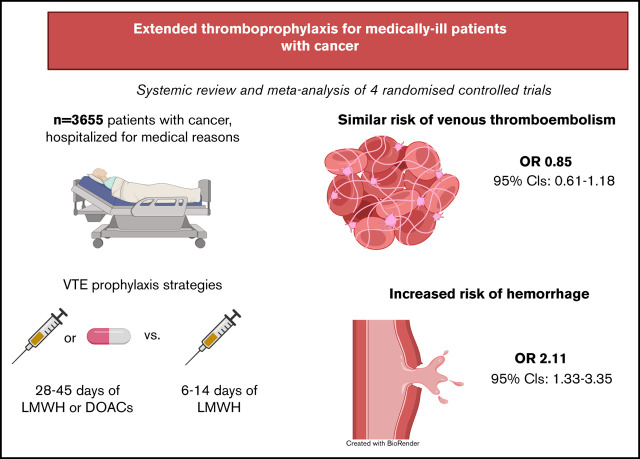
Abstract
Hospitalized medically ill patients with cancer are at increased risk of both venous thromboembolism and bleeding. The safety and efficacy of extended thromboprophylaxis in patients with cancer are unclear. We conducted a systematic review and meta-analysis of the literature using of MEDLINE, EMBASE, and the Cochrane CENTRAL databases to identify cancer subgroups enrolled in randomized controlled trials evaluating extended thromboprophylaxis following hospitalization. The primary outcomes were symptomatic and incidental venous thromboembolic events and hemorrhage (major hemorrhage and clinically relevant nonmajor bleeding). Four randomized controlled trials reported the outcomes of extended thromboprophylaxis in 3655 medically ill patients with active or history of cancer. The rates of venous thromboembolic events were similar between the extended-duration and standard-duration groups (odds ratio [OR], 0.85; 95% confidence interval [CI], 0.61-1.18; I2 = 0%). However, major and clinically relevant nonmajor bleeding occurred significantly more frequently in the extended-duration thromboprophylaxis group (OR, 2.10; 95% CI, 1.33-3.35; I2 = 8%). Extended thromboprophylaxis in hospitalized medically ill patients with cancer was not associated with a reduced rate of venous thromboembolic events but was associated with increased risk of hemorrhage. This study protocol was registered on PROSPERO as #CRD42020209333.
Visual Abstract
Introduction
Despite the routine use of thromboprophylaxis during hospitalization, venous thromboembolism (VTE) after hospital discharge remains an important preventable cause of death.1,2 Randomized trials have demonstrated that extended thromboprophylaxis in medically ill patients can reduce the incidence of VTE, but this is counterbalanced by increased risk of hemorrhage.3,4 Accordingly, current guidelines recommend against the routine use of extended thromboprophylaxis in unselected medically ill patients.5
Patients with cancer are at particularly high risk of thrombosis through multiple distinct mechanisms.6 A meta-analysis of randomized controlled trials (RCTs) demonstrated clear benefit of extended (28-42 days) thromboprophylaxis after hospitalization over standard-duration (14 days) thromboprophylaxis in postoperative patients, particularly after major procedures related to cancer.7 Primary thromboprophylaxis has been shown to benefit certain higher-risk ambulatory patients with cancer receiving chemotherapy.8 The role of extended thromboprophylaxis among hospitalized medical patients with cancer to reduce postdischarge thrombosis is not known. Importantly, patients with cancer are also susceptible to increased risk of bleeding from anticoagulant therapies.9,10 To delineate the benefit of extended thromboprophylaxis in preventing VTE and risk of bleeding specifically in hospitalized patients with cancer, we conducted a systemic review of the literature and a meta-analysis.
Methods
Search strategy and study selection
We searched PubMed, EMBASE, and the Cochrane CENTRAL databases from inception to 14 September 2020. Two authors (S.O. and R.P.) independently screened titles and abstracts, reviewed the full texts for eligibility, and extracted data from included studies. Disagreements were resolved by consensus or adjudicated by a third author (T.C.). Eligible studies were RCTs of adults hospitalized with medical illness with active or history of cancer treated with either standard- (<14 days) or extended-duration (>28 days) anticoagulation. Studies were included if they reported a primary outcome of either symptomatic VTE (proximal deep vein thrombosis or pulmonary embolism) or asymptomatic proximal deep vein thrombosis with standardized tests such as compressive ultrasonogram or computed tomography pulmonary angiogram or ventilation/perfusion scan. In these trials, bleeding events were reported as either major or clinically relevant nonmajor bleeding (CRNMB) per the International Society of Thrombosis and Hemostasis guideline.11 In this study, clinically relevant bleeding was defined as the combined rate of major bleeding and CRNMB. All thrombotic and bleeding outcomes had to be reported for cancer subgroups separately to be included.
