Key Points
Risk factors include HLA mismatch, severe aplastic anemia or malignancy, prior calcineurin inhibitor, and cytomegalovirus seropositivity.
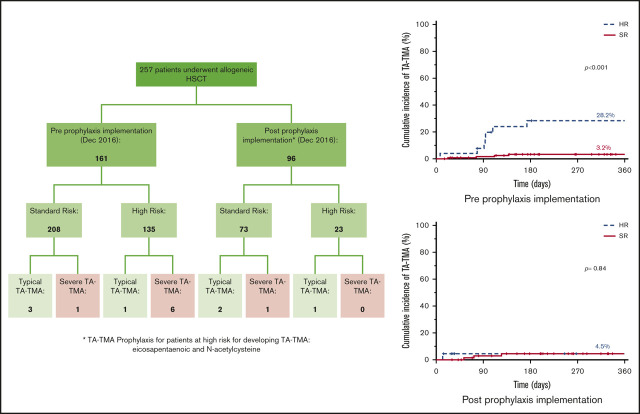
TA-TMA rates were decreased in high-risk patients from 28.2% to 4.5%, with the introduction of prophylaxis.
Abstract
Transplant-associated thrombotic microangiopathy (TA-TMA) is an endothelial injury syndrome that complicates hematopoietic stem cell transplant (HSCT). Morbidity and mortality from TA-TMA remain high, making prevention critical. We describe our retrospective single-center experience of TA-TMA after pediatric allogeneic HSCT and present a novel pre-HSCT risk-stratification system and prophylaxis regimen. From January 2012 through October 2019, 257 patients underwent 292 allogeneic HSCTs. Prospective risk stratification was introduced in December 2016. High-risk (HR) patients were treated with combination prophylaxis with eicosapentaenoic acid and N-acetylcysteine. The 1-year cumulative incidence of TA-TMA was 6.3% (95% confidence interval [CI], 3.2-9.4). Age ≥10 years, myeloablative conditioning with total body irradiation, HLA mismatch, diagnosis of severe aplastic anemia or malignancy, prior calcineurin inhibitor exposure, and recipient cytomegalovirus seropositivity were found to be pre-HSCT risk factors for development of TA-TMA. Before routine prophylaxis, TA-TMA rates were significantly different between the HR and standard-risk groups, at 28.2% (95% CI, 0-12.7) vs 3.2% (0.1-6.3), respectively (P < .001). After introduction of prophylaxis, the 1-year cumulative incidence of TA-TMA in the HR group decreased to 4.5% (95% CI, 0-13.1; P = .062, compared with the incidence before prophylaxis). Multicenter pediatric studies are needed to validate these risk criteria and to confirm the efficacy of the prophylactic regimen.
Visual Abstract
Introduction
Transplant-associated thrombotic microangiopathy (TA-TMA) is a multifactorial complication of hematopoietic stem cell transplant (HSCT) associated with endothelial injury caused by conditioning, immunosuppression, and other systemic insults that lead to microangiopathic hemolytic anemia, intravascular platelet activation, and formation of thrombi within the microcirculation.1-4 These adverse effects lead to end-organ injury from ischemia, particularly in the kidney, but also in the lungs and gastrointestinal tract. In addition to damaged endothelium, an activated complement cascade contributes significantly to the end-organ injury. Reported risk factors include those that are inherent and nonmodifiable (ie, genetic variants in complement genes), transplant-associated (ie, conditioning regimen), and associated with posttransplant events (ie, infections), leading to a “three-hit” hypothesis for the etiology of the disease.5
The diagnosis of TA-TMA is challenging, as systemic signs and symptoms of TA-TMA may overlap with other common transplant complications, such as medication-induced hypertension, sinusoidal obstructive syndrome, and expected cytopenias. Currently, there is no single diagnostic test, and there are various diagnostic consensus criteria, with the criteria proposed by Jodele et al6 generally adopted for the pediatric population.7-10 Because of these diagnostic challenges, the incidence of TA-TMA is poorly defined, with published reports ranging from 3% to 39%.11-13 There is no gold standard of treatment for TA-TMA. The initial approach consists of supportive care, including hypertension management, renal support, and treatment of infections, whereas treatment options with variable success include complement blockade, plasmapheresis, and defibrotide.2,5,14-19 With the complexity of diagnosis and lack of straightforward treatment, morbidity and mortality remain high, making prevention of TA-TMA critical.
There have been multiple single-center publications on pediatric TA-TMA, but only 1 large multicenter retrospective analysis, which incorporated both autologous and allogeneic HSCT.12,15,20-22 In addition, much of the literature focuses primarily on rates of TA-TMA and its complications and treatment, with few studies focused on how best to prevent it.23,24 In this report, we describe our single-center experience of TA-TMA in the allogeneic setting, with a focus on pretransplant risk and prophylaxis.
Methods
Patient data were retrospectively reviewed according to the principles set forth in the Declaration of Helsinki and were approved by the institutional review board. Data were manually extracted from the medical records of patients who underwent a conditioned allogeneic HSCT at University of California, San Francisco, Benioff Children’s Hospital, San Francisco from January 2012 through October 2019.
The patients’ charts were reviewed for pretransplant attributes, including age, sex, race/ethnicity, HSCT indication, and cytomegalovirus (CMV) serostatus, as well as transplant-related covariates, such as donor type (matched related, 9 of 10 mismatched related, 10 of 10 matched unrelated, ≤9 of 10 mismatched unrelated, and haploidentical), stem cell source, and conditioning (total body irradiation [TBI] containing myeloablative [MAC], non-TBI MAC, reduced intensity [RIC], and nonmyeloablative [NMA]).25 Graft-versus-host disease (GVHD) prophylaxis included a calcineurin inhibitor (CNI), primarily tacrolimus, for all patients except those who received an ex-vivo T-cell–depleted graft. No patient received sirolimus as initial, planned GVHD prophylaxis. Serotherapy was administered to most patients, with the exception of recipients of matched-sibling bone marrow (anti-thymocyte globulin, n = 162; alemtuzumab, n = 80; or none = 15). Use of CNI and serotherapy was not analyzed as a risk factor, given that their utilization was directly related to donor source and there was no control group for comparison. We did not collect posttransplant data on GVHD or infections, as these TA-TMA risk factors are not useful in developing a pretransplant risk-stratification system for a prophylactic regimen. Charts of patients who died of transplant-related mortality (TRM) were extensively reviewed and retrospectively assessed for a possible diagnosis of TA-TMA.
