Abstract
Context:
This study focuses on the marginal adaptation of a calcium silicate-based cement to the root dentin after retrieval of different intracanal medicaments.
Aim:
This study compared the marginal adaptation of a calcium silicate-based cement to radicular dentin in the apical third of the root canal following the use of three different intracanal medicaments.
Materials and Methods:
Forty single-rooted premolar teeth (n = 40) were decoronated 13 mm above the root apices; then, 3 mm of the root tips were resected to standardize the root length. Orthograde cleaning and shaping were done using the rotary files and apical enlargement using peeso reamers. Depending on the intracanal medicament used, the samples were equally divided into four groups: Group 1 - control, Group 2 - Metapex, Group 3 - triple antibiotic paste (TAP), and Group 4 - calcium hydroxide with Propolis. Subsequently, the medicament was removed and a 3 mm apical barrier of BiodentineTM was placed and later scanned using an ex vivo micro-computed tomography scanner.
Statistical Analysis Used:
One-way ANOVA F-test and Tukey's post hoc test were used.
Results:
Maximum adaptation was seen in control group (0.65) > Propolis (1.47) > TAP (4.37) > Metapex (5.25); a high statistically significant difference between the four groups was found (P < 0.001) with regard to the external voids between BiodentineTM and radicular dentin.
Conclusion:
On comparison of the marginal adaptation of Biodentine to root canal dentin following the use of three different intracanal medicaments, maximum adaptation was seen in Group 1, followed by Group 4, Group 3, and Group 2.
Keywords: Biodentine, calcium hydroxide, intracanal medicaments, marginal adaptation, micro-computed tomography, propolis
INTRODUCTION
The most important factor for root development and apical closure is the presence of a healthy pulp. Endodontic therapy is difficult in cases of thin fragile walls and open apex due to a lack of definite apical stop to achieve complete debridement and to limit obturation.[1] The treatment of choice for such teeth traditionally has been inducing the formation of calcified tissue barrier at the apex by a process termed apexification.[2] The main drawback of using calcium hydroxide (CH) to create a physiologic hard-tissue barrier is the prolonged duration of treatment, leading to susceptibility to root fracture and coronal microleakage.[3,4] To overcome this drawback, the creation of an artificial hard-tissue barrier using different materials such as dentinal chips, hydroxyapatite crystals, Portland cement, calcium sulfate, mineral trioxide aggregate (MTA), and Biodentine has been suggested in the literature.[5]
During biomechanical preparation, irrigants facilitate the removal of microorganisms, necrotic, and inflamed tissue.[6] However, this does not render the root canal completely free of bacteria. Hence, an interappointment medicament is recommended to significantly improve disinfection following chemomechanical debridement.[7] CH has been the prototype, but with the advancement in the field of endodontics, newer materials have evolved. A new composition of CH has recently been introduced, with the trade name of Metapex. It exhibits a more potent antibacterial activity within the root canal compared to pure CH.[8] Any single antibiotic cannot result in effective sterilization of the canal, due to the complexity of periradicular infections. A triple antibiotic paste (TAP) containing metronidazole, ciprofloxacin, and minocycline is an efficient regimen in controlling the root canal pathogens.[9] Of the newly found medications, Propolis (bee glue) has attracted attention as a natural antimicrobial agent. Global trends toward natural products have been the stimulus for further investigation of the medical potentials of propolis.[10]
After the canal disinfection and medication period, the intracanal medicament is removed before the placement of a root canal filling or repair material.[11] Contemporary irrigation modalities such as sonic irrigation with EndoActivator (EA) have been reported to effectively clean the debris and remove the smear layer.[12]
Investigators maintain that the quality of the apical seal is improved with the use of root-end filling material because the solubility of a sealer in periradicular tissue fluids can result in delayed leakage and long-term failure when a root-end filling is not placed. Johnson reported a potential relationship between long-term clinical success and properties for selecting an ideal root-end filling material, namely, biocompatibility, apical seal, and the physical properties.[13]
Newer material such as BiodentineTM(Septodont, France), a bioactive dentin substitute with mechanical properties similar to dentin and composition like MTA, is used as an apical barrier material.[14] Micro-computed tomography (micro-CT) analysis provides an accurate and rapid result with high-resolution images, without the need to section the roots, due to its nondestructive three-dimensional (3D) nature.[15] Limited studies are available on the subject of micro-CT in the analysis of marginal adaptation and for use of propolis as an intracanal medicament.
