Abstract
As we actively explore the environment, our motion relative to the world stimulates numerous sensory systems. Notably, proprioceptors provide feedback about body and limb position, while the vestibular system detects and encodes head motion. When the vestibular system is functioning normally, we are unaware of a distinct sensation because vestibular information is integrated with proprioceptive and other sensory inputs to generate our sense of motion. However, patients with vestibular sensory loss experience impairments that provide important insights into the function of this essential sensory system. For these patients, everyday activities such as walking become difficult because even small head movements can produce postural and perceptual instability. This review describes recent research demonstrating how the proprioceptive and vestibular systems effectively work together to provide us with our “6th sense” during everyday activities, and in particular considers the neural computations underlying the brain’s predictive sensing of head movement during voluntary self-motion.
Introduction
The vestibular system is an essential sensory system that makes important contributions to our subjective sense of movement and orientation in space. It comprises five sensory organs that are located in the petrous part of the temporal bone in close proximity to the cochlea.
Specifically, on each side of the head, three semicircular canals detect angular head acceleration about three orthogonal axes and two otolith organs (the saccule and utricle) detect linear head acceleration (i.e., gravity and translational movements). In turn, the head motion information that is sensed by the receptor cells within the semicircular canals and otolith sensory organs is transmitted via the afferent fibers of the vestibular nerve (a branch of the VIIIth nerve) to central vestibular pathways.
A unique feature of the vestibular system is that neurons in the vestibular nuclei, which receive direct peripheral afferent input, also send direct projections to motoneurons to generate essential reflexes mediated by the vestibular-ocular and vestibular-spinal pathways. An advantage of this streamlined circuitry is that vestibular sensorimotor reflexes have extraordinarily short latencies. For example, projections from vestibular afferents to the vestibular-ocular and vestibulo-spinal reflex pathways generate compensatory eye and head/body movement within ~5 and 35–40 ms, respectively, to ensure stable gaze and posture[1,2].To date, how vestibular afferents and central pathways encode and process head motion to generate these reflexes has been well studied [3,4]. Traditionally, single-unit recording experiments have largely been performed in conditions where head motion stimuli are passively applied using a motion platform to rotate or translate the entire animal subject.
However, much (if not most) of the vestibular input we experience during our lives is actually the result of our own voluntary (active) behavior. As a result, the head movement stimuli sensed by the vestibular system during our everyday activities can, in turn, rapidly influence subsequent head-in-space motion (reviewed in [5,6•]). Indeed, when vestibular stimuli are unexpected, the vestibulo-spinal reflex pathways generate robust responses that are essential for maintaining posture. Consequently, these compensatory motor commands directly influence head motion. Importantly, however, when vestibular stimuli are ‘expected’ as is generally the case during active movement, these same compensatory motor commands would be counterproductive. Notably, the compensatory motor commands generated by vestibulo-spinal reflexes would theoretically function to stabilize the head and body relative to space, thereby opposing the intended goal of active movement through the environment. This logic then raises the question of how vestibular pathways encode head motion during self-generated natural behaviors (active) versus passively applied (passive) vestibular stimulation?
The suppression of vestibular inputs during active head movements
A series of experiments performed in rhesus monkeys over the past two decades have revealed how the brain modulates vestibular pathways during active head movement (Figure 1). In particular, experiments have focused on a subclass of neurons in the vestibular nuclei that are commonly referred to as ‘vestibular-only’ (VO) neurons based on their lack of sensitivity to eye movements. VO neurons receive direct vestibular afferent input. VO neurons then send descending projections to the spinal cord to mediate vestibulo-spinal reflexes and ascending projections to the vestibular thalamus to contribute to self-motion perception (Figure 1A). Specifically, these vestibular nuclei neurons respond to passive stimulation of the vestibular system alone but are insensitive to passive stimulation of neck proprioceptors (Figure 1B, top and middle panels). Accordingly, they similarly encode head velocity during passive rotations of the head and body together in space (which stimulates only the vestibular system) and during passive rotations of the head-on-body (which simultaneously stimulates the vestibular and proprioceptive systems) (compare Figure 1B, top and Figure 1C left panels; [7]). When monkeys actively generate comparable head motion, these same neurons demonstrate a marked reduction (~70–80%) in modulation (Figure 1C, panel center; [7]). Importantly, this contrasts with individual vestibular afferents which respond identically to passive and active head motion [7–10]. Thus, vestibular afferents reliably encode head motion regardless of the behavioral context, yet the neurons they directly target in the vestibular nuclei display markedly suppressed modulation during active head motion.
Figure 1.
