Abstract
Rare cases of West Nile virus (WNV)‐associated inflammation outside the central nervous system (CNS) have been reported. We evaluated the systemic distribution of WNV in postmortem tissues during encephalitis in six patients using immunohistochemistry. WNV antigens were detected in neurons of CNS (all 6 cases), kidney (4 cases), lungs (2 cases), pancreas (2 cases), thyroid (2 cases), intestine (2 cases), stomach (1 case), esophagus (1 case), bile duct (1 case), skin (1 case), prostate (1 case) and testis (1 case). In systemic organs epithelial cells were infected. In none of the six cases were viral antigens identified in hepatocytes, heart, adrenal gland, nerves, skeletal muscles, bone, vessels and fat. All cases in which viral antigens were identified in systemic organs in addition to CNS were severely immunocompromised transplant recipients. With the exception of testis and brain, most foci of infection were not associated with inflammation. While the absence of inflammation may in part be due to patient immunosuppression or to possible transient nature of any host response, compartmentalization of viral antigen to the luminal region of epithelial cells may sequester WNV from immune recognition. Comparison of our findings with previous reports suggests that patients with WNV encephalitis can have widespread systemic infection.
INTRODUCTION
West Nile virus (WNV) was first isolated from the blood of a febrile woman in the West Nile province of Uganda in 1937. Historically, this mosquito‐borne flavivirus has been associated with periodic epidemics of febrile illness and sporadic encephalitis in Africa, the Mediterranean basin, Eastern Europe, southwest Asia and Australia (14). The virus first appeared in the western hemisphere during an outbreak of encephalitis in New York City in 1999 (35). Since then, it has spread dramatically across North America and southward into Latin America and the Caribbean (17).
The transmission of WNV in North America increases during warmer months, with peak activity from July through October (36). From 1999 through December 31, 2006, 23 886 cases of human WNV disease were reported in the USA (to the Centers for Disease Control, Atlanta, Georgia) with onset of illness as early as April and as late as December. Of these 23 886 cases, 9800 were reported to be neuro‐invasive disease, 13 439 were West Nile fever, and 647 had unspecified or other clinical presentations. There were 934 reported deaths because of WNV over the same period (Updates on reported human cases can be found at http://www.cdc.gov/ncidod/dvbid/westnile/index.htm).
The majority (about 80%) of human infections with WNV are clinically silent, and 20% of infections result in self‐limited fever. Typically, less than 1% of infected patients develop neuro‐invasive disease, which manifests as an overlapping spectrum of meningitis, encephalitis, and/or myeloradiculitis/poliomyelitis/flaccid paralysis (16, 50). Immunosuppressed and elderly persons are at higher risk of neuro‐invasive disease and long‐term sequelae (22, 36). Diabetes, hypertension and cerebrovascular disease have also been implicated as other possible risk factors for neuro‐invasive disease (17, 34). The higher incidence of WNV encephalitis during the current US infection suggests the introduced strain is more neurovirulent. Systemic infection is characterized by the acute onset of fever, headache, fatigue, malaise, muscle pain and weakness; gastrointestinal symptoms and a transient macular rash on the trunk and extremities are sometimes reported (17, 58). The clinical severity of WNV encephalitis ranges from mild disorientation to coma and death, and many patients manifest movement disorders, including severe tremors and Parkinsonism (50, 55). In approximately 13% of patients with neuro‐invasive WNV disease, WNV infection of spinal motor neurons causes acute asymmetric flaccid paralysis, similar to that seen with poliomyelitis (24).
The distribution of WNV in human tissues during infection has not been systematically described. Therefore, the cellular tropism of the virus in humans remains to be fully evaluated. Reports of WNV in non‐CNS tissues are rare. The presence of WNV in non‐CNS tissues of US patients was first reported in blood in 2002 by Huang et al, and since then only a few other cases have been reported. The 52 reported cases of fatal neuro‐invasive WNV disease in the USA are reviewed and summarized in the Supplementary Table S1.
Histopathologic examination of central nervous system (CNS) tissues from patients with WNV neuro‐invasive disease shows perivascular inflammation, microglial nodules, neuronophagia, and variable necrosis and neuronal loss, with pathologic changes concentrated in the brainstem, deep gray nuclei and anterior horns of the spinal cells (1, 3, 5, 6, 7, 9, 10, 12, 13, 15, 19, 20, 21, 25, 26, 28, 37, 40, 44, 45, 46, 47, 48, 52, 53). Spinal cord inflammation was seen in 17 of 23 patients who died with WNV neuro‐invasive disease (15). Inflammation was more prominent in the anterior horns than in the posterior horns of nine patients. Foci of demyelination, gliosis and occasional perivascular infiltrates may be found in persons with prolonged clinical courses (15).
Generally, the presence of WNV has been documented in persons who died early during the course of WNV infection (15, 21); however, persistence of WNV in human brain tissue samples 4 months after initial diagnosis has been recently documented (40). Using immunohistochemical (IHC) staining, WNV antigen has been demonstrated variably in brain, kidney, spleen, liver, lung, skin, bone marrow and intravascular mononuclear cells (1, 9, 10, 15, 19, 23, 37, 38, 40, 46, 53). IHC staining is more often positive than viral culture, showing WNV antigens in approximately 50% of fatal WNV neuro‐invasive disease cases. IHC staining is more commonly positive in patients who died during the first week of illness when viral antigen concentrations in CNS tissues are high (15), although these studies have been conducted primarily in immunocompetent persons. Persistence of viral antigen for considerably longer time periods has been demonstrated by IHC in patients with severe immunocompromising conditions, such as solid organ or bone marrow transplant recipients (15, 40). Viral antigens are found principally within neurons and neuronal processes, prominently in the brainstem, basal ganglia, thalami and spinal cord anterior horn cells, consistent with WNV’s neurotropic reputation. In general, viral antigens are focal and sparse, except in severely immunosuppressed patients where they are detected extensively throughout the CNS (15). However, there is one recent report suggesting glial cell infection (40). We have previously reported confocal florescent laser scanning microscopic visualization of WNV antigens in infected human brain tissue, but no infected systemic cells were visualized in screening sections of the lungs, heart, liver, spleen, kidneys and pancreas from this patient (37).
