Abstract
This historical review describes the evolution of the pathogenetic concepts associated with infection by the Human Immunodeficiency Virus (HIV), with emphasis on the pathology of the nervous system. Although the first descriptions of damage to the nervous system in the acquired immunodeficiency syndrome (AIDS) only appeared in 1982, the dramatic diffusion of the epidemic worldwide and the invariably rapidly fatal outcome of the disease, before the introduction of efficient treatment, generated from the beginning an enormous amount of research with rethinking on a number of pathogenetic concepts. Less than 25 years after the first autopsy series of AIDS patients were published and the virus responsible for AIDS was identified, satisfactory definition and classification of a number of neuropathological complications of HIV infection have been established, leading to accurate clinical radiological and biological diagnosis of the main neurological complications of the disease, which remain a major cause of disability and death in AIDS patients. Clinical and experimental studies have provided essential insight into the pathogenesis of CNS lesions and natural history of the disease. The relatively recent introduction of highly active antiretroviral therapy (HAART) in 1995–1996 has dramatically improved the course and prognosis of HIV disease. However, there remain a number of unsolved pathogenetic issues, the most puzzling of which remains the precise mechanism of neuronal damage underlying the specific HIV‐related cognitive disorders (HIV dementia). In addition, although HAART has changed the course of neurological complications of HIV infection, new issues have emerged such as the lack of improvement or even paradoxical deterioration of the neurological status in treated patients. Interpretation of these latter data remains largely speculative partly because of the small number of neuropathological studies related to the beneficial consequence of this treatment.
INTRODUCTION
Compared with other neurological diseases, an appreciation of involvement of the central nervous system (CNS) in the acquired immunodeficiency syndrome (AIDS) is a relatively recent event; however, because of the dramatic worldwide spread of the epidemic involving over 30 million individuals (107, 115, 126) and its dire consequences, it has generated an enormous amount of clinical and experimental research by physicians, radiologists, pathologists, virologists, immunologists and molecular biologists.
In the early eighties the medical community, initially in the USA, became alarmed by a new “epidemics” of Kaposi sarcomas and unusual opportunistic infections in the gay community and Haitians (10). A new disorder, referred to as “acquired immunodeficiency syndrome” (AIDS) with T‐cell depletion was identified; its viral origin was rapidly suspected and confirmed by the identification of a specific virus. Originally known as “lymphadenopathy associated virus” (LAV) (15) or “human T cell lymphotropic virus type III” (116), by consensus it was finally named “human immunodeficiency virus” (HIV).
Involvement of the nervous system was quickly recognized as a major cause of disability and death in AIDS patients (140). Because the disease was invariably fatal, the spectrum of HIV neuropathology was established at postmortem (62, 127). Concomitantly, a large number of experimental studies contributed to a better understanding of the pathogenesis of cerebral damage. The natural history of CNS involvement in HIV‐infected patients, particularly in the early stages of the disease, was eventually established.
However, there remained a number of unsolved issues; these include the stage of the infection at which HIV enters the central nervous system and the precise mechanism of neuronal damage underlying the specific HIV‐related cognitive disorders (HIV dementia); in addition, complications have emerged recently, following the introduction of therapeutic drugs.
In this review we will give an account of the following topics: (i) the virus, (ii) the definition and classification of the multiple neuropathological complications of HIV infection, (iii) the pathogenesis of the CNS changes and the mechanism of neuronal damage, (iv) the natural history of HIV infection of the CNS with particular emphasis to the changes in the early stages of the infection and the time of arrival of HIV into the CNS, (v) the physio‐pathological basis of HIV dementia, and (vi) the changing pattern of CNS lesions in patients receiving highly active antiretroviral treatment (HAART).
THE HUMAN IMMUNODEFICIENCY VIRUS (HIV)
A relationship between the disease and the virus was confirmed by the discovery of the human immune deficiency virus—HIV‐1 (15, 50) in these patients. A few years later, a second virus, HIV‐2, was found in West Africa. Both HIVs are RNA viruses and belong to the lentivirus group of the retrovirus family (156). HIV is a non‐transforming virus (ie, non‐oncogenic), which replicates through the generation of a proviral DNA intermediate by the action of a retroviral enzyme RNA‐directed DNA polymerase (reverse transcriptase). In vivo, a persistent infection is established following integration of proviral DNA into the host genome.
In parallel with the emergence of the AIDS epidemic, it became apparent, from 1982 to 1983, that a similar range of diseases affected macaques in primate centers in the USA and elsewhere (33). Indeed, serologic and molecular biological investigations gave strong support to the view that these viruses had first been acquired, about a century ago, from non‐human primates (51, 104, 157).
The biology of the virus has been described extensively and only some details, relevant to the scope of this paper will be mentioned here. HIV‐1 consists of two single‐stranded copies of RNA, non‐covalently linked to the core proteins, closely associated with the RNA‐directed DNA‐reverse transcriptase. The viral envelope contains a virally encoded trans‐membrane glycoprotein (gp41), which non‐covalently anchors the extravirion spike glycoprotein gp‐120. The HIV genome contains three principal structural genes, gag, pol and env, and the non‐structural genes tat, nef and rev (154). To enter cells, retroviruses must bind to a specific cellular receptor located on the surface of CD4 cells (32) and have been shown to productively infect T‐lymphocytes in vitro (83) as well as cells of the monocyte/macrophage lineage (97, 150). Subsequent studies have revealed that co‐receptors are involved in facilitating the infection. These include the chemokine receptors CXCR4 (47) and CCR5 (38). Presence of HIV within a cell does not necessarily evolve to its replication and formation of new virus (102); indeed, the HIV can take one of the following status within infected cells:
-
(i)
productive infection;
-
(ii)
latent infection, a situation in which the DNA provirus is blocked at the stage of transcription;
-
(iii)
non‐productive (or restricted) infection, in which the unintegrated or integrated viral DNA does not result in viral production.
HIV evolves continuously in vivo and this property has important implications with regard to the replication of the virus (41) and its cytopathic effects (41).
Although HIV infection appeared initially within the gay community, it soon spread to other groups, including intravenous drug users, patients receiving blood transfusion and organ transplants, women undergoing artificial insemination and children born to HIV‐positive parents or breast fed by seropositive women. With regard to the heterosexual route of infection, although it was well known from the outset, lately it has become the predominant form of infection, particularly in sub‐Saharan regions.
HIV AND THE NERVOUS SYSTEM: DEFINITION AND CLASSIFICATION OF THE MULTIPLE NEUROPATHOLOGICAL COMPLICATIONS OF HIV INFECTION
The severe deficit of cell‐mediated immunity makes AIDS patients prone to infections of virtually any organ in the body by a wide range of opportunistic organisms which include viruses, bacteria, fungi and protozoa. In addition, patients may develop, often in fulminant form, previously uncommon or unknown tumors, the most common of which are non‐Hodgkin’s lymphomas and Kaposi’s sarcoma (67).
With regard to the nervous system, an early paper (140) conveyed the impression that opportunistic organisms were the cause of all complications, including a subacute form of encephalitis, characterized by “subtle cognitive changes accompanied by malaise, lethargy . . .”.
Of the variety of organisms found in the nervous system, some were more common than others. Among the viruses, cytomegalovirus was present in between 10% (86) and 30% (101). An early report of progressive multifocal leukoencephalopathy (PML) in AIDS (111) was followed by a flurry of publications highlighting the increasing incidence of this previously rare viral encephalitis, because of a papovavirus: between 2% (111) and 5% (24, 153), or even 7% (86). Cryptococcosis represents the commonest mycosis in AIDS; it presents as meningitis in virtually all 27 patients reported by Kovacs et al (85). In large series, it was identified in 3%–8% of the cases (111, 153). A relatively uncommon disease before the advent of AIDS (74), toxoplasmosis became the commonest cause of intracerebral mass lesion, with a larger incidence among European (63, 69, 86) than American patients (90, 93, 111).
Lymphomas, either primary or secondary, are common in AIDS; they are usually high grade, non‐Hodgkin and appear as multiple masses, partly necrotic, particularly in the deep gray nuclei. Histologically they consist of B‐cell immunoblasts. A relationship with viruses, already found in patients undergoing transplants (66), was confirmed in AIDS patients, all of whom proved serologically positive to Epstein–Barr virus (46), including those with the cerebral form of the neoplasm (52).
The existence of a specific infection of the CNS by HIV became apparent only later and was confirmed by viral detection within the nervous tissue (133), together with the finding of multinucleated giant cells (MGC) (129) (Figure 1A). These cells, considered unique to AIDS and a hallmark of the syndrome (23), result from virus‐induced fusion of infected macrophages, thus reflecting both productive infection and a pathogenic role for the virus. MGC can be seen also in the gray matter, particularly in the deep gray nuclei and, together with microglia (Figure 1B), are seen to contain HIV protein (Figure 1C). “HIV‐induced” neuropathological changes were observed only in patients infected by the virus in the absence of any other cause. Further observations revealed three types of changes that may be variably associated:
Figure 1.
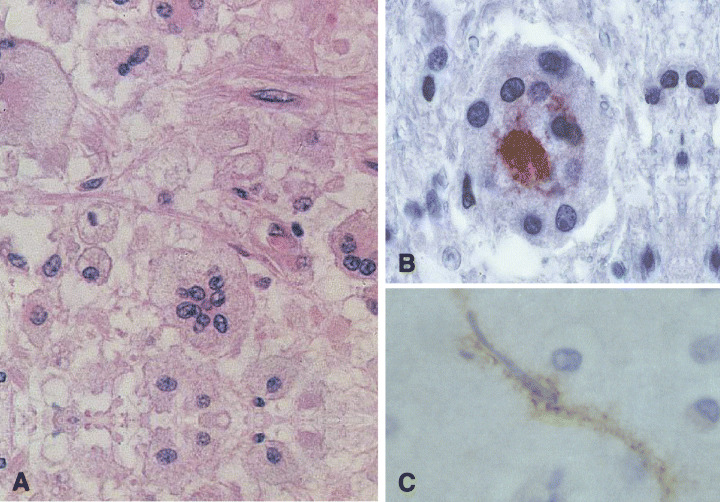
A. Multinucleated giant cells (MGC) in the cerebral white matter of a patient with HIV encephalitis. Note the centrally aggregated nuclei and the pale outer cytoplasm (hematoxylin and eosin [H&E]). B and C. In HIV‐encephalitis the cytoplasm of a number of MGC (B) and microglial cells (C) contains HIV. This can be revealed by a specific antibody (p24).
