Abstract
Purpose:
We examined whether an intervention combining pelvic floor muscle exercise and symptom self-management would improve urinary continence and quality of life in patients with prostate cancer.
Materials and Methods:
In a randomized, controlled, longitudinal clinical trial 279 patients with prostate cancer with persistent urinary incontinence were randomized to 1 of 3 groups, including biofeedback pelvic floor muscle exercise plus a support group, the biofeedback exercise plus telephone contact and usual care without intervention. The biofeedback plus support and plus telephone groups received 1 session of biofeedback assisted exercise and 6 biweekly sessions of problem solving therapy. This delivered symptom management skills through a peer support group or telephone contacts for 3 months. All subjects were assessed in blinded fashion at baseline, and 3 and 6 months for urinary leakage frequency, leakage amount and disease specific quality of life.
Results:
A total of 244 subjects completed the study. The biofeedback plus support and biofeedback plus telephone groups had a lower frequency of daily urinary leakage than the group with usual care without intervention at 3 months (p = 0.019 and p ≤0.001, respectively) but not at 6 months. The biofeedback plus support group but not the biofeedback plus telephone group had 13.3 gm lower leakage at 6 months than the usual care group (p = 0.003). Overall the biofeedback plus support and plus telephone groups reported less symptom severity (p ≤0.001) and fewer incontinence problems (p ≤ 0.01) than the usual care group at 6 months.
Conclusions:
Study findings show that pelvic floor muscle exercise practice plus symptom self-management in a peer support setting can significantly improve urinary continence and quality of life in patients with prostate cancer.
Keywords: prostatic neoplasms, urinary incontinence, pelvic floor, exercise, quality of life
IN the United States urinary incontinence affects more than 30% of patients with prostate cancer a year after surgery and 14% after 5 years.1,2 The effect of PFME on persistent incontinence remains inconclusive due to considerable variations in research methods.3 Tested interventions for persistent incontinence often rely on provider assistance (eg clinician assisted electronic stimulation and repeated BF sessions).3,4 To our knowledge their sustainability and cost-effectiveness have yet to be evaluated.
Strengthening pelvic floor muscles requires continuity of PFME.5 Patient knowledge of correct muscle contraction and adherence to the PFME regimen are critical.6,7 Self-management of life-style factors (eg fluid intake and bladder voiding schedule) has shown effectiveness in women8 but has yet to be applied to incontinent men. Because patients can acquire these skills through training, a patient centered intervention enabling patient activation and engagement is promising.
A patient centered approach requires that interventions be accessible and meet patient needs. Social support groups and individual telephone interventions have proved effective to promote adherence to therapeutic regimens.9,10 The support group has shown an effect size of 0.31 to improve QOL in patients with cancer.11 Because the telephone intervention is accessible and less expensive, it has broad appeal, especially for the elderly population.12 To our knowledge these interventions have not been studied to treat urinary incontinence. An evaluation of these interventions would fill a gap in the current knowledge and also contribute to developing treatment solutions that work in different patients and situations.
Thus, we investigated the Stay Dry program designed to teach PFME and self-management skills to patients with early stage prostate cancer who had persistent incontinence. Interventions were delivered through a support group or telephone contact. We addressed the question of whether the intervention groups would have significantly better urinary continence and QOL than the usual care group at 3 and 6-month assessments after controlling for sociodemographic and medical covariates.
MATERIALS AND METHODS
Design
A randomized, controlled, longitudinal clinical trial was performed from 2010 to 2013 in Cleveland, Ohio. Subjects were randomly assigned to 1 of 3 study groups, including BF PFME plus a support group, BF PFME plus telephone and UC. The BF plus support and BF plus telephone groups received study interventions for 3 months. Subjects were assessed at baseline, 3 months after intervention and at 6 months for followup.
Sampling
Eligibility included early stage (I, II or III) prostate cancer, completion of cancer treatment for at least 6 months and presenting incontinent symptoms. Men with dripping, a common and bothersome symptom that can progress without adequate care, were considered eligible irrespective of incontinence pad use. Study exclusion criteria included concurrent hormonal treatment, urinary tract infection or urinary retention, cognitive impairment and an implant to correct incontinence.
After obtaining institutional review board approval research staff used hospital databases to identify and contact patients by mail with physician permission. During a followup telephone call they obtained patient oral consent and administered ICSmaleSF (International Continence Society Male Short Form) questionnaire13 to screen for incontinence (cutoff 7 or greater). SPMSQ (Short Portable Mental Status Questionnaire) (cutoff 5 or greater)14 and a symptom list were used to screen for cognitive impairment, urinary infection and urinary retention. Medical charts were reviewed to ascertain patient disease and treatment status.
Randomization
Trained research personnel performed the randomization procedure using the minimization method, a computerized approach that has been shown to achieve a better balance between study group assignments within levels of stratification variables than the permuted blocks approach.15,16 We intended to balance study groups on key variables that can affect continence outcomes, including treatment type (surgery with and without radiotherapy vs radiotherapy alone), surgery type (open vs laparoscopic), radiotherapy type (brachytherapy vs external beam) and hospital site that was associated with surgeon expertise.
