Abstract
One avenue of progress toward understanding the neurobiological basis of autism is through the detailed study of the post‐mortem brain from affected individuals. The primary purpose of autism brain tissue banking is to make well‐characterized and optimally preserved post‐mortem brain tissue available to the neuroscience research community. In this paper we discuss our current understanding of the criteria for optimal characterization and preservation of post‐mortem brain tissue; the pitfalls associated with inadequate clinical and neuropathological characterization and the advantages and disadvantages of post‐mortem studies of the brain. We then describe the current status of the brain tissue bank supported by the Autism Tissue Program, including the demographic characteristics of the tissue donors, post‐mortem interval, sex, age and the method of preservation. Finally, we provide information on the policies and procedures that govern the distribution of brain specimens by this bank and the nature of the studies that are currently being supported directly by this program.
INTRODUCTION
The fundamental purpose of brain and tissue banking is to provide appropriate specimens for research studies (2, 5, 13, 17, 26, 35, 37, 54). These research studies generally explore basic neurobiological systems of the brain; such as neuroanatomical and neurochemical organization and function, cell structure and function, signal transduction etc.; or they seek to identify specific lesions and neurobiological properties that are associated with diverse diseases, such as autism, fragile‐X, schizophrenia, Alzheimer's disease, multiple sclerosis, Huntington's disease, etc. Understanding the basic typical and atypical structures and functions of the brain is a critical step to the understanding of these diseases and the development of rational treatment approaches for them. The developmental nature of autism makes this task more complex and highlights the importance of learning about brain development in normal as well as in disease states. Presently only a few brain banks collect and distribute a very small number of brain tissue specimens relevant to autism research, which limits the number and scope of studies that can be undertaken by the research community. Recent efforts by the NIH, advocacy groups and organizations such as Autism Speaks combined with the altruism and generosity of tissue donors have made significant strides in overcoming this critical roadblock to progress in autism neurobiology research. These efforts have made the future significantly brighter today than it was just 5 years ago and have made it possible to assemble the current mini‐symposium.
The relatively small literature on neuropathological findings in autism was reviewed by one of us (JP) recently (44, 45) and by others (eg, 11, 20, 30), and neuroanatomical findings are the subject of discussion by Dr Casanova in an article in this mini‐symposium. Although abnormalities within some brain structures such as the cerebellum and the neocortex have been appreciated and evidence for gross changes such as brain enlargement in a subset of cases has been found (10, 12, 50), unlike such neurological diseases as Alzheimer's disease, Huntington's disease, Parkinson's disease and multiple sclerosis, specific and invariant lesions associated with autism have not been recognized yet. However, the advances made in understanding the neurobiological substrates of many diseases of the brain have been caused in part by the availability of well‐characterized post‐mortem brain tissue for study (26, 38). The availability of banked brain tissues has not only permitted the identification of specific pathological hallmarks, but the study and elucidation of some of the prominent biochemical and molecular pathways whose dysfunction contributes to the identified neuropathology. It is likely that as the availability of well‐characterized post‐mortem brain tissue specimens expands, the use of these specimens by the research community will uncover critical lesions, brain regions and neurobiological pathways that will help us understand autism neuropathology and develop rational and evidence‐based approaches to its treatment. The autism brain atlas project initiative described below is an effort to jump‐start this quest by identifying, through systematic and quantitative approaches, the brain regions and architectural features of greatest vulnerability to the disease. The discovery of these vulnerable structures and cell groups will help guide neurobiological studies to the most affected brain regions and cell types, provide clues for animal model studies, help develop and evaluate treatment approaches, and perhaps reveal etiological culprits.
PHENOTYPIC CHARACTERIZATION
The most critical and basic aim of any brain tissue banking program must be attention to the phenotypic characteristics of the tissue donors. One of the foremost goals of a tissue bank must be to remain continuously vigilant and sensitive to the maxim of “garbage‐in/garbage‐out”. Failure to adhere to this dictum can invalidate any subsequent conclusions that are drawn from tissue‐based research studies. To satisfy this most basic brain banking need a series of requirements must be satisfied. These requirements include detailed knowledge of the psychological, neuropsychiatric, neurologic, neuropathological and medical characteristics of the tissue donors and the technical practices that promote optimal preservation of the specimens donated and banked. These essential requirements are interdependent and can rarely be satisfied completely. The true challenge is how close the bank can come to the ideal preservation methodology(ies) and to the complete description of the tissue donor.
In clinical and animal studies we have come to not only expect but to demand accurate, standardized and evidence‐based diagnosis, rigid control over extraneous variables and application of stringent inclusion and exclusion criteria to the study population. Patients with comorbid diseases and conditions are screened out and experimental confounds are obviated. When clear control over a variable cannot be achieved, sample sizes are increased to compensate and to minimize the contribution of uncontrolled variables. Adherence to these experimental issues has become second nature in clinical and animal studies, but too often they are ignored or overlooked in research with specimens from brain tissue banks. This is in part caused by the scarcity of available and well‐characterized brain tissue, and is in part caused by the lack of readily available ante‐mortem phenotypic information. As in most cases ante‐mortem assessment of post‐mortem tissue donors is impractical or even impossible, a detailed assessment and characterization of the specimen and the donor is not undertaken and the lack of evidence for confounding and intervening variables is accepted as the absence of such confounds. These concerns are as true, or even more true, for specimens derived from “control” cases as they are for specimens from persons identified as affected. Similarly, too often neuropathological and general autopsy studies are not undertaken and researchers rely on the accuracy of findings gleaned from the medical records and death certificates. Multiple studies have shown significant discrepancies, sometimes as large as 33%–50%, between for example clinically inferred diagnoses and causes of death vs. findings at autopsy (1, 36, 39, 47, 49, 51). In the experience of one of us (VH), 40% of cases derived from medical examiner sources and identified at initial screening as controls have been found on detailed medical record review and informant interviews to have suffered from one or more axis I psychiatric conditions. If a specimen is released for research as a normal control based on a neuropathological examination showing no evidence of significant neuropathological lesions, its use as a normal control specimen must be approached with extreme uncertainty as a large number of factors such as autism, mental illness, substance and alcohol use and abuse, renal and hepatic disease, etc. (24) can influence brain biochemistry and structure without clear neuropathological footprints.
The ante‐mortem information available for tissue donors must be complete enough to permit application of inclusion and exclusion criteria with at least as much rigor as that applied in clinical studies. It is the in‐depth characterization of the specimens used for research, both neuropathologically and clinically, that gives the specimens value for research and neither the clinical nor the neuropathological assessment of the specimens can be ignored without substantial cost. In one study comparing two post‐mortem psychological autopsy methods, concordance kappa scores for a diagnosis of schizophrenia were acceptable and high (0.94), but kappa scores for the diagnosis of major depression and bipolar disorder were poor at best (0.68 and 0.58, respectively) (14). These and similar studies suggest that without rigorous and meticulous attention to the characteristics of tissue specimens banked and distributed for research, neurobiological findings can be undermined significantly, leading to inconclusive results at best and erroneous and non‐replicable findings at worst. As described below, the Autism Tissue Program (ATP) has attempted to address these issues by conducting detailed and extensive semi‐structured interviews with the most knowledgeable informants and historians for the cases banked and distributed for research.