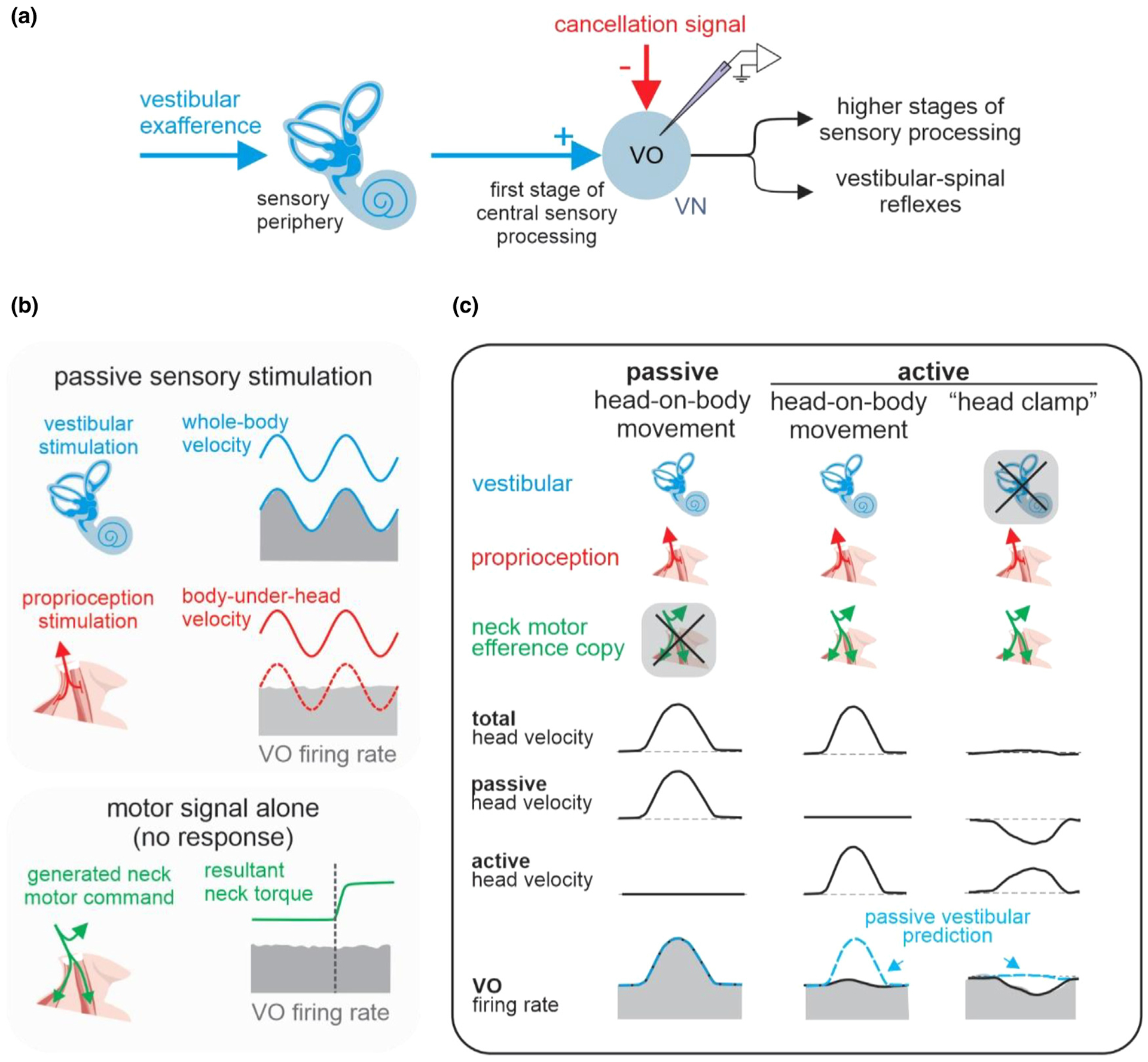
The response of the vestibular-only (VO) neurons in the vestibular nuclei (VN) to sensory and motor inputs. (A) A schematic illustrating the pathway of early vestibular processing. (B) The VO neurons respond to vestibular feedback (blue), but not proprioceptive feedback (red), and efference copy of motor command (green). (C) The response of VO neurons during active head-on-body movements (center) are suppressed compared to the response to passive head-on-body movements (left). During a “head clamp” experiment, in which the vestibular feedback of an active movement is cancelled by moving the whole-body, the VO neurons encode a negative image of expected vestibular feedback (right). Blue dashed traces show the predicted firing rate from a passive vestibular-only model.
Taken together, these results led to a series of experiments aimed at understanding the neural mechanism underlying the suppression of self-generated vestibular input. During active movements additional information from the animal’s motor system (i.e., a motor efference copy signal) is available for distinguishing between active and passive stimulation. Thus, one possibility is that neck efference copy signals provide direct inhibitory inputs to VO neurons to suppress their responses to vestibular afferent input during active head movements. To test whether this is the case, neuronal responses were recorded while a monkey attempted head movment (as confirmed by the measurement of neck torque) while its head was unexpectedly held stable. In this condition, neurons did not display any modulation in their responses (Figure 1B, bottom; [8]). Thus, this experiment ruled out the idea that a “negative image” of the active vestibular input estimated from an efferent copy of the neck motor command function to suppress the input to these neurons from the vestibular afferents. Thus, the mechanism underlying the cancellation of actively generated vestibular inputs (also termed vestibular reafference) differs from the “principle of reafference” originally proposed by Von Holst and Mittelstaedt [11]. In their influential model, Von Holst and Mittelstaedt proposed that the brain subtracts a copy of the expected sensory results of a motor command, termed “efference copy” (or “corollary discharge”, see [12]), from the sensory signal to eliminate a reafferent signal. While there is evidence for such a computation in model systems such as the electrosensory system of the electric fish[13,14], more recent experiments have revealed that vestibular reafference cancellation is accomplished via a more sophisticated mechanism in primates.
Specifically, we completed a subsequent series of experiments in which the correspondence between intended and actual head motion was experimentally controlled. In particular, this experiment included a condition in which a monkey generated active head movements, which were then sent to a vestibular motion platform controller to simultaneously passively rotate the monkey in the opposite direction. As a result of counterrotation of the motion platform, the monkeys head in space motion (ḢS, the stimulus for the vestibular system) was negated, while the movement of the monkey’s head relative to its body (ḢB) was not affected. Thus, the activation of neck proprioceptors was consistent with the motor commands. Importantly, VO neuron responses were inhibited in this condition in a manner consistent with a “negative image” of the head motion command (Figure 1C, right panel; [8]). Based on the logic of the experimental design, this result was taken as evidence to support the hypothesis that a cancellation signal is gated into the vestibular nuclei to suppress vestibular afferent input only in conditions where activation of proprioceptors matches the expected feedback (Figure 2). Indeed, this proposal is consistent with all data available to date. For example, it can explain why i) actively-generated vestibular inputs are selectively suppressed during simultaneous passive motion applied by rotating the entire animal (Figure 3A), but ii) actively-generated vestibular inputs are robustly encoded when the simultaneous application of passive motion (for example, head-on-body perturbations) results in activation of the same muscle proprioceptors (Figure 3B [15]).
Figure 2.
