Abstract
Even though SARS-CoV-2's primary transmission pathway is person-to-person, the role played by surfaces and food contact materials in carrying viral RNA should be further explored. For this purpose, the study aimed to investigate the persistence of SARS-CoV-2 using the strain ATCC® VR-1986HK™ on flow pack polyethylene (FPP) and polystyrene food trays (PFT). Samples of FPP and PFT were contaminated with heat-inactivated SARS-CoV-2 and were incubated at a temperature of 24 ± 1 °C and at controlled relative humidity (RH 65%). The experimental design included analyses at the time 0, 3, 6, 12, 24, 36, 48 and after every 24 h until the viral RNA was no longer detectable. The results showed a significant decrease (P < 0.001) in viral copy numbers on PFT within 3 h (24% reduction) and, at 72 h, the viral RNA had fallen below the limit of detection. Regarding the FPP, it was necessary to wait 24 h for a significant decrease (P = 0.015) in the viral load (14% reduction), while the detection threshold was reached at 96 h. These findings showed that the viral RNA persists longer on flow pack polyethylene samples than on polystyrene food trays, thus highlighting the importance of material characteristics in the persistence of SARS-CoV-2.
Keywords: COVID-19, Food packaging, Fomite, Surface-mediated transmission, Viral RNA detection
1. Introduction
The COVID-19 pandemic is to date a global health crisis that broke out in December 2019 in Wuhan, Hubei Province, China, and subsequently spread worldwide. Counting globally 131.309.792 confirmed cases and 2.854.276 deaths (WHO, 2020).
Currently, the main transmission routes are represented by contaminated droplets or aerosols from an infected individual (person-to-person transmission) (Rothan & Byrareddy, 2020; van Doremalen et al., 2020). Therefore, the diffusion can occur through direct contact with infected persons or indirectly by being in the same environment or coming into contact with objects and surfaces used by the infected person (surface-mediated transmission) (Razzini et al., 2020). About this, Anelich et al. (2020) reported that 10% of SARS-CoV-2 transmissions occur from contaminated surfaces and environments.
Based on the existing literature, there is no evidence of SARS-CoV-2 transmission being related to the consumption of contaminated food (Anelich et al., 2020; Kingsbury & Lake, 2020), consequently COVID-19 is not considered a foodborne disease. Nevertheless, food or contaminated food packaging should be considered an important carrier for the indirect transmission of the virus. In fact, the food industry could create an environment suitable for cross-contamination and the spread of SARS-CoV-2 infection. This is mainly due to its complex structure, which generally consists of several food business operators involved in every production phase, from handling to transporting the food. Hence, presymptomatic or asymptomatic food business operators could be a source of infection for the food they handle or the surfaces they come into contact with (Thippareddi et al., 2020). On the other hand, food chain-related cases of COVID-19 were diverse and numerous. The first outbreak occurred on the June 21, 2020 in Xinfadi, the biggest wholesale market in Beijing, where the SARS-CoV-2 was detected on the cutting board used to handle imported salmon (Global Times, 2020). The following genomic sequencing identified a European strain of SARS-CoV-2, pointing to frozen foods as possible virus carriers (Han et al., 2020). Subsequently, between July and August 2020, several COVID-19 clusters in China were repeatedly linked to imported frozen raw food. The presence of the virus was detected in food packaging materials (packages of frozen shrimps), food storage environment (shipping container), and food surfaces (frozen chicken wings) (Han et al., 2020).
In June 2020, an emerging COVID-19 outbreak related to the food industry was confirmed in the UK (BBC News, 2020a). Simultaneously, the Public Health Authorities confirmed 204 cases at a chicken factory in Llangefni (BBC News, 2020a) and 106 cases at Rowan Foods in Wrexham, UK (BBC News, 2020b). In addition, large clusters of COVID-19 positive cases, linked to the fish processing industry, were identified in Portugal (Fisher et al., 2020) and related to slaughterhouses in Germany as well as in Australia (ABC News, 2020a, 2020b; DW News, 2020).
