Abstract
Birth injury of the scalp, skull and central nervous system (CNS) is a well‐recognized complication of a difficult delivery. The rate of birth trauma has dropped precipitously and now accounts for less than 2% of neonatal deaths. Despite this dramatic decrease in birth‐trauma mortality significant injuries still occur. A variety of risk factors clearly predispose certain infants to birth‐related injury. Recent neuroradiology studies indicate that intracranial hemorrhage, even in asymptomatic infants, is not rare. Pathologists’ (neuropathologists and forensic pathologists) appreciation of the spectrum of birth injuries and their sequelae is critical in order to be able to distinguish these from inflicted injuries and post‐mortem changes.
Keywords: Birth trauma, neuropathology, central nervous system
INTRODUCTION
What is encompassed by the terms “birth injury” or “neonatal/perinatal injury” depends on the perspective of the author. Injuries related to birth may include discussions ranging from hypoxic‐ischemic brain injury—because of placental pathology—to traumatic injuries of the delivery. For the purposes of this article “birth injury” will refer to the pathology of traumatic (mechanical) injury and not include pathology‐related to non‐traumatic (eg, hypoxic‐ischemic brain injury and infections). Albeit traumatic injuries of the central nervous system (CNS) often include hypoxic‐ischemic injury (HII) and will be briefly mentioned in this context.
Injury of the cranium, the CNS and its coverings has dramatically decreased in the last several decades primarily because of better prenatal diagnostic tools (eg, ultrasound). The rate of birth trauma dropped precipitously from 1970 to 1985 and now accounts for less than 2% of neonatal deaths. Despite this dramatic decrease in birth trauma mortality significant injuries still occur at a rate of 6–8 deaths per 1000 live births. Risk factors that predispose infants to birth injury include macrosomia, malpresentation, cephalopelvic disproportion and forceps or vacuum delivery (20). Further complicating our understanding of birth injury is a recent neuroradiology study that indicates intracranial hemorrhage in asymptomatic infants following a vaginal delivery is not rare (22). In infants, inflicted trauma is the leading cause of injury death; half of which occur by 4 months of age (30). Therefore, appreciation of the spectrum of birth injuries and their sequelae is critical for medicolegal autopsies of infants and young children. This article will take an outside‐to‐inside approach to review birth injury of the cranium and the CNS and will discuss post‐mortem changes.
INJURY OF THE CRANIAL COVERINGS
Traumatic injury of the cranial coverings results in hemorrhage into the scalp [(caput succedaneum (CS)], subgaleal region [subgaleal hemorrhage (SGH)] and underneath the periosteum attached to the external surface of the skull [cephalhematoma (CH)]. The pathology of the injury reflects the nature of the tissues involved.
CS
CS is a serosanguinous collection of fluid within the scalp of the presenting portion of the head, which is most commonly the vertex (Figure 1). It is caused by compression of the scalp, which may occur from pressure against the cervix, or uterus or vacuum extraction. CS occurs in 20–40% of vacuum extractions (2). Grossly, CS is characterized by pitting edema of the effected scalp, crosses suture lines and often crosses the midline. CS gradually resolves over several days without treatment.
Figure 1.
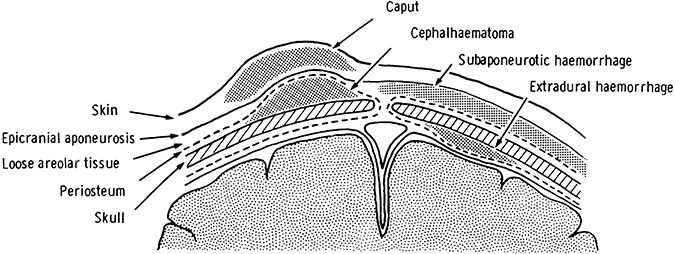
Diagram of sites of hemorrhage in the neonatal CNS coverings (reproduced from Pape KE, Wigglesworth JS (1979) Haemorrhage, Ischaemia and the Perinatal Brain. JB Lippincott: Philadelphia, with permission from JB Lippincott).
SGH
SGH is bleeding between the scalp galea aponeurosis and the periosteum (1, 2). The galea aponeurosis covers the vertex and the majority of the sides of the skull, and connects the frontalis and occipitalis muscles, which is collectively known as the epicranius (occipital frontalis). The continuity of the epicranius with the superficial fascia of the neck means subgaleal blood may track into the neck.
