Calcific aortic valve disease (CAVD) has taken center stage in recent years as an ever-growing public health epidemic. Calcification of the aortic valves, driven by endothelial dysfunction, ossification of valvular interstitial cells and immune infiltration is a slow and progressive pathology, notably the most common valvular heart disease, with a prevalence of about 2% in the aging population [1]. CAVD, characterized by the overall thickening of the valve leaflets and the increased prominence of fibrosis and calcium-rich nodules results in aortic stiffening and stenosis, eventually leading to myocardial dysfunction and heart failure [2]. While management of CAVD has improved significantly over the last sixty years, most notably with the switch to Doppler echocardiography from cardiac catheterization as the chosen method of diagnosis and follow-up, limited treatments exist, with symptomatic patients often undergoing either surgical or transcatheter valve replacements, both of which are associated with a significant morbidity/mortality rates and high cost [2]. Strikingly, although there has been a deluge of research papers focusing on the molecular signaling processes and mechanisms surrounding valvular calcification, progressing the belief that CAVD is an active-cellular driven disease as opposed to passive, degenerative disease, little in the way of pharmacological intervention has been developed, leaving patients often facing long waits and delays in intervention until the late stages of CAVD and thus resulting in a reduced quality of life in the interim.
In this issue of Circulation Research, Dutta et al [3] present an exciting pharmacological alternative for the treatment of CAVD, via repurposing a selective Exportin-1 inhibitor KTP-330 (Selinexor). Exportin-1 (XPO1), also known as Chromosome region maintenance 1, plays a crucial role in maintaining cellular homeostasis through its role as a transport protein, shuttling of over 200 proteins, including tumor suppressor proteins p53 and p27, from the nucleus to the cytoplasm. Interestingly, dysregulation of XPO1 plays an essential role in the development of various solid and hematological malignancies and is associated with resistance to several chemotherapies, making it a key druggable target for the oncology field [4], and here eloquently repurposed in the context of calcification. Previous work from the Lincoln lab has demonstrated the possible attenuation of calcification through an export inhibitor compound [5], however this is the labs first foray into repurposing of a Food and Drug Administration (FDA) approved drug for the modulation of calcification. The authors present a compelling argument for XPO1 inhibition to mitigate calcific nodule formation in both porcine and human valvular interstitial cells (VICs) and furthermore in prevention of CAVD development in Klotho-deficient mice. Through multi-omics analysis Dutta et al explore the beneficial role of inhibiting C/EBPβ in VICs, resulting in the repression of canonical Wnt signaling through activation of Axin1, subsequently reducing cell viability and pro-osteogenic markers.
Perhaps the most remarkable finding within this comprehensive study, is the way in which KPT-330 not only attenuates but also mitigates calcification nodule formation once formed within the valvular cells. Consequently, suggesting that KTP-330 could be used following the development of calcification. Therefore negating the need for a pre-emptive long-term treatment, which is known to have low levels of patient compliance and can incur detrimental outcomes for the patient, as seen by the recent statin SAMSON trial [6]. Porcine VICs, which were utilized for this study, were characterized into three categories, non-calcified (stage I), pre-calcific (stage II) and calcified (stage III) and their temporal differential transcriptomes analyzed. As expected, these profiles were unique at each stage, clearly emphasizing the difference in molecular signatures as these cells undergo osteogenesis. The authors then demonstrated that treatment with KPT-330 prior to the induction of calcification prevents calcific nodule formation. Once the control (dimethyl sulfoxide) treated cells reached pre-calcified stage II, they were then switched to treatment with KTP-330, which attenuated calcific nodule formation, highlighting the way XPO1 inhibition could arrest the molecular mechanism already in action mid-way through the development of calcification. Most interestingly, however, was the treatment of porcine VICs at calcified stage III, once the calcified nodules were already developed. Following 72 hours of KPT-330 treatment existing calcific nodules were mitigated, with a clear reduction in alizarin red staining, suggesting short term treatment of KPT-330 could acutely reduce calcification. Moreover, the expression of pro-osteogenic markers, characterized through transcriptomics, was reduced. Intriguingly however, neither the transcriptomic profile or proteome of calcified stage III KTP-330 treated cells aligned with that of non-calcified stage I or pre-calcified stage II, possibly suggesting a new post-calcification, quiescent phenotype the authors have yet to fully elucidate. Nevertheless, the authors present an undeniable argument that KTP-330 is a promising drug from the resolution of existing calcification nodules, promoting a shift to a mesoderm profile in these cells. Mechanistically, XPO1’s downstream target, C/EBPβ is increased within the nucleus following KTP-330 treatment, leading to decreased Wnt activity associated with the notable increase in Axin1 and the attenuation of calcification. Through increased interaction of Axin1 with β-catenin, the authors speculate that the reduction of β-catenin phosphorylation targets it for degradation, decreasing canonical Wnt signaling and reducing pro-osteogenic signaling cascades. Overall, the authors clearly demonstrates that pharmacological inhibition of XPO1 during any stage of mineral development can attenuate and even regress calcification in vitro and within the Klotho mouse model, providing critical rationale for developing more specific nuclear export molecules.