Data extraction and statistical analysis
The risk of bias for each study was assessed using the Cochrane Collaboration risk of bias assessment tool for randomized trials (version 2). Two reviewers independently assessed each study for the risk of bias. Disagreements between the 2 authors (S.O. and R.P.) were adjudicated by a third author (T.C.). Data analysis was performed using Comprehensive Meta-Analysis software (version 3.0; Eaglewood, NJ). Pooled odds ratios (ORs) of venous thrombosis events and bleeding events with respective 95% confidence intervals (CIs) were calculated using the generic inverse variance method with a random effects model. Interstudy heterogeneity was evaluated using the Cochran Q test and I2 statistic. A Cochran Q test P value of <.05 was considered significant for heterogeneity. An I2 value of 0% to 25% represents insignificant heterogeneity; 26% to 50%, low heterogeneity; 51% to 75%, moderate heterogeneity; and >75%, high heterogeneity. Funnel plots of event rates vs standard errors and the Egger regression test were used to assess for the presence of publication bias. Forest plots were generated to show the impact of thromboprophylaxis duration on thrombosis and bleeding in patients with active or history of cancer and in the noncancer population.
Results
Search results
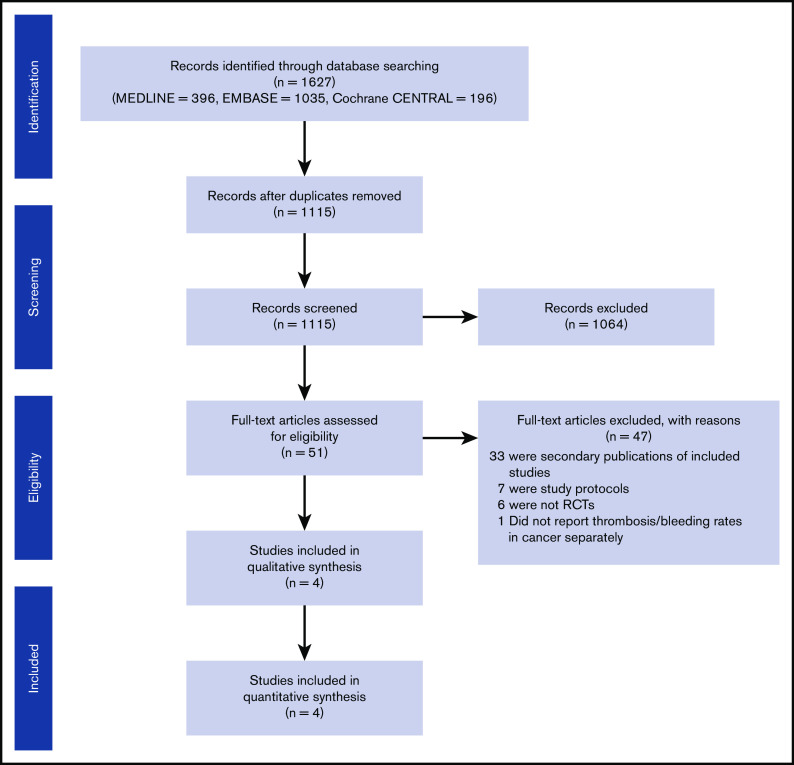
A total of 1115 publications were initially identified on the initial abstract screen. Full texts were obtained for 51 records. A total of 47 studies were excluded for the following reasons: secondary publication of an included study (n = 33), study protocol (n = 7), non-RCT (n = 6), and RCT (ADOPT) that did not report cancer subgroup analysis (n = 1; Figure 1). We separately contacted the ADOPT study investigators but were unable to obtain cancer subgroup data. Four RCTs (EXCLAIM, APEX, MAGELLAN, and MARINER12-16) compared extended prophylaxis (using enoxaparin, betrixaban, or rivaroxaban) against standard-duration prophylaxis using enoxaparin. All 4 trials included patients with cancer. In the 4 studies included, quality appraisal identified some overall concern for bias because of the use of data from subgroup analysis. However, the risk of bias was low for other measures, including measurement of outcomes, missing outcome data, deviation from intended intervention, and randomization processes (supplemental Table 1).
Figure 1.
PRISMA flow diagram of included studies.
