Abstract
Hormographiella aspergillata is a rare cause of invasive mold infection, mostly described in patients with hematological malignancies. We describe two cases of invasive H. aspergillata infections in patients with acute myeloid leukemia, successfully managed with complete surgical resection of the lesions and antifungal therapy of voriconazole alone or liposomal amphotericin B, followed by voriconazole, highlighting the key role of a multidisciplinary approach for the treatment of this rare and severe invasive mold infection.
Keywords: Coprinopsis cinerea, Hormographiella aspergillata, Voriconazole, Amphotericin B, Fungal PCR
Highlights
-
•
Hormographiella apergillata is a rare cause of invasive mold infection.
-
•
Early invasive procedures with tissue biopsies are crucial to obtain a diagnosis.
-
•
Surgical source control of all lesions should be attempted as soon as possible.
-
•
Liposomal amphotericin B is probably the best empirical antifungal therapy.
-
•
Maintenance with voriconazole can be considered after achieving source control.
1. Introduction
The mold Hormographiella aspergillata belongs to the Basidiomycota division of fungi, and is the asexual form of Coprinopsis cinerea, an edible mushroom usually found in compost and other nutrient-rich substrates [1].
H. aspergillata has been described as a rare cause of fungal infection in humans. Cases of prosthetic valve endocarditis and intraocular lens-implant-associated endophtalmitis have been described in immunocompetent patients [2,3], but the most severe cases have been reported as lung and disseminated infections in immunocompromised hosts, in particular those with hematological malignancies [[4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18]]. The elevated mortality rate (around 80%) may result from delayed diagnosis and therapy, in the absence of detection by common fungal biomarkers (e.g. 1,3-β-d-glucan, galactomannan) and sufficient culture yield. Moreover, there are limited clinical data on antifungal treatment efficacy. Most patients in previous case reports and series received multiple lines of antifungal therapy, including amphotericin B formulations in the majority of cases. While breakthrough infections have been reported under azole prophylaxis [[5], [6], [7], [8],18], the role of this antifungal drug class for the treatment of H. aspergillata infections remains unclear. Antifungal susceptibility testing data are scarce, but suggest a good in vitro activity of voriconazole, while resistance or trailing effect have been reported for other mold-active azoles (e.g. posaconazole, itraconazole) [13,14].
2. Cases
Case 1
A 46-year-old man was diagnosed with acute monoblastic leukemia with a FMS-like tyrosine kinase 3 (FLT3) internal tandem duplication (ITD) mutation, which was treated with an induction chemotherapy (cytarabine and idarubicine followed by high dose cytarabine and daunorubicine) combined with methylprednisolone and etoposide (at day 1 and 5) for a hemophagocytic syndrome. He was admitted to our hospital for a second cycle of induction therapy using the same regimen (day 0). Antifungal prophylaxis with posaconazole was started on the first day of neutropenia (day +9) and maintained during the entire neutropenic phase. After 10 days of persistent neutropenic fever despite broad-spectrum antibiotics and posaconazole prophylaxis (day +20), a thoraco-abdominal computerized tomography (CT) scan showed 3 nodular lung lesions (Fig. 1A). Serum galactomannan and 1,3-β-d-glucan were negative at this time. A bronchoscopy was performed on the following day (day +21), with sterile fungal culture and negative galactomannan on the bronchoalveolar lavage (BAL) sample. Empirical antifungal therapy with liposomal amphotericin B (L-AMB, 5 mg/kg/day) was initiated immediately after the bronchoscopy. A new chest CT performed two weeks after initiation of L-AMB (day +36) showed a reduction of all three lung lesions (73%, 55%, and 39% reduction, respectively, according to mass volumetry, Fig. 1A and B). Following neutrophil recovery, complete removal of the three lung lesions was performed by wedge resection of the right and the left lungs at week 2 (day +38) and 3 (day +44) after the start of L-AMB, respectively. The histopathological exam of the lung lesions showed bronchocentric pneumonia with narrow septate and branched mycelia compatible with an Aspergillus spp. (Fig. 2). Despite sterile fungal cultures, the panfungal PCR (targeting the 18S rDNA) [19] on the resected lung tissue was eventually positive for H. aspergillata on both the right inferior lobe and the left superior lobe lesions. Assessment of disease extension (abdominal and cerebral CT) did not show any other lesion. The antifungal therapy was changed to oral voriconazole (6 mg/kg twice a day on the first day, followed by 4 mg/kg twice a day) after 6 weeks of L-AMB (day +66) because of acute kidney injury attributed to L-AMB toxicity. Voriconazole therapy was continued for a total duration of 5 months without evidence of recurrent fungal disease on radiological follow-up (day +217). Voriconazole therapeutic drug monitoring was performed every two weeks and dosing was adjusted accordingly for a targeted trough concentration of 1 to 5 mg/l. In the meantime, the patient experienced relapsed AML, which was treated by two cycles of high dose cytarabine combined to cladribine and the FLT3-ITD inhibitor sorafenib, followed by a haploidentical allogeneic hematopoietic stem cell transplantation 4 months later (day +152). After 3 years, the patient is still alive and in complete remission.