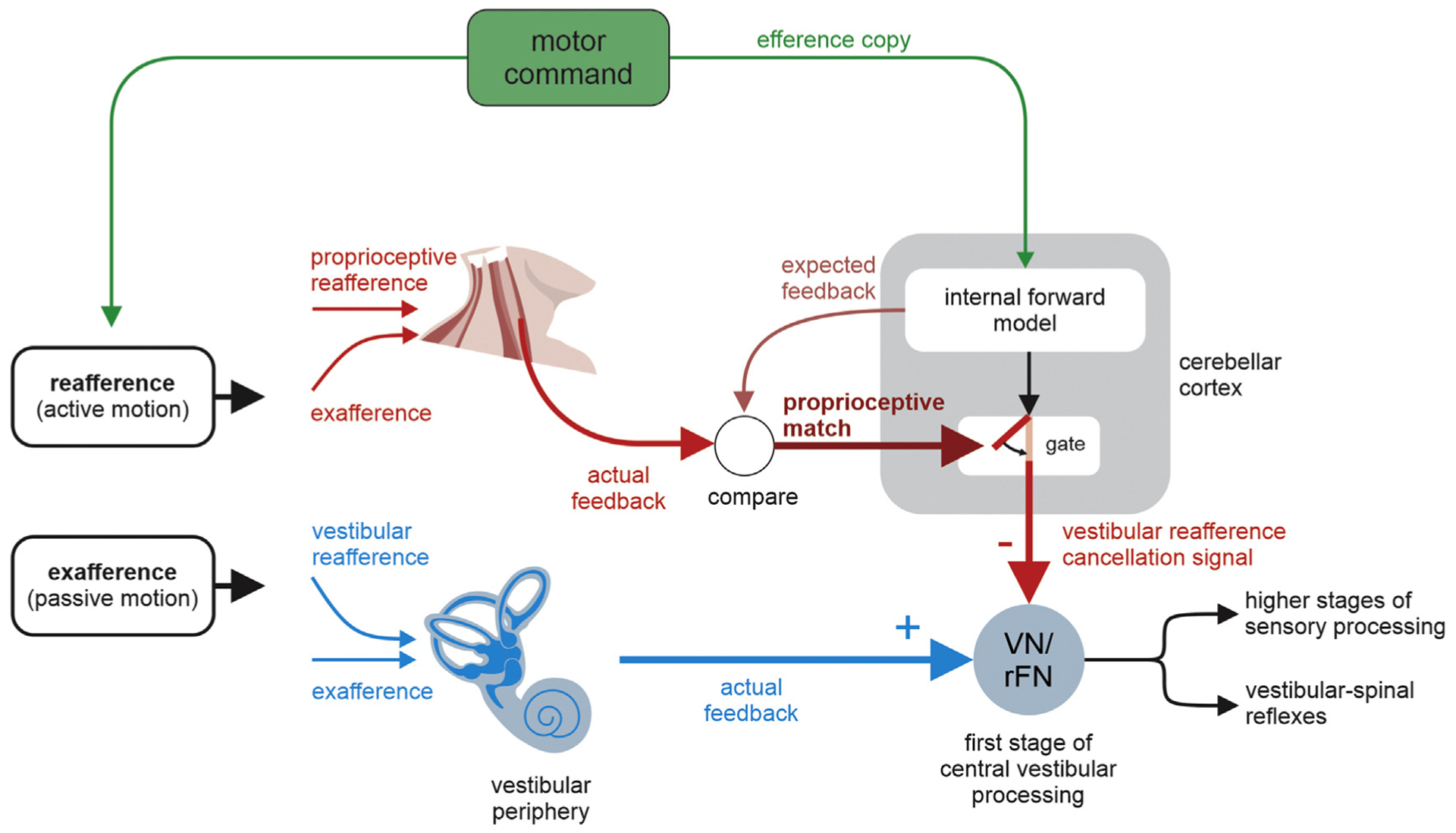
A theoretical framework for cancellation of sensory reafference in the vestibular system. In this model, a motor command is sent to 1) the neck muscle to actively move the head and 2) internal forward models of the sensory consequences of active movements, resulting in a prediction of the proprioceptive feedback expected as a result of the head movement command. In situations in which there is a match between expected and actual proprioceptive feedback as would be the case during normal active head movements, a vestibular reafference cancellation signal is sent to vestibular-only neurons in the vestibular nuclei (VN) and to the rostral fastigial nuclei (rFN) to suppress the self-generated vestibular inputs (closed gate). In situations in which there is a mismatch between the actual and predicted proprioceptive feedback, vestibular signals are not suppressed (open gate). It is notable that the brain uses a multimodal approach, combining inputs from the vestibular and proprioceptive systems to both sense self-motion and suppress the representation of actively generated self-motion.
Figure 3.
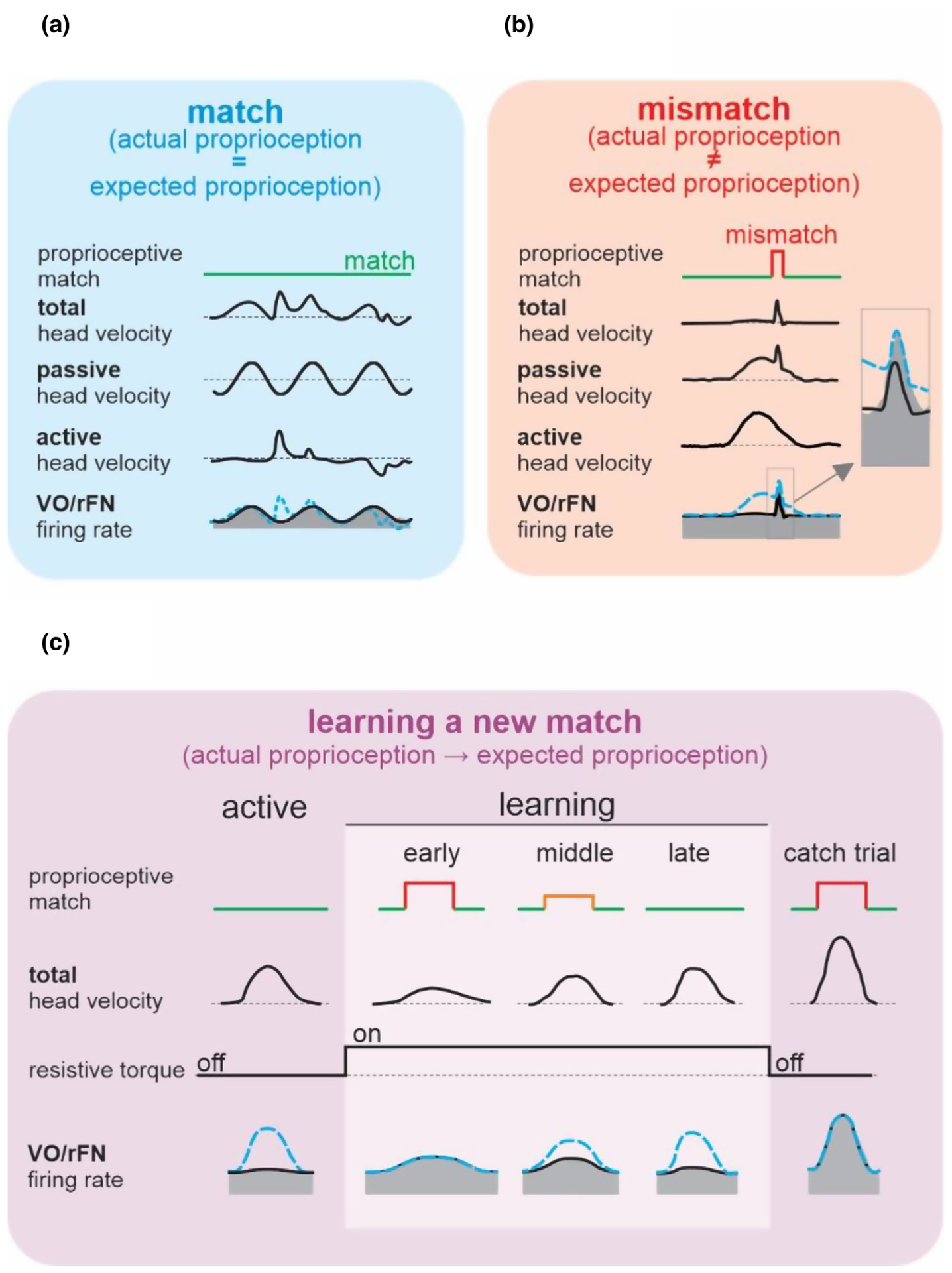
Comparisons of neural responses across conditions where there is a match versus mismatch between actual and expected proprioceptive input. (A) In the case where there is a match between actual and expected proprioceptive feedback, rFN and VO neurons selectively encode the passive part of self-motion. (B) In the case where there is a mismatch between actual and expected proprioceptive feedback, rFN and VO neurons encode total head velocity. Thus, the active component of self-motion is no longer cancelled. (C) The responses of rFN and VO neurons demonstrate the output of a neural computation that learns a new match between actual and expected proprioceptive inputs. A load was experimentally applied to the head that initially reduced active head-on-body velocity to 50% of its control value. In response, rFN and VO neurons increased their modulation to levels that were well predicted by their responsiveness to passive head motion (early learning). During learning, the neuronal sensitivities then gradually decreased from that measured during passive head motion to the suppressed response observed during active motion (compare early, middle, and late learning). Once learning had occurred, the applied load was then randomly removed for single catch trials, during which neurons again responded to active head motion as if it had been passively applied (far right: catch trial).
Finally, it is noteworthy that not all classes of neurons in the vestibular nuclei show the same reafferent suppression property as the VO neurons discussed above. Instead the behaviorally-dependent processing that occurs at the level of the vestibular nuclei is consistent with the functional roles of specific neuron classes (reviewed in [6•]). Two other distinct classes of neurons in the vestibular nuclei (i.e., position-vestibular pause and eye-head neurons) directly project to eye muscle motoneurons to mediate the generation and calibration of the vestibulo-ocular reflex (VOR), which generates compensatory eye movements to stabilize gaze during head motion. Notably, these VOR neurons do not differentially encode active and passive self-motion. Instead, their responses are suppressed in conditions where the goal is to redirect gaze (for example via an eye-head gaze shift), rather than stabilize gaze (the function of the VOR) (reviewed in [6•]). As a result, the VOR pathway is suppressed when it is specifically counterproductive – specifically, when it would evoke a compensatory eye movement in the direction opposite to the intended shift in gaze. Correspondingly, as reviewed above, vestibulo-spinal pathways are suppressed when they would be counterproductive, in conditions where compensatory postural stabilizing responses would oppose the intended voluntary self-motion relative to space.
