Abstract
Lewy bodies (LBs) and Lewy neurites (LNs) are the hallmarks of Parkinson's disease (PD). Although LBs and LNs, frequently coexistent, share some histological properties, their appearances are quite different under conventional two‐dimensional observation. In order to clarify how these apparently different structures (LBs and LNs) are related during their formation, we performed three‐dimensional observation on post‐mortem brainstem tissues with PD. Sixty‐µm thick floating sections were multi‐immunofluorolabeled for α‐synuclein (αS), ubiquitin (Ub) and neurofilament (NF). Serial confocal images were reconstructed with software. External three‐dimensional configuration of LBs, double‐labeled for αS and NF, exhibited frequent continuity with LNs (70%). Internally, αS and Ub formed the three‐dimensional concentric inner layers and NF rimmed these inner layers. This layered structure was shared among spherical LBs, rod‐shaped LNs and even convoluted forms of LBs/LNs. Furthermore, each layer exhibited continuity without interruption even in the convoluted form and around its junction to spherical LBs. This three‐layered structure shared among various Lewy pathologies and their layered continuity on three‐dimensional basis favor the hypothesis that LNs evolve into LBs. Besides progression from pale bodies to LBs, structural evolution from LNs into LBs may provide an alternative explanation for the variability of αS deposits and their interrelation.
Keywords: Lewy bodies, Lewy neurites, three‐dimensional reconstruction
INTRODUCTION
Lewy bodies (LBs) are the most distinctive neuropathological feature of idiopathic Parkinson's disease (PD) (21). On electron microscopy, LBs consist of filaments of approximately 10 nm in diameter, resembling 11, 12 but distinguishable from neurofilaments (NF) 14, 16, 22, 38. Although it is now considered that α‐synuclein (αS) is one of the major constituents of these filaments 23, 34, 40, 42, 46, immunocytochemical studies have demonstrated the presence of NF 14, 19, 33 and ubiquitin (Ub) epitopes in LBs (27) and Lewy neurites (LNs) (9), which are coexistent with a wide range of degenerative changes with LBs 15, 25. Frequent coexistence of LBs and LNs (10) and their antigenic and histological similarities suggest that these apparently distinct structures (5)are tightly related. It remains, still, to be explained how they are interrelated. The aim of the present study is to clarify how these apparently distinct structures (LBs and LNs) are related during their formation. For this purpose, three‐dimensional images of LBs and LNs were generated by using computer‐aided reconstruction of serial optical sections derived from laser confocal images of thick (60‐µm thick) triple‐immunolabeled sections.
MATERIALS AND METHODS
Subjects
Four adult brainstem tissue samples from post‐mortem specimens were obtained from Yokufukai Geriatric Hospital. Their ages at death were 73, 83, 91 and 100 years. The post‐mortem examination revealed marked neuronal loss and numerous LBs in the substantia nigra (SN), locus ceruleus (LC) and dorsal motor nucleus of vagus (DNV) and they were diagnosed as having PD. The formalin‐fixed brainstem blocks that include SN, LC or DNV were washed thoroughly with 0.1 M phosphate buffer and cryoprotected with 20% sucrose/0.1 M phosphate. Free‐floating sections with a thickness of 60 µm were obtained on a freezing microtome. During the following staining procedures, these sections were washed three times with phosphate‐buffered saline containing 0.3% polyoxyethylene (10) octylphenyl ether (Wako, Japan) (PBST) between each step.
Conventional immunohistochemistry
The free‐floating sections were treated with 1% hydrogen peroxidase for 30 minutes and then incubated with an anti‐αS antibody [LB509, 1:50, mouse monoclonal antibody immunoglobulin G (IgG), a kind gift from Professor Iwatsubo, University of Tokyo, Tokyo, Japan](24) diluted with PBST and the corresponding blocking serum. The sections were then incubated for 2 h with the biotinylated secondary antibody, followed by avidin–biotin–peroxidase complex (1:1000, ABC Elite, Vector, Burlingame, CA). The peroxidase labeling was visualized with diaminobenzidine‐nickel as chromogen.
Double immunofluorolabeling
The floating sections were incubated with 0.5% normal goat serum for 30 minutes at room temperature. We used an anti‐NF (SMI‐31, 1:1000, mouse monoclonal IgG, SMI, Baltimore, MD) and another anti‐αS (AB5038P, 1:500, rabbit polyclonal, Chemicon International, Temecula, CA) antibody. Because this combination is useful in visualizing external configurations of αS‐positive deposits, spatial relations between LBs and LNs are readily detectable when reconstructed on three‐dimensional basis. We incubated sections with these two primary antibodies diluted with PBST and the corresponding blocking serum for 7 days at 4°C. Because of the thickness (60 µm) of the floating sections, incubation with these primary antibodies was prolonged to 7 days or longer at 4°C to obtain a homogeneous staining throughout the entire thickness of the sections as we established previously (44). These two antibodies were visualized with an anti‐mouse IgG antibody made in goat conjugated with Alexa® 488 (1:200, Molecular Probes, Eugene, OR) and an anti‐rabbit IgG antibody made in goat conjugated with Alexa® 546 (1:200) for 2 h. After washing, they were mounted with buffered gylcerol containing p‐phenylenediamine to minimize photobleaching.