DOCUMENTATION AND DATA SHARING
Documentation, archiving, record keeping and database design and maintenance are more mundane, but critical, factors in the successful use of tissue banks. Ante‐mortem and post‐mortem assessment results must be documented in an accessible manner for the information to be of value from a research perspective. Databases must be maintained accurately, imparting the maximum of information to researchers accessibly, while at the same time safeguarding donor confidentiality and enforcing blinded distributions of tissue until data collection is completed. Just as the data derived from a tissue bank investigation is only as good as the characterization of the tissue and donor, even the most extensive characterization of the tissue and donor is of little practical use if the fruits of that characterization are not readily available to the tissue users. Although not commonly practiced, ideally all raw data derived from research on banked specimens should flow back to the bank and become archived. Such a system makes it possible for different tissue recipients and researchers to be cognizant of related research efforts and to maximize the gains from each research project by collaborating with each other. The ATP and others (eg, Harvard Brain Tissue Resource Center (HBTRC), The Mount Sinai School of Medicine Alzheimer's disease and Schizophrenia Brain Bank) have implemented programs to make extensive donor‐related information available to the brain tissue researchers through secure and privacy protected web‐based data portals and encourage data sharing activities between tissue recipients. Indeed, data sharing is now an obligate activity associated with ATP tissue distribution to research laboratories.
PLANNING FOR THE PRESENT AND THE FUTURE
Another general issue that confronts tissue banking is time. Most often, time constraints are viewed as the constraints on tissue and biochemical integrity posed by the time between death and when tissue is harvested and preserved—the post‐mortem interval (PMI). Some biomarkers have relatively short storage half‐lives, specific antigenicity can be lost over time and some neurochemicals deteriorate over prolonged periods of storage (6, 15, 16, 23, 31, 53). Additional constraints imposed by confounding variables and factors can significantly limit the numbers of specimens that are available for specific research studies. Factors such as PMI, peri‐mortem agonal state, licit and illicit drug exposure (19, 24), comorbid medical, psychiatric and neurological conditions all extract a significant cost to the numbers of cases and controls that can be used in a given study. For example, studies have shown that although post‐mortem brain specimens can be profitably used for gene expression research (18, 29, 48), agonal factors such as fever, coma, acidosis, etc., can profoundly influence the integrity of isolated RNA and compromise gene expression study findings (2, 6, 9, 25, 27, 33, 46, 55). Similar considerations apply to the influence of treatment drugs. Of course, these interpretive issues are not unique to post‐mortem tissue research and apply equally to the study of autism in living subjects. What is important to note from a brain banking perspective is that these considerations place significant constraints on the numbers of specimens that can be made available for specific studies. Although the brain banking experience with autism is not extensive enough to calculate accurately ratios for numbers of specimens collected vs. distributed for specific studies, experience in other brain diseases suggest that the gain‐to‐loss ratios for specific studies can range from 20% to 70%. Thus, sample attrition because of variables and conditions that can interfere with specific projects must be foreseen and accommodated. On the other hand, many of the constraints mentioned earlier are not universally applicable to all research projects. For example, the fact that the PMI was prolonged (e.g., 25 h) may preclude the study of biogenic amines in a given specimen, but studies that seek clinicopathological correlations are still feasible. Therefore, all specimens received by brain banks are invaluable, but the presence or absence of “interfering” factors affects the range of studies that a specific donation can support.
A more insidious and less tangible issue for brain banking is the interaction of time with the changing scientific landscape, the nature of research methodologies and the types of hypotheses that are posed over a period of years. Almost by definition a bank accumulates specimens over a protracted period of time. A bank with optimally designed policies and methods for the questions posed today must also be in a position to satisfy experimental and research questions of the future which may demand a different banking approach. As specimens accumulate slowly, a significant challenge to tissue banking is the need to anticipate the research needs of the future and to prepare to meet those needs, sometimes years in advance. These constraints and considerations require therefore adoption of banking protocols that accommodate as broad a set of experimental approaches and protocols as possible. A concrete example is the development of stereology methodologies for neuroanatomical studies (22, 41, 43, 52). At the heart of most stereological approaches is the availability of the entire structure of interest to permit implementation of specific sampling strategies. If the specimens are collected and subdivided to accommodate different research needs with different fixation and preservation protocols, then stereological analyses cannot be implemented in those specimens. At the level of donor characterization, the data gathered soon after death must anticipate the data needs of the future. For example, in Alzheimer's disease associated brain banking 15 years ago, we did not anticipate the need to know which exact cardiovascular drugs, steroid hormone or anti‐inflammatory agents the tissue donors were exposed to. With clinical studies now implicating these agents in disease progression the lack of drug exposure histories renders those specimens ineligible for some studies. Thus, in an optimal brain banking environment, donor characterization must extend beyond the known variables and issues of the day and anticipate the requirements of the research environment of the future. Many national brain banks continue to distribute brain tissue specimens today that were collected more than a decade or two ago. It is only through forward thinking and good preservation techniques that tissue longevity can be sustained. Just as 20 years ago we collected brain tissue specimens with the objective of their continued use with 21st century biotechnologies, we must collect, preserve and bank tissue today for the unforeseen technological advances and research questions of tomorrow. This challenge requires a certain degree of conservatism in applying preservation techniques that may be superior in the short‐run, but ones that may not have been shown to withstand the test of time. On the other hand, too conservative an approach toward tissue preservation runs the risk of making the banked specimens irrelevant to the needs of the future. The challenge is striking a balance between these potentially opposing forces. To this end, most brain banks, including the ATP, have adopted a fixed × frozen model where parts of each donated brain are fixed in formalin or paraformaldehyde while other regions are flash frozen and kept at –80°C until use. Precisely which brain regions are frozen and which are fixed varies from bank to bank, but frequently, as is the case with the ATP, one hemisphere is fixed and the other is coronally sectioned into ~1 cm. blocks and flash frozen whenever possible. This protocol has the advantage of preserving tissue for neuroanatomical and ex vivo neuroimaging studies (the fixed side or blocks), while permitting neurochemical and molecular biological studies to be performed in the same brain using the fresh‐frozen side or blocks. This protocol has the additional advantage of supporting a broad spectrum of neuroanatomical studies of the frozen blocks (28).
