CLINICAL HISTORY AND IMAGING STUDIES
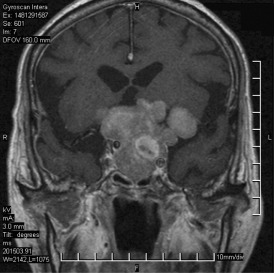
The patient is a man in his mid‐70s who had a subungual acral lentiginous melanoma (T3N1M0) of left big toe underwent amputation 15 months ago. Metastatic malignant melanoma of left inguinal lymph node was diagnosed with biopsy 14 months ago. Progressive visual disturbance was noted and a pituitary tumor was found with hypoglycemic episode 1 month ago. He presented to the hospital with falling down with initial loss of consciousness 2 days before admission. His past medical history was significant for chronic obstructive pulmonary disease. Endocrinologic analysis showed modest hyperprolactinemia, hypothyroidism and partial insufficiency of adrenocorticotropic hormone (ACTH). CT scans of the chest, abdomen and pelvis revealed enlarged lymph nodes in mediastinal, porta hepatis, para‐aortic, and bilateral inguinal regions. The largest one was 18 mm in the long axis at the left inguinal region. A 3 cm soft tissue mass was noted at the left renal hilum. Cerebral MRI demonstrated a 62 × 33 × 49 mm tumor occupying the enlarged pituitary fossa, extending upward to the suprasellar region and encasing both carotid arteries and the left middle cerebral artery (Figure 1). The tumor was mainly T1‐isointense and T2‐hyperintense to the gray matter, and homogeneously enhanced after contrast administration. Several small foci inside the tumor bulk had T1‐hyperintensity, T2‐hypointensity and no enhancement, indicating subacute hematoma or melanin. The patient underwent transsphenoidal surgical resection of his pituitary tumor.
Figure 1.
NEUROPATHOLOGICAL FINDINGS
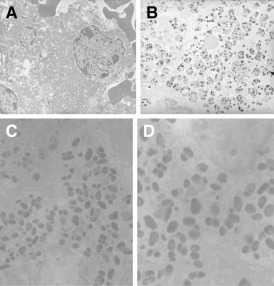
Histologic sections revealed an admixture of two tumors (Figure 2A). Juxtaposed to tumor 1 and focally infiltrating into it was the second tumor with hemorrhage. Tumor 2 consisted of sheets of large cells with prominent nucleoli and mitotic figures. Most of the cells of tumor 2 had heavily pigmented cytoplasm. Tumor 1 consisted of sheets and nests of smaller monomorphic cells without prominent nucleoli arranged in a delicate capillary network. These cells had round to oval nuclei, delicate chromatin, a moderate amount of chromophobic to eosinophilic cytoplasm with mild nuclear pleomorphism and no evidence of necrosis. There were also infiltrates of single and clusters of cells of tumor 2 in the capillaries and sheets of tumor 1 (Figure 2B). Tumor 1 was negative for prolactin, ACTH, GH, FSH, LH, TSH, S‐100 protein, HMB‐45, had a low MIB‐1 index (less than 1%), and was positive for CAM5.2 (Figure 2C). Tumor 2 was positive for S‐100 protein and HMB‐45 (Figure 2D), and negative for prolactin, ACTH, GH, FSH, LH, TSH and CAM5.2. The MIB‐1 proliferation index of tumor 2 was 10%. By electron microscopy, the cells of tumor 1 showed abundant mitochondria, sparse secretory granules, poorly developed RER and Golgi complex, and the other subcellular organelles were sparse (Figure 3A). Ultrastructure of tumor 2 revealed premelanosomes and melanosomes (Figure 3B–D).
Figure 2.
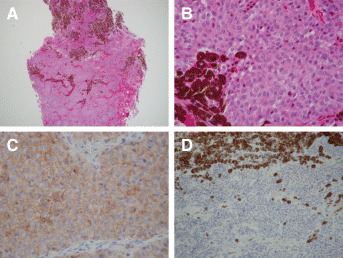
Figure 3.
DIAGNOSIS AND DISCUSSION
Diagnosis. Pituitary oncocytoma with metastatic malignant melanoma.
Discussion. The pituitary gland is an unusual site of metastatic tumor spread. The incidence of metastases to the normal pituitary gland, was 18 out of 1857 (1%) in autopsy cancer cases, with breast and lung representing the most common primary sites (3). Fewer than 15 cases of pituitary metastatic melanoma have been documented in the literature (4). Pituitary adenoma as recipient tumor for metastases represents an extremely rare condition. Eighteen cases of metastatic cancer to pituitary adenomas have been reported in the literature. The most common primary sites were breast and lung cancers. The other primary sites included stomach, renal, colorectum, carcinoid tumor of anterior mediastinum, pancreas, prostate and unknown origins (for references please refer to The International Society of Neuropathology website version of this case: available at http://www.intsoneuropathol.com/isn/cases.htm). To our knowledge, this is the first reported case of metastatic melanoma to a pituitary oncocytoma.