Rare cases of clinically and/or histologically confirmed WNV‐associated hepatitis, pancreatitis, myocarditis, cardiac dysrhythmia, myositis, rhabdomyolysis, orchitis, nephritis, chorioretinitis, uveitis, vitritis and optic neuritis have been reported (2, 10, 12, 15, 24, 27, 33, 37, 41, 42, 46, 53). There are few systematic surveys of WNV in human postmortem tissues, apart from the CNS, during neuro‐invasive WNV infection. WNV has been identified at autopsy in renal and splenic tissues by culture, IHC and reverse transcriptase polymerase chain reaction (RT‐PCR) (15, 37); in testicular tissue by electron microscopy (EM) (10, 53); and in liver, lung, bone marrow, intravascular mononuclear cells and skin by IHC (38). WNV infection of non‐CNS tissues has important implications for organ and blood donations. The aim of this study was to characterize the systemic distribution of WNV infection in human postmortem tissue in six patients and compare this with previously published reports.
MATERIALS AND METHODS
Case definition. A patient was considered to have neuro‐invasive WNV infection if there were clinical and histopathologic evidence of encephalitis and there was positive serology for WNV or presence of flavivirus antigens in brain tissue sections by IHC.
Histology. Hematoxylin and eosin (H&E) stained brain tissue sections were evaluated for inflammatory lesions in representative slides that were taken from the following CNS locations: cerebral cortex, hippocampus, thalamus, basal ganglia, midbrain, pons, medulla, cerebellum and spinal cord. The presence of meningitis and neuritis was also recorded. Histopathologic studies of non‐CNS tissues were also performed.
Immunohistochemistry. Paraffin‐embedded sections were deparaffinized in Histoclear 3X5 minutes (National Diagnostics, Atlanta, GA) and rehydrated through graded alcohols (100%, 95% and 75% each for two minutes) and PBS for 5 minutes. Endogenous peroxidase was inactivated by immersing in 3% H2O2 for 20 minutes. Antigen unmasking was performed by bringing sections to a 95°C 1X Target Retrieval Solution (Dako Corporation, Carpinteria, CA, USA) for 30 minutes, cooling 30 minutes to room temperature, tissue sections were washed twice in H2O and PBS, and tissue section were blocked with protein blocking agent (Thermo Shandon, 407501) for 30 minutes. Tissue sections were then incubated for 2 h at room temperature or over night at 4°C with Rabbit anti‐WNV (1:1000, Galveston, TX T35502) primary antibody (56). After being incubated with the primary antibody, slides were washed three times with PBS Tween 20 and goat anti‐Rabbit biotinylated link antibody (1:200, Dako Corporation) fluroprobe‐labeled streptavidin, and nuclear counterstain was sequentially applied. Positive controls included formalin‐fixed, paraffin‐embedded human CNS and non‐CNS tissue sections from patients with culture‐confirmed infections with WNV. Negative controls included CNS and non‐CNS tissue sections of patients with encephalitis caused by human immunodeficiency virus (HIV), or with meningitis caused by Streptococcus pneumoniae. Negative controls for each specimen also consisted of tissue sections incubated with normal mouse immunoglobulin or glial fibrillary acidic protein (GFAP) specific antisera rather than WNV specific antibodies. All the stained slides were independently evaluated by two authors (HBA and CAW), and then these two authors jointly assessed each slide to resolve whether a slide was positive or negative.
Confocal microscopy. Double or triple label confocal florescent laser scanning microscopy using antibodies for Pancytokeratin, CD3, CD68 and WNV was performed as previously described (37).
RESULTS
All six cases of neuro‐invasive WNV studied had serologic evidence of WNV infection. Table 1 lists the clinical and diagnostic characteristics for the six cases of neuro‐invasive WNV disease. Three of the six cases studied have been previously reported, and the respective citations are listed in Table 1. All six cases lived in states that reported animal or other human WNV infections in 2002. Their mean age was 57.3 years (range, 26 to 87 years); three (50%) were younger than 60 years of age, and four (66.7%) were male. Underlying medical conditions were reported in four patients, and three patients were severely immunocompromised transplant recipients. All patients presumably acquired the disease through mosquito bites.
Table 1.
Clinical and diagnostic characteristics of the six cases of neuro‐invasive WNV infection. Abbreviations: WNV = West Nile virus; WID = WNV illness duration; H&E = hematoxylin and eosin; IHC = immunohistochemistry; PCR = polymerase chain reaction; NR = not reported; M = male; F = female; ND = not done; CSF = cerebrospinal fluid.
| Case | Citation | Age/sex (years) | Predisposing history | WID (days) | WNV serology positive samples | H&E inflammatory lesions positive organs | WNV IHC positive organs | WNV PCR positive organs |
|---|---|---|---|---|---|---|---|---|
| 1* | 37 | 87/M | None | 11 | CSF, Serum | Brain | Brain | Brain, CSF, spleen, kidney |
| 2† | 10, 53 | 43/M | Renal transplant, diabetes mellitus | 14 | Serum | Brain, kidney, testes, skeletal muscle | Brain, kidney, lung, pancreas, thyroid, small bowel, prostate, testes, skin, bile duct, stomach | Brain |
| 3 | NR | 75/F | Diabetes mellitus, cerebrovascular disease, high blood pressure | 8 | CSF | Brain | Brain, kidney, esophagus | ND |
| 4 | NR | 26/M | Small bowel transplant | 142 | CSF, Serum | Brain | Brain, kidney, lung, pancreas, thyroid, colon | ND |
| 5 | 28 | 46/F | Non‐Hodgkin lymphoma, bone marrow transplant | 17 | CSF, Serum | Brain | Brain, kidney | Brain, blood |
| 6 | NR | 66/M | None | 18 | CSF, Serum | Brain | Brain | ND |
Summary of demographic and pathological findings in six cases of neuro‐invasive WNV presumed secondary to mosquito bite.
WNV culture was positive on the brain, CSF, spleen and kidney of Case 1.
WNV electron microscopy was positive in the testis of Case 2.
