Introduction
Metastasis to regional lymph nodes is recognized as a poor prognostic factor in urothelial bladder cancer. Outcomes of patients with pathologically node-positive (pN+) disease following radical cystectomy are well-characterized, with five-year overall survival (OS) rates of only 20–30%.1,2 Studies of patients with clinically node-positive (cN+) disease based on preoperative imaging have much more variable outcomes. For example, one recent series reported five-year OS of over 50% in patients with cN+ bladder cancer; whereas two other series based on cancer registry data reported five-year OS rates of only 12–30% depending on treatment modality.3–5 These variations are due, in part, to criteria used to stage patients as cN+. In one series, which used short-axis diameter ≥8 mm to define cN+ status, over 50% of patients staged as cN+ were found to be node-negative at surgery.4 Induction chemotherapy (ICT) prior to surgery improves outcomes in cN+ disease. Failure to respond to ICT is an especially poor prognostic factor, with five-year OS rates as low as 12–16%.6,7 We have felt that patients with cN+ disease and bulky (large or multiple) pelvic lymph nodes fare especially poorly. We sought to identify such a population within our practice and define their outcomes.
Methods
With IRB approval, we reviewed preoperative imaging reports of all patients undergoing radical cystectomy for bladder cancer at our institution from January 1, 2016 to December 31, 2019 to identify patients with cN+M0 disease. This period was selected to coincide with the availability of immuno-oncology (IO) treatments as salvage therapy. Patients were defined as cN+ if they had a single pelvic lymph node ≥1.5 cm in diameter or multiple pelvic lymph nodes ≥1.0 cm in diameter. These criteria were chosen based on our clinical experience, with a goal of identifying a subset of patients with bulky disease and a high probability of true N+ status. Patients with distant metastatic disease, including those with lymph nodes ≥1.0 cm above the aortic bifurcation, were excluded. Our primary outcome was two-year recurrence-free survival (RFS), starting from the date of surgery. RFS and OS were estimated using Kaplan-Meier curves. The log-rank test was used to compare survival between subgroups, with a two-sided p-value ≤0.05 deemed significant. Analyses were conducted using IBM SPSS Statistics version 27 (IBM, Armonk, NY, U.S.).
Results
A total of 612 patients underwent radical cystectomy during the study period, of whom 32 met our criteria for cN+M0 disease. Demographic factors and medical history are summarized in Table 1, and clinical staging factors in Table 2. Patients had bulky pelvic lymph nodes with a median largest node size of 2.0 cm (interquartile range [IQR] 2.4–2.7). Most patients were staged as cN2–3. High rates of hydronephrosis (66%) and variant histology (63%) were noted. Most patients (84%) initiated ICT, of whom, 89% completed three or more cycles. Reasons for not receiving ICT included patient refusal in three cases and treating clinican’s discretion in two cases; no patients were excluded from receiving chemotherapy on the basis of ineligibility. Two patients with disease progression after ICT received salvage IO before proceeding to cystectomy. All patients receiving ICT were re-staged prior to surgery; nodal response to treatment is summarized in Table 2. Clinical complete response, defined as reduction in all pathological nodes to <1 cm diameter, occurred in 41% of cases. Perioperative parameters, pathological outcomes, and 90-day complications are summarized in Table 3.
Table 1.
Patient demographics and history
| Characteristic | n (%) or median (25th–75th) |
|---|---|
| Total patients | 32 |
| Sex | |
| Male | 23 (72%) |
| Female | 9 (28%) |
| Age | 62 (58–69) |
| Race | |
| White | 20 (63%) |
| Black | 6 (19%) |
| Hispanic | 5 (16%) |
| Asian/Pacific Islander | 1 (3%) |
| Body mass index (BMI) | 27 (23–31) |
| ECOG performance status | |
| 0 | 15 (47%) |
| 1 | 17 (53%) |
| Charlson comorbidity index (CCI) | |
| 0 | 2 (6%) |
| 1 | 5 (16%) |
| 2 | 9 (28%) |
| 3 | 9 (28%) |
| 4 | 4 (13%) |
| 5 | 1 (3%) |
| 7 | 2 (6%) |
| Smoking history | |
| Current | 9 (28%) |
| Prior | 15 (47%) |
| Never | 8 (25%) |
| Prior history of bladder cancer | 8 (25%) |
| Interval since diagnosis (months) | 12.5 (9–48) |
Charlson comorbidity index calculations excluded the adjustment for concurrent malignancy.