Case 2
A 70-year-old woman was diagnosed with AML with mutated runt-related transcription factor 1 (RUNX1). The induction chemotherapy included fludarabine, high-dose cytarabine and idarubicin. Because complete remission was not achieved after the first cycle, she was admitted to our hospital (day 0) for a second cycle of chemotherapy including high-dose cytarabine and clofarabine with the addition of the B-cell lymphoma 2 (BCL2) inhibitor venetoclax. Antifungal prophylaxis with fluconazole (400 mg/day) was started on the first day of neutropenia (day +5). On the 13th day of neutropenia (day +17), a thoraco-abdominal CT-scan was performed because of persistent fever despite broad-spectrum antibiotics, which showed a lung mass in the right apex with a halo sign (Fig. 3A). The fluconazole prophylaxis was stopped and posaconazole was started (300 mg twice a day on the first day, then 300 mg once a day). A first bronchoscopy was rapidly performed (day +18) with negative fungal cultures and galactomannan in BAL, followed by a second bronchoscopy with ultrasound-guided transbronchial biopsies 5 days later (day +23). A CT of the chest, abdomen and pelvis performed 12 days after initiation of posaconazole therapy (day +29) showed a progression of the lung mass with a volume increase of 265% of the lesion (Fig. 3B), a new right pleural effusion, as well as a right gluteus minimus muscle abscess (Fig. 3C). The muscle abscess was drained by interventional radiology (day +29) and a lung wedge resection was performed four days later (day +33). An assessment of disease extension was made afterward with a normal brain magnetic resonance imaging (day +36) and a PET-CT (day +40) showing a persistent hypermetabolism around the right gluteus minimus muscle abscess (Fig. 3D). The histopathological analysis of both the lung tissue and muscle abscess showed branched septate mycelial elements and the panfungal PCR was positive for H. aspergillata, which was subsequently also recovered on culture. Antifungal susceptibility testing was performed by microbroth dilution method using the Sensititre YeastOneTM kit (Trek Diagnostics Systems, ThermoFisher Scientific, Cleveland, OH). Minimum inhibitory concentrations (MIC) for amphotericin B, voriconazole, posaconazole were 0.5, 0.5 and 2 mg/L, respectively. Based on these results, antifungal treatment was changed (day +31) to voriconazole (6 mg/kg twice a day on the first day, followed by 4 mg/kg twice a day). Voriconazole therapy was continued and the patient was eventually transferred home with a palliative treatment plan because of progression of the leukemia after 2 cycles of intensive chemotherapy. Eight months afterward, the patient is still alive with no clinical or radiological signs of recurrence of the fungal infection under voriconazole therapy.
Fig. 1.
Radiological assessment of infection at baseline and in follow-up (Case 1).
CT-scan images (sagittal view) of the lung lesions of Case 1. Pictures are taken at initial diagnosis (A) and at day +15 following introduction of liposomal amphotericin B and before the surgical intervention (B).
Volumes of the lung lesion are shown in the table with expression of the changes (in percent) in the follow-up image (B) compared to the baseline image (A).