LIMITATIONS AND CAVEATS
Despite the proven knowledge that can be gained from the study of post‐mortem brain tissue, the limitations of the approach must also be acknowledged. By its very nature, the study of post‐mortem tissue involves exploration of a static state. Most post‐mortem studies reveal a snapshot of the brain at the time of death. Of course some enduring lesions, such as neuritic plaques in Alzheimer's disease or substantia nigra neuronal loss in Parkinson's disease persist and can reveal changes that occurred long before death, but the dynamic abnormalities that led to the accumulation of the neuritic plaque or to the neuronal loss are difficult if not impossible to assess post‐mortem. Knowledge of the existence of such lesions can, however, spark other studies including animal models and in vitro approaches to reveal the dynamic processes that led to the generation of the lesion. The scarcity of available tissue for study also encourages studies that are limited in their sample size and sampling methodologies. Small sample sizes rob the study of statistical power to detect true effects when they exist, while at the same time increasing the likelihood of the false discovery of changes that are likely caused by idiosyncratic differences between donors and the identification of state‐dependent changes in tissue as opposed to trait markers of disease. These considerations are more acute in post‐mortem brain studies than they are in clinical research because more often than not the availability of tissue precludes application of selection criteria as stringently as in living subjects. In addition, the manner of death and agonal state are considerations that are specific to post‐mortem studies of the brain. Many of these considerations are valid for tissue specimens derived from control donors as they are for specimens derived from persons with autism, but the fact that some of these factors may be disproportionately represented in one group vs. the other can pose a significant interpretive dilemma. Comorbid neurological conditions such as seizure are common to autism (see Figure 1) and significant evidence suggests that seizures can contribute to neuronal loss (40). On the other hand, seizures, by definition are not common in controls and this discrepancy between study groups must be kept in mind when interpreting study results. The possibility of misidentifying state dependent changes as stable indices of disease is exaggerated further in autism by the developmental nature of the disease. Thus, considerations of central nervous system changes that occur in the course of the normal development of the brain and individual differences in the developmental trajectories and rate become issues of paramount importance in study design and data interpretation. For example, it is not unreasonable to hypothesize that the disease process might involve or influence myelination of axons in the brain. Myelination is, however, highly regulated developmentally and continues from infancy well into adulthood as well as varying significantly in males and females (21, 32, 42). A post‐mortem study of myelination in autism brain would need to exercise very precise control over age and sex and would need to involve a large enough sample of cases and controls to accommodate broad natural variations. Similar considerations apply to the study of neurotransmitter systems (8) and nearly every other aspect of the brain.
Figure 1.
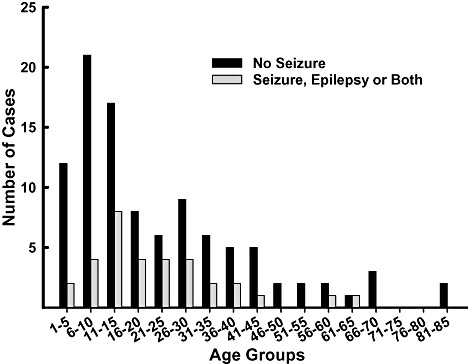
Seizure history of donors
THE AUTISM TISSUE BANK
History. The need for brain tissue as a critical resource to advance autism research was highlighted at a 1995 NIH‐sponsored meeting reviewing the “State of the Science in Autism” (4, 7). How to obtain tissue to maximize its use was not clear however, and the newly organized parent‐led National Alliance for Autism Research (NAAR) hosted a 2‐day meeting with experts in brain banking and tissue research to understand how it might best support brain collection. Deliberations addressed many of the brain banking standards described earlier to ensure that enough tissue of high quality went into the hands of qualified scientists. The result was the formation of the ATP (http://www.brainbank.org/) by NAAR with the Autism Society of America. At that time there were about 20 (autism) donor specimens housed in various US labs and banks and the initial thrust of the ATP was to launch a national publicity campaign to inform the autism community about the importance of brain donation to the NIH‐funded university brain banks (Harvard, Maryland and Miami). Further support for autism brain acquisition resulted from a mandate issued through the Children's Health Act of 2000 (http://frwebgate.access.gpo.gov/cgi‐bin/getdoc.cgi?dbname=106_cong_public_laws&docid=f:publ310.106) that authorized a program to collect and share brain tissue samples for autism research. The Act also mandated a federal Interagency Autism Coordinating Committee. In 2003, this body designated the HBTRC (http://www.brainbank.mclean.org) as the main autism brain repository and today the ATP continues to promote the donation of tissue to this established brain bank using tissue collection, processing and storage protocols that aim to maximize the research potential of each donation. An important feature of the ATP is that this rare resource is shared through an open application process and under the guidance of a specialized Tissue Advisory Board (TAB).
Current state of the collection. As of mid‐2007, the ATP had acquired 140 brain specimens in the USA and Canada. Of the 140 donors, 91 are reported to be autistic; home visits and records with Autism Diagnostic Interview‐Revised (ADI‐R, see below) have confirmed autism in 64 donors, three Asperger's, 27 relatives of a person with autism, 11 with other disorders and eight non‐affected controls. The ethnicity and sex of the ATP donors with autism spectrum disorders (autism and Asperger's) is provided in Table 1.
Table 1.
Ethnicity and sex of ATP tissue donors. Abbreviation: ASD = autism + Asperger's.
| ASD ethnicity | Female | Male | Total |
|---|---|---|---|
| African American | 2 | 11 | 13 |
| Asian | 0 | 4 | 4 |
| Hispanic | 1 | 3 | 4 |
| White | 15 | 58 | 73 |
| Total | 18 | 76 | 94 |
Ante‐mortem and post‐mortem characterization. There was early recognition that the ATP needed to establish diagnostic criteria and procedures to carefully document medical history and the neuropsychological attributes of the persons who donated tissue. Autism is characterized by abnormal behaviors in social, communication and repetitive‐motor domains as described by the DSM‐IV (3). Ante‐mortem characterization of the brain donor focused on the diagnosis of autism and a clinical protocol was established that used a recognized quantitative assessment of autism that relies on parent or close caregiver reporting, the ADI‐R (34). This assessment and a questionnaire are completed during a home visit with specially trained and certified clinicians who also collect medical records including medications and immunizations, other neuropsychological evaluations, educational evaluations with test scores, speech and hearing test reports, family history and genetic test results. The ATP makes a special effort to undertake ante‐mortem assessment of the unaffected, control brain donors. Home visits are arranged when possible and various tools that can reliably use parent or caregiver reports to measure social responsiveness and language development are being considered for addition to the clinical profile of all donors. The post‐mortem donor profile includes the autopsy report, conditions surrounding the death (agonal state) and amount of time elapsing from death to tissue retrieval (PMI) as well as details about the brain itself, such as fresh weight and any pathology observed during a neuropathology examination.
Conditions such as epilepsy are often present with autism and Figure 1 shows the ATP donors with and without seizure history grouped by age. The data also present a clear picture of the young age of the donors. Neuropathological examination of the tissue is performed by the HBTRC board‐certified neuropathologist. As it is important to discern the presence of neuropathological conditions in pediatric cases and controls, including pediatric complications, the ATP/HBTRC has the services of a pediatric neuropathologist for consultation on the young donors. Likewise, to obviate confounding of psychiatric conditions, records of donors are reviewed by a volunteer psychiatric consultant and author in this mini‐symposium (EL).