Interventions
The interventions consisted of 2 components. 1) At a 60-minute BF session BF plus support and BF plus telephone subjects learned PFME using a computerized BF machine. 2) Adapted PST17 was delivered through 6 biweekly sessions during 3 months after BF to teach self-management skills. BF plus support and BF plus telephone subjects were asked to practice PFME 3 times daily (primary goal) and meet the target in a certain area (secondary goal) as prioritized by individual, including 1) consuming 2,000 cc noncaffeinated fluid with 2 or fewer caffeine drinks daily, 2) setting bladder voiding schedules, 3) maintaining a diet balanced with fiber and fluid to avoid constipation and 4) performing daily exercise such as walking.
The BF plus support group consisted of 3 to 5 subjects each and lasted 60 to 75 minutes per session. The BF plus telephone group had an individual telephone contact with a therapist for approximately 45 minutes per session. UC subjects continued receiving usual care without receiving any intervention training sessions. They periodically received print materials unrelated to study interventions to minimize potential attention bias.
All BF sessions were performed by a BF technician trained elsewhere by a BF device manufacturer. The technician was experienced with teaching PFME. Two health psychologists and a nurse specialist delivered PST. They were trained in an initial trial with 9 subjects in which PST was manualized and reliability across therapists was examined to ensure consistency and adherence to the intervention. They led an equal number of support and telephone groups, and documented the number, duration, content and quality of intervention sessions in a log. Of the telephone and support group sessions 10% were randomly audiotaped. One of us (AYZ) and an independent evaluator listened to the audiotapes to check against the manual. Trained research staffers blinded to subject group assignment performed the assessments.
Measures
The primary outcome was urinary continence measured by the frequency and amount of urinary leakage. Leakage frequency was recorded in a diary for 3 days and average daily frequency was calculated.18 Leakage amount in gm was measured by the 1-hour pad test, a conventional objective measure.19
The secondary outcome was disease specific QOL measured by self-report. Severity of incontinence symptoms in the last month was measured on the 7-item I-PSS.20 Urinary function was assessed by the UCLA-PCI21 urinary function subscale and by a 6-point item of symptom bother. Subjects further rated incontinence as 10—“as bad as it could be” vs 0—no incontinence on a VAS22 for the last 7 days and 4 weeks. Demographic, socioeconomic and medical variables (eg cancer stage, treatment type, BMI and comorbidity) were collected at the baseline interview and verified against the medical chart.
Power analysis was performed using an effect size of 0.71 on daily leakage frequency obtained from a pilot study. A sample size of 78 per group had 99% power to detect a difference in 1-way ANOVA at a 1-sided α = 0.017 significance level for 3 group comparisons.
Statistical Analysis
Analyses were done in SAS®, version 9.2.23 We considered α = 0.05 significant in all statistical tests. ANOVA and the chi-square test were used to compare group differences in baseline characteristics. Linear mixed effects models24 were used to evaluate group effects on the mean change of the 7 outcome measures. We assumed a working autoregressive covariance structure because changes in continence function are physiologically accumulative and assessments are closely associated. We used adjusted means to correct the mean for missing data while adjusting for confounders in the model.23 Covariates of age, race, marital status, education, employment and BMI were controlled in all models.
RESULTS
Of 1,331 patients contacted for this study 1,078 (81%) responded, 339 (32%) were eligible, 289 consented and 10 withdrew prior to randomization. The remaining 279 patients were randomized at a consent rate of 82%. A total of 35 subjects dropped out after randomization for reasons of eligibility change or worsening health, family or economic conditions for a 13% attrition rate. The remaining 244 subjects, including 81 in the BF plus support group, 81 in the BF plus telephone group and 82 in the UC group, completed the study (fig. 1). There was no significant difference in sociodemographic and medical attributes between subjects included in study and dropouts.
Figure 1.
