Abstract
Heart disease is the leading cause of death in Asian Americans. Importantly, people of East Asian descent are more likely to carry a loss-of-function point mutation in aldehyde dehydrogenase 2 (ALDH2), ALDH2*2, which reduces ALDH2 enzymatic activity by at least 40% relative to wild type ALDH2. Given the role of ALDH2 in removing toxic aldehydes from the cell, ALDH2 is intimately involved in the cardioprotective mechanisms of ischemic preconditioning and the pathophysiology of ischemia reperfusion injury. The ALDH2*2 variant is associated with an increased incidence of coronary artery disease, myocardial infarction, and stroke. Furthermore, this variant is associated with insensitivity to nitroglycerin, which is commonly prescribed in patients with cardiovascular disease. In this review, we discuss the genetic susceptibility and pathophysiology associated with the ALDH2*2 variant in regards to cardiovascular disease. We also present the considerations for the management of heart disease and stroke specific to East Asians carrying the ALDH2*2 genetic variant.
Keywords: Aldehyde dehydrogenase, Aldehyde dehydrogenase 2 protein, Cardiotonic agents, Ischemia, Myocardial reperfusion injury, Nitroglycerin, Rat
INTRODUCTION
Precision medicine is the tailored approach to the prevention and management of disease based on the patient’s genetics, environment, and lifestyle. As we gain a better understanding of genetic susceptibility with regard to the pathophysiology of disease, we may expand the application of precision medicine to specific patient populations. The Obama Administration introduced the precision medicine initiative in 2015 with the goal of improving how we prevent and treat disease that is not just for the standard patient.[1] With the precision medicine initiative, it was also stressed there is an unmet need to improve the quality of health care for Asian Americans; a patient population with the highest projected growth in the United States over the next 50 years.[2,3]
For the Asian–American population, cardiovascular disease is the leading cause of death[4] with stroke and ischemic heart disease becoming the leading causes of years of life lost in China.[5] However, our primary understanding of cardiovascular disease has stemmed from an understanding of Western society genetics and lifestyles. For example, the pivotal Framingham Heart Study that defined the risk factors for cardiac disease that shaped much of our current public health guidelines consisted of a large cohort that was exclusively of European American ancestry.[6] Further, a large cohort study of patients undergoing emergent angiography that presented with acute coronary syndrome across the United States recently showed that Asians were more likely than other ethnicities to have arrhythmias, heart failure, shock, and in-hospital mortality.[7] However, the mechanism underlying this difference occurring for Asians versus non-Asian patients is not clear. Given than the Asian–American population will comprise nearly 10% of the United States population by 2060,[2] it is imperative to establish a better understanding of the genetic variants within the Asian population to better improve treatment strategies in the era of precision medicine.
A common genetic variant in the East-Asian population is the point mutation in the mitochondrial enzyme, aldehyde dehydrogenase 2 (ALDH2), and has been implicated in the pathophysiology of several diseases across many organ systems.[8] ALDH2 functions in the removal of toxic aldehydes from the cell. For example, acetaldehyde, an intermediate of ethanol metabolism is converted by ALDH2 to acetic acid. However, a glutamate to lysine substitution at residue 487 (denoted by ALDH2*2) leads to a dramatically decreased activity of ALDH2 in vivo.[9] Having the ALDH2*2 genetic variant results in facial flushing and tachycardia after consuming alcohol. The ALDH2*2 variant affects 8% of the world population and is most commonly found in the Han Chinese population, with a frequency of 40%, in addition to a prevalence of 40% in Japan and 20% in Korea.[10] Importantly, the ALDH2*2 variant is associated with an increased incidence of coronary artery disease,[11–14] myocardial infarction (MI),[15–17] and stroke[18] in East-Asian populations [Table 1]. The ALDH2*2 variant is also associated with an insensitivity to nitroglycerin treatment for angina.[28] To additionally complicate this treatment option, nitroglycerin can also inhibit aldheyde metabolism through ALDH2 and worsen ischemic cardiac damage if given during the ischemic event.[22]
Table 1:
The effect of aldehyde dehydrogenase*2 on cardiovascular disease
| Ischemic heart disease | ↑ Incidence of CAD[11–14] and MI[15–17] in East Asian populations ↑Ischemia/reperfusion injury[19–21] ↓ Cardioprotection from ischemic preconditioning[22,23] and remote ischemic pre/post-conditioning[24–27] ↓ Efficacy of nitroglycerin[28–31] |
| Stroke | ↑ Independent risk factor in Taiwanese men[18] ↑ Reactive aldehydes during ischemia[32] |
| Heart failure | ↓ Cardiac function after ischemic insult[33,34] ↑ ROS and myocyte cell death in diabetes[35–37] |
CAD: Coronary artery disease, MI: Myocardial infarction, ROS: Reactive oxygen species
In this review, we present the findings regarding the pathophysiology, genetic susceptibility, and management of myocardial and cerebral ischemia specific to the East-Asian patient population carrying the ALDH2*2 variant. The role of ALDH2 in ischemia reperfusion injury will be discussed, as this carries particular importance in patients with the ALDH2*2 variant that have baseline higher incidence of cardiovascular disease. Finally, we review the impact the ALDH2 mutation has on the pharmacodynamics of nitroglycerin given its ubiquitous use in the management of myocardial infarction.