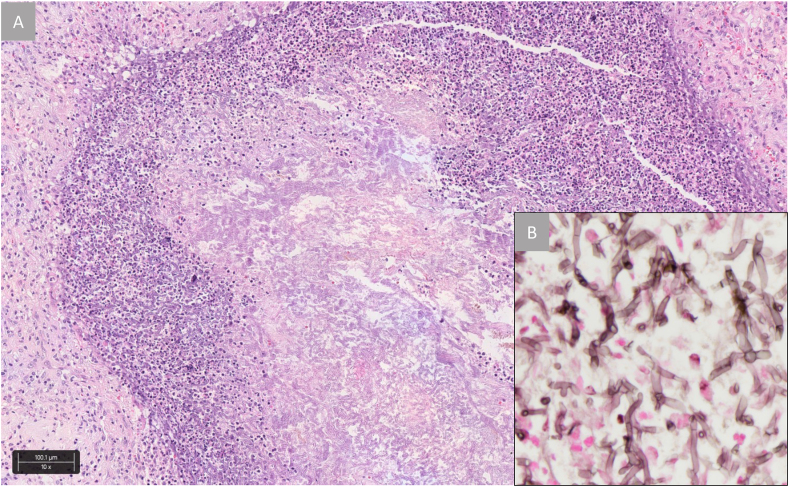
Fig. 2.
Histomorphology of the wedge resection with bronchocentric pneumonia (Case 1).
The central necrosis harbors closely packed fungal hyphae and surrounding acute inflammation and organization (A). The hyphae are narrow, septate and branched (B).
Hematoxylin and Eosin, x100 (A), Grocott's methylamine silver stain, x400 (B).
Fig. 3.
Radiological assessment of infection at baseline and in follow-up (Case 2)
CT-scan images (sagittal view) of the lung lesions of Case 2 (A and B), as well as PET-CT imaging of the right gluteus minimus abscess (C and D). Chest CT pictures are taken at initial diagnosis (A) and at day +12 following introduction of posaconazole therapy and before the surgical intervention (B).
Volume of the lung lesion is shown in the table with expression of the changes (in percent) in the follow-up image (B) compared to the baseline image (A).
3. Discussion
Hormographiella aspergillata is a rare, but possibly underreported cause of invasive fungal infection in neutropenic patients with hematological malignancies. Eighteen cases have been reported so far in this population with an associated mortality rate of 78% (14/18 cases, Table 1). Disseminated infection was observed in 30% of these cases with relatively frequent localization in brain or skin/soft tissues. The diagnosis was made on postmortem tissues or biopsy samples in most cases, stressing the difficulty to identify this pathogen and to initiate prompt appropriate antifungal therapy.
Table 1.
Literature review of invasive Hormographiella aspergillata infections in patients with hematological cancer (n = 20).
| General demographic characteristics | N patients = 20 |
|---|---|
| Male / Female | 10 (50%) / 10 (50%) |
| Age (median, range) | 44 (14 – 70) |
| Hematological disease | |
| Acute myeloid or lymphoid leukemia | 16 (80%) |
| Other a | 4 (20%) |
| Allogeneic stem cell transplantation | 14 (70%) |
| Site of infection | |
| Localized / disseminated (more than one site) | 14 (70%) / 6 (30%) |
| Lung | 19 (95%) |
| Brain | 4 (20%) |
| Skin and soft tissues | 3 (15%) |
| Other b | 3 (15%) |
| Microbiological identification of H. aspergillata | |
| Postmortem (autopsy) | 5 (25%) |
| Antemortem - biopsy sample only | 11 (55%) |
| Antemortem - non-biopsy sample c | 4 (20%) |
| Antifungal drugs | |
| Ongoing antifungal prophylaxis at time of diagnosis d | 5 (25%) |
| First-line treatment | |
| Amphotericin B formulation | 7 (35%) |
| Amphotericin B formulation + echinocandin | 2 (10%) |
| Voriconazole | 6 (30%) |
| Posaconazole | 1 (5%) |
| Echinocandin | 4 (20%) |
| Subsequent treatment lines | |
| Amphotericin B formulation | 7 (35%) |
| Voriconazole | 7 (35%) |
| Posaconazole | 3 (15%) |
| Itraconazole | 1 (5%) |
| Echinocandin | 3 (15%) |
| Outcome | |
| Overall mortality | 14 (70%) |
| Attributable to IFI | 9 (64%) |
| Partially attributable to IFI | 3 (21%) |
| Not related to IFI | 2 (14%) |
Literature review including previously published cases (4–18) and the two present case reports.
Refractory anemia with excess blasts (1), chronic myeloid leukemia with B-cell lymphoid blast phase (1), lymphoma (1), X-linked adrenoleukodystrophy (1).
Sinus (1), eye (1), intestine (1).
Bronchoalveolar lavage fluid (3), sinus fluid (1).