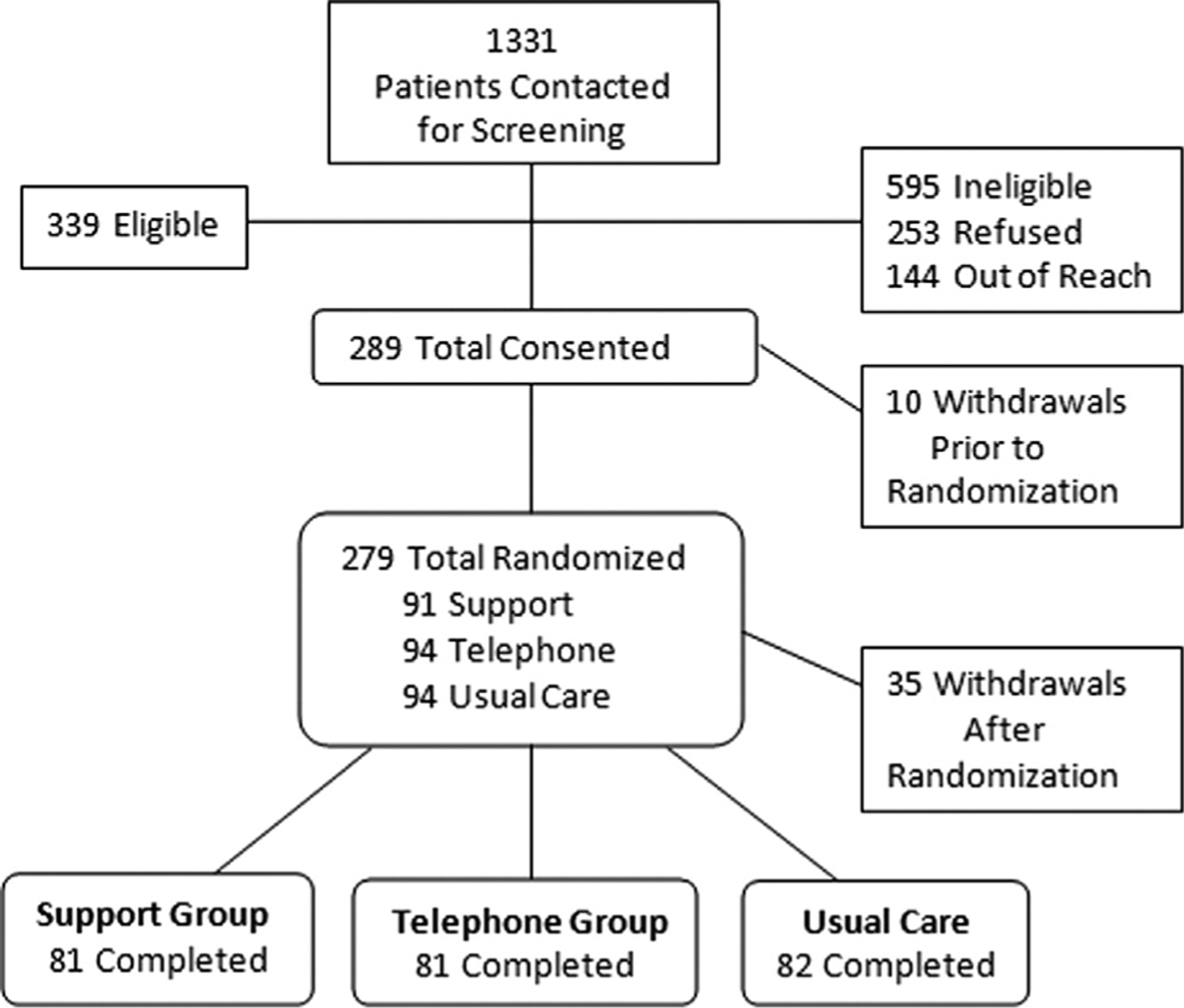
Subject enrollment flow chart
The 3 groups were similar in mean age (64 to 67 years), race (33% to 39% black), marital status (63% to 67% married) and education (33% to 43% college or greater). The majority had stage I or II cancer (greater than 94%), surgery (greater than 53%), radiation (48% to 56%), elevated comorbidity (Charlson index score range 0.63 to 0.95), a cancer diagnosis for 2 years or more and were overweight (mean BMI 28.3 kg/m2 or greater). Mean incontinence history was 26.9, 29.8 and 36.7 months in the UC, BF plus telephone and BF plus support groups, respectively. No significant group difference was found in any medical or demographic variables at baseline, including medication use, incontinence severity and urinary function, except employment status (p = 0.003, table 1).
Table 1.
Descriptive statistics
| Treatment Group | |||
|---|---|---|---|
| Support | Telephone | UC Control | |
| No. subjects | 81 | 81 | 82 |
| Mean ± SD age | 66.8 ± 7.2 | 64.3 ± 7.3 | 64.9 ± 8.2 |
| No. race (%): | |||
| Black | 31 (38.8) | 26 (32.5) | 28 (35) |
| White | 49 (61.3) | 53 (66.3) | 51 (63.8) |
| Other | 0 | 1 (1.3) | 1 (1.3) |
| No. married (%) | 54 (66.7) | 52 (64.2) | 52 (63.4) |
| No. education (%): | |||
| College or greater | 27 (33.3) | 35 (43.2) | 28 (34.2) |
| High school, associate degree | 46 (56.8) | 39 (48.2) | 47 (57.3) |
| Less than high school | 8 (9.9) | 7 (8.6) | 7 (8.5) |
| No. employed (%) | 28 (34.6) | 43 (53.1) | 36 (43.9)* |