The EXCLAIM, APEX, and MAGELLAN studies included both patients with active and history of cancer, with the exception of patients with intracerebral neoplasms or metastasis. The APEX study also excluded patients with active lung cancer. In contrast, the MARINER study only included patients with history of cancer in the past 5 years and did not include patients with active cancer (Tables 1 and 2). The EXCLAIM, APEX, and MAGELLAN studies used screening ultrasonography to identify asymptomatic as well as symptomatic VTE; however, only symptomatic VTE was included in the MARINER study. APEX, MAGELLAN, and MARINER used International Society of Thrombosis and Hemostasis criteria for their definition of bleeding events, including major bleeding and CRNMB.11 These results were combined and reported as clinically relevant bleeding in this meta-analysis. Intention-to-treat analysis was used to analyze the primary efficacy outcomes in all 4 studies. The EXCLAIM study did not report bleeding events in the cancer subgroup; study investigators were contacted, but data could not be obtained.
Table 1.
Designs of the included studies
| EXCLAIM 2010 | MAGELLAN 2013 | APEX 2016 | MARINER 2018 | |
|---|---|---|---|---|
| Authors | Hull et al15 | Cohen et al14 | Cohen et al16 | Spyropoulos et al13 |
| Year of publication | 2010 | 2013 | 2016 | 2018 |
| Setting | International, multicenter | International, multicenter | International, multicenter | International, multicenter |
| Study design | Randomized parallel placebo-controlled trial | Randomized active comparator–controlled trial | Randomized active comparator–controlled trial | Randomized placebo-controlled trial |
| Blinding | Double blind | Double blind | Double blind, double dummy | Double blind |
| Cancer status | Active cancer or history of cancer | History of cancer or active cancer | History of cancer or active cancer | History of cancer in the past 5 y |
| Excludes intracranial neoplasm or metastasis | Excludes intracranial neoplasm or metastasis | Excludes intracranial neoplasm or metastasis, active lung cancer with residual disease, and nonmelanoma skin cancer | Excludes all active cancers and nonmelanoma skin cancer | |
| Intervention | 40 mg enoxaparin SC daily for 10 ± 4 d then enoxaparin 40 mg SC daily for additional 28 ± 4 d | 10 mg rivaroxaban daily for 35 ± 4 d | 80 mg betrixaban orally daily for 35-42 d (loading dose, 160 mg); reduced-dose betrixaban (40 mg) for patients with severe renal insufficiency or receiving concomitant P-glycoprotein inhibitor | 10 mg rivaroxaban daily for 45 d after discharge |
| Control | 40 mg enoxaparin SC daily for 10 ± 4 d then placebo for additional 28 ± 4 d | 40 mg enoxaparin SC daily for 10 ± 4 d | 40 mg enoxaparin SC daily for 10 ± 4 d | Placebo for 45 d after discharge |
SC, subcutaneous.
Table 2.
Baseline characteristics of patients with cancer in the included studies
| Characteristic | EXCLAIM 2010 | MAGELLAN 2013 | APEX 2016 | MARINER 2018 | ||||
|---|---|---|---|---|---|---|---|---|
| Enoxaparin | Placebo | Rivaroxaban | Enoxaparin | Betrixaban | Enoxaparin | Rivaroxaban | Enoxaparin | |
| No. of participants | 2975 | 2988 | 4050 | 4051 | 3759 | 3754 | 6007 | 6012 |
| History of cancer | ||||||||
| % | 13.3 | 14.1 | 17.3 | 16.7 | 12.4 | 11.8 | 8.1 | 8.9 |
| n | 396 | 422 | 701 | 677 | 466 | 443 | 487 | 535 |
| Active cancer | ||||||||
| % | 1.7 | 1.5 | 7.3 | 7.3 | 0.7 | 0.2 | Excluded | Excluded |
| n | 51 | 49 | 296 | 296 | 26 | 11 | 0 | 0 |
| Mean age ± SD, y | NR | NR | NR | NR | 76.8 ± 9.3 | 76.0 ± 9.3 | NR | NR |
| Male sex, % | NR | NR | NR | NR | 48.1 | 50.9 | NR | NR |
| Weight, kg | NR | NR | NR | NR | 78.3 ± 9.3 | 81.2 ± 21.2 | NR | NR |
| White race, % | NR | NR | NR | NR | 93.3 | 91.5 | NR | NR |
| Mean BMI, kg/m2 | NR | NR | NR | NR | 28.6 ± 6.3 | 29.3 ± 7.3 | NR | NR |
| Acute medical condition, % | ||||||||
| Heart failure | NR | NR | NR | NR | 32.2 | 44.5 | NR | NR |
| Respiratory failure | NR | NR | NR | NR | 15 | 16.7 | NR | NR |
| Infection | NR | NR | NR | NR | 40.3 | 38.5 | NR | NR |
| Ischemic stroke | NR | NR | NR | NR | 6.8 | 8.7 | NR | NR |
| Inflammatory or rheumatic disease | NR | NR | NR | NR | 4.8 | 3.9 | NR | NR |
| VTE risk factors, % | ||||||||
| Age ≥75 y | NR | NR | NR | NR | 68.1 | 63.9 | NR | NR |
| History of VTE | NR | NR | NR | NR | 8.0 | 7.8 | NR | NR |
| Hormone therapy | NR | NR | NR | NR | 5.0 | 4.8 | NR | NR |
| History of heart failure (NYHA class 3 or 4) | NR | NR | NR | NR | 18.2 | 21.1 | NR | NR |
| Hereditary or acquired thrombophilia | NR | NR | NR | NR | 0.2 | 0.2 | NR | NR |
| Elevated D-dimer (≥2× ULN) | NR | NR | NR | NR | 56.7 | 59.6 | NR | NR |
| Acute infectious disease | NR | NR | NR | NR | 18 | 18.9 | NR | NR |
| Severe varicosis | NR | NR | NR | NR | 14.4 | 14.1 | NR | NR |
BMI, body mass index; NR, not reported; NYHA, New York Heart Association; SD, standard deviation; ULN, upper limit of normal.
