Abstract
Secondary aortoenteric fistula is a potentially lethal complication after aortic surgery. Traditional treatment consists of open graft excision with extra-anatomic bypass or in situ reconstruction. Patients who present in extremis, however, are generally poor candidates for re-do open aortic surgery. Endovascular repair has emerged as an alternative treatment modality for patients who would otherwise be unable to tolerate an extended operation. We report here a case of urgent endovascular repair of a juxtarenal secondary aortoenteric fistula via endovascular aneurysm repair with a renal artery chimney in a patient with a solitary kidney who presented in hemorrhagic and septic shock.
Keywords: Chimney, EVAR, Aortoenteric fistula, Juxtarenal, Abdominal aortic aneurysm
Secondary aortoenteric fistula (SAEF) is a devastating clinical entity. Definitive therapy consists of graft excision with extra-anatomic bypass or in situ reconstruction in addition to bowel repair.1, 2, 3 Such a major operative undertaking in patients who commonly present with hemorrhagic and/or septic shock often carries dismal results. Mortality ranges from 21% to 47% at 30 days, with higher rates noted in hemodynamically unstable patients.2,4, 5, 6, 7, 8, 9, 10, 11, 12 Because of unfavorable outcomes associated with open surgery, endovascular repair has emerged as a therapeutic option for treatment of SAEF.13,14
We report here a case of urgent endovascular repair of a juxtarenal SAEF via endovascular aneurysm repair (EVAR) with renal artery chimney stent graft in an unstable patient with a solitary kidney. The patient has consented to publication of this report.
Case report
A 68-year-old morbidly obese man with history of myocardial infarction, peripheral arterial disease, and a solitary kidney (owing to prior kidney donation to his wife) underwent open Dacron tube graft repair of a ruptured abdominal aortic aneurysm at another facility 30 days before his presentation.
His postoperative course was complicated by a large proximal anastomotic pseudoaneurysm with an aortocolonic fistula, managed nonoperatively. On postoperative day 30, he was transferred to our institution for worsening sepsis. Shortly after arrival, superimposed hemorrhagic shock developed after 1 L of lower gastrointestinal blood loss. Previous imaging demonstrated a fistula just proximal to the left renal artery ostium and a broad-based communication with the midtransverse colon (Fig 1). Given the patient's acutely deteriorating clinical status and hostile anatomy, a multidisciplinary team deemed him unsuitable for traditional definitive open surgery. A decision was made to proceed with a minimally invasive approach as a palliative measure.
Fig 1.
Computed tomography angiography demonstrating a broad-based, fistulous communication between the aorta just proximal to the left renal artery ostium and the midtransverse colon.
In the hybrid operating suite, transbrachial aortography demonstrated the juxtarenal proximal anastomosis abutting the left renal artery ostium and a patent tube graft sewn to a severely calcified distal aortic domain with extension of atherosclerosis into the proximal bilateral common iliac arteries.
After systemic heparinization via left brachial access, the left renal artery was selectively catheterized and a 90-cm 7F sheath was advanced for delivery of a VBX chimney stent graft (W.L. Gore & Associates, Flagstaff, Ariz) with ample proximal extension into unaffected proximal aorta in zone 7. Meanwhile, to seal the fistula and control active hemorrhage, an Endurant II proximal aortic cuff (Medtronic Aortic, Inc., Santa Rosa, Calif) was advanced and deployed into zone 8, covering the native left renal ostium with simultaneous placement of the VBX stent graft. Prompt improvement in hemodynamics was noted after this maneuver.