Triple immunofluorolabeling
An anti‐Ub antibody (DF2, 1:50, rat monoclonal IgM, a kind gift from Professor Mori, Osaka City University School of Medicine, Osaka, Japan) (30) was combined with the anti‐NF (SMI‐31) and the anti‐αS (AB5038P) antibodies for triple immunolabeling to investigate the internal structure of LBs and LNs. After treatment with 0.5% normal goat serum for 30 minutes at room temperature, we incubated sections with these three primary antibodies diluted with PBST and the corresponding blocking serum for 7 days or longer at 4°C. The sections were then incubated for 3 h with the biotinylated secondary antibody to rat IgM (µ‐chain specific, 1:200, goat polyclonal, KPL, Guildford, UK). The sections were then incubated with the avidin–biotin–peroxidase complex (1:200), the anti‐NF mouse monoclonal antibody (1:1000) and the anti‐αS rabbit polyclonal antibody (1:500) for another one night at 4°C. These three antibodies were visualized with a mixture of anti‐mouse IgG antibody made in goat conjugated with Alexa® 488 (1:200), anti‐rabbit IgG antibody made in goat conjugated with Alexa® 568 (1:200) and Alexa® 647‐conjugated streptavidin (s21374, 1:200) for 2 h. After washing, they were mounted with buffered glycerol containing p‐phenylenediamine to minimize photobleaching.
Laser confocal imaging and three‐dimensional reconstruction of confocal images
The fluorolabeled sections were observed under a fluorescence microscope combined with laser confocal system (Leica TCS/SP; Heidelberg, Germany or Olympus FV1000; Tokyo, Japan) equipped with multiple laser lines. Alexa® 488, which labeled the NF epitope, was excited by a 488‐nm beam and was detected through a light path ranging from 500–540 nm. Alexa® 546 or Alexa® 568, which labeled the αS epitope, was excited by a 546‐nm or 568‐nm beam and was detected through a light path ranging from 600–640 nm. Alexa® 647, which labeled Ub, was excited by a 647‐nm beam and was detected through a light path ranging from 690–730 nm. Serial optical sections with an interval of 0.1 µm or 0.2 µm were obtained and reconstructed for three‐dimensional observation on the software “Delta Viewer” (http://vivaldi.ics.nara‐wu.ac.jp/~wada/DeltaViewer/index‐j.html) provided by Professor Wada from Nara Women's University, Nara, Japan. In order to observe the internal structure of LBs, three‐dimensional reconstruction was also performed on another software, TRI/3D‐SRF II® (Ratoc, Tokyo, Japan), run on Windows XP (64 bit version).
RESULTS
Conventional immunohistochemistry with an anti‐αS antibody (LB509) demonstrated LBs (Figure 1, as indicated by the arrow), which are apparently distinct from LNs (Figure 1, indicated by the arrowhead), both observed in the DNV, LC and SN of the brain tissue from all the cases examined. In order to look for possible relations between LBs and LNs on a three‐dimensional basis, double‐labeled images for αS and NF were obtained serially at an interval of 0.2 µm so that the entire sphere of LBs was contained. These serial images were reconstructed to yield an external three‐dimensional view of 50 LBs and their relations to LNs. Among these 50 LBs, 35 LBs were in continuity with LNs (Figure 2).
Figure 1.
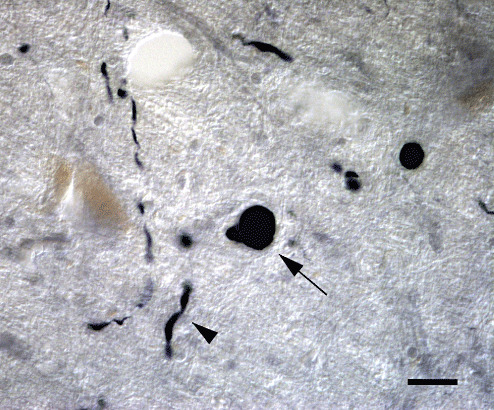
Lewy bodies (LBs) and Lewy neurites (LNs) in the locus ceruleus labeled with anti‐α‐synuclein (αS) antibody. αS immunohistochemical staining of brainstems demonstrated LBs (indicated by the arrow) and LNs (indicated by the arrowhead), which are apparently distinct from each other. Bar = 20 µm.
Figure 2.
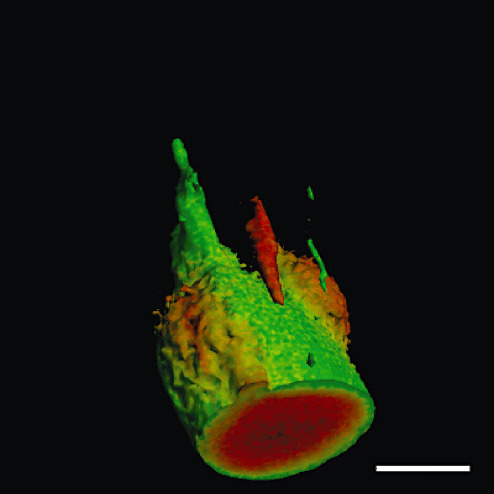
Serial optical sections of Lewy bodies (LBs) were obtained from thick floating sections double‐immunofluorolabeled for α‐synuclein (red) and neurofilament (green). They were reconstructed to yield external configurations of LBs, frequently in continuity with Lewy neurites. Bar = 20 µm.
