Abstract
MicroRNAs (miRNAs), a novel class of small non‐coding RNAs, are effective post‐transcriptional regulators of gene expression, exhibiting, when altered in human tumors, both oncogenic and tumor suppressive potential. Recently, miRNA involvement in the pathophysiology of brain cancer has been assessed. Aberrant gene expression is the main mechanism of miRNAs dysfunction in cancer, with abnormal expression levels of mature and/or precursor miRNA expression in tumor samples versus normal. MiRNA germline and somatic mutations or polymorphisms in the protein coding messenger RNA targeted by miRNAs may also occur, contributing to cancer predisposition, initiation and/or progression. If present in somatic cells, miRNA alterations may play a role in tumor initiation, while if present in germ line cells they could constitute a cancer predisposing event. MiRNA expression profiling of human tumors has led to the identification of signatures correlated with the tumor diagnosis, staging, progression, prognosis and response to treatment. MiRNA fingerprinting can therefore be added to the diagnostic and prognostic tools used by medical oncologists. Furthermore, new therapeutic strategies involving miRNA silencing or miRNA mimics could be proposed based on the roles of these small non‐coding RNAs as oncogenes and tumor suppressors in brain tumors.
Keywords: microRNAs, cancer
Data accumulated in the last 5 years clearly shows that alterations in microRNA (miRNAs) play a critical role in cancer initiation, progression and metastasis (8, 25, 51). MiRNAs are 19–25 nt in length non‐coding RNAs (ncRNAs) cleaved from 60–110 nt hairpin precursors (pre‐miRNAs) that are produced from large primary transcripts (pri‐miRNAs) sometimes few thousands bases in length (for review see (1, 56). MiRNAs act to regulate gene expression by reducing the levels of transcribed messenger RNA and/or of translated protein, and the spectrum of targets just started to be identified (36). Many miRNAs are conserved between distantly related organisms, suggesting that these molecules participate in essential biological processes, such as brain development (38) or synaptic development and maturation (68). MiRNAs are precisely regulated in time and space in diverse organisms, and specific patterns of miRNA expression can be recognized during brain development and neuronal differentiation (39, 54). In the present review we will discuss the new link between miRNAs and brain tumors. Such findings are important not only for scientists in general, but also for pathologists and clinicians, as the field of ncRNA will shortly touch every aspect of cancer pathology and medical practice in general.
ALTERATIONS OF MIRNAS ARE FOUND IN EVERY TYPE OF CANCER AND MAY CAUSE CANCER PREDISPOSITION AND PROGRESSION
Initially identified in B cell chronic lymphocytic leukemia (CLL) (12), the most common form of adult leukemia, miRNA alterations have been then detected in many types of human tumors (8, 25), including benign and malignant brain tumors (57). MiRNA main alteration in cancer cell is represented by aberrant expression, characterized by abnormal levels of expression for mature and/or precursor miRNA transcripts. The causes of the widespread differential expression of miRNA genes between malignant and normal cells can be explained by the genomic location of these genes in cancer‐associated regions and genomic alterations (10), by epigenetic mechanisms (50) as well as by alterations in the processing machinery (42).
Several developments for high‐throughput miRNAs profiling include oligonucleotide miRNA microarrays as described in (46), high‐throughput array‐based Klenow enzyme (RAKE) assay (58), quantitative RT‐PCR for precursor miRNA (67) or active miRNA (17) (65), miRAGE, a genome‐wide miRNA analysis with Serial Analysis of Gene Expression (24) or use of a bead‐based flow cytometric technique (48) (Table 1). MiRNAs differentially expressed between tumors and normal counterparts were identified for several tumor types like glioblastoma (19), pituitary adenoma (6), breast carcinoma (33), lung carcinoma (74), colorectal carcinoma (23), ovarian carcinoma (34), papillary thyroid carcinoma (30), hepatocellular carcinoma (HCC) (55), pancreatic endocrine (66) and exocrine tumors (4), cervical carcinomas (49), lymphoma (31), and CLL (14).
Table 1.
Examples of high‐throughput methods for miRNA expression. Abbreviations: miRNA = microRNA; qRT‐PCR = quantitative RT‐PCR.
| Type | Principle | Advantages | Disadvantages | References |
|---|---|---|---|---|
| MiRNA microarray custom one color | Thousands of oligonucleotide probes used to hybridize with mature / precursor miRNA probe cDNA | Ability to screen in parallel for the expression of a large number of miRNAs through extensive sample collections | Not able to discriminate easily various members of the same family | (46) |
| Array‐based Klenow enzyme (RAKE) | On‐slide application of the Klenow fragment of DNA polymerase I to extend unmodified miRNAs hybridized to immobilized DNA probes | Specificity and rapid expression profiling of all known miRNAs | Not useful to identify new miRNAs | (58) |
| Bead‐based technology | Single miRNA oligos coating polystyrene beads hybridized with biotin labeled double strand DNA target, followed by flow cytometry signal detection | Higher specificity and accuracy in respect with microarray microchips | Relatively low number of miRNAs that can be detected (less than 100) | (48) |
| MiRAGE (SAGE) | Serial analysis of gene expression adapted for small RNAs | Mixture of cloning and pexpression profiling: adequate to discover new miRNAs | High cost | (24) |
| Stem‐loop qRT‐PCR for mature product | Stem‐loop RT primer cDNA synthesiz followed by quantitative conventional TaqMan PCR | Specific quantification of the mature miRNA | Not useful to identify new miRNAs | (17) |
Glioblastoma multiforme (GBM) is the most common primary intracranial malignancy and the most aggressive type of brain tumor (53), consequently miRNA profiling in this hystotype is of high interest. The global expression in GBM of 245 microRNAs using a microarray technique was performed by Ciafre et al (19). This approach allowed the identification of miRNAs whose expression is significantly altered in tumors vs. peripheral brain area from the same patient, including miR‐221, strongly up‐regulated in glioblastoma and a set of brain‐enriched miRNAs, miR‐128, miR‐181a, miR‐181b and miR‐181c, which were down‐regulated in glioblastoma (Table 2). Chan et al (16) used oligonucleotide arrays printed on membranes with tri‐mer oligonucleotide probes specific for 180 human and mouse miRNAs and found marked elevation of miR‐21 levels in human primary GBM tissues and cell lines compared with non‐neoplastic fetal and adult brain tissues and with cultured non‐neoplastic glial cells. Furthermore, knockdown of miR‐21 in cultured GBM cells triggers activation of caspases and leads to increased apoptotic cell death, suggesting that the oncogenic miR‐21 may contribute to the malignant phenotype by blocking expression of critical apoptosis‐related genes (16) (Table 2). It is important to note that the same miRNA was found in the largest miRNA profiling study on human tumors published to date by Volinia et al (73) to be overexpressed in all six types of solid tumor analyzed.
