ABSTRACT
COVID-19, the disease caused by the novel severe acute respiratory syndrome-associated coronavirus 2 (SARS-CoV-2), was first detected in December 2019 and has since morphed into a global pandemic claiming over 2.4 million human lives and severely impacting global economy. The race for a safe and efficacious vaccine was thus initiated with government agencies as well as major pharmaceutical companies as frontrunners. An ideal vaccine would activate multiple arms of the adaptive immune system to generate cytotoxic T cell responses as well as neutralizing antibody responses, while avoiding pathological or deleterious immune responses that result in tissue damage or exacerbation of the disease. Developing an effective vaccine requires an inter-disciplinary effort involving virology, protein biology, biotechnology, immunology and pharmaceutical sciences. In this review, we provide a brief overview of the pathology and immune responses to SARS-CoV-2, which are fundamental to vaccine development. We then summarize the rationale for developing COVID-19 vaccines and provide novel insights into vaccine development from a pharmaceutical science perspective, such as selection of different antigens, adjuvants, delivery platforms and formulations. Finally, we review multiple clinical trial outcomes of novel vaccines in terms of safety and efficacy.
KEYWORDS: SARS-CoV-2, COVID-19, vaccine, clinical trial, formulation
Introduction
The first reported SARS-CoV-2 infected individuals were found in the Wuhan province of China in December of 2019. In a mere 3 months, on March 11,th 2020, the virus had infected 118,000 individuals in 114 countries and the disease caused by SARS-CoV-2 infection (COVID-19) was officially considered a pandemic (Cucinotta and Vanelli 2020; WHO 2020). Although other beta-coronavirus, such as MERS and SARS-CoV-1, have crossed the species barrier to cause outbreaks in the human population, these were quickly contained and caused relatively low numbers of infections/deaths. With high transmissibility, long incubation times, spread occurring in asymptomatic individuals, and relatively low death rate compared to previous zoonotic coronaviruses MERS and SARS, SARS-CoV-2 is a recipe for a global pandemic. As of February 15,th 2021, 108,484,802 (108 million) individuals have been infected and 2,394,323 (2.4 million) deaths have resulted worldwide from COVID-19. With such high case numbers 14 months after initial SARS-CoV-2 infection and a mortality rate approaching 2.3% globally, attempting to reach herd immunity would necessitate 180 million deaths worldwide. While improving therapeutics for the treatment of COVID-19, such as use antiviral agents, monoclonal antibodies (mAbs), and convalescent plasma, may reduce the mortality rate, a safe and effective vaccine is critical.
This review will primarily focus on clinical trial outcomes of SARS-CoV-2 vaccines and pharmaceutical aspects which are benefitting or hampering current approaches. We will describe the mechanism of SARS-CoV-2 infection, immunological determinants of protective immunity, and mechanistic differences in the approach being utilized by each vaccine candidate. With so much funding, companies and universities around the globe are racing to develop a vaccine. This is causing an unprecedented amount of literature on the subject being put out monthly. Reviews on COVID-19 vaccine development have recently been published therefore we will focus primarily on the most recent clinical and post-marketing data and provide novel insights into vaccine development from a pharmaceutical science point of view. A complete list of the status of the current COVID-19 vaccines is provided in Table 1.
Table 1.
Current vaccines in clinical development for COVID-19. The vaccines are categorized by platform technology (current as of 15th Feb, 2021)
| ID | Developer | Formulation Strategies | Current status | Note |
|---|---|---|---|---|
| Inactivated virus vaccines | ||||
| CoronaVac | Sinovac Biotech | Inactivated SARS-CoV-2 using β-propiolactone | Phase 3: NCT04456595 | EUA in China, U.A.E |
| BBIBP-CorV | Beijing Institute of Biological Products; Sinopharm | Inactivated SARS-CoV-2 (vero cell) | Phase 3:ChiCTR2000034780 NCT04560881 | EUA in China, UAE, Bharat |
| N.A | Wuhan Institute of Biological Products; Sinopharm | Inactivated SARS-CoV-2 using β-propiolactone | Phase 3: ChiCTR2000031809 | |
| N.A | Institute of Medical Biology; Chinese Academy of Medical Sciences | Inactivated SARS-CoV-2 vaccine | Phase 3: NCT04659239 | |
| Covaxin (BBV152) | Bharat Biotech; Indian Council of Medical Research (ICMR) | Inactivated SARS-CoV-2 vaccine with alum | Phase 3: NCT04641481 | EUA in India |
| ERUCOV-VAC | Erciyes University; Kocak Pharma | Inactivated SARS-CoV-2 | Phase 1: NCT04691947 | |
| VLA2001 | Valneva; Dynavax | Inactivated SARS-CoV-2 with ALUM plus CpG 1018 | Phase 1/2: NCT04671017 | |
| Live Attenuated Vaccine | ||||
| TMV-083 | Merck & Co.; Institute Pasteur; CEPI | measles-vector based vaccine candidate | Phase 1: NCT04497298 | |
| V591 | Institute Pasteur; University of Pittsburgh; Themis Bioscience; CEPI; ABL Europe; Merck | measles-vector based vaccine candidate | Phase 1: NCT04498247 | Discontinued due to poor efficacy |
| COH04S1 | City of Hope | Synthetic Modified Vaccinia Ankara (sMVA) vector expressing S and N proteins | Phase 1: NCT04639466 | |
| COVI-VAC | Codagenix; Serum Institute of India | Engineered Live-attenuated Virus | Phase 1: NCT04619628 | Intranasal Delivery |
| Protein subunit vaccines | ||||
| ZF2001 | Anhui Zhifei Longcom Biopharmaceutical | Adjuvanted recombinant RBD Dimer | Phase 3: NCT04646590 | |
| NVX-CoV2373 | Novavax | Full-length S-protein nanoparticle with Matrix-M adjuvant | Phase 3: NCT04611802 | Lower efficacy data in South Africa Seeking EUA in US |
| SCB-2019 | Clover Biopharmaceuticals; GSK; Dynavax | Recombinant SARS-CoV-2 Trimeric S Protein Subunit Vaccine using Trimer-Tag technology with AS03 adjuvant or CpG 1018 adjuvant plus Alum adjuvant | Phase 2/3: NCT04672395 | |
| Covax-19 | Vaxine Pty Ltd; Medytox | Recombinant S protein with Advax-CpG55.2 adjuvant (Advax-SM) | Phase 1: NCT04453852 | |
| MVC-COV1901 | Medigen Vaccine Biologics Corporation; NIAID; Dynavax | Prefusion form of the SARS-CoV-2 recombinant spike protein with CpG 1018 and ALUM | Phase 2: NCT04695652 | |
| Recombinant COVID-19 vaccine (Sf9 cells) | West China Hospital of Sichuan University | RBD | Phase 2: NCT04718467 | |
| FINLAY-FR-1 FINLAY-FR-1a (Soberana 1) FINLAY-FR-2 (Soberana 2) | BioCubaFarma; Institute Finlay de Vacunas (IFV); University of Havana | RBD-tetanus toxoid conjugate (Soberana 2) RBD dimer plus ALUM (Soberana 1) | Phase 2: RPCEC00000347 | |
| Abdala (CIGB 66) | Center for Genetic Engineering and Biotechnology of Cuba | RBD | Phase 2: RPCEC00000346 | |
| UB-612 | United Biomedical (COVAXX) | RBD peptide | Phase 2/3: NCT04683224 | |
| KBP-COVID-19 | Kentucky Bioprocessing, Inc | RBD Antigen inserted into tobacco plants for production followed by purification. Given with CpG adjuvant | Phase 1/2: NCT04473690 | |
| NANOCOVAX | Nanogen Biopharmaceutical | S protein (transmembrane domain deleted) with ALUM adjuvant | Phase 1/2: NCT04683484 | |
| COVAC-2 | University of Saskatchewan; National Research Council of Canada (NRC); Seppic; Vaccine Formulation Institute (VFI) | S1 domain with Sepivac SWE™ Adjuvant | Phase 1/2: NCT04702178 | |
| AKS-452 | Akston Biosciences | SARS-CoV-2-RBD-Fc fusion protein | Phase 1/2: NCT04681092 | |
| UQ-CSL v451 | University of Queensland; Seqiris; CSL; CEPI | S protein with MF59 adjuvant | Phase 1 | Did not progress to phase 2/3 due to interferen-ce with HIV tests |
| AdimrSC-2 f | Adimmune; JHL Biotech | RBD plus ALUM | Phase 1: NCT04522089 | |
| EpiVacCorona | Vector State Research Center of Virology and Biotechnology | SARS-CoV-2 Peptides | Phase 1/2: NCT04527575 | EUA in Russia |
| rVSV-∆G-spike | Israel Institute for Biological Research (IIBR) | Recombinant Vesicular Stomatitis Virus with S protein substitution for surface glycoprotein | Phase 1/2: NCT04608305 | |
| V590 | Merck | rVSV expressing S protein | Phase 1: NCT04569786 | Discontinu-ed due to poor efficacy |
| DNA Vaccines | ||||
| INO-4800 | Inovio Pharmaceuticals; International Vaccine Institute | DNA plasmid vaccine expressing >99.9 amino acid similarity with wild-type SARS-CoV-2 S protein sequence. | Phase 2/3: NCT04642638 | Intra Dermal administra-tion followed by electropor-ation (EP) using CELLECTRA® 2000 |
| AG0301-COVID19 | Osaka University; AnGes; Takara Bio | DNA plasmid vaccine (undisclosed target) | Phase2/3: NCT04655625 | |
| GLS-5310 | GeneOne Life Science | DNA plasmid vaccine | Phase 1/2: NCT04673149 | |
| GX-19 GX-19N | Genexine Consortium | DNA plasmid vaccine (S protein: GX-19) (N protein: GX-19N) | Phase 1/2: NCT04445389 Phase 1/2: NCT04715997 | Intramusc-ular via EP |
| ZyCoV-D | Zydus Cadila Healthcare | DNA plasmid vaccine | Phase 3: CTRI/2021/01/030416 | |
| CORVax12 | OncoSec Immunotherapies | DNA encoding stabilized S trimer co-administered with TAVO™ (plasmid IL-12) | Phase 1: NCT04627675 | EP for plasmid delivery |
| Covigenix VAX-001 | Aegis Life; Entos Pharmaceuticals | DNA plasmid vaccine | Phase 1/2: NCT04591184 | |
| bacTRL-Spike | Symvivo | Genetically modified probiotic bacteria from gut containing DNA plasmid encoded with SARS-CoV-2 spike protein | Phase 1: NCT04334980 | |
| RNA Vaccines | ||||
| mRNA-1273 | Moderna; NIAID | Ionizable Lipid nanoparticle encapsulated mRNA- S protein | Phase 3: NCT04470427 | EUA by FDA; multiple other countries |
| BNT162b2 BNT162b1 | BioNTech; Fosun Pharma; Pfizer | b2: Lipid nanoparticles with mRNA- prefusion stabilized S protein b1: lipid nanoparticles with mRNA-native trimeric RBD | Phase 3: NCT04368728 | b2: EUA by FDA; multiple other countries |
| ARCT-021 | Arcturus; Duke-NUS; Catalent | mRNA STARRTM Technology with LUNAR® RNA delivery technology | Phase 2: NCT04668339 | |
| LNP-nCoVsaRNA | Imperial College London | Needles free self-amplifying RNA SARS-CoV-2 lipid nanoparticle vaccine | Phase 1: ISRCTN17072692 | Abandoned: modifying RNA to address variants |
| CVnCoV | Curevac | mRNA based vaccines | Phase 3: NCT04652102 | |
| ARCoV | Suzhou Abogen Biosciences; Yunnan Walvax Biotechnology; Academy of Military Science | Ionizable lipid nanonparticles encapsulated mRNA vaccine targeting RBD of SARS-CoV-2 | Phase 2: ChiCTR2100041855 | |
| ChulaCov19 | Thailand’s Chulalongkorn University | mRNA based vaccines | Phase 1: NCT04566276 | |
| Non-replicating viral-vectored vaccines | ||||
| AZD1222 | University of Oxford; AstraZeneca | chimpanzee adenovirus vector encoded with SARS-CoV-2 S protein | Phase 3: NCT04516746 | EUA in UK and multiple other countries; Halted in South Africa |
| Ad5-nCoV | CanSino Biological Inc.; Beijing Institute of Biotechnology | Adenovirus type 5- encoded with SARS-CoV-2 S protein | Phase 3: NCT04526990 | EUA in China, Mexico |
| Sputnik V (Gam-COVID-Vac) | Gamaleya Research Institute | Ad5/Ad26 based vaccine encoding SARS CoV-2 S protein (liquid formulation) | Phase 3: NCT04656613 | EUA in Russia, multiple other countries |
| Gam-COVID-Vac Lyo | Ad5/Ad26 based vaccine encoding SARS CoV-2 S protein with lyophilization | Phase 2: NCT04437875 Completed | ||
| Sputnik-light | Ad26 encoding SARS-CoV-2 S protein | Phase 1/2: NCT04713488 | Single dose | |
| Ad26.COV2-S | Janssen Pharmaceutical Companies | Ad26 encoded with SARS-CoV-2 S protein | Phase 3: NCT04505722 | EUA in US; Lower efficacy in South Africa |
| Grad-Cov2 | ReiThera; LEUKOCARE; Univercells | Replication defective Gorilla Adenovirus encoding prefusion stabilized S protein. | Phase 1: NCT04528641 | |
| VXA-CoV2-1 | Vaxart | Oral Adenovirus type 5 based vaccine S protein and N protein | Phase 1: NCT04563702 | |
| hAd5-S-Fusion+N-ETSD | ImmunityBio | Second generation Adenovirus type 5- encoded with S and N proteins | Phase 1: NCT04591717 NCT04710303 | |
| NasoVax (AdCovid) | Altimmune | Ad5 Vector expressing RBD | Phase 2: NCT04442230 | |
| AdCLD-CoV19 | Cellid | Ad5/35 encoding S protein | Phase 1/2: NCT04666012 | |
| Virus like nanoparticle | ||||
| CoVLP | Mitsubishi Tanabe (Medicago); Laval University | Plant derived Coronavirus Virus like Particle with AS03 adjuvant | Phase 2/3: NCT04636697 | |
| RBD SARS-CoV-2 HBsAg VLP | SpyBiotech; Serum Institute of India | RBD conjugated to hepatitis B surface antigen on VLP | Phase 1/2: ACTRN12620000817943 | |
| Cellular Vaccines | ||||
| AV-COVID-19 | Aivita | Autologous dendritic cells loaded with antigens from SARS-CoV-2, with or without GM-CSF | Phase 1/2: NCT04386252 | |
| LV-SMENP | Shenzhen Geno-Immune Medical Institute | Injection of LV-SMENP-DC vaccine and infusion of antigen-specific CTLs | Phase 1/2: NCT04276896 | |
| Covid-19/aAPC | Shenzhen Geno-Immune Medical Institute | Pathogen-specific artificial APC | Phase 1: NCT04299724 | |
| Repurposed Vaccines | ||||
| BCG Vaccine | Multiple | Terberculosis Vaccine | Phase 3: NCT04328441 | |
| MMR vaccine | Multiple | Measles, Mumps, Rubella Vaccine | Phase 3: NCT04357028 | Suspended (Failure of Subject Recruitment) |
| VPM1002 | Vakzine Projekt Management; Serum Institute of India | Terberculosis Vaccine | Phase 3: NCT04387409 | |
| RUTI | Archivel Farma; Institute for Health Science Research Germans Trias i Pujol (IGTP) | Terberculosis Vaccine | Phase 3: NCT04453488 | |
Pathology of COVID-19 and immune response to SARS-CoV-2
COVID-19 is a respiratory disease caused by the coronavirus SARS-CoV-2, spread primarily through inhalation of respiratory droplets from infected individuals and contact with fomites (infectious surfaces) (Liu et al. 2020; Pastorino et al. 2020). Based on pathophysiology of the disease, the host immune response and associated symptoms, there are three consecutive phases of SARS-CoV-2 infection/COVID-19 with increasing severity (Mason 2020; Polak et al. 2020). The first phase, or asymptomatic phase, lasts for 1–2 days following exposure to the virus. During this early period, inhaled viruses bind to ciliated epithelial cells in the nasal cavity and start replicating. The detection of SARS-CoV-2 in tears and conjunctival specimen collected from infected individuals indicates the ocular route represents another route for its transmission (Xia et al. 2020; Zhang et al. 2020c). The viral structural spike (S) protein is critical for this early stage of infection, since attachment to target cells is dependent on its binding to angiotensin-converting enzyme 2 (ACE2) receptor (Hoffmann et al. 2020). The cleavage of ACE2 by type 2 transmembrane serine protease (TMPRSS2), also present in the host cell, contributes to viral entry (Hoffmann et al. 2020). Both ACE2 and TMPRSS2 have been found to be expressed on ocular tissue, with ACE3 expression reported to be high in the conjunctiva (Zhang et al. 2020a), explaining the ocular tropism of SARS-CoV-2. The S protein has two subunits S1 and S2, of which S1 harbours the receptor-binding domain (RBD), which attaches to ACE2 and triggers endocytosis of the virus. Upon exposure to proteases in the endosomal compartment, S1 is cleaved off and the S2 subunit, which bears a transmembrane domain, is released. Insertion of the S2 subunit within the host cell membrane facilitates membrane fusion and intracellular delivery of the viral package (Tay et al. 2020). The asymptomatic phase is characterized by a limited innate immune response, low viral loads, and local dissemination of the virus, which can be detected in nasal swabs as well as ocular tissues (Mason 2020; Zhang et al. 2020c).