-
(i)
HIV encephalitis (HIVE) (Figure 2A) is characterized by disseminated foci or more diffuse lesions including myelin loss, reactive astrocytosis and microglial activation with microglial nodules, macrophages and MGC. The viral load is high.
-
(ii)
HIV leukoencephalopathy (Figure 2B) shows diffuse myelin pallor, particularly severe in the centrum semiovale. It is frequently associated with HIVE. Both types of lesion were regarded by some authors as extremes of a spectrum of HIV‐induced pathology which may overlap in one‐third of cases (26). Abnormality of the brain microvasculature, seen in diffuse leukoencephalopathy (122, 138), leads to damage to the brain‐blood‐barrier (BBB), which is responsible for the diffuse myelin pallor (117). This was recently confirmed by evidence of loss of tight junction protein, zonula occludens‐1, in patients with HIV dementia (19). Widespread axonal damage identified by immunocytochemistry of beta‐amyloid protein precursor (Figure 3A) was also shown to be extremely frequent in AIDS (55).
-
(iii)
Involvement of the gray matter, although less obvious than the changes in the white matter, is also frequent in AIDS. “Diffuse poliodystrophy” (Figure 2C) characterized by diffuse reactive astrocytosis, and microglial activation in the cerebral gray matter was observed in about half of AIDS patients (24, 29); in many cases its severity is correlated with that of the leukoencephalopathy. Neuronal loss, suspected at microscopy, was confirmed by several morphometric studies (45). Later, neuronal damage in AIDS was correlated, at least in part, with apoptosis (4, 113, 135) (Figure 3B).
Figure 2.
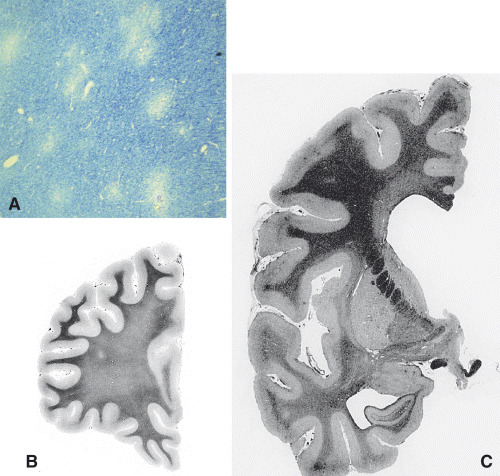
In HIV encephalitis (A) white matter shows the characteristic moth‐eaten appearance of the myelin. The multiple small foci of myelin loss are surrounded by normal‐looking white matter (luxol fast blue/Nissl’stain [LFB/N]). This pattern contrasts with the diffuse pallor of the centrum semiovale in HIV leukoencephalopathy (B). The less common form of HIV‐related poliodystrophy (C) shows brains diffusely reduced in size with wider sulci and enlarged ventricles. (B) and (C) are stained with Loyez method.
Figure 3.
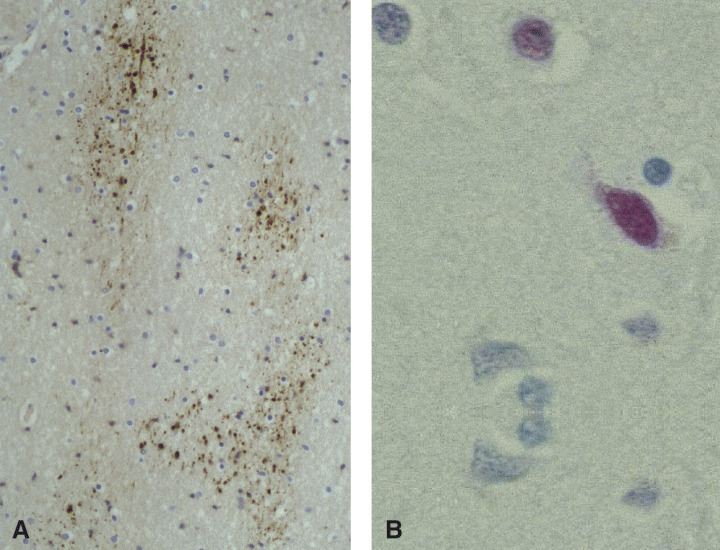
A. In a number of cases of HIV encephalitis and leukoencephalopathy, β‐amyloid precursor protein antibody reveals diffuse axonal damage. B. The TUNEL method shows DNA damage in the nerve cells. This can eventually lead to neuronal loss via apoptosis.
Unfortunately, it soon became apparent that HIV infection was not exclusive to adults, but that it could also affect children born to HIV‐positive parents. Of the three million children infected, 50% have already died of AIDS. Sub‐Saharan Africa is at present the area with most cases, approximately 1000 new cases every day. In fact, neurological complications in children are more common than in adults (39, 128). A number of them display delayed or arrested development, others develop microcephaly and progressive ataxia and cortico‐spinal tract signs. Pathological changes (131) include vascular mineralization in 92%, white matter changes (78%) with inflammatory infiltrates (75%), MGC (36%) and vascular inflammation (30%). More recently, Gelbard et al (54) confirmed the presence of neuronal apoptosis, already seen in adults. On the other hand, the lower incidence of opportunistic infections (14%) supports the view that most of these correspond to a reactivation of a previously latent infection.
Whereas the natural history of HIV infection is on the whole similar in all the various risk groups (see above), some differences were nevertheless observed: with regard to the haemophiliacs, Esiri et al (40) observed low prevalence of opportunistic infections and high rate of death from intracranial haemorrhage and liver cirrhosis. Similarly, the incidence of opportunistic infections is lower in drug users than in homosexuals, despite similar CD4 counts (17).
There exist, however, clinicopathologic conditions in HIV infection, for which no plausible pathogenesis has been provided. One of these is vacuolar myelopathy (VM). First described by Snider (140) and Goldstick et al (57), VM appears late in the course of the infection and represents the single most common spinal disorder in AIDS, with range from 1% (24, 79) to 55% (11). Rare in children (130), it presents with leg weakness, spastic paraparesis, sensory ataxia and incontinence. Changes (112) include intramyelinic and periaxonal vacuoles, some of which contain macrophages, within the lateral, posterior and, less prominently, anterior columns of the cord, though not limited to any specific tract. The brunt of the pathology was described as occurring at various levels; however, in a clinical and pathological study, it was noted (143) to start in the mid‐low thoracic cord with rostral and caudal spreading with increasing severity.
With regard to the pathogenesis of VM, its association with HIVE, the sporadic findings of MGC closely related to vacuoles (25), of MGCs and HIV antigens and HIV particles in the lesions (94) suggest a direct role by HIV. On the other hand, VM was found in non‐AIDS immunocompromised patients (79); although the similarities with subacute combined degeneration (SCD) suggested a possible vitamin B12 deficiency, this was not substantiated; it may, however, be possible that the requirements for methyl groups for membrane and myelin repair may lead to a secondary methyl group deficiency (142) and thus predispose the cord to a pattern of degeneration resembling SCD. Another possibility is that persistent immune activation in the CNS may lead to local production of myelin or membrane damaging cytokines, such as TNF‐α (147), toxins or oxygen radicals. Degenerating myelin sheaths may attract scavenging macrophages and those expressing gp‐120 may further cause a functional disorder of oligodendrocytes and thus could underlie the diffuse myelin loss seen in HIV encephalopathy (81). Such macrophages may be activated to secrete cytokines, or attempt to phagocytose myelin.
A further complication of HIV infection, albeit not exclusive of it, is HIV vasculitis. Lymphocytic (100) or granulomatous (161) infiltration of the walls of cerebral vessels with or without accompanying necrosis was described in rare cases sometimes associated with meningitis (121). The exact relationship between the vasculitis and HIV infection remains, to date, obscure but in a case, previously described as necrotizing vasculitis (152), ISH secondarily demonstrated Varicella zoster virus genome in the vessel wall (28).
PATHOGENESIS OF CNS CHANGES IN HIV INFECTION
Three issues appear to have been in the limelight for a number of years: (i) the type(s) of cell harboring HIV, (ii) the pathogenesis of neuronal damage and (iii) the time of arrival of HIV into the CNS.
Type of cell harboring HIV.
Microglial cells and macrophages have been known for some time to be the target for HIV (97, 150) and indeed several studies suggest the role of “Trojan horse” for HIV‐infected monocytes in the dissemination of infectious particles. Over the years there has been controversy as to whether neuroectodermal cells, and in particular neurons, plays a similar role. Moreover, various groups have found evidence of HIV infection in astrocytes (9, 120, 145, 157) and oligodendrocytes (9). Moreover, whereas all HIV‐positive demented patients show demonstrable HIV‐DNA and RNA in glial cells, it was found only in few non‐demented (9). With regard to cerebral endothelial cells, these also appear to be infected (9, 157). Subsequent work (14) showed that HIV envelope proteins induce uptake of HIV by brain endothelial cells of rodents; however, direct infection is not essential for endothelial cell loss, which may take place, with the mechanism of apoptosis, through exogenous HIV proteins (1). As for nerve cells, HIV‐1‐DNA was detected by Nuovo et al (108) and Bagasra et al (13), but not by others (9, 132). Despite the evidence that a number of cell types within the CNS appear to be infected, and that glial cells, in particular astrocytes, may represent a reservoir for HIV (120), it appears that in all these cell types HIV manages to establish a persistent, rather than productive, infection and that only in microglial cells and monocytes, which remain the undisputed reservoir and source of transmission of the infection in the CNS, HIV establishes a productive infection (18).
Pathogenesis of neuronal damage.
As productive HIV infection within nerve cells has never been conclusively shown, an indirect mechanism of neuronal damage, representing the basis of the cognitive disorder (see below), is considered more likely. Both virus‐ and glial cell‐derived proteins may contribute to neuronal damage (106). The following neurotoxic factors have been incriminated, which may act in combination (80): viral proteins, particularly gp120, gp41, tat, nef, or substances produced by activated glial and microglial cells such as cytokines, prostaglandins, proteases, arachidonic acid or quinolinic acid metabolites (45). In particular, as viral proteins, such as tat, can also be transported along neural pathways, they can cause neurotoxic damage at remote sites (22). A glutamate‐mediated excitotoxicity has been supported by a number of in vitro studies (37, 68, 92), as increased glutamate levels are found in the cerebrospinal fluid (CSF) and plasma of patients with HIV‐related dementia (48). Increased levels of quinolinic acid, a glutamate agonist, are present in the CSF of AIDS patients with cognitive and motor abnormalities (70). In addition, in HIV infection, activated microglial cells may express the high affinity glutamate transporter “excito amino acid transporter‐1”, while its normal expression by astrocytes is inhibited (58, 148). The involvement of the BBB has been mentioned above; the issue is reviewed by Petito and Cask (110).
