Abstract
Purpose:
The aim of this in vitro study was to investigate the effectiveness of a novel charcoal-containing whitening toothpaste and a mouthwash on tooth color change and the alterations of enamel that may be induced after toothbrushing, corresponding to a 90-day period.
Materials and Methods:
Forty human canines were used, stained with coffee, and divided into four groups (n = 10) as follows: Group 1 (control) submitted to toothbrushing with deionized water, Group 2 with a regular toothpaste, Group 3 with a whitening toothpaste (1% charcoal), and Group 4 with the same whitening toothpaste in combination with a mouthwash (1% charcoal and 0.5% H2O2). After the treatments, ΔΕ of the teeth was evaluated using an ultraviolet/Vis spectrophotometer, whereas the changes in surface morphology were observed by means of a confocal microscope.
Results:
The whitening toothpaste increased significantly ΔΕ (40.5%) compared to the control group (P < 0.001). In addition, the tested whitening toothpaste also increased ΔΕ (17.7%) compared to the regular toothpaste (P = 0.023). The whitening toothpaste presented smoother surfaces after toothbrushing, but more heterogeneous with numerous large craters, whereas the whitening mouthwash did not influence surface morphology changes.
Conclusions:
Charcoal-containing toothpastes may enhance the whitening of the teeth, but they should be used carefully due to changes that may induce on enamel. The patients should consult a dental professional for proper use. A charcoal-containing mouthwash in combination with whitening toothpastes probably cannot offer additional whitening effect.
Keywords: Charcoal, color change, enamel surface morphology, mouthwash, whitening toothpaste
INTRODUCTION
Tooth whitening is a very popular conservative method in dental esthetics, providing in many cases an appropriate alternative for restorative dentistry. There are two main approaches of tooth whitening: (a) tooth bleaching and (b) the use of over-the-counter products. Tooth bleaching can also be categorized into two types: in-office tooth bleaching, which is performed at a dental office with high-concentrated bleaching agents (25%–40% hydrogen peroxide (H2O2) or 35%–38% carbamide peroxide) for shorter application time, and at-home tooth bleaching, which is performed by the patient himself under dental supervision with low-concentrated bleaching agents (3%–6% H2O2 or 10%–16% carbamide peroxide) for longer application time.[1,2]
The mechanism of action of tooth bleaching treatments includes the interaction of H2O2 with the chromophores of the tooth tissues, while that of over-the-counter products includes mainly two mechanisms: (a) bleaching intrinsic stains using oxidizing agents such as H2O2 but in very low concentrations (0.5%–3%) that break down the superficial pigments in the tooth tissues and (b) removing extrinsic stains using various abrasive agents.[3]
Over-the-counter products are applied to the teeth by the patient using gum shields, strips, paint-on products, toothpastes, or mouthwashes. A very common whitening method is the use of the so-called “whitening” toothpastes that may contain abrasives (hydrated silica, perlite, alumina, calcium carbonate, calcium pyrophosphate, sodium bicarbonate, etc.), chemicals (H2O2, calcium peroxide, sodium citrate, sodium pyrophosphate, papain, etc.,), and/or optical brighteners, such as blue covarine, which is actually a dye that covers tooth surfaces and increases the perception of tooth whiteness.[1]
As mentioned before, whitening toothpastes include in their formulation a variation of abrasive components, responsible for removing extrinsic stains of the teeth, as well as dental biofilms and food debris.[4] Recently, charcoal-containing whitening toothpastes have become popular oral hygiene products, aiming to improve extrinsic stain removal and tooth whitening.[5] Charcoal-containing toothpastes work in a similar manner to regular toothpastes. Moreover, it has been claimed that activated charcoal binds to the tooth surface deposits such as microbial plaque and chromophores which are absorbed in the pores of the charcoal and then brushed away.[6]
The abrasivity of charcoal-containing toothpastes is considered to depend on the nature, method of preparation, and particle size distribution of the charcoal included in the formulation.[7] The abrasivity of the toothpaste affects its effectiveness to remove extrinsic stains and deposits resulting to color change. However, high abrasivity of toothpaste may induce surface loss and changes in surface morphology of tooth tissues.[7] Therefore, the objective of the present study was to investigate the effectiveness of a charcoal-containing whitening toothpaste on tooth color change and to evaluate the alterations of enamel surface that may be induced after simulation of toothbrushing corresponding to a 90-day period. Moreover, the influence of the use of a novel charcoal-containing whitening mouthwash on these properties was also examined in conjunction with the whitening toothpaste.
The first null hypothesis (H01) of the study was that the use of the tested charcoal-containing whitening toothpaste would not affect tooth color change, whereas the second null hypothesis (H02) was that the tested charcoal-containing whitening mouthwash would not improve color change when it was used in conjunction with the whitening toothpaste.
