Abstract
We have reviewed the features of two recently described intracranial tumors, which have been formally recognized as distinct entities by the 2007 WHO Classification of Brain Tumours: Papillary tumor of the pineal region and spindle cell oncocytoma of the pituitary gland. Their salient clinicopathological features, differential diagnosis, histogenetic hypothesis and outcome are discussed.
PAPILLARY TUMOR OF THE PINEAL REGION
Definition and general features. Described as a distinct entity in 2003 by Jouvet et al (12), papillary tumor of the pineal region (PTPR) has been formally codified in the 2007 edition of the World Health Organization (WHO) Classification of Tumours of the Central Nervous System (13), and assigned a provisional ICD‐O code of 9395/3. To date, only 41 examples have been documented; however, communication with neuropathologists at referral centers across the world confirms the existence of additional, unreported, examples. Moreover, it is clear that tumors with identical histology and clinicopathologic features as those of PTPR have previously been reported under various other names, including papillary pineocytoma, pineal parenchymal tumor, choroid plexus tumor, ependymoma and papillary meningioma. In addition, it is highly likely that additional unrecognized examples have been given a diagnosis of metastatic papillary carcinoma of unknown primary origin. The clinicopathologic features of PTPR have recently been reviewed by Fevre‐Montange et al (6) and by Dagnew et al (3). PTPR arises exclusively in the pineal region and occurs most commonly in adults, although the age at presentation ranges from 5 to 66 years (mean, 31.5 years). There is a slight predominance of females. Headache, usually of short duration, is the most common presenting symptom and occurs secondary to hydrocephalus resulting from compression of the cerebral aqueduct. PTPRs are usually well‐circumscribed, large (2.5–4.0 cm) mass lesions that sometimes feature a cystic component. On computerized tomographic (CT) imaging, they are hypodense and contrast‐enhancing. Magnetic resonance imaging (MRI) shows hyperintensity on T2‐weighted images and enhancement on T1‐weighted sequences following gadolinium administration.
Histological and immunohistochemical features. Papillary tumors of the pineal region characteristically show a discrete, compressive border with adjacent pineal gland and brain parenchyma. As the name indicates, a salient histological hallmark is papillary architecture (Figure 1A,B). The degree of papillary formation varies from case to case, with the other major architectural motif being solid cellular sheets of tumor cells (Figure 1C,D). The latter areas variably feature pseudorosettes, true rosettes, and/or tubules with small lumina. In the papillary component, vessels are often hyalinized and are covered by large, pale‐to‐eosinophilic cells arranged in a pseudostratified columnar layering (Figure 1B). In the solid cellular areas, tumor cells show clear or vacuolated cytoplasm (Figure 1D). Less commonly, eosinophilic and PAS‐positive cytoplasmic inclusions are encountered. As a rule, nuclei are regular, round‐to‐oval and contain stippled chromatin, although pleomorphic nuclei may be seen in some cases. Mitotic activity varies, ranging from 0 to 10 mitoses per 10 high power (×40) fields. Necrosis is usually found to some extent in most tumors. In contrast, vascular proliferation is consistently absent, although slight endothelial hyperplasia may be seen in some vessels. Recurrent tumors exhibit the same morphologic features as seen in the respective primary tumor, with the papillary component often becoming more prominent.
Figure 1.
