Abstract
The current methods of mechanical ventilation and pulmonary drug delivery do not account for the heterogeneity of acute respiratory distress syndrome or its dependence on gravity. The severe lung disease caused by severe acute respiratory distress syndrome coronavirus 2, coronavirus disease 2019, is one of the many causes of acute respiratory distress syndrome. Severe acute respiratory distress syndrome coronavirus 2 has caused more than three million deaths worldwide and has challenged all therapeutic options for mechanical ventilation. Thus, new therapies are necessary to prevent deaths and long-term complications of severe lung diseases and prolonged mechanical ventilation. The authors of the present report have developed a novel device that allows selective lobe ventilation and selective lobe recruitment and provides a new platform for pulmonary drug delivery. A major advantage of separating lobes that are mechanically heterogeneous is to allow for customization of ventilator parameters to match the needs of segments with similar compliance, a better overall ventilation perfusion relationship, and prevention of ventilator-induced lung injury of more compliant lobes. This device accounts for lung heterogeneity and is a potential new therapy for acute lung injury by allowing selective lobe mechanical ventilation using two novel modes of mechanical ventilation (differential positive end-expiratory pressure and asynchronous ventilation), and two new modalities of alveolar recruitment (selective lobe recruitment and continuous positive airway pressure of lower lobes with continuous ventilation of upper lobes). Herein the authors report their initial experience with this novel device, including a brief overview of device development; the initial in vitro, ex vivo, and in vivo testing; layout of future research; potential benefits and new therapies; and expected challenges before its uniform implementation into clinical practice.
Key Words: mechanical ventilation, acute respiratory distress syndrome, acute lung injury, lobe ventilation, lobar ventilation, heterogeneous lung disease, regional ventilation, pulmonary drug delivery, double-lumen tube, ventilator, split ventilation
SEVERE ACUTE respiratory syndrome coronavirus 2 (SARS-CoV-2) is one of the many causes of acute respiratory distress syndrome (ARDS). SARS-CoV-2 has caused more than three million deaths worldwide1 and has challenged all therapeutic options for mechanical ventilation. Poor outcomes have led clinicians to question the need, and best modality, for mechanical ventilation in these patients with severe respiratory failure and clinically significant hypoxemia.2, 3, 4
ARDS is a noncardiogenic form of pulmonary edema that results in bilateral parenchymal infiltrates. The disease now is recognized to have biologic and mechanical heterogeneity, with significant differences in compliance, opening pressure, and timeconstant among lung segments.5, 6, 7, 8, 9 Such differences make the selection of parameters for mechanical ventilation very challenging, and finding one parameter to fit all the different lung segments may not be possible.
The highly perfused, gravity-dependent zones are usually the most severely involved lung regions in ARDS.6 , 9, 10, 11 Ventilation with elevated airway pressure invariably results in preferential inspiratory flow to more compliant, nongravity–dependent zones,12 resulting in alveolar hyperdistention and causing ventilator-induced lung injury (VILI).13, 14, 15 Importantly, the current methods of mechanical ventilation and pulmonary drug delivery do not account for the mechanical heterogeneity of ARDS, its dependence on gravity, or the difference in alveolar timeconstant. Therefore, innovation in mechanical ventilation and new therapies are necessary to prevent deaths, long-term complications of severe lung diseases, and prolonged mechanical ventilation in ARDS.
The authors of the present report have developed a novel device that promotes effective and safe selective lobe ventilation and selective lobe recruitment and that provides a new platform for selective pulmonary delivery of medical gases and drugs. This device better accounts for lung heterogeneity and is a potential new therapy for acute lung injury by allowing for selective lobe mechanical ventilation using the following two novel modes of mechanical ventilation: (1) differential positive end-expiratory pressure (PEEP) and (2) asynchronous reverse cycle ventilation of upper and lower lung lobes. The device also uses the following two new modalities of alveolar recruitment: (1) selective lobe recruitment and (2) continuous lower lobe positive airway pressure.
Herein the authors report their preliminary experience with this novel device, including a brief overview of device development; the initial in vitro, ex-vivo, and in-vivo testing; layout of future research; potential benefits and new therapies; and expected challenges before its uniform implementation in clinical practice.
