Abstract
We present the case of a 45-year-old man who had presented with ubiquitous chylous reflux that manifested as a bilateral inguinal chylous cutaneous fistula and a voluminous right cervical chylous cyst. He had difficulty breathing owing to compression of the airway. Anastomosis of the chylous cyst wall with the external jugular vein was performed using a valvular vein segment to prevent blood reflux. Postoperatively, anticoagulant therapy was initiated. We found this derivative surgical procedure to be an effective and minimally invasive technique for complex lymphatic anomalies.
Keywords: Chylothorax, Chylous ascites, Chylous reflux, Lymphatic malformations, Thoracic duct
Complex lymphatic anomaly is an umbrella term that refers to high-risk lymphatic anomaly phenotypes and includes several entities with overlapping patterns of clinical symptoms, anatomic locations, imaging features, hematologic disorders, and complications.1,2 Any abnormality of the lymphatic system can trigger the emergence of lymphatic anomalies, composed of a rare diseases group consisting of Gorham-Stout disease, generalized lymphatic anomaly, cystic lymphatic malformation, and central conducting lymphatic anomaly (CCLA).
CCLA is classified by the International Society for the Study of Vascular Anomalies as a channel-type lymphatic malformation, consisting of a complicated lymphatic malformation that affects the paraxial lymphatic system, including the thoracic duct, cisterna chyli, and lumbar trunks and their tributaries.3 This group of complex lymphatic malformations with unclarified etiology results in abnormal function and structure of the involved lymphatic vessels, manifesting as lymphangiectasia, abnormal drainage of lymphatic fluid, lymphatic reflux, and effusion accumulation. The clinical manifestations can vary significantly, depending on the location and degree of involvement.
CCLA is difficult to distinguish from other lymphatic conditions owing to the common presenting characteristics and patterns that significantly overlap with those of other related groups such as generalized lymphatic anomaly. These include, but are not limited to, leakage and reflux of lymph fluid, pleural and pericardial effusions, chylothorax, pulmonary lymphangiectasia, chylous ascites, protein-losing enteropathy, subcutaneous effusions, and extremity swelling. Thus, the distinctive characteristic of CCLA is defined, not by dysmotility or lymphatic obstruction resulting in slow flow, stasis, high pressure, reflux, and chyle or by lymph leak into the pericardial, pleural, or peritoneal spaces, but by following the distinctive course of the orthotopic lymphatic channels.
The genetics of CCLA have yet not been fully established. EPHB4 and ARAF mutations have been identified as potential causes of the disease because activation of the signaling cascade of the gene is crucial to the differentiation process of the venous and lymphatic system and lymphatic valve development.4,5 Recently, a pathogenic variant corresponding to the JAG-1 gene has been reported as the cause of CCLA, broadening the spectrum of genes affected by the pathology, which remains a vast field for study.6
Medical treatment such as sirolimus (an mTOR [mechanistic target of rapamycin] inhibitor) has been shown in several trials to have good results in the management of lymphatic malformations.7, 8, 9, 10 However, to date, the evidence is not sufficient regarding its application to treat CCLAs. In addition, owing to the complex nature of these lesions, CCLAs have demonstrated a refractory response to some surgical treatments.11 Thus, we present an alternative surgical technique as a reasonable and palliative option for the treatment of cervical CC by creating a physiologic bypass from the chylous into the vein.
Case report
A 45-year-old patient, well known in our unit owing to his presentation with complex lymphatic anomalies of the CCLA type. He had a history of refractory chylothorax treated with pleurocentesis and pleurodesis with thoracic duct ligation and genital chylocutaneous fistulas. He presented for medical consultation with a right cervical tumor of recent appearance, which was diagnosed as a CC with extension into the upper mediastinum. The patient provided written informed consent for the report of his case.
He denied odynophagia, dysphagia, dyspnea, and any voice changes and had no history of previous neck surgery or trauma. On examination, a noncompressible, nontransilluminating, 8 × 12 × 5 cm swelling over the right supraclavicular fossa was found. Ultrasound examination of the neck confirmed a cystic mass ~8 × 12 × 5 cm and demonstrated enlarged hypoechoic compressible channels with absent blood flow on color flow Doppler. Aspiration puncture was performed, obtaining 300 mL of chyle/lymph, confirmed by biochemical analysis (Fig 1).
Fig 1.
Photographs showing aspiration puncture of the cyst, which obtained a white milky fluid confirmed as chyle by biochemical analysis.
