Abstract
Genetic modifiers of anemia in Plasmodium falciparum infection and sickle cell disease (SCD) are not fully known. Both conditions are associated with oxidative stress, hemolysis and anemia. The CYB5R3 gene encodes cytochrome b5 reductase 3, which converts methemoglobin to hemoglobin through oxidation of NADH. CYB5R3c.350C > G encoding CYB5R3T117S, the most frequent recognized African-specific polymorphism, does not have known functional significance, but its high allele frequency (23% in African Americans) suggests a selection advantage. Glucose-6-phosphate dehydrogenase (G6PD) is essential for protection from oxidants; its African-polymorphic X-linked A+ and A− alleles, and other variants with reduced activity, coincide with endemic malaria distribution, suggesting protection from lethal infection. We examined the association of CYB5R3c.350C > G with severe anemia (hemoglobin <5 g/dL) in the context of G6PD A+ and A− status among 165 Zambian children with malaria. CYB5R3c.350C > G offered protection against severe malarial anemia in children without G6PD deficiency (G6PD wild type or A+/A− heterozygotes) (odds ratio 0.29, P = .022) but not in G6PD A+ or A− hemizygotes/homozygotes. We also examined the relationship of CYB5R3c.350C > G with hemoglobin concentration among 267 children and 321 adults and adolescents with SCD in the US and UK and found higher hemoglobin in SCD patients without G6PD deficiency (β = 0.29, P = .022 children; β = 0.33, P = .004 adults). Functional studies in SCD erythrocytes revealed mildly lower activity of native CYB5R3T117S compared to wildtype CYB5R3 and higher NADH/NAD+ ratios. In conclusion, CYB5R3c.350C > G appears to ameliorate anemia severity in malaria and SCD patients without G6PD deficiency, possibly accounting for CYB5R3c.350C > G selection and its high prevalence.
1 |. INTRODCTION
Genetic modifiers of anemia severity in Plasmodium falciparum infection and sickle cell disease (SCD), conditions prevalent in sub-Saharan Africa, are not fully known. Both conditions are characterized by erythrocyte inclusions that lead to extravascular and intravascular hemolysis, the plasmodial parasite in malaria and polymerized hemoglobin S in SCD. The erythrocytes in both conditions also have increased oxidant stress,1–5 which may contribute to the degree of intravascular hemolysis and the degree of anemia. The mortality of malaria-infected children6,7 and children with SCD8,9 correlates with degree of anemia, and it is likely that protection from anemia contributes to positive selection of genetic polymorphisms in malarial endemic areas. For example, α-thalassemia due to single or double α-globin gene deletions (α3.7 deletion or α4.2 deletion allele frequency ~ 14% in African-Americans) is associated with protection from severe anemia in P. falciparum malaria10 and with reduced hemolysis in SCD.11
Cytochrome b5 reductase (CYB5R3, EC 1.6.2.2) participates in the transfer of electrons to cytochrome b5 from the NADH generated by glyceraldehyde-3-phosphate dehydrogenase (EC 1.2.1.12).12 Recessively inherited deficiency of CYB5R3 is responsible for congenital methemoglobinemia, with more than 40 methemoglobinemia-causing mutations reported to date.13 The CYB5R3c.350C > G variant (rs1800457 encoding CYB5R3T117S) is an African polymorphism14 that is not associated with methemoglobinemia. It has the highest prevalence of genetic polymorphisms yet known among African-Americans (allele frequency of 23%), and has not been detected in other ethnic and racial groups. It does not have known functional significance, but its high prevalence implies a selection benefit. We hypothesized that the possible advantage of CYB5R3c.350C > G may be protection from severe anemia under conditions of increased erythrocyte oxidant stress. From this perspective, we postulated that it may have parallel effects in patients with malaria and in patients with SCD.
X-linked glucose-6-phosphate dehydrogenase (G6PD EC 1.1.1.49) is the sole enzyme in erythrocytes that produces NADPH, the metabolic intermediate essential for maintenance of a high ratio of reduced to oxidized glutathione that protects erythrocytes from reactive oxygen species-mediated damage. The gene G6PD A+ (G6PDc. 376G) is an African specific polymorphism with slightly reduced activity15 but no apparent phenotype. G6PD A− (G6PDc. 202A/376G) is a related polymorphism that arose on the G6PD A+ chromosome, has decreased activity and enzyme stability, and increases the risk for oxidant-induced hemolysis.16 The worldwide distribution of all polymorphic G6PD deficient variants coincides with the geographic distribution of endemic malaria, suggesting protection from lethal malaria.6 Paradoxically, there is also evidence that G6PD A− increases the risk for severe malarial anemia.7,17 The nature of protection from malaria mortality by G6PD deficiency remains to be established. G6PD-deficient erythrocytes infested with malaria parasites may be phagocytized more efficiently than normal parasitized erythrocytes. Also, G6PD-deficient erythrocytes with impaired ability to maintain a high GSH/GSSG ratio may cause greater exposure of intra-erythrocytic parasites to reactive oxygen species. In addition, it is possible that the high prevalence of G6PD A alleles in the African population influences the effect of other polymorphisms that alter erythrocyte redox status.
In the present study, we examined the relationship of CYB5R3c.350C > G with the degree of anemia in children with falciparum malaria and in children and adults with SCD, and we examined whether the effect of this allele is influenced by G6PD A alleles.
2 |. METHODS
2.1 |. Cohorts
(a) A Zambian malarial cohort of children <5 years of age,18 (b) the Pulmonary Hypertension and Hypoxic Response in SCD (PUSH) cohort of children and adolescents with SCD,19 and (c) the Treatment of Pulmonary Hypertension and SCD with Sildenafil Therapy (Walk-PHaSST) cohort of adults and adolescents with SCD20 were employed. This was to analyze the effect of CYB5R3c.350C > G on the severity of anemia, and the degree of hemolysis in the setting of a propensity to low hemoglobin levels. Additionally, erythrocytes from Universtiy of Illinois (UIC) SCD patients and anonymous ARUP Laboratory (Salt Lake City, UT) hemoglobin S-positive erythrocytes were assessed for CYB5R3 activity and for NADH and NAD+ levels in some UIC samples. The Institutional Review Boards of the participating institutions approved the research and written informed consent was obtained for all participants.
