Highlights
-
•
Hematopoietic stem cell transplant (HSCT) patients are living longer.
-
•
Bone health is an important long-term comorbidity post-HSCT.
-
•
HSCT patients are at high risk of bone loss and fragility fracture.
-
•
HSCT patients are at high risk of bone loss and fragility fracture.
-
•
Recommendations are provided for better monitoring of bone health.
-
•
Recommendations include bone assessment, dietary advice and osteoporosis medication.
Keywords: Hematopoietic stem cell, Transplantation, Bone loss, Bone marrow, Fracture, Bone mineral density
Abstract
Background
Treatment advances have reduced the adverse events associated with hematopoietic stem cell transplant (HSCT) and led to an increased number of transplants performed. HSCT patients are living longer with concerns on long-term outcomes. Bone fragility and fracture are at the forefront for long-term morbidities post-HSCT.
Results
In HSCT recipients, evidence has accumulated to support recommendations for more extensive monitoring of bone fragility and more appropriate administration of osteoporosis pharmacotherapies for patients at high risk of bone loss and/or fracture.
Conclusion
This executive summary reports and summarizes the main recommendations published previously, including bone assessment, dietary and lifestyle recommendations and osteoporosis medication.
1. Introduction
The International Osteoporosis Foundation Committee of Scientific Advisors Working Group on Cancer and Bone Disease published a review paper on the management of bone in hematologic stem cell transplant patients in Osteoporosis International [1]. With the publication of this executive summary which reports and summarizes the main recommendations, we wish to propose an algorithm and recommendations to the hematology/oncology readership.
2. Bone loss risk factors
Substantial risk of bone loss exists in patients undergoing allogeneic hematopoietic stem cell transplant (HSCT) [2]. Risk factors for bone loss following HSCT include HSCT preparative regimen, induction of premature menopause, weight loss and medications used in the prevention and treatment of acute and chronic graft-versus-host disease (GVHD). Most women become menopausal subsequent to HSCT. Reduced intensity conditioning regimens are increasingly used and are associated with less organ toxicity but their impact on bone loss remains unknown. The integration of clinical risk factors with bone mineral density (BMD) has been operationalized into the Fracture Risk Assessment Tool (FRAX®), which provides a 10-year fracture risk probability for both women and men. FRAX® is not applicable in patients under the age of 40. FRAX® has not been validated in the HSCT population. In younger individuals undergoing HSCT, achievement of peak bone mass may be impaired with increases in fracture risk later in life. Low vitamin D levels have been reported in 49–70% of pre-HSCT [3], [4] and 58–100% post-HSCT patients [3], [4], [5].
3. When to test BMD
Most guidelines recommend dual energy X-ray absorptiometry (DXA) examination within one year of transplant [6], [7], [8]. The IOF Working Group on Cancer and Bone Disease also recommends the measurement of BMD before HSCT due to prior morbidity and exposure to chemotherapy agents potentially harmful to bone. Post-HSCT declines in BMD are not predictable. BMD measurement should be repeated in patients not given osteoporosis pharmacotherapy (for instance those with T score > −1.5 at the spine, total hip or femoral neck by DXA), approximately 3 months after HSCT. If patients have received osteoporosis pharmacotherapy, such as pamidronate or zoledronic acid, because of a T score < −1.5 at the spine, total hip or femoral neck as measured by DXA, a repeat BMD one year after HSCT is recommended. Individuals with specific clinical concerns may require follow-up evaluations at an earlier time point. BMD may be low in patients many years after HSCT. Thus, BMD should be monitored periodically in HSCT recipients [9].