During the second phase of infection, the virus propagates and starts disseminating to the upper airway. The virus infects multiple cells of the respiratory tract that express ACE2, such as bronchial and alveolar epithelial cells, and pneumocytes (Martines et al. 2020; Wiersinga et al. 2020). The epithelial-endothelial barrier integrity may be compromised, leading to infection of pulmonary endothelial capillary cells (Wiersinga et al. 2020). This phase coincides with clinical manifestation of COVID-19, and patients develop symptoms consistent with respiratory tract infections such as fever, dry cough and shortness of breath. The release of replicating virions by host cells results in their pyroptosis (Chen et al. 2019; Zhang et al. 2020b), a kind of programmed cell death associated with high inflammation that is commonly caused by intracellular pathogens such as cytopathic viruses, including SARS-CoV-2 (Fink and Cookson 2005). Heavier viral loads, together with pyroptosis of host cells, greatly accentuate the immune response. Pyroptosis is characterized by release of pathogen-associated molecular patterns (PAMP), including viral RNA, and damage-associated molecular patterns (DAMP), including ASC (apoptosis associated speck-like protein containing CARD) oligomers, nucleic acids and ATP. These are recognized by pattern recognition receptors (PRR) on macrophages, epithelial cells, and endothelial cells in the microenvironment, which respond by secreting pro-inflammatory cytokines and chemokines, such as CXCL10, IL-6, MCP1, MIP1α, and MIP1β (Tay et al. 2020). This inflammatory cytokine response recruits cells of both the innate (monocytes and macrophages) and adaptive branches of the immune system (T lymphocytes) to infected sites (Tian et al. 2020b; Xu et al. 2020). The pro-inflammatory milieu also skews the T lymphocyte response to a T helper 1 (TH1) response, which results in secretion of IFN-γ (Huang et al. 2005). Recruitment of T cells from circulation to the airways, impairment of lymphopoiesis brought about by the anti-viral inflammatory response, and possible direct killing of lymphocytes by the virus is thought to contribute to lymphopenia observed in about 80% of SARS-CoV-2 infected patients (Qin et al. 2020). In a majority of patients, the disease is mild being restricted to the upper airways, and infection is cleared by recruited immune cells and the ensuing protective immune response (Wu and McGoogan 2020). Most children infected with SARS-CoV-2 are asymptomatic and have reduced symptoms, representing a less severe form of the disease (Zimmermann and Curtis 2020). Lower levels of expression, differential tissue distribution and lower binding affinity of ACE2 receptors to SARS-CoV-2 are some of the numerous hypotheses that have been proposed to explain this phenomenon (Zimmermann and Curtis 2020)
Both T and B cell responses to SARS-CoV-2 ensue within approximately one week from onset of clinical symptoms (Thevarajan et al. 2020). Virus-specific CD8 + T cells that are recruited to the site of infection clear infected cells (identified by presentation of viral peptides in the context of MHC I) by their cytotoxic activity, limiting spread of the virus. CD4 + T cells secrete cytokines to drive immune cell recruitment, in addition to activating virus-specific B cells and driving their proliferation and differentiation into antibody-secreting plasma cells. Different classes of antibodies play important roles in the immune response to SARS-CoV-2 and have differential kinetics. Anti-SARS-CoV-2 IgM and IgA are detected in circulation first and peak early, followed by IgG as is typically seen during infections through the mucosal route. (Guo et al. 2020). IgM, usually the first class of antibody detected during a primary humoral response, is highly efficient at fixing complement, and therefore plays a pro-inflammatory role. IgG antibodies on the other hand are most effective at neutralization, a phenomenon where antibodies bind the virus and neutralize its ability to attach to host cells. While initial B cell responses are often directed towards the nucleocapsid (Tay et al. 2020), most neutralizing antibodies to coronaviruses bind the S protein, which is not surprising considering its role in host cell attachment of the virus (Temperton et al. 2005). Anti-S neutralizing antibodies can be detected in patients 2–3 weeks following onset (Nie et al. 2004). IgA also has neutralizing activity, and its abundance on mucosal surfaces of the respiratory tract represents a critical line of defence against SARS-CoV-2. This binding also targets the viruses for clearance (a process termed opsonization) and recruits phagocytes through the Fc region of the antibody. In a protective response, the opsonized virus particles are detected by alveolar macrophages and cleared by phagocytosis. An optimal immune response thus requires multiple branches of the immune system to act in unison in order to clear the virus while causing minimal lung damage, and thus lead to recovery. These patients are usually treated symptomatically (Mason 2020) and often do not require hospitalization.
Unfortunately, a dysfunctional immune response ensues in some cases, and about 20% of the patients (Mason 2020) progress to phase 3 of the disease, which is characterized by thickening of alveolar walls, endothelialitis, interstitial mononuclear inflammatory “ground glass” pulmonary infiltrates and pulmonary edema, leading to acute respiratory distress syndrome (ARDS) and often requiring respiratory support (Wiersinga et al. 2020). A pathogenic immune response to SARS-CoV-2 is associated with systemic cytokine storm involving excessive release of pro-inflammatory cytokines, such as IL-2, IL-6, IL-7, IL-10, G-CSF, CXCL10, MCP1, MIP1α and TNF-α (Tay et al. 2020). Such a cytokine storm has a multi-organ impact and can result in complications leading to circulatory failure, gastrointestinal dysfunction, and multi-organ dysfunction syndrome (MODS) with associated co-morbidities especially in older patients (Ruan et al. 2020), who are often hampered by a diminished immune response (Ho et al. 2001). Multiple factors across the innate and adaptive branches may contribute to a dysfunctional immune response, including increased T cell exhaustion (Zheng et al. 2020) and presence of non-neutralizing antibodies (Wang et al. 2016b). Pre-existing non-neutralizing antibodies can facilitate virus uptake and promote their entry into FcR expressing cells, resulting in antibody-dependent enhancement (ADE). ADE induced by non-neutralizing antibodies can also lead to lasting tissue and organ damage due to persistent inflammation (Arvin et al. 2020; Iwasaki and Yang 2020). Although neutralizing antibodies are largely thought to be protective in nature, some studies have shown a correlation between neutralizing antibodies and exacerbated inflammatory responses (Liu et al. 2019).
Vaccine design considerations
From the perspective of vaccine development, an ideal vaccine would generate an immune response that activates both branches of the adaptive immune system. It would require a robust T cell response, invoking cytotoxic CD8 + T cells that are capable of clearing infected cells to limit viral spread, as well as CD4 + T cells to prime B cells for a humoral response. An optimal antibody response would generate antibodies that neutralize viral entry into host cells by targeting the S protein (or more specifically the RBD), with little to no pro-inflammatory activity to minimize tissue damage and facilitate a rapid recovery. Figure 1 highlights components of novel vaccines that must be optimized prior to clinical trials to achieve these results.
Figure 1.
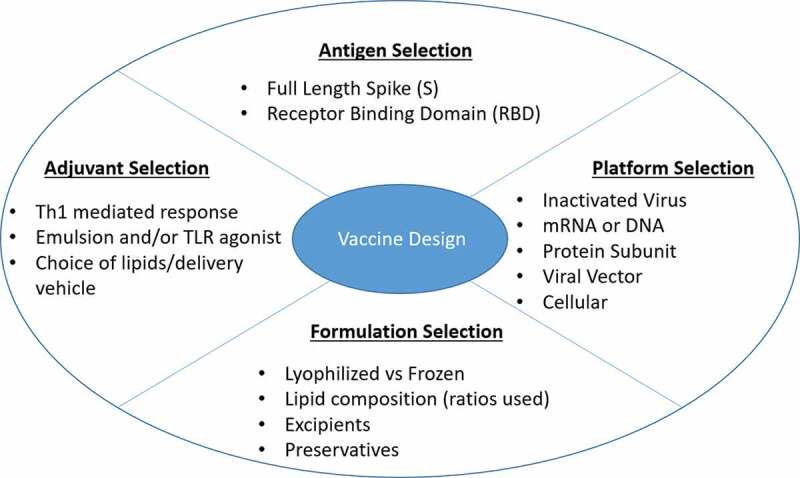
Critical aspects of novel vaccine design. The four most important considerations while developing a novel vaccine are shown. Each section lists bullet points of factors to be modified prior to initiating clinical trials
Platform selection
A platform is any broadly applicable technology or mechanism for the generation and/or delivery of vaccines. Currently a variety of vaccine platforms are in development around the world to fight COVID-19; inactivated virus, subunit, DNA, RNA, viral vector, and cellular vaccines are currently in clinical trials. Figure 2 highlights key vaccine candidates furthest along in clinical trials and those that have completed phase 3 trials as of February 2021. Each proposed platform may differ in delivery vehicle, antigen, adjuvant, delivery route, and ultimate immune response induced. Inactivated virus vaccines are dominant in current phase 3 trials with 3 different vaccines being developed in China and one in India (Kaabi, Limited 2020; Palacios 2020; Zhu et al. 2020). This is most likely due to past approval of inactivated vaccines for multiple viral pathogens, signifying a defined regulatory procedure for safety and scale up. Unlike inactivated vaccines, no mRNA vaccine has been approved for human use prior to development of mRNA vaccines for SARS-CoV-2 infection, yet there are 2 platform technologies that have been approved for Early Use Authorization (EUA) by the FDA in the United States (BioNtech 2020; ModernaTX 2020). Though these candidates had higher risk of investment (due to none being approved in the past), their utility may be far superior than inactivated or subunit vaccines. Apart from mechanistic differences, each platform technology requires a new set of scale-up procedures, regulations therein, and cold-chain requirements which may limit the number of vaccine doses produced and use in underdeveloped countries. Addressing the issue of scale-up, mRNA vaccines can be easily produced at large scale within weeks (Pardi et al. 2018). They also provide a framework to incorporate new antigens with ease making it an ideal platform to quickly produce a vaccine for viral variants and newly emerging viral outbreaks, COVID-19 being an example of this. With the widespread nature of this disease, it is essential to produce a vaccine that will be accessible to all who need it. Incorporating proteins into microneedle array (MNA) polymer matrices to form microneedle patches has been shown to improve formulation stability for Adenovirus and Influenza subunit vaccines, which demonstrate potent immunogenicity after 1 month or 1 year of storage at 25°C, respectively (Bachy et al. 2013; Mistilis et al. 2017). Use of the MNA patch is being attempted to avoid issues with cold-chain requirements for SARS-CoV-2. The patch incorporates antigen into mechanically strong water-soluble polymers to physically breach the outermost layer of skin (stratum corneum) and then rapidly dissolve in the underlying viable epidermis and dermis to deliver cargos to skin microenvironments (Balmert et al. 2020). The skin is an optimal vaccination target due to the presence of many antigen presenting cells (APCs) and ability to induce a robust humoral response (Kashem et al. 2017). This technology is still in the preclinical phase of development, and stability of SARS-CoV-2 spike protein in these patches remains under investigation (Kim et al. 2020). Another vaccine platform that has reached EUA in the UK, India, Argentina, Dominican Republic, El Salvador, Mexico and Morocco is a viral vector vaccine using a modified chimpanzee adenoviral vector encoding SARS-CoV-2 Spike protein (Folegatti et al. 2020b). A major concern for viral vector vaccines is pre-existing immunity toward the vector of choice, such as adenovirus type 5 (Ad5), which has been shown to limit vaccine immunogenicity in non-human primates and in Phase 1 trials for HIV (Casimiro et al. 2003; Catanzaro et al. 2006). This has inspired the use of chimpanzee-derived vector which has shown promise in phase 3 trials. Issues seen with inactivated virus vaccines such as scale-up and manufacturing capabilities may also impact viral vector vaccines in the future.
Figure 2.
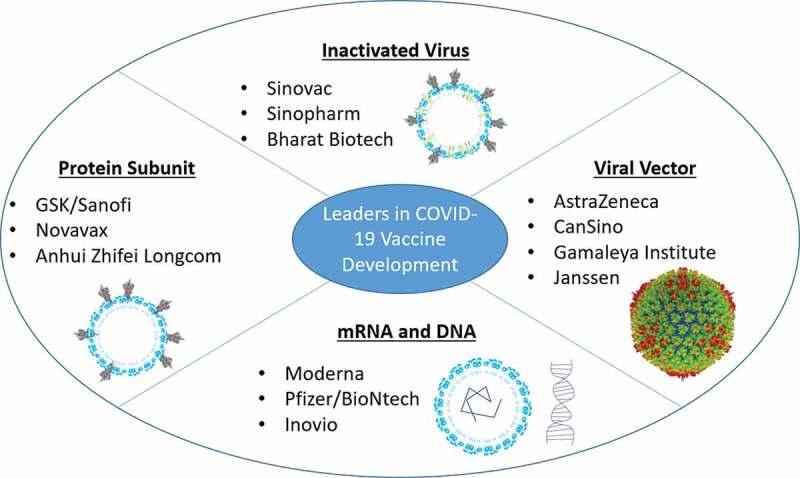
Leading platforms/companies in COVID-19 vaccine development. Bullet points highlight companies that are furthest along or have completed phase 3 clinical trials. Images are representative of each platform technology. Spike protein cryo-EM image was taken from (Wrapp et al. 2020) and adenoviral capsid cryo-EM image was taken from (Liu et al. 2010)
With the urgent need for a vaccine to be developed for COVID-19, the United States has opened funding for vaccine development at large scale through Operation Warp Speed. Operation Warp Speed has allocated 10 billion dollars in funding to help scale up manufacturing and prepare vaccine candidates for clinical trials with promising preclinical results. By mid-December 2020 they have provided a staggering 12.4B with 1.2B, 4.15, 1.6B, 1.95B, 2.0B, 1.456B given to AstraZeneca, Moderna, Novavax, Pfizer/BioNtech, and Sanofi/GSK, J&J, respectively (Barone 2020). Through this they have funded mRNA vaccines, subunit vaccines, and viral vector vaccines showing a balance of funding toward innovative technologies such as Moderna’s mRNA vaccine as well as established technologies like GSK’s subunit vaccine. GSK is currently enrolling for a Phase 2b clinical trial, and this subunit vaccine was developed from proprietary recombination techniques and adjuvant that has been used in past Influenza and H1N1 vaccines.(Ferguson et al. 2012; Liu 2020)
Antigen selection
The SARS-COV-2 virus has a single stranded RNA genome, surrounded by a nucleocapsid (Zhou et al. 2020). There are spike glycoproteins present on the surface, which are composed of homotrimers of S proteins. These glycoproteins consist of two subunits: S1 and S2; S refers to “Spike”. These S proteins are highly homologous and are valuable in determining virulence, tissue tropism, and the range of hosts it can infect(Chu et al. 2020). S proteins are class I viral fusion proteins; their main purpose is to bind to the receptor of the host cell (hACE2) and enable the virus entry into host. The S1 subunit shares approximately 64% of amino acid sequences with SARS-COV, suggesting that the immunogenic epitope is conserved (Ou et al. 2020; Walls et al. 2020). Once bound to the host ACE2 receptor, S protein is internalized and proteolytically cleaved by TMPRSS2 or endosomal cysteine proteases cathepsins B and L (CatB/L) (Hoffmann et al. 2020). This occurs between S1 and S2 at the S2ʹ site allowing for noncovalent linkage of the subunits. The use of TMPRSS2 inhibitors have been shown to prevent SARS-CoV-2 infection in-vitro (Hoffmann et al. 2020). The S2 subunit shares approximately 40% of amino acid identity with other SARS-COVs; it comprises a fusion peptide, transmembrane domain, and cytoplasmic domain. Once cleaved S2 follows irreversible conformational changes into a stabilized post-fusion state, allowing the fusion protein to interact with host cell membrane and ultimately release the viral genetic material into the cell (Fan et al. 2020).