After the puncture, contrast was injected under radioscopy guidance to evaluate the cyst extension and the possibility for subsequent sclerotherapy. The imaging study showed a voluminous cyst originating from the superior mediastinum, passing the limit of the right clavicle, and occupying the space of the supraclavicular fossa.
As a first step, the patient underwent sclerotherapy with bleomycin 0.5 mg/kg body weight, not exceeding 10 U at a time, with good tolerance to the procedure. After 3 months of follow-up, the cyst had increased in size. Thus, another intralesional bleomycin injection was performed but without satisfactory results and with persistence of the cervical CC. At 1 month after sclerotherapy, the patient reported the onset of pain and discomfort in the right supraclavicular region with the sensation of suffocation, which required the urgent development of another therapeutic alternative.
Inspired by the concept of lymphovenous anastomosis (LVA),12 the surgical alternative of creating a cystic–venous pathway was proposed, as a palliative treatment, to create a physiologic bridge that would allow for the reincorporation of the chylous and lymph content into the bloodstream. We proceeded with a venous Duplex ultrasound examination to determine which venous vessel would allow for sufficient flow and anatomic closeness to offer a valvular segment for anastomosis. The external jugular vein was selected for this purpose.
A lateral cervical right incision was performed, and the platysma muscle was sectioned and retracted to access the cyst. The external jugular vein was carefully dissected around its circumference (Fig 2) and sectioned, and the vein's proximal end was ligated (Fig 3). Subsequently, a thickened cystic wall was identified, opened, and sutured using a side-to-end technique at the distal end of the sectioned external jugular vein to create the cystic–venous bridge (Fig 4). The patient was discharged with instructions to continue anticoagulation therapy with apixaban for 6 months postoperatively.
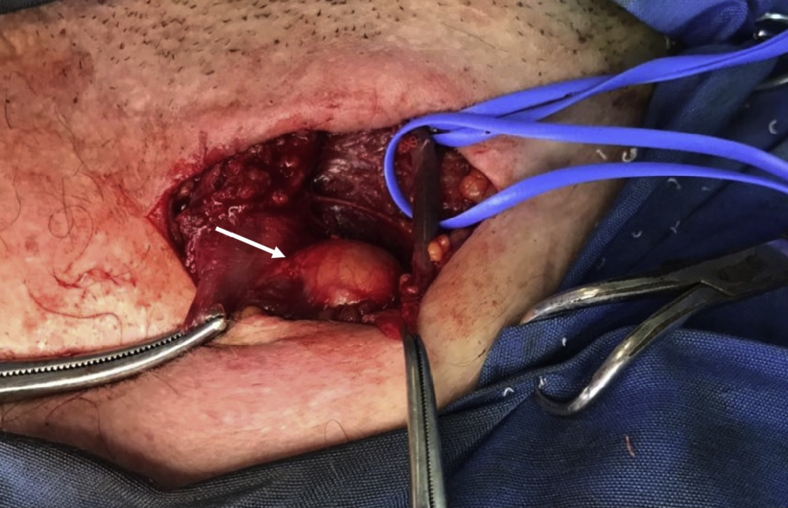
Fig 2.
The right external jugular vein was carefully dissected around its circumference and identified with a blue vessel loop. The chylous cyst (arrow) is adjacent to it.
Fig 3.
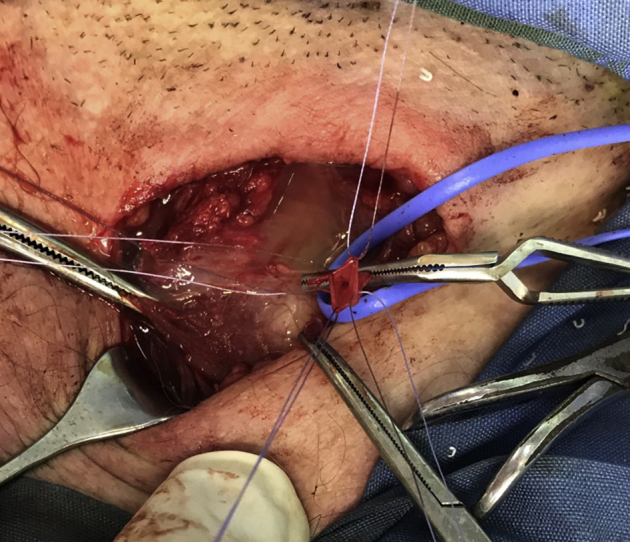
External jugular vein sectioned and prepared for anastomosis and cyst wall opened.