Formal prospective TA-TMA screening and risk stratification of all patients were implemented on 21 December 2016. Initial TA-TMA risk stratification was devised based on published risk factors. Patients were classified as high risk (HR) if they met 3 of the following criteria: ≥10 years of age, race/ethnicity other than White non-Hispanic, ABO blood group minor incompatibility, and haploidentical donor.17,26-34 All patients had daily complete blood counts, twice weekly lactate dehydrogenase determination, and spot urine protein monitoring. Laboratory screening for HR patients included twice weekly haptoglobin and D-dimers starting on day +14 through day +100.35,36 In addition, HR patients received a standard-of-care prophylactic regimen, with a combination of eicosapentaenoic acid (EPA, a component of fish oil; 30 mg/kg per day; maximum, 2000 mg/day) at least 2 weeks before conditioning through day +100 and N-acetylcysteine (NAC) 70 mg/kg every 8 hours from day +3 through day +42 (or discharge).23,37-39 Both drugs have low side-effect profiles: EPA has the potential to cause bad breath and nausea, and NAC may cause nausea, vomiting, and urticaria. NAC can be given IV if the patient is unable to tolerate oral or nasogastric administration. Studies have found that there is not an increased risk of bleeding with EPA, but doses were held when platelets were <20 000 × 109/L and restarted once the patient was platelet transfusion independent.40-42
Diagnosis of TA-TMA was made according to the Jodele criteria.6 TA-TMA was then classified as nonsevere or severe, based on the need for intensive care unit admission, surgical drainage of effusions, or dialysis or the presence of gastrointestinal bleeding or neurologic involvement. Patients who underwent transplant before the introduction of risk stratification were retrospectively assigned to a risk group according to the same criteria.
Statistical analysis
Kaplan-Meier analysis was used to estimate the 1-year cumulative incidence of TA-TMA for selected clinical variables in the overall cohort of patients, as well as the subgroups comprising patients who underwent HSCT before and after implementation of prophylaxis based on risk stratification. Univariate logistic regression was performed for each of the identified potential clinical risk factors; multivariable analyses were found to be underpowered due to the sample size. SPSS (version 26) was used to determine summary statistics and cumulative incidences, and STATA (version 16.1; College Station, TX) was used for univariate logistic regression.
Results
During the study period, 257 patients underwent 292 allogeneic HSCTs, 3 patients had prior autologous transplants, and 5 patients had a prior allogeneic conditioned transplant before January 2012. Patient and transplant characteristics are shown in Tables 1 and 2, respectively, and were similar before and after risk stratification and implementation of prophylaxis, with the exception of more mismatched unrelated donors before and more haploidentical donors after implementation.
Table 1.
Patient characteristics and incidence of TA-TMA
| Overall | Preprophylaxis implementation | Postprophylaxis implementation | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Overall, n (%) | One-year cumulative incidence of TA-TMA, % (95% CI) | P | Overall, n (%) | One-year cumulative incidence of TA-TMA, % (95% CI) | P | Overall, n (%) | One-year cumulative incidence of TA-TMA, % (95% CI) | P | |
| TA-TMA, Overall | 15/257 | 6.3 (3.2-9.4) | <.001 | 11/161 | 7.3 (3.2-11.4) | <.001 | 4/96 | 4.5 (0.2-8.8) | .84 |
| SR | 7/208 | 3.6 (1.1-6.1) | 4/135 | 3.2 (0.1-6.3) | 3/73 | 4.4 (0.1-9.3) | |||
| HR | 8/49 | 18.2 (6.8-29.6) | 7/26 | 28.2 (17.8-38.6) | 1/23 | 4.5 (0-13.1) | |||
| Age, y | .04 | .11 | .18 | ||||||
| <10 | 138 (54) | 3.1 (0.2-6) | 84 (52) | 3.8 (0.1-8.1) | 54 (56) | 2.1 (0.1-6.2) | |||
| ≥10 | 119 (46) | 9.8 (4.3-15.3) | 77 (48) | 10.9 (3.8-18) | 42 (44) | 7.8 (0.1-16.2) | |||
| Sex | .43 | .88 | .18 | ||||||
| Male | 151 (59) | 5.1 (1.4-8.8) | 94 (58) | 6.9 (1.6-12.2) | 57 (59) | 2 (0.1-5.9) | |||
| Female | 106 (41) | 7.9 (2.6-13.2) | 67 (42) | 7.9 (1.2-14.6) | 39 (41) | 8 (0.1-16.6) | |||
| Race | .34 | .30 | .12 | ||||||
| White | 181 (70) | 4.8 (1.5-7.9) | 111 (69) | 5.8 (1.3-10.3) | 70 (73) | 3.1 (0.1-7.4) | |||
| Black/African American | 20 (8) | 11.1 (0.1-25.5) | 10 (6) | 22.2 (0.1-49.4) | 10 (10) | 0 (0-32.1) | |||
| Asian American | 50 (20) | 10.5 (1.7-19.3) | 34 (21) | 9 (0.1-18.8) | 16 (17) | 14.1 (0.1-32.3) | |||
| Native American | 6 (2) | 0 (0-44.3) | 6 (4) | 0 (0-44.3) | 0 | — | |||
| Race/ethnicity | .98 | .75 | .9 | ||||||
| White | 83 (32) | 3.7 (0-9.2) | 53 (33) | 8.3 (0.5-16.1) | 30 (31) | 3.3 (2.7-3.9) | |||
| Hispanic and non-White | 174 (68) | 6.2 (2.5-9.9) | 108 (67) | 6.9 (2-11.8) | 66 (69) | 5 (0-10.5) | |||
| HSCT indication | .006 | .02 | .23 | ||||||
| Malignancy* | 157 (61) | 8.3 (3.8-12.8) | 94 (58) | 10.1 (3.8-16.4) | 63 (66) | 5.3 (0.1-11.2) | |||
| SAA | 17 (7) | 17.6 (0-35.6) | 10 (6) | 20 (0-44.7) | 7 (7) | 14.3 (0.1-40.2) | |||
| Other nonmalignant† | 83 (32) | 0 (0-5.3) | 57 (36) | 0 (0-7.6) | 26 (27) | 0 (0-15.2) | |||
| Recipient CMV serostatus | .03 | .12 | .14 | ||||||
| Positive | 120 (47) | 9.9 (4.2-15.6) | 80 (50) | 10.6 (3.7-17.5) | 40 (41) | 8.4 (0.1-17.6) | |||
| Negative/unknown‡ | 137 (53) | 3.1 (0.2-6) | 81 (50) | 4 (0-8.3) | 56 (58) | 1.9 (0.1-5.4) | |||
Three patients with lymphoma had prior autologous transplants (2008, 2013, and 2015).