ALDEHYDE DEHYDROGENASE 2 INVOLVEMENT IN MYOCARDIAL INFARCTION
Ischemic heart disease remains a leading cause of death worldwide. Treatment strategies for restoring blood flow to the area at risk, either by surgical or percutaneous intervention, paradoxically can induce cardiomyocyte death through reperfusion injury. As such, decades of research on the mechanisms underlying myocardial reperfusion injury have been performed in order to develop methods to protect the viable myocardium from damage.[38] Myocardial reperfusion injury typically manifests as an arrhythmia, reversible contractile dysfunction, microvascular obstruction, or lethal myocardial injury. The common themes underlying these pathologies are oxidative stress, calcium overload, inflammation, and mitochondrial dysfunction. Reactive aldehydes, such as 4-hydroxynonenal (4-HNE) play an important role in the pathology of ischemia-reperfusion and impair mitochondrial function, inhibit contractility, induce arrhythmias, and worsen damage after ischemia.[39,40] However, we do not currently have a treatment to confer cardioprotection during ischemia and reperfusion.
Murry et al. first demonstrated the concept of ischemic preconditioning (IPC), whereby the infarct size could be reduced by the cycling short periods of coronary occlusion and reperfusion immediately before a sustained occlusion.[41] Remote IPC applies the concept of IPC to a more accessible peripheral organ or tissue, such as a limb. Similarly, remote ischemic postconditioning elicits cardioprotection by the episodes of ischemia/reperfusion at the time of reperfusion. The cellular signaling pathways involved in cardioprotection have been extensively reviewed,[39,40,42] and this review will highlight some of these mechanisms.
It has been known for the decades that volatile anesthetics provide cardioprotective effects when given prior to an ischemia-reperfusion event.[43,44] Preconditioning with a volatile anesthetic, such as isoflurane, reduces infarct size and preserves contractile function following myocardial ischemia and reperfusion through protein kinase C epsilon (PKCε).[45] Data suggest that the cardioprotective effect of isoflurane is secondary to increased ALDH2 activity as a result of phosphorylation by PKCε after it translocates to the mitochondria.[46] However, the benefit of volatile anesthetics as cardioprotective agents in clinical trials is inconclusive.[47]
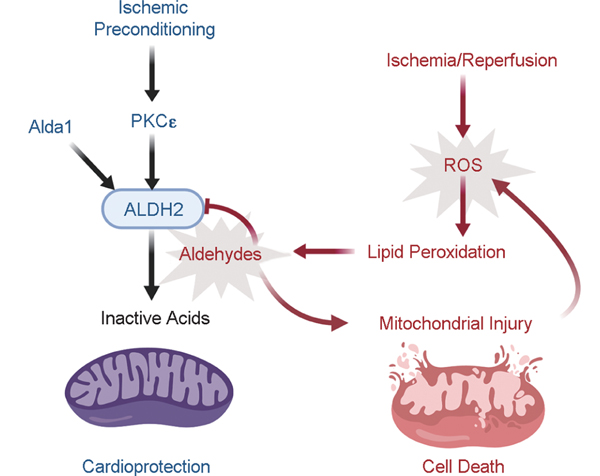
Mounting evidence supports that ALDH2 is intimately involved in the endogenous cytoprotection induced by IPC[23] [Figure 1]. PKCε is an important mediator of preconditioning, as its inhibition eliminates the protective effect of preconditioning and activation stimulates cardioprotection.[24,48] Phosphorylation of ALDH2 mediated by PKCε increases its catalytic activity by 33% with a 49% reduction of infarct size, and inhibition of PKCε decreases ALDH2 activity and worsens cardiac function after ischemia.[22] Ethanol mimics IPC-induced cardioprotection and ALDH2 is the downstream enzyme responsible.[25] Chen et al. demonstrated that the inhibition of ALDH2 by cyanamide significantly increased myocardial infarct size by 49% and abolished cardiac protection from ischemia.[22] Alda-1 is an activator of both wild-type ALDH2*1 and ALDH2*2 that increases its ability to metabolize aldehydes. Alda-1 reduced infarct size by 60% when given 5 min prior to ischemia, importantly solidifying an inverse correlation between ALDH2 activity and cardiac damage from ischemia. Furthermore, Alda-1 improves cardiac function postmyocardial arrest in a rat model of extracorporeal membrane oxygen cardiac bypass.[26]
Figure 1:
Aldehyde dehydrogenase 2 is protective against ischemia-reperfusion injury. Ischemia and reperfusion generate reactive oxygen species (ROS), which leads to the generation of toxic aldehydes, such as 4-HNE. The increased aldehydic load in the cell causes mitochondrial injury, further contributing to generation of ROS, and ultimately cell death. ALDH2 metabolizes aldehydes to inactive acids and is cytoprotective during ischemia/reperfusion. ALDH2 is activated by PKC to exert its protective effects during IPC. Alda-1 is a pharmacologic activator of ALDH2 that prevents the detrimental effects of ischemia-reperfusion injury.
In addition to a cardioprotective role during IPC, there is evidence that ALDH2 activity is necessary for remote, preconditioning, and postconditioning as well. The cardioprotection conferred by remote preconditioning is attenuated by pharmacologic and genetic inhibition of ALDH2 in both humans and rabbits.[27,49] Remote ischemia applied just before reperfusion is also cardioprotective, which is associated with upregulated ALDH2 expression.[19,50] These important studies solidified a role for ALDH2 in the pathophysiology of ischemic and remote preconditioning in small animals and humans. The discovery of Alda-1 also provides a potential pharmacologic agent to mimic IPC, especially to restore enzymatic activity to the E487K mutant ALDH2*2 at higher risk for ischemic injury.