A match between expected and actual proprioceptive feedback is required for predictive coding of self-motion
Thus, to summarize so far, the evidence available to date is consistent with a model in which vestibular signals that arise from self-generated head movements are inhibited by a mechanism that compares an internal prediction of the expected ‘proprioceptive’ consequences of self-motion with the actual resultant proprioceptive feedback (Figure 2). Importantly, a comparable theoretical model structure has also been used to understand motor learning. In this context, there are many reasons to believe that the brain (specifically the cerebellum) computes an estimate (internal model) of the sensory consequences of an action, which it then compares to the actual sensory feedback (reviewed in [16,17]). This comparison then results in the computation of a sensory prediction error signal that guides the updating of the motor program. Correspondingly, in the present context, the computation of sensory prediction error effectively enables the distinction between actively generated and passively applied vestibular stimulation.
Indeed, recent studies of the primate vestibular system have unified these two views of the role of sensory prediction error signals. Experiments in behaving monkeys recorded the responses of cerebellar output neurons in the most medial of the deep cerebellar nuclei (rostral fastigial nucleus (rFN)) – a structure which constitutes a major output target of the cerebellar cortex and in turn sends strong projections to the vestibular nuclei and spinal cord to ensure accurate posture and the maintenance of balance (reviewed in [18•]). The findings were consistent with the proposal that the cerebellum compares the expected and actual sensory consequences of active behaviors to compute a sensory prediction signal [19,20]. Specifically, expected and actual sensory inputs are well-matched (i.e., the sensory prediction error is minimal) during active head movements, and in turn these cerebellar output neurons are relatively unresponsive. In contrast, there is no expected sensory input during passive self-motion (i.e., there is a significant sensory prediction error signal) and accordingly the same neurons robustly encode passive head motion. It then follows that these same cerebellar output neurons should also fire during voluntary self-motion if the normal relationship between the brain’s motor command responsible for head movement and the actual resultant movement is altered. We tested this prediction in recent experiments by applying a load to the monkey’s head that initially decreased its voluntarily generated head velocity by 50% (Figure 3C, early learning) [21••]. Notably, the analysis of trial-by-trial changes in neuronal responses revealed the output of an elegant computation in which rapid updating of an internal model enabled the motor system to learn to expect previously unexpected sensory input. Further, the time course of this updating (~50 head movements) was consistent with that of the resultant behavioral learning (Figure 3C, compare behavioral and neural responses of late and early learning). Furthermore, the cerebellar output neurons again showed an instantaneous increase in vestibular sensitivity when the load was removed in catch trials (Figure 3C, right column). Thus, the responses of cerebellar output neurons demonstrate the computation of a sensory prediction error signal. Importantly, these cerebellar neurons project to vestibulo-spinal neurons in the vestibular nuclei, which likewise demonstrate comparable responses (Figure 1A). Overall, this cerebellar-based computation is logically consistent with the role of the vestibulo-spinal reflex pathways, in that it ensures the accurate and robust control of posture and balance by selectively encoding a continuously updated representation of unexpected motion.