Triple immunofluorolabeling ( Figure 3) for αS (red), Ub (blue) and NF (green) were performed in order to further examine the internal structure of LBs and LNs. In spherical LBs (Figure 3A), these epitopes were stratified into concentric inner layers with Ub in the center surrounded by αS. NF at the outermost layer rimmed these internal core structures. This three‐layered structure of spherical LBs was shared with LNs (Figure 3B). Serial optical sections with an interval of 0.1 µm were stacked ( Figure 4) and reconstructed. Reconstructed LBs exhibited the three‐layered structure regardless of the optical plane ( Figure 5A: XY plane; 5B: XZ plane; 5C: YZ plane). Negative control sections similarly processed in the absence of the three primary antibodies gave no significant signals when detected in parallel (data not shown). This three‐layered structure was shared with LNs (Figure 5D: XY plane; 5E: XZ plane; 5F: YZ plane). This three‐layered structure was consistently observed in whatever optical planes that can be displayed with this software. In addition to the internal views of the reconstructed LBs and LNs, external views of the reconstructed structures from arbitrary viewpoints are also provided in Figure 6. Longer incubation time allowed to immunolabelling the entire thickness of floating sections (60 µm in thickness) without a significant gradient. Intensities of the three fluorescent signals were strong enough to capture more than 300 optical sections, which were sufficient to encompass the entire structure of LBs in sufficient resolution along the Z‐axis (Figure 6; see also Video Clips S1–S3). NF (green) formed a shell‐like structure surrounding the concentric inner core composed of Ub and αS. Convoluted LNs were also organized into the same three‐layered structure ( Figure 7). Each of the layers was continuous with that of spherical LBs even at the junction between LN and LB.
Figure 3.
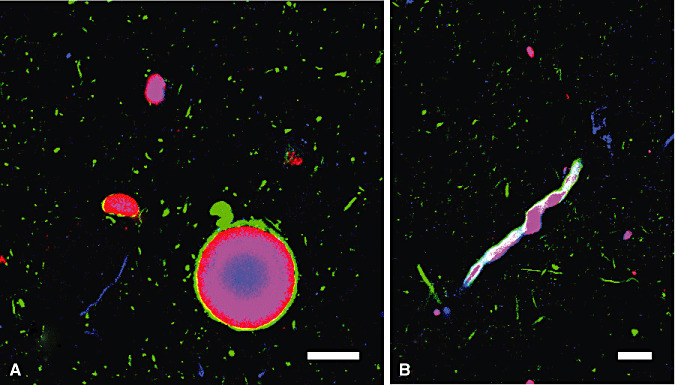
Triple immunofluorolabeling of LBs (A) and LNs (B) with anti‐αS (red), anti‐Ub (blue) and anti‐NF (green) antibodies. They are stratified into concentric layers. Ub epitope is in the center of inner layer and αS epitope is surrounding the Ub epitope. NF epitope at the outermost layer rims these internal core structures. This three‐layered structure of spherical LBs was shared with that of rod‐shaped LNs. Bars = 20µm. Abbreviations: LBs = Lewy bodies; LNs = Lewy neurites; αS = α‐synuclein; Ub = ubiquitin; NF = neurofilament.
Figure 4.
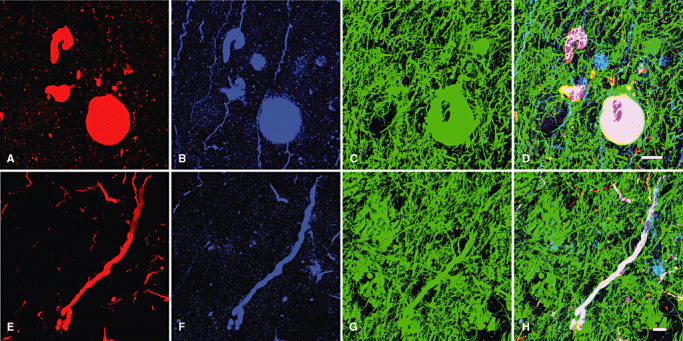
Stacked images of 300 serial optical sections of triple‐immunofluorolabeled LBs and LNs with an interval of 0.1 µm. LBs (A–D) and LNs (E–H) are immunopositive for α‐synuclein (red), ubiquitin (blue) and neurofilament (green). There is a significant overlap between the epitopes. Bars = 20 µm. Abbreviations: LBs = Lewy bodies; LNs = Lewy neurites.
Figure 5.
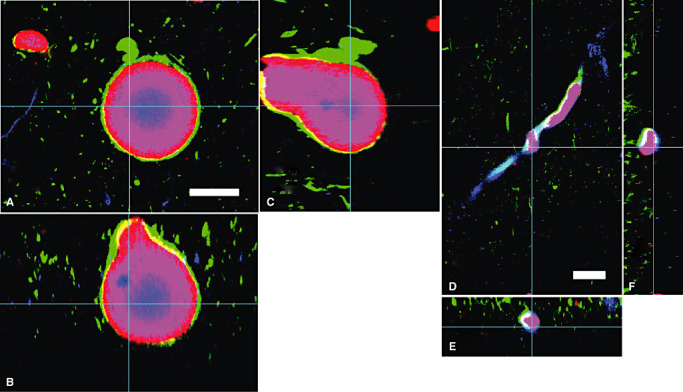
Cross sections of reconstructed LBs (A–C) and LNs (D–F). A,D. One of the optical sections (X–Y) at the depth indicated with blue lines in B,C and E,F. B,E. Cross‐sectional X–Z image along the blue lines indicated in A,D and C, F. C,F. Cross‐sectional Y–Z image along the blue lines indicated in A,D and B,E. LBs and LNs showed three‐layered structure in whatever optical planes. Red = α‐synuclein; blue = ubiquitin; green = neurofilament. Bars = 20 µm. Abbreviations: LBs = Lewy bodies; LNs = Lewy neurites.
