Abstract
Glucocorticoids are prenatally administered to promote the maturation of the lungs. They, however, can affect neuronal proliferation and differentiation. In newborn marmoset monkeys, intrauterine hyperexposure to dexamethasone (DEX) resulted in a significantly decreased proliferation rate in the hippocampal dentate gyrus without affecting neuronal differentiation. In this study, marmoset monkeys received 5 mg/kg body weight DEX either during early (days 42–48) or late (days 90–96) pregnancy. The volume of the dentate granule cell layer as well as the proliferation and neuronal differentiation in the dentate gyrus of their 2‐year‐old offspring were investigated. The density of proliferating cells (Ki‐67), apoptotic cells (in situ tailing) and cells differentiating to neurons (double cortin, TUC‐4 and calretinin) were determined immunohistochemically. Analysis of the dentate granule cell layer volume showed no significant differences between early or late DEX‐exposed marmosets and untreated control animals. Similarly, proliferation and neuronal differentiation in DEX‐treated animals was not significantly different in comparison with controls. In summary, the decreased proliferation rate observed in newborn marmosets after intrauterine exposure to DEX was no longer detectable in their 2‐year‐old siblings suggesting no long‐lasting effect of prenatal hyperexposure to DEX on neuronal proliferation and differentiation in the dentate gyrus of marmoset monkeys.
Keywords: dentate gyrus, marmoset, neurogenesis, prenatal dexamethasone, progenitor cell proliferation
INTRODUCTION
Different environmental influences during embryonic development such as nutrition, hormones or growth factors may exert long‐lasting effects on various body systems such as the regulation of hormones, blood pressure and glucose tolerance. The question whether there is a fetal origin for chronic diseases of adulthood is still a matter of debate.
In this context glucocorticoid hormones are of special interest as they are widely used both in the prenatal and in early neonatal period to enhance the maturation of the lungs in preterm infants. The brain is very sensitive for the effects of steroid hormones, and their receptors are especially abundant in the hippocampal formation as demonstrated in several mammalian species such as rat, mouse, marmoset monkey and humans 34, 37, 39, 41. Early environmental manipulations regulate glucocorticoid receptor gene expression in the hippocampal formation and alter the responsivity of the hypothalamic‐pituitary‐adrenal (HPA) axis: maternal separation, physical trauma and endotoxin administration enhanced HPA axis responsivity to stress while short periods of infantile stimulation and handling decreased it (31).
In general, glucocorticoid hormones and their synthetic derivates such as dexamethasone (DEX) are known to exert mostly detrimental effects on neuronal tissue such as reduction in brain volume, increased rate of apoptosis and impairment of neurons as well as changes in synapse formation 1, 18, 19. Furthermore, alterations in brain monoamine metabolism and gene expression of nuclear transcription factors were reported in rats after prenatal DEX exposure 35, 40. Studies of postnatal administration of DEX in rats revealed decreased body and brain weights (13). Furthermore, administration of DEX on postnatal day 7 resulted in behavioral alterations reflecting hippocampal deficits in young rats (12). Maternal treatment with DEX from gestational day 15 until delivery caused not only low birth weight and poor weight gain but also an impaired spatial learning in the rat offspring (3).
The hippocampus plays a crucial role in learning and memory. Furthermore, there is a growing body of data proposing a relationship between adult dentate neurogenesis and hippocampal learning and memory 11, 46. In adult neurogenesis, the transformation of neural progenitor cells into newly generated cells with neuronal properties occurs within the subgranular zone of the dentate gyrus and in the subventricular zone of the lateral ventricle. This phenomenon can still be observed in old age but its frequency declines rapidly with aging (25). In general, neurogenesis is influenced by various stimuli: environmental enrichment (22) and physical exercise such as running (47) cause an increase of neuronal proliferation and differentiation. Moreover, several antidepressant drugs appear to stimulate neurogenesis 29, 30. Conversely, DEX and stress decrease adult neurogenesis (48). Interestingly, voluntary wheel running led to increased progenitor cell proliferation despite elevated corticosterone levels because of the stress of physical exercise (46).
So far, there has been no study investigating the impact of prenatal DEX exposure on adult neurogenesis. Moreover, most of the studies on the effects of prenatal glucocorticoid administration were carried out in rodent models that can only partly be transferred to human. Therefore, the present study was performed in common marmoset monkeys, a species that closely reflects many aspects of human physiology. The pregnant marmosets received 5 mg/kg body weight DEX either during early (days 42–48, phase of organogenesis) or late pregnancy (days 90–96). In our former study, the neuronal proliferation and differentiation in the dentate gyrus of newborn marmosets after DEX exposure in utero were investigated. Both early and late exposure with DEX led to a significantly decreased proliferation rate of dentate granule cells without affecting neuronal differentiation (44). In the present study, the volume of the dentate granule cell layer as well as neuronal proliferation and differentiation of their 2‐year‐old siblings was determined.