Although each of these toxins can damage nerve cells, it has been shown that a combination of cellular and viral factors induces a more severe degree of neuronal apoptosis that each of them in isolation (160). This has led to the suggestion that neuronal apoptosis and consequent neuronal loss in HIV‐infected patients may be multifactorial (3). The mechanism of the final irreversible stage of neuronal DNA damage and eventual apoptosis appears to involve oxidative stress and glutamate‐receptor‐mediated toxicity (80). In vitro studies have suggested that combined HIV protein and cytokine neurotoxicity may be mediated by oxidative stress (2) and cause neuronal apoptosis (136). It has been shown in vivo that peroxinitrite activity resulting from the simultaneous production of super oxide anion and nitric oxide is significantly increased in demented, compared with non‐demented, AIDS patients (16). Finally, Nath and Geiger (105) have suggested that the continuous presence of HIV proteins may not be necessary for nerve cell damage, as neurotoxic proteins can initiate a cascade of events that self‐perpetuate in a positive feedback manner. The topic is reviewed and discussed by Li et al (91).
Time of arrival of HIV into the CNS.
This issue has wider than a mere academic relevance; indeed, a late arrival of the virus into the CNS would allow early treatment to prevent infection entering the CNS, thus potentially avoiding late complications such as HIV encephalitis and dementia.
Unfortunately, early data seemed to support the hypothesis that infection of the nervous system takes place at an early stage: the appearance of meningitis (71), encephalopathy (27) or myelopathy (36) during seroconversion; intrathecal synthesis of HIV‐1 antibody (118); the recovery of HIV in the CSF (71) as well as from the brain tissue of one patient accidentally injected with HIV, who died 15 days later (34).
These findings initiated a neuropathologic investigation of the brain of asymptomatic HIV‐positive individuals for evidence of HIV; in keeping with the hypothesis of an early entry of the virus, Gray et al (65) described lymphocytic meningitis (Figure 4A), perivascular mononuclear infiltrates, discrete myelin pallor, astrocytic gliosis and microgliosis. Additional findings were discrete neuronal loss via apoptosis (7) and diffuse axonal damage (8). Following the detection of HIV‐DNA in the brain tissue of asymptomatic individuals (6), An et al revealed a condition of immune activation of the cerebral tissue (Figure 4B), with presence of toxic cytokines (Figure 4C) (5). Finally, the same group (9) found that, at this stage, astrocytes and endothelial cells, in addition to microglia (Figure 4D), are infected. However, although HIV proviral DNA has been demonstrated in a number of brains, viral replication remains very low during this stage.
Figure 4.
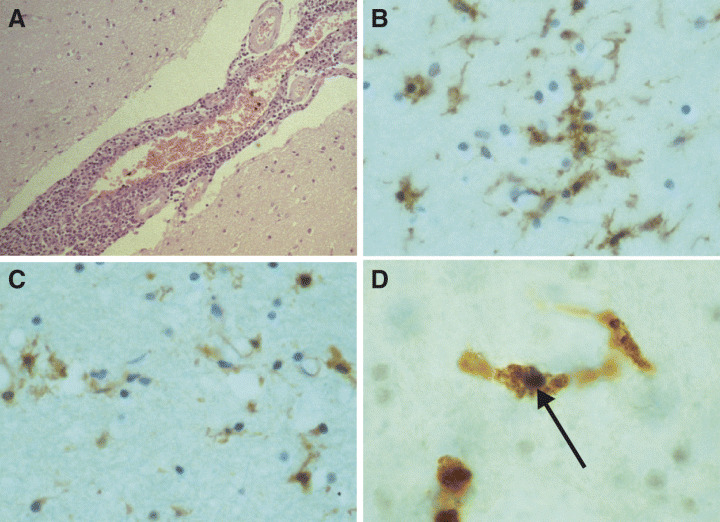
Lymphocytic meningitis may be detected in the brain of asymptomatic HIV‐positive patients who die from HIV‐unrelated causes (H&E) (A). Anti‐TN3 antibody highlights the conditions of immune activation (B) that may be the cause of the presence of increased amounts of cytokines (anti‐TNF‐α antibody) (C). In situ‐PCR allows detection of HIV‐1‐DNA within the nucleus (arrow) of a CD‐68 positive cell (D).
THE PATHOGENESIS OF HIV DEMENTIA
About 20% of patients with AIDS develop a specific progressive cognitive/motor syndrome “HIV dementia” or AIDS‐dementia complex (ADC), of which HIV‐encephalitis was initially considered the pathological counterpart. Some groups still support this correlation (158), despite the fact that other teams were unable to confirm it (3, 21, 64, 103), even when morphometry (56) and polymerase chain reaction (78, 88) were applied.
Other pathogenetic possibilities for the dementia were explored, including microglial activation (56). Indeed, this finding was successfully correlated by a number of observations (3, 9, 137); however, microglial activation remains a nonspecific finding which is not restricted to demented patients.
The role of abnormal astrocytic function was also taken into account, in the form either of an increased expression of adhesion molecules (125), enhanced astrocyte activation (53) or loss of astrocytic function (120, 145, 149). A convincing correlation could not be established also between dementia and leukoencephalopathy (3, 56) and equally disappointing was the attempt to blame axonal damage, despite its consistent association with HIV encephalitis (55). When poliodystrophy was considered as the possible culprit for dementia, expectations were dashed not only by the lack of correlation (43, 155), but also by the absence of cortical neuronal loss in a group of patients with cognitive disorders (124). The obvious conclusion of these investigations could be that a sizeable neuronal loss which could induce dementia is only a late event.
As the various disorders associated with organic dementia have specific localization for the cell loss, studies were carried out in the attempt at localizing the cell loss also in HIV dementia. However, whereas Everall et al (42) found cell loss in the putamen, Bell et al (17) detected productive infection preferentially in the frontal cortex and Petito et al (114) reported reactive gliosis in the hippocampus.
The lack of an exact correlation between the cognitive disorders and the different HIV‐induced changes together with recent reports of improvement of the cognitive disorders after HAART, suggest that HIV dementia (HIVD) might be the expression of a specific neuronal dysfunction resulting from the combined effects of several HIV‐related neurotoxic factors (cf. Pathogenesis of NS changes in HIV infection), involving different aetiopathogenetic mechanisms, some of which may be reversible.
If one accepts that HIVD reflects a specific neuronal dysfunction resulting from the combined action of HIV protein, glial and microglial activation, perhaps mediated by oxidative stress and glutamate‐mediated excitotoxicity (cf. Pathogenesis of NS changes in HIV infection), its relationship with the different HIV‐induced neuropathological changes is easier to understand (59). Indeed, these changes may result from the same mechanisms: HIVE reflects productive HIV infection, HIV leukoencephalopathy is secondary to an alteration of the BBB resulting either from the effect of circulating factors or factors locally produced by activated macrophages. The same applies for axonal damage. Finally, neuronal apoptosis and consequent DPD may result from the neurotoxicity of these combined factors, or from axonal damage through retrograde degeneration. It is also possible that deafferentation of neurons may induce apoptosis in nerve cells. This hypothesis is in keeping with the description of synaptic and dendritic simplification in the brains of AIDS patients with severe HIVD (96), and in those with mild to moderate neurocognitive disorders (44). The importance of the different factors may vary from one patient to another resulting in different histological features. This may explain why, although these lesions are more frequent in patients with HIVD, none can be strictly correlated to the cognitive impairment.
MODIFICATION OF CNS COMPLICATIONS OF AIDS FOLLOWING THE INTRODUCTION OF HAART
The introduction of antiretroviral treatment has produced two types of effects: on the one hand it has reduced both the morbidity and the incidence of several disorders, including those involving the nervous system; on the other it has modified the pathological pattern as it was originally known.
With regard to the pharmacological treatment, the history of neuro‐AIDS can be divided into four stages:
-
(i)
An early period to 1986, during which no specific treatment was available.
-
(ii)
Following the introduction of the first nucleoside reverse‐transcriptase inhibitor (NRTI), zidovudine, a second phase (1986–1992) was characterized by a decrease of the number of patients suffering from HIV‐related dementia.
-
(iii)
The third stage (to 1996) can be defined by the introduction of newer NRTIs, which led to the suppression of HIV replication in the CSF.
-
(iv)
The latest form of treatment, HAART, consists of the administration of 3 drugs, including NRTIs, non‐NRTI and protease inhibitors, following strict rules. More recently, and particularly in the USA, this regimen has been replaced by combined antiretroviral treatment, in which neither the class nor the type of drugs are taken into consideration.
The effects of HAART soon appeared dramatic and highly encouraging; sadly, they are in stark contrast with the bleak and relentless spread of the disease in countries, such as sub‐Saharan Africa, that cannot afford the drugs. HAART is successful in suppressing HIV replication (109) and improving cellular immunity, (12) with protection against opportunistic infections (76, 95, 119). Outside the nervous system, HAART has decreased the incidence of tuberculosis (77, 89) and of Pneumocystis carinii (146); prolonged the survival in patients with tuberculosis and fungal infections (16); decreased the rate of oral lesions (123) and allowed longer survival in patients with non‐Hodgkin lymphoma (72). In addition, it is hypothesized that several organ‐specific disorders, including HIV‐associated nephropathy, wasting syndrome and cardiomyopathy, all considered a direct consequence of HIV, could benefit from HAART treatment (146). This review will deal only with the effects of HAART on the nervous system.
However, despite a decrease in the number of AIDS‐related postmortems, brain disease remains a major cause of death. In the early stages, when HAART was administered to patients with severe immunodeficiency, the incidence of PML and primary non‐Hodgkin lymphomas remained unchanged, while toxoplasmosis and CMV and HIV encephalitis decreased. On the other hand, when it was given at an early stage of immunosuppression, PML, CMV and primary lymphomas decreased, whereas infections occurring in mildly immunodeficient patients (varicella zoster virus (VZV) and herpes zoster virus (HSV) encephalitis) became more frequent. These data are reviewed and summarized by Gray et al (61).