Table 2.
MicroRNAs as oncogenes and tumor suppressors in brain tumors. Abbreviations: miRNA = microRNA; B‐CLL = B cell chronic lymphocytic leukemia; DLBCL = diffuse large B cell lymphoma.
| MiRNA | Putative function | Deregulation in tumors | Molecular mechanism and regulation | Diagnostic and prognostic markers |
|---|---|---|---|---|
| Has_let‐7 family | Tumor suppressor | Let‐7 members generally display reduced expression in lung, breast, gastric, colon cancers and pituitary adenomas | Let‐7 overexpression represses cell proliferation and let‐7f promotes angiogenesis; Let‐7b is up‐regulated at the transcriptional level by MYCN in primary neuroblastoma | Let‐7a‐2 low expression correlates with poor survival in lung cancer patients; Loss of let‐7 expression identifies a less differentiated class of cancers |
| Hsa_miR‐16‐1 ‐15a cluster | Tumor suppressor | Reduced expression in CLL, DLBCLs, multiple myeloma, pituitary adenoma, prostate and pancreatic cancers | Exogenous restoration in leukemia cells induces apoptosis by directly targeting BCL2, while in solid cancer cells miR‐16 negatively regulates cellular growth and cell cycle progression by down‐regulating G0/G1 proteins (CDK6, CDC27, CARD10 and C10orf46) | MiR‐15a and miR‐16 discriminate between good and bad prognosis in CLL patients |
| Hsa_miR‐21 | Oncogenic | Elevated levels in glioblastoma primary tumors and cell lines, in breast and cervical carcinomas, uterine leiomyosarcoma, and DLBCL | Controls cell proliferation and apoptosis by regulating BCL2 expression in breast cancer cells; MiR‐21 knockdown in glioblastoma cells increases apoptosis; Induces invasion, intravasation and metastasis by targeting PDCD4 in colorectal cancers. Regulated at the transcriptional level by STAT3 | High expression is associated with longer relapse free survival in DLBCL |
| Hsa_miR‐181 family | Oncogenic | Overexpression in breast, pancreas, prostate cancers | Regulated at the transcriptional level by MYCN | Low expression in aggressive CLL with 11q deletions |
| Hsa_miR‐221‐222 cluster | Oncogenic | Highly expressed in CLL, thyroid papillary carcinoma and glioblastoma | Directly target p27 and promotes cancer cell proliferation; Positive transcriptional regulation of miR‐221 by MYCN in primary neuroblastoma | High expression of miR‐221 correlates with a bad prognosis in CLL patients |
Gene names as in NCBI at http://www.ncbi.nlm.nih.gov/sites/entrez
MicroRNA name according to mirBASE at http://microrna.sanger.ac.uk/sequences/.
Pituitary adenomas are the most common tumors in the central nervous system, and account for approximately 10% of all primary intracerebral tumors (32). An initial study from Bottoni et al (5) performed by Northern blot, individuated a cluster of two miRNAs, miR‐15a and miR‐16 to have a reduced expression in the majority of samples analyzed (Table 2). Interestingly, the miR‐15a‐16 cluster is located at chromosome 13q14.3 in a region frequently deleted in this type of tumor. Moreover, the expression inversely correlated with tumor diameter suggesting that these genes may, at least in part, influence tumor growth. A subsequent genome‐wide profiling performed by the same group using microarray and quantitative RT‐PCR (6) in 32 pituitary adenomas and in 6 normal pituitary gland samples found that 30 miRNAs are differentially expressed between normal pituitary and pituitary adenomas. Among them are the members of let‐7 family, well‐known tumor suppressor miRNAs in human lung cancers by regulating RAS oncogene expression (37) (Table 2).
In spite of decades of research, the molecular basis for the major fraction of familial cancers is unknown. As the thermodynamics of RNA–RNA binding play essential roles in the miRNA interaction with the target mRNA, sequence variations influencing this interaction may result in cancer predisposition (9). In fact, a germ line mutation in the pri‐miR‐16‐1/15a precursor in a patient with familial CLL and breast cancer in first‐degree members of family suggests a possible predisposing effect (14). Strongly supporting this paradigm are the results by Raveche et al describing abnormal miR‐16 locus with synteny to human 13q14 linked to CLL in NZB mice (64). Together these two studies, one in human CLL and the second in a mouse model of the human disease confirm that miR‐16 is the first example of a miRNA involved in cancer predisposition (9, 11). Recently, Sevignani et al (69) reported a statistically significant association between the chromosomal location of miRNAs and that of mouse cancer susceptibility loci influencing the development of solid tumors and identified distinct patterns of flanking DNA sequences for several miRNAs located at or near susceptibility loci in inbred strains with different tumor susceptibilities. Furthermore, germ line single nucleotide polymorphysms (SNP) were identified in two recognition sites in c‐KIT oncogene for miR‐221, miR‐222 and miR‐146, all strongly overexpressed in thyroid cancers (30), meaning that the SNPs in 3′UTR of messenger RNAs could not be simple evolution bystanders but active “players” in the cancer predisposition. All these data together, strongly suggest that miRNAs are involved in cancer initiation and progression at a much higher extent as initially believed, prompting the focus of hundreds of laboratories on the identification of molecular pathways involving miRNAs.
