Abstract
Uncontrolled cell growth, division, and lack of enough blood supply causes low oxygen content or hypoxia in cancerous tumor microenvironments. 17β-Estradiol (E2), an estrogen receptor (ER) ligand, can be incorporated on the surface of nanocarriers for targeted drug delivery to breast cancer cells overexpressing ER. In the present study, we synthesized estradiol-conjugated hypoxia-responsive polymeric nanoparticles (polymersomes) encapsulating the anticancer drug doxorubicin (E2-Dox-HRPS) for targeted delivery into the hypoxic niches of estrogen receptor-positive breast cancer microtumors. Estradiol-conjugated polymersomes released over 90% of their encapsulated Dox in a sustained manner within hypoxia (2% oxygen) after 12 hours. However, they released about 30% of Dox in normal oxygen partial pressure (21% oxygen, normoxia) during this time. Fluorescence microscopic studies demonstrated higher cytosolic and nuclear internalization of E2-Dox-HRPS (targeted polymersomes) compared to Dox-HRPs (non-targeted polymersomes). Monolayer cell viability studies on ER-positive MCF7 cells showed higher cytotoxicity of targeted polymersomes in hypoxia compared to normoxia. Cytotoxicity studies with hypoxic three-dimensional spheroid cultures of MCF7 cells treated with targeted polymersomes indicated significant differences compared to normoxic spheroids. The novel estradiol-conjugated hypoxia-responsive polymersomes described here have the potential for targeted drug delivery in estrogen receptor-positive breast cancer therapy.
Keywords: Hypoxia, Polymersomes, Estrogen receptor, Breast cancer, Targeted drug delivery
TOC Graphic:
Introduction
Breast cancer is the most common malignancy and the second leading cause of death among women in the United States.1 There are three significant biological markers in breast cancer: estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER-2). Based on these biomarkers, gene expressions, and molecular profiling, breast cancer is classified into different subtypes, including luminal A (ER+/PR+/HER2−), luminal B (ER+/PR+/HER2+), HER2 overexpression (ER−/PR−/HER2+), and basal-like tumors (ER−/PR−/HER2−).2,3 About 83% of breast cancer cases are hormone receptor-positive (ER+/PR+) and are susceptible to hormonal therapy for suppressing tumor growth.4
Nanotechnology is an emerging field for targeted drug delivery in cancer therapy. Polymersomes are polymeric bilayer nanovesicles prepared from amphiphilic block copolymers.5–7 They have several advantages compared to lipid-based nanocarriers, including controllable elasticity, permeability, and mechanical property that depend on the molecular weight of the hydrophobic block of the copolymers.6 Polymersomes have an aqueous core surrounded by a bilayer membrane. This membrane has a hydrophobic part in the interior side and hydrophilic coronas on the outside and inside surfaces.8 Polymersomes encapsulate hydrophilic therapeutics (such as proteins, peptides, nucleic acid fragments, anticancer drugs, and enzymes) in the aqueous core while hydrophobic molecules are integrated into the bilayer.9–16 Polymersomes are rendered long circulating in the blood by the introduction of polyethylene glycol (PEG) as one of the main copolymers in their structure, or by conjugating PEG (PEGylation) on their surface.17 The presence of PEG in polymersomes minimizes protein opsonization and protects the vesicles from degradation by dendritic cells or phagocytes.17
Uncontrolled and rapid cell proliferation along with insufficient blood supply produce low oxygen partial pressure or hypoxia in almost all solid tumors.17 Hypoxia increases cancer cell survival through aggressiveness, metastasis to different organs, and resistance to chemotherapy and radiation therapy, leading to poor clinical outcomes.18–20
The chemotherapeutic agents are toxic or ineffective above or below their optimal plasma level, respectively. Hence, the carrier nanoparticles should release all of their anticancer payloads selectively at the targeted tumor sites. To reach this requirement, polymersomes are reported which respond to physical, chemical, or biological stimuli such as temperature, light, magnetic field, pH, redox, hypoxia, enzymes, inflammation, etc.21–25 Since more than half of all tumors in breast cancer cases have less than 2.5 mm of Hg oxygen pressure,26 hypoxia-responsive polymersomes are one of the favored nanocarriers for selective drug release in hypoxic niches of tumors.22 Ligand-decorated polymersomes have been engineered for targeted drug delivery into microtumors to improve therapeutic efficacy and reduce cytotoxicity of the chemotherapeutic medicines.10
Estrogen is a hormone critical for the function of many organs such as bone, brain, cardiovascular, and reproductive systems. However, it is also associated with breast, ovarian, and uterine cancers.27 17β-Estradiol (E2) is the predominant hormone among the three forms of estrogen hormones.28 The cellular effects of estrogen are mediated by estrogen receptor α (ERα) and estrogen receptor β (ERβ), which are members of the nuclear hormone receptor superfamily of transcription factors. ERα expression is less than 10% in healthy breast cells but increases to 80% in breast cancer tissues.29 To improve the cellular uptake of anticancer drugs and genes to ER-overexpressed cancer cells, estrogen conjugated liposomes have been developed. The reported liposomes encapsulated anticancer agents for targeted delivery to tumor tissues.30–32
In this study, we engineered estradiol conjugated hypoxia-responsive polymersomes loaded with doxorubicin for targeted drug delivery to hypoxic niches of ER-positive breast cancer microtumors. To our knowledge, this is the first report of receptor-mediated hypoxia-responsive polymeric drug carriers targeting breast cancer cells through the overexpressed estrogen receptors. With further developments, these ER targeted polymeric nanoparticles have translational potential for delivering chemotherapeutics to cancer tumor tissues.
1. Materials and Methods
1.1. Materials
1-Ethyl-3-(3-dimethyl aminopropyl)-carbodiimide hydrochloride (EDC), N-hydroxysuccinimide (NHS), dimethylaminopyridine, tin (II) ethoxyhexanoate, CuCl2.2H2O, pentamethyl diethylenetriamine, tetrahydrofuran (THF), and 17β-ethynylestradiol were purchased from Millipore Sigma (St. Louis, MO, USA). Diazobenzenebenzene, 3,3′-dicarboxylic acid was purchased from TCI America (Montgomeryville, PA, USA). mPEG-NH2 (MW: 2000) was purchased from BiochemPeg (Watertown, MA, USA). The water used in experiments was prepared by a Millipore Sigma water purification system (Burlington, MA, USA). The NMR spectra were recorded using a Bruker Advance II HD instrument. Gel-permeation chromatography (GPC) was performed using a Tosoh Bioscience (King of Prussia, PA, USA) EcoSEC HLC-8320 instrument. THF was used as mobile phase in GPC, and polystyrene was the standard used for calibration.
1.2. Synthesis of the diblock copolymer PLA8500-didiazobenzenebenzene-PEG2000
Excess Diazobenzenebenzene, 3,3′-dicarboxylic acid (27 mg, 0.14 mmol, TCI chemicals) was dissolved in 5 mL of pyridine in a round bottom flask wrapped in aluminum foil. 1-Ethyl-3-(3-dimethyl aminopropyl)-carbodiimide hydrochloride (EDC.HCl, 27 mg, 0.14 mmol), N-hydroxysuccinimide (NHS, 11.5 mg, 0.14 mmol) and dimethylaminopyridine (DMAP, 0.6mg, 5%mol) were then added. The reaction mixture was stirred for 1 h under a nitrogen atmosphere. mPEG-NH2 (MW: 2000, 100 mg, 0.05 mmol) was dissolved dropwise in 1 mL CHCl3, added into this reagent mixture, and stirred overnight. Then, the solvent was removed under reduced pressure, the rest of the mixture was suspended in 50 mL water, and centrifuged at 2000 g for 10 min. The supernatant was filtered to remove the unreacted dicarboxylic acid, and the filtrate was dialyzed in 4 L water (molecular weight cut-off: 1000) for 2 days. The dialysate was dried under reduced pressure to obtain a yellow solid (yield: 62%). 1H NMR (400 MHz, chloroform-d) δ ppm: 8.00-8.23 (aromatic CH=CH-CH, m, 8 H), 3.67 ((CH2-CH2-O), t, 4 H), 3.40 ((CH3-O), s, 3 H).
The synthesized PEG–diphenyldiazobenzenecarboxylate (50 mg, 0.023 mmol) was dissolved in pyridine (1.25 mL). To this solution, 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide hydrochloride (EDC.HCl, 6.7 mg, 0.0345 mmol), and N-hydroxysuccinimide (NHS, 4 mg, 0.0345 mmol) were added followed by excess 3-aminopropanol (9 μL, 8.75 mg, 0.116 mmol). The reaction mixture was stirred overnight. The solvent was then evaporated. The residue obtained was dissolved in 10 mL dichloromethane and washed with water three times. The bottom organic layer was dried under vacuum to obtain the PEG conjugate (yield: 51%). 1H NMR (400 MHz, chloroform-d) δ ppm: 8.00-8.23 (aromatic CH=CH-CH, m, 8 H), 3.67 ((CH2-CH2-O), t, 4H), 3.40 ((CH3-O), s, 3 H), 0.88 ((NH-CH2-CH2-CH2-OH), m, 2 H).