Prior to binding to hACE2 on epithelial cells in the respiratory tract initiating an infection, SARS-CoV-2 circulates in air droplets with trimeric spike proteins protruding from the surface of the particle. Surface exposure and requirement for binding/uptake into cells makes S protein an ideal target for vaccine development. In theory, anti-Spike neutralizing antibodies would bind Spike protein on circulating virus particles, preventing interaction and uptake into cells, therefore increasing clearance of the virus. Also, it has been increasingly accepted that specific neutralizing antibodies are required to provide protection from infection rather than cause antibody-dependent enhancement of disease. ADE has been seen in SARS-CoV and MERS-CoV vaccine development and must be monitored in preclinical and clinical trials of vaccine candidates (Wang et al. 2014). It was shown that N and E protein-specific antibodies could exacerbate the disease and increase lung pathology (Wang et al. 2014). Directing antibodies toward the S protein could be a strategy to prevent ADE, although antibodies toward specific regions of the S protein may also induce ADE (Wang et al. 2016a). Vaccines currently in clinical trials are working to avoid this issue by utilizing S protein antigens chemically stabilized in their pre-fusion state by incorporating two proline residues (Corbett et al. 2020). In another approach, only small portions of the protein that are known to produce protective immunity are incorporated, such as the receptor-binding domain, while other regions that may cause ADE are eliminated (Mulligan et al. 2020). In other approaches, Foldon domains have been incorporated to form stable trimers of S or RBD antigens for multivalent display that would mimic protein conformation on the virus particle and increase immunogenicity (Mulligan et al. 2020). Apart from ADE, binding antibodies can lead to vaccine-associated enhanced respiratory disease (VAERD) through antibody complex dissemination and complement activation causing inflammation and obstruction of airways. In early vaccine trials of formalin inactivated respiratory syncytial virus (RSV) it was found that 80% of vaccinated individuals required hospitalization upon infection compared to 5% in the placebo group due to antibody complexes (Kim et al. 1969). Further assessment has shown Th2-skewed CD4 + T cell response, characterized in current trials by induction of cytokines IL-4, IL-5, and IL-13, were a likely factor that contributed to two children’s’ deaths in this trial (Graham 2020). Current trials are assessing CD4 T cells phenotypes to avoid Th2 responses and promote a Th1 mediated response seen during normal infection. Although ADE and VAERD theoretically are significant worries for vaccines being developed to SARS-CoV-2, no vaccines have shown evidence of this in clinical trials thus far.
One of the reasons for the ineffectiveness of vaccines is the mutability of the pathogens, which is a result of evolutionary pressure to evade immune responses. Multiple variant strains of SARS-CoV-2 are now being reported, the most prominent ones being B.1.1.7 (U.K.), B.1.351 (South Africa) and P.1 (Brazil) (Greaney et al. 2021; Vasques Nonaka et al. 2021; Weisblum et al. 2020), raising legitimate concerns about the cross-protective ability of the vaccines being developed. In the case of seasonal influenza vaccines, the majority of antibodies elicited recognize strain-specific hypermutating regions of the Hemagglutinin (HA), making yearly vaccinations with new formulations that have been incorporated with HA sequences from the most prevalent strains necessary. Choice of epitopes is absolutely critical for the success of vaccination strategies. Linear epitopes, defined as epitopes that consist of a sequence of amino acids are relatively easier to identify by conventional techniques. The majority of antibody responses generated in nature however, recognize conformational epitopes, which consist of discontinuous amino acid residues that come together as a result of protein folding to form a three-dimensional antibody-binding surface. The advent of immunoinformatics, reverse vaccinology and structural vaccinology has resulted in the identification of a wide variety of both linear and conformational epitopes as part of the new age rational vaccine design. Behmard and colleagues used an immunoinformatic approach to identify multiple T cell and B cell epitopes, including conformational epitopes (Behmard et al. 2020). A multi-epitope polypeptide was then constructed using 34 cytotoxic T lymphocyte epitopes and 12 helper T lymphocyte epitopes fused to each other using a linker. The same approach also yielded 7 conformational and 9 linear B lymphocyte epitopes. Docking studies revealed strong binding of the polypeptide to Toll-Like Receptor-3 (TLR-3), demonstrating promise (Behmard et al. 2020). Similar approaches have been reported by other groups (Naz et al. 2020; Sarkar et al. 2020).
In the context of protection from emerging variant strains it will be beneficial to induce both humoral and cellular immunity towards multiple sites on the S protein. As newly emerging variants have been shown to have over 10 genetic mutations from the wild-type virus causing significant structural changes, leading to increased affinity towards hACE2 and increased transmissibility (Kupferschmidt 2021; Tegally et al. 2020). Preliminary studies assessing neutralizing activity of mRNA vaccinated patient plasma towards emerging variants has shown 79% neutralizing ability towards the B.1.1.7 variant compared to wild-type, signifying a reduced but still prevalent neutralizing ability toward the variant (Muik et al. 2021; Wang et al. 2021).Targeting the RBD of the wild-type will likely have less protection from mutant strains than targeting the whole S protein. Using whole spike protein in vaccination approaches will allow for the development of polyclonal antibodies and cellular immunity to a wide array of S protein linear and conformational epitopes. Therefore, if mutations occur in a specific region of the S protein there will still be neutralizing antibodies and cellular immunity towards other regions of the protein.
Adjuvant selection
The choice of proper adjuvant for a vaccine is essential to produce a robust immune response. Adjuvants do not only propagate immunity toward antigen, but can also skew the adaptive immune response in a particular direction. An ideal adjuvant for vaccines against COVID-19 should confer a TH1 skewed adaptive immune response, generating high neutralizing ability and induce a potent long lasting CD8 + T cell response. Live attenuated, inactivated, and viral vector vaccines confer adjuvant activity alone and do not require additional adjuvant modalities due to the presence of immunogenic epitopes on the delivery vehicles themselves. Live attenuated and inactivated vaccines have been shown to induce immune response similar to their respective live pathogens alone (Zhu et al. 2020). Similarly, with viral vector technologies the delivery vehicle is a viral particle such as an adenovirus. Exposed on the Ad5 viral surface are immunogenic hexon, penton, and fiber proteins which activate the innate immune responses by binding pathogen recognition receptors (PRR) on the cell surface or after endocytosis in the endosome by TLR2 and 9, respectively (Appledorn et al. 2008). Also double stranded DNA (dsDNA) contained within the capsid may activate nucleotide oligomerization domain (NOD)-like receptors in the cytoplasm (Hendrickx et al. 2014). Activating innate immunity caused upregulation of pro-inflammatory cytokines TNFα, IL6, IL12, IFN-γ, IL1α, and IL1β (Hendrickx et al. 2014). Different adenoviruses have varying innate immunity induction and may therefore provide varying amounts of adjuvant effect (Barouch et al. 2004; Teigler et al. 2014). Although inducing a strong innate response while delivering the antigenic genetic material is desired to produce a robust adaptive immune response, it must be carefully monitored to prevent adverse effects to patients. High dose adenoviral gene therapies have caused cytokine storms and death in gene therapy trials due to over activation of innate immunity(Wilson 2009).
mRNA vaccines have utilized immunogenic liposomes as an adjuvant to boost immune response. Similar to adenoviral vectors, liposomes act as a delivery vehicle to obtain high antigen concentrations in target cells. Proper sizing of liposomes and modifications such as pegylation allow greater lymphatic uptake after SC injection and less nonspecific events (Gabizon et al. 2003; Mui et al. 2013; Zhuang et al. 2012). Antigen can then be processed by APCs to present peptide on MHC molecules in the presence of pro-inflammatory cytokines and costimulatory molecules to mount an adaptive immune response. The lipid composition may determine the cytokine milieu secreted by DCs and costimulatory molecules upregulated (Gaitonde et al. 2012; Nakanishi et al. 1999). Ionizable liposomes delivering mRNA achieve robust immune responses due to their ability to efficiently escape the endosome, therefore delivering their cargo to the cytoplasm (Hafez et al. 2001). The incorporation of an ionizable lipid that is positively charged at low pH also allows for high loading capabilities with the negatively charged mRNA, in addition to aiding in endosomal escape (Habrant et al. 2016; Love et al. 2010). Since mRNA vaccines produce antigenic protein within cell cytoplasm, the protein is typically processed and presented on MHC-1 molecules to induced a TH1 skewed adaptive response and robust CD8 T cell response (Sahin et al. 2014). However, addition of the chemical adjuvant alum has been shown to produce a balanced TH1/TH2 response for Moderna’s mRNA-1273 in mice (Corbett et al. 2020).
For subunit vaccines, preclinical studies have demonstrated that the antigen alone (soluble antigen) does not induce a substantial immune response compared to those with adjuvant. It must be packaged into a nanoparticle for efficient uptake into cells and proteolytic processing in order to mount a robust adaptive immune response. Classical adjuvants such as alum are under investigation, and although its mechanism is not fully understood, it is thought to create an emulsion retaining the antigen at the injection site allowing for increased antigen uptake by APCs and presentation to T cells (Brewer 2006). As mentioned for mRNA-1273, although ALUM can boost the immune response, it may tend to follow a Th2 phenotype therefore other adjuvants are being investigated (Corbett et al. 2020). Matrix-M1, a saponin-based adjuvant is being employed by Novavax for their full length, Sf9 derived, recombinant spike protein vaccine (Keech et al. 2020). Their vaccine has shown protection from upper or lower respiratory infection upon challenge in non-human primates via induction of neutralizing antibodies in a Th1 dependent manner (Tian et al. 2020a). Matrix-M adjuvant has been used in development of influenza vaccines with an acceptable safety profile in clinical trials (Cox et al. 2011). Clover Pharmaceuticals has partnered with GSK and Dynavax in order to utilize their proprietary adjuvants AS03 and CpG 1018. AS03, developed by GSK, is included in licensed H5N1 pre-pandemic and H1N1 pandemic influenza vaccines (Garcon et al. 2012). AS03, composed of α-Tocopherol and squalene in an oil-in-water emulsion, induced greater antibody levels as well as CD4 + T cell responses toward H1N1 in human subjects compared to vaccine without adjuvant (Roman et al. 2011).
CpG 1018, an adjuvant developed by Dynovax, is under investigation as an adjuvant for two subunit vaccine candidates currently in phase 2 clinical trials (by Clover Biopharmaceuticals and Medigen Vaccine Biologics) . CpG 1018 is currently used in a recombinant Hepatitis B vaccine HEPLISAV-B™ (Campbell 2017). Oligonucleotides containing unmethylated CpG sequences are known to activate the innate immune system by binding to TLR9 in the endosome (Hartmann and Krieg 2000; Krug et al. 2001). Clinical studies with HBsAg vaccination consistently demonstrate more rapid induction of protective antibody titers with CpG 1018 compared to alum in all populations studied, including groups that are harder to immunize such as the elderly and immunocompromised individuals (Campbell 2017). Clover Biopharmaceuticals are attempting a phase 1 trial utilizing CpG 1018 + Alum as a treatment group to further boost immunity toward the S protein. Another CpG adjuvant being investigated is CpG55.2, a component in Advax-SM adjuvant. Advax-SM is composed of the crystalized polysaccharide delta inulin and CpG. Delta Inulin has been shown to increase humoral and cellular responses to a wide variety of antigens, but unlike Alum or CpG, it does not require adsorption to antigen or triggering of inflammatory danger signals to boost immune response (Saade et al. 2013). It was proven to be safe and effective in human trials of influenza vaccines, hepatitis B vaccines, and insect sting allergy vaccines (Gordon et al. 2012; Petrovsky and Cooper 2015; Saade et al. 2013). More importantly, Advax-SM adjuvant system has demonstrated greater Th1-skewing properties when formulated with live RSV immunization (Eichinger et al. 2020) and ameliorated Th2-related airway eosinophilia in a model of immunization against SARS-associated coronavirus (Honda-Okubo et al. 2015; Petrovsky and Cooper 2015).
MF59 adjuvant is included in the subunit vaccine which made it to phase 3 clinical trials by the University of Queensland. Licensed for human use in 1997, MF59 was the first approved adjuvant since the use of alum salts dating back 70 years prior (Schultze et al. 2008). It is licensed as part of the influenza vaccine Fluad®, and extensive studies have demonstrated its safety and enhanced immune response in humans (Schultze et al. 2008). MF59 consists of an oil-in-water emulsion of squalene droplets stabilized by addition of 2 non-ionic surfactants (Tween 80 and Span 85) (Schultze et al. 2008). Although its mechanism of action has not been completely elucidated, it is shown to induce significant macrophage recruitment to the injection site and uptake into resident dendritic cells (Dupuis et al. 2001, 1998). Macrophage recruitment was significantly suppressed in mice deficient for CCR2 (Dupuis et al. 2001). Nevertheless, MF59 has shown to potentiate humoral and cellular immune response in preclinical and clinical studies. MF59 was the most potent immune activator compared to alum, calcium phosphate (CAP), poly-(lactide co-glycolide) (PLG) delivery system, and CpG in mouse models (Singh et al. 2006). In addition, when combined with CpG, MF59 induced a greater Th1 response represented by higher IgG2a titers and strongly enhanced IFN-γ response in splenocytes from immunized mice (Wack et al. 2008).
Formulation selection/considerations
With Moderna, BioNtech, and AstraZeneca vaccines now available for priority populations around the globe, data on adverse reactions to these vaccines continues to grow. Reactions such as lymphadenopathy and shoulder injury related to vaccine administration were witnessed in Phase 3 studies for BNT162b2, but occurrence of anaphylaxis was not observed. When given to the general population anaphylactic reactions are occurring and have prompted advisories by the CDC for the management of anaphylaxis when dosing. Providers of mRNA vaccines are required to monitor patients after vaccine administration and have treatments for immediate anaphylaxis reactions on hand. This has prompted inquiries on what is driving this anaphylaxis to provide insight for newly developing therapies as well as prevent occurrence when giving these products to the general population. While the active ingredient of BNT162b2 and mRNA-1273 therapies are mRNA encoding S protein, this is not likely the cause of anaphylaxis due to their rapid elimination by nucleases present throughout the body. Lipid nanoparticles have been implicated in the past as a cause of anaphylaxis. It has been shown complement can more efficiently bind to liposomes with increasing charge (Bradley et al. 1999). Though this is possible the more likely culprit of these reactions is incorporation of PEG moiety. BNT162b2, mRNA1273, and AZD122 incorporate 2-[(polyethylene glycol)-2000]-N,Nditetradecylacetamide (ALC-0159) and 1,2-dimyristoyl-racglycero3- methoxypolyethylene glycol-2000 (PEG2000- DMG), and Polysorbate 80 respectively. PEG moieties are present in many everyday products and used in liposomal formulations to prevent nonspecific events and opsonization. Pre-existing immunity to PEG has been shown to induce immediate hypersensitivity reactions dependent on molecular weight (MW) and cross react with Polysorbate 80 (increasing MW results in increased antibody binding) (Stone et al. 2019). It is not clear whether PEG in these formulations plays a role in anaphylactic reactions, but the incident rate is extremely low following vaccination and is effectively managed with common medications.
