Abstract
Neurotrophic factors (NTFs) have the unique potential to support neuronal survival and to augment neuronal function in the injured and diseased nervous system. Numerous studies conducted over the last 20 years have provided evidence for the potent therapeutic potential of NTFs in animal models of neurodegenerative diseases. However, major obstacles for the therapeutic use of NTFs are the inability to deliver proteins across the blood‐brain‐barrier, and dose‐limiting adverse effects resulting from the broad exposure of nontargeted structures to NTFs. Two recent developments have allowed NTFs’ promise to be truly tested for the first time: first, recent improvements in viral vectors that allow the targeted delivery of NTFs while providing a long‐lasting supply and sufficient therapeutic doses of NTFs; and second, improved animal models developed in recent years. In this review, we will discuss some of the potential therapeutic applications of NTFs in neurodegenerative diseases and the potential contribution of disturbed neurotrophic factor signaling to neurodegenerative diseases.
INTRODUCTION
Substantial progress has been made over the last two decades in elucidating the neurobiological function of neurotrophins and other neurotrophic factors (NTFs) during development and adulthood. Since the discovery of nerve growth factor (NGF), the first NTF described, it has become clear that these naturally produced neuron survival‐promoting factors are vital not only for nervous system development but also for the maintenance and functioning of the adult nervous system. The accompanying reviews focus on various aspects of neurotrophins in human neurological disorders (88, 127, 130, 150). In this review, I will also discuss other NTFs that have promise for treatment of neurodegenerative disorders.
The discovery that NTFs support neuronal survival and function in the adult central nervous system (CNS) generated broad interest in the use of these factors to intervene in neurodegenerative diseases. Numerous in vitro studies and in vivo studies in animal models of neuronal degeneration have provided proof‐of‐concept and preclinical data that have led to several clinical trials starting in the early 1990s using peripheral or intracerebroventricular (i.c.v.) protein administration, and continuing to date using more sophisticated means of NTF delivery. Indeed, it has become increasingly clear that the successful implementation of NTF therapy requires a targeted, localized delivery of NTFs to avoid unwanted adverse effects resulting from widespread receptor activation. These insights have led to the first promising clinical trials of NTFs in Alzheimer’s Disease (AD) and Parkinson’s Disease (PD). This review will highlight some of the preclinical studies conducted to date with NTFs in models of AD, PD and other neurodegenerative disorders, and summarize current and previous clinical trials.
More recently, several lines of research have indicated that changes in neurotrophin signaling may also contribute to neuronal degeneration in some CNS disorders (see accompanying review by Twiss et al) (150). Although defects in NTFs or their receptors have not been found to be the underlying cause of neurodegenerative disorders, recent studies suggest that changes in retrograde neurotrophin transport, decreased neurotrophin synthesis, altered processing of proneurotrophins or signaling through p75NTR may contribute as a secondary event to neuronal degeneration in some CNS disorders.
NGF AND AD
NGF was the first neurotrophin that was discovered in the search for neuron survival‐promoting factors in the nervous system. Although initial reports focused on its effects in development and in the peripheral nervous system (PNS), studies indicating expression of NGF in the adult neocortex and hippocampus (32, 84, 137) have concluded that NGF also has important activities in the adult CNS. In the mid‐80’s several groups reported concomitantly that i.c.v. infusions of NGF can prevent the lesion‐induced degeneration of cholinergic neurons in the medial septum (53, 57, 87, 157). As cholinergic neurons in the basal forebrain undergo severe degeneration in AD (122, 154, 155), and this degeneration is likely to contribute to the cognitive decline in AD (13, 123), it was speculated that NGF might have therapeutic potential in preventing or slowing the cognitive decline in AD by targeting the cholinergic component of neuronal degeneration in AD (58).
Subsequent studies confirmed the potent effects of NGF in primate models of lesion‐induced degeneration (40, 76, 79, 145, 146), and importantly also indicated that NGF infusions can ameliorate cholinergic neuronal atrophy and memory deficits in aged rodents, and increase cholinergic activity (49, 50, 99, 100).
Taken together, these reports indicate that NGF is highly potent in preventing cholinergic neuronal degeneration and in augmenting cholinergic function by increasing acetylcholine production. These studies led to a small clinical trial using i.c.v. infusions of NGF, but treatments had to be discontinued because of the development of a pain syndrome in some patients (46, 116). Several animal studies indicated similar adverse effects, including hypophagia, weight loss, Schwann cell hyperplasia, sprouting of sensory and sympathetic neurons, and pain syndromes (69, 92, 129, 156, 158) resulting from the broad exposure of nervous system structures to NGF.
In parallel studies, a means of localized, intraparenchymal NGF delivery was developed using cells genetically modified to express NGF. Using the same models of cholinergic neuronal degeneration described above, cellular grafts serving as biological minipumps next to cholinergic cell bodies were found to be equally effective in preventing lesion‐induced or neurotoxin‐induced degeneration in rodents (37, 71, 102, 126) and primates (40, 79, 147, 148). Cellular NGF delivery was further shown to prevent age‐related neuronal degeneration in rodents (26) and primates (28, 139), and to ameliorate memory deficits in aged memory‐impaired rats (26, 101, 104). Additional safety and dose‐escalation studies in primates confirmed that localized NGF delivery to the basal forebrain by genetically modified fibroblasts is safe and well tolerated (M.H. Tuszynski, unpub. data).
On the basis of these results, a phase I study of ex vivo NGF gene therapy was initiated, enrolling eight subjects with mild AD (149). The aim of this study was primarily to determine whether NGF gene transfer is safe, but secondary outcome measures included fluoro‐deoxy‐glucose positron emission tomography (PET) scans and cognitive testing. The rationale for the enrollment of patients in early to mid‐stage of AD was twofold: first, patients need to able to give informed consent to an invasive experimental treatment; and second, NGF needs to be administered at a time when neurons are still alive to be therapeutically effective. Primary autologous fibroblasts were cultivated from a skin biopsy from each patient and genetically modified to express NGF. The study design included a staggered entry with a 3‐month surgery delay between patients, and a dose escalation, with the first patients receiving only unilateral injections, followed by higher cell doses and bilateral cell injections. Cells were stereotactically implanted into the basal forebrain adjacent to the nucleus basalis of Meynert (NBM), which provides cholinergic input throughout the neocortex. Initially, surgeries were performed while patients were only sedated, and abrupt movements during the cell injection resulted in hemorrhages in two patients. General anesthesia in subsequent subjects allowed the safe completion of the study and no adverse events related to NGF delivery were observed. Cognitive testing indicated an improvement in the rate of cognitive decline, in particular after longer time periods post surgery. PET scans in bilaterally treated subjects also indicated an increase in metabolic activity throughout the neocortex, consistent with the widespread modulation of cortical activity from the NBM. In addition, histological analysis of the brain of one of the subjects, who died 5 weeks after the surgery, showed sprouting of cholinergic neurons from the NBM into NGF‐secreting grafts, to a similar extent as previously observed in the primate brain.
These data indicated for the first time that basal forebrain cholinergic neurons (BFCNs) in the Alzheimer’s brain remain responsive to NGF. Although definitive conclusions about the effectiveness of NGF cannot be drawn from this small open label trial, if effects of similar magnitude can be observed in placebo‐controlled, blinded trials, this would represent a significant improvement over current symptomatic treatments.
Since the initiation of the phase I trial described above, substantial improvements in gene therapy and vector design have eliminated the need for labor‐intensive preparation of autologous cells, in vitro gene transfer and cell characterization. Direct in vivo injection of replication‐incompetent viral vectors, such as adeno‐associated virus (AAV) or lentivirus, allows for the localized production of trophic factors in the CNS. Studies using in vivo NGF gene transfer in animal models of cholinergic neuronal degeneration have confirmed the neuroprotective effects of NGF on BFCNs (19, 21, 74, 75, 97, 159, 160). These studies, together with additional safety and toxicology studies (23), have led to a second phase I study sponsored by Ceregene, Inc. to evaluate the safety of AAV–NGF gene transfer in AD. Should this study, conducted at Rush University, Chicago, indicate that NGF delivery by in vivo gene transfer is safe, additional phaseII/III trials will likely provide an answer as to whether NGF gene therapy is a valuable means of reducing cholinergic neuronal degeneration, and whether targeting the cholinergic component of AD can delay the cognitive decline in AD patients.
GDNF FAMILY LIGANDS IN PD
Although a large number of trophic factors have been shown to enhance the in vitro survival of dopaminergic neurons affected in PD (10, 63, 66, 67, 86, 94, 107), recent studies have focused on glial cell‐line derived neurotrophic factor (GDNF) and other members of the same family, as these factors appear to have the most robust effects on dopaminergic neuronal survival (94).
GDNF, the first member of the GDNF family ligands (GFLs) was initially characterized as a highly specific NTF for midbrain dopaminergic neurons (15, 143). Since the discovery of GDNF, several other highly homologous members of this family have been discovered, including neurturin (85), persephin (107) and artemin (10). Each of these molecules signals through the receptor tyrosine kinase ret after binding preferentially to one specific GDNF family receptor of the four family members discovered to date (GDNF family receptor (GFR)‐alpha1‐4). Signal transduction of GDNF is mediated by binding to GFR‐alpha‐1 and to a smaller degree to GFR‐alpha‐2, followed by ret‐induced intracellular phosphorylation events.