Device Development
Rationale
The rationale for development of the device was based on the premise that gravity-dependent zones are located mostly in the lower lung lobes. This relationship becomes more evident during mechanical ventilation with patients in the supine position and some degree of head elevation, in whom oblique lung fissures assume a more horizontal position. A major advantage of separating lobes that are mechanically heterogeneous is to allow for customization of ventilator parameters to match the needs of segments with similar compliance, for a better overall ventilation-perfusion relationship (V/Q), and for prevention of VILI of more compliant lobes.
Methods
Multiple criteria and design specifications were taken into consideration to build a mechanical airway apparatus with at least three lumens of different lengths that could pass and fit into the airway, achieve final position using flexible bronchoscopy, and provide functional isolation and effective ventilation of the different lobes. The prototype developed started with a single-lumen tube that the authors named the “Shuttle” tube, which has a special contour and collapsed sheath in the posterior wall. The sheath is located outside the posterior tube wall but inside a cuff, creating an internal channel to allow placement of the distal lobar tubes (Fig 1 ). Computer-assisted design software, SolidWorks (Dassault Systèmes, Waltham, MA), was used for the design. Multiple iterations of design concepts, molds, connectors, and the anatomic model were printed three-dimensionally (3D) using a Formlabs 3D printer (Formlabs, Somerville, MA). Tubes, cuffs, cuff lines, and the sheath were purchased from different vendors and modified according to the design needs. The authors systematically examined several criteria for feasibility and clinical utility of the device and designed specific features to fulfill such criteria (Table 1 ).
Fig 1.
Lateral view of the shuttle tube. 1, Tracheal lumen; 2, tracheal connector; 3, sheath; 4, connector for the sheath; 5, tracheal cuff; 6, cuff line. (B) Perspective view of the assembly. 1, cuffs; 2, secondary tubes; 3, tracheal lumen; 4, cuffs; 5, sheath for the secondary tube; 6, connector for the sheath; 7, tracheal lumen connector; 8, secondary lobar tubes; 9, connector for the secondary lobar tubes. (C) Diagram tubes and ventilator connection for differential positive end-expiratory pressure. 1, tracheal lumen; 2, tracheal tube; 3, tube connector; 4, right lower lobe tube lumen; 5, right lower lobe tube; 6, left lower lobe tube lumen; 7, left lower lobe tube; 8, secondary lobar tube connectors; 9, respiratory circuit for the lobar tubes; 10, respiratory circuit for the upper lobes bilaterally and right middle lobe. LLL, left lower lobe; LUL, left upper lobe; RLL, right lower lobe, RML, right middle lobe; RUL, right upper lobe.
Table 1.
Specific Clinical Criteria and Design Features to Support Them in the Shuttle Tube
| Criteria | Design Feature |
|---|---|
| Passage through airway—pharynx, glottis, and trachea | Largest external dimension of the device of 12 mm |
| Effective ventilation | Internal area of the Shuttle tube of at least 45 mm2 and for the lobar tube at least 15 mm2 |
| Suction ports | 14 G suction to remove secretions |
| Placement in a difficult airway | Direct or video laryngoscopy, fiberoptic intubation |
| Positioning by bronchoscopy | Tracheal and lobar tubes internal dimmension at least 4.5 mm for passage of a 3.8 mm flexible bronchoscope |
| Material selection and final manufacturing process | Possible for tubing extrusion |
In Vitro Testing
In vitro testing with a 3D-printed anatomic model of the human tracheal bronchial tree allowed for evaluation of the placement, interaction, and positioning of the three separate tubes, and the initial instructions for device instrumentation were created.