Figure 2.
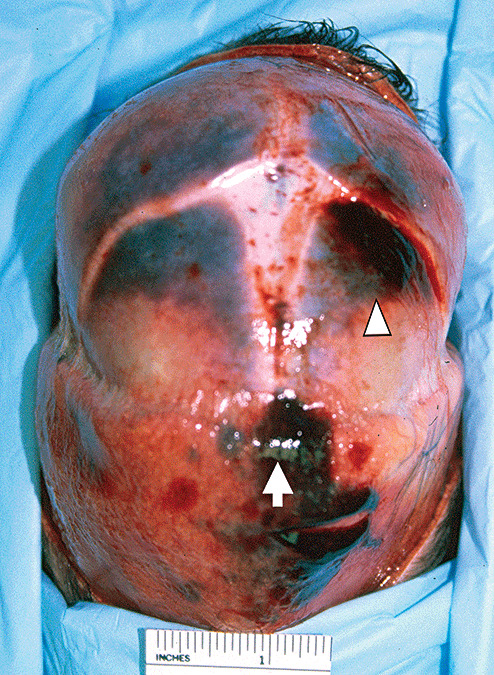
Acute subgaleal hemorrhage (arrow), acute cephalhematoma (arrowhead) of a 1‐day‐old infant (concealed pregnancy) delivered into a bathtub‐filled with water that was brought to the attention of authorities when the mother presented to a local Emergency Department because of postpartum bleeding.
The incidence of SGH is relatively rare, at 0.8/1000 of all deliveries. In vacuum‐assisted deliveries the incidence of SGH climbs to 6.4/1000 (29). SGH may not be clinically apparent immediately post‐partum, but develop over hours to days. Grossly, SGH is a firm mass that may continue to expand after birth. Because of blood loss it may result in hypovolemic shock and death. The neonate may also develop a coagulopathy or hyperbilirubinemia caused by blood loss from the SGH. Coagulopathies, such as vitamin K deficiency, can result in a delayed presentation. SGH may be a result of traumatic injury of soft tissue (emissary veins bridging the subgaleal space) or skull fracture (linear and diastatic fractures or fragmentation of the superior margin of the parietal bone). Blood loss can be significant enough to require transfusion. The reported mortality from severe SGH ranges from 2.7% to 22.8%. Kilani et al reported a mortality of 11.8% and good short‐term outcome in survivors (16).
CH
A CH is a well‐circumscribed subperiosteal collection of blood between the dense fibrous periosteal tissue covering the skull and the skull (Figure 1). It typically overlies one parietal bone and the cranial sutures limit its size (Figure 2). Rare posterior occipital midline CHs does occur, but are limited by the lamboid sutures. CH occurs in 1%–2% of live births and is more common with primiparous deliveries and in male infants. In forceps‐assisted deliveries the incidence of CH is up to 9.5%. In 10%–25% of CHs there is an associated linear skull fracture (15). Separation of the periosteum from the bone during delivery is another possible mechanism for CH development. Grossly, CHs may increase in size after birth, but in and of themselves are not associated with neurological symptoms. Rarely, they may result in significant blood loss and osteomyelitis 21, 27. Typically, CHs resolve in weeks to months, but may calcify and appear as bony protuberances of the skull (Figure 3). As the skull grows a calcified CH will diminish and/or disappear.
Figure 3.
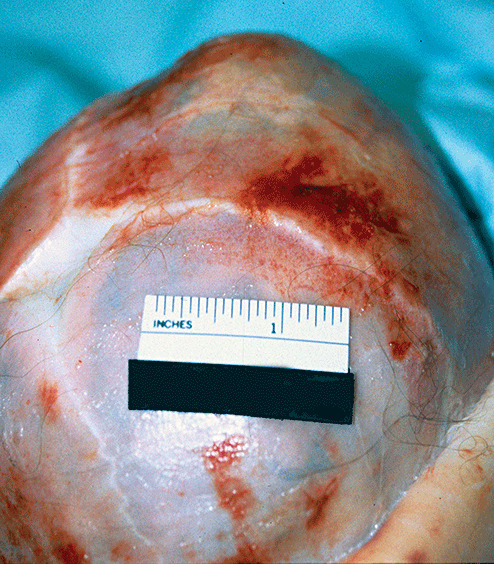
Healed cephalhematoma, 3‐month‐old infant delivered vaginally with postpartum diagnosis of cephalhematoma.