Glial nodules with variable loss of neurons and perivascular cuffing by mononuclear cells were observed in all six cases. In general, mononuclear inflammation and loss of neurons were most prominent in the gray matter of the thalamus, medulla, pons and midbrain, whereas the cerebral cortex and cerebellum showed fewer inflammatory nodules and less perivascular inflammatory cell cuffing. Inflammation was seen in all six spinal cord samples and was considered extensive in four cases. Inflammation was usually seen in both anterior and posterior horns, but inflammation was more prominent in the anterior horn in all cases. Lymphocytic meningitis was observed in two of the six cases. Lymphocytic neuritis of cranial or spinal nerves was present in one of the six cases of neuro‐invasive WNV infection studied. The histology of all non‐CNS tissues (heart, lung, liver, spleen, kidney, pancreas, stomach, intestine, bone, adrenal gland, testes, blood vessels and peripheral nerves) studied from the six cases with neuro‐invasive WNV infection were unremarkable, with the exception of the renal transplant patient (Case 2) whose testes, psoas muscle and kidney showed interstitial lymphocytic inflammatory infiltrates.
The distribution of WNV in systemic and CNS tissues is summarized in Table 2. Figure 1 illustrates examples of the immunofluorescence staining in one case with the most abundant immunofluorescence positivity (Case 2). Positive IHC staining of CNS tissue was obtained in all six cases. With respect to the various regions of the CNS, WNV antigens were detected by IHC in the brainstem (5 cases), spinal cord (5 cases), cerebellum (5 cases), thalamus (4 cases), basal ganglia (2 cases), hippocampus (2 cases) and neocortex (1 case). IHC staining showed viral antigens throughout the cytoplasm of neurons and neuronal processes, usually associated with mononuclear inflammation of variable intensity. In most cases, infected cells were sparse, with staining in only one or two neurons per section. However, cases with severe immunosuppression showed regions with extensive viral antigens in most of the neurons of the thalamus, pons, medulla, midbrain, cerebellum and cerebral cortex, not always commensurate with inflammation.
Table 2.
Immunohistochemical results in tissues of six cases of neuro‐invasive WNV infection. Abbreviations: CNS = central nervous system; ND = not done; NA = not applicable; WNV = West Nile virus.
| Organ | Case number | Summary | |||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | ||
| CNS | |||||||
| Neocortex | Positive | Negative | Negative | Negative | Negative | Negative | 1/6 |
| Hippocampus | Positive | Negative | Negative | Negative | ND | Positive | 2/5 |
| Thalamus | Positive | Positive | ND | Positive | ND | Positive | 4/4 |
| Basal ganglia | Positive | Positive | Negative | Negative | ND | Negative | 2/5 |
| Brainstem | Positive | Positive | Positive | Negative | Positive | Positive | 5/6 |
| Cerebellum | Positive | Positive | Positive | Negative | Positive | Positive | 5/6 |
| Spinal cord | Positive | ND | Positive | Positive | Positive | Positive | 5/5 |
| Kidney | |||||||
| Glomeruli | Negative | Negative | Negative | Negative | Negative | Negative | 0/6 |
| Tubules | Negative | Positive | Positive | Positive | Positive | Negative | 4/6 |
| Lung | |||||||
| Bronchi | Negative | Positive | Negative | Positive | Negative | Negative | 2/6 |
| Alveoli | Negative | Negative | Negative | Negative | Negative | Negative | 0/6 |
| Pancreas | |||||||
| Exocrine glands | Negative | Positive | Negative | Positive | Negative | Negative | 2/6 |
| Duct epithelia | Negative | Negative | Negative | Negative | Negative | Negative | 0/6 |
| Thyroid epithelia | ND | Positive | Negative | Positive | Negative | Negative | 2/5 |
| Intestinal epithelia | ND | Positive | Negative | Positive | Negative | ND | 2/4 |
| Prostate | ND | Positive | NA | ND | NA | ND | 1/1 |
| Testes | |||||||
| Epithelia | ND | Positive | NA | ND | NA | ND | 1/1 |
| Interstitial cells | ND | Negative | NA | ND | NA | ND | 0/1 |
| Skin | |||||||
| Epidermis | ND | Negative | ND | ND | Negative | ND | 0/2 |
| Eccrine glands | ND | Positive | ND | ND | Negative | ND | 1/2 |
| Liver hepatocytes | Negative | Negative | Negative | Negative | Negative | Negative | 0/6 |
| Bile duct epithelia | Negative | Positive | Negative | Negative | Negative | Negative | 1/6 |
| Spleen | |||||||
| Red pulp | Negative | Negative | ND | Negative | ND | Negative | 0/4 |
| White pulp | Negative | Negative | ND | Negative | ND | Negative | 0/4 |
| Lymph node | ND | Negative | ND | Negative | ND | Negative | 0/3 |
| Heart | Negative | Negative | Negative | Negative | Negative | Negative | 0/6 |
| Stomach | ND | Positive | ND | ND | Negative | ND | 1/2 |
| Esophagus | ND | ND | Positive | ND | ND | ND | 1/1 |
| Skeletal muscle | ND | Negative | ND | ND | Negative | ND | 0/2 |
| Bone | |||||||
| Osteocytes | ND | ND | ND | ND | Negative | Negative | 0/2 |
| Marrow | ND | ND | ND | ND | Negative | Negative | 0/2 |
| Peripheral nerves | Negative | Negative | ND | Negative | ND | ND | 0/3 |
| Blood vessels | Negative | Negative | Negative | Negative | Negative | Negative | 0/6 |
| Adipose tissue | Negative | Negative | Negative | Negative | Negative | Negative | 0/6 |
| Adrenal gland | ND | Negative | Negative | Negative | Negative | ND | 0/4 |
Positive, many WNV immunoreactive cells with complete filling of cytoplasm present; Negative, no WNV immunoreactive cells with complete filling of cytoplasm present.
Figure 1.