2.2 |. Genetic testing
2.2.1 |. Cytochrome b5 reductase
The SNP assay (C_2986212_20, rs1800457) Taqman genotyping for CYB5R3c.350C > G on genomic DNA was carried out on a StepOnePlus Real-Time PCR System (Applied Biosystems, Foster City, CA), a 7900HT Fast Real-Time PCR system (Applied Biosystems), or an LC480 (Roche). The genotypes were called with StepOne Software v2.3 or the Applied Biosystems SDS 2.2.2 software package. For the Zambian cohort, DNA was isolated from dried blood spots and from frozen serum samples and genotyping was performed using the StepOnePlus Real-Time PCR System for DNA from blood spots and the 7900HT Fast Real-Time PCR system for DNA from serum. There was 97.7% agreement in the genotyping between tests done on DNA isolated from dried blood spots and DNA isolated from serum. For the PUSH, Walk-PHaSST, ARUP and UIC samples DNA was isolated from venipuncture blood samples.
2.2.2 |. Alpha-globin and beta-globin
The α-thalassemia copy number genotyping was performed as described.21 For the PUSH cohort, hemoglobin S, C and E genotyping was conducted at ARUP Laboratories (Salt Lake City, UT) using loci-spanning probe PCR.22
2.2.3 |. G6PD genotypes
For the Zambian cohort and the UIC patients, Taqman genotyping for G6PD A+ and G6PD A− was carried out on a StepOnePlus Real-Time PCR System using GTXpress Master Mix (Applied Biosystems) and a fast thermal cycling protocol. The genotypes were called with StepOne Software v2.3. For the PUSH cohort G6PD genotyping was conducted as described.23 Both the PUSH and Walk-PHaSST cohorts had previously been genotyped on Illumina Human 610-Quad SNP array covering more than 500 k SNPs that do not include the investigated G6PD mutations.24 We therefore imputed G6PDA376G (rs1050829) and G6PDG202A (rs1050828) genotypes for the Walk-PHaSST cohort using the array genotyped SNPs on the X chromosome. Samples with <95% genotype rate and X chromosome SNPs with a minor allele frequency (MAF) <0.01 were removed. Population outliers were identified based on principal components analysis25 of autosome SNPs. Proportion of identity-by-descent was calculated pair-wise using PLINK26 to identify related individuals (π≥ 0.125) and potentially contaminated DNA samples (mean π ^≥ 0.033). The X chromosome SNP genotypes were imputed from the African reference panel haplotypes in the 1000 genomes project phase three data using Shapeit227 and Impute2.28 The imputation quality r2 for rs1050829 and rs1050828 was 0.92 and 0.90 respectively. A similar procedure was applied to impute the rs1050829 and rs1050828 genotypes for the PUSH cohort for an empirical estimation of the genotype concordance rate. Imputed genotypes for the PUSH cohort were compared to the genotypes obtained by TaqMan analysis. The genotype concordance rate was 0.90 for rs1050829 (imputation quality r2 = 0.91) and 0.93 for rs1050828 (imputation quality r2 = 0.89).
2.3 |. Cytochrome b5 reductase 3 activity in erythrocytes
We measured erythrocyte CYB5R3 activity in three groups of sickle cell patients. (a) Walk-PHaSST trial participants. Erythrocytes from Walk-PHaSST hemoglobin SS adults at steady-state were analyzed at the University of Pittsburgh (n = 40). There were 15 CYB5R3 wild type individuals, 16 CYB5R3c.350C > G heterozygotes and 9 CYB5R3c.350C > G homozygotes. (b) UIC patients. Adults with SCD at UIC were studied at steady state (n = 25; hemoglobin SS 17, hemoglobin SC 5, hemoglobin S-beta+-thalassemia 3). There were 15 CYB5R3 wild type individuals, 4 CYB5R3c.350C > G heterozygotes and 5 CYB5R3c.350C > G homozygotes. (c) Patient samples from ARUP laboratories. We obtained anonymous erythrocyte samples from ARUP Laboratories of 31 children who had hemoglobin S present on hemoglobin fractionation and who did not have a diagnosis of hemoglobin S trait. Three had hemoglobin SC disease and the remainder had unspecified sickle phenotype. There were 15 CYB5R3 wildtype individuals, 15 CYB5R3c.350C > G heterozygotes and 1 CYB5R3c.350C > G homozygote. The median (range) age of the ARUP patients was 6 (0.2–17) years and 14 (45%) were females.