| No.Ca stage (%): | |||
| I + II | 76 (95) | 76 (93.8) | 78 (95.1) |
| III | 4 (5) | 5 (6.2) | 4 (4.9) |
| No. surgery (%) | 43 (53.1) | 43 (53.1) | 47 (57.3) |
| No. radiation (%) | 45 (55.6) | 43 (53.1) | 39 (47.6) |
| No. surgery + radiation (%) | 8 (9.9) | 5 (6.2) | 6 (6.1) |
| Mean ± SD BMI (kg/m2) | 28.3 ± 4.0 | 29.3 ± 5.7 | 29.2 ± 5.4 |
| Mean ± SD Charlson comorbidity index score | 0.63 ± 1.1 | 0.78 ± 1.1 | 0.95 ± 1.8 |
| Mean ± SD incontinence history (mos) | 36.7 ± 50.2 | 29.8 ± 37.8 | 26.9 ± 28.5 |
| Mean ± SD mos after Ca diagnosis | 29.8 ± 24.7 | 25.6 ± 18 | 24.5 ± 16.4 |
| Median ± SD Gleason score | 6.0 ± 1.4 | 7.0 ± 0.8 | 7.0 ± 1.6 |
| Mean ± SD current prostate specific antigen (ng/ml) | 0.74 ± 1.9 | 0.71 ± 1.7 | 0.49 ± 1.3 |
| No. medication (%): | |||
| Anticholinergic | 6 (8.3) | 5 (7.4) | 1 (1.4) |
| α-Blocker | 16 (22.2) | 13 (19.1) | 17 (23.3) |
| Diuretic | 13 (18.1) | 15 (22.1) | 20 (27.4) |
| Mean ± SD baseline No. leaks/day: | |||
| Daily | 2.4 ± 3.0 | 2.8 ± 3.0 | 2.8 ± 3.1 |
| 3 Mos | 1.6 ± 2.5 | 1.9 ± 2.5 | 2.7 ± 3.3 |
| 6 Mos | 1.9 ± 3.1 | 1.6 ± 3.1 | 2.5 ± 2.9 |
| Mean ± SD leakage amount (gm): | |||
| Baseline | 33.9 ± 65.8 | 22.2 ± 49 | 21± 47.2 |
| 3 Mos | 17.9 ± 43.3 | 22.6 ± 72.4 | 11± 24.2 |
| 6 Mos | 18.1 ± 49.2 | 7.5 ± 21.8 | 13.8 ± 36.7 |
| Mean ± SD baseline incontinence severity | 13.9 ± 7.1 | 14.8 ± 8.4 | 14.7 ± 7.1 |
| Mean ± SD baseline VAS rating: | |||
| Last 7 days | 4.1 ± 3.0 | 3.9 ± 3.2 | 4.3 ± 3.1 |