Immunodeficiencies (n = 41), bone marrow failure syndromes (n = 12), hemoglobinopathies (n = 8), hemophagocytic lymphohistiocytosis (n = 8), metabolic disorders (n = 11), and osteopetrosis (n = 3).
Patients unable to make specific antibodies without prior CMV infection.
Table 2.
Transplant characteristics and incidence of TA-TMA
| Overall | Preprophylaxis implementation | Postprophylaxis implementation | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Overall, n (%) | One-year cumulative incidence of TA-TMA, % (95% CI) | P | Overall, n (%) | One-year cumulative incidence of TA-TMA, % (95% CI) | P | Overall, n (%) | One-year cumulative incidence of TA-TMA, % (95% CI) | P | |
| Conditioning | .17 | .29 | .02 | ||||||
| TBI-MAC | 43 (17) | 12.2 (2.2-22.2) | 32 (20) | 9.8 (0.3-19.3) | 11 (11) | 19.2 (0-43.1) | |||
| Non TBI-MAC* | 171 (66) | 5.7 (2-9.4) | 101 (63) | 8.5 (2.8-14.2) | 70 (73) | 1.6 (0-4.7) | |||
| RIC or NMA | 43 (17) | 2.4 (0-7.1) | 28 (17) | 0 | 15 (16) | 7.1 (0-20.6) | |||
| Stem cell source | .40 | .47 | .86 | ||||||
| Bone marrow | 88 (34) | 4.7 (0.2-9.2) | 46 (29) | 4.7 (0-11) | 42 (44) | 4.9 (0-11.6) | |||
| PBSCs† | 153 (60) | 7.8 (3.3-12.3) | 106 (66) | 9.1 (3.4-14.8) | 47 (49) | 4.6 (0-10.9) | |||
| Cord‡ | 16 (6) | 0 | 9 (5) | 0 | 7 (7) | 0 | |||
| Donor type | .13 | .02 | .82 | ||||||
| MRD | 60 (23) | 5 (0-10.5) | 32 (20) | 3.1 (0-9.1) | 28 (29) | 7.1 (0-16.7) | |||
| MMRD (9/10) | 3 (1) | 0 | 3 (2) | 0 | 0 | — | |||
| Haploidentical | 58 (23) | 14.2 (4.4-24) | 31 (19) | 22.1 (6.4-37.8) | 27 (28) | 3.7 (0-10.7) | |||
| MUD (10/10) | 92 (36) | 3.4 (0-7.1) | 58 (36) | 3.5 (0-8.4) | 34 (36) | 3.2 (0-9.5) | |||
| MMUD (≤9/10) | 44 (17) | 4.9 (0-11.6) | 37 (23) | 5.8 (0-13.6) | 7 (7) | 0 | |||
| HLA-mismatch (<10/10) | .08 | .04 | .79 | ||||||
| No | 152 (59) | 4.1 (1-7.2) | 90 (56) | 3.4 (0-7.1) | 62 (64) | 5 (0-10.5) | |||
| Yes | 105 (41) | 9.9 (3.8-16) | 71 (44) | 12.7 (4.5-20.9) | 34 (36) | 2.9 (0-8.6) | |||
| Minor ABO mismatch | .98 | .58 | .27 | ||||||
| No | 189 (74) | 6.3 (2.8-9.8) | 115 (71) | 6.5 (1.8-11.2) | 74 (77) | 5.9 (0.4-11.4) | |||
| Yes | 68 (26) | 6.3 (0.4-12.2) | 46 (29) | 9.1 (0.7-17.5) | 22 (23) | 0 | |||
| Pre-HSCT CNI exposure | .003 | .02 | .07 | ||||||
| No | 238 (93) | 5 (2.1-7.9) | 147 (91) | 5.8 (1.9-9.7) | 91 (95) | 3.6 (0-7.5) | |||
| Yes | 19 (7) | 22.4 (3-41.8) | 14 (9) | 22.6 (0.1-45.1) | 5 (5) | 20 (0-55) | |||
MMRD, mismatched related donor; MMUD, mismatched unrelated donor; MRD, matched related donor; MUD, matched unrelated donor.
Conditioning regimens for patients with Fanconi anemia were considered MAC.
One patient received PBSC + marrow.
Three patients received cord + marrow.
TA-TMA
Fifteen patients were diagnosed with TA-TMA, for a 1-year cumulative incidence of 6.3% (95% confidence interval [CI], 3.2-9.4; details in supplemental Table 1). All were clinically diagnosed during their HSCT course, except 1 case from 2013, in which diagnosis was retrospective; 8 (53.3%) had severe disease. Of the 7 patients with nonsevere TA-TMA, 1 received no treatment, 4 discontinued CNI alone, 1 discontinued CNI and received eculizumab, and 1 received eculizumab; none of those patients died of TA-TMA. Conversely, of the 8 patients with severe TA-TMA, all received multiple therapeutic interventions, including eculizumab, defibrotide, rituximab, steroids, and total plasma exchange, and 5 (62.5%) died of complications of TA-TMA at a median of 112 days (range, 56-542) from time of diagnosis, including the patient diagnosed retrospectively. The 3 surviving patients had chronic kidney disease, 2 of whom required long-term dialysis and kidney transplantation. The 1-year cumulative incidence of TMA-related mortality in the entire cohort was 2.2% (95% CI, 0.2-4.2); however, there was a 33.3% attributable mortality after a diagnosis of any TA-TMA, and a 30% incidence of severe renal morbidity in survivors.