A wide range of stability is observed in the currently approved vaccine products. BNT162b2 requires cold chain of −80°C to −60°C while mRNA1273 can be supplied at −25°C to −15°C. mRNA-1273 can be stored for 30 days at 2–8°C prior to first use and 6 hours once the first dose has been drawn. Similarly, BNT162b2 can be stored for 6 hours once diluted. Both products come frozen in multi-dose vials. The formulations of these products are quite similar in that they’re composed of mRNA, lipids, salts, and sugar. Salts are included to provides adequate isotonicity and pH of formulations while sugar is added as a stabilizer during freezing. Both products are labelled as preservative free to avoid public avoidance of vaccine administration. Major differences of product stability mainly come from the lipid components of the formulation. While both contain 1,2-Distearoyl-sn-glycero-3- phosphocholine (DSPC) as a backbone phospholipid allowing the formation of liposomes, and cholesterol to stabilize the liposomal bilayer, they differ in ionizable lipids and PEG moiety incorporated. The ratio of lipids in the formulations has not been released but is known to have effects on the stability and phase properties of liposomal formulations. Adjuvant activity of liposomal formulations depends on lipid composition in drug formulations. Therapeutics such as doxil and paclitaxel formulation do not contain adjuvant lipids. Cholesterol to lipids ratios have been shown to have significant effects on particle stability and release kinetics (Briuglia et al. 2015). PEG moieties differ between products but PEGylation of liposomes has been shown to cause small differences in liposomal stability in the case of paclitaxel(Yang et al. 2007). Pfizer vaccine formulation was recently approved by the FDA to be transported and stored at conventional temperatures commonly found in pharmaceutical freezers for a period of up to two weeks which will alleviate many logistical issues presently seen with vaccine rollout.
Clinical trial outcomes of novel COVID-19 vaccines
Viral vector vaccines
Viral vector vaccines are attractive vaccine candidates owing to their delivery capacity to many human tissues (tropism) as well as natural adjuvant activity of the vector itself. Viral vectors are modified by deleting regions of the viral cDNA making them replication deficient, while incorporating the gene of interest to produce antigen in vivo (Danthinne and Imperiale 2000). Once a stable non-replicating viral vector has been produced, it is easy to modify to accommodate new antigens via incorporation of novel antigenic genes. This minor change requires less changes to upstream manufacturing processes (isolation of adenovirus from cellular components in culture) than other vaccine technologies such as subunit vaccines. For these reasons AZD1222, Ad5-nCoV, and Ad26.CoV2-S by AstraZeneca, CanSino, and Janssen, respectively, have been some of the first vaccines into clinical trials and currently in Phase 3 clinical trials with AstraZeneca being the first of its class to receive EUA in the UK on Dec 30th, 2020.
AstraZeneca and CanSino were the first viral vector vaccines to release their phase 1/2 clinical results on July 20, 2020 in Lancet (Folegatti et al. 2020b; Zhu et al. 2020). AstraZeneca repurposed a modified Chimpanzee derived viral vector previously used in vaccine development for MERS. They had previously shown protection from MERS-CoV-mediated disease in non-human primates as well as safety across all dose levels in phase 1 clinical trials (Folegatti et al. 2020a; Van Doremalen et al. 2020). CanSino also repurposed a previously developed vaccine, using an Ad5 based vaccine for Ebola (Zhu et al. 2015). This vaccine was approved for Emergency Use and stockpiling in China and is currently the only Ad5 vaccine to pass regulatory approval (Li et al. 2018). This allowed CanSino to rapidly apply their technology to SARS-CoV-2 and be the first candidate to enter clinical trials on March 16, 2020. Meanwhile Janssen Pharmaceuticals is following a similar approach using Ad26 expressing a stabilized full length S protein (Mercado et al. 2020). Janssen is making use of its AdVac platform previously used for Janssen’s European Commission-approved Ebola vaccine and to construct its HIV, RSV and Zika vaccine candidates (Baden et al. 2013; Cox et al. 2018; Williams et al. 2020). This Ad26 vector had been previously used to express a modified HIV gp140 protein in non-human primates and humans (Baden et al. 2013). Although lagging behind AstraZeneca and CanSino, preclinical results were published on July 30, 2020 and Phase 3 clinical trials are underway. A complete list of current COVID-19 vaccines that have been granted EUA is listed in Table 2.
Table 2.
Vaccines which obtained or applied for EUA for COVID-19. (current as of 15th Feb, 2021)
| ID | Developer | Formulation Strategies | Countries with EUA | Platform |
|---|---|---|---|---|
| CoronaVac | Sinovac Biotech | Inactivated SARS-CoV-2 using β-propiolactone | China, U.A.E | Inactivated Virus |
| BBIBP-CorV | Beijing Institute of Biological Products; Sinopharm | Inactivated SARS-CoV-2 (vero cell) | China, U.A.E, Bharat | Inactivated Virus |
| Covaxin (BBV152) | Bharat Biotech; Indian Council of Medical Research (ICMR) | Inactivated SARS-CoV-2 vaccine with alum | India | Inactivated Virus |
| NVX-CoV2373 | Novavax | Full-length S-protein nanoparticle with Matrix-M adjuvant | Seeking EUA from FDA | Protein Subunit |
| EpiVacCorona | Vector State Research Center of Virology and Biotechnology | SARS-CoV-2 Peptides | Russia | Peptide |
| mRNA-1273 | Moderna; NIAID | Ionizable Lipid nanoparticle encapsulated mRNA- S protein | US; multiple other countries | mRNA |
| BNT162b2 | BioNTech; Fosun Pharma; Pfizer | b2: Lipid nanoparticles with mRNA- prefusion stabilized S protein | US; multiple other countries | mRNA |
| AZD1222 | University of Oxford; AstraZeneca | chimpanzee adenovirus vector encoded with SARS-CoV-2 S protein | UK; multiple other countries; Halted in South Africa | Viral Vector |
| Ad5-nCoV | CanSino Biological Inc.; Beijing Institute of Biotechnology | Adenovirus type 5- encoded with SARS-CoV-2 S protein | China, Mexico | Viral Vector |
| Sputnik V (Gam-COVID-Vac) | Gamaleya Research Institute | Ad5/Ad26 based vaccine encoding SARS CoV-2 S protein (liquid formulation) | Russia, multiple other countries | Viral Vector |
| Ad26.COV2-S | Janssen Pharmaceutical Companies | Ad26 encoded with SARS-CoV-2 S protein | US, seeking approval in EU and Canada | Viral Vector |
When assessing current clinical findings on viral vector vaccines we can see anticipated issues arise. In particular, Ad5 based vaccines show dampened and delayed adaptive immune response toward SARS-CoV-2 in individuals with pre-existing immunity toward Ad5. Patients with low pre-existing Ad5 immunity had nearly 2-fold higher neutralizing antibody titres toward SARS-CoV-2, although cellular immunity, which was observed as IFN-γ secreting PBMCs (in response to SARS-CoV-2), was not altered in 88–90% of vaccine treated patients (Zhu et al. 2020). Pre-existing Ad5 immunity as well as age affected overall immunity and tolerability of the vaccine. Increase in tolerability (seen as a decrease in adverse reactions) may offset the issue of decreased immune response if patients can receive a higher vector dose. Although robust antibody responses were seen after a single injection of Ad5-nCoV in an RBD-specific ELISA, with seroconversion rates at 96% and 97%, respectively, at day 28. seroconversion of neutralizing antibodies was only reported in 59% and 47% of high and low-dose groups (Zhu et al. 2020). A major drawback of this study was failure to compare results with convalescent plasma samples from patients that have recovered from SARS-CoV-2 infection as a positive control. It is difficult to quantitatively compare vaccine products produced by different companies due to changes in antigen for ELISA development and types of neutralization assay employed. The assay of convalescent plasma provides a metric for clinical utility as these samples can be gathered from around the world and are representative of response toward natural infection which vaccines are attempting to mimic. They did note, however, that both anti-RBD titers measured by ELISA and neutralizing antibody titers to pseudovirus significantly correlated with neutralizing antibody titers to live virus with a correlation coefficient of 0.75 and 0.72 (p < .0001) respectively. Thus, this metric could potentially be used to assess clinical utility of vaccines in the future. A Phase III trial for Ad5-nCoV using a single 5 × 1010 viral particles dose (low dose) is currently ongoing in Russia, Saudi Arabia, Pakistan, Argentina, and Mexico.
AstraZeneca’s AZD1222 is avoiding the issue of limited efficacy from pre-existing anti-viral vector immunity by incorporating a full-length S protein gene into replication-deficient simian adenovirus vector ChAdOx1. Since this is a simian virus, there is low transmissibility in humans, therefore prevalence of inhibitory titers is much lower than that of Ad5 (Dicks et al. 2012). This allows for greater uptake of the viral particle into cells resulting in enhanced antigen production in vivo and a more robust immune response. Only one individual in this study had pre-existing high titers toward the vector, and 18% of subjects had low titers. To combat decreased adjuvant effect provided by a simian virus particle compared to human, AZD1222 also incorporates a tissue plasminogen activator (tPA) sequence and optimized S protein sequencing to boost antigen production in vivo. tPA has been used in the past to boost expression and secretion of modified vaccinia virus Ankara (MVA)-based vaccine for the prevention of TB (Kou et al. 2017). Following a single intramuscular dose of AZD1222, S-specific T-cell responses peaked on day 14 which were observed in nearly all patients (Folegatti et al. 2020b). Anti-S IgG responses rose by day 28 and neutralizing titers were found in 91% of individuals using MNA80 assay (Folegatti et al. 2020b). In a smaller 10-person cohort, a subsequent booster immunization was given. This group showed increased IgG response as well as neutralization activity (100% via MNA80) similar to levels in convalescent plasma (Folegatti et al. 2020b). Of note, both single and boost administration showed neutralization activity in 100% of individuals using the PRNT50 assay. Interestingly, the booster immunization did not affect T-cell responses. Deciding whether or not to move forward with a single immunization or a prime-boost regimen depended on which outcome is correlated most with protective immunity. For example, if neutralizing titers are the best predictor of vaccine efficacy, then prime-boost regimens would be desired due to the increase in titers described above. If cellular immunity is the primary driver of protective immunity, then a single immunization would be preferred. This highlights the issues with working with a novel virus. Although high inhibitory titers prevented infection from SARS-CoV-2 challenge in non-human primates, there is increasing evidence that cellular immunity also plays a major role in protection (Ni et al. 2020a, 2020b). Within convalescent plasma samples in this study, there is one asymptomatic individual with low neutralization, while 3 individuals with severe disease had high neutralization activity, thus neutralization activity alone may not necessarily confer protection. They decided to use a two dose regimen for further evaluation.
AstraZeneca more recently published a Phase 2/3 study conducted in the UK on November 18th, 2020 to expand their vaccine candidate to older population (Ramasamy et al. 2021). They stratified groups based on age into 18–55 years, 56–69 years, and 70 years and older similar to Moderna and Pfizer trials (see below) in order to expand utility of their vaccine candidates. Reactogenicity decreased as age increased. Systemic reactions were present in 86% of the younger cohort and reduced to 65% in patients older than 70; fatigue, headache, feverishness, and myalgia were the most commonly solicited systemic adverse reactions. Of note, 13 serious adverse events occurred during the study period, none of which were considered to be related to either study vaccine. Binding IgG titers and neutralizing titers were similar across all age groups who received two doses. A slight reduction in binding antibody titers was observed when given one dose and a more substantial reduction in inhibitory titers was observed when given a single dose by day 53 post vaccination. This study confirmed that AZD-1222 is well tolerated in older populations, therefore older individuals could be included in phase 3 studies. On November 23rd, 2020 AstraZeneca announced that their Phase 3 trial had met the primary efficacy endpoint in preventing COVID-19 (Voysey et al. 2021b). Two dosing regimens were assessed in this trial, one being a half dose for priming the immune system followed by a full dose to boost and the other being two full doses. Surprisingly, the half-dose regimen resulted in 90% protection from SARS-CoV-2 infection 2 weeks after boost immunization while the full-dose regimen resulted in 62% efficacy. An explanation for the difference in protection between the dosing regimens has not been identified, and it will be interesting to see if these values hold true until study completion. One explanation could be lower induction of anti-ChAdOx1 antibodies upon receiving a half dose therefore allowing greater delivery of the subsequent full dose, but this hypothesis would require additional anti-ChAdOx1 titer data to verify. No hospitalizations or severe COVID-19 cases were reported in the vaccinated group, and from 21 days after the first dose, there were ten cases hospitalized for COVID-19, all in the control arm; two were classified as severe COVID-19, including one death. These studies prompted EUA of AZD1222 in the UK on Dec 30th, 2020 followed by EUA by India as well as Argentina, Dominican Republic, El Salvador, Mexico, and Morocco for the active immunization of adults on Jan 6th, 2021. More recently AZD1222 has been approved for early use in Canada on Feb 26th, 2021.
Since being approved for early use, AZD1222 has realized the issue of conferring protection against emerging variants. On Febuary 7th, the South African health minister announced that it would be halting the use of AZD1222 (University 2021). This is due to preliminary efficacy findings that prevention of mild disease occurred in only about 10% of individuals receiving the vaccine in South Africa (University, 2021). This is likely due to the novel South African SARS-CoV-2 variant responsible for 90% of current cases in South Africa. While these are troubling results, this study has a small sample size and only accounted for 40 COVID-19 cases (University, 2021). Prevention of moderate to severe COVID-19 has not been assessed in this population and is expected to have greater efficacy than prevention of mild disease as seen in other vaccine trials. To further boost their vaccine efficacy Astrazeneca conducted a study assessing the timing of a booster dose which is currently in pre-print. They found that giving a prime-boost regimen 12 weeks apart increased vaccine efficacy from 54.9% to 82.4% compared to the previously established <6 week interval (Voysey 2021a). This may prove beneficial for combating variants, but compared to efficacy of Janssen’s vaccine in the South African population (54% prevention of moderate to severe disease, discussed below) efficacy of about 10% in prevention of mild disease will likely make this vaccine a secondary option for vaccination of South Africans.
Janssen Pharmaceuticals has also joined the race for creating a vaccine utilizing a non-replicating Ad26 viral vector. Ad26 is a human adenovirus with less seroprevalence than other vectors such as Ad5, thus less of the population would have pre-existing antibodies towards this vector allowing improved delivery to target cells. This is a similar approach to AstraZeneca using a chimpanzee adenoviral vector to bypass the issue of pre-existing immunity. Janssen released interim results of a Phase 1/2a study assessing safety and efficacy of the vaccine in healthy adults aged 18–55 and elderly aged 65 and older (Sadoff et al. 2021). 5 × 1010 or 1 × 1011 viral particles per vaccination were administered as either a single dose or with boosting 56 days after primary vaccination. Interim data for this vaccine candidate only cover safety and immunogenicity up to 29 days following primary vaccination. Thus far, the elderly population have reduced reactogenicity to the vaccine demonstrated by lower prevalence of local and systemic adverse reactions. Local reactions were reduced from 58% to 27% and systemic reactions reduced from 64% to 36% in the elderly compared to younger individuals, respectively. Fever was also lower in the elderly with no elderly individuals having grade 3 fever. Immunogenicity profiles of the different dose levels gave interesting results. At both dose levels one dose showed neutralizing seroconversion rates of 92% in younger individuals, but in elderly individuals the lower dose caused greater seroconversion rates (100%) than the high dose (83%). Furthermore, geometric mean titer (GMT) of neutralizing antibodies was increased with increasing dose in younger individuals (214 to 243) while increasing dose resulted in a slight decrease in GMT (196 to 127) in the elderly. This same trend was more pronounced for binding IgG titers; younger individuals had increased GMT from 528 to 695 with increasing dose while the elderly had reduced GMT from 507 to 248 showing over a 2-fold reduction. In all participants, binding IgG and neutralizing antibody levels induced by both dose levels were comparable to convalescent plasma, although they are toward the lower end of titer distributions in convalescent plasma. Other vaccine candidates have shown similar or greater mean titer values compared to convalescent plasma which may limit this vaccine’s comparable efficacy. However, compared to those candidates requiring two vaccinations, the single-dose regimen for Janssen’s vaccine would be preferable if titer levels are sufficient to confer protection. Ad26.CoV2.S also elicits a Th1 skewed cellular response that was observed in 80% and 83% of young and elderly individuals, respectively. This is a weaker cellular response compared to other vaccine candidates, but like other vaccine candidates little to no Th2 immune response was observed. CD8 T cell responses were also induced in 51% and 64% of young individuals receiving low or high dose, respectively, and only 33% of elderly showed CD8 T cell responses in either dose group. Due to the lower titers in elderly individuals given the high dose, the low dose with a single-dose regimen was chosen to move forward to Phase 3 trials.