Device Instrumentation
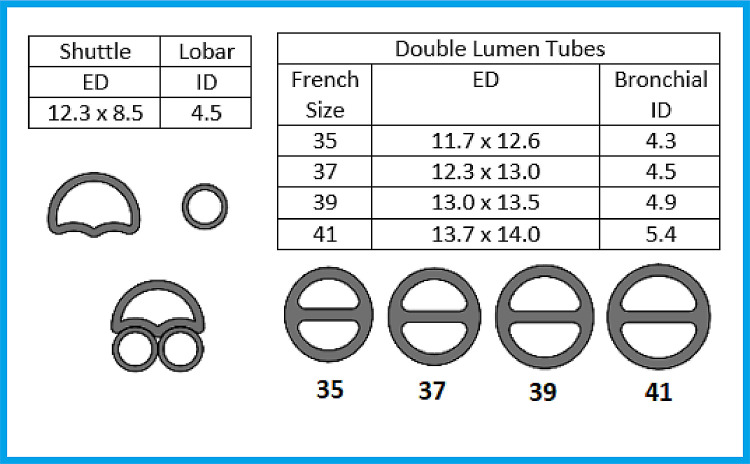
The Shuttle, the single-lumen tube, and the double-lumen tube of various sizes have been used to perform laryngoscopy, video laryngoscopy, and intubation in a high-quality airway mannequin (Anesthesia Human Patient Simulator, CAE Healthcare, Sarasota, FL). Placement was successful, and the authors believe that the level of difficulty was similar to that of a single-lumen tube and easier than that of a double-lumen tube. Shuttle placement is aimed to be performed by direct laryngoscopy, or alternatively by, video laryngoscopy or fiberoptic intubation in patients with a difficult airway. The dimensions of the Shuttle and lobar tubes and a comparison with double-lumen tubes (35-41 F) are referenced in Figure 2 . The cross-section of the Shuttle has a greater lateral than anteroposterior dimension. Therefore, the authors recommend aligning the lateral position of this tube with the anteroposterior dimension of the glottis aperture during intubation. After tracheal insertion, the tube is rotated 90 degrees (clockwise or counterclockwise) to achieve an anteroposterior orientation.
Fig 2.
Dimensions in millimeters and cross-section profiles of shuttle, lobar, and double-lumen tubes (35-to- 39 F). Abbreviations: ED, external dimension; ID, internal dimension.
After intubation and-two lung ventilation are established, a 3.8-mm diameter, or smaller, flexible bronchoscope is used to confirm and adjust the position of the distal tip at least 3 cm proximal to the main carina. The placement of the secondary tubes into lung lobes also is guided by the same-size bronchoscope. The final position of the lobar tubes on the mainstem bronchi allows for one-lung ventilation (OLV) or further advancement to a lung lobe, allowing for selective lobe ventilation. If bilateral selective lower lobe ventilation is desired, placement of the right lower tube should be performed before placement of the left lower tube to facilitate placement and prevent the lobar tubes from becoming crossed or kinked inside the sheath. Inflation of the cuffs on the lobar tubes also can be confirmed by bronchoscopy proximal to the cuffs to prevent overinflation and cuff herniation.
Ex Vivo Testing
The ex vivo testing was performed on a swine model (Video 1). In comparison with human anatomy, the following main macroscopic anatomic differences of the swine respiratory system are: (1) the trachea is approximately 15-to-20 cm longer than human trachea; (2) the right lung is divided into four lobes (upper, accessory, middle, and lower); (3) the right upper lobe bronchi come directly from the trachea very proximal to the carina; and (4) the accessory lobe is a small lobe located retro-cardiac, with a high takeoff from the right mainstem bronchi.16 For the sake of simplicity and comparison with the human lungs, the middle lobe was considered as being nondependent together with the upper lobes, and the accessory lobe was not considered because of the small size, location, and high takeoff. Placement of the device and selective ventilation of separate lung lobes without the surrounding chest wall showed the dynamics and differences in regional ventilation in separate lobes of new ventilation and recruitment modalities such as differential PEEP, selective lobe recruitment, continuous positive airway pressure (CPAP), and asynchronous ventilation. The tracheal portion of the device was connected to one ventilator (Aestiva 5, Datex-Ohmeda, WI), and the twos lobar tubes were advanced to the right and left lower lobes. The proximal end of the secondary tubes were attached to a “Y” connector, then connected to a second ventilator with the same specifications. This configuration divides the lung segments into two compartments; one is a less gravity-dependent compartment containing the upper lobes of both lungs and right middle lobe, and the second is a more gravity-dependent compartment encompassing the bilateral lower lobes.
In Vivo Testing and Plans for Current and Future Research
The protocol (A20-150) was approved by the Wake Forest Institutional Animal Care and Use Committee to test this new respiratory apparatus in a double-hit injury model of ARDS. In this model, after general anesthesia, ARDS was induced by (1) lung lavage to deplete surfactant and then (2) the administration of intravenous oleic acid to cause endothelial lesions. Bronchoscopy with successful positioning of the apparatus in swine lungs was performed with a 3.8-mm flexible bronchoscope (BFlex Scope; Verathon, Bothell, WA), and imaging acquisition was performed using the GlideScope Core (Verathon) unit (Fig 3 ). The preliminary results suggested that better ventilation and oxygenation could be achieved with the selective lobe ventilation strategy compared with the current protective lung strategy, but more experiments are required to achieve statistical power and, hopefully, will translate to clinical significance.