Tissue processing protocols. Donor tissues are referred to the ATP through a loose and disseminated network of parents and caregivers, physicians, pathologists, medical examiners and brain banks. Consent to donate brain tissue for research purposes is secured from the donor's next of kin. Every effort is made to comply with the standardized tissue handling procedures of the HBTRC (http://www.brainbank.mclean.org). The protocol calls for “fresh” preparations at the brain bank or by several referral sources at the recovery site. The fresh preparation calls for fixing the right whole brain hemisphere in formalin and snap freezing the left hemisphere following coronal sectioning at 1 cm. Strict adherence to this protocol is possible on a subset of cases that are received after relatively short PMI and from referral sources that have the resources to process the brain optimally. Alternative preparations are half fixed (right hemisphere) and the left whole hemisphere is frozen at the recovery site or as a last resort, both hemispheres are fixed when such resources are not available, or when the PMI exceeds 24 h. Table 2 shows the autism donor cases with age, sex, PMI, brain weight and fixation of right and left hemispheres.
Table 2.
Donor and banked brain tissue characteristics. Abbreviations: PMI = post‐mortem interval; F = female; M = male; NA = not available; L‐brain = left brain; R‐brain = right brain.
| Age (years) | Sex | PMI | Brain mass | L‐brain | R‐brain | |
|---|---|---|---|---|---|---|
| 1 | 2 | M | 4 | 1328 | Sliced snap frozen | Formalin‐fixed whole |
| 2 | 4 | M | NA | 1160 | Formalin‐fixed whole | Formalin‐fixed whole |
| 3 | 5 | F | 18 | NA | Sliced snap frozen | Formalin‐fixed whole |
| 4 | 5 | M | 25.5 | 1560 | Frozen whole | Formalin‐fixed whole |
| 5 | 5 | M | 15.6 | 1250 | Formalin‐fixed sections | Formalin‐fixed sections |
| 6 | 5 | M | 19.7 | 1420 | Formalin‐fixed sections | Formalin‐fixed sections |
| 7 | 7 | M | 41.8 | 1575 | Limited fixed tissue | Limited fixed tissue |
| 8 | 7 | M | 11.4 | 1560 | Formalin‐fixed sections | Formalin‐fixed sections |
| 9 | 7 | M | 25 | 1610 | Formalin‐fixed sections | Formalin‐fixed whole |
| 10 | 8 | M | 24 | 1150 | Limited fixed tissue | Limited fixed tissue |
| 11 | 8 | M | 22.2 | 1570 | Sliced snap frozen | Formalin‐fixed sections |
| 12 | 8 | M | 20.3 | 1525 | Formalin‐fixed sections | Formalin‐fixed sections |
| 13 | 9 | M | 27 | 1320 | Sliced snap frozen | Formalin‐fixed sections |
| 14 | 9 | M | 3.75 | 1690 | Formalin‐fixed sections | Formalin‐fixed sections |
| 15 | 10 | M | 74.5 | 1680 | Formalin‐fixed whole | Formalin‐fixed whole |
| 16 | 10 | F | 16.6 | 1050 | Frozen whole | Formalin‐fixed whole |
| 17 | 11 | F | 12.9 | 1460 | Formalin‐fixed whole | Frozen whole |
| 18 | 11 | M | 8.3 | 1500 | Limited fixed tissue | Limited fixed tissue |
| 19 | 11 | M | NA | NA | Sliced snap frozen | Formalin‐fixed sections |
| 20 | 13 | M | 8 | 1470 | Formalin‐fixed whole | Formalin‐fixed whole |
| 21 | 13 | M | 16 | 1900 | Formalin‐fixed sections | Formalin‐fixed sections |
| 22 | 13 | M | NA | NA | Formalin‐fixed sections | Formalin‐fixed sections |
| 23 | 14 | M | 10.3 | 1615 | Formalin‐fixed sections | Formalin‐fixed sections |
| 24 | 14 | M | 34.8 | 1770 | Formalin‐fixed sections | Formalin‐fixed whole |
| 25 | 14 | M | 26.8 | 1090 | Formalin‐fixed sections | Formalin‐fixed sections |
| 26 | 15 | M | 2.5 | 1390 | Formalin‐fixed whole | Formalin‐fixed whole |
| 27 | 15 | F | NA | NA | Formalin‐fixed whole | Formalin‐fixed whole |
| 28 | 15 | M | 24 | 1605 | Formalin‐fixed whole | Formalin‐fixed whole |
| 29 | 16 | M | NA | 1663 | Frozen whole | Formalin‐fixed whole |
| 30 | 16 | M | 22 | 1230 | Frozen whole | Formalin‐fixed whole |
| 31 | 17 | F | 25 | 1158 | Formalin‐fixed whole | Formalin‐fixed sections |
| 32 | 18 | F | 6.75 | 2100 | Formalin‐fixed sections | Frozen whole |
| 33 | 19 | M | NA | 1880 | Sliced snap frozen | Formalin‐fixed sections |
| 34 | 20 | M | 23.7 | 1144 | Frozen whole | Formalin‐fixed whole |
| 35 | 22 | M | 25 | 1375 | Frozen whole | Formalin‐fixed whole |
| 36 | 23 | M | 14 | 1610 | Formalin‐fixed whole | Formalin‐fixed whole |
| 37 | 25 | F | NA | ma | Formalin‐fixed sections | Formalin‐fixed sections |
| 38 | 26 | F | 28.7 | 1310 | Frozen whole | Formalin‐fixed whole |
| 39 | 27 | M | 8.3 | 1575 | Frozen whole | Formalin‐fixed whole |
| 40 | 29 | F | 17.8 | NA | Sliced snap frozen | Formalin‐fixed sections |
| 41 | 30 | M | 20.3 | 1230 | Frozen whole | Formalin‐fixed whole |
| 42 | 30 | M | NA | NA | Unknown Fixation | Formalin‐fixed sections |
| 43 | 30 | M | 16.1 | 1800 | Frozen whole | Formalin‐fixed whole |
| 44 | 31 | M | 99 | 1600 | Formalin‐fixed sections | Formalin‐fixed sections |
| 45 | 32 | M | 28.7 | 1694 | Formalin‐fixed whole | Formalin‐fixed whole |
| 46 | 32 | M | 23.1 | 1510 | Formalin‐fixed whole | Formalin‐fixed whole |
| 47 | 34 | M | 16.5 | 1367 | Sliced snap frozen | Formalin‐fixed sections |
| 48 | 36 | M | 24 | 1370 | Formalin‐fixed sections | Formalin‐fixed whole |
| 49 | 39 | M | 22.8 | 1440 | Frozen Sections | Formalin‐fixed whole |
| 50 | 39 | M | 14 | 1520 | Sliced snap frozen | Formalin‐fixed whole |
| 51 | 39 | M | 4 | 1545 | Sliced snap frozen | Formalin‐fixed sections |
| 52 | 40 | F | 32.7 | 890 | Formalin‐fixed whole | Formalin‐fixed whole |
| 53 | 41 | M | NA | 1385 | Sliced snap frozen | Formalin‐fixed sections |
| 54 | 44 | M | 30.8 | 1530 | Formalin‐fixed whole | Formalin‐fixed whole |
| 55 | 45 | M | 20.3 | 1530 | Formalin‐fixed whole | Formalin‐fixed sections |
| 56 | 45 | M | 40.2 | 1360 | Formalin‐fixed sections | Formalin‐fixed sections |
| 57 | 48 | M | NA | 1260 | Formalin‐fixed whole | Formalin‐fixed sections |
| 58 | 49 | F | 16.3 | 1675 | Frozen whole | Formalin‐fixed whole |
| 59 | 52 | M | 11.5 | 1324 | Formalin‐fixed whole | Sliced snap frozen |
| 60 | 56 | M | 19.5 | 1630 | Formalin‐fixed whole | Frozen whole |
| 61 | 56 | M | 3.35 | 1570 | Formalin‐fixed whole | Formalin‐fixed whole |
| 62 | 64 | M | 18.5 | 1450 | Formalin‐fixed whole | Formalin‐fixed whole |
| 63 | 66 | M | 13.4 | 1380 | Formalin‐fixed sections | Formalin‐fixed whole |
| 64 | 82 | M | 24.7 | 1345 | Frozen Sections | Formalin‐fixed sections |