It is likely that ALDH2 exhibits cardioprotective effects by removing toxic aldehydes, like 4-HNE, that develop during ischemia. ALDH2 catalyses the oxidation of 4-HNE to 4-hydroxynonenoic acid in cardiomyocyte mitochondria, and this reaction is compromised during ischemia by decreasing the NAD+/NADH ratio.[51] NAD plays a crucial role in energy metabolism, mitochondrial function, and the cellular reducing potential since NADH is a major electron donor to the electron transport chain. Importantly, NAD+ is a cofactor for aldehyde oxidation by ALDH2, so the imbalance of NAD+/NADH during ischemia creates a cycle of inactive ALDH2, buildup of reactive aldehydes and a further decrease of the NAD+/NADH ratio. As the concentration of 4-HNE increases during ischemia, ALDH2 is also inhibited by it, placing an additional oxidative stress on the cell. Hill et al. showed that 4-HNE exhausts cardiomyocyte mitochondrial reserve capacity and promotes bioenergetic stress.[35] Increased concentrations of 4-HNE promote glycolysis and aerobic respiration, placing further stress on cells that are under ischemic conditions. Thus, 4-HNE increases oxygen consumption/demand while at the same time decreasing efficiency by damaging mitochondrial respiratory complexes and overall depleting the bioenergetic reserve capacity. The genetic and pharmacologic inhibition of ALDH2 also reduces mitochondrial respiratory reserve capacity,[36] making it difficult for the vulnerable cardiomyocyte to handle further oxidative stress. The recent development of a compound that increases NAD+ may potentially allow for an additional pharmaceutical approach to enhance the metabolic and oxidative capacity of cardiomyocytes during ischemia.[37]
The oxidative stress placed on the cells is particularly important in diabetes, since it is responsible for many of the complications seen in the disease. Therefore, the models of diabetes or hyperglycemia can further elucidate the role of ALDH2 under physiologic stress. A study in diabetic rats demonstrated higher intracellular ROS from isolated cardiomyocytes is secondary to a decreased mitochondrial ALDH2 activity.[20] In a model of streptozotocin-induced diabetes, both the expression and activity of ALDH2 were reduced, when ALDH2 was induced by Alda1, the cardiac and mitochondrial function improved.[21] Furthermore, inhibition of ALDH2 with disulfiram increased the concentration of ROS and 4-HNE, reduced mitochondrial membrane potential and respiration, and induced cell death in cardiomyocytes subjected to 33 mM of D-glucose versus control (33 mM mannitol).[52]
ALDH2 also has a regulatory role on the signaling cascades activated upon ischemia and reperfusion. The detrimental effects of ischemia-reperfusion to the myocardium are augmented in a knockout ALDH2 mouse model,[53] and there was less activation of the cardioprotective autophagy signaling pathway compared to wild type. Periods of ischemia markedly increased AMPK phosphorylation in wild type mouse hearts, which was even more pronounced with ALDH2 overexpression, whereas Akt phosphorylation increased during the period of reperfusion. It was suggested that the regulatory role of ALDH2 in this autophagy pathway is related to its ability to detoxify 4-HNE that would otherwise contribute to the phosphorylation of AMPK/Akt upstream PTEN and LKB1. Under ischemic conditions, ALDH2*2 induced pluripotent stem cell-derived cardiomyocytes had significantly higher levels of ROS and apoptosis, with a strong increase in expression of c-Jun, likely by ROS-mediated activation of JNK and an increase in c-Jun-mediated cell death.[54] This was supported by the improvement of cell survival to wild type control levels with JNK inhibition in ALDH2*2-induced pluripotent stem cell-derived cardiomyocytes.
The cardioprotective effects of ALDH2 may also be related to its involvement in regulating the renin-angiotensin system (RAS). Phosphorylation of ALDH2 mediated by PKCε increases its catalytic activity, which then reduces aldehyde-induced cardiac mast cell renin release and prevention of RAS activation.[55,56] Importantly, the activation of RAS increases norepinephrine release and may be responsible for reperfusion-induced arrhythmias. Ischemia reperfusion in isolated guinea pig hearts caused large increases in renin and norepinephrine release with subsequent severe arrhythmias that was severely blunted with IPC.[56] In addition, the activation of the Gi-coupled sphingosine-1-phosphate receptor through AKT on the mast cell during ischemia-reperfusion demonstrated cardioprotective effects by opposing the activation of RAS.[33] Together, the activation of ALDH2 reduces norepinephrine release by preventing the activation of RAS.
ALDH2 is also involved in the cardiac remodeling and dysfunction following a myocardial infarction.[34,57] Genetic overexpression of ALDH2 in mice is protective against the development of post-MI heart failure, whereas an ALDH2 knockout mouse had significantly worse function post-MI as measured by echocardiography compared to wild type.[32] Chronic activation (6 weeks) of ALDH2 by Alda-1 in rats 4 weeks after an MI improved cardiac function and suppressed fibrosis and hypertrophy commonly seen in post-MI heart failure.[58] Both of these studies reported decreased 4-HNE-protein adducts with increased ALDH2 activity, which supports a role for aldehydes in the pathogenesis of postischemic heart dysfunction. Furthermore, Alda-1 improved mitochondrial bioenergetics and decreased ROS in failing hearts.[58] Together, ALDH2 is critical for cardioprotection both during ischemia-reperfusion and against the development of heart failure after the initial ischemic insult. The studies of ALDH2 as a cardioprotective contributor in rodents have paved the way for future studies to explore targeting ALDH2 activation in humans as a strategy to reduce aldehydic load.