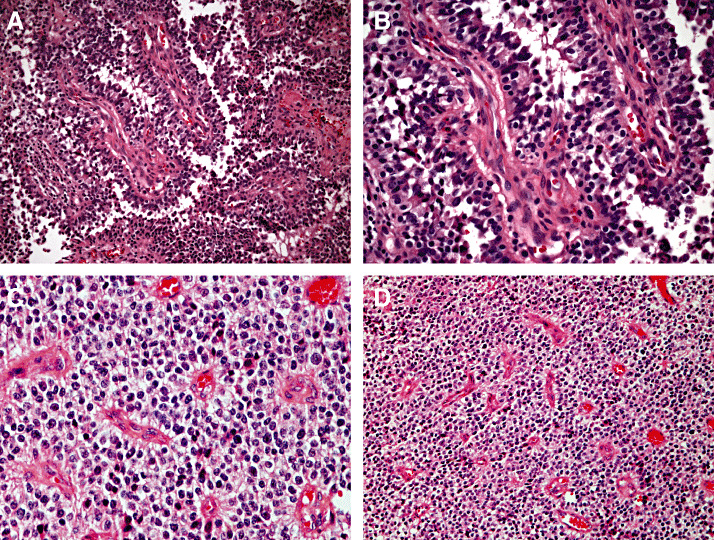
Papillary tumor of the pineal region (PTPR). PTPR exhibits a characteristic papillary architecture (A,B). At higher power (B), a pseudostratified columnar arrangement around vessels is seen. Also characteristic are areas of solid tumor, shown here at low (C) and high (D) power. (All panels, H&E)
The immunophenotype of PTPR has been extensively investigated (6). The most distinctive feature is immunoreactivity for a broad spectrum of cytokeratins, including KL1, AE1/AE3, CAM5.2 and cytokeratin 18, which is more evident in the papillary than in the solid component (Figure 2A). In particular, cytokeratin 18 expression is a constant finding and is always intense. Although examined in only a minority of cases, no CK20 staining has been reported, and only weak, focal staining and has been reported for CK7 and CK5/6. Epithelial membrane antigen (EMA) expression is seen in the majority of tumors, but is restricted to the cell surface, particularly that abutting vessels. Dot‐like staining similar to that seen in ependymoma may also be seen (12, 16, 20). In addition to epithelial marker staining, PTPRs have also been reported to express vimentin, S‐100 protein, NSE, MAP2, N‐CAM and transthyretin 10, 20). Cytoplasmic and often nuclear expression of S100 protein is present in nearly all tumor cells, and vimentin typically stains tumor cell cytoplasm adjacent to vessel walls (Figure 2B,C). Reactivity for glial fibrillary acidic protein (GFAP) has been reported in approximately 12% of tumors, but expression of this marker is typically restricted to focal perivascular areas at the tumour (Figure 2D). Synaptophysin and chromogranin reactivity may be seen, but only weakly and focally (12). No immunolabeling for neurofilament proteins has been reported. Focal transthyretin staining has been reported in approximately half of tumors tested and has also been observed in normal peritumoral pineal cells. Expression of the adhesion molecules NCAM and E‐cadherin has been examined in only two tumors; the former was present in both lesions, particularly on surface membranes, whereas no E‐cadherin reactivity was observed. The same two tumors also expressed nestin. The majority of PTPR are characterized by lack of membrane staining for Kir7.1 and lack of cytoplasmic reactivity for stanniocalcin‐1, both of which are seen in choroid plexus tumors (10). The MIB‐1 (Ki‐67 antigen) labeling index ranges from less than 5% to greater than 10%, with indices being higher in young patients.
Figure 2.
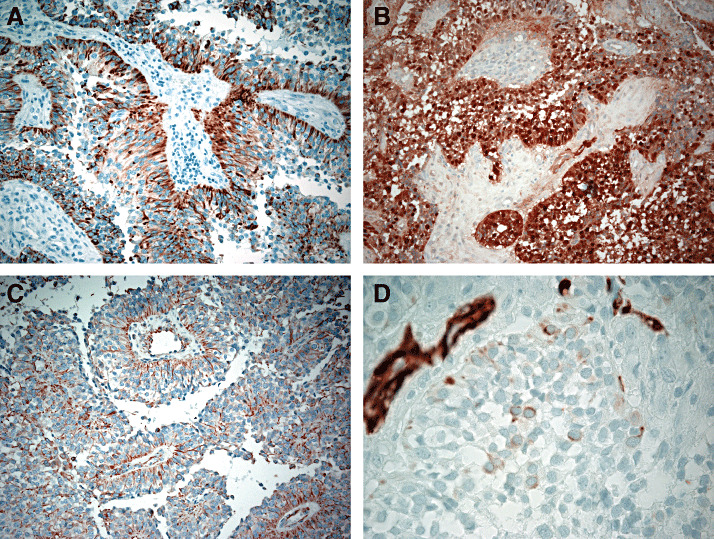
Papillary tumor of the pineal region. Characteristic papillary tumor of the pineal region expression patterns are illustrated for CAM5.2 (A), S‐100 protein (B), vimentin (C), and glial fibrillary acidic protein (D).