Given the many cases reported, several authors have investigated the persistence of SARS-CoV-2 on food packaging, surfaces and food. In particular, Chin et al. (2020) found that the infectivity of SARS-CoV-2 at controlled temperature and relative humidity (22 °C and RH 65%) was no longer detectable after 3 h on printing and tissue papers and after 2 days on treated wood and cloth. In contrast, the virus was more stable on smooth surfaces with a 4 days duration of infectivity on glass and banknotes and of 7 days on stainless steel and plastic.
Another study conducted with a virus closely related to SARS-CoV-2 (HuCoV-229E), showed that the human coronavirus 229E was able to persist at 21 °C and with a relative humidity that ranged from 30% to 40%, on silicone rubber for 3 days and on polytetrafluoroethylene (Teflon), polyvinyl chloride (PVC), ceramic tiles and glass for at least 5 days (Warnes et al., 2015). Regarding the virus infectivity in food, Fisher et al. (2020) reported that the SARS-CoV-2 titers did not decline until after 3 weeks in chicken, pork and salmon stored at respectively 4, -20 and −80 °C.
This study aimed at investigating, through molecular methods, the persistence of SARS-CoV-2 (ATCC® VR-1986HK™) on flow pack polyethylene (FPP) and polystyrene food trays (PFT), two different food contact materials, among those most frequently used for food packaging. In fact, as reported by Tiwari et al. (2006), infectivity has a faster biological decay rate than viral RNA persistence, and therefore, if it is possible to state that its presence may not be an indicator of viability, it is also possible to assert that its total absence defines a situation of absolute safety.
2. Materials and methods
Heat-inactivated SARS-CoV-2 (ATCC® VR-1986HK™) is a preparation of Severe Acute Respiratory Syndrome related Coronavirus 2 (SARS-CoV-2), strain 2019-nCoV/USAWA1/2020, which has been inactivated by heating at 65 °C for 30 min and is therefore unable to replicate.
2.1. Heat-inactivated SARS-CoV-2 (ATCC® VR-1986HK™) stability on different surfaces assay
FPP and PFT samples of 1 cm2 each were contaminated with 5 μL of heat-inactivated SARS-CoV-2 containing 3.75 × 105 copies/μL (ATCC® VR-1986HK™). The viral inoculum was evenly distributed over the entire surface through five droplets by 1 μL each to mimic airborne aerosols.
Throughout the experiment, samples were kept in an incubator that provided stable levels of temperature (24 °C ± 1) and relative humidity (RH 65%).
The experimental design included analyses at the time 0, 3, 6, 12, 24, 36, 48 and after every 24 h until the viral RNA was no longer detectable. For each desired time point, three (n=3) samples were analyzed and the inoculated virus was recovered by adding 135 μL of phosphate-buffered saline (PBS). For each sample, the viral RNA extraction was performed and then the extracts were analyzed in duplicate through the use of a real-time PCR.
2.2. Viral RNA extraction and Real Time PCR assay
Viral RNA was extracted with the QIAamp® Viral RNA Mini Kit (Qiagen, Milan, Italy), based on spin columns, according to the manufacturer's instructions and using Intype IC-RNA as process control (Indical Bioscence, Leipzig, Germany) for each sample to evaluate the efficiency of extraction method. The RNA obtained was amplified and quantified by real-time RT-PCR using the VETfinder “Detection of CoV-19 and SARS and Recovery control in environmental sample” detection kit (Generon Srl, San Prospero, Modena, Italy). Tenfold serial dilutions of heat-inactivated SARS-CoV-2 containing 3.75 × 105 copies/μL (ATCC® VR-1986HK™) were made out in PBS, frozen at −80 °C, and used only once. Duplicates of standard dilutions and RNA templates were simultaneously subjected to real-time analysis.
Each reaction with a final volume of 25 μL comprised 20 μL of Ready-to use Mastermixes and 5 μL of RNA sample or standard dilution. The thermal cycle protocol included RT at 55 °C for 10 min, activation of Taq Polymerase at 95 °C for 3 min followed by 45 cycles of 95 °C for 15 s and 58 °C for 30 s, according to the manufacturer's instructions. The RNA copy numbers of SARS-CoV-2 was automatically calculated by the CFX connect Real Time system, (Bio-Rad Laboratories Srl) based on standard curves, plotting the quantification cycle (Cq) values against each standard tenfold dilution. A sample was considered negative when the Cq value of the qRT-PCR was ≥ 40.