MATERIAL AND METHODS
Animals and experimental groups
Adult common marmoset monkeys (Callithrix jacchus) were obtained from the breeding colony at the German Primate Center in Göttingen, Germany and housed in pairs on a regular day/night cycle (lights on from 07:00 h to 19:00 h) at 26°C, 55% relative humidity, with free access to food and water. Animal experiments were conducted in accordance with the European Communities Council Directive of November 24, 1986 (86/EEC) and were approved by the Government of Lower Saxony, Germany. We used the minimum number of animals required to obtain consistent data. This study was performed within the European Commission funded project EUPEAH (glucocorticoid hormone programming in early life and its impact on adult health).
Pregnant marmosets were treated orally with 5 mg DEX/kg body weight daily either during early pregnancy from day 42 to day 48 or during late pregnancy from day 90 to day 96 post‐conceptionem, respectively. The average expected full term gestation for a marmoset monkey is 145 days. For this purpose DEX tablets (DEX 0.5, 1.5 or 4 mg Jenapharm®, Jena, Germany) were split and dosed according to the body weight of the individual animal. Each dose was dissolved in 0.4 mL of tap water and mixed with 1.6 mL Nutri‐Cal (Albrecht, Aulendorf, Germany) in a syringe. This mixture was taken voluntarily, control animals received vehicle only either during early or late pregnancy.
To study the long‐term effects of intrauterine treatment of DEX, the male littermates of the initially investigated newborn monkeys were brought up by their mothers and housed under the same conditions as adult common marmoset monkeys. The control group as well as the early DEX‐exposed group comprised 10 male animals and the late DEX‐exposed group consisted of 11 male common marmoset monkeys. Within the control group five animals received vehicle from day 42 to day 48 and five animals during late pregnancy from day 90 to day 96.
After having reached an average age of 27 months (median of controls 27 months, early treatment group 26.5 months and late treatment group 28 months, respectively) monkeys were killed in general anesthesia with an overdose of xylazine (Rompun®, Bayer, Leverkusen, Germany) and ketamine (Ketavet®, Pharmacia & Upjohn, Erlangen, Germany). The brain was removed and the left hippocampal formation was immersion fixed in 4% paraformaldehyde.
Volume of the granule cell layer of the dentate gyrus
The paraffin‐embedded hippocampal formation was cut in regular intervals of 10 × 5 µm sections (for immunohistochemical analysis), followed by an omission of 150 µm tissue and again 10 × 5 µm sections etc. until the end of the hippocampal formation was reached. The area of the dentate granule cell layer was measured planimetrically in the first of the 10 sections of each interval. The size of the dentate granule cell layer was evaluated in hemalum‐stained sections with a 2× objective. Briefly, the border of the granule cell layer was marked manually and the size was measured with a computer‐based imaging system (BX51; Olympus, Hamburg, Germany; software AnalySIS® 3.2; Soft Imaging System GmbH, Münster, Germany). By multiplication of the areas of the sections by the distances between the individual sections the granule cell layer volume was estimated.
Immunohistochemistry
Five‐micrometer‐thick deparaffinized and hydrated brain sections were pre‐treated with microwaving for 5 × 3 minutes in citric acid buffer, 10 mmol/L, pH 6.0. After blocking with 10% fetal calf serum/phosphate‐buffered saline (FCS/PBS) for 30 minutes, primary antibodies were applied at the concentrations indicated and allowed to bind overnight at 4°C (calretinin, TUC‐4) or for 90 minutes at room temperature (Ki‐67, doublecortin).
For quantification of cell proliferation, Ki‐67 was detected by a mouse monoclonal anti‐Ki‐67 antibody (1:100, Dako, Kopenhagen, Denmark). Calretinin was visualized by a rabbit polyclonal anti‐calretinin antibody (1:2000, Swant, Bellinzona, Switzerland), TUC‐4 by a 1:1000 diluted polyclonal rabbit anti‐TUC‐4 antibody (Chemicon, Temecula, CA, USA) and doublecortin by a 1:50 diluted polyclonal goat anti‐doublecortin antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA). For light microscopy, sections were incubated with appropriate concentrations of biotinylated secondary antibodies (Amersham, Little Chalfont, Buckinghamshire, UK) followed by treatment with avidin‐peroxidase (Sigma‐Aldrich, St. Louis, MO, USA). Diuminobenzidine (DAB) was used as the chromogenic substrate (Roche, Mannheim, Germany) for Ki‐67 and doublecortin. In slices incubated with the calretinin antibody, the color reaction was developed with newfuchsin (Dako, Kopenhagen, Denmark). Detection of TUC‐4 was performed with the “alkaline phosphatase/anti‐alkaline phosphatase” method, and newfuchsin was used for the color reaction. All slices were counterstained with hemalum (Merck, Darmstadt, Germany). Control sections were incubated with isotype control antibodies or without primary antibody.
