Abstract
Fluoropyrimidine-based chemotherapies are widely used to treat gastrointestinal tract, head and neck, and breast carcinomas. Severe toxicities mostly impact rapidly dividing cell lines and can occur due to the partial or complete deficiency in dihydropyrimidine dehydrogenase (DPD) catabolism. Since April 2020, the European Medicines Agency (EMA) recommends DPD testing before any fluoropyrimidine-based treatment. Currently, different assays are used to predict DPD deficiency; the two main approaches consist of either phenotyping the enzyme activity (directly or indirectly) or genotyping the four main deficiency-related polymorphisms associated with 5-fluorouracil (5-FU) toxicity. In this review, we focused on the advantages and limitations of these diagnostic methods: direct phenotyping by evaluation of peripheral mononuclear cell DPD activity (PBMC-DPD activity), indirect phenotyping assessed by uracil levels or UH2/U ratio, and genotyping DPD of four variants directly associated with 5-FU toxicity. The risk of 5-FU toxicity increases with uracil concentration. Having a pyrimidine-related structure, 5-FU is catabolised by the same physiological pathway. By assessing uracil concentration in plasma, indirect phenotyping of DPD is then measured. With this approach, in France, a decreased 5-FU dose is systematically recommended at a uracil concentration of 16 ng/ml, which may lead to chemotherapy under-exposure as uracil concentration is a continuous variable and the association between uracil levels and DPD activity is not clear. We aim herein to describe the different available strategies developed to improve fluoropyrimidine-based chemotherapy safety, how they are implemented in routine clinical practice, and the possible relationship with inefficacy mechanisms.
Key words: dihydropyrimidine dehydrogenase, 5-FU, toxicity, uracil, phenotype, genotype
Highlights
-
•
5-FU is the backbone of gastrointestinal tract and head and neck carcinoma treatment.
-
•
5-FU is mainly catabolised by DPD and decreased DPD activity can cause severe reactions.
-
•
Capecitabine, as a prodrug of 5-FU, may also induce severe toxicity in patients with DPD deficiency.
-
•
Assessing DPD status is recommended by the EMA since April 2020.
-
•
There are many strategies to measure DPD activity and some of them are used to tailor 5-FU dosage.
Introduction
Capecitabine and 5-fluorouracil (5-FU) are anti-neoplastic agents with activity against various tumours, including gastrointestinal tract, head and neck, and breast carcinomas. 5-FU displays a dose-response relationship regarding both its efficacy and its toxicity.1 The toxicity induced by 5-FU may lead to ulceration of the lining of the gastrointestinal tract, hand-foot syndrome, shortness of breath, neutropenia, thrombocytopenia, neurotoxicity, and even death. The reported frequency of severe toxicity is around 30% for grade 3, 3%-5% for grade 4, and 0.1%-1% for grade 5 toxicity based on the Common Terminology Criteria for Adverse Events (NCI-CTCAE).2
Over 80% of the administered 5-FU is catabolised by dihydropyrimidine dehydrogenase (DPD).3 Partial or complete DPD activity deficiency can cause severe adverse reactions.4 Following intravenous bolus administration, the elimination half-life varies from 8 to 22 min. Dihydrofluorouracil (the metabolite of 5-FU catabolised by DPD) can be detected within 5 min, demonstrating rapid catabolism.
Different strategies have been proposed to predict a DPD deficiency; the two main approaches are phenotyping the enzyme activity (directly or indirectly) or genotyping the four main polymorphisms associated with 5-FU toxicity.
In February 2018, the French medicines agency (Agence Nationale de Sécurité du Médicament et des Produits de Santé, ANSM) recommended DPD genotyping for all patients receiving fluoropyrimidine-based treatment to improve its safety. In December 2018, a new guideline from the French cancer institute (Institut National Du Cancer, INCa) and the French health authority (Haute Autorité de Santé, HAS) recommended the measurement of the uracil blood level and dose adaptation if this concentration was between 16 and 150 ng/ml.2 For levels >150 ng/ml, alternative regimens without 5-FU/capecitabine should be considered. At the request of the ANSM, the European Medicines Agency (EMA) conducted a review of the evidence concerning DPD deficiency and now also recommends DPD testing by assessing the presence of the four main variants.5 The Dutch Pharmacogenetics Working Group (DPWG) and other European scientific associations have published these same recommendations.6,7
In contrast, the US Food and Drug Administration has chosen not to require any regulatory review of laboratory or genetic tests for the use of 5-FU. Clinical laboratories may develop and validate tests in-house and market them as a laboratory service. Some may offer assays for DPYD and thymidylate synthase (TYMS) variant testing, and others will measure 5-FU area under the curve.8
The Clinical Pharmacogenetics Implementation Consortium (CPIC) dosing guideline for 5-FU and capecitabine assigned for each ∗ allele diplotype (DPYD genotype) the probable DPD enzyme activity score (DPD phenotype); and then linked genotype with fluoropyrimidine dosing. The CPIC recommends an alternative drug for patients who are poor DPYD metabolisers with an activity score of 0. In those who are poor metabolisers with an activity score of 0.5, an alternative drug is also recommended; however, if no other suitable therapeutic option is available, 5-FU or capecitabine should be administered at a strongly reduced dose with early therapeutic drug monitoring. Patients who are intermediate metabolisers with an activity score of 1 or 1.5 should receive a 50% dose reduction.9
Many clinical studies using different approaches have reported the association between a predicted DPD deficiency and a higher risk of toxicity.10,11 All the recommendations proposed a dose adaptation for patients at high risk; however, the choice of the optimal biomarker to implement in clinical practice remains unclear.
In this review, we summarise the different available strategies aiming to improve the safety of patients treated with fluoropyrimidine-based chemotherapy. We provide ‘take-home messages’ for each one of them. We hope that a better knowledge of these strategies and how they have been developed may facilitate their transfer from biologist to clinical practice.