GDNF has been tested in a significant number of animal models of PD [reviewed in (73)]. Starting in the mid‐1990s, GDNF injections had been shown to protect dopaminergic neurons in the substantia nigra (SN) from axotomy and from 1‐methyl‐4‐phenyl‐1,2,3,6‐tetrahydropyridine (MPTP)‐induced lesions in rodents (15, 143) and in nonhuman primates (54). Similar effects were obtained using encapsulated GDNF‐producing cells: dopaminergic neurons were rescued and amphetamine‐induced rotational abnormalities were normalized (42, 144).
A subsequent phase I trial injecting GDNF i.c.v. turned out to be not only ineffective but resulted in severe adverse effects, including anorexia, and severe nausea hours to several days after injections; weight loss occurred in the majority of subjects (115). Adverse effects encountered in this trial are not surprising, and clearly a result of the exposure of nontargeted structures to GDNF. As mentioned above, similar adverse effects have been observed with i.c.v. infusions of NGF in rodents and in AD patients. The lack of efficacy resulted from insufficient diffusion of GDNF from the lateral ventricle to the actual target area, the striatum. Thus, the majority of GDNF resided in the cerebrospinal fluid without ever reaching the affected neurons in the SN or their projections in caudate and putamen.
To achieve sufficient concentrations in the striatum, clinical trials of intrastriatal GDNF infusions were initiated showing much more promising outcomes (56, 95, 119, 138), including improvements in the Parkinson’s rating scale, increased dopamine uptake indicated by PET scans and morphological responses in one patient who had died, while adverse effects were mild or absent. A subsequent larger placebo‐controlled trial did not replicate these initial findings (89). However, the mode of GDNF delivery was changed, the dose was lower than in the previous trials, potentially too low to see any clinical benefit, and the patient population was atypical for PD (12). Thus, additional trials are needed to determine whether GDNF will benefit PD patients.
The relatively focused loss of dopaminergic neurons in the SN makes PD an ideal candidate disease for NTF gene therapy. Promising results in models of PD using GDNF delivery by recombinant adenoviral (18, 27, 91), adeno‐associated viral vectors (38, 96, 98) and lentiviral vectors (17, 83) support the view that GDNF gene therapy as a valuable alternative means to localized infusions, which are complicated by shifts in catheter positions, infections, stability of trophic factors and the need to refill infusion pumps.
In addition to GDNF, neurturin, a trophic factor with similar properties as GDNF (85), has been tested in animal models of PD showing efficacy comparable to GDNF (63, 128). Rodent and primate efficacy and toxicology data (14, 24, 34, 61) have led to a phase I trial to test the safety of AAV‐2 mediated neurturin delivery to the striatum of PD patients. Should the phase I trial currently underway at the University of California, San Francisco, and Rush University, Chicago indicate that neurturin gene therapy is safe, blinded, placebo‐controlled phase II/III trials will determine whether neurturin gene therapy can protect dopaminergic neurons from neuronal degeneration and improve motor dysfunction in PD.
NTFS IN HUNTINGTON’S DISEASE (HD)
A large number of NTFs have also been tested as neuroprotective agents in animal models of HD to prevent striatal degeneration of medium spiny neurons. Many studies have been conducted using striatal neurotoxic lesions that do not fully replicate the pathological changes of HD resulting from an autosomal dominant disorder with progressive motor, cognitive and psychiatric disturbances. Since the identification of the genetic defect causing HD (142), transgenic mouse models and viral expression of huntingtin with variable length of CAG poly‐glutamine repeats have allowed a better replication of the pathological changes underlying the disease.
In excitotoxic lesion models, all NTFs tested have indeed been reported to be neuroprotective to a variable degree, including NGF (6, 35, 39, 51, 52, 80, 81, 103, 151), BDNF (52, 103, 121, 152), NT‐3 (6), NT‐4/5 (3), GDNF (7, 120), transforming growth factor‐β (3) and the neuropoetic cytokine CNTF (6, 41, 44). The mechanism of some of the neuroprotective effects observed is not fully established and might be indirect (82). It might be necessary to re‐evaluate some NTFs reported to be neuroprotective after excitotoxic lesions in improved animal models that more closely resemble the human disease. For example, GDNF reported to be effective in excitotoxic lesion models (7, 120) appears to be ineffective in transgenic mouse models overexpressing mutant huntingtin (124).
More recent studies have also evaluated NTF gene transfer using AAV or lentivirus as a means to provide long‐term, localized NTF support in animal models of HD. Overexpression of BDNF, GDNF and CNTF using AAV (72), adenovirus (16, 112) or lentivirus (36, 106, 125) were found to be protective after excitotoxic lesions.
The only NTF tested in a clinical trial in HD patients is CNTF (9, 20). This trial was based on animal studies using CNTF protein delivery (6) or encapsulated CNTF‐producing cells in rodent (41, 43, 45) and primate models (44, 111). The phase I study delivered CNTF into the lateral ventricle of six patients using encapsulated CNTF‐secreting cells. After retrieval of the capsules, low cell survival was observed in about 60% of all retrieved capsules and no clinical benefit was observed in any of the treated subjects. The lack of any clinical benefit might partially be a result of the limited diffusion of CNTF through the ventricular wall into the adjacent putamen (82) similar to the limited diffusion of GDNF after intraventricular injection.
As mentioned earlier, BDNF has also been tested in several animal studies for its neuroprotective effects after neurotoxic lesions. Recent evidence points toward a role of huntingtin in influencing BDNF transport and BDNF expression, providing additional rationale to investigate BDNF as a potential therapeutic molecule in this devastating polyglutamine disease (see below).
NTFS IN ALS
Several NTFs have been found to have potent effects on motor neuron survival in vitro, during development, after injury to motor neuron systems and in genetic models of motor neuron degeneration, providing a rationale to develop NTFs as treatment for ALS, in which ventral motor neuron degeneration is extensive. BDNF, CNTF, insulin‐like growth factor‐1 (IGF‐1) and GDNF have been evaluated in animal models of motor neuron disease or ALS using direct protein delivery, with encouraging results (60, 65, 68, 77, 78, 93, 109, 110, 117, 118, 134, 135, 136, 161, 162, 163). Based on these studies, clinical trials with CNTF (5, 108, 140), BDNF (141) and IGF‐1 (22, 90) have been conducted. To date, these clinical trials have essentially failed, an outcome that is at least partially attributable to the mode of NTF delivery and/or the instability of the administered molecules. The mode of administration likely led to subtherapeutic levels in the CNS or dose‐limiting adverse effects caused by broad distribution centrally or peripherally, including weight loss, severe coughing, fever and muscle wasting.
Intrathecal infusions of CNTF or intrathecal delivery of CNTF using encapsulated heterologous cells producing CNTF in patients with ALS did not lead to the same adverse effects previously reported but also failed to deliver significant clinical benefits, potentially because of inefficient penetration of the spinal cord parenchyma (1, 2).
More recently, improvements in gene therapy vectors and the ability of viral vectors to be retrogradely transported to motor neurons after injection into peripheral muscle targets have shown some promise in transgenic mouse models of ALS. Expression of vascular endothelial growth factor (VEGF) in motor neurons via retrograde transport of a VEGF‐coding lentivirus from muscle (8) and expression of IGF‐1 in motor neurons after AAV‐2 injection into muscle (70) improved animal survival and delayed motor neuron death in transgenic mice overexpressing mutant superoxide dismutase‐1 (SOD‐1). GDNF expressed in muscle after AAV gene transfer was also shown to be retrogradely transported to motor neurons, to delay motor neuron degeneration and to prolong the lifespan of SOD‐1 expressing mice (153).
Re‐evaluation of some previously tested factors such as IGF‐1 in clinical trials with site‐specific delivery may therefore allow for therapeutic doses to be reached in ventral spinal cord motor neurons without adverse effects from widespread CNS and PNS exposure.
NEUROTROPHIN PROCESSING, SIGNALING AND TRANSPORT IN NEURODEGENERATIVE DISORDERS
As mentioned earlier, NTFs, including neurotrophins and GFLs, are vital for nervous system development, indicated by the lethality of many homozygous knockout mice for NTFs or severe developmental abnormalities in the nervous system and other organs of homozygous and heterozygous knockout animals [see (150)].
Although loss of NTF signaling during development and adulthood has clearly been shown to result in developmental deficits and neuronal degeneration, no direct evidence has been provided to link the loss of neurotrophin signaling to the etiology of any specific neurodegenerative disease. Despite the lack of any direct evidence for a role of NTF deficiency as the underlying cause for neurodegenerative diseases, impairment of target‐derived signaling by retrograde transport of NTFs may contribute to neuronal dysfunction and neurodegenerative diseases such as AD, ALS or Huntington’s disease (64, 131) (Figure 1).
Figure 1.
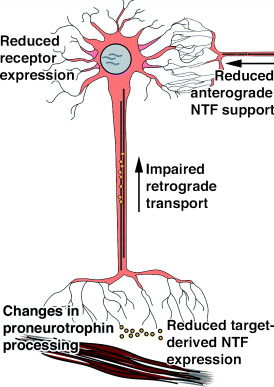
Schematic outline of potential changes in neurotrophic factor (NTF) signaling that could contribute to neuronal degeneration. Reduced NTF expression by peripheral targets or central nervous system target neurons, dysfunctional proneurotrophin processing, diminished receptor expression by affected neuronal populations, or decreased anterograde and retrograde transport of NTFs could impair NTF support and further aggravate neuronal dysfunction.