SKULL FRACTURES
The incidence of skull fractures has been reported to be 2.9% (14). Although skull fractures typically occur in the setting of a forceps delivery they can rarely occur in uncomplicated vaginal deliveries (12). When fractures do occur they are typically non‐displaced linear fractures of the parietal bone, cause no symptoms, and require no therapy (42). CH may be associated with a skull fracture; and leptomeningeal cysts may occur along the fracture line, but usually regress and only infrequently require surgical treatment 1, 5. Diastatic fractures (fractures along suture lines) have been described with instrument‐assisted deliveries and may result in “growing fractures,” which are fractures that expand with time 17, 26, 31. Calcification of birth‐associated fractures is visible on radiographs by 7 days of life. If healing is not apparent radiographically by 11 days of life then an injury after birth should be considered (4).
Depressed fractures
Depressed skull fractures are often referred to as “ping‐pong” fractures because on X‐ray they resemble an indented ping‐pong. Dupuis et al looked retrospectively at a series of 68 newborns with depressed skull fractures and found that 18 of the deliveries were not instrument‐associated fractures, and were thus classified as the so‐called spontaneous fractures. The fractures that were instrument‐associated were more likely to be associated with intracranial pathology, although long‐term sequelae were rare (6). Uncomplicated “ping‐pong” fractures may be monitored or treated with digital manipulation, breast pump, obstetric vacuum extractor or neurosurgical intervention (32).
Occipital osteodiastasis
Occipital osteodiastasis is the separation of the squamous and lateral parts of the occipital bone (Figure 4). The squamous occipital bone forms the posterior portion of the foramen magnum and is not fused with lateral occipital bone until about 2 years of age. Anterior displacement and upward rotation of the squamous portion of the occipital bone by suboccipital pressure, such as in a breech delivery, result in occipital osteodiastasis. Posterior cranial fossa subdural hemorrhage (SDH), contusion/laceration/compression of the cerebellum and medulla, tearing of the dura and damage of the sinuses are all reported complications of occipital osteodiastasis 3, 41. Visualization of occipital osteodiastasis at autopsy is greatly facilitated by a posterior approach to examination of the cervico–medullary junction and spinal cord.
Figure 4.
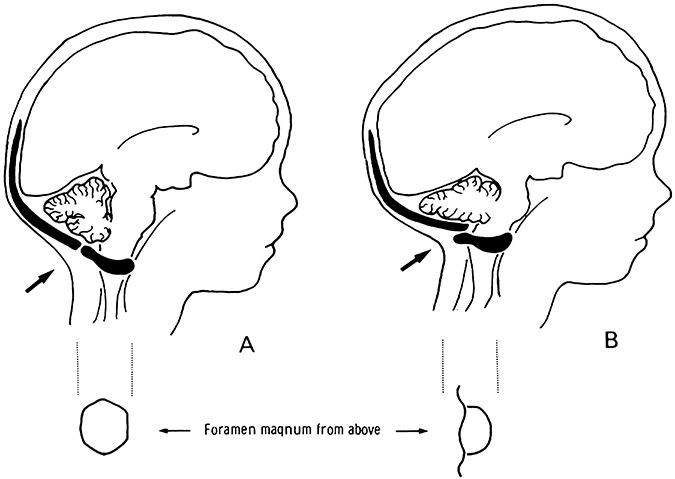
Diagram demonstrating occipital osteodiastasis. A. Normal unfused posterior cranial fossa bones of the neonate, B. Occipital osteodiastasis with compression of posterior cranial fossa structures (reproduced from Pape KE, Wigglesworth JS (1979) Haemorrhage, Ischaemia and the Perinatal Brain. JB Lippincott: Philadelphia, with permission from JB Lippincott).
INTRACRANIAL HEMORRHAGE
Epidural hemorrhage (EDH)
In neonates EDH accounts for 2% of intracranial hemorrhage (36). EDH in newborns is typically associated with an instrument‐assisted vaginal delivery in a nulliparous woman. As in adults, there may be a delay in the onset of symptoms (28). Treatment of the neonatal EDH is dependent on prompt recognition and typically requires surgical intervention (7).