2.3. Statistical analysis
The statistical analysis was performed using the “stats” package of the R software (R v.4.0.3). The copy numbers of the SARS-CoV-2 (ATCC® VR-1986HK™) was compared among the detected hours through the pairwise comparisons using t tests. The significance threshold was set at P < 0.05. Relative Repeatability Standard Deviation (RSDr) was calculated dividing the SDr by the mean of results and the data of PCR replicates were evaluated only where RSDr was ≤ 25% (Pierboni et al., 2020).
3. Results and discussion
The authors, in this first study conducted on two different food contact materials used for food packaging, investigated the persistence of SARS-CoV-2 on flow pack polyethylene and polystyrene food trays using the ATCC® VR-1986HK™ strain inactivated by heat treatment (65 °C for 30 min) provided by the American Type Culture Collection. The use of ATCC SARS-CoV-2 allowed us to perform our experiments in a BSL2 laboratory without the problem of viral infectivity (Lista et al., 2020). Moreover, as reported by several authors (Kim et al., 2020; Lista et al., 2020; Pastorino et al., 2020), the heat treatment used for ATCC does not interfere with the detection of viral RNA copy numbers.
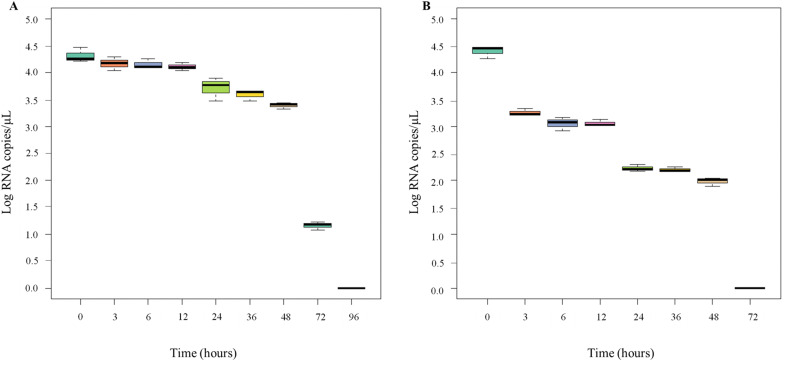
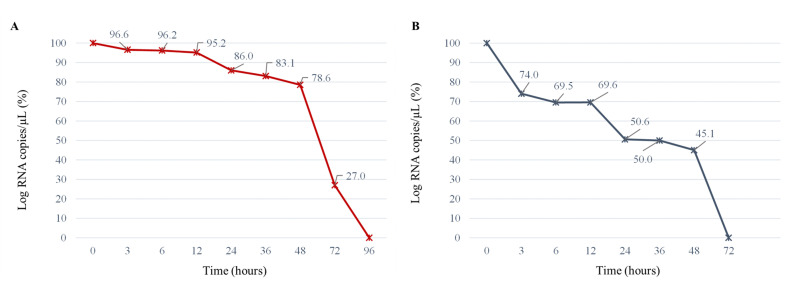
The mean detection values of SARS-CoV-2 on PFT and FPP, obtained in this study, are shown in Fig. 1 A-B, respectively. As expected, there was a significant reduction of the virus on both food contact materials during the observation period. However, the persistence of viral RNA at room temperature seems to be affected by the type of material on which it was deposited. Indeed, although the initial quantification (0 h) of dispersed viral nucleic acid was the same (4.32 ± 0.14 and 4.40 ± 0.12 Log copies/μL for FPP and PFT, respectively; P = 0.487), the mean values quantified at each time point on the FPP were always higher than on the PFT (P < 0.001). Notably, on the latter, a significant decrease in viral copies number was observed within 3 h (0 h vs 3 h: P < 0.001), while on FPP it was necessary to wait 24 h (0 h vs 24 h: P = 0.015). In percentage terms, the decrease of virus at 3 h on the PFT was 26.0% (1.14 Log reduction), compared with only 3.4% on the FPP (0.15 Log reduction) (Fig. 2 A–B). After 24 h, 86.0% of the virus copies initially quantified on the surface could still be recovered on flow packs polyethylene, while only 51.0% could be recovered on polystyrene food trays. Other authors have highlighted the importance of material type in the persistence of SARS-CoV-2 but only referred to differences concerning the infectious dose (TCID50). Specifically, van Doremalen et al. (2020) showed that the virus titer on plastic materials and stainless surface was detectable up to 72 h, while on cardboard and copper surface the persistence of infectivity was much shorter, 24 h and 4 h respectively, even if the authors themselves do not explore the mechanism by which these surfaces had an impact on infectivity.