Figure 6.
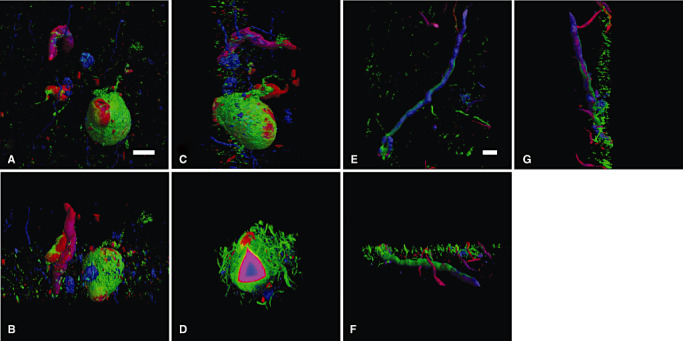
External and internal views of the three‐dimensional reconstructed LBs and LNs. Three‐dimensional reconstruction from serial optical sections with the interval of 0.1 µm along Z‐axis was performed on a software “Delta Viewer,” and the same LBs and LNs were observed from different angles: upper view (A,E); front view (B,F); and side view (C,G). The three‐layered structure was maintained when a part of LBs was removed after a three‐dimensional reconstruction (D). Red = α‐synuclein, blue = ubiquitin; green = neurofilament. Bars = 20 µm. Abbreviations: LBs = Lewy bodies; LNs = Lewy neurites.
Figure 7.
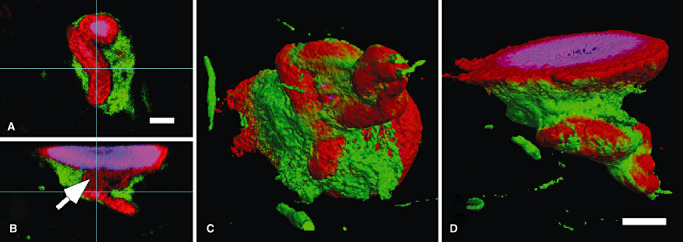
Internal (A,B) and external (C,D) views of reconstructed LNs/LBs with convolution. Three‐dimensional analyses demonstrated LNs/LBs with convolution. The same three‐layered structure is maintained in convoluted LNs/LBs reconstructed and shown along XY (A), XZ (B) and YZ (data not shown) planes. Each layer of the internal structure is in continuity (indicated by the arrow) with that of the adjacent spherical LB (C,D). Red = α‐synuclein, blue = ubiquitin; green = neurofilament. Bars = 20 µm (A–D). Abbreviations: LBs = Lewy bodies; LNs = Lewy neurites.
DISCUSSION
We successfully performed triple immunofluorolabelling for αS, Ub and NF, all of which are constituents of LBs. Longer incubation of up to 7 days (44) of thick floating sections (60 µm in thickness) followed by sensitive fluorescent dyes enabled us to immunolabel the entire thickness of the sections with sufficient intensities to resist photobleaching even after multiple laser scanning of different optical planes (up to 300 sections × two times for averaging in this study). Serial optical sections at an interval of 0.1 µm or 0.2 µm provided a sufficient resolution along the Z‐axis, when reconstructed on either software.
With this approach, it was possible to reconstruct entire LBs with a sufficient resolution on a three‐dimensional basis. Three‐dimensional analysis of LBs double‐labeled for αS and NF demonstrated that 70% (35/50) of LBs have a continuity with LNs (Figure 2). This frequent continuity between LBs and LNs based on their external configurations suggests that these apparently distinct structures (Figure 1) may have some relation and their formations are not completely independent. On triple‐labeled sections, a kind of a three‐layered structure was identified on two‐dimensional cut surface of LBs (Figure 2), which stratified into concentric inner layers with Ub in the center surrounded by αS and an outermost rim of NF. Because either software provides an internal view of the reconstructed objects at whatever optical plane, it was possible to confirm that this three‐layered structure was consistent throughout the LBs (Figure 5). Moreover, the same three‐layered structure was shared with LNs, which are apparently distinct from LBs on αS‐stained sections (Figure 1). Because Ub is colocalized not only with αS (43) but also with synphilin‐1 (47) and Pael‐R (31) in LBs, it remains to be clarified which are the pathological target molecules for ubiquitination during LB formation. Irrespective of the protein which undergoes pathological ubiquitination, Ub immunoreactivity in LBs and LNs provides further support for the similarity of the three‐layered structure shared by LBs and LNs on a three‐dimensional basis. This finding suggests that LBs and LNs are formed under a similar mechanism even though Ub‐immunoreactive LBs and LNs which we observed in the present study are confined to a subgroup at a relatively late stage of their evolution 20, 37, 46.