Fluoropyrimidine-based drug
Pharmacological activity of fluoropyrimidine
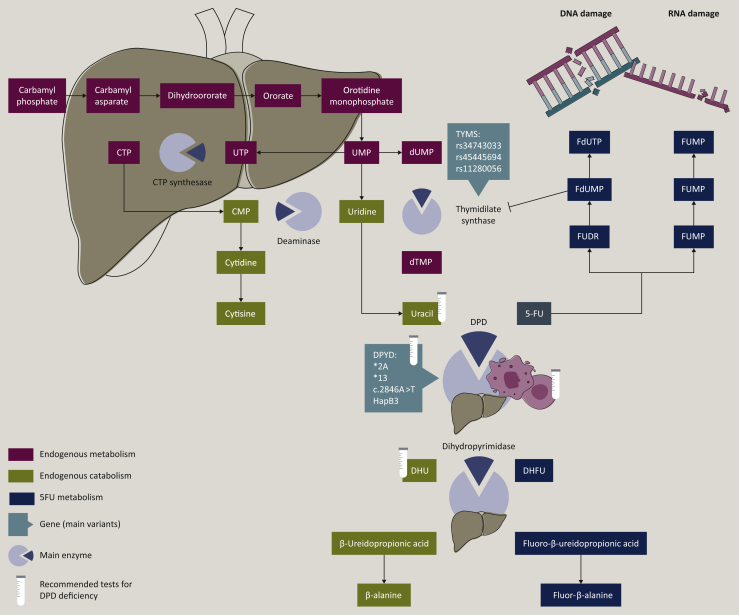
Different mechanisms of 5-FU activation into cytotoxic nucleotides have been described.12,13 One of them is the conversion of 5-FU to 5-fluoro-deoxyuridine monophosphate (FdUMP) that inhibits the enzyme thymidylate synthase (TS). Other cytotoxic mechanisms of fluoropyrimidines include the conversion of fluoro(deoxy)uridine diphosphate [F(d)UDP] to fluorouridine triphosphate and fluorodeoxyuridine triphosphate that are incorporated into RNA and DNA, respectively. All these mechanisms overwhelm DNA repair mechanisms which leads to cell death (Figure 1). Only up to 3% of the original dose of 5-FU mediates the cytotoxic effects, and which mechanism is preponderant in tumour cells remains unclear. However, the cytotoxic activity of 5-FU is highly dependent on the administration schedule: TS inhibition by FdUMP prevails when 5-FU is given as a continuous infusion, and fluorouridine triphosphate incorporation into RNA is considered as being the main mechanism of action when 5-FU is administered as a bolus.14,15
Figure 1.
5-FU mechanisms of action and pyrimidine metabolism. 5-FU is converted to FdUMP which inhibits the enzyme thymidylate synthase (TS). Other cytotoxic mechanisms of action of 5-FU include the conversion of FUDP and FdUDP to FUTP and FdUTP that are incorporated into RNA and DNA, respectively. All this mechanism overwhelms DNA repair mechanisms and eventually leads to cell death. 5-FU catabolism consists of three consecutive steps. Firstly, 5-FU is catalysed to 5,6 dihydrofluorouracil (DHFU) by the dihydropyrimidine dehydrogenase (DPD). Secondly, DHFU is catalysed to fluoro B-ureidopropionate by dihydropyrimidinase. And then fluoro B-ureidopropionate is catalysed to fluoro B-alanine by the ureidopropionase. 5-FU, 5-flourouracil; CMP, cytidine monophosphate; CTP, cytidine triphosphate; DHU, dihydrouracil; dTMP, deoxythymidine monophosphate; dUMP, deoxyuridine monophosphate; FdUDP, fluorodeoxyuridine diphosphate; FdUMP, fluorodeoxyuridine monophosphate; FdUTP, fluorodeoxyuridine triphosphate; FUDP, fluorouridine diphosphate; FUDR, fluorodeoxyuridine; FUMP, fluorouridine monophosphate; FUTP, fluorouridine triphosphate; UMP, uridine monophosphate; UTP, uridine triphosphate.
Following intravenous injection, conversion of 5-FU to FdUMP occurs via thymidylate phosphorylase (TP) to fluorodeoxyuridine (FUDR) and then by the action of thymidine kinase to FdUMP or indirectly via fluorouridine monophosphate (FUMP) or fluorouridine (FUR) to fluorouridine diphosphate and then ribonucleotide reductase (RNR) action to fluorodeoxyuridine diphosphate and FdUMP.16
Catabolism of fluoropyrimidine
After 5-FU infusion, nearly 20% of the dose is directly excreted in the urine and the vast majority is catabolised to inactive metabolites.3 5-FU has a chemical structure that is like endogenous pyrimidine molecules and is catabolised by the same pathway. It consists of three consecutive steps: firstly, thymine (uracil, 5-FU) is catalysed into dihydrothymine (dihydrouracil, dihydrofluorouracil) by DPD; secondly, dihydrothymine is catalysed into B-ureidoisobutyrate (B-ureidopropionate, fluoro B-ureidopropionate) by dihydropyrimidinase; thirdly, B-ureidoisobutyrate is metabolised into B-aminoisobutyrate (B-alanine, fluoro B-alanine) by ureidopropionase.16 Dihydrofluorouracil (FUH2) itself demonstrates potential toxicity in some tumour lines.17
Oral drugs: capecitabine and trifluridine/tipiracil
To be effective, capecitabine should be absorbed and converted by enzymes into deoxy-fluorocytidine (DFCR), FUDR, and 5-FU.18 Oral absorption can be modified by intake of food or partial/total gastrectomy, and a first-pass effect plays a role in the bioavailability of capecitabine metabolites as the enzymes carboxylesterase (capecitabine → DFCR), cytidine deaminase (DFCR → FUDR), and thymidine phosphorylase (FUDR → 5-FU) are highly active in liver tissue.19,20
Trifluridine (FTD) is a thymidine-based nucleoside analogue with antitumour activity by incorporation into DNA.21 When administered alone, FTD is rapidly metabolised by thymidine phosphorylase in the liver and the gastrointestinal tract to inactive forms. Tipiracil inhibits TP and has been added to the formulation to increase its efficacy. FTD is incorporated into cells, phosphorylated by thymidine kinase to FTD-triphosphate, and incorporated into DNA more efficiently than fluorodeoxyuridine triphosphate.22 While both FTD and 5-FU inhibit TS, this mechanism is not considered clinically relevant for FTD as continuous infusion is needed to do so and FTD has been developed to be given orally twice daily.23
FTD/tipiracil is not a substrate for DPD24 and some reports suggest that it can be safely used in DPD-deficient patients.25
Mechanisms of toxicity
Haematotoxicity, gastrointestinal toxicity, and hand-foot syndrome
Inhibition of TS or DNA/RNA incorporation of 5-FU metabolites leads to cell death in rapidly proliferating cell lines and explains toxicity such as ulceration of the lining of the gastrointestinal tract, hand-foot syndrome (HFS), and myelosuppression. HFS and myelosuppression are generally observed after 8-9 weeks and between 9 and 14 days of fluorouracil administration, respectively.3,16,26 HFS pathogenesis is not fully understood and is the most frequent side-effect of capecitabine.27
Mechanism of cardiotoxicity and neurotoxicity
Induced 5-FU cardiotoxicity and neurotoxicity are not well characterised. For cardiotoxicity, two pathophysiological mechanisms are proposed. The first is ischaemia secondary to coronary vasospasm28 and the second is related to the direct myocardial toxicity of fluoroacetate. In the latter, 5-FU is converted to α-fluoro-β-alanine (FBAL) and subsequently to fluoroacetate, the presence of which has been correlated with cardiotoxicity and neurotoxicity syndromes.29 DPD deficiency does not seem to be linked to cardiotoxicity.