Risk-stratification system and prophylaxis implementation
TA-TMA prophylaxis based on our risk-stratification system was implemented in December 2016, with 161 patients undergoing transplant before TA-TMA risk stratification and 96 patients after. The minority (19%) of patients were considered HR, with a similar proportion before (retrospectively assigned, 16.1%) and after (prospectively assigned, 24%) implementation (P = .14).
Before stratification, there were 11 cases of TA-TMA, for a 1-year cumulative incidence of 7.3% (95% CI, 3.2-11.4), compared with 4 cases that were diagnosed after stratification, for a 1-year cumulative incidence of 4.5% (95% CI, 0.2-8.8; P = .42). TA-TMA rates by risk stratification were statistically significant overall (1-year cumulative incidence: standard-risk [SR] 3.6% [95% CI, 1.1-6.1], and HR 18.2% [95% CI, 6.8-29.6; P < .001] and before stratification [1-year cumulative incidence: SR 3.2%; 95% CI, 0.1-6.3 and HR 28.2% [95% CI, 17.8-38.6; P < .001]; Figure 1A). Because SR patients were not treated differently, their TA-TMA rates did not change after risk stratification. Notably, the 1-year cumulative incidence of TA-TMA in the HR group, which received combination prophylaxis, decreased from 28.2% (95% CI, 17.8-38.6) to 4.5% (95% CI, 0-13.1; P = .062; Figure 1A-B; supplemental Table 2). Furthermore, the severity of TA-TMA declined with time (Table 3). Of the 8 patients with severe TA-TMA, 6 were HR and all were treated before risk stratification and prophylaxis. The HR patient who developed TA-TMA after risk stratification had nonsevere disease that resolved quickly with eculizumab and had no long-term TA-TMA–related sequalae.
Figure 1.
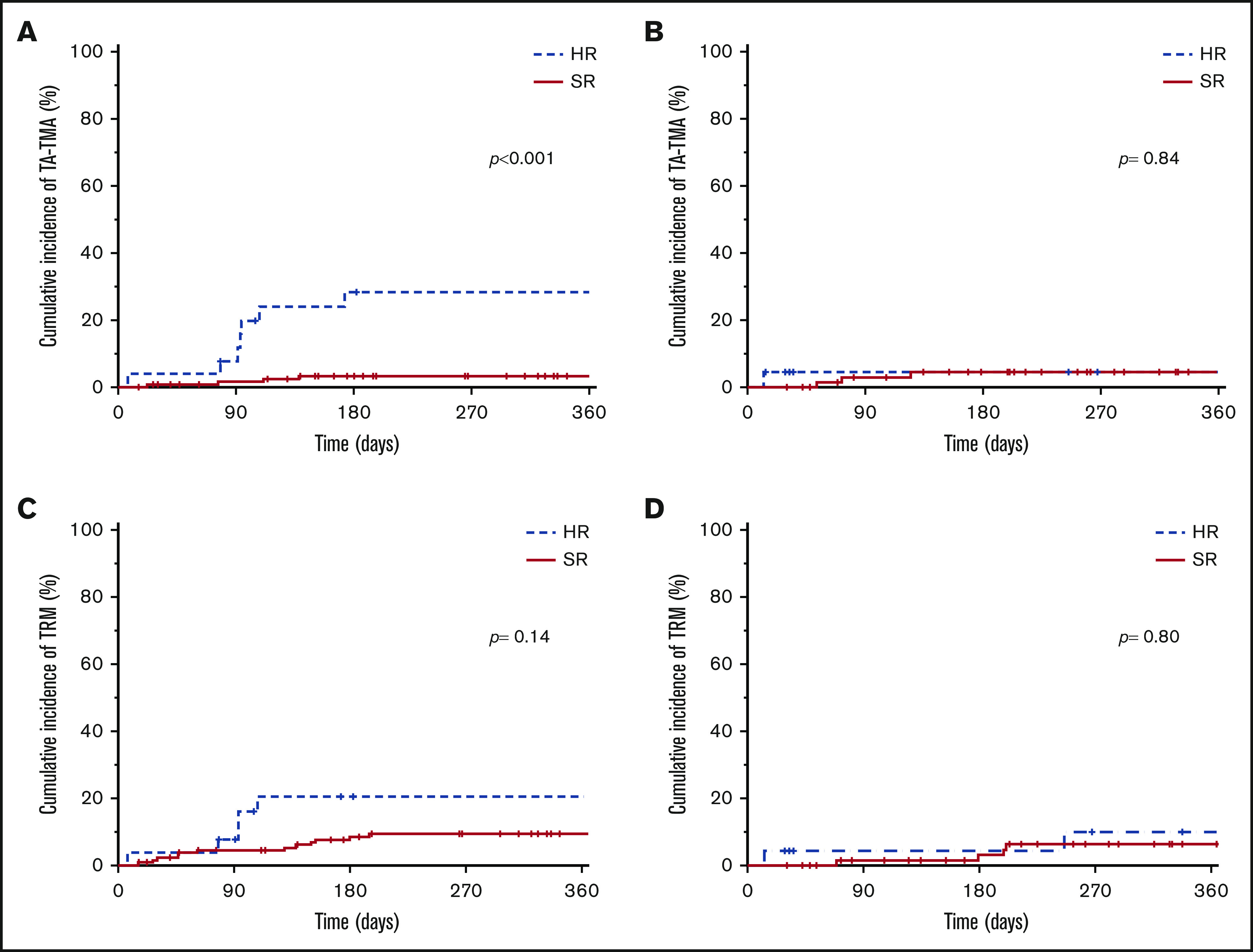
Cumulative incidence of TA-TMA and TRM by risk stratification. Cumulative incidence of TA-TMA before (A) and after (B) risk stratification. Cumulative incidence of TRM before (C) and after (D) risk stratification.
Table 3.