Multiple different mechanisms of injury may result in neonatal EDH. A linear skull fracture accompanies neonatal EDH in the majority of cases (36) and may be associated with a CH (7). The classically described middle meningeal artery laceration from the temporal bone fracture does occur, but neonatal EDH may also be caused by a sinus or vein injury (36). The pediatric dura is tightly adhered to the skull, and the meningeal arteries are not embedded within the skull (36), which creates another potential mechanism for EDH. In newborns, EDH may result from the dura mater separating from the skull secondary to compression (molding of the head) during delivery—usually at a suture line—and, by this mechanism, does not typically have an associated skull fracture (9).
SDH and subarachnoid hemorrhage (SAH)
SDH is the most common intracranial bleeding associated with birth trauma 22, 33. In vaginally delivered normal infants, thin SDH—typically infratentorial—may be seen and not associated with any neurological symptoms 13, 22. Neonates may show symptoms caused by SDH ranging from subtle irritability to seizures and bradycardia (33). Large SDH has been described with forceps delivery of term infants (10). Other research indicates that the rate of SDH in forceps, vacuum‐assisted and cesarian section deliveries is comparable, however (39). Substantial posterior cranial fossa SDH can also occur as a result of laceration of the vein of Galen (24). The tentorium cerebelli may be torn and result in SDH as well. SAH in the neonate is most often associated with intraventricular hemorrhage and is predominately basilar because intraventricular blood passes through the foramina of Luschka and the foramen of Magendie into the subarachnoid space.
SPINE AND SPINAL CORD INJURY
The exact incidence of birth‐associated spinal cord injury is not known. Studies have suggested that spinal cord injury is a factor in up to 10% of neonatal deaths 37, 38. However, post‐mortem interpretation of subtle findings such as intraparenchymal hemorrhage may be difficult, particularly without correlation with clinical symptoms. For example, cervical epidural blood is a post‐mortem artifact that can be misinterpreted as an injury (34).
Upper cervical cord injury has been reported to occur twice as frequently as cervicothoracic injury (23). The type of spine and spinal cord injury is associated with specific factors of the type of delivery. For example, upper cervical cord injury is related to a cephalic presentation, and cervicothoracic cord injury is associated with breech deliveries (23). Hyperextension of the head during a vaginal delivery of a breech infant puts the cervicothoracic cord at risk for injury (35). In a forceps–cephalic delivery, hyperextension and 90° rotation of the head are the mechanisms of injury of the upper cervical cord (25).
Newborns with spinal cord injury may present as stillborns/rapid death, severe respiratory failure or neurological sequelae in the neonatal period. Infants that survive may be diagnosed as having “cerebral palsy” or be evaluated for neuromuscular disease or hypoxic‐ischemic brain injury. Of course, the specific neurological symptoms of an infant correlates with the level and extent of cord injury. Further complicating the clinical evaluation of the newborn may be concomitant peripheral nerve injury. Magnetic resonance imaging provides the best ante–mortem technique to resolve the issue of cord injury (19), particularly in the subacute to chronic phase of injury. Ultrasound and computed tomography may be the best modalities to evaluate the newborn in the acute setting. The management and outcome of the neonate will depend on the specific nature of the injury.
HII OF THE CNS
HII of the CNS may accompany birth injury and complicate both the clinical symptoms and neuropathological findings. HII can be thought of as either a focal or a global injury, which can be complicated by concomitant secondary changes. Infectious processes may be superimposed on HII or be the initiating process that leads to HII. A detailed discussion of HII is beyond the scope of this article, but reviews of global injury (eg, periventricular leukomalacia) and focal (eg, presumed perinatal strokes) are available 11, 18.