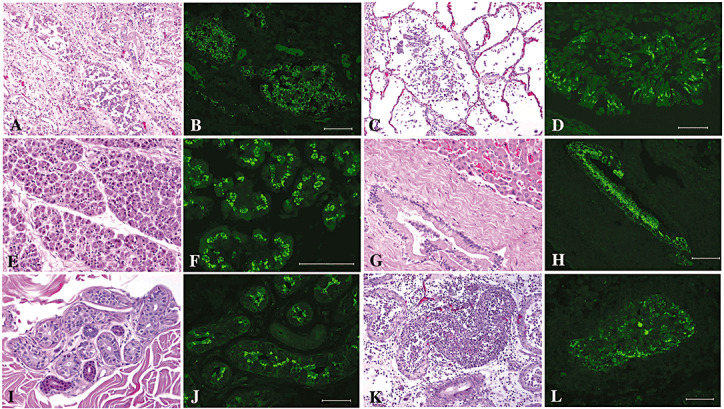
Examples of H&E (A,C,E,G,I,K) and immunofluorescent distribution of WNV antigen (Green; B,D,F,H,J,L) are demonstrated from Case 2. Kidney (Figure 1A,B) shows abundant viral antigen in select distal renal tubules. WNV antigen is particularly focused on the epithelial surface independent of the minimal inflammatory response present. Lung tissue (C,D) shows moderate autolysis and minimal inflammation despite abundant WNV antigen on apical surface of bronchial epithelium. Exocrine pancreas (E,F) showed no inflammation despite abundant regions of epithelial WNV infection. In the liver (G,H) viral antigen was limited to select regions of the bile duct epithelium. For the most part the skin (I,J) showed no inflammation despite abundant WNV antigen within eccrine glands. The testes (K,L) were severely inflamed and showed abundant WNV antigen within portions of the seminiferous tubules. H&E = hematoxylin and eosin; WNV = West Nile virus.
CD68 immunostaining revealed profuse microglial activation in the gray and white matter accentuated in regions delineated above. Gilial fibrillary acid protein (GFAP) immunostaining highlighted moderate global astrocytosis. CD20 and CD3 immunostaining revealed sparse perivascular B‐lymphocytes and variable parenchymal infiltration by T‐lymphocytes. CD4 and CD8 immunophenotypic characterization of the T‐lymphocytes revealed few CD4 positive T‐helper cells with a marked predominance of infiltrating CD8 positive T‐suppressor/cytotoxic cells. Confocal florescent laser scanning microscopy using antibodies for CD3, CD68 and WNV revealed diffuse positive cytoplasmic viral immunofluorescence of many neurons in the substantia nigra, spinal anterior horn cells, pontine neurons, neurons of the CA2 and 3 of the hippocampus, the entorhinal cortex and neurons of the thalamus with rare neurons in the caudate nucleus and arcuate nucleus. Triple label staining for WNV and leukocyte markers CD68 and CD3 confirmed H&E impression of T‐lymphocyte infiltration and macrophage engulfment of infected neurons (Figure 2).
Figure 2.

Top images: Paraffin section of human brain with WNV encephalitis immunostained for macrophages (CD68‐Red) and WNV (Green), show infected neurons in region of macrophage infiltration. Occasional neurons appear to be engulfed by ameboid macrophages. Bottom images: Paraffin section of human brain with WNV encephalitis immunostained for T‐cells (CD3‐Red) and WNV (Green), show infected neurons with infiltrating CD3 T‐cells. Occasional T‐cell nodules surround fragmented neurons. WNV = West Nile virus.
With respect to the non‐CNS tissues, WNV antigens were detected by immunofluorescence in the tubules of kidney (4 cases) (Figure 1A,B), bronchi of lung (2 cases) (Figure 1C,D), pancreas (2 cases) (Figure 1E,F), bile duct (1 case) (Figure 1G,H), skin (1 case) (Figure 1I,J), testis (1 case) (Figure 1K,L), thyroid (2 cases), intestine (2 cases), stomach (1 case), esophagus (1 case) and prostate (1 case). Except for the kidney and testes, most viral antigens were detected in non‐CNS locations with minimal inflammation. While this may be due to the subject’s immunosuppression, the distribution of viral antigens in the infected epithelium demonstrated a clear polarity for the apical surface, away from the basement membrane and thus distal to immune surveillance (Figure 3). In the testes where WNV antigen did not show a polar distribution, inflammation was more pronounced. In all six cases, no viral antigens were identified in; liver, spleen, heart, adrenal gland, lymph node, peripheral nerve, skeletal muscle, bone, blood vessel and fat. Occasional individual cells showed non‐specific staining in lymph nodes and spleen; however, these could not readily be distinguished from rare positive foci in negative control immunohistochemistry. These rare non‐specific staining was considered negative, as it did not demonstrate the characteristic staining of the complete filling of cell cytoplasm consistent with the positive staining seen on the positive control WNV case. WNV antigens were detected in 10 different non‐CNS organs in the renal transplant patient (Case 2), whose testes, kidney and psoas muscle showed interstitial lymphocytic inflammatory infiltrates. Of the other five cases, WNV antigens were detected in five different non‐CNS organs in one case (Case 4), two different non‐CNS organs (Case 3) in another case, one non‐CNS organ in one more case (Case 5), and in no non‐CNS organ in the remaining two cases with no underlying medical conditions (Cases 1 and 6). Cases with relatively severe immunosuppression following transplantation showed viral antigens in more systemic organs in addition to the CNS.
Figure 3.
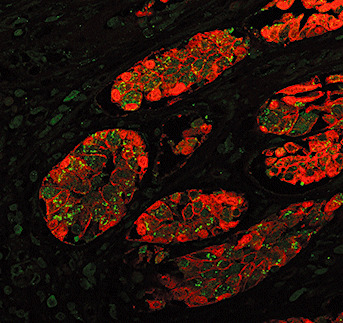
Paraffin section of intestine immunostained for epithelial cells (Pancytokeratin‐Red) and WNV (Green) shows infected epithelial cells containing viral antigen predominantly in cytoplasm on the apical side of the nuclei. WNV = West Nile virus.