For the synthesis of PLA8500-didiazobenzenebenzene-PEG2000, D, L-lactide (500 mg, 3.5 mmol) and tin (II) ethoxyhexanoate (3 μL, 0.009 mmol) were added to anhydrous toluene (5 mL) in a 35 mL high-pressure reaction vessel. The product (100 mg, 0.05 mmol) obtained from the previous step was then added to the reaction mixture. The high-pressure reaction vessel was purged with nitrogen for 5 minutes and stirred at 120° C for 24 hours. After cooling to 25° C, the reaction mixture was added dropwise to cold ether. The top clear supernatant was decanted and the precipitate was washed again with ether and dried (yield: 65%). The resultant polymer was analyzed by 1H-NMR spectroscopy and gel permeation chromatography for purity and molecular weight. 1H NMR (400 MHz, chloroform-d): 5.19 ((−CH-C=O), q, 1 H), 3.67 ((CH2-CH2-O), t, 4 H), 1.59 ((CH3-CH-C=O), d, 3 H). Polydispersity index: 1.17 (gel-permeation chromatography: Mn = 10,700; Mw = 12,500).
1.3. Synthesis of polymer PLA17000-PEG2000-Estradiol
Hydroxyl-PEG2000-azide (100 mg, 0.05 mmol), D, L-lactide (800 mg, 5.6 mmol), and tin (II) ethoxyhexanoate (3 μL, 0.009 mmol) were added to anhydrous toluene (5 mL) in a 35 mL glass high-pressure vessel and then purged with nitrogen. The solution was stirred at 120° C under nitrogen for 24 hours. After cooling to 25° C, the reaction mixture was added dropwise to cold ether. The top clear supernatant was decanted, the precipitate was washed with ether and dried under vacuum (yield: 72%). To characterize the purity and molecular weight, the product was analyzed by 1H NMR spectroscopy and gel permeation chromatography. 1H NMR (400 MHz, chloroform-d): 6.70((CH=CH-C=O), d, 1 H), 6.52((CH=CH-C=O), s, 1 H), 5.18 ((−CH-C=O), q, 1 H), 3.94 ((CH2-C=O), d, 2 H), 3.66 ((CH2-CH2-O), t, 4 H), 1.58 ((CH3-CH-C=O), d, 3 H), 0.89 ((CH3-C-), s, 6 H).
The ethynylestradiol was reacted with N3-PEG2000-PLA17000 polymer using [2 + 3]-cycloaddition reaction (Scheme 1). Briefly, a solution of Cu2+ complex (0.053 M) was prepared by mixing 3 mL of CuCl2.2H2O (90.3 mg, 0.53 mmol) and 3 mL of pentamethyl diethylenetriamine (PMDETA) (442 μL, 2.1 mmol) in water, diluting the mixture to 10 mL in water, and stirring for 2 hours. Ethynylestradiol (3 mg, 10 μmol) and N3-PEG2000-PLA17000 (100 mg, 5 μmol) were dissolved in 5 mL tetrahydrofuran (THF). Then, 100 μL of fresh sodium ascorbate water solution (27 mg/mL) and 100 μL copper complex (0.053 M) were added to the solution and stirred at 25° C for 24 hours. The reaction mixture was added into 40 mL water and the precipitate was pelleted using a centrifuge at 2906 g for 10 minutes. The precipitate was washed three times with water, added to an ion exchange resin (100 mg, Dowex.HCR-W2) in 20 mL water (to remove any remaining Cu2+ complex or amine), and stirred for 2 hours. Then, the mixture was dried under vacuum. 1H NMR (400 MHz, chloroform-d) δ ppm: 6.70((CH=CH-C=O), d, 1 H), 6.52((CH=CH-C=O), s, 1 H), 5.18 ((−CH-C=O), q, 1 H), 3.94 ((CH2-C=O), d, 2 H), 3.66 ((CH2-CH2-O), t, 4 H), 1.58 ((CH3-CH-C=O), d, 3 H), 0.89 ((CH3-C-), s, 6 H). Polydispersity index: 1.15 (gel-permeation chromatography; Mn = 16,400, Mw = 18,900). Percentage of estrogen attached: For 100% attachment of the estradiol, the integration of the 1H NMR signals at 3.66 ppm (PEG) and 6.52 ppm (benzene ring in estradiol) should be 188:1. From the calculated ratio in the 1H NMR spectrum, we estimate the attachment of estradiol to be 20%.
Scheme 1.
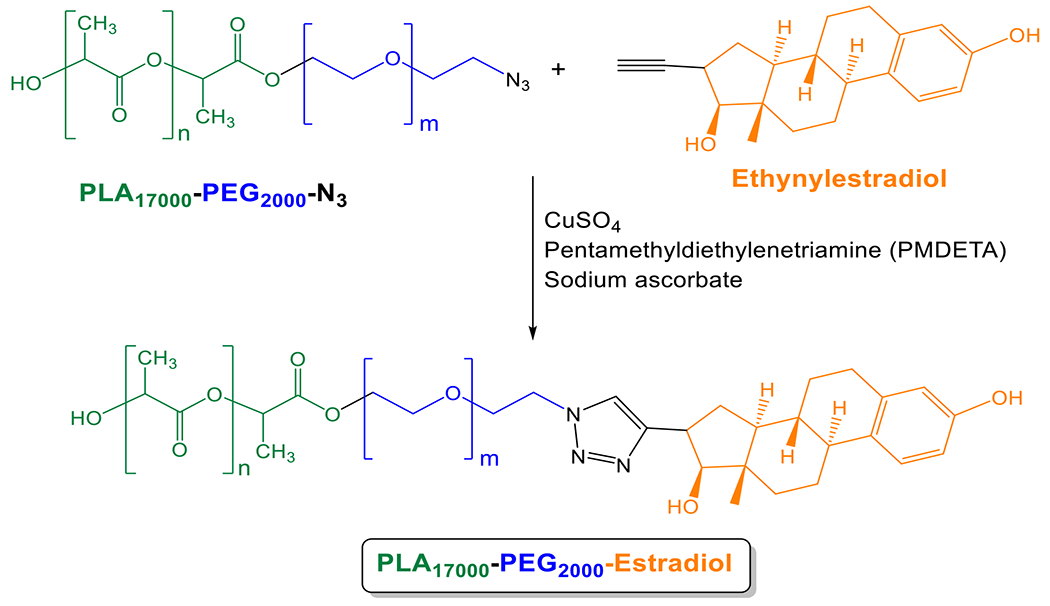
Synthesis of the polymer PLA17000-PEG2000-Estradiol
1.4. Preparation of Dox-encapsulated (non-targeted) polymersomes and Estradiol-conjugated Dox-encapsulated (targeted) polymersomes
PLA8500-diazobenzenebenzene-PEG2000 and PLA17000-PEG2000-Estradiol were separately dissolved in acetone (10 mg/mL). Doxorubicin hydrochloride was dissolved in deionized water (20 mg/mL) and 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine (DPPE)-N-lissamine rhodamine B sulfonyl ammonium salt (LR) lipid was dissolved in chloroform (0.01 mg/mL). PLA8500-diazobenzene-PEG2000 (95%), LR dye (5%), and doxorubicin hydrochloride were used to prepare non-targeted Dox-encapsulated polymersomes. For making targeted polymersomes, a combination of LR (5%), PLA8500-diazobenzene-PEG2000 (93%) and PLA17000-PEG2000-Estradiol (2%) were used. Doxorubicin was encapsulated in the nanocarriers with the pH gradient method.22 Briefly, 1 mL LR solution was added into two glass vials and chloroform was evaporated to make a thin film. The thin film in each glass vial was hydrated with 1 mL citrate buffer (20 mM, pH 4). One of the glass vials was used to prepare non-targeted polymersomes by adding PLA8500-Diazobenzene-PEG2000 polymer solution (100 μL) dropwise into the citrate buffer and stirring for 10 min at 25° C. The second glass vial was used for targeted polymersome preparation. The PLA8500-diazobenzene-PEG2000 polymer and PLA17000-PEG2000-Estradiol polymer solutions (100 μL and 3.6 μL, respectively) were added dropwise into the citrate buffer and stirred for 10 minutes at 25° C. Acetone in the resultant solutions was evaporated by passing air through the mixture for 45 minutes. Polymersomes were formed after sonicating the glass vials with polymer solution for 1 hour in a bath sonicator (Symphony 117V, 60 Hz, Level 9). The polymersomes were passed through a Sephadex G-100 gel filtration column to build the pH gradient across the membrane. Doxorubicin was added to these polymersomes (polymer: drug ratio 5:1) and the mixture was stirred for 4 hours at 25° C. The solutions were again passed through Sephadex G-100 column to remove unencapsulated drugs from the polymersomes. The drug encapsulation efficiency was determined by UV-vis spectroscopy at 480 nm.
1.5. Preparation of buffer-encapsulated polymersomes
Buffer-encapsulated polymersomes were prepared to assess the cytotoxicity of the vesicles without any doxorubicin. PLA8500-diazobenzene-PEG2000 solution and LR lipid dye were used in the molar ratio of 95:5. Briefly, the LR solution was evaporated into a glass vial. Then, PLA8500-diazobenzene-PEG2000 solution (10 mg/mL, 100 μL) was added into the vial and the mixture was added dropwise into another glass vial containing 1 mL HEPES buffer (25 mM, pH 7.4). The acetone solvent in the polymer solution was removed by passing the air through the vial for 45 minutes. The mixture was then sonicated for 60 minutes and passed through Sephadex G-100 chromatography column to separate unencapsulated Dox-loaded polymersomes from encapsulated ones.