LBs exhibit a peripheral halo and a concentric eosinophilic appearance with hematoxilin and eosin staining. The ultrastructural study of LBs in the SN and LC revealed that they contain filamentous material with a diameter of 10–20 nm with irregular contours, radiating out from a denser central core 11, 12 and the halo of classical LBs is composed of the radiating filaments (18). Identification of NF epitopes in LBs 19, 33 and the similar dimension of the filaments in LBs and LNs to that of NF raised a possibility that filaments in LBs were NF. Some researchers, however, had identified filaments representative of LBs (LB filaments) but distinguishable from NF based on the variable diameters ranging from 7 to 20 nm, decoration with dense granular material and the absence of side‐arms characteristic of NF 14, 16, 22, 32, 38. These morphological characteristics of LB filaments are readily detectable not only in the central pale area (pale body), where intervening other filamentous structures are sparse (22) but also in the neurites (1). It is now considered that αS is a major constituent of LBs and LNs because LB filaments, either isolated 2, 39 or in the tissue 1, 46, exhibit αS‐like immunoreactivity mainly in the periphery of LBs, which is in agreement with our findings based on three‐dimensional observation. However, one may wonder how these αS‐positive LB filaments are related to NF. Several researchers have identified NF‐like bundles running peripherally outside the radiating LB filaments in LBs 1, 14, 16, 22, 32, 38, 46. These electron microscopic features of LBs are shared with those of LNs and in good agreement with the layered structure of these epitopes similarly shared between LBs and LNs as we demonstrated on a three‐dimensional basis.
In addition to the similar layered structure shared between LBs and LNs, this three‐dimensional study confirmed frequent continuity (70%) between LBs and LNs. Lack of this continuity in 30% of LBs raises the possibility that some LBs and LNs are formed independently. Indeed, predominant neuritic deposition of αS is associated with the A53T mutation of αS gene in human brain 41, 48 and in its mouse model (17) as well. In contrast, perikaryal deposition of αS could be independent of its neuritic counterpart (26), suggesting that these two processes are not necessarily linked. Are these possibly independent structures united secondarily to form a more frequent (70%) continuous structure? Furthermore, we may then ask how these two distinct structures, both with the same three‐layered structure, are secondarily united to form a single structure with a continuous three‐layered structure. On the other hand, neuropathological studies 6, 8, 36, 41, 48 on human brains are in agreement that formation of LNs occurs prior to LBs. Moreover, Braak et al observed somatic LBs in close contact with voluminous LNs as we demonstrated in this three‐dimensional study (4). They speculated that abnormal LN material is not produced in the cellular processes of affected nerve cells but instead is either actively or passively transferred into them. Furthermore, molecules in non‐aggregated state may be locally concentrated when being transported into a restricted space as in narrow axons, where they are potentially more liable to aggregate to form LNs. Derangement of transport into axons may further accelerate this cascade and formation of LNs itself may aggravate this cascade. Interestingly, blockade of axonal transport in cultured rat cortical neurons transfected with the A30P mutant of αS results in early accumulation of αS at the proximal neurites prior to soma (35). These lines of evidence are compatible with the interpretation that some LNs extend into the soma to form LBs. However, it is also possible that some LNs and LBs are formed independently and remain independent throughout their evolution without interacting with each other as suggested by the observation that 30% of LBs lacked continuity with LNs. Another variety of αS deposits are convoluted LNs (Figure 7). Their interior was of the same three‐layered structure (Figure 7) as well and each layer was continuous with the corresponding layer of the adjacent spherical LB. Because the three‐layered structure was maintained in the convolution, it is likely that this three‐layered structure with a significant length is initially organized as we demonstrated in LNs followed by convolution of this long structure to form a very complicated three‐dimensional organization (Figure 7). Otherwise, this three‐layered structure should have been organized in the convoluted state, which is hard to imagine. This interpretation is again compatible with our hypothesis that some of the preceding LNs extend into soma to form LBs. The reverse possibility could not be ruled out completely from the present study, but this interpretation requires assumptions that (i) formed LBs are plastic and pliable enough to be transformed into more smaller and more slender LNs; and more curiously, (ii) these LBs should maintain their internal three‐layered structure during this transformation, both of which are not very plausible. We do not yet know whether visible aggregates in the neurons are transported along the neurites. However, colocalization of transfected αS with kinesin‐1 and dynein in rat cortical neurons (45) suggests that the αS molecule is transported along neurites. Blockade of axonal transport in cultured cells leads to aggregate formation of various molecules including αS (35). It remains to be clarified how this derangement is related to proteasomal functions (29), mutations of αS and relevant genes (28) and mitochondrial functions 3, 13, each claimed to be relevant to the pathogenesis of PD. The three‐layered internal structure, shared between LNs and LBs, not only spherical but also convoluted ones, and their continuity suggest that evolution from LNs to LBs may be one of the major ways, even if not the exclusive one. This is compatible with a series of experimental observations 35, 45 that some derangement of transport in neurites is closely related to formation of LNs and LBs. We do not yet know, however, why the deranged transport leads to aggregation of αS to form LNs and finally LBs. In parallel, it has also been proposed that pale bodies (PBs), deposits of αS with less aggregated form, are precursors of LBs 1, 18, 22. Because PBs are usually spherical, it is again hard to imagine that the three‐layered structure of the large sphere evolves into smaller convoluted forms of LBs with a layered structure (Figure 7). However, whether LBs are derived from PBs or from LNs is not mutually exclusive. Gómez‐Tortosa et al observed the less aggregated and less compact αS inclusions which were Ub‐negative and considered them as PBs or precursors of PBs (20). Because Ub‐like immunoreactivity in PBs is variable 7, 20, it is plausible that Ub‐like immunoreactivity in LNs is also variable as well, which means that some of αS‐positive, Ub‐negative LNs (23) represent premature LNs, just as some of αS‐positive, Ub‐negative spherical inclusions represent premature PBs, presumably with less aggregated αS. Nevertheless, immunohistochemical features of PBs and LBs are overlapping significantly in one way such that some PBs contain Ub‐like immunoreactivity 7, 20 as LBs do and in another way such that NF‐like filaments have also been identified at the periphery of PBs (46). It is, then, plausible that some of the spherical structures we observed in this study represent not only LBs but also PBs. It is needless to say that this progressive aggregation of αS is essential for initial deposition of PBs and subsequent formation of LBs. In addition to this progressive aggregation, structural evolution from LNs into LBs, with a uniform internal structure, may provide an alternative explanation for the variability of αS deposits and their interrelation in PD.