Cancer characteristics of included studies
Across these 4 trials, 3655 participants with active or history of cancer were identified. Among these, history of cancer accounted for 80% of the participants, and 20% had active cancer. MAGELLAN had the highest proportion of patients with cancer (active cancer, 7.3% and history of cancer, 17%), whereas the MARINER study had the lowest proportion (active cancer, 0% and history of cancer, 8.5%; Tables 1 and 2). The percentages of participants with either active or history of cancer were equally randomly assigned to receive either extended- or standard-duration thromboprophylaxis in all 4 trials. Data were not available for specific cancer types in the EXCLAIM, MAGELLAN, or MARINER studies. In the APEX study, the most common types of cancer were genitourinary (betrixaban group, 41.5% and enoxaparin group, 39.4%) and gastrointestinal (15.5% and 17.2%, respectively), followed by skin (8.0% and 8.8%, respectively) and respiratory (5.9% and 9.5%, respectively).17 Additional data on cancer stage or treatment were not reported in the 4 trials.
Study outcomes
Among 3655 participants, 1832 patients were randomly assigned to extended-duration prophylaxis with either enoxaparin, rivaroxaban, or betrixaban, whereas 1853 received standard-duration prophylaxis with enoxaparin. Pooled analysis showed no differences between the rates of venous thromboembolic events in the extended prophylaxis group when compared with standard prophylaxis in the cancer population (OR, 0.85; 95% CI, 0.61-1.18; I2 = 0%; Figure 2). However, the risk of clinically relevant bleeding was higher in the extended-duration thromboprophylaxis group (OR, 2.11; 95% CI, 1.33-3.35; I2 = 8%; Figure 3). The funnel plot was symmetrical, suggesting the absence of publication bias (supplemental Figure 1). Neither the risk of major bleeding nor the risk of CRNMB alone was statistically significant (major bleeding: OR, 1.98; 95% CI, 0.62-6.35 and CRNMB: OR, 1.85; 95% CI, 0.87-3.92; Figure 3). Given that the MARINER study excluded patients with active cancer, we also performed a sensitivity analysis excluding this study. These pooled analyses remained consistent, with no apparent differences in total VTEs (OR, 0.86; 95% CI, 0.61-1.20), but extended thromboprophylaxis led to a significant increase in clinically relevant bleeding (OR, 2.21; 95% CI, 1.02-4.80; supplemental Figure 2).
Figure 2.

Forest plot showing pooled risk ratio of venous thromboembolic events.
Figure 3.