In situ tailing
Deparaffinized 5‐µm sections were incubated with 200 µg/mL proteinase K (Sigma‐Aldrich, St. Louis, MO, USA) for 20 minutes at 37°C. Then they were treated for 1 h at 37°C with a mixture of 5× tailing buffer, 1 µL digoxigenin DNA labeling mix, 2 µL cobalt chloride, 12.5 U terminal transferase and distilled water to a final volume of 50 µL. Sections were incubated with 10% fetal calf serum for 20 minutes and then treated with anti‐digoxigenin antibody (1:250, Roche, Mannheim, Germany) for 90 minutes at room temperature. The color reaction was developed with 4‐nitroblue‐tetrazolium‐chloride/5‐bromine‐4‐chloride‐3‐indolyl‐phosphate, and sections were counterstained with red‐aluminium hydroxide (Roche, Mannheim, Germany).
Quantification of immunoreactive cells
To obtain data from different regions within the dentate gyrus, sections within the frontal, central and dorsal region of the dentate gyrus were evaluated: the median and 25%/75% percentiles of all sections available in each individual animal were determined. These sections were used for immunohistochemical analysis.
The slices were examined using a 40× objective and all immunoreactive cells within the region of interest were counted by a blinded investigator. Only cells within the granule cell layer and on the border of the subgranular zone were considered for analysis.
Statistical analysis
Values were expressed as medians ± 25%/75% percentiles except for body weight and hippocampal volume that were expressed as means ± standard deviation (SD). Data were compared by the Kruskal Wallis non‐parametric anova. Statistical analysis was performed using GraphPad Prism version 4.00 (GraphPad Software, San Diego, CA, USA).
RESULTS
Body and brain weights
The body weights of the early and late DEX‐exposed marmosets were not significantly different compared with control animals, though some of the late DEX‐exposed animals tended to have a slightly lower body weight compared with controls. Similarly, comparison of brain weights of the three groups did not show a significant difference (Table 1).
Table 1.
Body and brain weights as well as volume of the dentate gyrus of controls, early and late dexamethasone‐exposed 2‐year‐old common marmoset monkeys (mean ± standard deviation; P = 0.63 and P = 0.81, respectively).
| Control | Early treatment | Late treatment | |
|---|---|---|---|
| Body weights (g) | 430.5 ± 73.0 | 404.3 ± 64.23 | 396.6 ± 99.8 |
| Brain weights (g) | 7.93 ± 0.7 | 7.99 ± 0.3 | 7.85 ± 0.52 |
Volume of the granule cell layer of the dentate gyrus
The volume of the granule cell layer of the dentate gyrus estimated by planimetry was not significantly different in controls, early and late DEX‐exposed animals (Table 2). Furthermore, no differences in cell density were observed between the three groups. The number of neurons per mm2 dentate gyrus was determined in four animals per group and statistical analysis (nonparametric anova) was performed. The means ± SD for control, early and late DEX‐group were as followed: 14149 ± 2214 neurons/mm2, 14090 ± 2272 neurons/mm2 and 13261 ± 1212 neurons/mm2, respectively; P = 0.87.
Table 2.
Volume of the dentate gyrus of controls, early and late dexamethasone‐exposed 2‐year‐old common marmoset monkeys (mean ± standard deviation; P = 0.67).
| Control | Early treatment | Late treatment | |
|---|---|---|---|
| Volume of the dentate gyrus (mm3) | 1.06 ± 0.23 | 1.13 ± 0.28 | 1.0 ± 0.49 |
No changes in proliferation after exposure to DEX
Proliferating cells were stained with Ki‐67 that labels a specific nuclear antigen which is present in all proliferating cells during cell cycle phases G1, S, G2 and mitosis but is absent in the G0 phase (16). The density of Ki‐67‐immunolabeled cells in control animals that received vehicle early or late during pregnancy was not significantly different. Therefore, all control animals were combined in one group for statistical analysis.
In contrast to the observed reduced proliferation rate in newborn marmoset monkeys, determination of Ki‐67 immunoreactive cells of the 2‐year‐old animals revealed no significant differences in all three groups ( Figure 1A,B). Moreover, no morphological abnormalities of pyramidal neurons were observed in both groups.
Figure 1.
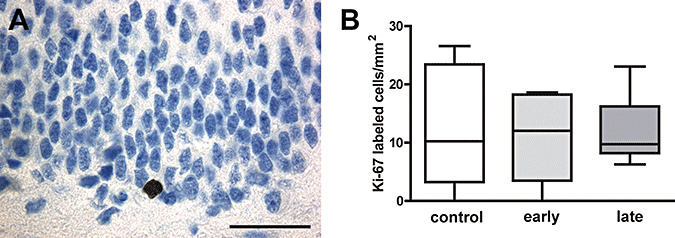
A. Ki‐67‐immunoreactive neuron in the subgranular cell layer of the dentate gyrus (scale bar = 50 µm). B. Density of proliferating cells in the dentate gyrus of untreated and early and late dexamethasone‐exposed 2‐year‐old marmosets (medians ± 25%/75% percentiles, P = 0.93).
