Abstract
To explore the validity of the criteria for dementia with Lewy bodies (DLB) revised in 2005, we examined community based consecutive autopsy cases. 10.3% of the non‐demented subjects and 31.2% of the demented subjects showed the Lewy body pathology. Applying the revised pathological criteria to the 205 demented subjects, the types of LB pathology of 11 cases (5.4%) were brainstem‐predominant, 24 cases (11.7%) were limbic type and 24 cases (11.7%) were diffuse neocortical type, although there were many subjects not to fit the criteria exactly. The prevalence of Lewy bodies (LBs) was almost same regardless of gender; however, the extent of the LB pathology among females was more severe than that in males. The likelihood of DLB being modified by concomitant Alzheimer's pathology was as follows: 27 cases (13.2%) showed low likelihood, 16 cases (7.8%) showed intermediate likelihood and 16 cases (7.8%) showed high likelihood. Since the numbers of clinical features of DLB were significantly higher in the pathological intermediate and high likelihood DLB groups than in the low likelihood DLB group or no LB group, both the intermediate and high likelihood groups of DLB should be considered as pathological DLB.
Keywords: dementia, diagnosis, DLB, Lewy body, pathology
INTRODUCTION
Dementia with Lewy bodies (DLB) has been suggested to be the third major dementia in older people, accounting for 15% to 25% of dementia cases 12, 16, 22, 24, 38); however, the history of the study of this type of dementia is still young. Recognition of DLB has become more widespread since the establishment of the first diagnostic criteria in 1996 (24) and the discovery of α‐synuclein as the major constituent of Lewy bodies (LBs) in 1997 30, 32). In particular, immunostaining of α‐synuclein makes it easy to identify neocortical type LBs. Consequently, with the liberal definition of the pathological criteria of DLB in 1996, no less than 60% of Alzheimer's disease (AD) cases may be considered to meet pathologic criteria for DLB (23). Virtually none of these patients show the clinical features of DLB, especially those cases with extensive neurofibrillary tangles (NFTs; 7, 26) and those with one or more LBs in the amygdala, but without significant Lewy related pathology in other brain regions (15). The inclusion of such cases as pathologically confirmed DLB may have contributed to the view that the clinical criteria have suboptimal sensitivity (20).
Taking these issues into consideration, new diagnostic criteria for DLB were proposed in 2005 (23). The new criteria took into account both the extent of Lewy related pathology and AD‐type pathology in assessing the degree of certainty that the neuropathologic findings explain the DLB clinical syndrome. Immunostaining of α‐synuclein was recommended to detect LBs and Lewy related pathology, and a semiquantitative grading of lesion density was recommended. As indicated by the authors, the revised criteria obviously require further research to test their validity; however, to date, almost no study concerning this subject has been performed.
This is the first report of a community‐based clinicopathological study of DLB, which verified the revised criteria.
MATERIALS AND METHODS
Subjects
The clinicopathological study of dementia, part of the Hisayama study, was previously described 13, 28, 36, 39). The Hisayama study investigated the epidemiology of cerebrovascular disease in the general Japanese population 17, 18, 34, 39). We carried out autopsies on most deceased subjects to confirm the causes of death and to examine brain pathology. We collected information about new neurological events, including stroke and cognitive impairment, through a daily monitoring system established by the study team, local practitioners and the town government. Members of our study group visited the town at least once a week to maintain contact with physicians and staff of the local Health and Welfare Office. At least once a week, we also surveyed the three major hospitals with geriatric or psychiatric wards near the town, to which Hisayama residents are usually admitted when necessary. Regular health checks and extensive neuropsychiatric evaluation, including medical history and physical examination, neurological history and examination, semi‐structured psychiatric interview and neuropsychological assessment, were given biennially to obtain information on any new neurological events missed by the monitoring network. When we suspected new neurological symptoms, including cognitive impairment, the study physicians carefully evaluated the subject, and an effort was made to obtain further diagnostic information, including brain CT and MRI. The diagnosis of dementia was made clinically based on the guidelines of the Diagnostic and Statistical Manual of Mental Disorders, Revised Third Edition (DSM‐III‐R) (3).
In this study, we analyzed two groups. Group 1 was the 102 consecutive autopsy series of the Hisayama study including both demented and non‐demented subjects who died between October 1, 1998 and March 31, 2001 and underwent the autopsy (autopsy rate: 70.5%) and explored the risk factors of synucleinopathy with the revised criteria of LB pathology. Group 2 was the 205 consecutive autopsy series of the Hisayama study including only demented subjects who died between January 1, 1986 and March 31, 2003, including the demented cases of Group 1, and underwent the autopsy and studied the practice of the revised criteria of DLB. Autopsy rate of Group 2 was 64.0% and this rate was very close to that of the whole autopsy rate of this period (62.1%).
Clinical features
The core features and suggestive features of the revised criteria for DLB (23) were retrospectively ascertained from our database including the medical records, the nurse records, the interview records of caregivers and facility staffs for each subject. The results of single photon emission computed tomography (SPECT) or positron emission tomography (PET) imagings were not included in this study because these imaging studies examining the dopamine transporter uptake were not popular in Japan. Also, we picked up such features as repeated vocalizing, flailing limbs and moving around the bed during sleep, and we described these features as “sleep behavior disorder” instead of “REM sleep behavior disorder” because it was very difficult to monitor the sleep brain waves in community based study. Then, we surveyed all clinical core features: fluctuating cognition, recurrent visual hallucinations and spontaneous features of Parkinsonism. However, only sleep behavior disorder and severe neuroleptic sensitivity were investigated as suggestive features. We excluded the visual hallucination and Parkinsonism when these features occurred after more than 5 years since the dementia onset, because these features are also common in the late stage of AD.