Nevertheless, it soon became clear that these were not the only differences observed in the HAART era. Notwithstanding that 25% of treated patients develop intolerance to the drugs, manifesting as nausea, anorexia, skin rash, hepatitis and neuropsychiatric disorders and present a relentless progression of the disease, other patients develop what has become known as “burnt‐out” lesions (61); these result from treated forms of VZV encephalitis (35), toxoplasmosis, HIVE and PML, and are remarkable for the absence of both inflammation and infectious agents. These “scar” lesions may be found in clinically and biologically cured patients who die from other causes as previously reported for toxoplasmosis (141). In other instances, despite efficient treatment, the neurological condition of the patient may continue to deteriorate. In those patients with multifocal extensive toxoplasmosis, or late treated HIVE or PML, it seems that, despite successful eradication of productive infection in the CNS, treatment was too late to prevent irreversible destructive lesions with, in some cases, secondary progressing Wallerian degeneration (61).
Unfortunately, it has to be remembered that of the several antiretroviral drugs presently used, only zidovudine attains effective therapeutic levels in the CNS. In addition, in a minority of patients, HAART‐induced partial restoration of specific immunity may unmask or worsen a pre‐existing disease. This complication, referred to as immune reconstruction inflammatory syndrome (IRIS), is defined as a “paradoxical deterioration in clinical status, attributable to the recovery of the immune system during HAART” (134). Mycobacterial infection (31, 49), CMV retinitis (75) and cryptococcal meningitis (82, 159) related to IRIS have been reported. In some patients with PML and receiving HAART, contrast enhancement revealed a florid inflammatory reaction, usually discrete in untreated individuals, which was confirmed by cerebral biopsy (99, 144). In most cases, this correlated with prolonged survival and was interpreted as a marker of both improved immune status and outcome (30, 73, 84); however in rare instances (99), it coincided with clinical and radiological deterioration.
Fatal cases, possibly representing this syndrome, are those described by Langford et al (87) in seven HAART‐treated patients. In these, postmortem revealed myelin loss, lympho‐monocytic perivascular exudates, axonal injury and astrogliosis. All these changes were considered more severe than in the pre‐HAART era. A pathologic and immunohistochemical account of the syndrome in the CNS was subsequently provided by Miller et al (98); they reported two patients who, despite HAART and a subsequent improved CD4 count and decreased HIV load in the CSF, developed fatal encephalopathy and diffuse myelin pallor (Figure 5A). In both, the brain tissue showed presence of HIV‐DNA by polymerase chain reaction; the salient pathology consisted of diffuse microglial hyperplasia, massive and diffuse perivascular (Figure 5B) and intra‐parenchymal (Figure 5C) infiltration by CD8+/CD4− lymphocytes. It was suggested that the rapid immune reconstruction induced by the treatment led to a redistribution of lymphocytes into the peripheral blood. This was followed by recruitment of CD8+ cells into the brain, resulting in diffuse infiltration and consequent tissue damage. The latter could take place in two ways: either by ligating TNF receptor‐like molecules by their corresponding ligands, triggering the apoptosis pathway, or by secreting the content of cytoplasmic vesicles (139).
Figure 5.
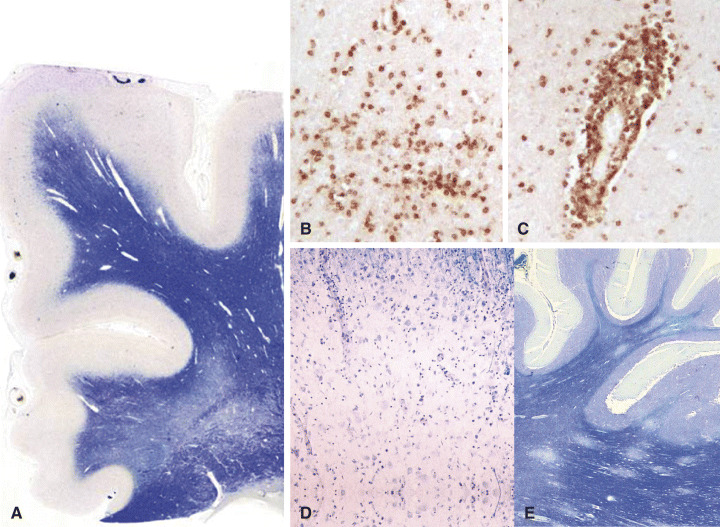
A–C. Neuropathological changes in association with IRIS include diffuse myelin pallor (H&E) (A) and massive infiltration by T, CD‐8‐positive lymphocytes both within the parenchyma (B) and around small blood vessels (C). D and E. IRIS may supervene in patients with previous PML (D); in addition, an appearance reminiscent of ADEM may also appear together with massive lymphocytic infiltration (E) (LFB/N).
Another fatal case in a patient with PML revealed, at neuropathological examination, two coexisting, and equally undesirable, effects of this therapy: on the one hand an active inflammatory PML with abundant JC virus (JCV) together with massive intraparenchymal and perivascular infiltration by CD8+ lymphocytes in the absence of CD4+ lymphocytes (Figure 4C); this is a possible reaction to a smouldering‐active infection. On the other hand, there was also an acute perivenous leukoencephalitis, similar to acute perivenous encephalomyelitis (Figure 4D) devoid of JCV (151), and considered a possible reaction to a latent antigen or inactive infectious agent (20). It is suggested that both patterns may relate to CD8+ lymphocyte cytotoxicity (60).
In conclusion, it now appears that introduction of HAART has dramatically modified the course and prognosis of HIV infection. However, these encouraging results are restricted to the developed world where treatment is widely available; in addition, their impact on the HIV‐associated cognitive disorders, which represent the most disabling complications of the disease, is not clearly established; finally new HAART‐related neurological complications have appeared. Therefore, it is crucial that further research continue both for a better understanding of the mechanisms of neurodegeneration and for wider prevention and treatment of HIV infection.
REFERENCES
- 1. Acheampong EA, Parveen Z, Muthoga LW, Lakayeh M, Mukhtar M, Pomerantz RJ (2005) Human immunodeficiency virus type 1 Nef potently induces apoptosis in primary human brain microvascular endothelial cells via the activation of caspases. J Virol 79:4257–4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Adamson DC, Wildemann B, Sasaki M (1996) Immunologic NO synthase: elevation in severe AIDS dementia and induction by HIV‐1 gp41. Science 274:1917–21. [DOI] [PubMed] [Google Scholar]
- 3. Adle Biassette H, Chrétien F, Wingertsmann L, Henry C, Ereau T, Scaravilli F, Tardieu M, Gray F (1999) Neuronal apoptosis does not correlate with dementia in HIV infection but is related to microglial activation and axonal damage. Neuropathol Appl Neurobiol 25:123–133. [DOI] [PubMed] [Google Scholar]
- 4. Adle‐Biassette H, Levy Y, Colombel M, Poron F, Natchev S, Keohane C, Gray F (1995) Neuronal apoptosis in HIV infection in adults. Neuopathol Appl Neurobiol 21:218–227. [DOI] [PubMed] [Google Scholar]
- 5. An SF, Ciardi A, Giometto B, Scaravilli T, Gray F, Scaravilli F (1996) Investigation on the expression of major histocompatibility complex class II and cytokines and detection of HIV‐1 DNA within brains of asymptomatic and symptomatic HIV‐1‐positive patients. Acta Neuropathol 91:494–503. [DOI] [PubMed] [Google Scholar]
- 6. An SF, Giometto B, Scaravilli F (1996) HIV‐1 DNA in brains in AIDS and pre‐AIDS: correlation with the stage of the disease. Ann Neurol 40:611–617. [DOI] [PubMed] [Google Scholar]
- 7. An SF, Giometto B, Scaravilli T, Tavolato B, Gray F, Scaravilli F (1996) Programmed cell death in brains of HIV‐1‐positive AIDS and pre‐AIDS patients. Acta Neuropathol 91:169–173. [DOI] [PubMed] [Google Scholar]
- 8. An SF, Giometto B, Groves M, Miller R, Beckett AAJ, Gray F, Tavolato B, Scaravilli F (1997) Axonal damage revealed by accumulation of β‐APP in HIV‐positive individuals without AIDS. J Neuropathol Exp Neurol 56:1262–1268. [DOI] [PubMed] [Google Scholar]
- 9. An SF, Groves M, Giometto B, Becket AAJ, Scaravilli F (1999) Detection and localisation of HIV‐1 DNA and RNA in fixed adult AIDS brain by polymerase chain reaction/in situ hybridisation technique. Acta Neuropathol 98:481–487. [DOI] [PubMed] [Google Scholar]
- 10. Anonymous (1982) Opportunistic infections and Kaposi’s sarcoma among Haitians in the United States. Conn Med 46:727–728. [PubMed] [Google Scholar]
- 11. Artigas J, Grosse G, Niedobitek F (1990) Vacuolar myelopathy in AIDS. A morphological analysis. Pathol Res Pract 186:228–237. [DOI] [PubMed] [Google Scholar]
- 12. Autran B, Carcelain G, Li TS, Blanc C, Mathez D, Tubiana R, Katlama C, Debre P, Leibowitch J (1997) Positive effects of combined antiretroviral therapy on CD4+ T cells homeostasis and function in advanced HIV cases. Science 277:112–116. [DOI] [PubMed] [Google Scholar]
- 13. Bagasra O, Lavi E, Bobroski L, Khalili K, Pestaner JP, Tawadros R, Pomerantz RJ (1996) Cell reservoirs of HIV‐1 in the central nervous system of infected individuals: identification by the combination of in situ polymerase chain reaction and immunohistochemistry. AIDS 10:573–585. [DOI] [PubMed] [Google Scholar]