ALDEHYDE DEHYDROGENASE 2 INVOLVEMENT IN STROKE
China has unusually the high levels of stroke compared with countries with similar sociodemographic indices. Since the treatment of infectious, nutritional, and maternal/neonatal conditions improved, stroke is now the leading cause of years of life lost in China.[5] Similar to the disparity of outcomes in cardiovascular disease, compared to Non-Hispanic Whites, Asian Americans experience higher proportionate mortality from cerebrovascular disease.[59] In fact, ALDH2*2 is an independent risk factor for ischemic stroke in Taiwanese men.[18] There is evidence that the activation of ALDH2 is protective against ischemic stroke by the removal of aldehydes, particularly 4HNE, during ischemia in the brain.[60] Interestingly, these results were confirmed in a small cohort where the levels of plasma 4-HNE were significantly higher in patients suffering from an ischemic stroke, both before and after the ischemic insult compared to controls. Similar to myocardial ischemia, ethanol provided protection against ischemic stroke by PKCε activation of ALDH2. Volatile anesthetics also demonstrate neuroprotective effects as isoflurane preconditioning decreased infarct volume and lessened damaging microglial activation.[29]
CONSIDERATIONS FOR MI TREATMENT
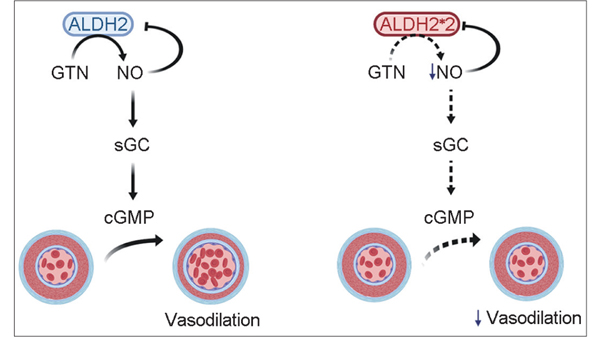
Nitroglycerin or glyceryl trinitrate (GTN), is widely used as a first line agent in management of acute angina in many different countries given its vasodilatory actions.[61] However, the efficacy of sublingual GTN in relieving angina is variable and many patients develop tolerance to chronic therapy, which may be related to impaired bioactivation of GTN to its active vasodilator metabolite, NO.[30,62,63] Chen et al. discovered that the reductase activity of ALDH2 is responsible for GTN bioactivation, and therefore the desired effect of NO release.[31] Specifically, GTN binds to the active site of ALDH2 in a position for nucleophilic attack of Cys-302 to catalyze its conversion to 1,2-GDN and NO.[64] Given the important role of ALDH2 in the formation of NO [Figure 2], it was necessary to study the efficacy of GTN with an inactive ALDH2*2 variant. Pharmacologic and genetic inhibition of ALDH2 in humans demonstrated a decrease in response to GTN measured by forearm blood flow,[65] which is similar to a decrease in GTN-induced vasodilation in a knockout ALDH2−/−mouse.[66] Importantly, this was not due to a change in efficacy of nitrates, as there was no difference in flow with the control vasodilator, nitroprusside. In addition, patients carrying at least one ALDH2*2 allele had a lower vasodilatory response to GTN compared to patients with wild-type ALDH2.[28] Interestingly, Alda-1 (ALDH2 activator) was unable to rescue the decreased efficacy of GTN in cells expressing ALDH2*2.[67] Beyond the vasodilatory effects of GTN, there is evidence to suggest that GTN also has an influence on the pathophysiology of ischemia-reperfusion in cardiomyocytes. In fact, continuous treatment with GTN during ischemia augments cardiac ischemic damage and infarct size in rodents.[22,68] The sustained treatment with GTN inhibits ALDH2 activity, which is the likely mechanism underlying the detrimental effects of GTN during ischemia. Although Alda-1 was unable to augment the vasodilatory effects of GTN, the pretreatment of Alda-1 was able to prevent GTN-induced cardiac damage during ischemia.[68] These observations highlight the importance of familiarity with ALDH2 and GTN metabolism, as patients with the ALDH2*2 enzyme may not respond to GTN and have worse cardiovascular outcomes following GTN treatment during a suspected myocardial infarction.
Figure 2:
Aldehyde dehydrogenase 2ALDH2 is required for the bioactivation of nitroglycerin. The release of nitric oxide (NO) from nitroglycerin, or glyceryl trinitrate (GTN), requires ALDH2 (Left). NO activates soluble guanylate cyclase to form cyclic guanosine monophosphate and produce vasodilation. There is negative feedback from NO to inhibit ALDH2 activity. The ALDH2*2 variant decreases the vasodilatory response of nitroglycerin compared to wild-type since it is unable to bioactivate GTN and release NO (Right).
Aside from treatment of acute angina, the vasodilatory effects of GTN can lower blood pressure during a suspected ischemic stroke. There have been conflicting studies in patients receiving GTN with the onset of stroke symptoms in terms of recovery of function. The Rapid Intervention with Glyceryl Trinitrate in Hypertensive Stroke (RIGHT) was a small (41 patients) randomized control ambulance-based trial of patients with hypertensive acute stroke given transdermal GTN for 7 days versus nothing (blinded with gauze dressing) and compared systolic blood pressure, death, function, and adverse events in the acute setting. The RIGHT study suggested that GTN improves functional outcome, however the sample was underpowered and the ethnicity of each patient was not reported.[69] The follow-up RIGHT-2 trial recently demonstrated that prehospital GTN had a tendency to harm, showing more severe stroke scales, intracerebral hemorrhage, and more acute stroke onset.[70] Another multicenter randomized control trial of patients with an acute ischemic or hemorrhagic stroke did not show harm with GTN treatment, although it did not show any benefit either.[71] There needs to be further studies of GTN use during an acute ischemic stroke, with particular attention paid to genetics. The ALDH2*2 variant may negatively impact the outcome of an ischemic stroke in patients treated with GTN given the evidence described in this review.