NGF is expressed in neocortex and hippocampus, and normally retrogradely transported to cholinergic cell bodies in the nucleus basalis and medial septum, respectively. In AD, several studies have indicated that cortical NGF levels cortex are stable (4, 114) or increased (33, 59, 133), whereas NGF levels in the nucleus basalis are decreased, providing evidence for a deficit in retrograde NGF transport from cortical targets back to the basal forebrain. Further support for this hypothesis comes form mouse models of Down’s syndrome, which demonstrate a marked age‐related atrophy of BFCNs, one that can be reversed by NGF administration (62). The degeneration of cholinergic neurons is strongly associated with a highly diminished retrograde transport of NGF in these animals (30), which can be directly linked to the presence of an extra copy of amyloid precursor protein (APP) (132). Diminished retrograde transport of NGF has also been shown in aged rodents (29), one correlational animal model for the cholinergic atrophy observed in AD. Furthermore, decreases in NGF receptor expression by BFCNs precede cholinergic neuronal loss in AD [reviewed in (31)]. Thus, early decreases in receptor expression could result in diminished retrograde NGF transport from the cortex to the nucleus basalis (29, 30, 113) and contribute to neuronal degeneration. Current strategies aimed at augmenting cholinergic function by NGF gene therapy in AD therefore provide NGF directly to cholinergic cell bodies, bypassing the need for long‐distance retrograde transport. One potential concern is that NGF delivery to the cell soma instead of the normal target (cortex) could result in the withdrawal of cortical cholinergic projections. However, data in aged primates indicate that NGF delivery at the cell soma in the basal forebrain increases cholinergic innervation density in the cortex (28).
In HD, recent studies point toward a role of mutant huntingtin in disrupting BDNF expression and transport. Reduced BDNF levels have been found in brain regions most affected in HD patients (caudate and putamen) (48) and in transgenic mice that express human mutant huntingtin (164). Two possible mechanisms underlying these changes have been suggested [reviewed in (105)]. Reduced BDNF gene transcription (164) caused by differential interaction of mutant huntingtin (containing expanded CAG repeats) with the transcription machinery (165) has been reported as one possible explanation. However, it is also possible that mutant huntingtin interferes with the anterograde transport of BDNF from cortex to striatum, contributing to BDNF depletion in the striatum (55). Supporting the hypothesis that defects in BDNF availability in the striatum could contribute to neuronal degeneration also comes from studies in conditional BDNF knockout animals which have shown that depletion of cortical BDNF results in neuronal atrophy of medium spiny neurons, followed by neuronal loss with aging (11). In addition, decreases in BDNF advance the onset of motor dysfunction and neuronal degeneration in a mouse model of HD (25). Taken together, these studies provide a rationale to deliver BDNF into caudate and putamen to prevent or slow the degeneration of medium spiny neurons in HD, but additional animal studies are needed.
Proneurotrophins may also play a role in neurodegeneration. As discussed in more detail in the previous review (150), proneurotrophins appear to have antagonistic functions compared with the mature form of neurotrophins. The significance of proneurotrophins in neurodegenerative diseases remains to be determined. It appears that the majority of NGF found in cortex is pro‐NGF and increased levels of pro‐NGF have been found in AD (47). Whether this increase in pro‐NGF is a result of decreased processing of pro‐NGF, changes in pro‐NGF transport or whether changes in expression underlie the increased levels is unknown. The possibility that proneurotrophins could induce cell death in neurodegenerative diseases following binding to p75NTR is a theoretical possibility that remains to be proven.
CONCLUSIONS
NTF delivery continues to be an attractive neuroprotective treatment strategy for neurodegenerative disorders. As outlined in this review, the means of targeted delivery is one key for the successful implementation of NTF therapy. Advances in viral vectors now allow for a localized, long‐term delivery of NTFs, thereby avoiding adverse effects from the broad exposure of CNS, PNS and other organ systems. Clinical trials to be conducted over the next years will allow us to truly determine whether NTFs are efficacious in delaying or slowing neuronal degeneration, thereby affecting some of the associated cognitive, psychiatric and motor dysfunctions.
ACKNOWLEDGMENTS
Supported by the California Roman Reed SCI Research Fund, International Spinal Research Trust, Wings for Life and the NIH (NS46466; NS047101). Thanks to Ron Alfa for his help in designing Figure 1.
Conflict of interest statement: The author is a scientific advisor of Ceregene, Inc.
REFERENCES
- 1. Aebischer P, Schluep M, Déglon N, Joseph JM, Hirt L, Heyd B, Goddard M, Hammang JP, Zurn AD, Kato AC, Regli F, Baetge EE (1996) Intrathecal delivery of CNTF using encapsulated genetically modified xenogeneic cells in amyotrophic lateral sclerosis patients. Nat Med 2:696–699. [DOI] [PubMed] [Google Scholar]
- 2. Aebischer P, Pochon NA, Heyd B, Deglon N, Joseph JM, Zurn AD, Baetge EE, Hammang JP, Goddard M, Lysaght M, Kaplan F, Kato AC, Schluep M, Hirt L, Regli F, Porchet F, De Tribolet N (1996) Gene therapy for amyotrophic lateral sclerosis (ALS) using a polymer encapsulated xenogenic cell line engineered to secrete hCNTF. Hum Gene Ther 7:851–860. [DOI] [PubMed] [Google Scholar]
- 3. Alexi T, Venero JL, Hefti F (1997) Protective effects of neurotrophin‐4/5 and transforming growth factor‐alpha on striatal neuronal phenotypic degeneration after excitotoxic lesioning with quinolinic acid. Neuroscience 78:73–86. [DOI] [PubMed] [Google Scholar]
- 4. Allen SJ, MacGowan SH, Treanor JJ, Feeney R, Wilcock GK, Dawbarn D (1991) Normal beta‐NGF content in Alzheimer’s disease cerebral cortex and hippocampus. Neurosci Lett 131:135–139. [DOI] [PubMed] [Google Scholar]
- 5. ALS CNTF Treatment Study Group (1996) A double‐blind placebo‐controlled clinical trial of subcutaneous recombinant human ciliary neurotrophic factor (rHCNTF) in amyotrophic lateral sclerosis. Neurology 46:1244–1249. [DOI] [PubMed] [Google Scholar]
- 6. Anderson KD, Panayotatos N, Corcoran TL, Lindsay RM, Wiegand SJ (1996) Ciliary neurotrophic factor protects striatal output neurons in an animal model of Huntington disease. Proc Natl Acad Sci USA 93:7346–7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Araujo DM, Hilt DC (1997) Glial cell line‐derived neurotrophic factor attenuates the excitotoxin‐induced behavioral and neurochemical deficits in a rodent model of Huntington’s disease. Neuroscience 81:1099–1110. [DOI] [PubMed] [Google Scholar]
- 8. Azzouz M, Ralph GS, Storkebaum E, Walmsley LE, Mitrophanous KA, Kingsman SM, Carmeliet P, Mazarakis ND (2004) VEGF delivery with retrogradely transported lentivector prolongs survival in a mouse ALS model. Nature 429:413–417. [DOI] [PubMed] [Google Scholar]
- 9. Bachoud‐Levi AC, Deglon N, Nguyen JP, Bloch J, Bourdet C, Winkel L, Remy P, Goddard M, Lefaucheur JP, Brugieres P, Baudic S, Cesaro P, Peschanski M, Aebischer P (2000) Neuroprotective gene therapy for Huntington’s disease using a polymer encapsulated BHK cell line engineered to secrete human CNTF. Hum Gene Ther 11:1723–1729. [DOI] [PubMed] [Google Scholar]
- 10. Baloh RH, Tansey MG, Lampe PA, Fahrner TJ, Enomoto H, Simburger KS, Leitner ML, Araki T, Johnson EM Jr, Milbrandt J (1998) Artemin, a novel member of the GDNF ligand family, supports peripheral and central neurons and signals through the GFRalpha3‐RET receptor complex. Neuron 21:1291–1302. [DOI] [PubMed] [Google Scholar]
- 11. Baquet ZC, Gorski JA, Jones KR (2004) Early striatal dendrite deficits followed by neuron loss with advanced age in the absence of anterograde cortical brain‐derived neurotrophic factor. J Neurosci 24:4250–4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Barker RA (2006) Continuing trials of GDNF in Parkinson’s disease. Lancet Neurol 5:285–286. [DOI] [PubMed] [Google Scholar]
- 13. Bartus RT, Dean RLd, Beer B, Lippa AS (1982) The cholinergic hypothesis of geriatric memory dysfunction. Science 217:408–414. [DOI] [PubMed] [Google Scholar]
- 14. Bartus RT, Herzog CD, Cunningham JJ, Brandon EP, Wilson A, Hofer EK, Kordower JH, Ostrove JM, Gasmi M (2005) Biological activity of CERE‐120, an AAV2 vector encoding human neurturin, in the rat 6—hydroxydopamine model of nigrostriatal degeneration and in the young intact and aged rat. Abstract Viewer/Itinerary Planner. Washington, DC: Soc Neurosci, 2005. Online: Progr No 545.541.