Modulation of 5-FU activity
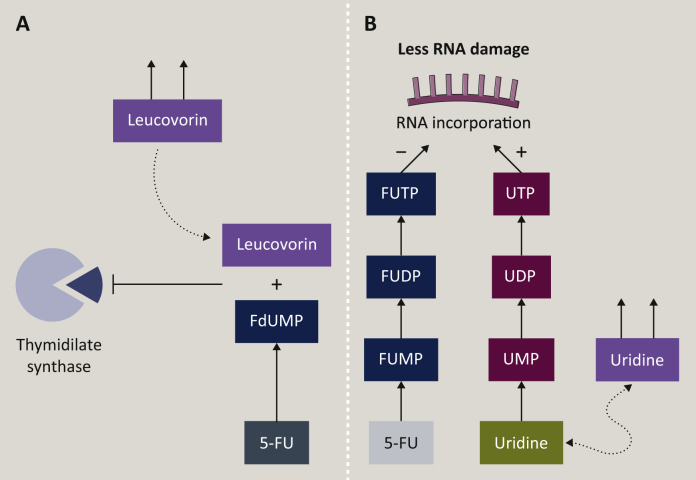
Leucovorin (LV) treatment and uridine metabolism can differentiate between two mechanisms of 5-FU (Figure 2). FdUMP forms a ternary covalent complex with 5,10-methylene tetrahydrofolate and TS, and this inhibits the formation of dTMP from dUMP; this ternary complex is stabilised by the administration of LV. If 5-FU toxicity is strengthened by the addition of LV then TS inhibition plays a significant role, whereas if 5-FU toxicity is not strengthened by LV then RNA toxicity is the main mechanism of 5-FU action. Adding exogenous uridine increases the amount of available uridine monophosphate for RNA incorporation. If the toxicity decreases after uridine addition, then 5-FU incorporation into RNA plays an important role.30
Figure 2.
Impact of exogenous factor on 5-FU metabolism. Leucovorin (LV) treatment and uridine metabolism are presented in two mechanisms of 5-FU action. The FdUMP, TS, and 5,10, methylene-THF ternary complex is stabilised by the administration of LV (A). Administration of uridine lowers UMP catabolisation; higher amounts of UMP, UDP, and UTP facilitate UTP on FUTP RNA incorporation (B). FdUMP, 5-fluoro-deoxyuridine monophosphate; FUTP, fluorouridine triphosphate; TS, thymidylate synthase; UMP, uridine monophosphate; UDP, uridine diphosphate; UTP, uridine triphosphate.
Mechanisms of resistance
As with other anticancer drugs, there are various mechanisms of both innate and acquired resistance that may be exhibited by tumour cells in response to 5-FU.
The entry into the cell can be impaired by membrane transporters such as ATP-binding cassette (ABC) and solute carrier transporters. ABCB1, also known as multidrug resistance 1 (MDR1), is a member of the ABC transporter superfamily and its protein product is called P-glycoprotein (P-gp). ABCB1 overexpression in tumours has been associated with multidrug resistance to cancer chemotherapy drugs.31 No relationship was established between capecitabine or 5-FU metabolism and ABCB1. However, patients homozygote or heterozygote for one of three single-nucleotide polymorphisms (SNPs) (rs1128503, rs1128503, or rs1045642) had a higher risk of developing neutropenia and HFS than homozygous wild-type.32 ABCB1 may be involved in the transport of capecitabine or derived metabolites. It has been described that ABCB5 expression was enhanced in colorectal cancer patients after 5-FU-based chemotherapy33 or that ABCB11 is involved in efflux transport of FdUMP.34
Lower TP or RNR expression contributes to lower active metabolites and correlates with resistance to 5-FU.35,36 The TYMS gene, coding for TS, can harbour polymorphisms in the promoter enhancer region (rs34743033, rs45445694, or rs11280056) that influence the translation efficiency of TYMS mRNA and lower TYMS mRNA expression levels in normal tissue and are associated with a higher risk of the cytotoxic effects of 5-FU.37
Many other pathways can be described that emphasise the complex activity of 5-FU. Methylene tetrahydrofolate reductase (MTHFR) is another gene of interest; MTHFR catalyses the conversion of 5,10-methylenetetrahydrofolate (5,10-methylene-THF) to 5-methyl-tetrahydrofolate (5-methyl-THF). The MTHFR c.C677T polymorphism can cause a 30% reduction in enzymatic activity and leads to an accumulation of 5,10-methylene-THF. This excess of 5,10-methylene-THF increases the stability of the ternary complex and is significantly associated with increased risk of developing grade 3/4 toxicity.38
Take-home message
Many factors can modulate 5-FU activity and its catabolism. The most described parameter is DPD deficiency; treating patients with completely deficient DPD activity with 5-FU may lead to death. Assessing DPD activity helps to predict 5-FU toxicity.9, 10, 11
Biomarkers of fluoropyrimidine-based chemotherapy toxicity
DPD activity in peripheral mononuclear cells (PBMC-DPD)
The metabolic activity of DPD is directly assessed by measuring the amounts of dihydrouracil, carbamyl-B-alanine, and B-alanine produced from exposure of patient lymphocytes to6, 7, 8, 9, 10, 11, 12, 13, 14 uracil. In-vitro studies have demonstrated that DPD activity is present in several normal and tumour cells (peripheral blood lymphocytes and liver).39,40
PBMC-DPD deficiency is defined by the proportion of activity compared with the mean PBMC-DPD activity in a reference population. As PBMC-DPD and liver-DPD activity display a similar Gaussian distribution in the population,39 the estimation for the overall DPD activity in the liver and other tissues is extrapolated from the estimation of the PBMC-DPD activity. One limitation of this method is that in normal peripheral blood lymphocytes and intestinal mucosa, the catabolism of the uracil cannot be detected beyond dihydrouracil, indicating a very low or a lack of dihydropyrimidinase activity.39,40 In vitro, other factors can inhibit DPD activity, such a high amount of uracil and/or reduced nicotinamide adenine dinucleotide phosphate (NADPH) before the reaction in rat or human liver.41
High DPD activity should also be considered, as survival and response rate was lower in patients with higher DPD activity probably due to lower drug exposure.42
However, the implementation of this method in routine care is highly limited as it is a time-consuming technique and it requires a large volume of blood and radiolabeled materials.