The proprioceptive system provides robust information during active and passive movements
Accordingly, the data available to date are consistent with a specific model in which self-generated vestibular signals are inhibited by a cerebellar-based mechanism that compares an internal prediction of the proprioceptive consequences of head motion to the actual resultant proprioceptive feedback (Figure 2). This model thus assumes that the proprioceptive system sends a robust signal to the cerebellum during active behaviors - raising the question of whether there is any evidence for this prediction. Indeed, recent work by Seki and colleagues provides clear support for this proposal. During active movements, the essential information provided about the position and movement of limbs by the proprioceptive system [22,23] is actually facilitated rather than suppressed at the level of the spinal cord [24••,25,26]. Further, movement-specific facilitation has been reported at subsequent stages of proprioceptive processing from the dorsal column–medial lemniscus pathway to the somatosensory thalamus [24••,27]. Further, in addition to the dorsal column–medial lemniscus system, spinocerebellar pathways demonstrate the integration of proprioceptive inputs with motor-related inputs [28,29]. Further studies of the specific circuits underlying the integration of motor signals and proprioceptive sensory signals at these early stages of somatosensory processing will be fundamental to understanding how the brain ensures accurate motor control during active self-motion.
Vestibular-proprioceptive integration: transforming vestibular input from a head- to body-centered reference frame for postural control
The integration of vestibular and proprioceptive signals is also vital for transforming vestibular input from head-centered to body-centered coordinates during everyday activities. Notably, the vestibular receptor organs are located in the inner ear, thus its native reference-frame is ‘head-centered’. In contrast, in order to accurately control the musculature to maintain postural stability, the brain must encode motor signals in a ‘head-centered’ or ‘body-centered’ reference frame that is relevant to the desired behavior. Experiments in rhesus monkeys have shown that neurons in the vestibular nuclei encode passively applied self-motion in a ‘head-centered’ referenced frame [15]. In contrast, a significant fraction of neurons in the rostral fastigial nucleus (rFN) represent self-motion signals in a body-centered reference frame [30,31,32•]. Importantly, the integration of vestibular and proprioceptive signals underlies this coordinate transformation. Indeed, two classes of rFN neurons, bimodal and unimodal, are involved in separate processing streams (Figure 4A, [19,33]). Bimodal neurons encode body-in-space motion in passive conditions and respond to both vestibular and proprioceptive stimulation (thus termed bimodal neurons; Figure 4A, [33]). In contrast, unimodal neurons encode head-in-space motion in passive condition and are only sensitive to vestibular inputs (thus termed unimodal neurons). Bimodal neurons further display tuning to both vestibular and proprioceptive input that similarly varies as a nonlinear function of static head-on-body position (Figure 4B, [33]). As a result, the integration of these two inputs leads to the accurate encoding of body motion relative to space by bimodal neurons.
Figure 4.
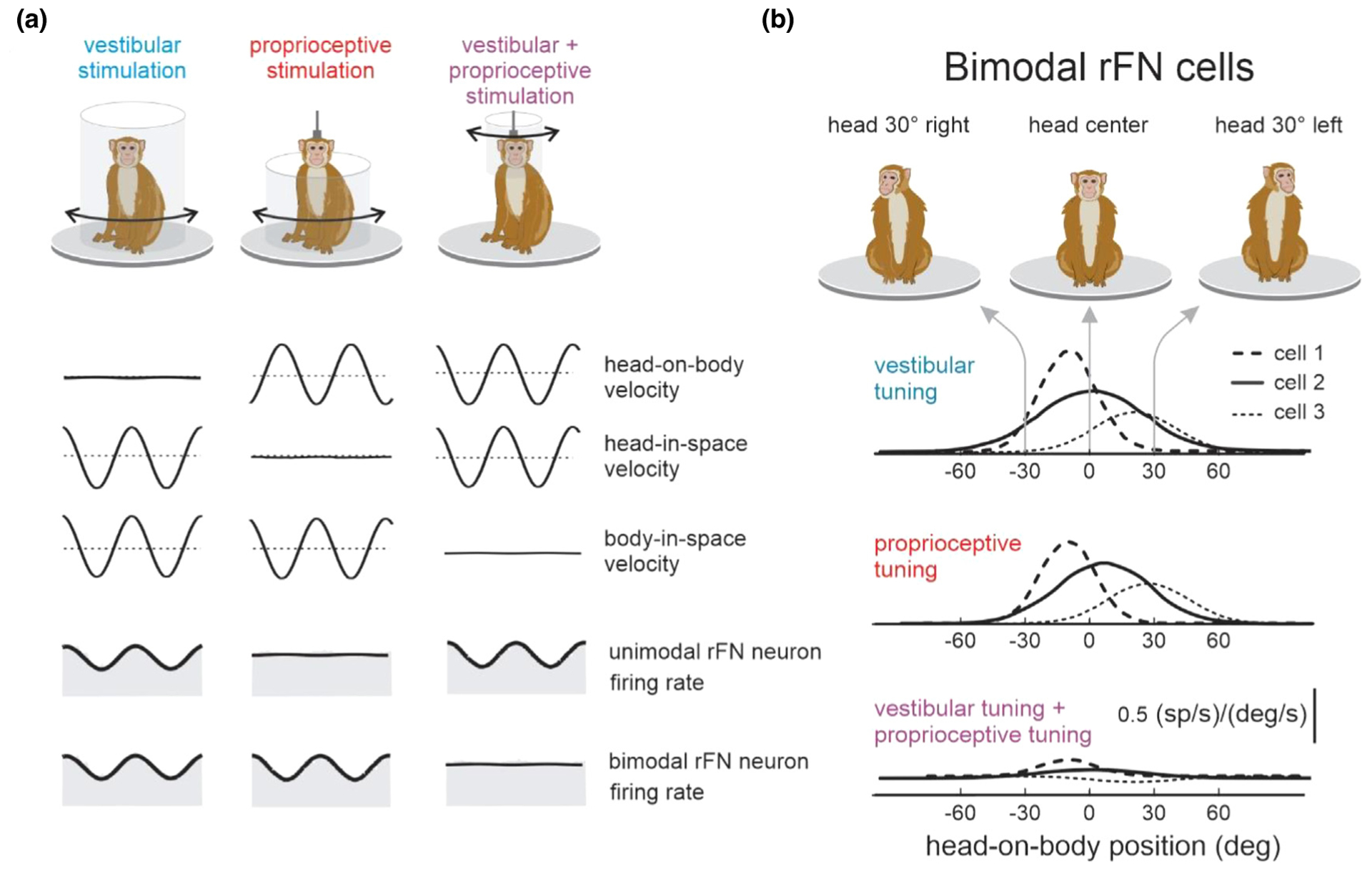
Multimodal integration within vestibular pathways. (A) Unimodal neurons in the rostral fastigial nuclei (rFN) only respond to vestibular stimulation during whole-body or head-on-body stimulations. Bimodal neurons in rFN respond to vestibular (left) as well as proprioceptive (center) stimulation. During head-on-body stimulation (right) the vestibular and dynamic proprioceptive inputs sum to produce complete response cancellation. This is consistent with encoding body motion in these neurons. (B) Tuning curves of 3 example cells showing the change in vestibular (blue) and proprioceptive (red) sensitivity as a function of static head-on-body position. For each cell, tuning curves for each modality sum linearly during combined stimulation such that bimodal neurons are not modulated during head-on-body motion (bottom).
