Abstract
There is a large amount of tissue stored in brain collections and brain banks, but little is known about whether formalin‐fixed tissues and paraffin blocks stored for years in brain banks are suitable for the retrospective genetic studies. The study was carried out in order to: (i) compare DNA preservation in frozen, formalin‐fixed and paraffin‐embedded tissues stored for different periods; (ii) study point mutations and triplet expansions in frozen, formalin‐fixed and paraffin‐embedded material stored for variable periods, and using different fixative solutions; (iii) compare different methods to optimize DNA extraction and DNA amplification from suboptimally preserved brain tissue. DNA preservation is suitable for genetic studies in samples stored at −80°C for several years. Formalin‐fixed, paraffin‐embedded tissue was inferior to frozen tissue, but did yield adequate results in many cases depending on the type of fixative solution and time of fixation before embedding. Prolonged fixation in formalin rarely yielded useful DNA. Similar results were obtained in samples from prion diseases. The best results were obtained by using the Qiagen kits (QIAmp DNA Micro) in frozen material, paraffin blocks and formalin‐fixed tissue. Genomiphi™ and TaKaRa Ex Taq™ methods were also assayed in paraffin blocks and in formalin‐fixed samples with limited success.
INTRODUCTION
DNA is relatively resistant to postmortem degradation under appropriate conditions. This property is valuable in forensic pathology and paleontology, allowing limited genetic studies in tissues maintained for thousand of years (2, 7, 11, 12, 13, 18). However, DNA is vulnerable to degradation in liquid solutions, particularly fixatives used for tissue preservation in current pathological practice. Brain tissues stored frozen, in formalin or embedded in paraffin, such as those in brain banks or brain collections are extremely useful for the study of human neurological diseases. Yet the quality of the material largely depends on a number of external factors that may have distinct impacts on proteins, lipids, RNA and DNA. It is the general belief that frozen material, even when stored for years, is suitable for DNA studies (9, 23). Suboptimal DNA preservation or even massive degradation, however, can be expected in tissues stored in formalin at room temperature for months or years (8, 10, 15). This is mainly because of the progressive acidification of formalin into formic acid. The use of buffered formalin stabilizes the solution for a longer time than non‐buffered formalin. Periodical renewal of the buffered fixative solution in samples stored for months or years reduces the deleterious effects of old formalin. DNA preservation in paraffin blocks is variable but probably dependent on the type of fixative solution and on the time of tissue storage in the fixative before paraffin embedding.
There is a tremendous amount of tissue stored in brain collections and brain banks, the most advantageous use of which remains to be discerned. We do not know, for example, to what extent the space‐occupying formalin‐fixed material accumulated in brain banks is suitable for genetic studies, either for the study of mutations or for the search of polymorphisms. This is an important issue, as it is increasingly recognized that sporadic neurodegenerative diseases are modulated by distinct genetic factors in addition to the environmental ones. Furthermore, whether formalin‐fixed samples and paraffin blocks stored in brain banks are suitable for the retrospective study of pathogenic point mutations and triplet expansion is barely understood.
For these reasons, we set up the present collaborative study aimed to: (i) compare DNA preservation in the same samples either frozen, formalin‐fixed or paraffin‐embedded considering short periods of formalin storage (less than 1 year), as well as long periods of storage (several years); (ii) study point mutations and triplet expansions in frozen as well as formalin‐fixed and paraffin‐embedded material preserved for variable time periods, and using different fixative solutions; (iii) compare different methods for optimizing DNA extraction and DNA amplification from suboptimally conserved brain tissue. Finally, brain samples from patients with genetic prion diseases have also been included as they usually are treated with formic acid prior to neuropathological study in order to prevent PrP infectivity.
MATERIAL AND METHODS
Effect of postmortem delay, fixation time, type of fixative and paraffin embedding on DNA preservation.
Brain samples from two patients aged 48 and 62 years with clinically proven sudden death (cardiac infarction) were used. Samples from each brain were frozen at the time of death (2 h), or stored for 3, 6, 21 and 48 h at 4°C and then frozen at −80°C to mimic progressive postmortem delay in tissue processing. In addition, samples from the same brains were fixed in 4% buffered formalin for 1 month and then embedded in paraffin, or stored in 4% buffered formalin for 1 year. Material analyzed for DNA preservation from the two cases included: (i) frozen tissue with increasing postmortem delay; (ii) buffered formalin‐fixed paraffin‐embedded sections; and (iii) formalin‐fixed tissue stored in buffered formalin for 1 year.
