CLINICAL HISTORY
An 80‐year‐old man was transferred to our institution with worsening heart failure. His past medical history was significant for type‐2 diabetes mellitus, hypertension, coronary artery disease, chronic obstructive pulmonary disease, emphysema, chronic renal insufficiency and recurrent exacerbations of congestive heart failure. In addition, approximately 5 weeks prior to this admission, he presented at another institution with acute onset of left hemiplegia. Radiographic imaging at the time revealed a right thalamic lesion interpreted to represent acute ischemic changes. He received thrombolytic therapy but showed little improvement in neurological symptoms.
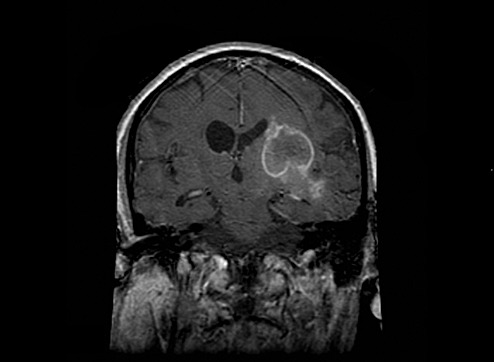
He was transferred to a rehabilitation program but there also showed no improvement in neurological symptoms. While in rehabilitation, recurrent heart failure required hospitalization and transfer to this institution. His cardiopulmonary function improved with treatment, but his neurologic status remained unchanged until hospital day ten when he exhibited progressive mental status deterioration. MRI of the brain showed a ring‐enhancing lesion similar to that seen 6 weeks earlier, but now observed to extend into the right basal ganglia and medial temporal lobe with right cerebral hemispheric edema, right‐to‐left midline shift and asymmetric ventricles (Figure 1—the image has been inverted vertically to correlate with the gross pathologic specimen). These changes were interpreted to be characteristic of a cerebral abscess, but because of the lack of systemic findings, a high‐grade intracerebral neoplasm was also considered. Because of his poor clinical status, the patient's family declined a brain biopsy. He died on the eleventh hospital day.
Figure 1.
GENERAL AUTOPSY FINDINGS
An autopsy, performed within 4 h of death, showed acute bronchopneumonia involving all lung lobes in the setting of marked emphysematous changes. Lung cultures demonstrated coagulase‐positive staphylococcus. The examination of the heart showed moderate‐to‐severe calcific atherosclerosis in all major coronary arteries. There was a remote myocardial infarction involving the anterior left ventricular wall and the anterior two‐thirds of the interventricular septum, extending from apex to base.
NEUROPATHOLOGIC AND MICROBIOLOGIC FINDINGS
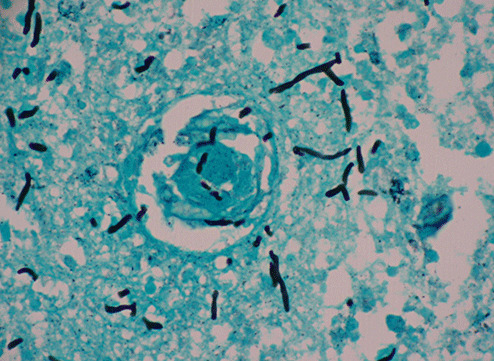
Gross examination of the brain demonstrated generalized cerebral edema and mild uncal grooving bilaterally. At the time of autopsy, the right parietal lobe was incised sterilely to reveal a 3.5‐cm gray‐white, hemorrhagic abscess cavity with shaggy walls in the right cerebral deep white matter and right basal ganglia (Figure 2). This cavity contained green‐grey necrotic debris and purulent material. Fungal and bacterial cultures were taken. Subsequent examination of the formalin‐fixed brain showed this cavity to extend into the anterior thalamus with multiple sub‐centimeter satellite lesions. Microscopic examination of hematoxylin and eosin stained sections of the abscess cavity showed abundant pigmented fungal hyphae invading the surrounding brain tissue (Figure 3). Gomori methenamine silver stain highlighted the irregularly swollen hyphae and conidia and demonstrated vascular invasion (Figure 4).