Fig 4.

Creation of the cyst–venous shunt with a side to end anastomosis. Chylous fluid can be bypassed to the external jugular vein from either side of the cyst.
The CC–venous anastomosis resulted in a significant reduction in the cyst size and improvement in the patient's symptoms. We had used a valvular vein segment to prevent venous blood reflux. The wound healed without any postoperative complications requiring exploration and met aesthetic criteria. The patient experienced complete resolution of the cervical mass (Fig 5). At 1 year of follow-up, he had been strictly compliant with our recommendations and had experienced significant improvement in his symptoms. Therefore, a more invasive approach was not considered.
Fig 5.

Photograph showing the healed wound with full resolution of the cervical mass.
Discussion
The analysis of the clinical, imaging, histologic, and hematologic features is often needed to reach a diagnosis. Aspiration of fluid collections can readily determine whether the fluid is chylous fluid.13 The presence of chyle indicates dysfunction at the mesenteric or retroperitoneal level or above the cisterna chyli owing to reflux.14 The imaging patterns of generalized lymphatic anomaly and Gorham-Stout disease have been segregated by their distinctive bone lesions and periosteum features.15,16 More aggressive histologic features, such as spindled lymphatic endothelial cells, clinical progression, hemorrhage, and/or moderate hematologic changes should raise suspicion of kaposiform lymphangiomatosis. A biopsy could be needed for the diagnosis, although avoidance of a rib biopsy is advised to prevent iatrogenic chronic pleural effusion. Lymphangiography and/or magnetic resonance imaging can be used to visualize the anatomy and function of the lymphatic system and might identify thoracic duct dysfunction in CCLAs.17, 18, 19, 20
Complex lymphatic anomalies are usually treated by either surgical resection or sclerotherapy after unsuccessful medical treatment. However, sclerotherapy has limited efficacy, and surgical resection has high complication rates. Although new targeted treatments such as sirolimus have recently been developed, the features of CCLA are not well known enough to define therapeutic behavior. Thus, the currently available therapeutic options are more of a palliative nature and provide a transitory response to the pathology.
Therefore, a novel approach is required to achieve better surgical results. Less invasive procedures, such as LVA, are widely accepted interventions for lymphedema. In the present case report, we assessed the outcomes of an LVA modification to treat a complex lymphatic malformation.
Conclusion
We found the venous anastomosis procedure determined from the findings of a chylous flow assessment is an effective and minimally invasive surgery for complex lymphatic anomalies. This type of malformation presents with significant diagnostic and therapeutic challenges. Future therapies will be developed from the results of clinical studies and a better understanding of the effects of combinations of multiple medications and medical and procedural treatment protocols.
Footnotes
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Fishman S.J., Burrows P.E., Upton J., Hendren W.H. Life-threatening anomalies of the thoracic duct: anatomic delineation dictates management. J Pediatr Surg. 2001;36:1269–1272. doi: 10.1053/jpsu.2001.25792. [DOI] [PubMed] [Google Scholar]
- 2.Papendieck C.M., Amore M.A. An atlas of neonatal and infantile lymphedema. In: Lee B.B., Rockson S.G., Bergan J., editors. Lymphedema: A Concise Compendium of Theory and Practice. 2nd edition. Springer; Cham, Switzerland: 2018. pp. 777–798. [Google Scholar]
- 3.International Society for the Study of Vascular Anomalies ISSVA Classification for Vascular Anomalies. Approved at the May 2018 General Assembly, Amsterdam, The Netherlands. http://issva.org/classification Available at:
- 4.Li D., March M.E., Gutierrez-Uzquiza A., Kao C., Seiler C., Pinto E. ARAF recurrent mutation causes central conducting lymphatic anomaly treatable with a MEK inhibitor. Nat Med. 2019;25:1116–1122. doi: 10.1038/s41591-019-0479-2. [DOI] [PubMed] [Google Scholar]