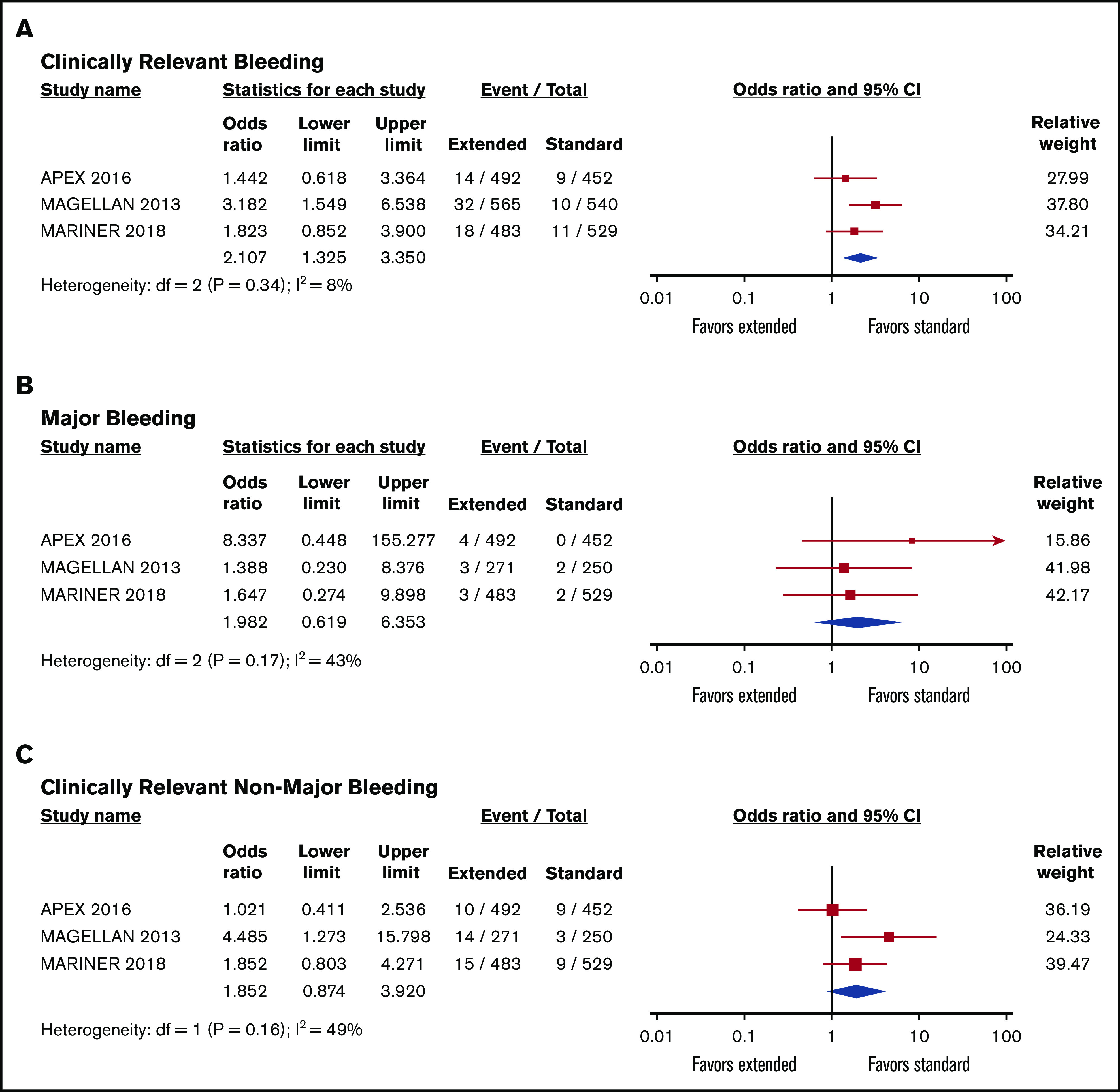
Forest plot showing pooled risk ratios of bleeding. Clinically relevant bleeding (A), major bleeding (B), and CRNMB (C) in patients receiving extended- vs standard-duration thromboprophylaxis.
Cancer vs noncancer population
To assess whether the diagnosis of cancer influenced outcomes with extended thromboprophylaxis in acutely ill medical patients, we compared outcomes of participants with and without cancer. Extended thromboprophylaxis was associated with significant reduction in VTE risk (OR, 0.72; 95% CI, 0.61-0.84) in patients without cancer but not in the cancer subpopulation (Figure 4A). However, the risk of clinically relevant bleeding was significantly higher with extended thromboprophylaxis in both groups (with and without cancer; Figure 4B).
Figure 4.

Forest plot showing pooled risk ratios of VTE and bleeding. VTE (A) and clinically relevant bleeding (B) in patients receiving extended- vs standard-duration thromboprophylaxis in cancer and noncancer populations.
Discussion
Patients with cancer have higher risks of bleeding and thrombosis, making clinical decisions around pharmacologic thromboprophylaxis particularly challenging. The randomized studies included in this meta-analysis did not show clear evidence of reduced VTE with extended-duration thromboprophylaxis (28-42 days) as compared with standard-duration low molecular weight thromboprophylaxis (14 days) in patients with active or history of cancer hospitalized for medical illness. There was, however, an increased incidence of clinically relevant bleeding as defined by combined major bleeding and CRNMB with extended thromboprophylaxis.