Fig 3.
Bronchoscopy and positioning of the tracheal and lobar tubes. (A) Swine tracheal bronchial tree anatomy illustration. (B) Right upper lobe in the three o'clock position; the carina is more distal in the center. (C) View from inside the Shuttle tube. (D) Shuttle tube above the right upper lobe takeoff in the three o'clock position. (E) Right mainstem bronchi trifurcation; from left to right, right middle lobe, accessory, right lower lobe. (F) Lobar tube placed on the right mainstem bronchi. (G) Both lobar tubes placed on the right and left mainstem bronchi. (H) Lobar tube placed into the right lower lobe, past the right middle lobe and accessory. (I) View from the inside of left lobar tube showing the left upper lobe takeoff at the nine o'clock position and left lower lobe in the center.
Expected Challenges Before Uniform Implementation in Clinical Practice
The use of this device in clinical practice will require continued preclinical in vitro, ex vivo, and in vivo animal model testing before a clinical trial to confirm safety and effectiveness in humans. The current plan is to continue preclinical work and test whether the novel device is equivalent to or better than the currently available double-lumen tubes. An important challenge to address is the need for two ventilators for one patient in order to implement selective lobe ventilation. The authors have considered that the need for two ventilators is not an ideal option, and if selective lung therapies prove to have clinical benefit in the near future, new respiratory circuits, new drug delivery systems, and, likely, new ventilators will need to be designed.
Potential Benefits and New Therapies
The lung lesions caused by SARS-CoV-2 show variable stages of inflammation, fluid accumulation, and decreased perfusion, which culminate with the clinical picture of ARDS. It also appears that SARS-CoV-2 produces fibroproliferative lung damage in the most severe cases, with progressive worsening of lung function, especially in patients with prolonged hospitalization and mechanical ventilation.17
Now more than ever, there is a need to explore new therapeutic options and potential improvements in the care of patients with severe lung injury. ARDS is an acute, diffuse, inflammatory form of lung injury18 with noncardiogenic pulmonary edema, characterized by bilateral lung infiltrates and hypoxemia caused by multiple etiologies. Before 2019, the incidence of ARDS in intensive care units was approximately 10%-to- 15% of admitted patients and up to 23% of mechanically ventilated patients.19 The mortality rates of ARDS is 27%, 32%, and 45% for mild, moderate, and severe disease, respectively.19 Mechanical ventilation with elevated airway pressures results in VILI.20 , 21 Protective ventilation strategies with low tidal volumes reduce complications but have no significant effect on mortality.22 In particular, heterogeneity constitutes a problem because the current methods of mechanical ventilation do not allow for regional ventilation of lung zones with different mechanical properties. Customization of ventilator parameters based on regional ventilation imaging by computed tomography (CT) or electrical impedance tomography may have important clinical benefit.23, 24, 25
Lung recruitment maneuvers are transient and designed to open up all the collapsed alveoli with sustained increases of transpulmonary pressures. The duration of the recruitment is important to open alveoli with different timeconstants, but sustained positive intrathoracic pressures reduce venous return (preload).26 Selective lobe ventilation is an interesting and appealing concept. However, placing multiple tubes in the airway seems unpractical, in particular in patients with low physiologic reserve in whom significant work within the airway may be risky. Moreover, having multiple ventilators seems to be clinically challenging and may not be feasible during ventilator shortages. On the other hand, the pandemic and ventilator shortages have forced clinicians to create means to perform split ventilation with different PEEP levels to treat more than one patient.27 , 28 The idea of split ventilation now may have a long-term application for patients who could benefit from selective lobe ventilation.
The design achieved for the apparatus allows the Shuttle to work as single-lumen tube, and placing one secondary tube in the mainstem bronchi would allow for OLV. If selective lobe ventilation is desired, one or more of the secondary tubes could be advanced to the respective lung lobe(s). Compared with the current double-lumen tubes used for thoracic surgery, the Shuttle is more malleable and has a smaller profile, which may be beneficial in patients with difficult airways. The placement of secondary tubes can be performed with no interruption of ventilation, and the secondary tubes slide inside the Shuttle sheath, preventing tracheal injuries. The double-lumen feature simply can be converted to a single lumen by removing the secondary tube without having to perform a tube exchange, which also may be a useful feature for extensive thoracic procedures such as esophagectomy and lung transplantation.