CONCLUSION
Awareness of the essential role for ALDH2 in cardiovascular health, beyond its effects on alcohol metabolism, will undoubtedly improve health care for the growing East-Asian population in the United States and globally. ALDH2 is both cardioprotective and neuroprotective, highlighting the important consequences of ischemia in the presence of an inactive ALDH2 enzyme, ALDH2*2 that is highly prevalent in those of East Asian descent. The treatment of heart disease and stroke with nitroglycerin is common practice, so it is critical to consider that the ALDH2 enzyme is responsible for converting nitroglycerin to the active metabolite NO. The pharmacologic activation of ALDH2 may prove beneficial in future management of cardiovascular disease, especially in patients carrying an inactive mutation of this enzyme, ALDH2*2.
BULLET POINTS.
Reactive aldehyde metabolism by ALDH2 is an important mediator of ischemic damage to the heart and brain
When considering cardiovascular health, physicians should account for the ALDH2 variant in East Asians which can severely limit reactive aldehyde metabolism in addition to the metabolism of certain drugs, such as nitroglycerin
Future clinical studies on modulating ALDH2 to improve reactive aldehyde metabolism during ischemia are warranted
Acknowledgments
Financial support and sponsorship
Supported by the National Institutes of Health, National Institutes of General Medical Science GM119522 (ERG) and GM089626 (CLG).
Footnotes
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Fact Sheet: President Obama’s Precision Medicine Initiative; 2015. Available from: https://obamawhitehouse.archives.gov/thepress-office/2015/01/30/fact-sheet-president-obama-s-precisionmedicine-initiative. [Last acessed on 2020 Jan 07].
- 2.Colby SL, Ortma JM. Projections of the Size and Composition of the U.S. Population: 2014 to 2060. Current Population Reports [EB/OL]. [2020-xx-xx]. https://www.researchgate.net/publication/312449644_Projections_of_the_Size_and_Composition_of_the_US_Population_2014_to_2060_Current_Population_Reports. [Google Scholar]
- 3.Initiative on Asian Americans and Pacific Islanders. The White House. Available from: https://obamawhitehouse.archives.gov/node/14486. [Last accessed on 2019 Dec 06].
- 4.Correction to: Heart disease and stroke statistics-2018 update: A report from the American Heart Association. Circulation 2018;137:e493. doi: 10.1161/CIR.0000000000000573. [DOI] [PubMed] [Google Scholar]
- 5.Zhou M, Wang H, Zeng X, Yin P, Zhu J, Chen W, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019;394:1145–58. doi: 10.1016/S0140-6736(19)30427-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsao CW, Vasan RS. Cohort Profile: The Framingham Heart Study (FHS): Overview of milestones in cardiovascular epidemiology. Int J Epidemiol 2015;44:1800–13. doi: 10.1093/ije/dyv337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shaw LJ, Shaw RE, Merz CN, Brindis RG, Klein LW, Nallamothu B, et al. Impact of ethnicity and gender differences on angiographic coronary artery disease prevalence and in-hospital mortality in the American College of Cardiology-National Cardiovascular Data Registry. Circulation 2008;117:1787–801. doi: 10.1161/CIRCULATIONAHA.107.726562. [DOI] [PubMed] [Google Scholar]
- 8.Gross ER, Zambelli VO, Small BA, Ferreira JC, Chen CH, Mochly-Rosen D. A personalized medicine approach for Asian Americans with the aldehyde dehydrogenase 2*2 variant. Annu Rev Pharmacol Toxicol 2015;55:107–27. doi: 10.1146/annurevpharmtox-010814-124915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Larson HN, Zhou J, Chen Z, Stamler JS, Weiner H, Hurley TD. Structural and functional consequences of coenzyme binding to the inactive Asian variant of mitochondrial aldehyde dehydrogenase: Roles of residues 475 and 487. J Biol Chem 2007;282:12940–50. doi: 10.1074/jbc.M607959200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li H, Borinskaya S, Yoshimura K, Kal’ina N, Marusin A, Stepanov VA, et al. Refined geographic distribution of the oriental ALDH2*504Lys (nee 487Lys) variant. Ann Hum Genet 2009;73:335–45. doi: 10.1111/j.1469-1809.2009.00517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang LL, Wang YQ, Fu B, Zhao SL, Kui Y. Aldehyde dehydrogenase 2 (ALDH2) polymorphism gene and coronary artery disease risk: A meta-analysis. Genet Mol Res 2015;14:18503–14. doi: 10.4238/2015.December.23.38. [DOI] [PubMed] [Google Scholar]
- 12.Gu J, Li L. Reply to: ALDH2 Glu504Lys polymorphism and susceptibility to coronary artery disease and myocardial infarction in East Asians: A meta-analysis. Arch Med Res 2014;45:281. doi: 10.1016/j.arcmed.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Han H, Wang H, Yin Z, Jiang H, Fang M, Han J. Association of genetic polymorphisms in ADH and ALDH2 with risk of coronary artery disease and myocardial infarction: A meta-analysis. Gene 2013;526:134–41. doi: 10.1016/j.gene.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 14.Xu F, Sun Y, Shang R, Li M, Cui L, Cui Z, et al. The Glu504Lys polymorphism of aldehyde dehydrogenase 2 contributes to development of coronary artery disease. Tohoku J Exp Med 2014;234:143–50. doi: 10.1620/tjem.234.143. [DOI] [PubMed] [Google Scholar]