Take-home message
The estimation of the DPD activity from PBMCs is accurate but difficult to implement in routine.
UH2/U and the plasma uracil concentration
Endogenous uracil is naturally metabolised in the liver into dihydrouracil (UH2) by DPD. In case of metabolic deficiency, uracil is increased and UH2 decreased.40
The endogenous plasma uracil level and 5-FU clearance were compared with PBMC-DPD activity, and a linear correlation was observed between DPD activity and 5-FU clearance, but DPD activity was poorly correlated with the plasma uracil levels. Likewise, the plasma uracil levels were poorly correlated with 5-FU clearance.43 Considering the phenotyping approaches, Boisdron-Celle et al. reported 252 patients with 5-FU infusion for the pretherapeutic detection of DPD deficiency:
-
•
A correlation was found between uracil plasma levels and 5-FU plasma clearance.
-
•
A correlation was found between uracil plasma levels and 5-FU toxicity (mean ± SD uracil concentrations increased with toxicity grade: grade 0, 14 ± 6.7 ng/ml; NCI-CTCAE grade 1, 14 ± 2.6 ng/ml; grade 2, 17.5 ± 4.8 ng/ml; grade 3, 25 ± 11 ng/ml; grade 4, 24.4 ± 10 ng/ml).
-
•
No correlation was found between the UH2/U ratio and 5-FU plasma clearance, but a significant correlation was found between UH2/U ratio and treatment toxicity (the mean ± SD UH2/U ratio decreased with toxicity grade: grade 0, 8 ± 2.5; grade 1, 6.5 ± 3.2; grade 2, 6.7 ± 1.7; grade 3, 5 ± 3.1; grade 4, 3.7 ± 2.7).10
In another study, plasma uracil level and 5-FU plasma clearance were poorly correlated. The UH2/U ratios were correlated to 5-FU plasma clearance.11
More recently, no relationship was found between 5-FU clearance and uracil concentration or UH2/U ratio; the authors also highlight that the mean area under the curve for patients with uracil <16 ng/ml was significantly lower than for those with uracil ≥16 ng/ml.44
Uracil is mainly catabolised by the DPD in the liver and 5-FU toxicity occurs in several tissues. Heterogeneity of DPD activity and different rate-limiting enzymes with a lack of dihydropyrimidinase in some cells may explain these results.39,40
Quantification of uracil and UH2 is based on high-performance liquid chromatography (HPLC) coupled with UV or more frequently with mass spectrometry detection.45, 46, 47 Preanalytical conditions (patient samples, stock solutions, and sample extracts) are essential for the reliability of the results owing to the high instability of uracil and its metabolites.40 Moreover, using an exact threshold such as 16 ng/ml does not allow a continuous adaptation of 5-FU dosage in clinical practice; for instance, the increased risk of toxicity for a uracil level at 15.9 ng/ml would be very close to that at 16.1 ng/ml, but the 5-FU dosing would be highly different based on the EMA recommendations. There is limited information concerning prospective validation (with its sensitivity and specificity) of uracil as a biomarker.
Toxicity was studied in 550 patients treated with 5-FU. Pre-treatment uracil was associated with significantly increased risk of overall severe toxicity (grade 3 and 4); overall response (OR) of 8.2 (P = 0.0004) for the group U ≥13.9-16 ng/ml, and OR of 5.3 (P = 0.0087) for the group U >16 ng/ml (in comparison with group U <13 ng/ml). Two of the 17 patients (12%) in the uracil >16 ng/ml group had fatal treatment-related toxicity.48
Based on a consensus in France, uracil concentration ≥150 ng/ml has been adopted to define complete DPD deficiency.
Take-home message
The risk of 5-FU toxicity increases with pre-treatment uracil concentration; however, using 16 ng/ml as the threshold for DPD deficiency with a systematic decreased 5-FU dose may lead to chemotherapy under-exposure as uracil concentration is a continuous variable and the association between uracil levels and DPD activity is not clear.
DPYD Genotyping
The DPD deficiency can be partial (the prevalence in European patients is estimated to be between 3% and 8%) or complete (the prevalence between 0.01% and 0.5%).2 DPD is encoded by a single-copy gene (DPYD) on each chromosome; it is 1100 kb in size and contains 23 exons (Chromosome 1; 817 347 pbs).49 In the COSMIC database, from 38 889 unique samples screened for DPYD, 3239 samples with unique mutations were detected. Most were missense substitutions (18.96%), synonymous substitutions (4.85%), or nonsense substitutions (1.48 %).50 So far, only a limited number of these are associated with a decreased DPD activity which may explain the occurrence of severe toxicities with fluoropyrimidines.