MATERIALS AND METHODS
Preparation of the specimens
Forty sound human canines were freshly extracted for periodontal reasons and stored in a 0.5% chloramine T solution at 6°C for up to 3 months. The patients were informed for the purpose of the study and gave their consent. The experiment was conducted respecting the rules of the local Ethical and Research Committee and the policies of the Aristotle University of Thessaloniki in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments. Immediately after the extractions, the periodontal remains were completely removed and the teeth were cleaned with slurry of pumice and water. The tooth specimens were prepared according to Wiegand et al.[8] The crowns were separated from the roots and each crown was sagittally sectioned into two halves [Figure 1] using a water-cooled diamond disk (Isomet, Buehler, Lake Bluff, IL, USA). The labial surfaces of the teeth were used for color change assessment, whereas the lingual surfaces were separately investigated for surface morphology changes. Subsequently, all the sections were examined by means of an optical microscope under ×10 magnification to confirm their surface integrity. Afterward, the labial sections were immersed into artificial saliva for storage at 37°C, whereas the lingual sections were embedded in self-cured acrylic resin (NT Newton, Toros Dental, Antalya, Turkey), and the enamel surfaces were ground on a polishing machine (Jean Wirtz TG 250, Dusseldorf, Germany) with 200 rpm under water cooling (50 ml/min) using gradually 600-, 800-, 1000-, and 1200-grit silicon carbide abrasive papers (Apex S system, Buehler, Lake Bluff, IL, USA) and a 0.4-μm alumina grinding suspension, in order to form parallel planar surfaces. After grinding, the specimens were checked for absence of dentin areas on the grinding surfaces, immersed in ultrasonic bath (Euronda Spa, Montecchio Precalcino, Vicenza, Italy) for 5 min, and stored in artificial saliva at 37°C. The composition of artificial saliva was as follows: 0.103 g/l of CaCl2, 0.019 g/l MgCl2·6H2O, 0.544 g/l KH2PO4, and 2.24 g/l KCl, and buffer (TCP-KOH) was added to adjust the pH to 7.[9]
Figure 1.
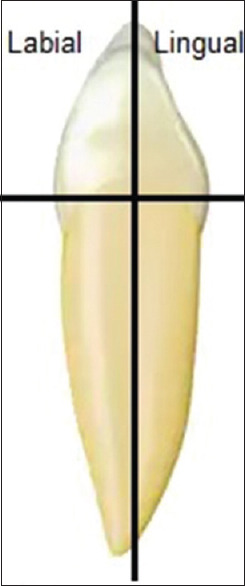
Preparation of the specimens. The crowns of each canine were separated from the roots and each crown was sagittally sectioned into two halves
Tooth staining method
The tooth specimens were stained according to Torres et al.[3] More specifically, the labial sections were placed into silicon molds in a way that only the enamel surface was exposed and were immersed into a 1% sodium hypochlorite (NaClO) solution for 30 min to remove any initial extrinsic stain. The enamel surfaces were then etched for 60 s using 35% phosphoric acid gel (Scotchbond™ Etchant, 3M ESPE, St. Paul, MN, USA) and rinsed with deionized water for 30 s. The dentin of the specimens was previously covered with an acid-resistant nail varnish. A solution of coffee was made by pouring 25 g of a commercial coffee product (Nescafe Classic, Nestle, Vevey, Switzerland) in 100 ml of distilled water and heated for 10 min until the water boiled. Then, the solution was allowed to cool and the specimens were immersed into the solution for 24 h. After 24 h, the specimens were removed from the coffee solution, rinsed with deionized water, and placed in ultrasonic bath for 2 min.
Experimental groups of the study
The specimens were randomly divided into four groups (n = 10) and were submitted to one of the following treatments:
Group 1 (control group) specimens were submitted to toothbrushing simulation only with deionized water and without the use of toothpaste or mouthwash during the procedure
Group 2 specimens were submitted to toothbrushing simulation using a commercial toothpaste (Colgate Total®, Colgate-Palmolive Company, Greece) with medium relative dentin abrasivity (RDA = 70)
Group 3 specimens were submitted to toothbrushing simulation using a whitening toothpaste (Black and Polish Toothpaste, Frezyderm, Greece), which contains charcoal as an active agent
Group 4 specimens were submitted to toothbrushing simulation using the same whitening toothpaste in combination with a whitening mouthwash (Black and Polish Mouthwash, Frezyderm, Greece), which contains charcoal and H2O2 as active agents.
The compositions of the tested products are presented in Table 1.
Table 1.