Twenty cases which were preserved in non‐buffered formalin for periods ranging from 10 to 20 years at room temperature were used. Paraffin blocks of the same cases produced between 6 and 10 months after non‐buffered‐formalin fixation were examined in parallel.
Effect of fixation time, type of fixative and paraffin embedding on (re‐) identification of point mutations and number of triplet repeats.
Two frozen brain samples stored for 14 and 20 years were analyzed for point mutations in PS1 (E318G) and MAPT (R406W). These cases had been afflicted with early onset familial Alzheimer’s disease and familial frontotemporal dementia, respectively. The characterization of mutations was done in affected relatives years later.
Archival brain samples from six cases with known mutations in PSEN1 (M139T, E318G, and V89L), APP (A713T) and MAPT (P301L, and delN296) genes were used. Mutations in the corresponding genes were first analyzed in frozen brain tissue obtained at the time of postmortem. The study was carried out in: (i) frozen samples stored at −80°C for 2–6 years; (ii) paraffin blocks which were produced with tissue samples fixed in 4% buffered formalin for 4–6 weeks, and then maintained at room temperature for 2–6 years; and (iii) formalin‐fixed samples which were stored in the initial fixative solution with no further changes, at room temperature for 2–6 years.
Archival brain samples from six cases with known CAG expansions causing SCA3 (three cases), dentatorubropallidoluysian atrophy: DRPLA (two cases) and SCA2 (one case) were used. The mutations were discovered during life in blood samples in three cases and in postmortem brain tissue in the other three. The study was carried out in: (i) paraffin blocks produced at the time of the neuropathological study (between three and 6 months of storage in 4% buffered formalin), and stored at room temperature for 3–6 years; and (ii) formalin‐fixed material from the same cases stored at room temperature in the initial fixative solution for 3–6 years.
Study of mutations in buffered formalin‐fixed samples, and in buffered formalin‐fixed paraffin‐embedded samples from different affected members of the same family, stored for variable periods: 13, 12, 11, 10 and 3 years. All patients had the K670N/M671L mutation in the APP gene.
Formalin‐fixed samples of ten cases with known mutations in APP (four cases) or in PS1 (six cases) stored for up to 10 years in 10% non‐buffered formalin at room temperature were analyzed.
Studies in prion diseases were carried out in 12 frozen samples from genetic Creutzfeldt–Jakob disease (CJD) cases that were kept stored in the freezer for 30–35 years (eight E200K‐129M, three D178N‐129V, one insertion of seven octapeptide repeats‐129M). In addition, point mutations in PRNP were analyzed in eight samples from genetic CJD and FFI (one D178N‐129M, three E200K‐129M, four V210I‐129M) stored in formalin (n = 5) or processed for paraffin embedding after a variable time of formalin fixation (n = 8).
Finally, formalin‐fixed, formic acid‐treated paraffin‐embedded samples from three cases with familial CJD (two E200K‐129M, one D178N‐129V) and nine cases with fatal familial insomnia (FFI) (D178N‐129M) were examined. Genetic diagnosis was made by using peripheral blood cells or frozen postmortem brain tissue. The present work was conducted by using formalin‐fixed, formic acid‐treated paraffin blocks produced at the time of the neuropathological study (between 4 and 8 weeks after death), and then stored at room temperature for variable periods (between 6 months and 7 years).
Comparison of several methods for optimizing DNA extraction and DNA amplification
DNA extraction and analysis.
DNA was extracted following several protocols. Initial phenol/chloroform extraction and ethanol precipitation was replaced by commercial methods, and procedures were carried out following the suggestions of the suppliers with modifications for frozen, paraffin‐embedded and formalin‐fixed samples. DNeasy® Tissue (Qiagen, Hilden, Germany) and QIAmp DNA Micro (Qiagen) were currently used.
The quality of extracted DNA was initially examined by agarose gel electrophoresis and ethidium bromide staining, but because of the poor precision of this method, it was replaced by the analysis with Bioanalyzer (Agilent, Wiesental, Germany). Basically, the data analysis procedures consist of the following steps: raw data are read and stored by the system for all individual wells, a software algorithm filters the data and plots the resulting electropherograms of all wells, peaks are identified for all wells and tabulated by migration time, a DNA ladder (a mixture of DNA fragments of different sizes) is run first from the ladder well, and a standard curve of migration time against DNA size is plotted by using linear interpolation. Two DNA fragments (50 bp and 17 000 bp) are run with each of the samples, bracketing the DNA sizing analysis. Called lower and upper markers, these are internal standards which are used to align the ladder analysis with the individual sample analysis.