POST‐MORTEM CHANGES
Post‐mortem artifacts, autolysis and decompositional changes (putrefaction) may mimic ante–mortem injury. In medicolegal autopsies the separation of intrauterine maceration, ante–mortem injury and decomposition of a live birth is critical to determine cause and manner of death. Intrauterine death of an infant results in a relatively standardized sequence of changes because of predominately autolysis (maceration). Initially there is skin slippage, generalized red‐brown discoloration of tissues and accumulation of similar fluid within body cavities. As maceration progresses the cranial sutures may overlap and appear suggestive of ante–mortem pathology (8). Opening of the skull may reveal red‐brown fluid and other changes mimicking ante–mortem injury. The histology of markedly soft autolyzed brain tissue may be surprisingly preserved, however. In contradistinction, compression of the skull of a macerated fetus during delivery may force the soft CNS tissue along the spinal cord and spinal nerves, creating a retroperitoneal mass (the so‐called primitive neuroectodermal tumor) (Figure 5) (40). Eventually, the intrauterine retained fetus will become mummified and little, if any, CNS tissue can be evaluated. Post‐mortem changes of liveborn infants will follow classically described post‐mortem changes (eg, livor mortis, Tardieu spots, decomposition). The folds of the neck soft tissue in infants may create a linear furrow suggestion of a ligature mark. Both stillborn and initially live birth deliveries may be concealed by the mother. Distinction of post‐mortem artifacts from birth injury and ante–mortem injury may be challenging and critical to the death investigation, but may ultimately be difficult if not impossible to determine.
Figure 5.
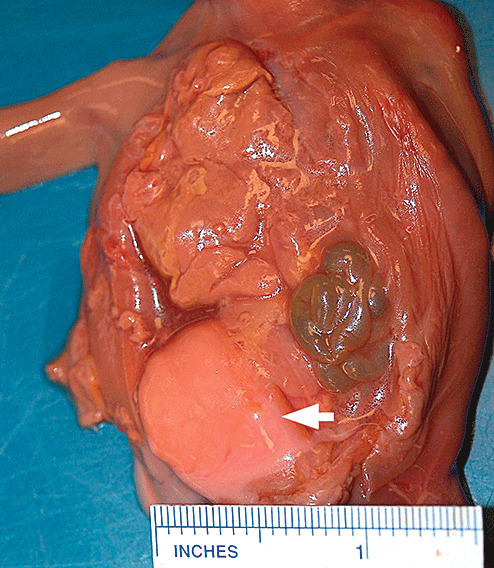
Photograph of macerated fetus with large retroperitoneal mass containing CNS tissue caused by post‐mortem changes (the so‐called primitive neuroectodermal tumor).
CONCLUSIONS
The exact prevalence of birth trauma of the CNS and its coverings is difficult to ascertain, but it has declined in recent decades. Technological improvements have likely been a major factor in the decreased incidence of birth trauma. Ironically, technological improvements have also helped recognize birth trauma that would not have previously been appreciated. It is essential to recognize the spectrum of possible birth injuries, its sequelae, and that autopsy findings may overlap with natural disease processes and other forms of traumatic injury.
REFERENCES
- 1. Bobinski L, Bostrom S, Zsigmond P, Theodorsson A (2007) Leptomeningeal cyst due to vacuum extraction delivery in a twin infant. Acta Neurochir (Wien) 149:319–323. [DOI] [PubMed] [Google Scholar]
- 2. Chenoy R, Johanson R (1992) A randomized prospective study comparing delivery with metal and silicone rubber vacuum extractor cups. Br J Obstet Gynaecol 99:360–363. [DOI] [PubMed] [Google Scholar]
- 3. Clement R, Bresson C, Marcorelles P, Rodat O, Lagarde N (2006) Cerebellar‐pulmonary embolism, cause of death in the newborn. J Clin Forensic Med 13:361–365. [DOI] [PubMed] [Google Scholar]