- 15. Beck KD, Valverde J, Alexi T, Poulsen K, Moffat B, Vandlen RA, Rosenthal A, Hefti F (1995) Mesencephalic dopaminergic neurons protected by GDNF from axotomy‐induced degeneration in the adult brain. Nature 373:339–341. [DOI] [PubMed] [Google Scholar]
- 16. Bemelmans AP, Horellou P, Pradier L, Brunet I, Colin P, Mallet J (1999) Brain‐derived neurotrophic factor‐mediated protection of striatal neurons in an excitotoxic rat model of Huntington’s disease, as demonstrated by adenoviral gene transfer. Hum Gene Ther 10:2987–2997. [DOI] [PubMed] [Google Scholar]
- 17. Bensadoun JC, Deglon N, Tseng JL, Ridet JL, Zurn AD, Aebischer P (2000) Lentiviral vectors as a gene delivery system in the mouse midbrain: cellular and behavioral improvements in a 6‐OHDA model of Parkinson’s disease using GDNF. Exp Neurol 164:15–24. [DOI] [PubMed] [Google Scholar]
- 18. Bilang‐Bleuel A, Revah F, Colin P, Locquet I, Robert JJ, Mallet J, Horellou P (1997) Intrastriatal injection of an adenoviral vector expressing glial‐cell‐line‐derived neurotrophic factor prevents dopaminergic neuron degeneration and behavioral impairment in a rat model of Parkinson disease. Proc Natl Acad Sci USA 94:8818–8823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Blesch A, Conner J, Pfeiffer A, Gasmi M, Britton W, Alfa R, Verma I, Tuszynski MH (2005) Regulated lentiviral NGF gene transfer controls rescue of medial septal cholinergic neurons. Mol Ther 11:916–925. [DOI] [PubMed] [Google Scholar]
- 20. Bloch J, Bachoud‐Levi AC, Deglon N, Lefaucheur JP, Winkel L, Palfi S, Nguyen JP, Bourdet C, Gaura V, Remy P, Brugieres P, Boisse MF, Baudic S, Cesaro P, Hantraye P, Aebischer P, Peschanski M (2004) Neuroprotective gene therapy for Huntington’s disease, using polymer‐encapsulated cells engineered to secrete human ciliary neurotrophic factor: results of a phase I study. Hum Gene Ther 15:968–975. [DOI] [PubMed] [Google Scholar]
- 21. Blömer U, Kafri T, Randolph‐Moore L, Verma IM, Gage FH (1998) Bcl‐xL protects adult septal cholinergic neurons from axotomized cell death. Proc Natl Acad Sci USA 95:2603–2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Borasio GD, Robberecht W, Leigh PN, Emile J, Guiloff RJ, Jerusalem F, Silani V, Vos PE, Wokke JH, Dobbins T (1998) A placebo‐controlled trial of insulin‐like growth factor‐I in amyotrophic lateral sclerosis. European ALS/IGF‐I Study Group. Neurology 51:583–586. [DOI] [PubMed] [Google Scholar]
- 23. Brandon EP, Bishop KM, Hofer EK, Brown L, Kruegel B, Austin M, Printz MA, Gasmi M, Ostrove JM, Tuszynski MH, Bartus RT (2004) Pre‐clinical safety and efficacy studies of adeno‐associated virus (AAV)‐based delivery of human nerve growth factor (NGF) to basal forebrain cholinergic neurons (BFCNS). Abstract Viewer/Itinerary Planner. Washington, DC: Soc Neurosci, 2004. Online: Progr No 216.218.
- 24. Brandon EP, Herzog CD, Wilson A, Stansell J, Loui T, Landers M, Kruegel B, Hofer K, Gammon D, Dass B, Brown L, Bolton A, Cunningham JJ, Gasmi M, Printz MA, Kordower JH, Ostrove JM, Bartus RT (2005) Controlled distribution of vector and neurturin following striatal administration of CERE‐120, an AAV2—based vector being developed for Parkinson’s disease. 2005 Abstract Viewer/Itinerary Planner. Washington, DC: Soc Neurosci Progr No 545.2.
- 25. Canals JM, Pineda JR, Torres‐Peraza JF, Bosch M, Martin‐Ibanez R, Munoz MT, Mengod G, Ernfors P, Alberch J (2004) Brain‐derived neurotrophic factor regulates the onset and severity of motor dysfunction associated with enkephalinergic neuronal degeneration in Huntington’s disease. J Neurosci 24:7727–7739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen KS, Gage FH (1995) Somatic gene transfer of NGF to the aged brain: behavioral and morphological amelioration. J Neurosci 15:2819–2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Choi‐Lundberg DL, Lin Q, Chang YN, Chiang YL, Hay CM, Mohajeri H, Davidson BL, Bohn MC (1997) Dopaminergic neurons protected from degeneration by GDNF gene therapy. Science 275:838–841. [DOI] [PubMed] [Google Scholar]
- 28. Conner JM, Darracq MA, Roberts J, Tuszynski MH (2001) Non‐tropic actions of neurotrophins: subcortical NGF gene delivery reverses age‐related degeneration of primate cortical cholinergic innervation. Proc Natl Acad Sci USA 98:1941–1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cooper JD, Lindholm D, Sofroniew MV (1994) Reduced transport of [125I]nerve growth factor by cholinergic neurons and down‐regulated TrkA expression in the medial septum of aged rats. Neuroscience 62:625–629. [DOI] [PubMed] [Google Scholar]
- 30. Cooper JD, Salehi A, Delcroix JD, Howe CL, Belichenko PV, Chua‐Couzens J, Kilbridge JF, Carlson EJ, Epstein CJ, Mobley WC (2001) Failed retrograde transport of NGF in a mouse model of Down’s syndrome: reversal of cholinergic neurodegenerative phenotypes following NGF infusion. Proc Natl Acad Sci USA 98:10439–10444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Counts SE, Mufson EJ (2005) The role of nerve growth factor receptors in cholinergic basal forebrain degeneration in prodromal Alzheimer disease. J Neuropathol Exp Neurol 64:263–272. [DOI] [PubMed] [Google Scholar]
- 32. Crutcher KA, Collins F (1982) In vitro evidence for two distinct hippocampal growth factors: basis of neuronal plasticity? Science 218:67–68. [DOI] [PubMed] [Google Scholar]
- 33. Crutcher KA, Scott SA, Liang S, Everson WV, Weingartner J (1993) Detection of NGF‐like activity in human brain tissue: increased levels in Alzheimer’s disease. J Neurosci 13:2540–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dass B, Herzog CD, Bakay RAE, Gasmi M, Chen E, Chu Y, Stansell JE, Folino GG, Anderson MV, Bartus RT, Kordower JH (2005) Adeno‐associated virus mediated gene delivery of neurturin (CERE‐120) prevents MPTP—induced motor disability following injection into the striatum of nonhuman primates. 2005 Abstract Viewer/Itinerary Planner. Washington, DC: Soc Neurosci: Program No. 545.546.