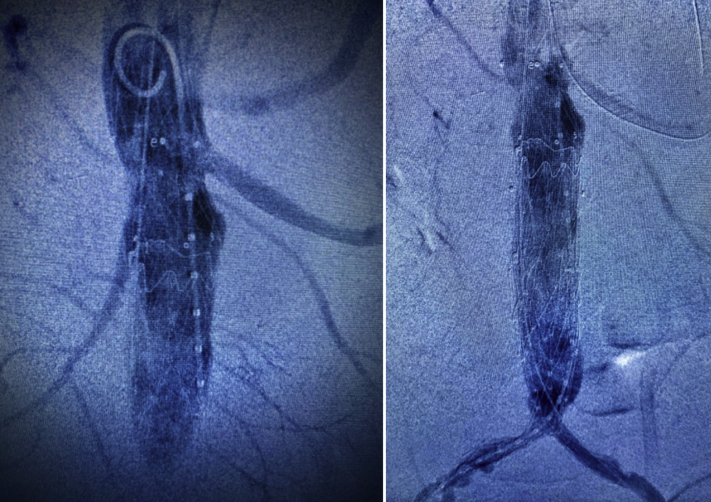
Next, to exclude the entire tube graft from the systemic circulation, avoid contralateral gate cannulation, and concomitantly address bilateral iliac occlusive disease, an anatomically fixated, a bifurcated unibody AFX aortic endograft (Endologix, Inc., Irvine, Calif) was deployed at the aortic bifurcation with overlap into the previously deployed proximal cuff (Fig 2). This strategy facilitated kissing balloon angioplasty with luminal augmentation of diseased bilateral iliac arteries. Completion angiography demonstrated no endoleak.
Fig 2.
Completion angiography demonstrating exclusion of the fistula after placement of an aortic cuff and left renal artery chimney stent graft (left) and complete exclusion of the infected tube graft via placement of an anatomically fixated, bifurcated unibody endograft (right).
Given the interval improvement in hemodynamics, the patient then underwent laparotomy for an extended left hemicolectomy and transverse colostomy. Total operative time was 8 hours, including 3 hours of vascular surgery. Of note, approximately 90 minutes of adhesiolysis was required to gain access to the fistula. The abdomen was closed primarily at the conclusion of the procedure.
Prompt postoperative improvement in sepsis and hemodynamic status was noted. Serial postoperative imaging to 18 months has shown near-resolution of periaortic inflammatory changes with widely patent aortic and left renal arterial repairs (Fig 3, Fig 4, Fig 5). The patient is active and fully functional. He initially received 6 weeks of intravenous antibiotics and remains on lifelong suppressive ciprofloxacin and amoxicillin/clavulanic acid (Augmentin).
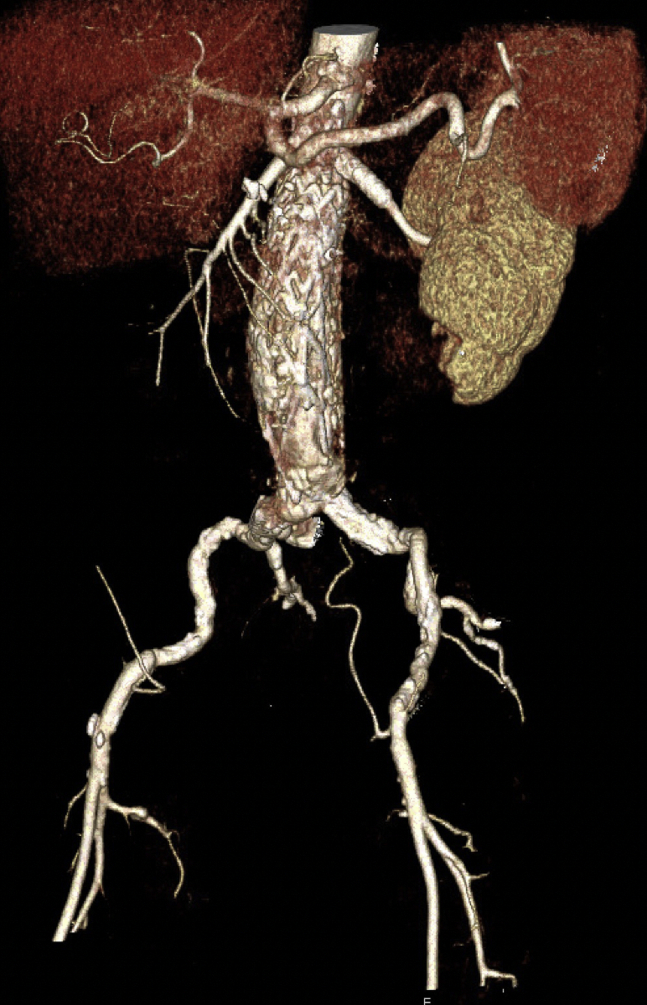
Fig 3.
Three-dimensional reconstruction of surveillance computed tomography angiography at 7 months demonstrating patent left renal artery and patent endograft.
Fig 4.
Noncontrast computed tomography scan at 18 months demonstrating minimal per-aortic inflammation.
Fig 5.