Altogether, the integration of vestibular and proprioceptive signals in bimodal neurons transforms coding from the ‘head-centered’ to ‘body-centered’ reference frame required to dynamically control the musculature to maintain postural stability. However, when head motion is actively generated (i.e., expected) rather than passively applied, neuronal responses are drastically (~70%) suppressed. Specifically, bimodal neurons no longer robustly encode body-in-space motion and unimodal neurons no longer robustly encode head-in-space motion [21••,34•]. Importantly, these neurons do continue to faithfully encode passively applied stimulation regardless when it occurs simultaneously with self-generated stimulation. This selective coding of unexpected vestibular / proprioceptive input underlies the brain’s ability to selectively adjust postural tone in response to unexpected motion experienced during voluntary movement. The responses of rFN bimodal and unimodal neurons to passive versus active self-motion are furthermore consistent with the view that the cerebellum builds a dynamic prediction (i.e., internal model) of the sensory consequences of self-motion [21••,34•].
Proprioceptive substitution in vestibular pathways following vestibular sensory loss
Finally, it is also noteworthy that following peripheral vestibular loss, proprioceptive inputs are unmasked at the level of the vestibular nuclei of rhesus monkeys [35–37]. Impressively, vestibular nuclei neurons are normally insensitive to passive stimulation of neck proprioceptors as reviewed above (Figure 1B, bottom panel). Yet these same neurons show robust responses to passive stimulation of neck proprioceptors within 24 hours of peripheral vestibular loss. Vestibular and proprioceptive inputs to vestibular nuclei neurons are mediated by AMPA and NMDA receptors, respectively [38,39]. Thus, the finding that these neurons are insensitive to neck rotation before vestibular loss in monkeys suggests that the proprioceptive NMDA dependent synapses are normally silent. One possibility is that a relative increase in the number of AMPA receptors following a lesion leads to the activation of “silent” NMDA synapses (see discussion [35]). Future work will be needed to fully understand the cellular mechanisms underlying the unmasking of inputs from neck proprioceptors in the vestibular nuclei following peripheral vestibular loss.
It is also noteworthy that this upweighting of extra-vestibular information follows an increase in the variability of the vestibular input [40], which ultimately constrains the reliability with which these neurons encode self-motion. Accordingly, to improve neural performance it appears the brain adapts a strategy in which inputs from the proprioceptive system are unmasked [40], thereby providing a neural substrate for improvements in posture and self-motion perception following vestibular loss (e.g., [41,42]). Interestingly, the time course of these changes corresponds to that of improvement in the sensorimotor performance of astronauts after transitioning to micro-gravity or returning to earth [43]. Thus, we speculate that experimental studies that further our understanding of the neural mechanisms underlying sensorimotor adaptation will provide important insights into how to optimize goal-oriented training programs both for patient rehabilitation and for astronauts before and after space exploration missions.
Conclusions and implications: consequences for motor control and perceptual stability
In the vestibular system, VO neurons at the first central stage of processing preferentially encode passive self-motion. Notably, the vestibular sensory responses of these neurons are only cancelled when there is a match between actual proprioceptive feedback and the brain’s expectation (i.e., expected proprioceptive feedback, Figure 2). Importantly, this strategy is not specific to rhesus monkeys but is shared across species, including mice [44], squirrel monkeys [45], and Cynomolgus monkeys [46]. Single unit recording experiments in monkeys have further shown that the output of the cerebellum reveals rapid updating of an internal model as unexpected proprioceptive inputs become expected. Notably, a comparable strategy underlies the suppression of vestibular inputs generated by active linear translations and combined translational and rotational motion [43,47]. In order to maintain accurate postural control and perceptual stability, the brain must also discriminate between passively and actively generated changes in the head’s orientation relative to gravity. We have also recently studied how the vestibular system accounts for the presence of gravity during everyday activities. Recording experiments in both the vestibular nuclei and rostral fastigial nuclei (rFN) have established that the gravity-driven responses of single neurons are similarly cancelled when changes in head orientation are the consequence of voluntarily generated self-motion [34•]. Thus, consistent with the model shown in Figure 2, the brain computes a dynamic estimate (e.g., an internal model) of the sensory consequences of gravity during active self-motion to ensure the preferential encoding of unexpected head motion required for postural and perceptual stability.