Neuropathological assessment
Brains were weighed, evaluated for grossly detectable lesions and abnormalities of the blood vessels, and fixed with 10% buffered formalin for at least 2 weeks. All infarcts (including status lacunaris and Binswanger's disease or leukoaraiosis) and hypertensive hemorrhages were registered with regard to their age, size and topographical location. Brain specimens were taken following the consensus guidelines for DLB, the Consortium to Establish a Registry for Alzheimer's Disease (CERAD) guidelines and Braak & Braak stage for NFT 8, 9, 23, 24, 25, 27). Thus, the specimens in each case included middle frontal gyrus, superior and middle temporal gyri, inferior parietal lobule, anterior cingulate gyrus, amygdala, hippocampus with entorhinal cortex and transentorhinal cortex (at the level of the lateral geniculate body, LGB), calcarine cortex, basal ganglia including the nucleus basalis of Meynert, thalamus, substantia nigra, locus coeruleus and dorsal vagal nucleus. Sections were embedded in paraffin and were routinely stained using hematoxylin‐eosin, Klüver‐Barrera and a modified Bielschowsky's method.
Specimens from every subject were immunostained with a panel of antibodies against α‐synuclein (LB509; monoclonal, mouse, 1:100; donated by Dr Iwatubo) 4, 36), tau (polyclonal, rabbit, 1:100; Dako, Demark) and ubiquitin (polyclonal, rabbit; 1:100, Dako). Immunolabeling was detected using a standard indirect immunoperoxidase method and viewed with diaminobenzidine (DAB; Dojindo, Japan). The sections were lightly counterstained with hematoxylin.
Neuritic plaques were estimated by a modified Bielschowsky's method. NFTs were assessed by tau immunostaining. In each case, the frequency of neuritic plaques and NFT were semiquantitatively evaluated, and converted to a plaque score according to CERAD criteria and Braak stage established by Braak and Braak 8, 9, 27). The CERAD score and the Braak stage were combined to estimate the likelihood that dementia was due to AD, according to the NIA‐RI criteria (1).
The extent of LB pathology was estimated based on the revised consensus guidelines for DLB (23) and the type of LB pathology (none, brainstem‐predominant, limbic, diffuse neocortical) and the likelihood of DLB (no, low, intermediate, high) were assigned for each of the 205 cases. In determining the type of LB pathology, first, we explored the middle frontal gyrus, superior and middle temporal gyri, inferior parietal lobule, anterior cingulate gyrus, transentorhinal cortex, substantia nigra, locus coeruleus, and dorsal vagal nucleus. In addition, we explored amygdala to distinguish the none, brainstem and limbic type of LB pathology. The nucleus basalis of Meynert was examined when needed (see Table 2). In determining the likelihood of DLB, we used the NIA‐RI criteria (1) as the assessment of Alzheimer type pathology. Those cases that did not fit the criteria of the type of LB pathology exactly were assigned according to the pattern of regional involvement rather than total LB count.
Table 2.
Distribution of LB. A. non‐demented individuals. B. demented subjects. Numbers refer to a semiquantitative scoring system: 1 = mild, with sparse LBs; 2 = moderate, with more than one LB in a low‐power field; 3 = severe, four or more LBs in a low power field; 4 = very severe, numerous LBs. The specimens that could not be sampled because of infarction or poor preservation are presented by NS. Abbreviation: LB = Lewy body; LC = locus coeruleus; SN = substantia nigra; TE = transentorhinal cortex.
| A. Non‐demented subjects with LB. | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case no. | Sex | Age at death | Type of LB pathology | Brainstem regions | Basal forebrain/limbic reguons | Neocortical regions | |||||||
| IX‐X | LC | SN | nbM | Amygdala | TE | Cingulate | Temporal | Frontal | Parietal | ||||
| 1 | M | 70 | Brainstem | 2 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | M | 85 | Brainstem | 3 | 3 | 3 | 3 | 0 | 0 | 0 | 0 | 0 | 0 |
| 3 | M | 90 | Brainstem | NS | 3 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 4 | M | 98 | Limbic | 1 | 1 | 1 | 2 | 2 | 0 | 1 | 0 | 1 | 0 |
| 5 | F | 84 | Brainstem | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 6 | F | 84 | Brainstem | 2 | 3 | 3 | 3 | 0 | 0 | 1 | 0 | 0 | 0 |
| 7 | F | 87 | Limbic | 0 | 2 | 3 | 0 | 2 | 0 | 2 | 1 | 0 | 0 |
| B. Demented subjects with LB. | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| case no. | Sex | Age at death | Type of LB pathology | Brainstem regions | Basal forebrain/limbic reguons | Neocortical regions | |||||||