DISCUSSION
This study confirms widely disseminated systemic WNV infection in patients with fatal neuro‐invasive WNV disease. Since the introduction of WNV to the USA, there has been concern about the spectrum and outcomes of this disease in immunosuppressed patients. Severe WNV disease and fatalities are more frequent in the elderly population, in whom underlying chronic, possibly immunocompromising, conditions are more common (22, 40). However, host factors that increase the risk of developing neuro‐invasive WNV disease with a fatal outcome, other than age and immunosuppression, have yet to be clearly identified (17, 34).
Computed tomography of the head is generally normal and not helpful in the diagnosis of neuro‐invasive WNV, except to exclude other etiologies of encephalopathy. Brain magnetic resonance imaging (MRI) may frequently be normal, even in severe encephalitis (8, 49). However, when abnormalities are noted on MRI, they include prominent signal abnormalities in the deep gray matter, predominantly in the basal ganglia, thalamus, brain stem and cerebellum of patients with encephalitis, and in the anterior spinal cord in patients with poliomyelitis‐like syndrome (50). These lesions are particularly notable on T2‐ and fluid attenuation inversion recovery (FLAIR) sequences (8, 49, 50). The location of these MRI abnormalities corresponds to regions of the CNS with prominent inflammation on histology and virus localization by IHC of reported cases of neuro‐invasive WNV disease (1, 3, 5, 6, 7, 9, 10, 12, 13, 15, 19, 20, 21, 25, 26, 28, 37, 40, 44, 45, 46, 47, 48, 52, 53), as confirmed in this series.
In patients with WNV neuro‐invasive disease, WNV‐specific IgM is almost always detectable in serum and/or cerebrospinal fluid (CSF) by the time CNS symptoms begin (31), as confirmed in this series. However, the utility of WNV seropositivity as a diagnostic tool is limited in immunosuppressed patients, where IgM antibody appears late in the course of the infection (28, 53). Real‐time RT‐PCR is the most sensitive nucleic acid amplification test for WNV, able to detect as few as 50 viral RNA copies per milliliter (approximately 0.1 PFU/mL), which is approximately 1000‐fold more sensitive than culture (39). The sensitivity of RT‐PCR among 28 patients with serologically confirmed neuro‐invasive WNV disease was 57% in CSF and 14% in serum (30). Using RT‐PCR, WNV RNA has been detected in serum, blood, CSF, brain, spleen and kidney (18, 51, 54).
Before 2001, attempts to culture WNV from postmortem tissues in the USA had been unsuccessful. Subsequently, WNV has been cultured from a blood sample obtained from a patient who died during the early stages of infection, before an antibody response could be mounted (21). But WNV is rarely isolated from the blood of patients with neuro‐invasive WNV disease, because IgM is present by the time neurologic symptoms develop and viremia is usually absent. WNV has been more frequently cultured from human CSF (37, 54). Recently, the virus has been isolated from postmortem human brain tissue of mostly immunosuppressed patients with high viral loads (9, 23, 37, 44). The other postmortem human tissues, apart from brain, from where the virus has been cultured are spleen and kidney (37). WNV is best isolated in cell culture or suckling mice and identified by indirect immunofluorescence assay with specific monoclonal antibodies or by RT‐PCR. Visualization of WNV particles by EM is rare, and when found, virions have been seen within the endoplasmic reticulum of neurons in the brain (9, 15, 37, 44) and testis (10, 53).
One report of WNV antigens in acutely fatal WNV hemorrhagic fever suggested WNV infection of endothelial cells (38). We did not observe endothelial infection in our subjects. This may be because our subjects survived longer and the virus may have been cleared from the endothelium or the difference may be accounted for on technical grounds. For obvious reasons human autopsies are seldom perfused to eliminate vascular blood. The kinetics of WNV viremia is such that by the time patients with encephalitis die, viremia is very low or absent. The reported WNV hemorrhagic fever autopsy showed very high terminal viremia (107 virions per milliliter of plasma) (38), thus it is possible that this vascular viremia generated the impression of endothelial infection as it has in other viral infections with high viremia (57, 59).
Because four of our six patients with WNV encephalitis were immunosuppressed, the distribution of WNV described here may not reflect systemic infection in non‐immunosuppressed patients with asymptomatic infection. Nevertheless, the pronounced epithelial tropism and polar distribution of WNV suggests a cautionary note regarding the potential for asymptomatic human carriers. WNV may evade the immune system after primary acute infection by budding toward the apical surface of glandular epithelium. This polar budding may permit individuals to be chronic carriers without immune or inflammatory clearance of virus. While excretion of virus in bile and urine is unlikely to transmit to new hosts, the presence of virus in the testes leaves open the possibility of sexual transmission. The presence of virus chronically in sweat glands may foster further arthropod dissemination. Our observation of a persistence of viral antigens in systemic organs and the CNS after the initial onset of symptoms (up to 142 days in our small bowel transplant patient, Case 4) confirms recent reports in transplant recipients (15, 40). As IHC positivity indicates productive viral infection, our observation suggests that WNV can persist in certain systemic organs and CNS for almost 5 months after the initial infection in patients with severe immunocompromising conditions. This may have treatment implications for transplantation patients who contract WNV. Additionally, the chronic persistence of the apical budded WNV in the kidney and skin without any inflammation or symptoms creates therapeutic challenges for kidney and skin transplantation programs.
Numerous animal models of WNV infection have been described (4, 29, 43, 60). For economic and experimental design reasons, rodents have been most commonly studied. The various animal models in immunocompetent animals show remarkable similarity to human infection, with most animals remaining asymptomatic, but developing a brief viremia that quickly abates with production of specific neutralizing antibody. Recent studies in hamsters (56) have shown chronic or persistent WNV infection may occur. Intriguingly, the persistently infected hamsters have shown WNV infection in organs similar to what we describe here (eg, kidney and lung). The mechanism of viral persistence in immunocompetent animals remains to be fully elucidated; however, some selected viral mutations may facilitate the chronic infection (11). In contrast, experimental infection of immunosuppressed hamsters with WNV (32) produced a fulminant encephalitis with multiple organ involvement and death, as observed in our immunosuppressed patients.
ACKNOWLEDGEMENTS
This project has been funded in whole or in part with Federal funds from the National Institute of Allergy and Infectious Disease, National Institutes of Health, Department of Health and Human Services, under Contract N01 50027 HHSN266200500027C.