To analyze the quality of DNA, we distinguished three different regions (Figure 1A):
Figure 1.
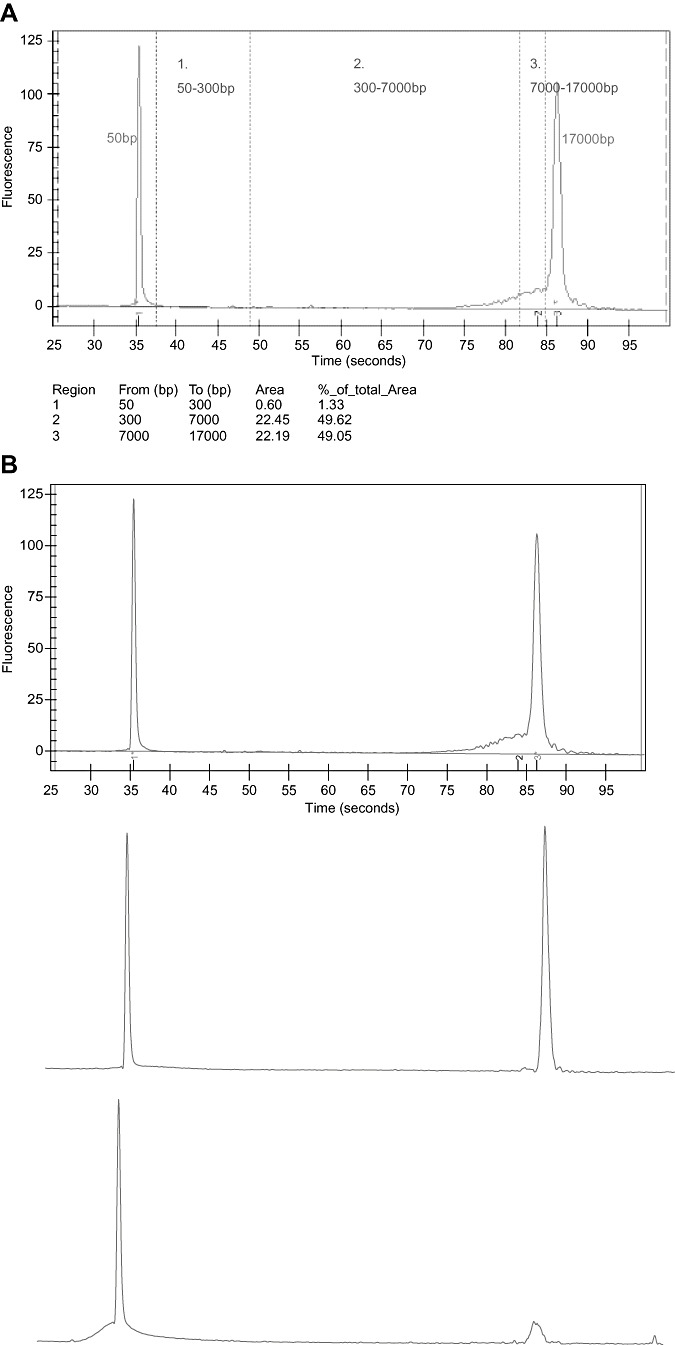
A. Quantification of DNA by Agilent Bioanalyzer. Analysis of DNA smears. Three regions can be considered: region 1: DNA fragments of 50–300 bp; region 2: DNA of 300–7000 bp; region 3: DNA of 7000–17 000 bp. B. Examples of DNA quality. Upper figure: good DNA preservation; middle: suboptimal DNA preservation; lower: poor DNA preservation.
-
•
Region 1: from 50 bp to 300 bp. We considered this region to be degraded DNA.
-
•
Region 2: from 300 bp to 7000 bp. We considered this region acceptable quality DNA for performing mutation analysis by polymerase chain reaction (PCR).
-
•
Region 3: from 7000 bp to 17 000 bp. The DNA in this region is considered to be of good quality.
The quality of the sample depends partially on the amount of DNA but more importantly on the size of DNA extracted. The sample was considered unsuitable for DNA studies when the majority of DNA was found in the first region—that is, the region in which the DNA was in the form of fragments smaller than 300 bp. In fact, samples smaller than 500 bp were rarely useful for mutation studies, even if treated with methods aimed to amplify DNA in samples of poor quality (see later).