MIRNAS AS ONCOGENES AND TUMOR SUPPRESSORS
The classical view of cancer as a genetic disease affecting oncogenes and tumor suppressor genes known to code for proteins is now expanding to include also miRNAs (1, 2, and Table 2). MiRNA genes are frequently located at fragile sites, in minimal regions of loss of heterozygosity, minimal regions of amplification (minimal amplicons), and common breakpoint regions (13). Many miRNA have been demonstrated to act either as tumor suppressor genes or oncogenes according to the mRNA they target and proven to accelerate the oncogenic process (25). MiRNAs themselves can act as tumor suppressors when down‐regulated (as is the case of miR‐15a and miR‐16‐1 (20) or let‐7 family (37)) or as oncogenes when overexpressed (as is the case of miR‐155 (73), miR17‐92 cluster (31) or miR‐21 (16, 73)).
Figure 1.
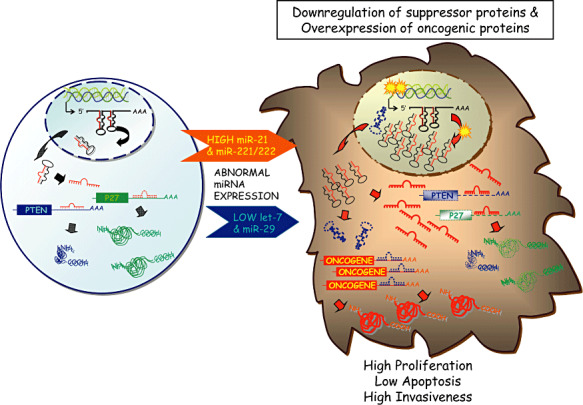
MicroRNAs as oncogenes and tumor suppressors. Cancer is a very complex genetic disease characterized by alterations in oncogenic and tumor‐suppressor protein‐coding genes and microRNAs. The abnormalities found to influence the activity of miRNAs includes chromosomal rearrangements, genomic amplifications or deletions, mutations and hypermethylation. Activation of oncogenic microRNAs reduced the levels of proteins blocking proliferation and activating apoptosis; by contrast, inactivation of suppressor miRNAs is followed by accumulation of proteins that stimulates proliferation and decrease apoptosis. The two oncogenic paradigms found in gliomas, the interaction between the oncogenic miR‐21 and the suppressor protein coding gene PTEN and between the oncogenic cluster miR‐221‐222 and the p27kip1 suppressor protein are schematically presented. The possibility that other miRNAs (such as members of let‐7 or miR‐29 families) are down‐regulated and, consequently oncogenic proteins (such as RAS) overexpressed, is also presented.
Figure 2.
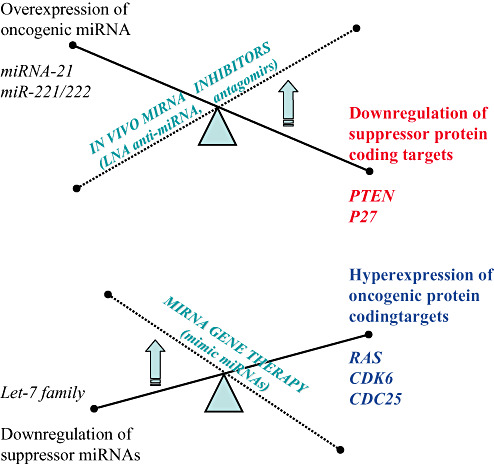
Principles of microRNA (miRNA)—based gene therapy and in vivo inhibition of miRNAs in cancer cells. The major types of microRNA manipulation with therapeutic goals in order to revert the malignant phenotype are presented in link with the main miRNA abnormalities found in human cancers.
The cluster miR‐221‐222 (19, 43) has been found to be up‐regulated and to have a pro‐tumorigenic effect in GBM, as well as in thyroid papillary carcinomas, pancreatic and prostate cancers (27, 30, 44, 72). The gears through which these two highly similar clustered miRNAs seem to work have been partially disclosed by the identification of the CDK inhibitor p27kip1 as their primary direct target (29, 43, 72), through different though convergent approaches (bioinformatic analyses, use of a miRNA expression library competed with sensor vector containing p27kip1 3′UTR, Dicer knockdown in GBM cell lines and luciferase 3′UTR complementarity assays). These different studies proved that miR‐221‐222 overexpression leads to cell cycle deregulation and their loss determines a G1 cell cycle arrest, distinctive of a p27kip1 block and which is not achieved in p27kip1 absence. Besides identifying the direct interaction responsible for p27kip1 reduction in GBM cell lines as well as in other tumor cell lines, Agami's group also found evidence for an in vivo inverse correlation between miR‐221‐222 and p27kip1 expression levels in GBM. P27kip1 is a well‐known tumor suppressor down‐regulated in many human tumors including GBM, and its expression levels in primary cancers are highly correlated with decreased patient survival (70). Similarly, miR‐221‐222 cluster has also been associated with poor prognostic miRNA signatures in thyroid papillary carcinomas (30, 61), pancreatic adenocarcinoma and GBM (19, 44), indeed reinforcing interconnection between miR‐221‐222 and p27kip1 in the pathogenesis of GBM. For this tumor type p27kip1 has also been suggested as possible candidate for adenoviral delivering gene therapy (62).