Table 2.
Clinical staging and treatment factors
| Characteristic | n (%) or median (25th–75th) |
|---|---|
| Clinical tumor stage (TURBT) | |
| cT1 | 7 (21%) |
| ≥cT2 | 25 (78%) |
| Clinical node stage (imaging) | |
| cN1 | 9 (28%) |
| cN2 | 18 (56%) |
| cN3 | 5 (16%) |
| Number of ≥1 cm pelvic nodes | |
| 1 | 10 (31%) |
| 2 | 9 (28%) |
| ≥3 | 12 (38%) |
| Size of largest pelvic node (cm) | 2.0 (2.4–2.7) |
| Confirmatory lymph node biopsy | 2 (6%) |
| Hydronephrosis (imaging) | 21 (66%) |
| Lymphovascular invasion | 10 (34%) |
| Variant histology | |
| Any | 20 (63%) |
| Squamous | 8 (25%) |
| Micropapillary | 4 (13%) |
| Sarcomatoid | 3 (9%) |
| Glandular | 2 (6%) |
| Plasmacytoid | 2 (6%) |
| Neuroendocrine | 1 (3%) |
| Microcystic | 1 (3%) |
| Induction systemic treatment | |
| Any | 27 (84%) |
| ≥3 cycles | 24 (75%) |
| MVAC | 13 (48%) |
| Gem/cis | 11 (41%) |
| Gem/carbo | 3 (11%) |
| Immuno-oncology | 2 (1%) |
| Clinical lymph node response to induction treatment | |
| Complete response (all nodes decreased to <1 cm) | 12 (44%) |
| Partial response (≥30% reduction in node size) | 2 (7%) |
| Stable disease | 9 (33%) |
| Progressive disease (≥20% increase in node size) | 4 (15%) |
Node counts and clinical stage are discordant in some cases, as the latter may have incorporated additional staging information, such as positron emission tomography scan. Data on lymphovascular invasion was not reported for three patients, resulting in a denominator of 29. Presence of variant histology is based on consensus between pathology at transurethral resection of bladder tumor (TURBT) and radical cystectomy (RC). Two patients with variant histology had mixed patterns (micropapillary/glandular and squamous/plasmacytoid) and were counted in two categories. Both patients who received immuno-oncology therapy had previously received chemotherapy. Definitions of complete response, partial response, and progressive disease were based on RECISTv1.1 criteria. MVAC: methotrexate, vinblastine sulfate, doxorubicin hydrochloride (adriamycin), and cisplatin.
Table 3.