For practical purposes, we have used the following classification for DNA preservation: good, all the DNA is found in regions 2 and 3; suboptimal, the vast majority of DNA is in region 2 and some in region 1; and poor, the majority of DNA is found in region 1. Examples of this instrumental classification are shown in Figure 1B.
Mutation analysis.
The study of mutations was carried out directly—that is, using no additional treatments for DNA, in case of good quality DNA, or using commercial amplification kits in case of suboptimal samples.
The GenomiPhi™ DNA Amplification kit (Amersham Biosciences, Cerdanyola, Spain) uses a simple isothermal whole genome amplification method for the representational amplification of human genomic DNA from a limited amount of sample. This method uses the unique biochemical properties of Phi29 DNA polymerase to amplify linear DNA.
The TaKaRa Ex Taq™ (Otsu‐Shiga, Japan) polymerase is an optimized enzyme for accurate PCR. This method provides more efficient amplification and higher fidelity than conventional Taq DNA polymerase under conventional PCR conditions. In trying to improve poor quality DNA, we have also used the “Repair DNA protocol” of TaKaRa Ex Taq™ prior to amplifying DNA using TaKaRa Ex Taq™. The method of “Repair DNA protocol” is to use a dNTPs mixture instead of random hexamers to anneal to the template DNA at multiple sites. After the repair protocol has been applied, TaKaRa Ex Taq™ initiates the polymerization using the repaired DNA as a template and generates new single‐stranded DNA. The subsequent priming and strand displacement replication of this DNA results in the formation of double‐stranded DNA.
The main variable parameters for PCR amplification were the annealing temperature (depending on the %CG of the primer) and the concentration of the Mg2+ ions. Conditions for the study of point mutations in PSN1, APP and MAPT are summarized in the supplemental Tables S1–S3. The PCR parameters were for each exon amplification, using primers and PCR conditions described elsewhere (5, 17, 21, 22, 24).
Conditions for the study of CAG triplet expansion‐related diseases (SCA2, SCA3 and DRPL) are summarized in the supplemental Table S4. The PCR parameters were optimized for each gene amplification, using primers and conditions described elsewhere (14, 19).
Conditions for the study of prion diseases are shown in supplemental Table S5. Conditions have been described elsewhere (3).
Runs were done on a DNA analyzer 3730 (Applied Biosystem, Nieuwerkerk, the Netherlands) and results were analyzed with the GeneMapper v3.0 (Applied Biosystem).
Sequence analysis.
Two programs available on the Internet were used to analyze the sequences: http://www.serachLauncher.bcm.tmc.edu and http://www.genomatix.de/cgi‐bin/dialign/dialign.pl. Usually, a text format was used to analyze the sequences, and a chromatogram format to view the sequences (http://www.technelysium.com.au/chromas.html).
As this was a collaborative study of BrainNet Europe, the same brain samples from the same brain bank (Barcelona) were supplied to the other members in order to randomize DNA extraction in the different laboratories. Then, tissue samples from different centers were shipped to Barcelona for genetic study of frozen, formalin‐fixed, and paraffin‐embedded brain tissues. Finally, genetic analysis of familial prion diseases was carried out in Barcelona and Bologna.
RESULTS
Effect of postmortem delay, fixation time, type of fixative and paraffin embedding on DNA preservation.
In the two cases examined, the material was well preserved and suitable for DNA studies whatever its origin (frozen, buffered formalin‐fixed and paraffin‐embedded, or buffered formalin‐fixed samples) within the time‐points of the study: 4% buffered formalin, paraffin blocks produced after 1 month of fixation and maximal time of storage in formalin before the DNA study up to 1 year.
Although variable from one case to another, suboptimal results were obtained in twenty cases fixed and stored in non‐buffered formalin and embedded in paraffin (as revealed by the majority of DNA in region 2, Figure 1). Poor results were obtained in the same cases in which the tissue was fixed in non‐buffered formalin for 10 to 20 years at room temperature (majority of DNA in region 1).
Effect of fixation time, type of fixative and paraffin embedding on (re‐) identification of point mutations and number of triplet repeats.
Genetic studies in frozen samples stored at −80°C for up to 20 years identified E318G as the PSN1 mutation. The product of the MAPT mutation was identified as R406W.