Hence, this in vitro study was planned to comparatively evaluate the marginal adaptation of Biodentine to radicular dentin in the apical third of root canals using micro-CT when three different intracanal medicaments were used.
MATERIALS AND METHODS
Tooth selection and preparation: This study was approved by the Institutional ethics committee. Forty single-rooted premolar teeth, extracted for periodontal or orthodontic reasons, were included. The inclusion criteria were well-developed single roots, straight root canals, no root caries, root resorption, cavity, fractures, and/or cracks. The exclusion criteria were grossly carious teeth involving root caries; teeth with developmental disturbances, cracks/fracture (complete/incomplete), caries/restorations, and more than one root. The teeth were stored in a normal saline solution containing 0.1% sodium azide following extraction. Thereafter, all the soft-tissue residue and calculus if any were removed and teeth stored in distilled water until used for the study. A power analysis was established by G*Power, version 3.0.1 (Franz Faul University, Kiel, Germany). A total sample size of 40 (10 in each experimental group) would yield 80% power to detect significant differences, with an effect size of 0.60 and a significance level at 0.05.
All teeth were firstly decoronated 13 mm above the root apices, thereafter 3 mm of the root tips were resected for standardization, using a diamond disc (Taboom DD001 4AHP, China) to obtain a final root length of 10 mm, under copious water irrigation. A 10/02 K file (Mani Inc, Japan) was introduced in the root canal to check for patency. The working length was measured using a #15 K-file (Mani Inc., Japan) until its tip was visible at the apical foramen and a glide path was established using a 15/02 ProGlider single-use rotary NiTi file (Dentsply, Tulsa Dental Specialities, Switzerland). The root canals were then shaped using the complete sequence of the ProTaper Gold rotary nickel-titanium files (Dentsply Sirona, USA) – S1, S2, F1, F2, and F3. They were rinsed with 3% NaOCl (Prime Dental Products Private Limited, Thane, India) solution between each file. The internal diameter was standardized using Pesso burs (Dentsply-Maillefer, Ballaigues, Switzerland) sequence #1 through #5 until a homogeneous diameter of the root apex of 1.5 mm was achieved. The final irrigation was performed with 20 ml 3% NaOCl (5 min), 20 ml 17% EDTA (Avue Prep, Dental Avenue), and 5 ml normal saline, using a 27-gauge side-vented needle (Scott's Dental Supply, USA), placed 2 mm from the apex. Subsequently, the canals were dried with paper points and the apical openings were blocked using unbonded composite resin (3M™ Filtek™ Z350 XT Universal Syringe). Depending on the intracanal medicament used, the samples were randomly divided into four experimental groups of ten teeth each: Group 1- control without any intracanal medicament, Group 2 - Metapex, Group 3 - CH with Propolis, and Group 4 - TAP.
Intracanal medicaments
TAP paste was prepared by mixing equal portions of metronidazole (Flagyl 200 mg, Sanofi), ciprofloxacin (Ciplox 100 mg, Cipla), and minocycline (Minoz 100 mg, SUN Pharmaceuticals) with macrogol and propylene glycol. The powder to liquid ratio of pastes was 7:1. Metapex (Meta Bio-Med CO LTD, Republic of Korea) was introduced into the canals through a disposable syringe and given by the manufacturer. CH powder (Prime Dental Products Private Limited, India) was mixed with Propolis Extract (Herb Pharm, U. S. A.) on a mixing pad using a plastic spatula. The medicaments were placed into the canals with a lentulo spiral. A small cotton pellet was then placed over the paste, and the coronal openings of the root canals were sealed with Cavit™, Temporary Filling Material (3MTM, USA). Sterile gauze dampened with sterile saline was placed around and on top of the sections to create a humid environment and prevent dehydration of the intracanal medicament. The specimens were incubated for 3 weeks at 370C in 100% humidity.
Then, the intracanal medicament from the various experimental groups was removed using sonic activation with the help of an EA (Dentsply Sirona, USA), following the removal of the temporary filling. The root canals were flushed with 20 ml 17% EDTA (5 min) and 5 ml normal saline for complete removal of the material and dried with paper points. The removal of the material was confirmed with a dental operating microscope using ×10 magnification.