The CYB5R3 enzymatic activity was measured in erythrocytes using the ferricyanide method.29 Leukocytes and platelets were depleted by filtering heparinized whole blood through a column of α-cellulose and Avicel pH 101 (Sigma-Aldrich, 11363) followed by three washes with cold 0.9% saline. The erythrocyte fraction was then resuspended in an equal volume of 0.9% saline. Then 100 μL of this suspension was lysed in 900 μL of chilled 2.7 mM EDTA, pH 7.0, 0.7 mM β-mercaptoethanol, frozen in liquid nitrogen and stored at −80 °C until assay. 20 μL of the hemolysate was mixed with 200 μL of potassium ferricyanide and allowed to stand for 1 minute. The NADH reduction reaction was carried out using potassium ferricyanide as a substrate using 1/10 volume of the hemolysate-ferricyanide mixture in a 96-well quartz plate or 1 mL quartz cuvette. The final reaction concentrations were 200 μM NADH, 200 μM potassium ferricyanide, 100 μM Tris-HCl, 5 mM EDTA pH 8.0. Enzymatic activity was calculated according to Beutler30 using 6.22 mM−1 cm−1 as the extinction coefficient for NADH. Data was collected on a Spectramax M3 plate reader (Molecular Devices). An aliquot of the erythrocytes was used for measuring the hemoglobin concentration by Drabkin’s solution.31
2.4 |. Concentrations of NADH and total NAD in erythrocytes
These levels were measured in 19 UIC SCD patients (hemoglobin SS 15, hemoglobin SC 3, hemoglobin S-beta+-thalassemia 1). Ten to 20 μL of leukocyte and platelet depleted erythrocytes were lysed in 10 mM nicotinamide, 20 mM sodium bicarbonate (NaHC03) and 100 mM sodium carbonate (Na2C03) to a total volume of 1 mL. The lysate was immediately flash frozen in liquid nitrogen and stored at −80° C until assay. On the day of the assay, the hemolysate was thawed in a water bath just until all ice melted, vortexed, and immediately split (500 μL each) to measure NADH and total NAD. The NADH was measured in hemolysate that was heated at 60° C for 30 minutes to destroy NAD+ in a dry heating block. During the heating process, the hemolysate for total NAD was kept on ice. After 30 minutes, the samples were spun at 16 000 g for 5 minutes at 4 °C. Then 50 μL of hemolysate was pre-incubated with alcohol dehydrogenase at 37 °C in Tris buffer. The reaction was initiated by adding a mixture of spectrometric grade ethanol (catalogue number 483511, Sigma Aldrich), WST-8 (catalogue number 18721, Cayman Chemical, MI, USA) and 1-methoxy-5-methylphenazinium (methyl sulfate) (catalogue number 21258, Cayman Chemical, MI, USA). The final reaction concentrations were 0.2 mg/mL alcohol dehydrogenase (ADH, LS001069, Worthington Biochemical Corporation, NJ), 0.1 M Tris-HCl pH 8.0, 5 mM EDTA, 0.6 M ethanol, 0.5 mM WST-8, 70 μM 1-methoxy-5-methylphenazinium (methyl sulfate). Absorbance was recorded at 460 nm using Spectramax M3 plate reader. For each hemolysate, blank reactions without ADH were carried out and the absorbance was subtracted from the reactions with ADH. Under these conditions, the rates were linear for at least 20 minutes. The NADH or total NAD concentrations were interpolated from rates obtained using NADH (N20100, RPI corp, IL) between 0–1.6 μM, and NAD+ was calculated by subtracting NADH from total NAD.
2.5 |. Statistical analysis
2.5.1 |. Severe malarial anemia
We examined the association of G6PD A+, G6PD A− and CYB5R3c.350C > G with severe malarial anemia in logistic regression models. We previously published a comprehensive analysis of the clinical features, laboratory tests and inflammatory cytokine measurements that were associated with severe anemia in this cohort.18 We found that weight-for-age z-score, splenomegaly, the presence of stool parasites, and plasma concentrations of interleukin-10 and tumor necrosis factor-alpha were the most significant independent predictors of severe anemia. Therefore, we adjusted for these variables in the logistic regression models of severe anemia in that we developed for the present study. Female G6PDA376G homozygotes who were G6PDG202A heterozygotes were considered to have G6PD A+ (Table 1).
TABLE 1.
G6PD mutation status in the three cohorts studied. Results in number (%)
|
G6PD mutation status Genotype |
Zambian children with malaria |
PUSH: US SCD children/ adolescents |
Walk-PHaSST: US/UK SCD adolescents/ adults |
||||
|---|---|---|---|---|---|---|---|
| Genotype | Allele | Males (N = 83) | Females (N = 82) | Males (N = 144) | Females (N = 123) | Males (N = 148) | Females (N = 173) |
| Wildtype | wt or wt/wt | 49 (59.0%) | 34 (41.5%) | 98 (68.1%) | 53 (43.1%) | 99 (66.9%) | 84 (48.6%) |
| Heterozygotes for A alleles | 376/wt | 9 (11.0%) | 38 (30.9%) | - | 45 (26.0%) | ||
| 376–202/wt | 20 (24.4%) | 17 (13.8%) | - | 26 (15.0%) | |||
| A+ hemizygotes (males) or homozygotes (females) | 376 or 376/376 | 17 (20.5%) | 4 (4.9%) | 29 (20.1%) | 5 (4.1%) | 37 (25.0%) | 7 (4.1%) |
| 376/376–202 | 11 (13.4%) | 7 (5.7%) | - | 8 (4.6%) | |||
| A- hemizygotes (males) or homozyogtes (females) | 376–202 or 376–202/376–202 | 17 (20.5%) | 4 (4.9%) | 17 (11.8%) | 3 (2.4%) | 12 (8.1%) | 3 (1.7%) |
2.5.2 |. Degree of anemia and hemolysis in PUSH and Walk-PHaSST
In the PUSH and Walk-PHaSST cohorts, principal components analysis of clinical site-adjusted values for lactate dehydrogenase, aspartate aminotransferase, reticulocyte percentage and total bilirubin was used to develop a hemolytic component. This hemolytic component expresses the shared variability of the four markers of hemolysis that were used for its development. We have used this variable to reflect the degree of hemolysis in sickle cell disease in a number of publications.19,32,33 The relationship of G6PD A+, G6PD A− and CYB5R3 genotypes with hemoglobin concentration and hemolytic component was examined with multiple linear regression in PUSH and Walk-PHaSST SCD participants who did not have a blood transfusion in the past 2 months. It was also examined in Walk-PHaSST participants whose hemoglobin A percent was 0 (except for hemoglobin S-beta+-thalassemia) and whose serum creatinine was less than 1.5 mg/dL. In these models, hemoglobin concentration and hemolytic index were adjusted for age, sex, severe sickling genotype, α-thalassemia genotype, and current treatment with hydroxyurea in the PUSH cohort, and for age, sex, severe sickling genotype, α-thalassemia, and hemoglobin F% in the Walk-PHaSST cohort.
2.5.3 |. Erythrocyte CYB5R3 activity and NADH to NAD+ ratio
The relationship of CYB5R3 genotypes with erythrocyte CYB5R3 activity and with the ratio of NADH to NAD+ in erythrocytes, was analyzed by multiple linear regression with adjustment for significant co-factors: laboratory of measurement in the case of CYB5R3 activity and G6PD A+ and A− in the case of erythrocyte NADH to NAD+ ratio.