What are the functional implications of this differential processing of active vs. passive self-motion? As noted above, the same vestibular neurons that show suppressed responses during active movements also send projections to the spinal cord. These projections drive compensatory vestibulo-spinal reflexes, which function to ensure the maintenance of balance. Accordingly, this suppression mechanism enables the selective gating (and calibration) of descending vestibulo-spinal reflexes during active movements where the goal is to move through the world. Furthermore, this proprioception-dependent mechanism also likely underlies our perceptual stability during self-motion (reviewed in [48,49]). Recent experiments have demonstrated that (unexpected) self-motion is similarly encoded by neurons in the vestibular thalamus, which receive direct input from the vestibular nuclei and contribute to self-motion perception [50•]. Thus, this result indicates that the ascending vestibular thalamocortical pathway preferentially transmits passive (unexpected) vestibular information to cortex. We speculate that this selectively plays a critical role in ensuring perceptual stability during our everyday activities.
Acknowledgments
We thank Robyn Mildren for critically reading the manuscript. This work was funded by the National Institute on Deafness and Other Communication Disorders at the National Institutes of Health (Grants R01-DC002390, R01-DC013069 to K.E.C.) and Brain Initiative Grant 1UF1NS11169
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Huterer M, Cullen KE: Vestibuloocular reflex dynamics during high-frequency and high-acceleration rotations of the head on body in rhesus monkey. J Neurophysiol 2002, 88:13–28. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell DE, Dai C, Rahman MA, Ahn JH, Santina CC, Cullen KE: Head movements evoked in alert rhesus monkey by vestibular prosthesis stimulation: implications for postural and gaze stabilization. PLoS One 2013, 8 e78767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldberg JM: Afferent diversity and the organization of central vestibular pathways. Exp brain Res 2000, 130:277–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cullen KE, Roy JE: Signal Processing in the Vestibular System During Active Versus Passive Head Movements. J Neurophysiol 2004, 91:1919–1933. [DOI] [PubMed] [Google Scholar]
- 5.Clark TK, Newman MC, Karmali F, Oman CM, Merfeld DM: Mathematical models for dynamic, multisensory spatial orientation perception. Prog Brain Res 2019, 248:65–90. [DOI] [PubMed] [Google Scholar]
- 6.•.Cullen KE: Vestibular processing during natural self-motion: implications for perception and action. Nat Rev Neurosci 2019, 20:346–363 [DOI] [PMC free article] [PubMed] [Google Scholar]; This review provides an integrative summary of sensory substitution in early vestibular pathways. Specifically, this work discusses how peripheral vestibular loss results in the unmasking of neck proprioceptive and motor inputs at the first stage of central vestibular processing.
- 7.Roy JE, Cullen KE: Selective processing of vestibular reafference during self-generated head motion. J Neurosci 2001, 21:2131–2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roy JE, Cullen KE: Dissociating Self-Generated from Passively Applied Head Motion: Neural Mechanisms in the Vestibular Nuclei. J Neurosci 2004, 24:2102–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cullen KE, Minor LB: Semicircular canal afferents similarly encode active and passive head-on-body rotations: implications for the role of vestibular efference. J Neurosci 2002, 22:RC226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jamali M, Sadeghi SG, Cullen KE: Response of vestibular nerve afferents innervating utricle and saccule during passive and active translations. J Neurophysiol 2009, 101:141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.von Holst E, Mittelstaedt H: Das Reafferenzprinzip. Naturwissenschaften 1950, 37:464–476. [Google Scholar]
- 12.Sperry RW: Neural basis of the spontaneous optokinetic response produced by visual inversion. J Comp Physiol Psychol 1950, 43:482. [DOI] [PubMed] [Google Scholar]
- 13.Bell CC: Sensory coding and corollary discharge effects in mormyrid electric fish. J Exp Biol 1989, 146:229–253. [DOI] [PubMed] [Google Scholar]
- 14.Roberts PD, Bell CC: Computational consequences of temporally asymmetric learning rules: II. Sensory image cancellation. J Comput Neurosci 2000, 9:67–83. [DOI] [PubMed] [Google Scholar]
- 15.Brooks JX, Cullen KE: Early vestibular processing does not discriminate active from passive self-motion if there is a discrepancy between predicted and actual proprioceptive feedback. J Neurophysiol 2014, 111:2465–2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miall RC, Wolpert DM: Forward Models for Physiological Motor Control. Neural Netw 1996, 9:1265–1279. [DOI] [PubMed] [Google Scholar]
- 17.Krakauer JW, Mazzoni P: Human sensorimotor learning: adaptation, skill, and beyond. Curr Opin Neurobiol 2011, 21:636–644. [DOI] [PubMed] [Google Scholar]
- 18.•.Fujita H, Kodama T, du Lac S: Modular output circuits of the fastigial nucleus for diverse motor and nonmotor functions of the cerebellar vermis. Elife 2020, 9 e58613. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study identified five major classes of glutamatergic neurons in the fastigial nucleus. The neurons of each class project to a distinct target, which suggests specific functional roles for the cerebellar modules that comprise these classes.
- 19.Brooks JX, Cullen KE: The primate cerebellum selectively encodes unexpected self-motion. Curr Biol 2013, 23:947–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cullen KE, Brooks JX: Neural Correlates of Sensory Prediction Errors in Monkeys: Evidence for Internal Models of Voluntary Self-Motion in the Cerebellum. Cerebellum 2015, 14:31–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.••.Brooks JX, Carriot J, Cullen KE: Learning to expect the unexpected: rapid updating in primate cerebellum during voluntary self-motion. Nat Neurosci 2015, 18:1310–1317 [DOI] [PMC free article] [PubMed] [Google Scholar]; This study, for the first time, revealed the neural evidence of sensory prediction error and the updating of an internal forward model in the cerebellum that can predict the sensory consequences of altered head movements.
- 22.Lackner JR, DiZio P: Vestibular, proprioceptive, and haptic contributions to spatial orientation. Annu Rev Psychol 2005, 56:115–147. [DOI] [PubMed] [Google Scholar]
- 23.Carriot J, Cian C, Paillard A, Denise P, Lackner JR: Influence of multisensory graviceptive information on the apparent zenith. Exp brain Res 2011, 208:569–579. [DOI] [PubMed] [Google Scholar]
- 24.••.Confais J, Kim G, Tomatsu S, Takei T, Seki K: Nerve-specific input modulation to spinal neurons during a motor task in the monkey. J Neurosci 2017, 37:2612–2626 [DOI] [PMC free article] [PubMed] [Google Scholar]; This study assessed the response to passive and active proprioceptive stimulation at the level of the spinal cord and showed that the activity of these neurons was facilitated rather than suppressed during relevant active movements.