Barrier materials
After a thorough inspection of the root canals, the barrier material was placed. BiodentineTM was mixed according to the instructions provided by the manufacturer. Five drops of Biodentine liquid from a single-dose container were added into a powder-containing capsule and mixed for 30 s. Approximately 3 mm of barrier material was placed in the apical third of the root canal with a sterile carrier, gently adapted with a hand plugger, and a moistened cotton pellet to the dentinal walls. The specimens were then wrapped in the moistened sterile gauze and incubated at 37°C for 4 days.
Micro-computed tomography analysis
The dental specimens were imaged using X-ray micro-CT (Xradia Versa 510 X-ray Microscope, Zeiss, Pleasanton, CA, USA) with X-ray energy of 90 kV/8 W, equipped with a lower energy filter. The tomographic image acquisitions were taken at 6.5 microns resolution, with 1601 projections at the exposure rate of 1 s per projection, over 360° rotational angle, at a field of view of 6.6 mm × 6.6 mm, using 0.4x optical magnification. Projections without the samples in the beam (reference images) were collected and averaged. A filtered back-projection algorithm was used for the reconstruction of the projections to generate two-dimensional virtual cross-sections of the specimens. Image processing software, Dragonfly Pro (Version 3.6, ZEISS, Oberkochen, Germany) was used to generate volume-rendered 3D images of specimens. This procedure was done to analyze the marginal adaptation of barrier materials concerning void occurrence along the previously medicated root canal walls. The region of interest was particularized as an annular area of the tooth in the apical third of the root canal over a length of 3 mm for measuring the volume of the gap between the radicular dentin surface and Biodentine.
Statistical analysis
Descriptive statistics including mean and standard deviation of external void measurement values and inferential statistics calculated using Statistical Product and Service Solution (SPSS) version 21 software IBM, New York, United States. Shapiro–Wilk test showed a normal distribution of data. One-way ANOVA F-test was used for overall comparison among the groups and Tukey's post hoc test was used for multiple comparisons of adaptation between groups. P < 0.05 was considered significant for all statistical tests.
RESULTS
The representative axial cross-sections obtained from each experimental group are shown in Figure 1a-d. The minimum and maximum values (percentage of volume) of the external voids between the dentin walls and Biodentine as an apical barrier material for each group are shown in Table 1 and Figure 2.
Figure 1.
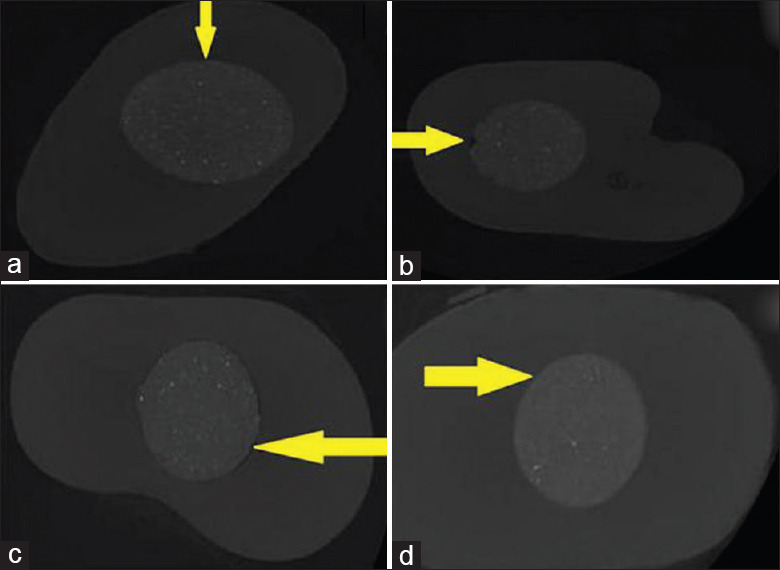
Representative micro-computed tomography photos of radicular dentin showing the external voids between the dentin walls and barrier material (Biodentine) marked with yellow arrows in axial view. (a) Control group; significant differences in the gap volumes were observed between the medicated and the control groups. (b) Metapex - Biodentine; decreased marginal adaptation than other test groups (c) calcium hydroxide - Propolis-Biodentine; less external voids when compared to Metapex and triple antibiotic paste group. (d) Triple antibiotic paste - Biodentine; more marginal adaptation compared to Metapex group
Table 1.