- 14. Banks WA, Freed EO, Wolf KM, Robinson SM, Franko M, Kumar VB (2001) Transport of human immunodeficiency virus type 1 pseudoviruses across the blood‐brain barrier: role of envelope proteins and adsorptive endocytosis. J Virol 75: 4681–4691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Barré‐Sinoussi F, Cherman JC, Rey F, Nugeyre M, Chamaret S, Gruest J, Dauguet C, Axler‐Blin C, Vezinet‐Brun F, Rouzioux C, Rozenbaum W, Montagnier L (1983) Isolation of T‐lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome. Science 220:868–871. [DOI] [PubMed] [Google Scholar]
- 16. Boven LA, Gomes L, Henry C, Gray F, Verhoef J, Portegies P, Tardieu M, Nottet HS (1999) Increased peroxynitrite activity in AIDS dementia complex: implications for the neuropathogenesis of HIV‐1 infection. J Immunol 162:4319–4327. [PubMed] [Google Scholar]
- 17. Bell JE, Brettle RP, Chiswick A, Simmonds P (1998) HIV encephalitis, proviral load and dementia in drug users and homosexuals with AIDS. Effect of neocortical involvement. Brain 121: 2043–2052. [DOI] [PubMed] [Google Scholar]
- 18. Bissel SJ, Wiley CA (2004) Human immunodeficiency virus infection of the brain: pitfalls in evaluating infected/affected cell populations. Brain Pathol 14:97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Boven LA, Middle J, Verhoef J, De Groot CJ, Nottet HS (2000) Monocyte infiltration is highly associated with loss of the tight junction protein zonula occludens in HIV‐a‐associated dementia. Neuropathol Appl Neurobiol 26:356–360. [DOI] [PubMed] [Google Scholar]
- 20. Breton G, Seilhean D, Chérin P, Herson S, Benveniste O (2002) Paradoxical intracranial cryptococcoma in a Human Immunodeficiency Virus‐infected man being treated with combination Antiretroviral Therapy. Am J Med 113:155–157. [DOI] [PubMed] [Google Scholar]
- 21. Brew BJ, Rosenblum M, Cronin K, Price RW (1995) AIDS dementia complex and HIV‐1 brain infection: clinical‐virological correlations. Ann Neurol 38:563–570. [DOI] [PubMed] [Google Scholar]
- 22. Bruce‐Keller AJ, Chauhan A, Dimayuga FO, Gee J, Keller JN, Nath A (2003) Synaptic transport of human immunodeficiency virus‐Tat protein causes neurotoxicityand gliosis in rat brain. J Neurosci 23:8417–8422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Budka H (1986) Multinucleated giant cells in brain: a hallmark of the acquired immune deficiency syndrome (AIDS). Acta Neuropathol 69:253–258. [DOI] [PubMed] [Google Scholar]
- 24. Budka H, Costanzi G, Cristina S, Lechi A, Parravicini C, Trabattoni R, Vago L (1987) Brain pathology induced by the infection with the human immunodeficiency virus (AIDS). Acta Neuropathol 75: 186–198. [DOI] [PubMed] [Google Scholar]
- 25. Budka H, Maier H, Pokl P (1988) Human immunodeficiency virus in vacuolar myelopathy of the acquired immunodeficiency syndrome. N Engl J Med 319:1667–1668. [DOI] [PubMed] [Google Scholar]
- 26. Budka H, Wiley CA, Kleihues P, Artigas J, Asbury AK, Cho ES, Cornblath DR, Dal Canto MC, De Girolami U, Dickson D, Epstein LG, Esiri MM, Giangaspero F, Gosztonyi G, Gray F, Griffin JW, Hénin D, Iwasaki Y, Janssen RS, Johnson RT, Lantos PL, Lyman WD, McArthur JC, Nagashima K, Peress N, Petito CK, Price RW, Rhodes RH, Rosenblum M, Said G, Scaravilli F, Sharer LR, Vinters HV (1991) HIV associated disease of the nervous system: review and nomenclature and proposal for neuropathology‐based terminology. Brain Pathol 1:143–152. [DOI] [PubMed] [Google Scholar]
- 27. Carne CA, Tedder RS, Smith A, Sutherland S, Elkington SG, Daly HM, Preston FE, Craskey J (1985) Acute encephalopathy coincident with seroconversion for anti‐HTLV‐III. Lancet ii:1206–1208. [DOI] [PubMed] [Google Scholar]
- 28. Chrétien F, Gray F, Lescs MC, Geny C, Dubreuil‐Lemaire ML, Ricolfi F, Baudrimont M, Levy Y, Sobel A, Vinters HV (1993) Acute varicella‐zoster virus ventriculitis and meningo‐myelo‐radiculitis in acquired immunodeficiency syndrome. Acta Neuropathol 86:659–665. [DOI] [PubMed] [Google Scholar]
- 29. Ciardi A, Sinclair E, Scaravilli F, Harcourt‐Webster NJ, Lucas S (1990) Involvement of the cerebral cortex in HIV‐encephalopathy. A morphological and immuno‐histochemical study. Acta Neuropathol 81:51–59. [DOI] [PubMed] [Google Scholar]
- 30. Collazos J, Mayo J, Martinez E, Blanco MS (1999) Contrast‐enhancing progressive multifocal leukoencephalopathy as an immune reconstitution event in AIDS patients. AIDS 13:426–428. [DOI] [PubMed] [Google Scholar]
- 31. Crump JA, Tyrer MJ, Lloyd‐Owen SJ, Han LY, Lipman MC, Johnson MA (1998) Miliary tuberculosis with paradoxical expansion of intracranial tuberculomas complicating immunodeficiency virus infection in a patient receiving highly active antiretroviral therapy. Clin Infect Dis 26:1008–1009. [DOI] [PubMed] [Google Scholar]
- 32. Dalgliesh AG, Beverley PCL, Clapman PR, Crawford DH, Greaves MF, Weiss RA (1984) The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature 312:767–768. [DOI] [PubMed] [Google Scholar]
- 33. Daniel MD, Letvin NL, King NW, Kannagi M, Sehgal PK, Hunt RD, Kanki PJ, Essex M, Desrosiers RC (1985) Isolation of t‐cell tropic HTLV‐III‐like retrovirus from macaques. Science 228:1201–1204. [DOI] [PubMed] [Google Scholar]
- 34. Davis LE, Hjelle BL, Miller VE, Palmer DL, Llewellyn AL, Merlin TL, Young SA, Mills RG, Wachsman W, Wiley CA (1992) Early viral brain invasion in iatrogenic human immunodefiency virus infection. Neurology 42:1736–1739. [DOI] [PubMed] [Google Scholar]
- 35. De la Grandmaison GL, Carlier R, Chrétien F, De Truchis P, Orlikowski D, Gray F (2005) “Burnt out” varicella‐zoster‐virus encephalitis in an AIDS patient following treatment by highly active antiretroviral therapy. Clin Radiol 60:613–617. [DOI] [PubMed] [Google Scholar]
- 36. Denning DW, Anderson J, Rudge P, Smith H (1987) Acute myelopathy associated with primary infection with human immunodeficiency virus. BMJ 294:143–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dewhurst S, Gelbard HA, Fine SM (1996) Neuropathogenesis of AIDS. Mol Med Today 2:16–23. [DOI] [PubMed] [Google Scholar]
- 38. Dragic T, Litwin V, Allaway GP, Martin SR, Huang Y, Nagashima KA, Cayanan C, Maddon PJ, Koup RA, Moore JP, Paxton WA (1996) HIV‐1 entry into CD4+ cells is mediated by the chemokine receptor CC‐CKR‐5. Nature 381:667–673. [DOI] [PubMed] [Google Scholar]
- 39. Epstein LG, Sharer LR, Goudsmit J (1988) Neurological and neuropathological features of human immunodeficiency virus infection in children. Ann Neurol 23:s19–23. [DOI] [PubMed] [Google Scholar]
- 40. Esiri MM, Scaravilli F, Millard PR, Harcourt‐Webster JN (1989) Neuropathology of HIV infection in haemophiliacs: comparative necropsy study. BMJ 299:1312–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Evans LA, Levy JA (1989) Characteristics of HIV infection and pathogenesis. Biochem Biophys Acta 989:237–254. [PubMed] [Google Scholar]
- 42. Everall I, Barnes H, Spargo E, Lantos P (1995) Assessment of neuronal density in the putamen in human immunodeficiency virus (HIV) infection. Application of stereology and spatial analysis of quadrats. J Neurovirol 1:126–129. [DOI] [PubMed] [Google Scholar]
- 43. Everall IP, Glass JD, McArthur J, Spargo E, Lantos P (1994) Neuronal density in the superior frontal and temporal gyri does not correlate with the degree of human immunodeficiency virus‐associated dementia. Acta Neuropathol 88:538–544. [DOI] [PubMed] [Google Scholar]
- 44. Everall IP, Heaton RK, Marcotte TD, Ellis RJ, McCutchan JA, Atkinson JH, Grant I, Mallory M, Masliah E (1999) Cortical synaptic density is reduced in mild to moderate human immunodeficiency virus neurocognitive disorder. HNRC Group. HIV Neurobehavioral Research Center. Brain Pathol 9:209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Everall IP, Luthert P, Lantos PL (1993) A review of neuronal damage in human immunodeficiency virus infection: its assessment, possible mechanism and relationship to dementia. J Neuropathol Exp Neurol 52:561–556. [DOI] [PubMed] [Google Scholar]
- 46. Fauci AS, Macher AM, Longo DL, Lane HC, Rook AH, Masur H, Gelmann EP (1984) Acquired immunodeficiency syndrome: epidemiologic, clinical, immunologic and therapeutic considerations. Ann Int Med 100:92–106. [DOI] [PubMed] [Google Scholar]
- 47. Feng Y, Broder CC, Kennedy PE, Berger EA (1996) HIV‐1 entry cofactor: functional cDNA cloning of a seven transmembrane, G protein‐coupled receptor. Science 272:872–877. [DOI] [PubMed] [Google Scholar]
- 48. Ferrarese C, Aliprandi A, Tremolizzo L, Stanzani L, De Micheli A, Dolara A, Frattola L (2001) Increased glutamate in CSF and plasma of patients with HIV dementia. Neurology 57:671–675. [DOI] [PubMed] [Google Scholar]
- 49. Foudraine NA, Hovenkamp E, Notermans DW, Meenhorst PL, Klein MR, Lange JM, Miedema F, Reiss P (1999) Immunopathology as a result of highly active antiretroviral therapy in HIV‐1‐infected patients. AIDS 13:177–184. [DOI] [PubMed] [Google Scholar]
- 50. Gallo RC, Salahuddin SZ, Popovic M, Shaerer GM, Kaplan M, Haynes BF, Palker TJ, Redfield R, Oleske J, Safai B, White G, Foster P, Markham PD (1984) Frequent detection and isolation of cytopathic retrovirus (HTLV‐III) from patients with AIDS and at risk for AIDS. Science 224:500–503. [DOI] [PubMed] [Google Scholar]