Supporting information
Video Clip S1. Convoluted LB in the dorsal vagal nucleus.
Video Clip S2. LB in continuity with LN in the locus ceruleus.
Video Clip S3. LB in continuity with LN in the substantia nigra.
This material is available as part of the online article from: http://www.blackwellsynergy.com
Please note: Blackwell Publishing is not responsible for the content or functionality of any supplementary materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item
Supporting info item
Supporting info item
ACKNOWLEDGMENT
This work is supported in part by the grant from Tokyo Metropolitan Government to the “Three‐dimension project.”
REFERENCES
- 1. Arima K, Uéda K, Sunohara N, Hirai S, Izumiyama Y, Tonozuka‐Uehara H, Kawai M (1998) Immunoelectron‐microscopic demonstration of NACP/alpha‐synuclein‐epitopes on the filamentous component of Lewy bodies in Parkinson's disease and in dementia with Lewy bodies. Brain Res 808:93–100. [DOI] [PubMed] [Google Scholar]
- 2. Baba M, Nakajo S, Tu PH, Tomita T, Nakaya K, Lee VM et al (1998) Aggregation of alpha‐synuclein in Lewy bodies of sporadic Parkinson's disease and dementia with Lewy bodies. Am J Pathol 152:879–884. [PMC free article] [PubMed] [Google Scholar]
- 3. Betarbet R, Sherer TB, MacKenzie G, Garcia‐Osuna M, Panov AV, Greenamyre JT (2000) Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nat Neurosci 3:1301–1306. [DOI] [PubMed] [Google Scholar]
- 4. Braak E, Sandmann‐Keil D, Rüb U, Gai WP, De Vos RA, Jansen Steur ENH et al (2001) Alpha‐synuclein immunopositive Parkinson's disease‐related inclusion bodies in lower brain stem nuclei. Acta Neuropathol (Berl) 101:195–201. [DOI] [PubMed] [Google Scholar]
- 5. Braak H, Sandmann‐Keil D, Gai WP, Braak E (1999) Extensive axonal Lewy neurites in Parkinson's disease: a novel pathological feature revealed by alpha‐synuclein immunocytochemistry. Neurosci Lett 265:67–69. [DOI] [PubMed] [Google Scholar]
- 6. Braak H, Del Tredici K, Rüb U, De Vos RA, Jansen Steur EN, Braak E (2003) Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging 24:197–211. [DOI] [PubMed] [Google Scholar]
- 7. Dale GE, Probst A, Luthert P, Martin J, Anderton BH, Leigh PN (1992) Relationships between Lewy bodies and pale bodies in Parkinson's disease. Acta Neuropathol(Berl) 83:525–529. [DOI] [PubMed] [Google Scholar]
- 8. Del Tredici K, Rüb U, De Vos RA, Bohl JR, Braak H (2002) Where does Parkinson disease pathology begin in the brain? J Neuropathol Exp Neurol 61:413–426. [DOI] [PubMed] [Google Scholar]
- 9. Dickson DW, Ruan D, Crystal H, Mark MH, Davies P, Kress Y, Yen SH (1991) Hippocampal degeneration differentiates diffuse Lewy body disease (DLBD) from Alzheimer's disease: light and electron microscopic immunocytochemistry of CA2‐3 neurites specific to DLBD. Neurology 41:1402–1409. [DOI] [PubMed] [Google Scholar]
- 10. Dickson DW, Schmidt ML, Lee VM, Zhao ML, Yen SH, Trojanowski JQ (1994) Immunoreactivity profile of hippocampal CA2/3 neurites in diffuse Lewy body disease. Acta Neuropathol (Berl) 87:269–276. [DOI] [PubMed] [Google Scholar]
- 11. Duffy PE, Tennyson VM (1965) Phase and electron microscopic observations of Lewy bodies and melanin granules in the substantia nigra and locus cearuleus in Parkinson's disease. J Neuropathol Exp Neurol 24:398–414. [Google Scholar]
- 12. Forno LS, Norville RL (1976) Ultrastructure of Lewy bodies in the stellate ganglion. Acta Neuropathol (Berl) 34:183–197. [DOI] [PubMed] [Google Scholar]
- 13. Forno LS, Langston JW, DeLanney LE, Irwin I, Ricaurte GA (1986) Locus ceruleus lesions and eosinophilic inclusions in MPTP‐treated monkeys. Ann Neurol 20:449–455. [DOI] [PubMed] [Google Scholar]
- 14. Forno LS, Sternberger LA, Sternberger NH, Strefling AM, Swanson K, Eng LF (1986) Reaction of Lewy bodies with antibodies to phosphorylated and non‐phosphorylated neurofilaments. Neurosci Lett 64:253–258. [DOI] [PubMed] [Google Scholar]
- 15. Gai WP, Blessing WW, Blumbergs PC (1995) Ubiquitin‐positive degenerating neurites in the brainstem in Parkinson's disease. Brain 118:1447–1459. [DOI] [PubMed] [Google Scholar]