Mean and standard deviation values (percentage of volume) in mm3 of the external voids between the dentin walls and barrier material (Biodentine)
| Mean | SD | Standard error | Minimum | Maximum | |
|---|---|---|---|---|---|
| Group1 (control) | 0.65 | 0.17 | 0.05 | 0.40 | 0.90 |
| Group 2 (Metapex) | 5.25 | 0.29 | 0.09 | 4.90 | 5.60 |
| Group 3 (Propolis + CH) | 1.47 | 0.23 | 0.07 | 1.20 | 1.90 |
| Group 4 (TAP) | 4.37 | 0.30 | 0.09 | 3.90 | 4.80 |
The findings between the groups are highly significant with P<0.001. CH: Calcium hydroxide, TAP: Triple antibiotic paste. SD: Standard deviation
Figure 2.
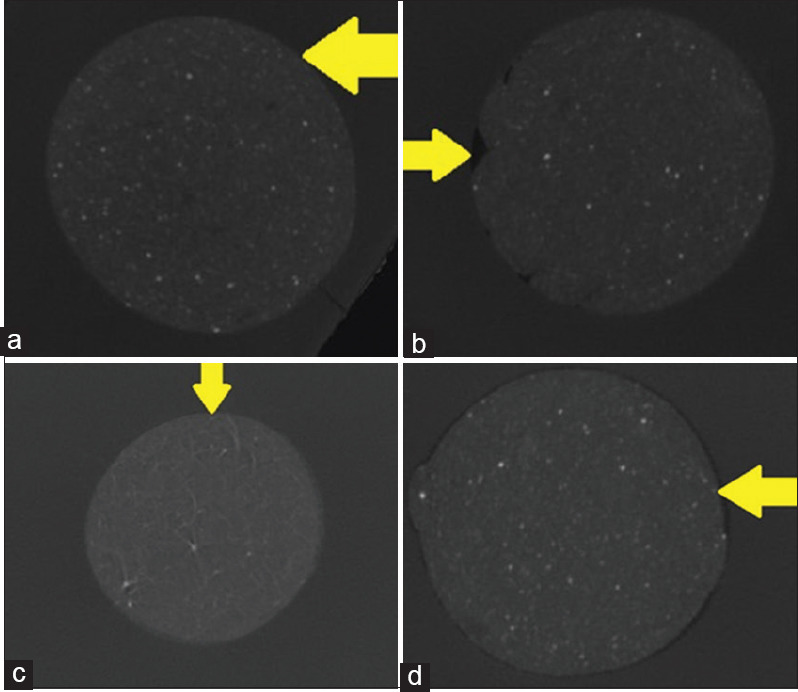
(a-d) Highlight the voids between Biodentine and radicular dentin in Group (a) Control group Group (b) Metapex Biodentine, Group (c) calcium hydroxide – Propolis – Biodentine and Group (d) triple antibiotic paste Biodentine respectively
On comparison of marginal adaptation of Biodentine to root canal dentin after removal of the three medicaments, maximum adaptation was seen in the control group (0.65) > Propolis with CH (1.47) > TAP (4.37) > Metapex (5.25). The findings were highly significant (P < 0.001).
Among the various intracanal medicaments, maximum voids were seen in Group 2 (Metapex)-5.25.
Intergroup comparisons
On the comparison between the control group and the various intracanal medicaments, a highly statistical difference was observed. It was 4.60 for the Metapex group, 0.82 for CH with Propolis group, and 3.72 for TAP. On the comparison between Groups 3 and 4, a difference of 2.90 was observed. Table 2 and Graph 1 give a graphical representation comparing the percentage of external voids present between dentin walls and barrier material between Group 1 (Control group), Group 2 (Metapex), Group 3 (Propolis and CH), and Group 4 (TAP).
Table 2.