- 35. Davies SW, Beardsall K (1992) Nerve growth factor selectively prevents excitotoxin induced degeneration of striatal cholinergic neurones. Neurosci Lett 140:161–164. [DOI] [PubMed] [Google Scholar]
- 36. De Almeida LP, Zala D, Aebischer P, Deglon N (2001) Neuroprotective effect of a CNTF‐expressing lentiviral vector in the quinolinic acid rat model of Huntington’s disease. Neurobiol Dis 8:433–446. [DOI] [PubMed] [Google Scholar]
- 37. Dekker AJ, Winkler J, Ray J, Thal LJ, Gage FH (1994) Grafting of nerve growth factor‐producing fibroblasts reduces behavioral deficits in rats with lesions of the nucleus basalis magnocellularis. Neuroscience 60:299–309. [DOI] [PubMed] [Google Scholar]
- 38. During MJ, Leone P (1997) Targets for gene therapy of Parkinson’s disease: growth factors, signal transduction, and promoters. Exp Neurol 144:74–81. [DOI] [PubMed] [Google Scholar]
- 39. Emerich DF, Hammang JP, Baetge EE, Winn SR (1994) Implantation of polymer‐encapsulated human nerve growth factor‐secreting fibroblasts attenuates the behavioral and neuropathological consequences of quinolinic acid injections into rodent striatum. Exp Neurol 130:141–150. [DOI] [PubMed] [Google Scholar]
- 40. Emerich DF, Winn SR, Harper J, Hammang JP, Baetge EE, Kordower JH (1994) Implants of polymer‐encapsulated human NGF‐secreting cells in the nonhuman primate: rescue and sprouting of degenerating cholinergic basal forebrain neurons. J Comp Neurol 349:148–164. [DOI] [PubMed] [Google Scholar]
- 41. Emerich DF, Lindner MD, Winn SR, Chen EY, Frydel BR, Kordower JH (1996) Implants of encapsulated human CNTF‐producing fibroblasts prevent behavioral deficits and striatal degeneration in a rodent model of Huntington’s disease. J Neurosci 16:5168–5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Emerich DF, Plone M, Francis J, Frydel BR, Winn SR, Lindner MD (1996) Alleviation of behavioral deficits in aged rodents following implantation of encapsulated GDNF‐producing fibroblasts. Brain Res 736:99–110. [PubMed] [Google Scholar]
- 43. Emerich DF, Cain CK, Greco C, Saydoff JA, Hu ZY, Liu H, Lindner MD (1997) Cellular delivery of human CNTF prevents motor and cognitive dysfunction in a rodent model of Huntington’s disease. Cell Transplant 6:249–266. [DOI] [PubMed] [Google Scholar]
- 44. Emerich DF, Winn SR, Hantraye PM, Peschanski M, Chen EY, Chu Y, McDermott P, Baetge EE, Kordower JH (1997) Protective effect of encapsulated cells producing neurotrophic factor CNTF in a monkey model of Huntington’s disease. Nature 386:395–399. [DOI] [PubMed] [Google Scholar]
- 45. Emerich DF, Bruhn S, Chu Y, Kordower JH (1998) Cellular delivery of CNTF but not NT‐4/5 prevents degeneration of striatal neurons in a rodent model of Huntington’s disease. Cell Transplant 7:213–225. [DOI] [PubMed] [Google Scholar]
- 46. Eriksdotter Jönhagen M, Nordberg A, Amberla K, Bäckman L, Ebendal T, Meyerson B, Olson L, Seiger A, Shigeta M, Theodorsson E, Viitanen M, Winblad B, Wahlund LO (1998) Intracerebroventricular infusion of nerve growth factor in three patients with Alzheimer’s disease. Dement Geriatr Cogn Disord 9:246–257. [DOI] [PubMed] [Google Scholar]
- 47. Fahnestock M, Michalski B, Xu B, Coughlin MD (2001) The precursor pro‐nerve growth factor is the predominant form of nerve growth factor in brain and is increased in Alzheimer’s disease. Mol Cell Neurosci 18:210–220. [DOI] [PubMed] [Google Scholar]
- 48. Ferrer I, Goutan E, Marin C, Rey MJ, Ribalta T (2000) Brain‐derived neurotrophic factor in Huntington disease. Brain Res 866:257–261. [DOI] [PubMed] [Google Scholar]
- 49. Fischer W, Wictorin K, Bjorklund A, Williams LR, Varon S, Gage FH (1987) Amelioration of cholinergic neuron atrophy and spatial memory impairment in aged rats by nerve growth factor. Nature 329:65–68. [DOI] [PubMed] [Google Scholar]
- 50. Fischer W, Björklund A, Chen K, Gage FH (1991) NGF improves spatial memory in aged rodents as a function of age. J Neurosci 11:1889–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Frim DM, Short MP, Rosenberg WS, Simpson J, Breakefield XO, Isacson O (1993) Local protective effects of nerve growth factor‐secreting fibroblasts against excitotoxic lesions in the rat striatum. J Neurosurg 78:267–273. [DOI] [PubMed] [Google Scholar]
- 52. Frim DM, Uhler TA, Short MP, Ezzedine ZD, Klagsbrun M, Breakefield XO, Isacson O (1993) Effects of biologically delivered NGF, BDNF and bFGF on striatal excitotoxic lesions. Neuroreport 4:367–370. [DOI] [PubMed] [Google Scholar]
- 53. Gage FH, Armstrong DM, Williams LR, Varon S (1988) Morphological response of axotomized septal neurons to nerve growth factor. J Comp Neurol 269:147–155. [DOI] [PubMed] [Google Scholar]
- 54. Gash DM, Zhang Z, Ovadia A, Cass WA, Yi A, Simmerman L, Russell D, Martin D, Lapchak PA, Collins F, Hoffer BJ, Gerhardt GA (1996) Functional recovery in parkinsonian monkeys treated with GDNF. Nature 380:252–255. [DOI] [PubMed] [Google Scholar]
- 55. Gauthier LR, Charrin BC, Borrell‐Pages M, Dompierre JP, Rangone H, Cordelieres FP, De Mey J, MacDonald ME, Lessmann V, Humbert S, Saudou F (2004) Huntingtin controls neurotrophic support and survival of neurons by enhancing BDNF vesicular transport along microtubules. Cell 118:127–138. [DOI] [PubMed] [Google Scholar]
- 56. Gill SS, Patel NK, Hotton GR, O’Sullivan K, McCarter R, Bunnage M, Brooks DJ, Svendsen CN, Heywood P (2003) Direct brain infusion of glial cell line‐derived neurotrophic factor in Parkinson disease. Nat Med 9:589–595. [DOI] [PubMed] [Google Scholar]
- 57. Hefti F (1986) Nerve growth factor promotes survival of septal cholinergic neurons after fimbrial transections. J Neurosci 6:2155–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hefti F, Weiner WJ (1986) Nerve growth factor and Alzheimer’s disease. Ann Neurol 20:275–281. [DOI] [PubMed] [Google Scholar]
- 59. Hellweg R, Gericke CA, Jendroska K, Hartung HD, Cervos‐Navarro J (1998) NGF content in the cerebral cortex of non‐demented patients with amyloid‐plaques and in symptomatic Alzheimer’s disease. Int J Dev Neurosci 16:787–794. [DOI] [PubMed] [Google Scholar]
- 60. Henderson CE, Phillips HS, Pollock RA, Davies AM, Lemeulle C, Armanini M, Simmons L, Moffet B, Vandlen RA, Simpson LC, Koliatsos VE, Rosenthal A (1994) GDNF: a potent survival factor for motoneurons present in peripheral nerve and muscle. Science 266:1062–1064. [DOI] [PubMed] [Google Scholar]
- 61. Herzog CD, Holden JE, Bakay RA, Stansell J, Folino G, Ostrove JM, Bartus RT, Kordower JH (2005) Enhanced 18F—dopa uptake in the striatum of aged rhesus monkeys following striatal delivery of CERE‐120, an AAV2 vector encoding human neurturin. 2005 Abstract Viewer/Itinerary Planner. Washington, DC: Soc Neurosci. Progr No 545.547.
- 62. Holtzman DM, Li Y, Chen K, Gage FH, Epstein CJ, Mobley WC (1993) Nerve growth factor reverses neuronal atrophy in a Down syndrome model of age‐related neurodegeneration. Neurology 43:2668–2673. [DOI] [PubMed] [Google Scholar]
- 63. Horger BA, Nishimura MC, Armanini MP, Wang LC, Poulsen KT, Rosenblad C, Kirik D, Moffat B, Simmons L, Johnson E Jr, Milbrandt J, Rosenthal A, Bjorklund A, Vandlen RA, Hynes MA, Phillips HS (1998) Neurturin exerts potent actions on survival and function of midbrain dopaminergic neurons. J Neurosci 18:4929–4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Howe CL, Mobley WC (2005) Long‐distance retrograde neurotrophic signaling. Curr Opin Neurobiol 15:40–48. [DOI] [PubMed] [Google Scholar]
- 65. Hughes RA, Sendtner M, Thoenen H (1993) Members of several gene families influence survival of rat motoneurons in vitro and in vivo . J Neurosci Res 36:663–671. [DOI] [PubMed] [Google Scholar]
- 66. Hyman C, Hofer M, Barde YA, Juhasz M, Yancopoulos GD, Squinto SP, Lindsay RM (1991) BDNF is a neurotrophic factor for dopaminergic neurons of the substantia nigra. Nature 350:230–232. [DOI] [PubMed] [Google Scholar]
- 67. Hyman C, Juhasz M, Jackson C, Wright P, Ip NY, Lindsay RM (1994) Overlapping and distinct actions of the neurotrophins BDNF, NT‐3, and NT‐4/5 on cultured dopaminergic and GABAergic neurons of the ventral mesencephalon. J Neurosci 14:335–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ikeda K, Wong V, Holmlund TH, Greene T, Cedarbaum JM, Lindsay RM, Mitsumoto H (1995) Histometric effects of ciliary neurotrophic factor in wobbler mouse motor neuron disease. Ann Neurol 37:47–54. [DOI] [PubMed] [Google Scholar]