Ultrasound examination at 18 months demonstrating widely patent aorta and left renal artery.
Discussion
Although open surgical repair remains the definitive therapy for SAEF, many unstable patients are unfit for this operation. An endovascular approach has been associated with decreased perioperative morbidity and mortality and shorter hospital stays, although some studies have reported that these early benefits are lost in longer follow-up owing to persistent/recurrent infection rates as high as 60% at 1 year.14, 15, 16, 17 Other investigators have contended, however, that long-term survival rates are similar in both open and endovascular groups.16 In fact, EVAR is now the preferred treatment for mycotic abdominal aortic aneurysms (MAAA) in Sweden and was associated with increased 3-month survival (74% open vs 96% EVAR; P < .001) without a higher incidence of serious infection-related complications or reoperations and comparable survival (60% open vs 58% EVAR; P = .771) at 5 years.18 A similar 5-year survival of 53% was reported in a Taiwanese cohort who underwent EVAR for MAAA.19 Another smaller study evaluating open vs endovascular repair for MAAA in the Netherlands found comparable outcomes between the two modalities at 1 year.20 Selection bias does exist in retrospective studies of SAEF, because patients who underwent endovascular treatment were likely more medically complex and unfit for open repair. Thus, continued debate revolves around whether endovascular management should serve strictly as a bridge to open surgery or if it can be considered a permanent solution.13 In our opinion, consideration should be given to an endovascular-first approach, regardless of the ultimate decision to pursue definitive open surgery.
A number of notable features are highlighted in this case. First, our patient presented in hemorrhagic and septic shock, both predictors of increased postoperative mortality.17 Second, the juxtarenal position of the SAEF and its communication with the transverse colon is unusual. The large majority of reports on SAEF describe infrarenal AEF, most commonly in communication with the third part of the duodenum secondary to long-term erosion.13,17,21 Upon laparotomy, the distal and descending colon were found to be frankly necrotic with extension of necrosis to the level of the proximal rectum. The early development of SAEF at the proximal anastomosis suggests colonic injury potentially secondary to ischemia and/or iatrogenic factors during the initial AAA repair. Last, open repair would have required a suprarenal cross-clamp. Delivery of a chimney stent graft allowed exclusion of the fistula without interrupting renal perfusion in this patient with a solitary kidney. There are two previous reports that use a similar strategy. One describes a SAEF initially treated with open aortic ligation and extra-anatomic bypass. Four months later, there was a recurrent SAEF, which was then treated via bilateral renal artery chimney stent grafts and endovascular coiling of the aortic stump.22 Another report describes SAEF at the proximal anastomosis of an elective open AAA repair from years prior.23 This patient had a known occluded left iliac limb; thus, he underwent bilateral renal artery chimney stent graft with aorto-uni-iliac stent graft placement into the right limb. In contrast, our patient required either a bifurcated device or an aorto-uni-iliac device with femoral-femoral bypass to maintain perfusion to bilateral lower extremities. In the context of the patient's hemodynamic instability and known iliac occlusive disease, the Endologix AFX aortic platform was selected, allowing us a low-profile bifurcated unibody device that obviates the need for contralateral gate cannulation.
This patient will continue on lifelong antibiotics, which has been advocated as an acceptable mode of treatment.13,24 Endovascular repair of SAEF without subsequent aortic reconstruction has been anecdotally reported with good outcomes ranging from 8 months to 4 years.25, 26, 27 Other reports caution against viewing EVAR as definitive therapy, because reinfection and recurrence have been reported in up to 60% of patients at 1 year in patients who initially presented with sepsis.14,15 In a select group of anatomically and medically challenging patients, however, this procedure may be the only option.