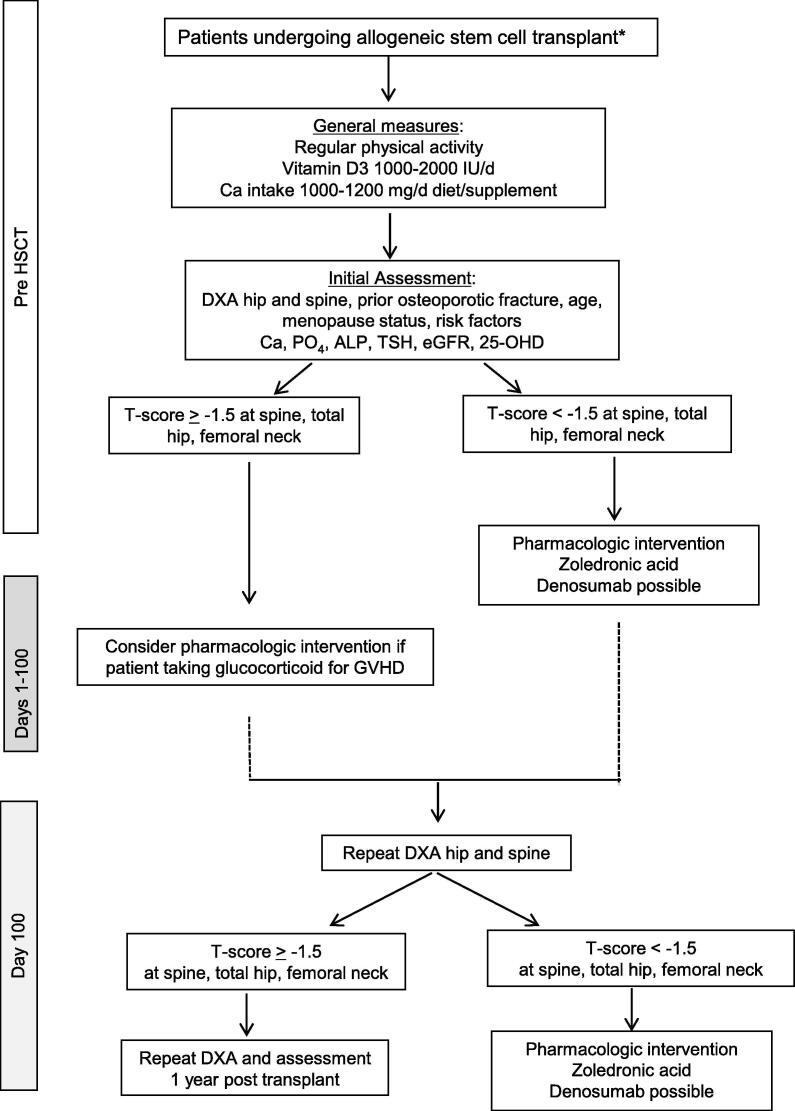
Secondary causes of bone loss may coexist in this HSCT population. Secondary causes should be evaluated with measurement of calcium, phosphate, alkaline phosphatase, thyroid-stimulating hormone (TSH), creatinine, estimated glomerular filtration rate (eGFR) and 25-hydroxyvitamin D (25-OH-VitD). In some clinical circumstances, parathyroid hormone (PTH), testosterone, estradiol, follicle-stimulating hormone (FSH), and luteinizing hormone (LH) could be measured too (Fig. 1).
Fig. 1.
Management algorithm for patients undergoing allogeneic stem cell transplant. The asterisk symbol represents patients receiving an autologous HSCT with underlying myeloma [26], [27]. DXA: dual energy X-ray absorptiometry; Ca: calcium; PO4: phosphate; ALP: alkaline phosphatase; 25-OHD: 25-hydroxy vitamin D; TSH: thyroid-stimulating hormone; GVHD: graft-versus-host disease; eGFR: estimated glomerular filtration rate.
4. Diet and lifestyle recommendations
A well balanced diet with adequate intakes of calcium, protein and Vitamin D is recommended [9]. Food intake and gastrointestinal (GI) absorption are reduced after HSCT and may be more problematic with GI GVHD. Because of frequent post-HSCT nutritional deficiency, attention to adequate caloric intake with the possible early addition of total parenteral nutrition (TPN) should be paid.