The efficacy of prolonged thromboprophylaxis in patients with cancer at increased risk of thrombosis has been observed both in the ambulatory setting and following cancer-related abdominopelvic surgery.18-20 On the basis of this meta-analysis, it seems that extended thromboprophylaxis increases major bleeding without reducing symptomatic VTE following hospitalization in patients with cancer. These results are consistent with a prior retrospective cohort study of predominantly cancer patients hospitalized at the Brigham and Women’s Hospital. Faniko et al21 retrospectively identified 461 hospitalized medical patients at high risk of VTE who received VTE prophylaxis at discharge; thromboprophylaxis did not reduce the rate of symptomatic VTE (5.0% vs 4.3%; P = .58), but it did increase major bleeding events (3.9% vs 1.9%; P = .03). Acutely ill hospitalized patients with cancer are at increased risk of developing major hemorrhage, which likely explains the increased rate of hemorrhage observed in the postdischarge period.22,23
Prior meta-analyses of randomized controlled studies comparing standard vs extended thromboprophylaxis in all patients (ie, hospitalized medical patients with and without cancer) identified a significant reduction in VTE but with an associated increased risk of bleeding.3,4,24 In contrast, we did not detect a statistical benefit for extended thromboprophylaxis among the subgroup with cancer. This finding is in keeping with subgroup analyses of cancer patients receiving standard-duration thromboprophylaxis, where a clear benefit of thromboprophylaxis relative to placebo was not found.25 A potential explanation for this apparent lack of efficacy is that acutely ill patients with cancer are so prothrombotic that standard prophylactic dosing of anticoagulants is insufficient for primary prevention. A recent phase 2 trial suggested that higher doses of low molecular weight heparin may be more effective in this population.26
One of the key limitations of this analysis is the lack of consistency in the criteria used to define active cancer. In the MARINER study specifically, patients with active cancer were excluded, and the study focused only on patients with history of cancer. A sensitivity analysis that excluded this study did not alter the primary findings regarding the lack of efficacy and increased hemorrhage in the cancer subgroups (supplemental Figure 2). There are reports on increased VTE risk in cancer survivors even up to 5 years from diagnosis compared with control populations,27,28 although the risk of thrombosis is greater in the time surrounding first diagnosis. We also acknowledge the limited available data for this analysis, with only 4 randomized trials evaluated, including a total of 3655 patients. Accordingly, it is possible that with additional studies, a modest statistical reduction in thrombosis will become apparent. Outcomes and safety data in either active cancer or history of cancer subgroups alone were also not available for analysis. Although we observed a greater risk of hemorrhage in patients with cancer, the relative safety and benefit of extended thromboprophylaxis specifically in patients with active cancer could not be established in this data set. Additionally, granular details regarding pertinent cancer-specific risk factors were not available, such as primary tumor type (solid vs hematologic malignancy), stage, and type of therapy, which limits our ability to draw conclusions regarding relative benefit among any specific cancer population. This study only included apixaban and rivaroxaban, which limits generalizability to other direct oral anticoagulants. Future studies focusing on patients with highly prothrombotic, active malignancies are likely to identify those who are most likely to benefit from extended thromboprophylaxis after hospitalization for medical reasons. Additionally, predictive models such as the Khorana score and HASBLED could be useful in identifying high-risk subpopulations.29,30 Finally, bleeding risks are different across anticoagulation agents, with low molecular weight heparin having been shown to be linked to lower bleeding rates compared with direct oral anticoagulants.31 A meta-analysis of 14 randomized controlled studies in cancer thrombosis showed that low molecular weight heparin had almost half the rate of major hemorrhage on pairwise comparison with direct oral agents (hazard ratio, 0.53; 95% CI, 0.40-0.70).31 Among direct oral anticoagulants, the risk of bleeding may also be lower with apixaban compared with rivaroxaban.32-34 Therefore, careful selection of the appropriate agent, duration, and population has the potential to identify a safe and effective regimen of extended thromboprophylaxis specifically in the cancer population.
In summary, extended thromboprophylaxis in patients with active and history of cancer hospitalized for medical reasons was associated with increased risk of bleeding, without clear evidence of reduction in thrombosis. Consistent with the guidance regarding the hospitalized noncancer population, these data argue against routine extended-duration thromboprophylaxis in patients with active or history of malignancy.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgment
This work was supported by the National Heart, Lung, and Blood Institute, National Institutes of Health (U01HL143365).
Authorship
Contribution: R.P., J.I.Z., and A.A.K. conceived the study; S.O., R.P., and T.C. contributed to the study design and search strategy; S.O. and R.P. performed study selection and data extraction; T.C. performed the statistical analysis; S.O. and R.P wrote the initial manuscript; and T.C., J.I.Z., and A.A.K. provided critical review of the manuscript and approved of the final draft.
