Abstract
Regulatory authorities have devoted increasing attention and resources to a range of issues surrounding the regulation of novel nicotine and tobacco products. This review highlights the inherent complexity of evaluating prospective policies that pertain to products that heat solutions containing nicotine, but not tobacco leaf, sometimes referred to as electronic nicotine delivery systems (ENDS). The US Food and Drug Administration (FDA) is compelled to incorporate a set of public health criteria in their decision making, collectively referred to as the Population Health Standard. Adherence to this standard is necessary to estimate the impact of prospective ENDS policy decisions on net population harm associated with nontherapeutic nicotine products. For policies that are expected to decrease or increase ENDS use, application of the Population Health Standard requires a comprehensive assessment of the status quo impact of ENDS use on population health. Accordingly, this review first assesses the state of the evidence on the direct harms of ENDS and the indirect effects of ENDS use on smoking, particularly rates of initiation and cessation. After that, the example of flavor restrictions is used to demonstrate the further considerations that are involved in applying the Population Health Standard to a prospective ENDS policy.
Implications: This narrative review aims to inform regulatory considerations about ENDS through the prism of the Population Health Standard. More specifically, this review (1) describes and explains the importance of this approach; (2) provides guidance on evaluating the state of the evidence linking ENDS to the net population harm associated with nontherapeutic nicotine products; and (3) illustrates how this framework can inform policymaking using the example of flavor restrictions.
The Population Health Standard
Regulatory authorities have devoted increasing attention and resources to a range of issues surrounding the regulation of novel nicotine and tobacco products. In the United States, Congress has delegated authority over all tobacco products that do not meet the definition of a drug or medical device to the Food and Drug Administration (FDA) with the passage of the Family Smoking Prevention and Tobacco Control Act (hereafter “Tobacco Control Act”) in 2009. This review pertains to products that heat solutions containing nicotine, but not tobacco leaf, sometimes referred to as electronic nicotine delivery systems (ENDS) or vaping products. Other novel products such as dissolvable oral tobacco products or heated tobacco products are beyond the scope of this review, as are more established products such as snus, although some of the broader concepts addressed here may extend to these products as well.
The FDA formally deemed ENDS to be included in the tobacco products regulated by the Tobacco Control Act in 2016. The Tobacco Control Act repeatedly references a set of criteria related to population health collectively referred to as the “Population Health Standard.” These criteria include consideration of the following:
“the risks and benefits to the population as a whole, including users and nonusers”;
“the increased or decreased likelihood that existing users of tobacco products will stop using such products”; and
“the increased or decreased likelihood that those who do not use tobacco products will start using such products.” 1
The Tobacco Control Act requires that the Population Health Standard be considered in reference to nearly all significant regulatory actions related to tobacco products, including restrictions on sale, distribution, access, and advertising [section 906(d)(1)]; promulgation of new tobacco products standards [section 907(a)(3)(B)(I)]; marketing authorization decisions on Pre-Market Tobacco Applications for new products [section 910(c)(4)]; and marketing authorization decisions for “Reduced Risk” [section 911(g)(1)] or “Reduced Exposure” [section 911(g)(2)(B)(iv)] tobacco product applications [section 911(g)(4)].
In addition to the ubiquity of these standards in the text of the Tobacco Control Act, the criteria outlined in the Population Health Standard correspond to the key parameters of population models that produce quantitative estimates on population impact.2–8 These criteria are also in alignment with the reasoning of scholarly work that addresses the net impact of ENDS on population health.8–10 The core rationale for the Population Health Standard is to ensure that regulatory decisions for tobacco and nicotine products are not made without comprehensive consideration of their potential and likely consequences. Public health groups pushed for these criteria to be written into the Tobacco Control Act due in part to an understanding of the damage that had accrued during past policymaking processes that had lacked this broader view.11,12 Although Congress wrote these criteria into the Tobacco Control Act for the explicit purpose of guiding FDA decisions on tobacco and nicotine, the rationale for these criteria is no less applicable to tobacco and nicotine policy decisions made by other governmental bodies at the state and local level or abroad. In addition, there is a growing recognition that FDA should put increased emphasis on population health considerations into their drug and device decision making.13
We focus on three core ways that ENDS use—and, thus, policies expected to reduce or increase ENDS use—might affect disease burden at the population level. These map to the key inputs used in population models that attempt to assess the net population health impact of ENDS.2–8
(1) Direct harms associated with ENDS use among nonsmokers, especially youth never smokers and adult former smokers. This would include harms associated with nicotine, metals, solvents, flavoring chemicals, contaminants, or other potentially hazardous chemicals—apart from any impact on subsequent smoking initiation or progression ([2] below).
(2) Indirect effects on the rate of smoking initiation or progression among nonsmokers, particularly youth and young adults.
(3) Indirect effects on the rate of smoking cessation, including complete switching from combustible tobacco use to ENDS-only use.
Levy et al.9 elaborate on causal pathways not emphasized above, including prolonged dual use among individuals who otherwise would have only smoked, direct harms on all never smokers including adults, and indirect effects on the rate of reinitiation of smoking among former smokers.
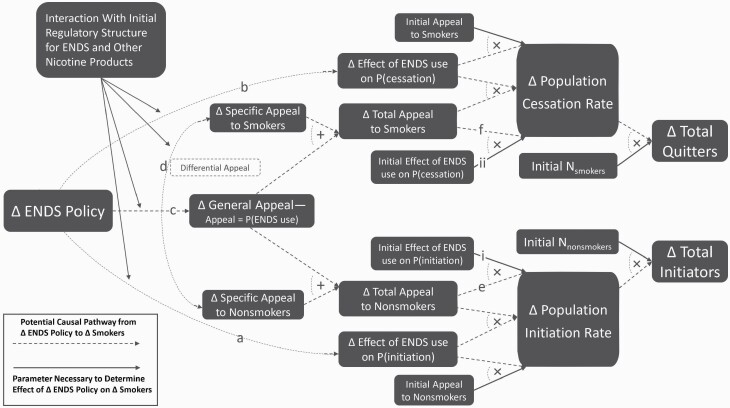
The indirect effects of ENDS use on smoking are described in terms of the effect of ENDS use on rates of initiation and cessation to account for how ENDS policy can affect smoking prevalence through changes in the prevalence of ENDS use among nonsmokers and smokers respectively (Figure 1). This is in addition to ways in which ENDS policy can affect the average probability that an individual ENDS user initiates or quits smoking. The core dilemma for policymakers is that situations often arise where it is difficult or impossible to design a policy that will affect ENDS prevalence among nonsmokers without similarly affecting smokers, and vice versa. As a result, policies that broadly affect the appeal, availability, or cost of ENDS and that cannot be easily tailored to affect only specific subpopulations to the exclusion of others—examples include premarket review decisions, excise taxes, nicotine levels, and flavor restrictions—are highly likely to cause unintended consequences. In such cases, it is especially important to adhere to a decision-making process that is grounded in the Population Health Standard criteria. Many causal mechanisms that might link such policies to disease rates are best described as amplifying or dampening the status quo harms and benefits of ENDS. Consequently, a general assessment of these initial harms and benefits—especially (1)–(3) above—is needed to evaluate the advisability of any such policy.