| IX‐X | LC | SN | nbM | Amygdala | T. E. | Cingulate | Temporal | Frontal | Parietal | ||||
| 1 | M | 83 | None | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | M | 91 | None | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| 3 | M | 78 | Brainstem | 3 | 3 | 2 | 0 | 0 | 1 | 0 | 0 | 0 | |
| 4 | M | 83 | Brainstem | 1 | 3 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 5 | M | 83 | Brainstem | 3 | 3 | 3 | NS | 0 | 0 | 0 | 0 | 0 | |
| 6 | M | 87 | Brainstem | 3 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 7 | M | 88 | Brainstem | 0 | 1 | 2 | NS | 0 | 1 | 0 | 0 | 0 | |
| 8 | M | 90 | Brainstem | 0 | 0 | 3 | 0 | 1 | 0 | 0 | 0 | 0 | |
| 9 | M | 93 | Brainstem | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 10 | M | 68 | Limbic | 3 | 2 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 |
| 11 | M | 71 | Limbic | 2 | 1 | 3 | 3 | 3 | 0 | 0 | 0 | ||
| 12* | M | 79 | Limbic | 0 | 3 | 3 | 4 | 2 | 3 | 1 | 0 | 0 | |
| 13 | M | 81 | Limbic | 0 | 0 | 1 | 0 | 3 | 0 | 0 | 0 | 0 | 0 |
| 14* | M | 83 | Limbic | 3 | 3 | 3 | 4 | 3 | 3 | 2 | 2 | 0 | 0 |
| 15 | M | 85 | Limbic | 2 | 2 | 1 | 0 | 3 | 2 | 0 | 0 | 0 | |
| 16 | M | 86 | Limbic | NS | NS | NS | NS | NS | 3 | NS | NS | 0 | NS |
| 17 | M | 86 | Limbic | 2 | 2 | 2 | 4 | 1 | 0 | 0 | 0 | 0 | |
| 18 | M | 86 | Limbic | 0 | 0 | 1 | 1 | 3 | 2 | 0 | 0 | 0 | 0 |
| 19 | M | 89 | Limbic | 3 | 3 | 3 | 4 | 2 | 3 | 0 | 1 | 0 | |
| 20 | M | 89 | Limbic | 3 | 3 | 2 | 3 | 0 | 0 | 0 | 0 | 0 | |
| 21 | M | 76 | Neocortical | 3 | 3 | 3 | 4 | 4 | 3 | 1 | 1 | ||
| 22 | M | 80 | Neocortical | 3 | 3 | 3 | 3 | 2 | 3 | 1 | 1 | 1 | |
| 23 | M | 83 | Neocortical | 2 | 3 | 3 | 0 | 2 | 2 | 1 | 1 | 1 | |
| 24 | M | 94 | Neocortical | 3 | 3 | 3 | 4 | 3 | 4 | 3 | 3 | 3 | |
| 25 | F | 91 | None | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| 26 | F | 95 | None | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 27 | F | 95 | None | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 28 | F | 83 | Brainstem | 3 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 29 | F | 84 | Brainstem | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 30 | F | 91 | Brainstem | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 31 | F | 99 | Brainstem | 3 | 3 | 2 | 0 | 1 | 0 | 0 | 0 | 0 | |
| 32 | F | 80 | Limbic | 3 | 2 | 2 | 4 | 3 | 0 | 0 | 0 | 0 | 0 |
| 33 | F | 82 | Limbic | 2 | NS | 3 | 4 | 2 | 3 | 1 | 0 | 0 | |
| 34 | F | 84 | Limbic | 0 | 1 | 0 | 0 | 3 | 2 | 0 | 0 | 0 | 0 |
| 35* | F | 84 | Limbic | 2 | 0 | 2 | 0 | 2 | 2 | 2 | 0 | 0 | |
| 36 | F | 87 | Limbic | 3 | 3 | 3 | 3 | 4 | 0 | 0 | 0 | ||
| 37 | F | 90 | Limbic | 2 | 3 | 2 | 3 | 3 | 2 | 2 | 0 | 0 | 0 |
| 38 | F | 90 | Limbic | 3 | 3 | 3 | NS | 3 | 0 | 0 | 0 | 0 | |
| 39 | F | 91 | Limbic | 0 | 1 | 1 | 3 | 1 | 1 | 0 | 0 | 0 | |
| 40 | F | 93 | Limbic | 0 | 0 | 3 | 4 | 2 | 3 | 2 | 0 | 0 | |
| 41 | F | 93 | Limbic | 3 | 3 | 2 | 2 | 2 | 1 | 0 | 0 | ||
| 42 | F | 95 | Limbic | 2 | 2 | 3 | 4 | 3 | 3 | 1 | 0 | 0 | |
| 43 | F | 96 | Limbic | 0 | 0 | 2 | 4 | 2 | 0 | 0 | 0 | 0 | |
| 44 | F | 97 | Limbic | 3 | 3 | 2 | 2 | 2 | 0 | 0 | 0 | ||
| 45 | F | 79 | Neocortical | 3 | 3 | 3 | 3 | 3 | 3 | 1 | 1 | ||
| 46 | F | 81 | Neocortical | 3 | NS | 3 | 4 | 4 | 4 | 3 | 2 | 2 | |
| 47 | F | 82 | Neocortical | 3 | 3 | 3 | 4 | 4 | 4 | 3 | 2 | 3 | |
| 48 | F | 82 | Neocortical | 3 | 3 | 3 | 4 | 2 | 2 | 1 | 1 | ||
| 49 | F | 82 | Neocortical | 3 | 3 | 3 | 4 | 4 | 3 | 3 | 3 | ||
| 50 | F | 84 | Neocortical | 3 | 3 | 3 | 4 | 4 | 4 | 3 | 3 | 3 | |
| 51* | F | 84 | Neocortical | 3 | 3 | 3 | 4 | 4 | 4 | 4 | 3 | 3 | 3 |
| 52 | F | 85 | Neocortical | NS | 3 | 3 | 4 | 4 | 3 | 1 | 1 | ||
| 53 | F | 86 | Neocortical | 3 | 3 | 3 | 4 | 3 | 3 | 3 | 2 | 1 | |
| 54 | F | 86 | Neocortical | 3 | 3 | 3 | 3 | 4 | 2 | 2 | 0 | ||
| 55 | F | 86 | Neocortical | 3 | 3 | 3 | 3 | 4 | 4 | 3 | 2 | 2 | 0 |
| 56 | F | 89 | Neocortical | 3 | 3 | 3 | 4 | 3 | 3 | 2 | 1 | 1 | |
| 57 | F | 89 | Neocortical | 3 | 3 | 2 | 2 | 3 | 2 | 1 | 1 | ||
| 58 | F | 89 | Neocortical | 3 | 3 | 3 | 1 | 2 | 2 | 1 | 0 | ||
| 59 | F | 90 | Neocortical | 3 | 3 | 3 | 4 | 2 | 2 | 1 | 1 | 1 | |
| 60 | F | 93 | Neocortical | 3 | 3 | 3 | 3 | 4 | 3 | 2 | 1 | ||
| 61 | F | 93 | Neocortical | 3 | 3 | 3 | 4 | 4 | 4 | 4 | 4 | 3 | 3 |
| 62 | F | 94 | Neocortical | 2 | 2 | 3 | 4 | 3 | 3 | 2 | 0 | 2 | |
| 63 | F | 94 | Neocortical | 3 | 3 | 3 | 4 | 4 | 3 | 3 | 2 | 2 | 2 |
| 64 | F | 95 | Neocortical | 4 | 3 | 3 | 3 | 4 | 3 | 1 | 0 | ||
Demented patients with pre‐existing Parkinsonism.