Conflict-of-interest disclosure: J.I.Z. reports research funding from Incyte and Quercegen; consultancy services for Sanofi, CSL Behring, and Parexel; and honoraria from/advisory board participation for Pfizer/Bristol-Myers Squibb, Portola, and Daiichi. A.A.K. reports personal fees from Bayer, Janssen, Bristol-Myers Squibb, Anthos, Medtronic, Medscape, Nektar, Parexel, Seattle Genetics, and TriSalus and grants to the Cleveland Clinic from Bristol-Myers Squibb, Leap, and Pfizer. The remaining authors declare no competing financial interests.
Correspondence: Jeffrey I. Zwicker, Beth Israel Deaconess Medical Center, 330 Brookline Ave, Boston, MA 02215; e-mail: jzwicker@bidmc.harvard.edu.
References
- 1.Heit JA, Crusan DJ, Ashrani AA, Petterson TM, Bailey KR. Effect of a near-universal hospitalization-based prophylaxis regimen on annual number of venous thromboembolism events in the US. Blood. 2017;130(2):109-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flanders SA, Greene MT, Grant P, et al. Hospital performance for pharmacologic venous thromboembolism prophylaxis and rate of venous thromboembolism: a cohort study. JAMA Intern Med. 2014;174(10):1577-1584. [DOI] [PubMed] [Google Scholar]
- 3.Chiasakul T, Evans CR, Spyropoulos AC, Raskob G, Crowther M, Cuker A. Extended vs. standard-duration thromboprophylaxis in acutely ill medical patients: a systematic review and meta-analysis. Thromb Res. 2019;184:58-61. [DOI] [PubMed] [Google Scholar]
- 4.Tao DL, Bien JY, DeLoughery TG, Shatzel JJ. Extended thromboprophylaxis with direct oral anticoagulants for medical patients: a systematic review and meta-analysis. Blood. 2017;129(5):653-655. [DOI] [PubMed] [Google Scholar]
- 5.Schünemann HJ, Cushman M, Burnett AE, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized medical patients. Blood Adv. 2018;2(22):3198-3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abdol Razak NB, Jones G, Bhandari M, Berndt MC, Metharom P. Cancer-associated thrombosis: an overview of mechanisms, risk factors, and treatment. Cancers (Basel). 2018;10(10):380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akl EA, Terrenato I, Barba M, et al. Low-molecular-weight heparin vs unfractionated heparin for perioperative thromboprophylaxis in patients with cancer: a systematic review and meta-analysis. Arch Intern Med. 2008;168(12):1261-1269. [DOI] [PubMed] [Google Scholar]
- 8.Di Nisio M, Porreca E, Candeloro M, De Tursi M, Russi I, Rutjes AW. Primary prophylaxis for venous thromboembolism in ambulatory cancer patients receiving chemotherapy. Cochrane Database Syst Rev. 2016;12(12):CD008500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stein PD, Beemath A, Meyers FA, Skaf E, Sanchez J, Olson RE. Incidence of venous thromboembolism in patients hospitalized with cancer. Am J Med. 2006;119(1):60-68. [DOI] [PubMed] [Google Scholar]
- 10.Prandoni P, Lensing AWA, Piccioli A, et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood. 2002;100(10):3484-3488. [DOI] [PubMed] [Google Scholar]
- 11.Kaatz S, Ahmad D, Spyropoulos AC, Schulman S; Subcommittee on Control of Anticoagulation . Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: communication from the SSC of the ISTH. J Thromb Haemost. 2015;13(11):2119-2126. [DOI] [PubMed] [Google Scholar]
- 12.Goldhaber SZ, Leizorovicz A, Kakkar AK, et al. ; ADOPT trial investigators . Apixaban versus enoxaparin for thromboprophylaxis in medically ill patients. N Engl J Med. 2011;365(23):2167-2177. [DOI] [PubMed] [Google Scholar]
- 13.Spyropoulos AC, Ageno W, Albers GW, et al. ; MARINER investigators . Rivaroxaban for thromboprophylaxis after hospitalization for medical illness. N Engl J Med. 2018;379(12):1118-1127. [DOI] [PubMed] [Google Scholar]
- 14.Cohen AT, Spiro TE, Büller HR, et al. ; MAGELLAN investigators . Rivaroxaban for thromboprophylaxis in acutely ill medical patients. N Engl J Med. 2013;368(6):513-523. [DOI] [PubMed] [Google Scholar]
- 15.Hull RD, Schellong SM, Tapson VF, et al. ; EXCLAIM (Extended Prophylaxis for Venous ThromboEmbolism in Acutely Ill Medical Patients With Prolonged Immobilization) study . Extended-duration venous thromboembolism prophylaxis in acutely ill medical patients with recently reduced mobility: a randomized trial. Ann Intern Med. 2010;153(1):8-18. [DOI] [PubMed] [Google Scholar]