Figure 1.
Relating change in ENDS policy to change in population-level smoking prevalence.
Direct Harms Associated With ENDS Use Among Nonsmokers
Assessing direct harms is necessary both to understand the absolute risks of ENDS use and to estimate the relative risks of ENDS compared with cigarette smoking. Exclusive use of ENDS exposes users to substantially lower exposures to many toxins known to contribute to the health risks of smoking.8 This reduction is visible both in comparisons of ENDS aerosol to cigarette smoke and in comparisons of the metabolites of these toxins in the urine of vapers and smokers.14 These exposure reductions suggest the promise of ENDS as a reduced-harm alternative to smoking. However, ENDS still present a set of established risks that are nontrivial—though they could potentially be minimized through regulation—as well as potential risks that could plausibly turn out to be quite severe.
First, ENDS deliver substantial doses of nicotine, which is widely recognized as addictive to users.8,15 Long-term harms from nicotine use have not been definitively established but cannot be ruled out. Animal studies have raised concerns about the possible impact of nicotine on the developing adolescent brain, and short-term effects of nicotine on heart rate and blood vessels has raised concerns about whether there might be longer term effects on cardiovascular health.8 Pod systems like JUUL and similar products deliver substantially higher levels of nicotine per puff compared with earlier ENDS products.16 The speed of delivery to the bloodstream and the relative physical tolerability of vaping compared with smoking may engender higher blood nicotine levels and greater symptoms of nicotine dependence.14,17 This could entail substantial nicotine-associated health risks among high-intensity vapers—the intensity/harm gradient of nicotine is not well established.
Second, researchers have identified several hazardous or potentially hazardous chemicals in ENDS flavoring or emissions from ENDS flavoring being heated and/or mixed with other e-liquid components. Cinnamaldehyde may present a particularly acute threat,18 and vanillin and pentanedione may also be especially harmful.18 There appear to be far greater hazards associated with some flavors than with others.19 Studies have shown the potential for wide variance in aldehydes in general,20 diacetyl, and acetyl propionyl21 across flavors. Notably, the substantial variance in free radicals across flavors bears seemingly no relationship to the actual user experience (eg, different “vanilla” flavors show up at the most and least harmful ends of the spectrum).22
Third, the interaction of ENDS aerosol with the highly sensitive and reactive tissues in the lungs and airways may entail substantial respiratory harm even at relatively low levels of exposure to toxins. ENDS use has been associated with irritation to the respiratory system,8 which might be caused by the nicotine itself, solvents such as propylene glycol or vegetable glycerin, flavoring chemicals, contaminants, or some combination.
Fourth, black market products or legal products with insufficient safety regulations may expose users to harmful contaminants or dangerous device malfunctions. Poisonings and accidental injuries have been an issue from the beginning,23 and the recent epidemic of e-cigarette or vaping product use-associated lung injury—which has been linked to illicit tetrahydrocannabinol cartridges diluted with vitamin-E acetate24—demonstrates the dangers associated with black market supply chains. E-cigarette or vaping product use-associated lung injury has not been directly connected to nicotine vaping, but this episode serves to highlight both the harms that contaminants can cause given the sensitivity of lung tissues and the relationship between the effectiveness of regulatory oversight and the level of danger that ENDS users are likely to encounter.
Lastly, it is also important to consider whether the direct harms might differ across subgroups. ENDS use has become more prevalent among youth and young adults than older adults,25–27 which means policymakers need to specifically consider whether there might be differential risks faced by these subpopulations. The marginal harm associated with the acquisition of an addiction to nicotine among never users of tobacco should be considered greater than the marginal harm associated with the perpetuation of nicotine addiction among former smokers who have completely switched to ENDS. Youth and young adults may also be more likely to encounter counterfeit products and their associated risks due to their higher likelihood of obtaining products through informal or social sources.28 Regulatory actions restricting product availability could exacerbate this problem.
The challenge for public health authorities is that the true severity of these risks will not be known for many years. There are clear opportunities for reducing harms associated with established risks. For risks that are plausible but unproven, regulators must make estimates and assumptions pending further research.
Indirect Effects on the Rate of Smoking Initiation or Progression Among Nonsmokers
The Population Health Standard requires consideration of a potential causal “gateway” role, which, if realized, would be very damaging to net population health because the hazards of smoking are so severe. The dramatic increase in ENDS initiation among youth,25,26 coupled with evidence that ENDS use is associated with subsequent initiation of cigarette smoking among nonsmokers,8 challenges researchers to carefully consider the underlying causal relationships between vaping and smoking in the youth and young adult populations.
Assessing potential gateway effects is challenging because it is difficult to identify an unbiased estimate of the actual causal effect of ENDS use on smoking initiation. Specifically, it is difficult to distinguish correlations between ENDS use and subsequent smoking that result from posited causal mechanisms,9,29 and those that result from “common-liability” factors that could affect both ENDS experimentation and smoking experimentation.30,31 These include individual-level factors related to curiosity, susceptibility, and exposure to marketing; family and peer factors including the number of smokers in an individual’s household and among their peer group; school-level factors related to social norms and surrounding retailer density; 32 and policy factors at the municipal, state, and national levels. Surveys often attempt to capture some of these factors, but many cannot be precisely measured, some change rapidly over time, and few, if any, data sets are rich enough to include all variables known to affect smoking initiation. ENDS use may be acting as a “signal” for some individuals if it correlates with unmeasured risk factors, making it easier to identify those who are at greatest risk for subsequent smoking but not independently increasing the risk that it reveals.
Given the obstacles to implementing experimental designs to examine the impact of ENDS use on youth populations, observational studies must be relied upon. One such approach involves cohort studies that look at whether ENDS users are more likely than nonusers to initiate smoking. Advantages of this approach include the ability to characterize the exposure and outcomes in detail and greater statistical power due to the direct focus on ENDS use and smoking. The disadvantage is the likelihood of biased estimates due to omitted variables that correlate with both pre-existing smoking propensity and the decision or opportunity to use ENDS.
Another approach is to conduct econometric studies that attempt to exploit exogenous (ie, unconfounded) variations in policies, prices, or other factors that can be expected to affect propensity to use ENDS but not directly affect smoking behaviors. The advantage of econometric studies is that they are explicitly designed to identify causal effects, typically estimating an association that can only be explained by a causal effect given a set of underlying assumptions. One disadvantage of this approach is that exposure is assessed indirectly. This makes it more difficult to assess precisely the magnitude of the relationship between ENDS use and smoking. Perhaps most importantly, the assumptions necessary for causal inference may not hold (ie, variables posited as being exogenous may not be), leading to biased estimates. For example, state and municipal legislative actions toward ENDS might be reflective of electorates with certain views and attitudes toward all nicotine products, including cigarettes.