- 15.Jo SA, Kim EK, Park MH, Han C, Park HY, Jang Y, et al. A Glu487Lys polymorphism in the gene for mitochondrial aldehyde dehydrogenase 2 is associated with myocardial infarction in elderly Korean men. Clin Chim Acta 2007;382:437. doi: 10.1016/j.cca.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 16.Takagi S, Iwai N, Yamauchi R, Kojima S, Yasuno S, Baba T, et al. Aldehyde dehydrogenase 2 gene is a risk factor for myocardial infarction in Japanese men. Hypertens Res 2002;25:677–81. doi: 10.1291/hypres.25.677. [DOI] [PubMed] [Google Scholar]
- 17.Yasue H, Mizuno Y, Harada E. Association of East Asian Variant Aldehyde Dehydrogenase 2 Genotype (ALDH2*2*) with Coronary Spasm and Acute Myocardial Infarction. Adv Exp Med Biol. 2019;1193:121–134. doi: 10.1007/978-981-13-62606_7. Available from: http://med.stanford.edu/grosslab.html. [Last accessed on 2020 Jan 21]. [DOI] [PubMed] [Google Scholar]
- 18.Sung YF, Lu CC, Lee JT, Hung YJ, Hu CJ, Jeng JS, et al. Homozygous ALDH2*2 is an independent risk factor for ischemic stroke in Taiwanese men. Stroke 2016;47:2174–9. doi: 10.1161/STROKEAHA.116.013204. [DOI] [PubMed] [Google Scholar]
- 19.Zhang ZX, Li H, He JS, Chu HJ, Zhang XT, Yin L. Remote ischemic postconditioning alleviates myocardial ischemia/reperfusion injury by up-regulating ALDH2. Eur Rev Med Pharmacol Sci 2018;22:6475–84. doi: 10.26355/eurrev_201810_16061. [DOI] [PubMed] [Google Scholar]
- 20.Wang J, Wang H, Hao P, Xue L, Wei S, Zhang Y, et al. Inhibition of aldehyde dehydrogenase 2 by oxidative stress is associated with cardiac dysfunction in diabetic rats. Mol Med 2011;17:1729. doi: 10.2119/molmed.2010.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y, Babcock SA, Hu N, Maris JR, Wang H, Ren J. Mitochondrial aldehyde dehydrogenase (ALDH2) protects against streptozotocin-induced diabetic cardiomyopathy: Role of GSK3β and mitochondrial function. BMC Med 2012;10:40. doi: 10.1186/1741-7015-10-40. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Chen CH, Budas GR, Churchill EN, Disatnik MH, Hurley TD, Mochly-Rosen D. Activation of aldehyde dehydrogenase-2 reduces ischemic damage to the heart. Science 2008;321:1493–5. doi: 10.1126/science.1158554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen CH, Sun L, Mochly-Rosen D. Mitochondrial aldehyde dehydrogenase and cardiac diseases. Cardiovasc Res 2010;88:51–7. doi: 10.1093/cvr/cvq192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ytrehus K, Liu Y, Downey JM. Preconditioning protects ischemic rabbit heart by protein kinase C activation. Am J Physiol 1994;266:H1145–52. doi: 10.1152/ajpheart.1994.266.3.H1145. [DOI] [PubMed] [Google Scholar]
- 25.Chen CH, Gray MO, Mochly-Rosen D. Cardioprotection from ischemia by a brief exposure to physiological levels of ethanol: Role of epsilon protein kinase C. Proc Natl Acad Sci U S A 1999;96:12784–9. doi: 10.1073/pnas.96.22.12784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woods C, Shang C, Taghavi F, Downey P, Zalewski A, Rubio GR, et al. In vivo post-cardiac arrest myocardial dysfunction is supported by Ca2+/calmodulin-dependent protein kinase II-mediated calcium long-term potentiation and mitigated by alda-1, an agonist of aldehyde dehydrogenase type 2. Circulation 2016;134:961–77. doi: 10.1161/CIRCULATIONAHA.116.021618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Contractor H, Støttrup NB, Cunnington C, Manlhiot C, Diesch J, Ormerod JO, et al. Aldehyde dehydrogenase-2 inhibition blocks remote preconditioning in experimental and human models. Basic Res Cardiol 2013;108:343. doi: 10.1007/s00395-013-03433. [DOI] [PubMed] [Google Scholar]
- 28.Li Y, Zhang D, Jin W, Shao C, Yan P, Xu C, et al. Mitochondrial aldehyde dehydrogenase-2 (ALDH2) Glu504Lys polymorphism contributes to the variation in efficacy of sublingual nitroglycerin. J Clin Invest 2006;116:506–11. doi: 10.1172/JCI26564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun M, Deng B, Zhao X, Gao C, Yang L, Zhao H, et al. Isoflurane preconditioning provides neuroprotection against stroke by regulating the expression of the TLR4 signalling pathway to alleviate microglial activation. Sci Rep 2015;5:11445. doi: 10.1038/srep11445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferreira JC, Mochly-Rosen D. Nitroglycerin use in myocardial infarction patients. Circ J 2012;76:15–21. doi: 10.1253/circj.cj11-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Z, Zhang J, Stamler JS. Identification of the enzymatic mechanism of nitroglycerin bioactivation. Proc Natl Acad Sci U S A 2002;99:8306–11. doi: 10.1073/pnas.122225199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun A, Zou Y, Wang P, Xu D, Gong H, Wang S, et al. Mitochondrial aldehyde dehydrogenase 2 plays protective roles in heart failure after myocardial infarction via suppression of the cytosolic JNK/p53 pathway in mice. J Am Heart Assoc 2014;3:e000779. doi: 10.1161/JAHA.113.000779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marino A, Sakamoto T, Robador PA, Tomita K, Levi R. S1P receptor 1-Mediated anti-renin-angiotensin system cardioprotection: Pivotal role of mast cell aldehyde dehydrogenase type 2. J Pharmacol Exp Ther 2017;362:230–42. doi: 10.1124/jpet.117.241976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pang J, Wang J, Zhang Y, Xu F, Chen Y. Targeting acetaldehyde dehydrogenase 2 (ALDH2) in heart failure-Recent insights and perspectives. Biochim Biophys Acta Mol Basis Dis 2017;1863:1933–41. doi: 10.1016/j.bbadis.