- 51. Gao F, Bailes E, Robertson DL, Chen Y, Rodenbug CM, Michael SF, Cummins LB, Arthur LO, Peters M, Shaw GM, Sharp PM, Hahn BH (1999) Origin of HIV‐1in the chimpanzee Pan troglodytes troglodytes. Nature 397:436–441. [DOI] [PubMed] [Google Scholar]
- 52. Geddes JF, Battacharjee MB, Savage K, Scaravilli F, McLaughlin JE (1992) Primary cerebral lymphoma: a study of 47 cases probed for Epstein‐Barr virus genome. J Clin Pathol 45:587–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Geiger KD, Stoldt P, Schlote W, Derouiche A (2006) Ezrin immunoreactivity reveals specific astrocyte activation in cerebral HIV. J Neuropathol Exp Neurol 65:87–96. [DOI] [PubMed] [Google Scholar]
- 54. Gelbard HA, James HJ, Sharer LR, Perry SW, Saito Y, Kazee AM, Blumberg BM, Epstein LG (1995) Apoptotic neurons in brains from paediatric patients with HIV‐1 encephalitis and progressive encephalopathy. Neuropathol Appl Neurobiol 21:208–217. [DOI] [PubMed] [Google Scholar]
- 55. Giometto B, An SF, Groves M, Scaravilli T, Geddes JF, Miller R, Tavolato B, Beckett AA, Scaravilli F (1997) Accumulation of beta‐amyloid precursor protein in HIV encephalitis: relationship with neurophysiological abnormalities. Ann Neurol 42:34–40. [DOI] [PubMed] [Google Scholar]
- 56. Glass JD, Wesselingh SL, Selnes OA, McArthur JC (1993) Clinical‐neuropathologic correlation in HIV‐associated dementia. Neurology 43:2230–2237. [DOI] [PubMed] [Google Scholar]
- 57. Goldstick L, Mandybur TI, Bode R (1985) Spinal cord degeneration in AIDS. Neurology 35:103–106. [DOI] [PubMed] [Google Scholar]
- 58. Gras G, Chrétien F, Vallat‐Decouvelaere AV, Le Pavec G, Porcheray F, Bousset C, Leone C, Mialocq P, Dereuddre‐Bosquet N, Clayette P, Le Grand R, Luminon C, Dormont D, Gray F (2003) Regulated expression of sodium‐dependent glutamate transporters and synthetase: a neuroprotective role for activated microglia and macrophages in HIV infection? Brain Pathol 13:211–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gray F, Adle‐Biassette H, Chrétien F, Lorin de la Grandmaison GL, Force G, Keohane C (2001) Neuropathology and neurodegeneration in human immunodeficiency virus infection. Pathogenesis of HIV‐induced lesions of the brain, correlations with HIV‐associated disorders and modifications according to treatments. Clin Neuropathol 20: 146–155. [PubMed] [Google Scholar]
- 60. Gray F, Bazille C, Adle‐Biassette H, Mikol J, Moulignier A, Scaravilli F (2005) Central nervous system immune reconstitution disease in AIDS patients receiving highly active antiretroviral treatment. J Neurovirol 11(Suppl. 3):16–22. [DOI] [PubMed] [Google Scholar]
- 61. Gray F, Chrétien F, Vallat‐Decouvelaere AV, Scaravilli F (2003) The changing pattern of HIV neuropathology in the HAART era. J Neuropathol Exp Neurol 62:429–440. [DOI] [PubMed] [Google Scholar]
- 62. Gray F, Geny C, Lionnet F, Dournon E, Fenelon G, Gherardi R, Poirier J (1991) Neuropathologic study of 135 adult cases of acquired immunodeficiency syndrome (AIDS). Ann Pathol 11:236–247 (in French). [PubMed] [Google Scholar]
- 63. Gray F, Gherardi R, Keohane C, Favolini M, Sobel A, Poirier J (1988) Pathology of the central nervous system in 40 cases of acquired immune deficiency syndrome (AIDS). Neuropathol Appl Neurobiol 14:365–380. [DOI] [PubMed] [Google Scholar]
- 64. Gray F, Haug H, Chimelli L, Geny C, Gaston A, Scaravilli F, Budka H (1991) Prominent cortical atrophy with neuronal loss as correlate of human immunodeficiency virus encephalopathy. Acta Neuropathol 82:229–233. [DOI] [PubMed] [Google Scholar]
- 65. Gray F, Lescs MC, Keohane C, Paraire F, Marc B, Durigon M, Gherardi R (1992) Early brain changes in HIV infection: neuropathological study of 11 HIV‐seropositive, no‐AIDS cases. J Neuropathol Exp Neurol 51:177–185. [DOI] [PubMed] [Google Scholar]
- 66. Hanto DW, Frizzera G, Purtilo DT, Sakamoto K, Sullivan JL, Saemundsen AK, Klein G, Simmons RL, Najarian JS (1981) Clinical spectrum of lymphoproliferative disorders in renal transplant recipients and evidence for the role of Epstein‐Barr virus. Cancer Res 41:4253–4261. [PubMed] [Google Scholar]
- 67. Harcourt‐Webster JN (1993) General pathology. In: The Neuropathology of HIV Infection. Scaravilli F (ed.), pp. 53–98. Springer: London. [Google Scholar]
- 68. Haughey NJ, Nath A, Mattson NR, Slevin JT, Geiger JD (2001) HIV‐1 Tat through phosphorylation of NMDA receptors petentiates glutamate excitotoxicity. J Neurochem 78:457–467. [DOI] [PubMed] [Google Scholar]
- 69. Hénin D, Duyckaerts C, Chaunu MP, Vazeux R, Brousse N, Rozenbaum W, Hauw JJ (1987) Etude neuropathologique de 31 cas de syndrome d’immunodépression acquise. Rev Neurol (Paris) 143:631–642. [PubMed] [Google Scholar]
- 70. Heyes MP, Ellis RJ, Ryan L, Childers ME, Grant I, Wolfson T, Archibald S, Jernigan TL, HNRC Group, HIV Neurobehavioural Research Center (2001) Elevated cerebro spinal fluid quinolinic acid levels are associated with region‐specific cerebral volume loss in HIV infection. Brain 124:1033–1042. [DOI] [PubMed] [Google Scholar]
- 71. Ho DD, Rota TR, Schooley RT, Kaplan JC, Allan JD, Groopman JE, Resnick L, Felsenstein D, Andrews CA, Hirsch MS (1985) Isolation of HTLV‐III from cerebrospinal fluid and neural tissues of patients with neurological syndromes related to the acquired immunodeficiency syndrome. N Engl J Med 313:1493–1497. [DOI] [PubMed] [Google Scholar]
- 72. Hoffmann C, Tabrizian S, Wolf E, Eggers C, Stoehr A, Plettenberg A, Buhk T, Stellbrink HJ, Horst HA, Jager H, Rosenkranz T (2001) Survival of AIDS patients with primary central nervous system lymphoma is dramatically improved by HAART‐induced immune recovery. AIDS 15:2119–2127. [DOI] [PubMed] [Google Scholar]
- 73. Hoffmann S, Horst HA, Albrecht H, Schlote W (2003) Progressive multifocal leukoencephalopathy with unusual inflammatory response during antiretroviral treatment. J Neurol Neurosurg Psychiat 74:1142–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hooper DC, Pruitt AA, Rubin RH (1982) Central nervous system infection in the chronically immunosuppressed. Medicine 61:166–188. [DOI] [PubMed] [Google Scholar]
- 75. Jacobson MA, Zegans M, Pavan PR, O’Donnell JJ, Sattler F, Rao N, Owens S, Pollard R (1997) Cytomegalovirus retinitis after initiation of highly active antiretroviral therapy. Lancet 349:1143–1145. [DOI] [PubMed] [Google Scholar]
- 76. Jellinger KA, Setinek U, Drilicek M, Bohm G, Steurer A, Lintner F (2000) Neuropathology and general autopsy findings in AIDS during the last 15 years. Acta Neuropathol 100:213–220. [DOI] [PubMed] [Google Scholar]
- 77. Jerene D, Naess A, Lindtjorn B (2006) Antiretroviral therapy at a district hospital in Ethiopia prevents death and tuberculosis in a cohort of HIV patients. AIDS Res Ther 3:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Johnson RT, Glass JD, McArthur JC, Chesebro BW (1996) Quantitation of human immunodeficiency virus in brains of demented and nondemented patients with acquired immunodeficiency syndrome. Ann Neurol 39:392–395. [DOI] [PubMed] [Google Scholar]
- 79. Kamin SS, Petito CK (1991) Idiopathic myelopathies with white matter vacuolation in non‐acquired immunodeficiency patients. Hum Pathol 22:816–824. [DOI] [PubMed] [Google Scholar]
- 80. Kaul M, Garden GA, Lipton SA (2001) Pathways to neuronal injury and apoptosis in HIV‐associated dementia. Nature 410:988–994. [DOI] [PubMed] [Google Scholar]
- 81. Kimura‐Kuroda J, Nagashima K, Yasui K (1994) Inhibition of myelin formation by HIV‐1 gp‐120 in rat cerebral cortex culture. Arch Virol 137:81–99. [DOI] [PubMed] [Google Scholar]
- 82. King MD, Perlino CA, Cinnamon J, Jernigan JA (2002) Paradoxical recurrent meningitis following therapy of cryptococcal meningitis: an immune reconstitution syndrome after initiation of highly active antiretroviral therapy. AIDS 13:724–726. [DOI] [PubMed] [Google Scholar]
- 83. Klatzmann D, Champagne E, Chamaret S, Gruest J, Guetard D, Hercend T, Gluckman JC, Montagnier L (1984) T‐lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature 312:767–768. [DOI] [PubMed] [Google Scholar]
- 84. Kotecha N, George MJ, Smith TW, Corvi F, Litofsky NS (1998) Enhancing progressive multifocal leukoencephalopathy: an indicator of improved immune status? Am J Med 105:541–543. [DOI] [PubMed] [Google Scholar]
- 85. Kovacs JA, Kovacs AA, Polis M, Wright WC, Gill VJ, Tuazon CV, Gelmann EP, Lane HC, Longfield R, Overturf G, Macher AM, Fauci AS, Parrillo JE, Bennett JE, Masur H (1985) Cryptococcosis in the acquired immunodeficiency syndrome. Ann Intern Med 103:533–538. [DOI] [PubMed] [Google Scholar]
- 86. Lang W, Miklossy J, Deruaz JP, Pizzolato GP, Schaffner T, Gessaga E, Kleihues P (1989) Neuropathology of the acquired immune deficiency syndrome (AIDS): a report of 135 consecutive autopsy cases from Switzerland. Acta Neuropathol 77:379–390. [DOI] [PubMed] [Google Scholar]