Statistical methods
The quantitative data obtained was compared between the groups by Mann‐Whitney's U‐test or Kruskal‐Wallis test, as appropriate. Correlation analysis was done using the Spearman nonparametric method. Statistical significance was defined as P < 0.05. In the nonparametric statistical process, the following scale was adopted: CERAD (0 = none; 1 = sparse; 2 = moderate; 3 = frequent), type of LB pathology (0 = none; 1 = brainstem‐predominant; 2 = limbic; 3 = diffuse neocortical), the likelihood of DLB (0 = no; 1 = low; 2 = intermediate; 3 = high).
RESULTS
Clinico‐neuropathological information of subjects
Group 1
The total number of Group 1 was 102. Among them, 68 subjects were non‐demented and 34 subjects were demented. The clinico‐neuropathological information on all the subjects of Group 1 is shown in Table 1. The age at death was significantly older in LB positive cases than in LB negative cases (Mann‐Whitney U‐test, P < 0.05) and Braak stage of NFT was more severe in LB positive cases than in LB negative cases (Mann‐Whitney U‐test, P < 0.01). Also, the extent of LB pathology got more severe along with aging and Braak stage of NFT (Spearman's rank correlation test, r = 0.43, P < 0.01; r = 0.41, P < 0.05, respectively). The LB pathology tended to spread wider among female than male but this difference did not reach statistical difference (Mann‐Whitney U‐test, P = 0.052).
Table 1.
Clinical and neuropathological information on the subjects of Group 1. CERAD values are presented according to the following scale; 0—none; 1—sparse; 2—moderate; 3—frequent. Significant difference between LB positive group and LB negative group. Abbreviation: LB = Lewy body.
| Group | n (male/female) | Age at death [mean ± SD] (years) | Brain weight [mean ± SD] (g) | CERAD [mean ± SD] | Braak & Braak stage [mean ± SD] |
|---|---|---|---|---|---|
| Total | 102 (51/51) | 80.2 ± 12.2 | 1221.6 ± 161.6 | 1.60 ± 1.16 | 3.31 ± 1.90 |
| LB negative group | 79 (43/36) | 78.5 ± 12.7* | 1235.6 ± 170.9 | 1.53 ± 1.16 | 3.13 ± 1.90** |
| LB positive group | 23 (8/15) | 86.4 ± 7.7* | 1173.5 ± 114.7 | 1.83 ± 1.15 | 4.00 ± 1.80** |
| Brainstem‐predominant | 8 (5/3) | 84.8 ± 8.5 | 1200.0 ± 100.6 | 1.50 ± 0.93 | 3.63 ± 1.85 |
| Limbic (transitional) | 5 (2/3) | 89.2 ± 11.1 | 1225.0 ± 72.6 | 2.40 ± 0.89 | 4.60 ± 0.55 |
| Diffuse neocortical | 10 (1/9) | 86.3 ± 5.1 | 1126.5 ± 131.9 | 1.80 ± 1.40 | 3.90 ± 2.18 |
P < 0.01 and
P < 0.05 (Mann‐Whitney's U test).
Group 2
The total number of Group 2 was 205. The mean age at death was 86.2 ± 6.7 years; 78 subjects were male and 127 were female. The mean age at death of females was significantly higher than that of males, and the extent of Alzheimer type pathology (neuritic plaque and NFT) was significantly more severe in females than in males (Mann‐Whitney U‐test, P < 0.01). On the other hand, the prevalence of LBs was almost the same between males and females (male: 30.8%, female: 31.5%, total: 31.2%).
Applying the revised pathologic criteria to the LB positive cases
The distribution of LB pathology among LB‐positive cases is shown in Table 2. Of the 68 non‐demented subjects, seven subjects exhibited the LB pathology, and the types of LB pathology of five subjects (7.4%) were brainstem‐predominant and two subjects (2.9%) were limbic type (but these seven subjects did not exhibit any clinical features related to LB pathology). Of the 205 demented subjects, 64 subjects had the LB pathology, and the types of LB pathology of 11 cases (5.4%) were brainstem‐predominant, 24 cases (11.7%) were limbic type and 24 cases (11.7%) were diffuse neocortical type. The types of LB pathology of five subjects (2.4%) were none because the LB pathology was slight.
Group 2 was allocated to likelihood of being DLB. Twenty‐seven cases (13.2%) were deemed to have a low likelihood of being DLB, 16 cases (7.8%) had intermediate likelihood of being DLB, and 16 cases (7.8%) had a high likelihood of being DLB. A comparison of LB pathology between the genders or ages is shown in Table 3. The LB pathology among males tended to occur younger than female and to be confined within the brainstem (37.5% of male LB‐positive cases) and limbic system (45.8%), although the LB pathology among female tended to occur in their ninth decade and to be spread throughout the neocortex (50.0% of female LB‐positive cases). Because the likelihood of DLB was greatly influenced by the associated AD pathology, the composition of each “likelihood of DLB” group was different between the genders. For example, among males, five (71%) of the seven high likelihood DLB cases showed limbic type LB pathology, but none of the high likelihood DLB cases among females showed limbic type LB pathology; all of these showed diffuse neocortical type LB pathology. In addition, the majority of LB‐positive cases among the oldest cases were classified as low likelihood DLB, because of the severe AD pathology associated with aging.
Table 3.