Pairwise Comparison of percentage of external voids present between dentin walls and barrier material in Group A (Control group), Group B (Metapex), Group C (Propolis and CaOH2) and Group D (TAP) visualised under MicroCT using Tukey’s Post Hoc test
| Tukey’s post hoc test to find pairwise comparison | |||
|---|---|---|---|
| Group | Comparison Group | Mean Difference | p value, Significance |
| Group 1 (CONTROL) | Group 2 (METAPEX) | 4.60 | P<0.001** |
| Group 3 (PROPOLIS + CH*) | 0.82 | P<0.001** | |
| Group 4 (TAP†) | 3.72 | P<0.001** | |
| Group 2 (METAPEX) | Group 3 (PROPOLIS + CH*) | 3.78 | P<0.001** |
| Group 4 (TAP†) | 0.88 | P<0.001** | |
| Group 3 (PROPOLIS + CH*) | Group 4 (TAP†) | 2.90 | P<0.001** |
P>0.05 – not significant, *P<0.05 – significant, **P<0.001 – highly significant. *Calcium Hydroxide, †Triple Antibiotic Paste
Graph 1.
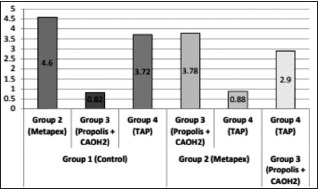
Graphical representation comparing the percentage of external voids (in mm3) present between dentin walls and barrier material between Group 1 (Control group), Group 2 (Metapex), Group 3 (Propolis and calcium hydroxide), and Group 4 (triple antibiotic paste)
DISCUSSION
Currently, there are a few methods of treating a tooth that has a necrotic pulp and an open apex. These methods include filling the root canal with the large (blunt) end of a gutta-percha cone or customized gutta-percha cones with a sealer; inducing apical closure by the formation of an apical stop (CH is generally used) against which a permanent root canal filling can subsequently be inserted; and placing a biologically acceptable substance in the apical portion of the root canal (apical plug) thus forming an apical barrier. This method to induce a calcific barrier across an open apex of an immature, pulpless tooth is termed apexification. Filling the root canal with the large end of a gutta-percha cone or a customized cone is not advisable because the apical foramen is generally wider than the root canal orifice which prevents the proper condensation of the gutta-percha, and proper preparation of the canal would weaken the tooth considerably.[16] It would also be difficult to assess the point of root development. Also due to the various drawbacks associated with CH, the use of an apical plug method is a suitable alternative treatment plan for such cases.[17] In our study, Biodentine is used as a compact material in the open area of the root-end to induce the formation of a calcified barrier in the periapical region. The advantages are a shorter treatment time and the development of a good apical seal. However, the findings of this study can only be compared with the studies in which the effects of various medicaments on the marginal adaptation of the cement were evaluated, but there are no data in the literature about the effects of medicaments on the marginal adaptation of a calcium silicate-based cement as an apical barrier, used in regeneration procedures. However, the medication with CH significantly improved the marginal adaptation of ProRoot MTA to the coronal third of root dentin walls, and the use of TAP or DAP decreased the marginal adaptation of both ProRoot MTA and Biodentine.[18]
CH and modified TAP, when used as intracanal medicaments, affect the sealing ability of Biodentine.[19] Our study showed that the sealing ability of Biodentine to the radicular dentin is best seen in the Propolis group, followed by the TAP and least in the Metapex group. Residues of medicaments decrease the micromechanical interlocking properties of Biodentine and deteriorate the integrity of the sealer dentin interface. They act as a physical barrier between radicular dentin and root canal sealer, negatively affecting sealer penetration into dentinal walls. Biodentine has higher dentine bond strength values than MTA after the use of various intracanal medicaments.[20] Furthermore, the dentine element uptake is more prominent for Biodentine than MTA.[21] Hence Biodentine was chosen as the barrier material in our study.