Differential diagnosis. The differential diagnosis of PTPR is broad and includes all pineal region lesions known to exhibit papillary architecture (3, 6). These include the pineal parenchymal tumors, papillary ependymoma, choroid plexus tumors, papillary meningioma and metastases. Unlike PTPR, pineal parenchymal tumors show strong immunoexpression of neuronal markers. Distinction of PTPR from metastatic papillary carcinomas of unknown primary can be challenging and is primarily based on the very low or absent expression of CK7/CK20 and low MIB‐1 labeling indices that are characteristic of PTPR. Nestin expression may also aid diagnosis in that it is usually absent in metastatic carcinoma. The distinction from choroid plexus tumors may also be difficult if based upon immunohistochemical findings alone. Both PTPR and choroid plexus papilloma express cytokeratins and transthyretin; however, most PTPRs show MAP‐2 staining and do not express Kir7.1, which is a marker for choroid plexus tumors (6). The distinction of PTPR from ependymoma can be problematic in that the former often shows immunohistochemical and ultrastructural features of ependymal differentiation. The relatively lesser degree of GFAP expression seen in PTPR is of marginal utility, and the presence of neuronal features at the immunohistochemical and ultrastructural levels (14) is of limited value because ependymomas may also exhibit neuronal differentiation (18). Papillary meningioma can usually be excluded by virtue of a general lack of cytokeratin expression.
Histogenesis. The histogenesis of PTPR remains to be confirmed, although it is likely that these distinctive tumors originate from remnants of specialized ependymal cells of the subcommissural organ (12), which is present in humans during embryologic development and persists in vestigial form in adults (8). Support for this notion comes from the expression of nestin and cytokeratin 18, as well as from DNA microarray analyses, which have shown high expression of the genes ZFH4, RFX3, TTR and CGRP, all of which are expressed by the subcommissural organ (5). The subcommissural organ is a member of the highly specialized circumventricular organ (CVO) group, which also includes the subfornical organ and the organum vasculosum of the lamina terminalis. The potential significance of the specialized ependymal covering associated with the CVOs with respect to the origin of several rare types of CNS tumor is increasingly recognized (8, 17).
Genetics. The genetic profile of PTPR has been investigated using comparative genomic hybridization. Of five lesions examined, four showed chromosomal imbalances, with the most common changes being loss of chromosomes 10 (four cases) and 22q (three cases), as well as gain of chromosomes 4 (four cases), 8, 9 and 12 (three cases each) (10).
Prognostic and predictive factors. Papillary tumors of the pineal region are characterized by frequent local recurrence but only occasional spinal dissemination. This prompted the WHO panel to suggest correspondence to grades II or III. Fevre‐Montange et al (6) reviewed the prognosis of PTRP. Detailed follow‐up information was obtained in 29 of 31 cases, with the mean follow‐up period being 4.2 years. Progression was seen in 72% of cases. Five‐year estimates of overall and progression‐free survival were 73% and 27%, respectively. Seven patients died of disease. The same authors also investigated the effect of age, gender, tumor size, gross total resection and adjuvant radiotherapy upon survival. Incomplete resection and a mitotic index higher than five per 10 high power fields (HPFs) seemed to correlate with decreased survival and recurrence (6), whereas age less than 30 years was unassociated with risk of progression or death. On univariate analysis, gross total resection was the only clinical factor strongly associated with overall survival and recurrence, but statistical significance was not achieved. Fifteen patients had received radiotherapy, nine after complete and six after incomplete resection; however, the therapeutic regimens differed. The observation that MIB‐1 labeling is higher in younger patients is of interest, but this parameter did not correlate with a less favorable outcome in this age group. Given the infrequent occurrence of PTPR, conclusions regarding behavior and optimal post‐operative treatment await additional experience and prospective study.
SPINDLE CELL ONCOCYTOMA
Definition and general features. Spindle cell oncocytoma (SCO), which was described as a new entity by Roncaroli et al in 2002 (19), is a non‐adenomatous sellar region tumor that has been codified in the 2007 WHO classification, in which it is defined as a spindled‐to‐epithelioid, oncocytic, non‐endocrine neoplasm of the anterior hypophysis that manifests in adults and follows a benign clinical course (9). It corresponds to WHO grade I and has a provisional ICD‐O code of 8290/0. SCO is an uncommon lesion of adults in the age range of 26–71 years (mean, 56 years). The actual incidence is difficult to determine, but in one single‐institution experience, SCO accounted for approximately 0.4% of all operated sellar tumors (15). Only 10 examples have been published to date (4, 15, 19, 22).