Classification of male subjects (A, C, E) and female subjects (B, D, F) of Group 2 according to the revised criteria of DLB. A–B age at death <80, C–D 80≤ age at death ≤89, E–F 89< age at death. Abbreviation: LB = Lewy body; NIA‐RI = National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer's Disease.
| A. Male (Age <80) | B. Female (Age <80) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| NIA‐RI (Alzheimer) | Total | NIA‐RI (Alzheimer) | Total | ||||||||
| Low | Intermediate | High | Low | Intermediate | High | ||||||
| Type of LB pathology | None | 13 | 3 | 1 | 17 | Type of LB pathology | None | 2 | 1 | 8 | 11 |
| Brainstem | 1 | 0 | 0 | 1 | Brainstem | 0 | 0 | 0 | 0 | ||
| Limbic | 2 | 0 | 1 | 3 | Limbic | 0 | 0 | 0 | 0 | ||
| Neocortical | 0 | 0 | 1 | 1 | Neocortical | 0 | 0 | 1 | 1 | ||
| Total | 16 | 3 | 3 | 22 | Total | 2 | 1 | 9 | 12 | ||
| C. Male (80≤ Age ≤89) | D. Female (80≤ Age ≤89) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| NIA‐RI (Alzheimer) | Total | NIA‐RI (Alzheimer) | Total | ||||||||
| Low | Intermediate | High | Low | Intermediate | High | ||||||
| Type of LB pathology | None | 15 | 5 | 9 | 29 | Type of LB pathology | None | 18 | 3 | 19 | 40 |
| Brainstem | 2 | 1 | 1 | 4 | Brainstem | 1 | 1 | 0 | 2 | ||
| Limbic | 3 | 1 | 4 | 8 | Limbic | 0 | 1 | 4 | 5 | ||
| Neocortical | 1 | 1 | 0 | 2 | Neocortical | 5 | 1 | 7 | 13 | ||
| Total | 21 | 8 | 14 | 43 | 24 | 6 | 30 | 60 | |||
| E. Male (89< Age) | F. Female (89< Age) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| NIA‐RI (Alzheimer) | Total | NIA‐RI (Alzheimer) | Total | ||||||||
| Low | Intermediate | High | Low | Intermediate | High | ||||||
| Type of LB pathology | None | 4 | 2 | 4 | 10 | Type of LB pathology | None | 7 | 3 | 29 | 39 |
| Brainstem | 0 | 1 | 1 | 2 | Brainstem | 1 | 1 | 0 | 2 | ||
| Limbic | 0 | 0 | 0 | 0 | Limbic | 0 | 1 | 7 | 8 | ||
| Neocortical | 0 | 0 | 1 | 1 | Neocortical | 2 | 1 | 3 | 6 | ||
| Total | 4 | 3 | 6 | 13 | Total | 10 | 6 | 39 | 55 | ||
Correlation between neuropathological and clinical assessments of DLB
To compare the neuropathological and clinical assessments of DLB, we excluded 52 cases from the 205 cases, because these 52 cases had been diagnosed as other types of dementia during life, based on the exclusive features of the revised criteria (23). The individual diagnoses were as follows: vascular dementia (38 cases), Parkinson's disease dementia (PDD, eight cases), tumor‐related dementia (two cases), head injury (two cases), carbon monoxide poisoning (one case) and alcoholic psychosis with dementia (one case). In diagnosing vascular dementia clinically, we used the National Institute of Neurological Disorders and Stroke – Association International pour la Recherche et l'Enseignement en Neurosciences (NINDS‐AIREN) criteria (31). Those cases that were undiagnosed the type of dementia during life and revealed to be vascular dementia after autopsies were included (many were small‐vessel disease with dementia cases without apparent focal neurologic signs). To distinguish PDD from DLB, we used the 1‐year rule (23). Thus, 153 cases were included in the study analyzing the correlation between neuropathological assessment and clinical assessment of DLB.
The presence rate of core and suggestive features in each type of LB pathology and the likelihood of DLB are shown in Figure 1. Statistically significant differences among the types of LB pathology or the likelihood of DLB were observed, especially in the core features. The average numbers of core features presented in 153 cases are shown in Table 4. The diffuse neocortical LB group showed the greatest number of core features compared with other groups, and reached statistical significance when compared with the limbic LB group (Mann‐Whitney U‐test, P < 0.05) and the no LB group (Mann‐Whitney U‐test, P < 0.01). However, among the groups classified on the basis of likelihood of DLB, the intermediate likelihood of DLB group presented with the highest number of core features, rather than high likelihood group. This is because the cases that showed diffuse neocortical LB pathology associated with severe AD pathology (NIA‐RI: high likelihood of AD), which was classified as having an intermediate likelihood of being DLB, presented with core features most often (Table 4). Among cases of high likelihood of DLB, two cases of subcategory in which type of LB pathology is limbic and NIA‐RI is low likelihood (cases no. 19 and no. 20 in Table 2) presented no core features, whereas two PDD cases excluded in this study corresponded to this subcategory. Suggestive features were not so common in every group.
Figure 1.
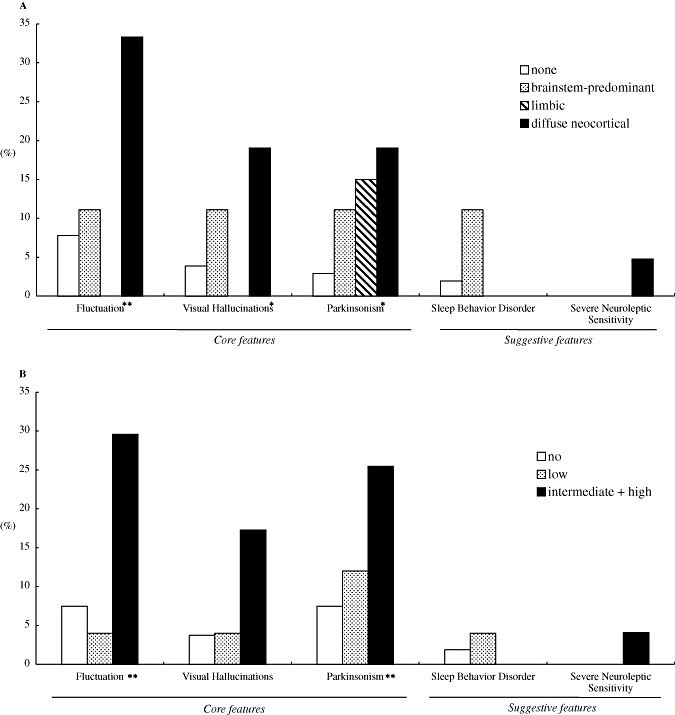
The presence rates of core and suggestive features with respect to each type of LB pathology (A) and each likelihood of having DLB (B). The intermediate and high likelihood cases of DLB are combined (see Discussion). There was a significant difference among the groups *P < 0.05 and **P < 0.01 (Kruskal‐Wallis test).