Operative and pathological outcomes
| Characteristic | n (%) or median (25th–75th) |
|---|---|
| Surgical approach | |
| Open | 17 (53%) |
| Robotic | 15 (47%) |
| Operative time (min) | 324 (290–379) |
| Estimated blood loss (mL) | 300 (250–575) |
| Postoperative length of stay (days) | 6 (5–12) |
| Total red blood cell transfused (units) | 0.5 (0–2) |
| Followup (months) | 17 (9–24) |
| 90-day complications | |
| Minor (Clavien-Dindo grade 1–2) | 16 (90%) |
| Major (Clavien-Dindo grade 3–5) | 8 (25%) |
| Emergency room visit | 12 (38%) |
| Re-admission | 8 (25%) |
| Re-operation | 1 (3%) |
| Death | 2 (6%) |
| Pathological stage | |
| pT0 | 5 (16%) |
| pTis | 1 (3%) |
| pT1 | 1 (3%) |
| pT2 | 7 (22%) |
| pT3 | 12 (38%) |
| pT4 | 6 (19%) |
| ≤pT1 | 7 (22%) |
| ≥pT2 | 25 (78%) |
| pNx | 2 (6%) |
| pN0 | 11 (34%) |
| pN1 | 5 (16%) |
| pN2 | 12 (38%) |
| pN3 | 2 (6%) |
| ≥pN1 | 21 (66%) |
| pT0N0 (pathological complete response) | 5 (16%) |
| Lymph node dissection | |
| Total nodes | 13.5 (8–20) |
| Positive nodes | 1 (0–2.8) |
| Residual disease at surgery | |
| Any | 9 (28%) |
| Gross residual disease (R2) | 7 (22%) |
| Positive soft tissue margin (R1) | 4 (13%) |
| Adjuvant treatment | |
| Any | 9 (29%) |
| Chemotherapy | 1 (3%) |
| Immuno-oncology | 7 (23%) |
| Radiation | 2 (6%) |
| Salvage treatment | |
| Any | 13 (42%) |
| Chemotherapy | 6 (19%) |
| Immuno-oncology | 9 (29%) |
| Radiation | 8 (26%) |
Of note, both patients staged as pNx had bulky, grossly unresectable nodal disease and were included in the calculation as ≥pN1. Data on subsequent treatment was not available for one patient. Adjuvant/salvage modalities do not sum to 100% because some patients received multiple treatments.
With regard to pathological outcomes, complete pathological response (pT0N0) was achieved in 5/32 (16%) patients, all of whom had received ≥3 cycles of ICT. Negative lymph nodes (pN0) were found in 11/32 (34%) patients, of whom all but one had received ICT. More than half (56%) of patients received additional treatment after surgery. Consistent with the patients’ aggressive underlying disease, oncological outcomes were poor (Fig. 1). The two-year RFS and OS rates were 37% and 42%, respectively. Patients with negative lymph nodes (pN0) or complete pathological response (pT0N0) at surgery had significantly better outcomes (two-year RFS 72% for pN0 vs. 18% for pN+, log-rank p=0.005; 80% for pT0N0 vs. 28% for pT+/N+, log-rank p=0.042). All observed recurrences occurred within the first 12 months of surgery.
Fig. 1.
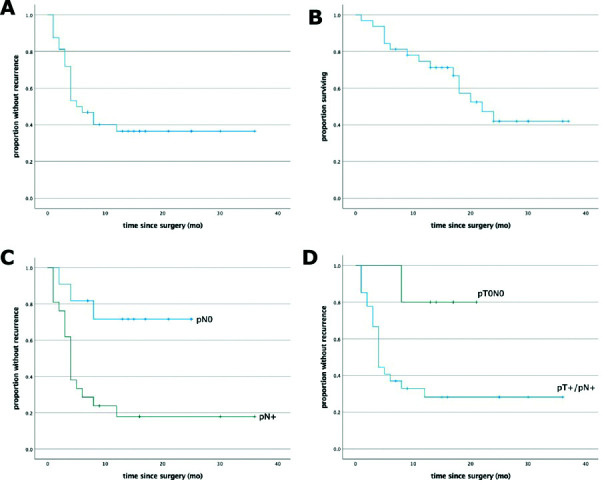
Oncological outcomes.
Discussion
Studies of patients with clinically node-positive bladder cancer have had widely varying outcomes. We sought to define the outcomes of a subset of patients with bulky cN+ disease undergoing radical cystectomy in our practice. Our cohort was notable for a high proportion of variant histology and by high levels of adverse prognostic factors. We used two-year RFS as a primary endpoint, which has been shown to be a good proxy for longer-term outcomes.8 We noted a high rate of early recurrence, with a two-year RFS rate of only 37%. OS at two years was similarly poor (42%), despite a large proportion of patients receiving additional treatment. Consistent with prior reports, pathological N0 status and complete response to ICT were strong predictors of improved outcomes.4,6,9
Our study is limited by its retrospective nature and small number of patients. Our analysis was confined to patients undergoing radical cystectomy, and it does not capture the outcomes of patients who presented as cN+ and did not undergo surgery. Most patients (84%) received ICT, and all of those who did not were clinically eligible for ICT. In light of reports that 25–50% of bladder cancer patients are chemo-ineligible,10 this observation suggests that our cohort represents a select subset of cN+ patients and implies that chemo-ineligible patients who present with cN+ disease are not being offered curative treatment.