3 |. RESULTS
3.1 |. Severe anemia in southern Zambian children <5 years of age with malaria
3.1.1 |. Zambian malarial anemia cohort
The study was conducted prospectively and involved 165 children of Tonga ethnicity admitted to Macha Mission Hospital in southern Zambia with the clinical diagnosis of malaria from 2000–2005.18 Inclusion criteria included age < 5 years and the presence of asexual forms of P. falciparum determined by light microscopic examination (1000×) of a thick smear of the peripheral blood stained with Giemsa. The hematocrit was measured by capillary tube centrifugation on a finger stick blood specimen and used to estimate the hemoglobin concentration by dividing by three. We prospectively defined severe malarial anemia as hemoglobin <5 g/dL or hematocrit <15% according to the World Health Organization definition for this condition in children.34,35 Sixty-seven children had severe malarial anemia, 66 had uncomplicated malaria defined as hemoglobin ≥6 g/dL and the absence of coma as defined by a Blantyre coma score of 5, and 33 had cerebral malaria defined as a Blantyre coma score of 2 or less and hemoglobin ≥6 g/dL. The mean ± SD age was 27 ± 13 months and 82 (49.7%) were females. A little over half of the children had been treated with chloroquine, sulfadoxine-pyrimethamine or quinine before presentation but none had received an artemisinin derivative. The mean corpuscular volume tended to be greater in the children with severe anemia, suggesting that iron deficiency was not a major factor.
3.1.2 |. Effects of G6PD A+ and A−
The G6PD genotypes are summarized in Table 1. G6PD A− hemizygosity or homozygosity increased the adjusted odds of severe malarial anemia (OR 6.9; 95% CI 1.6–34; P = .008), confirming the results of the MalariaGEN study,7 but a similar trend existed with G6PD A+ hemizygosity or homozygosity (OR 2.5, 95% CI 0.76–8.6; P = .13). No association with severe anemia was found with heterozygotes for the G6PD A alleles (P = .4). We therefore defined G6PD A allele categories as no-risk (G6PD deficiency not present, that is. wild type or heterozygote for G6PD A alleles), mid-risk (hemizygosity or homozygosity for G6PD A+), and high-risk (hemizygosity or homozygosity G6PD A−). We observed an additive effect of G6PD allele categories on the risk of severe anemia (P = .003) (Figure 1).
FIGURE 1.
Zambian early childhood malaria cohort. There is a progressive increase in the proportion of children with severe anemia in the two risk categories of G6PD A alleles: (1) hemizygosity or homozygosity for G6PD A+ and (2) hemizygosity or homozygosity for G6PD A− (P = .003 after adjustment for weight-for-age z-score, splenomegaly, stool parasites and plasma concentrations of interleukin-10 and tumor necrosis factor-alpha, the most significant independent predictors of severe malarial anemia in this cohort as we previously reported)18
3.1.3 |. Effect of CYB5R3c350C > G
The allele frequency of CYB5R3c.350C > G was 0.31 (Table 2). We found a trend to a progressive reduction in the adjusted risk of severe anemia with heterozygosity and homozygosity for CYB5R3c.350C > G (OR = 0.55, 95% CI 0.26–1.1; P = .11). In further analysis, we observed an interaction between CYB5R3c.350C > G and G6PD A allele categories (presence or absence of G6PD risk categories) on severe anemia (P = .017). We therefore stratified our analysis according to the presence or absence of G6PD risk categories. In the children without G6PD deficiency (G6PD wild type or heterozygote for A alleles), CYB5R3c.350C > G offered protection against severe anemia (OR 0.34, 95% CI 0.11–0.90, P = .030, Figure 2A) in an additive model. This model suggests that CYB5R3c.350C > G heterozygotes have a 2.9-fold reduction in the odds of severe anemia and CYB5R3c.350C > G homozygotes have a 5.9-fold reduction in the odds if G6PD deficiency is not present. In contrast, in G6PD A+ or G6PD A− hemizygotes or homozygotes, CYB5R3c.350C > G did not show protection from severe malarial anemia (Figure 2B).
TABLE 2.
CYB5R3 mutation status in the three cohorts studied. Results in number (%)
| Mutation status | Zambian children with malaria (N = 165) | PUSH: US SCD children/ adolescents (N = 267) | Walk-PHaSST: US/ UKSCD adolescents/ adults (N = 321) |
| CYB5R3 wildtype subjects | 75 (45.5%) | 136 (50.9%) | 148 (46.1%) |
| CYB5R3c350C > G heterozygotes | 78 (47.3%) | 109 (40.8%) | 135 (42.1%) |
| CYB5R3c350C > G homozygotes | 12 (7.3%) | 22 (8.2%) | 38 (11.8%) |
FIGURE 2.
Zambian early childhood malaria cohort. We observed an interaction between CYB5R3c.350C > G genotypes (wildtype, heterozygote, hemizygote) and risk categories of G6PD A alleles on severe anemia (P = .017). We therefore stratified our analysis according to the presence or absence of G6PD risk categories. A, In G6PD wildtype children or heterozygotes for A alleles, CYB5R3c.350C > G offered protection against severe anemia (P = .030 after adjustment for the covariates mentioned in Figure 1). B, In hemizygotes or homozyogtes for G6PD A+ or G6PD A−, CYB5R3c.350C > G did not show protection from severe malarial anemia
3.2 |. SCD Pediatric cohort (PUSH)
3.2.1 |. PUSH cohort
To determine if the findings observed in Zambian children with malaria could be confirmed in another clinical setting, we studied 267 children and adolescents with SCD who had not received a recent blood transfusion (within 2 months) from four US centers, the PUSH cohort.19 Children were recruited as outpatients at steady state (without pain crisis, fever or other acute complications) when they presented for routine care. The mean ± SD age was 11 ± 5 years and 123 (46.1%) were females.
3.2.2 |. Genotype frequencies
Hemoglobin SS was present in 179 (67.0%), SC in 69 (25.8%), S-beta+-thalassemia in 10 (3.8%), S-beta0-thalassemia in six (2.3%) and other major sickling genotypes in six (2.3%) subjects. The G6PD genotype frequencies are shown in Table 1. The allele frequency of CYB5R3c.350C > G was 0.29 (Table 2). Single gene deletion α-thalassemia was present in 28.5% while double deletion α-thalassemia was present in 3.0% of the children (α-thalassemia allele frequency 0.086).