Oily vehicles elevate the antimicrobial effects of CH against Enterococcus faecalis and other bacteria.[22] Thus, Metapex was used which is a silicone oil-based CH paste. Endodontic regenerative procedures, an alternative clinical approach to apexification, involve the use of TAP as a dressing and the induction of bleeding to create a matrix for the growth of vital tissue in the pulp canal space. Hoshino et al. demonstrated that alone, none of the drugs resulted in the complete elimination of bacteria. However, in combination, these drugs were able to consistently sterilize all samples.[23] Out of the newly found medications, propolis has attracted attention as an antimicrobial agent. Propolis was introduced into dentistry in 1996 by Krell and is a natural resinous mixture, which is produced by honeybees.[10] Various studies in the literature have proved the antimicrobial efficacy of propolis against E. faecalis.[24] de Rezende et al. evaluated two propolis pastes associated with CH with and without alcohol and concluded that the latter produced greater inhibition halos.[25] Thus, in our study, a nonalcoholic CH – propolis paste is used. The utilization of liquid EDTA (17%) shows effective retrieval of the intracanal medicaments in our study. One of the main purposes of our study was to devise a nondestructive approach. Micro-CT being a 3D imaging technique does not cause any severance to the samples and thus is used to evaluate the marginal adaptation of Biodentine. Techniques such as scanning electron microscope, fluid infiltration, and CLSM have their drawbacks.
CONCLUSION
Within the limitations of this in vitro study, it can be concluded that Propolis as an intracanal medication with CH significantly improved the marginal adaptation of Biodentine in the apical third of root dentin walls. However, the use of TAP or Metapex decreased the marginal adaptation of Biodentine. It should be emphasized that the present study was carried out using extracted human teeth and further in vivo studies are recommended to obtain clinically relevant results.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
We acknowledge the CSIR National Chemical Laboratory, Pune; for their support to conduct this research.
REFERENCES
- 1.Bakland L. Endodontic considerations in dental trauma. Endodontics. 2002;1:795–844. [Google Scholar]
- 2.Steiner JC. Inducing root end closure of nonvital permanent teeth. J Dent Child. 1968;35:47–54. [PubMed] [Google Scholar]
- 3.Andreasen JO, Farik B, Munksgaard EC. Long-term calcium hydroxide as a root canal dressing may increase risk of root fracture. Dent Traumatol. 2002;18:134–7. doi: 10.1034/j.1600-9657.2002.00097.x. [DOI] [PubMed] [Google Scholar]
- 4.Holden DT, Schwartz SA, Kirkpatrick TC, Schindler WG. Clinical outcomes of artificial root-end barriers with mineral trioxide aggregate in teeth with immature apices. J Endod. 2008;34:812–7. doi: 10.1016/j.joen.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 5.Tronstad L. Tissue reactions following apical plugging of the root canal with dentin chips in monkey teeth subjected to pulpectomy. Oral Surg Oral Med Oral Pathol. 1978;45:297–304. doi: 10.1016/0030-4220(78)90098-1. [DOI] [PubMed] [Google Scholar]
- 6.Haapasalo M, Shen Y, Wang Z, Gao Y. Irrigation in endodontics. Br Dent J. 2014;216:299–303. doi: 10.1038/sj.bdj.2014.204. [DOI] [PubMed] [Google Scholar]
- 7.Siqueira JF Jr, Magalhães KM, Rôças IN. acterial reduction in infected root canals treated with 25% NaOCl as an irrigant and calcium hydroxide/camphorated paramonochlorophenol paste as an intracanal dressing. J Endod. 2007;33:667–72. doi: 10.1016/j.joen.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Cwikla SJ, Bélanger M, Giguère S, Progulske-Fox A, Vertucci FJ. Dentinal tubule disinfection using three calcium hydroxide formulations. J Endod. 2005;31:50–2. doi: 10.1097/01.don.0000134291.03828.d1. [DOI] [PubMed] [Google Scholar]
- 9.Sato I, Ando-Kurihara N, Kota K, Iwaku M, Hoshino E. Sterilization of infected root-canal dentine by topical application of a mixture of ciprofloxacin, metronidazole and minocycline in situ. Int Endod J. 1996;29:118–24. doi: 10.1111/j.1365-2591.1996.tb01172.x. [DOI] [PubMed] [Google Scholar]