Figure 2.
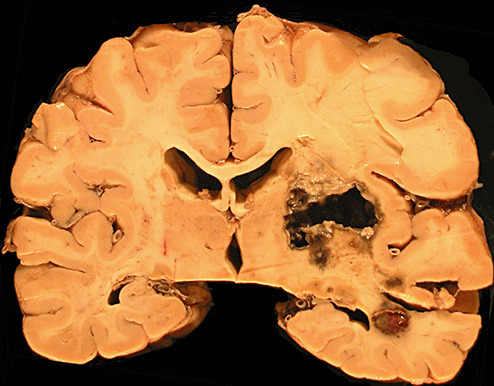
Figure 3.
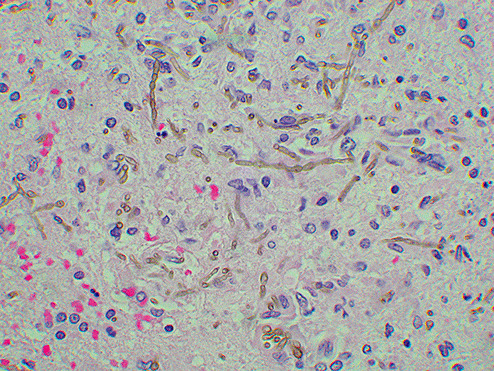
Figure 4.
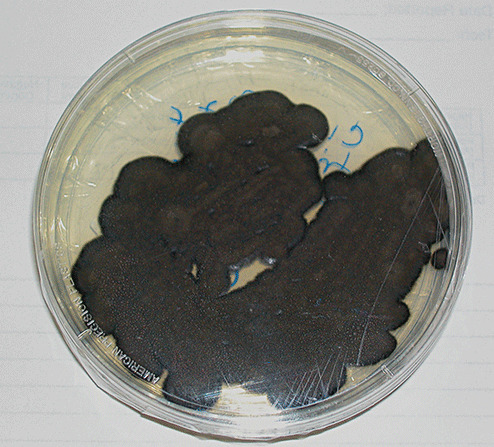
Microbiological studies revealed moderate growth of a black mold with a velvety appearance on Sabouraud's dextrose agar. There was good growth at 30, 37 and 42 degrees Celsius. Both the surface and the reverse side of the plate appeared jet‐black (Figure 5). Dissecting microscope exam of the plate showed hyphae made up of sparsely branched wavy chains of smooth oval conidia branching at several angles.
Figure 5.
DIAGNOSIS
Cerebral Phaeohyphomycosis.
DISCUSSION
The term “phaeohyphomycosis” was originally coined as a histopathologic term to designate subcutaneous and deep‐seated infections by dematiaceous fungi, that is, infections caused by any species of the mycotic genus Dematium. More recently, the term has come into broader use to describe infections at any site caused by species within the genus Dematium (9) including Xylohypha bantiana (previously known as Cladophialophora bantiana) 5, 6, 13, Wangiella dermatitidis (previously known as Exophiala dermatitidis) 8, 10, Onchronoconis gallopavum 14, 15 and Ramichloridium mackenziei 4, 11. An interesting characteristic of these organisms is neurotropism in humans, which, although well documented, is not understood (12).
Cerebral phaeohyphomycosis is a rare but well‐delineated disease entity. It is a clinical syndrome diagnosed in those patients with deep‐seated cerebral infections secondary to dematiaceous fungi. Such infections have been attributed to multiple species of dematiaceous (darkly pigmented) fungi, but X. bantiana (previously known as Cladophialophora species) is the most common (12). The pigmented nature of this organism has been shown to be due to melanin which it produces, and this feature may assist this organism in evading host defenses (7). W. dermatitidis and C. neoformans melanin‐negative mutants show reduced virulence when compared with their wild‐type counterparts. This may be, in part, secondary to melanin's inherent quality as a buffer against host oxidation killing mechanisms. The melanin‐specific stain, Fontana‐Masson, can be used for further identification of the organism. Other dematiaceous fungi known to cause cerebral infection include R. mackenziei, W. dermatitidis and Bipolaris sp. (2). R. mackenziei is the only organism showing a recurrent geographic distribution with all but one reported case occurring in the Middle East. The majority of cases reported in North America have occurred secondary to X. bantiana (12).