- 16. Galloway PG, Mulvihill P, Perry G (1992) Filaments of Lewy bodies contain insoluble cytoskeletal elements. Am J Pathol 140:809–822. [PMC free article] [PubMed] [Google Scholar]
- 17. Giasson BI, Duda JE, Quinn SM, Zhang B, Trojanowski JQ, Lee VM (2002) Neuronal alpha‐synucleinopathy with severe movement disorder in mice expressing A53T human alpha‐synuclein. Neuron 34:521–533. [DOI] [PubMed] [Google Scholar]
- 18. Gibb WR, Scott T, Lees AJ (1991) Neuronal inclusions of Parkinson's disease. Mov Disord 6:2–11. [DOI] [PubMed] [Google Scholar]
- 19. Goldman JE, Yen SH, Chiu FC, Peress NS (1983) Lewy bodies of Parkinson's disease contain neurofilament antigens. Science 221:1082–1084. [DOI] [PubMed] [Google Scholar]
- 20. Gómez‐Tortosa E, Newell K, Irizarry MC, Sanders JL, Hyman BT (2000) α‐synuclein immunoreactivity in dementia with Lewy bodies: morphological staging and comparison with ubiquitin immunostaining. Acta Neuropathol (Berl) 99:352–357. [DOI] [PubMed] [Google Scholar]
- 21. Greenfield JG, Bosanquet FD (1953) The brain‐stem lesions in Parkinsonism. J Neurol Neurosurg Psychiatry 16:213–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hayashida K, Oyanagi S, Mizutani Y, Yokochi M (1993) An early cytoplasmic change before Lewy body maturation: an ultrastructural study of the substantia nigra from an autopsy case of juvenile parkinsonism. Acta Neuropathol (Berl) 85:445–448. [DOI] [PubMed] [Google Scholar]
- 23. Irizarry MC, Growdon W, Gomez‐Isla T, Newell K, George JM, Clayton DF, Hyman BT (1998) Nigral and cortical Lewy bodies and dystrophic nigral neurites in Parkinson's disease and cortical Lewy body disease contain alpha‐synuclein immunoreactivity. J Neuropathol Exp Neurol 57:334–337. [DOI] [PubMed] [Google Scholar]
- 24. Iwatsubo T, Yamaguchi H, Fujimuro M, Yokosawa H, Ihara Y, Trojanowski JQ, Lee VM (1996) Purification and characterization of Lewy bodies from the brains of patients with diffuse Lewy body disease. Am J Pathol 148:1517–1529. [PMC free article] [PubMed] [Google Scholar]
- 25. Kim H, Gearing M, Mirra SS (1995) Ubiquitin‐positive CA2/3 neurites in hippocampus coexist with cortical Lewy bodies. Neurology 45:1768–1770. [DOI] [PubMed] [Google Scholar]
- 26. Kuusisto E, Parkkinen L, Alafuzoff I (2003) Morphogenesis of Lewy bodies: dissimilar incorporation of alpha‐synuclein, ubiquitin, and p62. J Neuropathol Exp Neurol 62:1241–1253. [DOI] [PubMed] [Google Scholar]
- 27. Kuzuhara S, Mori H, Izumiyama N, Yoshimura M, Ihara Y (1988) Lewy bodies are ubiquitinated. A light and electron microscopic immunocytochemical study. Acta Neuropathol (Berl) 75:345–353. [DOI] [PubMed] [Google Scholar]
- 28. Masliah E, Rockenstein E, Veinbergs I, Mallory M, Hashimoto M, Takeda A et al (2000) Dopaminergic loss and inclusion body formation in alpha‐synuclein mice: implications for neurodegenerative disorders. Science 287:1265–1269. [DOI] [PubMed] [Google Scholar]
- 29. McNaught KS, Perl DP, Brownell AL, Olanow CW (2004) Systemic exposure to proteasome inhibitors causes a progressive model of Parkinson's disease. Ann Neurol 56:149–162. [DOI] [PubMed] [Google Scholar]
- 30. Mori H, Kondo J, Ihara Y (1987) Ubiquitin is a component of paired helical filaments in Alzheimer's disease. Science 235:1641–1644. [DOI] [PubMed] [Google Scholar]
- 31. Murakami T, Shoji M, Imai Y, Inoue H, Kawarabayashi T, Matsubara E et al (2004) Pael‐R is accumulated in Lewy bodies of Parkinson's disease. Ann Neurol 55:439–442. [DOI] [PubMed] [Google Scholar]
- 32. Oyanagi S (1992) Lewy bodies and Lafora bodies. In: Denshikenbikyo ni yoru Shinkeibyourigaku no Susume [in Japanese]. Chapter 9, p. 183. Igaku‐Shoin: Tokyo. [Google Scholar]
- 33. Pappolla MA (1986) Lewy bodies of Parkinson's disease. Immune electron microscopic demonstration of neurofilament antigens in constituent filaments. Arch Pathol Lab Med 110:1160–1163. [PubMed] [Google Scholar]
- 34. Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A et al (1997) Mutation in the alpha‐synuclein gene identified in families with Parkinson's disease. Science 276:2045–2047. [DOI] [PubMed] [Google Scholar]