Fig. 1.
Distribution of the copy numbers of the SARS-CoV-2 (ATCC® VR-1986HK™) detected at different hours; A: flow pack polyethylene (FPP); B: polystyrene food trays (PFT).
Fig. 2.
Residual percentage of the copy numbers of the SARS-CoV-2 (ATCC® VR-1986HK™) revealed at different hours; A: flow pack polyethylene (FPP);B: polystyrene food trays (PFT).
Therefore, it would appear that, under the same environmental conditions, the composition of the material played a crucial role in the release of the viral particles over time.
For these reasons, we assume that the PFT structure may have absorbed more of the droplets containing the virus, allowing less SARS-CoV-2 RNA to be recovered at each analysis time. After 48 h, on PFT, more than half of the viral load (55.0%; 2.42 Log reduction) was no longer detectable, while 78.6% was still present on the FPP with a similar value to that recorded for PFT after 3 h of observation. At the 72 h, the amount of viral RNA on the polystyrene food trays had declined below the detection limit (Cq value > 40), whereas for the flow pack polyethylene this decay was reached after 96 h.
Chin et al. (2020) evaluated the stability of the infectious virus deposited on several porous and non-porous surfaces at 22 °C and 65% RH, concluding that the virus titer persisted from 2 to 4 days on non-porous surfaces and from 30 min to 2 days on porous surfaces, highlighting that infectivity would appear to be influenced by surface porosity. Indeed, as Tiwari et al. (2006) suggests, surface porosity could play a key role in the elution efficiency of the viral particles.
Therefore, the decrease in infectivity reported by the above-mentioned authors may not be linked to the effective inactivation of the virus, but simply to a lower capacity of virus recovery from the surfaces. The obtained results in our study support this hypothesis where, at equal temperature and RH, the material on which the SARS-CoV-2 is deposited would appear to influence the quantity of viral RNA recovered.
The PFT and FPP tested in this study are synthetic polymers that have different chemical structures, morphologies and properties. In particular, expanded polystyrene is not entirely waterproof; its conformation, made of interstitial gaps between the expanded pellets that create an open network of channels, allows the water to penetrate and to therefore become trapped (Gnip et al., 2006). Regarding polyethylene, used for plastic films in the food packaging industry, has an excellent mechanical strength, hydrophobicity and moisture barrier properties (Tankhiwale & Bajpai, 2012).
Based on these considerations, to precisely understand the role of surfaces as a fomite, the evaluation of infectious decay cannot be separated from an evaluation of the efficiency of virus recovery from surfaces (potential physical losses) (Biryukov et al., 2020).
4. Conclusion
Although COVID-19 infection mainly spreads through the conventional person-to-person route, during the current pandemic, SARS-CoV-2 was detected several times on food and mostly on the packaging materials. In this regard, our experiment showed that SARS-CoV-2 RNA is less detectable from PFT than FPP. The latter remains contaminated longer and is potentially infectious up to 72 h at room temperature. Moreover, the persistence and infectivity of the virus can be further prolonged in refrigerated and frozen conditions when these materials are exposed to the cold chain in the food industry. Indeed, the increased resistance of the virus on cold surfaces would appear to be one of the causes of the many outbreaks in slaughterhouses and meat processing facilities. For this reason, the strict application of good hygiene practices and the conscious use of personal protective equipment (PPE), such as masks, gloves, and other PPEs, is essential to limit the spread of infection in these contexts.