Another miRNA found overexpressed in GBM and revealed to have oncogenic potentials is miR‐21. The role of miR‐21 overexpression in glioblastomas was investigated by a loss of function approach. MiR‐21 was down modulated efficiently using sequence specific chemically modified oligonucleotides (2′‐O‐methyloligoribonucleotides and locked nucleic acids, LNA oligonucleotides). Mir‐21 downregulation determined a striking reduction in the total cell number and the effect was mainly caused by an increase in apoptosis with caspase activation rather than a result of decreased proliferation (16). At least in GBM, miR‐21 is an anti‐apoptotic factor, as in Hela cells its down‐regulation determines instead an increase in cell proliferation without affecting apoptosis (18). The different biological effect of miR‐21 in different cells is very likely to be dependent on the cell‐specific repertoire of target genes. MiR‐21 in GBM functions as an oncogenic miRNA by blocking expression of apoptosis‐enabling genes (16). Although in brain tumors, the exact mechanism of action has not been unveiled, it is possible that its oncogenic functions may be exerted in similar ways as found in other tumors. For example, Meng et al (52) found that miR‐21, highly overexpressed in hepatocellular carcinoma (HCC), directly interact to the 3′UTR of the PTEN gene and to reduce PTEN protein expression, with a consequent increase in phosphorylation of its downstream targets (FAK and AKT). Nevertheless PTEN was originally cloned as a tumor suppressor for brain tumors (45) with point mutations occurring in 25% of the cases (59), ulteriorly confirming the relevance of miR‐21 in GBM pathogenesis. Further work done by Frankel et al in breast cancer cells (26) and by Asangani et al (2) in colon cancer cells found that miR‐21 overexpression leads to PDCD4 reduction, by direct interaction with its 3′UTR and that anti‐miR‐21 treatment is followed by an increase in endogenous PDCD4 protein levels. PDCD4 is a tumor suppressor known to be up‐regulated during apoptosis (75, 76) reduced in different tumors (28) and evidence demonstrate that miR‐21 overexpression effects are at least in part due to its down‐regulation. Although this target has not been proven in brain tumors, it is possible that it may be relevant in these tumor types as well, as a recent study demonstrated that the majority of the glioma samples analyzed lacked PDCD4 protein expression, whereas adjacent normal glial tissues expressed high levels of PDCD4 (28). An additional molecular mechanism proving the oncogenic properties of miR‐21 in an in vivo xenograft breast cancer model consists in targeting of the tumor suppressor protein TPM1 at the translational level. TPM1 per se is able to reduce tumor cell proliferation and anchorage independence therefore explaining the effects on tumor growth exerted by miR‐21 (77).
It is well established that constitutive activation of Stat3 contributes to the pathogenesis of glioblastoma by promoting both proliferation and survival of GBM cells (63), and recent findings support that stat3 activation regulates among other anti‐apoptotic proteins (survivin, Bcl‐2, and Mcl‐1) also miR‐21 (28). In GBM, miR‐21 overexpression could be, to a certain extent, explained by Stat3 iperactivation therefore targeting Stat3 signaling may provide a potential therapeutic intervention for GBM (47), also because indirectly affecting miR‐21 transcription.
MIRNA PROFILING IS A NEW DIAGNOSTIC AND PROGNOSTIC TOOL FOR CANCER PATIENTS
As the cancer mortality did not drop significantly in the last two decades (35), any new tool for diagnosis and prognosis is welcomed in the panel of cancer patient care. Furthermore, the brain tumors represent a special medical condition not only because of the aggressiveness, but also because of their very specific location, that make more difficult any type of diagnostic and therapeutic intervention (7). In the study by Bottoni et al (6), 24 miRNAs were identified as a predictive signature of pituitary adenoma and 29 miRNAs were able to predict pituitary adenoma histotype. MiRNA expression could differentiate micro‐ from macro‐adenomas and treated from non‐treated patient samples. Several of the identified miRNAs are involved in cell proliferation and apoptosis, suggesting that their deregulated expression may be involved in pituitary tumorigenesis. MiR‐21 expression was found by Chan et al, (16) increased 5‐ to 100‐fold in human GBM tissue compared with control non‐neoplastic brain. Furthermore, they found a similarly robust increase in miR‐21 expression in six commonly used model cell lines derived from human glioblastomas. These represent only initial studies that should be followed by large trials in patients with brain cancers to support the biomarker roles for these miRNAs.
Large profiling studies using solid tissue and hematological tumors have proven the utility of miRNA profiling for diagnosis and prognosis (14, 48, 73). The diagnosis of the metastatic cancer of unknown primary site represent a special medical issue as these patients present with metastases without an established primary tumor, and therefore the therapeutically curative or palliative intervention are delayed (48). Lu et al, using a bead‐based flow‐cytometric profiling technology performed on a set of 334 including multiple human cancers mainly leukemias, found that the miRNA‐based classifier is better in establishing the correct diagnosis of poorly differentiated samples with non‐diagnostic histological appearance than the protein coding genes messenger RNA classifier. Volinia et al (73) described a large‐scale microarray analysis in 540 samples from six solid types of the most frequent human cancers and found a common signature composed by 61 miRNAs. Out of the 228 miRNA analyzed, 36 were overexpressed and 21 were down‐regulated in cancer cells vs. normal cells. Hierarchical clustering analysis showed that this miRNA signature enabled the tumor samples to be grouped based on their tissue of origin, or in other words to help in diagnosis (73). In the majority of CLL patients, the prognosis is relatively good and the treatment after diagnosis is started only if poor prognostic markers are evident. By performing a miRNA profiling screen on 144 CLL patients, a unique signature of 13 miRNAs was shown to differentiate cases on the basis of a good or bad prognosis or on the presence or absence of disease progression (14). Among them, miR‐16‐1 and miR‐15a were expressed at lower levels in patients with a good prognosis, in agreement with early reports that 13q14.3 genomic deletions at the locus harboring these genes are related with a favorable course of the disease. Thus, CLL miRNA profiling could help in deciding which patient should undergo treatment ab initio, avoiding the watchful waiting attitude.