The results of cohort studies have consistently shown that nonsmoking youth who use ENDS are more likely to initiate tobacco smoking than nonsmoking youth who do not.8,33 The robustness of these results across settings was considered to be “substantial” evidence for causality in the National Academies of Sciences, Engineering, and Medicine (NASEM) report.8 The research cited by NASEM firmly establishes the plausibility of a causal gateway, noting the variety of geographic and age settings that show a correlation between ENDS use and subsequent smoking among youth and young adults. Other reviews examining the same cohort-study evidence have not concluded that a causal link has been established using causal criteria that are more conservative and consistent with the criteria applied to studies of cessation impact in the NASEM report.30 Causal reasoning through correlations found in cohort studies requires the refutation of alternative noncausal explanations, especially “common-liability” factors, which can otherwise explain why ENDS use would be associated with subsequent smoking.31
There are several reasons why common-liability explanations cannot be easily dismissed. First, consistently observed associations between ENDS use and smoking susceptibility34–37 cast doubt on included studies that do not rigorously measure this at baseline. Second, measured associations between ENDS use and subsequent smoking consistently tend to shrink when previously unmeasured covariates are included.35 A study analyzing data from the Population Assessment of Tobacco and Health (PATH) survey, which includes the most rigorous set of confounders, showed that controlling for baseline marijuana use or smoking susceptibility reduces the observed association below statistical significance38 (see Supplementary Tables). Third, many empirical associations have been observed where causal explanations are relatively less likely compared to common-liability explanations, such as brief experimental ENDS use, and past but not current ENDS use at baseline.34,38,39 In sum, the cohort literature clearly establishes an association between ENDS use and subsequent smoking initiation, but we cannot infer that this entails a causal gateway without being able to rule out common-liability explanations.
Several econometric studies have attempted to identify a causal effect of ENDS use on smoking initiation by conducting “difference in differences” studies that utilize the exogenous policy variation in minimum age laws for ENDS across states. Most, with one exception40 have found that minimum age laws for ENDS lead to more smoking among youth41–44—the opposite of a causal gateway. However, one rigorous analysis instead found that minimum age laws for ENDS reduced youth smoking.40 It is not immediately clear what explains this discrepancy, and so it is difficult to draw firm conclusions from the existing econometric literature.
A related body of literature closely examines the trend in youth smoking prevalence before and after the emergence of ENDS. Levy et al.45 found that the downward trend in youth smoking prevalence accelerated during the period when youth vaping prevalence increased from 2014 to 2017, a result that is more consistent with a diversion effect than a gateway effect. Hammond et al.46 report a similar decrease in smoking more recently among youths aged 16–19 in the United States and Canada as vaping has spiked in each country. The causal meaning of these ecological trends is more speculative than that of econometric studies. Changes in prevalence result from a mix of factors including cohort replacement, changes in initiation rate, and changes in cessation rate that cannot be easily disentangled from one another. In addition, youth prevalence trends might primarily result from contemporaneous changes in the policy or cultural environment that are unrelated to ENDS. One important causal implication of these trends is that they are not consistent with the presence of a large causal gateway effect. If they continue, further consideration will need to be given to the prospect that the net impact of vaping may be to decrease smoking initiation.
The causal evidence on the gateway effect is weak overall, with no definitive answer as to whether it exists or not. If there is one, it is yet to produce a spike in smoking initiation, which suggests that the upper bound on what the net gateway effect could be is relatively low.
Indirect Effects on the Rate of Smoking Cessation
The Population Health Standard requires considering the impact of ENDS policymaking on smoking cessation. There are at least three distinct ways in which ENDS availability and use in general might have a causal impact on smoking cessation: elicitation, appeal, and efficacy. Elicitation entails increased odds of attempting to quit. Appeal entails a greater likelihood that smokers will use ENDS for cessation, which will increase population-level cessation rates if efficacy is above zero. Efficacy entails that ENDS increase the odds of cessation among smokers who use them for this purpose.
The potential for elicitation of quit attempts47 is a relatively distinct feature of ENDS in comparison to conventional cessation aids and approaches. Although consistent with population data that show increases in quit attempts in recent years,48,49 it is very difficult to determine the true effect of ENDS on quit attempts because there is a key source of bias—reverse causality—that is applicable to almost any cohort study that attempts to address this issue. It is difficult to distinguish instances of elicited quit attempts among smokers who would not otherwise have attempted to quit from instances of quit attempts that would have occurred anyway for which ENDS are used as a quit method.
Even though ENDS cannot lawfully be marketed for this cessation, evidence from studies that analyze PATH data from waves 1 and 2 have found that ENDS are an appealing method of smoking cessation compared with other quit aids such as conventional nicotine replacement or prescription medications.50,51 Increased use of ENDS as a quit method has been outpacing displacement of nicotine replacement therapy.52,53 This suggests that ENDS might be appealing more to a different set of smokers than do conventional nicotine replacement therapy products and increasing the overall share of quit attempts that involve quit aids.
A growing body of research has sought to determine whether ENDS are efficacious for smoking cessation. Randomized controlled trials (RCTs) of first-generation ENDS suggested cessation efficacy at or above that of conventional nicotine replacement therapies,54–57 but these estimates were imprecise. Two major RCTs have been published since the publication of most other meta-analyses and reviews including the NASEM report.58,59 The more powerful of these, Hajek et al.,58 was the first RCT to find a significantly higher cessation rate for a group receiving free tank-style ENDS than one receiving free nicotine replacement therapy. Both products were distributed in conjunction with smoking cessation counseling, and ENDS users were taught how to use the product. The majority of participants in the ENDS arm who successfully quit were still using ENDS at follow-up. In Halpern et al.,59 the only group to receive “free e-cigarettes” and no other aids or incentives had a higher cessation rate than did the only group that did not receive any free ENDS (the “usual care” group). The raw p-value, appropriate for researchers aiming to evaluate the efficacy of ENDS because only this single comparison could be used to assess this, was below the threshold of statistical significance (p = .040) for the primary ITT analysis. The effect size was very small due to poor compliance. The authors’ secondary analysis, which nonrandomly excluded participants that did not sign into the study web portal after treatment assignment, yielded a larger effect size but a higher raw p-value (p = .067).59
RCTs have inherent limitations in terms of generalizability. Real-world users will not be provided with free products, are rarely given individual instructions for use, and trials typically use products that contain outdated technology. The definition of compliance with the protocol could have a substantial impact on the interpretation of the trial outcome in real-world applications. For example, are subjects assigned to an ENDS arm or an RCT who do not follow the protocol and reject ENDS use altogether considered a failure of the product’s efficacy, an excluded participant because of noncompliance, or are they moved to the control group?
Econometric observational studies that are designed to identify causal effects have found effects consistent with product substitution.60–64 Cross-price elasticities have proven difficult to measure because ENDS sales can be difficult to track due to the preponderance of online and specialty store sales as well as the frequent introduction of novel product types. Generally, they have been shown to be positive, indicating substitution, when results are statistically significant.62,65–67 Similarly, higher e-cigarette prices have been shown to lead to higher monthly cigarette consumption among youth.64 Most of these results do not allow for the contribution of smoking reduction to be disentangled from that of smoking cessation.
