. Art. No: CD012575. [DOI: 10.1002/14651858.CD012575] [DOI] [PMC free article] [PubMed] [Google Scholar]