MiRNA expression could be exploited for better understanding the response to therapy, too. For example, as targeting PTEN suppressor gene, miR‐21 expression could be used as a predictor of response to AKT/PI3K pathway targeting therapies and the expression of downstream mediators of tumor growth and metastasis could be modulated by targeting mir‐21 (52).
MIRNAS AS NEW THERAPEUTIC TARGETS
The fact that miRNA overexpression in cancer cells has a pathogenic effect provides the rationale for using miRNAs as potential therapeutic targets in cancer. Several agents like LNAs anti‐miRNAs and antagomirs are now under development and ready to be tested for in vivo effects and if present, hopefully to be used in clinical trials (71). The LNAs anti‐miRNAs represents modified antisense single‐stranded oligonucleotide about 22 nt in length designed to be complementary to a selected miRNA and thereby specifically inhibit expression of that gene (60). The antagomirs are chemically modified and cholesterol‐conjugated single‐stranded 23‐nucleotide RNAs complementary to the targeted miRNA and represent the only yet RNA therapeutic molecule designed to specifically inhibit the miRNA (40). The modifications were introduced to increase the stability of the RNA and protect it from degradation. The only tissue where antagomirs did not act when injected systemically was the brain, probably because of the difficulty of crossing the blood‐brain barrier, but when injected locally into the mouse cortex they efficiently target miRNAs (41).
Several recently reported trials in gliomas have investigated novel approaches such as molecularly targeted therapy. Important for the potential clinical use, miR‐21 that was shown to be strongly overexpressed in glioblastomas, could be silenced in vitro by using LNA‐modified antisense oligonucleotides—leading to a significant reduction in cell viability, accompanied by elevated intracellular levels of caspases (16). Corsten et al (21), by using LNA‐anti‐miR‐21 oligonucleotides, showed that the combined suppression of miR‐21 and neural precursor cells expressing a secretable variant of the cytotoxic agent tumor necrosis factor‐related apoptosis inducing ligand (NPC‐S‐TRAIL) leads to a synergistic increase in caspase activity and significantly decreases cell viability in human glioma cells in vitro and in an in vivo brain tumor mouse xenograft.
Although exciting and full of promises, the field of RNA as a therapeutic molecule is not free of obstacles. A good practical option is to understand what could be the strategies used to optimize the efficiency of this new anti‐cancer strategy. One such possible strategy for glioblastoma should exploit the molecular interactions between miR‐21 and PTEN and between miR‐221‐222 cluster and p27kip1, respectively. Therefore, a mixed approach of targeting at the same time both oncogenics miRNA in association with chemotherapic drugs could be proposed. While an anti‐miR‐21 targeted therapy would increase GBM apoptotic rate, targeting miR‐221 and ‐222 by restoring normal p27kip1 levels would reduce their aggressive growth and also affect their invasive potential, considering p27kip1 novel role in inhibiting cell motility (3). Therefore, anti‐miR‐21 plus anti‐miR‐221/222 therapy could be tested in the near future in phase I trials in conjunction with established therapies. Much more, in light of the newly discovered interactions between various categories of non‐codingRNAs (15), targeting not miRNAs but ultraconserved genes regulated by miRNAs (eg, miR‐21 and miR‐221‐222 cluster) start to be an alternative option. The time of RNA therapeutics is not far away, and we are looking forward for clinical studies showing high efficiency of target inhibition with significant increase in patient survival and reduced toxic effects.
CONCLUSIONS
Instead of simply adding a new layer of complexity to cancer cell molecular machinery, miRNAs disrupted in cancer may be the dominant unifying players. Participation of miRNAs in cancer predisposition will be better understood in the near future by wider studies of familial cancer patients. MiRNAs, after half a century of being misclassified as the degraded form of “the messenger in the middle” (22, 57), have rapidly changed the dogma of cellular biology and are readily starting to be recognized as promising diagnostic and prognostic tools for cancer patients. Being the natural antisense regulators of multiple genes involved in fundamental eukaryotic survival and proliferation, the miRNA targeting perspective in cancer therapy is becoming not just an optimistic view of cancer research but a near and real future option for medical oncologists.
ACKNOWLEDGMENTS
Dr Calin is supported by an MD Anderson Trust grant and by a Regent scholarship and by the CLL Global Research Foundation. We apologize to our colleagues, whose work was not cited because of space limitations.