| Last 4 wks | 4.5 ± 3.2 | 4.3 ± 3.3 | 4.5 ± 2.9 |
| Mean ± SD baseline urinary function | 54.6 ± 26 | 51± 25.6 | 48.8 ± 25.2 |
| Mean ± SD baseline urinary function bother | 46.8 ± 28 | 42.2 ± 31 | 38.3 ± 25.6 |
| No. baseline urge to rush to toilet to urinate (%): | |||
| Most or all time (weekly or greater) | 45 (55.5) | 50 (61.8) | 52 (63.5) |
| Never-sometimes (biweekly or less) | 36 (44.5) | 31 (38.2) | 30 (36.4)† |
p = 0.03.
Question from ICSmaleSF.
Outcomes
Primary.
Based on diary data the BF plus support and BF plus telephone groups (p = 0.019 and ≤0.001, respectively) had significantly decreased daily urinary leakage frequency at 3 months but not at 6 months compared to the UC group. Based on pad tests the BF plus support group showed a decrease of 13.3 gm at 6 months, significantly lower than the UC group (p = 0.003). However, the UC group had a lower leakage amount at 3 months than the BF plus telephone group (p = 0.009). This trend reversed at 6 months but did not reach statistical significance (p = 0.224, figs. 2 and 3). We also analyzed both objective measures (leakage frequency and amount) using an intent to treat approach. Results confirmed the reported significant findings.
Figure 2.
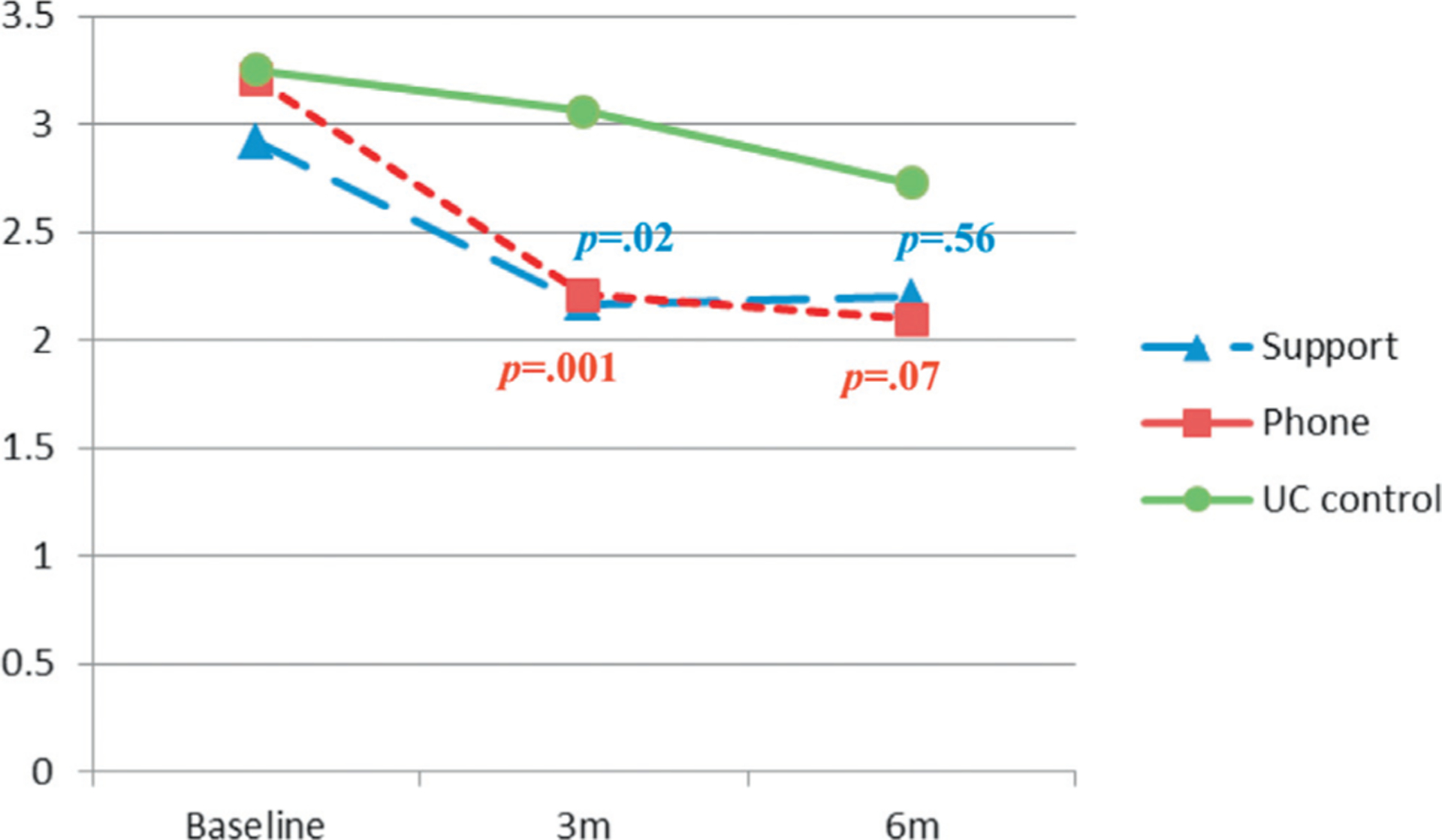
Adjusted mean frequency of daily urinary leakage from patient diary. m, months.
Figure 3.
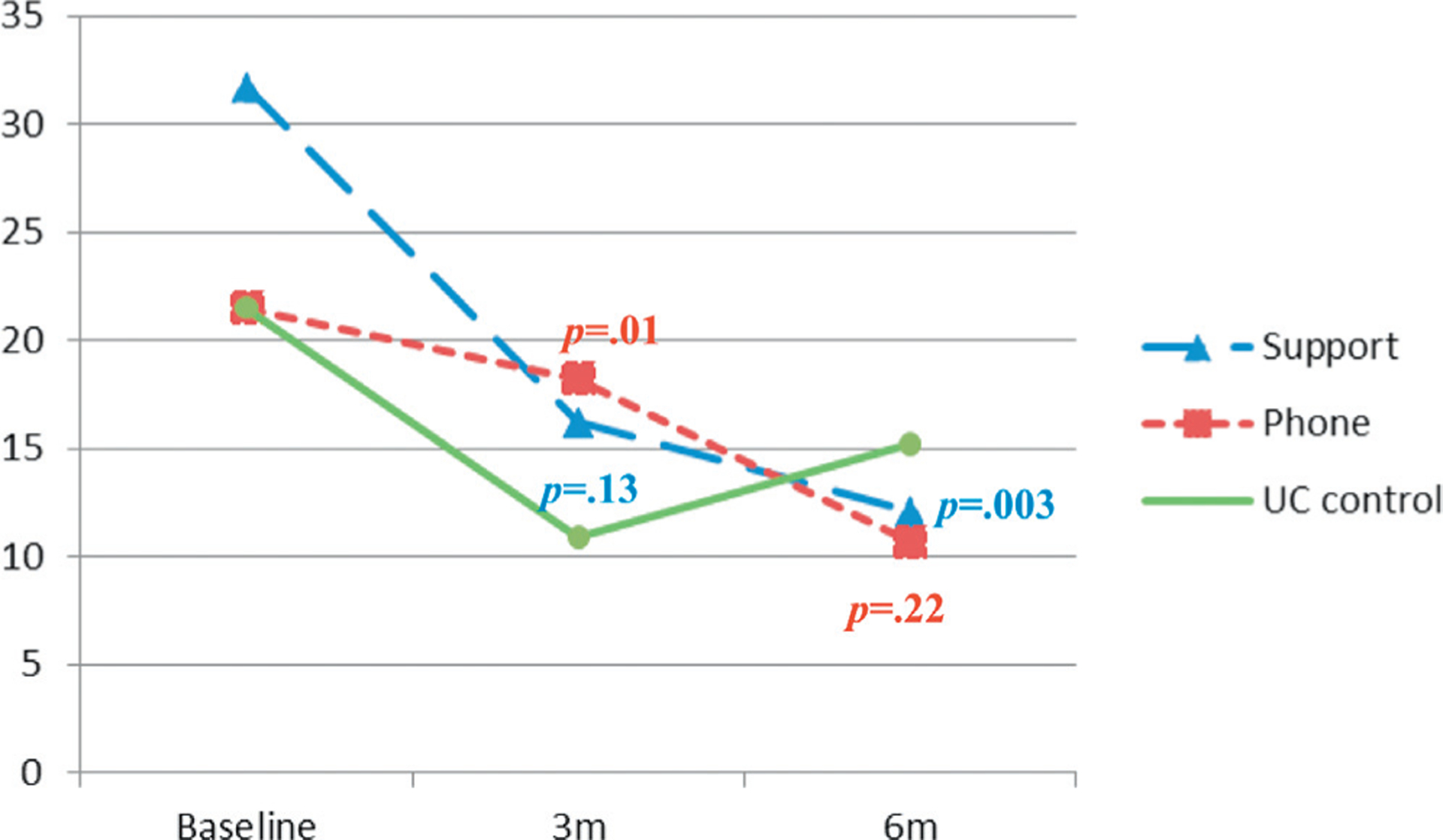
Adjusted average amount of urinary leakage from incontinence pad test. m, months.