Dairy intake should be encouraged, however calcium supplements could be used to help to meet patients' need for 1000–1200 mg elemental calcium from diet and supplement combined.
Baseline assessment of serum 25-OH-vit D may help to determine patients in need of a loading dose of vitamin D prior to transplant. A loading dose of vitamin D3 (for example 50,000–100,000 IU) prior to HSCT contributes to ensure vitamin D sufficiency throughout the critical first three months post-HSCT. Vitamin D is inexpensive and easy to administer orally.
All patients should be educated about physical exercise and lifestyle changes to reduce bone loss and to improve quality of life, such as regular weight-bearing and muscle strengthening exercises, avoidance of tobacco and excessive alcohol intake [10].
5. Intervention thresholds
Parenteral antiresorptive therapy is preferred due to the potential for poor absorption and adherence associated with oral therapy [11] as well as GI side effects in this population who are at particular risk. Bisphosphonates are the most studied drugs for the prevention of HSCT-induced bone loss. Bisphosphonates like pamidronate or zoledronic acid, prevent bone loss [12], [13] or even increase bone mass [14], [15]. A meta-analysis has confirmed that bisphosphonates, and in particular, zoledronic acid, are promising in the prevention of HSCT-induced bone loss [16]. Intervention with intravenous zoledronic acid should be considered if pre-HSCT BMD T-score < −1.5 at any one of the relevant sites (total hip, femoral neck, lumbar spine, one third radius), and if renal function permits. Though there are no data specific to this population, denosumab could be considered in patients with renal impairment, who are not candidates for bisphosphonates.
All patients receiving prolonged courses of glucocorticoid for GVHD are at high risk for bone loss and fracture. Therefore, prophylaxis with bisphosphonates, ideally zoledronic acid 5 mg, may be considered regardless of the T-score.
The major fractures due to osteoporosis are those of the hip, spine, wrist and proximal humerus and are associated with low BMD and other factors such as the tendency of falling, age, gender and race. They are called fragility fractures because they are occurring after a low-energy trauma, such as a fall from standing height. The risk of re-fracture is highest in the first two years following a first fracture [17], and thus a prior fragility fracture is a strong indication to treatment and should be initiated as soon as possible after fracture surgery, as secondary fracture prevention.
In younger women with treatment induced amenorrhea post-HSCT, estrogen-based Menopausal Hormone Therapy (MHT) may be an effective antiresorptive therapy and perhaps show additive benefits with regard to cardiovascular disease [18], although its efficacy in preventing glucocorticoid induced bone loss in this population has not been established [19], [20].
Denosumab, a monoclonal antibody to receptor activator of nuclear factor kappa-B ligand (RANKL), is a novel approach to prevent bone loss, though not yet specifically evaluated in HSCT recipients. Denosumab 60 mg subcutaneously twice yearly prevent clinical fractures in postmenopausal women with breast cancer, who receive an aromatase inhibitor irrespective of baseline age or BMD [21]. This therapy may be safer than bisphosphonates in the context of renal impairment (eGFR < 35 mL per minute) and may be more convenient to administer than intravenous therapy. In addition, there are recent data documenting a superior BMD response in patients on glucocorticoid treated with denosumab compared to risedronate [22].
Teriparatide is a parathyroid hormone fragment that is anabolic for bone, approved for the treatment of postmenopausal, male, and glucocorticoid osteoporosis. It is contraindicated in patients with prior cancer or with previous skeletal irradiation, including HSCT patients [23]. Abaloparatide, a parathyroid hormone-related peptide (PTHrP) analog and romosozumab, an inhibitor of sclerostin, are both biological anabolic treatments but no study has been published on abaloparatide or romosozumab in transplant recipients.
6. Obstacles to implementation of bone fragility guidelines
Despite convincing data that bone loss is common post-HSCT, many HSCT patients are not monitored for bone fragility and most do not receive prophylactic intervention, despite a possible increased long-term fracture risk.