3.2.3 |. Association of G6PD A+ and A− with hemoglobin concentration and hemolytic component
We previously reported an independent association of G6PD A− with lower hemoglobin concentration in children with sickle cell anemia that did not appear to be related to increased hemolysis.23 Given the progressive association of G6PD A+ and A− with severe anemia that we observed in children with malaria (Figure 1), we examined the relationship of G6PD A+ and A− with hemoglobin concentration in patients with SCD in a multiple linear regression model that adjusted for age, sex, severe sickling genotype, α-thalassemia, treatment with hydroxyurea and clinical site. We observed progressively lower adjusted hemoglobin concentrations in PUSH children with G6PD A+ (hemizygotes or homozygotes for A+) and G6PD A− (hemizygotes or homozygotes for A−) compared to those who did not have G6PD deficiency (G6PD wild type or heterozygote for A alleles) (β = −0.30 g/dL, P = .012, n = 256). The hemolytic component as a marker of hemolysis did not increase with these G6PD A+ and A− categories in a multiple linear regression analysis (P > .9, n = 243).
3.2.4 |. Association of CYB5R3c.350C > G with hemoglobin concentration and hemolytic component
Given our findings in children with malaria in Zambia, we stratified our analysis of the relationship of CYB5R3c.350C > G with hemoglobin concentration and hemolytic component according to G6PD status: absence of G6PD deficiency (G6PD wildtype or A allele heterozygotes) vs G6PD A+ and A− (A+ or A− hemizygotes or homozygotes). Among children without G6PD deficiency, CYB5R3c.350C > G heterozygotes and homozygotes had progressively higher hemoglobin concentrations compared to CYB5R3 wildtype subjects in multiple linear regression analysis that adjusted for age, sex, severe sickling genotype, α-thalassemia, treatment with hydroxyurea and clinical site (β = 0.29, P = .022, n = 203) (Figure 3A). This analysis suggests that, if G6PD A+ and A− are not present, CYB5R3c.350C > G heterozygotes with SCD have a hemoglobin concentration that averages 0.3 g/dL higher than CYB5R3c.350C > G wild type subjects and that CYB5R3c.350C > G homozygotes with SCD have a hemoglobin concentration that averages 0.6 g/dL higher than wild type subjects. Among patients with G6PD A+ or A−, a significant association of CYB5R3c.350C > G with hemoglobin concentration was not observed (Figure 3B). Furthermore, CYB5R3c.350C > G heterozygotes and homozygotes had progressively lower values for the hemolytic component in subjects without G6PD deficiency (β = −0.57, P < .001 n = 193) but not in those with G6PD A+ or A− (Figure 3C,D).
FIGURE 3.
Children and adolescents with SCD. A, The adjusted hemoglobin concentration is higher in PUSH CYB5R3c.350C > G heterozygotes and homozygotes who possess G6PD wildtype alleles or are heterozygotes for G6PD A alleles. B, This does not apply to those who are hemizygotes or homozygotes for G6PD A+ or A−. C, The adjusted hemolytic component (derived by principal components analysis from reticulocytes and serum LDH, AST and bilirubin concentrations) is progressively lower in CYB5R3c.350C > G heterozygotes and homozygotes who are G6PD wildtype subjects or heterozygotes for A alleles. D, This does not apply to those who are G6PD A+ or A− hemizygotes or homozygotes
3.3 |. SCD adult and adolescent cohort (walk-PHaSST)
3.3.1 |. Walk-PHaSST cohort20
Subjects with SCD were recruited for the Walk-PHaSST study33 at nine United States sickle cell centers and one United Kingdom sickle cell center. Subjects aged ≥12 years and at steady state were eligible to participate. Here we evaluated 321 participants who did not have a recent blood transfusion based on 0% hemoglobin A on HPLC hemoglobin fractionation (for genotypes other than hemoglobin S-beta+-thalassemia), and whose serum creatinine was less than 1.5 mg/dL. The mean ± SD age was 37 ± 13 years and 173 (53.9%) were females.
3.3.2 |. Genotype frequencies
Hemoglobin SS was present in 225 (70.1%), SC in 86 (26.8%), S-beta+-thalassemia in eight (2.5%) and S-beta0-thalassemia in two (0.6%) patients. The G6PD allele frequencies are summarized in Table 1. The allele frequency of CYB5R3c.350C > G was 0.33 (Table 2). Single gene deletion α-thalassemia was present in 33.6% while double deletion α-thalassemia was present in 1.9% of the patients.
3.3.3 |. Association of G6PD A+ and A− with hemoglobin and hemolytic component
We did not observe significant trends of additive effects of G6PD A+ and A− genotypes on lowering the hemoglobin concentration (β = −0.60, P = .7, n = 319) and increasing the hemolytic component (β = 0.20, P = .16, n = 270) in multiple linear regression analyses that adjusted for age, sex, severe sickling genotype, alpha-thalassemia, and hemoglobin F percent.
3.3.4 |. Association of CYB5R3c.350C > G with hemoglobin concentration and hemolytic component
CYB5R3c.350C > G heterozygotes and homozygotes had progressively higher adjusted hemoglobin concentrations in the subjects without G6PD deficiency (G6PD wildtype or A allele heterozygote subjects) (β = 0.33, P = .004, n = 260) (Figure 4A) but not in the G6PD A+ or A− hemizygotes or homozygotes (Figure 4B). In keeping with reduced hemolysis, CYB5R3c.350C > G heterozygotes and homozygotes had progressively lower values for the hemolytic component in both the patients without G6PD deficiency (G6PD wild type or A allele heterozygote subjects) (β = −0.23, P = .038, n = 227) (Figure 4C) and in the G6PD A+ or A− hemizygotes or homozygotes (β = −0.52, P = .036, n = 43) (Figure 4D).
FIGURE 4.