Point mutations were demonstrated in all frozen samples (6/6), in 4/6 samples obtained from paraffin blocks, and in only one sample in which buffered formalin‐fixed tissue from cases with PSEN1 (M139T, E318G, and V89L), APP (A713T) and MAPT (P301L and delN296) genes were used. Frozen samples were stored for 2–6 years; paraffin samples were produced 4–6 weeks after buffered‐formalin fixation, and then maintained at room temperature for 2–6 years, whereas formalin‐fixed samples were stored at room temperature for 2–6 years.
Triplet expansions were demonstrated in all available frozen samples (4/4), and in all available samples from paraffin blocks (5/5), but in none (0/5) from tissues fixed in buffered formalin for 3–6 years.
Studies were carried out in five affected members of the same family, harboring the K670N/M671L mutation in the APP gene, over a period of 13 years. Positive results were obtained in 5/5 frozen samples, in 5/5 paraffin blocks and in 4/5 samples stored in formalin. Surprisingly, negative results in the formalin‐fixed case corresponded to the case with shorter formalin fixation.
Brain samples of eight cases with known mutations in APP (four cases) and PSEN1 (four cases) which were stored in non‐buffered formalin for many years were analyzed. No mutations were detected in these cases.
PRNP mutations were detected in all frozen samples, even those stored for 35 years. Screened paraffin blocks from eight cases which were processed after 2, 3, 6, 8 and 13 weeks of formalin fixation resulted in positive results (mutation found) in all cases but the one with the longest time of formalin fixation. The positive detection of the mutation required the use of a nested PCR protocol (double amplification). Analyses of five tissue samples, fixed in buffered formalin for 8, 24, 25, 34 and 48 weeks without processing and paraffin embedding, led to the detection of the mutation in two (fixation times: 8 and 25 weeks) out of five cases.
Genetic studies in three cases with familial CJD (two E200K‐129M, one D178N‐129V) and nine cases with FFI (D178N‐129M) carried out in tissue obtained from paraffin blocks in which the tissue was fixed in 4% buffered formalin for months, treated with 95% formic acid for decontamination purpose (time of treatment between 10 to 30 minutes) and stored in formalin, were negative in every case of the present series.
Comparison of different methods to optimize DNA extraction and DNA amplification.
In order to compare different methods, parallel studies were carried out using Qiagen, Genomiphi™ and TaKaRa Ex Taq™ methods in paraffin blocks and in formalin‐fixed samples.
In samples obtained from paraffin blocks, point mutations were identified as follows: Qiagen, 4/6; Genomiphi™, 3/6 (positive cases as with Qiagen); and TaKaRa Ex Taq™, 0/6. Regarding formalin‐fixed tissue, the results were as follows: Qiagen, 1/6; Genomiphi™, 0/6; and TaKaRa Ex Tag™, 1/6 (the same as with Qiagen).
Expansions of CAG triplets in samples obtained from paraffin blocks (one DRPLA and one SCA2 were eliminated) were identified as follows: Qiagen, 4/4; Genomiphi™, 0/4; and TaKaRa Ex Taq™, 0/4. Regarding formalin‐fixed tissue (one DRPLA was eliminated), the results were the following: Qiagen, 0/5; Genomiphi™: 1/5; and TakaRa Ex Tag™: 1/5 (different from the case identified following Genomiphi™).
A summary of these observations is shown in Table 1.
Table 1.
Summary of results showing the effects of fixation time, type of fixative and paraffin embedding on (re‐)identification of point mutations and number of triplet repeats, and by using different methods: QIAmp MicroQuiagen, Genomiphi™ and TaKaRa Ex Taq™.
| Frozen QIAmp MicroQuiagen | Paraffin QIAmp MicroQuiagen | Formalin QIAmp MicroQuiagen | Paraffin Genomiphi™ | Paraffin TaKaRa Ex Taq™ | Formalin Genomiphi™ | Formalin TaKaRa Ex Taq™ | |
|---|---|---|---|---|---|---|---|
| M139T PSEN1 | Yes | Yes | No | Yes | No | No | No |
| A713T APP | Yes | Yes | No | Yes | No | No | No |
| delN296 MAPT | Yes | Yes | No | No | No | No | No |
| P301L MAPT | Yes | Yes | Yes | No | No | No | Yes |
| E318G PSEN1 | Yes | No | No | No | No | No | No |
| V89L PSEN1 | Yes | No | No | No | No | No | No |
| SCA3 | Yes | Yes | No | No | No | No | No |
| SCA3 | Yes | Yes | No | No | No | Yes | No |
| SCA3 | Yes | Yes | No | No | No | No | No |
| DRPLA | Yes | Yes | No | No | No | No | No |
| DRPLA | — | — | No | — | — | No | Yes |
| SCA2 | — | Yes | — | — | — | — | — |
DISCUSSION
Brain banking is crucial in providing tissue for the study of neurological diseases, particularly human neurodegenerative diseases for which experimental models, including genetically manipulated ones, are still barely appropriate counterparts for the vast majority of sporadic diseases. Molecular studies, including those on nucleic acids, are at increasing demand and essential for a better understanding of human neurodegenerative diseases. Thus there is a major concern about the present position of brain banks as brain tissue providers in relation to the quality of material stored and the methods for improving genetic and genomic analyses (1, 16).