Secondary.
The BF plus support group reported significantly less severe incontinence symptoms on I-PSS than the UC group at 3 and 6 months (p ≤0.001). The BF plus telephone group reported less severe symptoms than the UC group only at 6 months (p ≤0.001). Further, on UCLA-PCI the BF plus telephone group reported significantly better urinary function and less bother at 6 months than the UC group (p = 0.049, p = 0.009). At 6 months both intervention groups rated urinary incontinence as less problematic than the UC group on the VAS for the last 7 days (p = 0.014 and 0.015) and 4 weeks (p ≤0.001 and 0.002, respectively, table 2).
Table 2.
Treatment effects on incontinence and QOL
| 3 Months vs Baseline* | 6 Months vs Baseline* | |||||||
|---|---|---|---|---|---|---|---|---|
| Dependent Variables (treatment group) | No.Pts | Estimate | 95% CI | p Value | No.Pts | Estimate | 95% CI | p Value |
| Daily leakage frequency: | 191 | 187 | ||||||
| Support | −0.56 | −1.03, −0.09 | 0.019 | −0.20 | −0.85, 0.45 | 0.555 | ||
| Telephone | −0.82 | −1.29, −0.35 | 0.001 | −0.59 | −1.24, 0.06 | 0.069 | ||
| Leakage amount (gm): | 211 | 215 | ||||||
| Support | −4.90 | −11.27, −1.47 | 0.133 | −13.34 | −22.08, −4.60 | 0.003 | ||
| Telephone | 7.23 | 1.86, 12.6 | 0.009 | −4.56 | −11.91, 2.79 | 0.224 | ||
| Incontinence symptom severity: | 206 | 208 | ||||||
| Support | −2.30 | −3.20, −1.40 | 0.001 | −2.60 | −3.84, −1.37 | 0.001 | ||
| Telephone | −1.0 | −2.02, 0.02 | 0.055 | −2.55 | −3.92, −1.18 | 0.001 | ||
| Urinary function: | 209 | 213 | ||||||
| Support | 1.23 | −2.22, 4.68 | 0.483 | 1.42 | −3.30, 6.14 | 0.555 | ||
| Telephone | 2.01 | −1.54, 5.56 | 0.267 | 4.84 | 0.04, 9.64 | 0.049 | ||
| Urinary function bother: | 209 | 213 | ||||||
| Support | −0.32 | −4.36, 3.72 | 0.878 | 3.78 | −1.75, 9.31 | 0.181 | ||
| Telephone | 2.52 | −1.87, 6.91 | 0.262 | 7.89 | 1.95, 13.83 | 0.009 | ||
| VAS rating in last 7 days: | 205 | 212 | ||||||
| Support | −0.14 | −0.55, 0.27 | 0.511 | −0.70 | −1.27, −0.13 | 0.014 | ||
| Telephone | 0.07 | −0.34, 0.48 | 0.737 | −0.70 | −1.27, −0.13 | 0.015 | ||
| VAS rating in last 4 wks: | 205 | 212 | ||||||
| Support | −0.26 | −0.68, 0.17 | 0.237 | −1.02 | −0.84, 0.32 | 0.0006 | ||
| Telephone | −0.26 | −0.69, 0.17 | 0.234 | −0.90 | −1.48, −0.32 | 0.0024 | ||
In all linear mixed effect models UC group was referent with age, race, marital status, education, employment and BMI controlled to account for possible confounding effect on outcome variables.
Time Effect
When examining the within group intervention effect in the 7 mixed effects models, the 2 intervention groups showed significantly lower leakage frequency and amount at 3 and 6 months. All 3 groups demonstrated significantly reduced symptom severity and improved urinary function at 3 and 6 months vs baseline (almost all results p ≤0.001). However, the UC group did not show changes in leakage frequency at 3 and 6 months (p = 0.98 and 0.45), leakage amount (p = 0.34) or VAS ratings (p = 0.09 and 0.66, respectively) at 6 months compared to baseline. The BF plus telephone group demonstrated no change in leakage amount at 3 months compared to baseline (p = 0.71).