On January 29th, 2021 Janssen announced that primary and secondary endpoints have been reached for their phase 3 ENSEMBLE trial (J&J 2021). The vaccine was found to be 66% effective in preventing moderate to severe COVID-19 28 days after vaccination. Efficacy in preventing moderate to severe disease varied by geographic region with 72% in the US, 66% in Latin America, and 57% in South Africa. The vaccine had substantial effect in reducing severe disease with an overall efficacy of 85% from all regions studied, and no patients that received the vaccine required hospitalizations 28 days following vaccination. Data on the prevention of acquiring SARS-CoV-2 infection post vaccination has not been released, although considering the drop in efficacy from preventing severe disease compared to already approved mRNA products (100% and 90% for Moderna and Pfizer, respectively), it is expected that this vaccine will have a similar drop in efficacy for infection rates as well. Although these efficacy values are reduced compared to other approved products, it’s important to note that approximately 95% of COVID-19 infections in South Africa were caused by the B.1.351 escape variant. This variant did not emerge until after Moderna or Pfizer’s Phase 3 trials were concluded, therefore the reduced efficacy in the South African arm of this trial is likely due to the presence of the variant, and it may not be appropriate to compare this efficacy with outcomes seen in previous trials. From this data Janssen has submitted a request for EUA to the FDA that was reviewed on February 26th, 2021. Considering low rates of adverse events (9% fever and 0.2% grade 3 fever), lower severe adverse reactions than placebo group, and no signs of anaphylaxis observed, combined with a current shortage of vaccine’s on market, this vaccine was likely to be approved. The vaccine was approved for EUA by the FDA on February 27th, 2021 making it the first COVID-19 vaccine to be given with a single dose which will alleviate many logistical issues with vaccine distribution. Janssen has also primitively started recruiting for another phase 3 trial (ENSEMBLE 2: NCT04614948) which will assess the effectiveness of a 2 dose regimen given 2 months apart.
Sputnik V produced by the Gamaleya institute is the most recent competitor of adenoviral based vaccines to publish Phase 1/2 clinical findings on September 4th, 2020 (Logunov et al. 2020). Their approach utilized a heterologous prime-boost dosing of Ad26 and Ad5 viral vectors encoding full length S protein in frozen or lyophilized formulations. Acceptable systemic and local reactions were observed with the most common being pain at injection site (58%), hyperthermia (50%), headache (42%), asthenia (28%), and muscle and joint pain (24%) (Logunov et al. 2020). Adverse reactions reported were only mild to moderate with no severe reactions reported. RBD specific IgG titers were induced using single administration of either Ad5 or Ad26 vectors to levels comparable to convalescent plasma patients, and upon heterologous prime-boost, RBD specific IgG rose to nearly 10-fold that of convalescent plasma patients (Logunov et al. 2020). Only the prime-boost regimen induced neutralizing antibodies in 100% of individuals with similar values to that of convalescent plasma patients, furthermore the prime-boost group was unique in that CD4 + and CD8 + T cell proliferation were observed in all subjects (Logunov et al. 2020). Flow cytometry analysis of T cells following ex vivo stimulation with Spike protein was used to evaluate proliferation in response to antigen. This is not as reliable of a method as others using antigen-specific ELISpot assays to evaluate cellular responses. Another method used was fold increase in IFN-γ secretion by PBMCs which, again, would be improved by the use of an antigen-specific ELISpot Assay. IFN-γ response in circulation was observed at day 28 in individuals receiving Ad5 or Ad26 alone or in combination (Logunov et al. 2020). This study also explored the effect of pre-existing titers toward Ad5 or Ad26 on vaccine efficacy and cross neutralization capabilities of each. Contradictory to results for CanSino’s Ad5 based vaccine, pre-existing immunity to the vector did not affect vaccine efficacy here. Furthermore, they state “administration of rAd26 did not increase the titer of neutralizing antibodies to rAd5 on day 28, and vice versa, which indicates the absence of cross-reactivity with respect to vaccine components.” This is in some agreement with the literature where mice primed with Ad26 had boosted immune response when given Ad5 (Abbink et al. 2007), but will need to be validated in an ongoing larger scale phase 3 clinical trial. It is important to note that neutralizing titers toward the viral vectors were only assessed at day 0 and day 28. For the prime-boost group this means the measurement is taken 28 days after receiving rAd26 and 7 days after rAd5. 7 days is not sufficient time to assess the neutralizing antibody response induced by the boost administration. Typically, it takes 2–3 weeks to generate germinal centers and produce IgG antibodies with neutralizing ability, therefore another time point at 42 days (as was done for anti-SARS-CoV-2 neutralization) would give a better estimate of the cross reactivity of Ad5 to Ad26. Nevertheless, these results are promising for the use of heterologous prime-boost immunizations in vaccine development. Sputnik V is currently approved for use in many countries around the world with the most current being Hungary, the first from the EU to take part. In a press release provided by Gamaleya National Research Center on Dec 14th, 2020 interim results for the phase 3 trial showed 91.4% efficacy from infection with 78 individuals developing COVID-19 in the trial thus far. This was taken 21 days after a single dose and is expected to improve upon boost administration. The vaccine provided 100% protection from severe COVID-19 with 20 individuals recorded all in the placebo group. Though these infection numbers are lower than that of Pfizer and Moderna’s phase 3 trials (described below), this is very promising and has warranted AstraZeneca to partner with Gamaleya Institute in hopes to improve their vaccine’s efficacy. This new trial will consist of one dose ChAdOx1 vector and one dose of either Ad5 or Ad26 vector encoding S protein (NatureBiotechnology 2021).
Merck was developing two replication-competent viral vectors as vaccine candidates for SARS-CoV-2. The use of replication-competent vectors is thought to provide longer lasting production of antigen within cells, providing greater immune stimulation, allowing for a single dose to be given. On January 25th, 2021 they announced that while their vaccines were well tolerated in healthy adults, immune responses were below that of convalescent plasma and therefore greatly reduced compared to other vaccines already authorized for emergency use (Merck 2021). Due to this, Merck decided to drop both candidates and focus their efforts on developing 2 therapeutics for COVID-19 that are currently in clinical trials. Considering they dropped both vaccines with varying constructs, this could represent an overall limitation of replication-competent vectors as vaccine platforms. It will be interesting to see if results from an ongoing Phase 2 trial implemented by the Israeli Institute of Biological Research (IIBR) will support or refute the findings in Merck’s clinical trials. Similar with Merck’s construct, IIBR utilizes Vesicular stomatitis virus (VSV) as a delivery vehicle for their vaccine. They have replaced the original glycoprotein on the surface of VSV with a S protein (Yahalom-Ronen et al. 2020). Therefore, the particle has S-protein on the surface as well as genetic material to produce surface exposed S protein particles within target cells.
mRNA vaccines
Although there was no mRNA vaccine approved on market prior to COVID-19, this approach offers many advantages over other vaccine platforms in terms of production, versatility, and immune response induced. Since mRNA is simple genetic material it can be optimized to produce high yields via in-vitro transcription reactions. This process is relatively cheap compared to viral vector production and is easily scalable due to the absence of cell cultures and purification steps. mRNA constructs can be adapted to novel antigens once the genetic information of that antigen is available, providing a rapid method for vaccine candidate selection. For instance, within 5 days of sequence release, Current Good Manufacturing Practice (CGMP) production of mRNA/LNP expressing the SARS-CoV-2 S-2P was initiated in parallel with preclinical evaluation by Moderna (Corbett et al. 2020). An issue with mRNA vaccines is the stability of the construct. mRNA is required to pass the hydrophobic cell membrane and reach the cytoplasm for efficient transcription of genetic material. RNase and endonucleases are present in cells and throughout the body to regulate natural mRNA production and will readily degrade the genetic material before transcription can occur, therefore protection from these degradation pathways prior to reaching the cytoplasm is essential. mRNA vaccines currently in clinical trials are packaged into lipidic nanoparticles to achieve this. An ideal vehicle will deliver the mRNA construct into the cytoplasm of the cell, to allow translation of antigen, while also exerting adjuvant activity to induce a robust immune response. Modifications to the mRNA itself, such as addition of synthetic cap, regulatory elements, or nucleoside modifications can protect from degradation within the cell and help boost protein production (Andries et al. 2015; Gallie 1991; Holtkamp et al. 2006). A major advantage of mRNA vaccines over other platforms is the immunogenicity induced. Antigen is produced in the cytoplasm of the cells and therefore is processed as an intracellular pathogen and loaded onto MHC class 1 molecules for T cell recognition (Chahal et al. 2017). This has been shown to induce potent CD4 + T cell responses and effector CD8 + T cells (Sahin et al. 2020). The CD4 + T cell response to mRNA vaccines generally follows a Th1 pathway producing lFN-γ, IL-2, and TNF, but not IL-4 or IL-5 therefore reducing the chance of VAERD (Sahin et al. 2020).
Moderna was the second company to publish clinical findings for a COVID-19 vaccine on July 14th, 2020, and the first to be granted EUA on Dec 11th, 2020. Their vaccine, mRNA-1273, consists of a lipid nanoparticle containing nucleoside-modified messenger RNA that encodes the SARS-CoV-2 S stabilized in its prefusion conformation. Two proline residues were inserted above the heptad repeat in the S protein. Preclinical studies in mice demonstrated that this mutation induced potent neutralizing antibodies toward wild type SARS-CoV-2 and the prevalent D614G mutant (Corbett et al. 2020). In a phase 1 trial consisting of 45 healthy adults, all participants were scheduled to receive two vaccinations of 25, 100, or 250 µg 28 days apart (Jackson et al. 2020). No buffer or negative control was included in this study, but comparisons to convalescent plasma samples were used as a positive control. Binding antibody IgG geometric mean titers (GMTs) to S-2P increased rapidly after the first vaccination, with seroconversion in all participants by day 15 and similar results seen using an RBD specific ELISA. Upon the second vaccination, neutralizing activity was found in all individuals and a dose-response relationship was observed. Neutralizing ability was greater than that of convalescent plasma samples for all dose groups after the second vaccination. Cellular responses to 25 and 100 µg doses showed CD4 T cells had a Th1 skewed response with cells expressing TNFα, IL-2, and lFN-γ; no Th2 cytokines were observed. A weak CD8 T cell response was observed in the 100 µg dose group. This is supported by mouse studies demonstrating a Th1 skewed response induced by mRNA-1273 as well as activated CD8 T cells that clear pathogen with no evidence of immunopathology. Much of the high dose (250 µg) data was not provided but there was a higher prevalence of severe adverse reactions with this dose. Because two vaccinations of 100 µg induced potent immune response with greater tolerability, Moderna is progressed to Phase 2 and 3 clinical trials with 100 µg as the high dose.
Moderna more recently published results of a phase 1 study on September 29th, 2020 establishing safety and efficacy of mRNA-1273 in older adults (Anderson et al. 2020). Similar antibody titers and neutralizing capabilities were observed in participants aged 56–70 and greater than 70 years old compared to younger participants previously studied. An interesting finding in this population is that neutralization was negligible after a single dose of either 25 or 100 µg but matched that of younger patients and convalescent plasma 15 days after a second dose, demonstrating the need for two doses. T-cell responses also confirmed a Th1 phenotype with induction of IFN-γ, IL-2, and TNFα upon stimulation by peptide pools, coinciding with an absence of Th2 cytokines. Although this trial had only 40 participants, it provided the basis for Moderna to evaluate mRNA-1273 in older adults in trials moving forward. On November 30th, 2020, Moderna announced its primary efficacy analysis of the ongoing Phase 3 trial composed of 30,000 individuals and conducted at 99 sites (Baden et al. 2020). The primary endpoint was prevention of SARS-CoV-2 infection 2 weeks after second immunization. Astonishingly, out of 196 confirmed cases, only 11 were in the vaccine treated group therefore giving 94.1% efficacy from viral infection. As well as giving great protection from SARS-CoV-2 infection, interim results have shown 100% protection from severe disease; 30 individuals developed severe COVID-19, all of which were in the placebo group. This along with the observation that efficacy was consistent across age, race and ethnicity, and gender demographics makes this a robust vaccine platform that could be utilized in more at-risk populations. The testing on children, pregnant individuals, and immunocompromised individuals still must be assessed to ensure adequate safety and efficacy in these populations. An application for EUA by the FDA was submitted on the same day as release of these results and EUA was granted 11 days later.
Besides Moderna, BioNtech and Pfizer are the only other companies to currently have an mRNA vaccine in phase 3 clinical studies. An interim report for phase 1/2 clinical data on BNT162b1 was published on July 1st, 2020 with full results published on August 12th, 2020. Their vaccine platform is similar to that of Moderna in that they are using lipid nanoparticles to deliver a nucleoside-modified mRNA vaccine. A major difference in their vaccine platform is the antigen of interest. BNT162b1 encodes trimerized SARS-CoV-2 spike glycoprotein RBD. RBD antigen expressed by BNT162b1 is modified by the addition of a T4 fibritin-derived foldon trimerization domain to increase its immunogenicity by multivalent display. This was also a small-scale clinical trial including 45 individuals across 3 dose groups 10, 30, and 100 µg. These dose levels are lower than those assessed by Moderna, being 25, 100, and 250 µg, which may be due to the optimized antigen design or difference in adjuvant activity of the delivery vehicles. In this trial, low and medium doses were well tolerated and patients received two doses of vaccine 21 days apart. The high dose of 100 µg saw increased reactogenicity with one injection causing severe local and systemic adverse reactions in about 10% of individuals, and administration of a second dose was halted. 10 and 30 µg doses were well tolerated with no severe adverse reactions after a single vaccination, and less than 10% of individuals had severe fatigue or chills after a second vaccination. A transient decrease in lymphocyte counts were observed across all dose groups but returned to normal levels within 6–8 days. RNA vaccines are known to induce type I interferon, associated with transient migration of lymphocytes into tissues, which may explain the drop in lymphocyte counts. When assessing immunogenicity data, similar to Moderna, a single vaccination did not induce neutralizing titers toward SARS-CoV-2 except for the high dose which induced a weak neutralizing response below the level of convalescent plasma. Binding antibodies were also not induced in the 10 and 30 µg dose groups after a single vaccination, thus lack of a high ratio of binding/neutralizing titers could potentially avoid the issue of ADE. Upon second vaccination of 10 and 30 µg, potent binding and neutralizing titers were induced. For 10 and 30 µg doses, respectively, binding antibodies were 9.8 and 46-fold and neutralizing antibodies were 1.9 to 4.6-fold that of a panel of COVID-19 convalescent human sera at least 14 days after a positive SARS-CoV-2 PCR result. The low dose required and potent neutralizing activity observed make this an attractive vaccine candidate.
