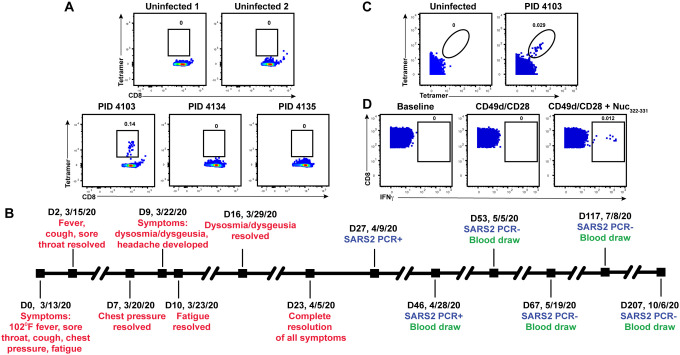
Figure 1. Identification and description of case study PID4103 with immunodominant CD8+ T cell response against Nuc331–331.
(A) A distinct population of Nuc322–331-specific CD8+ T cells is detected by FACS tetramer staining in convalescent donor PID4103. Top: PBMCs from uninfected individuals were analyzed by FACS for binding to the HLA-B*40:01/Nuc322–331 tetramer. Results are representative of 6 independent uninfected donors. Bottom: PBMCs from convalescent COVID-19 individuals from the CHIRP cohort were analyzed by FACS for binding to the HLA-B*40:01/Nuc322–331 tetramer. Participant PID4103, but not participants PID4134 and PID 4135, harbors cells binding to the tetramer. Numbers correspond to the percentage of cells within the gates. Results are gated on live, singlet CD3+CD8+ cells.
(B) Timeline of clinical course of PID4103’s SARS-CoV-2 infection and sampling. Red indicates the dates of specific symptom initiation and resolution, blue the dates and results of SARS-CoV-2 PCR tests, and green the dates of blood draws.
(C) A distinct population of Nuc322–331-specific CD8+ T cells is detected by CyTOF in PID4103 through dual tetramer staining. PBMCs from one uninfected individual and from PID4103 were stained with two sets of HLA-B*40:01/Nuc322–331 tetramers conjugated to different metal lanthalides, facilitating specific detection of Nuc322–331-specific CD8+ T cells. Numbers correspond to the percentage of cells within the gates. Results are gated on live, singlet CD3+CD8+ cells.
(D) Nuc322–331 specific CD8+ T cells can be stimulated by the Nuc322–331 peptide. PBMCs from PID4103 were phenotyped by CyTOF at baseline, or following 4 hours of co-stimulation with αCD49d/CD28 antibody in the absence or presence of the Nuc322–331 peptide. Stimulations were conducted in the presence of brefeldin A to enable the detection of IFNγ. Numbers correspond to the percentage of cells within the gates. Results are gated on live, singlet CD3+CD8+ cells.