Labeling of late mitotic neuronal progenitors and early postmitotic neurons
Detection of Double cortin
Double cortin (DCX) directs neuronal migration into the cerebral cortex by regulating the organization and stability of microtubules. It is transiently expressed for presumably 2–3 weeks by mitotic neuronal precursor cells and early postmitotic neurons 5, 7. Of all neuronal markers evaluated in this study, DCX was most abundantly expressed, especially in the subgranular layer of the dentate gyrus. Density of DCX‐immunoreactive cells in DEX‐exposed animals was not significantly different in comparison with controls ( Figure 2A,B).
Figure 2.
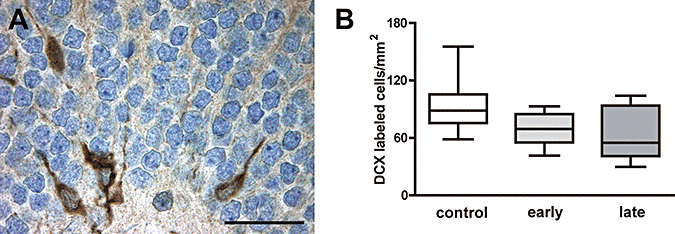
A. Four cells expressing the early neuronal marker doublecortin in the granule cell layer and subgranular cell layer of the dentate gyrus (scale bar = 50 µm). B. No significant changes in the density of doublecortin‐immunoreactive cells were observed between untreated and early and late dexamethasone‐exposed 2‐year‐old marmosets (medians ± 25%/75% percentiles, P = 0.09).
Detection of TUC‐4
TUC‐4 (TOAD‐64) is a membrane‐associated neural protein that is transiently expressed in the cytoplasm and processes of both late mitotic neuronal progenitors and early postmitotic neurons as they begin their migration 14, 33. Its expression peaks during axonal outgrowth and declines thereafter to almost undetectable levels in the adult brain (38). Comparison of TUC‐4‐immunoreactive neurons in the dentate gyrus of DEX‐exposed and control animals revealed a similar density ( Figure 3A,B).
Figure 3.
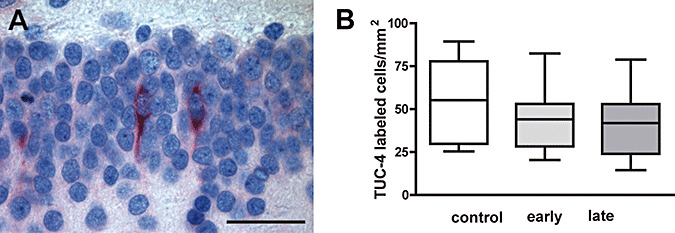
A. Cells expressing the early neuronal marker TUC‐4 in the granule cell layer of the dentate gyrus (scale bar = 50 µm). B. Comparison of the density of TUC‐4‐immunoreactive cells in the dentate gyrus of controls, early and late dexamethasone‐exposed 2‐year‐old marmoset revealed no significant differences (medians ± 25%/75% percentiles, P = 0.45).
Detection of calretinin
Calretinin is a calcium‐binding protein and marker for postmitotic granule cells. Its expression appears to be restricted to a short phase in which synaptogenesis takes place, supposedly approximately 2–3 weeks 4, 23, 32. Calretinin‐immunoreactive cells were most abundant in the subgranular layer of the dentate gyrus and especially the hilus of the hippocampus. The density of cells expressing calretinin in the subgranular layer of the dentate gyrus was not significantly different between control marmosets and DEX‐exposed animals ( Figure 4A,B).
Figure 4.
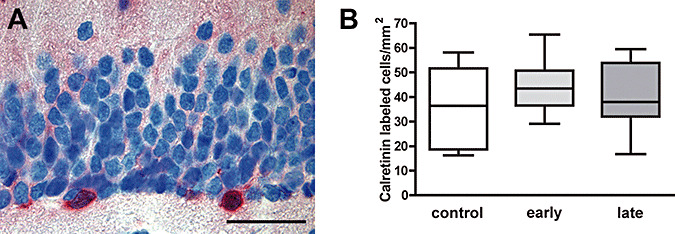
A. Calretinin‐expressing cells in the subgranular cell layer of the dentate gyrus reflecting immature granule cells (scale bar = 50 µm). B. Density of calretinin‐immunoreactive cells in the dentate subgranular cell layer of controls, early and late dexamethasone‐exposed marmoset monkeys at the age of 2 years (medians ± 25%/75% percentiles, P = 0.49).