- 87. Langford TD, Letendre SL, Marcotte TD, Ellis RJ, McCutchan JA, Grant I, Mallory ME, Hansen LA, Archibald S, Jernigan T, Masliah E (2002) Severe, demyelinating leukoencephalopathy in AIDS patients on antiretroviral therapy. AIDS 16:1019–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Lazarini F, Seilhean D, Rosenblum O, Suarez S, Conquy L, Uchihara T, Sazdovitch V, Mokhtari K, Maisonobe T, Boussin F, Katlama C, Bricaire F, Duyckaerts C, Hauw JJ (1997) Human immunodeficiency virus type 1 DNA and RNA load in brains of demented and nondemented patients with acquired immunodeficiency syndrome. J Neurovirol 3:299–303. [DOI] [PubMed] [Google Scholar]
- 89. Lawn SD, Badri M, Wood R (2005) Tuberculosis among HIV‐infected patients receiving HAART: long term incidence and risk factors in South African cohort. AIDS 19:2109–2116. [DOI] [PubMed] [Google Scholar]
- 90. Levy RM, Bredesen DE, Rosenblum ML (1988) Opportunistic central nervous system pathology in patients with AIDS. Ann Neurol 23(Suppl.):S7–S12. [DOI] [PubMed] [Google Scholar]
- 91. Li W, Galey D, Mattson MP, Nath A (2005) Molecular and cellular mechanisms of neuronal cell death in HIV dementia. Neurotox Res 8:119–134. [DOI] [PubMed] [Google Scholar]
- 92. Lipton SA (1991) HIV‐related neurotoxicity. Brain Pathol 1:193–199. [DOI] [PubMed] [Google Scholar]
- 93. Luft BJ, Brooks RG, Conley FK, McCabe RE, Remington JS (1984) Toxoplasmic encephalitis in patients with acquired immune deficiency syndrome. JAMA 252:913–917. [PubMed] [Google Scholar]
- 94. Maier H, Budka H, Lassmann H, Pohl P (1989) Vacuolar myelopathy with multinucleated giant cells in the acquired immunodeficiency syndrome (AIDS). Light and electron microscopic distribution of the human immunodeficiency virus (HIV) antigens. Acta Neuropathol 78:497–503. [DOI] [PubMed] [Google Scholar]
- 95. Maschke M, Kastrup O, Esser S, Ross B, Hengge U, Hufnagel A (2000) Incidence and prevalence of neurological disorders associated with HIV since the introduction of highly active antiretroviral therapy (HAART). J Neurol Neurosurg Psychiat 69:376–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Masliah E, Eaton RK, Marcotte TD, Ellis RJ, Wiley CA, Mallory M, Achim CL, McCutchan JA, Nelson JA, Atkinson JH, Grant I (1997) Dendritic injury is a pathological substrate for human immunodeficiency virus‐related cognitive disorders. HNRC Group. The HIV Neurobehavioral Research Center. Ann Neurol 42:963–972. [DOI] [PubMed] [Google Scholar]
- 97. Meltzer MS, Gendelman HE (1992) Mononuclear phagocytes as targets, tissue reservoirs, and immunoregulatory cells in human immunodeficiency virus disease. Curr Top Microbiol Immunol 181:239–263. [DOI] [PubMed] [Google Scholar]
- 98. Miller RF, Isaacson PG, Hall‐Craggs M, Lucas S, Gray F, Scaravilli F, An SF (2004) Cerebral CD8+ lymphocytosis in HIV‐1 infected patients with immune restoration induced by HAART. Acta Neuropathol 108:17–23. [DOI] [PubMed] [Google Scholar]
- 99. Miralles P, Berenguer J, Lacruz C, Cosin J, Lopez JC, Padilla B, Munoz L, Garcia‐de‐Viedma D (2001) Inflammatory reactions in progressive multifocal leukoencephalopathy after highly acive antiretroviral therapy. AIDS 15:190–192. [DOI] [PubMed] [Google Scholar]
- 100. Mizusawa H, Hirano A, Llena JF, Shintaku M (1988) Cerebrovascular lesions in acquired immunodeficiency syndrome (AIDS). Acta Neuropathol 76:451–457. [DOI] [PubMed] [Google Scholar]
- 101. Morgello S, Cho E‐S, Nielsen S, Devinsky O, Petito CK (1987) Cytomegalovirus encephalitis in patients with acquired immunodeficiency syndrome: an autopsy study of 30 cases and a review of the literature. Hum Pathol 18:289–297. [DOI] [PubMed] [Google Scholar]
- 102. Moore JP, Stevenson M (2000) New targets for inhibitors of HIV‐1 replication. Nat Rev Mol Cell Biol 1:40–49. [DOI] [PubMed] [Google Scholar]
- 103. Navia BA, Cho E‐S, Petito CK, Price RW (1986) The AIDS dementia complex: II. Neuropathology. Ann Neurol 19:525–535. [DOI] [PubMed] [Google Scholar]
- 104. Nahmias AJ, Weiss J, Yao X, Lee F, Kodsi R, Schanfield M, Matthews T, Bolognesi D, Durack D, Motulsky A, Kanki P, Essex M (1986) Evidence for human infection with an HTLVIII/LAV‐like virus in Central Africa, 1959. Lancet i:1279–1280. [DOI] [PubMed] [Google Scholar]
- 105. Nath A, Geiger J (1998) Neurobiological aspects of human immunodeficiency virus infection: neurotoxic mechanisms. Progr Neurobiol 54: 19–33. [DOI] [PubMed] [Google Scholar]
- 106. Nath A, Conant K, Chen P, Scott C, Major EO (1999) Transient exposure to HIV‐1 Tat protein results in cytokine production in macrophages and astrocytes. A hit and run phenomenon. J Biol Chem 274:17098–17101. [DOI] [PubMed] [Google Scholar]
- 107. Nathan DG (1998) Clinical research: perceptions, reality, and proposed solutions. National Institute of Health Director’s Panel on Clinical Research. JAMA 280:1427–1431. [DOI] [PubMed] [Google Scholar]
- 108. Nuovo GJ, Gallery F, MacConnell P, Braun A (1994) in situ detection of polymerase chain reaction‐amplified HIV‐1 nucleic acids and tumor necrosis factor‐alpha RNA in the central nervous system. Am J Pathol 144:659–666. [PMC free article] [PubMed] [Google Scholar]
- 109. Pakker NG, Ross MTL, Van Leeuwen R, De Long MD, Koot M, Reiss P, Lange JM, Miedema F, Danner SA, Shellekens PT (1997) Patterns of T cell repopulation, virus load reduction and restoration of T cell function in HIV‐infected persons during therapy with different antiretrovirals. J Acquir Immne Defic Syndr Hum Retrovirol 16:318–326. [DOI] [PubMed] [Google Scholar]
- 110. Petito CK, Cask KS (1992) Blood brain barrier abnormality in the acquired immunodeficiency syndrome: immunohistochemical localization of serum proteins in post‐mortem brain. Ann Neurol 32:658–666. [DOI] [PubMed] [Google Scholar]
- 111. Petito CK, Cho E‐S, Lemann W, Navia BA, Price RW (1986) Neuropathology of acquired immunodeficiency syndrome (AIDS): an autopsy review. J Neuropathol Exp Neurol 45:635–646. [DOI] [PubMed] [Google Scholar]
- 112. Petito CK, Navia BA, Cho E‐S, Jordan BD, George DC, Price RW (1985) Vacuolar myelopathy pathologically resembling subacute combined degeneration in patients with acquired immunodeficiency syndrome. N Engl J Med 312:874–879. [DOI] [PubMed] [Google Scholar]
- 113. Petito CK, Roberts B (1995) Evidence of apoptotic cell death in HIV encephalitis. Am. J Pathol 146:1121–1130. [PMC free article] [PubMed] [Google Scholar]
- 114. Petito CK, Roberts B, Cantando JD, Rabinstein A, Duncan R (2001) Hippocampal injury and alterations in neuronal chemochine co‐receptor expression in patients with AIDS. J Neuropathol Exp Neurol 60:377–385. [DOI] [PubMed] [Google Scholar]
- 115. Phoolcharoen W (1998) HIV/AIDS prevention in Thailand: success and challenges. Science 280:1873–1874. [DOI] [PubMed] [Google Scholar]
- 116. Popovic M, Sarngadharan MG, Read E, Gallo RC (1984) Detection, isolation and continuous production of cytopathic retrovirus (HTLV‐III) from patients with AIDS and pre‐AIDS. Science 224:497–500. [DOI] [PubMed] [Google Scholar]
- 117. Power C, Kong PA, Crawford TO, Wesselingh S, Glass JD, McArthur JC, Trapp BD (1993) Cerebral white matter changes in acquired immunodeficiency syndrome dementia: alterations of the blood‐brain‐barrier. Ann Neurol 34:339–350. [DOI] [PubMed] [Google Scholar]
- 118. Resnick L, Burger JR, Shapshak P, Tourtellotte WW (1988) Early penetration of the blood‐brain‐barrier by HIV. Neurology 38:9–14. [DOI] [PubMed] [Google Scholar]
- 119. Sacktor NC, Lyles RH, Skolasky R, Anderson DE, McArthur JC, Mcarlane G, Selnes OA, Becker JT, Cohen B, Wesch J, Miller EN (1999) The multicenter AIDS Cohort Study. HIV‐1‐related neurological disease incidence changes in the era of highly active antiretroviral therapy. Neurology 52:A252–A253. [Google Scholar]
- 120. Saito Y, Sharer LR, Epstein LG, Michaels J, Mintz M, Louder M, Golding K, Cvetkovich TA, Blumberg BM (1994) Overexpression of nef as a marker for restricted HIV‐1 infection of astrocytes in post‐mortem pediatric central nervous tissue. Neurology 44:474–481. [DOI] [PubMed] [Google Scholar]
- 121. Scaravilli F, Daniel SE, Harcourt‐Webster N, Guiloff RJ (1989) Chronic basal meningitis and vasculitis in acquired immunodeficiency syndrome. A possible role for human immunodeficiency virus. Arch Pathol Lab Med 113:192–195. [PubMed] [Google Scholar]
- 122. Schmidbauer M, Huemer M, Cristina S, Trabattoni GR, Budka H (1982) Morphological spectrum, distribution and clinical correlations of white matter lesions in AIDS brains. Neuropathol Appl Neurobiol 18:489–501. [DOI] [PubMed] [Google Scholar]
- 123. Schmidt‐Westhausen AM, Priepke F, Bergmann FJ, Reichart PA (2000) Decline in the rate of opportunistic infections following introduction of highly active antiretroviral therapy. J Oral Pathol Med 29:336–341. [DOI] [PubMed] [Google Scholar]