The ex vivo testing showed different possibilities for ventilation, lung recruitment, delivery of medical gases, and drug delivery. The selection of mechanical ventilation parameters, such as PEEP, minute ventilation, inspiratory/expiratory (I:E) ratio, and upper and lower lobe respiratory cycle synchrony deserve special consideration.
Differential PEEP
Using a higher PEEP on gravity-dependent lobes provides better recruitment, and using lower PEEP on nongravity–dependent lobes would prevent overdistention and avoid VILI of more compliant segments. There also is evidence from OLV studies that differential PEEP can shift perfusion among gravity-dependent and nongravity–dependent lungs and that higher PEEP on the diseased affected segments also could shift perfusion to nondependent segments, improving the V/Q relationship.29 , 30
Minute Ventilation
The selection of different tidal volumes and respiratory rates on isolated compartments allows for new options of volume and rate customization, leading to better carbon dioxide elimination without causing VILI. For example, more-compliant upper lobes can receive more volume with less PEEP; conversely, the less- compliant lower lobes will receive lower volumes but can receive either increased PEEP or higher respiratory rates or both.
I:E Ratio
Using longer expiratory times on the upper lobes favors better carbon dioxide elimination, and using an inverse I:E ratio on the lower lobes maintains the alveoli open for a longer period, favoring oxygenation.
Respiratory Cycle Synchrony
In positive-pressure ventilation, peak pressures are achieved by the limitations of distention of lung parenchyma and the physical limitations of the chest cavity. Having the ability to ventilate the upper and lower lobes at different times lessens the physical competition for space within the chest cavity, which may reduce airway pressure.
Equipment deadspace is an important factor to be considered, especially when mechanically ventilating stiff lungs with limited tidal volumes.31 Using the novel apparatus in a modified configuration potentially will eliminate equipment deadspace. Placing the secondary tubes in the trachea, removing the “Y” piece from the respiratory circuit, and connecting one limb directly to the Shuttle and the other limb to the secondary tubes technically would result in moving the “Y” piece into the airway. By reducing unnecessary deadspace, effective ventilation is increased, the work of breathing is reduced, and more effective carbon dioxide elimination is promoted.
The combination of high perfusion and collapsed alveoli creates a problem for the delivery of any pulmonary-delivered therapy for heterogeneous lung diseases. Drugs administered through tracheal injections or nebulized particles will take the path of least resistance to normal or less injured lung parenchyma, where they will be systemically absorbed. Therefore, treatment either will not reach the diseased tissue in need of therapy or will not have had sufficient exposure time to have a significant effect. This is likely the reason why surfactant administration has not shown benefit in treating adult ARDS.32 Certain therapies, such as lung lavage, may temporarily worse oxygenation by removing surfactant from normally ventilated areas. The novel apparatus allows the delivery of therapies to specific lobes that are injured and simultaneous ventilation of the remaining lobes to maintain gas exchange. Drugs that increase pulmonary perfusion, such as prostaglandin and nitric oxide, likely would improve oxygenation if delivered to the alveoli that are not shunting. Heliox or liquid ventilation could be an interesting option for lung zones, for which achieving aeration is difficult. Finally, the device has the potential to be a novel delivery platform for drugs, medical gases, and biologics.
The potential limitations of human use are related to the insertion, positioning, stability, effective ventilation, and safety. The passage of the apparatus across the narrow airway segments, such as the glottis and cricoid, is a potential limiting factor depending on the relative device available, device sizes, and the patient's airway dimensions. The lobar tubes are flexible and guided by flexible bronchoscopy so placement should be possible in all the different lobe segments; however, some lobar bronchi are very short with little margin for safe landing and cuff stabilization, in particular in the right upper lobe and right middle lobe. The main challenge foreseen is the safety of prolonged ventilation depending on required cuff pressure. An interval cuff deflation of the lobar tube probably is prudent to prevent bronchomalacia.