Pre-HSCT DXA examination can identify patients with osteopenia/osteoporosis, who may benefit from early interventions to prevent or reverse HSCT-related bone loss.
The risk of renal dysfunction in patients receiving intravenous zoledronic acid for osteoporosis is small. Transient increases in serum creatinine are observed 9–11 days after zoledronic acid infusion [24]. It is recommended that patients should be well-hydrated and not receive agents known to adversely affect renal function [25]. Zoledronic acid dosing pre-HSCT is best administered prior to HSCT conditioning and GVHD prophylaxis.
Denosumab is a promising agent for prevention of bone loss in HSCT patients. Inhibiting the immunomodulatory effect of RANKL on both the innate and adaptive immune systems theoretically could increase the risk of infection and/or disease relapse in HSCT patients. For this reason, controlled clinical trials are required.
The proposed algorithm represents expert opinion, analogous to similar algorithms created to aid clinicians in the management of glucocorticoid-induced osteoporosis.
Conflict of interest
P Hadji received honoraria, travel grants and scientific research grants from Amgen, Eli Lilly, MSD, Novartis, Pfizer, Procter & Gamble and Roche. D Kendler received honoraria, speakers bureau, and/or research grants from Amgen, Eli Lilly, MSD, Pfizer, AstraZeneca and Astellas. T de Villiers has acted as a consultant or speaker for the following companies: Abbott, Amgen, Aspen, MSD, Pfizer. R Rizzoli received fees for consultancy or lectures from Amgen, Danone, EffRx, Nestlé, ObsEva and Radius Health. PR Ebeling received research grants, honoraria and/or speakers’ fees from Amgen, Eli-Lilly, Novartis and Gilead. J Cannata-Andia received honoraria, and scientific research grants from ISCIII; PI19/00532, PI20/00633, PI20/00753), ISCIII REDinREN (RD06/0016/1013, RD12/0021/0023 and RD16/0009/0017), Fondo Europeo de Desarrollo Regional (FEDER), Plan Estatal de I+D+I 2013-2016, Plan de Ciencia, Tecnología e Innovación 2018-2022 del Principado de Asturias (GRUPIN14-028, IDI-2018-000152), University of Oviedo and Ministerio de Ciencia, Innovación y Universidade and from Amgen, Shire and VIFOR/Fresenius.
References
- 1.Kendler D.L., Body J.J., Brandi M.L. Bone management in hematologic stem cell transplant recipients. Osteoporos. Int. 2018;29:2597–2610. doi: 10.1007/s00198-018-4669-4. [DOI] [PubMed] [Google Scholar]
- 2.McClune B.L., Majhail N.S. Osteoporosis after stem cell transplantation. Curr. Osteoporos. Rep. 2013;11:305–310. doi: 10.1007/s11914-013-0180-1. [DOI] [PubMed] [Google Scholar]
- 3.Joseph R.W., Alousi A., Konda B. High incidence of vitamin D deficiency in patients undergoing allogeneic stem cell transplantation. Am. J. Hematol. 2011;86:954–956. doi: 10.1002/ajh.22143. [DOI] [PubMed] [Google Scholar]
- 4.Massenkeil G., Fiene C., Rosen O., Michael R., Reisinger W., Arnold R. Loss of bone mass and vitamin D deficiency after hematopoietic stem cell transplantation: standard prophylactic measures fail to prevent osteoporosis. Leukemia. 2001;15:1701–1705. doi: 10.1038/sj.leu.2402264. [DOI] [PubMed] [Google Scholar]