Walk-PHaSST adults and adolescents with SCD. A, The adjusted hemoglobin concentration is progressively higher in the CYB5R3c.350C > G heterozygotes and homozygotes compared to CYB5R3 wildtype subjects B, but not in the hemizygotes or homozygotes for G6PD A+ or A−. C, The hemolytic component is progressively lower in the CYB5R3c.350C > G heterozygotes and homozygotes compared to CYB5R3 wildtype subjects both in subjects who are G6PD wildtype or G6PD A allele heterozygotes and D, among those who are hemizygotes or homozygotes for G6PD A+ or A−
3.4 |. Enzymatic activity of CYB5R3 according to CYB5R3T117S genotype
Figure 5 shows that there was a trend to mildly lower enzymatic activity of CYB5R3T117S compared to wild type CYB5R3 in the erythrocytes of three groups of SCD patients: 40 adult participants in Walk-PHaSST, 25 adult UIC patients, and 31 ARUP children with sickle hemoglobin (P = .001 for test of trend). A lower kcat and a higher Km(NADH) for CYB5R3T117S than wild type CYB5R3 has been reported,36,37 compatible with a lower substrate turnover rate and weaker affinity implying lower enzyme activity. However, the data in reference 36 is from an artificial fusion protein of the microsomal soluble heme-containing fragment of cytochrome b5 and the soluble flavin-containing fragment of cytochrome b5 reductase. The fusion protein contains differences in sequence and length compared to CYB5R3.
FIGURE 5.
There is a progressive decrease in the erythrocyte CYB5R3 enzymatic activity in CYB5R3c.350C > G heterozygotes and homozygotes compared to wildtype in SCD patients. Shown are geometric mean and SE activity after adjustment for laboratory where assay was performed. Walk-PHaSST 40 patients, ARUP 31 patients, UIC 25 patients
3.5 |. Erythrocyte NADH/NAD+ ratio according to CYB5R3c.350C > G genotype
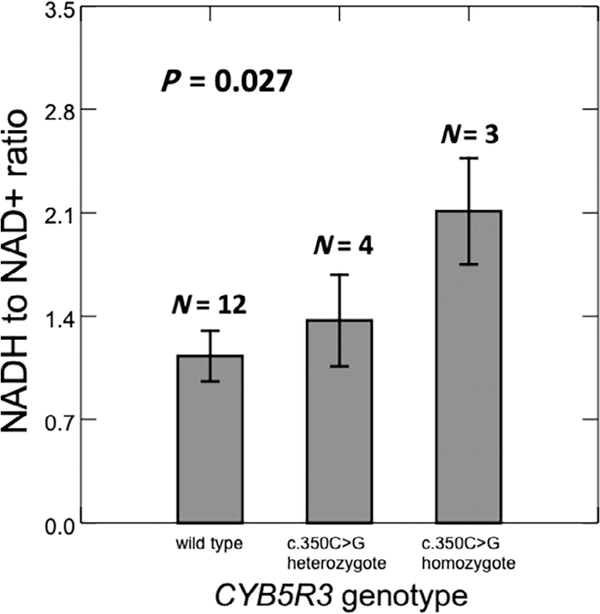
We measured erythrocyte NADH/NAD+ ratios in hemolysates of 19 SCD patients. Four were heterozygotes and three homozygotes for CYB5R3c.350C > G; two had G6PD A+, two had G6PD A−, and 15 did not have G6PD deficiency. The mean NADH/NAD+ ratio increased progressively in CYB5R3c.350C > G heterozygotes and homozygotes (P = .034) and in G6PD A+ and A− patients (P = .021). Figure 6 depicts the mean NADH/NAD+ ratio according to CYB5R3 genotype after adjustment for G6PD A+ and A−. The NADH/NAD+ ratio increased progressively in the CYB5R3c.350C > G heterozygotes and homozygotes (beta = 0.44, P = .027), compatible with lower enzyme activity of the CYB5R3c.350C > G variant.
FIGURE 6.
In multiple linear regression analysis, NADH/NAD+ ratio increases with CYB5R3c.350C > G genotype in 19 UIC SCD patients. Shown are mean and SE of the NADH/NAD+ ratio as adjusted for G6PD A+ and A−
4 |. DISCUSSION
In summary, (a) we confirm an association of G6PD A− with severe malarial anemia in southern Zambian children, (b) we observe an additive increased risk of severe malarial anemia of G6PD A+, (c) we report amelioration of severe malarial anemia with CYB5R3c.350C > G in children with wildtype G6PD or heterozygosity for A alleles, but not in those with hemizygosity or homozygosity for G6PD A+ or A−, (d) we observe similar patterns in pediatric and adult SCD patients with regard to progressively higher hemoglobin concentrations in CYB5R3c.350C > G heterozygotes and homozygotes who are G6PD wildtype or heterozygotes for G6PD A alleles, and (e) we demonstrate slightly decreased CYB5R3 activity and increased NADH to NAD+ ratios in CYB5R3c.350C > G sickle erythrocytes.
CYB5R3c.350C > G is the most common known African polymorphism and thus is present in a large proportion of patients with malaria and SCD. The results of the present study suggest that, in patients with malaria or SCD, CYB5R3c.350C > G may be associated with a lesser degree of anemia in G6PD wild type individuals or those who are heterozygotes for the G6PD A alleles. According to our models and compared to CYB5R3 wild type individuals, heterozygotes and homozygotes for CYB5R3c.350C > G have estimated 2.9-fold and 5.9-fold reductions in the odds of severe anemia in children with malaria and estimated 0.3 g/dL and 0.6 g/dL increases in hemoglobin concentration in patients with SCD. The degree of anemia is a determinant of mortality in children infected with P. falciparum malaria6,7 and the present study suggests that CYB5R3c.350C > G may protect from severe anemia in children with P. falciparum malaria, which may contribute to its high gene frequency in Africa. The observation that CYB5R3c.350C > G may ameliorate hemolysis in patients with SCD provides a surrogate independent argument for the beneficial effect of this common African-specific polymorphism on preventing severe malarial anemia in children. Nevertheless, with the small cohort of patients studied and realizing that the etiology of anemia in Africa children is complex, more studies are needed to critically establish the influence of G6PD and CYB5R3 genotypes on severe malarial anemia.
The results of the present study indicate that CYB5R3c.350C > G genotype should be considered in future studies of risk profile for hemolysis and its complications in patients with SCD.38 The high allele frequency points to the possibility that it could be a phenotype-modifying factor in about a half of patients with SCD. The findings of lower hemolytic parameters and higher hemoglobin concentration in SCD patients with the common CYB5R3c.350C > G allele in the present study are comparable to the effect of coexistent α-thalassemia.11 The lower hemolytic rate and higher hemoglobin concentration associated with α-thalassemia in the setting of SCD are balanced by a tendency to a higher rate of vaso-occlusive complications.39 Therefore investigations are needed to characterize potential beneficial and detrimental effects of CYB5R3c.350C > G in patients with SCD.