The composition of the tested dental products of the study according to manufacturers
| Product | Type | Composition | Active agents | Manufacturer |
|---|---|---|---|---|
| Black and Polish Toothpaste | Whitening toothpaste | Deionized water, sorbitol, sodium saccharine, sodium fluoride (0.32% w/w), sodium benzoate, polyglycol 1500s, Blanoz 7M1F Pharm, Tixosil 73, Tixosil 43, Pearlwhite 19, flavor spearmint frost EAB24297/00, biosol, citric acid, Speckare CAC3, sabosol L30 | Active charcoal (1% w/w) | Frezyderm Ltd, Athens, Greece |
| Black and Polish Mouthwash | Whitening mouthwash | Deionized water, sodium saccharine, sodium monofluorophosphate (0.2% w/w), sodium benzoate, Furdentyl, flavor spearmint frost EAB24297/00, biosol, sabowax ELH 40, glycerin, peroxydone K90, active charcoal CA3, citric acid | Active charcoal (1% w/w) Hydrogen peroxide (0.5% w/w) | Frezyderm Ltd, Athens, Greece |
| Colgate Total® | Regular toothpaste | Water, glycerin, hydrated silica, PVM/MA copolymer, sodium lauryl sulfate, cellulose gum, aroma, sodium hydroxide, carrageenan, sodium fluoride, triclosan, sodium saccharin, limonene, CI 77891 | Hydrated silica | Colgate-Palmolive Company, Piraeus, Greece |
Toothbrushing simulation
A commercial electric toothbrush was used in this study (Oral-B, Braun, France) for toothbrushing simulation. The following parameters were selected for the procedure: load of the toothbrush standardized at 250 g, medium hardness toothbrush head (Oral-B EB20, Procter and Gamble, Athens, Greece), rotation rate at 7500 rpm, and rotation sense changing every 30 s. The electric toothbrush was fixed in a constructed device that allowed the heads of the brushes to be aligned parallel to the surface of the specimens and to control the pressure by an electric system [Figure 2]. The toothbrush head was in direct contact with the specimen and 0.2 g of the tested toothpaste was weighted on a high precision scale, diluted in 500 μL of distilled water, and applied directly on the surface of the specimen with a syringe. In this investigation, it was assumed that individuals brush their teeth twice a day for an average of 2 min. There were a total of four quadrants with three surfaces of teeth to be brushed. As a toothbrush length covers approximately two or three teeth at the same time, each quadrant is brushed in two parts. Then, it is reasonable to assume that a 10-s brushing on each tooth surface effectively represents one person's daily toothbrushing habits. The duration of toothbrushing procedure for each tooth specimen was 15 min, which corresponds to a 3-month (90 days) daily toothbrushing. After the toothbrushing procedure, the specimens were rinsed with deionized water for 20 s, air-dried for 5 s, and immersed into artificial saliva at 37°C.[10]
Figure 2.

The electric toothbrush was fixed in a constructed device that allowed the heads of the brushes to be aligned parallel to the surface of the specimens and to control the pressure by an electric system
Mouth rinsing simulation
The use of the tested whitening mouthwash for the Group 4 specimens followed the instructions of the manufacturer. In particular, it is recommended to use 10 ml of the mouthwash for 30 s after each toothbrushing, corresponding to 90-min mouth rinsing during a 90-day period. For the experiment of the present study, each tooth specimen after toothbrushing simulation was immersed into the tested mouthwash for 90 min and then rinsed with deionized water for 20 s, air-dried for 5 s, and immersed into artificial saliva at 37°C. The whitening solution was refreshed every 10 min.
Evaluation of tooth color change
Color change evaluation was implemented using a double-beam ultraviolet/Vis spectrophotometer (Lambda 18, PerkinElmer, Waltham, MA, USA) equipped with an integrating sphere. The measurements were performed in a spectral range of 200–800 nm with an accuracy of 1 nm. Calibration of the spectrophotometer was carried out at the beginning of each set of measurements using a barium sulfate (BaSO4) standard. The data from the spectral reflectance curves of the visual spectrum (380–780 nm) were converted to L*, a*, and b * units of the perceptually uniform CIEL * a*b* color space. An L * a*b* color space is a color-opponent space with dimensions L * for lightness and a * and b * for the color-opponent dimensions, based on nonlinearly compressed coordinates. In particular, L * ranges from 0 (black) to 100 (white), a * from−128 (green) to +128 (red), and b * from?-128 (blue) to +128 (yellow). Total color changes (ΔΕ) were determined using the following equations:
ΔL* = L*f–L * i (1)
Δμ* = μ*f–μ*i (2)
Δb* = b*f–b * i (3)
ΔΕ = (ΔL*2+ Δμ*2+ Δb*2)½ (4)
Where “f” indicates the final value and “i” indicates the initial value. The perceptibility threshold (PT) of ΔΕ was set at 1.2 and the acceptability threshold (AT) of ΔΕ for color change of the teeth after the treatments was set at 2.7, according to Paravina et al.[11]
For customization and reproducibility of the specimens' position, a standardized mounting system was developed using the rectangular inner frame of the black Bakelite sample assembly that the spectrophotometer was equipped with. Black, nonpolychromatic, thermo-plasticized silicon (Schwarze Klebesticks, STEINEL, Germany) was applied to fill the cylindrical inner frame using a hot-melt glue applicator (Gluematic 3002, STEINEL, Germany), and each tooth specimen was fixed within the black silicon during setting to make individualized specimen carriers. All measurements were carried out before and 24 h after the treatments repeated twice and averaged. If Δ between two recordings of the same specimen exceeded 1, new measurements were obtained. The measurements were implemented at room temperature (23°C ± 1°C) by the same operator, who did not know which experimental group was measured. During measurements, the teeth were immediately (<1 min) transferred from their storage solution to the spectrophotometer to retain their humidity and prevent procedural tooth color changes attributed to drying.[12]
Evaluation of surface morphology changes
Surface morphology changes of the enamel of the lingual surfaces before and after toothbrushing simulation were observed by means of confocal microscope (three-dimensional [3D] Optical Surface Metrology System Leica DCM8, Leica Microsystems CMS GmbH, Mannheim, Germany) using vertical scanning interferometry. This method relies on light interferometry (bright field) and the microscope operates as a noncontact optical profiler in vertical scanning mode to produce 3D topography maps of the enamel surface. For each tooth specimen, four images were obtained from the four quadrants of the surface at ×20 magnification. The light source of the microscope was a green light-emitting diode irradiating at 530 nm.