REFERENCES
- 1. Ambros V (2003) MicroRNA pathways in flies and worms: growth death, fat, stress, and timing. Cell 113:673–676. [DOI] [PubMed] [Google Scholar]
- 2. Asangani IA, Rasheed SA, Nikolova DA, Leupold JH, Colburn NH, Post S, Allgayer H (2007) MicroRNA‐21 (miR‐21) post‐transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene [Epub ahead of print. [DOI] [PubMed]
- 3. Baldassarre G, Belletti B, Nicoloso MS, Schiappacassi M, Vecchione A, Spessotto P et al (2005) p27(Kip1)‐stathmin interaction influences sarcoma cell migration and invasion. Cancer Cell 7:51–63. [DOI] [PubMed] [Google Scholar]
- 4. Bloomston M, Frankel WL, Petrocca F, Volinia S, Alder H, Hagan JP et al (2007) MicroRNA expression patterns to differentiate pancreatic adenocarcinoma from normal pancreas and chronic pancreatitis. JAMA 297:1901–1908. [DOI] [PubMed] [Google Scholar]
- 5. Bottoni A, Piccin D, Tagliati F, Luchin A, Zatelli MC, Degli Uberti EC (2005) miR‐15a and miR‐16‐1 down‐regulation in pituitary adenomas. J Cell Physiol 204:280–285. [DOI] [PubMed] [Google Scholar]
- 6. Bottoni A, Zatelli MC, Ferracin M, Tagliati F, Piccin D, Vignali C et al (2007) Identification of differentially expressed microRNAs by microarray: a possible role for microRNA genes in pituitary adenomas. J Cell Physiol 210:370–377. [DOI] [PubMed] [Google Scholar]
- 7. Buckner JC, Brown PD, O'Neill BP, Meyer FB, Wetmore CJ, Uhm JH (2007) Central nervous system tumors. Mayo Clin Proc 82:1271–1286. [DOI] [PubMed] [Google Scholar]
- 8. Calin GA, Croce CM (2006) MicroRNA signatures in human cancers. Nat Rev Cancer 6:857–866. [DOI] [PubMed] [Google Scholar]
- 9. Calin GA, Croce CM (2006) MicroRNA‐cancer connection: the beginning of a new tale. Cancer Res 66:7390–7394. [DOI] [PubMed] [Google Scholar]
- 10. Calin GA, Croce CM (2006) MicroRNAs and chromosomal abnormalities in cancer cells. Oncogene 25:6202–6210. [DOI] [PubMed] [Google Scholar]
- 11. Calin GA, Croce CM (2007) Chromosomal rearrangements and microRNAs: a new cancer link with clinical implications. J Clin Invest 117:2059–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Calin GA, Dumitru CD, Shimizu M, Bichi R, Zupo S, Noch E et al (2002) Frequent deletions and down‐regulation of micro‐ RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci USA 99:15524–15529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Calin GA, Sevignani C, Dumitru CD, Hyslop T, Noch E, Yendamuri S et al (2004) Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc Natl Acad Sci USA 101:2999–3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Calin GA, Ferracin M, Cimmino A, Di Leva G, Shimizu M, Wojcik SE et al (2005) A MicroRNA signature associated with prognosis and progression in chronic lymphocytic leukemia. N Engl J Med 353:1793–1801. [DOI] [PubMed] [Google Scholar]
- 15. Calin GA, Liu CG, Ferracin M, Hyslop T, Spizzo R, Sevignani C et al (2007) Ultraconserved regions encoding ncRNAs are altered in human leukemias and carcinomas. Cancer Cell 12:215–229. [DOI] [PubMed] [Google Scholar]
- 16. Chan JA, Krichevsky AM, Kosik KS (2005) MicroRNA‐21 is an antiapoptotic factor in human glioblastoma cells. Cancer Res 65:6029–6033. [DOI] [PubMed] [Google Scholar]
- 17. Chen C, Ridzon DA, Broomer AJ, Zhou Z, Lee DH, Nguyen JT et al (2005) Real‐time quantification of microRNAs by stem‐loop RT‐PCR. Nucleic Acids Res 33:e179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cheng AM, Byrom MW, Shelton J, Ford LP (2005) Antisense inhibition of human miRNAs and indications for an involvement of miRNA in cell growth and apoptosis. Nucleic Acids Res 33:1290–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ciafre SA, Galardi S, Mangiola A, Ferracin M, Liu CG, Sabatino G et al (2005) Extensive modulation of a set of microRNAs in primary glioblastoma. Biochem Biophys Res Commun 334:1351–1358. [DOI] [PubMed] [Google Scholar]
- 20. Cimmino A, Calin GA, Fabbri M, Iorio MV, Ferracin M, Shimizu M et al (2005) miR‐15 and miR‐16 induce apoptosis by targeting BCL2. Proc Natl Acad Sci USA 102:13944–13949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Corsten MF, Miranda R, Kasmieh R, Krichevsky AM, Weissleder R, Shah K (2007) MicroRNA‐21 knockdown disrupts glioma growth in vivo and displays synergistic cytotoxicity with neural precursor cell delivered S‐TRAIL in human gliomas. Cancer Res 67:8994–9000. [DOI] [PubMed] [Google Scholar]
- 22. Crick FH (1958) On protein synthesis. Symp Soc Exp Biol 12:138–163. [PubMed] [Google Scholar]
- 23. Cummins JM, Velculescu VE (2006) Implications of micro‐RNA profiling for cancer diagnosis. Oncogene 25:6220–6227. [DOI] [PubMed] [Google Scholar]
- 24. Cummins JM, He Y, Leary RJ, Pagliarini R, Diaz LA Jr, Sjoblom T et al (2006) The colorectal microRNAome. Proc Natl Acad Sci USA 103:3687–3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Esquela‐Kerscher A, Slack FJ (2006) Oncomirs—microRNAs with a role in cancer. Nat Rev Cancer 6:259–269. [DOI] [PubMed] [Google Scholar]
- 26. Frankel LB, Christoffersen NR, Jacobsen A, Lindow M, Krogh A, Lund AH (2007) Programmed cell death 4 (PDCD4) is an important functional target of the microRNA miR‐21 in breast cancer cells. J Biol Chem [Epub ahead of print. [DOI] [PubMed]