If our observation of reduced CYB5R3 enzyme activity and increased NADH/NAD+ ratio in CYB5R3c.350C > G positive sickle erythrocytes is correct and also applies to malaria-infected erythrocytes, then an anti-oxidant effect might explain a beneficial effect on hemolysis. L-glutamine has been administered to patients with sickle cell anemia to decrease sickle erythrocyte susceptibility to oxidative stress by increasing the NADH/NAD+ ratio,40 and this approach has proven to have clinical benefit in a multicenter study.41 An increase in the NADH/NAD+ ratio in CYB5R3c.350C > G erythrocytes might favor less conversion of glyceraldehyde-3-phosphate to 1,3-diphosphogycerate by glyceraldehyde-3-phophate dehydrogenase with an accumulation of upstream metabolites including glucose-6-phosphate, the crucial substrate for the hexose monophosphate shunt (HMP) and anti-oxidant function of G6PD. On the other hand, with decreased activity of G6PD any such benefit of this variant CYB5R3T117S enzyme might be lost. Hemoglobin conformation controls the dominance of either the Embden-Meyerhof pathway (EMP) or the HMP. Deoxygenated hemoglobin favors EMP dominance whereas oxygenated hemoglobin favors HMP dominance.42 In sickle erythrocytes, sickle hemoglobin is hypothesized to bind to band 3 protein, thus favoring the EMP and disfavoring the HMP, making sickle erythrocytes vulnerable to oxidant attack.43 The enzyme that plays a key role in controlling the glycolytic flux is glyceraldehyde-3-phophate dehydrogenase, the direct donor of NADH for the methemoglobin-reductase pathway. Our data support the hypothesis that the increased erythrocyte NADH/NAD+ ratio among those with the CYB5R3c.350C > G mutation may lead to a dominance of HMP over EMP, thus favoring protection from hemolysis.
In summary, we provide the first evidence of a beneficial effect of CYB5R3T117 that may contribute to its high gene frequency among Africans.
Acknowledgments
Funding information
National Heart, Lung, and Blood Institute, Grant/Award Numbers: 1 R01 HL079912-02, 2 R25 HL003679-08; NCRR, Grant/Award Number: 2MOI RR10284-10
Footnotes
CONFLICT OF INTEREST
All authors declare no conflict of interest with the content of the manuscript.
REFERENCES
- 1.Becker K, Tilley L, Vennerstrom JL, Roberts D, Rogerson S, Ginsburg H. Oxidative stress in malaria parasite-infected erythrocytes: host-parasite interactions. Int J Parasitol. 2004;34(2):163–189. [DOI] [PubMed] [Google Scholar]
- 2.Das BS, Nanda NK. Evidence for erythrocyte lipid peroxidation in acute falciparum malaria. Trans R Soc Trop Med Hyg. 1999;93(1):58–62. [DOI] [PubMed] [Google Scholar]
- 3.Omodeo-Sale F, Motti A, Basilico N, Parapini S, Olliaro P, Taramelli D. Accelerated senescence of human erythrocytes cultured with Plasmodium falciparum. Blood. 2003;102(2):705–711. [DOI] [PubMed] [Google Scholar]
- 4.Hebbel RP, Eaton JW, Balasingam M, Steinberg MH. Spontaneous oxygen radical generation by sickle erythrocytes. J Clin Invest. 1982; 70(6):1253–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amer J, Ghoti H, Rachmilewitz E, Koren A, Levin C, Fibach E. Red blood cells, platelets and polymorphonuclear neutrophils of patients with sickle cell disease exhibit oxidative stress that can be ameliorated by antioxidants. Br J Haematol. 2006;132(1):108–113. [DOI] [PubMed] [Google Scholar]
- 6.Ruwende C, Khoo SC, Snow RW, et al. Natural selection of hemi- and heterozygotes for G6PD deficiency in Africa by resistance to severe malaria. Nature. 1995;376(6537):246–249. [DOI] [PubMed] [Google Scholar]
- 7.Rockett KA, Clarke GM, Fitzpatrick K, et al. Reappraisal of known malaria resistance loci in a large multicenter study. Nat Genet. 2014; 46(11):1197–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leikin SL, Gallagher D, Kinney TR, Sloane D, Klug P, Rida W. Mortality in children and adolescents with sickle cell disease. Cooperative Study of Sickle Cell Disease. Pediatrics. 1989;84(3):500–508. [PubMed] [Google Scholar]
- 9.Sebastiani P, Nolan VG, Baldwin CT, et al. A network model to predict the risk of death in sickle cell disease. Blood. 2007;110(7):2727–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams TN, Wambua S, Uyoga S, et al. Both heterozygous and homozygous alpha+ thalassemias protect against severe and fatal Plasmodium falciparum malaria on the coast of Kenya. Blood. 2005; 106(1):368–371. [DOI] [PubMed] [Google Scholar]
- 11.Embury SH, Dozy AM, Miller J, et al. Concurrent sickle-cell anemia and alpha-thalassemia: effect on severity of anemia. N Engl J Med. 1982;306(5):270–274. [DOI] [PubMed] [Google Scholar]
- 12.Strittmatter P The reaction sequence in electron transfer in the reduced nicotinamide adenine dinucleotide-cytochrome b5 reductase system. J Biol Chem. 1965;240(11):4481–4487. [PubMed] [Google Scholar]
- 13.Agarwal A, Prchal JT. In: Kaushansky K, Lichtman MA, Prchal JT, et al. , eds. Methemoglobinemia and Other Causes of Cyanosis. Chapter 50 Williams Hematology. 9th ed. New York, NY: McGraw Hill; 2015:789–800. [Google Scholar]
- 14.Jenkins MM, Prchal JT. A high-frequency polymorphism of NADH-cytochrome b5 reductase in African-Americans. Hum Genet. 1997;99 (2):248–250. [DOI] [PubMed] [Google Scholar]
- 15.Gomez-Manzo S, Marcial-Quino J, Vanoye-Carlo A, et al. Mutations of Glucose-6-Phosphate Dehydrogenase Durham, Santa-Maria and A + Variants Are Associated with Loss Functional and Structural Stability of the Protein. Int J Mol Sci. 2015;16(12):28657–28668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luzzatto L, Nannelli C, Notaro R. Glucose-6-Phosphate Dehydrogenase Deficiency. Hematol Oncol Clin North Am. 2016;30(2):373–393. [DOI] [PubMed] [Google Scholar]