1.6. Characterization
1.6.1. Atomic Force Microscopy (AFM)
Polymersomes were prepared before and after 24-hour exposure to hypoxia. For the hypoxic condition, 100 μM NADPH solution, and 100 μL human liver microsomes were added to 1 mL polymersome solution. Polymersome samples (1 mg/mL) were prepared by incubating 10 μL of each solution on silicon substrates (University Wafer) for 10 minutes in a sealed compartment. The samples were then rinsed with deionized water (Millipore) and dried under purified nitrogen flow. The imaging was performed using a commercial atomic force microscope (NT-MDT NTEGRA AFM). The samples were imaged under ambient conditions in semi-contact mode using an AFM tip with a resonant frequency of 190 kHz (Budget sensors).
1.6.2. Dynamic Light Scattering (DLS)
After polymersome preparation, the size and zeta potential of non-targeted and targeted Dox-encapsulated polymersomes were analyzed before and after 24-hour exposure to hypoxia (the same conditions as described for AFM imaging) by Dynamic Light Scattering (Malvern Zetasizer Nano-ZS90). Four measurements were carried out for each sample and an average of all measurements was recorded.
1.6.3. Transmission Electron Microscopy (TEM)
Polymersomes were prepared before and after 24-hour exposure to hypoxia (the same conditions as described for AFM imaging). To pretreat TEM grids, poly L-lysine (1%) was added on 300 mesh Formvar-carbon coated copper grids. After standing for 1 minute, grids were air-dried. A drop of polymersome suspension (1% diluted, 10 μL) was placed on the grids for 30 seconds and a filter paper was used to absorb the liquid. Phosphotungstic acid (0.1%, pH 7.0) was added to the grids, allowed to stand for 2 minutes, and then wicked away from the grids. Images were obtained (when the grids were dried) in a JOEL JEM-2100 LB6 transmission electron microscope (Peabody, Massachusetts, USA).
1.7. Drug loading and release from the polymersomes
The encapsulation efficiency and loading content of doxorubicin in Dox-encapsulated polymersomes were estimated by using a UV-vis spectrophotometer at 480 nm. A calibration curve was created by preparing different Dox concentrations dissolved in deionized water. To evaluate the hypoxia-mediated release behavior of the polymersomes, 1 mL of E2-Dox-HRPS (2 mg/mL), NADPH (100 μM), and 100 μL human liver microsomes were dispersed in 1 mL HEPES buffer (pH 7.4, 25 mM) within a cellulose membrane tube (MWCO: 10 KDa). The tube was immersed in 20 mL HEPES buffer containing 100 μM NADPH within a hypoxia chamber (2% oxygen). For the normoxic condition (control), the tube was immersed in 20 mL HEPES buffer containing 100 μM NADPH at standard atmospheric conditions (21% oxygen) (both hypoxic chambers and normoxic incubators contained 5% CO2 during all experiments). Each sample was gently shaken at 100 rpm. The release medium was withdrawn and replenished with the medium at predefined time intervals. Dox concentration was measured by absorption at 480 nm.
1.8. Cell culture
The MCF7 cell line (ER+ breast carcinoma) was purchased from American Type Culture Collection (ATCC, Manassas, VA). The MCF7 cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% Fetal Bovine Serum (Avantar Seradigm) and 1% v/v antibiotics (Penicillin, Streptomycin, Amphotericin B solution, Corning). For normoxia, a humidified incubator containing 5% CO2, 21% oxygen, 74% nitrogen, and 37° C was used. The Biospherix C21 hypoxic chamber supplemented with 2% oxygen, 93% nitrogen, and 5% CO2 was used to induce hypoxia for the cells.
1.9. Cellular uptake study
The MCF7 cells were cultured in two twelve-well plates (starting with 5000 cells per well) and allowed to grow in normoxia (21% oxygen) and hypoxia (2% oxygen) separately for one doubling time. Both the plates were treated with buffer-encapsulated polymersomes (non-targeted and targeted, 30 μL each well) for 3 hours. Subsequently, the cells were washed with PBS for 3 times and cytoskeleton actin filaments and nuclei of the cells were stained using Phalloidin (Biotium) and DAPI (NucBlue, Invitrogen) dyes, respectively. To demonstrate the role of receptor-mediated endocytosis, another cellular uptake study was performed using 3 μM free estradiol, equivalent amount of targeted Polymersomes, and a combination of 3 μM free estradiol and targeted polymersomes. A fluorescence microscope (Leica, 20x objective) was used for imaging.
1.10. Cytotoxicity studies with plain polymersomes
Different concentrations of the HEPES buffer-encapsulated polymersomes (20 to 100 μg/mL) were used to treat the MCF7 cells. The cells were seeded into two 96-well plates (5000 cells per well) and grew in normoxia (21% oxygen) and hypoxia (2% oxygen) separately. Then, buffer-encapsulated polymersomes were used to treat the cells. After 3 days, the cell viability was measured by the Alamar Blue assay. Briefly, a mixture of DMEM and Alamar Blue reagent (9:1 ratio) was prepared and added into each well of 96-well plates. After 5 hours, the fluorescence was recorded (excitation: 560 nm, emission: 595 nm), and the cell viability was calculated.
1.11. Monolayer cell viability assay
The MCF7 cells were seeded into two 96-well cell culture plates (5000 cells per well) and allowed to grow in normoxia (21% oxygen) and hypoxia (2% oxygen) (one plate under each condition). When the cells reached 85-90% confluency, they were divided into 4 different treatment groups: control (without treatment), Dox-encapsulated hypoxia-responsive polymersomes (Dox-HRPs, non-targeted polymersomes), estradiol-conjugated Dox-encapsulated hypoxia-responsive polymersomes (E2-Dox-HRPs, targeted polymersomes), and free Dox. The cells were treated for 3 days with 5, 10, and 15 μM of free Dox and the equivalent amount of Dox in both non-targeted and targeted polymersomes. Subsequently, the plates were washed with phosphate buffer saline (PBS) three times and the cell viability was assessed by the Alamar Blue assay.
1.12. Three-dimensional spheroidal cell viability assay
Three-dimensional spheroids of MCF7 cells were prepared using a magnetic 3D cell culture kit (Greiner-Bio-one, Monroe, NC). Briefly, 200 μL NanoShuttle-PL magnetic nanoparticles were added into an 85-90% confluent MCF7 cell culture flask and incubated overnight. Unattached nanoparticles were removed by washing the flask with PBS. Then, the cells were detached from the flask by trypsin and added into each well (15,000 cells per well) in a 96-well 3D culture plate and put on top of a magnetic spheroid drive to form spheroids within 15 min. The cells grew overnight in normoxia (21% oxygen) and hypoxia (2% oxygen) separately. Then, the cells were divided into 4 treatment groups (same as the monolayer cell culture) and treated for 3 days with the same concentrations of Dox (5, 10, and 15 μM) in both normoxia and hypoxia. The spheroids were washed with PBS, dislodged from the plates by removing the magnetic spheroid drive, transferred into a new cell culture plate, and cultured overnight. The following day, the cell viability was measured using Alamar Blue assay. To evaluate the effect of the drugs on the growth rate of the three-dimensional cultures, a different group of the 6-day old spheroids were treated with 10 μM free Dox, non-targeted and targeted Dox-encapsulated polymersomes for 72 hours in both normoxia (21% oxygen) and hypoxia (2% oxygen). Then, the spheroids were washed with PBS and their growth was monitored until day 16. NIH ImageJ software was used to analyze and measure the percent growth rate of the spheroids.
1.13. Statistical analysis
OriginPro software (version 9.3, Northampton, Massachusetts) was used for statistical analysis. The results were presented as mean ± SD (n = 3). The significant statistical difference (p < 0.05) between normoxia and hypoxia with different drug concentrations and polymersome treatments was analyzed by a one-way ANOVA Tukey HSD test.