- 5.Li D., Wenger T.L., Seiler C., March M.E., Gutierrez-Uzquiza A., Kao C. Pathogenic variant in EPHB4 results in central conducting lymphatic anomaly. Hum Mol Genet. 2018;27:3233–3245. doi: 10.1093/hmg/ddy218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li D., Sheppard S.E., Peroutka C., Barnes C., Reid J.R., Smith C.L. Expanded phenotypic spectrum of JAG1-associated diseases: central conducting lymphatic anomaly with a pathogenic variant in JAG1. Clin Genet. 2021 Jan 12 doi: 10.1111/cge.13915. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Papendieck C.M., Amore M.A. Treatment strategy on neonatal and infant CVMs. In: Kim Y.W., Lee B.B., Yakes W.F., Soo Do Y., editors. Congenital Vascular Malformations: A Comprehensive Review of Current Management. 1st ed. Springer; Cham, Switzerland: 2017. pp. 363–368. [Google Scholar]
- 8.Papendieck C.M., Amore M.A. What is difference in management of primary lymphedema between adults and children, and how much? In: Lee B.B., Gloviczki P., Blei F., editors. Vascular Malformations: Advances and Controversies in Contemporary Management. 1st ed. Taylor & Francis Ltd; Boca Raton, FL: 2019. pp. 283–286. [Google Scholar]
- 9.Papendieck C.M., Amore M.A. How much different should the management of lymphangioma among the pediatric/neonatal age group be? In: Lee B.B., Gloviczki P., Blei F., editors. Vascular Malformations: Advances and Controversies in Contemporary Management. 1st ed. Taylor & Francis Ltd; Boca Raton, FL: 2019. pp. 327–330. [Google Scholar]
- 10.Salvia S.A., Amore M.A., Papendieck C.M. 2020. Topical tacrolimus 0.1% for treatment of cutaneous microcystic lymphatic malformations, lymphology number #1692/20. [PubMed] [Google Scholar]
- 11.Fishman S.J., Burrows P.E., Upton J., Hendren W.H. Life-threatening anomalies of the thoracic duct: anatomic delineation dictates management. J Pediatr Surg. 2001;36:1269–1272. doi: 10.1053/jpsu.2001.25792. [DOI] [PubMed] [Google Scholar]
- 12.Papendieck C., Barbosa L., Amore M., Martinez Allende R., Mogollon G., Gomez Rueda S. A new combined operative technique using crossed inguinal lymphatic rescue for pediatric patients with mixed lymphatic and venous malformations. Lymphology. 2017;50:141–147. [PubMed] [Google Scholar]
- 13.Ross J.K. A review of the surgery of the thoracic duct. Thorax. 1961;16:12–21. doi: 10.1136/thx.16.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kwon S.S., Falk A., Mitty H.A. Thoracic duct injury associated with left internal jugular vein catheterization: anatomic considerations. J Vasc Interv Radiol. 2002;13:337–339. doi: 10.1016/s1051-0443(07)61730-8. [DOI] [PubMed] [Google Scholar]
- 15.Trenor C.C., III, Chaudry G. Complex lymphatic anomalies. Semin Pediatr Surg. 2014;23:186–190. doi: 10.1053/j.sempedsurg.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 16.Papendieck C.M., Amore M.A. Treatment strategy on chylolymphatic/CVMs. In: Kim Y.W., Lee B.B., Yakes W.F., Soo Do Y., editors. Congenital Vascular Malformations: A Comprehensive Review of Current Management. 1st ed. Springer; Cham, Switzerland: 2017. pp. 355–362. [Google Scholar]
- 17.Papendieck C.M., Amore M.A. How should aggressive chyloreflux (e.g. chyluria, chyloascites, chylothorax, chyle leakage) be handled? In: Lee B.B., Gloviczki P., Blei F., editors. Vascular Malformations: Advances and Controversies in Contemporary Management. 1st edition. Taylor & Francis Ltd; Boca Raton, FL: 2019. pp. 319–324. [Google Scholar]
- 18.Amore M., Bernárdez R., Enríquez R., Granja S., Romeo H. Anatomical variations of the thoracic duct: a preliminary report in adult and fetal specimens. Lymphology. 2016;49:205–209. [PubMed] [Google Scholar]
- 19.Schuenke M., Schulte E., Schumacher U., Ross L.M., Lamperti E.D., Voll M. Thieme; New York, NY: 2010. Atlas of Anatomy: Neck and Internal Organs. [Google Scholar]
- 20.Langford R.J., Daudia A.T., Malins T.J. A morphological study of the thoracic duct at the jugulo-subclavian junction. J Craniomaxillofac Surg. 1999;27:100–104. doi: 10.1016/s1010-5182(99)80021-3. [DOI] [PubMed] [Google Scholar]