- 17.Calis JC, Phiri KS, Faragher EB, et al. Severe anemia in Malawian children. Malawi Med J. 2016;28(3):99–107. [PMC free article] [PubMed] [Google Scholar]
- 18.Thuma PE, van Dijk J, Bucala R, et al. Distinct clinical and immunologic profiles in severe malarial anemia and cerebral malaria in Zambia. J Infect Dis. 2011;203(2):211–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Minniti CP, Sable C, Campbell A, et al. Elevated tricuspid regurgitant jet velocity in children and adolescents with sickle cell disease: association with hemolysis and hemoglobin oxygen desaturation. Haematologica. 2009;94(3):340–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Machado RF, Barst RJ, Yovetich NA, et al. Hospitalization for pain in patients with sickle cell disease treated with sildenafil for elevated TRV and low exercise capacity. Blood. 2011;118(4):855–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tan AS, Quah TC, Low PS, Chong SS. A rapid and reliable 7-deletion multiplex polymerase chain reaction assay for alpha-thalassemia. Blood. 2001;98(1):250–251. [DOI] [PubMed] [Google Scholar]
- 22.Pont-Kingdon G, Chou LS, Damjanovich K, et al. Multiplex genotyping by melting analysis of loci-spanning probes: beta-globin as an example. Biotechniques. 2007;42(2):193–197. [DOI] [PubMed] [Google Scholar]
- 23.Nouraie M, Reading NS, Campbell A, et al. Association of G6PD with lower haemoglobin concentration but not increased haemolysis in patients with sickle cell anaemia. Br J Haematol. 2010;150(2):218–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bae HT, Baldwin CT, Sebastiani P, et al. Meta-analysis of 2040 sickle cell anemia patients: BCL11A and HBS1L-MYB are the major modifiers of HbF in African Americans. Blood. 2012;120(9):1961–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang J, Lee SH, Goddard ME, Visscher PM. GCTA: a tool for genome-wide complex trait analysis. Am J Hum Genet. 2011;88(1):76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Purcell S, Neale B, Todd-Brown K, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Delaneau O, Marchini J, Zagury JF. A linear complexity phasing method for thousands of genomes. Nat Methods. 2011;9(2):179–181. [DOI] [PubMed] [Google Scholar]
- 28.Howie BN, Donnelly P, Marchini J. A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet. 2009;5(6):e1000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hegesh E, Calmanovici N, Avron M. New method for determining ferrihemoglobin reductase (NADH-methemoglobin reductase) in erythrocytes. J Lab Clin Med. 1968;72(2):339–344. [PubMed] [Google Scholar]
- 30.Beutler E Red Cell Metabolism: A Manual of Biochemical Methods. 3rd ed. New York, NY: Grune & Stratton, Inc; 1984. [Google Scholar]
- 31.Balasubramaniam P, Malathi A. Comparative study of hemoglobin estimated by Drabkin’s and Sahli’s methods. J Postgrad Med. 1992;38 (1):8–9. [PubMed] [Google Scholar]
- 32.Nouraie M, Lee JS, Zhang Y, et al. The relationship between the severity of hemolysis, clinical manifestations and risk of death in 415 patients with sickle cell anemia in the US and Europe. Haematologica. 2013;98(3):464–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sachdev V, Kato GJ, Gibbs JS, et al. Echocardiographic markers of elevated pulmonary pressure and left ventricular diastolic dysfunction are associated with exercise intolerance in adults and adolescents with homozygous sickle cell anemia in the United States and United Kingdom. Circulation. 2011;124(13):1452–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.WHO. Severe falciparum malaria. Trans R Soc Trop Med Hyg. 2000;94: S1–S90. [PubMed] [Google Scholar]
- 35.WHO. Severe malaria. Trop Med Int Health. 2014;19(Suppl 1):7–131. [DOI] [PubMed] [Google Scholar]
- 36.Davis CA, Barber MJ. Heterologous expression of enzymopenic methemoglobinemia variants using a novel NADH:cytochrome c reductase fusion protein. Protein Expr Purif. 2003;30(1):43–54. [DOI] [PubMed] [Google Scholar]
- 37.Roma GW. Systemic Analysis of Structure-Function Relationships of Conserved Sequence Motifs in the NADH-Binding Lobe of Cytochrome b5 Reductase. Tampa, FL: University of South Florida; 2008. [Google Scholar]
- 38.Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. Lancet. 2010; 376(9757):2018–2031. [DOI] [PubMed] [Google Scholar]
- 39.Renoux C, Connes P, Nader E, et al. Alpha-thalassaemia promotes frequent vaso-occlusive crises in children with sickle cell anaemia through haemorheological changes. Pediatr Blood Cancer. 2017;64: e26455. 1–8. [DOI] [PubMed] [Google Scholar]
- 40.Niihara Y, Zerez CR, Akiyama DS, Tanaka KR. Oral L-glutamine therapy for sickle cell anemia: I. Subjective clinical improvement and favorable change in red cell NAD redox potential. Am J Hematol. 1998;58(2):117–121. [DOI] [PubMed] [Google Scholar]
- 41.Niihara Y, Miller ST, Kanter J, et al. A phase 3 Trial of l-glutamine in sickle cell disease. N Engl J Med. 2018;379(3):226–235. [DOI] [PubMed] [Google Scholar]
- 42.Rogers SC, Said A, Corcuera D, McLaughlin D, Kell P, Doctor A. Hypoxia limits antioxidant capacity in red blood cells by altering glycolytic pathway dominance. FASEB J. 2009;23(9):3159–3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rogers SC, Ross JG, d’Avignon A, et al. Sickle hemoglobin disturbs normal coupling among erythrocyte O2 content, glycolysis, and antioxidant capacity. Blood. 2013;121(9):1651–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]