TA-TMA severity by stratification and risk group
| Total | SR | HR | |||
|---|---|---|---|---|---|
| Prestratification | Poststratification* | Prestratification | Poststratification† | ||
| Total TA-TMA Cases | 15/257 | 4/135 | 3/73 | 7/26 | 1/23 |
| Typical vs severe | |||||
| Typical | 7 | 3 | 2 | 1 | 1 |
| Severe‡ | 8 | 1§ | 1 | 6 | 0 |
No change in clinical practice other than enhanced screening for development of TA-TMA.
HR poststratification patients received prophylaxis with NAC and EPA.
Severe TA-TMA defined as the need for admission to the intensive care unit, surgical drainage of effusions, or dialysis and the presence of gastrointestinal bleeding or neurologic involvement.
This patient was diagnosed retrospectively and did not receive treatment.
The overall 1-year cumulative incidence of TRM was 9.7% (95% CI, 6-13.4), with 11.1% (95% CI, 6.2-16) and 7.3% (95% CI, 1.6-13; P = .41) dying before and after stratification, respectively. The overall 1-year cumulative incidence of TRM of SR patients was 8.3% (95% CI, 4.4-12.2), compared with 16.3% (95% CI, 5.1-27.5; P =.17) of HR patients. For HR patients, the 1-year cumulative incidence of TRM was 20.5% (95% CI, 4.4-36.6) and 10.2% (95% CI, 0-23.7) before and after stratification, respectively (P = .36; Figure 1C-D).
Patient characteristic risk factors
Univariate analysis showed that patients ≥10 years of age had higher rates of TA-TMA (Tables 1 and 4). Neither sex nor Hispanic ethnicity was found to be a risk factor. In the preliminary risk stratification, Hispanic ethnicity was included as a risk factor, but on formal analysis, it did not appear to be one; 1-year cumulative incidence in White non-Hispanic patients was 3.7% (95% CI, 0-9.2), whereas the incidence among those who identified as Hispanic was 6.2% (95% CI, 2.5-9.9; P = .98). When ethnicity was not considered, the 1-year cumulative incidence in White patients was 4.8% (95% CI, 1.5-7.9) and in patients of all other races was 9.9% (95% CI, 2.8-17; P = 0.12). After introduction of our risk stratification, we saw an improvement in the 1-year cumulative incidence of TA-TMA in the Black/African patients from 22.2% (95% CI, 0-49.4) to 0% (95% CI, 0-32.1), although small numbers prevented that result from attaining statistically significance (P = .13), and there was no difference in TA-TMA incidence before and after risk stratification in the Asian group. Underlying disease was a clear TA-TMA risk factor, with patients with severe aplastic anemia (SAA) having a 1-year cumulative incidence of 17.6% (95% CI, 0-35.6), compared with patients with malignancy or other nonmalignant diagnoses, who had 1-year cumulative incidences of 8.3% (95% CI, 3.8-12.8) and 0% (95% CI, 0-5.3), respectively (P = .006). Patients who were CMV IgG seropositive before transplant had a higher 1-year cumulative incidence at 9.9% (95% CI, 4.2-15.6) than those who were not seropositive, at 3.1% (95% CI, 0.2-6; P = 0.03).
Table 4.
Risk factors for TA-TMA by univariate regression
| Odds ratio | P | 95% CI | |
|---|---|---|---|
| Age ≥10 y | 3.65 | .03 | 1.13-11.8 |
| Non-White | 2.19 | .14 | 0.77-6.25 |
| HLA mismatched (≤9 of 10) | 1.99 | .20 | 0.69-5.75 |
| CMV seropositive recipient | 2.89 | .08 | 0.90-9.30 |
| Conditioning | |||
| RIC/NMA | 1 | ||
| Non-TBI MAC | 2.71 | .35 | 0.33-22.1 |
| TBI MAC | 9.00 | .05 | 1.04-77.6 |
| Diagnosis | |||
| Malignant | 1 | — | — |
| Nonmalignant other than SAA | — | — | — |
| SAA | 3.00 | .12 | 0.76-11.9 |
| Pre-HSCT CNI exposure | 2.29 | .18 | 0.69-7.55 |
Transplant characteristic risk factors
As shown in Table 2, the number of transplants, both autologous and allogeneic, did not increase risk of TA-TMA. Patients with CNI exposure before admission for HSCT had a 1-year cumulative incidence of TA-TMA of 22.4% (95% CI, 3-41.8), compared with 5% (95% CI, 2.1-7.9) in those without prior CNI exposure (P = .003). However, prior CNI exposure and a diagnosis of SAA were confounded, with 53% (9 of 17) of patients with SAA treated with CNIs before transplant, 3 of whom (33%) developed TA-TMA, whereas, of the non-SAA patients, only 1 of 33 (3%) who received CNI before HSCT admission developed TA-TMA.
The stem cell source was not a risk factor on univariate analysis, although, notably, no patients who received a cord blood transplant developed TA-TMA (n = 16). There was no significant effect of conditioning on risk of TA-TMA, with TBI-containing MAC regimens having a 1-year cumulative incidence of 12.2% (95% CI, 2.2-22.2), compared with non-TBI MAC regimens, or with RIC and NMA having 1-year cumulative incidences of 5.7% (95% CI, 2-9.4) and 2.4% (95% CI, 0-7.1), respectively (P = .17). Haploidentical donors and HLA-mismatched donors were identified as risk factors for patients before stratification, with this association no longer significant after implementation of prophylaxis. Minor ABO blood group incompatibility did not cause an increased risk of TA-TMA.
Discussion
TA-TMA is becoming increasingly recognized as a serious complication of pediatric HSCT.12,15,21,43,44 Previously reported pretransplant risk factors include female sex, African American race, SAA, CMV seropositive recipient, prior transplant, mismatched donors, minor ABO mismatch, myeloablative conditioning, and peripheral blood stem cells (PBSCs) as the donor source.5 Epperla et al analyzed >23 000 patients reported to the Center for International Blood and Marrow Transplant Research, including adults and pediatric patients with autologous and allogeneic transplants, and found a TA-TMA incidence by center designation of 3%. They identified MAC, African American race, SAA, and mismatched donors as risk factors for TA-TMA, similar to our findings. Other identified TA-TMA risk factors in that study that were not predictive in our pediatric patient population included female sex and prior autologous HSCT. Although their sample size was far larger, Center for International Blood and Marrow Transplant Research data rely on accurate reporting from centers that did not use uniform diagnostic criteria.13 Schoettler et al21 confirmed some of these risk factors in a pediatric population; however, their data require reconsideration of risk after the development of GVHD and posttransplant infections. We focused particularly on pretransplant risk factors, to be able to address modifiable risk factors and to identify patients at high risk for developing TA-TMA, thereby facilitating the use of up-front prophylactic strategies for preventing the occurrence of TA-TMA.