- 69. Isaacson LG, Saffran BN, Crutcher KA (1990) Intracerebral NGF infusion induces hyperinnervation of cerebral blood vessels. Neurobiol Aging 11:51–55. [DOI] [PubMed] [Google Scholar]
- 70. Kaspar BK, Llado J, Sherkat N, Rothstein JD, Gage FH (2003) Retrograde viral delivery of IGF‐1 prolongs survival in a mouse ALS model. Science 301:839–842. [DOI] [PubMed] [Google Scholar]
- 71. Kawaja MD, Rosenberg MB, Yoshida K, Gage FH (1992) Somatic gene transfer of nerve growth factor promotes the survival of axotomized septal neurons and the regeneration of their axons in adult rats. J Neurosci 12:2849–2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kells AP, Fong DM, Dragunow M, During MJ, Young D, Connor B (2004) AAV‐mediated gene delivery of BDNF or GDNF is neuroprotective in a model of Huntington disease. Mol Ther 9:682–688. [DOI] [PubMed] [Google Scholar]
- 73. Kirik D, Georgievska B, Bjorklund A (2004) Localized striatal delivery of GDNF as a treatment for Parkinson disease. Nat Neurosci 7:105–110. [DOI] [PubMed] [Google Scholar]
- 74. Klein RL, Muir D, King MA, Peel AL, Zolotukhin S, Möller JC, Krüttgen A, Heymach JV Jr, Muzyczka, N , Meyer EM (1999) Long‐term actions of vector‐derived nerve growth factor or brain‐derived neurotrophic factor on choline acetyltransferase and Trk receptor levels in the adult rat basal forebrain. Neuroscience 90:815–821. [DOI] [PubMed] [Google Scholar]
- 75. Klein RL, Hirko AC, Meyers CA, Grimes JR, Muzyczka N, Meyer EM (2000) NGF gene transfer to intrinsic basal forebrain neurons increases cholinergic cell size and protects from age‐related, spatial memory deficits in middle‐aged rats. Brain Res 875:144–151. [DOI] [PubMed] [Google Scholar]
- 76. Koliatsos VE, Nauta HJ, Clatterbuck RE, Holtzman DM, Mobley WC, Price DL (1990) Mouse nerve growth factor prevents degeneration of axotomized basal forebrain cholinergic neurons in the monkey. J Neurosci 10:3801–3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Koliatsos VE, Clatterbuck RE, Winslow JW, Cayouette MH, Price DL (1993) Evidence that brain‐derived neurotrophic factor is a trophic factor for motor neurons in vivo . Neuron 10:359–367. [DOI] [PubMed] [Google Scholar]
- 78. Koliatsos VE, Cayouette MH, Berkemeier LR, Clatterbuck RE, Price DL, Rosenthal A (1994) Neurotrophin 4/5 is a trophic factor for mammalian facial motor neurons. Proc Natl Acad Sci USA 91:3304–3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kordower JH, Winn SR, Liu YT, Mufson EJ, Sladek JR Jr, Hammang JP, Baetge EE, Emerich DF (1994) The aged monkey basal forebrain: rescue and sprouting of axotomized basal forebrain neurons after grafts of encapsulated cells secreting human nerve growth factor. Proc Natl Acad Sci USA 91:10898–10902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Kordower JH, Chen EY, Mufson EJ, Winn SR, Emerich DF (1996) Intrastriatal implants of polymer encapsulated cells genetically modified to secrete human nerve growth factor: trophic effects upon cholinergic and noncholinergic striatal neurons. Neuroscience 72:63–77. [DOI] [PubMed] [Google Scholar]
- 81. Kordower JH, Chen EY, Winkler C, Fricker R, Charles V, Messing A, Mufson EJ, Wong SC, Rosenstein JM, Björklund A, Emerich DF, Hammang J, Carpenter MK (1997) Grafts of EGF‐responsive neural stem cells derived from GFAP‐hNGF transgenic mice: trophic and tropic effects in a rodent model of Huntington’s disease. J Comp Neurol 387:96–113. [DOI] [PubMed] [Google Scholar]
- 82. Kordower JH, Isacson O, Emerich DF (1999) Cellular delivery of trophic factors for the treatment of Huntington’s disease: is neuroprotection possible? Exp Neurol 159:4–20. [DOI] [PubMed] [Google Scholar]
- 83. Kordower JH, Emborg ME, Bloch J, Ma SY, Chu Y, Leventhal L, McBride J, Chen E‐Y, Palfi S, Roitberg BZ, Brown WD, Holden JE, Pyzalski R, Taylor MD, Carvey P, Ling Z, Trono D, Hantraye P, Deglon N, Aebischer P (2000) Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson’s disease. Science 290:767–773. [DOI] [PubMed] [Google Scholar]
- 84. Korsching S, Auburger G, Heumann R, Scott J, Thoenen H (1985) Levels of nerve growth factor and its mRNA in the central nervous system of the rat correlate with cholinergic innervation. EMBO J 4:1389–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Kotzbauer PT, Lampe PA, Heuckeroth RO, Golden JP, Creedon DJ, Johnson EM Jr, Milbrandt J (1996) Neurturin, a relative of glial‐cell‐line‐derived neurotrophic factor. Nature 384:467–470. [DOI] [PubMed] [Google Scholar]
- 86. Krieglstein K, Suter‐Crazzolara C, Fischer WH, Unsicker K (1995) TGF‐beta superfamily members promote survival of midbrain dopaminergic neurons and protect them against MPP+ toxicity. EMBO J 14:736–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Kromer LF (1987) Nerve growth factor treatment after brain injury prevents neuronal death. Science 235:214–216. [DOI] [PubMed] [Google Scholar]
- 88. Krüttgen A, Schneider I, Weis J (2006) The dark side of the NGF family: neurotrophins in neoplasias. Brain Pathol 16:304–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Lang AE, Gill S, Patel NK, Lozano A, Nutt JG, Penn R, Brooks DJ, Hotton G, Moro E, Heywood P, Brodsky MA, Burchiel K, Kelly P, Dalvi A, Scott B, Stacy M, Turner D, Wooten VG, Elias WJ, Laws ER, Dhawan V, Stoessl AJ, Matcham J, Coffey RJ, Traub M (2006) Randomized controlled trial of intraputamenal glial cell line‐derived neurotrophic factor infusion in Parkinson disease. Ann Neurol 59:459–466. [DOI] [PubMed] [Google Scholar]
- 90. Lange DJ, Felice KJ, Festoff BW, Gawel MJ, Gelinas DF, Kratz R, Lai EC, Murphy MF, Natter HM, Norris FH, Rudnicki S (1996) Recombinant human insulin‐like growth factor‐I in ALS: description of a double‐blind, placebo‐controlled study. North American ALS/IGF‐I Study Group. Neurology 47:S94–S93; Discussion S94–S95. [DOI] [PubMed] [Google Scholar]
- 91. Lapchak PA, Araujo DM, Hilt DC, Sheng J, Jiao S (1997) Adenoviral vector‐mediated GDNF gene therapy in a rodent lesion model of late stage Parkinson’s disease. Brain Res 777:153–160. [DOI] [PubMed] [Google Scholar]
- 92. Levi‐Montalcini R (1987) The nerve growth factor 35 years later. Science 237:1154–1162. [DOI] [PubMed] [Google Scholar]
- 93. Li L, Wu W, Lin LF, Lei M, Oppenheim RW, Houenou LJ (1995) Rescue of adult mouse motoneurons from injury‐induced cell death by glial cell line‐derived neurotrophic factor. Proc Natl Acad Sci USA 92:9771–9775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F (1993) GDNF: a glial cell line‐derived neurotrophic factor for midbrain dopaminergic neurons. Science 260:1130–1132. [DOI] [PubMed] [Google Scholar]
- 95. Love S, Plaha P, Patel NK, Hotton GR, Brooks DJ, Gill SS (2005) Glial cell line‐derived neurotrophic factor induces neuronal sprouting in human brain. Nat Med 11:703–704. [DOI] [PubMed] [Google Scholar]
- 96. Mandel RJ, Spratt SK, Snyder RO, Leff SE (1997) Midbrain injection of recombinant adeno‐associated virus encoding rat glial cell line‐derived neurotrophic factor protects nigral neurons in a progressive 6‐hydroxydopamine‐induced degeneration model of Parkinson’s disease in rats. Proc Natl Acad Sci USA 94:14083–14088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Mandel RJ, Gage FH, Clevenger DG, Spratt SK, Snyder RO, Leff SE (1999) Nerve growth factor expressed in the medial septum following in vivo gene delivery using a recombinant adeno‐associated viral vector protects cholinergic neurons from fimbria‐fornix lesion‐induced degeneration. Exp Neurol 155:59–64. [DOI] [PubMed] [Google Scholar]
- 98. Mandel RJ, Snyder RO, Leff SE (1999) Recombinant adeno‐associated viral vector‐mediated glial cell line‐derived neurotrophic factor gene transfer protects nigral dopamine neurons after onset of progressive degeneration in a rat model of Parkinson’s disease. Exp Neurol 160:205–214. [DOI] [PubMed] [Google Scholar]
- 99. Markowska AL, Koliatsos VE, Breckler SJ, Price DL, Olton DS (1994) Human nerve growth factor improves spatial memory in aged but not in young rats. J Neurosci 14:4815–4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Markowska AL, Price D, Koliatsos VE (1996) Selective effects of nerve growth factor on spatial recent memory as assessed by a delayed nonmatching‐to‐position task in the water maze. J Neurosci 16:3541–3548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Martinez‐Serrano A, Fischer W, Bjorklund A (1995) Reversal of age‐dependent cognitive impairments and cholinergic neuron atrophy by NGF‐secreting neural progenitors grafted to the basal forebrain. Neuron 15:473–484. [DOI] [PubMed] [Google Scholar]