- 5.Sproat L., Bolwell B., Rybicki L., Dean R., Sobecks R., Pohlman B., Andresen S., Sweetenham J., Copelan E., Kalaycio M. Vitamin D level after allogeneic hematopoietic stem cell transplant. Biol Blood Marrow Transplant. 2011;17:1079–1083. doi: 10.1016/j.bbmt.2010.12.704. [DOI] [PubMed] [Google Scholar]
- 6.Hautmann S.H., Elad S., Lawitschka A., et al. Metabolic bone diseases in patients after allogeneic hematopoietic stem cell transplantation: report from the Consensus Conference on Clinical Practice in chronic graft-versus-host disease. Transpl. Int. 24:2011;867–879. [DOI] [PubMed]
- 7.McClune B.L., Polgreen L.E., Burmeister L.A., Blaes A.H., Mulrooney D.A., Burns L.J., Majhail N.S. Screening, prevention and management of osteoporosis and bone loss in adult and pediatric hematopoietic cell transplant recipients. Bone Marrow Transplant. 2011;46:1–9. doi: 10.1038/bmt.2010.198. [DOI] [PubMed] [Google Scholar]
- 8.Rizzo J.D., Wingard J.R., Tichelli A., Lee S.J., Van Lint M.T., Burns L.J., Davies S.M., Ferrara J.L.M., Socié G. Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation: joint recommendations of the European Group for Blood and Marrow Transplantation, Center for International Blood and Marrow Transplant Research, and the American Society for Blood and Marrow Transplantation (EBMT/CIBMTR/ASBMT) Bone Marrow Transplant. 2006;37:249–261. doi: 10.1038/sj.bmt.1705243. [DOI] [PubMed] [Google Scholar]
- 9.Tauchmanova L., Colao A., Lombardi G., Rotoli B., Selleri C. Bone loss and its management in long-term survivors from allogeneic stem cell transplantation. J. Clin. Endocrinol. Metab. 2007;92:4536–4545. doi: 10.1210/jc.2006-2870. [DOI] [PubMed] [Google Scholar]
- 10.Wiskemann J., Kuehl R., Dreger P., Schwerdtfeger R., Huber G., Ulrich C.M., Jaeger D., Bohus M. Efficacy of exercise training in SCT patients–who benefits most? Bone Marrow Transplant. 2014;49:443–448. doi: 10.1038/bmt.2013.194. [DOI] [PubMed] [Google Scholar]
- 11.Hadji P., Kyvernitakis I., Kann P.H. GRAND-4: the German retrospective analysis of long-term persistence in women with osteoporosis treated with bisphosphonates or denosumab. Osteoporos. Int. 2016;27:2967–2978. doi: 10.1007/s00198-016-3623-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chae Y.S., Kim J.G., Moon J.H., Kim S.N., Lee S.J., Kim Y.J., Sohn S.K. Pilot study on the use of zoledronic acid to prevent bone loss in allo-SCT recipients. Bone Marrow Transplant. 2009;44:35–41. doi: 10.1038/bmt.2008.414. [DOI] [PubMed] [Google Scholar]
- 13.Grigg A.P., Shuttleworth P., Reynolds J., Schwarer A.P., Szer J., Bradstock K., Hui C., Herrmann R., Ebeling P.R. Pamidronate reduces bone loss after allogeneic stem cell transplantation. J. Clin. Endocrinol. Metab. 2006;91:3835–3843. doi: 10.1210/jc.2006-0684. [DOI] [PubMed] [Google Scholar]
- 14.Tauchmanovà L., De Simone G., Musella T., Orio F., Ricci P., Nappi C., Lombardi G., Colao A., Rotoli B., Selleri C. Effects of various antireabsorptive treatments on bone mineral density in hypogonadal young women after allogeneic stem cell transplantation. Bone Marrow Transplant. 2006;37:81–88. doi: 10.1038/sj.bmt.1705196. [DOI] [PubMed] [Google Scholar]
- 15.Tauchmanova L., Ricci P., Serio B., Lombardi G., Colao A., Rotoli B., Selleri C. Short-term zoledronic acid treatment increases bone mineral density and marrow clonogenic fibroblast progenitors after allogeneic stem cell transplantation. J. Clin. Endocrinol. Metab. 2005;90:627–634. doi: 10.1210/jc.2004-0509. [DOI] [PubMed] [Google Scholar]