- 124. Seilhean D, Duyckaerts C, Vazeux R, Bolgert F, Brunet P, Katlama C, Gentilini M, Hauw JJ (1993) HIV‐associated cognitive/motor complex: absence of neuronal loss in the cerebral neocortex. Neurology 43:1492–1499. [DOI] [PubMed] [Google Scholar]
- 125. Seilhean D, Dzia‐Lepfoundzou A, Sazdovitch V, Cannella B, Raine CS, Katlama C, Bricaire F, Duyckaerts C, Hauw JJ (1997) Astrocytic adhesion molecules are increased in HIV‐associated cognitive/motor complex. Neuropathol Appl Neurobiol 23:83–92. [PubMed] [Google Scholar]
- 126. Shacker T, Collier AC, Hughes J, Shea T, Corey L (1996) Clinical and epidemiologic features of primary HIV infection. Ann Int Med 125:257–264. [DOI] [PubMed] [Google Scholar]
- 127. Sharer LR (1992) Pathology of HIV‐1 infection of the central nervous system. A review. J Neuropathol Exp Neurol 51:3–11. [DOI] [PubMed] [Google Scholar]
- 128. Sharer LR, Cho ES (1989) Neuropathology of HIV infection: adults vs. children. Progr AIDS Pathol 1:131–141. [PubMed] [Google Scholar]
- 129. Sharer LR, Cho E‐S, Epstein LG (1985) Multinucleated giant cells and HTLV‐III in AIDS encephalopathy. Hum Pathol 16:760. [DOI] [PubMed] [Google Scholar]
- 130. Sharer LR, Dowling PC, Michaels J, Cook SD, Menonna J, Blumberg BM, Epstein LG (1990) Spinal cord disease in children with HIV‐1 infection: a combined molecular biological and neuropathological study. Neuropathol Appl Neurobiol 16:317–331. [DOI] [PubMed] [Google Scholar]
- 131. Sharer LR, Epstein LG, Cho E‐S, Joshi VV, Meyenhofer MF, Rankin LF, Petito CK (1986) Pathologic features of AIDS encephalopathy in children: evidence for LAV/HTLV‐III infection of brain. Hum Pathol 17:271–284. [DOI] [PubMed] [Google Scholar]
- 132. Sharer LR, Saito Y, Epstein LG, Blumberg BM (1994) Detection of HIV‐1 DNA in pediatric AIDS brain tissue by two step ISPCR. Adv Neuroimmunol 4:283–285. [DOI] [PubMed] [Google Scholar]
- 133. Shaw GM, Harper ME, Hahn BH, Epstein LG, Gajdusek DC, Prive RW, Navia BA, Petito CK, O’Hara CJ, Groopman JE, Cho E‐S, Oleske JM, Wong‐Staal F, Gallo RC (1985) HTLV‐III infection in brains of children and adults with AIDS encephalopathy. Science 227:177–182. [DOI] [PubMed] [Google Scholar]
- 134. Shelburne SA, Hamill RJ, Rodriguez‐Barradas MC, Greenberg SB, Atmar RL, Musher DW, Gathe JC Jr, Visnegarwala F, Trautner BW (2002) Immune reconstruction inflammatory syndrome. Emergence of a unique syndrome during highly active antiretroviral therapy. Medicine 81:213–227. [DOI] [PubMed] [Google Scholar]
- 135. Shi B, De Girolami U, He J, Wang S, Lorenzo A, Busciglio J, Gabuzda D (1996) Apoptosis induced by HIV‐1 infection of the central nervous system. J Clin Invest 98:1979–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Shi B, Raina J, Lorenzo A, Busciglio J, Gabuzda D (1998) Neuronal apoptosis induced by HIV‐1 Tat protein and TNF‐alpha: potentiation of neurotoxicity mediated by oxidative stress and implication for HIV‐1 dementia. J Neurovirol 4:281–290. [DOI] [PubMed] [Google Scholar]
- 137. Sinclair E, Gray F, Ciardi A, Scaravilli F (1994) Immunohistochemical changes and PCR detection of HIV provirus DNA in brains of asymptomatic HIV‐positive patients. J Neuropathol Exp Neurol 53:43–50. [DOI] [PubMed] [Google Scholar]
- 138. Smith TW, De Girolami U, Hénin D, Bolgert F, Hauw JJ (1990) Human immunodeficiency virus (HIV) leukoencephalopathy and the microcirculation. J Neuropathol Exp Neurol 49:357–370. [DOI] [PubMed] [Google Scholar]
- 139. Smyth MJ, Kelly JM, Sutton VR, Davis JE, Browne KA, Sayers TJ, Trapani JA (2001) Unlocking the secrets of cytoplasmic granule proteins. J Leukoc Biol 70:18–29. [PubMed] [Google Scholar]
- 140. Snider WD, Simpson DM, Nielsen S, Gold JW, Metroka CE, Posner JB (1983) Neurological complications of acquired immune deficiency syndrome: analysis of 50 patients. Ann Neurol 14: 403–418. [DOI] [PubMed] [Google Scholar]
- 141. Strittmatter C, Lang W, Wiestler OD, Kleihues P (1992) The changing pattern of human immunodeficiency virus‐ associated cerebral toxoplasmosis: a study of 46 postmortem cases. Acta Neuropathologica 83:475–481. [DOI] [PubMed] [Google Scholar]
- 142. Surtees R, Hyland K, Smith I (1990) Central nervous system methyl‐group metabolism in children with neurological complications of HIV infections. Lancet 335:619–621. [DOI] [PubMed] [Google Scholar]
- 143. Tan SV, Guiloff RJ, Scaravilli F (1995) AIDS‐associated vacuolar myelopathy. A morphometric study. Brain 118:1247–1261. [DOI] [PubMed] [Google Scholar]
- 144. Tantisiriwat W, Tebas P, Clifford DB, Powderly WG, Fichtenbaum CJ (1998) Progressive multifocal leukoencephalopathy in patients with AIDS receiving highly active antiretroviral therapy. Clin Infect Dis 28:1152–1154. [DOI] [PubMed] [Google Scholar]
- 145. Tornatore C, Chandra R, Berger JR, Major EO (1994) HIV‐1 infection of subcortical astrocytes in the pediatric central nervous system. Neurology 44:481–487. [DOI] [PubMed] [Google Scholar]
- 146. Torre D, Speranza F, Martregani R (2005) Impact of highly active antiretroviral therapy on organ‐specific manifestations of HIV‐1 infection. HIV Med 6:66–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Tyor WR, Glass JD, Baumrind N, McArthur JC, Griffin JW, Becker PS, Griffin DE (1993) Cytokine expression of macrophages in HIV‐1‐associated vacuolar myelopathy. Neurology 43:1002–1009. [DOI] [PubMed] [Google Scholar]
- 148. Vallat‐Decouvelaere AV, Chrétien F, Gras G, Le Pavec G, Dormont D, Gray F (2003) Expression of excitatory amino acid‐transporter‐1 in brain macrophages and microglia of HIV patients. A neuroprotective role for microglia? J Neuropathol Exp Neurol 62:475–485. [DOI] [PubMed] [Google Scholar]
- 149. Vallat AV, De Girolami U, He J, Mhashilkar A, Marasco W, Shi B, Gray F, Bell J, Keohane C, Smith TW, Gabuzda D (1998) Localization of HIV‐1 co‐receptors CCR5 and CXCR4 in the brain of children with AIDS. Am J Pathol 152:167–178. [PMC free article] [PubMed] [Google Scholar]
- 150. Vazeux R, Brousse N, Jarry A, Hénin D, Marche C, Vedrenne C, Mikol J, Wolff M, Michou C, Rozenbaum W, Bureau J‐F, Montagnier L, Brahic M (1987) AIDS subacute encephalitis. Identification of HIV‐infected cells. Am J Pathol 126:403–410. [PMC free article] [PubMed] [Google Scholar]
- 151. Vendrely A, Bienvenu B, Gasnault J, Thiebault JB, Salmon D, Gray F (2005) Fulminant inflammatory leukoencephalopathy associated with HAART‐induced immune restoration in AIDS‐related progressive multifocal leukoencephalopathy. Acta Neuropathol 109:449–455. [DOI] [PubMed] [Google Scholar]
- 152. Vinters H, Guerra WF, Eppolito L, Keith PE (1988) Necrotizing vasculitis of the nervous system in a patient with AIDS‐related complex. Neuropathol Appl Neurobiol 14:417–424. [DOI] [PubMed] [Google Scholar]
- 153. Vinters H, Tomiyasu U, Anders KH (1989) Neuropathologic complications of infection with the human immunodeficiency virus (HIV). Adv AIDS Pathol 1:101–130. [PubMed] [Google Scholar]
- 154. Weber JN (1993) HIV infection: a cellular approach. In: The Neuropathology of HIV Infection. Scaravilli F (ed.), pp. 1–8. Springer: London. [Google Scholar]
- 155. Weis S, Haug H, Budka H (1993) Astroglial changes in the cerebral cortex of AIDS brains: a morphometric and immunohistochemical investigation. Neuropathol Appl Neurobiol 19:329–335. [DOI] [PubMed] [Google Scholar]
- 156. Weiss R (1985) Human T‐cell retroviruses. In: RNA Tumor Viruses, 2nd edn, vol 2, Supplements and appendices. Weiss R, Teich N, Varmus H, Coffin J (eds), pp. 405–485. Cold Spring Harbor Laboratory: New York. [Google Scholar]
- 157. Wiley CA, Schrier RD, Nelson JA, Lampert PW, Oldstone MG (1986) Cellular localisation of human immunodeficiency virus infection within the brains of acquired immunodeficiency syndrome patients. Proc Natl Acad Sci USA 83:7089–7093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Wiley CA, Achim C (1994) Human immunodeficiency virus encephalitis is the pathological correlate of dementia in acquired immunodeficiency syndrome. Ann Neurol 36:673–676. [DOI] [PubMed] [Google Scholar]
- 159. Wood ML, MacGinley R, Eisen DP, Allworth AM (1998) HIV combination therapy: partial immune restitution unmasking latent cryptococcal infection. AIDS 12:1491–1494. [DOI] [PubMed] [Google Scholar]
- 160. Xu Y, Kulkosky J, Acheampong E, Nunnari G, Sullivan J, Pomerantz RJ (2004) HIV‐1‐mediated apoptosis of neuronal cells: proximal molecular mechanisms of HIV‐1‐induced encephalopathy. Proc Natl Acad Sci USA 101:7070–7075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Yankner BA, Skolnik PR, Shoukimas GM, Gabuzda DH, Sobel RA, Ho DD (1986) Cerebral granulomatous angiitis associated with isolation of human lymphotropic virus type III from the central nervous system. Ann Neurol 20:362–364. [DOI] [PubMed] [Google Scholar]