Posaconazole (2), voriconazole (1), itraconazole (1), caspofungin (1).
We described here two cases of invasive H. aspergillata infection in patients with AML that were successfully managed with early surgery and antifungal treatment. Some key interventions were probably determinants for the favorable outcome of these fungal infections.
First, we performed a prompt and invasive diagnostic strategy with bronchoscopy and lung tissue biopsy. In our experience, mini probe-guided transbronchial lung biopsies can be performed safely in neutropenic and thrombocytopenic patients using platelet transfusion during the procedure, and is associated with a better yield than BAL alone for the diagnosis of invasive fungal diseases [19]. Molecular diagnostic tools, such as our broad panfungal PCR targeting the 18S rDNA [20], were used in addition to standard cultures for the rapid detection of the causal fungal pathogen. Indeed, H. aspergillata, as most other basidiomycetous molds, are usually not detected by fungal biomarkers, such as galactomannan or 1,3-β-d-glucan.
Second, the surgical approach consisting of complete wedge resection of the lesions was probably a key element in the successful outcome of these cases. This experience supports the crucial role of surgery following neutrophil recovery in the management of rare and fastidious mold infections in general, including H. aspergillata and other non-Aspergillus molds that are relatively resistant to antifungals and notoriously difficult to treat [21]. Indeed, previous reports of H. aspergillata invasive infections were associated with a very high mortality and it is noteworthy that surgical interventions were performed in a minority of cases (33%, Table 1).
Our review of literature showed that multiple lines of antifungal therapy were often used (Table 1). Most patients received first-line or second-line treatments of amphotericin B formulations, for which H. aspergillata exhibited relatively low MIC in most cases except one (Table 2). In our first case-report, liposomal amphotericin B was associated with a favorable response as illustrated by the significant reduction of the lung lesions after two weeks of therapy and before the surgical intervention.
Table 2.
Antifungal susceptibility profile of Hormographiella aspergillata clinical isolates reported in the literature (n = 16).
| 1st author [ref] N isolates Testing method |
Present case 1 isolate SYO |
Gené (1) 7 isolates BMiD |
Suarez (5) 1 isolate EUCAST |
Nanno (8) 1 isolate NA |
Bojic (12) 1 isolate E-test |
Conen (13) 3 isolates CLSI |
Verweij (14) 1 isolate Agar / BMaD |
Isabel Cristina (18) 1 isolate EUCAST |
|---|---|---|---|---|---|---|---|---|
| Itraconazole | 2 | 0.51 ± 0.1 | ≥8 | 0.25 | – | – | 32 / 8 | – |
| Voriconazole | 0.5 | – | 1 | 0.015 | 0.125 | 0.125 - 0.25 | 0.5 / NA | 0.06 |
| Posaconazole | 2 | – | – | – | 0.064 | 2 | – | 0.06 |
| Caspofungin | 0.5 | – | 2 | – | – | ≥32 | – | 32 |
| Anidulafungin | 0.12 | – | – | – | – | – | – | – |
| Micafungin | 0.25 | 2.3 ± 1.5 | – | ≥16 | – | – | – | – |
| Amphotericin B | 0.5 | 0.5/2.3 | 2 | 0.125 | 0.094 | 0.5 | 0.03 / 0.5 | 32 |
Values are minimum inhibitory concentration (MIC) expressed in mg/L.
SYO: Sensititre YeastOneTM; BMiD: broth microdilution; NA, not available; EUCAST: European Committee on antimicrobial susceptibility testing; CLSI: clinical and laboratory standards institute; BMaD: broth macrodilution.
The role of azoles in this setting is more debatable. Some breakthrough H. aspergillata infections have been reported under mold-active azoles (Table 1) and in vitro susceptibility of this mold to azoles is variable (Table 2) [[5], [6], [7], [8],18]. Notably, posaconazole MICs are relatively high and a trailing effect has been described, which suggests some level of tolerance of H. aspergillata to this drug [13]. Indeed, we observed a progression of the lung mass under posaconazole therapy in our second reported case. However, we observed a favorable outcome in the absence of relapsing infection with maintenance voriconazole therapy following surgery in both cases. In contrast to posaconazole, voriconazole displayed good in vitro activity against all tested isolates of the literature and in the present case for which antifungal susceptibility testing could be performed (i.e. median 0.125 mg/L, range 0.015 – 1 mg/L, Table 2) [1,5,8,[12], [13], [14],18]. Use of voriconazole as first-line or second-line treatments has been associated with variable outcomes [6,13,15,16]. Indeed, progression of the fungal disease under voriconazole therapy was observed in some cases [13,15] and one breakthrough H. aspergillata infection under voriconazole prophylaxis has also been reported [13]. Our data however suggest that voriconazole may have a place for the treatment of this rare mold disease, provided that an optimal source control is achieved by surgery. Whether the novel broad-spectrum azole drug isavuconazole could be used in this setting remains to be determined.