Table 4.
Mean number of 3 core features (fluctuating cognition, recurrent visual hallucinations and spontaneous features of parkinsonism) presented in the subjects within each subdivision. Abbreviation: LB = Lewy body; NIA‐RI = National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer's Disease.
| NIA‐RI (Alzheimer) | ||||
|---|---|---|---|---|
| Low | Intermdeiate | High | ||
| Type of LB pathology | None | 0.14 | 0.40 | 0.11 |
| Brainstem | 0.33 | 0.50 | 0.00 | |
| Limbic | 0.00 | 0.67 | 0.07 | |
| Neocortical | 0.71 | 0.50 | 0.75 | |
DISCUSSION
Here, we applied the new DLB criteria to the Hisayama pathological cohort study, and examined their validity from various pathological angles. We explored the proportions of the types of LB pathology and the likelihood of DLB in demented cases, as well as the correlations of the pathological diagnosis and clinical features of DLB with the minimum selection bias due to recruiting the 102 consecutive autopsy series and the 205 consecutive autopsy series with dementia from the general population.
The major problem in applying the revised pathological criteria of DLB was that there were many subjects not to fit the criteria of the type of LB pathology exactly. Among LB‐positive subjects except for five subjects with so slight LB pathology that was allocated to none type, 28 of 59 subjects (47.5%) revealed not to fit the criteria; specifically, 7 of 11 brainstem‐predominant cases (63.6%), 17 of 24 limbic type cases (70.8%) and 4 of 24 diffuse neocortical type cases (16.7%) showed conflicting distribution of LBs (see Table 2). Firstly, all of the three brainstem regions scarcely presented the LB pathology together in some brainstem‐predominant and limbic type cases, and secondly, the extent of LB pathology in the amygdala got very severe in some cases even though the LB pathology did not involve the neocortex. It is noteworthy that the latter pattern of LB distribution was reported as Alzheimer disease with amygdala Lewy bodies (35). The extension pattern of LB pathology in DLB may show great variability of distribution and it is not easy to determine a stage like NFT. Recently, the similar results were reported that almost half (49%) of Lewy related pathology positive cases were not classifiable according to the revised pathological criteria of DLB (19), and the authors suggested that modifying the published criteria by reducing the number of regions requiring examination and adding an amygdala predominant category permitted classification of 97% of Lewy related pathology positive cases from the referral‐based sample.
Although a large revision of the pathological criteria of the type of LB pathology was performed, the type of LB pathology was changed in a few cases only. The major changes observed were caused by the adoption of LBs in the amygdala as a hallmark of limbic pathology, resulting in eight brainstem‐predominant type cases based on 1996 criteria being reclassified as limbic type. However, these cases often did not present with the clinical core features and suggestive features of DLB. Recently, AD patients with LBs in the amygdala were reported to be susceptible to major depression (21), and this may also be true of DLB patients. The possible clinical correlation of LBs in the amygdala in DLB remains unclear and further studies are required to clarify this.
There were 8 subjects in the Group 2 who exhibited the PDD. Of eight subjects, one subject was diffuse neocortical type of LB pathology, three subjects were limbic type and four subjects had no LB pathology; two may be Parkinsonism due to infarction, one may be Parkinsonism induced by drug and one is unknown origin. The relationship between the duration of Parkinson disease prior to the onset of dementia and key neuropathologic and neurochemical characteristics were previously reported (6), but in our study, this relationship was not apparent probably because of the limitation of subjects.
The prevalence of LB was almost the same between sexes, but the severity of LB pathology differed. LBs among males were usually confined within the brainstem and limbic system, although those among females tended to spread throughout the regions of neocortex associated with AD pathology (see Table 3). We previously reported this similar sex‐related tendency (36), but the number of available studies was too small to determine the potential effect of sex on the result (40). The age difference of males and females makes comparisons difficult to interpret and statistical comparisons should be controlled for the age difference; however, the size of our samples was small for the statistical correction. Nevertheless, we must consider that there may be a difference in the population of cases classified as high likelihood of DLB between the sexes. This considerable difference may be of some inconvenience of further studies, for example, of risk factors.
The effect of age on LB pathology remains unclear. Some studies have concluded that the frequency of LBs becomes higher with age 14, 29, 36); others have reported that aging has no effect on the frequency of LBs (2). This is the first community based pathological study for the LB pathology with the most recently published criteria and the result is very similar to our previous report based on the first pathological criteria (36), that is, the extent of LB pathology got more severe along with aging.
It is important in this study to define the pathological likelihood of DLB. Of the types of LB pathology, the diffuse neocortical type of LB group showed the clinical features of DLB most often, but of the likelihood of DLB groups, the high likelihood DLB group showed fewer clinical features of DLB than the intermediate likelihood of DLB group. This is because the cases that showed diffuse neocortical LB pathology associated with severe AD pathology (NIA‐RI: high likelihood of AD) presented the core features most often, but these cases were assigned as having intermediate likelihood of being DLB (see Table 4). Certainly, the previous studies reported that those cases with extensive NFTs showed fewer clinical features of DLB, like visual hallucinations 7, 11, 26), but the difference in LB pathology burden between the mild AD pathology group and the severe AD pathology group was not taken into consideration in these studies. In addition, Alzheimer‐type pathology becomes more severe with aging, and as many as 66.2% of our subjects of 90 years old or more at death were assigned as having a high likelihood of Alzheimer's disease according to NIA‐RI so the likelihood of DLB tends to become lower at older ages (see Table 3). However, the age at death surely depends on medical aspects; in other words, the level of medical treatment that the subject got in life may have serious effects on the pathological diagnosis of DLB. Therefore, we propose the following amendments. First, cases with intermediate and high likelihood of DLB should be considered as pathological DLB. A diagnosis of “mixed dementia of DLB and Alzheimer's disease” may be the most appropriate for the intermediate likelihood of DLB group. The other suggested amendment is the introduction of some dividing system depending on the age at death, such as CERAD (27).