Four variants are currently screened in routine and have been directly tested for 5-FU toxicity (DPYD∗2A or rs3918290, DPYD∗13 or rs55886062, c.2846A>T or D949V or rs67376798, HapB3 or rs75017182 or rs56038477), (Table 1).51,52
-
•
These variants are related to 5-FU-induced toxicity but the association between known risk related to DPYD genotype heterozygote variants and uracil levels or UH2/U ratio are not clearly characterised.53,54
-
•
Patients have experienced grade 3/4 toxicity without any of the four well-known mutations (in a cohort of 546 colon cancer patients experiencing toxicity, only 64 carried a mutated DPYD).48,55 In view of certain in vitro and ex vivo data, it is very likely that other functional variants exist, resulting in a deficit of the DPD activity.
-
•
In populations of African origin, the allelic frequencies of the variants DPYD∗2A, DPYD∗13, c.2846A>T and HapB3 are estimated to be 0.1%, 0%, 0.1%, and 0%, respectively; in East Asian countries, these all were estimated to be 0%.49
Table 1.
Four main variants and their frequencies
| Variant | Frequency (%) |
|---|---|
| DPYD∗2; rs3918290 | 0.65 |
| DPYD∗13; rs55886062 | 0.03 |
| HapB3; rs75017182 + rs56038477 | 1.3% |
| D949V; rs67376798 | 0.32 |
For the DPYD∗2 variant, a GT to AT point mutation leads to a pre-mRNA splicing of exon 14. The transcript protein function is partially or totally impaired for heterozygous or homozygous mutations, respectively. The HapB3 variant is driven by the same mechanism, with a CA to GT point mutation and a pre-mRNA splicing of exon 11. For the DPYD∗13 and c.2846A>T variants, missense mutations leads, respectively, to the nucleic acid substitution I560S and D949V. Further studies are needed to discern which variants are most likely to impact DPD activity and induce toxicity.9,56
In 2015, Henricks et al. proposed the calculation of a gene activity score based on the functional impact attributed by these authors to each of the four main variants, with a 50% or more dose reduction for heterozygous variants and that homozygous variants should not be treated with 5-FU.57
Reported experience of systematic DPYD genotyping in routine practice showed that the administration of 5-FU at a reduced dose in patients heterozygous for DPYD∗2A is safe.58 There are also reported cases of severe toxicity (grade 3/4) induced by capecitabine that were found to be DPD deficient,59 and DPYD genotyping successfully reduced this risk in a prospective study for breast cancer patients.60
Variability in the genotype-phenotype relationship can be due to the regulation of DPD at the post-transcriptional level. Short RNAs associated with RNA-induced silencing complex (RISC) proteins can bind to DPD mRNA and inhibit its translation and increasing degradation.
Post-transcriptional regulation of DPD involves the microRNAs miR-27a and miR-27b. Regulation of DPD activity has been tested in mouse lines and tissue preparations. Mouse liver DPD activity was inversely correlated with expression levels of miR-27a and miR-27b.61 Data on the functional effects of miR-27 on DPD in vivo are limited.
Take-home message
Some DPYD variants are associated with a higher risk of toxicity. There is a consensus to adapt 5-FU dosage based on genotyping and reported experience showed that it has been implemented safely in routine practice. More variants are needed to be described because the four screened fail to explain all 5-FU-related toxicity.
Conclusion and perspectives
Fluoropyrimidine-based chemotherapies are the backbone in the treatment of many cancers and different strategies can be used to improve the safety of patients.54,62,63 Today, in France, the main strategy consists of determining uracil plasma concentration with dose-adapted treatment if uracil blood level is between 16 and 150 ng/ml; fluoropyrimidine-based chemotherapies should be avoided at levels above 150 ng/ml.2 In view of the literature, pre-treatment uracil concentrations are associated with overall toxicity but there is no clear correlation between uracil and DPD deficiency based on the four known associated DPYD variants or on PBMC-DPD activity.64 With these strategies, some patients with an impaired DPD activity and uracil level below 16 ng/ml can suffer from severe toxicities but also patients with uracil levels above 16 ng/ml without DPD decreased activity can be undertreated with a tailored dose of 5-FU.55 Many variants of DPYD with an impaired activity have been described with more or less clinical significance.56 Better modelling of factors explaining high levels of uracil and description of significant variant for DYPD could help in the near future to better predict 5-FU toxicity.
Moreover, 5-FU dose adaptation needs to be evaluated in routine clinical practice since the 2018 recommendations. Further studies are warranted to evaluate the proportion of patients who are tested before starting a 5-FU treatment; the techniques used to predict 5-FU toxicity; the proportion of patients predicted to have higher risk for 5-FU toxicity; the proportion of patients receiving an initial course of chemotherapy with a decreased dose of 5-FU; the proportion of the latter who receive an increase in 5-FU dose in the absence of toxicity during the first course of chemotherapy; the prognostic impact of the dose adaptation; and whether this monitoring allows less 5-FU induced toxicity.
Acknowledgments
Funding
None declared.
Disclosure
The authors have declared no conflicts of interest.
References
- 1.Van Kuilenburg A.B.P., Van Lenthe H., Blom M.J., Mul E.P.J., Gennip A.H.V. Profound variation in dihydropyrimidine dehydrogenase activity in human blood cells: major implications for the detection of partly deficient patients. Br J Cancer. 1999;79(3-4):620–626. doi: 10.1038/sj.bjc.6690097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Screening for dihydropyrimidine dehydrogenase deficiency to prevent fluoropyrimidines (5-fluorouracil) severe adverse reactions, Recommendations, INCa, HAS, December 2018. https://www.has-sante.fr Available at:
- 3.Heggie G.D., Sommadossi J.-P., Cross D.S., Huster W.J., Diasio R.B. Clinical pharmacokinetics of 5-fluorouracil and its metabolites in plasma, urine, and bile. Cancer Res. 1987;47(8):2203–2206. [PubMed] [Google Scholar]
- 4.Madi A., Fisher D., Maughan T.S. Pharmacogenetic analyses of 2183 patients with advanced colorectal cancer; potential role for common dihydropyrimidine dehydrogenase variants in toxicity to chemotherapy. Eur J Cancer. 2018;102:31–39. doi: 10.1016/j.ejca.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 5.EMA EMA recommendations on DPD testing prior to treatment with fluorouracil, capecitabine, tegafur and flucytosine. 2020. https://www.ema.europa.eu/en/news/ema-recommendations-dpd-testing-prior-treatment-fluorouracil-capecitabine-tegafur-flucytosine Available at: Accessed November 18, 2020. [DOI] [PubMed]
- 6.Roncato R., Cecchin E., Toffoli G. Improving decision making on DPYD and UGT1A1∗28 patients’ profiling with an innovative reimbursement strategy. Pharmacogenomics. 2018;19(4):301–304. doi: 10.2217/pgs-2017-0303. [DOI] [PubMed] [Google Scholar]
- 7.Martens F.K., Huntjens D.W., Rigter T., Bartels M., Bet P.M., Cornel M.C. DPD testing before treatment with fluoropyrimidines in the Amsterdam UMCs: an evaluation of current pharmacogenetic practice. Front Pharmacol. 2020;10:1609. doi: 10.3389/fphar.2019.01609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.USFDA Table of Pharmacogenetic Associations. FDA. 2020. https://www.fda.gov/medical-devices/precision-medicine/table-pharmacogenetic-associations Available at: Accessed February 22, 2020.