SCO originates in adenohypophyseal tissue, and accordingly may present as an intrasellar, suprasellar, or combined intrasellar/suprasellar mass. In this regard, five of the10 reported cases presented as intrasellar masses with suprasellar extension, three invaded into the cavernous sinus (4, 22), and one invaded the sellar floor (15). The clinical presentation of patients with SCO is indistinguishable from that of other non‐hormone‐producing sellar masses. Patients reported so far have exhibited primarily hypopituitarism and visual field defects; less frequently, headache, nausea and vomiting were present (4, 15, 19, 22). One of two reported patients with recurrent SCO had involvement of the skull base with epistaxis (15). The neuroimaging findings of SCO are indistinguishable from those of pituitary macroadenoma, with MRI studies showing contrast enhancement, circumscribed growth pattern and solid appearance. Calcification has not been reported.
Histopathological features. Grossly, SCO is indistinguishable from conventional pituitary adenoma. The texture varies from soft, creamy and amenable to removal by ultrasonic aspiration, to firm and tenaciously adherent to the surrounding structures. Although the imaging appearance is well circumscribed, a sharp margin with the surrounding pituitary parenchyma is usually absent.
Histologically, SCO is composed of compact, interwoven fascicles of spindled cells, with a variable admixture of epithelioid cells. Some tumors exhibit a vaguely lobular architecture. The cytoplasm is typically eosinophilic and finely granular (Figure 3A,B). As a rule, nuclei are only mildly to moderately atypical, but occasional cells may show nuclear hyperchromasia and marked pleomorphism (Figure 3C). Mitoses are usually rare‐to‐absent, although increased mitotic activity has been reported in one recurrent lesion (15). Focal myxoid changes may be present (Figure 3D). Many tumors display patchy infiltrates of mature lymphocytes.
Figure 3.
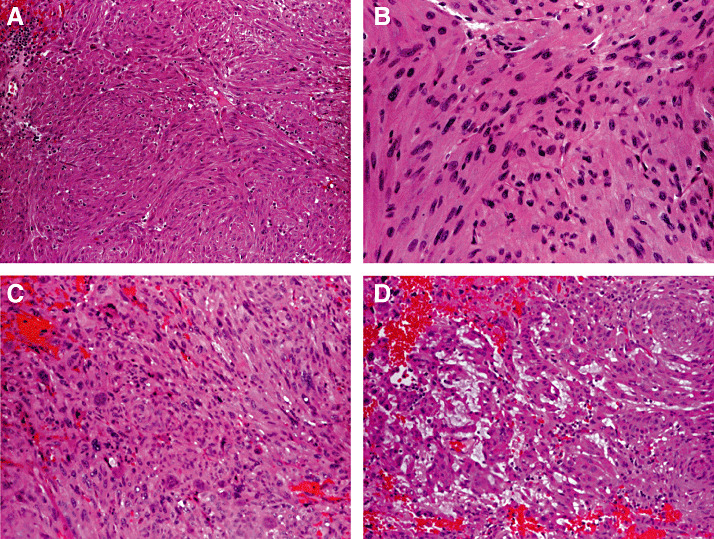
Spindle cell oncocytoma. These tumors typically consist of interwoven fascicles of spindled cells and, to a lesser extent, epithelioid cells (A) that exhibit finely granular eosinophilic cytoplasm and mild‐to‐moderate nuclear atypia (B). Marked nuclear hyperchromasia and pleomorphism may be seen (C), and focal myxoid changes may be present in some tumors (D).
SCOs have been investigated with a broad spectrum of antibodies. The typical immunophenotype includes expression of vimentin, S‐100 protein (Figure 4A), EMA (Figure 4B), anti‐mitochondrial antibody 113‐1 and galectin‐3 (Figure 4C). In contrast, immunostains for pituitary hormones and other antigens, including synaptophysin, chromogranin, cytokeratins, GFAP, CD34, bcl‐2, smooth muscle actin and desmin, are usually negative. Only one case reportedly showed expression of bcl‐2 and GFAP in occasional cells (22). The MIB‐1 labeling index of primary tumors has ranged from 1% to 8% (mean, 2.8%). Indices as high as 18%–20% have been seen in the reported recurrences of two aggressive tumors (15).