Policies and mechanisms for requesting tissue. It was understood from the start that autism brain tissue was and would most likely remain a rare resource; therefore, with HBTRC board approval, the ATP sets tissue request guidelines and authorizes the dissemination of all tissue to TAB‐approved projects. Investigators seeking tissue submit a proposal that includes a research plan stating hypotheses to be tested and outlining investigative techniques, equipment to be used, expertise of personnel provided in biosketches and specifics about tissue selection (donor profile, brain regions, fixation). The applicant describes quantitative and statistical analyses, and a numerical analysis to justify the number of samples to be examined in order to produce scientifically valid measures and conclusions. Data sharing is a requirement of the program whereby applicants sign an agreement that, if approved to receive tissue, they will provide the ATP TAB with results every 6 months following receipt of tissue. Pre‐publication data are shared with other investigators only at the discretion of the Principle Investigator; post‐publication data, slides and images or any tissue derivative that could be useful for further scientific exploration is expected to be provided to the ATP within 3 months. Proposals submitted to the ATP are reviewed by the ATP TAB every 4 months. By mid‐2007, 128 proposals had been submitted for review and 67 projects had been approved for brain tissue or derivative distribution.
Distributions to date. Autism tissue distribution. Table 3 shows Tissue Sample Distribution for years 2005, 2006 and the first quarter of 2007.
Table 3.
Tissue sample distributions (2005–2007). Abbreviation: Q1 = the first quarter.
| Year | Samples | Cases | Projects |
|---|---|---|---|
| 2005 | 157 | 48 | 8 |
| 2006 | 284 | 64 | 12 |
| 2007 Q1 | 112 | 31 | 4 |
Types of specimens available. Every effort is made to extend the tissue resource for as many uses as possible and thus, through tissue processing decisions and data‐sharing agreements, a variety of specimens are available for research. Fixed and frozen samples are available for distribution through the HBTRC and various investigators have generated post‐mortem MRI, tissue array slides, stereological series and Nissl‐stained series of limbic brain regions and whole hemispheres, and genomic and cDNA to share with other TAB‐approved investigators.
SELF‐SPONSORED PROJECTS
The availability of this range of biomaterials requires support of the ATP in the form of self‐sponsored projects. One of these is the Brain Atlas Project, a collaboration between two laboratories. The project resulted from two independent applications made to the ATP to perform comprehensive cell counting and volumetry, MRI and 3‐D reconstructions of whole formalin‐fixed brain hemispheres that, after a series of meetings with TAB representatives, evolved into a joint project by co‐PIs Christoph Schmitz, MD, Department of Psychiatry and Neuropsychology, Maastricht University, Netherlands and Jerzy Wegiel, PhD, NY Institute for Basic Research, Staten Island, NY. Stereologic analysis (morphometry and volumetry) are being performed or are planned for the following cortical and subcortical regions:
Cortical regions:
-
•
Entire cortical gray matter
-
•
Brodmann Area 17
-
•
Fusiform gyrus
-
•
Inferior temporal gyrus
-
•
Middle temporal gyrus
-
•
Pars opercularis in the inferior frontal gyrus
-
•
Fronto‐insular cortex
-
•
Anterior cingulate gyrus
Subcortical regions:
-
•
Paraventricular nucleus
-
•
Supraoptic nucleus (lateral, medial and dorsal)
-
•
Superchiasmatic nucleus
-
•
Preoptic area
-
•
Mammillary nucleus (medialis and lateralis)
Hippocampal complex and amygdala:
-
•
Entorhinal cortex: volume of six layers and the number of neurons in five layers
-
•
Subicular complex with the subiculum proper (molecular and pyramidal layer), presubiculum (molecular, parvopyramidal and pyramidal layers), and parasubiculum (molecular and pyramidal layers)
-
•
Cornu Ammonis: volume of the alveus and the strata: oriens, pyramidale, radiatum, lacunosum/moleculare; number of neurons: stratum pyramidale in the CA1, 2, 3 and 4 sectors
-
•
Dentate gyrus: volume of the molecular and granule cell layers; number of granule cells
-
•
Amygdaloid body: volume and number of neurons in the corticomedial group of nuclei (cortical, medial, and central nuclei), basolateral group of nuclei (basal, lateral, accessory basal) and anterior amygdaloid area
Cholinergic system—nucleus basalis of Meynert:
-
•
CH1 (medial septal nucleus)
-
•
CH2 (vertical limb nucleus of the diagonal band of Broca)
-
•
CH3 (horizontal limb nucleus of the diagonal band of Broca)
-
•
CH4 (anteromedial, anterolateral, intermediate and posterior part of the nucleus basalis of Meynert)
Motor system:
-
•
Substantia nigra (volumes and number of neurons with and without melanin)
-
•
Pars reticulata
-
•
Pars compacta: dorsal pars compacta—dorsal, dorso‐medial, dorso‐lateral rostral; lateral; Ventral pars compacta—ventro‐medial, ventro‐lateral part, Lateral nucleus
-
•
-
•
Striatum
-
•
Caudate nucleus (volume, number of large and small neurons)
-
•
Putamen (volume, number of large and small neurons)
-
•
Globus pallidus—internal and external (volume, number of neurons)
-
•
Nucleus accumbens (volume, number of neurons);
-
•
-
•
Cerebellum (volume)
-
•
Cortex (volume)
-
•
Molecular layer (volume)
-
•
Granule cell layer (volume, number of neurons)
-
•
Purkinje cells layer (number)
-
•
-
•
White matter (volume)
-
•
Dentate nucleus (volume, number of neurons)
-
•
Brainstem:
-
•
Nucleus of facial nerve (volume, number of neurons)
-
•
Inferior Olive (volume, number of neurons)
-
•
Principalis (volume, number of neurons)
-
•
Dorsalis (volume, number of neurons)
-
•
Medialis (volume, number of neurons)
-
•
As it stands in 2007, the Brain Atlas Project has been assigned 14 autism and 14 control cases (Table 4). Post‐mortem MRI is part of every brain examination. The research capacity of the Brain Atlas Project extends beyond its original clinicopathology aim to include other projects that use tissue from cases in the principal project. Seventeen investigators have various samples of brain atlas donor cases (stained sections, digital images, post‐mortem MRIs, samples of celloidin‐processed 200 µm tissue sections, frozen tissue and genetic material).