The present study was designed to: (i) compare DNA preservation in frozen, formalin‐fixed paraffin‐embedded, and formalin‐fixed samples stored for variable periods; (ii) study point mutations and triplet expansions in frozen, formalin‐fixed and paraffin‐embedded material for variable periods; and (iii) compare different methods for optimizing DNA extraction and DNA amplification from suboptimally conserved brain tissue. It was carried out as a multi‐center collaboration in the context of the BrainNet Europe consortium. The study covered about 400 determinations considering distinct samples and different methods.
We confirmed that DNA preservation is good and suitable for genetic studies in samples stored at −80°C for several years. The maximum time in the present series corresponds to a sample stored for up to 35 years. Therefore, fresh‐frozen tissue is the optimal method to preserve banked brain tissue for DNA recovery. Furthermore, the Alzheimer Disease Centers in the USA, as well as other brain banks in North America, Europe, Japan and Australia already use a system of storing both fresh‐frozen and fixed tissue. The present findings further emphasize the utilization of this model in the context of a multi‐institutional program for the banking of brain tissue.
We also found that acceptable results can be also obtained in paraffin blocks provided that these blocks were produced after a short time of fixation in 4% buffered formalin. Suboptimal or bad results, that is, degraded DNA, with the impossibility of analyzing DNA mutations, occur in formalin‐fixed tissues stored for long periods (longer than 6 months). The chance to obtain positive results in cases with point mutations, deletions and triplet expansions is almost null in tissue that has been formalin fixed for years.
Concerning the issue of optimizing DNA preservation in formalin‐fixed material, the present results have shown that a key factor is the fixative solution itself, as 4% buffered formalin is better than 10% non‐buffered formalin for DNA preservation. Furthermore, the time of permanence of the sample in the fixative solution is crucial. The shorter the time of buffered‐formalin fixation, the better the DNA preservation. Blocks produced up to 1 month after buffered‐formalin fixation are consistent with DNA preservation, whereas DNA quality is suboptimal or bad in samples from paraffin blocks produced several months or years after fixation.
Similar results were obtained by us in samples from prion diseases. Yet formic‐acid treatment, commonly utilized for prion decontamination, may contribute to DNA degradation. No PRNP mutations in familiar CJD and FFI cases were found in the present series, thus indicating that samples treated with formic acid are even less optimal for genetic studies. However, point mutation in PRNP has been found at least in two non‐related cases after formic acid treatment using nested PCR (personal observation; S. Capellari and P. Parchi). Samples of these cases were embedded after a short time of buffered‐formalin fixation and short formic acid treatment. Therefore, the duration of formalin fixation, duration of formic acid treatment, tissue thickness and further formalin fixation for long periods are probably additional contributory factors in the study of prion diseases.
Regarding protocols for DNA extraction, we obtained the best and most reproducible results by using the QIAmp Micro Qiagen method. This method has been successfully used in frozen, paraffin‐embedded and formalin‐fixed samples.
Although very helpful in other paradigms and tissues (tumors), little help can be expected from the use of DNA amplification methods to restore suboptimal DNA in brain samples stored in formalin. Yet mutations have been demonstrated using Genomiphi™ and TaKaRa Ex Tag™ in a few paraffin blocks and, exceptionally, in cases fixed in formalin in which QIAmp Micro Qiagen failed. Therefore, these methods can be considered as ad extremis candidates in current DNA study practice in brain banks.
Several studies have recently appeared with methods geared to improve the fixation of tissues with less damage to nucleic acids and to optimize DNA extraction (4, 6, 20, 25). In this line, the present results have practical implications for the management of tissues in brain banks, in helping to identify limitations and possible pitfalls, and in optimizing the study of DNA of stored material.