Figure 4.
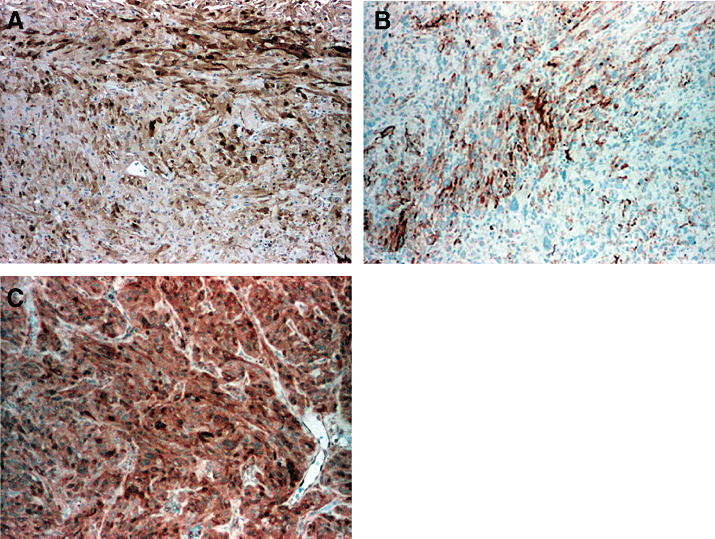
Spindle cell oncocytoma. Spindle cell oncocytomas are characterised by immunoreactivity for S‐100 protein (A), EMA (B), and galectin‐3 (C).
Ultrastructural study has been performed in eight cases (4, 12, 15, 18), and has proved helpful in distinguishing SCO from pituitary adenoma, in which the neoplastic cells of SCO are characteristically filled with abnormal mitochondria and, with rare exceptions, display scant, well‐formed desmosomes (15) or junctions of intermediate type. In addition, in contrast to pituitary adenoma, all reported cases except one have lacked secretory granules (4).
Histogenesis. The origin of SCO remains to be established. A derivation from the folliculostellate cell (FSC) of the anterior pituitary was postulated in the original description based upon similarities between the neoplastic cells and normal FSCs (19). FSCs comprise a heterogeneous population of non‐hormone‐secreting stellate cells of the anterior pituitary that form a three‐dimensional network surrounding the acini. They exert regulatory functions upon the hormone‐secreting cells, play a key role in intercellular communication by releasing signaling molecules and growth factors, and may function as antigen presenting cells (1). S‐100 protein is a reliable marker for human FSCs, and they are also known to express GFAP, vimentin, cytokeratin, galectin‐3, MHC class II antigen, interleukins 6 and 10, annexin‐1, follistatin, macrophage inhibiting factor and vascular endothelial growth factor in various combinations, supporting the concept that FSCs are functionally heterogeneous. Ultrastructurally, FSCs form desmosomal junctions along their lateral borders between themselves and with glandular cells. In contrast, cell‐to‐cell junctions are absent or rare between the cells of the latter population. Recent ultrastructural and immunohistochemical studies of normal and adenomatous human pituitary suggest that FSCs may represent an adult stem cell‐progenitor population (11).
Differential diagnosis. The differential diagnosis of SCO is broad and includes oncocytic pituitary adenoma, granular cell tumor, pituicytoma, intrasellar schwannoma, meningioma with oncocytic change, solitary fibrous tumor and paraganglioma. Criteria for distinguishing SCO from these tumors have been extensively discussed (4, 15, 19, 22, 23); however, the differential diagnosis between SCO and pituicytoma warrants further mention. Like SCO, pituicytomas are intensely immunoreactive for vimentin and S‐100 protein. Although the majority of pituicytomas exhibit reactivity for GFAP, the extent and intensity of expression is quite variable, with some examples being immunonegative. In addition, a few pituicytomas have shown focal EMA immunolabeling (2, 21), although when present this is usually patchy and cytoplasmic rather than membranous. Ultrastructurally, SCO and pituicytoma exhibit some shared features. Brat et al (2) described abundant cytoplasmic filaments and scattered “intermediate” junctions in pituicytoma. These authors also mentioned the presence of abundant mitochondria in the three tumors analyzed, and moderate accumulation of mitochondria in pituicytoma was noted by Figarella‐Branger et al (7). However, the hallmark feature of SCO, oncocytic change, is not a characteristic attribute of pituicytoma.