Model Statistics
Overall we observed a significant group × time interaction effect in 5 models (each p ≤0.01) but not in the 2 models of urinary function and bother (p ≤0.32 and ≤0.07, respectively, table 2). We also observed a significant time effect in all 7 models (each p ≤0.0001). We did not find a significant group effect in any model except in the analysis of symptom severity (p = 0.02). However, that group effect became statistically insignificant after adjusting for the experiment-wise error rate via the Bonferroni correction.
DISCUSSION
The significant decrease in leakage frequency at 3 months demonstrated intervention effectiveness. The waning effect at 6 months may be explained by some natural progress in the UC group during followup (fig. 2). It may also be explained by missing data at 6 months (6.2%), which could have reduced statistical power. This finding suggests that providing the study interventions beyond a 3-month time frame would be beneficial to help these patients continuously decrease the frequency of urinary leakage.
The UC group had a steep reduction in the leakage amount at 3 months due to a placebo effect or another unknown reason (fig. 3). However, the intervention effect was observed at 6 months when the BF plus support group demonstrated a significant reduction of 13.3 gm with an effect size of 0.20. The clinical significance of this result must be determined in the future due to the lack of literature on the matter. This reduction can provide symptom relief but it is most meaningful when the leakage amount is minimized to almost zero. This underscores the importance of extending the study interventions to enhance the intervention effect. It is not surprising that the BF plus support group showed superior outcomes on this measure since peer support has a known healing effect.25 The BF plus telephone group might have experienced interventional effects at a slower pace.
Further, subject self-reports confirmed the findings of objective measures. Symptom severity and QOL measured on I-PSS and VAS were significantly improved in both intervention groups at 6 months. This finding may reflect a delayed psychological response to physiological improvement at 6 months. However, I-PSS was created to assess symptoms of benign prostatic hyperplasia and it may not be the best measure for this patient population. UCLA-PCI mainly measures leakage frequency. The superior outcome on this measure in the BF plus telephone group may indicate the better decrease in leakage frequency. It is also possible that UCLA-PCI was less sensitive to changes because many subjects had minor baseline leakage for which improvements may be difficult to detect.
Sample attrition may explain discordance between the 2 objective measures. The rate of missing data at 6 months was 8.6%, 13.8% and 8.4% in the BF plus support, BF plus telephone and UC groups, respectively, for leakage amount but 2 to 3 times higher for leakage frequency. Surgeon high expertise contributed slightly differently to the BF plus support, BF plus telephone and UC groups (24%, 28% and 17%, respectively). This difference was statistically insignificant but it could influence the study outcome as patients of a highly experienced surgeon may recover better. The 3-month intervention duration limited our ability to assess intervention sustainability and long-term effect. Because many study subjects experienced minor incontinence problems at baseline, which are common but still bothersome, perceived changes could be difficult to detect on some study measures.
CONCLUSIONS
The UC group had a 3 to 10-month shorter history of incontinence and, thus, more potential for natural recovery. However, we found evidence that PFME practice plus symptom management can significantly improve urinary function and QOL in patients with prostate cancer who have long-term urinary incontinence. This patient centered approach requires minimal intrusive treatment (ie only a 1-time BF session), reduces reliance on technology and hospital facilities, and empowers patients to take charge of urinary health. The BF plus support intervention is effective and can be more efficacious with intervention extended beyond 3 months. The BF plus telephone intervention is promising and deserves more research. Future research could also address the issue of intervention delivery to areas where peer support is not readily available such as rural communities. Using technology, adjusting peer support to various settings and simplifying the intervention could enhance intervention delivery. Additional efforts to integrate the intervention into clinical care could make this behavioral treatment widely available through health care systems and across geographic areas to benefit those in need.
ACKNOWLEDGMENTS
John O’Neill assisted with project management. Neil Casey assisted with data management. Drs. Linien Chien and Nahida Gordon assisted with SAS programming. Matthew McManus provided editorial assistance. The BF technician was trained at Laborie.
Supported by National Institutes of Health/National Cancer Institute Grant R01CA127493 (AYZ), Cleveland Clinic, University Hospitals of Cleveland, Louis Stokes Cleveland Veterans Affairs Medical Center and MetroHealth System (affiliated with Case Western Reserve University).
Abbreviations and Acronyms
- BF
biofeedback
- BMI
body mass index
- I-PSS
International Prostate Symptom Score
- PFME
pelvic floor muscle exercise
- PST
problem solving therapy
- QOL
quality of life
- UC
usual care without intervention
- VAS
visual analog scale
Footnotes
ClinicalTrials.gov Identifier: NCT01365182.