Density of apoptotic neurons
Apoptotic neurons were detected by in situ tailing of fragmented DNA and by morphological criteria 15, 17. The total amount of apoptotic neurons was low and only very few cells within the subgranular zone were labeled. The density of apoptotic neurons/mm2 in the dentate gyrus in early and late DEX‐exposed and control animals revealed no major differences between these groups ( Figure 5A,B). The density of apoptotic neurons in 2‐year‐old marmosets was more than one order of magnitude lower than the density of apoptotic neurons in the newborn littermates investigated in our previous study (44).
Figure 5.
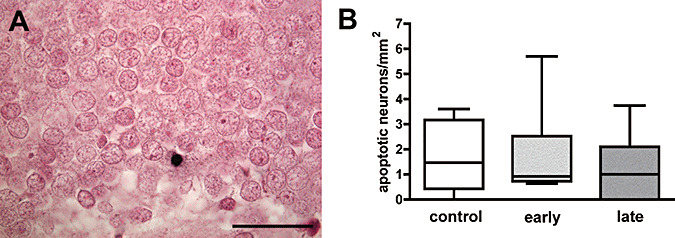
A. Apoptotic neuron (in situ tailing) in the subgranular cell layer of the dentate gyrus in 2‐year‐old marmoset monkey (scale bar = 50 µm). B. Density of apoptotic cells in the dentate gyrus of control marmosets and 2 years after early and late exposure to prenatal dexamethasone (medians ± 25%/75% percentiles, P = 0.8).
DISCUSSION
The question whether environmental influences during prenatal development—in the so‐called phase of “early life programming”—can be the origin of chronic diseases in adulthood is still a matter of debate.
Studies investigating long‐term neurological outcome after intrauterine or postnatal glucocorticoid exposure are scarce—especially in adults and higher mammals. In the present study, comparison of the volume of the dentate granule cell layer did not reveal any significant differences between DEX‐exposed and control animals at the age of 2 years. In contrast, exposure to 5 mg/kg body weight DEX for 2 days during late pregnancy led to a 30% decrease in hippocampal volume in 20‐month‐old rhesus monkeys (45). Moreover, prenatal stress during early and late pregnancy by an acoustical startle protocol resulted in a mild reduction of hippocampal volume in rhesus monkeys at the age of 36 months (6). Reasons for these variances could be species differences as well as methodological differences in volume determination: in comparison with the present study where the granule cell layer volume was assessed stereologically in new world primates, other studies measured the volume of the whole hippocampal formation in old world monkeys by radiographical means. In this study, the volume of the granule cell layer was assessed because adult neurogenesis is restricted to the dentate granule cell layer. However, the dentate gyrus accounts for only 6% of the volume of the human hippocampus (21). Possibly, volume decreases of other hippocampal areas such as CA1‐4 were responsible for the overall shrinkage of the hippocampal formation observed by others. For example, chronic psychosocial stress caused dendritic atrophy of CA3 neurons in tree shrews (28). When interpreting these data it has further to be taken into consideration that radiographically determined volume reductions, especially hippocampal volume loss, are reversible after recovery from stress or treatment with antidepressant drugs 8, 9. Reductions in the volume of the hippocampal formation were at least in part reversible in patients with Cushing's syndrome by treatment of the underlying disease (42). The structural correlate for the reduction of hippocampal volume has not been completely elucidated yet. It was proposed that alterations in axonal and synaptic components as well as glial changes could be a cause of the shrinkage (8).
Though there are data concerning changes of hippocampal volume because of antenatal glucocorticoids, information of the impact on neurogenesis is scarce. In our previous study with newborn marmosets, evaluation of neurogenesis revealed an impaired neuronal proliferation after DEX exposure during early and late pregnancy without affecting neuronal differentiation. In contrast to the reduced proliferation rate in newborn animals, differences in neuronal proliferation could no longer be detected in 2‐year‐old monkeys. One limiting factor in comparing both studies could be sex differences: in the present study exclusively male littermates were investigated while the majority of the newborn animals studied were female. However, previous studies on the impact of sex difference on neurogenesis only found differences when comparing female rats in a high estrogen state with males (43). Moreover, no substantial influence of gender differences and estradiol levels on hippocampal neurogenesis was observed in mice (26). In the present study, there are no additional correlative behavioral data which could elucidate if the unchanged rate of neurogenesis was accompanied by a physiological cognitive development in these monkeys. As our investigations are part of a European‐wide study on the impact of early life glucocorticoid programming on adult health, neuropsychological investigations were performed by a cooperation partner.
A time‐limited effect of glucocorticoid hormones on neuronal proliferation—like in this study—was also observed in rats. Pregnancy and motherhood are known to increase glucocorticoid levels during lactation. The number of proliferating cells in the dentate gyrus of primiparous female rat was decreased during lactation. This phenomenon reversed in parallel with reduction of hormone levels by removal of the pups (27). Positive stimuli on neurogenesis also are at least in part time‐limited: voluntary wheel running both under usual conditions (24) and during pregnancy (2) had acute stimulatory effects on precursor cell proliferation which declined with time. Long‐term running over 24 days even decreased the proliferation to one‐half the level of non‐running control rats (36).