On October 14th, 2020, Pfizer and BioNtech published a phase 1 study evaluating two of their mRNA vaccines in healthy adults and in the elderly population (Walsh et al. 2020b). The inclusion of individuals 65–85 years old is in line with Moderna’s approach to make this vaccine available to the elderly population who are at higher risk of severe COVID-19. The two vaccine candidates differed in antigen encoded by the nucleoside modified mRNA, which surprisingly had little to no effect on immunogenicity. A larger difference was seen in reactogenicity to the compounds. BNT162b2 encodes a full-length S protein with two proline residues inserted to stabilize its prefusion conformation, therefore mimicking the S protein on the natural virus. Similar to Moderna’s results, binding antibodies were induced after a single dose of either vaccine and were boosted upon the subsequent dose to levels within the range of convalescent plasma. Neutralizing titers were significantly enhanced 7 days after boost immunization and remained until 14 days. Both binding IgG and neutralizing titers were reduced in the elderly compare to younger subjects but remained 1.1 to 2.2 times the GMT of the convalescent serum panel. When investigating reactogenicity towards BNT162b1 and BNT162b2, a clear increase in systemic reactions to BNT162b1 over BNT162b2 is observed. 75% of younger individuals and 35% of the elderly had fever greater than 38°C after a second 30 µg dose of BNT162b1, while only 17% and 8% had fever greater than 38°C after a second 30 µg dose of BNT162b2. This trend could be seen for fatigue as well, and there was generally less reactogenicity in individuals greater than 65 than younger participants. Based on the decrease in reactogenicity paired with similar immunogenicity to BNT162b1, BNT162b2 advanced to Phase 2/3 clinical trials (Walsh et al. 2020a). On November 18th, 2020, Pfizer and BioNtech were the first to release their Phase 3 data as they met all primary efficacy endpoints. Over 40,000 individuals have received two doses of vaccine to date with 170 confirmed cases of COVID-19. Of the 170 cases, only 8 cases were in the BNT162b2 treatment group thus conferring protective efficacy of 95% (Polack et al. 2020). Similar results were seen in the elderly population with protective efficacy in 94% of individuals. 10 individuals in the study developed severe COVID-19 and only 1 was in the BNT162b2 group therefore giving 90% protection from severe disease. Although this is slightly less promising than Moderna’s 100% protection from severe disease, more cases must be monitored to gain true insight on the effectiveness of this vaccine in preventing severe disease. Considering Pfizer and BioNtech applied for early use authorization 2 days later, on November 20th, 2020, this will most likely be done in post-marketing surveillance. BNT162b2 was the second vaccine to be approved for EUA by the FDA on Dec 11th, 2020 and is currently being administered to the general public.
Protein subunit vaccines
Protein subunit vaccines are also at the forefront of vaccine development for COVID-19. 3 candidates have completed phase 1 and 2 trials, while only 2 are currently progressing to Phase 3 trials. Novavax, GSK/Sanofi, and Anhui Zhifei Longcom Biopharmaceutical products have differences in antigen as well as adjuvants used (as discussed in the adjuvant selection section above). Novovax’s NVX-CoV2373 is composed of recombinant SARS-CoV-2 (rSARS-CoV-2) nanoparticle vaccine, constructed from the full-length S protein (i.e., including the transmembrane domain) (Keech et al. 2020). This protein is derived from an Sf9 insect cell-expression system and incorporates mutations to confer protease resistance and stabilize the protein in prefusion conformation (Keech et al. 2020). GSK/Sanofi’s is composed of SARS-CoV-2 S-Trimer fusion protein produced in Chinese hamster ovary (CHO) cells (Richmond et al. 2020). Anhui Zhifei’s is RBD-dimer protein produced in Chinese hamster ovary (CHO) cells (Yang et al. 2020). While each of these have shown comparable anti-S titers in healthy adults compared to convalescent plasma samples after 2, 2, and 3 doses respectively, GSK/Sanofi has halted the start of phase 3 trials due to reduced neutralizing ability seen in the elderly population. GSK assessed the use of SCB-2019 trimeric protein formulated with AS03 or CpG/Alum. Anti-SCB-2019 antibodies and neutralization titers were absent after a single dose and were mild after multiple doses of antigen alone (Richmond et al. 2020). Incorporation of antigen with each of the adjuvants tested raised neutralizing activity to that of convalescent plasma samples with slightly reduced neutralizing activity observed in SCB-2019 + CpG/Alum compared to SCB-2019 + AS03 (Richmond et al. 2020). Interestingly, there was a clear dose dependence on neutralizing activity in healthy adults which was not present in elderly population. The elderly population had increased neutralization activity when increasing dose from 3 to 9 ug, but a lower response when comparing 9 and 30 ug for both adjuvanted formulations (Richmond et al. 2020). They plan on adjusting the dose given in a Phase 2b trial to increase immunogenicity in the elderly. Anhui Zhifei has not tested their vaccines in elderly populations but is advancing to Phase 3 trials nonetheless (Yang et al. 2020). Novavax has released interim data from an ongoing phase 3 trial in the UK which has shown efficacy of 89.3%, comparable with Moderna and Pfizer’s mRNA vaccines (Novavax 2021). Furthermore, this was the first phase 3 study that assessed variants in infected individuals which found that the vaccine was 95.6% effective in preventing infection from wild-type SARS-CoV-2 and 85.6% effective from the UK variant. In a separate Phase 2 study in South Africa, NVX-CoV2373 showed lower overall efficacy of about 49.4%, but when stratified to only include HIV-negative individuals, efficacy rose to 60%. 27 of the 44 COVID-19 events in this trial have been sequenced and identified, 92.6 were due to the South African variant. These findings demonstrate the increased ability of the South African variant to evade immune response compared to the UK variant. This may be due to increased mutations within the RBD and S protein compared to the UK variant. The South African trial also gives insight into vaccinating immunocompromised individuals. With lower efficacy in HIV-positive groups this may warrant a subsequent booster dose for immunocompromised individuals. All phase 2 trials for these vaccines were shown to have a large dependence on adjuvant to boost immune response. All 3 of these vaccines were considered to have acceptable safety profiles.
As previously mentioned the emergence of SARS-CoV-2 variant strains has been recognized as an issue for vaccine development and may require re-development to provide adequate protection. This may have the most profound effect on the development of subunit vaccines as all current approaches are mimicking the wild-type S or RBD conformation. Although a polyclonal antibody response and cell mediated response is observed in trials for subunit vaccines, which should still offer partial protection against developing variants as seen with mRNA vaccines, the already reduced immunogenicity observed compared to mRNA vaccines may pose an issue. A method our lab is investigation to boost immune response towards subunit vaccines is the generation of small native-like aggregates of S protein. The presence of aggregates in protein therapeutics can increase immunogenicity towards the therapeutic given (Rosenberg 2006). Native-like aggregates are aggregates are made of self‐assembled monomeric units that retain several conformational characteristics of native protein (Roberts 2007). We have demonstrated the ability of native-like aggregates to induce greater neutralizing titers than native protein (Pisal et al. 2012) (Fathallah et al. 2015). In preliminary studies using E. coli derived S1 subunits we have identified mild stress conditions by which these proteins form native-like aggregates (unpublished data). Use of nanoparticle tracking analysis generate native like aggregates in the size range comparable to that of SARS-CoV-2. Immune response towards these aggregates given via intramuscular, subcutaneous, and intranasal administration in the presence of various adjuvants to generate both humoral and cell mediated response are currently being assessed.
Conclusions and future directions
Only two weeks after releasing Phase 3 results, BNT162b2 was approved for use in the UK on December 2nd, 2020. This is a great breakthrough in vaccine development as the first approved mRNA vaccine on the market, coming just under a year since the first SARS-CoV-2 full genome sequence became available on January 10th, 2020. This was followed by FDA approval of Moderna and Pfizer vaccines for EUA shortly after. Considering a typical vaccine takes approximately 10 years to develop and produce, this is an unprecedented timeline for vaccine development. Besides being approved, manufacturing facilities have already been established and are able to provide up to 50 million doses worldwide within the next month, in-part due to Operation Warp Speed providing capital to allow companies to begin risk free manufacturing early during development. The next large obstacle Pfizer and BioNtech have to overcome is the logistics of distributing the vaccine throughout the world. Studies investigating the stability of liposomes encapsulating mRNA in various formulations should be conducted to avoid rollout logistical issues for future viral outbreaks. Although having greater than 90% efficacy in 3 different products produced within a year of SARS-CoV-2 viral sequencing is astonishing. In depth long term follow up of individuals receiving these vaccines must be conducted to assess the durability of the immune response. Answers to these questions will help guide vaccine rollout to be as efficient as possible and save as many lives as possible. Apart from this, vaccines need to be tested in multiple populations not assessed in current clinical trials such as paediatrics, pregnant women, and immunocompromised individuals. For both viral vector and mRNA vaccines questions regarding the interchangeability of these vaccines remains to be addressed although studies conducted between AstraZeneca and Gamaleya Institute are currently being designed (NatureBiotechnology 2021). Lastly, although current mRNA vaccines show protection (albeit slightly diminished) from emerging variant strains of SARS-CoV-2, as the virus continues to mutate this is protection is expected to continue to decline. It will be interesting to see the development time for companies which modify their construct and conduct separate clinical trials to address this issue for the long term. This timeline is expected to be shorter than initial development of vaccines targeting wild-type SARS-CoV-2. Overall, an immense amount of progress has been made in vaccine development in the past year, but there is still an abundant amount of questions to be addressed. What is becoming increasingly clear is that the benefits of vaccination with the current COVID-19 vaccines far outweigh the risks of possible adverse effects, reassuring that we are on track to overcome the pandemic. Enormous research effort at astonishing pace greatly increases our understanding of viruses and lead to effective therapies and vaccines for virus based infectious diseases in general.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Abbink P, Lemckert AA, Ewald BA, Lynch DM, Denholtz M, Smits S, Holterman L, Damen I, Vogels R, Thorner AR, et al. 2007. Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. J Virol. 81(9):4654–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson EJ, Rouphael NG, Widge AT, Jackson LA, Roberts PC, Makhene M, Chappell JD, Denison MR, Stevens LJ, Pruijssers AJ, et al. 2020. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med. 383(25):2427–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andries O, Mc Cafferty S, De Smedt SC, Weiss R, Sanders NN, Kitada T.. 2015. N(1)-methylpseudouridine-incorporated mRNA outperforms pseudouridine-incorporated mRNA by providing enhanced protein expression and reduced immunogenicity in mammalian cell lines and mice. J Control Release. 217:337–44. [DOI] [PubMed] [Google Scholar]
- Appledorn DM, Patial S, McBride A, Godbehere S, Van Rooijen N, Parameswaran N, Amalfitano A. 2008. Adenovirus vector-induced innate inflammatory mediators, MAPK signaling, as well as adaptive immune responses are dependent upon both TLR2 and TLR9 in vivo. J Immunol. 181(3):2134–44. [DOI] [PubMed] [Google Scholar]
- Arvin AM, Fink K, Schmid MA, Cathcart A, Spreafico R, Havenar-Daughton C, Lanzavecchia A, Corti D, Virgin HW. 2020. A perspective on potential antibody-dependent enhancement of SARS-CoV-2. Nature. 584(7821):353–63. [DOI] [PubMed] [Google Scholar]
- Bachy V, Hervouet C, Becker PD, Chorro L, Carlin LM, Herath S, Papagatsias T, Barbaroux JB, Oh SJ, Benlahrech A, et al. 2013. Langerin negative dendritic cells promote potent CD8+ T-cell priming by skin delivery of live adenovirus vaccine microneedle arrays. Proc Natl Acad Sci U S A. 110(8):3041–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, et al. 2020. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 384:2320–2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baden LR, Walsh SR, Seaman MS, Tucker RP, Krause KH, Patel A, Johnson JA, Kleinjan J, Yanosick KE, Perry J, et al. 2013. First-in-human evaluation of the safety and immunogenicity of a recombinant adenovirus serotype 26 HIV-1 Env vaccine (IPCAVD 001). J Infect Dis. 207(2):240–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balmert SC, Carey CD, Falo GD, Sethi SK, Erdos G, Korkmaz E, Falo LD Jr.. 2020. Dissolving undercut microneedle arrays for multicomponent cutaneous vaccination. J Control Release. 317:336–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone E. 2020. The Trump Administration's 'Operation Warp Speed' Has Spent $12.4 Billion on Vaccines. How Much Is That, Really? TIME 2020 December 14, 2020.
- Barouch DH, Pau MG, Custers JH, Koudstaal W, Kostense S, Havenga MJ, Truitt DM, Sumida SM, Kishko MG, Arthur JC, et al. 2004. Immunogenicity of recombinant adenovirus serotype 35 vaccine in the presence of pre-existing anti-Ad5 immunity. J Immunol. 172(10):6290–97. [DOI] [PubMed] [Google Scholar]
- Behmard E, Soleymani B, Najafi A, Barzegari E. 2020. Immunoinformatic design of a COVID-19 subunit vaccine using entire structural immunogenic epitopes of SARS-CoV-2. Sci Rep. 10(1):20864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BioNtech . 2020. Study to describe the safety, tolerability, immunogenicity, and efficacy of RNA vaccine candidates against COVID-19 in healthy individuals. ClinicalTrialsGov.
- Bradley AJ, Brooks DE, Norris-Jones R, Devine DV. 1999. C1q binding to liposomes is surface charge dependent and is inhibited by peptides consisting of residues 14-26 of the human C1qA chain in a sequence independent manner. Biochim Biophys Acta. 1418(1):19–30. [DOI] [PubMed] [Google Scholar]
- Brewer JM. 2006. (How) do aluminium adjuvants work? Immunol Lett. 102(1):10–15. [DOI] [PubMed] [Google Scholar]
- Briuglia ML, Rotella C, McFarlane A, Lamprou DA. 2015. Influence of cholesterol on liposome stability and on in vitro drug release. Drug Deliv Transl Res. 5(3):231–42. [DOI] [PubMed] [Google Scholar]
- Campbell JD. 2017. Development of the CpG adjuvant 1018: a case study. Methods Mol Biol. 1494:15–27. [DOI] [PubMed] [Google Scholar]
- Casimiro DR, Chen L, Fu TM, Evans RK, Caulfield MJ, Davies ME, Tang A, Chen M, Huang L, Harris V, et al. 2003. Comparative immunogenicity in rhesus monkeys of DNA plasmid, recombinant vaccinia virus, and replication-defective adenovirus vectors expressing a human immunodeficiency virus type 1 gag gene. J Virol. 77(11):6305–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catanzaro AT, Koup RA, Roederer M, Bailer RT, Enama ME, Moodie Z, Gu L, Martin JE, Novik L, Chakrabarti BK, et al. 2006. Phase 1 safety and immunogenicity evaluation of a multiclade HIV-1 candidate vaccine delivered by a replication-defective recombinant adenovirus vector. J Infect Dis. 194(12):1638–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahal JS, Fang T, Woodham AW, Khan OF, Ling J, Anderson DG, Ploegh HL. 2017. An RNA nanoparticle vaccine against Zika virus elicits antibody and CD8+ T cell responses in a mouse model. Sci Rep. 7(1):252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen IY, Moriyama M, Chang MF, Ichinohe T. 2019. Severe acute respiratory syndrome coronavirus viroporin 3a activates the NLRP3 inflammasome. Front Microbiol. 10:1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu H, Chan JF, Yuen TT, Shuai H, Yuan S, Wang Y, Hu B, Yip CC, Tsang JO, Huang X, et al. 2020. Comparative tropism, replication kinetics, and cell damage profiling of SARS-CoV-2 and SARS-CoV with implications for clinical manifestations, transmissibility, and laboratory studies of COVID-19: an observational study. Lancet Microbe. 1(1):e14–e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinical Trial of Efficacy and Safety of Sinovac 's Adsorbed COVID-19 (Inactivated) Vaccine in Healthcare Professionals (PROFISCOV). 2020. at https://clinicaltrials.gov/ct2/show/NCT04456595?term=NCT04456595&draw=2&rank=1.)