- 102. Martinez‐Serrano A, Lundberg C, Horellou P, Fischer W, Bentlage C, Campbell K, McKay RD, Mallet J, Bjorklund A (1995) CNS‐derived neural progenitor cells for gene transfer of nerve growth factor to the adult rat brain: complete rescue of axotomized cholinergic neurons after transplantation into the septum. J Neurosci 15:5668–5680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Martinez‐Serrano A, Björklund A (1996) Protection of the neostriatum against excitotoxic damage by neurotrophin‐producing, genetically modified neural stem cells. J Neurosci 16:4604–4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Martinez‐Serrano A, Fischer W, Soderstrom S, Ebendal T, Bjorklund A (1996) Long‐term functional recovery from age‐induced spatial memory impairments by nerve growth factor gene transfer to the rat basal forebrain. Proc Natl Acad Sci USA 93:6355–6360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Marx J (2005) Neurodegeneration. Huntington’s research points to possible new therapies. Science 310:43–45. [DOI] [PubMed] [Google Scholar]
- 106. McBride JL, During MJ, Wuu J, Chen EY, Leurgans SE, Kordower JH (2003) Structural and functional neuroprotection in a rat model of Huntington’s disease by viral gene transfer of GDNF. Exp Neurol 181:213–223. [DOI] [PubMed] [Google Scholar]
- 107. Milbrandt J, De Sauvage FJ, Fahrner TJ, Baloh RH, Leitner ML, Tansey MG, Lampe PA, Heuckeroth RO, Kotzbauer PT, Simburger KS, Golden JP, Davies JA, Vejsada R, Kato AC, Hynes M, Sherman D, Nishimura M, Wang LC, Vandlen R, Moffat B, Klein RD, Poulsen K, Gray C, Garces A, Johnson EM Jr (1998) Persephin, a novel neurotrophic factor related to GDNF and neurturin. Neuron 20:245–253. [DOI] [PubMed] [Google Scholar]
- 108. Miller RG, Petajan JH, Bryan WW, Armon C, Barohn RJ, Goodpasture JC, Hoagland RJ, Parry GJ, Ross MA, Stromatt SC (1996) A placebo‐controlled trial of recombinant human ciliary neurotrophic (rhCNTF) factor in amyotrophic lateral sclerosis. rhCNTF ALS Study Group. Ann Neurol 39:256–260. [DOI] [PubMed] [Google Scholar]
- 109. Mitsumoto H, Ikeda K, Holmlund T, Greene T, Cedarbaum JM, Wong V, Lindsay RM (1994) The effects of ciliary neurotrophic factor on motor dysfunction in wobbler mouse motor neuron disease. Ann Neurol 36:142–148. [DOI] [PubMed] [Google Scholar]
- 110. Mitsumoto H, Ikeda K, Klinkosz B, Cedarbaum JM, Wong V, Lindsay RM (1994) Arrest of motor neuron disease in wobbler mice cotreated with CNTF and BDNF. Science 265:1107–1110. [DOI] [PubMed] [Google Scholar]
- 111. Mittoux V, Joseph JM, Conde F, Palfi S, Dautry C, Poyot T, Bloch J, Deglon N, Ouary S, Nimchinsky EA, Brouillet E, Hof PR, Peschanski M, Aebischer P, Hantraye P (2000) Restoration of cognitive and motor functions by ciliary neurotrophic factor in a primate model of Huntington’s disease. Hum Gene Ther 11:1177–1187. [DOI] [PubMed] [Google Scholar]
- 112. Mittoux V, Ouary S, Monville C, Lisovoski F, Poyot T, Conde F, Escartin C, Robichon R, Brouillet E, Peschanski M, Hantraye P (2002) Corticostriatopallidal neuroprotection by adenovirus‐mediated ciliary neurotrophic factor gene transfer in a rat model of progressive striatal degeneration. J Neurosci 22:4478–4486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Mufson EJ, Conner JM, Kordower JH (1995) Nerve growth factor in Alzheimer’s disease: defective retrograde transport to nucleus basalis. Neuroreport 6:1063–1066. [DOI] [PubMed] [Google Scholar]
- 114. Mufson EJ, Ikonomovic MD, Styren SD, Counts SE, Wuu J, Leurgans S, Bennett DA, Cochran EJ, DeKosky ST (2003) Preservation of brain nerve growth factor in mild cognitive impairment and Alzheimer disease. Arch Neurol 60:1143–1148. [DOI] [PubMed] [Google Scholar]
- 115. Nutt JG, Burchiel KJ, Comella CL, Jankovic J, Lang AE, Laws ER Jr, Lozano AM, Penn RD, Simpson RK Jr, Stacy M, Wooten GF (2003) Randomized, double‐blind trial of glial cell line‐derived neurotrophic factor (GDNF) in PD. Neurology 60:69–73. [DOI] [PubMed] [Google Scholar]
- 116. Olson L, Nordberg A, Von Holst H, Backman L, Ebendal T, Alafuzoff I, Amberla K, Hartvig P, Herlitz A, Lilja A, Lundqvist H, Langström B, Meyerson B, Persson A, Vittanen M, Winblad B, Seiger A (1992) Nerve growth factor affects 11C‐nicotine binding, blood flow, EEG, and verbal episodic memory in an Alzheimer patient (case report). J Neural Transm Park Dis Dement Sect 4:79–95. [DOI] [PubMed] [Google Scholar]
- 117. Oppenheim RW, Yin QW, Prevette D, Yan Q (1992) Brain‐derived neurotrophic factor rescues developing avian motoneurons from cell death. Nature 360:755–757. [DOI] [PubMed] [Google Scholar]
- 118. Oppenheim RW, Houenou LJ, Johnson JE, Lin LF, Li L, Lo AC, Newsome AL, Prevette DM, Wang S (1995) Developing motor neurons rescued from programmed and axotomy‐induced cell death by GDNF. Nature 373:344–346. [DOI] [PubMed] [Google Scholar]
- 119. Patel NK, Bunnage M, Plaha P, Svendsen CN, Heywood P, Gill SS (2005) Intraputamenal infusion of glial cell line‐derived neurotrophic factor in PD: a two‐year outcome study. Ann Neurol 57:298–302. [DOI] [PubMed] [Google Scholar]
- 120. Pérez‐Navarro E, Arenas E, Reiriz J, Calvo N, Alberch J (1996) Glial cell line‐derived neurotrophic factor protects striatal calbindin‐immunoreactive neurons from excitotoxic damage. Neuroscience 75:345–352. [DOI] [PubMed] [Google Scholar]
- 121. Perez‐Navarro E, Canudas AM, Akerund P, Alberch J, Arenas E (2000) Brain‐derived neurotrophic factor, neurotrophin‐3, and neurotrophin‐4/5 prevent the death of striatal projection neurons in a rodent model of Huntington’s disease. J Neurochem 75:2190–2199. [DOI] [PubMed] [Google Scholar]
- 122. Perry EK, Perry RH, Blessed G, Tomlinson BE (1977) Necropsy evidence of central cholinergic deficits in senile dementia. Lancet 1:189. [DOI] [PubMed] [Google Scholar]
- 123. Perry EK, Tomlinson BE, Blessed G, Bergmann K, Gibson PH, Perry RH (1978) Correlation of cholinergic abnormalities with senile plaques and mental test scores in senile dementia. Br Med J 2:1457–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Popovic N, Maingay M, Kirik D, Brundin P (2005) Lentiviral gene delivery of GDNF into the striatum of R6/2 Huntington mice fails to attenuate behavioral and neuropathological changes. Exp Neurol 193:65–74. [DOI] [PubMed] [Google Scholar]
- 125. Regulier E, Pereira de Almeida L, Sommer B, Aebischer P, Deglon N (2002) Dose‐dependent neuroprotective effect of ciliary neurotrophic factor delivered via tetracycline‐regulated lentiviral vectors in the quinolinic acid rat model of Huntington’s disease. Hum Gene Ther 13:1981–1990. [DOI] [PubMed] [Google Scholar]
- 126. Rosenberg MB, Friedmann T, Robertson RC, Tuszynski M, Wolff JA, Breakefield XO, Gage FH (1988) Grafting genetically modified cells to the damaged brain: restorative effects of NGF expression. Science 242:1575–1578. [DOI] [PubMed] [Google Scholar]
- 127. Rosenberg SS, Ng BK, Chan JR (2006) The quest for remyelination: a new role for neurotrophins and their receptors. Brain Pathol 16:288–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Rosenblad C, Kirik D, Devaux B, Moffat B, Phillips HS, Björklund A (1999) Protection and regeneration of nigral dopaminergic neurons by neurturin or GDNF in a partial lesion model of Parkinson’s disease after administration into the striatum or the lateral ventricle. Eur J Neurosci 11:1554–1566. [DOI] [PubMed] [Google Scholar]
- 129. Saffran BN, Woo JE, Mobley WC, Crutcher KA (1989) Intraventricular NGF infusion in the mature rat brain enhances sympathetic innervation of cerebrovascular targets but fails to elicit sympathetic ingrowth. Brain Res 492:245–254. [DOI] [PubMed] [Google Scholar]
- 130. Sahenk Z (2006) Neurotrophins and peripheral neuropathies. Brain Pathol 16:311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Salehi A, Delcroix JD, Mobley WC (2003) Traffic at the intersection of neurotrophic factor signaling and neurodegeneration. Trends Neurosci 26:73–80. [DOI] [PubMed] [Google Scholar]
- 132. Salehi A, Delcroix JD, Belichenko PV, Zhan K, Wu C, Valletta JS, Takimoto‐Kimura R, Kleschevnikov AM, Sambamurti K, Chung PP, Xia W, Villar A, Campbell WA, Kulnane LS, Nixon RA, Lamb BT, Epstein CJ, Stokin GB, Goldstein LS, Mobley WC (2006) Increased App expression in a mouse model of Down’s syndrome disrupts NGF transport and causes cholinergic neuron degeneration. Neuron 51:29–42. [DOI] [PubMed] [Google Scholar]