2. Results and Discussion:
2.1. Synthesis of the polymers and preparation of polymersomes
We synthesized the estradiol-conjugated polymer PLA17000-PEG2000-Estradiol from ethynyl estradiol and the azide polymer using [2 + 3]-cycloaddition reaction (Scheme 1). The hypoxia-responsive PLA8500-diazobenzene-PEG2000 polymer was synthesized by following an analogous protocol developed in our laboratory.22
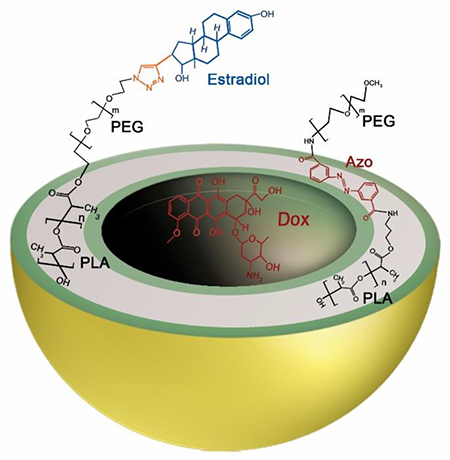
The synthesized polymers were characterized by NMR spectroscopy and gel permeation chromatography (GPC). GPC analysis indicated the polydispersity indices to be 1.17 for PLA8500-diazobenzene-PEG2000 and 1.15 for PLA17000-PEG2000-Estradiol. We performed GPC analysis for PLA8500-diazobenzene-PEG2000 polymer before and after exposure to hypoxia (Supporting Information, Figures S10, S11). We observed substantial changes to the GPC chromatogram, indicating smaller polymer fragments. The diazobenzenebenzene linker group between PEG and PLA molecules is responsible for the polymersome disintegration in hypoxic conditions and acts as a hypoxia-sensitive moiety.33 The surface PEG groups help in the prolonged blood circulation of the polymersomes. The ratio of molecular weights for the hydrophilic block to the whole polymer of 20-40% is critical for the polymersome formation.34 In the synthesized polymers, this ratio was 20%. As it was mentioned in the polymer synthesis, the yield of estradiol attachment to PLA-PEG-N3 polymer was around 20%. To prepare targeted polymersomes with high efficiency to bind the surface receptors, we optimized our polymersomes to contain 2% of the PLA-PEG-Estradiol polymer conjugate. (2% PLA-PEG-Estradiol, 93% PEG-diazobenzene-PLA, 5% lissamine-rhodamine dye) (Figure 1).
Figure 1.
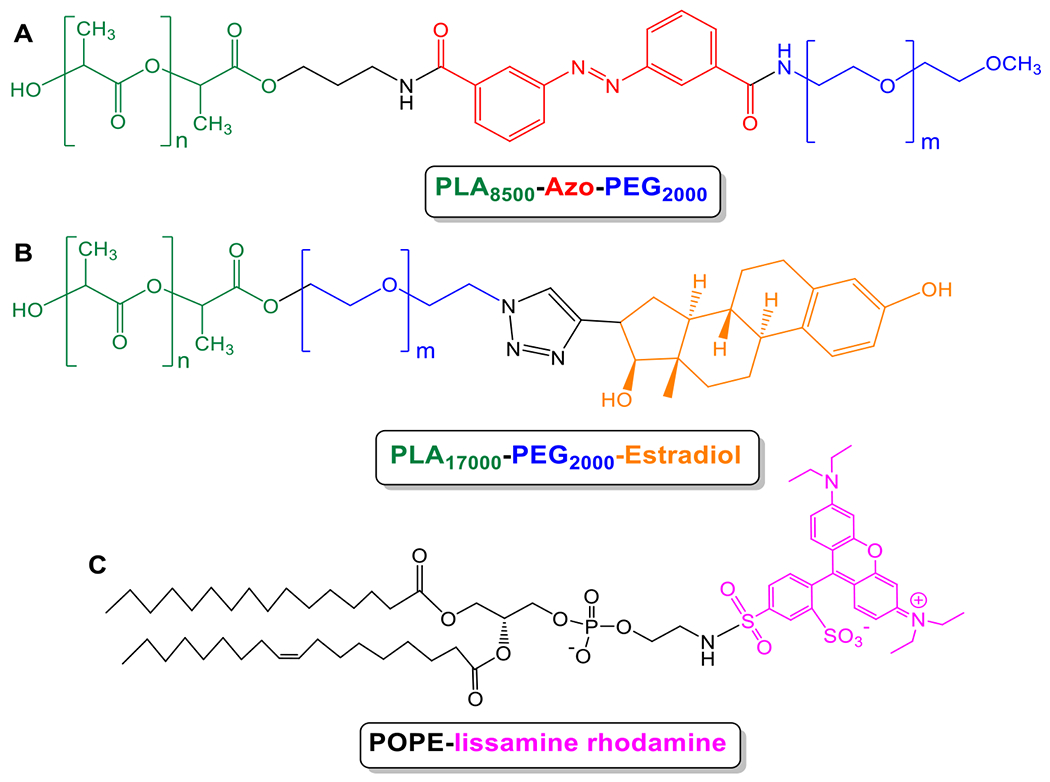
Chemical structures of polymers and fluorescent lipid used for polymersome preparation. (A) PLA8500–diazobenzene–PEG2000 polymer, (B) PLA17000–PEG2000–Estradiol polymer, and (C) 1, 2-dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-lissamine rhodamine B sulfonyl ammonium salt.
Both non-targeted and targeted polymersomes accumulate within the tumors via enhanced permeability and retention (EPR) effect which is due to the leaky vasculature and impaired lymphatic drainage present in the solid tumors.25 Subsequently, the targeted polymersomes enter the cells mediated by the interaction between estradiol groups of the polymersomes and estrogen receptors on the surface of breast cancer cells (Figure 2).30
Figure 2.
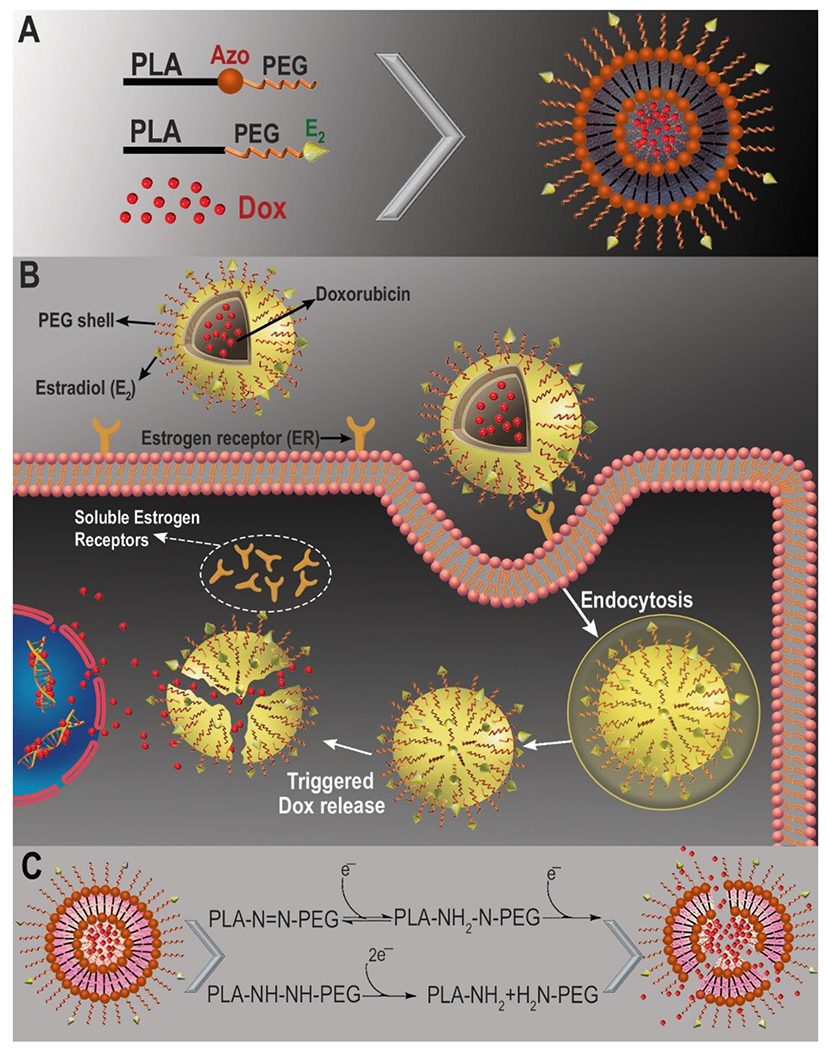
Schematic illustration of the targeted Dox-loaded polymersomes and internalization into ER-positive breast cancer cells. (A) Components of the ER targeted polymersomes; (B) ligand-receptor mediated endocytosis of ER targeted polymersomes into the cells.29 (C) Mechanism of Dox release in hypoxia.
The polymersomes were prepared using the solvent exchange method35 and characterized by dynamic light scattering, transmission electron microscopy, and atomic force microscopy. The average encapsulation efficiency and loading content of Dox within Dox-HRPs were found to be 48% and 9 weight percent, respectively. The average encapsulation efficiency and loading content of Dox within targeted E2-Dox-HRPS were 59% and 11.3 weight percent, respectively. The hydrodynamic diameter and zeta potential of the non-targeted and targeted polymersomes were measured before and after exposure to hypoxia. The zeta potential of the polymersomes was slightly positive and did not change significantly under normoxia and hypoxia (Table 1). We observed that the average hydrodynamic diameters of the non-targeted polymersomes changed from 126 ± 2 nm in normoxia to 41 ± 5 nm and 439 ± 25 nm in hypoxia (Figure 3A, Supporting information Figure S12). However, the targeted polymersomes with 17β-estradiol on the surface were larger, likely due to the incorporation of the higher molecular weight PLA17000-PEG2000-Estradiol polymer conjugate (168 ± 3 nm in normoxia and 52 ± 6 nm and 695 ± 32 nm in hypoxia, respectively; Figure 3B, Supporting information Figure S13, Table 1).
Table 1.