The present cases of H. aspergillata invasive fungal infections in leukemic patients are among the rare ones reported in the literature that were successfully treated. While we cannot draw firm conclusions and recommendations on the basis of only two cases, we attributed this therapeutic success to the extensive diagnostic work-up for early detection of the pathogen using mini probe-guided transbronchial lung biopsies and molecular diagnostic tools, and the aggressive surgical management of the lesions in conjunction with antifungal therapy of liposomal amphotericin B and/or voriconazole. Based on this experience, we suggest that similar approach could be used in the future for the management of such cases.
Declaration of competing interest
F. Lamoth reports research grants from Novartis, Merck and Pfizer, and speaker honoraria from Gilead.
Acknowledgements
We are grateful to all the medical staff who has been involved in the management of these patients.
References
- 1.Gene J., Guillamon J.M., Guarro J., Pujol I., Ulfig K. Molecular characterization, relatedness and antifungal susceptibility of the basidiomycetous Hormographiella species and Coprinus cinereus from clinical and environmental sources. Antonie Leeuwenhoek. 1996;70(1):49–57. doi: 10.1007/BF00393569. [DOI] [PubMed] [Google Scholar]
- 2.Speller D.E., MacIver A.G. Endocarditis caused by a Coprinus species: a fungus of the toadstool group. J. Med. Microbiol. 1971;4(3):370–374. doi: 10.1099/00222615-4-3-370. [DOI] [PubMed] [Google Scholar]
- 3.Jain N., Jinagal J., Kaur H., Ghosh A., Gupta S., Ram J. Ocular infection caused by Hormographiella aspergillata: a case report and review of literature. J. Mycol. Med. 2019;29(1):71–74. doi: 10.1016/j.mycmed.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 4.Surmont I., Van Aelst F., Verbanck J., De Hoog G.S. A pulmonary infection caused by Coprinus cinereus (Hormographiella aspergillata) diagnosed after a neutropenic episode. Med. Mycol. 2002;40(2):217–219. doi: 10.1080/mmy.40.2.217.219. [DOI] [PubMed] [Google Scholar]
- 5.Suarez F., Olivier G., Garcia-Hermoso D., Randriamalala E., Ghez D., Bruneau J. Breakthrough Hormographiella aspergillata infections arising in neutropenic patients treated empirically with caspofungin. J. Clin. Microbiol. 2011;49(1):461–465. doi: 10.1128/JCM.01213-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pang K.A., Godet C., Fekkar A., Scholler J., Nivoix Y., Letscher-Bru V. Breakthrough invasive mould infections in patients treated with caspofungin. J. Infect. 2012;64(4):424–429. doi: 10.1016/j.jinf.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 7.Heiblig M., Bozzoli V., Saison J., Thomas X., De Croze D., Traverse-Glehen A. Combined medico-surgical strategy for invasive sino-orbito-cerebral breakthrough fungal infection with Hormographiella aspergillata in an acute leukaemia patient. Mycoses. 2015;58(5):308–312. doi: 10.1111/myc.12305. [DOI] [PubMed] [Google Scholar]
- 8.Nanno S., Nakane T., Okamura H., Nishimoto M., Koh H., Nakamae H. Disseminated Hormographiella aspergillata infection with involvement of the lung, brain, and small intestine following allogeneic hematopoietic stem cell transplantation: case report and literature review. Transpl. Infect. Dis. 2016;18(4):611–616. doi: 10.1111/tid.12561. [DOI] [PubMed] [Google Scholar]
- 9.Chauhan A., Gruenberg J., Arbefeville S., Mettler T., Brent C.H., Ferrieri P. Disseminated Hormographiella aspergillata infection with lung and brain involvement after allogenic hematopoietic stem-cell transplantation in a 54-year-old man. Lab. Med. 2019;50(4):426–431. doi: 10.1093/labmed/lmz018. [DOI] [PubMed] [Google Scholar]