The prophylactic regimen of EPA and NAC was chosen because of its properties in endothelial health. EPA has been shown in a small trial to decrease TA-TMA, potentially via reduction of cytokines and stimulation of nitric oxide production.23 NAC is a synthetic antioxidant derived from cysteine that acts by removing reactive oxygen species, either directly or indirectly, by increasing glutathione biosynthesis. NAC significantly limited endothelial injury in a mouse model of shiga toxin–mediated atypical hemolytic uremic syndrome, and, in another study, it reduced neutrophil extracellular trap formation.37,45 These prophylactic interventions appeared to decrease the occurrence of TA-TMA in our cohort, with a significant improvement in rates of TA-TMA in the treated HR population. Both EPA and NAC are well-tolerated with minimal side-effect profile and low cost. Because the SR patients from the preliminary stratification system were not given specific prophylaxis, their incidence of TA-TMA was identical before and after stratification. Further optimization of our risk classification to identify those patients previously classified as SR who develop TA-TMA has the potential to further decrease the incidence and severity of TA-TMA in our patient population.
The risk factors identified in our analysis have raised additional questions. After implementation of prophylaxis, there was a trend toward improvement in TA-TMA incidence in the Black/African population that was not seen in the Asian population. A potential explanation for this finding is that there may be different mechanisms by which genetic ancestry increases risk for TA-TMA. For example, Black/African patients may have increased TA-TMA risk secondary to activating complement genetic polymorphisms.26 Conversely, Asian patients may have increased TA-TMA risk secondary to increased endothelial damage from increased chemotherapy exposure related to genetic polymorphisms in drug metabolism enzymes.46,47 Similarly, it is unclear whether age is a direct risk factor or simply a surrogate for other factors, such as chemotherapy dosing (weight-based vs body surface area–based; ideal vs actual body weight). Careful analysis of the pharmacokinetics and pharmacogenetics of various endothelial-toxic conditioning agents may provide further insight to answer these questions.46,47
There is a question of whether SAA itself is a risk factor for TA-TMA or the increased risk is solely secondary to prior use of a CNI.6 In this cohort, patients receiving CNI therapy before HSCT for SAA had a higher incidence of TA-TMA compared with patients without SAA. The numbers are small, but the result may indicate that SAA is an independent risk factor regardless of CNI exposure, although the mechanism for this risk remains unclear. Further larger studies are needed to evaluate the incidence of TA-TMA among those patients with SAA who proceed straight to HSCT vs those who first undergo immunosuppressive therapy with CNIs. Finally, HLA mismatch and CMV seropositivity may simply be surrogate markers for the risk of developing GVHD or infection after HSCT; however, they are amenable to use in a pre-HCT risk-stratification system.
As this is a retrospective analysis, there are limitations to the study. Formal TA-TMA screening was not implemented until December 2016, and the patients who underwent transplant before this date may not have had the necessary testing for retrospective diagnosis of TA-TMA. It is possible that there were cases of TA-TMA that were not diagnosed during the clinical course, therefore making the rates of TA-TMA falsely low in the earlier patients. Although if cases of TA-TMA were missed before implementation of prophylaxis and proactive screening, it would only increase the incidence in the earlier cohort, which would further support that the prophylactic regimen was beneficial in decreasing TA-TMA. We could not evaluate genetic ancestry directly in this study, as we relied on race and ethnicity as documented in the medical record. Because of the way the race and ethnicity questions are asked in our medical records, they are poor surrogates for ancestry and are confounded by social factors. It is, therefore, important to note that the higher risk of TA-TMA among non-White patients could also have been attributable to social determinants of health and structural racism. Furthermore, because this was a single-center study, sample numbers were small, making multivariable analysis challenging.
In conclusion, after implementation of prophylaxis in the HR patient population, it is encouraging to see a dramatic decrease in TA-TMA incidence and severity. The goal of our novel risk-stratification scoring system is to ensure that prophylaxis is provided to those patients who are at the highest risk of developing TA-TMA. Additional refinements of the risk stratification appear to be warranted, based on our additional analysis, and would benefit from further refinement in a large multicenter cohort that incorporates different HSCT approaches, to further investigate other potential risk factors, such as CNIs, sirolimus, or serotherapy, and that allow for multivariable analyses and formal prediction modeling. An appropriate next step would be a randomized controlled trial of EPA and NAC in HR patients that uses a validated risk-stratification system developed from the criteria found in this analysis (age, ≥10 years; MAC with total body irradiation, HLA mismatch, diagnosis of SAA or malignancy, prior CNI exposure, and recipient CMV seropositivity) and in other reports. Future efforts should also focus on identifying the safest and most effective prophylactic regimens, including promising medications such as statins. Similarly, a pilot trial of defibrotide prophylaxis in patients at high risk for development of TA-TMA is currently underway (registered on www.clinicaltrials.gov as NCT#03384693). With improved risk stratification and prophylaxis strategies, we hope to continue to decrease the morbidity and mortality associated with TA-TMA in the pediatric HSCT population.
Supplementary Material
The full-text version of this article contains a data supplement.
Footnotes
Send data sharing requests via e-mail to the corresponding author, Christine S. Higham (christine.higham@ucsf.edu).
Authorship
Contribution: C.S.H. and C.C.D. designed the study and analysis and wrote the manuscript; G.C. performed statistical analyses and edited the manuscript; and all other authors provided significant feedback and editing during manuscript preparation.