Localized superficial infections with dematiaceous fungi are not uncommon and produce subcutaneous involvement, otitis and sinusitis (1). Cerebral phaeohyphomycosis is the most common as well as the most serious and devastating of the deeper forms of dematiaceous fungal infection although other deep localized infections such as arthritis and endocarditis have been described (2).
Several dematiaceous fungi are known for their neurotropism including X. bantiana, the causative organism in this case (7). While it seems logical that central nervous system (CNS) infections may occur from direct spread from paranasal sinuses, only one case recorded in the literature had concomitant colonization of paranasal sinuses (3). It therefore seems likely, although yet unproven, that CNS seeding occurs by the hematogenous route, probably initiated by a respiratory colonization consequent to inhalation. However, the low frequency of CNS infections by X. bantiana has thus far obviated elucidation of this mechanism. In our case, overwhelming acute broncho‐pneumonia obscured clear assessment or confirmation of pulmonary fungal colonization.
Successful treatment regimens for cerebral phaeohyphomycosis have been described to combine early, aggressive surgical debridement with long‐term anti‐fungal therapy. Despite these therapies, however, the mortality rate is dismal approaching approximately 75% in reported cases (12).
A typical but particularly confounding and peculiar feature of this infection is that it occurs primarily in immunocompetent men with no obvious risk factors, whereas most other deep‐seated fungal/mold infections occur in immunosuppressed individuals. It should be noted, however, that a few cases of cerebral X. bantiana infection have been described in immunocompromised individuals(12).
In the microbiology lab, the differential diagnosis for X. bantiana by culture‐plate morphology is primarily Aspergillus Niger because of the velvety‐black growth. However, in A. Niger, the reverse side of the plate should be creamy‐tan and not black like that seen in dematiaceous fungal growth. In carrying out laboratory analysis of specimens from ring‐enhancing CNS lesions in immunocompetent patients (especially males), it is important to consider cerebral phaeohyphomycosis in the differential diagnosis, and particular laboratory precautions are recommended when dematiaceous fungal infection is suspected. Specimens should be handled with Biosafety Level 2 containment because of the known pathogenesis of these organisms in immunocompetent hosts and their ability to be aerosolized, and when cerebral phaeohyphomycosis is suspected or considered, slide cultures should not be made.
ABSTRACT
Cerebral phaeohyphomycosis is a rare diagnosis that designates a central nervous system (CNS) infection by dematiaceous fungi. These organisms most commonly cause cutaneous infections in humans, but much less commonly, they cause CNS disease with evidence of neurotropism. We describe here the clinical course and post‐mortem findings in a fatal case of cerebral phaeohyphomycosis occurring in an 80‐year‐old man. He had a long and complex past medical history and approximately 7 weeks prior to his death, he presented to an outside institution with imaging findings reported to be consistent with a cerebrovascular accident. He was treated with thrombolytic therapy and sent to a rehabilitation program. Approximately 2 weeks prior to his death, he was transferred to our institution with worsening chronic heart failure symptoms. Imaging after admission showed a ring‐enhancing lesion and the differential diagnosis shifted to include a primary neoplasm vs. an abscess. There was a downward clinical course and neurosurgical biopsy was declined secondary to predicted poor outcome. A full autopsy was performed and confirmed the pre‐mortem imaging findings of a cerebral abscess with multiple satellite lesions. The histologic and microbiologic findings were characteristic of cerebral phaeohyphomycosis. Microbiological features and disease characteristics of these organisms as well as incidence and populations affected are also discussed.