Conclusion
Herein a new apparatus for selective lobe ventilation and pulmonary delivery of drugs is presented. Differential lobar ventilation is the next frontier in the management of ARDS, as well as lobar pneumonias, and this novel device, when associated with new ventilatory strategies and drug delivery, potentially can expedite the availability of such therapeuties. The Shuttle tube starts as a single-lumen tube that can be used to easily perform OLV by insertion of a secondary bronchial tube, or it can be used to perform selective lobe ventilation by futher advancing secondary tubes to a lobar position. Animal studies and clinical trials are necessary to test the potential bennefits of the new concept in heterogeneous lung diseases. The new apparatus likely will provide clinical benefit in critical situations by providing better ventilation for heterogeneous lung diseases such as lobar pneumonia or atelectasis, ARDS, and bronchopleural fistulae. Moreover, new selective therapies, such as selective lavage and drug delivery, particularly of pulmory arterial dilators, potentially can provide a means to achieve better direct perfusion, V/Q, and oxygenation.
Conflict of Interest
Luiz Maracaja is the inventor of the apparatus for selective lobe ventilation. Founder of Pneumocyte LLC. The other authors declare no conflicts of interest.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1053/j.jvca.2021.04.041.
Appendix. Supplementary materials
References
- 1.Worldometer. Coronavirus death toll. Available at: https://www.worldometers.info/coronavirus/coronavirus-death-toll/. Accessed April 21, 2021.
- 2.Telias I, Katira BH, Brochard L. Is the prone position helpful during spontaneous breathing in patients with COVID-19? JAMA. 2020;323:2265–2267. doi: 10.1001/jama.2020.8539. [DOI] [PubMed] [Google Scholar]
- 3.Rahmanzade R, Rahmanzzadeh R, Tabarsi P, et al. Noninvasive versus invasive ventilation in COVID-19: One size does not fit all! Anesth Analg. 2020;131:e114–e115. doi: 10.1213/ANE.0000000000004943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caputo ND, Strayer RJ, Levitan R. Early self-proning in awake, non-intubated patients in the emergency department: A single ED's experience during the COVID-19 pandemic. Acad Emerg Med. 2020;27:375–378. doi: 10.1111/acem.13994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wellman TJ, De Prost N, Tucci M, et al. Lung metabolic activation as an early biomarker of acute respiratory distress syndrome and local gene expression heterogeneity. Anesthesiology. 2016;125:992–1004. doi: 10.1097/ALN.0000000000001334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shaver CM, Bastarache JA. Clinical and biological heterogeneity in acute respiratory distress syndrome: Direct versus indirect lung injury. Clin Chest Med. 2014;35:639–653. doi: 10.1016/j.ccm.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maley JH, Thompson BT. Embracing the heterogeneity of ARDS. Chest. 2019;155:453–455. doi: 10.1016/j.chest.2018.11.016. [DOI] [PubMed] [Google Scholar]
- 8.Wilson JG, Calfee CS. ARDS subphenotypes: Understanding a heterogeneous syndrome. Crit Care (London, Engl) 2020;24:102. doi: 10.1186/s13054-020-2778-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hurtado DE, Erranz B, Lillo F, et al. Progression of regional lung strain and heterogeneity in lung injury: Assessing the evolution under spontaneous breathing and mechanical ventilation. Ann Intensive Care. 2020;10:107. doi: 10.1186/s13613-020-00725-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thompson BT, Chambers RC, Liu KD. Acute respiratory distress syndrome. N Engl J Med. 2017;377:562–572. doi: 10.1056/NEJMra1608077. [DOI] [PubMed] [Google Scholar]
- 11.Stevens JP, Law A, Giannakoulis J. Acute respiratory distress syndrome. JAMA. 2018;319:732. doi: 10.1001/jama.2018.0483. [DOI] [PubMed] [Google Scholar]
- 12.Haudebourg AF, Perier F, Tuffet S, et al. Respiratory mechanics of COVID-19- versus non-COVID-19-associated acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020;202:287–290. doi: 10.1164/rccm.202004-1226LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beitler JR, Malhotra A, Thompson BT. Ventilator-induced lung injury. Clin Chest Med. 2016;37:633–646. doi: 10.1016/j.ccm.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med. 2013;369:2126–2136. doi: 10.1056/NEJMra1208707. [DOI] [PubMed] [Google Scholar]