Although the present study was focused on brain banks, similar conclusions can be applied to brain collections with material stored for years in formalin. Unfortunately, this material is probably no longer suitable to study mutations and polymorphisms. Provision of frozen material or, as a minimum, production of paraffin blocks after a short buffered‐formalin fixation is strongly recommended.
Supporting information
Table S2. APP PCR conditions.
Table S3. MAPT PCR conditions.
Table S4. SCA3, DRPLA and SCA2 PCR conditions.
Table S5. PRNP PCR conditions.
Supporting info item
ACKNOWLEDGEMENTS
This study was supported by the European Commission under the Sixth Framework Programme (BrainNet Europe II, LSHM‐CT‐2004‐503039). The paper reflects only the authors’ views and the Community is not liable for any use that may be made of it. Frozen tissues from genetic prion disease stored for 30–35 years were kindly provided by P. Brown MD, Bethesda, MA, USA. We thank T. Yohannan for editorial assistance.
REFERENCES
- 1. Buesa C, Maes T, Subirada F, Barrachina M, Ferrer I (2004) DNA chip technology in brain banks: confronting a degrading world. J Neuropathol Exp Neurol 63:1003–1014. [DOI] [PubMed] [Google Scholar]
- 2. Calacal GC, Delfin FC, Tan MM, Roewer L, Magtanong DL, Lara MC, Fortun R, De Ungria MC (2005) Identification of exhumed remains of fire tragedy victims using conventional methods and autosomal/Y‐chromosomal short tandem repeat DNA profiling. Am J Forensic Med Pathol 26:285–291. [DOI] [PubMed] [Google Scholar]
- 3. Capellari S, Vital C, Parchi P, Petersen RB, Ferrer X, Jarnier D, Pegoraro E, Gambetti P, Julien J (1997) Familial prion disease with a novel 144‐bp insertion in the prion protein gene in a Basque family. Neurology 49:133–141. [DOI] [PubMed] [Google Scholar]
- 4. Coura R, Prolla JC, Meurer L, Ashton‐Prolla P (2005) An alternative protocol for DNA extraction from formalin fixed and paraffin wax embedded tissue. J Clin Pathol 58:894–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Goate A, Chartier‐Harlin MC, Mullan M, Brown J, Crawford F, Fidani L, Giuffra L, Haynes A, Irving N, James L, Mant R, Newton P, Rooke K, Roques P, Talbot C, Pericak‐Vance M, Roses A, Williamson R, Rossor M, Owen M, Hardy J (1991) Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer’s disease. Nature 349:704–706. [DOI] [PubMed] [Google Scholar]
- 6. Kline MC, Duewer DL, Redman JW, Butler JM (2005) Results from the NIST 2004 DNA quantitation study. J Forensic Sci 50:570–578. [PubMed] [Google Scholar]
- 7. Konomi N, Lebwohl E, Zhang D (2002) Comparison of DNA and RNA extraction methods for mummified tissues. Mol Cell Probes 16:445–451. [DOI] [PubMed] [Google Scholar]
- 8. Koppelstaetter C, Jennings P, Hochegger K, Perco P, Ischia R, Karkoszka H, Mayer G (2005) Effect of tissue fixatives on telomere length determination by qualitative PCR. Mech Ageing Dev 126:1331–1333. [DOI] [PubMed] [Google Scholar]
- 9. Krajick K (2002) Glacial research. Melting glaciers release ancient relics. Science 296:2142. [DOI] [PubMed] [Google Scholar]
- 10. Kunkle RA, Miller JM, Alt DP, Cutlip RC, Cockett NE, Wang S, Richt JA, Thomsen BV, Hall SM (2006) Determination of sheep prion gene polymorphisms from paraffin‐embedded tissues. J Vet Diagn Invest 18:443–447. [DOI] [PubMed] [Google Scholar]
- 11. Luciani S, Fornaciari G, Rickards O, Labarga CM, Rollo F (2006) Molecular characterization of a pre‐Columbian mummy and in situ coprolite. Am J Phys Anthropol 129:620–629. [DOI] [PubMed] [Google Scholar]