- 10.Corzo-Leon D.E., Satlin M.J., Soave R., Shore T.B., Schuetz A.N., Jacobs S.E. Epidemiology and outcomes of invasive fungal infections in allogeneic haematopoietic stem cell transplant recipients in the era of antifungal prophylaxis: a single-centre study with focus on emerging pathogens. Mycoses. 2015;58(6):325–336. doi: 10.1111/myc.12318. [DOI] [PubMed] [Google Scholar]
- 11.Lagrou K., Massonet C., Theunissen K., Meersseman W., Lontie M., Verbeken E. Fatal pulmonary infection in a leukaemic patient caused by Hormographiella aspergillata. J. Med. Microbiol. 2005;54(Pt 7):685–688. doi: 10.1099/jmm.0.46016-0. [DOI] [PubMed] [Google Scholar]
- 12.Bojic M., Willinger B., Rath T., Tobudic S., Thalhammer F., Bohm A. Fatal skin and pulmonary infection caused by Hormographiella aspergillata in a leukaemic patient: case report and literature overview. Mycoses. 2013;56(6):687–689. doi: 10.1111/myc.12087. [DOI] [PubMed] [Google Scholar]
- 13.Conen A., Weisser M., Hohler D., Frei R., Stern M. Hormographiella aspergillata: an emerging mould in acute leukaemia patients? Clin. Microbiol. Infect. 2011;17(2):273–277. doi: 10.1111/j.1469-0691.2010.03266.x. [DOI] [PubMed] [Google Scholar]
- 14.Verweij P.E., van Kasteren M., van de Nes J., de Hoog G.S., de Pauw B.E., Meis J.F. Fatal pulmonary infection caused by the basidiomycete Hormographiella aspergillata. J. Clin. Microbiol. 1997;35(10):2675–2678. doi: 10.1128/jcm.35.10.2675-2678.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Godet C., Cateau E., Rammaert B., Grosset M., Le Moal G., Beraud G. Nebulized liposomal amphotericin B for treatment of pulmonary infection caused by Hormographiella aspergillata: case report and literature review. Mycopathologia. 2017;182(7–8):709–713. doi: 10.1007/s11046-017-0117-9. [DOI] [PubMed] [Google Scholar]
- 16.Abuali M.M., Posada R., Del Toro G., Roman E., Ramani R., Chaturvedi S. Rhizomucor variabilis var. regularior and Hormographiella aspergillata infections in a leukemic bone marrow transplant recipient with refractory neutropenia. J. Clin. Microbiol. 2009;47(12):4176–4179. doi: 10.1128/JCM.00305-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moniot M., Lavergne R.A., Morel T., Guieze R., Morio F., Poirier P. Hormographiella aspergillata: an emerging basidiomycete in the clinical setting? A case report and literature review. BMC Infect. Dis. 2020;20(1):945. doi: 10.1186/s12879-020-05679-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Isabel Cristina R.S., Diana A., Karen A. Breakthrough Hormographiella aspergillata infection in a patient with acute myeloid leukemia receiving posaconazole prophylaxis: a case report and review. Mycopathologia. 2020;185(6):1069–1076. doi: 10.1007/s11046-020-00488-z. [DOI] [PubMed] [Google Scholar]
- 19.Greub G., Sahli R., Brouillet R., Jaton K. Ten years of R&D and full automation in molecular diagnosis. Future Microbiol. 2016;11(3):403–425. doi: 10.2217/fmb.15.152. [DOI] [PubMed] [Google Scholar]
- 20.Bernasconi M., Casutt A., Koutsokera A., Letovanec I., Tissot F., Nicod L.P. Radial ultrasound-assisted transbronchial biopsy: a new diagnostic approach for non-resolving pulmonary infiltrates in neutropenic hemato-oncological patients. Lung. 2016;194(6):917–921. doi: 10.1007/s00408-016-9947-3. [DOI] [PubMed] [Google Scholar]
- 21.Lamoth F., Kontoyiannis D.P. Therapeutic challenges of non-Aspergillus invasive mold infections in immunosuppressed patients. Antimicrob. Agents Chemother. 2019;63(11) doi: 10.1128/AAC.01244-19. [DOI] [PMC free article] [PubMed] [Google Scholar]