- 10.Zare Jahromi M, Toubayani H, Rezaei M. Propolis: A new alternative for root canal disinfection. Iran Endod J. 2012;7:127–33. [PMC free article] [PubMed] [Google Scholar]
- 11.Alsubait S, Alsaad N, Alahmari S, Alfaraj F, Alfawaz H, Alqedairi A. The effect of intracanal medicaments used in Endodontics on the dislocation resistance of two calcium silicate-based filling materials. BMC Oral Health. 2020;20:57. doi: 10.1186/s12903-020-1044-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gu LS, Kim JR, Ling J, Choi KK, Pashley DH, Tay FR. Review of contemporary irrigant agitation techniques and devices. J Endod. 2009;35:791–804. doi: 10.1016/j.joen.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 13.Johnson BR. Considerations in the selection of a root-end filling material. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;87:398–404. doi: 10.1016/s1079-2104(99)70237-4. [DOI] [PubMed] [Google Scholar]
- 14.Kokate SR, Pawar AM. An in vitro comparative stereomicroscopic evaluation of marginal seal between MTA, glass inomer cement and biodentine as root end filling materials using 1% methylene blue as tracer. Endodontology. 2012;24:36–42. [Google Scholar]
- 15.Al Fouzan K, Awadh M, Badwelan M, Gamal A, Geevarghese A, Babhair S, et al. Marginal adaptation of mineral trioxide aggregate (MTA) to root dentin surface with orthograde/retrograde application techniques: A microcomputed tomographic analysis. J Conserv Dent. 2015;18:109–13. doi: 10.4103/0972-0707.153069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morse DR, O'Larnic J, Yesilsoy C. Apexification: Review of the literature. Quintessence Int. 1990;21:589–98. [PubMed] [Google Scholar]
- 17.Sheehy EC, Roberts GJ. Use of calcium hydroxide for apical barrier formation and healing in non-vital immature permanent teeth: A review. Br Dent J. 1997;183:241–6. doi: 10.1038/sj.bdj.4809477. [DOI] [PubMed] [Google Scholar]
- 18.Ozturk TY, Guneser MB, Taschieri S, Maddalone M, Dincer AN, Venino PM, et al. Do the intracanal medicaments affect the marginal adaptation of calcium silicate-based materials to dentin? J Dent Sci. 2019;14:157–62. doi: 10.1016/j.jds.2019.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shaheen NA, Ghoneim WM. Effect of two intracanal medicaments on the sealing ability and push-out bond strength of Biodentine apical plug. Tanta Dent J. 2018;15:111. [Google Scholar]
- 20.Nagas E, Cehreli ZC, Uyanik MO, Vallittu PK, Lassila LV. Effect of several intracanal medicaments on the push-out bond strength of ProRoot MTA and Biodentine. Int Endod J. 2016;49:184–8. doi: 10.1111/iej.12433. [DOI] [PubMed] [Google Scholar]
- 21.Han L, Okiji T. Uptake of calcium and silicon released from calcium silicate-based endodontic materials into root canal dentine. Int Endod J. 2011;44:1081–7. doi: 10.1111/j.1365-2591.2011.01924.x. [DOI] [PubMed] [Google Scholar]
- 22.Gomes BP, Ferraz CC, Vianna ME, Rosalen PL, Zaia AA, Teixeira FB, et al. In vitro antimicrobial activity of calcium hydroxide pastes and their vehicles against selected microorganisms. Braz Dent J. 2002;13:155–61. doi: 10.1590/s0103-64402002000300002. [DOI] [PubMed] [Google Scholar]
- 23.Hoshino E, Kurihara-Ando N, Sato I, Uematsu H, Sato M, Kota K, et al. In vitro antibacterial susceptibility of bacteria taken from infected root dentine to a mixture of ciprofloxacin, metronidazole and minocycline. Int Endod J. 1996;29:125–30. doi: 10.1111/j.1365-2591.1996.tb01173.x. [DOI] [PubMed] [Google Scholar]
- 24.Basrani B, Tjäderhane L, Santos JM, Pascon E, Grad H, Lawrence HP, et al. Efficacy of chlorhexidine- and calcium hydroxide-containing medicaments against Enterococcus faecalis in vitro. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;96:618–24. doi: 10.1016/s1079-2104(03)00166-5. [DOI] [PubMed] [Google Scholar]
- 25.de Rezende GP, da Costa LR, Pimenta FC, Baroni DA. In vitro antimicrobial activity of endodontic pastes with propolis extracts and calcium hydroxide: A preliminary study. Braz Dent J. 2008;19:301–5. doi: 10.1590/s0103-64402008000400003. [DOI] [PubMed] [Google Scholar]