This manuscript, however, brings up a wider question in the field of drug repurposing. From discovery and development of a drug candidate to post-market monitoring following FDA approval can take up to 15-20 years and cost upwards of $2 billion dollars [7] (Figure 1). This cost is often passed on to the patient, clearly seen through the increasingly high health insurance premiums and the disturbing statistic that nearly 1 in 4 Americans struggle to afford prescribed medicines [8]. This begs the questions why more pharmaceutical companies do not opt to repurpose already approved FDA drugs, negating the need to undergo expensive and complex drug development phases and clinical development?
Figure 1. Industry-academia collaboration for drug repurposing.
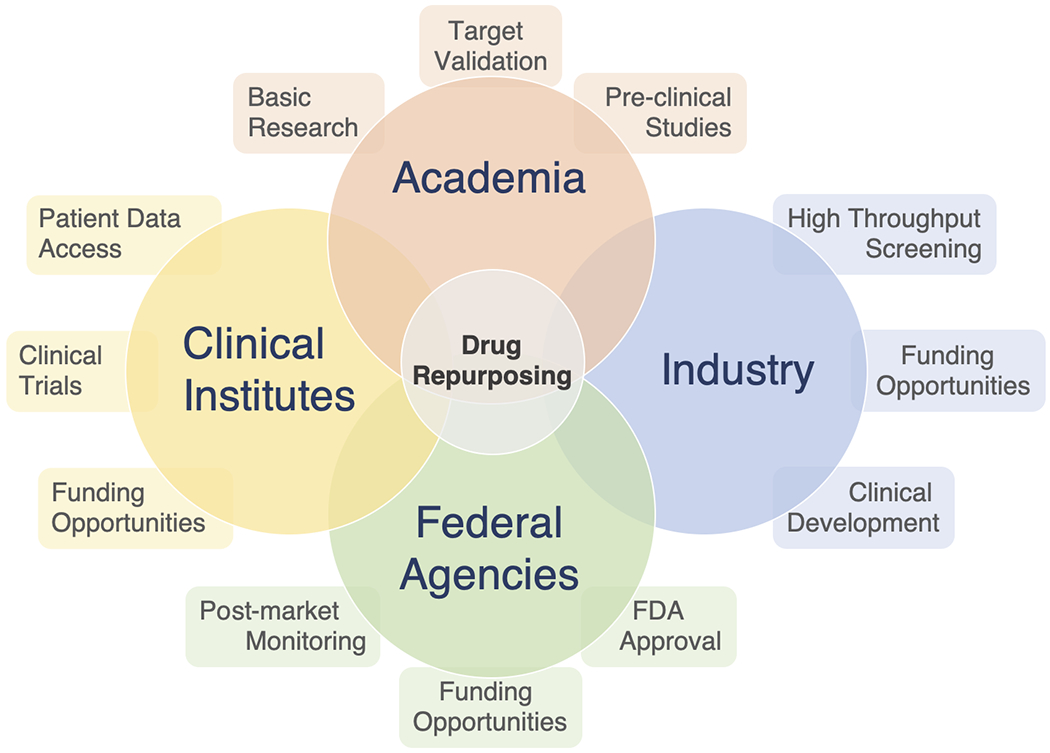
Collaborative relations between, academia, industry, federal agencies and clinical institutes are required to effectively repurpose drugs.
With the recent surge of biomedical research, genetic data and the move towards embracing big data as a key source in patient information [9], the shift from the single to multi-target paradigm in drug discovery to find novel indications for existing compounds has provided a promising alternative to overcome drug development bottlenecks, allowing approved drugs to be used off-label at a faster rate. This, coupled with the explosion of network-based methods [10] used to elucidate the heterogenous drug-disease relationship for more complex diseases; evidenced by more than 4000 publications on ‘drug repositioning’ in the last decade on PubMed, clearly demonstrates the push towards drug repurposing within the scientific community. What is more, drug repurposing has been shown to work, most notably in the cardiovascular field with Canakinumab [11] and more recently Colchicine [12], both originally anti-inflammatory drugs, which significantly lowered the risk of cardiovascular events compared to those who received the placebo.
If academia can demonstrate the effectiveness of repurposing a drug in vitro or in pre-clinical animal studies through mechanistic insight, then this would bypass the need to conduct expensive clinical trials looking at the tolerability of these drugs in humans. Moreover, with the ability of academia to unbiasedly assess previously detrimental compounds, such as in the case of cyclic retinoids vs acyclic retinoids [13] and determine new treatment avenues through more selective compounds, the adverse effects associated with the compounds predecessors can be negated. Therefore, drugs which were once ruled out for treatment due to unfavorable outcomes may now be offered as treatment for pathologies that have no pharmaceutical intervention currently on the market, much like in calcific aortic valve disease. Of note, transport protein inhibition, such as the work presented here by Dutta et al. has long been discussed as a therapeutic option of pathological cell conditions [14]. It is therefore feasible, that through the precise switching of transcriptional profiles within calcified VICs that compounds such as KTP-330 may indeed be the meticulous and controlled treatment required for to address this old pathology. It is worth noting however, the recent overwhelming failures of repurposing drugs in the current coronavirus pandemic. Most notably, resdesivir showed no benefit in treatment for COVID-19 [15], highlighting the high-risk nature of drug repurposing. It must be mentioned, however, that resdesivir did not come through the usual channels of drug repurposing, which consists or large database screens and high-throughput testing. Rather, it was suggested due to its ability to inhibit RNA-dependent polymerases, such as the those found in SARS-Cov-2 and should therefore not be used as the exemplary for drug repurposing.