Neuronal differentiation was not different from that of control animals neither in newborn nor in adult DEX‐exposed marmosets. Several studies showed that elevated corticosteroid hormones themselves—or as a result of acute or chronic stress—affected the proliferation and differentiation of dentate granule cells 6, 10, 45, 48. Again, many of these changes were time‐limited and reversible—already after a few days or after weeks 9, 20. Considering a time span of several weeks until 2 years between exposure to DEX and evaluation of neuronal proliferation and differentiation, our observation of no substantial long‐term changes in basal adult neurogenesis in this model appears to be in accordance with others.
Both in newborn and young adult marmoset monkeys, no influence of intrauterine DEX‐exposure on the rate of apoptosis within the hippocampal formation was evident. Similarly, apoptosis in the rat dentate gyrus was decreased during chronic stress only, whereas these changes were reversible within 3 weeks of recovery (20). Given the low numbers of apoptotic cells in the dentate gyrus that even decline with age, significant differences are hard to find. It can be speculated whether there may have been changes in the rate of hippocampal apoptosis during the acute phase of prenatal exposure but apparently long‐lasting effects of glucocorticoid exposure were absent.
In summary, our investigations suggest no long‐lasting effect of short‐term prenatal exposure to DEX on neuronal proliferation and differentiation in common marmoset monkeys. Considering the consequences on the basal rate of adult neurogenesis, short‐term prenatal therapy with DEX to induce lung maturation of the infant appears to be a justifiable therapeutic approach.
ACKNOWLEDGMENT
This work was supported by the European Commission Grant QLRT‐2001‐02758 (EUPEAH).
REFERENCES
- 1. Antonow‐Schlorke I, Schwab M, Li C, Nathanielsz PW (2003) Glucocorticoid exposure at the dose used clinically alters cytoskeletal proteins and presynaptic terminals in the fetal baboon brain. J Physiol 547:117–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bick‐Sander A, Steiner B, Wolf SA, Babu H, Kempermann G (2006) Running in pregnancy transiently increases postnatal hippocampal neurogenesis in the offspring. Proc Natl Acad Sci USA 103:3852–3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brabham T, Phelka A, Zimmer C, Nash A, Lopez JF, Vazquez DM (2000) Effects of prenatal dexamethasone on spatial learning and response to stress is influenced by maternal factors. Am J Physiol Regul Integr Comp Physiol 279:R1899–R1909. [DOI] [PubMed] [Google Scholar]
- 4. Brandt MD, Jessberger S, Steiner B, Kronenberg G, Reuter K, Bick‐Sander A et al (2003) Transient calretinin expression defines early postmitotic step of neuronal differentiation in adult hippocampal neurogenesis of mice. Mol Cell Neurosci 24:603–613. [DOI] [PubMed] [Google Scholar]
- 5. Brown JP, Couillard‐Despres S, Cooper‐Kuhn CM, Winkler J, Aigner L, Kuhn HG (2003) Transient expression of doublecortin during adult neurogenesis. J Comp Neurol 467:1–10. [DOI] [PubMed] [Google Scholar]
- 6. Coe CL, Kramer M, Czeh B, Gould E, Reeves AJ, Kirschbaum C, Fuchs E (2003) Prenatal stress diminishes neurogenesis in the dentate gyrus of juvenile rhesus monkeys. Biol Psychiatry 54:1025–1034. [DOI] [PubMed] [Google Scholar]
- 7. Couillard‐Despres S, Winner B, Schaubeck S, Aigner R, Vroemen M, Weidner N et al (2005) Doublecortin expression levels in adult brain reflect neurogenesis. Eur J Neurosci 21:1–14. [DOI] [PubMed] [Google Scholar]
- 8. Czeh B, Lucassen PJ (2007) What causes the hippocampal volume decrease in depression? Are neurogenesis, glial changes and apoptosis implicated? Eur Arch Psychiatry Clin Neurosci 257:250–260. [DOI] [PubMed] [Google Scholar]
- 9. Czeh B, Michaelis T, Watanabe T, Frahm J, De Biurrun G, Van Kampen M et al (2001) Stress‐induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proc Natl Acad Sci USA 98:12796–12801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Czeh B, Welt T, Fischer AK, Erhardt A, Schmitt W, Muller MB et al (2002) Chronic psychosocial stress and concomitant repetitive transcranial magnetic stimulation: effects on stress hormone levels and adult hippocampal neurogenesis. Biol Psychiatry 52:1057–1065. [DOI] [PubMed] [Google Scholar]