Statistical analysis
Having preliminarily checked that data distribution was normal and that group variances were homogeneous (Kolmogorov–Smirnov test and Levene's test, respectively), the one-way analysis of variance was applied to verify the existence of statistically significant between-group differences, followed by the Tukey's test for post hoc comparisons (Bonferroni corrected). The level of significance was set at α = 0.05.
RESULTS
Tooth color change
The mean values and standard deviations of ΔΕ (color change) of each experimental group of the study after the toothbrushing simulation are presented in Table 2. The use of the tested whitening toothpaste increased significantly color change (40.5%) compared to the control group (P < 0.001). In addition, the tested whitening toothpaste also increased color change (17.7%) compared to the regular toothpaste with medium RDA (P = 0.023), which exhibited approximately 27.6% higher ΔΕ compared to the control group. The difference in the mean ΔΕ between the whitening (11.17 ± 3.11) and the regular (9.19 ± 1.69) toothpaste was 1.98. The use of the whitening mouthwash in combination with the whitening toothpaste did not improve color change of the teeth (P = 0.125). Finally, the color change in all the experimental groups exceeded the established perceptibility and ATs.
Table 2.
Means and standard deviations of color change (ΔΕ) the experimental groups of the study
| Group | ΔΕ | Significance | %ΔΕ increase | Exceeds PT | Exceeds ΑT |
|---|---|---|---|---|---|
| No toothpaste (control) | 6.65±1.29 | a | - | Yes | Yes |
| Colgate total toothpaste | 9.19±1.69 | b | 27.6 | Yes | Yes |
| Black and polish toothpaste | 11.17±3.11 | c | 40.5 | Yes | Yes |
| Black and polish toothpaste+mouthwash | 11.88±5.11 | c | 44.0 | Yes | Yes |
Same lowercase letter indicates no statistically significant difference (P<0.05). %ΔΕ increase: The % increase of ΔΕ of each experimental group in comparison with the control group. PT: Perceptibility threshold (ΔΕ=1.2), AT: Acceptability threshold (ΔΕ=2.7)
Surface morphology changes
Topographic surface maps (magnification ×20) of representative specimens of each experimental group before and after the treatments are illustrated in Figures 3 and 6a, b. Observations of the representative images of the enamel surfaces revealed alterations of surface morphology in all the experimental groups after toothbrushing. More specifically, in the control [Figure 3b] and regular toothpaste [Figure 4a-b] groups, the enamel surfaces appeared uniform and rougher, but in the latter group, the roughness was more intense with increased peak-to-valley height. On the other hand, the whitening toothpaste groups [Figures 5a-b and 6b] presented smoother surfaces after the toothbrushing, but more heterogeneous with numerous large and deep craters and other surface features. The use of the whitening mouthwash in conjunction with the whitening toothpaste did not influence surface morphology of the enamel.
Figure 3.
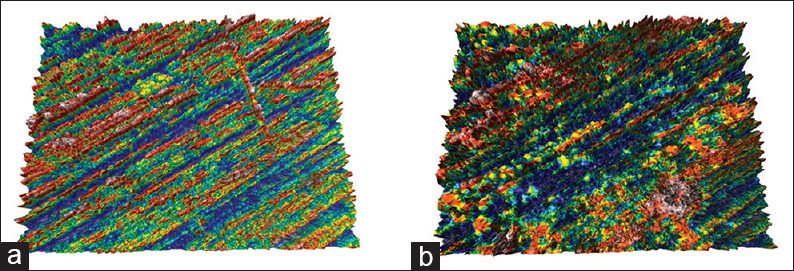
Topographic surface maps (magnification ×20) of representative specimens of Group 1 (control) before (a) and after (b) the treatments. After toothbrushing a rougher surface appears compared to a smoother and uniform surface before treatment
Figure 6.