- 16.Pundole X., Cheema H.I., Sanchez-Petitto G., Lopez-Olivo M.A., Suarez-Almazor M.E., Lu H. Prevention and treatment of bone loss and fractures in patients undergoing a hematopoietic stem cell transplant: a systematic review and meta-analysis. Bone Marrow Transplant. 2017;52:663–670. doi: 10.1038/bmt.2016.312. [DOI] [PubMed] [Google Scholar]
- 17.Johansson H., Siggeirsdóttir K., Harvey N.C., Odén A., Gudnason V., McCloskey E., Sigurdsson G., Kanis J.A. Imminent risk of fracture after fracture. Osteoporos. Int. 2017;28:775–780. doi: 10.1007/s00198-016-3868-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mucowski S.J., Mack W.J., Shoupe D., Kono N., Paulson R., Hodis H.N. Effect of prior oophorectomy on changes in bone mineral density and carotid artery intima-media thickness in postmenopausal women. Fertil. Steril. 2014;101:1117–1122. doi: 10.1016/j.fertnstert.2013.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castelo-Branco C., Pons F., Martinez de Osaba M.J., Garrido J., Fortuny A. Menstrual history as a determinant of current bone density in young hirsute women. Metabolism. 1996;45:515–518. doi: 10.1016/s0026-0495(96)90229-2. [DOI] [PubMed] [Google Scholar]
- 20.Kananen K., Volin L., Laitinen K., Alfthan H., Ruutu T., Välimäki M.J. Prevention of bone loss after allogeneic stem cell transplantation by calcium, vitamin D, and sex hormone replacement with or without pamidronate. J. Clin. Endocrinol. Metab. 2005;90:3877–3885. doi: 10.1210/jc.2004-2161. [DOI] [PubMed] [Google Scholar]
- 21.Gnant M., Pfeiler G., Dubsky P.C. Adjuvant denosumab in breast cancer (ABCSG-18): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet. 2015;386:433–443. doi: 10.1016/S0140-6736(15)60995-3. [DOI] [PubMed] [Google Scholar]
- 22.Saag K.G., Wagman R.B., Geusens P., Adachi J.D., Messina O.D., Emkey R., Chapurlat R., Wang A., Pannacciulli N., Lems W.F. Denosumab versus risedronate in glucocorticoid-induced osteoporosis: a multicentre, randomised, double-blind, active-controlled, double-dummy, non-inferiority study. Lancet Diabetes Endocrinol. 2018;6:445–454. doi: 10.1016/S2213-8587(18)30075-5. [DOI] [PubMed] [Google Scholar]
- 23.Subbiah V., Madsen V.S., Raymond A.K., Benjamin R.S., Ludwig J.A. Of mice and men: divergent risks of teriparatide-induced osteosarcoma. Osteoporos. Int. 2010;21:1041–1045. doi: 10.1007/s00198-009-1004-0. [DOI] [PubMed] [Google Scholar]
- 24.Boonen S., Sellmeyer D.E., Lippuner K., Orlov-Morozov A., Abrams K., Mesenbrink P., Eriksen E.F., Miller P.D. Renal safety of annual zoledronic acid infusions in osteoporotic postmenopausal women. Kidney Int. 2008;74:641–648. doi: 10.1038/ki.2008.193. [DOI] [PubMed] [Google Scholar]
- 25.Miller P.D. The kidney and bisphosphonates. Bone. 2011;49:77–81. doi: 10.1016/j.bone.2010.12.024. [DOI] [PubMed] [Google Scholar]
- 26.Gertz M.A., Dingli D. How we manage autologous stem cell transplantation for patients with multiple myeloma. Blood. 2014;124:882–890. doi: 10.1182/blood-2014-03-544759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moreau P., San Miguel J., Sonneveld P. Multiple myeloma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017;28:iv52–iv61. doi: 10.1093/annonc/mdx096. [DOI] [PubMed] [Google Scholar]