- 27. Galardi S, Mercatelli N, Giorda E, Massalini S, Frajese GV, Ciafre SA, Farace MG (2007) miR‐221 and miR‐222 expression affects the proliferation potential of human prostate carcinoma cell lines by targeting p27Kip1. J Biol Chem 282:23716–23724. [DOI] [PubMed] [Google Scholar]
- 28. Gao F, Zhang P, Zhou C, Li J, Wang Q, Zhu F et al (2007) Frequent loss of PDCD4 expression in human glioma: possible role in the tumorigenesis of glioma. Oncol Rep 17:123–128. [PubMed] [Google Scholar]
- 29. Gillies JK, Lorimer IA (2007) Regulation of p27Kip1 by miRNA 221/222 in glioblastoma. Cell Cycle 6:2005–2009. [DOI] [PubMed] [Google Scholar]
- 30. He H, Jazdzewski K, Li W, Liyanarachchi S, Nagy R, Volinia S et al (2005) The role of microRNA genes in papillary thyroid carcinoma. Proc Natl Acad Sci USA 102:19075–19080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. He L, Thomson JM, Hemann MT, Hernando‐Monge E, Mu D, Goodson S et al (2005) A microRNA polycistron as a potential human oncogene. Nature 435:828–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Heaney AP (2005) Pituitary tumour pathogenesis. Br Med Bull 75–76:81–97. [DOI] [PubMed] [Google Scholar]
- 33. Iorio MV, Ferracin M, Liu CG, Veronese A, Spizzo R, Sabbioni S et al (2005) MicroRNA gene expression deregulation in human breast cancer. Cancer Res 65:7065–7070. [DOI] [PubMed] [Google Scholar]
- 34. Iorio MV, Visone R, Di Leva G, Donati V, Petrocca F, Casalini P et al (2007) MicroRNA signatures in human ovarian cancer. Cancer Res 67:8699–8707. [DOI] [PubMed] [Google Scholar]
- 35. Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ (2007) Cancer statistics, 2007. CA Cancer J Clin 57:43–66. [DOI] [PubMed] [Google Scholar]
- 36. Johnson CD, Esquela‐Kerscher A, Stefani G, Byrom M, Kelnar K, Ovcharenko D et al (2007) The let‐7 microRNA represses cell proliferation pathways in human cells. Cancer Res 67:7713–7722. [DOI] [PubMed] [Google Scholar]
- 37. Johnson SM, Grosshans H, Shingara J, Byrom M, Jarvis R, Cheng A et al (2005) RAS is regulated by the let‐7 microRNA family. Cell 120:635–647. [DOI] [PubMed] [Google Scholar]
- 38. Kapsimali M, Kloosterman WP, De Bruijn E, Rosa F, Plasterk RH, Wilson SW (2007) MicroRNAs show a wide diversity of expression profiles in the developing and mature central nervous system. Genome Biol 8:R173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Krichevsky AM, King KS, Donahue CP, Khrapko K, Kosik KS (2003) A microRNA array reveals extensive regulation of microRNAs during brain development. RNA 9:1274–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Krutzfeldt J, Rajewsky N, Braich R, Rajeev KG, Tuschl T, Manoharan M, Stoffel M (2005) Silencing of microRNAs in vivo with 'antagomirs. Nature 438:685–689. [DOI] [PubMed] [Google Scholar]
- 41. Krutzfeldt J, Kuwajima S, Braich R, Rajeev KG, Pena J, Tuschl T et al (2007) Specificity, duplex degradation and subcellular localization of antagomirs. Nucleic Acids Res 35:2885–2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kumar MS, Lu J, Mercer KL, Golub TR, Jacks T (2007) Impaired microRNA processing enhances cellular transformation and tumorigenesis. Nat Genet 39:673–677. [DOI] [PubMed] [Google Scholar]
- 43. Le Sage C, Nagel R, Egan DA, Schrier M, Mesman E, Mangiola A et al (2007) Regulation of the p27(Kip1) tumor suppressor by miR‐221 and miR‐222 promotes cancer cell proliferation. EMBO J 26:3699–3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lee EJ, Gusev Y, Jiang J, Nuovo GJ, Lerner MR, Frankel WL et al (2007) Expression profiling identifies microRNA signature in pancreatic cancer. Int J Cancer 120:1046–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI et al (1997) PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science 275:1943–1947. [DOI] [PubMed] [Google Scholar]
- 46. Liu CG, Calin GA, Meloon B, Gamliel N, Sevignani C, Ferracin M et al (2004) An oligonucleotide microchip for genome‐wide microRNA profiling in human and mouse tissues. Proc Natl Acad Sci USA 101:9740–9744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Loffler D, Brocke‐Heidrich K, Pfeifer G, Stocsits C, Hackermuller J, Kretzschmar AK et al (2007) Interleukin‐6 dependent survival of multiple myeloma cells involves the Stat3‐mediated induction of microRNA‐21 through a highly conserved enhancer. Blood 110:1330–1333. [DOI] [PubMed] [Google Scholar]
- 48. Lu J, Getz G, Miska EA, Alvarez‐Saavedra E, Lamb J, Peck D et al (2005) MicroRNA expression profiles classify human cancers. Nature 435:834–838. [DOI] [PubMed] [Google Scholar]
- 49. Lui WO, Pourmand N, Patterson BK, Fire A (2007) Patterns of known and novel small RNAs in human cervical cancer. Cancer Res 67:6031–6043. [DOI] [PubMed] [Google Scholar]
- 50. Lujambio A, Esteller M (2007) CpG island hypermethylation of tumor suppressor microRNAs in human cancer. Cell Cycle 6:1455–1459. [PubMed] [Google Scholar]
- 51. Ma L, Teruya‐Feldstein J, Weinberg RA (2007) Tumour invasion and metastasis initiated by microRNA‐10b in breast cancer. Nature 449:682–688. [DOI] [PubMed] [Google Scholar]
- 52. Meng F, Henson R, Wehbe‐Janek H, Ghoshal K, Jacob ST, Patel T (2007) MicroRNA‐21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology 133:647–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Miller CR, Perry A (2007) Glioblastoma. Arch Pathol Lab Med 131:397–406. [DOI] [PubMed] [Google Scholar]