- 25.Oya T, Takei T, Seki K: Distinct sensorimotor feedback loops for dynamic and static control of primate precision grip. Commun Biol 2020, 3:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tomatsu S, Kim G, Confais J, Takei T, Seki K: Two distinct proprioceptive representations of voluntary movements in primate spinal neurons. bioRxiv 2018. [Google Scholar]
- 27.Leiras R, Velo P, Martín-Cora F, Canedo A: Processing afferent proprioceptive information at the main cuneate nucleus of anesthetized cats. J Neurosci 2010, 30:15383–15399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hantman AW, Jessell TM: Clarke’s column neurons as the focus of a corticospinal corollary circuit. Nat Neurosci 2010, 13:1233–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Azim E, Jiang J, Alstermark B, Jessell TM: Skilled reaching relies on a V2a propriospinal internal copy circuit. Nature 2014, 508:357–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kleine JF, Guan Y, Kipiani E, Glonti L, Hoshi M, Buttner U: Trunk position influences vestibular responses of fastigial nucleus neurons in the alert monkey. J Neurophysiol 2004, 91:2090–2100. [DOI] [PubMed] [Google Scholar]
- 31.Shaikh AG, Meng H, Angelaki DE: Multiple reference frames for motion in the primate cerebellum. J Neurosci 2004, 24:4491–4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.•.Martin CZ, Brooks JX, Green AM: Role of rostral fastigial neurons in encoding a body-centered representation of translation in three dimensions. J Neurosci 2018, 38:3584–3602 [DOI] [PMC free article] [PubMed] [Google Scholar]; This work studied the 3D transformation of the reference frame from head-centered to body-centered translational self-motion in rFN neurons. The results showed that while the activity of single rFN neurons reflect partial transformation, a linear combination of the response of 5–7 neurons can encode motion in a body-centered frame.
- 33.Brooks JX, Cullen KE: Multimodal integration in rostral fastigial nucleus provides an estimate of body movement. J Neurosci 2009, 29:10499–10511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.•.Mackrous I, Carriot J, Jamali M, Cullen KE: Cerebellar Prediction of the Dynamic Sensory Consequences of Gravity. Curr Biol 2019, 29:2698–2710 [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper showed that the effect of gravity in central vestibular neurons that mediate postural reflexes is canceled during self-generated movements. This finding shows that the brain detects unexpected consequences of gravity to ensure postural and perceptual stability.
- 35.Sadeghi SG, Minor LB, Cullen KE: Neural correlates of motor learning in the vestibulo-ocular reflex: dynamic regulation of multimodal integration in the macaque vestibular system. J Neurosci 2010, 30:10158–10168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sadeghi SG, Minor LB, Cullen KE: Multimodal integration after unilateral labyrinthine lesion: single vestibular nuclei neuron responses and implications for postural compensation. J Neurophysiol 2011, 105:661–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sadeghi SG, Minor LB, Cullen KE: Neural correlates of sensory substitution in vestibular pathways following complete vestibular loss. J Neurosci 2012, 32:14685–14695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith PF, de Waele C, Vidal P-P, Darlington CL: Excitatory amino acid receptors in normal and abnormal vestibular function. Mol Neurobiol 1991, 5:369. [DOI] [PubMed] [Google Scholar]
- 39.Straka H, Dieringer N: Basic organization principles of the VOR: lessons from frogs. Prog Neurobiol 2004, 73:259–309. [DOI] [PubMed] [Google Scholar]
- 40.Jamali M, Mitchell DE, Dale A, Carriot J, Sadeghi SG, Cullen KE: Neuronal detection thresholds during vestibular compensation: contributions of response variability and sensory substitution. J Neurophysiol 2014, 592:1565–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cousins S, Kaski D, Cutfield N, Seemungal B, Golding JF, Gresty M, Glasauer S, Bronstein AM: Vestibular perception following acute unilateral vestibular lesions. PLoS One 2013, 8 e61862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gauchard GC, Parietti-Winkler C, Lion A, Simon C, Perrin PP: Impact of pre-operative regular physical activity on balance control compensation after vestibular schwannoma surgery. Gait Posture 2013, 37:82–87. [DOI] [PubMed] [Google Scholar]
- 43.Carriot J, Jamali M, Cullen KE: Rapid adaptation of multisensory integration in vestibular pathways. Front Syst Neurosci 2015, 9:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Medrea I, Cullen KE: Multisensory integration in early vestibular processing in mice: the encoding of passive vs. active motion. J Neurophysiol 2013, 110:2704–2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McCrea RA, Gdowski GT, Boyle R, Belton T: Firing behavior of vestibular neurons during active and passive head movements: vestibulo-spinal and other non-eye-movement related neurons. J Neurophysiol 1999, 82:416–428. [DOI] [PubMed] [Google Scholar]
- 46.Sadeghi SG, Mitchell DE, Cullen KE: Different neural strategies for multimodal integration: comparison of two macaque monkey species. Exp Brain Res 2009, 195:45–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carriot J, Brooks JX, Cullen KE: Multimodal integration of self-motion cues in the vestibular system: active versus passive translations. J Neurosci 2013, 33:19555–19566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cullen KE,Taube JS:Oursenseofdirection: progress, controversies and challenges. Nat Neurosci Perspect 2017, 20:1465–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cullen KE, Wang L: Predictive coding in early vestibular pathways: Implications for vestibular cognition. Cogn Neuropsychol 2020:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.•.Dale A, Cullen KE: The ventral posterior lateral thalamus preferentially encodes externally applied versus active movement: implications for self-motion perception. Cereb Cortex 2019, 29:305–318 [DOI] [PMC free article] [PubMed] [Google Scholar]; This study showed that similar to the neurons at early stages of vestibular processing, the responses of the vestibular neurons in the ventral posterior lateral thalamus is attenuated during active compared to passive self-motion. The selective encoding of unexpected self-motion in these neurons, which are part of the vestibular thalamocortical pathway, provide a neural correlate for ensuring perceptual stability.