Prognostic and predictive factors. Overall, SCO is benign tumor with little tendency to recur, even after incomplete excision. Updated follow‐up of the five originally reported cases found no recurrence (follow‐up range, 6–11 years; mean 7.8 years). Combining these data with the reported follow‐up of other cases, eight patients were alive and well after follow‐up ranging from 6 to 16 years (mean, 7.8 years), and two experienced recurrence within 3 years of initial surgery. Of these, one patient was clinically stable for 8 years and then became symptomatic because of optic chiasm compression (4). The other patient experienced an aggressive second recurrence, with destruction of the clivus, sphenoid sinus and ethmoid sinus, as well as nasopharyngeal and nasal cavity extension (15). In both patients, the primary tumors showed no features that were predictive of aggressive behavior. Both of the recurrent tumors exhibited high MIB‐1 labeling indices (18% and 20%).
Treatment. Surgery is the treatment of choice for SCO, with small size and lack of invasion being factors that favor gross total resection. When this cannot be achieved, radiation therapy might be considered. The role of radiation therapy has been discussed by Dahiya et al (4); one of their two patients received proton beam irradiation and was recurrence‐free after 7 years. However, experience is very limited and long‐term follow‐up of additional cases is needed to determine the significance of potential prognostic factors such as invasion and proliferation activity.
REFERENCES
- 1. Allaerts W, Vankelecom H (2005) History and perspective of pituitary folliculo‐stellate cell research. Eur J Endocrinol 153:1–12. [DOI] [PubMed] [Google Scholar]
- 2. Brat DJ, Scheithauer BW, Staugaitis SM, Holtzman RNN, Morgello S, Burger PC (2000) Pituicytoma: a distinctive low‐grade glioma of the neurohypophysis. Am J Surg Pathol 24:362–368. [DOI] [PubMed] [Google Scholar]
- 3. Dagnew E, Langford LA, Lang FF, DeMonte F (2007) Papillary tumors of the pineal region: case report. Neurosurgery 60:E953–E955. [DOI] [PubMed] [Google Scholar]
- 4. Dahiya S, Sarkar C, Hedley‐Whyte ET, Sharma MC, Zervas NT, Sridhar E, Louis DN (2005) Spindle cell oncocytoma of the adenohypophysis: report of two cases. Acta Neuropathol (Berl) 110:97–99. [DOI] [PubMed] [Google Scholar]
- 5. Fevre‐Montange M, Champier J, Szathmari A, Wierinckx A, Mottolese C, Guyotat J, Figarella‐Branger D, Jouvet A, Lachuer J (2006) Microarray analysis reveals differential gene expression patterns in tumors of the pineal region. J Neuropathol Exp Neurol 65:675–684. [DOI] [PubMed] [Google Scholar]
- 6. Fevre‐Montange M, Hasselblatt M, Figarella‐Branger D, Chauveinc L, Champier J, Saint‐Pierre G, Taillandier L, Coulon A, Paulus W, Fauchon F, Jouvet A (2006) Prognosis and histopathologic features in papillary tumors of the pineal region: a retrospective multicenter study of 31 cases. J Neuropathol Exp Neurol 65:1004–1011. [DOI] [PubMed] [Google Scholar]
- 7. Figarella‐Branger D, Dufour H, Fernandez C, Bouvier‐Labit C, Grisoli F, Pellissier JF (2002) Pituicytomas, a mis‐diagnosed benign tumor of the neurohypophysis: report of three cases. Acta Neuropathol (Berl) 104:313–319. [DOI] [PubMed] [Google Scholar]
- 8. Fuller GN, Burger PC (2006) The central nervous system. In: Histology for Pathologists, Chapter 11, 3rd edn. Mills SE (ed.), pp. 273–319. Raven Press: New York. [Google Scholar]