- 4. Cumming WA (1979) Neonatal skeletal fractures. Birth trauma or child abuse? J Can Assoc Radiol 30:30–33. [PubMed] [Google Scholar]
- 5. Djientcheu VD, Rilliet B, Delavelle J, Argyropoulo M, Gudinchet F, De Tribolet N (1996) Leptomeningeal cyst in newborns due to vacuum extraction: report of two cases. Childs Nerv Syst 12: 399–403. [DOI] [PubMed] [Google Scholar]
- 6. Dupuis O, Silveira R, Dupont C, Mottolese C, Kahn P, Dittmar A, Rudigoz RC (2005) Comparison of “instrument‐associated” and “spontaneous” obstetric depressed skull fractures in a cohort of 68 neonates. Am J Obstet Gynecol 192:165–170. [DOI] [PubMed] [Google Scholar]
- 7. Gama CH, Fenichel GM (1985) Epidural hematoma of the newborn due to birth trauma. Pediatr Neurol 1:52–53. [DOI] [PubMed] [Google Scholar]
- 8. Genest DR, Singer DB (1992) Estimating the time of death in stillborn fetuses: III. External fetal examination; a study of 86 stillborns. Obstet Gynecol 80:593–600. [PubMed] [Google Scholar]
- 9. Hamlat A, Heckly A, Adn M, Poulain P (2006) Pathophysiology of intracranial epidural haematoma following birth. Med Hypotheses 66:371–374. [DOI] [PubMed] [Google Scholar]
- 10. Hayashi T, Hashimoto T, Fukuda S, Ohshima Y, Moritaka K (1987) Neonatal subdural hematoma secondary to birth injury. Clinical analysis of 48 survivors. Childs Nerv Syst 3:23–29. [DOI] [PubMed] [Google Scholar]
- 11. Haynes RL, Baud O, Li J, Kinney HC, Volpe JJ, Folkerth DR (2005) Oxidative and nitrative injury in periventricular leukomalacia: a review. Brain Pathol 15:225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Heise RH, Srivatsa PJ, Karsell PR (1996) Spontaneous intrauterine linear skull fracture: a rare complication of spontaneous vaginal delivery. Obstet Gynecol 87:851–854. [PubMed] [Google Scholar]
- 13. Holden KR, Titus MO, Van Tassel P (1999) Cranial magnetic resonance imaging examination of normal term neonates: a pilot study. J Child Neurol 14:708–710. [DOI] [PubMed] [Google Scholar]
- 14. Hughes CA, Harley EH, Milmoe G, Bala R, Martorella A (1999) Birth trauma in the head and neck. Arch Otolaryngol Head Neck Surg 125:193–199. [DOI] [PubMed] [Google Scholar]
- 15. Kendall N, Woloshin H (1952) Cephalhematoma associated with fracture of the skull. J Pediatr 41:125–132. [DOI] [PubMed] [Google Scholar]
- 16. Kilani RA, Wetmore J (2006) Neonatal subgaleal hematoma: presentation and outcome–radiological findings and factors associated with mortality. Am J Perinatol 23:41–48. [DOI] [PubMed] [Google Scholar]
- 17. Kingsley D, Till K, Hoare R (1978) Growing fractures of the skull. J Neurol Neurosurg Psychiatry 41:312–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kirton A, Deveber G, Pontigon AM, Macgregor D, Shroff M (2008) Presumed perinatal ischemic stroke: vascular classification predicts outcomes. Ann Neurol 63:436–443. [DOI] [PubMed] [Google Scholar]
- 19. Lanska MJ, Roessmann U, Wiznitzer M (1990) Magnetic resonance imaging in cervical cord birth injury. Pediatrics 85:760–764. [PubMed] [Google Scholar]
- 20. Laroia N (2008) Birth trauma. Available at: http://www.emedicine.com/ped/topic2836.htm (accessed June 10, 2008).