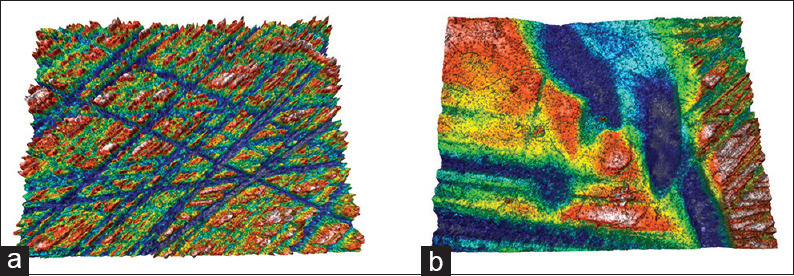
Topographic surface maps (magnification ×20) of representative specimens of Group 4 (whitening toothpaste + mouthwash) before (a) and after (b) the treatments. Both groups (3 and 4) presented similar morphology after toothbrushing with smoother surfaces but more heterogeneous with numerous large and deep craters and other surface features
Figure 4.
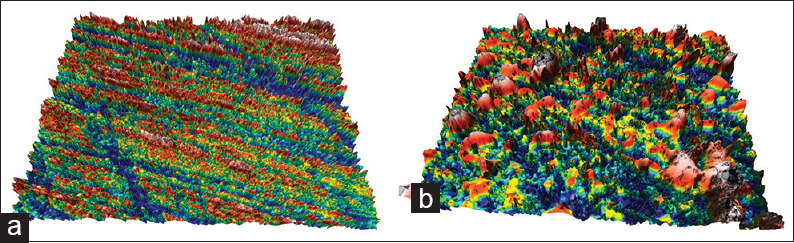
Topographic surface maps (magnification ×20) of representative specimens of Group 2 (regular toothpaste) before (a) and after (b) the treatments. After toothbrushing a rougher surface appears compared to a smoother and uniform surface before treatment, but the roughness was more intense with increased peak-to-valley compared to Group 1
Figure 5.
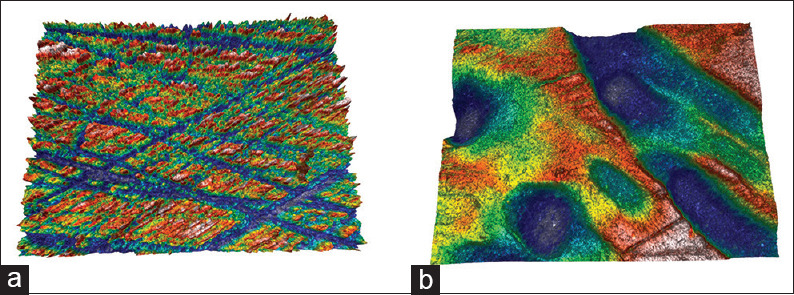
Topographic surface maps (magnification ×20) of representative specimens of Group 3 (whitening toothpaste) before (a) and after (b) the treatments. After toothbrushing with smoother surfaces were observed, but more heterogeneous numerous large and deep craters and other surface features
DISCUSSION
According to the results of the current study, H01, which stated that the use of the tested charcoal-containing whitening toothpaste would not affect tooth color change, was rejected. The data regarding the effectiveness of whitening toothpastes containing charcoal are scarce. Vaz et al.[6] demonstrated that a charcoal-containing toothpaste was effective for whitening treatment compared to a regular toothpaste which was the control group of the study, but less effective than other toothpastes which contained microbeads, H2O2, or blue covarine as whitening agents. On the other hand, in another recent study, it was reported that the use of an activated charcoal powder did not improve color change when combined with the tested regular and whitening toothpastes.[13] Moreover, in a recent literature review, the authors concluded that there is no sufficient evidence for whitening effect of charcoal-containing toothpastes.[5] It is interesting to mention that although in the current investigation, the charcoal-containing toothpaste presented statistically significant higher color change (ΔΕ = 11.17 ± 3.11) than the regular toothpaste (ΔΕ = 9.19 ± 1.69), this difference (×1.98) may not be clearly perceivable by the human eye.