Conflict-of-interest disclosure: C.C.D. has consulted for Alexion Inc. and Omeros Corporation. C.S.H. receives research funding from Jazz Pharmaceuticals. K.A.S. receives research funding from Novartis, Pfizer, and Daiichi-Sankyo and has served on an Ad Board for Dova Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Christine S. Higham, University of California, San Francisco, 550 16th St, Box 0434, San Francisco, CA 94158; e-mail: christine.higham@ucsf.edu.
References
- 1.Goldberg RJ, Nakagawa T, Johnson RJ, Thurman JM. The role of endothelial cell injury in thrombotic microangiopathy. Am J Kidney Dis. 2010;56(6):1168-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khosla J, Yeh AC, Spitzer TR, Dey BR. Hematopoietic stem cell transplant-associated thrombotic microangiopathy: current paradigm and novel therapies. Bone Marrow Transplant. 2018;53(2):129-137. [DOI] [PubMed] [Google Scholar]
- 3.Laskin BL, Goebel J, Davies SM, Jodele S. Small vessels, big trouble in the kidneys and beyond: hematopoietic stem cell transplantation-associated thrombotic microangiopathy. Blood. 2011;118(6):1452-1462. [DOI] [PubMed] [Google Scholar]
- 4.Rosenthal J. Hematopoietic cell transplantation-associated thrombotic microangiopathy: a review of pathophysiology, diagnosis, and treatment. J Blood Med. 2016;7:181-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dvorak CC, Higham C, Shimano KA. Transplant-associated thrombotic microangiopathy in pediatric hematopoietic cell transplant recipients: a practical approach to diagnosis and management. Front Pediatr. 2019;7:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jodele S, Davies SM, Lane A, et al. Diagnostic and risk criteria for HSCT-associated thrombotic microangiopathy: a study in children and young adults. Blood. 2014;124(4):645-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cho BS, Yahng SA, Lee SE, et al. Validation of recently proposed consensus criteria for thrombotic microangiopathy after allogeneic hematopoietic stem-cell transplantation. Transplantation. 2010;90(8):918-926. [DOI] [PubMed] [Google Scholar]
- 8.Ho VT, Cutler C, Carter S, et al. Blood and marrow transplant clinical trials network toxicity committee consensus summary: thrombotic microangiopathy after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2005;11(8):571-575. [DOI] [PubMed] [Google Scholar]
- 9.Ruutu T, Barosi G, Benjamin RJ, et al. ; European LeukemiaNet . Diagnostic criteria for hematopoietic stem cell transplant-associated microangiopathy: results of a consensus process by an International Working Group. Haematologica. 2007;92(1):95-100. [DOI] [PubMed] [Google Scholar]
- 10.Moiseev IS, Tsvetkova T, Aljurf M, et al. Clinical and morphological practices in the diagnosis of transplant-associated microangiopathy: a study on behalf of Transplant Complications Working Party of the EBMT. Bone Marrow Transplant. 2019;54(7):1022-1028. [DOI] [PubMed] [Google Scholar]
- 11.Jodele S, Laskin BL, Dandoy CE, et al. A new paradigm: Diagnosis and management of HSCT-associated thrombotic microangiopathy as multi-system endothelial injury. Blood Rev. 2015;29(3):191-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elfeky R, Lucchini G, Lum S-H, et al. New insights into risk factors for transplant-associated thrombotic microangiopathy in pediatric HSCT. Blood Adv. 2020;4(11):2418-2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Epperla N, Li A, Logan B, et al. Incidence, Risk Factors for and Outcomes of Transplant-Associated Thrombotic Microangiopathy. Br J Haematol. 2020;189(6):1171-1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corti P, Uderzo C, Tagliabue A, et al. Defibrotide as a promising treatment for thrombotic thrombocytopenic purpura in patients undergoing bone marrow transplantation. Bone Marrow Transplant. 2002;29(6):542-543. [DOI] [PubMed] [Google Scholar]
- 15.Jodele S, Dandoy CE, Lane A, et al. Complement blockade for TA-TMA: lessons learned from a large pediatric cohort treated with eculizumab. Blood. 2020;135(13):1049-1057. od.2019004218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martínez-Muñoz ME, Forés R, Lario A, et al. Use of defibrotide to treat adult patients with transplant-associated thrombotic microangiopathy. Bone Marrow Transplant. 2019;54(1):142-145. [DOI] [PubMed] [Google Scholar]
- 17.Oran B, Donato M, Aleman A, et al. Transplant-associated microangiopathy in patients receiving tacrolimus following allogeneic stem cell transplantation: risk factors and response to treatment. Biol Blood Marrow Transplant. 2007;13(4):469-477. [DOI] [PubMed] [Google Scholar]
- 18.Yeates L, Slatter MA, Bonanomi S, et al. Use of defibrotide to treat transplant-associated thrombotic microangiopathy: a retrospective study of the Paediatric Diseases and Inborn Errors Working Parties of the European Society of Blood and Marrow Transplantation. Bone Marrow Transplant. 2017;52(5):762-764. [DOI] [PubMed] [Google Scholar]
- 19.Gavriilaki E, Sakellari I, Anagnostopoulos A, Brodsky RA. Transplant-associated thrombotic microangiopathy: opening Pandora’s box. Bone Marrow Transplant. 2017;52(10):1355-1360. [DOI] [PubMed] [Google Scholar]
- 20.Dandoy CE, Rotz S, Alonso PB, et al. A pragmatic multi-institutional approach to understanding transplant-associated thrombotic microangiopathy after stem cell transplant. Blood Adv. 2021;5(1):1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schoettler M, Lehmann LE, Margossian S, et al. Risk factors for transplant-associated thrombotic microangiopathy and mortality in a pediatric cohort. Blood Adv. 2020;4(11):2536-2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tolbert VP, Dvorak CC, Golden C, et al. Risk factors for transplant-associated thrombotic microangiopathy after autologous hematopoietic cell transplant in high-risk neuroblastoma. Biol Blood Marrow Transplant. 2019;25(10):2031-2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takatsuka H, Takemoto Y, Iwata N, et al. Oral eicosapentaenoic acid for complications of bone marrow transplantation. Bone Marrow Transplant. 2001;28(8):769-774. [DOI] [PubMed] [Google Scholar]