REFERENCES
- 1. Ajello L, Georg LK, Steigbigel RT, Wang CJ (1974) A case of phaeohyphomycosis caused by a new species of Phialophora . Mycologia 66:490–498. [PubMed] [Google Scholar]
- 2. Brandt ME, Warnock DW (2003) Epidemiology, clinical manifestations and therapy of infections caused by dematiaceous fungi. J Chemother 15(Suppl 2):36–47. [DOI] [PubMed] [Google Scholar]
- 3. Brown JW III, Nadell J, Sanders CV, Sardenga L (1976) Brain abscess caused by Cladosporium trichoides (bantianum): a case with paranasal sinus involvement. South Med J 69:1519–1521. [DOI] [PubMed] [Google Scholar]
- 4. Campbell CK, Al‐Hedaithy SSA (1993) Phaeohyphomycosis of the brain cause by Ramichloridium mackenziei sp. Nov. in Middle Eastern countries. J Med Vet Mycol 31:325–332. [Google Scholar]
- 5. Dixon DM, Walsh TJ, Merz WG, McGinnis MR (1989) Infections due to Xylohypha bantiana (Cladosporium trichoides). Rev Infect Dis 11:515–525. [DOI] [PubMed] [Google Scholar]
- 6. Heney C, Song E, Kellen A, Raal F, Miller SD, Davis V (1989) Cerebral phaeohyphomycosis caused by Xylohypha bantiana . Eur J Clin Microbiol Infect Dis 8:984–988. [DOI] [PubMed] [Google Scholar]
- 7. Jacobsen ES (2000) Pathogenic roles for fungal melanins. Clin Microbiol Rev 13:708–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kenney RT, Kwon‐Chung JK, Waytes AT, Melnick DA, Pass HI, Merino MJ, Gallin JI (1992) Successful treatment of systemic Exophiala dermatitidis infection in a patient with chronic granulomatous disease. Clin Infect Dis 14:235–242. [DOI] [PubMed] [Google Scholar]
- 9. Koneman EW, Allen SD, Janda WM, Schreckenberger PC, Winn WC (1997) Mycology. In: Color Atlas and Textbook of Diagnostic Microbiology, 5th edn, Chapter 19, pp. 1012–1014. ChapterLippincott‐Raven Publishers: New York, NY. [Google Scholar]
- 10. Matsumoto T, Padhye AA, Ajello L, Standard PG (1984) Critical review of human isolates of Wangiella dermatitidis . Mycologia 76:232–249. [Google Scholar]
- 11. Musella RA, Collins BH (1968) Cerebral chromoblastomycosis. J Neurosurg 35:219–222. [DOI] [PubMed] [Google Scholar]
- 12. Revenkar SG, Sutton DA, Rinaldi MG (2004) Primary central nervous system phaeohyphomycosis: a review of 101 cases. Clin Infect Dis 38:206–216. [DOI] [PubMed] [Google Scholar]
- 13. Sekhon AS, Galbraith SJ, Mielke BW, Garg AK, Sheehan G (1992) Cerebral phaeohyphomycosis caused by Xylohypha bantiana, with a review of the literature. Eur J Epidemiol 8:387–390. [DOI] [PubMed] [Google Scholar]
- 14. Sides EH, Benson JD, Padhye AA (1991) Phaeohyphomycotic brain abscess due to Onchroconis gallpavum in a patient with malignant lymphoma of the large cell type. J Med Vet Mycol 29:317–322. [PubMed] [Google Scholar]
- 15. Terreni AA, DisSalvo AF, Baker AS Jr, Crymes WB, Morris PR, Dowda H Jr (1990) Disseminated Dactylaria gallopava infection in a diabetic patient with chronic lymphocytic leukemia of the T‐cell type. Am J Clin Pathol 94:104–107. [DOI] [PubMed] [Google Scholar]