Finally, to assess the actual risk of transmission via fomites, we need to address the knowledge gaps regarding the stability of SARS-CoV-2 in the environment while also obtaining more information on the role of food contact materials in viral particle release and persistence of infectivity. We suggest that further studies are necessary to understand whether the decay in infectivity of the virus, when released into the environment, is due to (i) its inactivation, (ii) less release in relation to the material characteristic, or (iii) a combination of both.
CRediT authorship contribution statement
Marta Castrica: Conceptualization, Formal analysis, Data curation, Writing – original draft, Writing – review & editing, Visualization, Supervision, Project administration. Claudia Balzaretti: Resources, Writing – review & editing, Project administration. Dino Miraglia: Conceptualization, Formal analysis, Data curation, Writing – original draft, Writing – review & editing, Visualization, Supervision, Project administration. Patrizio Lorusso: Methodology, Validation, Formal analysis, Writing – review & editing. Annamaria Pandiscia: Writing – review & editing. Giuseppina Tantillo: Writing – review & editing. Francesca Romana Massacci: Formal analysis, Writing – review & editing. Valentina Terio: Methodology, Validation, Formal analysis, Writing – review & editing.
Declaration of competing interest
All authors declare no competing interests.
Acknowledgments
The authors wish to thank Margherita Angelucci for English revision.
References
- ABC News Coronavirus shutdown at Cedar Meats over as abattoir resumes full operations. 2020. https://www.abc.net.au/news/2020-05-27/coronavirus-shutdown-melbourne-cedar-meats-workers-return/12289970
- ABC News Why was there a COVID-19 outbreak in Colac's abattoir, but not one in Warrnambool? 2020. https://www.abc.net.au/news/2020-08-06/covid-19-abattoir-outbreak-raises-questions-about-dhhs-response/12526816
- Anelich L.E.C.M., Lues R., Farber J.M., Parreira V.R. SARS-CoV-2 and risk to food safety. Frontiers in Nutrition. 2020;7(November):1–8. doi: 10.3389/fnut.2020.580551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BBC News Coronavirus: Almost 100 staff at food factories test positive. 2020. https://www.bbc.com/news/uk-wales-53091149
- BBC News Coronavirus cases at Rowan foods, Wrexham, rise to 166. 2020. https://www.bbc.com/news/uk-wales-53194358
- Biryukov J., Boydston J.A., Dunning R.A., Yeager J.J., Wood S., Reese A.L., Ferris A., Miller D., Weaver W., Zeitouni N.E., Phillips A., Freeburger D., Hooper I., Ratnesar-Shumate S., Yolitz J., Krause M., Williams G., Dawson D.G., Herzog A., Altamura L.A. Increasing temperature and relative humidity Accelerates inactivation of SARS-CoV-2 on surfaces. mSphere. 2020;5(4):1–9. doi: 10.1128/msphere.00441-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin A.W.H., Chu J.T.S., Perera M.R.A., Hui K.P.Y., Yen H.-L., Chan M.C.W., Peiris M., Poon L.L.M. Stability of SARS-CoV-2 in different environmental conditions. The Lancet Microbe. 2020 doi: 10.1016/s2666-5247(20)30003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., Tamin A., Harcourt J.L., Thornburg N.J., Gerber S.I., Lloyd-Smith J.O., de Wit E., Munster V.J. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. New England Journal of Medicine. 2020;382(16):1564–1567. doi: 10.1056/nejmc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DW News Coronavirus: Gütersloh mayor Henning Schulz slams Tönnies meat producer after massive outbreak forces lockdown. 2020. https://www.dw.com/en/coronavirus-gütersloh-mayor-henning-schulz-slams-tönnies-meat-producer-after-massive-outbreak-forces-lockdown/a-53932101