Hydrodynamic diameter and polydispersity index (PDI) of the polymersomes
| Polymersome | Average diameter (nm) | Zeta potential (mV) | PDI | |||
|---|---|---|---|---|---|---|
| Normoxia | Hypoxia | Normoxia | Hypoxia | Normoxia | Hypoxia | |
| Non-targeted | 126 ± 2 | 41 ± 5, 439 ± 25 | 0.12 ± 0.12 | 0.23 ± 0.17 | 0.12 ± 0.03 | 0.71 ± 0.14 |
| Targeted | 168 ± 3 | 52 ± 6, 695 ± 32 | 0.21 ± 0.11 | 0.32 ± 0.14 | 0.13 ± 0.02 | 0.77 ± 0.18 |
Figure 3.
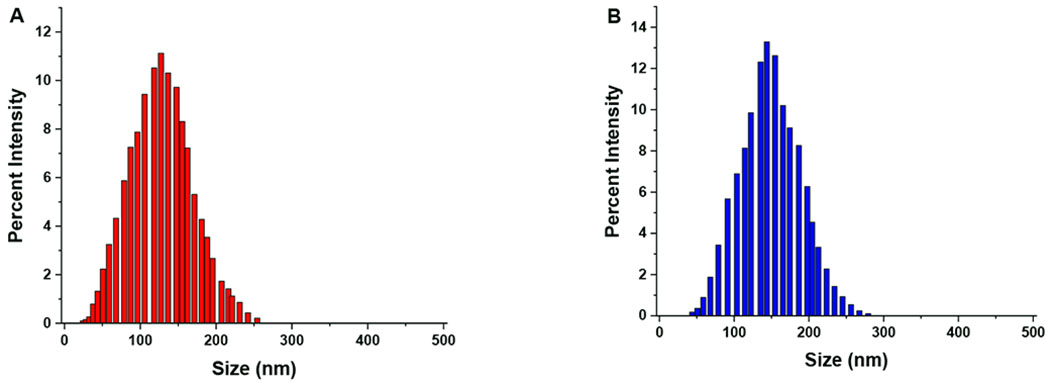
Hydrodynamic diameters of the polymersomes in normoxia. (A) Non-targeted, Dox-encapsulated and (B) ER-targeted Dox-encapsulated polymersomes.
2.2. Hypoxia-mediated drug release from polymersomes
The hypoxic microenvironment of solid tumors is rich in reductase enzymes and other reducing agents.36 Based on literature reports, we expect that the diazobenzene groups of the polymers will undergo four-electron reduction by the reductase enzymes under hypoxia to separate the hydrophobic PLA and the hydrophilic PEG blocks of the polymer (illustrated in Figure 2C). 37–39 Previously, we demonstrated that such reduction leads to the disintegration of the polymersome structure and release of the encapsulated drug.22
To test the release of drug under hypoxia, doxorubicin was encapsulated into the estradiol-conjugated hypoxia-responsive polymersomes (E2-Dox-HRPS). Dox release was measured as a function of time in hypoxia and normoxia (Figure 4).
Figure 4.
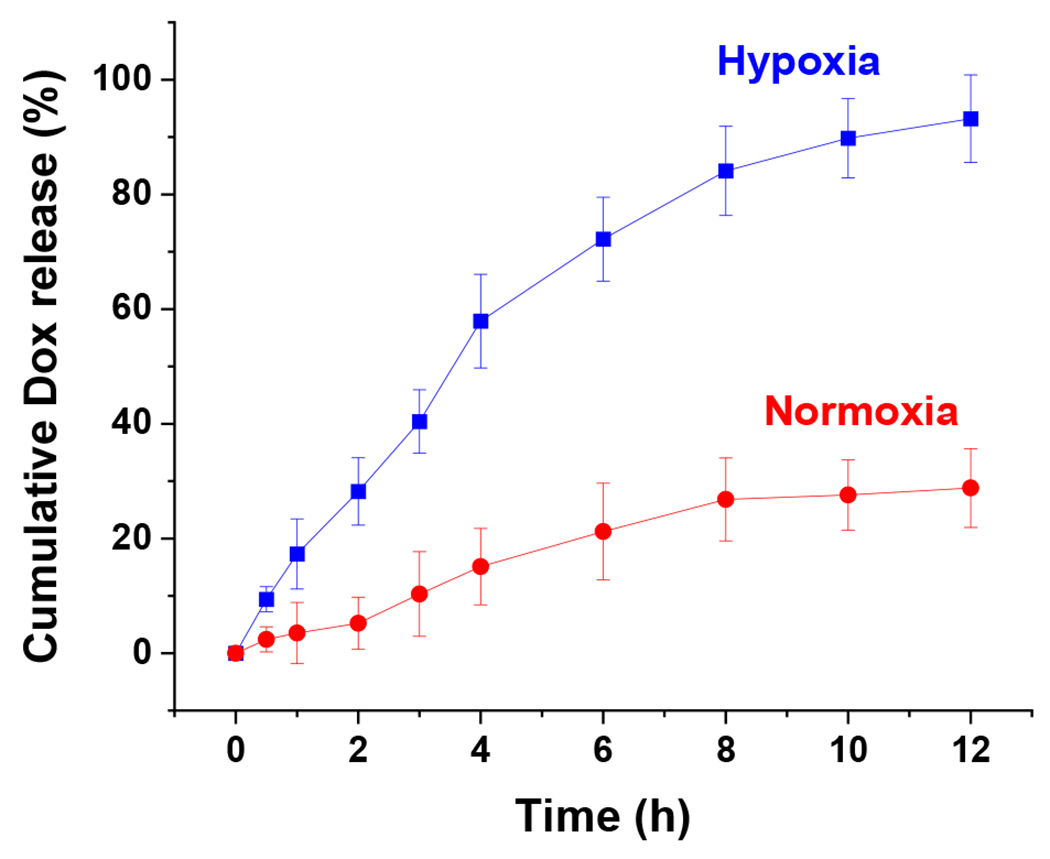
Cumulative release of encapsulated Dox from the targeted polymersomes under hypoxia (2% oxygen, blue squares) and normoxia (21% oxygen, red circles) (n = 3).
The release rate of Dox from polymersomes in hypoxia was significantly higher than Dox release in normoxia. The polymersomes released more than 90% of Dox in a sustained manner under hypoxia after 12 hours, while about 30% of Dox was released in normoxia during this time. The results demonstrated that hypoxia-responsive polymersomes preferentially release the encapsulated drug in hypoxic conditions due to the reduction of diazobenzenebenzene moiety within the polymer structure (Figure 2C).37,38 Polymersomes lost their spherical shape after exposure to hypoxic conditions, demonstrating vesicular disintegration (Figure 5A–D). Dynamic light scattering experiments showed a reduction in hydrodynamic diameter of the polymersomes and an increase in the polydispersity index under hypoxia (Table 1).
Figure 5.
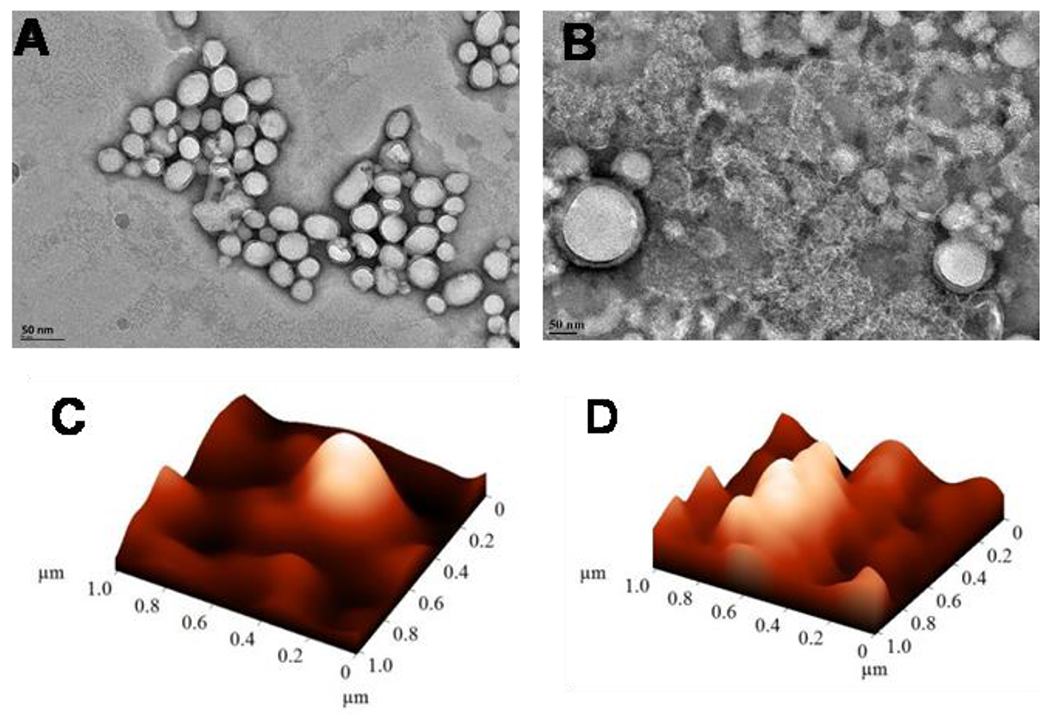
Transmission electron microscopic (TEM) and atomic force microscopic (AFM) images of estradiol-polymersomes under normoxia (A, C) and hypoxia (B, D).