- 133. Scott SA, Mufson EJ, Weingartner JA, Skau KA, Crutcher KA (1995) Nerve growth factor in Alzheimer’s disease: increased levels throughout the brain coupled with declines in nucleus basalis. J Neurosci 15:6213–6221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Sendtner M, Kreutzberg GW, Thoenen H (1990) Ciliary neurotrophic factor prevents the degeneration of motor neurons after axotomy. Nature 345:440–441. [DOI] [PubMed] [Google Scholar]
- 135. Sendtner M, Holtmann B, Kolbeck R, Thoenen H, Barde YA (1992) Brain‐derived neurotrophic factor prevents the death of motoneurons in newborn rats after nerve section. Nature 360:757–759. [DOI] [PubMed] [Google Scholar]
- 136. Sendtner M, Schmalbruch H, Stöckli KA, Carroll P, Kreutzberg GW, Thoenen H (1992) Ciliary neurotrophic factor prevents degeneration of motor neurons in mouse mutant progressive motor neuronopathy. Nature 358:502–504. [DOI] [PubMed] [Google Scholar]
- 137. Shelton DL, Reichardt LF (1986) Studies on the expression of the beta nerve growth factor (NGF) gene in the central nervous system: level and regional distribution of NGF mRNA suggest that NGF functions as a trophic factor for several distinct populations of neurons. Proc Natl Acad Sci USA 83:2714–2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Slevin JT, Gerhardt GA, Smith CD, Gash DM, Kryscio R, Young B (2005) Improvement of bilateral motor functions in patients with Parkinson disease through the unilateral intraputaminal infusion of glial cell line‐derived neurotrophic factor. J Neurosurg 102:216–222. [DOI] [PubMed] [Google Scholar]
- 139. Smith DE, Roberts J, Gage FH, Tuszynski MH (1999) Age‐associated neuronal atrophy occurs in the primate brain and is reversible by growth factor gene therapy. Proc Natl Acad Sci USA 96:10893–10898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. The ALS CNTF Treatment Study (ACTS) Phase I‐II Study Group (1995) A phase I study of recombinant human ciliary neurotrophic factor (rHCNTF) in patients with amyotrophic lateral sclerosis. Clin Neuropharmacol 18:515–532. [DOI] [PubMed] [Google Scholar]
- 141. The BDNF Study Group (Phase III) (1999) A controlled trial of recombinant methionyl human BDNF in ALS: the BDNF Study Group (Phase III). Neurology 52:1427–1433. [DOI] [PubMed] [Google Scholar]
- 142. The Huntington’s Disease Collaborative Research Group (1993) A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. The Huntington’s Disease Collaborative Research Group. Cell 72:971–983. [DOI] [PubMed] [Google Scholar]
- 143. Tomac A, Lindqvist E, Lin LF, Ogren SO, Young D, Hoffer BJ, Olson L (1995) Protection and repair of the nigrostriatal dopaminergic system by GDNF in vivo . Nature 373:335–339. [DOI] [PubMed] [Google Scholar]
- 144. Tseng JL, Baetge EE, Zurn AD, Aebischer P (1997) GDNF reduces drug‐induced rotational behavior after medial forebrain bundle transection by a mechanism not involving striatal dopamine. J Neurosci 17:325–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Tuszynski MH, U HS, Amaral DG, Gage FH (1990) Nerve growth factor infusion in the primate brain reduces lesion‐induced cholinergic neuronal degeneration. J Neurosci 10:3604–3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Tuszynski MH, Sang H, Yoshida K, Gage FH (1991) Recombinant human nerve growth factor infusions prevent cholinergic neuronal degeneration in the adult primate brain. Ann Neurol 30:625–636. [DOI] [PubMed] [Google Scholar]
- 147. Tuszynski MH, Senut MC, Ray J, Roberts J (1994) Somatic gene transfer to the adult primate central nervous system: in vitro and in vivo characterization of cells genetically modified to secrete nerve growth factor. Neurobiol Dis 1:67–78. [DOI] [PubMed] [Google Scholar]
- 148. Tuszynski MH, Roberts J, Senut MC, U HS, Gage FH (1996) Gene therapy in the adult primate brain: intraparenchymal grafts of cells genetically modified to produce nerve growth factor prevent cholinergic neuronal degeneration. Gene Ther 3:305–314. [PubMed] [Google Scholar]
- 149. Tuszynski MH, Thal L, Pay M, Salmon DP, U HS, Bakay R, Patel P, Blesch A, Vahlsing HL, Ho G, Tong G, Potkin SG, Fallon J, Hansen L, Mufson EJ, Kordower JH, Gall C, Conner J (2005) A phase 1 clinical trial of nerve growth factor gene therapy for Alzheimer disease. Nat Med 11:551–555. [DOI] [PubMed] [Google Scholar]
- 150. Twiss JL, Chang JH, Schanen NC (2006) Pathopysiological mechanisms for actions of neurotrophins. Brain Pathol 16:320–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Venero JL, Beck KD, Hefti F (1994) Intrastriatal infusion of nerve growth factor after quinolinic acid prevents reduction of cellular expression of choline acetyltransferase messenger RNA and trkA messenger RNA, but not glutamate decarboxylase messenger RNA. Neuroscience 61:257–268. [DOI] [PubMed] [Google Scholar]
- 152. Volpe BT, Wildmann J, Altar CA (1998) Brain‐derived neurotrophic factor prevents the loss of nigral neurons induced by excitotoxic striatal‐pallidal lesions. Neuroscience 83:741–748. [DOI] [PubMed] [Google Scholar]
- 153. Wang LJ, Lu YY, Muramatsu S, Ikeguchi K, Fujimoto K, Okada T, Mizukami H, Matsushita T, Hanazono Y, Kume A, Nagatsu T, Ozawa K, Nakano I (2002) Neuroprotective effects of glial cell line‐derived neurotrophic factor mediated by an adeno‐associated virus vector in a transgenic animal model of amyotrophic lateral sclerosis. J Neurosci 22:6920–6928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Whitehouse PJ, Price DL, Clark AW, Coyle JT, DeLong MR (1981) Alzheimer disease: evidence for selective loss of cholinergic neurons in the nucleus basalis. Ann Neurol 10:122–126. [DOI] [PubMed] [Google Scholar]
- 155. Whitehouse PJ, Price DL, Struble RG, Clark AW, Coyle JT, Delon MR (1982) Alzheimer’s disease and senile dementia: loss of neurons in the basal forebrain. Science 215:1237–1239. [DOI] [PubMed] [Google Scholar]
- 156. Williams LR (1991) Hypophagia is induced by intracerebroventricular administration of nerve growth factor. Exp Neurol 113:31–37. [DOI] [PubMed] [Google Scholar]
- 157. Williams LR, Varon S, Peterson GM, Wictorin K, Fischer W, Bjorklund A, Gage FH (1986) Continuous infusion of nerve growth factor prevents basal forebrain neuronal death after fimbria fornix transection. Proc Natl Acad Sci USA 83:9231–9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Winkler J, Ramirez GA, Kuhn HG, Peterson DA, Day‐Lollini PA, Stewart GR, Tuszynski MH, Gage FH, Thal LJ (1997) Reversible Schwann cell hyperplasia and sprouting of sensory and sympathetic neurites after intraventricular administration of nerve growth factor. Ann Neurol 41:82–93. [DOI] [PubMed] [Google Scholar]
- 159. Wu K, Meyers CA, Guerra NK, King MA, Meyer EM (2004) The effects of rAAV2‐mediated NGF gene delivery in adult and aged rats. Mol Ther 9:262–269. [DOI] [PubMed] [Google Scholar]
- 160. Wu K, Meyer EM, Bennett JA, Meyers CA, Hughes JA, King MA (2005) AAV2/5‐mediated NGF gene delivery protects septal cholinergic neurons following axotomy. Brain Res 1061:107–113. [DOI] [PubMed] [Google Scholar]
- 161. Yan Q, Elliott J, Snider WD (1992) Brain‐derived neurotrophic factor rescues spinal motor neurons from axotomy‐induced cell death. Nature 360:753–755. [DOI] [PubMed] [Google Scholar]
- 162. Yan Q, Elliott JL, Matheson C, Sun J, Zhang L, Mu X, Rex KL, Snider WD (1993) Influences of neurotrophins on mammalian motoneurons in vivo . J Neurobiol 24:1555–1577. [DOI] [PubMed] [Google Scholar]
- 163. Yan Q, Matheson C, Lopez OT (1995) In vivo neurotrophic effects of GDNF on neonatal and adult facial motor neurons. Nature 373:341–344. [DOI] [PubMed] [Google Scholar]
- 164. Zuccato C, Ciammola A, Rigamonti D, Leavitt BR, Goffredo D, Conti L, MacDonald ME, Friedlander RM, Silani V, Hayden MR, Timmusk T, Sipione S, Cattaneo E (2001) Loss of huntingtin‐mediated BDNF gene transcription in Huntington’s disease. Science 293:493–498. [DOI] [PubMed] [Google Scholar]
- 165. Zuccato C, Tartari M, Crotti A, Goffredo D, Valenza M, Conti L, Cataudella T, Leavitt BR, Hayden MR, Timmusk T, Rigamonti D, Cattaneo E (2003) Huntingtin interacts with REST/NRSF to modulate the transcription of NRSE‐controlled neuronal genes. Nat Genet 35:76–83. [DOI] [PubMed] [Google Scholar]