Activated charcoal is a nanocrystalline form of carbon (C) with a high specific surface area and high porosity in the nanometer range. Activated charcoal is commonly used as an adsorbent in various applications.[6] Due to this ability, charcoal may absorb extrinsic stains of the teeth in its pores and then brushed away providing tooth color change. Nevertheless, there is no sufficient scientific evidence for this action, and as a result, it has been assumed that charcoal does not change the color of the teeth other than by abrasive action similar to that of regular toothpastes. This is the reason that some authors recommend this kind of toothpastes only for color maintenance by delaying the recurrence of surface staining on tooth surfaces after tooth bleaching treatment.[14] In addition, it has been postulated that the high absorptive capacity of activated charcoal may reduce the availability of fluoride ions in the formula of the toothpaste, leading to limited capacity to remineralize the tooth tissues and as a consequence to decreased resistance to caries and tooth wear.[15]
The color of the teeth is mainly depended on the color of dentin and modified by the thickness and translucence of enamel. The mineral phase of enamel consists of calcium phosphate in the form of hydroxyapatite which is colorless. As a result, natural enamel usually has white color with some translucency.[16] Nevertheless, the continuous wear of enamel, which is attributed mainly to erosion and abrasion, may reduce the thickness of enamel, leading to a darker and more yellowish color because the dentin becomes more visible.[17] In addition, the deposition of various chromophores into and onto the tooth tissues from the diet (coffee, black tea, red wine, etc.) or other daily habits, such as smoking, may change the color creating intrinsic or extrinsic stains. This deposition may be accelerated by the increased porosity and surface roughness of enamel.[1] This fact may explain the differences in the degree of discoloration among the teeth after the staining method.
It has been claimed that the type of toothbrush used, the toothbrushing method, and the duration of brushing may be more important to the cleaning and whitening effect than the composition of the toothpaste.[7] As a matter of fact, in this in vitro study, these parameters were standard during toothbrushing in all the experimental groups focusing on the impact of the use of the tested toothpastes and mouthwash.
In the current investigation, acceptability and PT were established according to Paravina et al.[18] Visual thresholds are of great importance in dentistry as qualitative parameters for the evaluation and interpretation of clinical outcome and comparison of different treatments.[11] The PT refers to the smallest color difference that can be detected by an observer, while the AT is the color change that is acceptable by an observer in comparison with a reference color. This is important in tooth whitening when dental restorations are existed in anterior teeth. The question that defines PT is usually “Do you see any difference in the color of these two specimens?” and for AT is “Would you consider this difference acceptable under clinical conditions?” Of course, if the answer to the first question is negative, the second question is not needed. Industry tolerance is the difference between those two thresholds (perceptibility and acceptability) and determines how far from the perceptible threshold there is still an acceptable color match.[11] Considering that in tooth whitening, the demand of the patients is to achieve high color change, it is desirable to exceed the acceptable threshold. In fact, the success of a whitening treatment depends on how much the color change overcome AT. In the present study, the industry tolerance was 1.5 and all the experimental groups of the study exceeded perceptibility and ATs, even the control group (toothbrushing with deionized water). This means that the abrasive action of the toothbrush itself contributes to color change by removing some extrinsic stains of the enamel surface.
There are multiple parameters that affect the abrasive action of toothpastes on tooth surface, including the hardness, shape, size, distribution, and concentration of the abrasive particles that contain.[19] The composition of whitening toothpastes in abrasive substances should allow therapeutic action without damaging the hard tissues of the teeth, resulting in improved cleaning and reduction of tooth wear.[1,20] It has been postulated that the abrasive potential of charcoal-containing toothpastes depends on the nature, method of preparation, and particle size distribution of the charcoal included in the product.[7] Previous studies reported a relatively high abrasivity of oral hygiene charcoal-based products.[21] In particular, in a clinical case, the use of a whitening toothpaste containing charcoal resulted in loss of the surface luster of the enamel and a dull appearance, implying a loss of surface tissue and increased surface roughness. In the present study, the charcoal-containing toothpaste affected the surface morphology of enamel by leaving a heterogeneous surface with numerous large and deep craters and increased roughness. Brushing of the enamel with the charcoal-containing toothpaste induced different surface morphology patterns compared to the regular toothpaste which contains hydrated silica. This may be attributed to the different characteristics of their abrasive agents.
It is well documented that a more abrasive composition of a toothpaste offers better cleaning and whitening properties but on the other side a more aggressive abrasive action to tooth surface, which leads to faster and increased tooth wear. Despite the claims of low abrasivity for various charcoal-containing toothpastes, these claims have not yet been confirmed by scientific evidence.[5] This fact seems to comply with the results of the present investigation with regard to the changes of enamel surface morphology. A more comprehensive analysis of surface roughness and loss should be useful to evaluate the safety of this kind of products for daily use.
On the basis of the results reported in the current study, the H02, stating that the tested charcoal-containing whitening mouthwash would not improve color change when it was used in conjunction with the whitening toothpaste, should be accepted. The manufacturers of the tested charcoal-containing mouthwash recommend its use in combination with the tested charcoal-containing toothpaste in purpose to enhance the whitening effect on tooth surface. However, the outcomes of the current study did not justify their expectations. Nevertheless, the tested whitening mouthwash may be effective when it is applied in combination with regular toothpastes with lower whitening effect or alone.