- 9. Fuller GN, Scheithauer BW, Roncaroli F, Wesseling P (2007) Spindle cell oncocytoma. In: World Health Organization Classification of Tumours of the Central Nervous System. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK (eds), IARC: Lyon. In press. [Google Scholar]
- 10. Hasselblatt M, Blumcke I, Jeibmann A, Rickert CH, Jouvet A, Van De Nes JA, Kuchelmeister K, Brunn A, Fevre‐Montange M, Paulus W (2006) Immunohistochemical profile and chromosomal imbalances in papillary tumours of the pineal region. Neuropathol Appl Neurobiol 32:278–283. [DOI] [PubMed] [Google Scholar]
- 11. Horvath E, Kovacs K (2002) Folliculo‐stellate cells of the human pituitary: a type of adult stem cell? Ultrastruct Pathol 26:219–228. [DOI] [PubMed] [Google Scholar]
- 12. Jouvet A, Fauchon F, Liberski P, Saint‐Pierre G, Didier‐Bazes M, Heitzmann A, Delisle MB, Biassette HA, Vincent S, Mikol J, Streichenberger N, Ahboucha S, Brisson C, Belin MF, Fevre‐Montange M (2003) Papillary tumor of the pineal region. Am J Surg Pathol 27:505–512. [DOI] [PubMed] [Google Scholar]
- 13. Jouvet A, Nakazato Y, Scheithauer BW, Paulus W (2007) Papillary tumour of the pineal region. In: world Health Organization Classification of Tumours of the Central Nervous System. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK (eds), IARC: Lyon. In press. [Google Scholar]
- 14. Kern M, Robbins P, Lee G, Watson P (2006) Papillary tumor of the pineal region: a new pathological entity. Clin Neuropathol 25:185–192. [PubMed] [Google Scholar]
- 15. Kloub O, Perry A, Tu PH, Lipper M, Lopes MB (2005) Spindle cell oncocytoma of the adenohypophysis: report of two recurrent cases. Am J Surg Pathol 29:247–253. [DOI] [PubMed] [Google Scholar]
- 16. Kuchelmeister K, Hugens‐Penzel M, Jodicke A, Schachenmayr W (2006) Papillary tumour of the pineal region: histodiagnostic considerations. Neuropathol Appl Neurobiol 32:203–208. [DOI] [PubMed] [Google Scholar]
- 17. Leeds NE, Lang FF, Ribalta T, Sawaya R, Fuller GN (2006) Origin of chordoid glioma of the third ventricle. Arch Pathol Lab Med 130:460–464. [DOI] [PubMed] [Google Scholar]
- 18. Rodriguez FJ, Scheithauer BW, Robbins PD, Burger PC, Hessler RB, Perry A, Abell‐Aleff PC, Mireau GW (2007) Ependymomas with neuronal differentiation: a morphological spectrum. Acta Neuropathol 113:313–324. [DOI] [PubMed] [Google Scholar]
- 19. Roncaroli F, Scheithauer BW, Cenacchi G, Horvath E, Kovacs K, Lloyd RV, Abell‐Aleff P, Santi M, Yates AJ (2002) Spindle cell oncocytoma’ of the adenohypophysis: a tumor of folliculostellate cells? Am J Surg Pathol 26:1048–1055. [DOI] [PubMed] [Google Scholar]
- 20. Shibahara J, Todo T, Morita A, Mori H, Aoki S, Fukayama M (2004) Papillary neuroepithelial tumor of the pineal region. A case report. Acta Neuropathol (Berl) 108:337–340. [DOI] [PubMed] [Google Scholar]
- 21. Ulm AJ, Yachnis AT, Brat DJ, Rhoton AL Jr (2004) Pituicytoma: report of two cases and clues regarding histogenesis. Neurosurgery 54:753–757; discussion 757–758. [DOI] [PubMed] [Google Scholar]
- 22. Vajtai I, Sahli R, Kappeler A (2006) Spindle cell oncocytoma of the adenohypophysis: report of a case with a 16‐year follow‐up. Pathol Res Pract 202:745–750. [DOI] [PubMed] [Google Scholar]
- 23. Wesseling P, Brat DJ, Fuller GN (2007) Pituicytoma. In: World Health Organization Classification of Tumours of the Central Nervous System. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK (eds), IARC: Lyon. In press. [Google Scholar]