- 11. Drapeau E, Mayo W, Aurousseau C, Le Moal M, Piazza PV, Abrous DN (2003) Spatial memory performances of aged rats in the water maze predict levels of hippocampal neurogenesis. Proc Natl Acad Sci USA 100:14385–14390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ferguson SA, Paule MG, Holson RR (2001) Neonatal dexamethasone on day 7 in rats causes behavioral alterations reflective of hippocampal, but not cerebellar, deficits. Neurotoxicol Teratol 23:57–69. [DOI] [PubMed] [Google Scholar]
- 13. Flagel SB, Vazquez DM, Watson SJ Jr., Neal, CR Jr. (2002) Effects of tapering neonatal dexamethasone on rat growth, neurodevelopment, and stress response. Am J Physiol Regul Integr Comp Physiol 282:R55–R63. [DOI] [PubMed] [Google Scholar]
- 14. Gaetano C, Matsuo T, Thiele CJ (1997) Identification and characterization of a retinoic acid‐regulated human homologue of the unc‐33‐like phosphoprotein gene (hUlip) from neuroblastoma cells. J Biol Chem 272:12195–12201. [DOI] [PubMed] [Google Scholar]
- 15. Gerber J, Raivich G, Wellmer A, Noeske C, Kunst T, Werner A et al (2001) A mouse model of Streptococcus pneumoniae meningitis mimicking several features of human disease. Acta Neuropathol (Berl) 101:499–508. [DOI] [PubMed] [Google Scholar]
- 16. Gerdes J (1990) Ki‐67 and other proliferation markers useful for immunohistological diagnostic and prognostic evaluations in human malignancies. Semin Cancer Biol 1:199–206. [PubMed] [Google Scholar]
- 17. Gold R, Schmied M, Giegerich G, Breitschopf H, Hartung HP, Toyka KV, Lassmann H (1994) Differentiation between cellular apoptosis and necrosis by the combined use of in situ tailing and nick translation techniques. Lab Invest 71:219–225. [PubMed] [Google Scholar]
- 18. Hassan AH, Von Rosenstiel P, Patchev VK, Holsboer F, Almeida OF (1996) Exacerbation of apoptosis in the dentate gyrus of the aged rat by dexamethasone and the protective role of corticosterone. Exp Neurol 140:43–52. [DOI] [PubMed] [Google Scholar]
- 19. Haynes LE, Griffiths MR, Hyde RE, Barber DJ, Mitchell IJ (2001) Dexamethasone induces limited apoptosis and extensive sublethal damage to specific subregions of the striatum and hippocampus: implications for mood disorders. Neuroscience 104:57–69. [DOI] [PubMed] [Google Scholar]
- 20. Heine VM, Maslam S, Zareno J, Joels M, Lucassen PJ (2004) Suppressed proliferation and apoptotic changes in the rat dentate gyrus after acute and chronic stress are reversible. Eur J Neurosci 19:131–144. [DOI] [PubMed] [Google Scholar]
- 21. Joelving FC, Billeskov R, Christensen JR, West M, Pakkenberg B (2006) Hippocampal neuron and glial cell numbers in Parkinson's disease—a stereological study. Hippocampus 16:826–833. [DOI] [PubMed] [Google Scholar]
- 22. Kempermann G, Kuhn HG, Gage FH (1997) More hippocampal neurons in adult mice living in an enriched environment. Nature 386:493–495. [DOI] [PubMed] [Google Scholar]
- 23. Kempermann G, Jessberger S, Steiner B, Kronenberg G (2004) Milestones of neuronal development in the adult hippocampus. Trends Neurosci 27:447–452. [DOI] [PubMed] [Google Scholar]
- 24. Kronenberg G, Bick‐Sander A, Bunk E, Wolf C, Ehninger D, Kempermann G (2006) Physical exercise prevents age‐related decline in precursor cell activity in the mouse dentate gyrus. Neurobiol Aging 27:1505–1513. [DOI] [PubMed] [Google Scholar]
- 25. Kuhn HG, Dickinson‐Anson H, Gage FH (1996) Neurogenesis in the dentate gyrus of the adult rat: age‐related decrease of neuronal progenitor proliferation. J Neurosci 16:2027–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lagace DC, Fischer SJ, Eisch AJ (2007) Gender and endogenous levels of estradiol do not influence adult hippocampal neurogenesis in mice. Hippocampus 17:175–180. [DOI] [PubMed] [Google Scholar]
- 27. Leuner B, Mirescu C, Noiman L, Gould E (2007) Maternal experience inhibits the production of immature neurons in the hippocampus during the postpartum period through elevations in adrenal steroids. Hippocampus 17:434–442. [DOI] [PubMed] [Google Scholar]
- 28. Magarinos AM, McEwen BS, Flugge G, Fuchs E (1996) Chronic psychosocial stress causes apical dendritic atrophy of hippocampal CA3 pyramidal neurons in subordinate tree shrews. J Neurosci 16:3534–3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Malberg JE, Duman RS (2003) Cell proliferation in adult hippocampus is decreased by inescapable stress: reversal by fluoxetine treatment. Neuropsychopharmacology 28:1562–1571. [DOI] [PubMed] [Google Scholar]