In the present study, a novel charcoal-containing (1%) mouthwash was evaluated, which also contains 0.5% H2O2 as a whitening agent. The manufacturers support that this formula, which combines the whitening action of charcoal with H2O2, may be beneficial for the whitening of the teeth. To the authors' knowledge, there is no other study in literature that evaluated the properties of this product in order to compare it with the results of the current study. In general, the effectiveness of whitening mouthwashes has not been discussed adequately in literature. Potgieter and Grobler[22] demonstrated no significant bleaching effect after the use of various peroxide-based whitening mouthwashes on stained teeth after a 4-week treatment period. In contrast, Hasturk et al.,[23] who focused on the effectiveness of a 1.5% H2O2 mouthwash to reduce gingivitis and increase tooth whitening, reported positive results after 6 months of treatment period. Furthermore, Torres et al.[3] also reported whitening effect of two whitening mouthwashes containing 1.5% and 2% H2O2, which was similar to that of a 10% carbamide peroxide whitening gel.
The effect of a whitening mouthwash on toothbrush abrasion should also be considered. In a recent study,[24] the authors concluded that the continuous use of whitening mouthwashes is able to increase the enamel abrasion potential promoted by daily toothbrushing. Tooth whitening of charcoal-containing mouthwashes is presumably attributed to the inclusion of charcoal particles which act as abrasive agents to mechanically remove extrinsic stains from the tooth surfaces. However, it has been claimed that such products may raise the risk of enamel damage and caries formation due to the absence of fluoride or charcoal-induced fluoride degradation.[25] In the current study, the use of the tested charcoal-containing mouthwash did not deteriorate the surface damage of the enamel after brushing with the charcoal-containing toothpaste.
CONCLUSIONS
Within the limitations of this in vitro study, it could be concluded that the charcoal-containing toothpaste presented higher whitening effect on the teeth than the regular toothpaste, but the use of the charcoal-containing mouthwash in combination with the whitening toothpaste did not improve the color change. In addition, the use of the toothpastes during brushing influenced differently the surface morphology of the enamel, while the whitening mouthwash did not influence these morphological alterations. The patients that desire whiter teeth should be well informed by the dental professionals for the indications of the over-the-counter whitening products in order to be aware of the hazards of their daily use. Although the charcoal-containing whitening toothpaste may offer desirable tooth color change, it should be used carefully following the instructions of a dental professional.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors would like to thank Dr. Avraam Konstantinidis and Alexia Tsigarida for their technical support to use the confocal microscope for the purposes of this study, which were performed at the Department of Civil Engineering, Division of Structural Engineering, Faculty of Engineering, Aristotle University of Thessaloniki, Greece. In addition, the authors acknowledge with thanks Prof. Philomela Komninou for her guidance in evaluation of color change, which was implemented in the Department of Solid State Physics, School of Physics, Faculty of Sciences, Aristotle University of Thessaloniki, Greece. Finally, we would like to thank Panagiota Saraki (Professional & Scientific Relations Oral B) for the provision of the device for toothbrushing simulation. Partial financial support was received from Frezyderm S.A., Athens, Greece.
REFERENCES
- 1.Joiner A. The bleaching of teeth: A review of the literature. J Dent. 2006;34:412–9. doi: 10.1016/j.jdent.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 2.White DJ, Kozak KM, Zoladz JR, Duschner H, Götz H. Peroxide interactions with hard tissues: Effects on surface hardness and surface/subsurface ultrastructural properties. Compend Contin Educ Dent. 2002;23:42–8. [PubMed] [Google Scholar]
- 3.Torres CR, Perote LC, Gutierrez NC, Pucci CR, Borges AB. Efficacy of mouth rinses and toothpaste on tooth whitening. Oper Dent. 2013;38:57–62. doi: 10.2341/11-360-L. [DOI] [PubMed] [Google Scholar]
- 4.Barbieri GM, Mota EG, Rodrigues-Junior SA, Burnett LH Jr. Effect of whitening dentifrices on the surface roughness of commercial composites. J Esthet Restor Dent. 2011;23:338–45. doi: 10.1111/j.1708-8240.2011.00426.x. [DOI] [PubMed] [Google Scholar]