Observing the ecological trends in population smoking behaviors allows for a more precise focus on metrics of cessation, although the causal interpretation of these trends is far less straightforward. Following an increase in adult vaping in 2013,68 quit attempt rates and cessation rates increased during the period between 2014–2016 compared with previous years48,49 paralleling a similar finding in England.69 The elevated quit attempt and cessation rates were concentrated among smokers who reported past year48 or current49 ENDS use. Rates for nonusers of ENDS remained near the pre-2014 baseline. However, these trends do not imply a causal effect of increased vaping rates. Several contextual factors likely played roles in increased attempt rates. For example, mass media antismoking efforts such as the CDC “Tips” campaign aimed at adults and the FDA “Real Cost” campaign aimed at youth were on the air for much of this period. Moreover, smokers who were more likely to make a quit attempt or succeed at quitting might also have been more likely to initiate and sustain ENDS use. Further studies that can provides more recent data on quit attempt rates and cessation rates with more granular age subgroups would be welcome, especially considering the large increase in young adult vaping after 2016.
Observational cohort studies have shown wide-ranging results, due in part to inconsistent study populations, inclusion restrictions, and various aspects of study quality.70 Additionally, Villanti et al. emphasize that “non-randomized studies must be scrutinized with respect to the appropriateness of comparison groups used and assessment of the most plausible confounders relevant to the question at hand (ie, beyond demographics) to rule out other likely sources of bias.” 70 Unlike cohort studies of nonsmokers focused on initiation where one specific type of bias is nearly always applicable—unmeasured or unobservable common-liability factors—cohort studies of cessation may be biased in various ways.
Observational cohort studies that compare cessation rates among smokers who initiate ENDS use after baseline and those who do not71,72 will be biased toward a finding of greater cessation rates for ENDS users because of the likelihood that ENDS use selects for time-varying quit intention that cannot be controlled at baseline, similar to what would be expected if we compared smokers who initiated nicotine replacement therapy or other cessation aids after baseline with those who did not.
Several other design approaches would be biased toward a finding of reduced cessation. Studies that compare ever or dual users at baseline with never or nonusers of ENDS at baseline73–77 will be biased toward a finding of reduced cessation rates for ENDS users because ENDS users who quit before baseline will be excluded from the study entirely; filtering out these quitters will overpopulate the sample of included ENDS users with quit failures. Studies that focus only on success rates among quit attempters will be biased if they do not measure whether ENDS are being used as a quit method; otherwise, ENDS use may signal for lack of success using other methods. Similarly, studies that compare the cessation or success rates of ENDS users against nonusers in a clinical setting, especially as part of a secondary analysis of a smoking cessation trial designed to test the impact of a different intervention,78,79 will be biased toward a finding of reduced cessation because of the likelihood that ENDS use reveals relative dissatisfaction with the recommended intervention.
This list of likely and potential biases is far from exhaustive, and it is doubtful that any of these biases can be fully addressed through the inclusion of control variables at baseline because the selection mechanisms generating these biases are either unobservable or would occur after baseline. Given these limitations, there are still some consistent patterns that can be observed across the cohort-study literature. Both prospective71,72,80–83 and retrospective47,51,84 cohort studies show that some measures of ENDS use—for example, intense use, daily use, and use of tank-style products—are much more likely to be associated with increased cessation, whereas others—for example, intermittent use, nondaily use, and use of disposable products—are much more likely to be associated with reduced cessation. This includes a recent prospective study using PATH data.71 Another recent cohort study that uses PATH data compares success rates among quit attempters across quit methods, finding that smokers who used ENDS as a quit method were more likely to quit smoking (with many continuing to use ENDS) than smokers who did not.50 Although focusing on success rates among attempters using ENDS as a quit method manages to avoid the biases mentioned previously, it is still possible that these results are influenced by unmeasured confounders.
Giving priority to randomized studies, as others have,55,70 suggests that ENDS may increase a smokers’ odds of quitting. Unlike the previous section, the evidence base for cessation allows for a more straightforward causal interpretation due to its foundation in controlled experiments. Agreement between these experiments and the higher quality observational studies that lack clear threats to internal validity further supports this inference. The primary utility of the remaining studies, which give mixed answers about the efficacy of ENDS, are that they suggest when and how ENDS might lead to cessation and what mediating or moderating factors should be considered.
Relating ENDS Policy to Indirect Harms and Benefits Associated With Smoking: The Case of Flavors
Projecting the indirect impact of an ENDS policy on tobacco cigarette smoking (Figure 1) requires an assessment of (1) how such a policy is likely to modify or amplify the status quo relationships between ENDS and smoking and (2) whether the impact of such a policy is likely to be asymmetrical across subpopulations. As an illustrative example, consider a policy that intends to restrict nontobacco ENDS flavors.
First, use of a nontobacco-flavored ENDS may have differential causal effects on smoking initiation probability (path “a” in Figure 1) or cessation efficacy (path “b”) in comparison to tobacco-flavored ENDS use. This is an emerging focus for tobacco control researchers, but there is not strong evidence of differential effects at present. For initiation, there are not yet any published studies that directly address this question. For cessation, prospective cohort studies of smokers using PATH data have found a significant correlation between use of nontobacco flavor,85 or flavors other than tobacco and menthol,82 and the probability of subsequent cessation. These associations may be driven disproportionately by young adult smokers (defined as 18–34 for Chen et al.),82 who are more likely to prefer flavors than older adults (defined as 30 and older in Harrell et al.).86
Second, ENDS flavors may amplify the impacts of the causal effects of ENDS on initiation probability or cessation efficacy by increasing the appeal of ENDS use. Evidence suggests that the availability of menthol and other nontobacco flavors is a major contributor to the appeal of ENDS to youths 86–90 as well as adult smokers.86,91,92 Increasing the appeal of ENDS in general (path “c”) simultaneously amplifies (through paths “e” and “f”) the population-level impact of any pre-existing individual-level effect of ENDS on the probability of initiation (parameter “i”) or cessation (parameter “ii”) by expanding the affected share nonsmokers and smokers respectively. This point is worth underscoring. If a causal gateway were to be established, then flavors would be expected to increase the population-level smoking initiation rate even if nontobacco-flavored products had the exact same effect as tobacco-flavored products on an individual’s transition to smoking (ie, even if path “a” were eliminated). This is correspondingly true of population-level cessation (ie, eliminating path “b”). Given the strong evidence connecting flavors to appeal, and the thin evidence that connects flavors to an individual’s probability of smoking initiation or cessation, this appeal-related amplification should be the primary consideration of how flavors affect the overall rates of smoking initiation and cessation at the population level.
As such, determining the advisability of a policy measure pertaining to ENDS flavors cannot be disentangled from an assessment of the status quo impact of ENDS on net population harm, especially an assessment of the status quo gateway and cessation effects (parameters “i” and “ii”). Population models can serve as a useful guide here. As one might expect, the outputs of these models generally reflect their inputs. Models showing very little cessation effect and a very strong gateway effect6 estimate that ENDS are a detriment to public health—and thus permissive ENDS policy is detrimental as well. Models that parametrize causal gateway and cessation effects at levels commensurate with the evaluations in the preceding sections3,5,7,8 estimate that ENDS are likely to reduce smoking rates and improve public health in their baseline scenarios. Notably, these estimates tend to be much more sensitive to changes in the cessation effect parameter than that of the gateway effect.7,8 If policymakers believe there to be a substantial cessation effect, then the gateway effect would need to be very large to yield a net loss in life-years from increased ENDS use, suggesting that the greater concern at this juncture is the potential for direct harms from ENDS.