2.3. Cellular uptake study
To determine the internalization of the estradiol-conjugated polymersomes within ER-positive breast cancer cells, 5% POPE-lissamine rhodamine (Figure 1C) was incorporated into the bilayer of the vesicles. The MCF7 cells were incubated with 3 μM targeted and non-targeted polymersomes (without Dox) for 3 hours under normoxia and hypoxia (Figure 6A). Cellular localization was observed after 3-hour treating the cells with the polymersomes. The fluorescence intensity of the microscopic images was normalized with respect to the number of the MCF7 cells. The images were analyzed using the NIH ImageJ software and the quantitative fluorescence integral density per unit area of targeted and non-targeted treatments in both normoxia and hypoxia were calculated (Figure 6B). The integral density of the targeted polymersomes was 9.7 times higher compared to non-targeted vesicles under hypoxia and 3.5 times higher compared to targeted polymersomes in normoxia, respectively.
Figure 6.

(A) Fluorescence microscopic images of cellular internalization of non-targeted and targeted polymersomes in monolayer cultures of MCF7 cells in normoxia and hypoxia (scale bar: 50 μm). (B) Quantitative fluorescence integral density indicating cellular uptake after treating the MCF7 cells with targeted polymersomes in normoxia and hypoxia (n = 3, *p < 0.05, **p < 0.01).
Nanoparticle retention within the cancer cells depends on various processes, including the size of nanoparticles, endo- and exocytosis, cellular homeostasis, and the degree and duration of hypoxia exposure. Based on previous studies, nanoparticle uptake in breast cancer cells changes in a dynamic way in hypoxia.40 A prior study on MCF7 breast cancer cells demonstrated an elevated uptake of gold nanoparticles in hypoxia.41 Since the MCF7 cells overexpress the estrogen receptors on surface, we anticipated that the targeted polymersomes would show increased cellular internalization compared to the non-targeted counterparts.42 We observed that 3-hour exposure to hypoxia led to increased internalization of the E2-targeted polymersomes in the MCF7 cells (Figure 6). To further demonstrate the role of receptor-mediated endocytosis, we performed another uptake study using 3 μM free estradiol, equivalent amount of targeted polymersomes (E2-HRPs), and a combination of 3 μM both free estradiol and targeted polymersomes. The results demonstrated that estradiol decreased cellular uptake of the targeted polymersomes (Supporting Informations, Figures S8–9). This might be due to a competitive binding of free estradiol molecules to estrogen receptors. Thus, there would be less free estrogen receptors on cell surface to bind to the targeted E2-polymersomes.
2.4. Cytotoxicity and cell viability in monolayer cultures
To determine the toxicity, we incubated MCF7 cells with varying amounts of the HEPES buffer-encapsulated estradiol-targeted polymersomes. The polymersomes showed minimal toxicity with more than 87% cell viability after 72 hours in both normoxic and hypoxic conditions up to a total polymer concentration of 100 μg/mL (Figure 7A). To determine the effectiveness of the drug-loaded polymersomes, we incubated the MCF7 monolayer cell cultures for 72 hours with four treatments: non-targeted Dox-encapsulated hypoxia-responsive polymersomes (Dox-HRPs), targeted estradiol-conjugated Dox-encapsulated hypoxia-responsive polymersomes (E2-Dox-HRPs), free Dox, and control (no treatment) (Figure 7B). In hypoxic conditions, treating the MCF7 cells with 15 μM (Dox concentration) targeted E2-Dox-HRPS reduced the viability to 14%. We also observed that non-targeted Dox-HRPs with 15 μM doxorubicin reduced the cell viability to 39% under hypoxia. There was a significant difference (p < 0.05) between normoxia and hypoxia when the cells were treated with targeted E2-Dox-HRPS. A substantial reduction in the viability was also observed when the cells were treated with non-targeted Dox-HRPs in hypoxia compared to non-targeted Dox-HRPs in normoxia.
Figure 7.
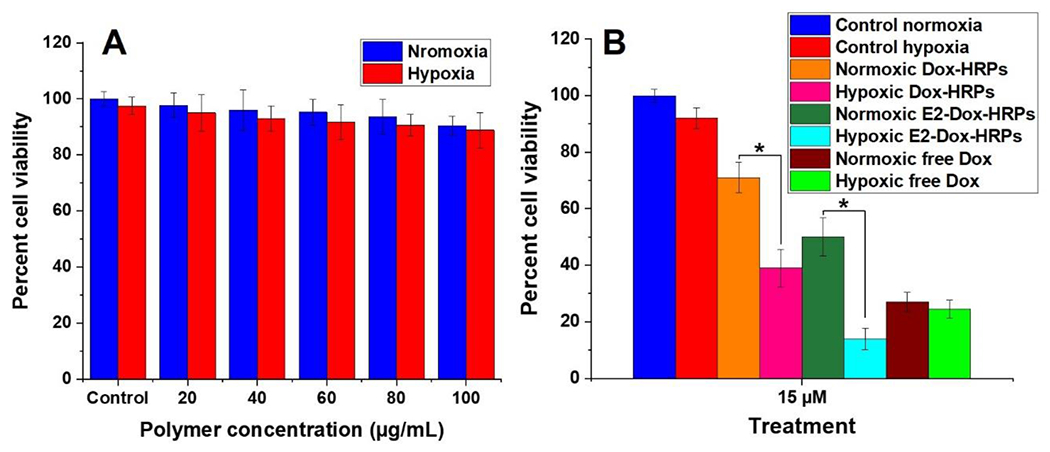
(A) Toxicity of the HEPES buffer encapsulated estradiol-polymersomes in normoxia and hypoxia for the MCF7 cells. (B) Viability of the MCF7 monolayer culture after 72 h treatment with free Dox, non-targeted Dox-encapsulated polymersomes (Dox-HRP), and targeted Dox-encapsulated polymersomes (E2-Dox-HRPS) under normoxia and hypoxia (n = 6, *p < 0.05).
The results demonstrated that both targeted and non-targeted polymersomes could lower the viability of the breast cancer cells in hypoxia compared to normoxia. However, the targeted Dox-polymersomes were more effective compared to the free drug (Figure 7B). Also, the targeted Dox-polymersomes were significantly more effective in killing breast cancer cells under hypoxia compared to normoxia. We did not observe such a trend for free doxorubicin. Based on the Dox release profile (Figure 4), the polymersomes will release about 40% of the encapsulated drug in 3 hours and about 80% after 8 hours. Hence, 72-hour treatment of the breast cancer cells with the polymersomes ensures enough time for the vesicles to enter the cells, undergo structural destabilization, and release the encapsulated drug in the cytosol.
2.5. Viability of breast cancer cells in three-dimensional spheroid cultures
Although the monolayer cultures offer valuable information about the cells, it does not mimic the three-dimensional structure of the tumors. To provide a better model system, MCF7 cells were cultured as three-dimensional spheroids. Hypoxic cells within the core of the spheroids can simulate hypoxic niches within tumors for in vitro hypoxia-responsive targeted drug delivery studies. The spheroids were treated for 72 hours with the two polymersome formulations and free drug the same as monolayer cell cultures (15 μM free Dox and equivalent amount of non-targeted and targeted Dox-encapsulated polymersomes) and the cell viability was determined (Figure 8A). Under hypoxia, treating the breast cancer cell spheroids with targeted E2-Dox-HRPS (15 μM Dox) significantly (p < 0.05) reduced the cell viability to 21% compared to targeted E2-Dox-HRPS in normoxia (55%). However, we did not observe significant differences in cell viability under hypoxia and normoxia when the cells were treated with non-targeted polymersomes or free Dox.
Figure 8.

(A) Viability of MCF7 cell spheroids after 72-hour treatment with free Dox, non-targeted (Dox-HRPs) and targeted polymersomes (E2-Dox-HRPS) under normoxia and hypoxia (n = 6, *p < 0.05). (B) Representative images of three-dimensional spheroid cultures of the MCF7 cells before and after 72-hour treatment with free Dox, non-targeted and targeted polymersomes in normoxia and hypoxia (scale bar: 100 μm).
Due to the enhanced permeability and retention effect, both targeted and non-targeted polymersomes can pass through the leaky vasculature and reach the tumor site.43 However, the targeted polymersomes will show enhanced tumor penetration and cytotoxicity based on receptor-mediated endocytosis. However, we note that some polymersomes may release their encapsulated Dox before cellular entry. In this case, free Dox will diffuse into the cell through the plasma membrane.44 The passive diffusion would be less predominant when Dox is delivered to the cancer cells via the targeted hypoxia-responsive polymersomes. We observed that majority of the polymersomes internalized into the breast cancer cells in 3 hours under hypoxia (Figure 6), while less than 40% of the drug was released after 3 hours (Figure 4).
To further evaluate the efficacy of non-targeted and targeted polymersomes, the 6-day old spheroids were treated with 10 μM free Dox and equivalent amount of non-targeted Dox-HRPs and targeted E2-Dox-HRPS for 72 hours in both hypoxia and normoxia and their growth was monitored until day 16 (Figures 8B, C).