Of core features, Parkinsonism was often observed even among the brainstem and limbic type of LB pathology groups; however, cognitive fluctuation and visual hallucinations were not constant among none to limbic type of LB pathology groups and were more characteristic symptoms of the diffuse neocortical type of LB pathology group. It is highly suggested that neocortical involvement of LB pathology at certain degree is a prerequisite for cognitive fluctuation and visual hallucinations, and probably for severe neuroleptic sensitivity (Figure 1A).
The limitation of our study was that we did not include the results of SPECT/PET imaging examinations and sleep waves, and did not adopt the objective scaling systems of core features recommended in the DLB clinical criteria in 2005, such as the Clinician Assessment of Fluctuation scale (37), the semistructured One Day Fluctuation Assessment scale (37), the Mayo Fluctuations Composite Scale, the Neuropsychiatric Inventory (NPI) (10) and the Unified Parkinson's disease Rating Scale (UPDRS) (5). With the addition of the results of these imaging studies and scaling protocols, better sensitivity and specificity may be expected. Further prospective clinicopathological studies including these data and novel examinations such as MIBG myocardial scintigraphy (33) are required.
ACKNOWLEDGMENTS
This study was supported in part by a Grant‐in‐Aid for the 21st Century COE program and Grant‐in‐Aid for Scientists (No 19300125) from the Ministry of Education, Culture, Sports, Science and Technology of Japan. We are grateful to Dr Takeshi Iwatsubo, Department of Neuropathology and Neuroscience, University of Tokyo, for generously donating the α‐synuclein antibody LB509. We thank Mr. S. Mawatari and Ms. S. Nagae for their technical assistance.
REFERENCES
- 1. Anonymous (1997) Consensus recommendations for the postmortem diagnosis of Alzheimer's disease. The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer's Disease. Neurobiol Aging 18(Suppl.4):S1–S2. [PubMed] [Google Scholar]
- 2. Anonymous (2001) Pathological correlates of late‐onset dementia in a multicentre, community‐based population in England and Wales. Neuropathology Group of the Medical Research Council Cognitive Function and Ageing Study (MRC CFAS). Lancet 357:169–175. [DOI] [PubMed] [Google Scholar]
- 3. Association AP (1987) Diagnositic and Statistical Manual of Mental Disorders, 3rd edn., revised. American Psychiatric Association: Washington, DC. [Google Scholar]
- 4. Baba M, Nakajo S, Tu PH, Tomita T, Nakaya K, Lee VM et al (1998) Aggregation of alpha‐synuclein in Lewy bodies of sporadic Parkinson's disease and dementia with Lewy bodies. Am J Pathol 152:879–884. [PMC free article] [PubMed] [Google Scholar]
- 5. Ballard C, McKeith I, Burn D, Harrison R, O'Brien J, Lowery K et al (1997) The UPDRS scale as a means of identifying extrapyramidal signs in patients suffering from dementia with Lewy bodies. Acta Neurol Scand 96:366–371. [DOI] [PubMed] [Google Scholar]
- 6. Ballard C, Ziabreva I, Perry R, Larsen JP, O'Brien J, McKeith I et al (2006) Differences in neuropathologic characteristics across the Lewy body dementia spectrum. Neurology 67:1931–1934. [DOI] [PubMed] [Google Scholar]
- 7. Ballard CG, Jacoby R, Del Ser T, Khan MN, Munoz DG, Holmes C et al (2004) Neuropathological substrates of psychiatric symptoms in prospectively studied patients with autopsy‐confirmed dementia with lewy bodies. Am J Psychiat 161:843–849. [DOI] [PubMed] [Google Scholar]
- 8. Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K (2006) Staging of Alzheimer disease‐associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol 112:389–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Braak H, Braak E (1991) Neuropathological stageing of Alzheimer‐related changes. Acta Neuropathol 82:239–259. [DOI] [PubMed] [Google Scholar]
- 10. Cummings JL, Mega M, Gray K, Rosenberg‐Thompson S, Carusi DA, Gornbein J (1994) The Neuropsychiatric Inventory: Comprehensive assessment of psychopathology in dementia. Neurology 44:2308–2314. [DOI] [PubMed] [Google Scholar]
- 11. Del Ser T, Hachinski V, Merskey H, Munoz DG (2001) Clinical and pathologic features of two groups of patients with dementia with Lewy bodies: Effect of coexisting Alzheimer‐type lesion load. Alz Dis Assoc Dis 15:31–44. [DOI] [PubMed] [Google Scholar]
- 12. Dickson DW, Ruan D, Crystal H, Mark MH, Davies P, Kress Y et al (1991) Hippocampal degeneration differentiates diffuse Lewy body disease (DLBD) from Alzheimer's disease: Light and electron microscopic immunocytochemistry of CA2‐3 neurites specific to DLBD. Neurology 41:1402–1409. [DOI] [PubMed] [Google Scholar]
- 13. Fujimi K, Noda K, Sasaki K, Wakisaka Y, Tanizaki Y, Iida M et al (2007) Altered expression of COX‐2 in subdivisions of the hippocampus during aging and in Alzheimer's disease: The Hisayama study. Dement Geriatr Cogn 23:423–431. [DOI] [PubMed] [Google Scholar]
- 14. Gibb WR, Lees AJ (1988) The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson's disease. J Neurol Neurosur Ps 51:745–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hamilton RL (2000) Lewy bodies in Alzheimer's disease: A neuropathological review of 145 cases using alpha‐synuclein immunohistochemistry. Brain Pathol 10:378–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hansen L, Salmon D, Galasko D, Masliah E, Katzman R, DeTeresa R et al (1990) The Lewy body variant of Alzheimer's disease: A clinical and pathologic entity. Neurology 40:1–8. [DOI] [PubMed] [Google Scholar]