Footnotes
Author conflict of interest: N.N. is on the speakers' bureau and a consultant for Endologix, Inc. and W. L. Gore & Associates; and is an advisor, consultant, and on the advisory board for Medtronic Aortic, Inc.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Feo C.F., Ginesu G.C., Pinna A., Galotti F., Paliogiannis P., Fancellu A. In situ reconstruction with autologous graft in the treatment of secondary aortoenteric fistulas: a retrospective case series. Ann Med Surg (Lond) 2020;49:53–56. doi: 10.1016/j.amsu.2019.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gnus J., Ferenc S., Koscielna M., Paprocka-Borowicz M., Dawidczyk P., Dziewiszek Secondary aortoenteric fistula after abdominal aortic graft implementation in our own material. Adv Clin Exp Med. 2016;25:1265–1271. doi: 10.17219/acem/66621. [DOI] [PubMed] [Google Scholar]
- 3.Kuestner L.M., Reilly L.M., Jicha D.L., Ehrenfeld W.K., Goldstone J., Stoney R.J. Secondary aortoenteric fistula: contemporary outcome with use of extraanatomic bypass and infected graft excision. J Vasc Surg. 1995;21:184–196. doi: 10.1016/s0741-5214(95)70261-x. [DOI] [PubMed] [Google Scholar]
- 4.Chopra A., Cieciura L., Modrall J.G., Valentine R.J., Chung J. Twenty-year experience with aorto-enteric fistula repair: gastrointestinal complications predict mortality. J Vasc Surg. 2017;66:1625. doi: 10.1016/j.jamcollsurg.2017.01.050. [DOI] [PubMed] [Google Scholar]
- 5.Montgomery R.S., Wilson S.E. The surgical management of aortoenteric fistulas. Surg Clin North Am. 1996;76:1147–1157. doi: 10.1016/s0039-6109(05)70503-x. [DOI] [PubMed] [Google Scholar]
- 6.Dorigo W., Pulli R., Azas L., Pratesi G., Innocenti A.A., Pratesi C. Early and long-term results of conventional surgical treatment of secondary aorto-enteric fistula. Eur J Vasc Endovasc Surg. 2003;26:512–518. doi: 10.1016/s1078-5884(03)00379-4. [DOI] [PubMed] [Google Scholar]
- 7.Argyriou C., Georgiadis G.S., Lazarides M.K., Georgakarakos E., Antoniou G.A. Endograft infection after endovascular abdominal aortic aneurysm repair: a systematic review and meta-analysis. J Endovasc Ther. 2017;24:688–697. doi: 10.1177/1526602817722018. [DOI] [PubMed] [Google Scholar]
- 8.Kahlberg A., Rinaldi E., Piffaretti G., Speziale F., Trimarchi S., Bonardelli S. Results from the Multicenter Study on Aortoenteric Fistulization After Stent Grafting of the Abdominal Aorta (MAEFISTO) J Vasc Surg. 2016;64:313–320.e1. doi: 10.1016/j.jvs.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 9.O'Mara C.S., Williams G.M., Ernst C.B. Secondary aortoenteric fistula: a 20 year experience. Am J Surg. 1981;142:203–209. doi: 10.1016/0002-9610(81)90275-0. [DOI] [PubMed] [Google Scholar]
- 10.Biro G., Szabo G., Fehervari M., Munch Z., Szeberin Z., Acsady G. Late outcome following open surgical management of secondary aortoenteric fistula. Langenbecks Arch Surg. 2011;396:1221–1229. doi: 10.1007/s00423-011-0807-6. [DOI] [PubMed] [Google Scholar]
- 11.Armstrong P.A., Back M.R., Wilson J.S., Shames M.L., Johnson B.L., Bandyk D.F. Improved outcomes in the recent management of secondary aortoenteric fistula. J Vasc Surg. 2005;42:660–666. doi: 10.1016/j.jvs.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 12.Bergqvist D., Björck M. Secondary arterioenteric fistulation - a systematic literature analysis. Eur J Vasc Endovasc Surg. 2009;37:31–42. doi: 10.1016/j.ejvs.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 13.Baril D.T., Carroccio A., Ellozy S.H., Palchik E., Sachdev U., Jacobs T.S. Evolving strategies for the treatment of aortoenteric fistulas. J Vasc Surg. 2006;44:250–257. doi: 10.1016/j.jvs.2006.04.031. [DOI] [PubMed] [Google Scholar]
- 14.Danneels M.I.L., Verhagen H.J.M., Teijink J.A.W., Cuypers P., Nevelsteen A., Vermassen F.E.G. Endovascular repair for aorto-enteric fistula: a bridge too far or a bridge to surgery? Eur J Vasc Endovasc Surg. 2006;32:27–33. doi: 10.1016/j.ejvs.2005.11.031. [DOI] [PubMed] [Google Scholar]