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 35.Hill BG, Dranka BP, Zou L, Chatham JC, Darley-Usmar VM. Importance of the bioenergetic reserve capacity in response to cardiomyocyte stress induced by 4-hydroxynonenal. Biochem J 2009;424:99–107. doi: 10.1042/BJ20090934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mali VR, Deshpande M, Pan G, Thandavarayan RA, Palaniyandi SS. Impaired ALDH2 activity decreases the mitochondrial respiration in H9C2 cardiomyocytes. Cell Signal 2016;28:1–6. doi: 10.1016/j.cellsig.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 37.Yang Y, Mohammed FS, Zhang N, Sauve AA. Dihydronicotinamide riboside is a potent NAD+concentration enhancer in vitro and in vivo. J Biol Chem 2019;294:9295–307. doi: 10.1074/jbc.RA118.005772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hausenloy DJ, Yellon DM. Ischaemic conditioning and reperfusion injury. Nat Rev Cardiol 2016;13:193–209. doi: 10.1038/nrcardio.2016.5. [DOI] [PubMed] [Google Scholar]
- 39.Heusch G Molecular basis of cardioprotection: Signal transduction in ischemic pre-, post-, and remote conditioning. Circ Res 2015;116:674–99. doi: 10.1161/CIRCRESAHA.116.305348. [DOI] [PubMed] [Google Scholar]
- 40.Yellon DM, Downey JM. Preconditioning the myocardium: From cellular physiology to clinical cardiology. Physiol Rev 2003;83:1113–51. doi: 10.1152/physrev.00009.2003. [DOI] [PubMed] [Google Scholar]
- 41.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: A delay of lethal cell injury in ischemic myocardium. Circulation 1986;74:1124–36. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 42.Gerd H, Kerstin B, Rainer S. Cardioprotection. Circulation 2008;118:1915–9. doi: 10.1161/CIRCULATIONAHA.108.805242. [DOI] [PubMed] [Google Scholar]
- 43.Toller WG, Kersten JR, Gross ER, Pagel PS, Warltier DC. Isoflurane preconditions myocardium against infarction via activation of inhibitory guanine nucleotide binding proteins. Anesthesiology 2000;92:1400–7. doi: 10.1097/00000542200005000-00031. [DOI] [PubMed] [Google Scholar]
- 44.Toller WG, Gross ER, Kersten JR, Pagel PS, Gross GJ, Warltier DC. Sarcolemmal and mitochondrial adenosine triphosphate-dependent potassium channels: Mechanism of desflurane-induced cardioprotection. Anesthesiology 2000;92:1731–9. doi: 10.1097/00000542-200006000-00033. [DOI] [PubMed] [Google Scholar]
- 45.Ludwig LM, Weihrauch D, Kersten JR, Pagel PS, Warltier DC. Protein kinase C translocation and Src protein tyrosine kinase activation mediate isoflurane-induced preconditioning in vivo: Potential downstream targets of mitochondrial adenosine triphosphate-sensitive potassium channels and reactive oxygen species. Anesthesiology 2004;100:532–9. doi: 10.1097/00000542200403000-00011. [DOI] [PubMed] [Google Scholar]
- 46.Lang XE, Wang X, Zhang KR, Lv JY, Jin JH, Li QS. Isoflurane preconditioning confers cardioprotection by activation of ALDH2. PLoS One 2013;8:e52469. doi: 10.1371/journal.pone.0052469. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 47.Kunst G, Klein AA. Peri-operative anaesthetic myocardial preconditioning and protection – Cellular mechanisms and clinical relevance in cardiac anaesthesia. Anaesthesia 2015;70:467–82. doi: 10.1111/anae.12975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mitchell MB, Meng X, Ao L, Brown JM, Harken AH, Banerjee A. Preconditioning of isolated rat heart is mediated by protein kinase C. Circ Res 1995;76:73–81. doi: 10.1161/01.res.76.1.73. [DOI] [PubMed] [Google Scholar]
- 49.Ormerod JO, Evans JD, Contractor H, Beretta M, Arif S, Fernandez BO, et al. Human second window pre-conditioning and post-conditioning by nitrite is influenced by a common polymorphism in mitochondrial aldehyde dehydrogenase. JACC Basic Transl Sci 2017;2:13–21. doi: 10.1016/j.jacbts.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu Y, Jia XJ, Zong QF, Zhang GJ, Ye HW, Hu J, et al. Remote ischemic postconditioning protects the heart by upregulating ALDH2 expression levels through the PI3K/Akt signaling pathway. Mol Med Rep 2014;10:536–42. doi: 10.3892/mmr.2014.2156. [DOI] [PubMed] [Google Scholar]
- 51.Hill BG, Awe SO, Vladykovskaya E, Ahmed Y, Liu SQ, Bhatnagar A, et al. Myocardial ischaemia inhibits mitochondrial metabolism of 4-hydroxy-trans-2-nonenal. Biochem J 2009;417:513–24. doi: 10.1042/BJ20081615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pan G, Deshpande M, Thandavarayan RA, Palaniyandi SS. ALDH2 inhibition potentiates high glucose stress-induced injury in cultured cardiomyocytes. J Diabetes Res 2016;2016:1390861. doi: 10.1155/2016/1390861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ma H, Guo R, Yu L, Zhang Y, Ren J. Aldehyde dehydrogenase 2 (ALDH2) rescues myocardial ischaemia/reperfusion injury: Role of autophagy paradox and toxic aldehyde. Eur Heart J 2011;32:1025–38. doi: 10.1093/eurheartj/ehq253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ebert AD, Kodo