- 30. Malberg JE, Eisch AJ, Nestler EJ, Duman RS (2000) Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci 20:9104–9110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Meaney MJ, Diorio J, Francis D, Widdowson J, LaPlante P, Caldji C et al (1996) Early environmental regulation of forebrain glucocorticoid receptor gene expression: implications for adrenocortical responses to stress. Dev Neurosci 18:49–72. [DOI] [PubMed] [Google Scholar]
- 32. Ming GL, Song H (2005) Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci 28:223–250. [DOI] [PubMed] [Google Scholar]
- 33. Minturn JE, Geschwind DH, Fryer HJ, Hockfield S (1995) Early postmitotic neurons transiently express toad‐64, a neural specific protein. J Comp Neurol 355:369–379. [DOI] [PubMed] [Google Scholar]
- 34. Morimoto M, Morita N, Ozawa H, Yokoyama K, Kawata M (1996) Distribution of glucocorticoid receptor immunoreactivity and mRNA in the rat brain: an immunohistochemical and in situ hybridization study. Neurosci Res 26:235–269. [DOI] [PubMed] [Google Scholar]
- 35. Muneoka K, Mikuni M, Ogawa T, Kitera K, Kamei K, Takigawa M, Takahashi K (1997) Prenatal dexamethasone exposure alters brain monoamine metabolism and adrenocortical response in rat offspring. Am J Physiol 273:R1669–R1675. [DOI] [PubMed] [Google Scholar]
- 36. Naylor AS, Persson AI, Eriksson PS, Jonsdottir IH, Thorlin T (2005) Extended voluntary running inhibits exercise‐induced adult hippocampal progenitor proliferation in the spontaneously hypertensive rat. J Neurophysiol 93:2406–2414. [DOI] [PubMed] [Google Scholar]
- 37. Pryce CR, Feldon J, Fuchs E, Knuesel I, Oertle T, Sengstag C, Spengler M, Weber E, Weston A, Jongen‐Relo A (2005) Postnatal ontogeny of hippocampal expression of the mineralocorticoid and glucocorticoid receptors in the common marmoset monkey. Eur J Neurosci 21:1521–1535. [DOI] [PubMed] [Google Scholar]
- 38. Quinn CC, Gray GE, Hockfield S (1999) A family of proteins implicated in axon guidance and outgrowth. J Neurobiol 41:158–164. [PubMed] [Google Scholar]
- 39. Seckl JR, Dickson KL, Yates C, Fink G (1991) Distribution of glucocorticoid and mineralocorticoid receptor messenger RNA expression in human postmortem hippocampus. Brain Res 561:332–337. [DOI] [PubMed] [Google Scholar]
- 40. Slotkin TA, Zhang J, McCook EC, Seidler FJ (1998) Glucocorticoid administration alters nuclear transcription factors in fetal rat brain: implications for the use of antenatal steroids. Brain Res Dev Brain Res 111:11–24. [DOI] [PubMed] [Google Scholar]
- 41. Speirs HJ, Seckl JR, Brown RW (2004) Ontogeny of glucocorticoid receptor and 11beta‐hydroxysteroid dehydrogenase type‐1 gene expression identifies potential critical periods of glucocorticoid susceptibility during development. J Endocrinol 181:105–116. [DOI] [PubMed] [Google Scholar]
- 42. Starkman MN, Giordani B, Gebarski SS, Berent S, Schork MA, Schteingart DE (1999) Decrease in cortisol reverses human hippocampal atrophy following treatment of Cushing's disease. Biol Psychiatry 46:1595–1602. [DOI] [PubMed] [Google Scholar]
- 43. Tanapat P, Hastings NB, Reeves AJ, Gould E (1999) Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. J Neurosci 19:5792–5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tauber SC, Schlumbohm C, Schilg L, Fuchs E, Nau R, Gerber J (2006) Intrauterine exposure to dexamethasone impairs proliferation but not neuronal differentiation in the dentate gyrus of newborn common marmoset monkeys. Brain Pathol 16:209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Uno H, Eisele S, Sakai A, Shelton S, Baker E, DeJesus O, Holden J (1994) Neurotoxicity of glucocorticoids in the primate brain. Horm Behav 28:336–348. [DOI] [PubMed] [Google Scholar]
- 46. Van Praag H, Christie BR, Sejnowski TJ, Gage FH (1999) Running enhances neurogenesis, learning, and long‐term potentiation in mice. Proc Natl Acad Sci USA 96:13427–13431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Van Praag H, Kempermann G, Gage FH (1999) Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci 2:266–270. [DOI] [PubMed] [Google Scholar]
- 48. Yu IT, Lee SH, Lee YS, Son H (2004) Differential effects of corticosterone and dexamethasone on hippocampal neurogenesis in vitro . Biochem Biophys Res Commun 317:484–490. [DOI] [PubMed] [Google Scholar]