We observed that targeted E2-Dox-HRPS were more toxic to the MCF7 cells by shrinking the volume of the spheroids up to 68%, while non-targeted polymersomes reduced the volume of the spheroid by 24% in hypoxia (Figure 9A). The targeted polymersomes significantly reduced the spheroid volume compared to non-targeted Dox-HRPs (p < 0.05) and control group (p < 0.01) in hypoxia (Figure 9A). A significant difference (p < 0.01) was also observed between targeted E2-Dox-HRPs and the control group in normoxia (Figure 9B), although not as pronounced as the cells under hypoxia. In addition, non-targeted polymersomes demonstrated less toxicity toward spheroids compared to free Dox in both hypoxia and normoxia. Overall, the results demonstrated that targeted polymersomes have the potential to shrink the microtumors in hypoxia compared to non-targeted polymersomes. Targeted polymersomes could also shrink the spheroids compared to the control group in both hypoxia and normoxia.
Figure 9.
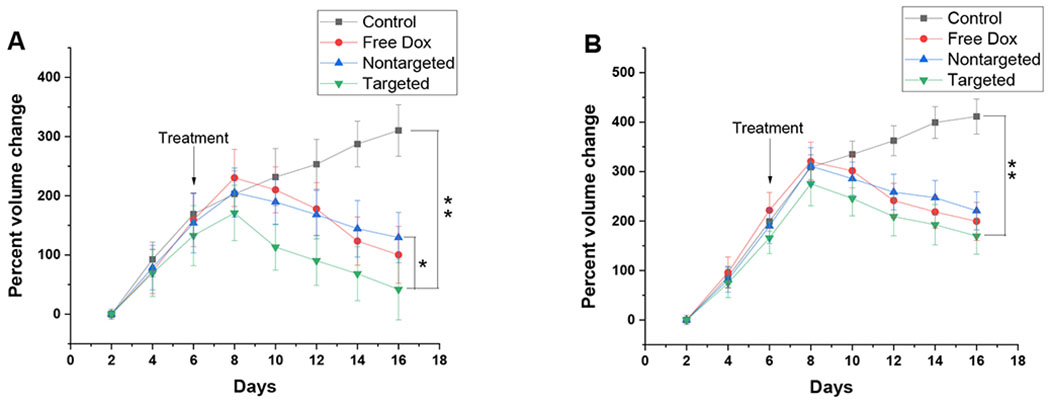
Growth curve for the MCF7 spheroids in hypoxia (A) and normoxia (B) treated with free Dox, non-targeted and targeted Dox-encapsulated polymersomes (n = 6, *p < 0.05, **p < 0.01).
3. Conclusion
The 17β-estradiol-conjugated polymersomes described here are the first reported targeted polymeric hypoxia-responsive drug delivery nanocarriers for reducing ER-positive breast cancer cell viability. Targeted polymersomes significantly reduced the volume of MCF7 spheroids in hypoxic conditions compared to non-targeted polymersomes and free drugs. Due to the presence of 17β-estradiol on the surface of targeted polymersomes, they can bind to the ER on the surface of the breast cancer cells and internalize. The targeted polymersomes encapsulated doxorubicin with an efficiency of 59%. The hypoxia-responsive group employed in polymer structure allowed the release of Dox within hypoxic breast cancer cells and enhanced doxorubicin therapeutic efficiency against ER-positive breast cancer cells. The merits of our targeted polymeric nanoparticles are; ability to selectively target the estrogen receptor-positive breast cancer cells, break in the hypoxic niches of microtumors, release the encapsulated anticancer drug, and reduce cancer cell viability. With further developments, the 17β-estradiol-conjugated hypoxia-responsive polymersomes have the translational potential for targeted drug delivery in ER-positive breast cancer therapy.
Supplementary Material
Acknowledgments:
This research was supported by NIH grant 1 R01GM 114080 (NIGMS) and a Ready-to-Go award from the DaCCoTA Center (NIGMS U54 GM128729) to SM. SM also acknowledges support from the Grand Challenge Initiative and the Office of the Dean, College of Health Professions, North Dakota State University. The authors thank Parinaz Ghanbari for designing all schematic illustrations in this manuscript.
Footnotes
Associated content
Supporting information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/
1H and 13C NMR spectra, FT-IR spectra, fluorescence microscopic images of cellular uptake studies, hydrodynamic diameter of the polymersomes under hypoxia, and GPC of the synthesized polymers under hypoxia and normoxia.
References
- (1).DeSantis CE; Ma J; Gaudet MM; Newman LA; Miller KD; Sauer AG; Jemal A; Siegel RL Breast Cancer Statistics, 2019. CA. Cancer J. Clin 2019, 69 (6), 438–451. 10.3322/caac.21583. [DOI] [PubMed] [Google Scholar]
- (2).Sørlie T; Perou CM; Tibshirani R; Aas T; Geisler S; Johnsen H; Hastie T; Eisen MB; van de Rijn M; Jeffrey SS; Thorsen T; Quist H; Matese JC; Brown PO; Botstein D; Lønning PE; Børresen-Dale A-L Gene Expression Patterns of Breast Carcinomas Distinguish Tumor Subclasses with Clinical Implications. Proc. Natl. Acad. Sci. U. S. A 2001, 98(19), 10869–10874. 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Dai X; Li T; Bai Z; Yang Y; Liu X; Zhan J; Shi B Breast Cancer Intrinsic Subtype Classification, Clinical Use and Future Trends. Am. J. Cancer Res 2015, 5(10), 2929–2943. [PMC free article] [PubMed] [Google Scholar]
- (4).Street W Breast Cancer Facts & Figures 2019-2020. 44. [Google Scholar]
- (5).Brinkhuis RP; Rutjes FPJT; Hest J. C. M. van. Polymeric Vesicles in Biomedical Applications. Polym. Chem 2011, 2(7), 1449–1462. 10.1039/C1PY00061F. [DOI] [Google Scholar]
- (6).Discher BM; Won Y-Y; Ege DS; Lee JC-M; Bates FS; Discher DE; Hammer DA Polymersomes: Tough Vesicles Made from Diblock Copolymers. Science 1999, 284 (5417), 1143–1146. 10.1126/science.284.5417.1143. [DOI] [PubMed] [Google Scholar]
- (7).Confeld MI; Mamnoon B; Feng L; Jensen-Smith H; Ray P; Froberg J; Kim J; Hollingsworth MA; Quadir M; Choi Y; Mallik S Targeting the Tumor Core: Hypoxia-Responsive Nanoparticles for Delivery of Chemotherapy to Pancreatic Tumors. Mol. Pharm 2020. 10.1021/acs.molpharmaceut.0c00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Chen W; Meng F; Cheng R; Zhong Z PH-Sensitive Degradable Polymersomes for Triggered Release of Anticancer Drugs: A Comparative Study with Micelles. J. Controlled Release 2010, 142 (1), 40–46. 10.1016/j.jconrel.2009.09.023. [DOI] [PubMed] [Google Scholar]
- (9).Lomas H; Canton I; MacNeil S; Du J; Armes SP; Ryan AJ; Lewis AL; Battaglia G Biomimetic PH Sensitive Polymersomes for Efficient DNA Encapsulation and Delivery. Adv. Mater 2007, 19 (23), 4238–243. 10.1002/adma.200700941. [DOI] [Google Scholar]
- (10).Nahire R; Haldar MK; Paul S; Ambre AH; Meghnani V; Layek B; Katti KS; Gange KN; Singh J; Sarkar K; Mallik S Multifunctional Polymersomes for Cytosolic Delivery of Gemcitabine and Doxorubicin to Cancer Cells. Biomaterials 2014, 35 (24), 6482–6497. 10.1016/j.biomaterials.2014.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Karandish F; Mamnoon B; Feng L; Haldar MK; Xia L; Gange KN; You S; Choi Y; Sarkar K; Mallik S Nucleus-Targeted, Echogenic Polymersomes for Delivering a Cancer Stemness Inhibitor to Pancreatic Cancer Cells. Biomacromolecules 2018, 19 (10), 4122–4132. 10.1021/acs.biomac.8b01133. [DOI] [PubMed] [Google Scholar]
- (12).Christian DA; Cai S; Bowen DM; Kim Y; Pajerowski JD; Discher DE Polymersome Carriers: From Self-Assembly to SiRNA and Protein Therapeutics. Eur. J. Pharm. Biopharm 2009, 71 (3), 463–74. 10.1016/j.ejpb.2008.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Anajafi T; Mallik S Polymersome-Based Drug-Delivery Strategies for Cancer Therapeutics. Ther. Deliv 2015, 6(4), 521–534. 10.4155/tde.14.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Lee JS; Feijen J Polymersomes for Drug Delivery: Design, Formation and Characterization. J. Controlled Release 2012, 161 (2), 473–483. 10.1016/j.jconrel.2011.10.005. [DOI] [PubMed] [Google Scholar]
- (15).Liu G; Ma S; Li S; Cheng R; Meng F; Liu H; Zhong Z The Highly Efficient Delivery of Exogenous Proteins into Cells Mediated by Biodegradable Chimaeric Polymersomes. Biomaterials 2010, 31 (29), 7575–7585. 10.1016/j.biomaterials.2010.06.021. [DOI] [PubMed] [Google Scholar]
- (16).Ahmed F; Pakunlu RI; Brannan A; Bates F; Minko T; Discher DE Biodegradable Polymersomes Loaded with Both Paclitaxel and Doxorubicin Permeate and Shrink Tumors, Inducing Apoptosis in Proportion to Accumulated Drug. J. Controlled Release 2006, 116 (2), 150–158. 10.1016/j.jconrel.2006.07.012. [DOI] [PubMed] [Google Scholar]