Most of the patients presented with symptoms similar to that of an ordinary pituitary adenoma. Diabetes insipidus, visual disturbances, cranial nerve palsy and anterior hypopituitarism are major clinical presentations noted in patients with pituitary metastases (2). The current case had visual disturbance, hypothyroidism and partial insufficiency of the adrenocorticotropic hormone. The interruption of the pathway of inhibitory influence of the hypothalamus on anterior pituitary prolactin secretion may have induced the mild hyperprolactinemia in this case. There were 10 cases of metastatic tumor to a nonfunctioning pituitary adenoma, six cases of metastases to functional pituitary adenomas (two secreted prolactin, two adrenocorticotropic hormone, two growth hormone), and two cases of metastases to oncocytic adenoma (including ours). The intraoperative findings were unremarkable in this case, although unusual consistency of the tumor found during resection has been reported (1, 7). The prognosis of these patients has been poor, as most patients had widespread metastases. Surgical intervention has been reported to be a prognostic factor for survival in cerebral metastatic melanoma (6). However, this patient died 1 month after operation.
The differential diagnosis of pigmented tumors includes melanoma, melanotic schwannoma, and melanotic meningioma. The histologic features of melanoma in this case consisted of typical epithelioid cells with pleomorphic nuclei and large nucleoli. Because primary intrasellar melanomas are rare, and the cell type was similar to the operated melanoma on the left big toe, a metastasis from the previous cutaneous lesion was diagnosed. The presence of typical melanoma infiltrating in pituitary adenomatous cells with disruption of reticular fibers in this specimen was clearly established with a reticulin stain under microscopic examination. The disruption of acinar structure indicated a pituitary adenoma instead of normal pituitary gland.
Immunohistochemical studies are useful in distinguishing metastatic melanoma from the pituitary adenoma component. Melanomas contain S‐100 protein and HMB‐45 reactivity. In contrast, the adenomatous cells are negative for S‐100 protein and HMB‐45. The MIB‐1 monoclonal antibody is a cell proliferation marker (Ki‐67 antigen). Significantly higher MIB‐1 labeling index was observed in a metastatic adenocarcinoma while low index was noted in the adenomatous component (5). The labeling index in the current case similarly showed a high index in the metastatic melanoma component and low index in the pituitary adenoma, indicating that the pituitary metastatic melanoma was a neoplasm with higher proliferation.
The pathogenetic explanation for the development of metastases in pituitary adenoma has not been established yet. Abnormalities of pituitary vasculature and non‐portal vessels or neovascularization in adjacent areas of pituitary adenomas have been the most commonly proposed mechanism for metastatic seeding to the pituitary adenoma (2). Metastases to the posterior lobe via direct systemic arterial blood supply with later metastases to the anterior lobe by direct extension from posterior lobe or from the portal vessels may have occurred in this case.
In conclusion, this report documents metastatic melanoma to a pituitary oncocytoma. Because the clinical presentation and imaging studies of the patient was similar to those of pituitary metastatic tumor, the correct diagnosis was established on the basis of pathological examination with immunostaining and ultrastructure.
REFERENCES
- 1. Abe T, Matsumoto K, Iida M, Hayashi M, Sanno N, Osamura Y (1997) Malignant carcinoid tumor of the anterior mediastinum metastasis to a prolactin‐secreting pituitary adenoma: a case report. Surg Neurol 48:389–394. [DOI] [PubMed] [Google Scholar]
- 2. Jin L, Lloyd RV (1993). Metastatic neoplasms to the pituitary gland. In: Surgical Pathology of the Pituitary Gland. Lloyd RV (ed.), Chapter 12, pp. 137–140. WB Saunders: Philadelphia. [Google Scholar]
- 3. Kovacs K (1973) Metastatic cancer of the pituitary gland. Oncology 27:533–542. [DOI] [PubMed] [Google Scholar]
- 4. Leung GKK, Chow WS, Tan KCB, Fan YW, Lam KSL (2003) Metastatic melanoma of the pituitary gland. J Neurosurg 99:913–915. [DOI] [PubMed] [Google Scholar]
- 5. Noga C, Prayson RA, Kowalski R, Sweency PJ, Mayberg M (2001) Metastatic adenocarcinoma to a pituitary adenoma. Ann Diagn Pathol 5:354–360. [DOI] [PubMed] [Google Scholar]
- 6. Stevens G, Firth I, Coates A (1992) Cerebral metastases from malignant melanoma. Radiother Oncol 23:185–191. [DOI] [PubMed] [Google Scholar]
- 7. Van Seters AP, Bots GTAM, Van Dulken H, Luyendijk W, Jan Viel Voye G (1985) Metastasis of an occult gastric carcinoma suggesting growth of a prolactinoma during bromocriptine therapy: a case report with a review of the literature. Neurosurgery 16:813–817. [DOI] [PubMed] [Google Scholar]