- 9.van Kuilenburg A.B.P., Haasjes J., Richel D.J. Clinical implications of dihydropyrimidine dehydrogenase (DPD) deficiency in patients with severe 5-fluorouracil-associated toxicity: identification of new mutations in the DPD gene. Clin Cancer Res. 2000;6(12):4705–4712. [PubMed] [Google Scholar]
- 10.Boisdron-Celle M., Remaud G., Traore S. 5-Fluorouracil-related severe toxicity: a comparison of different methods for the pretherapeutic detection of dihydropyrimidine dehydrogenase deficiency. Cancer Lett. 2007;249(2):271–282. doi: 10.1016/j.canlet.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 11.Gamelin E., Boisdron-Celle M., Guérin-Meyer V. Correlation between uracil and dihydrouracil plasma ratio, fluorouracil (5-FU) pharmacokinetic parameters, and tolerance in patients with advanced colorectal cancer: a potential interest for predicting 5-FU toxicity and determining optimal 5-FU dosage. J Clin Oncol. 1999;17(4):1105. doi: 10.1200/JCO.1999.17.4.1105. [DOI] [PubMed] [Google Scholar]
- 12.Longley D.B., Harkin D.P., Johnston P.G. 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3(5):330–338. doi: 10.1038/nrc1074. [DOI] [PubMed] [Google Scholar]
- 13.Blondy S., David V., Verdier M., Mathonnet M., Perraud A., Christou N. 5-Fluorouracil resistance mechanisms in colorectal cancer: from classical pathways to promising processes. Cancer Sci. 2020;111(9):3142–3154. doi: 10.1111/cas.14532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ibrahim T., Di Paolo A., Amatori F. Time-dependent pharmacokinetics of 5-fluorouracil and association with treatment tolerability in the adjuvant setting of colorectal cancer. J Clin Pharmacol. 2012;52(3):361–369. doi: 10.1177/0091270010396710. [DOI] [PubMed] [Google Scholar]
- 15.Katsumata K., Tomioka H., Sumi T. Correlation between clinicopathologic factors and kinetics of metabolic enzymes for 5-fluorouracil given to patients with colon carcinoma by two different dosage regimens. Cancer Chemother Pharmacol. 2003;51(2):155–160. doi: 10.1007/s00280-002-0526-3. [DOI] [PubMed] [Google Scholar]
- 16.Diasio R.B., Harris B.E. Clinical pharmacology of 5-fluorouracil. Clin Pharmacokinet. 1989;16(4):215–237. doi: 10.2165/00003088-198916040-00002. [DOI] [PubMed] [Google Scholar]
- 17.Diasio R.B., Schuetz J.D., Wallace H.J., Sommadossi J.P. Dihydrofluorouracil, a fluorouracil catabolite with antitumor activity in murine and human cells. Cancer Res. 1985;45(10):4900–4903. [PubMed] [Google Scholar]
- 18.Lunar N., Etienne-Grimaldi M.-C., Macaire P. Population pharmacokinetic and pharmacodynamic modeling of capecitabine and its metabolites in breast cancer patients. Cancer Chemother Pharmacol. 2021;87(2):229–239. doi: 10.1007/s00280-020-04208-8. [DOI] [PubMed] [Google Scholar]
- 19.Jacobs B.A.W., Deenen M.J., Joerger M. Pharmacokinetics of capecitabine and four metabolites in a heterogeneous population of cancer patients: a comprehensive analysis. CPT Pharmacometrics Syst Pharmacol. 2019;8(12):940–950. doi: 10.1002/psp4.12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tabata T., Katoh M., Tokudome S. Bioactivation of capecitabine in human liver: involvement of the cytosolic enzyme on 5’-deoxy-5-fluorocytidine formation. Drug Metab Dispos. 2004;32(7):762–767. doi: 10.1124/dmd.32.7.762. [DOI] [PubMed] [Google Scholar]
- 21.Lenz H.-J., Stintzing S., Loupakis F. TAS-102, a novel antitumor agent: a review of the mechanism of action. Cancer Treat Rev. 2015;41(9):777–783. doi: 10.1016/j.ctrv.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sakamoto K., Yokogawa T., Ueno H. Crucial roles of thymidine kinase 1 and deoxyUTPase in incorporating the antineoplastic nucleosides trifluridine and 2’-deoxy-5-fluorouridine into DNA. Int J Oncol. 2015;46(6):2327–2334. doi: 10.3892/ijo.2015.2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zaniboni A., Bertocchi P., Barni S., Petrelli F. TAS-102 (Lonsurf) for the treatment of metastatic colorectal cancer. A concise review. Clin Colorectal Cancer. 2016;15(4):292–297. doi: 10.1016/j.clcc.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 24.Peters G.J. Therapeutic potential of TAS-102 in the treatment of gastrointestinal malignancies. Ther Adv Med Oncol. 2015;7(6):340–356. doi: 10.1177/1758834015603313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bolzacchini E., Luchena G., Giordano M. Safety report of TAS-102 in a patient with reduced DPD activity. Clin Colorectal Cancer. 2019;18(4):310–312. doi: 10.1016/j.clcc.2019.07.008. [DOI] [PubMed] [Google Scholar]
- 26.Lévy E., Piedbois P., Buyse M. Toxicity of fluorouracil in patients with advanced colorectal cancer: effect of administration schedule and prognostic factors. J Clin Oncol. 1998;16(11):3537–3541. doi: 10.1200/JCO.1998.16.11.3537. [DOI] [PubMed] [Google Scholar]
- 27.Saif M.W. Capecitabine and hand-foot syndrome. Expert Opin Drug Saf. 2011;10(2):159–169. doi: 10.1517/14740338.2011.546342. [DOI] [PubMed] [Google Scholar]
- 28.Tajik R., Saadat H., Taherkhani M., Movahed M.R. Angina induced by 5-fluorouracil infusion in a patient with normal coronaries. Am Heart Hosp J. 2010;8(2):E111–E112. doi: 10.15420/ahhj.2010.8.2.111. [DOI] [PubMed] [Google Scholar]