H. Vinters is supported in part by the Daljit D. and Elaine Sarkaria Chair in Diagnostic Medicine.
Supporting information
Table S1. Reported cases of neuroinvasive WNV deaths in the United States.
Supporting info item
REFERENCES
- 1. Agamanolis DP, Leslie MJ, Caveny EA, Guarner J, Shieh WJ, Zaki SR (2003) Neuropathological findings in West Nile virus encephalitis: a case report. Ann Neurol 54:547–551. [DOI] [PubMed] [Google Scholar]
- 2. Anninger WV, Lomeo MD, Dingle J, Epstein AD, Lubow M (2003) West Nile virus‐associated optic neuritis and chorioretinitis. Am J Ophthalmol 136:1183–1185. [DOI] [PubMed] [Google Scholar]
- 3. Armstrong WS, Bashour CA, Smedira NG, Heupler FA, Hoeltge GA, Mawhorter SD, Sudheendra V, Gordon SM (2003) A case of fatal West Nile virus meningoencephalitis associated with receipt of blood transfusions after open heart surgery. Ann Thorac Surg 76:605–607. [DOI] [PubMed] [Google Scholar]
- 4. Austgen LE, Bowen RA, Bunning ML, Davis BS, Mitchell CJ, Chang GJ (2004) Experimental infection of cats and dogs with West Nile virus. Emerg Infect Dis 10:82–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barshes NR, Agee EE, Zgabay T, Brunicardi FC, Goss JA, Debakey ME (2006) West Nile virus encephalopathy following pancreatic islet transplantation. Am J Transplant 6:3037. [DOI] [PubMed] [Google Scholar]
- 6. Batalis NI, Galup L, Zaki SR, Prahlow JA (2005) West Nile virus encephalitis. Am J Forensic Med Pathol 26:192–196. [PubMed] [Google Scholar]
- 7. Bosanko CM, Gilroy J, Wang AM, Sanders W, Dulai M, Wilson J, Blum K (2003) West Nile virus encephalitis involving the substantia nigra: neuroimaging and pathologic findings with literature review. Arch Neurol 60:1448–1452. [DOI] [PubMed] [Google Scholar]
- 8. Brilla R, Block M, Geremia G, Wichter M (2004) Clinical and neuroradiologic features of 39 consecutive cases of West Nile Virus meningoencephalitis. J Neurol Sci 220:37–40. [DOI] [PubMed] [Google Scholar]
- 9. Cushing MM, Brat DJ, Mosunjac MI, Hennigar RA, Jernigan DB, Lanciotti R, Petersen LR, Goldsmith C, Rollin PE, Shieh WJ, Guarner J, Zaki SR (2004) Fatal West Nile virus encephalitis in a renal transplant recipient. Am J Clin Pathol 121:26–31. [DOI] [PubMed] [Google Scholar]
- 10. DeSalvo D, Roy‐Chaudhury P, Peddi R, Merchen T, Konijetti K, Gupta M, Boardman R, Rogers C, Buell J, Hanaway M, Broderick J, Smith R, Woodle ES (2004) West Nile virus encephalitis in organ transplant recipients: another high‐risk group for meningoencephalitis and death. Transplantation 77:466–469. [DOI] [PubMed] [Google Scholar]
- 11. Ding X, Wu X, Duan T, Siirin M, Guzman H, Yang Z, Tesh RB, Xiao SY (2005) Nucleotide and amino acid changes in West Nile virus strains exhibiting renal tropism in hamsters. Am J Trop Med Hyg 73:803–807. [PubMed] [Google Scholar]
- 12. Doron SI, Dashe JF, Adelman LS, Brown WF, Werner BG, Hadley S (2003) Histopathologically proven poliomyelitis with quadriplegia and loss of brainstem function due to West Nile virus infection. Clin Infect Dis 37:e74–e77. [DOI] [PubMed] [Google Scholar]
- 13. Fratkin JD, Leis AA, Stokic DS, Slavinski SA, Geiss RW (2004) Spinal cord neuropathology in human West Nile virus infection. Arch Pathol Lab Med 128:533–537. [DOI] [PubMed] [Google Scholar]
- 14. Granwehr BP, Lillibridge KM, Higgs S, Mason PW, Aronson JF, Campbell GA, Barrett AD (2004) West Nile virus: where are we now? Lancet Infect Dis 4:547–556. [DOI] [PubMed] [Google Scholar]
- 15. Guarner J, Shieh WJ, Hunter S, Paddock CD, Morken T, Campbell GL, Marfin AA, Zaki SR (2004) Clinicopathologic study and laboratory diagnosis of 23 cases with West Nile virus encephalomyelitis. Hum Pathol 35:983–990. [DOI] [PubMed] [Google Scholar]
- 16. Hayes EB, Gubler DJ (2006) West Nile virus: epidemiology and clinical features of an emerging epidemic in the United States. Annu Rev Med 57:181–194. [DOI] [PubMed] [Google Scholar]
- 17. Hayes EB, Sejvar JJ, Zaki SR, Lanciotti RS, Bode AV, Campbell GL (2005) Virology, pathology, and clinical manifestations of West Nile virus disease. Emerg Infect Dis 11:1174–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hiatt B, DesJardin L, Carter T, Gingrich R, Thompson C, De Magalhaes‐Silverman M (2003) A fatal case of West Nile virus infection in a bone marrow transplant recipient. Clin Infect Dis 37:e129–e131. [DOI] [PubMed] [Google Scholar]
- 19. Hollander H, Schaefer PW, Hedley‐Whyte ET (2005) Case records of the Massachusetts General Hospital. Case 22‐2005. An 81‐year‐old man with cough, fever, and altered mental status. N Engl J Med 353:287–295. [DOI] [PubMed] [Google Scholar]
- 20. Hong DS, Jacobson KL, Raad II, De Lima M, Anderlini P, Fuller GN, Ippoliti C, Cool RM, Leeds NE, Narvios A, Han XY, Padula A, Champlin RE, Hosing C (2003) West Nile encephalitis in 2 hematopoietic stem cell transplant recipients: case series and literature review. Clin Infect Dis 37:1044–1049. [DOI] [PubMed] [Google Scholar]