- Corbett KS, Edwards D, Leist SR, Abiona OM, Boyoglu-Barnum S, Gillespie RA, Himansu S, Schafer A, Ziwawo CT, DiPiazza AT, et al. 2020. SARS-CoV-2 mRNA vaccine development enabled by prototype pathogen preparedness. Nature. 586(7530): 567–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox F, van der Fits L, Abbink P, Larocca RA, van Huizen E, Saeland E, Verhagen J, Peterson R, Tolboom J, Kaufmann B, et al. 2018. Adenoviral vector type 26 encoding Zika virus (ZIKV) M-Env antigen induces humoral and cellular immune responses and protects mice and nonhuman primates against ZIKV challenge. PLoS One. 13(8):e0202820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RJ, Pedersen G, Madhun AS, Svindland S, Saevik M, Breakwell L, Hoschler K, Willemsen M, Campitelli L, Nostbakken JK, et al. 2011. Evaluation of a virosomal H5N1 vaccine formulated with matrix M adjuvant in a phase I clinical trial. Vaccine. 29(45):8049–59. [DOI] [PubMed] [Google Scholar]
- Cucinotta D, Vanelli M. 2020. WHO declares COVID-19 a pandemic. Acta Biomed. 91(1):157–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danthinne X, Imperiale MJ. 2000. Production of first generation adenovirus vectors: a review. Gene Ther. 7(20):1707–14. [DOI] [PubMed] [Google Scholar]
- Dicks MD, Spencer AJ, Edwards NJ, Wadell G, Bojang K, Gilbert SC, Hill AV, Cottingham MG, Kremer EJ. 2012. A novel chimpanzee adenovirus vector with low human seroprevalence: improved systems for vector derivation and comparative immunogenicity. PLoS One. 7(7):e40385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuis M, Denis-Mize K, LaBarbara A, Peters W, Charo IF, McDonald DM, Ott G. 2001. Immunization with the adjuvant MF59 induces macrophage trafficking and apoptosis. Eur J Immunol. 31(10):2910–18. [DOI] [PubMed] [Google Scholar]
- Dupuis M, Murphy TJ, Higgins D, Ugozzoli M, Van Nest G, Ott G, McDonald DM. 1998. Dendritic cells internalize vaccine adjuvant after intramuscular injection. Cell Immunol. 186(1):18–27. [DOI] [PubMed] [Google Scholar]
- Eichinger KM, Kosanovich JL, Gidwani SV, Zomback A, Lipp MA, Perkins TN, Oury TD, Petrovsky N, Marshall CP, Yondola MA, et al. 2020. Prefusion RSV F immunization elicits Th2-mediated lung pathology in mice when formulated with a Th2 (but not a Th1/Th2-balanced) adjuvant despite complete viral protection. Front Immunol. 11:111673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X, Cao D, Kong L, Zhang X. 2020. Cryo-EM analysis of the post-fusion structure of the SARS-CoV spike glycoprotein. Nat Commun. 11(1):3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fathallah AM, Chiang M, Mishra A, Kumar S, Xue L, Russell Middaugh C, Balu-Iyer SV. 2015. The effect of small oligomeric protein aggregates on the immunogenicity of intravenous and subcutaneous administered antibodies. J Pharm Sci. 104(11):3691–702. [DOI] [PubMed] [Google Scholar]
- Ferguson M, Risi G, Davis M, Sheldon E, Baron M, Li P, Madariaga M, Fries L, Godeaux O, Vaughn D. 2012. Safety and long-term humoral immune response in adults after vaccination with an H1N1 2009 pandemic influenza vaccine with or without AS03 adjuvant. J Infect Dis. 205(5):733–44. [DOI] [PubMed] [Google Scholar]
- Fink SL, Cookson BT. 2005. Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect Immun. 73(4):1907–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folegatti PM, Bittaye M, Flaxman A, Lopez FR, Bellamy D, Kupke A, Mair C, Makinson R, Sheridan J, Rohde C, et al. 2020a. Safety and immunogenicity of a candidate Middle East respiratory syndrome coronavirus viral-vectored vaccine: a dose-escalation, open-label, non-randomised, uncontrolled, phase 1 trial. Lancet Infect Dis. 20(7):816–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folegatti PM, Ewer KJ, Aley PK, Angus B, Becker S, Belij-Rammerstorfer S, Bellamy D, Bibi S, Bittaye M, Clutterbuck EA, et al. 2020b. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 396(10249):467–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabizon A, Shmeeda H, Barenholz Y. 2003. Pharmacokinetics of pegylated liposomal doxorubicin: review of animal and human studies. Clin Pharmacokinet. 42(5):419–36. [DOI] [PubMed] [Google Scholar]
- Gaitonde P, Peng A, Straubinger RM, Bankert RB, Balu-Iyer SV. 2012. Downregulation of CD40 signal and induction of TGF-beta by phosphatidylinositol mediates reduction in immunogenicity against recombinant human factor VIII. J Pharm Sci. 101(1):48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallie DR. 1991. The cap and poly(A) tail function synergistically to regulate mRNA translational efficiency. Genes Dev. 5(11):2108–16. [DOI] [PubMed] [Google Scholar]
- Garcon N, Vaughn DW, Didierlaurent AM. 2012. Development and evaluation of AS03, an adjuvant system containing alpha-tocopherol and squalene in an oil-in-water emulsion. Expert Rev Vaccines. 11(3):349–66. [DOI] [PubMed] [Google Scholar]
- Gordon DL, Sajkov D, Woodman RJ, Honda-Okubo Y, Cox MM, Heinzel S, Petrovsky N. 2012. Randomized clinical trial of immunogenicity and safety of a recombinant H1N1/2009 pandemic influenza vaccine containing Advax polysaccharide adjuvant. Vaccine. 30(36):5407–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham BS. 2020. Rapid COVID-19 vaccine development. Science. 368(6494):945–46. [DOI] [PubMed] [Google Scholar]
- Greaney AJ, Loes AN, Crawford KHD, Starr TN, Malone KD, Chu HY, Bloom JD. 2021. Comprehensive mapping of mutations to the SARS-CoV-2 receptor-binding domain that affect recognition by polyclonal human serum antibodies. [DOI] [PMC free article] [PubMed]
- Guo L, Ren L, Yang S, Xiao M, Chang YF, Yang, F, Dela Cruz CS, Wang Y, Wu C, Xiao Y, et al. 2020. Profiling early humoral response to diagnose novel coronavirus disease (COVID-19). Clin Infect Dis. 71(15):778–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habrant D, Peuziat P, Colombani T, Dallet L, Gehin J, Goudeau E, Evrard B, Lambert O, Haudebourg T, Pitard B. 2016. Design of ionizable lipids to overcome the limiting step of endosomal escape: application in the intracellular delivery of mRNA, DNA, and siRNA. J Med Chem. 59(7):3046–62. [DOI] [PubMed] [Google Scholar]
- Hafez IM, Maurer N, Cullis PR. 2001. On the mechanism whereby cationic lipids promote intracellular delivery of polynucleic acids. Gene Ther. 8(15):1188–96. [DOI] [PubMed] [Google Scholar]
- Hartmann G, Krieg AM. 2000. Mechanism and function of a newly identified CpG DNA motif in human primary B cells. J Immunol. 164(2):944–53. [DOI] [PubMed] [Google Scholar]
- Hendrickx R, Stichling N, Koelen J, Kuryk L, Lipiec A, Greber UF. 2014. Innate immunity to adenovirus. Hum Gene Ther. 25(4):265–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho JC, Chan KN, Hu WH, Lam WK, Zheng L, Tipoe GL, Sun J, Leung R, Tsang KW. 2001. The effect of aging on nasal mucociliary clearance, beat frequency, and ultrastructure of respiratory cilia. Am J Respir Crit Care Med. 163(4):983–88. [DOI] [PubMed] [Google Scholar]
- Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, et al. 2020. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 181(2):271–80 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtkamp S, Kreiter S, Selmi A, Simon P, Koslowski M, Huber C, Tureci O, Sahin U. 2006. Modification of antigen-encoding RNA increases stability, translational efficacy, and T-cell stimulatory capacity of dendritic cells. Blood. 108(13):4009–17. [DOI] [PubMed] [Google Scholar]
- Honda-Okubo Y, Barnard D, Ong CH, Peng BH, Tseng CT, Petrovsky N. 2015. Severe acute respiratory syndrome-associated coronavirus vaccines formulated with delta inulin adjuvants provide enhanced protection while ameliorating lung eosinophilic immunopathology. J Virol. 89(6):2995–3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang KJ, Su IJ, Theron M, Wu YC, Lai SK, Liu CC, Lei HY. 2005. An interferon-gamma-related cytokine storm in SARS patients. J Med Virol. 75(2):185–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki A, Yang Y. 2020. The potential danger of suboptimal antibody responses in COVID-19. Nat Rev Immunol. 20(6):339–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- J&J . 2021. Johnson & Johnson Announces Single-Shot Janssen COVID-19 Vaccine Candidate Met Primary Endpoints in Interim Analysis of its Phase 3 ENSEMBLE Trial. Online: Johnson & Johnson Services, Inc. [Google Scholar]
- Jackson LA, Anderson EJ, Rouphael NG, Roberts PC, Makhene M, Coler RN, McCullough MP, Chappell JD, Denison MR, Stevens LJ, et al. 2020. An mRNA vaccine against SARS-CoV-2 - preliminary report. N Engl J Med. 383(20):1920–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashem SW, Haniffa M, Kaplan DH. 2017. Antigen-presenting cells in the skin. Annu Rev Immunol. 35:469–99. [DOI] [PubMed] [Google Scholar]
- Keech C, Albert G, Cho I, Robertson A, Reed P, Neal S, Plested JS, Zhu M, Cloney-Clark S, Zhou H, et al. 2020. Phase 1-2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N Engl J Med. 83:2320–2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E, Erdos G, Huang S, Kenniston TW, Balmert SC, Carey CD, Raj VS, Epperly MW, Klimstra WB, Haagmans BL, et al. 2020. Microneedle array delivered recombinant coronavirus vaccines: immunogenicity and rapid translational development. EBioMedicine. 55:102743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HW, Canchola JG, Brandt CD, Pyles G, Chanock RM, Jensen K, Parrott RH. 1969. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 89(4):422–34. [DOI] [PubMed] [Google Scholar]
- Kou Y, Xu Y, Zhao Z, Liu J, Wu Y, You Q, Wang L, Gao F, Cai L, Jiang C. 2017. Tissue plasminogen activator (tPA) signal sequence enhances immunogenicity of MVA-based vaccine against tuberculosis. Immunol Lett. 190:51–57. [DOI] [PubMed] [Google Scholar]
- Krug A, Rothenfusser S, Hornung V, Jahrsdorfer B, Blackwell S, Ballas ZK, Endres S, Krieg AM, Hartmann G. 2001. Identification of CpG oligonucleotide sequences with high induction of IFN-alpha/beta in plasmacytoid dendritic cells. Eur J Immunol. 31(7):2154–63. [DOI] [PubMed] [Google Scholar]
- Kupferschmidt K. 2021. Fast-spreading U.K. virus variant raises alarms. Science. 371(6524):9–10. [DOI] [PubMed] [Google Scholar]
- Li Y, Wang L, Zhu T, Wu S, Feng L, Cheng P, Liu J, Wang J. 2018. Establishing China’s national standard for the recombinant adenovirus type 5 vector-based Ebola vaccine (Ad5-EBOV) virus titer. Hum Gene Ther Clin Dev. 29(4):226–32. [DOI] [PubMed] [Google Scholar]
- Liu A. Top vaccine players Sanofi, GSK win $2.1B Warp Speed funding for COVID-19 shot. 2020. July 31.
- Liu H, Jin L, Koh SB, Atanasov I, Schein S, Wu L, Zhou ZH. 2010. Atomic structure of human adenovirus by cryo-EM reveals interactions among protein networks. Science. 329(5995):1038–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Liao X, Qian S, Yuan J, Wang F, Liu Y, Wang Z, Wang FS, Liu L, Zhang Z. 2020. Community transmission of severe acute respiratory syndrome Coronavirus 2, Shenzhen, China, 2020. Emerg Infect Dis. 26(6):1320–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Wei Q, Lin Q, Fang J, Wang H, Kwok H, Tang H, Nishiura K, Peng J, Tan Z, et al. 2019. Anti-spike IgG causes severe acute lung injury by skewing macrophage responses during acute SARS-CoV infection. JCI Insight. 2019 Feb 21; 4(4): e123158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logunov DY, Dolzhikova IV, Zubkova OV, Tukhvatullin AI, Shcheblyakov DV, Dzharullaeva AS, Grousova DM, Erokhova AS, Kovyrshina AV, Botikov AG, et al. 2020. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: two open, non-randomised phase 1/2 studies from Russia. Lancet. 396(10255):887–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love KT, Mahon KP, Levins CG, Whitehead KA, Querbes W, Dorkin JR, Qin J, Cantley W, Qin LL, Racie T, et al. 2010. Lipid-like materials for low-dose, in vivo gene silencing. Proc Natl Acad Sci U S A. 107(5):1864–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martines RB, Ritter JM, Matkovic E, Gary J, Bollweg BC, Bullock H, Goldsmith CS, Silva-Flannery L, Seixas JN, Reagan-Steiner S, et al. 2020. Pathology and pathogenesis of SARS-CoV-2 associated with fatal coronavirus disease, United States. Emerg Infect Dis. 26(9):2005–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason RJ. 2020. Pathogenesis of COVID-19 from a cell biology perspective. Eur Respir J. 2020 Apr 16;55(4):2000607. doi: 10.1183/13993003.00607-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercado NB, Zahn R, Wegmann F, Loos C, Chandrashekar A, Yu J, Liu J, Peter L, McMahan K, Tostanoski LH, et al. 2020. Single-shot Ad26 vaccine protects against SARS-CoV-2 in rhesus macaques. Nature. 586(7830):583–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merck . 2021. Merck Discontinues Development of SARS-CoV2/COVID-19 Vaccine Candidates; Continues Development of Two Investigational Therapeutic.
- Mistilis MJ, Joyce JC, Esser ES, Skountzou I, Compans RW, Bommarius AS, Prausnitz MR. 2017. Long-term stability of influenza vaccine in a dissolving microneedle patch. Drug Deliv Transl Res. 7(2):195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ModernaTX . 2020. A study to evaluate efficacy, safety, and immunogenicity of mRNA-1273 vaccine in adults aged 18 years and older to prevent COVID-19. ClinicalTrialsGov.
- Mui BL, Tam YK, Jayaraman M, Ansell SM, Du X, Tam YY, Lin PJ, Chen S, Narayanannair JK, Rajeev KG, et al. 2013. Influence of polyethylene glycol lipid desorption rates on pharmacokinetics and pharmacodynamics of siRNA lipid nanoparticles. Mol Ther Nucleic Acids. 2:e139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muik A, Wallisch A-K, Sänger B, Swanson KA, Mühl J, Chen W, Cai H, Sarkar R, Türeci Ö, Dormitzer PR, et al. 2021. Neutralization of SARS-CoV-2 lineage B.1.1.7 pseudovirus by BNT162b2 vaccine-elicited human sera. 2021.01.18.426984. [DOI] [PMC free article] [PubMed]
- Mulligan MJ, Lyke KE, Kitchin N, Absalon J, Gurtman A, Lockhart S, Neuzil K, Raabe V, Bailey R, Swanson KA, et al. 2020. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature. 586(7830):589–93. [DOI] [PubMed] [Google Scholar]
- Nakanishi T, Kunisawa J, Hayashi A, Tsutsumi Y, Kubo K, Nakagawa S, Nakanishi M, Tanaka K, Mayumi T. 1999. Positively charged liposome functions as an efficient immunoadjuvant in inducing cell-mediated immune response to soluble proteins. J Control Release. 61(1–2):233–40. [DOI] [PubMed] [Google Scholar]
- NatureBiotechnology . 2021. AstraZeneca joins Russia to boost coronavirus vaccine. Nat Biotechnol. 39(1):11. [DOI] [PubMed] [Google Scholar]
- Naz A, Shahid F, Butt TT, Awan FM, Ali A, Malik A. 2020. Designing multi-epitope vaccines to combat emerging Coronavirus disease 2019 (COVID-19) by employing immuno-informatics approach. Front Immunol. 11:1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni L, Cheng M-L, Zhao H, Feng Y, Liu J, Ye F, Ye Q, Zhu G, Li X, Wang P, et al. 2020a. Impaired cellular immunity to SARS-CoV-2 in severe COVID-19 patients. medRxiv. 2020.08.10.20171371. [DOI] [PMC free article] [PubMed]
- Ni L, Ye F, Cheng ML, Feng Y, Deng YQ, Zhao H, Wei P, Ge J, Gou M, Li X, et al. 2020b. Detection of SARS-CoV-2-specific humoral and cellular immunity in COVID-19 convalescent individuals. Immunity. 52(6):971–77 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie Y, Wang G, Shi X, Zhang H, Qiu Y, He Z, Wang W, Lian G, Yin X, Du L, et al. 2004. Neutralizing antibodies in patients with severe acute respiratory syndrome-associated coronavirus infection. J Infect Dis. 190(6):1119–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novavax . 2021. Novavax COVID-19 Vaccine Demonstrates 89.3% Efficacy in UK Phase 3 Trial. Online: Novavax, Inc.