A further, related consideration is whether there is a differential impact of flavors on appeal across subpopulations (path “d”). Nontobacco flavors appear to disproportionately interest nonsmokers, who are less interested in tobacco-flavored products than smokers.93 At the same time, the absolute number of current smokers and recent former smokers that use nontobacco-flavored ENDS is far greater than that of never smokers or long-term former smokers.93 Similarly, youth nicotine-containing ENDS prevalence is currently much higher than adult prevalence,26,27,94 and there is an even greater prevalence disparity for use of flavored ENDS because youth ENDS users are more likely to be using nontobacco-flavored liquid than adult users.86 However, because a larger absolute number of adults than youth report past 30-day ENDS use (over two and a half times as many in 201826,95), the absolute number of adults who use nontobacco-flavored ENDS or cite flavors as a reason why they use ENDS is a multiple of the absolute number of youth saying the same thing. Projecting the net impact of a policy to restrict or remove ENDS flavors requires several considerations beyond the general impact on the safety and appeal of ENDS, including the relative priority of the subpopulations of nonsmoking youth and smoking adults, the disproportionate appeal of nontobacco flavors to youth and nonsmokers, and the disproportionate reach of nontobacco-flavored ENDS into the populations of current smokers and recent former smokers.
Concluding Discussion
Applying the Population Health Standard to ENDS regulations requires policymakers to incorporate a comprehensive assessment of the direct and indirect effects of their decisions. Several policies relating to direct harms would be easy to justify by this standard, especially regulations to better protect ENDS users from unscrupulous suppliers and dangerous manufacturing practices. Restrictions on certain flavoring chemicals that pose excess risks are needed as well, as are standards for batteries and other potentially volatile or faulty device components.
Direct harms can be further reduced at the population level by reducing ENDS use among never smokers and youths. Presuming the NASEM report assessment that “current evidence points to e-cigarettes being less harmful than combustible tobacco cigarettes” 8 is correct, the risks of ENDS use are arguably acceptable for individuals seeking to quit smoking, but that does not extend to never smokers. Recently, policies aimed at restricting the sale of flavored ENDS have been proposed at the federal level, following similar policies that have been enacted or proposed in an increasing number of states and localities, all with the aim of responding to the rapid escalation in youth ENDS use. The relationship between flavors and appeal among youth suggests that a more restrictive policy on flavors is worthy of consideration.
Applying the Population Health Standard here—or to any other policy decision that would be expected to broadly affect the appeal, availability, or cost of ENDS—requires assessing indirect effects as well. An assessment that ENDS use is increasing the odds of smoking initiation among ENDS users would give more credence to a restrictive policy. A determination that ENDS use is increasing cessation rates among smokers would present a major drawback to a restrictive policy. In such cases, strong consideration must be given to more tailored policies, such as increasing the minimum age of sale to 21 (“Tobacco 21”),96,97 which can potentially reduce youth vaping with minimal spillover impact on adult smokers.
If tailored policies are insufficient, the Population Health Standard does not provide a simple roadmap for how to balance competing considerations. Population models can offer guidance, but by their nature, they cannot encompass the various nuances of a policy decision as it confronts a regulator or politician. An overly aggressive push toward a restrictive or permissive policy may engender so much opposition from either advocates for smokers seeking alternatives or advocates for protection of youth from harmful products that the entire endeavor gets blown off course. The issue of youth vaping has demonstrated such strong political salience in the United States that the scope for permissive ENDS regulation has been effectively narrowed at this time. An overly aggressive push against flavors might similarly engender strong salience among opponents that in turn narrows the scope for reducing the appeal of ENDS to youth. More than anything, the Population Health Standard requires careful deliberation on the part of policymakers because incautious policymaking is unlikely to be successful in any context.
Lastly, designing policies for ENDS must consider the wider policy environment for nicotine products, especially combustible tobacco products but also including less dangerous conventional nicotine replacement products. It is possible that restricting ENDS flavors will increase the odds of transitioning to smoking by creating a situation where nonsmoking ENDS users are much more likely to encounter tobacco flavoring, or else by encouraging dual use versus complete switching. Certainly, removing ENDS flavors including menthol while permitting menthol cigarettes to remain on the market would seem likely to shift some menthol ENDS users toward menthol cigarettes. Proliferation of flavored cigars would need to be addressed as well.98 The prospect of shifting ENDS users toward smoking could be reduced through the simultaneous introduction of new policies aimed at tobacco cigarettes, such as a menthol-flavoring ban99 or the reduction of nicotine to nonaddictive levels in cigarettes and other combustible tobacco products.100 ENDS policies should never be more restrictive than those for combustible tobacco or less restrictive than those for medicinal nicotine.
Supplementary Material
A Contributorship Form detailing each author’s specific involvement with this content, as well as any supplementary data, are available online at https://academic.oup.com/ntr.
Funding
Professor Berg’s research is supported by the following grants: National Cancer Institute (R01 CA215155-01A1; R01 CA179422-01; PI: Berg); Fogarty International Center (R01 TW010664-01; MPIs: Berg, Kegler). D.L.A. and M.P.E. were supported by the National Institute on Drug Abuse and the Food and Drug Administration (FDA) Center for Tobacco Products (CTP) (D.L.A. and M.P.E.: grant number P50DA036128; D.L.A.: R01DA047397).
Declaration of Interests
M.P.E. received unrestricted research funding support from Pfizer, Inc. (“Diffusion of Tobacco Control Fundamentals to Other Large Chinese Cities,” Michael P. Eriksen, Principal Investigator). D.L.A. has received funds for work done for the World Health Organization Tobacco Free Initiative, as a Special Government Employee of the US Food and Drug Administration, as a consultant for Pfizer, and as an independent contractor for McKing Consulting. Any views expressed here are those of the authors and do not necessarily represent those of the American Cancer Society or the American Cancer Society—Cancer Action Network, the National Institutes of Health, or the Food and Drug Administration.
References
- 1. 111 P.L. 31; 123 Stat. 1776; 2009.