- Fisher D., Reilly A., Kang A., Zheng E., Cook A.R., Anderson D.E. 2020. Seeding of outbreaks of COVID-19 by contaminated fresh and frozen food. (BioRxiv) [Google Scholar]
- Global Times Beijing supermarkets stop selling salmon after wholesalers test positive for coronavirus. 2020. https://www.globaltimes.cn/content/1191462.shtml
- Gnip I.Y., Kersulis V., Vejelis S., Vaitkus S. Long-term water absorption of expanded polystyrene boards. Polymer Testing. 2006 doi: 10.1016/j.polymertesting.2006.04.002. [DOI] [Google Scholar]
- Han J., Zhang X., He S., Jia P. Can the coronavirus disease be transmitted from food? A review of evidence, risks, policies and knowledge gaps. Environmental Chemistry Letters. 2020 doi: 10.1007/s10311-020-01101-x. 0123456789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.I., Casel M.A.B., Kim S.M., Kim S.G., Park S.J., Kim E.H., Jeong H.W., Poo H., Choi Y.K. Development of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) thermal inactivation method with preservation of diagnostic sensitivity. Journal of Microbiology. 2020;58(10):886–891. doi: 10.1007/s12275-020-0335-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury J., Lake R. 2020. Potential for foodborne transmission of Covid-19: Literature review update. In food safety science and research Center. [Google Scholar]
- Lista M.J., Page R., Sertkaya H., Matos P., Ortiz-Zapater E., Maguire T., Poulton K., O'Byrne A., Bouton C., Dickenson R., Ficarelli M., Howard M., Betancor G., Galao R.P., Pickering S., Signell A., Wilson H., Cliff P., Patel A., Martinez-Nunez R.T. Resilient SARS-CoV-2 diagnostics workflows including viral heat inactivation. 2020. [DOI] [PMC free article] [PubMed]
- Pastorino B., Touret F., Gilles M., Lamballerie X. De. What Protocols for Biosafety; 2020. pp. 6–13. (Samples). [Google Scholar]
- Pierboni E., Rondini C., Zampa S., Tovo G.R., Altissimi S., Haouet N. Evaluation of rice as unregulated hidden allergen by fast real-time PCR. Journal of Cereal Science. 2020;92 doi: 10.1016/j.jcs.2020.102929. (November 2019), 102929. [DOI] [Google Scholar]
- Razzini K., Castrica M., Menchetti L., Maggi L., Negroni L., Orfeo N.V., Pizzoccheri A., Stocco M., Muttini S., Balzaretti C.M. SARS-CoV-2 RNA detection in the air and on surfaces in the COVID-19 ward of a hospital in Milan, Italy. The Science of the Total Environment. 2020;742 doi: 10.1016/j.scitotenv.2020.140540. 140540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. Journal of Autoimmunity. 2020;109(February) doi: 10.1016/j.jaut.2020.102433. 102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tankhiwale R., Bajpai S.K. Preparation, characterization and antibacterial applications of ZnO-nanoparticles coated polyethylene films for food packaging. Colloids and Surfaces B: Biointerfaces. 2012 doi: 10.1016/j.colsurfb.2011.09.031. [DOI] [PubMed] [Google Scholar]
- Thippareddi H., Balamurugan S., Patel J., Singh M., Brassard J. Coronaviruses – potential human threat from foodborne transmission? Lebensmittel-Wissenschaft & Technologie. 2020;134(July) doi: 10.1016/j.lwt.2020.110147. 110147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari A., Patnayak D.P., Chander Y., Parsad M., Goyal S.M. Survival of two avian respiratory viruses on porous and nonporous surfaces. Avian Diseases. 2006;50(2):284–287. doi: 10.1637/7453-101205R.1. [DOI] [PubMed] [Google Scholar]
- Warnes S.L., Little Z.R., Keevil C.W. Human coronavirus 229E remains infectious on common touch surface materials. mBio. 2015 doi: 10.1128/mBio.01697-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO WHO coronavirus disease (COVID-19) Dashboard. 2020. https://covid19.who.int/?source=content_type:react%7Cfirst_level_url:article%7Csection:main_content%7Cbutton:body_link