- Ou X, Liu Y, Lei X, Li P, Mi D, Ren L, Guo L, Guo R, Chen T, Hu J, et al. 2020. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun. 11(1):1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardi N, Hogan MJ, Porter FW, Weissman D. 2018. mRNA vaccines - a new era in vaccinology. Nat Rev Drug Discov. 17(4):261–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastorino B, Touret F, Gilles M, De Lamballerie X, Charrel RN. 2020. Prolonged infectivity of SARS-CoV-2 in fomites. Emerg Infect Dis. 26(9):2256–2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovsky N, Cooper PD. 2015. Advax, a novel microcrystalline polysaccharide particle engineered from delta inulin, provides robust adjuvant potency together with tolerability and safety. Vaccine. 33(44):5920–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisal DS, Kosloski MP, Middaugh CR, Bankert RB, Balu-Iyer SV. 2012. Native-like aggregates of factor VIII are immunogenic in von Willebrand factor deficient and hemophilia a mice. J Pharm Sci. 101(6):2055–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Perez Marc G, Moreira ED, Zerbini C, et al. 2020. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 383(27):2603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polak SB, Van Gool IC, Cohen D, Von Der Thusen JH, Van Paassen J. 2020. A systematic review of pathological findings in COVID-19: a pathophysiological timeline and possible mechanisms of disease progression. Mod Pathol. 383: 2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, Xie C, Ma K, Shang K, Wang W, et al. 2020. Dysregulation of immune response in patients with Coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 71(15):762–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasamy MN, Minassian AM, Ewer KJ, Flaxman AL, Folegatti PM, Owens DR, Voysey M, Aley PK, Angus B, Babbage G, et al. 2021. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 396(10267):1979–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randomized, Double Blind, Parallel Placebo Controlled, 2020. Phase III Clinical Trial to Evaluate the Safety and Protective Efficacy of Inactivated SARS-CoV-2 Vaccine in Healthy Population Aged 18 Years and above Wuhan Institute of Biological Products co., LTD. at http://www.chictr.org.cn/hvshowproject.aspx?id=43780
- Richmond P, Hatchuel L, Dong M, Ma B, Hu B, Smolenov I, Li P, Liang P, Han HH, Liang J, et al. 2020. A first-in-human evaluation of the safety and immunogenicity of SCB-2019, an adjuvanted, recombinant SARS-CoV-2 trimeric S-protein subunit vaccine for COVID-19 in healthy adults; a phase 1, randomised, double-blind, placebo-controlled trial. medRxiv. 2020.12.03.20243709.
- Roberts CJ. 2007. Non-native protein aggregation kinetics. Biotechnol Bioeng. 98(5):927–38. [DOI] [PubMed] [Google Scholar]
- Roman F, Clement F, Dewe W, Walravens K, Maes C, Willekens J, De Boever F, Hanon E, Leroux-Roels G. 2011. Effect on cellular and humoral immune responses of the AS03 adjuvant system in an A/H1N1/2009 influenza virus vaccine administered to adults during two randomized controlled trials. Clin Vaccine Immunol. 18(5):835–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg AS. 2006. Effects of protein aggregates: an immunologic perspective. Aaps J. 8(3):E501–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan Q, Yang K, Wang W, Jiang L, Song J. 2020. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 46(5):846–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saade F, Honda-Okubo Y, Trec S, Petrovsky N. 2013. A novel hepatitis B vaccine containing Advax, a polysaccharide adjuvant derived from delta inulin, induces robust humoral and cellular immunity with minimal reactogenicity in preclinical testing. Vaccine. 31(15):1999–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadoff J, Le Gars M, Shukarev G, Heerwegh D, Truyers C, De Groot AM, Stoop J, Tete S, Van Damme W, Leroux-Roels I, et al. 2021. Interim results of a phase 1-2a trial of Ad26.COV2.S Covid-19 vaccine. N Engl J Med. 2021 Jan 13;NEJMoa2034201. doi: 10.1056/NEJMoa2034201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin U, Kariko K, Tureci O. 2014. mRNA-based therapeutics–developing a new class of drugs. Nat Rev Drug Discov. 13(10):759–80. [DOI] [PubMed] [Google Scholar]
- Sahin U, Muik A, Derhovanessian E, Vogler I, Kranz LM, Vormehr M, Baum A, Pascal K, Quandt J, Maurus D, et al. 2020. Concurrent human antibody and TH1 type T-cell responses elicited by a COVID-19 RNA vaccine. medRxiv. 2020.07.17.20140533.
- Sarkar B, Ullah MA, Araf Y, Rahman MS. 2020. Engineering a novel subunit vaccine against SARS-CoV-2 by exploring immunoinformatics approach. Inform Med Unlocked. 21:100478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultze V, D’Agosto V, Wack A, Novicki D, Zorn J, Hennig R. 2008. Safety of MF59 adjuvant. Vaccine. 26(26):3209–22. [DOI] [PubMed] [Google Scholar]
- Singh M, Ugozzoli M, Kazzaz J, Chesko J, Soenawan E, Mannucci D, Titta F, Contorni M, Volpini G, Del Guidice G, et al. 2006. A preliminary evaluation of alternative adjuvants to alum using a range of established and new generation vaccine antigens. Vaccine. 24(10):1680–86. [DOI] [PubMed] [Google Scholar]
- Stone CA Jr., Liu Y, Relling MV, Krantz MS, Pratt AL, Abreo A, Hemler JA, Phillips EJ. 2019. Immediate hypersensitivity to polyethylene glycols and polysorbates: more common than we have recognized. J Allergy Clin Immunol Pract. 7(5):1533–40 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay MZ, Poh CM, Renia L, MacAry PA, Ng LFP. 2020. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 20(6):363–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegally H, Wilkinson E, Giovanetti M, Iranzadeh A, Fonseca V, Giandhari J, Doolabh D, Pillay S, San EJ, Msomi N, et al. 2020. Emergence and rapid spread of a new severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) lineage with multiple spike mutations in South Africa. medRxiv. 2020.12.21.20248640.
- Teigler JE, Kagan JC, Barouch DH. 2014. Late endosomal trafficking of alternative serotype adenovirus vaccine vectors augments antiviral innate immunity. J Virol. 88(18):10354–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temperton NJ, Chan PK, Simmons G, Zambon MC, Tedder RS, Takeuchi Y, Weiss RA. 2005. Longitudinally profiling neutralizing antibody response to SARS coronavirus with pseudotypes. Emerg Infect Dis. 11(3):411–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thevarajan I, Nguyen THO, Koutsakos M, Druce J, Caly L, Van De Sandt CE, Jia X, Nicholson S, Catton M, Cowie B, et al. 2020. Breadth of concomitant immune responses prior to patient recovery: a case report of non-severe COVID-19. Nat Med. 26(4):453–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian J-H, Patel N, Haupt R, Zhou H, Weston S, Hammond H, Lague J, Portnoff AD, Norton J, Guebre-Xabier M, et al. 2020a. SARS-CoV-2 spike glycoprotein vaccine candidate NVX-CoV2373 elicits immunogenicity in baboons and protection in mice. bioRxiv. 2020.06.29.178509. [DOI] [PMC free article] [PubMed]
- Tian S, Hu W, Niu L, Liu H, Xu H, Xiao S-Y. 2020b. Pulmonary pathology of early-phase 2019 novel Coronavirus (COVID-19) pneumonia in two patients with lung cancer. J Thoracic Oncol. 15(5):700–04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- University W. Oxford Covid-19 vaccine trial results. Online: University of the Witwatersrand, Johannesburg; 2021.
- Van Doremalen N, Haddock E, Feldmann F, Meade-White K, Bushmaker T, Fischer RJ, Okumura A, Hanley PW, Saturday G, Edwards NJ, et al. 2020. A single dose of ChAdOx1 MERS provides protective immunity in rhesus macaques. Sci Adv. 6(24):eaba8399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasques Nonaka CK, Miranda Franco M, Gräf T, Almeida Mendes AV, Santana de Aguiar R, Giovanetti M, Solano de Freitas Souza B. 2021. Genomic evidence of a Sars-Cov-2 reinfection case with E484K spike mutation in Brazil.
- Voysey M, Clemens S, Madhi S, Weckx L, Folegatti P, Aley P, Angus B, Bailie V, Barnabas S, Bhorat Q, et al. 2021a. Single dose administration, and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine. The Lancet. 397:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, Angus B, Baillie VL, Barnabas SL, Bhorat QE, et al. 2021b. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 397(10269):99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wack A, Baudner BC, Hilbert AK, Manini I, Nuti S, Tavarini S, Scheffczik H, Ugozzoli M, Singh M, Kazzaz J, et al. 2008. Combination adjuvants for the induction of potent, long-lasting antibody and T-cell responses to influenza vaccine in mice. Vaccine. 26(4):552–61. [DOI] [PubMed] [Google Scholar]
- Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D. 2020. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 181(2):281–92 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh EE, Frenck R, Falsey AR, Kitchin N, Absalon J, Gurtman A, Lockhart S, Neuzil K, Mulligan MJ, Bailey R, et al. 2020a. RNA-based COVID-19 vaccine BNT162b2 selected for a pivotal efficacy study. [DOI] [PMC free article] [PubMed]
- Walsh EE, Frenck RW Jr., Falsey AR, Kitchin N, Absalon J, Gurtman A, Lockhart S, Neuzil K, Mulligan MJ, Bailey R, et al. 2020b. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med. 383(25):2439–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Zhang L, Kuwahara K, Li L, Liu Z, Li T, Zhu H, Liu J, Xu Y, Xie J, et al. 2016a. Immunodominant SARS coronavirus epitopes in humans elicited both enhancing and neutralizing effects on infection in non-human primates. ACS Infect Dis. 2(5):361–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Zhang L, Kuwahara K, Li L, Liu Z, Li T, Zhu H, Liu J, Xu Y, Xie J, et al. 2016b. Immunodominant SARS Coronavirus epitopes in humans elicited both enhancing and neutralizing effects on infection in non-human primates. ACS Infect Dis. 2(5):361–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SF, Tseng SP, Yen CH, Yang JY, Tsao CH, Shen CW, Chen KH, Liu FT, Liu WT, Chen YM, et al. 2014. Antibody-dependent SARS coronavirus infection is mediated by antibodies against spike proteins. Biochem Biophys Res Commun. 451(2):208–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Schmidt F, Weisblum Y, Muecksch F, Barnes CO, Finkin S, Schaefer-Babajew D, Cipolla M, Gaebler C, Lieberman JA, et al. 2021. mRNA vaccine-elicited antibodies to SARS-CoV-2 and circulating variants. bioRxiv. 2021.01.15.426911. [DOI] [PMC free article] [PubMed]
- Weisblum Y, Schmidt F, Zhang F, DaSilva J, Poston D, Lorenzi JCC, Muecksch F, Rutkowska M, Hoffmann HH, Michailidis E, et al. 2020. Escape from neutralizing antibodies by SARS-CoV-2 spike protein variants. eLife. 9. doi: 10.7554/eLife.61312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . 2020. Director-General's opening remarks at the media briefing on COVID-19-11 March 2020. WHO. [Google Scholar]
- Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. 2020. Pathophysiology, transmission, diagnosis, and treatment of Coronavirus disease 2019 (COVID-19): a review. JAMA. 324(8):782–93. [DOI] [PubMed] [Google Scholar]
- Williams K, Bastian AR, Feldman RA, Omoruyi E, De Paepe E, Hendriks J, Van Zeeburg H, Godeaux O, Langedijk JPM, Schuitemaker H, et al. 2020. Phase 1 safety and immunogenicity study of a respiratory syncytial virus vaccine with an adenovirus 26 vector encoding prefusion F (Ad26.RSV.preF) in adults aged >/=60 Years. J Infect Dis. 222(6):979–88. [DOI] [PubMed] [Google Scholar]
- Wilson JM. 2009. Lessons learned from the gene therapy trial for ornithine transcarbamylase deficiency. Mol Genet Metab. 96(4):151–57. [DOI] [PubMed] [Google Scholar]
- Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh CL, Abiona O, Graham BS, McLellan JS. 2020. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 367(6483):1260–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, McGoogan JM. 2020. Characteristics of and important lessons from the Coronavirus disease 2019 (COVID-19) outbreak in China. Jama. 323(13):1239. [DOI] [PubMed] [Google Scholar]
- Xia J, Tong J, Liu M, Shen Y, Guo D. 2020. Evaluation of coronavirus in tears and conjunctival secretions of patients with SARS-CoV-2 infection. J Med Virol. 92(6):589–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, et al. 2020. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 8(4):420–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahalom-Ronen Y, Tamir H, Melamed S, Politi B, Shifman O, Achdout H, Vitner EB, Israeli O, Milrot E, Stein D, et al. 2020. A single dose of recombinant VSV-G-spike vaccine provides protection against SARS-CoV-2 challenge. Nat Commun. 11(1):6402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Li Y, Dai L, Wang J, He P, Li C, Fang X, Wang C, Zhao X, Huang E, et al. 2020. Safety and immunogenicity of a recombinant tandem-repeat dimeric RBD protein vaccine against COVID-19 in adults: pooled analysis of two randomized, double-blind, placebo-controlled, phase 1 and 2 trials. medRxiv. 2020.12.20.20248602. [DOI] [PMC free article] [PubMed]
- Yang T, Cui FD, Choi MK, Cho JW, Chung SJ, Shim CK, Kim DD. 2007. Enhanced solubility and stability of PEGylated liposomal paclitaxel: in vitro and in vivo evaluation. Int J Pharm. 338(1–2):317–26. [DOI] [PubMed] [Google Scholar]
- Zhang BN, Wang Q, Liu T, Dou SQ, Qi X, Jiang H, Qi BX, Zhang B, Zhou QJ. 2020a. A special on epidemic prevention and control: analysis on expression of 2019-nCoV related ACE2 and TMPRSS2 in eye tissues. Zhonghua Yan Ke Za Zhi. 56(6):438–46. [DOI] [PubMed] [Google Scholar]
- Zhang H, Zhou P, Wei Y, Yue H, Wang Y, Hu M, Zhang S, Cao T, Yang C, Li M, et al. 2020b. Histopathologic changes and SARS-CoV-2 immunostaining in the lung of a patient with COVID-19. Ann Intern Med. 172(9):629–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Chen X, Chen L, Deng C, Zou X, Liu W, Yu H, Chen B, Sun X. 2020c. The evidence of SARS-CoV-2 infection on ocular surface. Ocul Surf. 18(3):360–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng HY, Zhang M, Yang CX, Zhang N, Wang XC, Yang XP, Dong XQ, Zheng YT. 2020. Elevated exhaustion levels and reduced functional diversity of T cells in peripheral blood may predict severe progression in COVID-19 patients. Cell Mol Immunol. 17(5):541–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, et al. 2020. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 579(7798):270–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu FC, Guan XH, Li YH, Huang JY, Jiang T, Hou LH, Li JX, Yang BF, Wang L, Wang WJ, et al. 2020. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 396(10249):479–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu FC, Hou LH, Li JX, Wu SP, Liu P, Zhang GR, Hu YM, Meng FY, Xu JJ, Tang R, et al. 2015. Safety and immunogenicity of a novel recombinant adenovirus type-5 vector-based Ebola vaccine in healthy adults in China: preliminary report of a randomised, double-blind, placebo-controlled, phase 1 trial. Lancet. 385(9984):2272–79. [DOI] [PubMed] [Google Scholar]
- Zhuang Y, Ma Y, Wang C, Hai L, Yan C, Zhang Y, Liu F, Cai L. 2012. PEGylated cationic liposomes robustly augment vaccine-induced immune responses: role of lymphatic trafficking and biodistribution. J Control Release. 159(1):135–42. [DOI] [PubMed] [Google Scholar]
- Zimmermann P, Curtis N. 2020. Why is COVID-19 less severe in children? A review of the proposed mechanisms underlying the age-related difference in severity of SARS-CoV-2 infections. Arch Dis Child. doi: 10.1136/archdischild-2020-320338 [DOI] [PubMed] [Google Scholar]