- 2. Kalkhoran S, Glantz SA. Modeling the health effects of expanding e-cigarette sales in the United States and United Kingdom: a Monte Carlo analysis. JAMA Intern Med. 2015;175(10):1671–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cherng ST, Tam J, Christine PJ, Meza R. Modeling the effects of e-cigarettes on smoking behavior: implications for future adult smoking prevalence. Epidemiology. 2016;27(6):819–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hill A, Camacho OM. A system dynamics modelling approach to assess the impact of launching a new nicotine product on population health outcomes. Regul Toxicol Pharmacol. 2017;86:265–278. [DOI] [PubMed] [Google Scholar]
- 5. Levy DT, Borland R, Villanti AC, et al. The application of a decision-theoretic model to estimate the public health impact of vaporized nicotine product initiation in the United States. Nicotine Tob Res. 2017;19(2):149–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Soneji SS, Sung HY, Primack BA, Pierce JP, Sargent JD. Quantifying population-level health benefits and harms of e-cigarette use in the United States. PLoS One. 2018;13(3):e0193328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Warner KE, Mendez D. E-cigarettes: comparing the possible risks of increasing smoking initiation with the potential benefits of increasing smoking cessation. Nicotine Tob Res. 2019;21(1):41–47. [DOI] [PubMed] [Google Scholar]
- 8. National Academies of Sciences, Engineering, and Medicine. 2018. Public Health Consequences of E-cigarettes. Washington, DC: The National Academies Press. doi: 10.17226/24952. [DOI] [PubMed] [Google Scholar]
- 9. Levy DT, Cummings KM, Villanti AC, et al. A framework for evaluating the public health impact of e-cigarettes and other vaporized nicotine products. Addiction. 2017;112(1):8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Drope J, Cahn Z, Kennedy R, et al. Key issues surrounding the health impacts of electronic nicotine delivery systems (ENDS) and other sources of nicotine. CA Cancer J Clin 2017;67(6):449–471. [DOI] [PubMed] [Google Scholar]
- 11. Banthin CN, Daynard RA. Room for two in tobacco control: limits on the preemptive scope of the proposed legislation granting FDA oversight of tobacco current issues in tobacco regulation, litigation, and policy – symposium – safer tobacco products: reducing harm or giving false hope. J Health Care Law Policy. 2008;11( 1):57–82. [Google Scholar]
- 12. Fairchild A, Colgrove J. Out of the ashes: the life, death, and rebirth of the “safer” cigarette in the United States. Am J Public Health. 2004;94(2):192–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zettler PJ, Riley MF, Kesselheim AS. Implementing a public health perspective in FDA drug regulation. Food Drug Law J. 2018;73( 2):221–256. [Google Scholar]
- 14. Shahab L, Goniewicz ML, Blount BC, et al. Nicotine, carcinogen, and toxin exposure in long-term E-cigarette and nicotine replacement therapy users: a cross-sectional study. Ann Intern Med. 2017;166(6):390–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. U.S. Department of Health and Human Services, Office of the Surgeon General. E-Cigarette Use Among Youth and Young Adults: A Report of the Surgeon General. 2016. https://e-cigarettes.surgeongeneral.gov/documents/2016_SGR_Full_Report_non-508.pdf. Accessed August 28, 2017.
- 16. Goniewicz ML, Boykan R, Messina CR, Eliscu A, Tolentino J. High exposure to nicotine among adolescents who use Juul and other vape pod systems (‘pods’). Tob Control. 2019;28(6):676–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jankowski M, Krzystanek M, Zejda JE, et al. E-cigarettes are more addictive than traditional cigarettes – a study in highly educated young people. Int J Environ Res Public Health. 2019;16(13):2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Muthumalage T, Prinz M, Ansah KO, Gerloff J, Sundar IK, Rahman I. Inflammatory and oxidative responses induced by exposure to commonly used e-cigarette flavoring chemicals and flavored e-liquids without nicotine. Front Physiol. 2018;8:1130.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gerloff J, Sundar IK, Freter R, et al. Inflammatory response and barrier dysfunction by different e-cigarette flavoring chemicals identified by gas chromatography-mass spectrometry in e-liquids and e-vapors on human lung epithelial cells and fibroblasts. Appl In Vitro Toxicol. 2017;3(1):28–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Khlystov A, Samburova V. Flavoring compounds dominate toxic aldehyde production during e-cigarette vaping. Environ Sci Technol. 2016;50(23):13080–13085. [DOI] [PubMed] [Google Scholar]
- 21. Farsalinos KE, Kistler KA, Gillman G, Voudris V. Evaluation of electronic cigarette liquids and aerosol for the presence of selected inhalation toxins. Nicotine Tob Res. 2015;17(2):168–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bitzer ZT, Goel R, Reilly SM, et al. Effect of flavoring chemicals on free radical formation in electronic cigarette aerosols. Free Radic Biol Med. 2018;120:72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tzortzi A, Kapetanstrataki M, Evangelopoulou V, Behrakis P. A systematic literature review of e-cigarette-related illness and injury: not just for the respirologist. Int J Environ Res Public Health. 2020;17(7) :2248.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Blount BC, Karwowski MP, Shields PG, et al. ; Lung Injury Response Laboratory Working Group . Vitamin E acetate in bronchoalveolar-lavage fluid associated with EVALI. N Engl J Med. 2020;382(8):697–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Miech RA, Schulenberg JE, Johnston LD, Bachman JG, O’Malley PM, Patrick ME. National Adolescent Drug Trends in 2018. 2018. http://www.monitoringthefuture.org/data/18data.html#2018data-drugs. Accessed December 20, 2018.
- 26. Cullen KA, Ambrose BK, Gentzke AS, Apelberg BJ, Jamal A, King BA. Notes from the field: use of electronic cigarettes and any tobacco product among middle and high school students – United States, 2011–2018. MMWR Morb Mortal Wkly Rep. 2018;67(45):1276–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dai H, Leventhal AM. Prevalence of e-cigarette use among adults in the United States, 2014–2018. JAMA. 2019;322(18):1824–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Committee on the Public Health Implications of Raising the Minimum Age for Purchasing Tobacco Products, Board on Population Health and Public Health Practice, Institute of Medicine. Restrictions on youth access to tobacco products. In: Bonnie RJ, Stratton K, Kwan LY, eds. Public Health Implications of Raising the Minimum Age of Legal Access to Tobacco Products. Washington, DC: National Academies Press (US); 2015. https://www.ncbi.nlm.nih.gov/books/NBK310404/. Accessed May 15, 2020. [PubMed] [Google Scholar]
- 29. Schneider S, Diehl K. Vaping as a catalyst for smoking? An initial model on the initiation of electronic cigarette use and the transition to tobacco smoking among adolescents. Nicotine Tob Res. 2016;18(5):647–653. [DOI] [PubMed] [Google Scholar]