Conclusions
In highlighting the outcomes of this cohort of patients with bulky cN+M0 bladder cancer, we hope to draw attention to a population of patients who have limited treatment options and who suffer early, rapid recurrence of disease. It is possible that more aggressive initial treatment of these patients may improve outcomes. We anticipate clinical trials in this space in the years to come.
Footnotes
Competing interests: Dr. Howard co-authored a publication of a clinical trial involving enhanced cystoscopy in NMIBC sponsored by Karl Storz. The remaining authors report no competing personal or financial interests related to this work.
This paper has been peer-reviewed.
References
- 1.Stein JP, Lieskovsky G, Cote R, et al. Radical cystectomy in the treatment of invasive bladder cancer: Long-term results in 1054 patients. J Clin Oncol. 2001;19:666–75. doi: 10.1200/JCO.2001.19.3.666. [DOI] [PubMed] [Google Scholar]
- 2.Hautmann RE, De Petriconi RC, Pfeiffer C, et al. Radical cystectomy for urothelial carcinoma of the bladder without neoadjuvant or adjuvant therapy: Long-term results in 1100 patients. Eur Urol. 2012;61:1039–47. doi: 10.1016/j.eururo.2012.02.028. [DOI] [PubMed] [Google Scholar]
- 3.Galsky MD, Stensland K, Sfakianos JP, et al. Comparative effectiveness of treatment strategies for bladder cancer with clinical evidence of regional lymph node involvement. J Clin Oncol. 2016;34:2627–35. doi: 10.1200/JCO.2016.67.5033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moschini M, Mattei A, Cornelius J, et al. World J Urol. 2018;36:639–44. doi: 10.1007/s00345-018-2190-1. [DOI] [PubMed] [Google Scholar]
- 5.Staník M, Poprach A, Zapletalová M, et al. Comparison of different treatment modalities outcomes in clinically node-positive bladder cancer: Analysis of a population-based cancer registry. Clin Genitourin Cancer. 2019;17:e759–67. doi: 10.1016/j.clgc.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 6.Ho PL, Willis DL, Patil J, et al. Outcome of patients with clinically node-positive bladder cancer undergoing consolidative surgery after preoperative chemotherapy: The MD Anderson Cancer Center experience. Urol Oncol Semin Orig Investig. 2016;34:59.e1–59.e8. doi: 10.1016/j.urolonc.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 7.Ploussard G, Pradere B, Beauval J-B, et al. Survival outcomes of patients with pathologically proven positive lymph nodes at time of radical cystectomy with or without neoadjuvant chemotherapy. J Clin Med. 2020;9:1962. doi: 10.3390/jcm9061962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim HS, Jeong CW, Kwak C, et al. Disease-free survival at 2 and 3 years is a significant early surrogate marker predicting the 5-year overall survival in patients treated with radical cystectomy for urothelial carcinoma of the bladder. Front Oncol. 2015;5:1–8. doi: 10.3389/fonc.2015.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hermans TJN, Fransen van de Putte EE, Horenblas S, et al. Pathological downstaging and survival after induction chemotherapy and radical cystectomy for clinically node-positive bladder cancer-results of a nationwide population-based study. Eur J Cancer. 2016;69:1–8. doi: 10.1016/j.ejca.2016.09.015. [DOI] [PubMed] [Google Scholar]
- 10.Dash A, Galsky MD, Vickers AJ, et al. Impact of renal impairment on eligibility for adjuvant cisplatin-based chemotherapy in patients with urothelial carcinoma of the bladder. Cancer. 2006;107:506–13. doi: 10.1002/cncr.22031. [DOI] [PubMed] [Google Scholar]