- 21. Leonard S, Anthony B (1961) Giant cephalhematoma of newborn with hemorrhagic disease and hyperbilirubinemia. Am J Dis Child 101:170–173. [DOI] [PubMed] [Google Scholar]
- 22. Looney CB, Smith JK, Merck LH, Wolfe HM, Chescheir NC, Hamer RM, Gilmore JH (2007) Intracranial hemorrhage in asymptomatic neonates: prevalence on MR images and relationship to obstetric and neonatal risk factors. Radiology 242:535–541. [DOI] [PubMed] [Google Scholar]
- 23. MacKinnon JA, Perlman M, Kirpalani H, Rehan V, Sauve R, Kovacs L (1993) Spinal cord injury at birth: diagnostic and prognostic data in twenty‐two patients. J Pediatr 122:431–437. [DOI] [PubMed] [Google Scholar]
- 24. Menezes AH, Smith DE, Bell WE (1983) Posterior fossa hemorrhage in the term neonate. Neurosurgery 13:452–456. [DOI] [PubMed] [Google Scholar]
- 25. Menticoglou SM, Perlman M, Manning FA (1995) High cervical spinal cord injury in neonates delivered with forceps: report of 15 cases. Obstet Gynecol 86:589–594. [DOI] [PubMed] [Google Scholar]
- 26. Miranda P, Vila M, Alvarez‐Garijo JA, Perez‐Nunez A (2007) Birth trauma and development of growing fracture after coronal suture disruption. Childs Nerv Syst 23:355–358. [DOI] [PubMed] [Google Scholar]
- 27. Mohon RT, Mehalic TF, Grimes CK, Philip AG (1986) Infected cephalhematoma and neonatal osteomyelitis of the skull. Pediatr Infect Dis 5:253–256. [DOI] [PubMed] [Google Scholar]
- 28. Negishi H, Lee Y, Itoh K, Suzuki J, Nishino M, Takada S, Yamasaki S (1989) Nonsurgical management of epidural hematoma in neonates. Pediatr Neurol 5:253–256. [DOI] [PubMed] [Google Scholar]
- 29. Ng PC, Siu YK, Lewindon PJ (1995) Subaponeurotic haemorrhage in the 1990s: a 3‐year surveillance. Acta Paediatr 84:1065–1069. [DOI] [PubMed] [Google Scholar]
- 30. Overpeck MD, Brenner RA, Trumble AC, Trifiletti LB, Berendes HW (1998) Risk factors for infant homicide in the United States. N Engl J Med 339:1211–1216. [DOI] [PubMed] [Google Scholar]
- 31. Papaefthymiou G, Oberbauer R, Pendl G (1996) Craniocerebral birth trauma caused by vacuum extraction: a case of growing skull fracture as a perinatal complication. Childs Nerv Syst 12:117–120. [DOI] [PubMed] [Google Scholar]
- 32. Pollak L, Raziel A, Ariely S, Schiffer J (1999) Revival of non‐surgical management of neonatal depressed skull fractures. J Paediatr Child Health 35:96–97. [DOI] [PubMed] [Google Scholar]
- 33. Pollina J, Dias MS, Li V, Kachurek D, Arbesman M (2001) Cranial birth injuries in term newborn infants. Pediatr Neurosurg 35:113–119. [DOI] [PubMed] [Google Scholar]
- 34. Rutty GN, Squier WM, Padfield CJ (2005) Epidural haemorrhage of the cervical spinal cord: a post‐mortem artefact? Neuropathol Appl Neurobiol 31:247–257. [DOI] [PubMed] [Google Scholar]
- 35. Svenningsen NW, Westgren M, Ingemarsson I (1985) Modern strategy for the term breech delivery—a study with a 4‐year follow‐up of the infants. J Perinat Med 13:117–126. [DOI] [PubMed] [Google Scholar]
- 36. Takagi T, Nagai R, Wakabayashi S, Mizawa I, Hayashi K (1978) Extradural hemorrhage in the newborn as a result of birth trauma. Childs Brain 4:306–318. [DOI] [PubMed] [Google Scholar]
- 37. Towbin A (1964) Spinal cord and brain stem injury at birth. Arch Pathol 77:620–632. [PubMed] [Google Scholar]
- 38. Towbin A (1969) Latent spinal cord and brain stem injury in newborn infants. Dev Med Child Neurol 11:54–68. [DOI] [PubMed] [Google Scholar]
- 39. Towner D, Castro MA, Eby‐Wilkens E, Gilbert WM (1999) Effect of mode of delivery in nulliparous women on neonatal intracranial injury. N Engl J Med 341:1709–1714. [DOI] [PubMed] [Google Scholar]
- 40. Van Noort G, De La Fuente AA (1988) So‐called primitive neuroectodermal tumor in macerated fetuses: a confusing artifact. Pediatr Pathol 8:359–365. [DOI] [PubMed] [Google Scholar]
- 41. Wigglesworth JS, Husemeyer RP (1977) Intracranial birth trauma in vaginal breech delivery: the continued importance of injury to the occipital bone. Br J Obstet Gynaecol 84:684–691. [DOI] [PubMed] [Google Scholar]
- 42. Zelson C, Lee SJ, Pearl M (1974) The incidence of skull fractures underlying cephalhematomas in newborn infants. J Pediatr 85:371–373. [DOI] [PubMed] [Google Scholar]