- 30. Glasser A, Abudayyeh H, Cantrell J, Niaura R. Patterns of e-cigarette use among youth and young adults: review of the impact of E-Cigarettes on cigarette smoking. Nicotine Tob Res. 2019;21(10):1320–1330. [DOI] [PubMed] [Google Scholar]
- 31. Kozlowski LT, Warner KE. Adolescents and e-cigarettes: objects of concern may appear larger than they are. Drug Alcohol Depend. 2017;174:209–214. [DOI] [PubMed] [Google Scholar]
- 32. Corsi DJ, Lippert AM. An examination of the shift in school-level clustering of US adolescent electronic cigarette use and its multilevel correlates, 2011–2013. Health Place. 2016;38:30–38. [DOI] [PubMed] [Google Scholar]
- 33. Soneji S, Barrington-Trimis JL, Wills TA, et al. Association between initial use of e-cigarettes and subsequent cigarette smoking among adolescents and young adults: a systematic review and meta-analysis. JAMA Pediatr. 2017;171(8):788–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wills TA, Knight R, Sargent JD, Gibbons FX, Pagano I, Williams RJ. Longitudinal study of e-cigarette use and onset of cigarette smoking among high school students in Hawaii. Tob Control. 2017;26(1):34–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Leventhal AM, Strong DR, Kirkpatrick MG, et al. Association of electronic cigarette use with initiation of combustible tobacco product smoking in early adolescence. JAMA. 2015;314(7):700–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dutra LM, Glantz SA. E-cigarettes and national adolescent cigarette use: 2004–2014. Pediatrics. 2017;139(2):e20162450.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Barrington-Trimis JL, Urman R, Berhane K, et al. E-Cigarettes and future cigarette use. Pediatrics. 2016;138(1):e20160379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Watkins SL, Glantz SA, Chaffee BW. Association of noncigarette tobacco product use with future cigarette smoking among youth in the Population Assessment of Tobacco and Health (PATH) Study, 2013–2015. JAMA Pediatr. 2018;172(2):181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Leventhal AM, Stone MD, Andrabi N, et al. Association of e-cigarette vaping and progression to heavier patterns of cigarette smoking. JAMA. 2016;316(18):1918–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Abouk R, Adams S. Bans on electronic cigarette sales to minors and smoking among high school students. J Health Econ. 2017;54:17–24. [DOI] [PubMed] [Google Scholar]
- 41. Friedman AS. How does electronic cigarette access affect adolescent smoking? J Health Econ. 2015;44:300–308. [DOI] [PubMed] [Google Scholar]
- 42. Pesko MF, Hughes JM, Faisal FS. The influence of electronic cigarette age purchasing restrictions on adolescent tobacco and marijuana use. Prev Med. 2016;87:207–212. [DOI] [PubMed] [Google Scholar]
- 43. Dave D, Feng B, Pesko MF. The effects of e-cigarette minimum legal sale age laws on youth substance use. Health Econ. 2019;28(3):419–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pesko MF, Currie JM. E-cigarette minimum legal sale age laws and traditional cigarette use among rural pregnant teenagers. J Health Econ. 2019;66:71–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Levy DT, Warner KE, Cummings KM, et al. Examining the relationship of vaping to smoking initiation among US youth and young adults: a reality check. Tob Control. 2018. doi: 10.1136/tobaccocontrol-2018-054446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hammond D, Rynard VL, Reid JL. Changes in prevalence of vaping among youths in the United States, Canada, and England from 2017 to 2019. JAMA Pediatr 2020;174(8):797–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Levy DT, Yuan Z, Luo Y, Abrams DB. The relationship of e-cigarette use to cigarette quit attempts and cessation: insights from a large, nationally representative U.S. survey. Nicotine Tob Res. 2018;20(8):931–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhu SH, Zhuang YL, Wong S, Cummins SE, Tedeschi GJ. E-cigarette use and associated changes in population smoking cessation: evidence from US current population surveys. BMJ. 2017;358:j3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Johnson L, Ma Y, Fisher SL, et al. E-cigarette usage is associated with increased past-12-month quit attempts and successful smoking cessation in two us population-based surveys. Nicotine Tob Res. 2019;21(10):1331–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Benmarhnia T, Pierce JP, Leas E, et al. Can e-cigarettes and pharmaceutical aids increase smoking cessation and reduce cigarette consumption? Findings from a nationally representative cohort of American smokers. Am J Epidemiol. 2018;187(11):2397–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rodu B, Plurphanswat N. Quit methods used by American smokers, 2013–2014. Int J Environ Res Public Health. 2017;14(11) :1403.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Beard E, West R, Michie S, Brown J. Association between electronic cigarette use and changes in quit attempts, success of quit attempts, use of smoking cessation pharmacotherapy, and use of stop smoking services in England: time series analysis of population trends. BMJ. 2016;354:i4645. [DOI] [PubMed] [Google Scholar]
- 53. Beard E, Brown J, McNeill A, Michie S, West R. Has growth in electronic cigarette use by smokers been responsible for the decline in use of licensed nicotine products? Findings from repeated cross-sectional surveys. Thorax. 2015;70(10):974–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bullen C, Howe C, Laugesen M, et al. Electronic cigarettes for smoking cessation: a randomised controlled trial. Lancet. 2013;382(9905):1629–1637. [DOI] [PubMed] [Google Scholar]
- 55. Adriaens K, Van Gucht D, Declerck P, Baeyens F. Effectiveness of the electronic cigarette: an eight-week Flemish study with six-month follow-up on smoking reduction, craving and experienced benefits and complaints. Int J Environ Res Public Health. 2014;11(11):11220–11248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Caponnetto P, Campagna D, Cibella F, et al. EffiCiency and Safety of an eLectronic cigAreTte (ECLAT) as tobacco cigarettes substitute: a prospective 12-month randomized control design study. PLoS One. 2013;8(6):e66317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hartmann-Boyce J, McRobbie H, Bullen C, Begh R, Stead LF, Hajek P. Electronic cigarettes for smoking cessation. Cochrane Database Syst Rev. 2016;9:CD010216.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hajek P, Phillips-Waller A, Przulj D, et al. A randomized trial of e-cigarettes versus nicotine-replacement therapy. N Engl J Med. 2019;380(7):629–637. [DOI] [PubMed] [Google Scholar]
- 59. Halpern SD, Harhay MO, Saulsgiver K, Brophy C, Troxel AB, Volpp KG. A pragmatic trial of e-cigarettes, incentives, and drugs for smoking cessation. N Engl J Med. 2018;378(24):2302–2310. doi: 10.1056/NEJMsa1715757 [DOI] [PubMed] [Google Scholar]
- 60. Tuchman AE. Advertising and demand for addictive goods: the effects of e-cigarette advertising. Mark Sci. 2019;38(6):913–1084.