K, Liang P, Wu H, Huber BC, Riegler J, et al. Characterization of the molecular mechanisms underlying increased ischemic damage in the aldehyde dehydrogenase 2 genetic polymorphism using a human induced pluripotent stem cell model system. Sci Transl Med 2014;6:255ra130. doi: 10.1126/scitranslmed.3009027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aldi S, Takano K, Tomita K, Koda K, Chan NY, Marino A, et al. Histamine H4-receptors inhibit mast cell renin release in ischemia/reperfusion via protein kinase C ε-dependent aldehyde dehydrogenase type-2 activation. J Pharmacol Exp Ther 2014;349:508–17. doi: 10.1124/jpet.114.214122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Koda K, Salazar-Rodriguez M, Corti F, Chan NY, Estephan R, Silver RB, et al. Aldehyde dehydrogenase activation prevents reperfusion arrhythmias by inhibiting local renin release from cardiac mast cells. Circulation 2010;122:771–81. doi: 10.1161/CIRCULATIONAHA.110.952481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yavari A, Ashrafian H. Potentiating mitochondrial aldehyde dehydrogenase 2 to treat post-infarction heart failure. Cardiovasc Res 2014;103:429–31. doi: 10.1093/cvr/cvu175. [DOI] [PubMed] [Google Scholar]
- 58.Gomes KM, Campos JC, Bechara LR, Queliconi B, Lima VM, Disatnik MH, et al. Aldehyde dehydrogenase 2 activation in heart failure restores mitochondrial function and improves ventricular function and remodelling. Cardiovasc Res 2014;103:498–508. doi: 10.1093/cvr/cvu125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jose PO, Frank AT, Kapphahn KI, Goldstein BA, Eggleston K, Hastings KG, et al. Cardiovascular disease mortality in Asian Americans. J Am Coll Cardiol 2014;64:2486–94. doi: 10.1016/j.jacc.2014.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guo JM, Liu AJ, Zang P, Dong WZ, Ying L, Wang W, et al. ALDH2 protects against stroke by clearing 4-HNE. Cell Res 2013;23:915–30. doi: 10.1038/cr.2013.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thadani U Management of stable angina – Current guidelines: A critical appraisal. Cardiovasc Drugs Ther 2016;30:419–26. doi: 10.1007/s10557-016-6681-2. [DOI] [PubMed] [Google Scholar]
- 62.Mayer B, Beretta M. The enigma of nitroglycerin bioactivation and nitrate tolerance: News, views and troubles. Br J Pharmacol 2008;155:170–84. doi: 10.1038/bjp.2008.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Daiber A, Wenzel P, Oelze M, Schuhmacher S, Jansen T, Münzel T. Mitochondrial aldehyde dehydrogenase (ALDH-2)--maker of and marker for nitrate tolerance in response to nitroglycerin treatment. Chem Biol Interact 2009;178:40–7. doi: 10.1016/j.cbi.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 64.Lang BS, Gorren AC, Oberdorfer G, Wenzl MV, Furdui CM, Poole LB, et al. Vascular bioactivation of nitroglycerin by aldehyde dehydrogenase-2: Reaction intermediates revealed by crystallography and mass spectrometry. J Biol Chem 2012;287:38124–34. doi: 10.1074/jbc.M112.371716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mackenzie IS, Maki-Petaja KM, McEniery CM, Bao YP, Wallace SM, Cheriyan J, et al. Aldehyde dehydrogenase 2 plays a role in the bioactivation of nitroglycerin in humans. Arterioscler Thromb Vasc Biol 2005;25:1891–5. doi: 10.1161/01.ATV.0000179599.71086.89. [DOI] [PubMed] [Google Scholar]
- 66.Chen Z, Foster MW, Zhang J, Mao L, Rockman HA, Kawamoto T, et al. An essential role for mitochondrial aldehyde dehydrogenase in nitroglycerin bioactivation. Proc Natl Acad Sci U S A 2005;102:12159–64. doi: 10.1073/pnas.0503723102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Beretta M, Gorren AC, Wenzl MV, Weis R, Russwurm M, Koesling D, et al. Characterization of the East Asian variant of aldehyde dehydrogenase-2: Bioactivation of nitroglycerin and effects of Alda-1. J Biol Chem 2010;285:943–52. doi: 10.1074/jbc.M109.014548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sun L, Ferreira JC, Mochly-Rosen D. ALDH2 activator inhibits increased myocardial infarction injury by nitroglycerin tolerance. Sci Transl Med 2011;3:107ra111. doi: 10.1126/scitranslmed.3002067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ankolekar S, Fuller M, Cross I, Renton C, Cox P, Sprigg N, et al. Feasibility of an ambulance-based stroke trial, and safety of glyceryl trinitrate in ultra-acute stroke: The rapid intervention with glyceryl trinitrate in Hypertensive Stroke Trial (RIGHT, ISRCTN66434824). Stroke 2013;44:3120–8. doi: 10.1161/STROKEAHA.113.001301. [DOI] [PubMed] [Google Scholar]
- 70.Bath PM, Scutt P, Anderson CS, Appleton JP, Berge E, Cala L, et al. Prehospital transdermal glyceryl trinitrate in patients with ultra-acute presumed stroke (RIGHT-2): An ambulance-based, randomised, sham-controlled, blinded, phase 3 trial. Lancet 2019;393:1009–20. doi: 10.1016/S0140-6736(19)30194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.ENOS Trial Investigators. Efficacy of nitric oxide, with or without continuing antihypertensive treatment, for management of high blood pressure in acute stroke (ENOS): A partialfactorial randomised controlled trial. Lancet 2015;385:617–28. doi: 10.1016/S0140-6736(14)61121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]