Table 4.
Characteristics of cases and controls used in the Autism Brain Atlas project. Abbreviations: A = autism; C = control; F = female; M = male; H = hemisphere; R = right; L = left; BW = birth weight; PMI = post‐mortem interval; * = not available.
| No | Case | Age (years) | Sex | H | BW (g) | PMI (h) | Fix (days) | Cause of death |
|---|---|---|---|---|---|---|---|---|
| A1 | IBR‐425‐02 | 4 | M | R | 1160 | 30 | 4560 | Drowning |
| C1 | B‐6736 | 4 | F | R | 1530 | 17 | 126 | Acute bronchopneumonia after tonsillectomy |
| A2 | UMB‐1627 | 5 | F | R | 1390 | 13 | 1568 | Auto trauma |
| C2 | UMB‐1499 | 4 | F | R | 1222 | 21 | 233 | Lymphocytic myocarditis |
| A3 | B‐6403 | 7 | M | R | 1610 | 25 | 330 | Drowning |
| C3 | UMB‐4898 | 7 | M | R | 1240 | 12 | 130 | Drowning |
| A4 | B‐5666 | 8 | M | R | 1570 | 22 | 196 | Sarcoma |
| C4 | UMB‐1708 | 8 | F | R | 1222 | 20 | 650 | Traumatic multiple injury |
| A5 | B‐5535 | 13 | M | L | 1470 | 8 | 75 | Seizure? |
| C5 | BTB‐3638 | 14 | M | R | 1464 | 20 | 1067 | Electrocution |
| A6 | B‐6115 | 17 | F | L | 1158 | 25 | 470 | Dilated cardiomyopathy |
| C6 | UMB‐1843 | 15 | F | R | 1250 | 9 | 372 | Multiple injuries |
| A7 | UMB‐1638 | 21 | F | R | 1108 | 50 | 136 | Obstructive pulmonary disease |
| C7 | UMB‐1846 | 20 | F | R | 1340 | 9 | 245 | Multiple injuries |
| A8 | IBR‐93‐01 | 23 | M | R | 1610 | 14 | 505 | Drowning |
| C8 | UMB‐1646 | 23 | M | R | 1520 | 6 | 95 | Ruptured spleen |
| A9 | B‐5947 | 32 | M | L | 1510 | 23 | 288 | Respiratory failure |
| C9 | UMB‐4543 | 29 | M | R | 1514 | 13 | 89 | Traumatic multiple injury |
| A10 | B‐6212 | 36 | M | R | 1370 | 24 | * | Circulatory failure of cardiac origin; renal failure |
| C10 | UMB‐1576 | 32 | M | R | * | 24 | * | Compressional asphyxia |
| A11 | B‐6202 | 48 | M | L | 1260 | * | 875 | Oxygen deprivation‐chocking on food |
| C11 | BTB‐3899 | 48 | M | L | 1412 | 24 | 215 | Atherosclerotic heart disease |
| A12 | BB‐1376 | 52 | M | L | 1324 | 11 | 84 | Heart attack |
| C12 | IBR‐252‐02 | 51 | M | L | 1450 | 18 | 1819 | Myocardial infarct |
| A13 | B‐6276 | 56 | M | R | 1570 | 3 | 692 | Arteriosclerotic heart disease |
| C13 | BTB‐3983 | 52 | M | R | 1430 | 12 | 158 | atherosclerotic cardiovascular disease |
| A14 | B‐6862 | 66 | M | R | 1380 | 13 | * | Suspected drowning |
| C14 | B‐6874 | 64 | M | R | 1250 | 28 | * | Cardiac arrest |
THE DATA AND RESEARCH PORTAL
The Autism Information Portal, http://www.atpportal.org, was established in 2003 to provide researchers, ATP TAB and/or administrators with an easily searchable repository of all information collected by ATP on registrants, donors, tissues and projects. The Oracle Relational Database system stores both structured and unstructured data and can easily accommodate additional information resources such as imaging and software tools for investigators. The Oracle web server uses Secure Socket Layer with an Entrust certificate to ensure encryption of the data when accessed through the http://www.atpportal.org. All applications for tissue, and application reviews by the TAB, are made online on the portal site. The Portal has a brain slice viewer provided by VH used by applicants to show the precise brain regions needed for the proposed study.
Table 5 shows an example of the type of data on donors in the portal. Next to “Donor case list” in the upper left cell, there is a list of donor and tissue attributes. Then, for Individual case summaries, there are 10 tables with more data, and so on. Donor documentation (reports, medical evaluations, neuropathology reports, etc.) are on the portal; all documents are reviewed for compliance with the Health Insurance Portability and Accountability Act de‐identification guidelines before posting. Similar organization of data applies to registrant data and research projects.
Table 5.
Examples and classes of data collected on Autism Brain Bank cases and made available to investigators through the ATP‐data portal. Abbreviations: PMI = post‐mortem interval; ADI‐R = Autism Diagnostic Interview‐Revised; PI = Principal Investigator.
| Donor case list | Primary diagnosis, sex, ethnicity, age, primary cause death, secondary cause death, PMI, mass, pH, left fix, right fix, tissue quality, storage (days), notes | |
| Individual Case Summary | Demographic data | Sex, ethnicity, age, PMI, brain mass, brain pH, primary diagnosis, control, primary cause death, secondary cause death |
| Case tissue summary | Left fix, left notes, right fix, right notes, tissue quality, other tissue observations | |
| Similar donors (by age) | Age, sex, control, ethnicity, primary diagnosis, primary cause death | |
| Perinatal | ||
| Disorders | Disorder, diagnosis notes | |
| Medications | Medication, dose, unit, frequency, comment | |
| Immunizations | Case, number, immunization, notes | |
| Genetic testing | Genetic test, comment | |
| Researchers utilizing donor tissue | Primary PI, contact, research project | |
| Tissue distribution to researchers | PI contact, research project, region, lat, fix, distributed | |
| Disorders (all list) | Case #, age, ethnicity, control, disorder, diagnosis notes | |
| Medications (all list) | Case #, medication name, dose, med. unit, frequency, medication comment | |
| Immunizations (all list) | Case #, immunization, notes | |
| Genetic tests (all list) | Case #, genetic test, test comment | |
| Perinatal (all list) | Case #, perinatal condition, perinatal comment | |
| ADI‐R and IQ, etc. (all list) | Case #, type of test, test scores for ADI‐R, vineland, cars and IQ | |
| Ethnicity distribution (all list) | ||
| Tissue distribution (all list) | Case #, primary PI, research project, region code, region, lat, fix, distributed | |
| Secondary distributions (all list) | Case #, sending PI, receiving PI, tissue transferred date | |
ADI‐R, autopsy, neurology, neuropathology, psychiatry, vision, occupational therapy/physical therapy, aud, EEG, family, education, immunization, genetics, gross image, stained image, medical history, MRI doc, MRI DICOM, MRI available
ACKNOWLEDGMENTS
We express our gratitude and appreciation to the selfless families who donate tissue for research at a time of great sorrow.