- 61.Dave DM, Dench D, Grossman M, Kenkel DS, Saffer H. Does E-Cigarette Advertising Encourage Adult Smokers to Quit? J Health Econ. 2019;68:102227.; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zheng Y, Zhen C, Nonnemaker J, Dench D. Advertising, habit formation, and U.S. tobacco product demand. Am J Agric Econ. 2016;98(4):1038–1054. [Google Scholar]
- 63. Cooper MT, Pesko MF. The effect of e-cigarette indoor vaping restrictions on adult prenatal smoking and birth outcomes. J Health Econ. 2017;56:178–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Pesko M, Warman C.. Re-exploring the early relationship between teenage cigarette and e-cigarette use using price and tax changes. Rochester, NY: Social Science Research Network; 2021. https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3077468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Huang J, Gwarnicki C, Xu X, Caraballo RS, Wada R, Chaloupka FJ. A comprehensive examination of own- and cross-price elasticities of tobacco and nicotine replacement products in the U.S. Prev Med. 2018;117:107–114.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Stoklosa M, Drope J, Chaloupka FJ. Prices and e-cigarette demand: evidence from the European Union. Nicotine Tob Res. 2016;18(10):1973–1980. [DOI] [PubMed] [Google Scholar]
- 67. Huang J, Tauras J, Chaloupka FJ. The impact of price and tobacco control policies on the demand for electronic nicotine delivery systems. Tob Control. 2014;23(suppl 3):iii41–iii47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Levy DT, Yuan Z, Li Y, Mays D, Sanchez-Romero LM. An examination of the variation in estimates of e-cigarette prevalence among U.S. Adults. Int J Environ Res Public Health. 2019;16(17) :3164.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Beard E, West R, Michie S, Brown J. Association of prevalence of electronic cigarette use with smoking cessation and cigarette consumption in England: a time-series analysis between 2006 and 2017. Addiction. 2020;115(5):961–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Villanti AC, Feirman SP, Niaura RS, et al. How do we determine the impact of e-cigarettes on cigarette smoking cessation or reduction? Review and recommendations for answering the research question with scientific rigor. Addiction. 2018;113(3):391–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Berry KM, Reynolds LM, Collins JM, et al. E-cigarette initiation and associated changes in smoking cessation and reduction: the Population Assessment of Tobacco and Health Study, 2013–2015. Tob Control. 2019;28(1):42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hitchman SC, Brose LS, Brown J, Robson D, McNeill A. Associations between E-Cigarette type, frequency of use, and quitting smoking: findings from a longitudinal online panel survey in Great Britain. Nicotine Tob Res. 2015;17(10):1187–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Grana RA, Popova L, Ling PM. A longitudinal analysis of electronic cigarette use and smoking cessation. JAMA Intern Med. 2014;174(5):812–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Al-Delaimy WK, Myers MG, Leas EC, Strong DR, Hofstetter CR. E-cigarette use in the past and quitting behavior in the future: a population-based study. Am J Public Health.. 2015;105(6):1213–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Brose LS, Hitchman SC, Brown J, West R, McNeill A. Is the use of electronic cigarettes while smoking associated with smoking cessation attempts, cessation and reduced cigarette consumption? A survey with a 1-year follow-up. Addiction. 2015;110(7):1160–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Manzoli L, Flacco ME, Ferrante M, et al. ; ISLESE Working Group . Cohort study of electronic cigarette use: effectiveness and safety at 24 months. Tob Control. 2017;26(3):284–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Weaver SR, Huang J, Pechacek TF, Heath JW, Ashley DL, Eriksen MP. Are electronic nicotine delivery systems helping cigarette smokers quit? Evidence from a prospective cohort study of U.S. adult smokers, 2015–2016. PLoS One. 2018;13(7) :e0198047.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Pearson JL, Stanton CA, Cha S, Niaura RS, Luta G, Graham AL. E-cigarettes and smoking cessation: insights and cautions from a secondary analysis of data from a study of online treatment-seeking smokers. Nicotine Tob Res. 2015;17(10):1219–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Rigotti NA, Chang Y, Tindle HA, et al. Association of e-cigarette use with smoking cessation among smokers who plan to quit after a hospitalization: a prospective study. Ann Intern Med. 2018;168(9):613–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Kalkhoran S, Chang Y, Rigotti NA. Electronic cigarette use and cigarette abstinence over 2 years among U.S. smokers in the population assessment of tobacco and health study. Nicotine Tob Res. 2020;22(5):728–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Cahn Z, Haardörfer R, Lewis M, Wang Y, Berg CJ. Examining e-cigarette purchases and cessation in a consumer panel of smokers. J Smok Cessat. 2019;14(1):32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Chen JC. Flavored e-cigarette use and cigarette smoking reduction and cessation – a large national study among young adult smokers. Subst Use Misuse. 2018;53(12):2017–2031. doi: 10.1080/10826084.2018.1455704 [DOI] [PubMed] [Google Scholar]
- 83. Biener L, Hargraves JL. A longitudinal study of electronic cigarette use among a population-based sample of adult smokers: association with smoking cessation and motivation to quit. Nicotine Tob Res. 2015;17(2):127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Giovenco DP, Delnevo CD. Prevalence of population smoking cessation by electronic cigarette use status in a national sample of recent smokers. Addict Behav. 2018;76:129–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Buu A, Hu YH, Piper ME, Lin HC. The association between e-cigarette use characteristics and combustible cigarette consumption and dependence symptoms: results from a national longitudinal study. Addict Behav. 2018;84:69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Harrell MB, Weaver SR, Loukas A, et al. Flavored e-cigarette use: characterizing youth, young adult, and adult users. Prev Med Rep. 2017;5:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Pepper JK, Ribisl KM, Brewer NT. Adolescents’ interest in trying flavoured e-cigarettes. Tob Control. 2016;25(suppl 2):ii62–ii66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Shang C, Huang J, Chaloupka FJ, Emery SL. The impact of flavour, device type and warning messages on youth preferences for electronic nicotine delivery systems: evidence from an online discrete choice experiment. Tob Control. 2018;27(e2):e152–e159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Berg CJ. Preferred flavors and reasons for e-cigarette use and discontinued use among never, current, and former smokers. Int J Public Health. 2016;61(2):225–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Kong G, Morean ME, Cavallo DA, Camenga DR, Krishnan-Sarin S. Reasons for electronic cigarette experimentation and discontinuation among adolescents and young adults. Nicotine Tob Res. 2015;17(7):847–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Coleman BN, Rostron B, Johnson SE, et al. Electronic cigarette use among US adults in the Population Assessment of Tobacco and Health (PATH) Study, 2013–2014. Tob Control. 2017;26(e2):e117–e126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Buckell J, Marti J, Sindelar JL. Should flavours be banned in cigarettes and e-cigarettes? Evidence on adult smokers and recent quitters from a discrete choice experiment. Tob Control. 2019;28:168–175.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Bonhomme MG, Holder-Hayes E, Ambrose BK, et al. Flavoured non-cigarette tobacco product use among US adults: 2013–2014. Tob Control.. 2016;25(suppl 2):ii4–ii13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Miech R, Johnston L, O’Malley PM, Bachman JG, Patrick ME. Trends in adolescent vaping, 2017–2019. N Engl J Med. 2019;381(15):1490–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Centers for Disease Control and Prevention. 2018 National Health Interview Survey (NHIS) Public Use Data Release. https://www.cdc.gov/nchs/nhis/nhis_2018_data_release.htm. Accessed August 19, 2020.
- 96. Winickoff JP, Gottlieb M, Mello MM. Tobacco 21 – an idea whose time has come. N Engl J Med. 2014;370(4):295–297. [DOI] [PubMed] [Google Scholar]
- 97. Sindelar JL. Regulating vaping – policies, possibilities, and perils. N Engl J Med. 2020;382(20):e54. [DOI] [PubMed] [Google Scholar]
- 98. Viola AS, Giovenco DP, Miller Lo EJ, Delnevo CD. A cigar by any other name would taste as sweet. Tob Control. 2016;25(5):605–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Wackowski OA, Delnevo CD, Pearson JL. Switching to E-cigarettes in the event of a menthol cigarette ban. Nicotine Tob Res. 2015;17(10):1286–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Hatsukami DK, Benowitz NL, Donny E, Henningfield J, Zeller M. Nicotine reduction: strategic research plan. Nicotine Tob Res. 2013;15(6):1003–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.