- 21. Huang C, Slater B, Rudd R, Parchuri N, Hull R, Dupuis M, Hindenburg A (2002) First Isolation of West Nile virus from a patient with encephalitis in the United States. Emerg Infect Dis 8:1367–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huhn GD, Austin C, Langkop C, Kelly K, Lucht R, Lampman R, Novak R, Haramis L, Boker R, Smith S, Chudoba M, Gerber S, Conover C, Dworkin MS (2005) The emergence of West Nile virus during a large outbreak in Illinois in 2002. Am J Trop Med Hyg 72:768–776. [PubMed] [Google Scholar]
- 23. Iwamoto M, Jernigan DB, Guasch A, Trepka MJ, Blackmore CG, Hellinger WC, Pham SM, Zaki S, Lanciotti RS, Lance‐Parker SE, DiazGranados CA, Winquist AG, Perlino CA, Wiersma S, Hillyer KL, Goodman JL, Marfin AA, Chamberland ME, Petersen LR (2003) Transmission of West Nile virus from an organ donor to four transplant recipients. N Engl J Med 348:2196–2203. [DOI] [PubMed] [Google Scholar]
- 24. Jeha LE, Sila CA, Lederman RJ, Prayson RA, Isada CM, Gordon SM (2003) West Nile virus infection: a new acute paralytic illness. Neurology 61:55–59. [DOI] [PubMed] [Google Scholar]
- 25. Kelley TW, Prayson RA, Isada CM (2003) Spinal cord disease in West Nile virus infection. N Engl J Med 348:564–566; author reply 564–566. [DOI] [PubMed] [Google Scholar]
- 26. Kelley TW, Prayson RA, Ruiz AI, Isada CM, Gordon SM (2003) The neuropathology of West Nile virus meningoencephalitis. A report of two cases and review of the literature. Am J Clin Pathol 119:749–753. [DOI] [PubMed] [Google Scholar]
- 27. Khairallah M, Ben Yahia S, Ladjimi A, Zeghidi H, Ben Romdhane F, Besbes L, Zaouali S, Messaoud R (2004) Chorioretinal involvement in patients with West Nile virus infection. Ophthalmology 111:2065–2070. [DOI] [PubMed] [Google Scholar]
- 28. Kleinschmidt‐DeMasters BK, Marder BA, Levi ME, Laird SP, McNutt JT, Escott EJ, Everson GT, Tyler KL (2004) Naturally acquired West Nile virus encephalomyelitis in transplant recipients: clinical, laboratory, diagnostic, and neuropathological features. Arch Neurol 61:1210–1220. [DOI] [PubMed] [Google Scholar]
- 29. Komar N, Langevin S, Hinten S, Nemeth N, Edwards E, Hettler D, Davis B, Bowen R, Bunning M (2003) Experimental infection of North American birds with the New York 1999 strain of West Nile virus. Emerg Infect Dis 9:311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lanciotti RS, Kerst AJ, Nasci RS, Godsey MS, Mitchell CJ, Savage HM, Komar N, Panella NA, Allen BC, Volpe KE, Davis BS, Roehrig JT (2000) Rapid detection of West Nile virus from human clinical specimens, field‐collected mosquitoes, and avian samples by a TaqMan reverse transcriptase‐PCR assay. J Clin Microbiol 38:4066–4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Martin DA, Noga A, Kosoy O, Johnson AJ, Petersen LR, Lanciotti RS (2004) Evaluation of a diagnostic algorithm using immunoglobulin M enzyme‐linked immunosorbent assay to differentiate human West Nile Virus and St. Louis Encephalitis virus infections during the 2002 West Nile Virus epidemic in the United States. Clin Diagn Lab Immunol 11:1130–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mateo R, Xiao SY, Guzman H, Lei H, Da Rosa AP, Tesh RB (2006) Effects of immunosuppression on West Nile Virus infection in hamsters. Am J Trop Med Hyg 75:356–362. [PubMed] [Google Scholar]
- 33. Montgomery SP, Chow CC, Smith SW, Marfin AA, O’Leary DR, Campbell GL (2005) Rhabdomyolysis in patients with West Nile encephalitis and meningitis. Vector Borne Zoonotic Dis 5:252–257. [DOI] [PubMed] [Google Scholar]
- 34. Murray K, Baraniuk S, Resnick M, Arafat R, Kilborn C, Cain K, Shallenberger R, York TL, Martinez D, Hellums JS, Hellums D, Malkoff M, Elgawley N, McNeely W, Khuwaja SA, Tesh RB (2006) Risk factors for encephalitis and death from West Nile virus infection. Epidemiol Infect 134:1325–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nash D, Mostashari F, Fine A, Miller J, O’Leary D, Murray K, Huang A, Rosenberg A, Greenberg A, Sherman M, Wong S, Layton M (2001) The outbreak of West Nile virus infection in the New York City area in 1999. N Engl J Med 344:1807–1814. [DOI] [PubMed] [Google Scholar]
- 36. O’Leary DR, Marfin AA, Montgomery SP, Kipp AM, Lehman JA, Biggerstaff BJ, Elko VL, Collins PD, Jones JE, Campbell GL (2004) The epidemic of West Nile virus in the United States, 2002. Vector Borne Zoonotic Dis 4:61–70. [DOI] [PubMed] [Google Scholar]
- 37. Omalu BI, Shakir AA, Wang G, Lipkin WI, Wiley CA (2003) Fatal fulminant pan‐meningo‐polioencephalitis due to West Nile virus. Brain Pathol 13:465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Paddock CD, Nicholson WL, Bhatnagar J, Goldsmith CS, Greer PW, Hayes EB, Risko JA, Henderson C, Blackmore CG, Lanciotti RS, Campbell GL, Zaki SR (2006) Fatal hemorrhagic fever caused by West Nile virus in the United States. Clin Infect Dis 42:1527–1535. [DOI] [PubMed] [Google Scholar]