- 35. Saha AR, Hill J, Utton MA, Asuni AA, Ackerley S, Grierson AJ et al (2004) Parkinson's disease alpha‐synuclein mutations exhibit defective axonal transport in cultured neurons. J Cell Sci 117:1017–1024. [DOI] [PubMed] [Google Scholar]
- 36. Saito Y, Kawashima A, Ruberu NN, Fujiwara H, Koyama S, Sawabe M et al (2003) Accumulation of phosphorylated alpha‐synuclein in aging human brain. J Neuropathol Exp Neurol 62:644–654. [DOI] [PubMed] [Google Scholar]
- 37. Sakamoto M, Uchihara T, Hayashi M, Nakamura A, Kikuchi E, Mizutani T et al (2002) Heterogeneity of nigral and cortical Lewy bodies differentiated by amplified triple‐labeling for alpha‐synuclein, ubiquitin, and thiazin red. Exp Neurol 177:88–94. [DOI] [PubMed] [Google Scholar]
- 38. Schmidt ML, Murray J, Lee VM, Hill WD, Wertkin A, Trojanowski JQ (1991) Epitope map of neurofilament protein domains in cortical and peripheral nervous system Lewy bodies. Am J Pathol 139:53–65. [PMC free article] [PubMed] [Google Scholar]
- 39. Spillantini MG (1998) Alpha‐synuclein in filamentous inclusions of Lewy bodies from Parkinson's disease and dementia with Lewy bodies. Proc Natl Acad Sci USA 95:6469–6473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M (1997) Alpha‐synuclein in Lewy bodies. Nature 388:839–840. [DOI] [PubMed] [Google Scholar]
- 41. Spira PJ, Sharpe DM, Halliday G, Cavanagh J, Nicholson GA (2001) Clinical and pathological features of a Parkinsonian syndrome in a family with an Ala53Thr alpha‐synuclein mutation. Ann Neurol 49:313–319. [PubMed] [Google Scholar]
- 42. Takeda A, Mallory M, Sundsmo M, Honer W, Hansen L, Masliah E (1998) Abnormal accumulation of NACP/alpha‐synuclein in neurodegenerative disorders. Am J Pathol 152:367–372. [PMC free article] [PubMed] [Google Scholar]
- 43. Tofaris GK, Razzaq A, Ghetti B, Lilley KS, Spillantini MG (2003) Ubiquitination of alpha‐synuclein in Lewy bodies is a pathological event not associated with impairment of proteasome function. J Biol Chem 278:44405–44411. [DOI] [PubMed] [Google Scholar]
- 44. Uchihara T, Nakamura A, Nakayama H, Arima K, Ishizuka N, Mori H, Mizushima S (2003) Triple immunofluorolabeling with two rabbit polyclonal antibodies and a mouse monoclonal antibody allowing three‐dimensional analysis of cotton wool plaques in Alzheimer disease. J Histochem Cytochem 51:1201–1206. [DOI] [PubMed] [Google Scholar]
- 45. Utton MA, Noble WJ, Hill JE, Anderton BH, Hanger DP (2005) Molecular motors implicated in the axonal transport of tau and alpha‐synuclein. J Cell Sci 118:4645–4654. [DOI] [PubMed] [Google Scholar]
- 46. Wakabayashi K, Hayashi S, Kakita A, Yamada M, Toyoshima Y, Yoshimoto M, Takahashi H (1998) Accumulation of alpha‐synuclein/NACP is a cytopathological feature common to Lewy body disease and multiple system atrophy. Acta Neuropathol (Berl) 96:442–452. [DOI] [PubMed] [Google Scholar]
- 47. Wakabayashi K, Engelender S, Tanaka Y, Yoshimoto M, Mori F, Tsuji S et al (2002) Immunocytochemical localization of synphilin‐1, an α‐synuclein‐associated protein, in neurodegenerative disorders. Acta Neuropathol (Berl) 103:209–214. [DOI] [PubMed] [Google Scholar]
- 48. Yamaguchi K, Cochran EJ, Murrell JR, Polymeropoulos MH, Shannon KM, Crowther RA et al (2005) Abundant neuritic inclusions and microvacuolar changes in a case of diffuse Lewy body disease with the A53T mutation in the alpha‐synuclein gene. Acta Neuropathol (Berl) 110:298–305. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video Clip S1. Convoluted LB in the dorsal vagal nucleus.
Video Clip S2. LB in continuity with LN in the locus ceruleus.
Video Clip S3. LB in continuity with LN in the substantia nigra.
This material is available as part of the online article from: http://www.blackwellsynergy.com
Please note: Blackwell Publishing is not responsible for the content or functionality of any supplementary materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item
Supporting info item
Supporting info item