- 16.Cohen AT, Harrington RA, Goldhaber SZ, et al. ; APEX investigators . Extended thromboprophylaxis with betrixaban in acutely ill medical patients. N Engl J Med. 2016;375(6):534-544. [DOI] [PubMed] [Google Scholar]
- 17.Ageno W, Lopes RD, Yee MK, et al. Extended prophylaxis of venous thromboembolism with betrixaban in acutely ill medical patients with and without cancer: insights from the APEX trial. J Thromb Thrombolysis. 2020;49(2):214-219. [DOI] [PubMed] [Google Scholar]
- 18.Khorana AA, Soff GA, Kakkar AK, et al. ; CASSINI investigators . Rivaroxaban for thromboprophylaxis in high-risk ambulatory patients with cancer. N Engl J Med. 2019;380(8):720-728. [DOI] [PubMed] [Google Scholar]
- 19.Carrier M, Abou-Nassar K, Mallick R, et al. ; AVERT investigators . Apixaban to prevent venous thromboembolism in patients with cancer. N Engl J Med. 2019;380(8):711-719. [DOI] [PubMed] [Google Scholar]
- 20.Felder S, Rasmussen MS, King R, et al. Prolonged thromboprophylaxis with low molecular weight heparin for abdominal or pelvic surgery. Cochrane Database Syst Rev. 2018;11(11):CD004318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fanikos J, Rao A, Seger AC, et al. Venous thromboembolism prophylaxis for medical service-mostly cancer-patients at hospital discharge. Am J Med. 2011;124(12):1143-1150. [DOI] [PubMed] [Google Scholar]
- 22.Di Nisio M, Candeloro M, Rutjes AWS, Galli V, Tritto M, Porreca E. Bleeding and venous thromboembolic events in patients with active cancer hospitalized for an acute medical illness. Thromb Res. 2018;169:44-49. [DOI] [PubMed] [Google Scholar]
- 23.Patell R, Gutierrez A, Rybicki L, Khorana AA. Identifying predictors for bleeding in hospitalized cancer patients: a cohort study. Thromb Res. 2017;158:38-43. [DOI] [PubMed] [Google Scholar]
- 24.Neumann I, Izcovich A, Zhang Y, et al. DOACs vs LMWHs in hospitalized medical patients: a systematic review and meta-analysis that informed 2018 ASH guidelines. Blood Adv. 2020;4(7):1512-1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carrier M, Khorana AA, Moretto P, Le Gal G, Karp R, Zwicker JI. Lack of evidence to support thromboprophylaxis in hospitalized medical patients with cancer. Am J Med. 2014;127(1):82-86.e1. [DOI] [PubMed] [Google Scholar]
- 26.Zwicker JI, Roopkumar J, Puligandla M, et al. Dose-adjusted enoxaparin thromboprophylaxis in hospitalized cancer patients: a randomized, double-blinded multicenter phase 2 trial. Blood Adv. 2020;4(10):2254-2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Madenci AL, Weil BR, Liu Q, et al. Long-term risk of venous thromboembolism in survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2018;36(31):3144-3151. [DOI] [PubMed] [Google Scholar]
- 28.Strongman H, Gadd S, Matthews A, et al. Medium and long-term risks of specific cardiovascular diseases in survivors of 20 adult cancers: a population-based cohort study using multiple linked UK electronic health records databases. Lancet. 2019;394(10203):1041-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patell R, Rybicki L, McCrae KR, Khorana AA. Predicting risk of venous thromboembolism in hospitalized cancer patients: utility of a risk assessment tool. Am J Hematol. 2017;92(6):501-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJGM, Lip GYH. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138(5):1093-1100. [DOI] [PubMed] [Google Scholar]
- 31.Rossel A, Robert-Ebadi H, Combescure C, et al. Anticoagulant therapy for acute venous thrombo-embolism in cancer patients: a systematic review and network meta-analysis. PLoS One. 2019;14(3):e0213940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee JH, Lee JH, Jo K-W, Huh J-W, Oh Y-M, Lee JS. Comparison of rivaroxaban and dalteparin for the long-term treatment of venous thromboembolism in patients with gynecologic cancers. J Gynecol Oncol. 2020;31(1):e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McBane RD II, Wysokinski WE, Le-Rademacher JG, et al. Apixaban and dalteparin in active malignancy-associated venous thromboembolism: the ADAM VTE trial. J Thromb Haemost. 2020;18(2):411-421. [DOI] [PubMed] [Google Scholar]
- 34.Agnelli G, Becattini C, Meyer G, et al. ; Caravaggio investigators . Apixaban for the treatment of venous thromboembolism associated with cancer. N Engl J Med. 2020;382(17):1599-1607. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.