- 15.Antoniou G.A., Koutsias S., Antoniou S.A., Georgiakakis A., Lazarides M.K., Giannoukas A.D. Outcome after endovascular stent graft repair of aortoenteric fistula: a systematic review. J Vasc Surg. 2009;49:782–789. doi: 10.1016/j.jvs.2008.08.068. [DOI] [PubMed] [Google Scholar]
- 16.Kakkos S.K., Bicknell C.D., Tsolakis I.A., Bergqvist D. Editor's choice - management of secondary aorto-enteric and other abdominal arterio-enteric fistulas: a review and pooled data analysis. Eur J Vasc Endovasc Surg. 2016;52:770–786. doi: 10.1016/j.ejvs.2016.09.014. [DOI] [PubMed] [Google Scholar]
- 17.Spanos K., Kouvelos G., Karathanos C., Matsagkas M., Giannoukas A.D. Current status of endovascular treatment of aortoenteric fistula. Semin Vasc Surg. 2017;30:80–84. doi: 10.1053/j.semvascsurg.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Sorelius K., Wanhainen A., Furebring M., Bjorck M., Gillgren P., Mani K. Nationwide study of the treatment of mycotic abdominal aortic aneurysms comparing open and endovascular repair. Circulation. 2016;134:1822–1832. doi: 10.1161/CIRCULATIONAHA.116.024021. [DOI] [PubMed] [Google Scholar]
- 19.Luo C.M., Chan C.Y., Chen Y.S., Wang S.S., Chi N.H., Wu I.H. Long-term outcome of endovascular treatment for mycotic aortic aneurysm. Eur J Vasc Endovasc Surg. 2017;54:464–471. doi: 10.1016/j.ejvs.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 20.Dang Q., Statius van Eps R.G., Wever J.J., Veger H.T.C. Dutch Society of Vascular Surgery, Steering Committee of the Dutch Surgical Aneurysm Audit, Dutch Institute for Clinical Auditing.. Nationwide study of the treatment of mycotic abdominal aortic aneurysms comparing open and endovascular repair in The Netherlands. J Vasc Surg. 2020;72:531–540. doi: 10.1016/j.jvs.2019.09.060. [DOI] [PubMed] [Google Scholar]
- 21.Osman M.F., Lyden S., Farivar B., Nazzal M., Srivastava S. Contemporary outcomes in the management of aortoenteric fistula. J Vasc Surg. 2018;68:e47–e48. [Google Scholar]
- 22.Colombi D., Bodini F.C., Sverzellati N., Morelli N., Capelli P., Michieletti E. A case of recurrent secondary aortoenteric fistula 4 months after surgery treated by endovascular coiling of the aortic stump and bilateral chimney stent grafts to renal arteries. Ann Vasc Surg. 2019;59:310.e1–310.e5. doi: 10.1016/j.avsg.2018.12.104. [DOI] [PubMed] [Google Scholar]
- 23.Tan G.W.L., Wong D., Punamiya S., Tan B.P., Vu C., Ang B. Aortoenteric fistula treated with endovascular aortic stent-graft and bilateral chimney stent-grafts to renal arteries. Ann Vasc Surg. 2012;26:422.e13–422.e136. doi: 10.1016/j.avsg.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 24.Burks J.A., Faries P.L., Gravereaux E.C., Hollier L.H., Marin M.L. Endovascular repair of bleeding aortoenteric fistulas: a 5-year experience. J Vasc Surg. 2001;34:1055–1059. doi: 10.1067/mva.2001.119752. [DOI] [PubMed] [Google Scholar]
- 25.Biancari F., Romsi P., Perälä J., Koivukangas V., Cresti R., Juvonen T. Staged endovascular stent-grafting and surgical treatment of a secondary aortoduodenal fistula. Eur J Vasc Endovasc Surg. 2006;31:42–43. doi: 10.1016/j.ejvs.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 26.Sörelius K., Sundbom M., Mani K., Wanhainen A. Hybrid treatment of a post-EVAR aortoenteric fistula. Vascular. 2013;22:385–389. doi: 10.1177/1708538113501661. [DOI] [PubMed] [Google Scholar]
- 27.Kotsis T., Lioupis C., Tzanis A., Nasiopoulou P., Goumasas K., Bakoyiannis K. Endovascular repair of a bleeding secondary aortoenteric fistula with acute leg ischemia: a case report and review of the literature. J Vasc Interv Radiol. 2006;17:563–567. doi: 10.1097/01.RVI.0000202745.36419.5A. [DOI] [PubMed] [Google Scholar]