- 15.Katira BH. Ventilator-induced lung injury: Classic and novel concepts. Respir Care. 2019;64:629–637. doi: 10.4187/respcare.07055. [DOI] [PubMed] [Google Scholar]
- 16.Judge EP, Hughes JML, Egan JJ, et al. Anatomy and bronchoscopy of the porcine lung. A model for translational respiratory medicine. Am J Respir Cell Mol Biol. 2014;51:334–343. doi: 10.1165/rcmb.2013-0453TR. [DOI] [PubMed] [Google Scholar]
- 17.Mauad T, Duarte-Neto AN, da Silva LFF, et al. Tracking the time course of pathological patterns of lung injury in severe COVID-19. Respir Res. 2021;22:32. doi: 10.1186/s12931-021-01628-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fanelli V, Fanelli V, Vlachou A, et al. Acute respiratory distress syndrome: new definition, current and future therapeutic options. J Thorac Dis. 2013;5:326–334. doi: 10.3978/j.issn.2072-1439.2013.04.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Estenssoro E, Dubin A, Laffaire E, et al. Incidence, clinical course, and outcome in 217 patients with acute respiratory distress syndrome. Crit Care Med. 2002;30:2450–2456. doi: 10.1097/00003246-200211000-00008. [DOI] [PubMed] [Google Scholar]
- 20.de Prost N, Ricard J-D, Saumon G, et al. Ventilator-induced lung injury: Historical perspectives and clinical implications. Ann Intensive Care. 2011;1:28. doi: 10.1186/2110-5820-1-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beitler JR, Sands SA, Loring SH, et al. Quantifying unintended exposure to high tidal volumes from breath stacking dyssynchrony in ARDS: The BREATHE criteria. Intensive Care Med. 2016;42:1427–1436. doi: 10.1007/s00134-016-4423-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petrucci N, De Feo C. Lung protective ventilation strategy for the acute respiratory distress syndrome. Cochrane Database System Rev. 2013;2 doi: 10.1002/14651858.CD003844.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pelosi P, Rocco PR, de Abreu MG. Use of computed tomography scanning to guide lung recruitment and adjust positive-end expiratory pressure. Curr Opin Crit Care. 2011;17:268–274. doi: 10.1097/MCC.0b013e328344ddbc. [DOI] [PubMed] [Google Scholar]
- 24.Zompatori M, Ciccarese F, Fasano L. Overview of current lung imaging in acute respiratory distress syndrome. Eur Respir Rev. 2014;23:519–530. doi: 10.1183/09059180.00001314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bachmann MC, Morais C, Bugedo G, et al. Electrical impedance tomography in acute respiratory distress syndrome. Crit Care. 2018;22:263. doi: 10.1186/s13054-018-2195-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Das A, Haque M, Chikhani M, et al. Hemodynamic effects of lung recruitment maneuvers in acute respiratory distress syndrome. BMC Pulm Med. 2017;17:34. doi: 10.1186/s12890-017-0369-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bunting L, Roy S, Pinson H, et al. A novel inline PEEP valve design for differential multi-ventilation. Am J Emerg Med. 2020;38:2045–2048. doi: 10.1016/j.ajem.2020.06.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levin MA, Shah A, Shah R, et al. Differential ventilation using flow control valves as a potential bridge to full ventilatory support during the COVID-19 crisis. Anesthesiology. 2020;133:892–904. doi: 10.1097/ALN.0000000000003473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brodsky JB. Approaches to hypoxemia during single-lung ventilation. Curr Opin Anaesthesiol. 2001;14:71–76. doi: 10.1097/00001503-200102000-00012. [DOI] [PubMed] [Google Scholar]
- 30.Hedenstierna G, Baehrendtz S, Kingstedt J, et al. Ventilation and perfusion of each lung during differential ventilation with selective PEEP. Anesthesiology. 1984;61:369–376. doi: 10.1097/00000542-198410000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Robertson HT. Dead space: The physiology of wasted ventilation. Eur Respir J. 2015;45:1704–1716. doi: 10.1183/09031936.00137614. [DOI] [PubMed] [Google Scholar]
- 32.Meng S-S, Chang W, Lu ZH, et al. Effect of surfactant administration on outcomes of adult patients in acute respiratory distress syndrome: A meta-analysis of randomized controlled trials. BMC Pulm Med. 2019;19:9. doi: 10.1186/s12890-018-0761-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.