Supports to this paper are AG02219 (VH), MH064673 (VH), MH066392 (VH), VA‐MIRECC (VH), and VA‐Merit Review (VH).
REFERENCES
- 1. Aalten CM, Samson MM, Jansen PA (2006) Diagnostic errors; the need to have autopsies. Neth J Med 64:186–190. [PubMed] [Google Scholar]
- 2. Alafuzoff I, Winblad B (1993) How to run a brain bank: potentials and pitfalls in the use of human post‐mortem brain material in research. J Neural Transm Suppl 39:235–243. [PubMed] [Google Scholar]
- 3. American Psychiatric Association (2007) Diagnostic and Statistical Manual of Mental Disorders DSM‐IV, vol. 4. American Psychiatric Association: Arlington. [Google Scholar]
- 4. Bauman ML (1996) Brief report: neuroanatomic observations of the brain in pervasive developmental disorders. J Autism Dev Disord 26:199–203. [DOI] [PubMed] [Google Scholar]
- 5. Bird ED, Vonsattel JP (1993) The development of a brain bank. J Neural Transm Suppl 39:17–23. [PubMed] [Google Scholar]
- 6. Bowen DM, Smith CB, White P, Davison AN (1976) Neurotransmitter‐related enzymes and indices of hypoxia in senile dementia and other abiotrophies. Brain 99:459–496. [DOI] [PubMed] [Google Scholar]
- 7. Bristol MM, Cohen DJ, Costello EJ, Denckla M, Eckberg TJ, Kallen R, Kraemer HC, Lord C, Maurer R, McIlvane WJ, Minshew N, Sigman M, Spence MA (1996) State of the science in autism: report to the National Institutes Health. J Autism Dev Disord 26:121–154. [DOI] [PubMed] [Google Scholar]
- 8. Brooks‐Kayal AR, Pritchett DB (1993) Developmental changes in human gamma‐aminobutyric acidA receptor subunit composition. Ann Neurol 34:687–693. [DOI] [PubMed] [Google Scholar]
- 9. Butterworth J, Tennant MC (1989) Postmortem human brain pH and lactate in sudden infant death syndrome. J Neurochem 53:1494–1499. [DOI] [PubMed] [Google Scholar]
- 10. Carper RA, Courchesne E (2005) Localized enlargement of the frontal cortex in early autism. Biol Psychiatry 57:126–133. [DOI] [PubMed] [Google Scholar]
- 11. Casanova MF (2006) Neuropathological and genetic findings in autism: the significance of a putative minicolumnopathy. Neuroscientist 12:435–441. [DOI] [PubMed] [Google Scholar]
- 12. Courchesne E, Pierce K (2005) Brain overgrowth in autism during a critical time in development: implications for frontal pyramidal neuron and interneuron development and connectivity. Int J Dev Neurosci 23:153–170. [DOI] [PubMed] [Google Scholar]
- 13. Cruz‐Sanchez FF, Mordini E, Ravid R (1997) Ethical aspects to be considered in brain banking. Ann Ist Super Sanita 33:477–482. [PubMed] [Google Scholar]
- 14. Deep‐Soboslay A, Akil M, Martin CE, Bigelow LB, Herman MM, Hyde TM, Kleinman JE (2005) Reliability of psychiatric diagnosis in postmortem research. Biol Psychiatry 57:96–101. [DOI] [PubMed] [Google Scholar]
- 15. Dodd PR, Hambley JW, Cowburn RF, Hardy JA (1988) A comparison of methodologies for the study of functional transmitter neurochemistry in human brain. J Neurochem 50:1333–1345. [DOI] [PubMed] [Google Scholar]
- 16. Ellison DW, Beal MF, Martin JB (1987) Amino acid neurotransmitters in postmortem human brain analyzed by high performance liquid chromatography with electrochemical detection. J Neurosci Methods 19:305–315. [DOI] [PubMed] [Google Scholar]
- 17. Esiri MM (1993) Brain banks: the Oxford experience. J Neural Transm Suppl 39:25–30. [PubMed] [Google Scholar]
- 18. Franz H, Ullmann C, Becker A, Ryan M, Bahn S, Arendt T, Simon M, Paabo S, Khaitovich P (2005) Systematic analysis of gene expression in human brains before and after death. Genome Biol 6:R112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Freund G, Anderson KJ (1996) Glutamate receptors in the frontal cortex of alcoholics. Alcohol Clin Exp Res 20:1165–1172. [DOI] [PubMed] [Google Scholar]
- 20. Gadea A, Lopez‐Colome AM (2001) Glial transporters for glutamate, glycine, and GABA III. Glycine transporters. J Neurosci Res 64:218–222. [DOI] [PubMed] [Google Scholar]
- 21. Graaf‐Peters VB, Hadders‐Algra M (2006) Ontogeny of the human central nervous system: what is happening when? Early Hum Dev 82:257–266. [DOI] [PubMed] [Google Scholar]
- 22. Gundersen H, Bagger P, Bendtsen T, Evans S, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard J, Pakkenberg B, Sorensen F, Vesterby A, West M (1988) The new sterological tools: disector, fractionator, nucleator and point sampled intercepts and their use in pathological research and diagnosis. APMIS 96:857–881. [DOI] [PubMed] [Google Scholar]
- 23. Hardy JA, Wester P, Winblad B, Gezelius C, Bring G, Eriksson A (1985) The patients dying after long terminal phase have acidotic brains; implications for biochemical measurements on autopsy tissue. J Neural Transm 61:253–264. [DOI] [PubMed] [Google Scholar]
- 24. Harper C, Matsumoto I (2005) Ethanol and brain damage. Curr Opin Pharmacol 5:73–78. [DOI] [PubMed] [Google Scholar]
- 25. Harrison PJ, Heath PR, Eastwood SL, Burnet PW, McDonald B, Pearson RC (1995) The relative importance of premortem acidosis and postmortem interval for human brain gene expression studies: selective mRNA vulnerability and comparison with their encoded proteins. Neurosci Lett 200:151–154. [DOI] [PubMed] [Google Scholar]
- 26. Hulette CM (2003) Brain banking in the United States. J Neuropathol Exp Neurol 62:715–722. [DOI] [PubMed] [Google Scholar]
- 27. Johnston NL, Cervenak J, Shore AD, Torrey EF, Yolken RH, Cerevnak J (1997) Multivariate analysis of RNA levels from postmortem human brains as measured by three different methods of RT‐PCR. Stanley Neuropathology Consortium. J Neurosci Methods 77:83–92. [DOI] [PubMed] [Google Scholar]