- 54. Miska EA, Alvarez‐Saavedra E, Townsend M, Yoshii A, Sestan N, Rakic P et al (2004) Microarray analysis of microRNA expression in the developing mammalian brain. Genome Biol 5:R68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Murakami Y, Yasuda T, Saigo K, Urashima T, Toyoda H, Okanoue T, Shimotohno K (2006) Comprehensive analysis of microRNA expression patterns in hepatocellular carcinoma and non‐tumorous tissues. Oncogene 25:2537–2545. [DOI] [PubMed] [Google Scholar]
- 56. Nelson P, Kiriakidou M, Sharma A, Maniataki E, Mourelatos Z (2003) The microRNA world: small is mighty. Trends Biochem Sci 28:534–540. [DOI] [PubMed] [Google Scholar]
- 57. Nelson PT, Keller JN (2007) RNA in brain disease: no longer just “the messenger in the middle”. J Neuropathol Exp Neurol 66:461–468. [DOI] [PubMed] [Google Scholar]
- 58. Nelson PT, Baldwin DA, Scearce LM, Oberholtzer JC, Tobias JW, Mourelatos Z (2004) Microarray‐based, high‐throughput gene expression profiling of microRNAs. Nat Methods 1:155–161. [DOI] [PubMed] [Google Scholar]
- 59. Ohgaki H, Kleihues P (2007) Genetic pathways to primary and secondary glioblastoma. Am J Pathol 170:1445–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Orom UA, Kauppinen S, Lund AH (2006) LNA‐modified oligonucleotides mediate specific inhibition of microRNA function. Gene 372:137–141. [DOI] [PubMed] [Google Scholar]
- 61. Pallante P, Visone R, Ferracin M, Ferraro A, Berlingieri MT, Troncone G et al (2006) MicroRNA deregulation in human thyroid papillary carcinomas. Endocr Relat Cancer 13:497–508. [DOI] [PubMed] [Google Scholar]
- 62. Park KH, Lee J, Yoo CG, Kim YW, Han SK, Shim YS et al (2004) Application of p27 gene therapy for human malignant glioma potentiated by using mutant p27. J Neurosurg 101:505–510. [DOI] [PubMed] [Google Scholar]
- 63. Rahaman SO, Harbor PC, Chernova O, Barnett GH, Vogelbaum MA, Haque SJ (2002) Inhibition of constitutively active Stat3 suppresses proliferation and induces apoptosis in glioblastoma multiforme cells. Oncogene 21:8404–8413. [DOI] [PubMed] [Google Scholar]
- 64. Raveche ES, Salerno E, Scaglione BJ, Manohar V, Abbasi F, Lin YC et al (2007) Abnormal microRNA‐16 locus with synteny to human 13q14 linked to CLL in NZB mice. Blood 109:5079–5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Raymond CK, Roberts BS, Garrett‐Engele P, Lim LP, Johnson JM (2005) Simple, quantitative primer‐extension PCR assay for direct monitoring of microRNAs and short‐interfering RNAs. RNA 11:1737–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Roldo C, Missiaglia E, Hagan JP, Falconi M, Capelli P, Bersani S et al (2006) MicroRNA Expression Abnormalities in Pancreatic Endocrine and Acinar Tumors are associated with distinctive pathological features and clinical behavior. J Clin Oncol 24:4677–4684. [DOI] [PubMed] [Google Scholar]
- 67. Schmittgen TD, Jiang J, Liu Q, Yang L (2004) A high‐throughput method to monitor the expression of microRNA precursors. Nucleic Acids Res 32:e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Schratt GM, Tuebing F, Nigh EA, Kane CG, Sabatini ME, Kiebler M, Greenberg ME (2006) A brain‐specific microRNA regulates dendritic spine development. Nature 439:283–289. [DOI] [PubMed] [Google Scholar]
- 69. Sevignani C, Calin GA, Nnadi SC, Shimizu M, Davuluri RV, Hyslop T et al (2007) MicroRNA genes are frequently located near mouse cancer susceptibility loci. Proc Natl Acad Sci USA 104: 8017–8022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Slingerland J, Pagano M (2000) Regulation of the cdk inhibitor p27 and its deregulation in cancer. J Cell Physiol 183:10–17. [DOI] [PubMed] [Google Scholar]
- 71. Tili E, Michaille JJ, Gandhi V, Plunkett W, Sampath D, Calin GA (2007) MiRNAs and their potential for use against cancer and other diseases. Future Oncology 3:521–537. [DOI] [PubMed] [Google Scholar]
- 72. Visone R, Russo L, Pallante P, De Martino I, Ferraro A, Leone V et al (2007) MicroRNAs (miR)‐221 and miR‐222, both overexpressed in human thyroid papillary carcinomas, regulate p27Kip1 protein levels and cell cycle. Endocr Relat Cancer 14:791–798. [DOI] [PubMed] [Google Scholar]
- 73. Volinia S, Calin GA, Liu CG, Ambs S, Cimmino A, Petrocca F et al (2006) A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci USA 103: 2257–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Yanaihara N, Caplen N, Bowman E, Seike M, Kumamoto K, Yi M et al (2006) Unique microRNA molecular profiles in lung cancer diagnosis and prognosis. Cancer Cell 9:189–198. [DOI] [PubMed] [Google Scholar]
- 75. Yang HS, Knies JL, Stark C, Colburn NH (2003) Pdcd4 suppresses tumor phenotype in JB6 cells by inhibiting AP‐1 transactivation. Oncogene 22:3712–3720. [DOI] [PubMed] [Google Scholar]
- 76. Zhang H, Ozaki I, Mizuta T, Hamajima H, Yasutake T, Eguchi Y et al (2006) Involvement of programmed cell death 4 in transforming growth factor‐beta1‐induced apoptosis in human hepatocellular carcinoma. Oncogene 25:6101–6112. [DOI] [PubMed] [Google Scholar]
- 77. Zhu S, Si ML, Wu H, Mo YY (2007) MicroRNA‐21 targets the tumor suppressor gene tropomyosin 1 (TPM1). J Biol Chem 282:14328–14336. [DOI] [PubMed] [Google Scholar]