- 17. Katsuki S (1966) Epidemiological and clinicopathological study on cerebrovascular disease in Japan. Prog Brain Res 21:64–89. [DOI] [PubMed] [Google Scholar]
- 18. Kiyohara Y, Yoshitake T, Kato I, Ohmura T, Kawano H, Ueda K et al (1994) Changing patterns in the prevalence of dementia in a Japanese community: the Hisayama study. Gerontology 40(Suppl.2): 29–35. [DOI] [PubMed] [Google Scholar]
- 19. Leverenz JHR, Tsuang DW, Schantz A, Vavrek D, Larson EB, Kukull WA et al (2008) Empiric refinement of the pathologic assessment of lewy‐related pathology in the dementia patient. Brain Pathol 18:220–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Litvan I, Bhatia KP, Burn DJ, Goetz CG, Lang AE, McKeith I et al (2003) Movement Disorders Society Scientific Issues Committee report: SIC Task Force appraisal of clinical diagnostic criteria for Parkinsonian disorders. Mov Disord 18:467–486. [DOI] [PubMed] [Google Scholar]
- 21. Lopez OL, Becker JT, Sweet RA, Martin‐Sanchez FJ, Hamilton RL (2006) Lewy bodies in the amygdala increase risk for major depression in subjects with Alzheimer disease. Neurology 67:660–665. [DOI] [PubMed] [Google Scholar]
- 22. McKeith I, Mintzer J, Aarsland D, Burn D, Chiu H, Cohen‐Mansfield J et al (2004) Dementia with Lewy bodies. Lancet Neurol 3:19–28. [DOI] [PubMed] [Google Scholar]
- 23. McKeith IG, Dickson DW, Lowe J, Emre M, O'Brien JT, Feldman H et al (2005) Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 65:1863–1872. [DOI] [PubMed] [Google Scholar]
- 24. McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA et al (1996) Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of theconsortium on DLB international workshop. Neurology 47:1113–1124. [DOI] [PubMed] [Google Scholar]
- 25. McKeith IG, Perry EK, Perry RH (1999) Report of the second dementia with Lewy body international workshop: Diagnosis and treatment. Consortium on Dementia with Lewy Bodies. Neurology 53:902–905. [DOI] [PubMed] [Google Scholar]
- 26. Merdes AR, Hansen LA, Jeste DV, Galasko D, Hofstetter CR, Ho GJ et al (2003) Influence of Alzheimer pathology on clinical diagnostic accuracy in dementia with Lewy bodies. Neurology 60:1586–1590. [DOI] [PubMed] [Google Scholar]
- 27. Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM et al (1991) The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology 41:479–486. [DOI] [PubMed] [Google Scholar]
- 28. Noda K, Sasaki K, Fujimi K, Wakisaka Y, Tanizaki Y, Wakugawa Y et al (2006) Quantitative analysis of neurofibrillary pathology in a general population to reappraise neuropathological criteria for senile dementia of the neurofibrillary tangle type (tangle‐only dementia): The Hisayama Study. Neuropathology 26:508–518. [DOI] [PubMed] [Google Scholar]
- 29. Parkkinen L, Soininen H, Laakso M, Alafuzoff I (2001) Alpha‐synuclein pathology is highly dependent on the case selection. Neuropathol Appl Neurobiol 27:314–325. [DOI] [PubMed] [Google Scholar]
- 30. Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A et al (1997) Mutation in the alpha‐synuclein gene identified in families with Parkinson's disease. Sci 276:2045–2047. [DOI] [PubMed] [Google Scholar]
- 31. Roman GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH et al (1993) Vascular dementia: diagnostic criteria for research studies. Report of the NINDS‐AIREN International Workshop. Neurology 43:250–260. [DOI] [PubMed] [Google Scholar]
- 32. Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M (1997) Alpha‐synuclein in Lewy bodies. Nature 388:839–840. [DOI] [PubMed] [Google Scholar]
- 33. Taki J, Yoshita M, Yamada M, Tonami N (2004) Significance of 123I‐MIBG scintigraphy as a pathophysiological indicator in the assessment of Parkinson's disease and related disorders: It can be a specific marker for Lewy body disease. Ann Nucl Med 18:453–461. [DOI] [PubMed] [Google Scholar]
- 34. Tanizaki Y, Kiyohara Y, Kato I, Iwamoto H, Nakayama K, Shinohara N et al (2000) Incidence and risk factors for subtypes of cerebral infarction in a general population: The Hisayama study. Stroke 31:2616–2622. [DOI] [PubMed] [Google Scholar]
- 35. Uchikado H, Lin W, DeLucia M, Dickson D (2006) Alzheimer disease with amygdala Lewy bodies: A distinct form of alpha‐synucleinopathy. J Neuropathol Exp Neurol 65:685–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wakisaka Y, Furuta A, Tanizaki Y, Kiyohara Y, Iida M, Iwaki T (2003) Age‐associated prevalence and risk factors of Lewy body pathology in a general population: the Hisayama study. Acta Neuropathol 106:374–382. [DOI] [PubMed] [Google Scholar]
- 37. Walker MP, Ayre GA, Cummings JL, Wesnes K, McKeith IG, O'Brien JT, Ballard CG (2000) The Clinician Assessment of Fluctuation and the One Day Fluctuation Assessment Scale. Two methods to assess fluctuating confusion in dementia. Br J Psychiatry 177:252–256. [DOI] [PubMed] [Google Scholar]
- 38. Weiner MF, Risser RC, Cullum CM, Honig L, White C, 3rd , Speciale S et al (1996) Alzheimer's disease and its Lewy body variant: A clinical analysis of postmortem verified cases. Am J Psychiatry 153:1269–1273. [DOI] [PubMed] [Google Scholar]
- 39. Yoshitake T, Kiyohara Y, Kato I, Ohmura T, Iwamoto H, Nakayama K et al (1995) Incidence and risk factors of vascular dementia and Alzheimer's disease in a defined elderly Japanese population: the Hisayama Study. Neurology 45:1161–1168. [DOI] [PubMed] [Google Scholar]
- 40. Zaccai J, McCracken C, Brayne C (2005) A systematic review of prevalence and incidence studies of dementia with Lewy bodies. Age Ageing 34:561–566. [DOI] [PubMed] [Google Scholar]