- 28. Jones EG, Hendry SH, Liu XB, Hodgins S, Potkin SG, Tourtellotte WW (1992) A method for fixation of previously fresh‐frozen human adult and fetal brains that preserves histological quality and immunoreactivity. J Neurosci Methods 44:133–144. [DOI] [PubMed] [Google Scholar]
- 29. Katsel P, Davis KL, Gorman JM, Haroutunian V (2005) Variations in myelin and oligodendrocyte‐related gene expression across multiple brain regions: a gene ontology study. Schizophr Res 17:241–252. [DOI] [PubMed] [Google Scholar]
- 30. Kemper TL, Bauman M (1998) Neuropathology of infantile autism. J Neuropathol Exp Neurol 57:645–652. [DOI] [PubMed] [Google Scholar]
- 31. Kontur PJ, Al‐Tikriti M, Innis RB, Roth RH (1994) Postmortem stability of monoamines, their metabolites, and receptor binding in rat brain regions. J Neurochem 62:282–290. [DOI] [PubMed] [Google Scholar]
- 32. Levitt P (2003) Structural and functional maturation of the developing primate brain. J Pediatr 143:S35–S45. [DOI] [PubMed] [Google Scholar]
- 33. Lipska BK, Deep‐Soboslay A, Weickert CS, Hyde TM, Martin CE, Herman MM, Kleinman JE (2006) Critical factors in gene expression in postmortem human brain: focus on studies in schizophrenia. Biol Psychiatry 60:650–658. [DOI] [PubMed] [Google Scholar]
- 34. Lord C, Leventhal BL, Cook EH (2001) Quantifying the phenotype in autism spectrum disorders. Am J Med Genet 105:36–38. [PubMed] [Google Scholar]
- 35. Mahy N (1993) Brain banks and research in neurochemistry. J Neural Transm Suppl 39:119–126. [PubMed] [Google Scholar]
- 36. Mant J, Wilson S, Parry J, Bridge P, Wilson R, Murdoch W, Quirke T, Davies M, Gammage M, Harrison R, Warfield A (2006) Clinicians didn't reliably distinguish between different causes of cardiac death using case histories. J Clin Epidemiol 59:862–867. [DOI] [PubMed] [Google Scholar]
- 37. McKee AC (1999) Brain banking: basic science methods. Alzheimer Dis Assoc Disord 13(Suppl.1):S39‐S44. [PubMed] [Google Scholar]
- 38. Murphy DD, Ravina B (2003) Brain banking for neurodegenerative diseases. Curr Opin Neurol 16:459–463. [DOI] [PubMed] [Google Scholar]
- 39. Mushtaq F, Ritchie D (2005) Do we know what people die of in the emergency department? Emerg Med J 22:718–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Olney JW, Collins RC, Sloviter RS (1986) Excitotoxic mechanisms of epileptic brain damage. Adv Neurol 44:857–877. [PubMed] [Google Scholar]
- 41. Pakkenberg B, Gundersen HJ (1989) New stereological method for obtaining unbiased and efficient estimates of total nerve cell number in human brain areas. Exemplified by the mediodorsal thalamic nucleus in schizophrenics. APMIS 97:677–681. [DOI] [PubMed] [Google Scholar]
- 42. Paus T (2005) Mapping brain maturation and cognitive development during adolescence. Trends Cogn Sci 9:60–68. [DOI] [PubMed] [Google Scholar]
- 43. Perl DP, Good PF, Bussiere T, Morrison JH, Erwin JM, Hof PR (2000) Practical approaches to stereology in the setting of aging‐ and disease‐ related brain banks. J Chem Neuroanat 20:7–19. [DOI] [PubMed] [Google Scholar]
- 44. Pickett J (2001) Current investigations in autism brain tissue research. J Autism Dev Disord 31:521–527. [DOI] [PubMed] [Google Scholar]
- 45. Pickett J, London E (2005) The neuropathology of autism: a review. J Neuropathol Exp Neurol 64:925–935. [DOI] [PubMed] [Google Scholar]
- 46. Preece P, Cairns NJ (2003) Quantifying mRNA in postmortem human brain: influence of gender, age at death, postmortem interval, brain pH, agonal state and inter‐lobe mRNA variance. Brain Res Mol Brain Res 118:60–71. [DOI] [PubMed] [Google Scholar]
- 47. Pritt BS, Hardin NJ, Richmond JA, Shapiro SL (2005) Death certification errors at an academic institution. Arch Pathol Lab Med 129:1476–1479. [DOI] [PubMed] [Google Scholar]
- 48. Purcell AE, Jeon OH, Zimmerman AW, Blue ME, Pevsner J (2001) Postmortem brain abnormalities of the glutamate neurotransmitter system in autism. Neurology 57:1618–1628. [DOI] [PubMed] [Google Scholar]
- 49. Ravakhah K (2006) Death certificates are not reliable: revivification of the autopsy. South Med J 99:728–733. [DOI] [PubMed] [Google Scholar]
- 50. Redcay E, Courchesne E (2005) When is the brain enlarged in autism? A meta‐analysis of all brain size reports. Biol Psychiatry 58:1–9. [DOI] [PubMed] [Google Scholar]
- 51. Roulson J, Benbow EW, Hasleton PS (2005) Discrepancies between clinical and autopsy diagnosis and the value of post mortem histology; a meta‐analysis and review. Histopathology 47:551–559. [DOI] [PubMed] [Google Scholar]
- 52. Schmitz C, Hof PR (2000) Recommendations for straightforward and rigorous methods of counting neurons based on a computer simulation approach. J Chem Neuroanat 20:93–114. [DOI] [PubMed] [Google Scholar]
- 53. Spokes E (1979) An analysis of factors influencing measurements of dopamine, noradrenaline, glutamate decarboxylase and choline acetylase in human post‐mortem brain tissue. Brain 102:333–346. [DOI] [PubMed] [Google Scholar]
- 54. Tourtellotte WW, Rosario IP, Conrad A, Syndulko K (1993) Human neuro‐specimen banking 1961–1992. The National Neurological Research Specimen Bank (a donor program of pre‐ and post‐mortem tissues and cerebrospinal fluid/blood; and a collection of cryopreserved human neurological specimens for neuroscientists). J Neural Transm Suppl 39:5–15. [PubMed] [Google Scholar]
- 55. Trotter SA, Brill LB II, Bennett JP Jr (2002) Stability of gene expression in postmortem brain revealed by cDNA gene array analysis. Brain Res 942:120–123. [DOI] [PubMed] [Google Scholar]