- 12. Marota I, Rollo F (2002) Molecular paleontology. Cell Mol Life Sci 59:97–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Marota I, Basile C, Ubaldi M, Rollo F (2002) DNA decay rate in papyri and human remains from Egyptian archaeological sites. Am J Phys Anthropol 117:310–318. [DOI] [PubMed] [Google Scholar]
- 14. Maruyama H, Kawakami H, Nakamura S (1996) Reevaluation of the exact CAG repeat length in hereditary cerebellar ataxias using highly denaturing conditions and long PCR. Hum Genet 97:591–595. [DOI] [PubMed] [Google Scholar]
- 15. Miething F, Hering S, Hanschke B, Dressler J (2006) Effect of fixation for the degradation of nuclear and mitochondrial DNA in different tissues. J Histochem Cytochem 54:371–374. [DOI] [PubMed] [Google Scholar]
- 16. Murphy DD, Ravina B (2003) Brain banking for neurodegenerative diseases. Curr Opin Neurol 16:459–463. [DOI] [PubMed] [Google Scholar]
- 17. Nishiwaki Y, Kamino K, Yoshiiwa A, Nagano K, Takeda M, Tanabe H, Nishimura T, Kobayashi T, Yamamoto H, Nonomura Y, Yoneda H, Sakai T, Imagawa M, Miki T, Ogihara T (1996) Mutational screening of APP gene in patients with early‐onset Alzheimer disease utilizing mismatched PCR‐RFLP. Clin Genet 49:119–123. [DOI] [PubMed] [Google Scholar]
- 18. Paabo S, Poinar H, Serre D, Jaenicke‐Despres V, Hebler J, Rohland N, Kuch M, Krause J, Vigilant L, Hofreiter M (2004) Genetic analyses from ancient DNA. Annu Rev Genet 38:645–679. [DOI] [PubMed] [Google Scholar]
- 19. Pulst SM, Nechiporuk A, Nechiporuk T, Gispert S, Chen XN, Lopes‐Cendes I, Pearlman S, Strakman S, Orozco‐Diaz G, Lunkes A, DeJong P, Rouleau GA, Auburger G, Korenberg JR, Figueroa C, Sahba S (1996) Moderate expansion of a normally biallelic trinucleotide repeat in spinocerebellar ataxia type 2. Nat Genet 14:237–238. [DOI] [PubMed] [Google Scholar]
- 20. Rivero ER, Neves AC, Siklva‐Valenzuela MG, Sousa SO, Nunes FD (2006) Simple salting‐out method for DNA extraction from formalin‐fixed, paraffin‐embedded tissues. Pathol Res Pract 202:523–529. [DOI] [PubMed] [Google Scholar]
- 21. Rizzu P, Van Swieten JC, Joosse M, Hasegawa M, Stevens M, Tibben A, Niermeijer MF, Hillebrand M, Ravid R, Oostra BA, Goedert M, Van Duijn CM, Heutink P (1999) High prevalence of mutations in the microtubule‐associated protein tau in a population study of frontotemporal dementia in the Netherlands. Am J Hum Genet 64:414–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rogaev EI, Sherrington R, Wu C, Levesque G, Liang Y, Rogaeva EA, Ikeda M, Holman K, Lin C, Lukiw WJ, De Jong PJ, Fraser PE, Rommens JM, St George‐Hyslop P (1997) Analysis of the 5′ sequence, genomic structure, and alternative splicing of the presenilin‐1 gene (PSEN1) associated with early onset Alzheimer disease. Genomics 40:415–424. [DOI] [PubMed] [Google Scholar]
- 23. Rollo F, Ermini L, Luciani S, Marota I, Olivieri C, Luiselli D (2006) Fine characterization of the Iceman’s mtDNA haplogroup. Am J Physiol Anthropol 130:557–564. [DOI] [PubMed] [Google Scholar]
- 24. Sherrington R, Froelich S, Sorbi S, Campion D, Chi H, Rogaeva EA, Levesque G, Rogaev EI, Lin C, Liang Y, Ikeda M, Mar L, Brice A, Agid Y, Percy ME, Clerget‐Darpoux F, Piacentini S, Marcon G, Nacmias B, Amaducci L, Frebourg T, Lannfelt L, Rommens JM, St George‐Hyslop PH (1996) Alzheimer’s disease associated with mutations in presenilin 2 is rare and variably penetrant. Hum Mol Genet 5:985–988. [DOI] [PubMed] [Google Scholar]
- 25. Stanta G, Mucelli SP, Petrera F, Bonin S, Bussolati G (2006) A novel fixative improves opportunities of nucleic acids and proteomic analysis in human archive’s tissues. Diagn Mol Pathol 15:115–123. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S2. APP PCR conditions.
Table S3. MAPT PCR conditions.
Table S4. SCA3, DRPLA and SCA2 PCR conditions.
Table S5. PRNP PCR conditions.
Supporting info item