- 5.Brooks JK, Bashirelahi N, Reynolds MA. Charcoal and charcoal-based dentifrices: A literature review. J Am Dent Assoc. 2017;148:661–70. doi: 10.1016/j.adaj.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Vaz VT, Jubilato DP, Oliveira MR, Bortolatto JF, Floros MC, Dantas AA, et al. Whitening toothpaste containing activated charcoal, blue covarine, hydrogen peroxide or microbeads: Which one is the most effective? J Appl Oral Sci. 2019;27:e20180051. doi: 10.1590/1678-7757-2018-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greenwall LH, Greenwall-Cohen J, Wilson NHF. Charcoal-containing dentifrices. Br Dent J. 2019;226:697–700. doi: 10.1038/s41415-019-0232-8. [DOI] [PubMed] [Google Scholar]
- 8.Wiegand A, Vollmer D, Foitzik M, Attin R, Attin T. Efficacy of different whitening modalities on bovine enamel and dentin. Clin Oral Investig. 2005;9:91–7. doi: 10.1007/s00784-004-0291-2. [DOI] [PubMed] [Google Scholar]
- 9.Dionysopoulos D, Strakas D, Koliniotou-Koumpia E, Koumpia E. Effect of Er, Cr: YSGG laser irradiation on bovine enamel surface during in-office tooth bleaching ex vivo. Odontology. 2017;105:320–8. doi: 10.1007/s10266-016-0273-2. [DOI] [PubMed] [Google Scholar]
- 10.Dionysopoulos D, Tolidis K, Sfeikos T. Effect of air-abrasion pre-treatment with bioactive glass 45S5 on enamel surface loss after erosion/abrasion challenge. Dent Mater. 2019;35:e193–203. doi: 10.1016/j.dental.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 11.Paravina RD, Ghinea R, Herrera LJ, Bona AD, Igiel C, Linninger M, et al. Color difference thresholds in dentistry. J Esthet Restor Dent. 2015;27(Suppl 1):S1–9. doi: 10.1111/jerd.12149. [DOI] [PubMed] [Google Scholar]
- 12.Ioannidis K, Beltes P, Lambrianidis T, Kapagiannidis D, Karagiannis V. Validation and spectrophotometric analysis of crown discoloration induced by root canal sealers. Clin Oral Investig. 2013;17:1525–33. doi: 10.1007/s00784-012-0850-x. [DOI] [PubMed] [Google Scholar]
- 13.Palandi SDS, Kury M, Picolo MZD, Coelho CSS, Cavalli V. Effects of activated charcoal powder combined with toothpastes on enamel color change and surface properties. J Esthet Restor Dent. 2020;32:783–90. doi: 10.1111/jerd.12646. [DOI] [PubMed] [Google Scholar]
- 14.Haywood VB. Tooth whitening is not always tooth bleaching. Inside Dent. 2018;14:80. [Google Scholar]
- 15.Tembhurkar AR, Dongre S. Studies on fluoride removal using adsorption process. J Environ Sci Eng. 2006;48:151–6. [PubMed] [Google Scholar]
- 16.Epple M, Meyer F, Enax J. A critical review of modern concepts for teeth whitening. Dent J (Basel) 2019;7:79. doi: 10.3390/dj7030079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Algarni AA, Ungar PS, Lippert F, Martínez-Mier EA, Eckert GJ, González-Cabezas C, et al. Trend-analysis of dental hard-tissue conditions as function of tooth age. J Dent. 2018;74:107–12. doi: 10.1016/j.jdent.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 18.Paravina RD, Pérez MM, Ghinea R. Acceptability and perceptibility thresholds in dentistry: A comprehensive review of clinical and research applications. J Esthet Restor Dent. 2019;31:103–12. doi: 10.1111/jerd.12465. [DOI] [PubMed] [Google Scholar]
- 19.Ferreira MC, Ramos-Jorge ML, Delbem AC, Vieirac Rde S. Effect of toothpastes with different abrasives on eroded human enamel: An in situ/ex vivo Study. Open Dent J. 2013;7:132–9. doi: 10.2174/1874210601307010132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Moraes Rego Roselino L, Tirapelli C, de Carvalho Panzeri Pires-de-Souza F. Randomized clinical study of alterations in the color and surface roughness of dental enamel brushed with whitening toothpaste. J Esthet Restor Dent. 2018;30:383–9. doi: 10.1111/jerd.12379. [DOI] [PubMed] [Google Scholar]
- 21.Yaacob HB, Park AW. Dental abrasion pattern in a selected group of Malaysians. J Nihon Univ Sch Dent. 1990;32:175–80. doi: 10.2334/josnusd1959.32.175. [DOI] [PubMed] [Google Scholar]
- 22.Potgieter E, Grobler SR. Whitening efficacy of three over-the-counter oral rinses. S Afr Dent J. 2011;66:128–131. [PubMed] [Google Scholar]
- 23.Hasturk H, Nunn M, Warbington M, Van Dyke TE. Efficacy of a fluoridated hydrogen peroxide-based mouthrinse for the treatment of gingivitis: A randomized clinical trial. J Periodontol. 2004;75:57–65. doi: 10.1902/jop.2004.75.1.57. [DOI] [PubMed] [Google Scholar]
- 24.Torres CRG, Bonício GC, Crastechini É, Mailart MC, Borges AB. Effect of whitening mouthrinses on enamel toothbrush abrasion. Am J Dent. 2018;31:285–9. [PubMed] [Google Scholar]
- 25.Brooks JK, Bashirelahi N, Hsia RC, Reynolds MA. Charcoal-based mouthwashes: A literature review. Br Dent J. 2020;228:290–4. doi: 10.1038/s41415-020-1265-8. [DOI] [PubMed] [Google Scholar]