- 29.Arellano M., Malet-Martino M., Martino R., Gires P. The anti-cancer drug 5-fluorouracil is metabolized by the isolated perfused rat liver and in rats into highly toxic fluoroacetate. Br J Cancer. 1998;77(1):79–86. doi: 10.1038/bjc.1998.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Codacci-Pisanelli G., Noordhuis P., van der Wilt C.L., Peters G.J. Selective protection by uridine of growth inhibition by 5-fluorouracil (5FU) mediated by 5FU incorporation into RNA, but not the thymidylate synthase mediated growth inhibition by 5FU-leucovorin. Nucleosides Nucleotides Nucleic Acids. 2008;27(6):733–739. doi: 10.1080/15257770802145496. [DOI] [PubMed] [Google Scholar]
- 31.Wang H., Li J., Wei W. Regulation of ATP-binding cassette subfamily B member 1 by snail contributes to chemoresistance in colorectal cancer. Cancer Sci. 2020;111(1):84–97. doi: 10.1111/cas.14253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gonzalez-Haba E., García M.I., Cortejoso L. ABCB1 gene polymorphisms are associated with adverse reactions in fluoropyrimidine-treated colorectal cancer patients. Pharmacogenomics. 2010;11(12):1715–1723. doi: 10.2217/pgs.10.159. [DOI] [PubMed] [Google Scholar]
- 33.Wilson B.J., Schatton T., Zhan Q. ABCB5 identifies a therapy-refractory tumor cell population in colorectal cancer patients. Cancer Res. 2011;71(15):5307–5316. doi: 10.1158/0008-5472.CAN-11-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oguri T., Bessho Y., Achiwa H. MRP8/ABCC11 directly confers resistance to 5-fluorouracil. Mol Cancer Ther. 2007;6(1):122–127. doi: 10.1158/1535-7163.MCT-06-0529. [DOI] [PubMed] [Google Scholar]
- 35.Fukushima M., Fujioka A., Uchida J., Nakagawa F., Takechi T. Thymidylate synthase (TS) and ribonucleotide reductase (RNR) may be involved in acquired resistance to 5-fluorouracil (5-FU) in human cancer xenografts in vivo. Eur J Cancer. 2001;37(13):1681–1687. doi: 10.1016/s0959-8049(01)00174-5. [DOI] [PubMed] [Google Scholar]
- 36.Petrioli R., Bargagli G., Lazzi S. Thymidine phosphorylase expression in metastatic sites is predictive for response in patients with colorectal cancer treated with continuous oral capecitabine and biweekly oxaliplatin. Anticancer Drugs. 2010;21(3):313–319. doi: 10.1097/CAD.0b013e328334d88a. [DOI] [PubMed] [Google Scholar]
- 37.Lecomte T., Ferraz J.-M., Zinzindohoué F. Thymidylate synthase gene polymorphism predicts toxicity in colorectal cancer patients receiving 5-fluorouracil-based chemotherapy. Clin Cancer Res. 2004;10(17):5880–5888. doi: 10.1158/1078-0432.CCR-04-0169. [DOI] [PubMed] [Google Scholar]
- 38.Nahid N.A., Apu M.N.H., Islam MdR. DPYD∗2A and MTHFR C677T predict toxicity and efficacy, respectively, in patients on chemotherapy with 5-fluorouracil for colorectal cancer. Cancer Chemother Pharmacol. 2018;81(1):119–129. doi: 10.1007/s00280-017-3478-3. [DOI] [PubMed] [Google Scholar]
- 39.Lu Z., Zhang R., Diasio R.B. Dihydropyrimidine dehydrogenase activity in human peripheral blood mononuclear cells and liver: population characteristics, newly identified deficient patients, and clinical implication in 5-fluorouracil chemotherapy. Cancer Res. 1993;53(22):5433–5438. [PubMed] [Google Scholar]
- 40.Gamelin E., Boisdron-Celle M., Larra F., Robert J. A simple chromatographic method for the analysis of pyrimidines and their ehydrogenated metabolites. J Liq Chrom Relat Tech. 1997;20(19):3155–3172. [Google Scholar]
- 41.Naguib F.N., el Kouni M.H., Cha S. Enzymes of uracil catabolism in normal and neoplastic human tissues. Cancer Res. 1985;45(11 Pt 1):5405–5412. [PubMed] [Google Scholar]
- 42.Chamorey E., Francois E., Etienne M.-C. DPD status and fluoropyrimidines-based treatment: high activity matters too. BMC Cancer. 2020;20(1):436. doi: 10.1186/s12885-020-06907-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fleming R.A., Milano G., Thyss A. Correlation between dihydropyrimidine dehydrogenase activity in peripheral mononuclear cells and systemic clearance of fluorouracil in cancer patients. Cancer Res. 1992;52(10):2899–2902. [PubMed] [Google Scholar]
- 44.Dolat M., Macaire P., Goirand F. Association of 5-FU therapeutic drug monitoring to DPD phenotype assessment may reduce 5-FU under-exposure. Pharmaceuticals (Basel) 2020;13(11):416. doi: 10.3390/ph13110416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tafzi N., Woillard J.-B., Fleytoux A., Picard N., Marquet P. Phenotyping of uracil and 5-fluorouracil metabolism using LC-MS/MS for prevention of toxicity and dose adjustment of fluoropyrimidines. Ther Drug Monit. 2020;42(4):540–547. doi: 10.1097/FTD.0000000000000768. [DOI] [PubMed] [Google Scholar]
- 46.Jacobs B.A.W., Rosing H., de Vries N. Development and validation of a rapid and sensitive UPLC-MS/MS method for determination of uracil and dihydrouracil in human plasma. J Pharm Biomed Anal. 2016;126:75–82. doi: 10.1016/j.jpba.2016.04.039. [DOI] [PubMed] [Google Scholar]
- 47.Neto O.V., Raymundo S., Franzoi M.A. DPD functional tests in plasma, fresh saliva and dried saliva samples as predictors of 5-fluorouracil exposure and occurrence of drug-related severe toxicity. Clin Biochem. 2018;56:18–25. doi: 10.1016/j.clinbiochem.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 48.Meulendijks D., Henricks L.M., Jacobs B.A.W. Pretreatment serum uracil concentration as a predictor of severe and fatal fluoropyrimidine-associated toxicity. Br J Cancer. 2017;116(11):1415–1424. doi: 10.1038/bjc.2017.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wei X., Elizondo G., Sapone A. Characterization of the human dihydropyrimidine dehydrogenase gene. Genomics. 1998;51(3):391–400. doi: 10.1006/geno.1998.5379. [DOI] [PubMed] [Google Scholar]
- 50.Cosmic DPYD Gene – COSMIC. https://cancer.sanger.ac.uk/cosmic/gene/analysis?ln=DPYD#variants Available at: Accessed December 25, 2020.