- 24.Zeisbrich M, Becker N, Benner A, et al. Transplant-associated thrombotic microangiopathy is an endothelial complication associated with refractoriness of acute GvHD. Bone Marrow Transplant. 2017;52(10):1399-1405. [DOI] [PubMed] [Google Scholar]
- 25.Bacigalupo A, Ballen K, Rizzo D, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15(12):1628-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jodele S, Zhang K, Zou F, et al. The genetic fingerprint of susceptibility for transplant-associated thrombotic microangiopathy. Blood. 2016;127(8):989-996. 2015-08-663435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grube M, Wolff D, Ahrens N, Herzberg PY, Herr W, Holler E. ABO blood group antigen mismatch has an impact on outcome after allogeneic peripheral blood stem cell transplantation. Clin Transplant. 2016;30(11):1457-1465. [DOI] [PubMed] [Google Scholar]
- 28.Uderzo C, Bonanomi S, Busca A, et al. Risk factors and severe outcome in thrombotic microangiopathy after allogeneic hematopoietic stem cell transplantation. Transplantation. 2006;82(5):638-644. [DOI] [PubMed] [Google Scholar]
- 29.Balassa K, Andrikovics H, Remenyi P, et al. The potential role of HLA-DRB1*11 in the development and outcome of haematopoietic stem cell transplantation-associated thrombotic microangiopathy. Bone Marrow Transplant. 2015;50(10):1321-1325. [DOI] [PubMed] [Google Scholar]
- 30.Nakamae H, Yamane T, Hasegawa T, et al. Risk factor analysis for thrombotic microangiopathy after reduced-intensity or myeloablative allogeneic hematopoietic stem cell transplantation. Am J Hematol. 2006;81(7):525-531. [DOI] [PubMed] [Google Scholar]
- 31.Willems E, Baron F, Seidel L, Frère P, Fillet G, Beguin Y. Comparison of thrombotic microangiopathy after allogeneic hematopoietic cell transplantation with high-dose or nonmyeloablative conditioning. Bone Marrow Transplant. 2010;45(4):689-693. [DOI] [PubMed] [Google Scholar]
- 32.Logan AC, Wang Z, Alimoghaddam K, et al. ; Center for International Blood and Marrow Transplantation . ABO mismatch is associated with increased nonrelapse mortality after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2015;21(4):746-754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Worel N, Greinix HT, Leitner G, et al. ABO-incompatible allogeneic hematopoietic stem cell transplantation following reduced-intensity conditioning: close association with transplant-associated microangiopathy. Transfus Apheresis Sci. 2007;36(3):297-304. [DOI] [PubMed] [Google Scholar]
- 34.Worel N, Kalhs P, Keil F, et al. ABO mismatch increases transplant-related morbidity and mortality in patients given nonmyeloablative allogeneic HPC transplantation. Transfusion. 2003;43(8):1153-1161. [DOI] [PubMed] [Google Scholar]
- 35.Seeber C, Hiller E, Holler E, Kolb HJ. Increased levels of tissue plasminogen activator (t-PA) and tissue plasminogen activator inhibitor (PAI) correlate with tumor necrosis factor alpha (TNF alpha)-release in patients suffering from microangiopathy following allogeneic bone marrow transplantation (BMT). Thromb Res. 1992;66(4):373-383. [DOI] [PubMed] [Google Scholar]
- 36.Tatekawa S, Kohno A, Ozeki K, et al. A novel diagnostic and prognostic biomarker panel for endothelial cell damage-related complications in allogeneic transplantation. Biol Blood Marrow Transplant. 2016;22(9):1573-1581. [DOI] [PubMed] [Google Scholar]
- 37.Gomez SA, Abrey-Recalde MJ, Panek CA, et al. The oxidative stress induced in vivo by Shiga toxin-2 contributes to the pathogenicity of haemolytic uraemic syndrome. Clin Exp Immunol. 2013;173(3):463-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rottenstreich A, Hochberg-Klein S, Rund D, Kalish Y. The role of N-acetylcysteine in the treatment of thrombotic thrombocytopenic purpura. J Thromb Thrombolysis. 2016;41(4):678-683. [DOI] [PubMed] [Google Scholar]
- 39.Tolar J, Orchard PJ, Bjoraker KJ, Ziegler RS, Shapiro EG, Charnas L. N-acetyl-L-cysteine improves outcome of advanced cerebral adrenoleukodystrophy. Bone Marrow Transplant. 2007;39(4):211-215. [DOI] [PubMed] [Google Scholar]
- 40.Harris WS. Expert opinion: omega-3 fatty acids and bleeding-cause for concern? Am J Cardiol. 2007;99(6A):44C-46C. [DOI] [PubMed] [Google Scholar]
- 41.Akintoye E, Sethi P, Harris WS, et al. Fish oil and perioperative bleeding. Circ Cardiovasc Qual Outcomes. 2018;11(11):e004584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bays HE. Safety considerations with omega-3 fatty acid therapy. Am J Cardiol. 2007;99(6A):35C-43C. [DOI] [PubMed] [Google Scholar]
- 43.Gavriilaki E, Sakellari I, Batsis I, et al. Transplant-associated thrombotic microangiopathy: Incidence, prognostic factors, morbidity, and mortality in allogeneic hematopoietic cell transplantation. Clin Transplant. 2018;32(9):e13371. [DOI] [PubMed] [Google Scholar]
- 44.Li A, Wu Q, Davis C, et al. Transplant-associated thrombotic microangiopathy is a multifactorial disease unresponsive to immunosuppressant withdrawal. Biol Blood Marrow Transplant. 2019;25(3):570-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Craver BM, Ramanathan G, Hoang S, et al. N-acetylcysteine inhibits thrombosis in a murine model of myeloproliferative neoplasm. Blood Adv. 2020;4(2):312-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klein K, Zanger UM. Pharmacogenomics of cytochrome P450 3A4: recent progress toward the “missing heritability” problem. Front Genet. 2013;4:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zanger UM, Klein K. Pharmacogenetics of cytochrome P450 2B6 (CYP2B6): advances on polymorphisms, mechanisms, and clinical relevance. Front Genet. 2013;4:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.