Perhaps the crucial part missing in the complex mosaic of drug repurposing in the symbiotic relationship between academia and big pharma, giving academic researchers the opportunity to explore multiple compounds without the pressures of patents and patient welfare hanging over them. Only then, with the willingness and full economic power of big pharma behind academia, will translational research be able to push through repurposed drugs to the benefit of the patient’s health.
Acknowledgments
Sources of Funding
E.A. lab is supported by NIH grants R01 HL136431, R01 HL141917 and R01 HL147095.
Footnotes
Conflict of Interest
None declared.
References
- [1].Nkomo VT, Gardin JM, Skelton TN, Gottdiener J, Scott C, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. 368. 1005–1010. 2006 [DOI] [PubMed] [Google Scholar]
- [2].Brescia AA, Deeb GM, Sang SLW, Tanaka D, Grossman PM, Sukul D, He C, Theurer PF, Clark M. et al. Surgical Explantation of Transcatheter Aortic Valve Bioprostheses: A Statewide Experience. Circ Cardiovasc Interv. Online ahead of print. 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Dutta P, Kodigepalli KM, LaHaye S, Thompson W, Rains S, Nagel C, Thatcher K, Hinton R, Lincoln J. KPT-330 Prevents Aortic Valve Calcification via a Novel C/EBPβ Signalling Pathway. Circ Res. 2021; 128: xx-xxx. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Azmi AS, Uddin MH, Mohammad RM. The nuclear export protein XPO1-From biology to targeted therapy. Nat Rev Clin Oncol. 18. 152–169. 2021 [DOI] [PubMed] [Google Scholar]
- [5].Huk DK, Austin B, Horne TE, Hinton RB, Ray WC, Heistad DD, Lincoln J. Valve Endothelial Cell-Derived TGFb1 Signaling Promotes Nuclear Localization of Sox9 in Interstitial Cells Associated with Attenuated Calcification. ATVB. 36. 328–338. 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wood FA, Howard JP, Finegold JA, Nowbar AN, Thompson DM, Arnold AD Rajkumar CA. Connolly S. Cegla J. et al. N-of-1 trial of a statin, placebo, or no treatment to assess side effects. NEJM. 383. 2182–2184. 2020 [DOI] [PubMed] [Google Scholar]
- [7].Wouters OJ, McKee M, Luyten J. Estimated Research and development Investment Needed to Bring a New Medicine to Market, 2009-2018. JAMA. 323. 884–853. 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kirzinger A US Poll on costs of Prescription Drugs. PharmacoEconomics & Outcomes News. 833. 34–39. 2019 [Google Scholar]
- [9].Schlotter F, Halu A, Goto S, Blaser M, Body SC, Lee LH, Higashi H, DeLaughter DM, Hutcheson JD. et al. Spatiotemporal Multi-Omics Mapping Generates a Molecular Atlas of the Aortic Valve and Reveals Networks Driving Disease. Circulation. 138. 377–393. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hopkins AL. Network pharmacology: the next paradigm in drug discovery. Nat Chem Biol. 4. 682–690. 2008. [DOI] [PubMed] [Google Scholar]
- [11].Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W. et al. Anti-inflammatory therapy with canakinumab for atherosclerotic disease. NEJM. 377. 1119–1131. 2017. [DOI] [PubMed] [Google Scholar]
- [12].Nidorf SM, Fiolet ATL, Mosterd A, Eikelboom JW, Schut A, Opstal TSJ, The SHK, Xu XF, Ireland MA. et al. Colchicine in Patients with Chronic Coronary Disease. NEJM. 383. 1938–1847. 2020. [DOI] [PubMed] [Google Scholar]
- [13].Rogers MA, Chen J, Nallamshetty S, Pham T, Goto S, Muehischlegel J, Libby P, Aikawa M, Aikawa E, Plutzky J. Retinoids Repress Human Cardiovascular Cell Calcification with Evidence for Distinct Selective Retinoid Modulator Effects. ATVB. 40. 656–669. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Mirna NC, Pierce GN. Therapeutic Targeting of Nuclear Protein Import in Pathological Cell Conditions. Pharm Rev. 61. 358–372. 2009. [DOI] [PubMed] [Google Scholar]
- [15].Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, Fu S, Gao L, Cheng Z. et al. Remdesivir in adults with severe COVID-19: A randomized, double-bind, placebo-controlled, multicentre trial. Lancet. 395. 1569–1578. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]