- 51.Hishinuma E., Narita Y., Saito S. Functional characterization of 21 allelic variants of dihydropyrimidine dehydrogenase identified in 1070 Japanese individuals. Drug Metab Dispos. 2018;46(8):1083–1090. doi: 10.1124/dmd.118.081737. [DOI] [PubMed] [Google Scholar]
- 52.Caudle K.E., Thorn C.F., Klein T.E. Clinical pharmacogenetics implementation consortium guidelines for dihydropyrimidine dehydrogenase genotype and fluoropyrimidine dosing. Clin Pharmacol Ther. 2013;94(6):640–645. doi: 10.1038/clpt.2013.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van Kuilenburg A.B.P., Meijer J., Tanck M.W.T. Phenotypic and clinical implications of variants in the dihydropyrimidine dehydrogenase gene. Biochim Biophys Acta. 2016;1862(4):754–762. doi: 10.1016/j.bbadis.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 54.Etienne-Grimaldi M.-C., Boyer J.-C., Beroud C. New advances in DPYD genotype and risk of severe toxicity under capecitabine. PLoS One. 2017;12(5):e0175998. doi: 10.1371/journal.pone.0175998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Meulendijks D., Cats A., Beijnen J.H., Schellens J.H.M. Improving safety of fluoropyrimidine chemotherapy by individualizing treatment based on dihydropyrimidine dehydrogenase activity – ready for clinical practice? Cancer Treat Rev. 2016;50:23–34. doi: 10.1016/j.ctrv.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 56.Amstutz U., Farese S., Aebi S., Largiadèr C.R. Dihydropyrimidine dehydrogenase gene variation and severe 5-fluorouracil toxicity: a haplotype assessment. Pharmacogenomics. 2009;10(6):931–944. doi: 10.2217/pgs.09.28. [DOI] [PubMed] [Google Scholar]
- 57.Henricks L.M., Opdam F.L., Beijnen J.H., Cats A., Schellens J.H.M. DPYD genotype-guided dose individualization to improve patient safety of fluoropyrimidine therapy: call for a drug label update. Ann Oncol. 2017;28(12):2915–2922. doi: 10.1093/annonc/mdx411. [DOI] [PubMed] [Google Scholar]
- 58.Jolivet C., Nassabein R., Soulières D. Implementing DPYD∗2A genotyping in clinical practice: the Quebec, Canada, experience. Oncologist. 2021;26(4):e597–e602. doi: 10.1002/onco.13626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Saif M.W., Diasio R. Is capecitabine safe in patients with gastrointestinal cancer and dihydropyrimidine dehydrogenase deficiency? Clin Colorectal Cancer. 2006;5(5):359–362. doi: 10.3816/CCC.2006.n.007. [DOI] [PubMed] [Google Scholar]
- 60.Stavraka C., Pouptsis A., Okonta L. Clinical implementation of pre-treatment DPYD genotyping in capecitabine-treated metastatic breast cancer patients. Breast Cancer Res Treat. 2019;175(2):511–517. doi: 10.1007/s10549-019-05144-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Offer S.M., Butterfield G.L., Jerde C.R., Fossum C.C., Wegner N.J., Diasio R.B. microRNAs miR-27a and miR-27b directly regulate liver dihydropyrimidine dehydrogenase expression through two conserved binding sites. Mol Cancer Ther. 2014;13(3):742–751. doi: 10.1158/1535-7163.MCT-13-0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pizzorno G., Diasio R.B., Cheng Y.-C. Pyrimidine Analogs. In: Kufe D.W., Pollock R.E., Weichselbaum R.R., editors. Holland-Frei Cancer Medicine. 6th edition. BC Decker; Hamilton, ON: 2003. https://www.ncbi.nlm.nih.gov/books/NBK13287/ Available at: Accessed June 24, 2020. [Google Scholar]
- 63.Defossez G., Le Guyader-Peyrou S., Uhry Z. National estimates of cancer incidence and mortality in mainland France between 1990 and 2018 – Volume 1: Solid Tumors: Study based on the cancer registers of the Francim network. Santé Publique France. 2019. https://www.santepubliquefrance.fr/maladies-et-traumatismes/cancers Available at: Accessed January 7, 2020.
- 64.Offer S.M., Fossum C.C., Wegner N.J., Stuflesser A.J., Butterfield G.L., Diasio R.B. Comparative functional analysis of DPYD variants of potential clinical relevance to dihydropyrimidine dehydrogenase activity. Cancer Res. 2014;74(9):2545–2554. doi: 10.1158/0008-5472.CAN-13-2482. [DOI] [PMC free article] [PubMed] [Google Scholar]