- (17).Photos PJ; Bacakova L; Discher B; Bates FS; Discher DE Polymer Vesicles in Vivo: Correlations with PEG Molecular Weight. J. Controlled Release 2003, 90 (3), 323–334. 10.1016/S0168-3659(03)00201-3. [DOI] [PubMed] [Google Scholar]
- (18).Yotnda P; Wu D; Swanson AM Hypoxic Tumors and Their Effect on Immune Cells and Cancer Therapy. In Immunotherapy of Cancer: Methods and Protocols; Yotnda P, Ed.; Methods in Molecular Biology; Humana Press: Totowa, NJ, 2010; pp 1–29. 10.1007/978-1-60761-786-0_1. [DOI] [PubMed] [Google Scholar]
- (19).Lu X; Kang Y Hypoxia and Hypoxia-Inducible Factors: Master Regulators of Metastasis. Clin. Cancer Res 2010, 16 (24), 5928–5935. 10.1158/1078-0432.CCR-10-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Wilson WR; Hay MP Targeting Hypoxia in Cancer Therapy. Nat. Rev. Cancer 2011, 11 (6), 393–410. 10.1038/nrc3064. [DOI] [PubMed] [Google Scholar]
- (21).Ding Y; Kang Y; Zhang X Enzyme-Responsive Polymer Assemblies Constructed through Covalent Synthesis and Supramolecular Strategy. Chem. Commun 2014, 51 (6), 996–1003. 10.1039/C4CC05878J. [DOI] [PubMed] [Google Scholar]
- (22).Kulkarni P; Haldar MK; You S; Choi Y; Mallik S Hypoxia-Responsive Polymersomes for Drug Delivery to Hypoxic Pancreatic Cancer Cells. Biomacromolecules 2016, 17 (8), 2507–2513. 10.1021/acs.biomac.6b00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Liu J; Huang Y; Kumar A; Tan A; Jin S; Mozhi A; Liang X-J PH-Sensitive Nano-Systems for Drug Delivery in Cancer Therapy. Biotechnol. Adv 2014, 32 (4), 693–710. 10.1016/j.biotechadv.2013.11.009. [DOI] [PubMed] [Google Scholar]
- (24).Karandish F; Froberg J; Borowicz P; Wilkinson JC; Choi Y; Mallik S Peptide-Targeted, Stimuli-Responsive Polymersomes for Delivering a Cancer Stemness Inhibitor to Cancer Stem Cell Microtumors. Colloids Surf. B Biointerfaces 2018, 163, 225–235. 10.1016/j.colsurfb.2017.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Thambi T; Park JH; Lee DS Stimuli-Responsive Polymersomes for Cancer Therapy. Biomater. Sci 2015, 4(1), 55–69. 10.1039/C5BM00268K. [DOI] [PubMed] [Google Scholar]
- (26).Vaupel P; Höckel M; Mayer A Detection and Characterization of Tumor Hypoxia Using PO2 Histography. Antioxid. Redox Signal 2007, 9 (8), 1221–1235. 10.1089/ars.2007.1628. [DOI] [PubMed] [Google Scholar]
- (27).Mechanisms for estrogen receptor expression in human cancer | Experimental Hematology & Oncology | Full Text https://ehoonline.biomedcentral.com/articles/10.1186/s40164-018-0116-7 (accessed Jan 11,2020). [DOI] [PMC free article] [PubMed]
- (28).Estrogen synthesis and signaling pathways during aging: from periphery to brain - ScienceDirect https://www.sciencedirect.com/science/article/pii/S1471491412002444?via%3Dihub (accessed Jan 11, 2020). [DOI] [PMC free article] [PubMed]
- (29).Differential expression of estrogen receptor α, β1, and β2 in lobular and ductal breast cancer | PNAS https://www.pnas.org/content/111/5/1933 (accessed Jan 11, 2020).
- (30).Estrogen-Anchored pH-Sensitive Liposomes as Nanomodule Designed for Site-Specific Delivery of Doxorubicin in Breast Cancer Therapy | Molecular Pharmaceutics https://pubs.acs.org/doi/full/10.1021/mp200439z (accessed Jan 11, 2020). [DOI] [PubMed]
- (31).Estrogen-functionalized liposomes grafted with glutathione-responsive sheddable chotooligosaccharides for the therapy of osteosarcoma: Drug Delivery: Vol 25, No 1 https://www.tandfonline.com/doi/full/10.1080/10717544.2018.1458920 (accessed Jan 11, 2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).17β-Estradiol-Associated Stealth-Liposomal Delivery of Anticancer Gene to Breast Cancer Cells - Reddy - 2005 - Angewandte Chemie International Edition - Wiley Online Library; https://onlinelibrary.wiley.com/doi/full/10.1002/anie.200501793 (accessed Jan 11, 2020). [DOI] [PubMed] [Google Scholar]
- (33).Chevalier A; Piao W; Hanaoka K; Nagano T; Renard P-Y; Romieu A Diazobenzenebenzene-Caged Sulforhodamine Dyes: A Novel Class of ‘turn-on’ Reactive Probes for Hypoxic Tumor Cell Imaging. Methods Appl. Fluoresc 2015, 3 (4), 044004. 10.1088/2050-6120/3/4/044004. [DOI] [PubMed] [Google Scholar]
- (34).Letchford K; Burt H A Review of the Formation and Classification of Amphiphilic Block Copolymer Nanoparticulate Structures: Micelles, Nanospheres, Nanocapsules and Polymersomes. Eur. J. Pharm. Biopharm 2007, 65 (3), 259–269. 10.1016/j.ejpb.2006.11.009. [DOI] [PubMed] [Google Scholar]
- (35).Discher BM; Won Y-Y; Ege DS; Lee JC-M; Bates FS; Discher DE; Hammer DA Polymersomes: Tough Vesicles Made from Diblock Copolymers. Science 1999, 284 (5417), 1143–1146. 10.1126/science.284.5417.1143. [DOI] [PubMed] [Google Scholar]
- (36).Patel A; Sant S Hypoxic Tumor Microenvironment: Opportunities to Develop Targeted Therapies. Biotechnol. Adv 2016, 34 (5), 803–812. 10.1016/j.biotechadv.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Kiyose K; Hanaoka K; Oushiki D; Nakamura T; Kajimura M; Suematsu M; Nishimatsu H; Yamane T; Terai T; Hirata Y; Nagano T Hypoxia-Sensitive Fluorescent Probes for in Vivo Real-Time Fluorescence Imaging of Acute Ischemia. J. Am. Chem. Soc 2010, 132 (45), 15846–15848. 10.1021/ja105937q. [DOI] [PubMed] [Google Scholar]
- (38).Zbaida S; Levine WG A Novel Application of Cyclic Voltammetry for Direct Investigation of Metabolic Intermediates in Microsomal Diazobenzene Reduction. Chem. Res. Toxicol 1991, 4(1), 82–88. 10.1021/tx00019a011. [DOI] [PubMed] [Google Scholar]
- (39).Confeld MI; Mamnoon B; Feng L; Jensen-Smith H; Ray P; Froberg J; Kim J; Hollingsworth MA; Quadir M; Choi Y; Mallik S Targeting the Tumor Core: Hypoxia-Responsive Nanoparticles for the Delivery of Chemotherapy to Pancreatic Tumors. Mol. Pharm 2020, 17(8), 2849–2863. 10.1021/acs.molpharmaceut.0c00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Brownlee WJ; Seib FP Impact of the Hypoxic Phenotype on the Uptake and Efflux of Nanoparticles by Human Breast Cancer Cells. Sci. Rep 2018, 8 (1), 12318. 10.1038/s41598-018-30517-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Neshatian M; Chung S; Yohan D; Yang C; Chithrani DB Determining the Size Dependence of Colloidal Gold Nanoparticle Uptake in a Tumor-like Interface (Hypoxic). Colloids Interface Sci. Commun 2014, 1, 57–61. 10.1016/j.colcom.2014.07.004. [DOI] [Google Scholar]
- (42).Jain AS; Goel PN; Shah SM; Dhawan VV; Nikam Y; Gude RP; Nagarsenker MS Tamoxifen Guided Liposomes for Targeting Encapsulated Anticancer Agent to Estrogen Receptor Positive Breast Cancer Cells: In Vitro and in Vivo Evaluation. Biomed. Pharmacother 2014, 68 (4), 429–438. 10.1016/j.biopha.2014.03.004. [DOI] [PubMed] [Google Scholar]
- (43).Das RP; Gandhi VV; Singh BG; Kunwar A Passive and Active Drug Targeting: Role of Nanocarriers in Rational Design of Anticancer Formulations. Curr. Pharm. Des 2019, 25 (28), 3034–3056. 10.2174/1381612825666190830155319. [DOI] [PubMed] [Google Scholar]
- (44).Dalmark M The Physicochemical Properties and Transmembraneous Transport of Doxorubicin. In Anthracycline Antibiotics in Cancer Therapy: Proceedings of the International Symposium on Anthracycline Antibiotics in Cancer Therapy, New York, New York, 16–18 September 1981; Muggia FM, Young CW, Carter SK, Eds.; Developments in Oncology; Springer; Netherlands: Dordrecht, 1982; pp 165–172. 10.1007/978-94-009-7630-6_15. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