REFERENCES
- 1.Penson DF, McLerran D, Feng Z et al. : 5-year urinary and sexual outcomes after radical prostatectomy: results from the prostate cancer outcomes study. J Urol 2005; 173: 1701. [DOI] [PubMed] [Google Scholar]
- 2.Potosky AL, Davis WW, Hoffman RM et al. : Five-year outcomes after prostatectomy or radiotherapy for prostate cancer: the prostate cancer outcomes study. J Natl Cancer Inst 2004; 96: 1358. [DOI] [PubMed] [Google Scholar]
- 3.Campbell SE, Glazener CMA, Hunter KF et al. : Conservative management for postprostatectomy urinary incontinence (Review). Cochrane Database Syst Rev 2012; CD001843. [DOI] [PubMed] [Google Scholar]
- 4.Goode PS, Burgio KL, Johnson TM et al. : Behavioral therapy with or without biofeedback and pelvic floor electrical stimulation for persistent postprostatectomy incontinence: a randomized controlled trial. JAMA 2001; 305: 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wells TJ: Pelvic (floor) muscle exercise. J Am Geriatr Soc 1990; 38: 333. [DOI] [PubMed] [Google Scholar]
- 6.Bump RC, Hurt WG, Fantl JA et al. : Assessment of Kegel pelvic muscle exercise performance after brief verbal instruction. Am J Obstet Gynecol 1991; 165: 322. [DOI] [PubMed] [Google Scholar]
- 7.Non-surgical treatment of stress incontinence. Lancet 1992; 340: 643. [PubMed] [Google Scholar]
- 8.Du Moulin MF, Hamers JP, Paulus A et al. : The role of the nurse in community continence care: a systematic review. Int J Nurs Stud 2005; 42: 479. [DOI] [PubMed] [Google Scholar]
- 9.Horvath KJ, Oakes JM, Rosser BR et al. : Feasibility, acceptability and preliminary efficacy of an online peer-to-peer social support ART adherence intervention. AIDS Behav 2013; 17: 2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castro CM and King AC: Telephone-assisted counseling for physical activity. Exerc Sport Sci Rev 2002; 30: 64. [DOI] [PubMed] [Google Scholar]
- 11.Rehse B and Pukrop R: Effects of psychosocial interventions on quality of life in adult cancer patients: meta analysis of 37 published controlled outcome studies. Patient Educ Couns 2003; 50: 179. [DOI] [PubMed] [Google Scholar]
- 12.Ekeland AG, Bowes A and Flottorp S: Effectiveness of telemedicine: a systematic review of reviews. Int J Med Inform 2010; 79: 736. [DOI] [PubMed] [Google Scholar]
- 13.Donovan JL, Peters TJ, Abrams P et al. : Scoring the short form ICS male SF questionnaire. International Continence Society. J Urol 2000; 164: 1948. [PubMed] [Google Scholar]
- 14.Pfeiffer E, Johnson TM and Chiofolo RC: Functional assessment of elderly subjects in four service settings. J Am Geriatr Soc 1981; 29: 433. [DOI] [PubMed] [Google Scholar]
- 15.Conlon M and Anderson GC: Three methods of random assignment: comparison of balance achieved on potentially confounding variables. Nurs Res 1990; 39: 376. [PubMed] [Google Scholar]
- 16.Hu F, Hu Y, Ma Z et al. : Adaptive randomization for balancing over covariates. WIREs Comput Stat 2014; 6: 288. [Google Scholar]
- 17.Nezu AM: Helping Cancer Patients Cope: A Problem-Solving Approach. Washington, D.C.: American Psychological Association; 1998. [Google Scholar]
- 18.Brown JS, McNaughton KS, Wyman JF et al. : Measurement characteristics of a voiding diary for use by men and women with overactive bladder. Urology 2003; 61: 802. [DOI] [PubMed] [Google Scholar]
- 19.Peterson AC, Amundsen CL and Webster GD: The 1-hour pad test is a valuable tool in the initial evaluation of women with urinary incontinence. J Pelvic Med Surgery 2005; 11: 251. [Google Scholar]
- 20.Barry MJ, Fowler FJ Jr, O’Leary MP et al. : The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol 1992; 148: 1549. [DOI] [PubMed] [Google Scholar]
- 21.Litwin MS, Hays RD, Fink A et al. : The UCLA Prostate Cancer Index: development, reliability, and validity of a health-related quality of life measure. Med Care 1998; 36: 1002. [DOI] [PubMed] [Google Scholar]
- 22.Van Kampen M, De Weerdt W, Van Poppel H et al. : Effect of pelvic-floor re-education on duration and degree of incontinence after radical prostatectomy: a randomised controlled trial. Lancet 2000; 355: 98. [DOI] [PubMed] [Google Scholar]
- 23.Enhancements in SAS/STAT 9.2 Software. Cary, North Carolina: SAS Institute; 2008. [Google Scholar]
- 24.Fitzmaurice GM, Laird NM and Ware JH: Applied Longitudinal Analysis. New York: John Wiley and Sons; 2004. [Google Scholar]
- 25.Proudfoot JG, Jayawant A, Whitton AE et al. : Mechanisms underpinning effective peer support: a qualitative analysis of interactions between expert peers and patients newly-diagnosed with bipolar disorder. BMC Psychiatry 2012; 12: 196. [DOI] [PMC free article] [PubMed] [Google Scholar]