- 39. Parida M, Posadas G, Inoue S, Hasebe F, Morita K (2004) Real‐time reverse transcription loop‐mediated isothermal amplification for rapid detection of West Nile virus. J Clin Microbiol 42:257–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Penn RG, Guarner J, Sejvar JJ, Hartman H, McComb RD, Nevins DL, Bhatnagar J, Zaki SR (2006) Persistent neuroinvasive West Nile virus infection in an immunocompromised patient. Clin Infect Dis 42:680–683. [DOI] [PubMed] [Google Scholar]
- 41. Perelman A, Stern J (1974) Acute pancreatitis in West Nile Fever. Am J Trop Med Hyg 23:1150–1152. [DOI] [PubMed] [Google Scholar]
- 42. Pergam SA, Delong CE, Echevarria L, Scully G, Goade DE (2006) Myocarditis in West Nile virus infection. Am J Trop Med Hyg 75:1232–1233. [PubMed] [Google Scholar]
- 43. Ratterree MS, Gutierrez RA, Travassos da Rosa AP, Dille BJ, Beasley DW, Bohm RP, Desai SM, Didier PJ, Bikenmeyer LG, Dawson GJ, Leary TP, Schochetman G, Phillippi‐Falkenstein K, Arroyo J, Barrett AD, Tesh RB (2004) Experimental infection of rhesus macaques with West Nile virus: level and duration of viremia and kinetics of the antibody response after infection. J Infect Dis 189:669–676. [DOI] [PubMed] [Google Scholar]
- 44. Reddy P, Davenport R, Ratanatharathorn V, Reynolds C, Silver S, Ayash L, Ferrara JL, Uberti JP (2004) West Nile virus encephalitis causing fatal CNS toxicity after hematopoietic stem cell transplantation. Bone Marrow Transplant 33:109–112. [DOI] [PubMed] [Google Scholar]
- 45. Sampson BA, Armbrustmacher V (2001) West Nile encephalitis: the neuropathology of four fatalities. Ann NY Acad Sci 951:172–178. [PubMed] [Google Scholar]
- 46. Sampson BA, Ambrosi C, Charlot A, Reiber K, Veress JF, Armbrustmacher V (2000) The pathology of human West Nile Virus infection. Hum Pathol 31:527–531. [DOI] [PubMed] [Google Scholar]
- 47. Sampson BA, Nields H, Armbrustmacher V, Asnis DS (2003) Muscle weakness in West Nile encephalitis is due to destruction of motor neurons. Hum Pathol 34:628–629. [DOI] [PubMed] [Google Scholar]
- 48. Schafernak KT, Bigio EH (2006) West Nile virus encephalomyelitis with polio‐like paralysis & nigral degeneration. Can J Neurol Sci 33:407–410. [DOI] [PubMed] [Google Scholar]
- 49. Sejvar JJ, Marfin AA (2006) Manifestations of West Nile neuroinvasive disease. Rev Med Virol 16:209–224. [DOI] [PubMed] [Google Scholar]
- 50. Sejvar JJ, Haddad MB, Tierney BC, Campbell GL, Marfin AA, Van Gerpen JA, Fleischauer A, Leis AA, Stokic DS, Petersen LR (2003) Neurologic manifestations and outcome of West Nile virus infection. JAMA 290:511–515. [DOI] [PubMed] [Google Scholar]
- 51. Shepherd JC, Subramanian A, Montgomery RA, Samaniego MD, Gong G, Bergmann A, Blythe D, Dropulic L (2004) West Nile virus encephalitis in a kidney transplant recipient. Am J Transplant 4:830–833. [DOI] [PubMed] [Google Scholar]
- 52. Shieh WJ, Guarner J, Layton M, Fine A, Miller J, Nash D, Campbell GL, Roehrig JT, Gubler DJ, Zaki SR (2000) The role of pathology in an investigation of an outbreak of West Nile encephalitis in New York, 1999. Emerg Infect Dis 6:370–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Smith RD, Konoplev S, DeCourten‐Myers G, Brown T (2004) West Nile virus encephalitis with myositis and orchitis. Hum Pathol 35:254–258. [DOI] [PubMed] [Google Scholar]
- 54. Solomon T, Fisher AF, Beasley DW, Mandava P, Granwehr BP, Langsjoen H, Travassos Da Rosa AP, Barrett AD, Tesh RB (2003) Natural and nosocomial infection in a patient with West Nile encephalitis and extrapyramidal movement disorders. Clin Infect Dis 36:e140–e145. [DOI] [PubMed] [Google Scholar]
- 55. Solomon T, Ooi MH, Beasley DW, Mallewa M (2003) West Nile encephalitis. BMJ 326:865–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tesh RB, Siirin M, Guzman H, Travassos da Rosa AP, Wu X, Duan T, Lei H, Nunes MR, Xiao SY (2005) Persistent West Nile virus infection in the golden hamster: studies on its mechanism and possible implications for other flavivirus infections. J Infect Dis 192:287–295. [DOI] [PubMed] [Google Scholar]
- 57. Ward JM, O’Leary TJ, Baskin GB, Benveniste R, Harris CA, Nara PL, Rhodes RH (1987) Immunohistochemical localization of human and simian immunodeficiency viral antigens in fixed tissue sections. Am J Pathol 127:199–205. [PMC free article] [PubMed] [Google Scholar]
- 58. Watson JT, Pertel PE, Jones RC, Siston AM, Paul WS, Austin CC, Gerber SI (2004) Clinical characteristics and functional outcomes of West Nile Fever. Ann Intern Med 141:360–365. [DOI] [PubMed] [Google Scholar]
- 59. Wiley CA, Schrier RD, Nelson JA, Lampert PW, Oldstone MBA (1986) Cellular localization of human immunodeficiency virus infection within the brains of acquired immune deficiency syndrome patients. PNAS 83:7089–7093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Xiao SY, Guzman H, Zhang H, Travassos da Rosa AP, Tesh RB (2001) West Nile virus infection in the golden hamster (Mesocricetus auratus): a model for West Nile encephalitis. Emerg Infect Dis 7:714–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Reported cases of neuroinvasive WNV deaths in the United States.
Supporting info item


